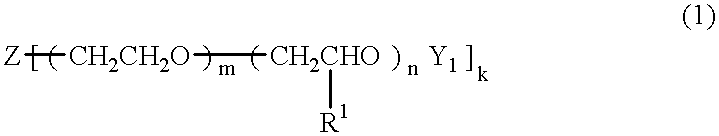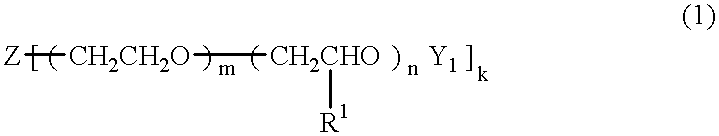Patents
Literature
35results about How to "Low self-discharge" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Electromechanical pricking aid for taking liquid samples
InactiveUS20080015623A1Easy to operateSafer designDiagnostic recording/measuringSensorsEngineeringPhysical limitations
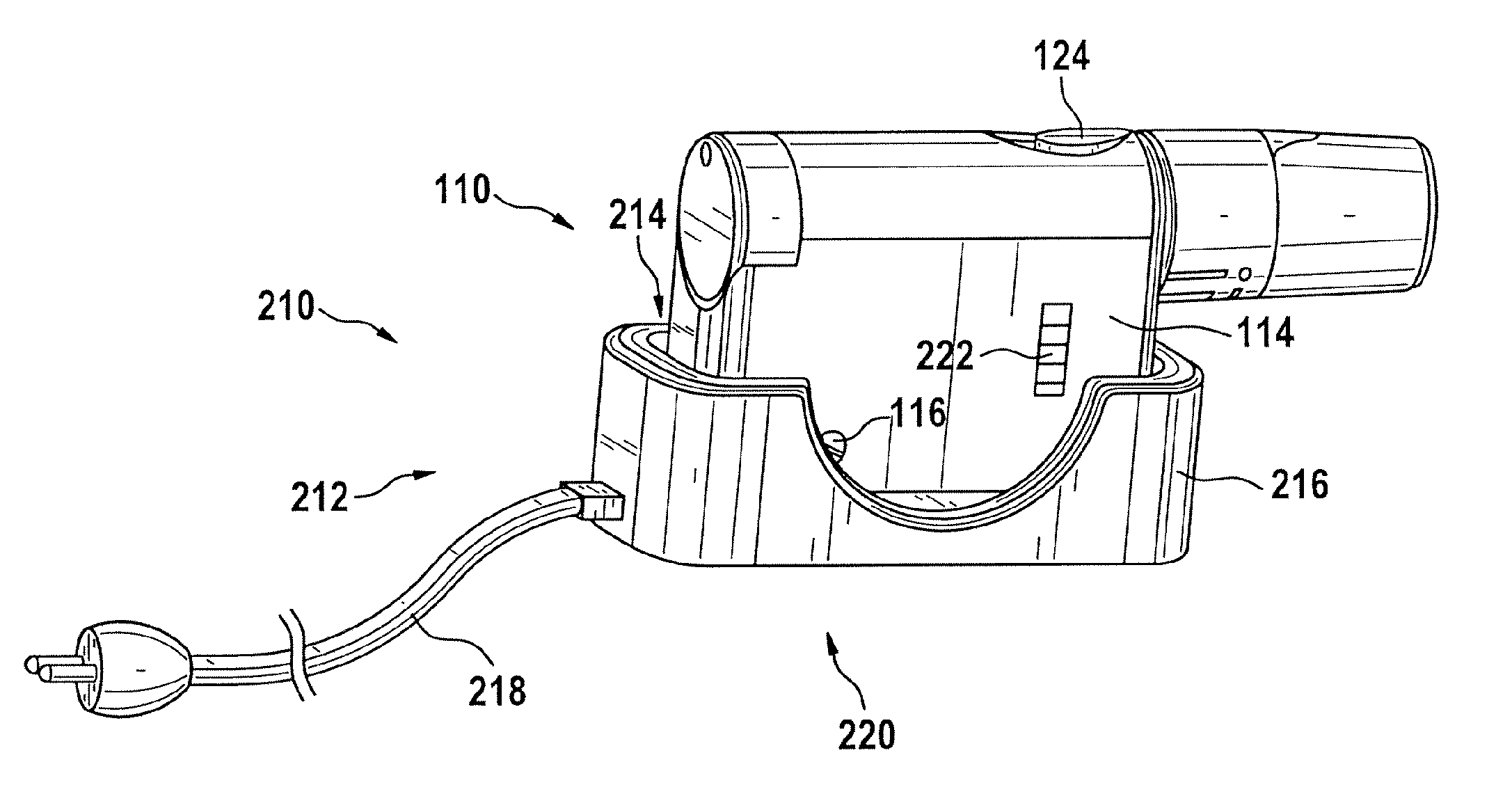
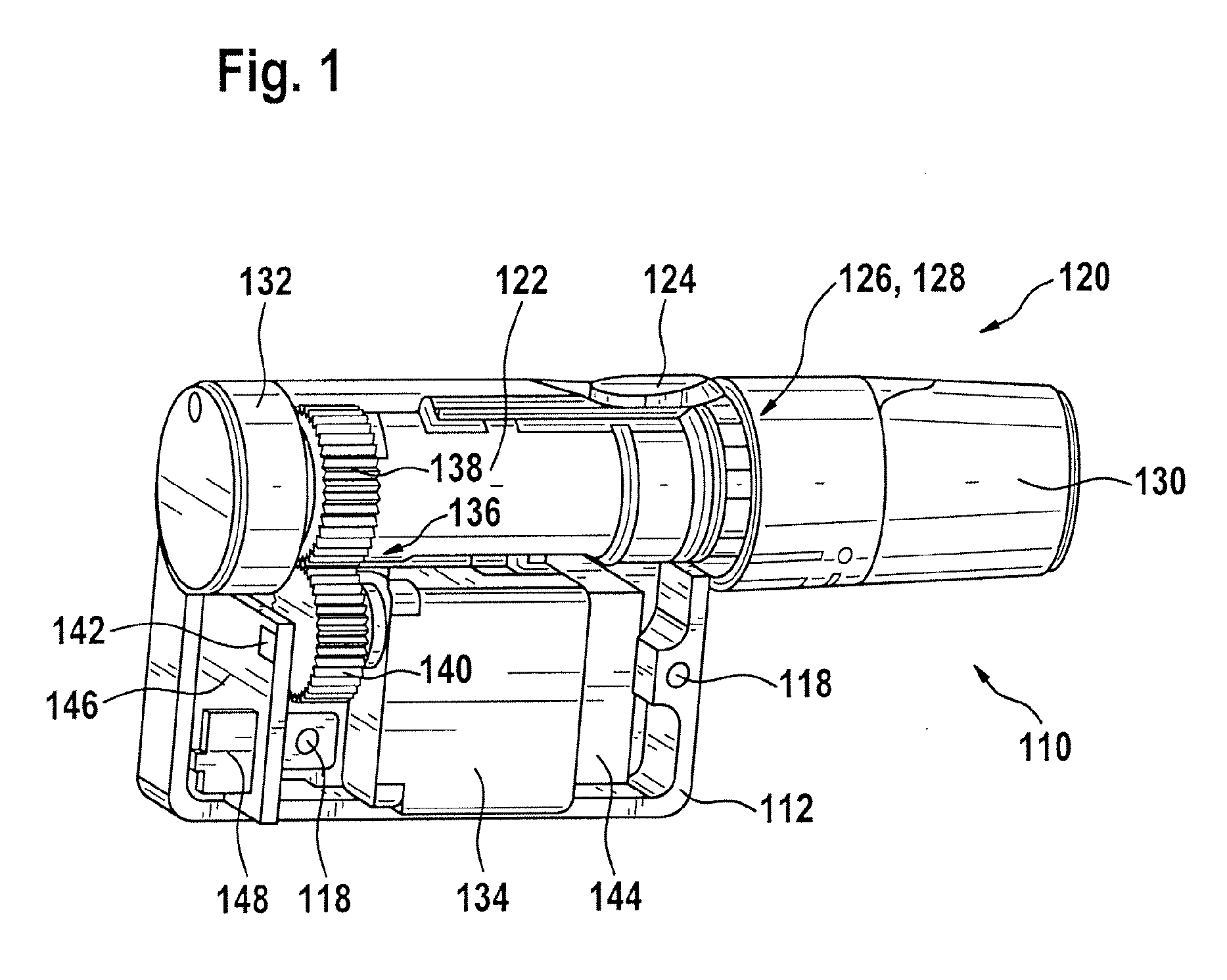
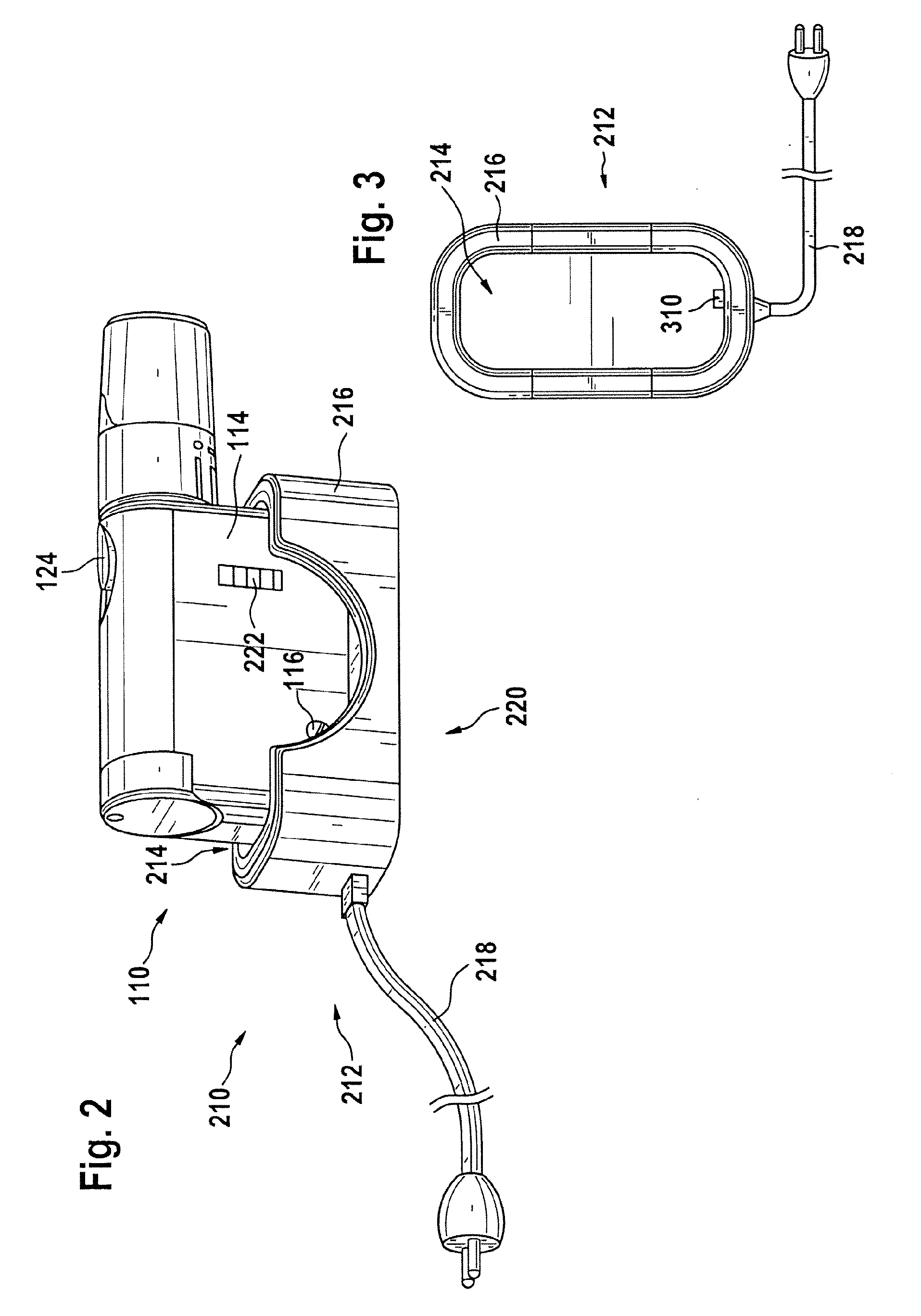
A portable lancing aid for providing liquid samples comprises a lancet system having at least one lancet, a tensioning device, and an electromechanical actuator. The tensioning device can be tensioned by the electromechanical actuator. The portable lancing aid may further include an energy source for storing electrical energy that is connected to the electromechanical actuator. Additionally, the portable lancing aid may include an interface for charging the energy source where the interface is externally accessible from the lancet system. The invention is ergonomical and easy to handle for children and patients with physical limitations. Furthermore, a lancing system for collecting liquid samples is provided with a portable lancing aid that is detachably mountable to a charging station for charging the portable lancing aid.
Owner:ROCHE DIABETES CARE INC
Novel enhanced electrochemical cells with solid-electrolyte interphase promoters
InactiveUS20060154144A1Improve protectionGood flexibilityAlkaline accumulatorsOrganic electrolyte cellsLithium metalCarbonate
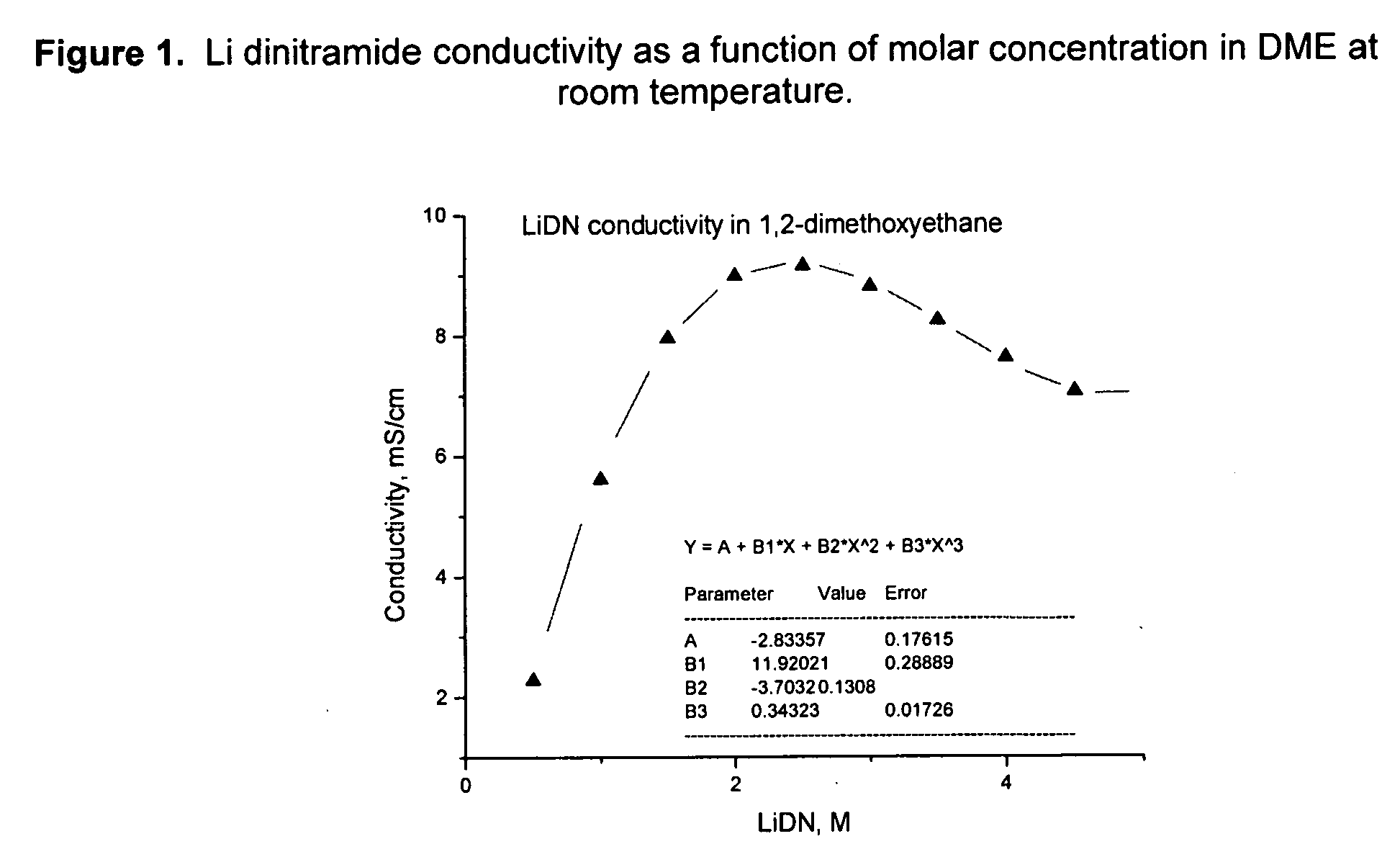
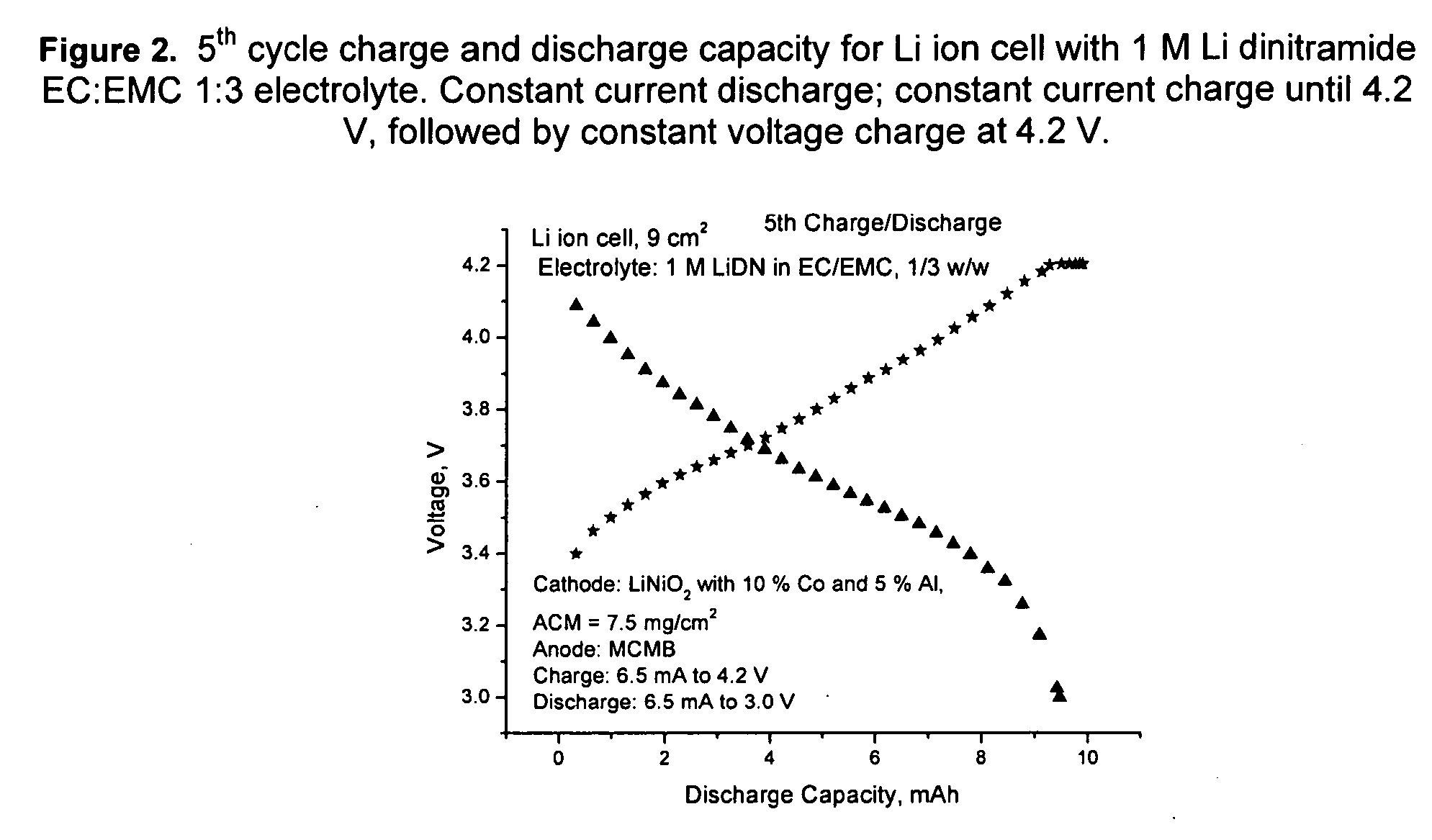
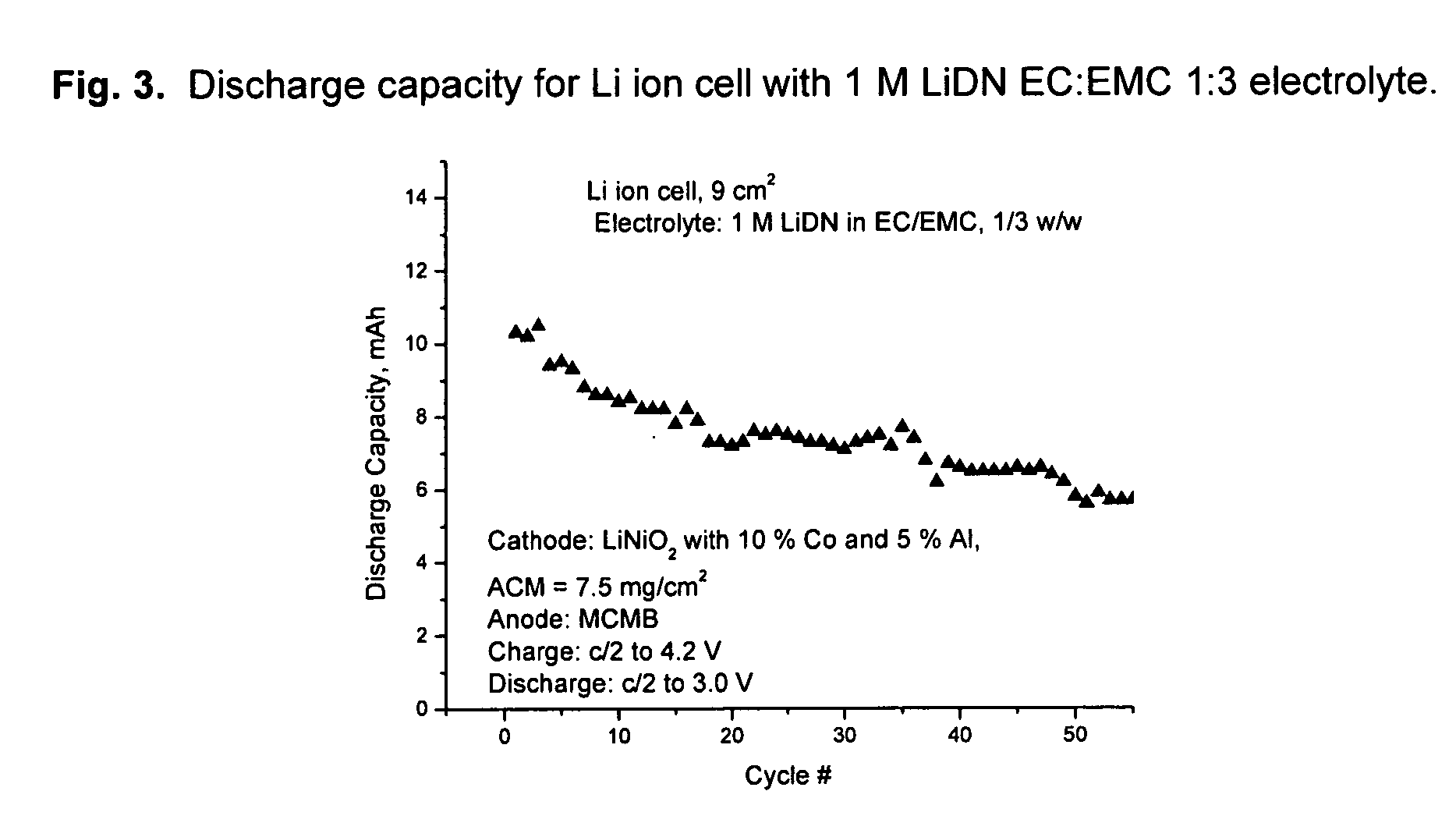
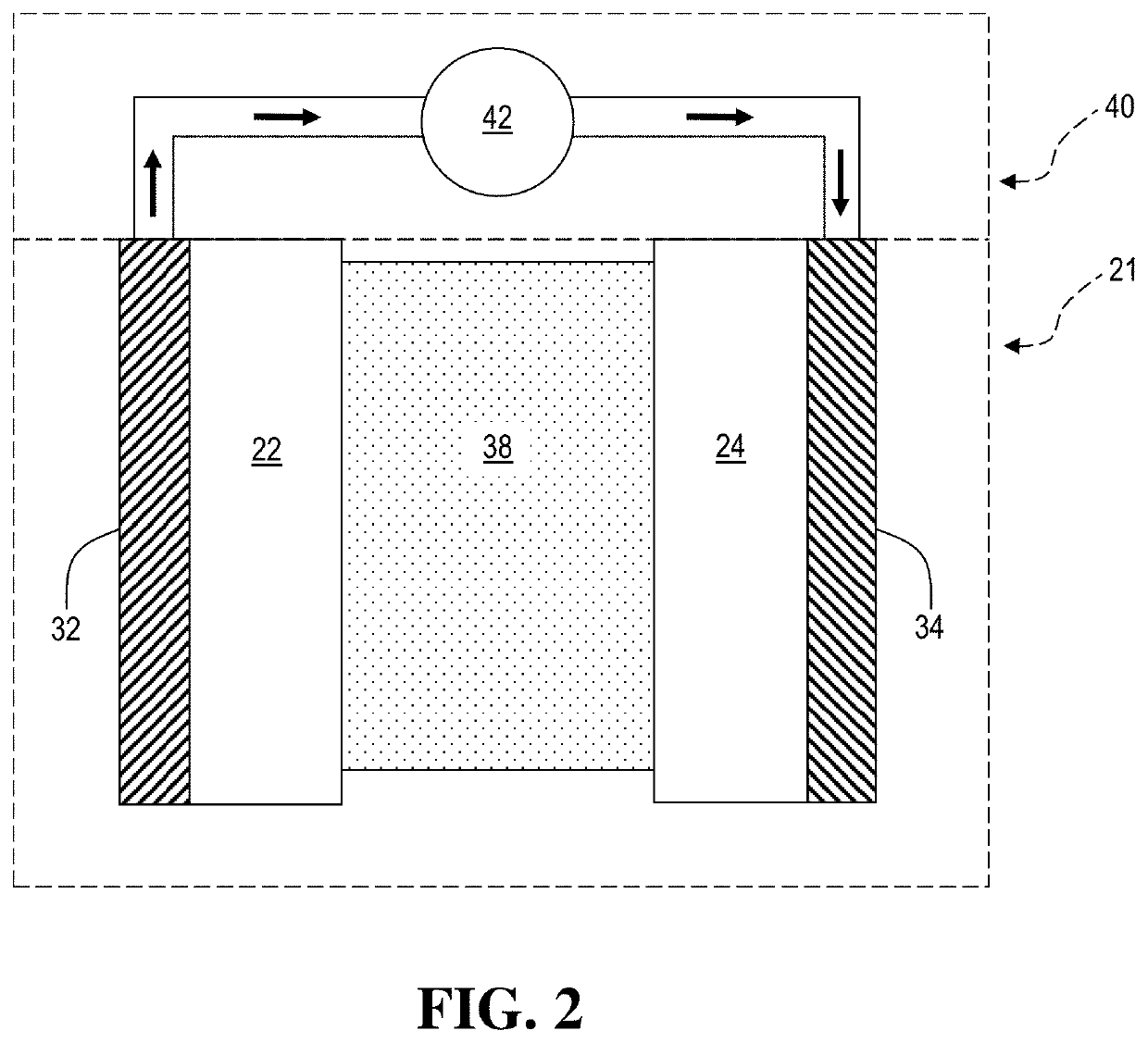
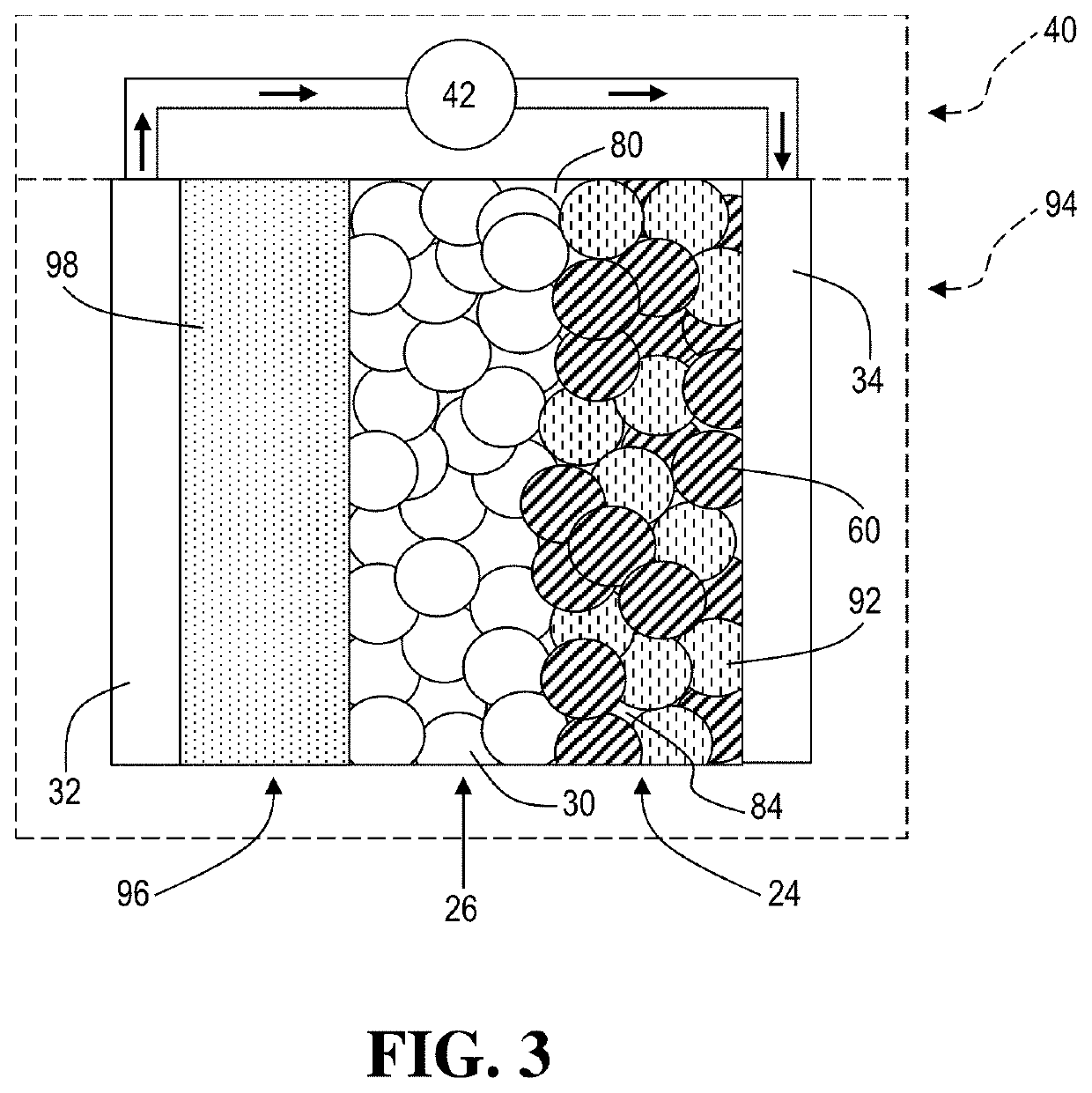
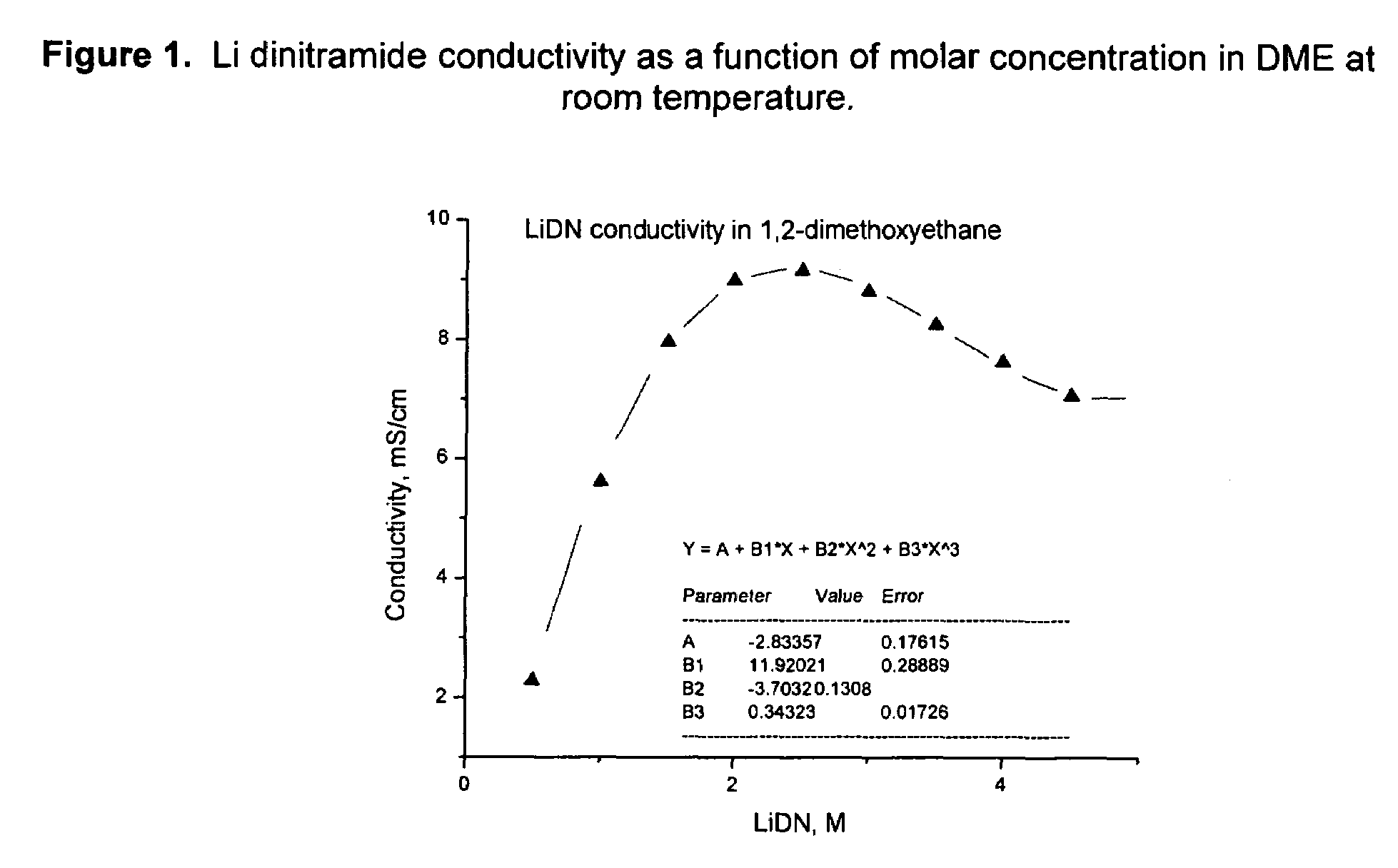
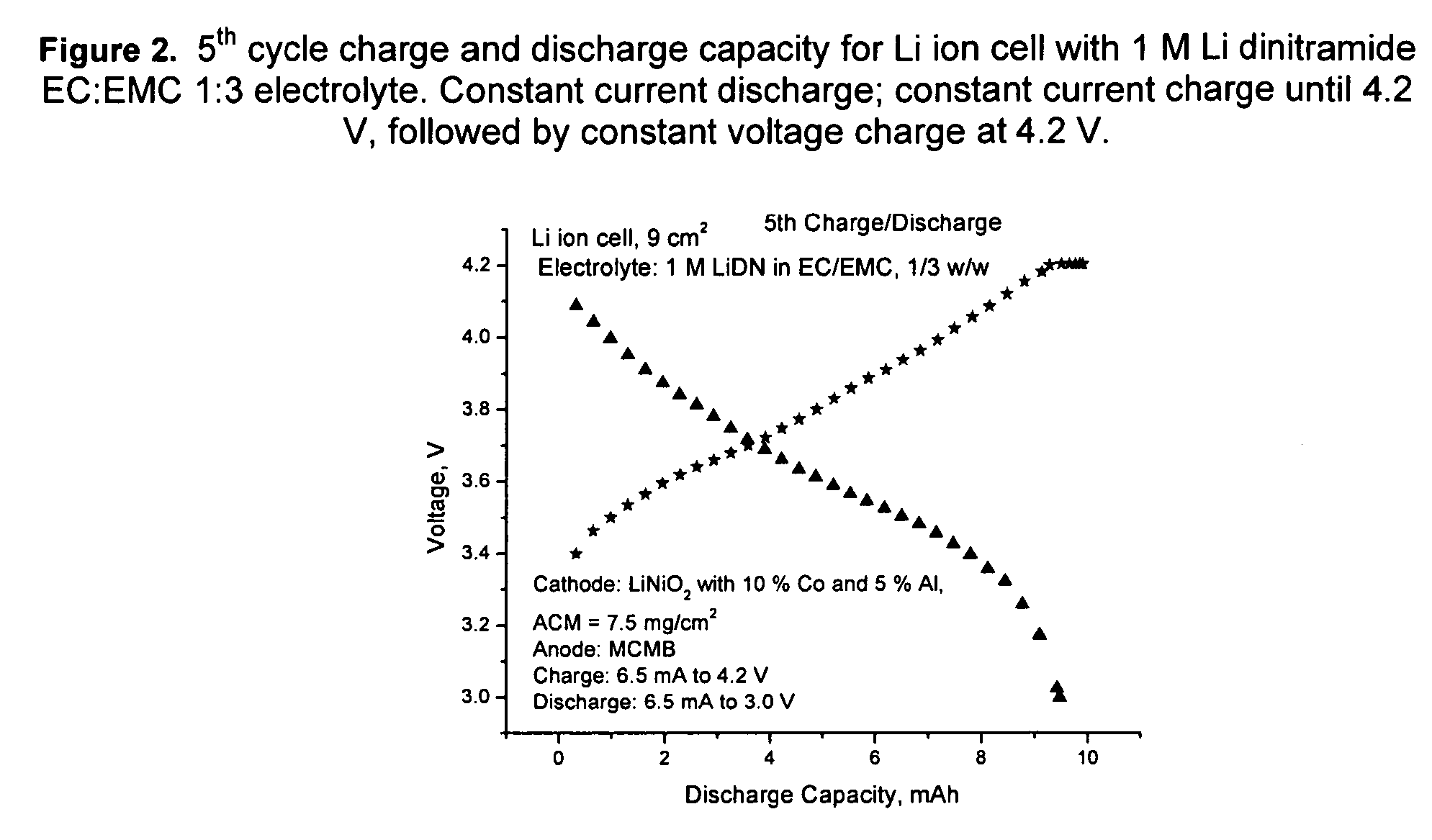
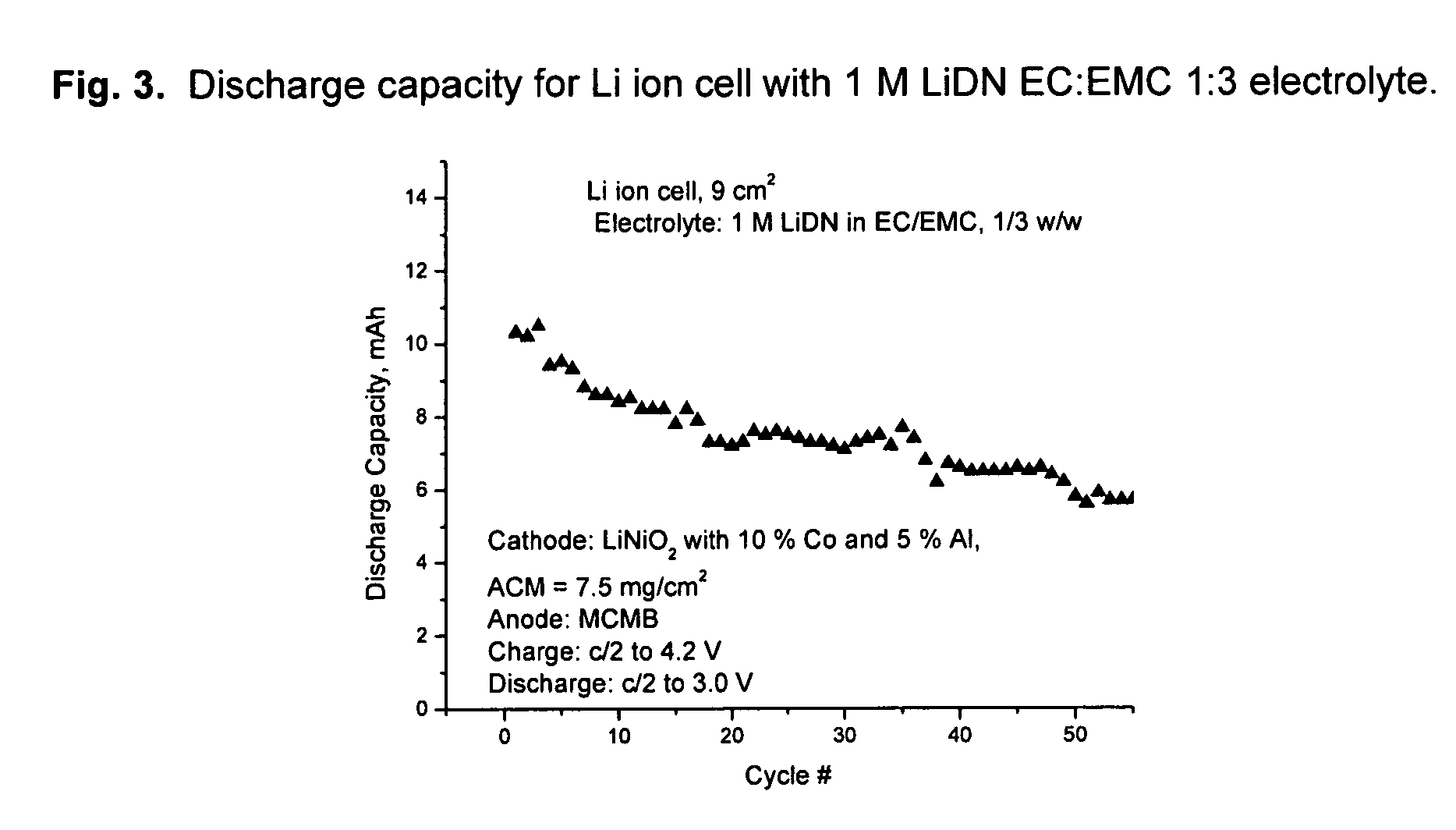
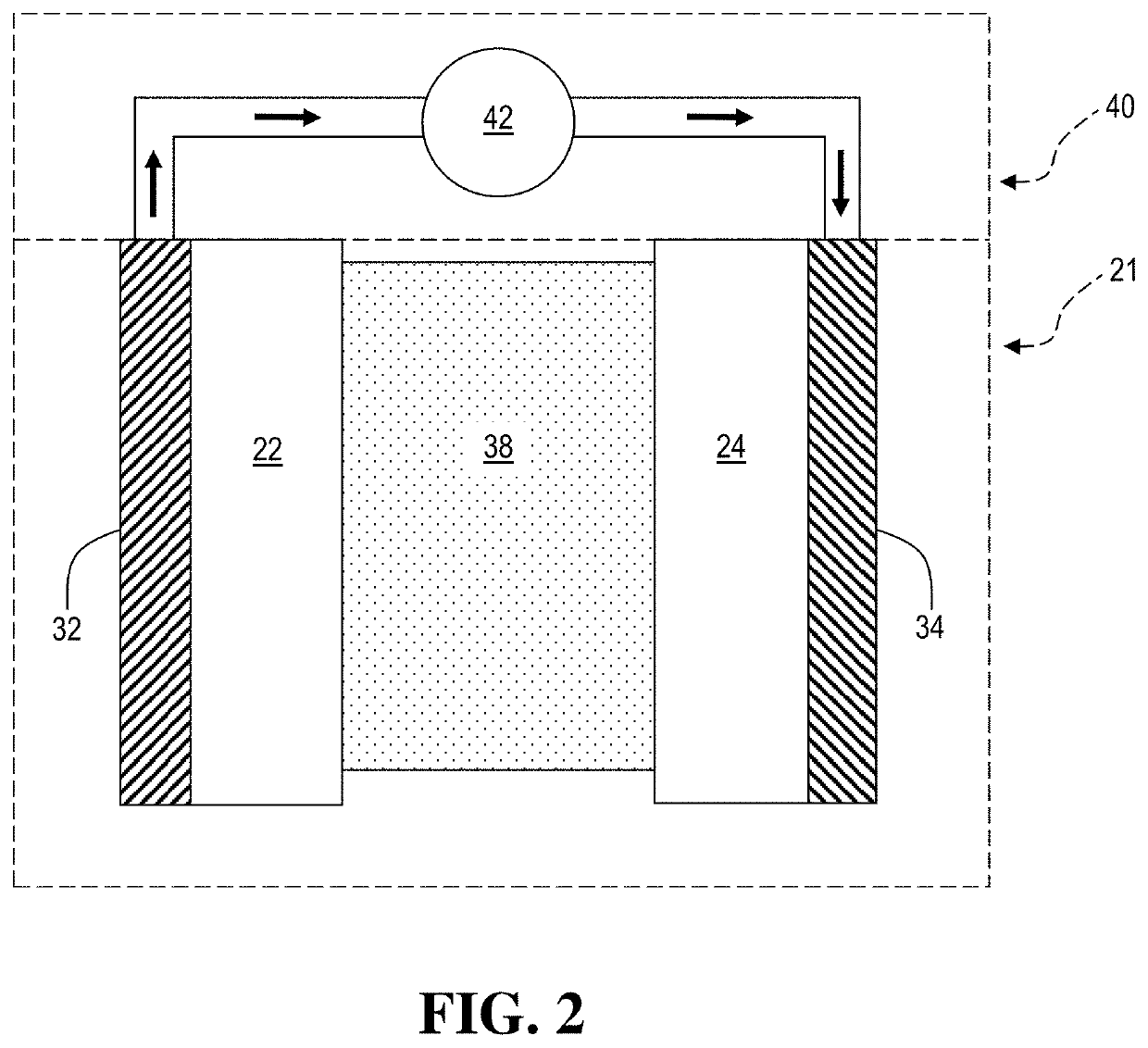
An electrochemical cell with an alkali metal containing anode having high discharge capacity, charge efficiency and low self-discharge. The addition of at least one nitramide or dinitramide salt of a metal cation to the electrochemical cell electrolyte unexpectedly lowers first cycle irreversible capacity, adds higher cycle life, lowers self-discharge and beneficially addresses several additional degrees of freedom with respect to electrolyte solvent selection while providing higher charge capacity. Additives include the lithium metal salts of nitramide or dinitramide, and the electrolyte consists essentially of a lithium metal salt dissolved in a at least one of an aqueous solvent, molten salt solvent system and a non-aqueous solvent mixture of at least one of organic ethers, esters, carbonates, acetals.
Owner:MATERIAL METHODS
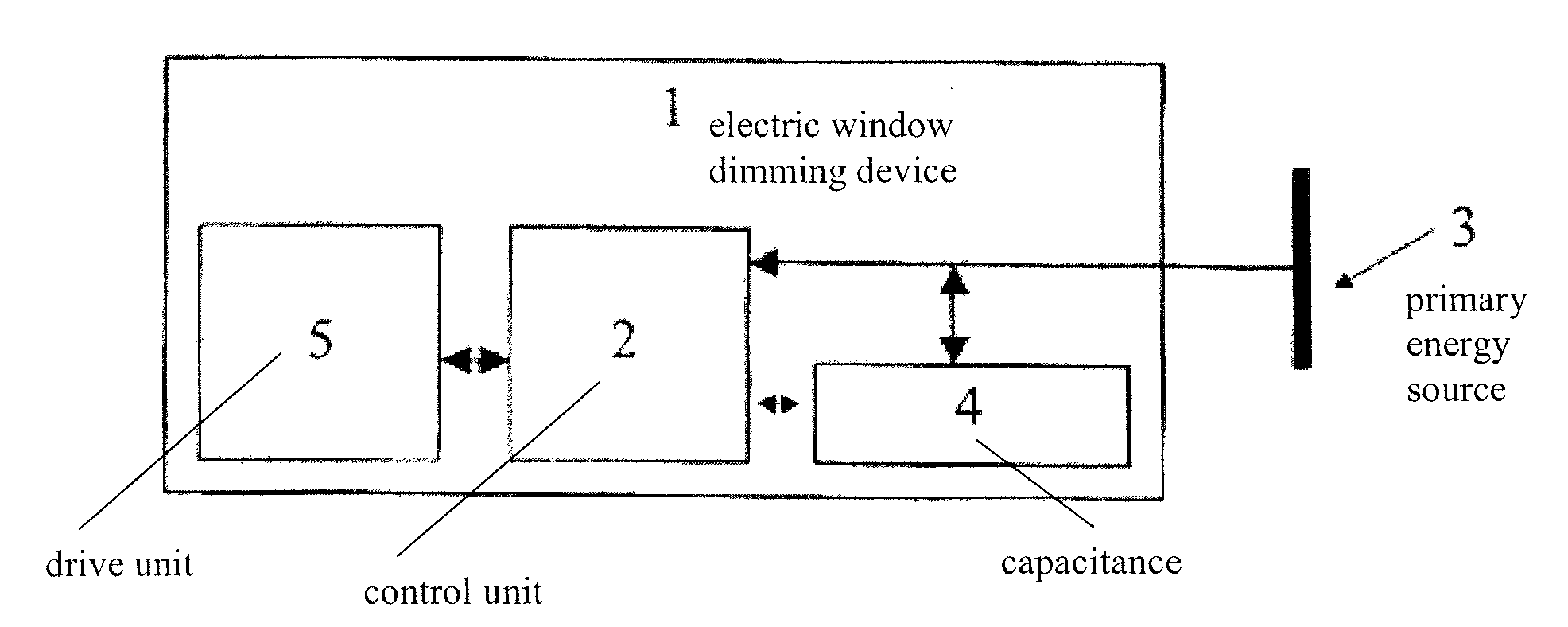
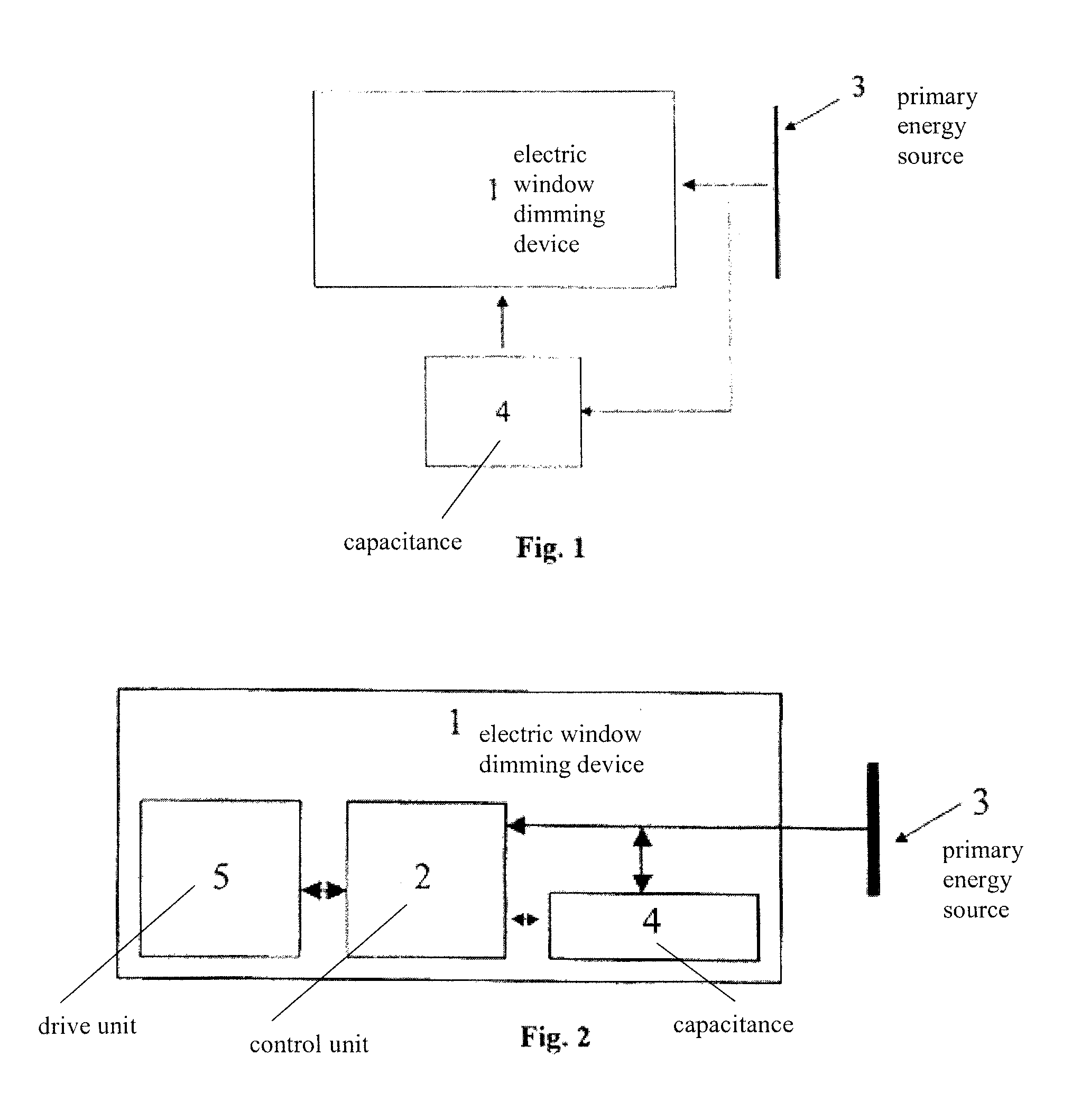
Power supply for an electric window dimming device
ActiveUS20080144158A1Ensure transparencyImprove safetyBatteries circuit arrangementsAntiglare equipmentSupply energyCapacitance
An electric window dimming device for reducing the light transmission of a window for an aircraft. The electric window dimming device comprises a capacitance. The electric window dimming device is adapted for being connected to the capacitance and to a primary energy source that provides energy. If the primary energy source fails, the capacitance supplies energy for electric window dimming device.
Owner:AIRBUS OPERATIONS GMBH
Method for the production of thin-film lithium-ion microbatteries and resulting microbatteries
ActiveUS20140308570A1Electrophoretic deposition can be facilitatedFunction increaseElectrolysis componentsCell electrodesAll solid stateElectrophoresis
Process for fabrication of all-solid-state thin film batteries, said batteries comprising a film of anode materials (anode film), a film of solid electrolyte materials (electrolyte film) and a film of cathode materials (cathode film) in electrical contact with a cathode collector, characterized in that:a first electrode film (cathode or anode) is deposited by electrophoresis on a conducting substrate or a substrate with at least one conducting zone, said substrate or said at least one conducting zone possibly being used as a collector of said electrode current (anode or cathode current) of the micro-battery,the electrolyte film is deposited by electrophoresis on said first electrode film,a second electrode film (anode or cathode) is deposited on the electrolyte film either by electrophoresis or by a vacuum deposition process.
Owner:I TEN
Sulfur-Carbon Composite Material, Its Application in Lithium-Sulfur Battery and Method for Preparing said Composite Material
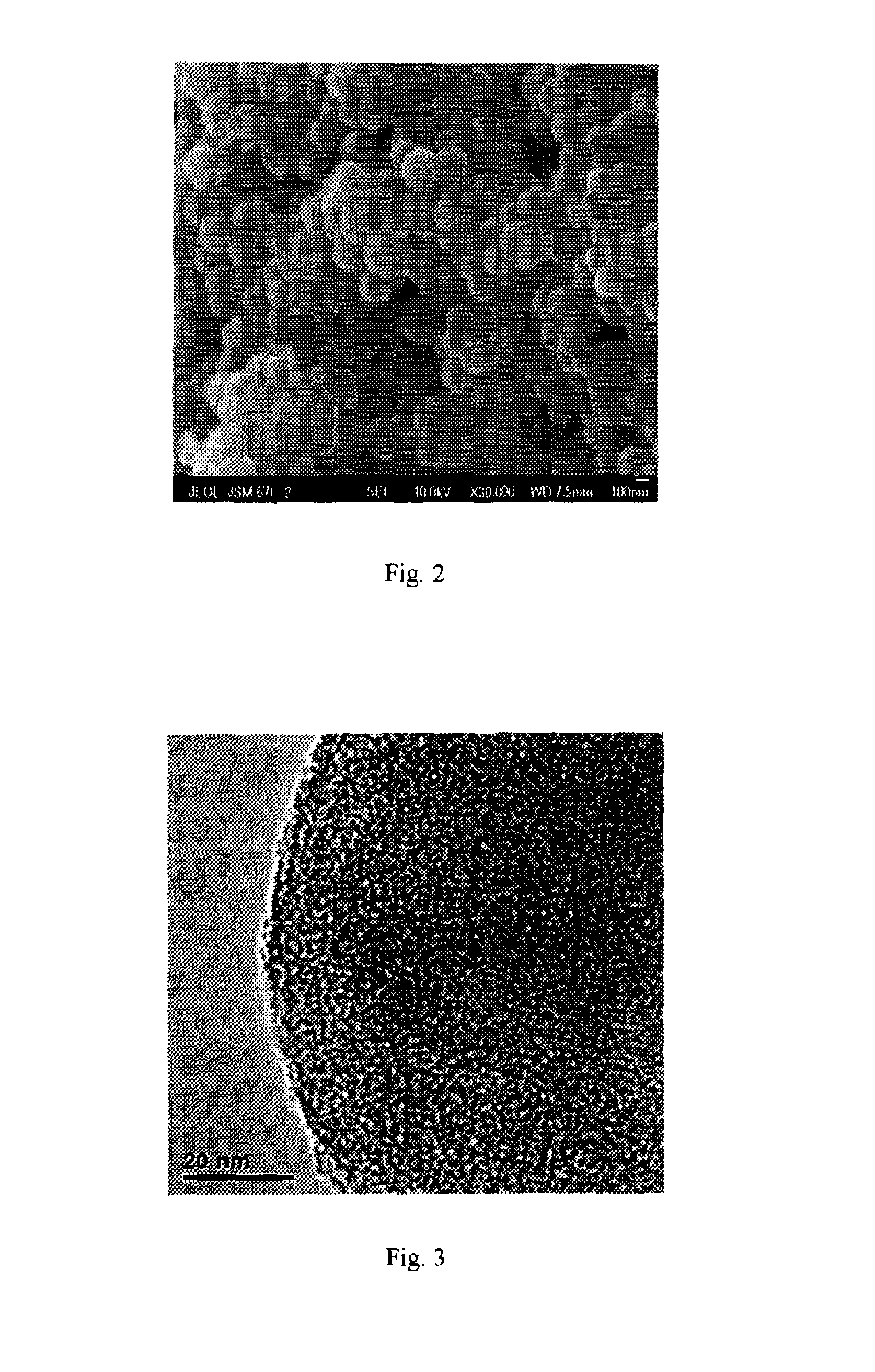
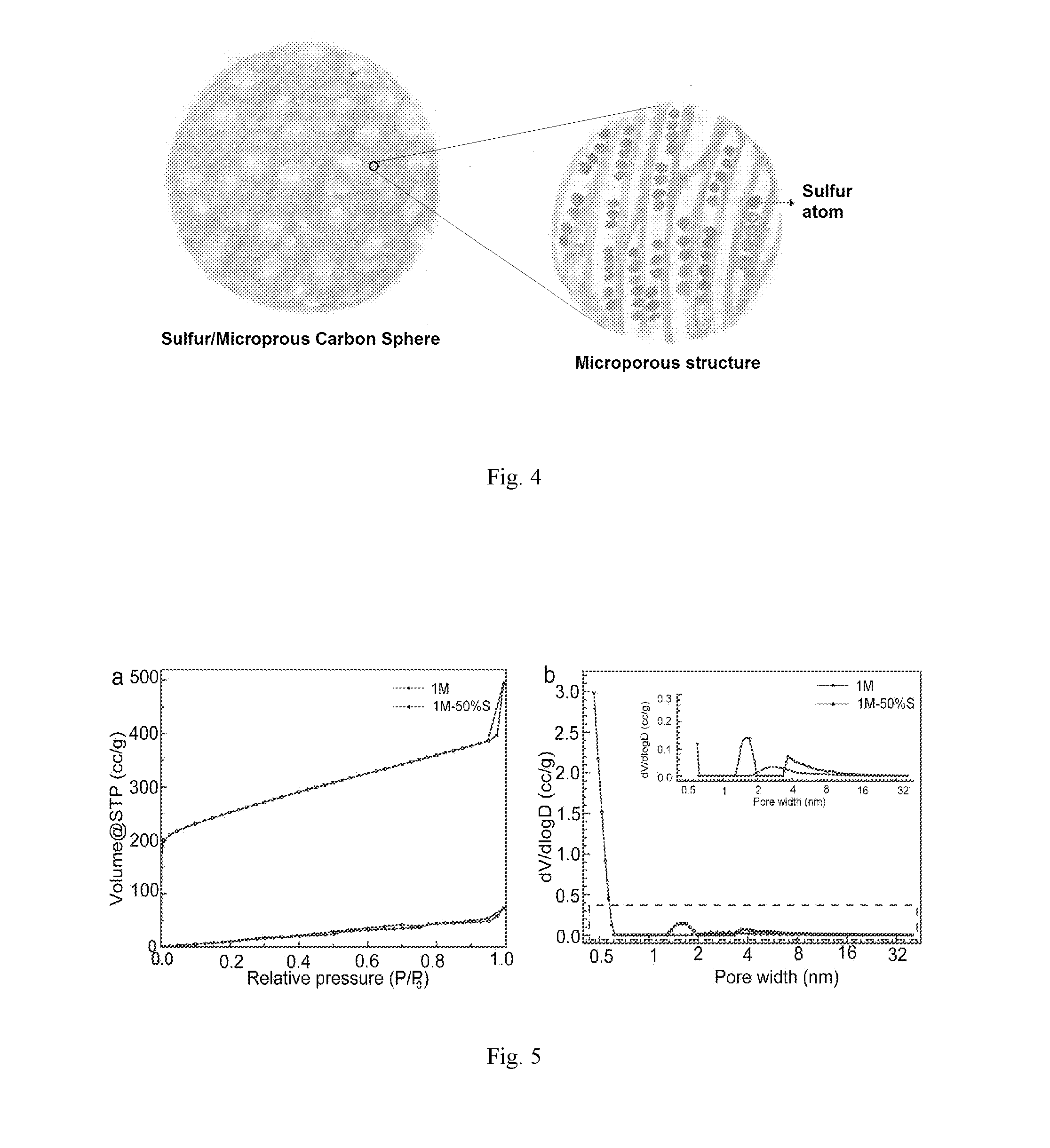
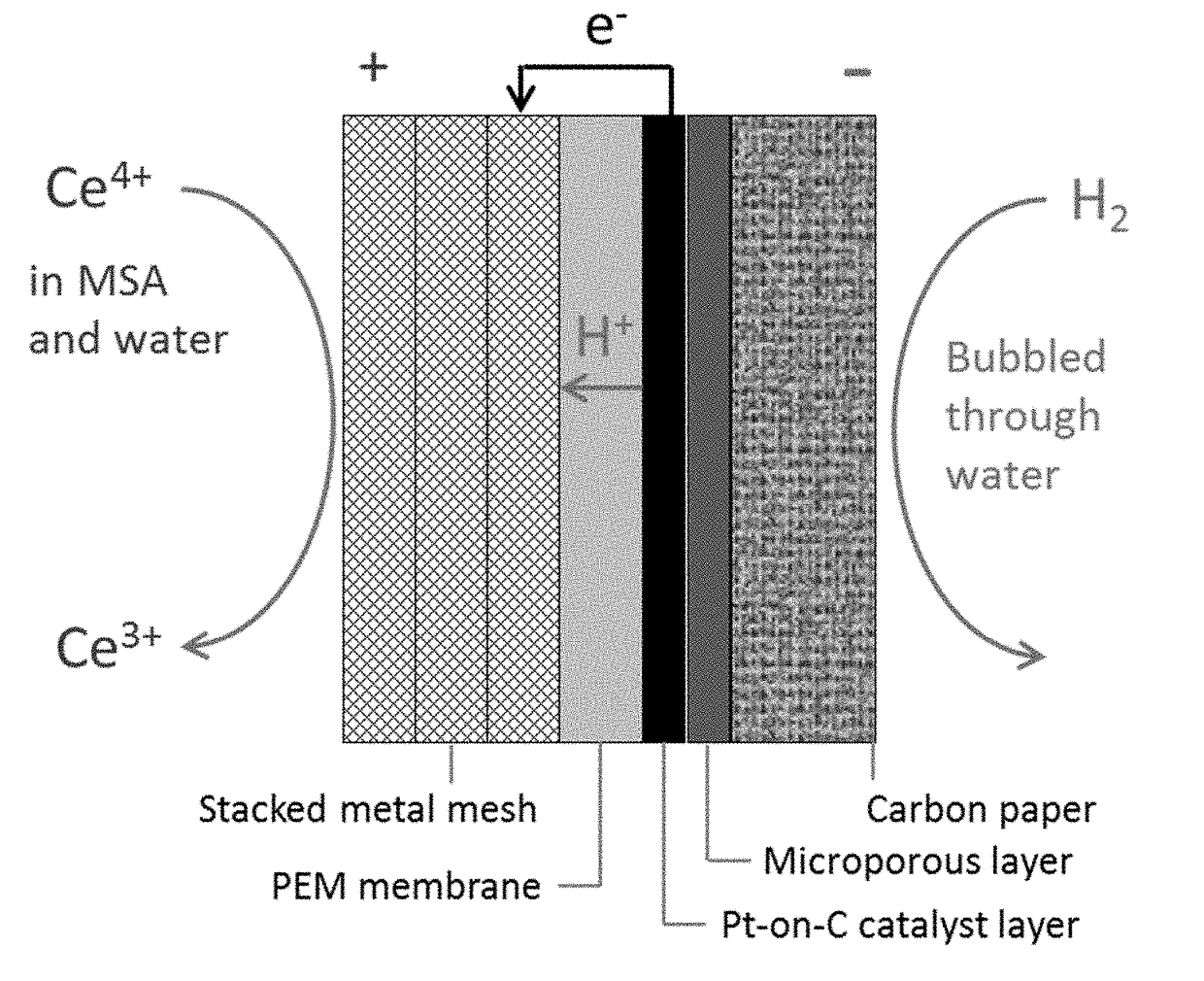
ActiveUS20150303458A1Large capacityImprove mechanical stabilityCarbon compoundsConductive materialCarbon compositesPorous carbon
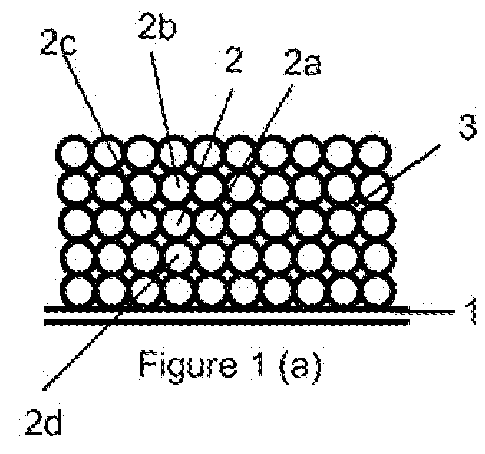
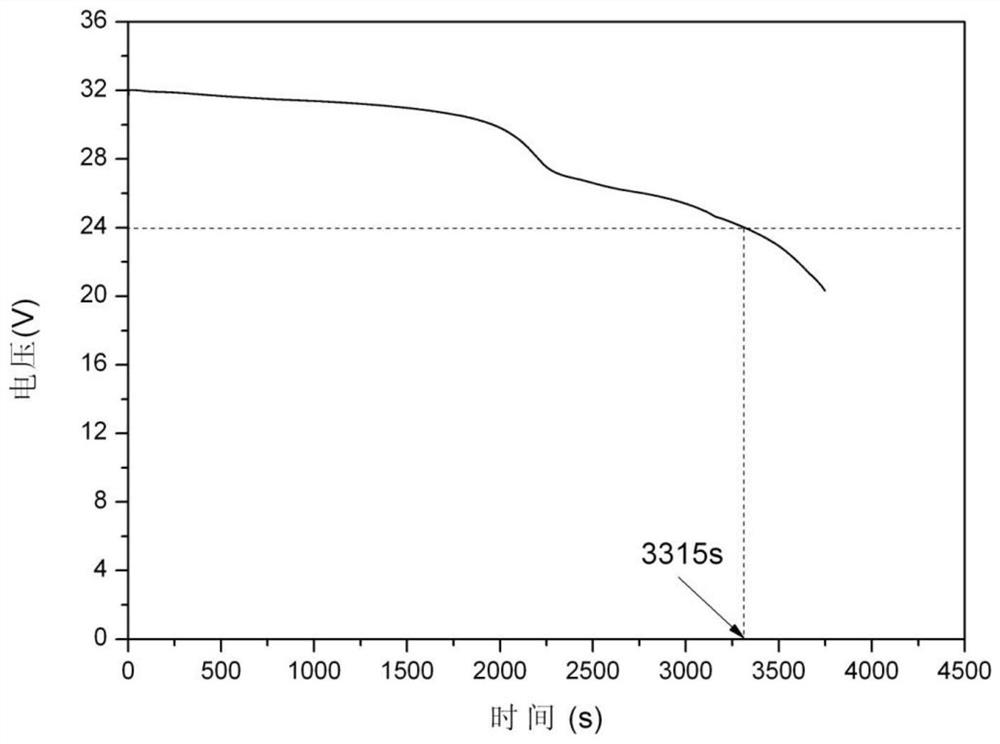
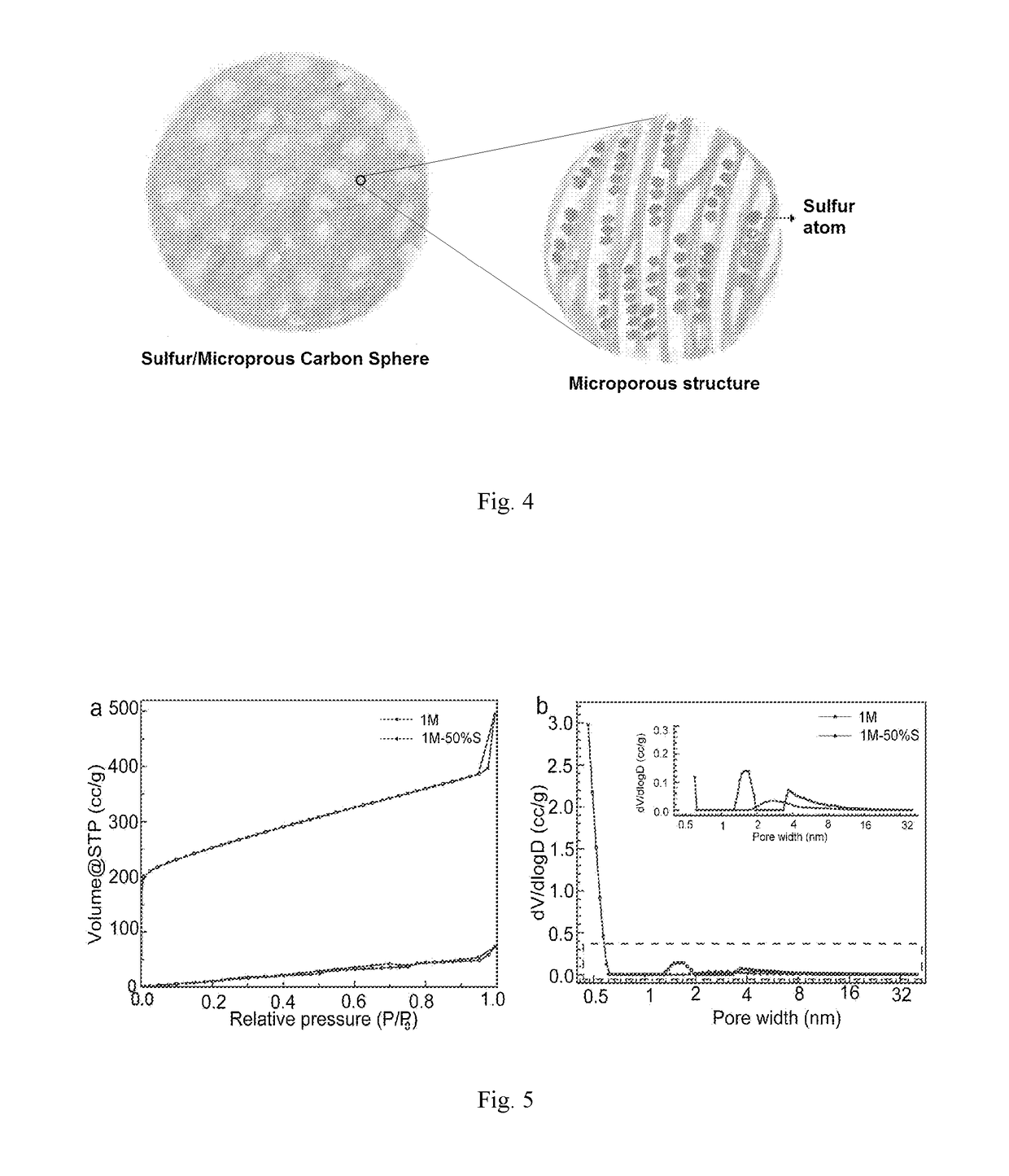
The present invention provides a sulfur-carbon composite material, said composite material comprising a porous carbon substrate containing both micropores and mesopores and sulfur, wherein the sulfur is only contained in the micropores of the carbon substrate. Moreover, a lithium-sulfur battery with its cathode comprising said sulfur-carbon composite material and the method for preparing such material is also provided.
Owner:ROBERT BOSCH GMBH +1
Optimization of the Cerium-Hydrogen Redox Flow Cell
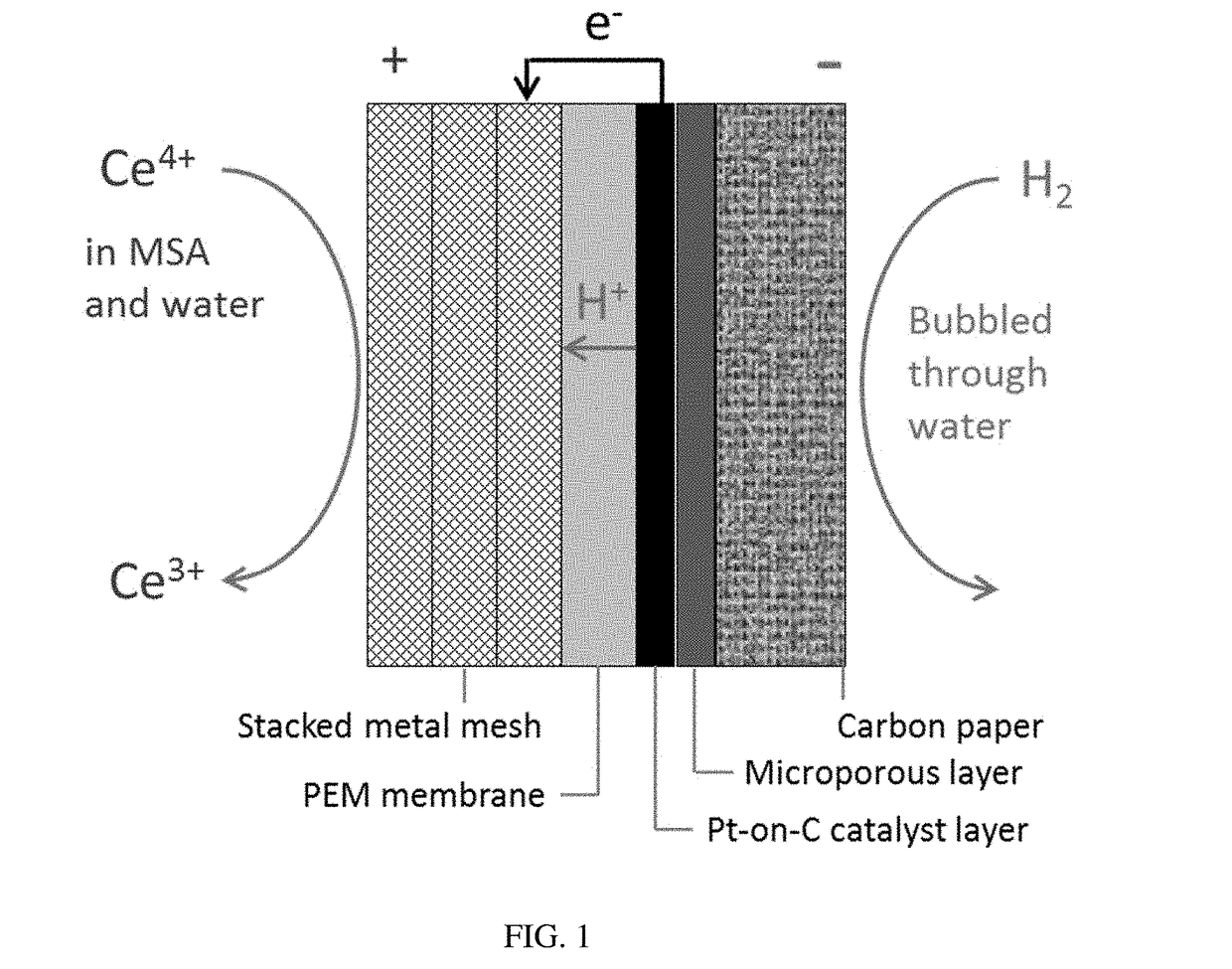
ActiveUS20170338508A1Improve efficiencyReduction in coulombic efficiencyHydrogenCell electrodesChemistryVoltage
The Ce—H2 redox flow cell is optimized using commercially-available cell materials. Cell performance is found to be sensitive to the upper charge cutoff voltage, membrane boiling pretreatment, methanesulfonic-acid concentration, (+) electrode surface area and flow pattern, and operating temperature. Performance is relatively insensitive to membrane thickness, Cerium concentration, and all features of the (−) electrode including hydrogen flow. Cell performance appears to be limited by mass transport and kinetics in the cerium (+) electrode. Maximum discharge power of 895 mW cm−2 was observed at 60° C.; an energy efficiency of 90% was achieved at 50° C. The Ce—H2 cell is promising for energy storage assuming one can optimize Ce reaction kinetics and electrolyte.
Owner:RGT UNIV OF CALIFORNIA
Ionic liquid additive-containing gel battery electrolyte
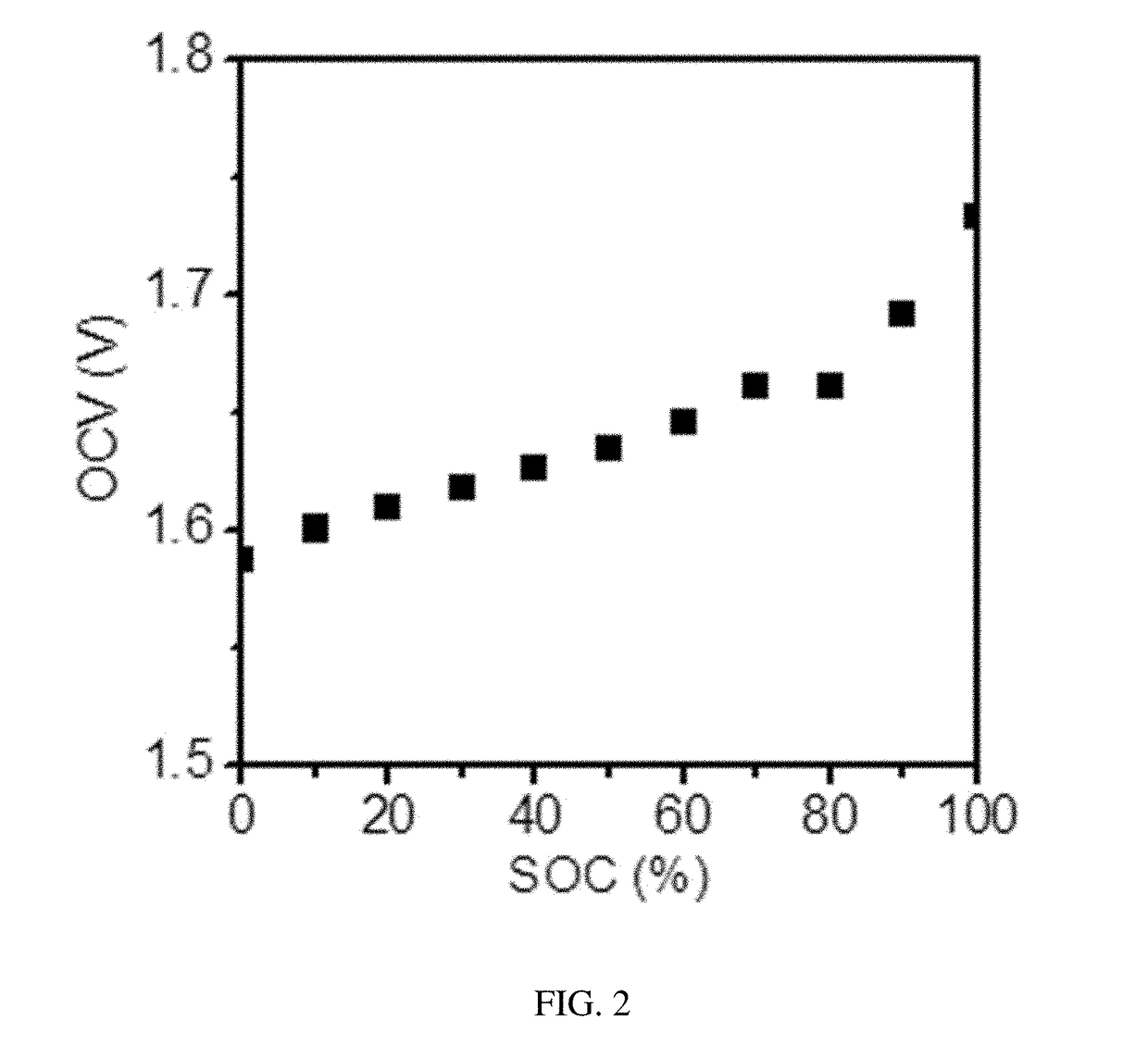
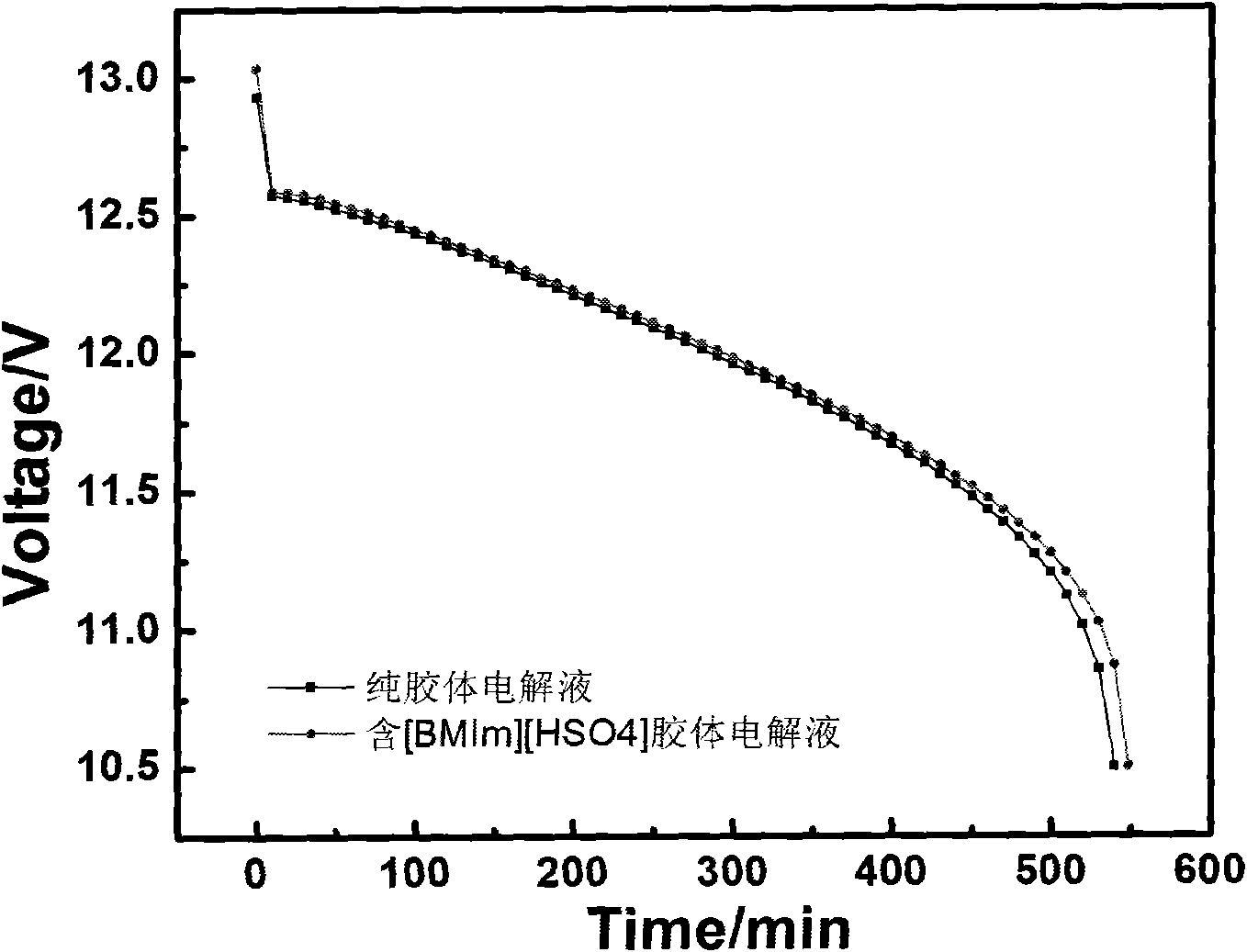
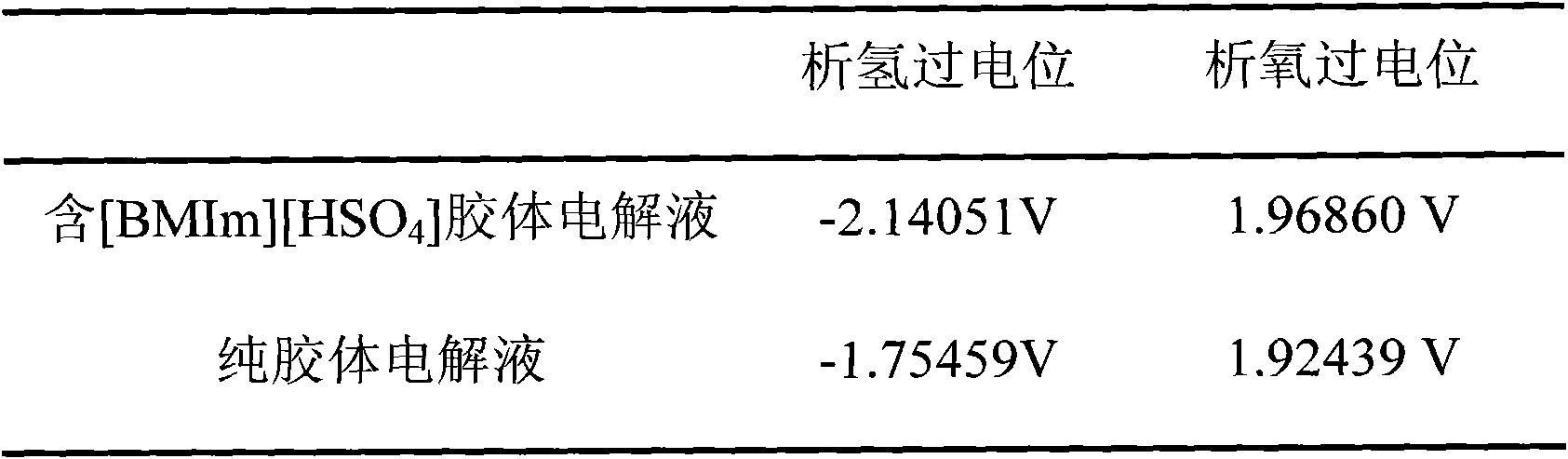
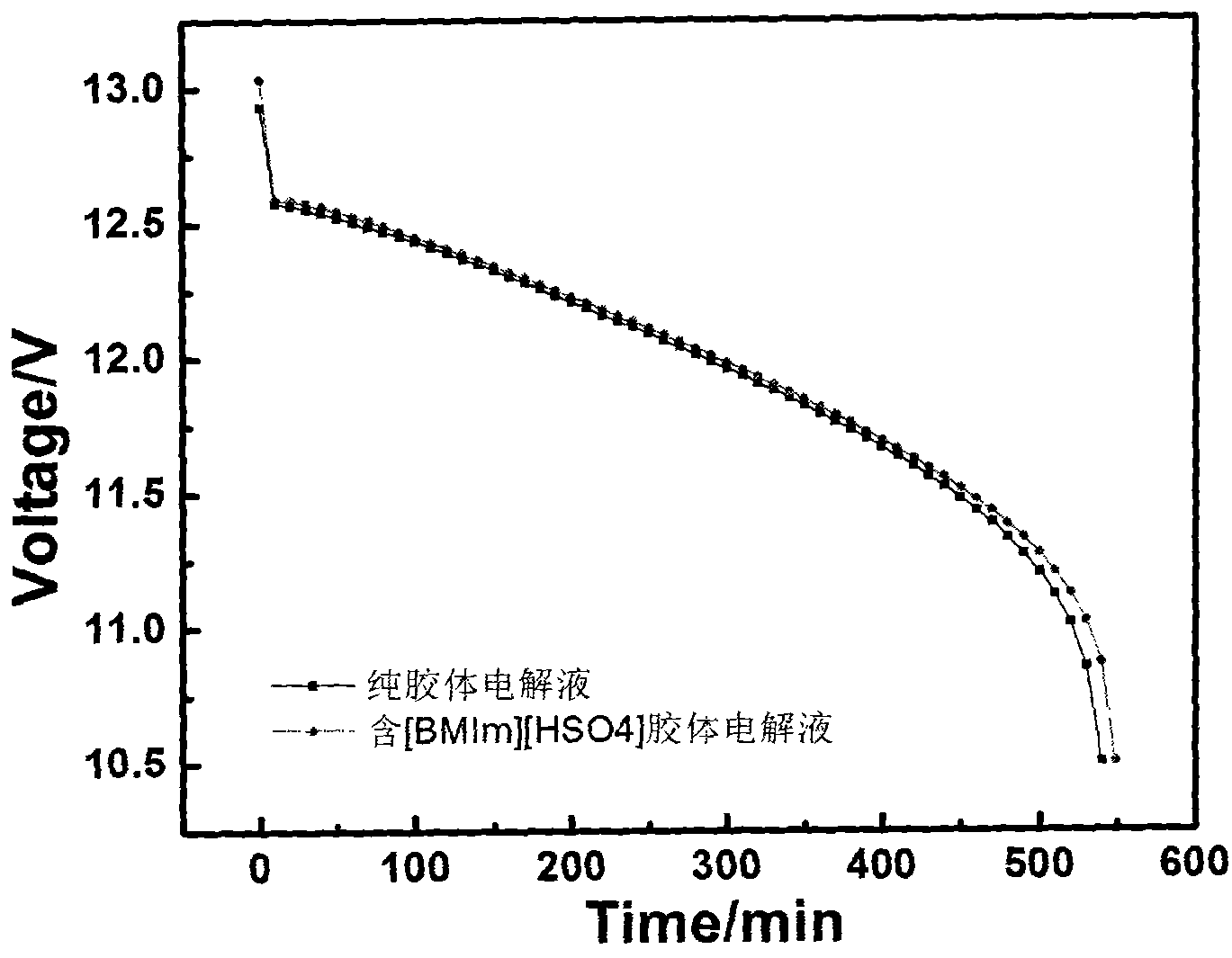
The invention discloses ionic liquid additive-containing gel battery electrolyte. The gel battery electrolyte comprises 5 to 10 weight percent of silica sol, 30 to 40 weight percent of sulfuric acid, 52 to 62 weight percent of water and 0.01 to 0.03 weight percent of 1-butyl-3-methylimidazole bisulfate ionic liquid. The electrolyte does not flow or acid does not overflow, the charging time is shortened, the discharging time is prolonged, the self-discharge is extremely low, the service life is prolonged, the electrolyte is easy and convenient to maintain, pure water generally needs be added for several times only and acid is not needed. In the production of a gel battery, the gel can be conveniently filled under normal pressure, has reliable performance and is safe to use.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Electric double layer capacitor
InactiveUS6299790B1Low self-discharge rateReduce incidenceSolid electrolytesHybrid capacitor electrolytesHydrogen compoundsSolvent
An electric double layer capacitor capable of functioning over a broad temperature range with a reduced incidence of electrolyte leakage and a low rate of self discharge is formed from a solid electrolyte containing (A) a crosslinking product of a polyoxyalkylene having a double bond terminally and / or in its side chain, (B) an electrolyte salt, (C) a low molecular weight polar solvent, and (D) polyacrylonitrile. The above-mentioned polyoxyalkylene (A) is, for example, a compound of the formula (1) below. In formula (1), Z is an active hydrogen compound residue; k is an integer from 1 to 6; R1 is an alkyl group having 1 to 8 carbon atoms; Y1 is an acryloyl or methacryloyl group; m is an integer from 0 to 460 and n is an integer from 0 to 350, excluding the case in which both m and n are simultaneously equal to 0.
Owner:DAI ICHI KOGYO SEIYAKU CO LTD
Additive to ceramic ion conducting material to mitigate the resistive effect of surface carbonates and hydroxides
ActiveUS20210083249A1Promote sinteringPrevent and inhibit and minimize dendrite formationElectrode thermal treatmentSecondary cellsCarbonate esterPhysical chemistry
A method of modifying a carbonate layer formed on a surface of an electrochemical cell component is provided. The surface includes a ceramic oxide. The carbonate layer includes a carbonate and is substantially non-conductive to lithium ions and sodium ions. The method includes contacting the carbonate layer with a modifying agent to form a mixture and causing the modifying agent to incorporate into the carbonate layer and form a modified hybrid layer including a eutectic mixture of the modifying agent and the carbonate. The modified hybrid layer is conductive to lithium ions and sodium ions.
Owner:GM GLOBAL TECH OPERATIONS LLC
Enhanced electrochemical cells with solid-electrolyte interphase promoters
InactiveUS7598002B2Reduce first cycle irreversible capacityIncrease capacityAlkaline accumulatorsOrganic electrolyte cellsLithium metalCarbonate
Owner:MATERIAL METHODS
Composition for negative electrode of alkaline electrolyte battery
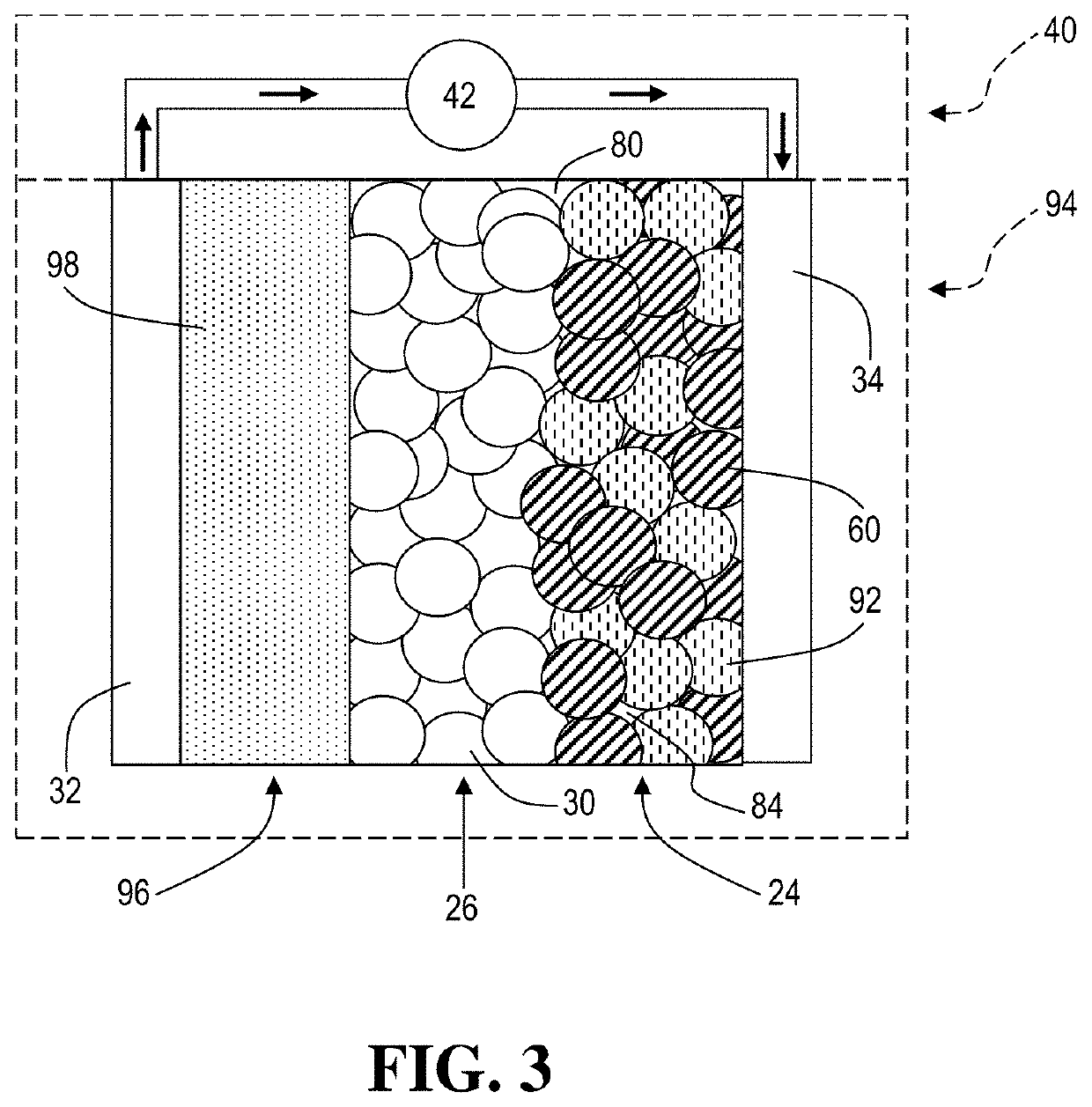
InactiveUS20080070117A1Long life-timeLow self-dischargeAlkaline accumulatorsNon-aqueous electrolyte accumulator electrodesHydrogenManganese
The invention proposes a composition comprising: a) a hydrogen-fixing alloy of formula R1-tMgtNis-zMz in which: R represents one or more elements chosen from the group comprising La, Ce, Nd and Pr; M represents one or more elements chosen from the group comprising Mn, Fe, Al, Co, Cu, Zr and Sn; 0.1≦t≦0.4; 3.0≦s≦4.3; z≦0.5; b) a manganese compound in a proportion such that the mass of manganese represents from 1 to 5.5% of the mass of the alloy, c) an yttrium compound in a proportion such that the mass of yttrium represents from 0.1% to 2% of the mass of the alloy. The invention also relates to an anode comprising the composition according to the invention. The invention also relates to an alkaline electrolyte battery comprising said at least one anode.
Owner:SAFT GRP SA
Solid-state electrolytes and methods for making the same
ActiveUS20210111426A1Improves Structural IntegrityExtended shelf lifeSolid electrolytesHybrid capacitor electrolytesSolid state electrolyteSulfate
The present disclosure relates to solid-state electrolytes and methods of making the same. The method includes admixing a sulfate precursor including one or more of Li2SO4 and Li2SO4.H2O with one or more carbonaceous capacitor materials. The first admixture is calcined to form an electrolyte precursor that is admixed with one or more additional components to form the solid-state electrolyte. When a ratio of the sulfate precursor to the one or more carbonaceous capacitor materials in the first admixture is about 1:2, the electrolyte precursor consists essentially of Li2S. When a ratio of the sulfate precursor to the one or more carbonaceous capacitor materials in the first admixture is less than about 1:2, the electrolyte precursor is a composite precursor including a solid-state capacitor cluster including the one or more carbonaceous capacitor materials and a sulfide coating including Li2S disposed on one or more exposed surfaces of the solid-state capacitor cluster.
Owner:GM GLOBAL TECH OPERATIONS LLC
Lithium phosphate coating for lithium lanthanum zirconium oxide solid-state electrolyte powders
InactiveUS20210257656A1Smooth connectionIncrease volumeSolid electrolytesSecondary cellsSolid state electrolyteNanowire
An electrochemical cell that cycles lithium ions is provided. The electrochemical cell includes a positive electrode, a negative electrode, a separator disposed between the positive electrode and the negative electrode, and a lithium phosphate (Li3PO4)-coated lithium lanthanum zirconium oxide (LLZO) material. The Li3PO4-coated LLZO material is a particle having a substantially spherical core comprising the LLZO and a layer comprising the Li3PO4 directly coating at least a portion of the substantially spherical core, the substantially spherical core having a diameter of less than or equal to about 100 μm; a nanowire having an elongate core comprising the LLZO and a layer comprising the Li3PO4 directly coating at least a portion of the elongate core, the elongate core having a length of less than or equal to about 10 mm and a diameter of less than or equal to about 100 μm; or a combination thereof.
Owner:GM GLOBAL TECH OPERATIONS LLC
Solid-state chemical current source and a method for increasing a discharge power
InactiveUS20080268332A1Prolong high power discharge timeReduce heat lossDeferred-action cellsSmall-sized cells cases/jacketsElectrical conductorThermodynamics
The invention relates to a solid-state chemical current source and to a method for increasing a discharge power thereof. The inventive current source can be used in electrochemical engineering, in particular for primary and secondary solid-state chemical power sources, which are based on solid ion conductors and exhibit a high discharge power and for a method for increasing the said discharge power. The solid-state chemical current source comprises a body provided with current leading-out wires and solid-state galvanic cells which are arranged therein, are connected to the current leading-out wires, are based on solid ion conductors and perform the function of heating elements. A heat insulation for reducing heat losses of the heated galvanic cells is arranged inside and\or outside the body. The inventive method for increasing the discharge power of the solid-state chemical current source by heating it consists in using the heat produced by the electric current flowing through the galvanic cells and in maintaining the hot state of the said galvanic cells during the discharge. The said invention makes it possible to obtain a solid-state chemical current source exhibiting a high discharge power, a low self-discharge (about 1-3% per year), a long-term power storage and to increase energy characteristics in such a way that they are equal to or greater than 600 Watt-hour / dm3.
Owner:THE POTANIN INST
Additive to ceramic ion conducting material to mitigate the resistive effect of surface carbonates and hydroxides
ActiveUS11094996B2Reduce needExtended shelf lifeElectrode thermal treatmentSecondary cellsPhysical chemistryElectrochemical cell
A method of modifying a carbonate layer formed on a surface of an electrochemical cell component is provided. The surface includes a ceramic oxide. The carbonate layer includes a carbonate and is substantially non-conductive to lithium ions and sodium ions. The method includes contacting the carbonate layer with a modifying agent to form a mixture and causing the modifying agent to incorporate into the carbonate layer and form a modified hybrid layer including a eutectic mixture of the modifying agent and the carbonate. The modified hybrid layer is conductive to lithium ions and sodium ions.
Owner:GM GLOBAL TECH OPERATIONS LLC
Solid-state chemical current source and a method for increasing a discharge power
InactiveUS20120119706A1Reduce heat lossSimplification of the current source structureBatteries circuit arrangementsSmall-sized cells cases/jacketsElectrical conductorGalvanic cell
A solid-state chemical current source and a method for increasing a discharge power thereof are disclosed. The current source can be used in electrochemical engineering, in particular for primary and secondary solid-state chemical power sources. The solid-state chemical current source comprises a body provided with current leading-out wires and solid-state galvanic cells which are arranged therein, are connected to the current leading-out wires, are based on solid ion conductors and perform the function of heating elements. A heat insulation for reducing heat losses of the heated galvanic cells is arranged inside and\or outside the body. The inventive method for increasing the discharge power of the solid-state chemical current source by heating it consists in using the heat produced by the electric current flowing through the galvanic cells and in maintaining the hot state of the said galvanic cells during the discharge.
Owner:THE POTANIN INST
Self-heating bipolar solid-state battery
PendingUS20220302526A1Reduce needLow self-dischargeFinal product manufactureElectrode carriers/collectorsElectrical batteryEngineering
The present disclosure provides a solid-state battery including at least one current collector that is in communication with one or more switches configured to move between open and closed positions, where the open position corresponds to a first operational state of the solid-state battery and the closed position corresponds to a second operational state of the solid-state battery; one or more electrodes disposed adjacent to the one or more current collectors; and one or more electrothermal material foils including a resistor material that is in electrical communication with that at least one current collector, where in the first operational state electrons may flow through the one or more electrothermal material foils during cycling of the solid-state battery so as to initiate a heating mode, and in the second operational state electrons may flow through the current collector during cycling of the solid-state battery so as to initiate a non-heating mode.
Owner:GM GLOBAL TECH OPERATIONS LLC
In-situ gelation method to make a bipolar solid-state battery
PendingUS20220181685A1Reduce needReduce impactSolid electrolytesFinal product manufactureEngineeringActive particles
A method for forming a bipolar solid-state battery includes preparing a mixture of gel precursor solution and solid electrolyte. The gel precursor includes a polymer, a first solvent, and a liquid electrolyte. The liquid electrolyte includes a second solvent, a lithium salt, and electrolyte additive. The method includes loading the mixture onto at least one of a first electrode, a second electrode, and a third electrode. Each of the first, second, and third electrodes includes a plurality of solid-state electroactive particles. The method includes removing at least a portion of the first solvent from the mixture to form a gel and positioning one of the first, second, and third electrodes with respect to another of the first, second, and third electrodes. The method includes applying a polymer blocker to a border of the first, second, or third electrodes.
Owner:GM GLOBAL TECH OPERATIONS LLC
High rate primary lithium battery with solid cathode
InactiveUS20060234124A1High discharge rateLow self-dischargeElectrochemical processing of electrodesSmall-sized cells cases/jacketsHigh rateChemistry
Owner:LITHDYNE +1
Solid state battery with uniformly distributed electrolyte, and methods of fabrication relating thereto
PendingUS20220181598A1Reduce needExtended shelf lifeSolid electrolytesNon-aqueous electrolyte accumulator electrodesPhysicsSolid-state battery
The present disclosure relates to a solid-state electrochemical cell having a uniformly distributed solid-state electrolyte and methods of fabrication relating thereto. The method may include forming a plurality of apertures within the one or more solid-state electrodes; impregnating the one or more solid-state electrodes with a solid-state electrolyte precursor solution so as to fill the plurality of apertures and any other void or pores within the one or more electrodes with the solid-state electrolyte precursor solution; and heating the one or more electrodes so as to solidify the solid-state electrolyte precursor solution and to form the distributed solid-state electrolyte.
Owner:GM GLOBAL TECH OPERATIONS LLC
Bipolar solid-state battery with enhanced interfacial contact
PendingUS20220263055A1Reduce needReduce impactSolid electrolytesFinal product manufactureChemical physicsPhysical chemistry
A method for forming a bipolar solid-state battery may include preparing a plurality of freestanding gels each comprising a polymer, a solvent, and a lithium salt and, also, positioning a first freestanding gel between a first electrode and a second electrode and a second freestanding gel between the second electrode and a third electrode. Each of the first electrode, the second electrode, and the third electrode may include a plurality of electroactive particles. The method may also include infiltrating at least a portion of the first free-standing gel into a space between particles of the first electrode and the second electrode and at least a portion of the second free-standing gel into a space between the particles of second electrode and the third electrode.
Owner:GM GLOBAL TECH OPERATIONS LLC
Cell frame for accommodating pouch cells
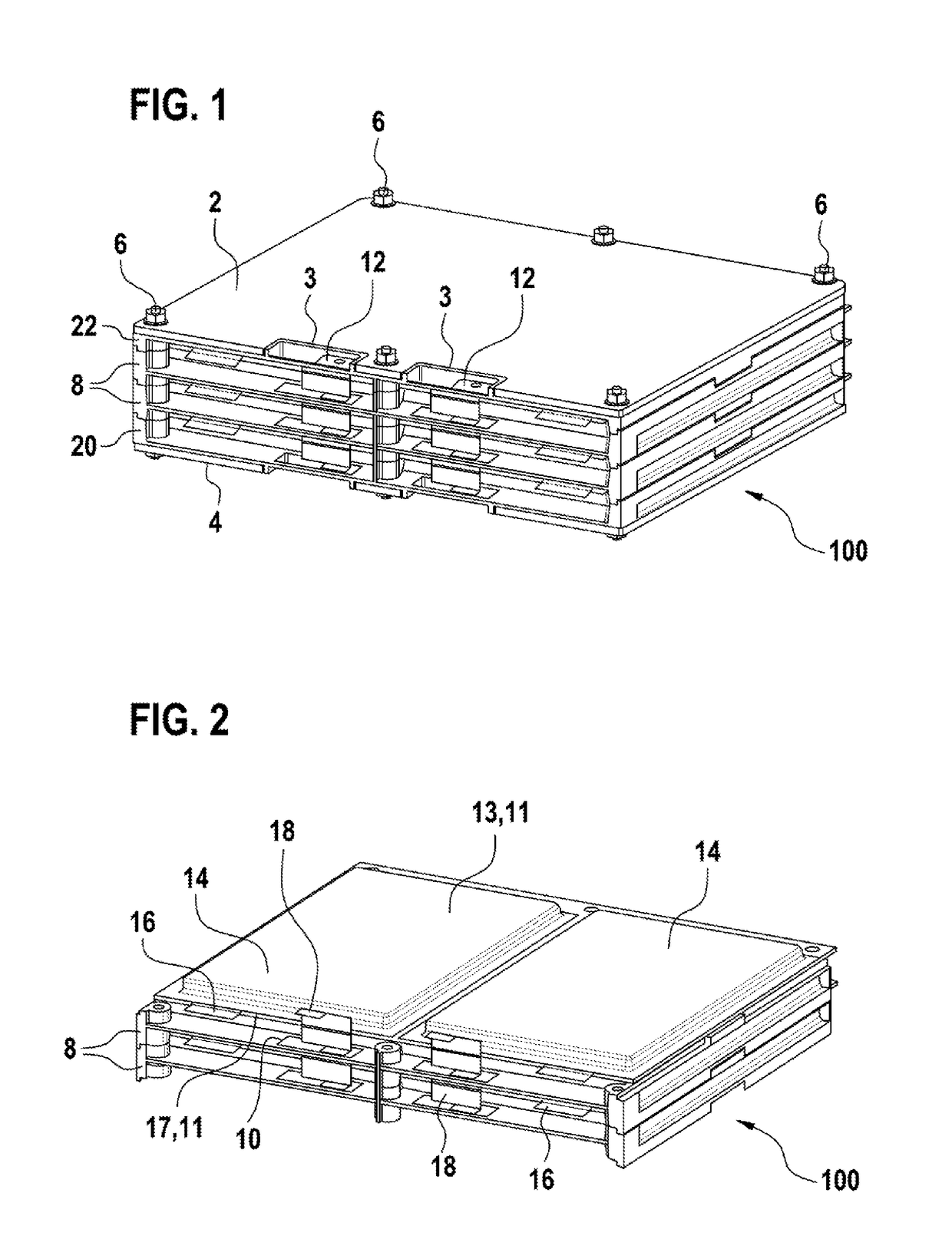
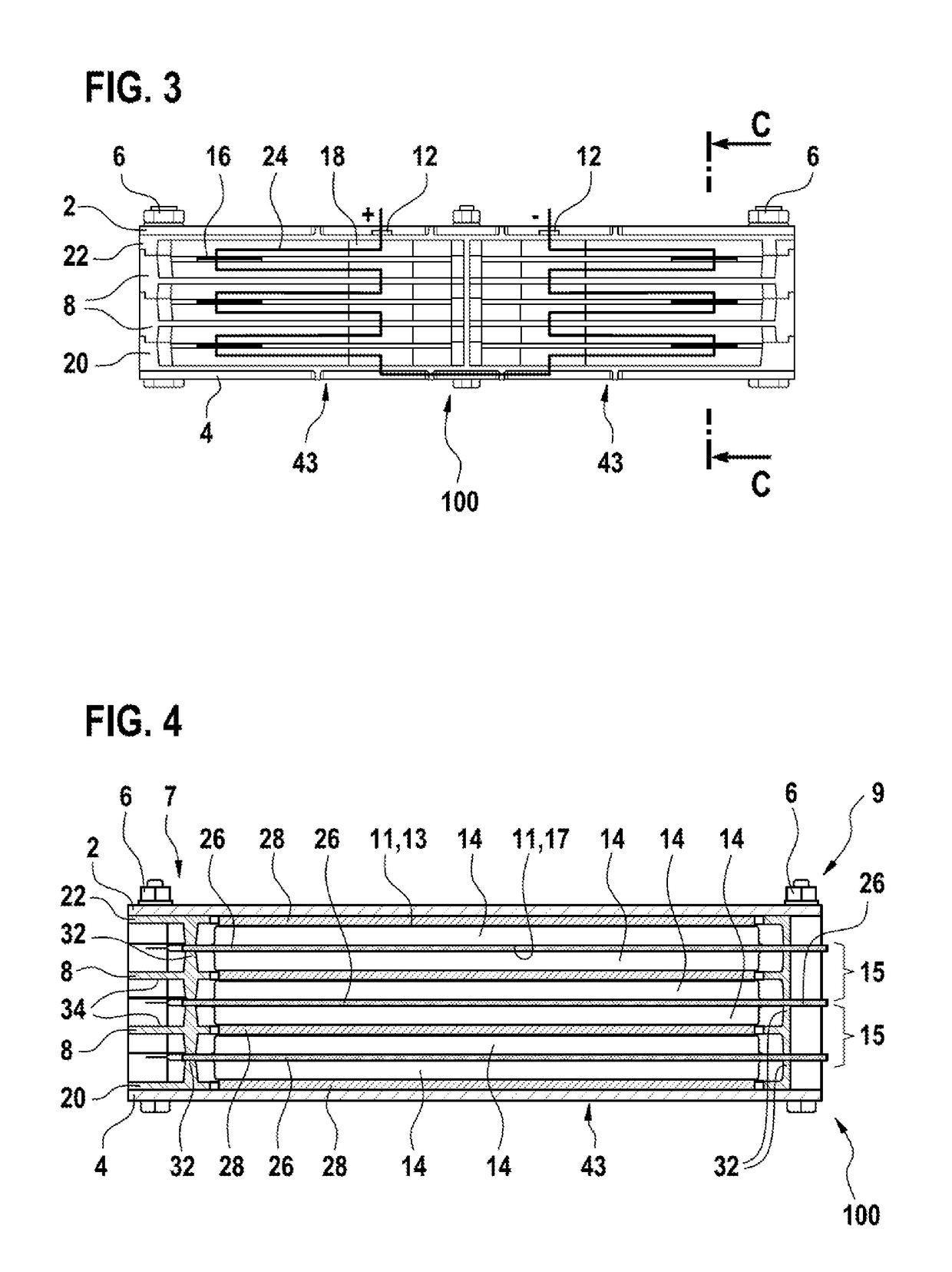
InactiveUS20180123108A1Freedom in isolationImprove accessibilitySecondary cellsCell component detailsContact padEngineering
A cell frame (8) for accommodating a specific number of pouch cells (14), having an integrated cell collector (10), wherein the cell connector (10) provides contact pads for clinching. A battery pack and a method for producing a battery pack are also specified.
Owner:ROBERT BOSCH GMBH
Solid-state bipolar battery having thick electrodes
PendingUS20220166031A1Reduce needLower capability requirementsSolid electrolytesElectrode carriers/collectorsSolid state electrolyteActive particles
The present disclosure provides a solid-state bipolar battery that includes negative and positive electrodes having thicknesses between about 100 μm and about 3000 μm, and a solid-state electrolyte layer disposed between the negative electrode and the positive electrode and having a thickness between about 5 μm and about 100 μm. The first electrode includes a plurality of negative solid-state electroactive particles embedded on or disposed within a first porous material. The second electrode includes plurality of positive solid-state electroactive particles embedded on or disposed within a second porous material that is the same or different from the first porous material. The solid-state bipolar battery includes a first current collector foil disposed on the first porous material, and a second current collector foil disposed on the second porous material. The first and second current collector foils may each have a thickness less than or equal to about 10 μm.
Owner:GM GLOBAL TECH OPERATIONS LLC
HIGH-CAPACITY AND LONG-LIFE NEGATIVE ELECTRODE HYDROGEN STORAGE MATERIAL OF La-Mg-Ni TYPE FOR SECONDARY RECHARGEABLE NICKEL-METAL HYDRIDE BATTERY AND METHOD FOR PREPARING THE SAME
ActiveUS20210218020A1Improve discharge capacityImprove cycle lifeElectrode melt handlingNegative electrodesMaterials scienceNickel–metal hydride battery
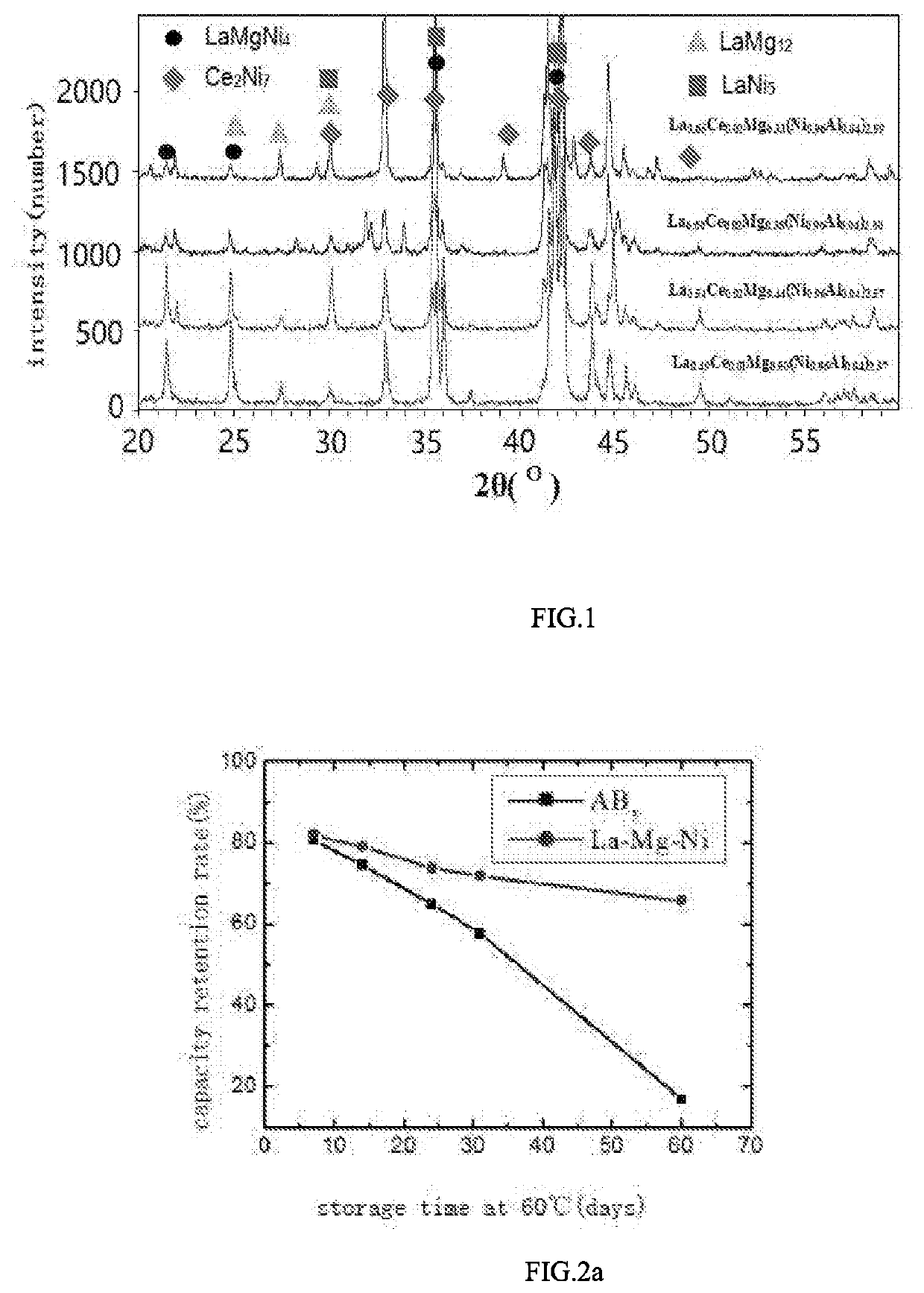
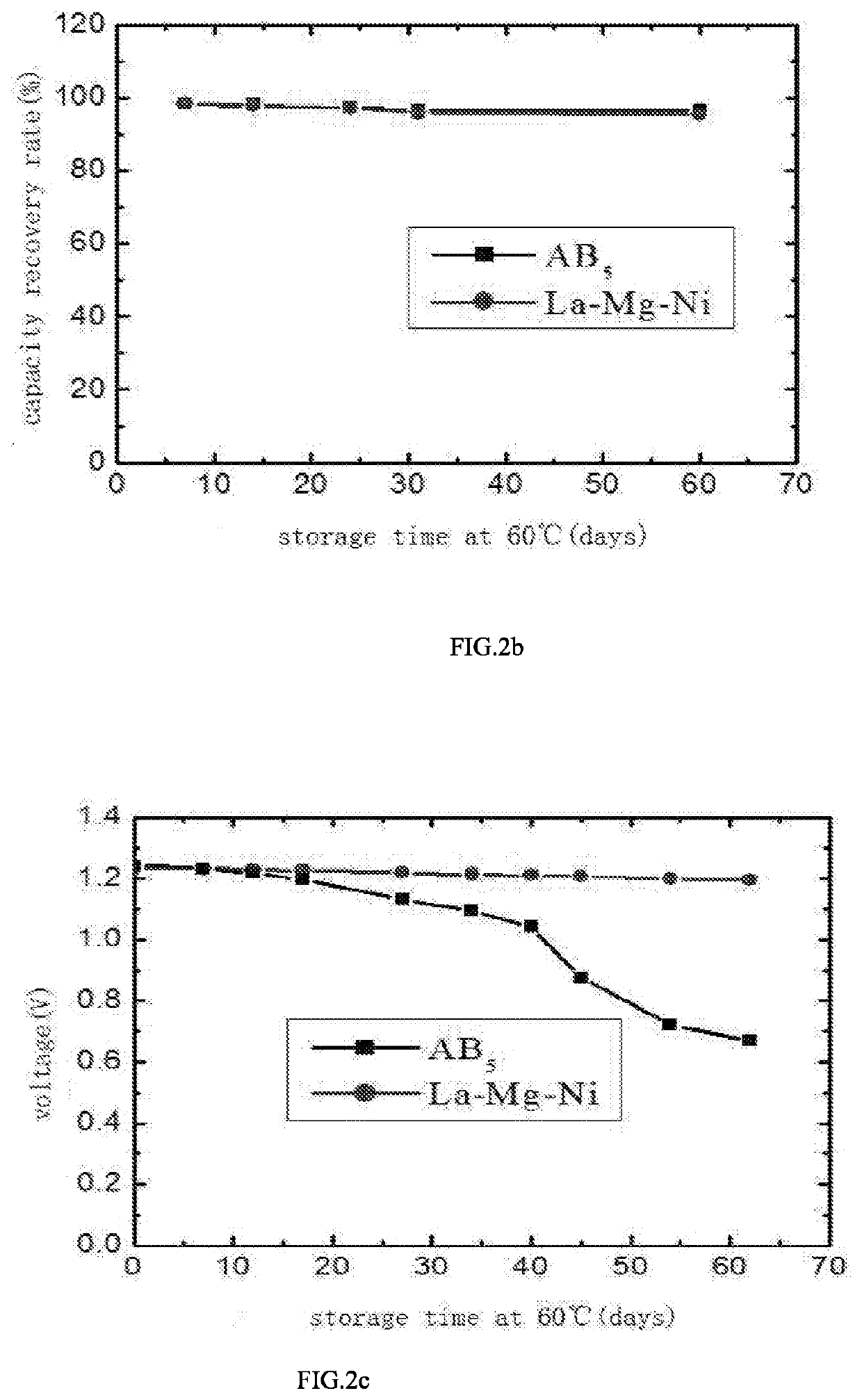
A high-capacity and long-life negative electrode hydrogen storage material of La—Mg—Ni type for secondary rechargeable nickel-metal hydride battery and a method for preparing the same are provided in the present invention. A chemical formula of the negative electrode hydrogen storage material of La—Mg—Ni type is La1-x-yRexMgy(Ni1-a-bAlaMb)z, wherein Re is at least one of Ce, Pr, Nd, Sm, Y, and M is at least one of Ti, Cr, Mo, Nb, Ga, V, Si, Zn, Sn; 0≤x≤0.10, 0.3≤y≤0.5, 0<a≤0.05, 0≤b≤0.02, 2.3≤z<3.0. The negative electrode hydrogen storage material of La—Mg—Ni type in the present invention has excellent charge-discharge capacity and cycle life. The negative electrode hydrogen storage material of La—Mg—Ni type can be applied in both common secondary rechargeable nickel-metal hydride battery and secondary rechargeable nickel-metal hydride battery with ultra-low self-discharge and long-term storage performance.
Owner:JIANGSU JICUI ANTAI CHUANGMING ADVANCED ENERGY MATERIALS RES INST CO LTD
Battery cell with monitoring device, and corresponding operating method
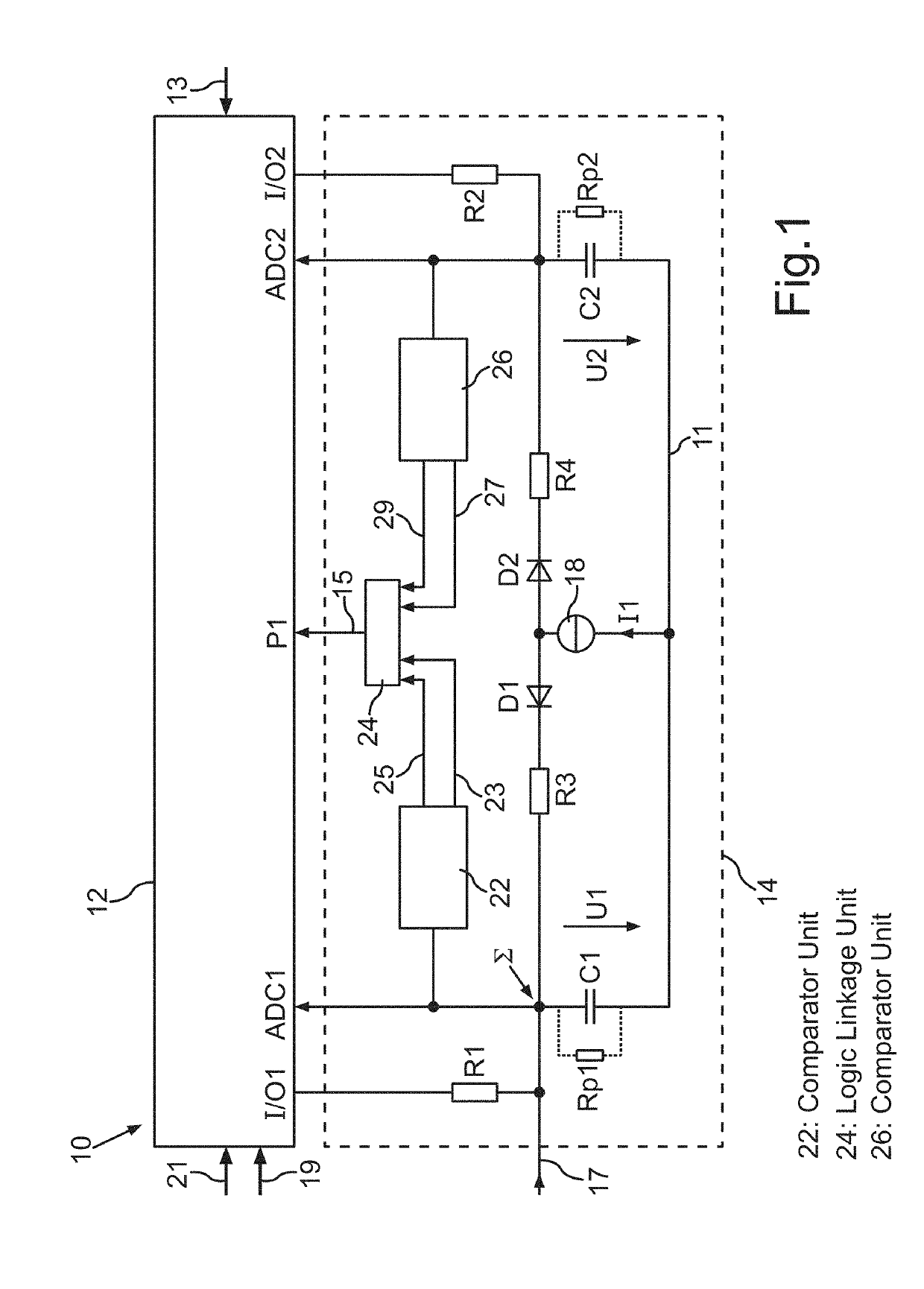
ActiveUS10340558B2Improve processingSpeed up the processBatteries circuit arrangementsTelemetry/telecontrol selection arrangementsProcess stateElectric energy
Owner:AUDI AG
Construction method of mixed type drainage lithium ion battery system based on lithium cobalt oxide and activated carbon
ActiveCN109768329AImprove cycle performanceImprove stabilityFinal product manufactureCell electrodesHybrid typeActivated carbon
The invention discloses a construction method of a mixed type drainage lithium ion battery system based on lithium cobalt oxide and activated carbon. The construction method comprises the following steps: S1, configuring electrolyte; S2, activating positive electrode and negative electrode; S3, determining the LiCoO2 specific capacity; S4, determining the activated carbon specific capacity; S5, determining the positive material load and the negative material load; and S6, constructing a battery system. The construction method disclosed by the invention has the advantages that the acquired electrolyte greatly improves the stability of the negative electrode in comparison with the conventional drainage lithium ion battery, and the cyclic performance of the prepared battery is greatly improved from the whole; the capacitive character AC of the battery constructed by adopting the method greatly improves the rate capability of the negative electrode, and the entire rate capability of the battery is only from the LiCoO2, but not depends on the dual function of the positive electrode and the negative electrode as the existing drainage lithium ion battery, thereby conveniently analyzing the performance of the battery at later period; the cyclicity of the LiCoO2 is improved in the construction method processed, the possibility of producing the residual current since the AC generates theH2 by decomposing the water at the negative electrode is reduced, and the self discharge rate of the system is reduced.
Owner:TAIYUAN UNIVERSITY OF SCIENCE AND TECHNOLOGY +2
Power supply for an electric window dimming device
ActiveUS7746037B2Provide energyReduce weightBatteries circuit arrangementsAntiglare equipmentCapacitanceEngineering
An electric window dimming device for reducing the light transmission of a window for an aircraft. The electric window dimming device comprises a capacitance. The electric window dimming device is adapted for being connected to the capacitance and to a primary energy source that provides energy. If the primary energy source fails, the capacitance supplies energy for electric window dimming device.
Owner:AIRBUS OPERATIONS GMBH
Methods for forming ionically conductive polymer composite interlayers in solid-state batteries
PendingUS20220344700A1Reduce needExtended shelf lifeSolid electrolytesElectrode thermal treatmentSolid state electrolytePolymer science
The present disclosure provides a method for forming an ionically conductive polymer composite interlayer. The method may include forming a precursor layer between a first surface of an electroactive material layer and a first surface of a solid-state electrolyte layer and converting the precursor layer to the ionically conductive polymer composite interlayer. The at least one of the electroactive material layer or solid-state electrolyte may include lithium. The first surface of the electroactive material layer and the first surface of the solid-state electrolyte layer may be substantially parallel. The precursor layer may include one or more fluoropolymers comprising carbon and fluorine. The ionically conductive polymer composite layer may have an ionic conductivity greater than or equal to about 1.0×10−8 S·cm−1 to less than or equal to about 1.0 S·cm−1 and may include a lithium fluoride embedded in a carbonaceous matrix.
Owner:GM GLOBAL TECH OPERATIONS LLC
A kind of composite cathode material that reduces self-discharge degree and its preparation method and application
ActiveCN110120495BIncrease output specific capacityReduce active positive polarizationDeferred-action cellsPositive electrodesElectrical batteryThermal Battery
The invention discloses a composite positive electrode material for reducing the self-discharge degree, which is made of thermal battery active positive electrode material, polar thiophilic fixing material, potassium-containing electrolyte and high-conductivity conductive agent, and the mass ratio of the above raw materials is: heat Battery active positive electrode material: polar thiophilic fixing material: potassium-containing electrolyte: high-conductivity conductive agent = 50-90: 2-40: 10-30: 0.1-5. The preparation method of the present invention can reduce the self-discharge degree of thermal battery composite positive electrode material with excellent performance and high consistency, and the prepared composite positive electrode material can significantly reduce the self-discharge degree of thermal battery positive electrode material at low current density, which can significantly reduce The capacity loss caused by the self-discharge of the sulfide positive electrode of the thermal battery at a lower current density improves the output specific capacity of the positive electrode of the thermal battery at a lower current density, significantly prolongs the working time, and is suitable for thermal batteries that work for a long time.
Owner:GUIZHOU MEILING POWER SUPPLY CO LTD
Sulfur-carbon composite material, its application in lithium-sulfur battery and method for preparing said composite material
ActiveUS10109847B2Efficient transportLarge capacityCarbon compoundsCell electrodesCarbon compositesSulfur
The present invention provides a sulfur-carbon composite material, said composite material comprising a porous carbon substrate containing both micropores and mesopores and sulfur, wherein the sulfur is only contained in the micropores of the carbon substrate. Moreover, a lithium-sulfur battery with its cathode comprising said sulfur-carbon composite material and the method for preparing such material is also provided.
Owner:ROBERT BOSCH GMBH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com