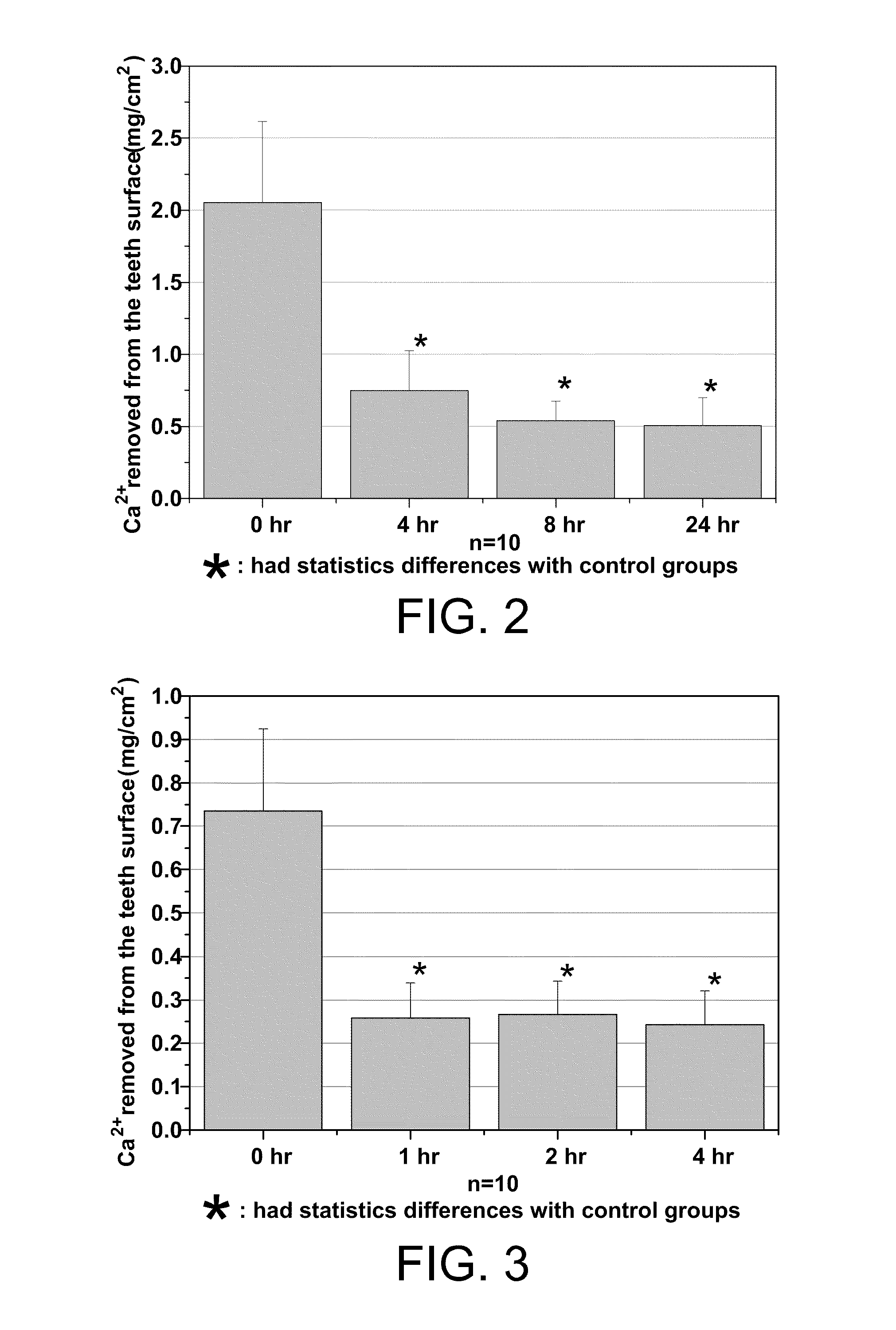
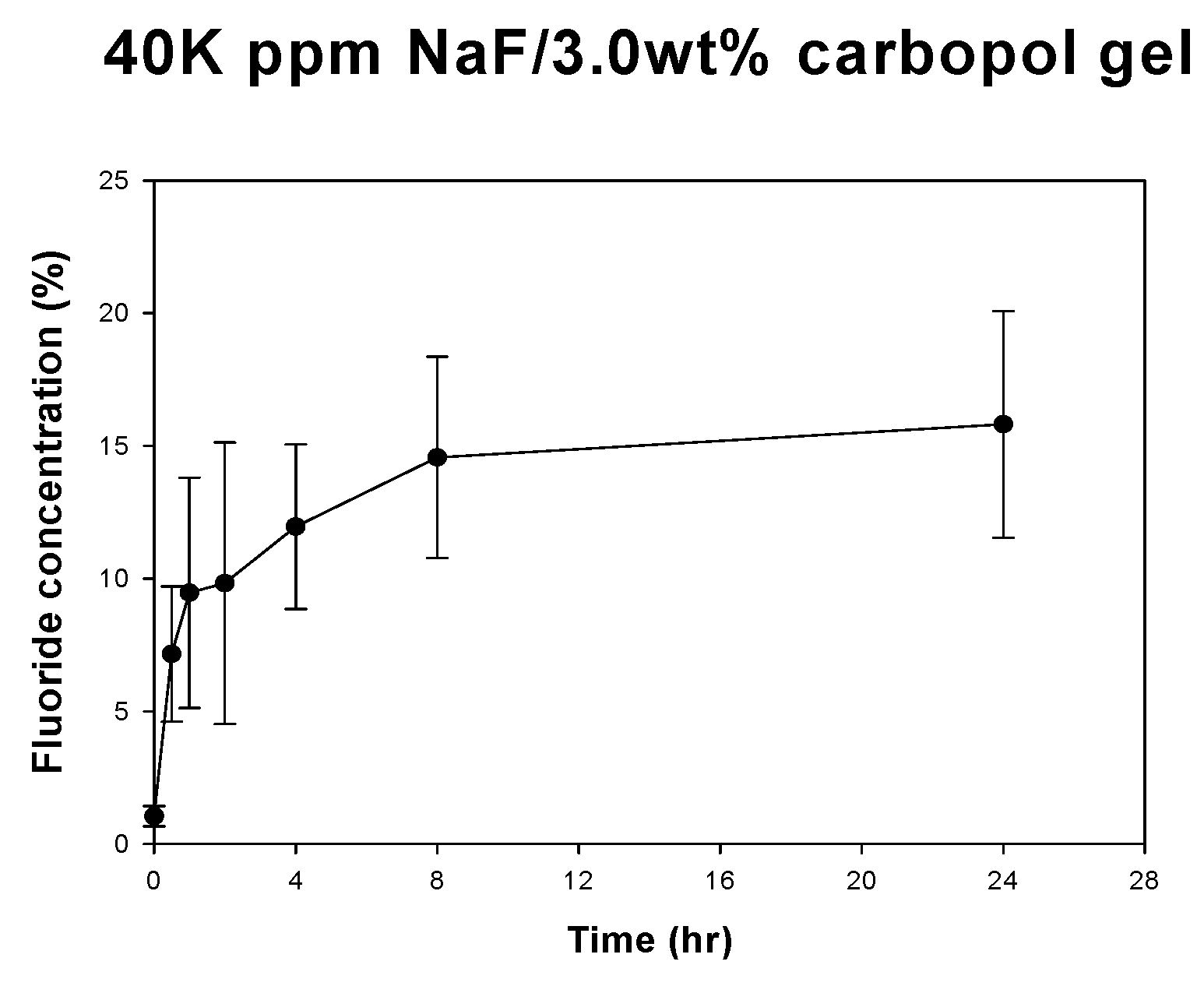
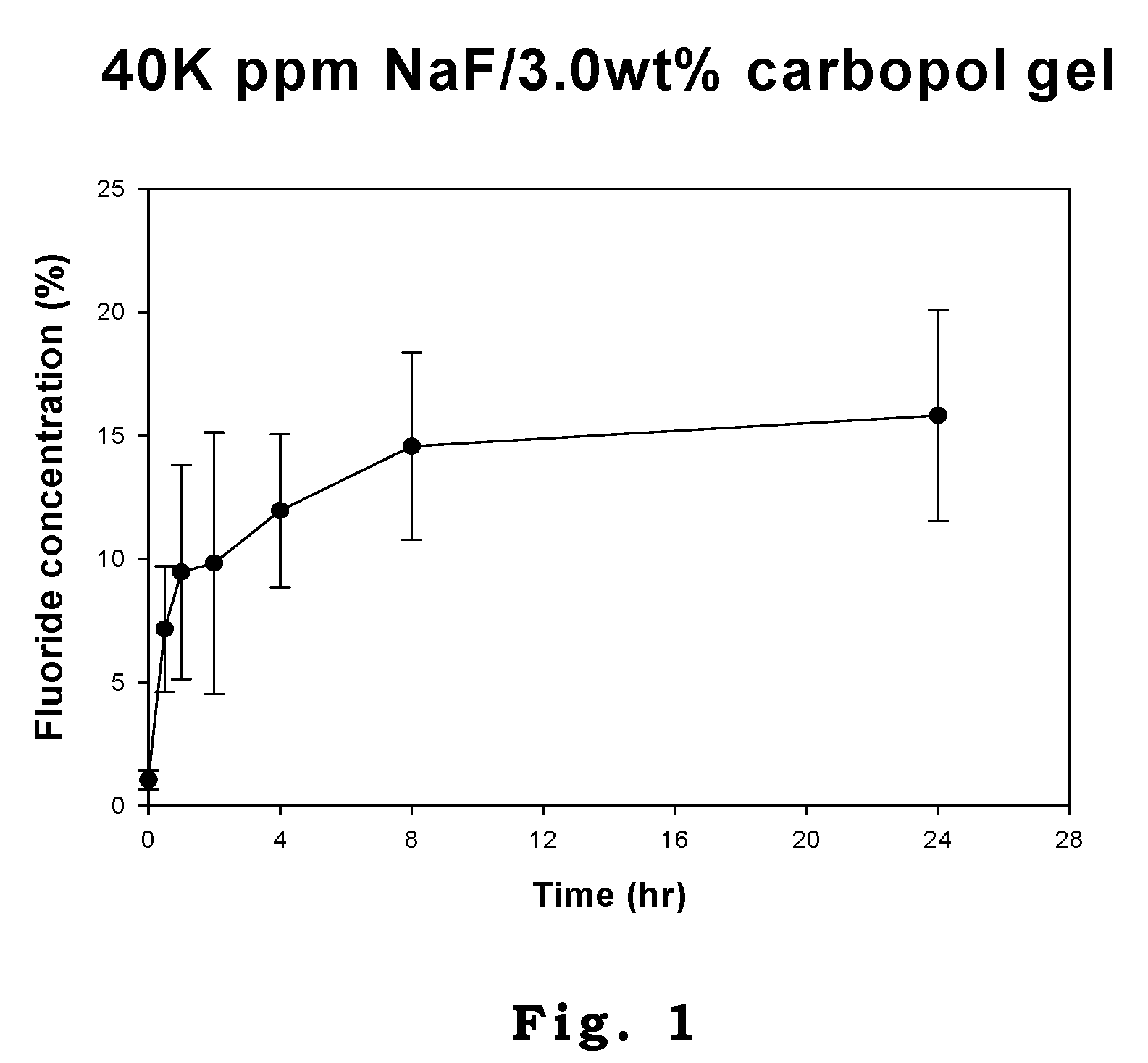
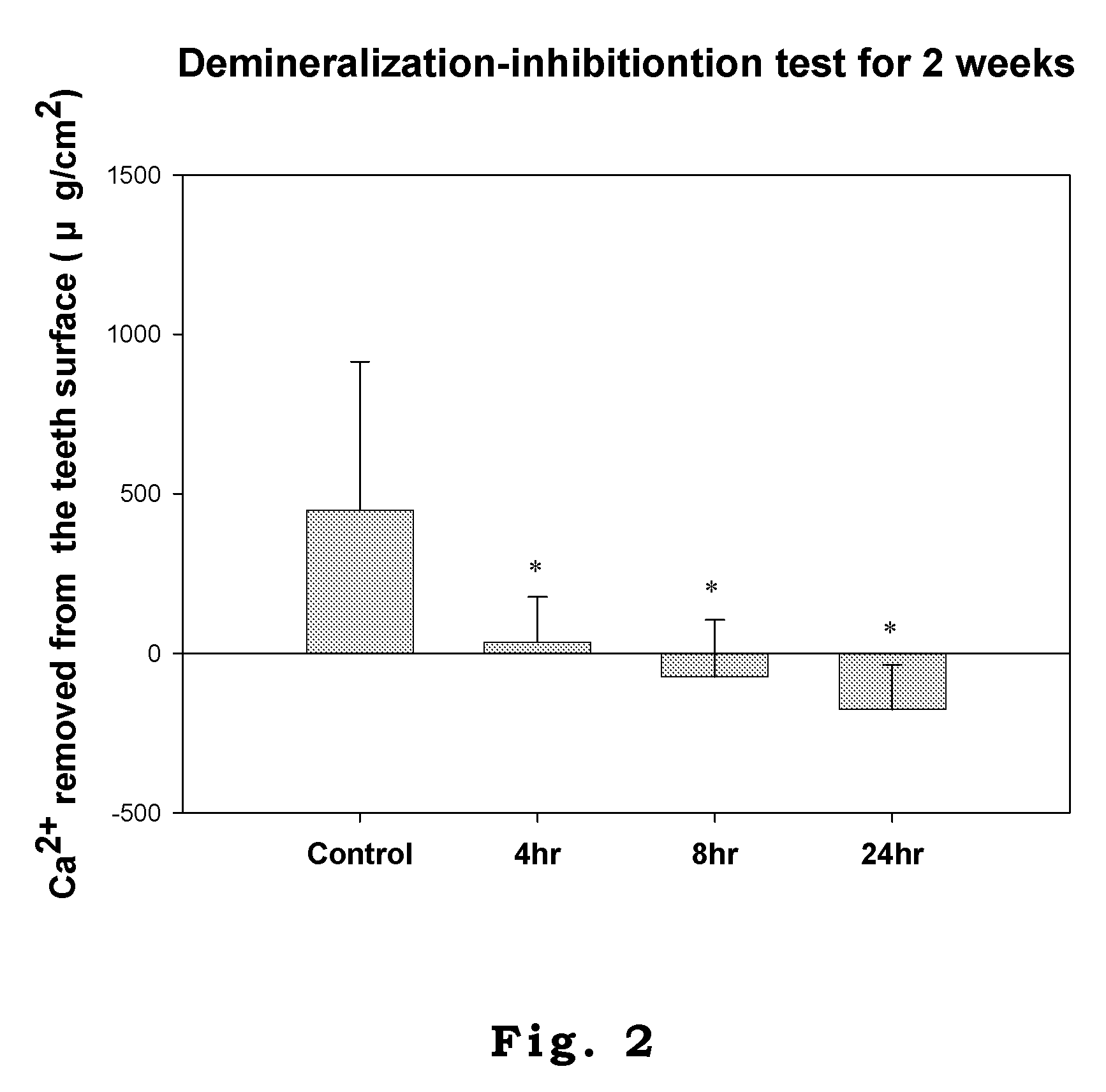
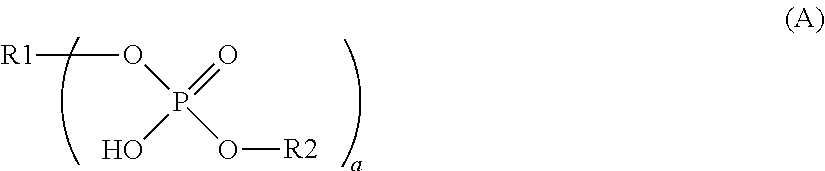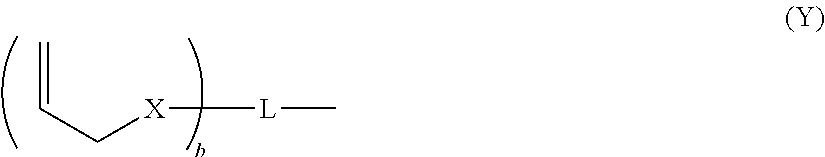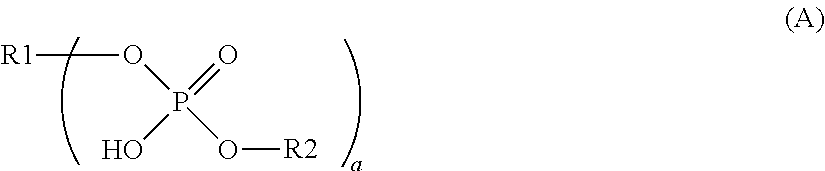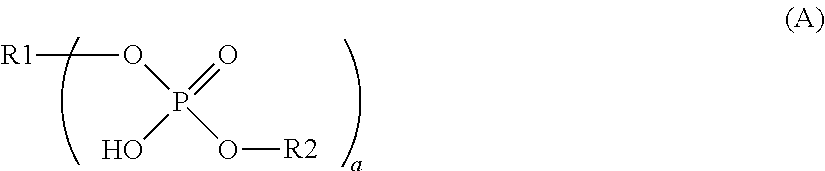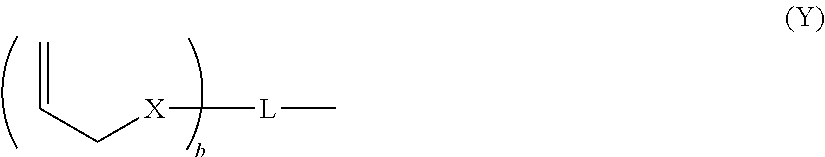Patents
Literature
30 results about "Fluoride release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
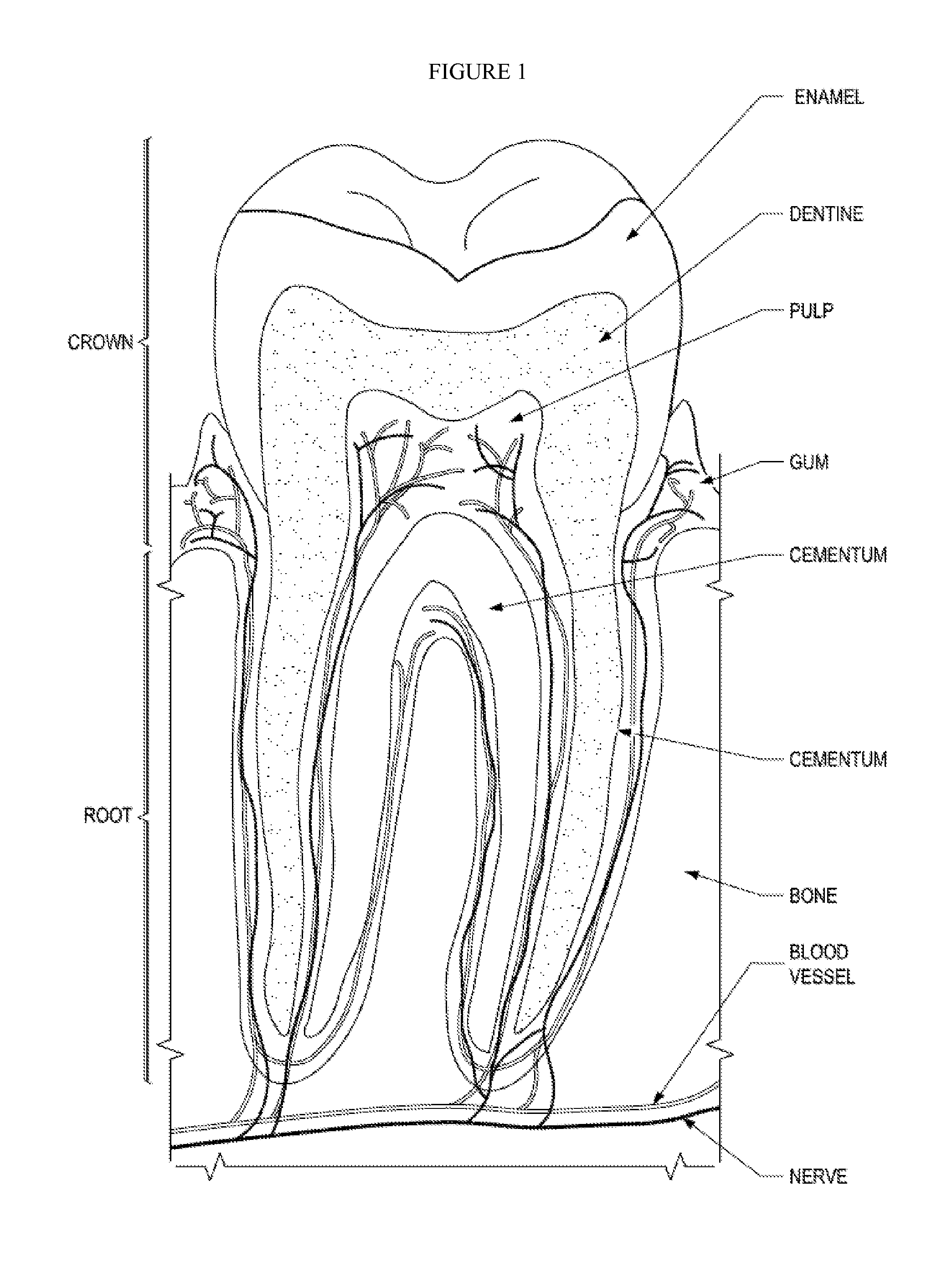
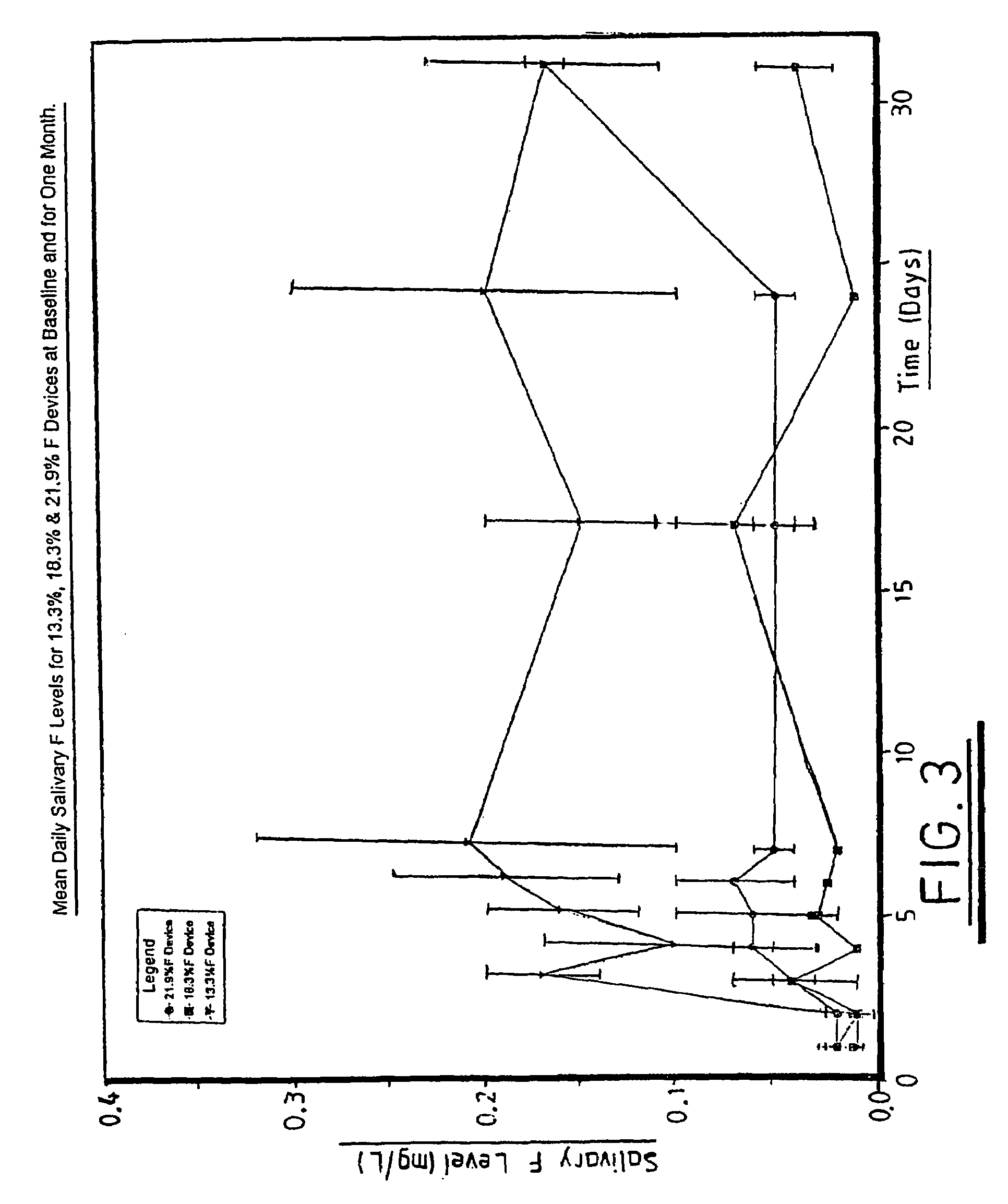
Fluoride release. Compomers release some fluoride ions, like a glass ionomer cement. The level of this fluoride release however is only around 10% of that of a glass ionomer, which makes it less useful for deciduous restorations.
Method and product for phosphosilicate slurry for use in dentistry and related bone cements
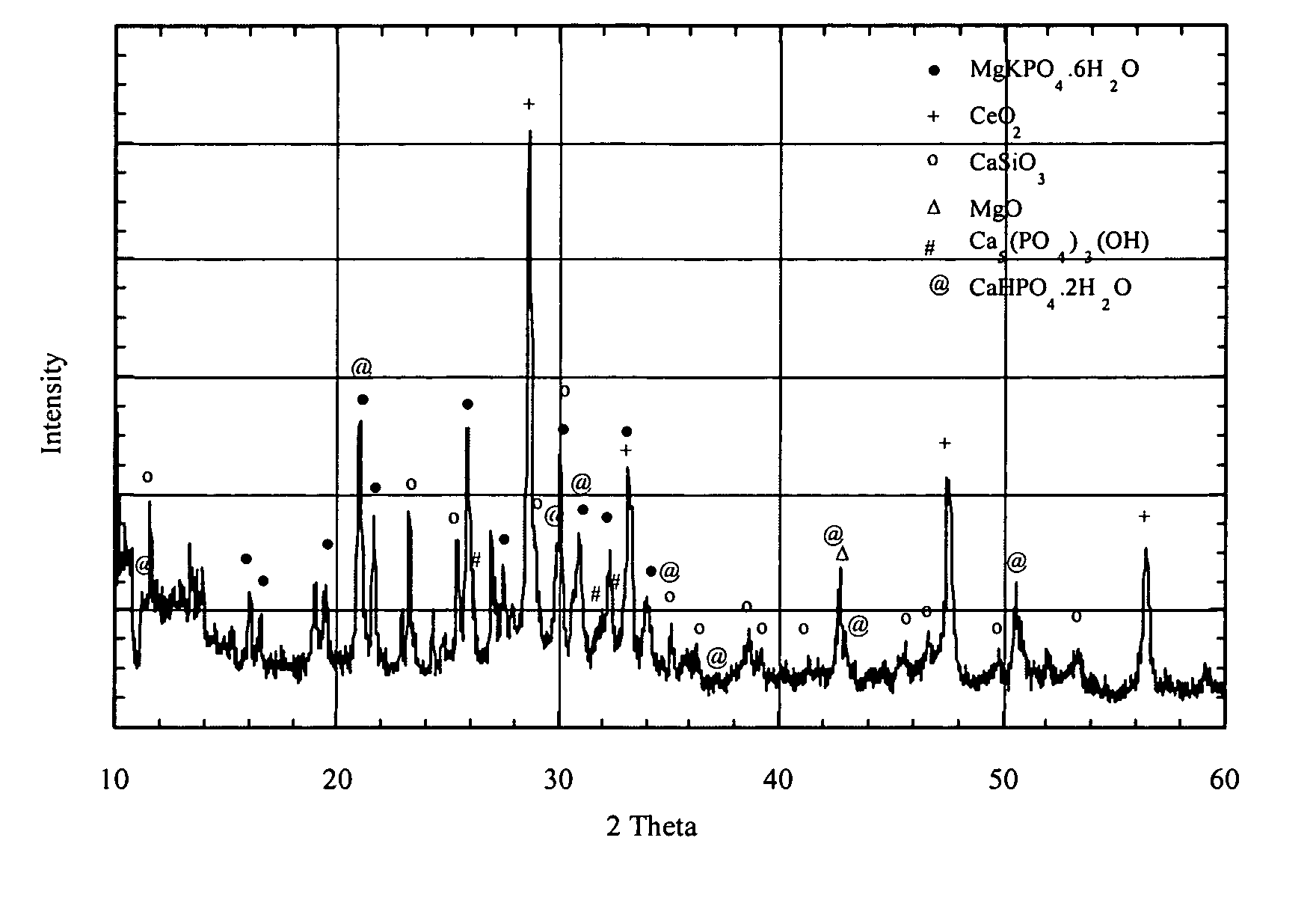
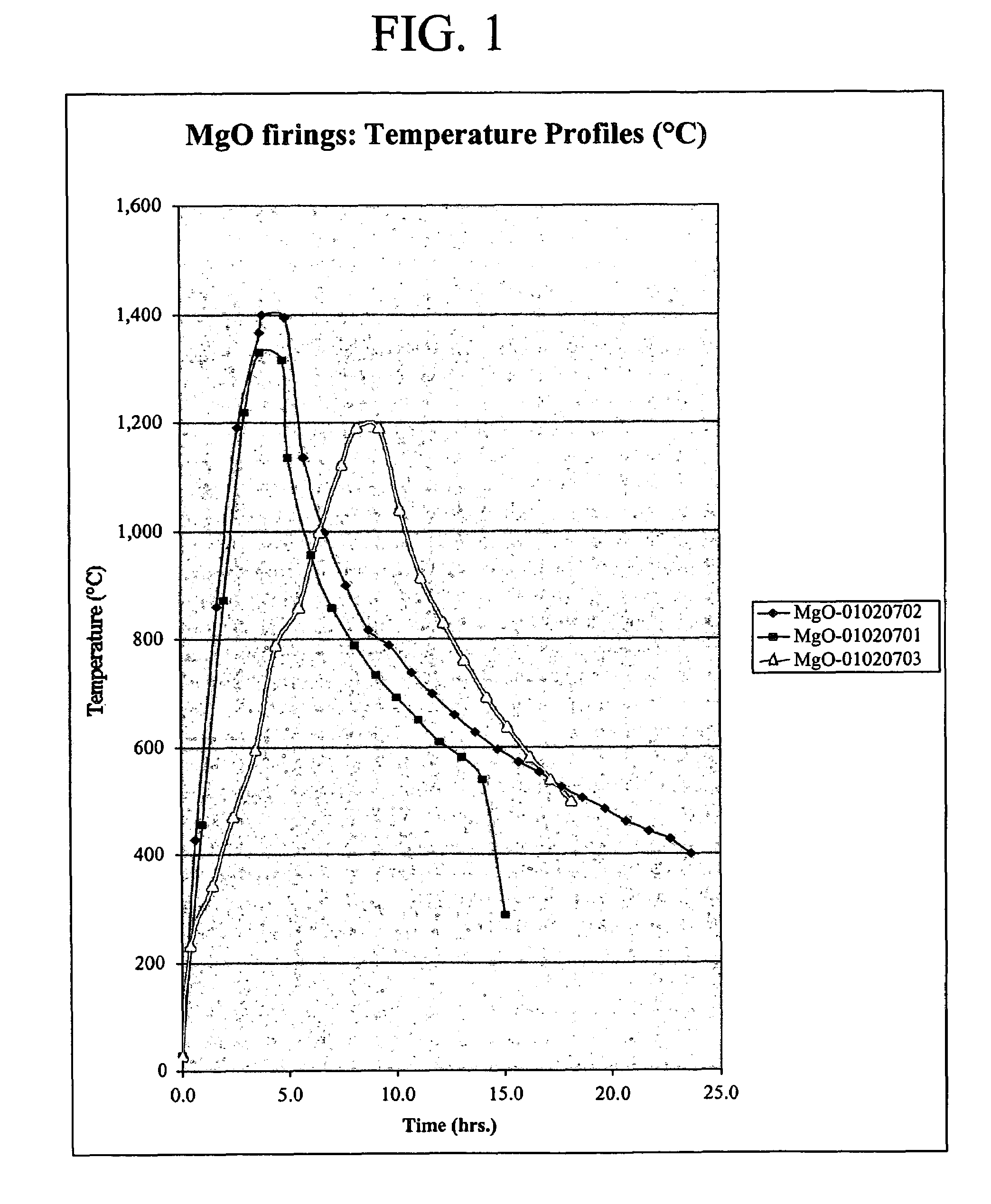
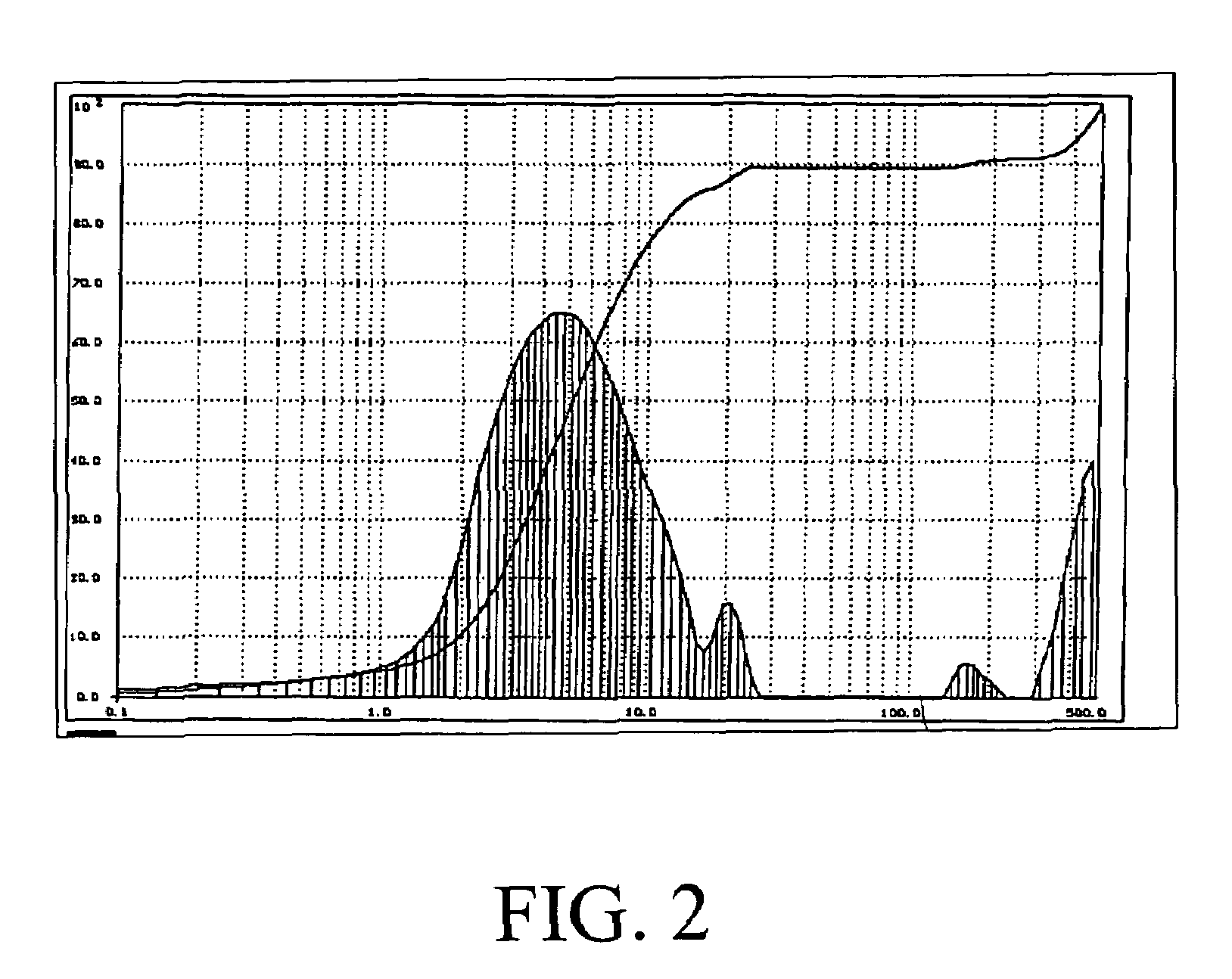
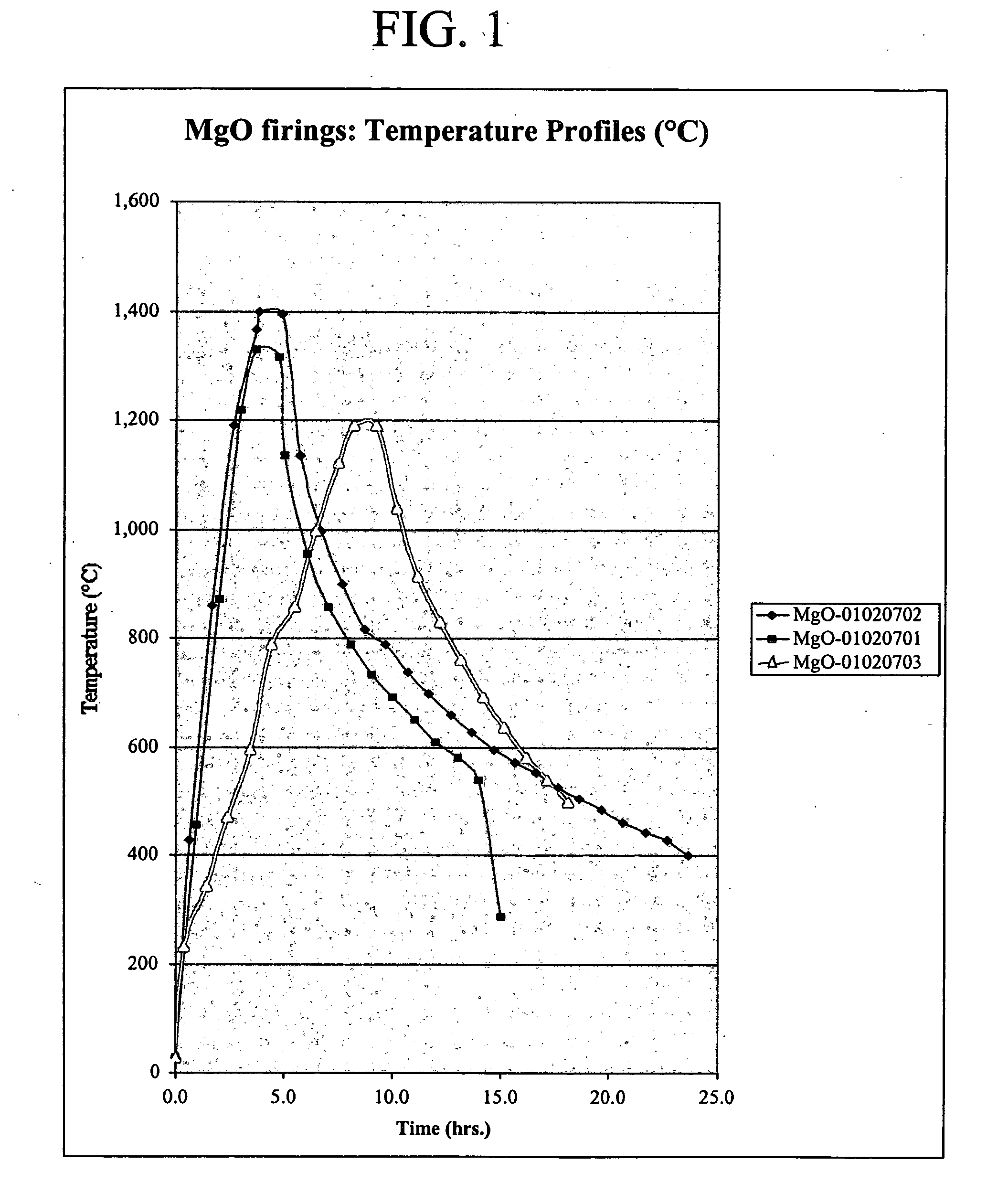
The present invention is directed to magnesium phosphate ceramics and their methods of manufacture. The composition of the invention is produced by combining a mixture of a substantially dry powder component with a liquid component. The substantially dry powder component comprises a sparsely soluble oxide powder, an alkali metal phosphate powder, a sparsely soluble silicate powder, with the balance of the substantially dry powder component comprising at least one powder selected from the group consisting of bioactive powders, biocompatible powders, fluorescent powders, fluoride releasing powders, and radiopaque powders. The liquid component comprises a pH modifying agent, a monovalent alkali metal phosphate in aqueous solution, the balance of the liquid component being water. The use of calcined magnesium oxide as the oxide powder and hydroxylapatite as the bioactive powder produces a self-setting ceramic that is particularly suited for use in dental and orthopedic applications.
Owner:UCHICAGO ARGONNE LLC +1
Method and product for phosphosilicate slurry for use in dentistry and related bone cements
ActiveUS20050028705A1Strong and long-lasting bondHigh strengthBiocideImpression capsMagnesium phosphateApatite
The present invention is directed to magnesium phosphate ceramics and their methods of manufacture. The composition of the invention is produced by combining a mixture of a substantially dry powder component with a liquid component. The substantially dry powder component comprises a sparsely soluble oxide powder, an alkali metal phosphate powder, a sparsely soluble silicate powder, with the balance of the substantially dry powder component comprising at least one powder selected from the group consisting of bioactive powders, biocompatible powders, fluorescent powders, fluoride releasing powders, and radiopaque powders. The liquid component comprises a pH modifying agent, a monovalent alkali metal phosphate in aqueous solution, the balance of the liquid component being water. The use of calcined magnesium oxide as the oxide powder and hydroxylapatite as the bioactive powder produces a self-setting ceramic that is particularly suited for use in dental and orthopedic applications.
Owner:UCHICAGO ARGONNE LLC +1
Filler containing composition and process for production and use thereof
ActiveUS7968617B2Produced cost-effectivelyEliminate needCosmetic preparationsImpression capsSilanesSolvent
The invention relates to a composition comprising an ethylenically unsaturated acidic compound, water, a functionalized silane, an initiator, optionally comprising a sensitising agent, a non-surface treated filler, optionally a solvent, optionally an ethylenically unsaturated compound, optionally additives selected from the group consisting of stabilizer(s), photobleachable colorant(s), fluoride release agent(s), pigments. The invention also relates to a process of producing such a composition by in-situ silanization of the non-surface treated filler.
Owner:3M INNOVATIVE PROPERTIES CO
Mineral trioxide aggregate (MTA) composition and use
ActiveUS20120156308A1Good strength developmentFast setting timeHeavy metal active ingredientsBiocideDiseaseHigh fluoride
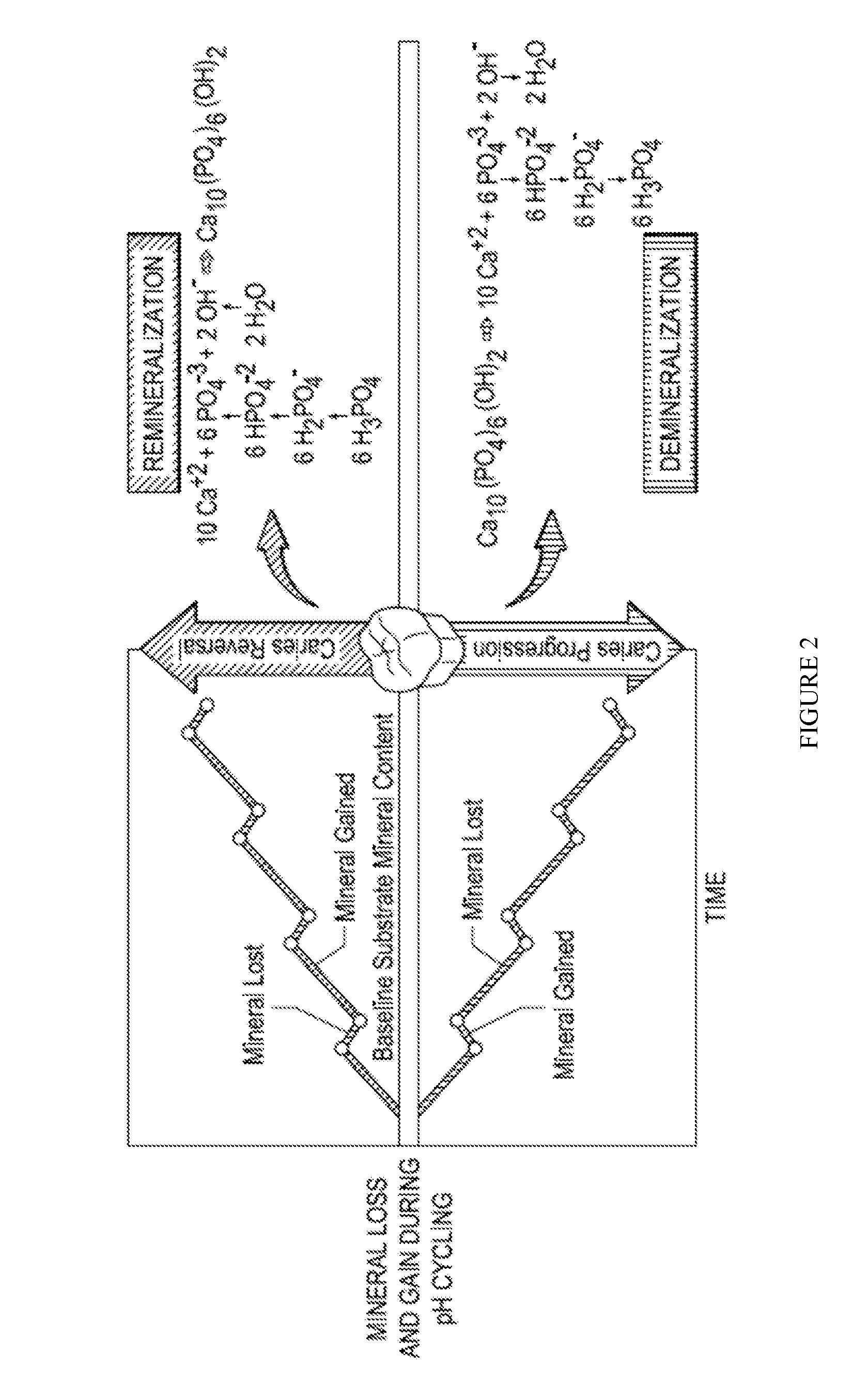
The present application discloses a fast-setting mineral trioxide aggregate (MTA) with fluoride release for practical treatment of diseases in teeth and bone, e.g. for caries treatment and / or prevention. The cariostatic MTA contain calcia-silica-alumina cement with moderately increased tricalcium aluminate content allowing high calcium hydroxide release. The MTA composition support remineralization and biomineralization, and it is suitable for stimulation of hard tissue regeneration. MTA embodiments contain superplasticizerand nanosilicate for improved mechanical properties. The MTA compositions include optional radiocontrast and nano-enriched leachable fluorine, nitrate, strontium, and phosphate. The fast-setting MTA paste exhibits flow-to-clay-like consistency, which allows new practical applications including cavity lining, temporary restoration, bonding, and cementations in one MTA embodiment. The high calcium hydroxide and high fluoride release are suitable for caries prevention and treatment, and per se inhibition of dental symptoms.
Owner:DENTOSOLVE APS
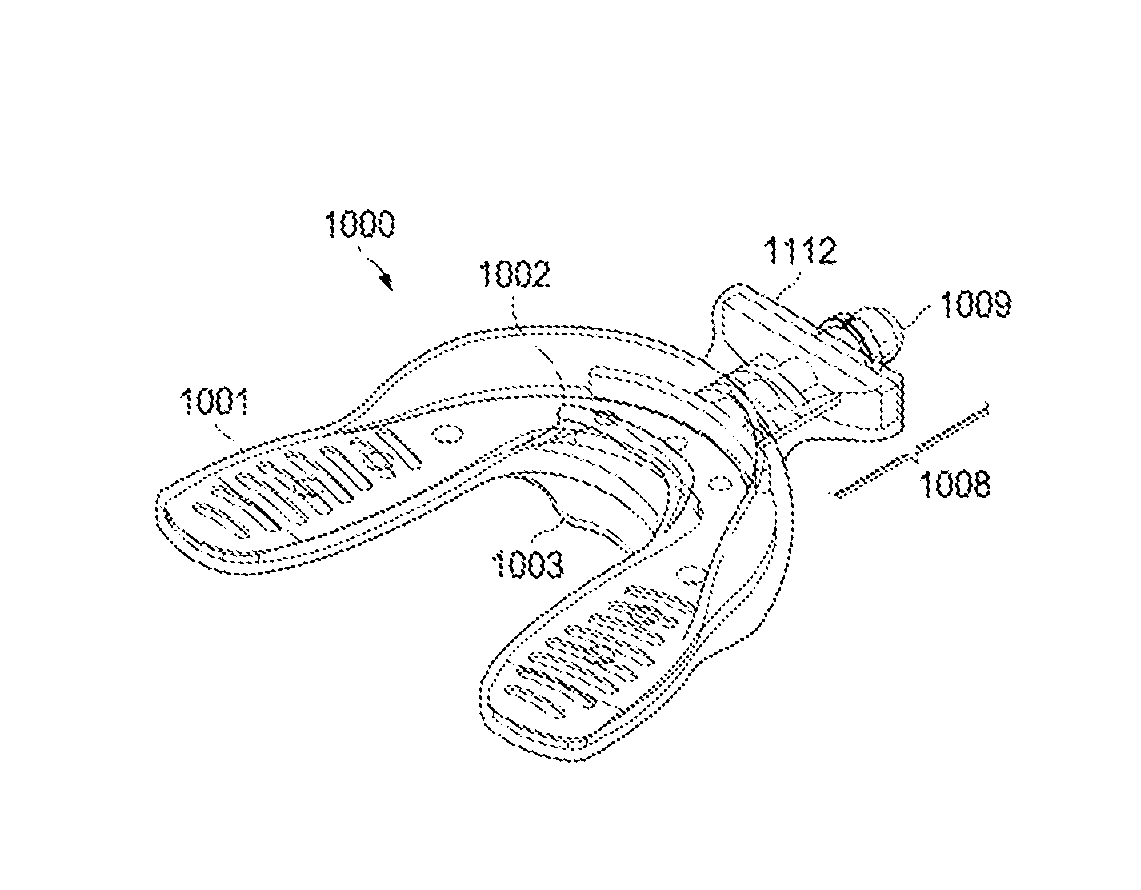
Fluoride releasing bite plate
The present disclosure relates to biteplates for use with various orthodontic remodeling devices, wherein the bite plate releases fluoride during use, thus encouraging remineralization on enamel. Additionally, bite plates with a colorant or color-changing matrix that serve as indicators for fluoride concentration are also described.
Owner:ADVANCED ORTHODONTICS & EDUCATION ASSOC LLC
Long-lasting, flavored dosage forms for sustained release of beneficial agents within the mouth
ActiveUS8236348B2Good sustained releaseSustained releaseBiocideCosmetic preparationsZinc compoundsHydrophilic polymers
Flavored dosage forms, e.g., lozenges and gums, are provided for sustained release of a flavoring agent in the mouth. The dosage forms provide sustained release by virtue of a wet matrix formed by admixture of a biocompatible, hydrophilic, water-insoluble polymer such as ethylcellulose and a flavoring agent, particularly an essential oil or a constituent thereof, e.g., a terpene or sesquiterpene. The dosage forms may also include a second beneficial agent in addition to the flavoring agent. Exemplary such beneficial agents include ionizable zinc compounds and other cold remedies, local anesthetic and anti-infective agents, diet aids, fluoride-releasing compounds, and nicotine. The dosage forms, when formulated as lozenges, may be somewhat adhesive or substantially nontacky, depending primarily on the molecular weight of the hydrophilic polymer. Adhesive lozenges can serve as dosage forms that adhere to the teeth or gums for delivery of a beneficial agent thereto. Methods for using the dosage forms to provide sustained release of a flavoring agent and optionally deliver a second beneficial agent are also provided, as are methods for treating the common cold, treating a sore throat, facilitating weight loss, and assisting in smoking cessation.
Owner:BENNES
Mitigation of Membrane Degradation by Multilayer Electrode
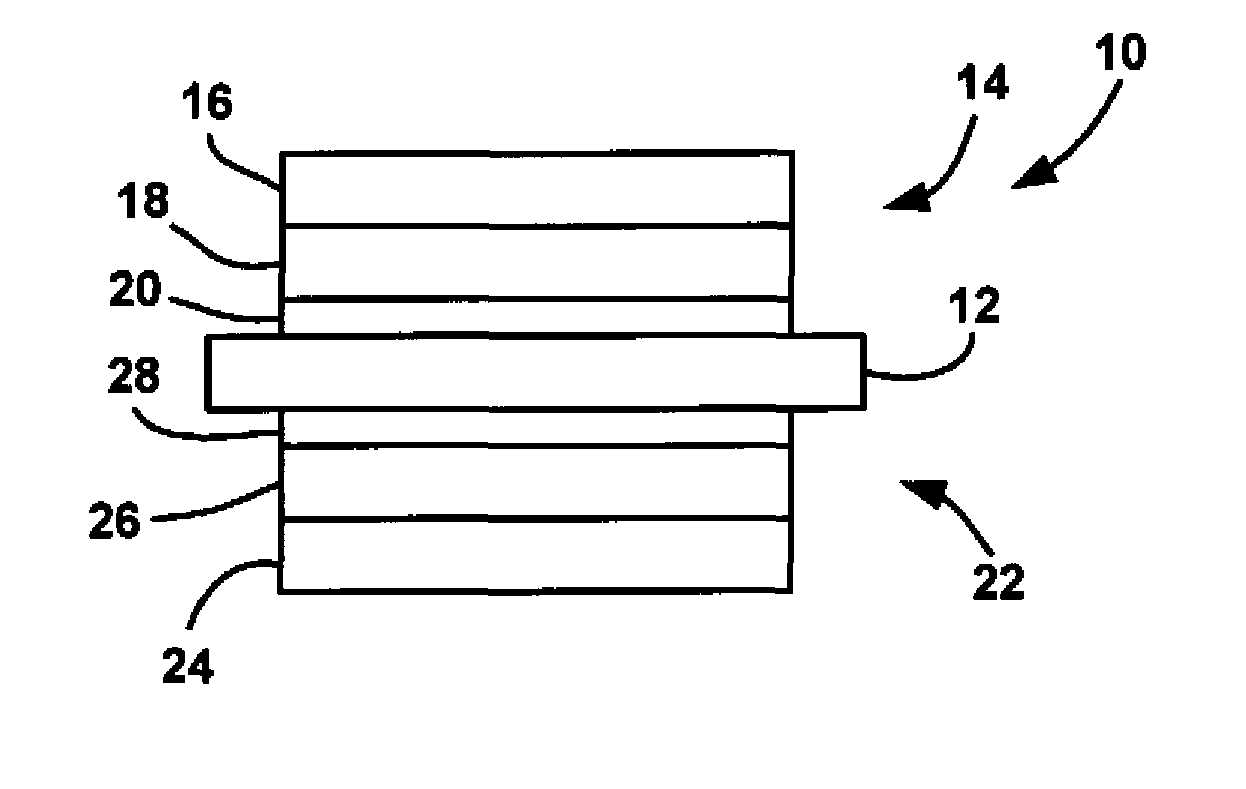
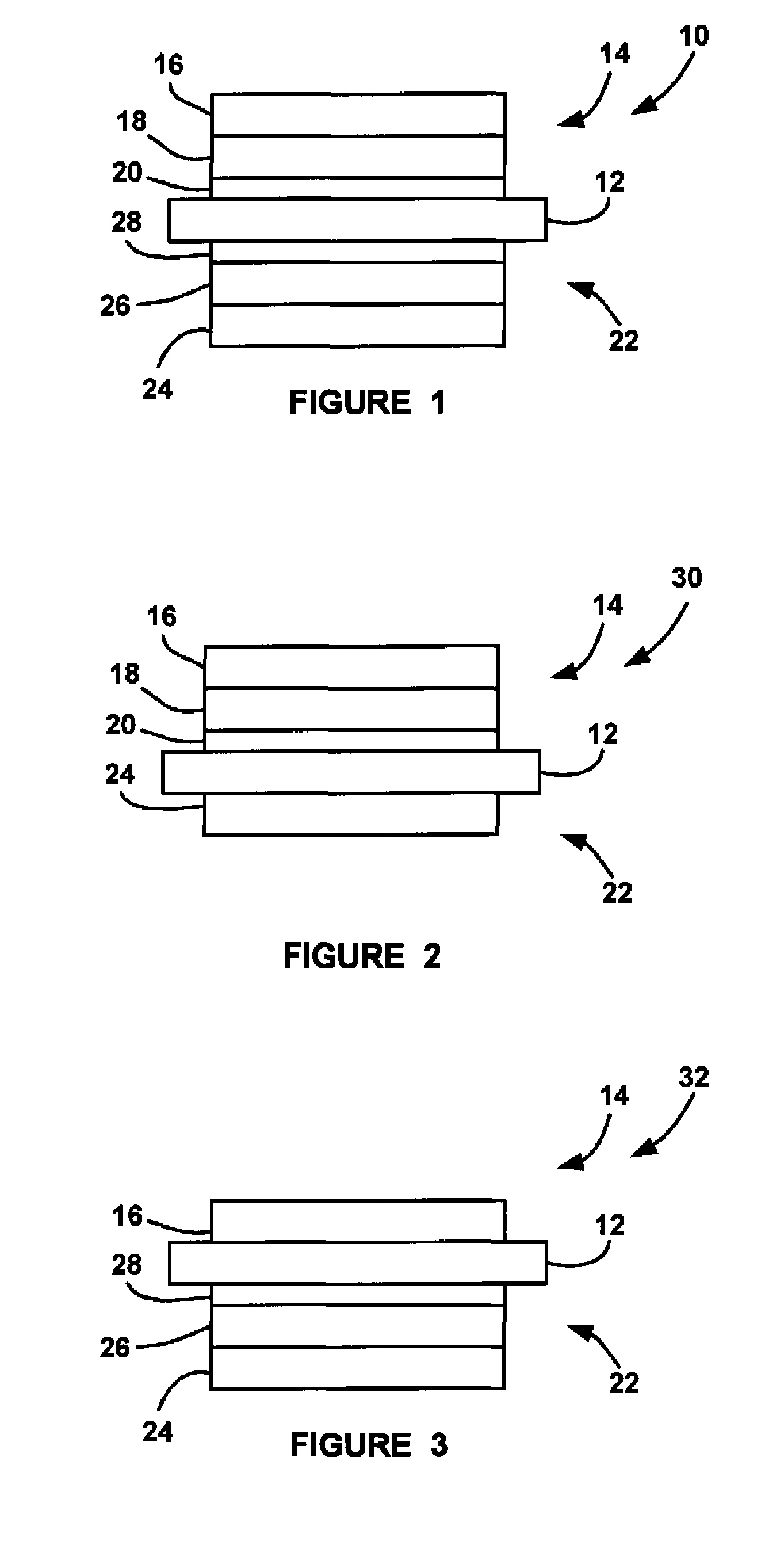
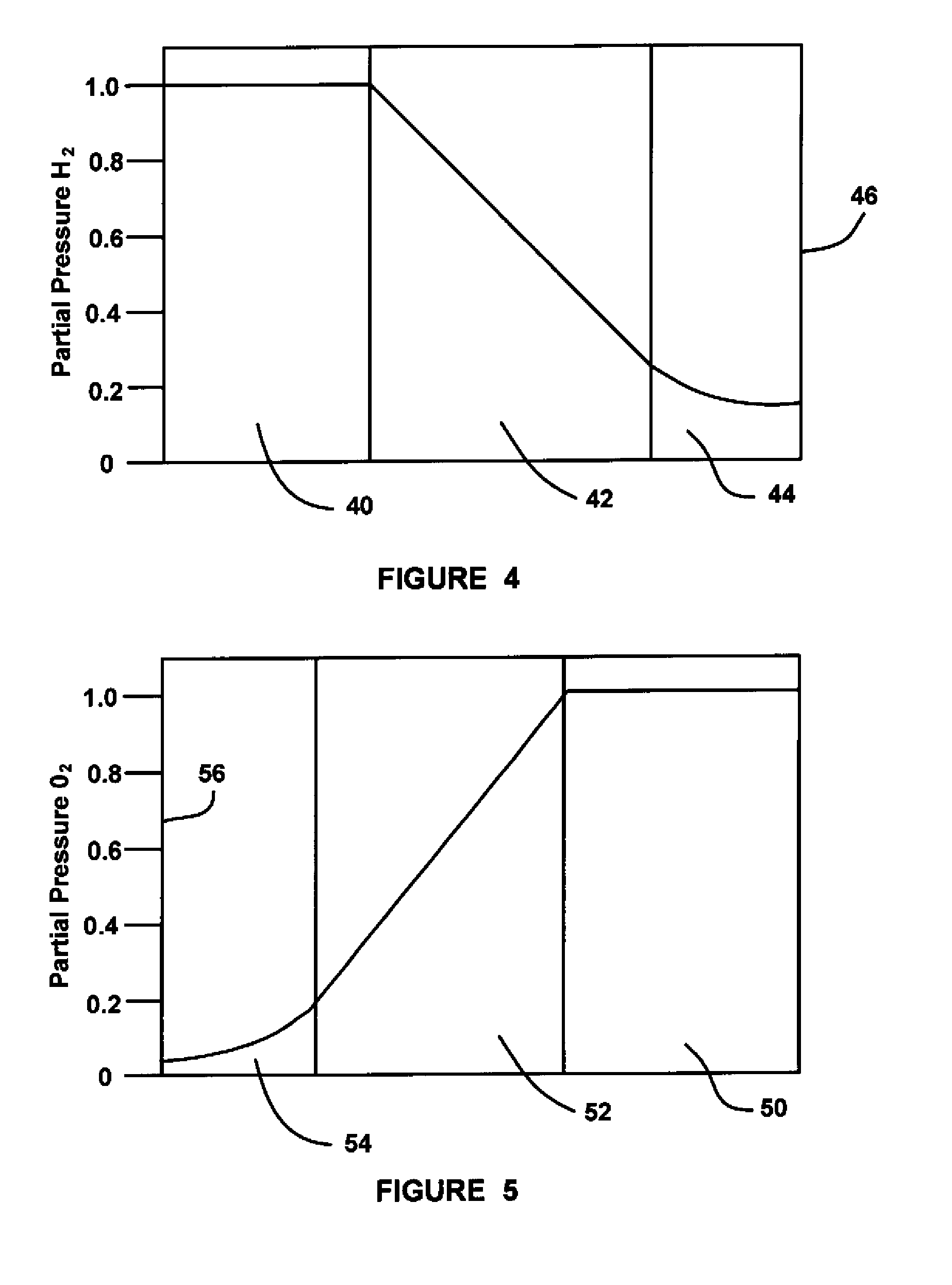
ActiveUS20090029235A1Reduce pressureLow release rateSolid electrolytesActive material electrodesHydrogenFuel cells
An MEA for a fuel cell that employs multiple catalyst layers to reduce the hydrogen and / or oxygen partial pressure at the membrane so as to reduce the fluoride release rate from the membrane and reduce membrane degradation. An anode side multi-layer catalyst configuration is positioned at the anode side of the MEA membrane. The anode side multi-layer catalyst configuration includes an anode side under layer positioned against the membrane and including a catalyst, an anode side middle layer positioned against the anode side under layer and not including a catalyst and an anode side catalyst layer positioned against the anode side middle layer and opposite to the anode side under layer and including a catalyst, where the amount of catalyst in the anode side catalyst layer is greater than the amount of catalyst in the anode side under layer.
Owner:GM GLOBAL TECH OPERATIONS LLC
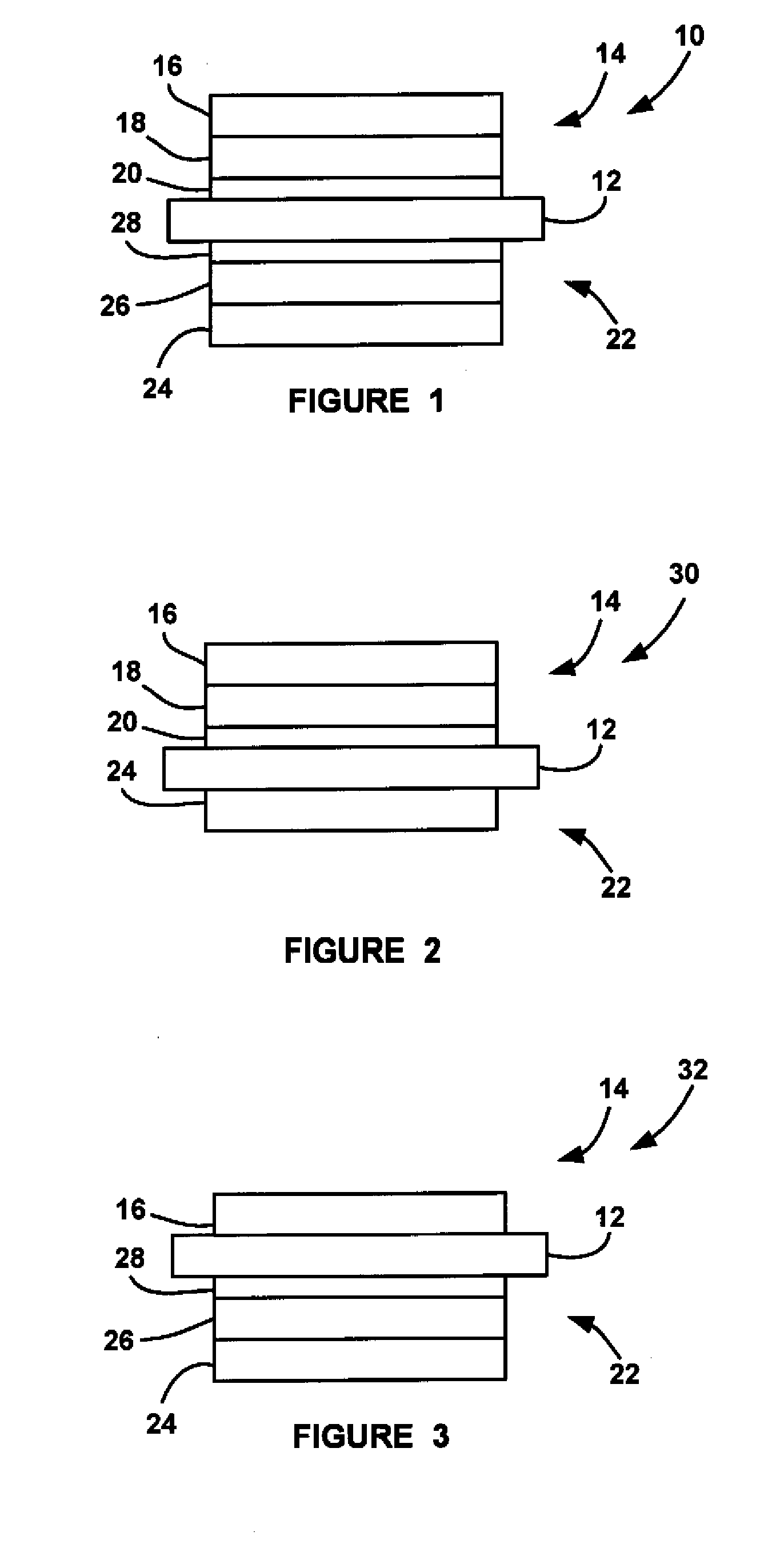
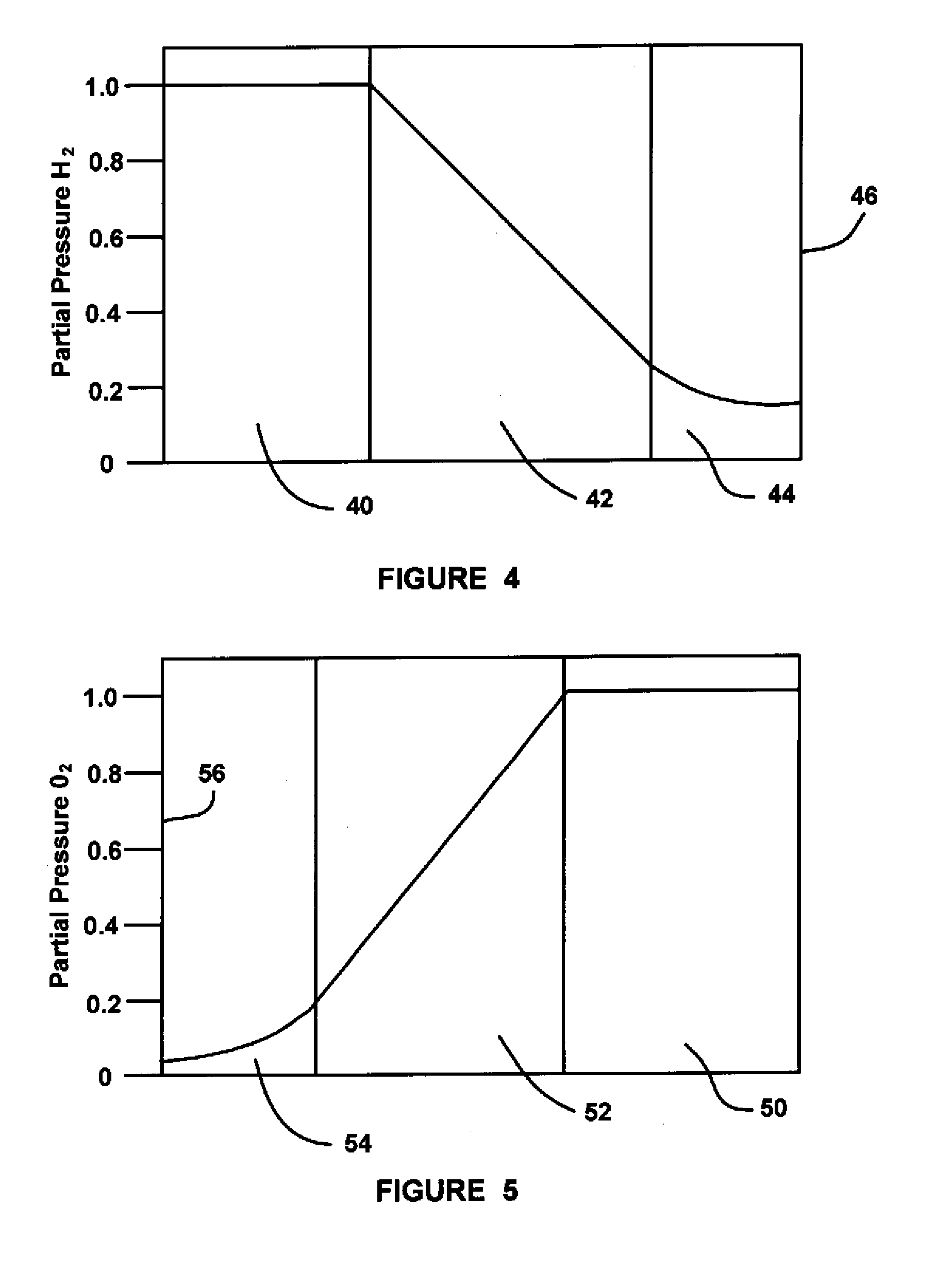
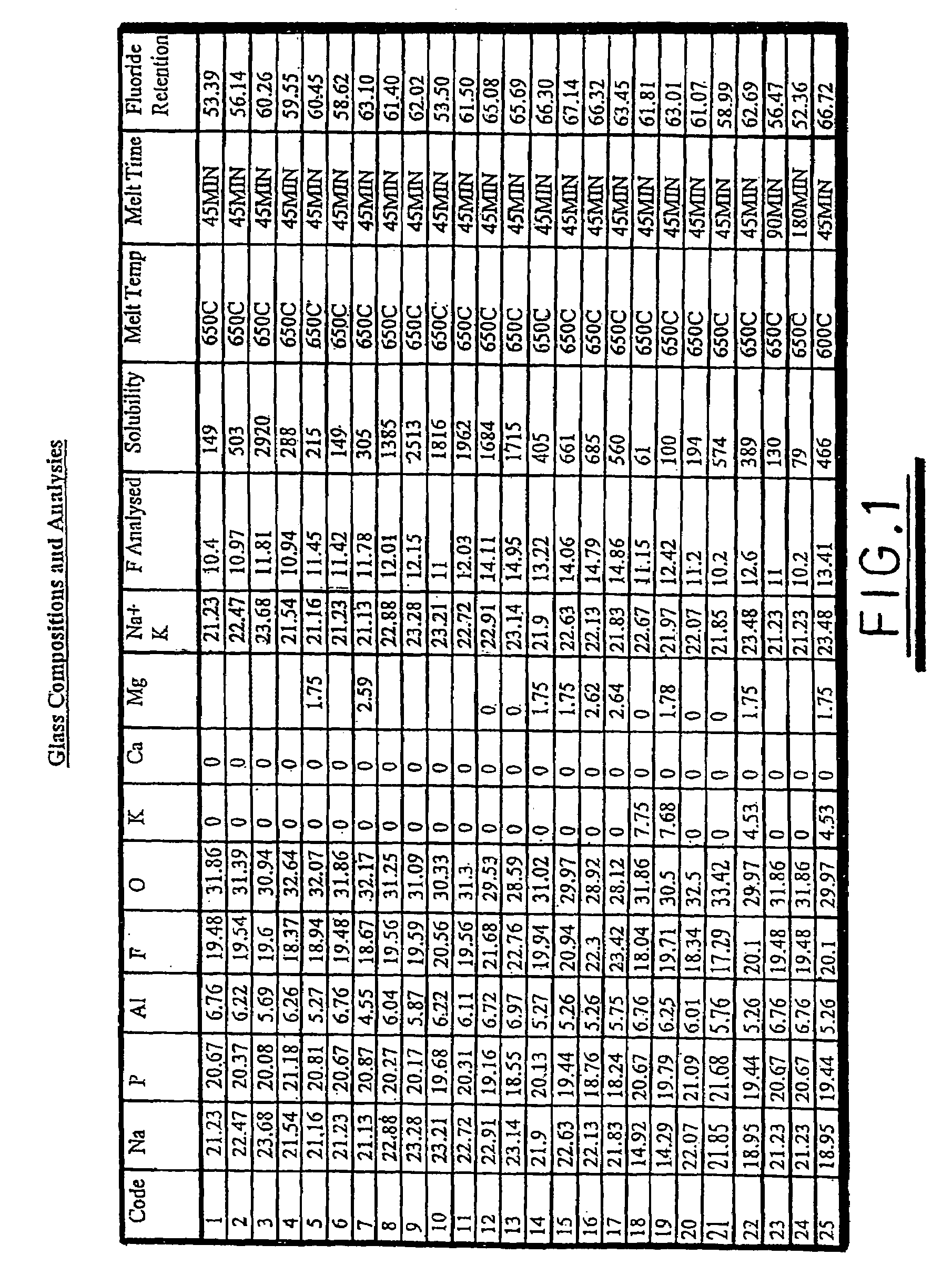
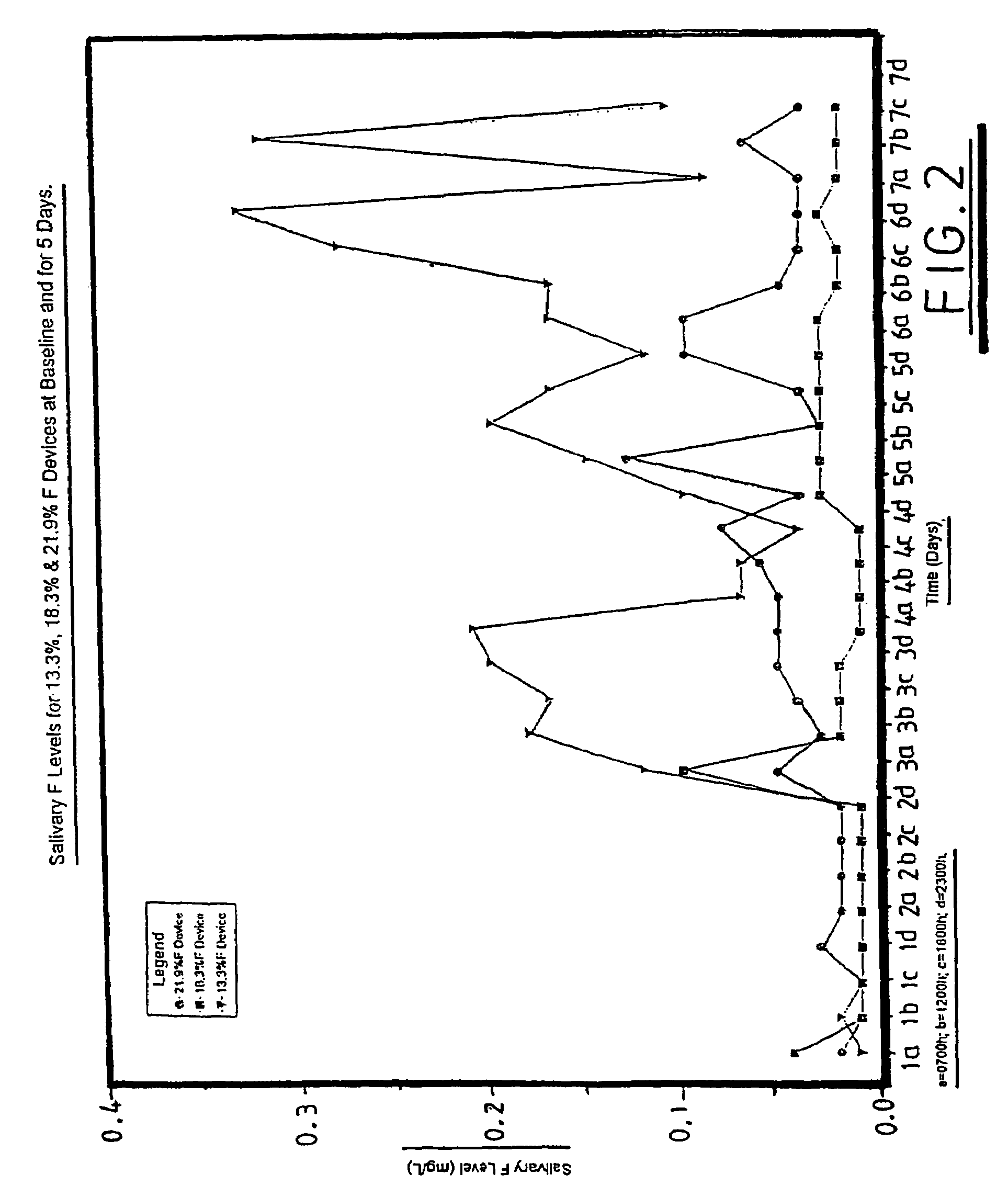
Glass composition
InactiveUS7175833B1Improve treatmentIncrease blockingBiocideCosmetic preparationsDentistryEmpirical formula
A glass composition having the general empirical formula given below, expressed in weight percent of the element: P: 16–24, F: 5–30, O: 20–40 and at least one of Na, K, Li or Al in an amount up to a total of 40 wt. % and optionally, up to 5 wt. % of boron and / or silicon. The composition may be used for the treatment and / or prevention of dental caries by providing a slow fluoride releasing device that may be attached to a tooth to release fluoride into the saliva or an individual.
Owner:TELDENT
Mineral trioxide aggregate (MTA) composition and use
ActiveUS8722100B2Good effectImprove mechanical propertiesHeavy metal active ingredientsBiocideHigh fluoridePhosphate
The present application discloses a fast-setting mineral trioxide aggregate (MTA) with fluoride release for practical treatment of diseases in teeth and bone, e.g. for caries treatment and / or prevention. The cariostatic MTA contain calcia-silica-alumina cement with moderately increased tricalcium aluminate content allowing high calcium hydroxide release. The MTA composition support remineralization and biomineralization, and it is suitable for stimulation of hard tissue regeneration. MTA embodiments contain superplasticizer and nanosilicate for improved mechanical properties. The MTA compositions include optional radiocontrast and nano-enriched leachable fluorine, nitrate, strontium, and phosphate. The fast-setting MTA paste exhibits flow-to-clay-like consistency, which allows new practical applications including cavity lining, temporary restoration, bonding, and cementations in one MTA embodiment. The high calcium hydroxide and high fluoride release are suitable for caries prevention and treatment, and per se inhibition of dental symptoms.
Owner:DENTOSOLVE APS
Filler containing composition and process for production and use thereof
ActiveUS20090247665A1Produced cost-effectivelyEliminate needCosmetic preparationsImpression capsSilanesSolvent
The invention relates to a composition comprising an ethylenically unsaturated acidic compound, water, a functionalized silane, an initiator, optionally comprising a sensitising agent, a non-surface treated filler, optionally a solvent, optionally an ethylenically unsaturated compound, optionally additives selected from the group consisting of stabilizer(s), photobleachable colorant(s), fluoride release agent(s), pigments. The invention also relates to a process of producing such a composition by in-situ silanization of the non-surface treated filler.
Owner:3M INNOVATIVE PROPERTIES CO
Fluoride varnish
ActiveUS20140162208A1Prevent rotRemineralize tooth surfaceCosmetic preparationsToilet preparationsTin FluoridesFluoride varnish
A tooth varnish that is free from pinus extracts, free of substantial undesired coloring agents, with a reduced viscosity, delivered in a user-friendly, flow-through, unit dose applicator and having improved fluoride release, uptake, and remineralization properties.
Owner:ELEVATE ORAL CARE
Fluoride releasing bite plate
The present disclosure relates to bite plates for use with various orthodontic remodeling devices. A bite plate that releases fluoride during use, thus encouraging remineralization on enamel is described. Additionally, bite plates with a colorant or color-changing matrix that serve as indicators for fluoride concentration are also described.
Owner:ADVANCED ORTHODONTICS & EDUCATION ASSOC LLC
Soft touch screen protective film
InactiveCN109968778AAvoidance of injuryImprove cleanlinessSynthetic resin layered productsInput/output processes for data processingPolyethylene terephthalateEngineering
The invention discloses a soft touch screen protective film. The protective film comprises a polyethylene terephthalate (PET) film layer, a supporting layer, a silica gel layer, a fluoride release film and a buffer layer; the supporting layer is attached to the bottom of the PET film layer; the silica gel layer is attached to the bottom of the supporting layer; one side, far away from the supportlayer, of the silica gel layer is connected with the buffer layer; and one side, far away from the silica gel layer, of the buffer layer is connected with the fluoride release film, wherein the thickness of the fluoride release film is 50 microns. The buffer layer comprises a first high-transparency PET film, a second high-transparency PET film and a third high-transparency PET film, wherein the first high-transparency PET film is attached to the bottom of the silica gel layer, the second high-transparency PET film is connected to the bottom of the first high-permeability PET film, and the third high-transparency PET film is connected to the bottom of the second high-permeability PET film. The soft touch screen protective film can well prevent a situation that bubbles is generated when thefilm is adhered to a mobile phone touch panel, so that adhesion is facilitated, and meanwhile, a situation that damage to a human bodies due to the fact that a conventional toughened glass film is easily broken is avoided.
Owner:NINGGUO QIANHONG ELECTRONIC CO LTD
Mitigation of membrane degradation by multilayer electrode
ActiveUS8206872B2Reduce pressureLow release rateSolid electrolytesActive material electrodesFuel cellsHydrogen
An MEA for a fuel cell that employs multiple catalyst layers to reduce the hydrogen and / or oxygen partial pressure at the membrane so as to reduce the fluoride release rate from the membrane and reduce membrane degradation. An anode side multi-layer catalyst configuration is positioned at the anode side of the MEA membrane. The anode side multi-layer catalyst configuration includes an anode side under layer positioned against the membrane and including a catalyst, an anode side middle layer positioned against the anode side under layer and not including a catalyst and an anode side catalyst layer positioned against the anode side middle layer and opposite to the anode side under layer and including a catalyst, where the amount of catalyst in the anode side catalyst layer is greater than the amount of catalyst in the anode side under layer.
Owner:GM GLOBAL TECH OPERATIONS LLC
Fluoride-releasing strips for caries prevention
InactiveUS20090257964A1Cosmetic preparationsToilet preparationsCarboxymethyl celluloseNursing caries
The present invention discloses fluoride-releasing strips for caries prevention, wherein the fluoride-releasing strips comprises a colloidal fluoride-containing solution for releasing fluorine ion and a support substrate. A colloidal fluoride-containing solution comprises a fluoride solution, at least one buffer, at least one moisturizer and a carboxymethyl cellulose (CMC). The concentration of fluoride ions in said colloidal fluoride-containing solution ranges from 2500 ppm to ppm. The support substrate is waterproof material and the colloidal fluoride-containing solution is applied on the support substrate.
Owner:NAT TAIWAN UNIV
Dental adhesive composition
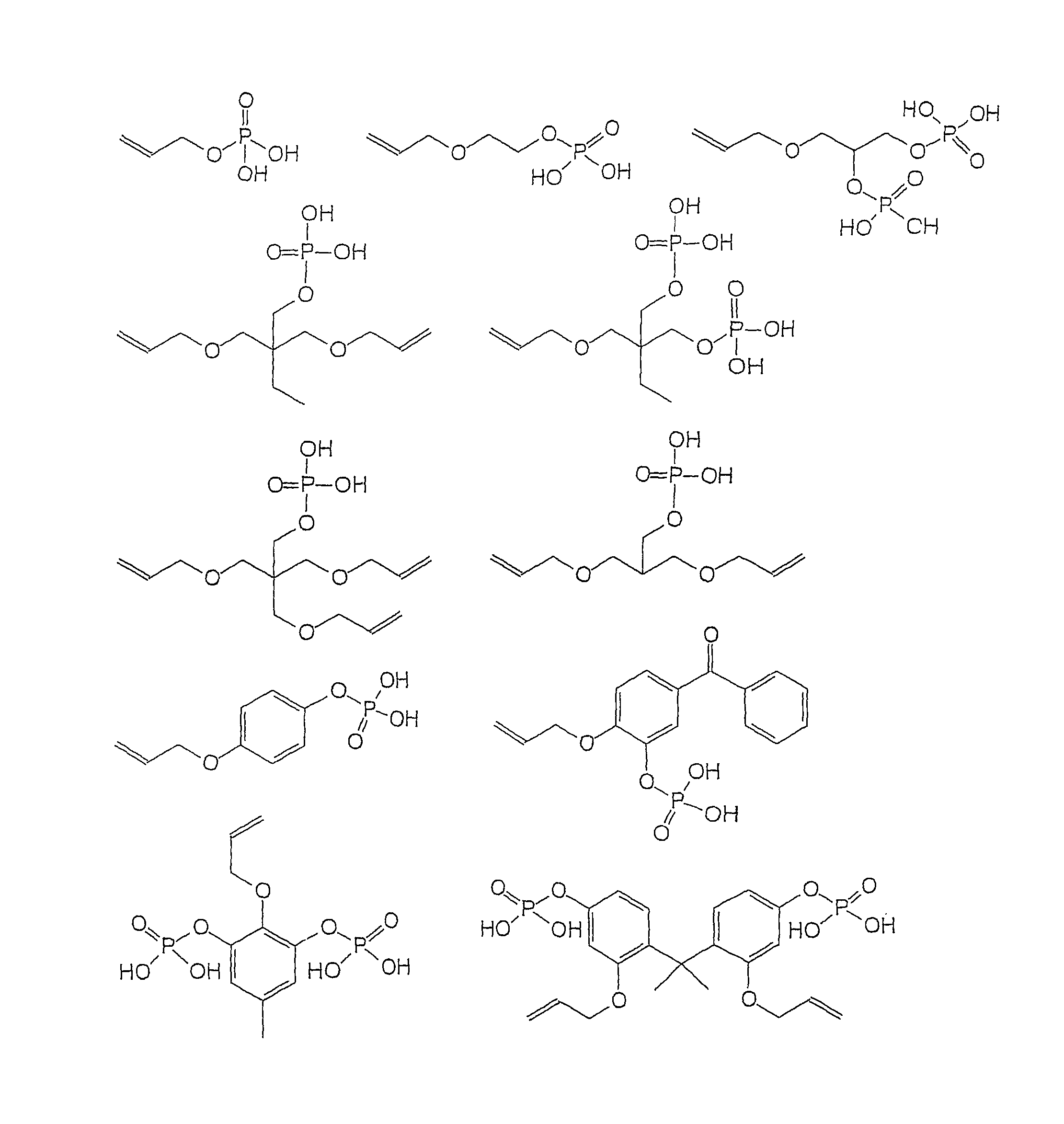
Dental composition comprising an aqueous mixture containing(i) a polymerizable acidic phosphoric acid ester monomer of the following formula (A):whereina is an integer of from 1 to 10; R1 represents a hydrogen atom or a moiety of the following formula (Y)wherein X independently represent an oxygen atom, a sulfur atom, or a group NR, wherein R may be a hydrogen atom, a C1-6 alkyl group or an acyl group; L represents an (a+b)-valent organic residue containing 1 to 20 carbon atoms and optionally including ether, thioether or amino groups or further acidic groups, whereby the carbon atoms comprise at least a+b carbon atoms selected from primary and secondary aliphatic carbon atoms, secondary alicyclic carbon atoms, and aromatic carbon atoms, each of said a+b carbon atoms linking a phosphate or 2-(oxaallyl) derivative group; b is an integer of from 1 to 10;R2 which may be the same or different, independently may be hydrogen, an allyl group or a moiety R1 wherein b is 1; provided that at least one of R1 and R2 is not hydrogen;(ii) one or more polymerizable N-substituted alkyl acrylic or acrylic acid amide monomers;(iii) an organic water-miscible solvent and / or water;(iv) a polymerization initiator;(v) an inhibitor and / or a stabilizer;(vi) optionally an organic or inorganic acid; and(vii) optionally a filler and / or a fluoride releasing compound.
Owner:DENTSPLY SIRONA INC
Self hardening glass carbomer composition
InactiveCN1809329AHigh hardnessReduce sensitivityImpression capsSurgical adhesivesFluorosilicate saltAcid water
The present invention relates to a self hardening glass carbomer composition obtainable by treating a fluorosilicate glass powder with: (a) a poly(dialkylsiloxane) having terminal hydroxyl groups, wherein the alkyl groups contain 1 to 4 carbon atoms, (b) an aqueous acid solution, and (c) separating the treated fluorosilicate glass powder from the aqueous acid solution. The glass carbomer compositions according to the invention have for example good toughness and strength and excellent fluoride release, In addition, the glass carbomer compositions according to the invention do not show shrinkage or expansion, an essential property for providing fillings for cavities having high strength and long durability. Moreover, the glass carbomer composition according to the present invention has a lower sensitivity towards abrasion and wear, a greater stiffness, a smoother surface, a better colourfastness, a better adherence to e.g. bone tissue and a lower water sensitivity.
Owner:健康玻璃基金会
Fluoride varnish
ActiveUS9107838B2Prevent rotRemineralize the tooth surfaceCosmetic preparationsToilet preparationsTin FluoridesFluoride varnish
A tooth varnish that is free from pinus extracts, free of substantial undesired coloring agents, with a reduced viscosity, delivered in a user-friendly, flow-through, unit dose applicator and having improved fluoride release, uptake, and remineralization properties.
Owner:ELEVATE ORAL CARE
Fluoride-releasing strips for tooth
InactiveUS20080305053A1Prevent dental cariesCosmetic preparationsToilet preparationsFluoride releaseFluorine
The present invention discloses fluoride-releasing strips for tooth, wherein the fluoride-releasing strips comprises a fluoride-containing solution for releasing fluorine ion and a support substrate. A formula of fluoride-containing solution comprises a fluoride solution, at least one buffer, at least one moisturizer and a tackiness agent. The support substrate is waterproof material and a fluoride-containing solution is applied on the support substrate.
Owner:NAT TAIWAN UNIV
Fluoride varnish
InactiveUS20150342844A1Prevent rotRemineralize tooth surfaceCosmetic preparationsBiocideFluoride varnishDental fluoride varnish
A tooth varnish that is free from pinus extracts, free of substantial undesired coloring agents, with a reduced viscosity, delivered in a user-friendly, flow-through, unit dose applicator and having improved fluoride release, uptake, and remineralization properties.
Owner:ELEVATE ORAL CARE
In-situ fluoride adsorption method from waste lithium battery via high-ferro slag
ActiveCN110010993ASolve stacking problemsAchieve reductionGas treatmentDispersed particle separationSorbentSlag
The invention discloses an in-situ fluoride adsorption method from a waste lithium battery via high-ferro slag. According to the method, the high-ferro slag serves as an adsorbent to absorb fluoride released in the high-temperature thermal decomposition process of an organic binder of polyvinylidene fluoride in an anode piece of the waste lithium battery. Compared with the prior art, in the method, the industrial solid waste can be converted into a functional material capable of in-situ fluoride adsorption, the difficulty in stacking the industrial solid waste is overcome, the industrial solidwaste is reduced, made into resource and made harmless, and the in-situ adsorption rate for fluorine in the polyvinylidene fluoride of high-ferro slag is as high as 99.0wt% and even higher.
Owner:TSINGHUA UNIV
Self hardening glass carbomer composition
InactiveUS20060217455A1Improve toughnessHigh strengthImpression capsSurgical adhesivesFluoride releaseHydroxy compound
The present invention relates to a self hardening glass carbomer composition obtainable by treating a fluorosilicate glass powder with: (a) a poly(dialkylsiloxane) having terminal hydroxyl groups, wherein the alkyl groups contain 1 to 4 carbon atoms, (b) an aqueous acid solution, and (c) separating the treated fluorosilicate glass powder from the aqueous acid solution. The glass carbomer compositions according to the invention have for example good toughness and strength and excellent fluoride release, In addition, the glass carbomer compositions according to the invention do not show shrinkage or expansion, an essential property for providing fillings for cavities having high strength and long durability. Moreover, the glass carbomer composition according to the present invention has a lower sensitivity towards abrasion and wear, a greater stiffness, a smoother surface, a better colourfastness, a better adherence to e.g. bone tissue and a lower water sensitivity.
Owner:STICHTING GLASS FOR HEALTH
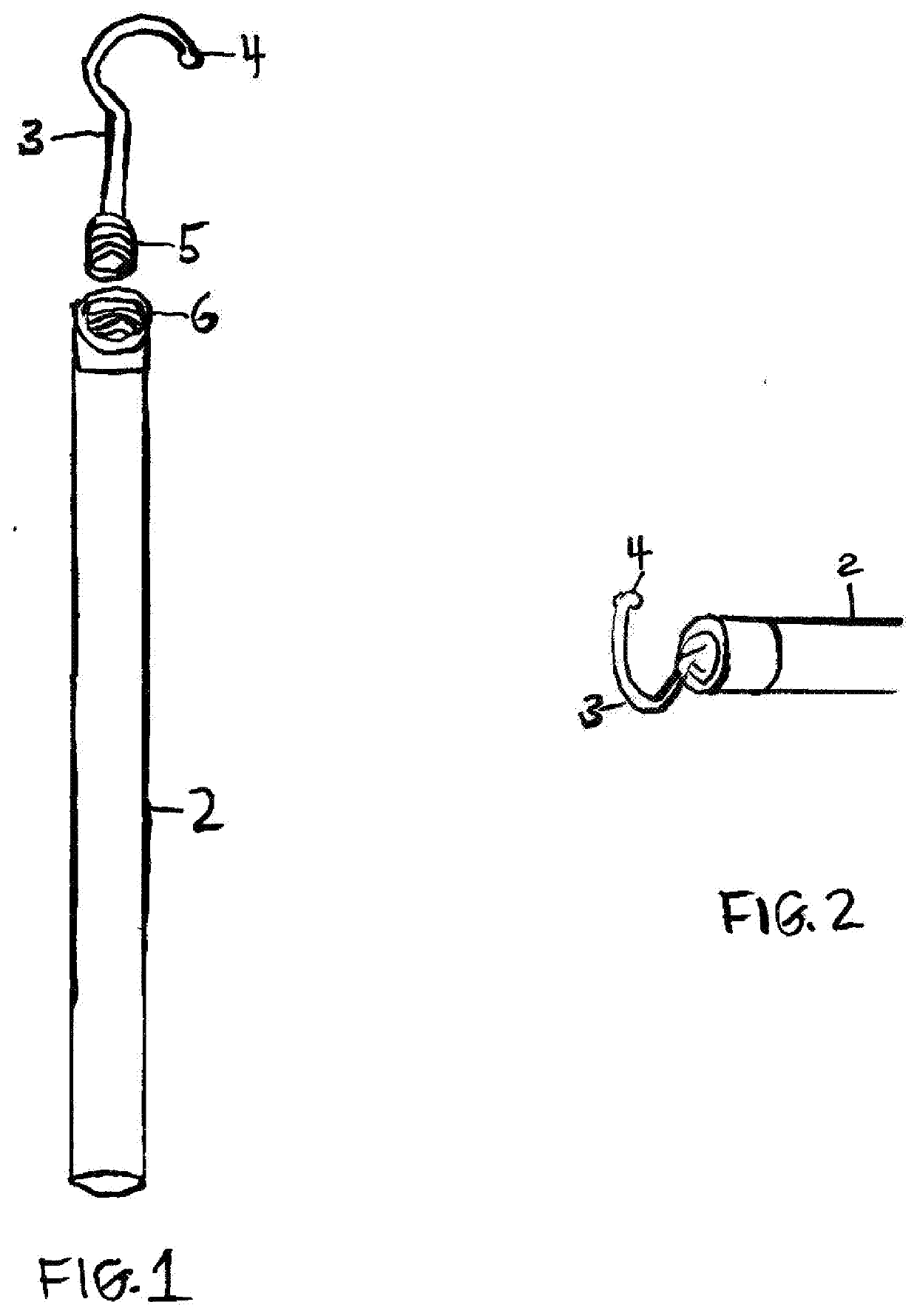
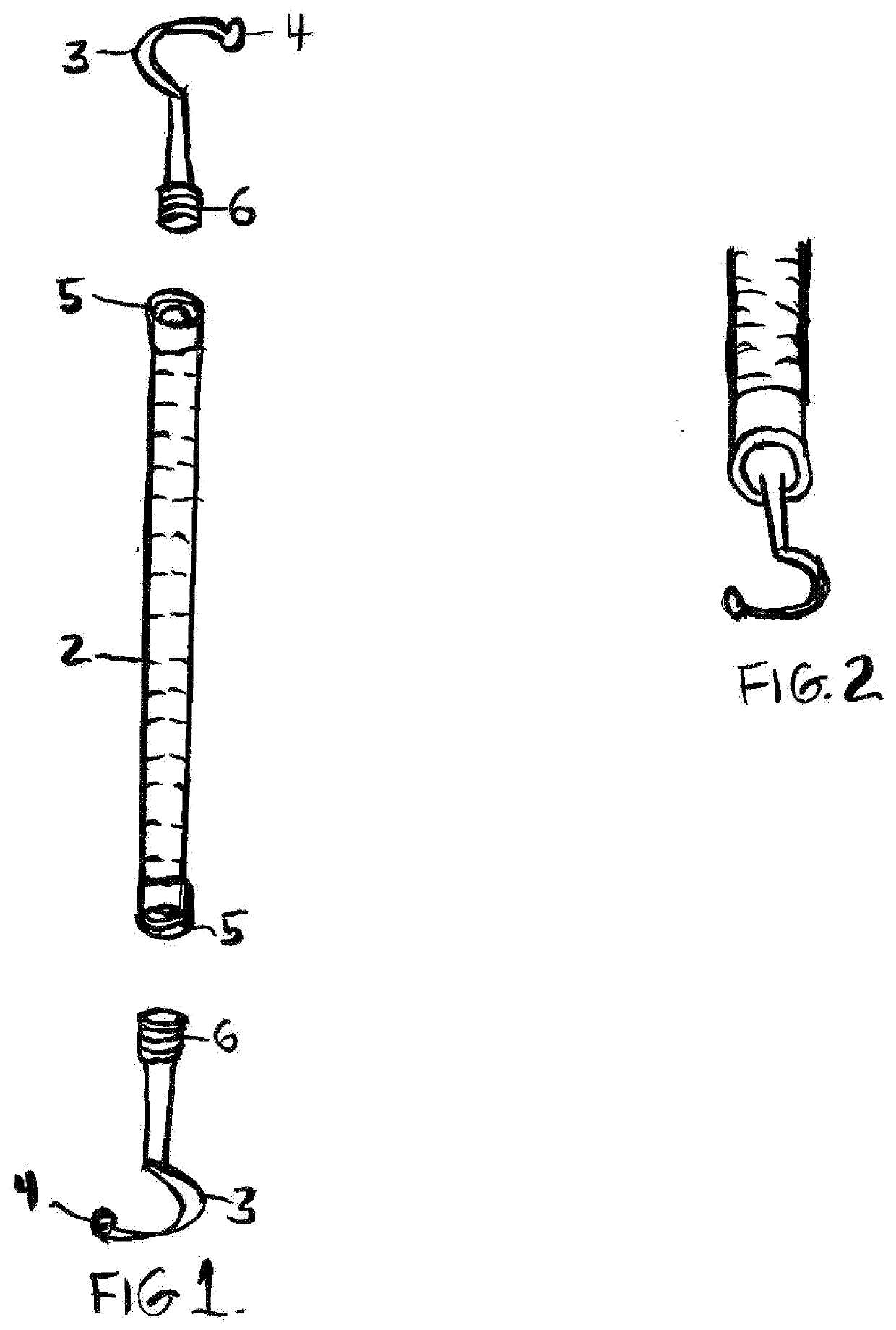
Interim therapeutic restoration and cavity liner placement (ITR) instrument description
This invention is a classic dental shepherd's hook explorer that has been fitted with a dycal style ball on the end, for a completely new instrument designed to facilitate exacting placement of cavity liners and bases, spreading flowable composite underneath fillings by the Dentist, and placing Interim Therapeutic Restorations (ITR) such as fluoride releasing glass ionomer by the Dentist or Registered Dental Hygienist Expanded Functions (RDHEF).Doctors then can reach over matrix bands and apply dental bases and liners, or spread flowable composite directly to the axial wall and pulp floor of their preps, and RDHEF's can access hard to reach interproxirnal, root and furcation areas to apply glass ionomer or other Interim Therapeutic Restorations where incipient caries have been hand excavated.
Owner:AYERS EVELYN ANNE BAUSCHKA
Dental adhesive composition
Dental composition comprising an aqueous mixture containing(i) a polymerizable acidic phosphoric acid ester monomer of the following formula (A):whereina is an integer of from 1 to 10; R1 represents a hydrogen atom or a moiety of the following formula (Y)wherein X independently represent an oxygen atom, a sulfur atom, or a group NR, wherein R may be a hydrogen atom, a C1-6 alkyl group or an acyl group; L represents an (a+b)-valent organic residue containing 1 to 20 carbon atoms and optionally including ether, thioether or amino groups or further acidic groups, whereby the carbon atoms comprise at least a+b carbon atoms selected from primary and secondary aliphatic carbon atoms, secondary alicyclic carbon atoms, and aromatic carbon atoms, each of said a+b carbon atoms linking a phosphate or 2-(oxaallyl) derivative group; b is an integer of from 1 to 10;R2 which may be the same or different, independently may be hydrogen, an allyl group or a moiety R1 wherein b is 1; provided that at least one of R1 and R2 is not hydrogen;(ii) one or more polymerizable N-substituted alkyl acrylic or acrylic acid amide monomers;(iii) an organic water-miscible solvent and / or water;(iv) a polymerization initiator;(v) an inhibitor and / or a stabilizer;(vi) optionally an organic or inorganic acid; and(vii) optionally a filler and / or a fluoride releasing compound.
Owner:DENTSPLY SIRONA INC
Interim Provisional Restoration Placement Instrument-#1
InactiveUS20200046457A1Hard to areaPrecise curvatureTooth pluggers/hammersTeeth fillingGlass ionomersIonomer
This invention is a classic dental cow horn explorer that has been fitted with dycal style balls on the ends, for a completely new instrument designed to facilitate exacting placement of cavity liners and bases, spreading flowable composite underneath fillings by the Dentist, and placing Interim Therapeutic Restorations (ITR) such as fluoride releasing glass ionomer by the Dentist or Registered Dental Hygienist Expanded Functions (RDHEF).Doctors then can reach over matrix bands and apply dental bases and liners, or spread flowable composite directly to the axial wall and pulp floor of their preps, and RDHEF's can access hard to reach interproximal, root and furcation areas to apply glass ionomer or other Interim Therapeutic Restorations where incipient caries have been hand excavated.
Owner:AYERS EVELYN ANNE BAUSCHKA
Nano fluororesin dental material and preparation method thereof
ActiveCN101721316BHas the function of anti-dental cariesPerformance improvements and enhancementsImpression capsDentistry preparationsMethacrylateSilanes
The nanometer fluorine-containing resin dental material and the preparation method thereof belong to the technical field of photocurable resin dental material and powder dispersion method. The resin dental material is composed of methacrylate monomers, initiators and inorganic fillers. The inorganic fillers contain fluorine-containing glass powder, nano-silica powder, dyes, strontium glass or / and barium glass; nano-YbF3 and Bi2O3 can also be included. The preparation involves silanization treatment of fluorine-containing glass powder and nano-silicon powder, and ultrasonic dispersion technology of nano-silicon powder in an organic medium. The resin dental material of the present invention has biological activity, can resist dental caries, and has relatively ideal effects in properties such as radiation resistance, transparency, tensile strength, wear resistance, density, rheology and the like. In particular, the combination of fluorine-containing glass powder and preferably methacrylate monomers has the best effect on fluorine release and mechanical properties at the same time.
Owner:吉林省登泰克牙科材料有限公司
Fluoride varnish
InactiveUS20130288194A1Prevent rotRemineralize the tooth surfaceCosmetic preparationsImpression capsFluoride varnishDental fluoride varnish
A tooth varnish that is free from pinus extracts, free of substantial undesired coloring agents, with a reduced viscosity, delivered in a user-friendly, flow-through, unit dose applicator and having improved fluoride release, uptake, and remineralization properties.
Owner:ELEVATE ORAL CARE
Preparation method of anti-secondary caries cavity repair material
ActiveCN101690696BNot easy to peel offGentle releaseImpression capsDentistry preparationsMethacrylateSilanes
The invention relates to a preparation method of an anti-secondary caries cavity restoration material, which belongs to the field of medical and dental preparations. The existing fluorine-containing anti-secondary caries composite resin caries filling materials have the problems of too fast release of fluorine, poor interface compatibility and no rigid support for the caries filling body. This case aims to solve the above problems. The steps of the method in this case include using a silane coupling agent to modify the surface of the nano-inorganic powder, and combining the modified nano-inorganic powder with monomers such as bisphenol A glycidyl methacrylate, dimethacrylic acid Materials such as diluents such as triethylene glycol ester, photoinitiators such as camphorquinone, and co-initiators such as dimethylaminoethyl methacrylate are mixed evenly under dark conditions, and are removed under vacuum conditions. Bubbles, the main point of this case is that the nano-inorganic powder contains fluorine-loaded zirconia nanotubes accounting for 10% to 80% of its own weight. The fluorine-loaded zirconia nanotubes have both the functions of slow-release fluorine to prevent secondary caries and structural reinforcement.
Owner:江苏南田工机有限公司
A controllable fluoride slow-release calcium phosphate bioactive material and preparation method thereof
InactiveCN105748510BGood biocompatibilityImprove biological activityDigestive systemInorganic non-active ingredientsApatiteBiocompatibility Testing
The present invention discloses a fluorine controlled-release calcium phosphate bioactive material and a preparation method thereof. Fluorinion loaded in apatite structure is used as a fluorine source, and beta-tricalcium phosphate (beta-TCP) and carbonate ions are used to substitute apatite as a carrier for fluoride release; a co-precipitation method is employed for the synthesis of a fluorine and carbonate ion co-doped apatite and beta-TCP biphasic bioactive materials; and by controlling the phase ratio of beta-TCP and apatite, and substitution of carbonate ions in apatite, the regulation of dissolution and absorption of apatite is achieved, thereby regulating the slow release of fluorinion in the apatite structure. The material not only has good biocompatibility and bioactivity, but also can maintain local effective fluorine concentration for a long time, and can be widely used in osteoporosis treatment and dental treatment; therefore, the material has significant economic benefits.
Owner:JINGDEZHEN CERAMIC INSTITUTE
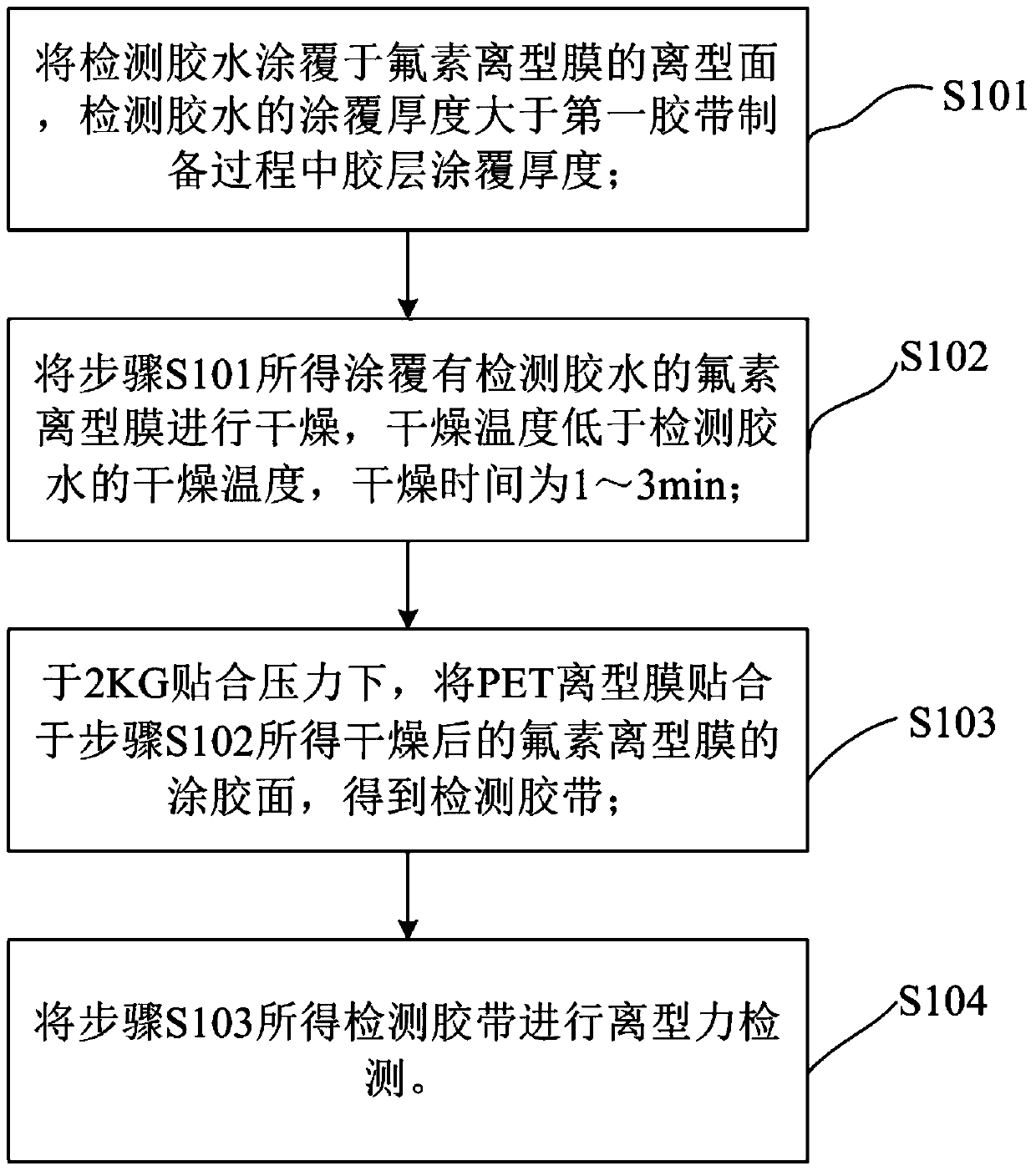
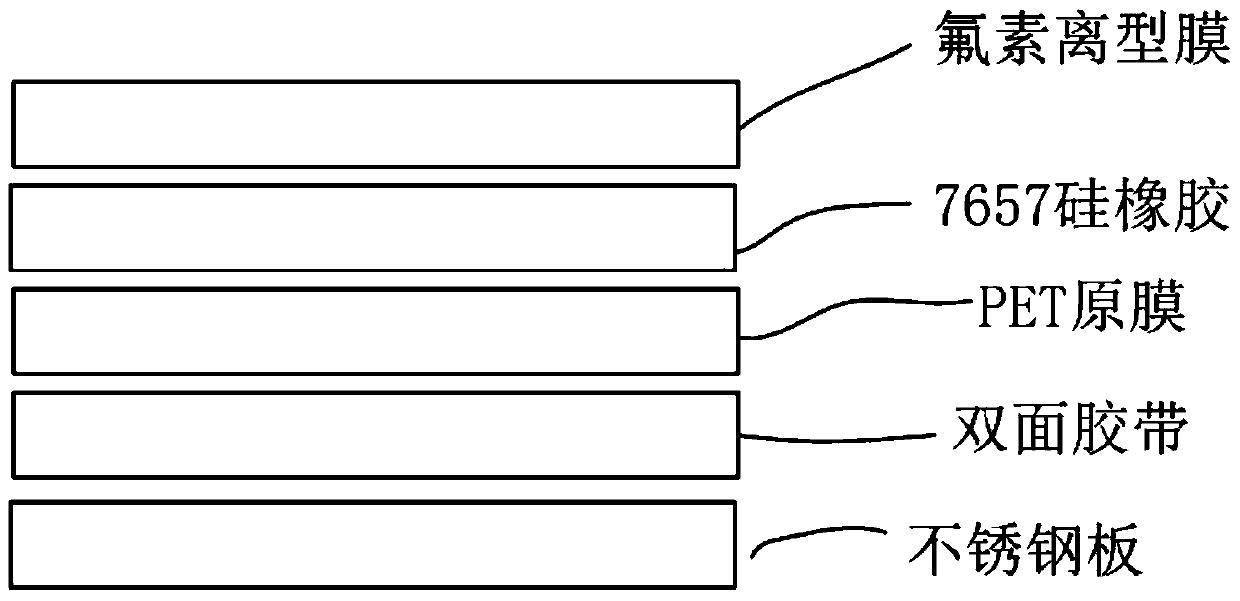
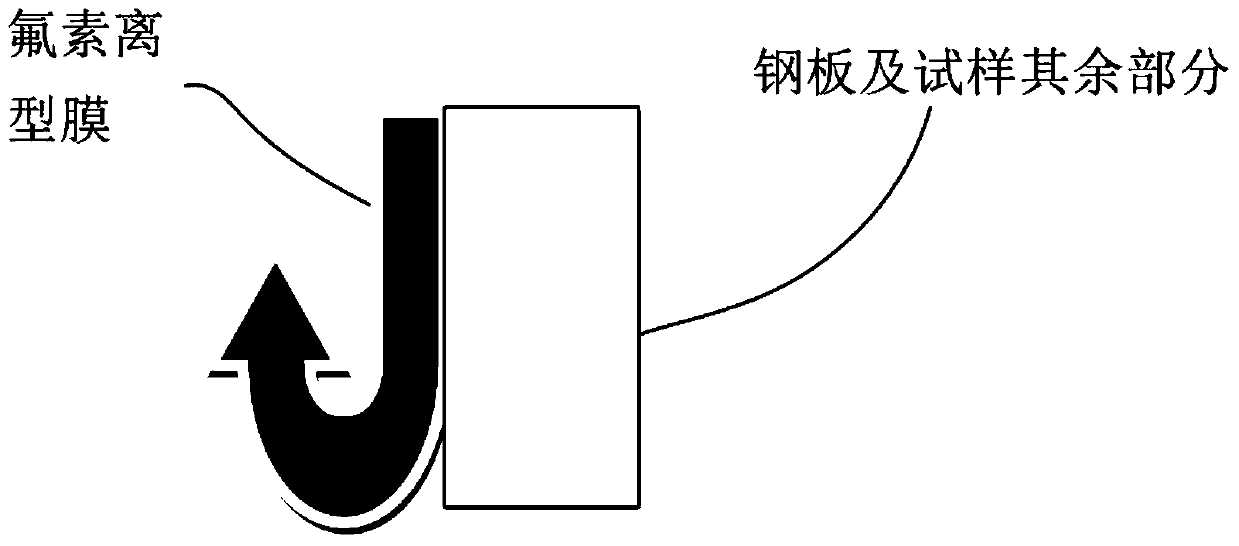
Detection method of fluorine release film
ActiveCN106153535BUsing mechanical meansMaterial analysisPolyethylene terephthalatePolyethylene terephthalate glycol
The invention relates to the technical field of release force detection, in particular to a fluoride-containing release film detection method which includes the steps: 1 coating detection glue on a release surface of a fluoride-containing release film; 2 drying the fluoride-containing release film coated with the detection glue in the step 1; 3 fitting a PET (polyethylene terephthalate) release film on the glued surface of the dried fluoride-containing release film obtained in the step 2 under 2KG fitting pressure; 4 detecting the release force of a detection tape obtained in the step 3. According to the detection method, based on tests after simulation tests more strict than practical application conditions, the influence of solvents or residual solvents in wet glue on the fluoride-containing release film in the production process can be effectively evaluated, and whether the fluoride-containing release film meets production requirements or not is timely discovered to avoid the problem that the release force of a coated product is increased or the coated product cannot be released.
Owner:SHENZHEN MOMA TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com