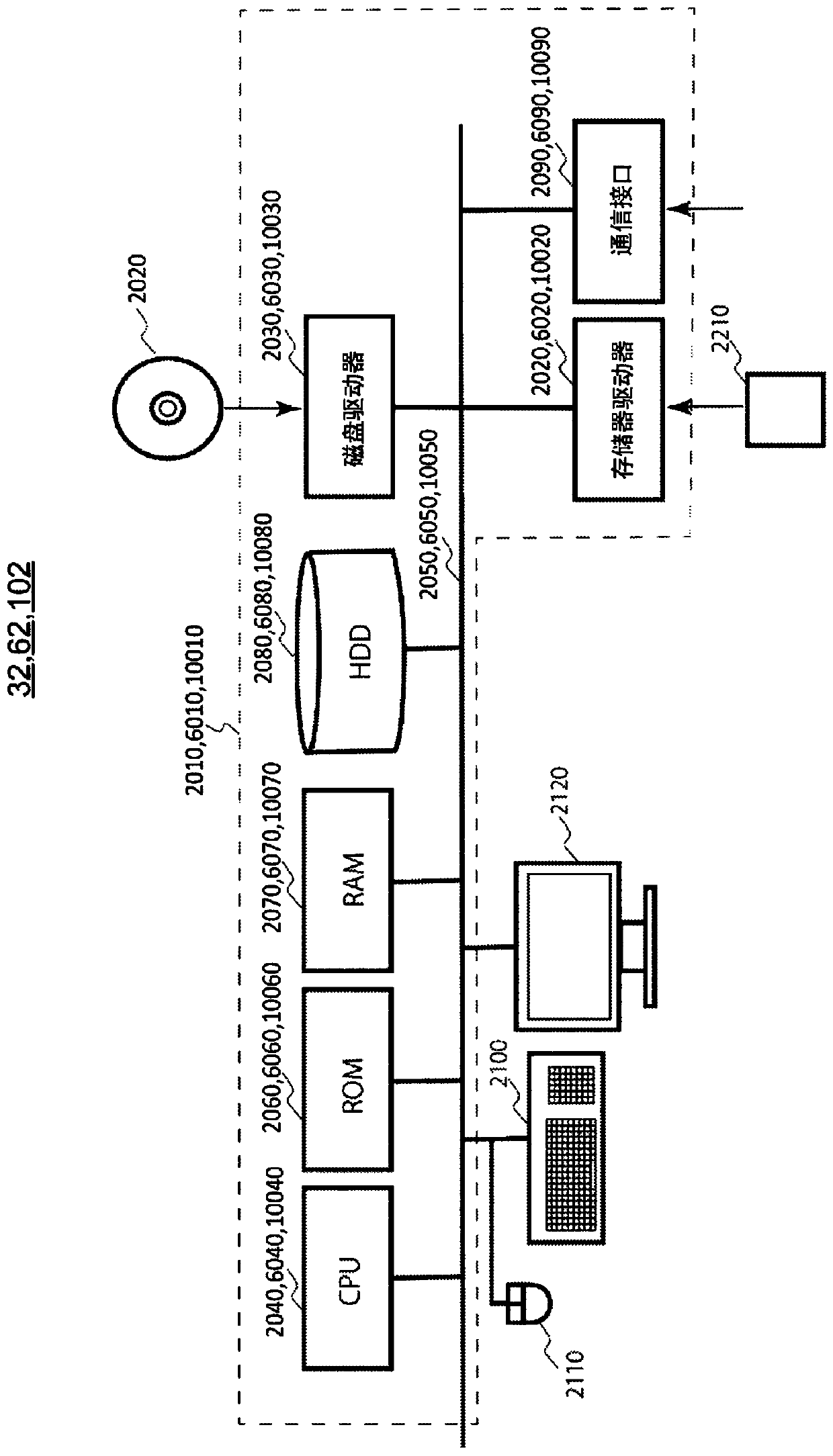
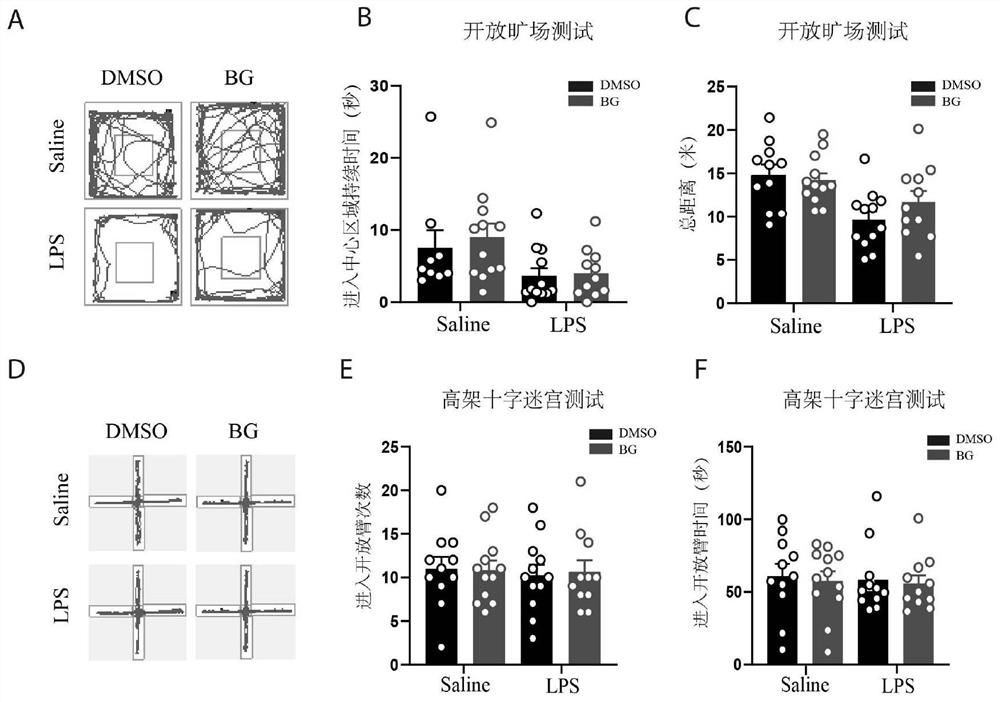
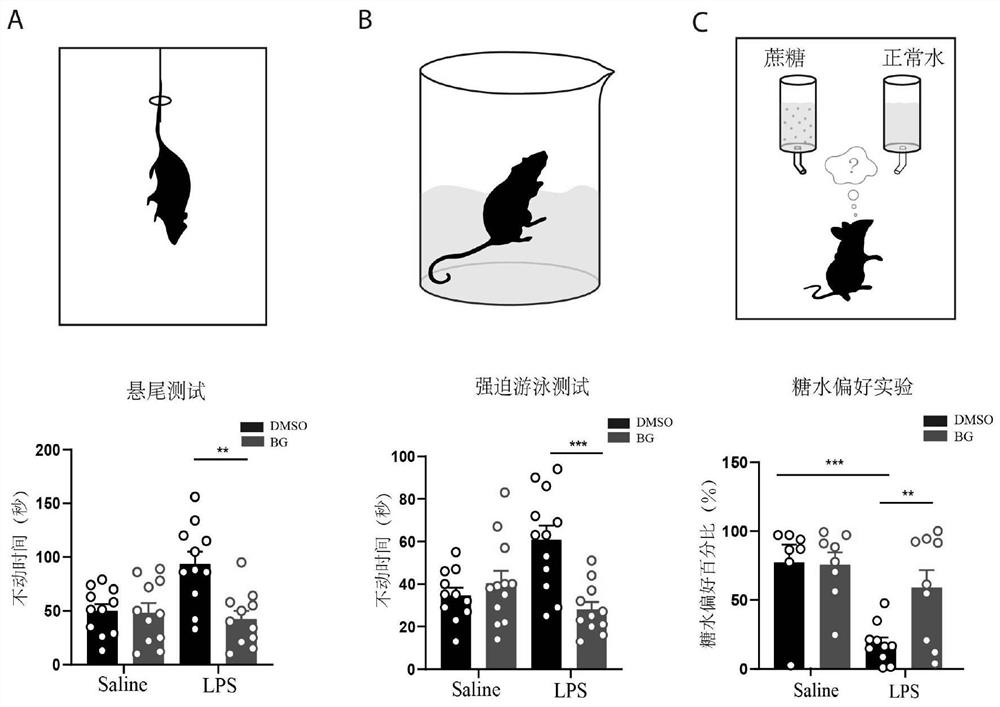
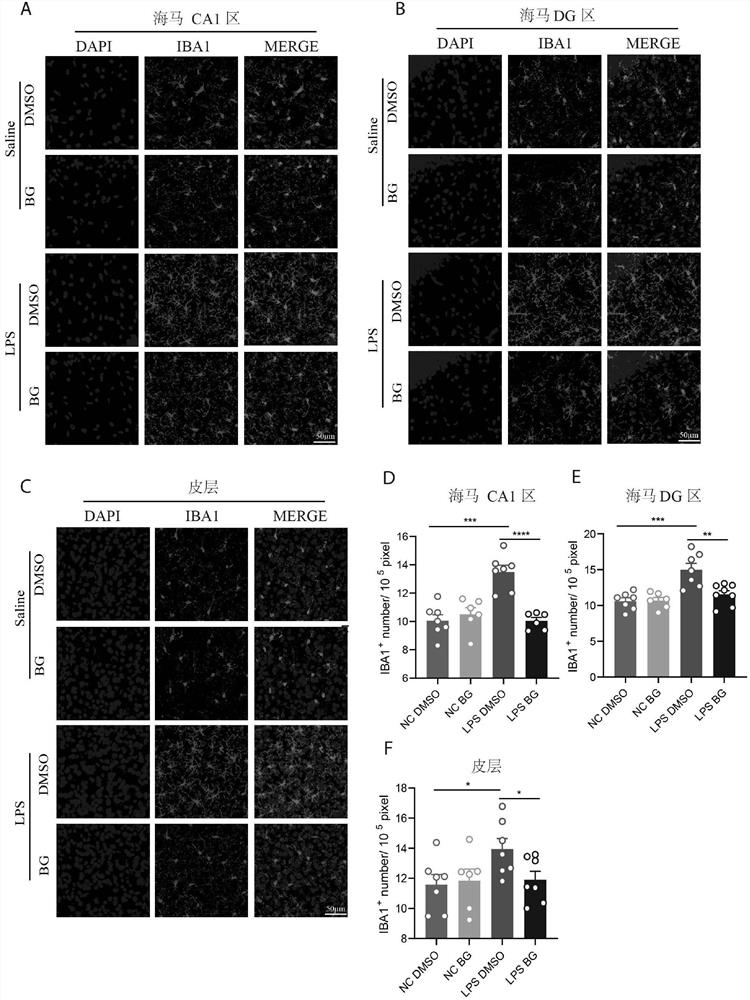
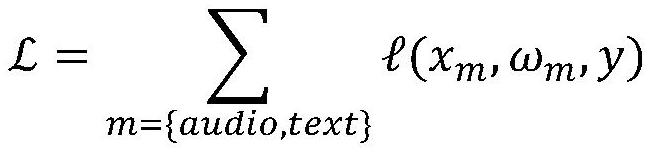Patents
Literature
36 results about "Depressive symptomatology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
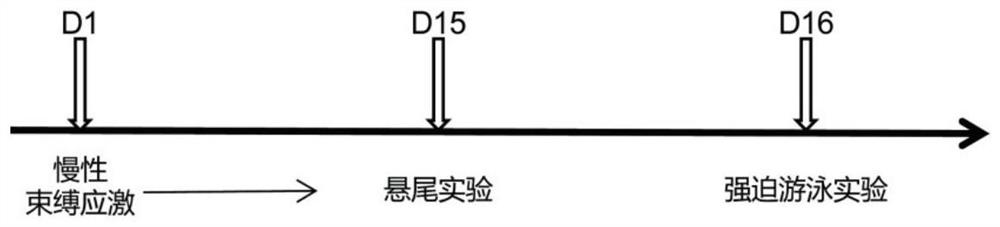
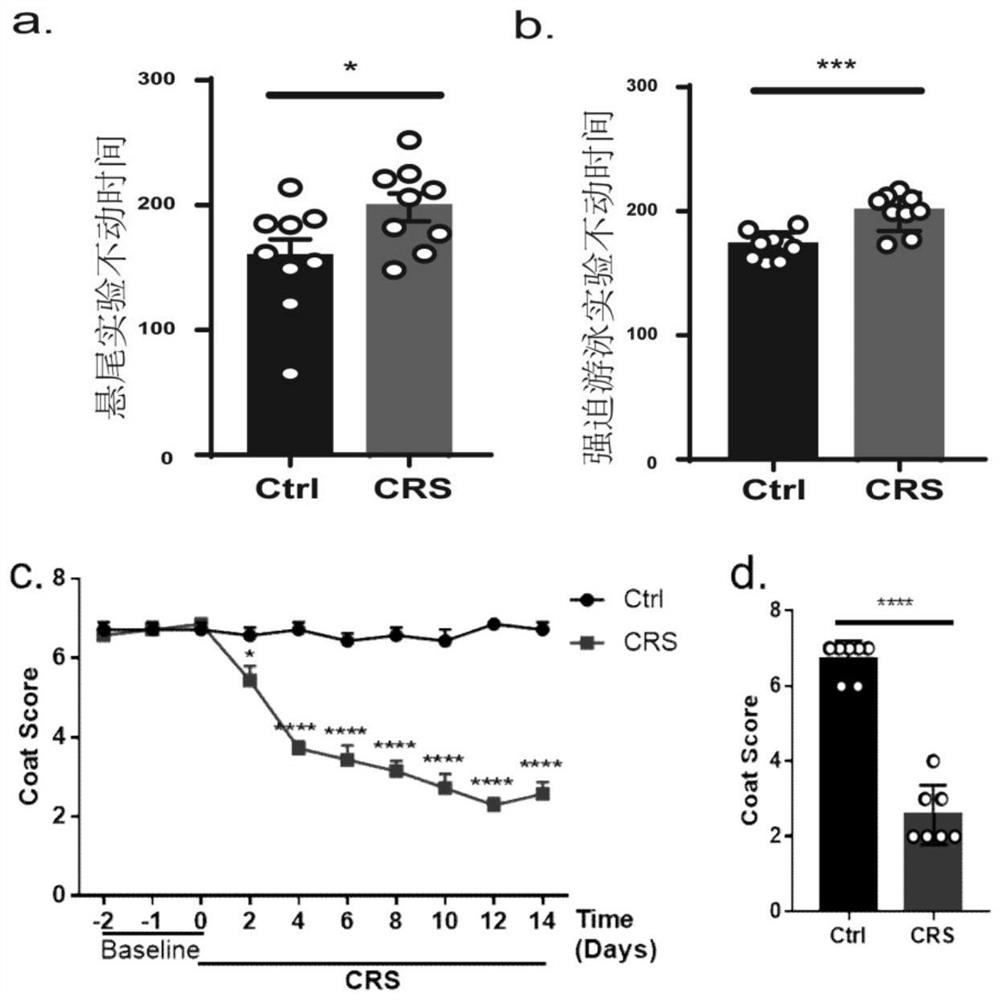
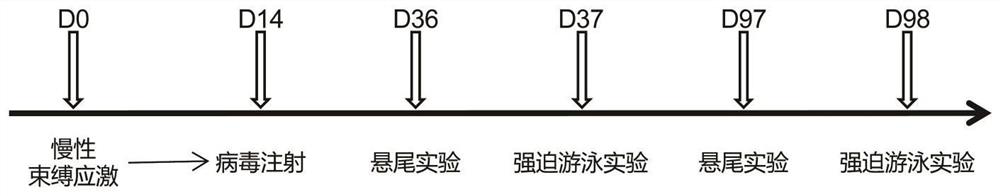
The Quick Inventory of Depressive Symptomatology is a short screening tool based on the larger Inventory of Depressive Symptomatology (IDS).[1] It is a self-report tool designed to screen for depression and measure changes in severity of symptoms.
Method of treating stress-mediated depression
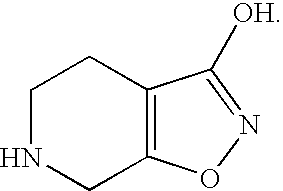
The present invention relates to a method for the treatment of depression comprising administering a therapeutically effective amount of gaboxadol to a patient, wherein the level of one or more inflammatory markers in said patient is increased or abnormal. The present invention also relates to a method for the treatment of stress-mediated depression comprising administering a therapeutically effective amount of gaboxadol to a patient, wherein the level of one or more inflammatory markers is increased or abnormal in said patient. The present invention also relates to a method for the treatment of depression or the amelioration of one or more depressive symptoms comprising administering a therapeutically effective amount of gaboxadol to a patient, wherein the clinical presentation of one or more symptoms of depression are the physiological effect of a general medical condition. Furthermore the present also relates to a method for testing the therapeutic effectiveness of a compound in the treatment of depression or reducing the symptoms of depression comprising measuring the amount of one or more inflammatory markers in a sample from a patient before said compound is administered to the patient and comparing with the amount of said one or more inflammatory markers in a sample from the same patient after administration of said compound to the patient.
Owner:H LUNDBECK AS
Differentiation device, differentiation method for depression symptoms, determination method for level of depression symptoms, stratification method for depression patients, determination method for effects of treatment of depression symptoms, and brain activity training device
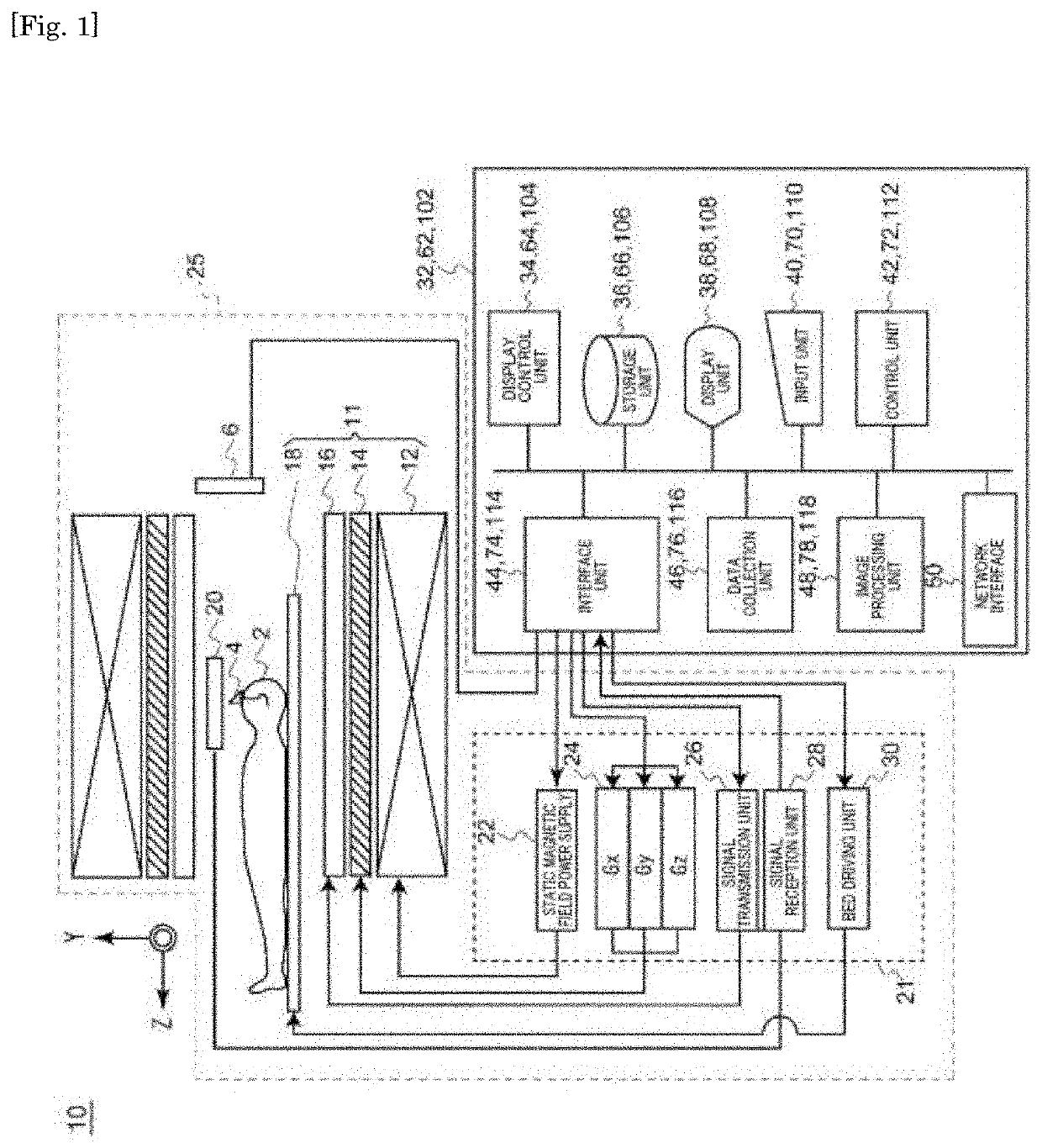
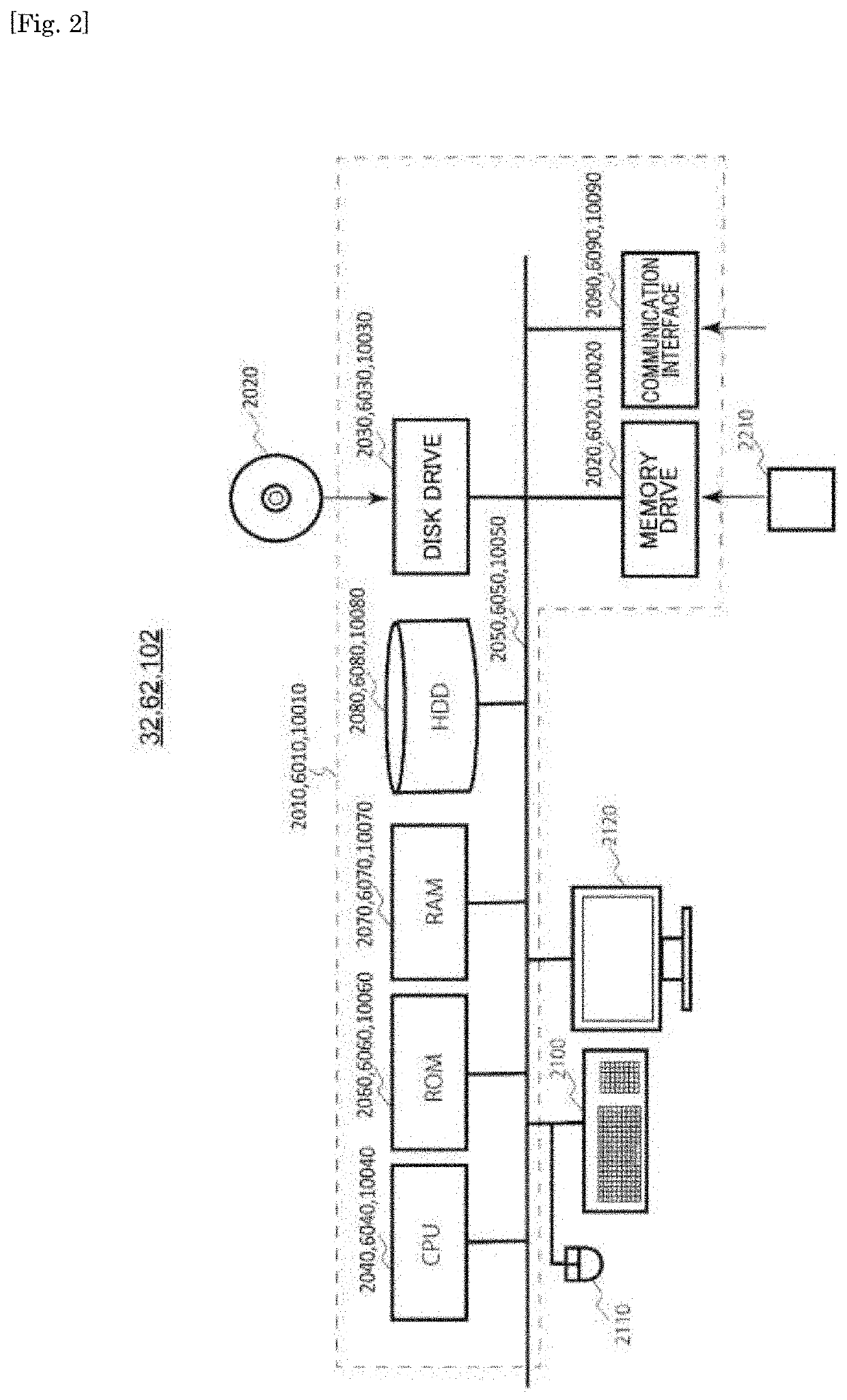
PendingUS20210034912A1Discriminate the level of the depressive symptomImage enhancementMedical data miningFunctional connectivityCerebral activity
Objective discrimination of a disease label of a depressive symptom with respect to an active state of a brain is achieved. One means for solving the problems of the present invention is to provide a discriminating device for assisting in determination of whether a subject has a depressive symptom. The discriminating device includes a storage device for storing information for identifying a classifier generated by classifier generation processing based on a signal obtained by using a brain activity detecting apparatus to measure, in advance and time-sequentially, a signal indicating a brain activity of a plurality of predetermined regions of each brain of a plurality of participants in a resting state, the plurality of participants including healthy individuals and patients with depression. The classifier is generated so as to discriminate a disease label of a depressive symptom based on a weighted sum of a plurality of functional connectivities selected by feature selection as being relevant to the disease label of the depressive symptom through machine learning from among functional connectivities of the plurality of predetermined regions. The discriminating device further includes a processor configured to execute discriminating processing of generating a classification result for the depressive symptom of the subject by using the classifier.
Owner:ATR ADVANCED TELECOMM RES INST INT +1
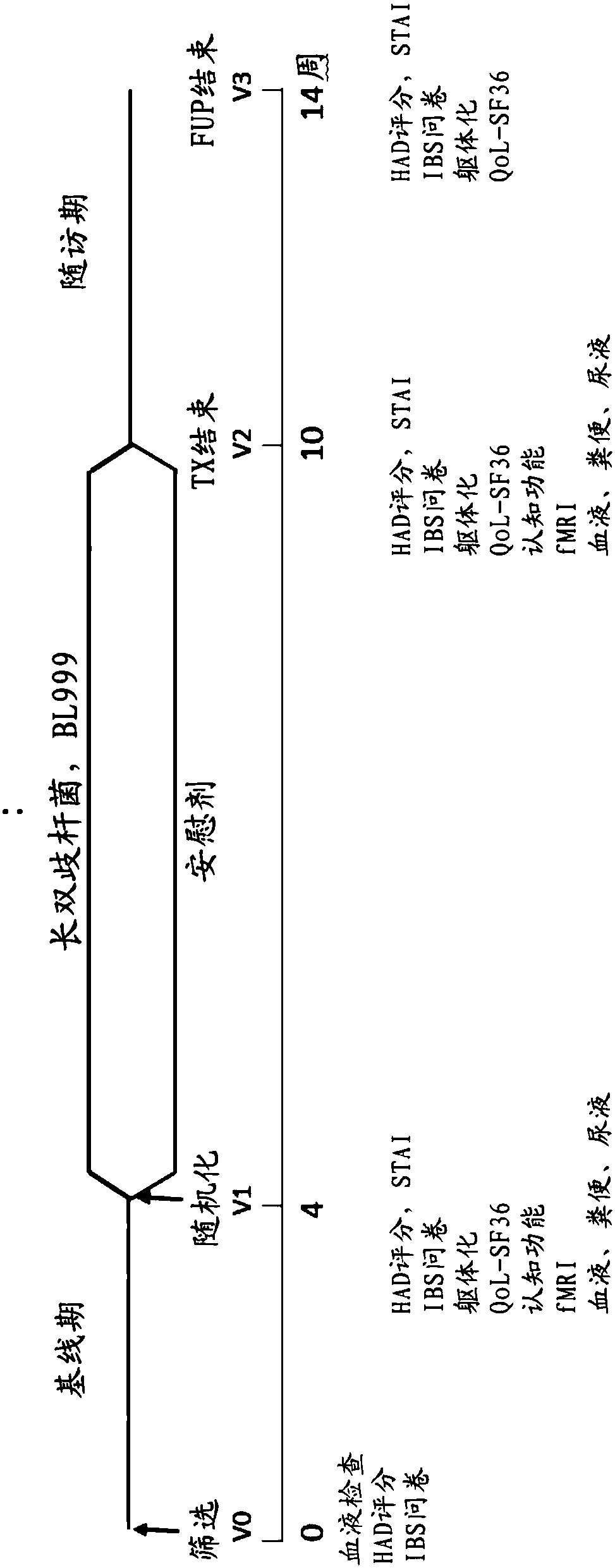
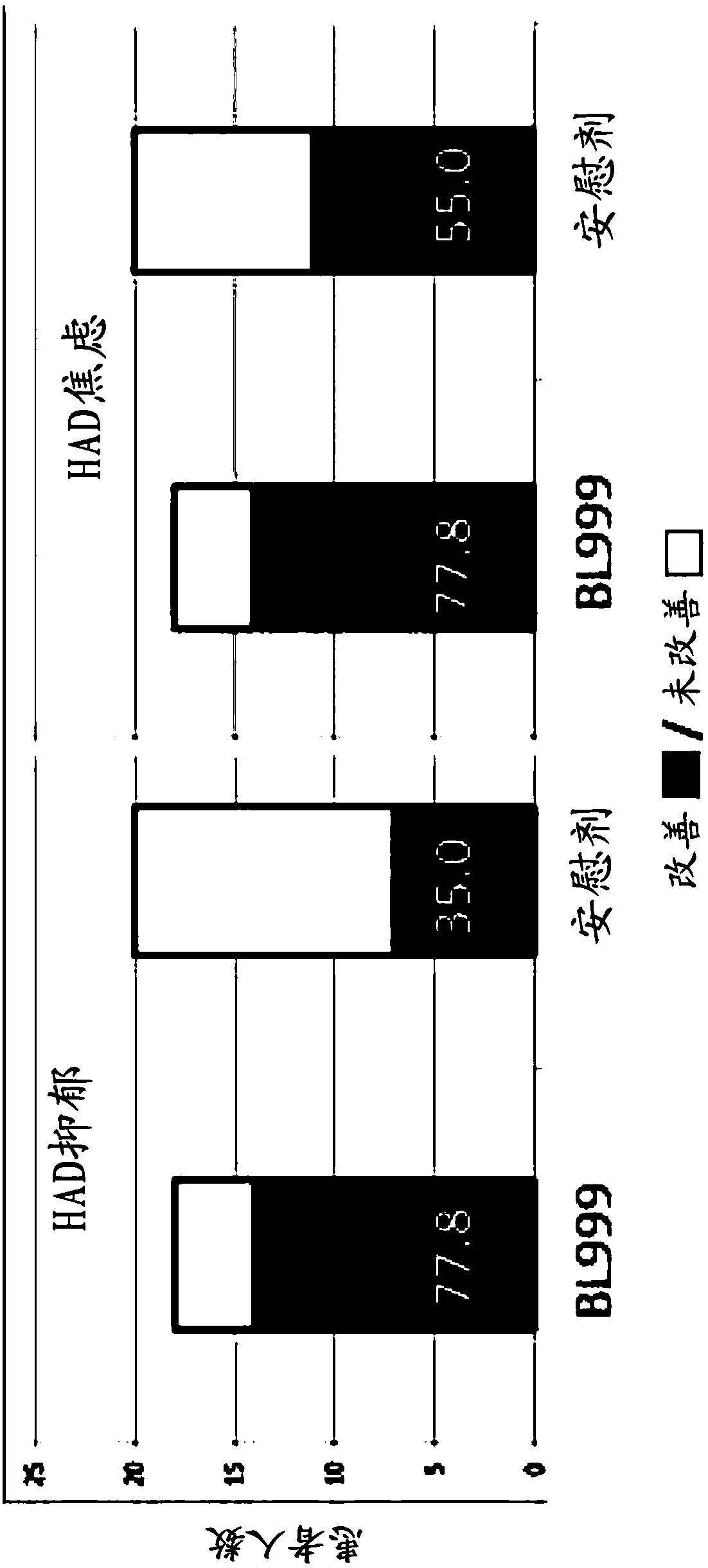
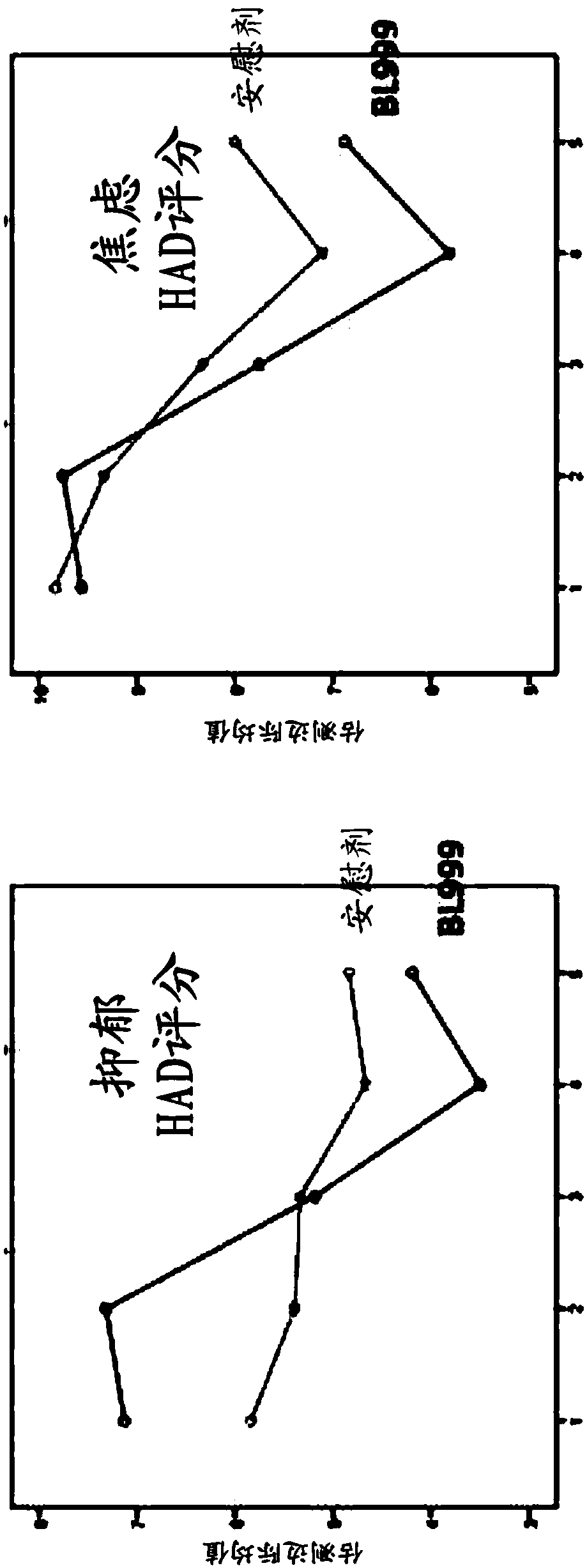
Methods and compositions using bifidobacterium longum to treat or prevent depressive symptoms
PendingCN108367033AApplication securityImprove securityNervous disorderUnknown materialsPhobiasDepression prevention
Compositions and methods use Bifidobacterium longum NCC3001 (ATCC BAA-999) to treat or prevent a depressive symptom. Prolonged anti-depressive effects can continue after administration of the compositions is ended. Non-limiting examples of a depressive symptom which can be treated or prevented include depressed mood; sadness; anxiety; "empty" feelings; loss of interest or pleasure; irritability; restlessness; changes in appetite or weight; sleep disturbances; lack of or decreased energy; feelings of worthlessness; guilt; helplessness; anger and hostility; difficulty in thinking, concentrating,or making decisions; hopelessness; tiredness; fatigue; memory difficulties; tearfulness; brooding; phobias; excessive worry over physical health; sexual dysfunction; persistent physical symptoms thatdo not respond to treatment; and combinations thereof. These depressive symptoms can be associated with a depressive state or depressive disorder or can be found sub-clinically or not associated withthese depressive states / disorders (e.g., not part of a syndrome or psychiatric disorder).
Owner:SOC DES PROD NESTLE SA
Differentiation device, differentiation method for depression symptoms, determination method for level of depression symptoms, stratification method for depression patients, determination method for effects of treatment of depression symptoms, and brain activity training device
The present invention differentiates objective disease labels for depression symptoms relative to brain activity states. As one means to address the problem to be addressed thereby, the present invention provides a differentiation device that is for helping to determine whether a subject has depression symptoms. The differentiation device comprises a storage device that is for storing informationthat specifies a classifier that has been generated by classifier generation processing from signals obtained by using a brain activity detection device to chronologically premeasure signals that indicate brain activity in a plurality of prescribed regions of the resting brains of each of a plurality of participants that include healthy and depressive patients. The classifier: is generated on thebasis of a weighted sum of a plurality of functional connectivities that, from among functional connectivities between the plurality of prescribed regions, have, by means of machine learning, been selected by feature selection as being related to disease labels for depression symptoms; and is generated to differentiate the disease labels for depression symptoms. The differentiation device also comprises a computing device. Using the classifier, the computing device executes differentiation processing that generates classification results for the depression symptoms of the subject.
Owner:ATR ADVANCED TELECOMM RES INST INT +1
Novel pharmaceutical compositions and methods for menopause related anxiety and depression
InactiveUS20210069209A1Lower estrogen levelsNervous disorderPharmaceutical delivery mechanismDuring menopauseAzelastine
Pharmaceutical compositions comprising azelastine or a pharmaceutically acceptable salt of azelastine and alprazolam are disclosed. Methods of using the pharmaceutical compositions for treating perimenopausal or menopausal patients, such as patients suffering from, experiencing, exhibiting and / or having one or more symptoms of anxiety or depression, are also disclosed.
Owner:LA PHARMATECH INC
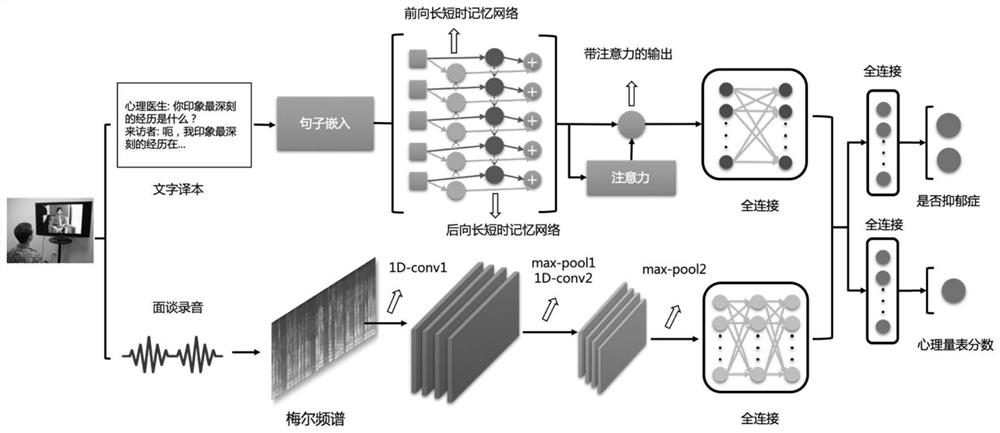
Depression symptom information evaluation method
InactiveCN112164459AStrong generalizationThe test results are accurate and stableSemantic analysisSpeech analysisDepressive symptomatologyKnowledge management
The invention discloses a depression symptom information evaluation method, which analyzes interview contents of a visitor and a psychologist, fuses voice features and character embedding features ininterview audio and character translations, scores depression-related psychological scale corresponding to the visitor, and improves the evaluation accuracy under the condition of not limiting the interview contents. According to the invention, an objective depression auxiliary evaluation method and rapid, effective and economic depression symptom self-evaluation can be provided for psychologists.
Owner:TONGJI UNIV
Maintenance therapy using tianeptine
The present application provides a method for treating treatment-resistant depression in a patient in need thereof, comprising administering to said patient a therapeutically effective amount of tianeptine, subsequent to a ketamine treatment; also provided is a method of treating suicidal ideation (SI), post-traumatic stress disorder (PTSD), mild cognitive impairment (MCI) or pre-dementia co-morbid with symptoms of depression; and further provided is maintenance therapy for depression remission.
Owner:GENOMIND
Application of all-cannabinoid in preparation of drugs for treating depression
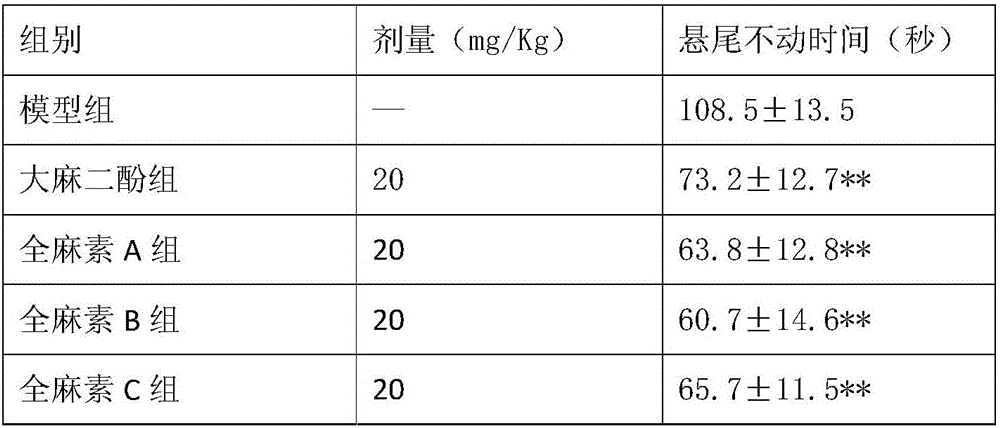
InactiveCN105963359AShorten immobility timeIncrease the number of voluntary activitiesNervous disorderPlant ingredientsEndogenous depressionReactive Depression
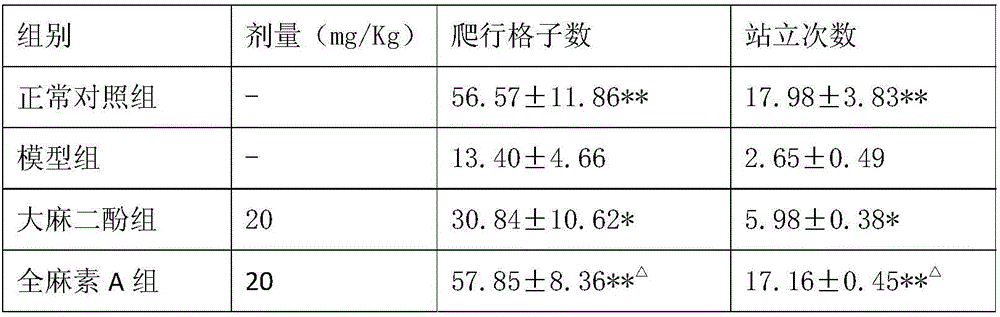
The invention discloses application of all-cannabinoid in preparation of drugs for treating depression. The all-cannabinoid is prepared by uniformly mixing, by weight, 0.3-99.7 parts of an industrial hemp seed extract with 99.7-0.3 parts of industrial cannabinoids. The all-cannabinoid has the obvious relieving effect on the symptoms such as secondary learning memory deterioration of the depression and can be used for preparing the drugs for treating various depression symptoms including endogenous depression, reactive depression, latent depression, secondary depression caused by drugs, climacteric or postnatal depression, depression induced by cerebral trauma or stroke, diabetes-complicated depression and depressive neurosis and used for preparing the drugs for treating the symptoms such as secondary learning memory deterioration and anhedonia which are caused by the depression, and the effect of the all-cannabinoid is better than that of cannabidiol. The all-cannabinoid can be prepared into the clinical drugs of various dosage forms through a conventional preparation method and is convenient to use.
Owner:云南瑞酚生物科技有限公司
Application of 2,3,5,4-tetrahydroxystilbene-2-O-beta-D-glucoside to preparing medicaments for treating depression
InactiveCN101966193ADecreased learning and memoryLow toxicityOrganic active ingredientsNervous disorderEndogenous depressionReactive Depression
The invention discloses application of 2,3,5,4-tetrahydroxystilbene-2-O-beta-D-glucoside to preparing medicaments for treating depression, in particular to application of 2,3,5,4-tetrahydroxystilbene-2-O-beta-D-glucoside as an active ingredient to preparing medicaments for treating depression. The invention provides the application of the 2,3,5,4-tetrahydroxystilbene-2-O-beta-D-glucoside as the medicaments for treating depression, and the 2,3,5,4-tetrahydroxystilbene-2-O-beta-D-glucoside is hardly innoxious and is applied to preparing medicaments for preventing or treating various depression symptoms including endogenous depression, reactive depression, masked depression, secondary depression caused by medicaments, menopausal or post-natal depression, depression induced by traumatic brain injury or brain death and depressive neurosis and medicaments for treating diabetes and depression complications and related diseases.
Owner:SOUTHWEST JIAOTONG UNIV
Application of emodin in preparation of drugs for treating depression
InactiveCN101961325AOrganic active ingredientsNervous disorderEndogenous depressionPost-pregnancy depression
The invention discloses an application of emodin as an active ingredient in the preparation of drugs for treating depression, wherein The emodin is used as an active ingredient to prepare drugs for treating the depression. The invention provides an application of the emodin as an active ingredient in the production of drugs for preventing and treating the depression. Emodin has the characteristic of low toxicity and is used for preparing drugs for preventing or treating various kinds of depression symptoms comprising endogenous depression, reactive depression, secretiveness depression, drug induced secondary depression, climacteric or postpartum depression, cerebral trauma or cerebral apoplexy induced depression and depressive neurosis, and can also be applied to drugs for treating diabetes accompanied by depression and related diseases.
Owner:SOUTHWEST JIAOTONG UNIV
Natural product compositions for treating or managing symptoms of add, adhd, anxiety, and depression
ActiveUS20180339008A1Relieve symptomsReducing and ameliorating symptomHydroxy compound active ingredientsPlant ingredientsDiseaseNatural product
Owner:KLELE LUKE
A method for differentially diagnosing in vitro a bipolar disorder and a major depressive disorder
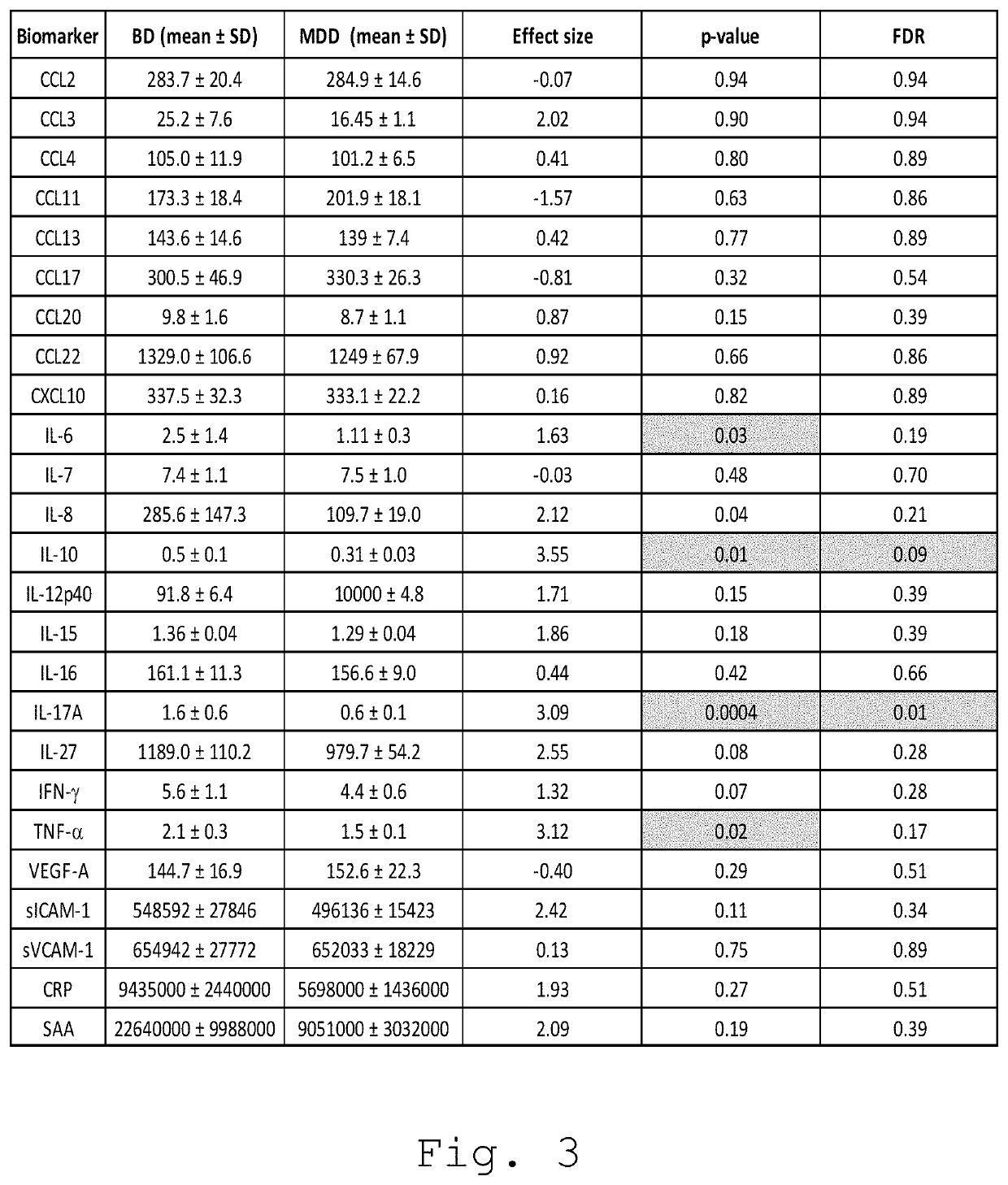
The invention relates to an in vitro or ex vivo method for differentially diagnosing a bipolar disorder and a major depressive disorder in a human patient in a need thereof presenting depressive symptoms, comprising the following steps: providing a biological sample from said patient; determining, from said biological sample, the abundance of at least one of the following cytokines TNF-α, IFN-γ, IL-6, IL-10, IL-12p40, IL-15, IL-16, IL-17A and IL-27; and diagnosing a bipolar disorder or a major depressive disorder from the determination of the abundance of the at least one of the following cytokines TNF-α, IFN-γ, IL-6, IL-10, IL-12p40, IL-15, IL-16, IL-17A and IL-27.
Owner:CENT NAT DE LA RECHERCHE SCI +7
Probiotic composition and application thereof in preparation of medicines for treating co-disease constipation and depression
ActiveCN113583923AImprove depressive symptomsRelieves symptoms of depressionNervous disorderBacteriaLactobacillus rhamnosusProbiotic bacterium
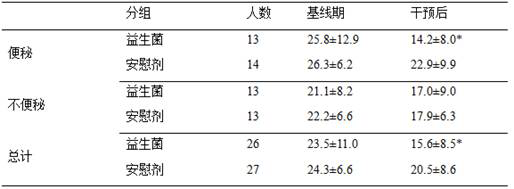
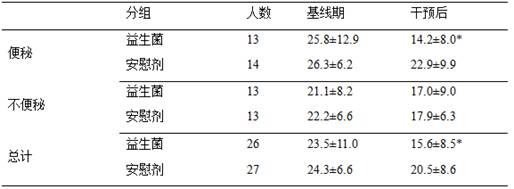
The invention provides a probiotic composition and an application thereof in preparation of medicines for treating co-disease constipation and depression. The composition comprises bifidobacterium animalis subsp. Lactis U9, lactobacillus paracasei L55, lactobacillus rhamnosus LL23, bifidobacterium adolescentis BQ23 and lactobacillus acidophilus RA15. According to the invention, a random double-blind test is carried out to carry out probiotic intervention on depression patients. According to the invention, 63 depression patients are recruited in total, and 31 of the patients suffer from constipation. The result shows that after 8 weeks of intervention, the HAMD score of the probiotic group of the depression crowd with co-disease constipation is reduced to 14.2 + / -8.0, which is significantly lower than that of a placebo group (22.9 + / -9.9, P is less than 0.05), and the HAMD score of the probiotic group of the depression crowd without constipation has no significant difference from that of the placebo group. The probiotic composition disclosed by the invention can be used for effectively relieving the depressive symptoms of depressive people with co-disease constipation.
Owner:CHINA AGRI UNIV
Natural product compositions for treating or managing symptoms of ADD, ADHD, anxiety, and depression
ActiveUS10624939B2Reducing and ameliorating symptomLower Level RequirementsHydroxy compound active ingredientsMagnoliophyta medical ingredientsDiseaseNatural product
Owner:KLELE LUKE
Application of rat model with depression related to chronic pain in researching on action mechanism and targets of antidepressant on pain
The invention discloses application of a rat model with depression related to a chronic pain in researching on an action mechanism and targets of an antidepressant on pain. According to the model, lumbar 5 spinal nerve of a rat is ligated to cause distinct mechanical hyperalgesia and abnormal pain, accomplished with depression symptom. Then the ligation is released, and the abnormal pain behaviors of animals gradually disappear, but the psychiatric symptoms continue to exist. The depression model prepared by the invention can simulate a clinical phenomenon which is that pains disappear but emotional disorders still exist after treatment for the chronic pain patients, and then the action mechanism and the targets of the antidepressant on pain is understood from the source.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
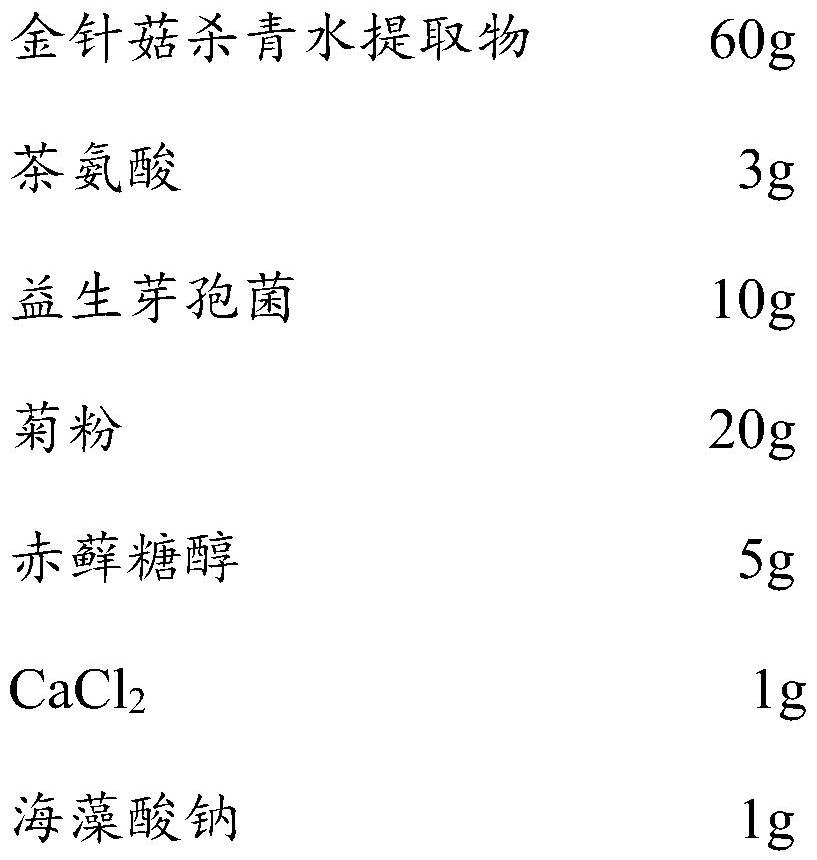
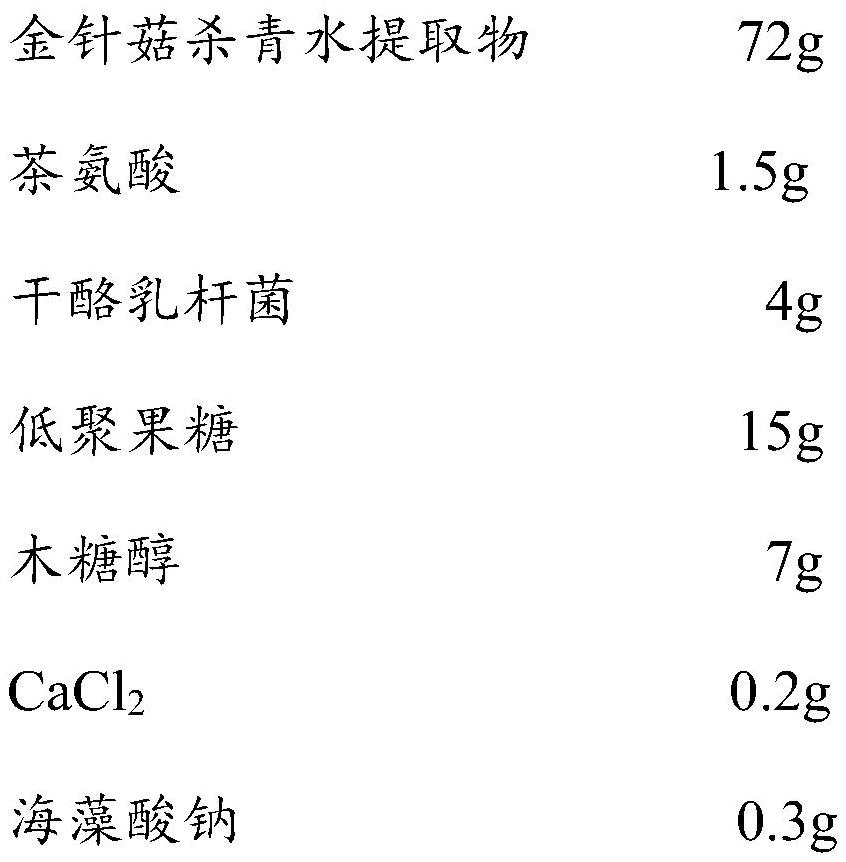
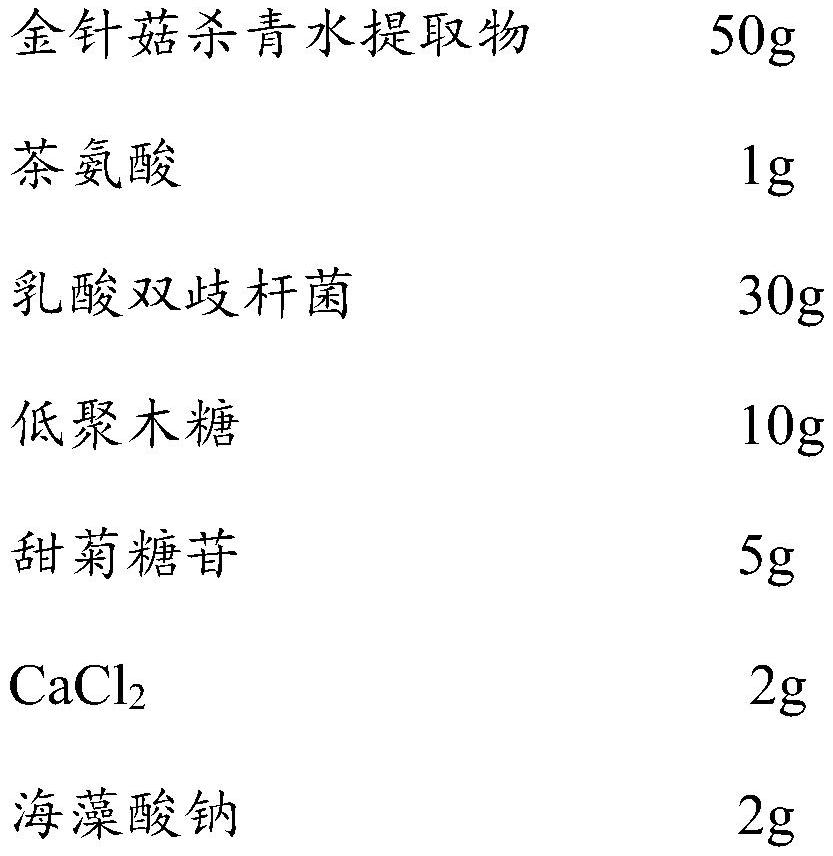
Pharmaceutical composition with effect of improving depressive symptoms and preparation method thereof
ActiveCN113332318AImprove depressive symptomsImprove sleepingNervous disorderHydroxy compound active ingredientsEfficacyPharmaceutical drug
The invention relates to a pharmaceutical composition capable of improving depressive symptoms. The composition is prepared from 50-80% of a flammulina velutipes enzyme-deactivated water extract, 1-3% of theanine, 1-10% of probiotics, 5-30% of prebiotics, 5-10% of sugar substitutes and a pharmaceutically acceptable carrier. The composition has the beneficial effects of well improving depressive symptoms, improving sleep, improving anxiety, improving memory, enhancing immunity and the like.
Owner:四川省食用菌研究所
Application of miR-129 in preparation of product for treating depression
ActiveCN113134011AImprove depressive symptomsSafe and long-lasting antidepressant effectOrganic active ingredientsNervous disorderHabenular nucleiDepressive symptomatology
The invention discloses an application of miR-129 in preparation of a product for treating depression. It is found in the invention that the depressive symptom of a mouse can be obviously improved by inner side habenular nucleus overexpression of the miR-129 of a depression mouse model, which indicates that the miR-129 or a substance for regulating and controlling the content or expression quantity of the miR-129 can be used for treating depression. Therefore, the micromolecule miR-129 can be used as a novel antidepressant drug, a safe and durable antidepressant effect can be achieved by single injection in an inner side habenular nucleus area, and the micromolecule miR-129 has a good clinical application prospect.
Owner:ACADEMY OF MILITARY MEDICAL SCI
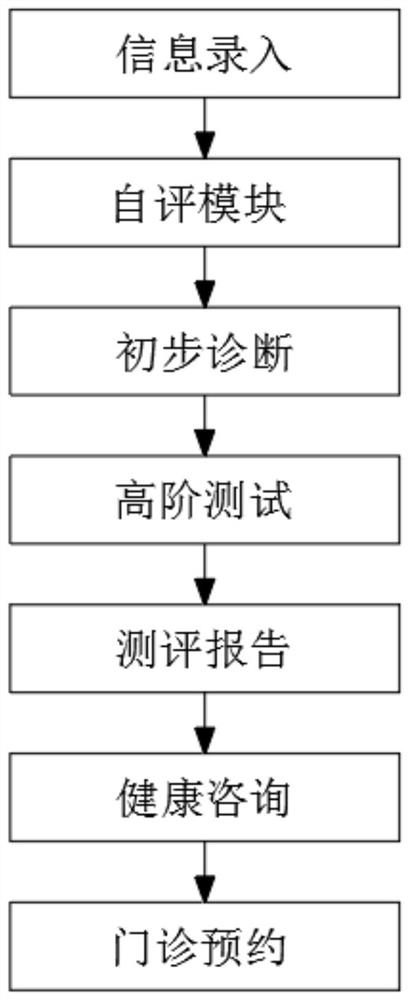
Depression patient symptom evaluation report system
InactiveCN113393914ASeek medical attention quicklyImprove accuracyMedical communicationMedical data miningPatients symptomsAnxiety
The invention discloses a depression patient symptom evaluation report system, and belongs to the technical field of health consultation evaluation. The method comprises the steps of 1, information input: establishing a personal health record for each patient, establishing an information sharing mechanism based on the Internet, and collecting and investigating nursing requirements and health consultation requirements of the patients; 2, a self-evaluation module: carrying out psychological record self-evaluation at any time, including depression evaluation, anxiety evaluation, suicide risk evaluation and the like; and 3, carrying out preliminary diagnosis. A personalized health evaluation report can be automatically generated according to self-evaluation, medication, medical treatment, basic conditions and the like of a patient, and then is pushed to the patient, family members and nursing personnel, so that the depressive symptom severity of the patient is judged according to the pushing result, the judgment accuracy is improved, and misjudgment and missed judgment are eliminated. Therefore, the psychological risk level of the depression patient is judged, the psychological condition of a detector is accurately grasped, the depression patient is timely reminded to go to a hospital to see a doctor, and symptomatic treatment is timely performed.
Owner:苏州市广济医院
A probiotic composition and its application in the preparation of comorbid constipation and depression medicine
ActiveCN113583923BImprove depressive symptomsRelieves symptoms of depressionNervous disorderBacteriaBiotechnologyDouble blind
The invention provides a probiotic composition and its application in the preparation of comorbid constipation and depression medicines, said composition comprising Bifidobacterium animalis subspecies U9, Lactobacillus paracasei L55, Lactobacillus rhamnosus LL23 , Bifidobacterium adolescentis BQ23 and Lactobacillus acidophilus RA15. The present invention has carried out a randomized double-blind test to perform probiotic intervention on depressed patients. A total of 63 depressed patients were recruited, 31 of whom had comorbid constipation. The results showed that after 8 weeks of intervention, the Hamilton depression (HAMD) score of the probiotic group decreased to 14.2±8.0 in the depressed group with comorbid constipation, which was significantly lower than that in the placebo group (22.9±9.9, P<0.05). There was no significant difference in HAMD scores between the population probiotic group and the placebo group. The probiotic composition of the invention can effectively alleviate the depression symptoms of depressed people with comorbid constipation.
Owner:CHINA AGRI UNIV
Application of physcion in preparing medicines for treating depression
InactiveCN101961326BOrganic active ingredientsNervous disorderEndogenous depressionPost-pregnancy depression
The invention discloses application of physcion used as an active component in preparing medicines for treating depression. The physcion has the characteristic of little toxicity, and can be used for preparing medicines for preventing or treating various kinds of depression, including endogenous depression, reactive depression, masked depression, secondary depression caused by medicines, climacteric depression or postpartum depression, depression induced by cerebral trauma or cerebrovascular disorder and depressive neurosis as well as diabetes companied by depression and related diseases.
Owner:SOUTHWEST JIAOTONG UNIV
Application of PF429242 in preparation of depression treatment medicine
PendingCN112933083AAvoid damageInhibit neuroinflammationNervous disorderAntipyreticIntraperitoneal routeStaining
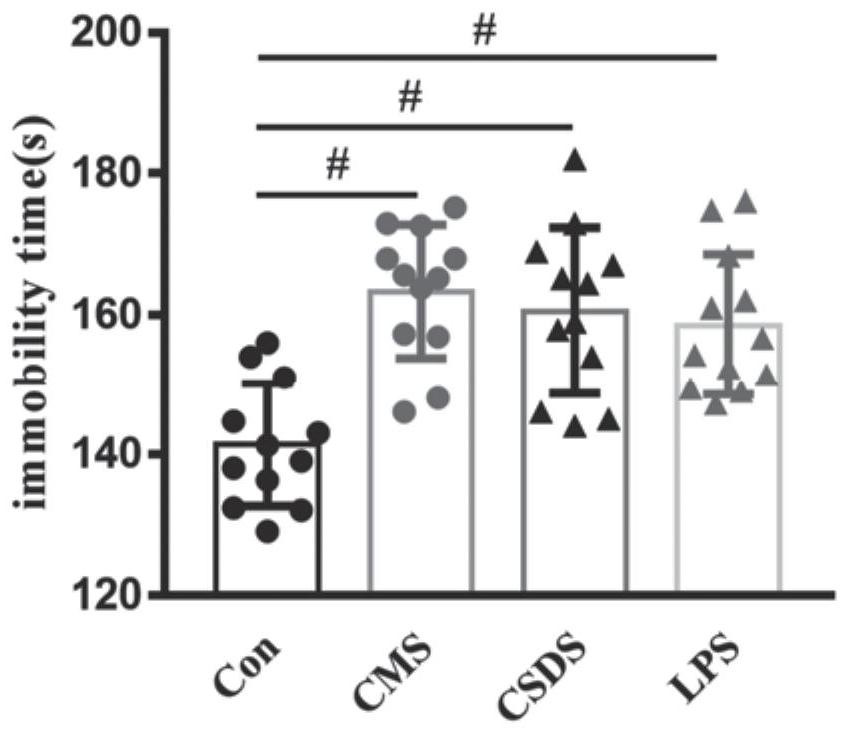
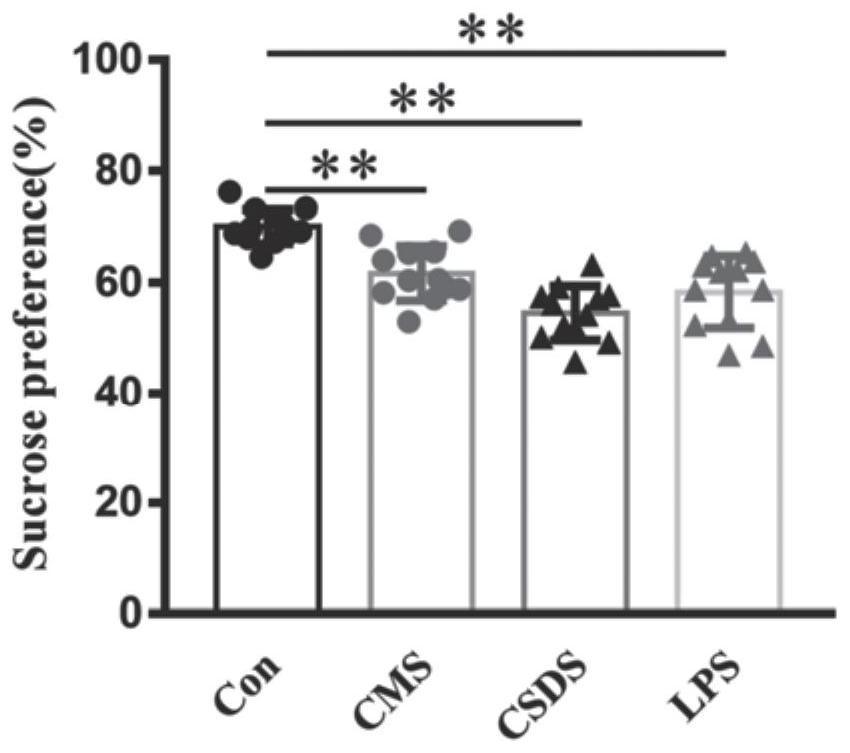
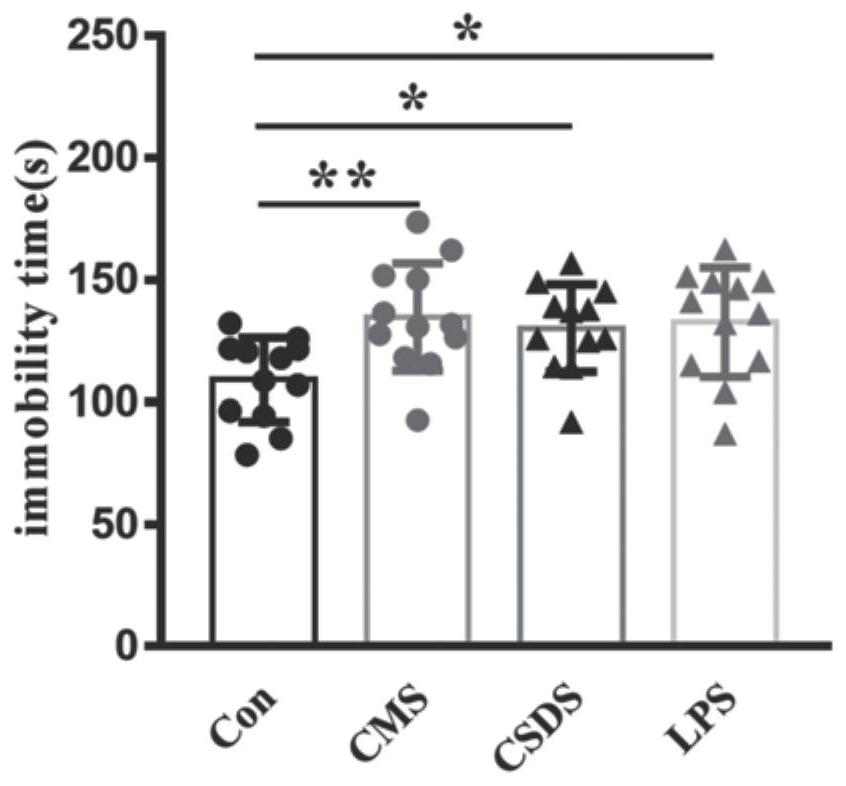
The invention discloses an application of PF429242 in preparation of a depression treatment medicine. Through detection of three models including a chronic unpredictable stress (CMS) model, a social failure model (CSDS) and an LPS intraperitoneal injection induced depression model, it is found that the appropriate dosage of PF429242 has an obvious improvement effect on depressive symptoms; meanwhile, an immunofluorescence staining experiment finds that the damage of 5-hydroxytryptamine neurons can be effectively improved by using a proper amount of PF429242, so that the depression-like behavior is inhibited; moreover, according to electrophysiological experiments, the improvement of the depression symptom by the PF429242 is that the treatment effect of the PF429242 is achieved through an IL-1R1 target spot. Therefore, the PF429242 has a good clinical application prospect when being applied to the medicine for treating the depression.
Owner:XUZHOU MEDICAL UNIV
Application of 5, 6-didehydro-pythoncidere in preparation of antidepressant drug
InactiveCN112263574ADespair reliefState of despair reversedOrganic active ingredientsNervous disorderPharmaceutical drugDepressive symptomatology
The invention discloses application of 5, 6-didehydro-pythoncidere in preparation of an antidepressant drug. A tail suspension test and a forced swimming test are that a depression model animal is placed in a severe environment and cannot escape so as to enter an intermittent motionless despair state. The antidepressant drug can relieve the despair degree of the depression model animal, so that the motionless state can be reversed, and the depression symptom is relieved. The experimental study finds that the 5, 6-didehydro-pythoncidere can relieve the despair degree of a depression model mouse, has an antidepressant effect, and has a prospect of being developed into the antidepressant drug.
Owner:南京温博生物科技有限公司
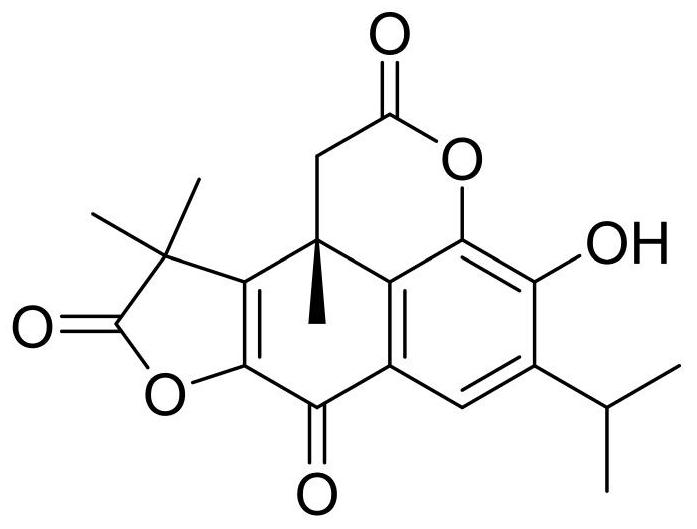
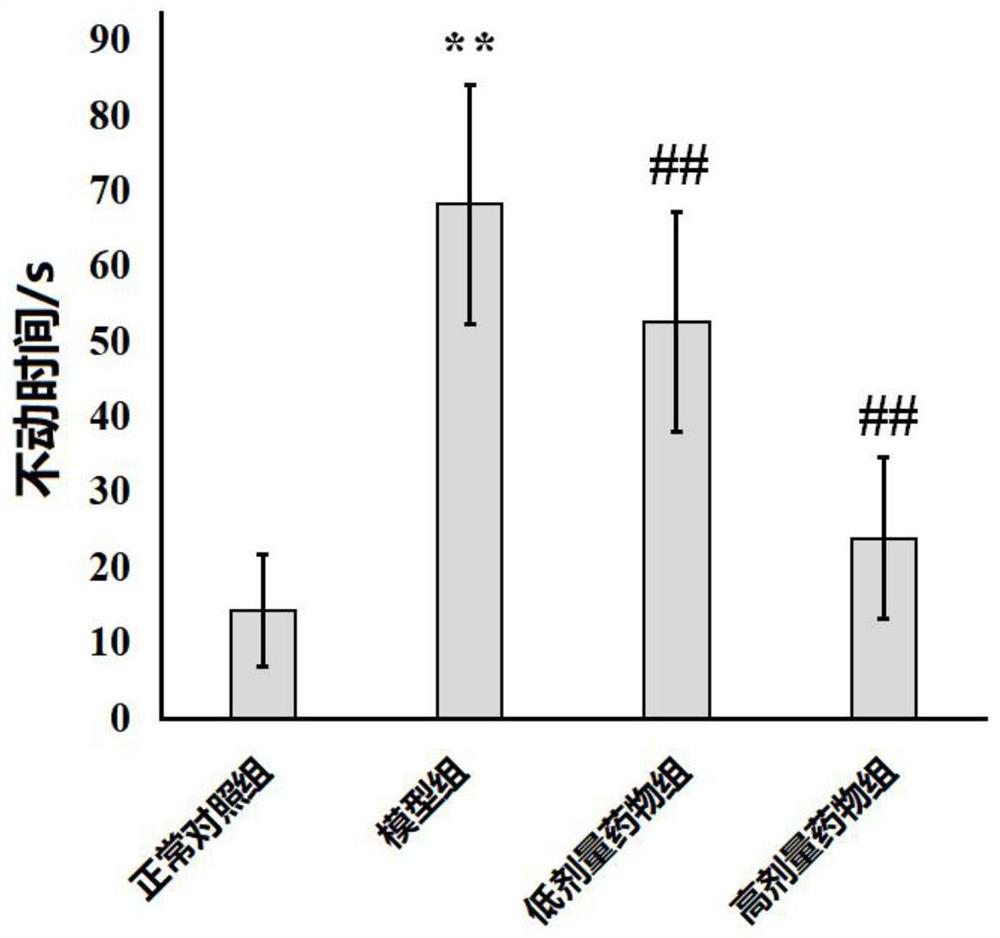
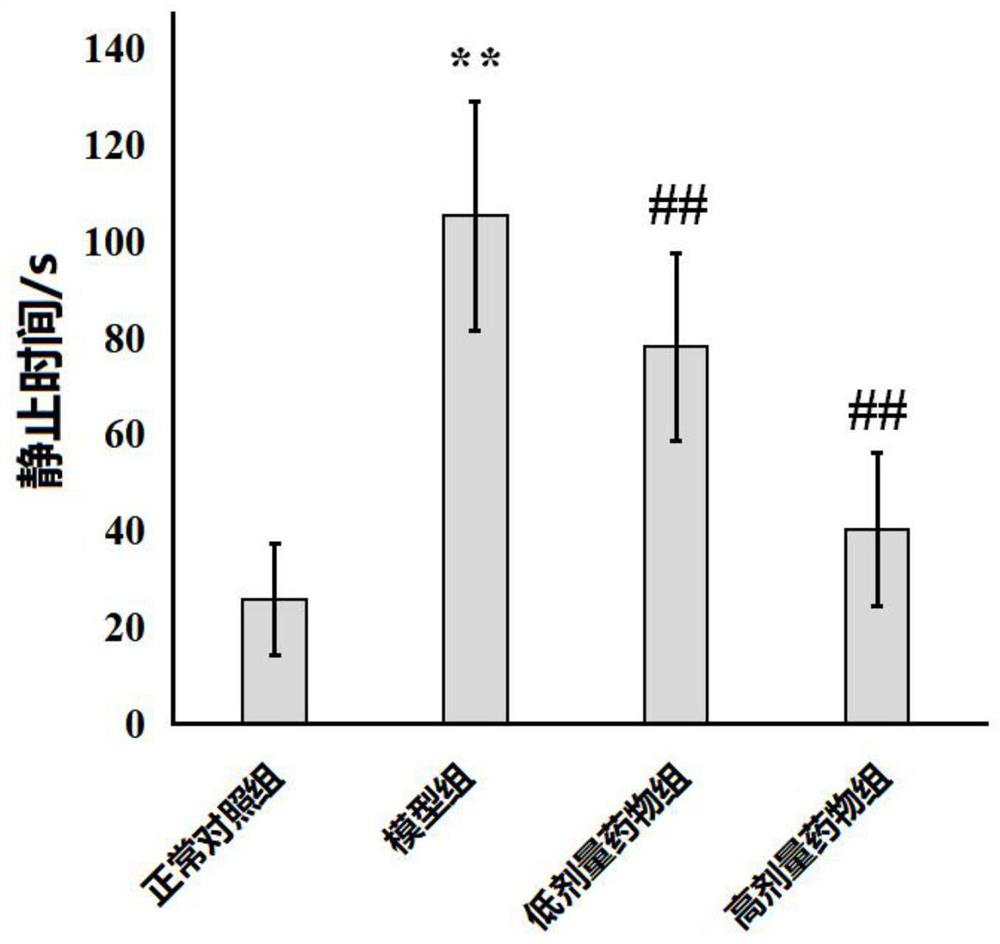
Application of bergapten in treatment of depression
PendingCN113797196AShorten immobility timeIncreased voluntary activityNervous disorderNatural extract food ingredientsGlial activationDrug discovery
The invention discloses application of bergapten in treatment of depression, and belongs to the technical field of medicines. The invention provides a natural medicine bergapten for preventing and treating depression by regulating inflammatory response for the first time, and the depression symptom is remarkably improved. The bergapten enables the immobility time of lipopolysaccharide (LPS)-induced anti-depression mice in a tail suspension test and a forced swimming test to be obviously reduced; the sweet water preference percentage is increased, and the autonomous activity is enhanced; and the action mechanism is related to the neuroinflammatory response mediated by the activation of depression mouse microglial cells. The invention provides a new way for improving the current depression treatment method. According to the scheme, new use of old medicines can be realized, and the time from medicine discovery to clinical transformation can be greatly shortened.
Owner:MINZU UNIVERSITY OF CHINA
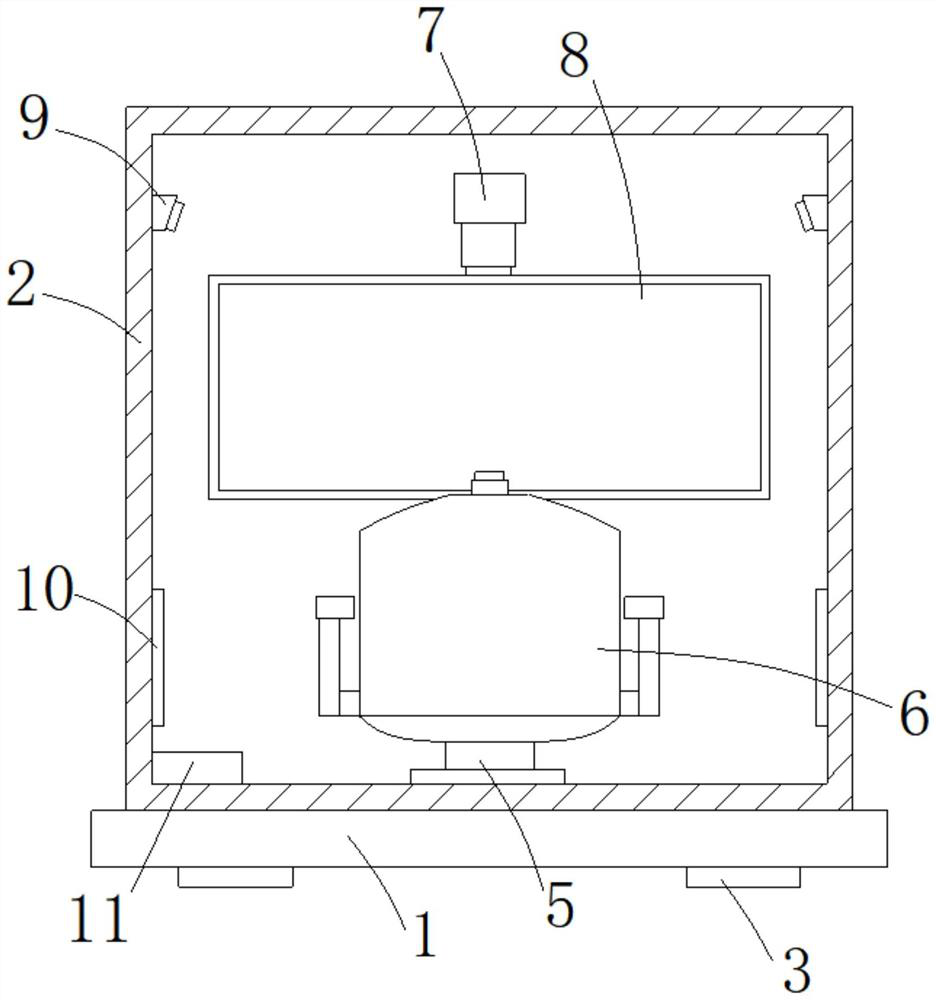
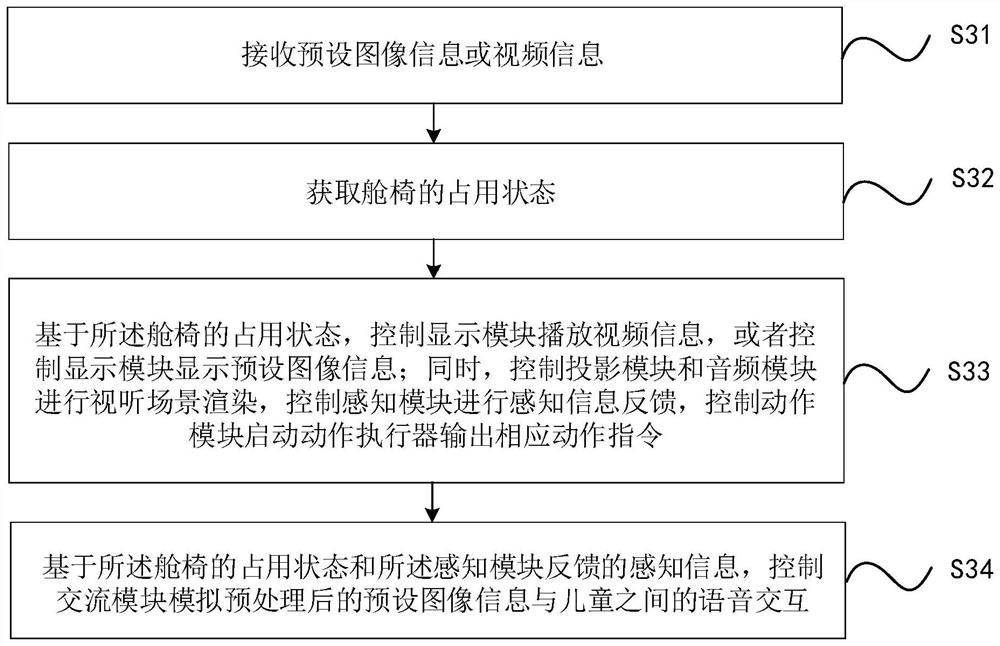
Children depression treatment shelter and control method thereof
PendingCN114191680AEnhanced multi-sensory perception experienceImprove sealingBreathing protectionTreatment roomsPhysical medicine and rehabilitationDepressive symptomatology
The invention relates to the technical field of household medical treatment, in particular to a child depression treatment shelter and a control method thereof. The child depression treatment square cabin comprises a cabin body, wherein a treatment cavity is formed in the cabin body; an action actuator is arranged at the bottom end of the treatment cavity, a cabin chair is arranged on the upper portion of the action actuator, and the action actuator can control the cabin chair to execute corresponding action instructions; a display screen is arranged right in front of the cabin chair, the display screen is connected with a telescopic rod, and the telescopic rod drives the display screen to move through stretching out and drawing back; a projector is arranged in the cabin body and is suitable for projecting to the inner wall of the cabin body; and a loudspeaker is arranged on the side wall of the cabin body. According to the children depression treatment square cabin provided by the invention, the family interaction mode is constructed and rendered in the isolated space to meet the conditions of family psychotherapy, so that the depression symptom of children is relieved.
Owner:JIANGXI INST OF FASHION TECH
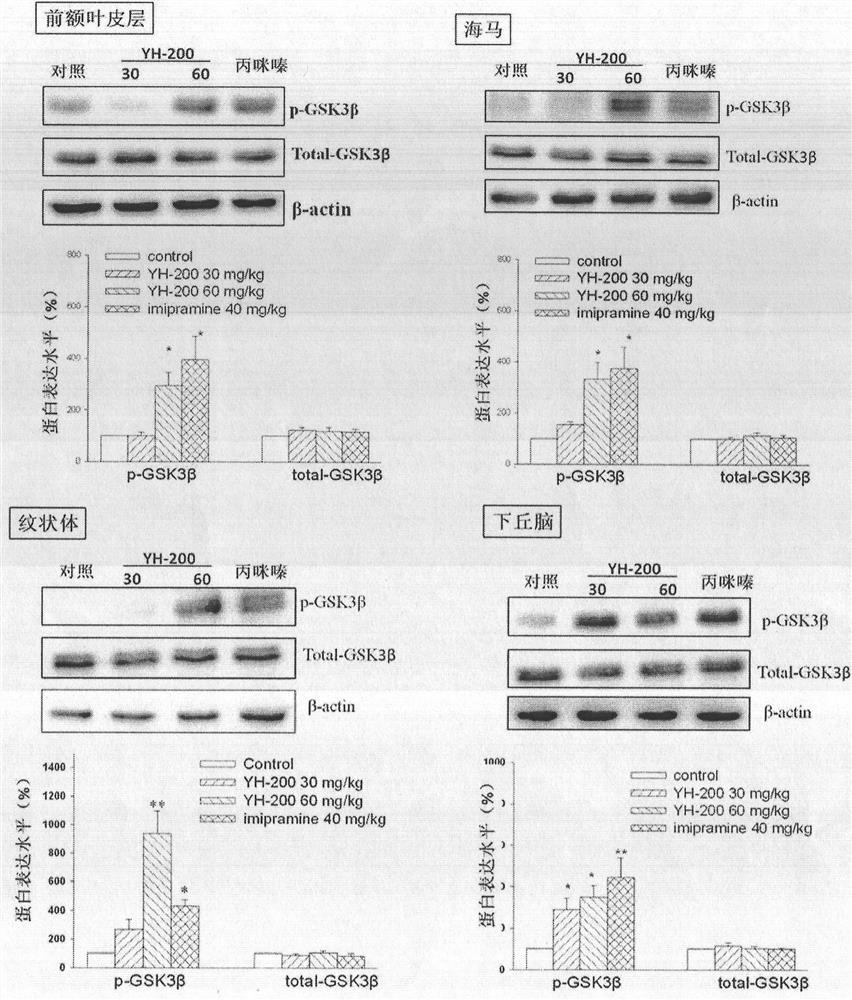
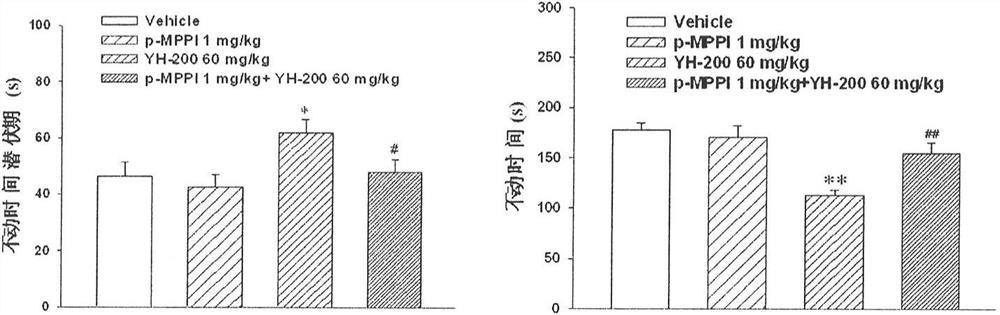
Use of 7-alkoxy fangchinoline base compounds in the preparation of medicines for treating and improving depressive symptoms
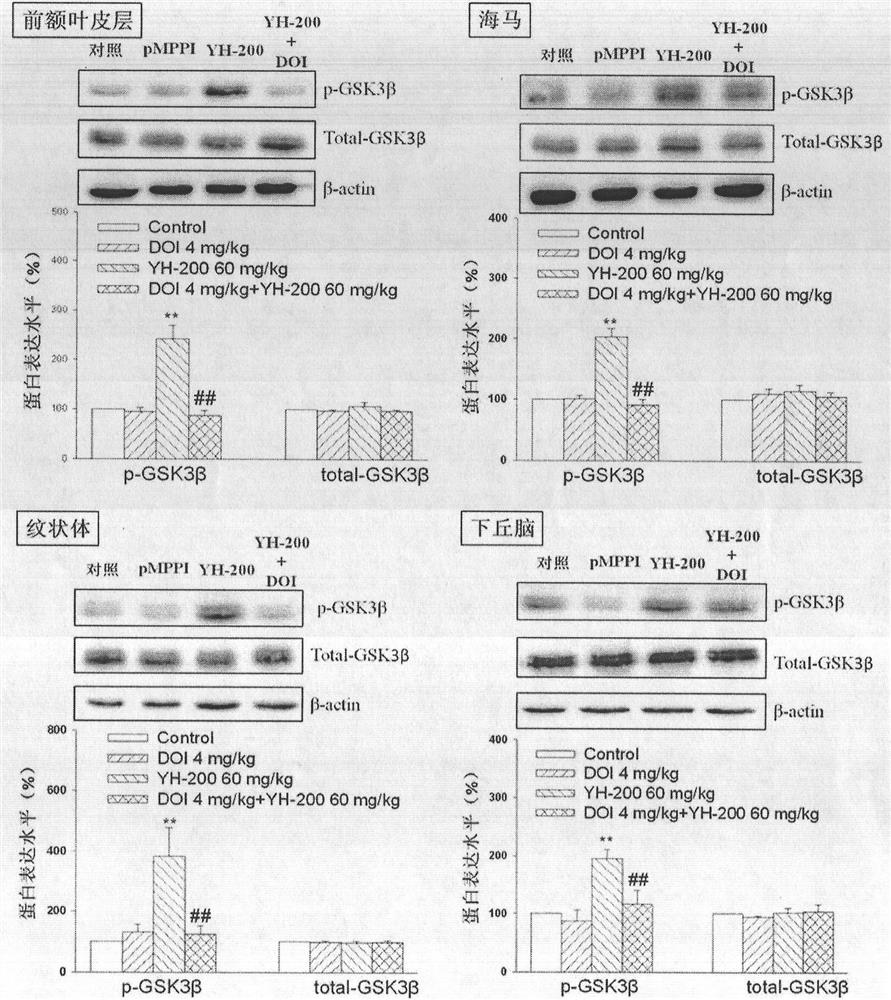
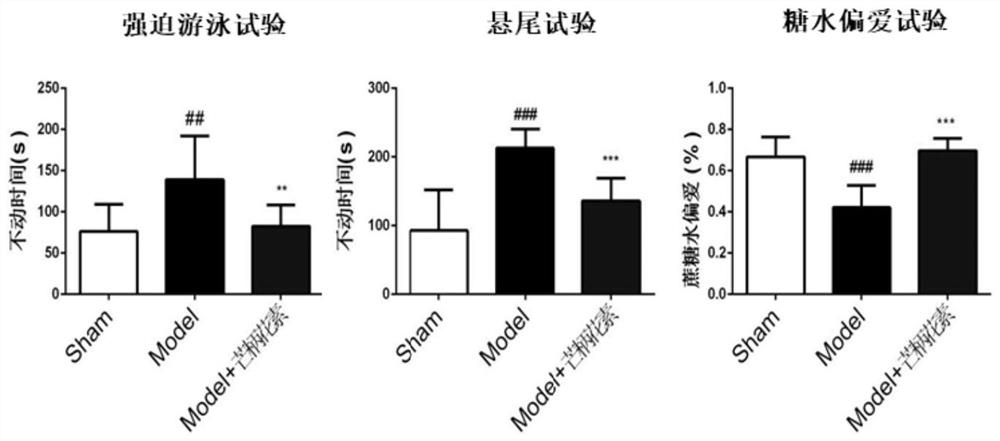
The invention provides a new application of 7-alkoxy fangchinoline derivatives or pharmaceutically acceptable salts thereof in the preparation of GSK-3β inhibitors and in the treatment and improvement of depressive symptoms. The compound 1‑8 with the structure of formula (I) has shown the effect of promoting the phosphorylation of glycogen synthesis kinase ‑3β (GSK‑3β) at the level of cells and whole animals, and then inhibiting its activity; the results of animal experiments found that compound 1‑8 May be enhanced by 5‑HT 1A Receptor and melatonin receptor function, inhibits 5‑HT 2A Receptor function, inhibit GSK‑3β activity, improve depressive symptoms such as hopelessness, loss of pleasure, anxiety and sleep disorders.
Owner:PEKING UNIV
Application of formononetin in preparation of drugs for preventing and treating myocardial infarction combined with depression
ActiveCN113332273AImprove depressive behaviorFunction increaseOrganic active ingredientsNervous disorderHippocampal regionSide effect
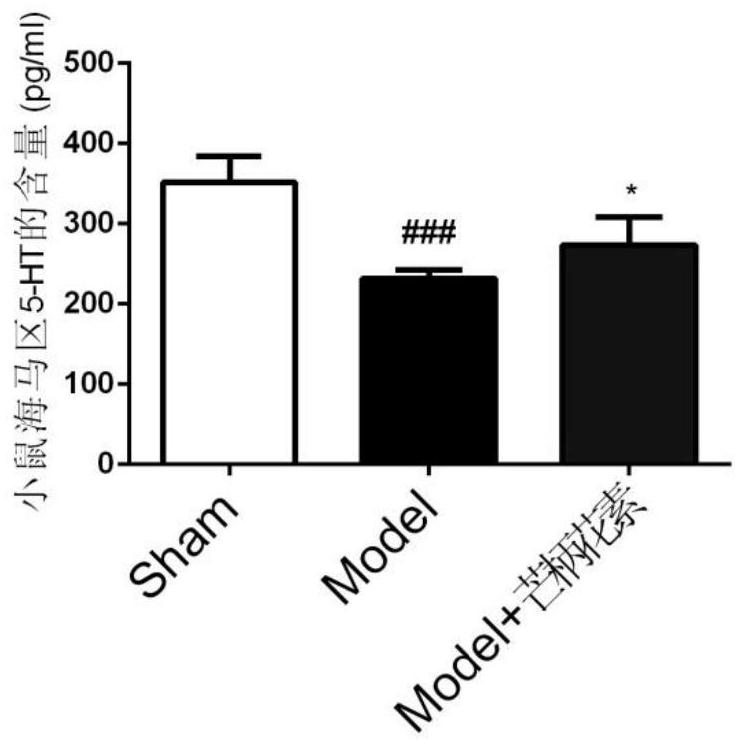
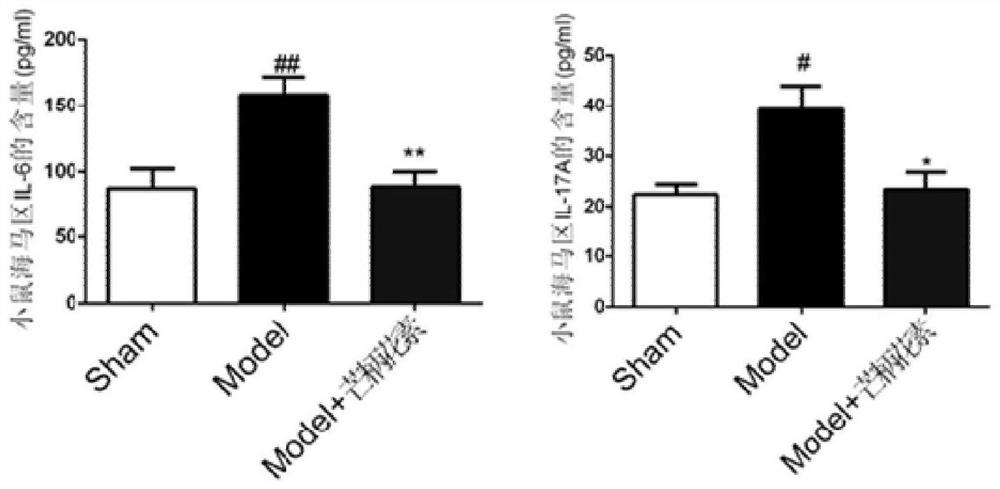
The invention belongs to the field of biological medicines and discloses application of formononetin in preparation of drugs for preventing and treating myocardial infarction combined with depression. The formononetin can obviously improve depression symptoms and myocardial dysfunction of mice suffering from myocardial infarction combined with depression. The formononetin can improve hypomotion, thought retardation, hypoactivity of willpower and inappetence, resist heart tissue apoptosis and / or resist brain tissue inflammation, can obviously increase expression of 5-HT and BDNF in the hippocampus, reduce expression of IL-6 and IL-17A in the hippocampus and serum, inhibit expression of in-vivo and in-vitro macrophage and microglial cell M1 type markers and promote expression of M2 type markers. The invention creatively provides application of formononetin in preparation of drugs for preventing and treating myocardial infarction combined with depression, interaction and side effects existing in drug combination are avoided, the probability of adverse reactions of patients is reduced, and a basis is provided for clinical practice of treating myocardial infarction combined with depression by formononetin.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Application of 2,3,5,4-tetrahydroxystilbene-2-O-beta-D-glucoside to preparing medicaments for treating depression
InactiveCN101966193BDecreased learning and memoryLow toxicityOrganic active ingredientsNervous disorderEndogenous depressionReactive Depression
The invention discloses application of 2,3,5,4-tetrahydroxystilbene-2-O-beta-D-glucoside to preparing medicaments for treating depression, in particular to application of 2,3,5,4-tetrahydroxystilbene-2-O-beta-D-glucoside as an active ingredient to preparing medicaments for treating depression. The invention provides the application of the 2,3,5,4-tetrahydroxystilbene-2-O-beta-D-glucoside as the medicaments for treating depression, and the 2,3,5,4-tetrahydroxystilbene-2-O-beta-D-glucoside is hardly innoxious and is applied to preparing medicaments for preventing or treating various depression symptoms including endogenous depression, reactive depression, masked depression, secondary depression caused by medicaments, menopausal or post-natal depression, depression induced by traumatic braininjury or brain death and depressive neurosis and medicaments for treating diabetes and depression complications and related diseases.
Owner:SOUTHWEST JIAOTONG UNIV
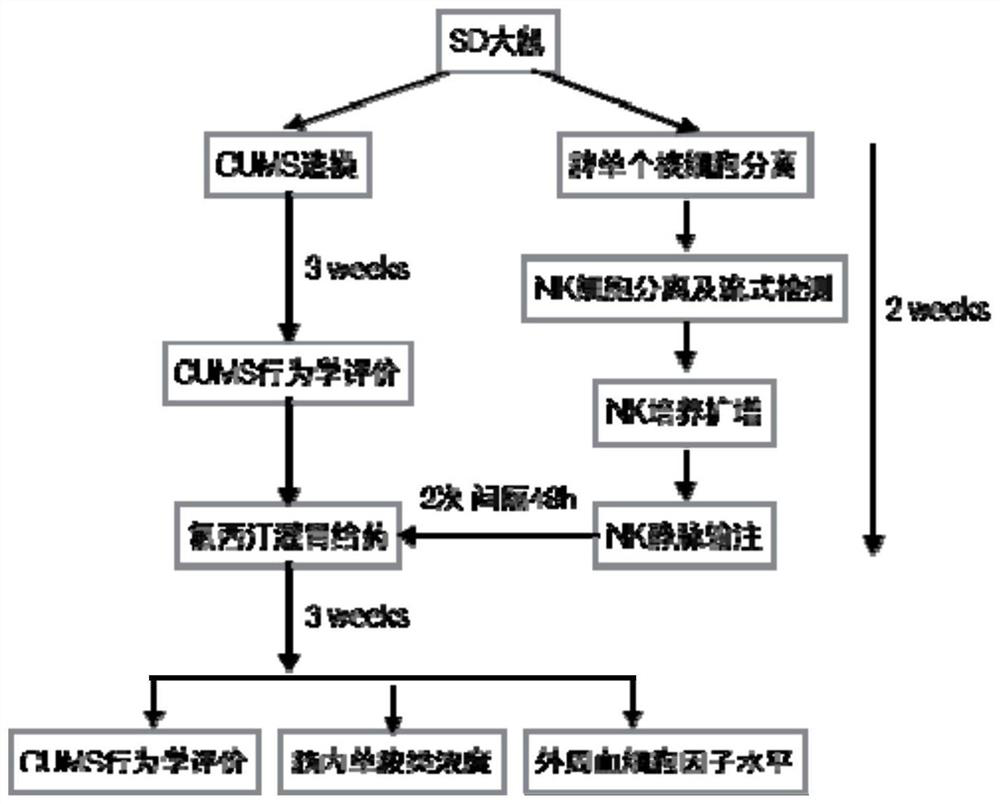
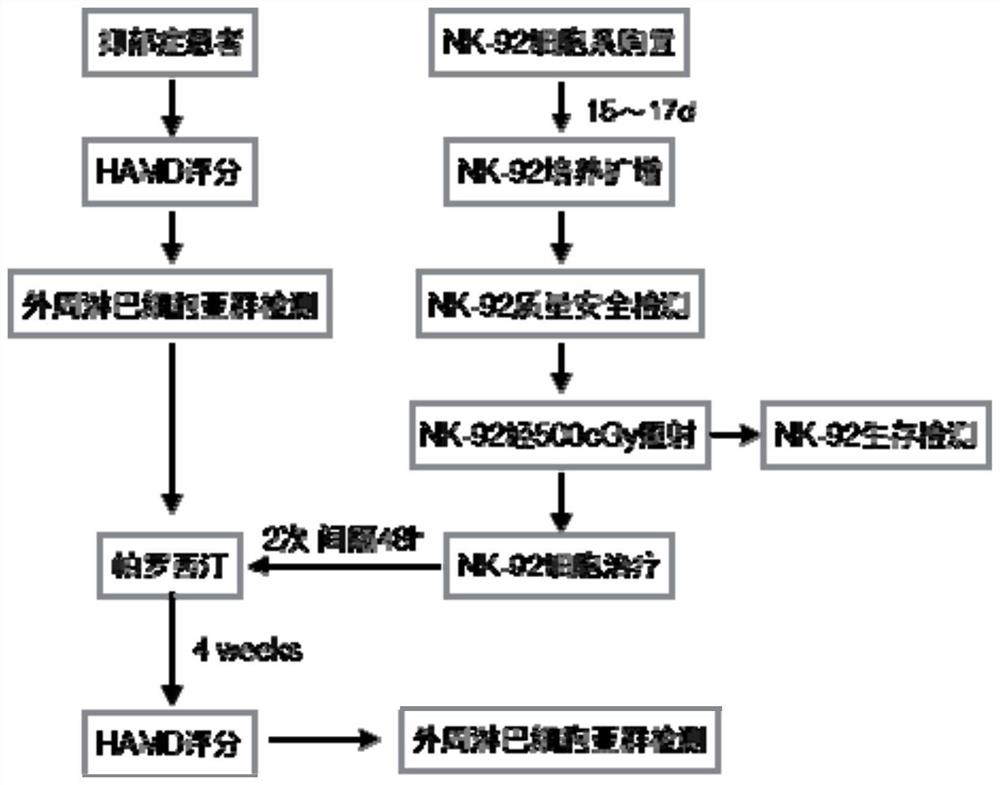
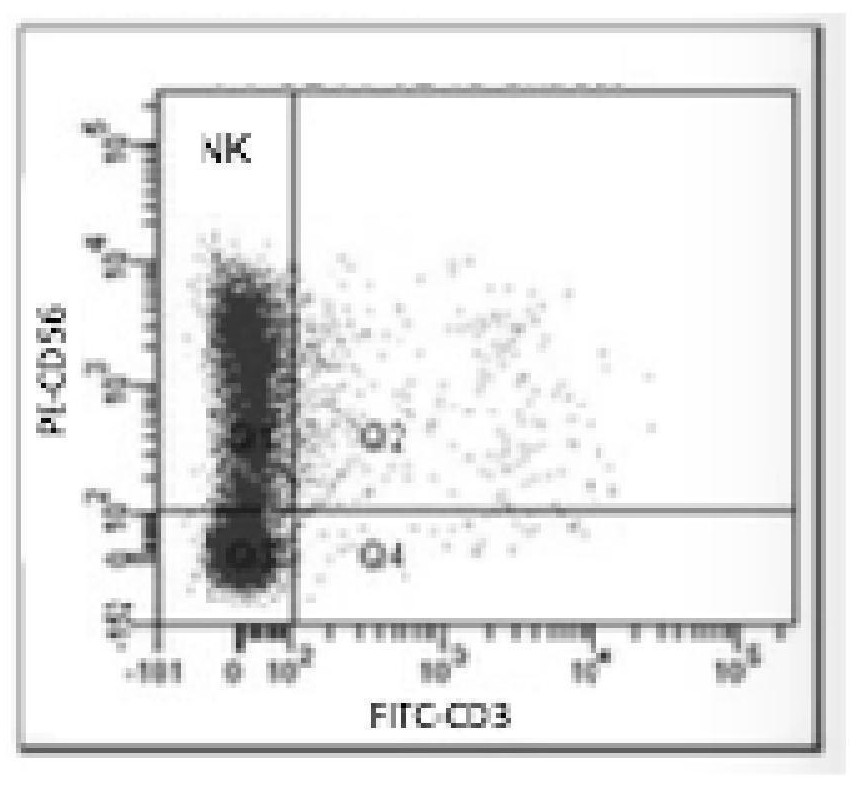
Application of natural killer cells combined with antidepressants in improving depressive symptoms
ActiveCN106138098BNervous disorderMammal material medical ingredientsNatural Killer Cell Inhibitory ReceptorsCytokine
The invention discloses a new application of natural killer (NK) cellular immunotherapy. An animal model is utilized to research the influence of NK cell in combination with fluoxetine for treating monoamine neurotransmitter, peripheral blood cell factor level and depression action in brain of a rat having chronic unpredictable depression; clinically, improvement of depression symptoms of a patient and adjustment of immunity index by irradiating NK-92 cell in combination with paroxetine are evaluated, and remarkable improvement on the depression symptom by an NK cell immunotherapy is proved. Therefore, the NK cell immunotherapy in combination with the antidepressant can remarkably improve the depression symptoms.
Owner:南京和乃安健康科技有限公司
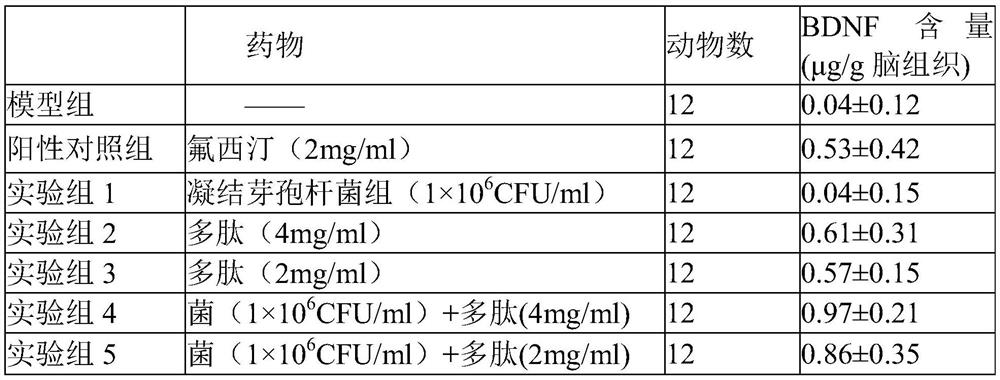
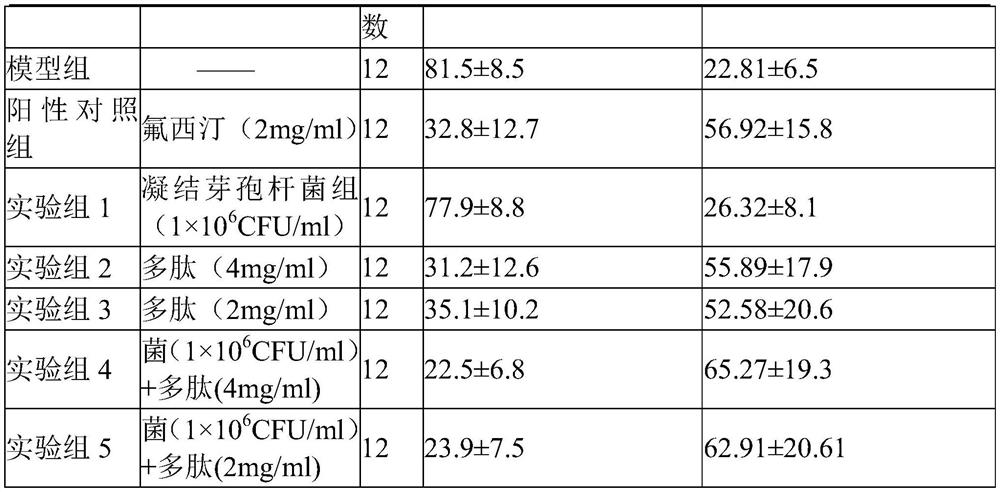
Bacillus coagulans preparation for treating depression
ActiveCN113181342AElevated BDNF contentImprove depressive symptomsNervous disorderPeptide/protein ingredientsNutritionPharmacology
The invention provides a pharmaceutical composition for treating depression. The composition comprises bacillus coagulans and a nutritional polypeptide, wherein the pharmaceutical composition provided by the invention can up-regulate the level of brain-derived neurotrophic factors and improve the depressive symptom of depression model animals. The pharmaceutical composition provided by the invention can be used as an effective candidate therapeutic drug for clinical treatment of depression, and has potential drug development value.
Owner:天津市宝恒生物科技有限公司
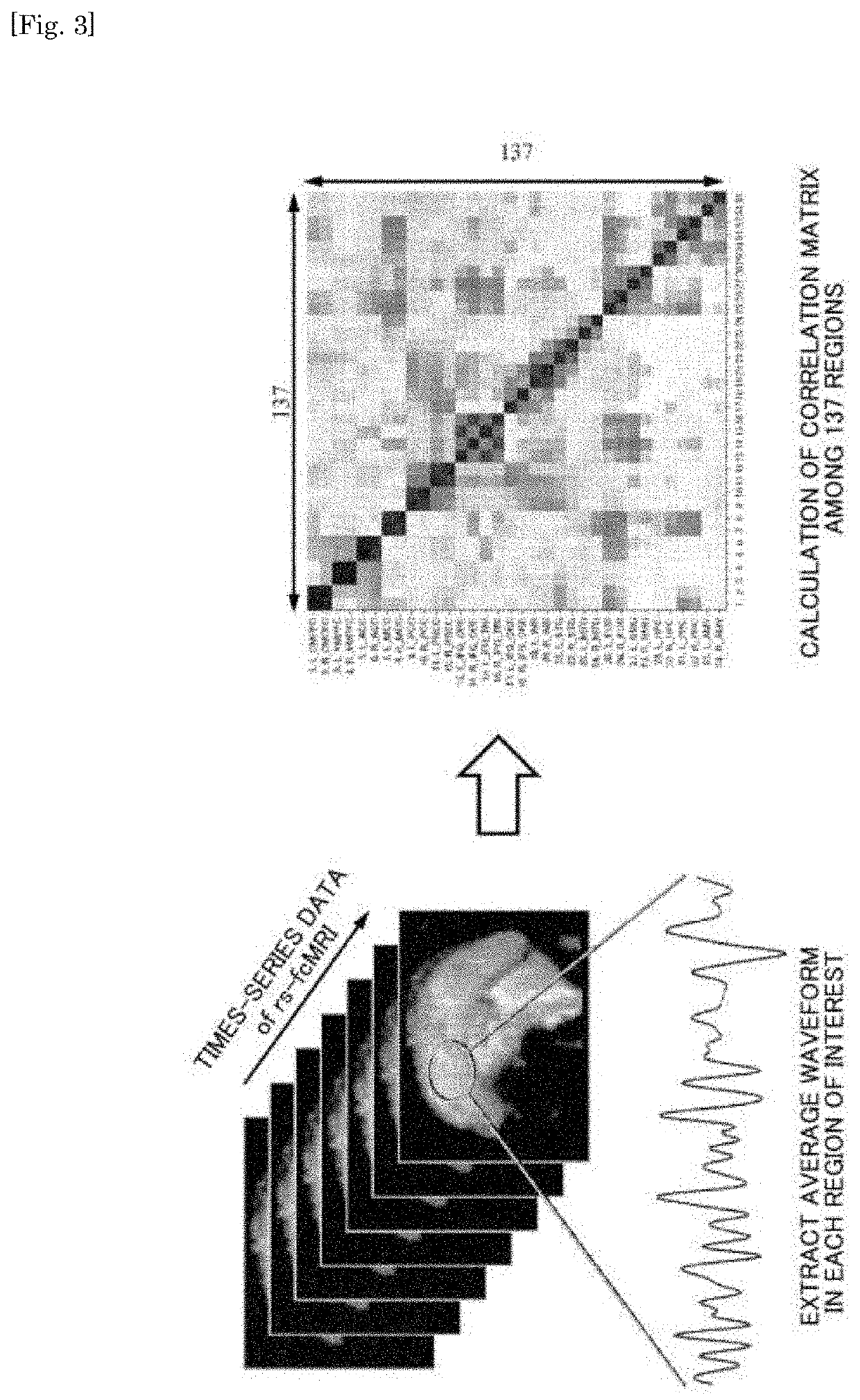
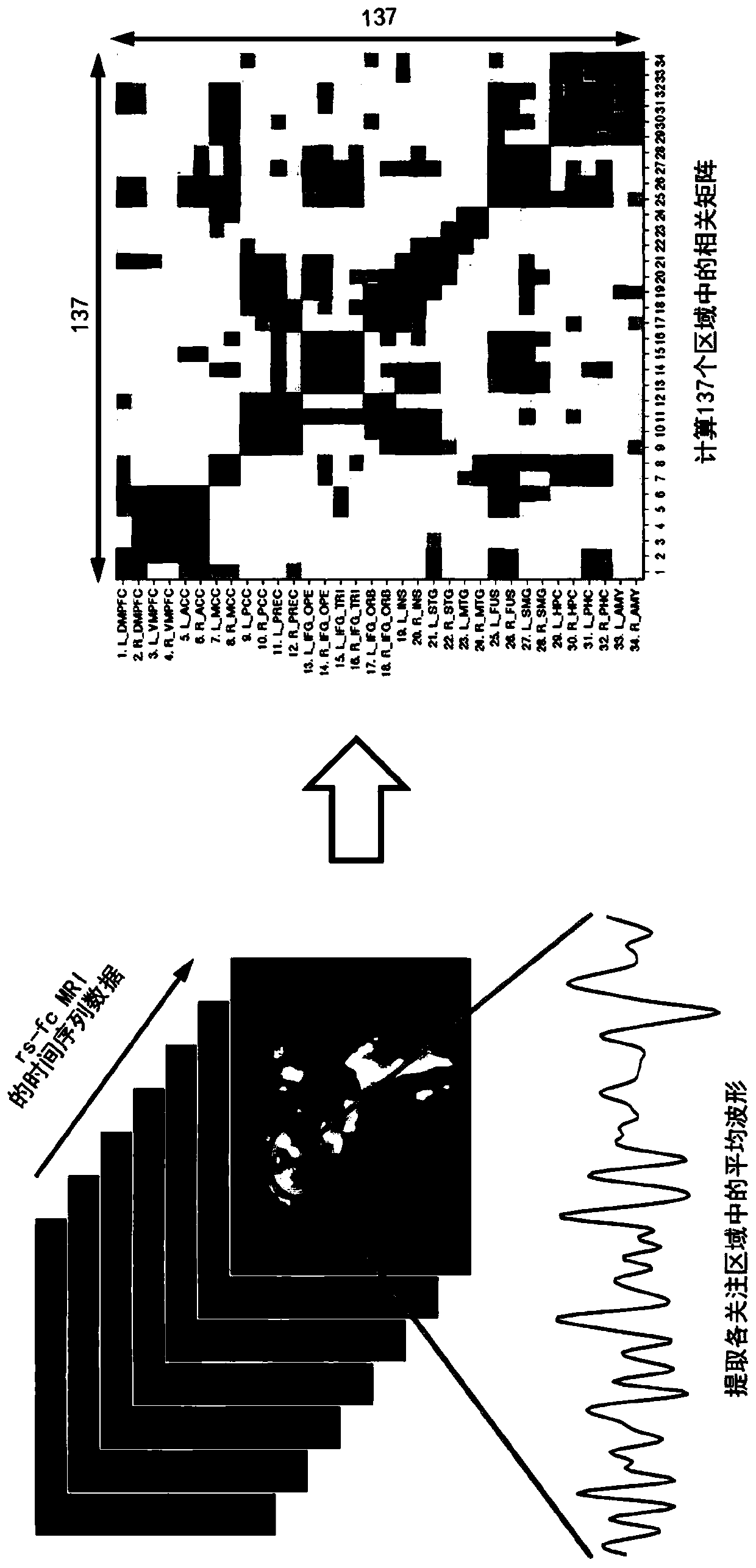
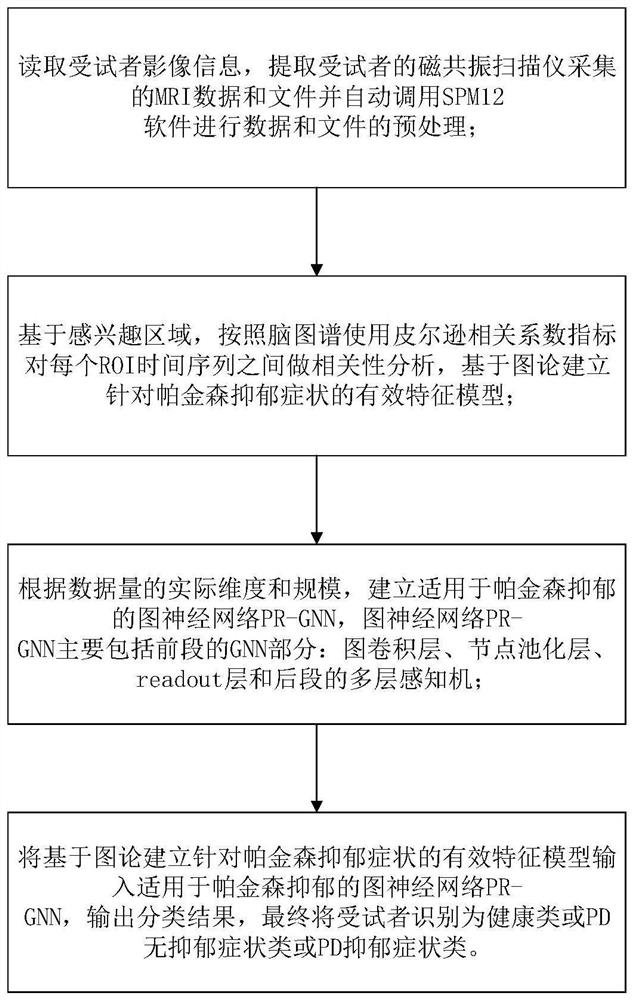
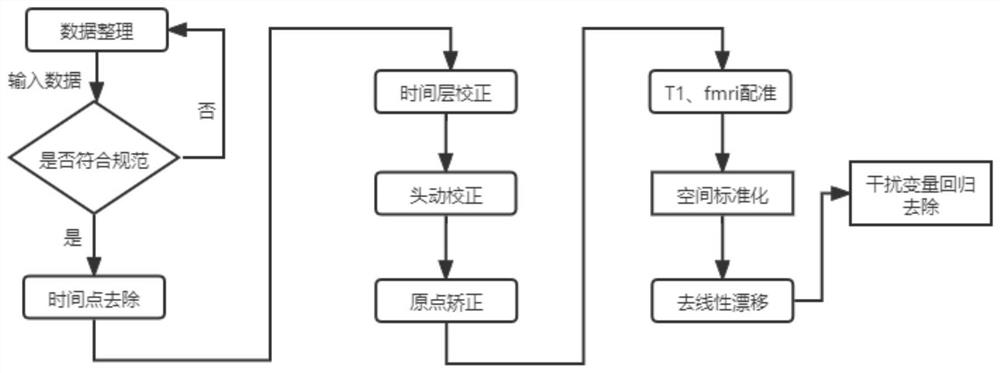
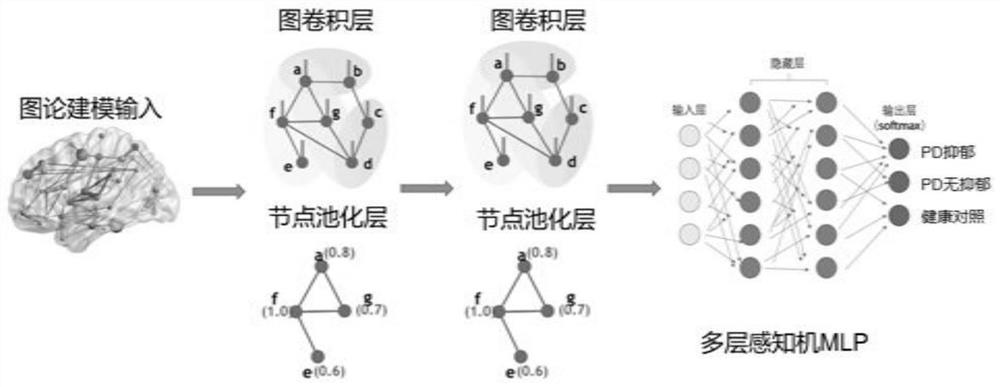
Parkinson depression auxiliary identification method based on fMRI graph neural network
The invention provides a Parkinson depression auxiliary recognition method based on an fMRI graph neural network. The Parkinson depression auxiliary recognition method comprises the steps that image information of a subject is read, MRI data and files collected by a magnetic resonance scanner of the subject are extracted, and SPM12 software is automatically called for data and file preprocessing; based on the region of interest, performing correlation analysis on each ROI time sequence by using a Pearson's correlation coefficient index according to a brain map, and establishing an effective feature model for the Parkinson depression symptom based on a graph theory; and establishing a graph neural network PR-GNN suitable for Parkinson's depression, inputting the effective feature model into the graph neural network PR-GNN suitable for Parkinson's depression, outputting a classification result, and finally identifying the subject as a healthy type or a PD non-depressive symptom type or a PD depressive symptom type. The three-classification prediction of unknown subjects is realized by using the PR-GNN, and the method is efficient and practical.
Owner:南京伯睿生命科学研究院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com