Patents
Literature
234 results about "Microbiological material" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
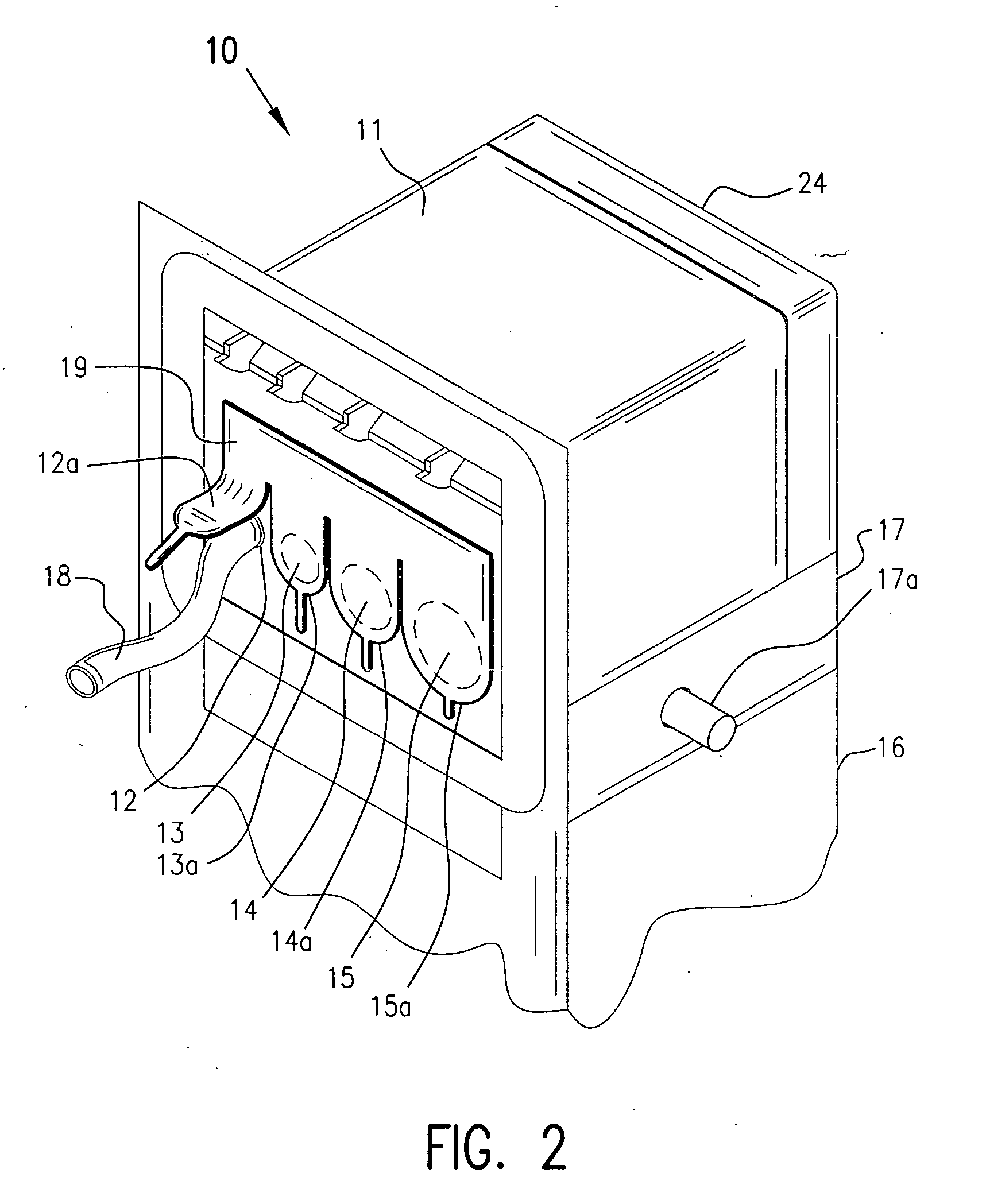
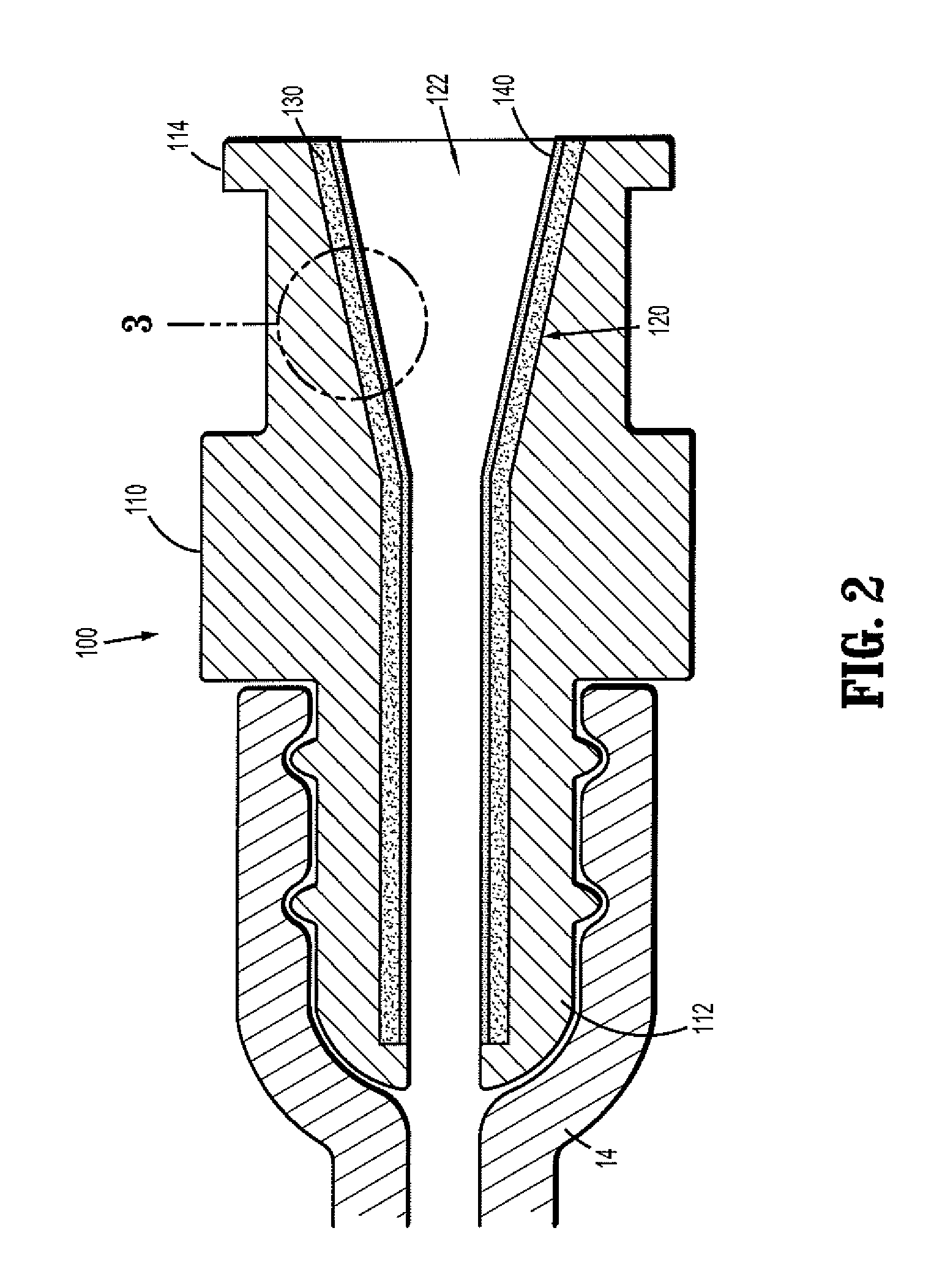
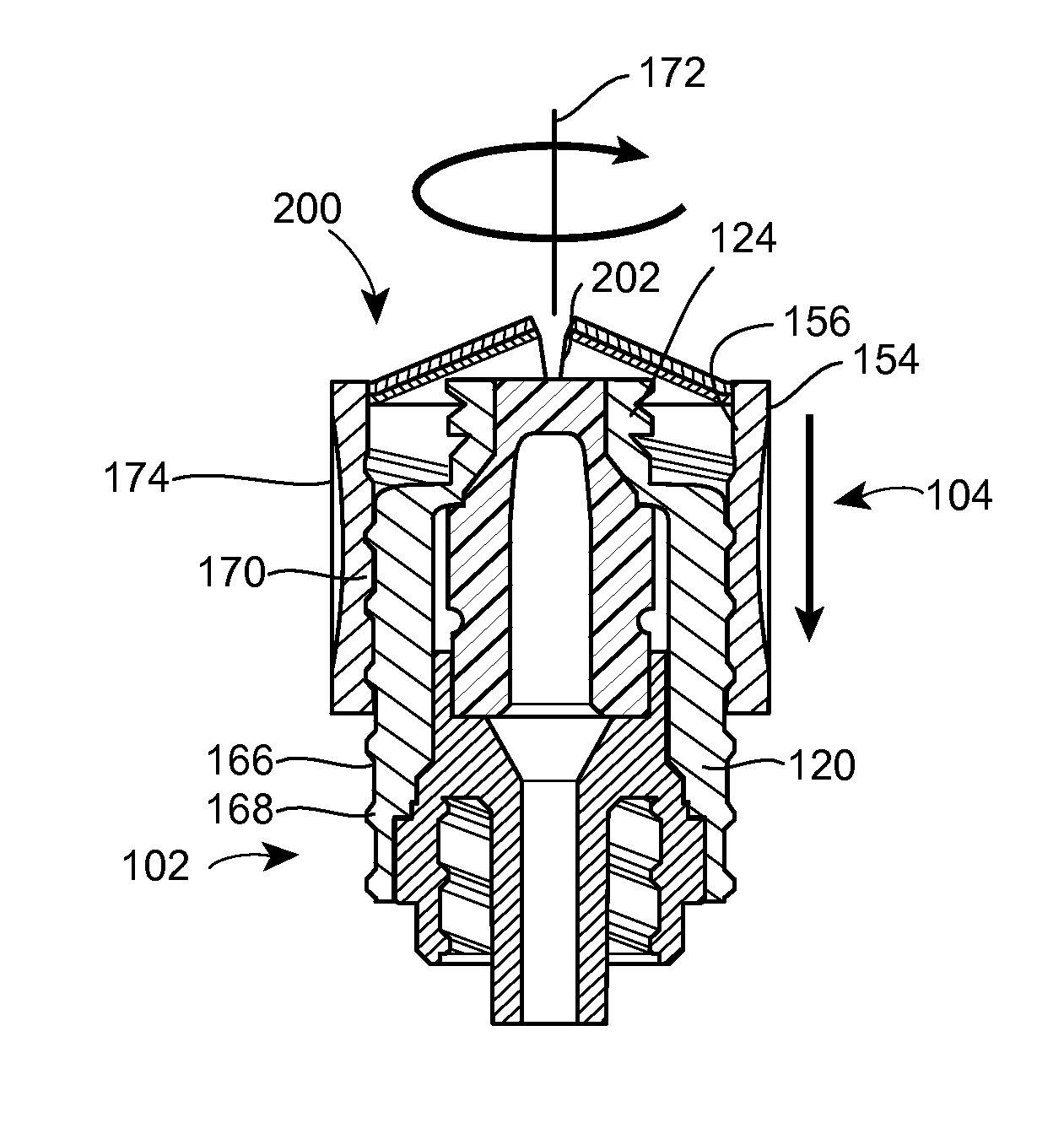
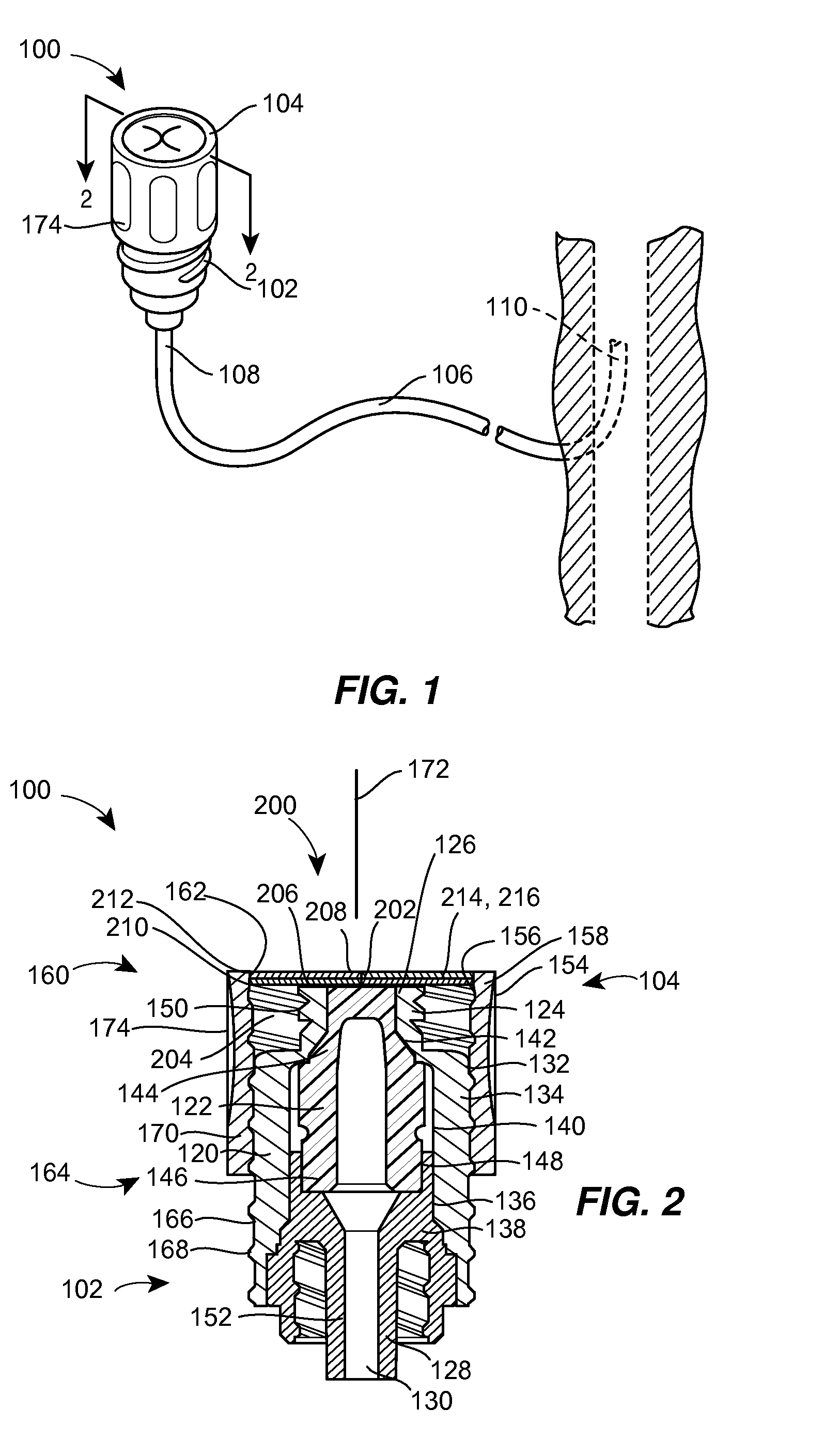
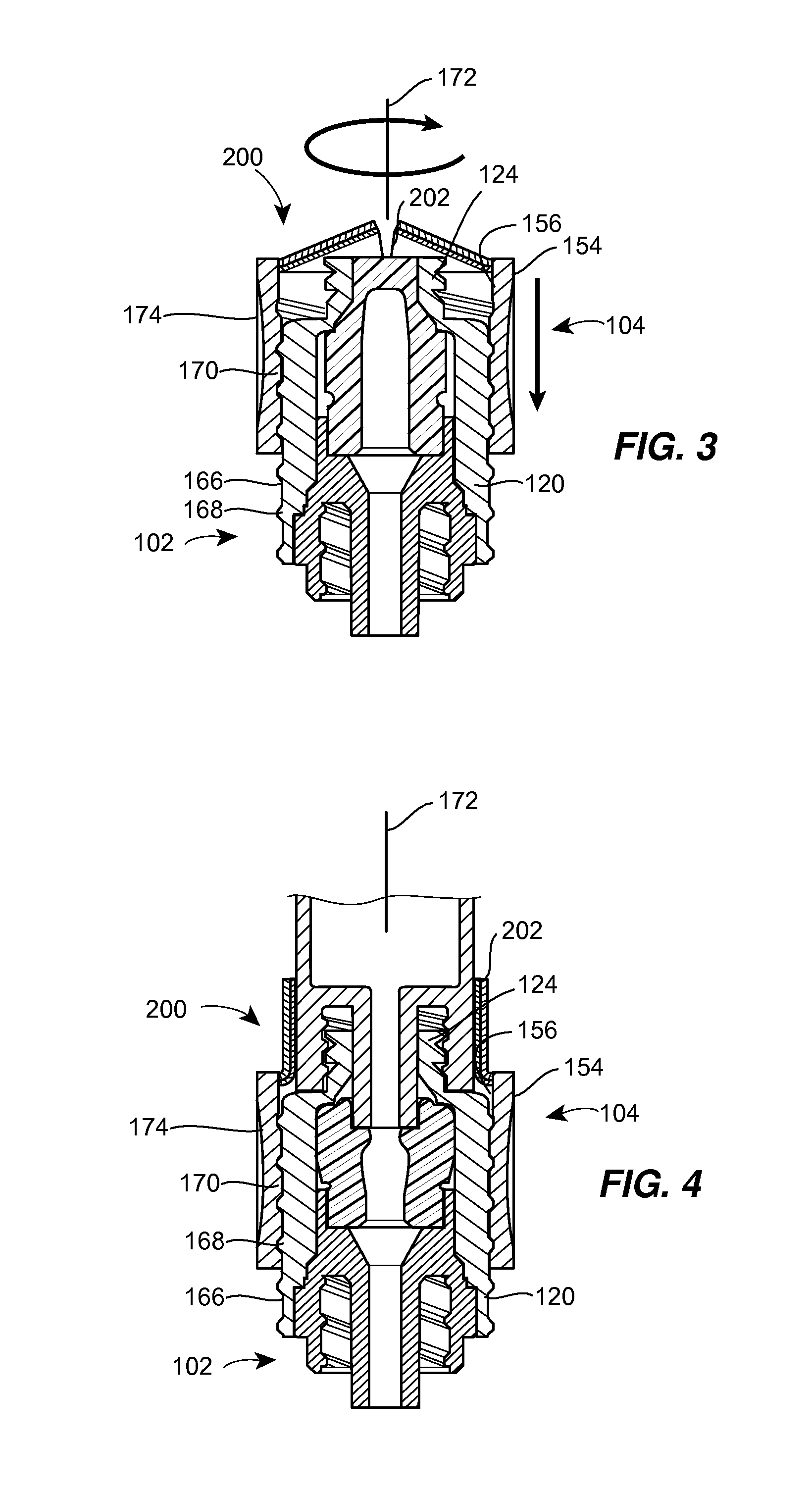
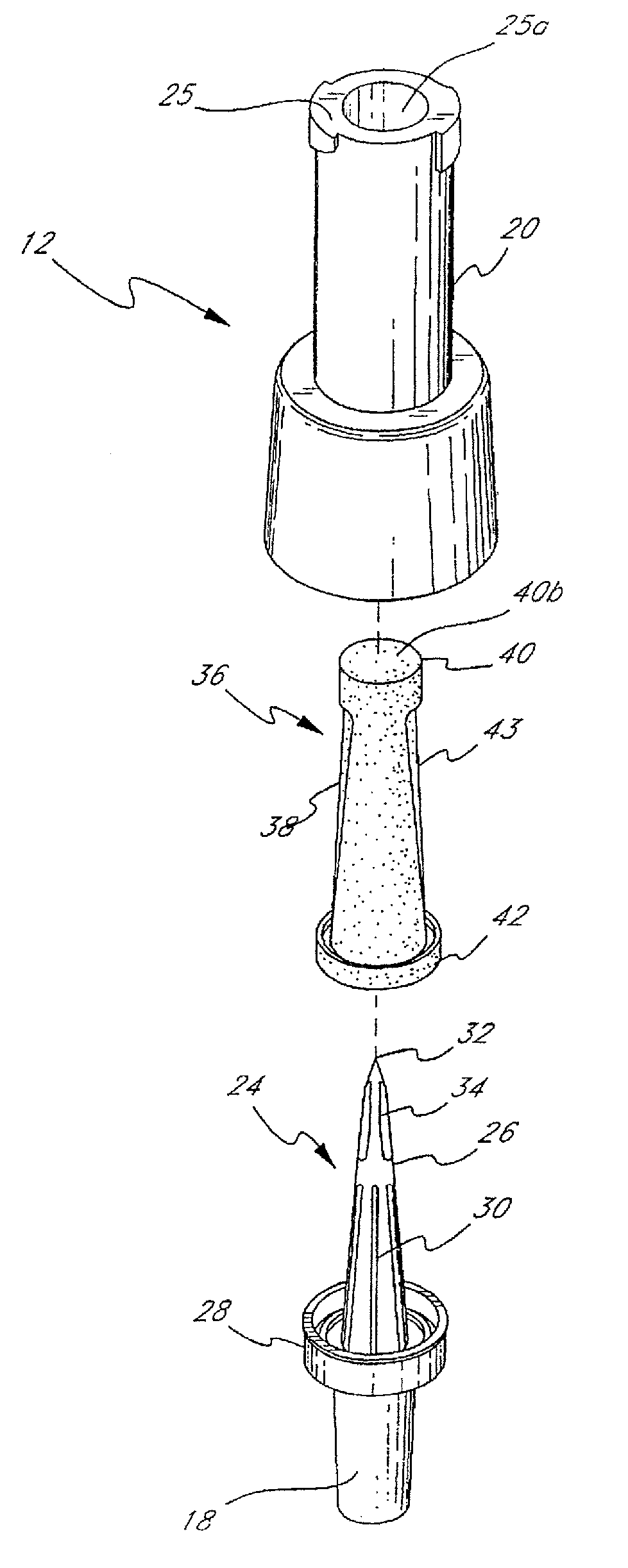
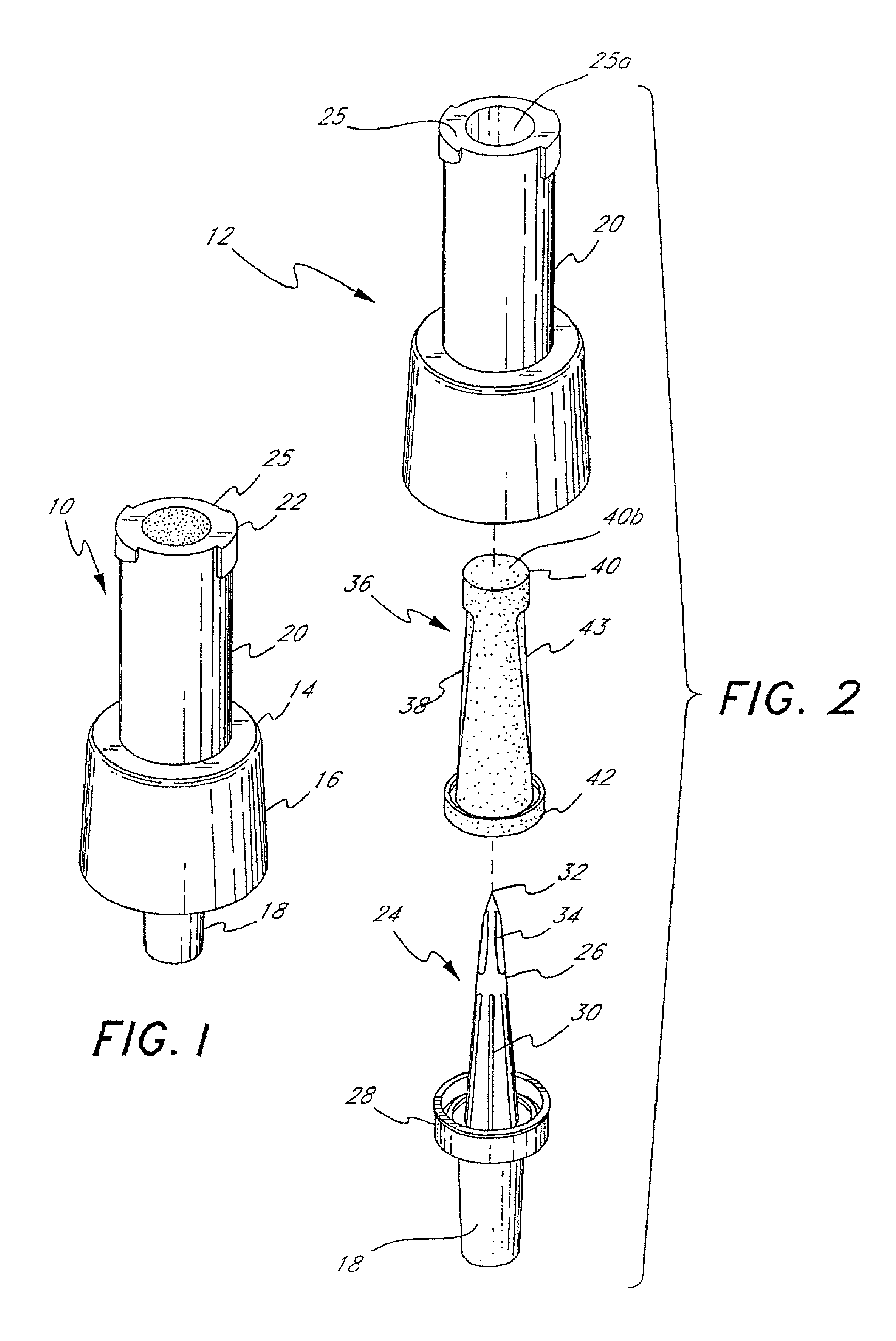
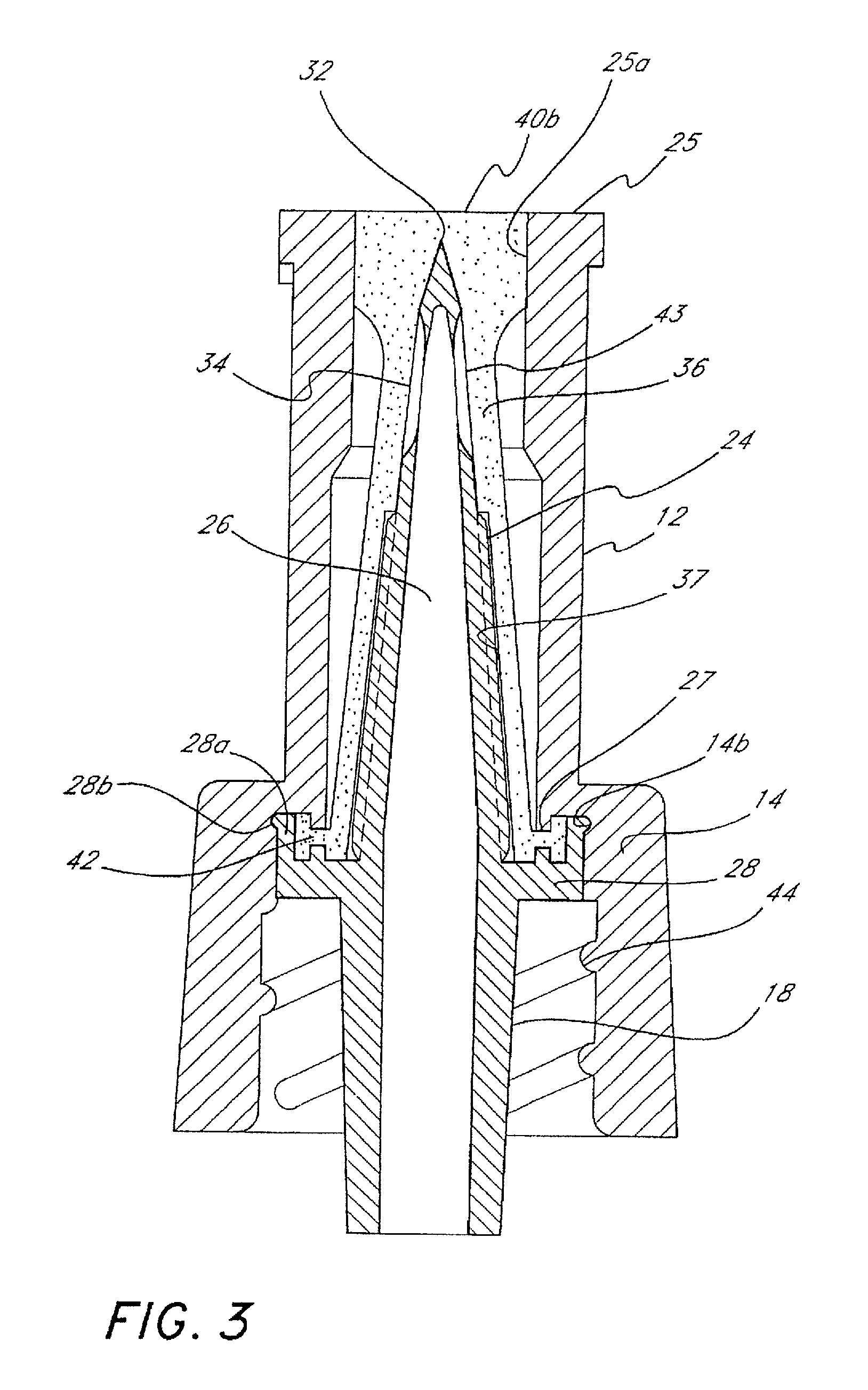
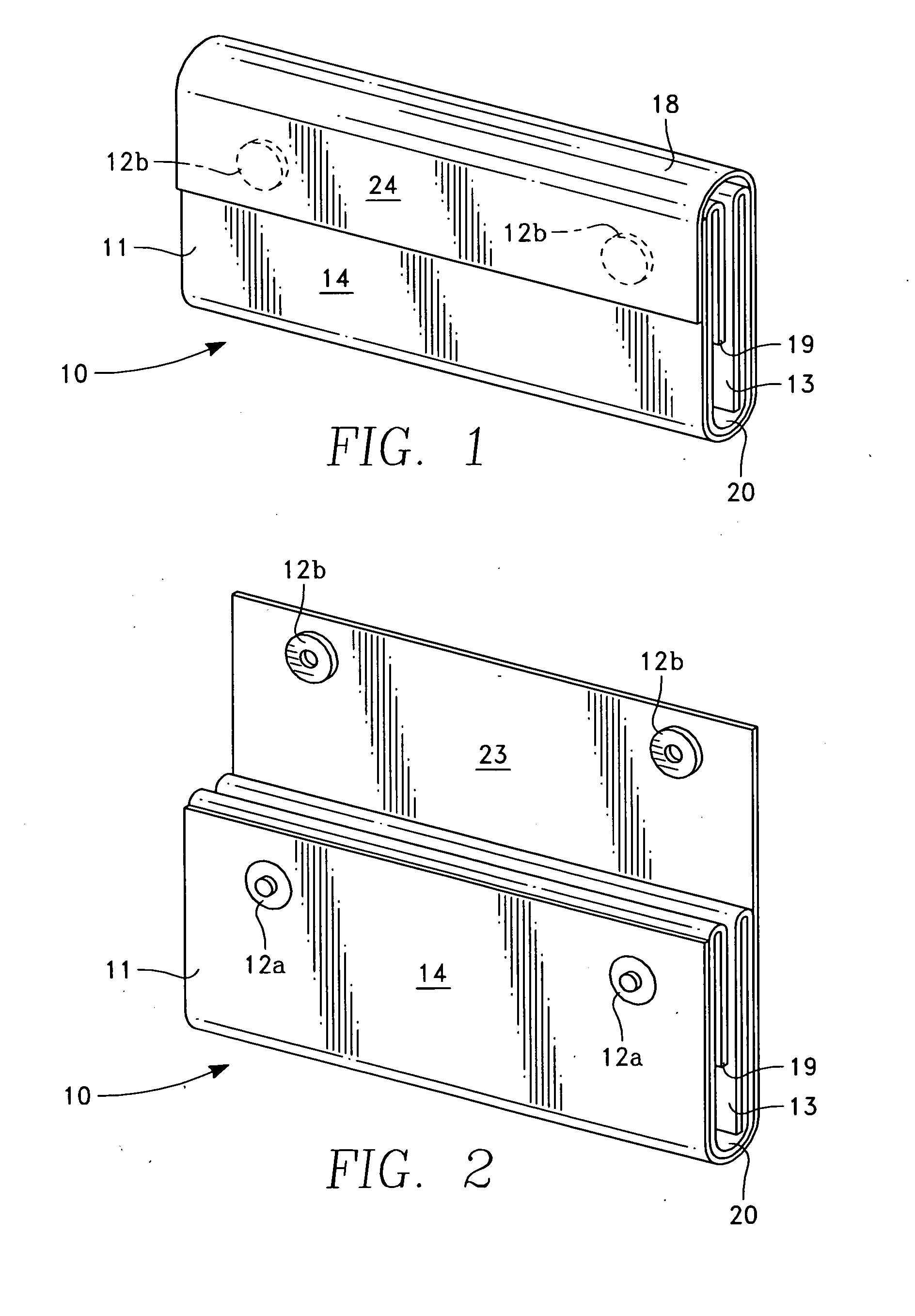
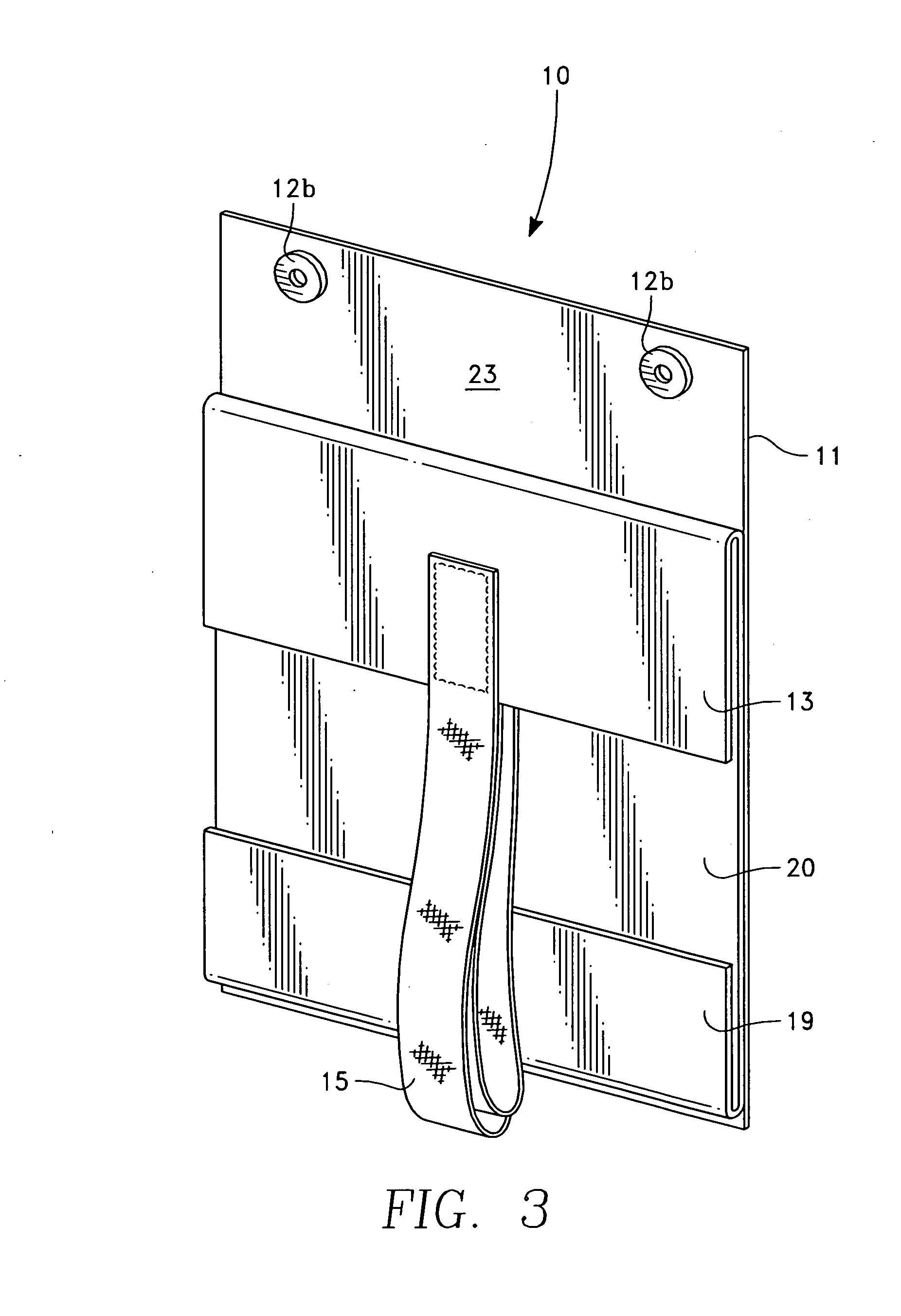
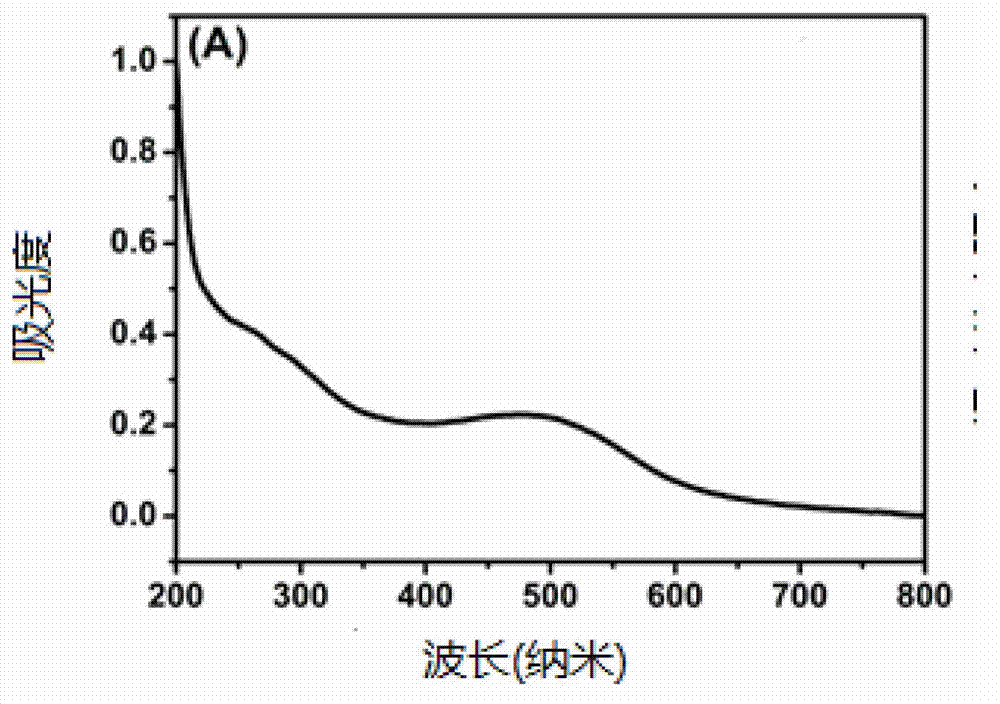
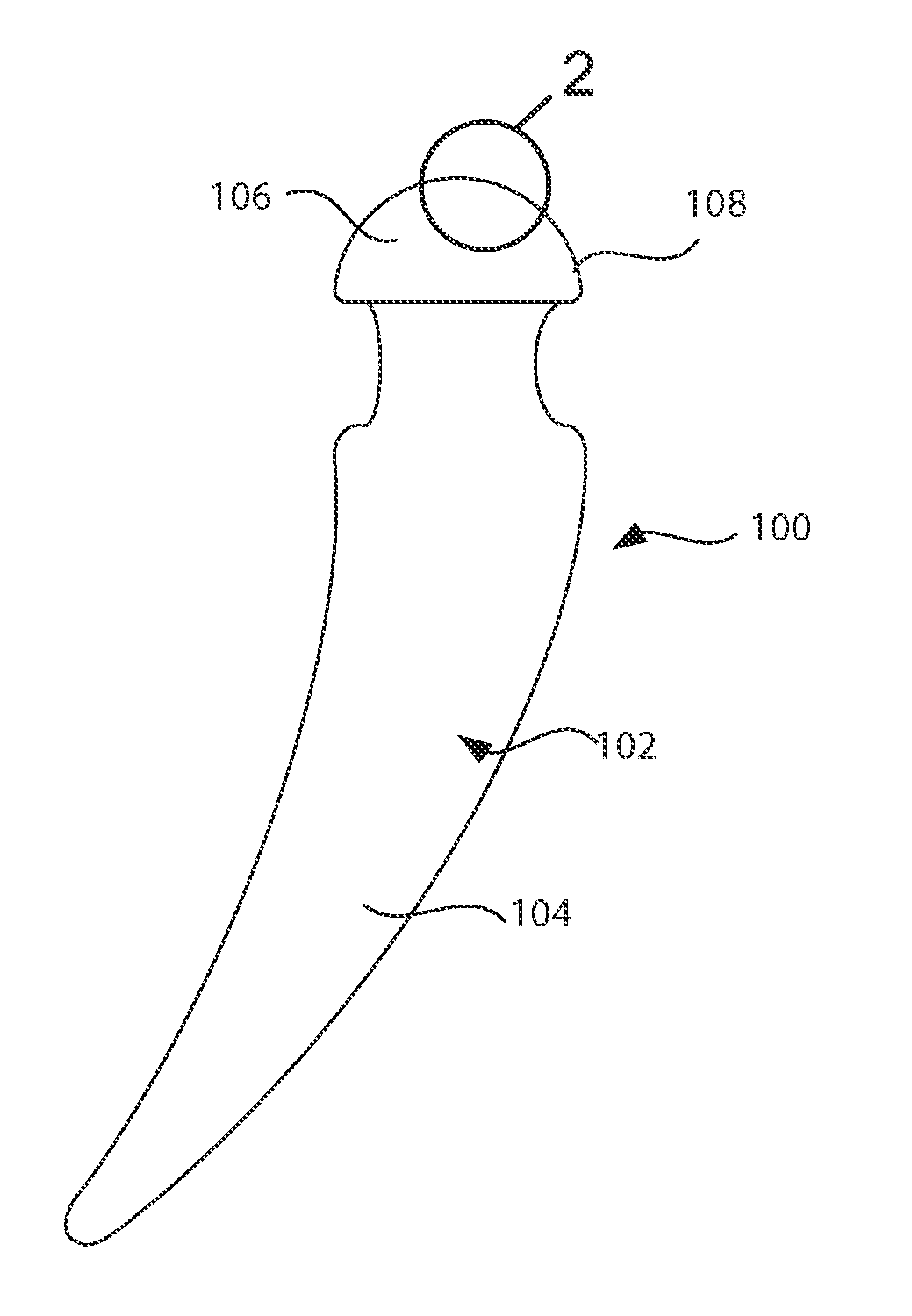
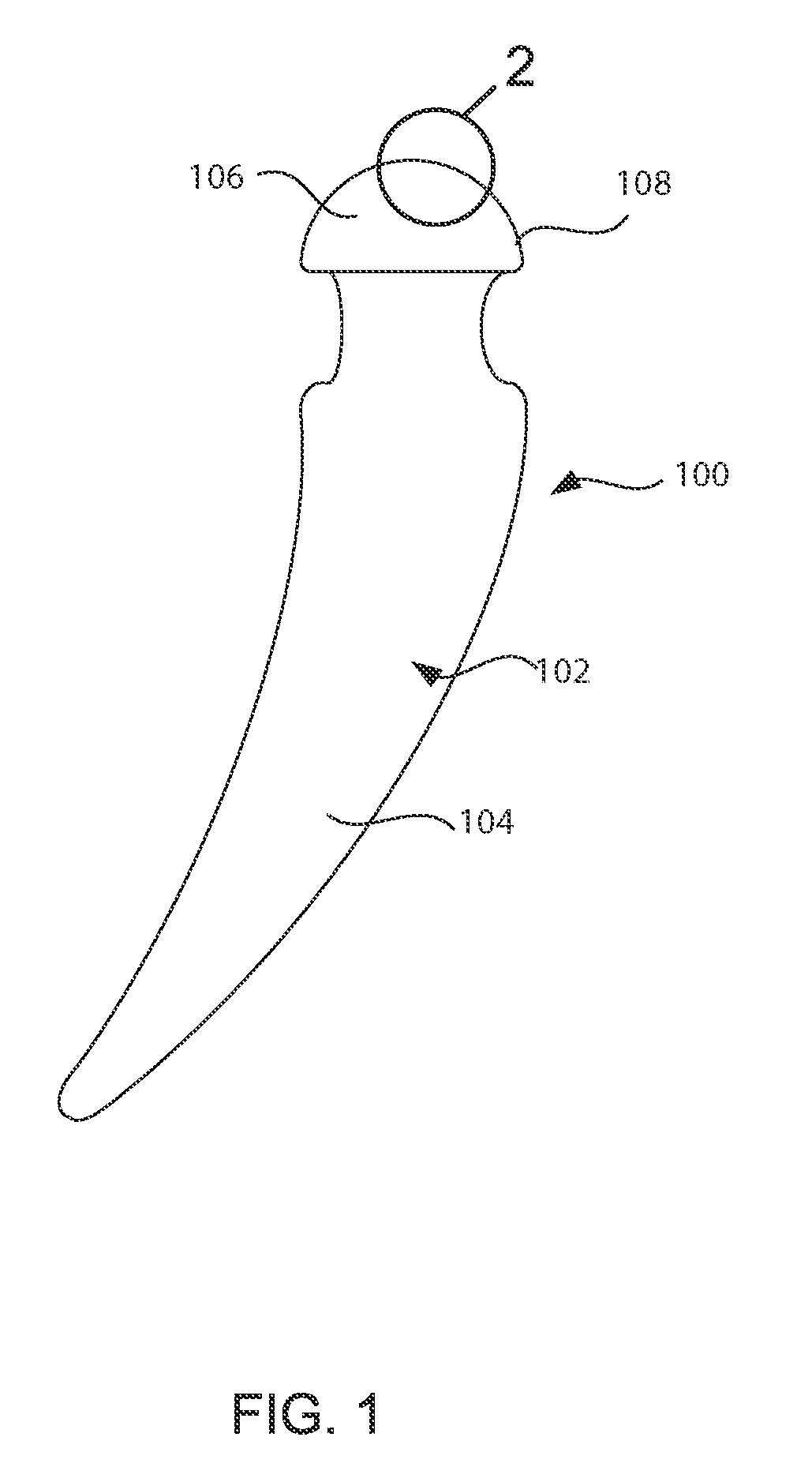
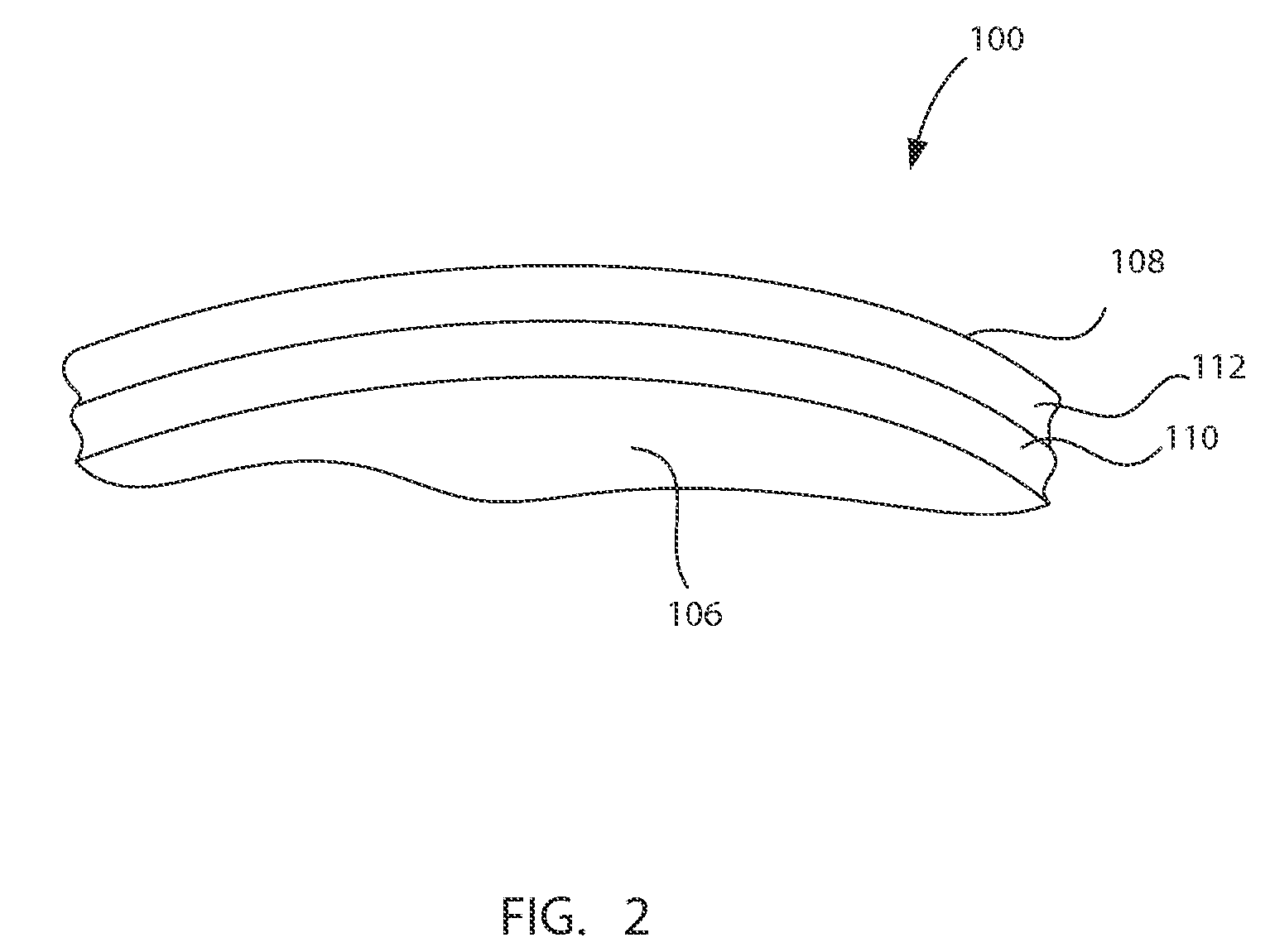
Barrier assembly for use with needleless connector
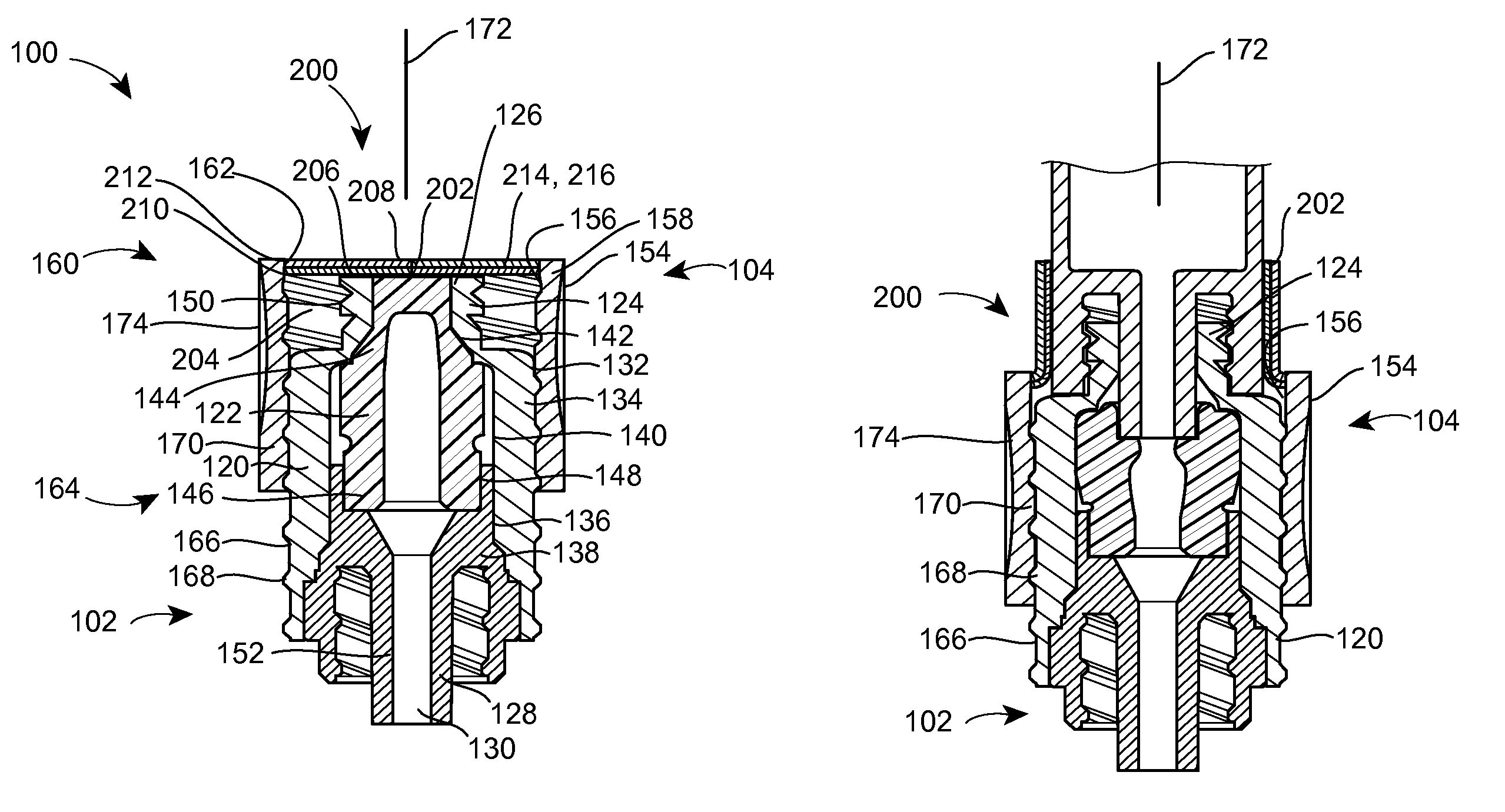
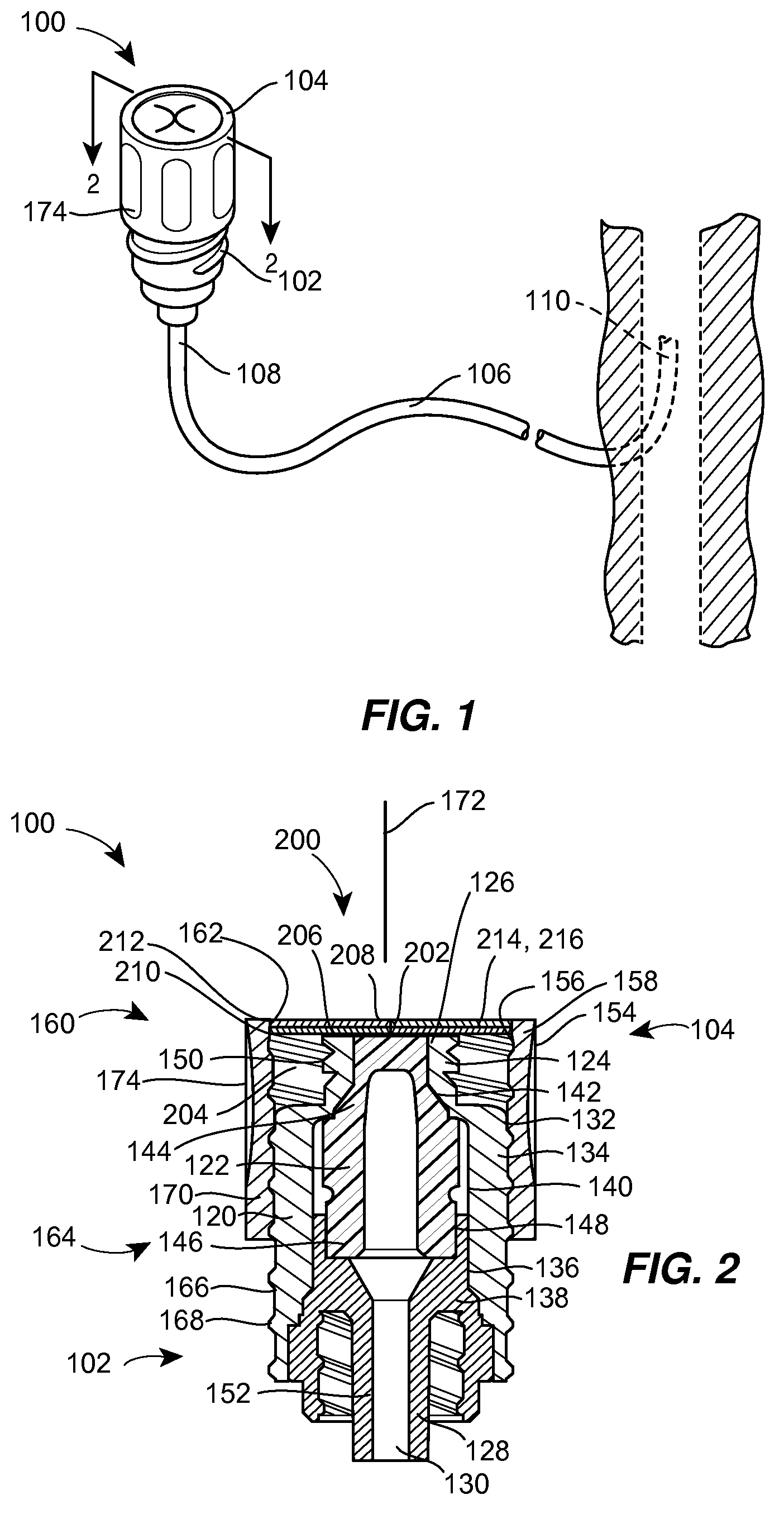
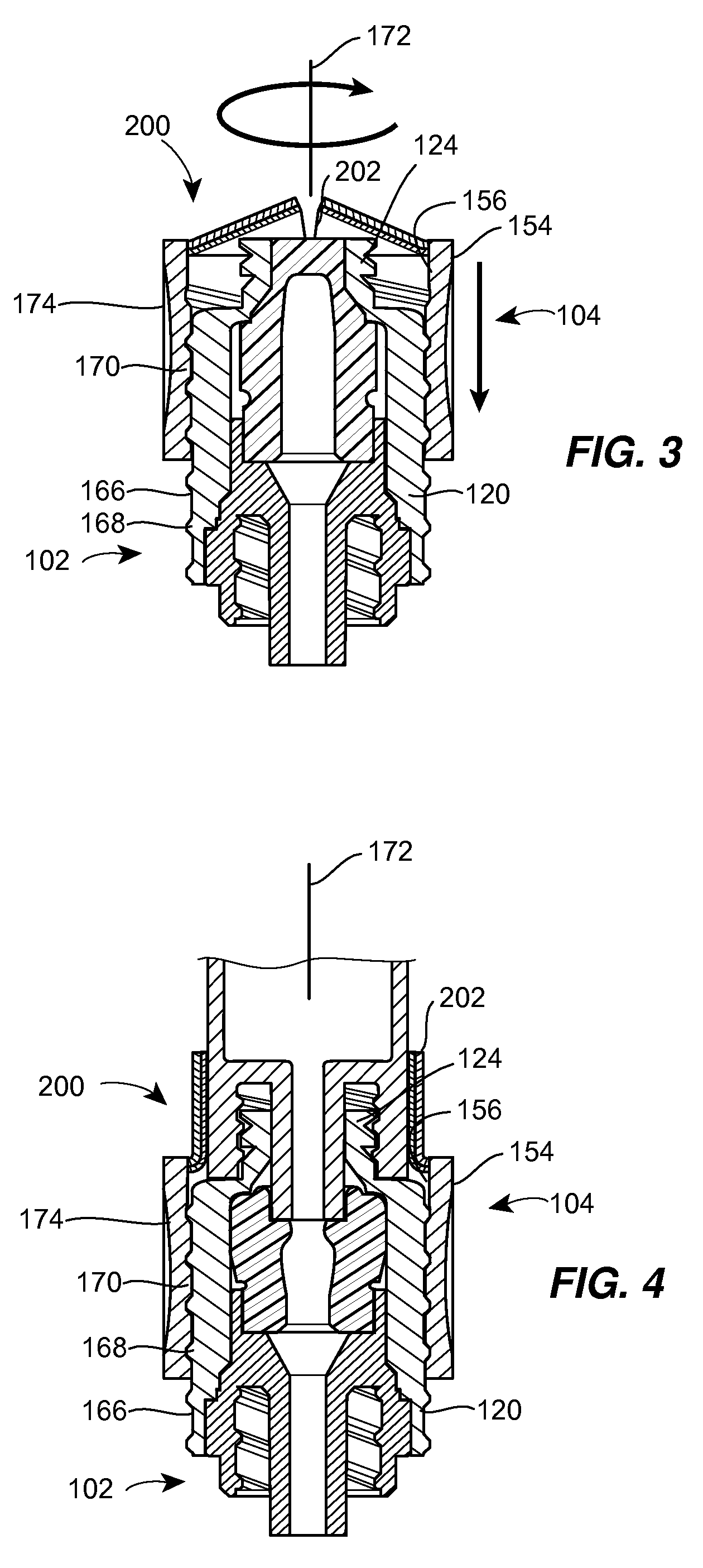
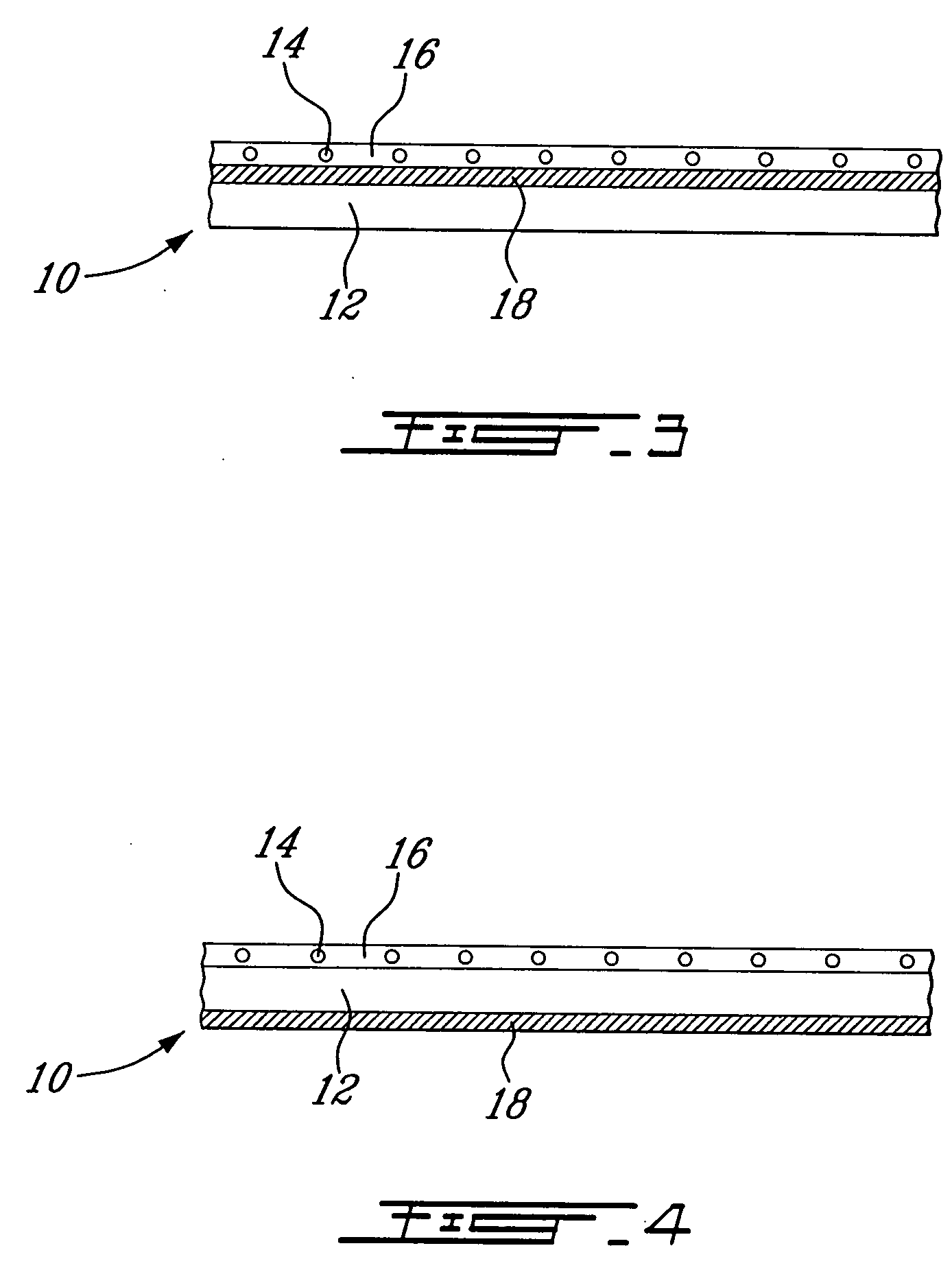
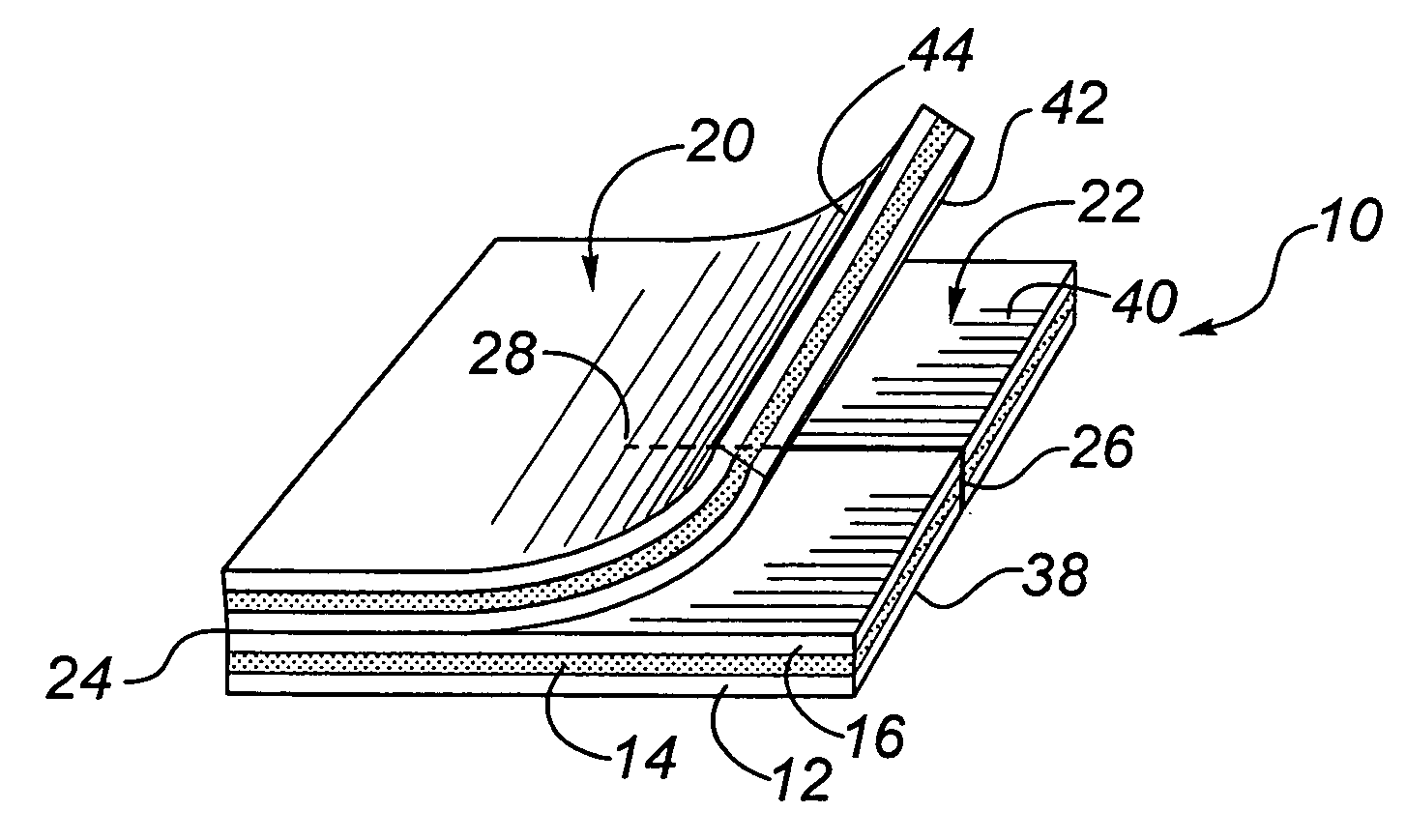
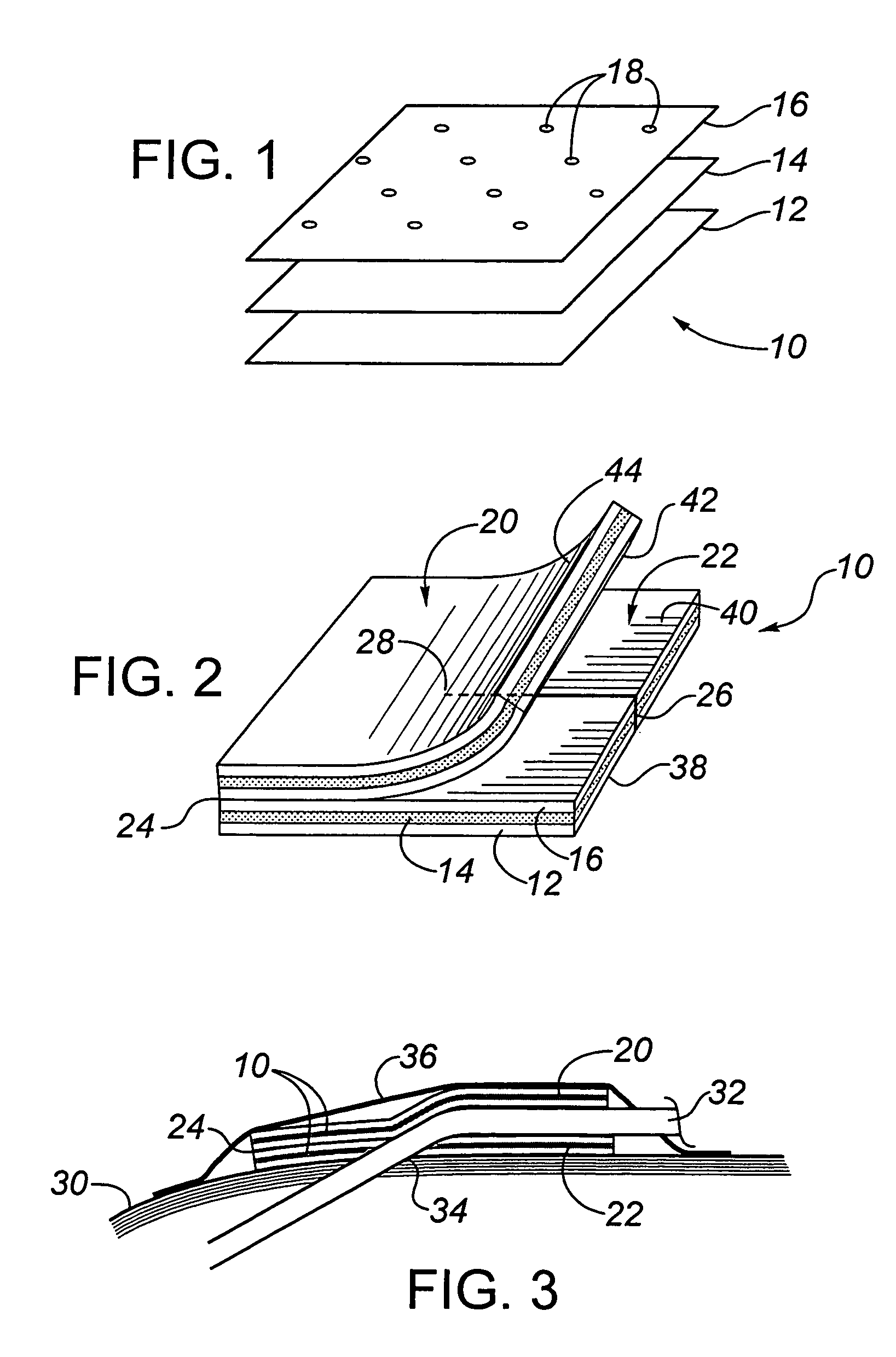
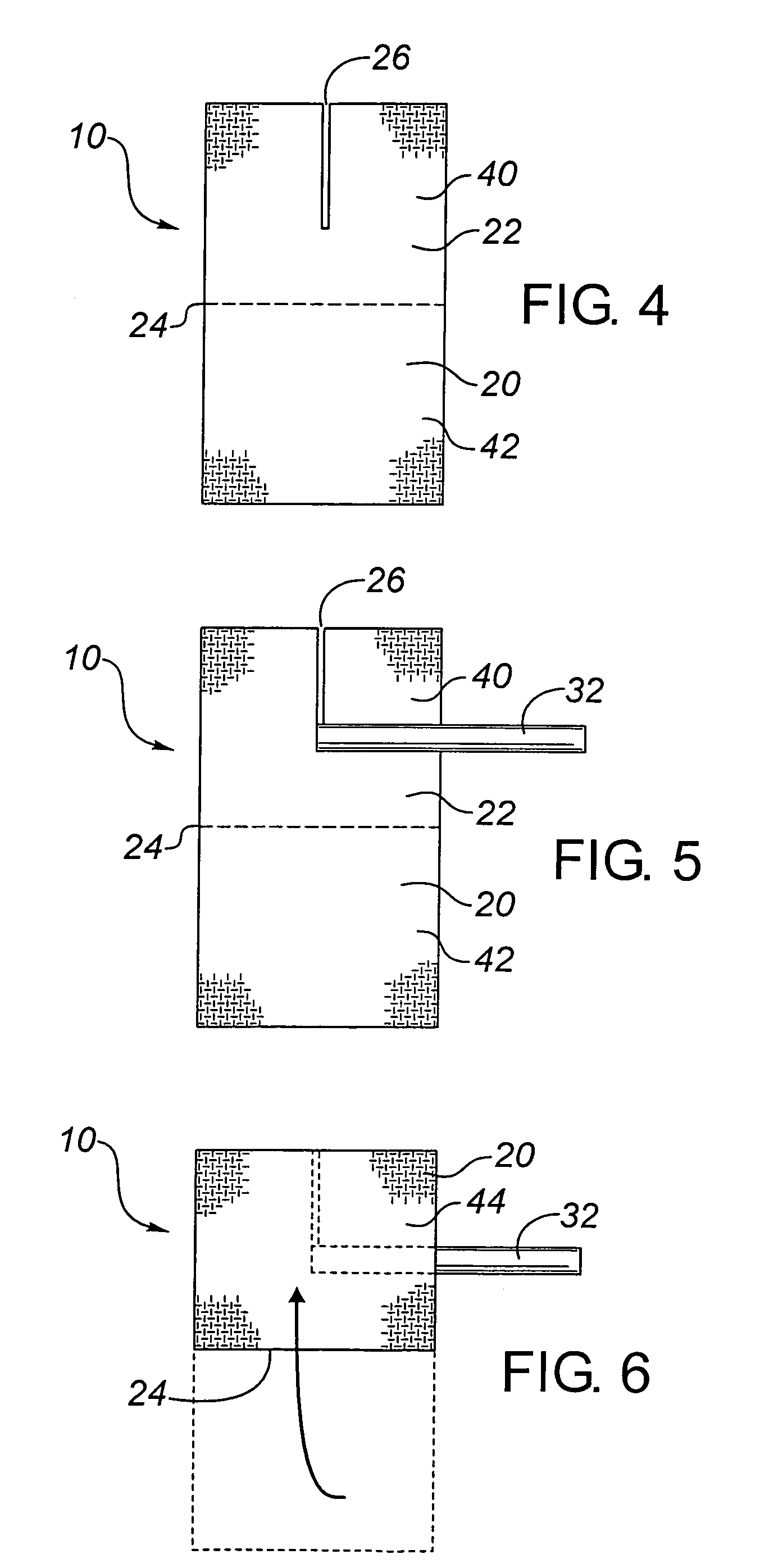
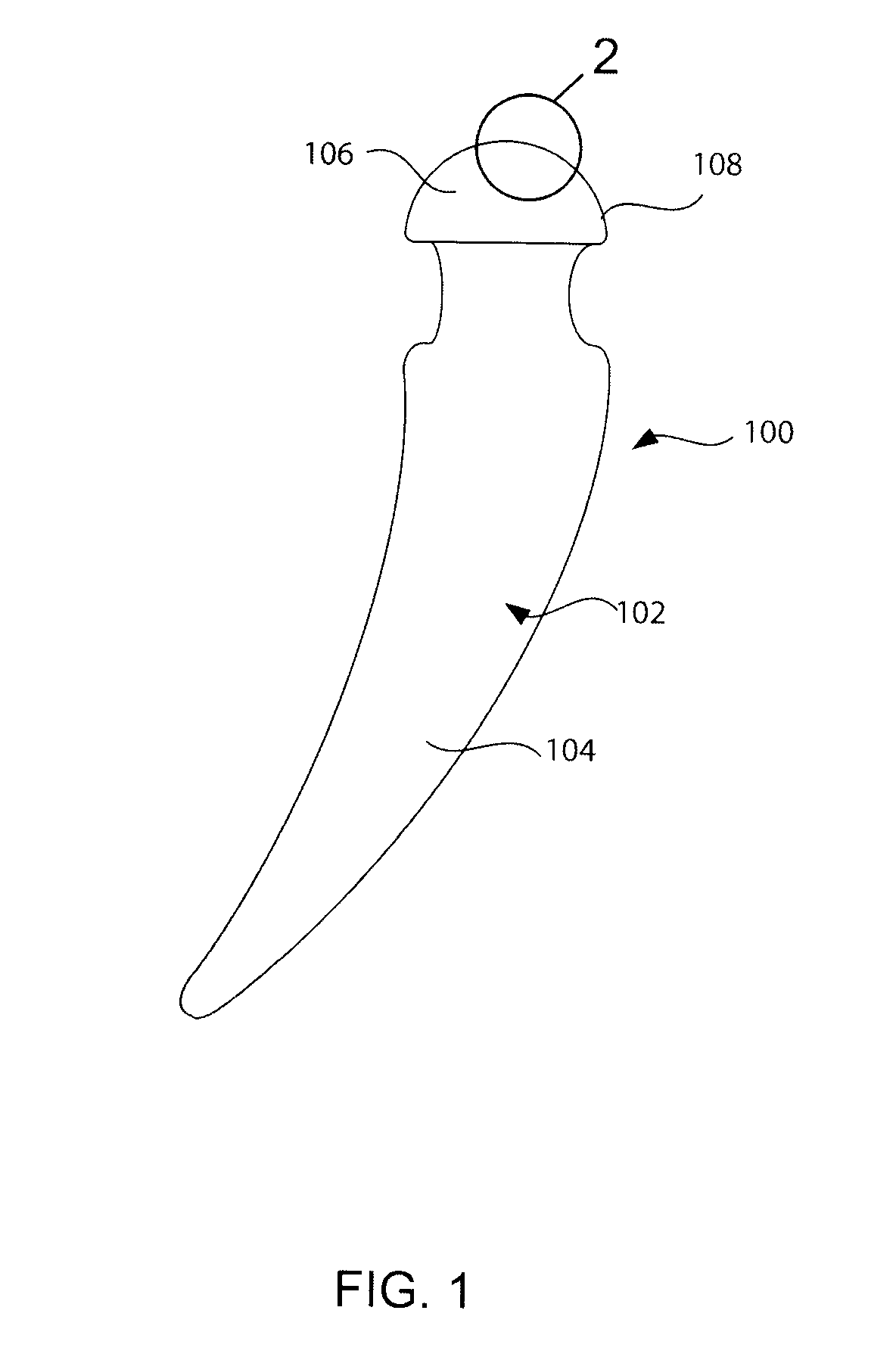
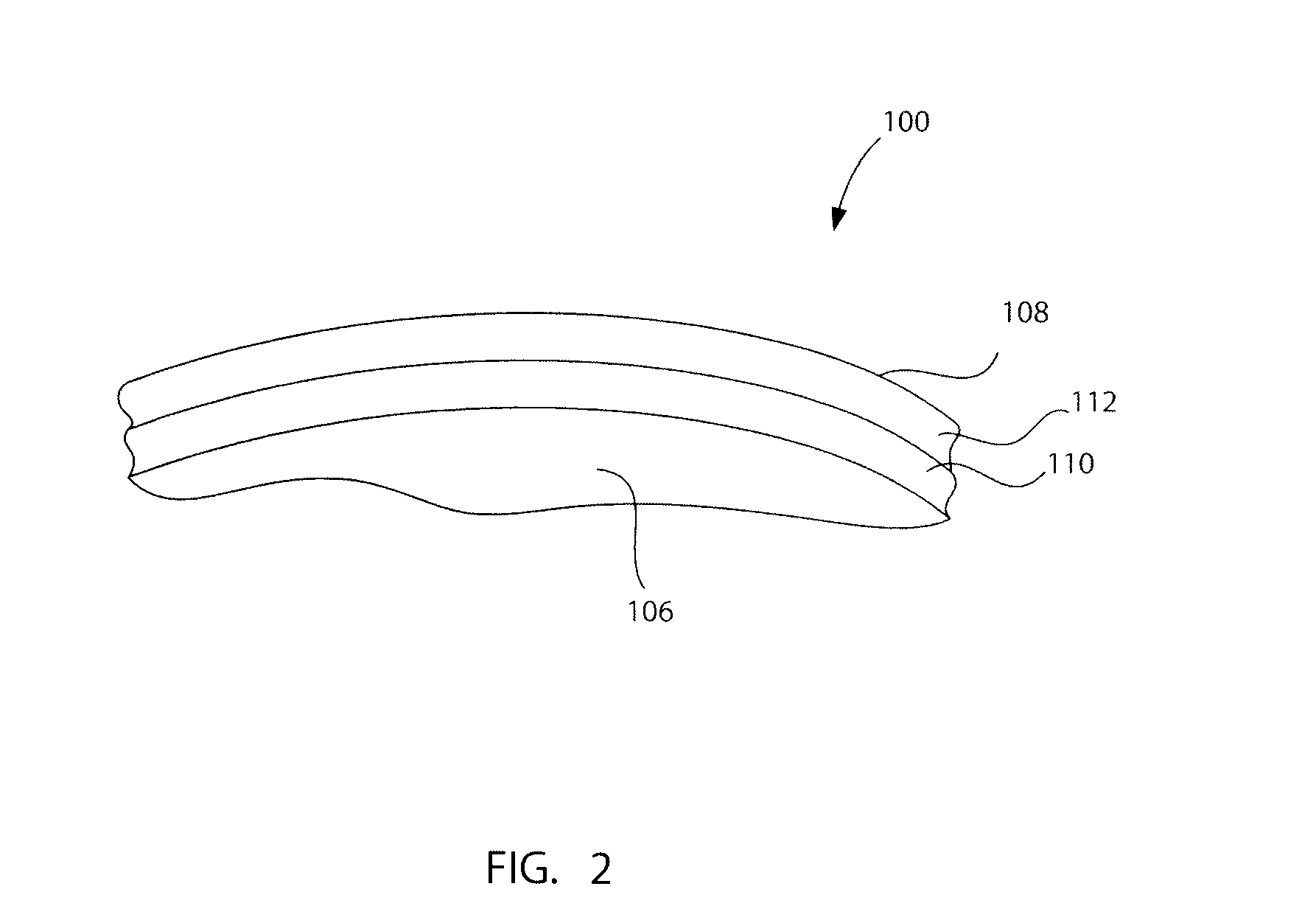
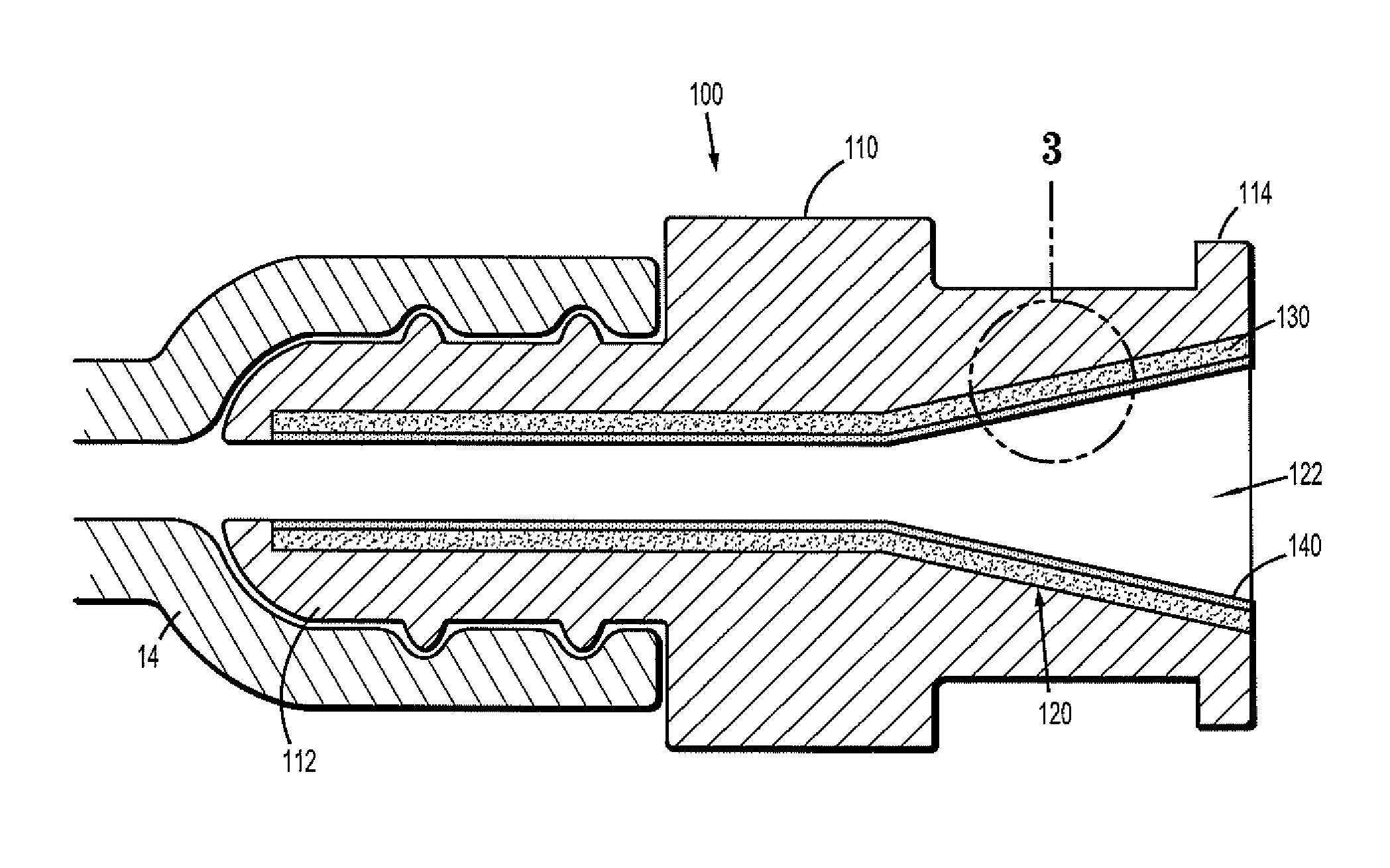
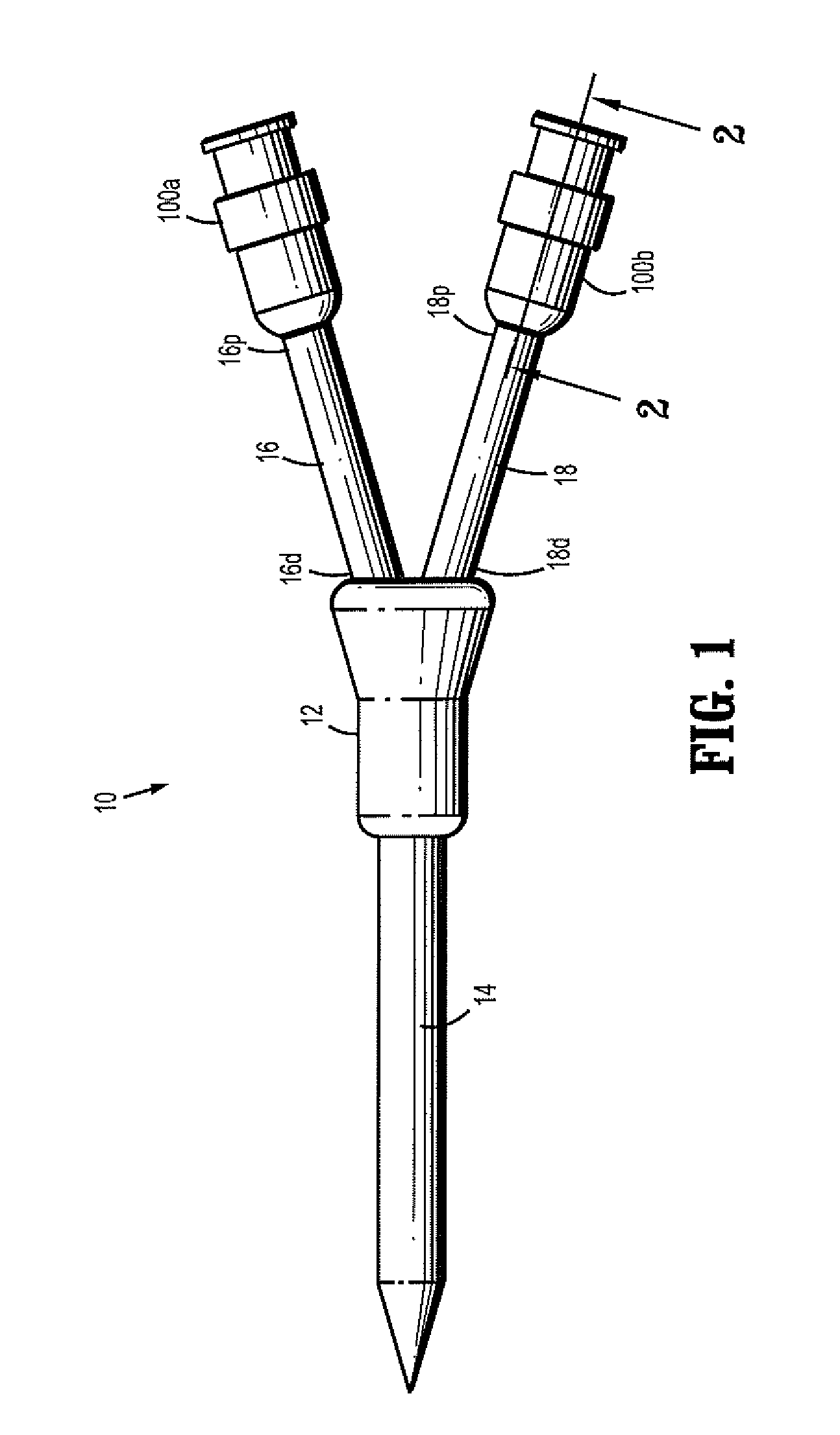
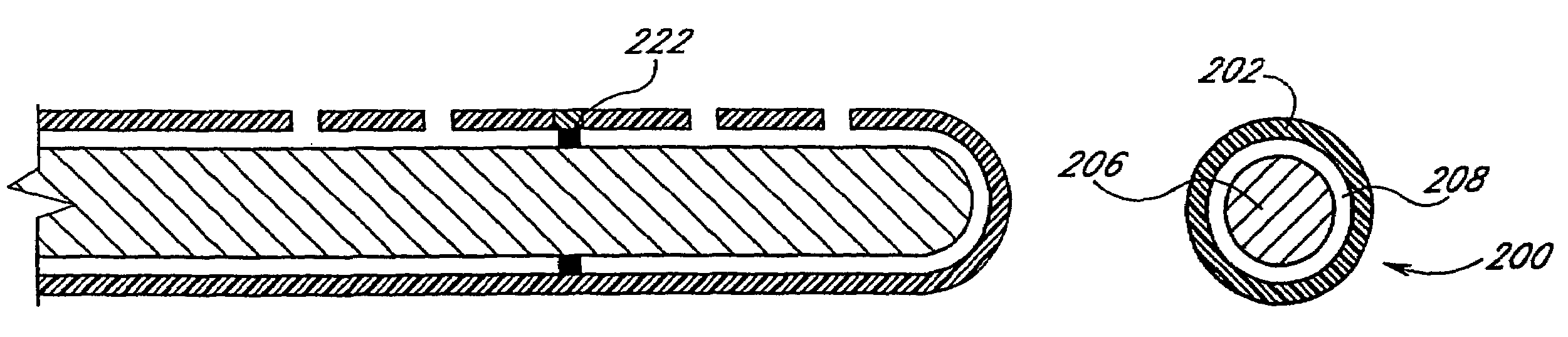
A needleless valve assembly includes a needleless connector having a housing with an inlet end with an inlet opening and an outlet end with an outlet opening, and a valve disposed in the housing to control passage between the openings. The assembly also includes a collar having an exterior surface and an interior surface, and a first end with a collar opening communicating between the interior surface and the exterior surface and a second end moveably attached to the housing. Further, the assembly includes a barrier disposed across the collar opening with a slit therethrough and including an anti-microbial material. The collar is moveable relative to the housing so that the inlet end of the housing depends past the exterior surface of the collar through the slit in the barrier. A kit may include the assembly and a catheter having a first end attached to the outlet opening.
Owner:BAXTER INT INC +1
Antimicrobial material
InactiveUS20060141015A1Lower surface energyImproved surface distributionBiocideSynthetic resin layered productsPolymer scienceAdhesive materials
The present invention relates to an antimicrobial material comprising sheet of fabric and- metallic salt crystals embedded in an adhesive material covering the sheet of fabric.
Owner:GROUPE CTT
Transcutaneous medical device dressings and method of use
InactiveUS7137968B1Coloring be generateAdvantage of easeCatheterInfusion needlesSkin contactAdhesive
A transcutaneous device dressing and method for its use with a transcutaneous medical device, such as an intravascular catheter, which punctures the skin of a patient and which has a portion of the medical device protruding from the skin which can lead to infection. The dressing includes a top and a bottom dressing, both being formed from a flexible material and having upper and lower surfaces, with the lower surface being the skin facing surface in use. The bottom dressing has a slit formed therein extending from one edge inwardly to a termination point within the confines of the bottom dressing. An anti-microbial material is provided without the use of adhesives at the upper and lower surfaces of the bottom dressing, and at least at the lower surface of the top dressing. In use, the bottom dressing is placed next to the skin, the slit allowing the bottom dressing to surround the puncture site such that the lower surface of the bottom dressing is in contact with the skin while the upper surface of the bottom dressing is in contact with a portion of the medical device protruding from the skin. The top dressing is placed above the puncture site such that its lower surface is in contact with a portion of the medical device protruding from the skin. In this way, there is exposure of the portion of the medical device protruding from the skin to the anti-microbial activity of the anti-microbial material.
Owner:NUCRYST PHARMA
Antimicrobial composition for medical articles
InactiveUS20050048124A1Inhibition formationImprove the lubrication effectPowder deliveryBiocideSolventCalcium Chelator
An antimicrobial composition is formed from about 5 to about 25 wt % of an antimicrobial formulation and about 75 to about 95 wt % of a silicone resin. The antimicrobial formulation is formed from about 60 to about 95 wt % of an antimicrobial material, about 1 to about 30 wt % calcium chelator, about 0.001 to about 0.25 wt % pigment, and about 0.5 to about 3.5 wt % lubricant. The silicone resin may be a dispersion of about 40 to about 60 w / v % of an RTV silicone resin in a solvent, a liquid silicone resin, or a solid silicone resin. An antimicrobial coating may be formed on the surface of an article by applying an antimicrobial composition to the article and permitting the solvent to evaporate. It may also be formed by making a mixture of about 5 to about 12 wt % of an antimicrobial formulation and about 88 to about 95 wt % of a liquid or solid silicone resin and molding, overmolding, or extruding the article from the compounded mixture.
Owner:ICET
Operating room smoke evacuator with integrated vacuum motor and filter
The present invention broadly comprises a multiport filter having at least three intake ports and at least two layers—a first layer for containing particles 3 microns or greater and a second layer that contains particles 3 microns or less in size. In alternate embodiments, the filter includes a third layer for trapping odors and gases and a fourth layer for trapping fines. The second layer may be comprised of ULPA material and may have antimicrobial material. The invention also includes a contaminant removal system for the filtering and recirculation of air contaminated with smoke from cautery-type surgical procedures. The invention also broadly comprises a surgical assemblies control service head designed to house powered surgical devices and a vacuum-filtering system for the treatment of air contamination caused by surgical procedures.
Owner:BUFFALO FILTER
Antimicrobial coating methods
ActiveUS20060198903A1Eliminate needSustained releaseHeavy metal active ingredientsBiocideMetallurgyIn vivo
The invention is directed to efficient methods for depositing highly adherent anti-microbial materials onto a wide range of surfaces. A controlled cathodic arc process is described, which results in enhanced adhesion of silver oxide to polymers and other surfaces, such as surfaces of medical devices. Deposition of anti-microbial materials directly onto the substrates is possible in a cost-effective manner that maintains high anti-microbial activity over several weeks when the coated devices are employed in vivo.
Owner:NORTHEASTERN UNIV
Laser based metal deposition (LBMD) of antimicrobials to implant surfaces
InactiveUS20070287027A1Improved bearing propertyImprove propertiesFinger jointsDental implantsWear resistantBearing surface
A method is provided for depositing a hard wear resistant surface onto a porous or non-porous base material of a medical implant. The wear resistant surface of the medical implant device may be formed by a Laser Based Metal Deposition (LBMD) method such as Laser Engineered Net Shaping (LENS). The wear resistant surface may include a blend of multiple different biocompatible materials. Further, functionally graded layers of biocompatible materials may be used to form the wear resistant surface. Usage of a porous material for the base may promote bone ingrowth to allow the implant to fuse strongly with the bone of a host patient. The hard wear resistant surface provides device longevity, particularly when applied to bearing surfaces such as artificial joint bearing surfaces or a dental implant bearing surfaces. An antimicrobial material such as silver may be deposited in combination with a metal to form an antimicrobial surface deposit.
Owner:MEDICINELODGE
Antimicrobial Luer Adapter
ActiveUS20120083750A1Material is facilitatedPharmaceutical delivery mechanismCatheterSurgeryMedical device
A luer adapter includes a body including an inner surface defining a lumen and first and second ends. Each end is adapted to connect to a medical device. An antimicrobial material is positioned at least on a portion of the inner surface. A barrier is positioned between the lumen and the antimicrobial material and permits the passage of the antimicrobial material therethrough upon exposure to moisture. The barrier may include hydrophilic material.
Owner:MOZARC MEDICAL US LLC
Treatment device
InactiveUS7122046B2Improve comfortImprove utilizationPlastersAdhesive dressingsDeodorantNitric oxide
A treatment device having a first portion in a first plane, a treatment portion with a cover in a second plane that defines a treatment volume extending between the first and second planes, and a flexible transition portion that connects the first portion to the treatment portion. The treatment portion including a polymeric foam ring having interior and exterior walls extending from the first plane to the second plane. The cover spanning the interior wall thereby forming a closed treatment volume. The polymeric foam ring may be impregnated with a medicament selected from the group including an antibiotic material, an antifungal material, an antimicrobrial material, a deodorant material and nitric oxide. The cover may include a pocket for use with an accessory, such as a heater or mapping grid.A method of treating a treatment area on a patient's skin with the treatment device. The treatment device includes a closed treatment volume having polymeric foam ring impregnated with a medicament. The treatment device is attached to the patient's skin, such that the closed treatment volume encloses the treatment area. The medicament is released into the treatment volume to bring the medicament into contact with the treatment area. Additional steps may include inserting a heater into the pocket or inserting the mapping grid into the pocket and tracking the treatment area with the mapping grid.
Owner:3M INNOVATIVE PROPERTIES CO +1
Saline land improvement method
InactiveCN104919931AImprove permeabilityImprove nutritional statusSoil-working methodsFertilizer mixturesLand improvementFungicide
The invention provides a saline land improvement method and belongs to the technical field of saline land improvement. The saline land improvement method comprises the following steps of locally smashing straws of plants growing in a saline land and then spreading the straws on the saline land, continuing to sequentially spread a composite microbiological material for renovation and urea on the surface of the saline land spread with smashed straws, and plowing and watering the saline land spread with the smashed straws, the composite microbiological material for renovation and the urea on the surface, wherein the composite microbiological material for renovation comprises raw material components: 4-6 parts by weight of organic manure, 2-4 parts by weight of humic acid, 0.7-3 parts by weight of agricultural organic waste, 0.2-0.4 part by weight of marine organic waste, 0.01-0.02 part by weight of liquid fungicide and 0.005-0.01 part by weight of solid fungicide. The saline land improvement method has the advantages of being low in renovation cost, small in working amount, convenient to operate and free of secondary pollution.
Owner:刘长生 +2
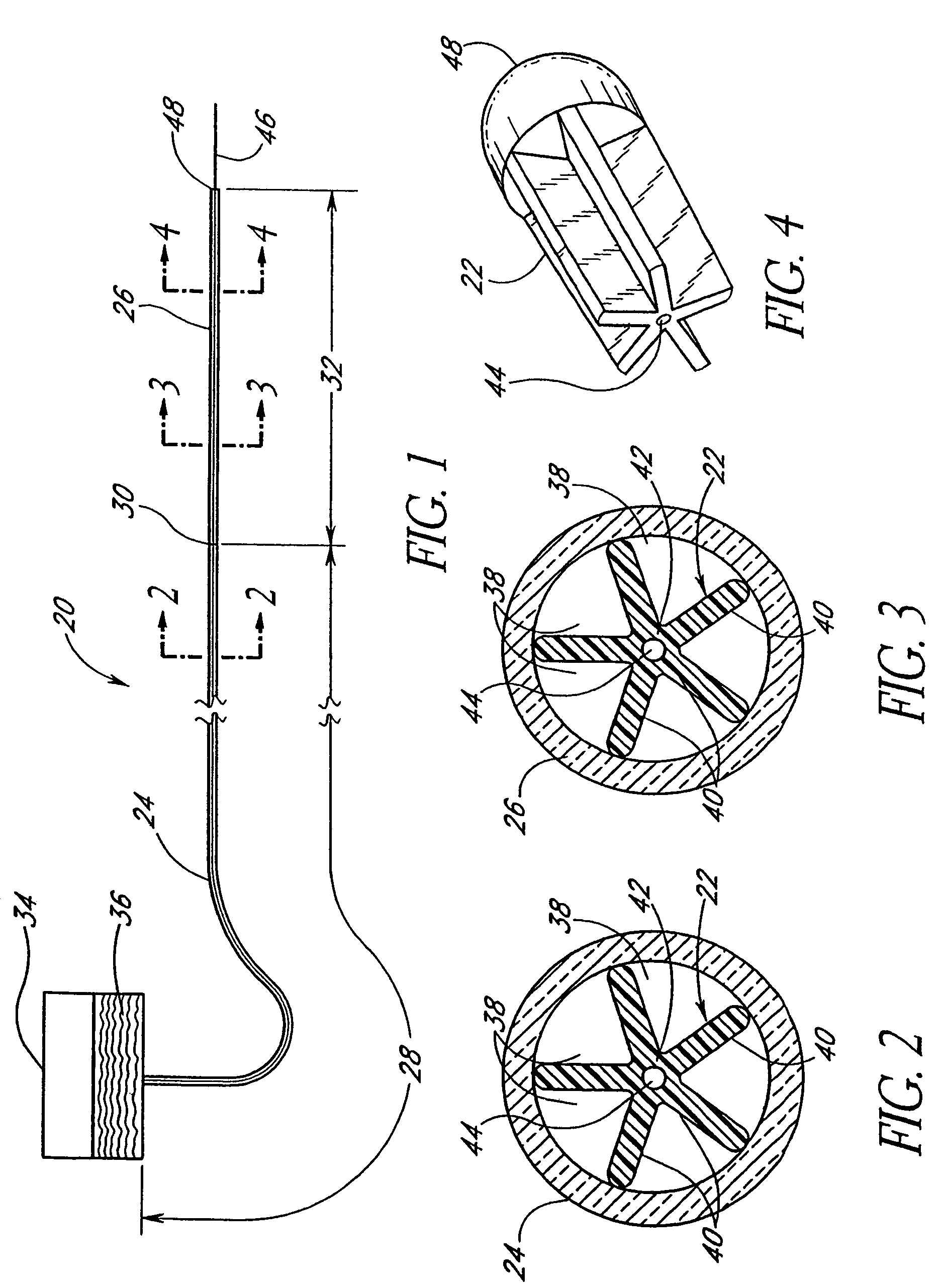
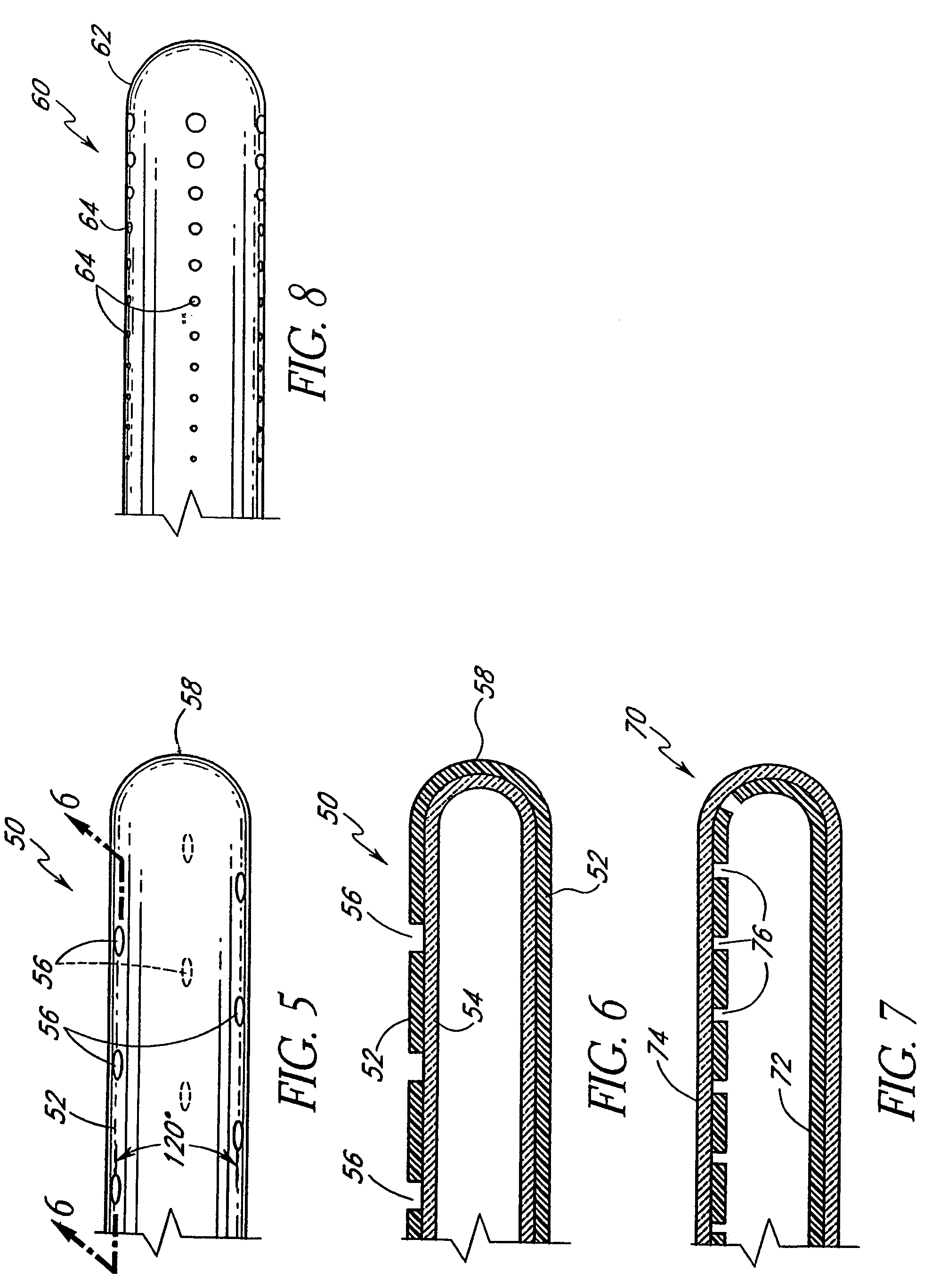
Anti-microbial catheter
A catheter having features configured to provide a substantially uniform flow rate of a fluid exiting the catheter and also exhibits anti-microbial properties. The uniform flow rate features may include one or more of a flow restricting membrane or flow restricting component within an infusion section of the catheter. In other arrangements, exit holes defining the infusion section of the catheter may be configured to provide the desired uniform flow rate over the length of the infusion section. Furthermore, the catheter also includes anti-microbial properties to inhibit the growth of microbes on or within the catheter and, preferably, to inhibit microbe growth in an anatomical region surrounding the catheter. The desired anti-microbial properties may be provided by an anti-microbial layer, anti-microbial materials dispersed within the material from which components of the catheters are constructed, or a combination of anti-microbial layers and embedded anti-microbial materials. In some arrangements, one or more portions of the catheter may be bio-absorbable.
Owner:AVENT INC
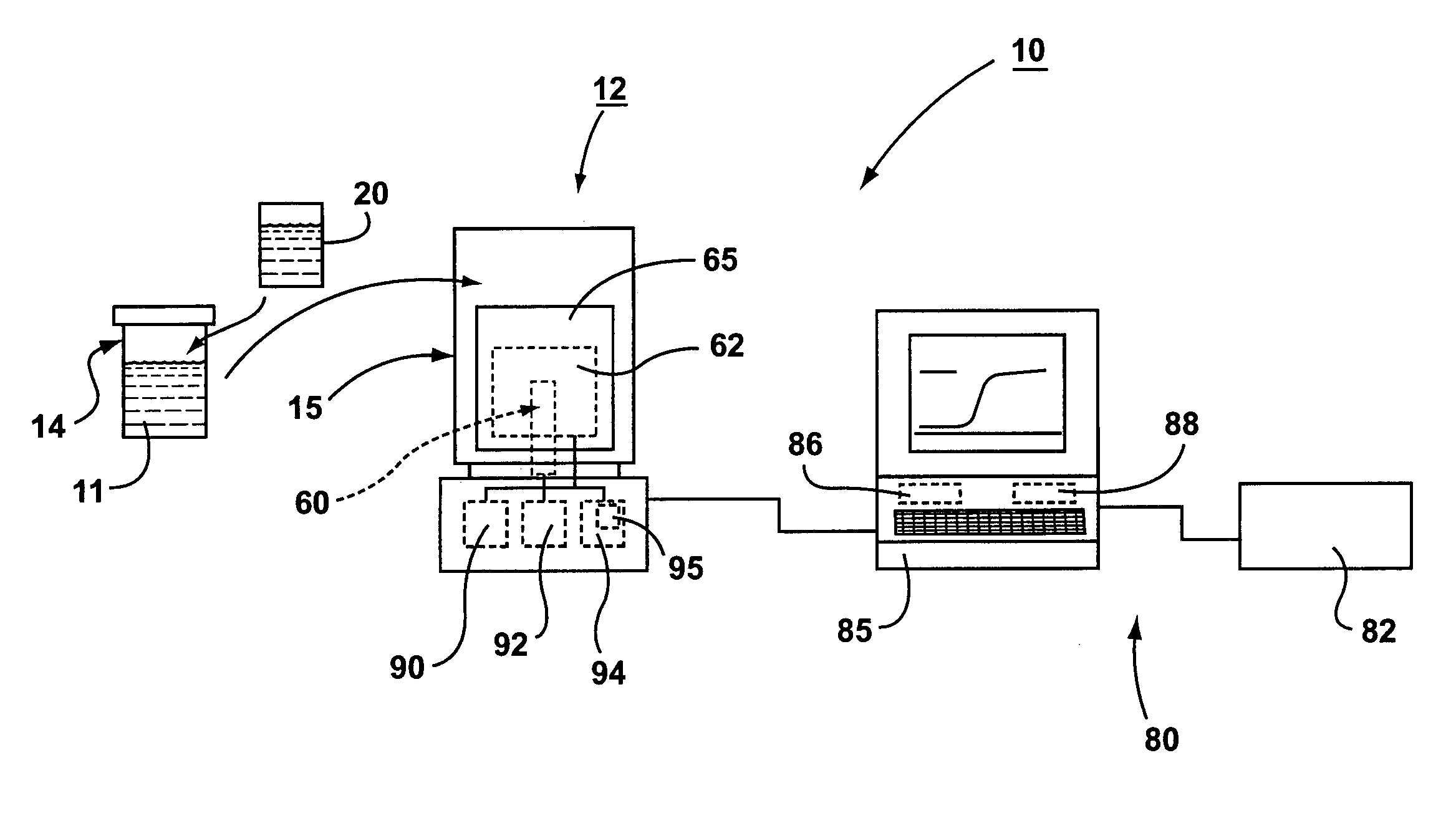
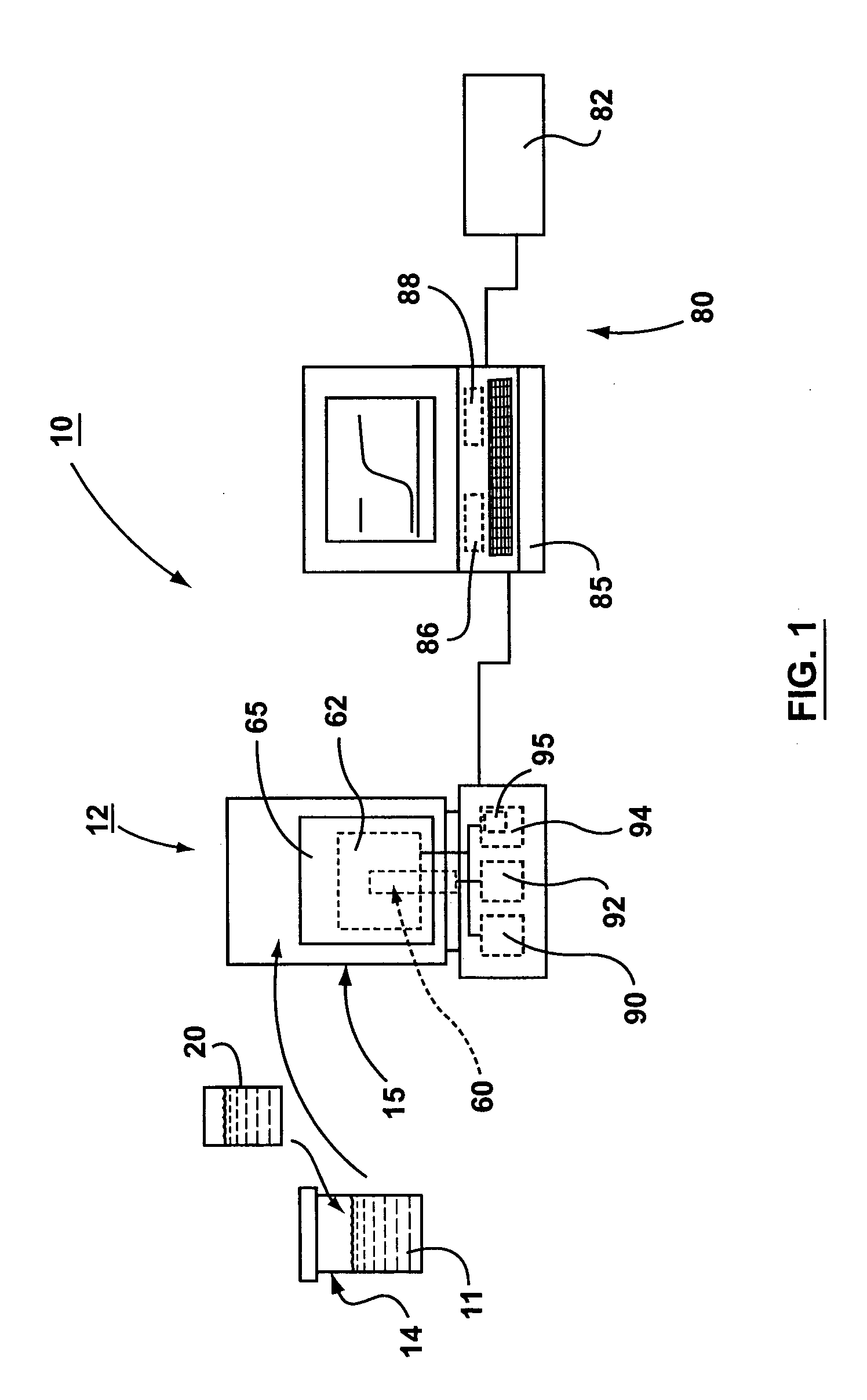
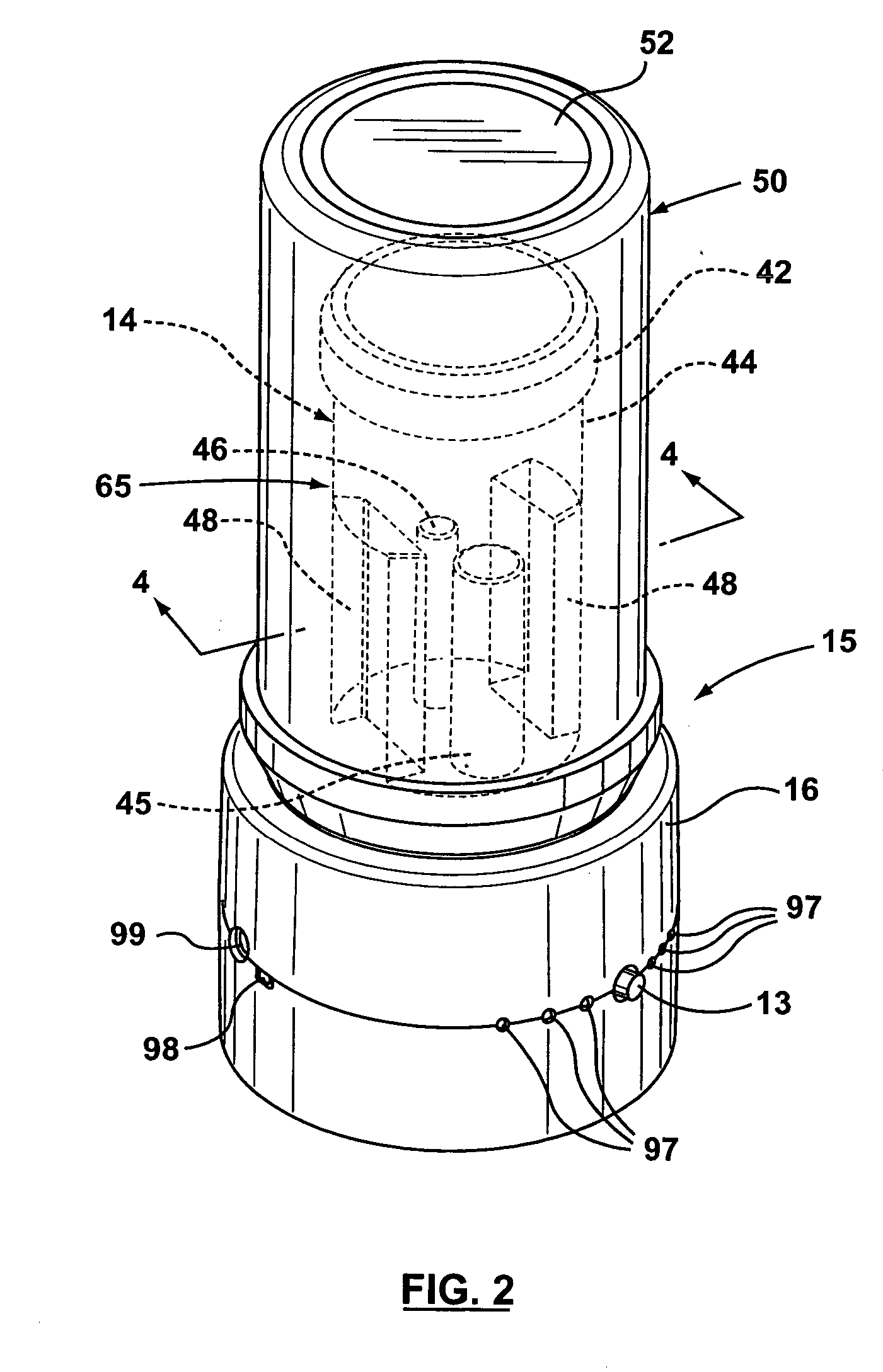
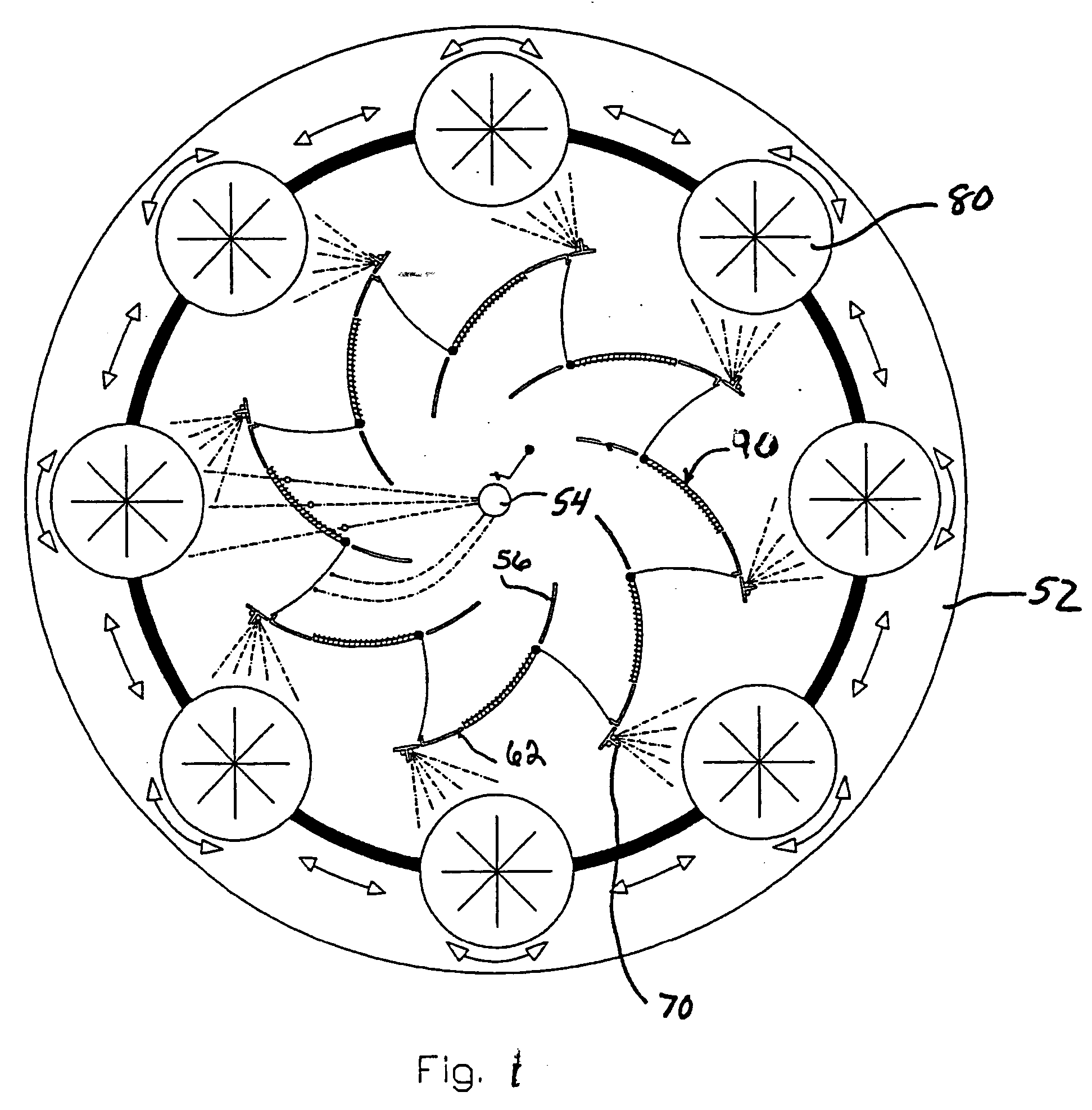
System for rapid analysis of microbiological materials in liquid samples
InactiveUS20050266516A1Bioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringSpecimen containers
A system for the rapid analysis of microbiological parameters includes a specimen container for containing a liquid sample, a housing having an enclosable chamber shaped for receiving the specimen container, an incubating system mounted within the housing for incubating microbiological materials within the liquid sample, and a spectrophotometer system mounted within the housing for measuring light absorbed, emitted or scattered by the liquid samples as the microbiological materials are incubated by the incubating system over time. The specimen container is filled with a liquid sample to be tested and mixed with a reagent that provides a detectable parameter, and placed inside the apparatus. The incubation system heats and maintains the temperature of the liquid sample within a preset range while the spectrophotometer system propagates light within the specimen container, and monitors and records changes in the light as the light propagates through the container. A continuous non-intrusive monitoring and recording of the test parameter is achieved as the incubation progresses. Any significant deviation of the signal output is an indication of presence of the detectable parameter, while the time taken to reach the significant deviation provides quantification of the microbiological parameter under investigation.
Owner:AQUASURE TECH
Antimicrobial compositions for use in products for petroleum extraction, personal care, wound care and other applications
ActiveUS20140271757A1Improve viabilityProvide benefitsBiocideCosmetic preparationsSolubilityPersonal care
Compositions having antimicrobial activity contain surface functionalized particles comprising an inorganic copper salt which has low water solubility. These types of inorganic salts may also be introduced in porous particles to yield antimicrobial compositions. The compositions may optionally comprise additional antimicrobial agents, salts with high water solubility, organic acids, salts of organic acids and their esters. The compositions may be added to various fluids used in the petroleum extraction industry, or used as coatings on components used in this industry. These antimicrobial materials may be used for reducing both anaerobic and aerobic bacteria and are also useful for reducing corrosion of ferrous components caused by anaerobic bacteria. Although such compositions may be used for any antimicrobial application, and some of the other important uses of these compositions are in wound care, personal care and waste processing.
Owner:AGIENIC
Antimicrobial coating methods
ActiveUS8066854B2Eliminate needSustained releaseBiocideHeavy metal active ingredientsMetallurgyIn vivo
The invention is directed to efficient methods for depositing highly adherent anti-microbial materials onto a wide range of surfaces. A controlled cathodic arc process is described, which results in enhanced adhesion of silver oxide to polymers and other surfaces, such as surfaces of medical devices. Deposition of anti-microbial materials directly onto the substrates is possible in a cost-effective manner that maintains high anti-microbial activity over several weeks when the coated devices are employed in vivo.
Owner:NORTHEASTERN UNIV
Replaceable tandem filter cartridge assembly
InactiveUS20070241045A1Easy to insertMinimizing water leakageWater/sewage treatment by ion-exchangeMembrane filtersFilter mediaEngineering
A two-stage, tandem, replaceable filter cartridge assembly includes an outer filter cartridge having a cylindrical shape and defining an axial bore therein, and an inner filter cartridge, also having a cylindrical shape which is received within the axial bore of the outer filter cartridge. The inner filter cartridge may be collapsible so that it can be easily inserted inside the axial bore of the outer filter cartridge, and is expandable so that in normal applications, it rests up against the inner surface of the outer filter cartridge's core, if such is included, or filter medium. One or both of the filter media of the inner and outer cartridges may be formed from an antimicrobial material. In a second embodiment, each of the inner and outer filter cartridges has top and bottom end caps which respectively are structured to mate with one another to form seals therebetween to prevent or minimize the egress of water between the mating top and bottom end caps.
Owner:PLEATCO
Antimicrobial composition for medical articles
InactiveUS7381751B2Inhibition formationImprove the lubrication effectBiocidePowder deliverySolventCalcium Chelator
An antimicrobial composition is formed from about 5 to about 25 wt % of an antimicrobial formulation and about 75 to about 95 wt % of a silicone resin. The antimicrobial formulation is formed from about 60 to about 95 wt % of an antimicrobial material, about 1 to about 30 wt % calcium chelator, about 0.001 to about 0.25 wt % pigment, and about 0.5 to about 3.5 wt % lubricant. The silicone resin may be a dispersion of about 40 to about 60 w / v % of an RTV silicone resin in a solvent, a liquid silicone resin, or a solid silicone resin. An antimicrobial coating may be formed on the surface of an article by applying an antimicrobial composition to the article and permitting the solvent to evaporate. It may also be formed by making a mixture of about 5 to about 12 wt % of an antimicrobial formulation and about 88 to about 95 wt % of a liquid or solid silicone resin and molding, overmolding, or extruding the article from the compounded mixture.
Owner:ICET
Microorganism-removing filter medium having high isoelectric material and low melt index binder
ActiveUS7303683B2Improve antimicrobial effectiveness of filterProlong lifeApparatus sterilizationEnergy based wastewater treatmentActivated carbonMicroorganism
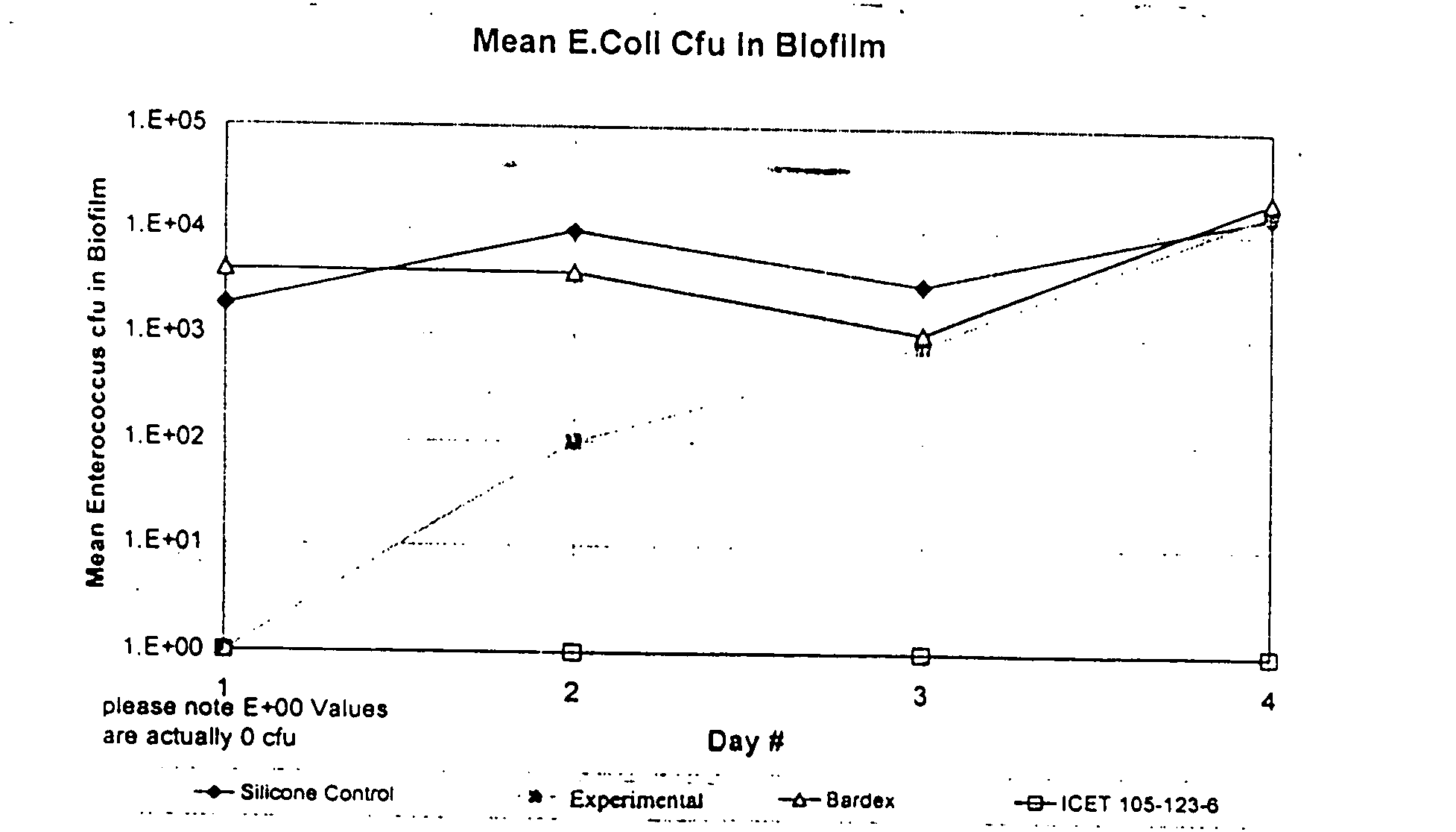
A filter medium capable of removing microorganisms from a fluid such as water. The filter medium includes particles of activated carbon, particles of a substantially insoluble inorganic material having an isoelectric point greater than the fluid being filtered. A low melt index binder, preferably with a melt index of less than about 1 gram per 10 minutes, binds the particles of activated carbon and particles of inorganic material, such that the binder will become tacky at elevated temperatures without becoming sufficiently liquid to substantially wet the particles of activated carbon and inorganic material. An antimicrobial material can be incorporated into the filter to prevent biofilm growth. The use of a biocidal material in combination with the high isoelectric point material provides a trap-and-kill mechanism for microorganism removal.
Owner:THE CLOROX CO
Ionic plasma deposition of anti-microbial surfaces and the anti-microbial surfaces resulting therefrom
InactiveUS20050003019A1Eliminate needEffective levelingCosmetic preparationsBiocidePlasma particlePlasma deposition
A process for depositing anti-microbial materials into or onto the surface of a substrate using ionic plasma deposition. The process includes the steps of providing a cathode of target material having anti-microbial potential which is disposed within a partial vacuum, powering the cathode to generate a plasma discharge for ionizing the target material into a plasma of constituent particles. The plasma particles are reacted with ionized gas, and are selected, controlled and directed toward the substrate by electromagnetic fields generated by at least one first anode adjacent to the cathode and at least one second anode positioned adjacent the first anode. Additional anode structures and charged screens provide further control of the plasma constituents. The plasma constituents, comprising the anti-microbial materials, are deposited on the substrate as dispersed ordered structures which form an anti-microbial surface into and onto the substrate.
Owner:PETERSEN JOHN H
Barrier assembly for use with needleless connector
InactiveUS20100100056A1Operating means/releasing devices for valvesInfusion devicesEngineeringVALVE PORT
A needleless valve assembly includes a needleless connector having a housing with an inlet end with an inlet opening and an outlet end with an outlet opening, and a valve disposed in the housing to control passage between the openings. The assembly also includes a collar having an exterior surface and an interior surface, and a first end with a collar opening communicating between the interior surface and the exterior surface and a second end moveably attached to the housing. Further, the assembly includes a barrier disposed across the collar opening with a slit therethrough and including an anti-microbial material. The collar is moveable relative to the housing so that the inlet end of the housing depends past the exterior surface of the collar through the slit in the barrier. A kit may include the assembly and a catheter having a first end attached to the outlet opening.
Owner:BAXTER INT INC +1
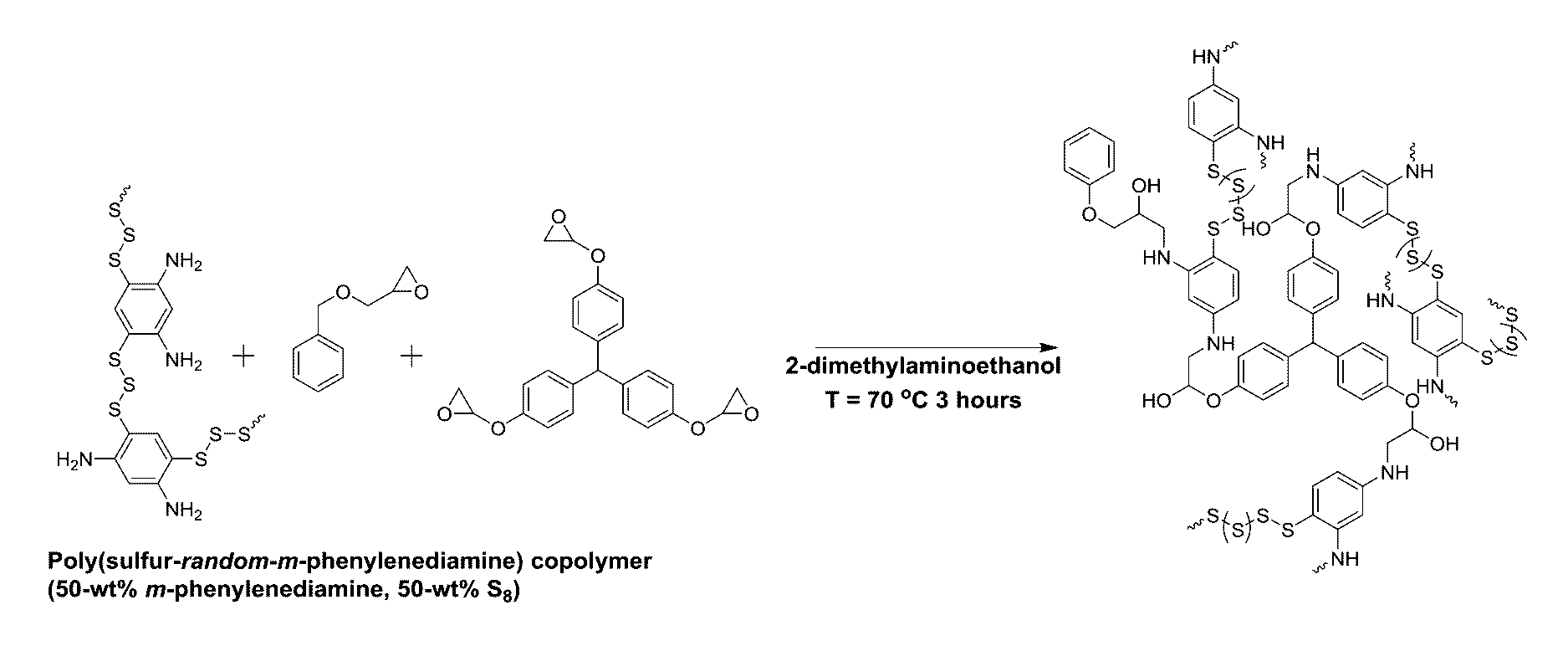
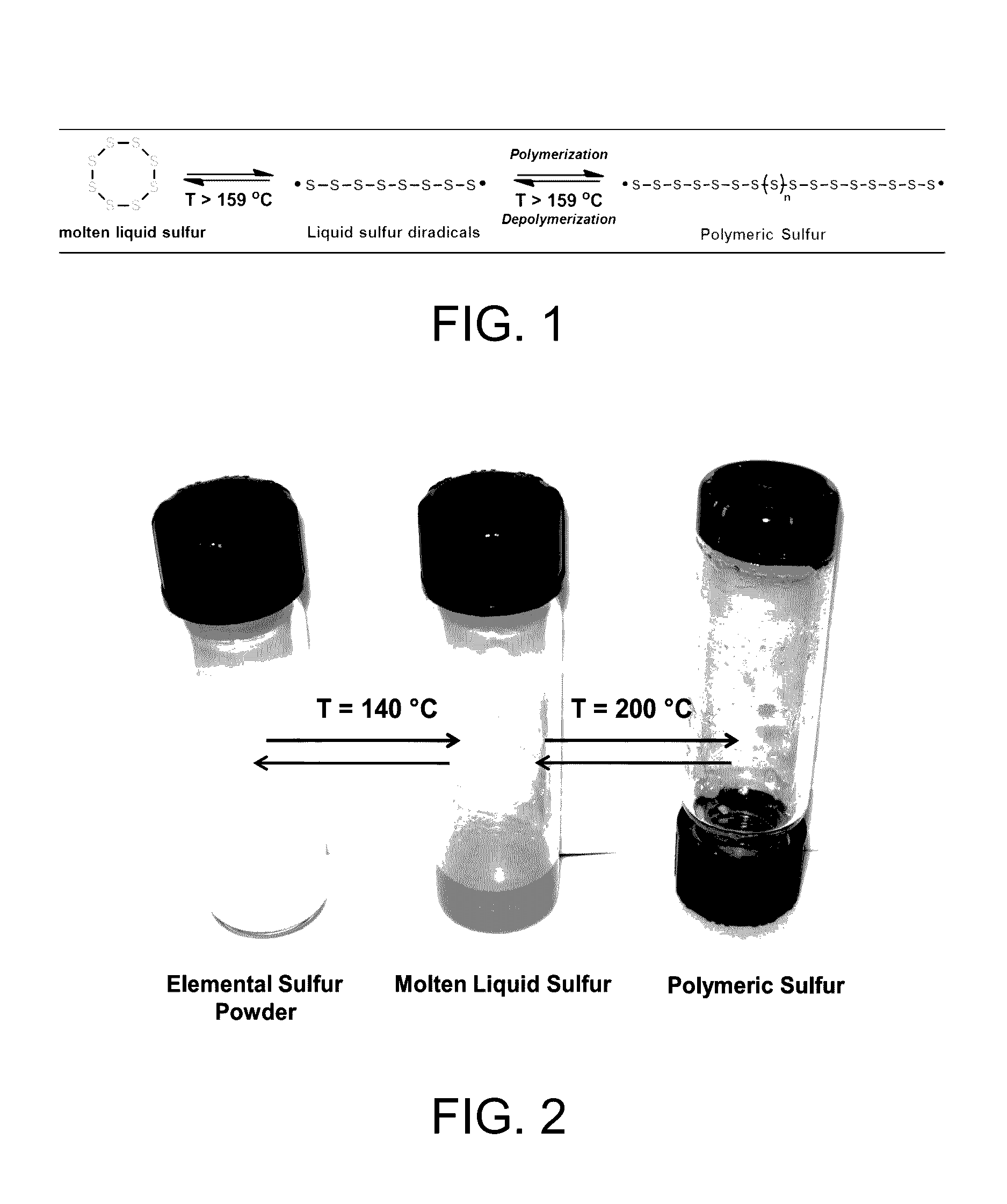
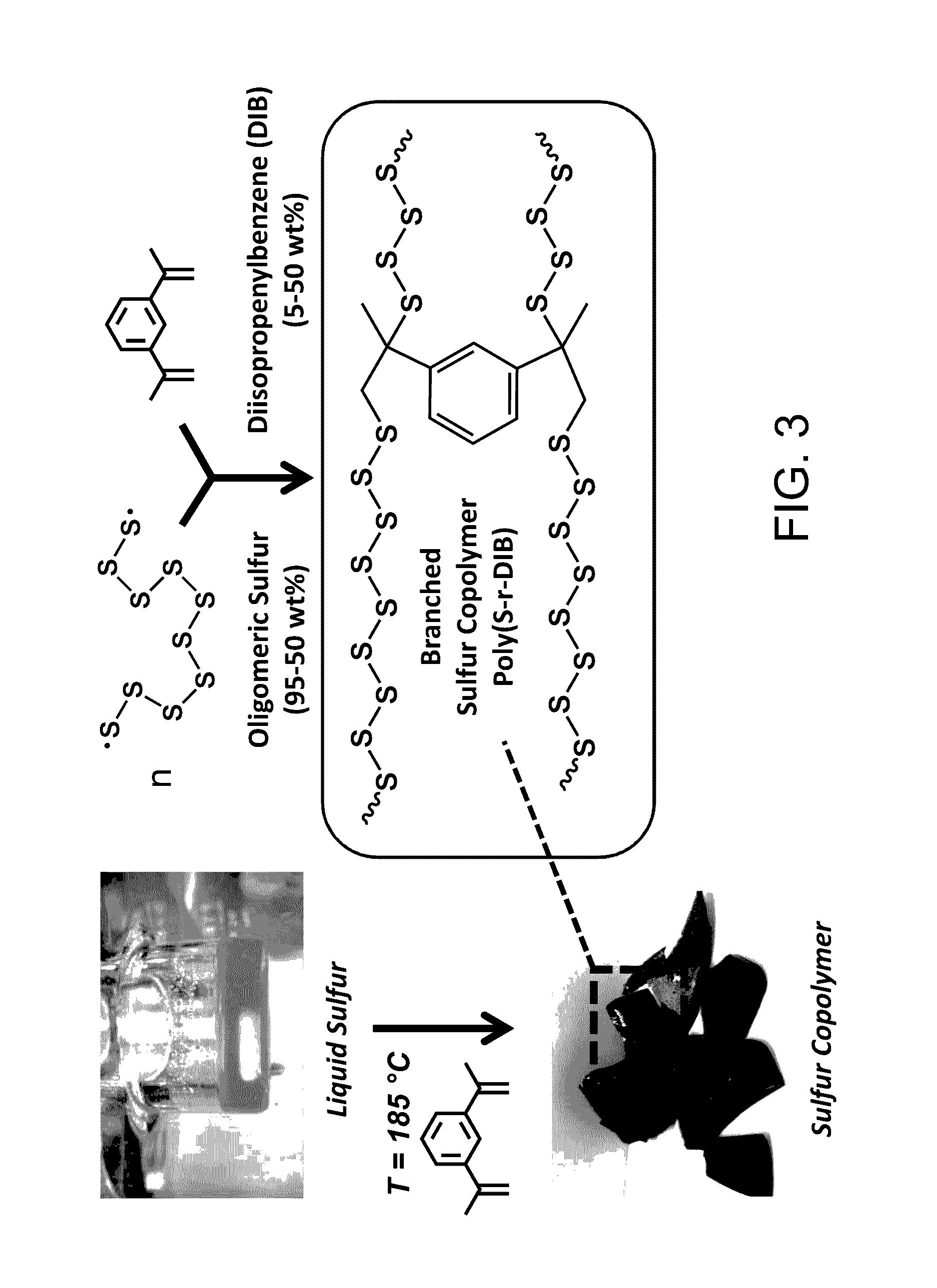
Sulfur composites and polymeric materials from elemental sulfur
ActiveUS9567439B1Increase heightSimple materialBiocideOrganic active ingredientsCeramic compositeSulfide
Sulfur composites and polymeric materials having a high sulfur content and prepared from elemental sulfur as the primary chemical feedstock. The sulfur copolymers are prepared by the polymerization of elemental sulfur with one or more monomers of amines, thiols, sulfides, alkynylly unsaturated monomers, nitrones, aldehydes, ketones, thiiranes, ethylenically unsaturated monomers, or epoxides. The sulfur copolymers may be further dispersed with metal or ceramic composites or copolymerized with elemental carbon, photoactive organic chromophores, or reactive and solubilizing / biocompatible moieties. The sulfur composites and polymeric materials feature the ability self-healing through thermal reformation. Applications utilizing the sulfur composites and polymeric materials may include electrochemical cells, optics, H2S donors and antimicrobial materials.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Contact-killing antimicrobial devices
InactiveUS7288264B1High activityMaintains long-term efficacyUltrasonic/sonic/infrasonic diagnosticsBiocideMicroorganismDisinfectant
Contact killing antimicrobial articles, devices and formulations are described which kill microorganisms on contact. The articles, devices or formulations contain a non-leaching antimicrobial material which is a unique combination of an organic matrix having biocidal metallic materials non-leachably associated with the matrix. The antimicrobial material may used to form an antimicrobial coating or layer on a surface of the article or device, or may be dispersed in a vehicle or carrier to form a topical antiseptic or disinfectant, or solid shape having contact killing antimicrobial properties. When a microorganism contacts the article, device, or formulation, the biocidal metallic material is transferred to the microorganism in amounts sufficient to kill it.
Owner:SURFACINE DEV COMPANY
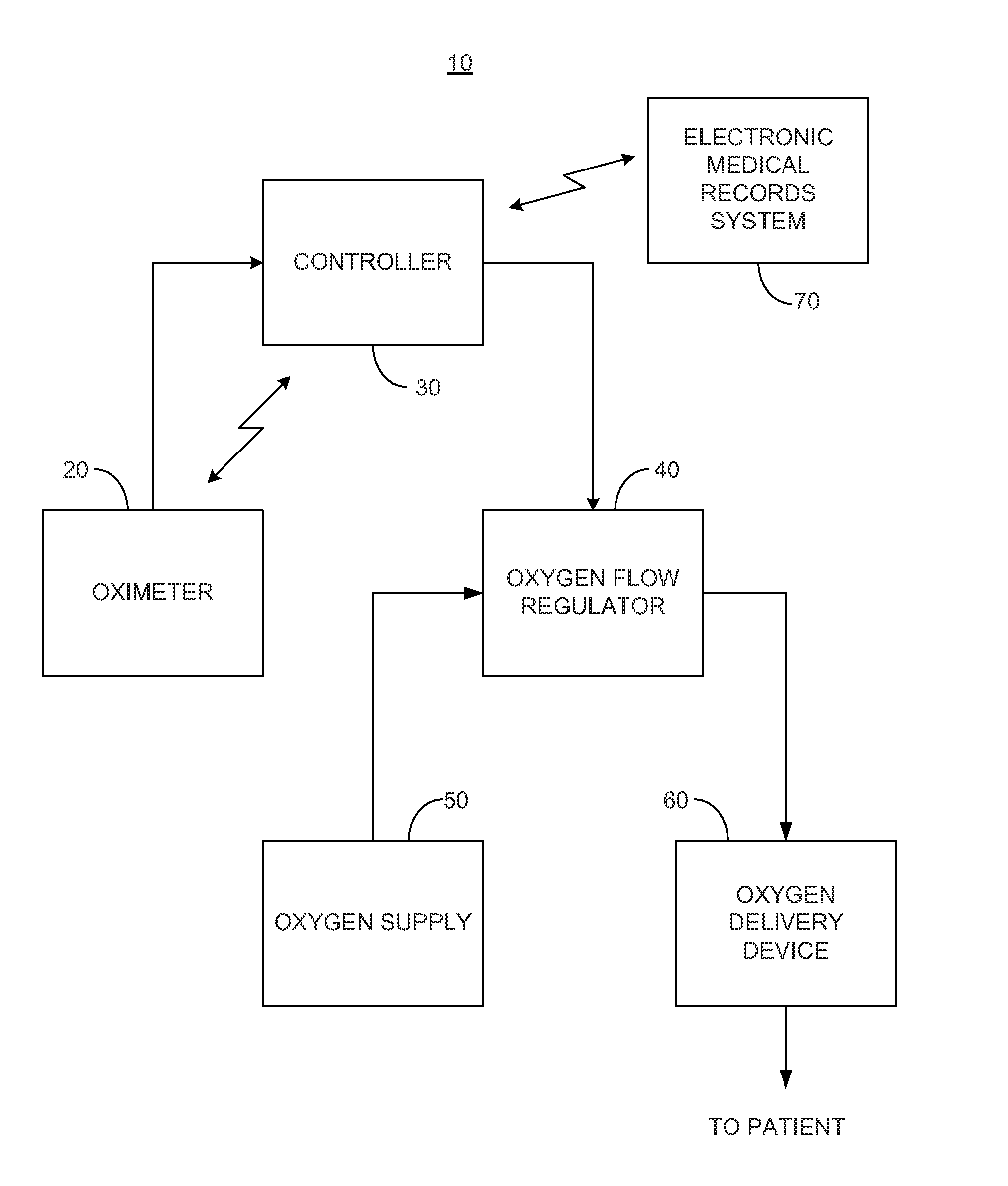
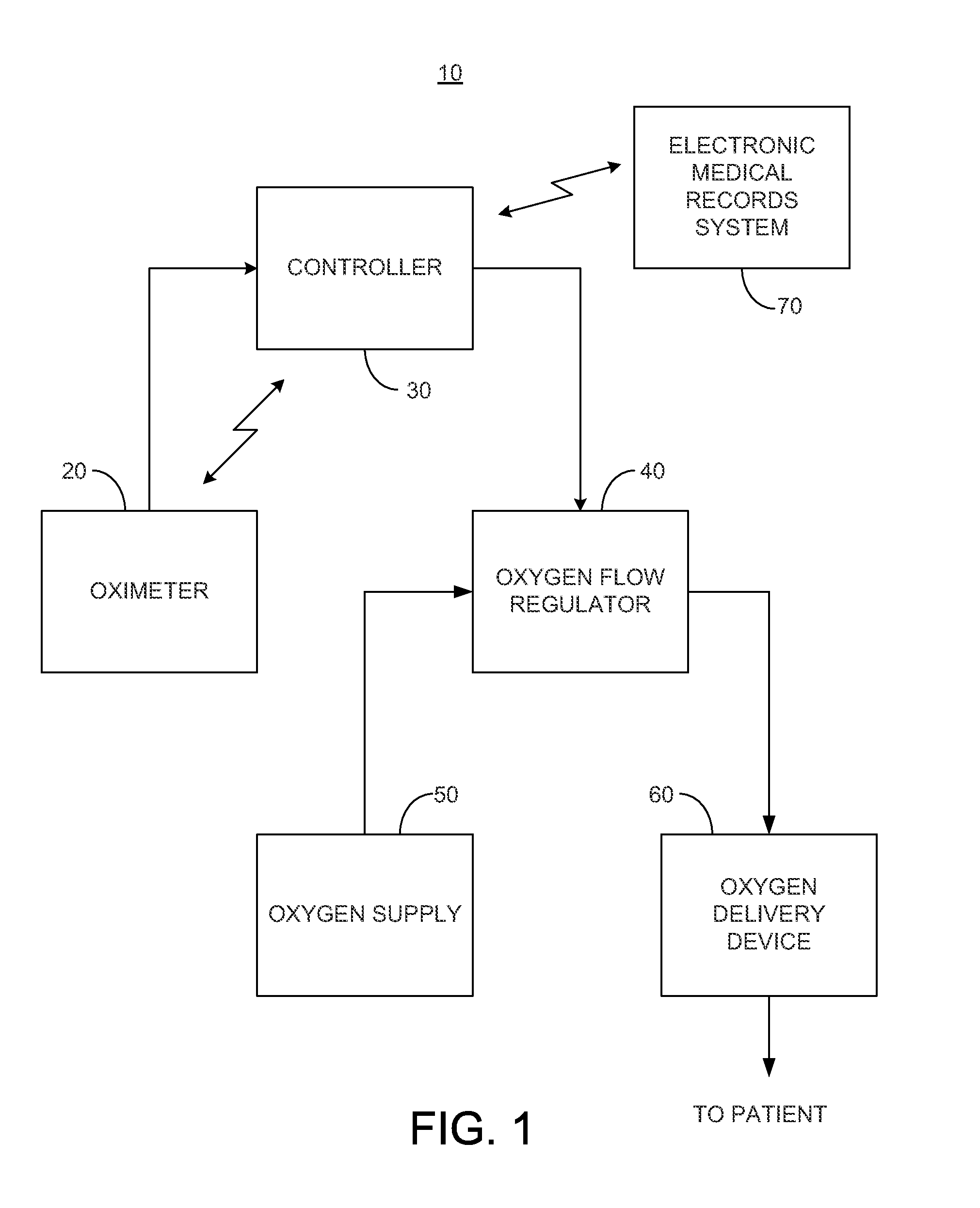
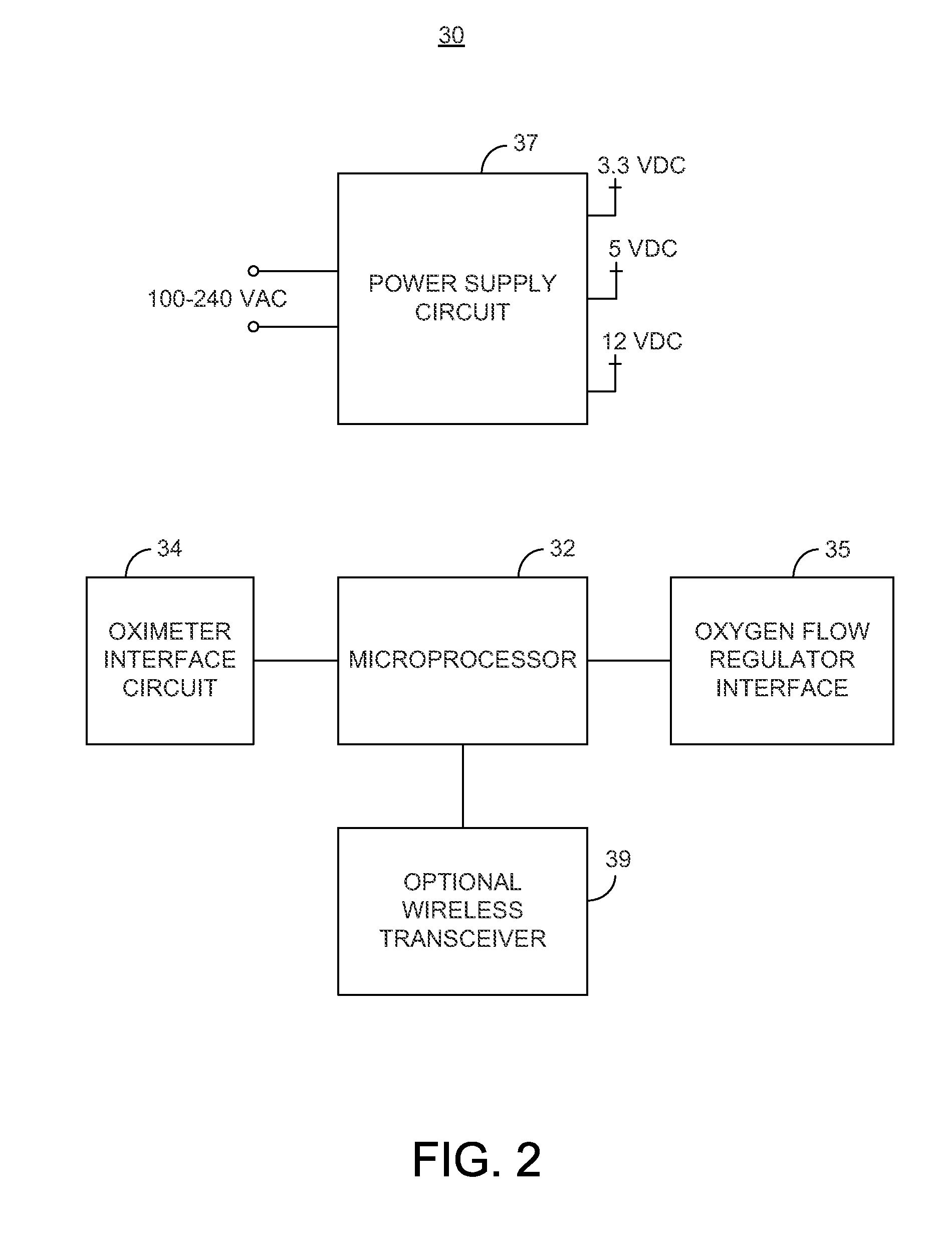
System and method for dynamic regulation of oxygen flow responsive to an oximeter
A system is provided for regulating a flow of oxygen to a patient where the system may include an oximeter for measuring the patient's blood oxygen level and / or heart rate; a controller for determining a desired oxygen flow rate based on the patient's blood oxygen level and / or heart rate, and generating a control signal representative of the desired oxygen flow rate; an oxygen flow regulator for dynamically regulating a flow rate of oxygen supplied to the patient responsive to the control signal provided by the controller; an oxygen delivery device; and a transmitter for wirelessly communicating with an electronic medical records system. The oxygen flow regulator may receive oxygen from an oxygen bottle, oxygen mixer, a wall oxygen supply of a healthcare facility, or an oxygen concentrator. One or more of the components of the system may be treated with an antimicrobial material.
Owner:EDDY PATRICK E
Antimicrobial transfer substrates and methods of use therewith
InactiveUS6454813B1Highly effective transfer mechanismEfficient transferInorganic/elemental detergent compounding agentsBiocideFiberAntimicrobial compound
Specific transfer methods and articles to impart a metal-ion based antimicrobial finish to recipient textile surfaces. Such treatments preferably comprise silver ions, particularly as constituents of inorganic metal salts or zeolites. In particular, the inventive method involves the application of a solid, inorganic antimicrobial material to a donor substrate (such as a dryer sheet), and the subsequent placement of such a substrate within a tumble drying machine containing textile fabrics and operating the machine. The donor substrate, upon contact with the recipient textile fabrics, transfers antimicrobially effective amounts of the metal-ion based compounds to such recipient fabrics thereby imparting at least a temporary antimicrobial finish over at least a portion of such fabrics. The donor substrates, with either the antimicrobial compound alone or mixed with standard tumble dryer additives (such as perfumes, fabric softeners, fiber lubricants, and the like) are also contemplated within this invention.
Owner:MILLIKEN & CO
Antimicrobial Resin Coated Proppants
InactiveUS20160032180A1Good economic viabilityProvide benefitsBiocideCosmetic preparationsActive agentAqueous solubility
The invention relates to polymeric coatings on proppants. These coatings have antimicrobial materials incorporated within these coatings. Preferably the antimicrobial materials have low water solubility. These antimicrobial agents are incorporated as particles whose surfaces are modified or these may also be incorporated within porous particles which are then added to the coating formulations. The antimicrobially active agents are incorporated in a fashion so that they can be released from these coatings in the environment of the proppants.
Owner:AGIENIC
Intravenous connector having antimicrobial treatment
An intravenous connector is provided having a valve body having first end configured to receive a syringe for administering medicine to a patient; a spike element having a tapered hollow spike terminating in a tip at a first end and having a second end configured for attaching to an intravenous tube, the spike element secured in the valve body such that the tip is disposed proximate the first end of the valve body and the second end of the spike element is located at a second end of the valve body; and a pliable seal member disposed in the valve body around the tapered hollow spike and exposed at the first end of the valve body. An antimicrobial material comprising a silane quaternary ammonium salt is incorporated on or within the seal member and is coated on the valve body and the spike element.
Owner:PARASOL MEDICAL
Composition
InactiveUS20080124368A1Improve the lubrication effectInhibition formationBiocideAntifouling/underwater paintsPolyesterSolvent
An antimicrobial composition is formed from about 5 to about 25 wt % of an antimicrobial formulation and about 75 to about 95 wt % of a polyurethane resin or polyurethane hybrids, copolymers, or mixtures with other polymers such as polyesters, nitrites, PVC, and synthetic rubber latexes. The antimicrobial formulation is formed from about 60 to about 90 wt % of an antimicrobial material, about 1 to about 30 wt % calcium chelator, about 0.001 to about 2 wt % color and appearance enhancing pigments, about 0.001 to about 2 wt % of surfactants, and about 0.5 to about 10 wt % lubricant. The polyurethane resin may be a nonaqueous or aqueous latex dispersion or a prepolymer that polymerizes when exposed to moisture. An antimicrobial coating may be formed on the surface of an article by applying an antimicrobial composition to the article; if a polyurethane prepolymer is used, the composition is exposed to moisture and if an aqueous or nonaqueous dispersion is used, the water or solvent is evaporated. A coating may also be formed by making a mixture of the antimicrobial formulation and a resin and molding, overmolding, or extruding the article from the compounded mixture.
Owner:SARANGAPANI SHANTHA
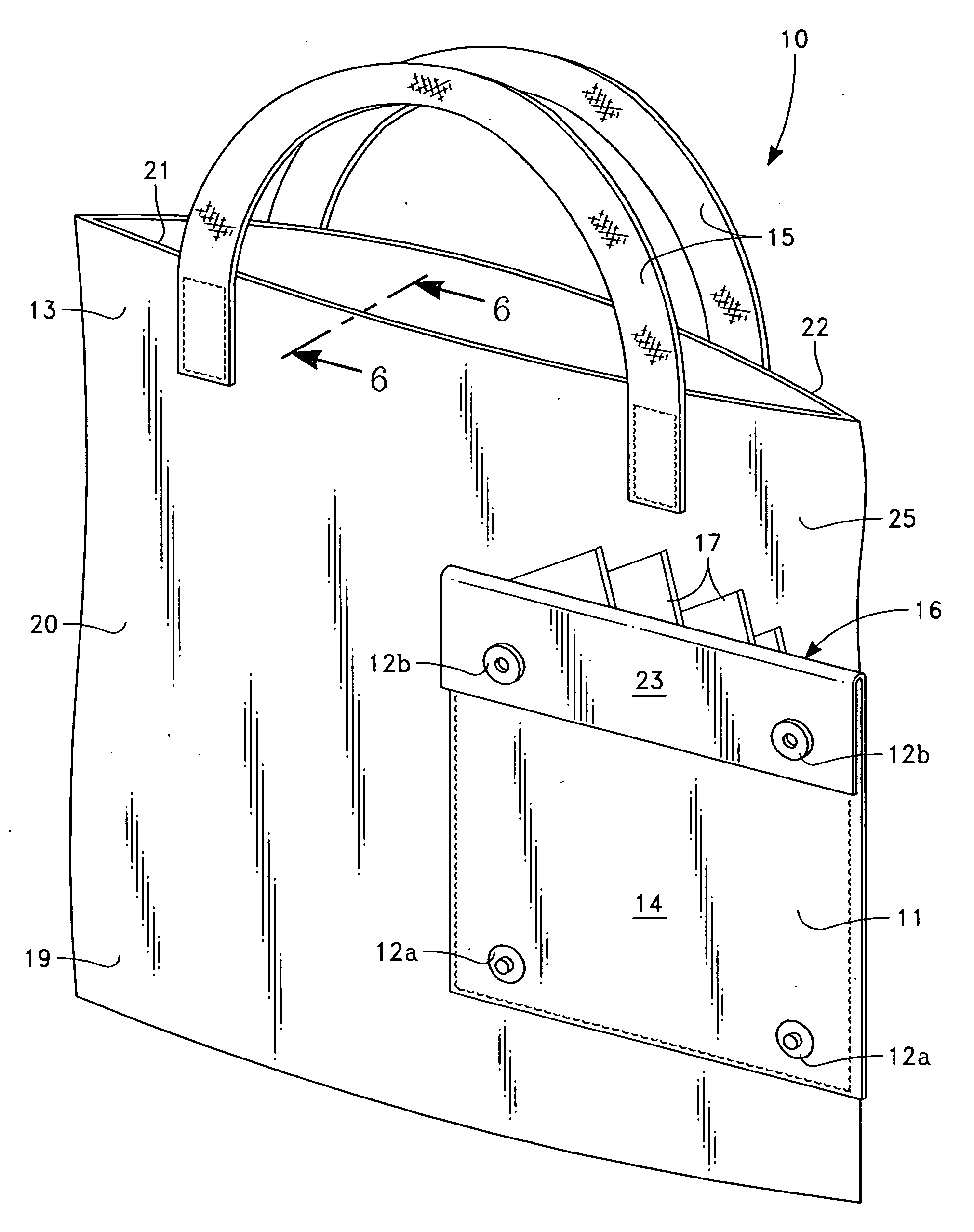
Compact foldable shopping bag
InactiveUS20100086237A1Easy to carryDomestic cooling apparatusLighting and heating apparatusEngineeringMagnet
An expandable, foldable compact shopping bag that includes a decorative outer face that converts into a pocket that holds further expandable, foldable compact shopping bags. The bag decor can match any other article of clothing or bags used by the consumer. The bag can include a thermal layer for the transport of perishable foodstuffs or made of an anti-microbial material. The bag is secured shut when folded through attachment means, such as magnets, snaps, buttons, zippers, hook and loop systems and the like.
Owner:DIEPEN MONA VAN
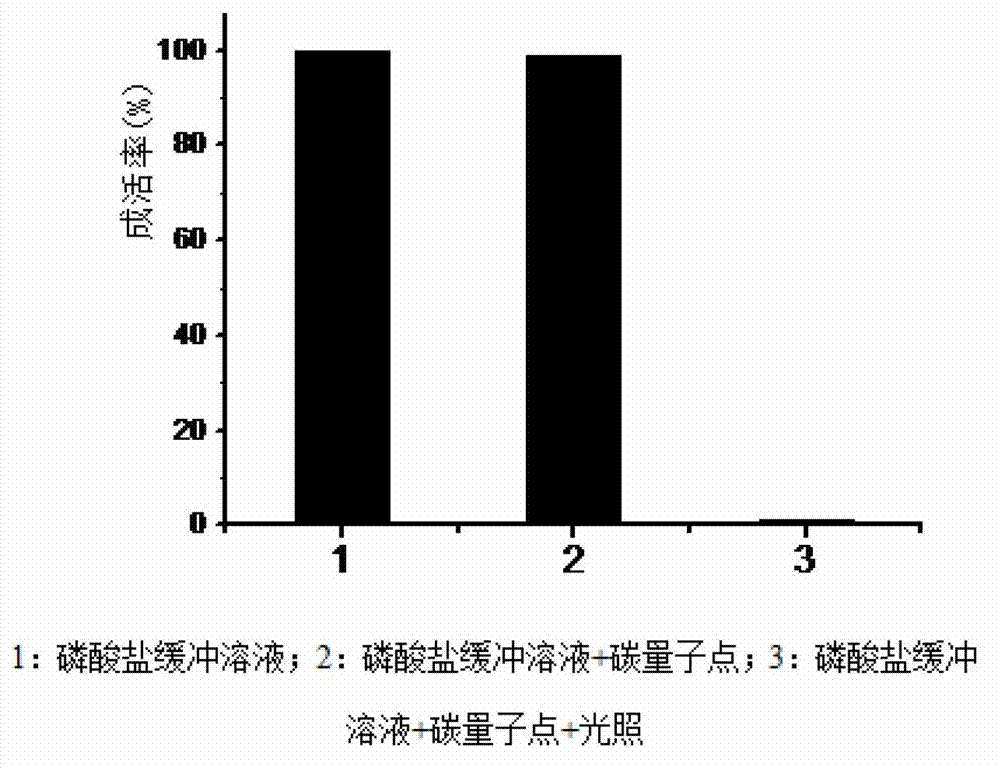
Application of heteroatom doped multifunctional carbon quantum dot serving as photosensitizer in antimicrobial material
ActiveCN103109867ABroad absorption spectrumEffective absorptionBiocideFungicidesOxygenMaterials science
The invention describes a usage of a heteroatom doped water-soluble carbon quantum dot serving as a photosensitizer in an antimicrobial material. Various types of heteroatom doped water-soluble carbon quantum dots contain one or more of such heteroatoms as N, S, Si, Se, P, As, Ge, Gd, B, Sb and Te, and the absorption spectrum wavelength of the heteroatom doped water-soluble carbon quantum dot is 300-850 nm. As shown in a research, the heteroatom doped water-soluble carbon quantum dots can efficiently generate active oxygen under the irradiation of visible light, so that the heteroatom doped water-soluble carbon quantum dot has a huge development potential in the application field of photodynamic (PDT) sterilization and disinfection.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Laser Based Metal Deposition LBMD of Antimicrobials to Implant Surfaces
InactiveUS20110208304A1Minimize adverse effectsHigh hardnessFinger jointsDental implantsWear resistantBone ingrowth
A method is provided for depositing a hard wear resistant surface onto a porous or non-porous base material of a medical implant. The wear resistant surface of the medical implant device may be formed by a Laser Based Metal Deposition (LBMD) method such as Laser Engineered Net Shaping (LENS). The wear resistant surface may include a blend of multiple different biocompatible materials. Further, functionally graded layers of biocompatible materials may be used to form the wear resistant surface. Usage of a porous material for the base may promote bone ingrowth to allow the implant to fuse strongly with the bone of a host patient. The hard wear resistant surface provides device longevity, particularly when applied to bearing surfaces such as artificial joint bearing surfaces or a dental implant bearing surfaces. An antimicrobial material such as silver may be deposited in combination with a metal to form an antimicrobial surface deposit.
Owner:MEDICINELODGE
Polymeric composites with functional surfaces
InactiveUS20190091950A1Easy and efficient to prepareLow costLayered productsPhotomechanical apparatusPolymer compositesMaterials science
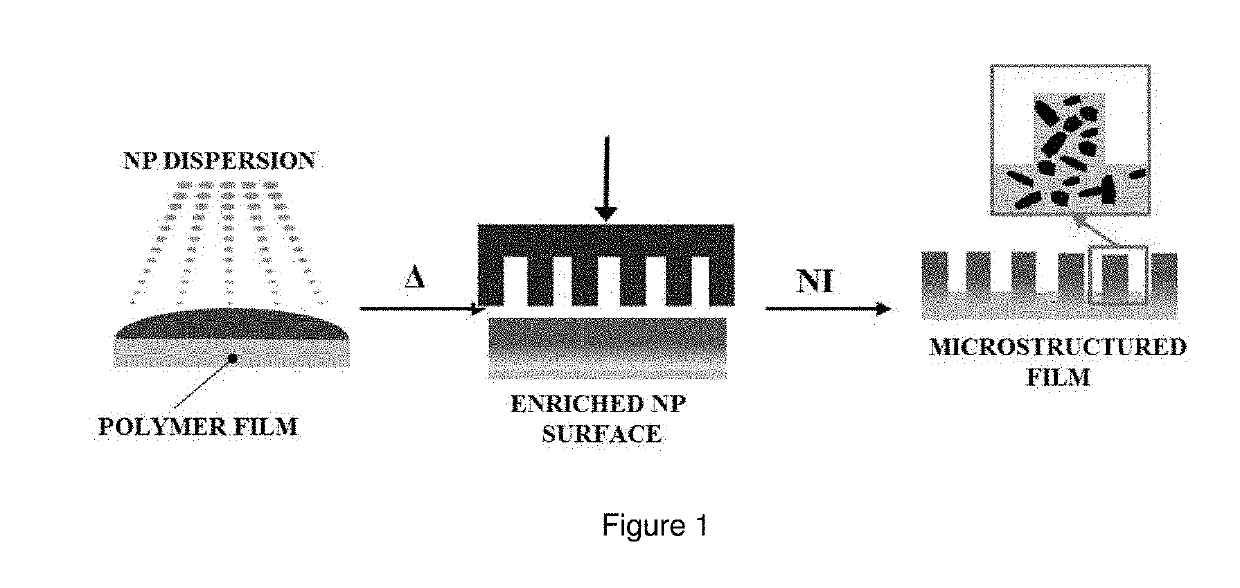
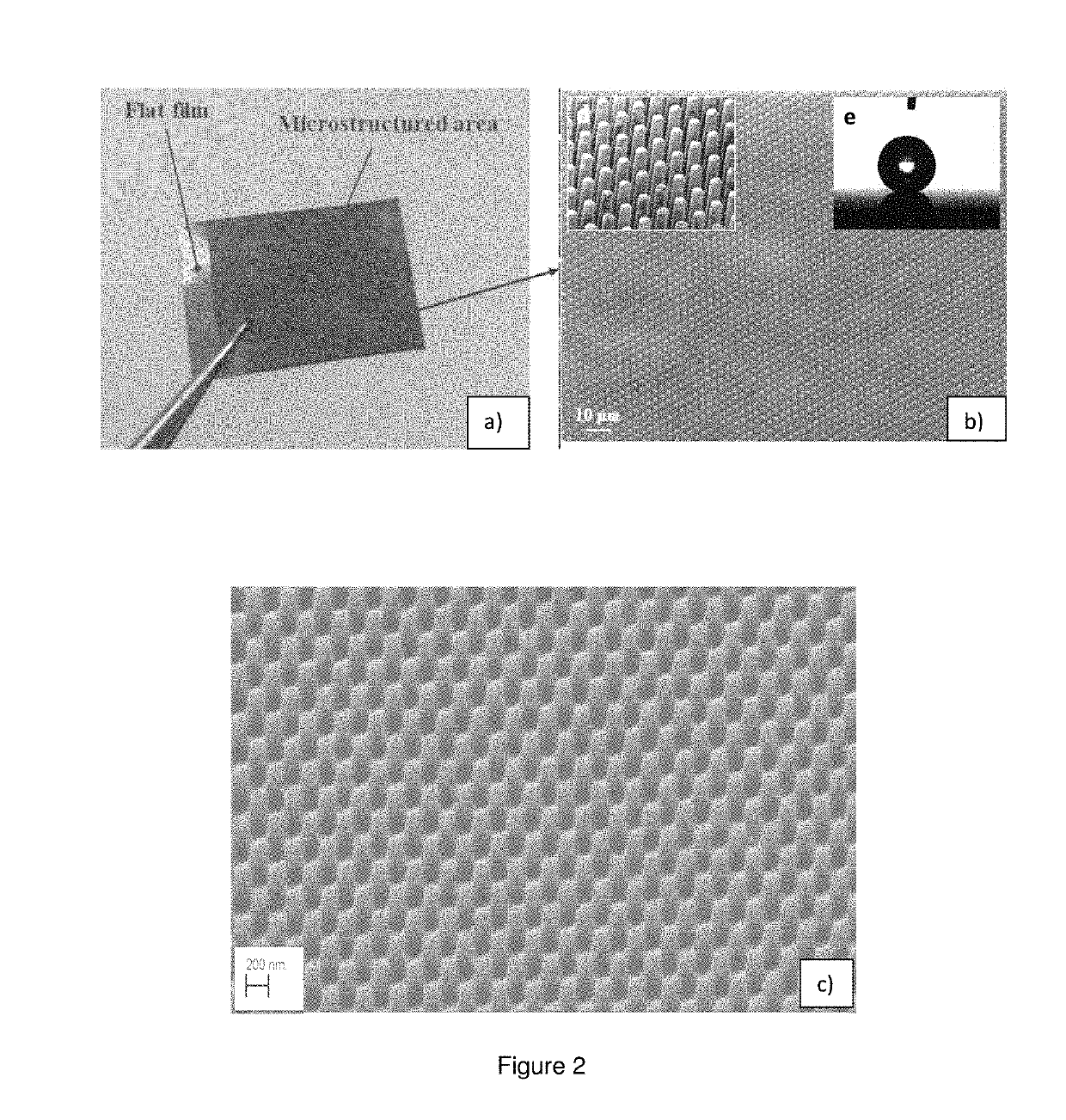
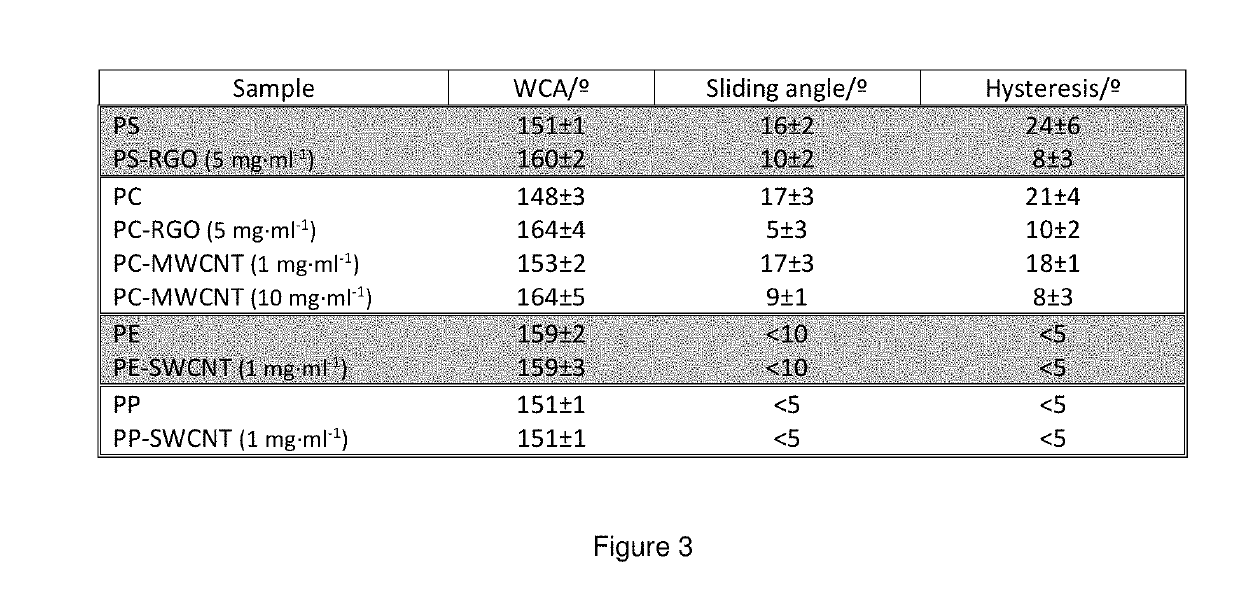
A composite having a textured surface with multiple protrusions and a bulk portion. The composite includes a polymeric matrix and particles, the major amount of particles being concentrated into the textured surface. The composite can be used as a structural coating, super-repellent material, antireflective material, or antimicrobial material among others.
Owner:FUNDACION IMDEA NANOCIENCIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com