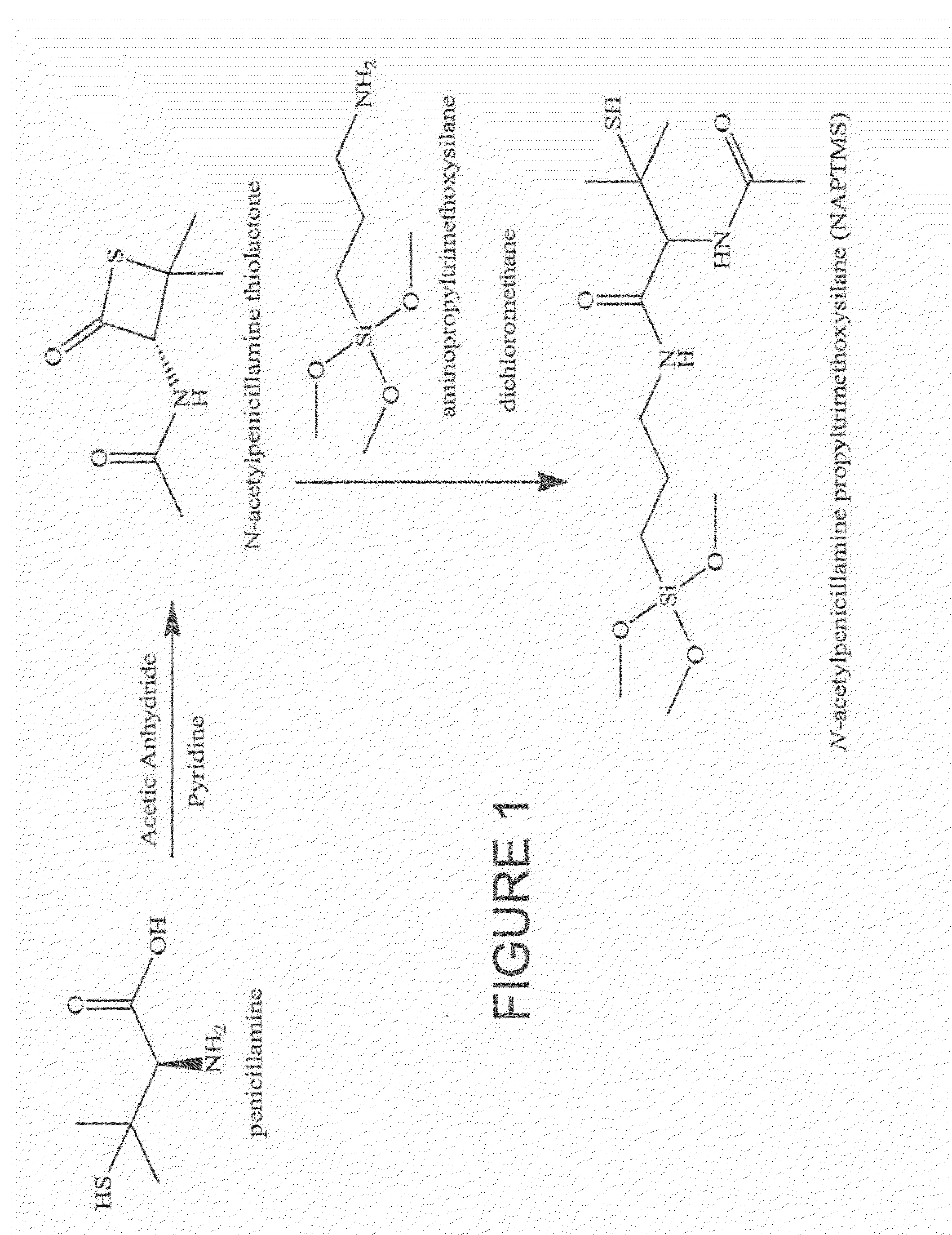
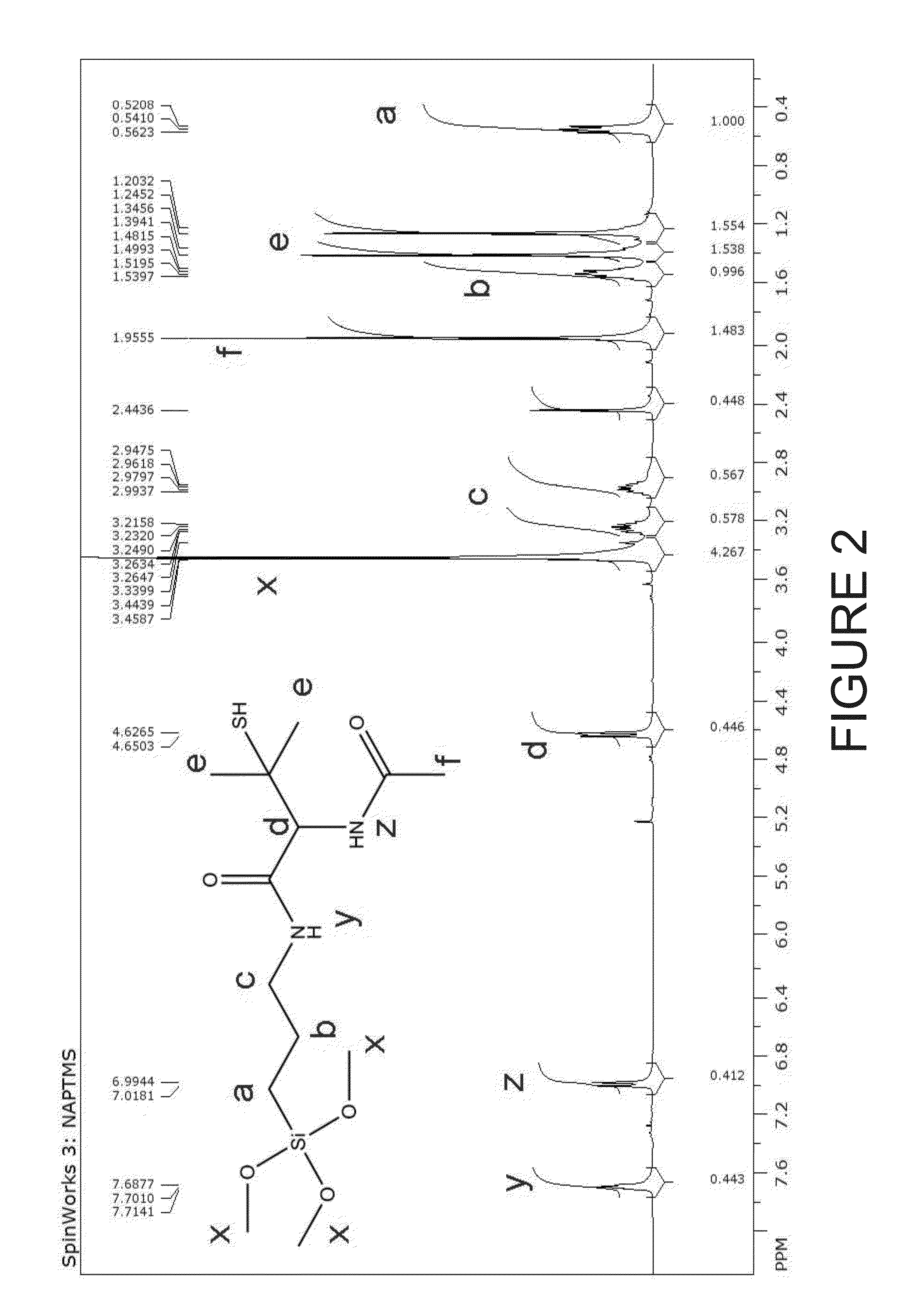
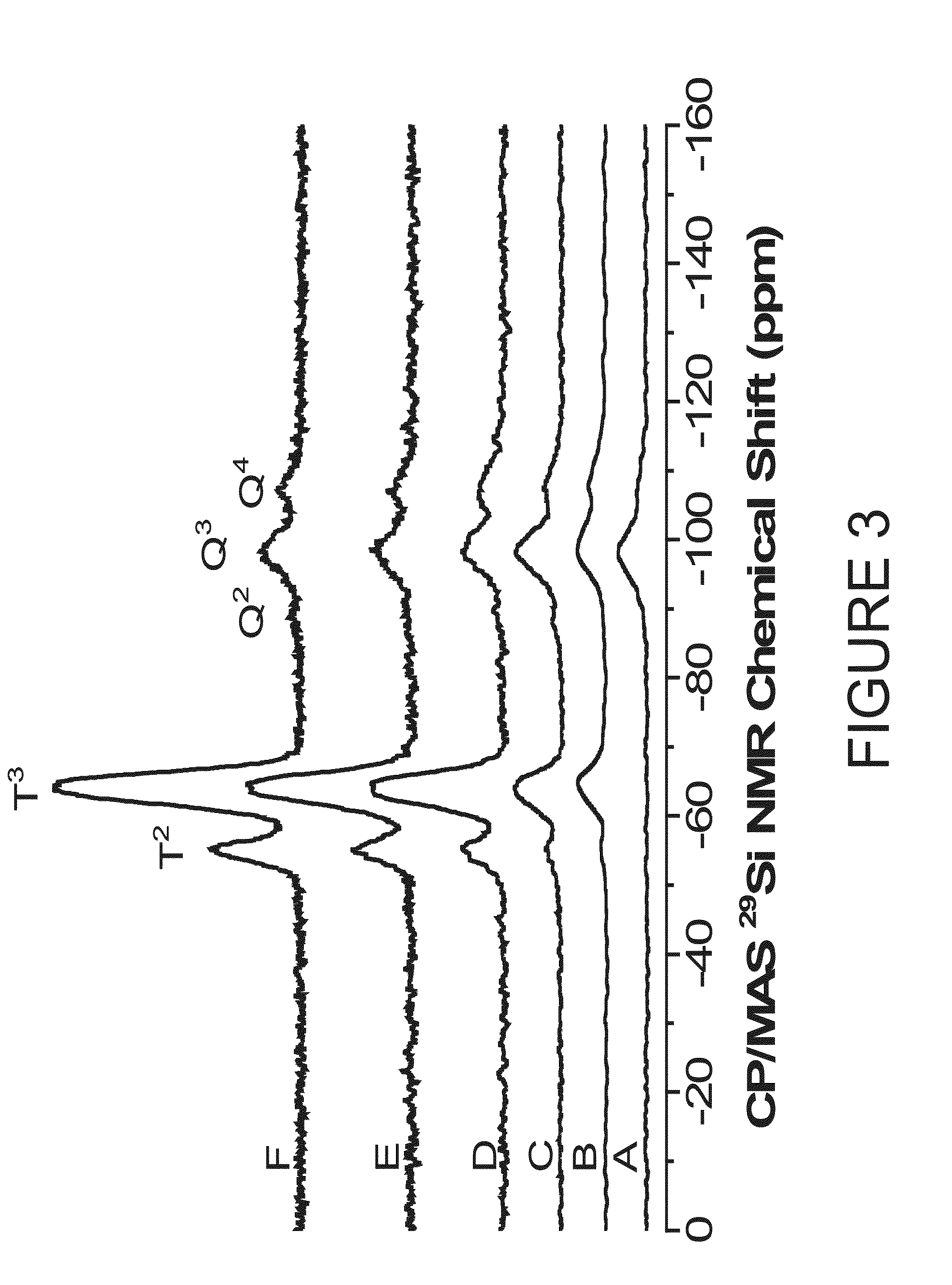
Patents
Literature
42 results about "S-Nitrosothiol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
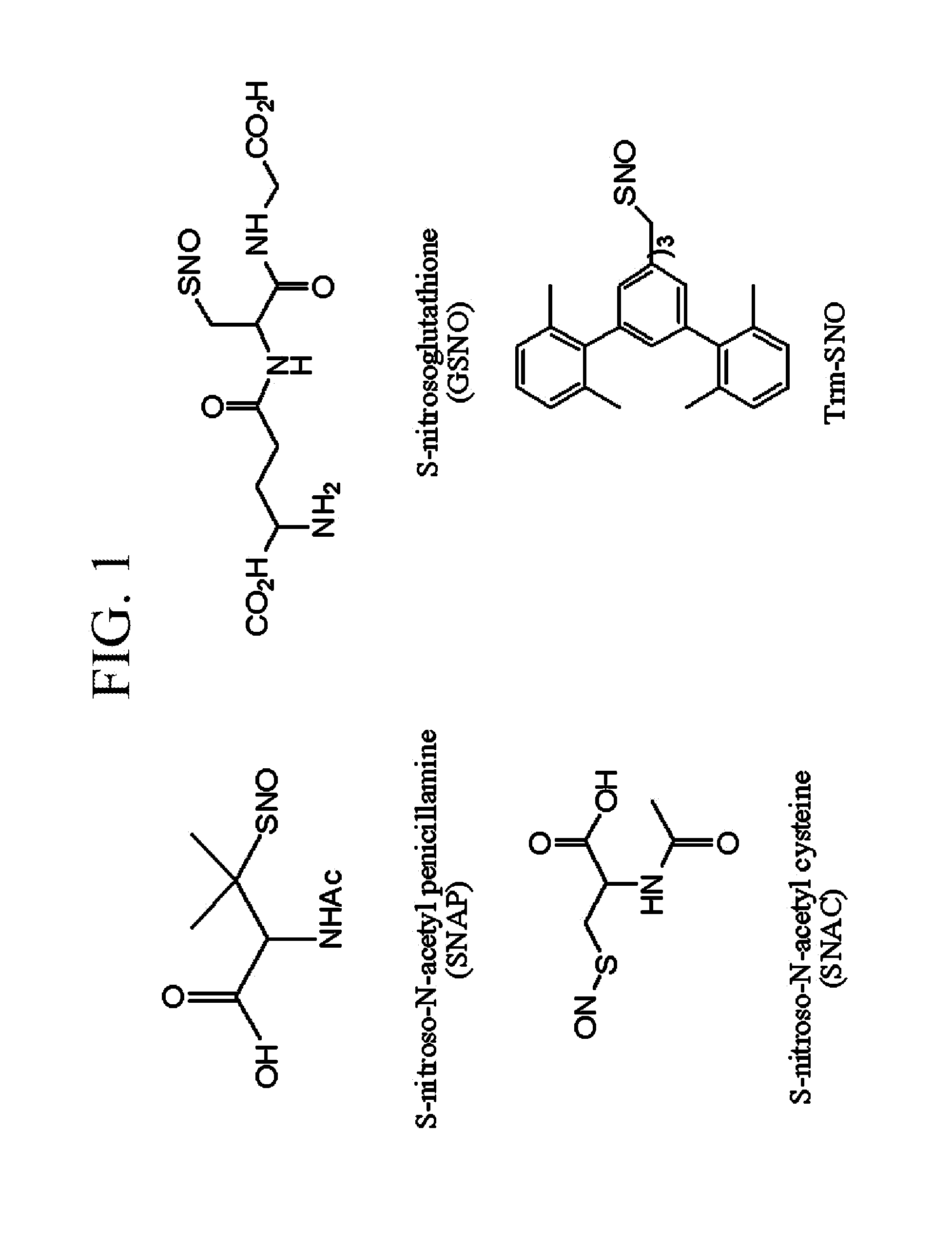
S-Nitrosothiols, also known as thionitrites, are organic compounds or functional groups containing a nitroso group attached to the sulfur atom of a thiol. S-Nitrosothiols have the general formula RSNO, where R denotes an organic group. Originally suggested by Ignarro to serve as intermediates in the action of organic nitrates, endogenous S-nitrosothiols were discovered by Stamler and colleagues (S-nitrosoalbumin in plasma and S-nitrosoglutathione in airway lining fluid) and shown to represent a main source of NO bioactivity in vivo. More recently, S-nitrosothiols have been implicated as primary mediators of protein S-nitrosylation, the oxidative modification of Cys thiol that provides ubiquitous regulation of protein function.
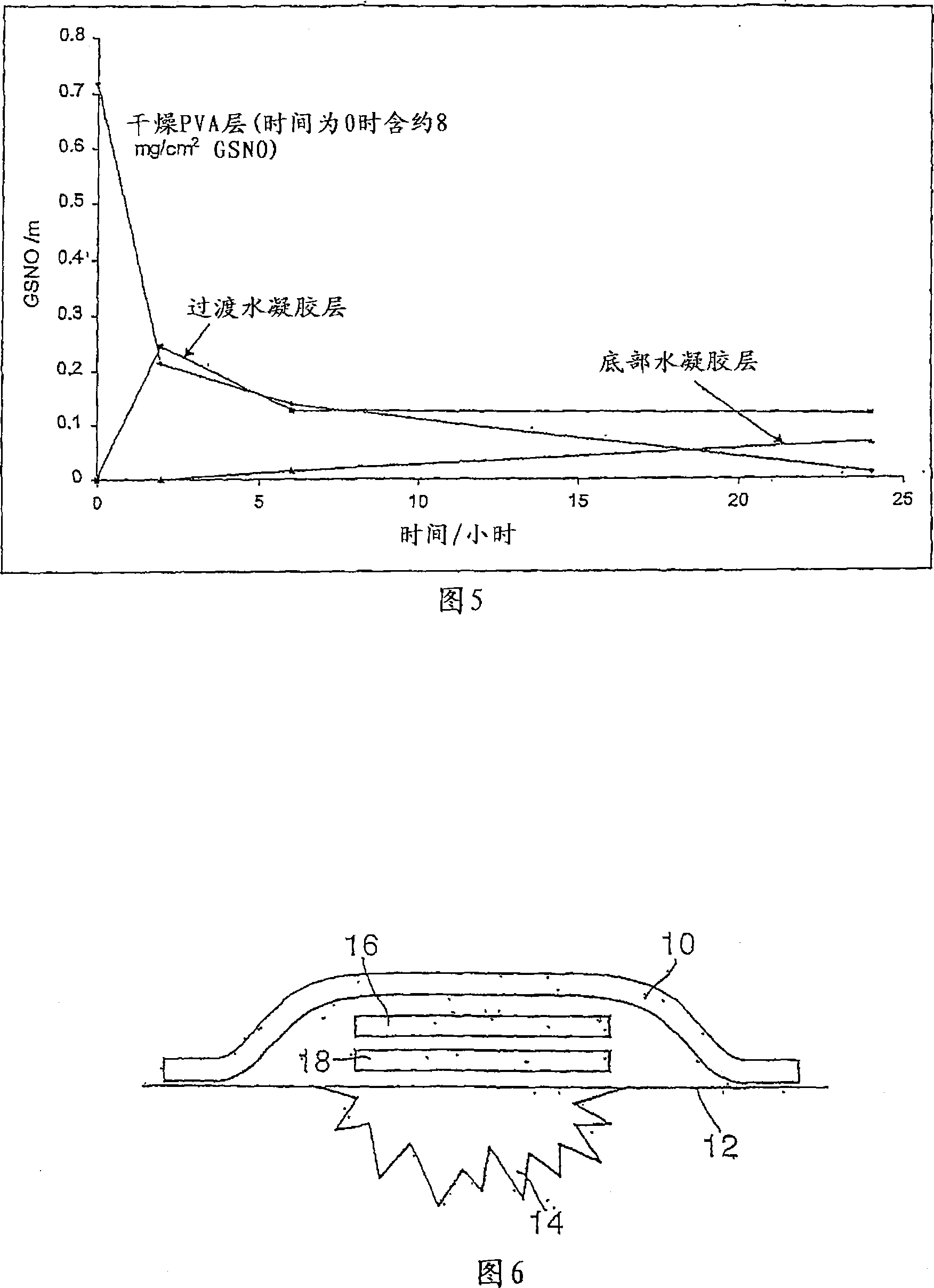
Skin Dressings
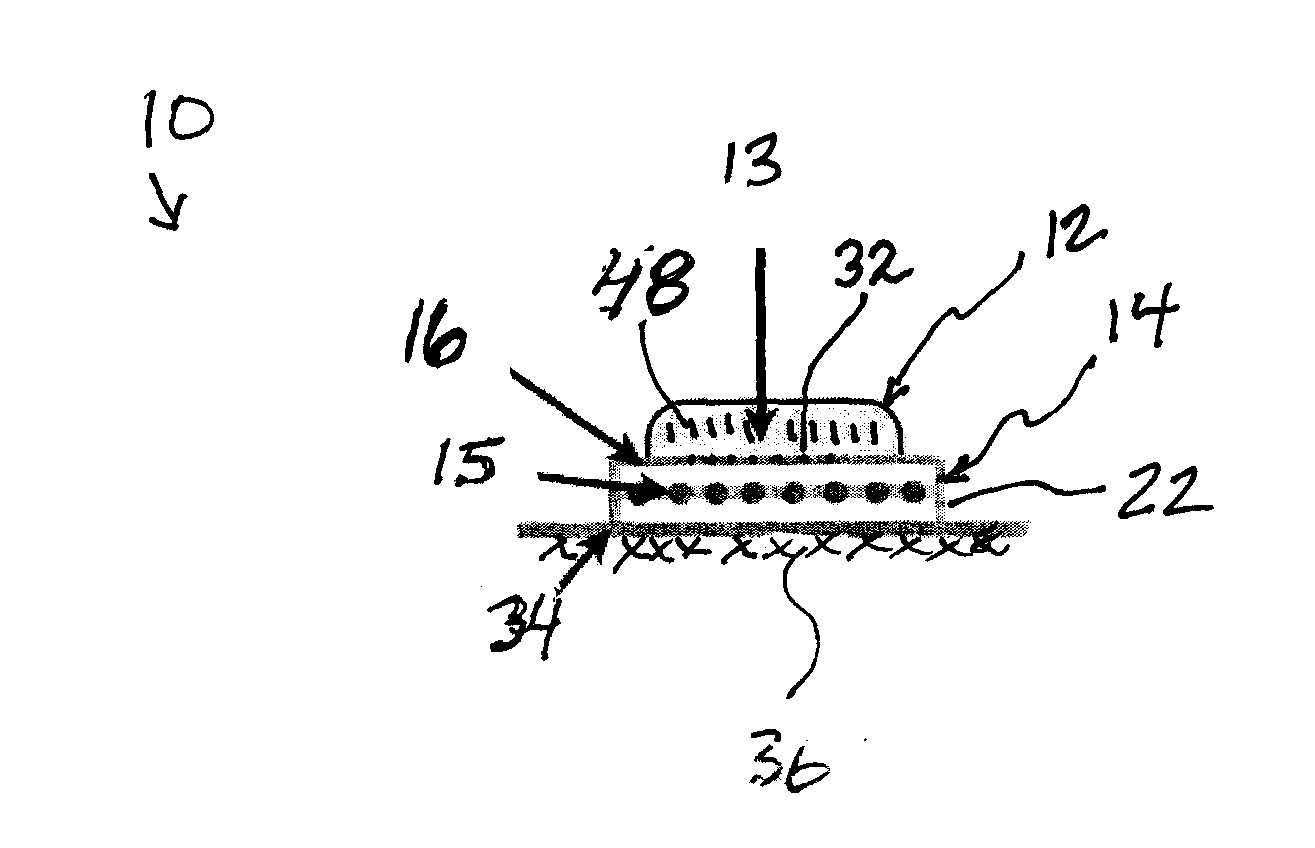
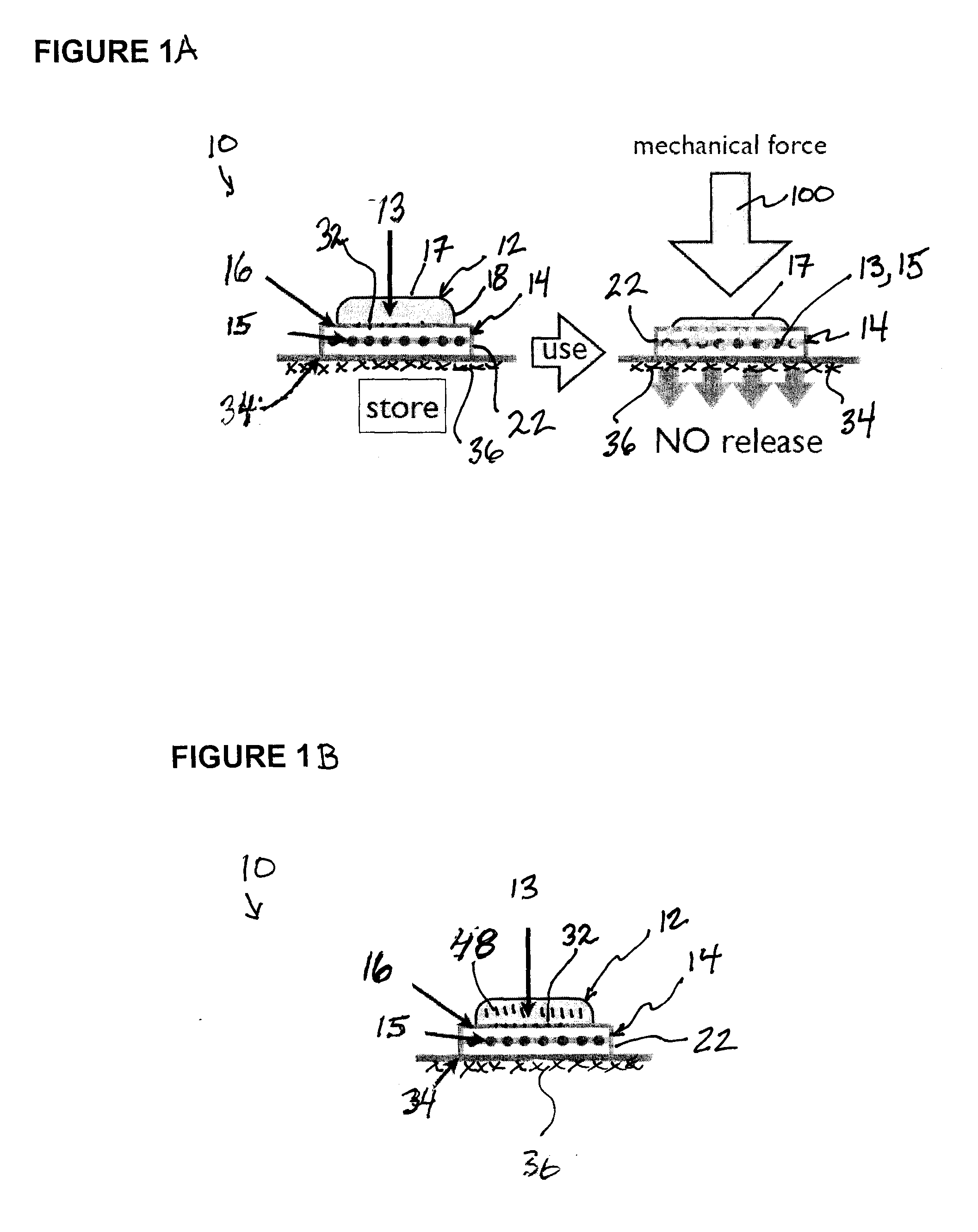
InactiveUS20090081279A1Increased collagen formationPromote cell differentiationBiocidePeptide/protein ingredientsWound healingNitroso
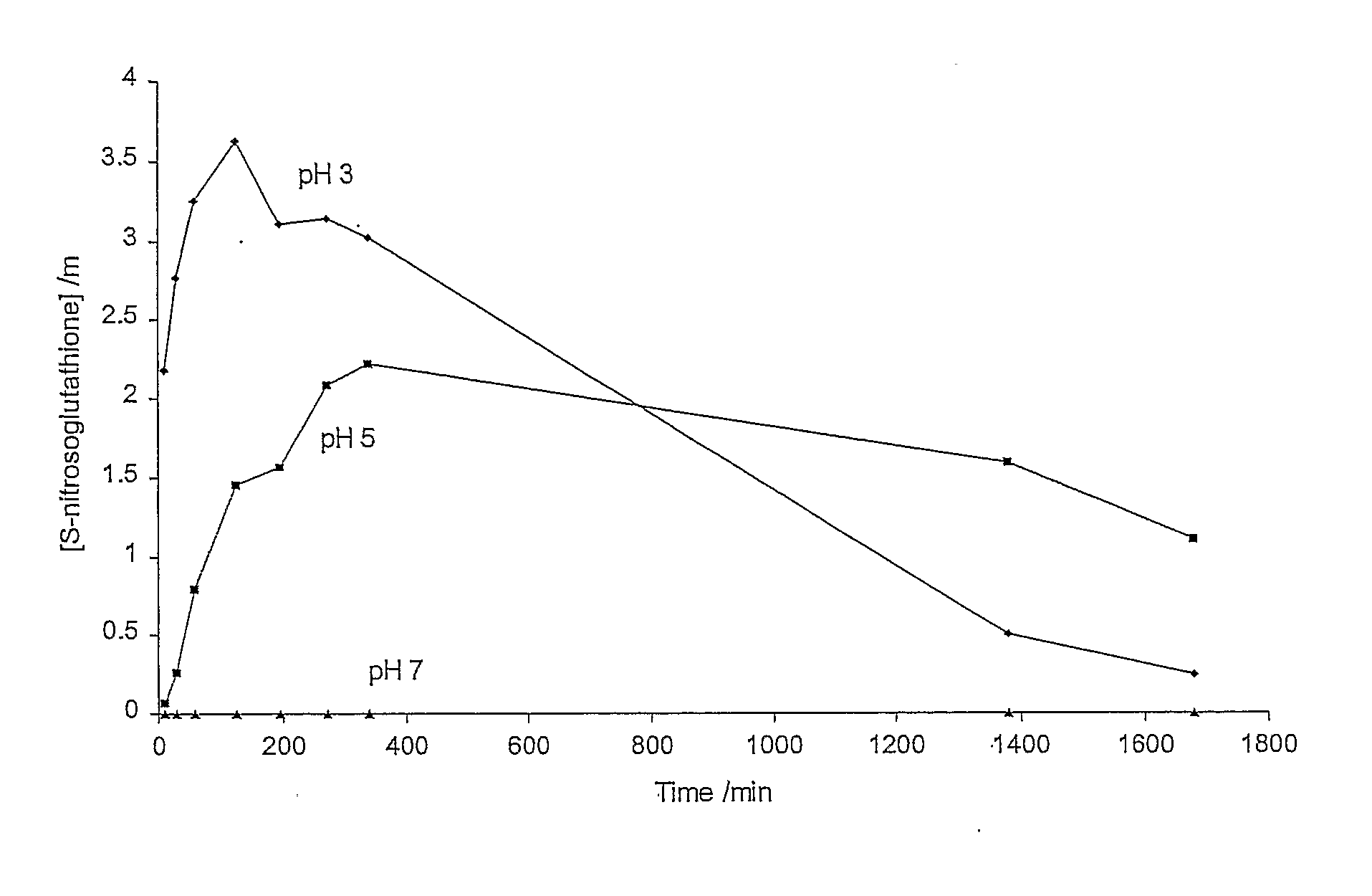
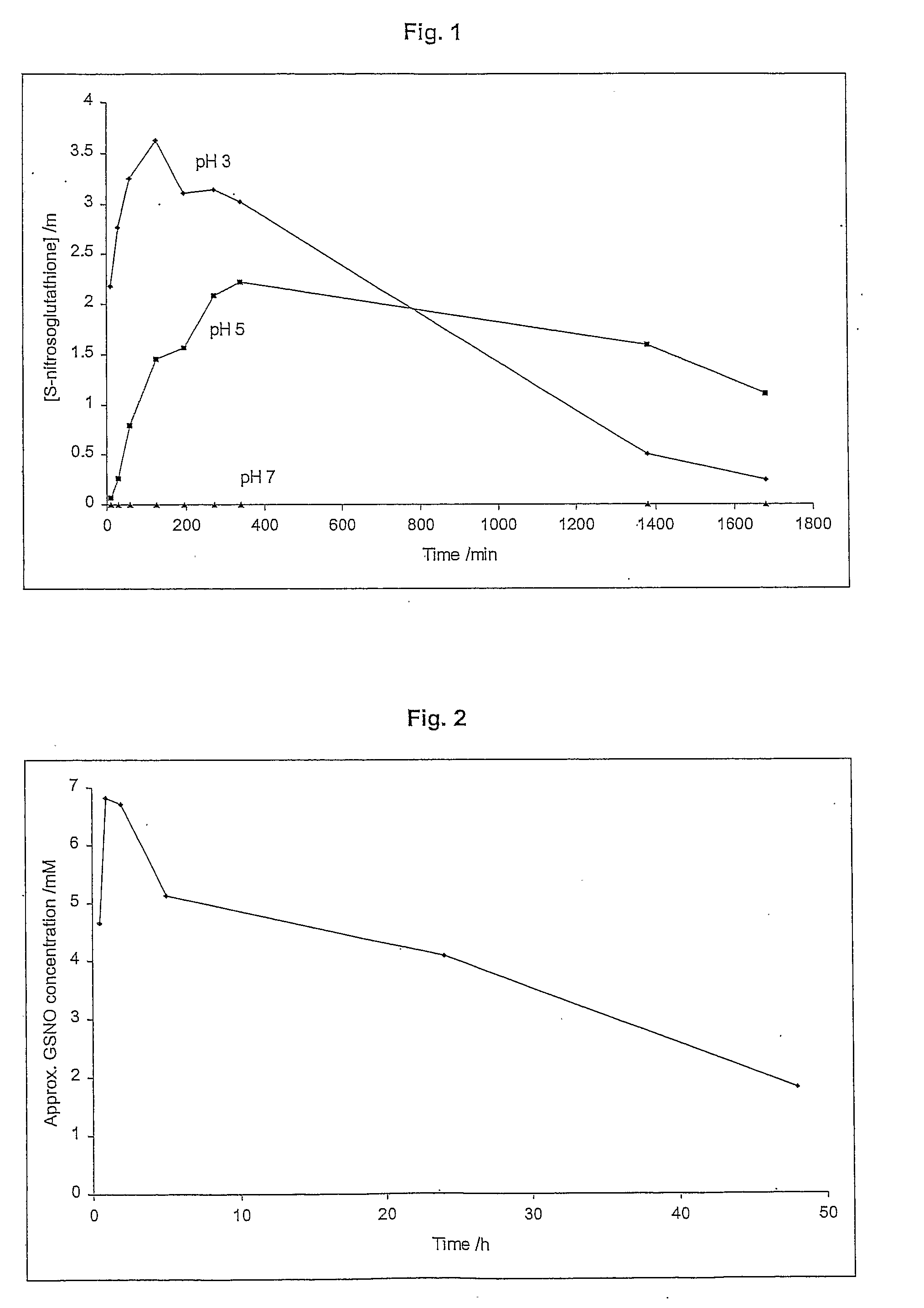
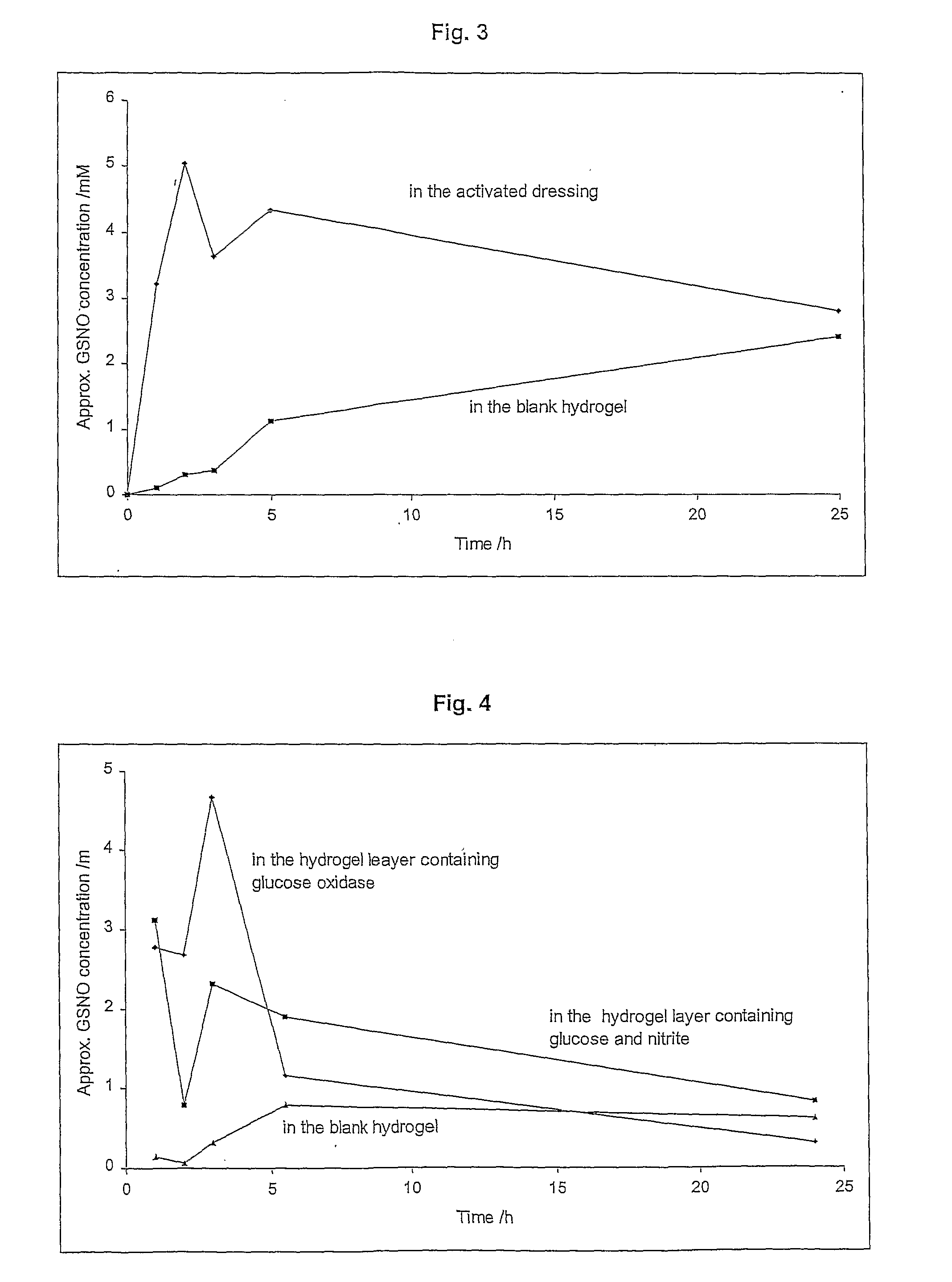
A skin dressing is adapted, on activation, to release one or more S-nitrosothiols, preferably S-nitroso-L-glutathione. S-nitrosothiols decompose spontaneously to produce nitric oxide, which has beneficial effects on tissues, particularly in wound healing.
Owner:INSENSE LIMITED
Magnetic Nanoparticles and Uses Thereof
InactiveUS20130089614A1Increase profitGood flexibilityPowder deliveryMaterial nanotechnologyMagnetite NanoparticlesSilica gel
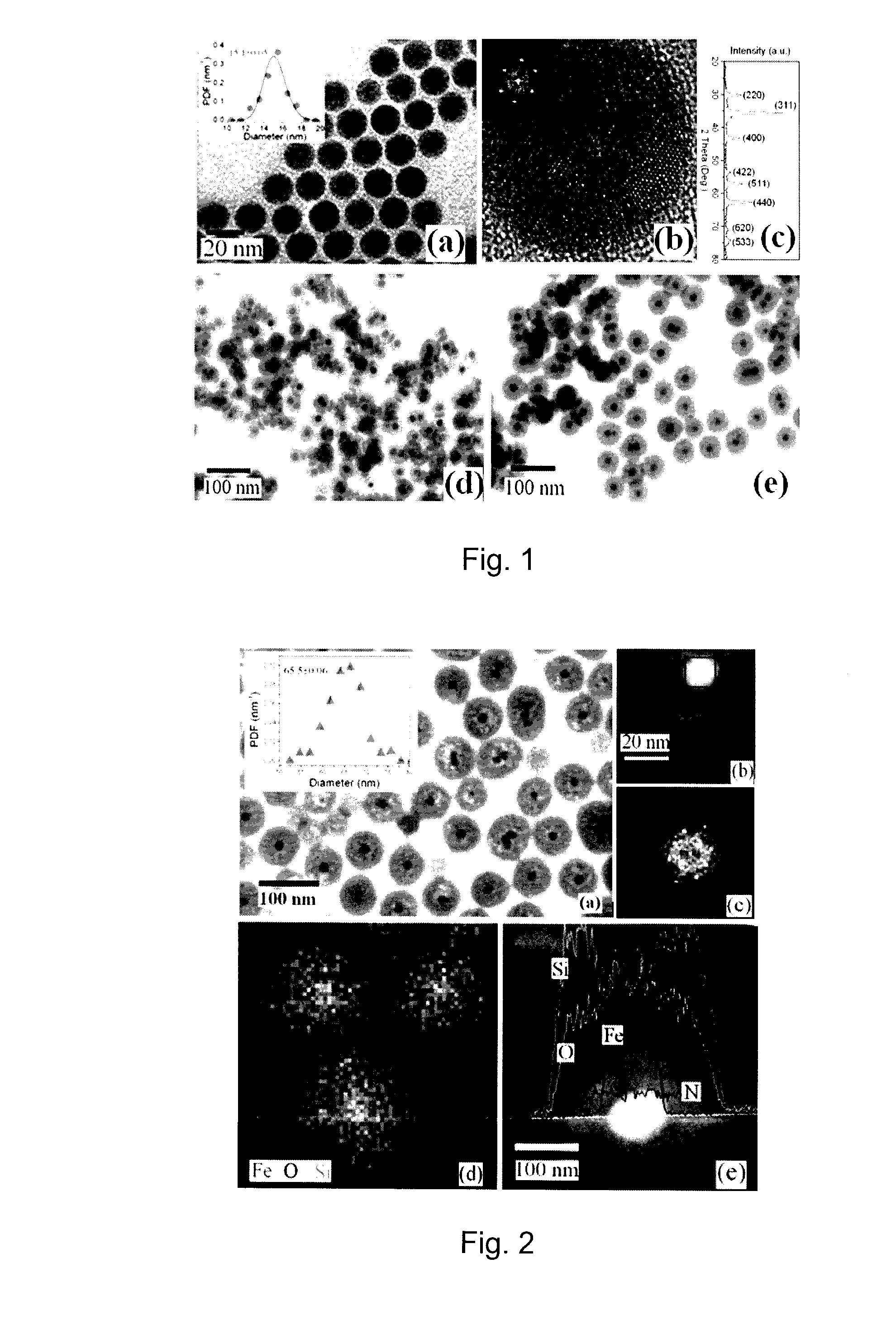
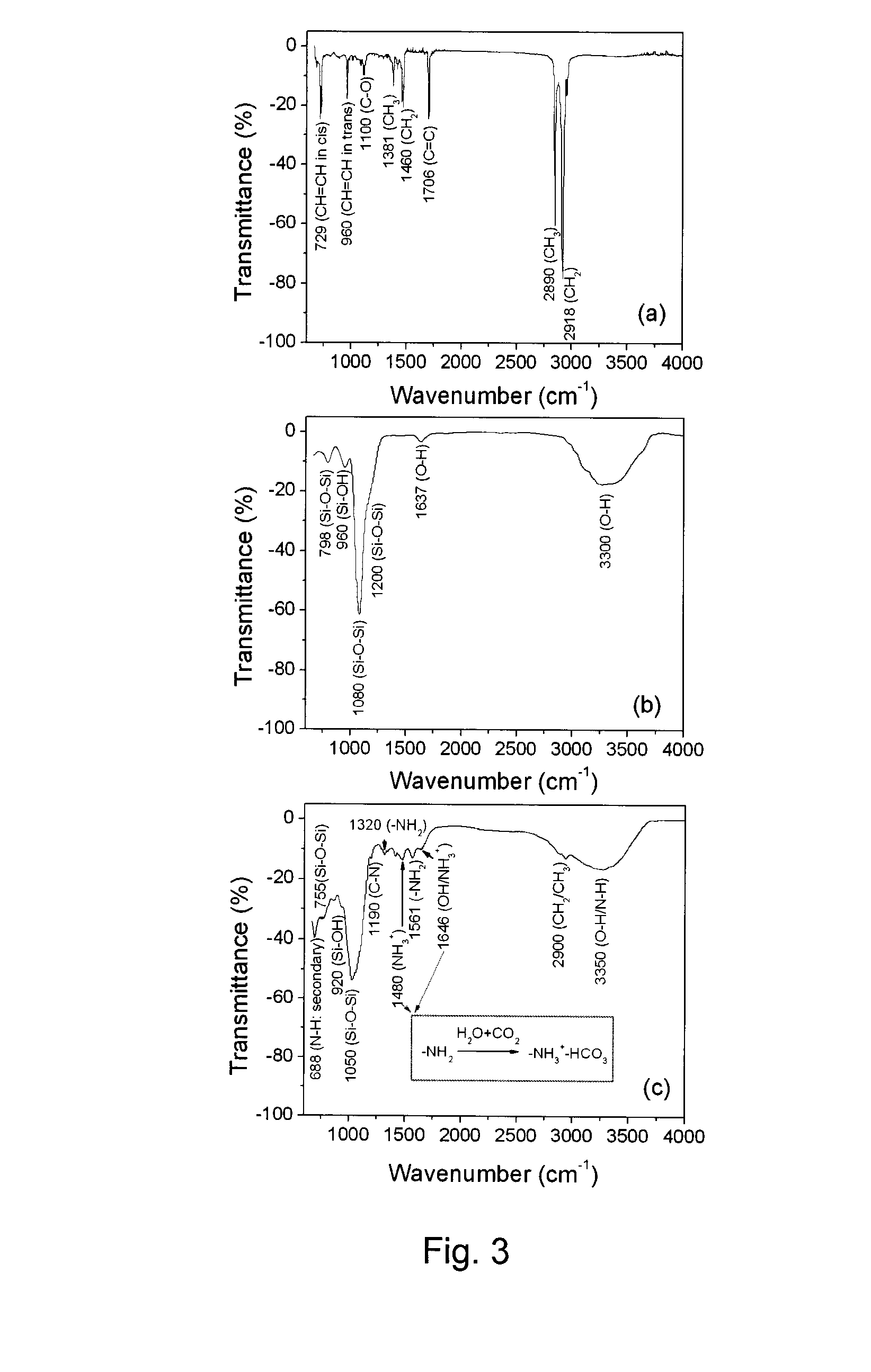
Magnetic nanoparticles are provided that have a superparamagnetic core and a nanoporous silica shell surrounding the core. The shell is functionalized with amine or S-nitrosothiol groups both inside and outside the nanopores. A process to provide such nanoparticles involves hydrolyzing tetraethoxysilane (TEOS) in a microemulsion of a superparamagnetic nanoparticle to form a superparamagnetic nanoparticle encapsulated by an incompletely hydrolyzed nanoporous silica shell, and hydrolyzing an amine-containing compound or a thiol-containing compound in situ in the presence of the incompletely hydrolyzed nanoporous silica shell before hydrolysis and densification of the silica shell is complete to functionalize the nanoporous silica shell with amine or thiol groups both inside and outside the nanopores and to maintain nanoporosity of the shell. Such magnetic nanoparticles are useful as carriers for chemical or biological species, particularly for magnetic resonance imaging, optical imaging, targeted drug delivery, cell delivery and magnetic separation applications.
Owner:ZHANG XUEFENG +1
Nitrosated and nitrosylated prostaglandins, compositions and methods of use
The invention describes novel nitrosated and / or nitrosylated prostaglandins, and novel compositions comprising at least one nitrosated and / or nitrosylated prostaglandin, and, optionally, at least one compound that donates, transfers or releases nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor, stimulates endogenous synthesis of nitric oxide or is a substrate for nitric oxide synthase, and / or at least one vasoactive agent. The invention also provides novel compositions comprising at least one prostaglandin and at least one S-nitrosothiol compound, and, optionally, at least one vasoactive agent. The prostaglandin is preferably a prostaglandin E1 compound, more preferably alprostadil, and the S-nitrosothiol compound is preferably S-nitrosoglutathione. The invention also provides methods for treating or preventing sexual dysfunctions in males and females, for enhancing sexual responses in males and females, and for treating or preventing cerebrovascular disorders, cardiovascular disorders, benign prostatic hyperplasia (BPH), glaucoma, peptic ulcers or for inducing abortions.
Owner:NITROMED
Skin dressings
InactiveUS20100197802A1Increase ratingsQuick buildBiocideSulfur/selenium/tellurium active ingredientsNitrateDermal dressing
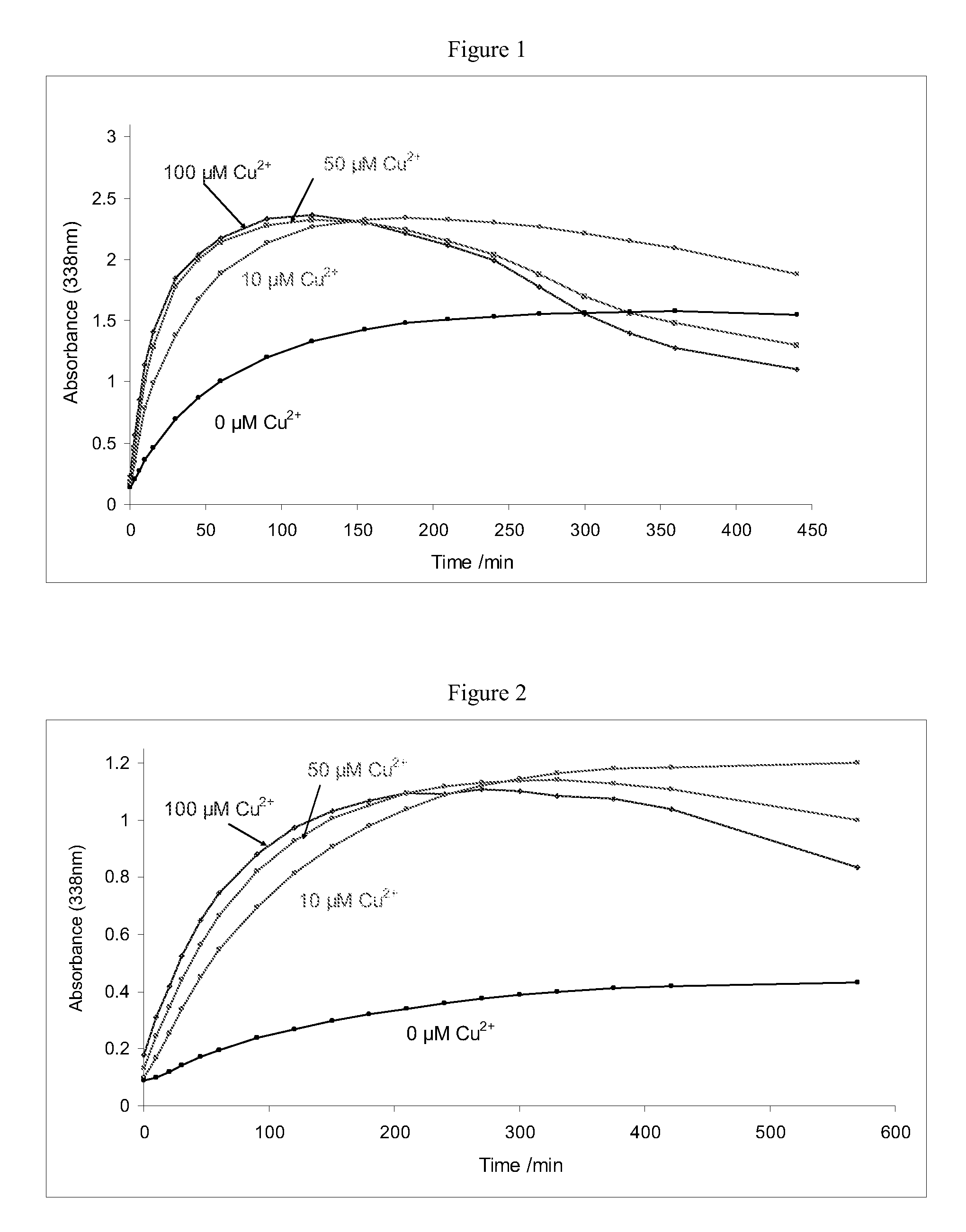
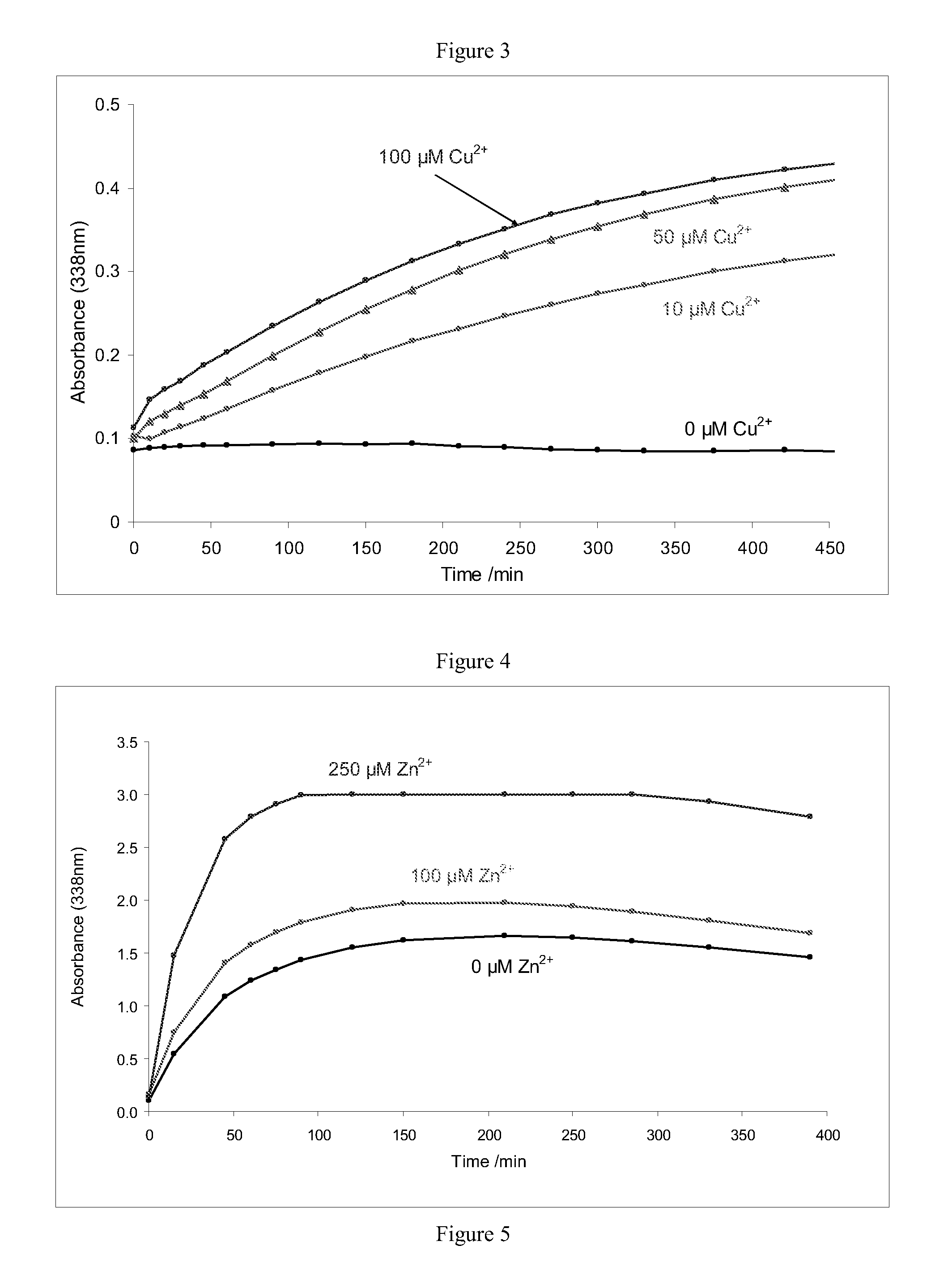
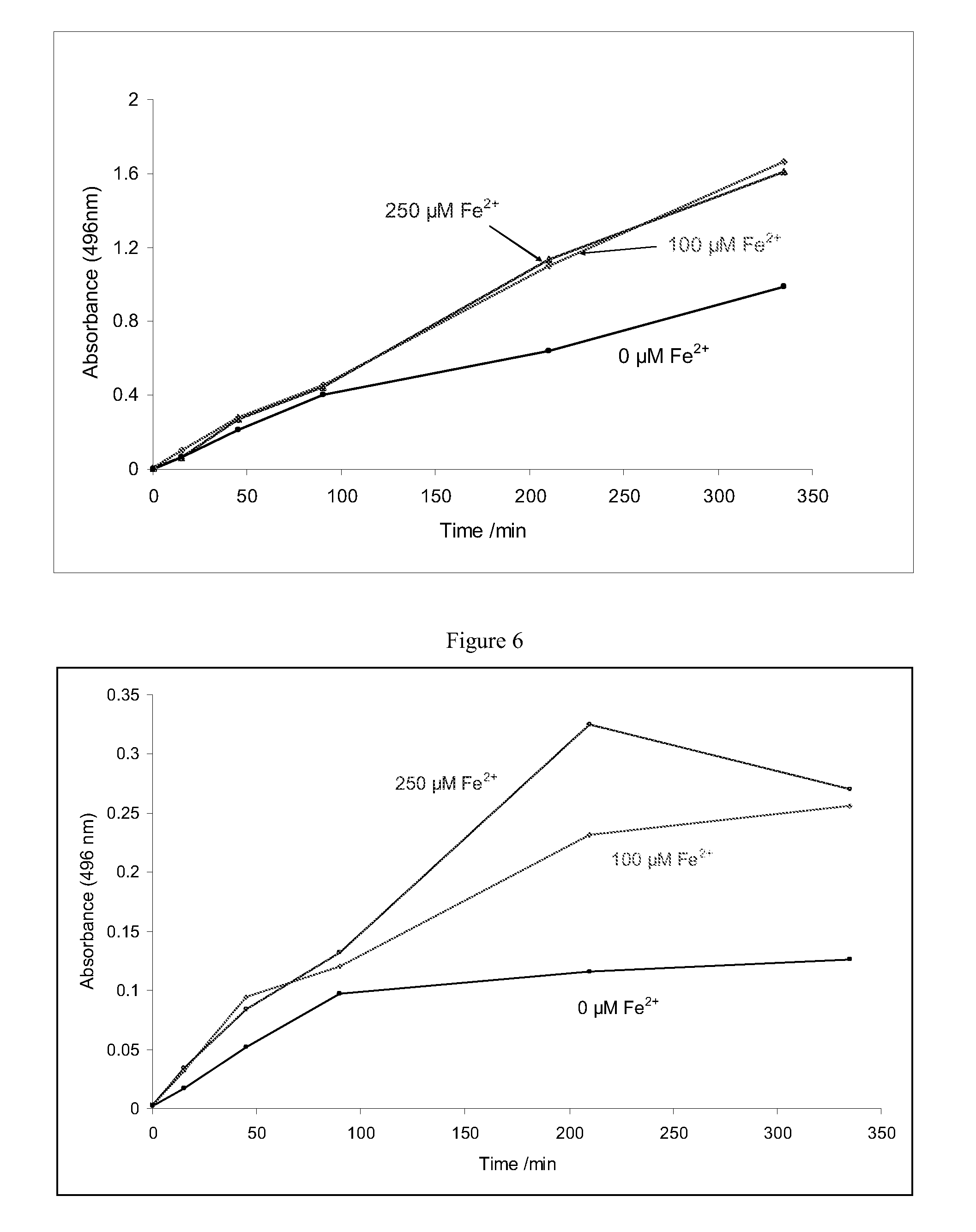
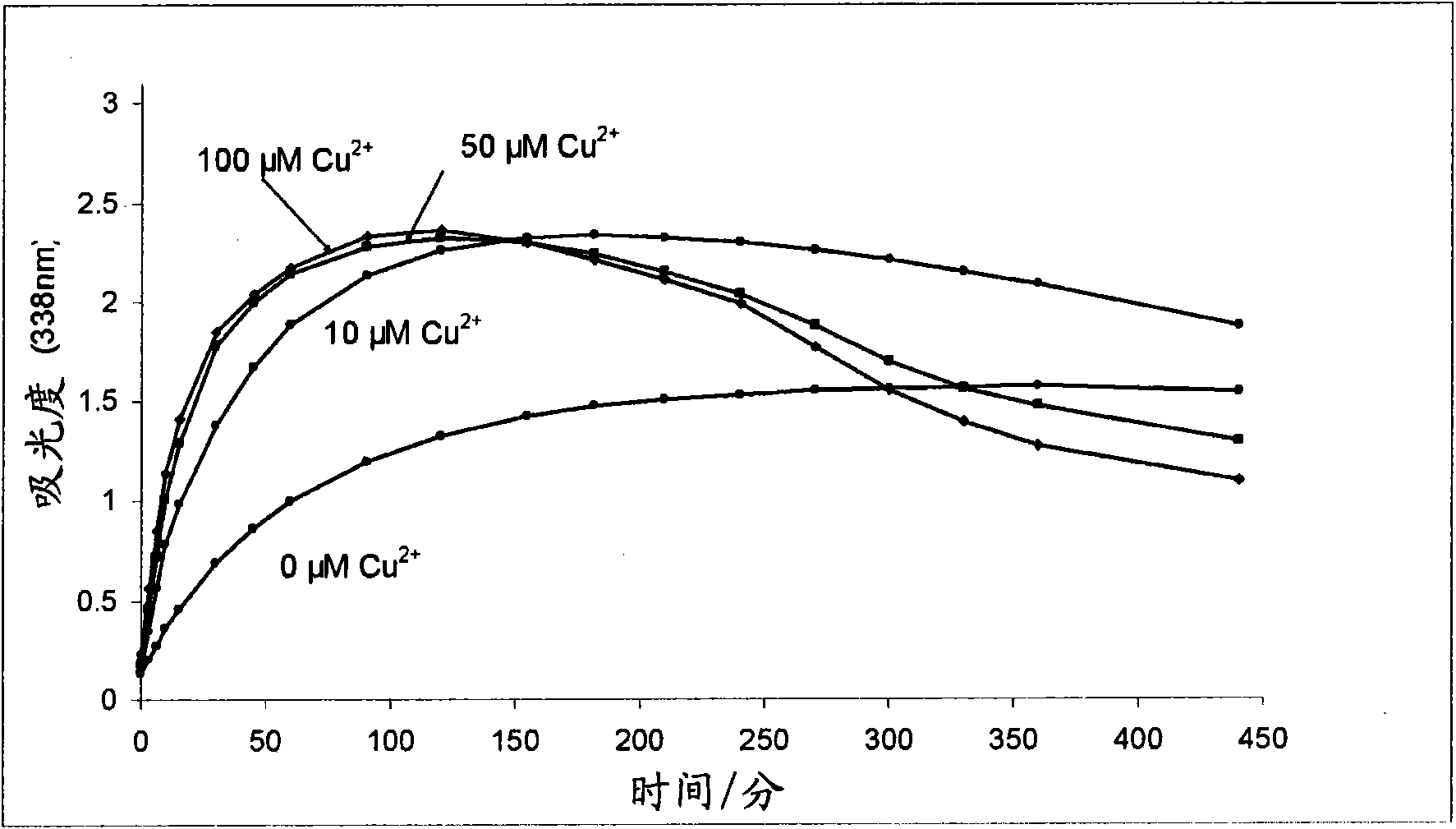
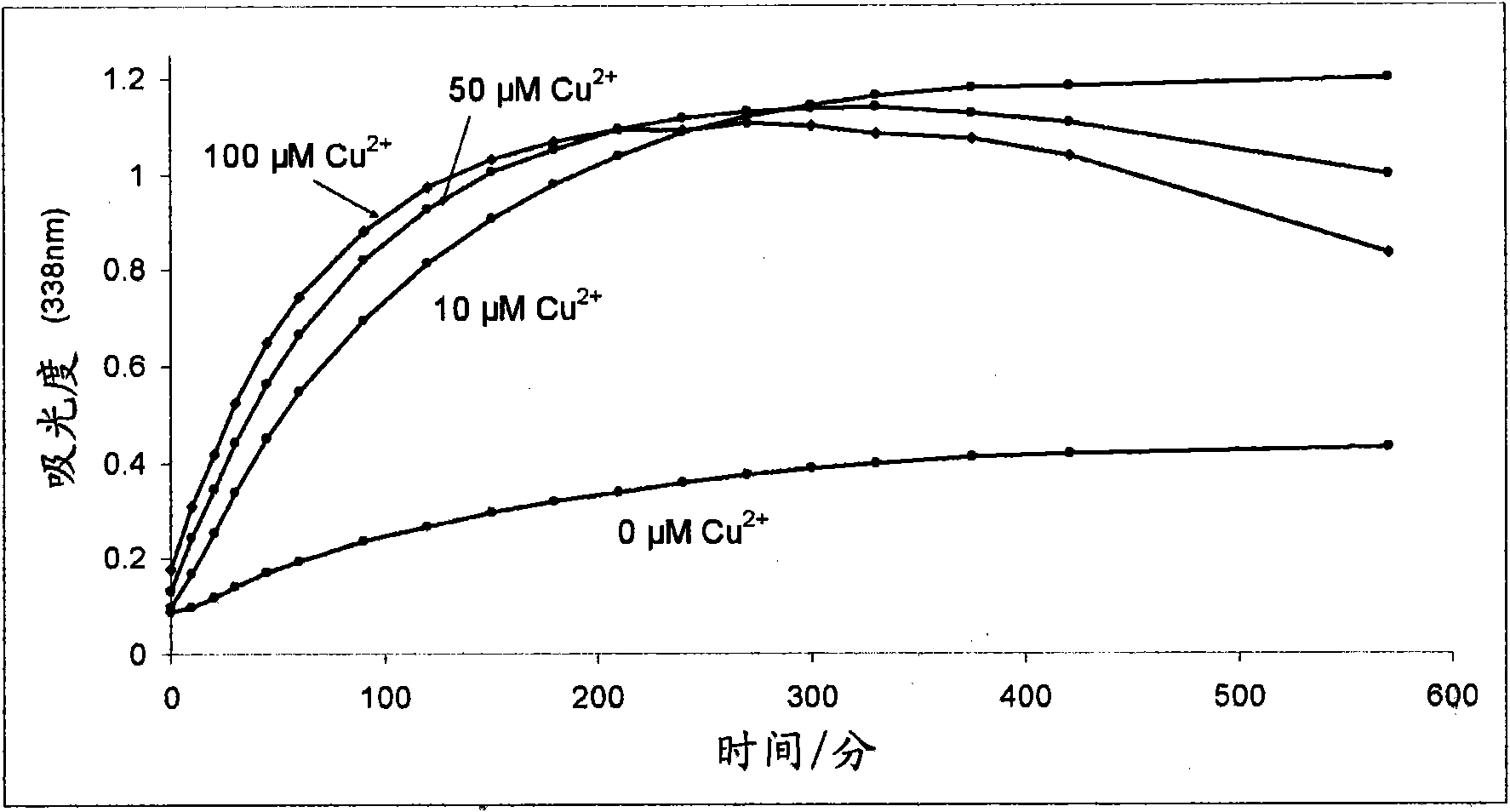
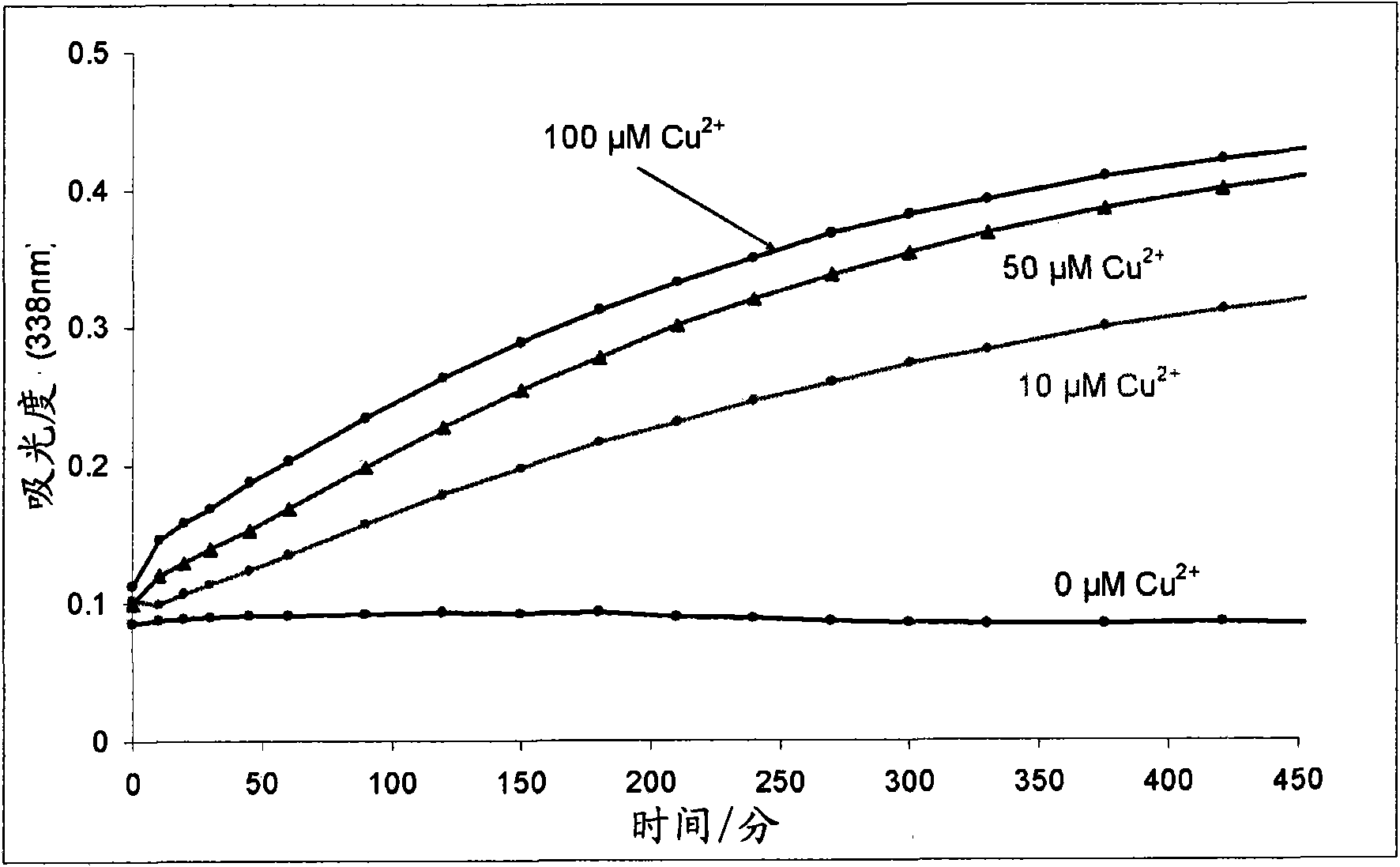
Improvements relating to skin dressing A skin dressing adapted, on activation, to generate one or more S-nitrosothiols by reaction between a thiol and a nitrate salt in the dressing, the skin dressing comprising a source of Cu2+, Zn2+ and / or Fe2+ ions for delivery of nitric oxide to a body site is provided.
Owner:INSENSE LIMITED
Nitric oxide-releasing s-nitrosothiol-modified silica particles and methods of making the same
ActiveUS20130344334A1Silicon organic compoundsSynthetic resin layered productsSilica particleSilanes
Provided according to some embodiments of the invention are methods of forming co-condensed silica particles. In some embodiments, the methods include reacting a thiol-containing silane and a backbone alkoxysilane in a reaction solution that comprises water to form thiol-functionalized co-condensed silica particles, wherein the thiol-functionalized co-condensed silica particles include a polysiloxane matrix and at least some of thiol groups are present within the polysiloxane matrix; and reacting the thiol-functionalized co-condensed silica particles with a nitrosating agent to provide the S-nitrosothiol-functionalized co-condensed silica particles. In some embodiments, provided are S-nitrosothiol-functionalized co-condensed silica particles.
Owner:NAVAN INC +1
Stents coated with no- and s-nitrosothiol-eluting hydrophlic polymeric blends
InactiveUS20100112033A1Efficient outcomeBiocideInorganic active ingredientsHydrophilic polymersPolyvinyl alcohol
This invention relates to stents coated with hydrophilic polymers containing S-nitrosothiols, which are able to provide local delivery of both nitric oxide and S-nitrosothiols by diffusion. This device is intended for coronary angioplasty applications with the purpose of inhibiting acute and chronic restenosis and refers to processes of stent coating with hydrophilic polymers containing incorporated S-nitrosothiols. This invention refers to an intracoronary implant device used in medical procedures, and introduces new S nitrosothiol-eluting stents coated with hydrophilic polymer multilayers. The hydrophilic polymers used for coating are polyvinyl alcohol, polyvinylpirrolidone and polyvinyl alcohol / polyvinylpirrolidone, polyvinyl alcohol / polyethylene glycol, polyvinylpirrolidone / polyethylene glycol and polyvinyl alcohol / polyvinylpirrolidone / polyethylene glycol blends. The S-nitrosothiols incorporated to the polymer coatings are mainly primary S-nitrosothiols, characterized by the fact of the nitric oxide (NO) molecule being covalently bound to a sulfur (S) atom which, in turn, is linked to a primary carbon in the molecule's structure, thus constituting the S—NO chemical group. The coating processes include immersion of the stents in polymer solutions containing S-nitrosothiols and nebulization processes of the polymer solutions containing S-nitrosothiols onto the stent surface.
Owner:UNIV ESTADUAL DE CAMPINAS UNICAMP +1
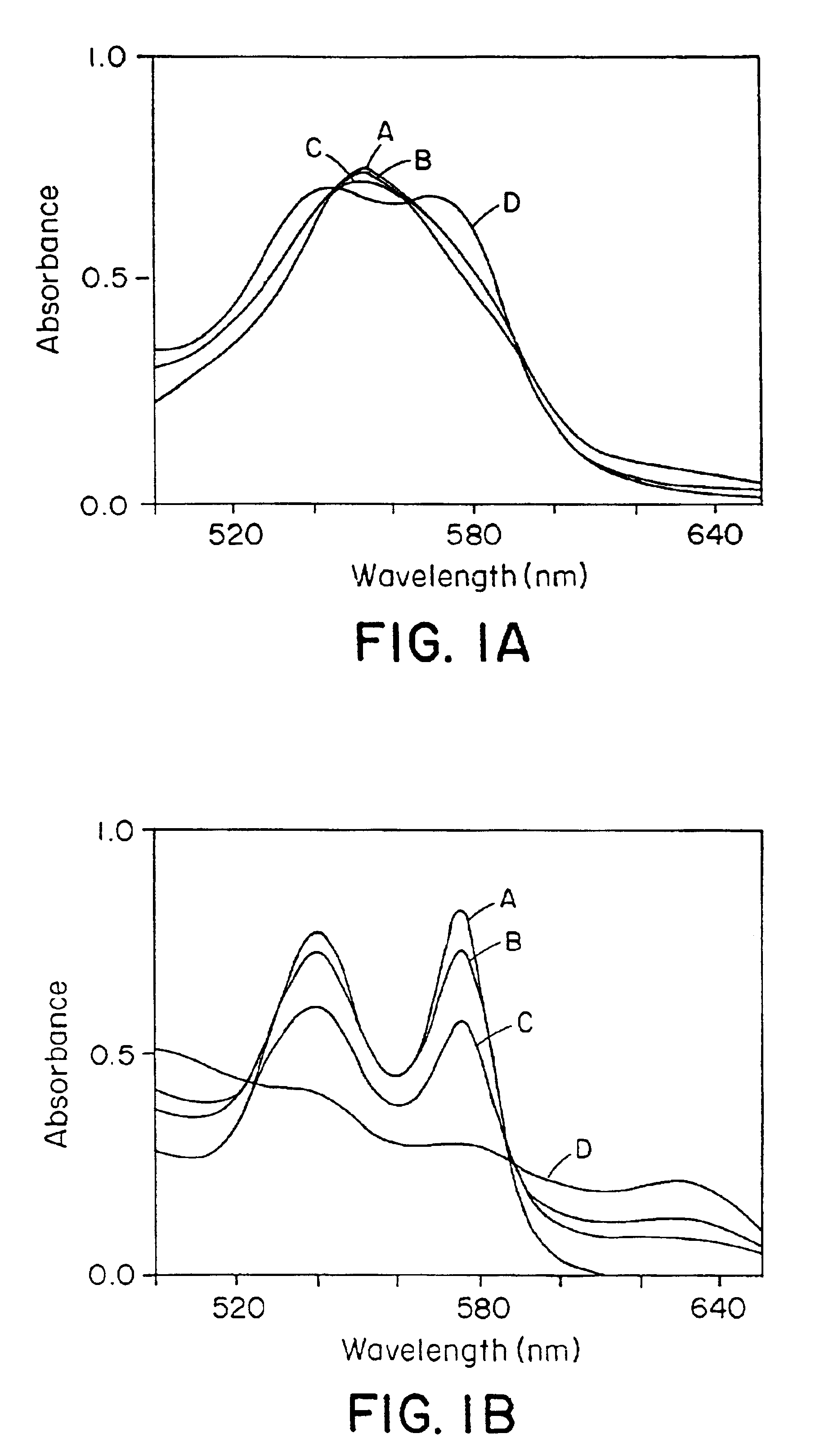
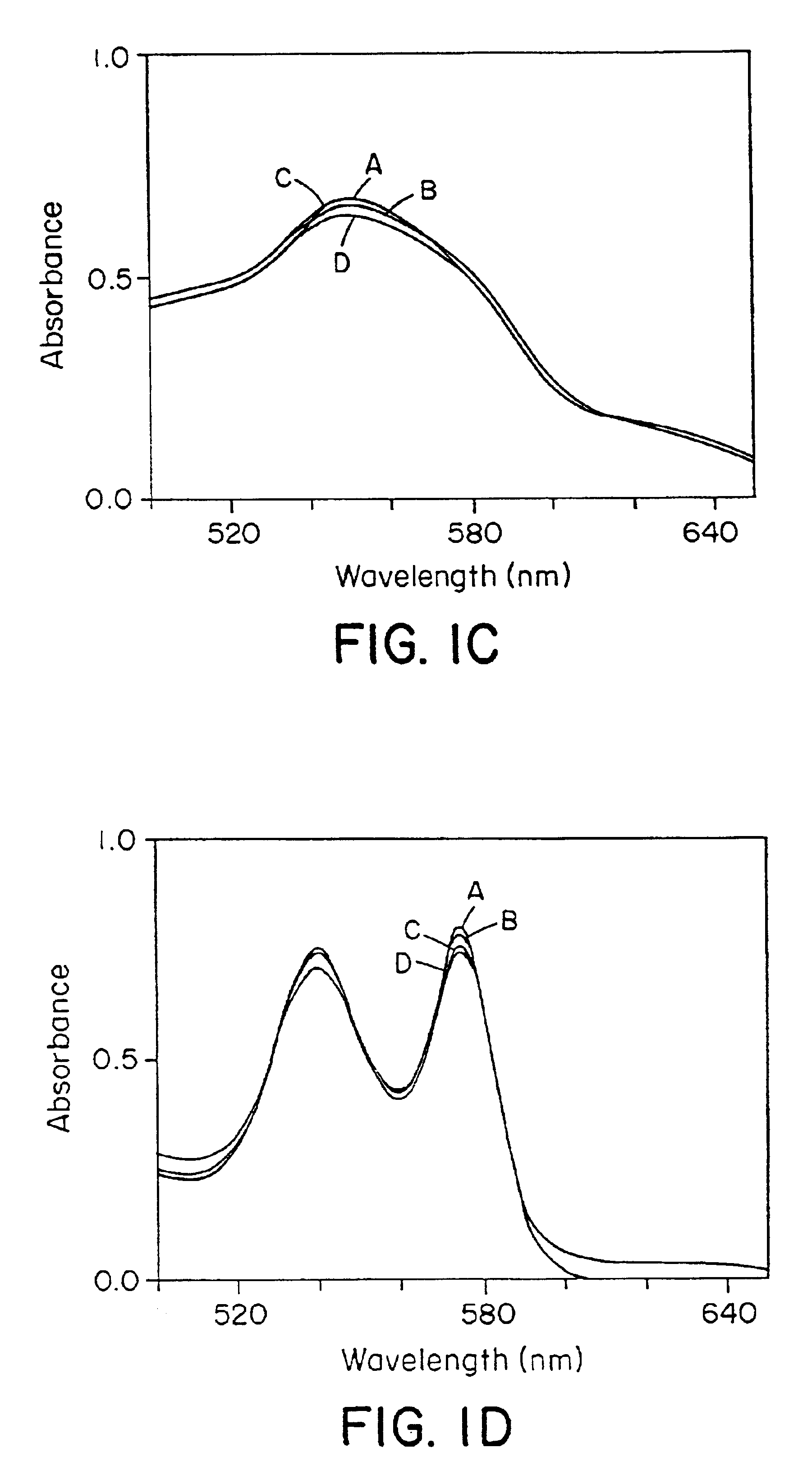
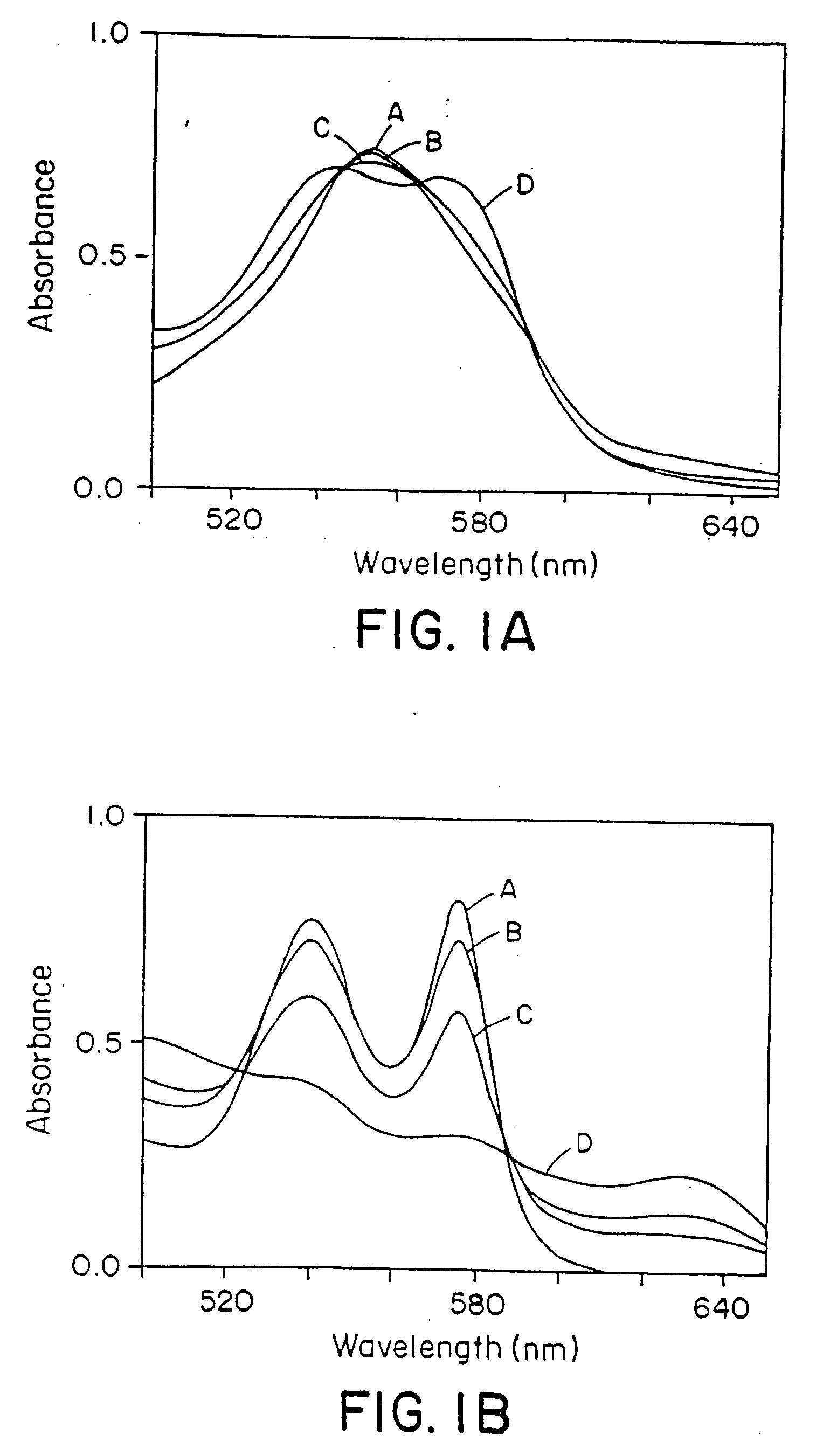
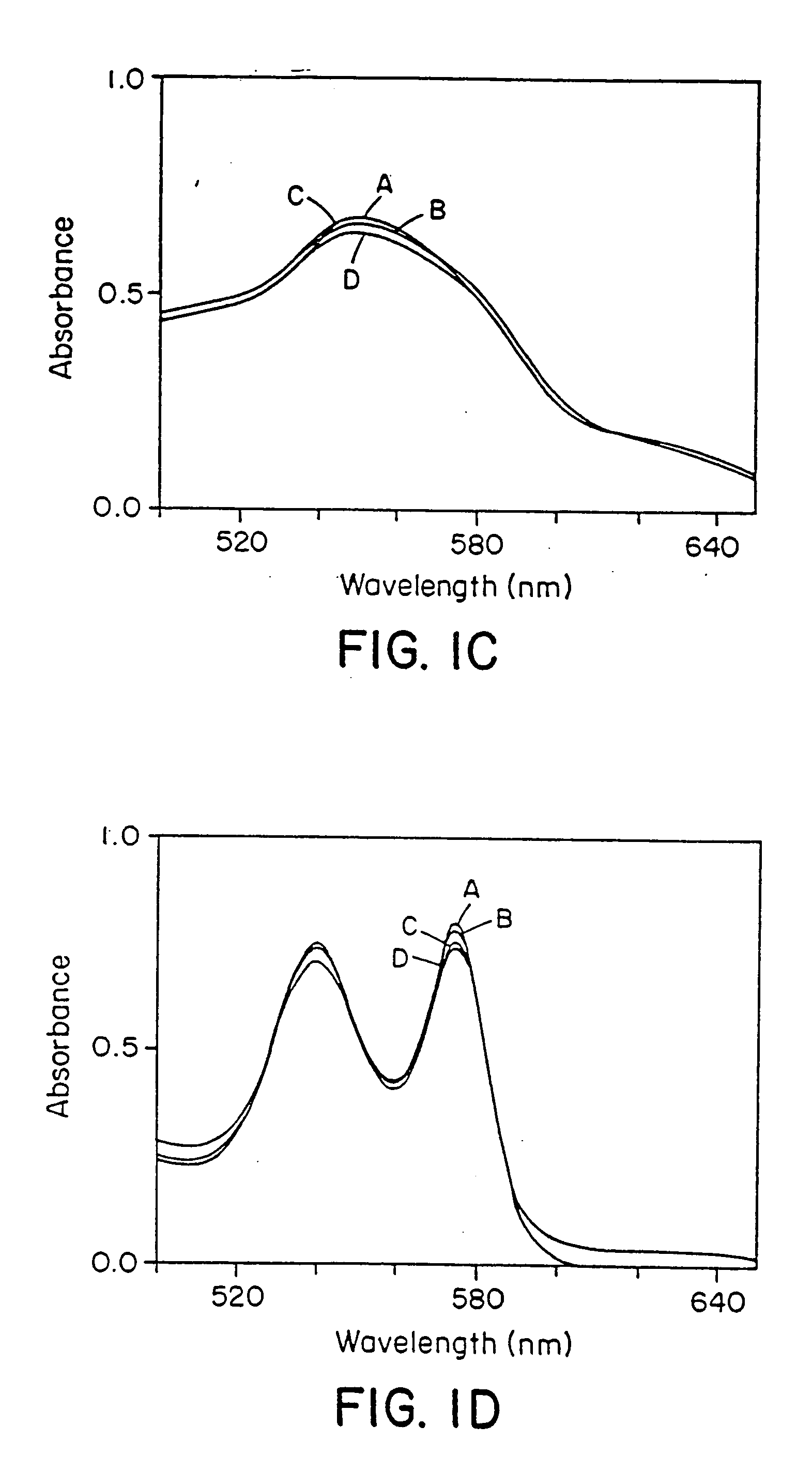
Use of S-nitrosothiol signaling to treat disordered control of breathing
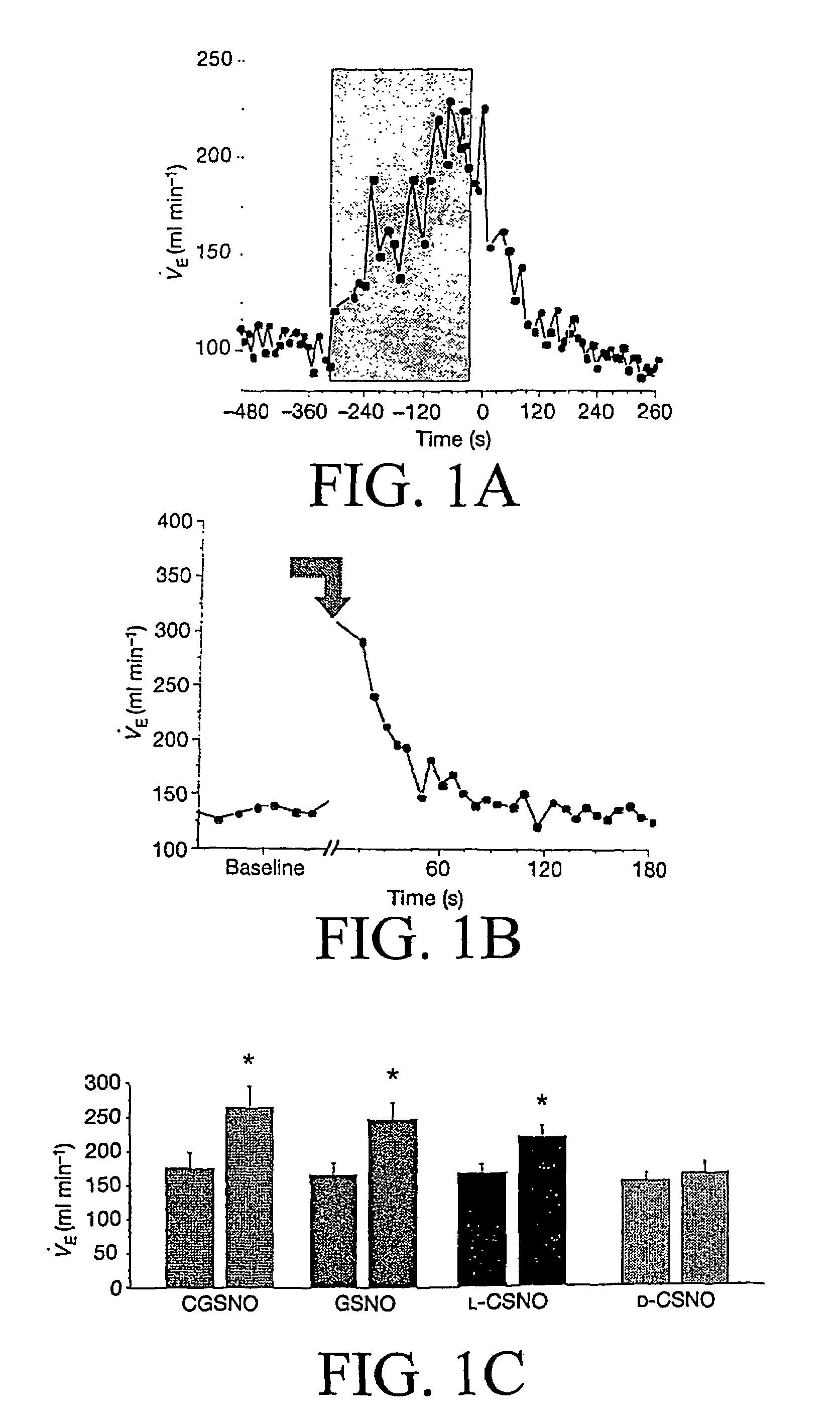
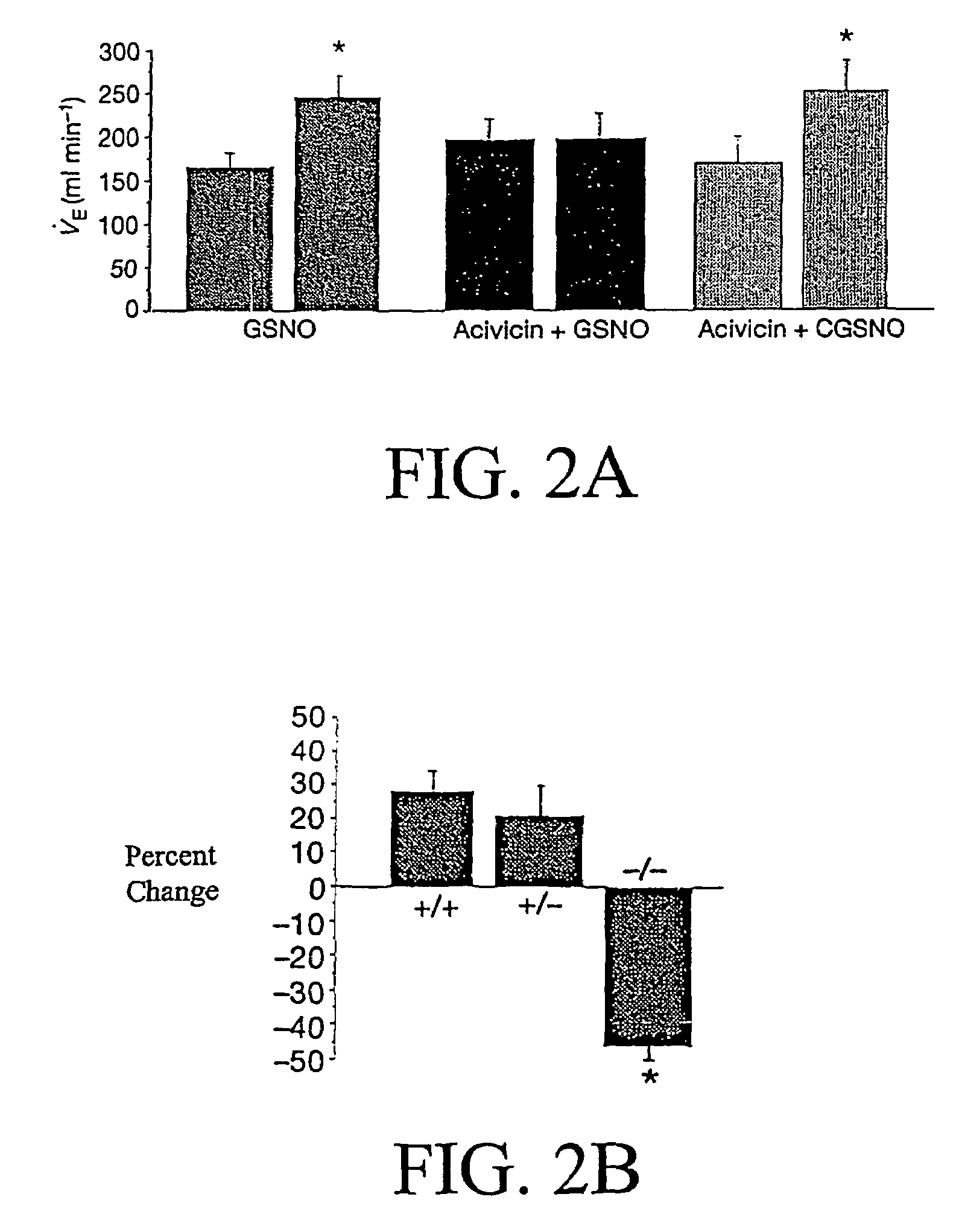
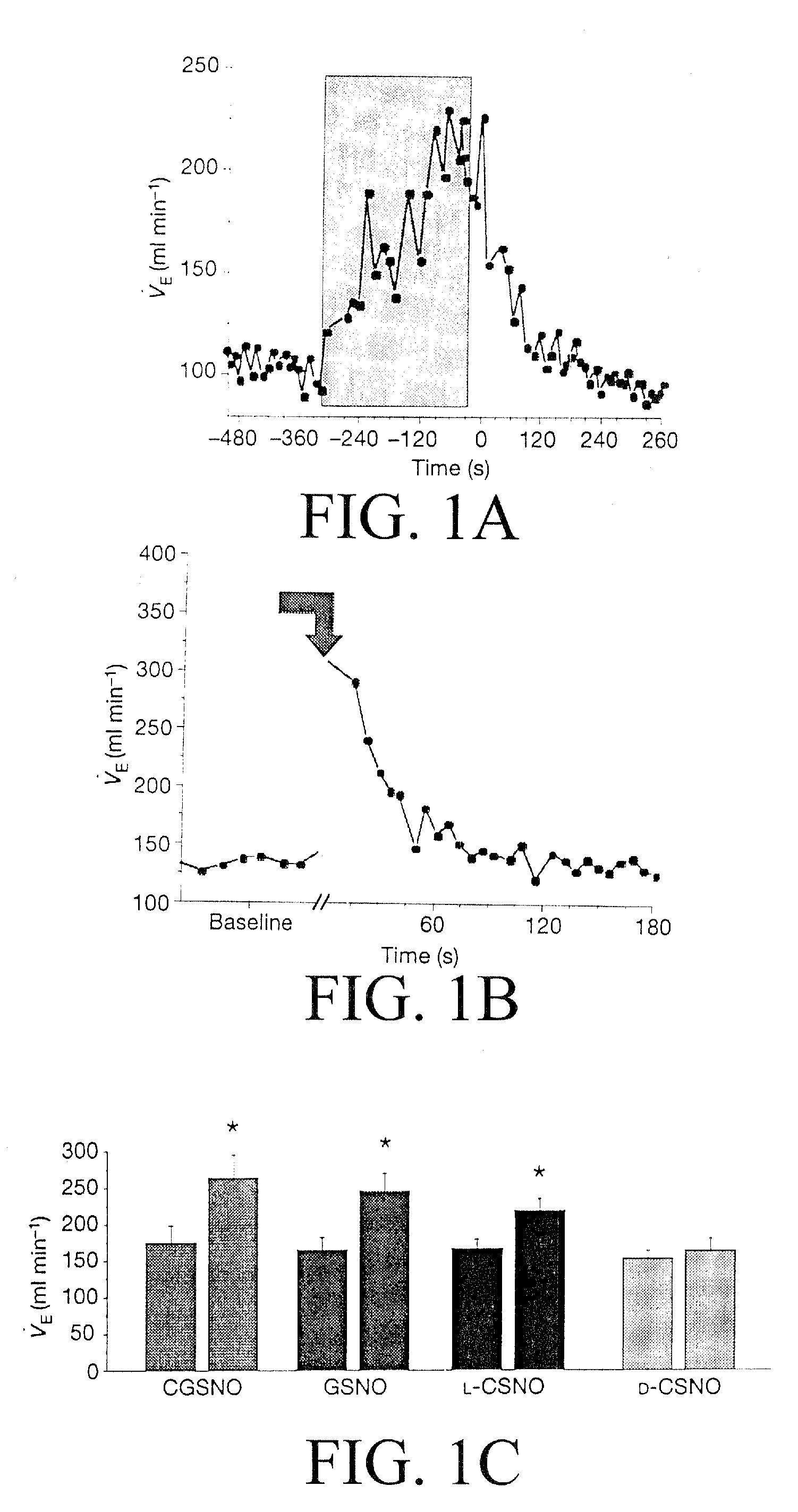
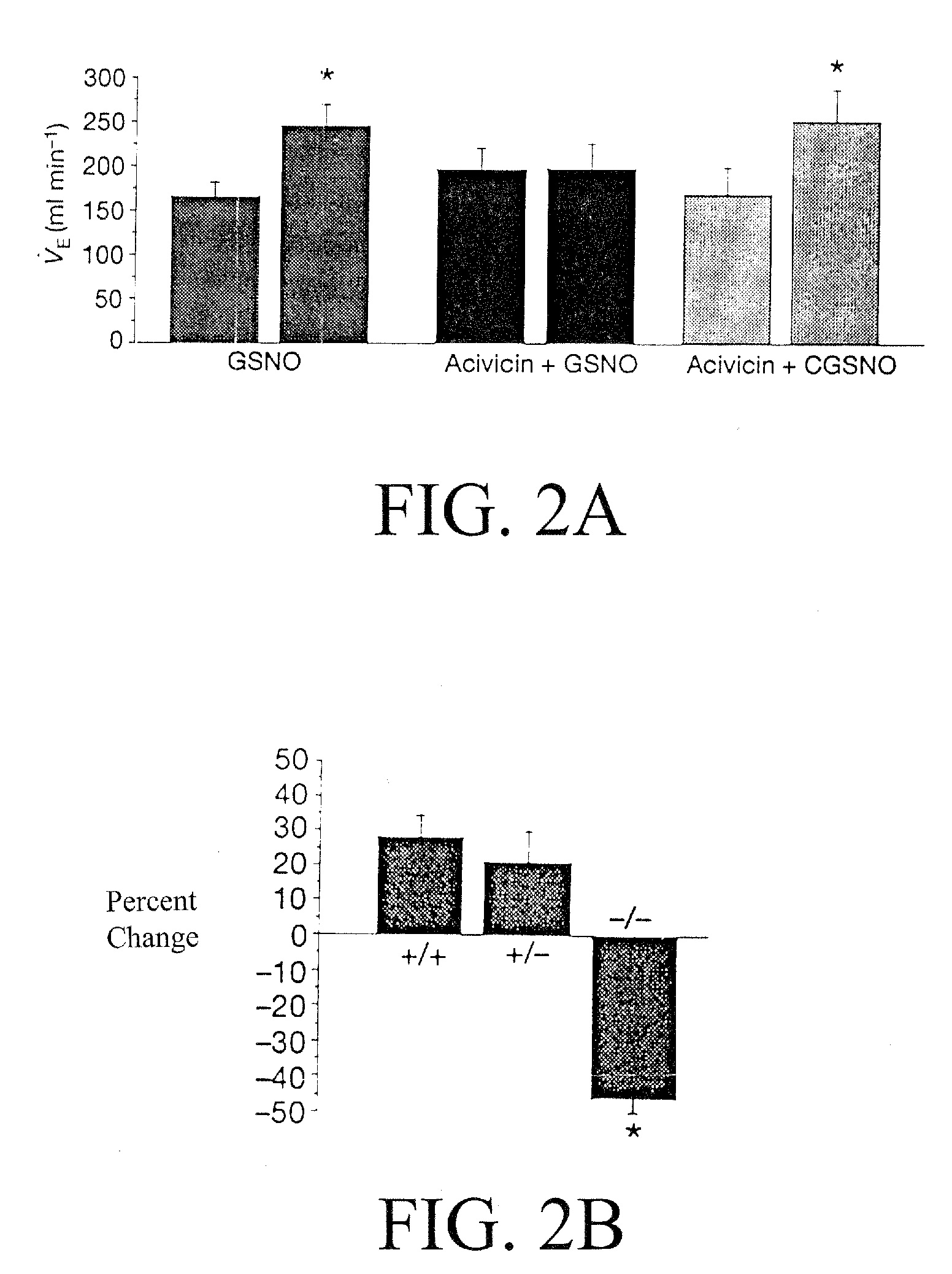
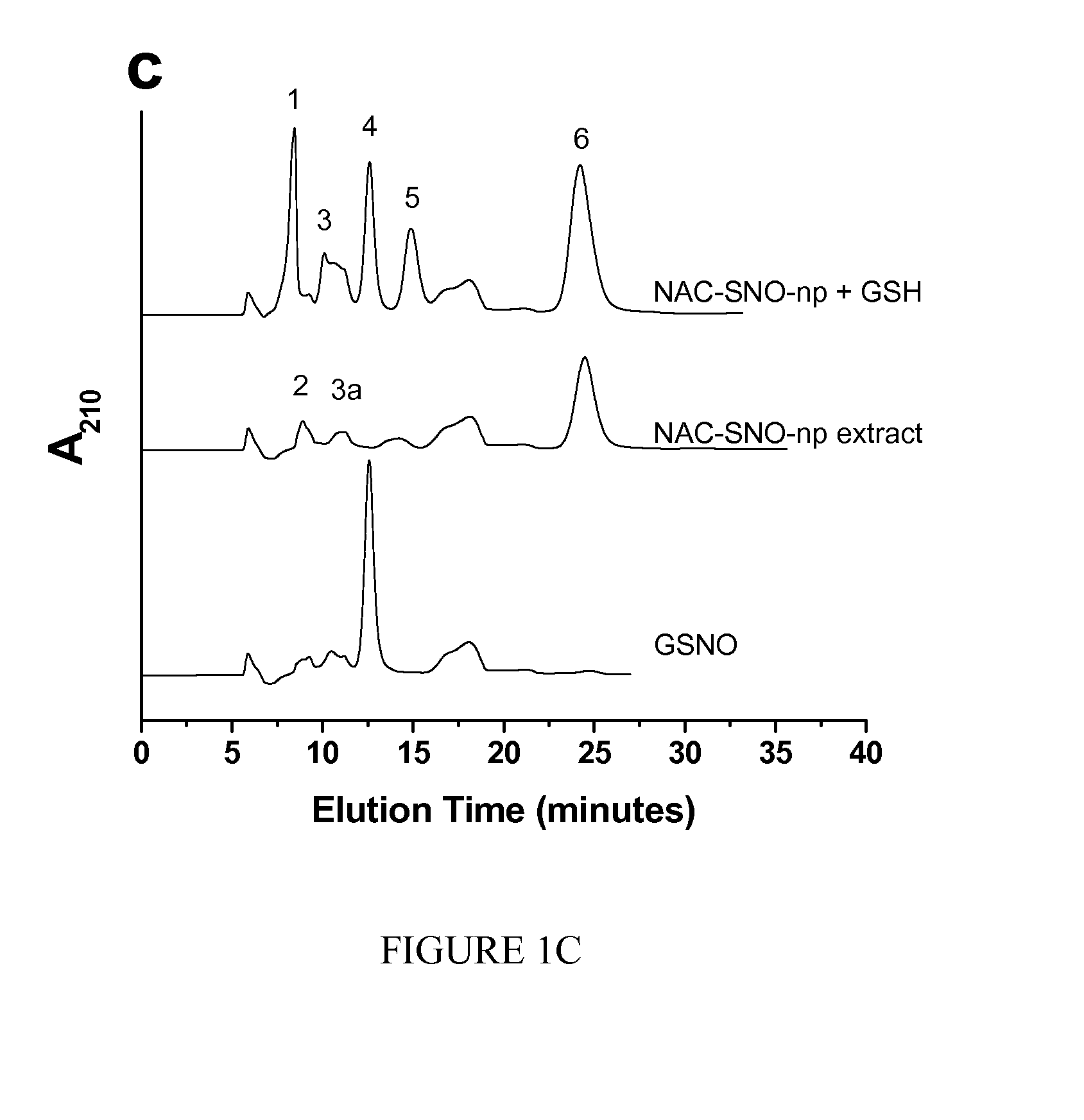
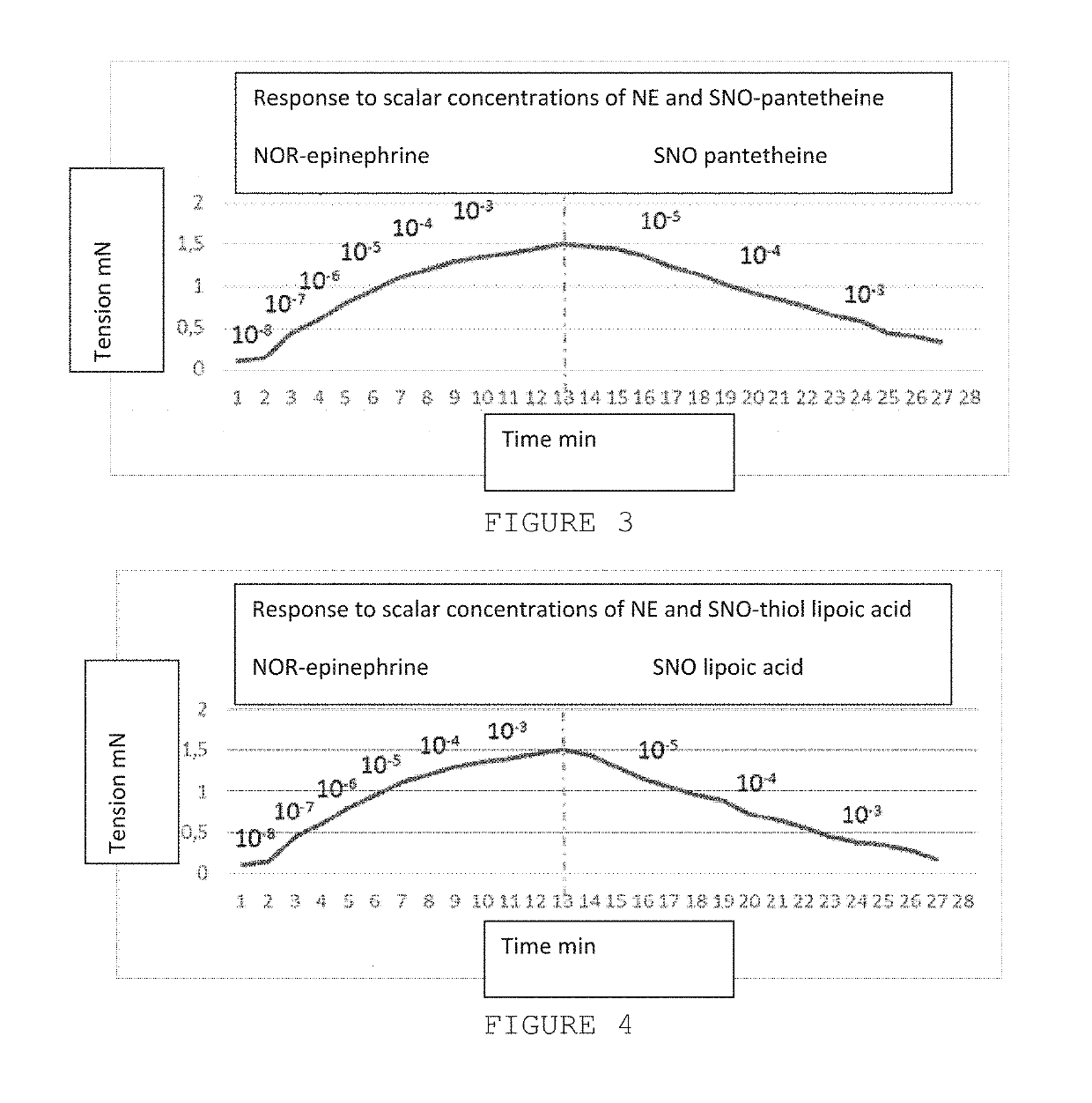
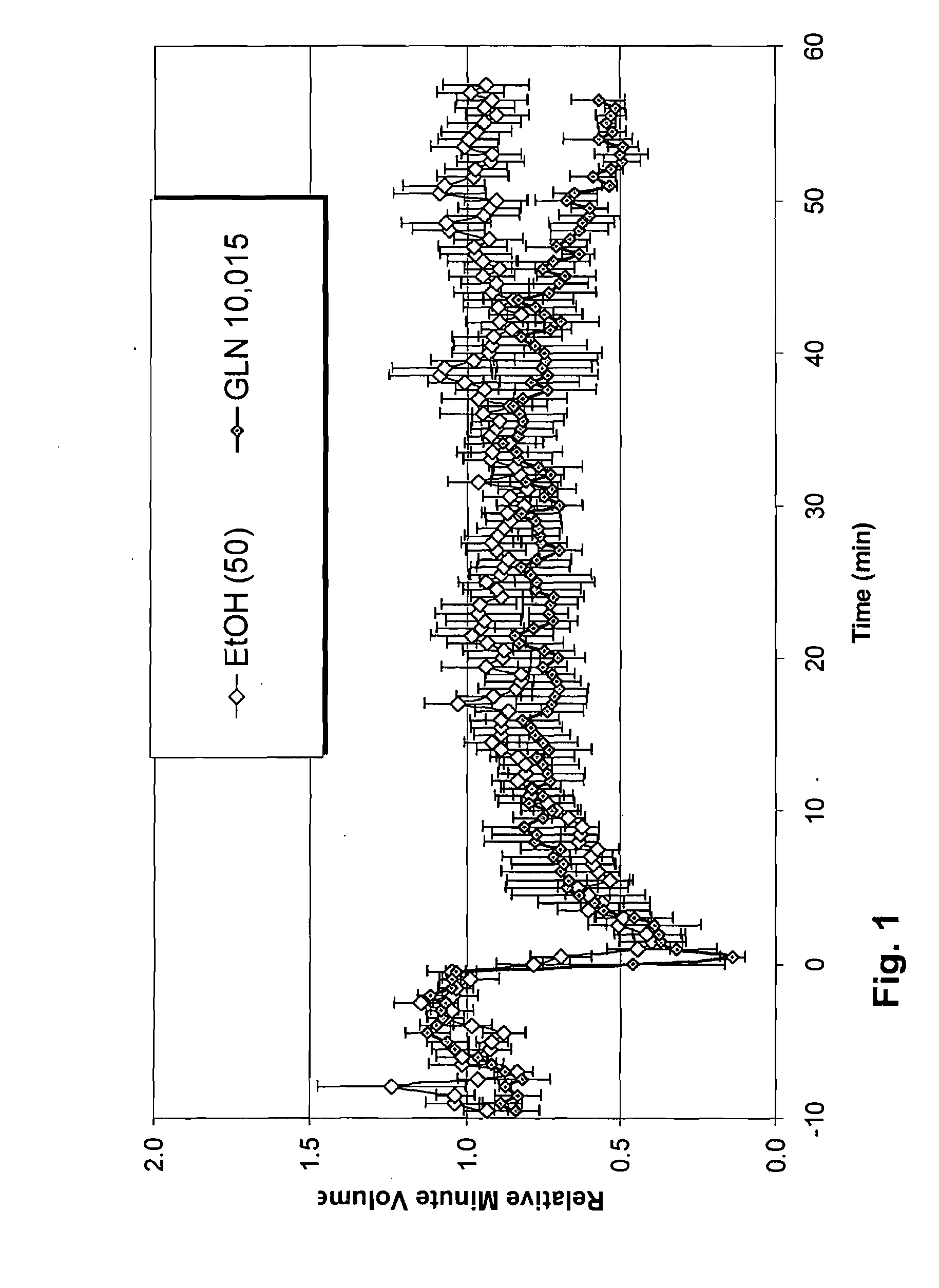
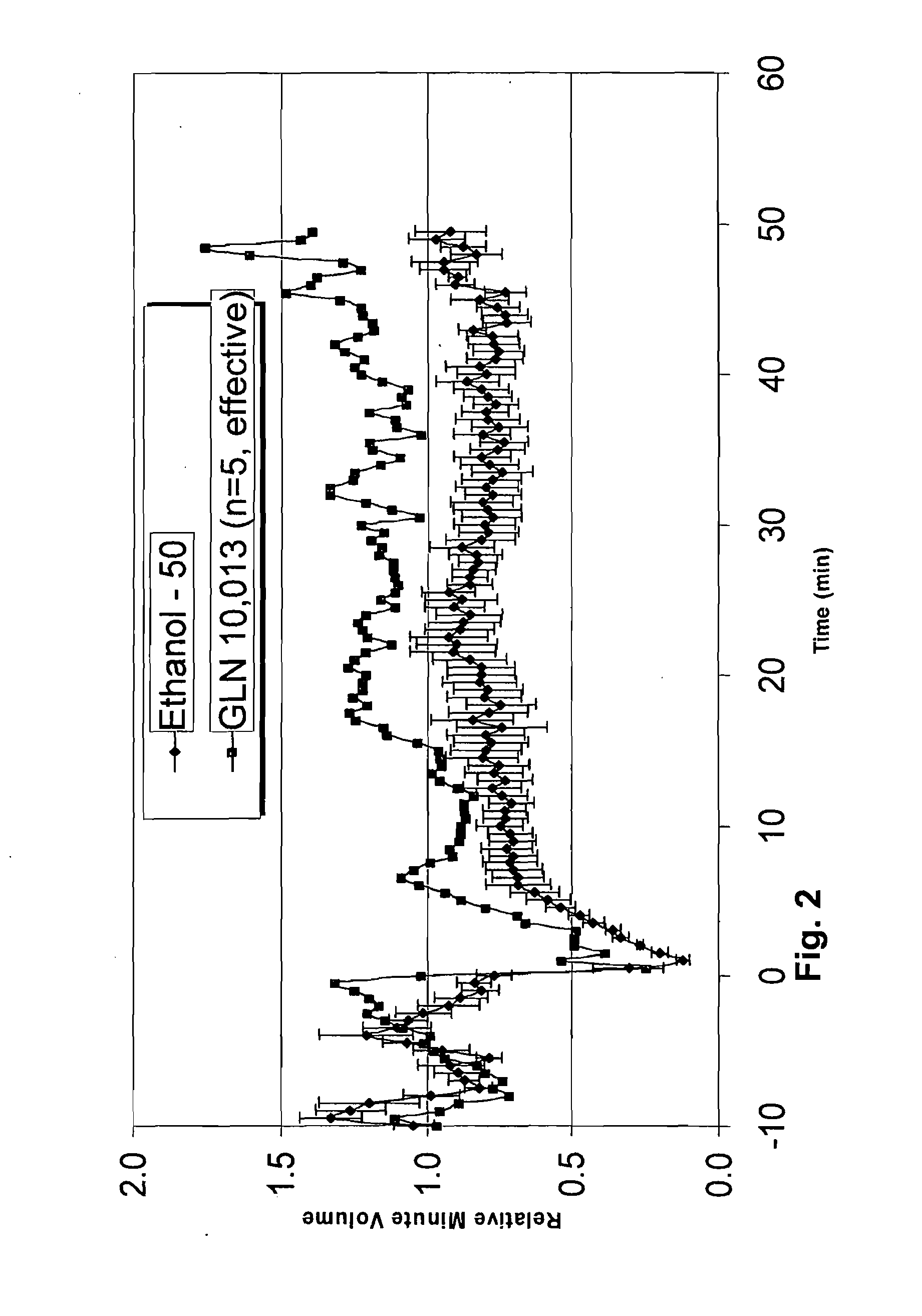
The present invention is directed to a method of treating disordered control of breathing including the treatment of apnea and hypoventilation associated with congenital or acquired brain stem abnormalities. Specifically the invention is directed to treating disordered control of breathing by administering an S-nitrosylating agent selected from the group consisting of ethyl nitrite, glutathione, nitric oxide, S-nitrosocysteine, S-nitrosoglutathione, S-nitro-N-acetyl cysteine. As shown in FIG. 1C the ability of endogenous SNOg to increase VE in freely behaving, conscious rates using whole-body plethysmography revealed that CSNO, GSNO and CGSNO (1 nmol each) caused equivalent increases in VE, whereas D-CSNO had no effect (left bar graph is the equivalent increases in VE, whereas D-CSNO had no effect (left bar graph is the control whereas the right bar represents administration of the respective SNO).
Owner:VIRGINIA UNIV OF PATENT FOUND +1
Use of S-Nitrosothiol Signaling to Treat Disordered Control of Breathing
Owner:GASTON BENJAMIN M +1
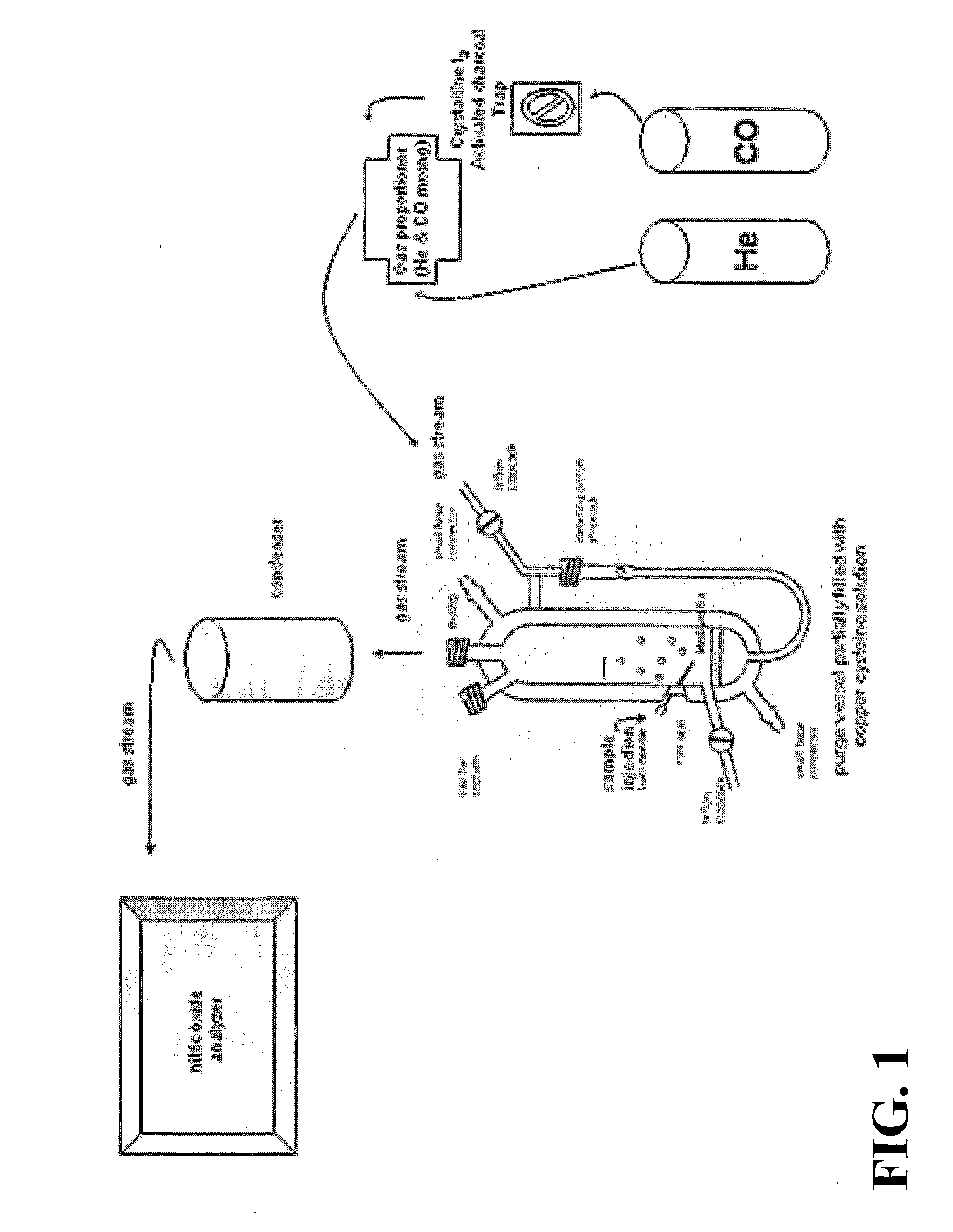
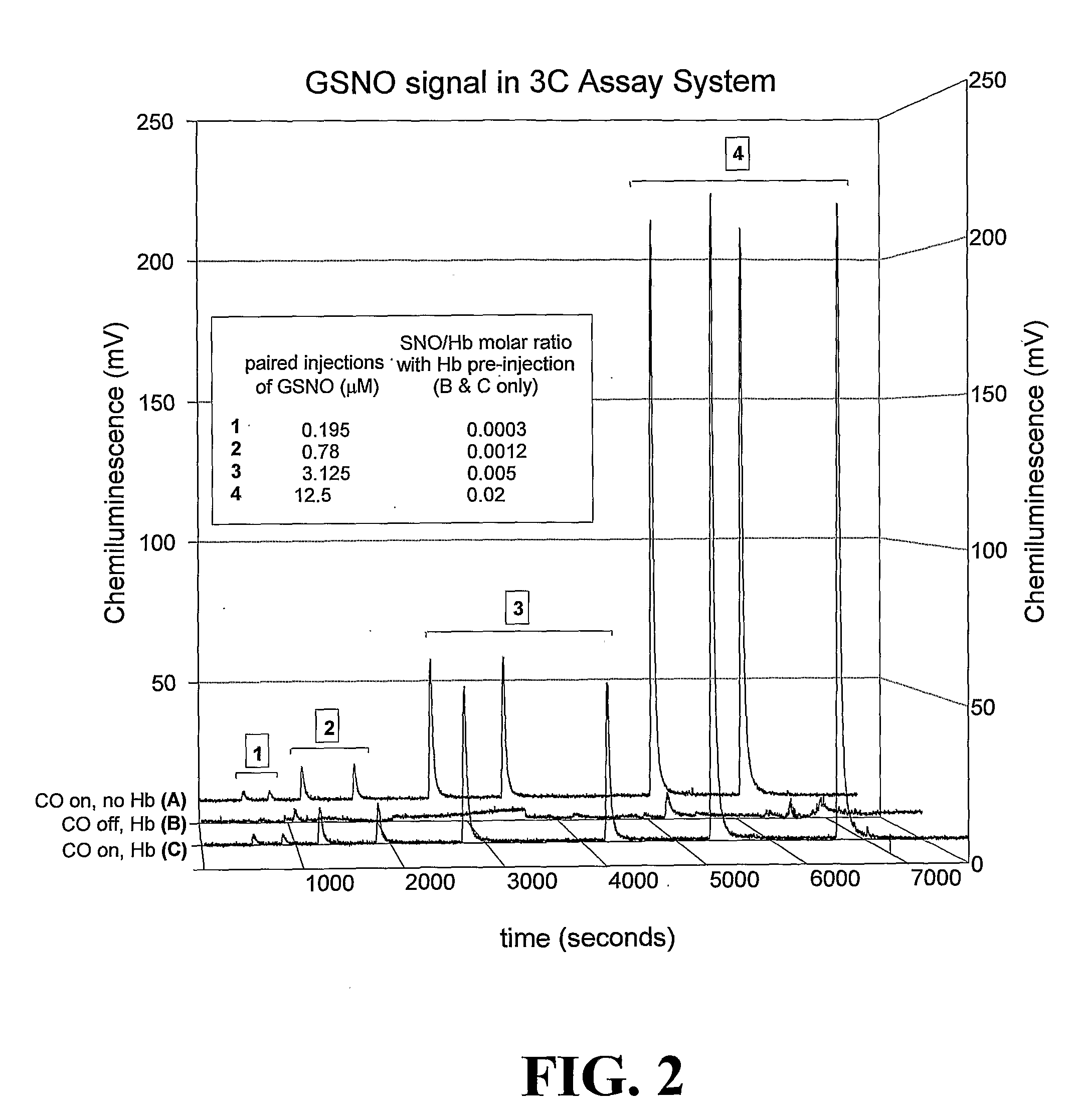
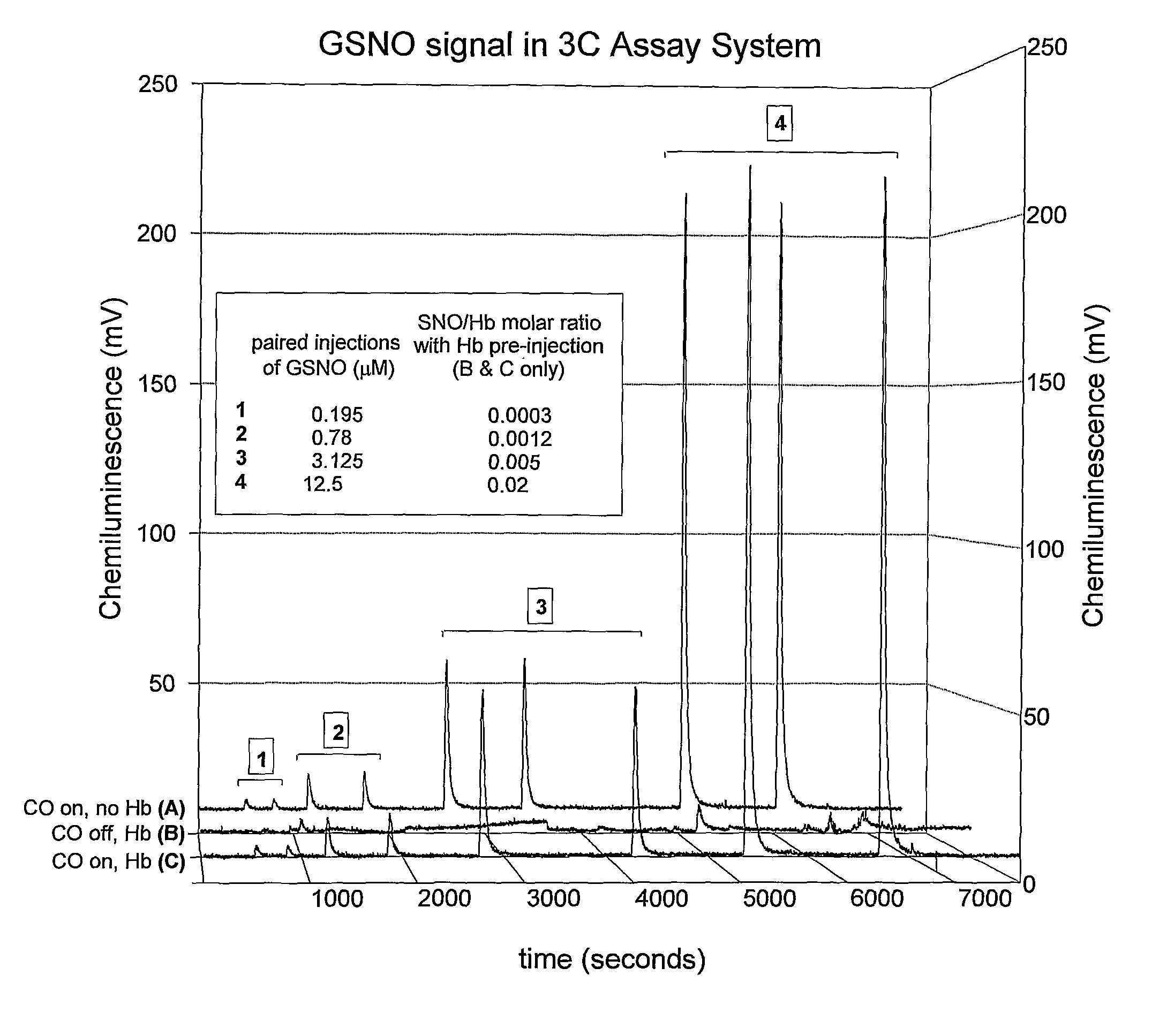
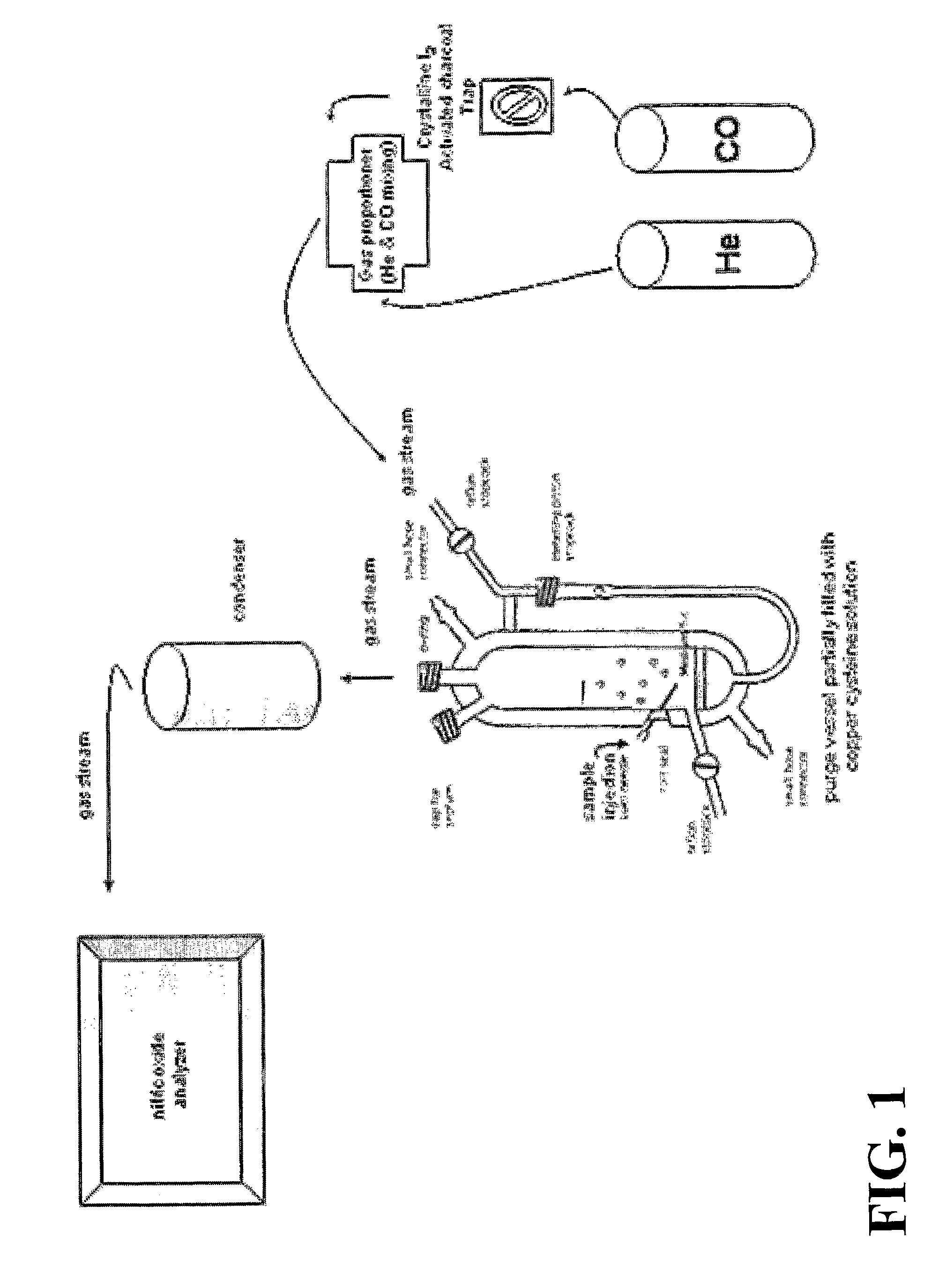
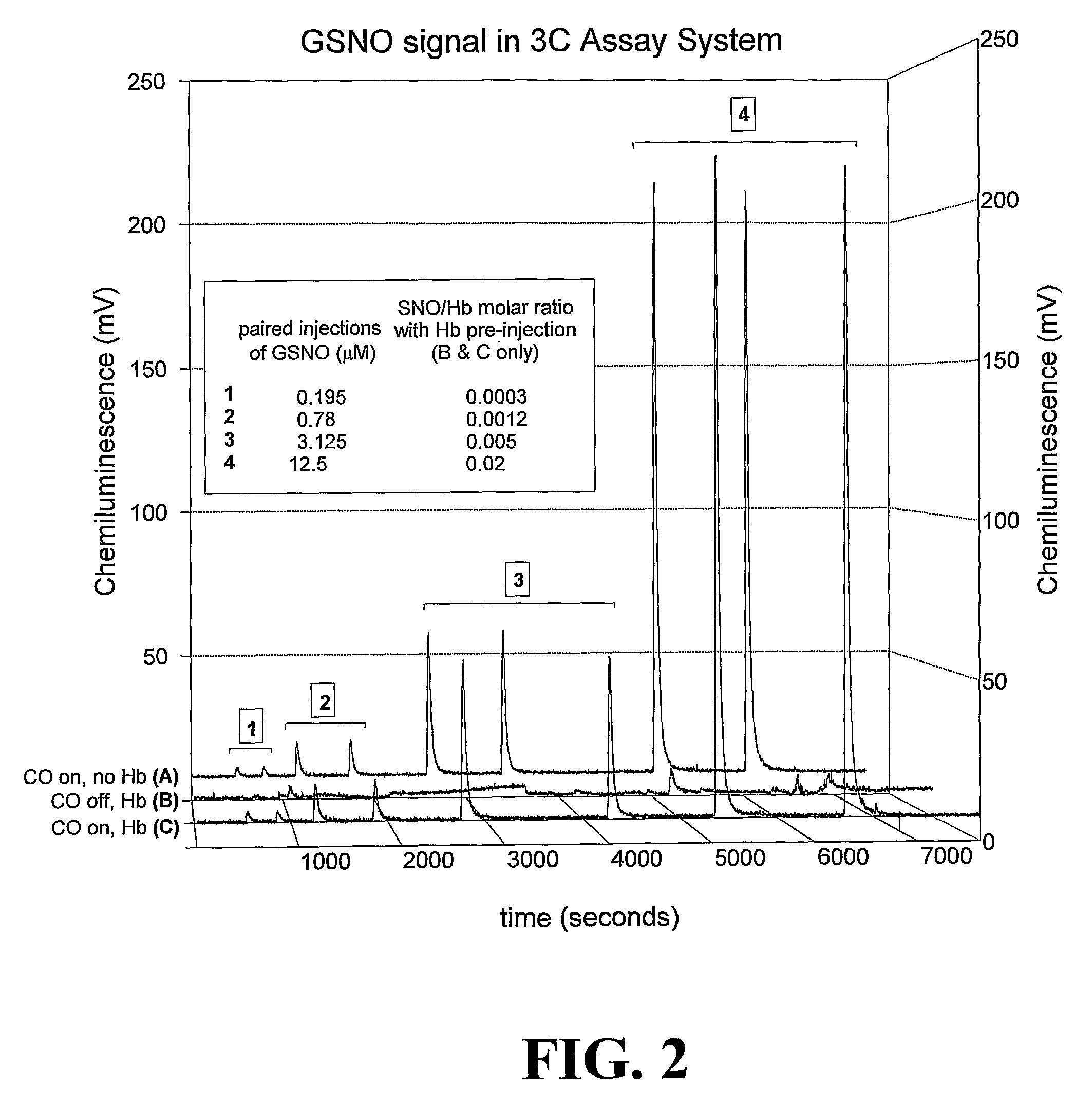
Methods For Identifying And Measuring S-Nitrosothiol Bonds In Heme-Containing Cells And Molecules
ActiveUS20070202605A1Chemiluminescene/bioluminescenceWithdrawing sample devicesCysteine thiolateHeme
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Stable S-nitrosothiol formulations
InactiveUS20070243262A1For long-term storageEffective meanPowder deliveryDispersion deliveryS-NitrosoglutathioneIn vivo
The invention provides stable S-nitrosothiol, such as S-nitrosoglutathione, formulations for long term storage and in vivo delivery of S-nitrosothiols. The invention provides stable aerosol formulations comprising S-nitrosothiol, such as S-nitrosoglutathione, and methods of treating patients in need of S-nitrosothiol, such as S-nitrosoglutathione, and / or nitric oxide treatment.
Owner:NITROX
S-Nitrosothiols Containing Composition for the Treatment of Fatty Liver Diseases, Obesity and Other Diseases Associated with the Metabolic Syndrome and the Use of Such Compositions
InactiveUS20100062059A1Avoid developmentEasy to returnAntibacterial agentsBiocideFatty liverRectal administration
The present invention refers to pharmaceutical compositions containing S-nitrosothiols as active principle. The referred compositions are intended for the treatment of the fatty liver disease and other diseases associated with the metabolic syndrome. The composition is administered either orally or rectally.
Owner:PROTAGEN
Methods for identifying and measuring S-nitrosothiol bonds in heme-containing cells and molecules
The invention provides a method of detecting an S-nitrosothiol in a sample which includes treating the sample with a transition metal such as copper (I) and cysteine in the presence of a substance capable of blocking interactions between iron-containing compounds and NO and detecting the generated NO.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Apparatus for the controlled release of topical nitric oxide
ActiveUS20140296773A1Portability suitableSuitable shelf lifePeptide/protein ingredientsInorganic active ingredientsControl releaseMedicine
A stable delivery system for the therapeutic release and application of nitric oxide to a patient suffering from a cutaneous injury or wound includes a S-nitrosothiol and transition element nanoparticles. The transition metal nanoparticles are selected to react with the S-nitrosothiol to release and diffuse nitric oxide into the injury or wound.
Owner:UNIVERSITY OF WINDSOR
Methods and systems for indentifying and monitoring S-nitrosothiols in biological samples
InactiveUS20060154376A1Easy to modifyReduced activityEnzyme stabilisationTissue cultureFormazanBiology
The present invention describes methods and kits for determining the level of S-nitrosothiols in biological fluid samples. The method includes separating tissue or cellular matter from the biological fluid sample and then contacting the biological fluid sample with paraformaldehyde in an amount sufficient to fix free thiols thereby essentially eliminating diaphorase activity of the free thiols. The biological fluid sample is then contacted with NADPH and nitro blue tetrazolium (NBT) wherein S-nitrosothiols in the biological fluid sample facilitate the reduction of NBT to NBT formazan or diformazan in the presence of paraformaldehyde. The amount of reduced NBT is measured and the determined levels correlate to the amount of S-nitrosothiols in the biological fluid sample.
Owner:WHALEN ERIN J
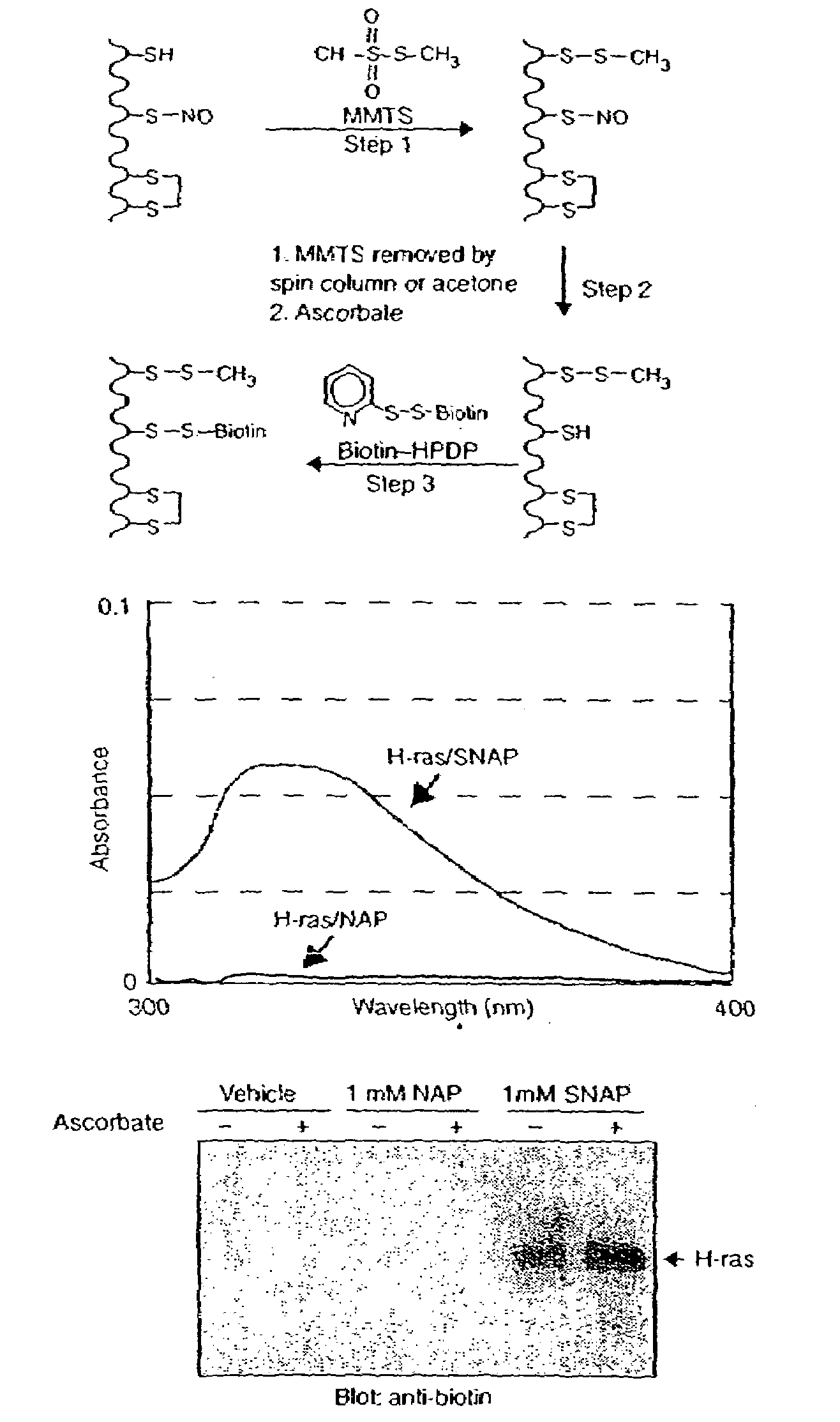
Method for assaying protein nitrosylation
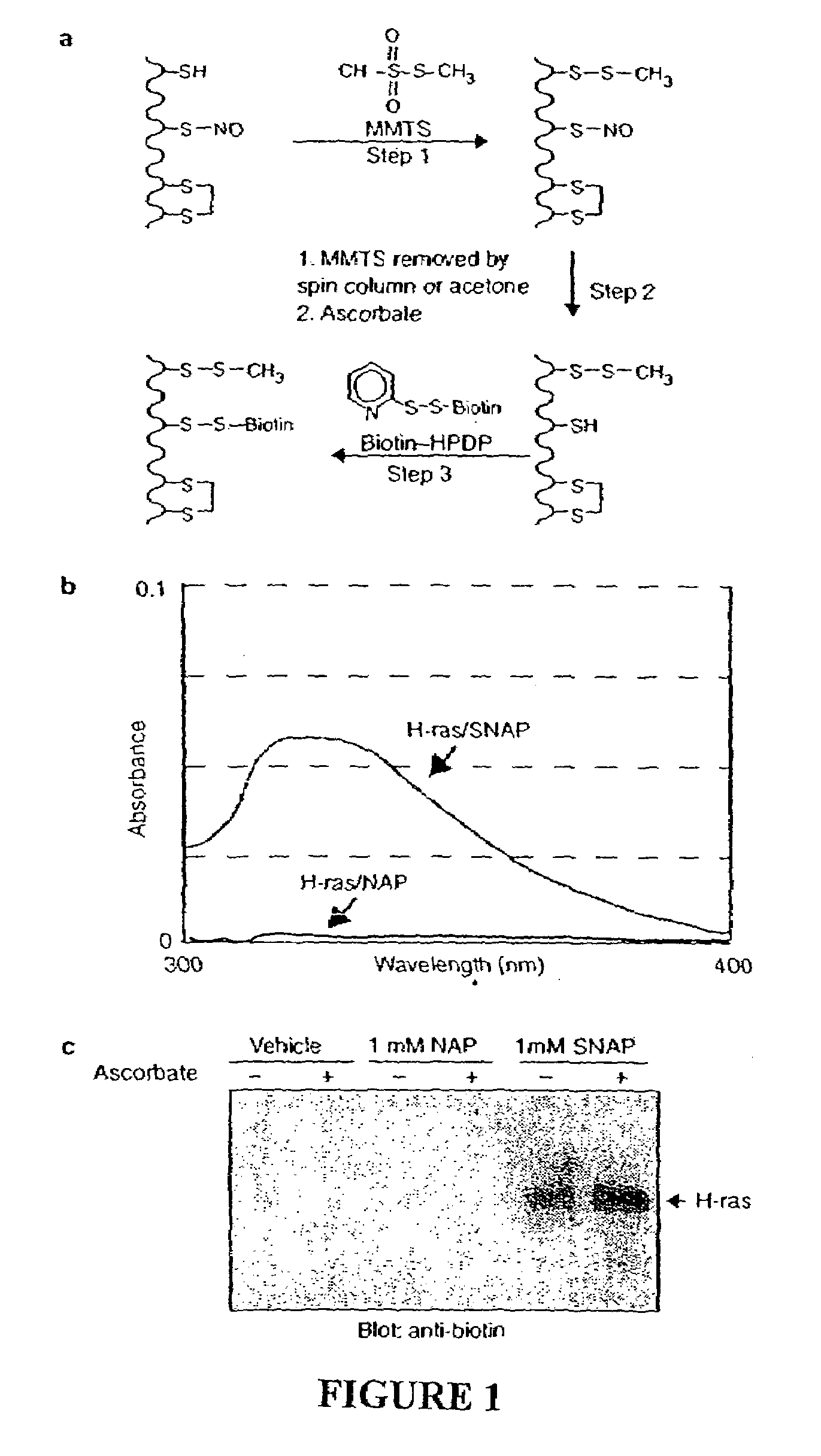
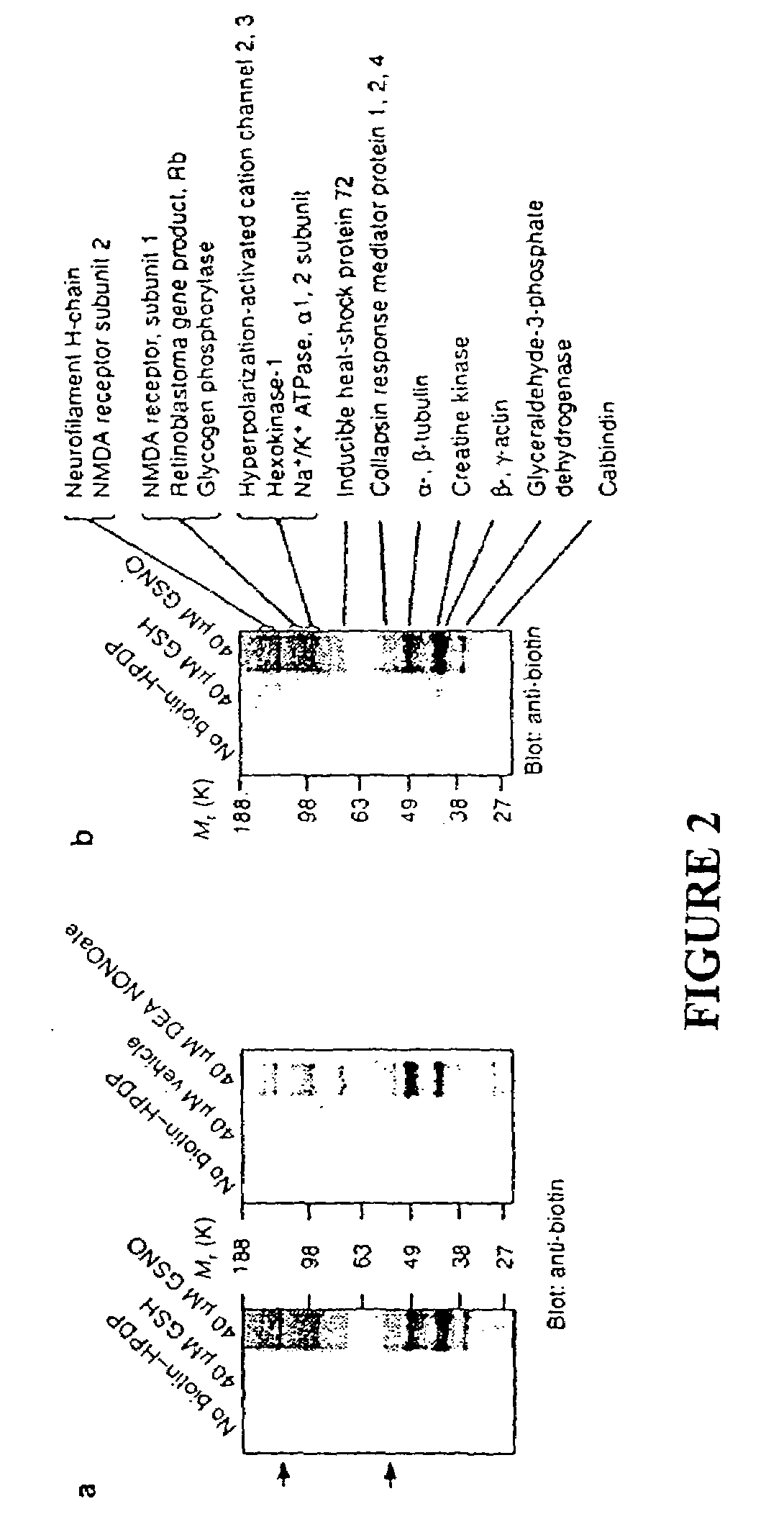
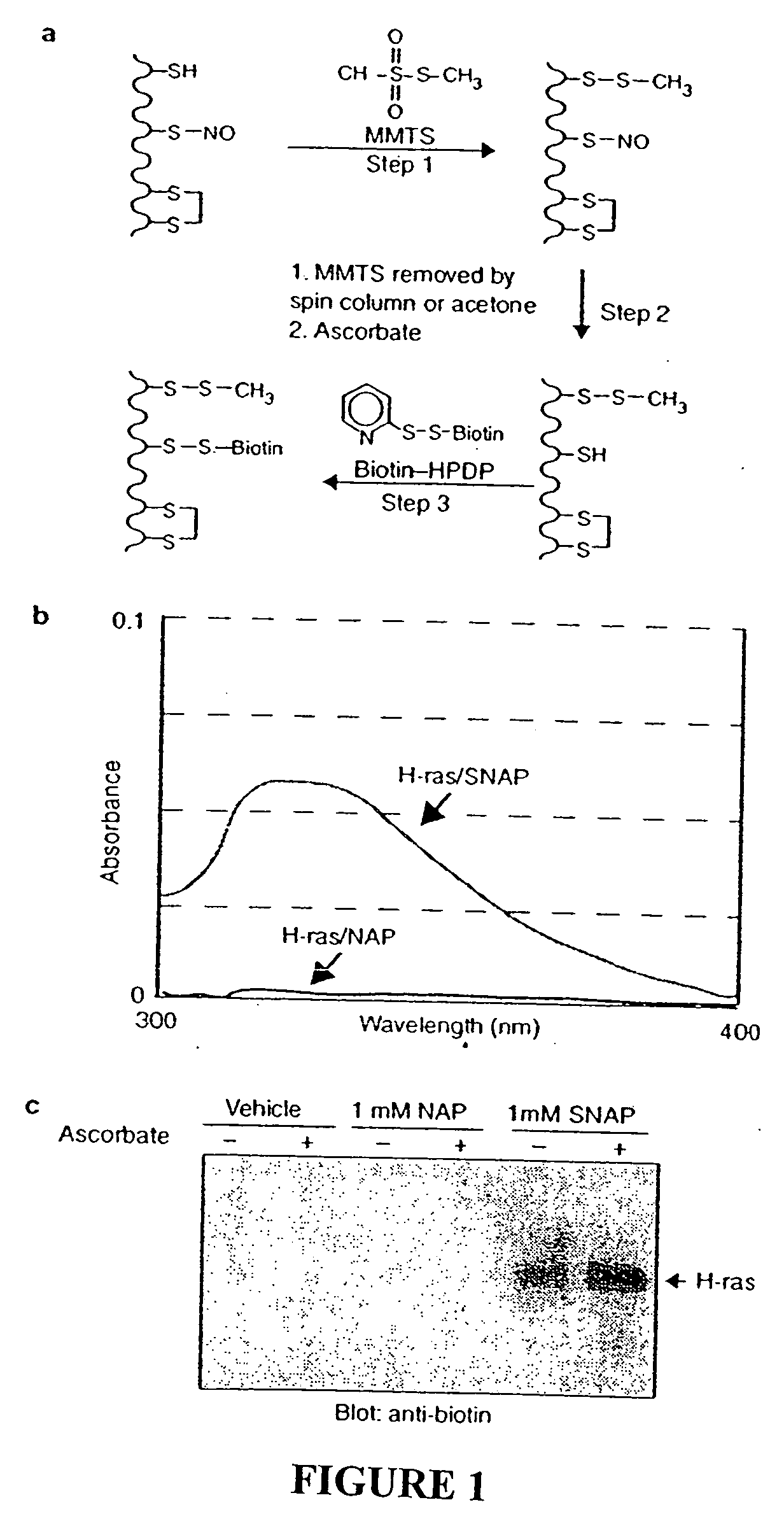
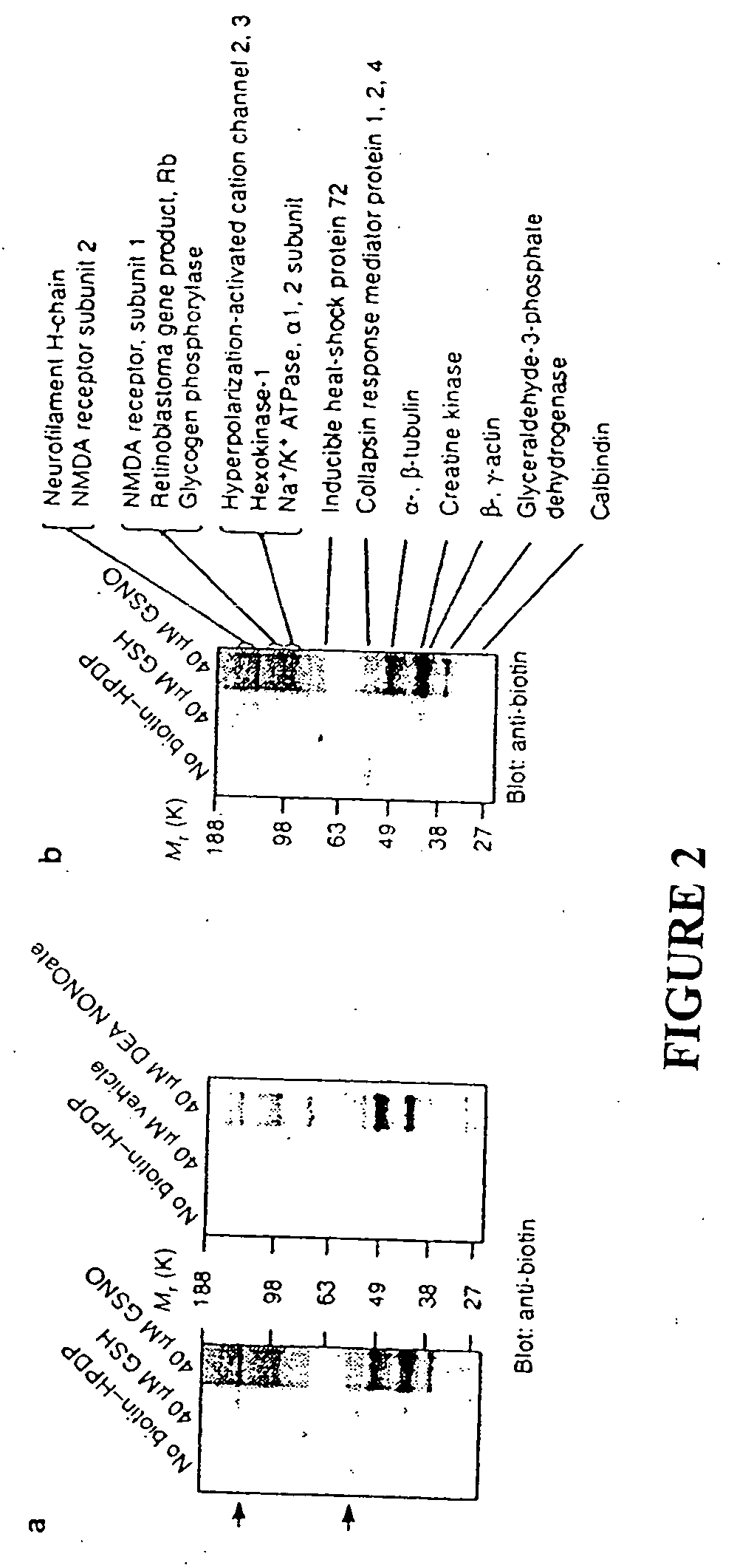
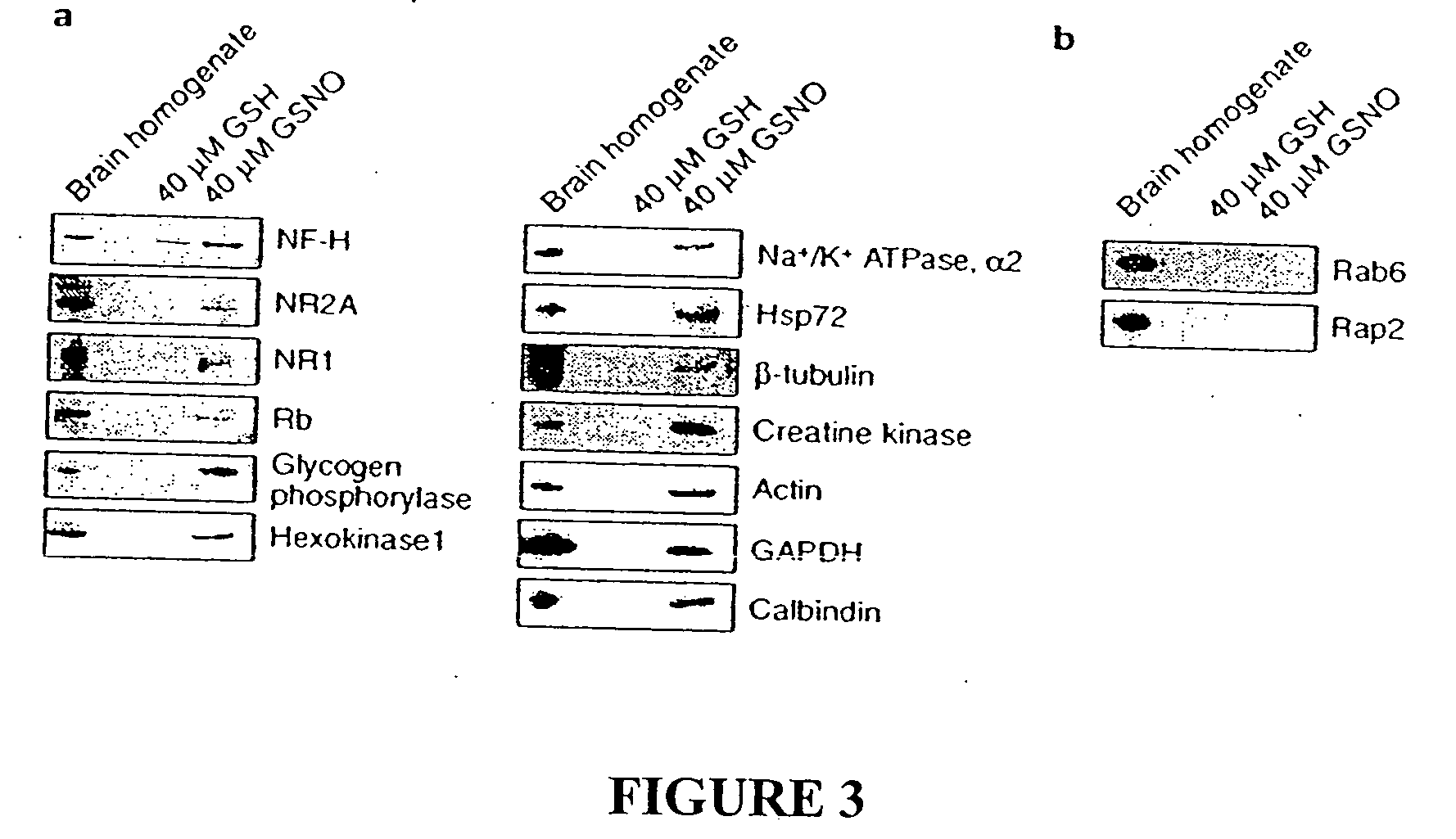
Many of the effects of nitric oxide are mediated by the direct modification of cysteine residues resulting in an adduct called a nitrosothiol. A method to detect proteins which contain nitrosothiols involves several steps. Nitrosylated cysteines are converted to tagged cysteines. Tagged proteins can then be detected, for example, by immunoblotting and / or can be purified by affinity chromatography. The method is applicable to the detection of S-nitrosylated proteins in cell lysates following in vitro S-nitrosylation, as well as to the detection of endogenous S-nitrosothiols in selected protein substrates.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Improvements relating to skin dressings
A skin dressing is adapted, on activation, to release one or more S-nitrosothiols, preferably S-nitroso-L-glutathione. S-nitrosothiols decompose spontaneously to produce nitric oxide, which has beneficial effects on tissues, particularly in wound healing.
Owner:INSENSE LIMITED
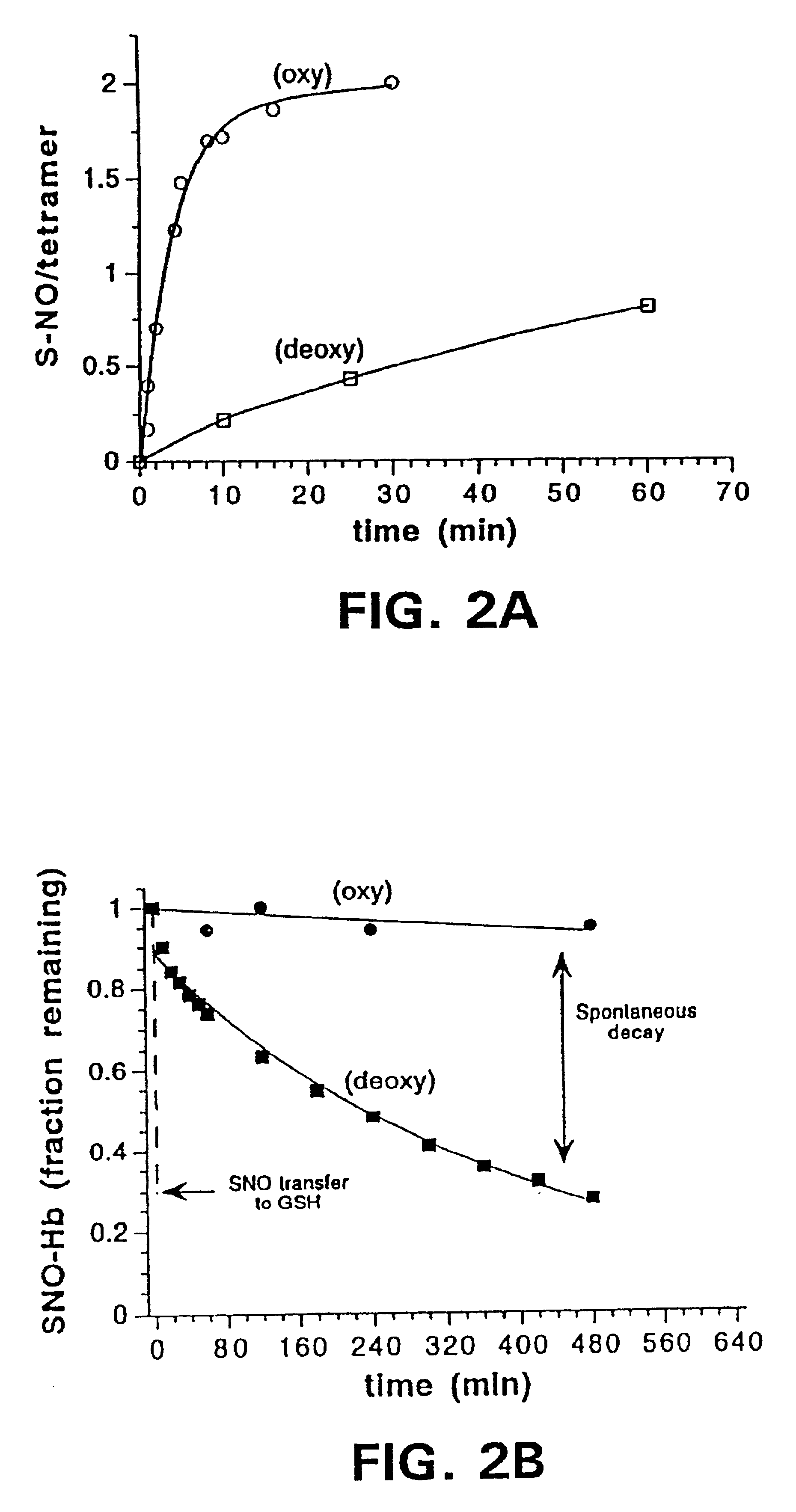
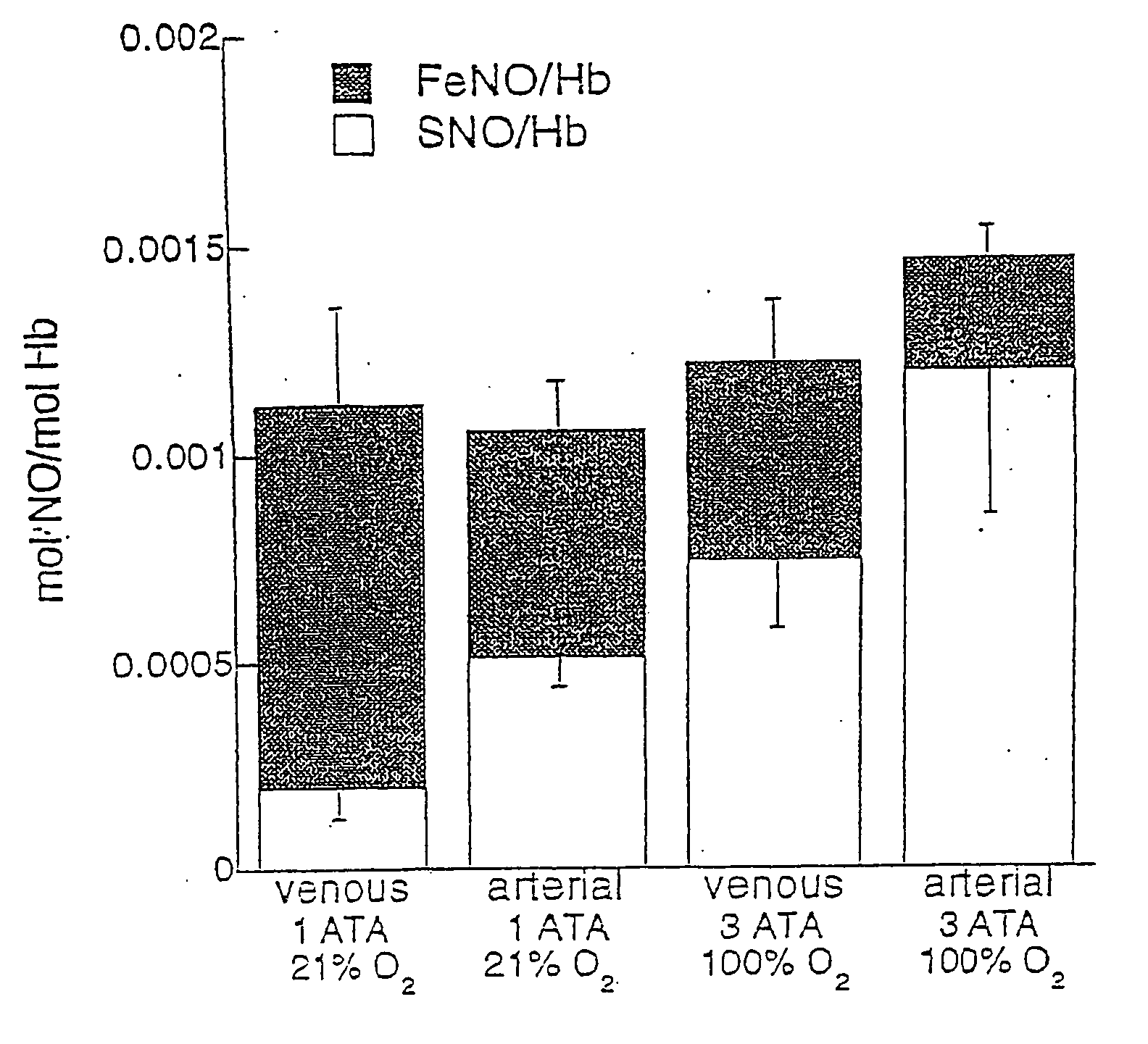
No-modified hemoglobins and uses therefore
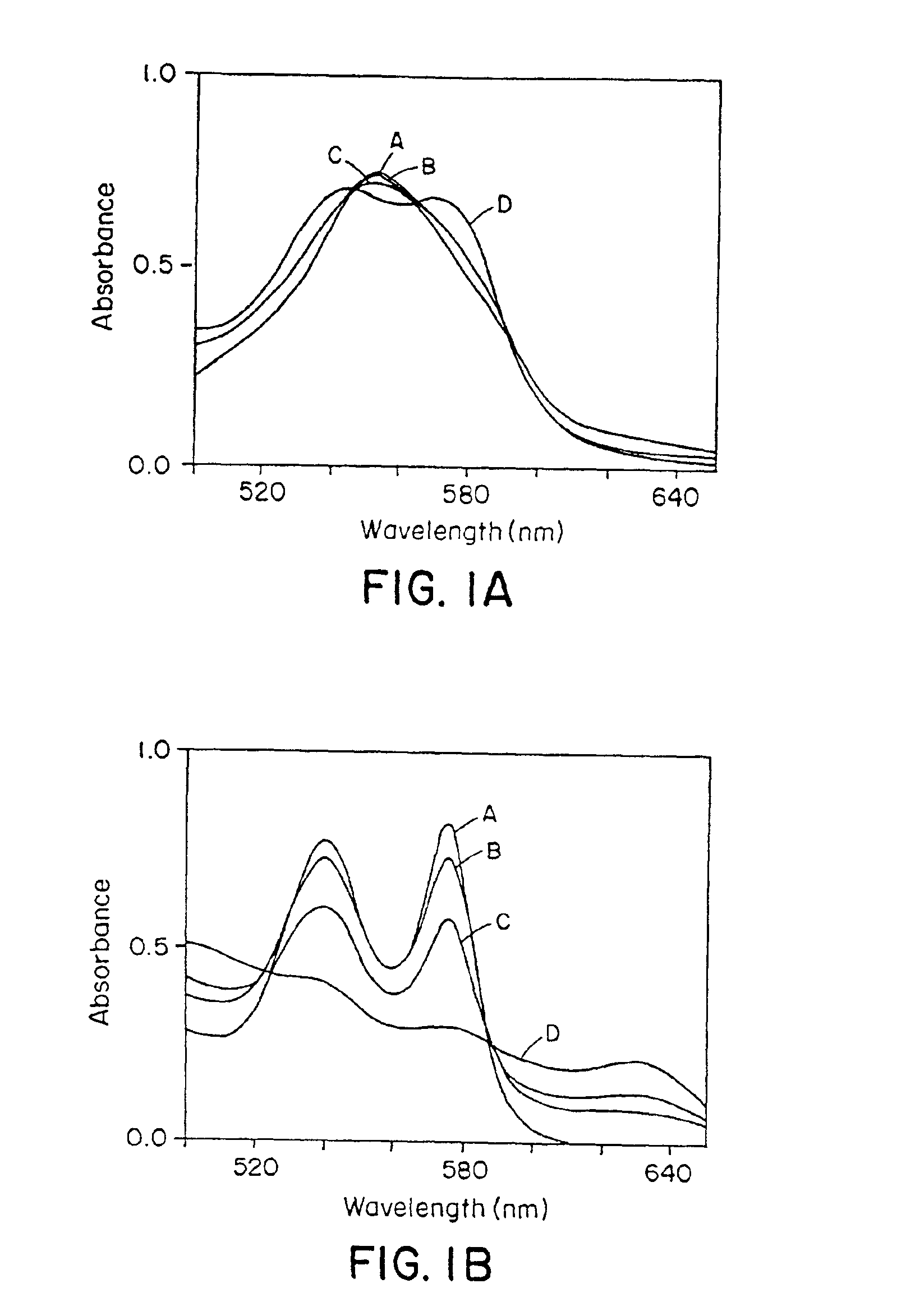
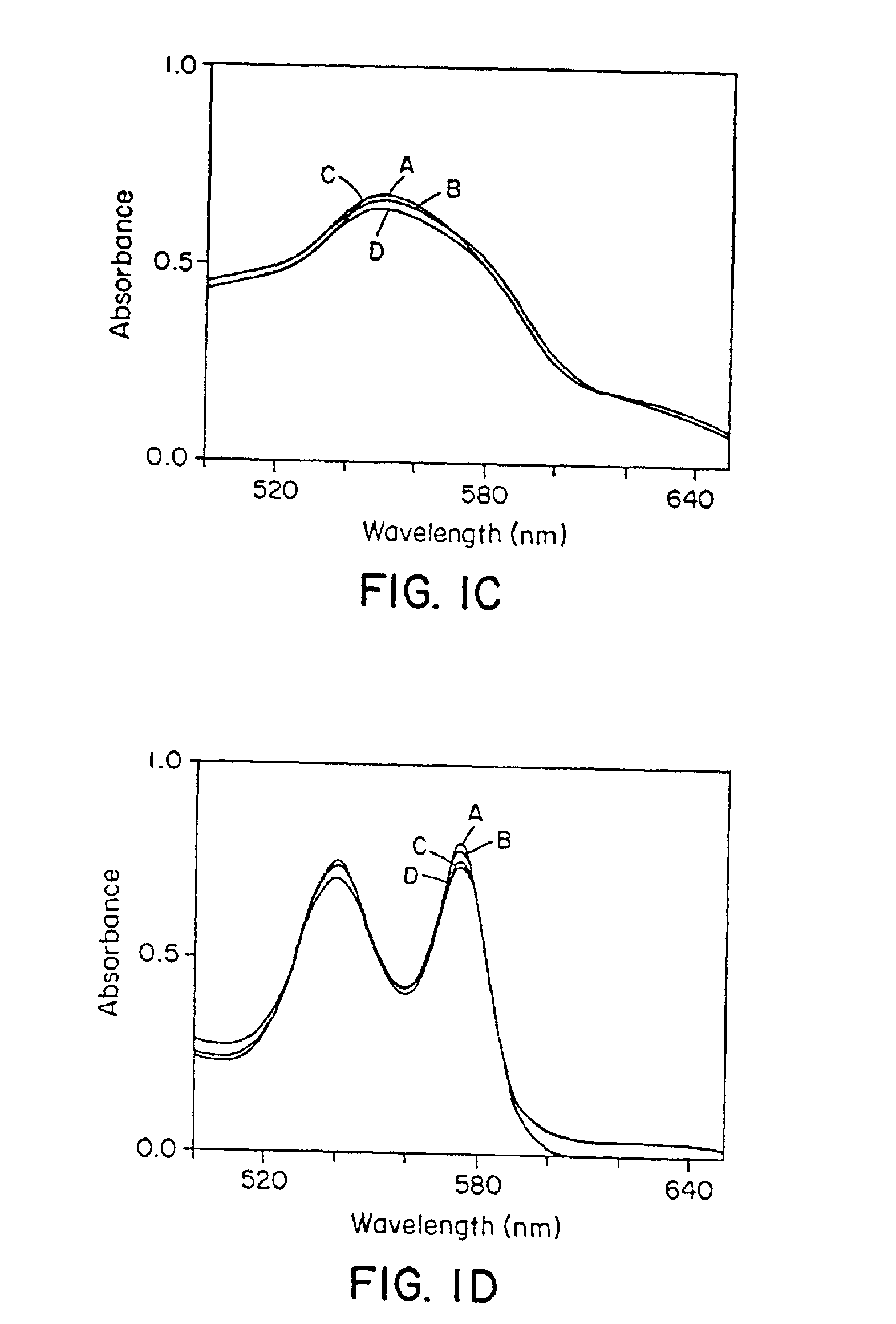
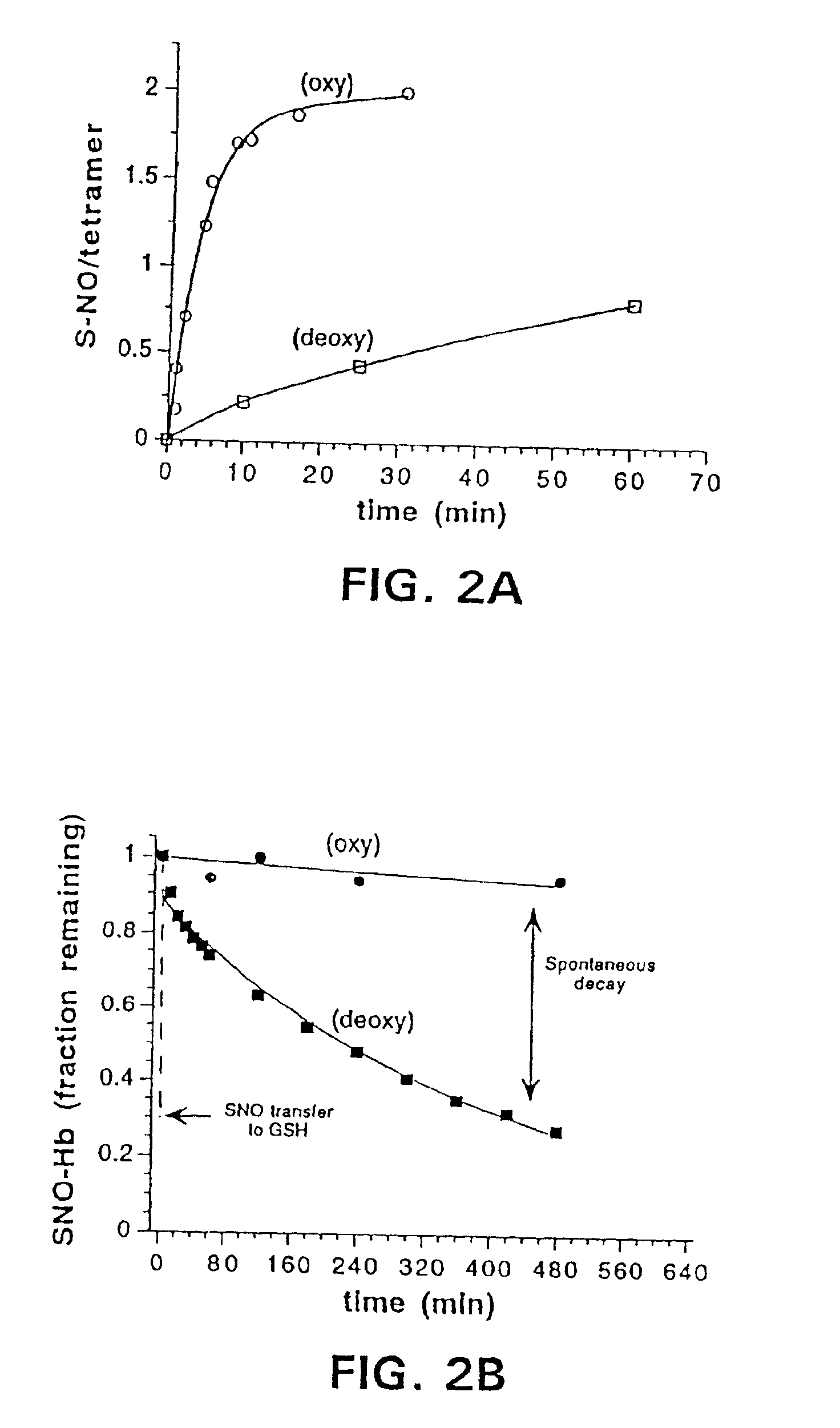
S-nitrosohemoglobin (SNO-Hb) can be formed by reaction of Hb with S-nitrosothiol and by other methods described herein which do not result in oxidation of the heme Fe. Other methods can be used which are not specific only for thiol groups, but which nitrosate Hb more extensively, and may produce polynitrosated metHb as a product or intermediate product of the method. SNO-Hb in its various forms and combinations thereof (oxy, deoxy, met; specifically S-nitrosylated, or nitrosated or nitrated to various extents) can be administered to an animal or human where it is desired to oxygenate, to scavenge free radicals, or to release NO+ groups to tissues. Thiols and / or NO donating agents can also be administered to enhance the transfer of NO+ groups. Examples of conditions to be treated by SNO-Hbs or other nitrosated or nitrated forms of Hb include ischemic injury, hypertension, angina, reperfusion injury and inflammation, and disorders characterized by thrombosis. Further embodiments of the invention are methods for assessing oxygen delivery to the tissues-of a mammal by measuring SNO-Hb and nitrosylhemoglobin in blood.
Owner:DUKE UNIV
Method for assaying protein nitrosylation
InactiveUS20050026227A1Microbiological testing/measurementBiological testingCysteine thiolateS-Nitrosylation
Many of the effects of nitric oxide are mediated by the direct modification of cysteine residues resulting in an adduct called a nitrosothiol. A method to detect proteins which contain nitrosothiols involves several steps. Nitrosylated cysteines are converted to tagged cysteines. Tagged proteins can then be detected, for example, by immunoblotting and / or can be purified by affinity chromatography. The method is applicable to the detection of S-nitrosylated proteins in cell lysates following in vitro S-nitrosylation, as well as to the detection of endogenous S-nitrosothiols in selected protein substrates.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Nanoparticle delivery vehicle for s-nitroso-n-acetyl cysteine and uses thereof
Nanoparticles are provided that comprise S-nitrosothiol (SNO) groups covalently bonded to the nanoparticles and / or S-nitrosothiol containing molecules encapsulated within the nanoparticles, as well as methods of making and using the nanoparticles. The invention also provides methods of preparing nanoparticles comprising Snitrosothiol (SNO) groups covalently bonded to the nanoparticles, where the methods comprise a) providing a buffer solution comprising chitosan, polyethylene glycol, nitrite, glucose, and hydrolyzed 3-mercaptopropyltrimethoxysilane (MPTS); b) adding hydrolyzed tetramethoxysilane (TMOS) to the buffer solution to produce a sol-gel; and c) lyophilizing and ball milling the sol-gel to produce nanoparticles of a desired size.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV +1
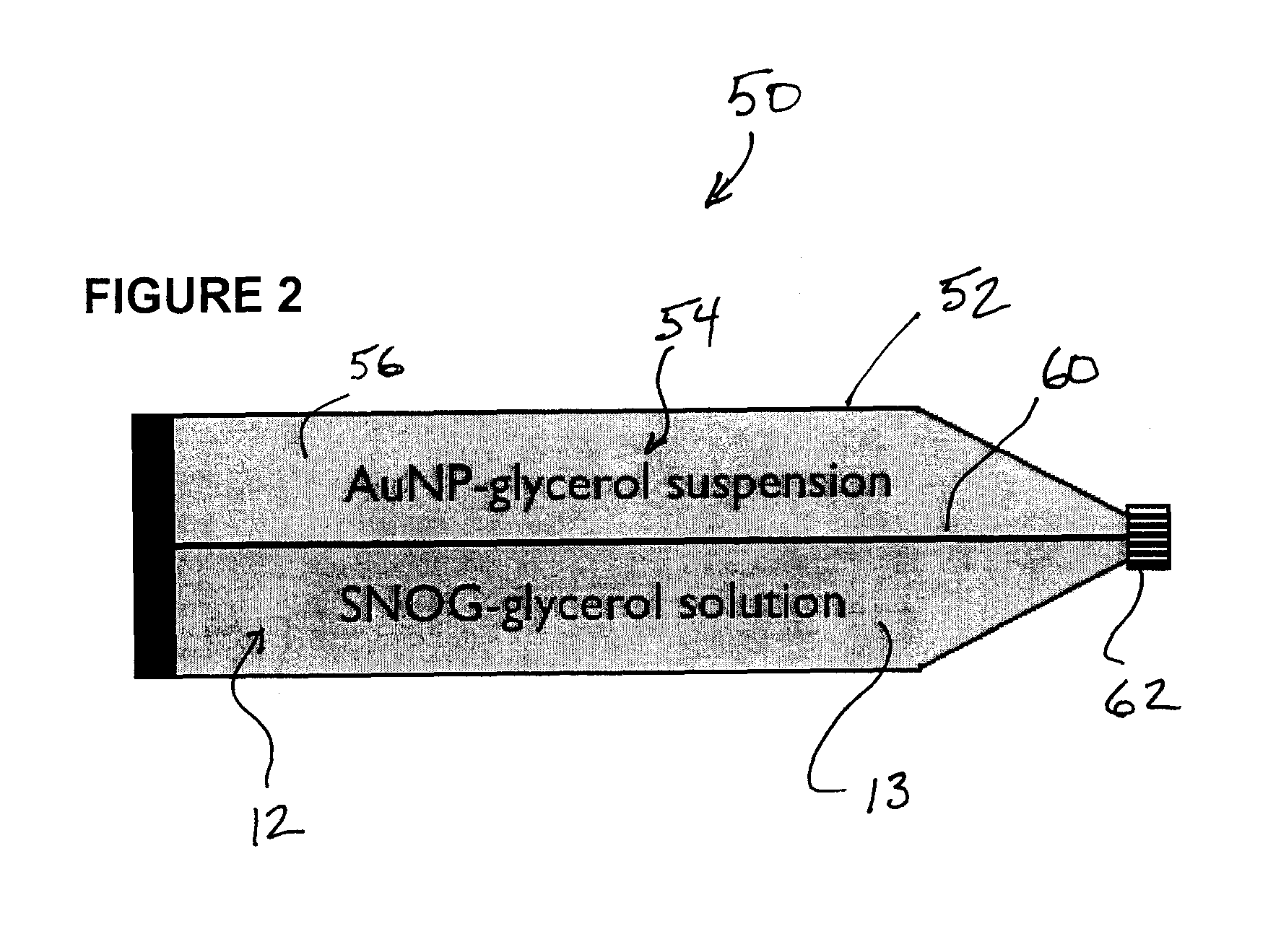
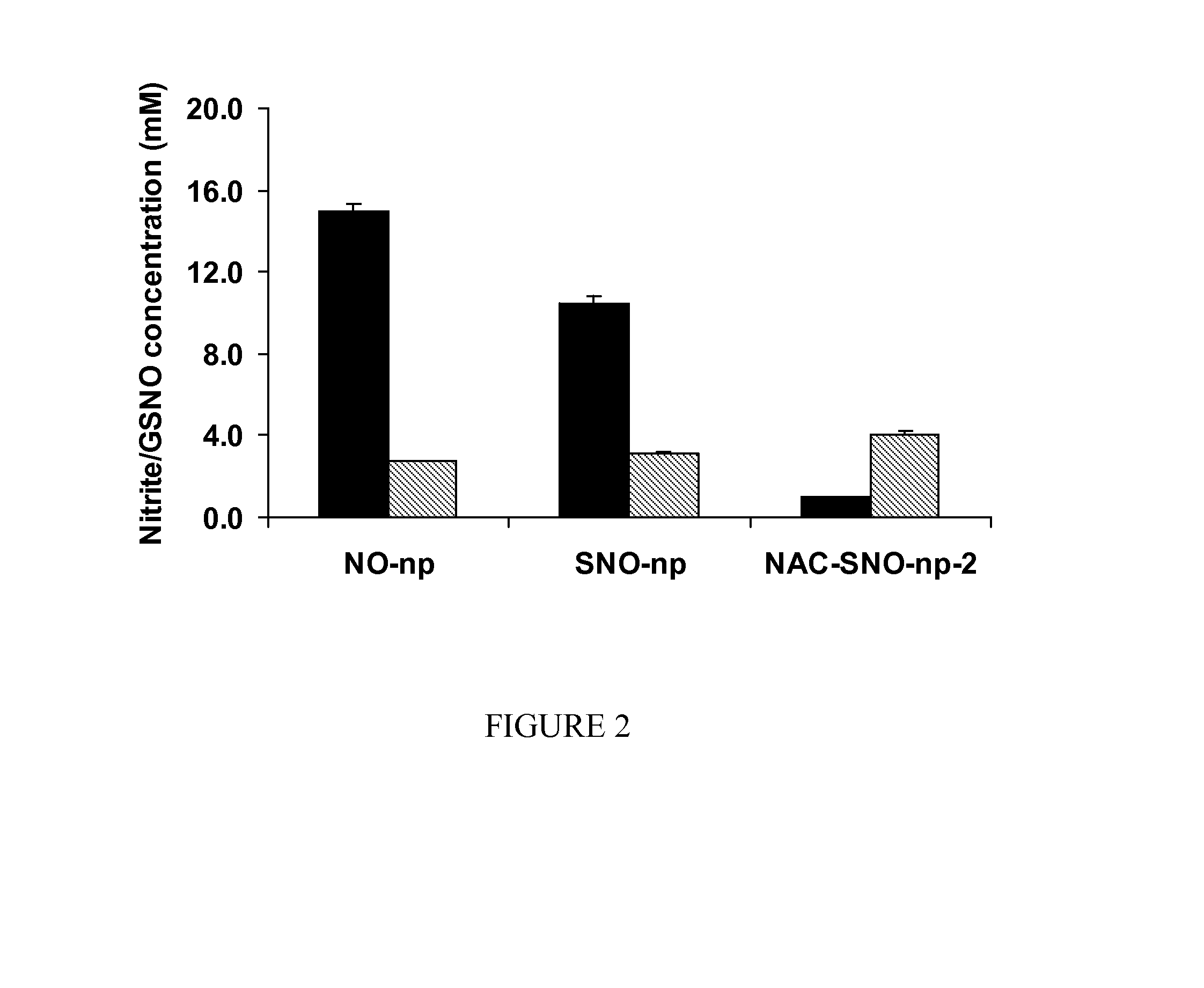
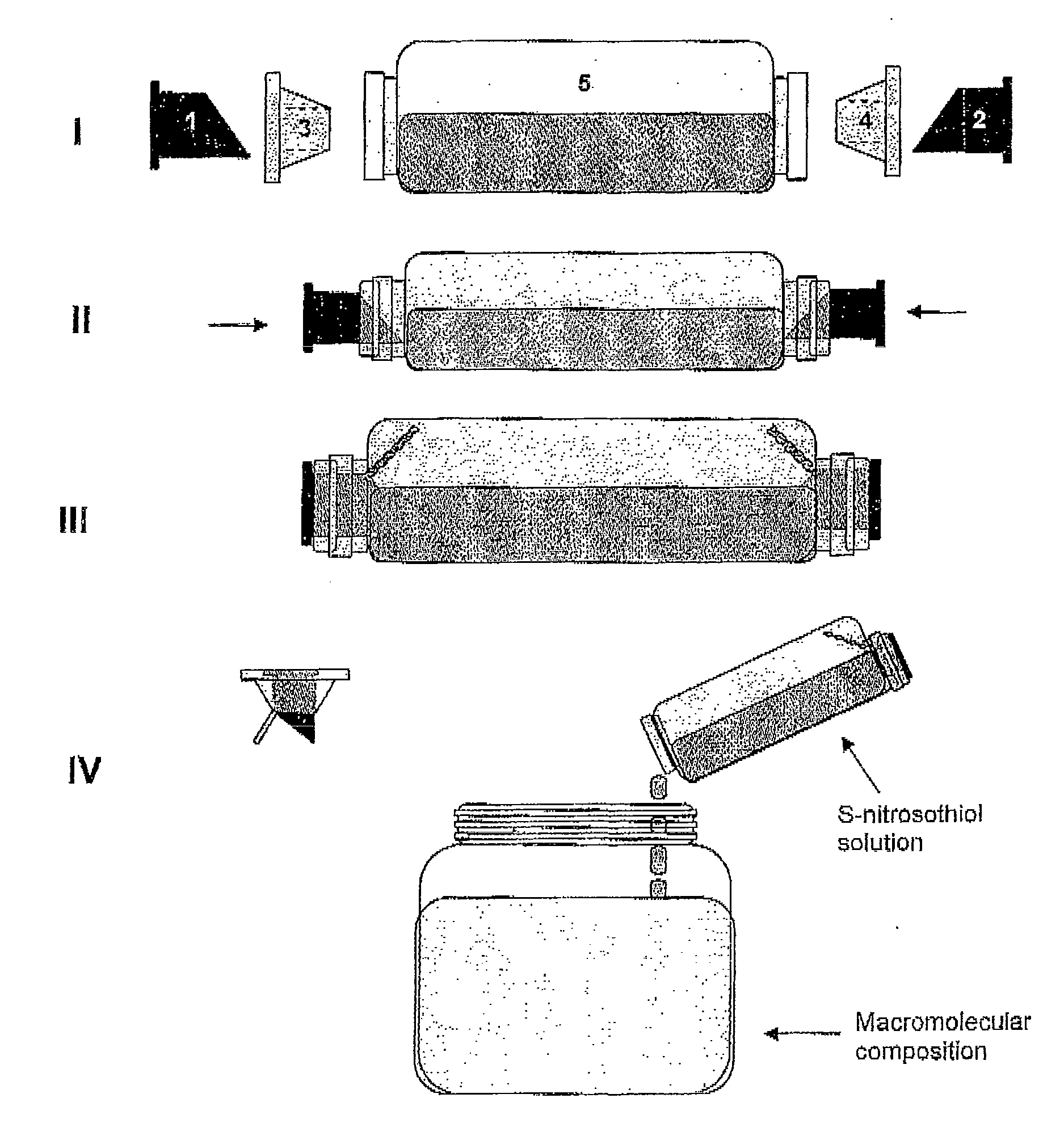
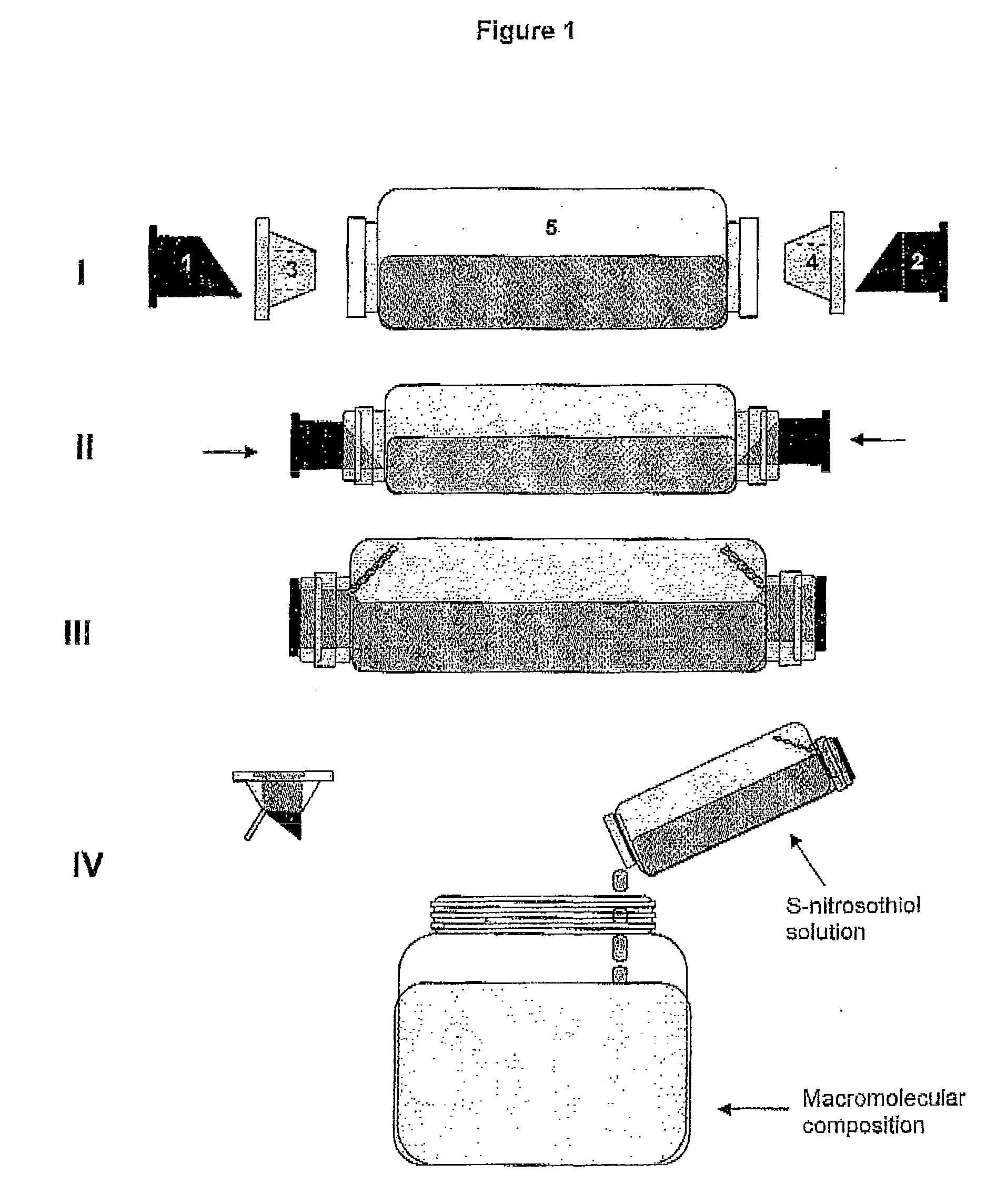
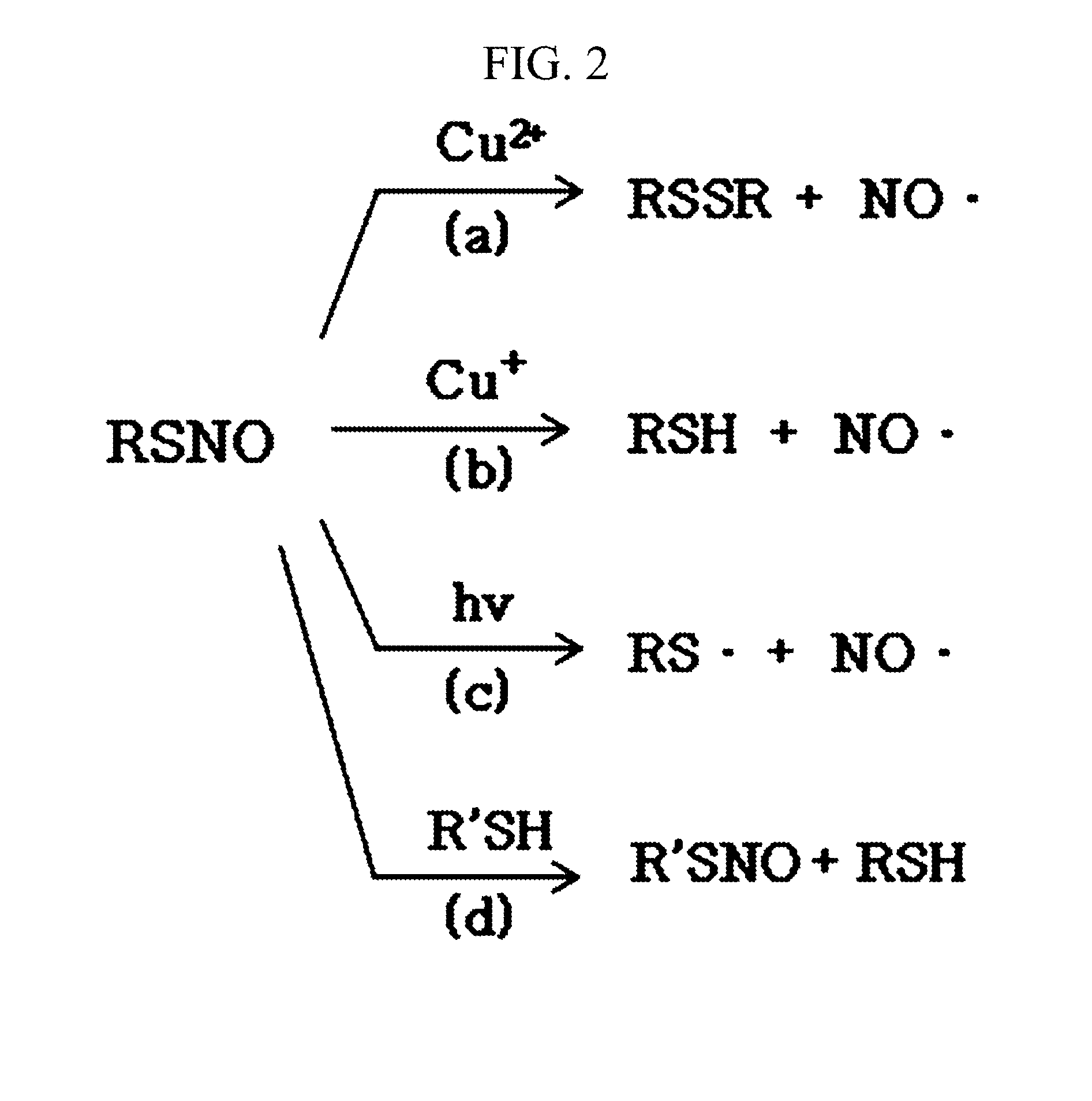
Process for synthesis and incorporation of nitric oxide donors in macromolecular compositions
InactiveUS20090311292A1Improve thermal stabilityPharmaceutical delivery mechanismTripeptide ingredientsSufficient timeDelivery vehicle
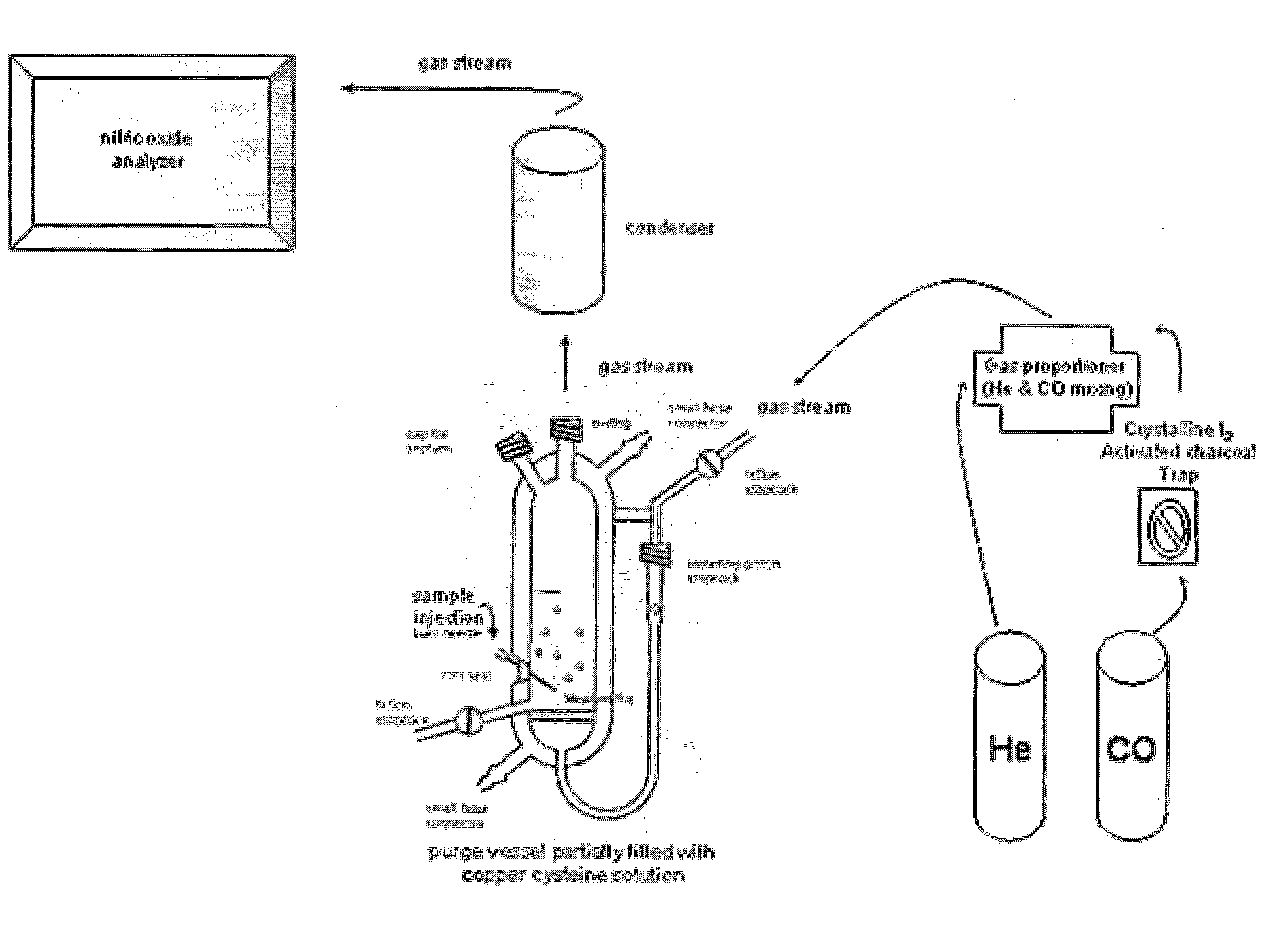
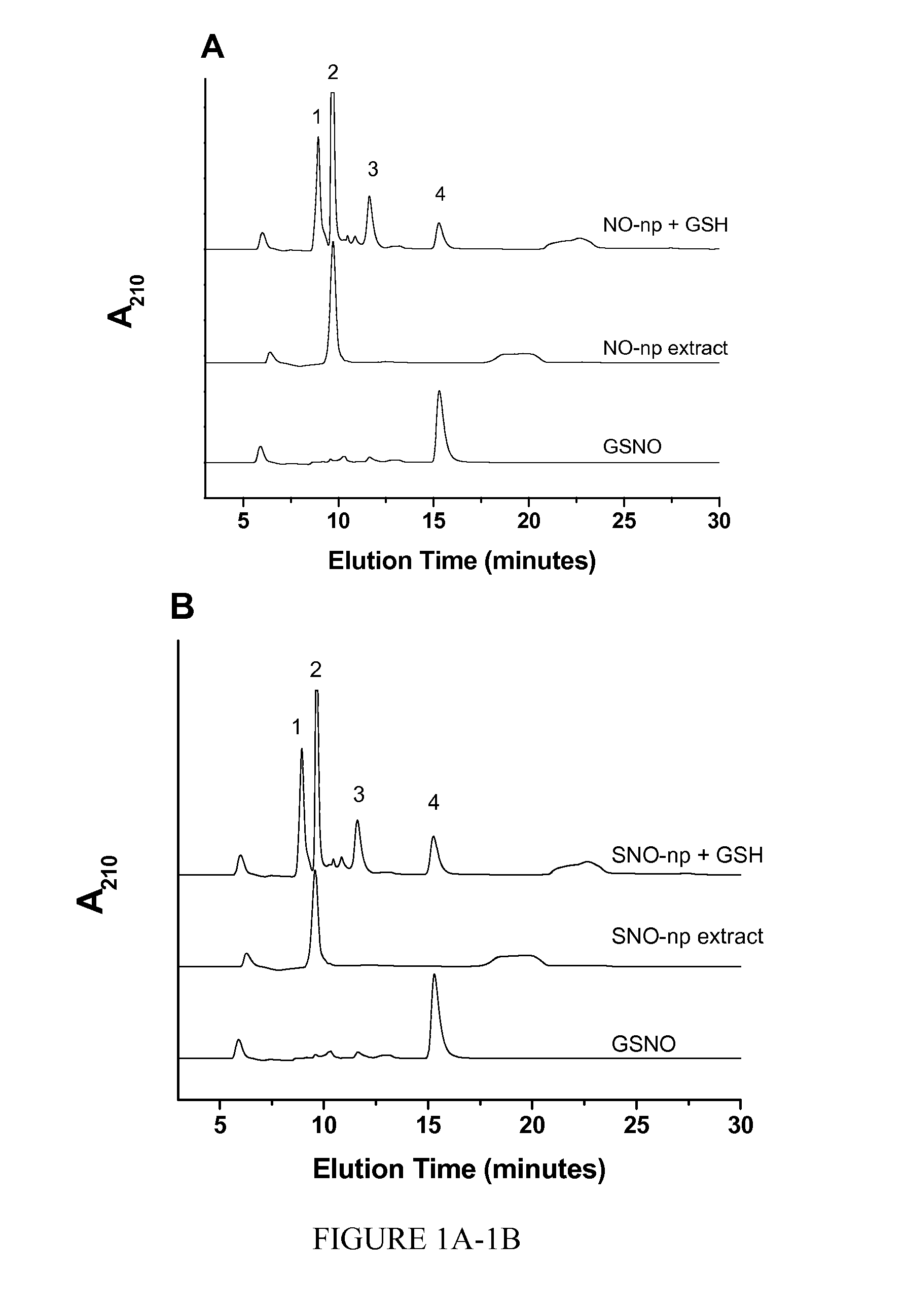
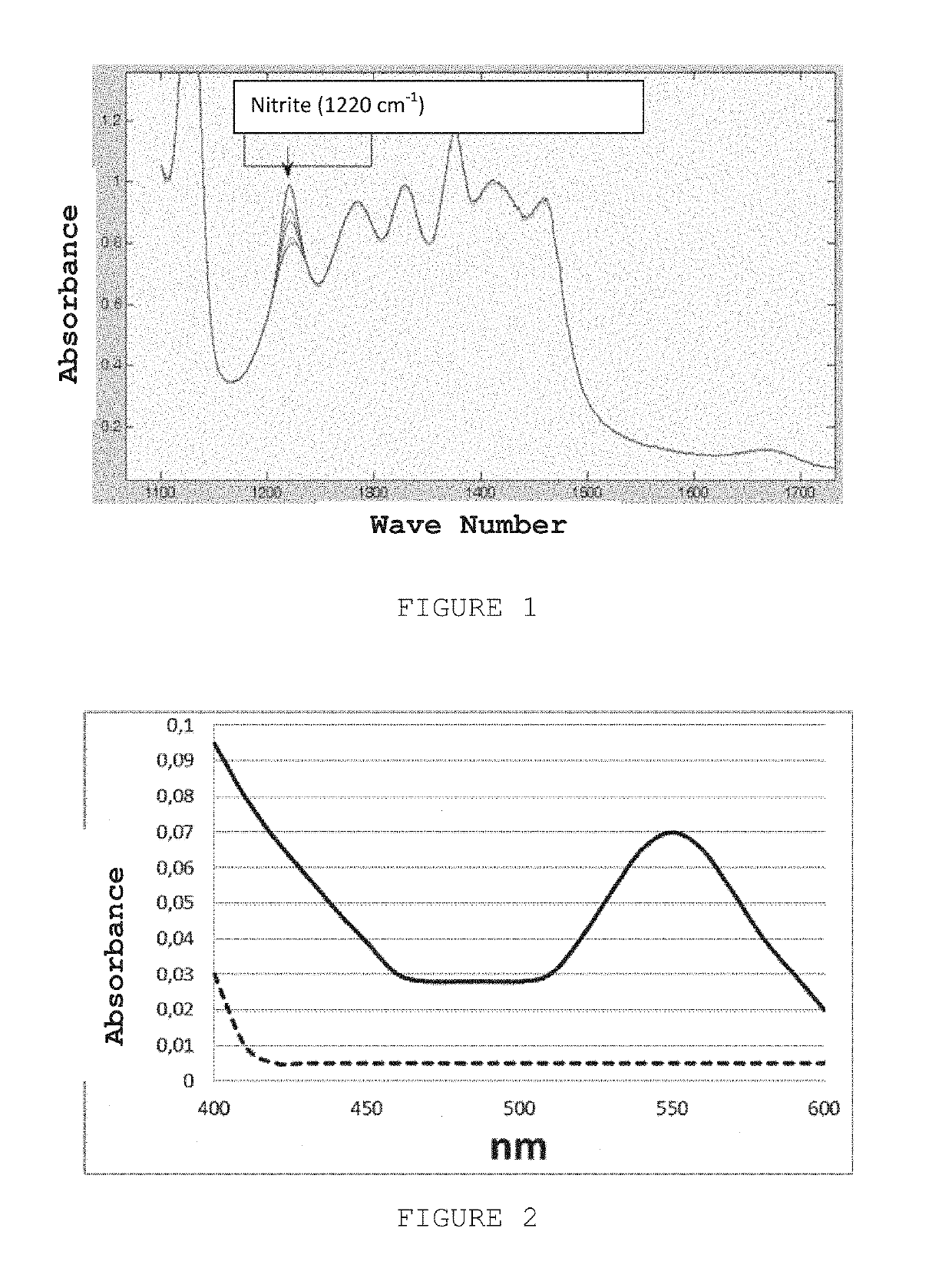
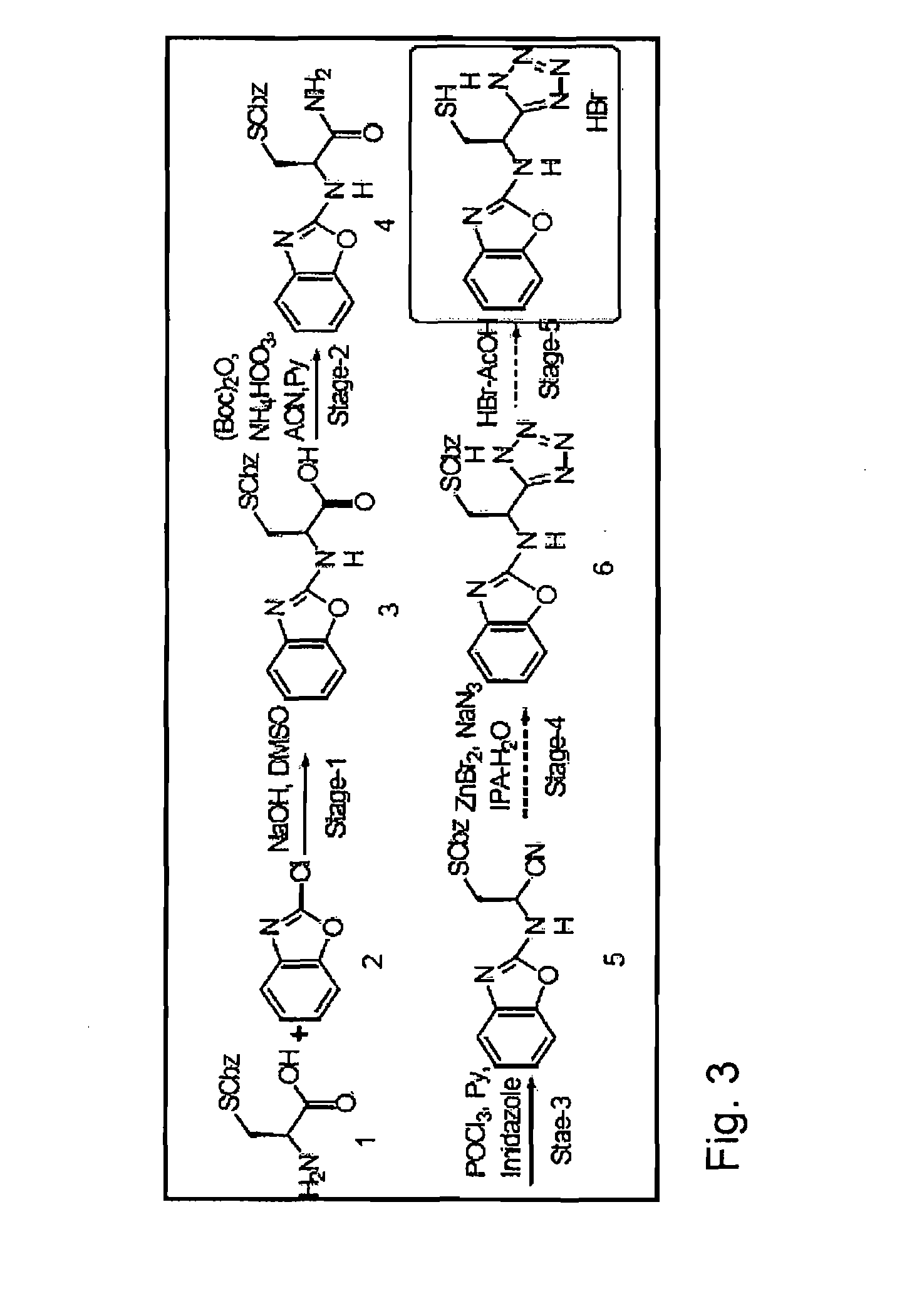
The present invention describes a process for the synthesis of S-nitrosothiols and the subsequent incorporation of these compounds in hydrophilic macromolecular compositions. By the process described herein, the S-nitrosothiols are synthesized in a device (FIG. 2) in a first step from the S-nitrosation reaction of their respective precursor thiols (A), promoted by a mechanical action that puts the thiols in contact with the nitrous acid formed from nitrite anions in acidic medium (B), and in a second mechanical operation, the freshly formed S-nitrosothiols are incorporated in an application vehicle (C) based on hydrophilic macromolecular compositions that increases their thermal stability. Therefore, the process under consideration combine the pre-application synthesis of S-nitrosothiols with their subsequent incorporation in delivery vehicles, with provide a relative stabilization of the S-nitrosothiols for sufficient periods so that the formulations prepared by this process may be stored in a domestic refrigerator during its time of use in its several possible applications.
Owner:CRISTALIA PROD QUI FARM LTDA +1
Improvements relating to skin dressings
InactiveCN101878046AIncrease spawn rateIncrease speedAbsorbent padsDermatological disorderMedicineBiological activation
Owner:INSENSE LIMITED
Red blood cells loaded with S-nitrosothiol and uses therefor
Red blood cells can be loaded with low molecular weight nitrosylating agents, such as S-nitrosothiols, to act as a delivery system for NO+ groups to tissues. Loaded red blood cells can be used in methods of therapy for conditions which are characterized by abnormal O2 metabolism of tissues, oxygen-related toxicity, abnormal vascular tone, abnormal red blood cell adhesion, or abnormal O2 delivery by red blood cells. Such treatment of red blood cells can be extended to in vivo therapies, with the object to achieve an increase in the ratio of red blood cell S-nitrosothiol to hemoglobin.
Owner:DUKE UNIV
Formulation For Release Of Nitric Oxide
ActiveUS20190282610A1Facilitates inversionImprove microcirculationInorganic active ingredientsSexual disorderSolventReducing agent
A composition for releasing nitric oxide which provides a rapid release of nitrogen monoxide, and also allows formation of S-nitrosothiol compounds which provide a slower extended release of nitrogen monoxide. A composition kit includes two components that are mixed together to initiate nitric oxide release. The kit includes: a liquid phase comprising a solvent and at least one reducing agent; and a solid phase comprising a nitrate and / or nitrite salt, copper ions and at least one thiol. A method of treating sexual dysfunction involves topically applying the composition to an area of the subject to be treated.
Owner:GLANOTECH LTD
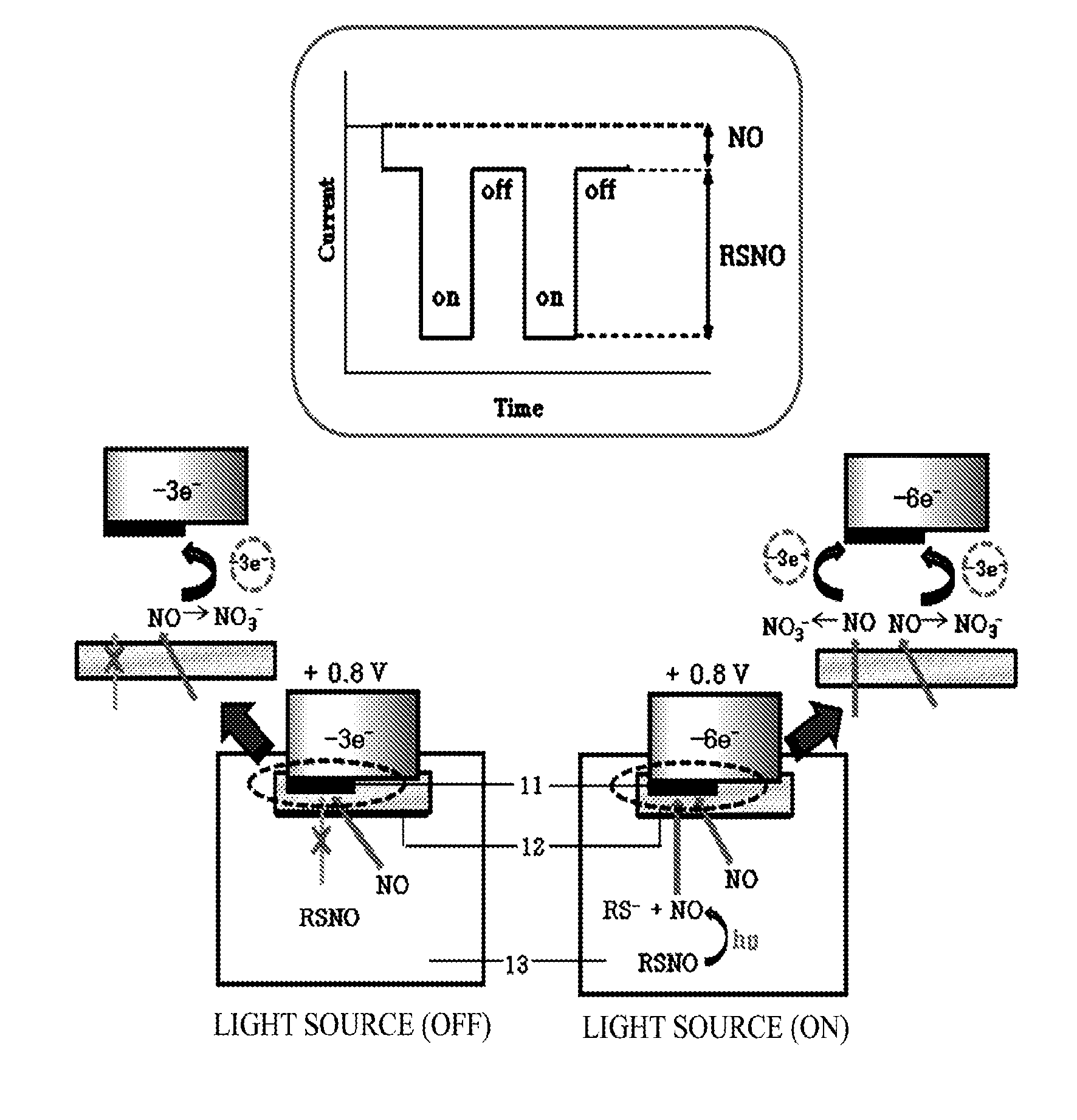
Amperometric sensors and devices for measuring concentration of s-nitrosothiols based on photo-induced decomposition of s-nitrosothiols
ActiveUS20140014512A1Reliably measureEasy to manufactureCurrent/voltage measurementMaterial electrochemical variablesDecompositionCurrent sensor
Provided are an amperometric sensor and device for electrochemically quantifying nitrosothiol (RSNO), which is associated with storage and delivery of nitric oxide (NO) in the human body. The amperometric sensor for measuring a concentration of RSNO includes an electrode configured to measure an electric current generated by an oxidation reaction of NO, and a means configured to start and stop photo-induced decomposition of RSNO. Here, the electric current is measured by the oxidation reaction of NO before and after the photo-induced decomposition of RSNO. The amperometric sensor may measure separate signals of RSNO and NO, which are present in the sample at the same time, using one electrode, thereby preventing an inhibition action caused by NO during the measurement of RSNO. Also, the amperometric sensor is simple in structure and easy to manufacture and may be applied to manufacture of a small electrode, and thus may be developed as a sensor for in vivo measurements.
Owner:I SENS INC
Red blood cells loaded with s-nitrosothiol and uses therefor
Red blood cells can be loaded with low molecular weight nitrosylating agents, such as S-nitrosothiols, to act as a delivery system for NO+ groups to tissues. Loaded red blood cells can be used in methods of therapy for conditions which are characterized by abnormal O2 metabolism of tissues, oxygen-related toxicity, abnormal vascular tone, abnormal red blood cell adhesion, or abnormal O2 delivery by red blood cells. Such treatment of red blood cells can be extended to in vivo therapies, with the object to achieve an increase in the ratio of red blood cell S-nitrosothiol to hemoglobin.
Owner:STAMLER JONATHAN S +3
Nitroso mercaptan fluorescent quantitative determination kit
InactiveCN101393126ASimplify complicated stepsSimple processChemiluminescene/bioluminescenceFluorescence/phosphorescenceNitrosoFluorescence
The invention relates to a fluorescent quantitative detection reagent kit for s-nitrosothiol, which comprises four reagent bottles which are filled with four reagents and are a reagent bottle 1, a reagent bottle 2, a reagent bottle 3 and a reagent bottle 4 respectively, a kit body (6), a kit cover (7), retaining plates provided with holes (5), and a reagent kit specification (8), wherein the reagent bottles are separated and fixed through the retaining plates provided with holes (5); a descriptive label is arranged on each reagent bottle; and the accurately weighed reagents with fixed weight proportions are hermetically enclosed into the reagent bottles, wherein 1 millimeter of 2, 3-diaminonaphthalene solution which is dissolved into 0.62 mass of HCl is filled into the reagent bottle 1; 1 millimeter of 2, 3-diaminonaphthalene solution which is dissolved into 0.62 mass of HCl and contains mercuric chloride is filled into the reagent bottle 2; a NaNO2 standard solution is filled into the reagent bottle 3; and 2.8 masses of NaOH solution is filled into the reagent bottle 4; and the reagent bottle 1 and the reagent bottle 2 are 1.5 millimeters of EP tubes which are packaged by black plastic bags. The reagent kit is simple, convenient and flexible to detect the s-nitrosothiol level of various cell samples.
Owner:HUAZHONG NORMAL UNIV
Methods and systems for indentifying and monitoring S-nitrosothiols in biological samples
InactiveUS7507585B2Reduced activityHigh activityEnzyme stabilisationTissue cultureFormazanParaformaldehyde
The present invention describes methods and kits for determining the level of S-nitrosothiols in biological fluid samples. The method includes separating tissue or cellular matter from the biological fluid sample and then contacting the biological fluid sample with paraformaldehyde in an amount sufficient to fix free thiols thereby essentially eliminating diaphorase activity of the free thiols. The biological fluid sample is then contacted with NADPH and nitro blue tetrazolium (NBT) wherein S-nitrosothiols in the biological fluid sample facilitate the reduction of NBT to NBT formazan or diformazan in the presence of paraformaldehyde. The amount of reduced NBT is measured and the determined levels correlate to the amount of S-nitrosothiols in the biological fluid sample.
Owner:WHALEN ERIN J
S-Nitrosothiol Compounds and Related Derivatives
InactiveUS20100105685A1Stabilizing breathing rhythm of a mammalConvenient and smoothBiocideOrganic chemistryS-NitrosylationMedicine
The present invention is directed to a method of treating a lack of normal breathing control including the treatment of apnea and hypoventilation associated with sleep, obesity, certain medicines and other medical conditions. In an aspect, the invention is directed to treating disordered control of breathing by administering an composition comprising a single compound which treats lack of normal breathing. In another aspect, the invention is directed to treating disordered control of breathing by administering an composition comprising a combination of two or more compounds, at least one of which treats lack of normal breathing. In an aspect, a compound is an S-nitrosylating agent.
Owner:GALLEON PHARMA INC
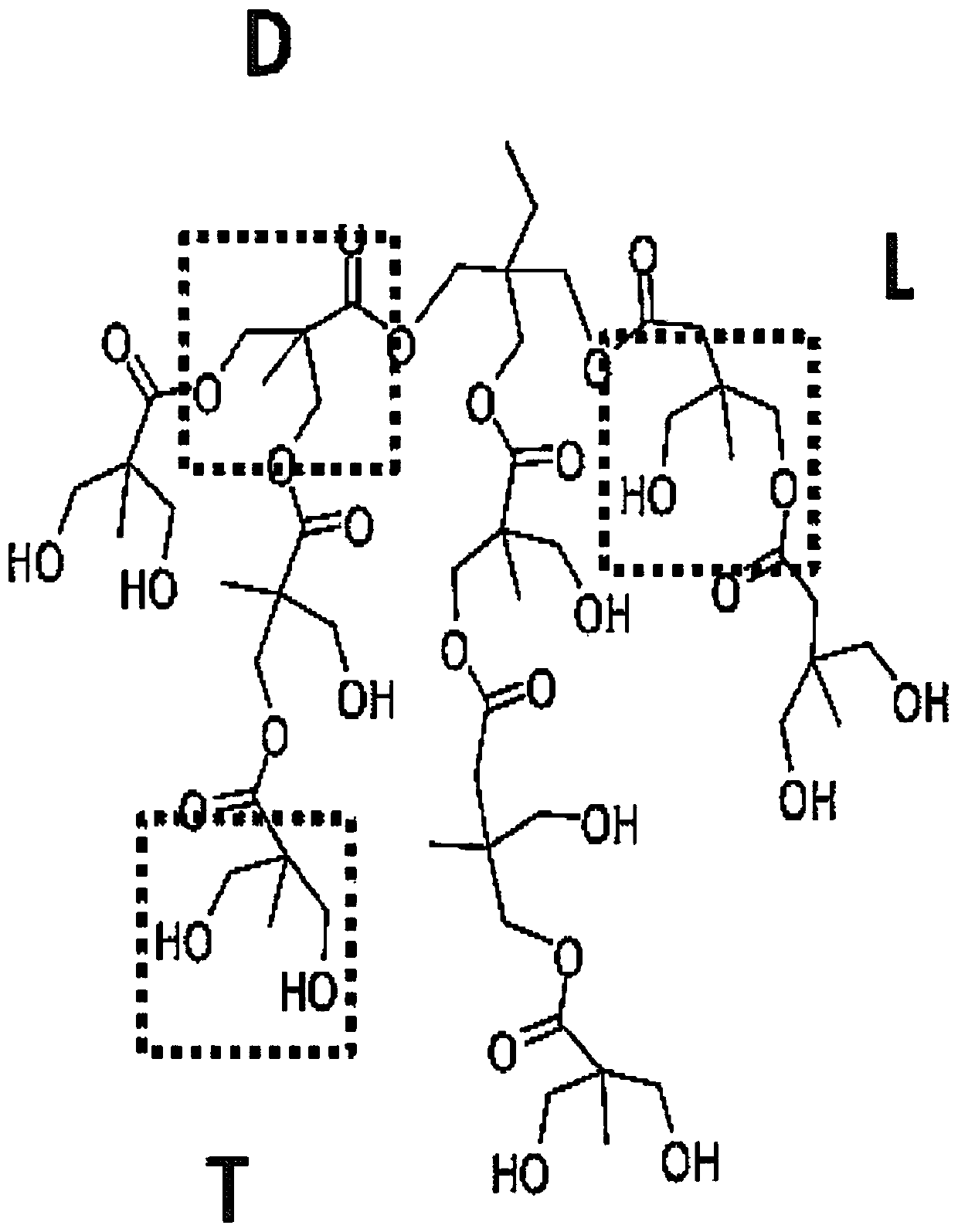
S-nitrosothiol-mediated hyperbranched polyesters
ActiveCN109937234AGroup 4/14 element organic compoundsInorganic active ingredientsHyperbranched polyesterBiodegradable polymer
The invention generally relates to compositions comprising degradable polymers and methods of making degradable polymers. Specifically, the disclosed degradable polymers comprise a biodegradable polymer backbone, a nitric oxide linker moiety, and a nitric oxide molecule. This abstract is intended as a scanning tool for purposes of searching in the particular art and is not intended to be limitingof the present invention.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Process for determining S-nitrosothiols in biological fluids
InactiveUS8148099B2Cheaply and easilyLow costMicrobiological testing/measurementBiological material analysisNitrosoDecomposition
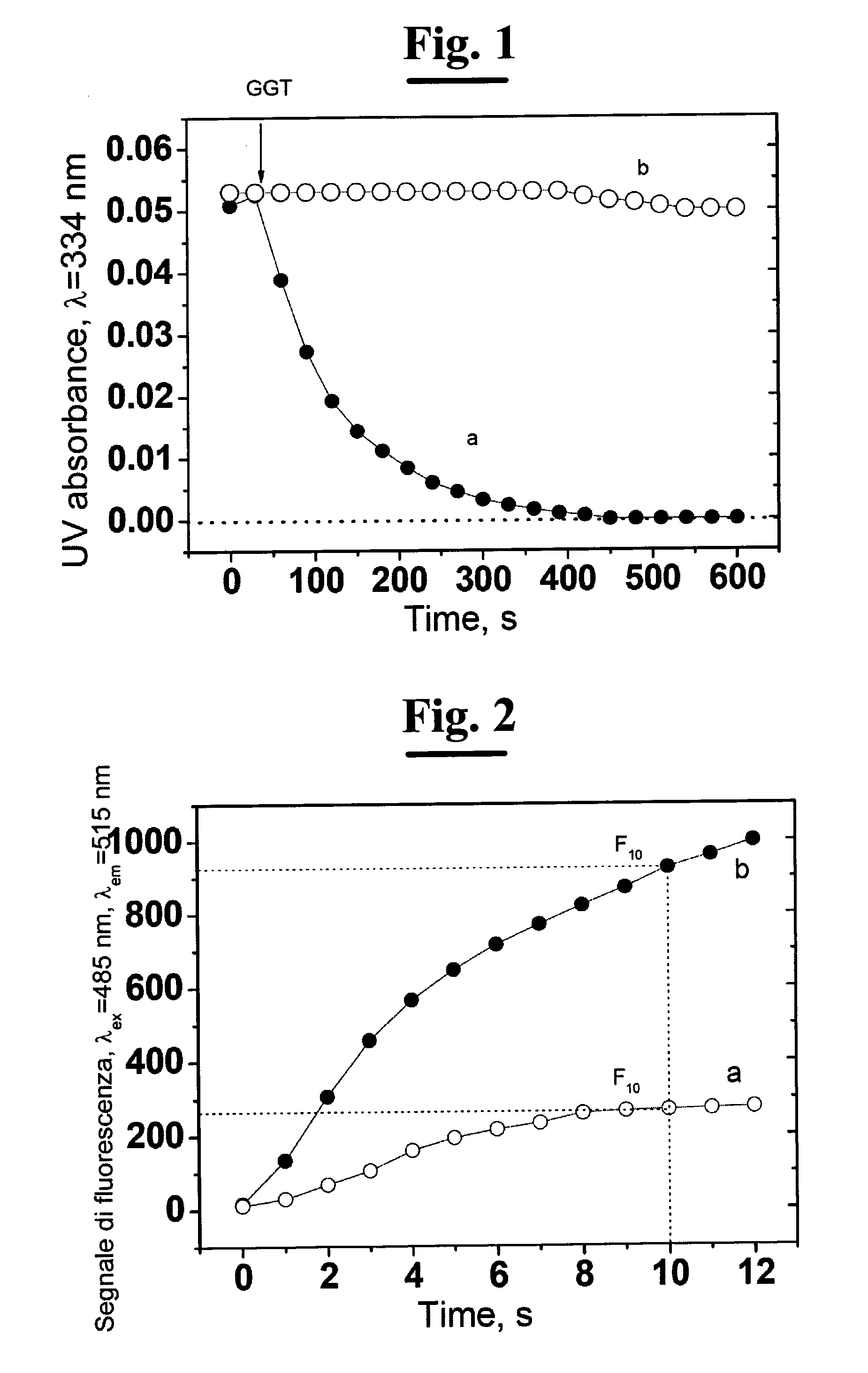
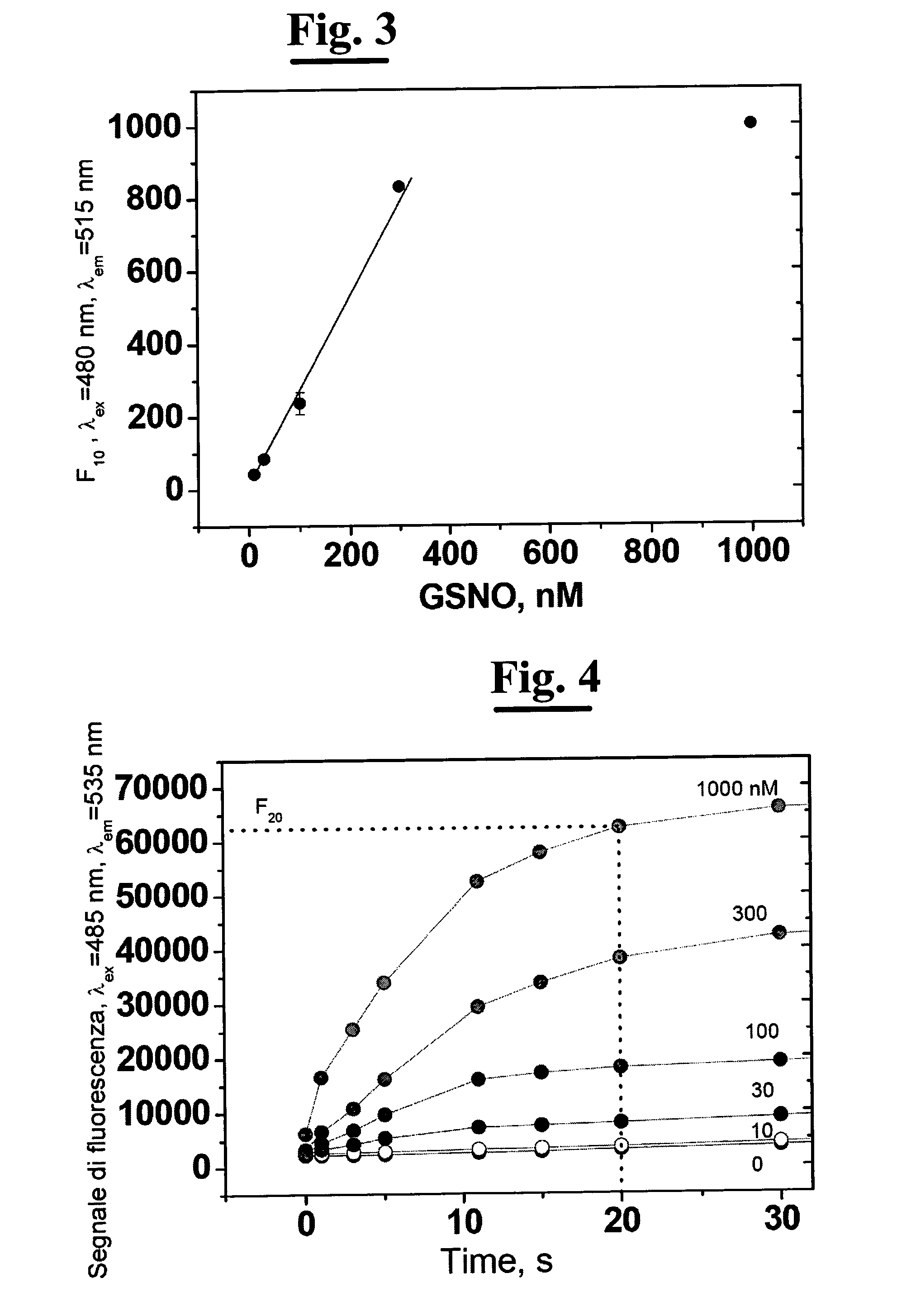
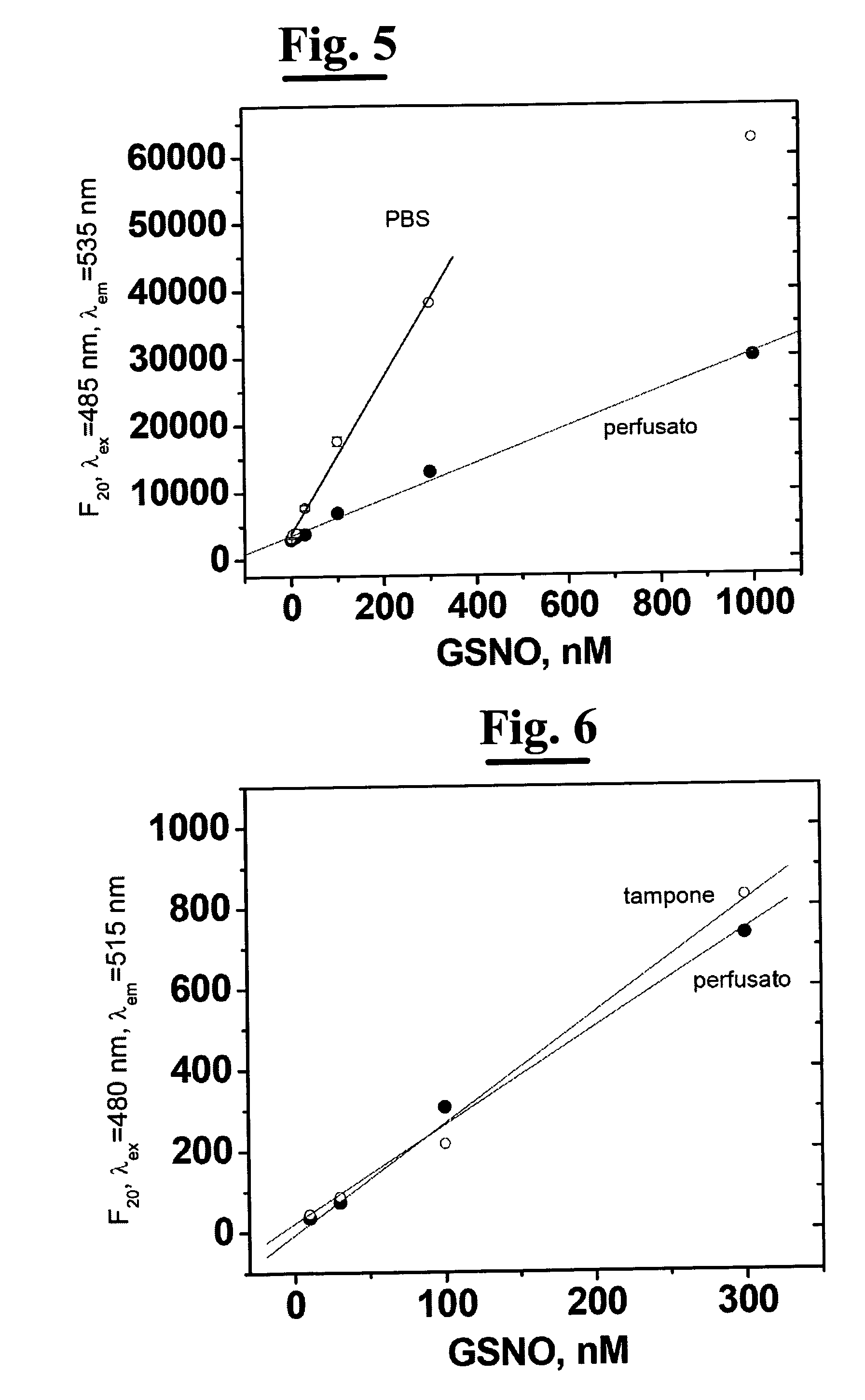
A process for determining S-nitrosothiols, in particular S-nitrosoglutathione, in biological fluids that is easy, selective, cheap with respect to the prior art, which requires the use of equipment commonly available in laboratories, at low cost, which can be used by not qualified operators. The process is based on the hydrolysis of S-nitrosoglutathione (GSNO) by an enzyme, in particular γ-glutamyltranspeptidase (GGT). This enzyme hydrolizes the residual γ-glutamyl of GSNO for giving glutamate (GIu) and S-nitroso-cysteinylglycine (GIyCySNO). In the presence of ions of transition metals GGT speeds up the release of NO since the intermediate that is formed, the GIyCySNO, is much more sensitive to a metal-dependent decomposition. Advantageously, the amount of nitric oxide present in the sample is measured through a reaction thereof with 4,5 diaminof luorescein (DAF-2), said reaction creating a fluorescent compound in an amount proportional to the S-nitrosothiol amount present in the sample. Alternatively, the amount of released NO can be measured by a chemiluminescence analyser, commercially available. In the presence of biological fluids having complex matrix, the introduction of the enzyme is done after separation of the S-nitrosothiol from the other components of the fluid.
Owner:UNIV DI PISA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com