Patents
Literature
45 results about "Percent fat" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
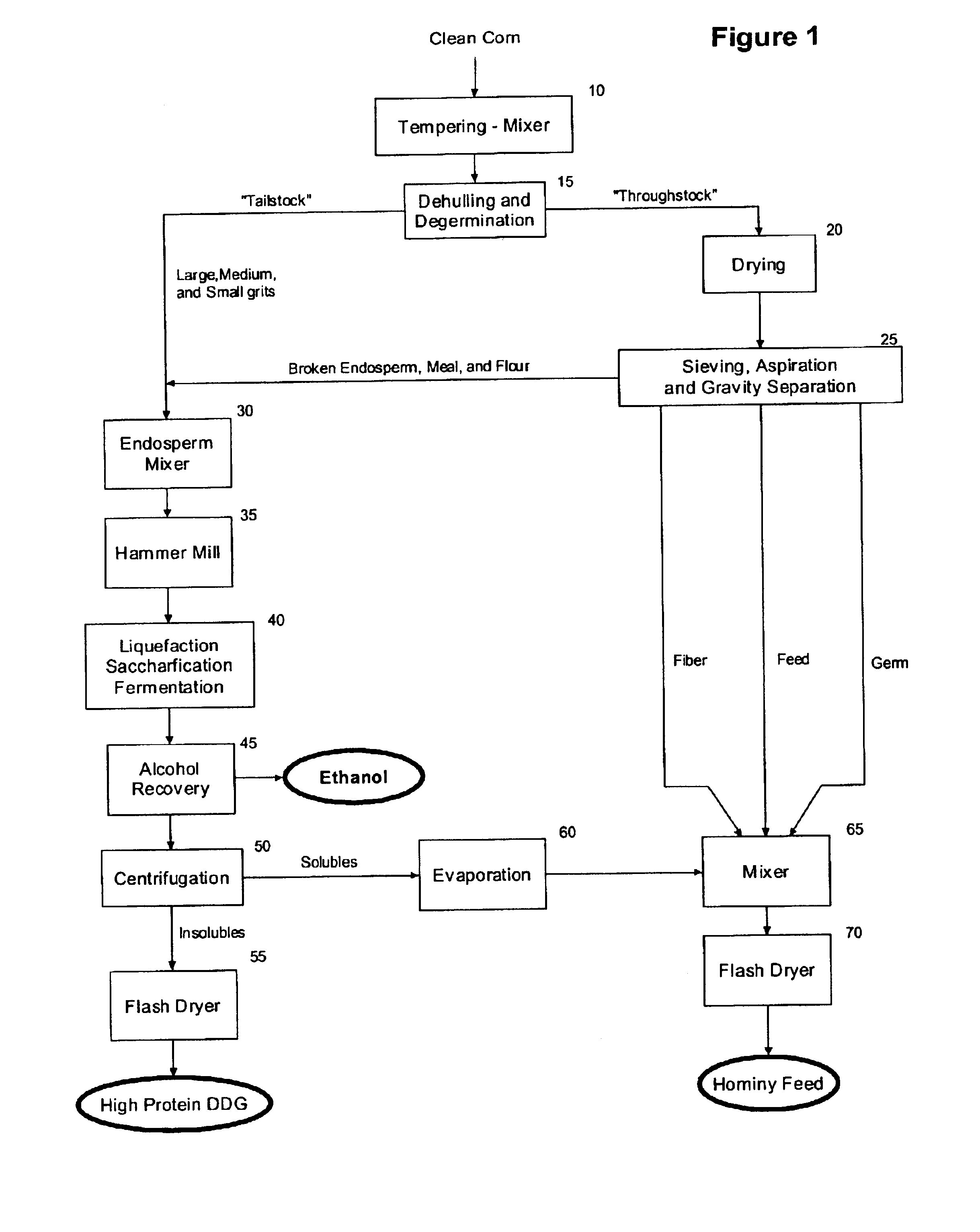
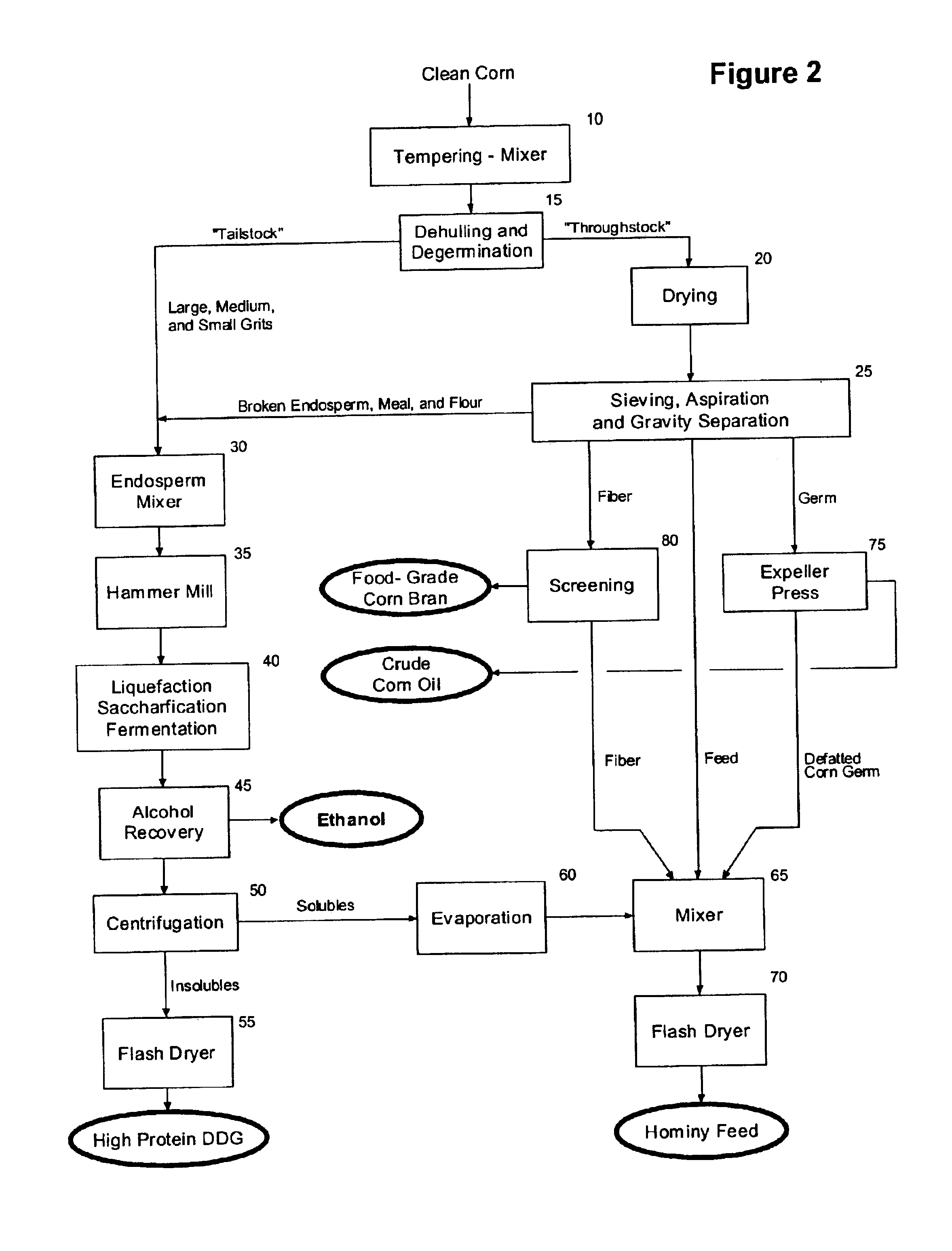
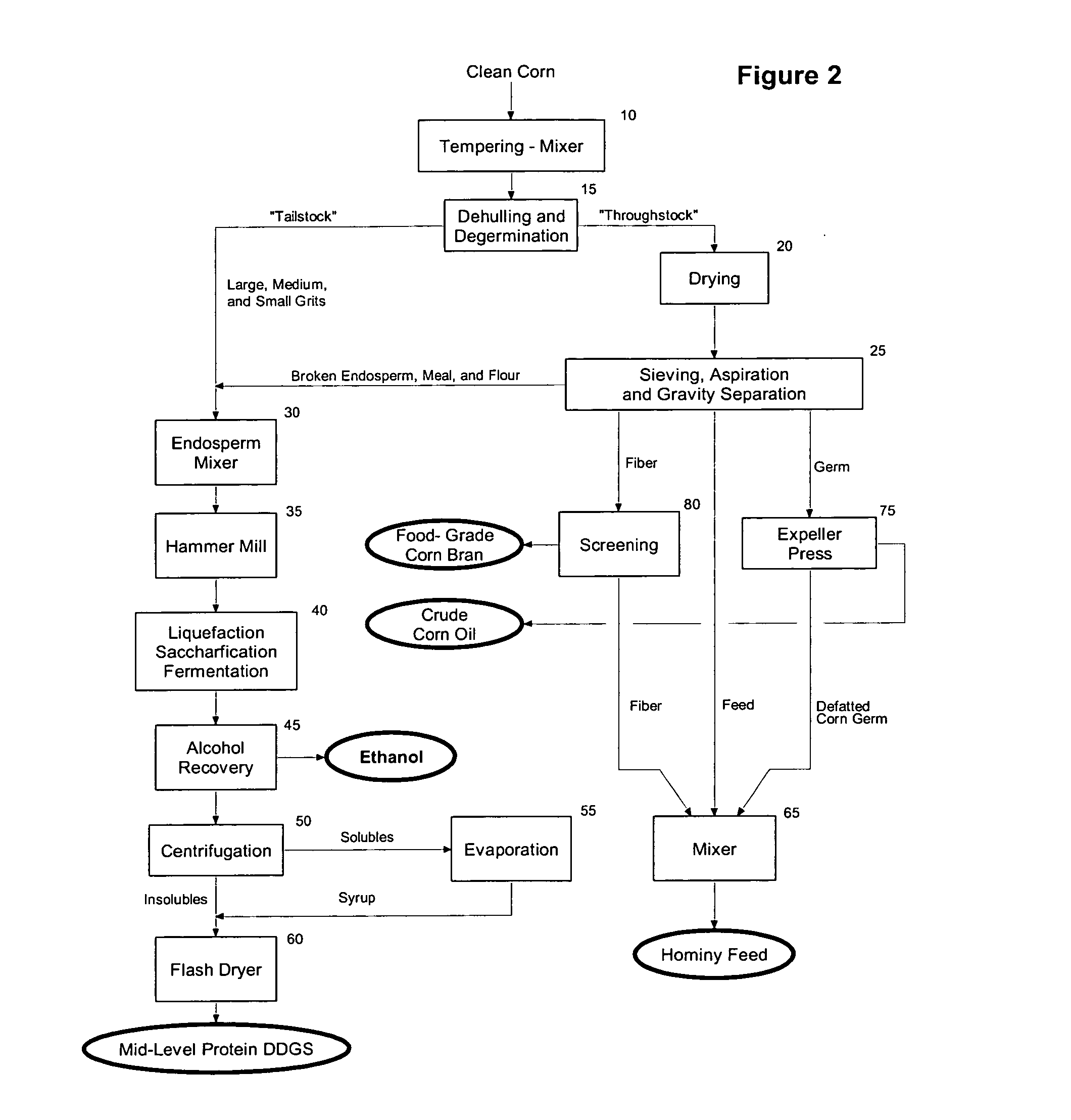
High protein corn product production and use
InactiveUS6962722B2Improve palatabilityImprove digestibilityFood processingClimate change adaptationWeight gainingFiber
The present invention relates to the production of a highly digestible, high protein product (high protein distillers dried grains or high protein DDG) from corn endosperm, and more particularly to a method for the recovery of high protein DDG by using: (i) dehulling and degermination to isolate a low fat, low fiber corn endosperm fraction, (ii) enzymatic hydrolysis to solubilize and alcoholic fermentation to assimilate the starch and non-starch carbohydrates present in the corn endosperm, and (iii) filtration and / or centrifugation to recover the dealcoholized insoluble solids that remain after fermentation of the corn endosperm. The present invention provides an alternative to the traditional dry mill method of processing corn to produce ethanol, and results in the production and recovery of a distillers' by-product (high protein DDG) with increased value and range of use as an ingredient in feeds for farm-raised ruminants and non-ruminants and pet foods. The product of the present invention contains less than about 2.0 weight percent starch, from about 55.0 to about 65.0 weight percent protein, from about 4.5 to about 7.5 weight percent fat, from about 3.0 to about 5.0 weight percent crude fiber, and from about 78.0 to about 90.0 percent total digestible nutrients, and improves the palatability and digestibility of animal feeds and / or pet foods into which it is incorporated, and aids in the management of the health and weight gain of the animal.
Owner:GREENSTOCK RESOURCES
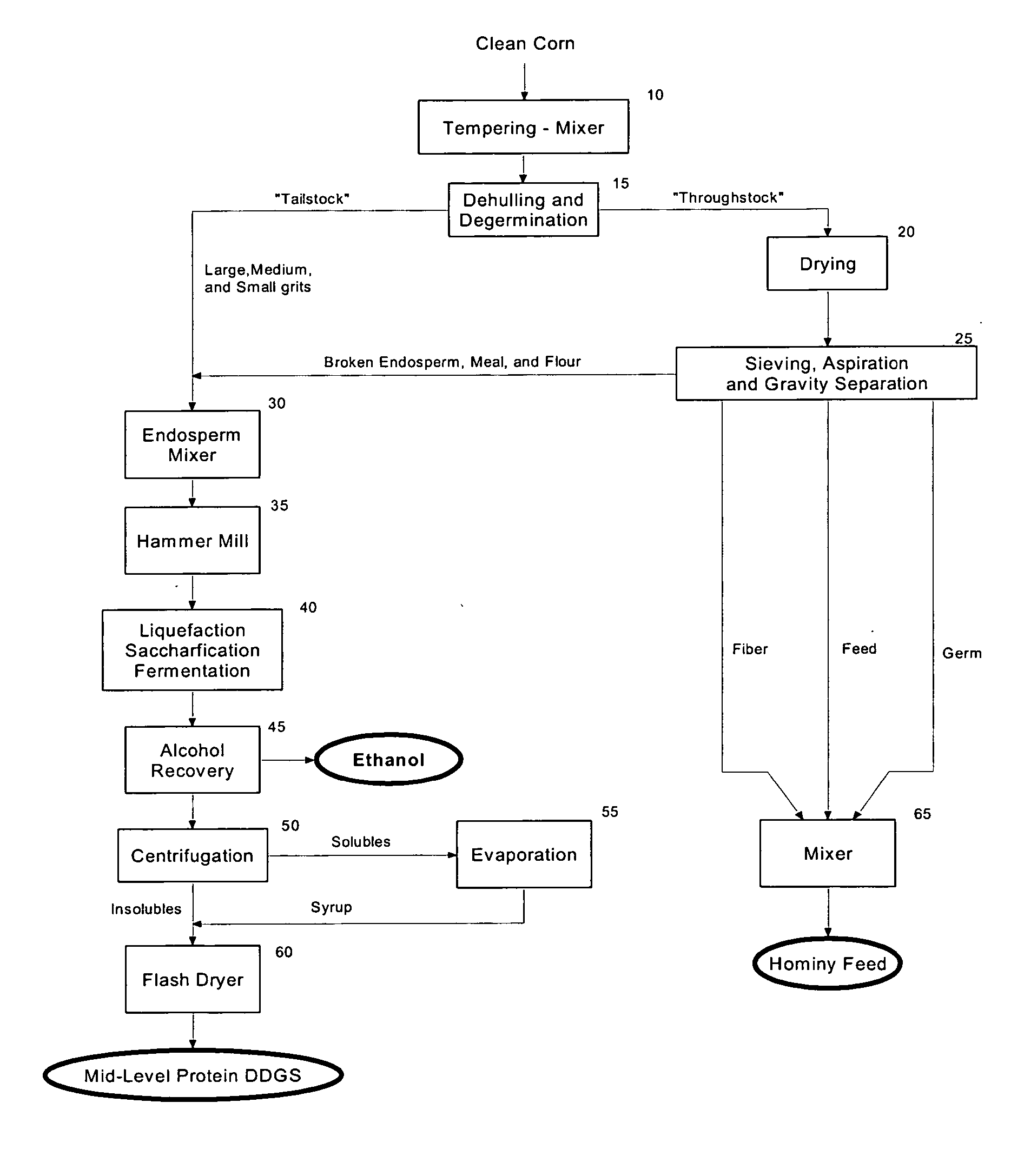
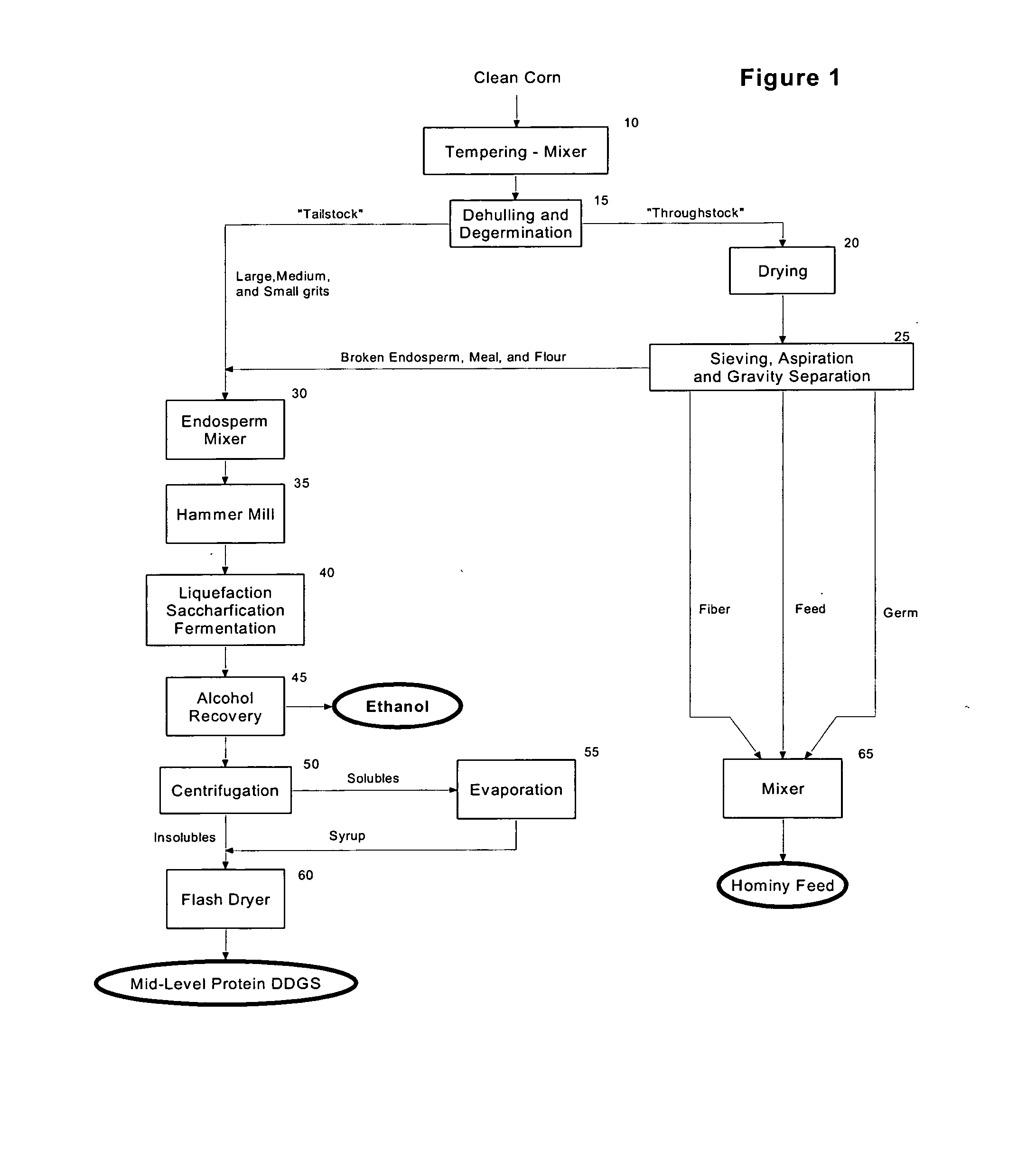
Mid-level protein distillers dried grains with solubles (DDGS) - production and use
InactiveUS20060057251A1Improve palatabilityImprove digestibilityFood processingClimate change adaptationFiberFiltration
The present invention relates to the production of a highly digestible, mid-level protein DDGS from corn endosperm, and more particularly to a method for the recovery of mid-level protein DDGS by using: (i) dehulling and degermination to isolate a low fat, low fiber corn endosperm fraction, (ii) enzymatic hydrolysis to solubilize and alcoholic fermentation to assimilate the starch and non-starch carbohydrates present in the corn endosperm, and (iii) filtration, centrifugation and / or evaporation to recover the dealcoholized insoluble and soluble solids that remain after fermentation of the corn endosperm. The product of the present invention contains less than about 5.0 weight percent starch, from about 40.0 to about 52.5 weight percent protein, from about 4.5 to about 8.5 weight percent fat, from about 3.0 to about 6.0 weight percent crude fiber, and from about 78.0 to about 90.0 percent total digestible nutrients.
Owner:GREENSTOCK RESOURCES
Sarms and method of use thereof
InactiveUS20100249228A1Increasing lean massReducing fat mass/percent fat massBiocideOrganic chemistryCarcass compositionReduced fat
This invention is directed to a feed composition and method of affecting the carcass composition by increasing the lean mass, reducing the fat mass, and / or reducing the percent fat mass comprising SARM compounds.
Owner:UNIV OF TENNESSEE RES FOUND
Reduced-fat flavored coating and methods of using same
Owner:NESTEC SA
Dairy products with reduced average particle size
InactiveUS6861080B2Improve the immunityIncrease pressureMilk preparationCheese manufactureCream cheeseMilk products
The present invention relates to superior dairy products which have firmness qualities and textural qualities not observed in conventional dairy products. The dairy products of this invention have average fat particle sizes of less than about 0.8 microns, preferably of about 0.1 to about 0.8 microns, and more preferably about 0.2 to about 0.6 microns. The dairy products which may be manufactured using this process are cream cheese, sour cream, and dairy products containing at least 4 percent fat. The present invention also provides a process for making a cream cheese product without the removal of whey and having average fat particle sizes of less than about 0.8 microns, preferably of about 0.1 to about 0.8 microns, and more preferably about 0.2 to about 0.6 microns.
Owner:KRAFT FOODS GRP BRANDS LLC
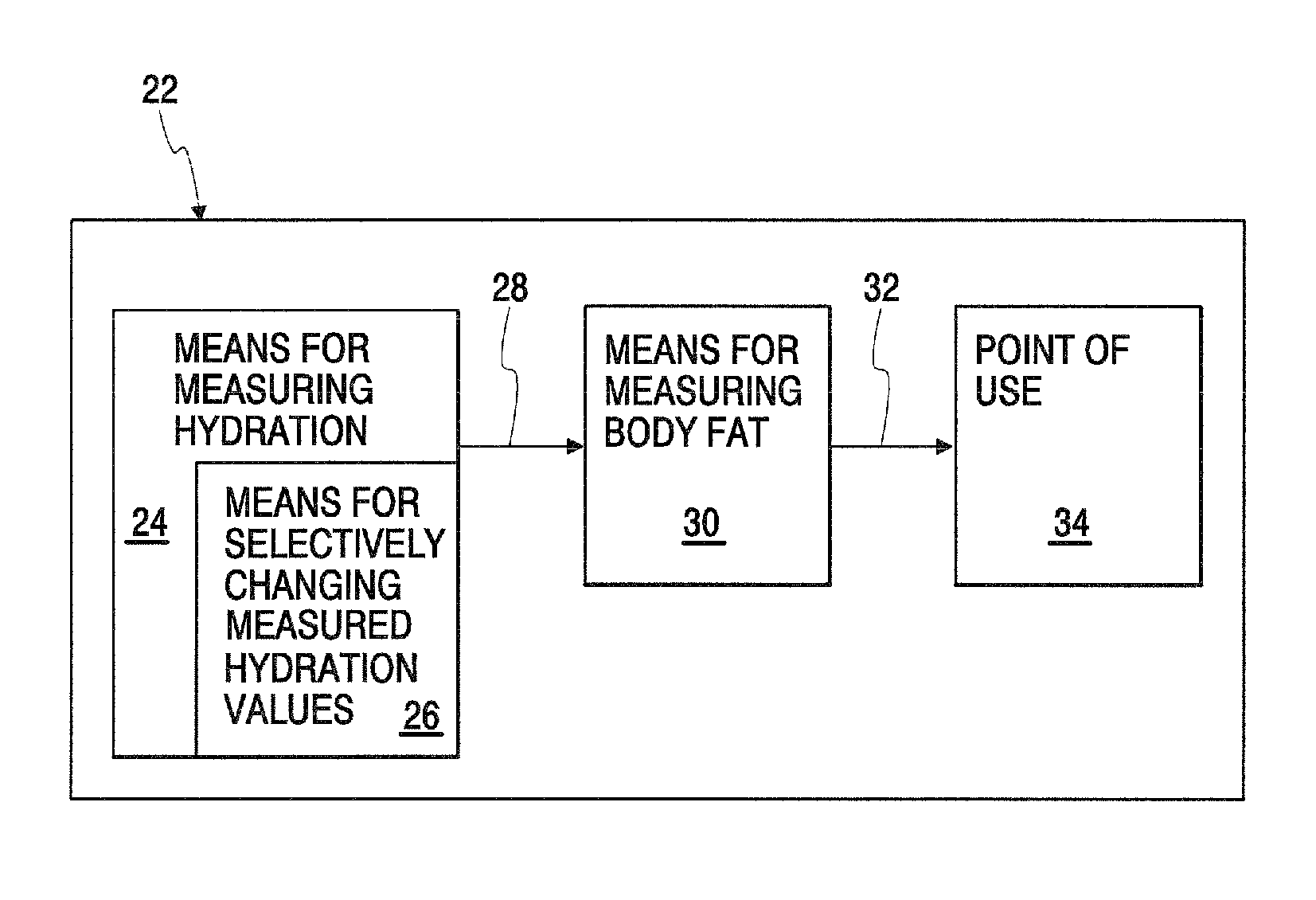
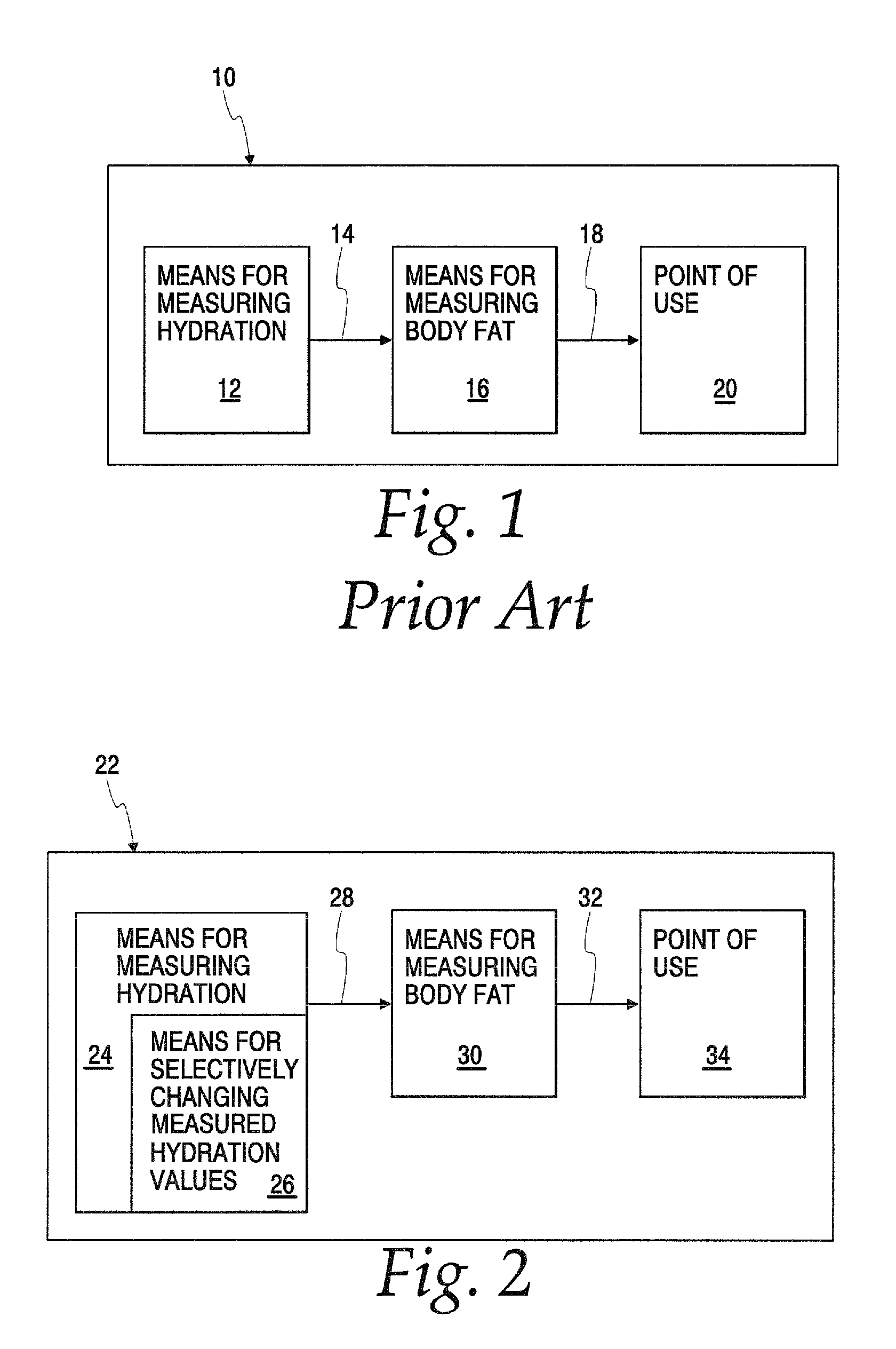
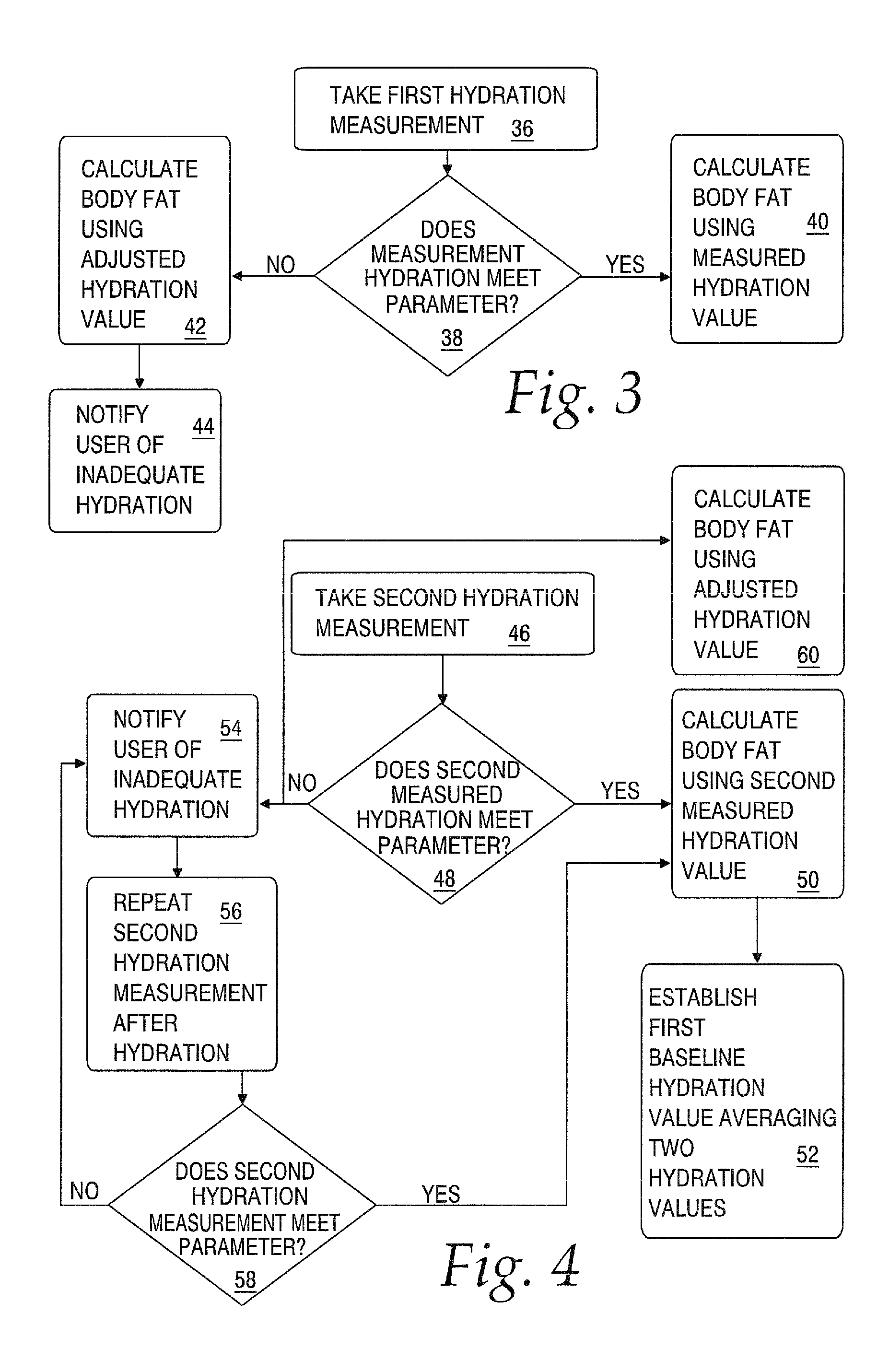
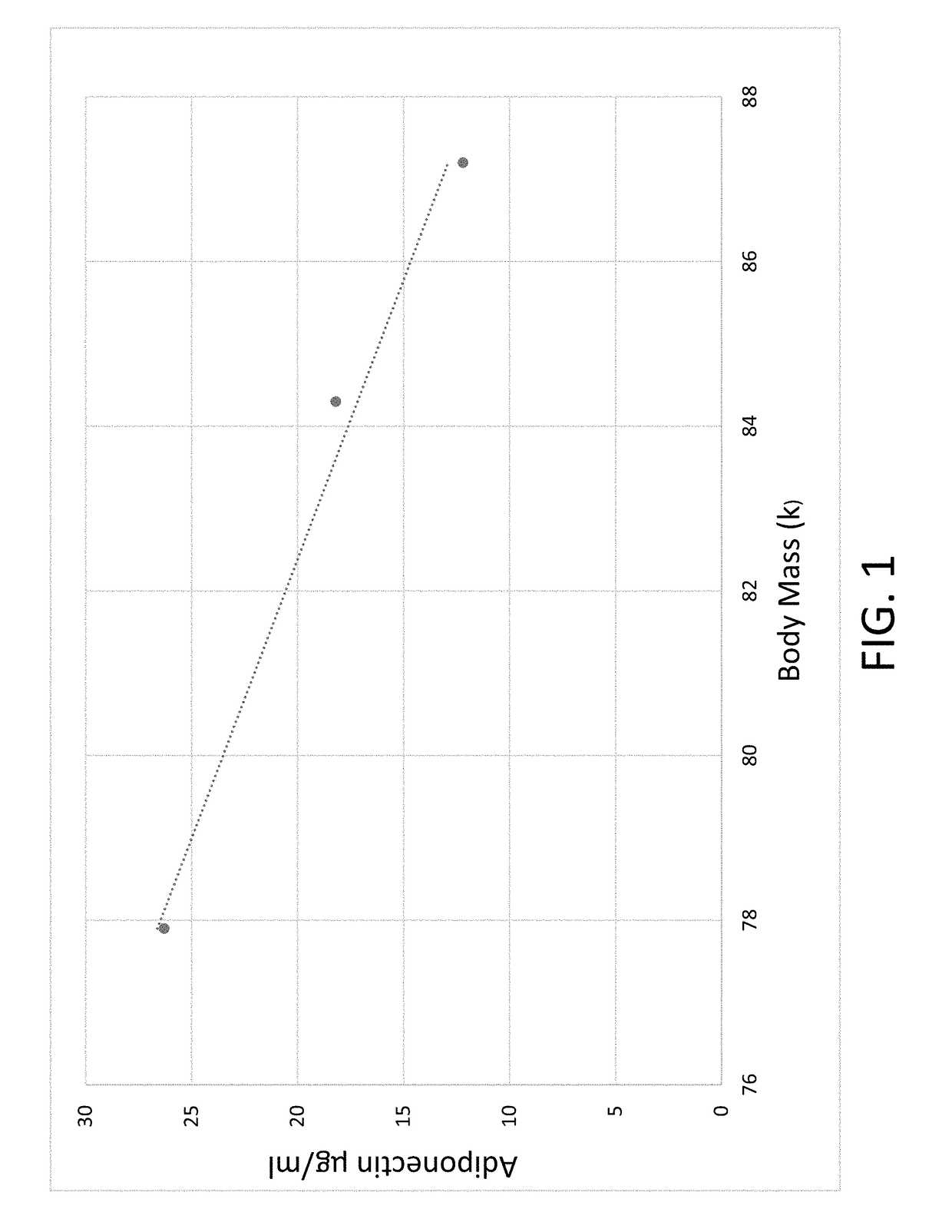
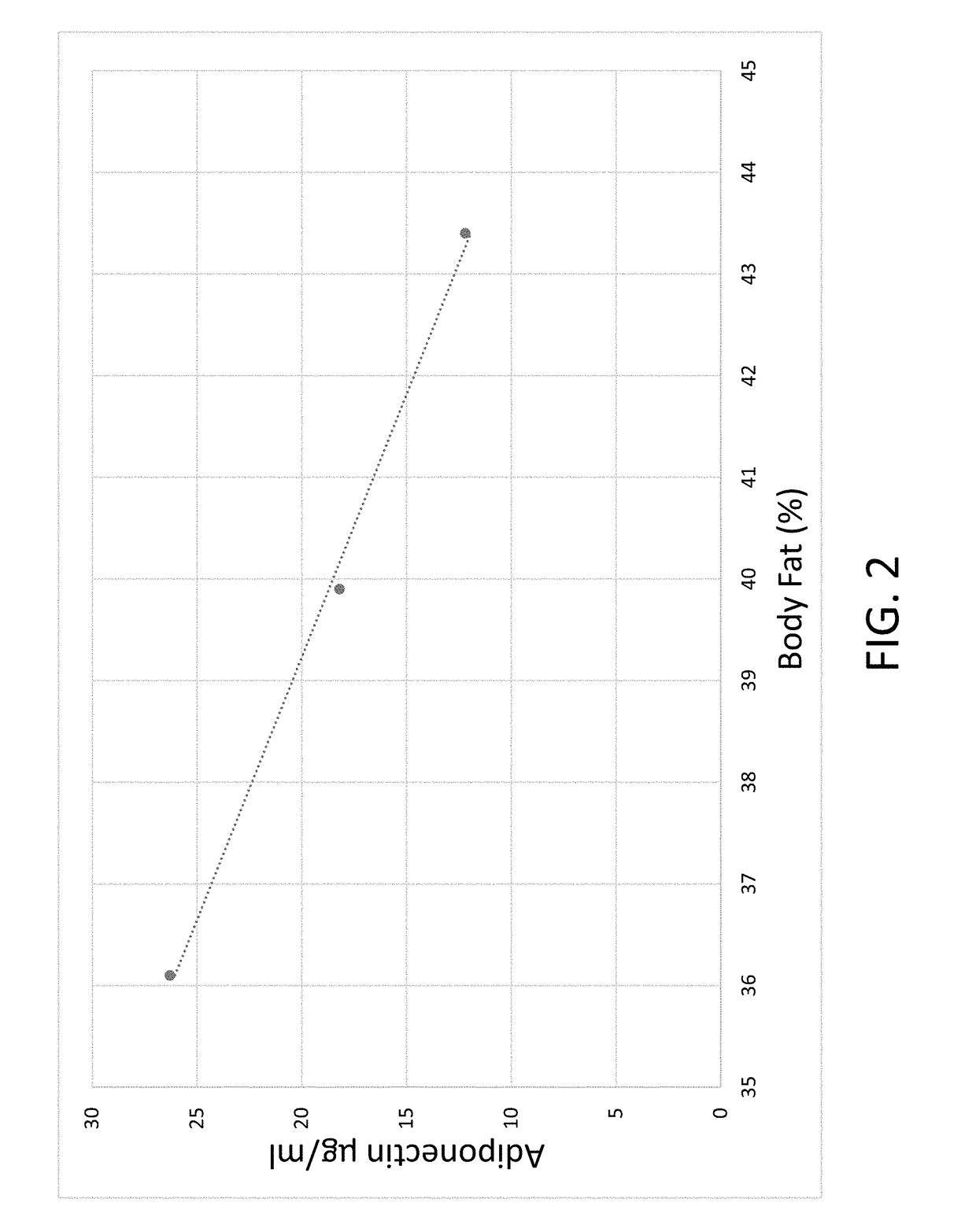
Body composition measuring device capable of comparing current measured value with past measured value
InactiveUS20050209528A1Easy to confirmCovering/liningsBuilding componentsData displayBody composition measure
In a display section, a measured value of information of a living body is displayed on a measured data display section, and a figure representing a human body including body parts such as arm and leg is displayed on a body display section. For example, a percentage of muscle and a percentage of body fat measured 30 days ago and a current percentage of muscle and percentage of body fat are compared with each other, and respective increase / decrease values are displayed on the measured data display section as well as the measured values. Further, a color of illumination in the background of the body display section is changed depending on a degree of the increase / decrease value. For example, the background of the body display section is illuminated in red when the percentage of muscle is decreased by a % or more, blue when increased by a % or more, and green when a percentage of variation is less than a % in comparing current data with data measured 30 days ago.
Owner:OMRON HEALTHCARE CO LTD
Decontamination methods for meat using carbonic acid at high pressures
InactiveUS20050260311A1Avoid separationEfficient separationMeat/fish preservation using liquidsGaseous food ingredientsHigh densityLean meat
A method for separating lean meat from lean meat-containing material, includes combining a particulate material with fluid, subcritical carbon dioxide at a pH of about 7 or less and a pressure of about 600 psig. The material and fluid is introduced into the vessel and is separated into low density and high density fractions. The material from the low density fraction is removed via an outlet and has a higher percentage of fat than the material introduced into the vessel. The material from the high density fraction is removed via an outlet and has a higher percentage of lean meat than the material introduced into the vessel. The vessel can include a centrifuge or a vessel disposed toward the vertical having an upper and lower outlet, wherein the separation is achieved by the respective densities of the material, and the natural or an artificial gravity field, such as in a centrifuge.
Owner:STONE MICHAEL
Method and bottle for infant feeding
A method for feeding an infant by non-human milk including the steps of (a) feeding the infant by fore-milk equivalent having a volume of 30-60% of a total meal and a percentage of fat of 2.5-3.5%; and (b) feeding the infant by hind-milk equivalent having a volume of 40-70% of the total meal and a percentage of fat of 3.7-5.5%.
Owner:SHAPIRA NIVA
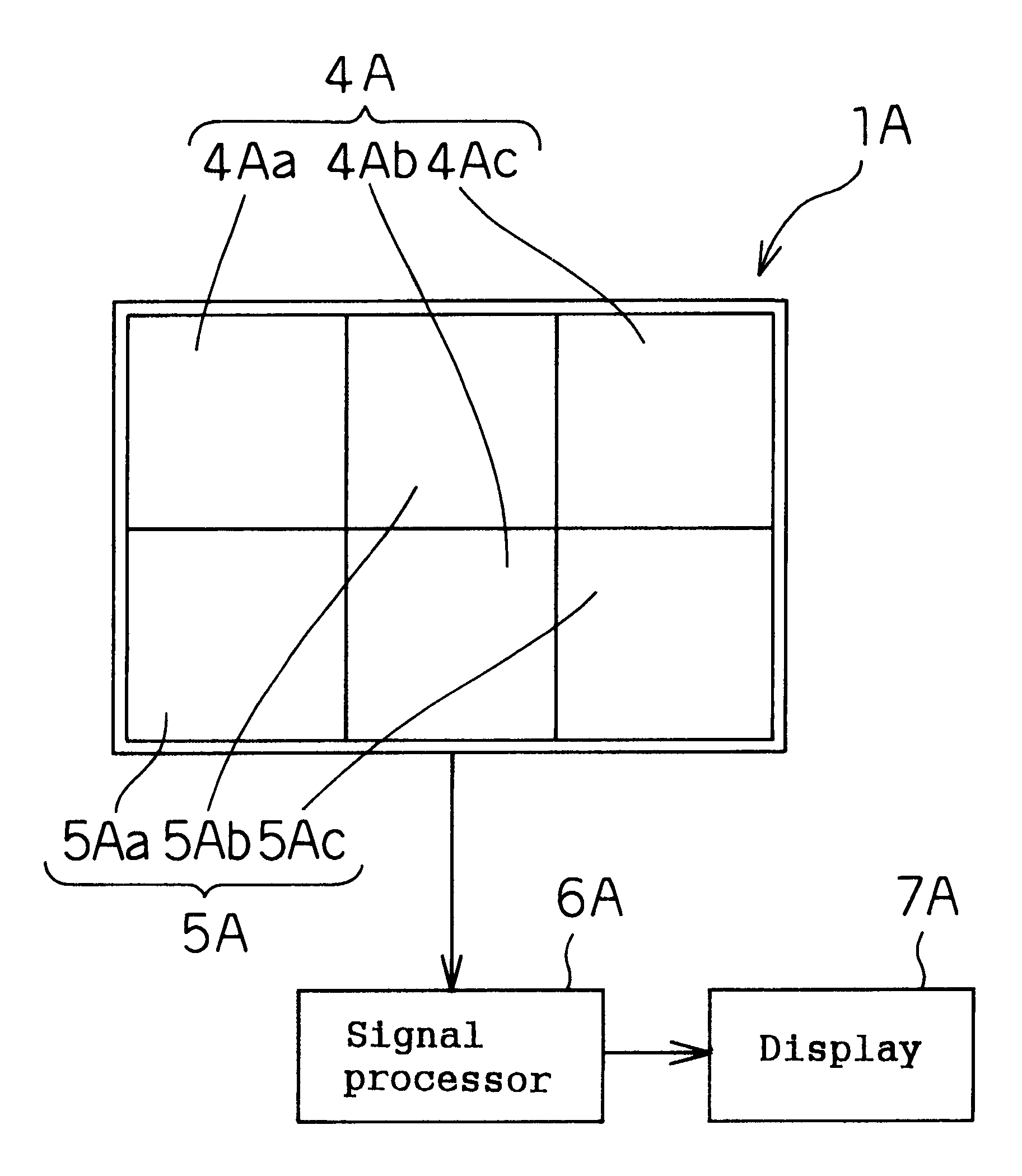
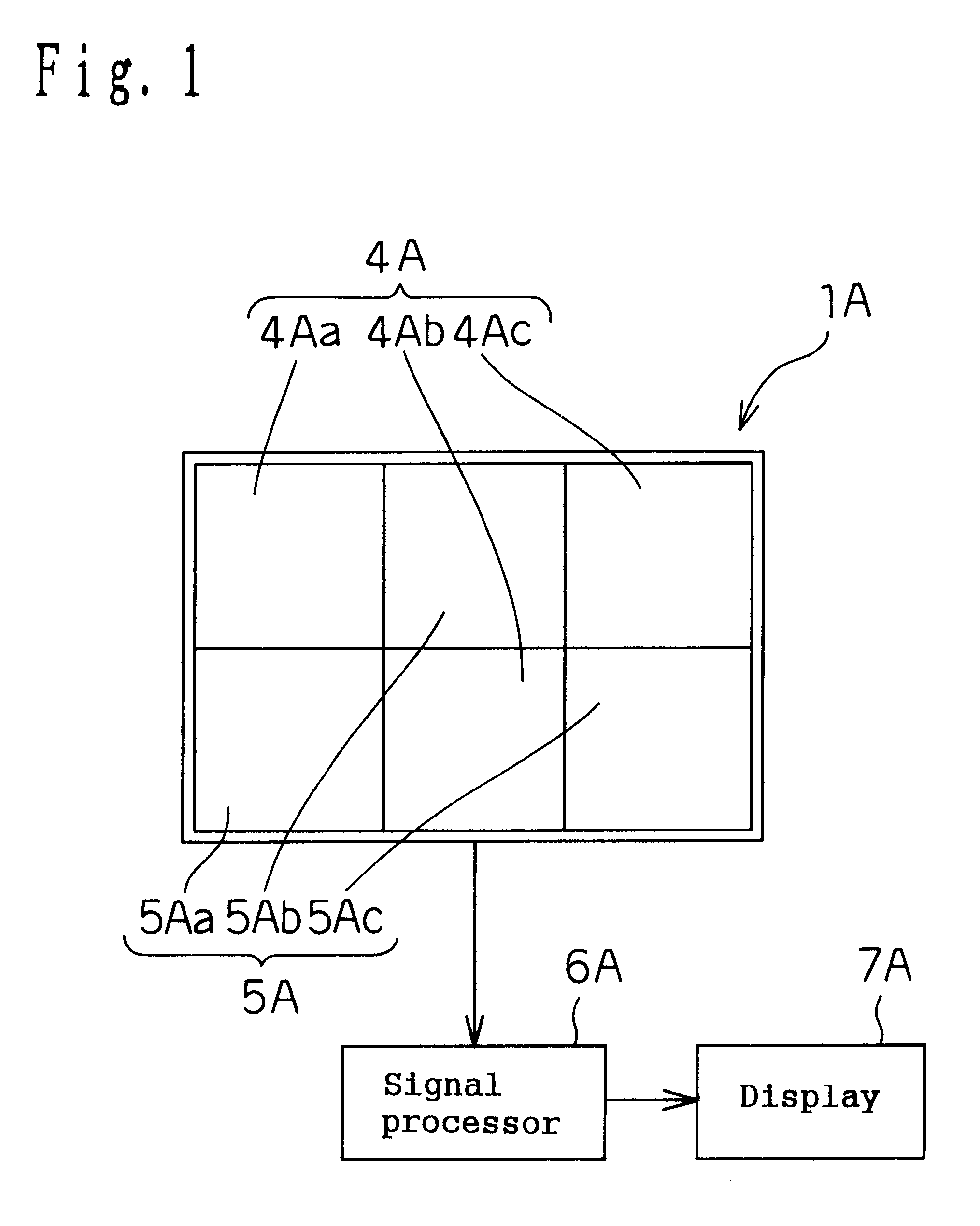
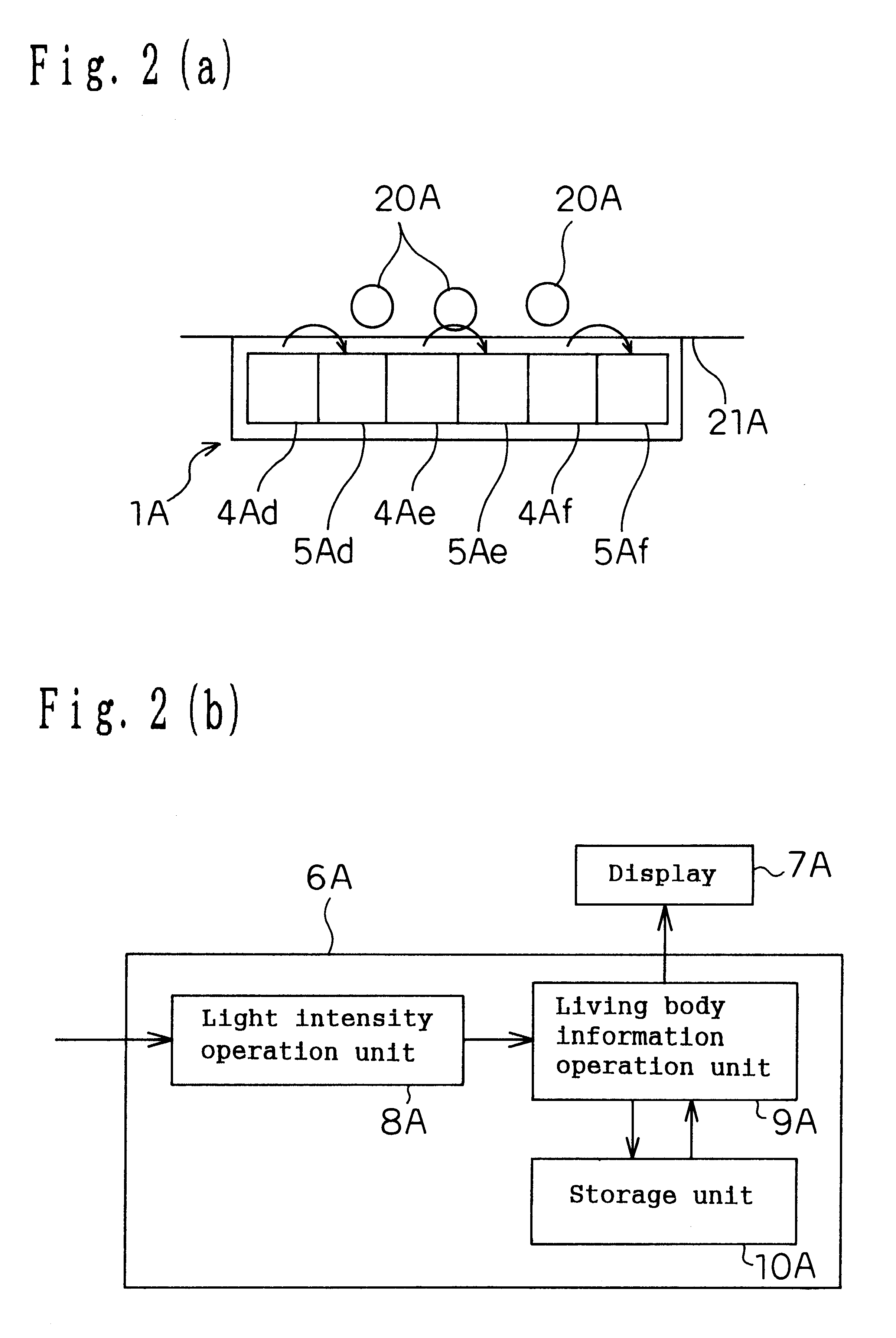
Living body information measuring apparatus living body information measuring method body fat measuring apparatus body fat measuring method and program recording medium
InactiveUS6584340B1Improve accuracySurgeryMaterial analysis by optical meansBiological bodyMeasurement device
A body fat measuring apparatus is provided with a light emitting device 1 for projecting light rays to a subject's tissue, light receiving devices 3 and 4 for detecting a transmitted light ray having passed through the subject's tissue and / or a reflected light ray reflected inside the subject's body, and a CPU 6 for calculating the subject's subcutaneous fat thickness and / or body fat percentage by performing an operation by use of the detection results of the light receiving devices 3 and 4. The light receiving devices 3 and 4 are situated at different distances from the light emitting device 1.
Owner:MATSUSHITA ELECTRIC WORKS LTD
Method and bottle for infant feeding
A method for feeding an infant by non-human milk including the steps of (a) feeding the infant by fore-milk equivalent having a volume of 30-60 % of a total meal and a percentage of fat of 2.5-3.5 %; and (b) feeding the infant by hind-milk equivalent having a volume of 40-70% of the total meal and a percentage of fat of 3.7-5.5 %.
Owner:SHAPIRA NIVA
System and method for lean recovery using non invasive sensors
InactiveUS20120128838A1Accurate recoveryRind cutting-off apparatusCharacter and pattern recognitionNon invasiveFat content
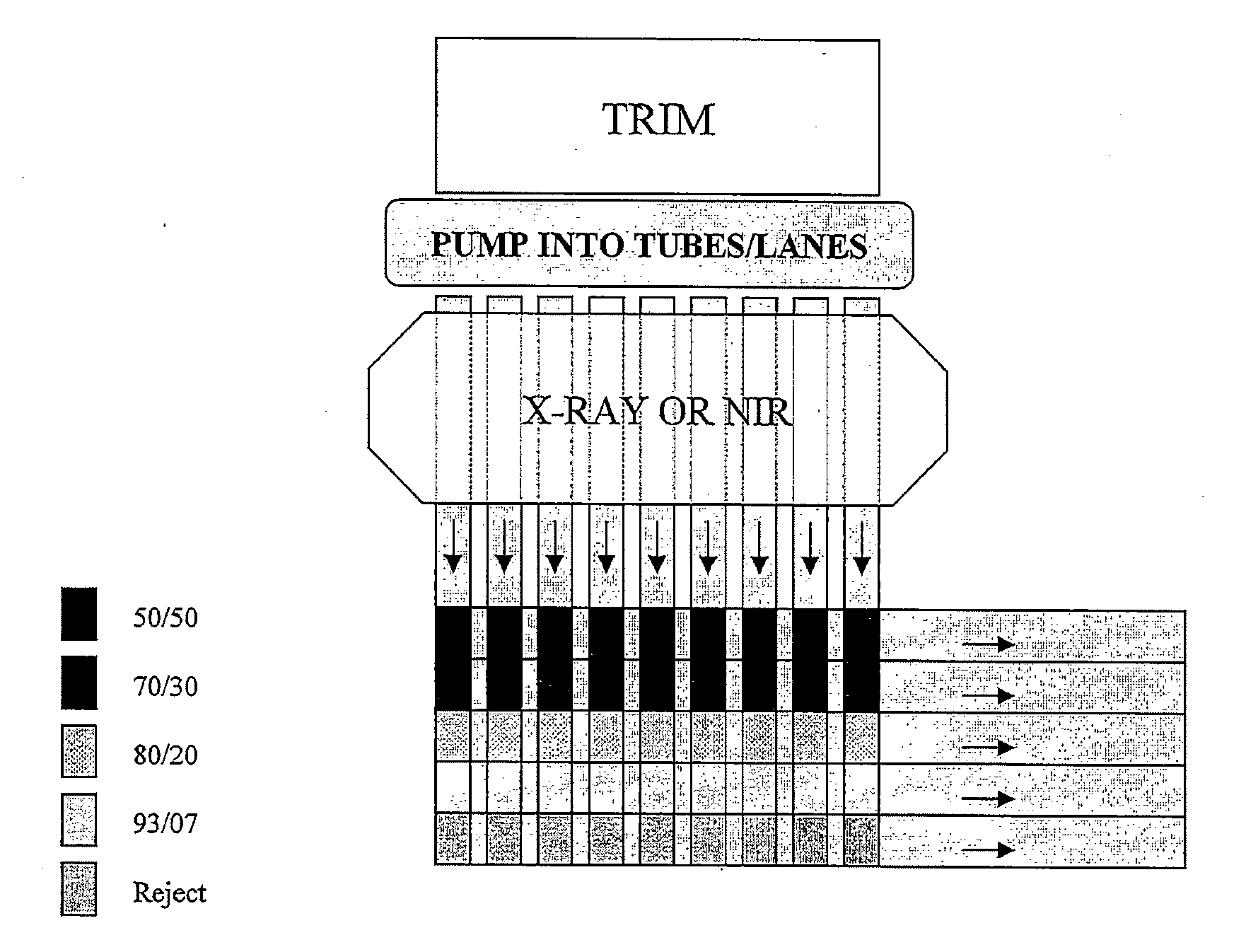
An apparatus and method for non-invasive lean recovery from a sparse lean product, where the method can include conveying ground sparse lean product through a conveyance channel where the conveyance channel extends along a path that extends through a scanning position adjacent a scanner. The process includes scanning along a predetermined length with the scanner the ground sparse lean product traveling through the conveyance channel and further analyzing the scan and determining the percent fat content for each ground sparse lean product segment which is defined by the predetermined length of the ground sparse lean product within the volume of the conveyance channel and the cross section areas of the conveyance channel. The process can further include directing each ground sparse lean product segment down one of a plurality of processing paths corresponding to the one defined fat content range in which the corresponding percent fat content falls.
Owner:TYSON FOODS
Computerized tomography device using X rays and image processing method
InactiveUS7526061B2Accurate identificationGood reproducibilityMaterial analysis using wave/particle radiationImage analysisImaging processingX-ray
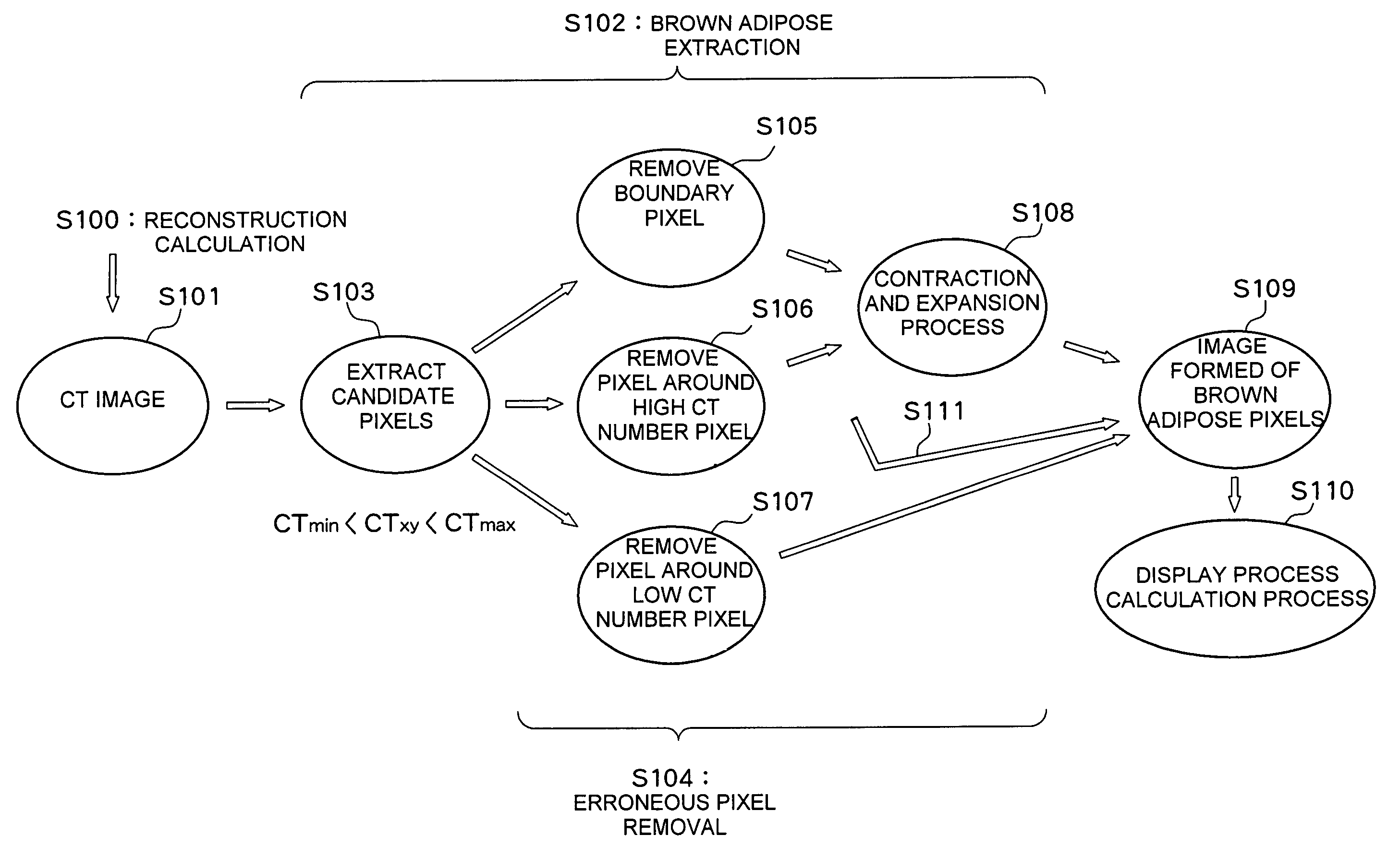
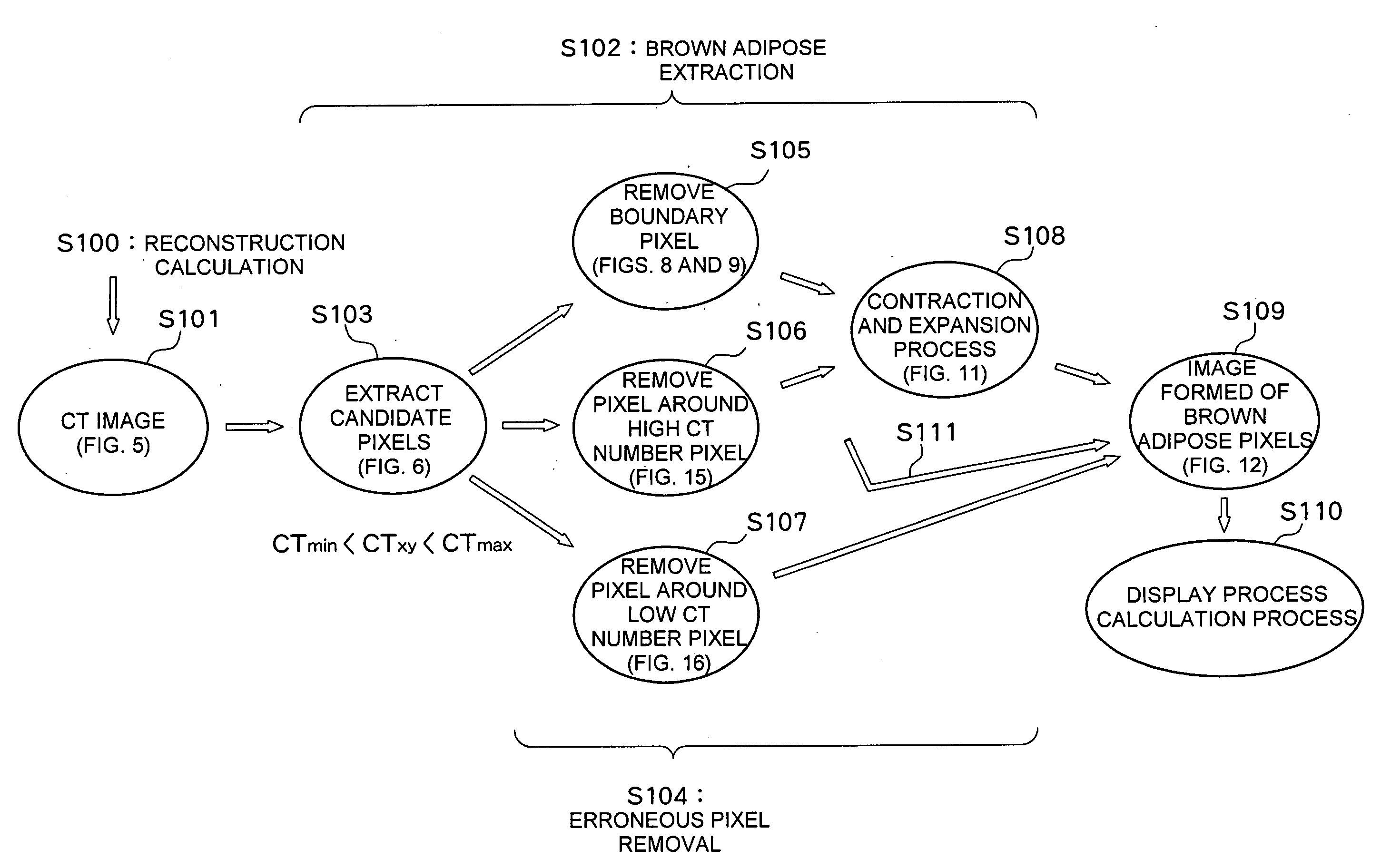
A computerized tomography device using an X-ray reconstructs a CT image of a sample and processes the CT image. Brown adipose candidate pixels are extracted based on a CT number of each pixel reconstructing the CT image. An erroneous pixel removal process is applied to the candidate pixels. In the erroneous pixel removal process, a boundary pixel removal process, a contraction and expansion process, or the like are applied. With this process, only the brown adipose pixels are extracted. An amount of brown adipose is determined from the brown adipose pixels and an evaluation value such as a brown adipose percentage is calculated based on the amount of brown adipose and amounts of other tissues.
Owner:HITACHI LTD
Chicken feed
InactiveCN101228926ARich varietyIncrease proteinFood processingAnimal feeding stuffArginineAnimal protein
The invention relates to a chicken feed pertaining to a poultry feedstuff, which solves the problems in the existing chicken feed of single source of high qualified animal protein, high price and unstable quality. The chicken feed is composed of the following weight proportion: 60-65 percent corn, 20-25 percent soybean meal, 3-7 percent dried earthworms, 8.5-9 percent worm cast, 0.8-1.2 percent calcium hydrogen phosphate, 0.1-0.5 percent salt and 0.8-3 percent premix feed; wherein, the dried earthworms is dried with Eisenia fetida. The invention uses the earthworm as the protein source of the chicken feed, which broadens the high qualified animal protein resources in the chicken feed. Earthworm is a low-priced animal with high protein content and abundant nutrition, while the dried Eisenia fetida selected by the invention has more than 59 percent protein content, 18 percent fat content, wherein, the proportion of leucine, arginine and lysine is higher, and the nutritional value is equivalent to that of the imported qualified fishmeal.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Computerized tomography device using X rays and image processing method
InactiveUS20070053485A1Accurate identificationGood reproducibilityMaterial analysis using wave/particle radiationImage analysisSoft x rayImaging processing
A computerized tomography device using an X-ray reconstructs a CT image of a sample and processes the CT image. Brown adipose candidate pixels are extracted based on a CT number of each pixel reconstructing the CT image. An erroneous pixel removal process is applied to the candidate pixels. In the erroneous pixel removal process, a boundary pixel removal process, a contraction and expansion process, or the like are applied. With this process, only the brown adipose pixels are extracted. An amount of brown adipose is determined from the brown adipose pixels and an evaluation value such as a brown adipose percentage is calculated based on the amount of brown adipose and amounts of other tissues.
Owner:HITACHI LTD
Gel-form composition for supplying protein and calcium
InactiveUS20050244543A1Highly suitableSafely ingestableAnimal feeding stuffAcidic food ingredientsHydrolysateCalcium supplementation
The present invention provides a gel composition for protein and calcium supplementation having a pH in the range of 3 to 4 and containing the following components: 3 to 8 wt. % of Protein or its hydrolysate that does not coagulate at pH 3 to pH 4; 0.1 to 0.5 wt. % of calcium; 0.5 to 3 wt. % of acidulant; 4 to 20 wt. % of carbohydrate; 0 to 5 wt. % of fat; 0 to 0.5 wt. % of emulsifying agent; 0.1 to 1 wt. % of agar; and 65 to 90 wt. % of water.
Owner:OTSUKA PHARM CO LTD
Water soluble animal muscle protein product
InactiveCN1751062AProtein composition from fishPeptide preparation methodsMuscle tissueMuscle protein
A water soluble peptide composition derived from animal muscle tissue proteins is provided. The peptide composition contains less than about 1 weight percent fats and oils based upon the weight of the peptide composition and less than about 2 weight percent ash based on the weight of the peptide composition.
Owner:PROTEUS INDUSTRIES INC
Frozen Aerated Confections
ActiveUS20080206425A1Good health benefitsGood processing propertyFrozen sweetsButter cocoaTriglyceride
A frozen aerated confection having an overrun of at least 40% and a fat component in an amount of 2 to 20% (by weight of the frozen aerated confection), said fat component comprising triglycerides of fatty acids wherein less than 70% (by weight of the fatty acids) of the fatty acids in the triglycerides are saturated, less than 8% (by weight of the triglycerides) of the triglycerides are SSS triglycerides; characterized in that the ratio of the percentage of fat that is solid at 5° C. to the percentage of the fatty acids in the triglycerides that are saturated (by weight of the fatty acids) is greater than 1 and in that the fat component comprises at most 60% (by weight) cocoa butter or shea nut oil.
Owner:CONOPCO INC D B A UNILEVER
System and method for lean recovery using non invasive sensors
An apparatus and method for non-invasive lean recovery from a sparse lean product or other animal muscle trimming, which can include conveying ground sparse lean through a conveyance channel where the conveyance channel extends along a path that extends through a scanning position adjacent a scanner. The process includes scanning along a predetermined length with the scanner the ground sparse lean product traveling through the conveyance channel and further analyzing the scan and determining the percent fat content for each ground sparse lean product segment which is defined by the predetermined length of the ground sparse lean product within the volume of the conveyance channel and the cross section areas of the conveyance channel. The process can further include directing each ground sparse lean product segment down one of a plurality of processing paths corresponding to the one defined fat content range in which the corresponding percent fat content falls.
Owner:TYSON FOODS
Large-scale process for the preparation of thylakoids
InactiveCN102088986AReduce intakeReduce blood fatty acidAntipyreticMetabolism disorderBiotechnologyBlood insulin
The present invention relates to a process on a large industrial scale for the production of thylakoids, from photosynthetic organisms, such as from green plant leaf material, to be used as ingredients in food, or additions to food, or dietary supplements, or pharmaceuticals for the purpose of preventing overweight, promoting satiety, reducing food intake, reducing bodyweight, reducing blood insulin concentration and reducing blood fats and percentage body fat in humans and animals.
Owner:THYLABISCO
Compound evening primrose and perilla herb oil fat emulsion oral solution, beverage and preparation method
The invention provides a compound evening primrose and perilla herb oil fat emulsion oral solution, beverage and a preparation method. The oral solution comprises raw materials of evening primrose seed oil, perilla herb oil, lecithin, glycerin, sterile pure water, an antioxidant, a stabilizing agent, a pH regulating agent, a flavoring agent, essence, and the like. The preparation process comprises the following steps of: in the presence of the protection of nitrogen, heating, dissolving and mixing the evening primrose seed oil, the perilla herb oil and the lecithin into an oil phase; mixing the glycerin, the antioxidant, the stabilizing agent, the flavoring agent, the essence and the sterile pure water into a water phase; slowly adding the oil phase into the strongly stirred water phase and shearing into a primary emulsion at high speed; regulating the pH value of the primary emulsion to be 6.5-8.5 by using a few pH regulating agent; emulsifying by using a high-pressure homogenizer; filtering by using a microporous membrane; and filling, sealing and sterilizing to obtain the intravenous fluid the oil content of which is 1-50 percent. The compound evening primrose and perilla herb oil fat emulsion oral solution is used for the field of healthy food, a 5-50 percent fat emulsion oral solution can be used as nutritional functional health-care beverage, and a 1-20 percent fat emulsion oral solution can be used as table beverage and popular beverage.
Owner:王京南
Magnetic nanometer particulate of liposoluble photosensitizer and method for preparing the same
InactiveCN101112361AAchieve active targetingGood magnetic responsePowder deliveryPharmaceutical non-active ingredientsParticulatesSide effect
The present invention relates to a magnetic nanoparticle of a fat-soluble photosensitizer and a preparation method thereof; the present invention is the composite nanoparticle with the size of 5nm to 300nm which consists of 30 percent to 70 percent polysaccharide polymer, 5 percent to 40 percent fat-soluble photosensitizer and 10 percent to 50 percent iron-containing magnetic particles which have the magnetic response under the function of external magnetic field. The preparation method thereof is that the polysaccharide polymer is dissolved in alkaline solution, the emulsifier is added, the organic solvents which dissolves the fat-soluble photosensitizer are dropped during the agitating, so as to form the emulsion; the hydrate of ferrous salts and ferric salts is dropped into the emulsion during the agitating, so as to generate the black precipitate, then the magnetic nanoparticle of the fat-soluble photosensitizer can be obtained through the heating, pH valve regulation, getting the solid phase, washing, freezing and drying sequentially. The present invention has the advantages of safety, no toxicity, strong magnetic response and good biological acceptability, can entirely achieve the intravenous administration and significantly improve the targeting of the tumor under the magnetic guide function, so as to improve the photosensitive activity of the photosensitizer and achieve the purposes of reducing the dosage and lowering the toxic and side effects.
Owner:INST OF FIELD OPERATION SURGERY NO 3 MILITARY MEDICL UNIV PLA
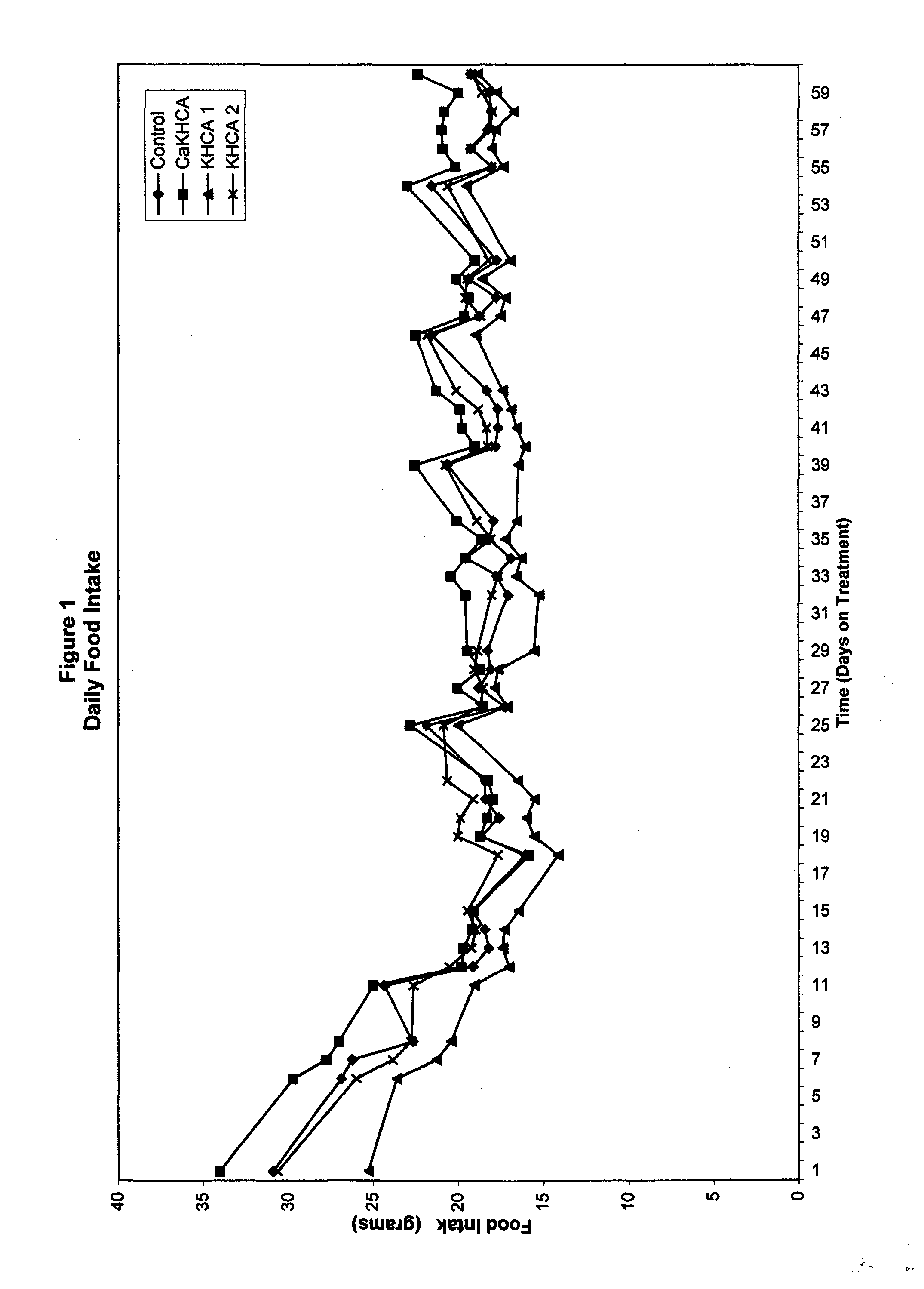
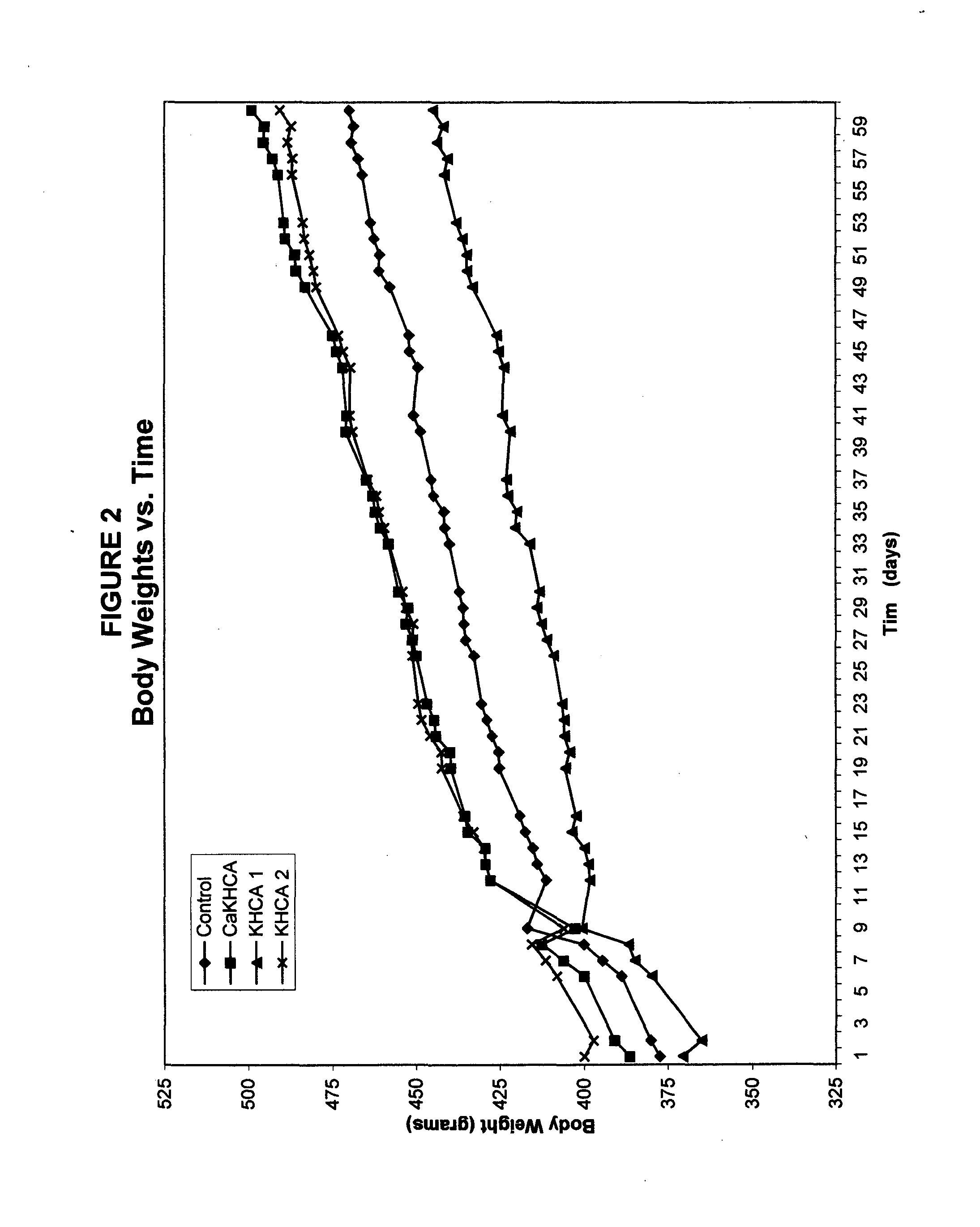
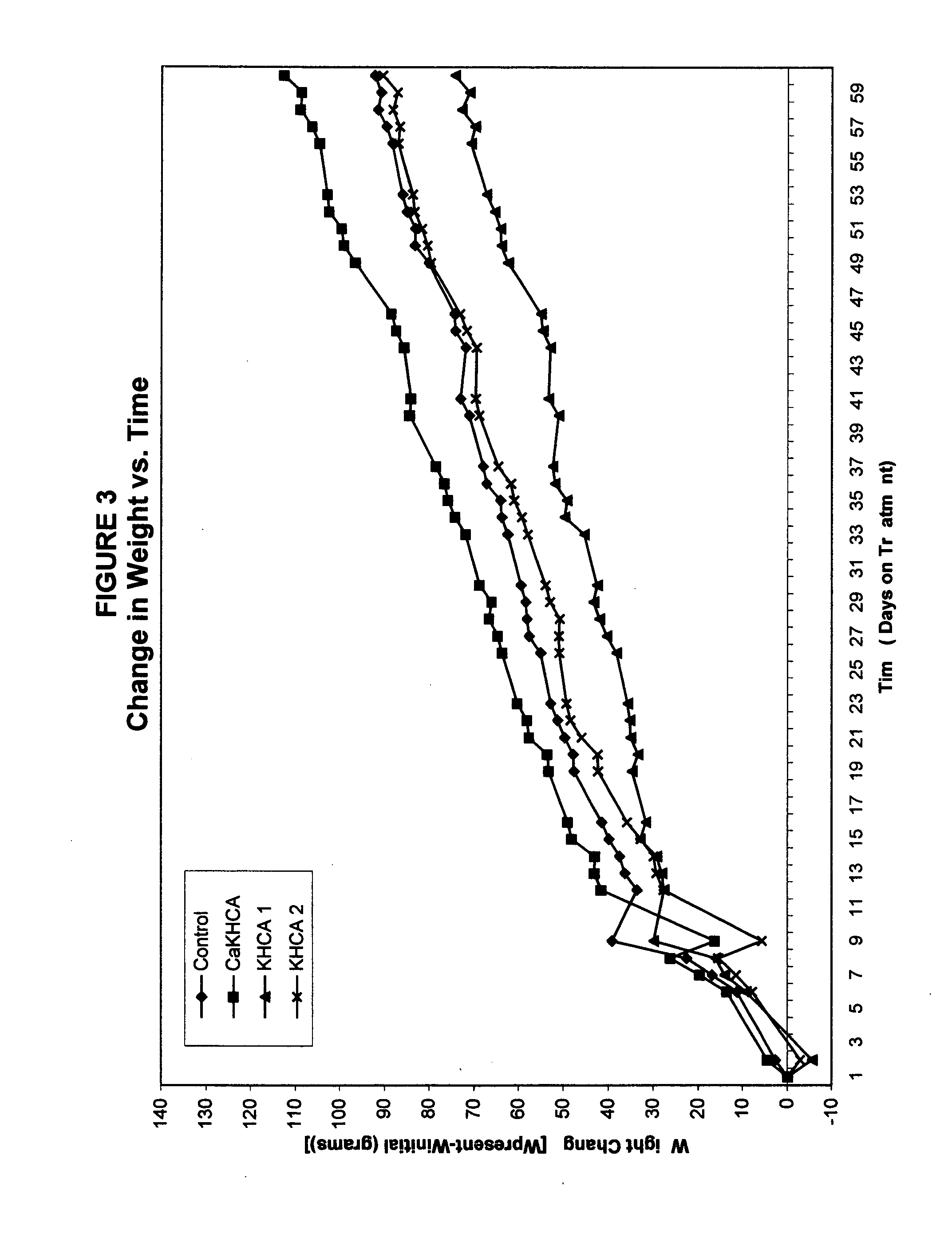
Treating cachexia and excessive catabolism with (-)-hydroxycitric acid
InactiveUS20050009919A1Maintaining proper metabolic functioningMaintaining energy expenditureBiocideCarbohydrate active ingredientsEphedrineWeight decreasing
The inventor has discovered that (−)-hydroxycitric acid (including the forms of its various salts) is useful for treating and ameliorating cachexia, health-threatening catabolism and unhealthful weight loss, such as is characteristic of sarcopenia. The dosage will depend on factors such as the starting weight of the individual and the percentage of the calories in the diet derived from fats. On a 30 percent fat diet, an efficacious daily dosage for most individuals will be between 250 mg and 3 grams per day. It may prove beneficial to deliver the desired dosage only once per day, preferably prior to the noon meal. The weight-gain effects of HCA are compromised by the actions compounds such as caffeine and ephedrine, hence these should be avoided. Due to the biphasic characteristics of HCA, there is an obvious overlap between dosages that can lead to weight gain and the higher dosages that can lead to weight loss in those who are above their ideal body weights. There is little or no evidence that HCA ingested even in quite large amounts causes significant weight loss in individuals who are at or below their idea weights or exhibit a body mass index (BMI) at or below 20. It is to be expected that dosage will need to be matched to the current state of a given individual suffering from cachexia, catabolism or sarcopenia.
Owner:GLYKON TECH GRP
System for measuring a user's percentage of body fat
ActiveUS8380297B2Diagnostic recording/measuringSensorsPhysical medicine and rehabilitationEngineering
Owner:HIGI SH
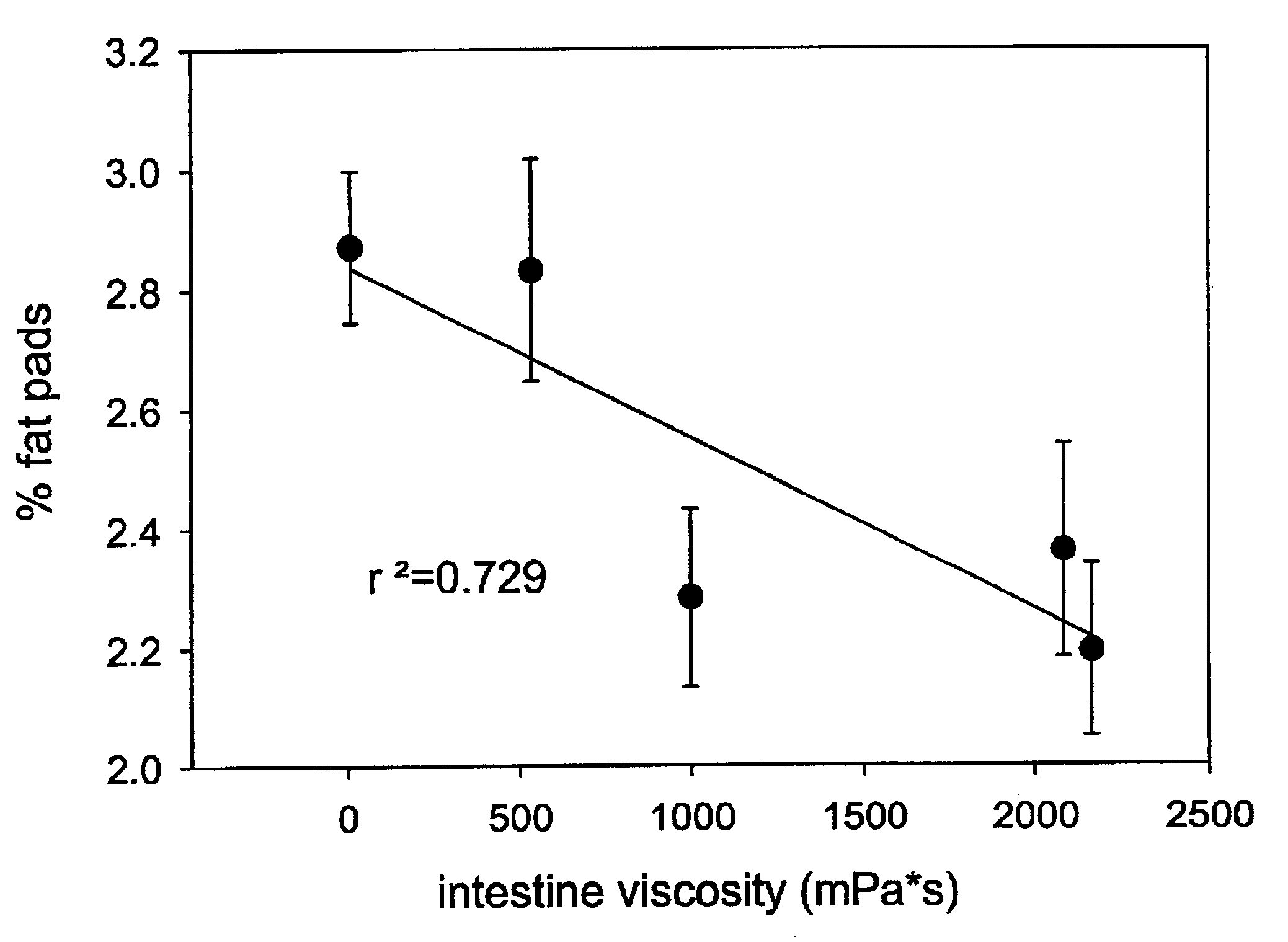
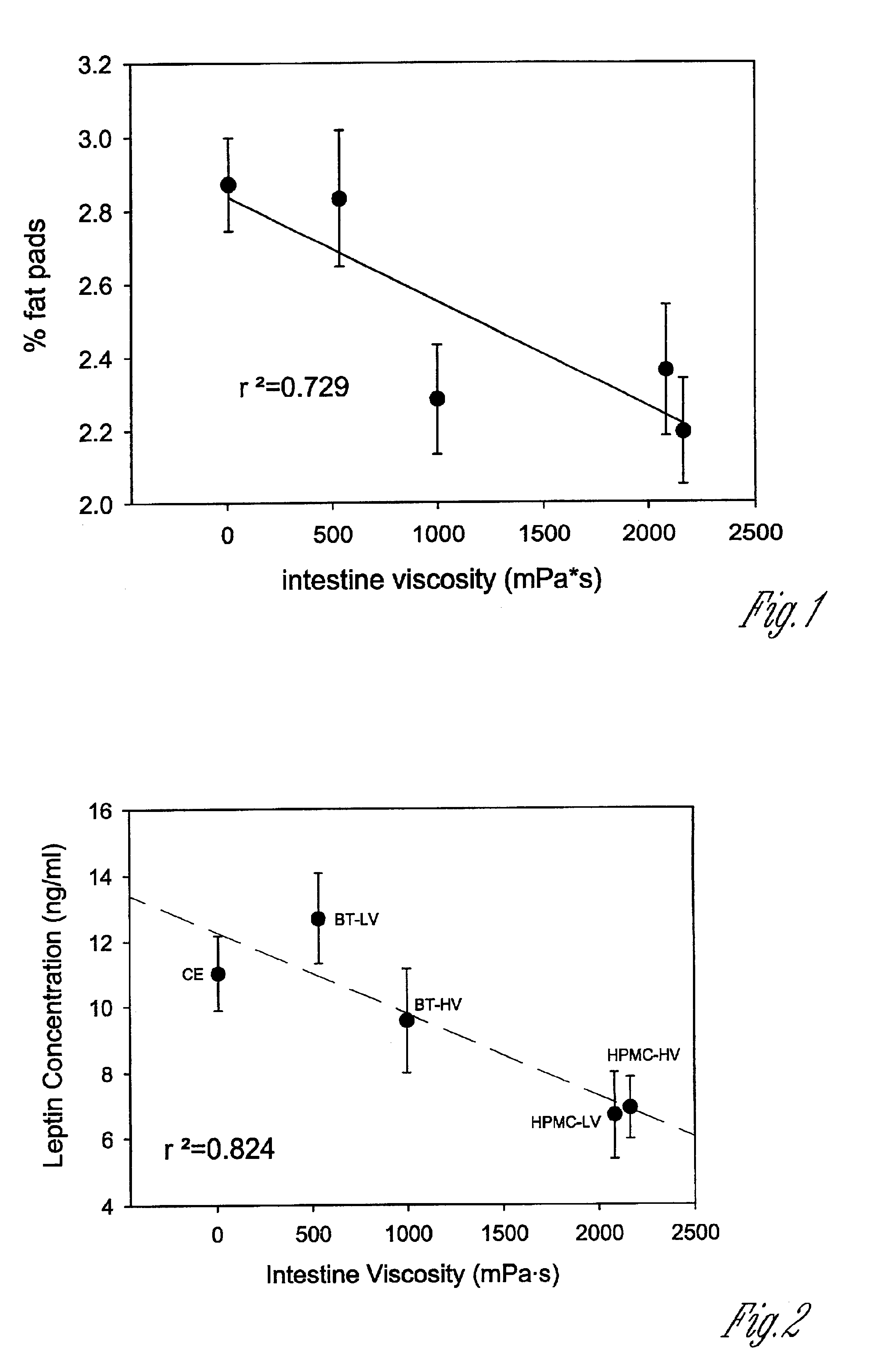
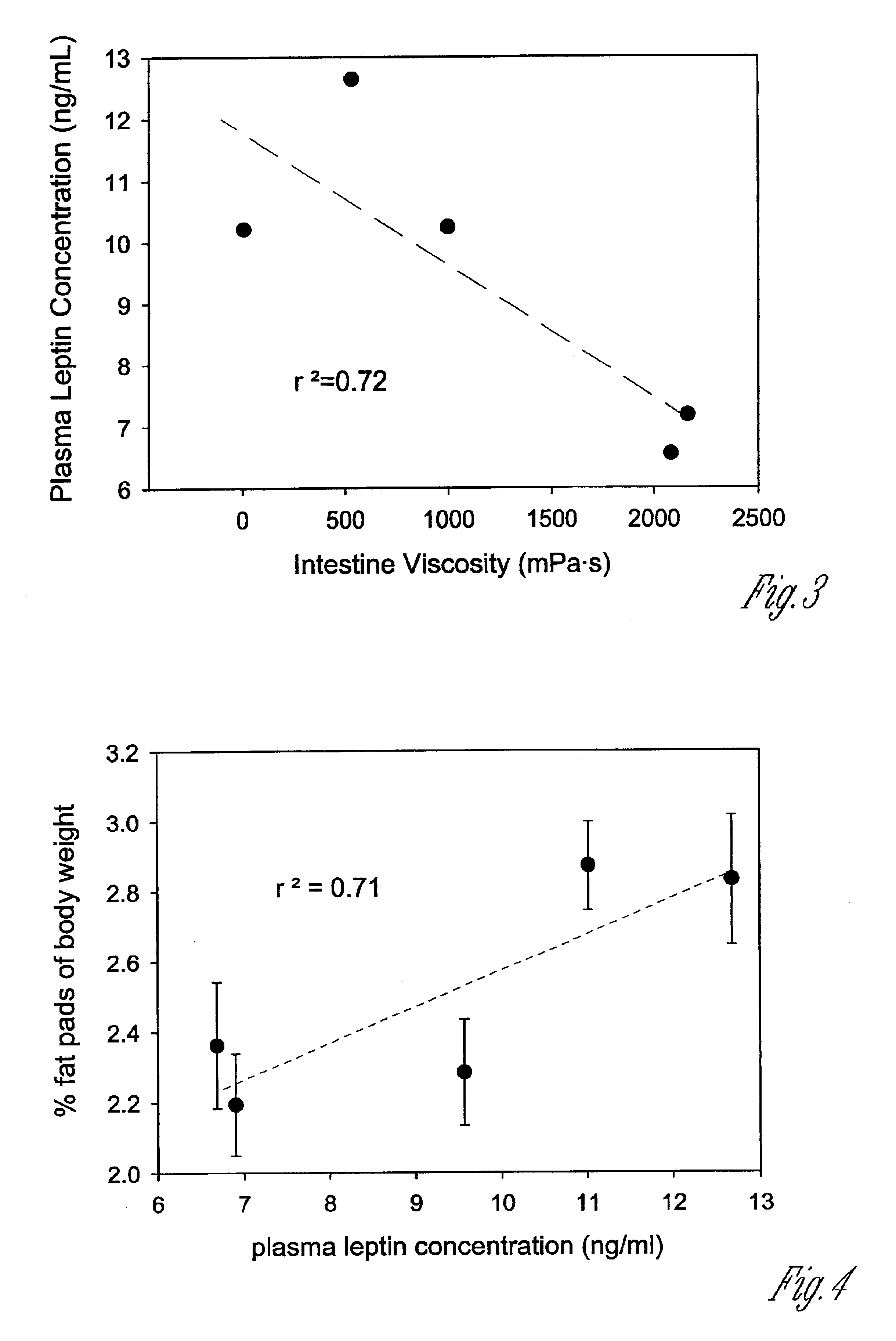
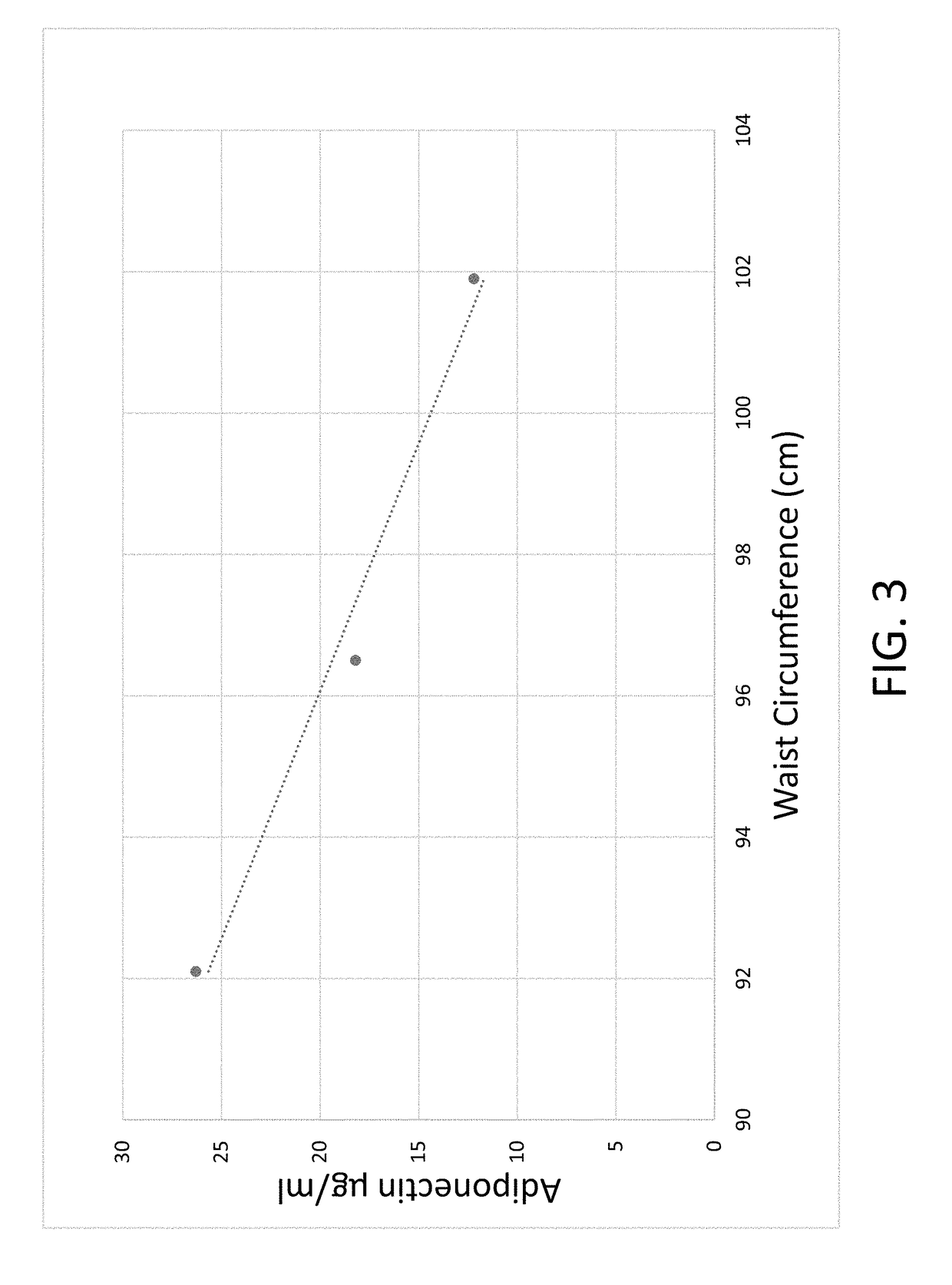
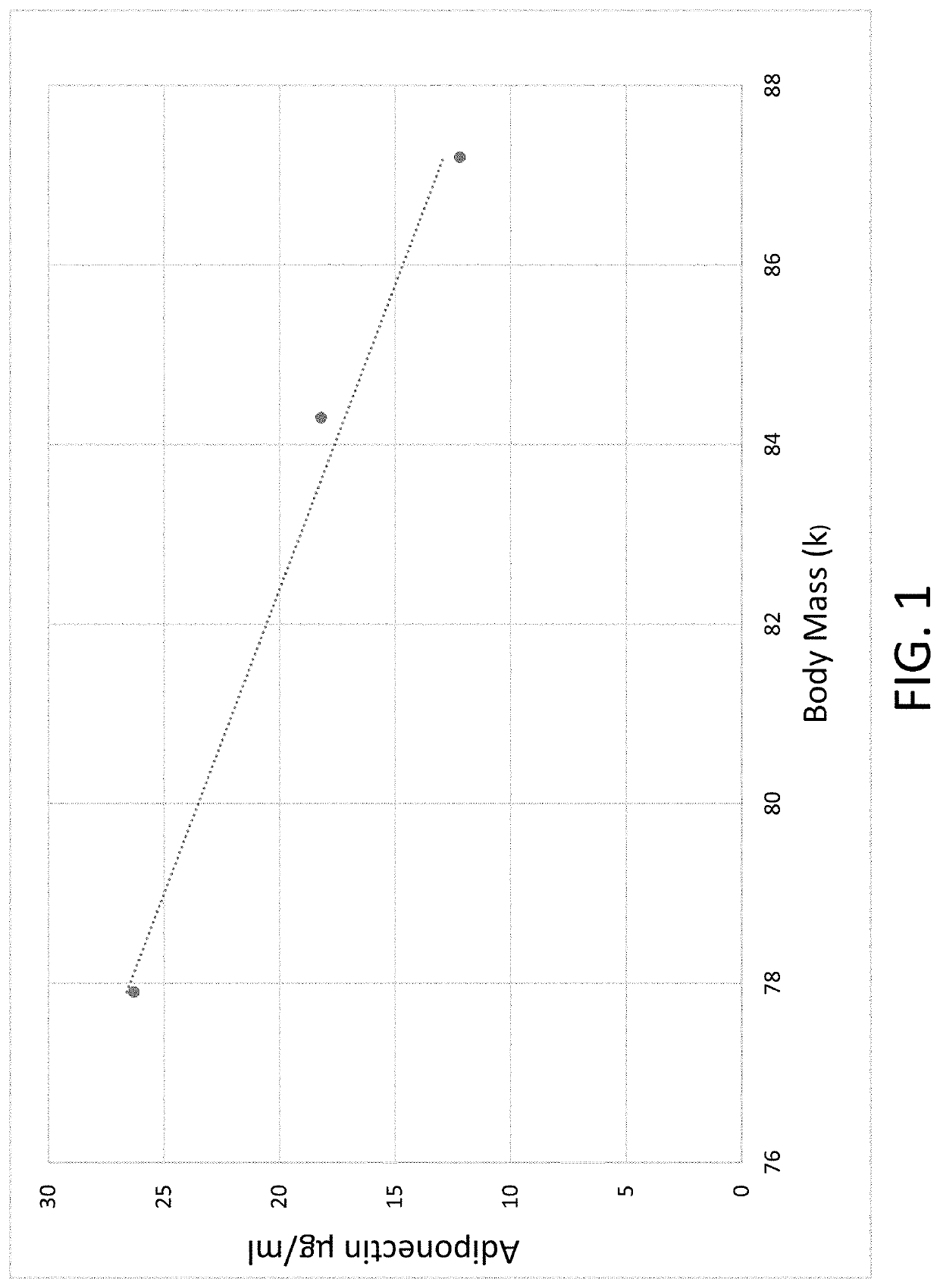
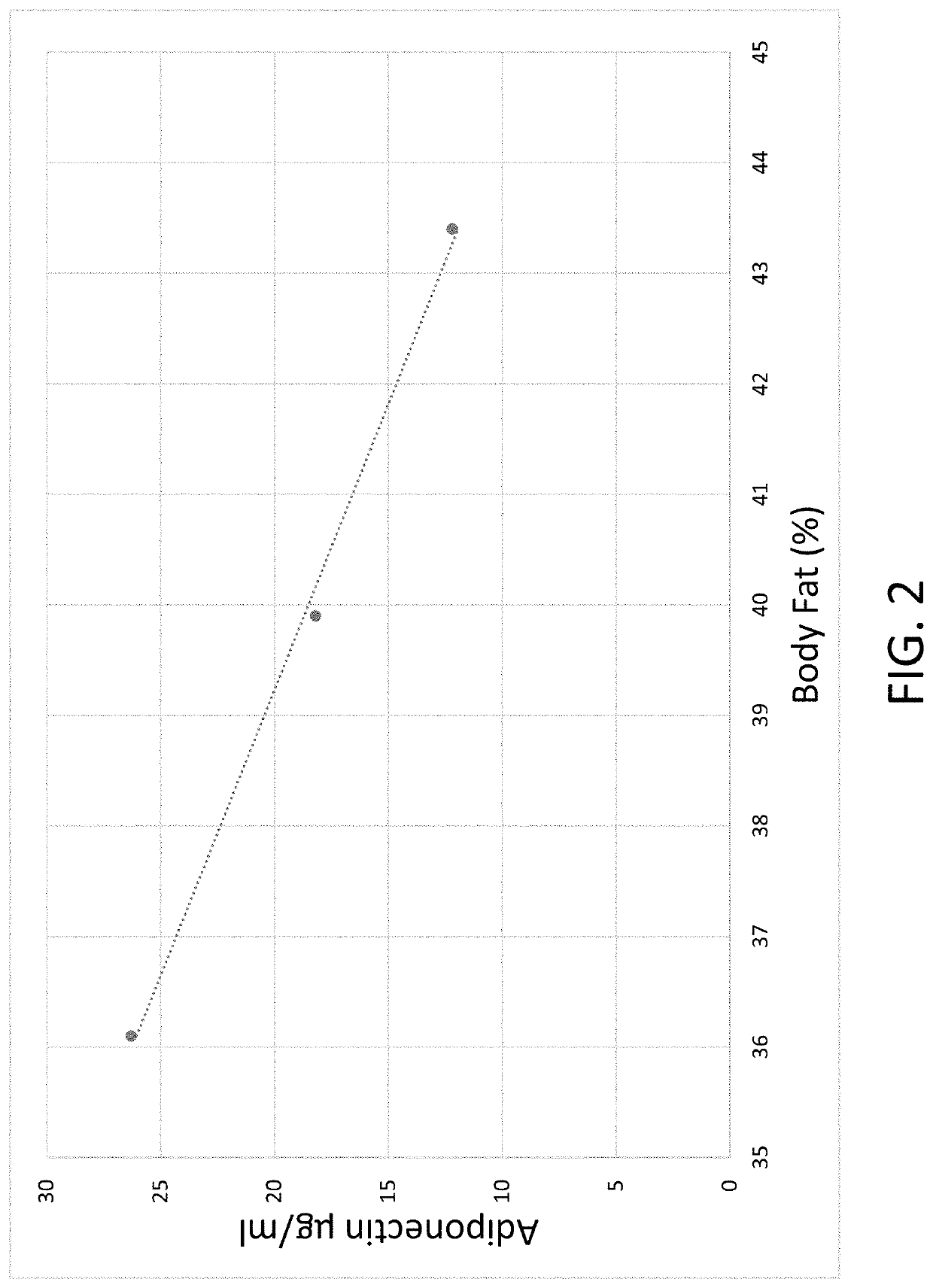
Composition for and method to increase serum adiponectin and reduce body fat
ActiveUS20190060267A1Increase in adiponectinReduce percentageMetabolism disorderPharmaceutical non-active ingredientsSerum adiponectinMyristic acid
The invention provides methods and compositions for reducing the percentage of body fat in an animal or human and increasing the level of adiponectin in an animal or human. Such methods include administering to an animal or human sufficient levels of a compound comprising a tri blend of HPMC (K15, K100, and K200) and myristic fatty acid.
Owner:FIRST FRUITS BUSINESS MINIST
Composition for and method to increase serum adiponectin and reduce body fat
ActiveUS10632092B2Increase in adiponectinReduce percentageMetabolism disorderPharmaceutical non-active ingredientsSerum adiponectinPhysiology
The invention provides methods and compositions for reducing the percentage of body fat in an animal or human and increasing the level of adiponectin in an animal or human. Such methods include administering to an animal or human sufficient levels of a compound comprising a tri blend of HPMC (K15, K100, and K200) and myristic fatty acid.
Owner:FIRST FRUITS BUSINESS MINIST
Method of producing a heat stable oil-in-water emulsion and the products made therefrom
A method of forming a heat stable oil-in-water emulsion comprises providing a selected amount of an aqueous component comprising more than 50 weight percent water. The aqueous component is optionally heated and a selected amount of a solids component is added to the aqueous component under agitation to form a first intermediate. A selected amount of a milk fat containing component is heated to a temperature sufficient to predominantly melt the fat prior to being to the first intermediate to form a second intermediate. The second intermediate is optionally heated for a selected period of time. The second intermediate is homogenized at between about 250 psig and 5000 psig to form the heat stable oil-in-water emulsion comprising at least 20 weight percent milk fat. The second intermediate may be heated to between about 130° F. and 150° F. for a selected period of time. The second intermediate is homogenized at between about 250 psig and 5000 psig to form a third intermediate oil-in-water emulsion comprising at least 20 weight percent fat. A selected amount of shear sensitive component is added to the third intermediate to form the heat stable oil-in-water emulsion on the present invention.
Owner:LAND O'LAKES
SARMs and method of use thereof
InactiveUS8309603B2Add additional massReduce the total massBiocideOrganic chemistryCarcass compositionReduced fat
This invention is directed to a feed composition and method of affecting the carcass composition by increasing the lean mass, reducing the fat mass, and / or reducing the percent fat mass comprising SARM compounds.
Owner:UNIV OF TENNESSEE RES FOUND
Ready-to-eat fudge dessert
The present invention provides a water containing, heat processable firm RTE fudge dessert. The RTE fudge dessert of this invention is prepared from an emulsified blend of water, fat, milk, lecithin, and a water binder. Sweeteners and / or flavorings may be added to provide the desired level of sweetness or desired flavor profile. A smooth firm fudge candy textured dessert comprising about 20 to about 60 percent water, about 15 to about 45 percent fat, about 1 to about 15 percent non-fat dry milk (or equivalent milk protein), about 0.1 to about 3 percent lecithin, and about 0.1 to about 20 percent water binder is provided.
Owner:KRAFT FOODS GRP BRANDS LLC
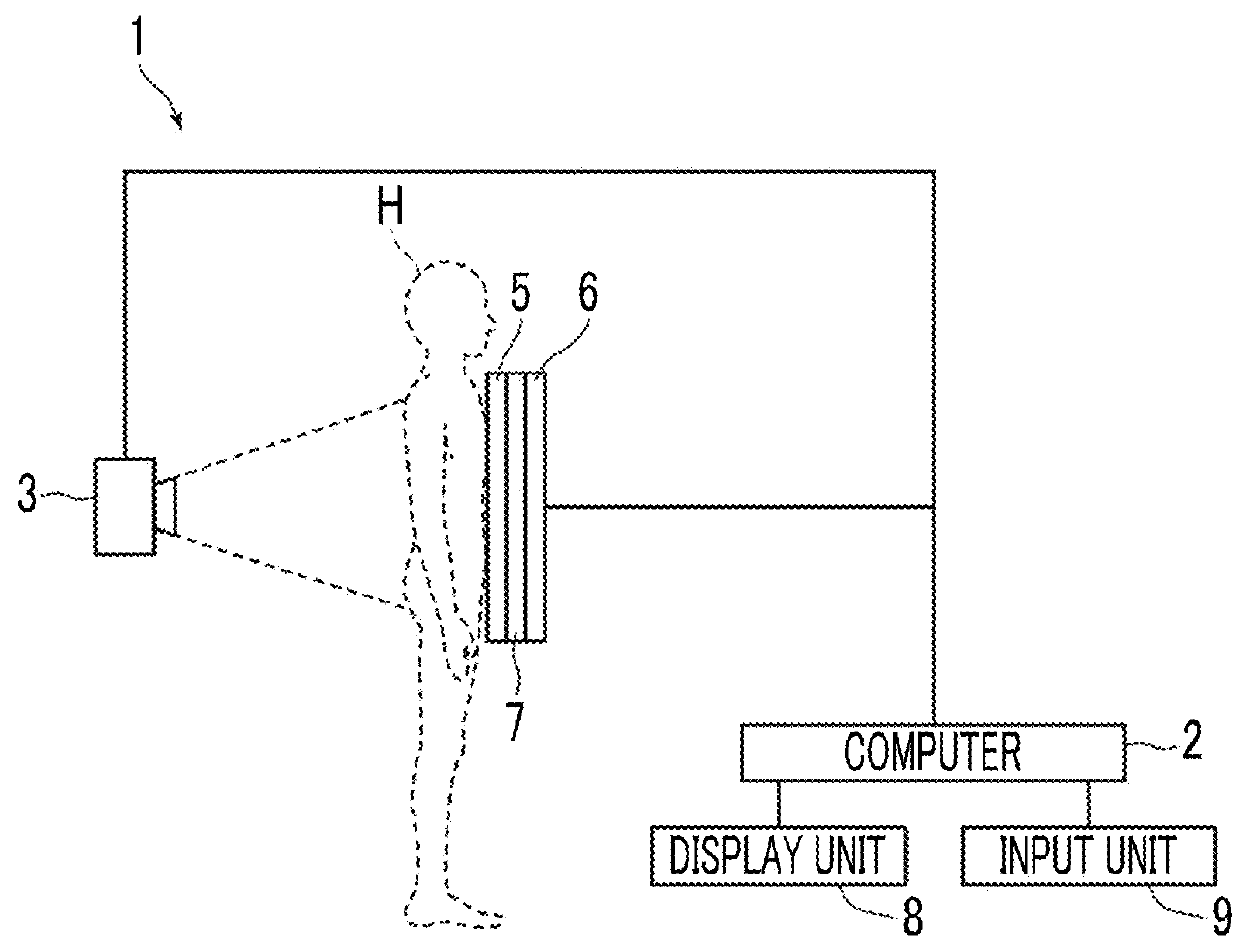
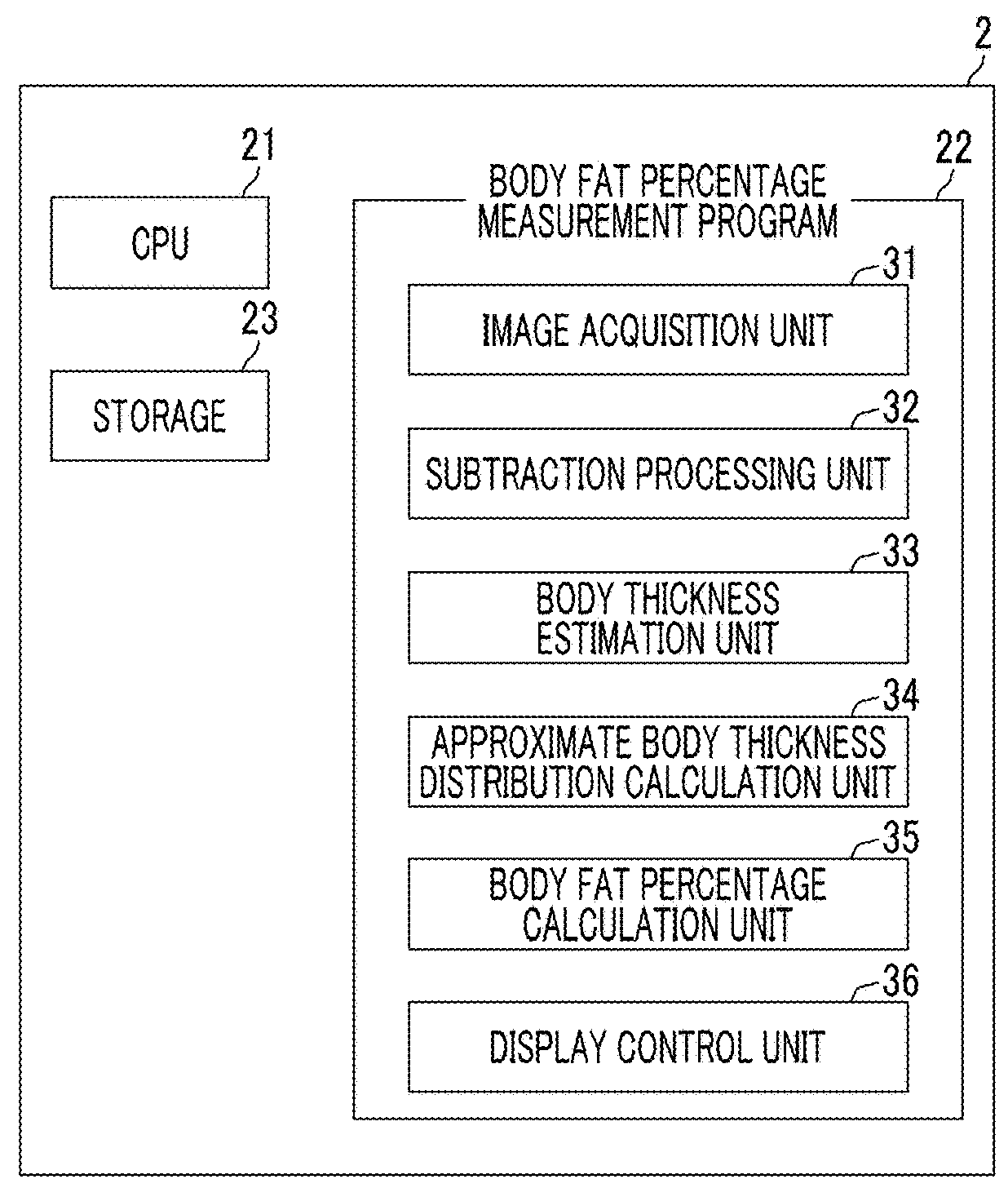
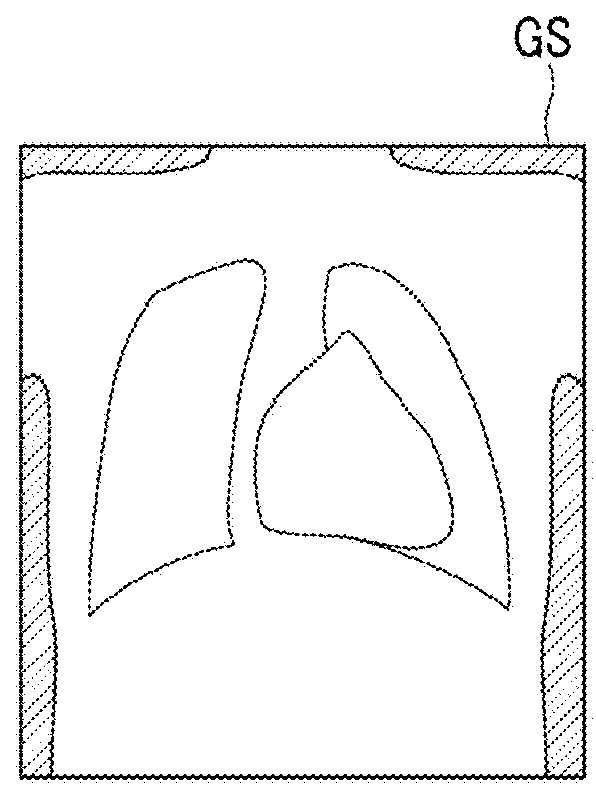
Body fat percentage measurement device, method and program
ActiveUS20180263559A1Reduce the amount requiredEasy to measureImage enhancementImage analysisImaging conditionMeasurement device
A subtraction processing unit generates a soft portion image representing a soft portion tissue of a subject from first and second radiation images. A body thickness estimation unit estimates a body thickness distribution of the subject on the basis of imaging conditions in a case where the soft portion image and the first and second radiation images are acquired. An approximate body thickness distribution calculation unit calculates an approximate body thickness distribution obtained by approximating the estimated body thickness distribution to a model corresponding to a human body, and a body fat percentage calculation unit calculates a distribution of a body fat percentage in the subject on the basis of the approximate body thickness distribution.
Owner:FUJIFILM CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com