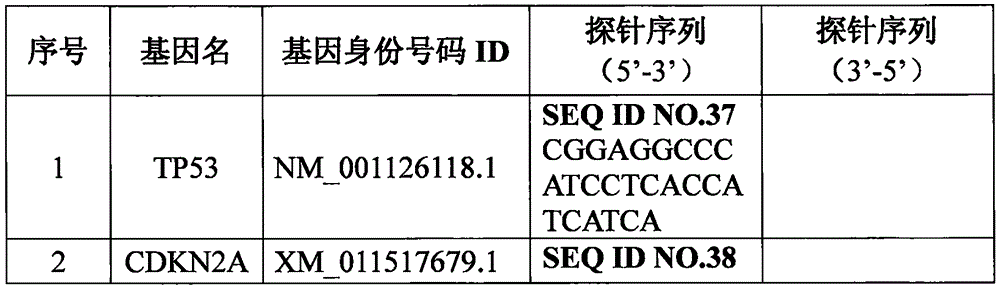
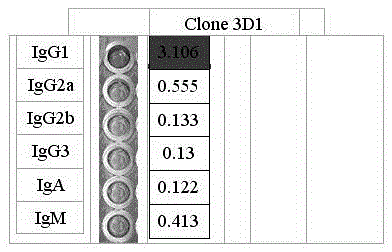
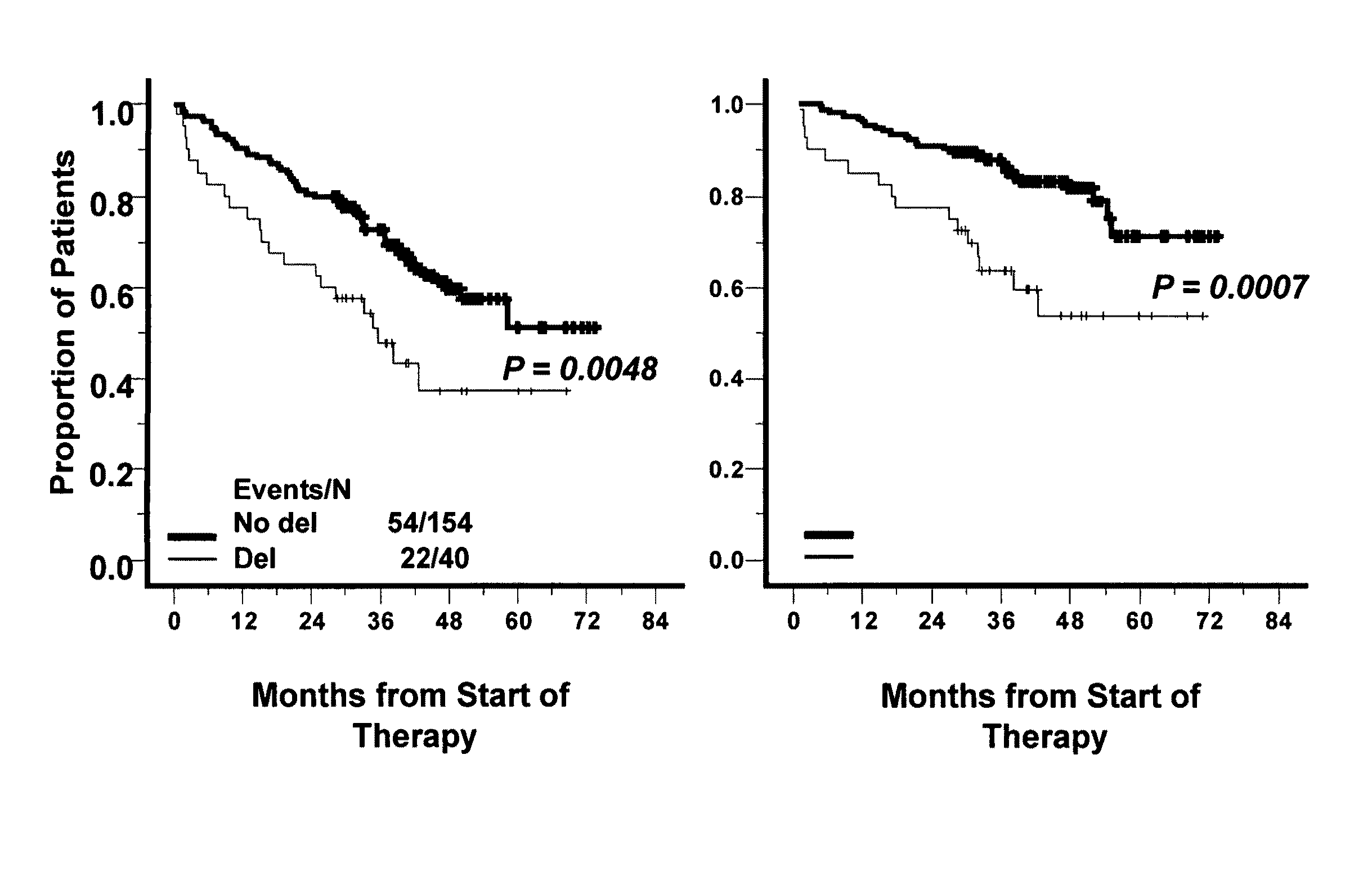
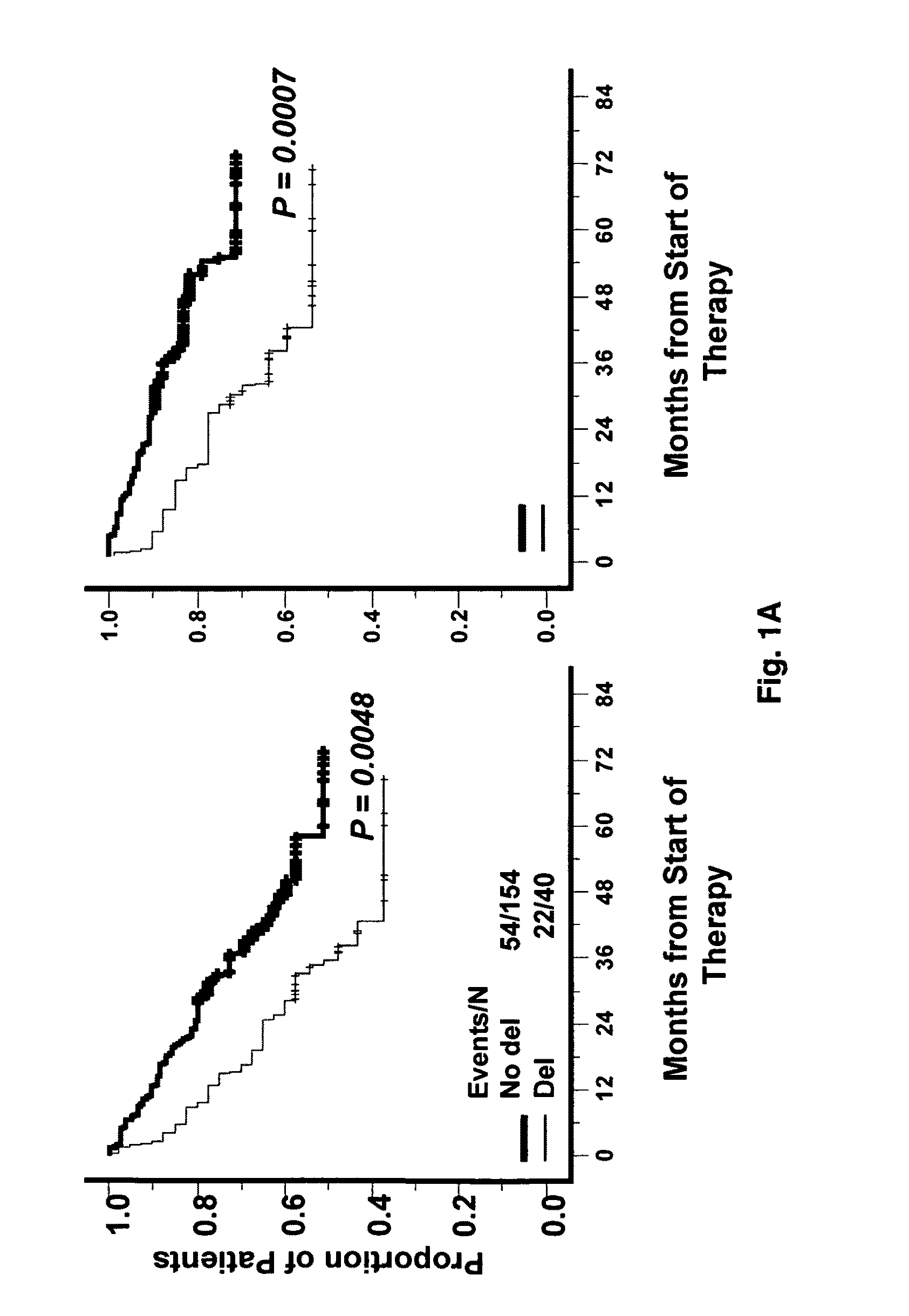
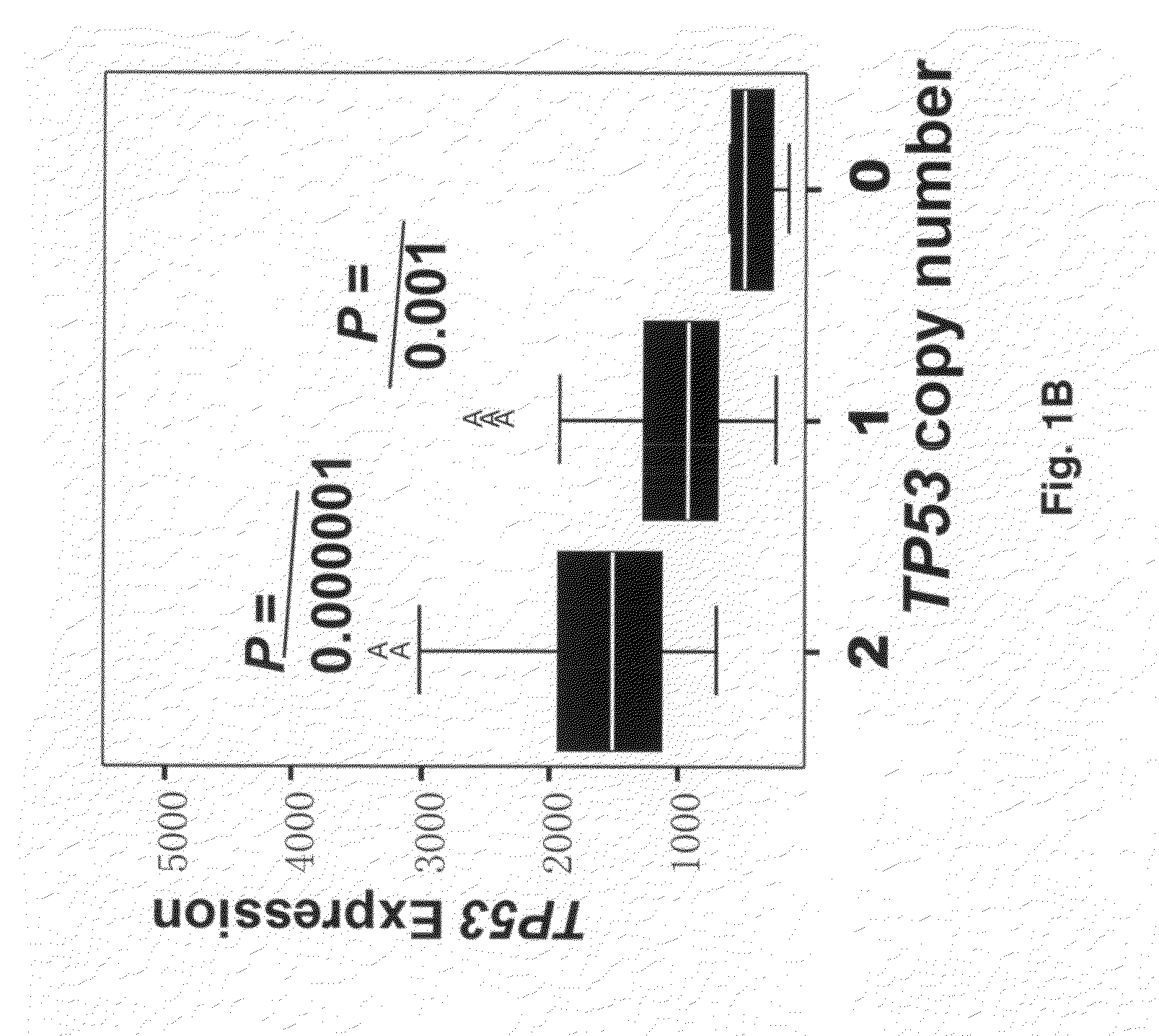
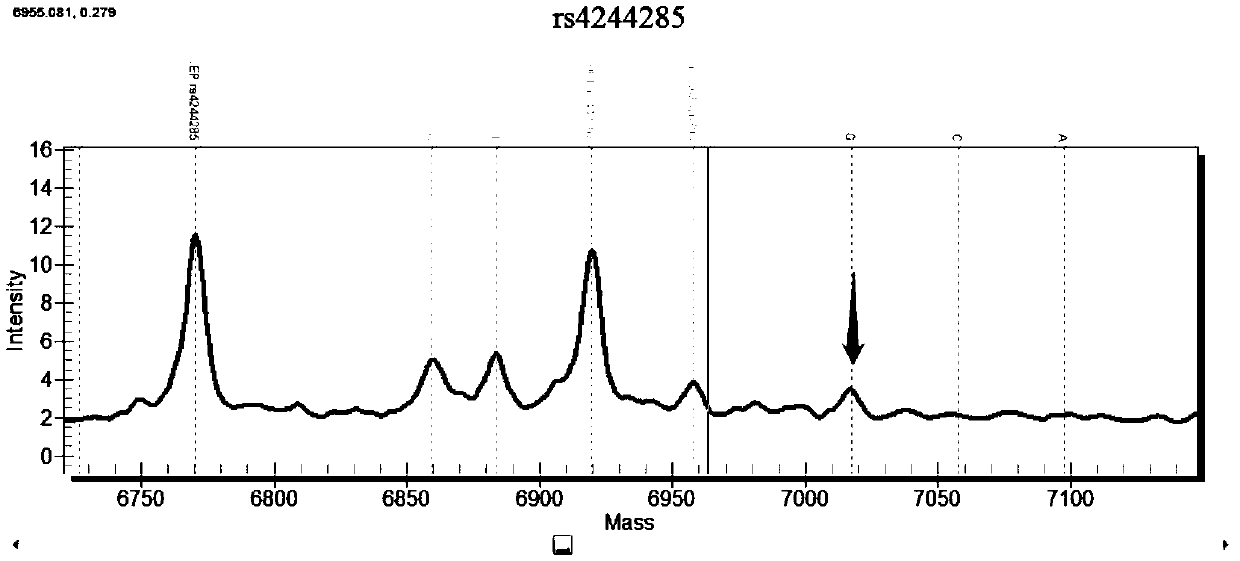
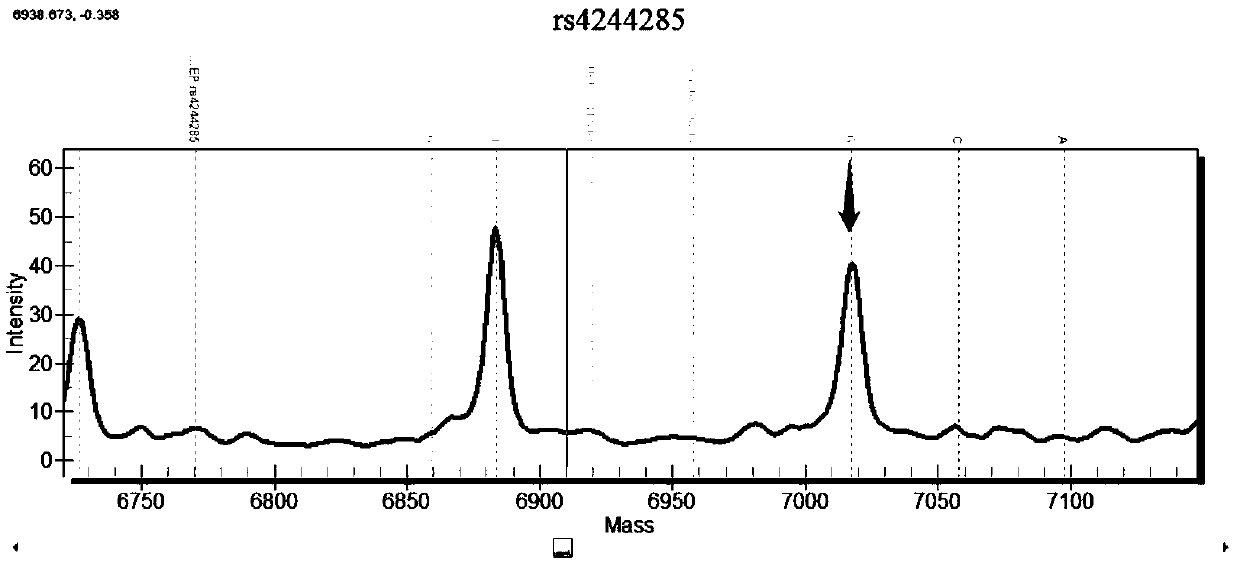
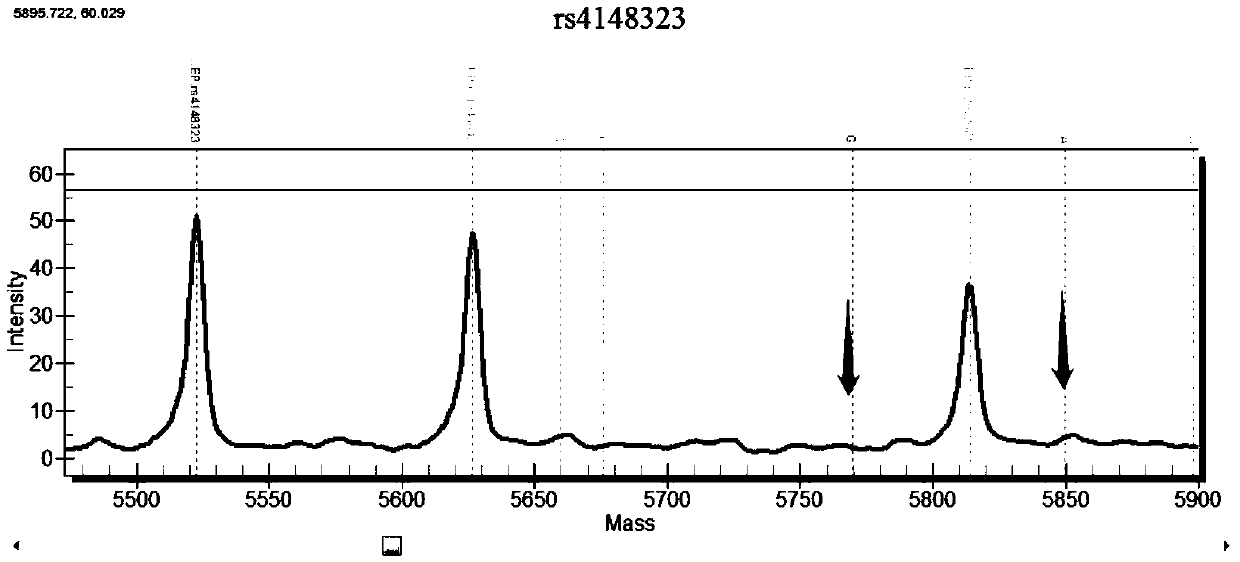
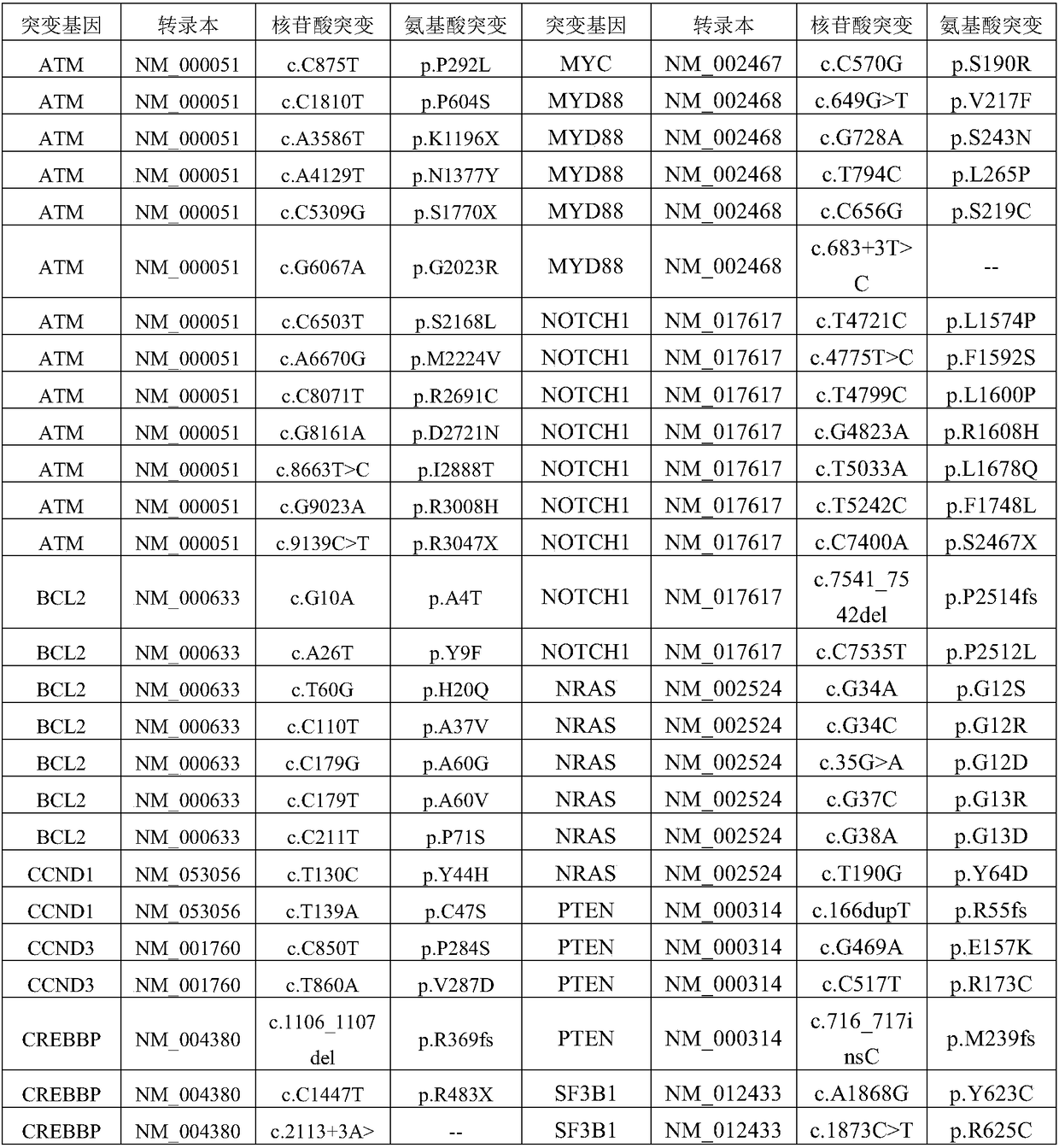
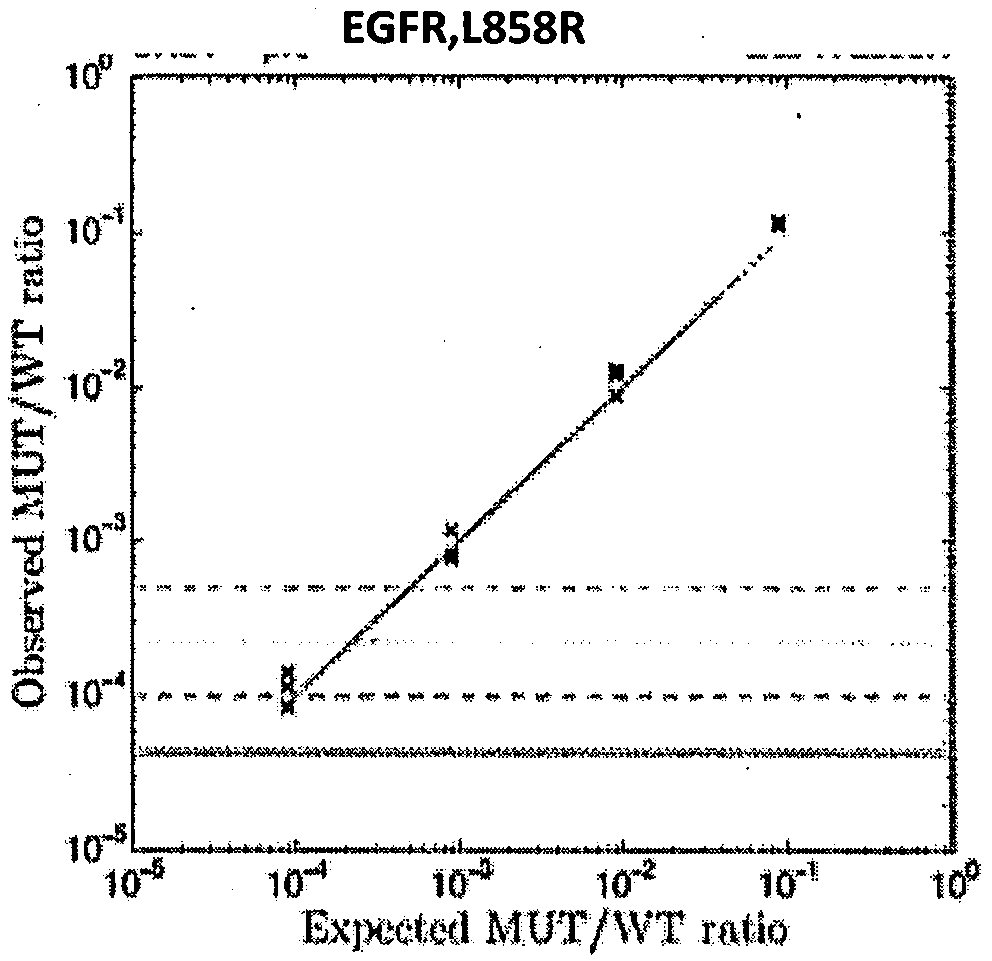
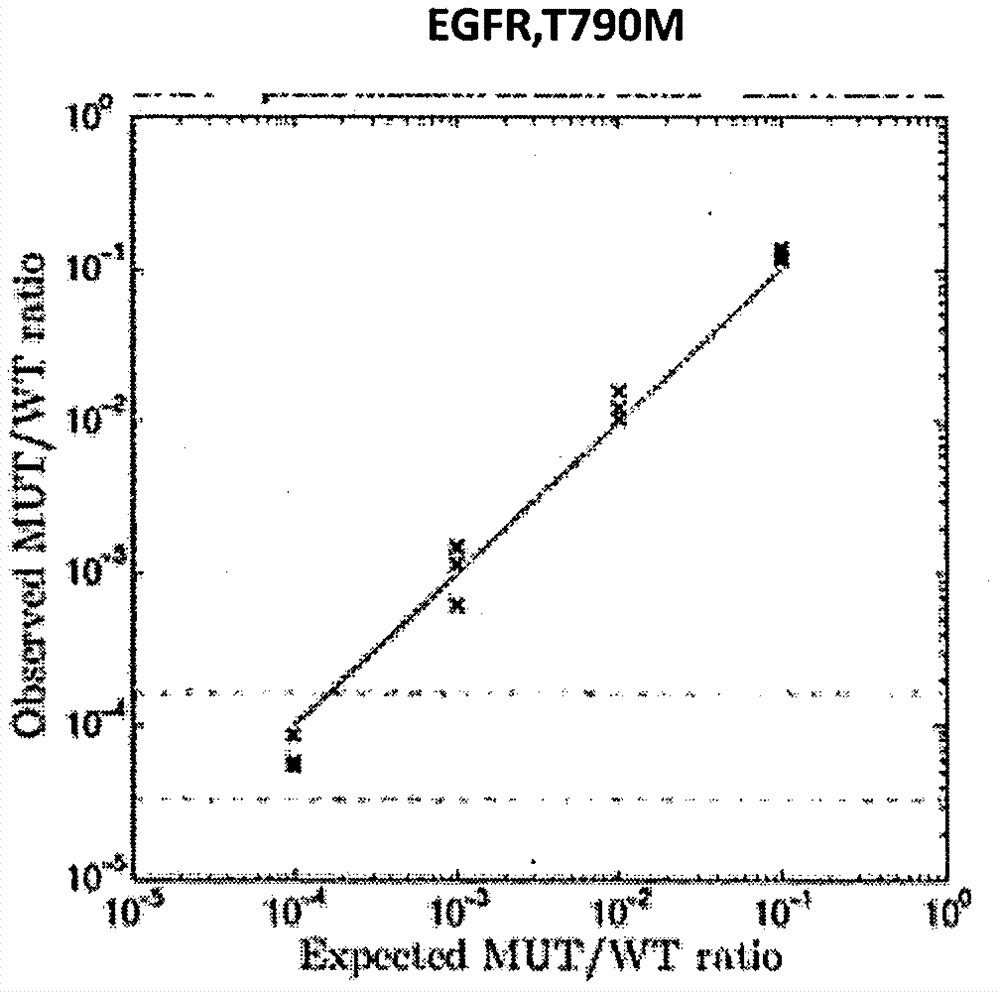
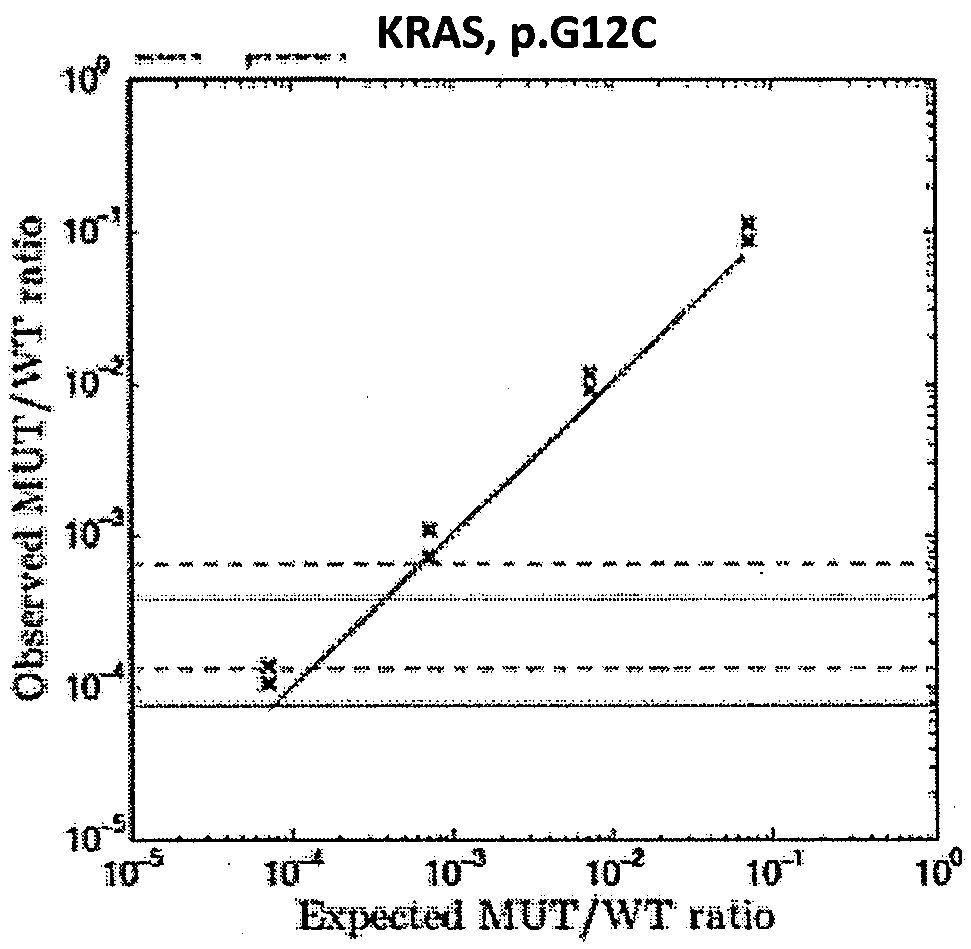
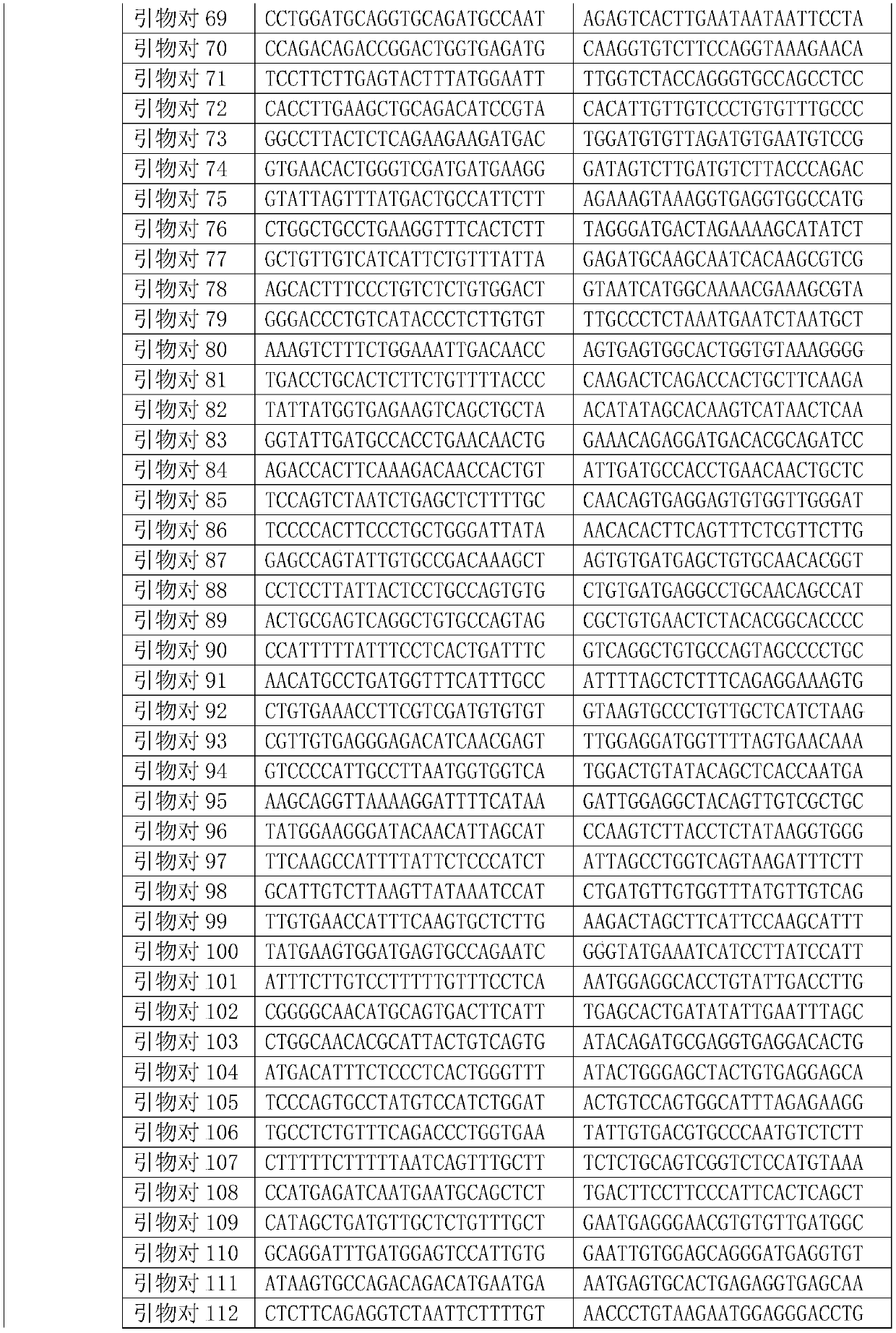
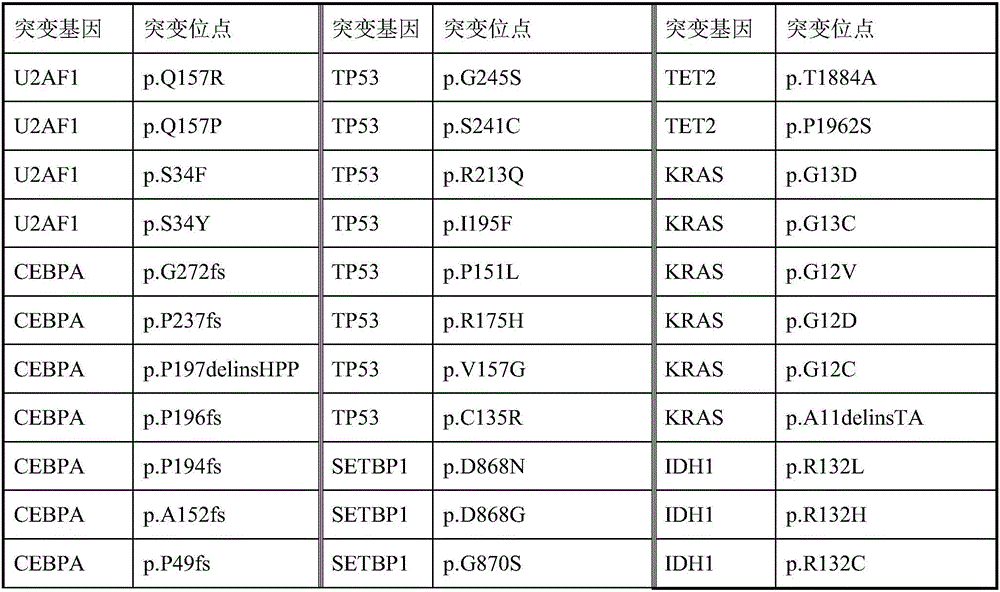
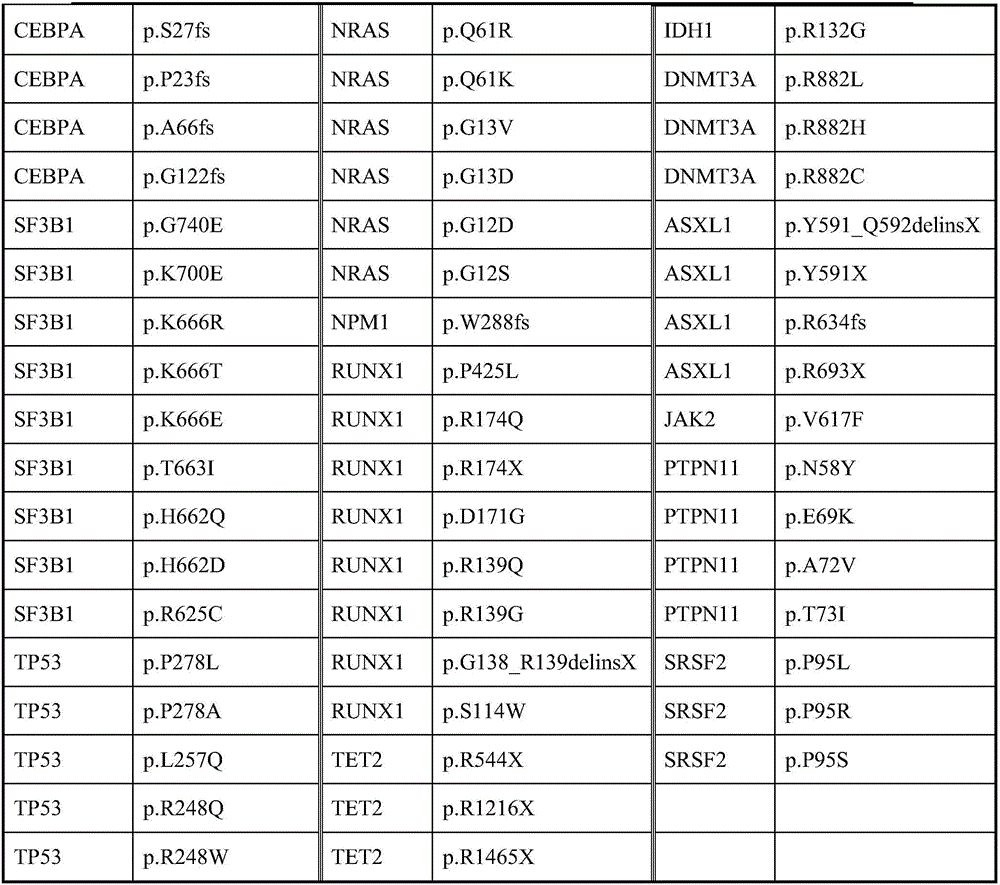
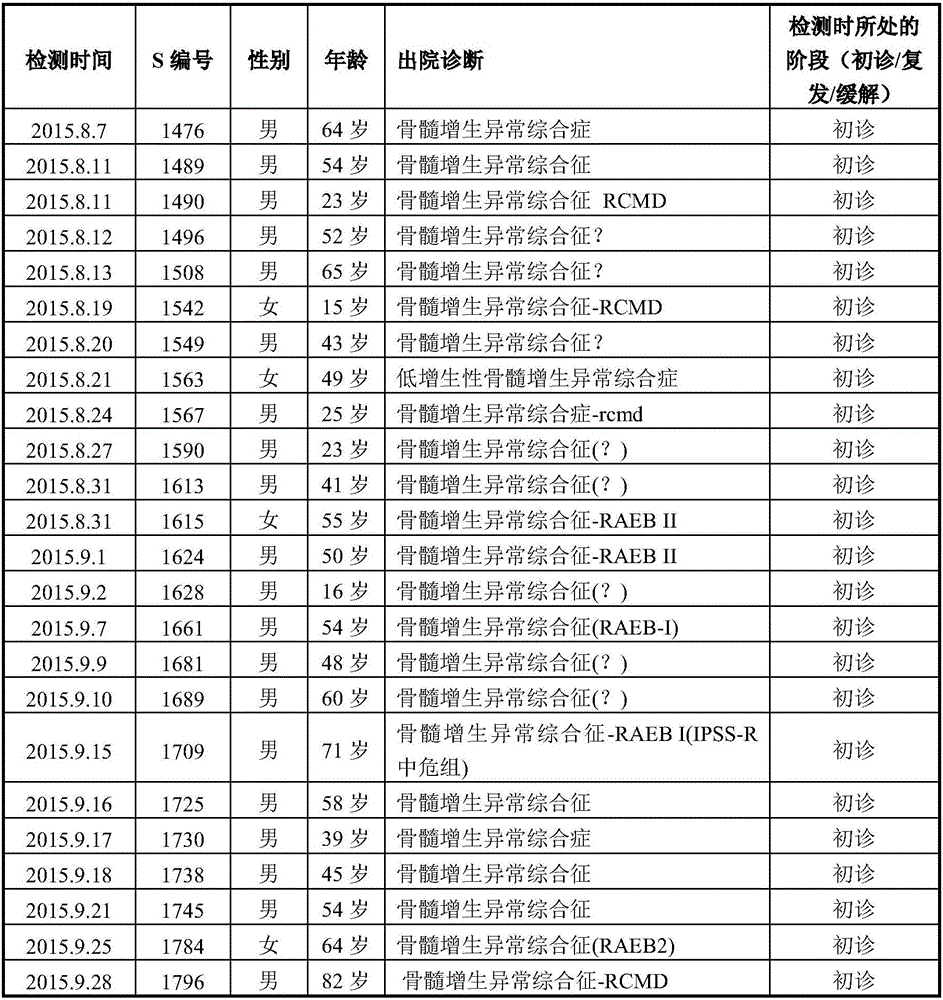
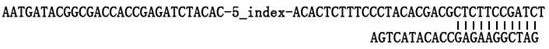Patents
Literature
73 results about "TP53 Genes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
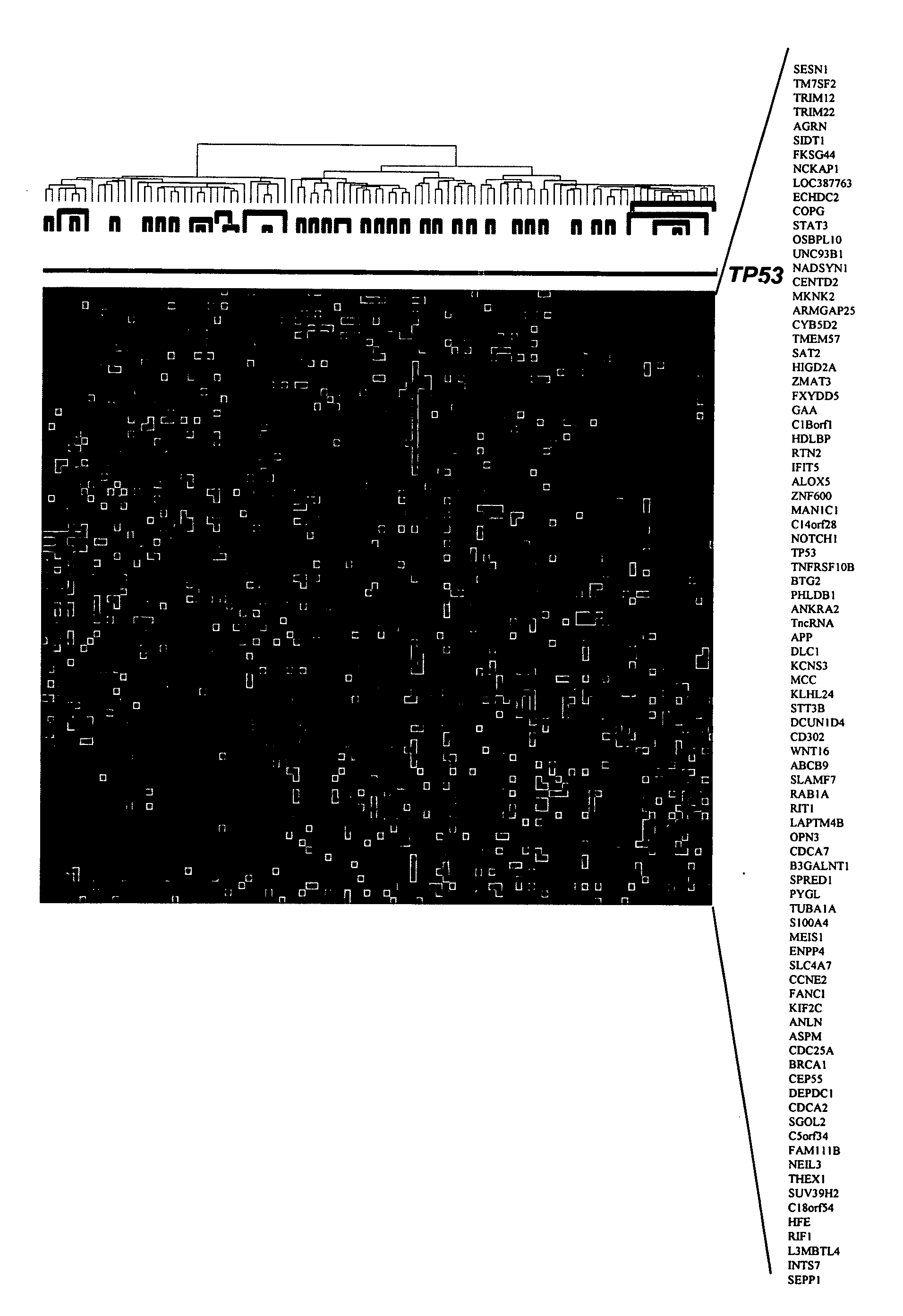
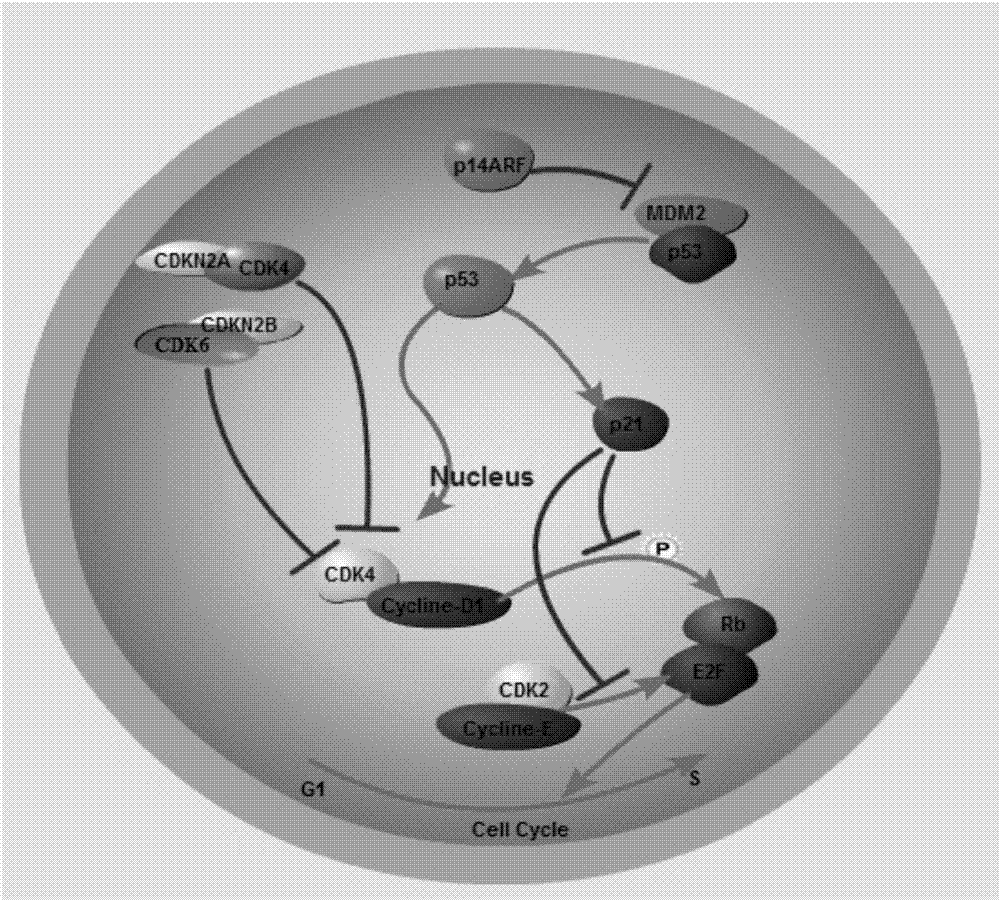
Tumor protein p53, also known as p53, cellular tumor antigen p53 (UniProt name), phosphoprotein p53, tumor suppressor p53, antigen NY-CO-13, or transformation-related protein 53 (TRP53), is any isoform of a protein encoded by homologous genes in various organisms, such as TP53 (humans) and Trp53 (mice).
TP53 gene expression and uses thereof
InactiveUS20080234138A1Microbiological testing/measurementLibrary screeningDisease progressionCancer research
The present invention is drawn to diagnosis, prognosis and treatment of multiple myeloma. In this regard, the present invention discloses importance of down-regulation of TP3 gene in multiple myeloma and its use as an independent progostic indicator of multiple myeloma. Additionally, the present invention also discloses novel-TP53 associated genes and demonstrates the clinical relevance of these alterations to disease progression.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
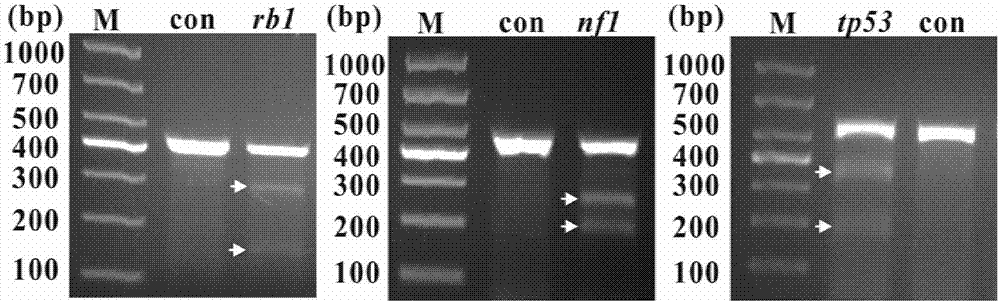
Gene knockout vector and zebra fish glioma model thereof
The invention provides a gene knockout vector and a zebra fish glioma model thereof. The gene knockout vector is obtained by connecting a target sequence to plasmids capable of expressing CRISPR-Cas9gene editing system related enzymes, and the target gene is a p53, Rb1 or Nf1 gene. The CRISPR / CAS9 technology is utilized for performing targeted knockout of rb1, nf1 and tp53 genes, an established transgene-induced malignant glioma zebra fish model can observe occurrence of malignant gliomas, tumor-induced angiogenesis and glioma stem cell generating processes through real-time fluorescence, andthe difference of pathogenesis of gliomas under different genetic background conditions is studied through the molecular biotechnology.
Owner:SHANTOU UNIV MEDICAL COLLEGE
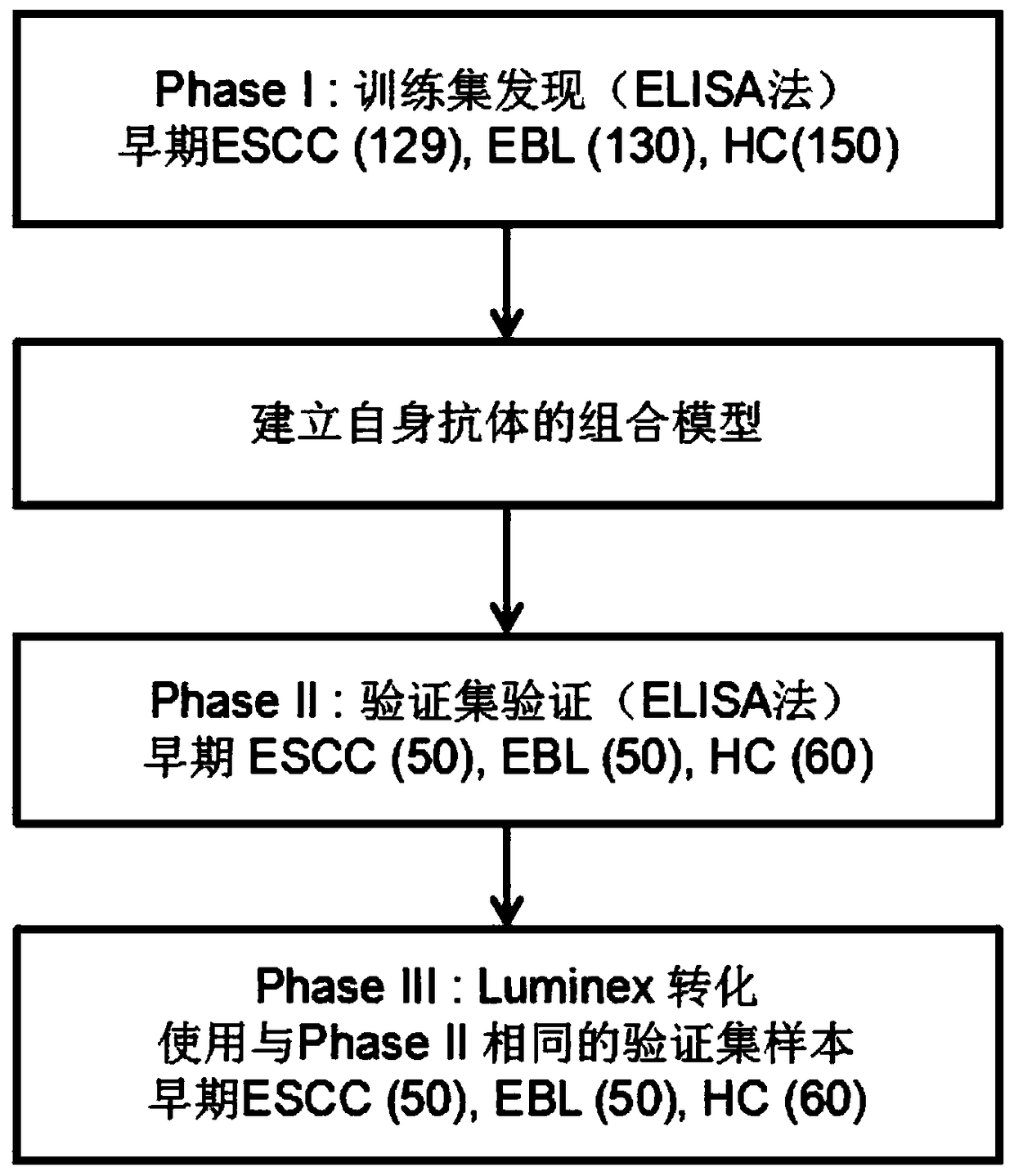
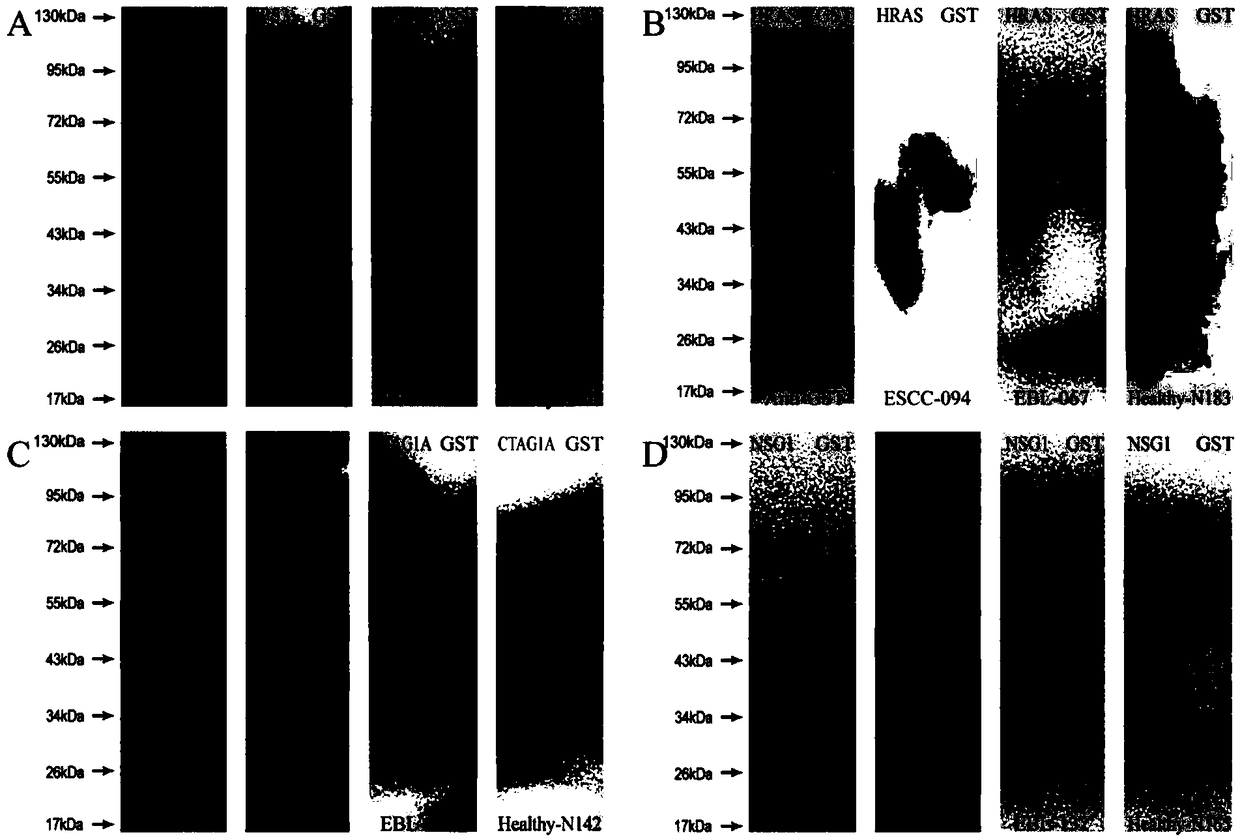
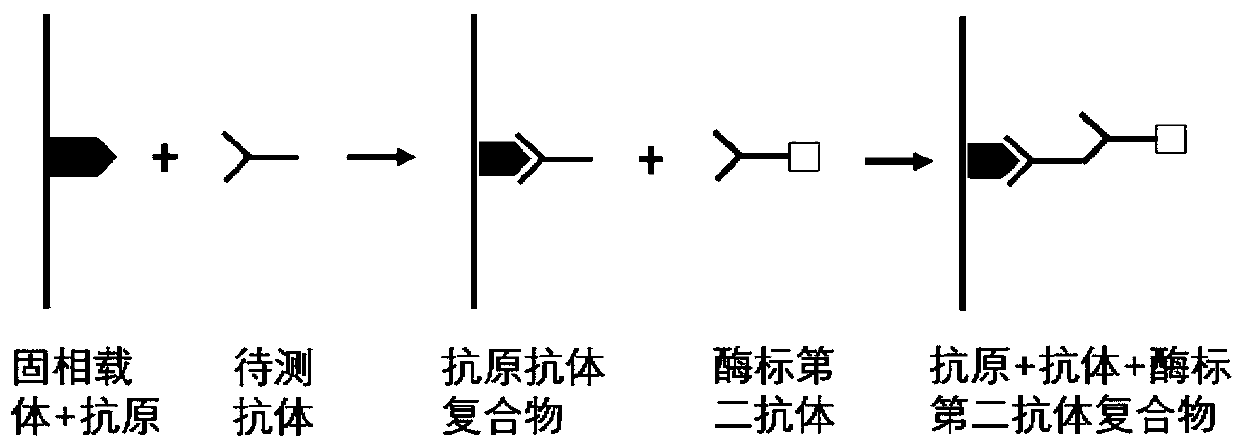
Four-autoantibody combined detection kit for diagnosing early esophageal squamous cancer and application thereof
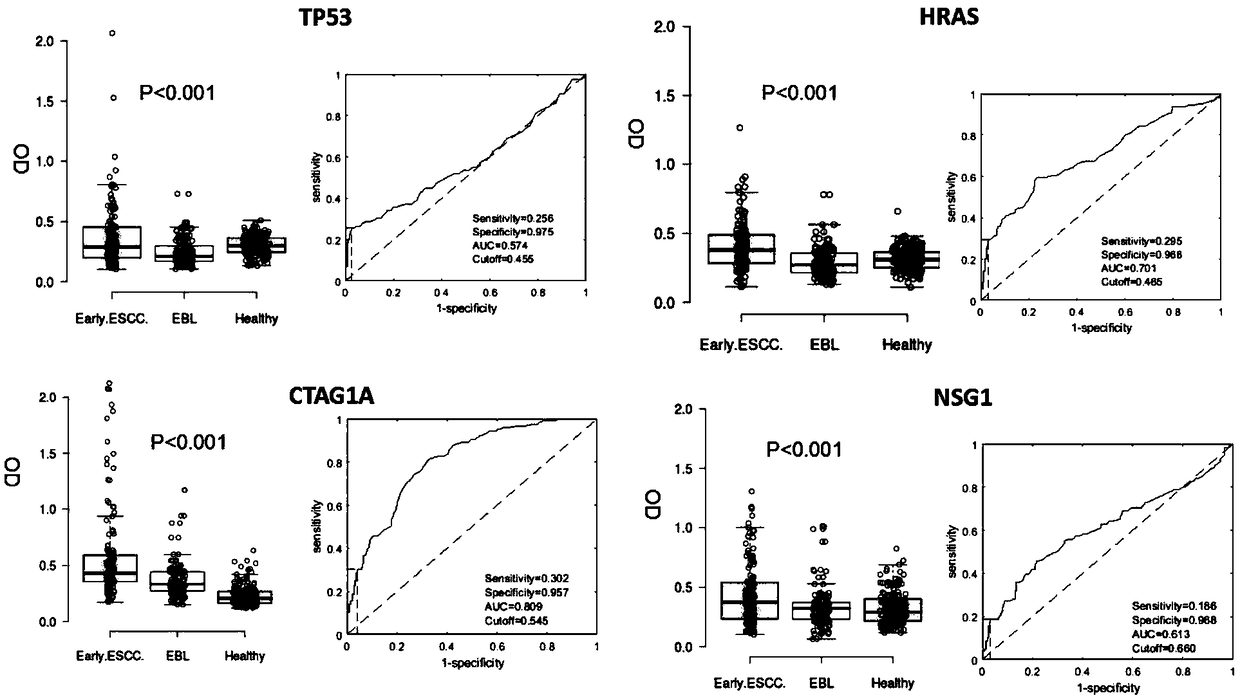
The invention provides a four-autoantibody combined detection kit for diagnosing an early esophageal squamous cancer and application thereof. The four-autoantibody combined detection kit for diagnosing the early esophageal squamous cancer comprises a solid substrate and antigen protein which coats the solid substrate; and the antigen protein comprises TP53, HRAS, CTAG1A and NSG1. The four-autoantibody combined detection kit for diagnosing the early esophageal squamous cancer, provided by the invention, has the advantages that the TP53, the HRAS, the CTAG1A and the NSG1 are firstly adopted as acombination to be used for early diagnosis of ESCC, higher sensibility and higher specificity are realized, the detection kit is conducive to further improvement of serologic screening quality of ESCC early diagnosis, and therefore, the cure rate and survival rate of patients are further promoted.
Owner:FUJIAN PROVINCIAL HOSPITAL
Method for predicting sensitivity of patients with NSCLC (non-small cell lung cancer) to immunotherapies
ActiveCN110305965AAccurate predictionAvoid blind medicationMicrobiological testing/measurementProteomicsOncologyBiomarker (petroleum)
The invention discloses a method for predicting sensitivity of patients with a cancer (non-small cell lung cancer) to immunotherapies, such as immunotherapy using an immune checkpoint inhibitor, by using KMT2C and TP53 genes as biomarkers, and also discloses application of a reagent, which detects the biomarkers specifically, in the preparation of kits to predict the sensitivity of patients with acancer (non-small cell lung cancer) to immunotherapies, such as immunotherapy using an immune checkpoint inhibitor. Through the combinatory consideration of TP53 and KMT2C gene mutation states herein, groups sensitive to ICIs (immune checkpoint inhibitors) can be predicted accurately from patients with NSCLC (non-small cell lung cancer), blind medication is avoided, and economy of ICI therapy isimproved.
Owner:SHANGHAI ORIGIMED CO LTD
Algorithms for outcome prediction in patients with node-positive chemotherapy-treated breast cancer
InactiveUS20110166838A1Reduce chanceReduce deathMicrobiological testing/measurementBiostatisticsCentromere protein JSOX4
The invention relates to methods for predicting an outcome of cancer in a patient suffering from cancer, said patient having been previously diagnosed as node positive and treated with cytotoxic chemotherapy, said method comprising determining in a biological sample from said patient an expression level of a plurality of genes selected from the group consisting of ACTG1, CAl2, CALM2, CCND1, CHPT1, CLEC2B, CTSB, CXCL13, DCN, DHRS2, EIF4B, ERBB2, ESR1, FBXO28, GABRP, GAPDH, H2AFZ, IGFBP3, IGHG1, IGKC, KCTD3, KIAA0101, KRT17, MLPH, MMP1, NAT1, NEK2, NR2F2, OAZ1, PCNA, PDLIM5, PGR, PPIA, PRC1, RACGAP1, RPL37A, SOX4, TOP2A, UBE2C and VEGF; ABCB1, ABCG2, ADAM15, AKR1C1, AKR1C3, AKT1, BANF1, BCL2, BIRC5, BRMS1, CASP10, CCNE2, CENPJ, CHPT1, EGFR, CTTN, ERBB3, ERBB4, FBLN1, FIP1L1, FLT1, FLT4, FNTA, GATA3, GSTP1, Herstatin, IGF1R, IGHM, KDR, KIT, CKRT5, SLC39A6, MAPK3, MAPT, MKI67, MMP7, MTA1, FRAP1, MUC1, MYC, NCOA3, NFIB, OLFM1, TP53, PCNA, PI3K, PPERLD1, RAB31, RAD54B, RAF1, SCUBE2, STAU, TINF2, TMSL8, VGLL1, TRA@, TUBA1, TUBB, TUBB2A.
Owner:SIVIDON DIAGNOSTICS
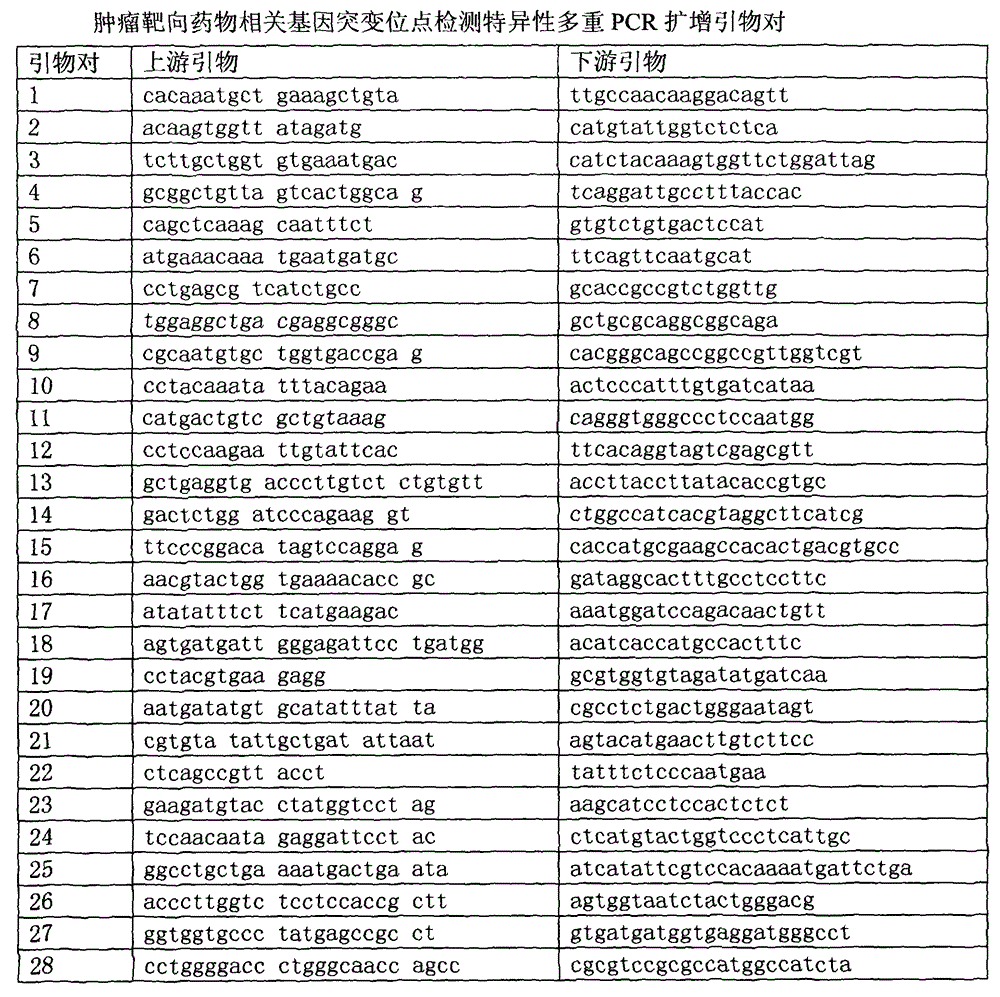
High flux detection method for tumor-targeted drugs related genes mutation, primers and reagent thereof
ActiveCN105624274AKnowing about genetic mutationsHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationTumor targetWilms' tumor
The invention relates to the field of tumor gene, and discloses a high-flux sequencing detection method for tumor-targeted drugs related genes mutation, a group of primer pairs and a reagent thereof. The method is used for detecting mutation of tumor-targeted drugs related genes in an acceptor, the related genes comprise BRAF, EGFR, KRAS, NRAS, TP53, FGFR3, KIT, PIK3CA, RET, PTEN, and CTNNB1. The method comprises the following steps: extraction of sample DNA, target gene enrichment, primer sequence removal, joint connection, PCR amplification, library homogenization, library detection and high flux sequencing. The disclosed primer pairs enable multiple PCR enrichment and amplification on the tumor-targeted drugs related genes, the method can be used for detecting 115 mutation sites in the above 11 genes, a detection result can be a medication guidance for the tumor-targeted drugs, and provides a basis for exploitation of novel cancer drugs.
Owner:绍兴积准生物科技有限公司
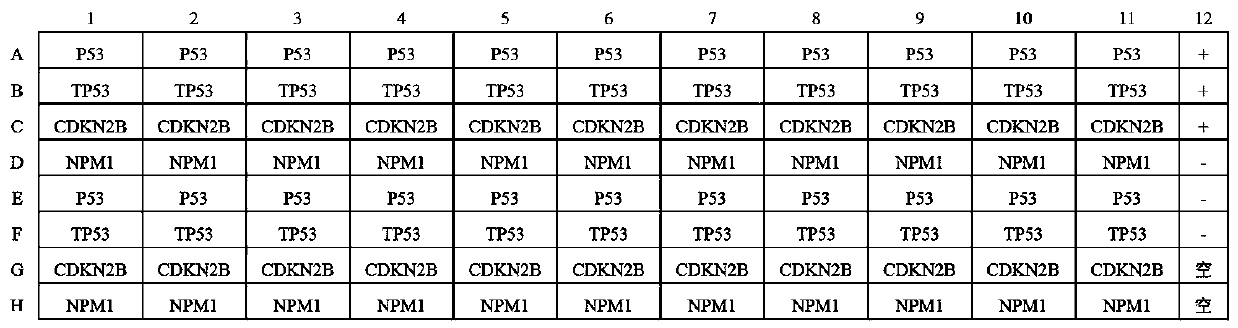
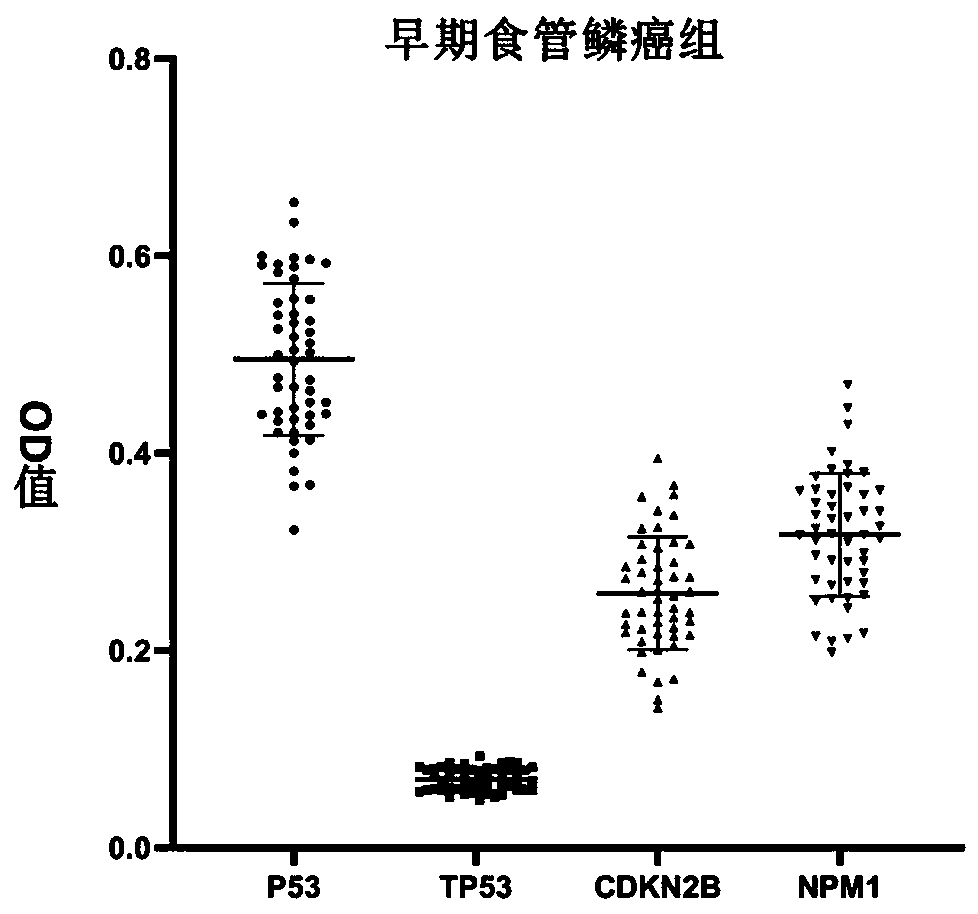
Autoantibody joint detection ELISA kit for early screening of esophageal squamous carcinoma
The invention belongs to the technical field of medical oncology, and particularly discloses an autoantibody joint detection ELISA kit for early screening of esophageal squamous carcinoma. The kit comprises a solid-phase carrier and a tumor-associated antigen coated on the solid-phase carrier, wherein the tumor-associated antigen consists of P53, TP53, CDKN2B and NPM1. Furthermore, the kit comprises sample diluent, a second antibody, second antibody diluent, positive control serum, negative control serum, developing solution, stop solution and washing solution. The ELISA kit is capable of effectively detecting the esophageal squamous carcinoma, especially the early esophageal squamous carcinoma, has detection sensitivity up to 88.8% and specificity up to 86.3%, can be used for the large-scale screening of asymptomatic groups in high-risk areas of the esophageal squamous carcinoma, and is beneficial for the esophageal squamous carcinoma screening and early discovery of the asymptomatichigh-risk groups.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Compositions and methods for inhibiting expression of pro-apoptotic genes
InactiveUS20100222409A1Reducing and preventing symptomInhibit and reduce expressionOrganic active ingredientsSugar derivativesDiseaseKEAP1
The invention relates to one or more inhibitors, in particular siRNA compounds, which down-regulate the expression of a pro-apoptotic gene selected from the group consisting of TP53; HTRA2; KEAP1; SHC1-SHC, ZNHIT1, LGALS3 and HI95. The invention also relates to a pharmaceutical composition comprising the compound, and a pharmaceutically acceptable carrier. The present invention further provides methods of treating a subject afflicted with a disease or a condition associated with those genes, comprising administering to the subject a pharmaceutical composition in a therapeutically effective dose so as to thereby treat the subject.
Owner:QUARK FARMACUITIKALS INC
Biomarkers of miR-34 activity
InactiveCN105473742AOrganic active ingredientsMicrobiological testing/measurementCancer cellMdm2 Protein
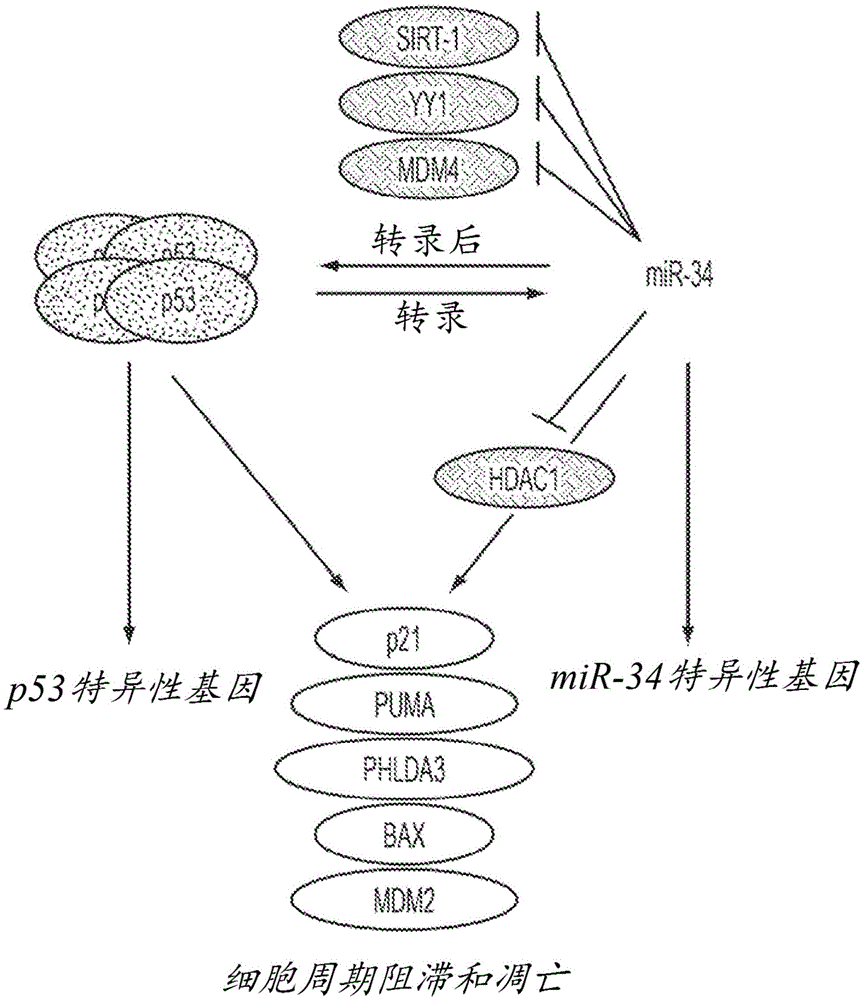
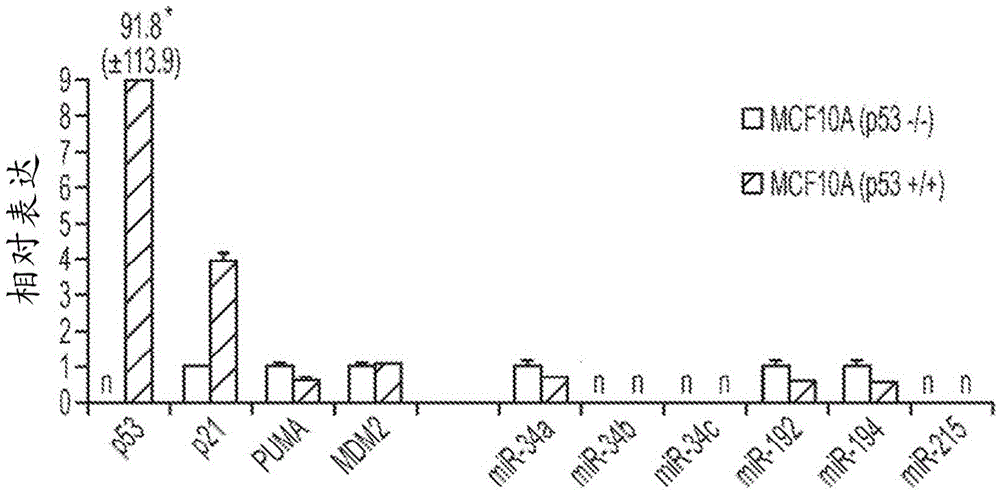
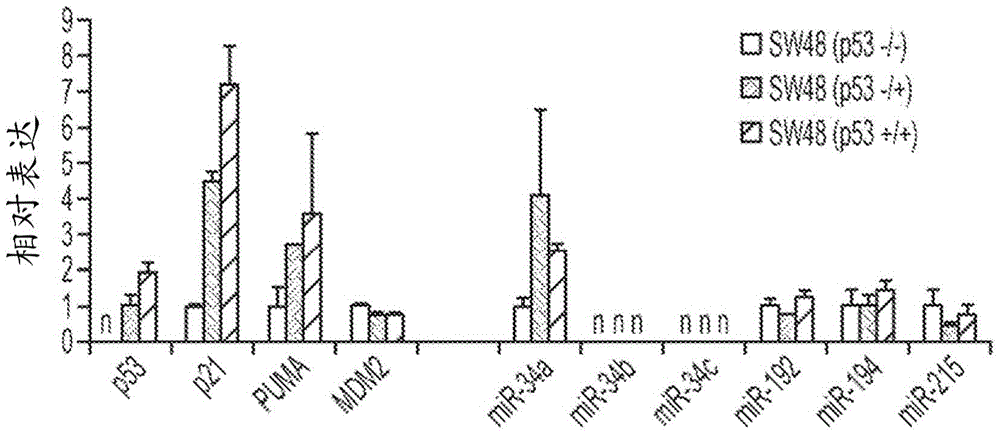
This invention is based in part on the discovery that miR-34 is independent of p53. It has been discovered that miR-34 functions in a TP53 -independent tumor suppression pathway. Specifically, miR-34-induced inhibition of cancer cell growth was found to be the same in p53-normal and p53-deficient cells. Thus, miR-34 has a more central role during tumor suppression that is uncoupled from p53. In the absence of p53, miR-34, unlike certain other miRNAs, is sufficient to induce an up-regulation of genes known to be regulated by p53, including but not limited to p21<CIP1 / WAF1>(CDKN1A), PUMA, BAX, NOXA, PHLDA3, and MDM2 and a down-regulation of HDAC1. Therefore, these biomarkers can be used as biomarkers of miR-34 activity. The invention is further based on the discovery that some of these biomarkers are indispensable for a therapeutic response to miR-34 activity, and are thus prerequisite biomarkers of miR-34 activity.
Owner:MIRNA THERAPEUTICS
Genetic Screening for Polymorphisms in Human Genes that Increase or Decrease Sensitivity to Toxic Agents
InactiveUS20070207195A1Reduce exposureData processing applicationsMicrobiological testing/measurementGeographic regionsIncreased risk
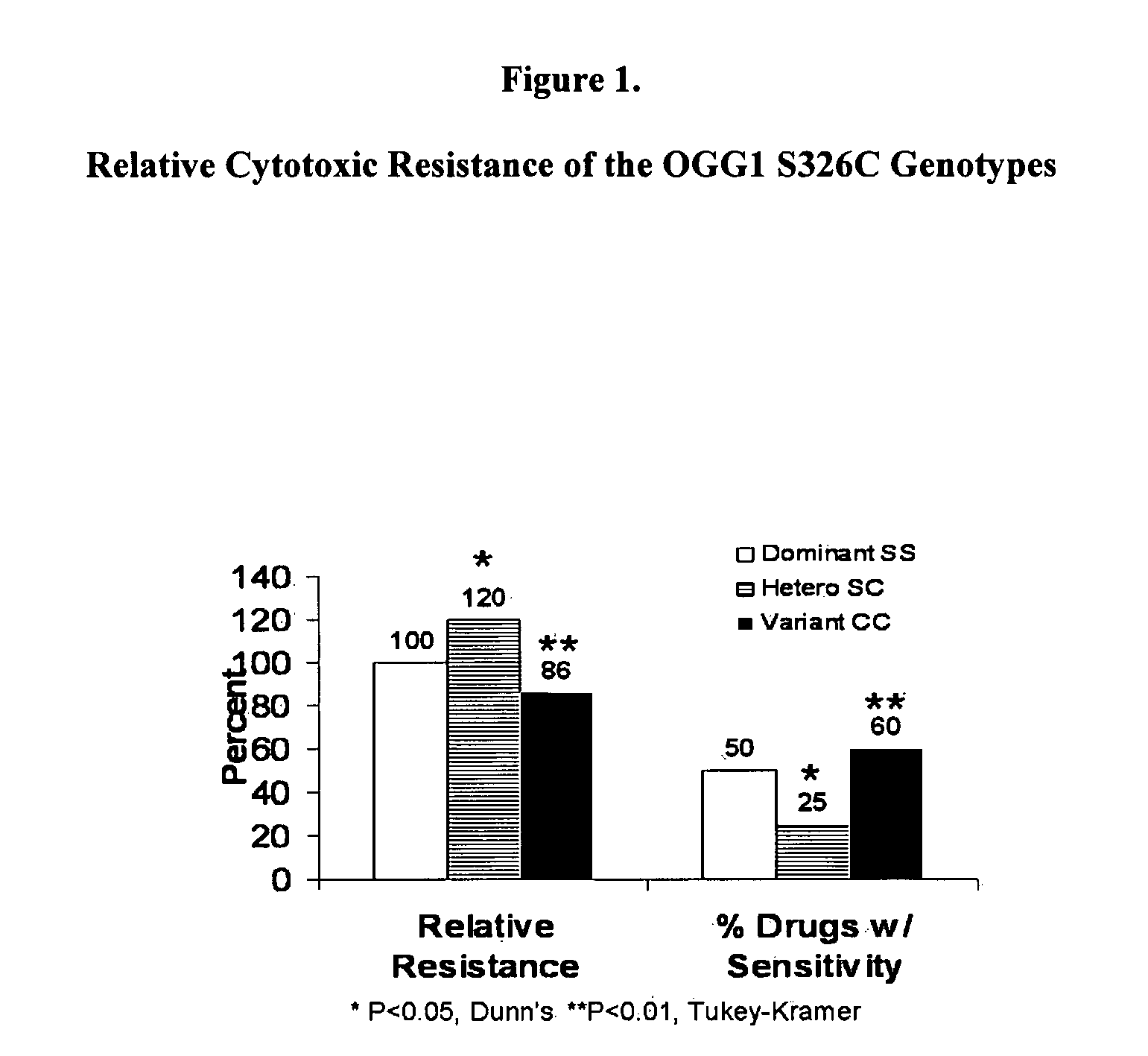
Methods are disclosed for genetically counseling a person based on one or more polymorphisms in his or her genes that sensitize him or her to toxic agents. Methods are also disclosed for genetically screening a group of individuals and / or a human population, based on, for example, ethnicity, race, religion or geographic region, to identify individuals with such polymorphisms for counseling. The methods can be used to counsel a person who has not been genetically tested for polymorphisms but who might have increased risk for sensitivity to toxic agents due to his or her membership in a particular group and / or population. The methods use correlations between genotypes of polymorphic alleles in a panel of cell lines and sensitivity of the cell lines to toxic agents. As examples, the methods are used to identify genotypes of allelic forms of the genes TP53, OGG1, ERCC2, XRCC1, and NOS3 that increase sensitivity or resistance of cells to toxic agents.
Owner:APPLIED GENETICS DERMATICS
Hormone receptor-positive breast cancer recurrence monitoring gene mutation library construction method
The invention discloses a hormone receptor-positive breast cancer recurrence monitoring gene mutation library construction method, which is characterized that the library covers the total 1357 somatic cell mutations on human genes such as KRAS, JAK3, AKT1, CREBBP, PTEN, RB1, TP53, CTNNB1, TSC2, TSC1, ERBB2, PIK3CA, SF3B1, JAK2, CDH1, SMAD4, GATA3, MAP2K4, MAP3K1 and ESR1. According to the present invention, a plurality of the target sequences are subjected to single tube amplification with the construction method to rapidly complete the library construction, wherein the whole library construction process takes only 2-3 h and the manual time only needs 45 min, such that the difficulty that the multi-gene and multi-target detection of somatic cells on the basis of the small amount of the peripheral blood sample is required in the clinical breast cancer recurrence monitoring is required can be effectively solved, and the lost is low.
Owner:XIAMEN SPACEGEN BIOTECH CO LTD
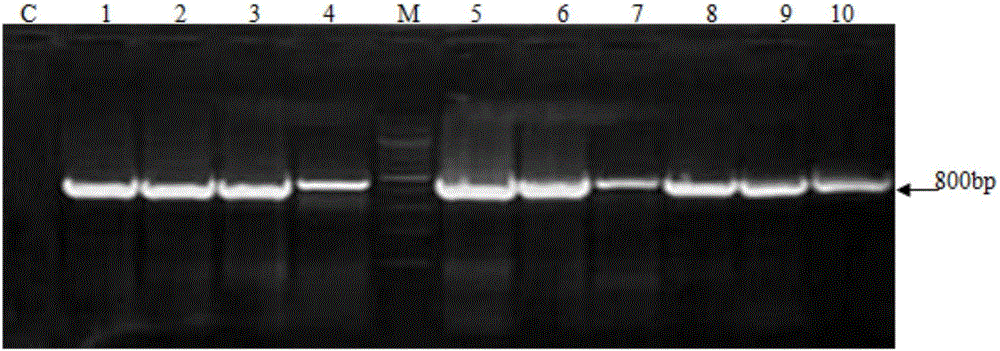
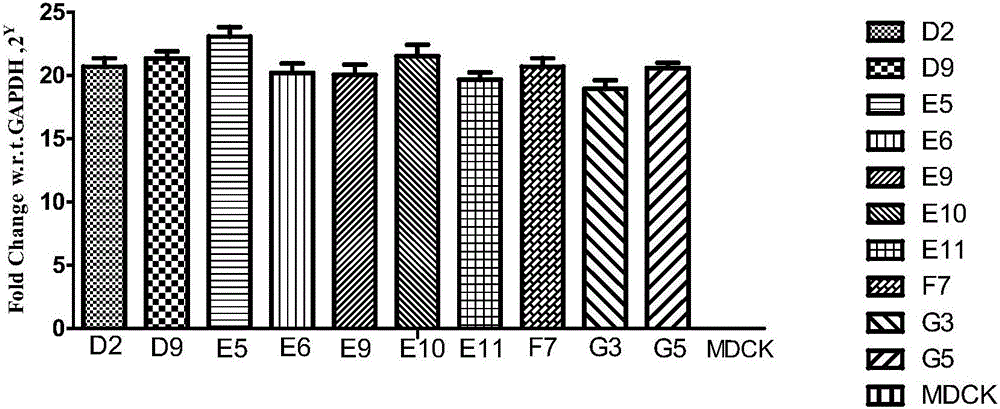
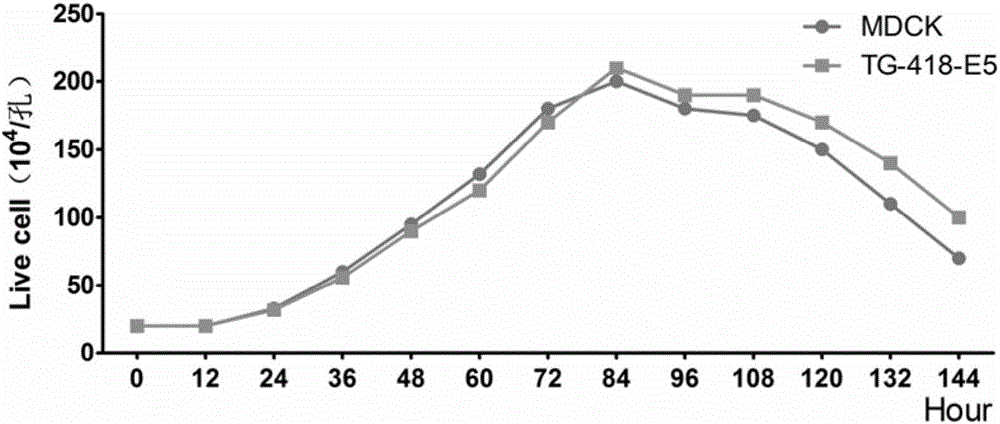
MDCK (Madin-Darby canine kidney) cell line capable of stably expressing human-derived TIGAR (TP53-induced glycolysis and apoptosis regulator) gene
ActiveCN106479983AImprove anti-apoptotic abilityReduce adhesionSsRNA viruses negative-senseCompound screeningCanine kidneyBiology
The invention belongs to the technical field of biology, and particularly relates to an MDCK (Madin-Darby canine kidney) cell line capable of stably expressing a human-derived TIGAR (TP53-induced glycolysis and apoptosis regulator) gene. The cell line is an MDCK cell line TG-418-E5 capable of stably expressing the human-derived TIGAR gene and contains a TIGAR coding gene sequence, and the preservation number of the cell line is CGMCC NO:12983. Through stable expression of the TIGAR gene in MDCK cells, anti-apoptosis capacity of the cells is improved, and the survival time of recombinant cells is prolonged. The method not only lays the foundation for research of application of MDCK serum-free fully-suspended culture in mass production of cell culture vaccines, but also increases breeding titer of avian influenza vaccine strains and saves the cost of a production process. Besides, the cell line TG-418-E5 has better application prospect in aspects of screening of anti-avian influenza virus drugs, screening of vaccine strains and production of the cell culture vaccines.
Owner:ZHONGCHONG XINNUO BIOTECH TAIZHOU CO LTD
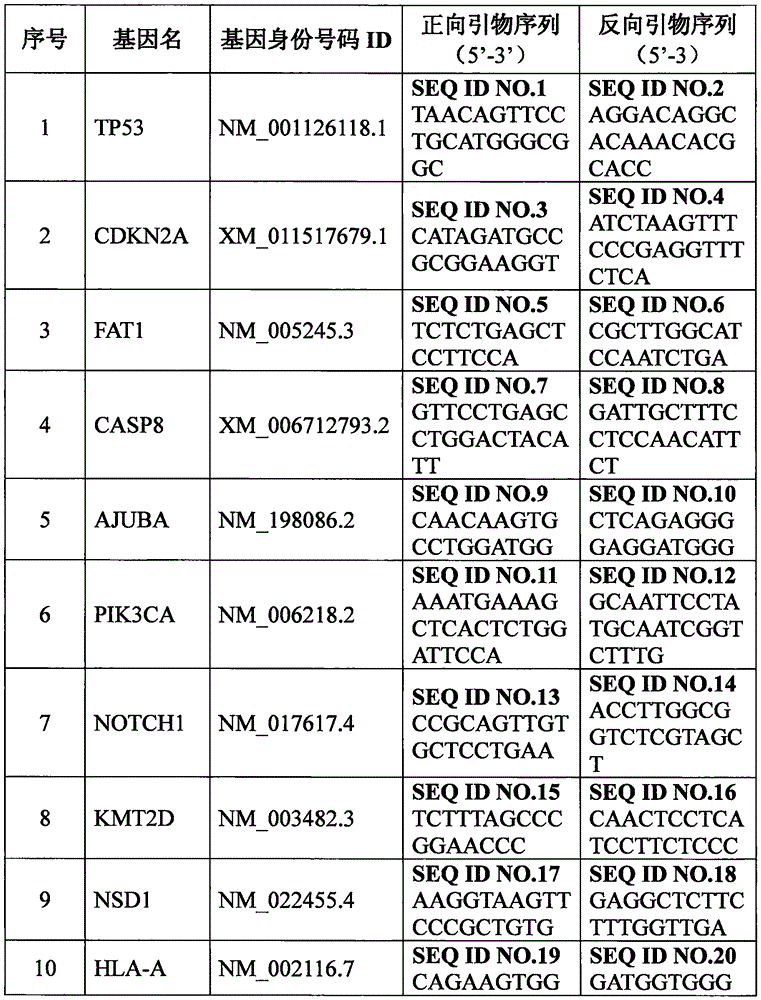
Set of genes for head and neck squamous cell carcinoma (HNSCC) molecular typing and application thereof
ActiveCN105671187AImprove rationalityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationKMT2D geneIndividualized treatment
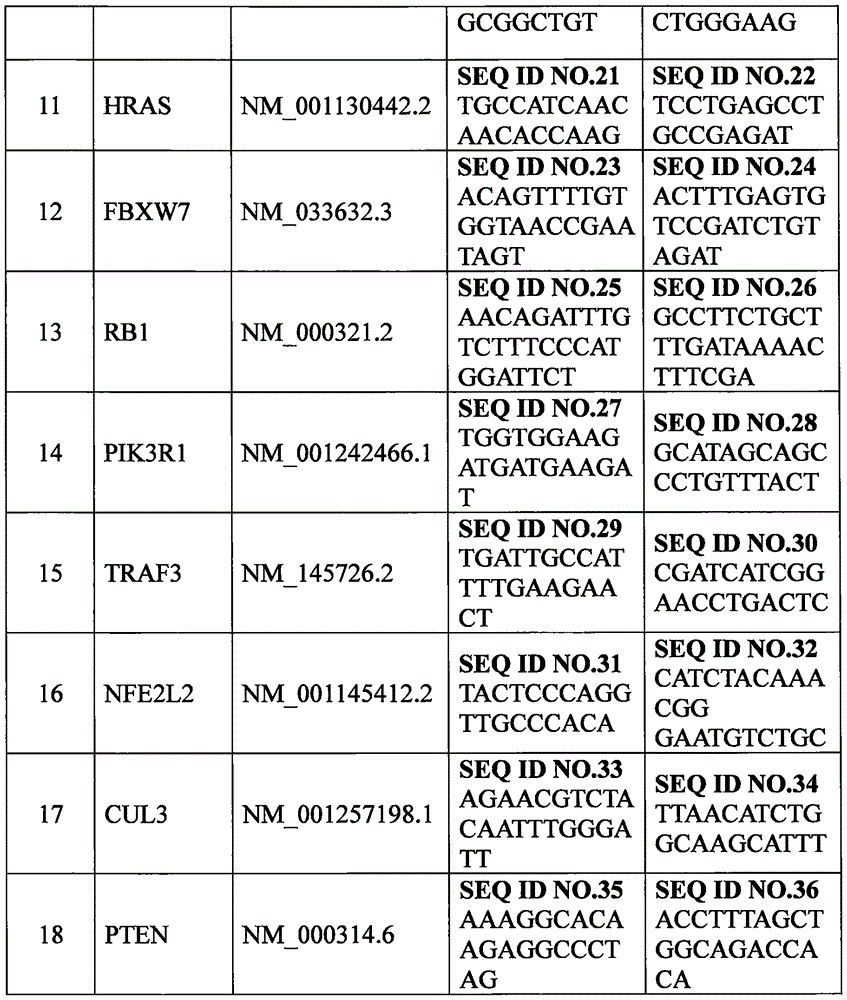
The invention provides a set of genes for head and neck squamous cell carcinoma (HNSCC) molecular typing. The set of genes comprises TP53 gene, CDKN2A gene, FAT1 gene, CASP8 gene, AJUBA gene, PIK3CA gene, NOTCH1 gene, KMT2D gene, NSD1 gene, HLA-A gene, HRAS gene, FBXW7 gene, RB1 gene, PIK3R1 gene, TRAF3 gene, NFE2L2 gene, CUL3 gene and PTEN gene. The invention also provides application of the genes in preparing a kit and gene chip for HNSCC molecular typing, and a kit and gene chip for HNSCC molecular typing prepared by using the genes. The genes can enhance the HNSCC typing rationality and accuracy, thereby providing key technical supports for implementing early diagnosis, effective interception and individualized treatment on HNSCC.
Owner:SOUTHERN MEDICAL UNIVERSITY
Epitope peptide monoclonal antibody for tumor-related gene TP53 and application thereof
ActiveCN105461807AStrong specificityIncreased sensitivityImmunoglobulins against animals/humansBiological material analysisProtein targetCarrier protein
The invention provides a monoclonal antibody for a gene TP53. A highly-conserved protein sequence of the gene TP53 is target protein. Epitope peptide is synthesized through design and coupled with carrier protein to be used as immunogen. The monoclonal antibody is prepared from the immunogen. According to the monoclonal antibody and the epitope peptide synthetized through design, the monoclonal antibody can be used for detecting quantitative expression of human blood or tissue TP53 antibodies, has the advantages of being high in specificity and sensitivity and can be widely applied in clinical detection and experimental research.
Owner:JILIN UNIV
TP53 Gene expression and uses thereof
The present invention is drawn to diagnosis, prognosis and treatment of multiple myeloma. In this regard, the present invention discloses importance of down-regulation of TP3 gene in multiple myeloma and its use as an independent progostic indicator of multiple myeloma. Additionally, the present invention also discloses novel-TP53 associated genes and demonstrates the clinical relevance of these alterations to disease progression.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Kit for screening colorectal cancer hereditary susceptibility genes
InactiveCN106244692AImprove detection efficiencyImprove accuracyMicrobiological testing/measurementCell sensitivityCYP1A2
The invention discloses a kit for screening colorectal cancer hereditary susceptibility genes. The kit comprises colorectal cancer susceptibility gene specificity primers for amplifying a plurality of target regions in a to-be-detected sample, wherein the colorectal cancer susceptibility genes include at least one of rs10795668, MMP2, SMAD7, ADH2, ALDH2, CYP1A2, MMP-1, MTHFR, TP53, VEGF, COX-2, DNMT3B, hMLH1, LOC727677, MMP9, MTRR and TGF-beta 1. The kit can detect a plurality of regions of the colorectal cancer susceptibility genes at the same time, detecting efficiency is improved, detecting cost is reduced, and detecting accuracy and sensitivity are high.
Owner:SICHUAN KINGMED DIAGNOSTICS CENT
Primer group, reagent and/or kit and system for detecting lung cancer chemotherapy related genes, and application
ActiveCN109517900AClustering is clearImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationCYP2C8Biology
The invention relates to a primer group, reagent and / or kit and system for detecting lung cancer chemotherapy related genes, and application. The primer group contains primers for detecting gene mutation of ERCC1, MTHFR, GSTP1, XRCC1, DYNC2H1, ABCB1, CYP2C8*3, TP53, NQO1, CBR3, SOD2, CYP2C19, UGT1A1*6, TYMS, NT5C2 and CDA. The primer group, the reagent and / or the kit, the system and the application have the advantages of high accuracy, high specificity, high sensitivity, good precision and the like, and meanwhile, further have the advantages of sample saving, short detection period, easy operation, convenient analysis and the like.
Owner:SIMCERE DIAGNOSTICS CO LTD +2
Detection kit for detecting lymphoma related gene group
InactiveCN108251527ARealize Auxiliary DiagnosisImprove detection efficiencyMicrobiological testing/measurementDNA/RNA fragmentationExonMultiplex pcrs
The invention discloses a sequencing kit for screening lymphoma related gene mutation and a later medical interpretation database. A lymphoma related gene group comprises 22 genes such as MYD88 and TP53. The sequencing kit comprises multi-PCR (Polymerase Chain Reaction) primers for amplifying all exons of the 22 lymphoma related genes. The kit for screening lymphoma related gene mutation, which isdisclosed by the invention, has the characteristics of being high in detection efficiency, wide in mutation gene covering, high in detection rate, and the like, and a provided medical interpretationreport has the characteristics of being accurate and authoritative.
Owner:天津见康华美医学诊断技术有限公司
Method for detecting multi-locus low-frequency mutation of free target DNA of lung cancer plasma and kit
InactiveCN107881230AReduce dosageHigh detection throughputMicrobiological testing/measurementBlood plasmaLung cancer
The invention relates to the technical field of biology and the field of nucleic acid detection and relates to method for detecting multi-locus low-frequency mutation of free target DNA of lung cancerplasma and a kit. The invention provides high-sensitivity and high-flux method for detecting multi-locus mutation of free DNA of lung cancer plasma once and a kit. According to the detection method,a ddPCR technique and a next generation sequencing technique are combined for detecting the mutation of lung cancer crDNA. The detection method comprises the following steps: (1) preparing a digital PCR mixed liquid including a to-be-tested sample DNA template, a primer and a PCR premixing liquid; (2) preparing a digital PCR micro-reaction drop, and carrying out PCR amplified reaction; (3) recycling and purifying a PCR product; (4) preparing and amplifying PCR premixing liquid for the second time; (5) carrying out purification and library quality control on the PCR product; and (6) carrying out computer sequencing and data analysis. According to the detection method, hotspot mutation information of 10 genes including EGFR / BRAF / KRAS / PIK3A / MET2 / ERBB2 / AKT1 / NRAS / TP53 / PTEN of lung cancer can bedetected for one time, the cost can be remarkably lowered, and the high-sensitivity and high-flue detection of mutation information of lung cancer ctDNA can be realized.
Owner:FUDAN UNIV
Blood plasma specific fragment free DNA (deoxyribonucleic acid) quantitative detection kit
ActiveCN104073554AEliminate errorsEliminate distractionsMicrobiological testing/measurementForward primerBlood plasma
The invention discloses a blood plasma specific fragment free DNA (deoxyribonucleic acid) quantitative detection kit comprising the following components: two primer pairs for amplifying TP53 gene fragments of which the lengths are respectively 400bp and 150bp, a primer pair for amplifying a reference gene Beta-actin, and fetal genome DNA, wherein the two primer pairs are consistent in backward primer sites, and the lengths of the amplified TP53 gene fragments are determined by virtue of a forward primer. According to the kit disclosed by the invention, a calibration system is introduced based on a fluorescent quantitative PCR (polymerase chain reaction) technology, the fetal genome DNA is used as background DNA, a house-keeping gene Beta-actin is used as the reference gene, a cancer suppressor gene TP53 is used as a target gene, an amplification primer is designed aiming at different lengths of fragments of the cancer suppressor gene TP53, different lengths of the fragments of the Beta-actin gene and the TP53 gene are synchronously amplified by adopting a real-time fluorescent quantitative PCR process, and the content of free DNA with specific fragment sizes in blood plasma can be quantitatively detected.
Owner:JIANGSU MICRODIAG BIOMEDICINE TECH CO LTD
Probe combination for detection of cancer
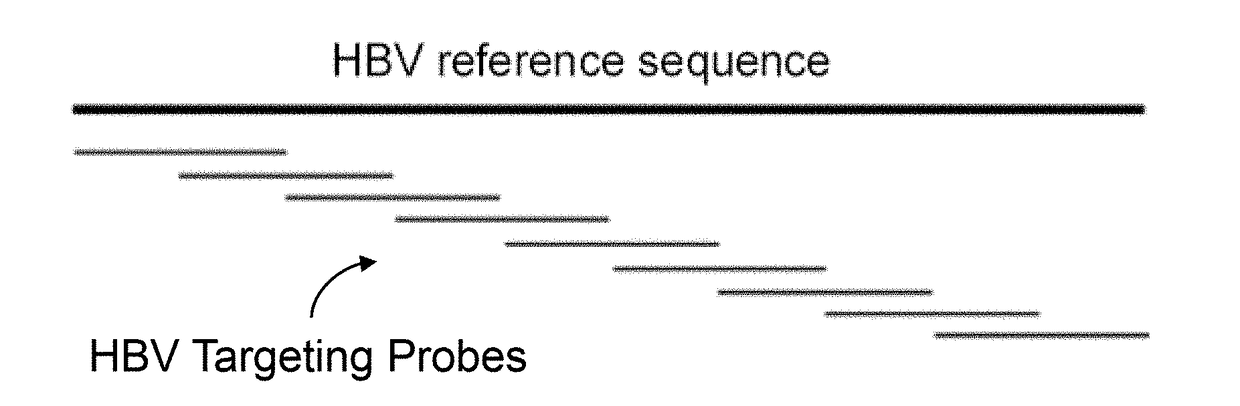
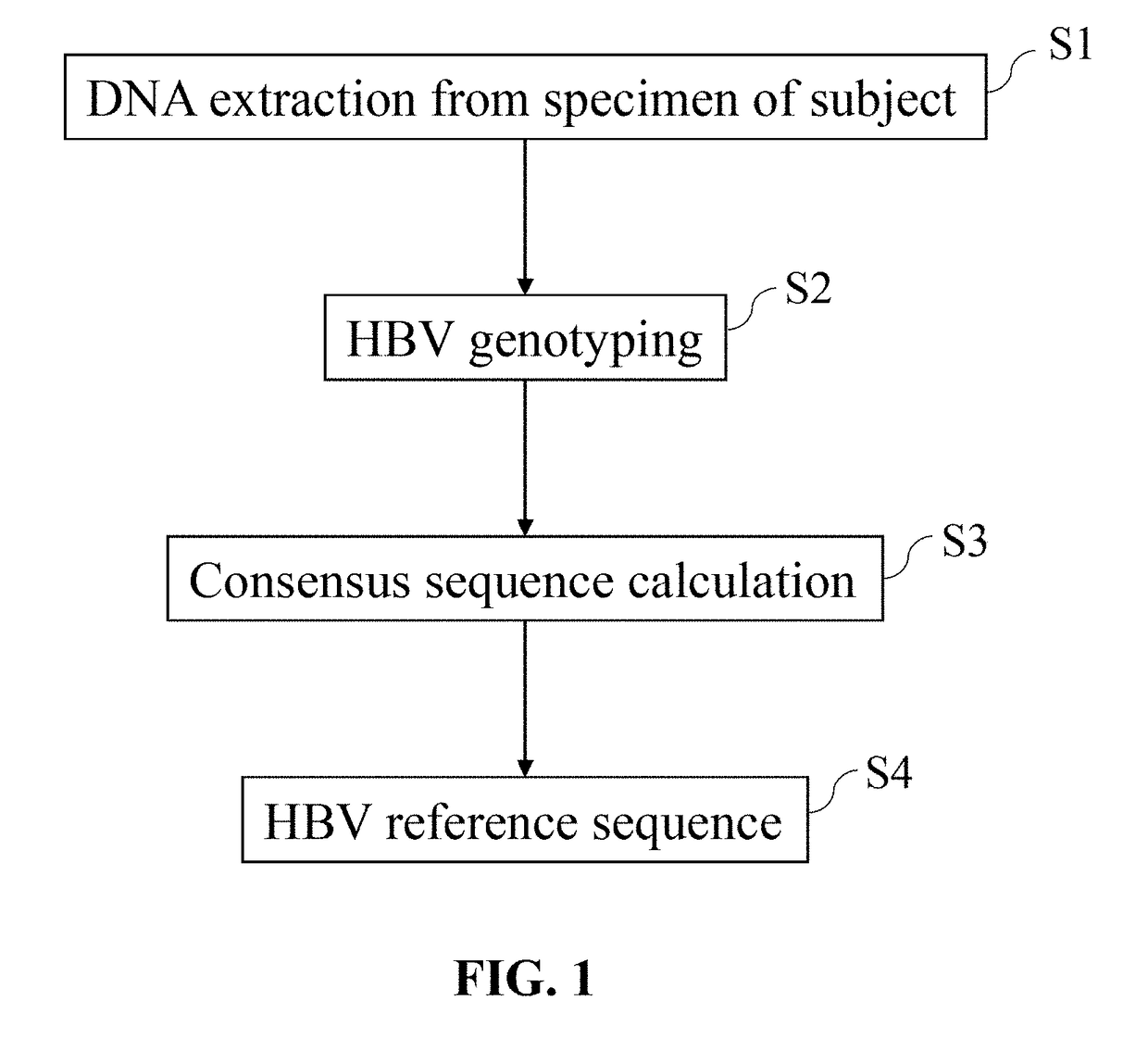
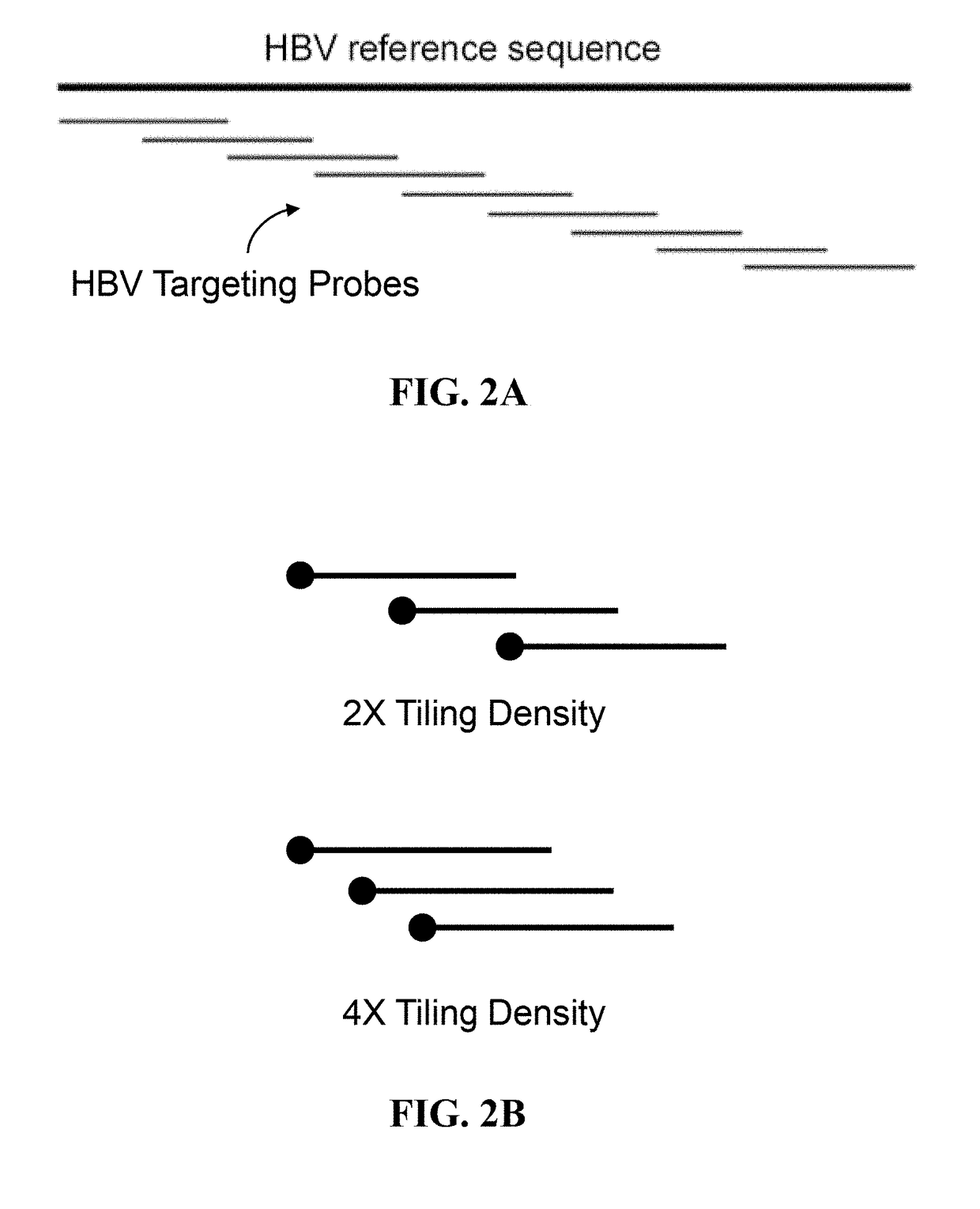
ActiveUS20180223380A1Detect presenceHighly sensitive, efficient, and reliableMicrobiological testing/measurementGene targetsHbv genotype
A probe combination for detecting cancer includes one or more sets of partial hepatitis B virus (HBV) targeting probes. When sequences of each of the sets of partial HBV targeting probes are aligned, an overall sequence of the aligned set of probes matches a reference sequence of a genome of a HBV genotype or a direct repeat (DR) region on the genome. In the aligned set of probes, each of the probes overlap with one or two adjacent probes by a portion of a length of the probe. The probe combination may further includes one or more sets of hotspot gene targeting probes targeting cancer hotspot genes such as CTNNB1, TERT, and TP53 genes, one or more sets of exogenous gene targeting probes targeting portions of a lambda phage genome, and endogenous gene targeting probes targeting endogenous genes such as GAPDH and GdX genes.
Owner:TCM BIOTECH INT CORP
Gene probe composition and kit for detecting clone evolution of multiple myeloma
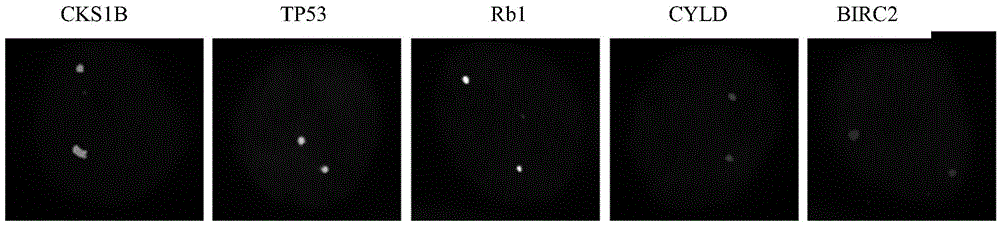
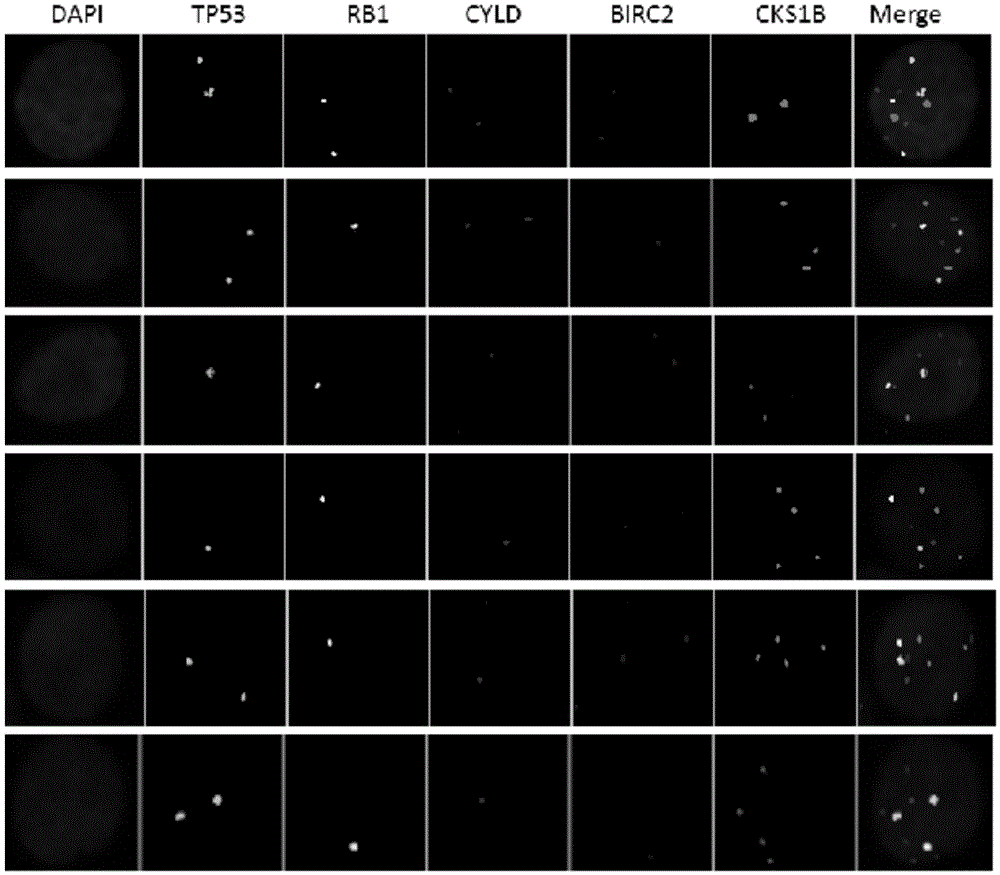
ActiveCN105603087AEasy to detectImprove efficiencyMicrobiological testing/measurementDNA/RNA fragmentationGene probeHeteroplasmy
The invention relates to a gene probe composition and a kit for detecting clone evolution of multiple myeloma. The gene probe composition comprises a TP53 gene probe, an Rb1 gene probe, a CKS1B gene probe, a CYLD gene probe and a BIRC2 gene probe. The kit can be applied to the analysis of clone structures and clone heterogeneity of the multiple myeloma.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Therapeutic, diagnostic, and prognostic methods for cancer
InactiveUS20170306418A1Slow changeIncrease changeOrganic active ingredientsMicrobiological testing/measurementTP53 GenesOncology
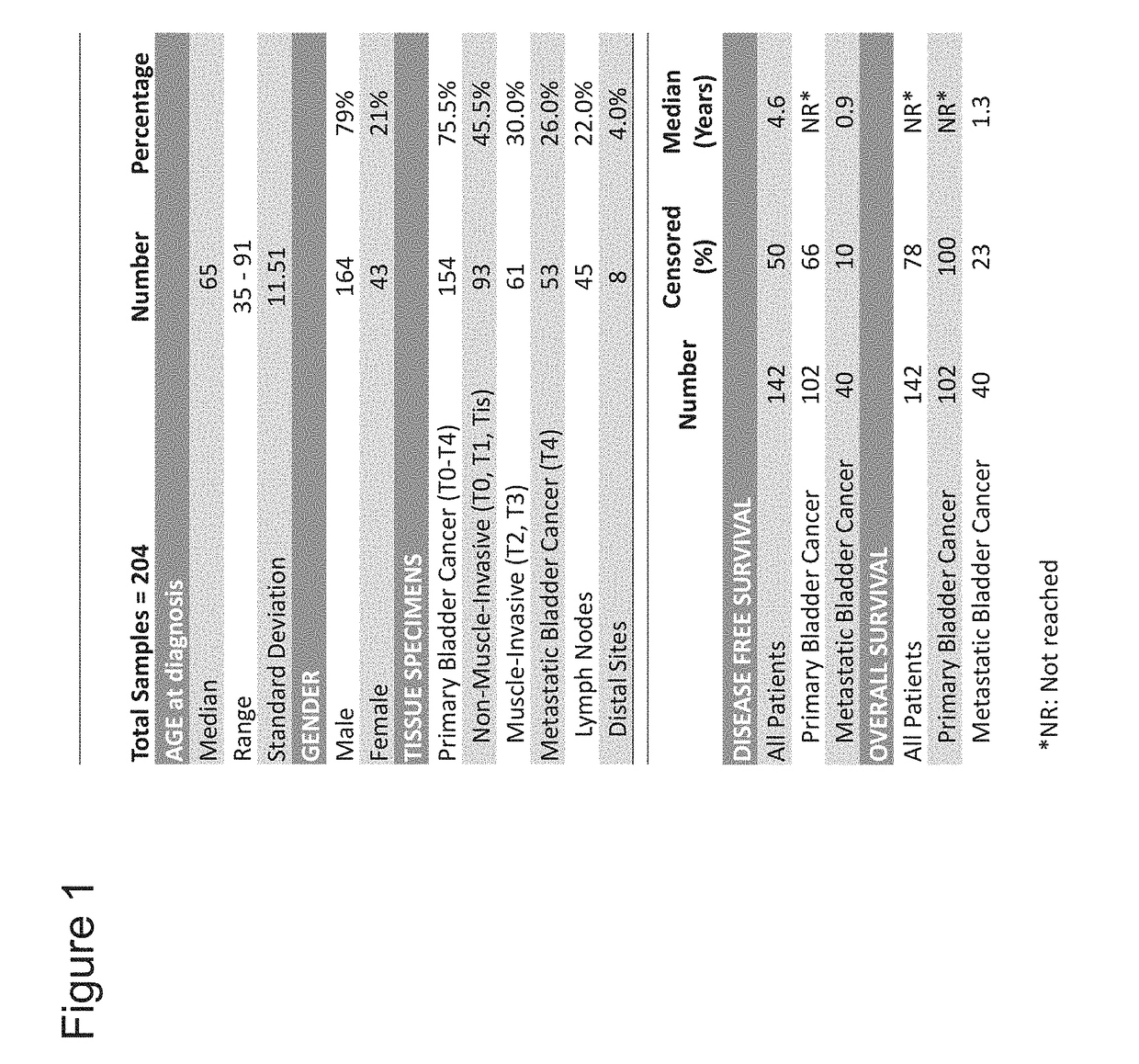
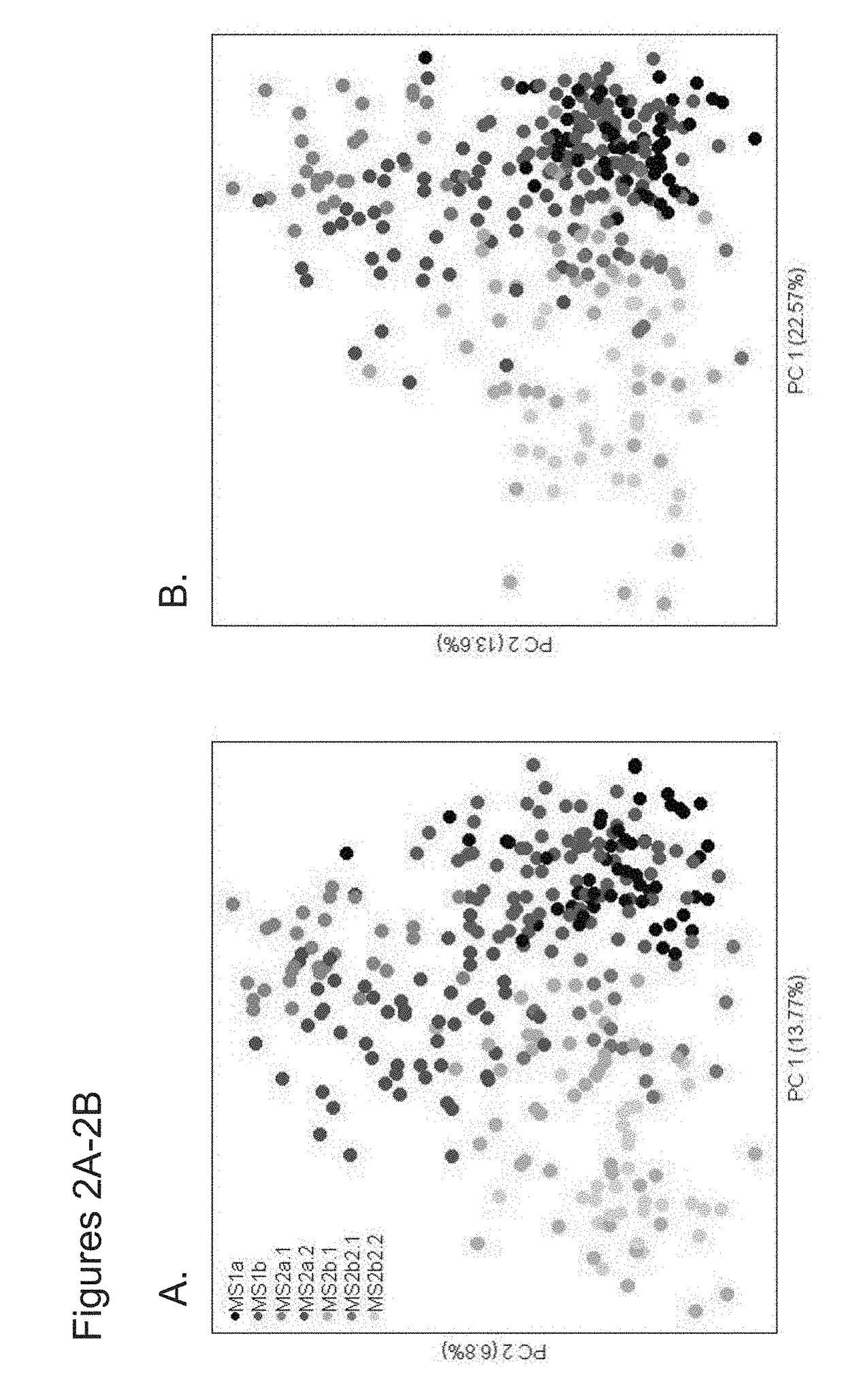
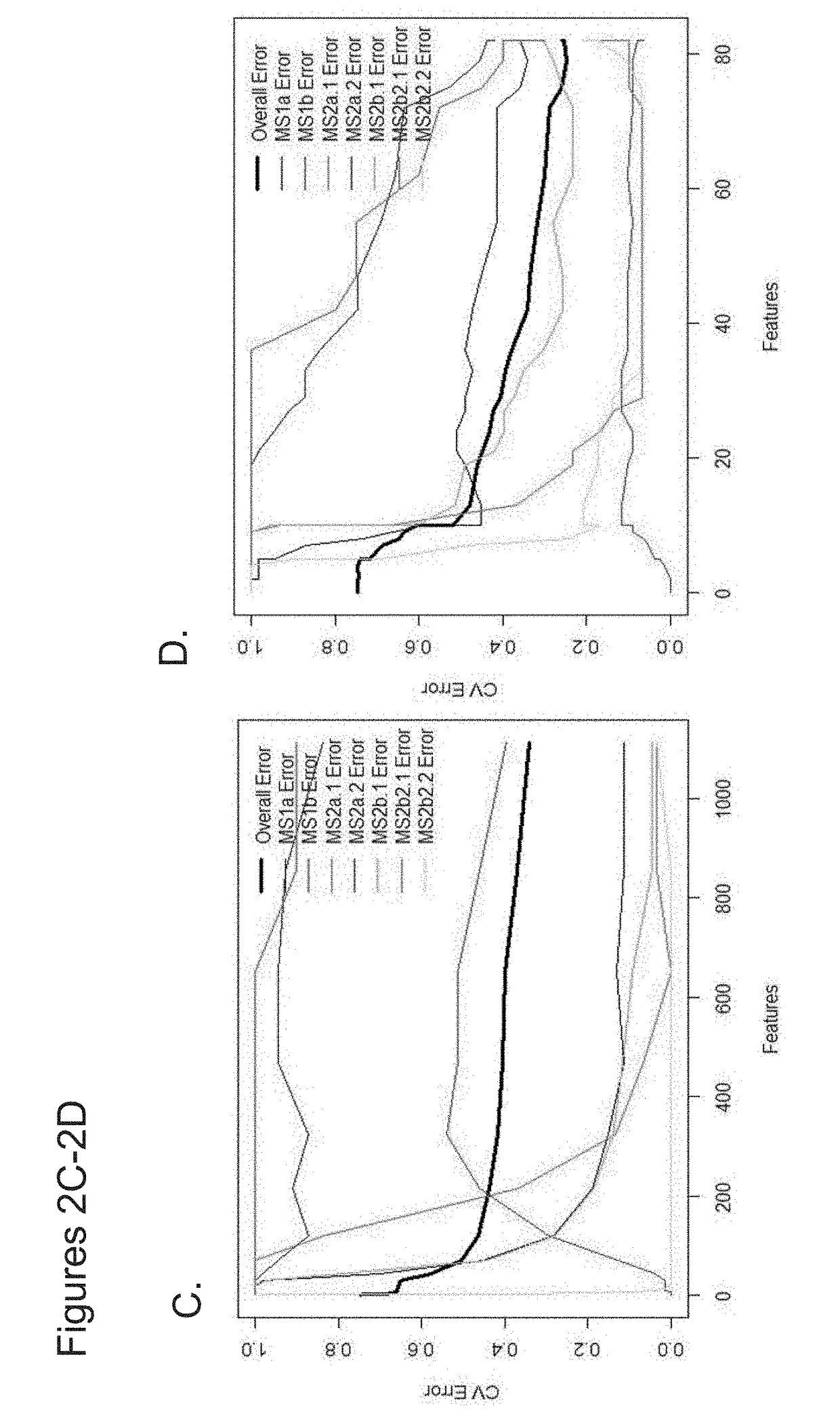
The invention provides methods and compositions to detect expression of one or more biornarkers, including FGFR3, TP53, and / or EGFR, for treating, diagnosing, and providing a prognosis for cancer, e.g., bladder cancer. The invention also provides kits and articles of manufacture for use in the methods.
Owner:GENENTECH INC
Kit for combined detection of acute myeloid leukemia
The invention provides a kit for combined detection of acute myeloid leukemia. The kit comprises a special linker for DNA library construction, a specific composite primer for detecting gene rearrangement and gene whole exon mutation, and a composite primer for library amplification. The special linker is 8 dimers formed by a linker primer 1 and eight different i5-terminal primers respectively; the types of the gene rearrangement and the gene whole exon mutation are CBFB rearrangement, RUNX1 rearrangement, MLL rearrangement, RARA rearrangement, MECOM rearrangement and TP53 gene whole exon mutation; and the composite primer for library amplification has different i7 terminals. The kit provided by the invention can be used for detecting a plurality of common molecular genetic variations of acute myeloid leukemia at one time, and is simple to operate, short in time and high in detection efficiency.
Owner:苏州科贝生物技术有限公司
Combination of nelfinavir, metformin and rosuvastatin for treating cancer caused by aberrations in pten/tp53
The present disclosure relates to a method of treating cancer using a composition comprising Nelfinavir, Metformin, Rosuvastatin, optionally along with a pharmaceutically acceptable excipient. The said composition is used for the treatment of cancer caused due to aberration in PTEN gene, optionally along with aberration in TP53 gene and related genes selected from group comprising PI3K, CDKN2A, MDM2 and MDM4.
Owner:PRECISION PHARM INC
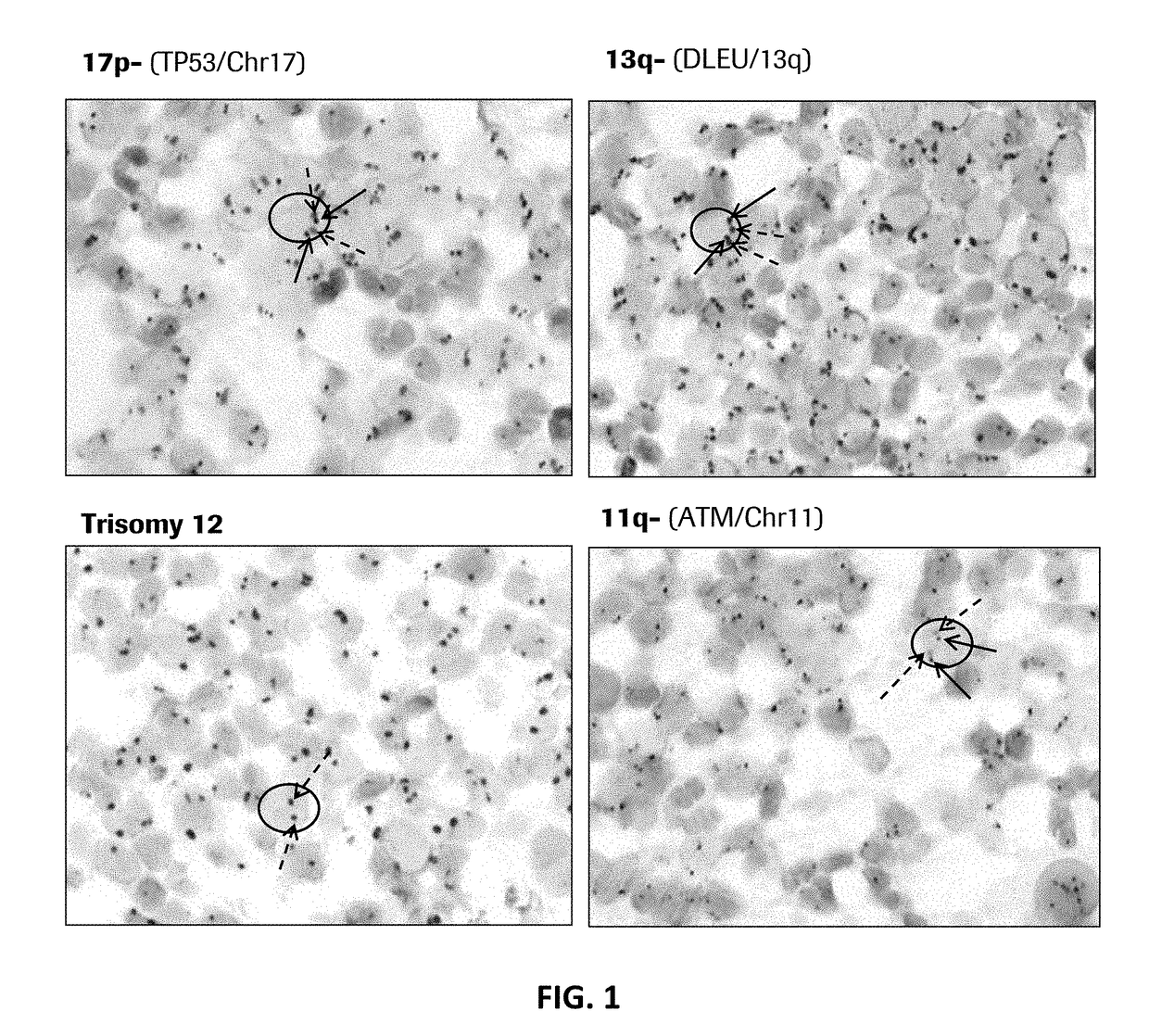
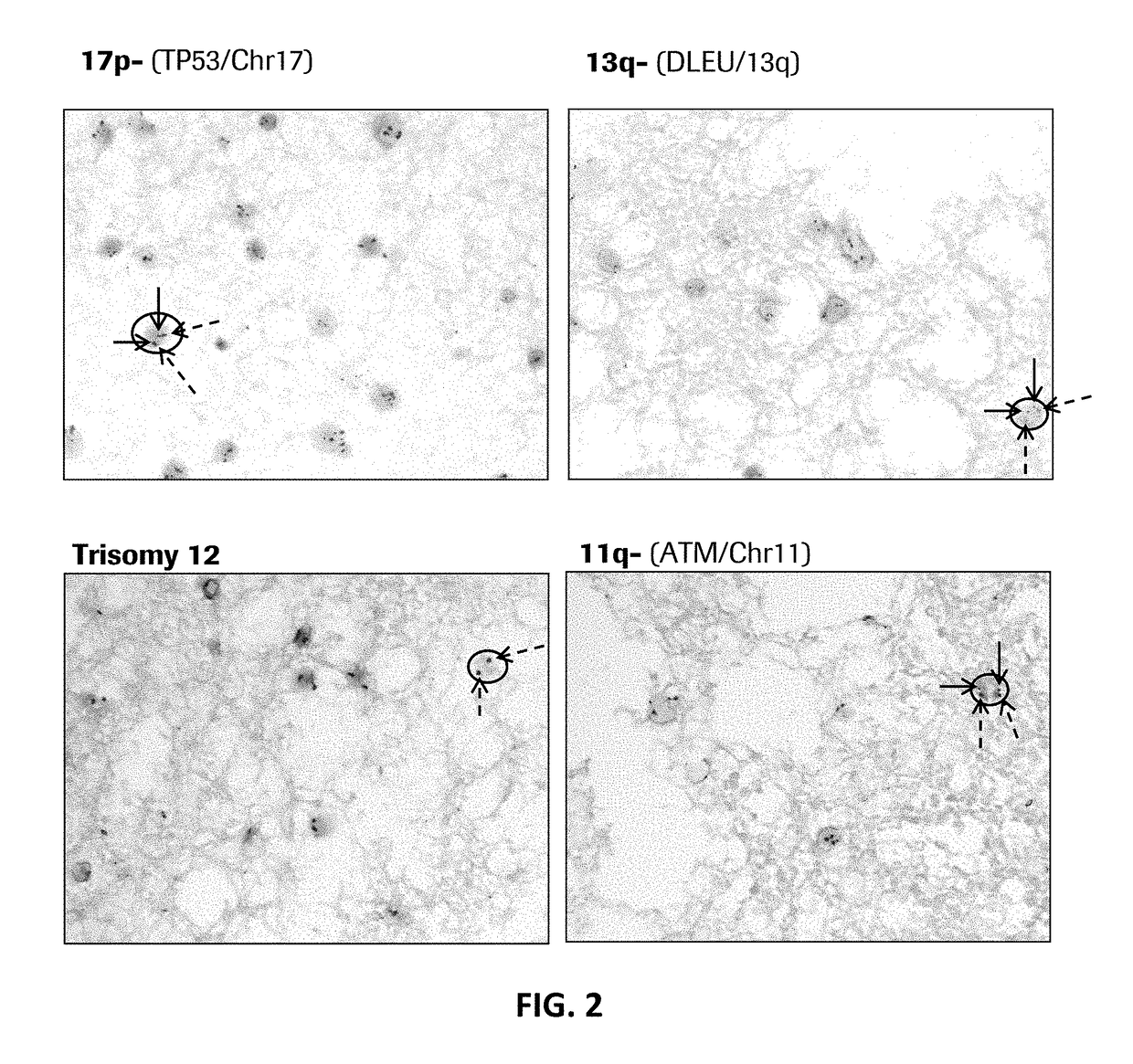
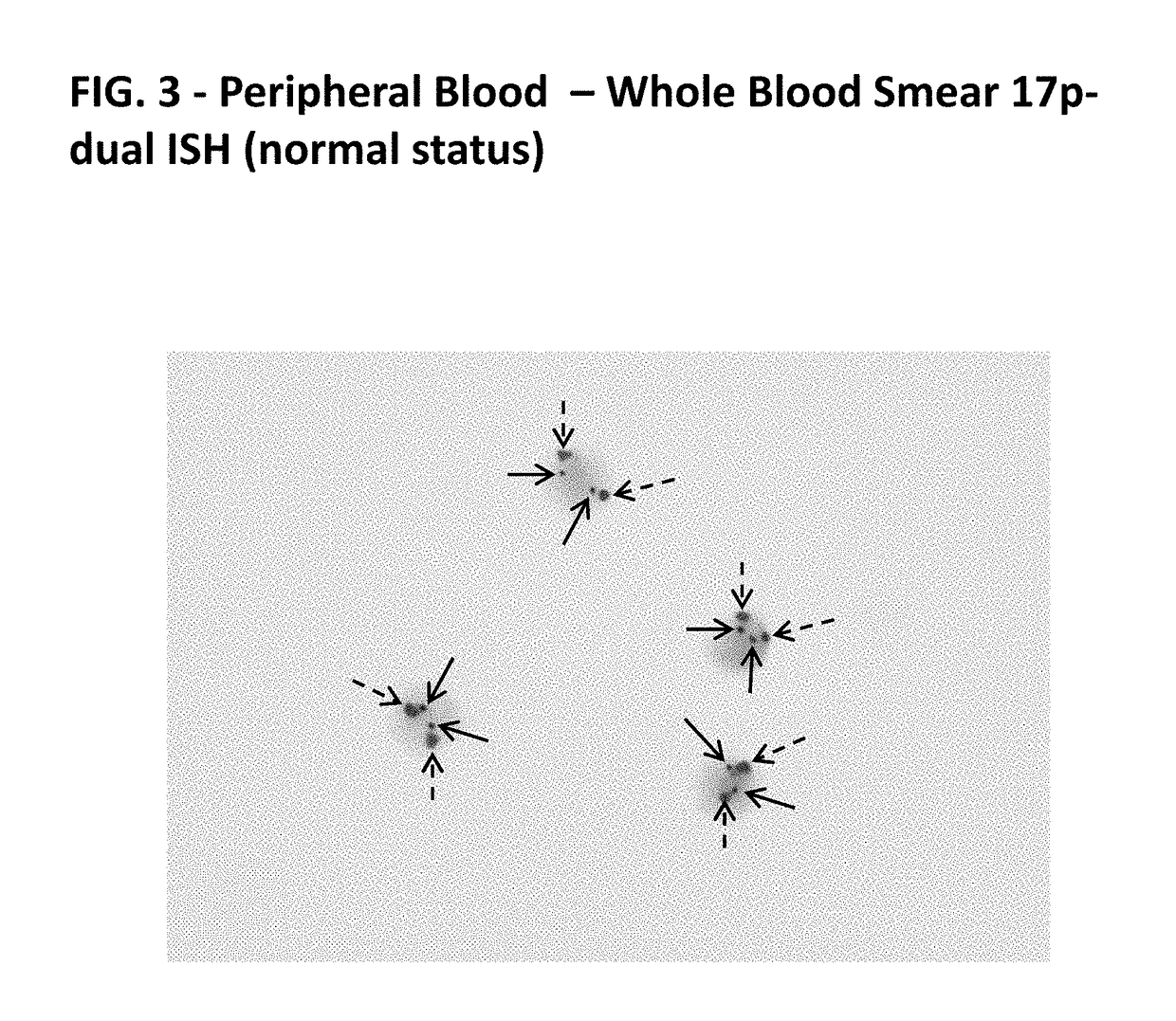
MULTIPLEX TP53/CEN17/B CELL GENE-PROTEIN CO-DETECTION ASSAY AND UNIQUELY SPECIFIC PROBES FOR 19q12, INSR, ATM, DLEU2, TP53, AND 13q12
InactiveUS20170212122A1Quick identificationMicrobiological testing/measurementPreparing sample for investigationSingle sampleCD79A
Disclosed herein are multiplex methods for co-detecting a B cell marker, TP53 nucleic acid, and Chromosome 17 centromere DNA in a single sample. Samples stained for the B cell marker (e.g., CD79a protein), TP53 nucleic acid, and Chromosome 17 centromere DNA may allow for the identification of the subtype of chronic lymphocytic leukemia (CLL) with the 17p deletion. The methods feature staining the B cell marker (e.g., CD79a) a first distinct color, TP53 in a second distinct color, and chromosome 17 centromere DNA in a third distinct color. Further disclosed are nucleic acid probes specific for 19q12, INSR, ATM, DLEU2, TP53, and 13q12.
Owner:VENTANA MEDICAL SYST INC
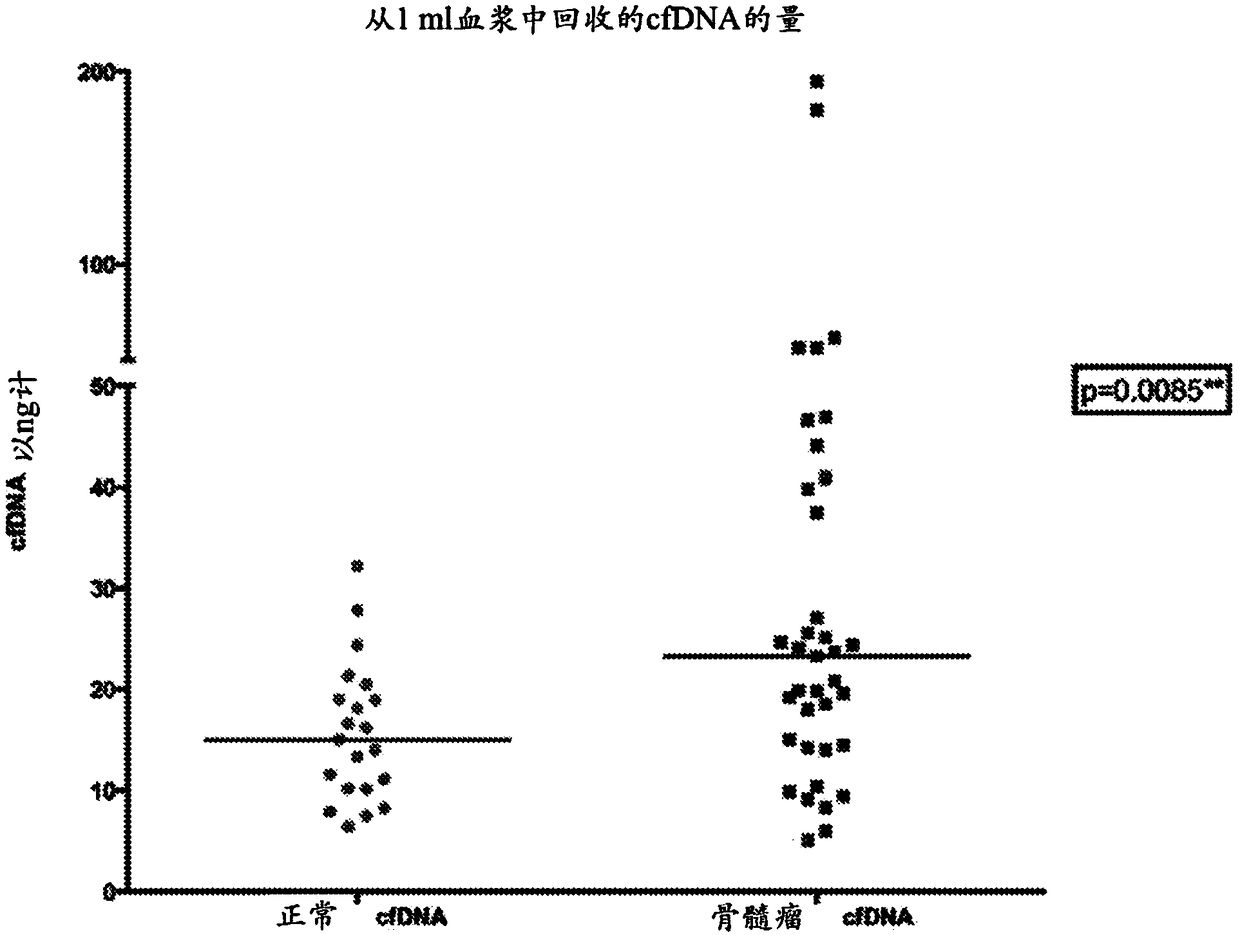
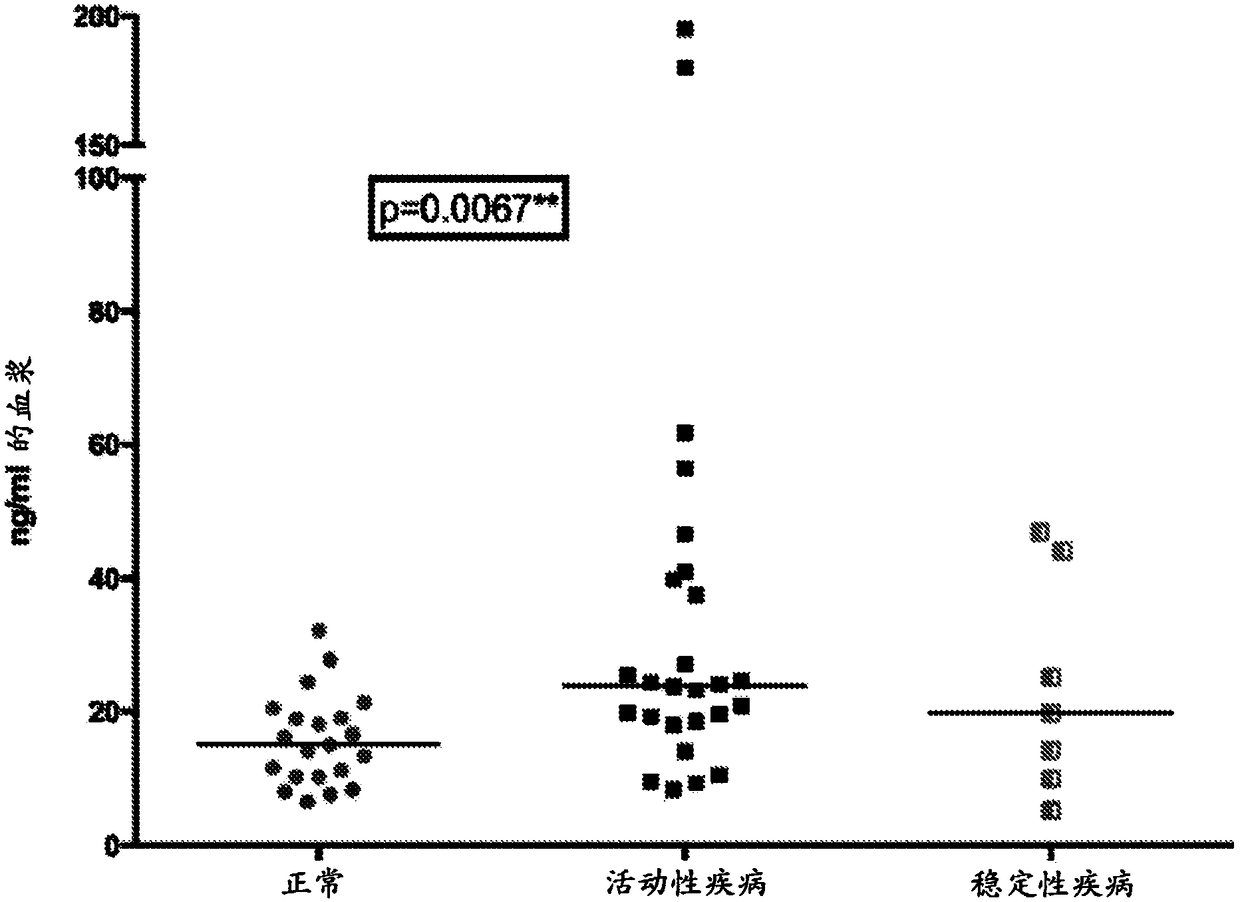
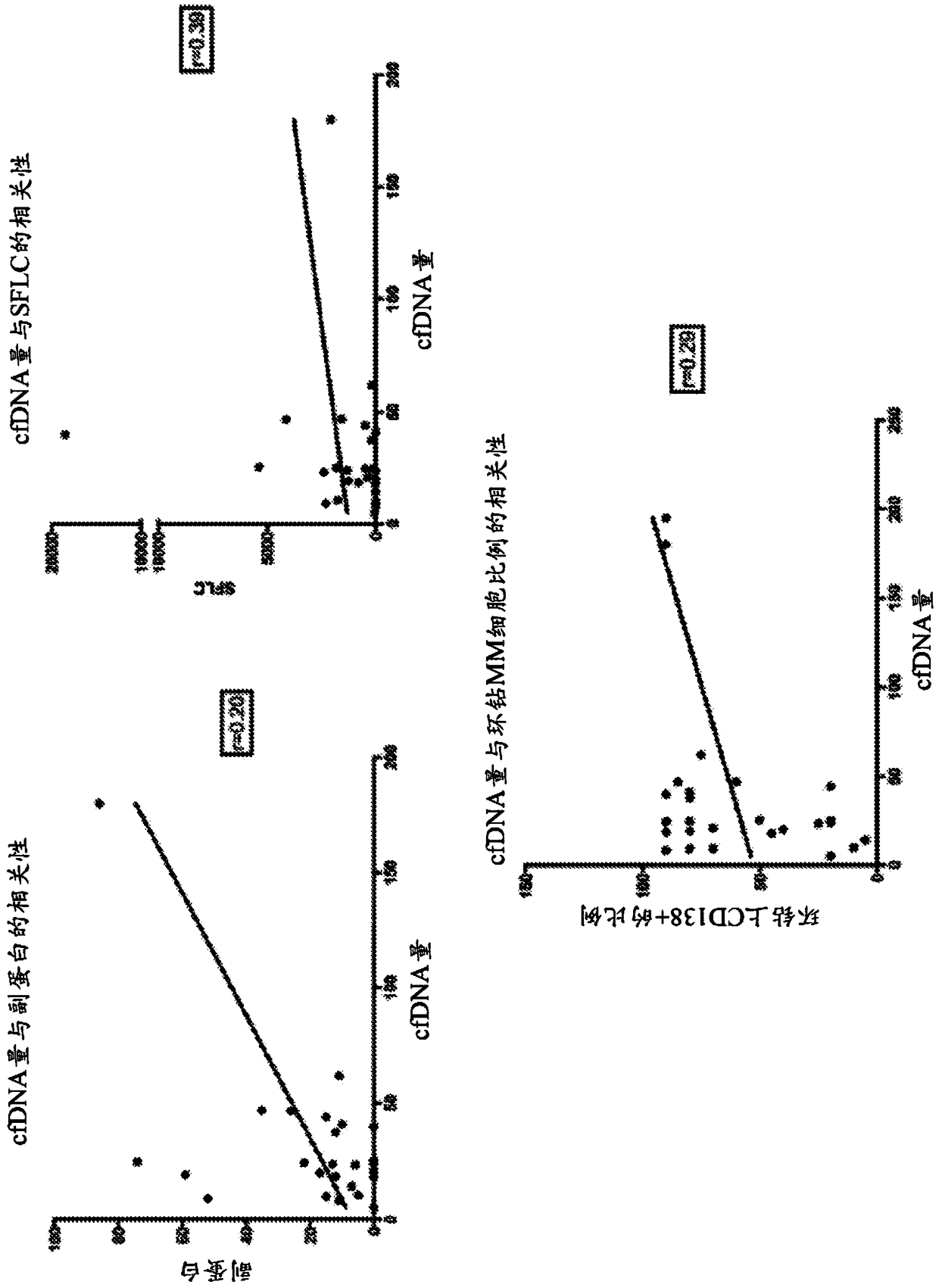
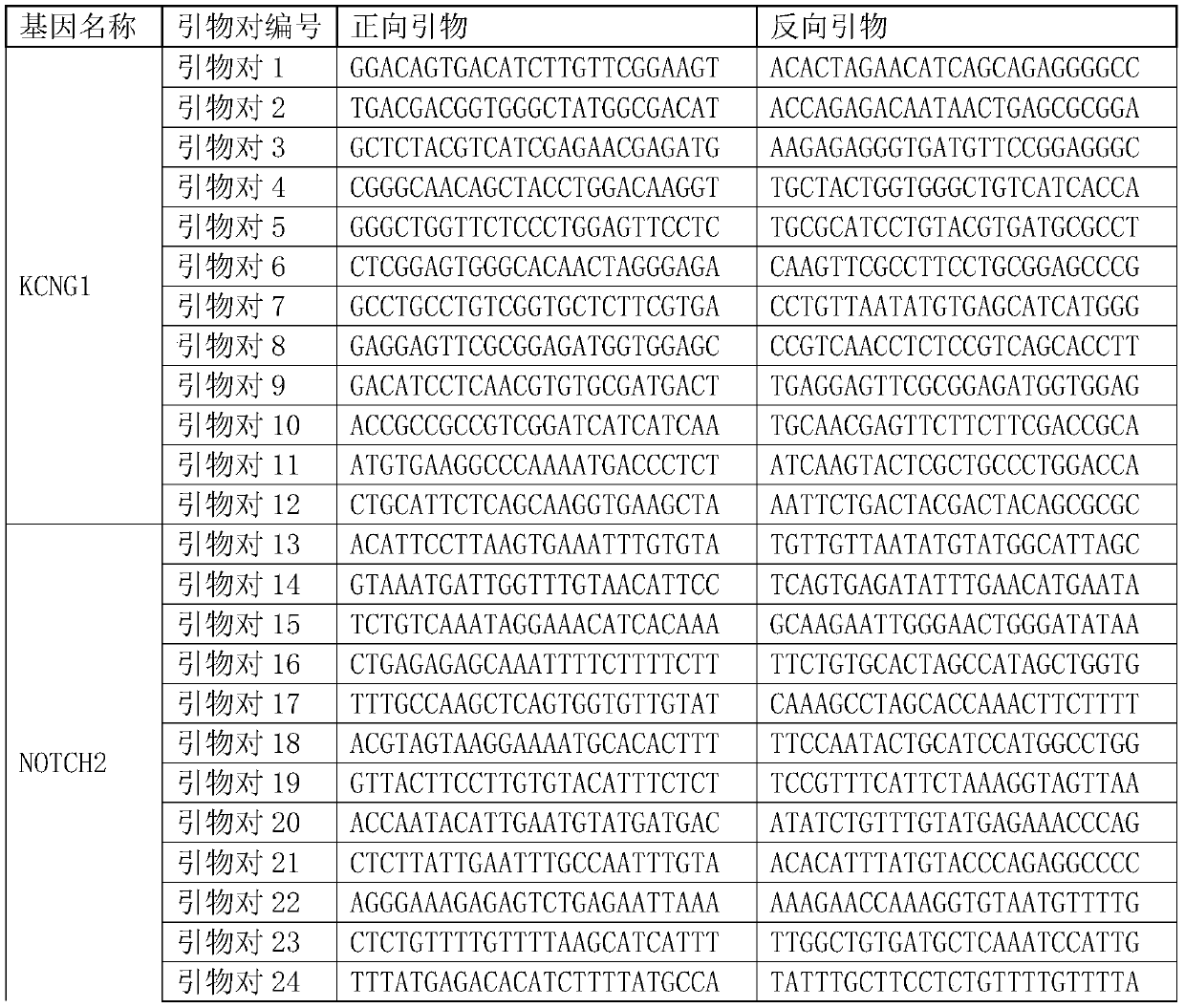
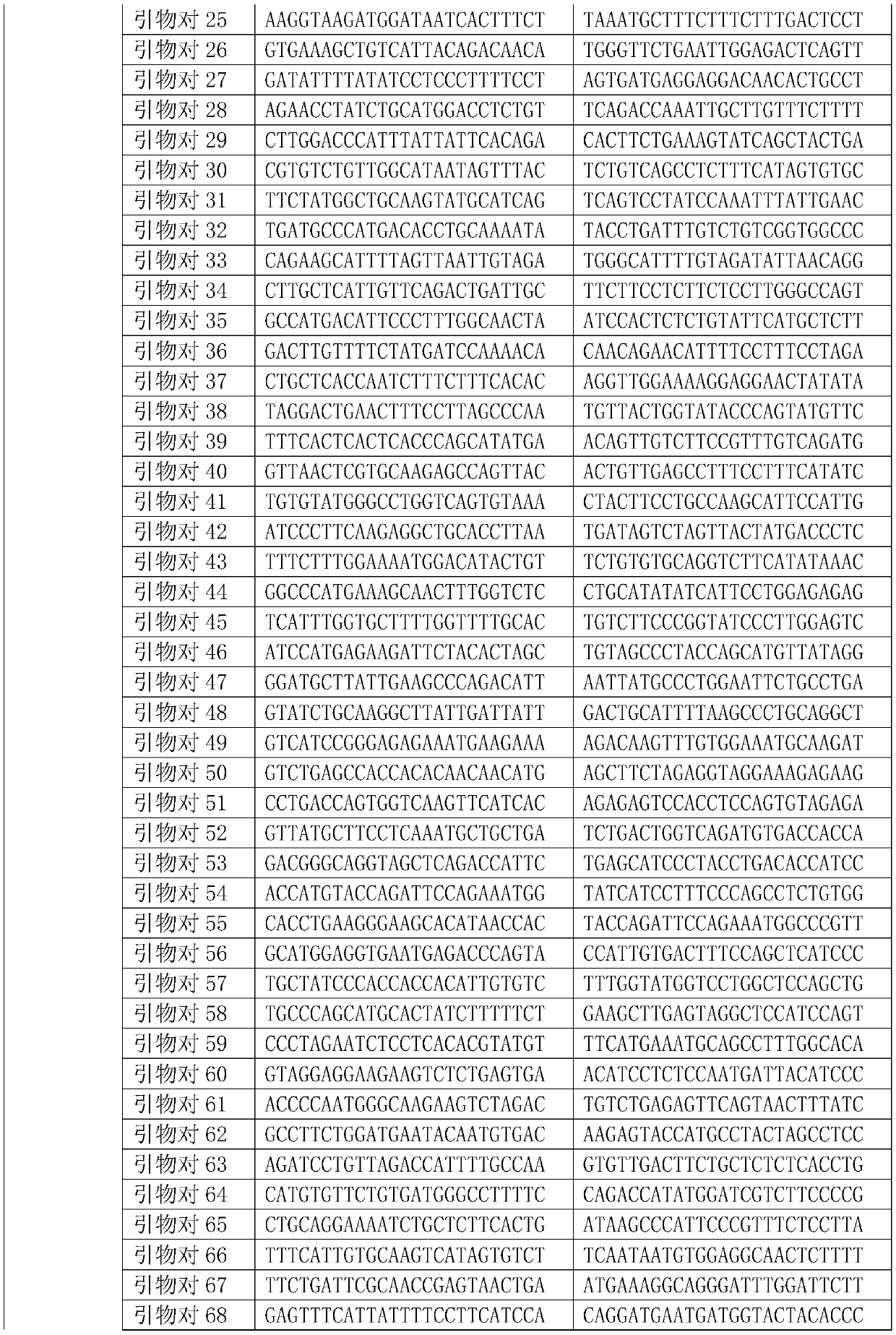
Monitoring treatment or progression of myeloma
The present invention relates to methods and kits for diagnosing myeloma, monitoring disease progression or treatment efficacy in an individual having a myeloma. In one aspect, the invention relates to A method for monitoring the response of an individual to treatment for multiple myeloma, the method comprising providing cell-free nucleic acids derived from a sample of peripheral blood from an individual that has undergone treatment for multiple myeloma; assessing the cell-free nucleic acids for a mutation in any one or more nucleotide sequences from a KRAS, NRAS, BRAF and / or TP53 gene; wherein an absence of, or reduction in the number of, mutations in a nucleotide sequence from a KRAS, NRAS, BRAF and / or TP53 gene indicates a response of the individual to treatment for multiple myeloma.
Owner:阿尔佛雷德医疗集团
Kit for detecting gene mutation of diffuse large B-cell lymphoma
The invention discloses a kit for detecting gene mutation of diffuse large B-cell lymphoma and belongs to the field of gene detection and disease diagnosis. It is found that a certain connection exists between KCNG1 gene and diffuse large B cell lymphoma (DLBCL), and the kit can be used for auxiliary diagnosis of DLBCL by detecting whether G185A mutation exists in KCNG1 gene or not. The genes detected by the kit for detecting DLBCL gene mutation include a KCNG1 gene and MYD88, CD79B, EZH2, B2M, NOTCH2, STAT6 and TP53 genes which are determined to be related to DLBCL generation in the prior art. The invention provides a new material for auxiliary diagnosis of DLBCL; the kit can detect the mutation condition of the gene at one time, and provides a basis for early diagnosis, typing judgment,prognosis stratification and medication guidance of DLBCL.
Owner:珠海铂华生物工程有限公司
Detection reagent kit for detecting MDS (myelodysplastic syndrome)-related gene group
PendingCN106521012AImprove detection efficiencyImprove the detection rateMicrobiological testing/measurementDNA/RNA fragmentationExonMultiplex pcrs
The invention discloses a groups of sequencing reagent kits for screening MDS (myelodysplastic syndrome)-related gene mutation and subsequence medical interpretation databases. The MDS-related gene group includes 16 genes of U2AF1, TP53 and the like. The sequencing reagent kits comprise multiple PCR (polymerase chain reaction) primers for amplifying all exons of the 16 MDS-related genes. The sequencing reagent kits and the medical interpretation databases have the advantages that the reagent kits for screening the MDS gene mutation are high in detection efficiency and detection rate and wide in mutant gene coverage; given medical interpretation reports are accurate and authoritative, and the like.
Owner:天津见康华美医学诊断技术有限公司
Method for constructing lung cancer multi-gene mutation library
The invention discloses a method for constructing a lung cancer multi-gene mutation library. The method can realize mutation of 1435 somatic cells of human genes EGFR, K-ras, B-raf, N-ras, PIK3CA, c-MET, TP53, c-Kit, PDGFRA, ALK, ROS1, RET, ERBB2, NTRK1 and NTRK3. The method can individually manage multiple target sequences and fast finish library construction. The whole library construction process is finished in 2-3h and the manual operation time is only 45min. The method can be used for Illumina and Ion torrent platforms. Through combination of the method and a high-throughput sequencing platform, the problem that a small amount of paraffin tissue slices and peripheral blood samples of a lung cancer patient need multi-gene and multi-target detection of somatic cells is solved and a costis low.
Owner:CHONGQING CANCER INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com