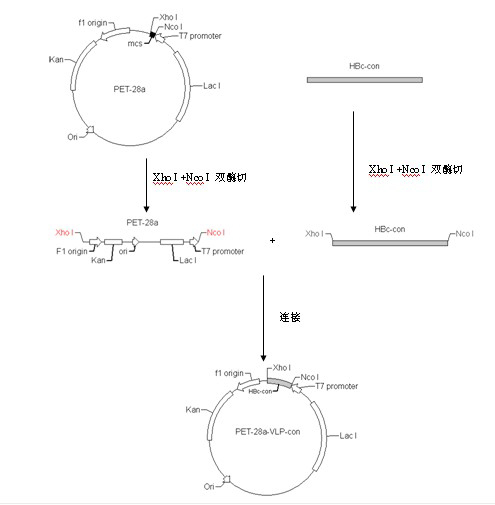
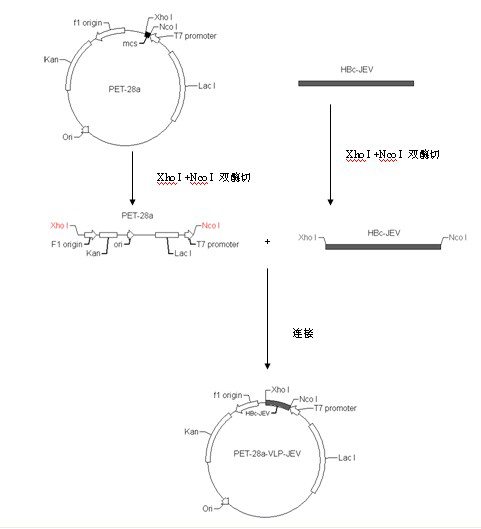
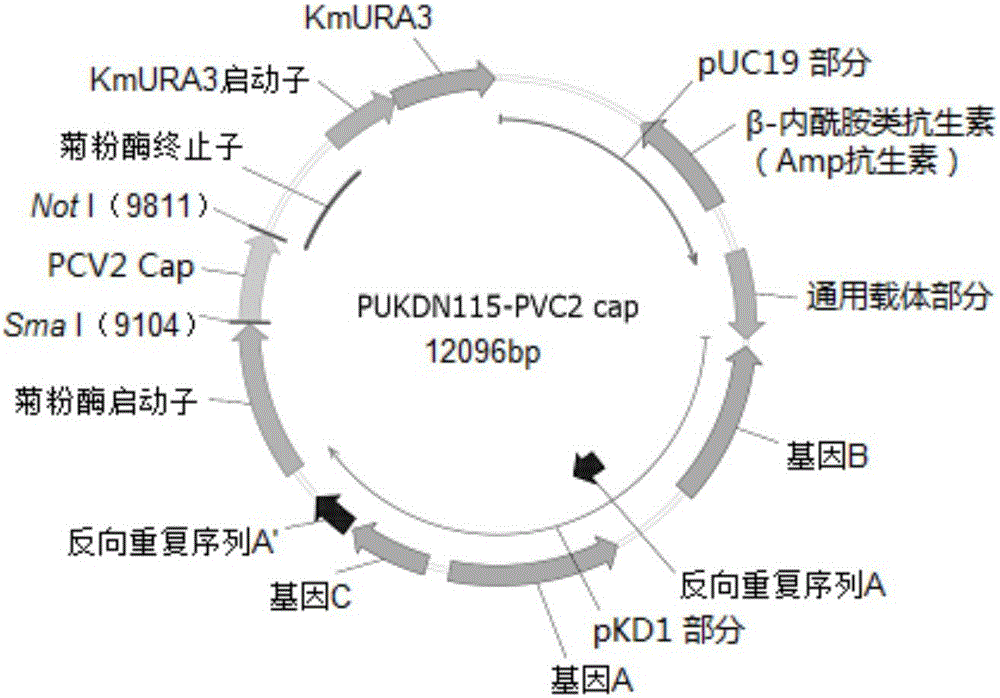
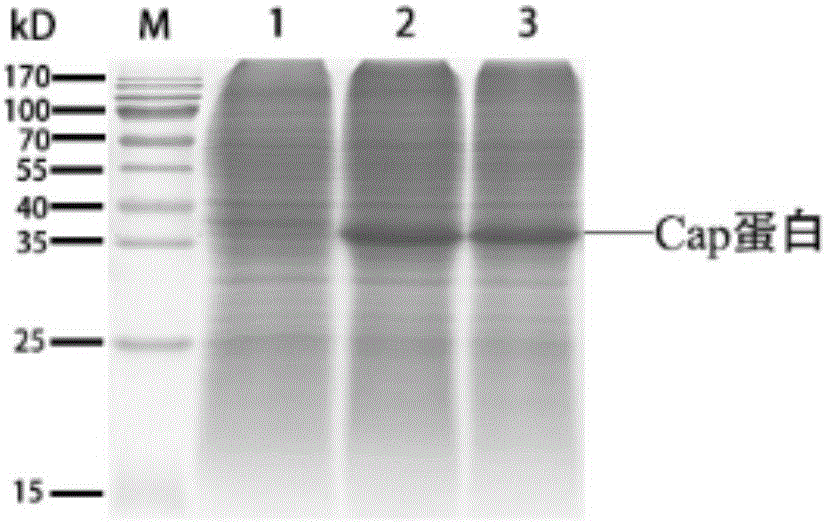
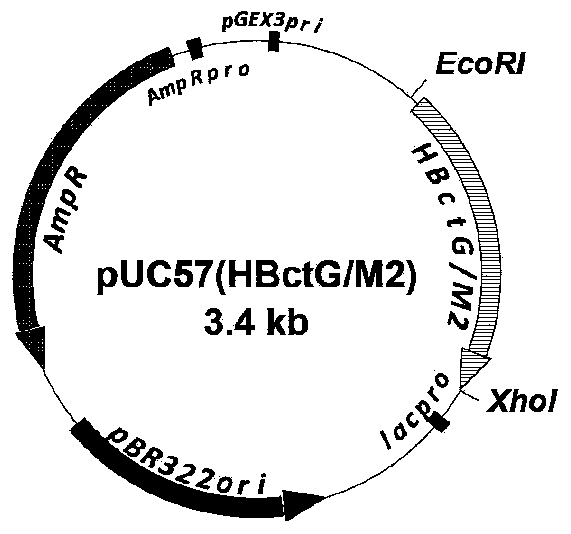
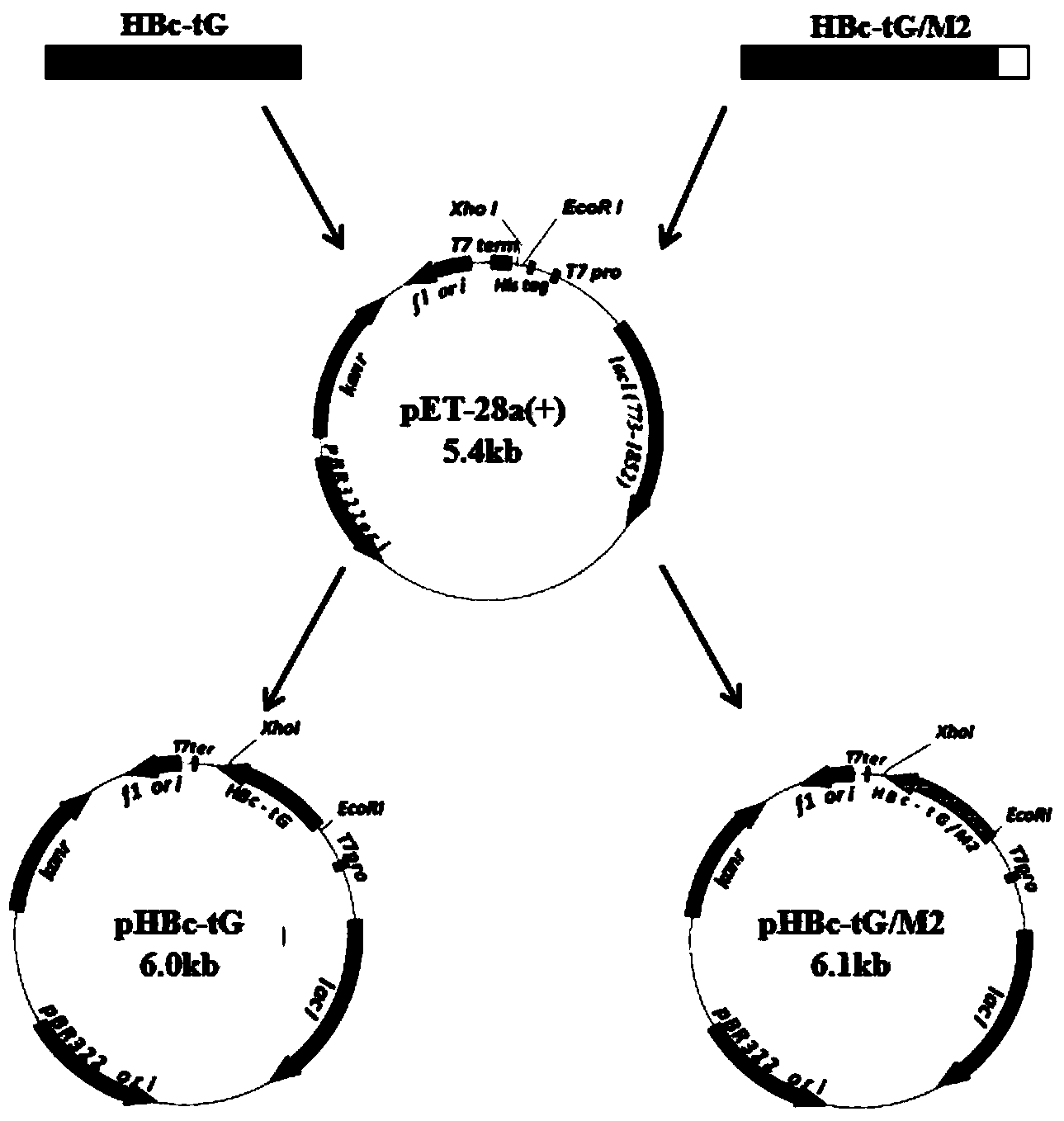
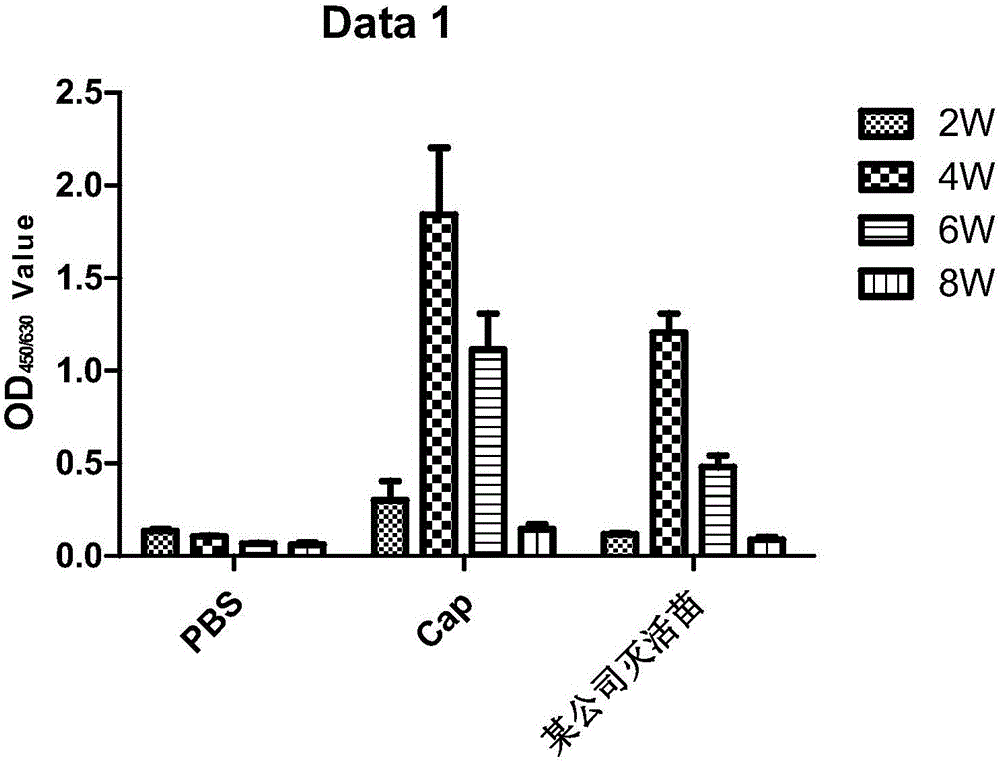
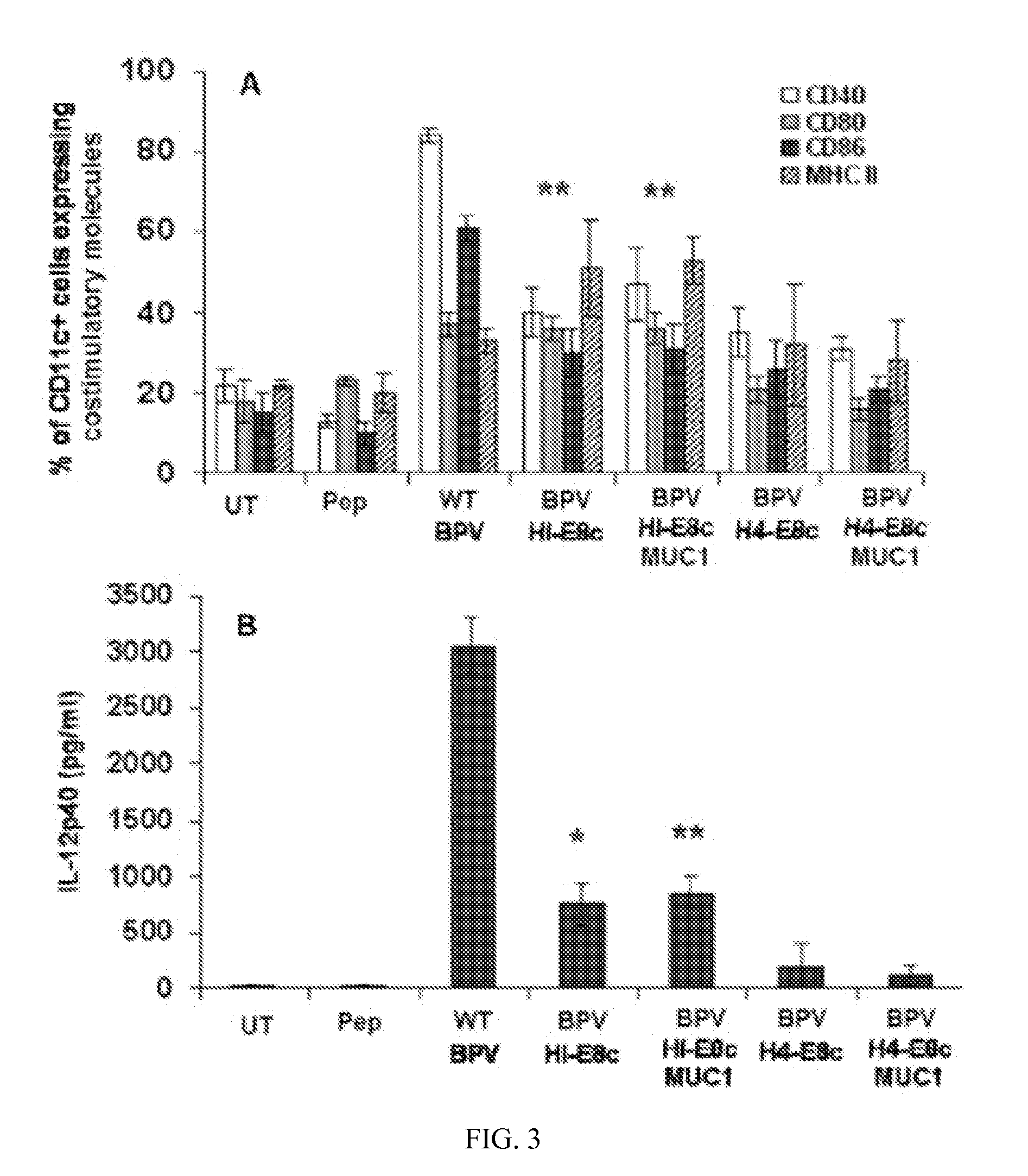
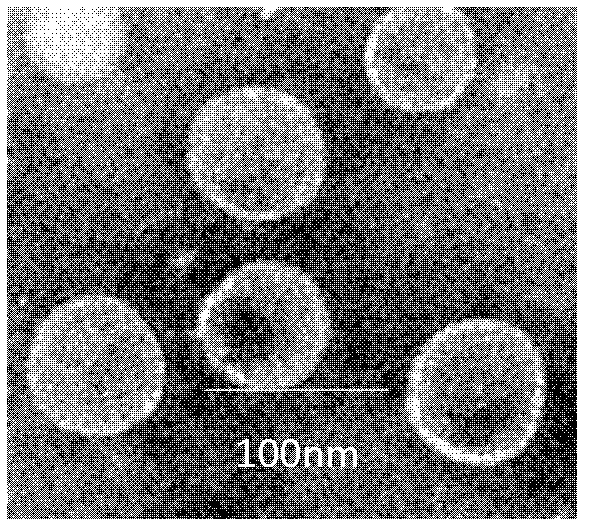
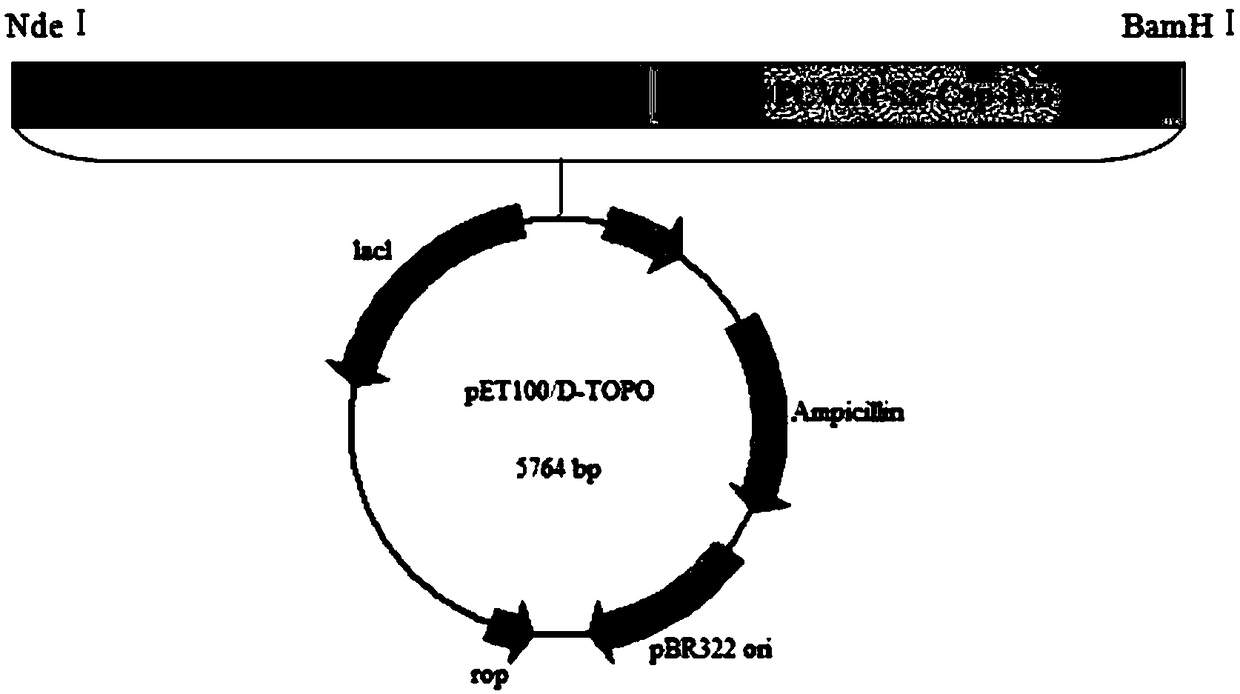
Patents
Literature
58 results about "Virus-Like Particle Vaccines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A virus-like particle vaccine can prime the body’s immune response and prevent the severe respiratory disease that results when patients given an early form of a vaccine for respiratory syncytial virus (RSV) are exposed to RSV, according to a study.
Virus-Like paramyxovirus particles and vaccines
InactiveUS20100040650A1SsRNA viruses negative-senseSsRNA viruses positive-senseStructural proteinIn vivo
The present invention is directed to alphavirus virus-like particles produced by synthesizing in cell, including in vivo, structural proteins in the absence of other alphavirus proteins. In particular, these virus-like particules vaccines induce cellular and humoral immune responses that can block or inhibit alphavirus infections. Also disclosed are methods of vaccinating subjects with virus-like particles and vectors encoding the same.
Owner:VANDERBILT UNIV
Porcine circovirus, porcine parvovirus and porcine reproductive and respiratory syndrome virus triple virus-like particle vaccine and its preparation method
The purpose of the invention is to disclose a porcine circovirus, porcine parvovirus and porcine reproductive and respiratory syndrome virus triplex virus-like particle vaccine and its preparation method. The triple virus-like particle vaccine (Triple VLP vaccine) of the invention contains VLP which is composed of PCV-2 major structural protein CAP protein, PPV VP2 protein epitope and PRRSV Gp5 protein epitope. It is proved by experiment that the vaccine can stimulate good double cellular and humoral immune response. It is shown by pharmacodynamic test that after immunization of different animal groups, the vaccine of injection, nose drops and water forms prepared by VLP antigen formed by the method with or without adjuvants can safely and effectively prevent the infection of PCV-2, PPV and PRRSV. The invention provides an ideal vaccine for the security of sows, piglets and fattening pigs to effectively prevent mixed infection of PCV-2, PPV and PRRSV.
Owner:CHONGQING UNIV
Coxsackie virus A16 type virus-like particle vaccine
InactiveCN102465144AHigh neutralizing activityAntiviralsViruses/bacteriophagesCoxsackievirus a16Spatial configuration
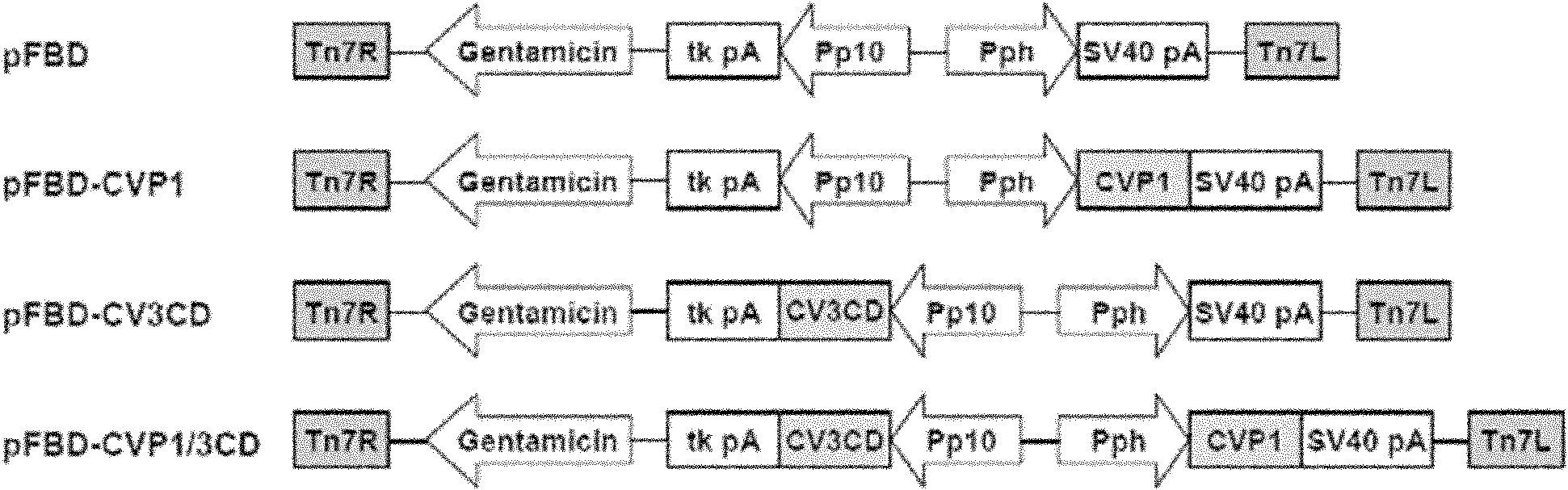
The invention relates to a coxsackie virus A16 type virus-like particle vaccine. The inventor fortuitously finds that proteins which have better spatial configurations and are suitably cut can be obtained by expressing P1 protein of CVA16 and 3CD protein of CVA16 by infecting insect cells with rhabdovirus. The proteins can be automatically assembled into a virus-like particle which has high immunogenicity.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Porcine reproductive and respiratory syndrome virus-like particle vaccine and preparation method thereof
A porcine reproductive and respiratory syndrome virus-like particle vaccine and a preparation method thereof relate to the field of biological medicines and aims at disclosing the porcine reproductive and respiratory syndrome virus-like particle vaccine (PRRS VLP vaccine) and the preparation method the vaccine. The PRRS VLP vaccine contains a VLP comprising three structure proteins of porcine reproductive and respiratory viruses M, N and GP5 and can excite favorable dual cell and humoral immune response. By adding or not adding an adjuvant into the formed VLP protein antigen, the pharmacodynamic test shows the prepared injection type, nasal drop type and water agent type vaccines are immunized with different animal groups so as to safely and effectively prevent the PRRSV infection and provide ideal vaccines for safely and effectively immunizing, preventing and controlling the PRRSV infection on different groups of sows, piglets and fat pigs.
Owner:CHONGQING UNIV
Japanese encephalitis particle vaccine and preparation method and application thereof
InactiveCN102127554AImprove the level ofStrong immune memoryViral antigen ingredientsVirus peptidesHepatitis B virus core AntigenEscherichia coli
The invention belongs to the field of biotechnology, and relates to a vaccine embedded with virus-like particles expressing multi-epitope antigen for Japanese encephalitis, and a preparation method and application thereof. The antigen of the vaccine is virus-like particles formed through spontaneous assembly of hepatitis B virus core antigen embedded with neutralizing antigen epitope expressing Japanese encephalitis virus and cytotoxic lymphocyte (CTL) antigen epitope, and is prepared through soluble expression of escherichia coli and purification. The Japanese encephalitis virus-like particle antigen is properly diluted with physiological saline, or is compatible with immunologic adjuvants to be prepared into the Japanese encephalitis particle vaccine. Animal experiments show that: the vaccine is safe and high-efficiency; mice inoculate with the vaccine generate high-level neutralizing antigen for the Japanese encephalitis virus to protect the mice against the attack of strong Japanese encephalitis virus by 100 percent.
Owner:NANJING AGRICULTURAL UNIVERSITY
Porcine circovirus type II virus-like particle vaccine and preparation method thereof
ActiveCN106399350AReduce clinical symptoms associated with infection with PCV2Reduce associated clinical symptomsFungiViral antigen ingredientsHigh densityGenus Kluyveromyces
The invention provides a kluyveromyces marxianus engineering bacterium obtained from recombinant expression of porcine circovirus type II capsid protein; and porcine circovirus type II virus-like particles are obtained by cloning porcine circovirus type II capsid protein genes into an expression vector and conducting recombinant expression in kluyveromyces marxianus. The invention also provides a preparation method of a porcine circovirus type II virus-like particle vaccine, wherein the preparation method comprises the following steps: preparing the porcine circovirus type II virus-like particles through high-density fermentation of the recombinant kluyveromyces marxianus, and then conducting separation and purification so as to prepare the vaccine. The porcine circovirus type II virus-like particle vaccine provided by the invention, which can generate a high-level serum IgG antibody just by implementing injection immunization once, has the advantages of being good in immunizing effect, high in safety, simple in culture operation, high in yield, low in production cost and the like, and the porcine circovirus type II virus-like particle vaccine can achieve large-scale enlarged production; and the porcine circovirus type II virus-like particle vaccine can be used for relieving and preventing related clinical symptoms caused by the infection of porcine circovirus type II (PCV2).
Owner:FUDAN UNIV
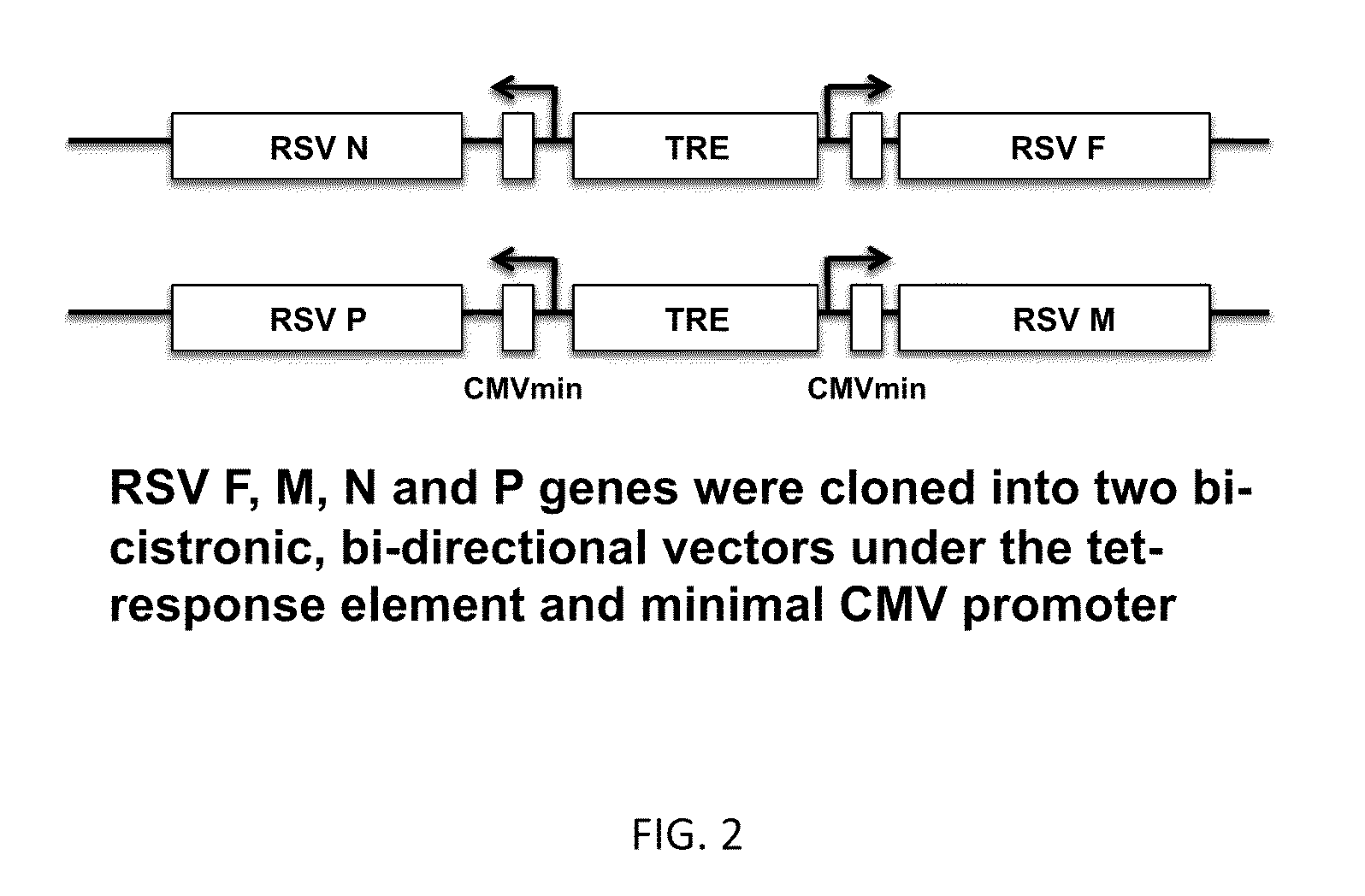
Respiratory syncytial virus virus-like particle vaccine as well as preparation method and application thereof
InactiveCN104293741AStrong immune memoryBacteriaInactivation/attenuationEscherichia coliRespiratory syncytial virus antibody
The invention belongs to the field of biotechnology, and particularly relates to a respiratory syncytial virus (RSV) virus-like particle (VLPs) subunit vaccine as well as a preparation method and application thereof. The component of the vaccine is chimeric antigen protein which is fusion-expressed together with neutral antigenic epitope of an RSVG protein or simultaneously with the antigenic epitope of T cells of an M2 protein by taking hepatitis B virus core protein as a carrier. The high-purity antigen component is prepared by efficiently expressing antigen protein in escherichia coli and then performing in-vitro purification, degeneration and renaturation and self assembling into virus-like particles (VLPs). The RSVG protein contained in the VLPs and the antigenic epitope of the T cells of the M2 protein are simultaneously expressed, so that the capability of the vaccine for introducing specific immunity response and anti-RSV infection immunity protection can be enhanced, the balanced Th1 / Th2 immunity response can be induced and the RSV vaccine is prevented from enhancing the incidence of diseases. Animals are immunized and inoculated with VLPs vaccines to induce organisms to generate high-level RSV neutral antigens, enhanced Th1 cell factor level and effective protection on RSV attack infection.
Owner:WUHAN UNIV
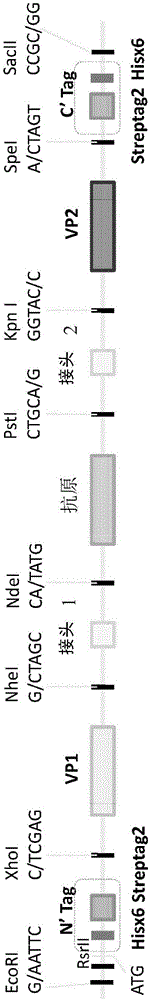
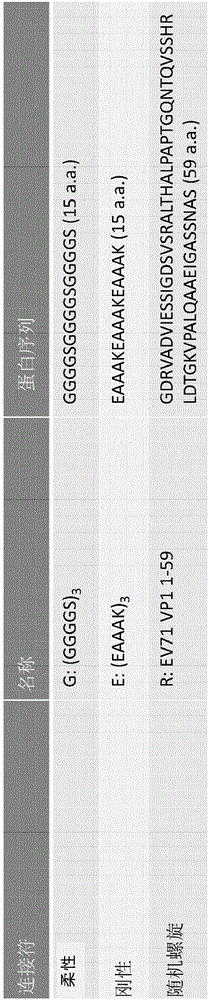
Preparation method of EV71 vaccine and vaccine prepared by preparation method
The invention discloses a preparation method of an EV71 vaccine and the vaccine prepared by the preparation method, relating to the preparation method of taking a Pichia yeast as an expression system for co-expression of P1 and 3CD proteins of the EV71 after codon optimization by utilizing different promoters, and obtaining the viral like particle vaccine with immunogenicity, and also relating to the vaccine prepared by the preparation method.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Porcine circovirus-like particle, and vaccine and preparation method thereof
InactiveCN103436499ASafe infectionEffective infectionViral antigen ingredientsInactivation/attenuationAdjuvantStructural protein
The invention discloses a porcine circovirus-like particle, a vaccine and a preparation method thereof. The virus-like particle is composed of a main structural protein-nucleocapsid protein of 2-type porcine circovirus, can excite cell and humoral immune response, can be used as a virus-like particle vaccine (VLP vaccine) to immune different fauna and can safely and effectively prevent PCV-2 infections after being used. The VLP can be made into injections, nose drops and drinking preparations by adding adjuvants or not. An ideal vaccine is provided for security of different populations of sows, piglets, fattening pigs and for effective immune prevention and control of PCV-2 infections.
Owner:CHONGQING AULEON BIOLOGICALS
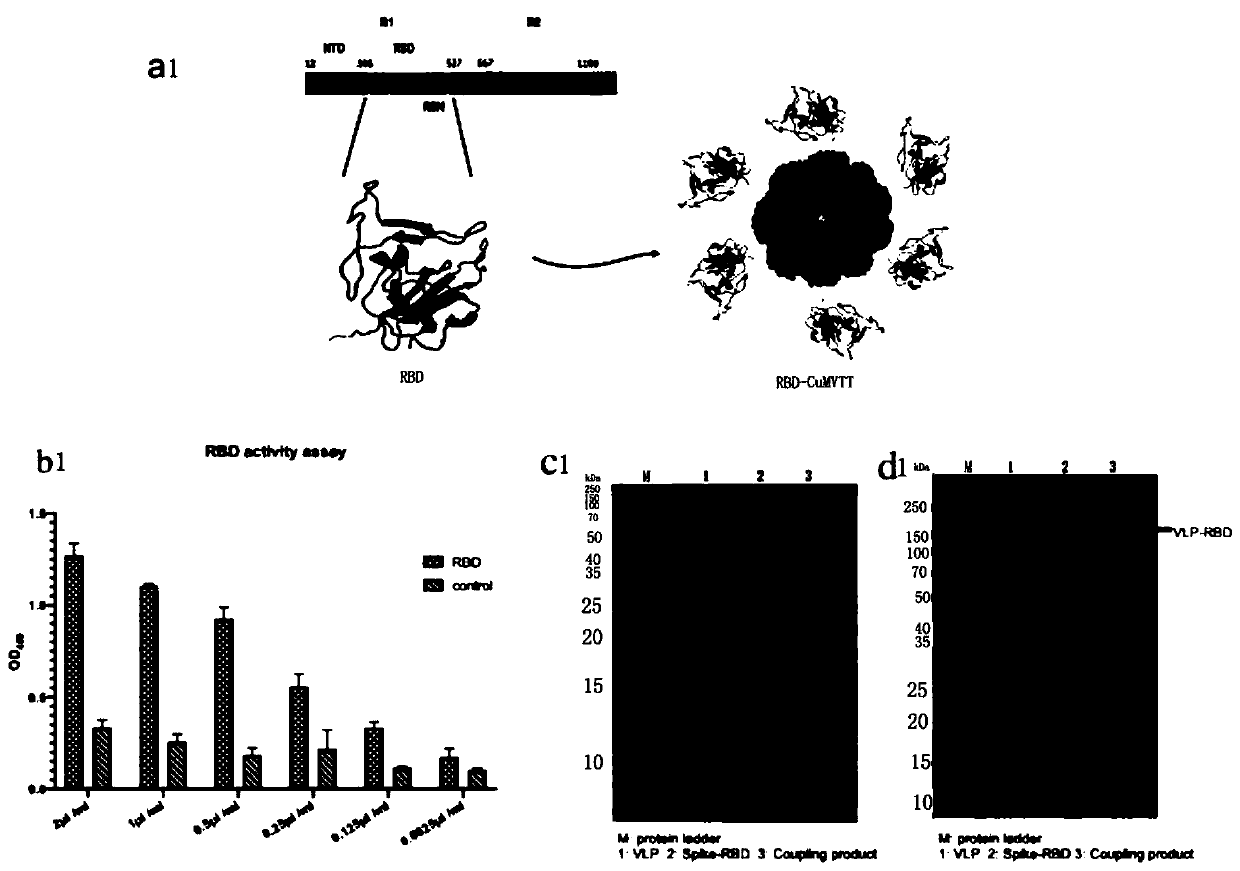
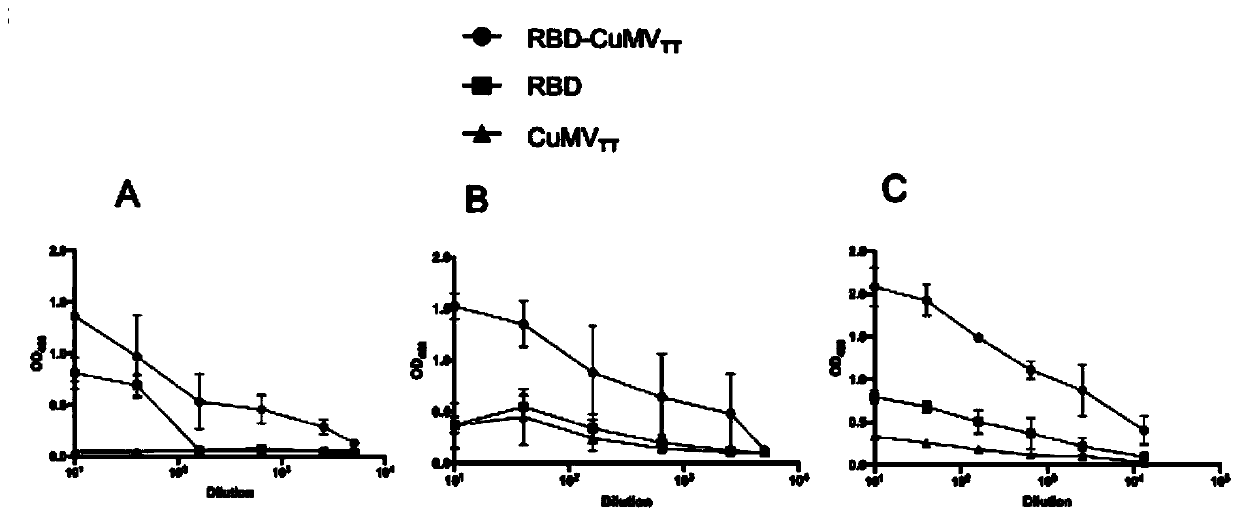
COVID-19-S-RBD virus-like particles and vaccine, and preparation methods of virus-like particles and vaccine
ActiveCN111303255ASuitable for industrial productionEasy to purifySsRNA viruses positive-senseViral antigen ingredientsEscherichia coliImmunity
The invention discloses COVID-19-S-RBD virus-like particles and vaccine, and preparation methods of the virus-like particles and the vaccine. pET28a-CuMVTT recombinant plasmid is constructed after a CuMVTT gene is used to connect pET28a plasmid; pFUSE-COVID-19-S-RBD recombinant plasmid is constructed by a COVID-19-S-RBD gene and pFUSE plasmid; the recombinant plasmid is respectively transferred into the expression strains of escherichia coli and the expression cell lines of 293 F cells; the expression strains of escherichia coli are cultivated, biomass is separated through centrifugation, andthe virus-like particles are obtained; COVID-19-S-RBD protein is obtained by cultivating the expression cell lines of the 293 F cells; and the virus-like particles are coupled to the COVID-19-S-RBD through a chemical coupling reagent SMPH. The virus-like particles and the vaccine can be easily obtained through bacteria culture; yield can be higher than that of chimeric expression; and thus, industrial production and fast immunity can be achieved.
Owner:深圳赫兹生命科学技术有限公司
Virus-like particle vaccines
InactiveUS20160185826A1Easy to foldStimulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseMultivalent VaccineViral structural protein
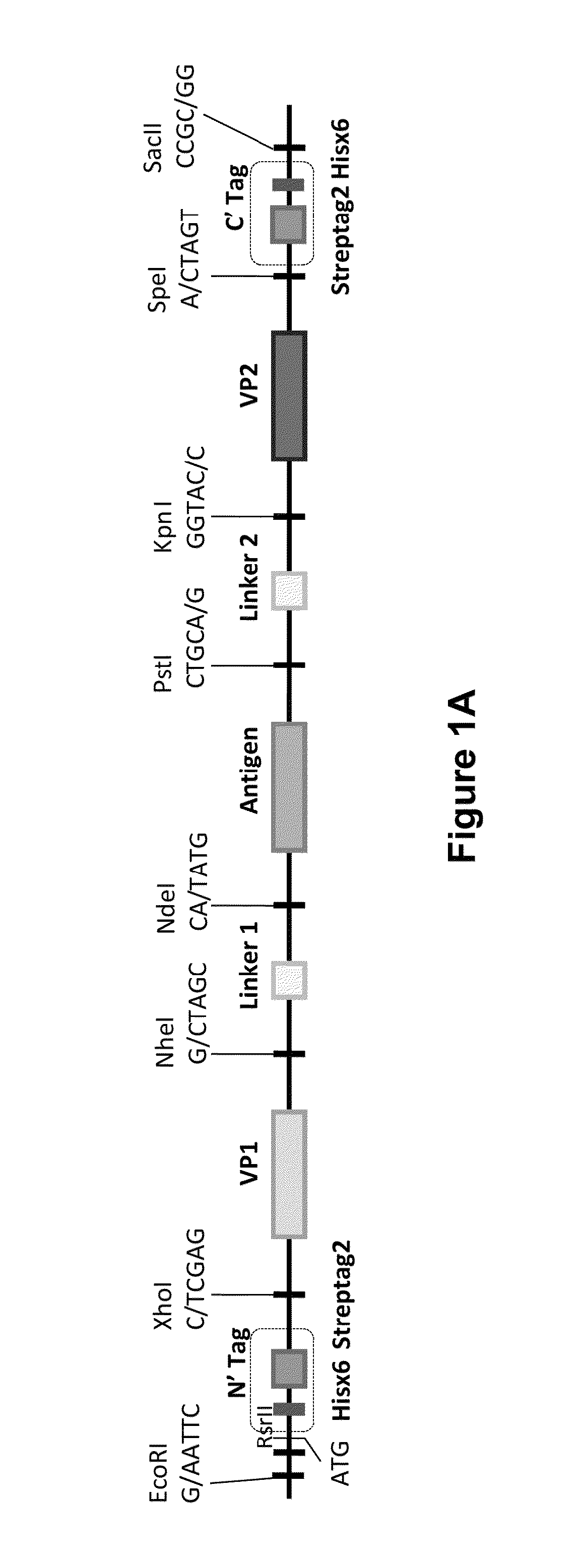
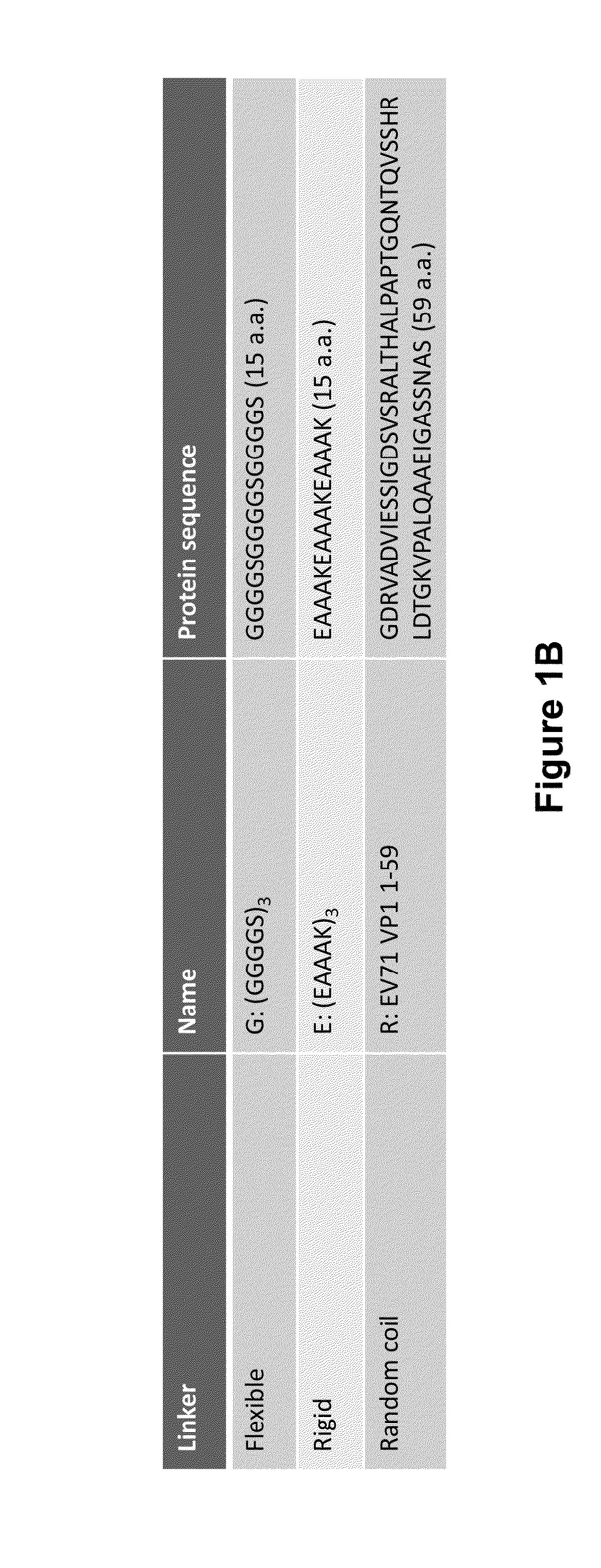
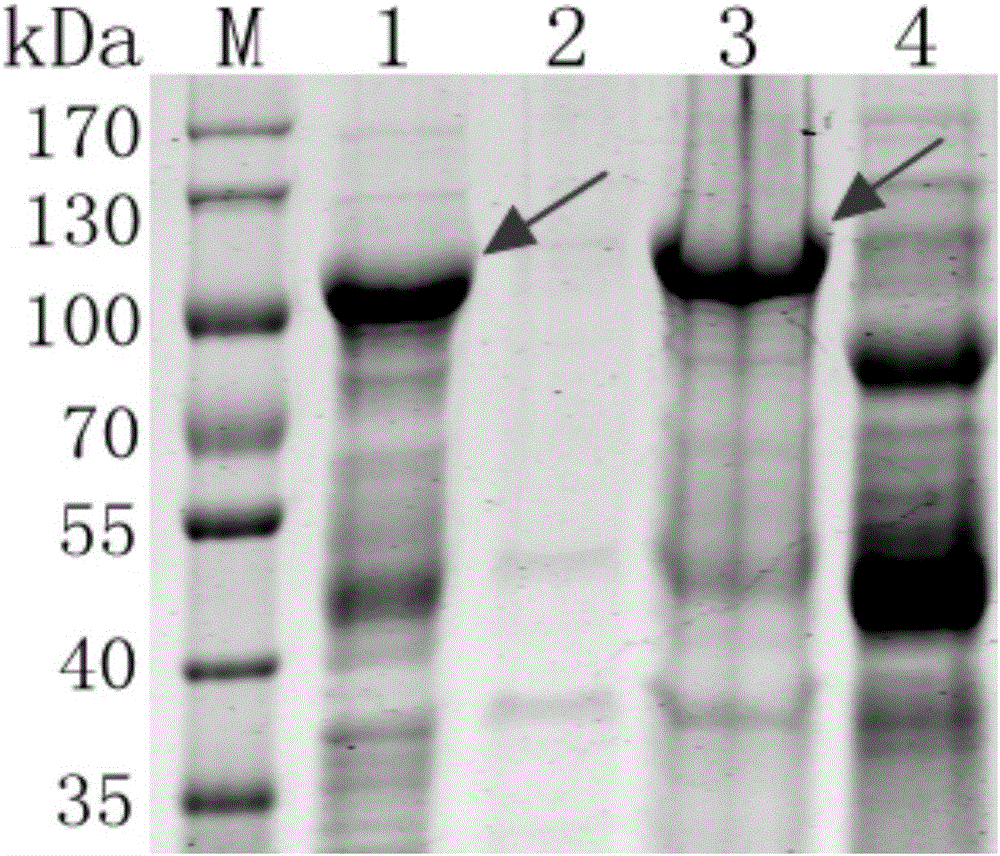
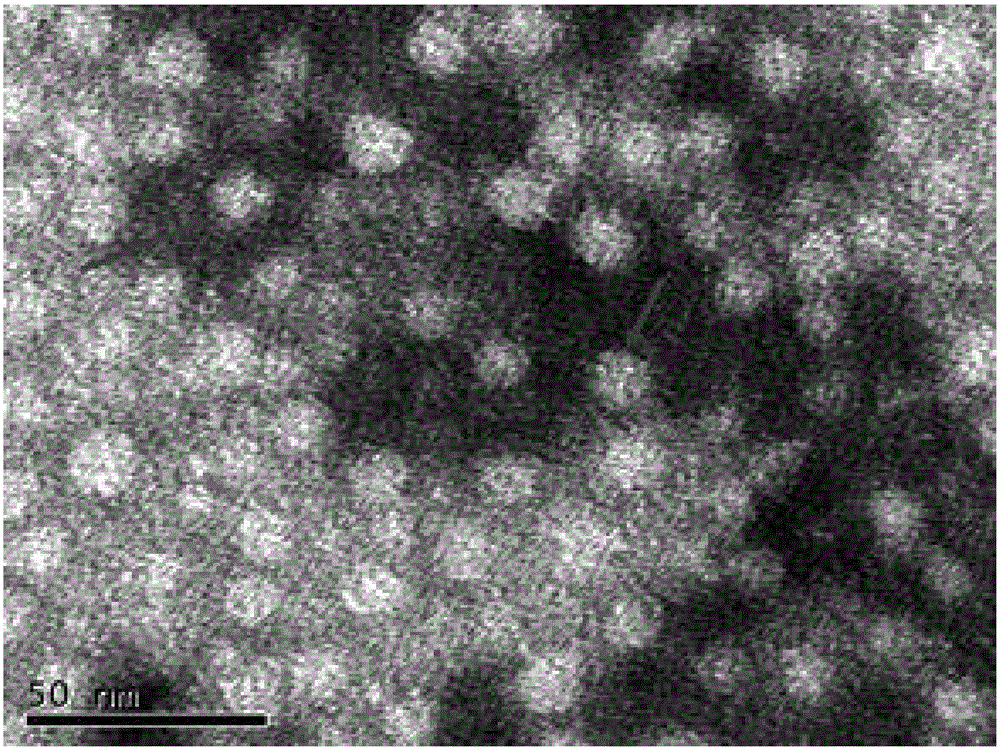
The invention is directed to dimeric fusion proteins and virus-like particles comprising such dimeric fusion proteins. These dimeric fusion proteins comprise an antigen or antigenic fragment carried between two viral structural proteins or fragments thereof, with or without linkers, in a manner that, relative to traditional monomeric platforms, minimizes steric hindrance among the antigen or antigenic fragment and the viral structural proteins or fragments thereof. This novel design provides for multivalent vaccines and enhanced immunogenicity. The invention also relates to nucleic acids encoding such dimeric fusion proteins and host cells comprising such nucleic acids. The invention further relates to pharmaceutical compositions comprising the dimeric fusion proteins and / or virus-like particles of the invention, and methods of prevention or treatment using such compositions.
Owner:MEDIGEN BIOTECH
Virus-like particle vaccine for PCV2 (porcine circovirus 2) as well as preparation method and application of virus-like particle vaccine
InactiveCN105999255AComplete epitopeImproving immunogenicityViral antigen ingredientsAntiviralsEscherichia coliPorcine circovirus
The invention discloses a virus-like particle vaccine for PCV2 (porcine circovirus 2) as well as a preparation method and an application of the virus-like particle vaccine, and belongs to the technical field of bio-pharmaceuticals. The virus-like particle vaccine comprises soluble protein which is obtained through expression of a PCV2 complete capsid protein gene in escherichia coli, wherein the capsid protein gene is screened from capsid protein genes of domestic PCV2 prevalent strains at present and is subjected to codon optimization, therefore, the capsid protein gene can be expressed substantively in the escherichia coli, and the expressed soluble protein can form virus-like particles. The PCV2 subunit vaccine prepared with the method has characteristics of high antigen purity, high immunogenicity and the like; besides, the vaccine preparation process is simple, the production cost is low, and quite good application prospect is realized.
Owner:SOUTH CHINA AGRI UNIV
Polyionic papilloma virus-like particle (VLP) vaccines
The present invention relates to the field of vaccines. In particular, the present invention provides compositions and methods relating to virus-like particle (VLP) vaccines. In one embodiment, a chimeric papillomavirus virus-like particle (VLP) comprises the L1 protein, wherein the HI loop of the L1 protein comprises negatively charged amino acids. In a more specific embodiment, a chimeric bovine papillomavirus VLP comprises the L1 protein, wherein the amino acid sequence EEEEEEEEC is inserted into the HI loop of the L1 protein.
Owner:VISCIDI RAPHAEL
Vaccine for chimeric virus-like particles and preparation method thereof
ActiveCN102153656AStrong immune-inducing activityInactivation/attenuationAntiviralsEpitopeChimerin Proteins
The invention discloses chimeric protein. The chimeric protein is obtained by inserting an epitope E7 of a human papillomavirus type 16 at a ring position of human papillomavirus type 16 (HPV 16) protein L1, namely between the 140th amino acid and the 141st amino acid, wherein the epitope E7 of the human papillomavirus type 16 has genes of protein (a) with the following codes: the protein is formed by an amino acid sequence shown by an MLDLQPETT epitope (12-20E7 for short), a RAHYNIVTF epitope(49-57E7 for short), an LLMGTLGIV epitope (82-90E7 for short) or a TLGIVCPI epitope (86-93E7 for short). The invention also discloses virus-like particles formed by aggregating the chimeric protein, a mixture formed by the virus-like particles and application of the virus-like particles and the mixture of the virus-like particles to the preparation of the vaccine for HPV 16 L1-virus-like particles.
Owner:SOUTH CHINA UNITED VACCINE INST
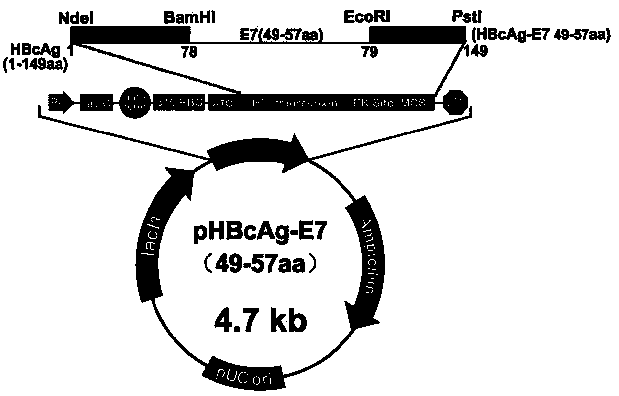
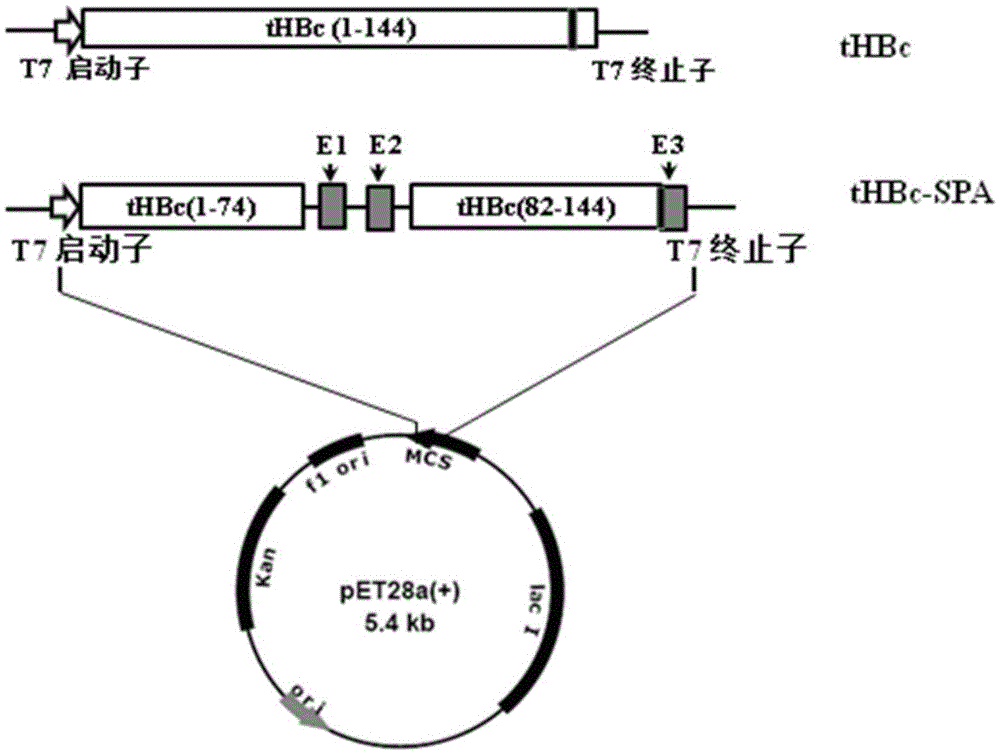
Application of HBcAg (hepatitis B core antigen) virus-like particle serving as cancer therapeutic vaccine carrier
InactiveCN105497886AImprove the level ofViral antigen ingredientsAntineoplastic agentsHepatitis B virus core AntigenEscherichia coli
The invention relates to the field of molecular biology and immunology, in particular to an application of an HBcAg (hepatitis B core antigen) virus-like particle serving as a cervical cancer therapeutic vaccine carrier. A preparation method comprises steps as follows: an HPV16 E749-57CTLs epitope peptide fragment is selected, a DNA (deoxyribose nucleic acid ) fragment of the HPV16 E749-57CTLs epitope peptide fragment is inserted between 78 and 79 amino acids of the HBcAg through genetic recombination, an obtained recombinant plasmid pHBcAg-E749-57 is converted into Escherichia coli DH5alpha, and an HBcAg virus-like particle vaccine presenting E749-57 is obtained after induction expression and purification. After a tumor-bearing mouse is immunized with the virus-like particle vaccine, the body of the mouse can be induced to generate a higher HPV16E7 specific cellular immunologic response, and growth of tumors is remarkably inhibited.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Respiratory syncytial virus-like particle vaccine and preparation method thereof
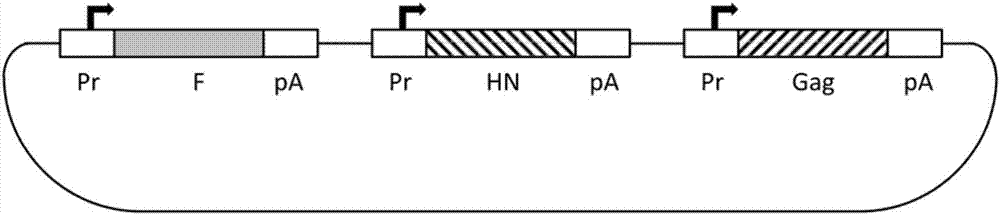
The invention discloses a virus-like particle vaccine (NDV / RSV VLP vaccine) of which the centre is from NVD (Newcastle disease virus) and the surface protein is from RSV (respiratory syncytial virus), and also discloses a preparation method of the virus-like particle vaccine. The RSV VLP vaccine provided by the invention comprises a VLP composed of four structural proteins of respiratory syncytial virus M, F, NP and G; and the NDV / RSV VLP vaccine comprises four structural proteins of Newcastle disease virus M, F, NP and NH and VLP, wherein the VLP is formed by two surface proteins which are a NDV F / RSV F fusion protein composed of Newcastle disease virus F protein and respiratory syncytial virus F protein and an NDV HN / RSV G fusion protein composed of Newcastle disease virus HN protein and respiratory syncytial virus G protein. The test of pesticide effectiveness shows that RSV infection can be safely and effectively prevented after various dosage forms prepared by the VLP protein antigen formed with the method by adding or not adding adjuvants are used for processing immunization for different animals or the crowd, and the vaccine for processing immune prevention for the RSV inflection is supplied for the crowd with different ages.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
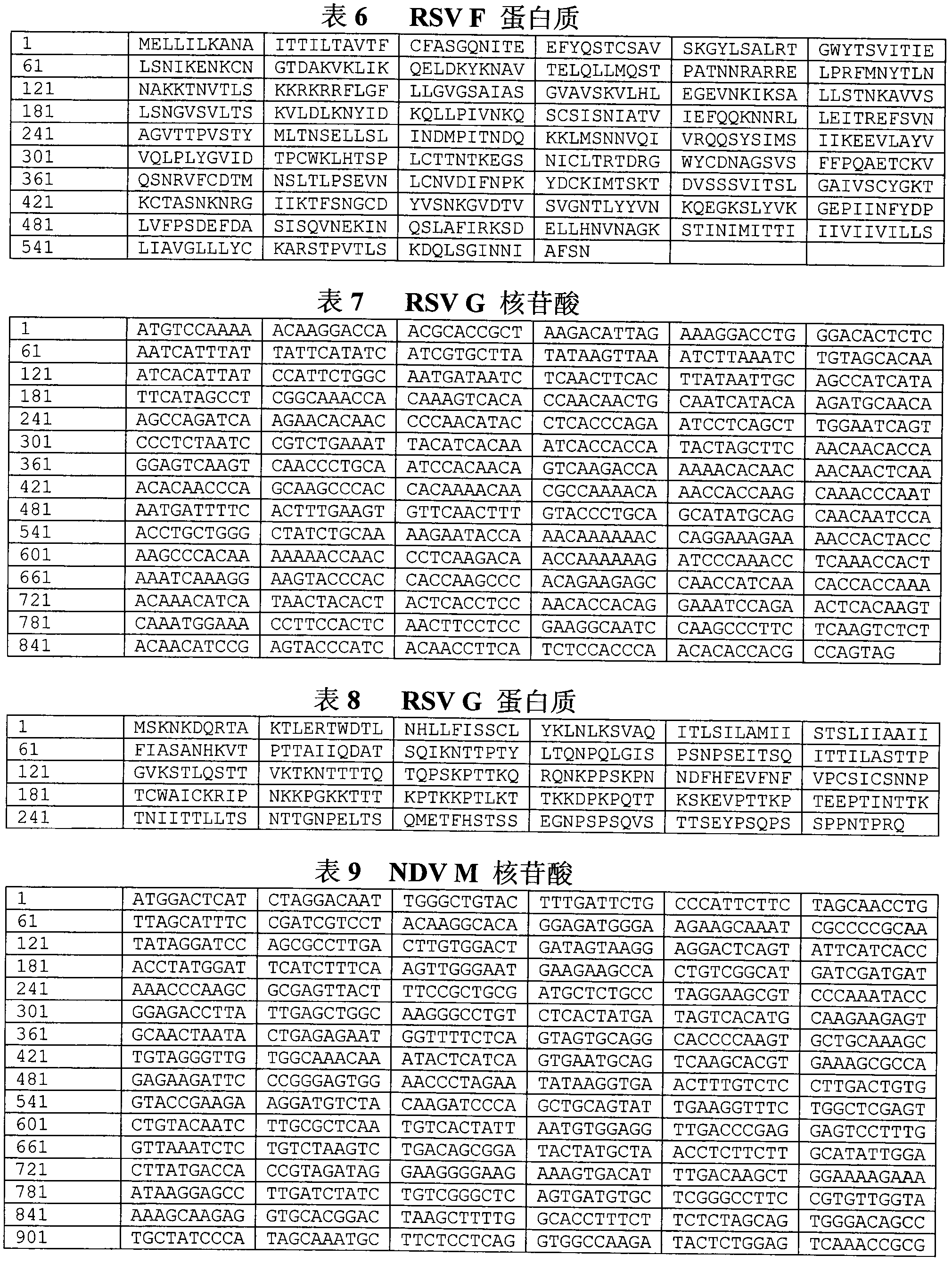
Method for constructing IL-33 presentation VLP (Virus-Like Particle) vaccine used in active immunotherapy of chronic asthma
ActiveCN104001168AEasy to makeEfficient purificationPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsDiseaseHepatitis B virus core Antigen
The invention provides a method for constructing an IL-33 presentation VLP (Virus-Like Particle) vaccine used in active immunotherapy of chronic asthma. The method comprises the following steps: extracting IL-33 total RNA (Ribonucleic Acid) from a mouse; performing reverse transcription to obtain IL33 total cDNA (complementary deoxyribonucleic acid); performing PCR (Polymerase Chain Reaction) amplification on the obtained total cDNA with a designed specific primer to obtain a coded IL-33 mature segment gene; inserting the gene between 78-bit amino acid and 79-bit amino acid of a hepatitis B virus core antigen HBcAg to obtain a recombinant plasmid pHBcAg33; transferring the plasmid onto escherichia coli DH5alpha or a BL21 competent cell; inducing by using IPTG (isopropyl-beta-d-thiogalactoside) and purifying to obtain the IL-33 presentation VLP vaccine. A strong neutralizing antibody which is specific to own molecules and has a durable action can be induced by inoculating the vaccine repeatedly in order to regulate and control immune response, thereby fulfilling the aim of regulating and controlling the progress of asthma.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Newcastle disease virus chimeric virus like particles, vaccine and preparing method
ActiveCN106867975AQuality improvementHigh antigen contentSsRNA viruses negative-senseViral antigen ingredientsPost translationalNewcastle disease virus NDV
The invention discloses virus like particles of newcastle disease virus, a vaccine and a preparing method. The vaccine is a virus like particle vaccine. The virus like particles are chimeric newcastle disease virus like particles. The vaccine is a novel vaccine prepared by the adoption of modern biology principles and methods. The vaccine adopts currently popular virulent newcastle disease virus strain gene type VII as a vaccine strain, and solves the problem that existing vaccine strains are not matched with epidemic strains; besides, the vaccine is high in antigen content and easy to produce; post-translational processing of an expression product is similar to that of structural protein of newcastle disease virus, and newcastle disease virus membrane protein on the surfaces of the particles maintains a natural structure, biological activity and immunogenicity.
Owner:NOVARTIS BIOTECH WUHAN +1
Virus-like particle of senecavirus A and preparation method and application thereof
ActiveCN108642021AHigh expressionImprove assembly efficiencySsRNA viruses positive-senseVirus peptidesSolubilityProtein target
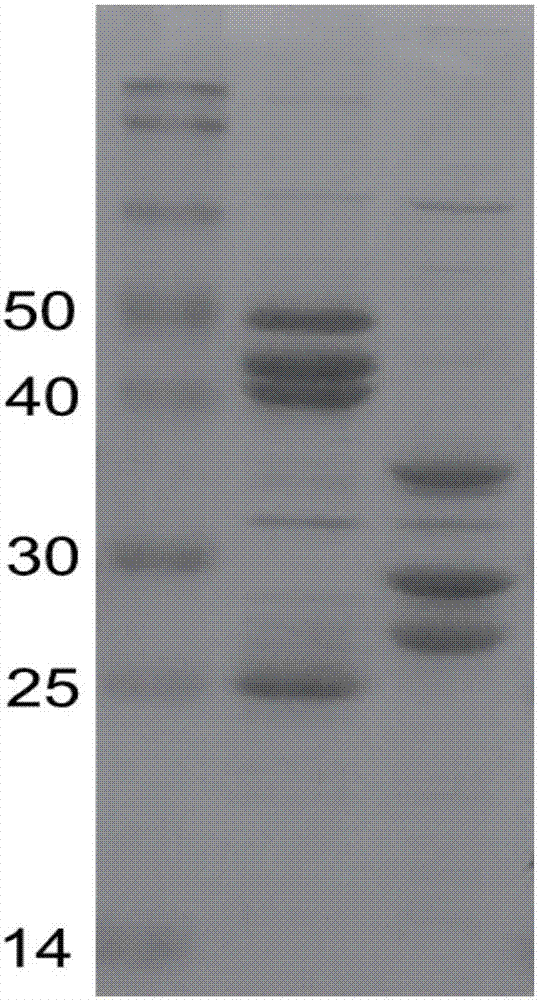
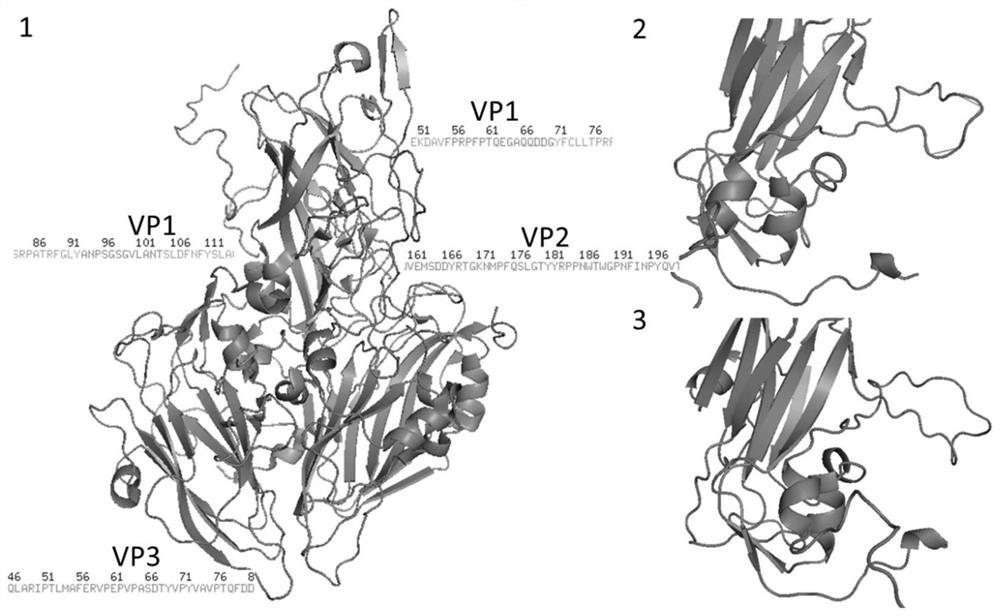
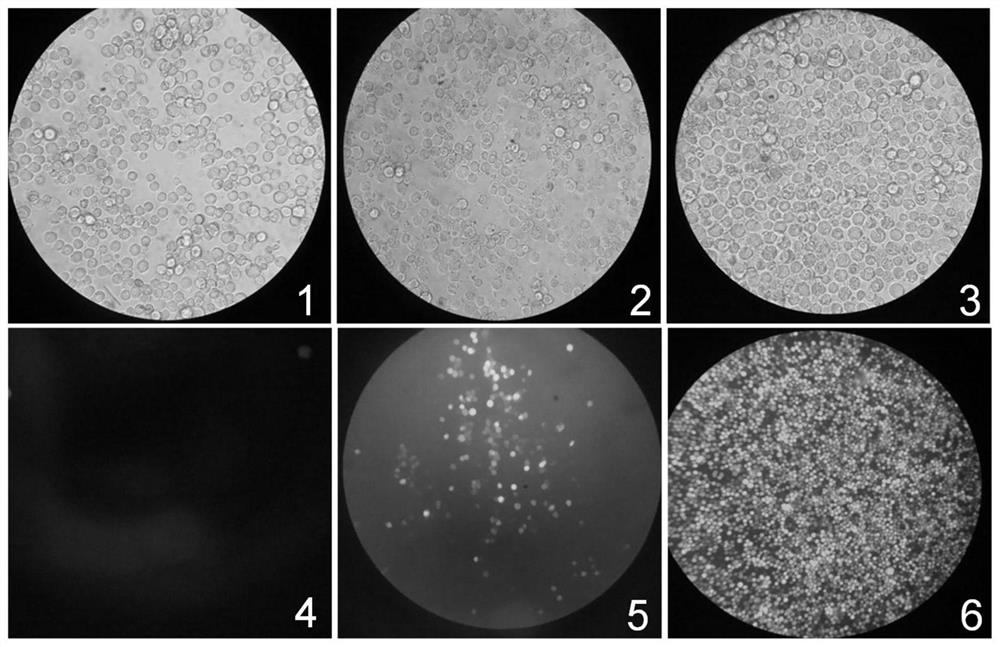
The invention discloses a virus-like particle of senecavirus A and a preparation method and application thereof. The virus-like particle of the senecavirus A is assembled by structural protein VP0, structural protein VP1 and structural protein VP3 of the senecavirus A, wherein the gene sequence of the encoding structural protein VP0 is shown in SEQ ID NO.1, the gene sequence of the encoding structural protein VP1 is shown in SEQ ID NO.2, and the gene sequence of the encoding structural protein VP3 is shown in SEQ ID NO.3. The method tries to combine different fusion tags to use so as to improve expression quantity of target proteins and assembling efficiency of the virus-like particle, and the result shows that after the N end of an SUMO VP1 gene combines with GST again, the solubility ofproteins expressed by expression bacterium which is transfected jointly by the combination of the obtained recombinant vector pGST / VP1, pSMK / VP0 and pSMC / VP3 is best, and the assembling efficiency ofVLPS is highest. The method provides technical support for further research and application of virus-like particle vaccines and accelerated transformation of animal vaccines from traditional inactivated vaccines to genetic engineering vaccines.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Anti-foot-and-mouth disease type O virus-like particle vaccine and preparation method thereof
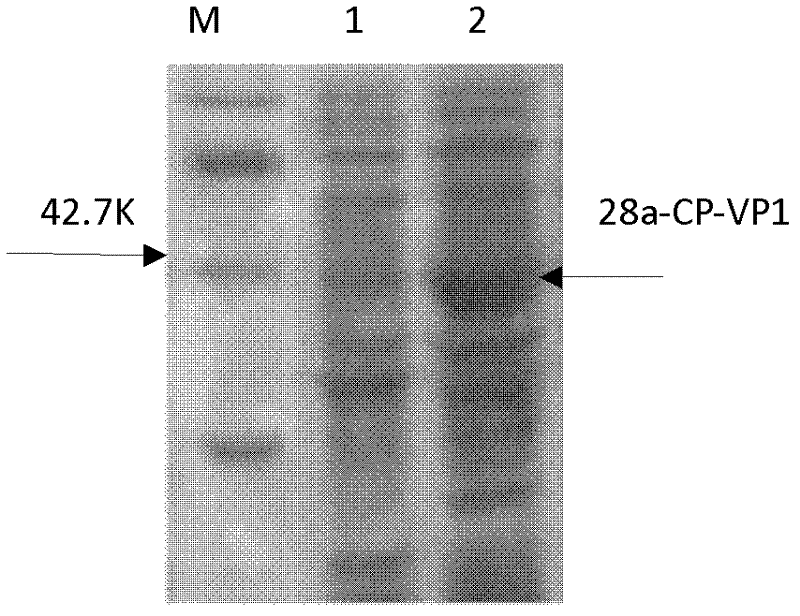
InactiveCN102512671ASafe preparationNo pathogenic effectBacteriaInactivation/attenuationGenetic engineeringImmunologic function
The invention discloses an anti-foot-and-mouth disease type O virus-like particle vaccine and a preparation method thereof, relating to the field of genetic engineering and immunology. The invention provides a safe and reliable anti-foot-and-mouth disease type O virus-like particle vaccine capable of effectively preventing the viruses of foot-and-mouth diseases, a preparation method of the particle vaccine, an amino acid sequence and a deoxyribonucleic acid (DNA) sequence of the anti-foot-and-mouth disease type O virus-like particle vaccine, a safe and stable prokaryotic expression vector 28a-CP-VP1 and the application of the anti-foot-and-mouth disease type O virus-like particle vaccine. The anti-foot-and-mouth disease type O virus-like particle vaccine has, shown in SEQ ID NO.1, the amino acid sequence which forms the anti-foot-and-mouth disease type O virus-like particle vaccine, and can be expressed by escherichia col and independently packed to be a virus-like particle structure in escherichia coli cells and exist as the virus-like particles. The vaccine can induce animal to generate anti-foot-and-mouth disease antibodies at the same time after immunization; the preparation is safe; and the vaccine has no pathogenic action probably caused by attenuated vaccine and inactivated vaccine.
Owner:XIAMEN UNIV
PCV2d (porcine circovirus type 2) virus-like particle vaccine and preparation method thereof
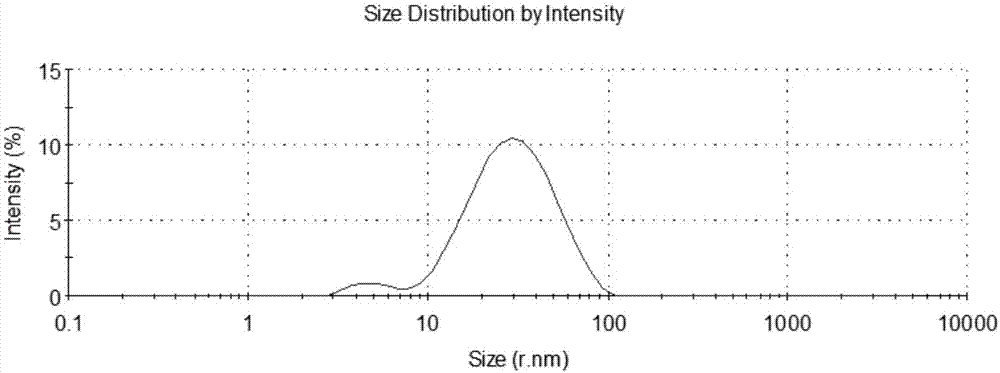
InactiveCN108355131ADoes not affect formDoes not affect sizeViral antigen ingredientsVirus peptidesEscherichia coliPhylogenetic tree
The invention relates to a PCV2d (porcine circovirus type 2) virus-like particle vaccine and a preparation method thereof. By means of epidemiological analysis for PCV2d strains of China, comparison of amino acid sequence information of a large quantity of collected strains as well as phylogenetic tree analysis, a Cap gene of a currently epidemic PCV2d strain in China is selected, PCV2d Cap protein is effectively expressed by an Escherichia coli prokaryotic expression system through codon sequence optimization, PCV2d virus-like particles are prepared successfully by purification and assembly in an in-vitro assembly and dialysis buffer solution, and form, size and concentration of the virus-like particles are not affected when the virus-like particles are placed for 6 months in a storage buffer solution at 4 DEG C and subzero 20 DEG C; the prepared PCV2d virus-like particle vaccine immunizes 21-day-old piglets, and a PCV2d challenge test proves that the vaccine has a good protecting effect for the piglets.
Owner:JIANGSU NANNONG HI TECH +1
Human papilloma virus sample particle vaccines
ActiveCN101116745AImprove the effectiveness of preventionAvoid infectionAntibody medical ingredientsAntineoplastic agentsHuman papilloma virus infectionEpidemiology
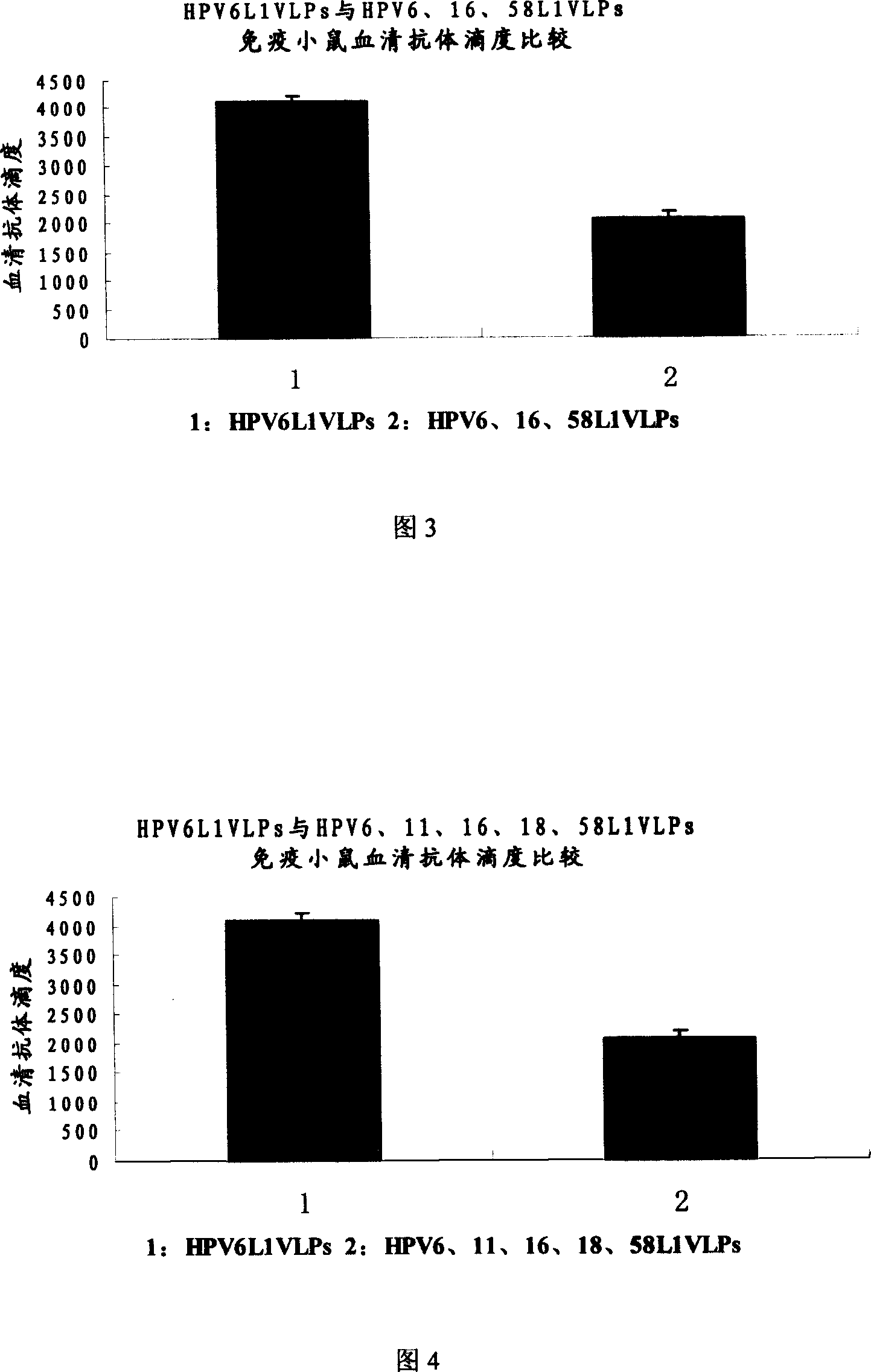
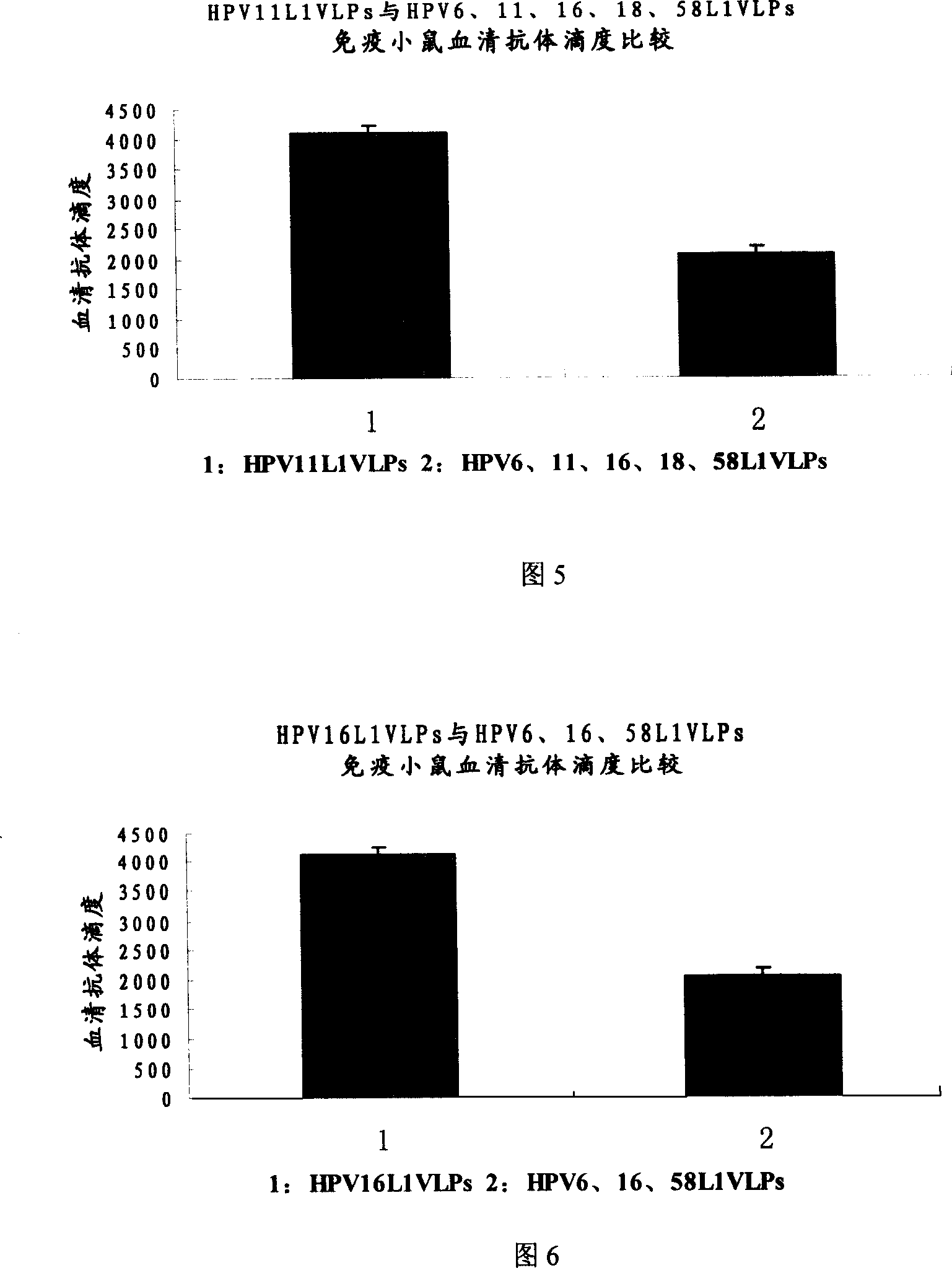
The invention relates to a virus-like particle vaccine against human papilloma virus, belonging to the biotechnology field. One milliliter vaccine contains the following components: HPV6L1VLPs: 10-60 ug, HPV16L1VLPs: 5-40 ug and HPV58L1VLPs10-60 ug. The invention has the advantages that HPV6L1, HPV16L1 and HPV58L1 proteins are secreted and expressed respectively, and the L1 proteins can be self-assembled to be VLPs. According to the infection epidemiology investigation result of human papilloma virus in some placed in Asia and China, the vaccine prepared by VLPs which is obtained by L1 proteins of HPV6, HPV16 and HPV58 can prevent 60 percent of the inventions resulting from HPV. The additional HPV type can further increases preventive effects of the vaccine.
Owner:CHANGCHUN BCHT BIOTECH
Enteroviral chimeric virus-like particle vaccine and preparation method and application thereof
The invention discloses an enteroviral chimeric virus-like particle vaccine and a preparation method and application thereof and belongs to the field of biotechnologies. Chimeric virus-like particles are hepatitis b virus core protein based recombinant enteroviral multi-epitope chimeric antigen protein, and the amino acid sequence of the particles is shown as SEQ ID NO.1. The nucleotide sequence of a DNA fragment encoding the virus-like particles is shown as SEQ ID NO.2. The DNA fragment shown as SEQ ID NO.2 is cloned to an escherichia coli expression vector to establish recombinant expression plasmids. The recombinant expression plasmids are converted into escherichia coli to obtain engineering bacteria. The engineering bacteria are cultured, and enteroviral chimeric virus-like particles are obtained through the operations, such as inducing expression and protein purification. The virus-like particles can induce a body to produce specific body fluid and cellular immune response, can be used for immune prevention of enterovirus EV71 and CA16 infection of people and susceptible animals and is used for preparation of the enteroviral vaccine.
Owner:河南杰瑞生物科技研究院有限公司
Virus-like particle vaccines
InactiveCN106573988ASsRNA viruses negative-senseSsRNA viruses positive-senseMultivalent VaccineViral structural protein
Owner:MEDIGEN BIOTECH
Application of gold nanoparticle as adjuvant to preparation of virus-like particle (VLP) vaccine
ActiveCN107261135AImprove immunityEnhance cellular immunitySsRNA viruses positive-senseViral antigen ingredientsAdjuvantT lymphocyte
The invention discloses application of a gold nanoparticle as an adjuvant to preparation of a VLP vaccine. According to the invention, the gold nanoparticle is used as the adjuvant for preparation of the VLP vaccine, and the gold nanoparticle is a gold nanocage or gold nanostar, preferably; results show that a conjugation product of the gold nanoparticle and VLPs can release carried VLPs after entering an animal body via injection and thus induce specific antibody immune response and T-lymphocyte immune response; and thus, it is proved that the gold nanoparticle is applicable as the adjuvant of VLPs for effective stimulation of specific immune response in animal bodies. The gold nanoparticle provided by the invention is applied as the adjuvant capable of improving the immune effect of VLPs against foot and mouth disease viruses and better promoting generation of cellular immunity and humoral immunity of VLP vaccines for preparation of the VLP vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Specific multivalent virus-like particle vaccines and uses thereof
InactiveUS20160206715A1Overcomes existing immune toleranceStrong immune responseNervous disorderAntipyreticLipopeptideVirology
The invention provides a VLP free of a viral genome comprising two or more display polypeptides, nucleic acid molecules, polymers of the nucleic acid, lipopolysaccharides, lipopeptides, peptidoglycans and / or small molecules.
Owner:BULLET BIOTECH +1
Chimeric virus-like particle vaccine and preparation method therefor and application of chimeric virus-like particle vaccine
ActiveCN111647087AIncrease productionHigh puritySsRNA viruses positive-senseAntibody mimetics/scaffoldsDiseaseEpitope
The invention relates to a chimeric virus-like particle vaccine and a preparation method therefor and application of the chimeric virus-like particle vaccine, in particular to a porcine Seneca valleyvirus and porcine circovirus-2 chimeric virus-like particle vaccine. According to the chimeric virus-like particle vaccine, sequences of VP0, VP3 and VP1 are connected in tandem for expression, wherein part of the VP3 sequences are replaced with porcine circovirus-2 ORF2 gene C-terminal epitope sequences to form a recombination sequence; the recombination sequence transfects sf9 cells after beingconstructed on recombinant bacmid for further expression to obtain porcine Seneca valley virus and porcine circovirus-2 chimeric virus-like particles. It is the first time for the invention to developthe porcine Seneca valley virus and porcine circovirus-2 chimeric virus-like particle vaccine by utilizing the chimeric virus-like particles, and the porcine Seneca valley virus and porcine circovirus-2 chimeric virus-like particle vaccine can exert a relatively good immune protection effect on the porcine Seneca valley virus and the porcine circovirus-2; and the vaccine of the invention is economical and practical, can effectively reduce the epidemic prevention cost of diseases, and provides a new method of preventing two diseases at the same time for breeding enterprises in China.
Owner:CHINA ANIMAL HUSBANDRY IND
A type A Seneca virus virus-like particle and its preparation method and application
ActiveCN108642021BHigh expressionImprove assembly efficiencySsRNA viruses positive-senseVirus peptidesStructural proteinSenecavirus A
A virus-like particle of Senecavirus A, the particle including a structural protein VP0, a structural protein VP1 and a structural protein VP3. The structural protein VP0 is encoded by a gene sequence represented by SEQ ID NO: 1. The structural protein VP1 is encoded by a gene sequence represented by SEQ ID NO: 2. The structural protein VP3 is encoded by a gene sequence represented by SEQ ID NO: 3.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
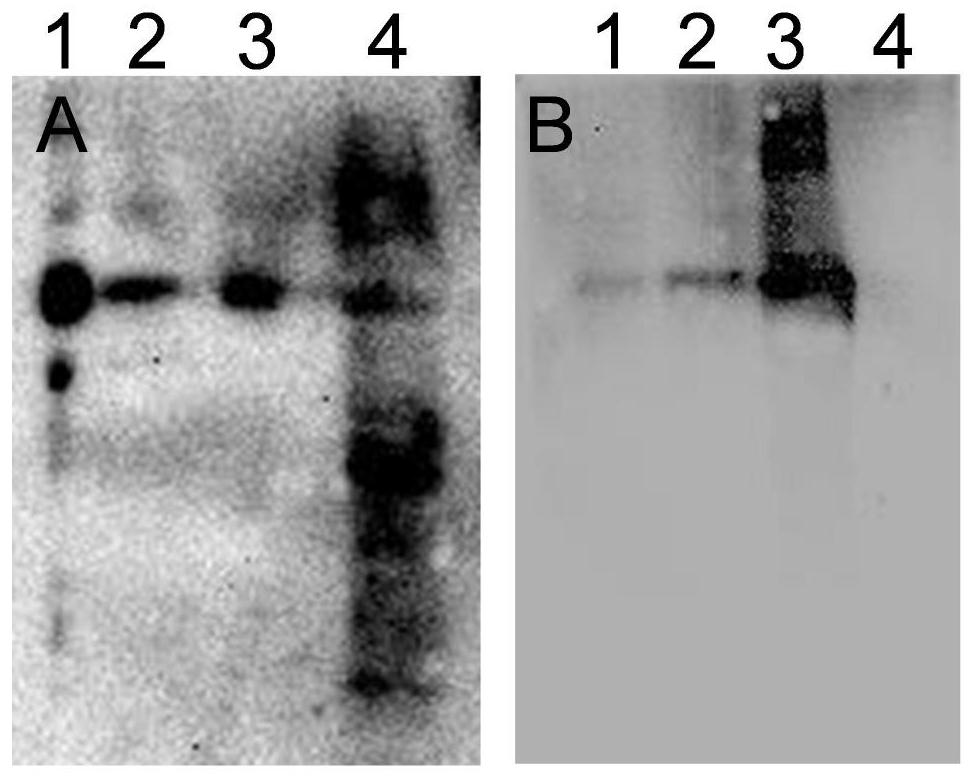
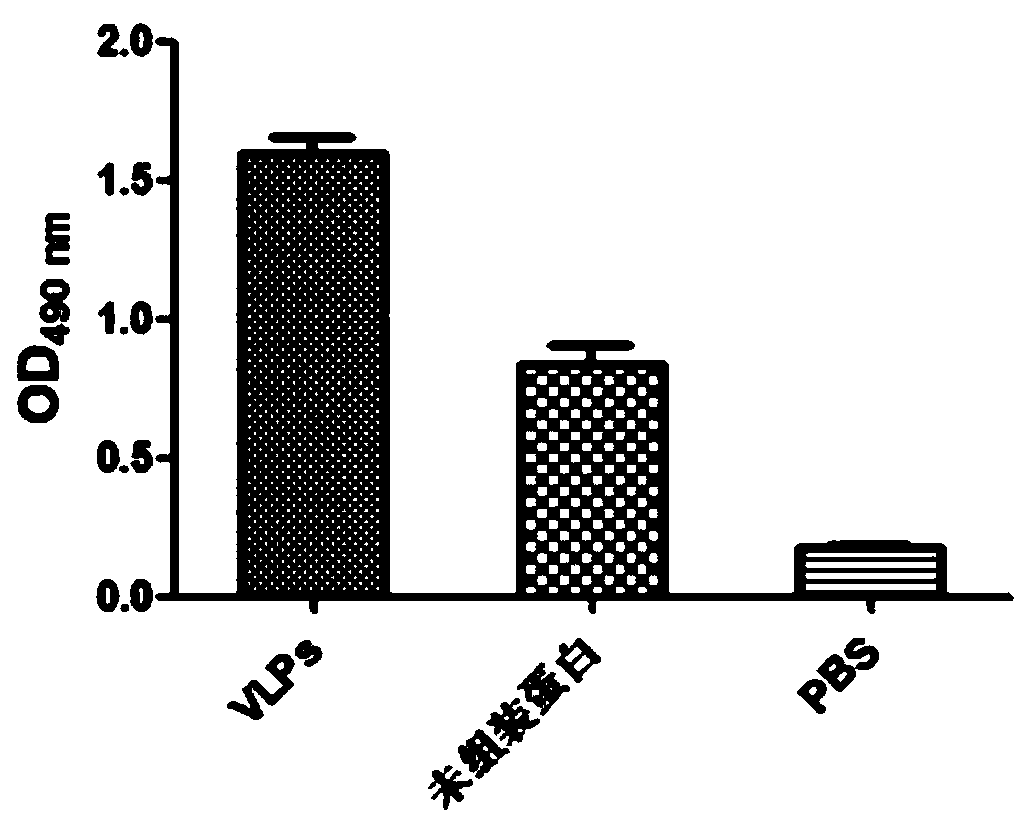
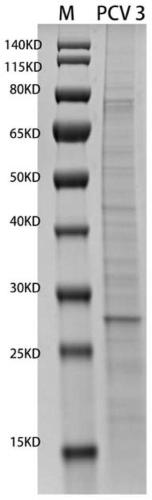
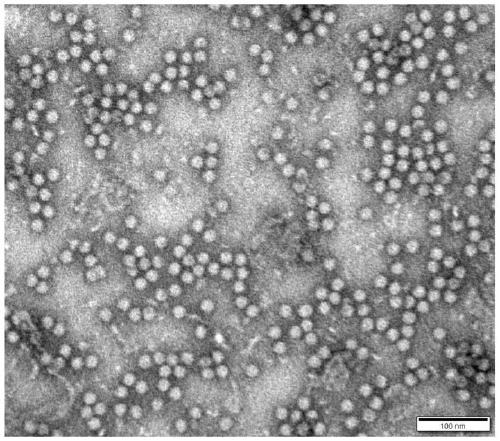
Porcine circovirus 3 Cap protein, nucleic acid, virus-like particles, vaccine, and preparation method and application of porcine circovirus 3 Cap protein
ActiveCN111253477AImprove expression efficiencyGood immune effectViral antigen ingredientsVirus peptidesProtein targetPorcine Circoviruses
The invention provides a porcine circovirus 3 Cap protein, nucleic acid, virus-like particles, vaccine, and a preparation method and application of the porcine circovirus 3 Cap protein, and relates tothe technical field of molecular biology. The porcine circovirus 3 Cap protein provided by the invention has an amino acid sequence shown as SEQ ID NO.2, can be expressed in a soluble manner in a yeast expression system, and effectively improves yeast expression efficiency of the porcine circovirus 3 Cap protein. In addition, the porcine circovirus 3 Cap protein provided by the invention can be self-assembled into the virus-like particles, the vaccine prepared by using the virus-like particles has the characteristics of cell immunity and humoral immunity, and immunologic related experiments show that the immune effect is good. The method for expressing the porcine circovirus type 3 Cap protein by utilizing the yeast expression system has the advantages that production cost is low, the production process is simple, and a large amount of target proteins can be obtained through high-density fermentation, has no endotoxin pollution, and can be used for large-scale production.
Owner:天康制药股份有限公司
Bursal subviral particle vaccine and preparation method thereof
PendingCN114058524AShort cycleIncrease productionFungiVirus peptidesSubviral particleInfectious bursitis
The invention belongs to the technical field of biological medicines, and particularly relates to a bursal subviral particle vaccine and a preparation method thereof. Directed at chicken infectious bursal disease virus variants, Kluyveromyces marxianus is adopted for recombinant expression of engineering strains of bursal subvirus-like particles, and the effective bursal subvirus-like particle vaccine is prepared. The method comprises the following steps that: firstly, a Kluyveromyces marxianus engineering strain for recombinant expression of virus subvirus-like particles is provided; the recombinant strain is obtained by transforming Kluyveromyces marxianus KM-YDQ1 by a recombinant expression bursal virus capsid protein carrier; according to the bursal virus subvirus-like particle vaccine, bursal subvirus-like particles obtained by culturing, fermenting, separating and purifying Kluyveromyces marxianus recombinant engineering strains are used as active ingredients. The protective effects of the chick immune bursal subviral particle vaccine on a bursal virus variant strain FJ-18 and a classic virulent strain BC6 / 85 both reach 100%.
Owner:FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com