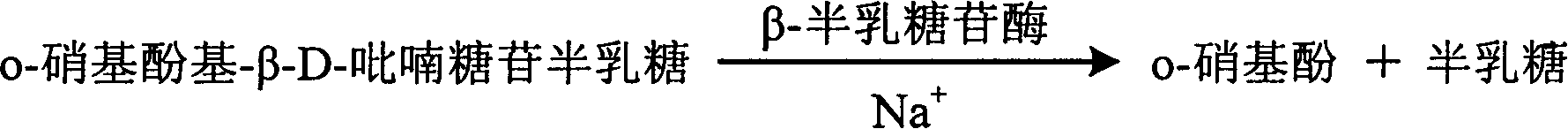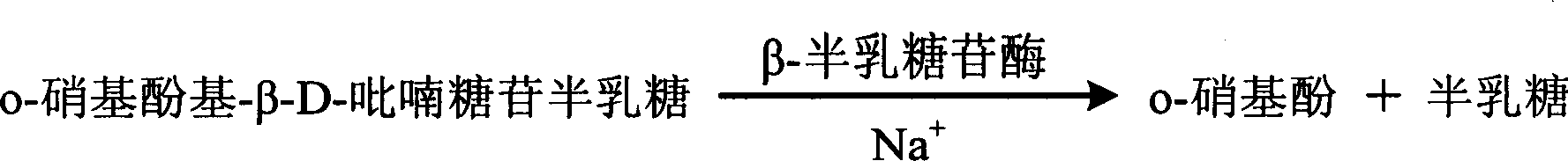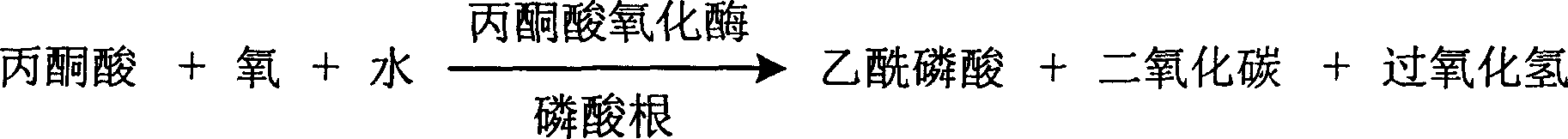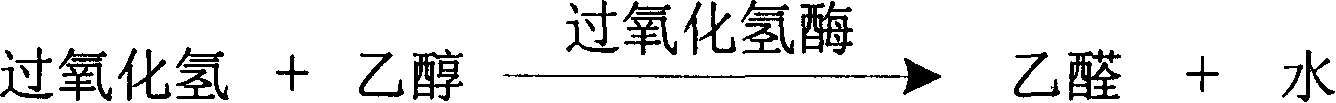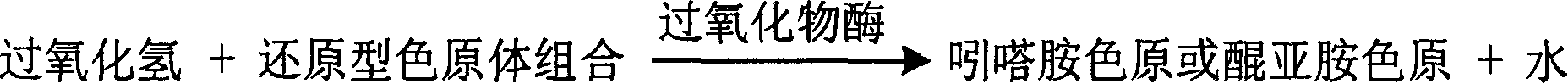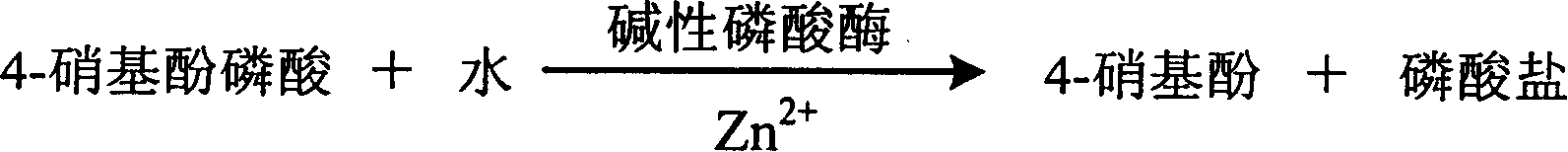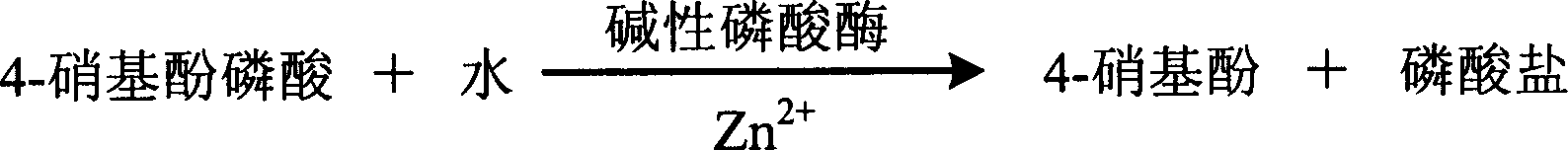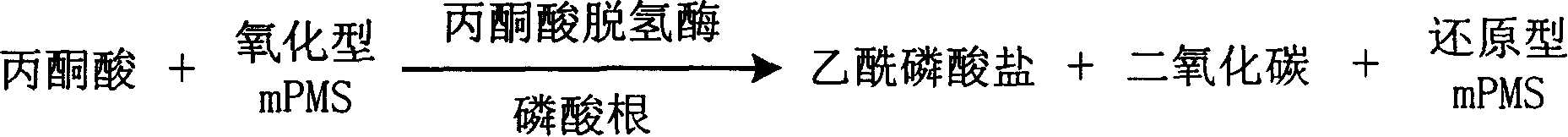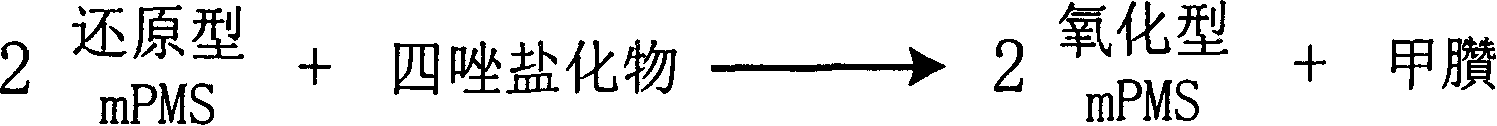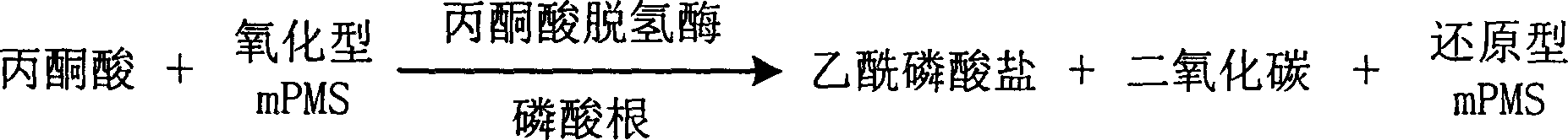Patents
Literature
30results about How to "Complete three-dimensional structure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Determination method of proangiotension transferase activity and proangiotension transferase diagnosis kit
InactiveCN1786185AActive responseReflect activityMicrobiological testing/measurementAngiotensin-converting enzymeBiochemistry
The invention relates to angiotensin converting enzyme activity measuring method and its diagnosis kit. It is applied artificial synthesis substrate FAPGG to directly react with angiotensin converting enzyme to form furyl-acryloyl-phenylalanine, and to measure its activity by 340nm absorbance lowering speed. The method has high specificity, good accuracy. And it can be quickly measured by ultraviolet / visible light analyzer or semi / full automatic biochemical analysis. Its measuring cost is low; and it is convenient for generalization and application.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
CMC nanofiber membrane loaded with silver nanoparticles and preparation method thereof
ActiveCN105332163AEasy to prepareNo pollution in the processFilament/thread formingNon-woven fabricsCelluloseFiber
The invention discloses a carboxymethylcellulose (CMC) nanofiber membrane loaded with silver nanoparticles and a preparation method thereof and aims at providing a silver-loaded antibacterial nanofiber and a preparation method which have the characteristics that the preparation method is simple and pollution-free, the prepared fiber membrane has excellent antibacterial property and biocompatibility and only CMC serves as a base material. The preparation method comprises the following steps: performing electrostatic spinning by taking a mixed solution of polyethylene oxide (PEO) and CMC as a spinning solution; soaking a fiber membrane in a silver nitrate solution of a certain concentration, taking out the membrane, drying in the dark, and reducing in moist air at normal temperature; and finally, preparing the CMC nanofiber membrane loaded with the silver nanoparticles. The prepared fiber membrane has excellent antibacterial property and biocompatibility, is good in structure and mechanical property retention and is a preferred material in the field of wound coating, and the preparation method is simple, economic and environment-friendly.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY +1
Method for preparing cell-free medical implant material
PendingCN110404113ANo damage to the three-dimensional structureComplete three-dimensional structureProsthesisProtease preparationTissue repair
The invention belongs to the field of medical repair materials and relates to a method for preparing a cell-free medical implant material. In the method for preparing the cell-free materials, neitherchemically synthesized detergents nor protease products are used to remove cells; but a plant-derived nonionic surfactant is used as a decellularizing agent, thus avoiding the damage of chemical detergents, protease preparations and the like to an ECM three-dimensional structure and the loss of effective active ingredients in ECM. The cell-free medical material prepared by the method can not onlybetter maintain the three-dimensional structure of ECM, but also allow more effective active ingredients to be remained in ECM and endowed with good mechanical properties. Thereby, the ability to induce chemotaxis and differentiation of host cells is stronger, and the effect of promoting tissue repair and regeneration will be better.
Owner:上海白衣缘生物工程有限公司
Enzymatic method for determining sodium ion content and sodium ion diagnosis kit
InactiveCN1786186AQuantitative reflection of contentHighlight substantive featuresMicrobiological testing/measurementIon contentPotassium
The invention relates to enzyme method measuring sodium ion content method and its diagnosis kit. It is adopted galactosidase reaction colorimetry to use sodium ion to activate beta-galactosidase enzymolysis o-nitrophenol group-beta-D- pyranoside galactose to make 405nm dominant wavelength absorbance rise. Its absorbance amplitude of fluctuation is proportional to sample sodium ion. The method has high specificity, good accuracy. The diagnosis kit can be made into bi-agent to reduce cross influence. The method can be quickly measured by ultraviolet / visible light analyzer or semi / full automatic biochemical analysis. Its measuring cost is low; and it is convenient for generalization and application.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Process for determining content of carbon dioxide and kit for diagnosing carbon dioxide therefor
InactiveCN1786694AHighlight substantive featuresSignificant progressAnalysis by subjecting material to chemical reactionColor/spectral properties measurementsUltravioletAbsorbance
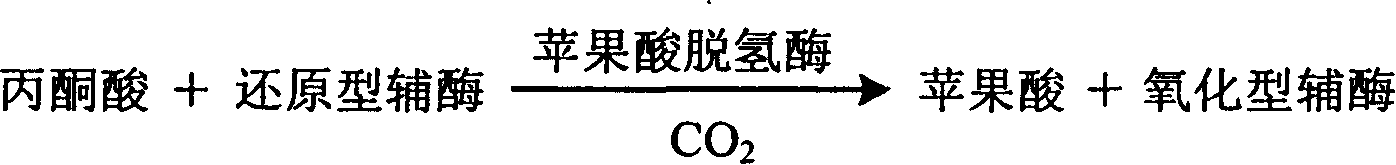
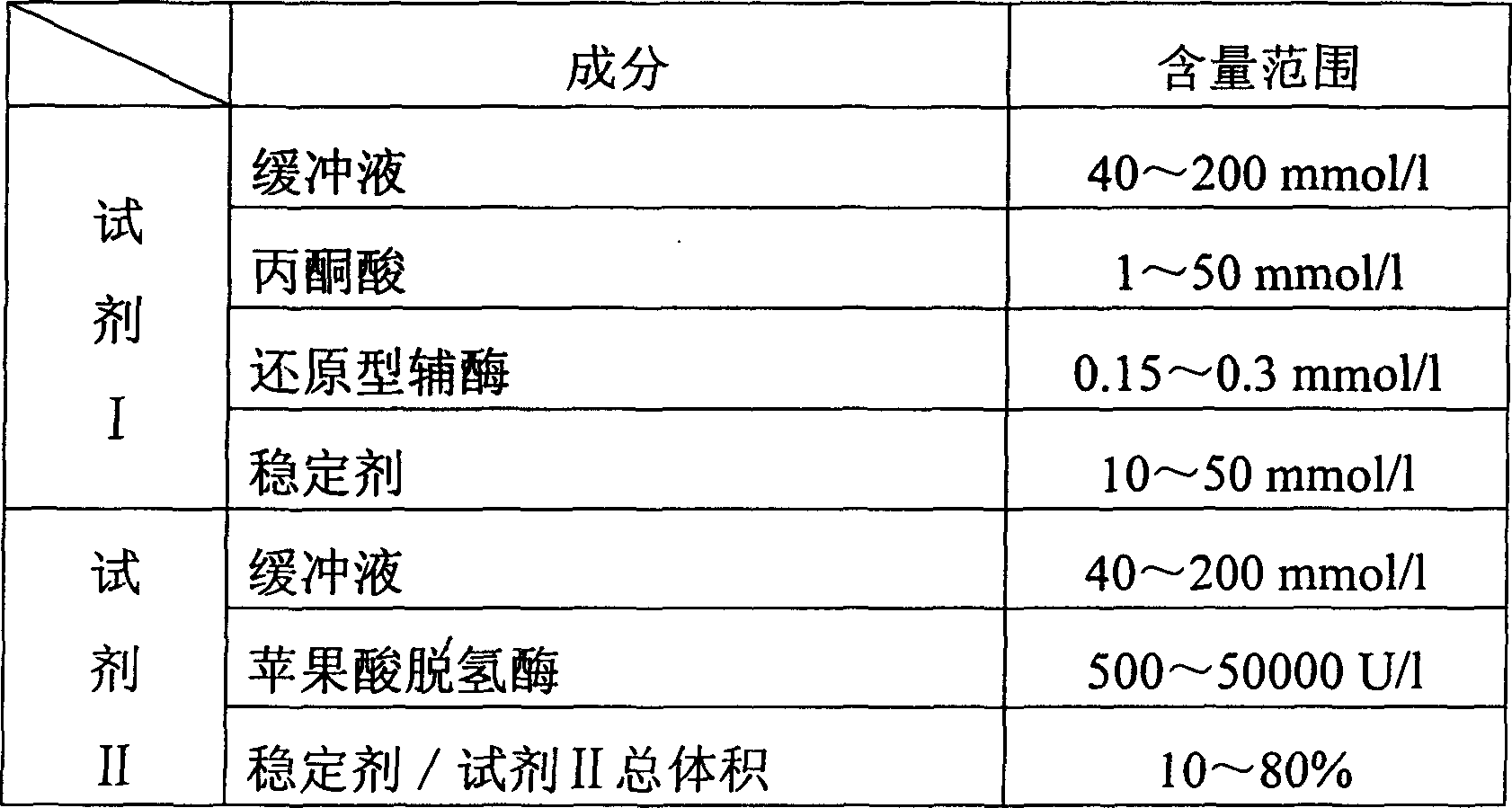
The invention relates to measuring method for carbon dioxide content and diagnosing kit. The invention directly uses carbon dioxide( bicarbonate radical) and pyruvic acid to produce malic acid by decarboxylation of malic dehydrogenase and oxidizes reduced coenzyme into oxidized type coenzyme, measuring the decent rate of dominant wavelength 340nm absorbance could quantitatively response the carbon dioxide content in sample, thus, the strength of bicarbonate radical could be extrapolated. The method has high specificity, has no pollution from inside and outside material, and accurate test result. The diagnose kit is made up into bi-agent or tri-agent that could reduce cross influence from each component and keeps stability of the agent that is convenient for long-term storage. The method could quickly test on commonness ultraviolet / visible light, analysis or semi-transfer / full automatically biochemical study instrument, and doesn't need particularity or extra instrument. The testing cost is cheap, and is convenient for popularization.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778955AReflect contentHighlight substantive featuresMicrobiological testing/measurementUltravioletWavelength
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Monoamine oxidase activity determination method and monoamine oxidase diagnostic kit
InactiveCN1789427AStrong specificityQuantitatively reflect the activityMicrobiological testing/measurementMonoamine oxidase APeroxidase
The invention relates measuring method of monoamine oxidase activity and its diagnostic kit, comprising the following steps: using the benzylamine, p-toluidine red-ª‰-azo naphthol, butyl amine, amyl amine, ª‰-phenylethylamine, tyramine and other aminated compounds as substrate, it reacted with monoamine oxidase to produce auricome, then auricome carried out coupling reaction with hydrogen peroxidase and aldehyde dehydrogenase, carrying out the reaction to turn the oxidation type coenzyme to reduction type coenzyme, detecting the ascending velocity of absorbance of main wavelength 340nm to reflect the activity of monoamine oxidase in sample by definite quantity. The method has high specificity, good accuracy. The diagnostic kit is made to double agents or tri-agent to reduce the across impact and keep the agent stability and be good for long term storage. The method can be rapidly detected by the ultra-violet / visible light analysis meter or semi-automatic / full-automatic biochemical analysis meter, so the method is easy to spread and use.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Monoamine oxidase activity determination method and monoamine oxidase diagnostic kit
InactiveCN1789428AHigh specificityThe test results are accurateMicrobiological testing/measurementAmount of substanceTyramine
The invention relates measuring method of monoamine oxidase activity and its diagnostic kit. The invention comprises the following steps: using the monoamine oxidase to hydrolyze benzylamine, p-toluidine red-ª‰-azo naphthol, butyl amine, amyl amine, ª‰-phenylethylamine, tyramine and other aminated compounds to produce the ammonia, then the ammonia carried out coupling reaction with glutamate dehydrogenase to turn the oxidation type coenzyme to reduction type coenzyme, detecting the reducing velocity of absorbance of main wavelength 340nm to reflect the activity of monoamine oxidase in sample by definite quantity. The method has high specificity and good accuracy. The diagnostic kit is made to double agents or tri-agent to reduce the across impact and keep the agent stability and be good for long term storage. The method can be rapidly detected by the ultra-violet / visible light analysis meter or semi-automatic / full-automatic biochemical analysis meter, so the method is easy to spread and use.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Monoamine oxidase activity determination method and monoamine oxidase diagnostic kit
InactiveCN1789426AStrong specificityThe test result is accurateMicrobiological testing/measurementQuinoneHydrogen peroxide
The invention relates measuring method of monoamine oxidase activity and its diagnostic kit. The method comprises the following steps: carrying out reaction with benzylamine, tyramine and monoamine oxidase to produce the hydrogen dioxide, then taking it with peroxidase to carry out enzyme coupling reaction, oxygenizing the achromatic color reduction type chromogen to become the quinone imines chromogen or indamine chromogen dye, detecting the change of absorbance of main wavelength 400-600nm to reflect the activity of monoamine oxidase in sample by definite quantity. The method has high specificity and good accuracy. The diagnostic kit is made to double agents or tri-agent to reduce the across impact and keep the agent stability and be good for long term storage. The method can be rapidly detected by the ultra-violet / visible light analysis meter or semi-automatic / full-automatic biochemical analysis meter, so the method is easy to spread and use.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778941AHighlight substantive featuresSignificant progressMicrobiological testing/measurementPeroxidaseUltraviolet
The invention is about the measuring method of inorganic Phosphates and its diagnosis reagent box. Producing hydroperoxide by reacting pyruvate oxidase with pyruvate under the existence of Inorganic Phosphates , then causing enzyme-coupled reaction with peroxidase and oxidating the colorless reduced chromogen combination to quinoneimine chromogen or indamide chromogen dyer with color, testing the variation of dominant wave-length 400ú¡600nm absorbance during the reaction and finally measuring the content of Inorganic Phosphates . This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
InactiveCN1778956AStrong specificityThe test result is accurateMicrobiological testing/measurementUltravioletWavelength
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of hexokinase, and producing glucose-6-phosphate by reacting glucose under the existence of adenosine triphosphate, and then causing coupled reaction with phosphoglucose dehydrogenase and transferring oxidized coenzyme to reduced coenzyme. Testing the ascending range of dominant wave-length340nm absorbance and finally measuring the content of magnesium ion in the sample. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778943AContent reflectionHighlight substantive featuresMicrobiological testing/measurementPeroxidaseUltraviolet
The invention is about the measuring method of Inorganic Phosphates and its diagnosis reagent box. Producing hypoxanthine by reacting nucleoside phosphorylase with carnine under the existence of Inorganic Phosphates in the sample of plasma, serum and so on, then producing urate and hydroperoxide by reactinghypoxanthine withxanthine oxidase, and then reacting bimolecular hydroperoxide with peroxidaseand oxidating the colorless reduced chromogen combination to quinoneimine chromogen or indamide chromogen dyer with color, testing the variation of dominant wave-length400ú¡600nmabsorbance during the reaction and finally measuring the content of Inorganic Phosphates . This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
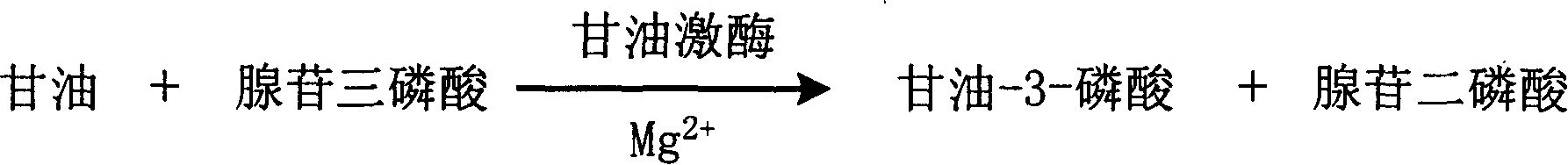
InactiveCN1778962AStrong specificityThe test result is accurateMicrobiological testing/measurementGlycerolUltraviolet
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of glycerokinase, and producing glycerol-3-phosphoryl by reacting glycerol under the existence of adenosine triphosphate, and then causing coupled reaction with glycerol-3-phosphoryl dehydrogenase, hydroperoxide enzyme and aldehyde dehydrogenase and transferring oxidized coenzyme to reduced coenzyme. Testing the ascending range of dominant wave-length 340nm absorbance and finally measuring the content of magnesium ion in the sample. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
InactiveCN1778961AStrong specificityThe test result is accurateMicrobiological testing/measurementUltravioletGlycerol
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of glycerokinase, and producing glycerol-3-phosphoryl by reacting glycerol under the existence of adenosine triphosphate, and then causing coupled reaction with glycerol-3-phosphoryl dehydrogenase, peroxidase and oxidating the colorless reduced chromogen combination to quinoneimine chromogen or indamide chromogen dyer with color, testing the ascending range of 400ú¡600nmabsorbance and finally measuring the content of Inorganic Phosphates This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Enzymatic method for determining potassium ion content and potassium ion diagnosis kit
InactiveCN1786187AStrong specificityThe test result is accurateMicrobiological testing/measurementIon content6-Phosphogluconolactone
The invention relates to enzyme method measuring kalium ion content method and its diagnosis kit. It is utilized kalium ion activation pyruvate kinase specificity to couple hexokinase, glucose phosphate dehydrogenase, 6-phosphogluconolactone and phosphogluconate dehydrogenase to react, reduce dichotomy type oxidizing coenzyme to dichotomy reducing type. To measure dominant wavelength 340nm absorbance rising speed can quantificationally reflect sample kalium ion content. The method has high specificity, good accuracy. The diagnosis kit can be made into bi-agent or tri-agent to reduce cross influence. The method can be quickly measured by ultraviolet / visible light analyzer or semi / full automatic biochemical analysis. Its measuring cost is low; and it is convenient for generalization and application.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
InactiveCN1778957AHigh activityStrong specificityMicrobiological testing/measurementLactate dehydrogenaseUltraviolet
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of hexokinase, and producing adenosine diphosphate by reacting glucose under the existence of adenosine triphosphate, and then causing coupled reaction with pyruvate kinase and lactate dehydrogenase and transferring oxidized coenzyme to reduced coenzyme. Testing the descending range of dominant wave-length340nm absorbance and finally measuring the content of magnesium ion in the sample. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Preparation method and application of dopamine/calcium phosphate hybrid micron flower
ActiveCN113521307AHigh specific surface areaHigh porosityPowder deliveryOrganic active ingredientsBiophysicsCalcium biphosphate
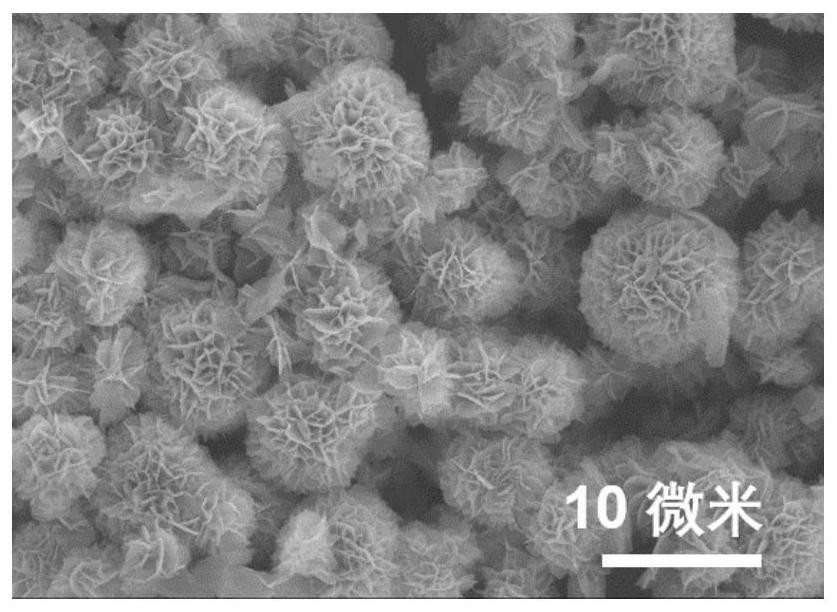
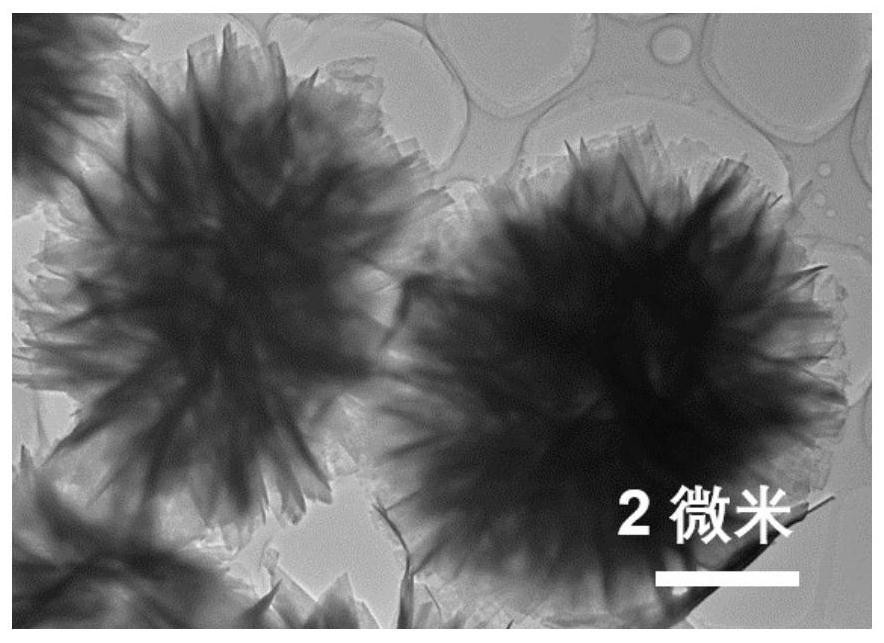
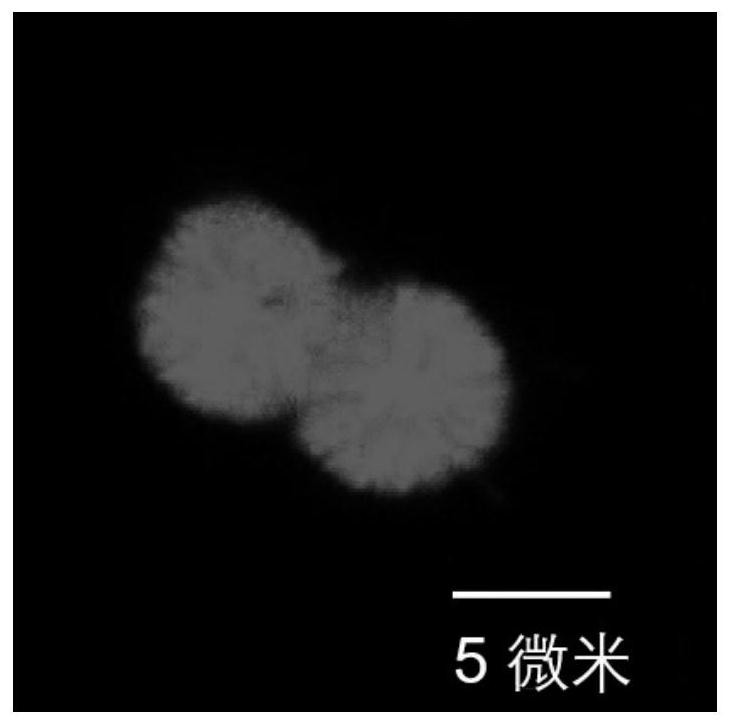
The invention provides a preparation method and application of a dopamine / calcium phosphate hybrid micron flower. The diameter of the dopamine / calcium phosphate hybrid micron-flower material is 3-10 microns, the dopamine / calcium phosphate hybrid micron-flower material is composed of regular porous nanosheets with the thickness of about 20 nanometers and the width of about 300-800 nanometers, and the dopamine / calcium phosphate hybrid micron-flower material has a complete three-dimensional structure, an extremely high specific surface region and high porosity. The material can be used as a drug carrying material or a drug carrier for preparing a drug delivery system. The drug delivery system has high drug loading rate, and can controllably release drugs under different pH conditions.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778954AReflect contentHighlight substantive featuresMicrobiological testing/measurementPhosphoenolpyruvate carboxylaseUltraviolet
The invention is about the measuring method of Inorganic Phosphates and its diagnosis reagent box. Producing carbon dioxide by reacting pyruvate oxidase with pyruvate under the activation ofInorganic Phosphates in the sample of plasma or serum and so on, and producing oxaloacetic acid by reacting carbon dioxide and phosphoenolpyruvate under the existence of phosphoenolpyruvate carboxylase, and then transferring oxidized coenzyme to reduced coenzyme by reacting oxaloacetic acid and malic acid dehydrogenase. Testing the descending range of dominant wave-length340nm absorbance and finally measuring the content of Inorganic Phosphates. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Process for determining content of zinc ion by enzyme method and kit for diagnosing zine ion thereof
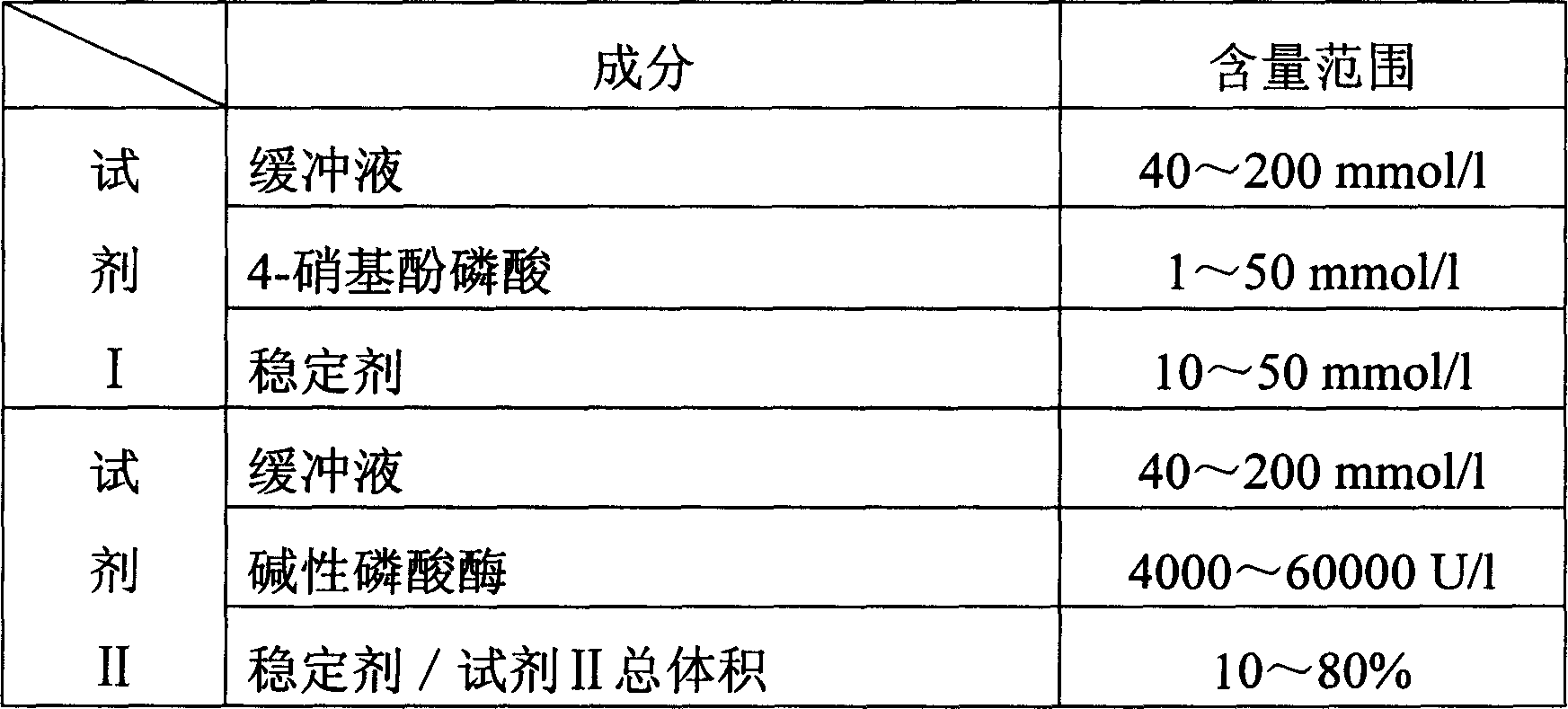
InactiveCN1786695AStrong specificityThe test result is accurateAnalysis by subjecting material to chemical reactionColor/spectral properties measurementsUltravioletPhosphoric acid
The invention relates to enzyme method measuring zinc ion concentration and zinc ion diagnose kit. The invention uses alkaline phosphatase reaction colorimetry method that uses alkaline phosphatase to enzymolysis four-nitrophenol phosphoric acid to form into four-nitrophenol under the activation of zinc ion, by detecting the ascending speed of 405nm dominant wavelength absorbance the zinc ionic content in sample would be quantitatively responsed. The method has high specificity, has no influence from inside and outside metal material, accurate test result. The diagnose kit is made up into bi-agent or tri-agent that could reduce cross influence from each component and keeps stability of the agent that is convenient for long-term storage. The method could quickly test on commonness ultraviolet / visible light, analyzer or semi-transfer / full automatic biochemical study instrument, and doesn't need particularity or extra instrument. The testing cost is cheap, and is convenient for popularization.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
InactiveCN1778960AStrong specificityThe test result is accurateMicrobiological testing/measurementGlycerolUltraviolet
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of glycerokinase, and producing glycerol-3-phosphoryl by reacting glycerol under the existence of adenosine triphosphate, and then causing coupled reaction with glycerol-3-phosphoryl dehydrogenase and transferring oxidized coenzyme to reduced coenzyme. Testing the descending range of dominant wave-length340nm absorbance and finally measuring the content of magnesium ion in the sample. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of magnesium ion content from enzyme method and magnesium ion diagnostic reagent kit
InactiveCN1778958AStrong specificityThe test result is accurateMicrobiological testing/measurementChemistryEnzyme method
The invention is about the enzyme method of measuring magnesium ion and its diagnosis reagent box. Making use of the peculiarity that magnesium ion in the sample of plasma or serum and so on can activate the activation of hexokinase, and producing glucose-6-phosphate by reacting glucose under the existence of adenosine triphosphate, and then causing coupled reaction with phosphoglucose dehydrogenase, 6-phosphogluconic acid lactone enzyme and phosphogluconate dehydrogenase, and transferring dimolecule oxidized coenzyme to dimolecule reduced coenzyme. Testing the ascending range of dominant wave-length340nm absorbance and finally measuring the content of magnesium ion in the sample. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Process for determining contect of potassium ion by enzyme method and kit for diagnosing potassium ion thereof
InactiveCN1786691AStrong specificityThe test result is accurateAnalysis by subjecting material to chemical reactionColor/spectral properties measurementsForeign matterPhosphoric acid
The invention relates to potassium ion content measuring method by enzyme method and potassium ion diagnosis kit. It is utilized potassium ion to activate pyruvate kinase to make adenosine diphosphate react with phosphoric acid enol pyruvic acid to generate adenosine triphosphate, couple hexokinase glucose phosphate dehydrogenase to react, and reduce oxidized coenzyme to reduced coenzyme, and quantitatively response potassium ion content by measuring main wavelength 340nm absorbance vertical speed. The method is not influence for sodium ion in sample, and not polluted by internal and foreign matter. And it has high specificity, precise testing result, and good accuracy. The diagnosis kit is formed to double or three reagents to reduce cross influence, keep reagent stability to be convenient for storing for long time. The method can realize fast testing by common ultraviolet / visible light analyzer or semi / full automatic biochemical analyzer. Thus its testing cost is low; and it is convenient for popularizing and applying in industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778953AReflect contentHighlight substantive featuresMicrobiological testing/measurementChemistryAmount of substance
The invention is about the measuring method of Inorganic Phosphates and its diagnosis reagent box. Producing hypoxanthine by reacting nucleoside phosphorylase with carnine under the activation ofInorganic Phosphates in the sample of plasma or serum and so on, and producing hydroperoxide by reacting hypoxanthine and xanthine oxidase, and producing acetaldehyde by reacting hydroperoxide with alcohol under the existence of hydroperoxide enzyme, and then transferring oxidized coenzyme to reduced coenzyme by reacting acetaldehyde and aldehyde dehydrogenase. Testing the ascending range of dominant wave-length340nm absorbance and finally measuring the content of Inorganic Phosphates. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778952AHighlight substantive featuresSignificant progressMicrobiological testing/measurementFormazanTetrazole
The invention is about the measuring method of Inorganic Phosphates and its diagnosis reagent box. Reacting pyruvate dehydrogenase with pyruvate under the activation of Inorganic Phosphates in the sample of plasma or serum and so on, transferring oxidized mPVS to reduced mPVS, and transferring the colorless tetrazole salinization to formazan dyer with color with reduced mPVS, testing the ascending range of dominant wave-length380ú¡800nmabsorbance before and after the reaction and finally measuring the content of Inorganic Phosphates . This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
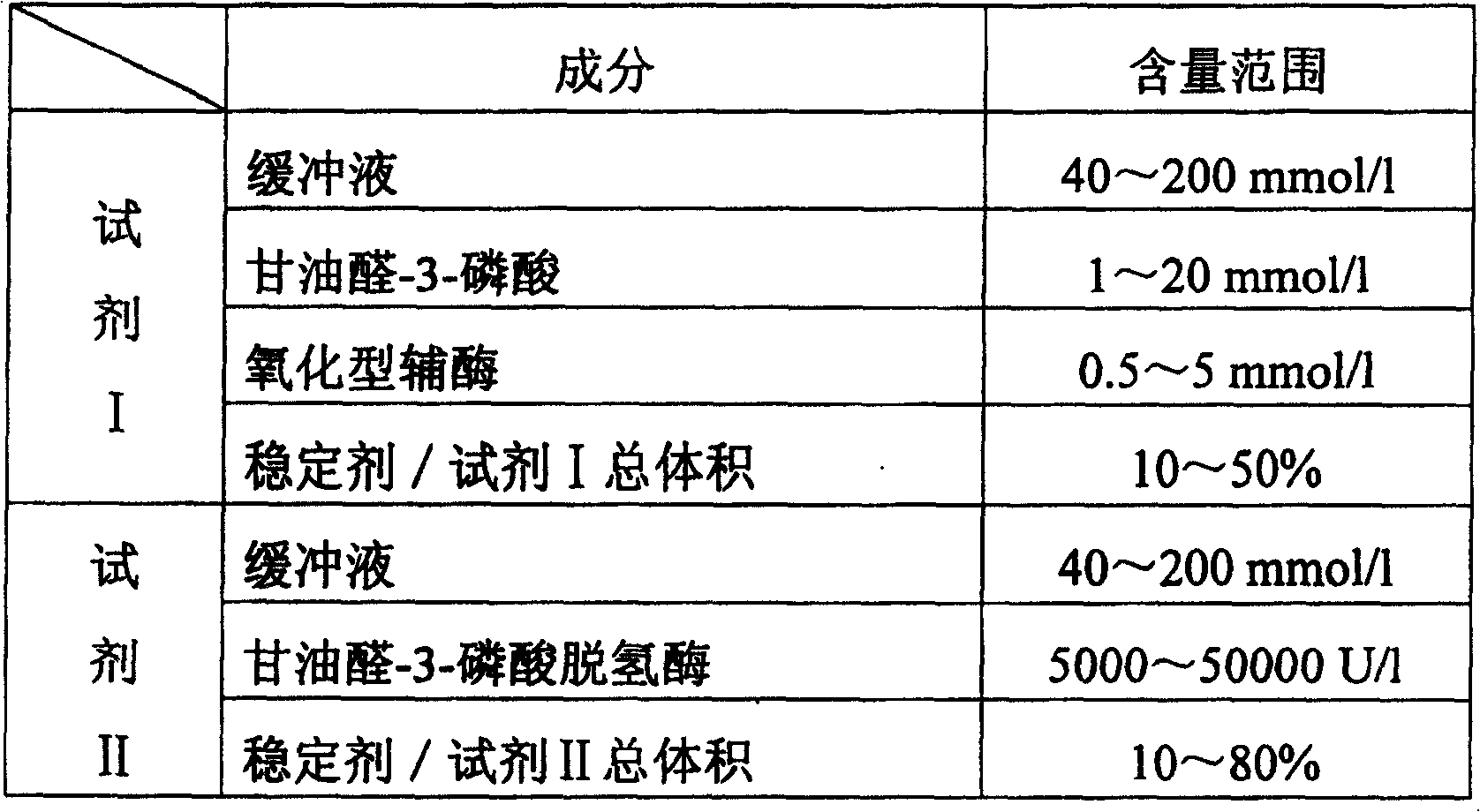
Determination of inorganic phosphorus and its diagnostic kit
InactiveCN100451656CReflect contentHighlight substantive featuresColor/spectral properties measurementsBiological testingSerum igeSerum samples
A method for determining inorganic phosphorus includes utilizing glyceraldehyde - 3 - phosphate dehydrogenase to react with glyceraldehyde - 3 - phosphate under activation of inorganic phosphorus in serum sample to make oxidized coenzyme be reduced coenzyme then detecting rise amplitude of absorbance at main wavelength of 340nm before and after reaction. A diagnostic kit is also disclosed.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
A metallographic method for displaying the three-dimensional structure of the silicon-rich phase in the aluminum-silicon coating
ActiveCN112945688BComplete three-dimensional structureFully display the three-dimensional structurePreparing sample for investigationMaterial analysis by optical meansMetal coatingMicroscopic observation
The invention belongs to the technical field of metal coating detection, and provides a method for displaying the three-dimensional structure of a silicon-rich phase in an aluminum-silicon coating by a metallographic method, including sample preparation, chemical corrosion and microscope observation. Coated steel plate, cut a part of it as a sample, and then inlaid and polished the sample in turn; (2) Chemical corrosion: put the sample prepared in step (1) into a corrosion solution for chemical corrosion, and the corrosion solution is made of an aqueous hydrochloric acid solution. (3) Microscope observation: After the chemical corrosion is completed, rinse the sample, dry it, and observe it with a ZISS metallographic microscope. The method provided by the invention can obtain a complete and clear three-dimensional structure of the silicon-rich phase, can fully display the growth of the silicon-rich phase, and has the characteristics of low detection cost, rapidity and simplicity.
Owner:TANGSHAN IRON & STEEL GROUP +1
An administer orally mixed nucleus glycosides as well as its preparing technics
ActiveCN101156936BComplete three-dimensional structureImprove biological activityOrganic active ingredientsMetabolism disorderMalt GrainSpatial structure
The invention belongs to the biology and pharmaceutical technical field, and relates to oral mixed nucleoside and the preparation process thereof. The invention mainly provides the oral mixed nucleoside and the preparation process thereof in order to overcome the problems existing in the prior art that the spatial structure of the product is damaged to a certain degree, the production cycle is longer, the operation is difficult, and the integrated cost is high. In order to overcome the problems existing in the prior art, the invention has the technical proposal that the oral mixed nucleoside is obtained through the following preparation processes, and the preparation processes comprise the steps that step one, malt roots are prepared; step two, pre-treatment: the malt roots are extracted through water, and filtered, after being preheated, phosphatase liquid is added to be prepared to be used; step three, hydrolytic reaction: purified water is preheated, and ribonucleic acid is added into the purified water, the pH value is adjusted, the mixed fluid in the step two is added into the purified water, the pH value is adjusted again, and the purified water is insulated and reacts; stepfour, the fluid is inactivated, the pH value is adjusted, and the fluid is filtered; step five, the fluid is concentrated and dried.
Owner:西安万隆制药股份有限公司
Method for displaying three-dimensional structure of silicon-rich phase in aluminum-silicon coating by metallographic method
ActiveCN112945688AComplete three-dimensional structureFully display the three-dimensional structurePreparing sample for investigationMaterial analysis by optical meansMetal coatingMicroscopic observation
The invention belongs to the technical field of metal coating detection, and provides a method for displaying a three-dimensional structure of a silicon-rich phase in an aluminum-silicon coating by a metallographic method, which comprises the steps of sample preparation, chemical corrosion and microscope observation, and specifically comprises the following steps: (1) sample preparation: taking an aluminum-silicon coating steel plate, cutting a part of the aluminum-silicon coating steel plate as a sample, and then sequentially carrying out embedding and polishing treatment on the sample; (2) chemical corrosion: putting the sample prepared in the step (1) into a corrosive liquid for chemical corrosion, wherein the corrosive liquid is prepared from a hydrochloric acid aqueous solution and a sodium chloride aqueous solution; and (3) microscope observation: after the chemical corrosion is completed, washing and drying the sample, and observing by using a ZISS metallographic microscope. According to the method provided by the invention, a complete and clear three-dimensional structure of the silicon-rich phase can be obtained, the growth condition of the silicon-rich phase can be fully displayed, and the method has the characteristics of low detection cost, rapidness and simplicity.
Owner:TANGSHAN IRON & STEEL GROUP +1
A kind of cmc nanofibrous film loaded with silver nanoparticles and preparation method thereof
ActiveCN105332163BEasy to prepareNo pollution in the processFilament/thread formingNon-woven fabricsFiberEnvironmental resistance
The invention discloses a carboxymethyl cellulose (CMC) nanofiber membrane loaded with silver nanoparticles and a preparation method thereof. The purpose is to provide a simple and pollution-free preparation method, and the prepared fiber membrane has excellent antibacterial properties and Biocompatible silver-loaded antibacterial nanofiber with only CMC as substrate and preparation method thereof. In the present invention, the mixed solution of polyethylene oxide (PEO) and CMC is used as the spinning stock solution to carry out electrospinning, and then the fiber film is soaked in a silver nitrate solution of a certain concentration, taken out and dried in the dark and placed in the The reduction is carried out in humid air at room temperature, and finally a nanofiber membrane of CMC nanofibers loaded with silver nanoparticles is prepared. The fiber membrane prepared by the invention has excellent antibacterial properties and biocompatibility, and the structure and mechanical properties of the fiber membrane itself are well maintained, and is a preferred material in the field of wound dressing, and the preparation method is simple, economical and environment-friendly.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY +1
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778942AStrong specificityQuantitatively reflect contentMicrobiological testing/measurementUltravioletWavelength
The invention is about the measuring method of Inorganic Phosphates and its diagnosis reagent box. Producing hypoxanthine by reacting nucleoside phosphorylase with carnine under the existence of Inorganic Phosphates , then producing hydroperoxide by reactinghypoxanthine with xanthine oxidase, and then reacting hydroperoxide with peroxidaseand oxidating the colorless reduced chromogen combination to quinoneimine chromogen or indamide chromogen dyer with color, testing the variation of dominant wave-length400ú¡600nmabsorbance during the reaction and finally measuring the content of Inorganic Phosphates . This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com