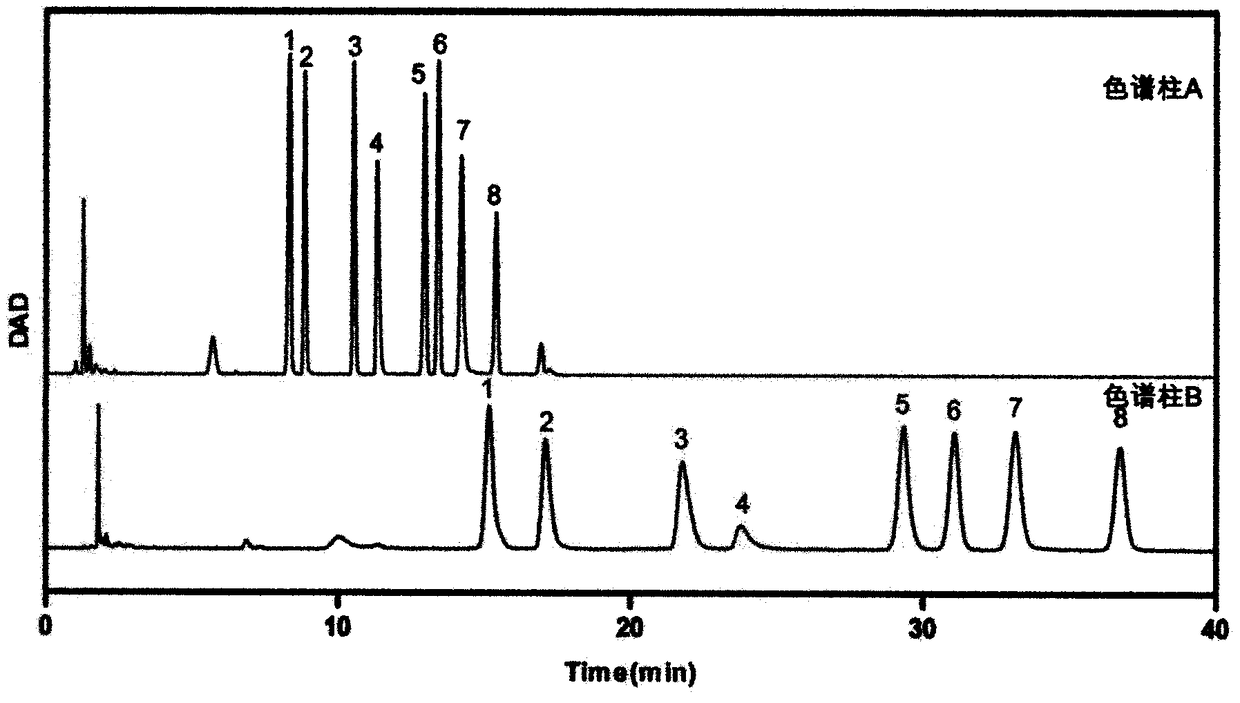
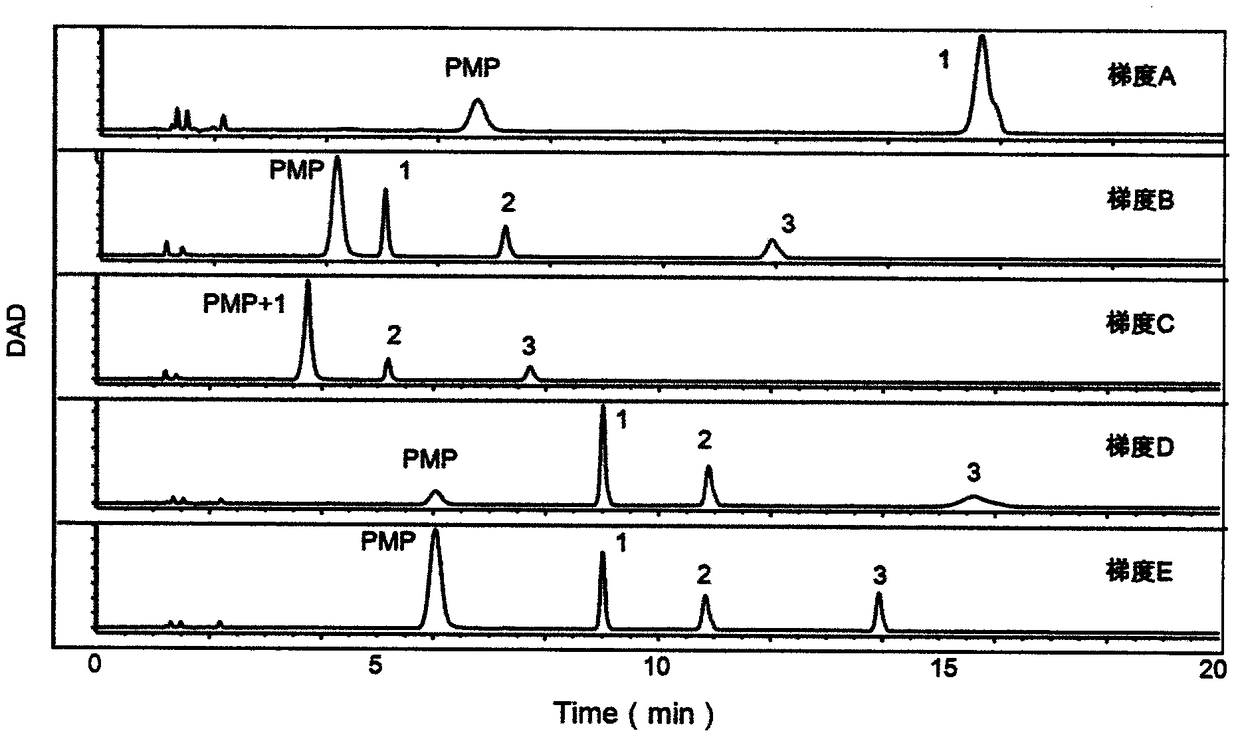
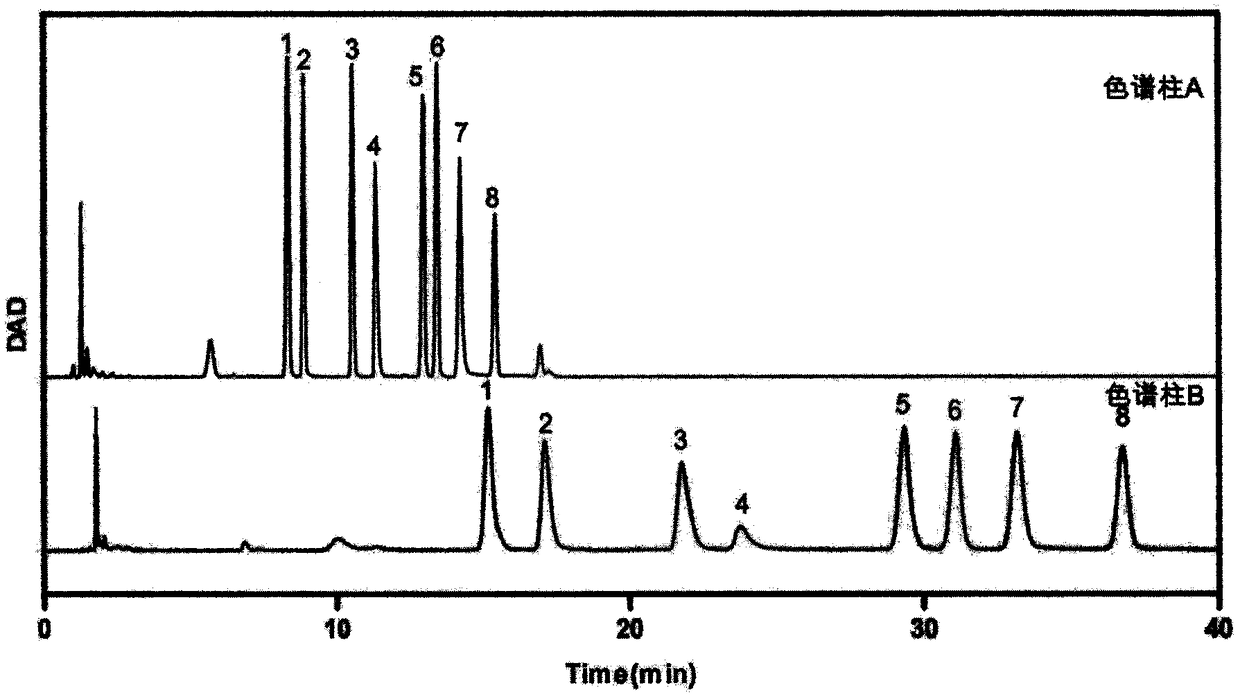
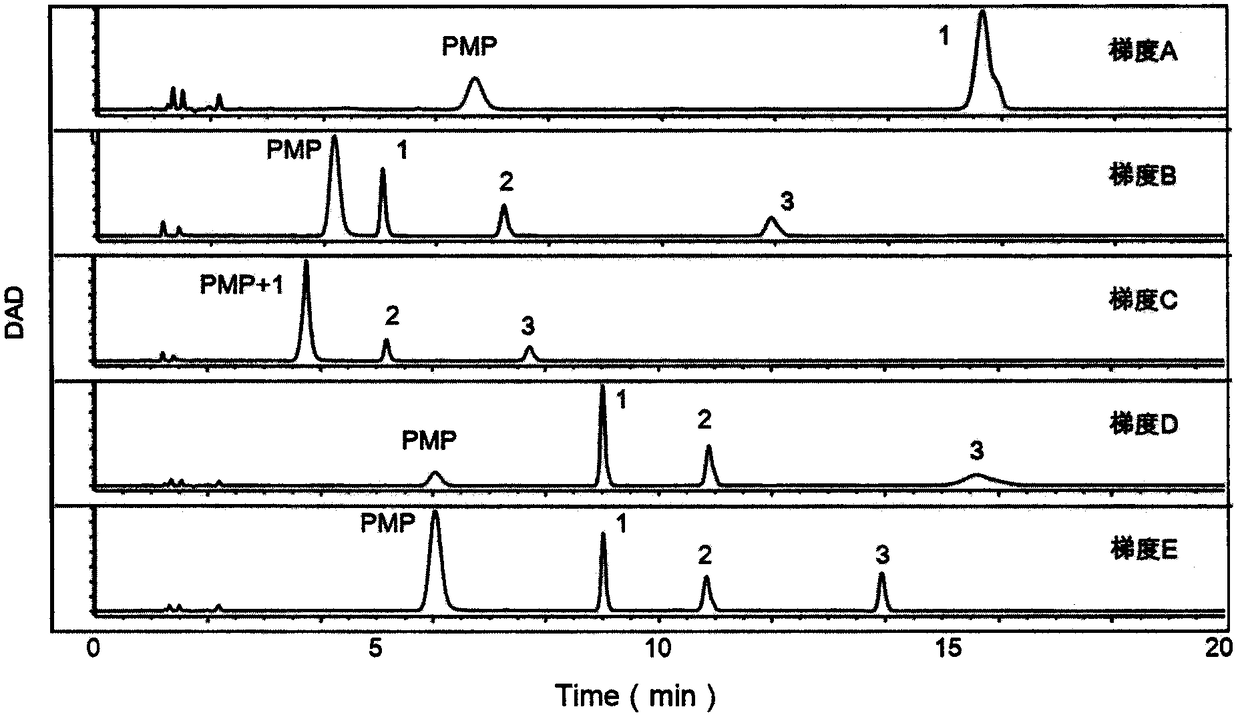
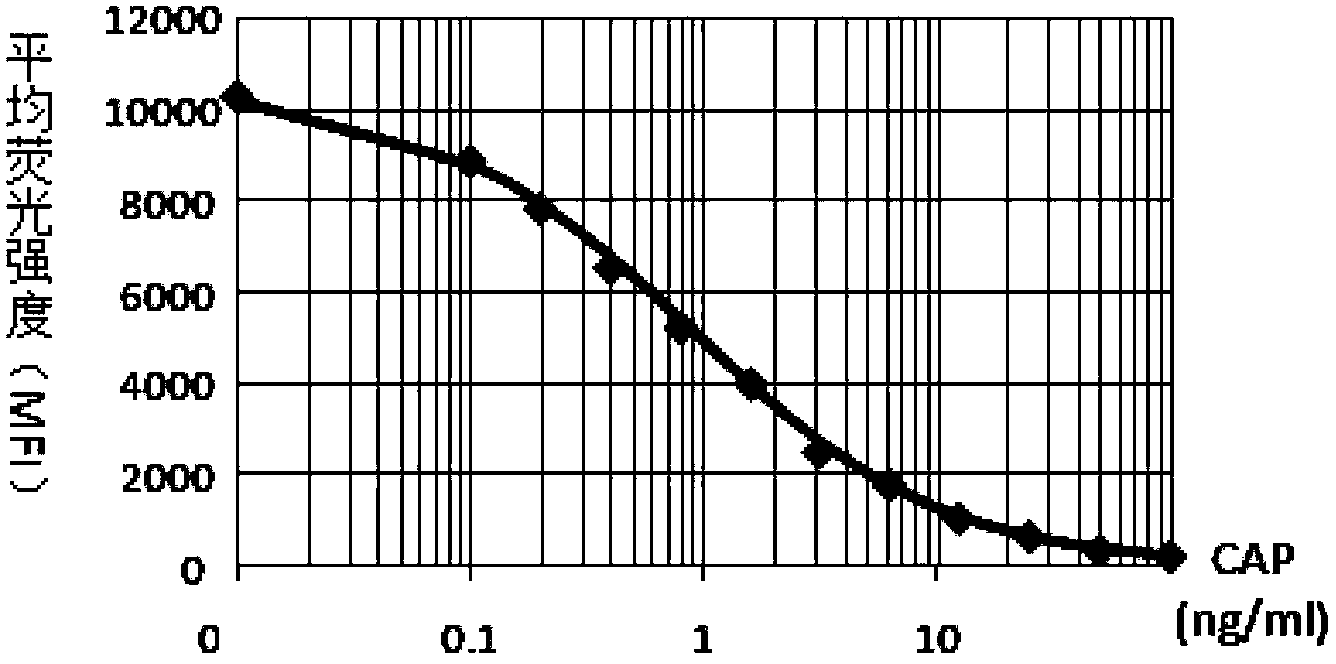
Patents
Literature
36results about How to "Less serum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Serum copper, iron and zinc rapid sensitive detection kit and preparing method thereof
InactiveCN1635379ALess serumSimple and fast operationMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsTest agentLength wave
This invention discloses a test agent box to measure the contents of copper, iron and zinc in blood serum and provides the process method of the agent box. The agent box in this invention dissociates the protein with copper, iron and zinc in blood serum in the buffer liquid system with the surface activity agent, which is compound with the pyridylazo organic compound to make color. The color complex compound has the longest wavelength at 550-600nm.
Owner:浙江东瓯诊断产品有限公司
Porcine reproductive and respiratory syndrome virus antibody competitive AlphaLISA detection kit and detection method thereof
The invention discloses a porcine reproductive and respiratory syndrome virus (PRRSV) antibody competitive AlphaLISA detection kit and a detection method thereof. The PRRSV antibody competitive AlphaLISA detection kit comprises a donor bead, an acceptor bead, a PRRSV N protein monoclonal antibody and an N protein antibody having a His label. The competitive AlphaLISA detection method for detecting a PRRSV antibody is established by optimizing test reaction conditions comprising the donor bead, the acceptor bead, the monoclonal antibody, the antigen and serum is established in the invention. The PRRSV antibody detection kit has the advantages of good specificity, high sensitivity, less serum consumption, no washing, no hemolysis influence, low detection cost and short detection time.
Owner:INSPECTION & QUARANTINE TECH CENT OF CHONGQING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Competitive Alpha LISA (linked immuno sorbent assay) detection kit for classical swine fever virus (CSFV) antibody and detection method thereof
InactiveCN103499693AStrong specificityHigh sensitivityBiological testingImmunoassaysAntigenSerum ige
The invention discloses a competitive Alpha LISA (linked immuno sorbent assay) detection kit for a classical swine fever virus (CSFV) antibody and a detection method thereof. The detection kit comprises donor microspheres, receptor microspheres, a swine fever virus E2 protein monoclonal antibody and an E2 protein antigen with a His label. A competitive Alpha LISA detection method for the CSFV antibody is created by optimizing test reaction conditions such as the donor microspheres, the receptor microspheres, the monoclonal antibody, the antigen and serum. The kit for detecting the CSFV antibody is good in specificity, high in sensitivity, low in usage amount of the serum, low in detection cost and short in detection time, does not need to be washed and can not be influenced by hemolysis.
Owner:INSPECTION & QUARANTINE TECH CENT OF CHONGQING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Competitive Alpha LISA (linked immuno sorbent assay) detection kit for porcine circovirus (PCV) 2 antibody and detection method thereof
The invention discloses a competitive Alpha LISA (linked immuno sorbent assay) detection kit for a porcine circovirus (PCV) 2 antibody and a detection method thereof. The detection kit comprises donor microspheres, receptor microspheres, a PCV 2 Cap protein monoclonal antibody and a Cap protein antigen with a His label. A competitive Alpha LISA detection method for the PCV antibody is created by optimizing test reaction conditions such as the donor microspheres, the receptor microspheres, the monoclonal antibody, the antigen and serum. The kit for detecting the PCV antibody is good in specificity, high in sensitivity, low in usage amount of the serum, low in detection cost and short in detection time, does not need to be washed and can not be influenced by hemolysis.
Owner:INSPECTION & QUARANTINE TECH CENT OF CHONGQING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Reagent kit for detecting immunoglobulin m (IgM) AlphaLISA for resisting enterovirus71 capsid protein1
The invention provides a reagent kit for detecting immunoglobulin m (IgM) AlphaLISA for resisting enterovirus71 capsid protein1, which comprises donor microbeads, receptor microbeads and biotinylated enterovirus71 capsid protein1 antibodies. Optimal dose of biotinylated vitamin P (VP)1 is fished out to be 37.5ng through the enzyme-linked immuno sorbent assay (ELISA), and a detecting method is built based on the IgM AlphaLISA for resisting enterovirus71 (EV71) VP1 through optimization of the donor microbeads, the receptor microbeads, blood serum and other test reaction conditions. The reagent kit is used for detecting the enterovirus71, is good in specificity, high in sensitivity and small in blood serum dosage, does not need to be washed, and cannot be influenced by hemolysis.
Owner:中国疾病预防控制中心病毒病预防控制所
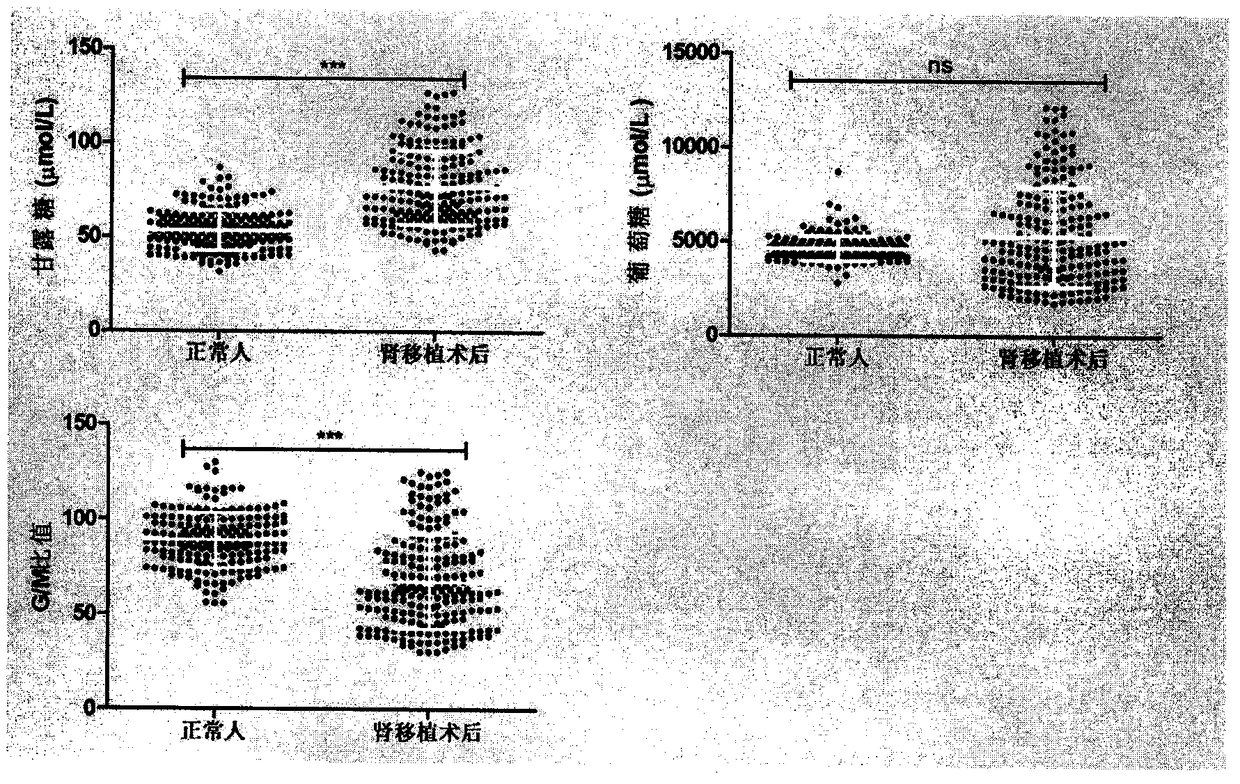
Method of biomarker for identifying renal transplantation prognosis and detection kit for identifying renal transplantation prognosis
InactiveCN109187814AThe pre-processing process is simpleEasy to handleComponent separationBlood collectionOrganism
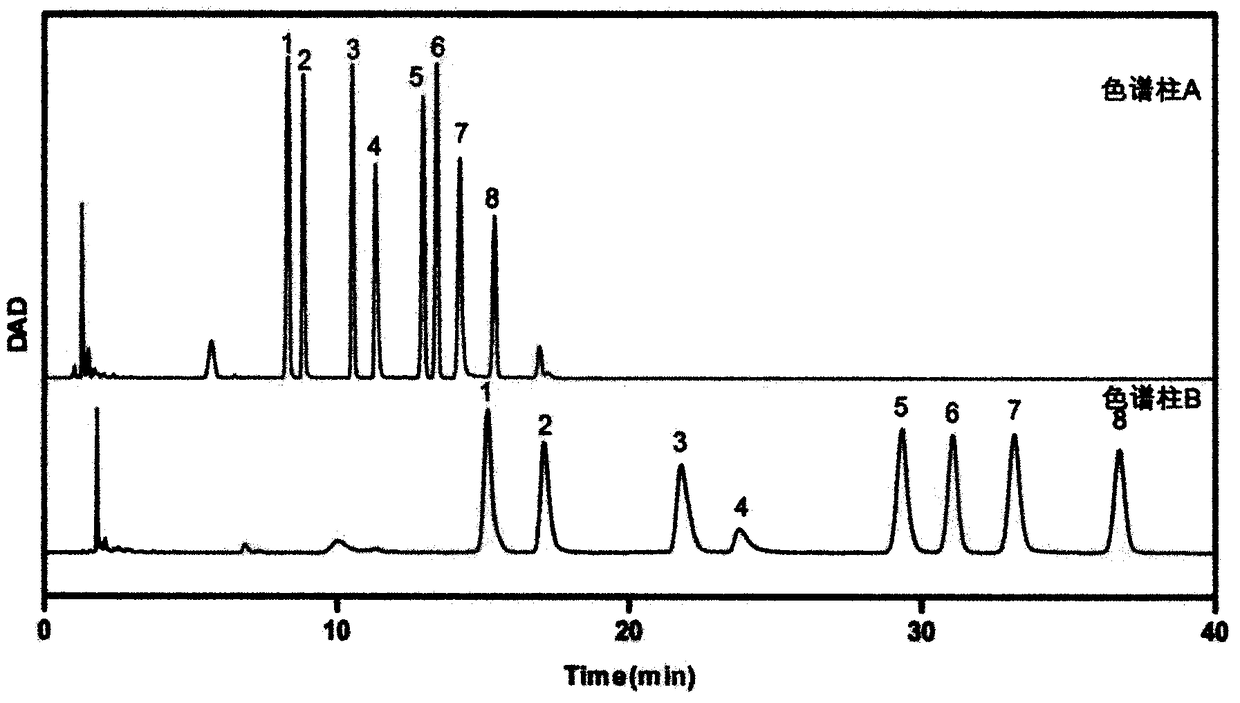
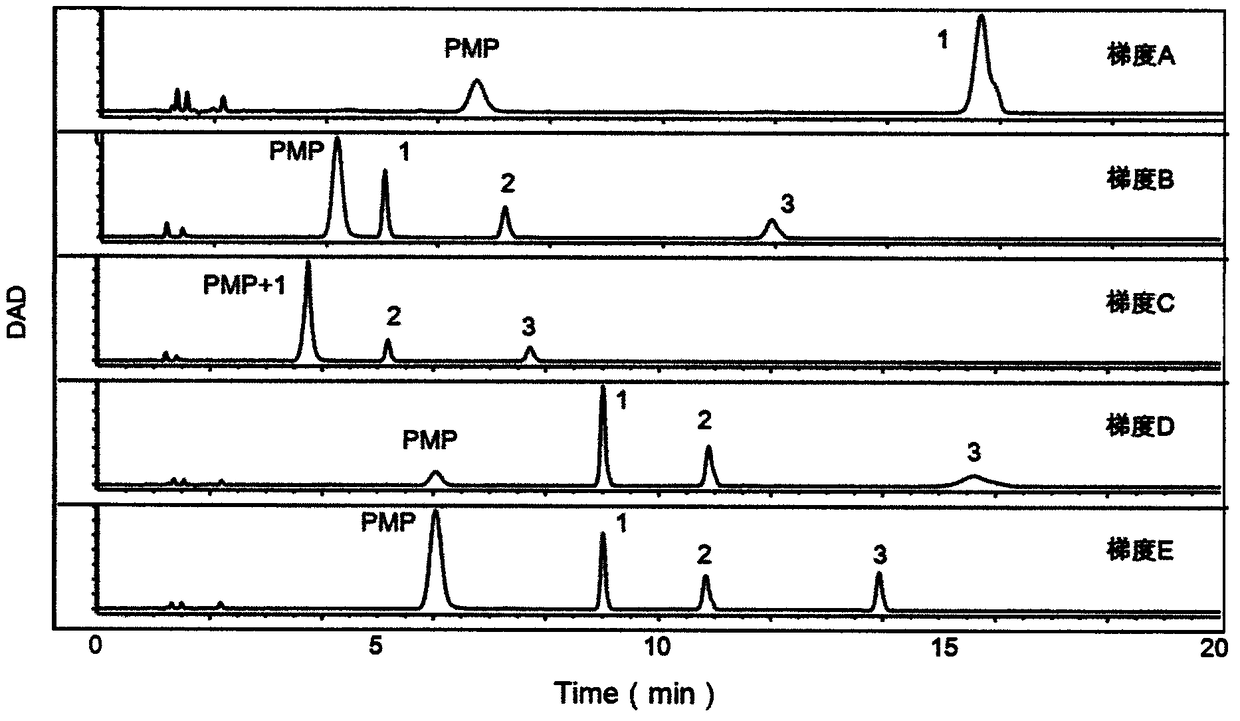
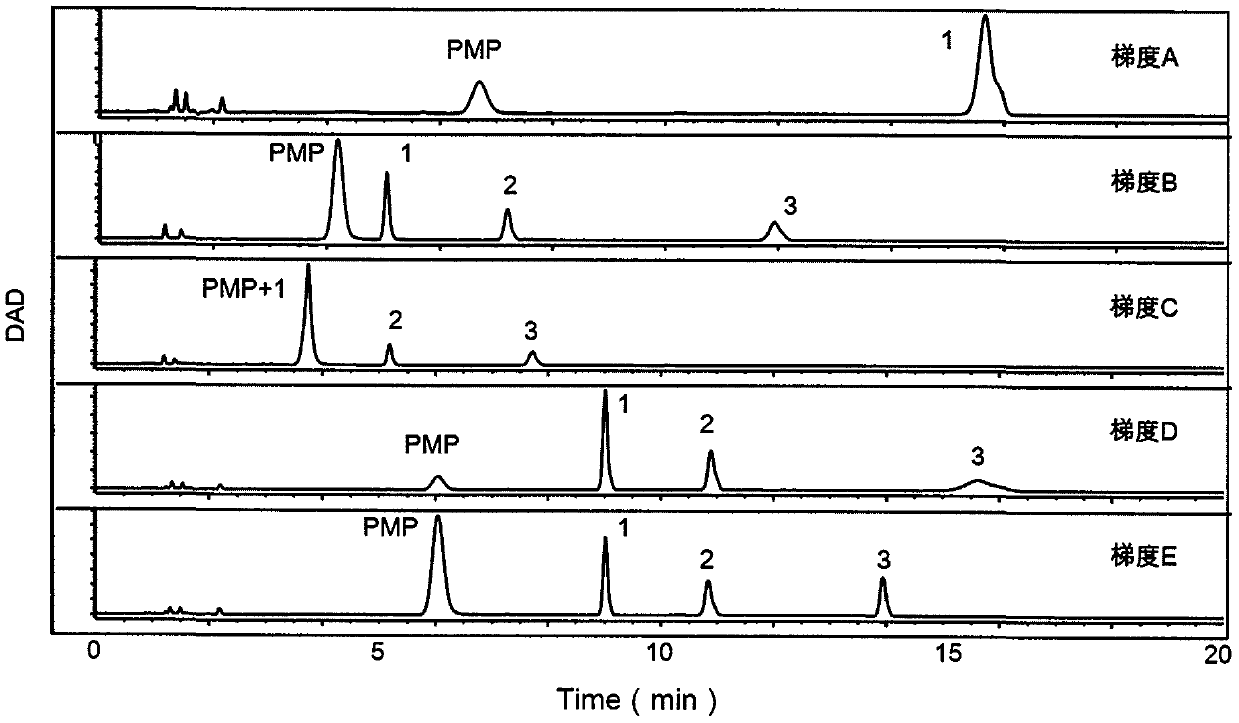
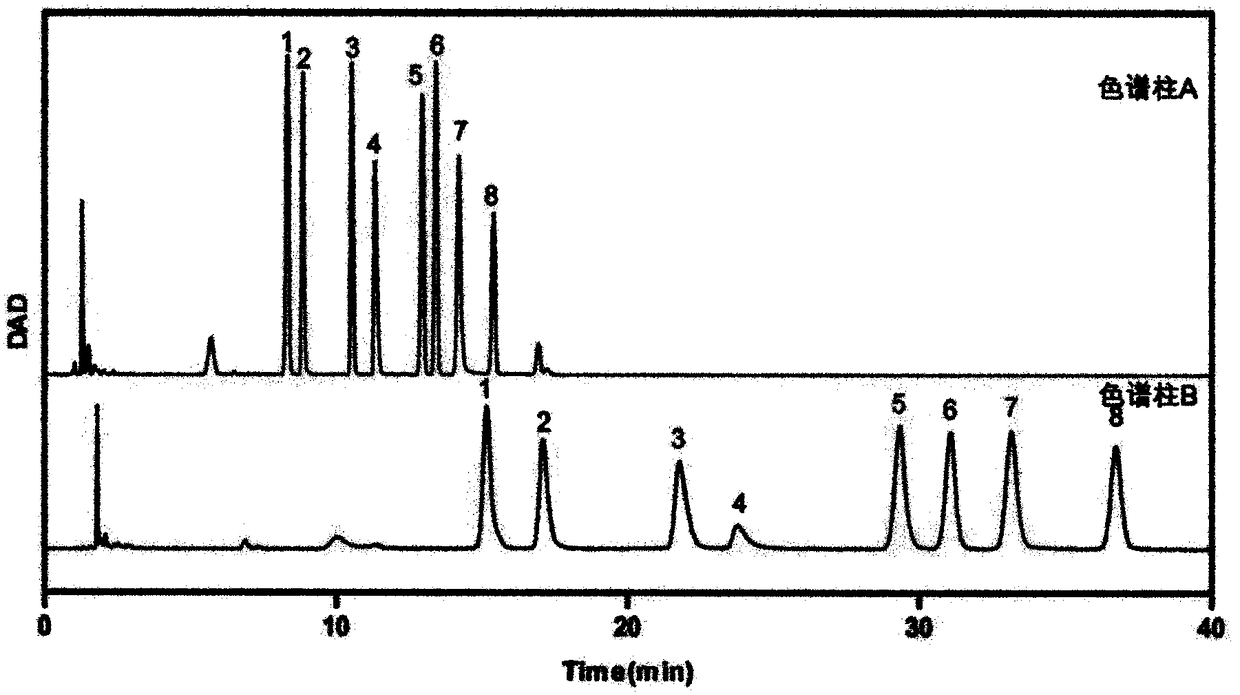
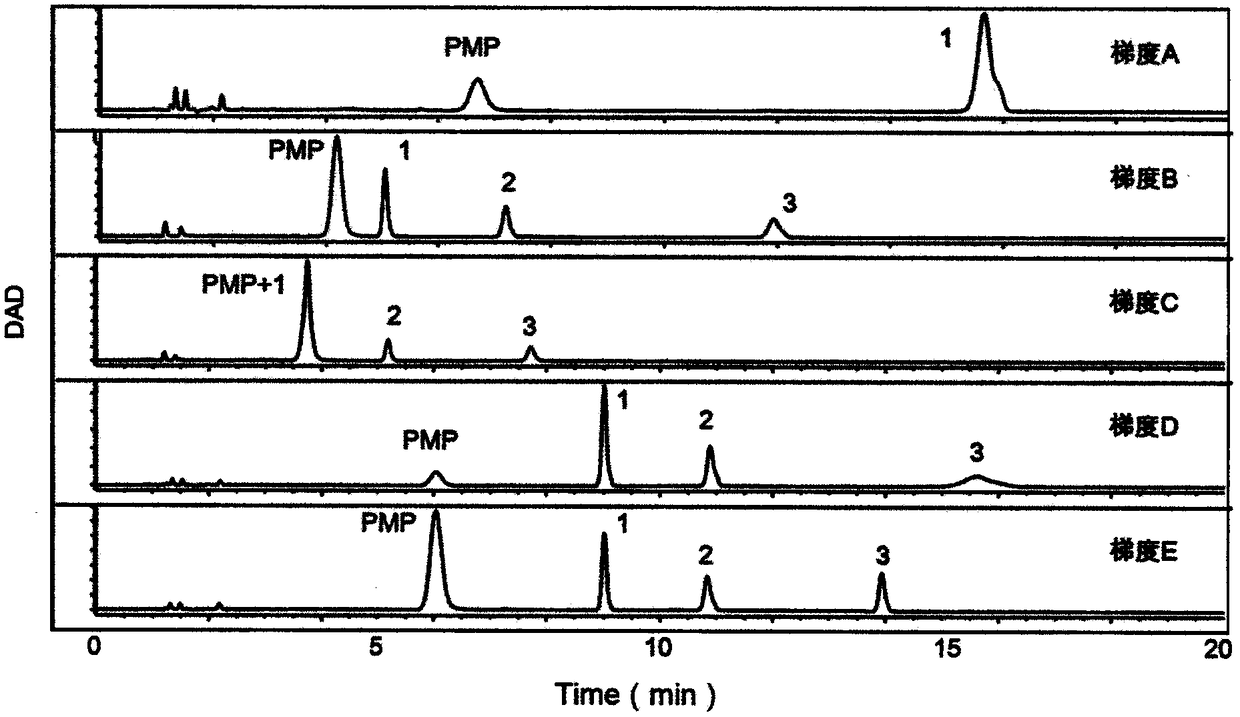
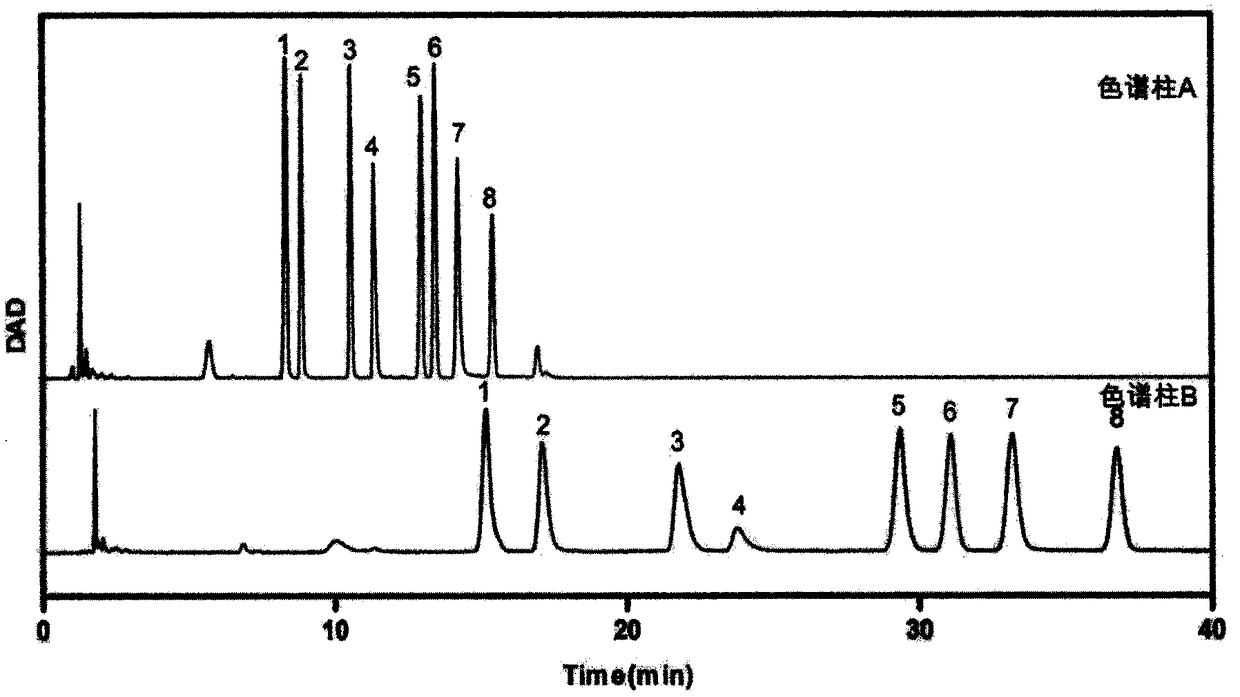
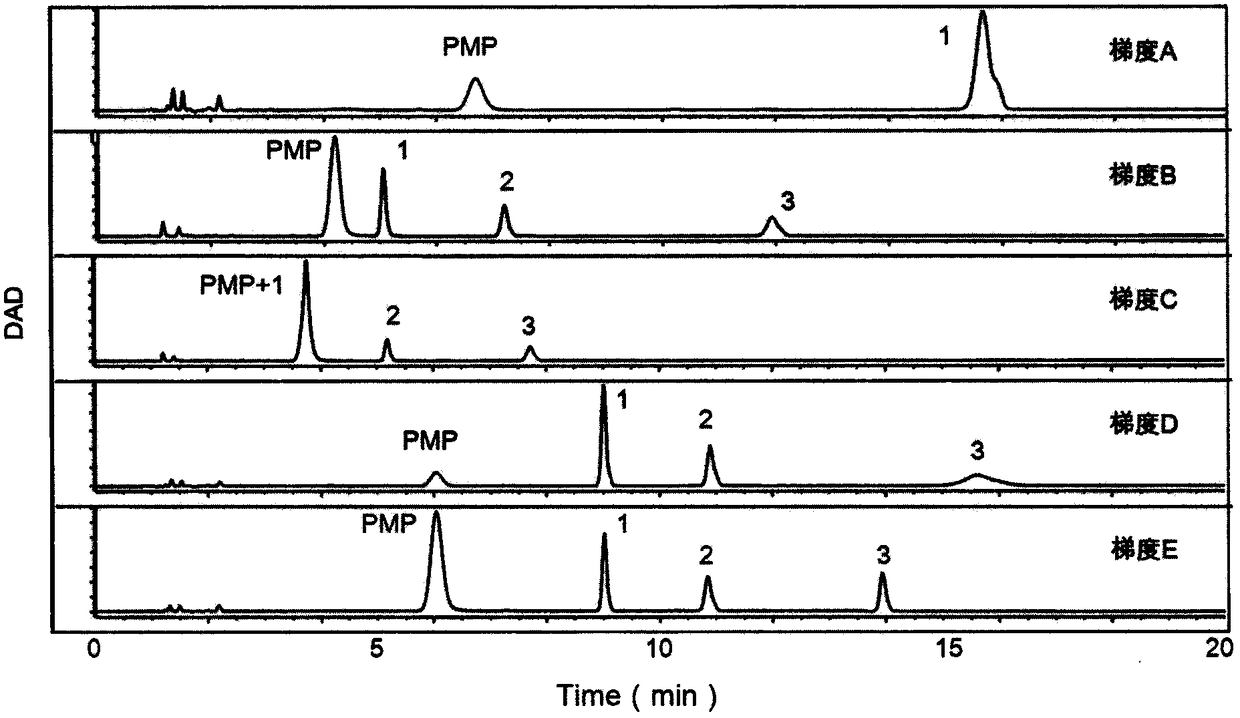
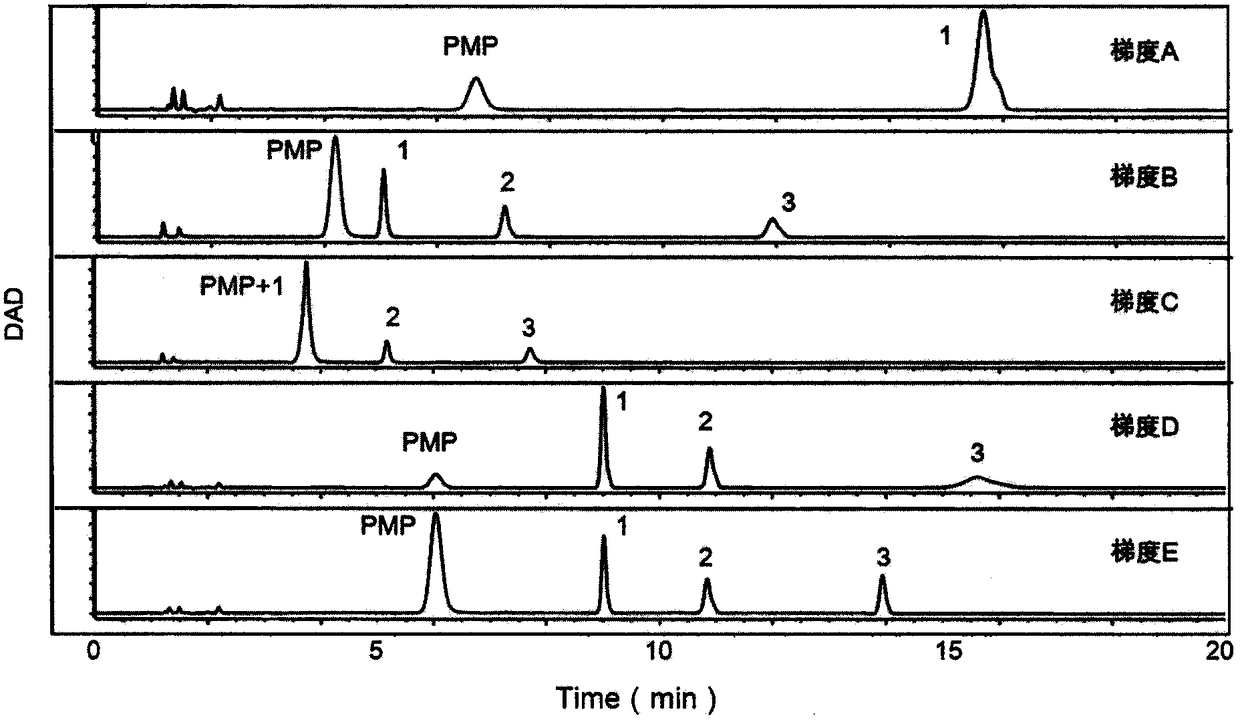
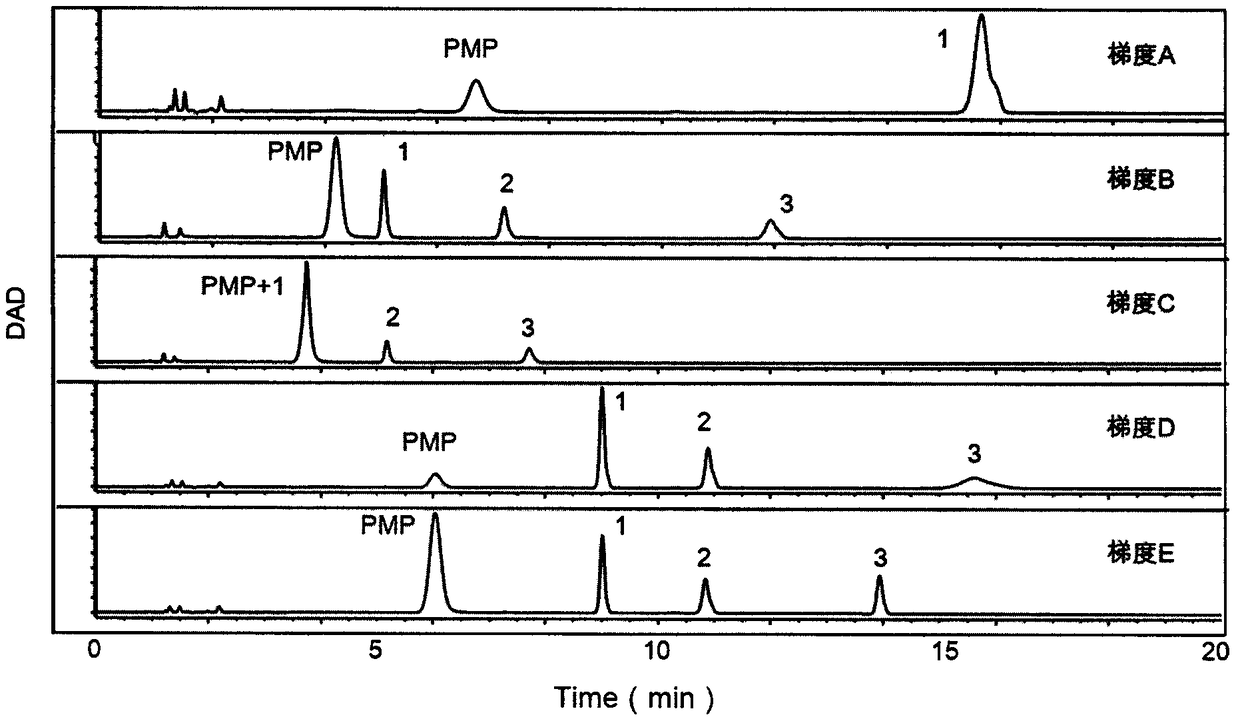
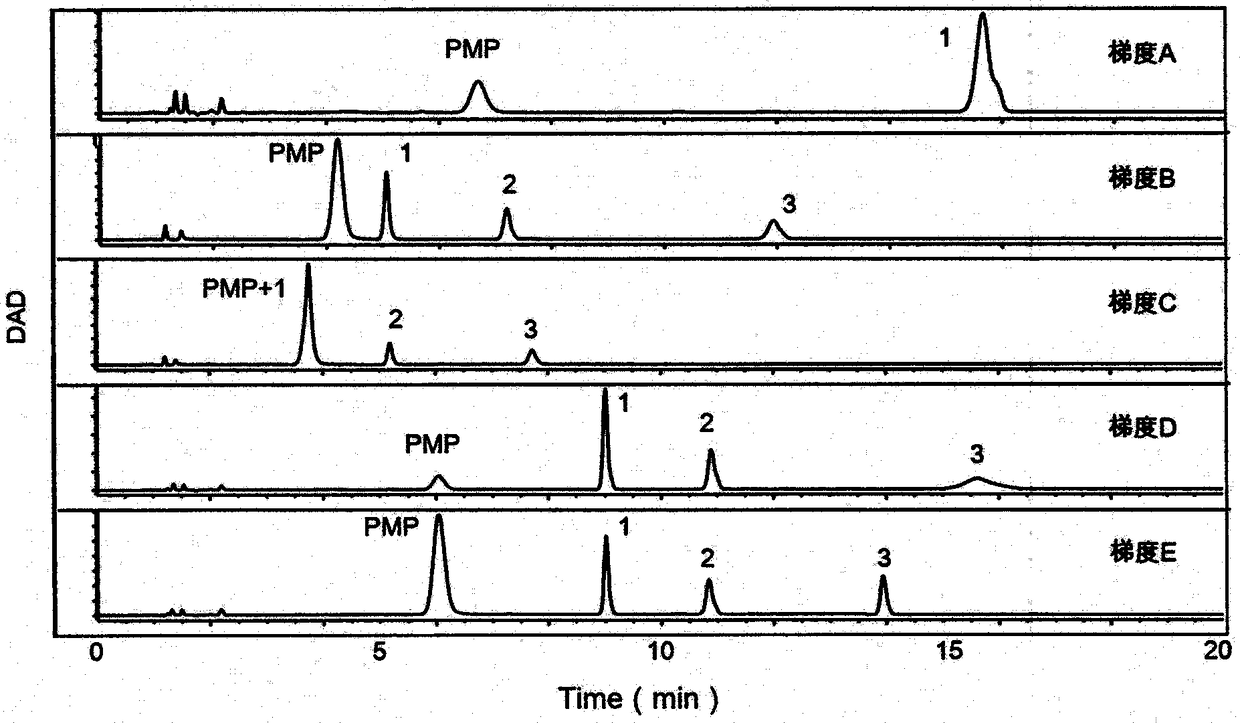
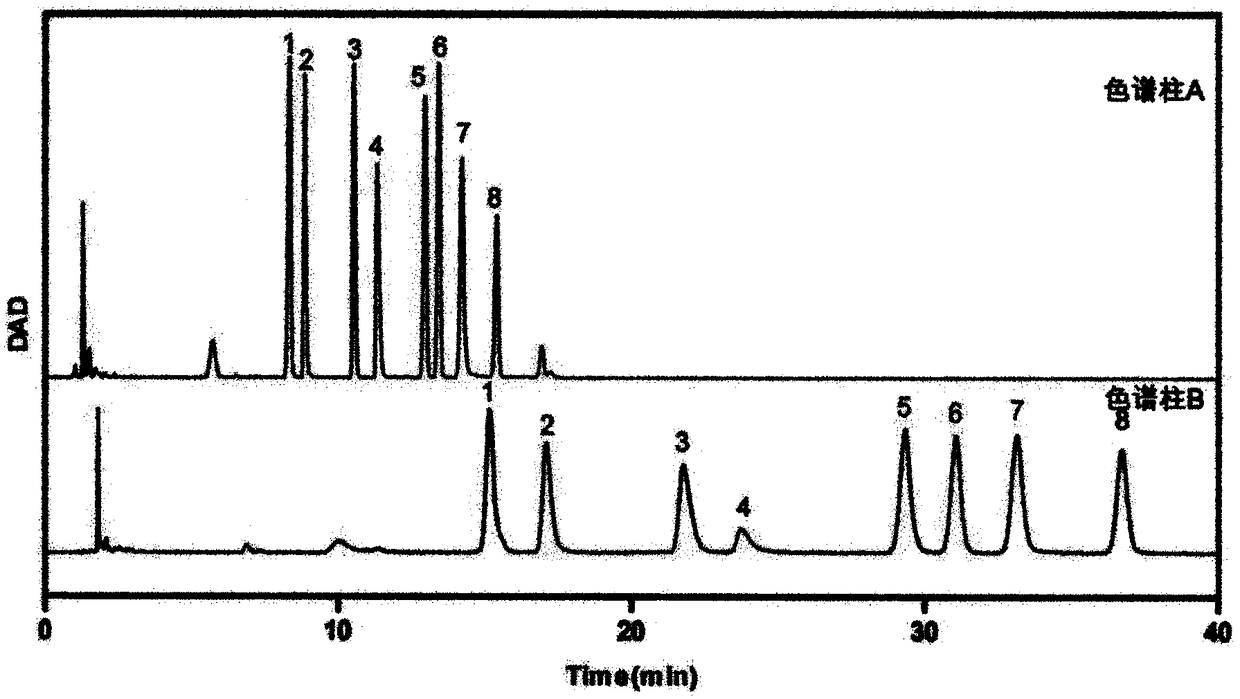
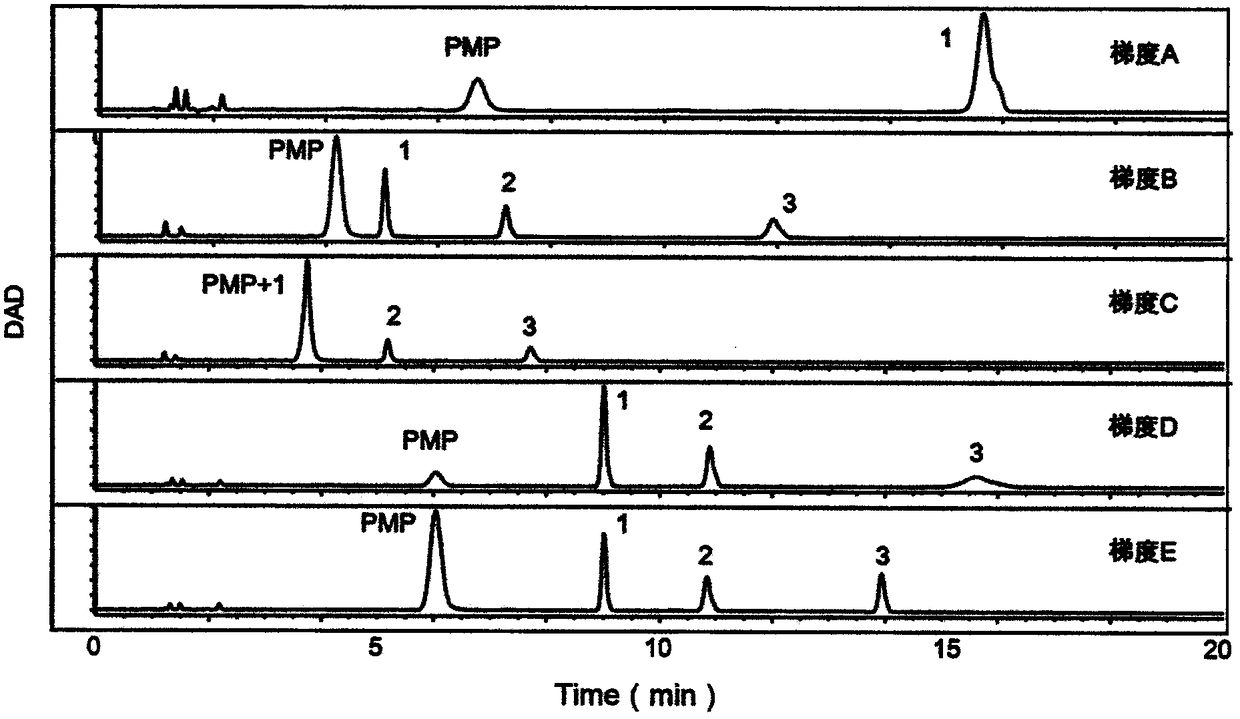
The invention provides a method of a biomarker for identifying renal transplantation prognosis and a detection kit for identifying the renal transplantation prognosis. The biomarker is a ratio of freemannose and glucose obtained by pre-column 1-phenyl-5-methyl pyrazolone (PMP) derivatization high performance liquid chromatography in serum. The detection method is a pre-column PMP derivatization high performance liquid chromatography method. The technical scheme has the advantages that the pretreatment is simple, the analysis time is short, the instrument price is reasonable, the conventionaluse requirements are met, the operation steps are simple and easy to learn, the accuracy of a detection result is high, only blood collection is needed, the needed serum amount is extremely small, theblood collection amount is smaller than 1 mL, and the like. The obtained result shows that the analysis method can quickly quantify the free mannose and glucose in the serum of a renal transplantation prognosis patient, and has very important significance for researching a relation between the free mannose and glucose in the serum and the renal transplantation prognosis, and finding a novel clinical prognosis monitoring marker for the renal transplantation prognosis.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Low serum culture medium for BHK-21 cell culture and corresponding virus production
InactiveCN110643568APromote growthGood growthCulture processMicroorganism based processesHydrolysateArginine
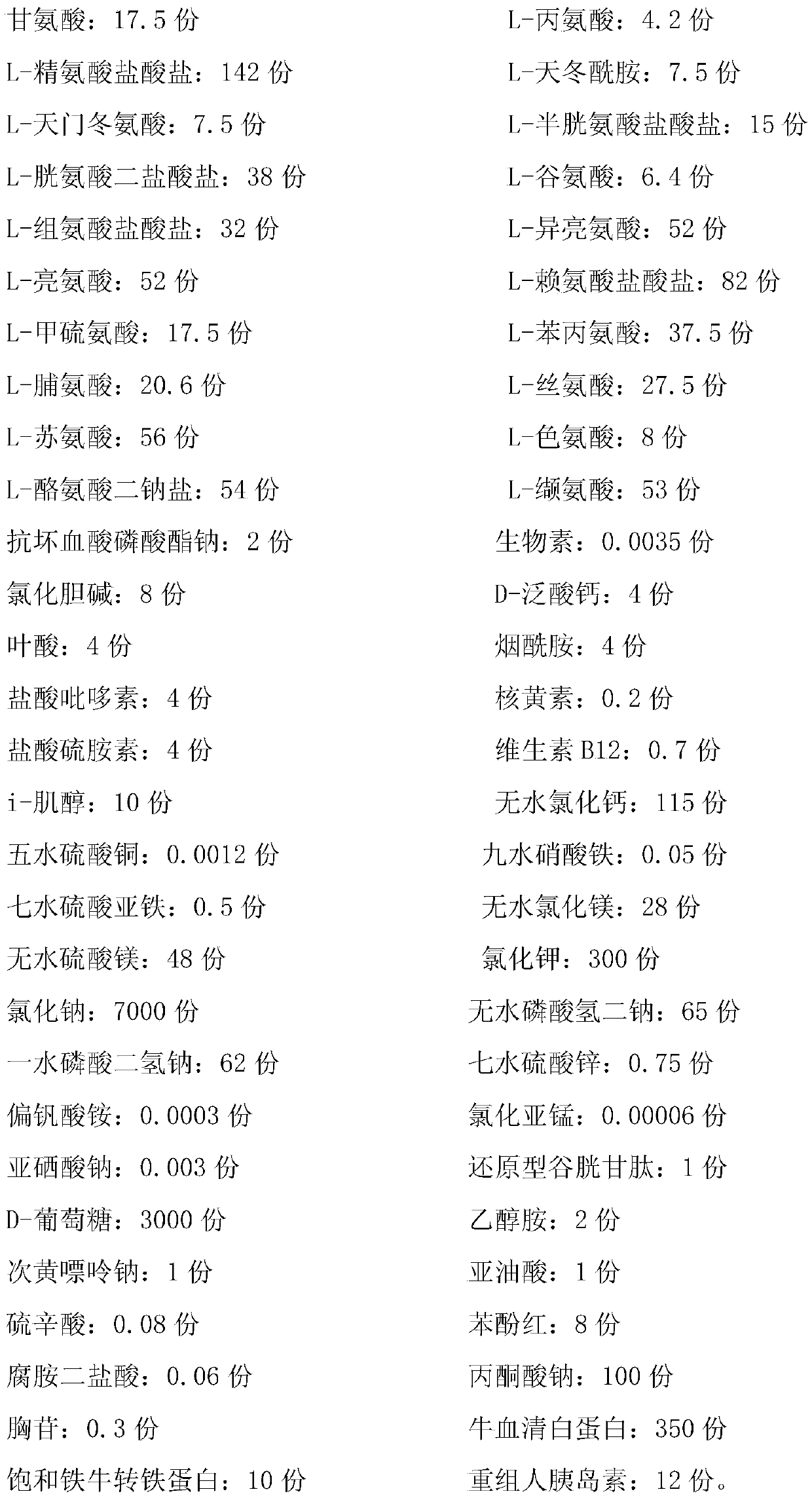
The invention discloses a low serum culture medium for BHK-21 cell culture and corresponding virus production. The low serum culture medium is prepared from the following components: amino acid, a vitamin, inorganic salts, a protein, microelements and auxiliary components; the amino acid comprises glycine, L-arginine monohydrochloride, L-asparaginate, L-glutamic acid, L-glutamine, L-lysine hydrochloride and L-serine; and the protein comprises 4-HEPES, sodium dihydrogen phosphate monohydrate, milk protein hydrolysate, bovine serum albumin and recombinant human insulin. According to the low serum culture medium for the BHK-21 cell culture and the corresponding virus production, serum is no longer needed to be added in the maintenance stage of a pseudorabies vaccine produced from a BHK-21 cell, dosage of the serum is further reduced, the good growth state of cells can still be maintained, virus titer is improved, reduction of poison value of a semi-finished product is small, and preservation time of the semi-finished product is prolonged.
Owner:山东巨山能源科技有限公司
Method for united typing detection of porcine contagious pleuropneumonia antibody and kit
InactiveCN101858912AMaintain native conformationHigh sensitivityFluorescence/phosphorescenceStatistical analysisBOAR
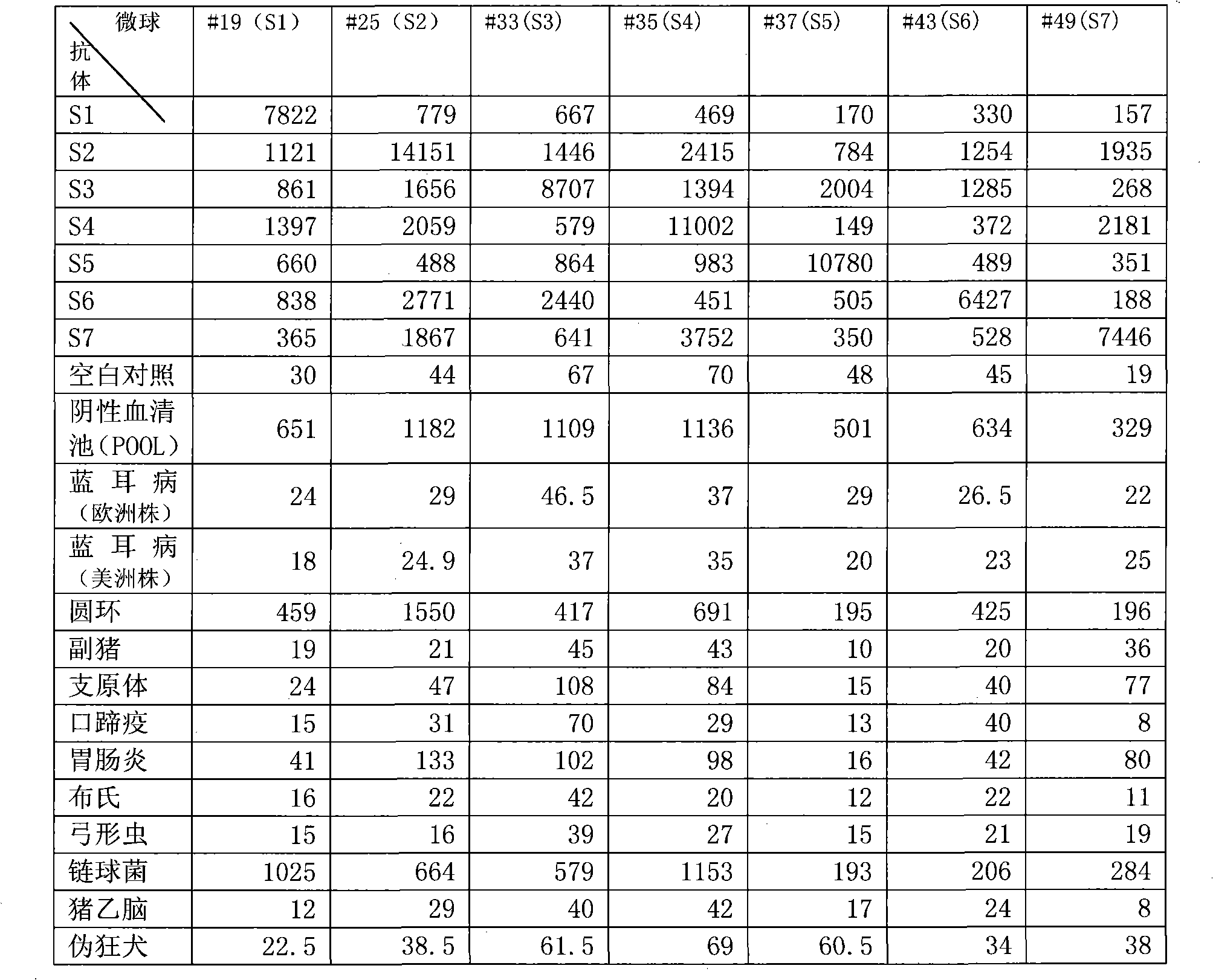
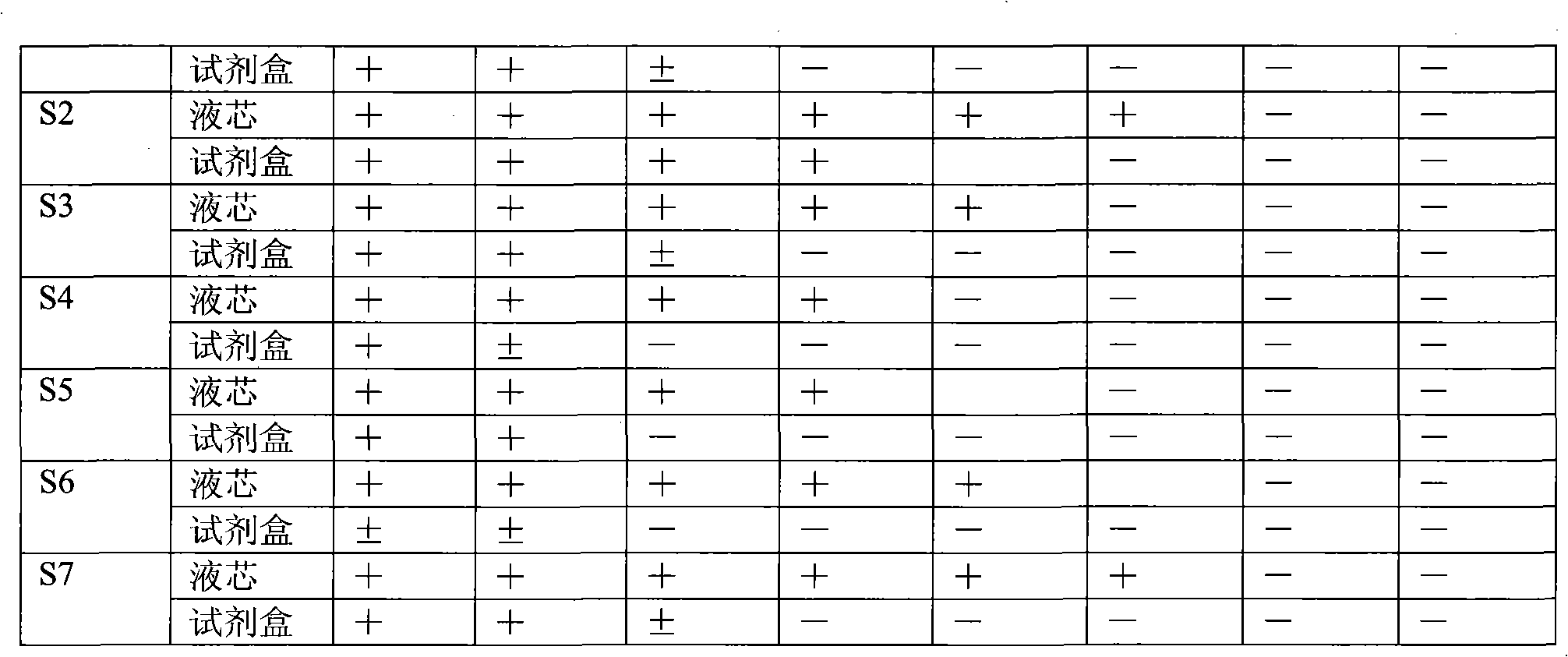
The invention discloses a method for united typing detection of a porcine contagious pleuropneumonia antibody. The method comprises the following steps: firstly preparing actinobacillus pleuropneumoniae polysaccharide antigen and rabbit anti-actinobacillus pleuropneumonia hyper-immune serum, and then purifying; coupling a liquid-phase chip microballoon by utilizing the purified rabbit anti-actinobacillus pleuropneumoniae hyper-immune serum, building a method for typing detection of the liquid-phase chip of the porcine pleuropneumonia antibody according to a double-sandwich ELISA principle, and determining the optimum experimental condition; and finally determining the threshold for positive and negative judgment of the liquid-phase chip through statistical analysis. In addition, the invention also discloses a kit for united typing detection of the liquid-phase chip of the porcine contagious pleuropneumoniae antibody. The invention can simultaneously carry out typing detection of the S1-S7-type serum antibody of porcine contagious pleuropneumonia, and the whole reaction can be completed within 3 hours; and the method has the characteristics of rapidly, sensitively, specifically andsimultaneously detecting a plurality of serum types, thus the method can be used for preliminarily screening entry and exit boars and diagnosing and monitoring porcine contagious pleuropneumoniae in hogpens of China.
Owner:SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C
A method of simultaneous detection of medroxyprogesterone acetate and diethylstilbestrol in aquatic products
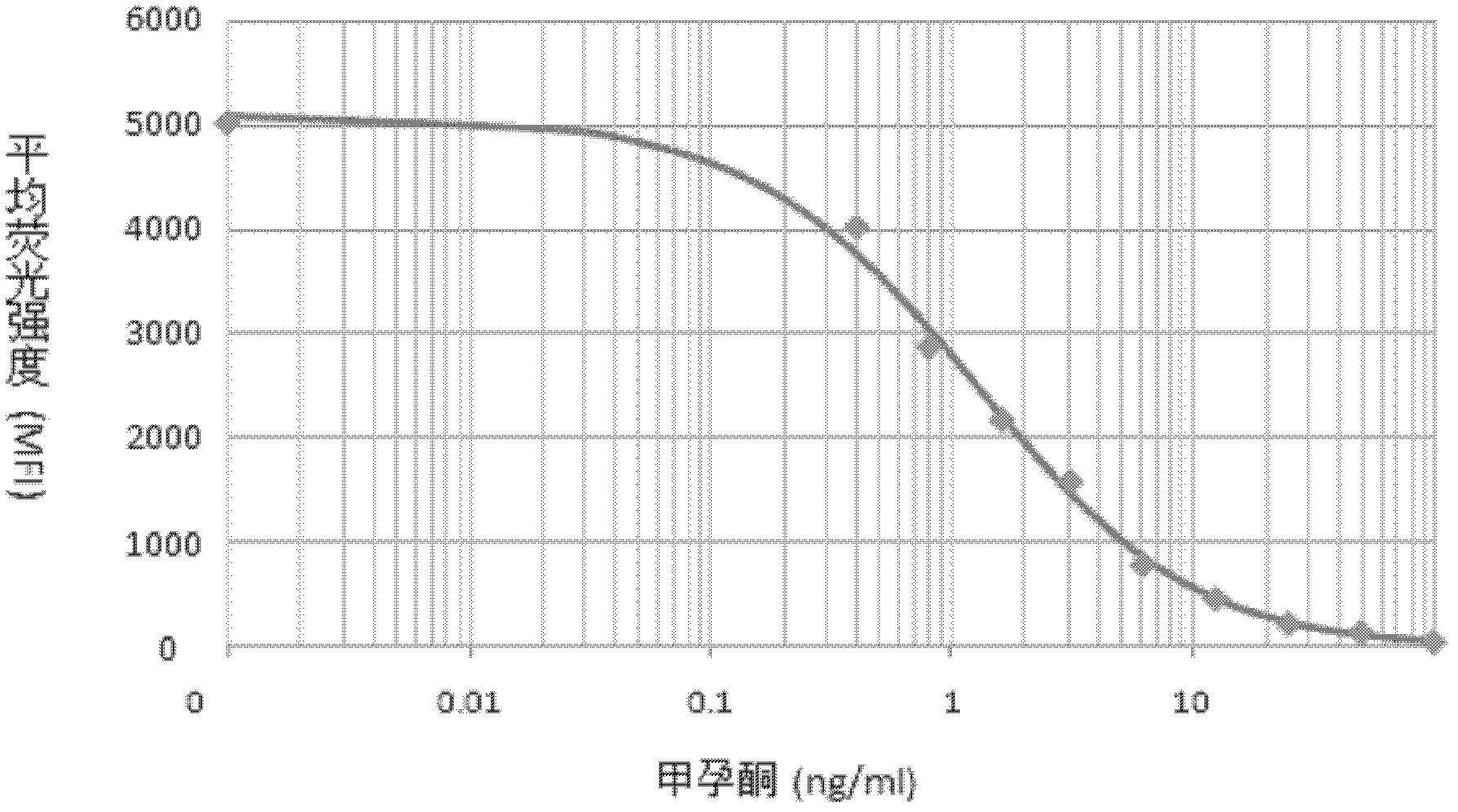
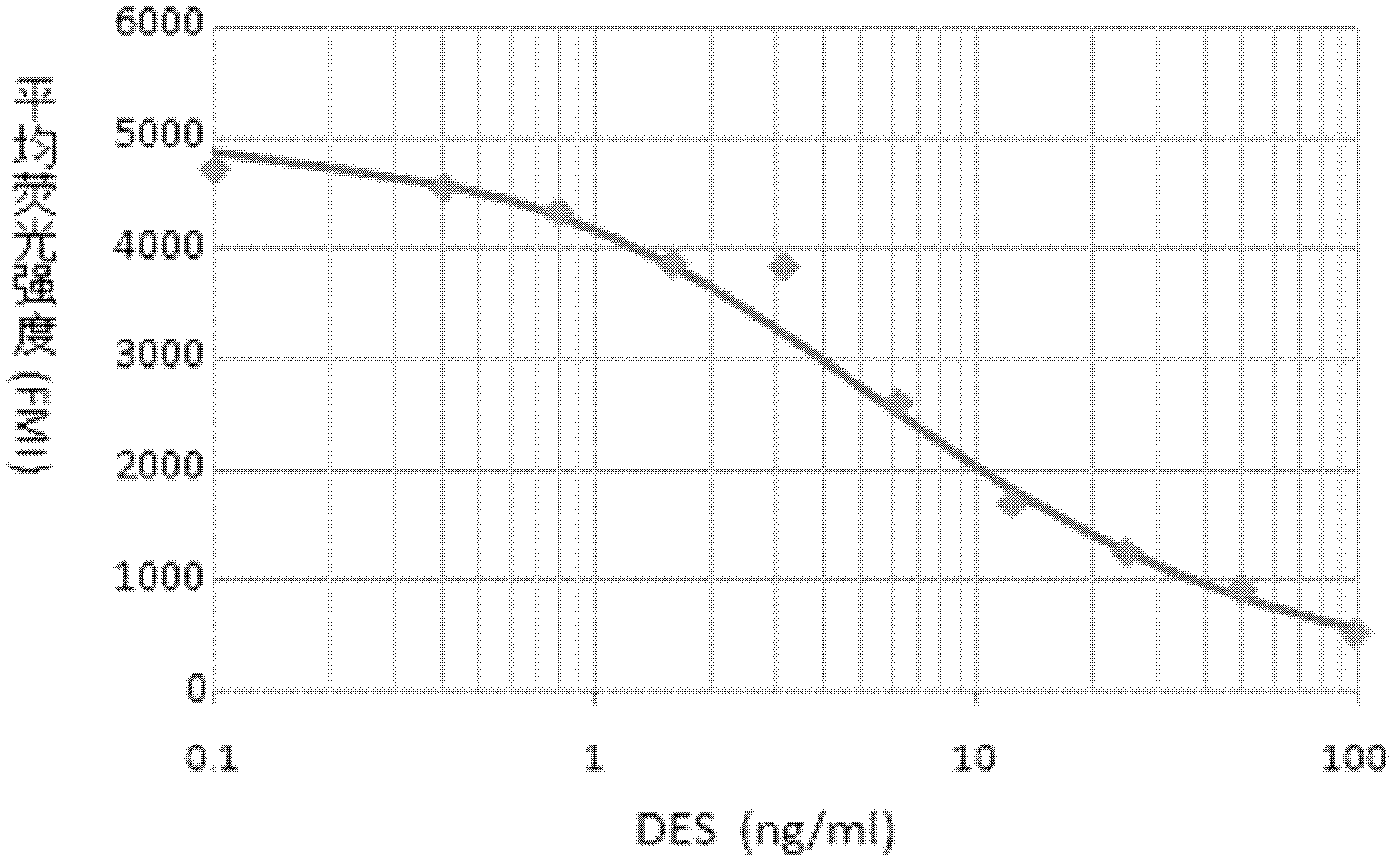
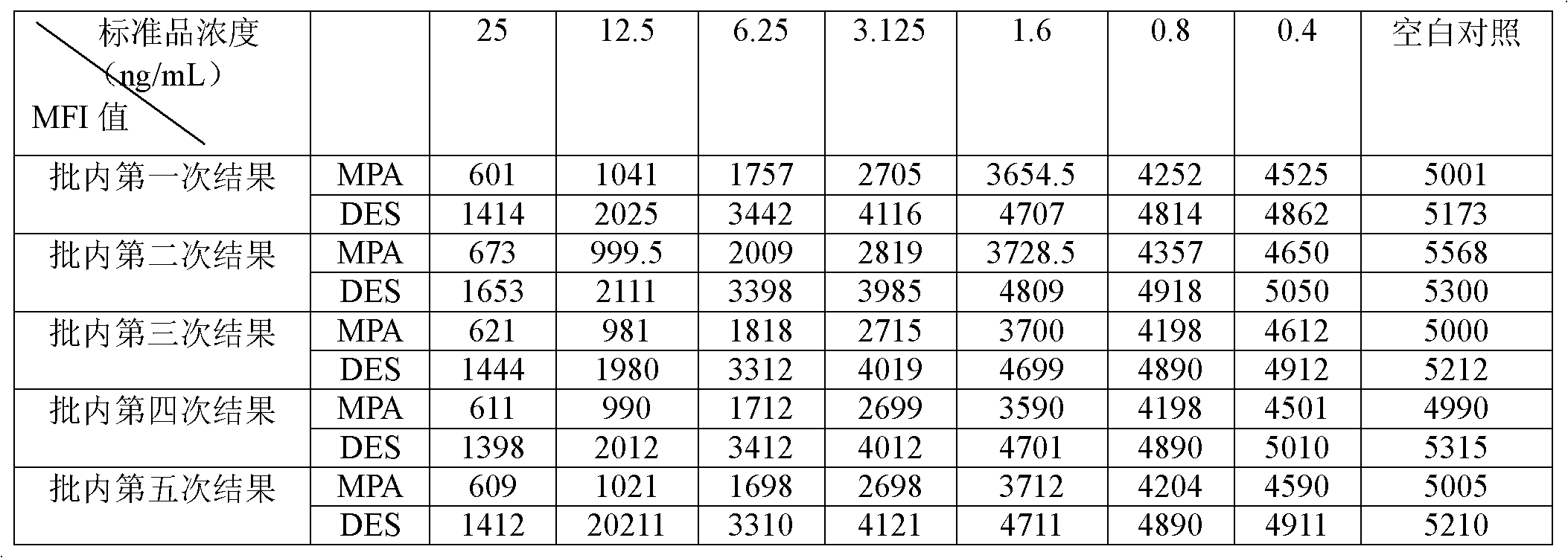
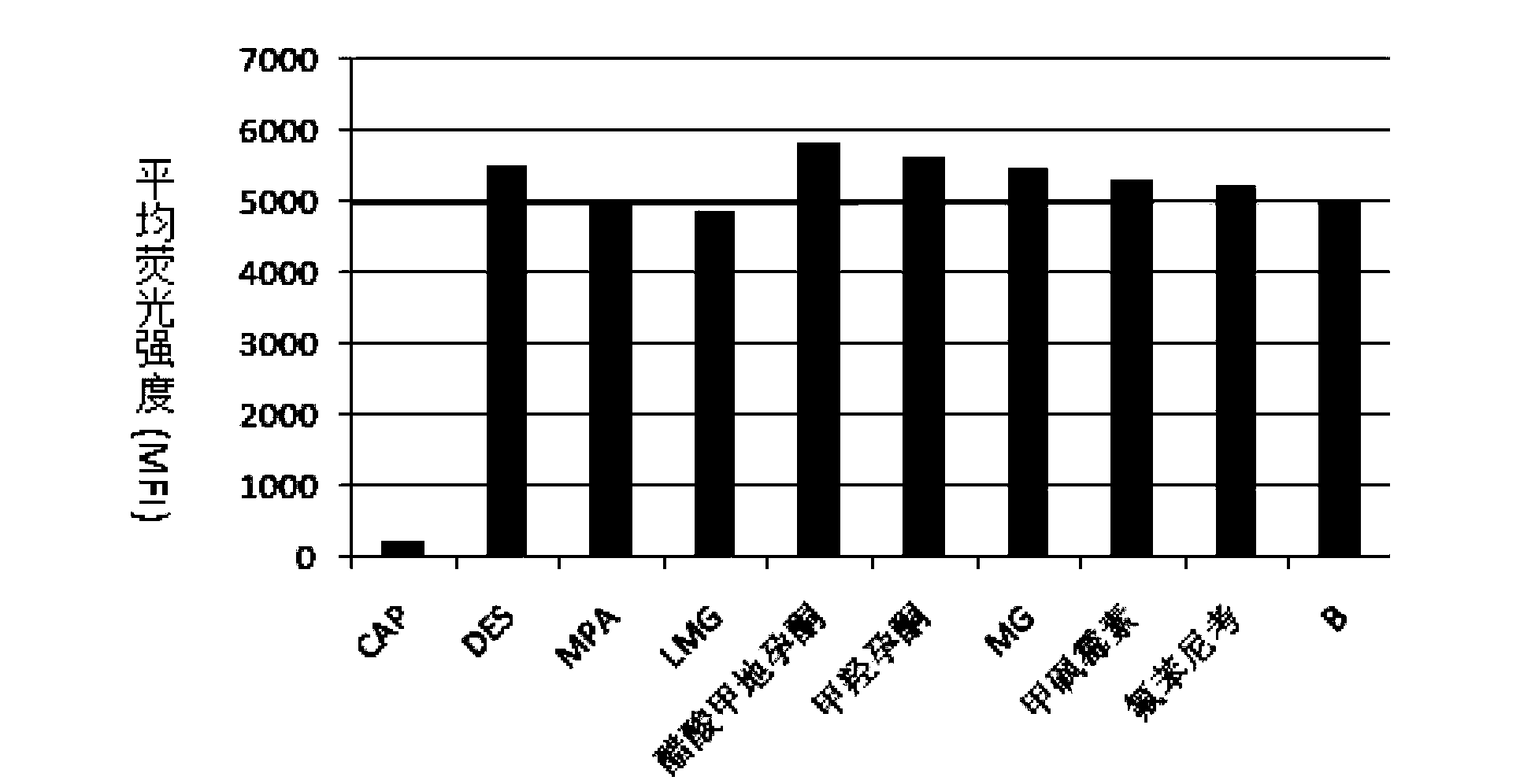
The invention belongs to the field of bio-engineering techniques, and relates to a suspension array method of simultaneous detection of medroxyprogesterone acetate and diethylstilbestrol in aquatic products. The method comprises the following steps: (1) preparing artificial antigens of medroxyprogesterone acetate and diethylstilbestrol individually; (2) preparing and purifying monoclonal antibodies against medroxyprogesterone acetate and diethylstilbestrol; (3) conjugating suspension array fluorescent microspheres to the artificial antigens obtained in step (1) separately; (4) biotinylating monoclonal antibodies obtained in step (2); and (5) simultaneously detecting medroxyprogesterone acetate and diethylstilbestrol residues in aquatic products by using conjugated fluorescent microspheres obtained in step (3) and biotinylated antibodies obtained in step (4) through the suspension array method. . The method provided by the invention can simultaneously detect two indicators of medroxyprogesterone acetate and diethylstilbestrol residues, and is fast, sensitive, and highly specific; wherein the minimum detection limits of both medroxyprogesterone acetate and diethylstilbestrol are 0.4ng / ml.
Owner:SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C +2
Method of authenticating pancreatitis biomarker and detection kit thereof
InactiveCN109187816AThe pre-processing process is simpleEasy to handleComponent separationPancreatitisAnalysis method
The invention provides a method of authenticating a pancreatitis biomarker and a detection kit thereof. The biomarker is free mannose and glucose that are obtained from serum through pre-column PMP-derived high performance liquid chromatography. The detection method refers to the pre-column PMP-derived high performance liquid chromatography. The method has the advantages that preprocessing is simple, analysis time is short, instrument price is reasonable and can be used routinely, operation steps are simple to learn, the detection result is highly accurate, a normal person and a patient suffering pancreatitis can be distinguished by sampling blood, and very little serum, less than 1mL, is needed. The obtained result shows that the analysis method can rapidly quantify free mannose and glucose in serum of a pancreatitis patient, and the method is significant for research of a relationship between free mannose and glucose in serum and pancreatitis and seeking of novel pancreatitis clinical detection markers.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Carp spinal cord cell line and its application
ActiveCN110305844AReduce generation costLess serumClimate change adaptationMicroorganism based processesHerpes simplex virusCarassius auratus gibelio
The invention provides a carp spinal cord cell line, which is named as a spinal cord cell line C8C37 of carassius auratus gibelio, which is preserved on the Chinese Culture Collection Center on November 15, 2018, and a preservation number is CCTCC NO: C2018204. The cell line can be rapidly propagated at 37 DEG C and the virus be can stably passed. The invention also provides a breeding method based on the spinal cord cell lines with similar properties. The cell line of the present invention can be well applied to the research of herpes simplex virus type 2 and the production of related vaccines, and has the advantages of low cost, low time consumption, and good effect.
Owner:成都史纪生物制药有限公司
Method for identifying colorectal cancer biomarker and detection kit thereof
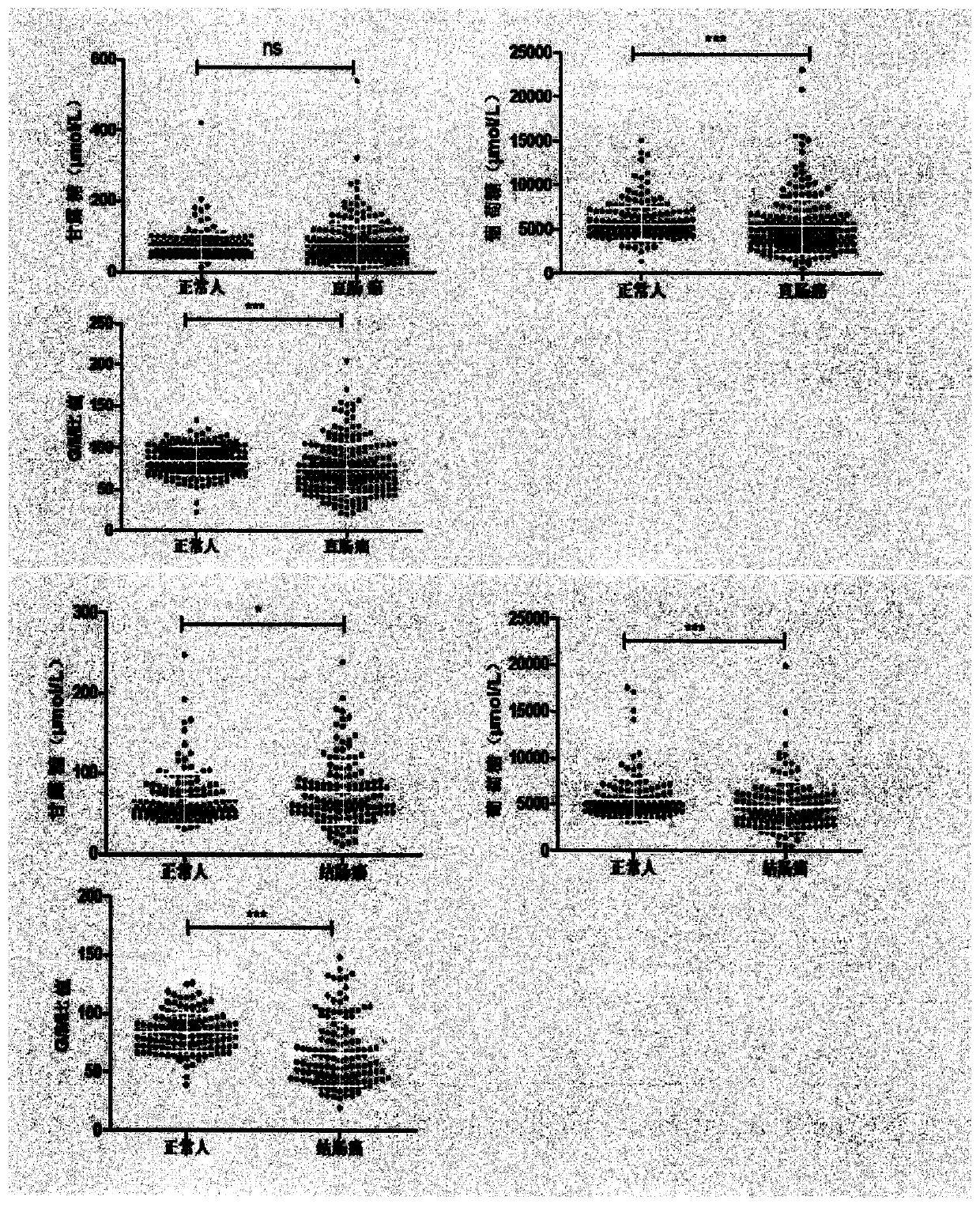
InactiveCN110261495AThe pre-processing process is simpleEasy to handleComponent separationBlood collectionHplc mass spectrometry
The invention provides a method for identifying a colorectal cancer biomarker and a detection kit thereof. The biomarker is free mannose and glucose obtained by high performance liquid chromatography of pre-column 1-phenyl-5-methylpyrazolone (PMP) derivation in a serum. A detection method of the invention is high performance liquid chromatography of pre-column PMP derivation. The technical scheme of the invention has advantages that pre-treatment is simple; analysis time is short; an apparatus price is reasonable and an apparatus accords with routine use; operation steps are simple and are easy to learn; accuracy of a detection result is high; normal people and a patient with a colorectal cancer can be distinguished only through blood sampling; and very little serum is required, a blood collection amount is less than 1mL and so on. An obtained result shows that the analysis method can rapidly quantify the free mannose and the glucose in the serum of the patient with the colorectal cancer. The method is very important to study a relationship between the free mannose and the glucose of the serum and the colorectal cancer, and search a novel colorectal cancer clinical detection marker.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Method and detection kit for identifying psoriasis biomarker
InactiveCN109406656AThe pre-processing process is simpleEasy to handleComponent separationPsoriasis patientMethyl palmoxirate
The invention provides a method and a detection kit for identifying a psoriasis biomarker. The biomarker is a ratio of free glucose to free mannose, wherein the free glucose and the free mannose are obtained by subjecting serum to pre-column 3-methyl-1-phenyl-2-pyrazolin-5-one (PMP) derivatization high-performance liquid chromatography. A detection method is a pre-column PMP derivatization high-performance liquid chromatography. According to the technical scheme, the method and the detection kit for identifying the psoriasis biomarker have the advantages that the pre-treatment is simple, the analysis time is short, the instrument price is reasonable, the conventional use is met, the operation steps are simple and easy to learn, the detection result is high in accuracy, a normal person anda psoriasis patient can be distinguished by just blood sampling, moreover, the required serum amount is very few, the amount of sampled blood is less than 1 mL, and the like. An obtained result showsthat by means of the analysis method, the free mannose and the free glucose in the serum of the psoriasis patient can be fast quantified, so that the method and the detection kit for identifying the psoriasis biomarker have significant meaning in studying a relation between free glucose and the free mannose in the serum and psoriasis and finding a novel psoriasis clinical detection marker.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Method for identifying biomarkers of asthma and detection kit
InactiveCN109212101AThe pre-processing process is simpleEasy to handleComponent separationBlood collectionMedicine
The invention provides a method for identifying biomarkers of asthma and a detection kit. The biomarkers are free mannose, free glucose in serum and the ratio of the glucose to the mannose, wherein the free mannose and the free glucose are obtained through pre-column PMP derivation high performance liquid chromatography. A detection method is the pre-column 1-phenyl-5-methyl pyrazolone (PMP) derivation high performance liquid chromatography. According to the technical scheme, the advantages that pretreatment is simple, the analysis time is short, the price of instruments is reasonable, conventional use is met, the operation steps are simple and easy to learn, the detection results have high accuracy, healthy person and asthmatic patients can be distinguished only through blood collection,the very little serum amount is required, and the blood collection amount is less than 1 mL are achieved. The obtained results show that the analysis method can rapidly quantify the free mannose and the free glucose in the serum of the asthma patients, and has great significance to study the relationship between the free mannose in the serum and asthma as well as the free glucose in the serum andasthma and to search for novel asthma clinical detection markers.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
A kind of mycoplasma bovis culture medium and preparation method
ActiveCN106399206BGrowth status can be judgedAvoid pollutionAntibacterial agentsBacterial antigen ingredientsPenicillinHydrolysate
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method and detection kit for identifying lymphoma biomarkers
InactiveCN109406657AThe pre-processing process is simpleEasy to handleComponent separationSerum ige3-methyl-1-phenyl-2-pyrazolin-5-one
The invention provides a method and a detection kit for identifying lymphoma biomarkers. The biomarkers are free glucose and free mannose which are obtained by subjecting serum to pre-column 3-methyl-1-phenyl-2-pyrazolin-5-one (PMP) derivatization high-performance liquid chromatography. A detection method is pre-column PMP derivatization high-performance liquid chromatography. According to the technical scheme, the method and the detection kit for identifying the lymphoma biomarkers have the advantages that the pre-treatment is simple, analysis time is short, the instrument price is reasonable, the conventional use is met, the operation steps are simple and easy to learn, the detection result is high in accuracy, a normal person and a lymphoma patient can be distinguished by just blood sampling, moreover, the required serum amount is very few, the amount of sampled blood is less than 1 mL, and the like. An obtained result shows that by means of the analysis method, the free mannose andthe free glucose in the serum of the lymphoma patient can be fast quantified, so that the method and the detection kit for identifying the lymphoma biomarkers have significant meaning in studying a relation between the free mannose and the free glucose in the serum and lymphoma and finding a novel lymphoma clinical detection marker.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Kit for testing acute coronary artery complex by insulin growth factor combing protein-4 hydrolase
InactiveCN1266478COvercome the shortcoming that indicators usually rise after a period of time after onsetSimple and fast operationBiological testingInsulin-like growth factorImmunosorbents
A reagent kit is composed of standard products, multiple clone antibody encapsulated plate, enzyme single clone antibody, quality control productand auxiliary reagent. The present invention also provides preparation method of kit and inspection method. The reaction method of quantitative biantibody sandwich enzyme linked immunosorbent is applied by the kit.
Owner:国家人口和计划生育委员会出生缺陷干预工程技术中心 +1
A low-serum cell culture medium with wide adaptability and preparation method thereof
ActiveCN106676053BWide adaptabilityEasy to adaptCulture processCell culture mediaBiotechnologyCell culture media
The invention discloses a low-serum cell culture medium with universal adaptability and a preparation method thereof, and belongs to the technical field of biochemistry, the preparation method comprises the following steps: (1), compounding solvent: dissolving trace materials into 940 to 1060 parts by mass of ultrapure water, compounding to be a solvent; (2), drying: mixing the solvent obtained in the step (1) with 1000 to 4500 parts by mass of D-glucose and drying; (3), ball milling: taking other raw materials and mixing uniformly with dried mixed materials in the step (2), then performing ball-milling, sieving through a 100-mesh screen, taking the sieved parts to prepare the culture medium. The culture medium has universal adaptability, can be applied to various cell lines such as VERO, BHK-21, A549, ST, PK-15; transition is not needed when cells is transferred from a high-serum culture medium to the low-serum cell culture medium provided by the invention during a culture process, the cells can adapt quickly, and the operation is simple and convenient.
Owner:山东巨山能源科技有限公司
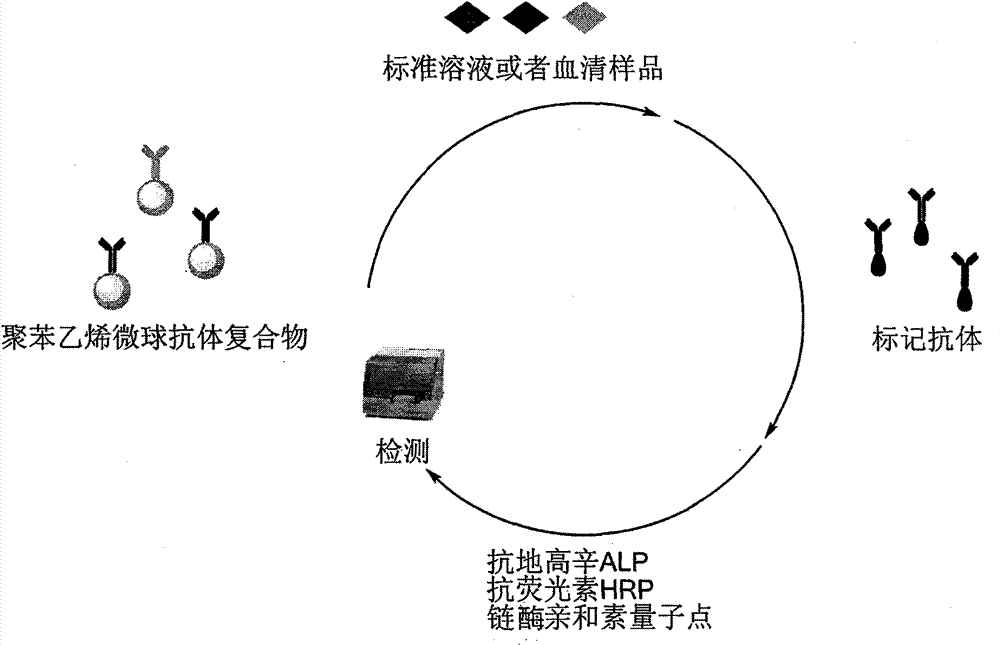
Homogeneous phase multi-index fluorescence/chemiluminescence measuring method and application thereof
InactiveCN101995396BLess serumShort detection timeChemiluminescene/bioluminescenceFluorescence/phosphorescenceCritical illnessFluorescence
The invention belongs to the field of blood testing, and relates to a homogeneous phase multi-index fluorescence / chemiluminescence measuring method and application thereof. The method comprises the steps of: fixing a plurality of specificity recognition monoclonal antibodies on a polystyrene microsphere, and reacting with a plurality of labeled antibody cores modified by haptens after combining with carcinoembryonic antigens, cell keratin fragments 19, neuron specificity enolase and other multiple markers; reacting with horse radish peroxidase modified by corresponding hapten antibodies, alkaline phosphatase and quantum dots, and measuring a plurality of markers on the same instrument by combining with detections of chemiluminescence, fluorescence and the like. The method has the advantages of small blood serum consumption, rapid detection time, low diagnosis cost and simple operation, is suitable for detecting a plurality of popularized indexes simultaneously, and can provide auxiliary judgment basis for the early diagnosis of critical illnesses of tumor and the like.
Owner:FUDAN UNIV
Method and detection kit for identifying ovarian cancer biomarkers
InactiveCN109406663AThe pre-processing process is simpleEasy to handleComponent separationMedicine3-methyl-1-phenyl-2-pyrazolin-5-one
The invention provides a method and a detection kit for identifying ovarian cancer biomarkers. The biomarkers are free glucose and free mannose which are obtained by subjecting serum to pre-column 3-methyl-1-phenyl-2-pyrazolin-5-one (PMP) derivatization high-performance liquid chromatography. A detection method is a pre-column PMP derivatization high-performance liquid chromatography. According tothe technical scheme, the method and the detection kit for identifying the ovarian cancer biomarkers have the advantages that the pre-treatment is simple, analysis time is short, the instrument priceis reasonable, the conventional use is met, the operation steps are simple and easy to learn, the detection result is high in accuracy, a normal person and an ovarian cancer patient can be distinguished by just sampling blood and subjecting the blood to the high-performance liquid chromatography for analysis, moreover, the required serum amount is very few, the amount of sampled blood is less than 1 mL, and the like. An obtained result shows that by means of the analysis method, the free mannose and glucose in the serum of the ovarian cancer patient can be fast quantified, so that the methodand the detection kit for identifying the ovarian cancer biomarkers have significant meaning to studying a relation between the free mannose and the free glucose in the serum and ovarian cancer and finding a novel ovarian cancer clinical detection marker.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Serum copper, iron and zinc rapid sensitive detection kit and preparing method thereof
InactiveCN1261766CLess serumSimple and fast operationMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsTest agentMedicine
This invention discloses a test agent box to measure the contents of copper, iron and zinc in blood serum and provides the process method of the agent box. The agent box in this invention dissociates the protein with copper, iron and zinc in blood serum in the buffer liquid system with the surface activity agent, which is compound with the pyridylazo organic compound to make color. The color complex compound has the longest wavelength at 550-600nm.
Owner:浙江东瓯诊断产品有限公司
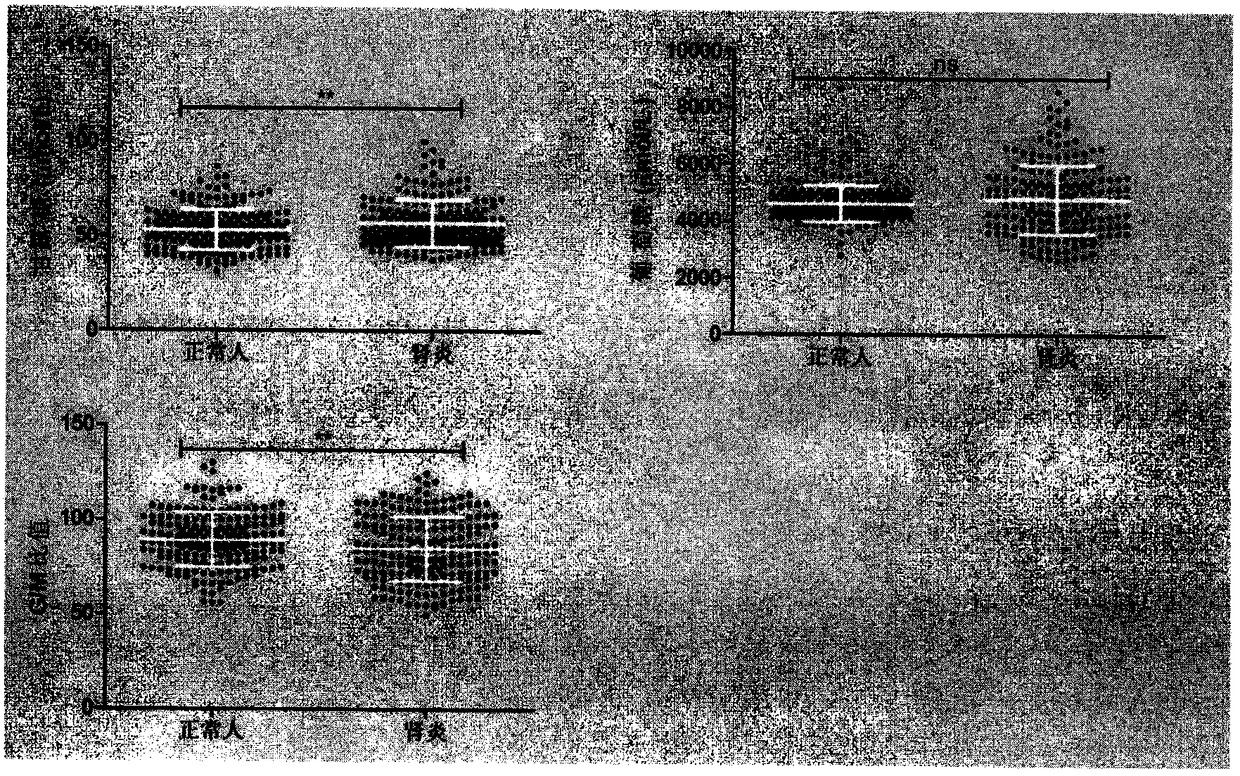
Method and detection kit for identifying nephritis biomarkers
InactiveCN109406661AThe pre-processing process is simpleEasy to handleComponent separationConcentration ratio3-methyl-1-phenyl-2-pyrazolin-5-one
The invention provides a method and a detection kit for identifying nephritis biomarkers. The biomarkers is free mannose, which is obtained by subjecting serum to pre-column 3-methyl-1-phenyl-2-pyrazolin-5-one (PMP) derivatization high-performance liquid chromatography, and a concentration ratio of glucose to mannose. A detection method is pre-column PMP derivatization high-performance liquid chromatography. According to the technical scheme, the method and the detection kit for identifying the nephritis biomarkers have the advantages that the pre-treatment is simple, the analysis time is short, the instrument price is reasonable, the conventional use is met, the operation steps are simple and easy to learn, the detection result is high in accuracy, a normal person and a nephritis patientcan be distinguished by just blood sampling, moreover, the required serum amount is very less, the amount of sampled blood is less than 1 mL, and the like. An obtained result shows that by means of the analysis method, the free mannose and the free glucose in the serum of the nephritis patient can be fast quantified, so that the method and the detection kit for identifying the nephritis biomarkershave significant meaning in studying a relation between the free mannose and the free glucose in the serum and nephritis and finding a novel nephritis clinical detection marker.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Liquid-phase chip method for detecting chloromycetin in aquatic product
InactiveCN103389377AStrong specificityImprove stabilityMaterial analysisAquatic productMonoclonal antibody
The invention belongs to the technical field of biological engineering, and relates to a liquid-phase chip kit used for detecting chloromycetin in an aquatic product. The method comprises the steps that: (1) a chloromycetin artificial antigen is prepared; (2) chloromycetin monoclonal antibody is prepared and purified; (3) the artificial antigen obtained in the step (1) is used for coupling liquid-phase chip fluorescent microspheres; (4) the monoclonal antibody obtained in the step (2) is used for biotinylation; and (5) the coupled microspheres obtained in the step (3) and the biotinylated antibody obtained in the step (4) are used for detecting chloromycetin in an aquatic product through the liquid-phase chip method. The method provided by the invention can be used for detecting chloromycetin residue index with high speed, good specificity, and high specificity. A chloromycetin detection limit is lower than 0.1ng / L.
Owner:SHANGHAI UNIV +1
Method of biomarker for identifying cervical cancer and detection kit for identifying cervical cancer
InactiveCN109406668AThe pre-processing process is simpleEasy to handleComponent separationOrganismHplc mass spectrometry
The invention provides a method of a biomarker for identifying cervical cancer and a detection kit for identifying cervical cancer. The biomarker comprises free mannose and glucose obtained by high performance liquid chromatography derived from pre-column 1-phenyl-5-methyl pyrazolone(PMP) in serum. The detection method is a pre-column PMP derived high performance liquid chromatography method. According to the technical scheme, the method and the detection kit have the advantages that pretreatment is easy, the analysis time is short, the instrument price is reasonable, the method is compliancewith conventional use, operation steps are simple and easy to learn, the accuracy of detection results is high, normal people can be distinguished from patients with cervical cancer only by collectingblood, the amount of required serum is small, and the amount of the collected blood is less than 1mL . The results show that the analytical method can rapidly quantify the free mannose and glucosein the serum of patients with cervical cancer, the method and the detection kit are of great significance for studying the relationship between serum free mannose and glucose and cervical cancer and finding new clinical detection markers for cervical cancer.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
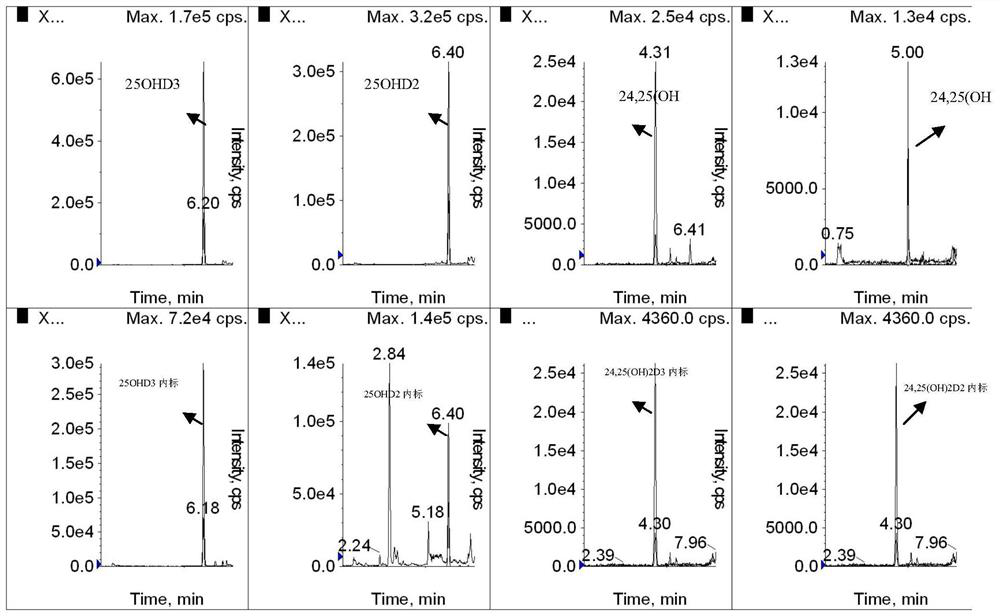
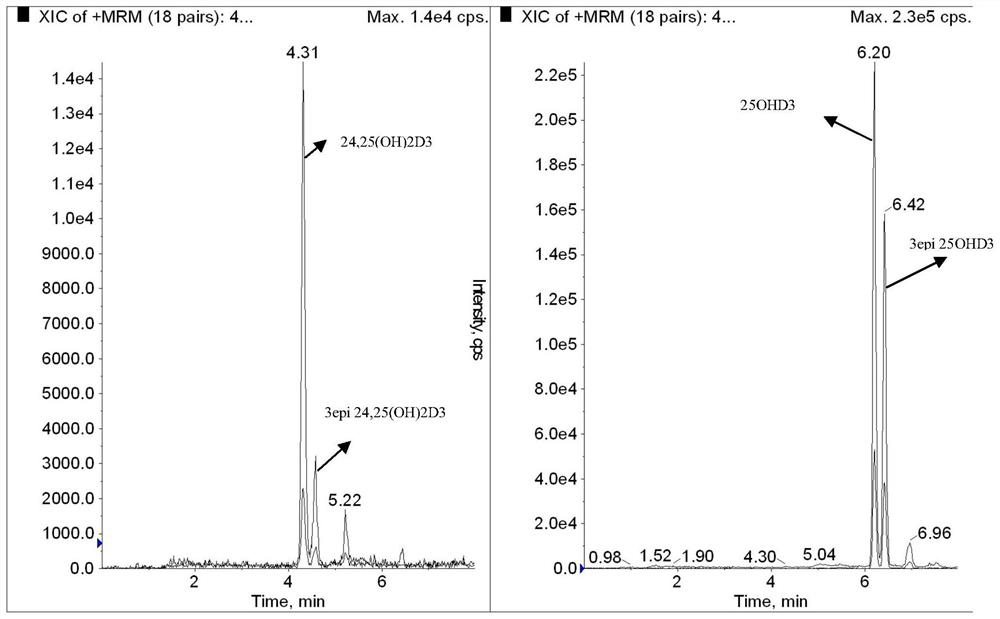
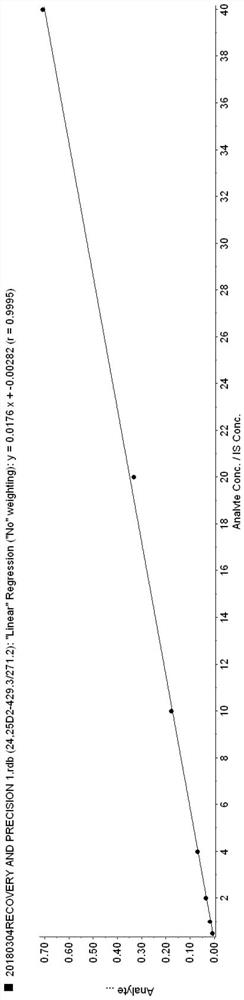
Simultaneous detection of serum 24,25(oh)2d and 25ohd
ActiveCN108593790BAccurate measurementEasy to handleComponent separationChromatographic separationSerum neutralization
Owner:北京豪思生物科技股份有限公司
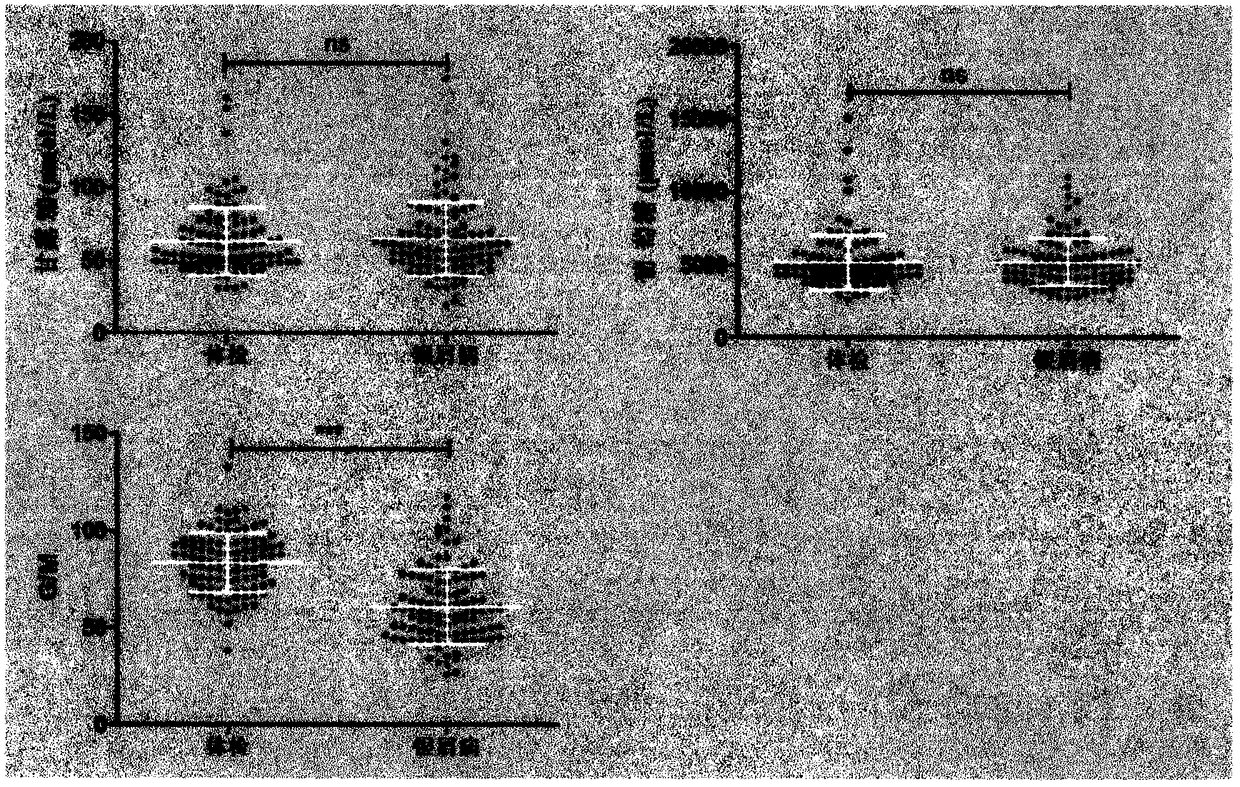
Method for identifying biomarker of esophageal cancer and detection kit thereof
InactiveCN109596724AThe pre-processing process is simpleEasy to handleComponent separationBlood collectionMedicine
The invention provides a method for identifying a biomarker of esophageal cancer and a detection kit thereof. The biomarker is the ratio of free glucose and mannose in serum obtained through pre-column 1-methyl-5-methyl pyrazolone (PMP)-derived high-performance liquid chromatography. The detection method in the invention is pre-column PMP-derived high-performance liquid chromatography. The technical scheme provided by the invention has the advantages of being simple in preprocessing, short in analysis time, and reasonable in instrument price, the conventional use is conformed to, the operationsteps are simple and easy to learn, the accuracy of the detection result is high, an average person and an esophagus cancer patient can be distinguished only through blood sampling, the number of needed serum is extremely small, the blood collection amount is smaller than 1 mL, and the like. The result shows that the analysis method can quickly quantify free mannose and glucose in serum of esophageal cancer patients and has very important significance in studying the relation between the free glucose and mannose in the serum and the esophagus cancer and finding a novel esophagus cancer clinical detection marker.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Method for identifying biomarkers of pneumonia and detection kit thereof
InactiveCN109212098AThe pre-processing process is simpleEasy to handleComponent separationBlood collectionPeak area
The invention provides a method for identifying biomarkers of pneumonia and applications thereof. The biomarker is a free glucose and a mannose obtained by high performance liquid chromatography (HPLC) derived from 1-phenyl-5-methylpyrazolone (PMP) from the serum of the patient. The method for identifying biomarkers of pneumonia has the advantages of being simple in pretreatment, short in analysistime, reasonable price of the instrument, in line with normal use, easy to learn, high in detection result accuracy, only blood sampling for HPLC analysis can distinguish between normal people and patients, and small in required serum amount, less than 1 mL in blood collection amount and the like. The results show that the analysis method can analyze the free mannose and the glucose in the serumquickly, and can be calculated to obtain the mannose, the glucose sugar concentrationm and the peak area, and has a very important significance in researching the relationship between the serum free mannose and the glucose and the diseases, and finding the biomarker for clinical detection of novel pneumonia diseases.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Method and detection kit for identifying biomarker of leukemia
InactiveCN109342599AThe pre-processing process is simpleEasy to handleComponent separationBlood collectionAnalysis method
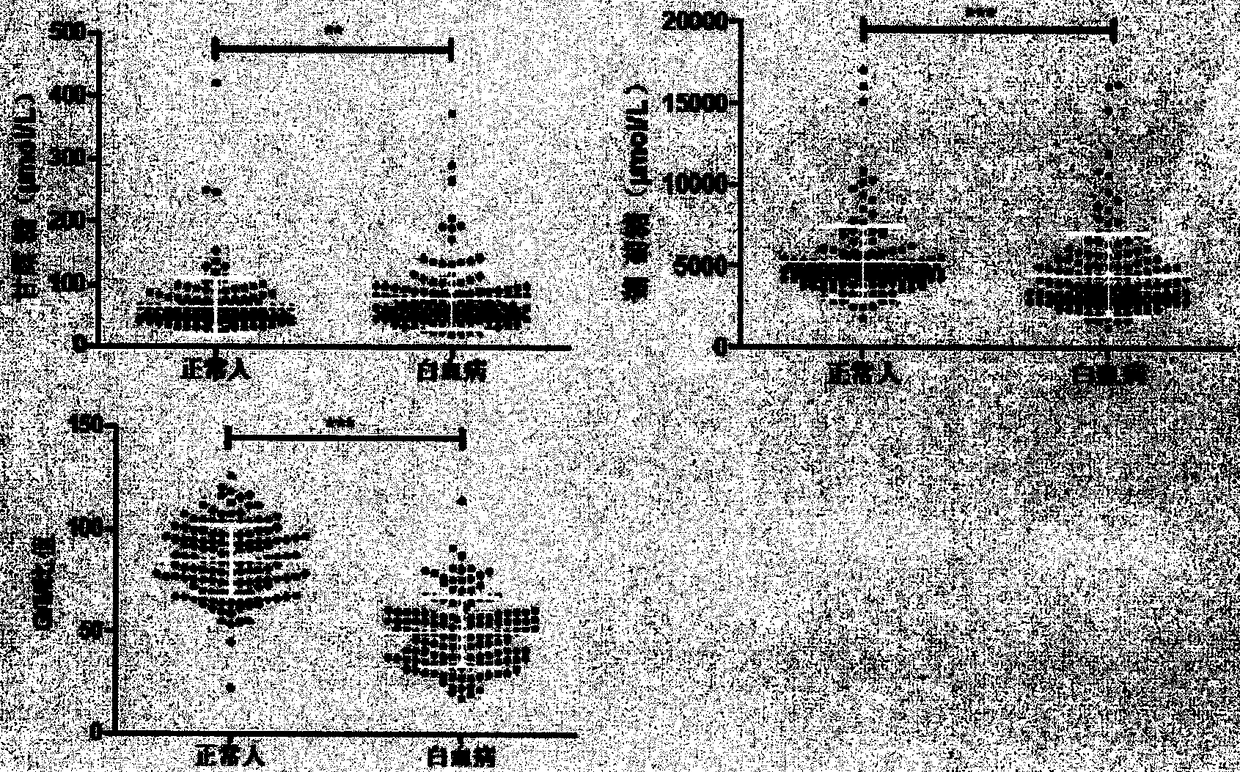
The invention provides a method and a detection kit for identifying a biomarker of leukemia. The biomarker is free mannose and glucose obtained by high performance liquid chromatography based on pre-column 1-phenyl-3-methyl-5-pyrazolone (PMP) derivatization in serum. The detection method is the high performance liquid chromatography method based on the pre-column PMP derivatization. The technicalscheme has the advantages that the pretreatment is simple, the analysis time is short, the instrument price is reasonable, the requirements of conventional use are met, the operation steps are simpleand easy to learn, the accuracy of a detection result is high, a normal person and a leukemia patient can be distinguished only by blood collection, the amount of the needed serum is extremely small,the blood collection amount is smaller than 1 mL, and the like. The obtained result shows that the analysis method can be used for rapidly determining the content of the free mannose and glucose in the serum of the leukemia patient, so that the method has very important significance for researching a relation between the free mannose and glucose in the serum and the leukemia, and looking for a novel leukemia clinical detection marker.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Method for authenticating liver cancer biomarker and detection kit thereof
InactiveCN109187815AThe pre-processing process is simpleEasy to handleComponent separationHplc mass spectrometryMedicine
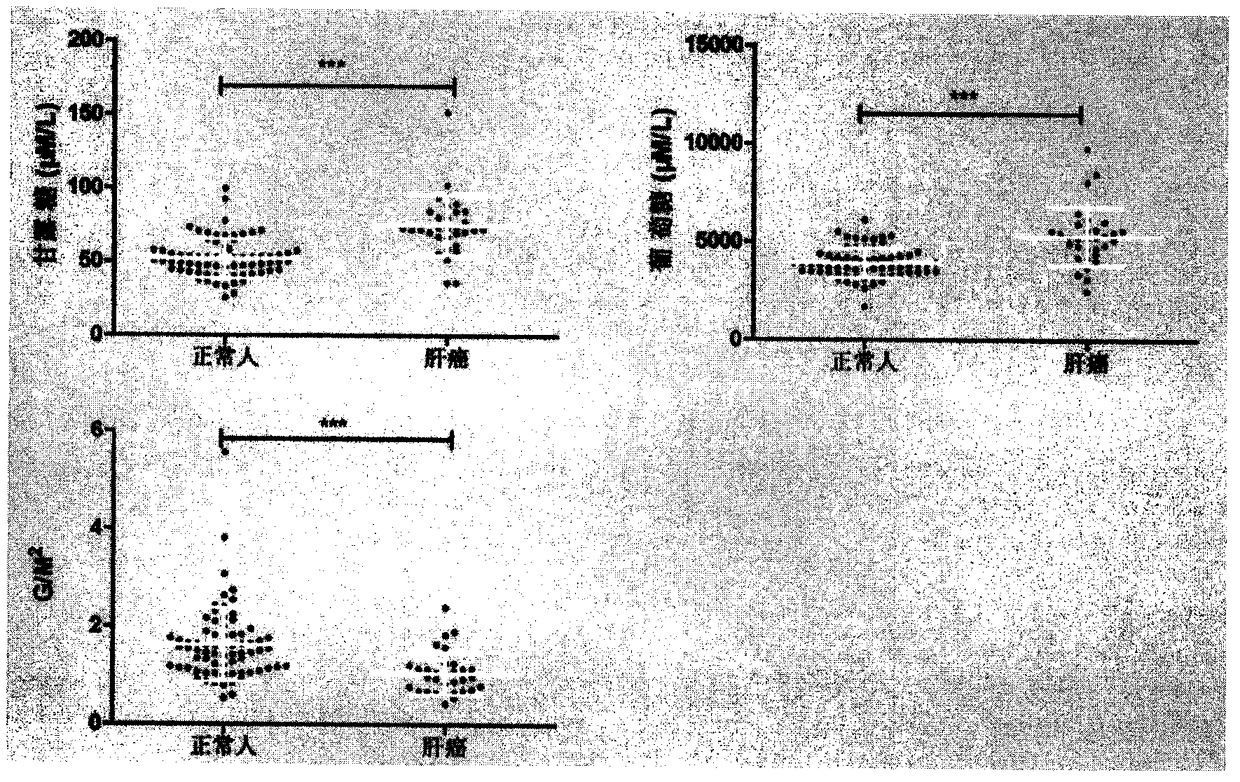
The invention provides a method for authenticating a liver cancer biomarker and a detection kit thereof. The biomarker comprises free mannose and glucose, obtained by high performance liquid chromatography through derivation of pre-column PMP, in patient serum. The detection method is high performance liquid chromatography derived by pre-column 1-phenyl-5-methyl pyrazolone (PMP). Through the technical scheme, the method has the advantages of simple pretreatment, short analysis time and reasonable instrument cost, conventional use is met, the operating steps are simple and easy to learn, the detection result is high in accuracy, normal persons and liver caner patients can be distinguished just by needing blood sampling, the quantity of required serum is extremely small, and the blood sampling quantity is smaller than 1 mL. The obtained result indicates that the analysis method can quickly quantify free mannose and glucose in the serum of a liver caner patient, and the biomarker is significant for researching the relation between the free mannose and glucose in the serum and liver caner and searching novel markers for liver cancer clinical detection.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Method and detection kit for identifying degenerative osteoarthropathy biomarkers
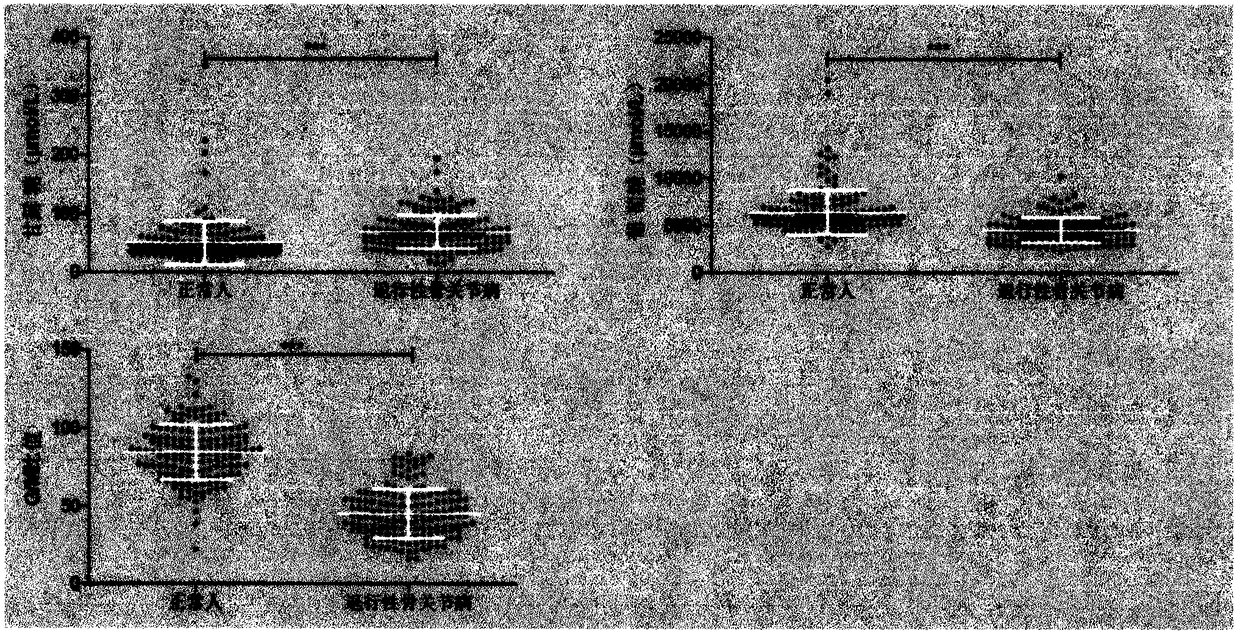
InactiveCN109406662AThe pre-processing process is simpleEasy to handleComponent separation3-methyl-1-phenyl-2-pyrazolin-5-onePre treatment
The invention provides a method and a detection kit for identifying degenerative osteoarthropathy biomarkers. The biomarkers are free mannose and free glucose, which are obtained by subjecting serum to pre-column 3-methyl-1-phenyl-2-pyrazolin-5-one (PMP) derivatization high-performance liquid chromatography, and a ratio of the free glucose to the free mannose. A detection method is pre-column PMPderivatization high-performance liquid chromatography. According to the technical scheme, the method and the detection kit for identifying the degenerative osteoarthropathy biomarkers have the advantages that pre-treatment is simple, the analysis time is short, the instrument price is reasonable, the conventional use is met, the operation steps are simple and easy to learn, the detection result ishigh in accuracy, only blood sampling is needed, moreover, the required serum amount is very few, the amount of sampled blood is less than 1 mL, and the like. An obtained result shows that by means of the analysis method, the free mannose and the free glucose in the serum of a degenerative osteoarthropathy patient can be fast quantified, so that the method and the detection kit for identifying the degenerative osteoarthropathy biomarkers have significant meaning in studying a relation between free mannose and free glucose in the serum and degenerative osteoarthropathy and finding a novel degenerative osteoarthropathy clinical detection marker.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com