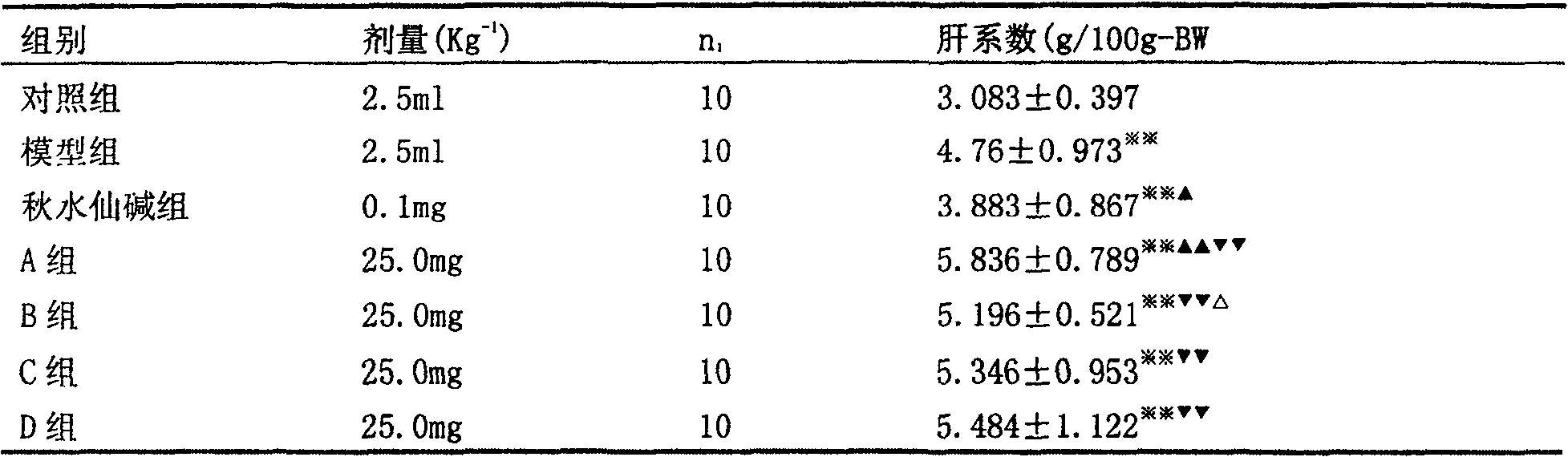
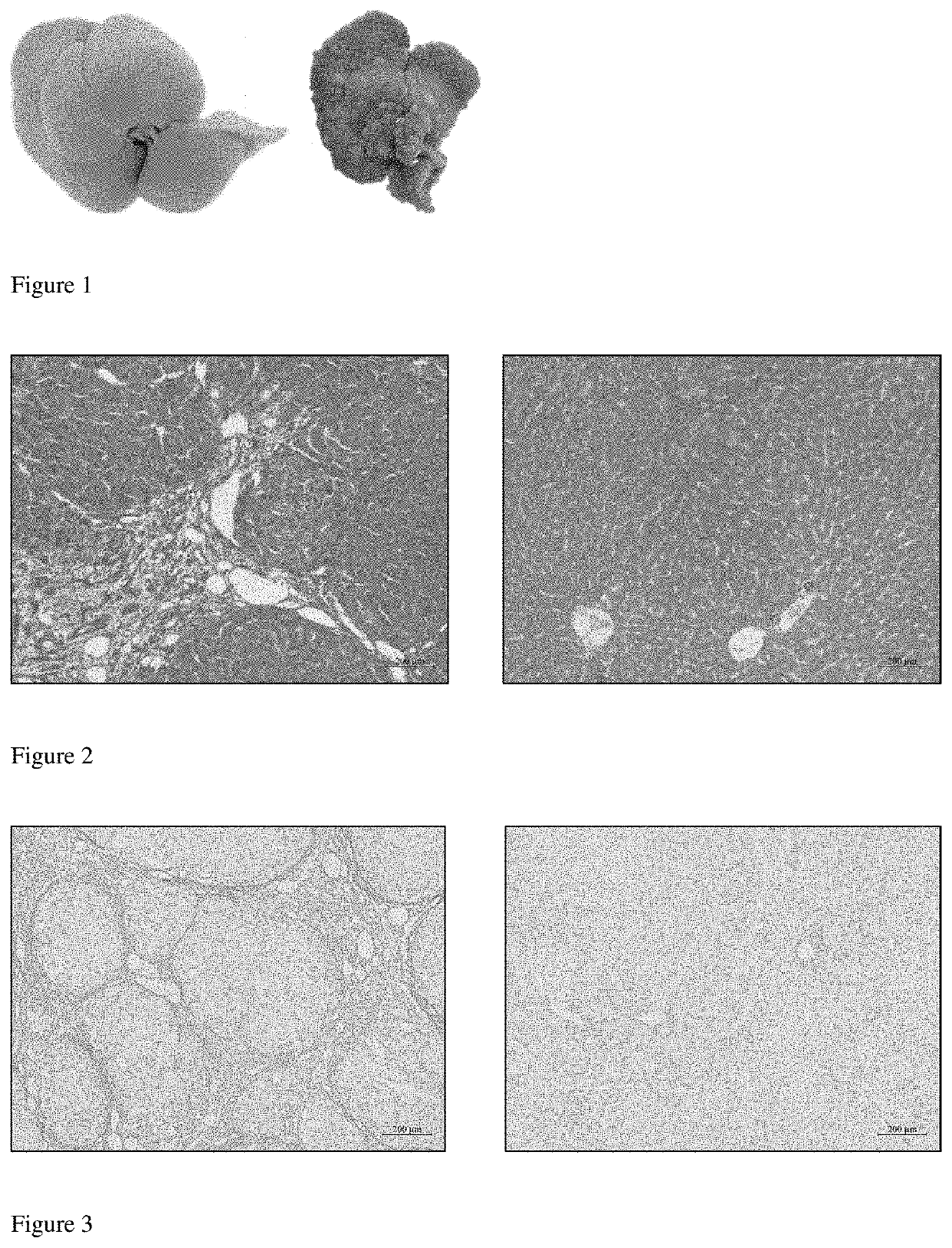
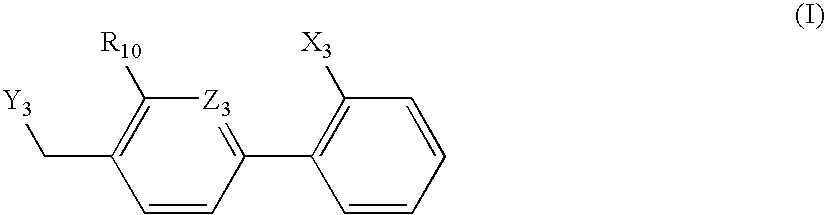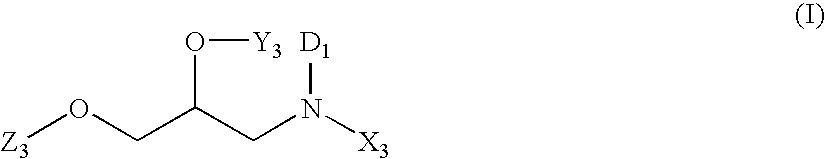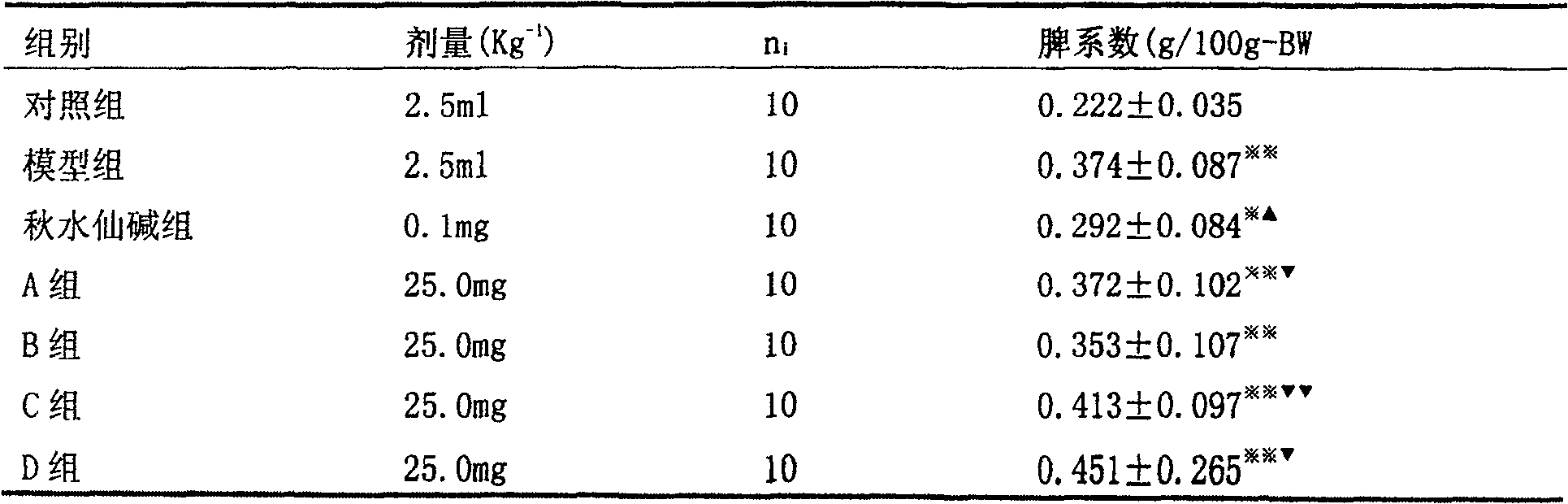Patents
Literature
51 results about "HYPERTENSION PORTAL" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
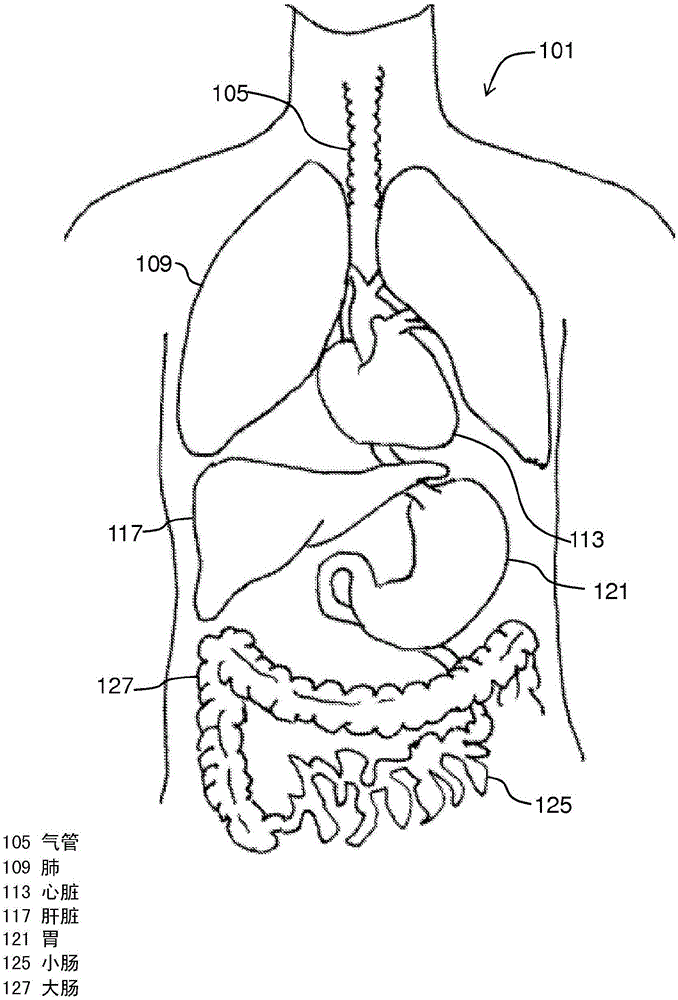
Portal hypertension is hypertension (high blood pressure) in the hepatic portal system – made up of the portal vein and its branches, that drain from most of the intestine to the liver. Portal hypertension is defined as a hepatic venous pressure gradient.
Nitric oxide enhancing diuretic compounds, compositions and methods of use
InactiveUS20060189603A1Antibacterial agentsSenses disorderRenovascular diseaseOsteoporosis treatment
The invention describes novel compositions and kits comprising at least one nitric oxide enhancing diuretic compound, or pharmaceutically acceptable salts thereof, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating conditions resulting from excessive water and / or electrolyte retention; (b) treating cardiovascular diseases; (c) treating renovascular diseases; (d) treating diabetes; (e) treating diseases resulting from oxidative stress; (f) treating endothelial dysfunctions; (g) treating diseases caused by endothelial dysfunctions; (h) treating cirrhosis; (j) treating pre-eclampsia; (k) treating osteoporosis; (l) treating nephropathy; (m) treating peripheral vascular diseases; (n) treating portal hypertension; (o) treating central nervous system disorders; (p) treating metabolic syndrome; (q) treating sexual dysfunctions; and (r) hyperlipidemia. The nitric oxide enhancing diuretic compounds comprise at least one nitric oxide enhancing group linked to the diuretic compound through one or more sites such as carbon, oxygen and / or nitrogen via a bond or moiety that cannot be hydrolyzed.
Owner:NICOX SA
Sulfonylurea derivative and pharmaceutical composition and application thereof
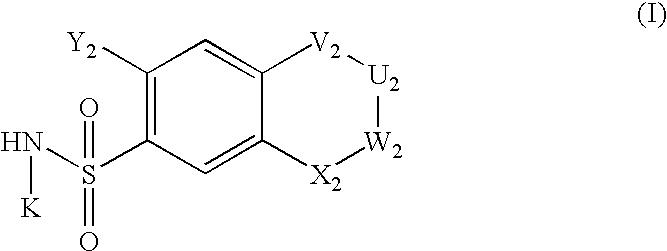
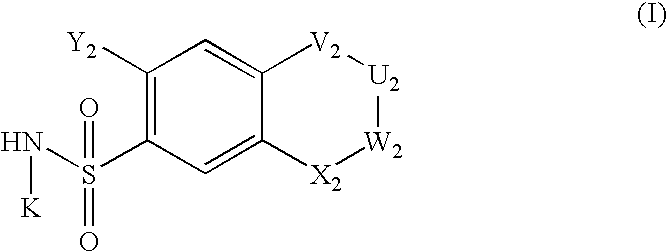
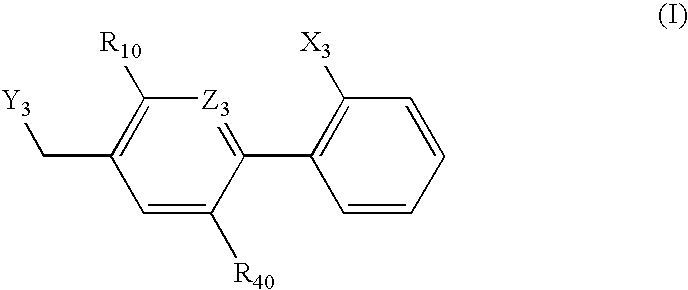
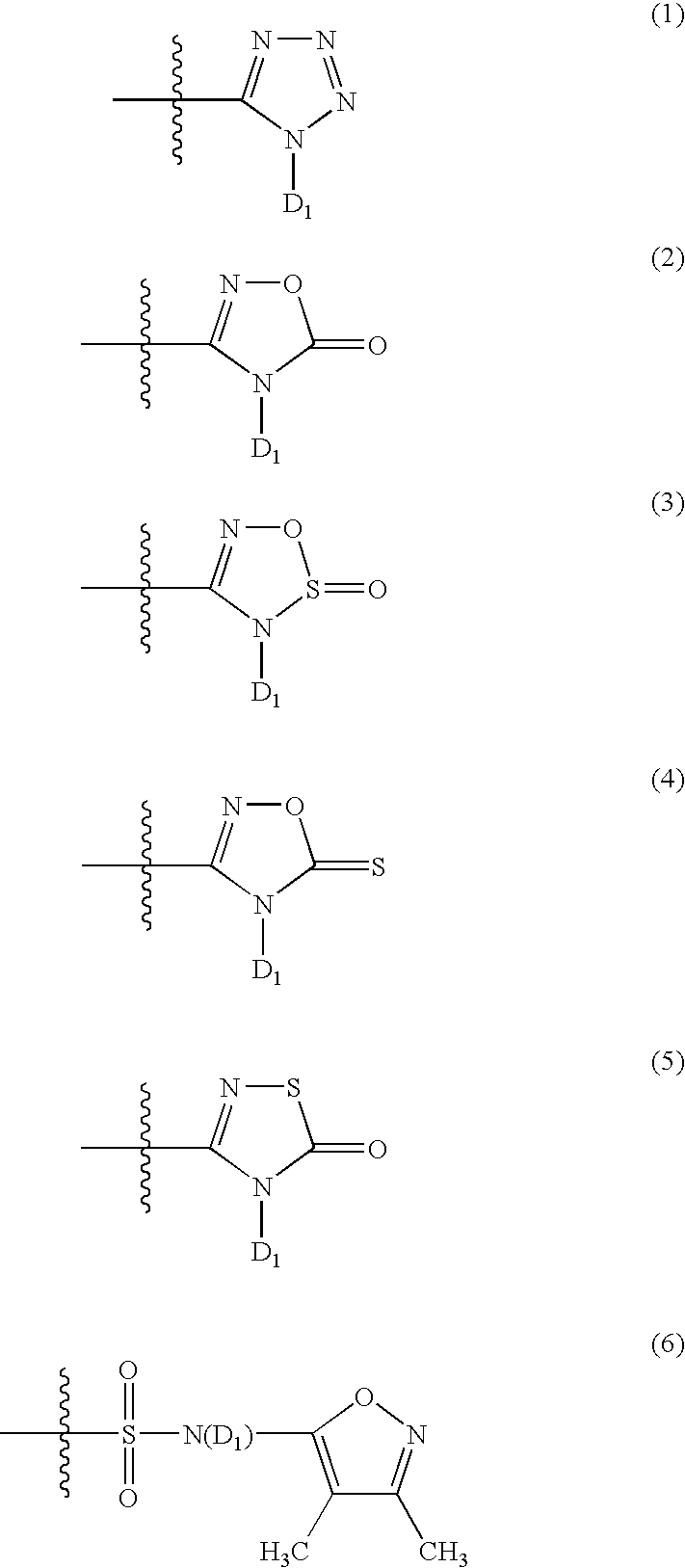
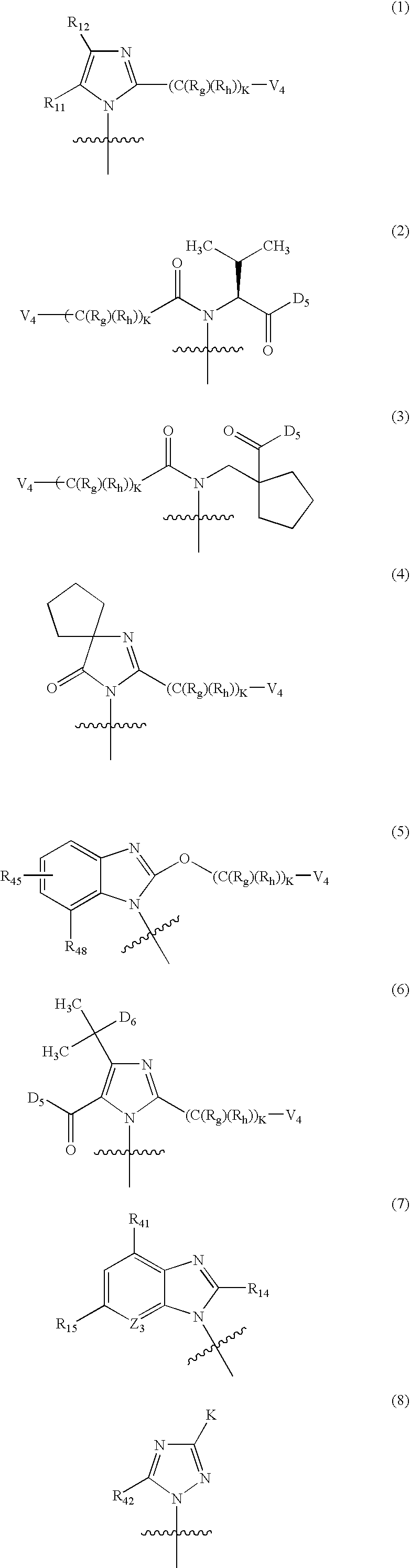
The invention relates to a preparation method and application of a sulfonylurea compound and a composition containing the same component as FXR and / or TGR5 agonist, the FXR and / or TGR5 agonist is a compound shown as a formula (I), or a pharmaceutically acceptable salt, a solvate, a prodrug, an isomer and a stable isotope derivative thereof. The compounds can be used for treatment of FXR and / or TGR5 mediated diseases including primary biliary cirrhosis, nonalcoholic fatty liver, portal hypertension, bile acid diarrhea and cholestasis, type II diabetes and obesity and other field.
Owner:SHANGHAI DE NOVO PHARMA
Nitric oxide enhancing diuretic compounds, compositions and methods of use
The invention describes novel compositions and kits comprising at least one nitric oxide enhancing diuretic compound, or pharmaceutically acceptable salts thereof, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating conditions resulting from excessive water and / or electrolyte retention; (b) treating cardiovascular diseases; (c) treating renovascular diseases; (d) treating diabetes; (e) treating diseases resulting from oxidative stress; (f) treating endothelial dysfunctions; (g) treating diseases caused by endothelial dysfunctions; (h) treating cirrhosis; (j) treating pre-eclampsia; (k) treating osteoporosis; (l) treating nephropathy; (m) treating peripheral vascular diseases; (n) treating portal hypertension; (o) treating central nervous system disorders; (p) treating metabolic syndrome; (q) treating sexual dysfunctions; and (r) hyperlipidemia. The nitric oxide enhancing diuretic compounds comprise at least one nitric oxide enhancing group linked to the diuretic compound through one or more sites such as carbon, oxygen and / or nitrogen via a bond or moiety that cannot be hydrolyzed.
Owner:NICOX SA
Nitric oxide enhancing angiotensin II antagonist compounds, compositions and methods of use
The invention describes compositions and kits comprising at least one nitric oxide enhancing angiotensin II antagonist compound, or pharmaceutically acceptable salts thereof, and novel compositions comprising at least one nitric oxide enhancing angiotensin II antagonist compound, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; (j) treating osteoporosis; (k) treating nephropathy; (l) treating peripheral vascular diseases; (m) treating portal hypertension (o) treating central nervous system disorders; (p) treating metabolic syndrome; and (q) treating hyperlipidemia. The nitric oxide enhancing angiotensin II antagonist compounds comprise at least one nitric oxide enhancing group linked to the angiotensin II antagonist compound through one or more sites such as carbon, oxygen and / or nitrogen via a bond or moiety that cannot be hydrolyzed.
Owner:NICOX SA
Organic nitric oxide enhancing salts of angiotensin ii antagonists, compositions and methods of use
The invention describes compositions and kits comprising at least one organic nitric oxide enhancing salt of an angiotensin π antagonist, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; (j) treating osteoporosis; (k) treating nephropathy; (l) treating peripheral vascular diseases; (m) treating portal hypertension; (n) treating ophthalmic disorders; (o) treating metabolic syndrome; and (p) treating hyperlipidemia. The organic nitric oxide enhancing compounds that form salts with the angiotensin II antagonists are organic nitrates, organic nitrites, nitrosothiols, thionitrites, thionitrates, NONOates, heterocyclic nitric oxide donors and / or nitroxides. The heterocyclic nitric oxide donors are furoxans, sydnonimines, oxatriazole-5-ones and / or oxatriazole-5-imines.
Owner:NICOX SA
Sulphonylaminocarbonyl derivatives, and pharmaceutical compositions and use thereof
The invention relates to preparation methods of sulphonylaminocarbonyl derivatives and compositions containing the same component and a use of the sulphonylaminocarbonyl derivatives as FXR and / or TGR5 agonists; the agonists are the sulphonylaminocarbonyl derivatives represented by the formula I, or pharmaceutically acceptable salts, prodrugs, solvates, hydrates, polymorphs, isomers, and stable isotope derivatives thereof. The compounds can be used for treatment of diseases and symptoms mediated by FXR and / or TGR5 and other therapeutic fields, wherein the diseases and symptoms include primary biliary cirrhosis, nonalcoholic fatty liver, portal hypertension, bile acid diarrhea and cholestasis, type II diabetes and obesity.
Owner:SHANGHAI DE NOVO PHARMA
Cardiovascular Compounds Comprising Nitric Oxide Enhancing Groups, Compositions and Methods of Use
The invention describes compositions and kits comprising at least one cardiovascular compound comprising at least one nitric oxide enhancing group, or pharmaceutically acceptable salts thereof, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; Q) treating osteoporosis; (k) treating nephropathy; (l) treating peripheral vascular diseases; (m) treating portal hypertension; (n) treating ophthalmic disorders; (o) treating metabolic syndrome; and (p) treating hyperlipidemia. The cardiovascular compounds are angiotensin II antagonists, aldosterone antagonists, endothelin antagonists, hydralazine compounds, neutral endopeptidase inhibitors and renin inhibitors. The nitric oxide enhancing groups are nitroxides and / or heterocyclic nitric oxide donors.
Owner:NICOX SA
Use of a vegetable drug composition in the manufacturing of pharmaceutical preparation for the treatment of portal hypertension caused by hepatocirrhosis
ActiveUS20100119541A1Accelerating degenerationEnhance body resistanceBiocideDigestive systemPortal veinPollen
Use of a vegetable drug composition in the manufacturing of a pharmaceutical preparation for the treatment of portal hypertension caused by hepatocirrhosis, said preparation comprises 25-38% of extract of radix Salviae Miltiorrhizae, 20-25% of extract of herb Gynostemmae Pentaphylli, 1-6% of alcoholic extract of fructus Schisandrae Chinensis, 19-26% of extract of Cordyceps, 6-8% of extract of pollen Pini, 6-10% of extract of semen Persicae. Said preparation can improve hepatic cell degeneration, necrosis, intraheptic hemorrhage, fibre proliferation, and reduce pressure of portal vein in mammal. A preparation method of said preparation is disclosed.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV
Pharmaceutical compositions for combination therapy
The present invention relates to a pharmaceutical composition comprising a combination of an FXR agonist and at least one lipid lowering agent (e.g., PPAR-alpha agonist, PPAR-delta agonist, PPAR-alpha and delta dual agonist, and / or statin). Also disclosed is use of the combination for the treatment or prevention of a FXR mediated disease or condition, such as primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), portal hypertension, bile acid diarrhea, NAFLD (nonalcoholic fatty liver disease), NASH (non-alcohol-induced steatohepatitis), and other chronic liver diseases. The combination of the present invention is useful for the treatment or prevention of conditions related to elevated lipid and liver enzyme levels. The present invention also relates to packs or kits including the pharmaceutical combination.
Owner:INTERCEPT PHARMA INC
Radiomics-based hepatic venous pressure gradient (rHVPG) calculation model construction method
ActiveCN107480675AIncrease operational difficultyInvasive riskImage enhancementImage analysisMedicineQuality of life
The invention provides a radiomics-based hepatic venous pressure gradient (rHVPG) calculation model construction method, on this basis, a more advantageous hepatic venous pressure gradient calculation model can be constructed, and a new way is provided for the calculation of the early stage non-invasive index of a patient with portal hypertension. The invention also provides a radiomics-based hepatic venous pressure gradient calculation system. The invention overcomes the many limitations of current non-invasive measurement methods. The rHVPG calculation system proposed by the invention is feasible, is expected to provide a new idea for noninvasive measurement of portal pressure, and plays a positive role in improving the quality of life of the patient with portal hypertension and alleviating the disease burden of families and society.
Owner:祁小龙
Nucleic acid constructs, cells transformed therewith and methods utilizing same for inducing liver regeneration and alleviation of portal hypertension
A method of inducing liver regeneration in a damaged liver tissue region of an individual is provided. The method including the step of providing at least two distinct growth factors to the damaged liver tissue region of the individual, at least one of the at least two distinct growth factors being an angiogenic factor.
Owner:MULTI GENE VASCULAR SYST
Cardiovascular Compounds Comprising Heterocyclic Nitric Oxide Donor Groups, Compositions and Methods of Use
InactiveUS20080306041A1Improve propertiesAntibacterial agentsBiocideVascular dilatationRenovascular disease
The invention describes compositions and kits comprising at least one cardiovascular compound comprising at least one heterocyclic nitric oxide donor group, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; (j) treating osteoporosis; (k) treating nephropathy; (l) treating peripheral vascular diseases; (m) treating portal hypertension and (n) treating ophthalmic disorders. The cardiovascular compounds are preferably β-adrenergic antagonists, angiotensin-converting enzyme (ACE) inhibitors, anti-hyperlipidemic compounds, and antithrombotic and vasodilator compounds. The heterocyclic nitric oxide donor groups are preferably furoxans, sydnonimines, oxatriazole-5-ones and / or oxatriazole-5-imines.
Owner:NICOX SA
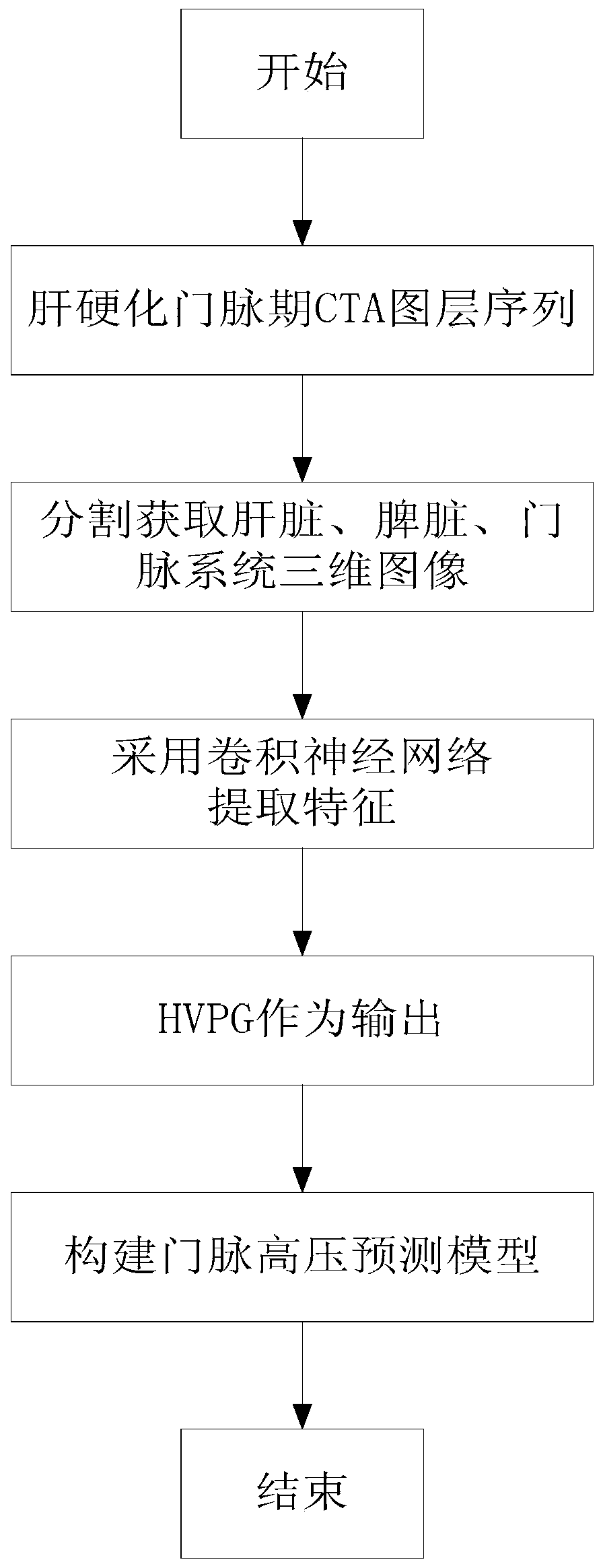
Cirrhotic portal hypertension non-invasive evaluation method based on 3D multi-channel convolutional neural network
PendingCN110755104AHigh sensitivityImprove accuracyComputerised tomographsTomography3d imageMedicine
The invention relates to the field of non-invasive measurement, discloses a cirrhotic portal hypertension non-invasive evaluation method based on a 3D multi-channel convolutional neural network, and solves the problems that in the prior art, an invasive measurement method is high in risk, high in cost and high in operation difficulty, and the accuracy of a current clinical non-invasive predictionmodel is still affected by various interference factors. The method comprises the following steps: S1, acquiring a CTA layer sequence image of a patient with liver cirrhosis and portal hypertension ina portal period; S2, preprocessing the CTA layer sequence image; S3, performing segmentation based on the preprocessed CTA layer sequence image to obtain three-dimensional images of the liver, the spleen and the portal vein system; S4, extracting the characteristics of the three-dimensional images of the liver, spleen and portal system by adopting a multi-channel 3D convolutional neural network,and training by taking a measured HVPG grading value of the patient with liver cirrhosis portal hypertension as output to obtain a stable portal hypertension prediction model; and S5, evaluating the portal pressure of the patient through the stable portal high-pressure prediction model.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
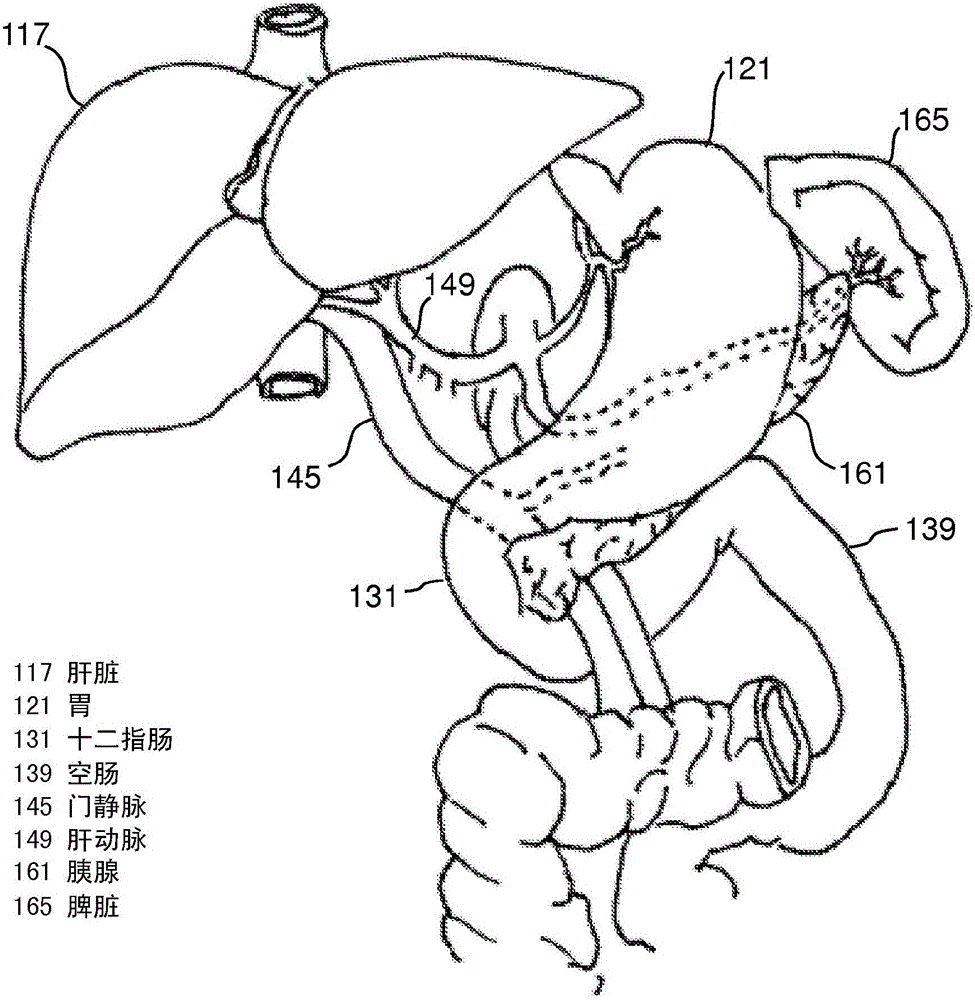
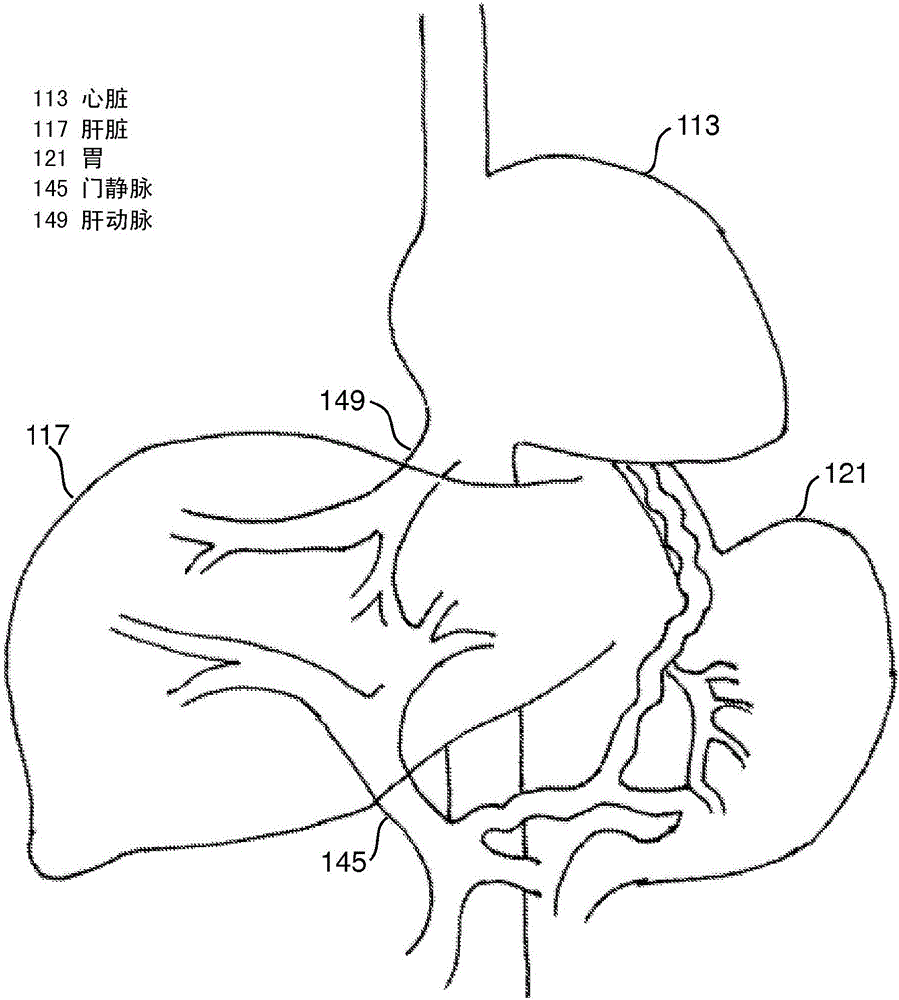
Devices and methods for intrahepatic shunts
The invention provides methods and devices for treating liver cirrhosis or portal hypertension by creating an intrahepatic shunt, or new passage, from a portal vein of a patient to a hepatic vein using a device with intravascular imaging capabilities and pressure sensing capabilities or positioning mechanisms. The integration of intravascular imaging aids in the precise placement of the shunt and pressure measurement may verify successful shunt creation. An apparatus may include a catheter with an extended body for insertion into a hepatic vein of a patient, an intravascular imaging device and a needle exit port on the distal portion of the extended body, and a needle disposed within a lumen in the catheter and configured to be pushed out of the exit port and extend away from a side of the extended body, in which the needle includes a pressure sensor.
Owner:KONINKLJIJKE PHILIPS NV +1
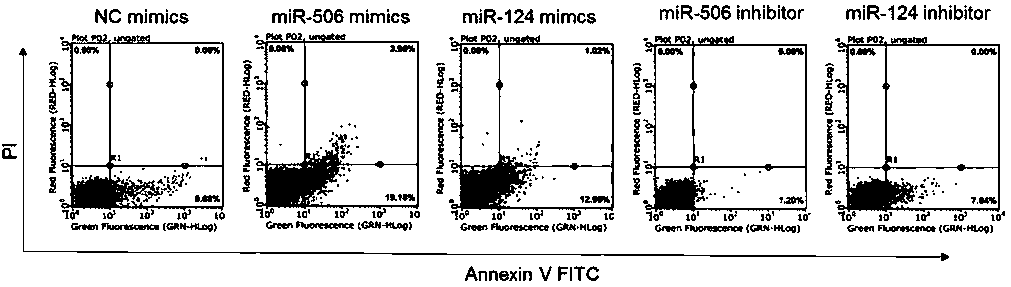
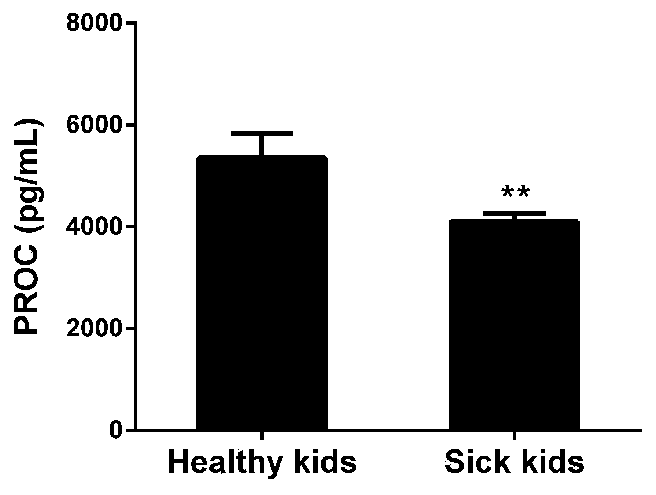
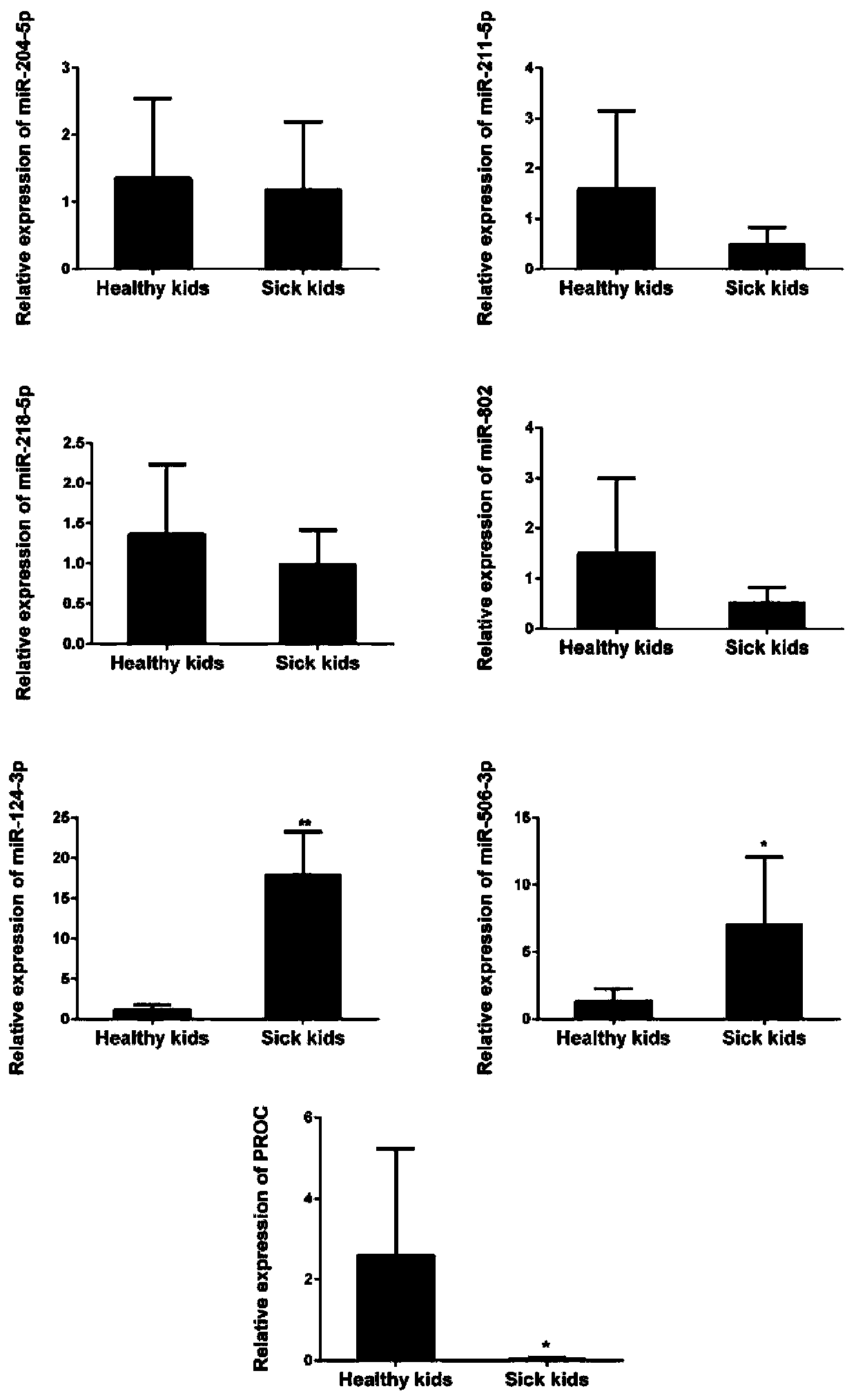
Application of protein C in treating hepatic portal hypertension
ActiveCN110157706APrevent proliferationPromote apoptosisOrganic active ingredientsVector-based foreign material introductionMedicineProtein C inhibitor
The invention relates to application of protein C in treating hepatic portal hypertension, relates to the relevant research of the protein C and portal hypertension, and also relates to protein C inhibitors including miRNA. The protein C inhibitor (miRNA) can inhibit the proliferation of hepatocytes and promote the apoptosis of the hepatocytes, which can reduce hyperplasia tissue of hepatic sinusoids to make portal venous flow smooth and reduce the portal pressure, thereby opening up new research prospects for the treatment of the portal hypertension (especially portal vein cavernous transformation) and providing new ideas for the deep development of miRNA drugs and clinical treatment.
Owner:首都儿科研究所
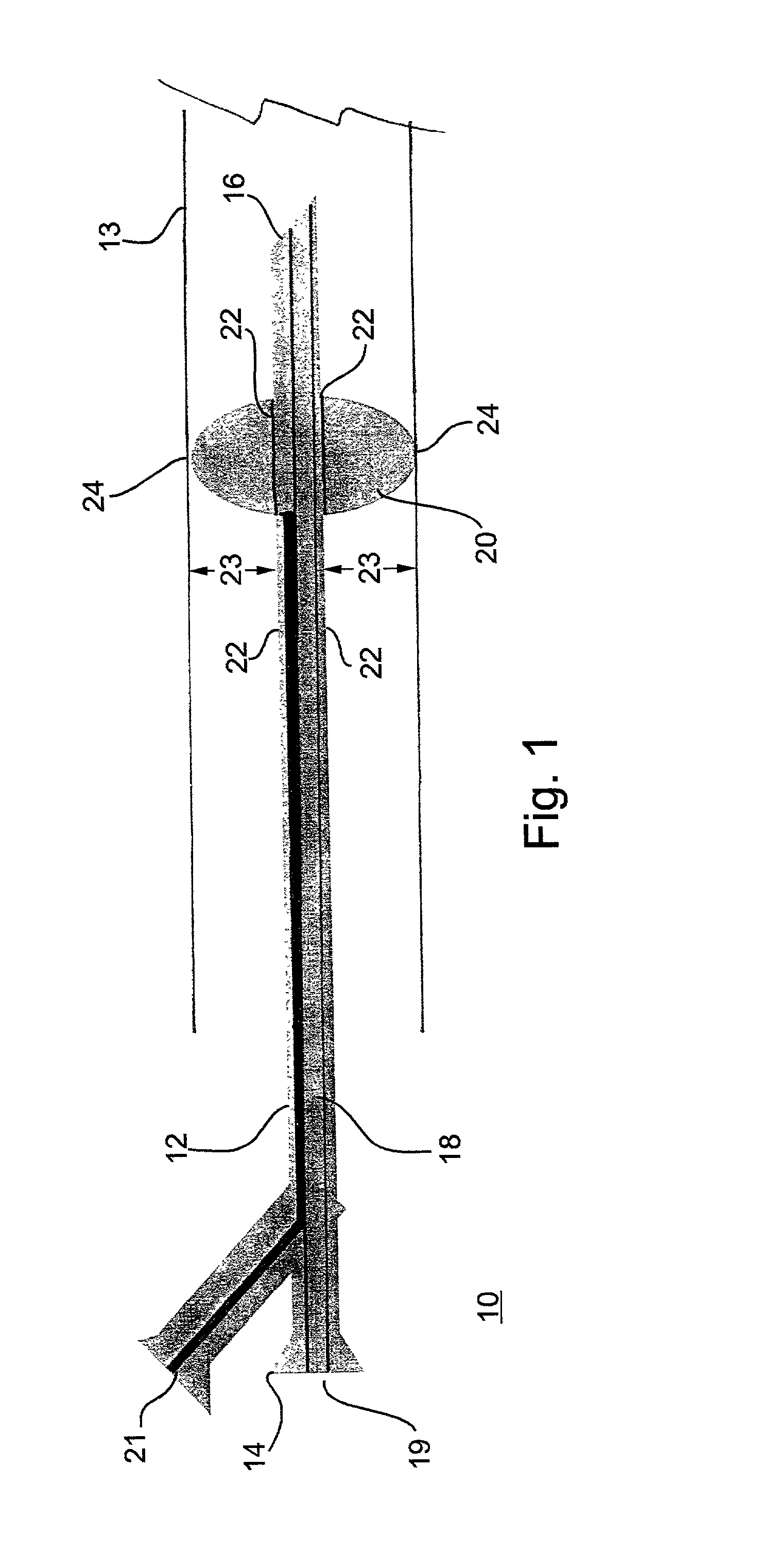
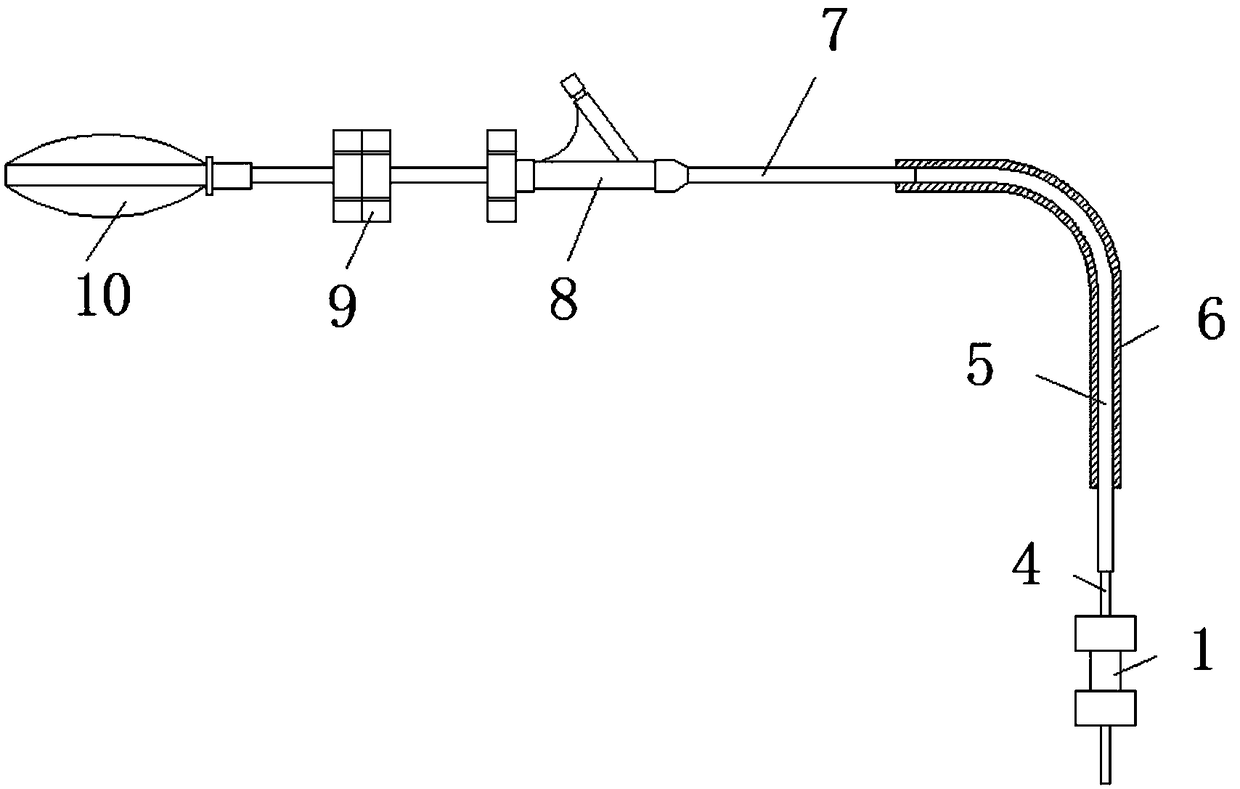
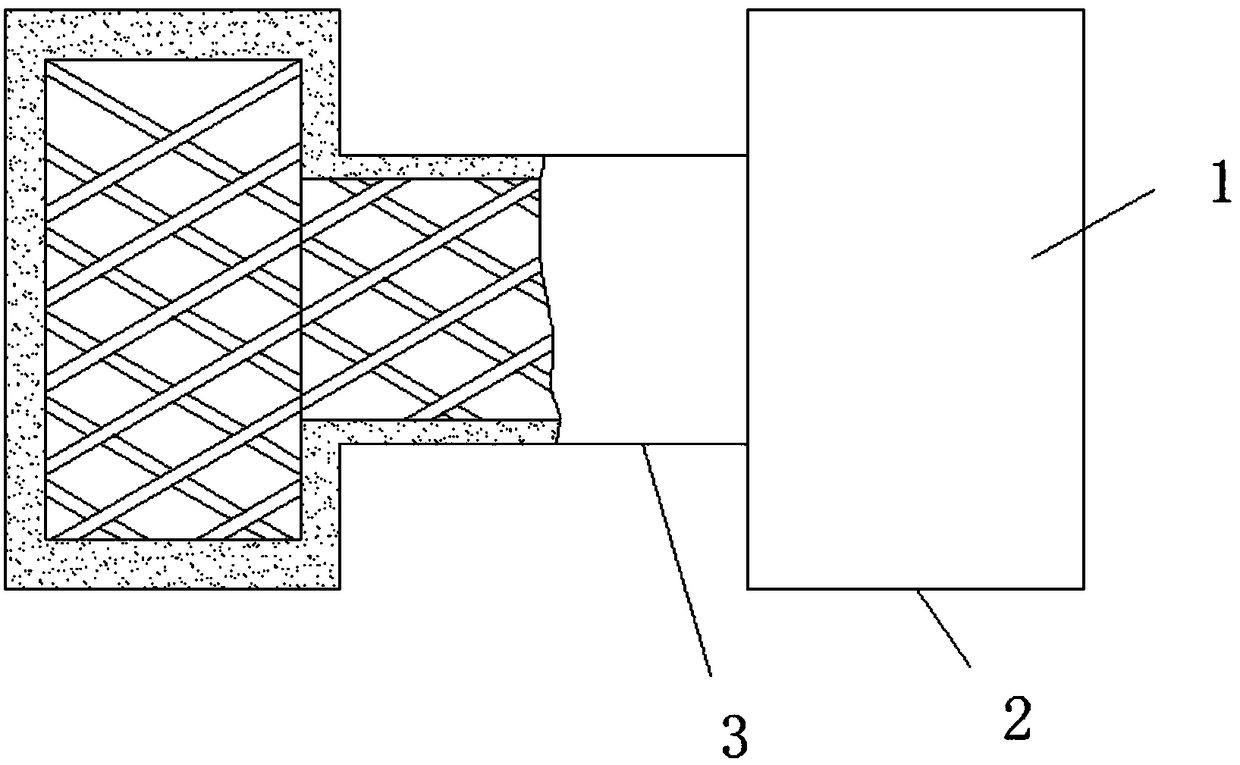
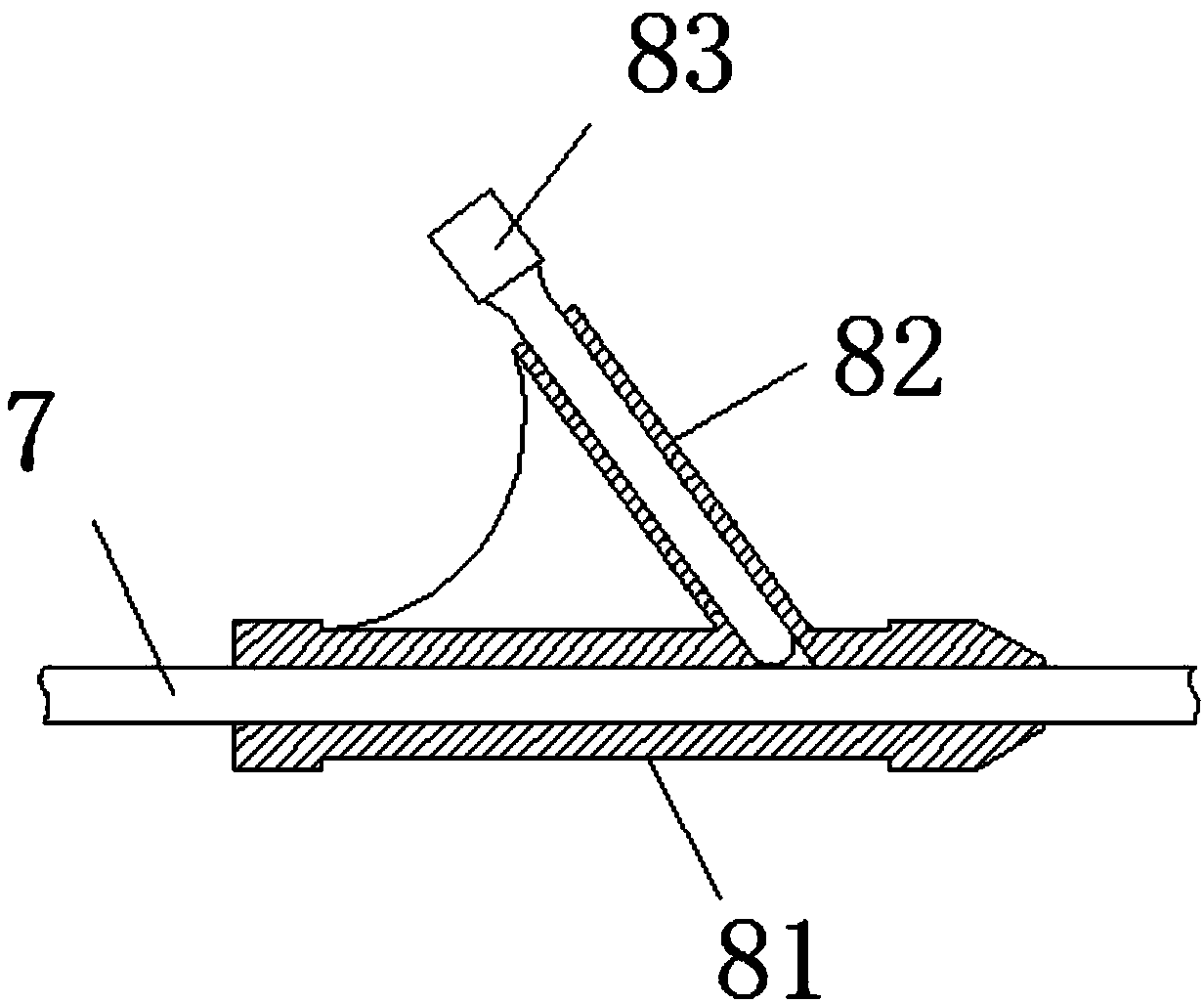
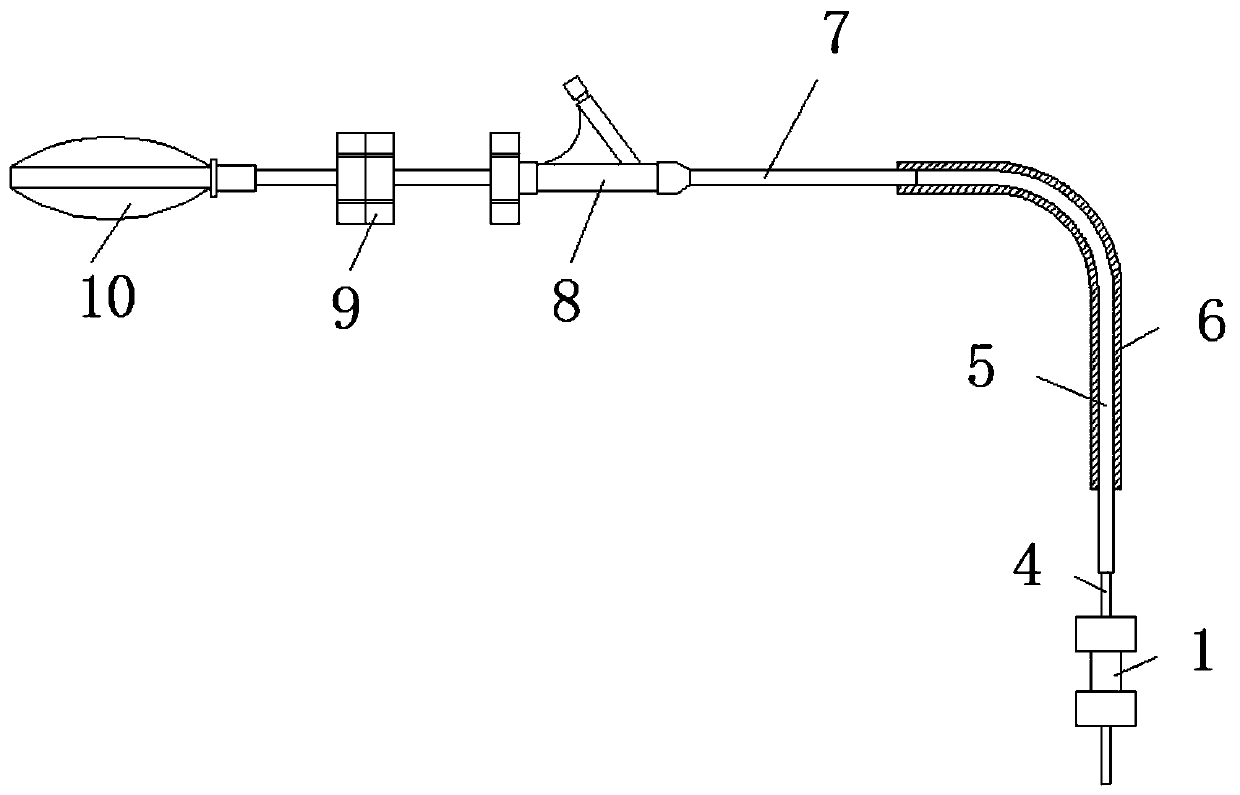
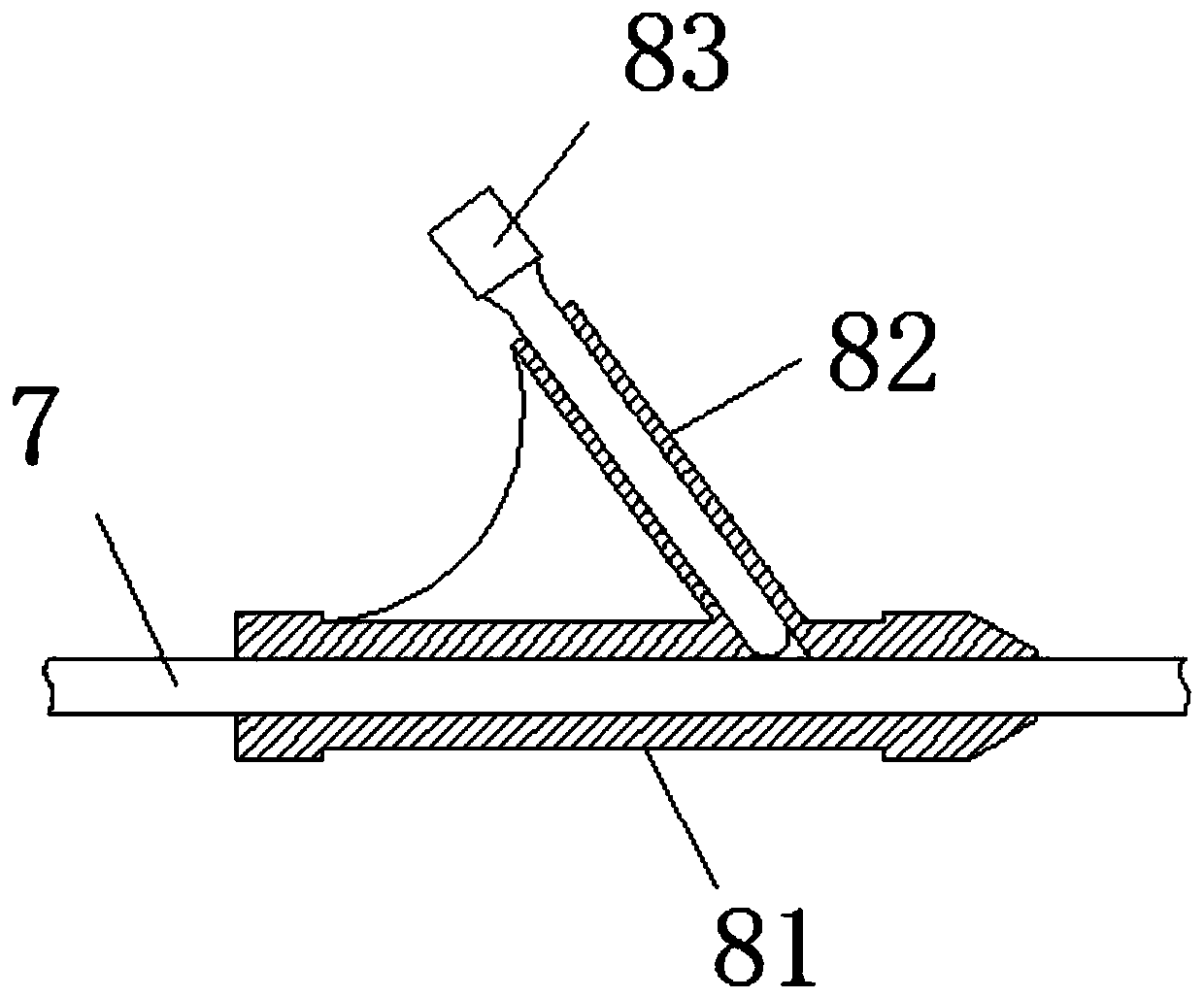
Extrahepatic vascular anastomosis stent and conveying system thereof
The invention discloses an extrahepatic vascular anastomosis stent and a conveying system thereof. The extrahepatic vascular anastomosis stent includes a stent body, a lead-in pipe, a soft pipe, a sleeve pipe, a metal pipe, a fixing device, a screw and a handle; the metal pipe is mounted at one end of the handle, the fixing device and the screw are mounted on the metal pipe, the screw is located between the handle and the fixing device, and the soft pipe is connected to the end, away from the handle, of the metal pipe; the lead-in pipe is mounted at the end, away from the metal pipe, of the soft pipe; the soft pipe is sleeved with the sleeve pipe, and the stent body is mounted on the lead-in pipe. By the adoption of the extrahepatic vascular anastomosis stent and the conveying system thereof, two blood vessels can be drawn close and anchored, and therefore the parallel blood vessels are subjected to anastomosis; the extrahepatic vascular anastomosis stent and the conveying system thereof are used for treating portal hypertension, history that portacaval and mesocaval shunt needs surgical laparotomy operations is changed so that the portacaval and mesocaval shunt can be conducted inan interventional and minimally invasive mode, pain of patients suffering from the portal hypertension is greatly reduced, and the therapeutic effect of the extrahepatic vascular anastomosis stent isimproved.
Owner:SHANDONG PROVINCIAL HOSPITAL AFFILIATED TO SHANDONG FIRST MEDICAL UNIVERSITY
Salt of bile acid derivative, crystal form structure thereof, and preparation method and application of bile acid derivative salt and crystal form structure thereof
InactiveCN113493485AFacilitate depositionImprove solubilityOrganic active ingredientsMetabolism disorderBile JuiceCholestasis
The invention provides a salt of a bile acid derivative, a crystal form of the salt and a preparation method and application of the salt and the crystal form. The salt, the crystal form and a composition of the salt and the crystal form can improve cholestasis, reduce portal pressure, improve liver functions and improve liver functions, and can be used for preparing medicines for treating or relieving chronic liver diseases, metabolic diseases or portal hypertension and related diseases thereof.
Owner:XI AN BIOCARE PHARMA LTD
Non-invasive portal vein pressure gradient measurement method based on medical image
The invention relates to a non-invasive portal vein pressure gradient measurement method based on a medical image. A portal vein simulation model with a scientific and reasonable structure, no clinical invasive surgery, good effect, low cost and easy implementation is used. Through the experiment, the simulation study of the population with cirrhosis and a normal group is carried out in the model,and the non-invasive portal vein pressure gradient measurement method based on the medical image is provided. The results show that the patient with cirrhosis has higher stress than the normal groupunder the method. In the experiment, as an end area decreases, the relative pressure increases correspondingly, an appropriate area is found to divide the thresholds the two types of population, whichis beneficial to the subsequent simulation calculation. The method uses three-dimensional modeling to combine with a hemodynamic simulation calculation method to analyze a continuous high-pressure principle of portal vein, avoids the high cost and personal injury caused by experiment or blind design, and has certain guiding significance for clinical detection of portal hypertension.
Owner:SHANGHAI UNIV
Chinese herbal medicine composition for treating cirrhosis and preparation method thereof
InactiveCN102872424AReduce inflammation and necrosisPromote regenerationAnthropod material medical ingredientsDigestive systemCentipedeTherapeutic effect
Owner:黄庆德
Prophylaxis and/or treatment of portal hypertension
InactiveCN1871010AReduce portal pressureAntibacterial agentsOrganic active ingredientsDiseaseSurgery
The invention relates to a medicament for the prophylaxis and / or treatment of diseases or complications associated with portal hypertension, especially haemorrhagic complications.
Owner:弗赖堡大学综合诊所
Agent for preventing or treating portal hypertension
InactiveUS20060173059A1Preventing and portal hypertensionGood treatment effectBiocideOrganic chemistrySide effectTherapeutic effect
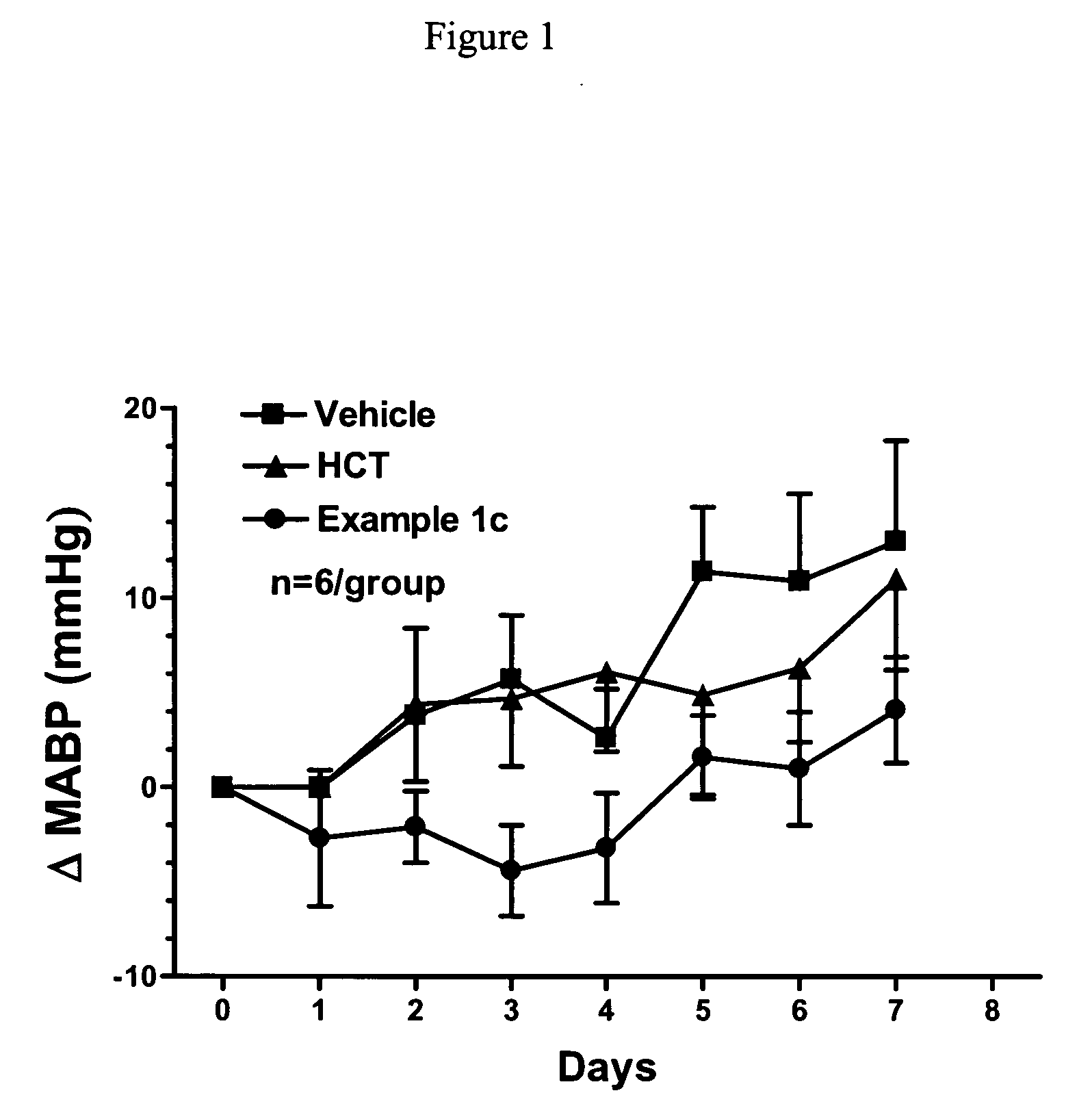
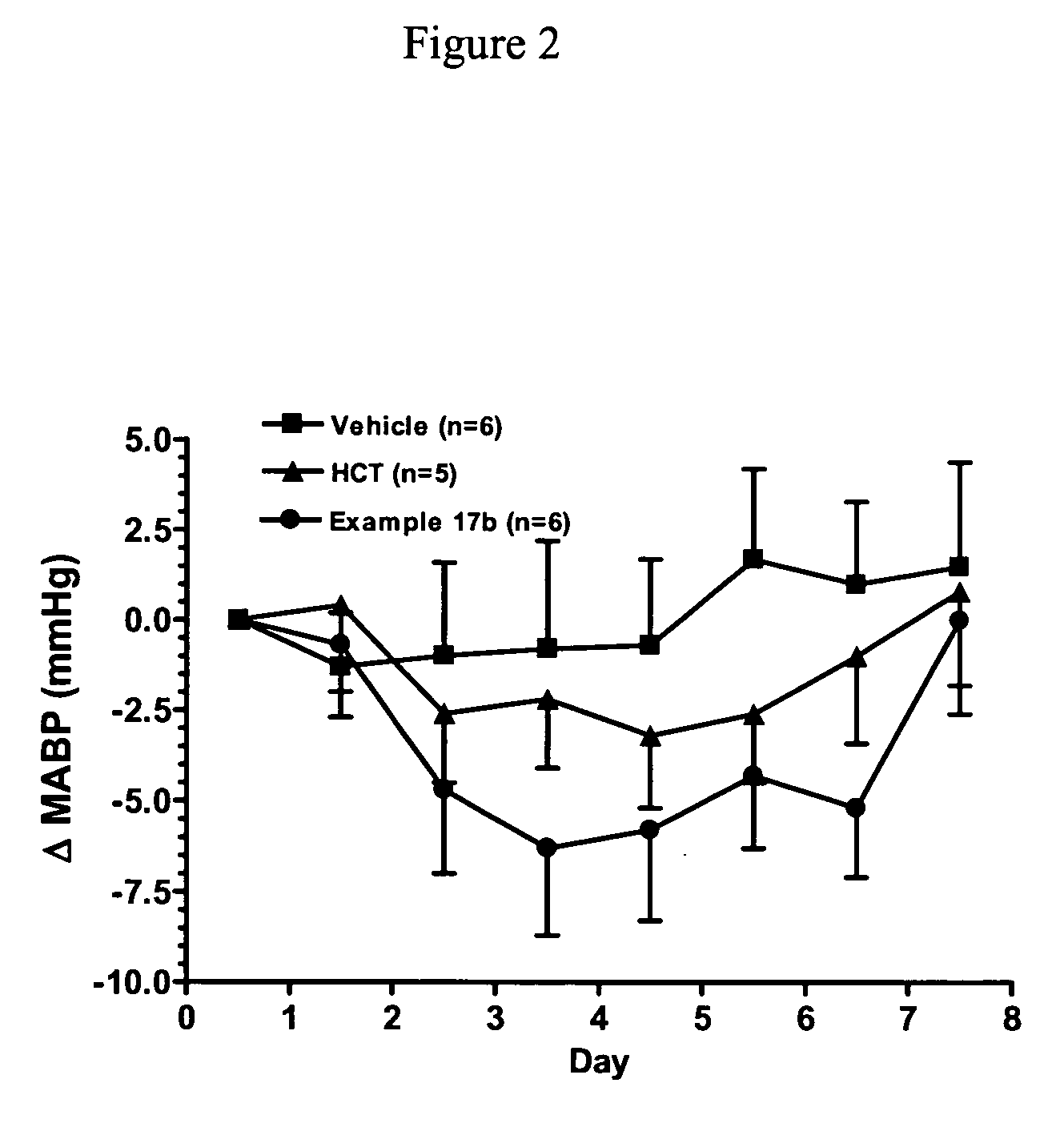
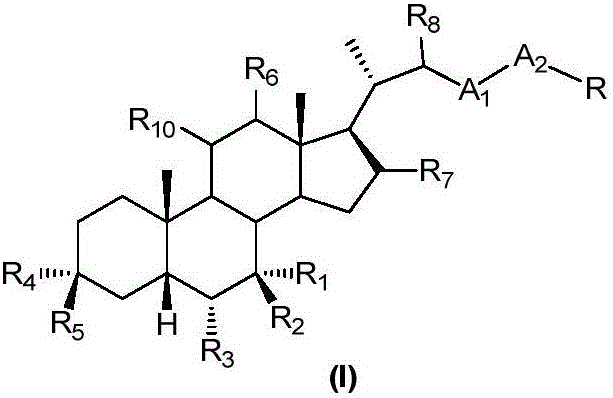
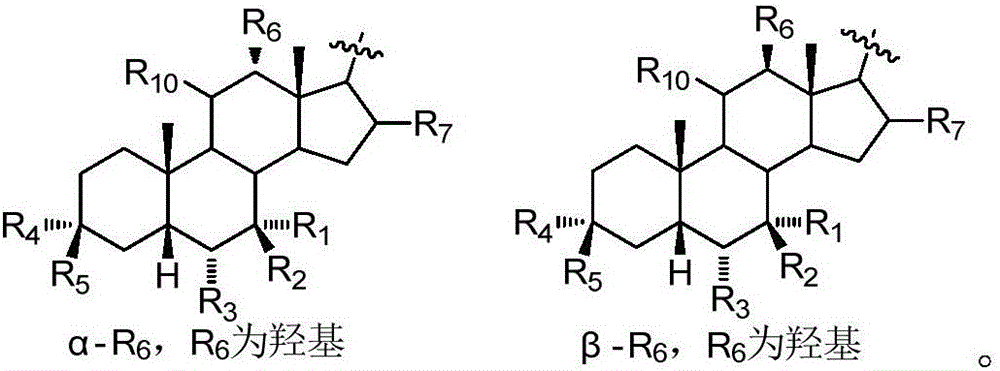
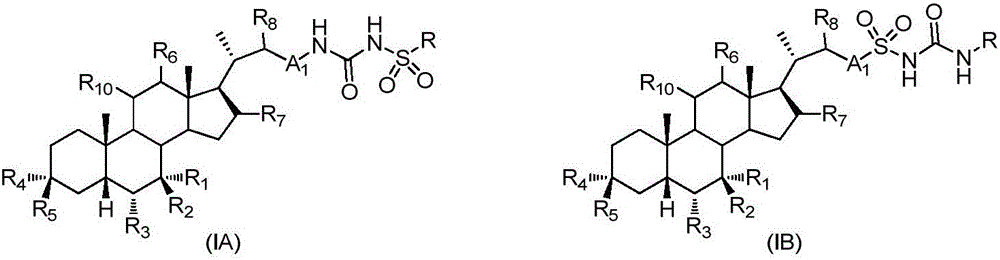
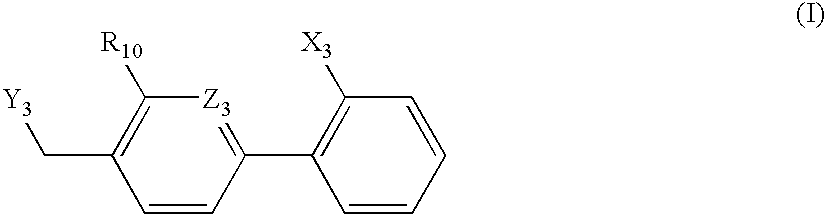
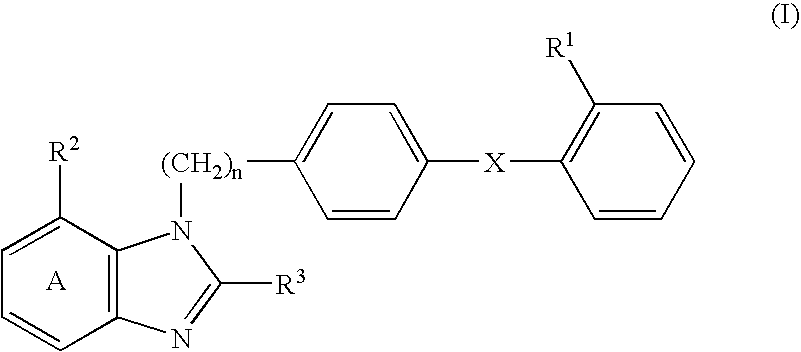
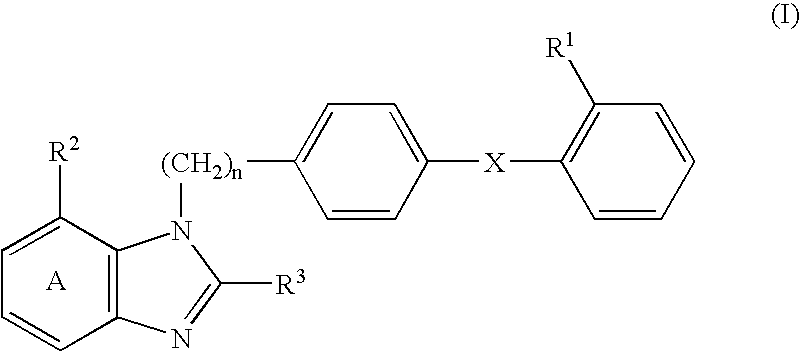
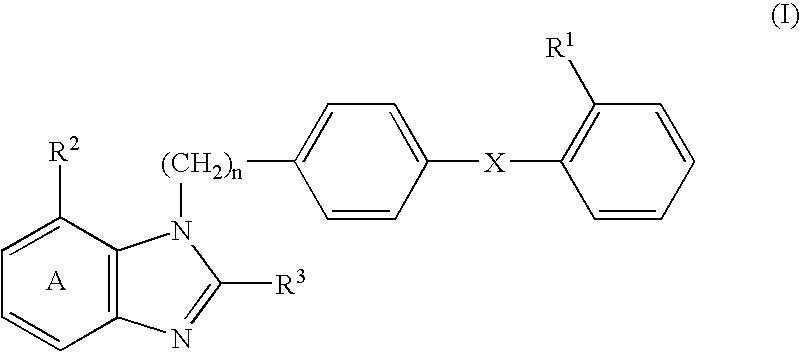
An agent for preventing or treating portal hypertension comprising a compound represented by the general formula (I): wherein R1 represents a group capable of forming an anion, etc.; X represents a bond or a spacer; n is an integer of 1 or 2; ring A is benzene ring which may be further substituted; R2 represents a group capable of forming an anion, etc.; and R3 represents a hydrocarbon group which may bonded via a hetero atom, and may be substituted, a salt thereof or a prodrug thereof, is provided. This agent has sufficiently excellent properties as medicine, since it has excellent prophylactic and therapeutic effects on portal hypertension without any side effect, etc.
Owner:WATANABE TOSHIFUMI +2
Compound for treatment of metabolic diseases, preparation method and application thereof
ActiveCN109929005BHigh potencyLittle side effectsOrganic active ingredientsMetabolism disorderDiseaseMetabolite
The present invention provides a compound for the treatment of metabolic diseases, which has a structure represented by formula (I) or formula (II), or its racemate, stereoisomer, geometric isomer, tautomer , solvates, hydrates, metabolites, pharmaceutically acceptable salts or prodrugs thereof. The compound provided by the invention is an activator of FXR and / or TGR5 receptors, which has the activity of activating FXR and / or TGR5 receptors, and can be used for preparing medicines for treating chronic liver diseases, metabolic diseases or portal hypertension.
Owner:XI AN BIOCARE PHARMA LTD
Cannabis based compositions and methods of treating hypertension
InactiveUS20180104213A1Improve stabilityOrganic active ingredientsMagnoliophyta medical ingredientsPheochromocytomaCannabis
The invention relates to a Cannabis-based pharmaceutical composition for the treatment of hypertensive disorders by submucosal delivery comprising a pharmaceutically acceptable base and an effective amount of at least one cannabinoid or endocannabinoid containing extract of a cloned hybrid of the plant Cannabis sativa, subspecies sativa and Cannabis sativa, subspecies indica of the CTSX-ISS lineage; and methods of treatment of primary and secondary hypertension, the secondary hypertension resulting from pheochromocytoma, primary hyperaldosteronism, adrenal hyperplasia, pulmonary hypertension, portal hypertension, folate deficiency hypertension, arterial hypertension or familial hypertension by administration between one and eight times per day.
Owner:KUBBY PATENT & LICENSES LLC
Nine-raw-material blood stanching powder and preparing method and application thereof
InactiveCN106361984AStrong hemostatic effectGood hemostatic effectPowder deliveryDigestive systemVeinHemoptyses
The invention belongs to the technical field of traditional Chinese medicine, and particularly relates to a nine-raw-material blood stanching powder and a preparing method and application thereof. The nine-raw-material blood stanching powder is prepared from, by weight, 10-20 parts of rhizoma bletillae, 25-35 parts of radix notoginseng, 10-20 parts of charred radix et rhizoma rhei, 15-25 parts of calcined marmor serpentinatum, 15-25 parts of cuttlebone, 15-25 parts of carbonized hair, 15-25 parts of Chinese arborvitae twigs and leaves (carbonized), 15-25 parts of radix rubiae and 15-25 parts of medicinal cyathula roots. The nine-raw-material blood stanching powder is capable of stanching blood and dispersing blood stasis and used for obstruction of collaterals by blood stasis, spontaneous external bleeding, hematemesis, hemoptysis and metrorrhagia embolism in blood caused by blood arterie-and-vein overflowing and cirrhotic portal hypertension patients with the syndromes.
Owner:单晓春
An extrahepatic vascular anastomosis stent and its delivery system
The invention discloses an extrahepatic vascular anastomosis stent and a conveying system thereof. The extrahepatic vascular anastomosis stent includes a stent body, a lead-in pipe, a soft pipe, a sleeve pipe, a metal pipe, a fixing device, a screw and a handle; the metal pipe is mounted at one end of the handle, the fixing device and the screw are mounted on the metal pipe, the screw is located between the handle and the fixing device, and the soft pipe is connected to the end, away from the handle, of the metal pipe; the lead-in pipe is mounted at the end, away from the metal pipe, of the soft pipe; the soft pipe is sleeved with the sleeve pipe, and the stent body is mounted on the lead-in pipe. By the adoption of the extrahepatic vascular anastomosis stent and the conveying system thereof, two blood vessels can be drawn close and anchored, and therefore the parallel blood vessels are subjected to anastomosis; the extrahepatic vascular anastomosis stent and the conveying system thereof are used for treating portal hypertension, history that portacaval and mesocaval shunt needs surgical laparotomy operations is changed so that the portacaval and mesocaval shunt can be conducted inan interventional and minimally invasive mode, pain of patients suffering from the portal hypertension is greatly reduced, and the therapeutic effect of the extrahepatic vascular anastomosis stent isimproved.
Owner:SHANDONG PROVINCIAL HOSPITAL AFFILIATED TO SHANDONG FIRST MEDICAL UNIVERSITY
Chinese medicine composition for treating liver fibrosis and portal vein hypertension and its preparation method
InactiveCN100546595CBlock formationEffective treatmentDigestive systemPharmaceutical delivery mechanismSalvia miltiorrhizaAdjuvant
The invention discloses a traditional Chinese medicine composition for liver fibrosis and portal hypertension. The composition is prepared by taking 1 weight part of licorice extract and 2-7 weight parts of salvia miltiorrhiza as raw materials. The preparation method is to take Danshen and grind it, extract it with hot water; combine the extracts, freeze, and filter; pass the filtrate through a macroporous adsorption resin column, wash the column with water, discard the eluent; then elute with 40% ethanol, and collect the eluate; Concentrate under reduced pressure to dryness to obtain the dry extract of Danshen; mix the dry extract of Danshen and licorice extract, add conventional auxiliary materials, and prepare a clinically acceptable dosage form. In the present invention, glycyrrhizic acid salt and salvianolic acid salt can also be prepared directly as raw materials. The composition of the present invention can significantly reduce the portal vein pressure of liver fibrosis, which has very important clinical value for portal hypertension associated with chronic hepatitis, liver fibrosis and liver cirrhosis, and fills the gap in drug treatment of this pathological state. blank.
Owner:BEIJING KYY BIOSCI
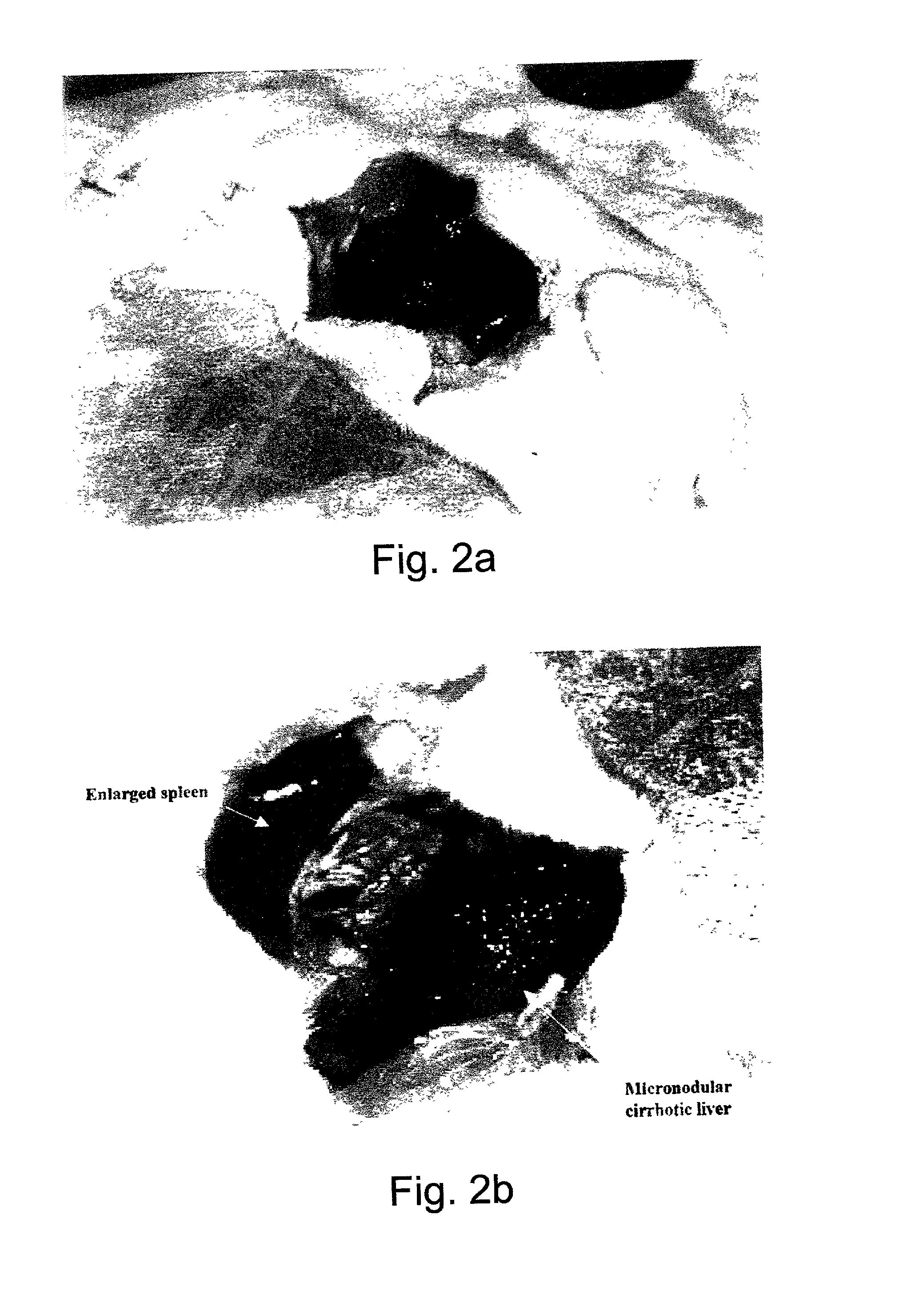
Composite reagent for fast preparation of portal hypertension animal models and preparation process
Composite reagent for a fast preparation of portal hypertension animal models is composed of granulated medical grade polyurethane, analytical pure tetrahydrofuran, and tetrachloromethane solution. A preparation process includes the following steps: (1) cleaning and drying the granulated medical grade polyurethane and placing into a sealable glass container, (2) adding the analytical pure tetrahydrofuran to the glass container, sealing the container, and placing the container still at a room temperature, (3) adding the tetrachloromethane solution, shaking and mixing evenly, (4) puncturing a portal vein under guide of B-ultrasound or injecting the composite reagent at a time by puncturing the portal vein on a hypogastric small incision, and therefore the portal hypertension animal models are built instantly. As organic solvent in the composite reagent is compatible with water in blood, polyurethane constituents are rapidly separated out to prevent pressure of the portal vein from rising due to blood flow of the portal vein. Meanwhile, tetrachloromethane enables liver to become cirrhosis so that the increasing pressure of the portal vein is stabilized. The composite reagent for the fast preparation of the portal hypertension animal models and the preparation process have the advantages of being fast in speed, little in damage, simple to operate and high in success rate.
Owner:XI AN JIAOTONG UNIV
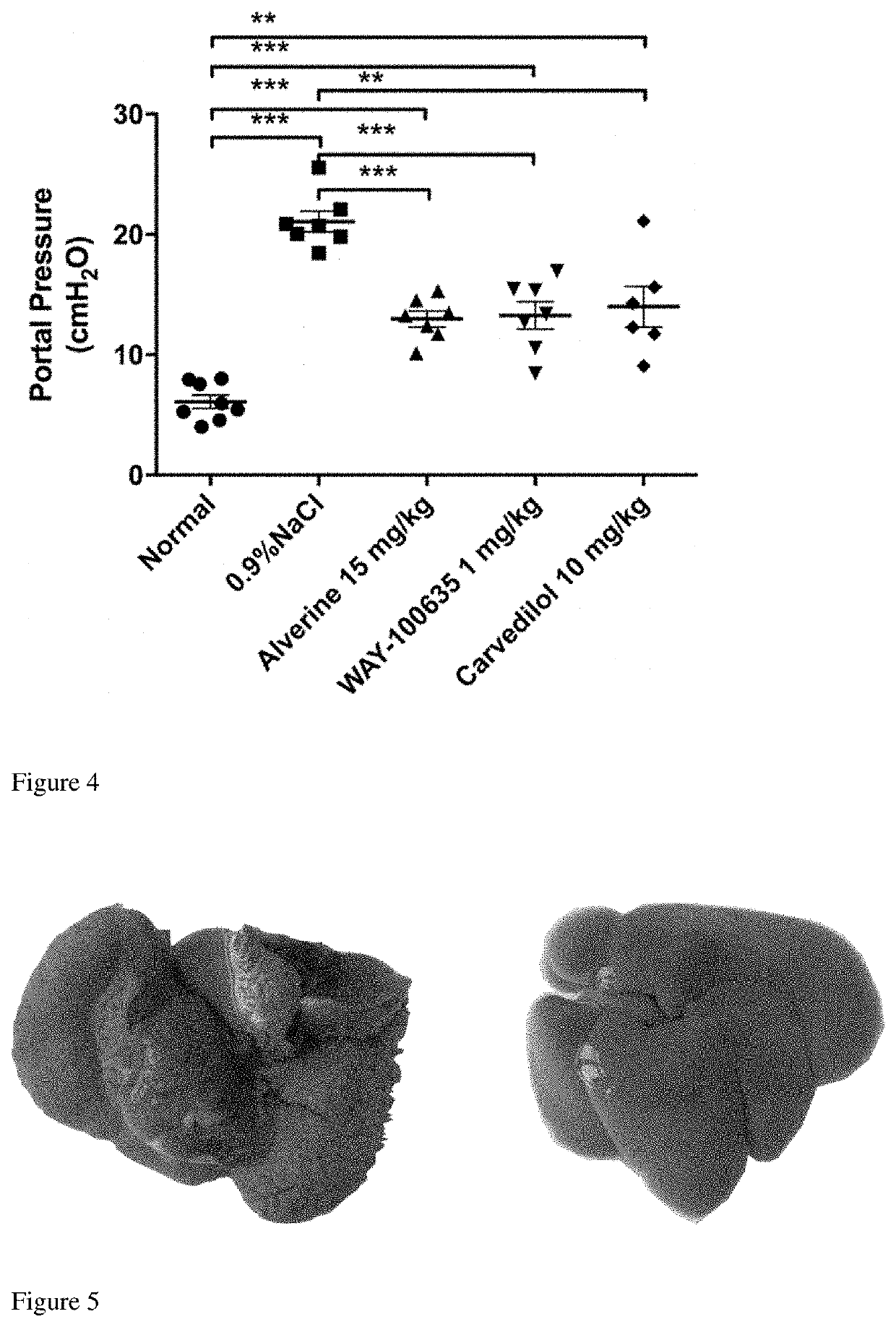
Application of 5-hydroxytryptamine receptor 1a in preparing drug for portal hypertension
InactiveUS20210038595A1Decrease portal pressureIncrease portal pressureOrganic active ingredientsCardiovascular disorderIntraperitoneal routeVena porta
The present invention relates to the technical field of pharmaceuticals and specifically describes an application of a 5-hydroxytryptamine receptor 1A in preparing a drug for portal hypertension. The present invention achieved a significant decrease in portal venous pressure in model rats with cirrhotic portal hypertension by means of an HTR1A inhibitor WAY100635 administered at 1 mg / kg / day by intraperitoneal injection or alverine administered at 15 mg / kg / day by oral gavage. A 5-hydroxytryptamine receptor 1A antagonist can be used to prepare an experimental drug for the treatment of cirrhotic portal hypertension. Furthermore, the 5-hydroxytryptamine receptor 1A antagonist can also be used to prepare drugs for symptoms of portal hypertension syndrome, such as esophageal and fundal varices, rupture and bleeding, ascites, or hepatic encephalopathy. The present invention provides a novel treatment means to treat the manifestations of portal hypertension syndrome, such as esophageal and fundal varices, rupture and bleeding, ascites, or hepatic encephalopathy.
Owner:SECOND AFFILIATED HOSPITAL SECOND MILITARY MEDICAL UNIV
Biomarker composite test for hepatic vein pressure gradient and cirrhosis treatment
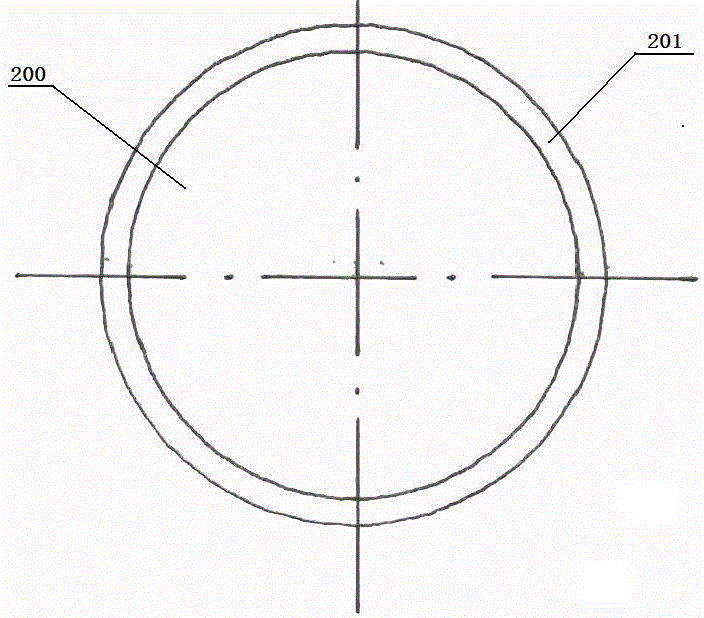
Diagnostic biomarker panel, method, kit, and device for diagnosing the severity and / or prognosis of cirrhosis are provided. More specifically, the invention provides a novel biomarker panel correlating to HVPG and esophageal varices. The invention further provides a biomarker panel and non-invasive test methods that predict non-clinically significant portal hypertension HVPG and non-clinically significant esophageal varices when the expression of the biomarker panel correlates with HVPG of less than 12 mmHg. The invention further provides that the patients with the expression of the biomarker panel correlating to non-clinically significant HVPG and esophageal varices can be excluded from undergoing esophagogastroduodenoscopy (EGD) screening and those correlating to HVPG equal to or greater than 12 mmHg are indicated for EGD.
Owner:RGT UNIV OF CALIFORNIA
Magnetic module, and magnet therapy group and bellyband containing the same
ActiveCN105056401AImprove applicabilityGood effectElectrotherapyMagnetotherapyCondensed matter physicsMagnet therapy
The invention relates to a magnetic module containing a circular-cake-shaped lower magnet and a circular-ring-shaped upper magnet. The circular-ring-shaped upper magnet is arranged above the circular-cake-shaped lower magnet; and the circular-ring-shaped upper magnet and the circular-cake-shaped lower magnet are divided into a split mode and a combined mode. Selectively, a central hole is formed or is not formed in the circular-cake-shaped lower magnet, wherein the size of the central hole meets a respective proper formula; and characteristics of proper size and good effect are realized. A health-care bellyband formed by the magnetic module has characteristics of convenient usage and convenient wearing; objectives of illness elimination, heath care, and recovery can be achieved; effects of liver stagnate dredging and portal hypertension alleviation can be realized; and the effects are obvious and unique.
Owner:魏奎珠
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com