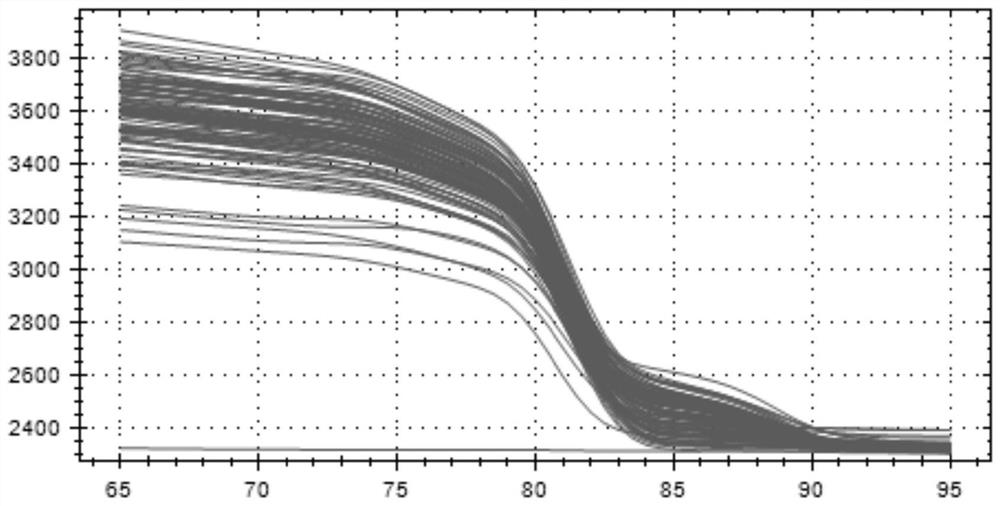
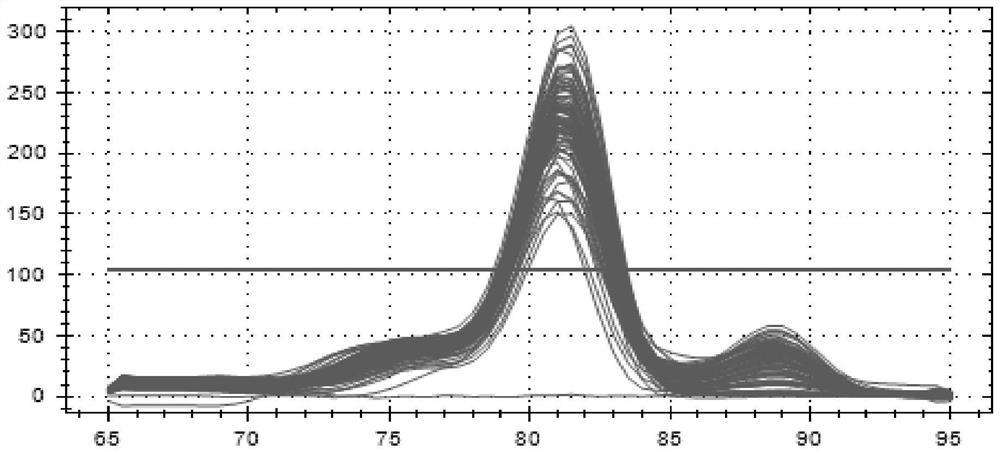
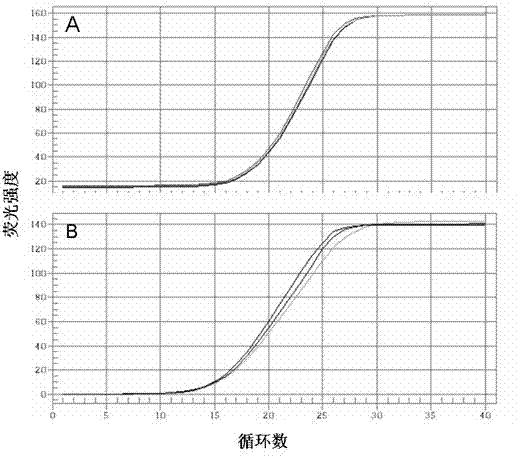
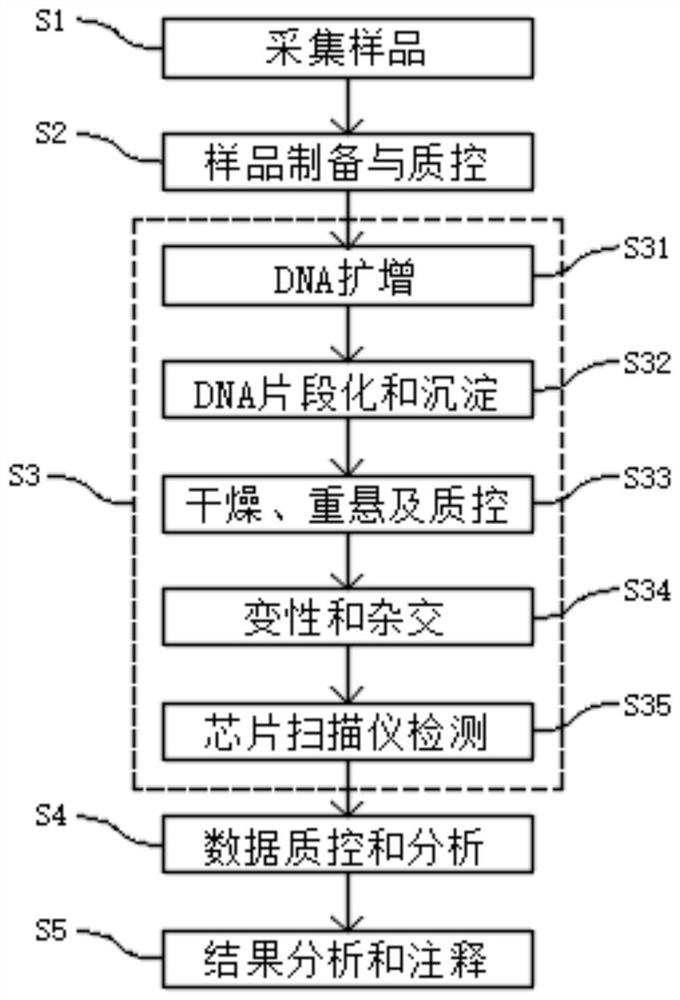
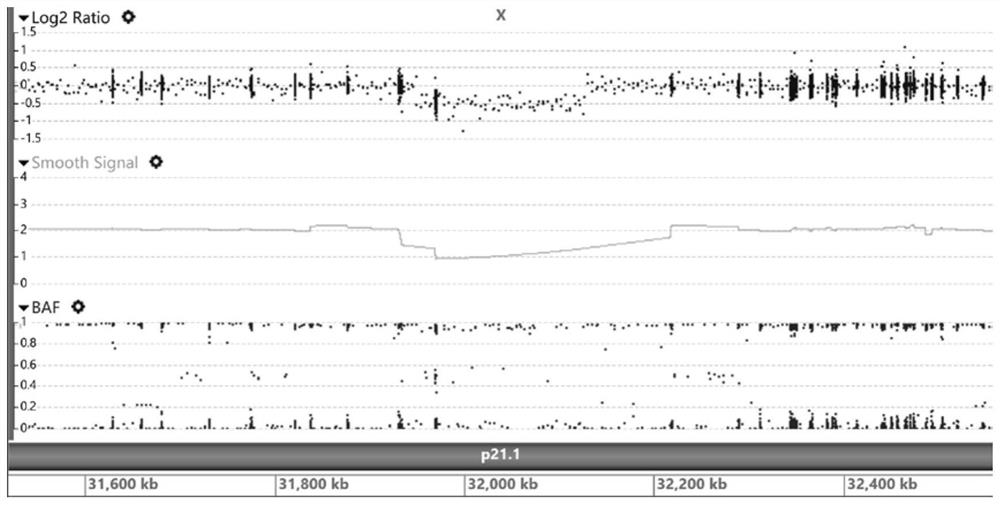
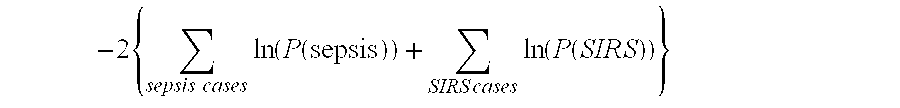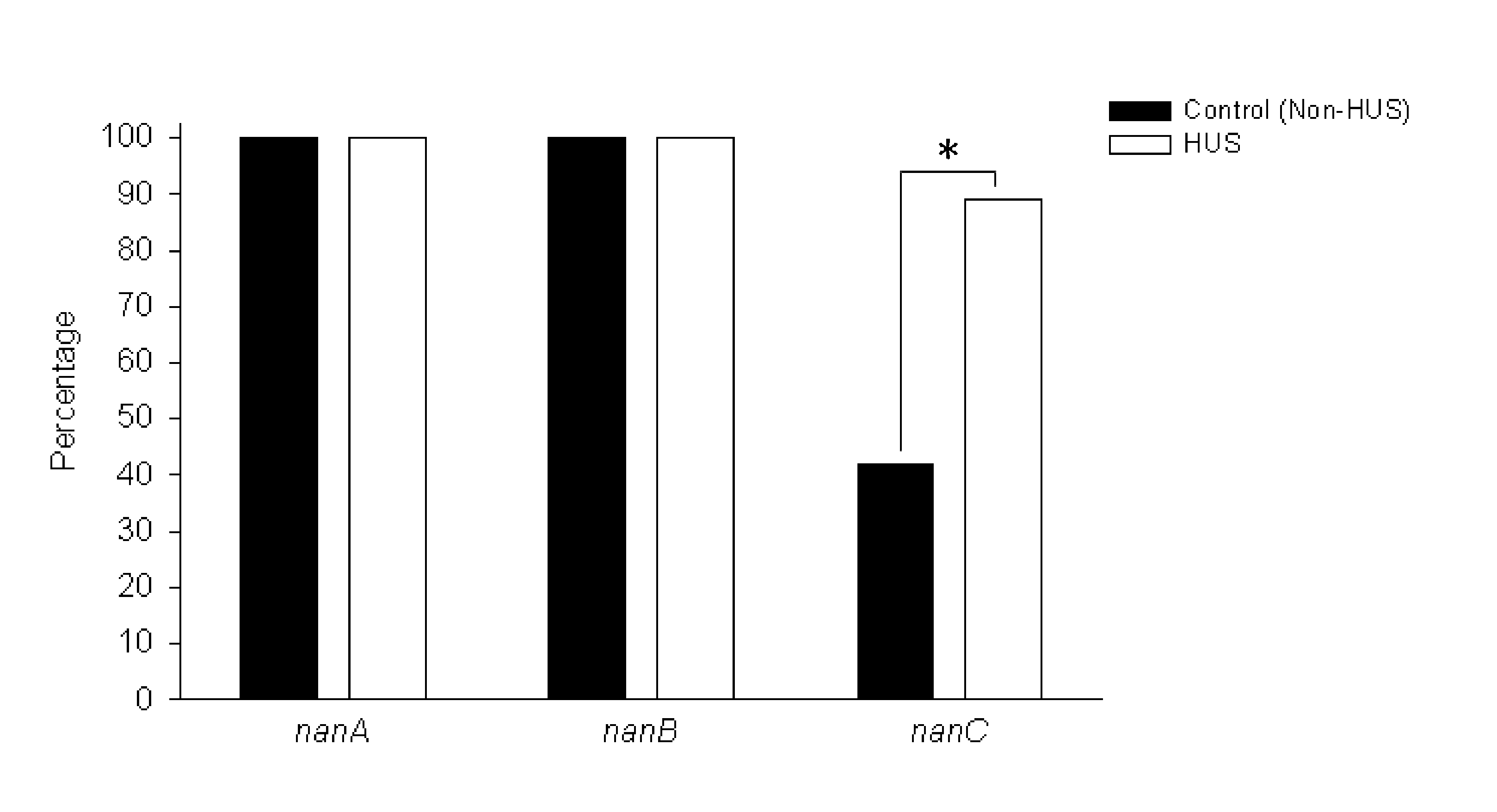Patents
Literature
49 results about "Molecular Diagnostic Method" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods for diagnosis or monitoring of disease predisposition by translation and validation of molecular discoveries in medicine into the clinical diagnostic setting.
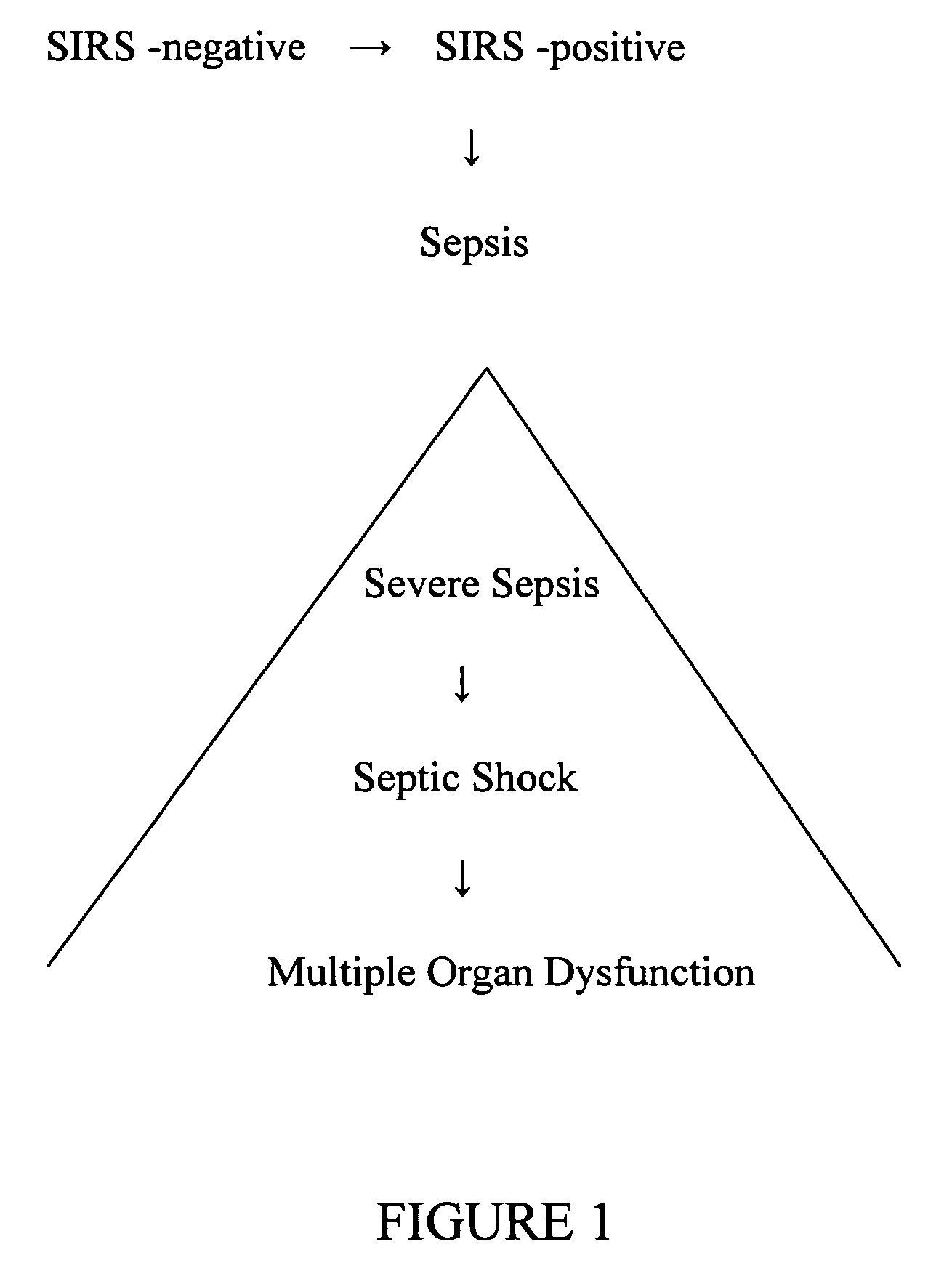
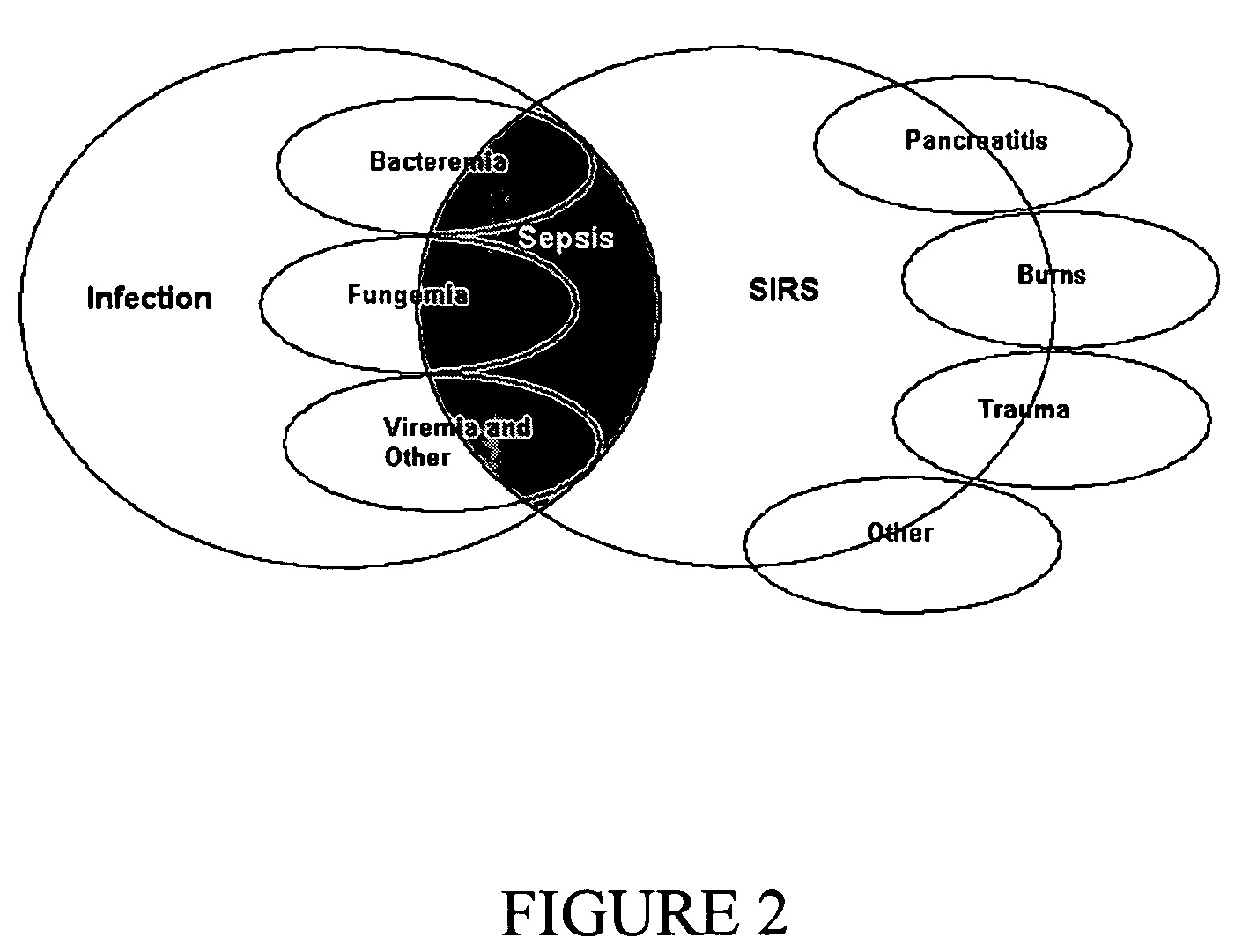
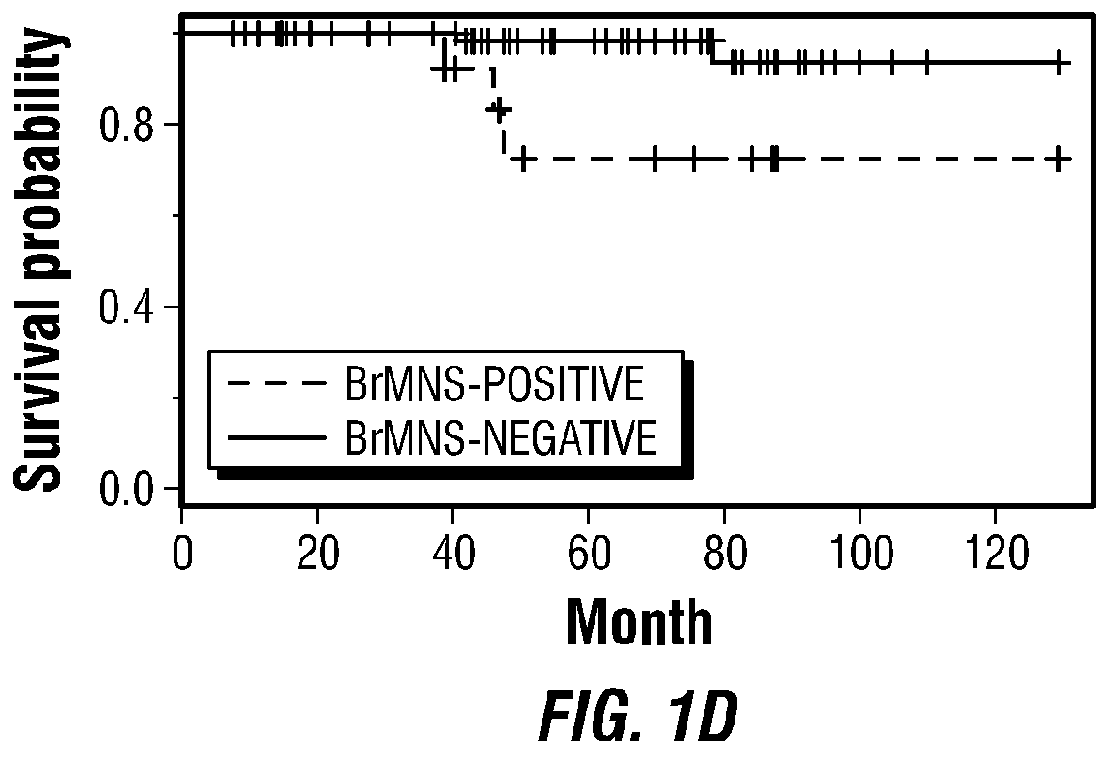
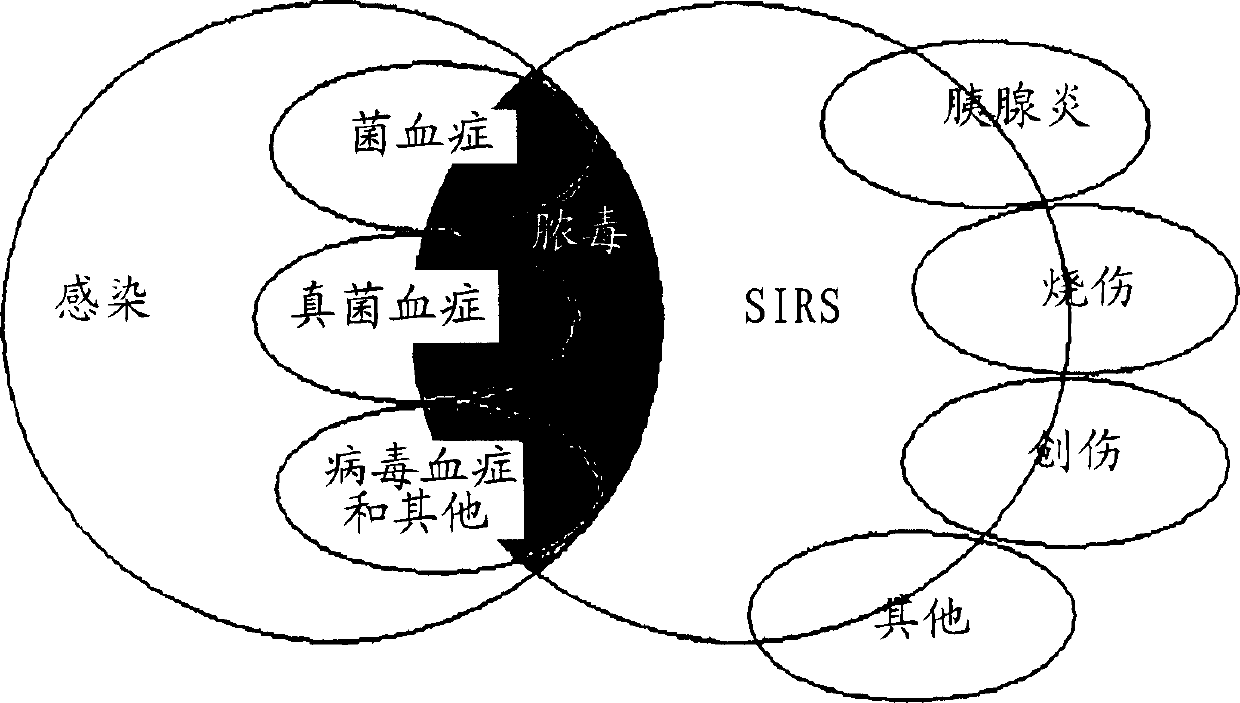
Diagnosis of sepsis or SIRS using biomarker profiles
ActiveUS7645573B2Accurate and rapid and sensitive prediction and diagnosisMicrobiological testing/measurementDisease diagnosisDiseaseEarly prediction
The early prediction or diagnosis of sepsis advantageously allows for clinical intervention before the disease rapidly progresses beyond initial stages to the more severe stages, such as severe sepsis or septic shock, which are associated with high mortality. Early prediction or diagnosis is accomplished using a molecular diagnostics approach, involving comparing an individual's profile of biomarker expression to profiles obtained from one or more control, or reference, populations, which may include a population who develops sepsis. Recognition of features in the individual's biomarker profile that are characteristic of the onset of sepsis allows a clinician to diagnose the onset of sepsis from a bodily fluid isolated at the individual at a single point in time. The necessity of monitoring the patient over a period of time is, therefore, avoided, advantageously allowing clinical intervention before the onset of serious symptoms. Further, because the biomarker expression is assayed for its profile, identification of the particular biomarkers is unnecessary. The comparison of an individual's biomarker profile to biomarker profiles of appropriate reference populations likewise can be used to diagnose SIRS in the individual.
Owner:BECTON DICKINSON & CO
Method for the Molecular Diagnosis of Prostate Cancer and Kit for Implementing Same
InactiveUS20100227317A1Microbiological testing/measurementImmunoglobulinsProstate sampleIn vitro analysis
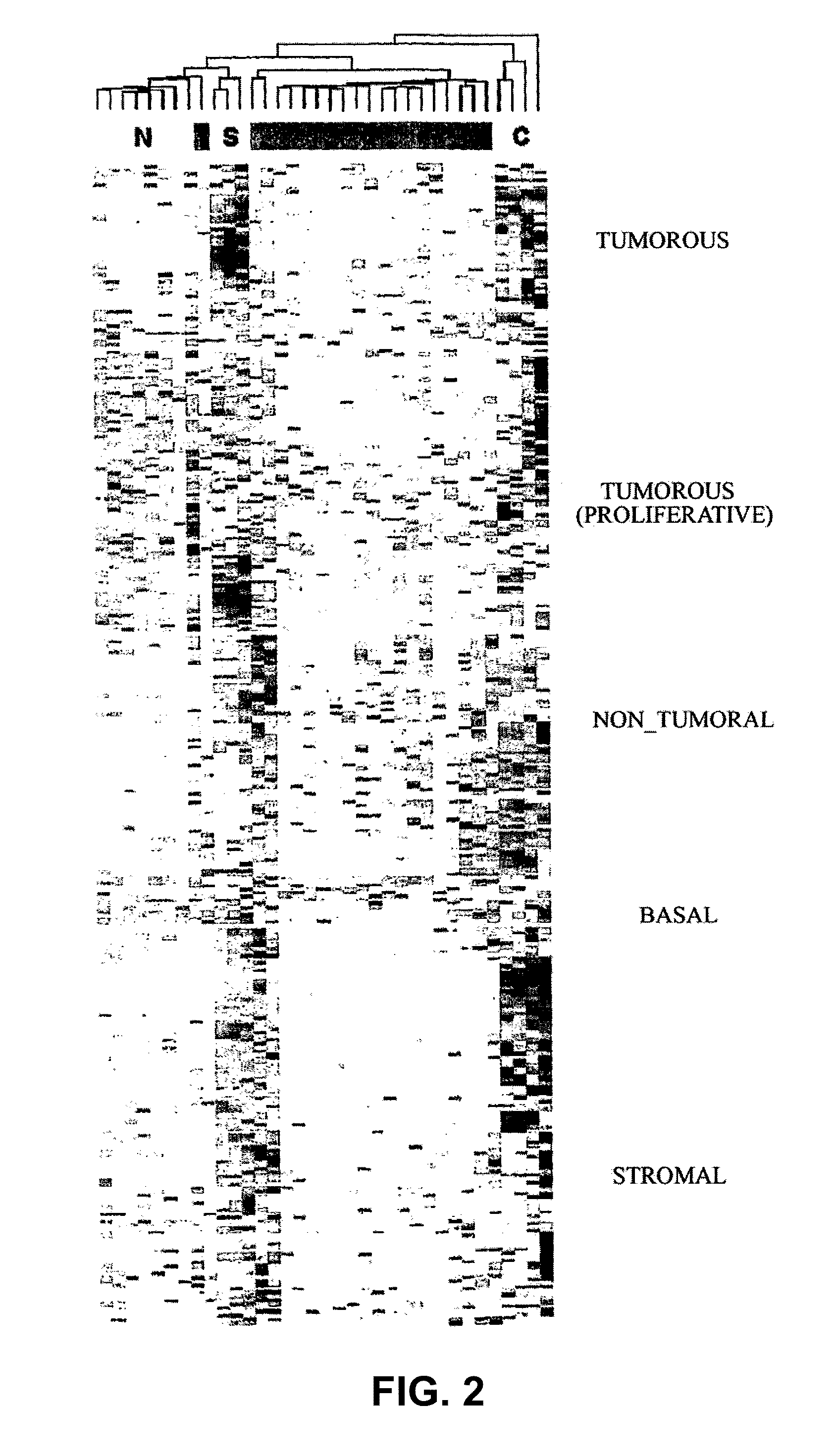
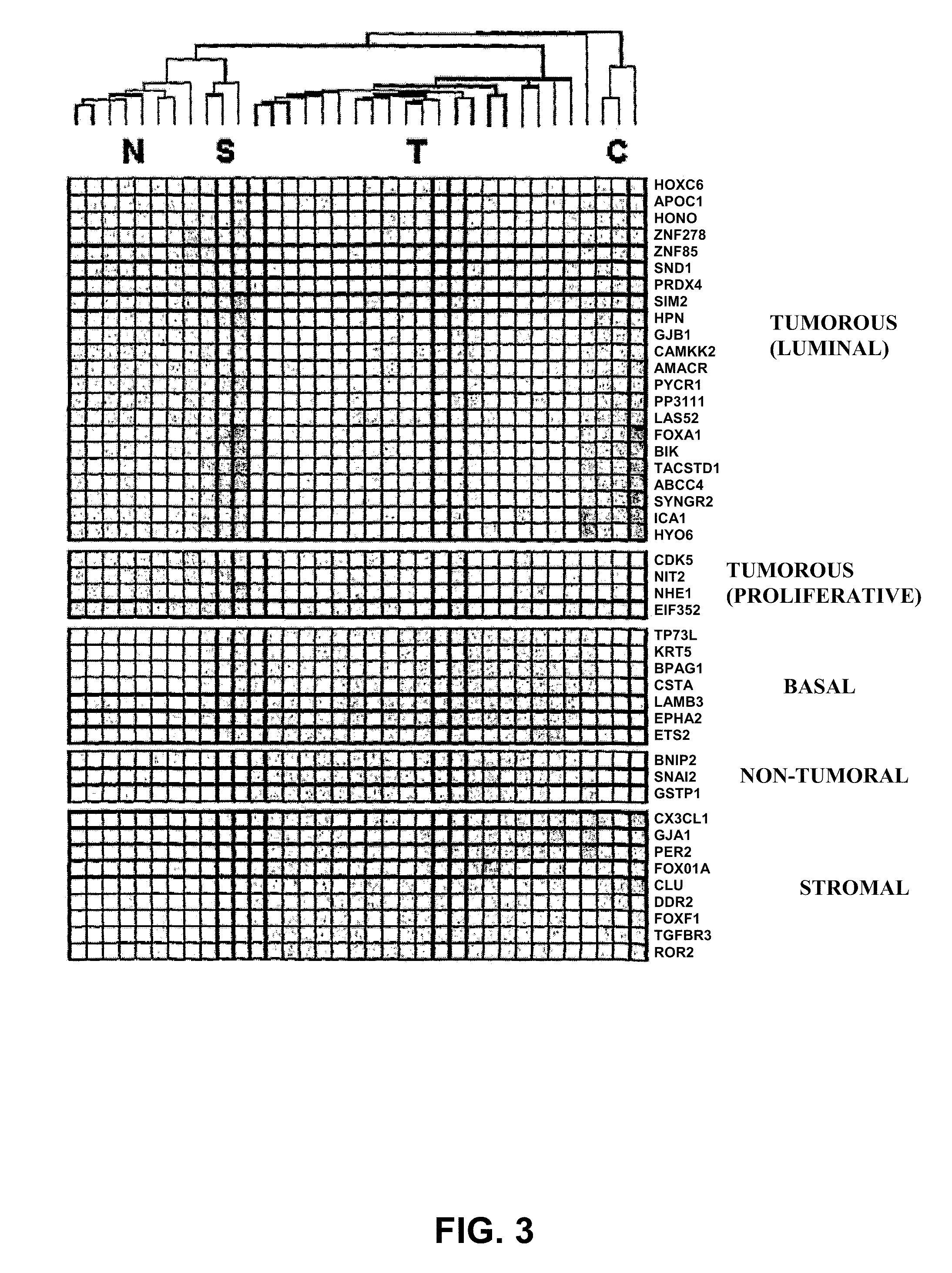
The invention relates to a method for the molecular diagnosis of prostate cancer, comprising the in vitro analysis of the overexpression or underexpression of combinations of genes that can distinguish, with high statistical significance, tumorous prostate samples from non-tumorous prostate samples. The invention also relates to a kit for the molecular diagnosis of prostate cancer, which can perform the above-mentioned detection.
Owner:FUNDACIO PRIVADA CLINIC PER A LA RECERCA BIOMEDICA +1
Approach to molecular diagnosis of human papillomavirus-related diseases
ActiveUS7361460B2Accurate sensitive toolReduce in quantitySugar derivativesMicrobiological testing/measurementProtein markersSequential method
The present invention relates to an accurate, sensitive, and efficient sequential or concurrently sequential method for molecular diagnosis of human papillomavirus (HPV)-based disease, where the method improves the accuracy and reliability of diagnostic and prognostic assessments of HPV-based disease. The method of the invention comprises a primary screen of a sample for HPV nucleic acids, followed by a secondary screen for molecular markers, such as proliferation and cell cycle control group protein markers. The sequential or concurrently sequential method significantly reduces the number of false positive results.
Owner:DIGENE CORP
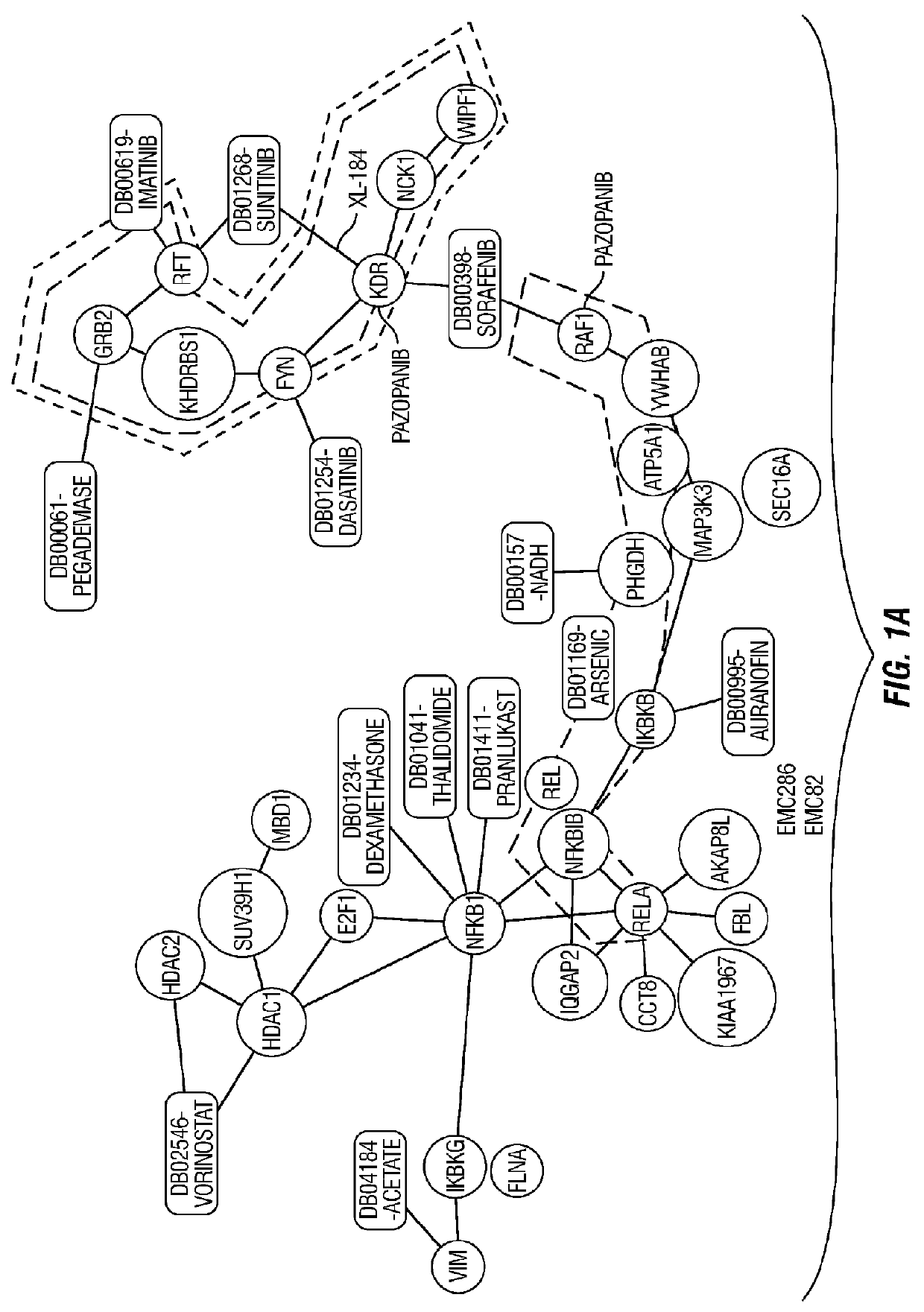
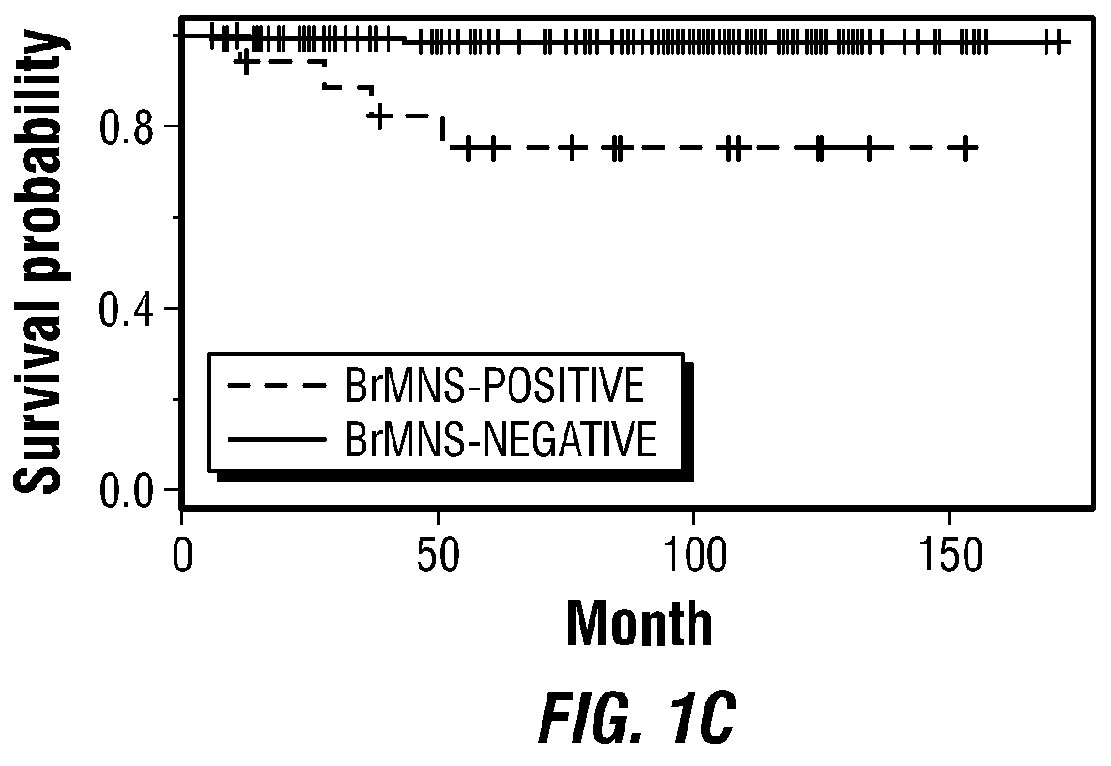
Molecular diagnostic methods for predicting brain metastasis of breast cancer
InactiveUS20120184560A1Improve personalizationEfficient targetingChemical property predictionOrganic active ingredientsPharmaceutical drugOncology
Disclosed are molecular diagnostic compositions and methods for predicting brain metastasis of breast cancer, as well as methods for drug repositioning to identify existing and new therapeutics for use in developing individualized, patient-specific treatment regimens for improving diagnoses and patient outcomes in individuals at risk for brain metastasis of breast cancer.
Owner:THE METHODIST HOSPITAL RES INST
Molecular diagnosis of sex-linked dwarf chicken and its uses in dwarf chicken breeding
InactiveCN1995395AImprove efficiencyImprove accuracyMicrobiological testing/measurementTibiaGrowth hormone
The invention discloses a molecular diagonizing method of sex chained dwarf chick and application to breed dwarf chick, which is characterized by the following: judging the caused reason from four mutation points of growth hormone acceptor; obtaining the mutation number; purifying the dwarf chicken; improving weight and tibia length and evenness.
Owner:SOUTH CHINA AGRI UNIV
Diagnosis of sepsis or SIRS using biomarker profiles
InactiveCN1738908AMicrobiological testing/measurementDisease diagnosisEarly predictionMortality rate
The early prediction or diagnosis of sepsis advantageously allows for clinical intervention before the disease rapidly progresses beyond initial stages to the more severe stages, such as severe sepsis or septic shock, which are associated with high mortality. Early prediction or diagnosis is accomplished using a molecular diagnostics approach, involving comparing an individual's profile of biomarker expression to profiles obtained from one or more control, or reference, populations, which may include a population that develops sepsis. Recognition of features in the individual's biomarker profile that are characteristic of the onset of sepsis allows a clinician to diagnose the onset of sepsis from a bodily fluid isolated at the individual at a single point in time. The necessity of monitoring the patient over a period of time is, therefore, avoided, advantageously allowing clinical intervention before the onset of serious symptoms of sepsis. Further, because the biomarker expression is assayed for its profile, identification of the particular biomarkers is unnecessary. The comparison of an individual's biomarker profile to biomarker profiles of appropriate reference populations likewise can be used to diagnose SIRS in the individual.
Owner:BECTON DICKINSON & CO
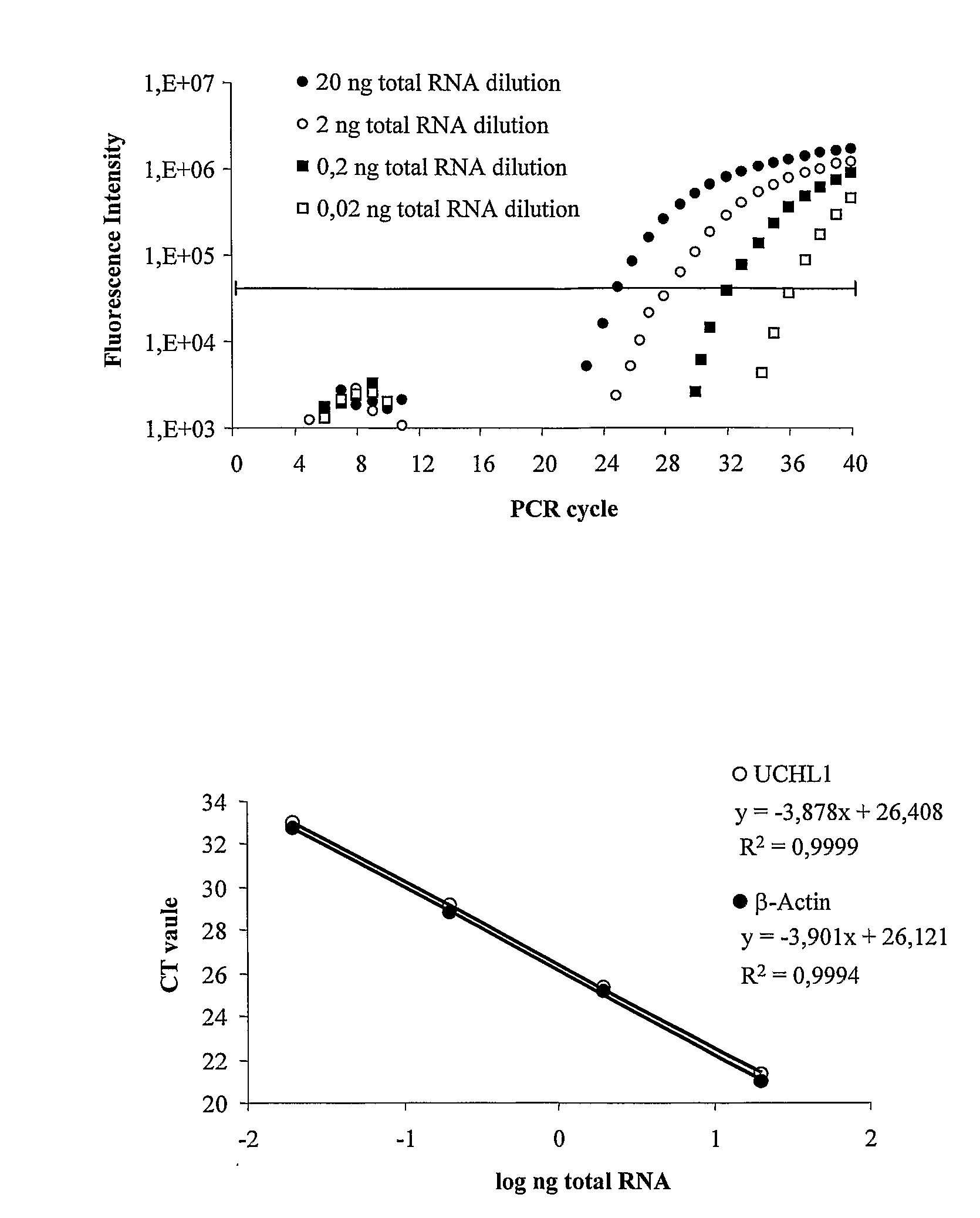
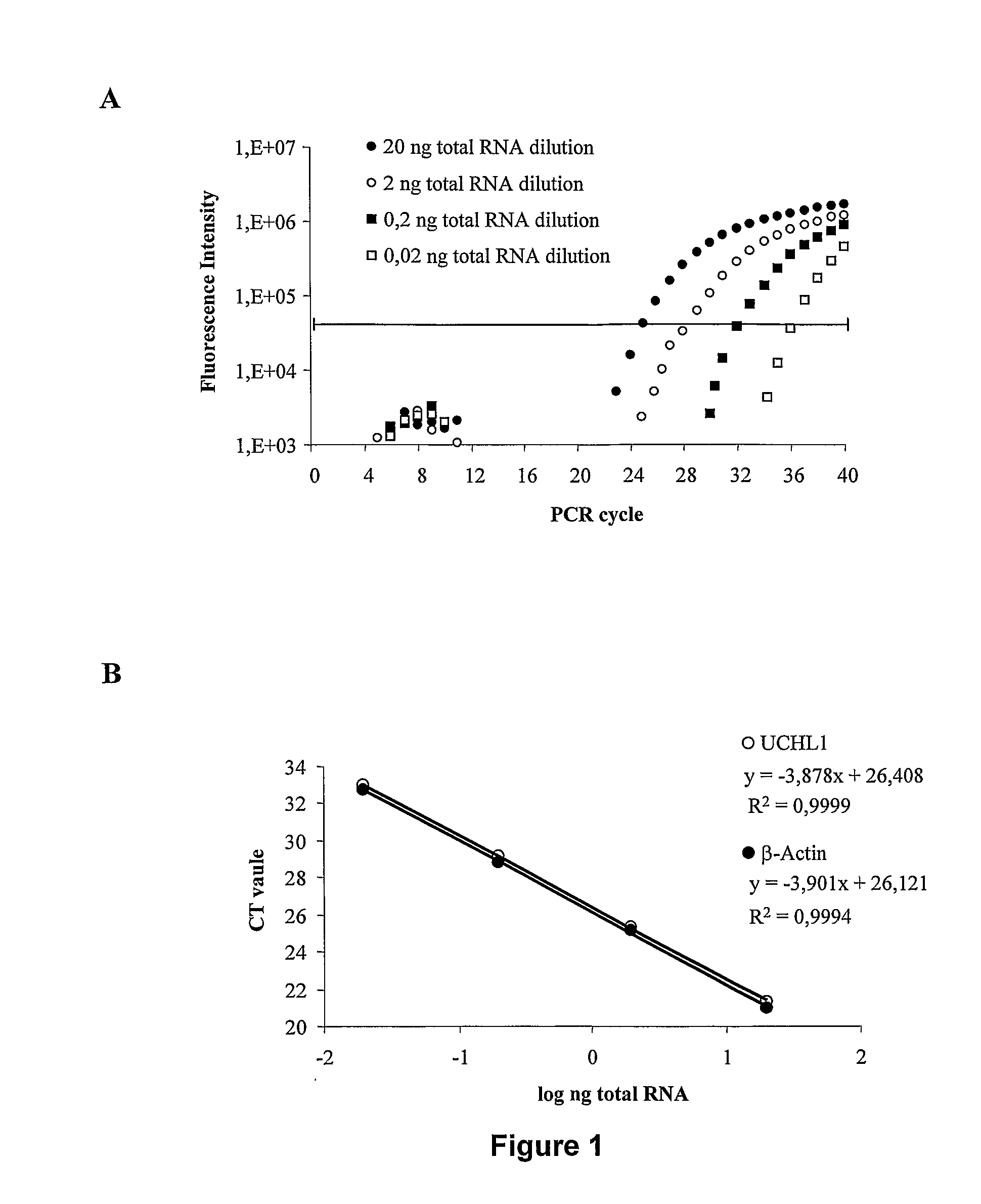
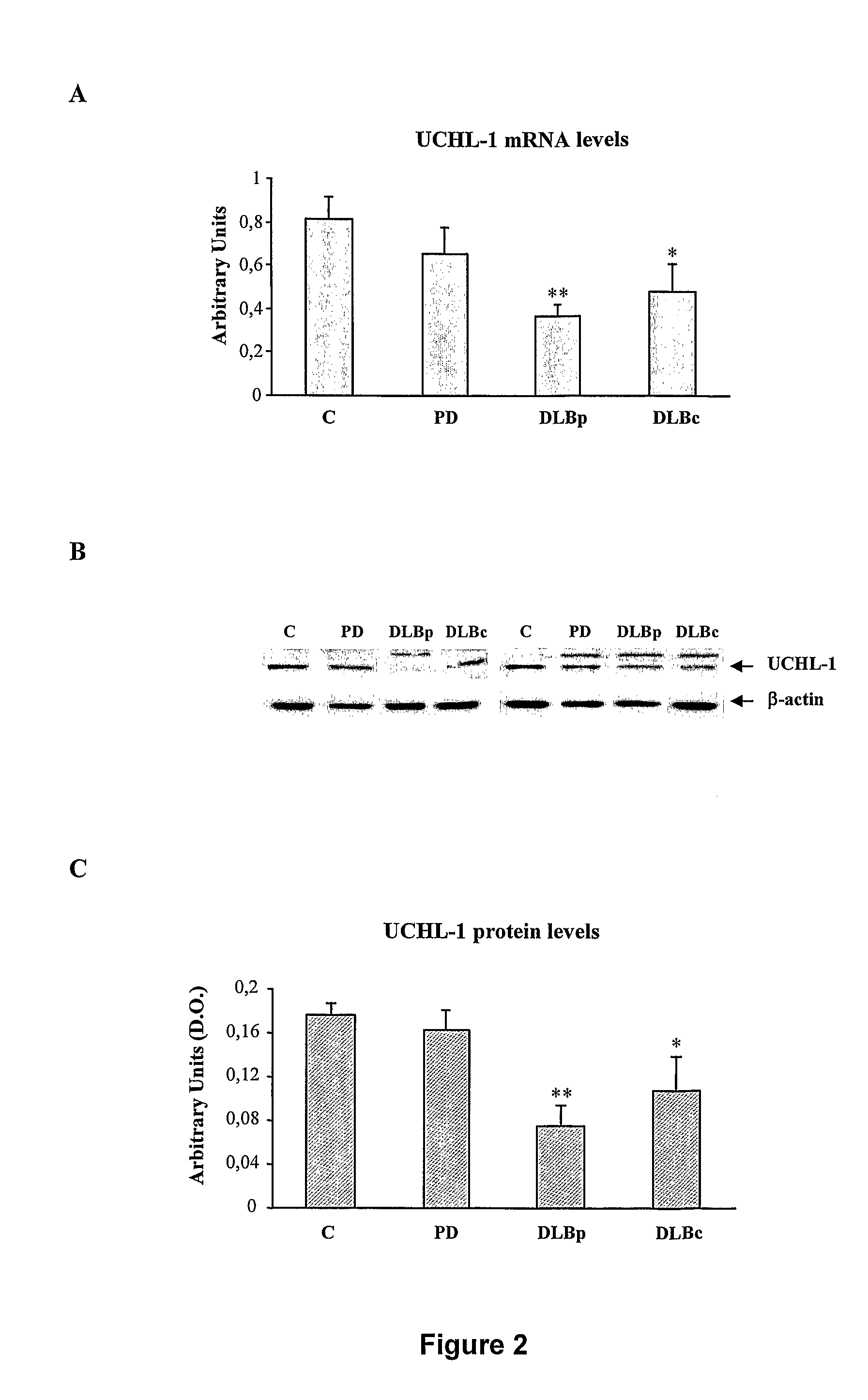
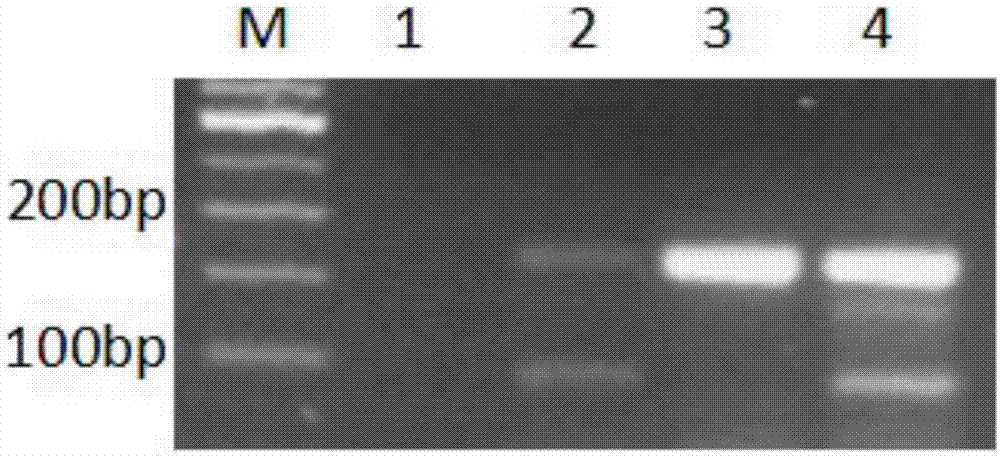
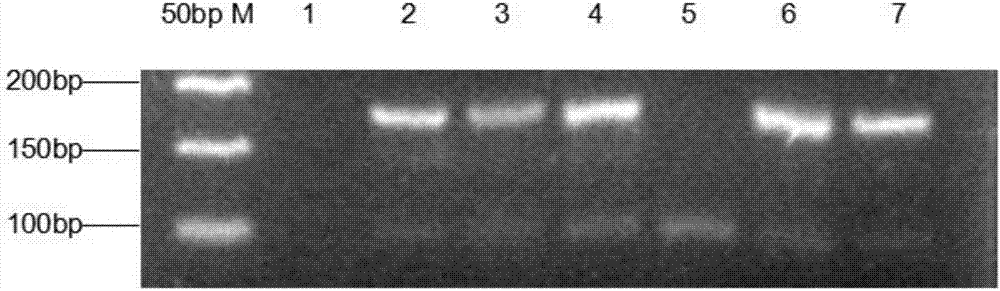
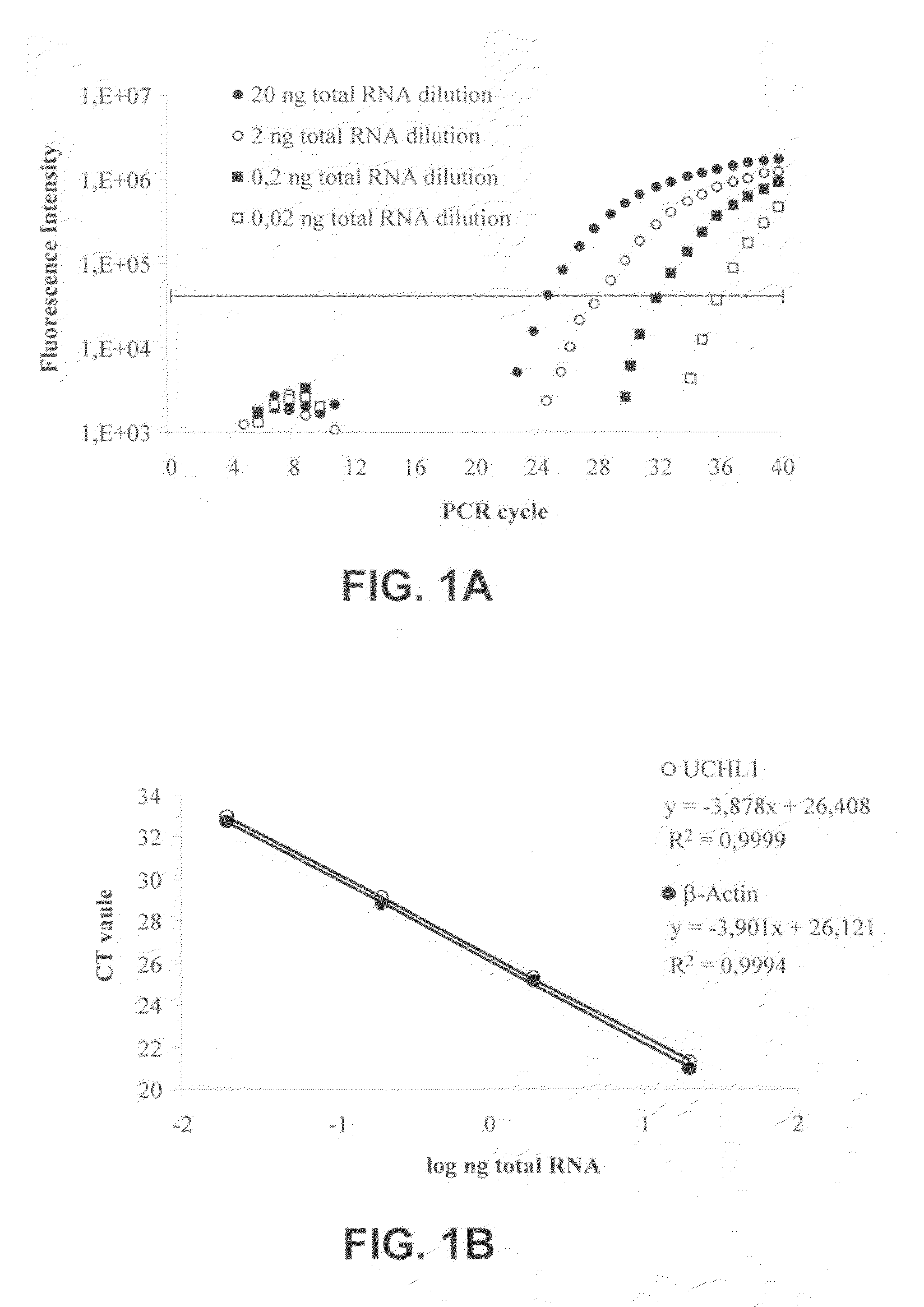
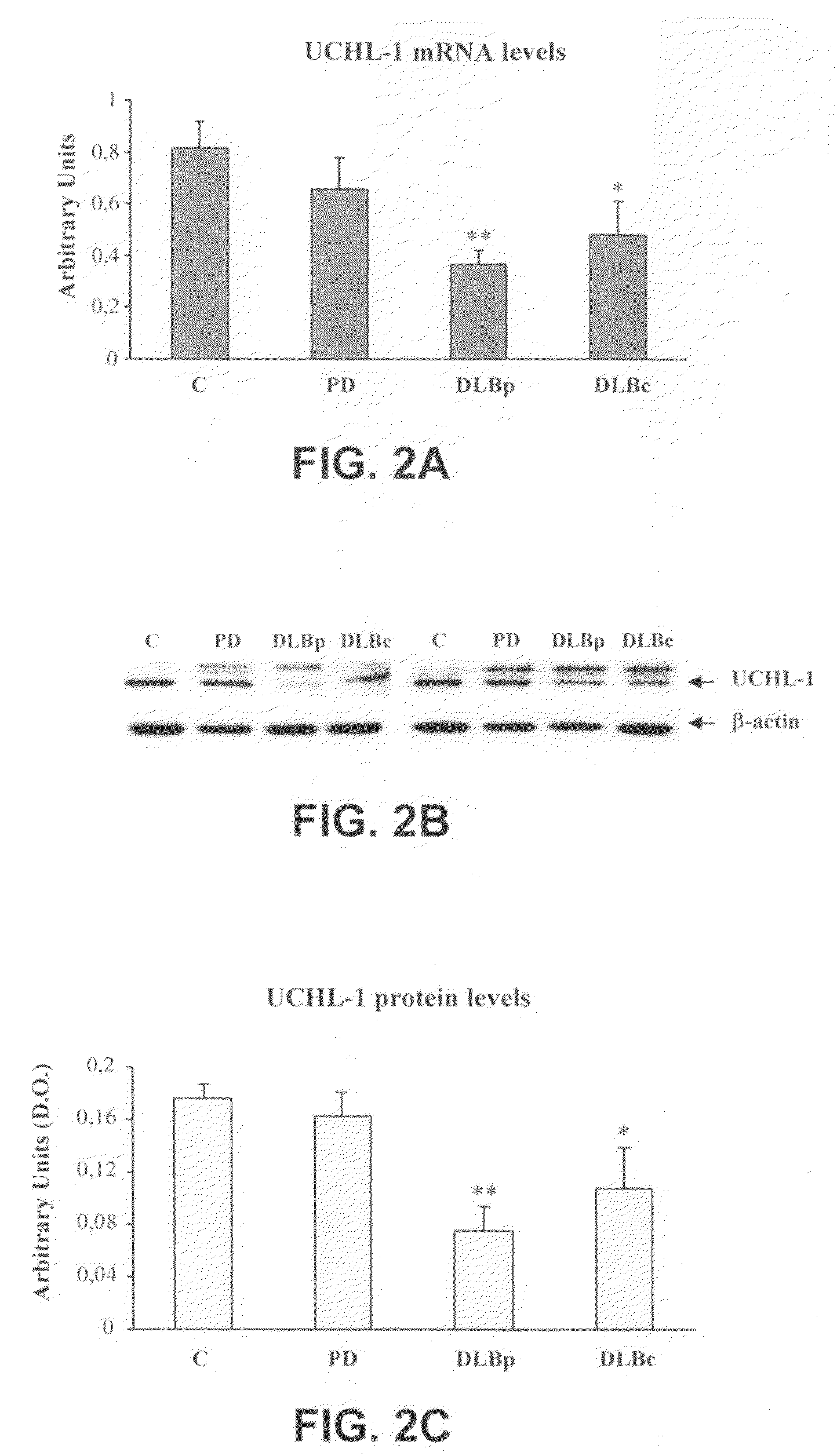
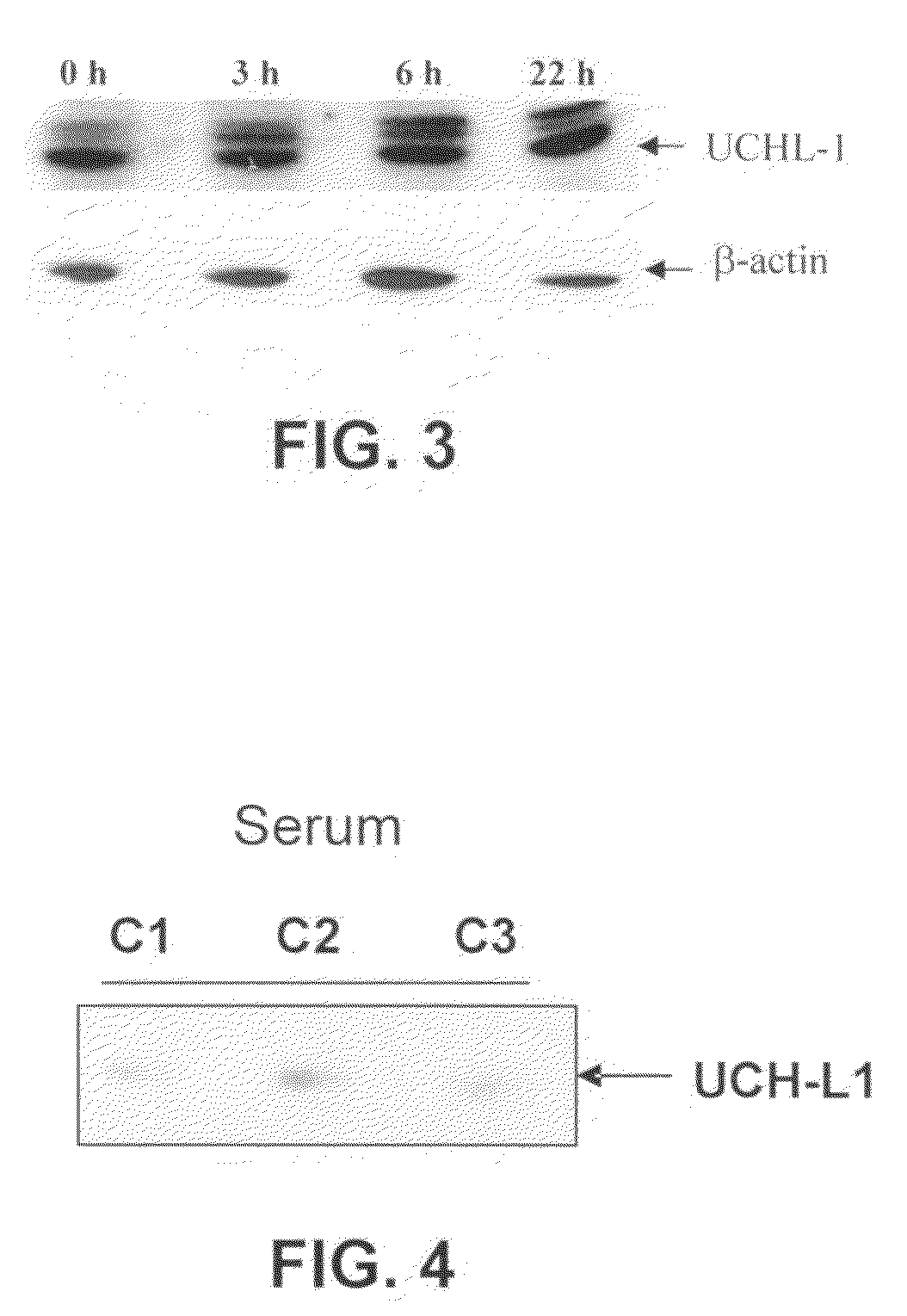
Molecular Diagnostic Method and Treatment in Dementia With Lewy Bodies
InactiveUS20090028845A1Organic active ingredientsNervous disorderUbiquitin carboxy-terminal hydrolase L1Dementia with Lewy bodies
The present invention describes methods of molecular diagnosis of a concrete form of a-synucleinopathy, the dementia with Lewy bodies (DLB), associated to the levels of ubiquitin carboxy-terminal hydrolase L1 (UCH-1) or the alteration of its ubiquityl-ligase activity. It also refers to the use of compounds that permit the modification of the UCH-L1 levels or of the enzymatic activity of UCH-L1. This invention has application in the diagnosis and treatment of patients suffering from DLB.
Owner:ORYZON GENOMICS SA
Molecule diagnosis method for detecting sheep high fecundity gene BMPR1B
InactiveCN103333948AMicrobiological testing/measurementDNA/RNA fragmentationForward primerGenes mutation
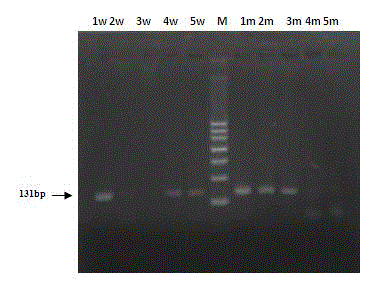
The present invention discloses a genotype analysis method for sheep high fecundity gene BMPR1B mutation site A746G. According to the present invention, an allele specific PCR method is adopted to design two groups of primers (the first group of the primers comprises common forward primer and wild type reverse primer, and the second group of the primers comprises common forward primer and mutant reverse primer), a genotype of detected sheep is ++ if a 131 bp DNA fragment is only amplified by using the first group of the primers, a genotype of detected sheep is BB if a 131 bp DNA fragment is only amplified by using the second group of the primers, and a genotype of detected sheep is B+ if a 131 bp DNA fragment is amplified by using the first group of the primers and the second group of the primers. With the present invention, uses of restriction endonucleases and DNA chips in the traditional detection method are avoided, the PCR product is directly subjected to agarose gel electrophoresis analysis to obtain a genotype analysis result, and the genotype analysis method is an accurate, simple and rapid sheep high fecundity gene BMPR1B molecule diagnosis method.
Owner:赵兴波
Method and special primers for fast identifying phytophthora capsici leonian resistance to pyrimorph
ActiveCN102851363AQuick checkSensitive detectionHydrolasesMicrobiological testing/measurementCelluloseDisease
The invention relates to identification of nucleotide point mutation of a phytophthora capsici leonian-cellulose synthase-related gene CesA3 and a use of the phytophthora capsici leonian-cellulose synthase-related gene CesA3 in detection of resistance to pyrimorph as a fungicide, and discloses a molecular detection method and special primers for identifying phytophthora capsici leonian gene CesA3 3365-site nucleotide mutation-caused resistance to pyrimorph. The pair of special primers is composed of DNA molecules shown in the formulas of SEQ ID NO: 2 and SEQ ID NO: 3. The molecular diagnosis method for detecting phytophthora capsici leonian resistance to pyrimorph as a fungicide has high sensitivity, good stability and a short detection period, can be used for high-flux detection of generation and development of field phytophthora capsici leonian resistance to pyrimorph as a fungicide, realizes early warning of diseases with resistance and has an important meaning for timely and effective control of development of diseases with resistance.
Owner:CHINA AGRI UNIV
Zika virus nucleic acid detection method based on electrochemical luminescence amplification principle
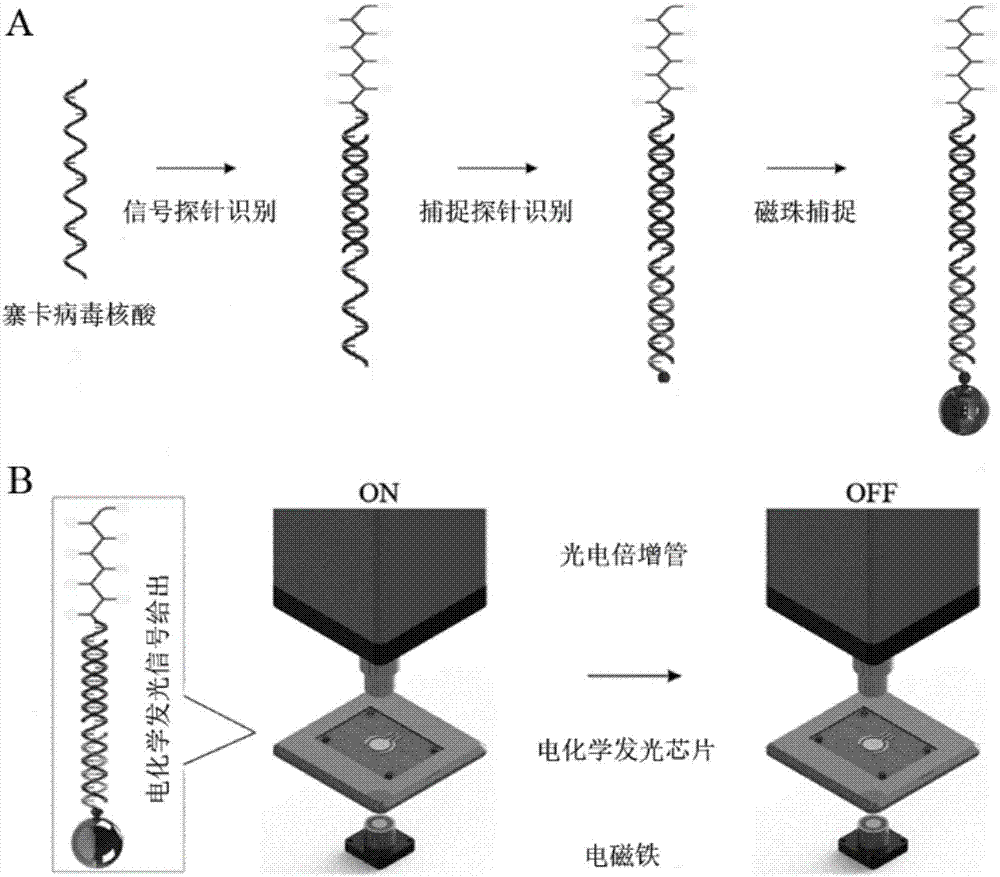
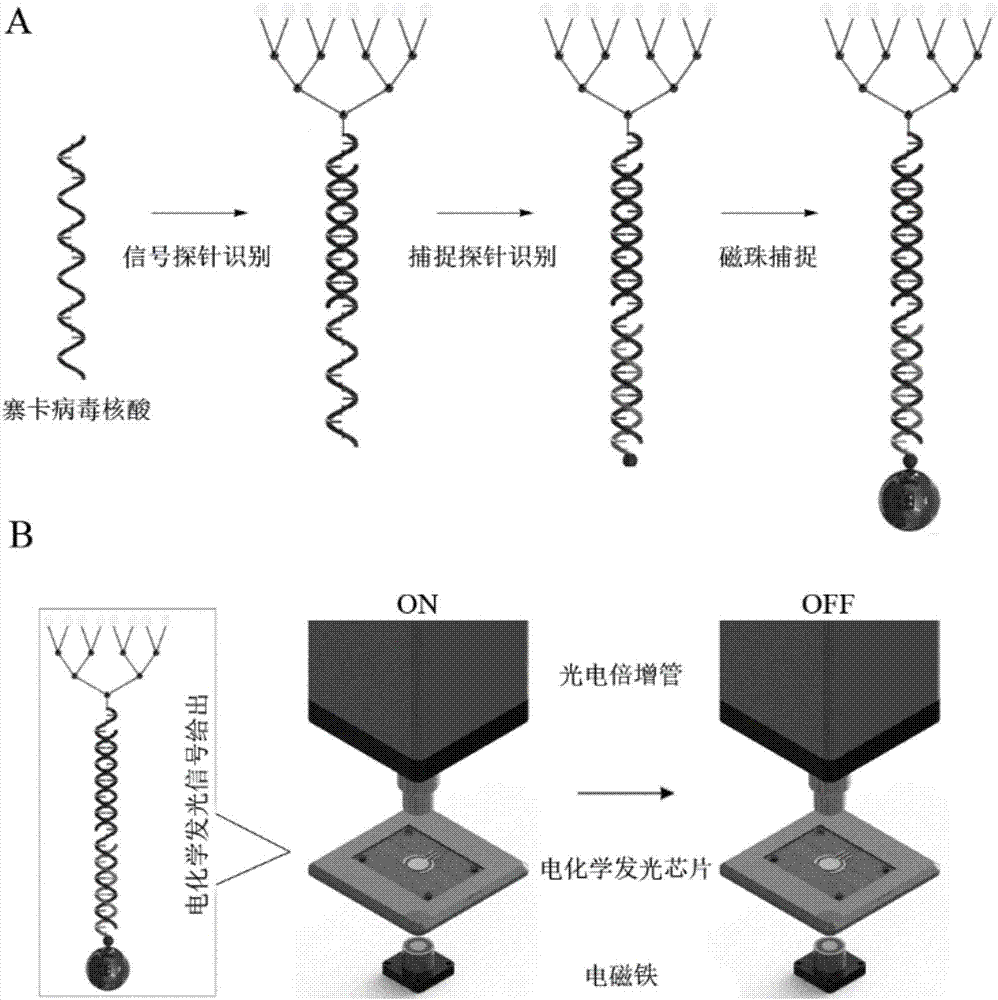
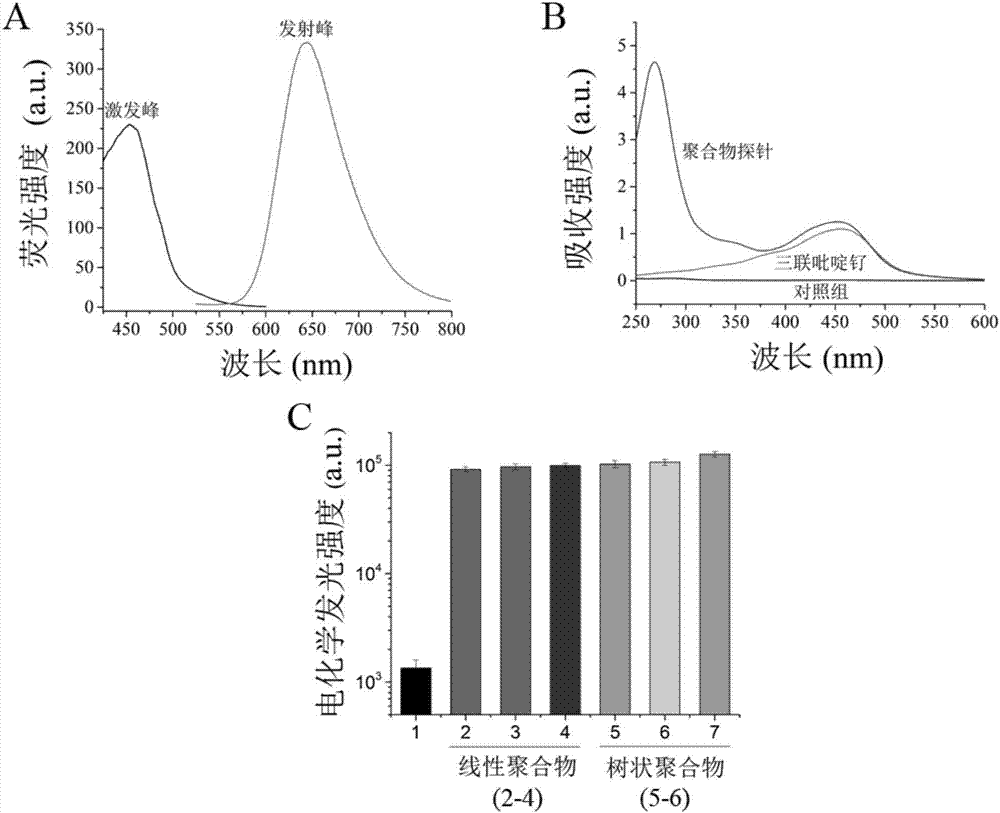
ActiveCN107475454AHigh sensitivityGuaranteed SensitivityMicrobiological testing/measurementMicroorganism based processesLuminous intensityElectrochemiluminescence
The invention discloses a zika virus nucleic acid detection method based on an electrochemical luminescence amplification principle and belongs to the technical field of zika virus molecular diagnosis. The invention respectively provides the zika virus nucleic acid detection method based on a linear and tree-shaped terpyridyl ruthenium polymer electrochemical luminescence amplification method. The method has the following advantages: 1) high sensitivity; 2) stable probe: the linear and tree-shaped terpyridyl ruthenium polymer used as an electrochemical luminescence amplification part of the probe, stable performance, and uniform degree of polymerization and luminous intensity; 3) simple and quick detection process: the zika virus detection is performed through simple sample pre-treatment and nucleic acid extraction process, time consumption is less, the amplification step is avoided and the detection is quick; 4) low cost. The method disclosed by the invention is a technical system for detecting zika virus nucleic acid; a novel molecular diagnosis method based on the electrochemical luminescence method technique is provided; the defect of single technique for detecting zika virus at present is effectively made up; a zika virus detection method requiring no amplification is supplied.
Owner:SUN YAT SEN UNIV
Kit for predicting prostate screening and lymphatic metastasis
InactiveCN107988365AReliable test resultsHigh clinical positive predictive valueMicrobiological testing/measurementDNA preparationFluorescenceProstate cancer
The invention relates to the molecular diagnosis field and relates to a molecular diagnosis method and reagent for non-invasive detection of prostate cancer and lymphatic metastasis of prostate cancerby virtue of urine. The method comprises the following steps: extracting and purifying urine nucleic acid; (2) carrying out DNA hydrosulphite conversion reaction and subsequent purification; (3) detecting a plurality of gene promoter regions by virtue of methylation-specific real-time fluorescence PCR; and (4) analyzing multiple gene results, wherein the steps (1) and (2) can be combined into onestep, and multiple genes contain the promoter regions of the following genes: CRMP4, PITX2, RASSF1A, SOSTDC, CYBA, EFEMP1, KNM5D, GSTP1, WFDC2, TACSTD2 and the like. By combining the detection results of the genes, the diagnosis of prostate cancer and the lymphatic metastasis of prostate cancer can be realized.
Owner:上海纽思格生物科技有限公司
Method for identifying nucleotide point mutation of phytophthora sojae beta-microtubulin gene and use thereof for diagnosing zoxamide resistance
InactiveCN102925565ATimely adjustment of disease control strategiesResistance delay and controlMicrobiological testing/measurementDepsipeptidesResistance developmentPhytophthora sojae
The invention relates to the cloning of a phytophthora sojae beta-microtubulin gene, the detection of the mutation site of the gene and the use of the gene in monitoring the zoxamide resistance, and particularly discloses a molecular detection method for zoxamide resistance caused by mutation of site-716 in the phytophthora sojae beta-microtubulin and special primers thereof. The primer pair consists of DNA molecules represented by SEQID NO:1 and SEQID NO:2, and the polymerase chain reaction (PCR) annealing temperature is 67DEG C. The invention provides a molecular diagnosis method for zoxamide resistance of phytophthora sojae, which has the characteristics of high sensitivity, simplicity, speed and stability, can be used for detecting the zoxamide resistance development trend of field phytophthora sojae with high pass, so that an early warning for medicine resistance can be given. The method has a great significance for control of medicine resistance in pathogenic bacteria.
Owner:CHINA AGRI UNIV
Molecular reagent for screening bladder cancer by using urine
InactiveCN108130371AReliable test resultsHigh clinical positive predictive valueMicrobiological testing/measurementDNA preparationBiotechnologyFluorescence
The invention belongs to the field of biotechnology molecular diagnosis, and provides a molecular diagnosis method of noninvasive detection of bladder cancer by using urine. The method comprises the following steps of 1, urine nucleic acid extraction and purification; 2, DNA hydrosulphite conversion reaction and subsequent purification; 3, methylation specific real-time fluorescence PCR detectionon a plurality of gene promotor regions; 4, multi-gene result analysis. The steps 1 and 2 can be merged into one step. The multi-gene comprises the promotor regions of the following genes including VIM, TMEFF2, HSPA2, GDF15, ABL1, CRH, IGF2, ANXA10, UPK1B, ACTB and the like; the gene combination detection results can be used for bladder cancer diagnosis and bladder cancer recurrence rate prediction.
Owner:上海纽思格生物医学科技有限公司
Rapid detection method of PWS and AS
InactiveCN107245519AEasy to operateDoes not involve repeated freeze-thaw cyclesMicrobiological testing/measurementQuality controlPcr method
The invention relates to a rapid detection method of PWS and AS. The PWS / AS is detected by adopting an MS-PCR method. The rapid detection method has the benefits that a DNA sample is treated by using bisulfite to complete within a short time, and meanwhile, the whole operation flow is simpler and more convenient by adopting a method of removing a sulfuric acid group on a column; meanwhile, more details are treated and adjusted, so that a complete set of inspection system is formed; multiple quality control points are set; the rapid detection method is reliable through clinical verification. MS-PCR is a quick and efficient molecular diagnostic method with optimal specificity and sensitivity; meanwhile, the detection cost is lower, and the majority of PWS / AS clinical cases can be diagnosed.
Owner:杭州博圣医学检验实验室有限公司
Molecular diagnostic method for dementia with lewy bodies
InactiveUS7745127B2Nervous disorderMicrobiological testing/measurementDementia with Lewy bodiesUbiquitin carboxy-terminal hydrolase L1
Owner:ORYZON GENOMICS SA
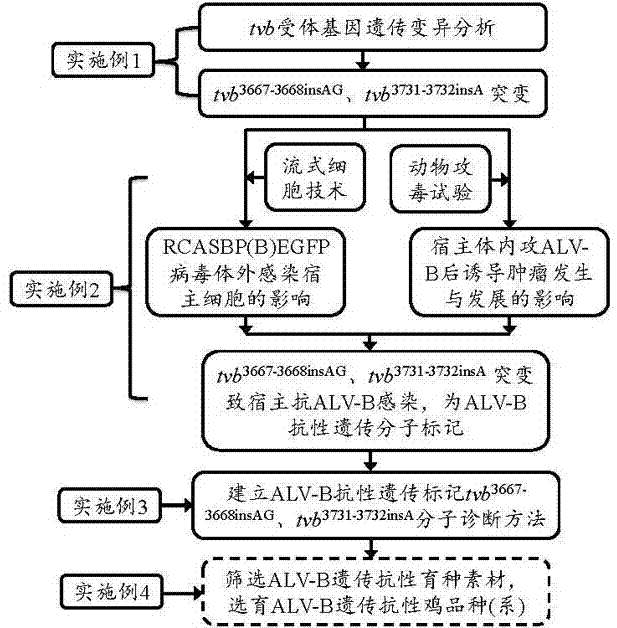
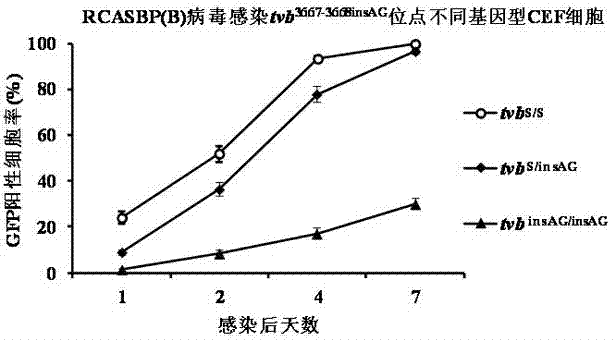
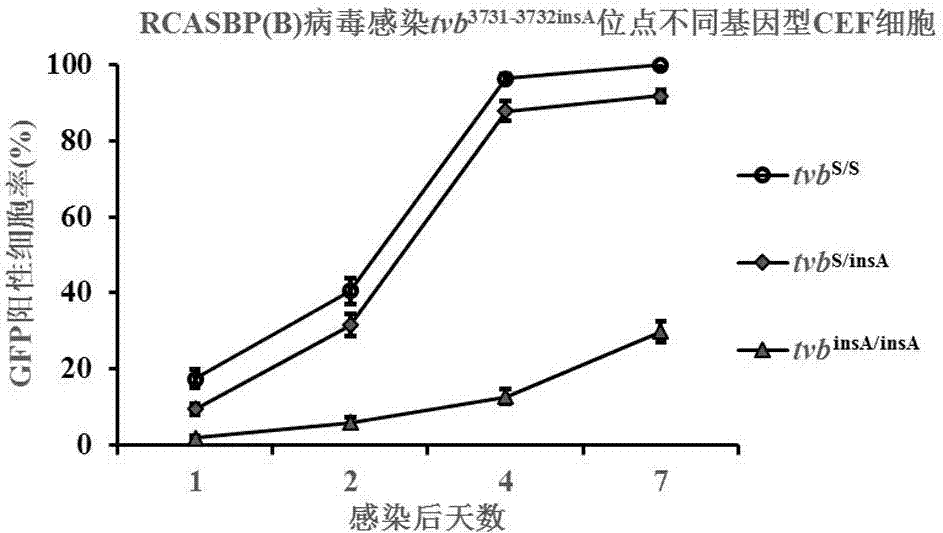
Chicken B subgroup avian leukosis resistantmolecular marker tvb<3731-3732insA> and molecular diagnosis method thereof
ActiveCN105441552AMicrobiological testing/measurementDNA/RNA fragmentationIn vivoMolecular Diagnostic Method
The invention belongs to the technical field of resistant variety breeding, and in particular discloses a chicken B subgroup avian leukosis resistantmolecular marker tvb<3731-3732insA> and a molecular diagnosis method thereof. The invention analyzes genetic variation of a Chinese chicken breed tvb receptor gene for the first time and discovers that the 3731st and the 3732nd sites of the Chinese chicken breed tvb receptor gene have A insertion mutation, and the inventor proves, from in vivo and in vitro experiments two aspects, that the natural mutation causes a host to generate genetic resistance to infection of ALV-B; moreover, the inventor establishes a molecular diagnosis method for a B subgroup avian leukosis resistant genetic marker, and the method can be applied to screening breeding materials of ALV-B genetic resistant chicken varieties (strains) so as to carry out breeding of the ALV-B genetic resistant chicken varieties (strains).
Owner:SOUTH CHINA AGRI UNIV
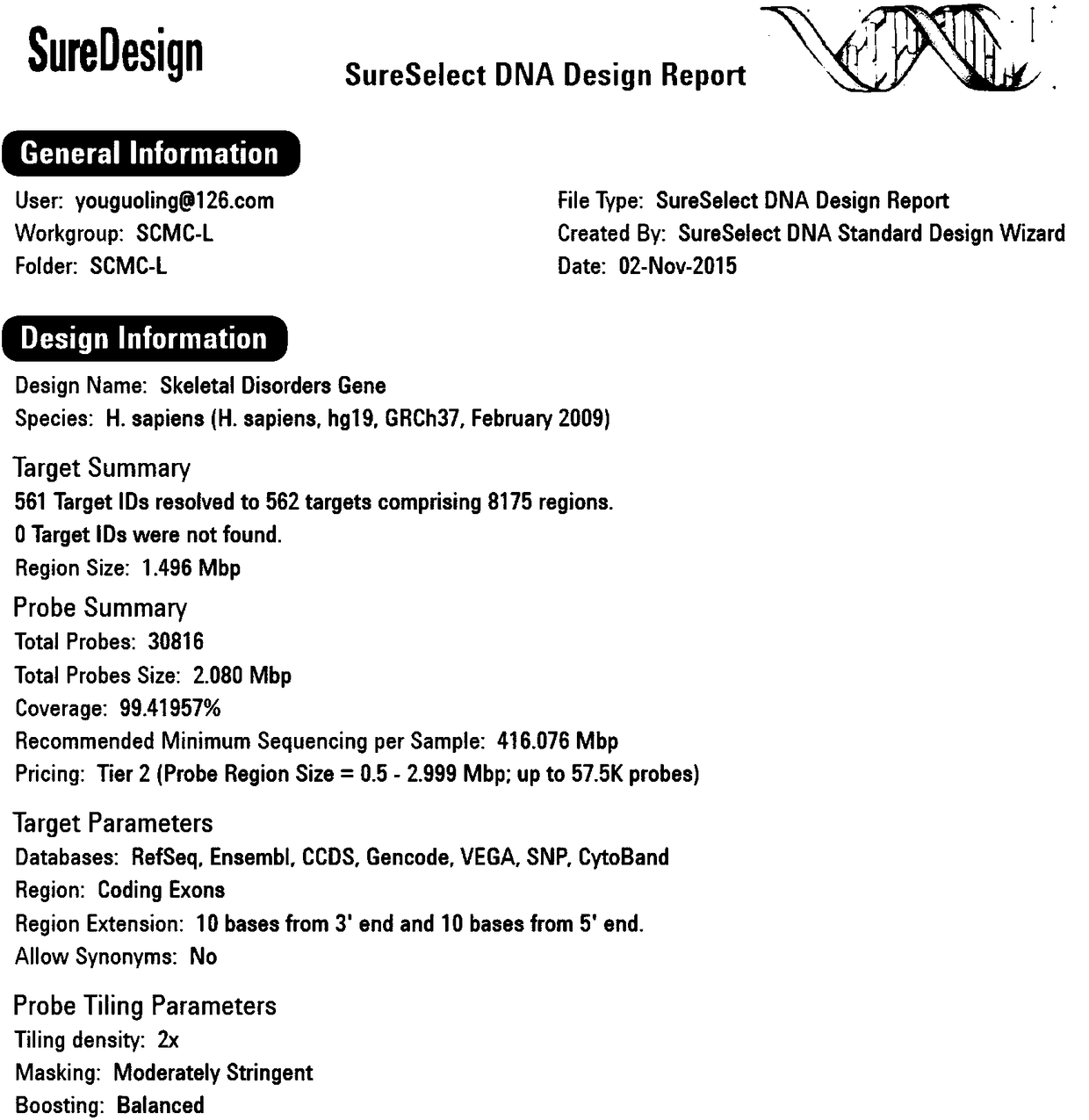
Molecular diagnostic method and kit of genetic bone diseases
InactiveCN108570496ALow costAccurate diagnosisMicrobiological testing/measurementPrenatal diagnosisGene mutation analysis
The invention relates to a molecular diagnostic method and kit of genetic bone diseases. The method includes using a capture probe aiming at 561 genetic bone disease related genes and applying a target gene high throughput parallel targeting sequencing method to determine the sequences of the corresponding genes of subjects. The kit includes the capture probe which aims at 561 genetic bone diseaserelated genes. The method has advantages of high throughput, high sensitivity and high singularity; the cost of analyzing pathogenic gene mutation of the genetic bone diseases can be rapidly reduced;and the method has practical clinical significance, is helpful for the drawing of genetic bone disease gene mutation spectrums and the explication of the molecular pathogenesis of the genetic bone diseases, and provides theoretical foundations of early accurate diagnosis, precision treatment, prenatal diagnosis and genetic counseling for the genetic bone diseases.
Owner:SHANGHAI CHILDRENS MEDICAL CENT AFFILIATED TO SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
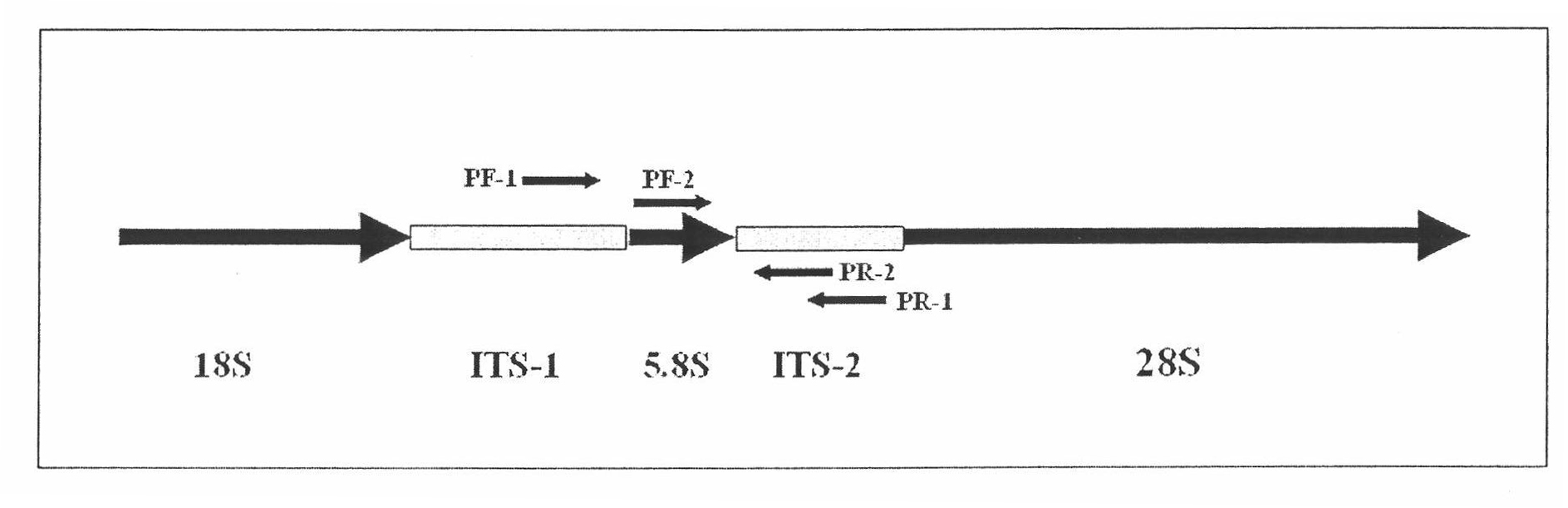
Molecular diagnosis method for rapid detection of bactrocera dorsalis ovum in fruits
InactiveCN101792796AQuick checkReliable resultsMicrobiological testing/measurementSequence analysisBactrocera dorsalis
The invention relates to a molecular diagnosis method for the rapid detection of a bactrocera dorsalis ovum in fruits, which adopts a nested type PCR to amplify ITS break sequence in bactrocera dorsalis rDNA, amplifies specific ITS segments in the bactrocera dorsalis rDNA through designing a nested type specific primer to accurately and rapidly identify the bactrocera dorsalis ovum in the fruits, and determines the differences of the ovum and the resembling variety thereof. The method has reliable result, can rapidly detect the existence of the bactrocera dorsalis ovum in the fruits, and determine the species evolution relation of the sample through sequence analysis.
Owner:SHANGHAI ACAD OF AGRI SCI
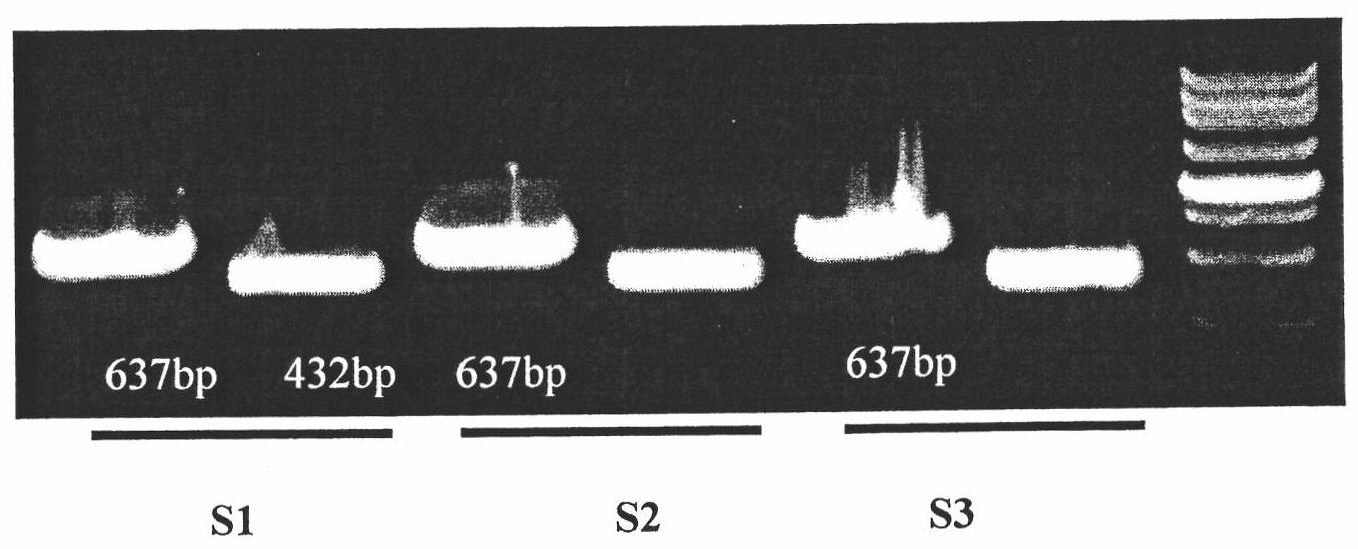
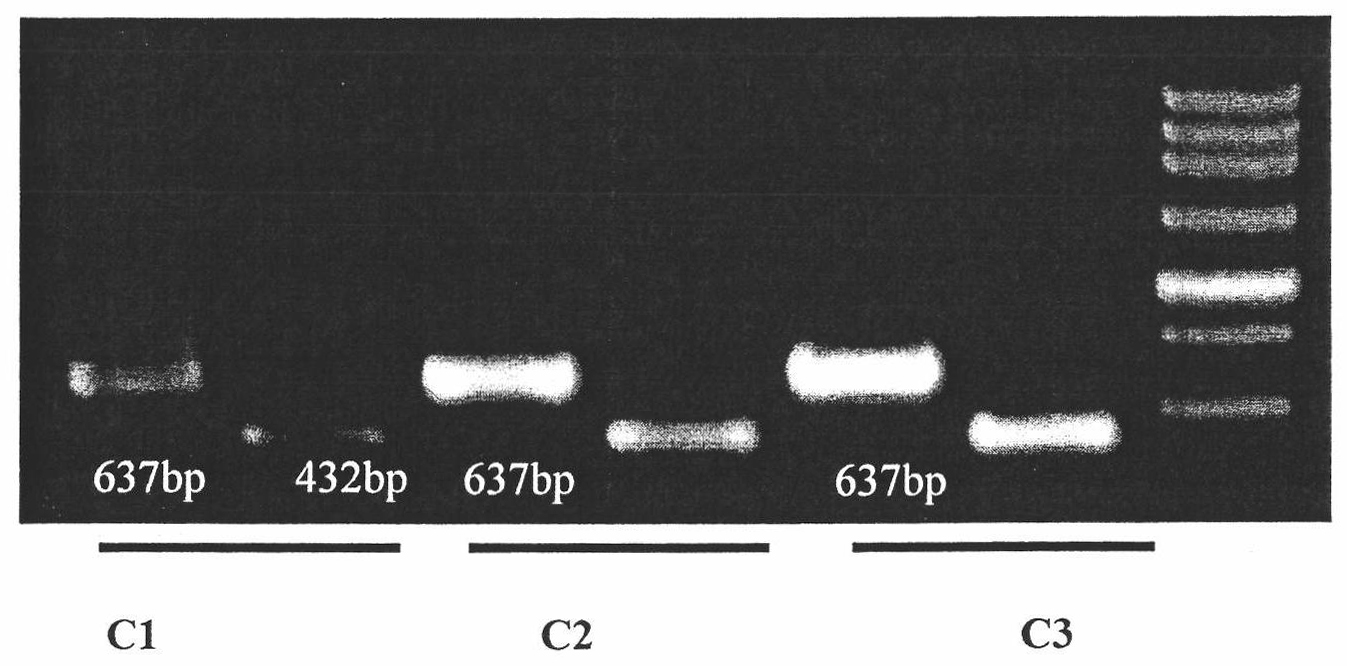
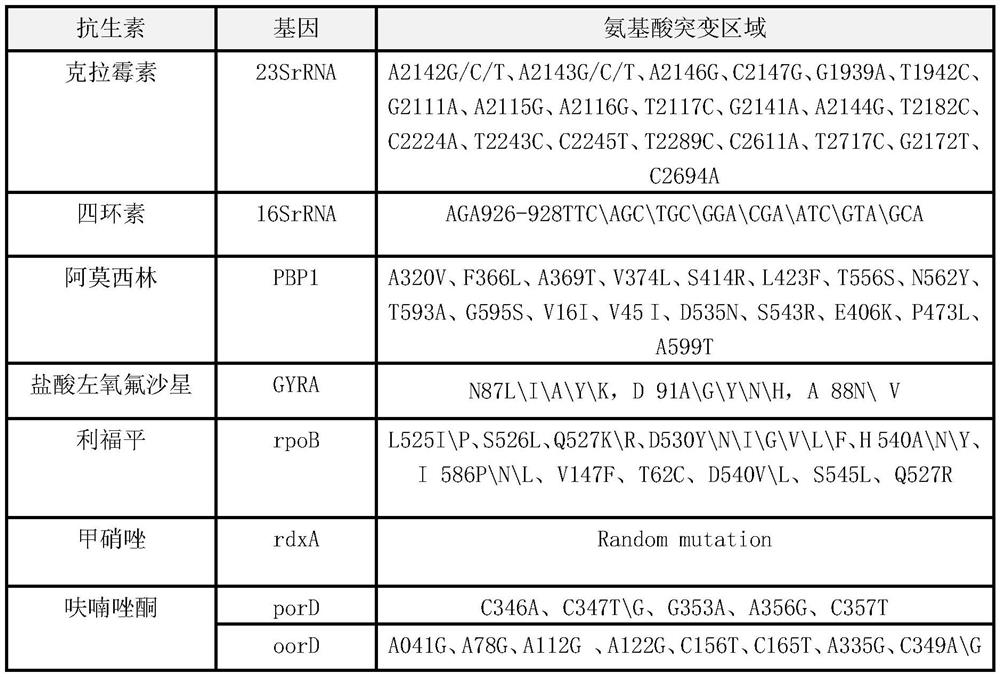
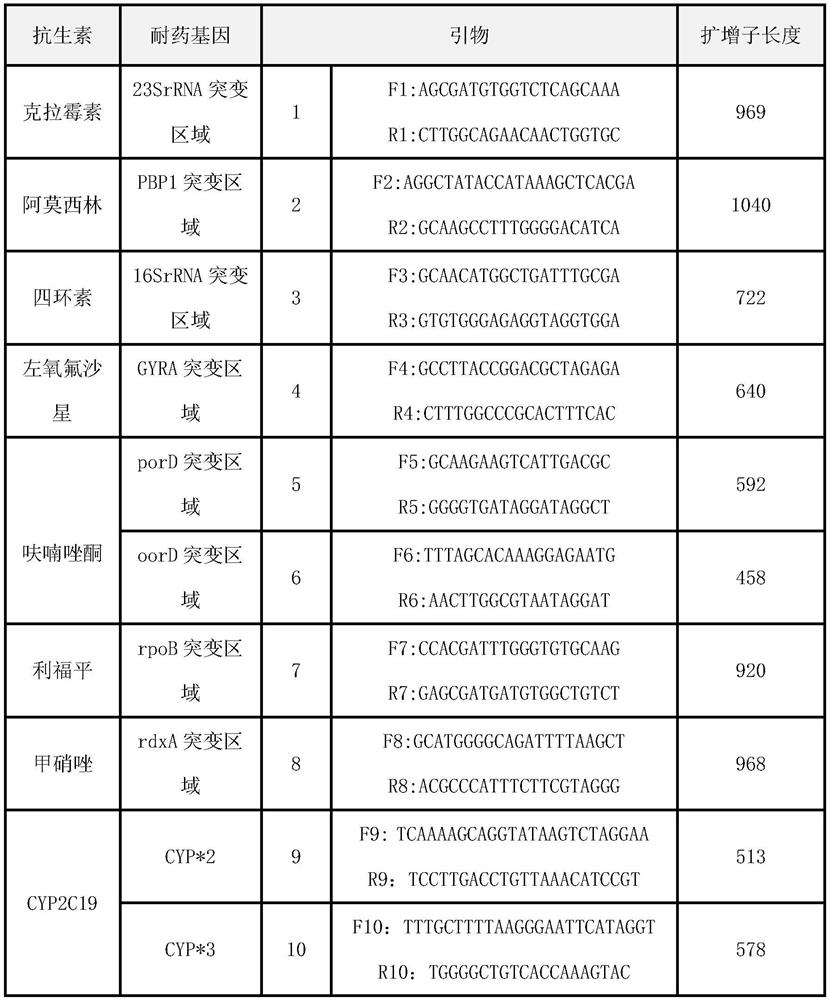
Quick molecular diagnosis method and reagent kit for individualized medication guidance for eradication therapy of helicobacter pylori
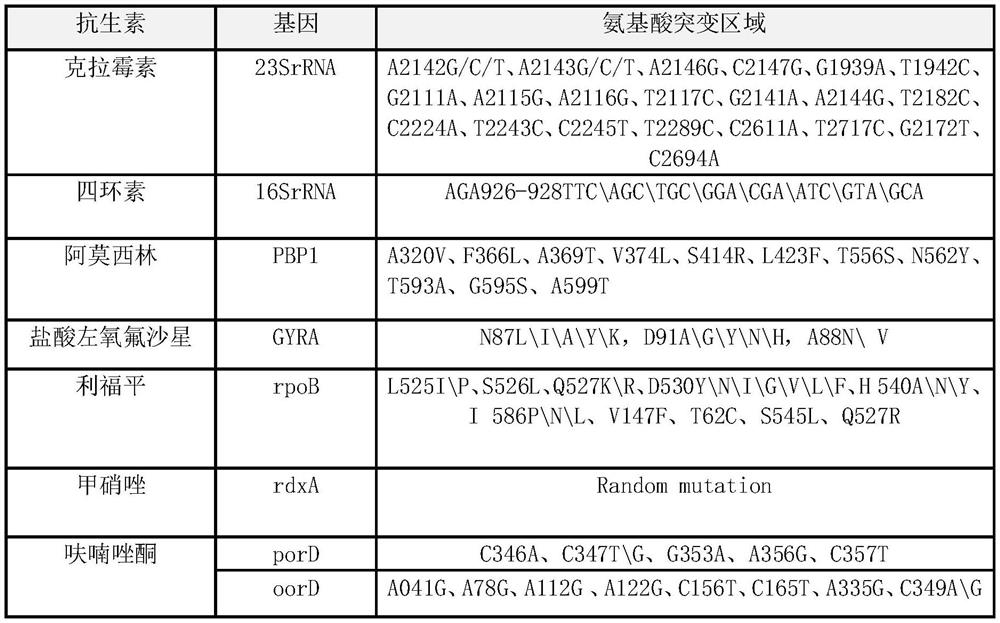
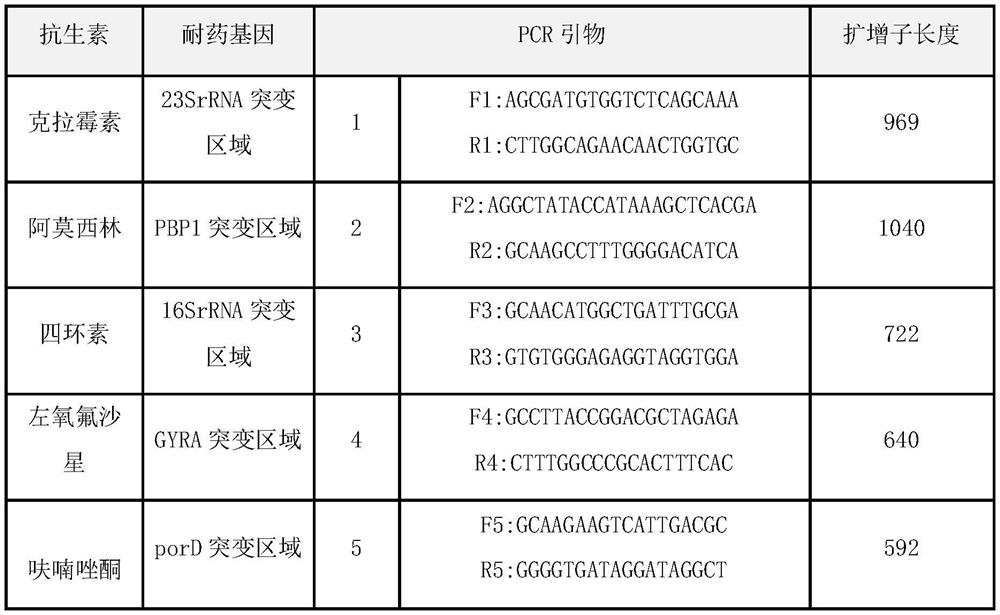
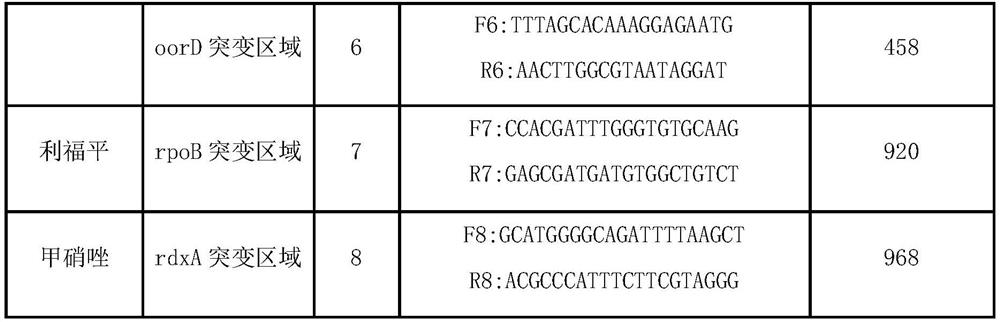
The invention relates to a diagnosing method and reagent kit for detecting and determining reverse tolerance of helicobacter pylori in gastric biopsy samples. The method can perform a downstream PCR reaction only by simply and directly performing complete genome extraction on biopsy samples of a patient. 23 pairs of primers of which the nucleotide sequences are as shown in figures 2 and 3 are usedfor joint detection of 101 mutation types of common mutation sites of 8 reverse tolerance genes of 23SrRNA,gyrA, PBP1, 16SrRNA, porD, oorD, rpoB and rdxA and two mutation types of a host drug metabolism enzyme CYP2C19 gene so as to perform fast detection and accurate analysis on mutation situation of reverse tolerance genes and drug metabolic enzyme genes of 7 antibiotics which are clinically andfrequently used at present through combination of a nested PCR technique with a sequencing technique, and the specificity and the accuracy are high. Compared with traditional minimal inhibitory concentration culture experiments, the diagnosing method is quicker and acuter in detection, is more suitable for clinical application, and has great clinical application prospects in the respects of guiding individualized diagnosis and treatment of patients being positive in helicobacter pylori.
Owner:深圳艾普斯金基因检测合伙企业(有限合伙)
SNP markers related to assessment of incidence risks of NK/T cell lymphoma and application of SNP markers
PendingCN111304323AEasy to get materialsEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationTissue sampleMolecular Diagnostic Method
The invention provides a set of SNP markers related to assessment of the incidence risks of NK / T cell lymphoma. The SNP markers comprise rs13015714, rs9271588 and rs9277378 sites. Sampling of testersis convenient, only peripheral blood is needed, no other tissue samples are needed, SNP is detected through simplified and specific amplification primers so that the operation is simple, and then theincidence risks of individuals are assessed through SNP typing. High-risk groups of NK / T cell lymphoma can be confirmed more accurately through a molecular diagnosis method so that the groups are guided to take preventive intervention measures, and the applicability and sensitivity are increased at the same time, so that the SNP markers are further applied to scientific research and clinical work.
Owner:SUN YAT SEN UNIV CANCER CENT
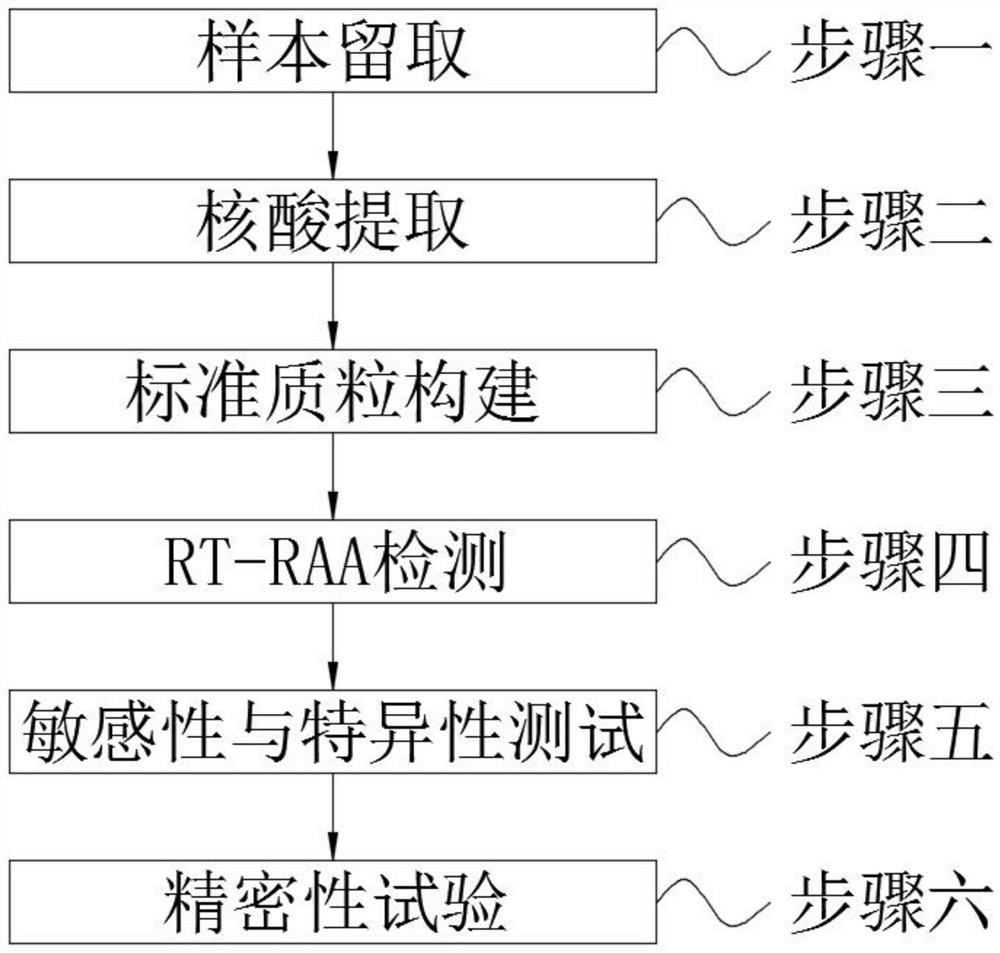
Method for detecting novel coronavirus virus based on real-time fluorescence RT-RAA
PendingCN111979304AMeet detectionHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesBronchial LavageBronchial epithelium
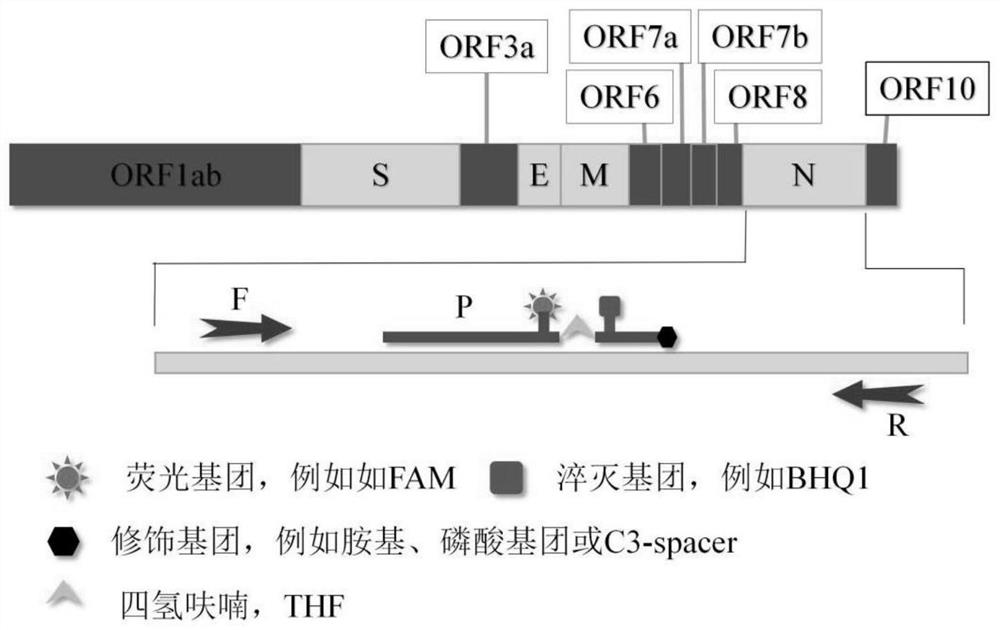
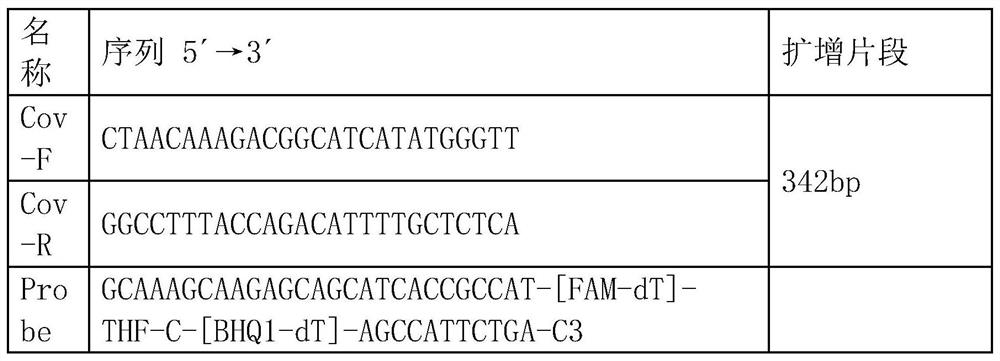
The invention discloses a method for detecting novel coronavirus virus based on real-time fluorescence RT-RAA. The method comprises the following steps: step 1, reserving a sample; 2, extracting nucleic acid; step 3, constructing standard plasmids; step 4, carrying out RT-RAA detection; step 5, testing sensitivity and specificity; step 6, carrying out a precision test, wherein in the step 1, a throat swab, a nasopharynx extract or a respiratory tract extract, a deep expectoration solution, a bronchial lavage solution, an alveolar lavage solution, a blood specimen, a serum specimen and an eye conjunctival swab are manually collected. According to the method for detecting the novel coronavirus virus based on the real-time fluorescence RT-RAA, a novel real-time fluorescence RT-RAA method fordetecting SARS-CoV-2 is constructed based on a recombinase mediated isothermal nucleic acid amplification technology, and compared with other molecular diagnosis methods, the method is high in detection sensitivity, easy, convenient and rapid to operate and suitable for large-scale industrial production. No special equipment is needed, the result is accurate, clinical sample detection can be met,and the method is particularly suitable for mobile emergency detection of resource-deficient areas or airports, schools and other units.
Owner:HANSHAN NORMAL UNIV +1
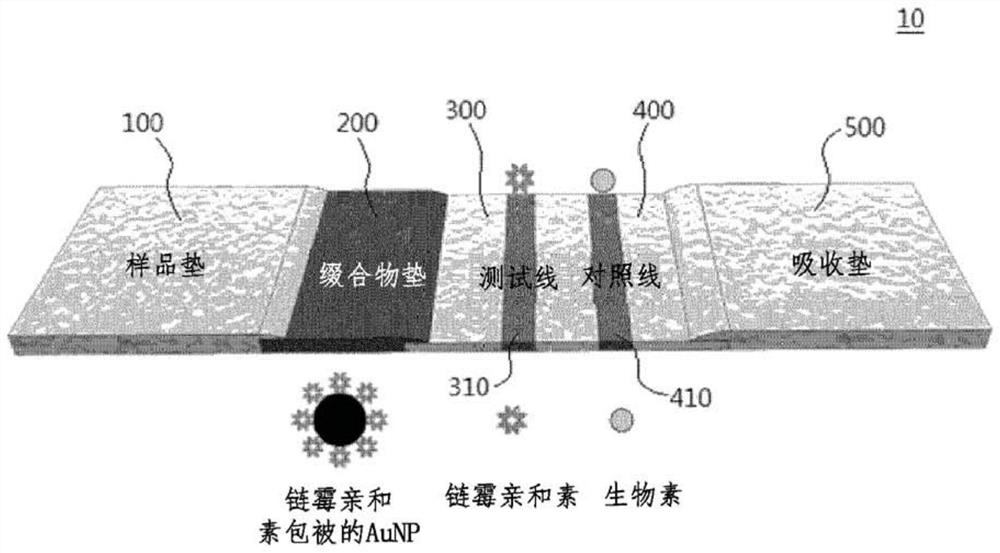
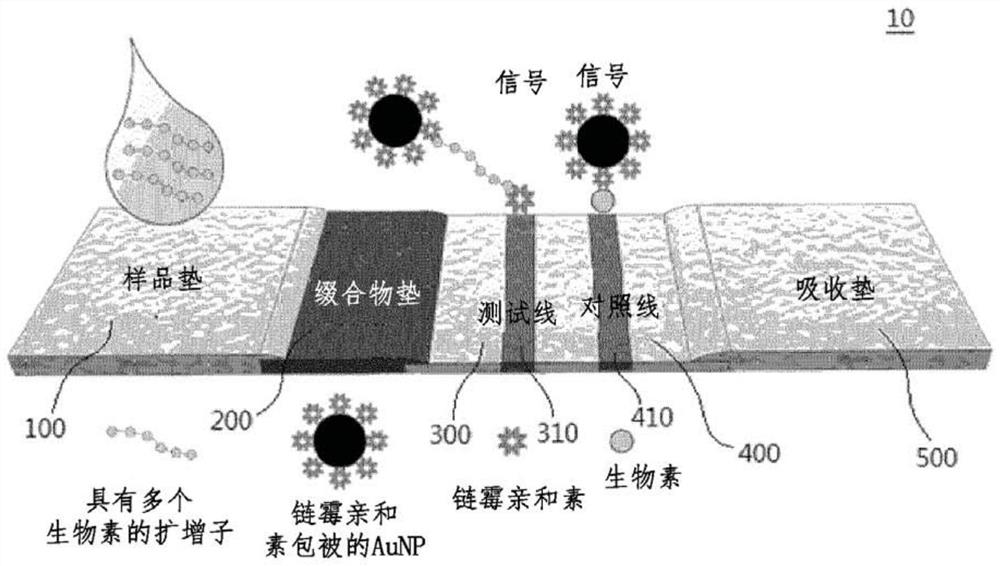
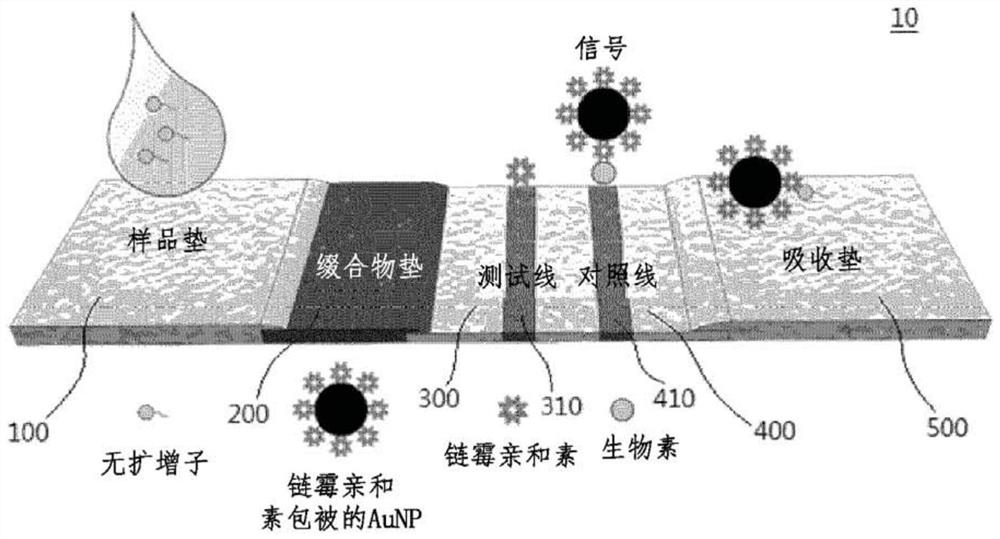
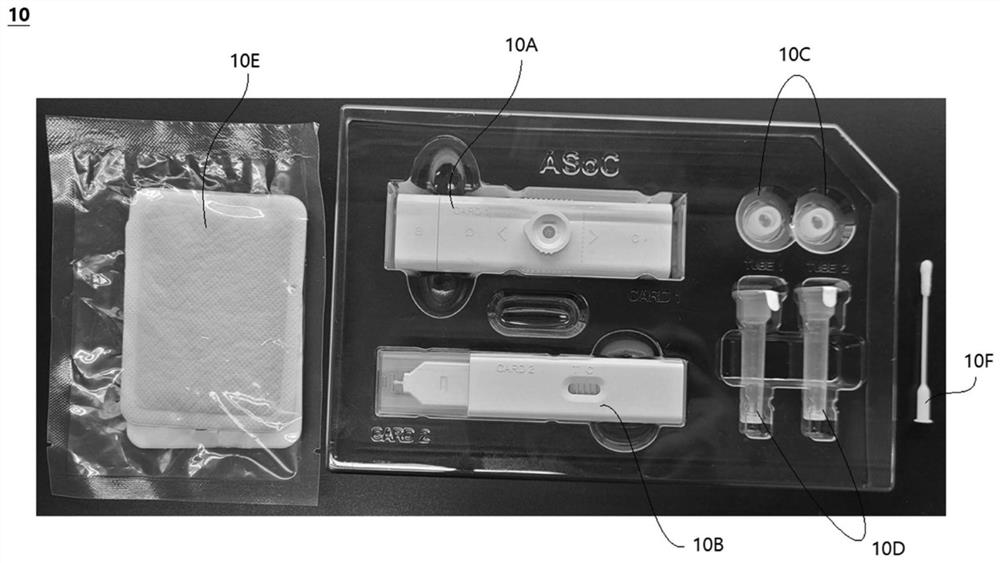
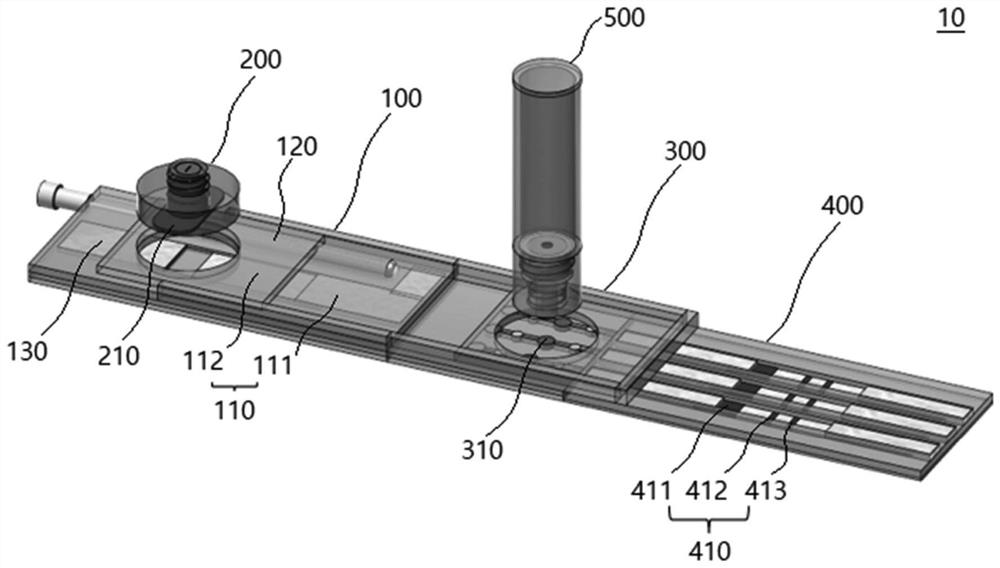
Lateral flow analysis strip and molecular diagnostic method using same
PendingCN113841050AHigh sensitivityMicrobiological testing/measurementMicroorganism based processesMolecular diagnosticsMolecular Diagnostic Method
Lateral flow analysis strips and molecular diagnostic methods using the same are disclosed. The lateral flow analysis strip may comprise a sample pad (100) into which a sample comprising at least one of an amplicon labeled with a label and an amplicon precursor labeled with a label is to be introduced; a conjugate pad (200) comprising a conjugate (C1), the conjugate (C1) having an indicator and a detection agent bound to a surface of the indicator, and the conjugate (C1) being non-fixedly adsorbed to the conjugate pad (200); a test pad (300) comprising a test line (310) to which a first trapping agent is fixed; a control pad (400) comprising a control line (410) to which a second trapping agent is immobilized; and an absorbent pad (500). The lateral flow analysis strip of the present invention can detect amplicons (e.g., nucleic acids) amplified by using an amplicon precursor labeled with a type of label (e.g., a primer or dNTP) and can determine the results directly with the naked eye. Furthermore, the lateral flow analysis strip advanlabel eously has high sensitivity by using the label having a strong binding force, the trapping agent bound to the label , a detection agent, and a trapping agent bound to the detection agent. Further, by using the label having a certain binding force, the trapping agent bound to the label, the detection agent, and the trapping agent bound to the detection agent, the lateral flow analysis strip has reproducible and stable effects.
Owner:菲尔梅迪株式会社
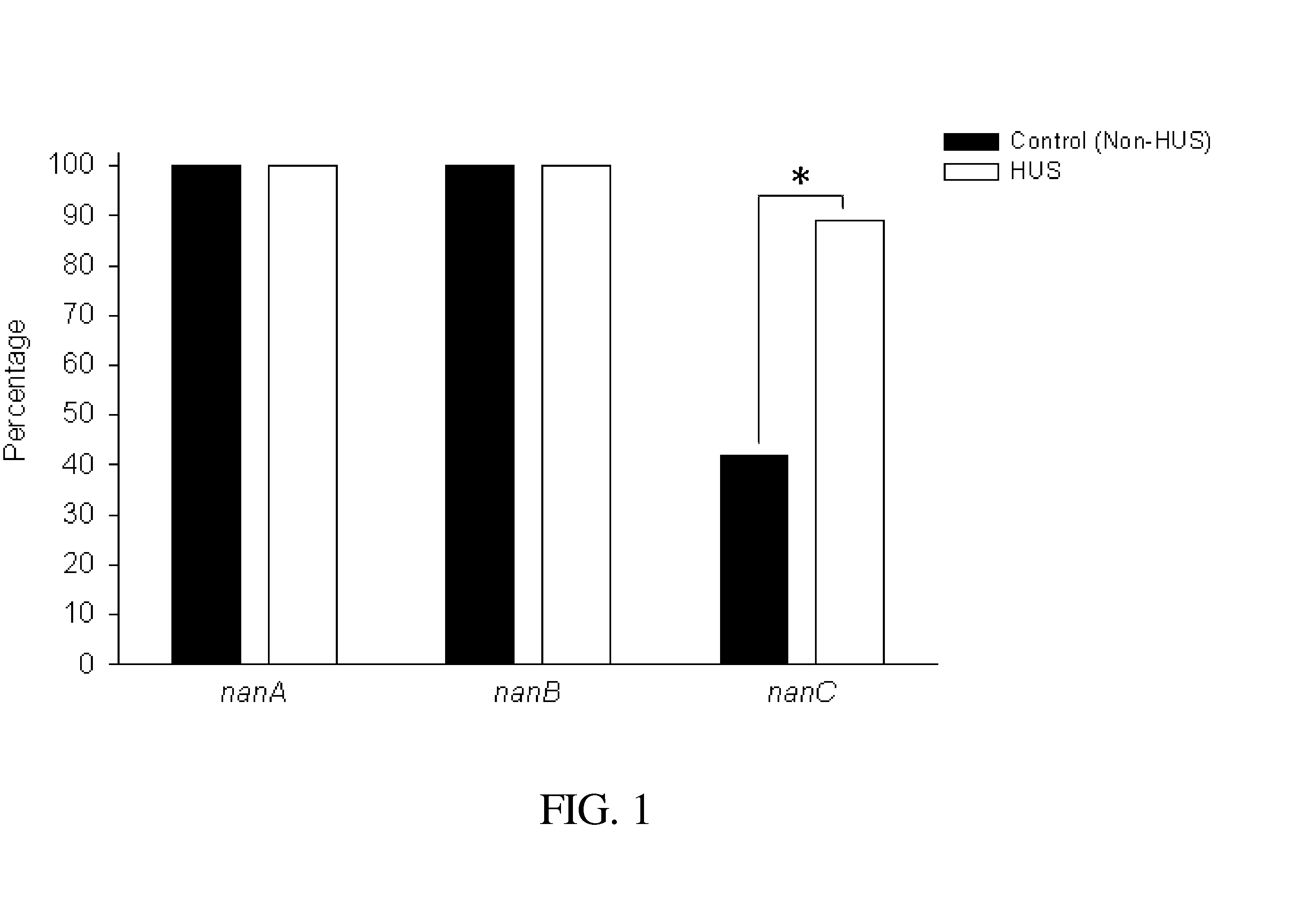
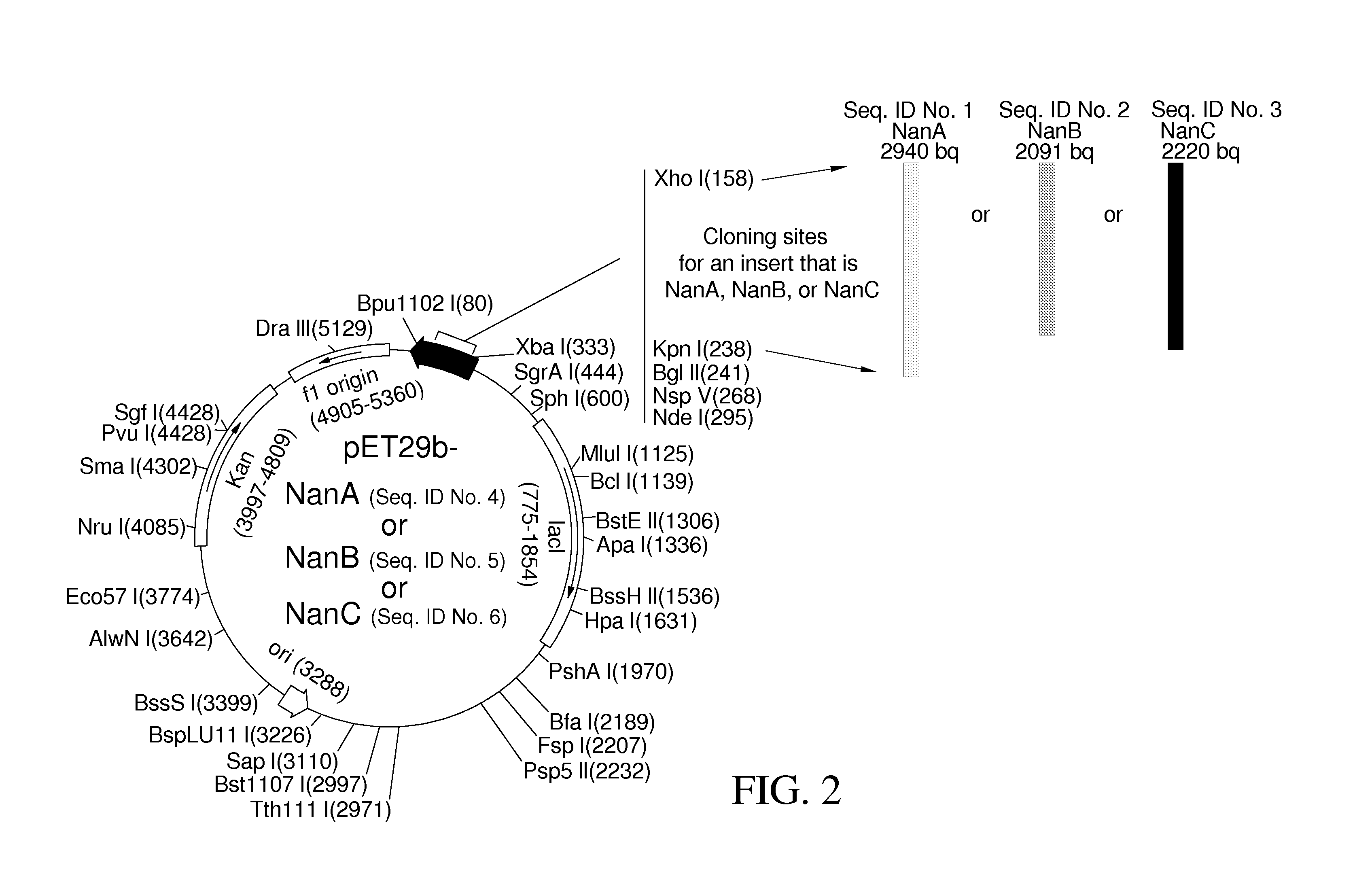
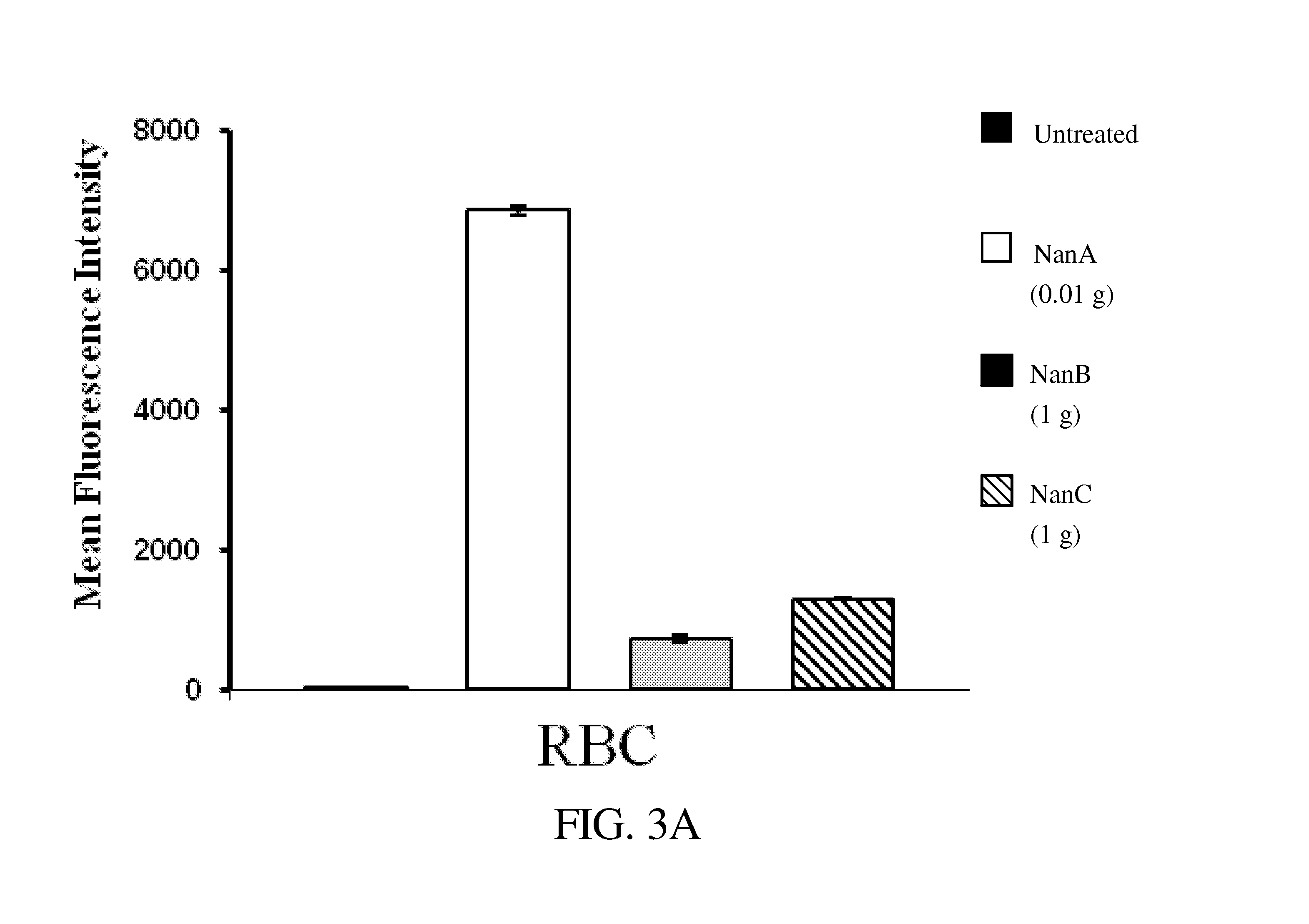
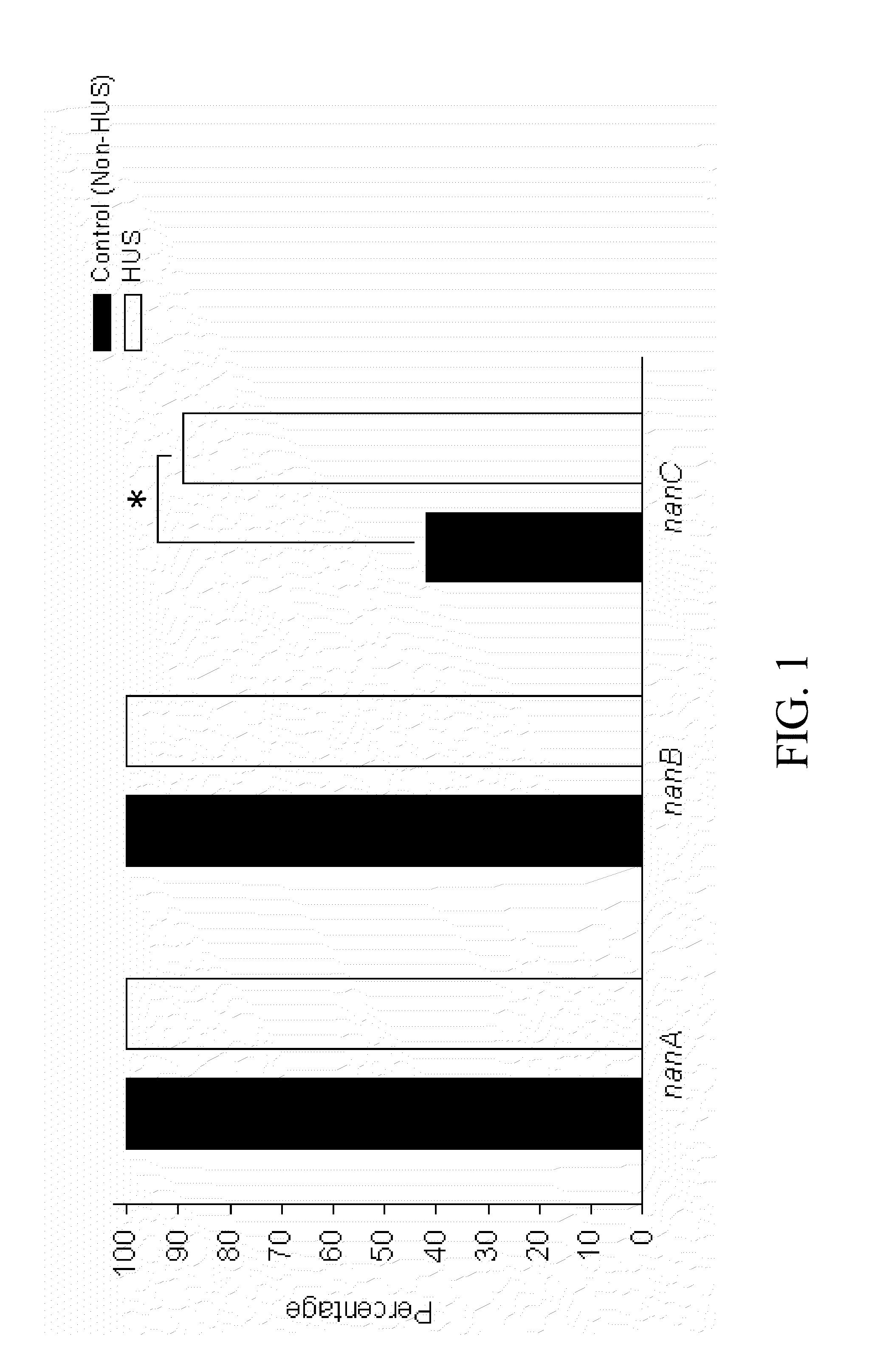
Method of diagnosing and preventing pneumococcal diseases using pneumococcal neuraminidases
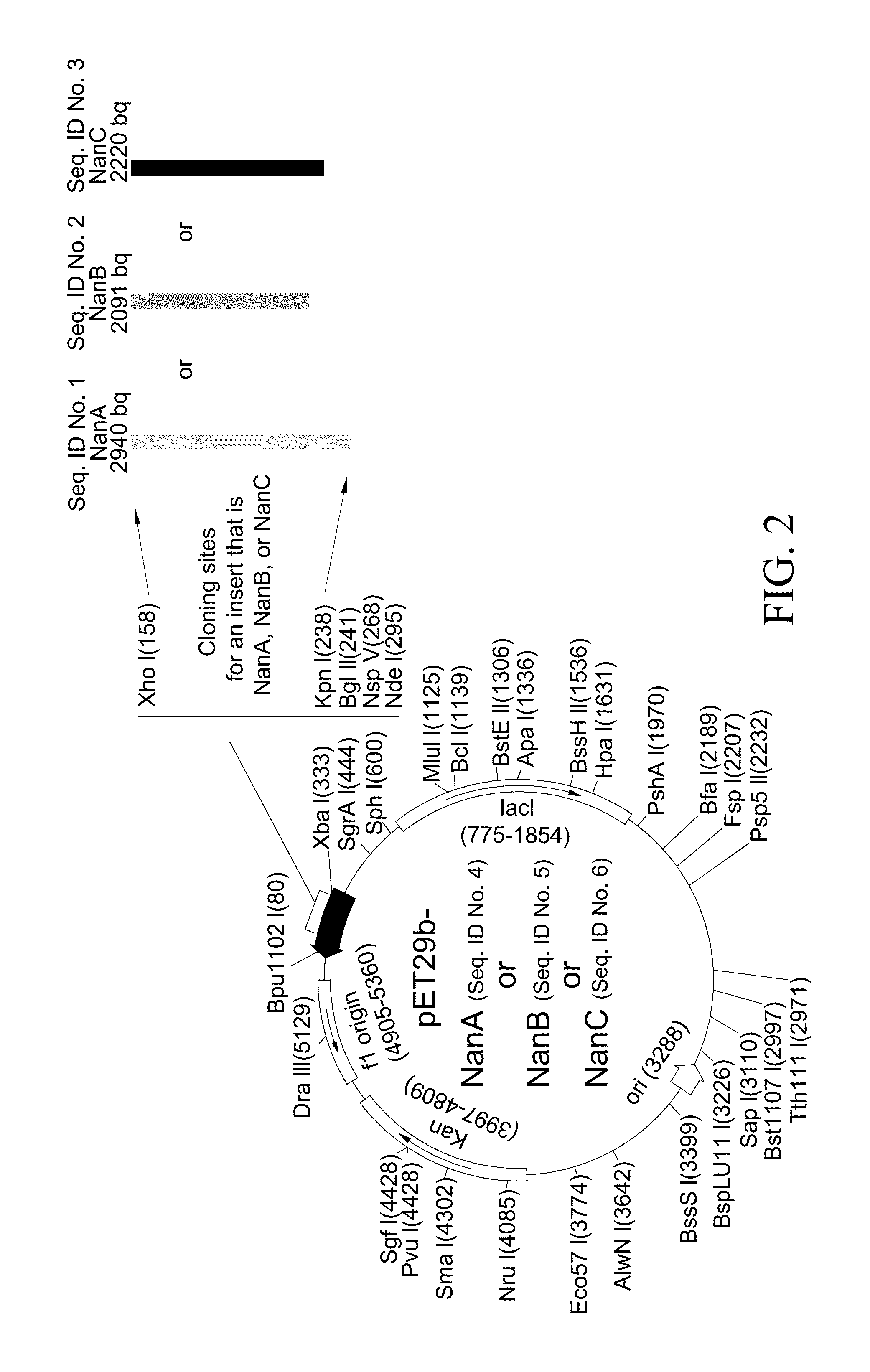
A method of providing protection against pneumococcal infection in a subject is disclosed. The method includes steps of administering to the subject a composition that includes combination of three recombinant pneumococcal neuraminidases: NanA, NanB, and NanC of S. pneumoniae strains CGSP14, wherein administration of the recombinant pneumococcal neuraminidases elicits an immune response to S. pneumoniae, and treats the subject. In one embodiment, the method further includes a step of adding adjuvants to enhance the immune response. The method also includes a step of using passive antibodies, wherein said passive antibodies are anti-neuraminidase antibodies generated from neuraminidases-immunized humanized animals: NanA, NanB, and NanC. Meanwhile, this invention also provides a method for the molecular diagnosis of pneumococcal infection.
Owner:CHUNG GUNG MEDICAL FOUNDATION LINKOU BRANCH
Molecular diagnosis method for evaluating growth traits based on copy number variation (CNV) marker of cattle ZNF146 gene and application of molecular diagnosis method
ActiveCN113151489ASimple and fast operationMicrobiological testing/measurementDNA/RNA fragmentationMedicineA-DNA
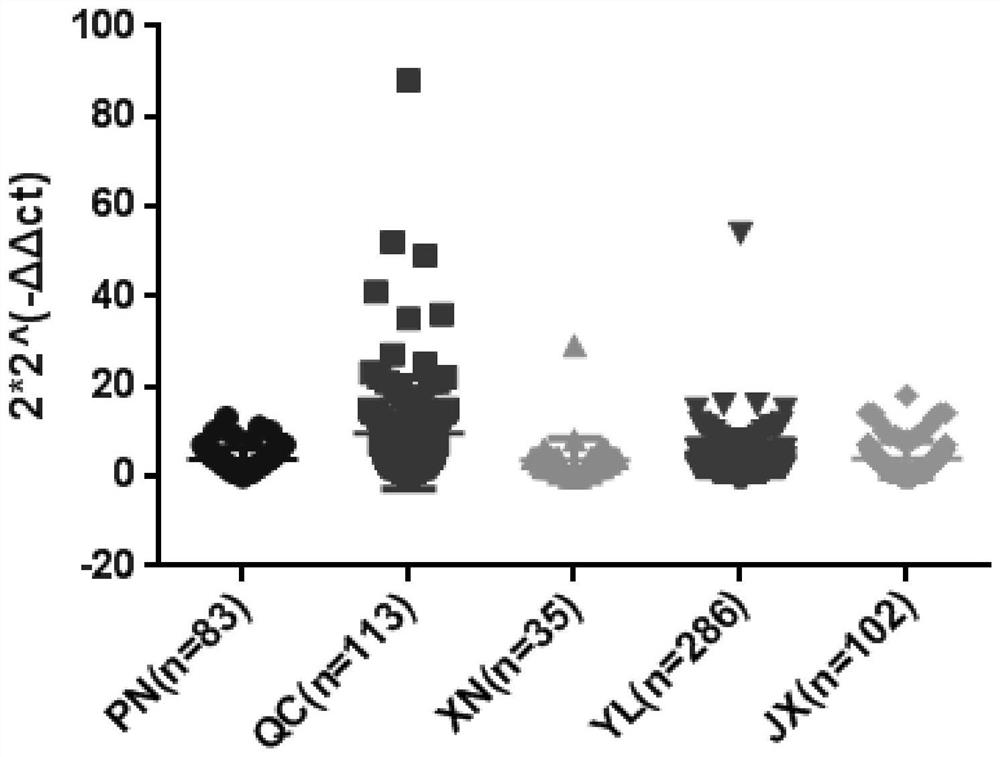
The invention discloses a molecular diagnosis method for evaluating growth traits based on a copy number variation (CNV) marker of a cattle ZNF146 gene and application of the molecular diagnosis method. Based on real-time quantitative polymerase chain reaction (PCR), by taking cattle genome DNA to be detected as a template, a CNV region of the ZNF146 gene and a partial fragment of the internal reference gene BTF3 are amplified by using two pairs of primers P1 and P2, and finally, a copy number variation type of an individual is calculated and judged by using a 2*2<-delta delta Ct> method. The invention can detect the CNV marker closely related to the cattle growth traits on a DNA level, and the method is simple, is rapid and is convenient to apply and popularize.
Owner:河南省畜牧总站
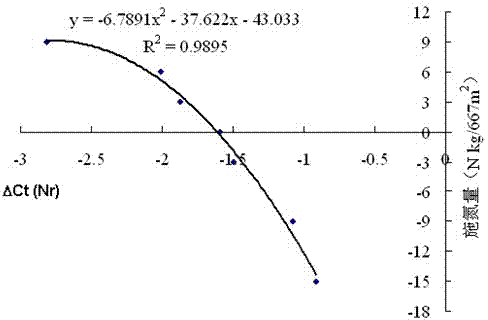
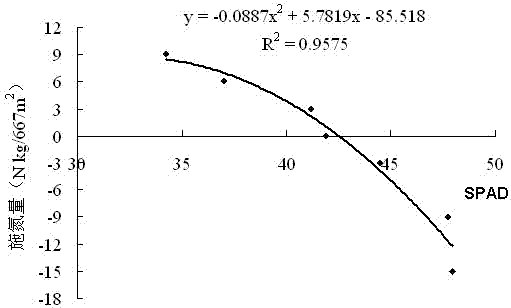
Molecular diagnostic method for nitrogen nutrition of field potato plants and application of method in fertilization
ActiveCN103923978AEasy to operateMicrobiological testing/measurementFertilising methodsBiotechnologyNutrition
The invention discloses a molecular diagnostic method for nitrogen nutrition of field potato plants. Specifically speaking, a nitrate reductase (NR) gene related to nitrogen absorption by potato plants is used as a molecular marker for judging the states of nitrogen nutrition of field potato plants. During diagnosis, RNA of a field potato plant is used as a template, reverse transcription is carried out to obtain cDNA, the expression level of the NR gene is detected by using real-time PCR, the state of nitrogen nutrition of the field potato plant is judged according to the expression level of the NR gene, and whether to carry out topdressing of a nitrogenous fertilizer and the amount of topdressing of the nitrogenous fertilizer can be further determined. The diagnostic method has the advantages of good accuracy in judgment, high sensitivity, etc.
Owner:HUNAN AGRICULTURAL UNIV
Kit and method for integrally and comprehensively detecting five complex genetic diseases
PendingCN114150051AIntegrated comprehensive detection comprehensiveIntegrated comprehensive detection and high efficiencyMicrobiological testing/measurementDNA/RNA fragmentationNucleotideThalassemia
The invention provides a method and a kit for integrally and comprehensively detecting five complex genetic diseases. The detection method and the kit are used for detecting all common known pathogenic variations of related genes of Duchenne muscular dystrophy, thalassemia, spinal muscular atrophy, hereditary hearing loss and congenital adrenal hyperplasia. According to the invention, only one experiment and one technical platform are needed to simultaneously detect single exon level copy number deletion / repetition and common clinical related known pathogenicity single nucleotide variation of the disease, so that the defects that a plurality of technical platforms are needed to be combined, the diagnosis efficiency is low and the detection cost is high in the existing gene detection method can be effectively overcome; the standardization degree of clinical detection of the diseases by doctors can be remarkably improved, and an integrated, high-timeliness, high-accuracy and high-operability molecular diagnosis method is provided for diagnosis and genetic intervention of similar complex genetic diseases, so that the diagnosis, treatment, prognosis and genetic counseling levels of the diseases are remarkably improved.
Owner:上海源赏生物科技有限公司
Molecular diagnostic method of a cancer tissue or a cancer cell
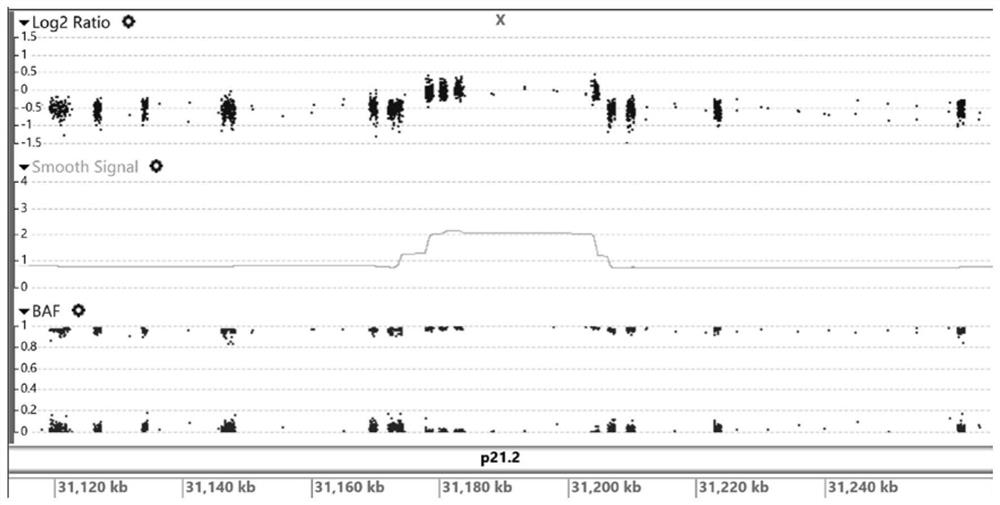
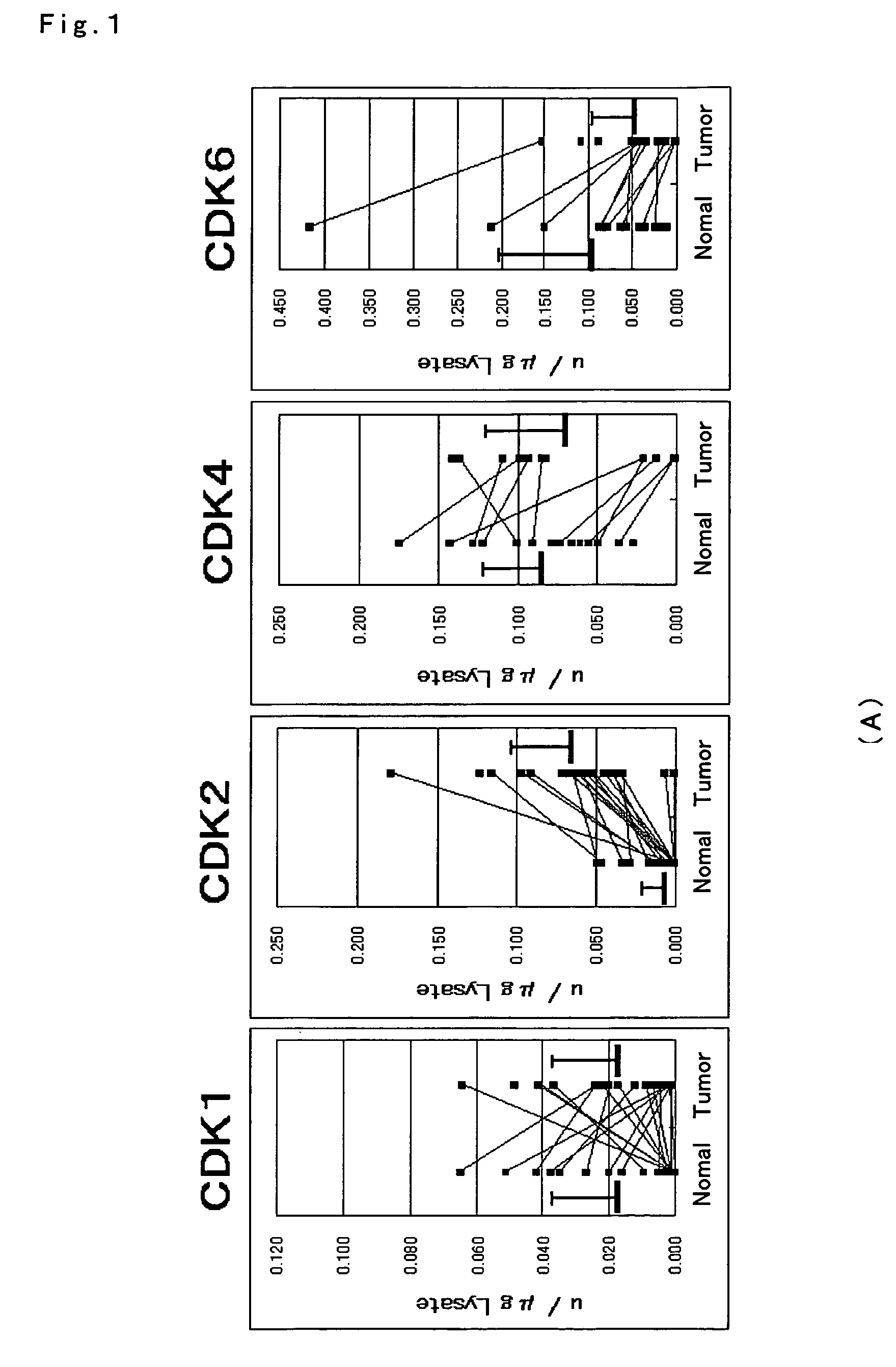
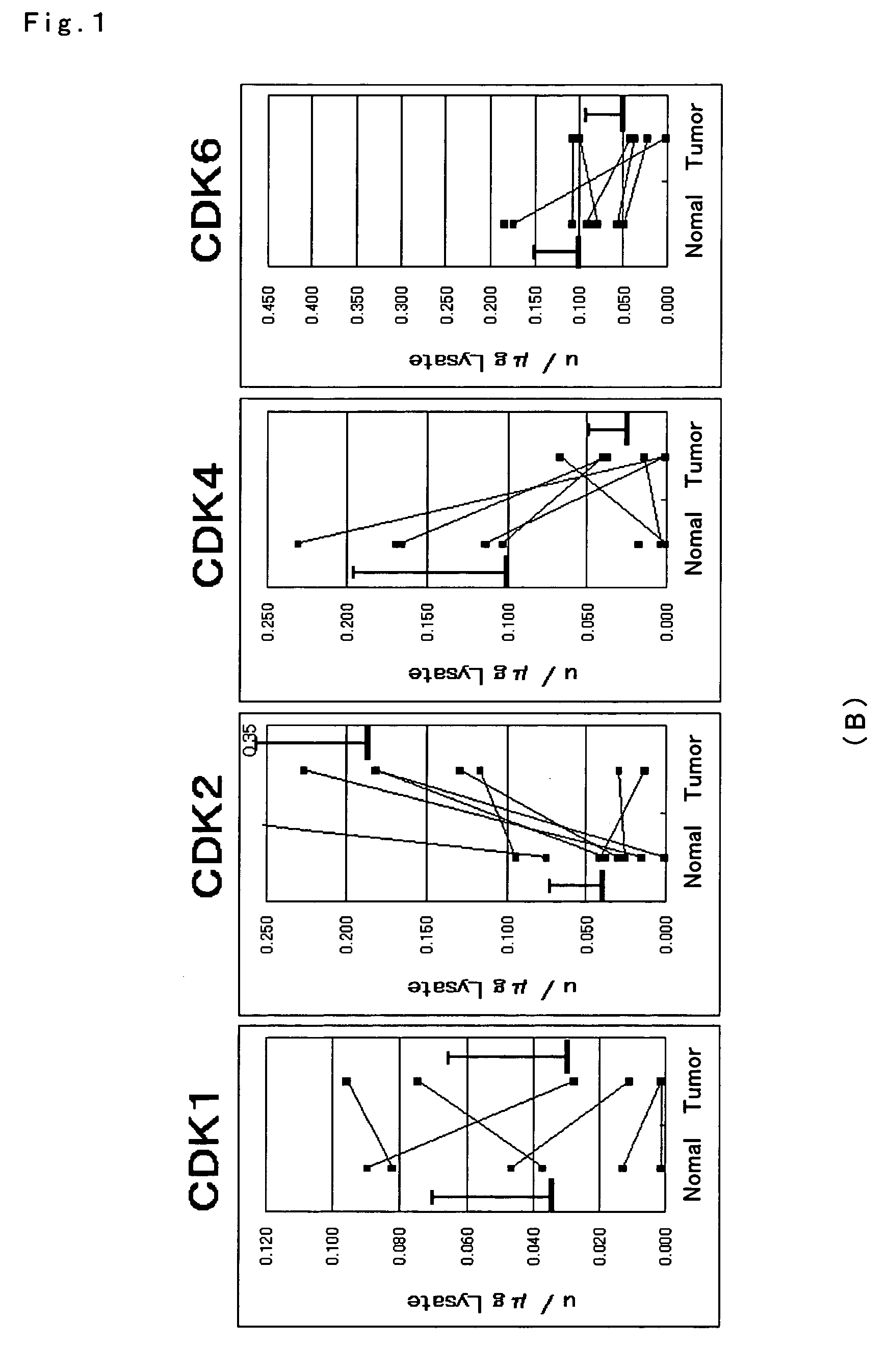
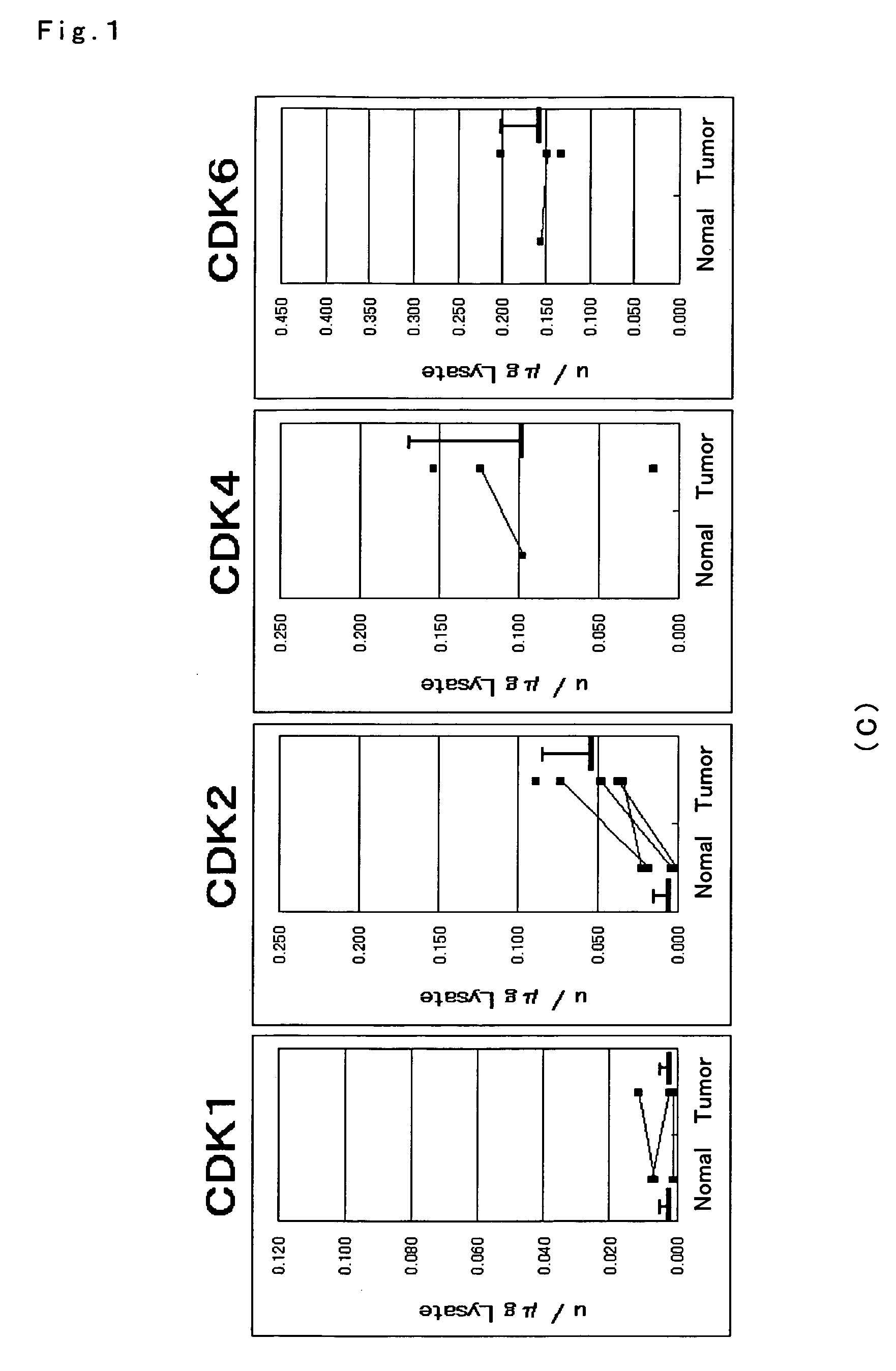
ActiveUS7501257B2Accurate diagnosisMicrobiological testing/measurementDisease diagnosisCancer cellMolecular Diagnostic Method
The present invention relates to a more accurate method of diagnosing cancer in a tissue or a cell by measuring the expression and activity of cell cycle related proteins and analyzing a cell cycle profile. In a cell cycle profile, at least two values of expression or activity of cell cycle related proteins are concurrently measured. The measurement of the cell cycle related protein is not limited as to the measurement method.
Owner:SYSMEX CORP
Method and kit for typing detection of drug resistance of helicobacter pylori in oral cavity
Because gastric biopsy samples are difficult in endoscope sampling and high in cost, helicobacter pylori (Hp) is hard to culture under harsh growth conditions and an invasive diagnosis method of the drug resistance of the Hp is not ideal, the invention provides a molecular diagnosis method for detecting drug resistance of non-invasive helicobacter pylori. An excrement sample is quickly detected and analyzed by combining a nested PCR technology and a sequencing technology; mutation of 52 sites of seven drug-resistant genes, such as 23SrRNA, gyrA, PBP1, 16SrRNA, porD, oorD and rpoB, and random mutation of an rdxA drug-resistant gene are jointly detected by utilizing primers with nucleotide sequences shown in a table 2 and a table 3; and thus,, the drug resistance conditions of seven antibiotics commonly used in clinic at present are judged. The invention belongs to the technical field of biology, is rapid and sensitive, is suitable for clinical application, and has a great application prospect in the aspect of guiding personalized diagnosis, treatment and eradication of helicobacter pylori patients.
Owner:深圳艾普斯金基因检测合伙企业(有限合伙)
Integrated kit for in-situ molecular diagnosis and molecular diagnosis method using same
PendingCN114644977ALow pollution operationCost-effectiveBioreactor/fermenter combinationsBiological substance pretreatmentsMolecular diagnosticsMolecular Diagnostic Method
Proposed are an integrated kit for in-situ molecular diagnosis and a molecular diagnosis method using the same. The integrated kit for on-site molecular diagnosis comprises a first assembly and a second assembly, the first assembly comprises a sample pretreatment part, a nucleic acid absorption part and a nucleic acid amplification part, and the second assembly comprises a detection part. The sample pretreatment section includes a transfer member configured to transfer a lysis buffer in which nucleic acid is dissolved, and is configured to pretreat a sample. The nucleic acid absorption portion is positioned on the sample pretreatment portion and configured to be slidable onto the nucleic acid amplification portion. The nucleic acid amplification part is connected to the sample pretreatment part, and is configured to elute the nucleic acid from the nucleic acid absorption part and amplify the eluted nucleic acid. The detection section is configured to transfer the nucleic acid amplified by the nucleic acid amplification section and detect the nucleic acid. The integrated kit for in-situ molecular diagnosis and the molecular diagnosis method using the same according to the present disclosure are advantageous in that the kit enables ordinary people to perform molecular diagnosis without specialized devices (such as a centrifuge or a pipettor), ensures low-pollution operation, and has cost effectiveness.
Owner:菲尔梅迪株式会社
Method of diagnosing and preventing pneumococcal diseases using pneumococcal neuraminidases
A method of providing protection against pneumococcal infection in a subject is disclosed. The method includes steps of administering to the subject a composition that includes combination of three recombinant pneumococcal neuraminidases: NanA, NanB, and NanC of S. pneumoniae strains CGSP14, wherein administration of the recombinant pneumococcal neuraminidases elicits an immune response to S. pneumoniae, and treats the subject. In one embodiment, the method further includes a step of adding adjuvants to enhance the immune response. The method also includes a step of using passive antibodies, wherein said passive antibodies are anti-neuraminidase antibodies generated from neuraminidases-immunized humanized animals: NanA, NanB, and NanC. Meanwhile, this invention also provides a method for the molecular diagnosis of pneumococcal infection.
Owner:CHUNG GUNG MEDICAL FOUNDATION LINKOU BRANCH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com