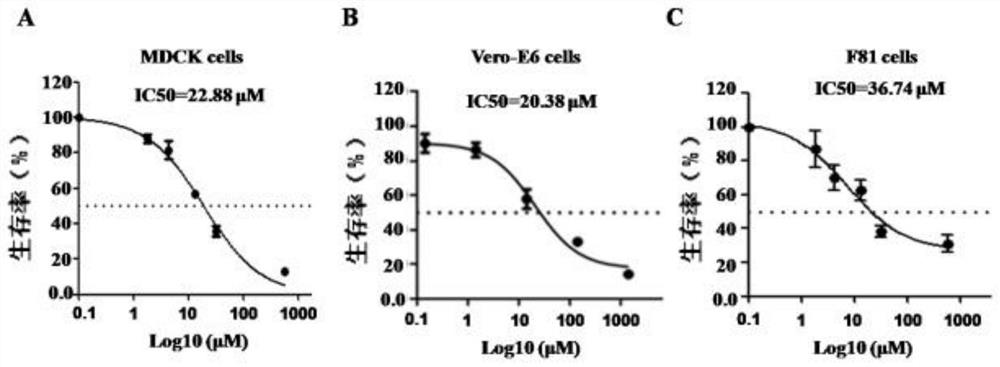
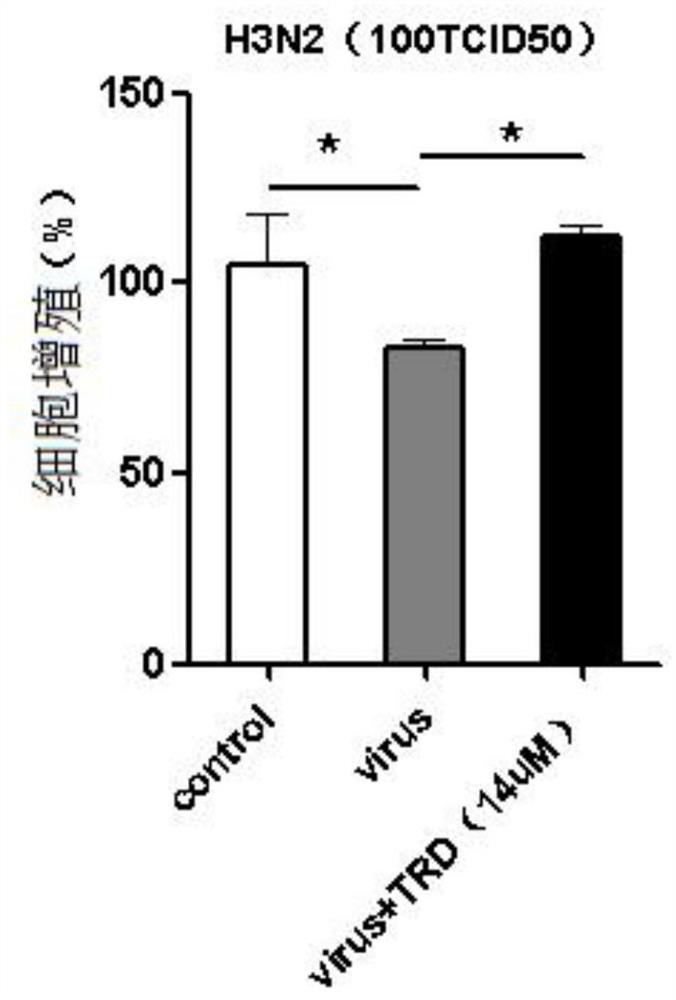
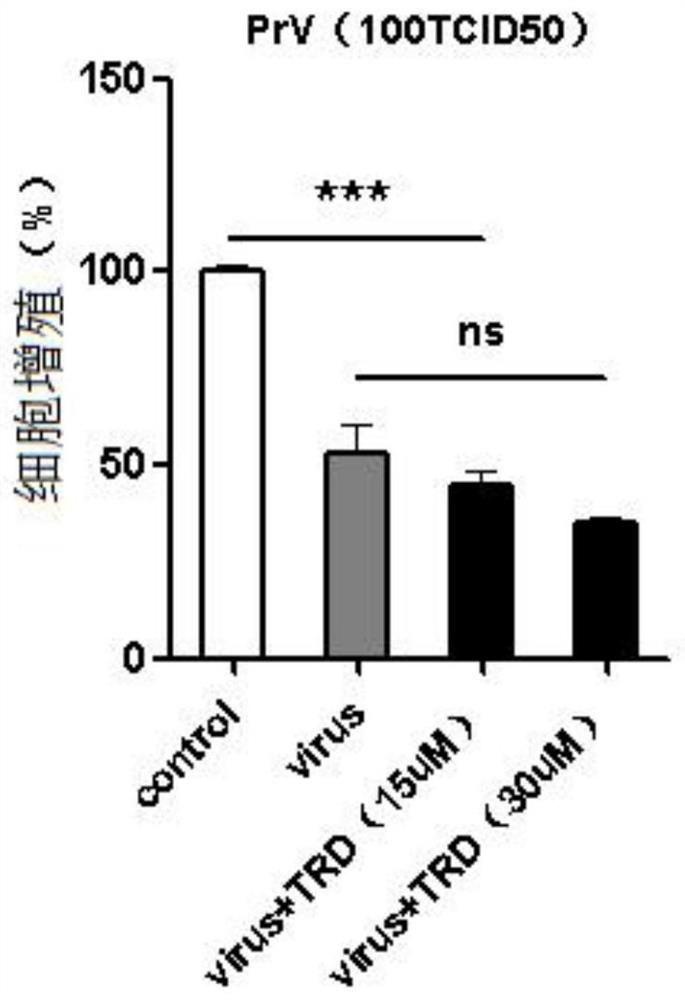
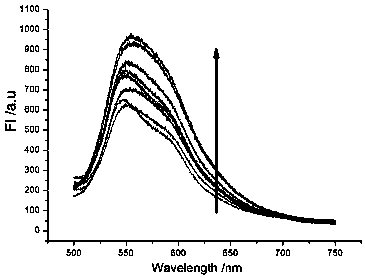
Patents
Literature
52 results about "Para-influenza viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
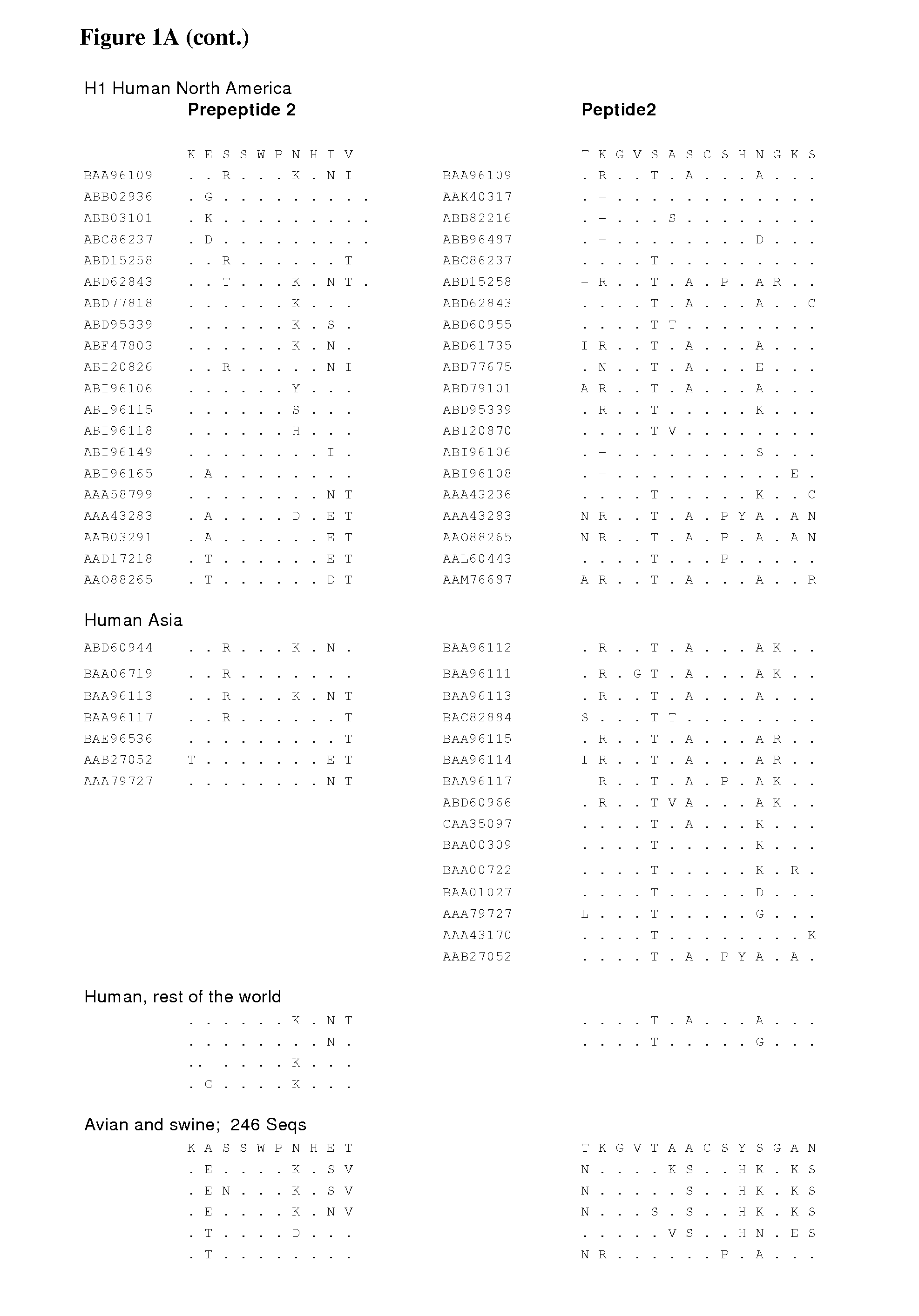
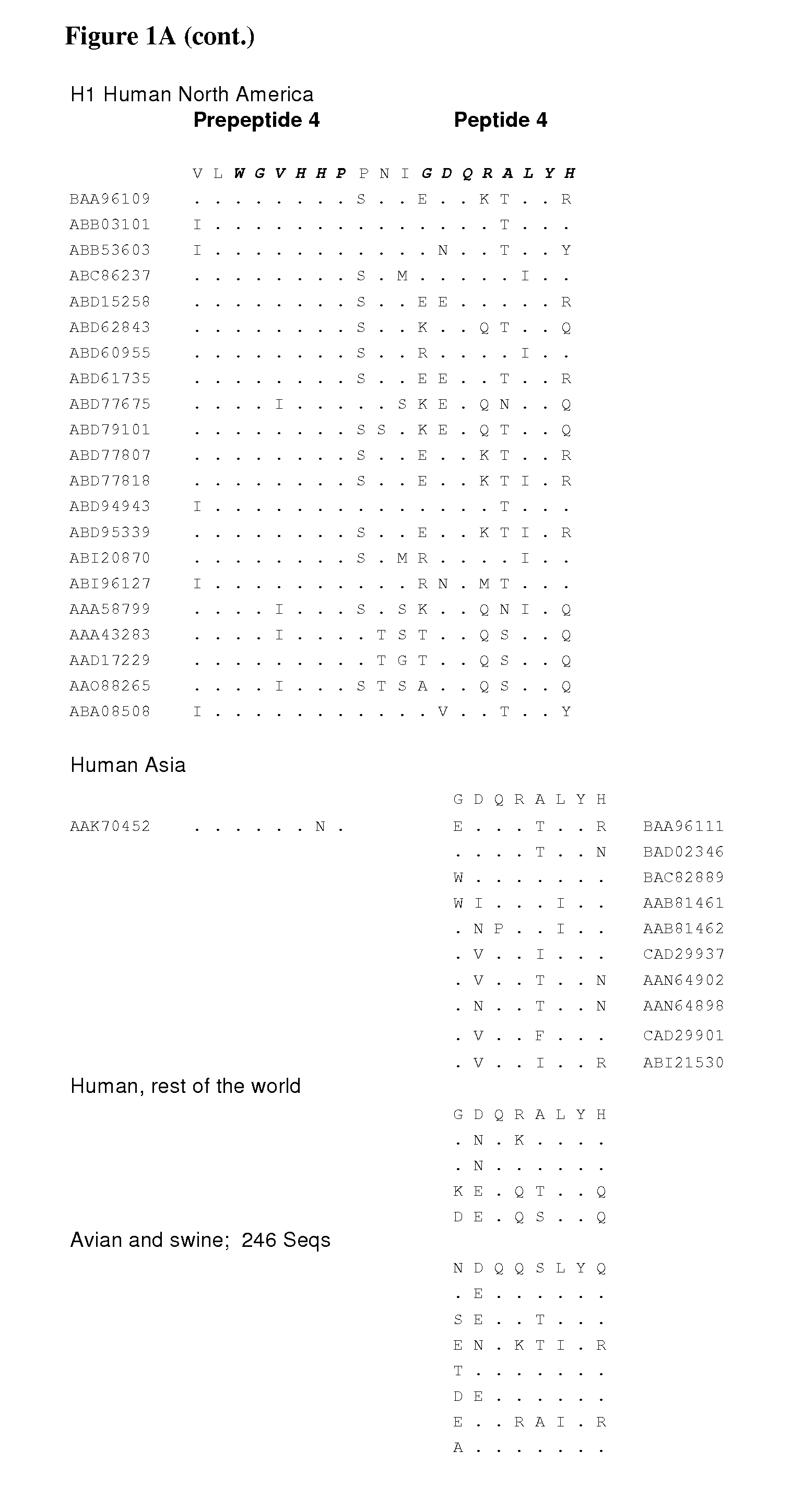
Signal for packaging of influenza virus vectors
InactiveUS7226774B2SsRNA viruses negative-senseViral antigen ingredientsPara-influenza virusesViral vector
The invention provides a packaging (incorporation) signal for influenza virus vectors, and methods of using the signal to transmit and maintain influenza viral and foreign nucleic acid in virus and cells.
Owner:WISCONSIN ALUMNI RES FOUND
Decreasing potential iatrogenic risks associated with influenza vaccines
InactiveUS20120034600A1Increased risk of contaminationSsRNA viruses negative-senseViral antigen ingredientsFlu immunizationInfluenza virus culture
Influenza viruses for use in preparing human vaccines have traditionally been grown on embryonated hen eggs, although more modern techniques grow the virus in mammalian cell culture e.g. on Vero, MDCK or PER.C6 cell lines. The inventor has realised that the conditions used for influenza virus culture can increase the risk that pathogens other than influenza virus may grow in the cell lines and have identified specific contamination risks. Suitable tests can thus be performed during manufacture in order to ensure safety and avoid iatrogenic infections.
Owner:NOVARTIS AG
Peptide vaccine for influenza virus
The invention provides peptide epitopes for use in the prevention and / or treatment of influenza or for the development of such treatment or vaccine against influenza. The invention also relates to a method for evaluating the potential of a chemical entity, such as an antibody, to bind to a peptide epitope derived from the divalent sialoside binding site of hemagglutinin protein of influenza virus, and to conjugates containing one or more such peptide epitopes. The peptide epitopes of the invention are cyclic peptides comprising a 7-mer peptide derived from H1, H3 or H5 hemagglutinin of influenza virus. The 7-mer peptide has a sequence corresponding to the loop sequence at positions 220-226 of X31-hemagglutinin.
Owner:GLYKOS FINLAND
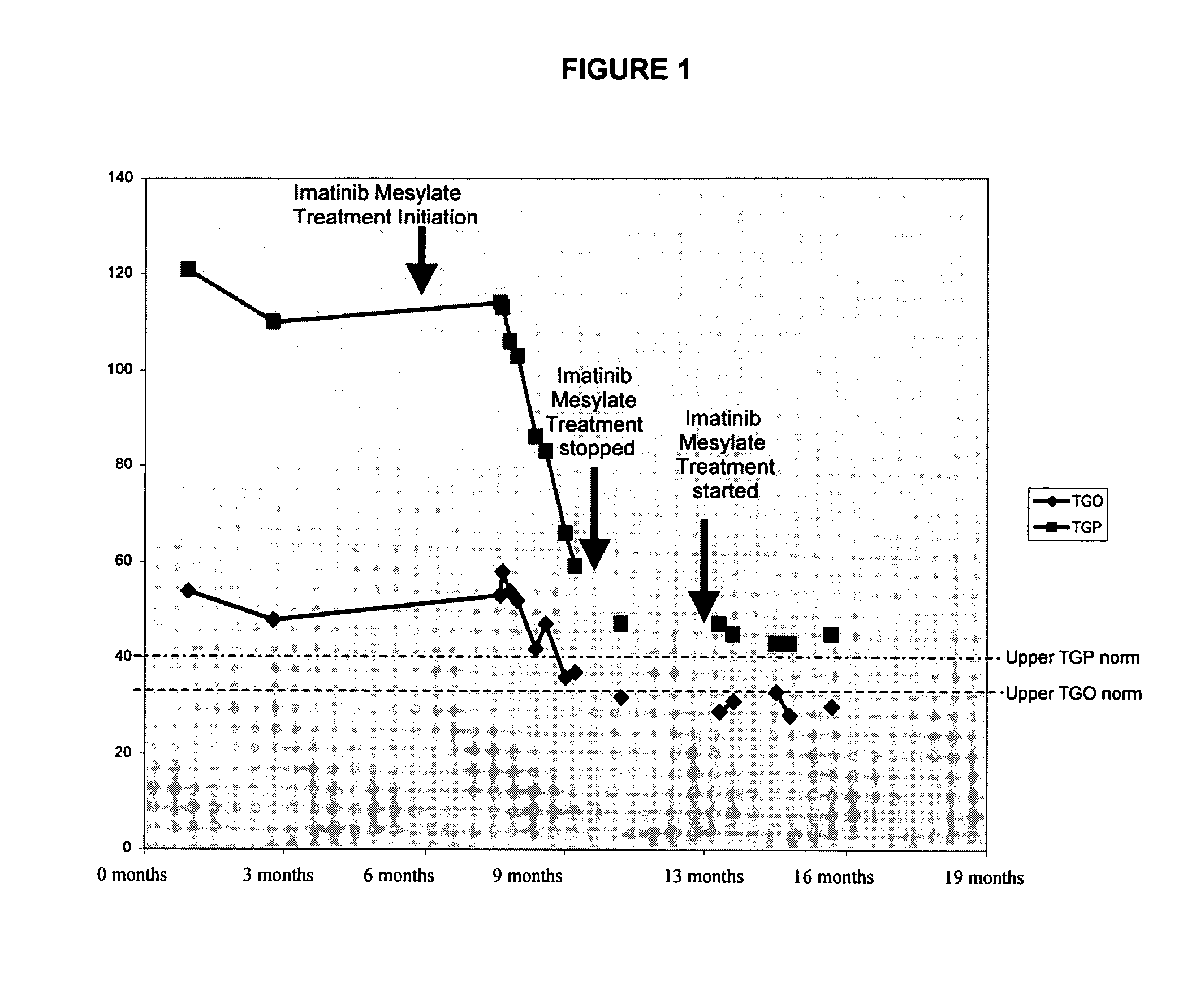
Use of imatinib to treat liver disorders and viral infections
The present invention relates to the use of imatinib for treating viral liver diseases and in particular for viral hepatitis. The invention provides the use of imatinib for inhibiting replication, transmission or both of hepatitis viruses. The invention further relates to the use of imatinib for inhibiting replication, transmission or both of other viruses including herpes virus, poxvirus, influenza virus, para influenza virus, respiratory syncytial virus, rhinovirus, yellow fever virus, west nile virus, and encephalitis virus.
Owner:BIONICHE LIFE SCI
Preparation method of pseudotype virus for nucleic acid detection of 2019-nCoV
ActiveCN111471717AEasy loadingSsRNA viruses positive-senseVirus peptidesViral nucleic acidViral vector
The invention discloses a preparation method of a pseudotype virus for nucleic acid detection of 2019-nCoV, and relates to the technical field of biology. The preparation method comprises a method forpreparing standard quality control products which contain but are not limited to a 2019-nCoV nucleotide sequence and are used for nucleic acid detection through a non-integrated slow virus vector system, a method which is used for preparing the standard quality control products for nucleic acid detection, and a method for constructing a non-integrated slow virus through mutating 64-locus amino acid Asp of integrase of slow virus auxiliary plasmid into Asn, and amino acids Arg, Arg and Lys at 262-264 loci into Ala, Ala and His. In the manner of removing elements of promoters and the like of slow viruses, the load quantity of virus vectors can be promoted, a pseudotype virus which can be used for nucleic acid detection and complete flow quality control is constructed, and the preparation method can be used for constructing a pseudotype virus for corona viruses and can also be used for constructing a pseudotype virus for SARS-COV, MERS-COV, influenza viruses and the like.
Owner:复百澳(苏州)生物医药科技有限公司
Flu vaccines and methods of use thereof
InactiveUS20090060949A1Amount of chicken red blood cell hemadsorptionReduce the amount requiredSsRNA viruses negative-senseOrganic active ingredientsFlu vaccinesPara-influenza viruses
Owner:THE ROCKEFELLER UNIV +1
Sample pretreatment solution for immunological test and method for using the same
InactiveUS20090269735A1Good backgroundSure easyMicrobiological testing/measurementBiological material analysisPara-influenza virusesImmunological tests
Sample pretreatment solutions for influenza virus tests by immunochromatography are described.Methods detecting influenza virus by immunochromatography using the sample pretreatment solutions and Kits comprising the sample pretreatment solution and an immunochromatographic device are also described.
Owner:SYSMEX CORP
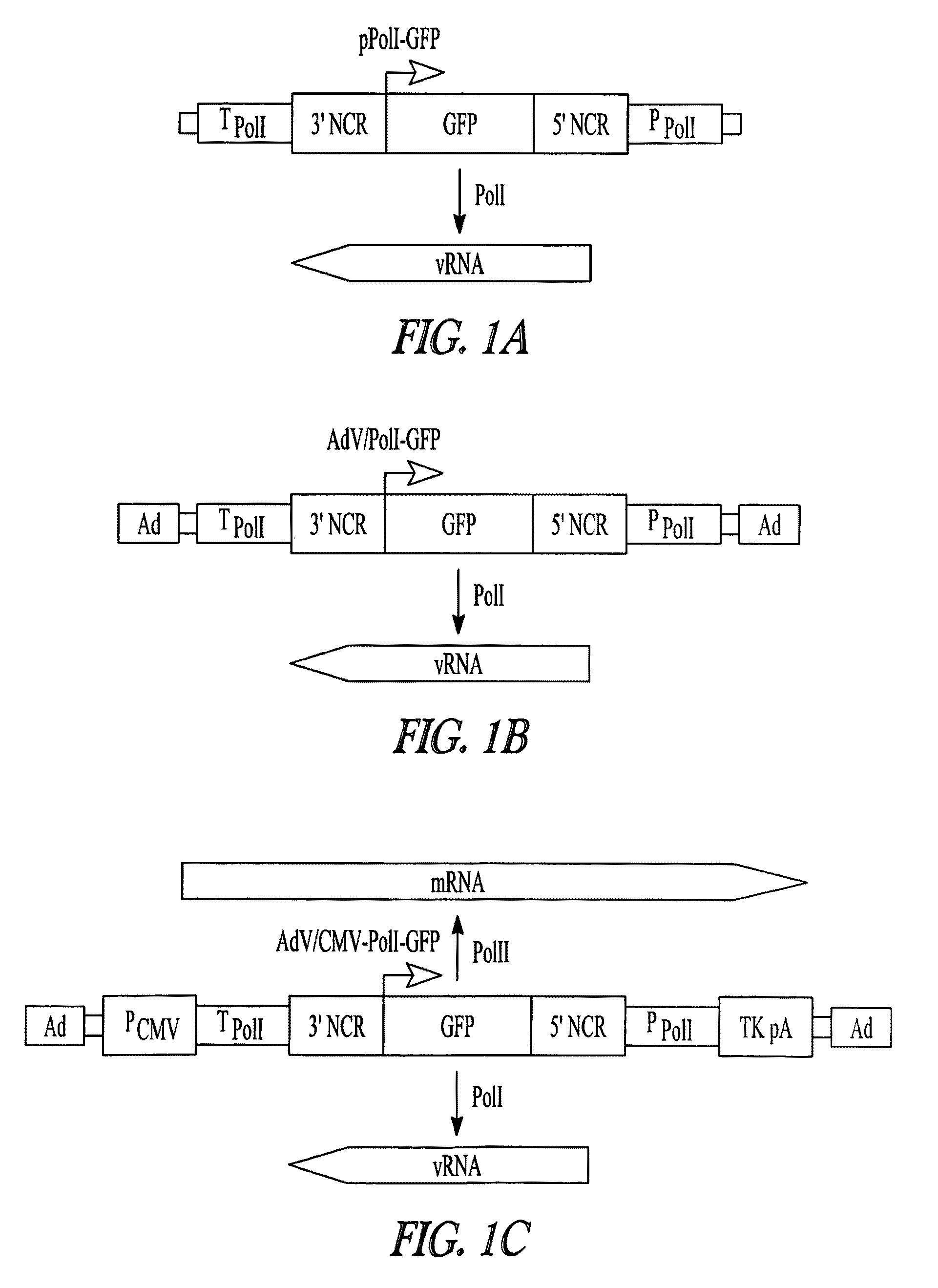
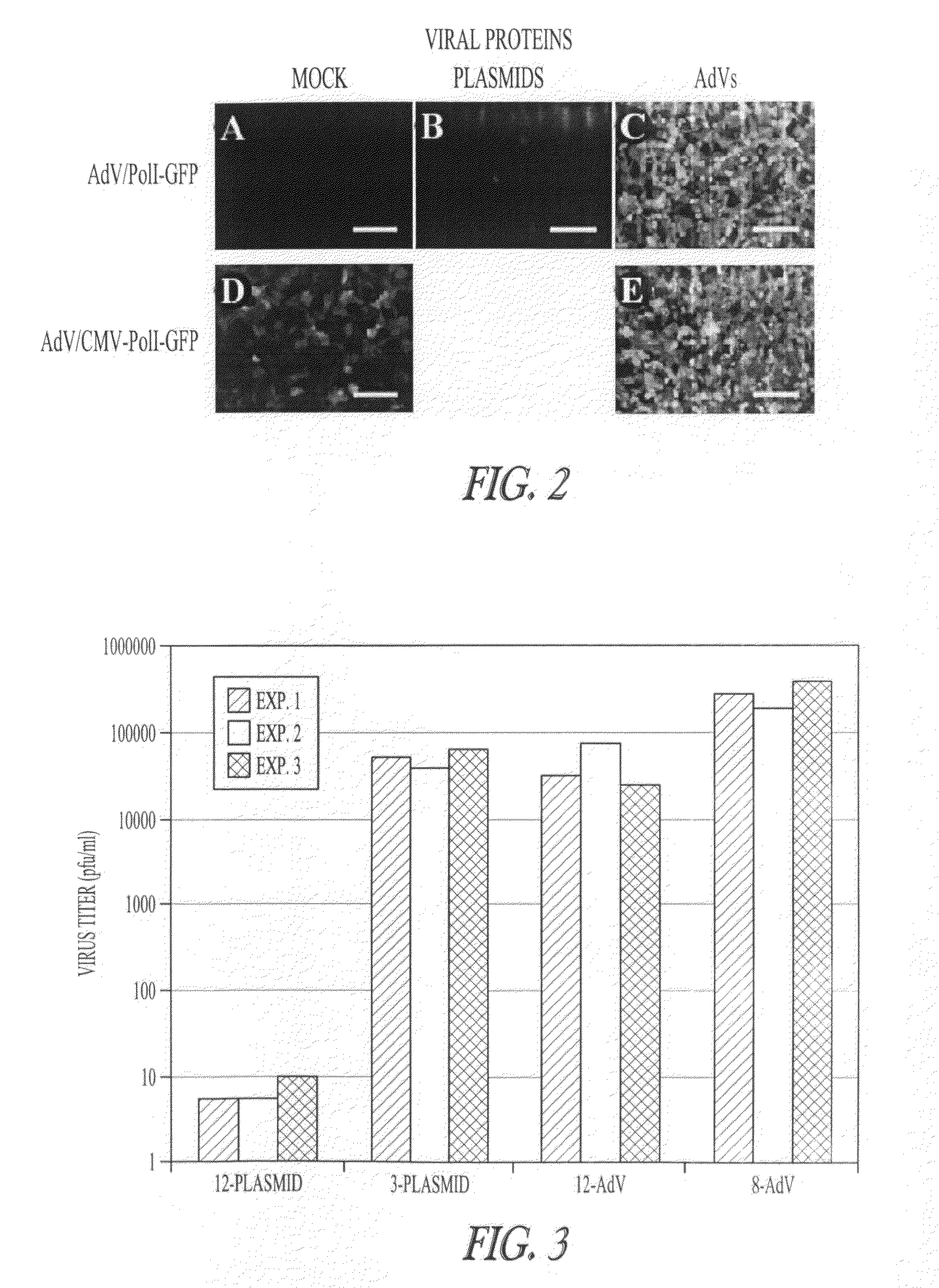
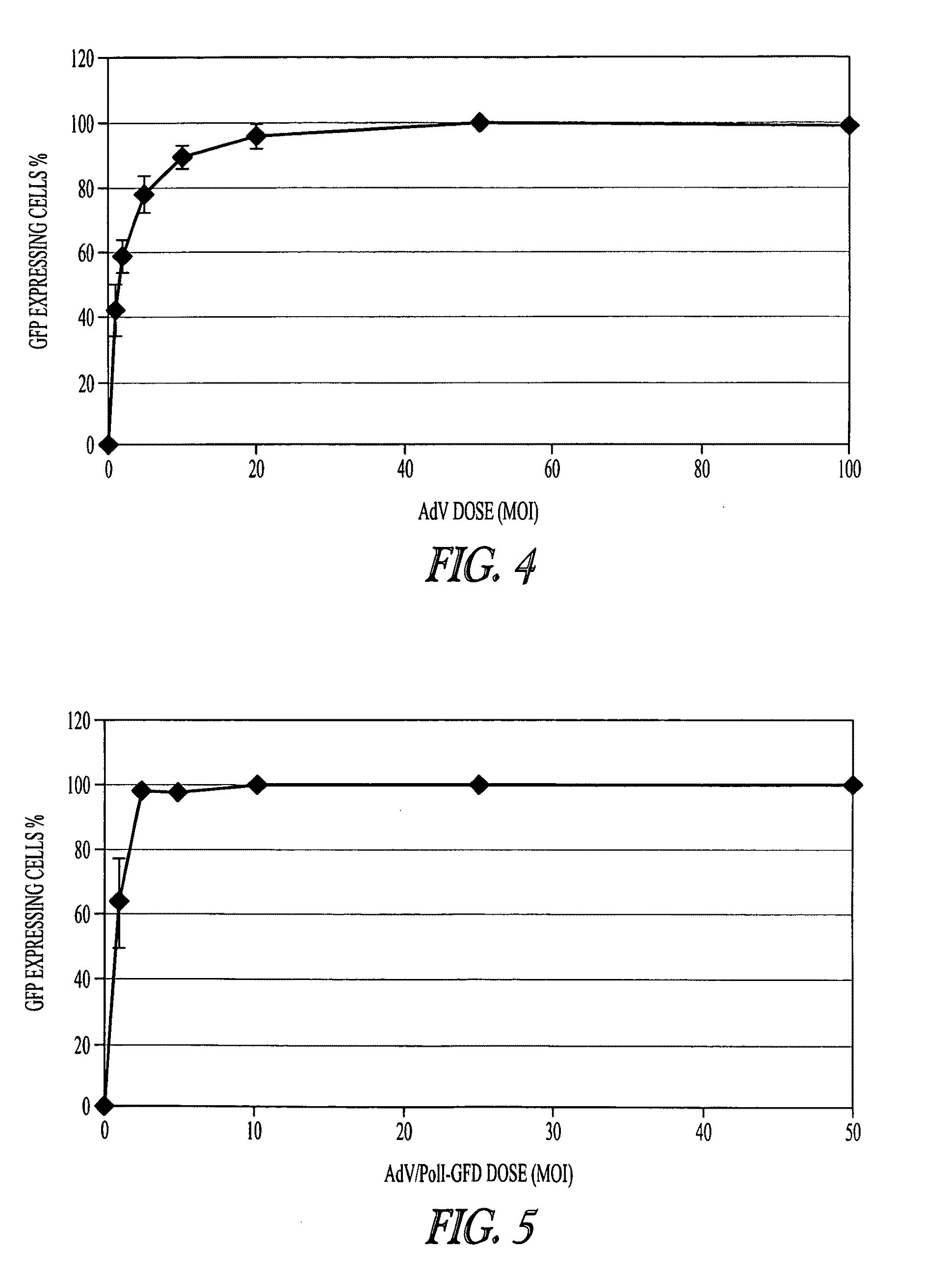
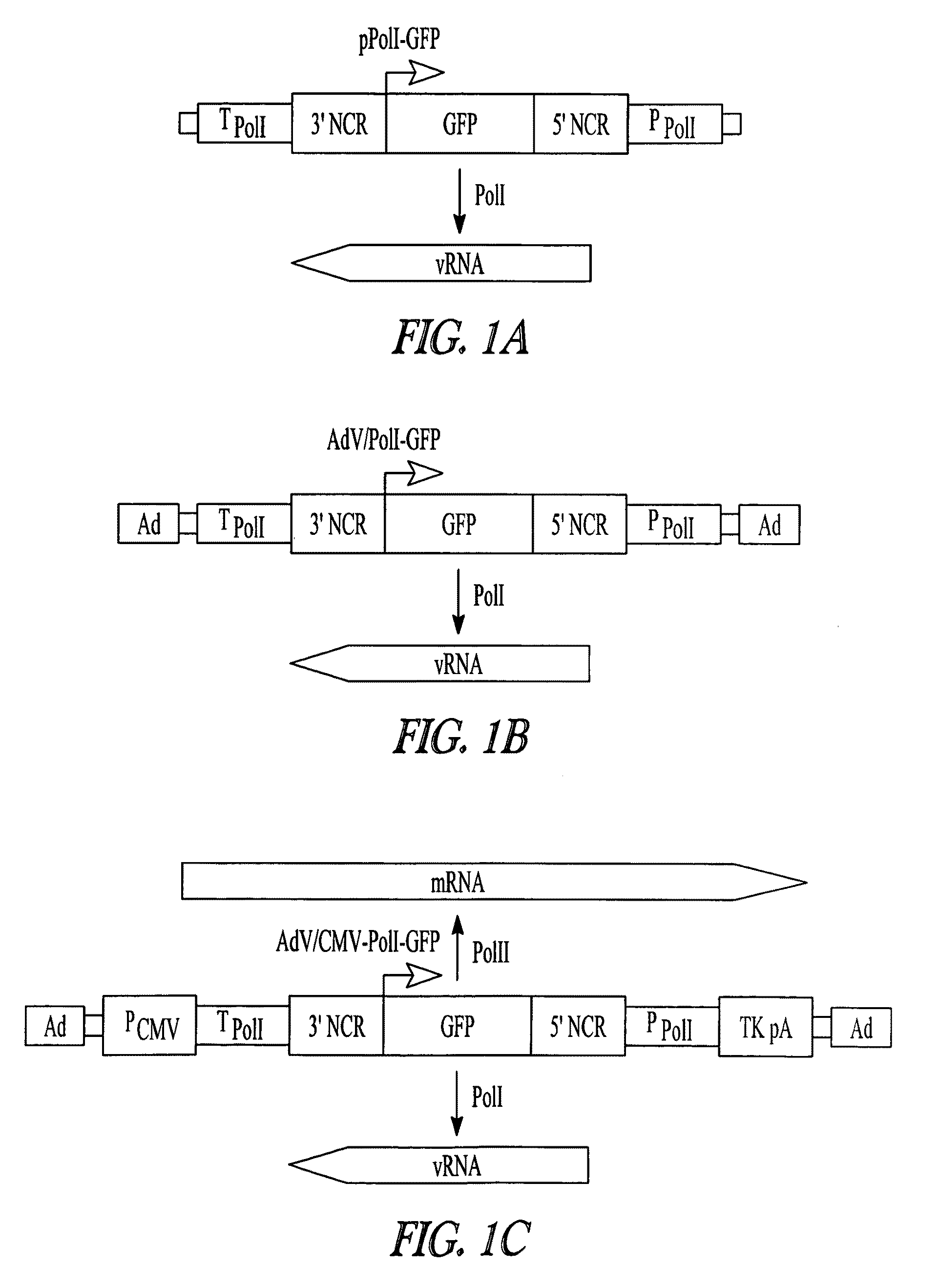
Adenoviral vectors for influenza virus production
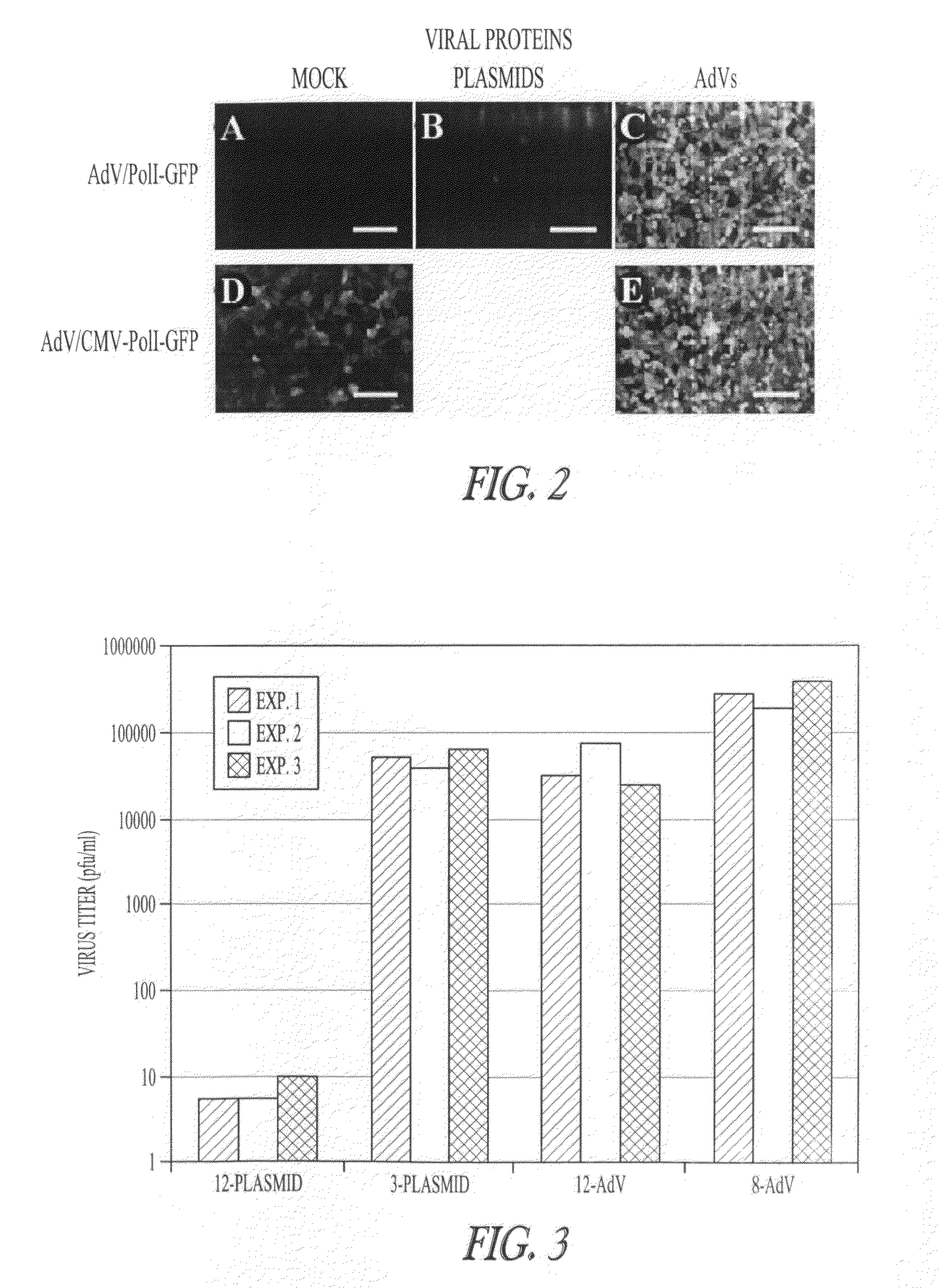
ActiveUS8043856B2High expressionIncrease productionSsRNA viruses negative-senseAnimal cellsVirus-RetrovirusD'Aguilar virus
The invention provides adenovirus and retrovirus vectors useful to prepare influenza virus. Also provided is a canine RNA polymerase I promoter and vectors having that promoter.
Owner:WISCONSIN ALUMNI RES FOUND
Piperazine derivatives for influenza virus inhibitions
ActiveUS20200010459A1Neutralizing activityCompetitive binding activityOrganic chemistryAntiviralsHemagglutininPharmaceutical drug
The present invention provides piperazine derivatives exhibiting high affinity to the stem region (viral membrane proximal part) of influenza hemagglutinin as determined through competition binding and high virus neutralization activity while having low cytotoxity. Furthermore, the present invention relates to pharmaceutical compositions comprising said piperazine derivatives, methods of preparing said piperazine derivatives, as well as said piperazine derivatives for use in medical prevention or treatment, especially for preventing or treating influenza.
Owner:JANSSEN VACCINES & PREVENTION BV
Flu vaccines and methods of use thereof
InactiveUS7981428B2Amount of chicken red blood cell hemadsorptionReduce the amount requiredSsRNA viruses negative-senseGenetic material ingredientsFlu vaccinesPara-influenza viruses
This disclosure is directed, inter alia, to polynucleotides, polypeptides, vectors, cells and compositions comprising the same, and their use in affecting viral pathogenesis, in particular for influenza viral infection.
Owner:ACAD SINIC +1
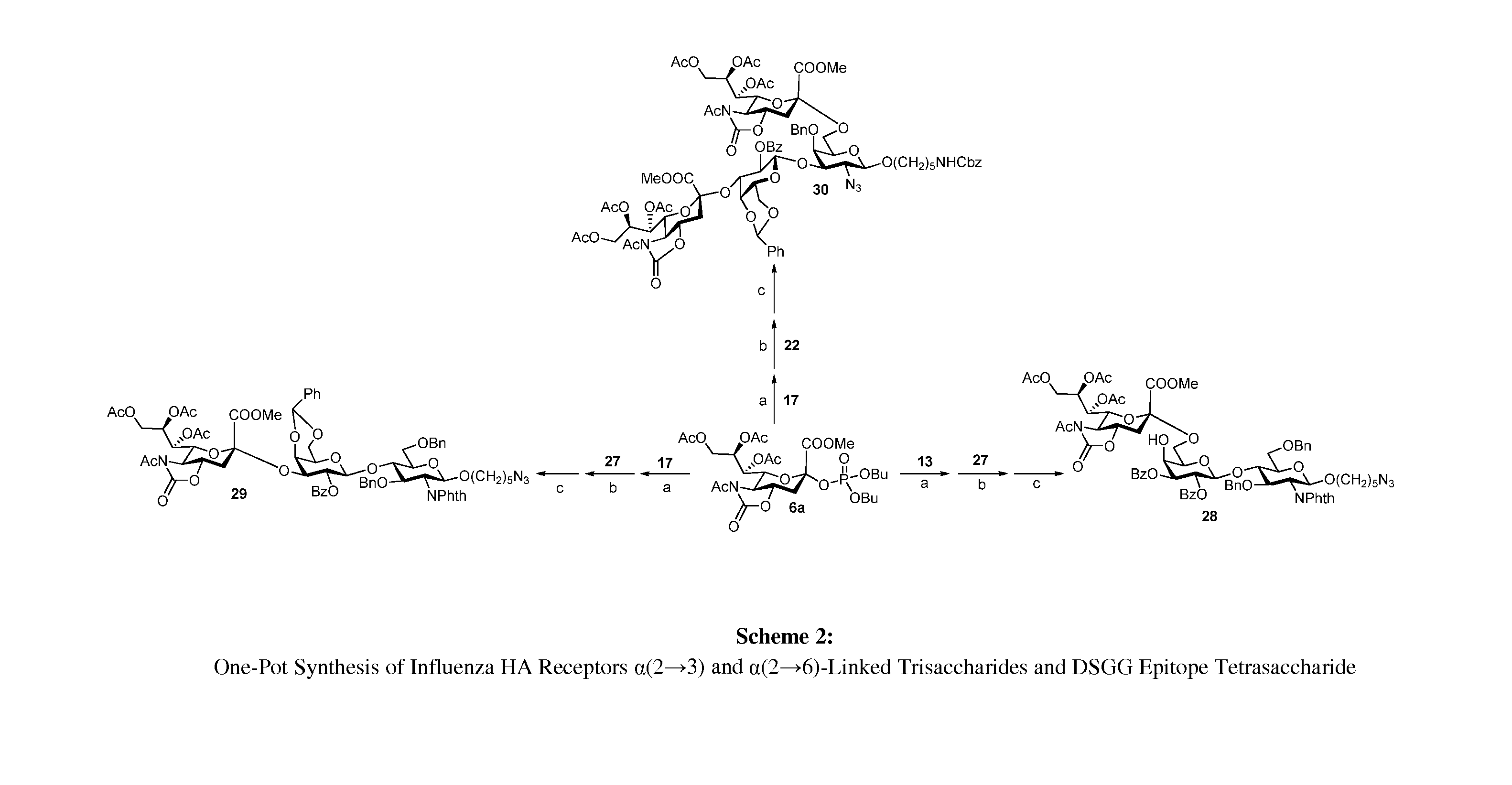
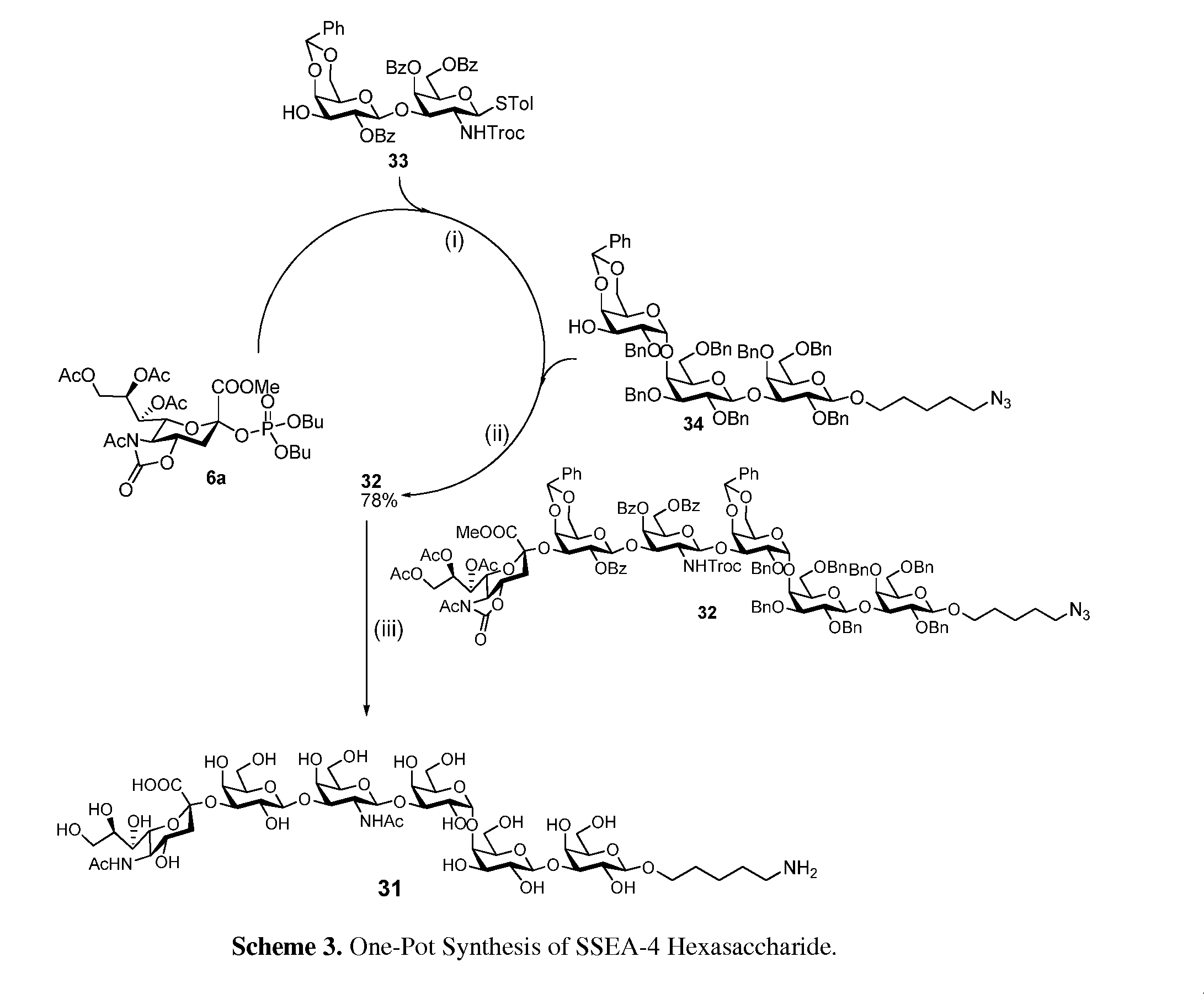
Alpha-selective sialyl phosphate donors for preparation of sialosides and sialoside arrays for influenza virus detection
A novel N-acetyl-5-N,4-O-carbonyl-protected dibutyl sialyl phosphate donor for sialylation of both primary and sterically hindered secondary acceptors to prepare sialosides with high yield and α-selectivity is disclosed. Methods for making disaccharide building blocks comprising α(2→3), α(2→6), α(2→8), α(2→8) / α(2→9) alternate, and α(2→9) sialosides are provided. methods for one-pot synthesis of complex sialosides are disclosed. Libraries of sialosides and methods for using the libraries for detection and receptor binding analysis of surface glycoproteins or pathogens and cancer cells are disclosed. Methods for distinguishing between hemagglutinin (HA) from various strains of influenza are provided.
Owner:ACAD SINIC
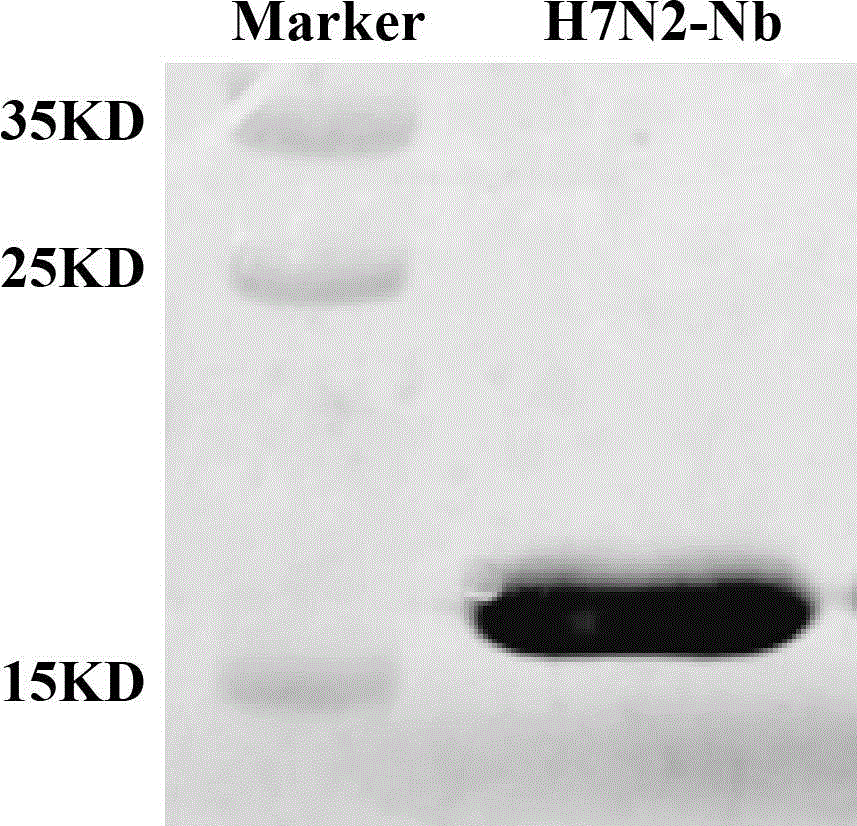
Nanometer antibody for avian influenza virus H7N2, and application of nanometer antibody
ActiveCN106188283AGood linear relationshipBacteriaMicroorganism based processesEscherichia coliEpitope
The invention discloses a nanometer antibody in accordance with epitope of avian influenza virus H7N2, and a gene sequence encoding the nanometer antibody, and besides, further discloses a host cell capable of expressing the nanometer antibody for the influenza virus H7N2. The nanometer antibody for the avian influenza virus H7N2 and the encoding sequence of the nanometer antibody are obtained for the first time, the nanometer antibody can be efficiently expressed in escherichia coli, can specifically recognize the avian influenza virus H7N2, is high in detection sensitivity, presents favorable linear relationship when being used for detecting the avian influenza virus H7N2, and has the application value of diagnosing the avian influenza virus H7N2.
Owner:北京科卫临床诊断试剂有限公司
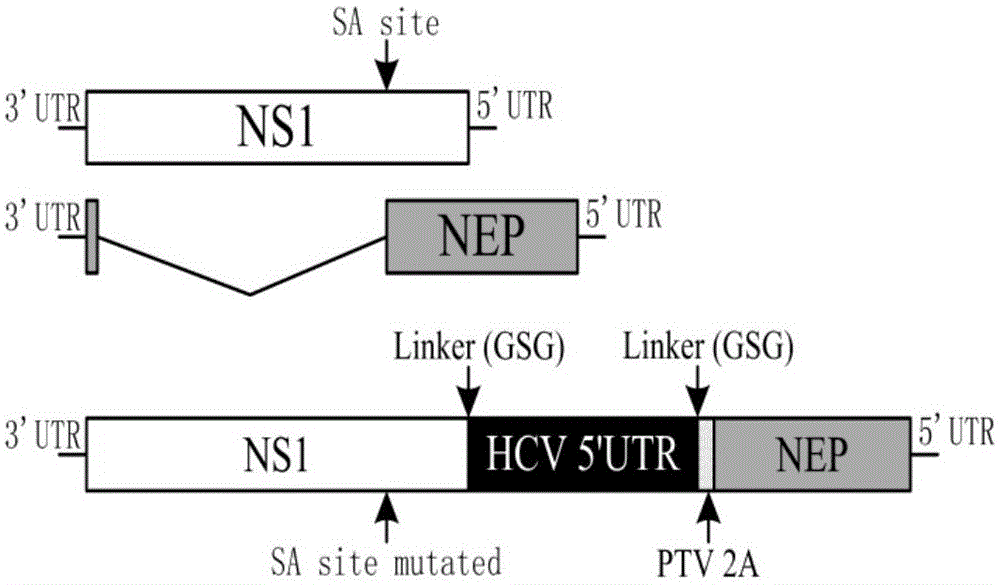
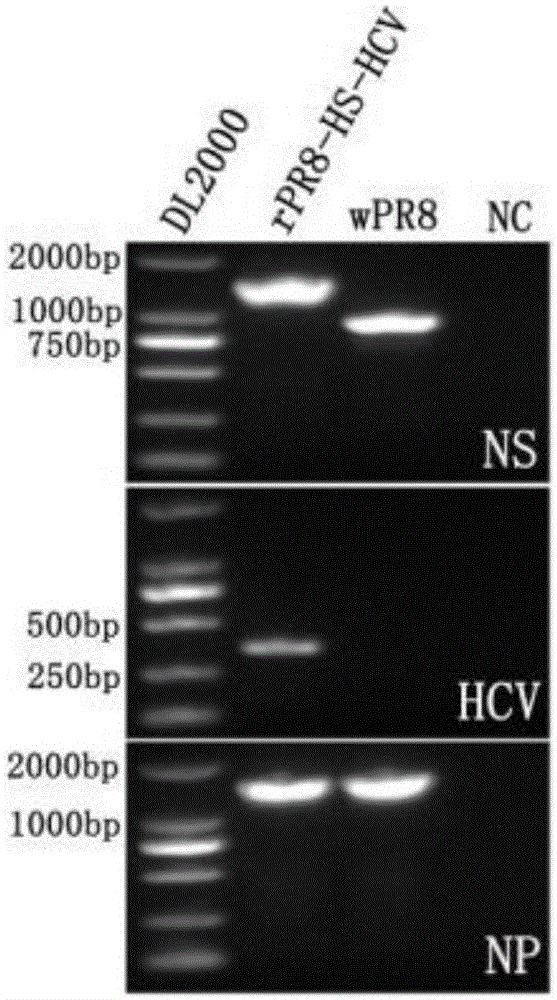
Influenza virus carried HCV nucleic acid test quality control product and preparation method thereof
ActiveCN105132583ALow costGood for mass manufacturingMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionTranscriptional expression
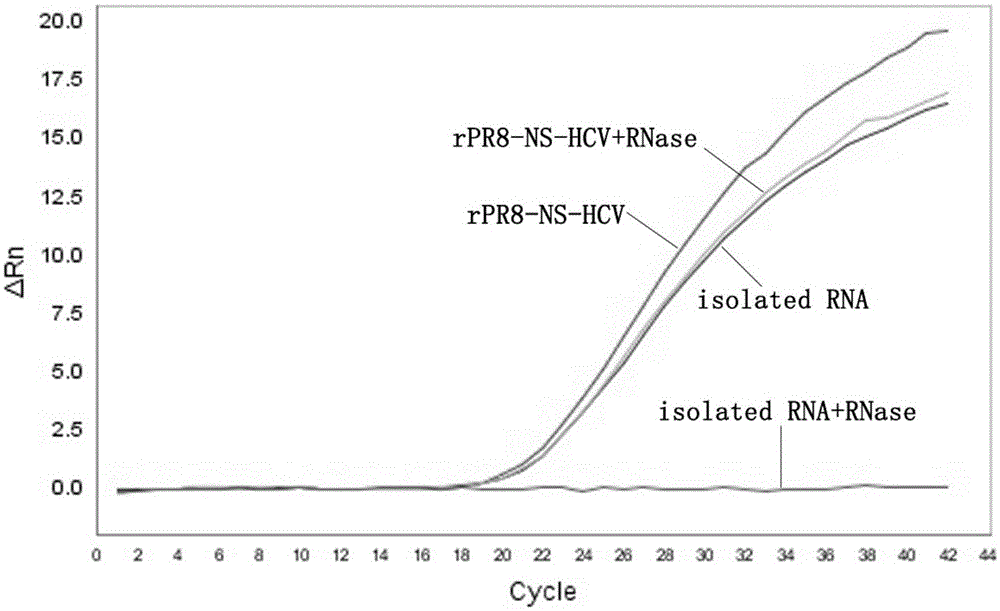
The invention relates to an influenza virus carried HCV nucleic acid test quality control product and a preparation method thereof; the quality control product is prepared from HCV conserved gene carried recombinant influenza virus through enlarged culture, inactivation and purification; the preparation method comprises the following steps: (1) constructing a plasmid which is used for rescuing influenza virus and is interpolated with HCV conserved gene in PR8 virus NS gene; (2) co-transfecting 293T cell to the constructed plasmid with plasmids which are respectively used for transcriptional expression of PR8 viruses PB2, PB1, PA, HA, NP, NA and M, so as to rescue and obtain HCV 5' UTR carried recombinant influenza virus; and (3) enlarged-culturing, inactivating and purifying the recombinant influenza virus so as to obtain the quality control product. The quality control product disclosed by the invention can be used for really simulating HCV pathogens and achieving all-around monitoring on HCV detection; and the quality control product is easy in large-scale preparation, low in cost, good in stability, easy in storage and transportation, and good in application prospect.
Owner:上海市临床检验中心
General nucleic acid detection method for influenza viruses
The invention provides a general nucleic acid detection method for influenza A virus, influenza B virus, influenza C virus and other recently discovered influenza viruses, belonging to the technical field of biology. The general nucleic acid detection method comprises the following three technical main points: 1) primer sequences needed in nucleic acid detection are determined; 2) detection reaction kinds are determined; and 3) a detection reaction system and reaction conditions are determined. The general nucleic acid detection method is applicable to scientific research on influenza viruses and to clinical diagnosis and detection of animals or human beings.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
Application of compound to preparation of drug for treating and preventing novel coronaviruses and influenza viruses
InactiveCN112641789AEnhanced inhibitory effectPositive inhibitory effectOrganic active ingredientsAntiviralsPulmonary infectionDisease
The invention discloses application of a compound in the aspect of antiviral drugs, and particularly relates to application of taurolidine to a drug for treating and preventing novel coronaviruses or influenza viruses. The drug is a drug for preventing and treating pulmonary diseases caused by viruses. The viruses include RNA viruses such as influenza viruses and coronaviruses. According to the invention, the effect range of the taurolidine is expanded, it is proved that replication of influenza viruses and SARS-CoV-2 can be remarkably inhibited at the cellular level, it is indicated that the taurolidine has a very positive inhibiting effect on the influenza viruses and SARS-CoV-2, and a research basis is provided and a new direction is opened up for preparing the drug for preventing and treating pulmonary infections caused by the influenza viruses and novel coronaviruses. Moreover, It is proved by in-vitro experiments that the taurolidine has a remarkable protection effect on lungs, can prolong the survival time of mice infected with the influenza viruses, and can be used for preventing and treating the pulmonary diseases caused by the influenza viruses.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE +1
96-hole ELISA (enzyme linked immunosorbent assay) plate-immobilized influenza virus imprint fluorescent sensor and application
The invention relates to a 96-hole ELISA (enzyme linked immunosorbent assay) plate-immobilized influenza virus imprint fluorescent sensor and application, and belongs to the technical field of biological molecular detection. The 96-hole ELISA plate-immobilized influenza virus imprint fluorescent sensor is specifically characterized in that an influenza virus particle is used as a template moleculeand is immobilized onto a 96-hole ELISA plate, a thin layer of polyaniline is polymerized to imprint the template virus, the template molecule is eluted, and the left two-dimensional imprinting cavity can be used for specifically identifying the target virus; HRP (horse raddish peroxidase) is used as a biological marker and is directly marked onto the surface of the influenza virus immobilized onto an imprinting film, the HRP can effectively catalyze a H2O2-OPDA (hydrogen peroxide and o-phenylenediamine) system to produce sensitive fluorescent effect, and the produced fluorescent intensity has direct relationship with the concentration of the influenza virus, so as to build a novel influenza virus fluorescent sensor; the prepared imprinting polymer film has the characteristic of an artificial antibody, is used for identifying and detecting the influenza virus, and has been successfully applied to detect a serum sample. The 96-hole ELISA plate-immobilized influenza virus imprint fluorescent sensor provides an influenza virus identifying and detection method with high stability, high specificity and high sensitivity.
Owner:MINJIANG UNIV
New use of 5-(1,1-dimethylallyl propyl)-4,4'-dihydroxyl-2-methoxyl chalcone
InactiveCN103405402AInhibition of infection replicationImprove survival rateAntiviralsKetone active ingredientsHand foot mouth diseasePara-influenza viruses
The invention relates to new use of a small molecule compound, and specifically relates to uses of 5-(1,1-dimethylallyl propyl)-4,4'-dihydroxyl-2-methoxyl chalcone or a pharmaceutically acceptable salt thereof in preparing medicaments for treating hand-foot-mouth disease virus infection related diseases caused by EV71 and the like and medicaments for influenza virus infection related diseases.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
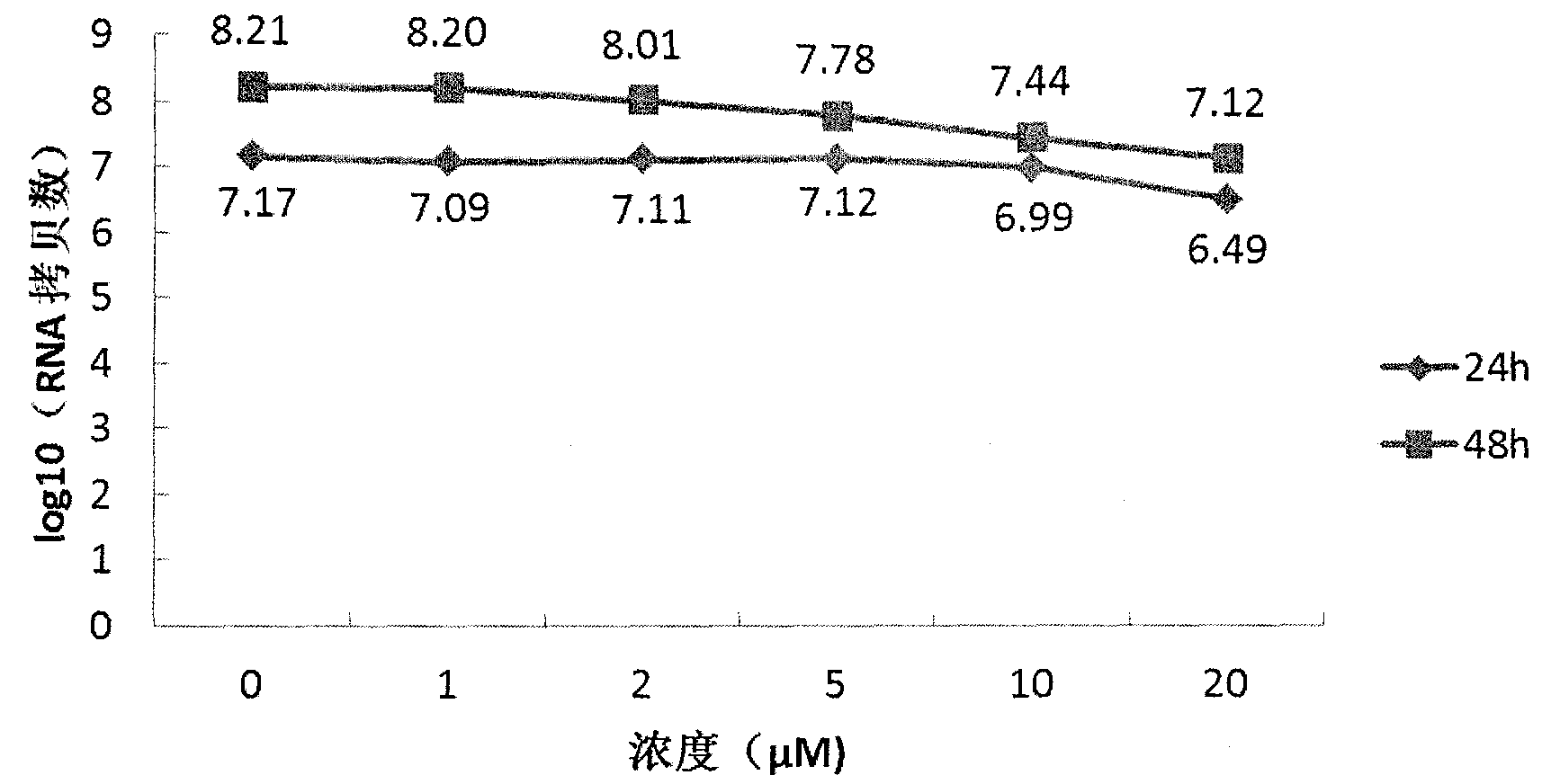
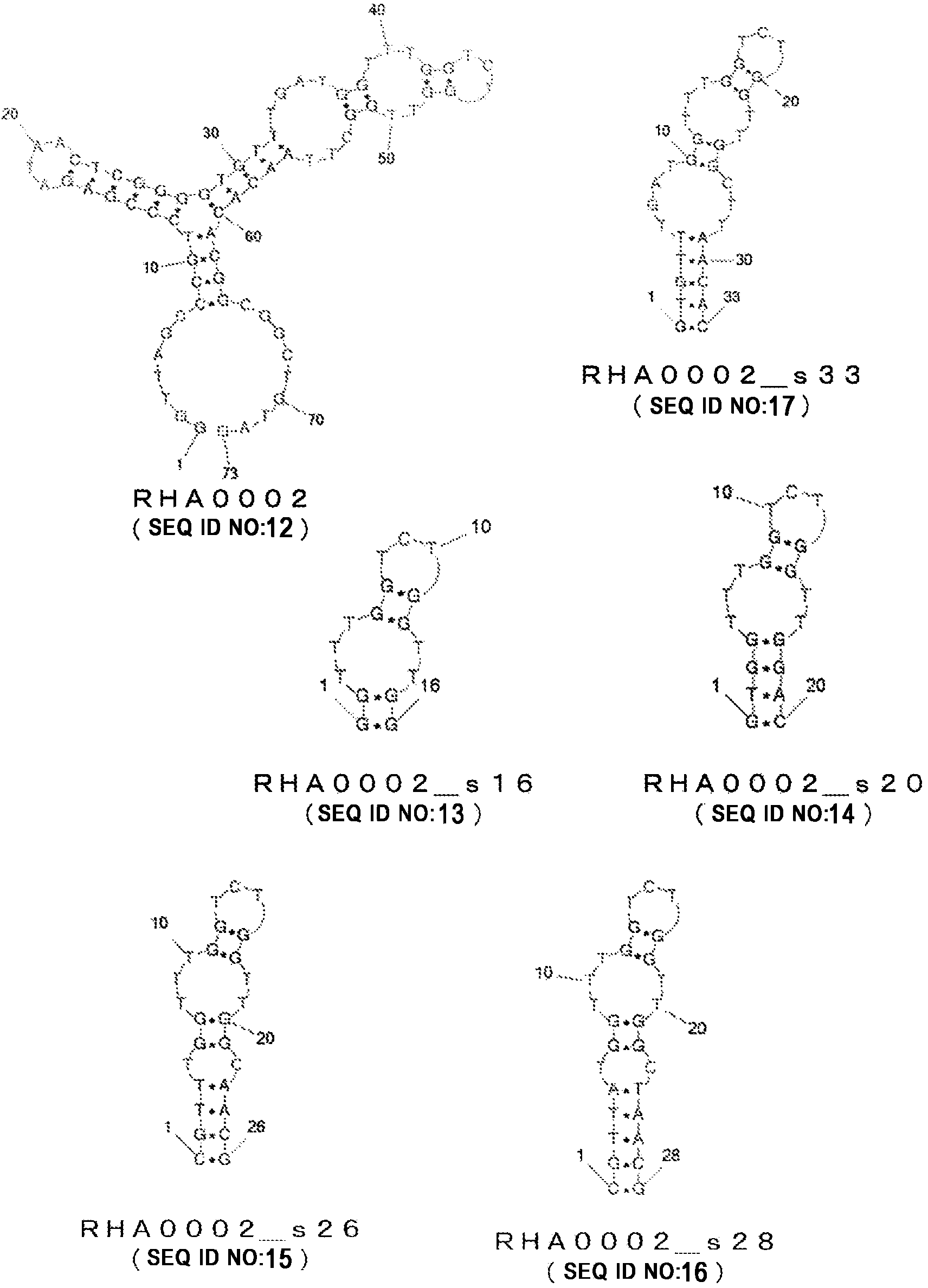
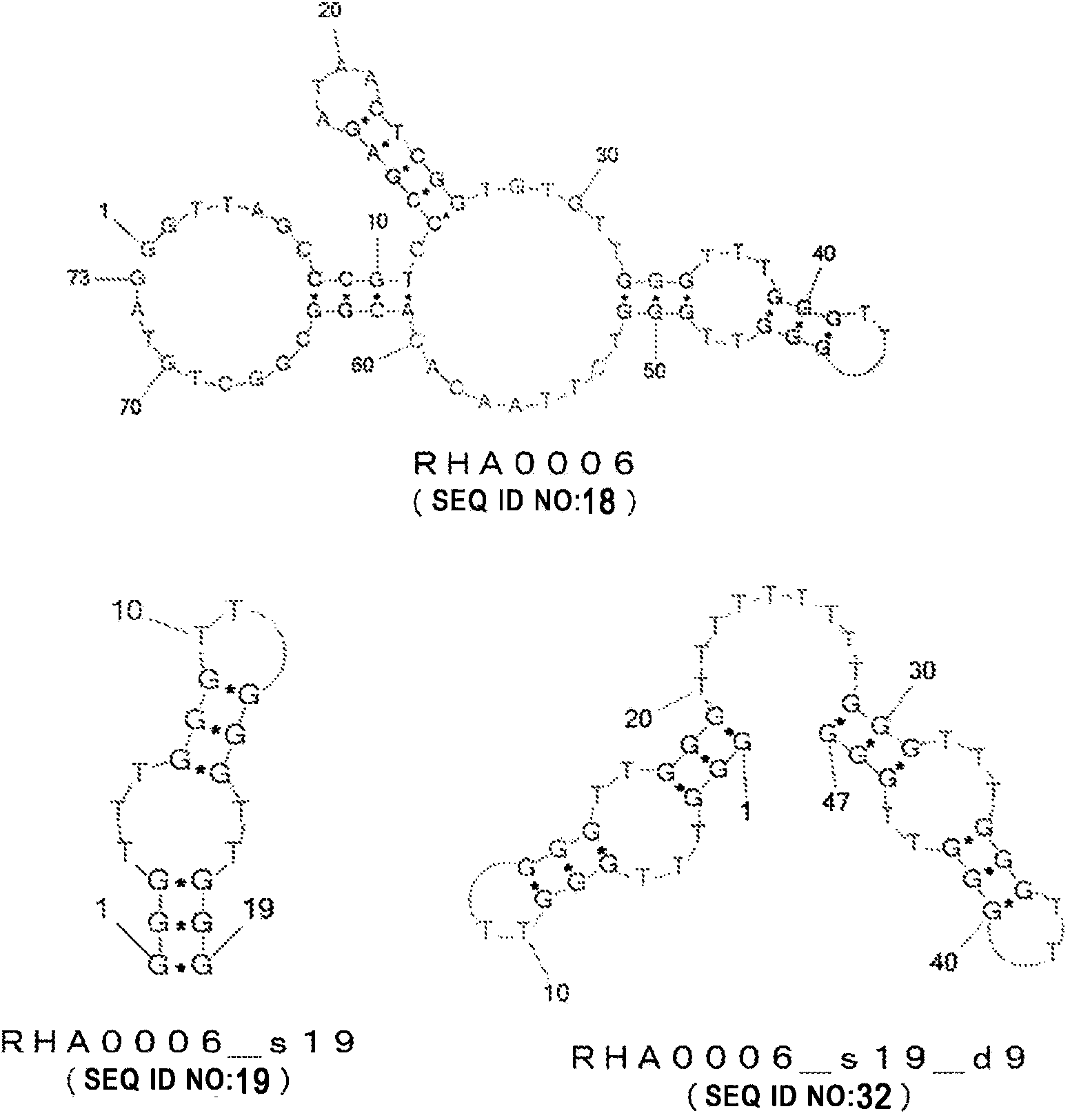
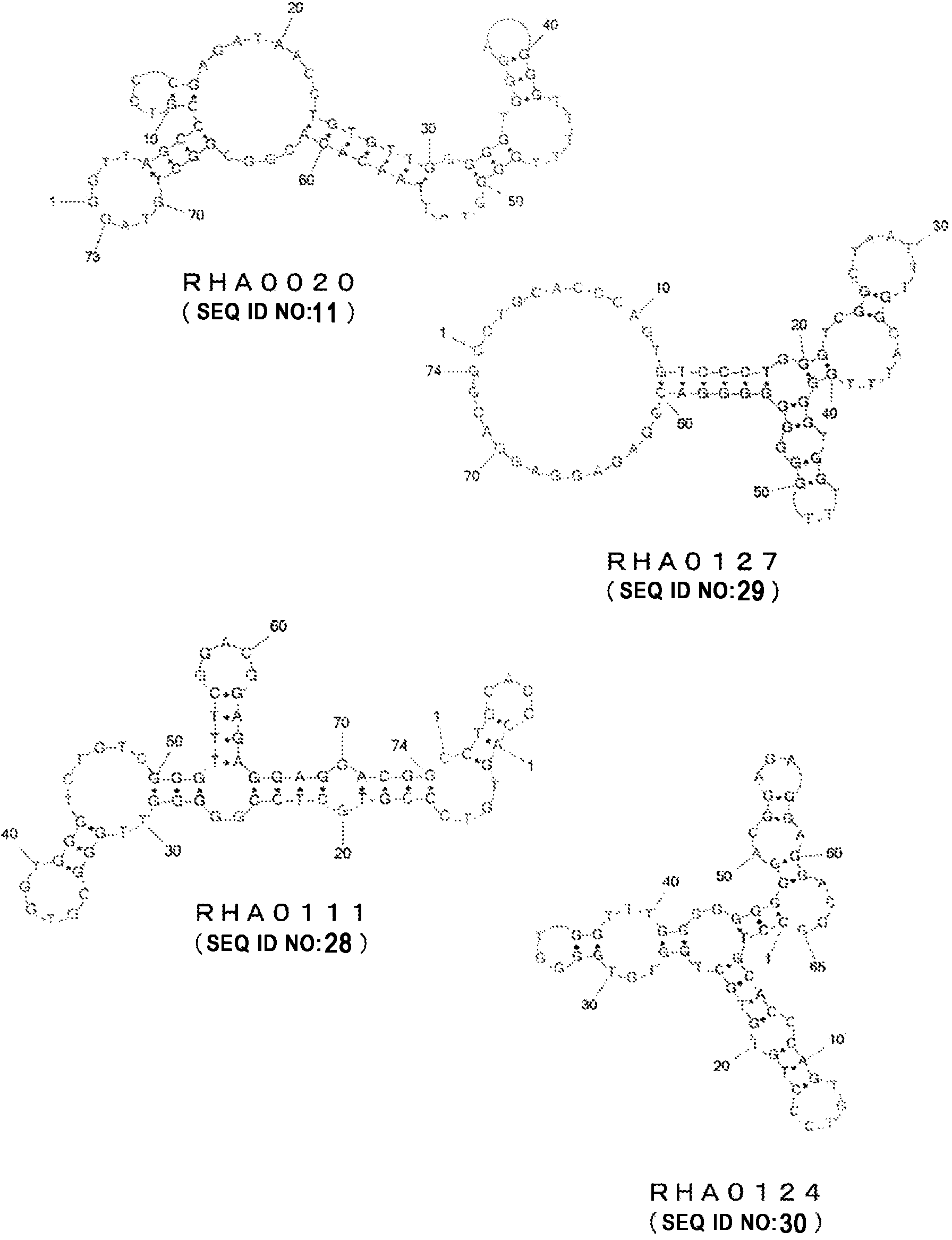
Nucleic acid molecule binding to influenza virus and use therefor
InactiveCN104364375AMicrobiological testing/measurementDNA/RNA fragmentationNucleotidePara-influenza viruses
Provided is a nucleic acid molecule that can be utilized to detect the influenza virus. A nucleic acid molecule containing any of the polynucleotides (a)-(d) below is taken as a nucleic acid molecule that binds to the influenza virus. (a) A polynucleotide composed of a base sequence of any of SEQ ID NO: 1-30; (b) a polynucleotide that binds to the influenza virus, composed of a base sequence in which one or more bases have been deleted, substituted, inserted and / or added in any of the base sequences of (a) above; (c) a polynucleotide that binds to the influenza virus, composed of a base sequence having 80% or higher identity with any of the base sequences of (a) above; (d) a polynucleotide that binds to the influenza virus, composed of a base sequence complementary to a polynucleotide that hybridizes with a polynucleotide composed of any of the base sequences of (a) above under stringent conditions.
Owner:NEC SOLUTION INNOVATORS LTD
Screening and identification method of influenza virus attenuated live vaccine strain
InactiveCN106939355AShorten the development cycleImprove throughputMicrobiological testing/measurementHigh densityBacteriophage
The invention relates to a screening and identification method of an influenza virus attenuated live vaccine strain, and belongs to the technical field of biological pharmacy. A method is researched and developed, and the method is used for obtaining an attenuated live vaccine candidate strain by construction and screening of a Mu phage transposon-mediated virus gene high density random insertion mutant library. A technical system for systematic evaluation of the obtained attenuated live vaccine strain is developed. The method can be applied directly to the screening and evaluation of the influenza virus attenuated live vaccine strain, and has wide referential significance for development and development of other virus attenuated live vaccines.
Owner:SUZHOU INST OF SYST MEDICINE
Application of taurolidine in virus resistance
ActiveCN113491700AExpand the scope of efficacyEnhanced inhibitory effectOrganic active ingredientsAntiviralsPulmonary infectionPharmaceutical medicine
The invention provides an application of taurolidine in virus resistance. Specifically, the invention provides use of the taurolidine or a derivative thereof, a prodrug, a solvate or a pharmaceutically acceptable salt thereof, or a composition containing the taurolidine or the derivative thereof, the prodrug, the solvate or the pharmaceutically acceptable salt thereof in preparation of antiviral drugs. Research results show that the taurolidine can significantly inhibit influenza virus and coronavirus at a cellular level. In addition, in-vivo experiments prove that taurolidine has a remarkable protection effect on lungs and can prolong the survival time of mice infected with influenza viruses or SARS-CoV-2 viruses. Therefore, the taurolidine can be used for preventing and treating lung diseases caused by the influenza virus or the coronavirus. The research result of the application expands the efficacy range of the taurolidine, and provides a research basis and opens up a new direction for research and development of drugs for preventing or treating pulmonary infection caused by the viruses.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE +1
Sample processing method for influenza virus immunoassay, and immunoassay method
ActiveUS20160223540A1High sensitivityBiological material analysisAntigen Binding FragmentAntigen-antibody reactions
Means for enabling an immunoassay with a sufficient sensitivity in an immunoassay for measuring influenza virus in a sample using influenza virus M1 protein as an antigen is provided. A sample processing method in an immunoassay for influenza virus, which method comprises, in an immunoassay for influenza virus using an antibody which undergoes antigen-antibody reaction with influenza virus matrix 1 protein, or an antigen-binding fragment thereof, bringing a sample containing influenza virus into contact with a sample processing liquid containing a surfactant having at least one group selected from the group consisting of palmityl, stearyl, and oleyl, is provided.
Owner:DENKA CO LTD
Use of imatinib to treat liver disorders and viral infections
The present invention relates to the use of imatinib for treating viral liver diseases and in particular for viral hepatitis. The invention provides the use of imatinib for inhibiting replication, transmission or both of hepatitis viruses. The invention further relates to the use of imatinib for inhibiting replication, transmission or both of other viruses including herpes virus, poxvirus, influenza virus, para influenza virus, respiratory syncytial virus, rhinovirus, yellow fever virus, west nile virus, and encephalitis virus.
Owner:BIONICHE LIFE SCI
Therapeutic agent for influenza virus infection diseases
InactiveUS20120202771A1Promote healingGood effectBiocideOrganic active ingredientsMicroorganismNatural product
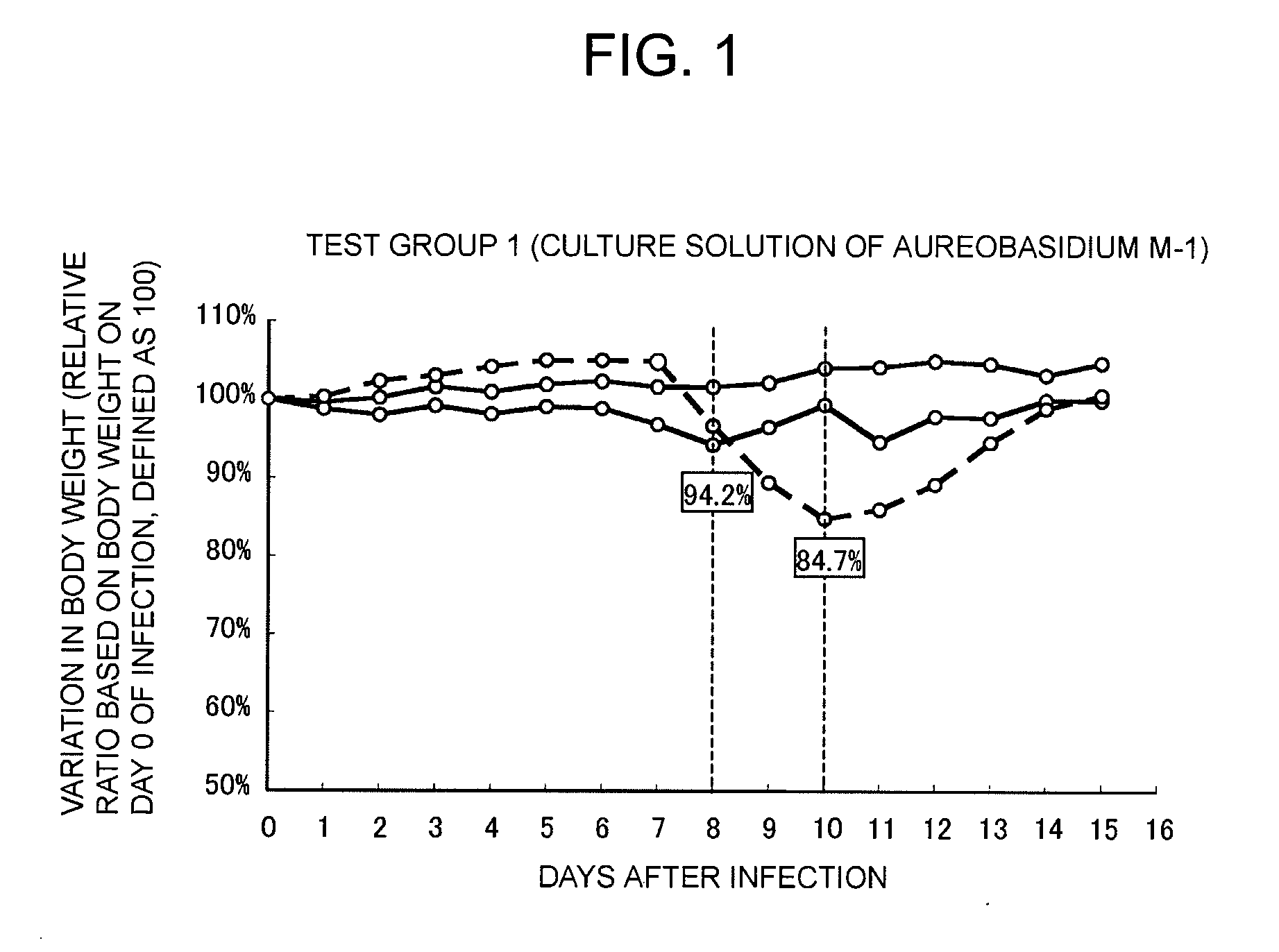
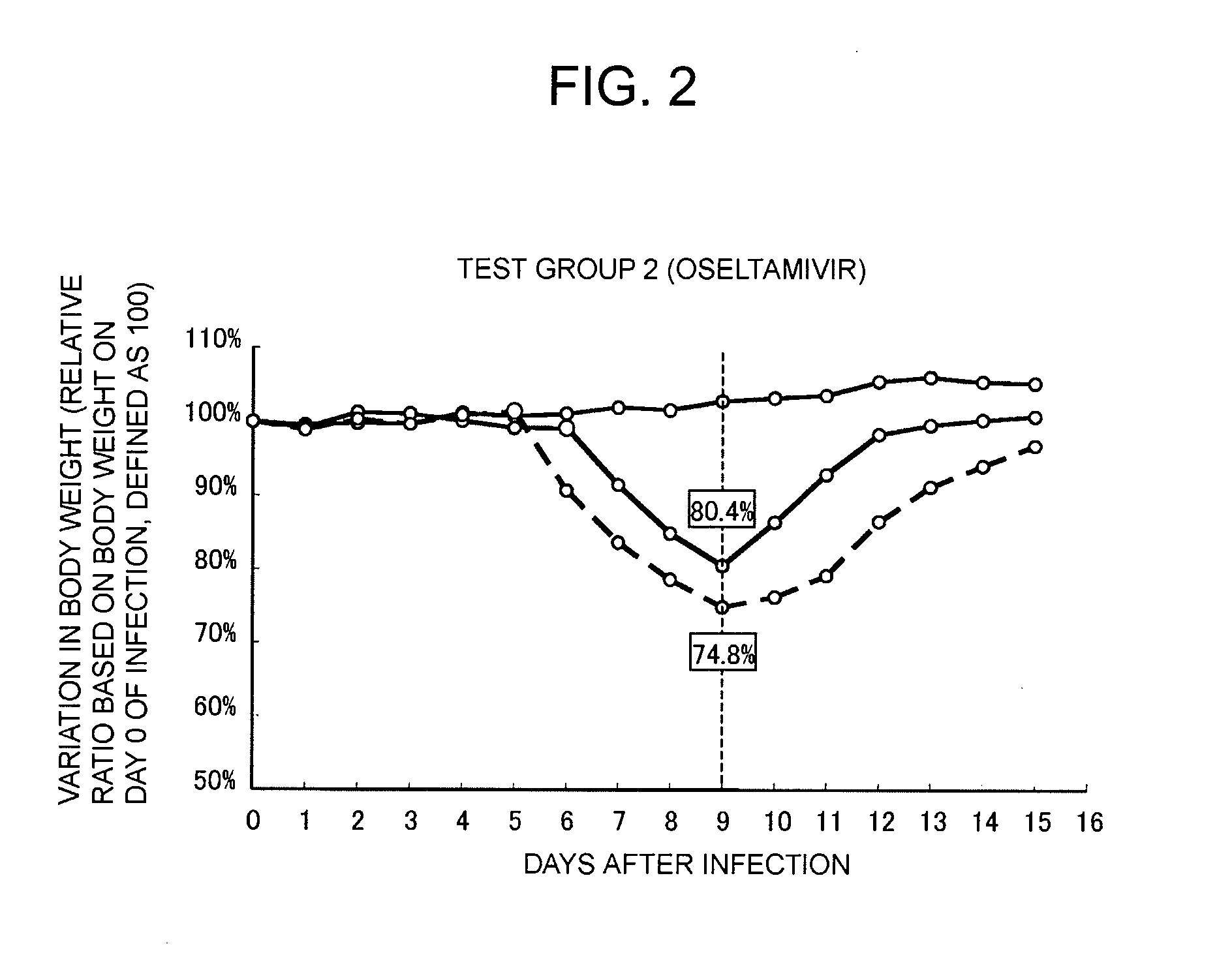
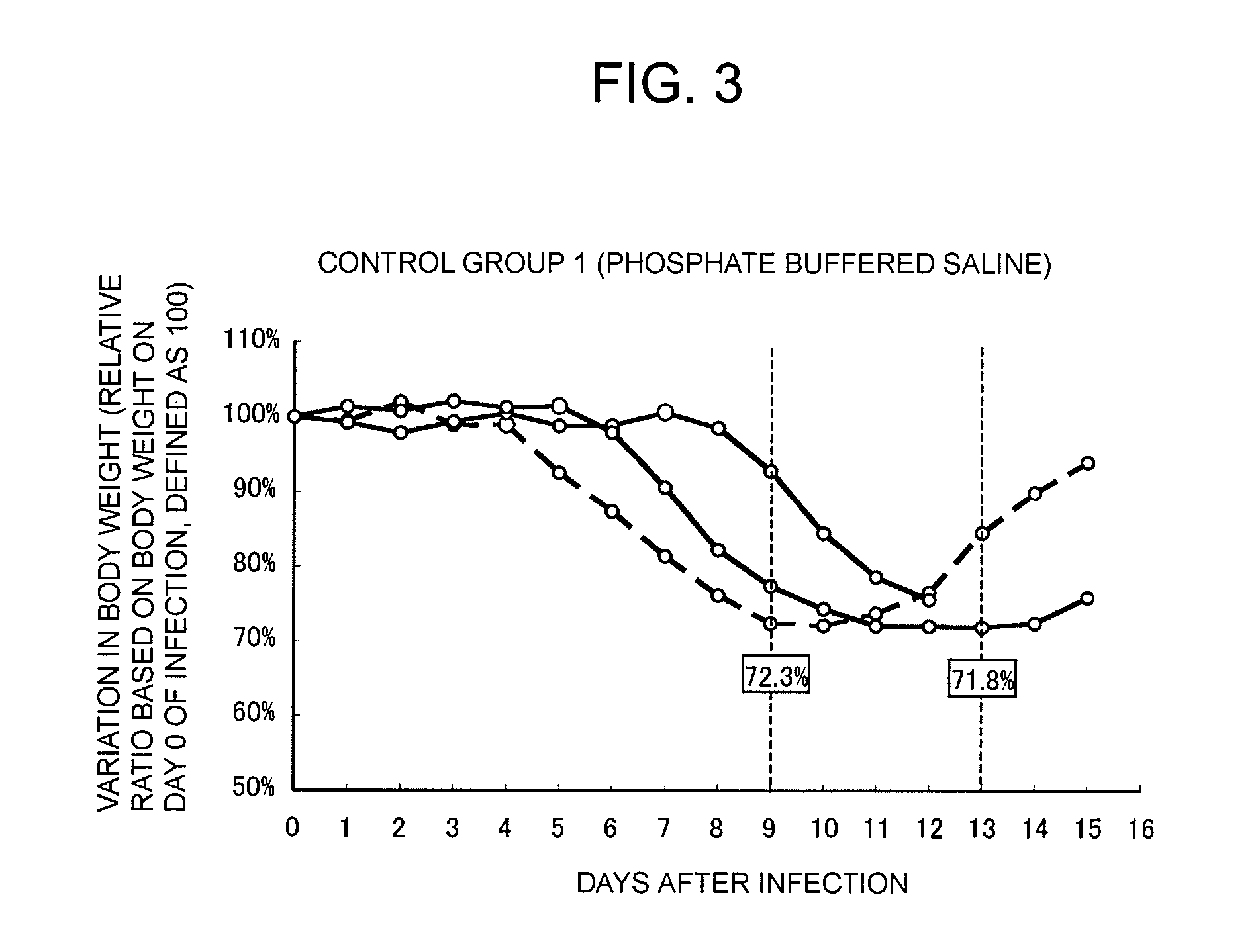
Provided is a therapeutic agent for an influenza virus infectious disease, which utilizes an active ingredient derived from a natural product and has an excellent effect. The therapeutic agent for an influenza virus infectious disease includes, as an active ingredient, a β-glucan-containing composition obtained from a culture of a microorganism belonging to Aureobasidium sp. The therapeutic agent is capable of preventing an influenza virus infectious disease from becoming serious and is capable of promoting the healing of the influenza virus infectious disease.
Owner:AUREO CO LTD
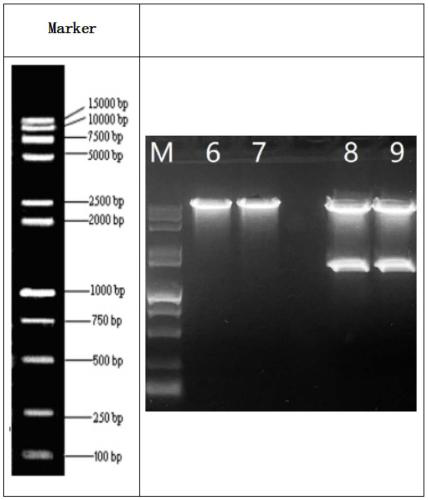
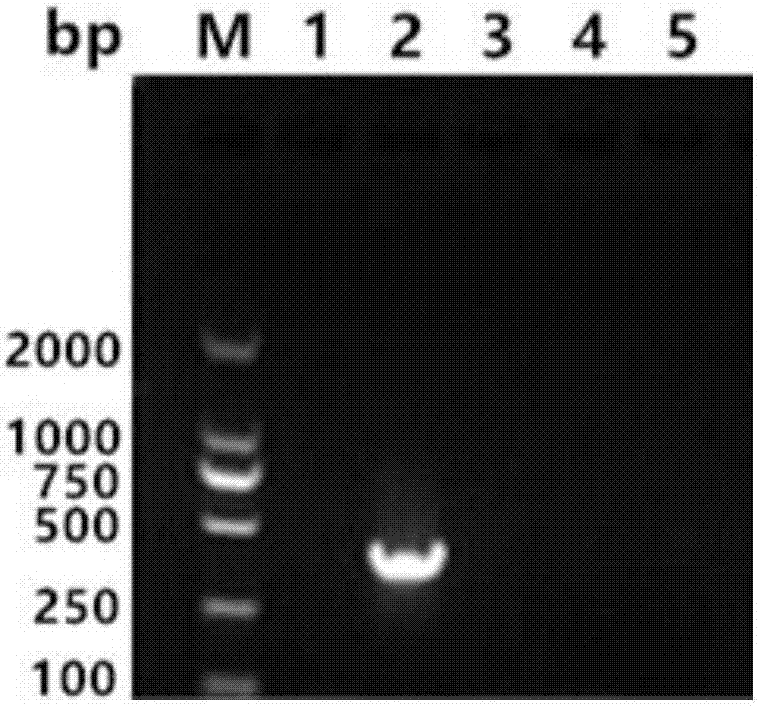
Bovine-para-influenza virus 3 nano-PCR (polymerase chain reaction) detection kit and preparation method thereof
InactiveCN106906307ASpecific and accurateAccurate specificityMicrobiological testing/measurementMicroorganism based processesBovine parainfluenza virusPositive control
The invention discloses a bovine-para-influenza virus 3 nano-PCR (polymerase chain reaction) detection kit and a preparation method thereof, relates to the field of bovine para-influenza virus detection, and solves the problems that an existing bovine-para-influenza virus 3 detection method is low in sensitivity, serological detection is high in false positive and foreign importing reagents are expensive. The kit comprises a MightyAmp enzyme, 2xBuffer Mix solution, gold nanoparticle sol, aseptic double-distilled water, a pair of specific primers P1 (5'-GCTCTTCTCTTTTTGTCCCATTCTT-3') and P2 (5'-AACCCCTTCCTCAATCCTGATATAC-3') and a bovine-para-influenza virus 3 positive control plasmid. The sequence of the bovine-para-influenza virus 3 positive control plasmid is as shown in SEQ ID NO: 2. The kit is high in sensitivity, simple, convenient, rapid, efficient and low in cost.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
Screening method of influenza virus related host gene mutants
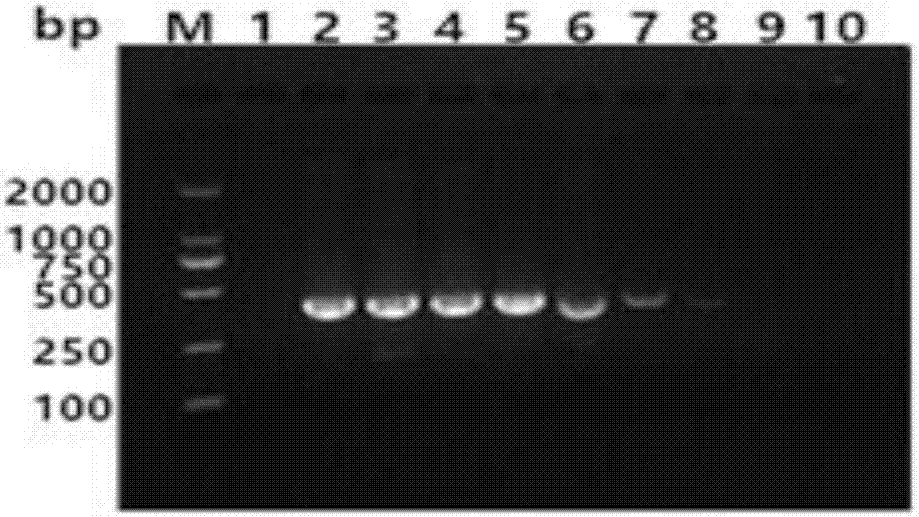
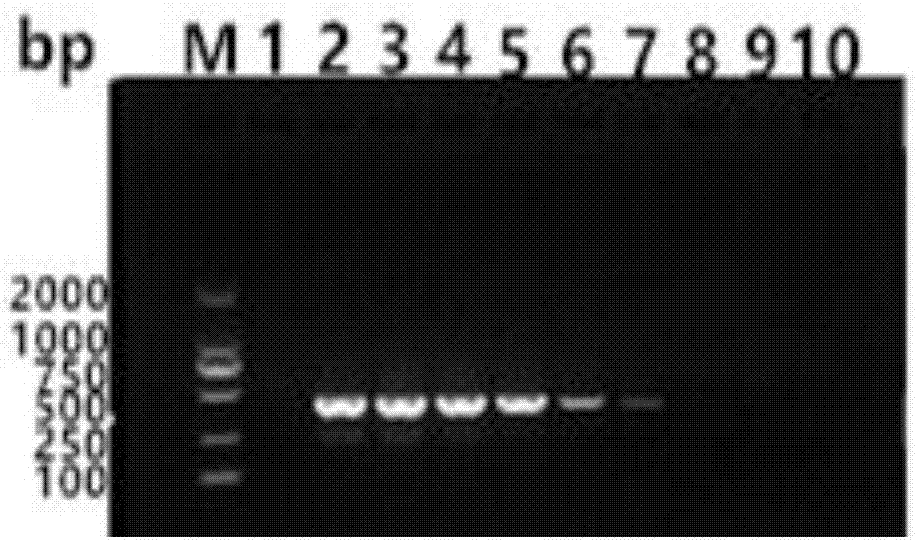
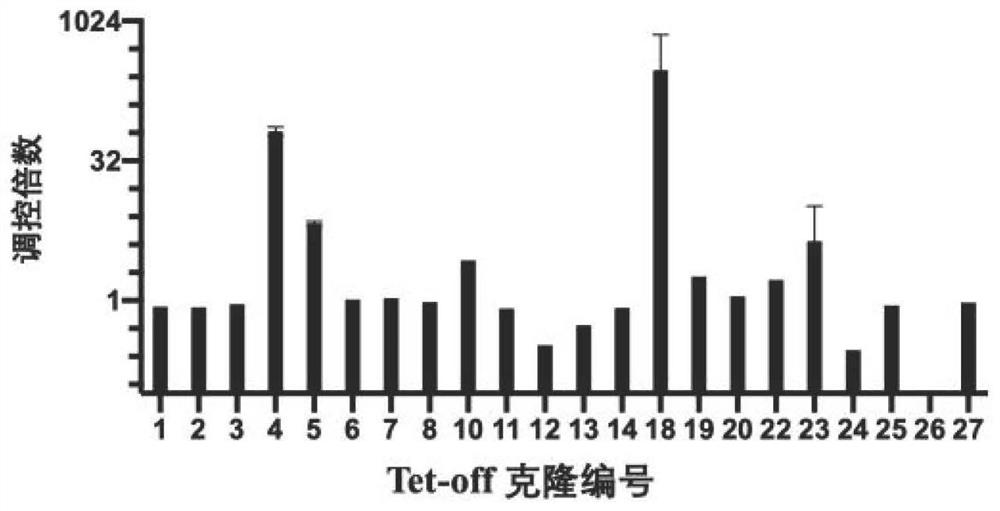
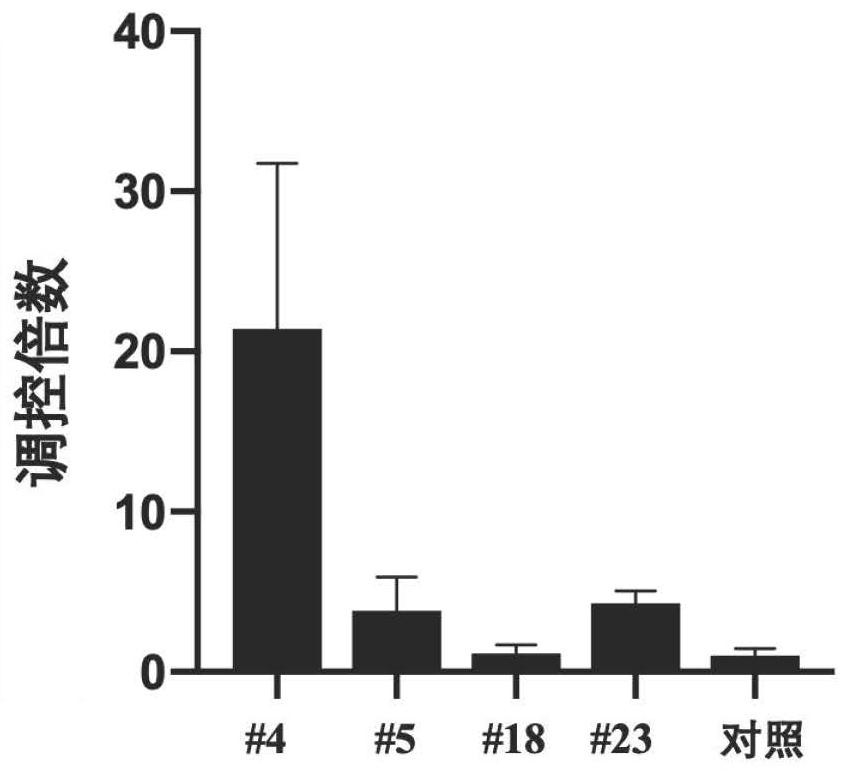
ActiveCN112553251AMicrobiological testing/measurementMicroorganism based processesMutantThelial cell
The invention discloses a screening method of influenza virus related host gene mutants, and belongs to the technical field of random mutation. In order to screen out host genes related to influenza viruses, the invention provides a screening method. The screening method comprises the following steps: 1) transfecting a human lung cancer cell strain with CAG-tTA plasmids to obtain a Tel-off cell strain of a transcription activator stably expressed and regulated by tetracycline; co-transfecting the Tel-off cell strain with a gene search vector and plasmids that effectively express transposase toobtain a whole-genome pulmonary epithelial cell gene mutation library; 2) infecting the mutation library obtained in the step 1) by using the H1N1-WSN influenza virus; and 3) extracting the genome DNA of the cell strain, resisting the H1N1-WSN influenza virus, obtained in the step 2), and carrying out sequencing by Splinkette PCR to obtain the influenza virus related host gene mutants. The screening method can be used for screening the influenza virus related host gene mutants.
Owner:THE THIRD MEDICAL CENT OF THE CHINESE PEOPLES LIBERATION ARMY GENERAL HOSPITAL
Application of human-derived monocytic series U937 in preparation of anti-influenza virus and drug screening model of inflammation factor induced by virus
InactiveCN107034263AIncreased sensitivityWide applicabilityMicrobiological testing/measurementMaterial analysisInflammatory factorsHigh-Throughput Screening Methods
The invention discloses application of a human-derived monocytic series U937 in preparation of an anti-influenza virus and a drug screening model of an inflammation factor induced by the virus. In the application, an influenza virus can effectively infect a U937 cell line and can induce a series of important inflammatory factor expressions including MCP-1, IP-10, IL-8, IL-6 and the like, and the toxicity and antiviral and anti-inflammatory activity of drugs can be comprehensively evaluated in a round of experiments by detecting the activity of injected cells, the virus level in supernatant and the level of relevant inflammatory factors. The established cell screening model is accurate, efficient, low in cost, short in consumed time and free of deviation property and can be used for high throughput screening of antiviral and anti-inflammatory drugs for treating the influenza virus.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Prophylactic/therapeutic agent for influenza virus infection
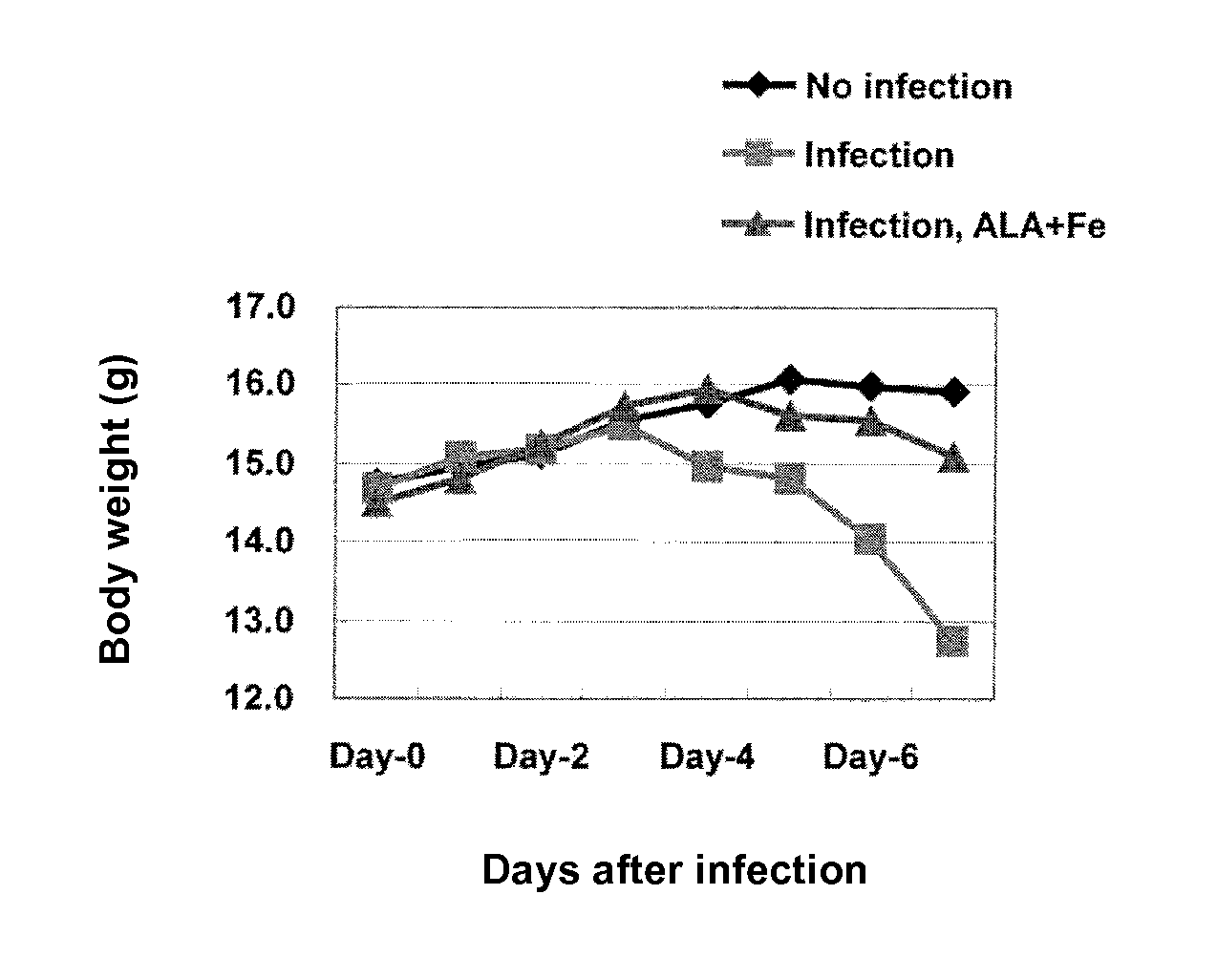
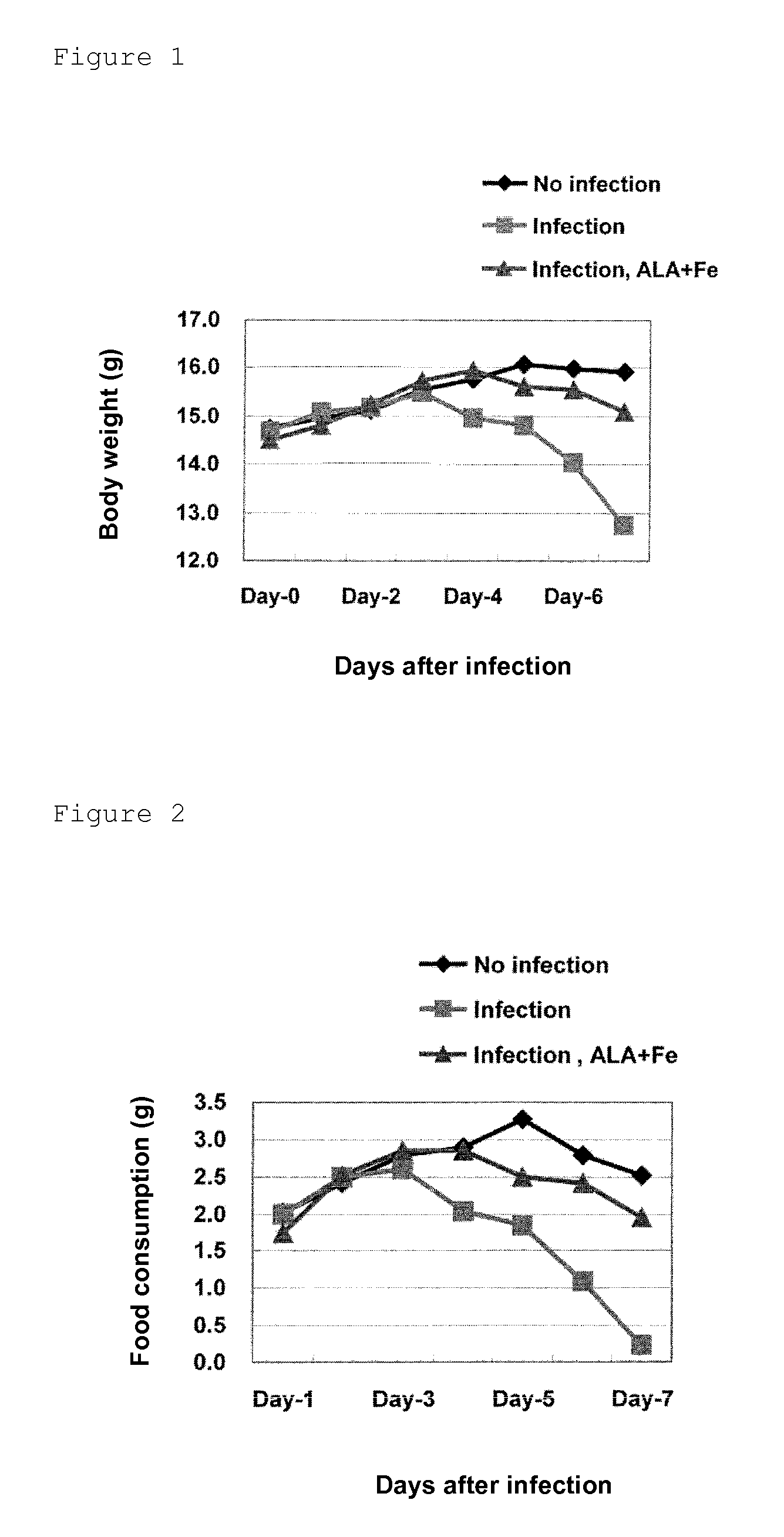
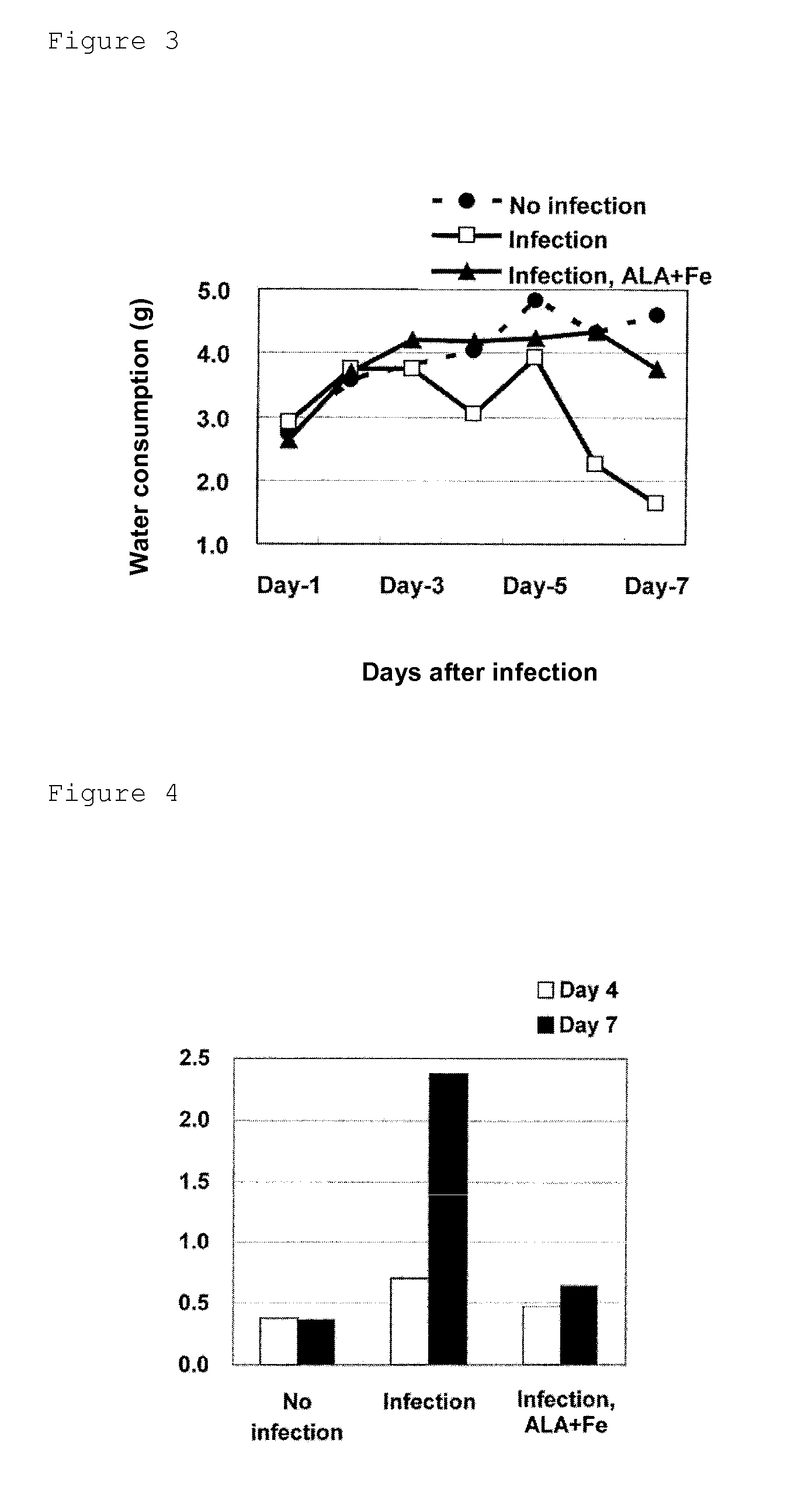
ActiveUS9351949B2Inhibit deteriorationSuppressing increase in ketone body levelHeavy metal active ingredientsOrganic active ingredientsVirus influenzaFood consumption
Provided is a prophylactic / therapeutic agent for influenza viral infection that is effective not only before and at an early stage of infection with influenza virus but also at an intermediate or late stage of the infection and is highly safe for human bodies. A prophylactic / therapeutic drug for influenza viral infection comprising, as active ingredients, 5-aminolevulinic acid (5-ALA), a derivative thereof or a salt of the 5-ALA or the derivative, and an iron compound is prepared. This prophylactic / therapeutic agent can be used for ameliorating (preventing) depression in food consumption, water consumption and body weight, for ameliorating (decreasing) increase in ketone body levels in blood that may otherwise cause ketosis, for ameliorating (preventing) depression in ATP levels in blood, or for ameliorating (increasing) a survival rate and depression in a body surface temperature.
Owner:UNIVERSITY OF TOKUSHIMA +1
Adenoviral vectors for influenza virus production
ActiveUS20090047728A1High expressionIncrease productionSsRNA viruses negative-senseVirus peptidesPara-influenza virusesRNA polymerase I
The invention provides adenovirus and retrovirus vectors useful to prepare influenza virus. Also provided is a canine RNA polymerase I promoter and vectors having that promoter.
Owner:WISCONSIN ALUMNI RES FOUND
Flu vaccines and methods of use thereof
InactiveUS8030029B2High expressionImprove survivabilitySsRNA viruses negative-senseGenetic material ingredientsFlu vaccinesPara-influenza viruses
This disclosure is directed, inter alia, to polynucleotides, polypeptides, vectors, cells and compositions comprising the same, and their use in affecting viral pathogenesis, in particular for influenza viral infection.
Owner:THE ROCKEFELLER UNIV +1
Live attenuated vaccines for influenza viruses
ActiveUS9827304B2Easily and quickly producedSsRNA viruses negative-senseViral antigen ingredientsPara-influenza virusesCell Membrane Proteins
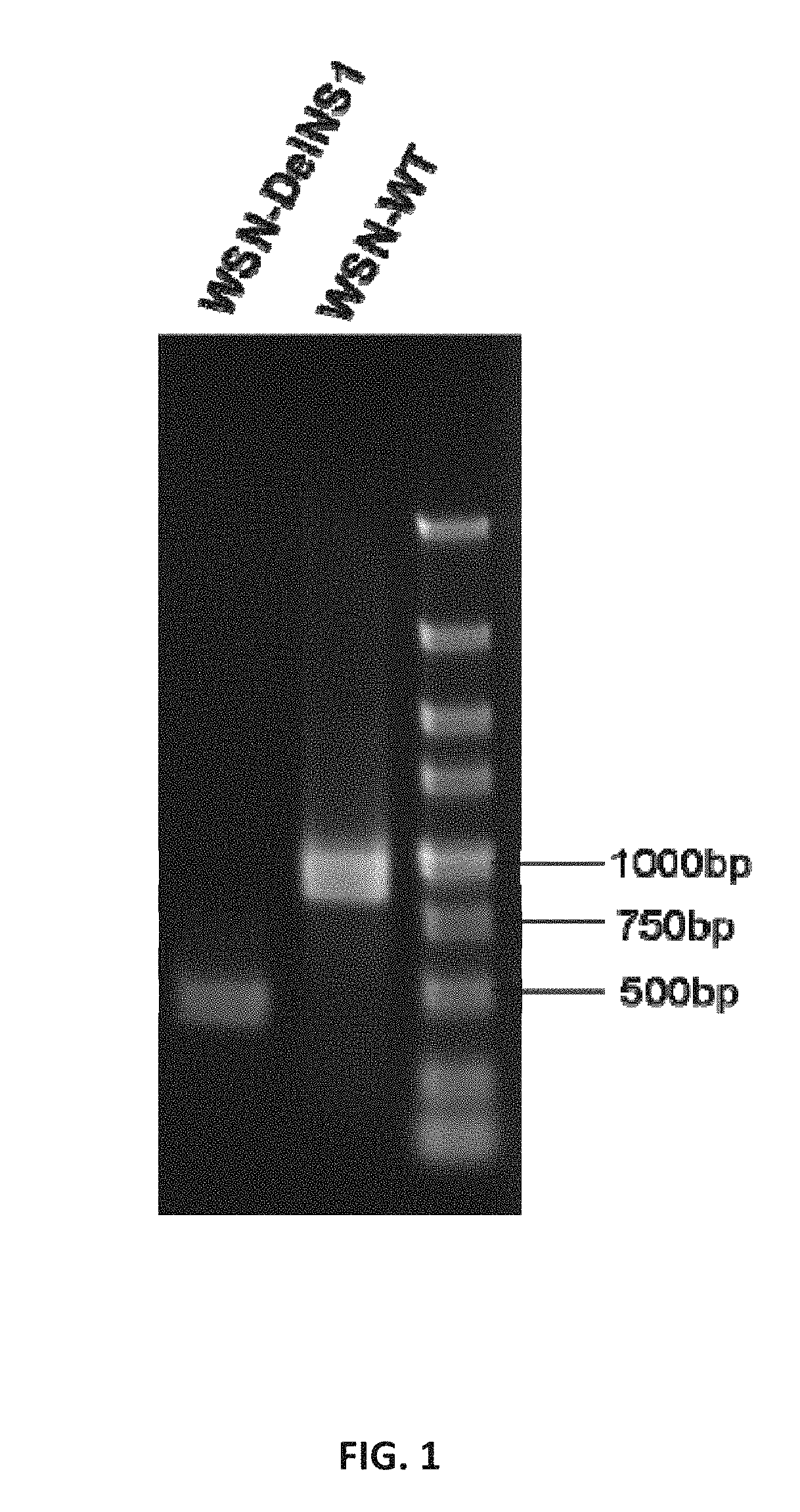
The subject invention pertains to attenuated influenza viruses, and related vaccines and methods, comprising a genetically modified viral genome. The genetically modified viral genome comprises a disruption in the non-structural (NS1) coding segment and one or more base substitution in the matrix membrane protein coding segment. The genetic modifications result in viruses that lose NS1 functionality, yet remain replication competent.
Owner:VERSITECH LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com