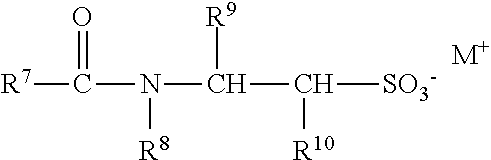
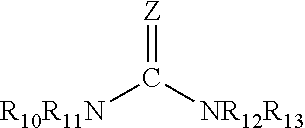
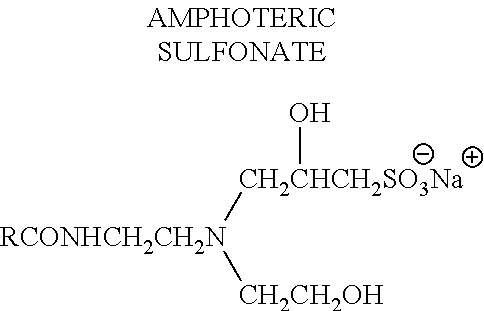
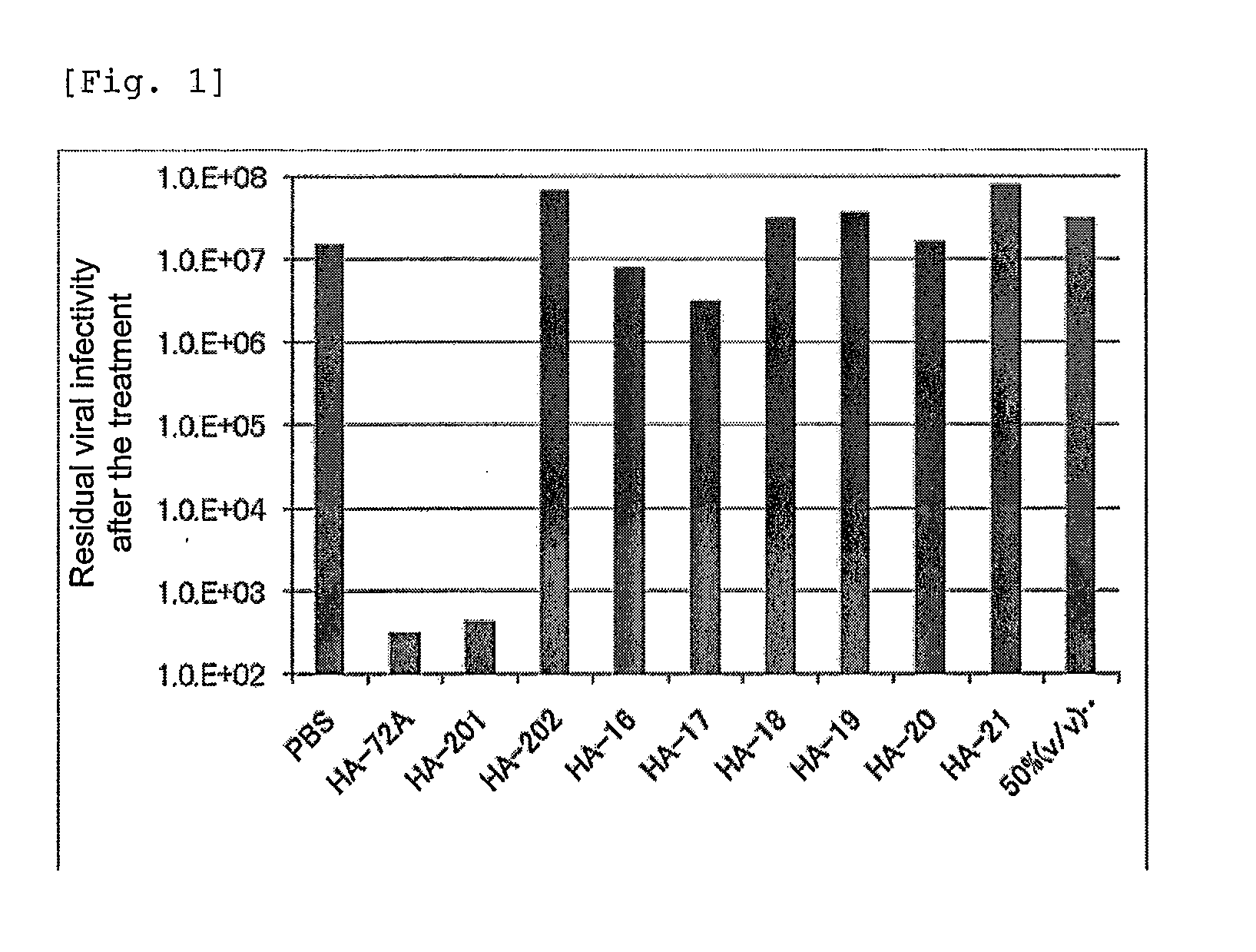
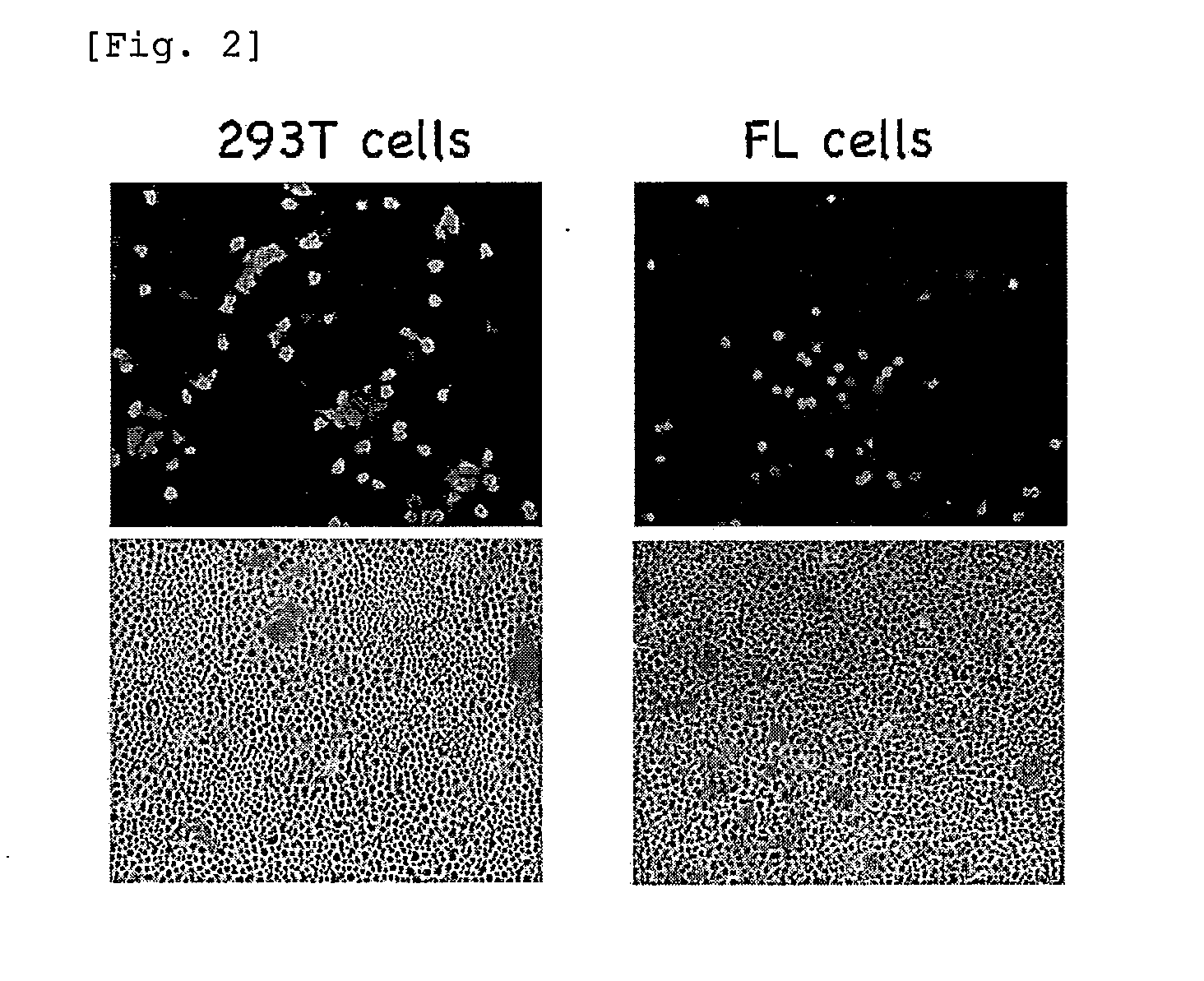
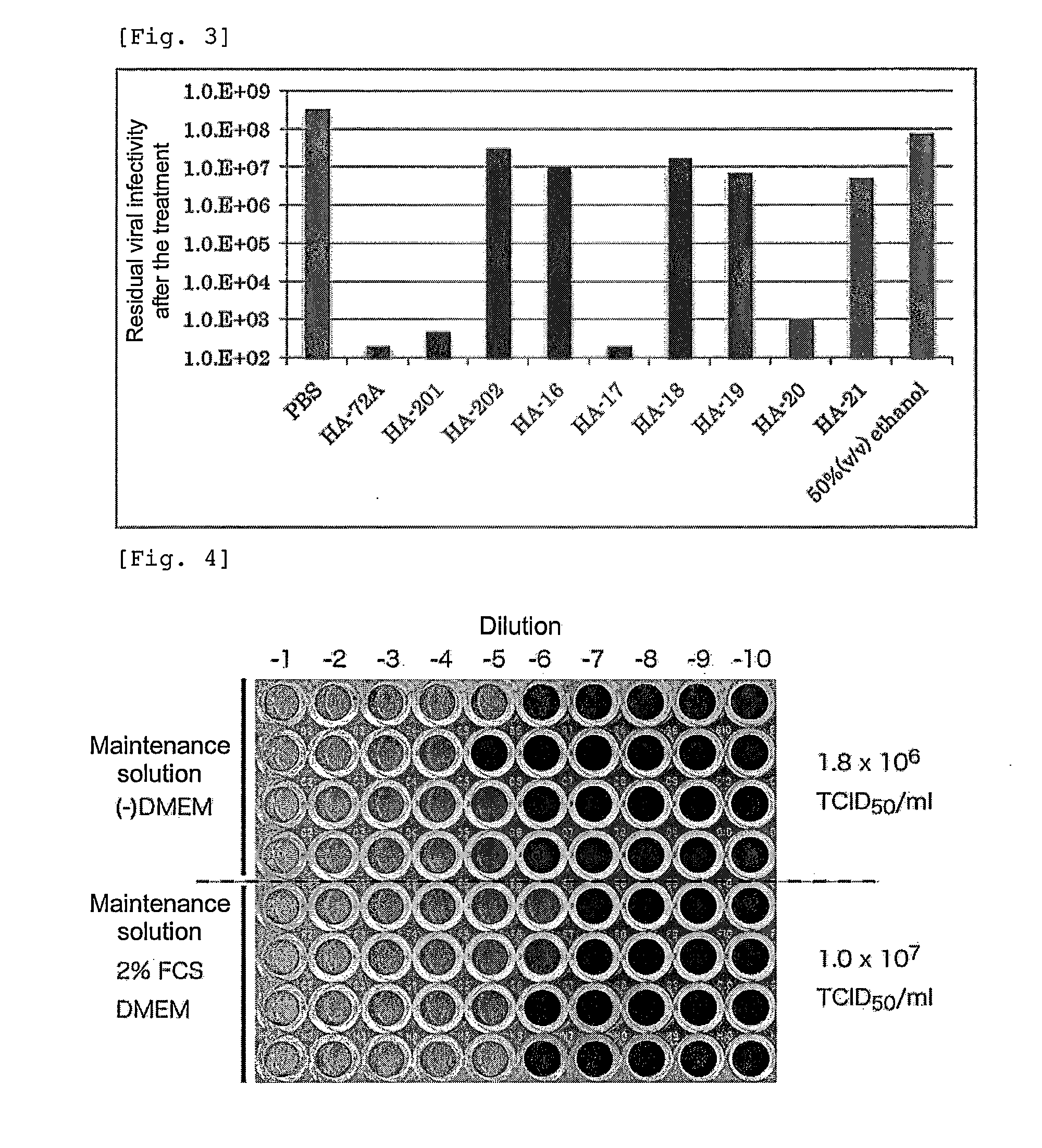
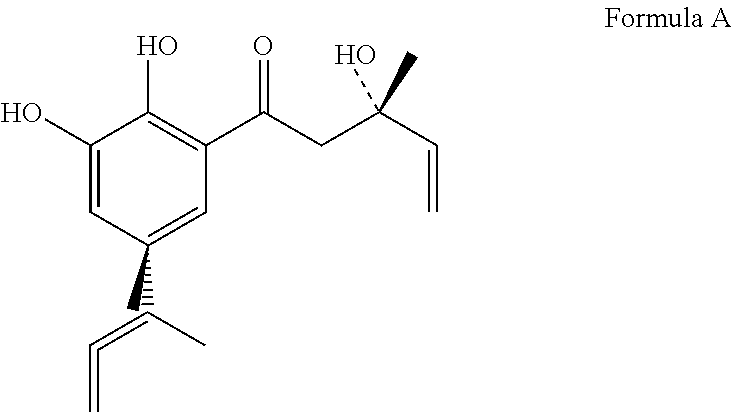
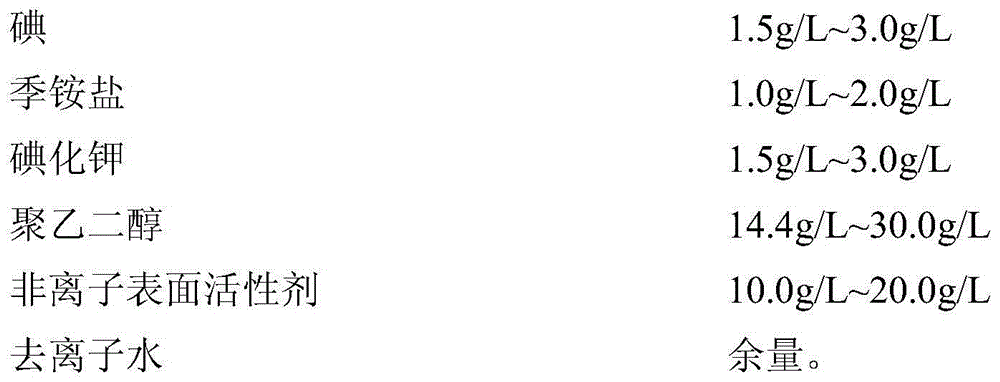Patents
Literature
40 results about "Non enveloped virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel reagents for transfection of eukaryotic cells
ActiveUS20090023215A1Improve efficiencyPeptide/protein ingredientsVirus peptidesDendrimerTransfection
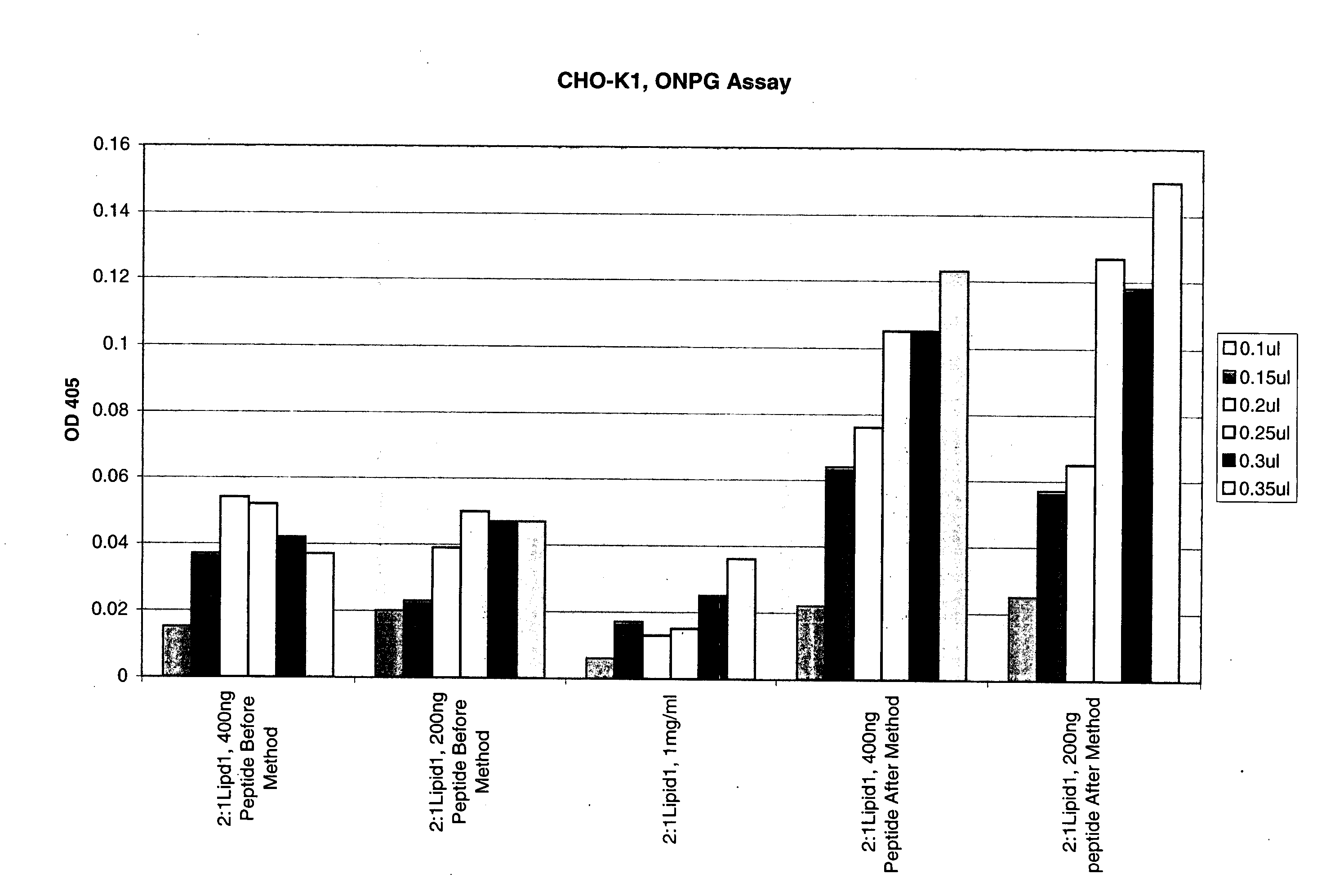
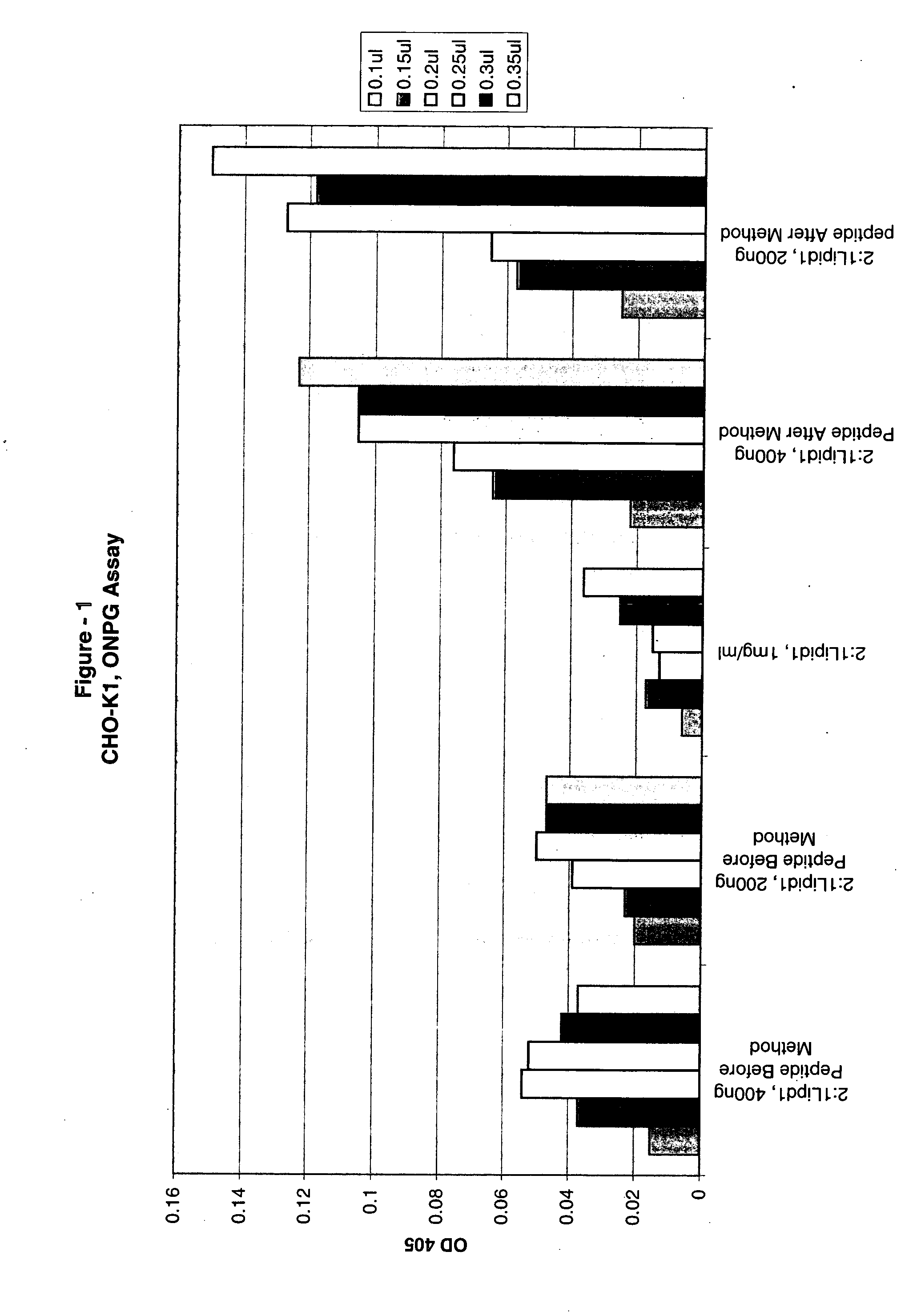
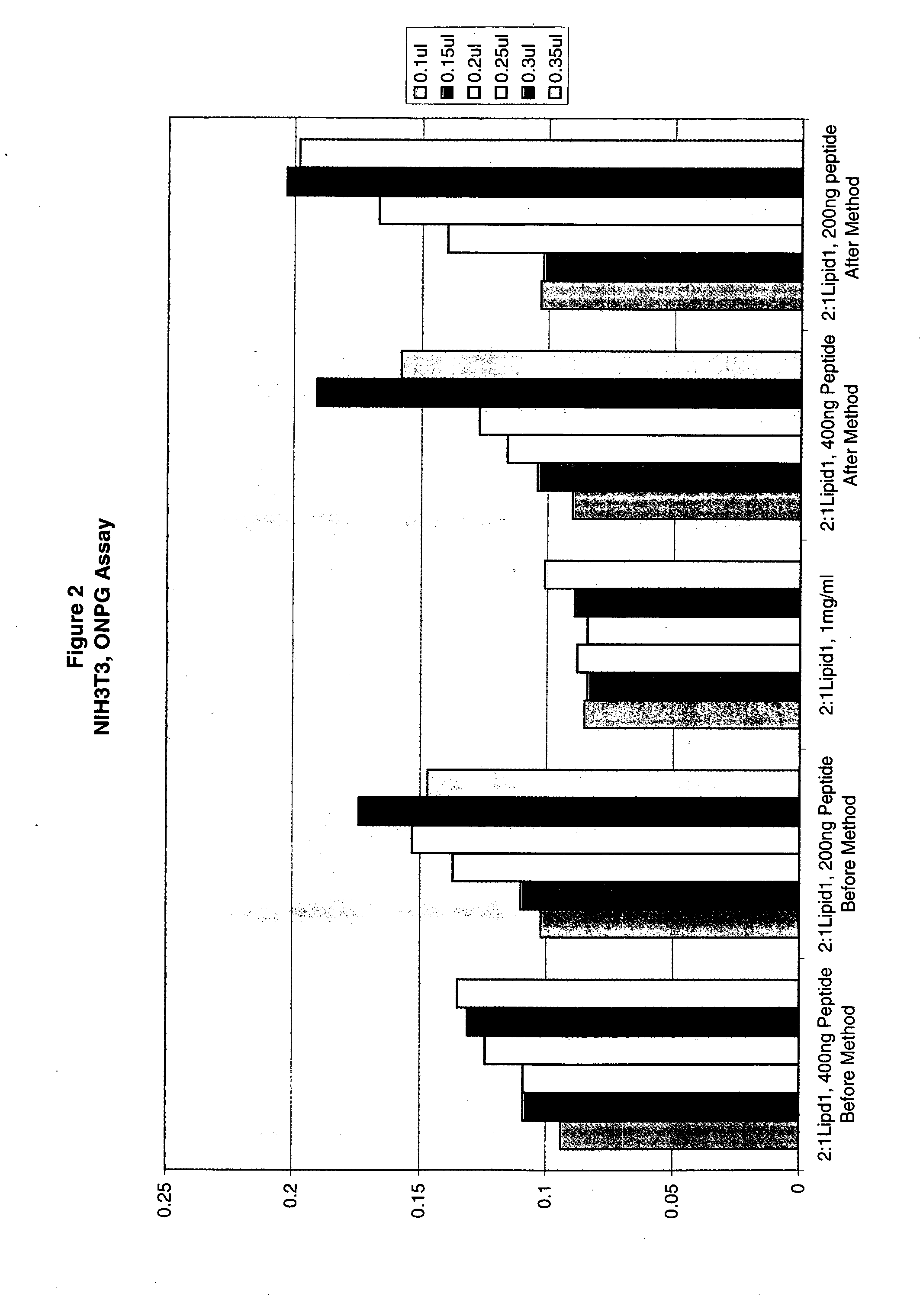
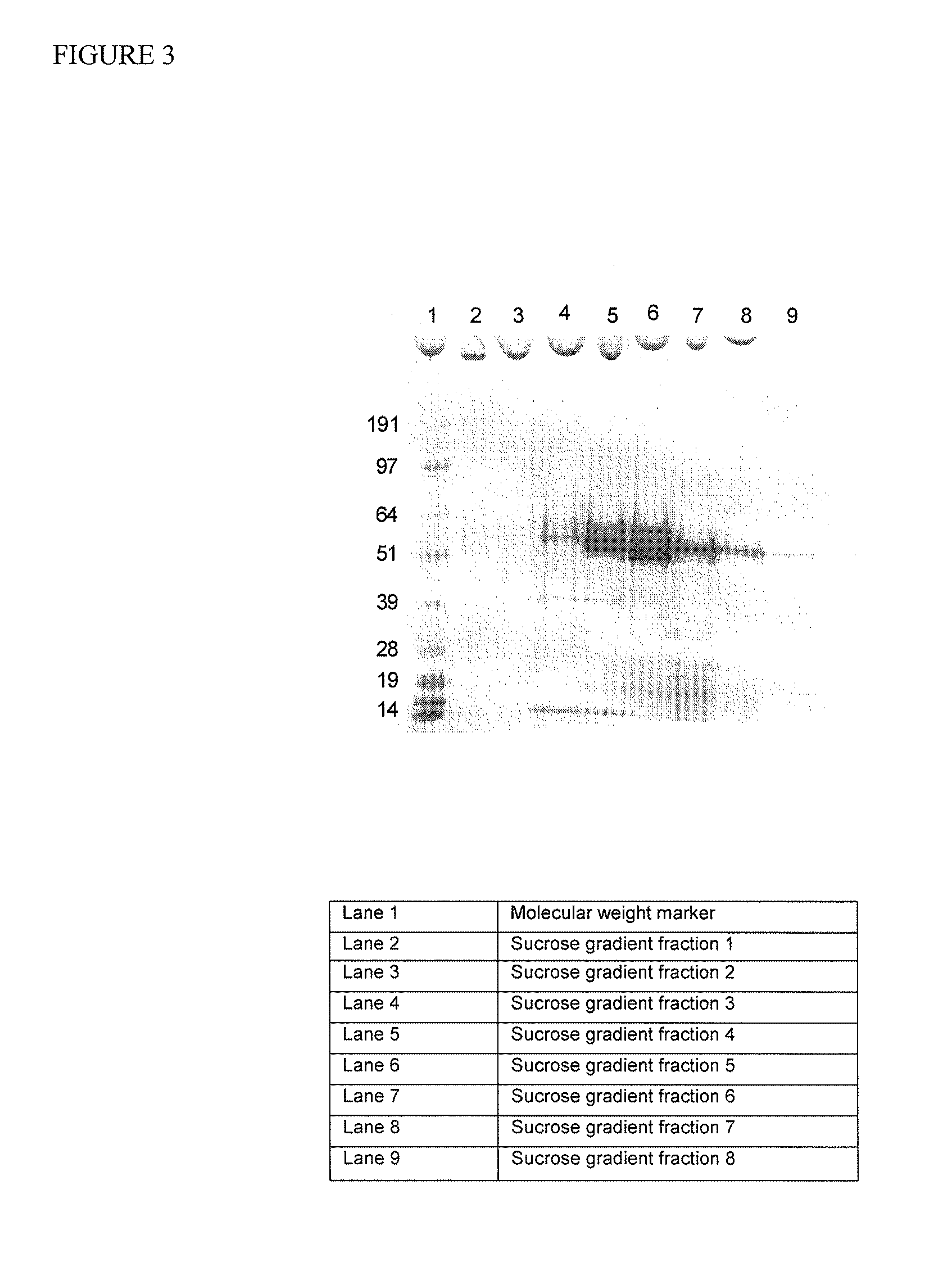
Compositions and methods for improved delivery of macromolecules into eukaryotic cells are provided. Fusogenic peptides from fusion proteins of non-enveloped viruses enhance the efficiency of transfection of eukaryotic cells mediated by transfection agents such as cationic lipids, polycationic polymers such as PEI and dendrimers. These fusogenic peptides are used as part of a transfection complex that efficiently delivers a macromolecule, for example, a nucleic acid, into a eukaryotic cell. Novel cationic lipids and compositions of cationic lipids also are provided that may be used for the introduction of macromolecules such as nucleic acids, proteins and peptides into a variety of cells and tissues. The lipids can be used alone, in combination with other lipids and / or in combination with fusogenic peptides to prepare transfection complexes.
Owner:MOLECULAR TRANSFER
Antiviral method
This invention provides a method of inactivating non-enveloped virus particles. The method includes the step of contacting the virus with a virucidally-enhanced alcoholic composition that includes an alcohol, and an enhancer selected from the group consisting of cationic oligomers and polymers, proton donors, chaotropic agents, and mixtures thereof.
Owner:GOJO IND INC
Antiviral method
This invention provides a method of inactivating non-enveloped virus particles. The method includes the step of contacting the virus with a virucidally-enhanced alcoholic composition that includes an alcohol, and an enhancer selected from the group consisting of cationic oligomers and polymers, proton donors, chaotropic agents, and mixtures thereof.
Owner:GOJO IND INC
Virus-like particles comprising composite capsid amino acid sequences for enhanced cross reactivity
ActiveUS8841120B2Strong immune responseImprove protectionVirusesMicroorganismsEpitopeVirus-like particle
The present invention provides polypeptides having a composite amino acid sequence derived from a consensus sequence representing the capsid proteins of two or more circulating strains of a non-enveloped virus. In particular, the invention provides virus-like particles comprising at least one composite polypeptide. Such virus-like particles have antigenic epitopes of two or more circulating strains of a non-enveloped virus and produce an increase in antisera cross-reactivity to one or more circulating strains of the non-enveloped virus. Methods of making composite virus-like particles and vaccine formulations comprising composite virus-like particles are also disclosed.
Owner:TAKEDA VACCINES INC
Virus-like particles comprising composite capsid amino acid sequences for enhanced cross reactivity
ActiveUS20110195113A1Enhance immune responseHigh IgG levelSsRNA viruses positive-senseMicroorganismsEpitopeVirus-like particle
The present invention provides polypeptides having a composite amino acid sequence derived from a consensus sequence representing the capsid proteins of two or more circulating strains of a non-enveloped virus. In particular, the invention provides virus-like particles comprising at least one composite polypeptide. Such virus-like particles have antigenic epitopes of two or more circulating strains of a non-enveloped virus and produce an increase in antisera cross-reactivity to one or more circulating strains of the non-enveloped virus. Methods of making composite virus-like particles and vaccine formulations comprising composite virus-like particles are also disclosed.
Owner:TAKEDA VACCINES INC
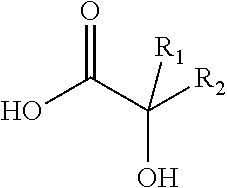
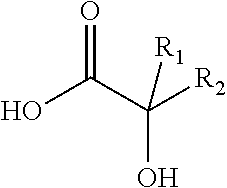
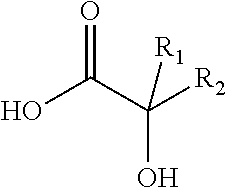
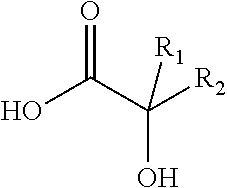
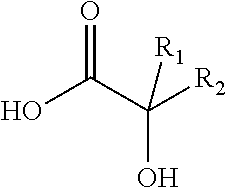
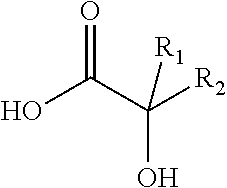
Antiviral compositions and methods for inactivating non-enveloped viruses using alkyl 2-hydroxycarboxylic acids
ActiveUS20140275255A1Transmission can be interruptedEffective controlBiocideHydroxy compound active ingredientsSurface cleaningHand sanitizer
The present invention is directed to antiviral compositions that provide efficacy against non-envelope viruses such as noroviruses. The antiviral compositions comprise an alkyl 2-hydroxycarboxylic acid and an effective amount of a sulfonated surfactant. The composition may be used as a topical on human skin, as a hand sanitizer or as a hard surface cleaning composition.
Owner:ECOLAB USA INC
Production method for non-enveloped virus particles
ActiveUS20170166871A1High purityNo laborious operationRecovery/purificationVector-based foreign material introductionBULK ACTIVE INGREDIENTActive ingredient
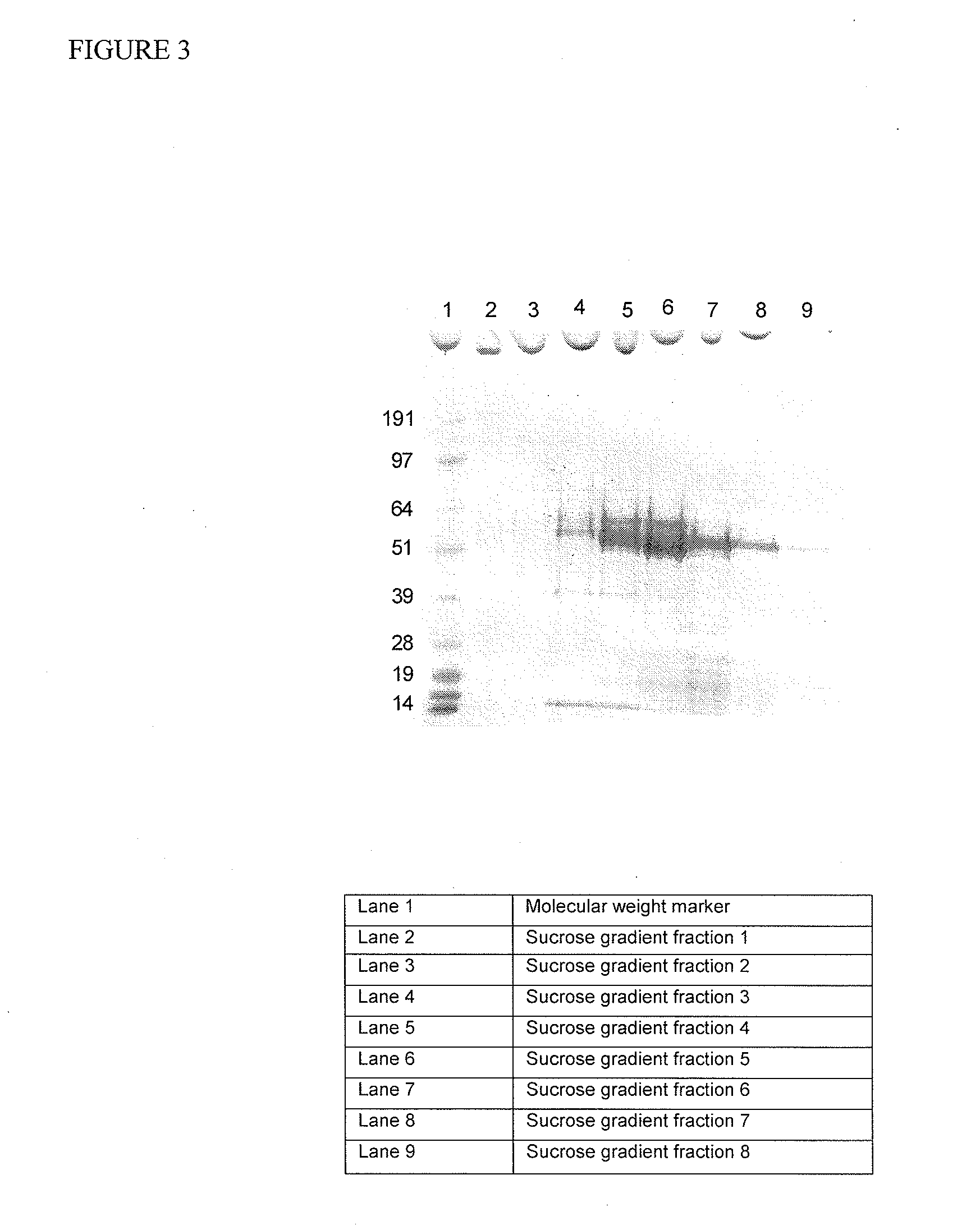
Provided are a production method for non-enveloped virus particles which is characterized in that a sample including non-envelopes virus particles is treated with PEG in at least two concentrations; a kit used in said production method; non-enveloped virus particles produced using said production method; and a pharmaceutical composition having the non-envelopes virus particles as an active ingredient.
Owner:TAKARA HOLDINGS
Antiviral method
This invention provides a method of inactivating non-enveloped virus particles. The method includes the step of contacting the virus with a virucidally-enhanced alcoholic composition that includes an alcohol, and an enhancer selected from the group consisting of cationic oligomers and polymers, proton donors, chaotropic agents, and mixtures thereof.
Owner:GOJO IND INC
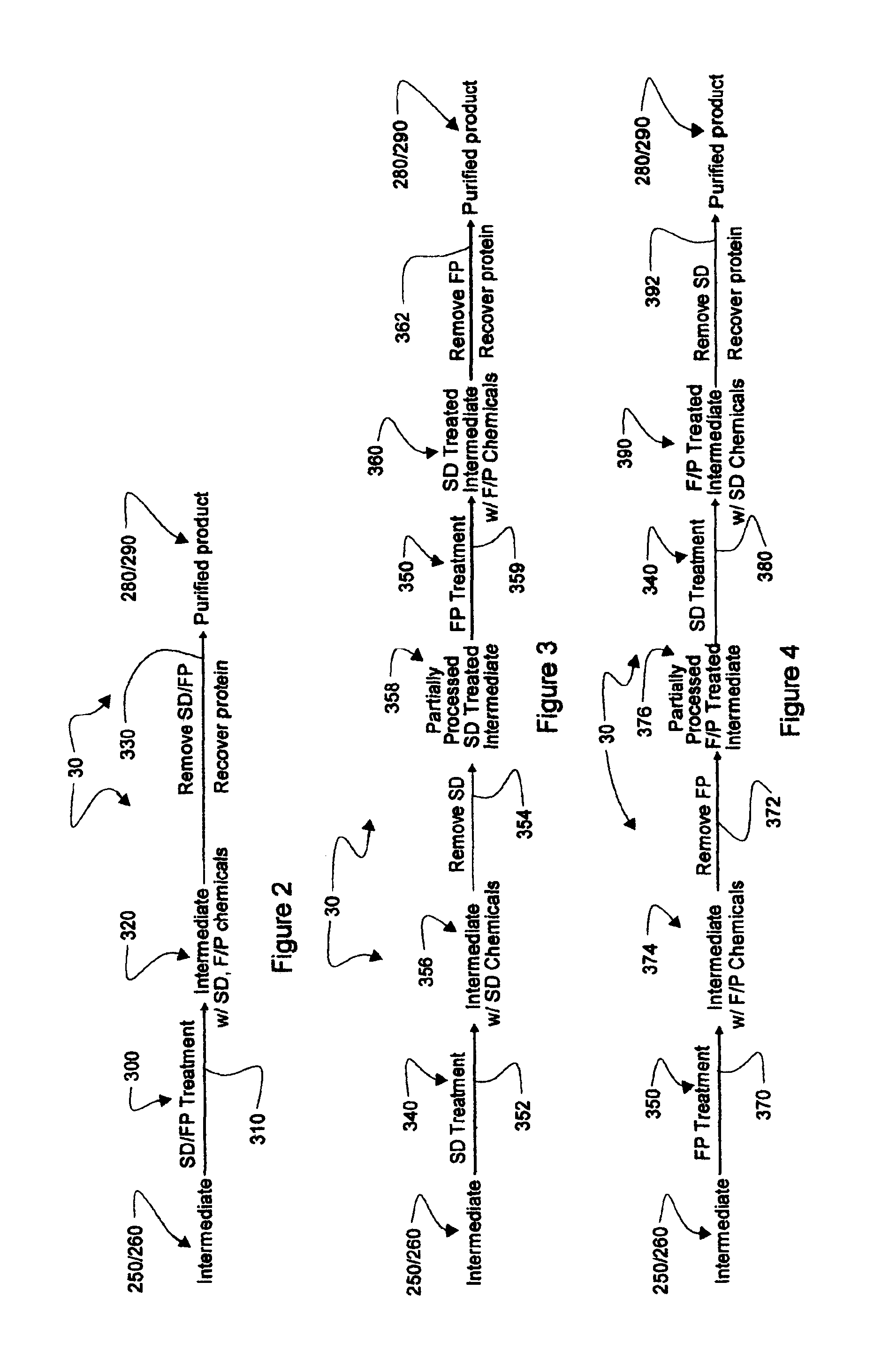
Augmented solvent/detergent method for inactivating enveloped and non-enveloped viruses
InactiveUS6881573B2Immunoglobulins against blood group antigensSugar derivativesSolvent detergentPhenol
Owner:LOUDERBACK ALLAN L
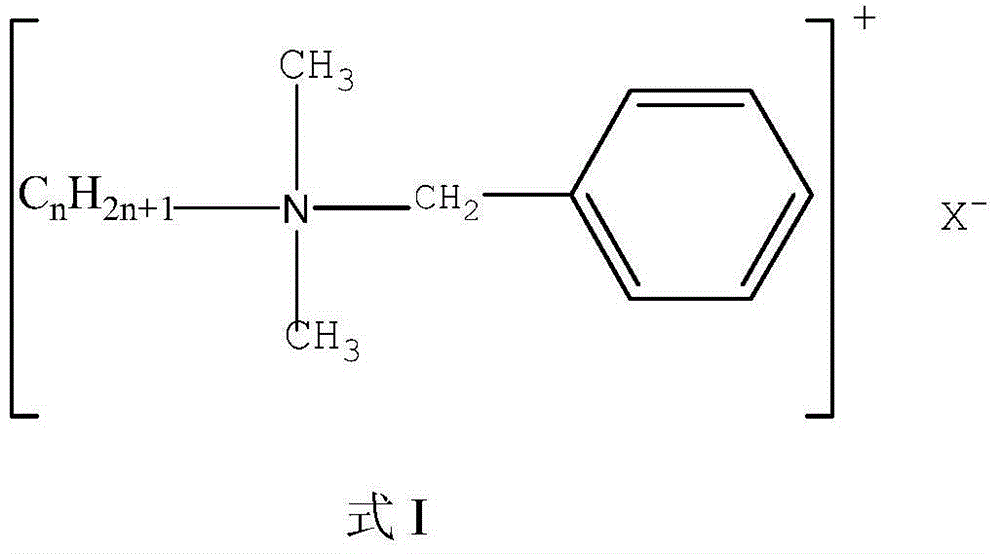
Compound skin mucous membrane disinfectant, preparation method and application thereof
The invention provides a compound disinfectant and a preparation method, the compound disinfectant comprises the following components: 0.5-10.0g / L of iodine, 0.5-2.0g / L of quaternary ammonium salt, 0.2-3.0g / L of potassium iodide, 10g / L-30g / L of polyethylene glycol, 2.5-25.0g / L of nonionic surfactant and the balance of deionized water. The quaternary ammonium salt preferably selects a mixture of single chain quaternary ammonium salt and double-chain quaternary ammonium salt. The invention also provides an application of the compound disinfectant for killing virus and bacteria on skin, mucous membrane, wound, hand and object surface. While in use, the compound skin mucous membrane disinfectant has no irritation on skin mucous membrane, no toxic and side effect, no anaphylactic reaction, convenient usage, wide application scope and rapid effect, and has microbe killing effect. The compound skin mucous membrane disinfectant has reliable effect for various pathogenic microorganism, can kill envelope-free virus in 3 minutes, and is an important means for preventing and controlling various infectious diseases.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Verification method of low pH incubation virus inactivation
InactiveCN108342368AImprove scalabilitySimple purification processSsRNA viruses negative-senseMicrobiological testing/measurementVirus inactivationValidation methods
The invention relates to a verification method of low pH incubation virus inactivation. The method comprises the steps of selecting indicator viruses and corresponding host cells; amplifying and purifying the indicator viruses; titering virus working primary liquid and samples; optimizing the pH value of virus inactivation verification experiments; estimating the low pH incubation virus inactivation effect. By means of the method, the full process of genetically engineered drug production technology virus inactivation can be simulated in a laboratory, and the amplifying and purifying technology of the indicator viruses is optimized, so that the titer of the working primary liquid of the indicator viruses is increased, the pH value is unified to 7.0, the quality is stable, and subsequent operation is facilitated; meanwhile, the specific technological details like the determination of the optimal incubation pH value and the optimization of the pH adjusting mode of low pH incubation are optimized, and the efficiency and the effectiveness of the inactivation of common DNA viruses, RNA viruses, enveloped viruses and non-enveloped viruses are improved.
Owner:CANVEST WUHAN BIOTECH
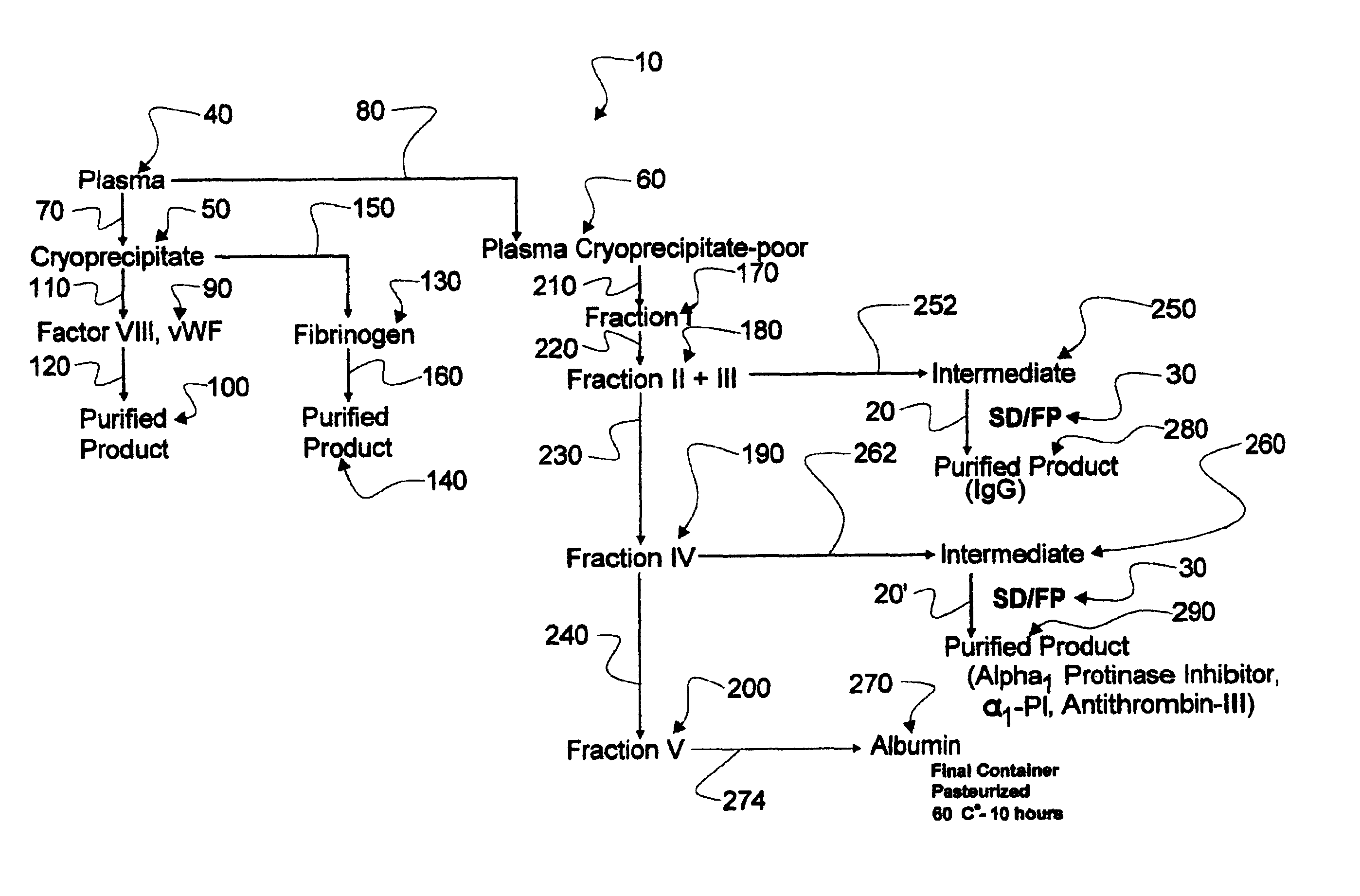
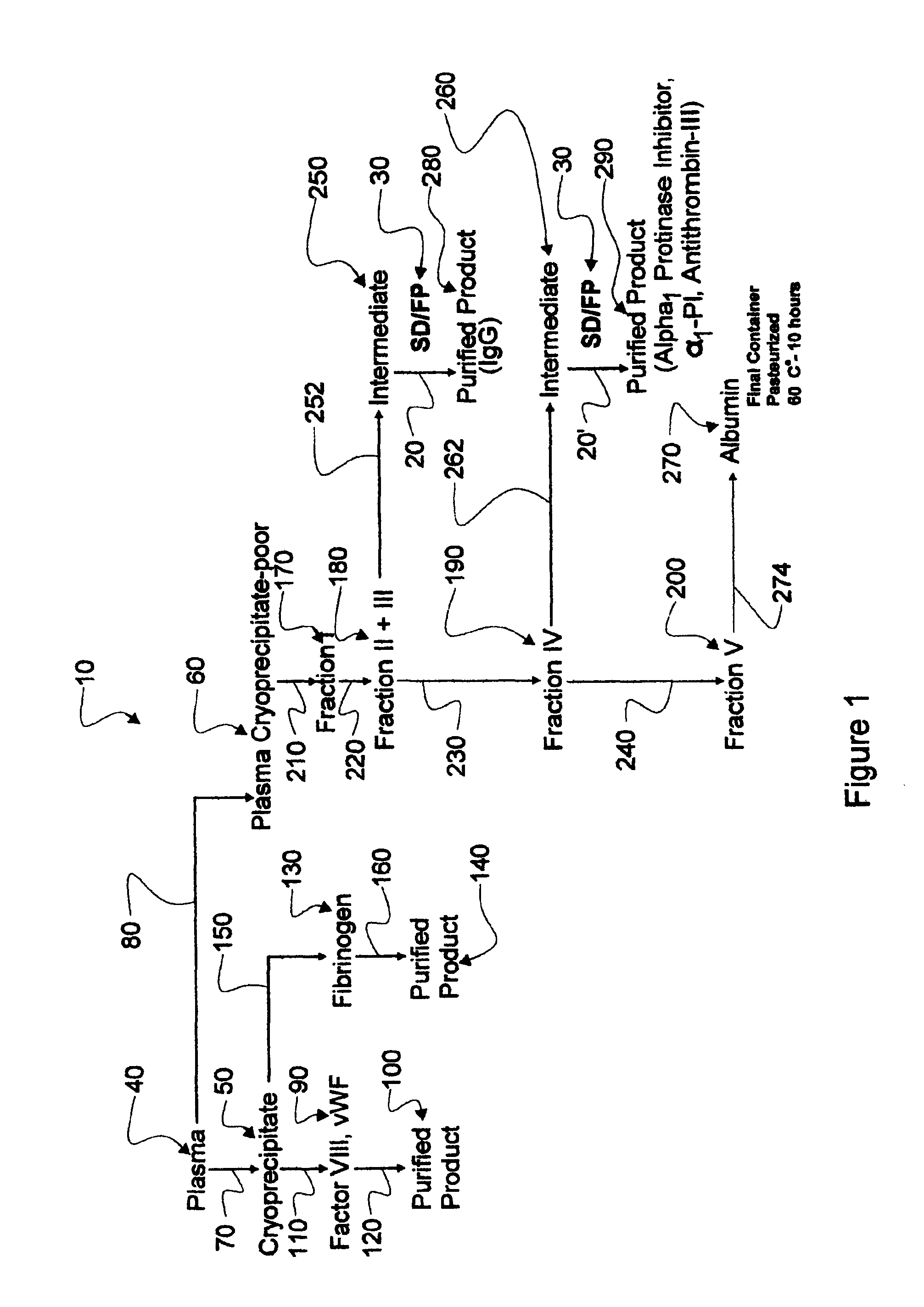
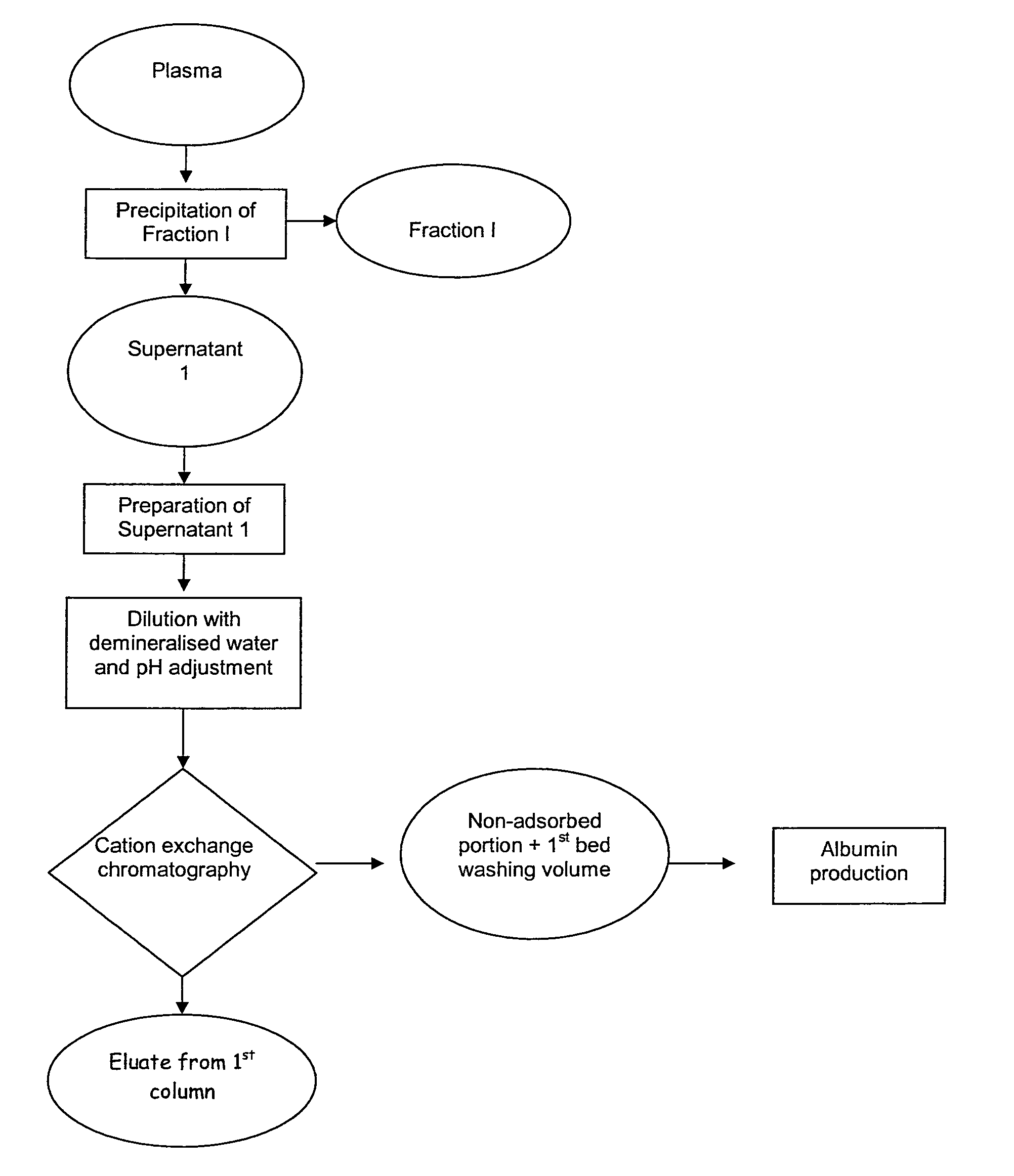
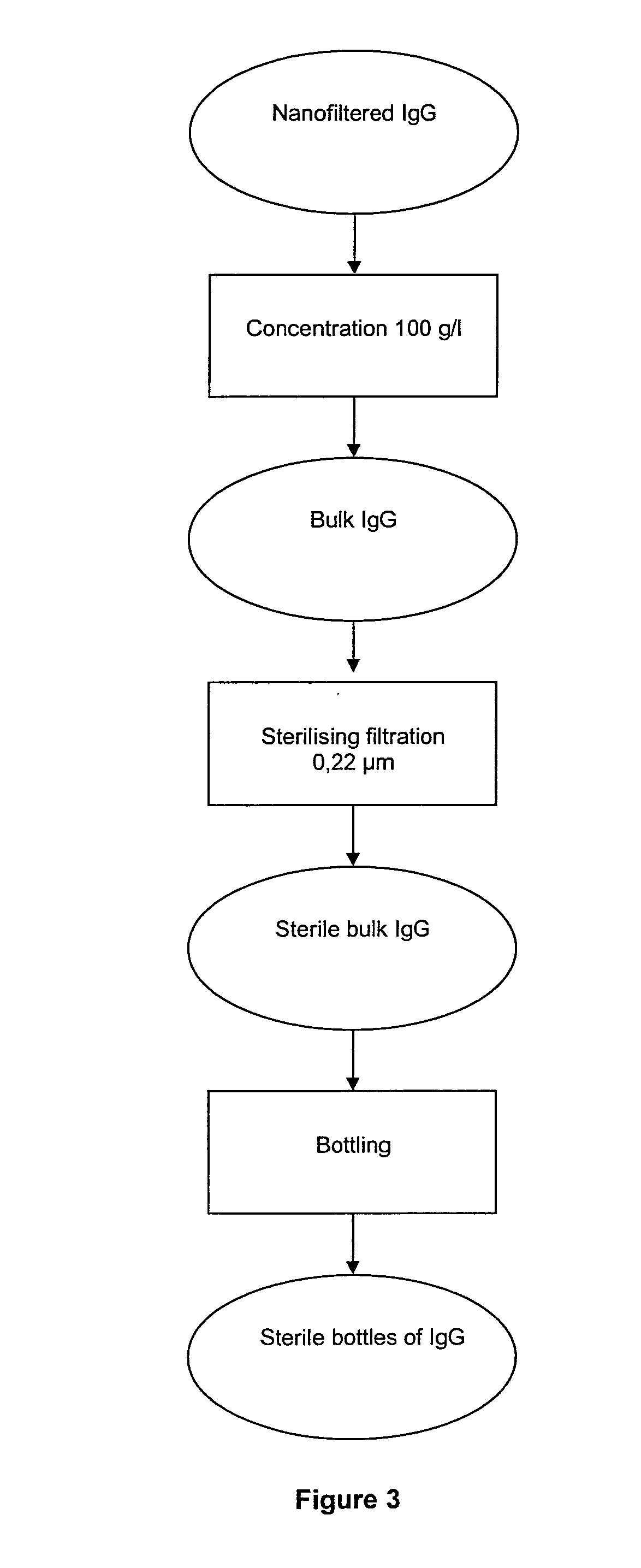
New process for the industrial-scale purification of gamma globulins from human plasma for industrial applications
ActiveUS20120316323A1High yieldImprove stabilityPeptide preparation methodsImmunoglobulinsAnion-exchange chromatographyBlood plasma
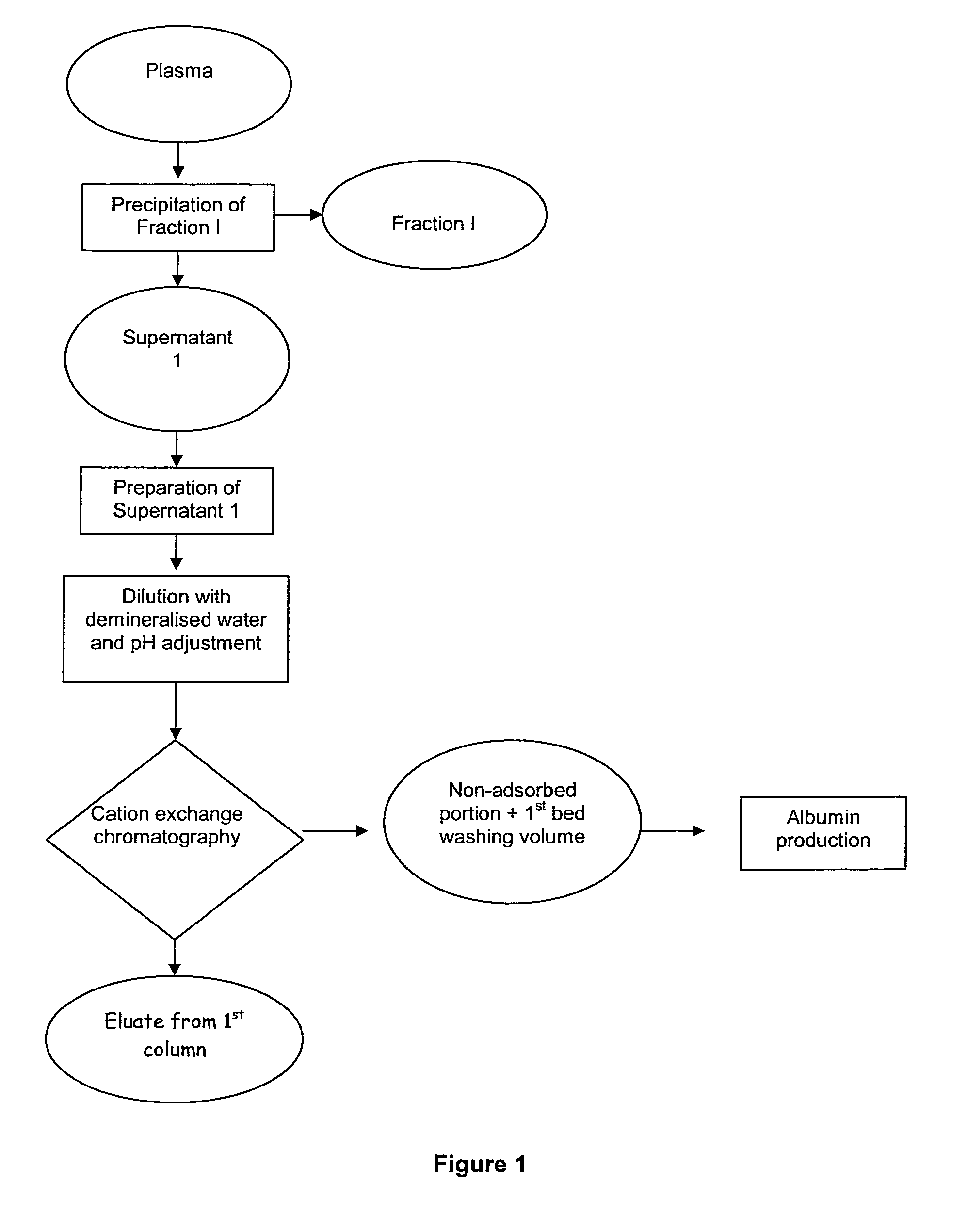
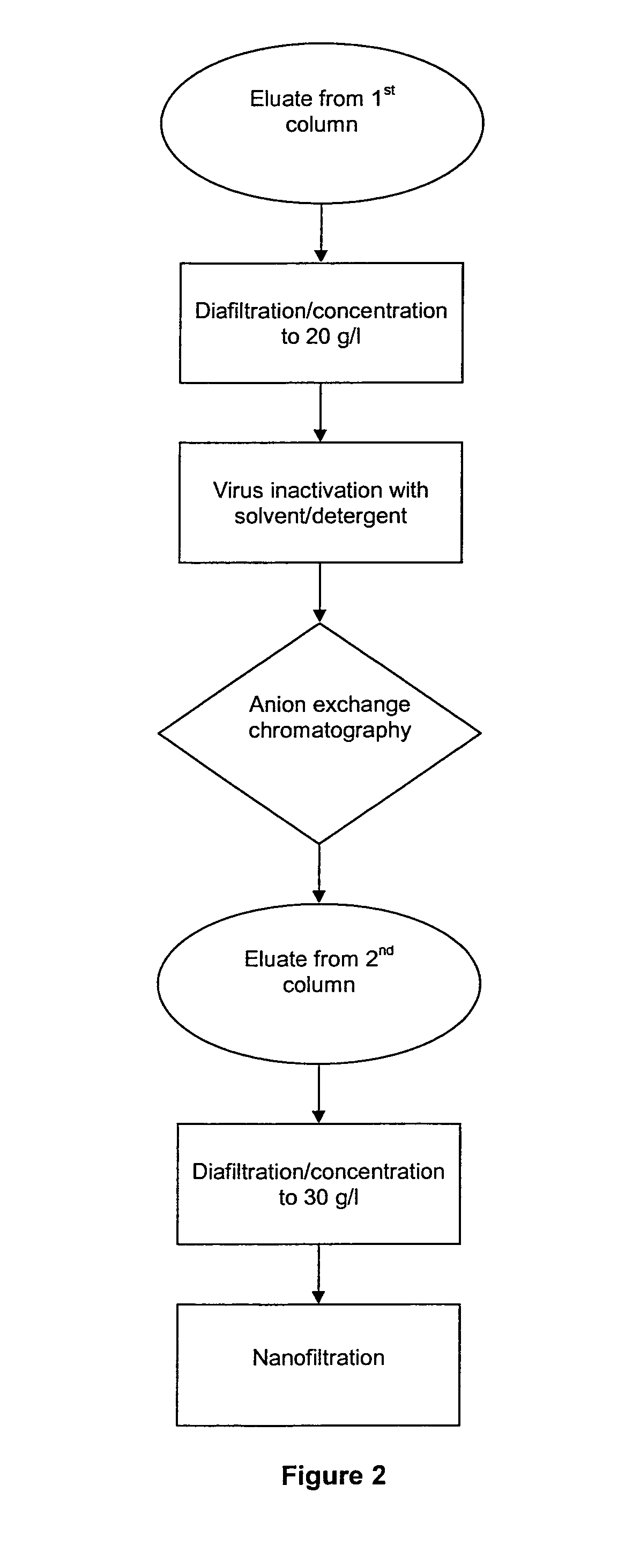
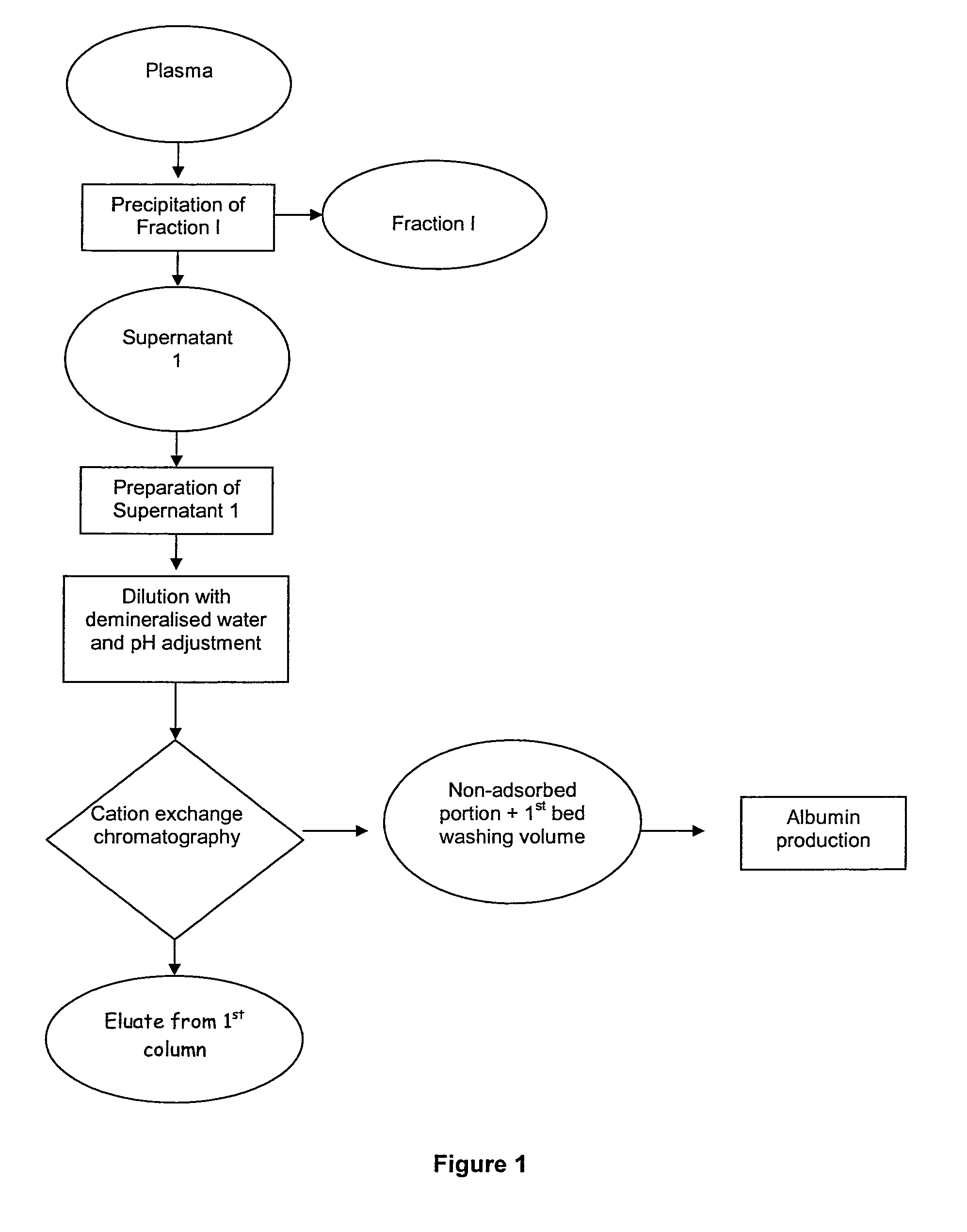
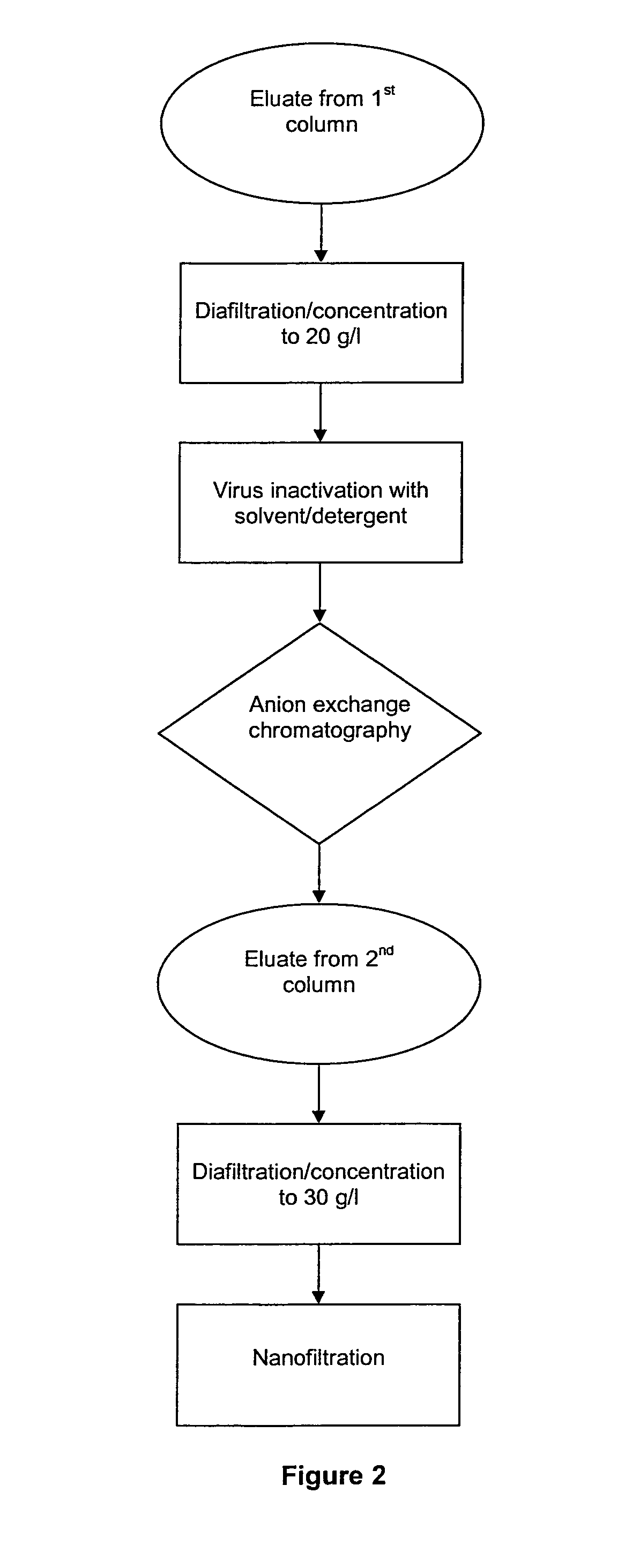
The invention relates to a novel, industrial-scale process for the purification of gamma-immunoglobulins (IgG) starting from plasma or fractions thereof. The method involves two chromatographic steps, i.e. a cation exchange capture chromatography, and then a polishing anion exchange chromatography, ensuring a highly purified end product, which contains no aggregates, and high yields. The process also involves a virus inactivation step by means of a solvent / detergent treatment to inactivate the viruses with a lipid envelope, and a virus removal step by nanofiltering to ensure the removal of the non-enveloped viruses.
Owner:KEDRION
Reagents for transfection of eukaryotic cells
ActiveUS7915230B2Improve efficiencyPeptide/protein ingredientsGenetic material ingredientsDendrimerLipid formation
Owner:MOLECULAR TRANSFER
Virus-free plasma protein compositions treated with porous membrane and process for producing the same
InactiveUS6867285B2Efficient removalSafer plasma protein preparationAntibacterial agentsPeptide/protein ingredientsFiberProtein composition
Contaminant viruses can be efficiently removed almost without losing the activity of protein by subjecting a plasma protein composition having a high risk of viral contamination to a treatment with a porous membrane having a pore size greater than a single-particle size of the virus, particularly by subjecting a plasma protein composition to a fractionation treatment by precipitation, before the porous membrane treatment. Particularly, a fibrinogen composition substantially free of non-enveloped viruses, Parvovirus among others, can be provided. By the application of the present invention, a safe plasma protein preparation free of viruses can be conveniently provided.
Owner:JAPAN BLOOD PROD ORG
Preparation method of antibacterial and antiviral nano water-based slurry
PendingCN111789131AImprove stabilityTo kill virusBiocideMaterial nanotechnologyEscherichia coliImplant
The invention provides a preparation method of antibacterial and antiviral nano water-based slurry. The preparation method comprises the following steps: dropwise adding an inorganic zinc salt solution into a sodium hydroxide solution, controlling the temperature and pH value to prepare columnar nano ZnO powder; then, mixing columnar nano ZnO powder, a Cu2SO4 water solution, and a certain amount of NaOH, CTAB, a GO water solution and hydrazine hydrate, carrying out an ultrasonic reaction to prepare Cu2O-ZnO-GO nano composite powder, finally adding the Cu2O-ZnO-GO nano composite powder into a mixed dispersion liquid, and stirring to obtain the antibacterial and antiviral nano water-based slurry. The slurry is characterized in that columnar nano ZnO and Cu2O simulate a dragonfly wing nano bulge structure; and ZnO and Cu2O are uniformly loaded by virtue of the large surface area of GO. The formed nano composite powder can effectively inactivate bacteria such as escherichia coli, staphylococcus aureus and streptococcus pneumoniae and non-enveloped viruses such as influenza viruses and SARS, and has a wide application prospect in the fields of coatings, paints, implants, household appliances and the like.
Owner:宿迁空天新材料有限公司
Method of inhibiting influenza infection with antiviral peptides
This invention relates to peptides having antiviral properties. The antiviral peptides comprise membrane transiting peptides, and active fragments and derivatives of such peptides. The antiviral peptides exhibit activity against a broad spectrum of viruses, including enveloped and non-enveloped viruses, and are used in pharmaceutical compositions to prevent and / or treat viral infections.
Owner:WISCONSIN ALUMNI RES FOUND
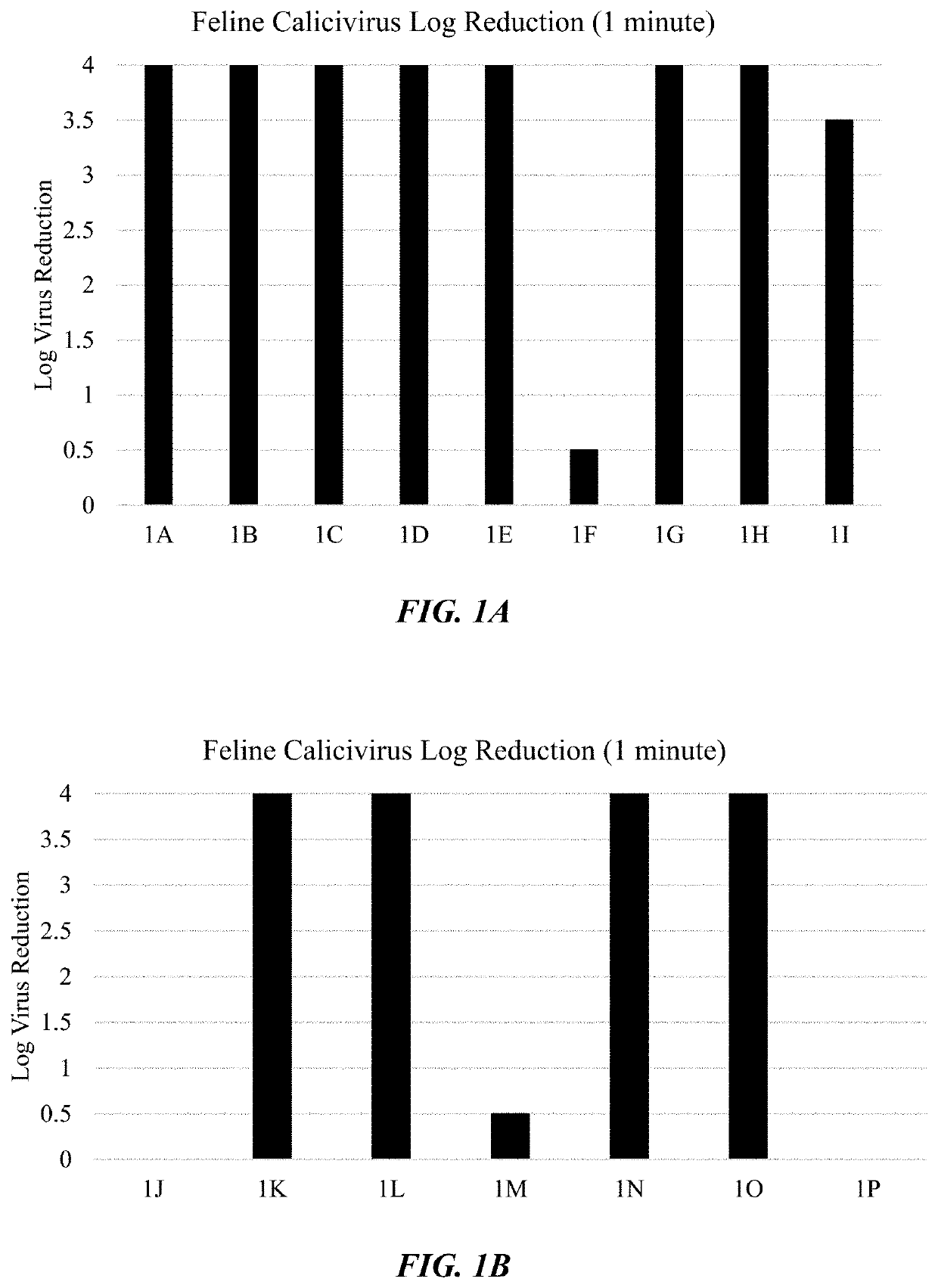
Disinfectant Composition with Rapid Antiviral Efficacy
InactiveUS20180200397A1Reduction in virus infectivityOrganic active ingredientsAntiviralsAlcoholAliphatic amine
A disinfectant composition that contains a unique combination of a primary antiviral agent and a secondary antiviral agent is provided. More particularly, the primary antiviral agent includes a polyprotic acid having a low first acid dissociation constant and the secondary antiviral agent is selected from the group consisting of an anionic N-acyl compound, metal halide salt, amine compound, amine compound, aliphatic amine oxide, alkyl glycoside, alcohol ethoxylate, and combinations thereof. By selectively controlling the particular nature of each antiviral agent and their relative concentration, the present inventors have discovered that a synergistic affect can be achieved in which the disinfectant composition is capable of exhibiting an antiviral efficacy against non-enveloped viruses.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Composition prepared from saikosaponin, the use and the preparation method thereof
InactiveCN105358220APrevent intrusionAvoid infectionAntiviralsAgainst vector-borne diseasesVesicular stomatitis virusHepacivirus
This invention relates to a pharmaceutical composition having saikosaponin as a main component, the use of the pharmaceutical composition, as well as the preparation method thereof, wherein the pharmaceutical composition is used to prevent or treat the infection of one virus selected from the hepatitis C virus, the measles virus, the respiratory syncytial virus, the vesicular stomatitis virus, the dengue virus, and the non-enveloped viruses.
Owner:KAOHSIUNG MEDICAL UNIVERSITY +1
High throughput quantification and characterization of viruses and products thereof
ActiveUS20170198332A1Improve accuracyImprove throughputComponent separationMicrobiological testing/measurementPresent methodProcess quality
Owner:BIOGENESIS BAGO URUGUAY
Antiviral compositions and methods for inactivating non-enveloped viruses using alkyl 2-hydroxycarboxylic acids
ActiveUS9808435B2Rapid reduction in microbial populationReduce microbial countBiocideCosmetic preparationsSurface cleaningHand sanitizer
The present invention is directed to antiviral compositions that provide efficacy against non-envelope viruses such as noroviruses. The antiviral compositions comprise an alkyl 2-hydroxycarboxylic acid and an effective amount of a sulfonated surfactant. The composition may be used as a topical on human skin, as a hand sanitizer or as a hard surface cleaning composition.
Owner:ECOLAB USA INC
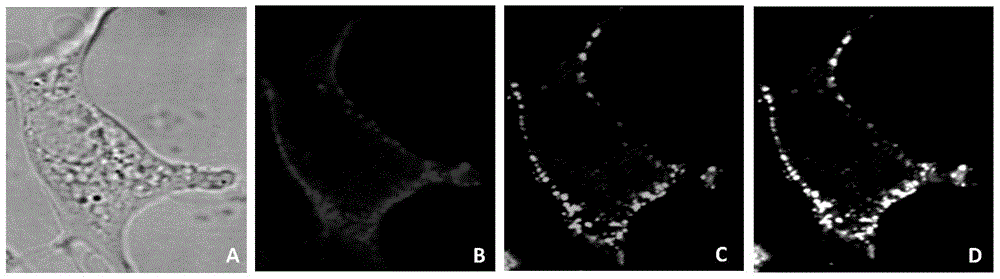
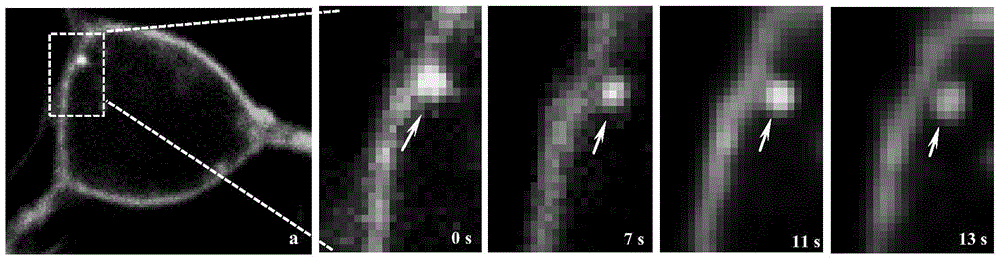
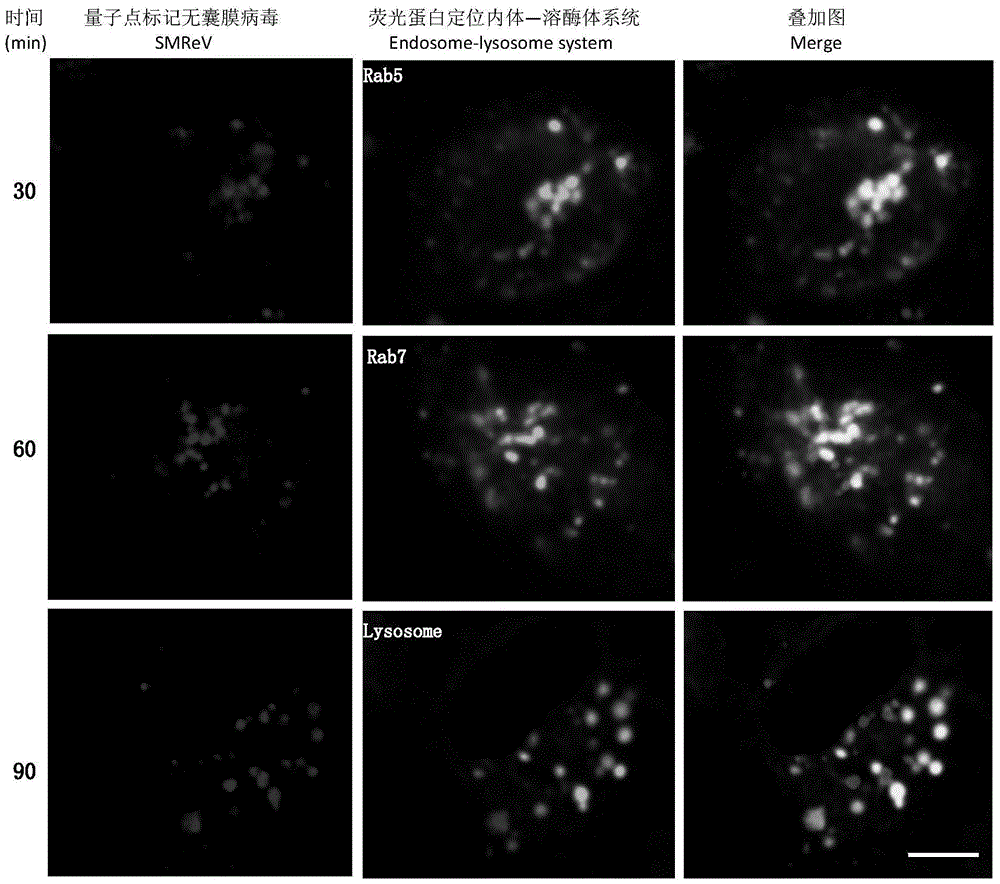
Non-enveloped virus quantum dot marking method and application
PendingCN105717084AImprove performanceImprove efficiencyFluorescence/phosphorescenceCell specificImmunofluorescent labeling
The invention discloses a non-enveloped virus quantum dot marking method and application.The method comprises the steps that biotinylation virus suspension is centrifuged, and biotinylation non-enveloped viruses of the uniform structure are obtained; the viruses are inoculated into susceptible cells, quantum dots are added for incubation, and non-enveloped viruses marked with the quantum dots can be obtained.By means of the marked non-enveloped viruses, non-enveloped virus outer capsid protein immunofluorescence marking and cell specific component fluorescent protein positioning, dynamic tracking of a non-enveloped virus living body in the invasion process and positioning of interaction components of the non-enveloped viruses and host cells are achieved.The method can also be used for monitoring the host cell invasion path of the viruses in real time, and is particularly applicable to identifying the interaction components of fish non-enveloped viruses and host cells and also applicable to screening, research and development of fish reovirus resistance preparations.
Owner:INST OF AQUATIC LIFE ACAD SINICA
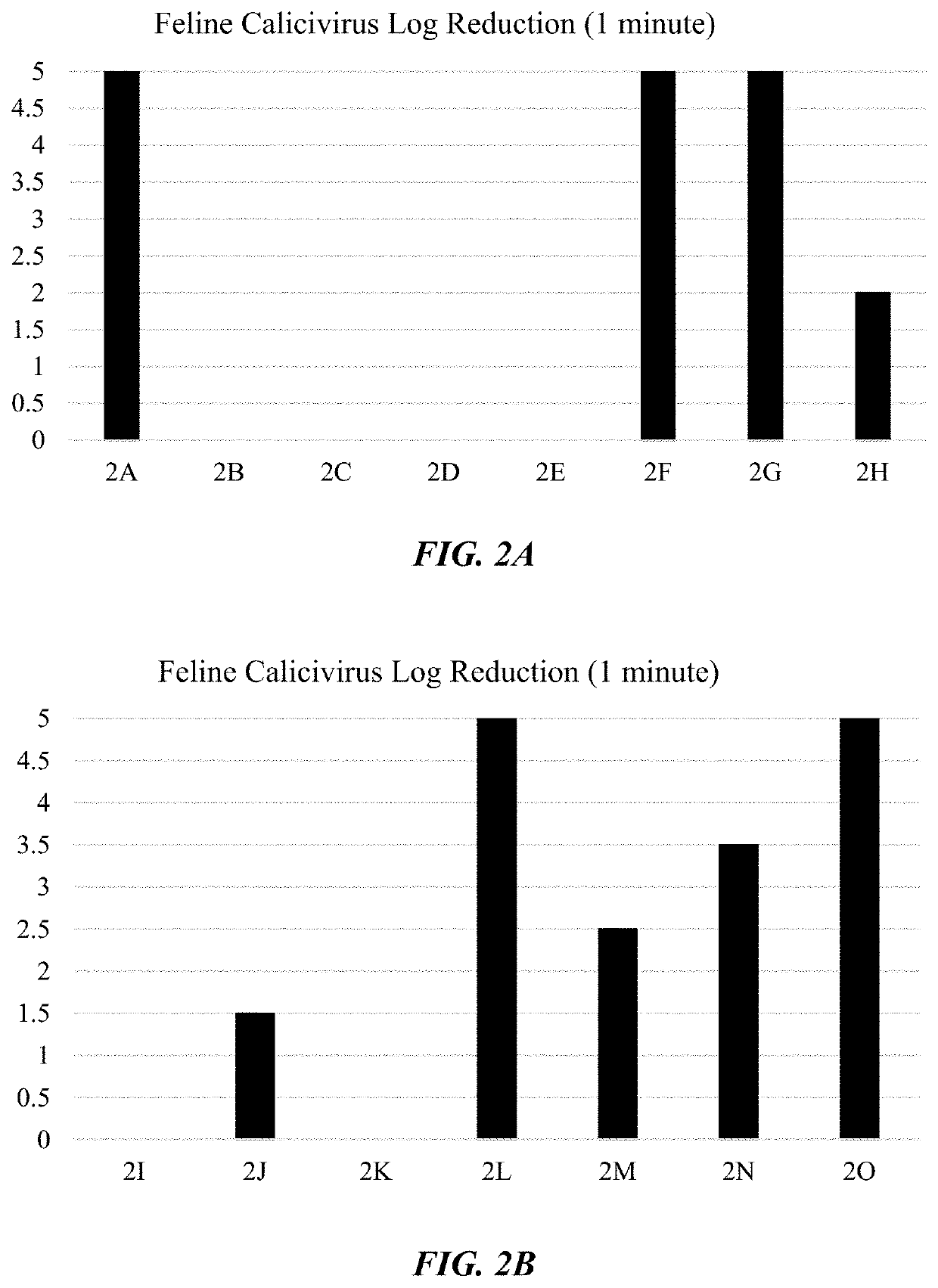
Hard surface cleaning solution with rapid viricidal activity
PendingUS20200323199A1Enhance killing activityStrong kill rateBiocideSurface-active detergent compositionsBiotechnologyPoliomyelitis
The invention relates to fast-acting viricidal compositions and methods of using the same, wherein the compositions are capable of removing soil and effectively inactivating a wide variety of viral pathogens, particularly small, non-enveloped viruses such as poliovirus and norovirus. The compositions are especially useful on hard surfaces, and can easily be applied to a service to provide rapid viricidal efficacy.
Owner:ECOLAB USA INC
"Method for Disinfection or Infection Control Against a Non-Enveloped Virus"
ActiveUS20130302453A1Good effectReduce morbiditySalicyclic acid active ingredientsBiocideEnterovirusRotavirus RNA
Provided herein is a method for disinfection or infection control against a non-enveloped virus using a composition including, as an active ingredient, a persimmon extract containing tannin from an astringent fruit of a plant of the genus Diospyros, such as the plant Diospyros kaki. The non-enveloped virus is a non-enveloped virus belonging to the genus Betanodavirus, Aquavirnavirus, Ranavirus, Enterovirus, Mastadenovirus, Vesivirus, or Rotavirus. In certain embodiments, the persimmon extract is prepared by heating a squeezed juice or an extract from an astringent fruit of a plant of the genus Diospyros, or obtained by treating a squeezed juice or an extract from an astringent fruit of the plant of the genus Diospyros with an alcohol, in order to inactivate an enzyme contained therein. The persimmon extract may also contain at least condensed tannin.
Owner:HIROSHIMA UNIVERSITY +1
Production method for non-enveloped virus particles
ActiveUS10023846B2High purityNo laborious operationRecovery/purificationVector-based foreign material introductionBULK ACTIVE INGREDIENTActive ingredient
Provided are a production method for non-enveloped virus particles which is characterized in that a sample including non-envelopes virus particles is treated with PEG in at least two concentrations; a kit used in said production method; non-enveloped virus particles produced using said production method; and a pharmaceutical composition having the non-envelopes virus particles as an active ingredient.
Owner:TAKARA HOLDINGS
Antiviral method and composition
The invention relates to an antiviral method and a composition. The invention provides a method of inactivating non-enveloped virus particles. The method includes the step of contacting the virus with a virucidally-enhanced alcoholic composition that includes an alcohol, and enhancers selected from cationic oligomers and polymers, proton donors, chaotropic agents, and mixtures thereof.
Owner:GOJO IND INC
Viral inhibitor compositions for in vivo therapeutic use comprising a combination of (-) -carvone, geraniol and a further essential oil component
ActiveUS20130225676A1Avoid mergingEffectively treat and preventBiocideHydroxy compound active ingredientsDiseaseGeraniol
The present invention concerns an antiviral composition comprising the following components: R-(−)-2-methyl-5-(prop-1-en-2-yl)-cyclohex-2-enone (also called (−) carvone) and S-(+)-2-methyl-5-(prop-1-en-2-yl)-cyclohex-2-enone (also called (+) carvone) and (2E)-3,7-dimethylocta-2,6-dien-1-ol (also called trans-geraniol) in combination with at least one more component chosen among essential oils components for use in treatment and prevention of diseases caused by DNA enveloped viruses, DNA non-enveloped viruses, RNA enveloped viruses and RNA non-enveloped viruses.
Owner:CESA ALLIANCE
Process for the industrial-scale purification of gamma globulins from human plasma for industrial applications
ActiveUS8933204B2High yieldImprove stabilitySerum immunoglobulinsDepsipeptidesAnion-exchange chromatographyBlood plasma
The invention relates to a novel, industrial-scale process for the purification of gamma-immunoglobulins (IgG) starting from plasma or fractions thereof. The method involves two chromatographic steps, i.e. a cation exchange capture chromatography, and then a polishing anion exchange chromatography, ensuring a highly purified end product, which contains no aggregates, and high yields. The process also involves a virus inactivation step by means of a solvent / detergent treatment to inactivate the viruses with a lipid envelope, and a virus removal step by nanofiltering to ensure the removal of the non-enveloped viruses.
Owner:KEDRION
Anti-non-enveloped virus agent and composition containing same, and Anti-viral product and method for producing same
InactiveCN112638158ASuitable for inactivationBiocideAntifouling/underwater paintsAlkaline earth metalMedicine
The purpose of the present invention is to provide: an anti-viral agent suitable for the inactivation of a non-enveloped virus; a composition containing the anti-viral agent; an anti-viral product; and a method for producing the anti-viral product. The anti-non-enveloped virus agent according to the present invention contains at least one component selected from (A) a compound containing an Al element, an Mg element and an O element, (B) a compound containing an alkali metal element or an alkaline earth metal element, and also containing an Si element and an O element, and (C) a hydroxide containing a bivalent metal element.
Owner:TOAGOSEI CO LTD
Antiviral methods and compositions
The invention relates to an antiviral method and a composition. The invention provides a method of inactivating non-enveloped virus particles. The method includes the step of contacting the virus with a virucidally-enhanced alcoholic composition that includes an alcohol, and enhancers selected from cationic oligomers and polymers, proton donors, chaotropic agents, and mixtures thereof.
Owner:GOJO IND INC
Method for manufacturing non-enveloped virus
The present invention provides a method for efficiently manufacturing a non-enveloped virus with high purity without laborious operation by cultivating cells having the ability to produce a non-enveloped virus and bringing the cells and an acidic solution into contact with each other. A non-enveloped virus vector manufactured by the method of the present invention and a composition having the non-enveloped virus vector as an active ingredient are very useful as gene transfer methods in the fields of basic research and clinical application gene therapy.
Owner:TAKARA HOLDINGS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com