Patents
Literature
33 results about "Campylobacter coli" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Campylobacter coli is a Gram-negative, microaerophilic, non-endospore-forming, S-shaped bacterial species within genus Campylobacter, that appears in most stool sample with diarrhea. But that does not exclude some strains of E. coli that are known to be harmless and residents of the human gut.
Fourteen-food-borne pathogenic bacterium multiplex PCR detection primer set and kit
ActiveCN103484546ATo achieve the detection effectMicrobiological testing/measurementAgainst vector-borne diseasesBacteroidesEscherichia coli
The invention discloses a fourteen-food-borne pathogenic bacterium multiplex PCR detection primer set and a kit. The detection primer set is composed of primer pairs for detecting salmonella, Shigella, Vibrio parahemolyticus, campylobacter jejuni, campylobacter coli, staphylococcus aureus, bacillus cereus, Listeria monocytogenes, yersinia enterocolitica, enterobacter sakazakii, Escherichia coli, vibrio cholerae, Escherichia coli O157, aeromonas hydrophila and internal positive control. A multiplex PCR detection method based on an ordinary PCR platform is built, multiplex PCR reactions are carried out on genomic DNA, extracted from a sample to be tested, of bacteria in the same reaction system through the primer pairs acquired through analysis and design, and whether the food-borne pathogenic bacteria are contained in the sample or not is judged through the electrophoretic analysis of reaction products.
Owner:北京卓诚惠生生物科技股份有限公司
Salmonella enterica presenting c. jejuni n-glycan or derivatives thereof
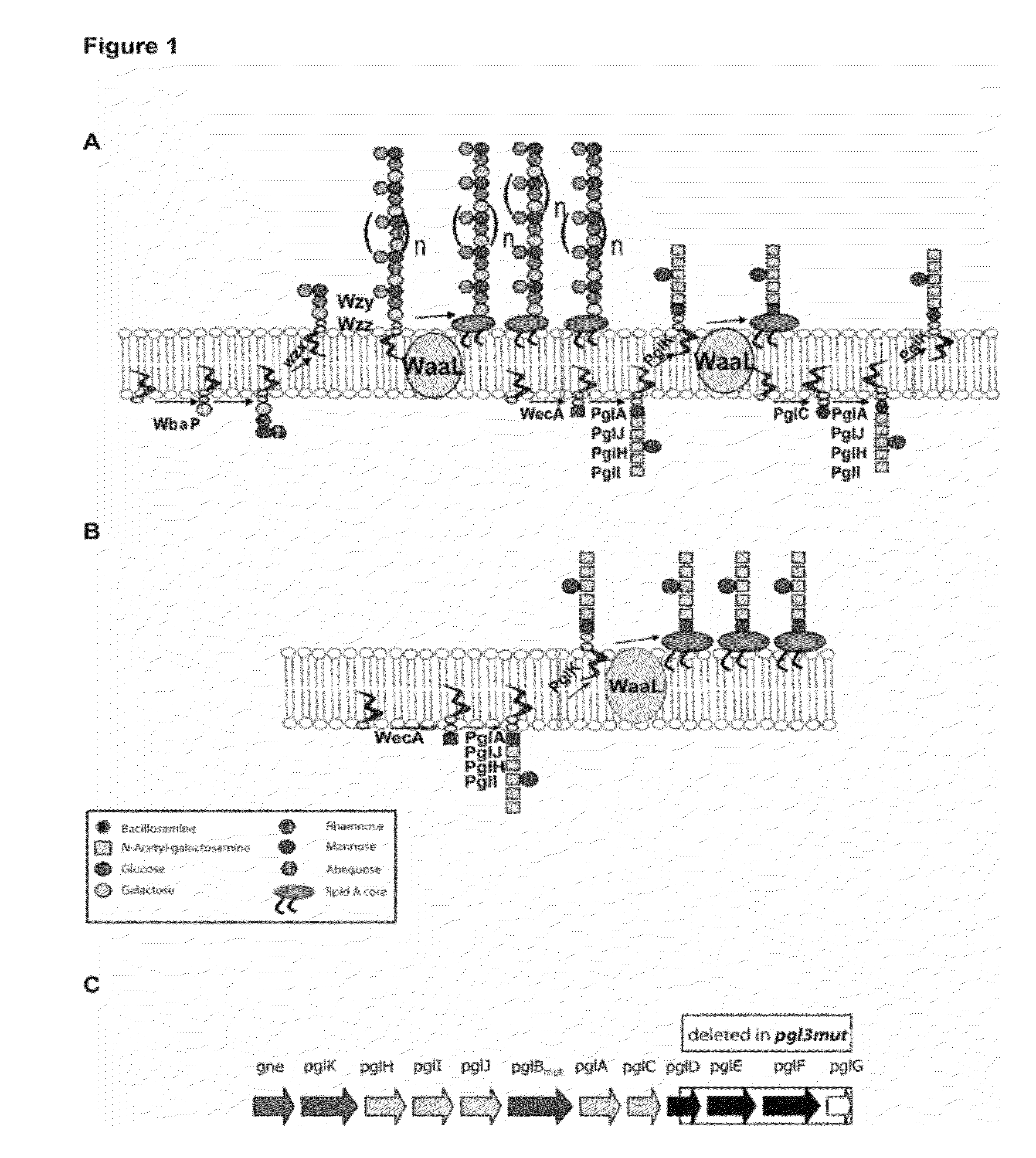
The present invention relates to Salmonella enterica comprising at least one pgl operon of Campylobacter jejuni or a functional derivative thereof and presenting at least one N-glycan of Campylobacter jejuni or N-glycan derivative thereof on its cell surface. In addition, it is directed to medical uses and pharmaceutical compositions thereof as well as methods for treating and / or preventing Campylobacter and optionally Salmonella infections and methods for producing these Salmonella strains.
Owner:ETH ZZURICH
Double-antibody sandwich ELISA (enzyme-linked immuno sorbent assay) method for detecting listeria in food on basis of monoclonal antibodies
ActiveCN105527441ANo cross reactionImmunoglobulins against bacteriaMaterial analysisEscherichia coliImmune profiling
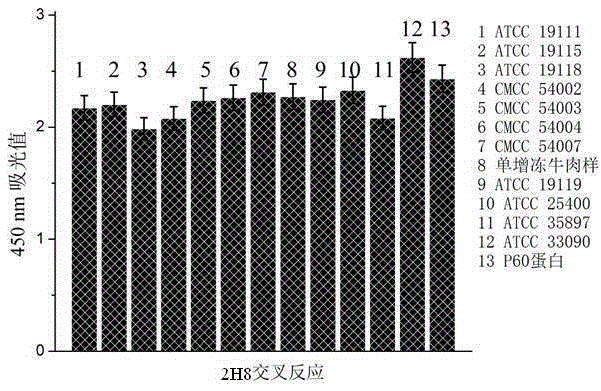
The invention discloses a double-antibody sandwich ELISA (enzyme-linked immuno sorbent assay) method for detecting listeria in food on the basis of monoclonal antibodies, belonging to the technical field of immunoassay. The method comprises the following steps: immunizing 8-week BALB mouse with prokaryotically expressed listeria monocytogenes P60 protein, and obtaining 15 strains of listeria specific monoclonal antibodies by immunization, fusion and multiple screening; respectively marking horse radish peroxidase HRP, and carrying out pairwise coupling by taking the listeria monocytogenes CMCC54003 as targets. The sandwich ELISA method which is established by screening different paired cross reactions and taking 2G7 as a coating antibody and 2H8-HRP as an enzyme labelled antibody has cross reactions to listeria including the listeria monocytogenes, and has no cross reactions to tested staphylococcus aureus, escherichia coli, escherichia coli O157, salmonella, enterobacter sakazakii, campylobacter jejuni and campylobacter coli. According to the method disclosed by the invention, the monoclonal antibodies having listeria specificity are prepared and the double-antibody sandwich ELISA method is established, and the method provides a technical means for specific rapid detection of the listeria in food.
Owner:JIANGNAN UNIV
Methods for detecting Campylobacter bacteria or antibodies to Campylobacter bacteria with an immunoassay
This invention relates generally to in vitro methods for inducing or enhancing expression of enteric bacterial antigens and / or virulence factors thereby producing antigenically enhanced enteric bacteria, to methods for using antigenically enhanced enteric bacteria and to vaccines comprising antigenically enhanced enteric bacteria. A Campylobacter bacterium having enhanced antigenic property is disclosed. A culture medium useful for culturing the Campylobacteria comprises 0.05% to 3% bile or 0.025% to 0.6% of one or more bile acids or salts. In addition a divalent cation chelator can also be included in the culture medium. The bacteria culture is in a growth phase at early log phase and between early log phase and stationary phase. The enhanced antigenic property is a higher level of an immunogenic antigen when compared to the antigenic property of the same bacteria grown on brain heart infusion broth. Also, antibodies to Campylobacter bacteria or Campylobacteria in samples are detected by immunoassays.
Owner:BIOPORT R&D
Campylobacteria sixtuple PCR detection kit
InactiveCN105695585AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesTrue positive rateMicrobiology
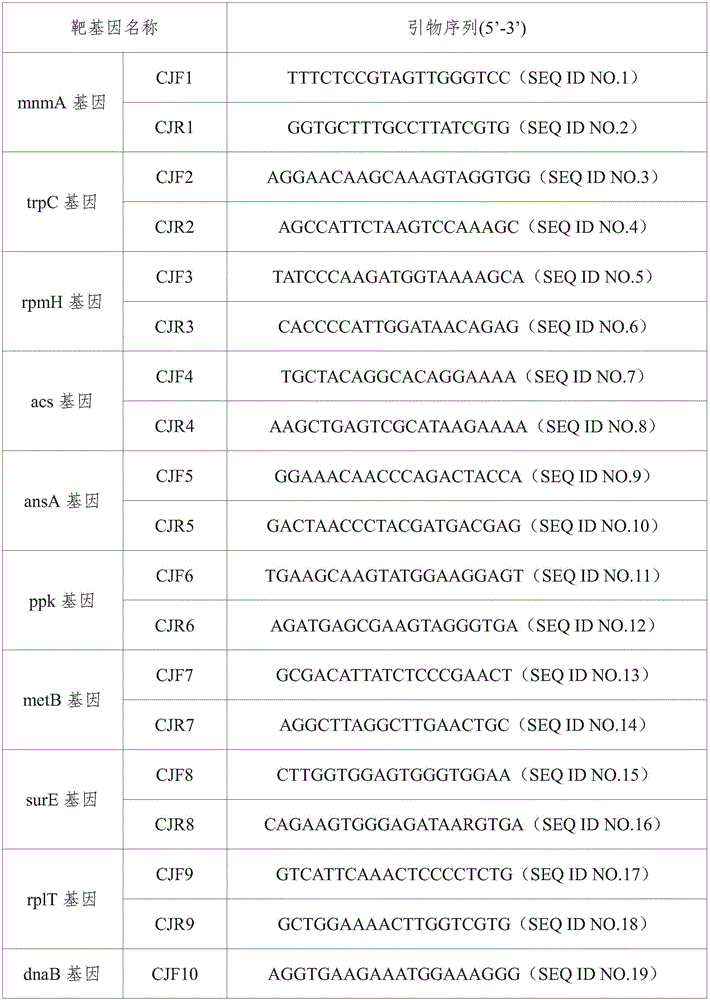
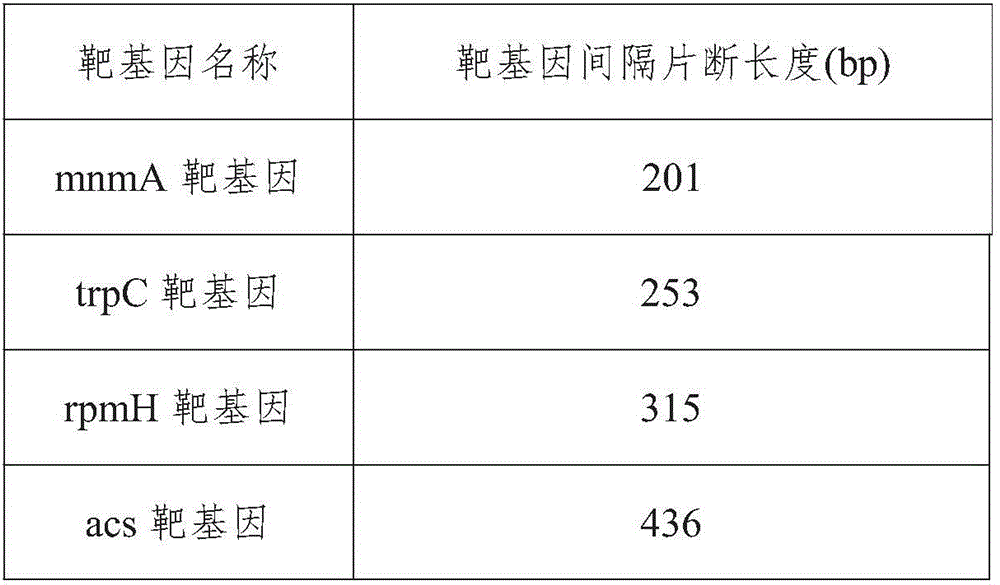
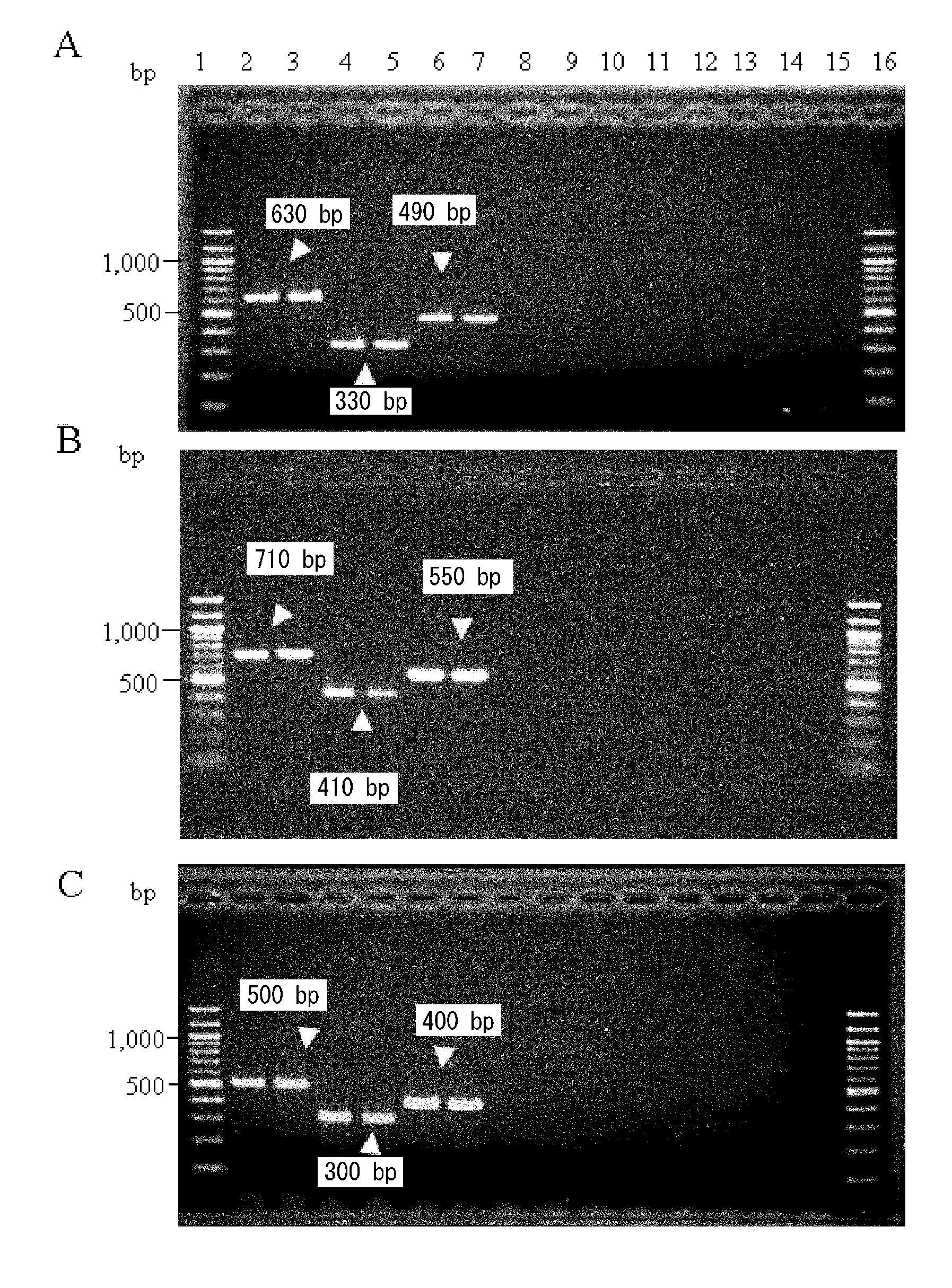
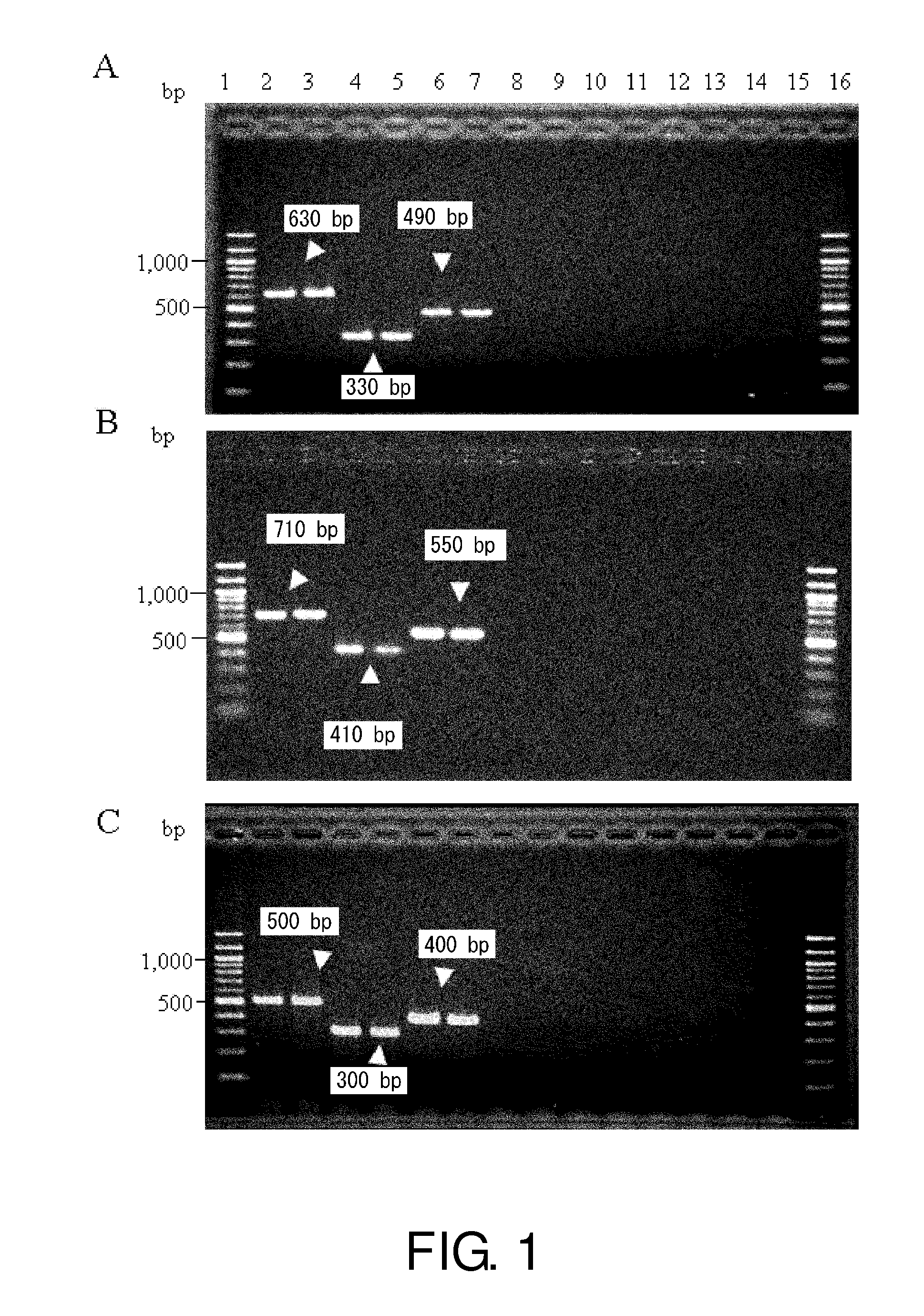
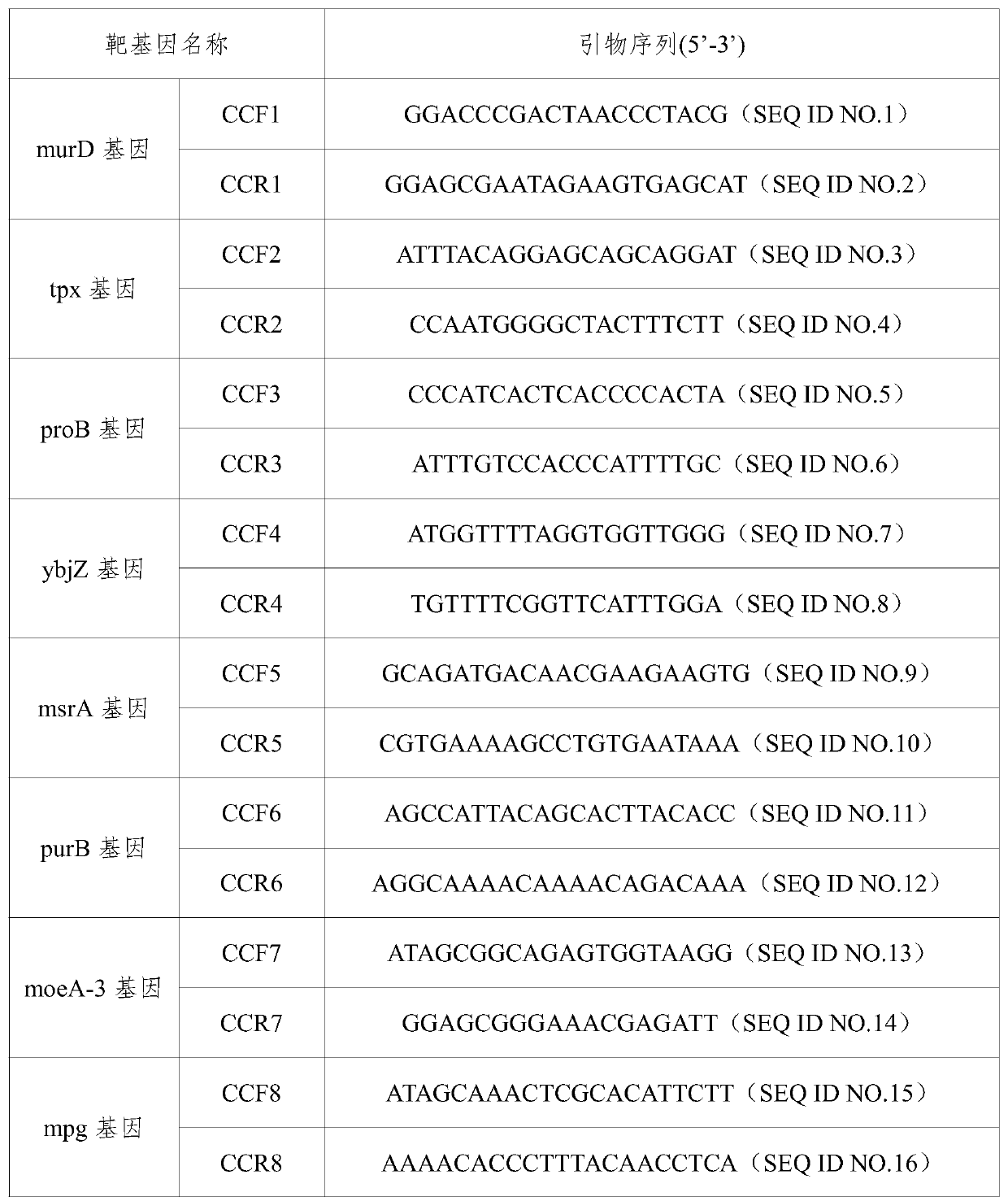
The invention provides a campylobacteria sixtuple PCR detection kit, and belongs to the technical field of bacterium PCR detection. The kit contains six pairs of primers which have nucleotide sequences shown in SEQ ID NO.1 to SEQ ID NO.12. The kit can authenticate whether a to-be-detected strain is campylobacteria or not and authenticate the five frequently-seen strains of campylobacteria including campylobacter jejuni, campylobacter coli, campylobacter fitus, campylobacter loridis and campylobacter liensis. The kit has sensitivity and specificity of 100% on detecting the five strains of campylobacteria, is easy to operate, greatly reduces detection cost and operation difficulty, and has good clinical application value.
Owner:ICDC CHINA CDC
Cytolethal distending toxins and detection of campylobacter bacteria using the same as a target
ActiveUS20100047797A1Quick checkRapid and convenient determinationSugar derivativesMicrobiological testing/measurementEscherichia coliTyping
The present inventors succeeded in cloning the CDT genes of C. coli and C. fetus, which were previously unknown, and in determining their sequences. In addition, the inventors also developed specific primers and primers common to the two species by comparing the CDTs of C. jejuni and C. fetus. Furthermore, the inventors demonstrated that these primers were applicable to multiplex PCR that simultaneously allows for the rapid and convenient determination of the presence of Campylobacter CDT and identification of species, and that they can also be used in PCR-RFLP-based typing.
Owner:FUSO PHARMA INDS
Antibodies against flagellin and uses thereof
InactiveUS20090191208A1Avoid loweringIncrease intakeSnake antigen ingredientsImmunoglobulins against bacteriaEscherichia coliCitrobacter
The present invention provides a novel class of monoclonal antibodies which have a high affinity, broad spectrum neutralizing reactivity to flagellin from various Gram-negative bacteria including, but not limited to, E. coli, Salmonella, Serratia, Proteus, Enterobacter, Citrobacter, Campylobacter and Pseudomonas. The present invention further provides methods of treating inflammatory bowel disease (IBD) and methods of treating enterobacterial infections using anti-flagellin antibodies in humans, other animals and birds.
Owner:INOTECK PHARMA CORP
Campylobacter Jejuni Outer Membrane Protein Immunogenic Composition
InactiveUS20080131453A1Reduce probabilityBacterial antigen ingredientsImmunoglobulins against bacteriaEpitopeMicrobiology
The field of this invention is the development of therapeutic agents having immunogenic efficacy against Campylobacter. The present invention is directed to a method of producing monoclonal antibodies that are highly specific for epitopes of Campylobacter jejuni outer membrane proteins; to specific monoclonal antibodies made by using the epitopes; and to uses thereof. The invention is drawn further to immunogens comprising certain outer membrane proteins or portions thereof from C. jejuni.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Mammalian animal compositon
InactiveUS20060134082A1Reduce and prevent Campylobacter infectionReduces and prevents zoonotic riskBiocideBacteria material medical ingredientsMicroorganismMicrobiology
The present invention relates to the use of probiotic microorganism in the manufacture of a composition for the prevention or reduction of gastrointestinal Camplylobacter infection in a mammalian animal. It also relates to a method for the prevention or reduction of gastrointestinal Campylobacter infection in a mammalian animal, the method comprising administering to said animal, a probiotic microorganism. The invention also relates to a probiotic microorganism, for use in preventing or reducing gastrointestinal Campylobacter infection in a mammalian animal.
Owner:MARS INC
Primer and method for fast typing campylobacter jejuni
ActiveCN105886622AAccurate typingGood typing abilityMicrobiological testing/measurementMicroorganism based processesDiseaseMicroorganism
The invention belongs to the field of microorganism molecular typing and particularly relates to a primer and a method for fast typing campylobacter jejuni. The primer includes 13 pairs of campylobacter jejuni primers. The primer is good in typing capacity, high in distinguishing index and capable of accurately typing the campylobacter jejuni. In addition, the method for fast typing the campylobacter coli is simple and fast to operate, good in stability and repeatability, capable of effectively assisting the clinical diagnosis of diseases infected by the campylobacter jejuni and ideal.
Owner:GUANGDONG PHARMA UNIV
Detection Of Bacteria Belonging to the Genus Campylobacter By Targeting Cytolethal Distending Toxin
ActiveUS20100248238A1Microbiological testing/measurementAgainst vector-borne diseasesEscherichia coliBacteroides
Multiplex PCR primers that can amplify the cdt genes of C. jejuni, C. coli, and C. fetus in a bacterial species-specific manner were prepared. Multiplex PCR with the primers was assessed using Campylobacter bacteria, other cdt gene-positive bacteria, and representative bacteria responsible for enteric infection. As a result, the present inventors' multiplex PCR using cdtB amplification primers was proven to enable simultaneous detection of different Campylobacter bacteria with high specificity. The methods of the present invention can identify Campylobacter at the bacterial species level in a single manipulation even when domestic animals or humans are infected with different bacterial species of Campylobacter.
Owner:FUSO PHARMA INDS +1
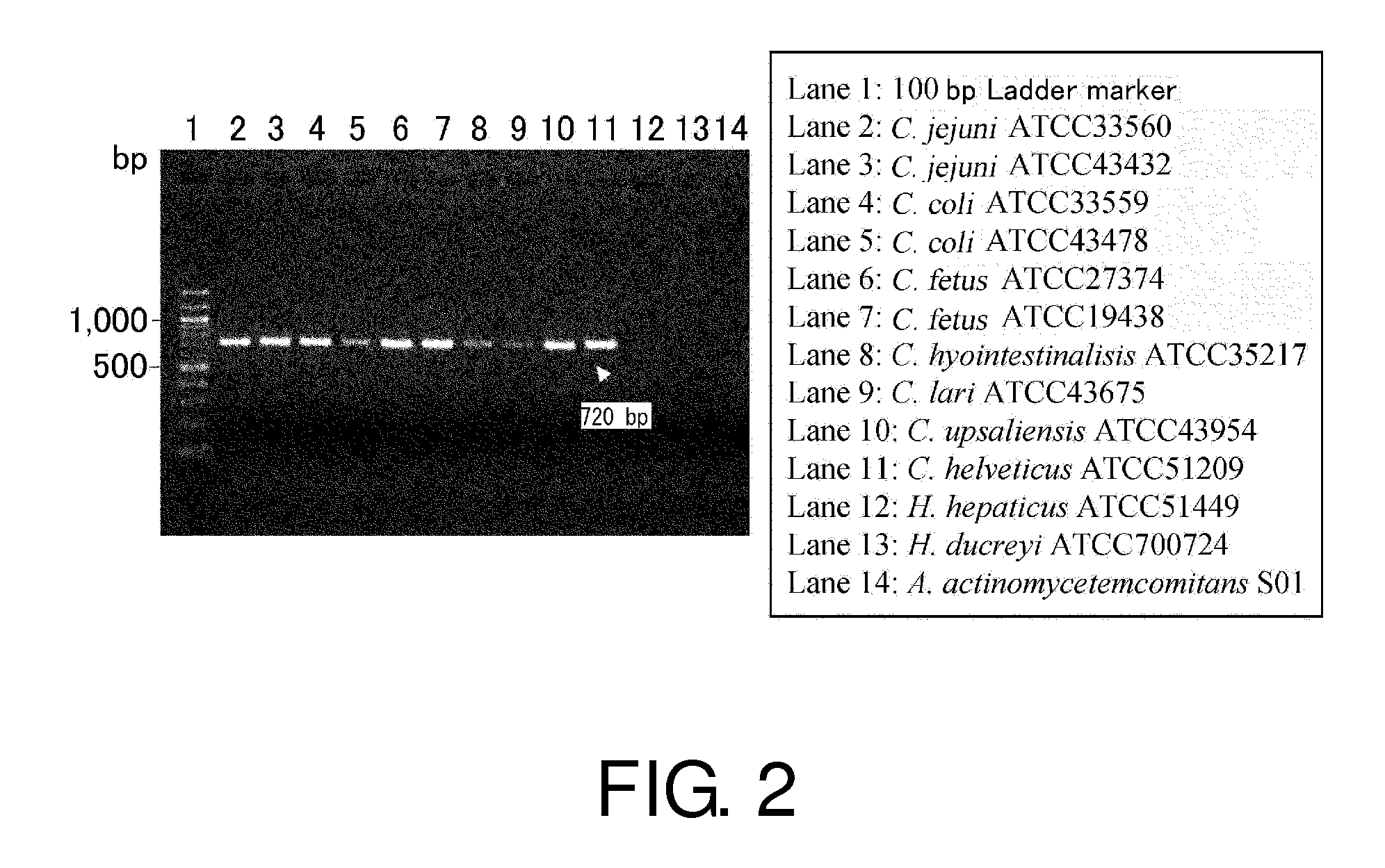
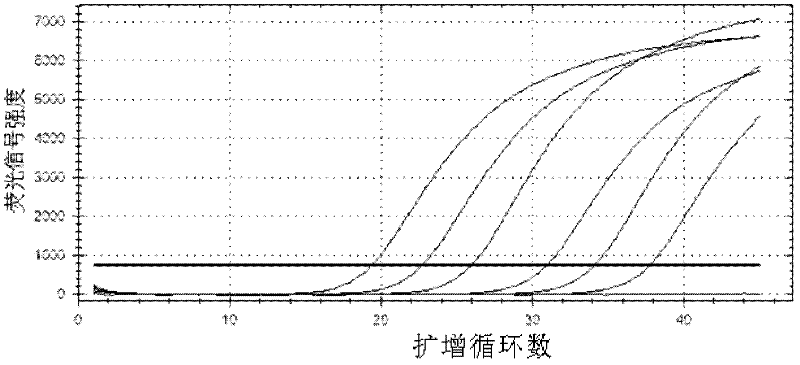
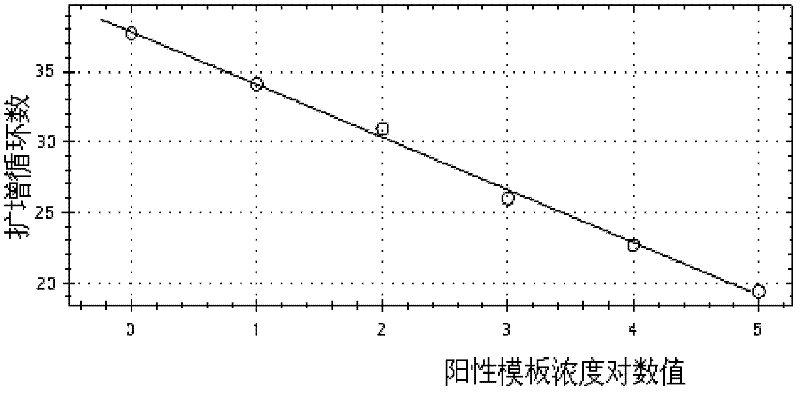
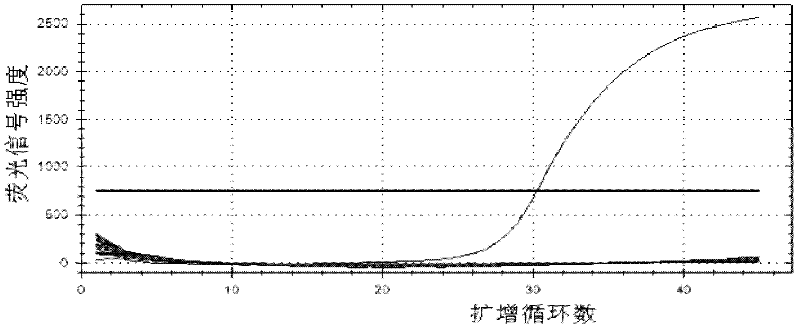
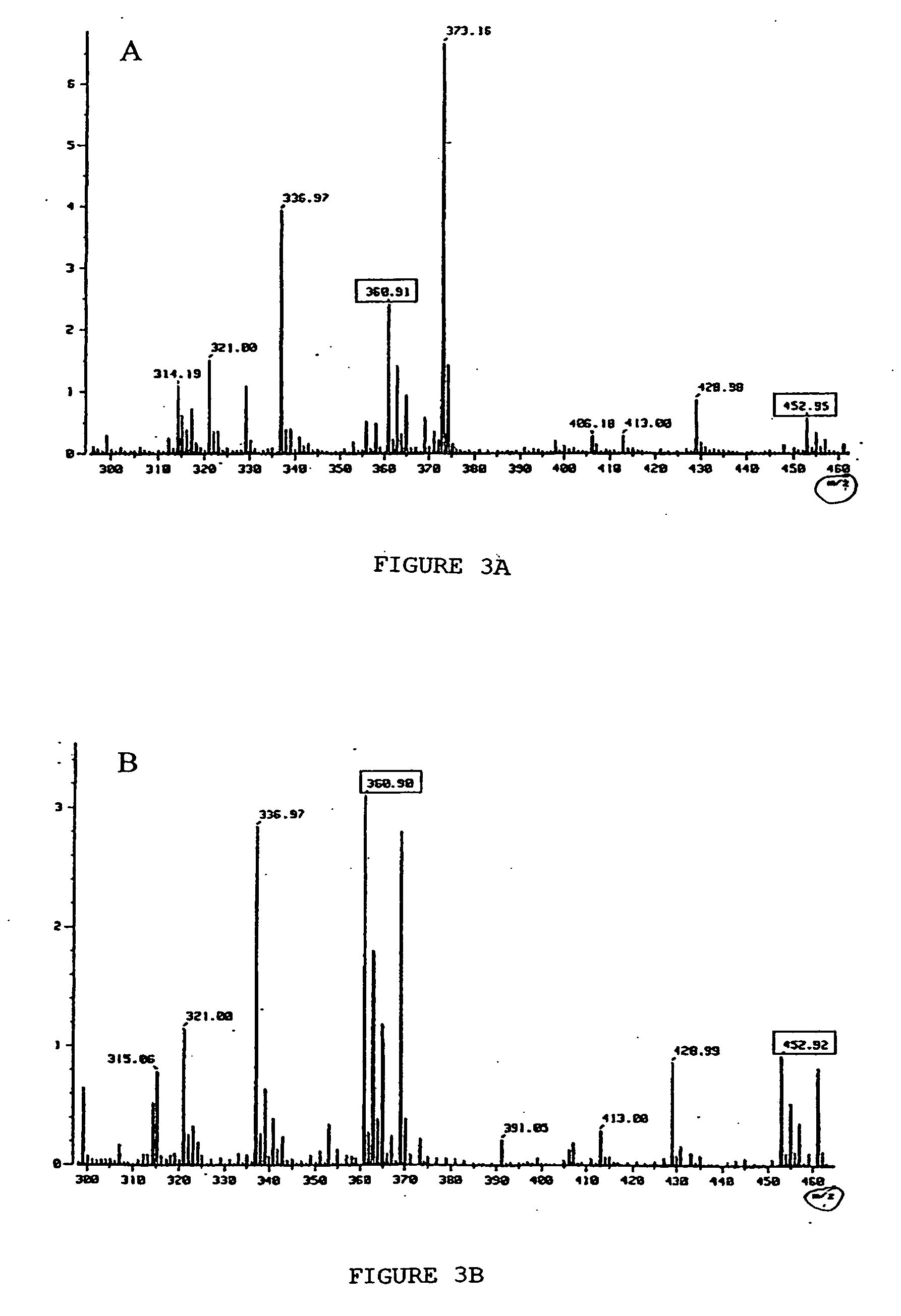
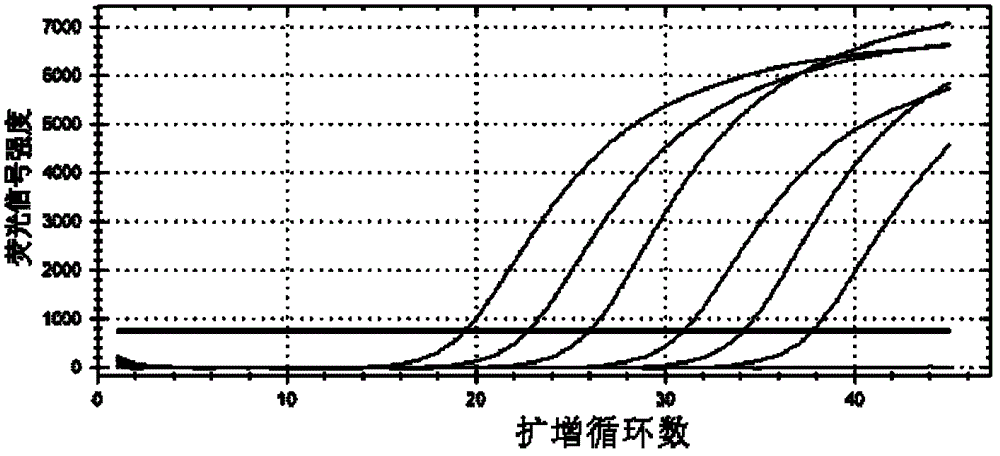
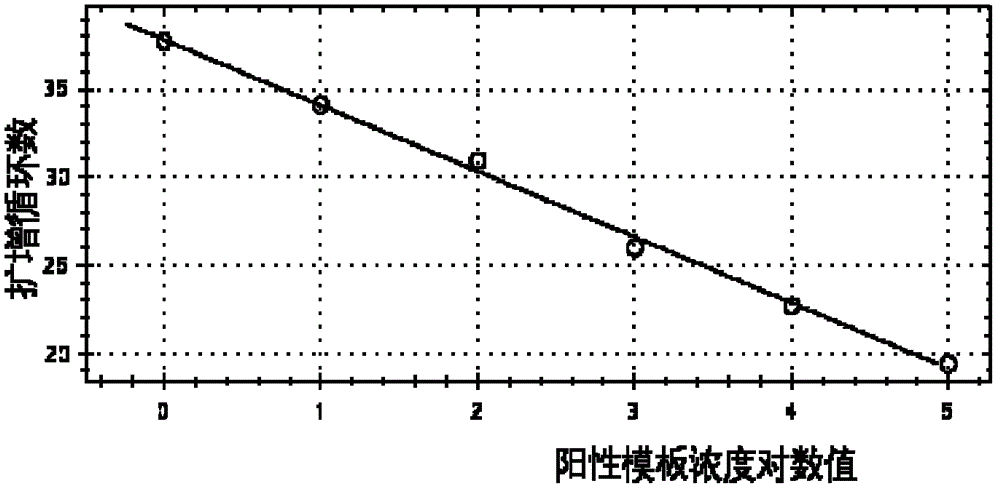
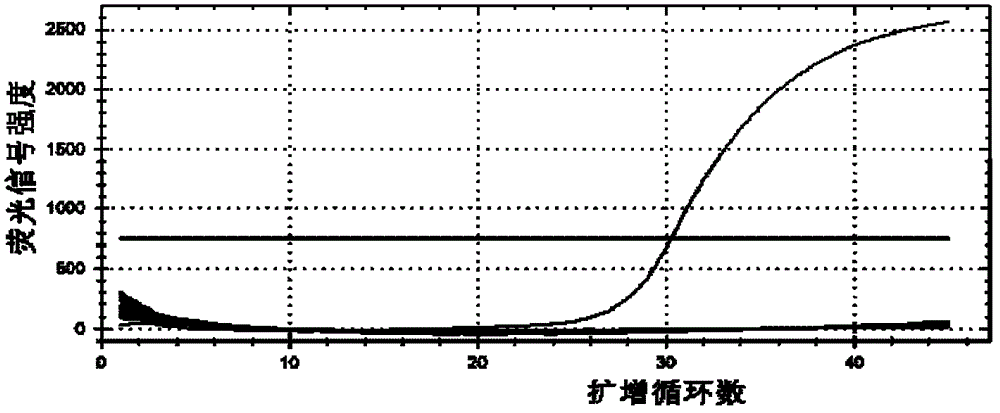
Primers, probes, methods and kits for detecting Campylobacter coli
InactiveCN102268479AHigh detection sensitivityGood repeatabilityMicrobiological testing/measurementFluorescence/phosphorescenceFluorescencePolymerase chain reaction
The invention provides real-time fluorescence quantitative PCR (Polymerase Chain Reaction) primers and probe for detecting campylobacter coli. The sequences of the primers and probe are respectively shown in SEQ ID NO.2, SEQ ID NO.3 and SEQ ID NO.4. The invention also provides a method for carrying out fluorescence quantitative PCR detection on campylobacter coli by using the primers and probe, and a kit for detecting campylobacter coli. The detection method and the kit for detecting campylobacter coli provided by the invention have the advantages of accurate detection, high sensitivity, strong specificity, simplicity, convenience, quickness and good clinical specimen detection capability.
Owner:ICDC CHINA CDC
Method for detecting a microorganism in a fecal specimen
The invention relates to detecting microorganisms in specimens for the purposes of inter alia diagnoses, screenings, quarantine inspections, and clinical tests. Specifically, it relates to detecting and quantifying enteropathogenic bacteria in a fecal specimen, including Shigella species, Salmonella species, Campylobacter species, enterohemorrhagic E. coli or Verocytotoxin-producing E. coli, Vibrio cholerae, and Clostridium perfringens. Provided is a method for detecting a microorganism in a fecal specimen, wherein the method comprises preparing a 25-50% (wt / vol) fecal lysate, optionally followed by isolating a nucleic acid from the fecal lysate; subjecting the nucleic acid to a nucleic acid amplification assay using a set of at least two nucleic acid amplification primers specific for the microorganism and detecting amplified nucleic acid to determine the presence of the microorganism in the specimen.
Owner:STICHTING LABUM VOOR INFECTIEZIEKTEN
Chicken meat product and method of making
A chicken meat product rendered substantially free of pathogenic bacterial species by having an external coating of sodium diacetate substantially free of an adhesive material. The wet coating is retained on the external surfaces of the dressed chicken meat by the surface tension of the sodium diacetate solution after the chicken meat is coated with the solution and is hung freely to permit excess liquid to drain away. The amount of sodium diacetate present in the product of the invention depends upon the degree of pathogenic bacterial contamination of the chicken meat. The sodium diacetate is included in each case in sufficient quantity to preserve the meat from growth of all pathogenic bacteria of the order and species of salmonella, E. coli, and campylobacter.
Owner:GLABE ELMER F
Chicken meat product
InactiveCN1291079AMeat/fish preservation by coatingMeat/fish preservation using chemicalsEscherichia coliSodium diacetate
A chicken meat product rendered substantially free of pathogenic bacterial species by having an external coating of sodium diacetate substantially free of an adhesive material. The wet coating is retained on the external surfaces of the dressed chicken meat by the surface tension of the sodium diacetate solution after the chicken meat is coated with the solution and is hung freely to permit excess liquid to drain away. The amount of sodium diacetate present in the product of the invention depends upon the degree of pathogenic bacterial contamination of the chicken meat. The sodium diacetate is included in each case in sufficient quantity to preserve the meat from growth of all pathogenic bacteria of the order and species of salmonella, E. coli, and campylobacter.
Owner:埃尔默・F・格拉贝
Campylobacter Immunogenic Compositions and Uses Thereof
ActiveUS20160184422A1Reduce colonizationAntibacterial agentsBacterial antigen ingredientsMicrobiologyImmunogenicity
The present invention provides immunogenic compositions against Campylobacter and methods for using the immunogenic composition to generate an immune response against Campylobacter and / or reduce intestinal colonization by Campylobacter.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
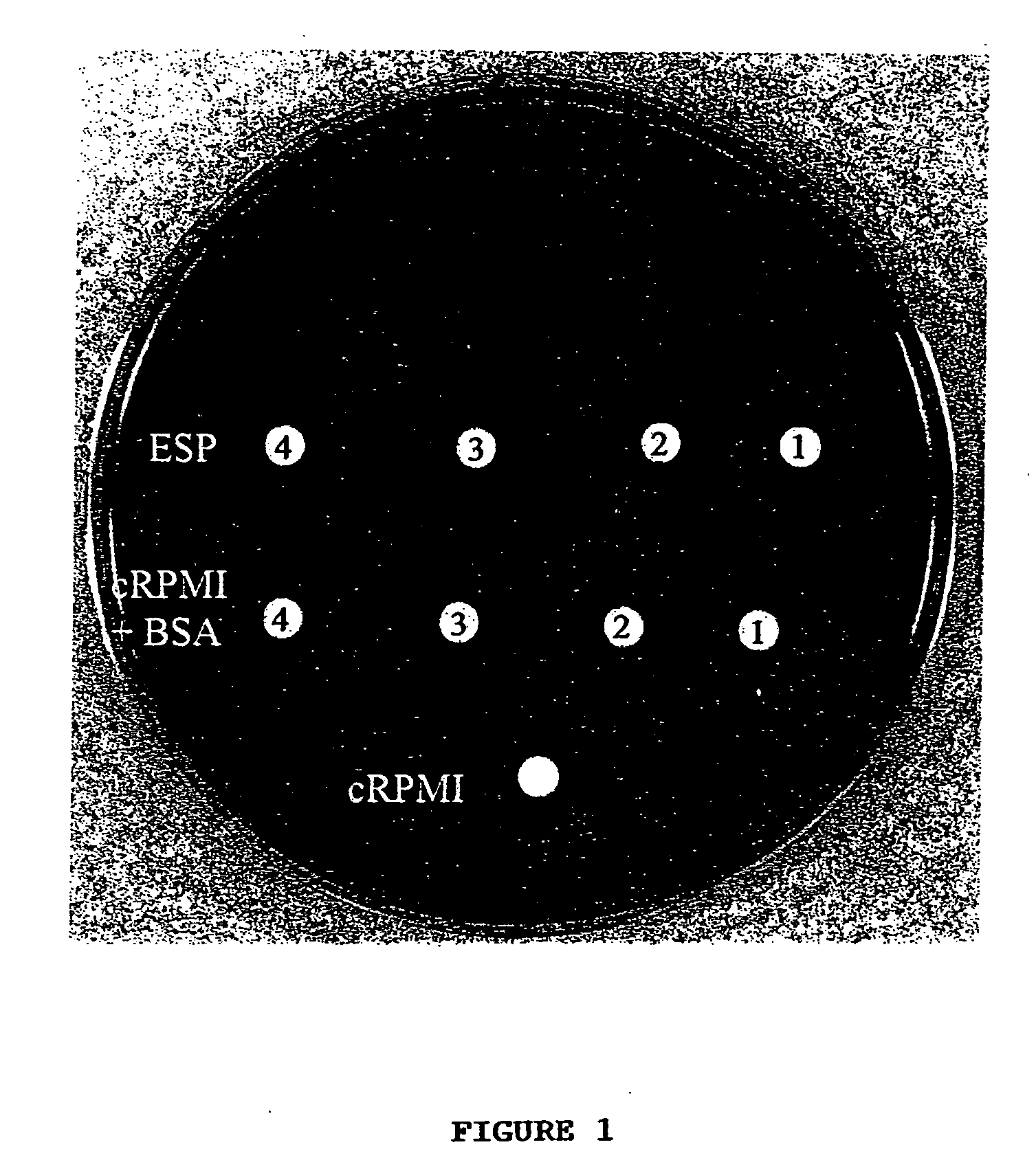
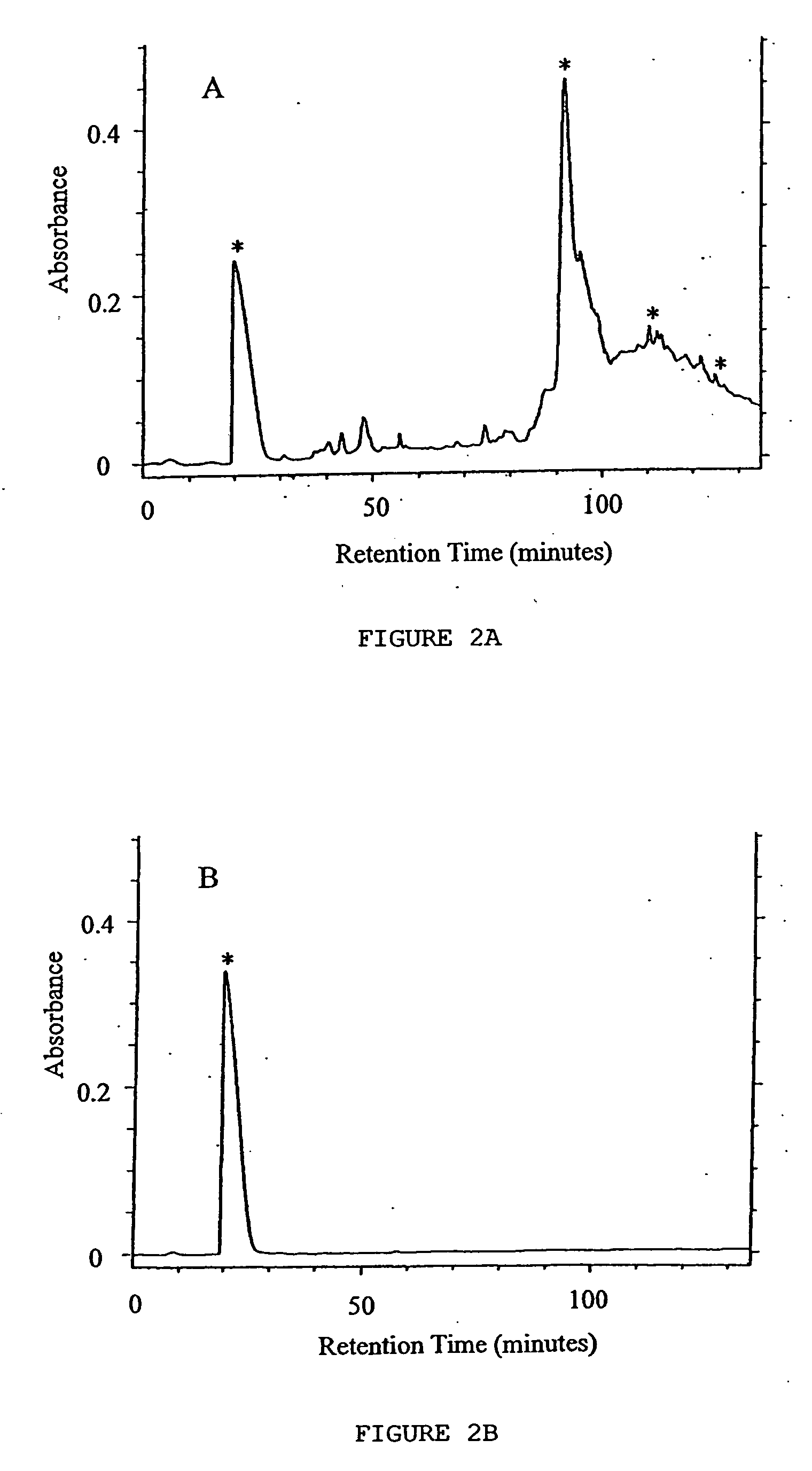
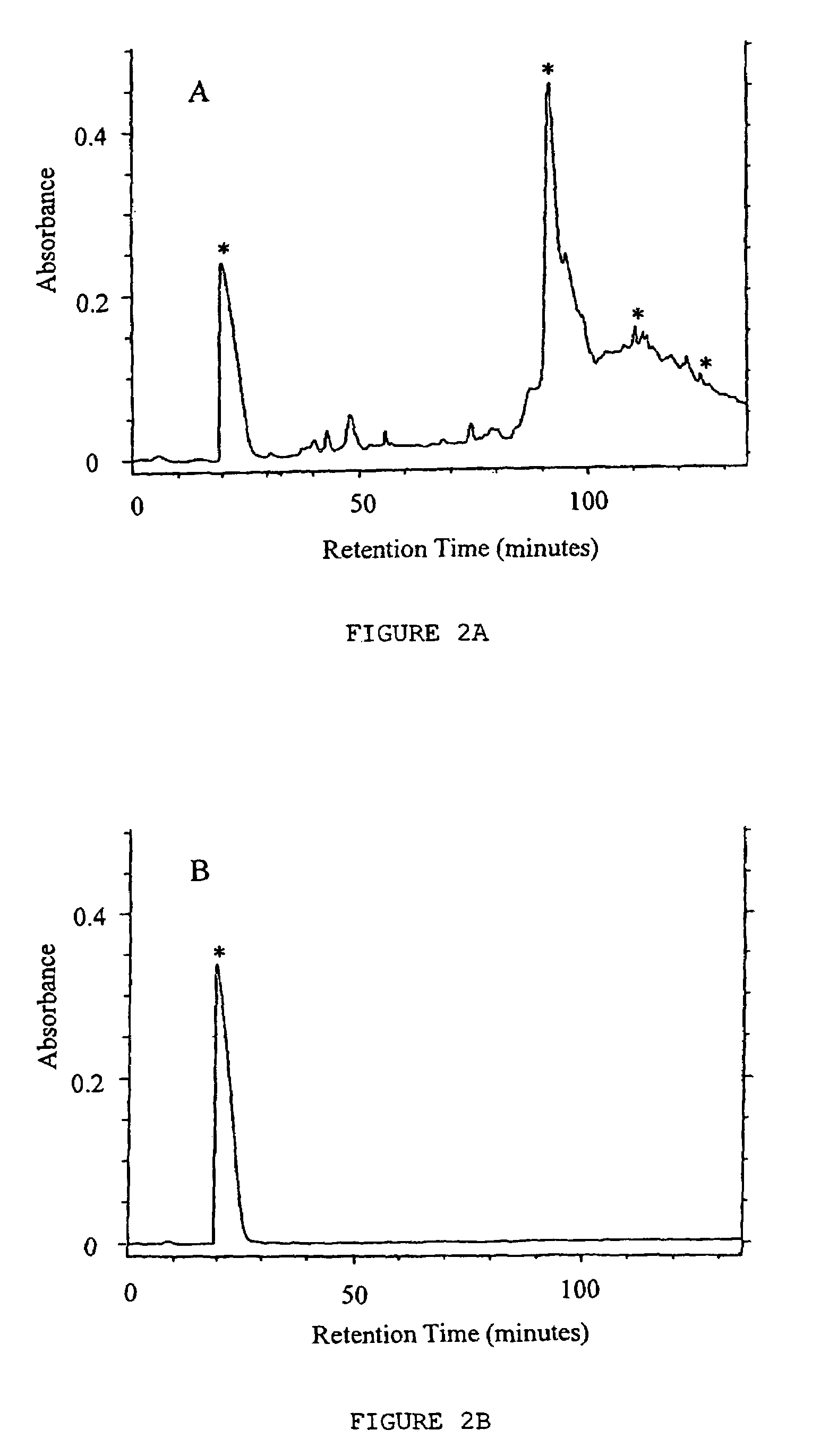
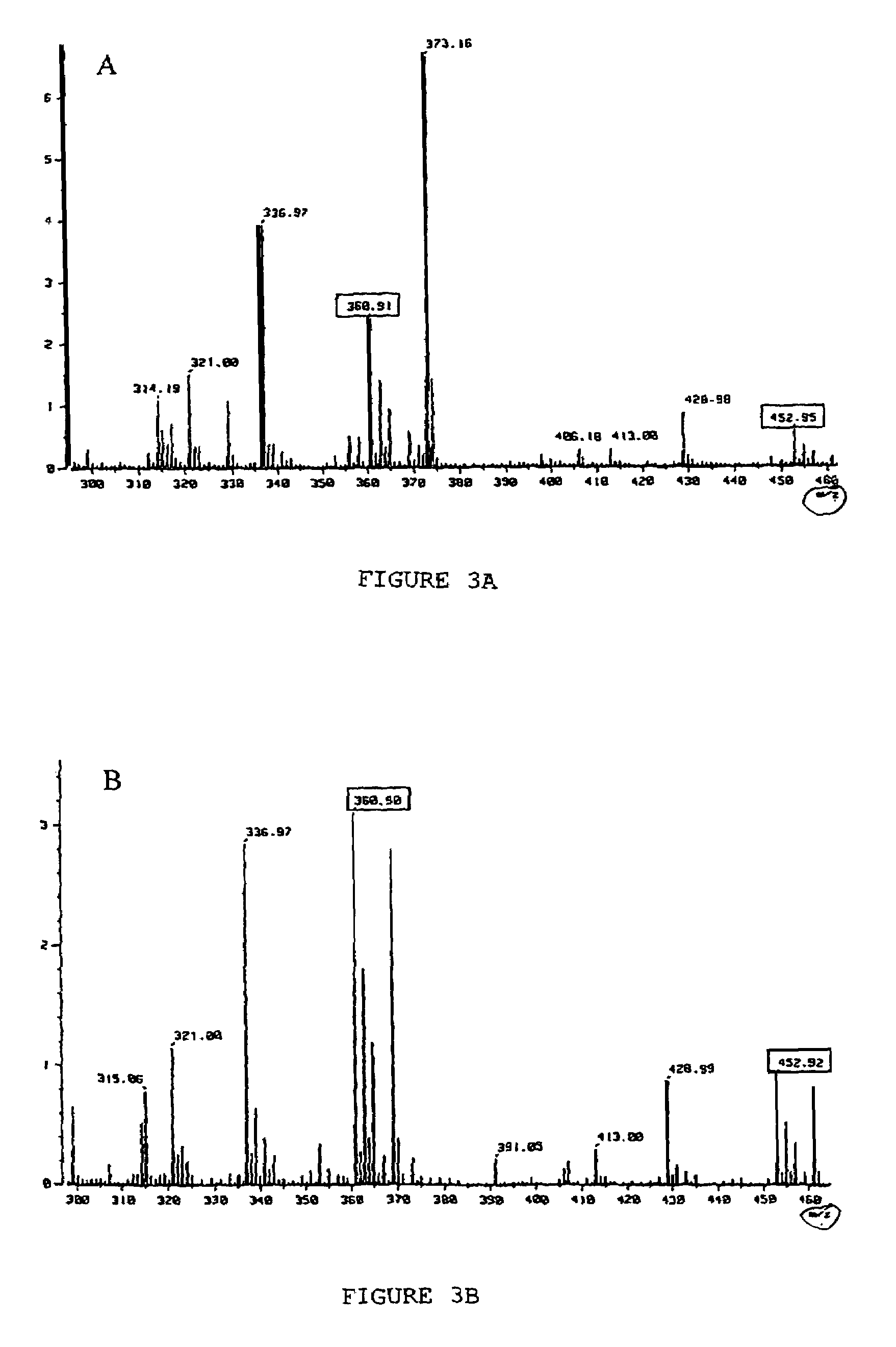
Detection of antibacterial activity in excretory secretory product of adult trichuris suis
The present invention provides a heat-stable and protease-resistant antibacterial activity in excretory-secretory products (ESP) of Trichuris suis. The antibacterial activity is not more than 10,000 MW; is resistant to boiling, trypsin, and pronase E; has a bactericidal mode of action; and is effective against Gram positive and Gram negative bacteria, including Escherichia coli, Campylobacter jejuni, Campylobacter coli, and Staphylococcus aureus. The antibacterial activity is useful in applications for killing or inhibiting the growth of microorganisms, in particular bacteria.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Primers, probe, method and kit for detecting campylobacter coli
InactiveCN102268479BIncrease positive rateHigh detection sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceBiochemistryCampylobacter coli
The invention provides real-time fluorescence quantitative PCR (Polymerase Chain Reaction) primers and probe for detecting campylobacter coli. The sequences of the primers and probe are respectively shown in SEQ ID NO.2, SEQ ID NO.3 and SEQ ID NO.4. The invention also provides a method for carrying out fluorescence quantitative PCR detection on campylobacter coli by using the primers and probe, and a kit for detecting campylobacter coli. The detection method and the kit for detecting campylobacter coli provided by the invention have the advantages of accurate detection, high sensitivity, strong specificity, simplicity, convenience, quickness and good clinical specimen detection capability.
Owner:ICDC CHINA CDC
Triplex PCR detection primers and triplex PCR detection kit for cytolethal distending toxins A, B and C of Campylobacter
ActiveCN108913794AQuick checkAccurate detectionMicrobiological testing/measurementMicroorganism based processesToxin typesCytolethal distending toxin
The invention discloses triplex PCR detection primers and a triplex PCR detection kit for cytolethal distending toxins A, B and C of Campylobacter. The Campylobacter involved in the invention comprises Campylobacter coli, Campylobacter jejuni and Campylobacter fitus, and the nucleotide sequences of corresponding primers thereof are as shown in SEQ ID No. 1 to SEQ ID No. 18. A triplex PCR detectionmethod provided by the invention can quickly and accurately detect the cytolethal distending toxins of Campylobacter, and can realize detection of a single toxin or all the three toxins selected fromthe cytolethal distending toxins A, B and C of Campylobacter in one operation, so time and effort are saved, and the detection can be completed in on shot without three PCR operations and three electrophoresis operations; a conventional single PCR method usually takes 2-3 days, and the triplex PCR detection method of the invention shortens detection time to 4 h, so work efficiency is greatly improved; and the method can be used for general investigation of the toxin types of Campylobacter, molecular epidemiological investigation and vaccine screening and detection.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Pyrococcus furiosus strains and methods of using same
InactiveUS20120135411A1MicroorganismsMicrobiological testing/measurementOrigin of replicationPolynucleotide
Provided herein are methods for transforming a Pyrococcus furiosus with a polynucleotide. In one embodiment, the method includes contacting a P. furiosus with a polynucleotide under conditions suitable for uptake of the polynucleotide by the P. furiosus, and identifying transformants at a frequency of, for instance, at least 103 transformants per microgram DNA. Also provided are isolated Pyrococcus furiosus having the characteristics of Pyrococcus furiosus COM1, and plasmids that include an origin of replication that functions in a Pyrococcus furiosus. The plasmid is stable in a recipient P. furiosus without selection for more than 100 generations and is structurally unchanged after replication in P. furiosus for more than 100 generations.
Owner:UNIV OF GEORGIA RES FOUND INC
Kit for simultaneous detection of multiple food-borne pathogenic bacteria and its application
ActiveCN107502672BQuick screeningPrecise screeningMicrobiological testing/measurementMicroorganism based processesBiotechnologyDisease
The invention discloses a kit capable of simultaneously detecting various food-borne pathogenic bacteria and an application of the kit, and belongs to the technical field of detection of food-borne pathogenic bacteria. The kit comprises parallel detection gene chips of the food-borne pathogenic bacteria fixed 19 pathogenic bacterium specificity probes, specific primers and universal primers. According to the kit, 19 typical food pathogenic bacteria such as campylobacter coli, campylobacter jejuni and enterobacter sakazakii can be simultaneously, rapidly and sensitively detected through reaction once, and detection data combined multiple amplification and multiple detection which cannot be provided by a traditional method are provided. According to a detection method, the time from sample treatment to generation of detection results only needs about 1-2 hours, accuracy rate is 100%, the method is simple in operation step, short in detection time, high in specificity, high in sensitivity and good in repeatability, 19 pathogenes causing foodborne diseases can be accurately detected in a classification manner, and the food-borne pathogenic bacteria are rapidly screened.
Owner:JINAN KAICHEN BIOTEC
Campylobacter Vaccines and Methods of use
InactiveUS20100074846A1Eliminate health hazardsProtect normal transmissionAntibacterial agentsBacterial antigen ingredientsDiseaseBacterial Gastritis
Porcine models for studying bacterial gastritis and gastric and duodenal ulcer disease caused by Campylobacter pathogens, such as C. coli are described, as well as methods of identifying vaccines and compounds for treating and / or preventing Campylobacter infection using the animal models. Also described are methods of preventing Campylobacter infection in swine, such as infection caused by C. coli, using immunogenic proteins and nucleic acids derived from Campylobacter pathogens, such as C. coli.
Owner:CEREBUS BIOLOGICALS
Plankton metabolite, plankton fermentation solution and application thereof
ActiveCN109431962AAnti-inflammatoryMuscle-building and lipid-loweringCosmetic preparationsAntipyreticMetaboliteFermentation
The invention provides a plankton metabolite. The plankton metabolite is obtained through taking mixed thalli of flavobacterium aquatile, rhizomonas and campylobacter as fermentation bacteria, and a water solution of algal polysaccharide as a culture medium, and fermenting. The invention further provides a plankton fermentation solution containing the plankton metabolite; the plankton metabolite and the plankton metabolite have the effects of resisting inflammations, increasing muscles and lowering lipid, remodeling a facial form and tightening skin.
Owner:仙婷(广州)科技研发有限公司
Campylobacter bacteria and detection
InactiveUS20150284674A1Attenuated or inactivatedStimulate immune responseBacterial antigen ingredientsSugar derivativesBacteroidesNucleotide
There is provided a polynucleotide comprising a sequence at least 98.5% identical to SEQ ID NO: 1 and cells comprising this sequence, for example ECACC Deposit Reference 12022102. The polynucleotides and cells are useful in the detection of spotty liver syndrome and spotty liver disease.
Owner:THE UK SEC FOR ENVIRONMENT FOOD & RURAL AFFAIRS
Detection of antibacterial activity in excretory secretory product of adult Trichuris suis
The present invention provides a heat-stable and protease-resistant antibacterial activity in excretory-secretory products (ESP) of Trichuris suis. The antibacterial activity is not more than 10,000 MW; is resistant to boiling, trypsin, and pronase E; has a bactericidal mode of action; and is effective against Gram positive and Gram negative bacteria, including Escherichia coli, Campylobacter jejuni, Campylobactercoli, and Staphylococcus aureus. The antibacterial activity is useful in applications for killing or inhibiting the growth of microorganisms, in particular bacteria.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
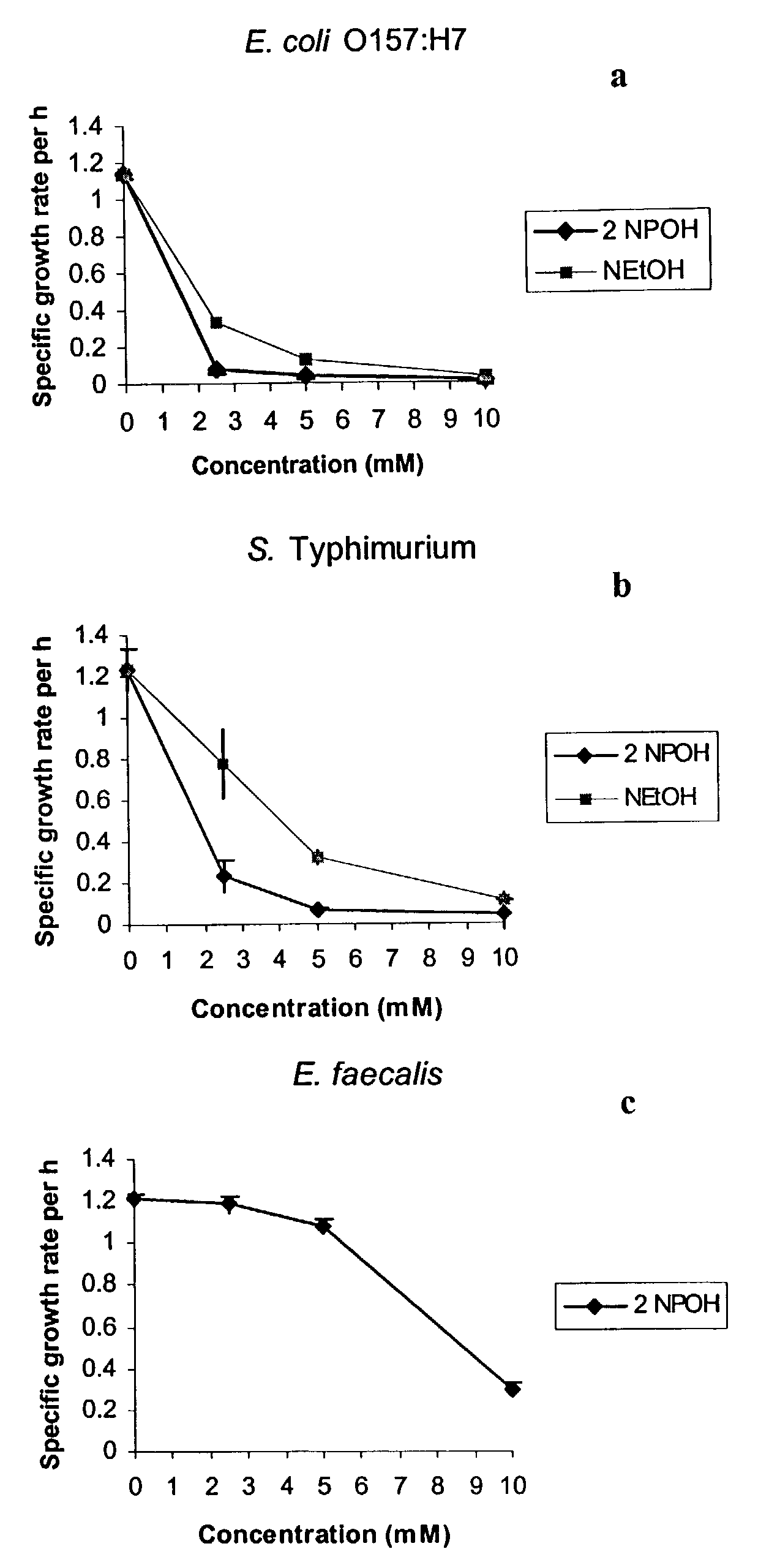
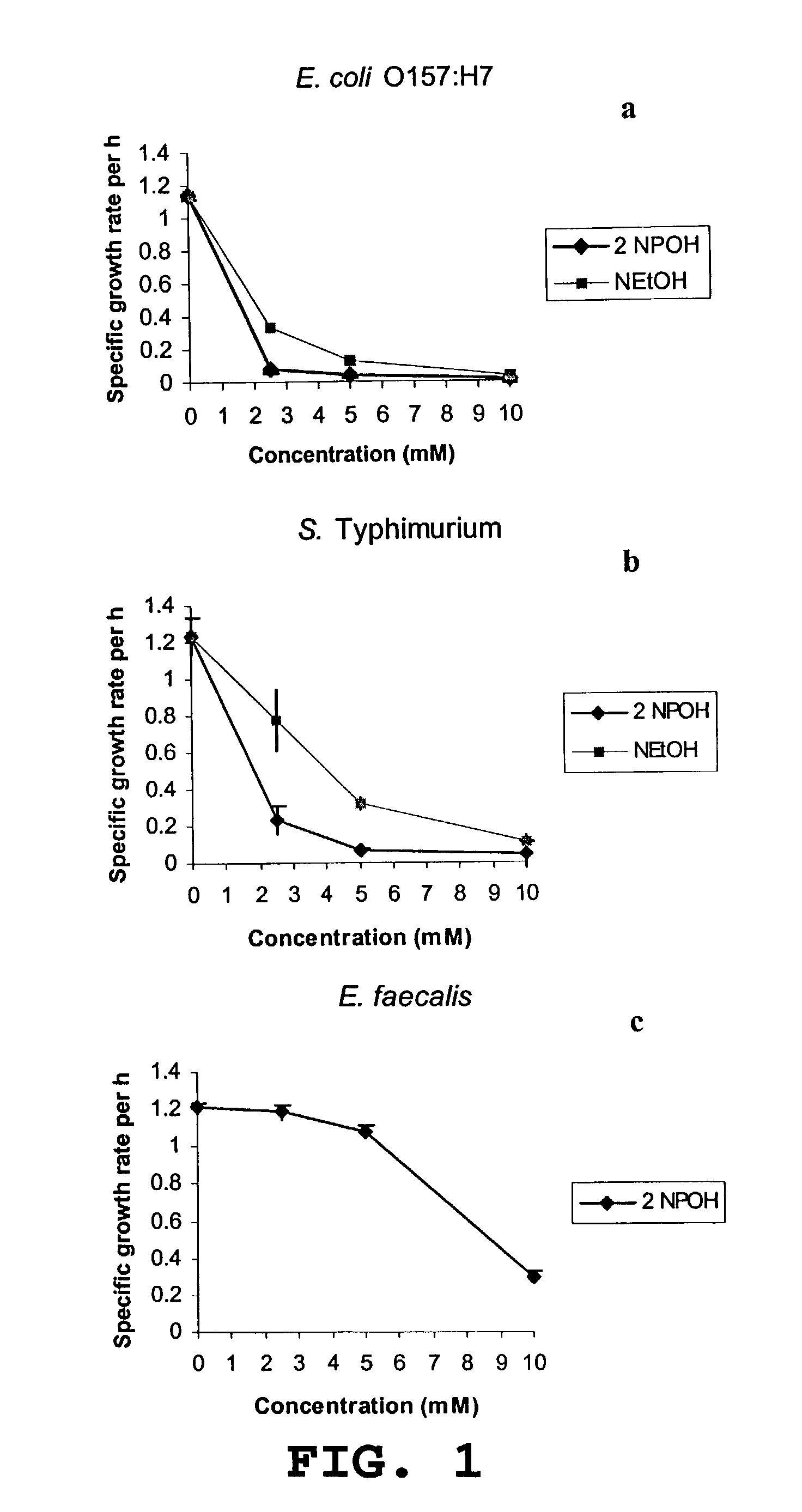
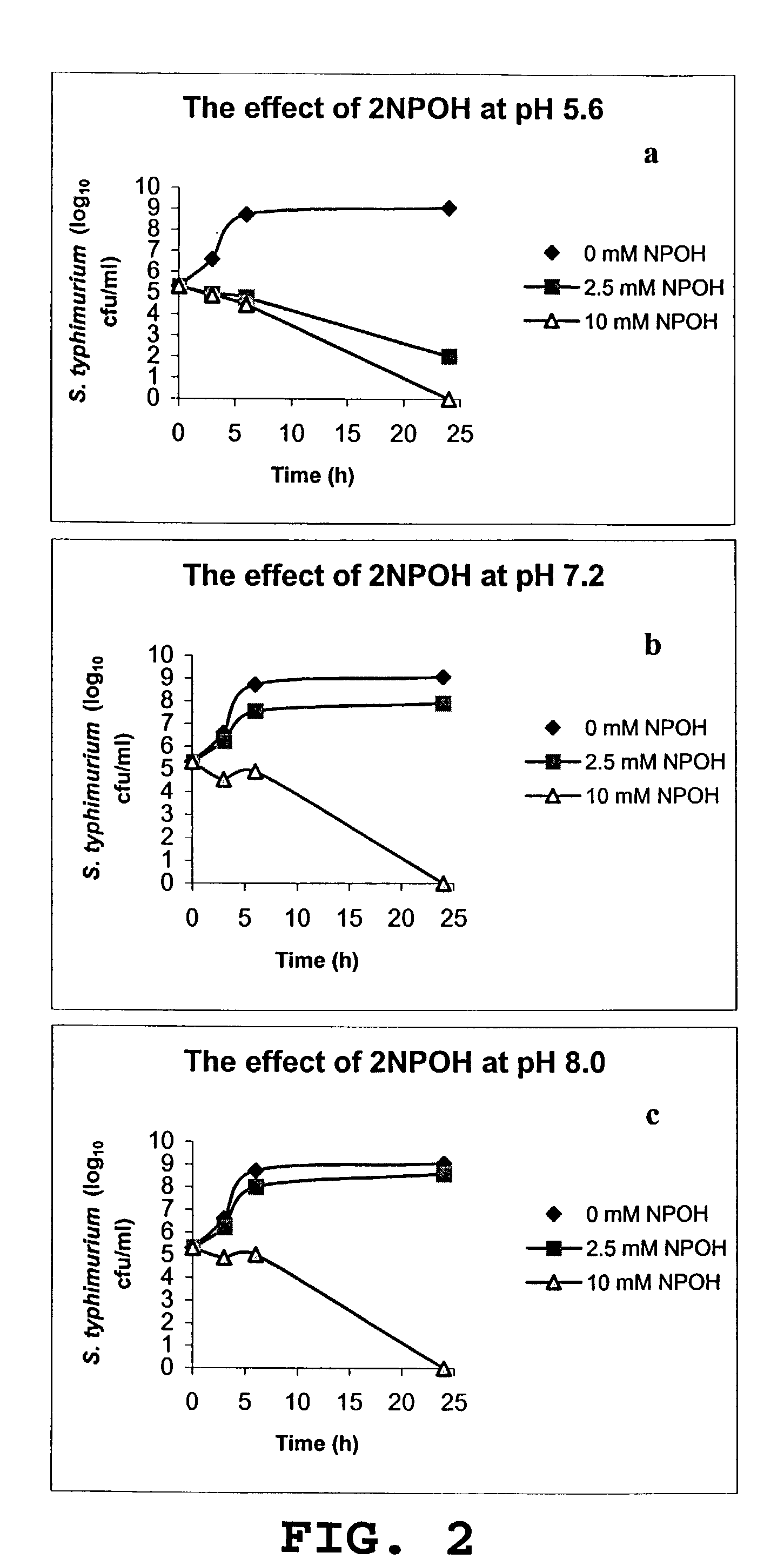
Use of 2-nitropropanol, 2-nitroethane, and 2-nitroethanol for control of microbial pathogens
ActiveUS7176244B1Reduce the populationPopulations of enteropathogenic bacteria may be substantially reducedBiocideHydroxy compound active ingredientsEscherichia coliBacteroides
The invention provides a method and compositions for controlling food borne enteric bacterial pathogens in animals. Populations of enteropathogenic bacteria may be substantially reduced or eliminated by treatment of animals with an effective amount of 2-nitropropanol, 2-nitroethane or 2-nitroethanol. The compounds may be administered orally, providing a reduction in the populations of the enteropathogenic bacteria in the alimentary tract of the animal, or they may be applied externally onto the animal to reduce the populations of any such bacteria which may be present as contaminants on the surface of the animal. The method and compositions are particularly useful for the control of Salmonella species, enteropathogenic Escherichia coli, Campylobacter species, and Listeria monocytogenes.
Owner:UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF AGRI THE
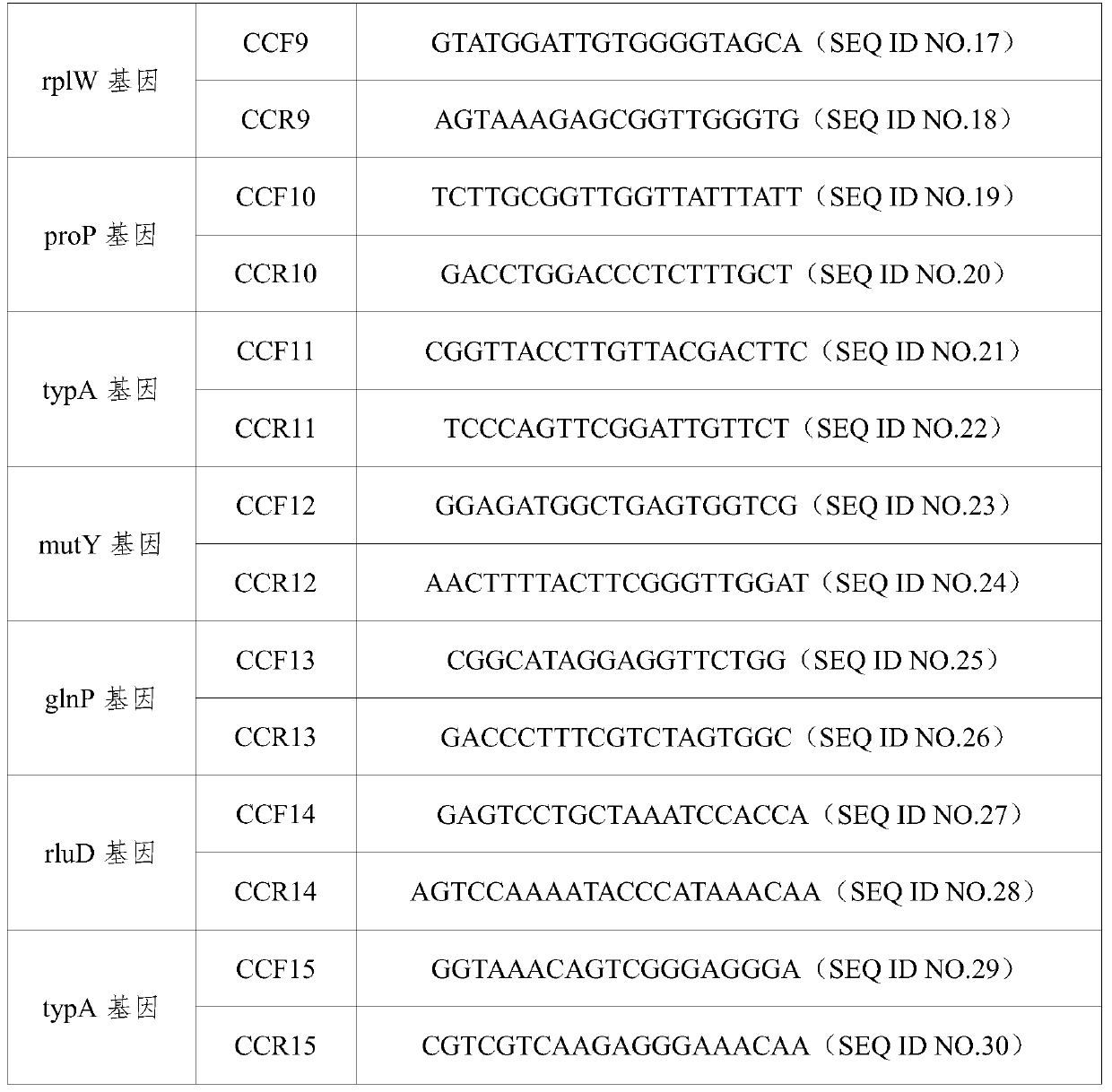
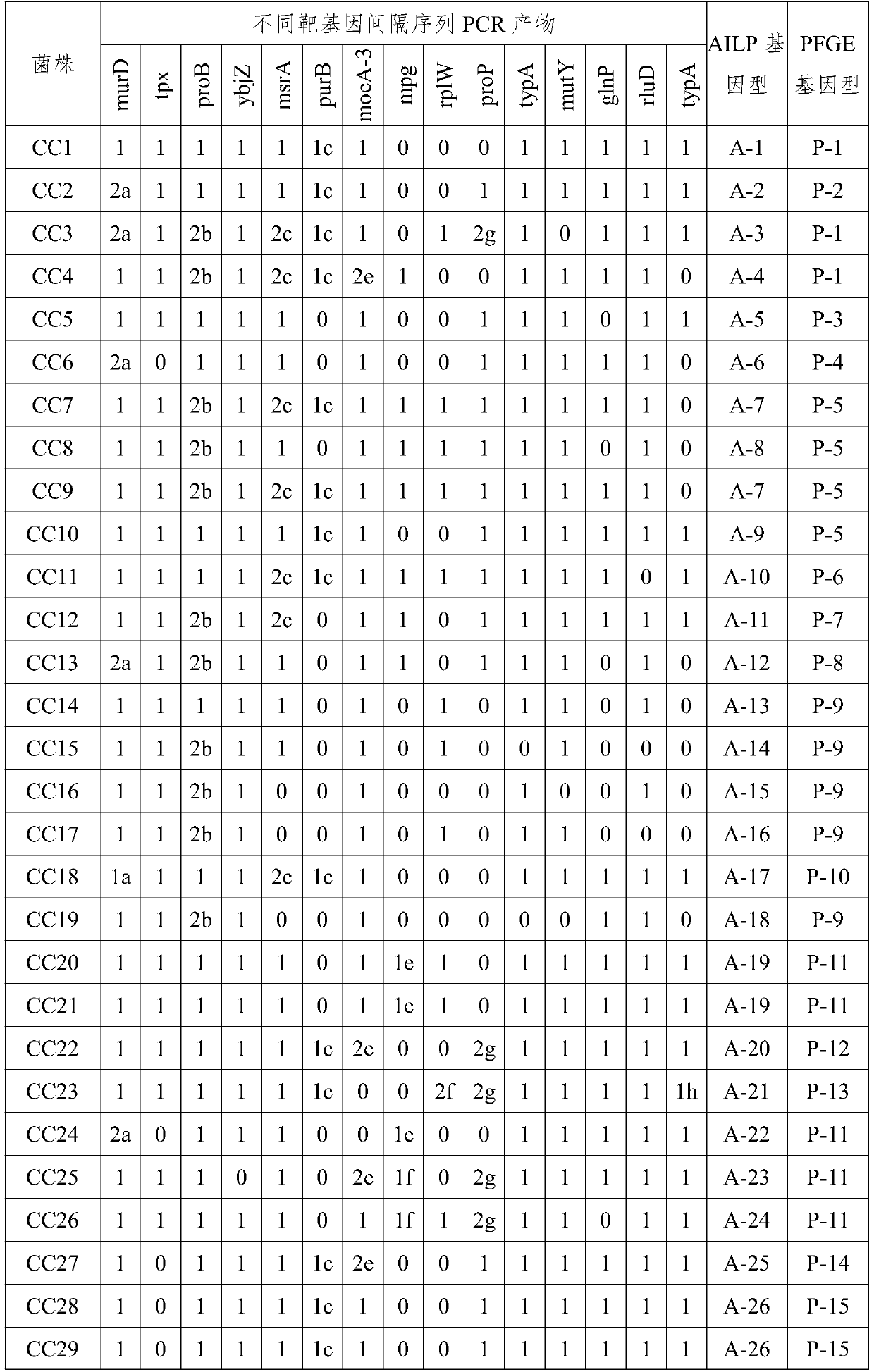
A primer and method for rapid typing of Campylobacter coli
ActiveCN105886623BAccurate typingGood resolution indexMicrobiological testing/measurementDNA/RNA fragmentationDiseaseMicroorganism
The invention belongs to the field of microorganism molecular typing and particularly relates to a primer and a method for fast typing campylobacter coli. The primer includes 15 pairs of campylobacter coli primers. The primer is good in typing capacity, high in distinguishing index and capable of accurately typing the campylobacter coli. In addition, the method for fast typing the campylobacter coli is simple and fast to operate, good in stability and repeatability, capable of effectively assisting the clinical diagnosis of diseases infected by the campylobacter coli and ideal.
Owner:GUANGDONG PHARMA UNIV
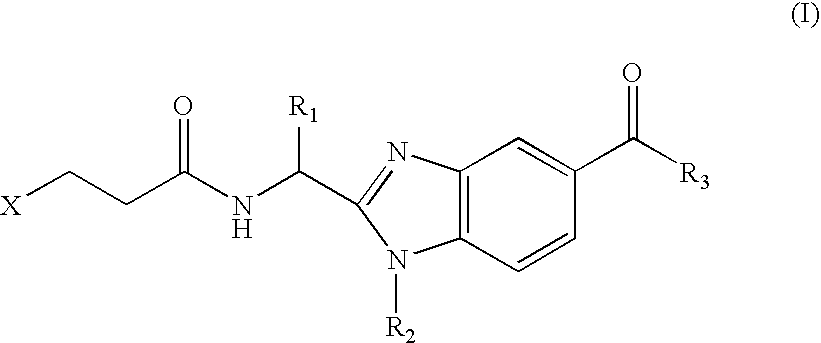
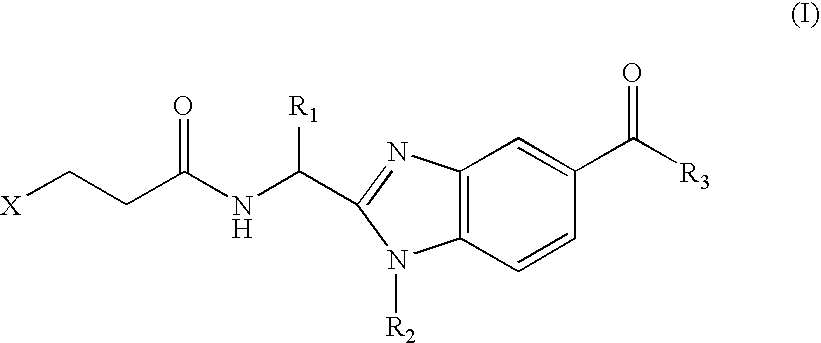
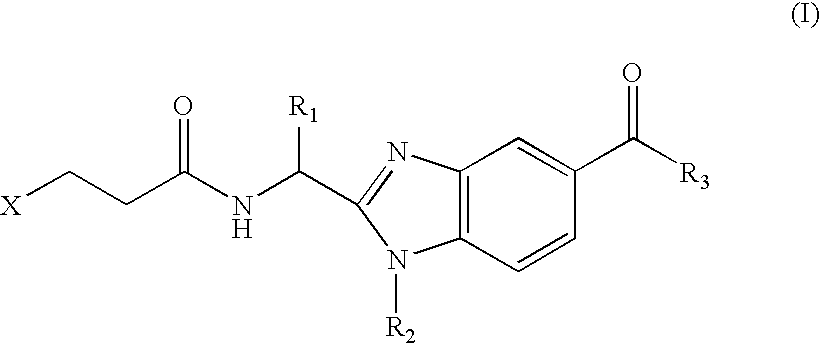
Benzimidazole derivatives and use thereof as peptide deformylase inhibitors
Benzimidazole compounds of the general formula (I) and pharmaceutically acceptable salts or esters thereof are peptide deformylase inhibitors useful in the treatment or prevention of infections and other diseases in which peptide deformylases are involved, especially in the treatment of bacterial and parasitic infections, for example infections fully or partly caused by microorganisms belonging to Staphylococcus, Enterococcus, Streptococcus, Haemophilus, Moraxella, Escherichia, Mycobacterium, Mycoplasma, Pseudomonas, Chlamydia, Rickettsia, Klebsiella, Shigella, Salmonella, Bordetella, Clostridium, helicobacter, Campylobacter, Legionella, or Neisseria.
Owner:ARPIDA AG
Fourteen-food-borne pathogenic bacterium multiplex PCR detection primer set and kit
ActiveCN103484546BTo achieve the detection effectMicrobiological testing/measurementAgainst vector-borne diseasesBacteroidesEscherichia coli
The invention discloses a fourteen-food-borne pathogenic bacterium multiplex PCR detection primer set and a kit. The detection primer set is composed of primer pairs for detecting salmonella, Shigella, Vibrio parahemolyticus, campylobacter jejuni, campylobacter coli, staphylococcus aureus, bacillus cereus, Listeria monocytogenes, yersinia enterocolitica, enterobacter sakazakii, Escherichia coli, vibrio cholerae, Escherichia coli O157, aeromonas hydrophila and internal positive control. A multiplex PCR detection method based on an ordinary PCR platform is built, multiplex PCR reactions are carried out on genomic DNA, extracted from a sample to be tested, of bacteria in the same reaction system through the primer pairs acquired through analysis and design, and whether the food-borne pathogenic bacteria are contained in the sample or not is judged through the electrophoretic analysis of reaction products.
Owner:北京卓诚惠生生物科技股份有限公司
Tagged sialyltransferase proteins
The present invention provides modified Campylobacter sialyltransferase proteins that exhibit enhanced expression as compared to its unmodified form. Nucleic acids that encode the sialyltransferase proteins are also included, as are methods to produce and use the sialyltransferase proteins.
Owner:RATIOPHARM GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com