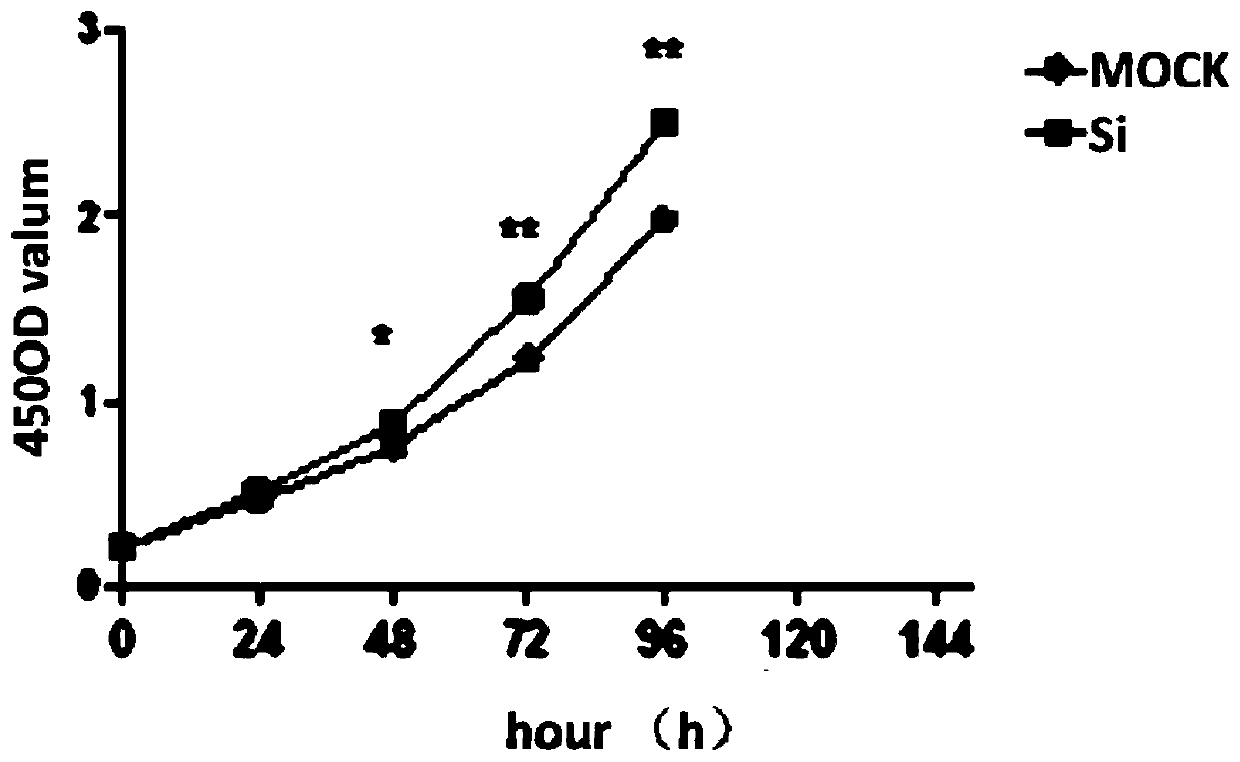
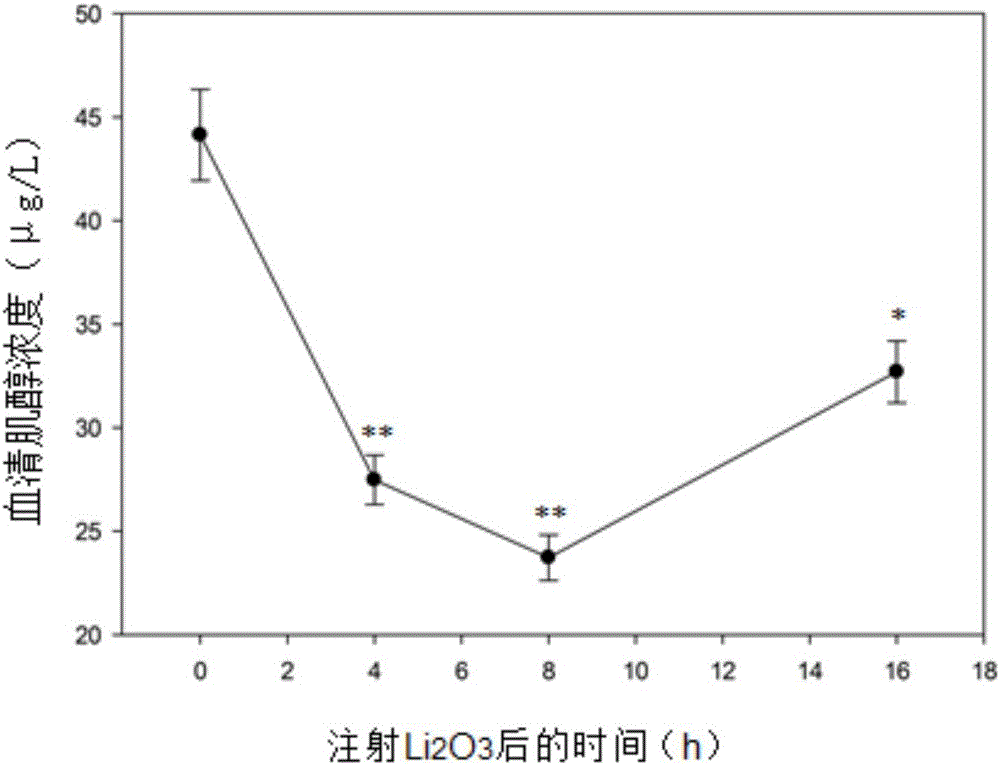
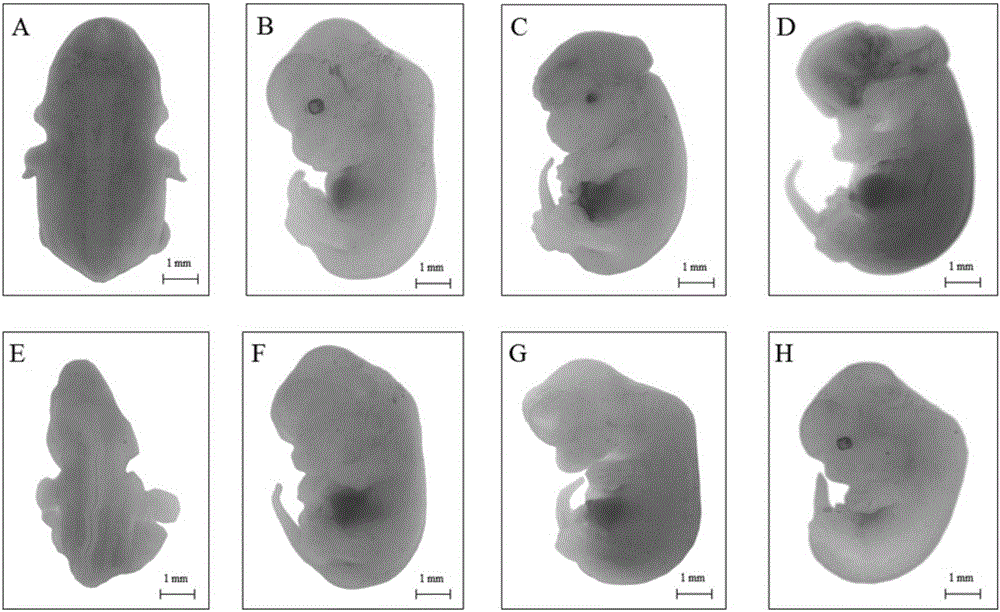
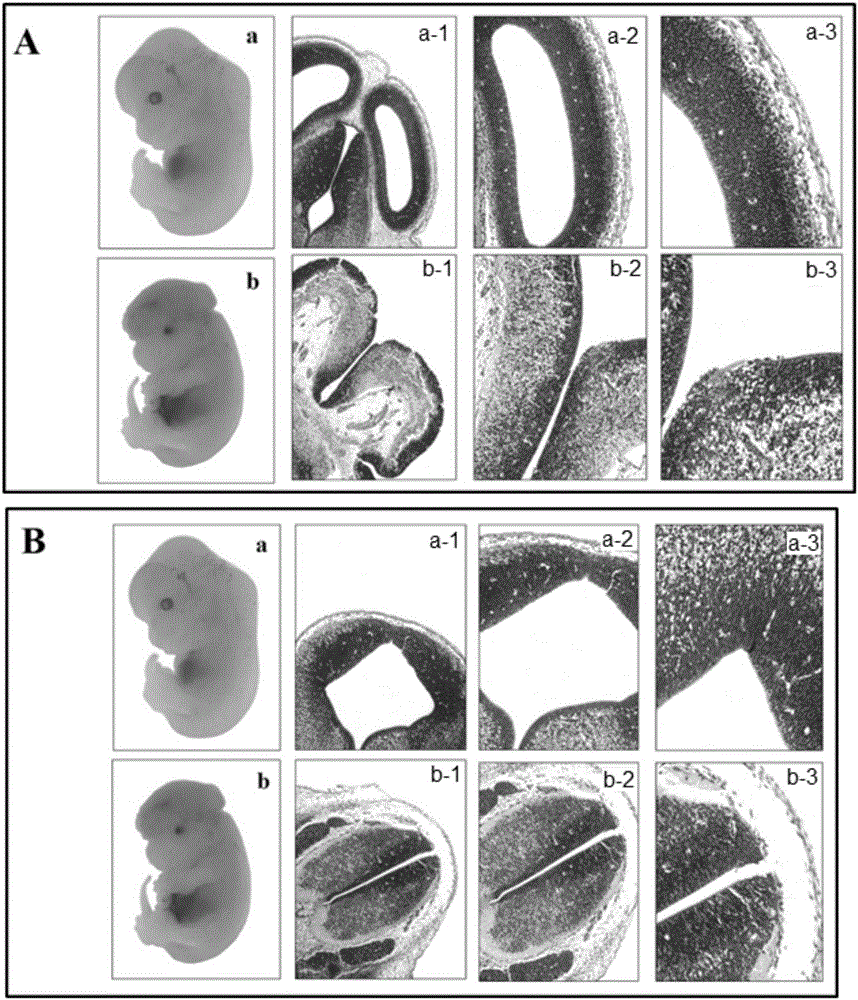
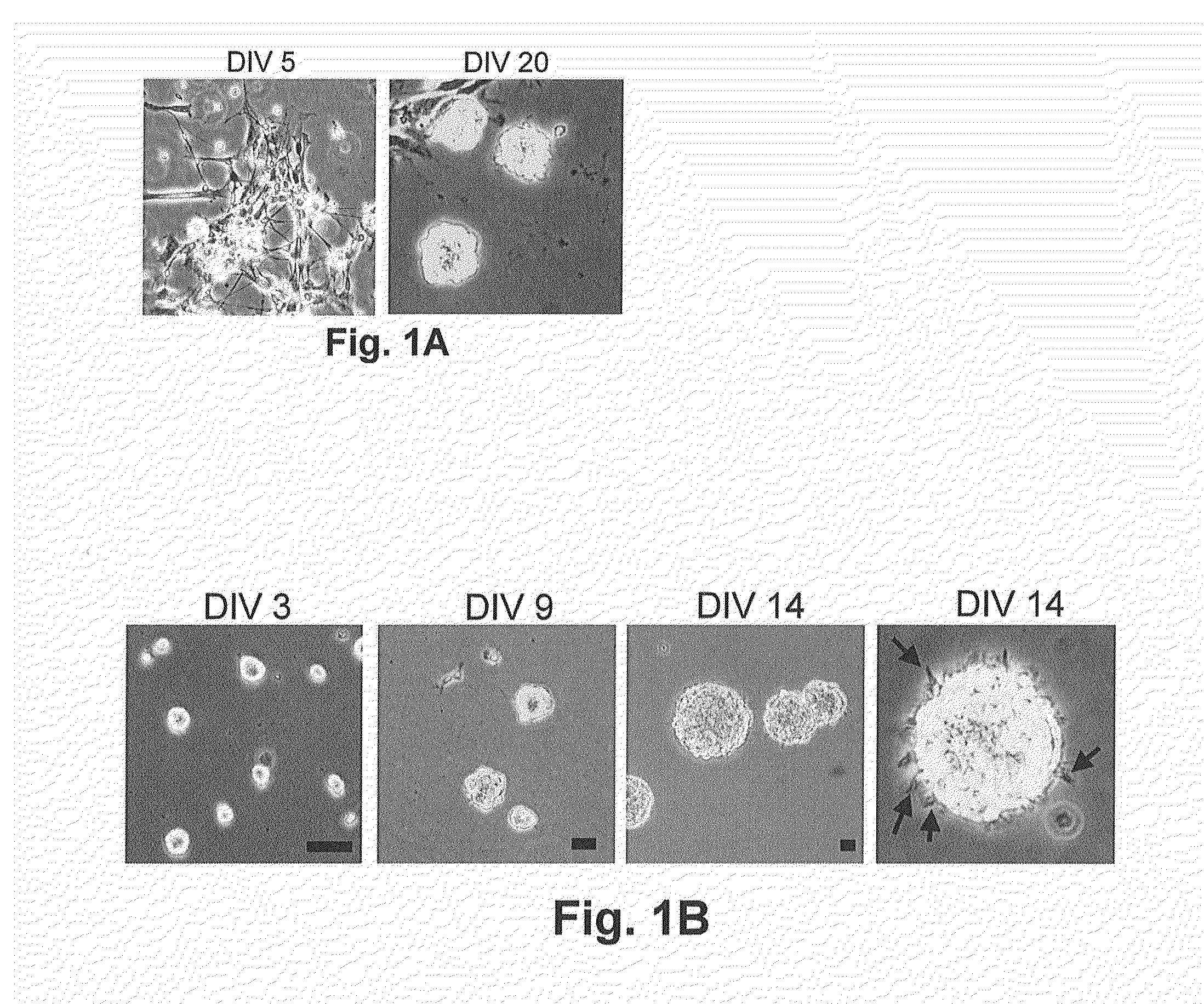
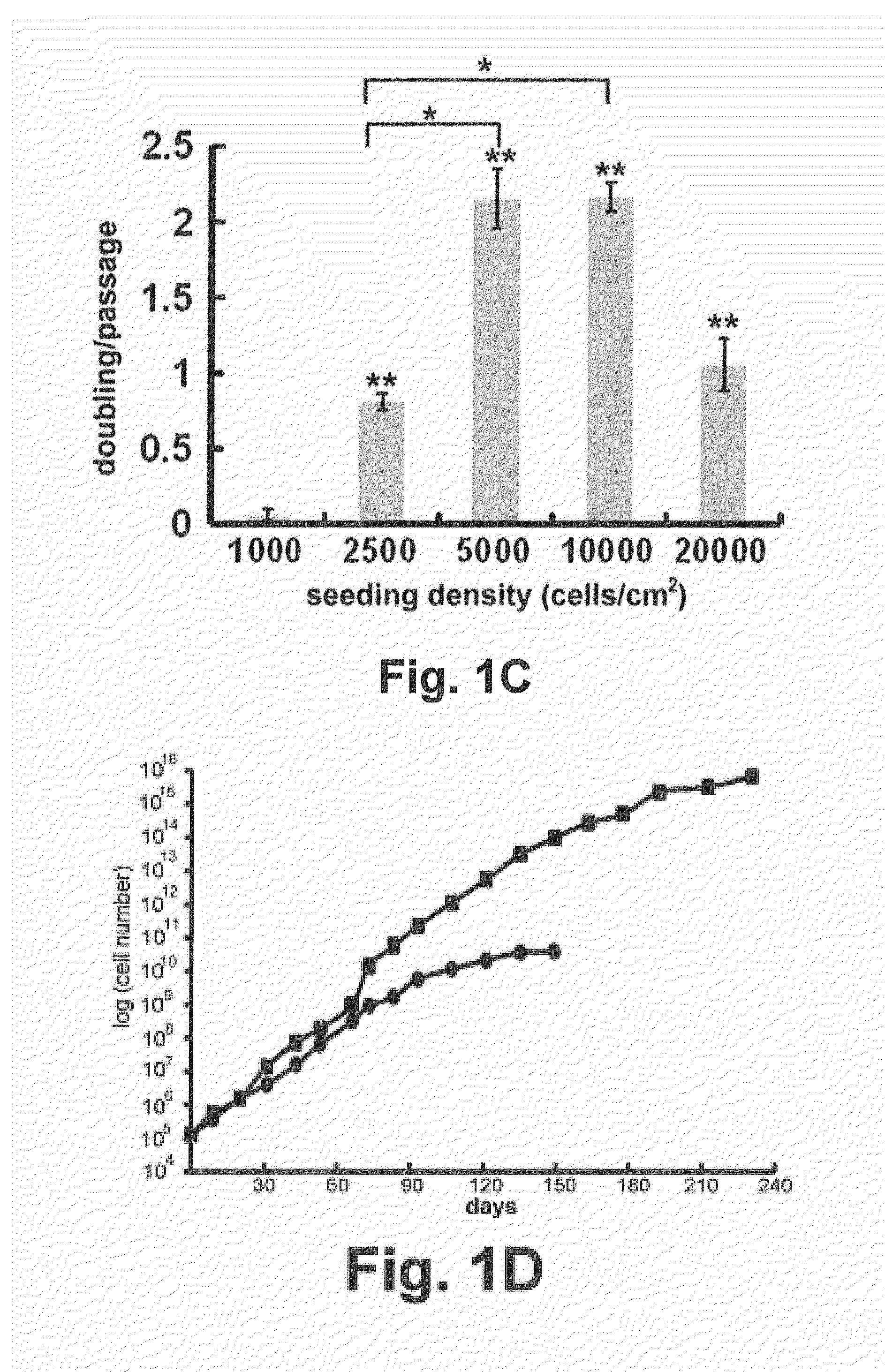
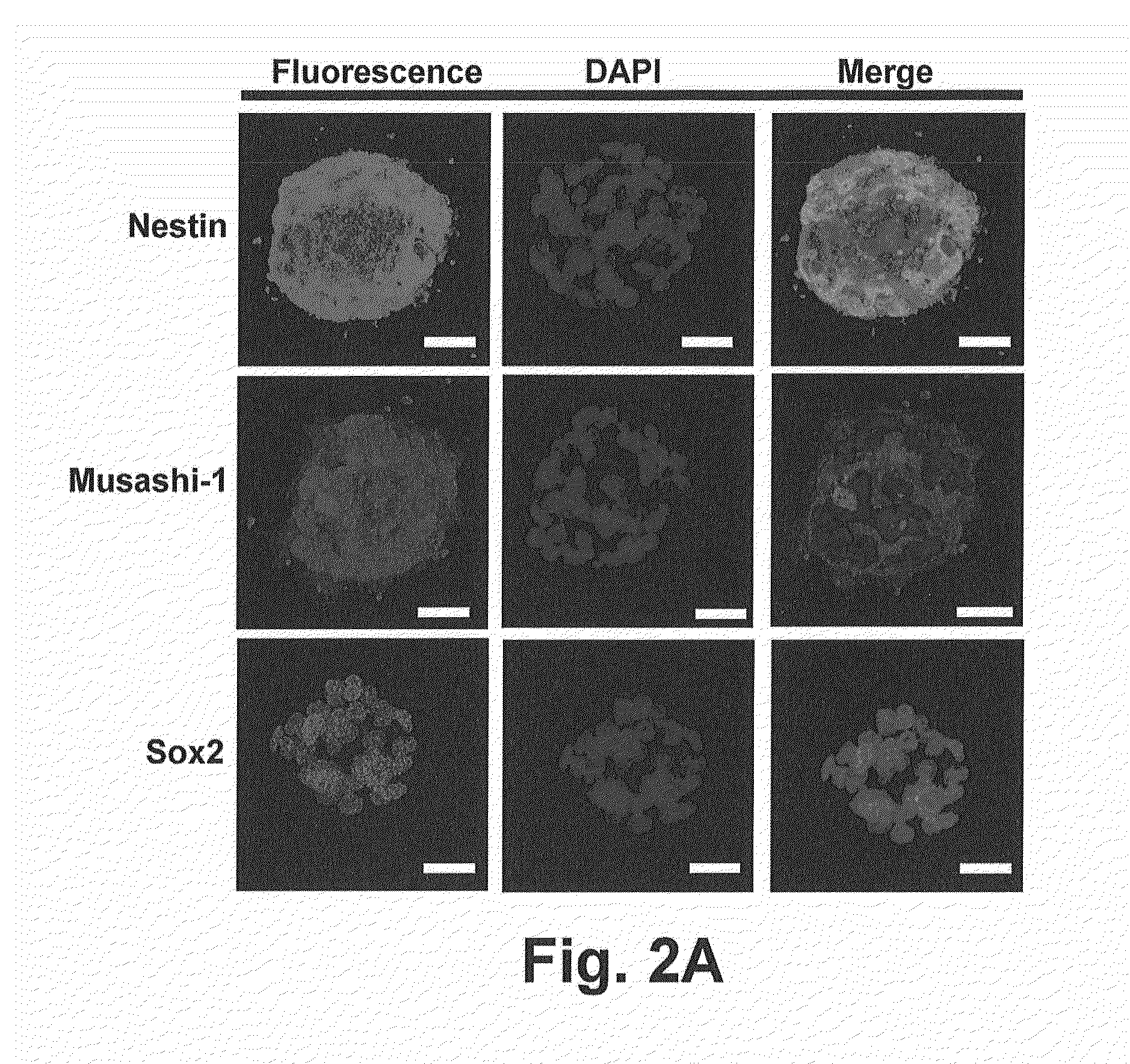
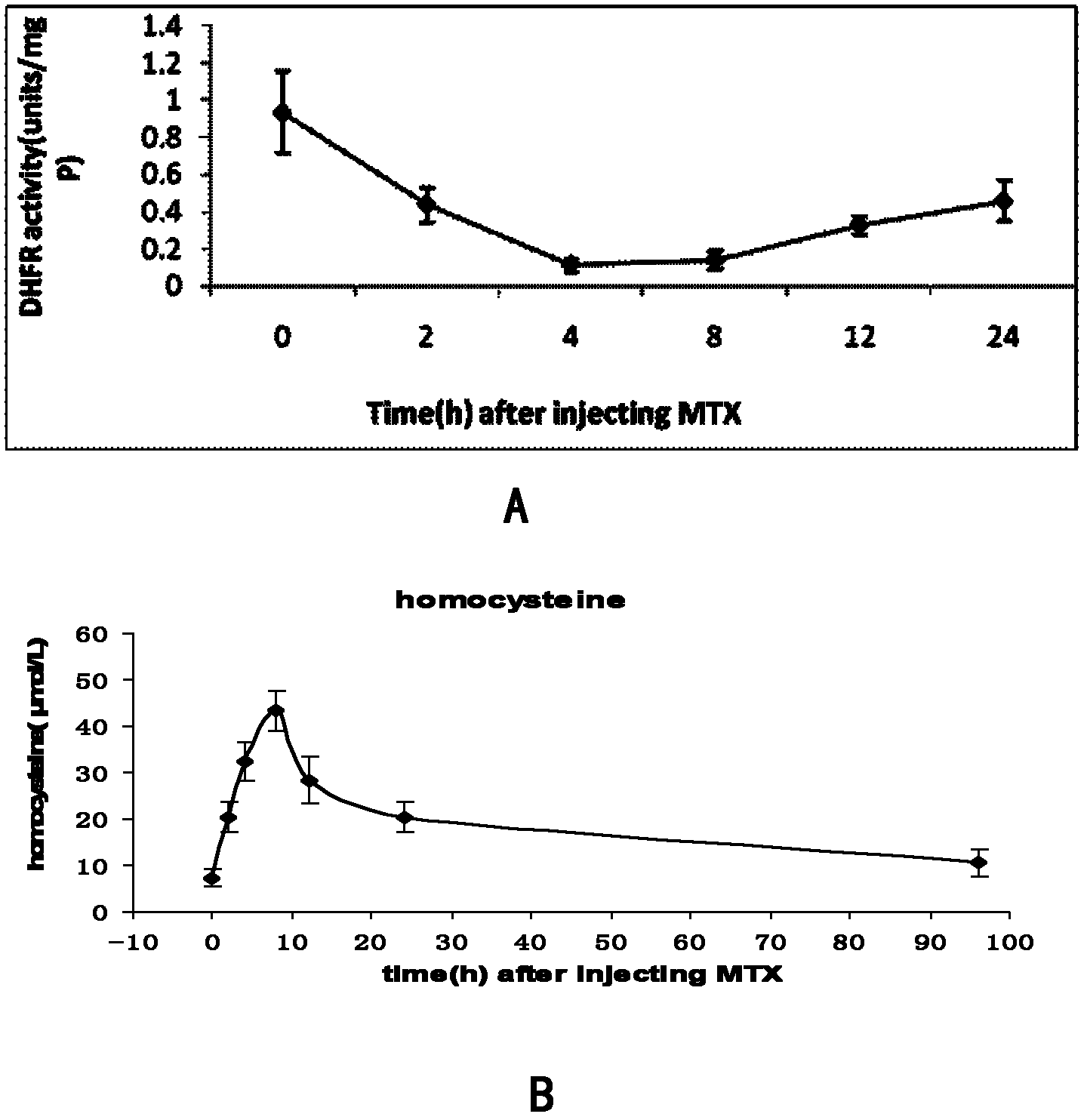
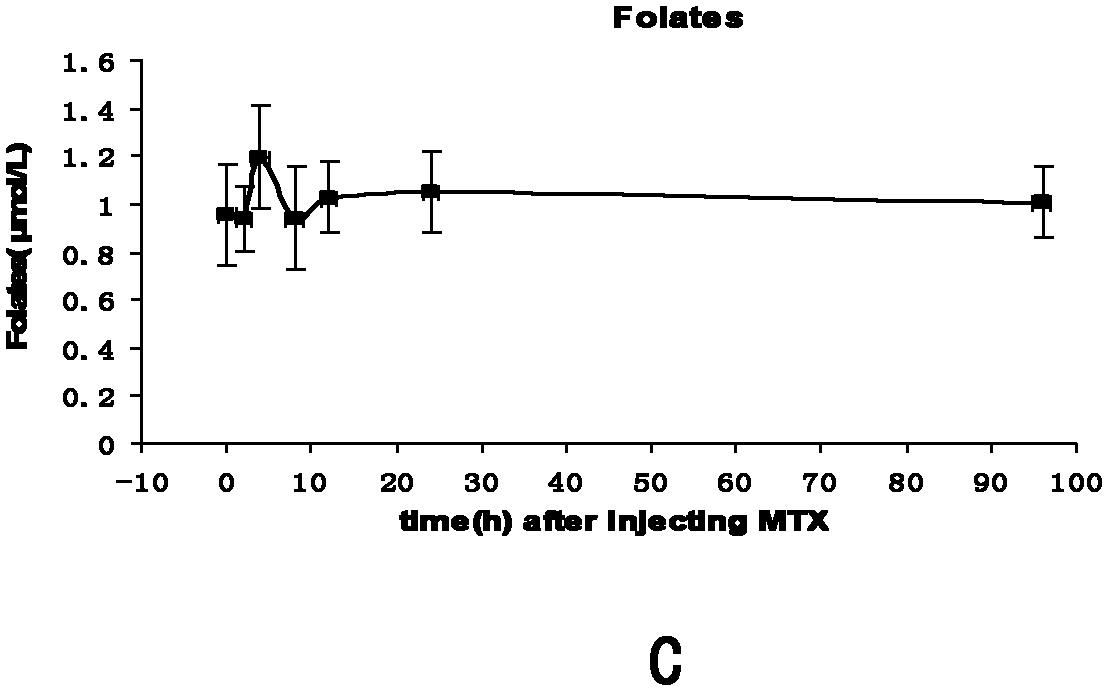
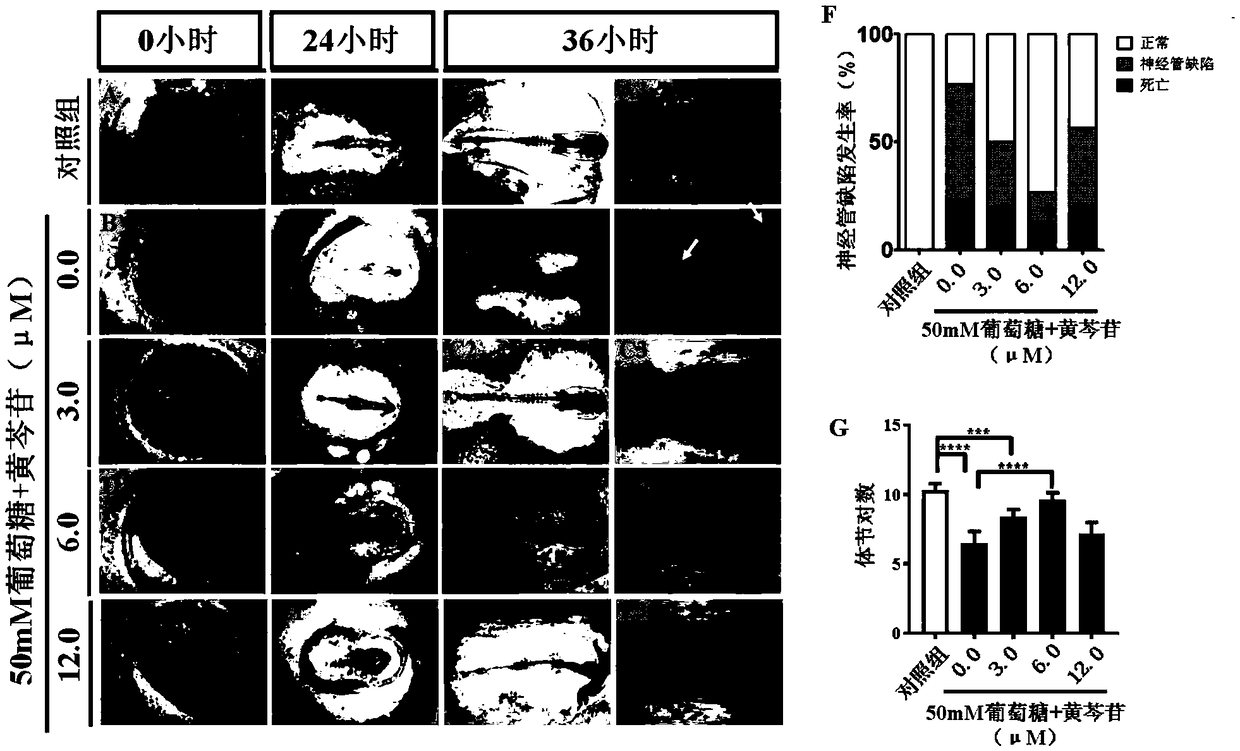
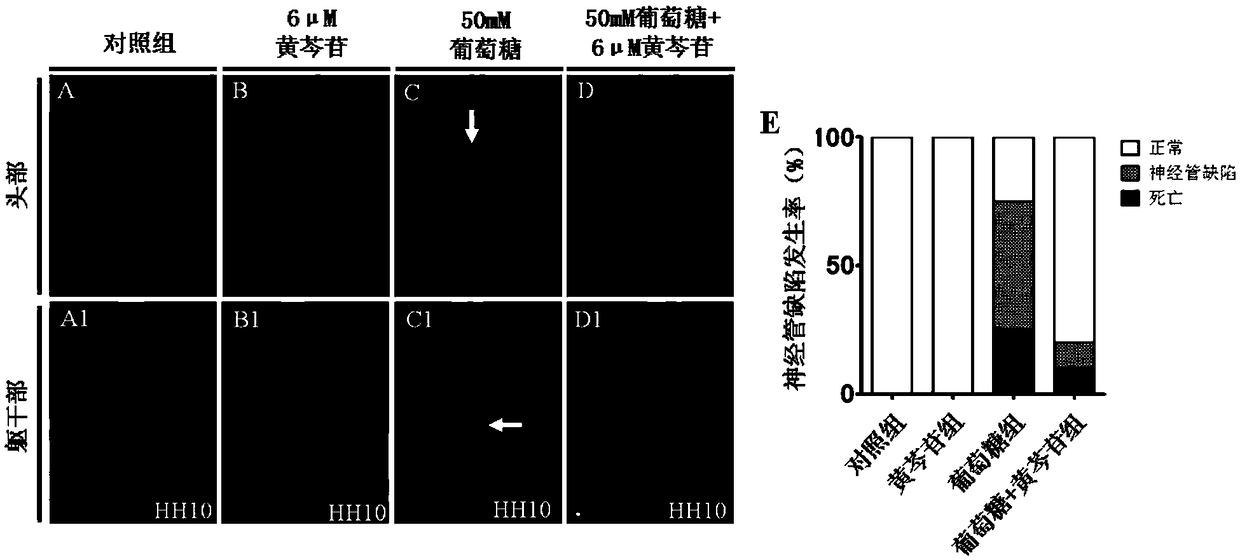
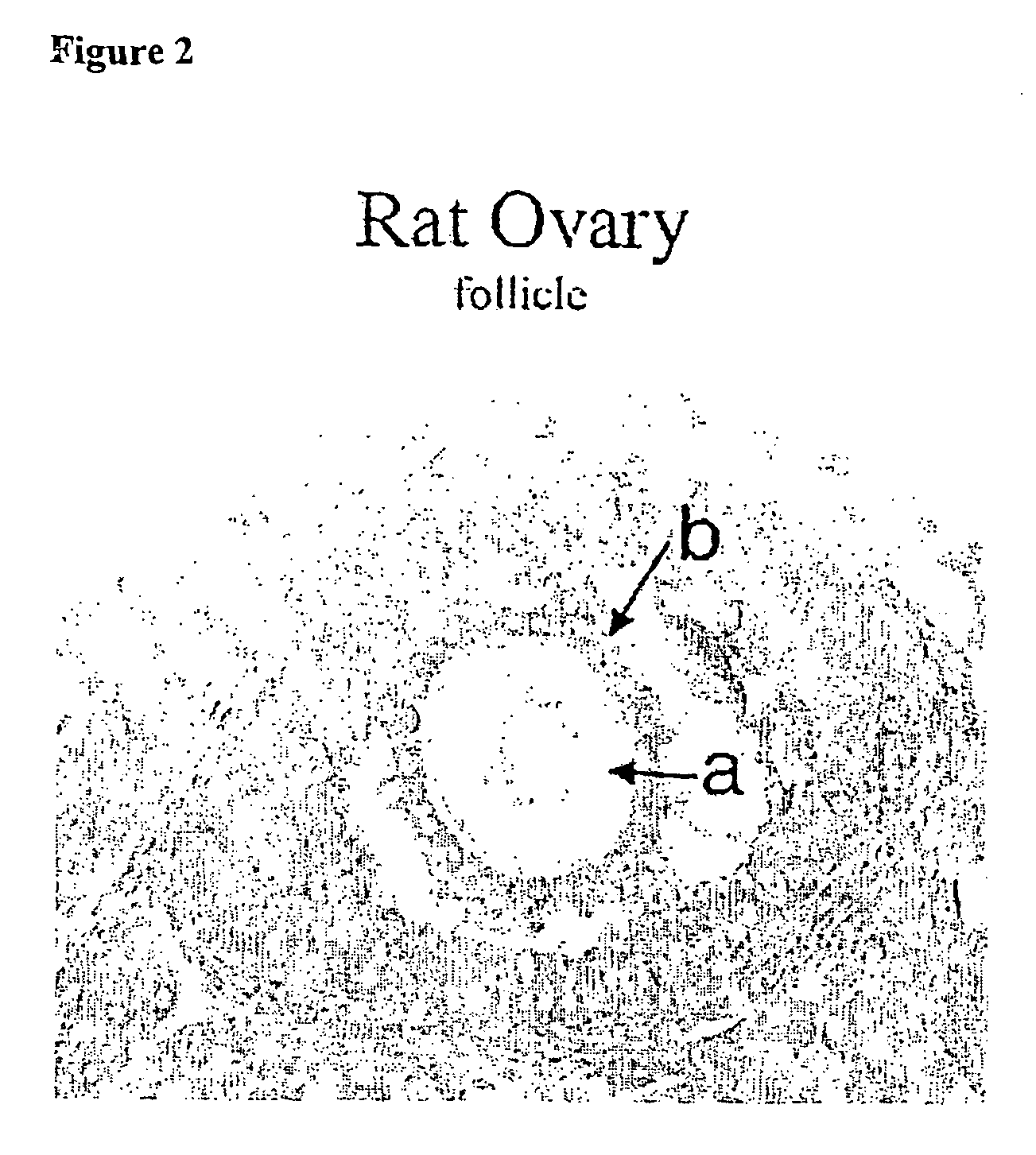
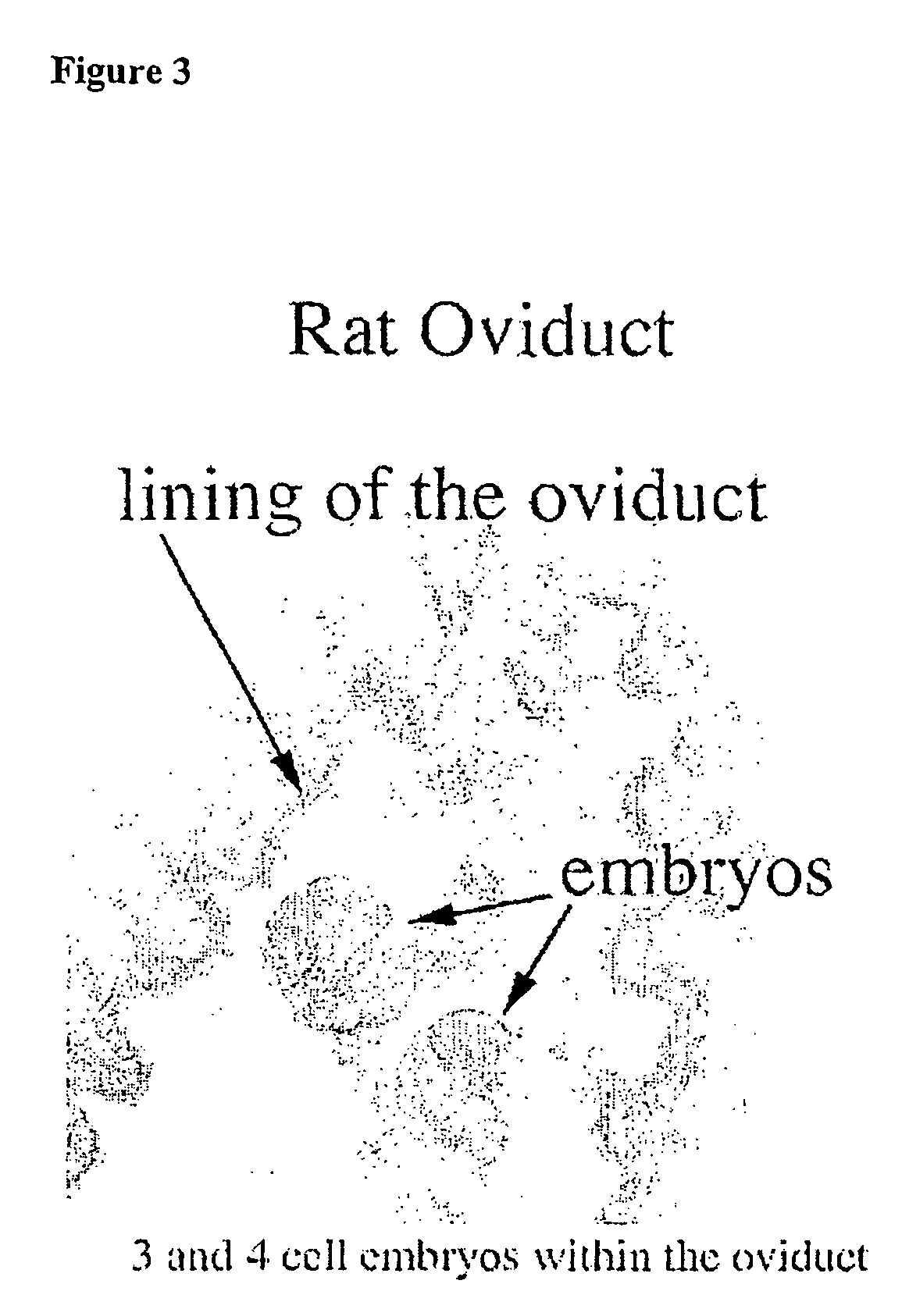
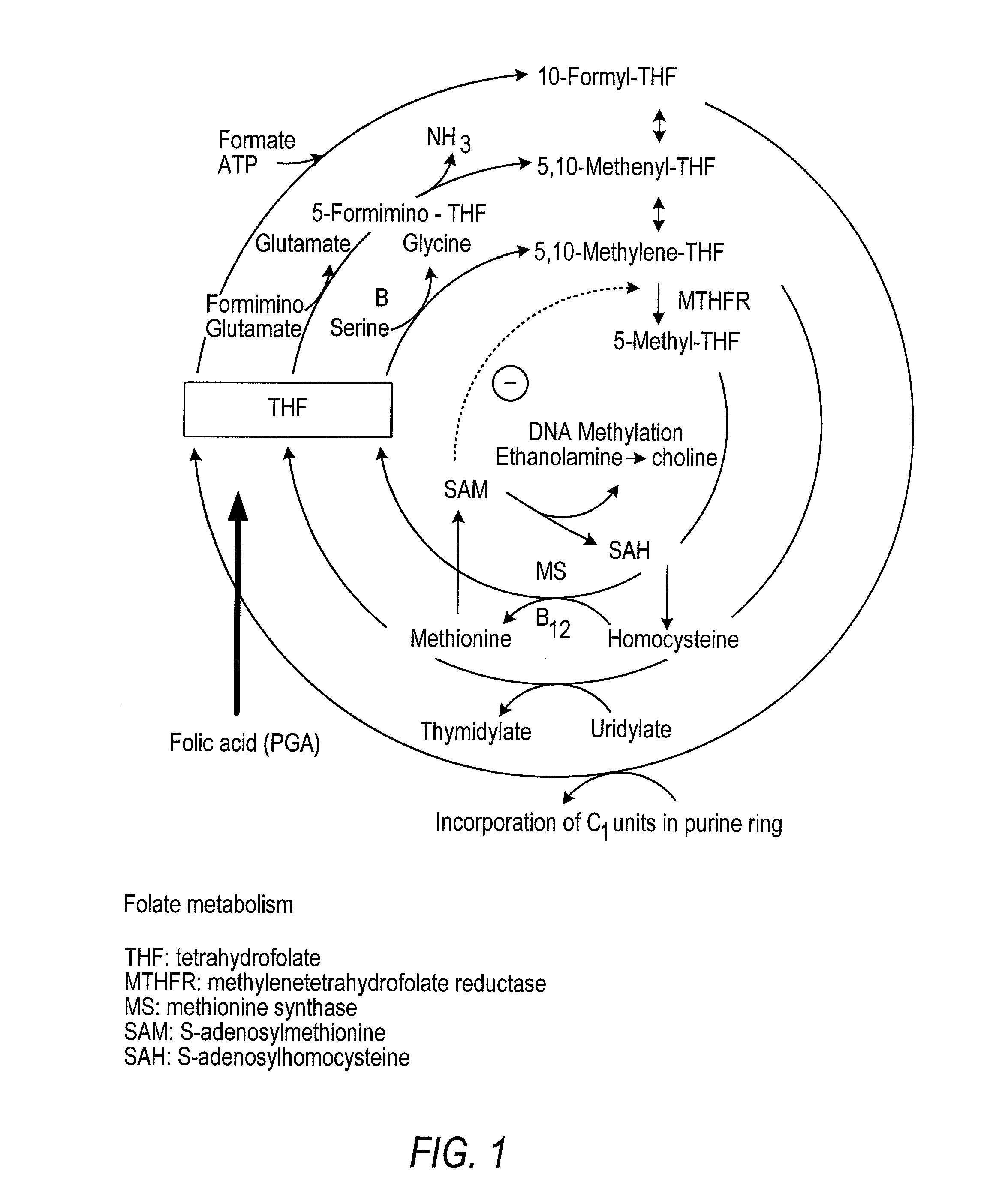
Patents
Literature
47 results about "Neural tube defect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
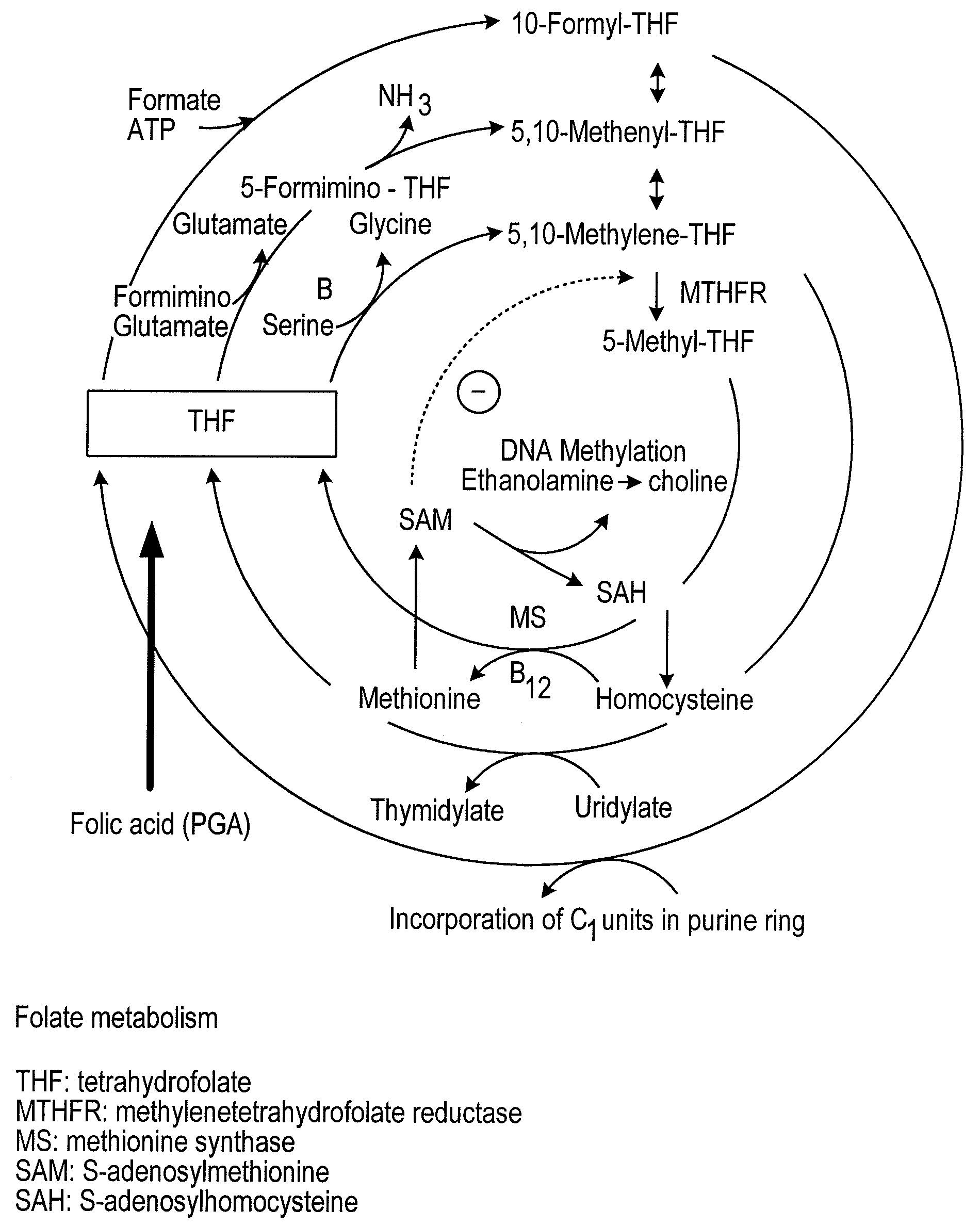
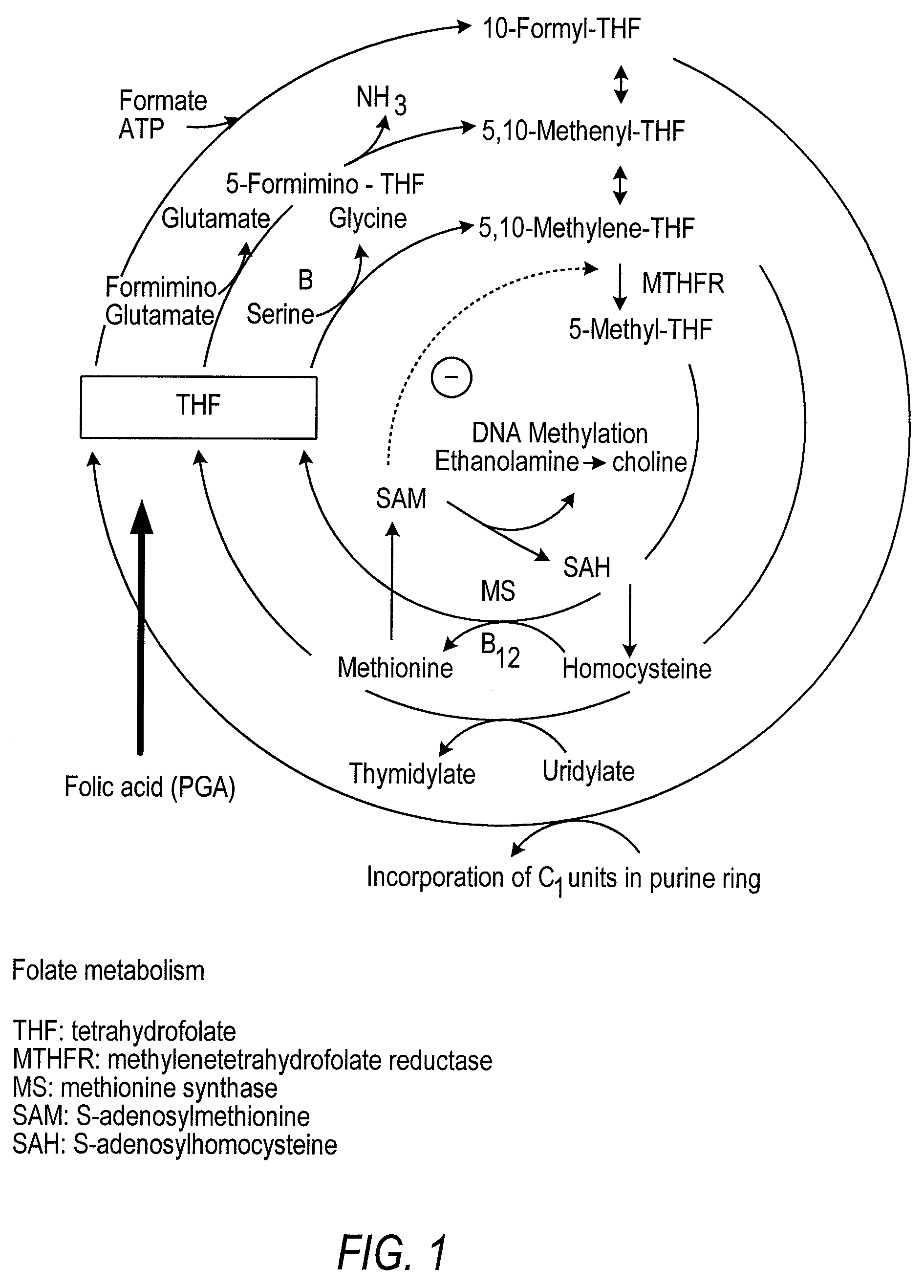
Neural tube defects (NTDs) are a group of birth defects in which an opening in the spine or cranium remains from early in human development. In the third week of pregnancy called gastrulation, specialized cells on the dorsal side of the embryo begin to change shape and form the neural tube. When the neural tube does not close completely, an NTD develops.
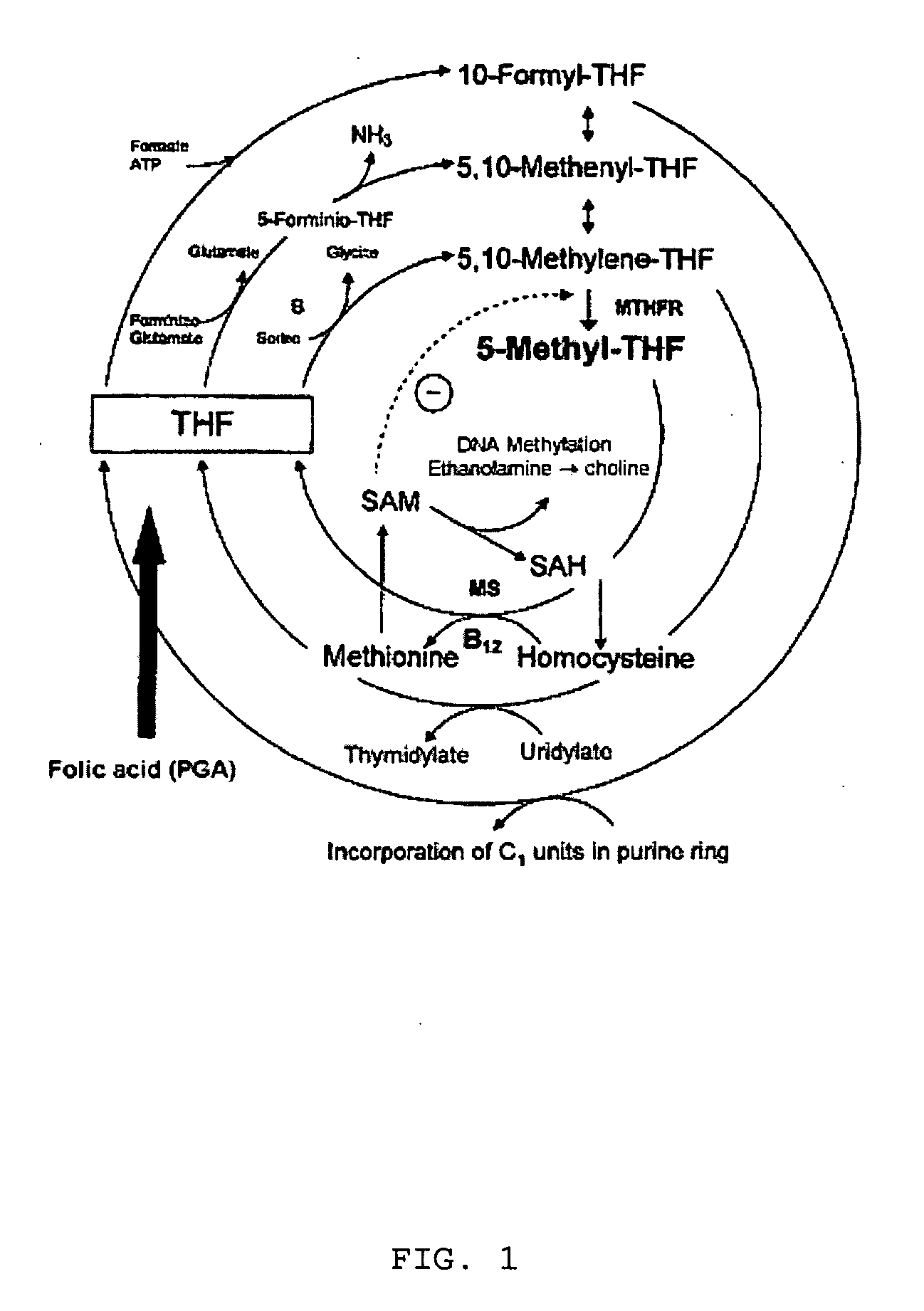
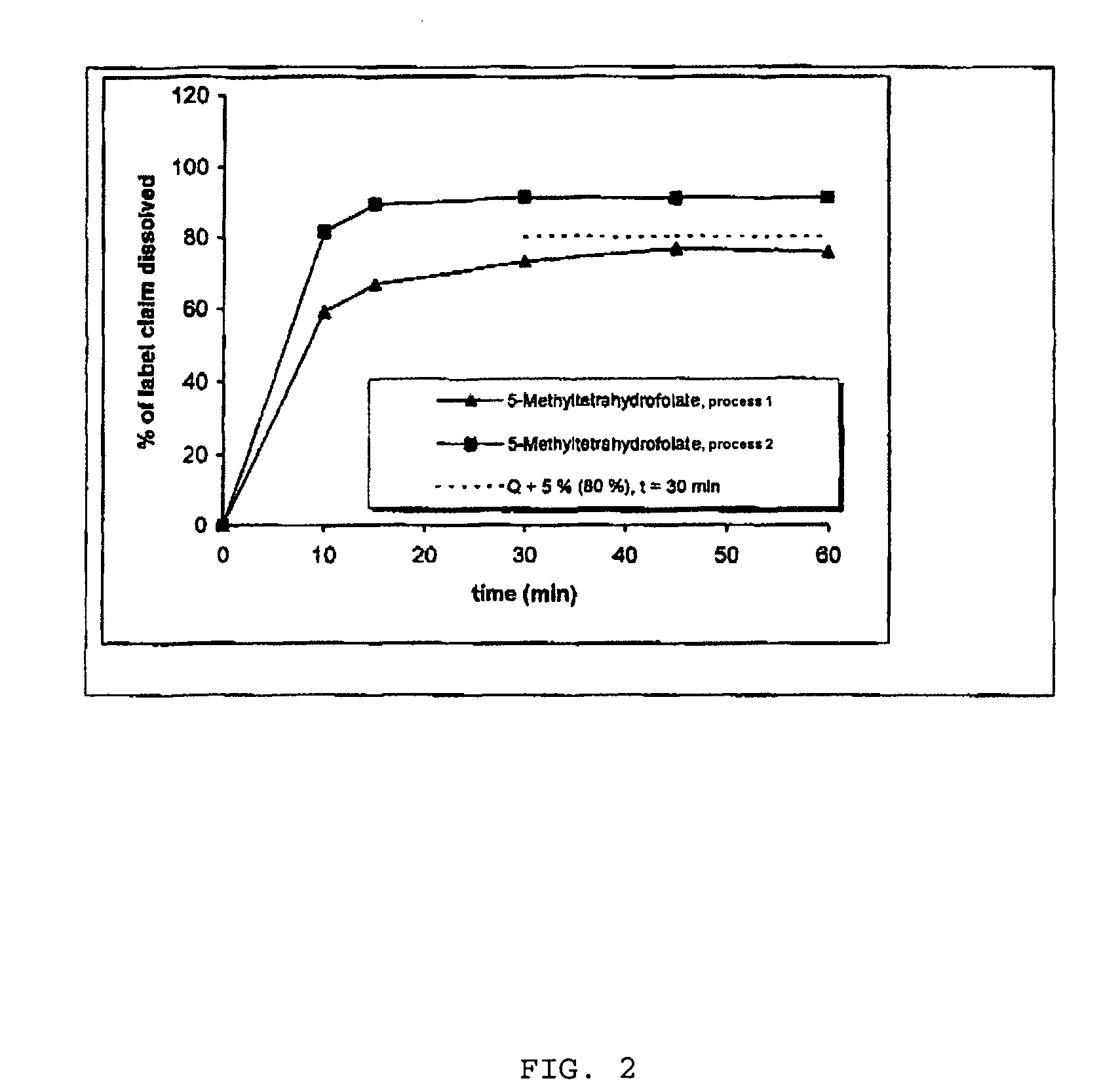
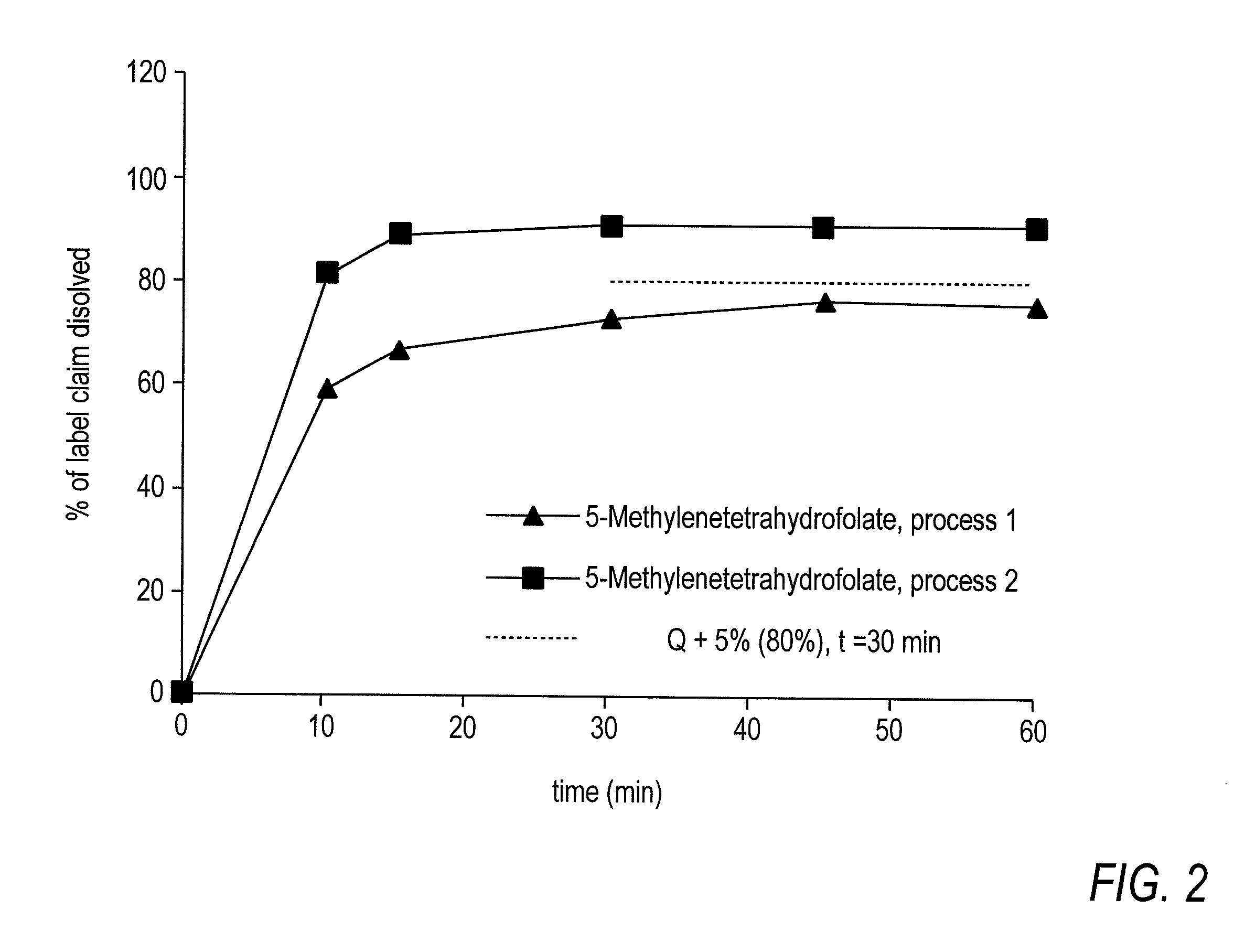
Pharmaceutical composition comprising progestogens and/or estrogens and 5-methyl- (6S)-tetrahydrofolate
The present invention relates to a pharmaceutical composition which may comprise progestogens, preferably drospirenone, estrogens, preferably ethinylestradiol and 5-methyl-(6S)-tetrahydrofolate, which may be employed as oral contraceptive and moreover prevents disorders caused by folate deficiency in the consumers, in particular cardiovascular disorders and, after conception of the embryo, congenital malformations caused by folate deficiency such as, for example, neural tube defects, ventricular valve defects, urogenital defects, and cleft lip, jaw and palate, without masking the symptoms of vitamin B12 deficiency, and at the same time even in the case of homozygous or heterozygous polymorphism of methylenetetrahydrofolate reductase facilitates unimpaired utilizability of the folate component 5-methyl-(6S)-tetrahydrofolate by the body and thus its biological activity for preventing the abovementioned congenital malformations caused by folate deficiency. In addition, a prolonged protective effect is maintained after discontinuation of the contraceptive.
Owner:SCHERING AG +1
Kit for detecting disease-causing genic mutation of neural tube defect of neonatus and application thereof
InactiveCN102181569AReduce morbidityQuick and easy detectionMicrobiological testing/measurementFluorescence/phosphorescenceDiseasePrenatal diagnosis
The invention discloses a kit for detecting whether mutation occurs at a relevant single nucleotide polymorphism (SNP) locus on a disease-causing gene of a neural tube defect of a neonatus. The kit mainly comprises a specific primer pair and a specific fluorescent probe pair which are used for detecting a No.rs1801133 SNP locus polymorphism genotype and a No.rs1801131 SNP locus polymorphism genotype on a methylenetetrahydrofolate reductase (MTHFR) gene and a No.rs1801394 SNP locus polymorphism genotype on a methionine synthase reductase (MTRR) gene, and the conventional fluorescent quantitative PCR reaction reagent. The kit is used for detecting a mutant of the disease-causing gene of the neural tube defect of the neonatus, can be used for quickly and conveniently searching a carrier with the disease-causing gene, can be applied to antenatal diagnosis and timely treatment, and reduces the morbidity of the neutral tube defect of the neonatus so as to fulfill the aims of promoting good prenatal and postnatal care.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Gene noninvasive detection kit for preventing neural tube defects of newborns
The invention provides a gene noninvasive detection kit for preventing neural tube defects of newborns. The kit comprises specific primers, DNA sequencing primers, a PCR (Polymerase Chain Reaction) reaction assembly, a PCR product purification assembly, a DNA sequencing reaction assembly and the like, wherein the specific primers are used for detecting No. rs1801133 SNP locus (MTHFR C677T) and No. rs1801131 SNP locus (MTHFR A1298C) on 5, 10-methylene tetrahydrofolate reductase (MTHFR) and No. rs1801394 SNP locus (MTRR A66G) on 5, 10-methylene methyl transferase reductase (MTRR). The kit evaluates the risk level of pregnant women giving birth to infants with neural tube defects by detecting the genotype of a single nucleotide polymorphism locus closely related to the main genetic factor causing newborn defects, namely female folic acid metabolism ability, and the pregnant women are individually instructed to supplement folic acid according to the gene detection result of each client. The method provided by the invention accords with the situation of our country, oral mucosa cell sampling is adopted as a sampling method which is painless and noninvasive, and cross infection is avoided. The sequencing detection results are accurate and reliable, expensive imported special instruments are not needed, and the method is easy to popularize and spread.
Owner:解码(上海)生物医药科技有限公司
Application of antifolic in constructing mouse model with neural tube defects (NTDs)
InactiveCN102228690AIncreased sensitivityTeratogenicity test period is shortIn-vivo testing preparationsPharmaceutical active ingredientsFolic acid metabolismRepeatability
The invention relates to an application of antifolic in constructing a mouse model with neural tube defects (NTDs). Specifically, an anti-folic acid metabolism drug is adopted for intervening a pregnant mouse to generate folic acid dysbolism and cause abnormal embryonic development of the mouse, thereby constructing a mouse model with NTDs. The method adopted by the invention has simplicity of operation, good repeatability, high teratogenesis rate and stable effect, and simulates an animal model close to human on the pathogenesis of NTDs by obstructing normal metabolism of folic acid, thereby facilitating further studies on congenital anomalies, especially NTDs.
Owner:首都儿科研究所
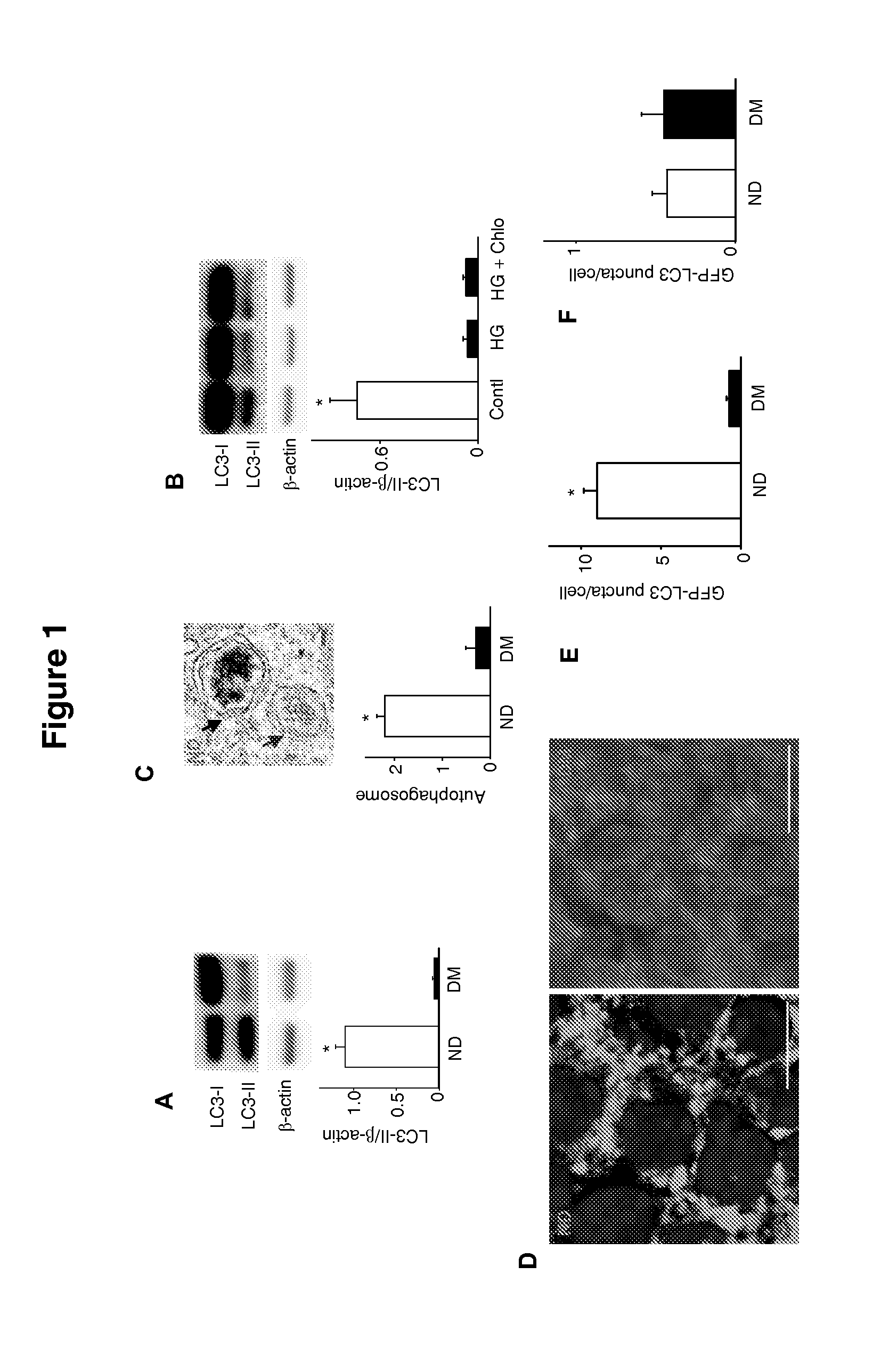
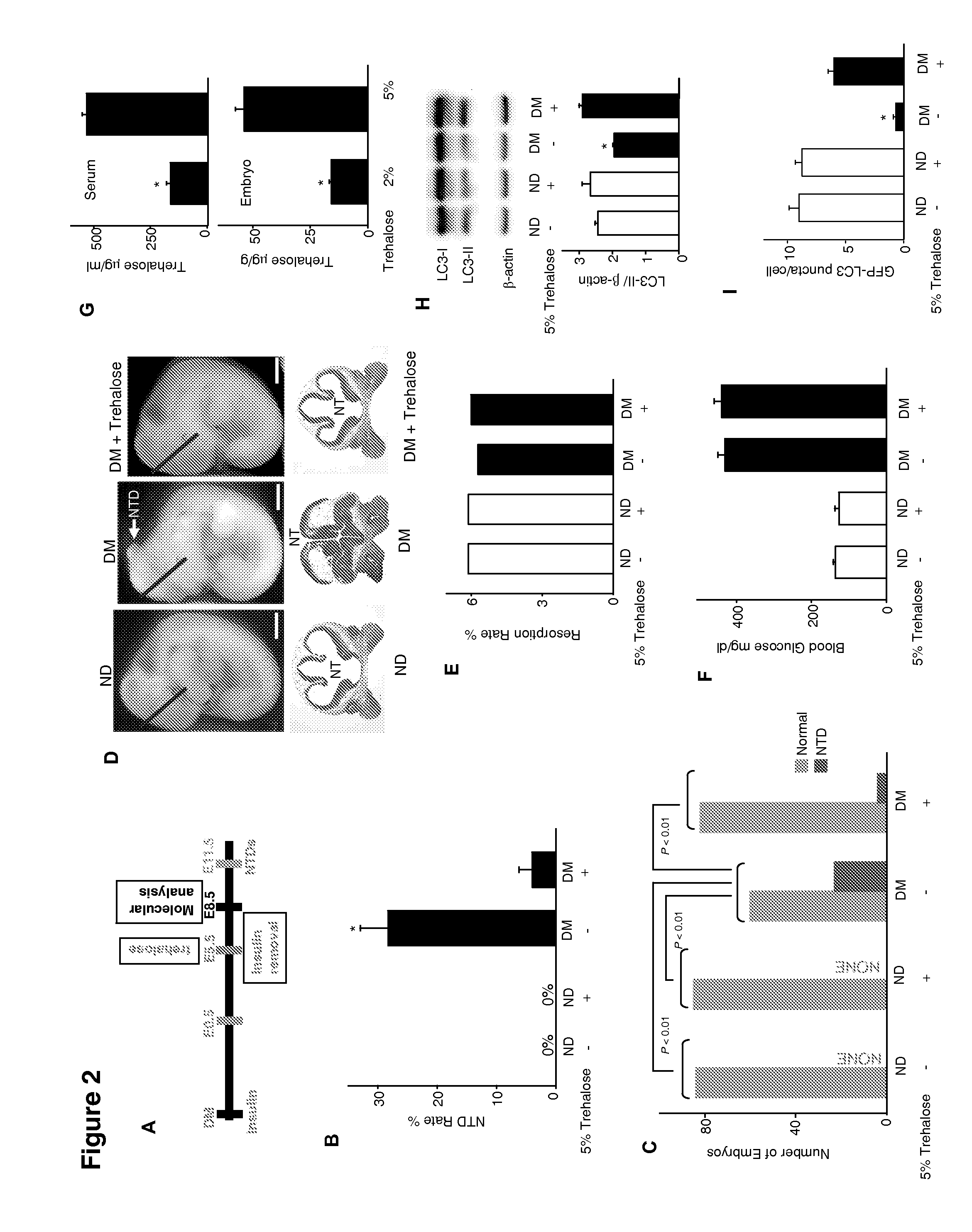
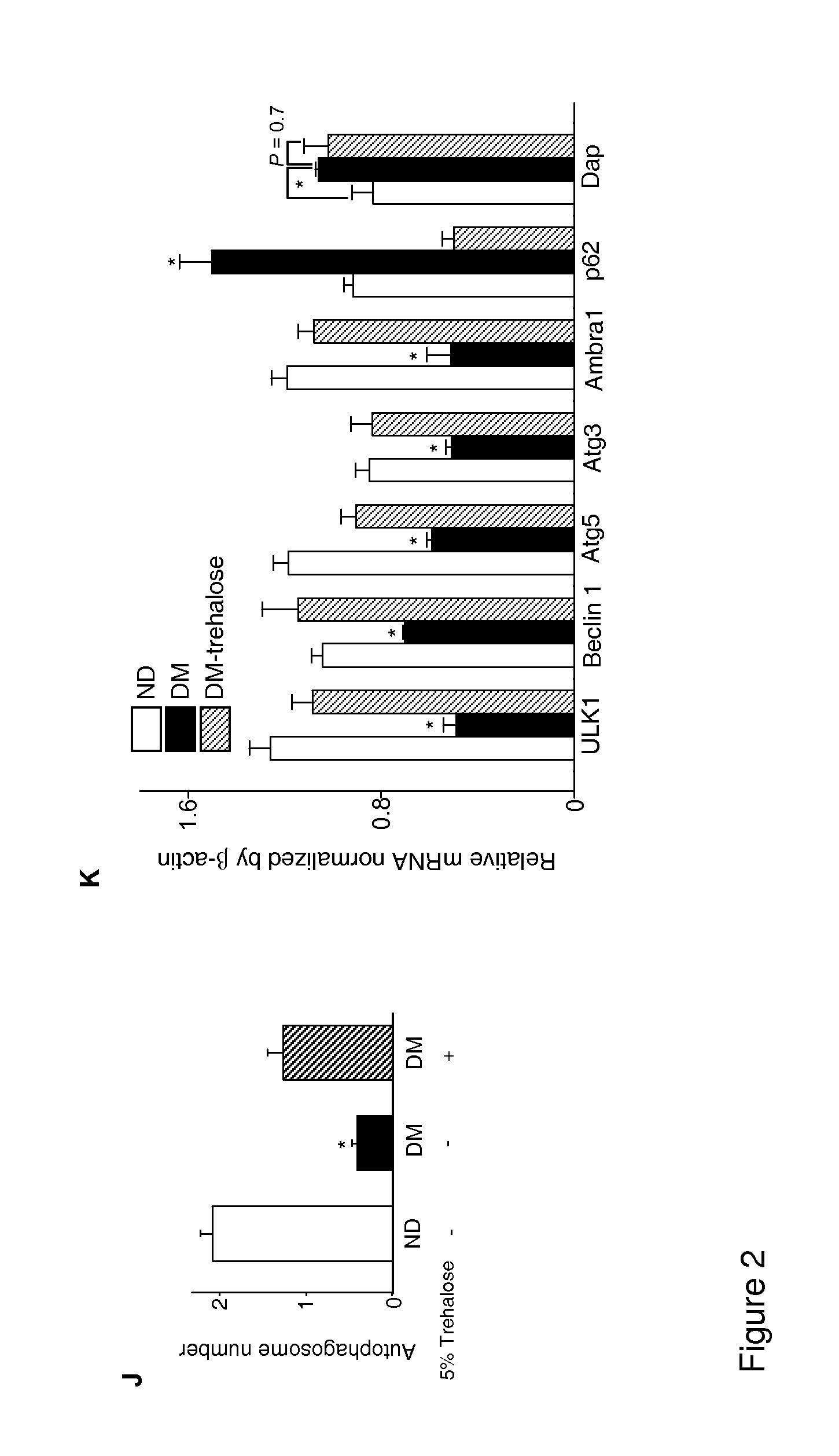
Use of trehalose for prevention of neural tube defects
ActiveUS20130316971A1Preventing hyperglycemia-induced embryonic neural tube defectsRestores cellular homeostasisOrganic active ingredientsBiocideAcute hyperglycaemiaNeurogenesis
Maternal diabetes suppresses autophagy in neuroepithelial cells of the developing neural tube which leads to neural tube defect formation. Trehalose treatment reversed autophagy impairment and prevented neural tube defects in diabetic pregnancies. Trehalose resolved homeostatic imbalance by correcting mitochondrial defects, dysfunctional proteins, ER stress, apoptosis, and delayed neurogenesis in the neural tubes exposed to hyperglycemia. Methods of using trehalose as an intervention against hyperglycemia-induced neural tube defects are provided herein.
Owner:UNIV OF MARYLAND
Pharmaceutical composition comprising progestogens and/or estrogens and 5-methyl-(6S)-tetrahydrofolate
The present invention relates to a pharmaceutical composition which comprises progestogens, preferably drospirenone, estrogens, preferably ethinylestradiol and 5-methyl-(6S)-tetrahydrofolate, can be employed as oral contraceptive and moreover prevents disorders caused by folate deficiency in the consumers, in particular cardiovascular disorders and, after conception of the embryo, congenital malformations caused by folate deficiency such as, for example, neural tube defects, ventricular valve defects, urogenital defects, and cleft lip, jaw and palate, without masking the symptoms of vitamin B12 deficiency, and at the same time even in the case of homozygous or heterozygous polymorphism of methylenetetrahydrofolate reductase facilitates unimpaired utilizability of the folate component 5-methyl-(6S)-tetrahydrofolate by the body and thus its biological activity for preventing the abovementioned congenital malformations caused by folate deficiency. In addition, a prolonged protective effect is maintained after discontinuation of the contraceptive.
Owner:MERCK & CIE KG +1
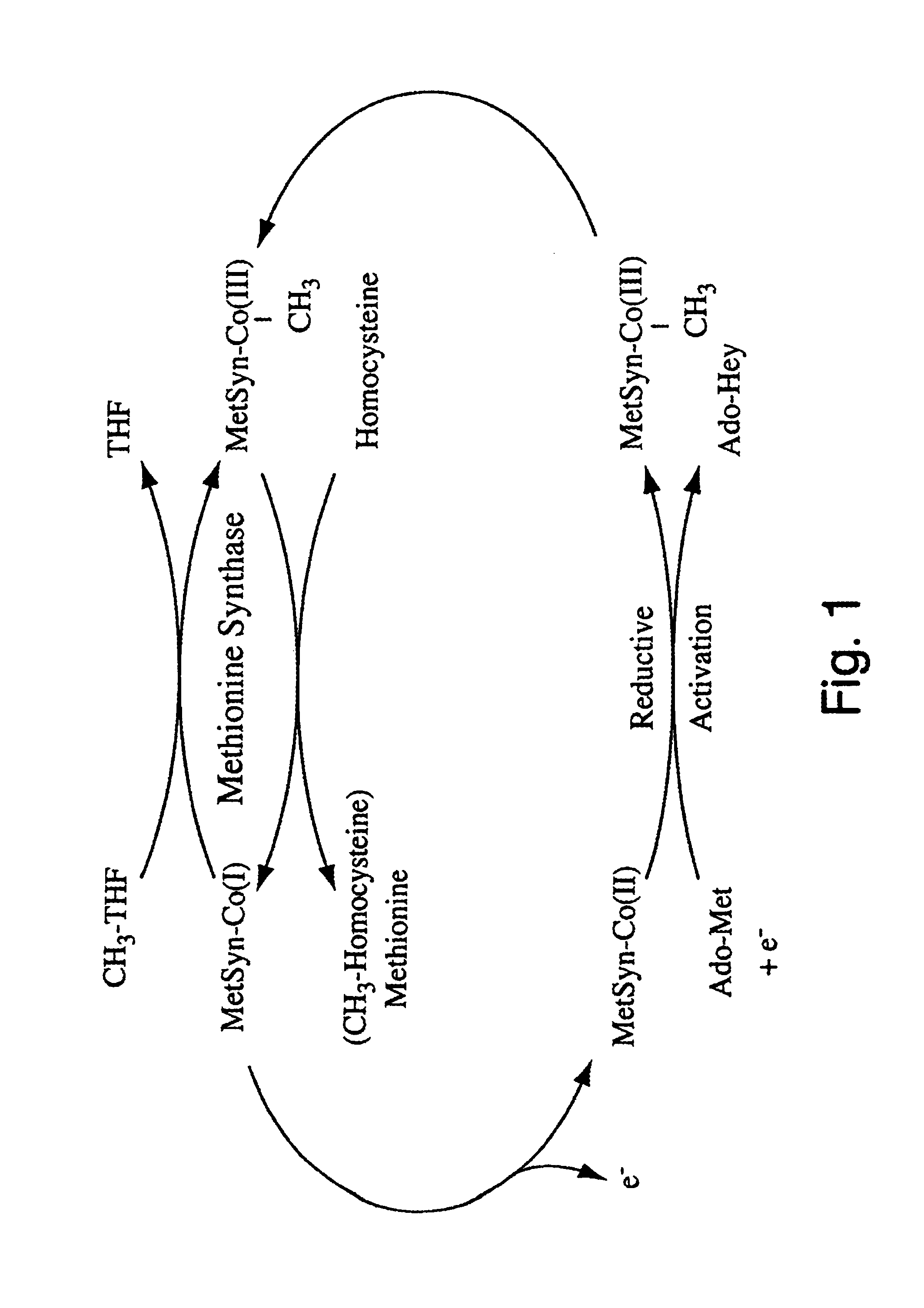
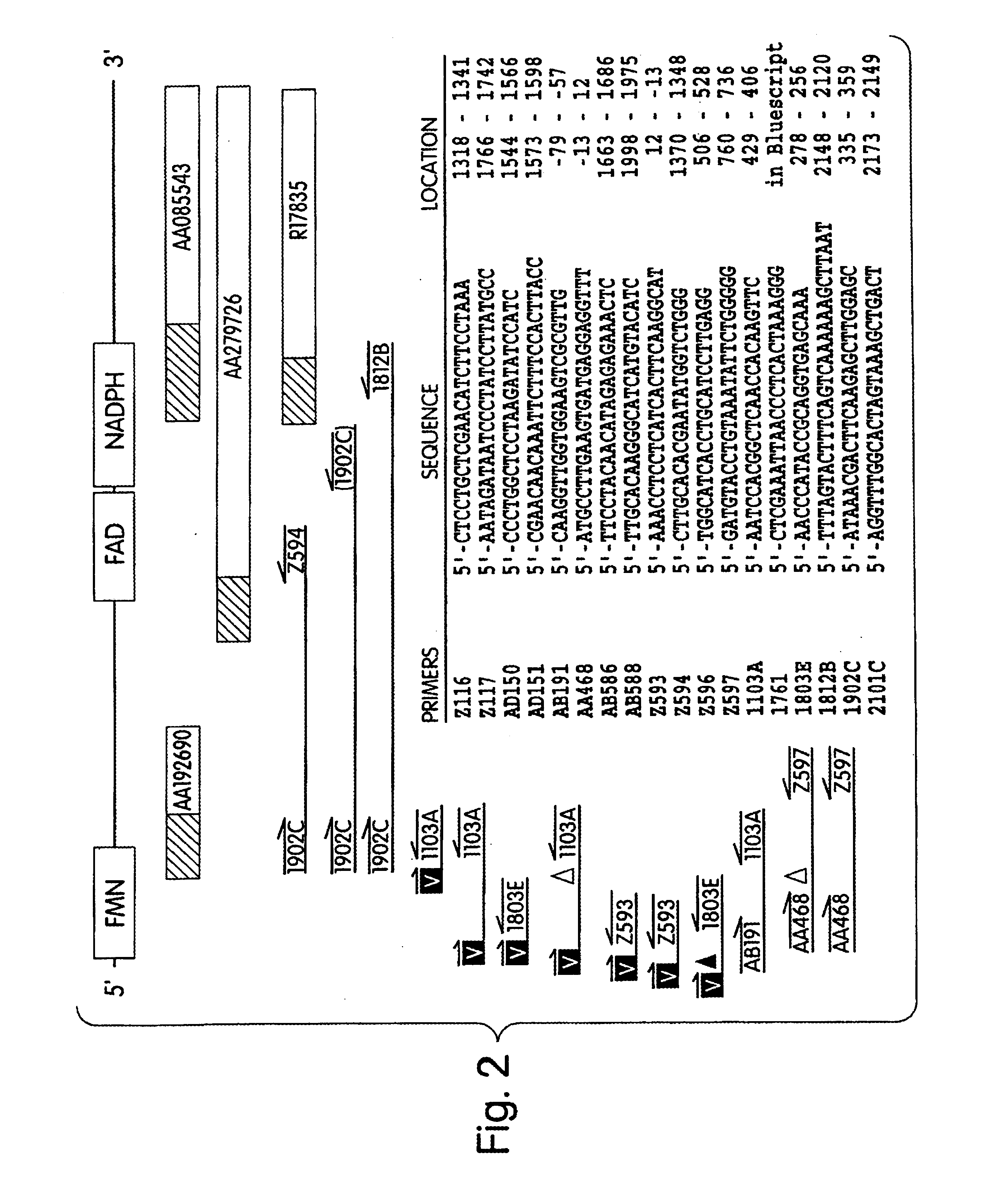
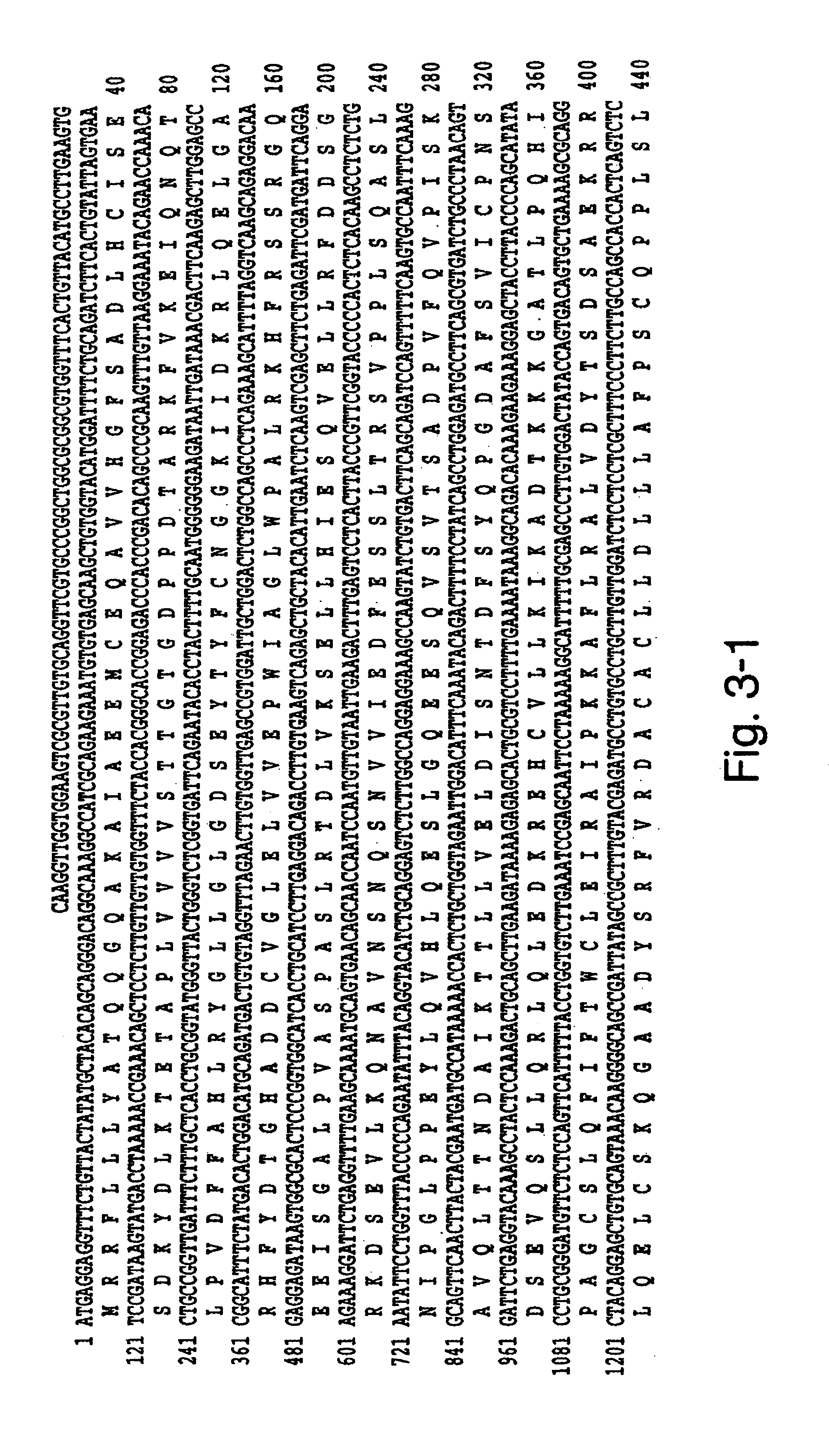
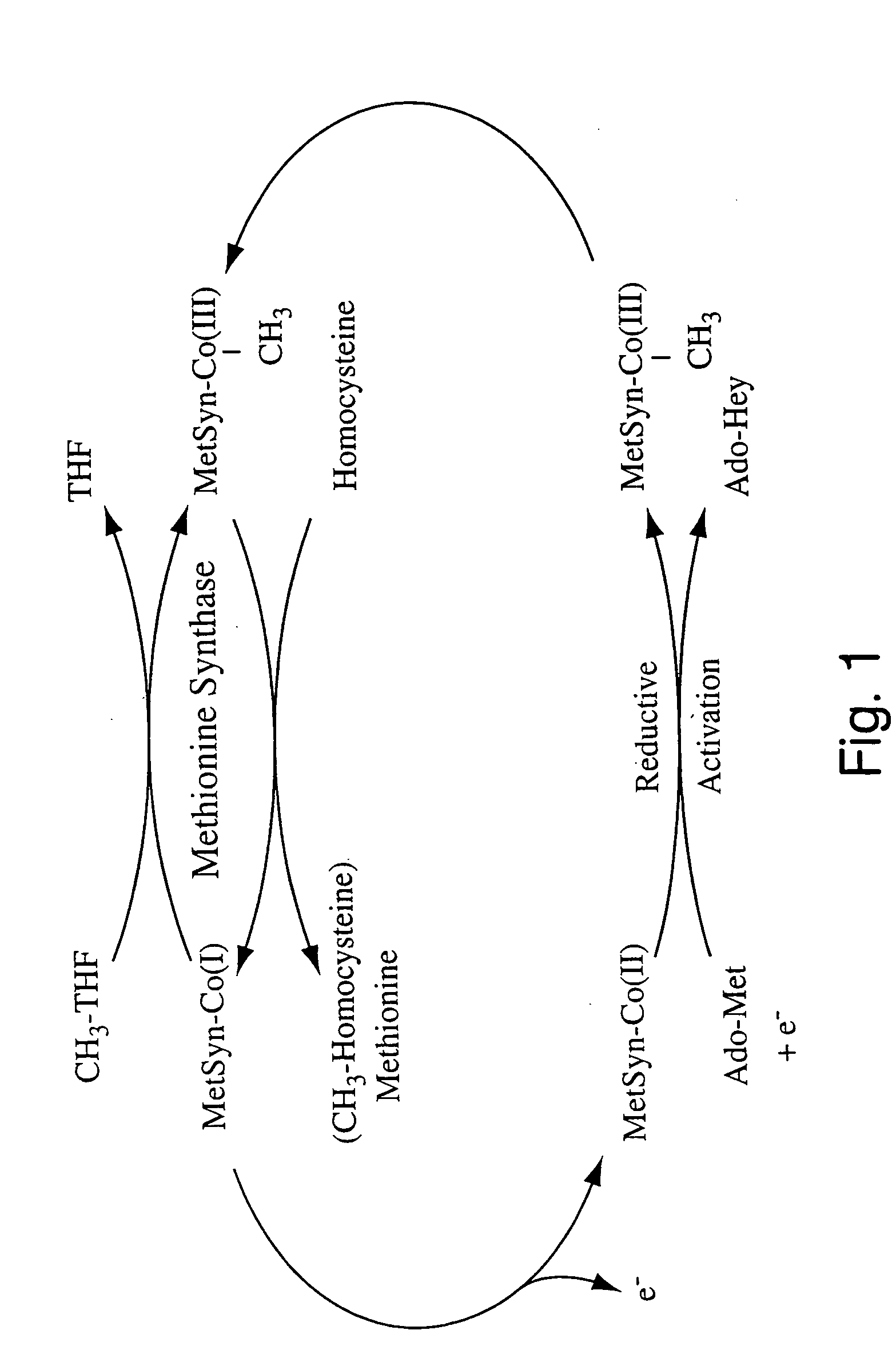
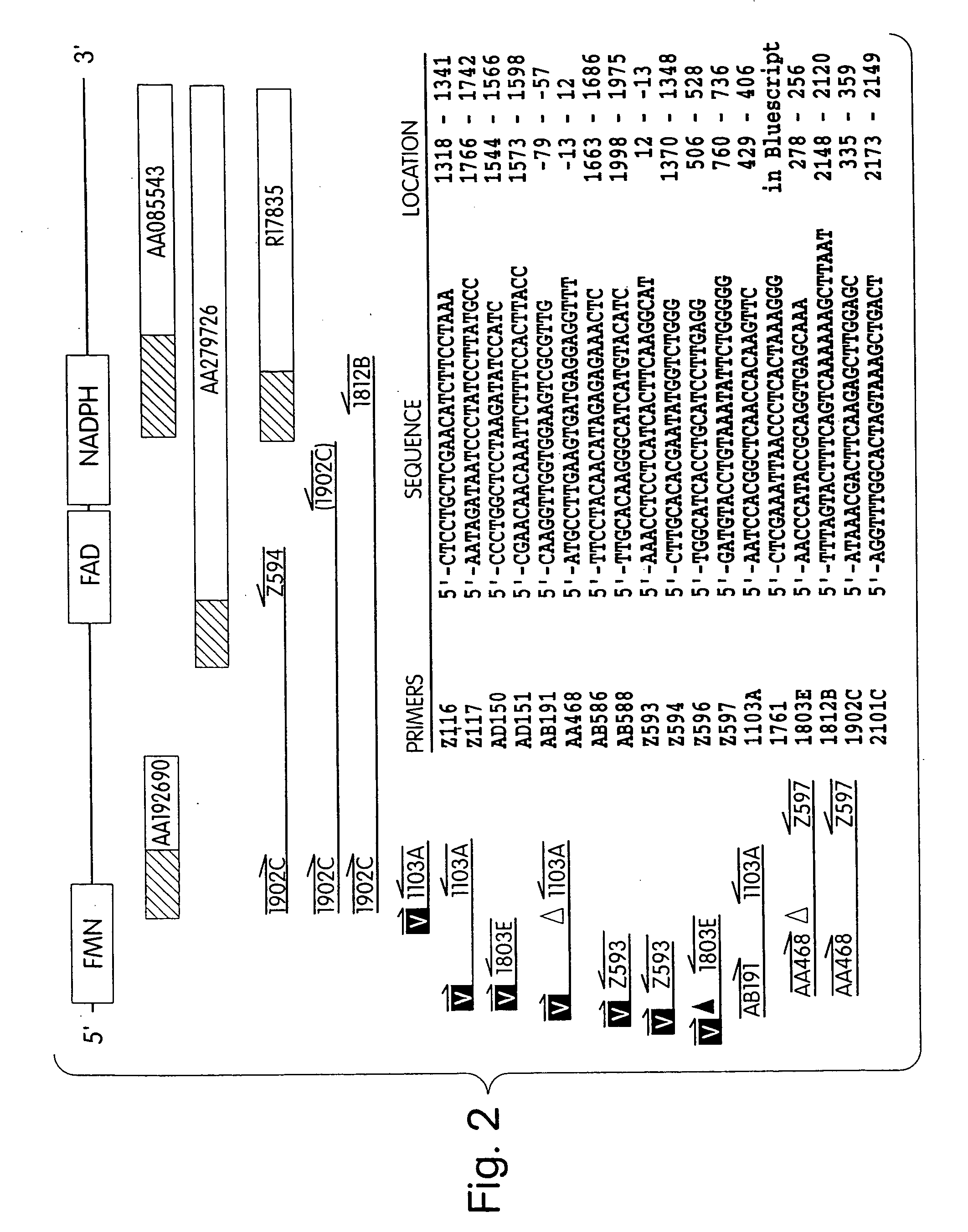
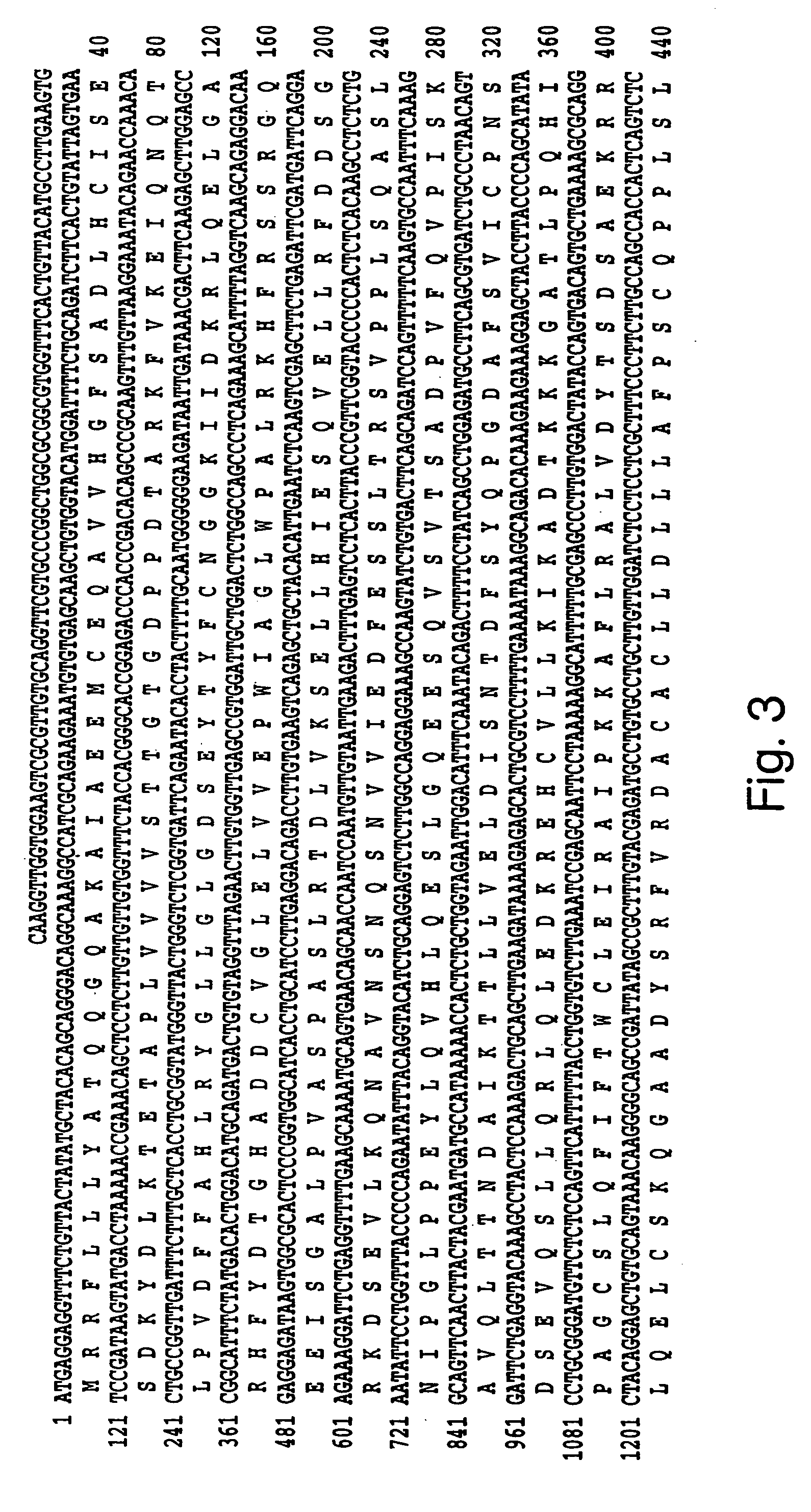
Human methionine synthase reductase: cloning, and methods for evaluating risk of, preventing, or treating neural tube defects, cardiovascular disease, cancer, and down's syndrome
InactiveUS7063944B1Easy to produceIncrease or decrease stabilitySugar derivativesMicrobiological testing/measurementHyperhomocysteinemia(Methionine synthase) reductase
The invention features a novel gene encoding methionine synthase reductase. The invention also features a method for detecting an increased likelihood of hyperhomocysteinemia and, in turn, an increased or decreased likelihood of neural tube defects, cardiovascular disease, Down's Syndrome or cancer. The invention also features therapeutic methods for treating and / or reducing the risk of cardiovascular disease, Down's Syndrome, cancer, or neural tube defects. Also provided are the sequences of the human methionine synthase reductase gene and protein and compounds and kits for performing the methods of the invention.
Owner:MCGILL UNIV
Human methionine synthase reductase: cloning, and methods for evaluating risk of neural tube deffects, cardiovascular disease, cancer and Down's Syndrome
InactiveUS20050191701A1Increase or decrease stabilityEasy to produceMicrobiological testing/measurementTransferasesADAMTS ProteinsHyperhomocysteinemia
The invention features a novel gene encoding methionine synthase reductase. The invention also features a method for detecting an increased likelihood of hyperhomocysteinemia and, in turn, an increased or decreased likelihood of neural tube defects, cardiovascular disease, Down's Syndrome or cancer. The invention also features therapeutic methods for treating and / or reducing the risk of cardiovascular disease, Down's Syndrome, cancer, or neural tube defects. Also provided are the sequences of the human methionine synthase reductase gene and protein and compounds and kits for performing the methods of the invention.
Owner:MCGILL UNIV
Reduction of embryonic neural tube defects by pre-or early-administration of germination-activated sporoderm-broken ganoderma lucidum spores to pregnant female
InactiveUS20060269568A1Promote proliferationPromote differentiationBiocideNervous disorderSporePregnancy
The present invention provides a method for preventing and reducing embryonic neural tube defects (NTDs) caused by cell cycle arrest in the neuroepithelial cells of the embryo. The method comprises administering germination activated sporoderm-broken Ganoderma lucidum spores (“GLSs”) to a female mammal capable of becoming pregnant an effect amount of GLSs. GLSs are preferred to be administered to the female mammal prior to or at an early stage of the pregnancy. The GLSs reduce embryonic NTDs caused by cell cycle arrest in the neuroepithelial cells.
Owner:KINDWAY INT
Method of treatment and compositions of D-chiro inositol and phosphates thereof
InactiveUS20080103116A1Prevent and reduce chanceReduce or prevent fetal malformation occurrenceBiocideHydroxy compound active ingredientsD-chiro-InositolPhosphate
The present invention relates to the use of D-chiroinositol or a phosphate thereof in combination with folate for the reduction or prevention of congenital deformations such as anorectal malformations, neural tube defects, cleft-lip, cleft palate, and other birth defects. The invention further relates to the use of D-chiroinositol or a phosphate thereof in quieting or preventing the sensitivity of breast tissue to estrogenic, progestogenic, and or anti-androgenic insult, whether from environmental, dietary, or medicinal sources. Co-therapies as well as combination products of D-chiro-inositol (or a phosphate thereof) with at least one of (a) a folate source and (b) one or more of an estrogenic substance, a progestogenic substance, and / or an antiandrogenic substance are also claimed.
Owner:JENNINGS SPRING BARBARA L
Dietary supplement formula of nutritional supplement food for pregnant women and method thereof
The invention relates to a dietary supplement formula of a nutritional supplement food for pregnant women and a method thereof. The dietary supplement formula is characterized in that the total dose formula of the nutritional supplement food eaten with meals daily comprises the following components: 20 grams of protein powder, 9 calcium-magnesium tablets, 6 pieces of natural vitamin B, 3 pieces of vitamin C complex, 6 iron folic acid tablets, 3 grains of carotenoid, 6 wheat germ oil capsules, and 6 echinacea tablets. The dietary supplement formula has a health-care function of enhancing immunity and alleviating physical fatigues, is more reasonable for supplementing the quantities and the types of vitamins, proteins, mineral matters and microelements. By using the dietary supplement formula, fetal neural tube defects can be thoroughly avoided, the immunological competence of a pregnant woman can be in the optimum state, and the pregnant woman can live through the duration of pregnancy without disasters and diseases and create a good developmental condition for a fetus.
Owner:刘建敏
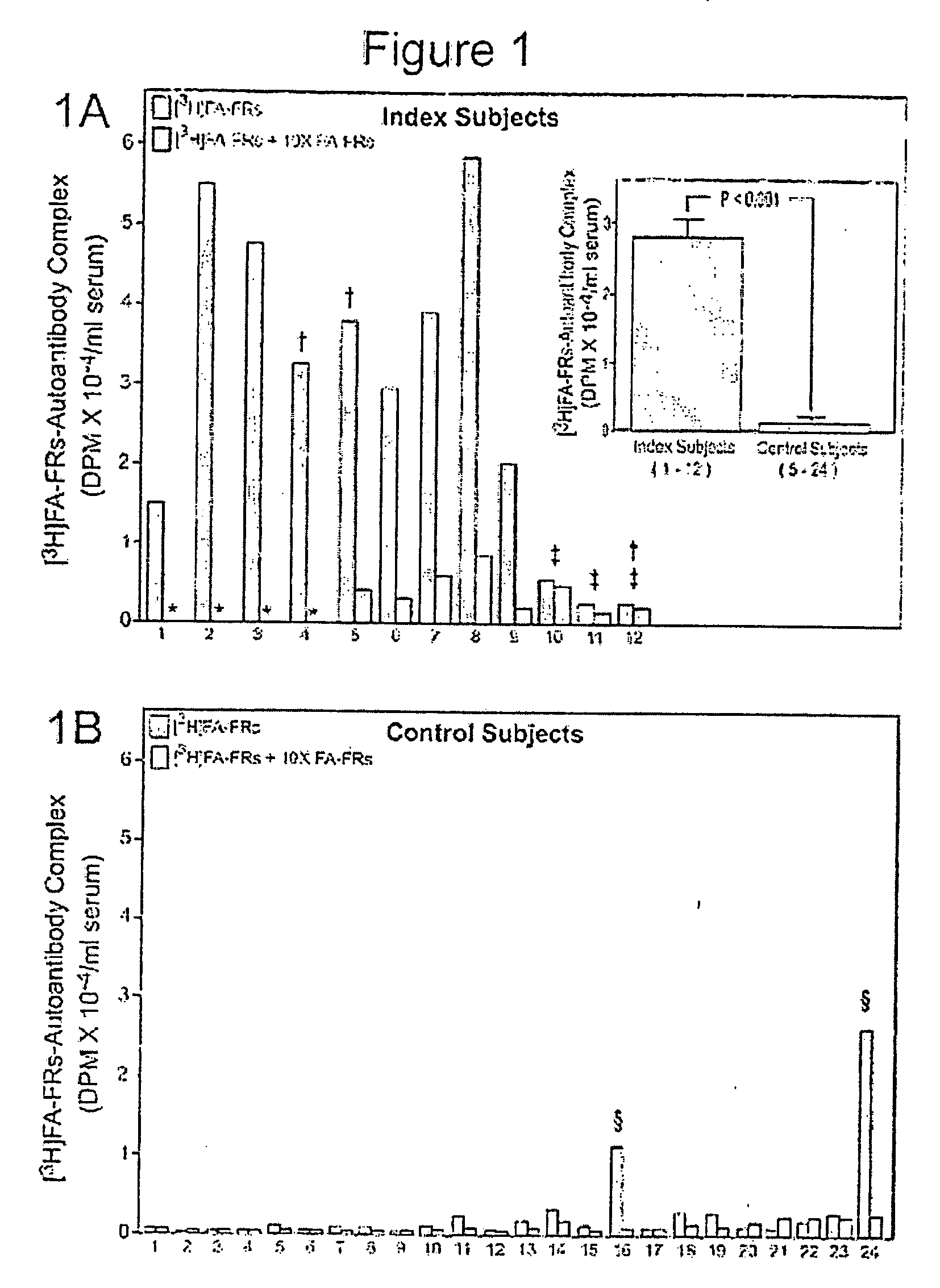
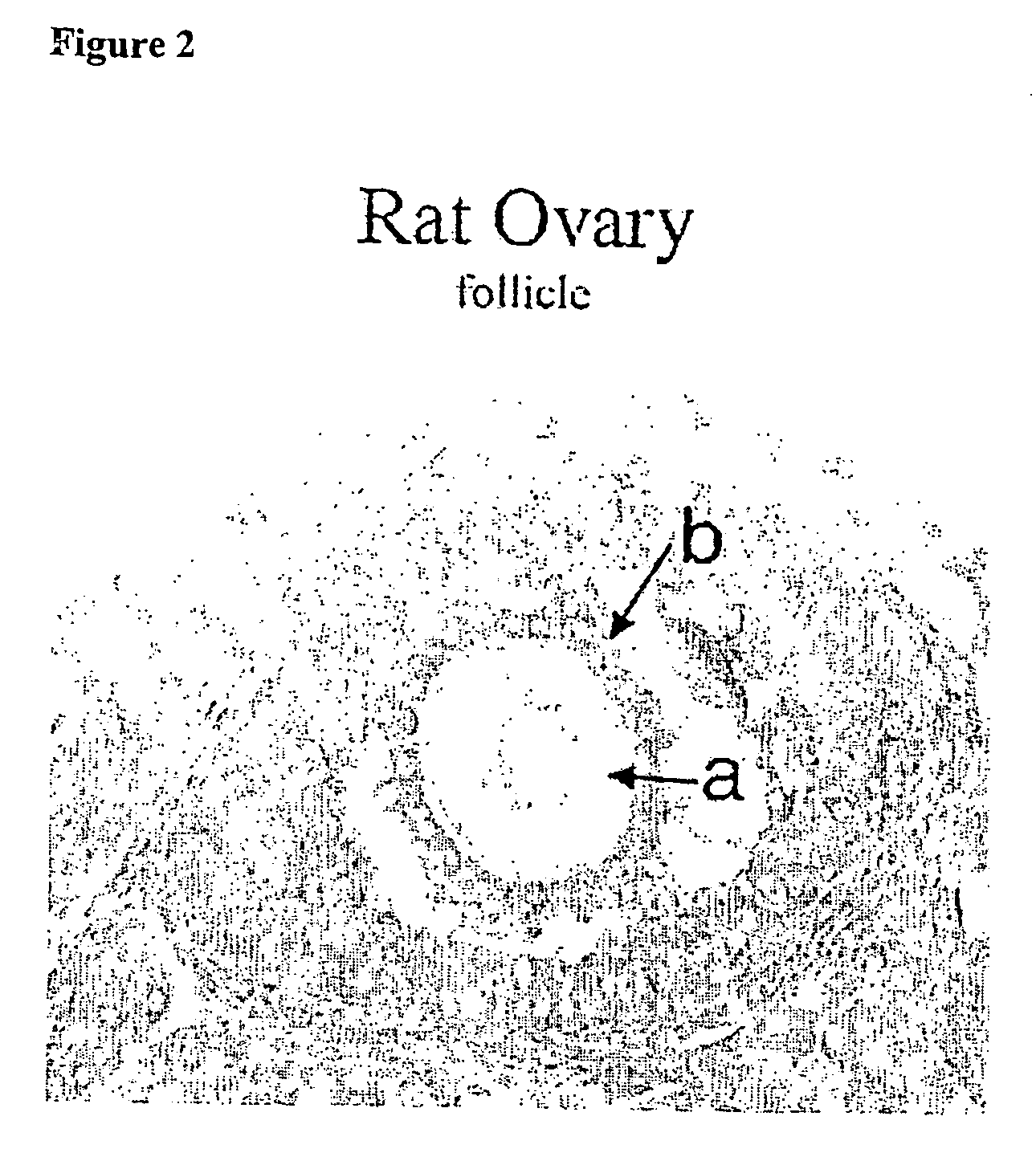
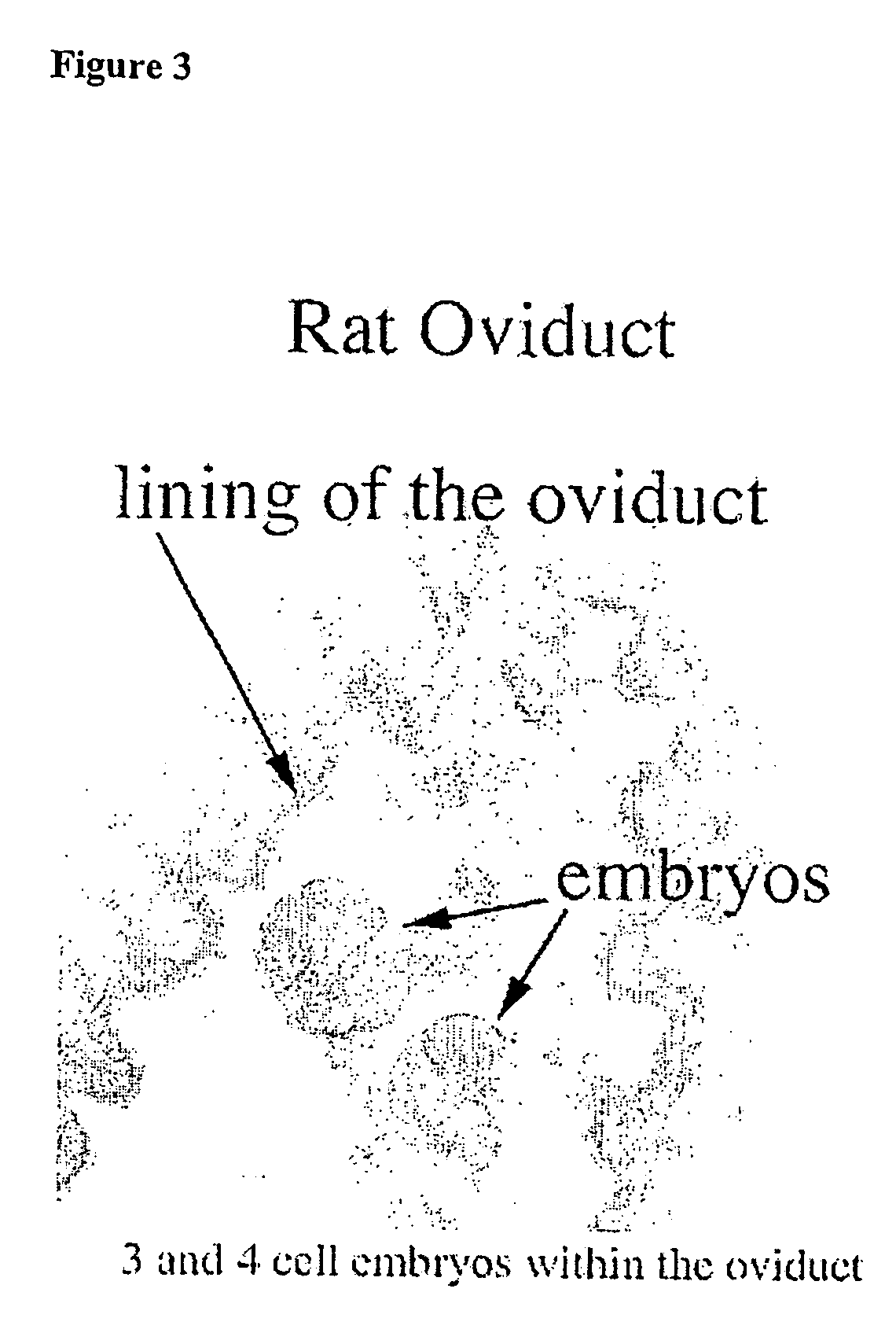
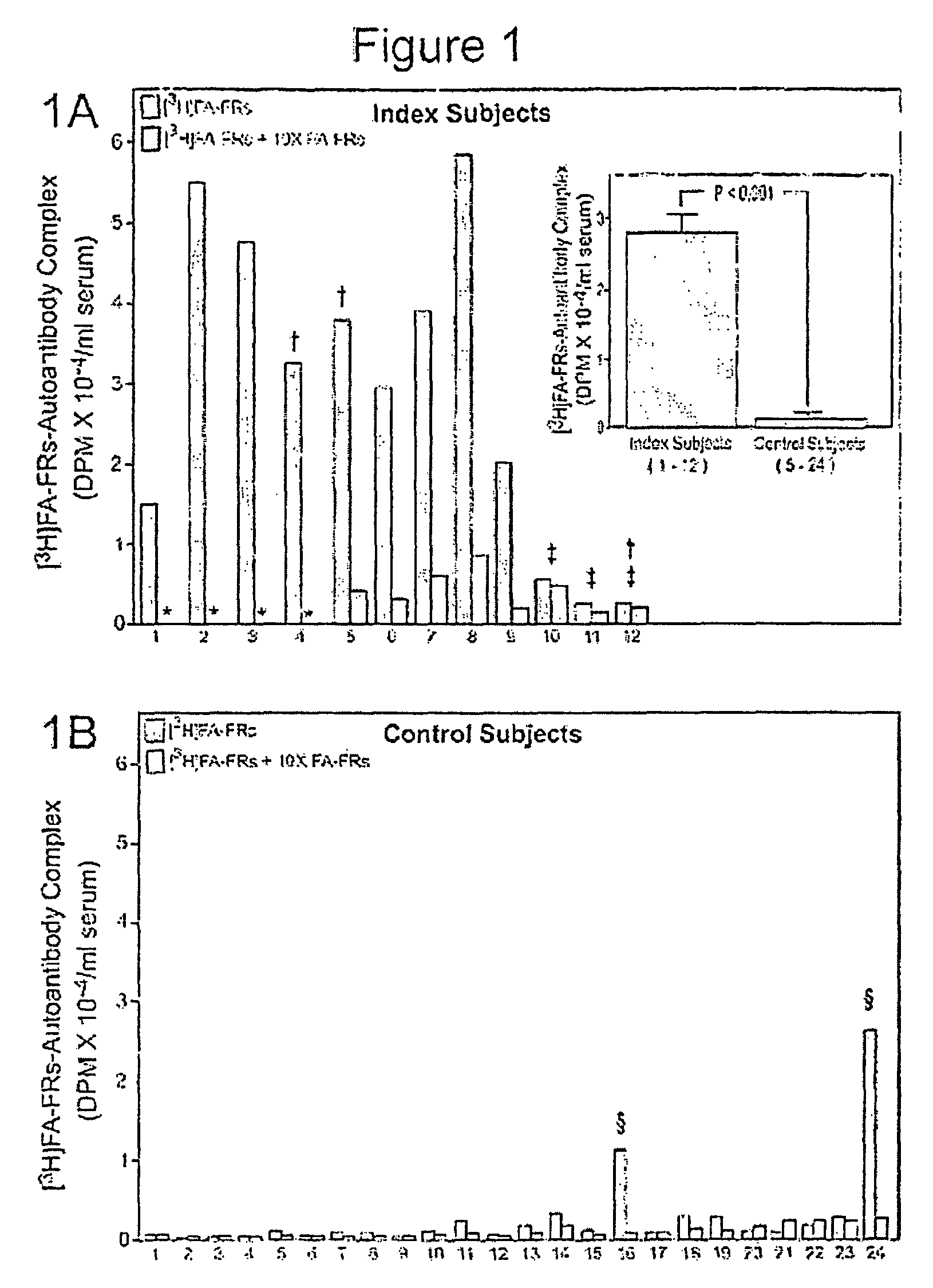
Assay for autoantibodies to folate receptors
The present invention identifies autoantibodies to folate receptors. Methods to identify these autoantibodies to the human folate receptors are also provided. The present invention also contemplates diagnostic methods and test kits to be used in a clinical setting for identifying a subject at risk of folate-sensitive abnormalities or disorders as observed in neural tube defect complicated pregnancies. In addition, infertility, spontaneous abortion, male sterility, unsuccessful in vitro fertilization, neurologic disorders and impaired folate absorption may also be associated with these autoantibodiesto folate receptors.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
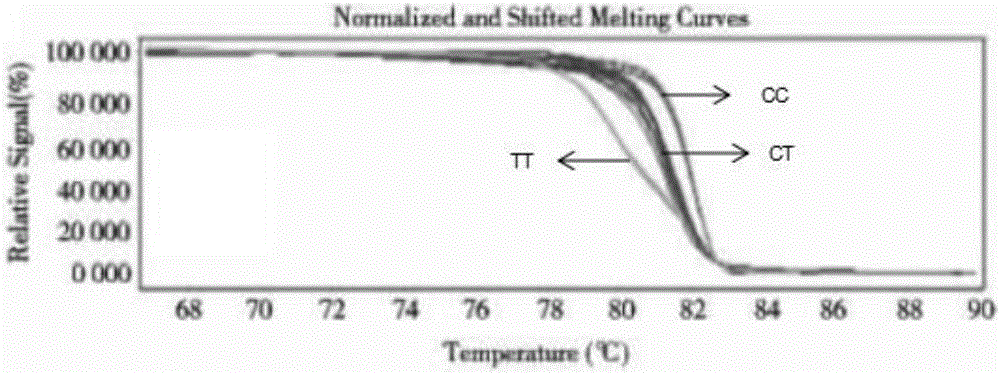
Kit for guiding folic acid taking dose for pregnant women and using method of kit
InactiveCN106566883ASafe to takeReduce spontaneous abortionMicrobiological testing/measurementPhysiologyAbortion
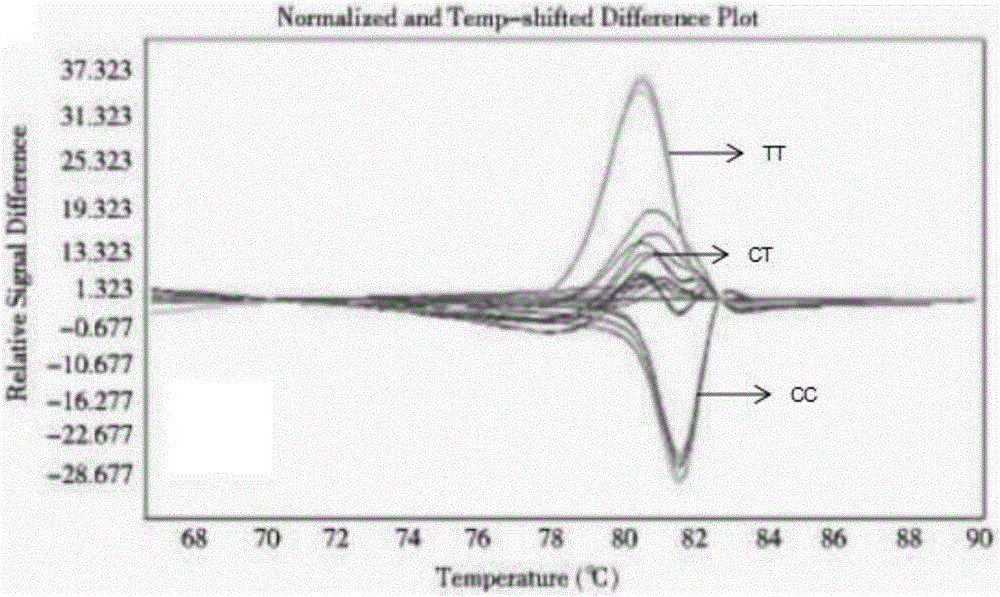
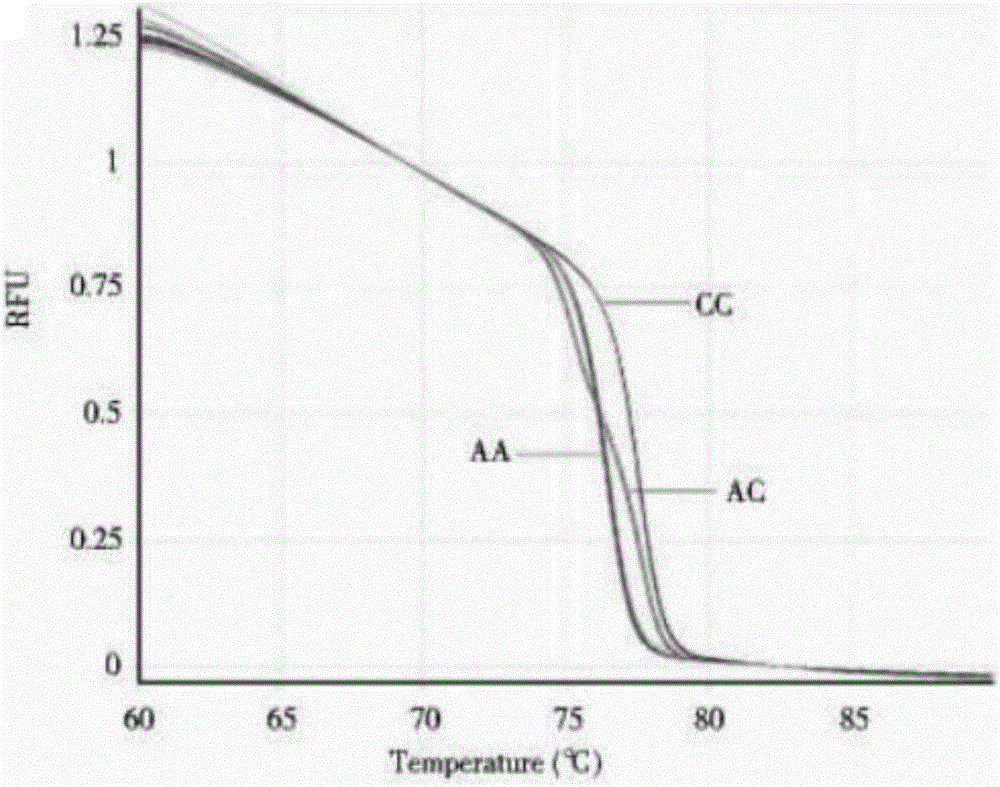
The invention provides a kit for guiding a folic acid taking dose for pregnant women. The kit consists of MTHFR C667T, MTHFR A1298C and MTRR A66G amplification primers and a PCR reaction reagent. The invention also provides a method of using the kit. According to the kit provided by the invention, folic acid metabolism-related genes of the pregnant women are detected by virtue of an HRM method, and then, folic acid supplementing and taking are guided for the pregnant women in accordance with genotype, so that the pregnant women can safely and effectively take folic acid and the occurrence of such circumstances as spontaneous abortion, premature delivery, neural tube defects and the like can be relieved.
Owner:成都睿杰森生物科技有限公司
Compositions and Methods of Detecting and Treating Neural Tube Defects
The present invention generally relates to compositions, reagents and methods for detecting and treating a neural tube defect in a fetus. One aspect of the invention provides a method including administrating a composition containing noggin or LDN-193189 to the fetus in utero. In certain embodiments, the composition is administrated if the maternal blood or amniotic fluid contains an elevated amount of BMP4 and / or a reduced amount of noggin. In another aspect of the invention, the method includes administrating a composition containing GDC-0449 to the fetus in utero. In certain embodiments, the GDC-0449 is administrated if the maternal blood or amniotic fluid contains an elevated amount of sonic hedgehog.
Owner:ANN & ROBERT H LURIE CHILDRENS HOSPITAL OF CHICAGO
Application of CDR1as to prenatal screening of neural tube defects, and product and method for performing prenatal screening of neural tube defects
ActiveCN110016504AEasy to detectReduce professional requirementsMicrobiological testing/measurementAgainst vector-borne diseasesCongenital malformationsRoutine screening
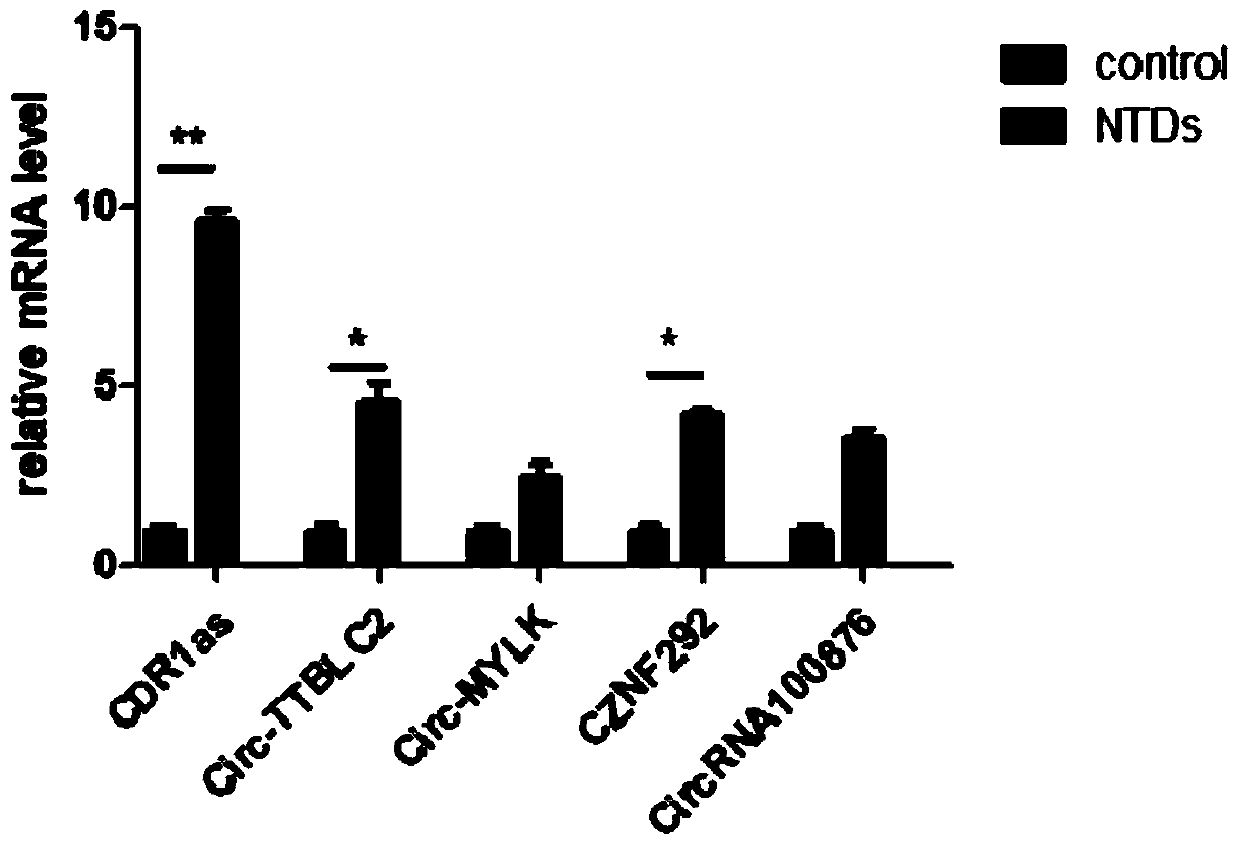
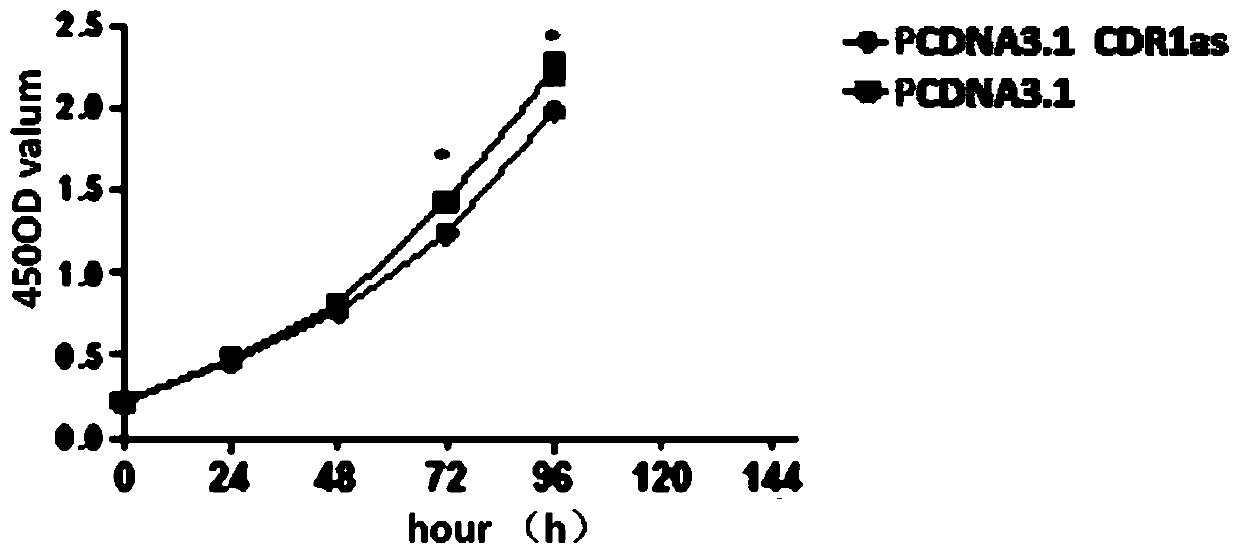
The invention provides an application of CDR1as to prenatal screening of neural tube defects, and a product and method for performing prenatal screening of neural tube defects, and relates to the technical field of clinical diagnosis. The invention provides an application of circRNA-CDR1as as a marker for prenatal screening of neural tube defects. Through research, the inventor finds out that the,circRNA-CDR1as is notably highly expressed in the tissue of NTDs child patients, the circRNA-CDR1as can be separately or can be united with other markers to be used for conventional screening of congenital malformation at the early pregnancy, and has great significance to families and society. According to the product and method for performing prenatal screening of neural tube defects, through detecting the expression number of the circRNA-CDR1as, NTDs is diagnosed, the detection cost is low, the detection is accurate and quick, and the NTDs can be diagnosed at the early pregnancy.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
Application of anti-inositol metabolism medicament to establishment of mouse model with NTDs
InactiveCN105920605ASmall side effectsHigh deformity rateInorganic active ingredientsSide effectBiological studies
The invention provides a method for obtaining a mouse model with neural tube defects (NTDs). The mouse model with the NTDs is established by applying an anti-inositol metabolism medicament to a pregnant mouse from the embryonic development of the pregnant mouse to the formation of neural tubes. The mouse model can be used for medical and biological research of the NTDs, is helpful to understand the causes and pathogenesis of human NTDs, and provides a model for further research on an action mechanism of inositol metabolic disorders in the NTDs. Moreover, according to the method, the neural tube defect rate is high, absorbed embryos are few, the embryo fatality rate is low, and toxic and side effects to the pregnant mouse are low.
Owner:王建华
Isolation of human neural stem cells from amniotic fluid of patients with neural tube defects
The present invention provides a method for isolating human neural stem cells from amniotic fluid of a patient whose fetus has been diagnosed to have a neural tube defect. Use of the isolated human neural stem cells in the treatment of neurological disorders is also provided.
Owner:FOOD IND RES & DEV INST
Application of antifolic in constructing mouse model with neural tube defects (NTDs)
InactiveCN102228690BIncreased sensitivityTeratogenicity test period is shortIn-vivo testing preparationsPharmaceutical active ingredientsFolic acid metabolismRepeatability
The invention relates to an application of antifolic in constructing a mouse model with neural tube defects (NTDs). Specifically, an anti-folic acid metabolism drug is adopted for intervening a pregnant mouse to generate folic acid dysbolism and cause abnormal embryonic development of the mouse, thereby constructing a mouse model with NTDs. The method adopted by the invention has simplicity of operation, good repeatability, high teratogenesis rate and stable effect, and simulates an animal model close to human on the pathogenesis of NTDs by obstructing normal metabolism of folic acid, thereby facilitating further studies on congenital anomalies, especially NTDs.
Owner:首都儿科研究所
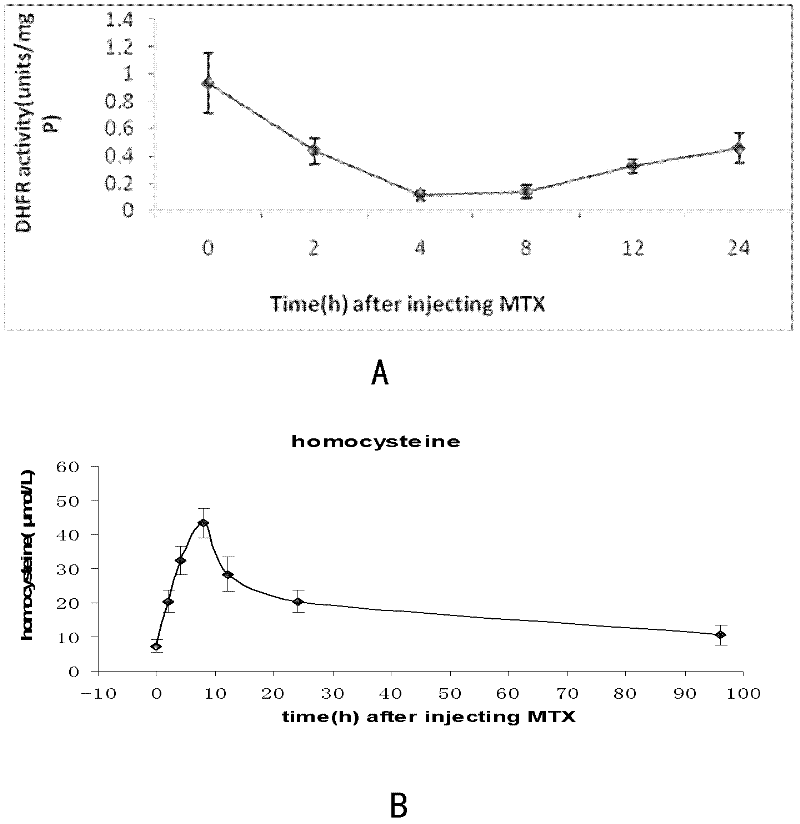
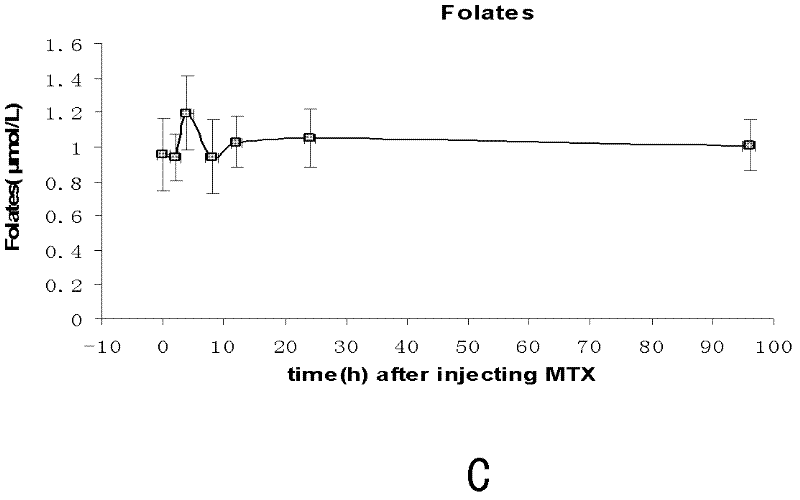
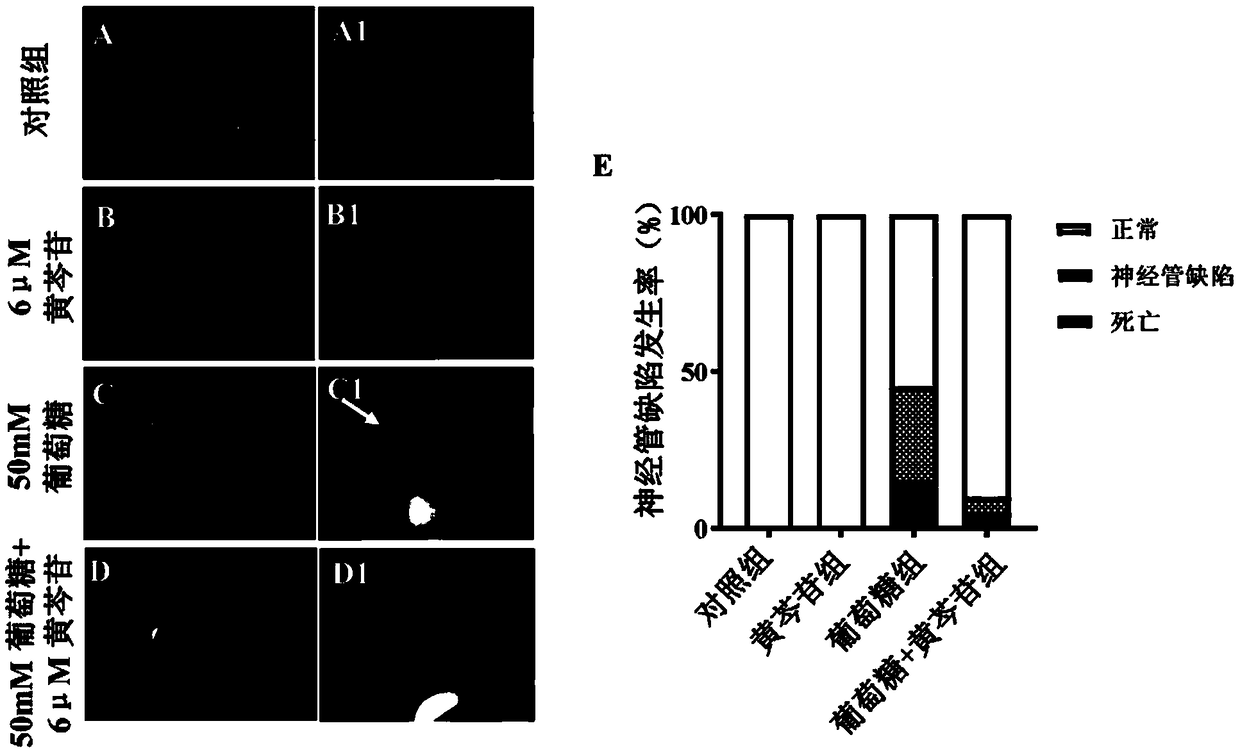
Application of baicalin in preparation of drug for treating fetal neural tube defect caused by gestational diabetes mellitus
ActiveCN109381473ADecreased retinoic acid levelsPromote self-renewalOrganic active ingredientsNervous disorderSide effectNeonatal diabetes
The invention provides application of baicalin in preparation of a drug for treating a fetal neural tube defect caused by gestational diabetes mellitus. Based on a model of a fetus with gestational diabetes mellitus, which is established by a chick embryo, a chick embryo experiment is performed by adopting a traditional Chinese medicine monomer baicalin, a result shows that a certain concentrationof baicalin can be used for effectively relieving the neural tube defect induced by a high glucose environment and effectively relieving poor neural tube cell differentiation, elevation of the levelof homocysteine, reduction of the level of retinoic acid and inhibition on self-renewing of neural stem cells caused by the high glucose environment. According to the application, the application range of the baicalin is enlarged, the application value of the baicalin is improved, and the development of new drugs can be further facilitated, for example, the activity of the baicalin is hopefully improved, or the side effect of the baicalin is reduced by structural modification or transformation by taking the baicalin as a lead compound; the safety drug is provided for preventing the neural tubedefect of the fetus with gestational diabetes mellitus, therefore, the application has good application prospect.
Owner:JINAN UNIVERSITY
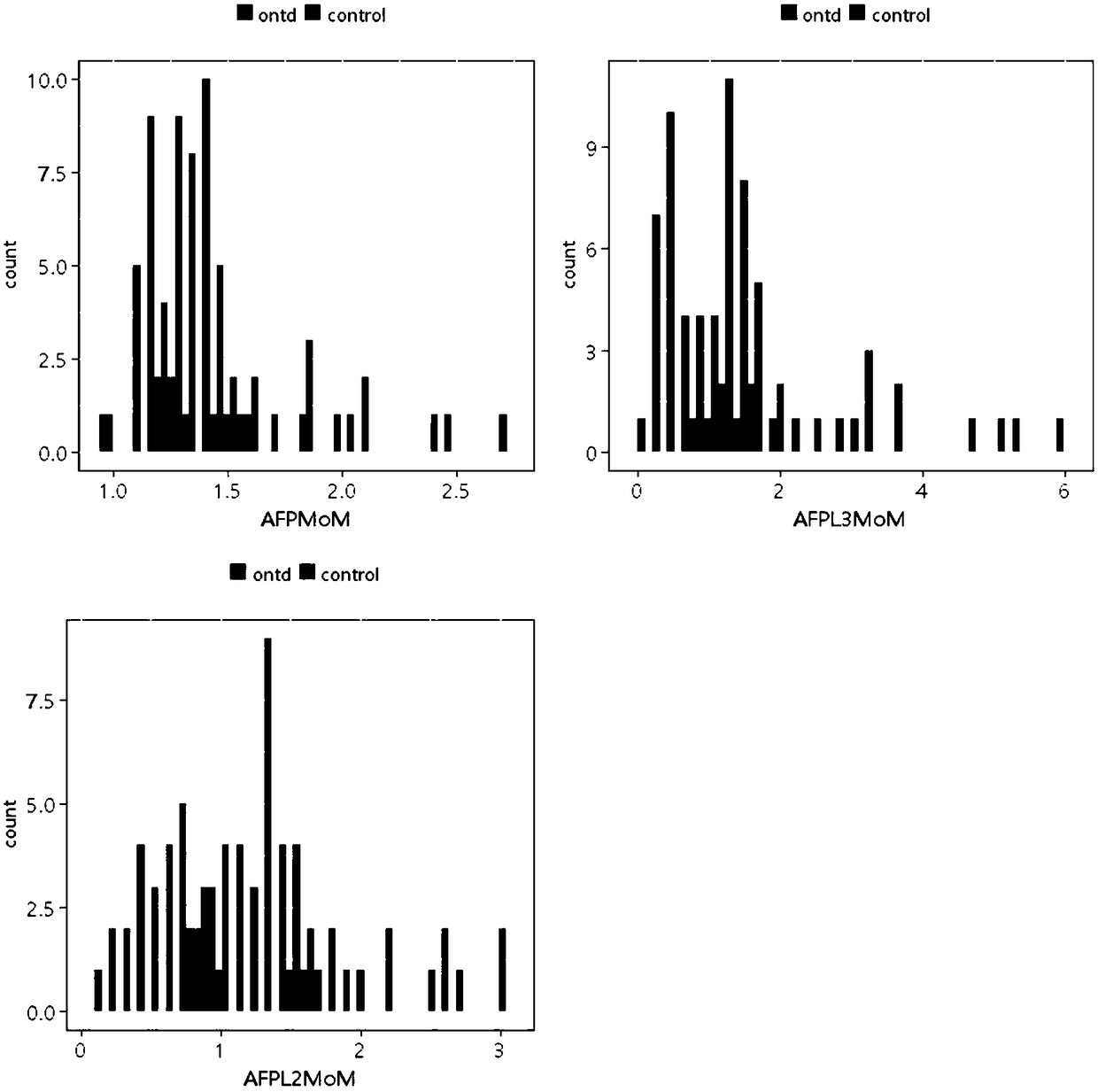
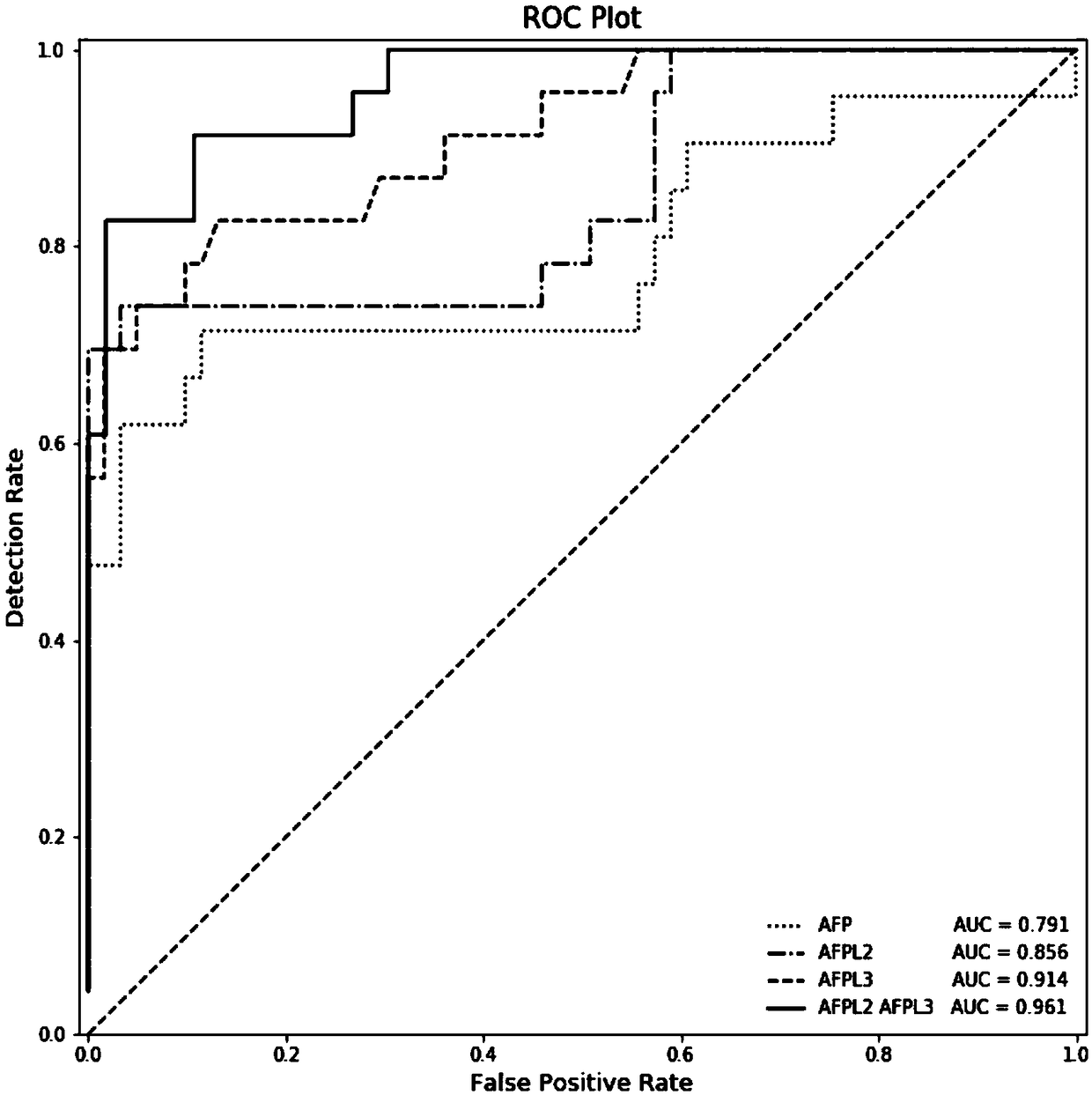
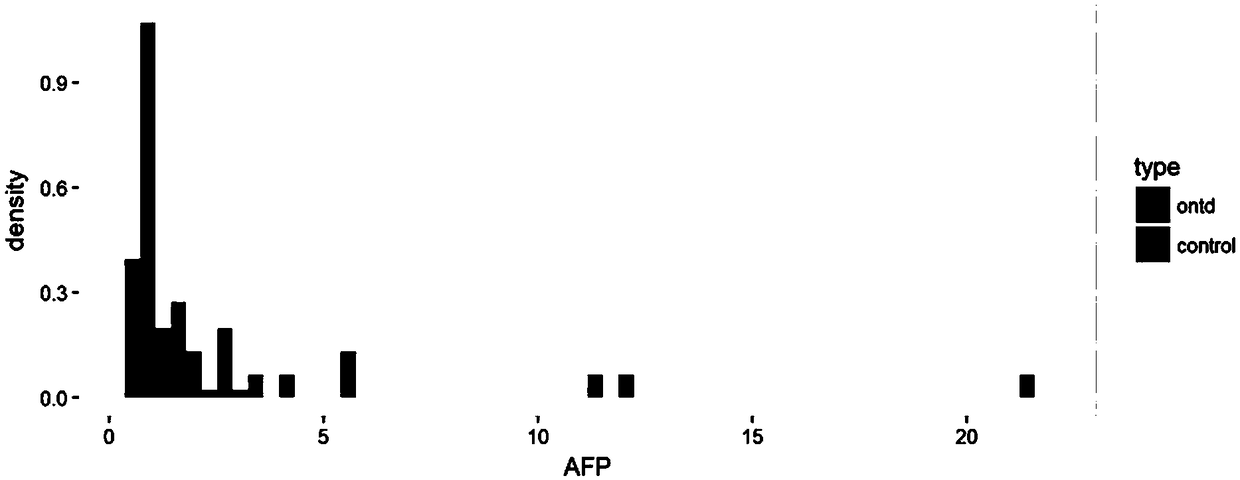
Method for screening open neural tube defects through maternal serum alpha-fetoprotein heteroplasmons L2 and L3 in second trimester
ActiveCN109187983AHigh sensitivityImprove featuresBiological testingTrue positive rateSecond trimester
The invention discloses a method for screening open neural tube defects (ONTD) of fetuses through maternal serum alpha-fetoprotein heteroplasmons L2 and L3 in a second trimester. The method comprisesthe following steps: (1) forming a case group by multiple pregnant women with fetuses determined as ONTD fetuses through ultrasonic tomography, and randomly forming a control group through multiple pregnant women with fetuses who grow normally; (2) detecting the levels of AFP-L2 and AFP-L3 of serums of two groups of the pregnant women by virtue of ELISA, carrying out statistical treatment on the correlation between the levels of AFP-L2 and AFP-L3 of the serums of the pregnant women in the second trimester and the ONTD of the fetuses, and analyzing the screening efficiency by virtue of different constructed risk calculation models; and (3) calculating the optimal critical values and areas under the curve of AFP-L2 and AFP-L, for screening the ONTD fetuses, of the serums of the pregnant women. The method has the beneficial effects that AFP-L2 and AFP-L of the serums of the pregnant women in the second trimester have relatively high sensitivity and specificity to the screening of the ONTDfetuses and are relatively good markers for screening the ONTD, and the screening efficiency of the risk calculation model constructed by virtue of AFP-L2 and AFP-L3 is better than that of an MoM value respectively corresponding to AFP, AFP-L2 and AFP-L3.
Owner:杭州市妇产科医院
Molecular marker for detecting neural tube malformation and application of marker
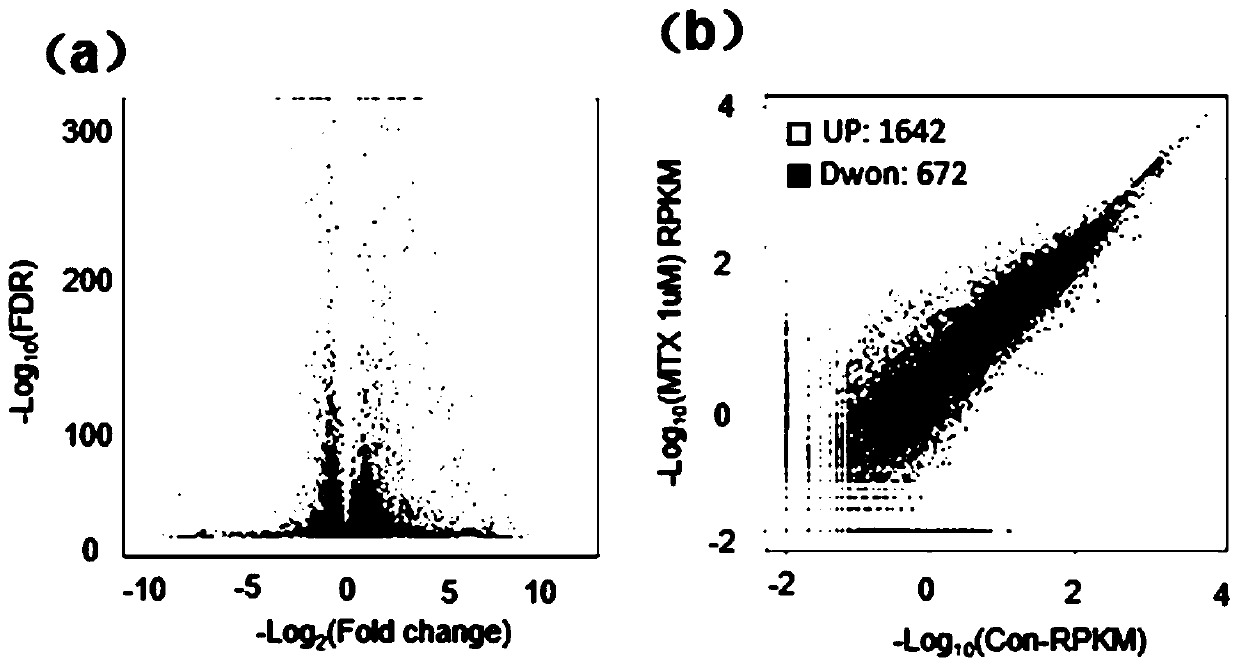
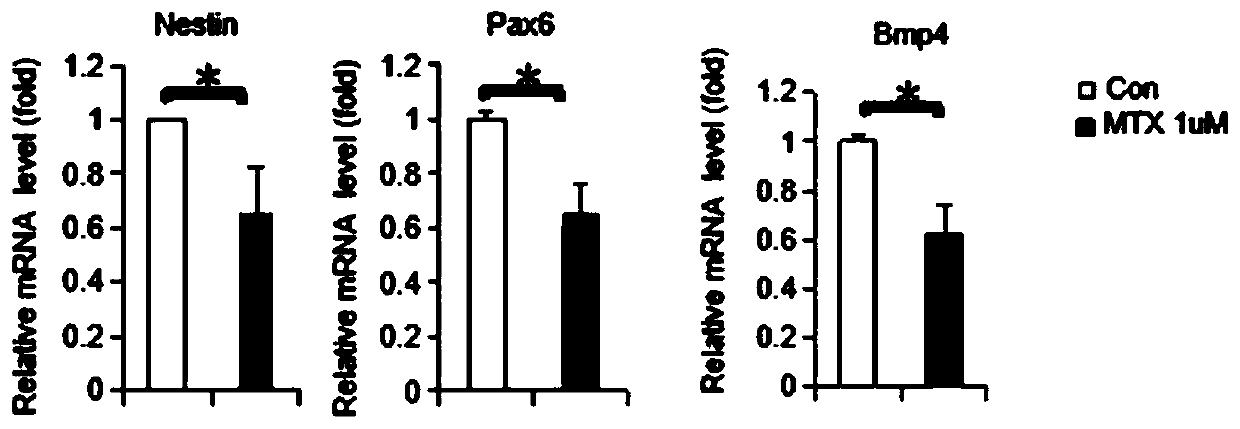
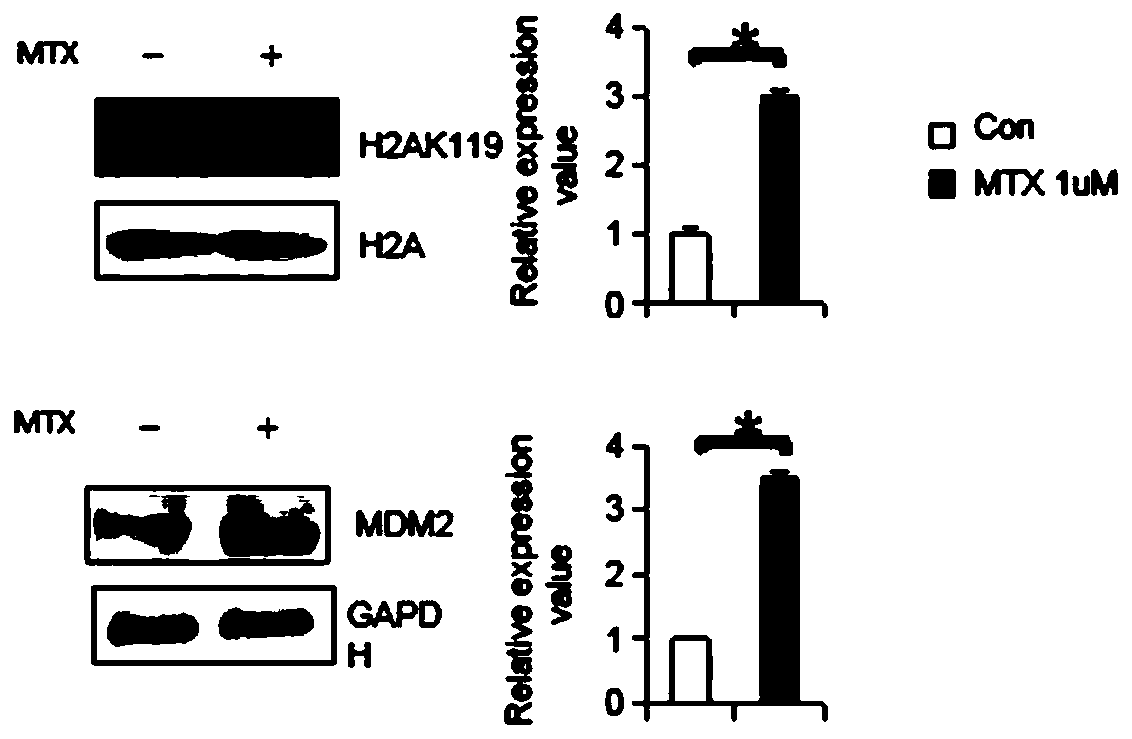
ActiveCN109988829AAchieve early diagnosisAchieve interventionMicrobiological testing/measurementDisease diagnosisPrenatal diagnosisPAX6
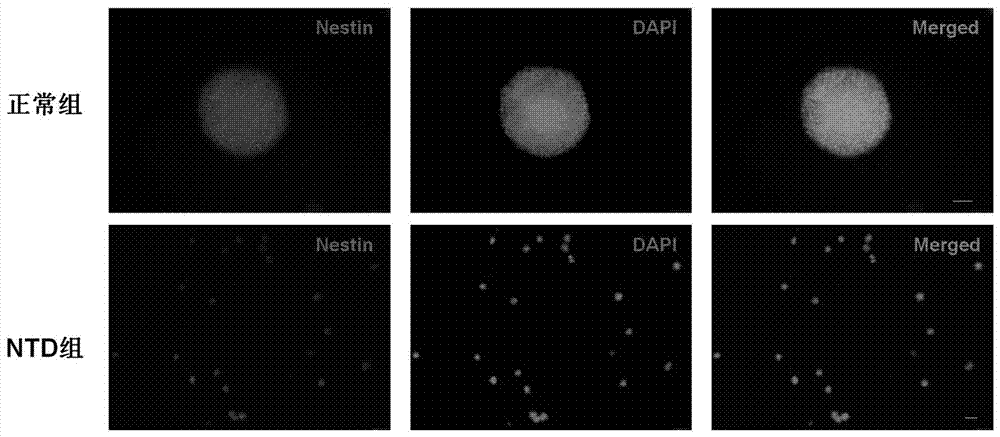
The invention belongs to the field of biology, in particular to a molecular marker for detecting neural tube malformation and an application of the marker. The molecular marker comprises specific marker gene and / or proteins, wherein the specific marker genes is at least one of Pax6, Nestin and Bmp4; and the proteins are H2AK119, Mdm2 and / or a gene encoding at least one of the H2AK119 and the Mdm2.The molecular marker can be used for preparing products for diagnosing or detecting neural tube defects, can quickly and conveniently detect changes of the molecular marker, and can be used for prenatal diagnosis, timely treatment and reduction of the incidence rate of neonatal neural tube malformation.
Owner:首都儿科研究所
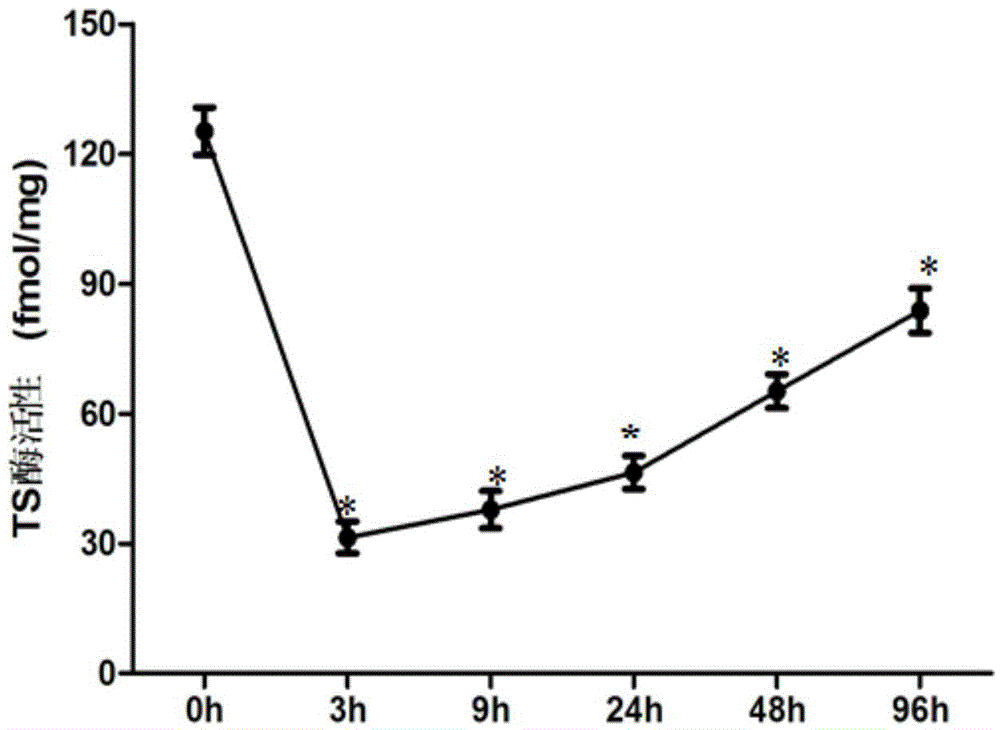
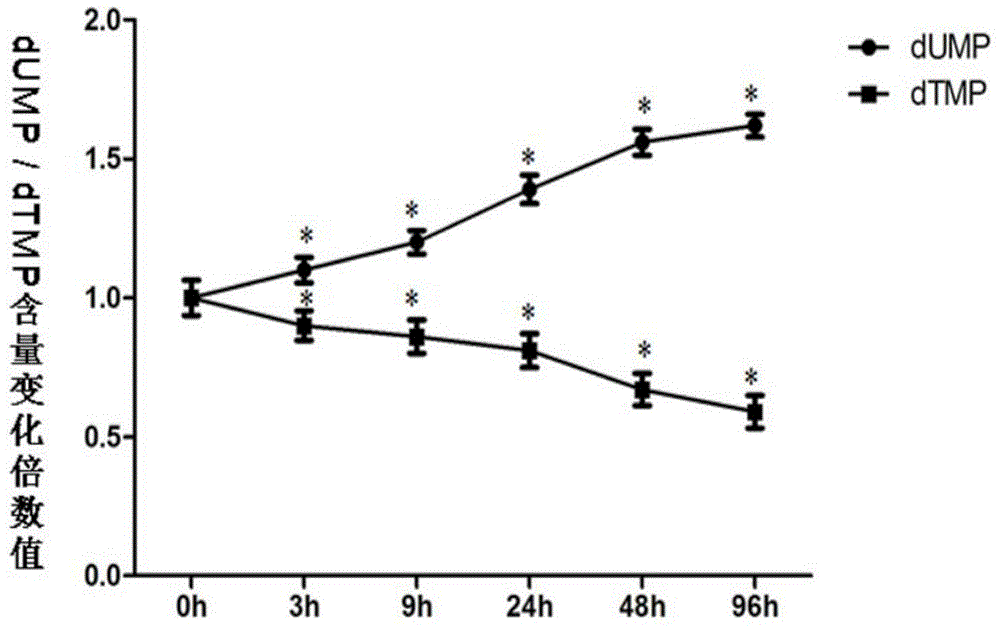
Application of thymidylate synthase inhibitor in preparation and construction of mouse model with NTDs (neural tube defects)
ActiveCN104800848AHigh deformity rateFetal fatality rate is lowOrganic active ingredientsThymidine monophosphateDysplasia
The invention relates to an application of a thymidylate synthase inhibitor in preparation and construction of a mouse model with NTDs (neural tube defects). Specifically, the thymidylate synthase inhibitor is used for intervening a pregnant mouse to cause dysbolism of thymidine monophosphate (dTMP) and dysplasia of a mouse embryo, so that the mouse model with the NTDs is constructed. The application is high in feasibility, easy to operate, good in repeatability, stable in effect and high in teratogenicity rate, the mouse model close to the pathogenesis of human NTDs is simulated through inhibition of metabolism of dTMP, and good animal model is provided for further research of birth defects, especially for research of the mechanism of all molecules and cells associated with the NTDs.
Owner:首都儿科研究所
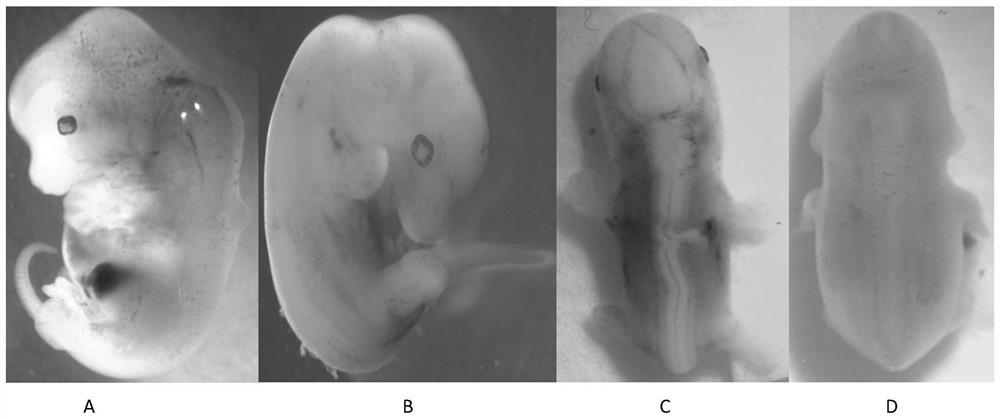
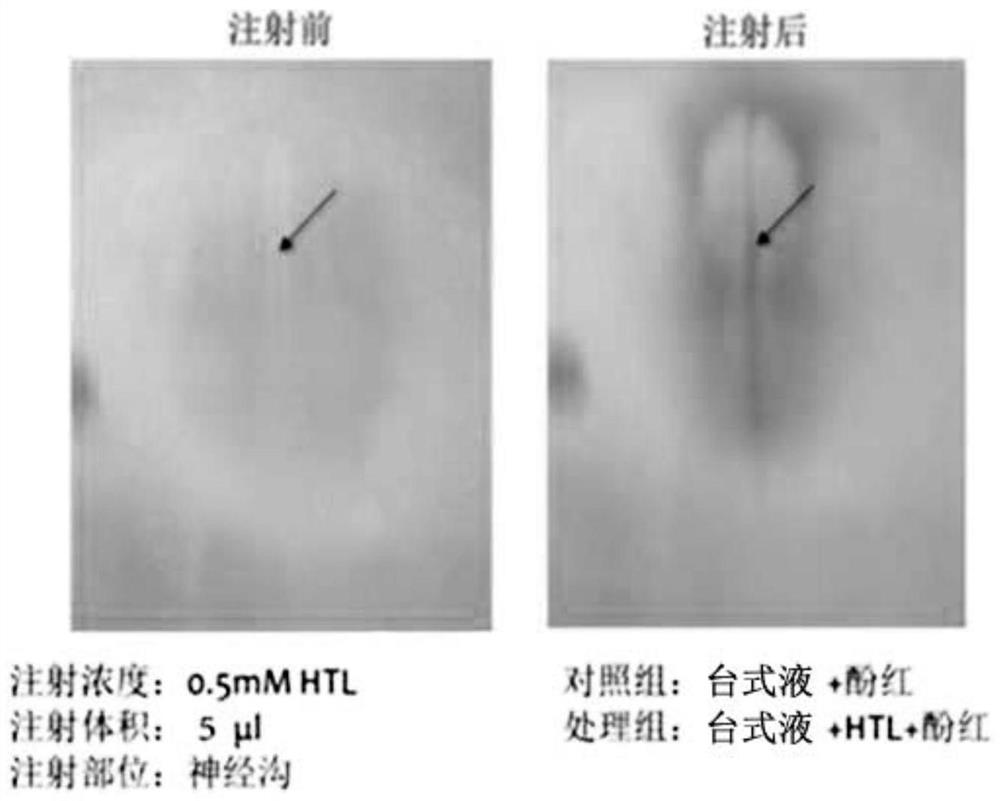
Construction method of neural tube defect model and application of construction method
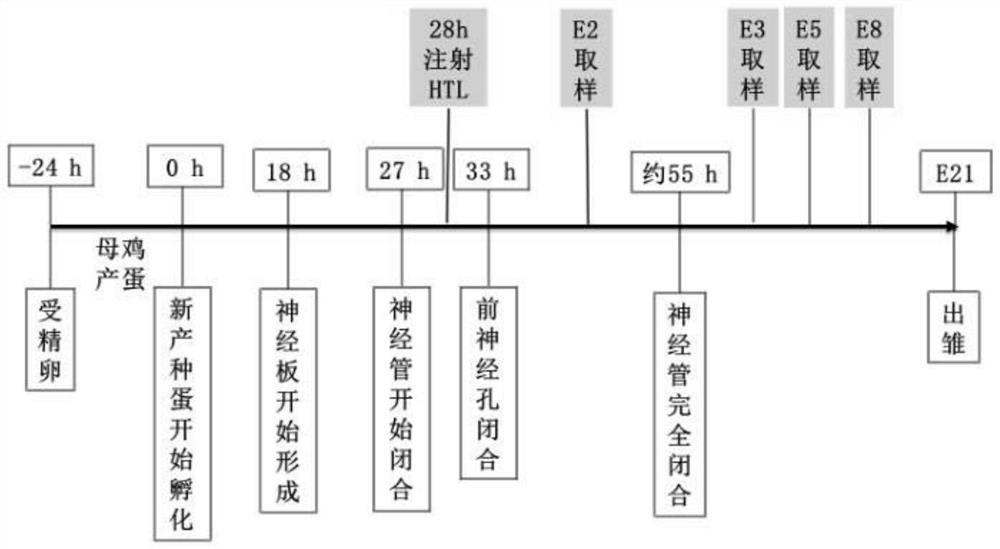
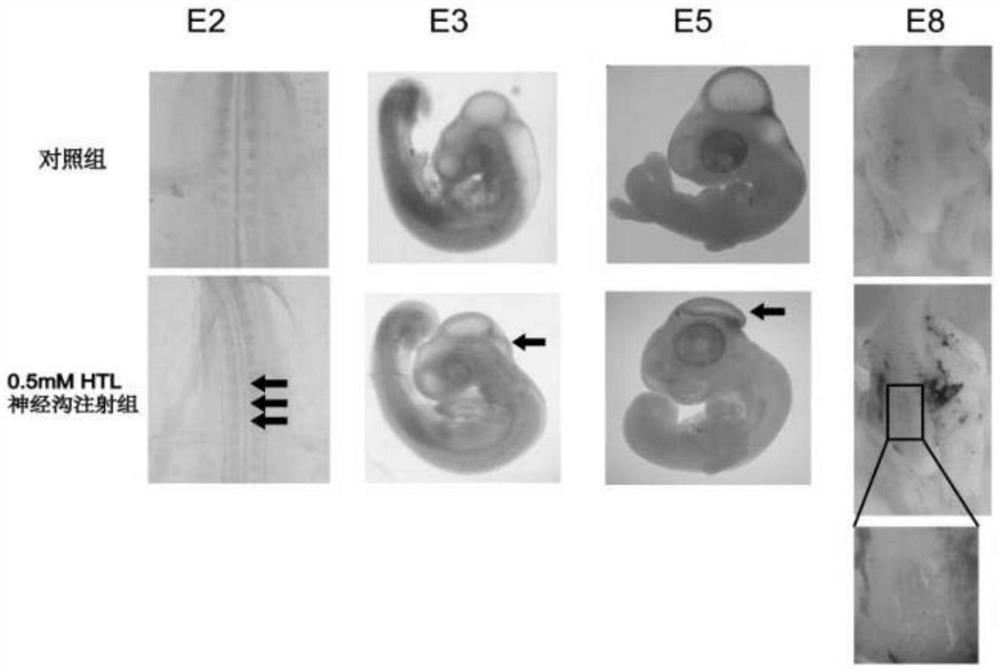
ActiveCN108853092AImprove survival rateEasy to observeOrganic active ingredientsNervous disorderNervous systemHyperhomocysteinemia
The invention discloses a construction method of a neural tube defect model. The construction method comprises the following steps of step 1, collecting artificially inseminated eggs, cleaning the eggs, and putting the eggs in an incubator for incubation; step 2, performing incubation on the eggs until neural plates of chicken embryos start to close, adopting a chicken embryo neural groove injection method to inject homocysteine thiolactone into the eggs; and step 3, performing continuous incubation on the treated eggs, observing phenotypes of the chicken embryos, and screening a successful neural tube defect model. On the other hand, the invention also discloses application of the construction method in studying pathogenesis of neural tube defects caused by hyperhomocysteinemia. By adopting the construction method of the neural tube defect model, the deformity rate of a nervous system reaches 50 percent, the survival rate of the embryos reaches up to 87.5 percent, and the constructionmethod is a best model making method of observing the neural tube defects at present.
Owner:首都儿科研究所
Method for detecting the affinity of folate receptor auto antibodies
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Construction of neural tube defect cell model and cell bank thereof under induction of hTERT recombinant
InactiveCN104419727AReduce injuriesIncrease success rateMicroorganism librariesVector-based foreign material introductionDiseaseEnzyme digestion
The invention discloses construction of a neural tube defect cell model and a cell bank thereof under induction of an hTERT recombinant and relates to medical field. The construction is characterized in that incision enzymes EcoRI and XhoI are used for carrying out double-enzyme digestion on a plasmid pCIneo-hTERT and a carrier pLXSNneo, and Ligation Mix is used for connecting hTERT and pLXSNneo enzyme-digested products subjected to PCR amplification and gel electrophoresis separation to construct a pLXSNneo-hTERT recombinant; DH5a competent cells are transformed to purify, amplify and extract plasmids; the neural tube defect cells in logarithmic growth during in-vitro passage are subjected to liposome transfection so that the recombinant and the cell DNA are integrated and subjected to enlarged cultivation; clones containing positive recombinants are subjected to G418 screening; the cells, of which cellular morphology, growth curves, karyotype, nude mouse tumorigenesis tests, cell telomerase activity, hTERT mRNA expression, immunohistochemistry, and cell generation cycles and apoptosis rate meet immortal cell characteristics, and which are the same or similar to primary cells, are screened to be used as the neural tube defect cell model constructed under induction of hTERT to be frozen in liquid nitrogen. Therefore, the foundation is laid for long-time in-vitro researches on pathogenesis of related diseases from cellular level.
Owner:翁炳焕
Pharmaceutical Composition Comprising Progestogens and/or Estrogens and 5-Methyl-(6S)-tetrahydrofolate
The present invention relates to a pharmaceutical composition which comprises progestogens, preferably drospirenone, estrogens, preferably ethinylestradiol and 5-methyl-(6S)-tetrahydrofolate, can be employed as oral contraceptive and moreover prevents disorders caused by folate deficiency in the consumers, in particular cardiovascular disorders and, after conception of the embryo, congenital malformations caused by folate deficiency such as, for example, neural tube defects, ventricular valve defects, urogenital defects, and cleft lip, jaw and palate, without masking the symptoms of vitamin B12 deficiency, and at the same time even in the case of homozygous or heterozygous polymorphism of methylenetetrahydrofolate reductase facilitates unimpaired utilizability of the folate component 5-methyl-(6S)-tetrahydrofolate by the body and thus its biological activity for preventing the abovementioned congenital malformations caused by folate deficiency. In addition, a prolonged protective effect is maintained after discontinuation of the contraceptive.
Owner:BAYER INTELLECTUAL PROPERTY GMBH +1
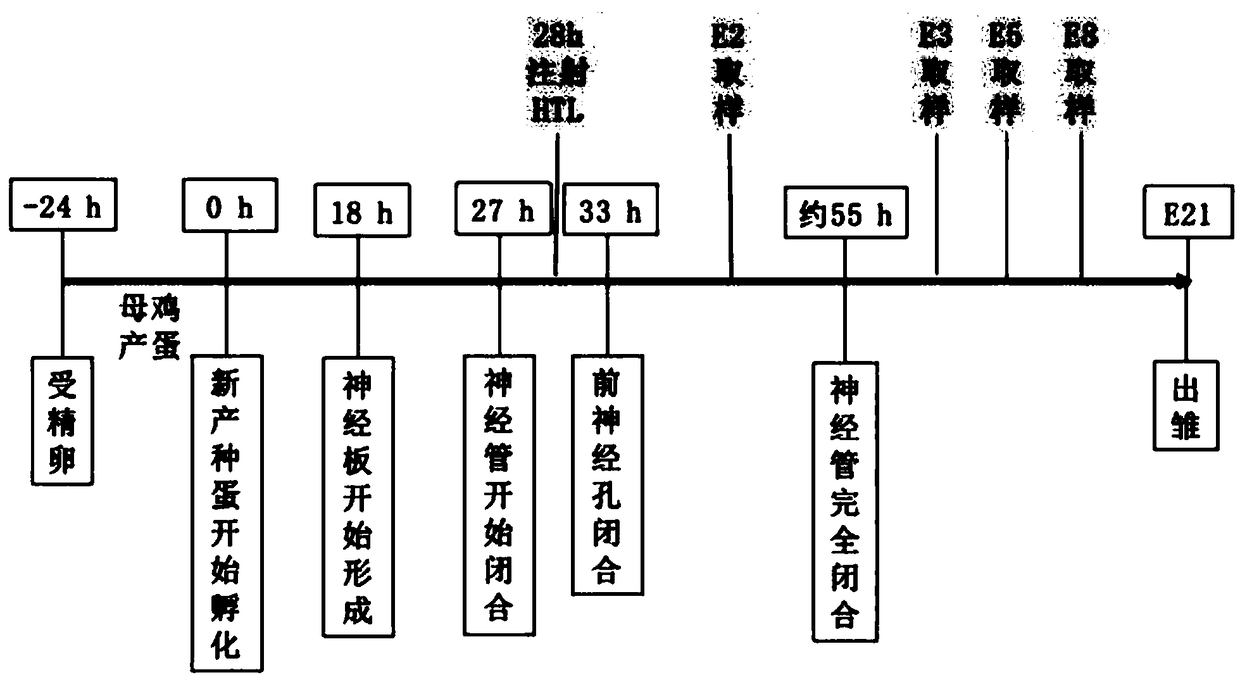
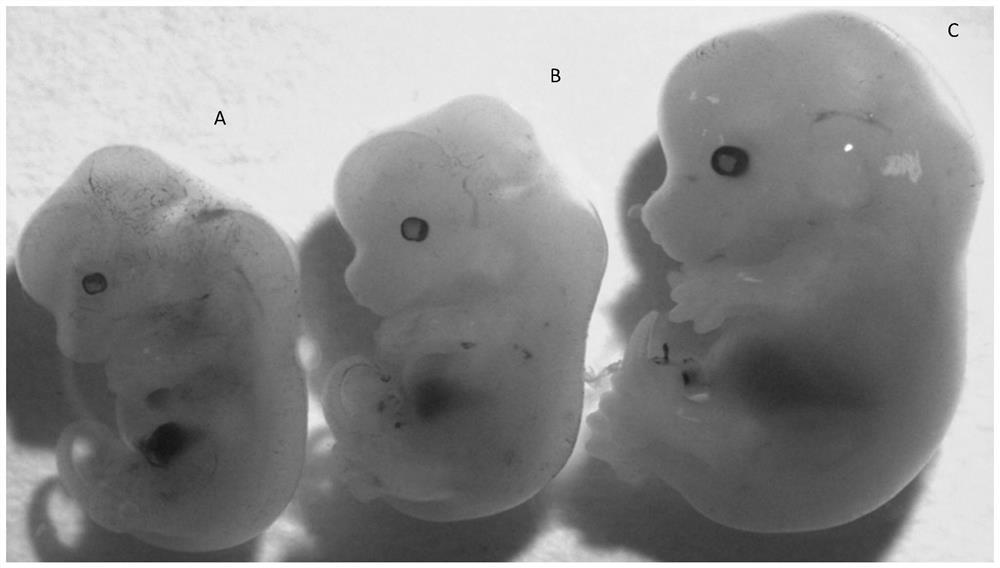
NTDs (neural tube defects) rat embryo animal model and constructing method thereof
The invention discloses an NTDs (neural tube defects) rat embryo animal model and a constructing method thereof. Normal pregnant rats which have been pregnant for 6 to 10 days are injected with 19-23 mg / kg of retinoic acid at one time for intragastric administration intervention, then construction of the stable and efficient NTDs rat embryo animal mode can be induced rapidly after pregnant 10.5 days, and further, rat embryo brain vesicle tissue is separated out for cell culture while an NTDs cell model can be built. The animal model and the cell model constructed by the method can provide good platforms for research on NTDs molecular mechanism and cellular mechanism in vivo and vitro.
Owner:SHANXI MEDICAL UNIV
A method for constructing a mouse model of neural tube defects
ActiveCN109331020BIncreased deformity rateIncrease deformity rateOrganic active ingredientsNervous disorderSide effectMedicine
The invention relates to the field of applied basic medical research, in particular to a method for constructing a neural tube defect mouse model. The present invention uses physical or chemical teratogenic factors to intervene pregnant mice fed with a low folic acid diet, uses low folic acid as a sensitizing factor, imitates the human physiological environment, induces neural tube defects in mice, and greatly increases the success rate of model construction; The method adopted in the invention is simple, has good repeatability, high deformity rate, little toxic and side effects in pregnant mice, and low embryo lethality rate.
Owner:首都儿科研究所
A method of constructing a neural tube defect model and its application
ActiveCN108853092BImprove survival rateEasy to observeOrganic active ingredientsNervous disorderAnimal scienceNervous system
The invention discloses a method for constructing a neural tube defect model, which comprises the following steps: step 1, collecting artificially inseminated eggs, cleaning them and placing them in an incubator for incubation; step 2, incubating the eggs to the neural plate of chicken embryos After the start of closure, inject homocysteine thiolactone into the eggs using the chicken embryo neural groove injection method; step 3, continue to incubate the treated eggs, observe the phenotype of the chicken embryos, and screen out successful neural tube defects Model. Another aspect of the present invention also discloses the application of the above construction method in the study of the pathogenesis of neural tube defects caused by high homocysteine. By adopting the neural tube defect model construction method provided by the invention, the deformity rate of the nervous system reaches 50%, and the embryo survival rate reaches 87.5%, which is currently the best modeling method for observing neural tube defects.
Owner:首都儿科研究所
Nucleic acid adaptor for specific identifying human cerebral acetylcholinesterase and its use
The invention offers oligonucleotide adapter of specific combining human-brain acetylcholinesterase containing SEQ ID: 1, SEQ ID: 2 or SEQ ID: 3 sequence, method of detecting or discriminating human-brain acetylcholinesterase, method and diagnostic reagent and medicine to diagnose or therapy the disease relating to human-brain acetylcholinesterase (especially neural tube defect and neurotic disorder), and so on.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com