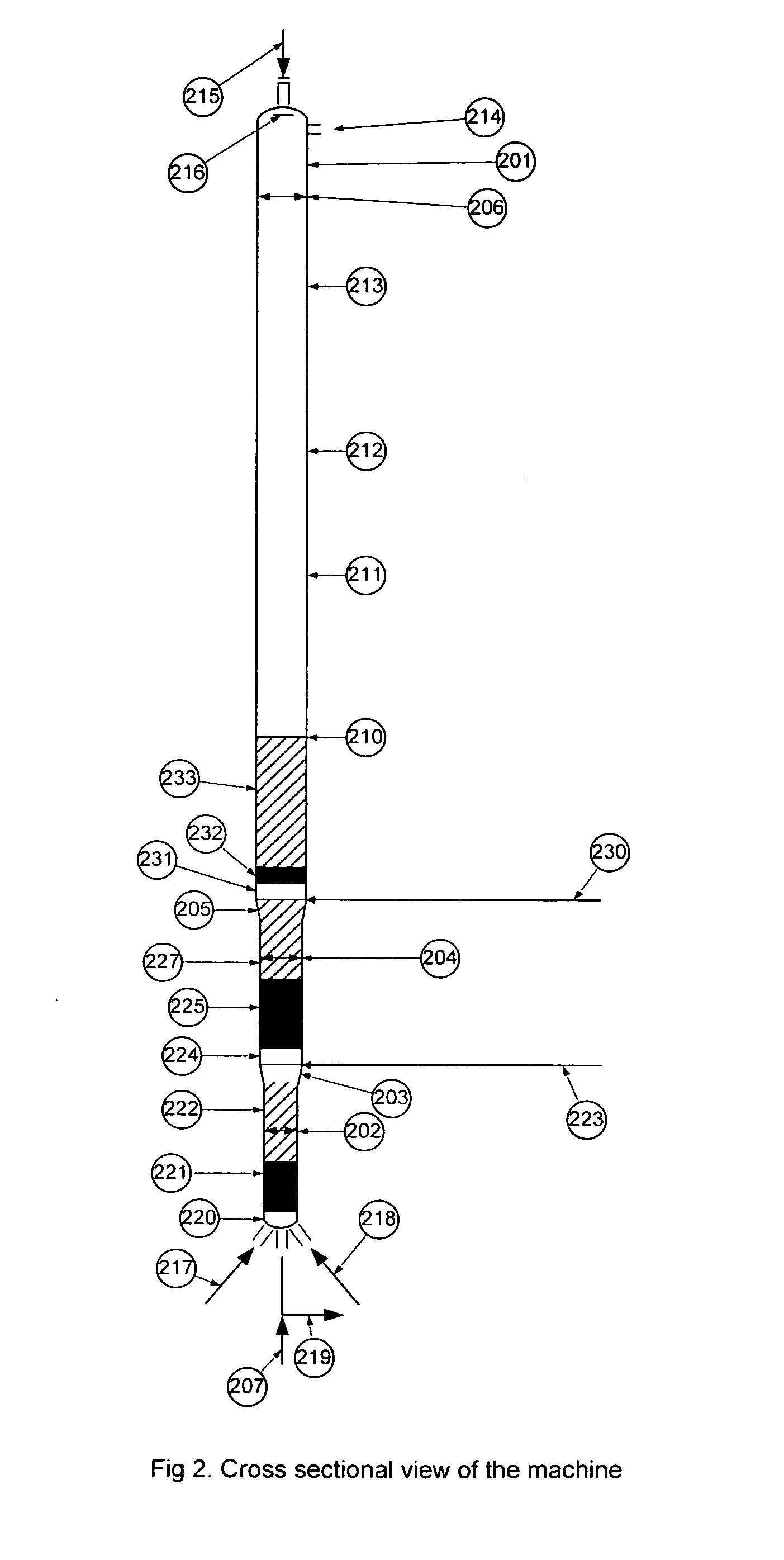
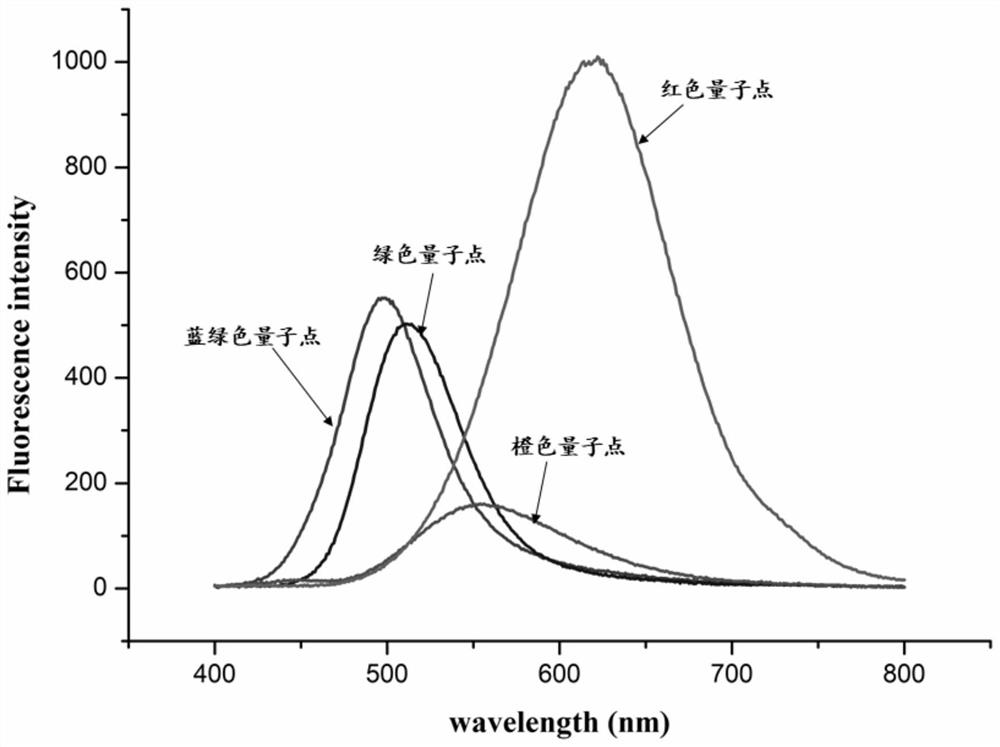
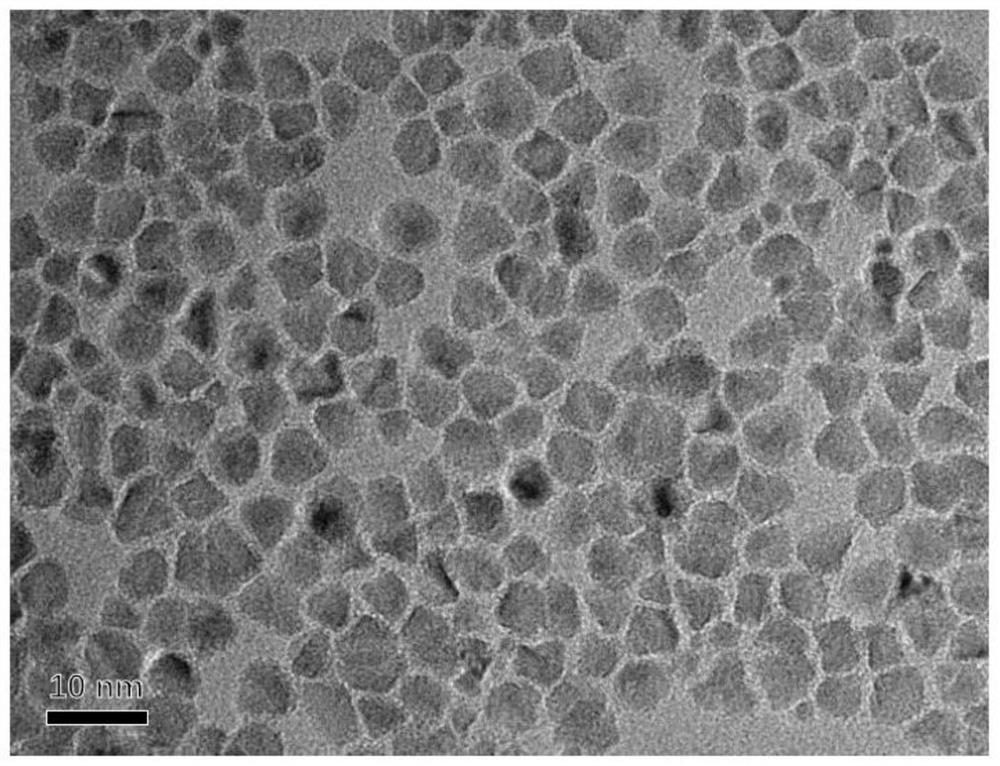
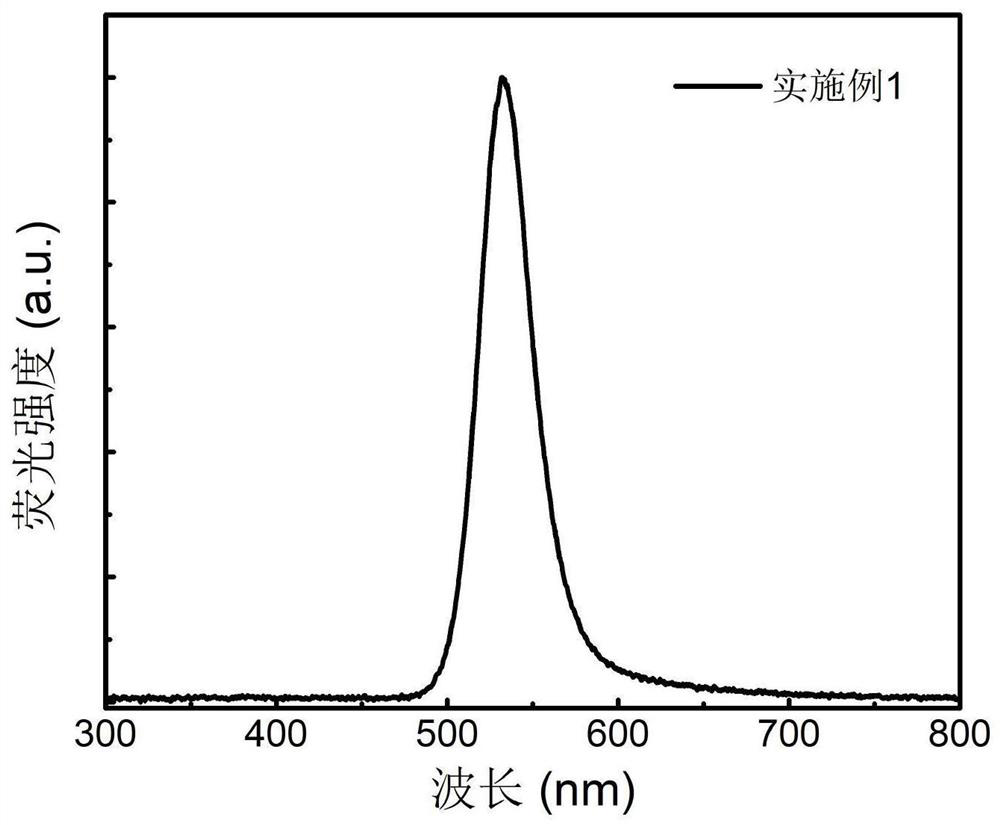
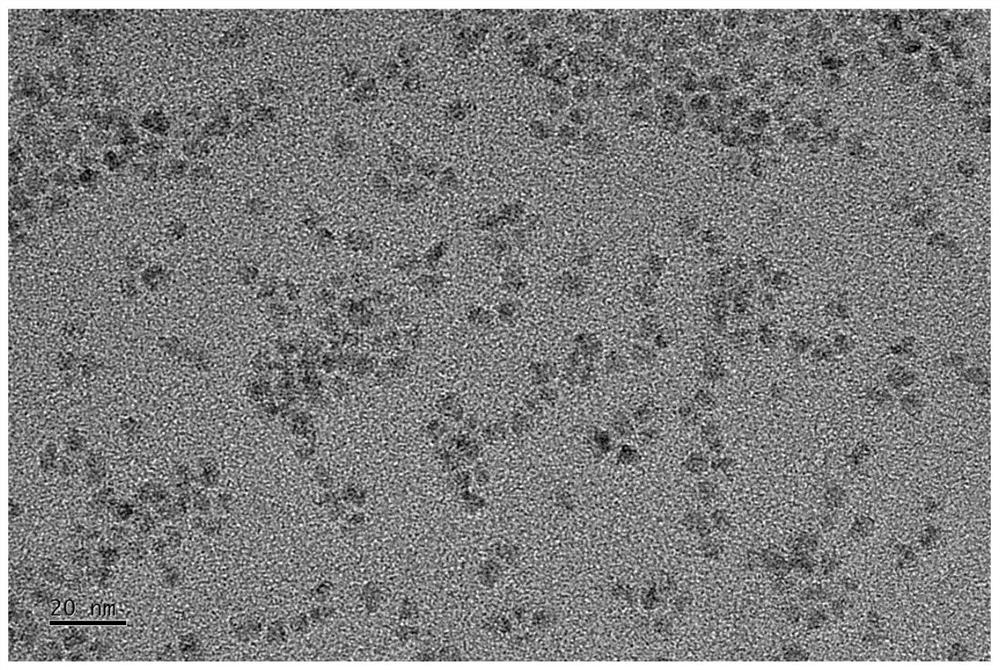
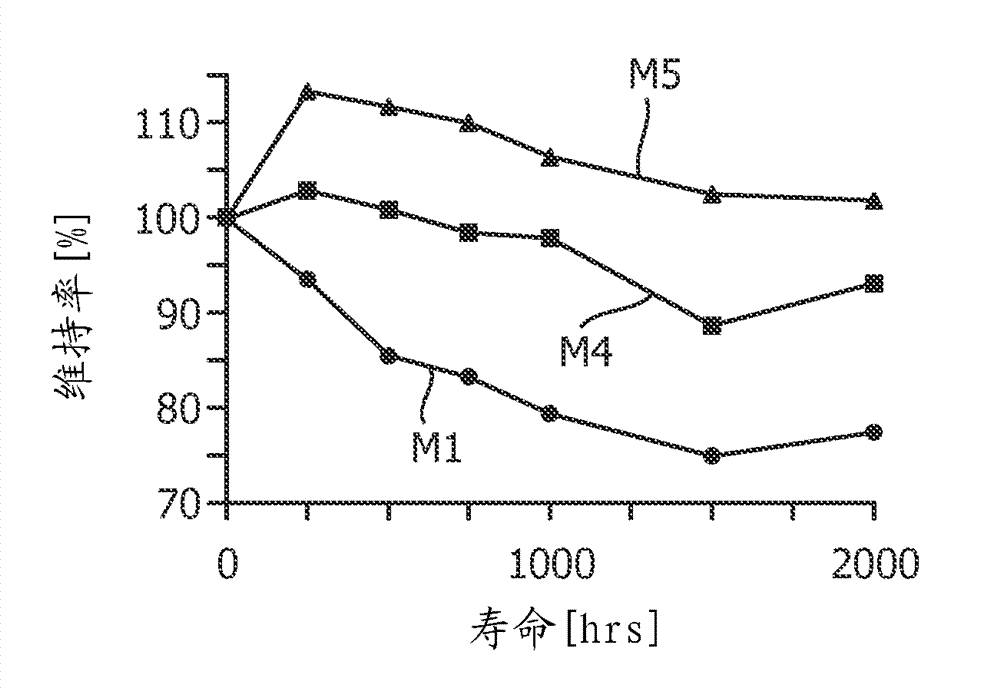
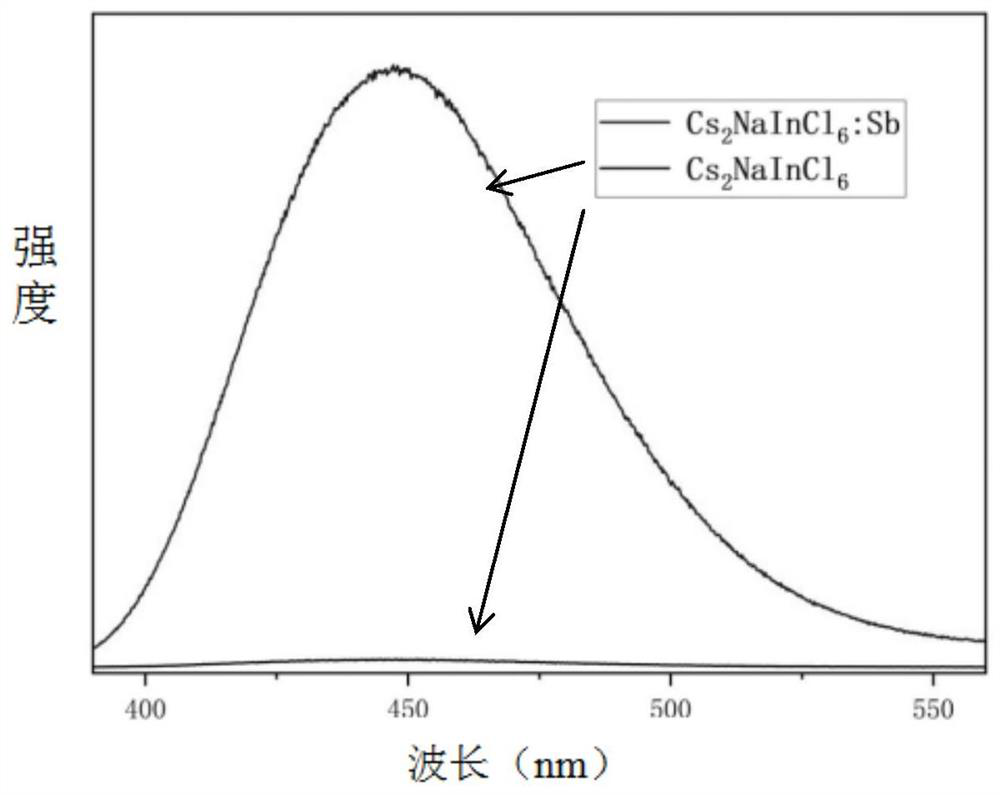
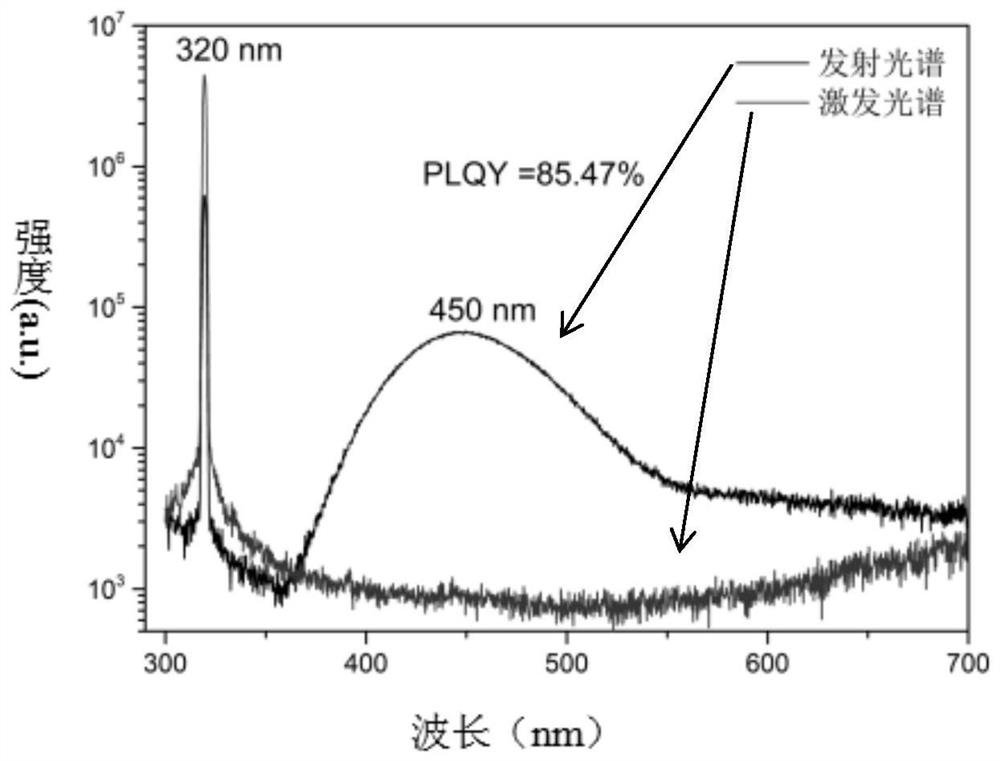
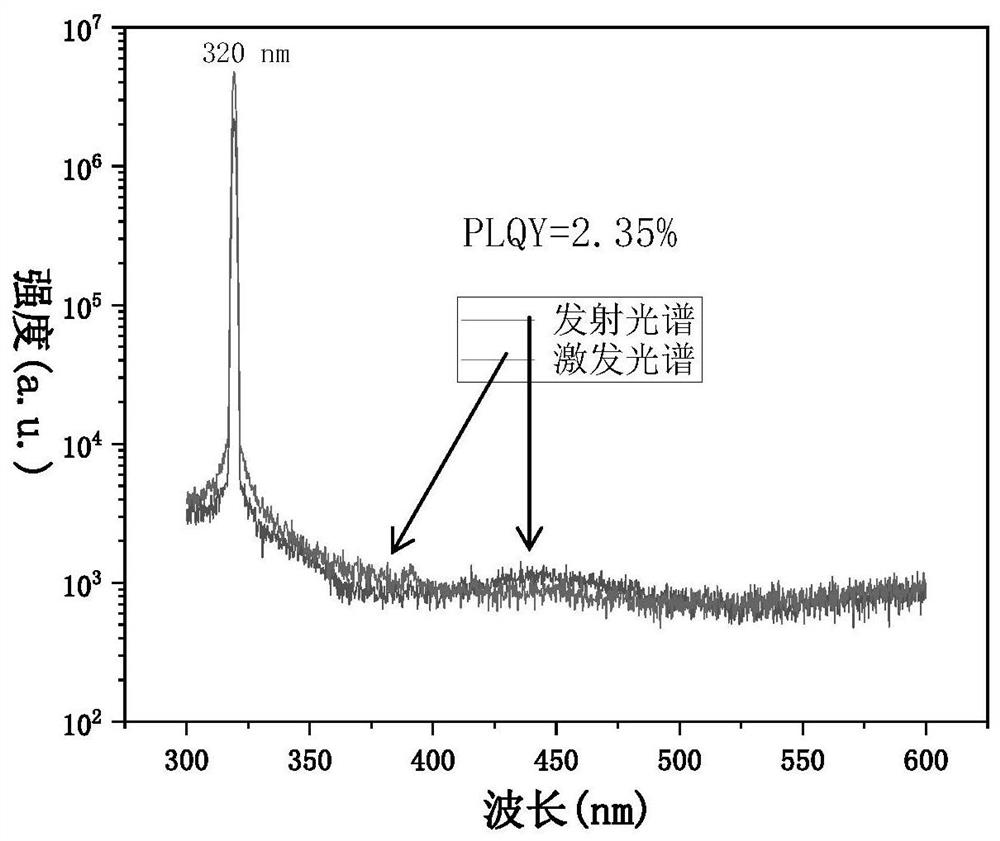
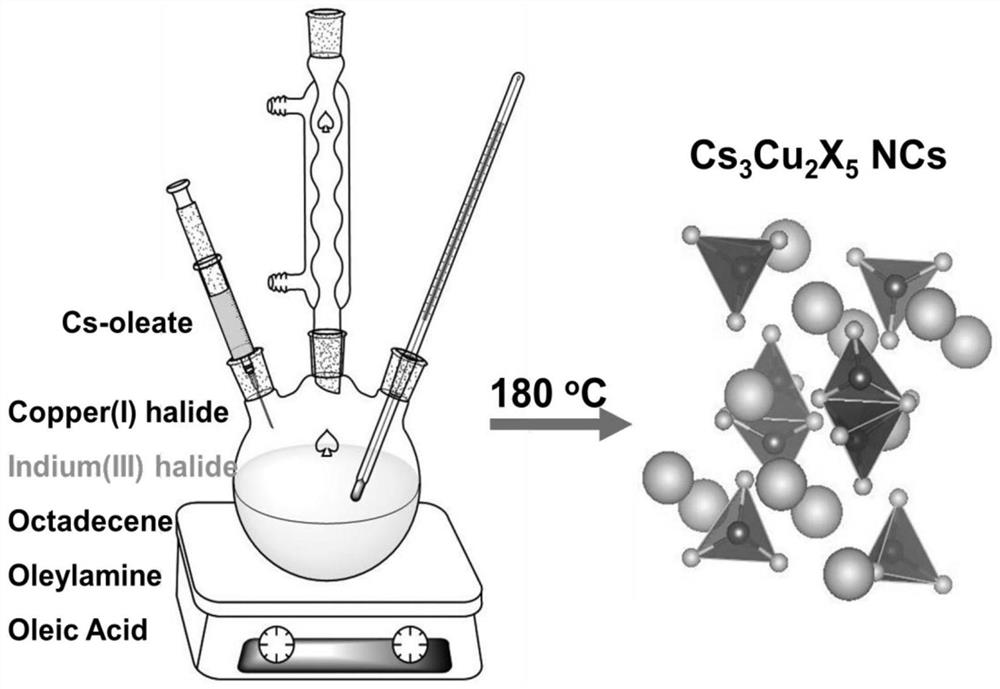
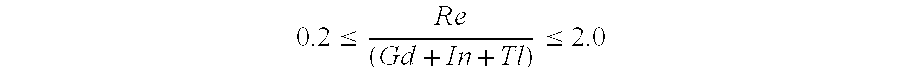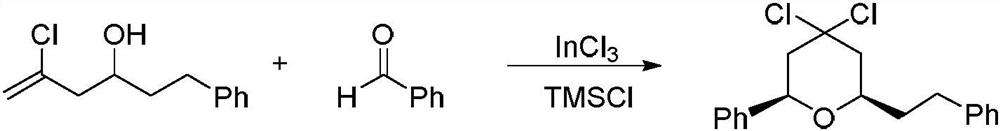Patents
Literature
34 results about "Indium halides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.
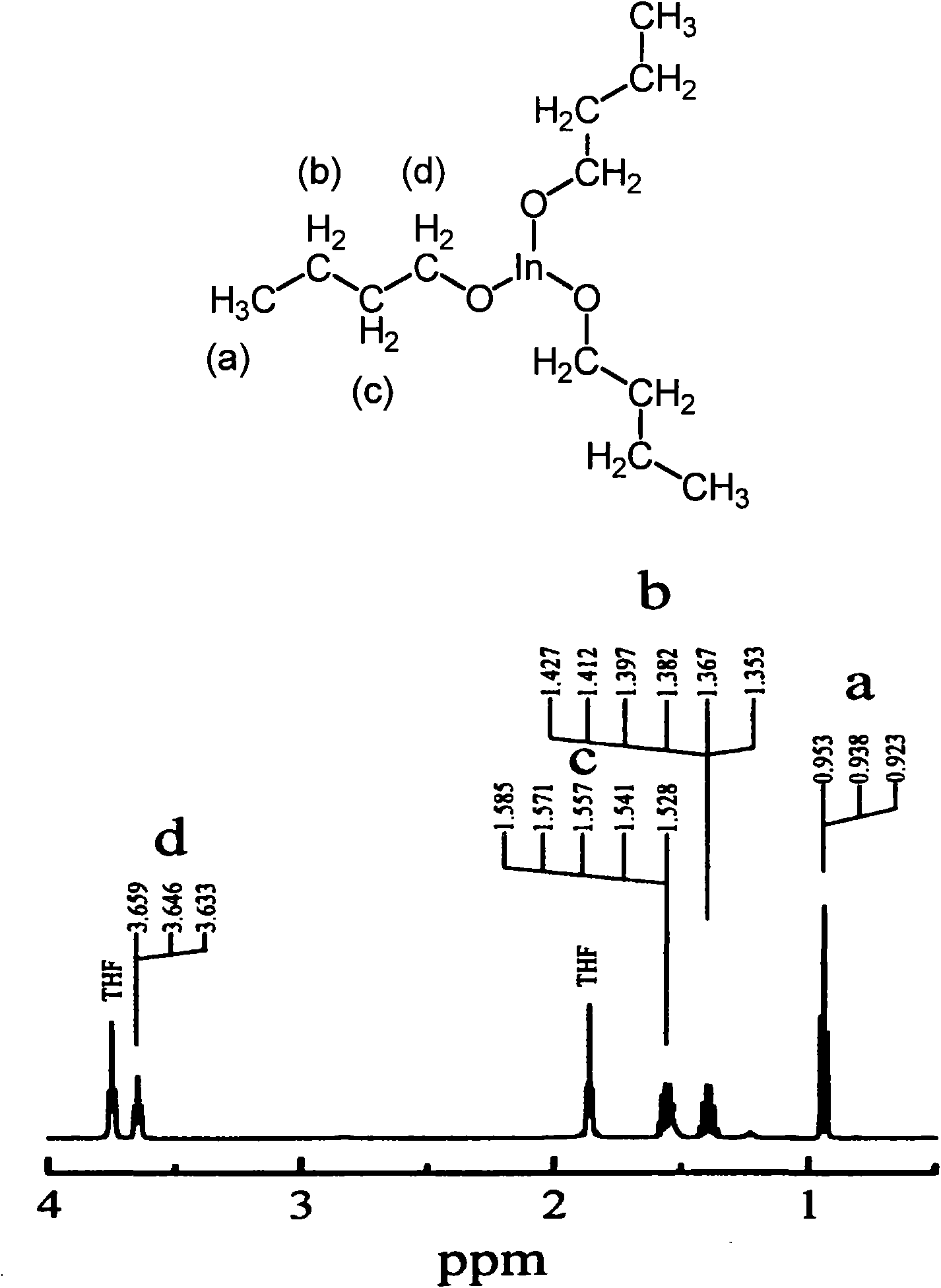
Synthesis of gallium and indium alkoxides
InactiveUS20090112012A1Eliminate chlorine contaminationGroup 4/14 element organic compoundsOrganic compound preparationAlkaline earth metalPotassium
A method of synthesising gallium or indium alkoxide from the corresponding gallium or indium halide, the method comprising the step of reacting the gallium or indium metal halide with the alkoxide of an alkali earth metal, to produce the desired metal alkoxide essentially free of chlorine contamination. The method provides a composition comprising a solution of gallium or indium alkoxide of high purity, the composition having a chloride content of less than 30 ppm, a barium or strontium content of less than 30 ppm, and a sodium or potassium content of less than 30 ppm, without requiring any additional steps.
Owner:MULTIVALENT
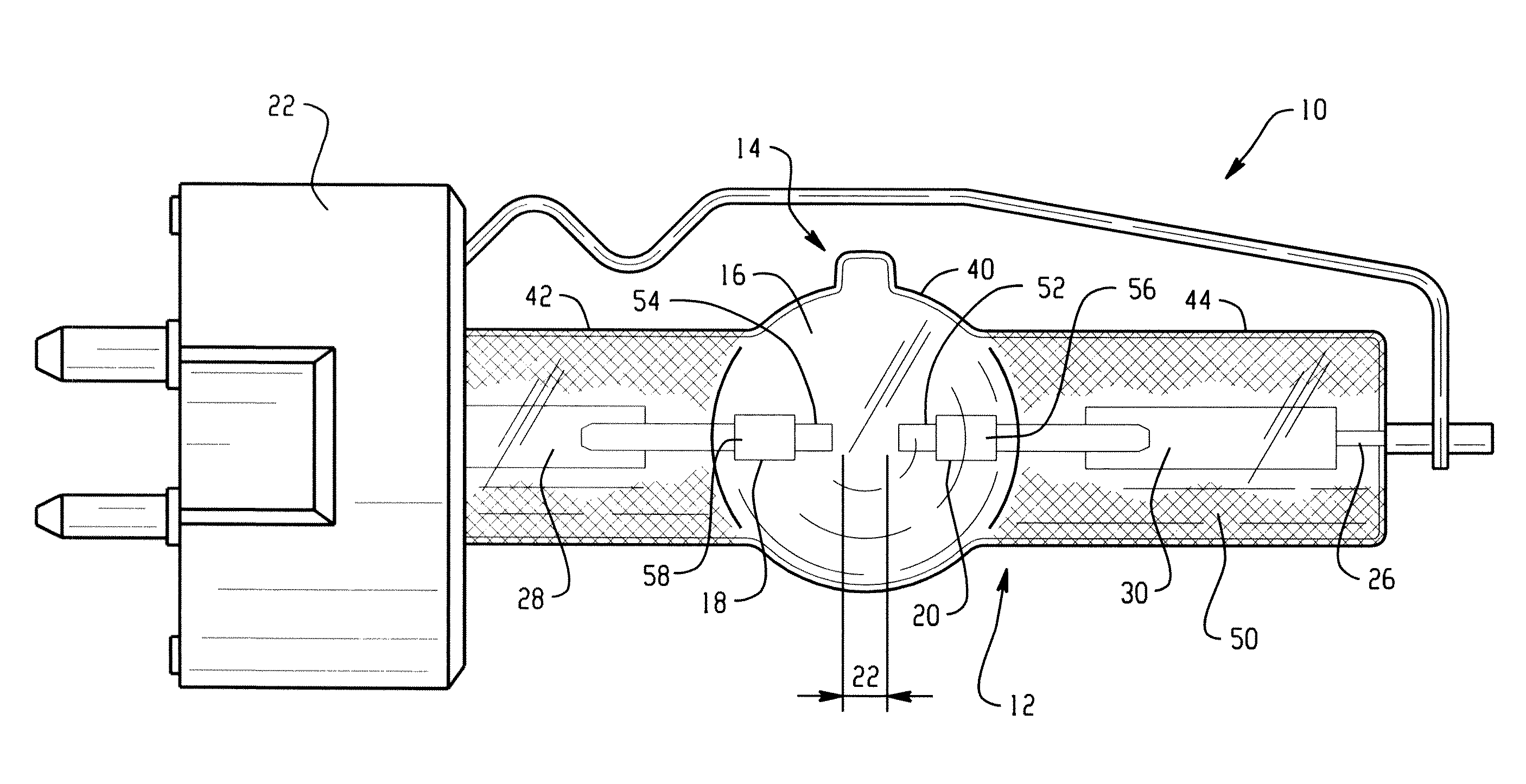
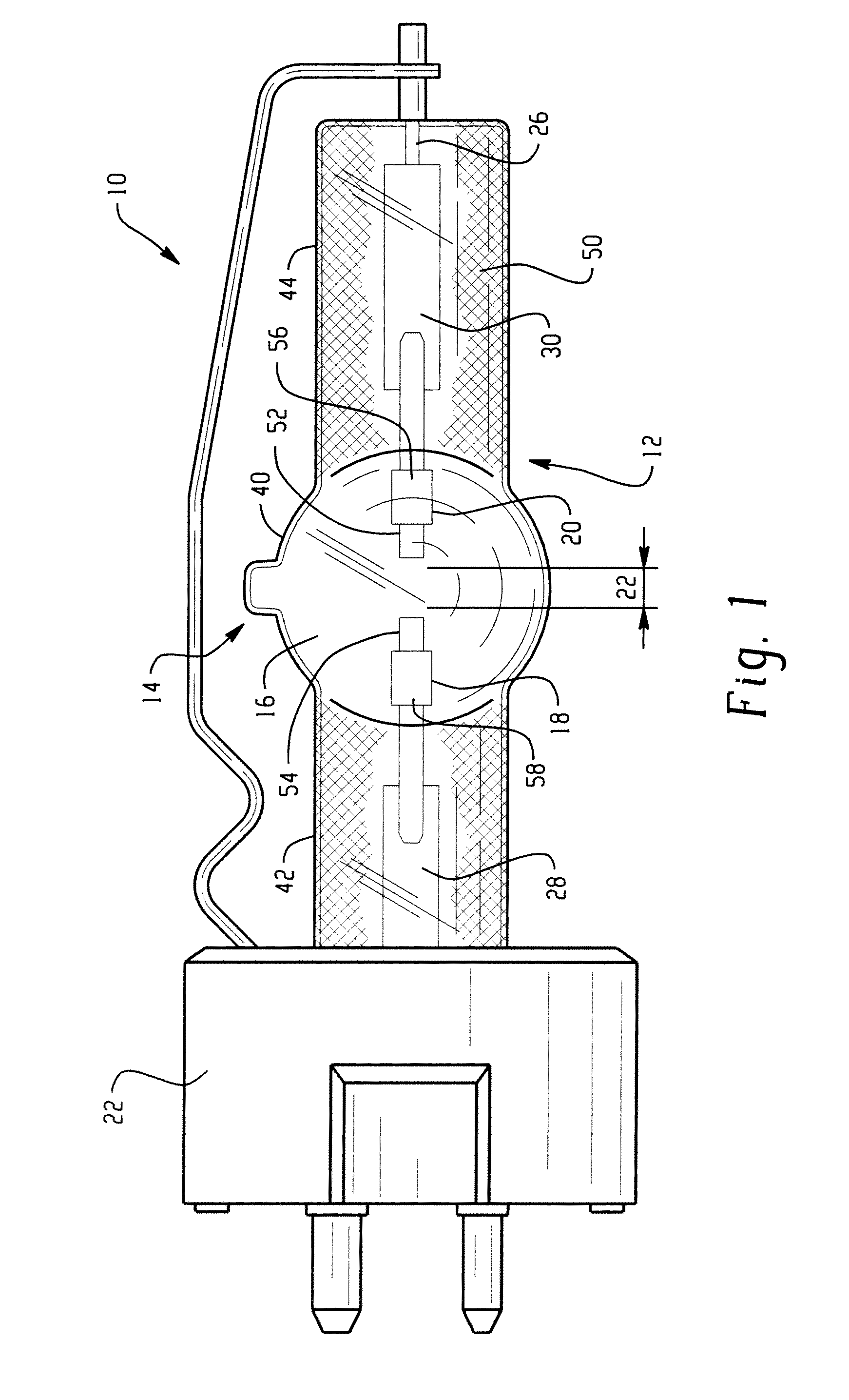
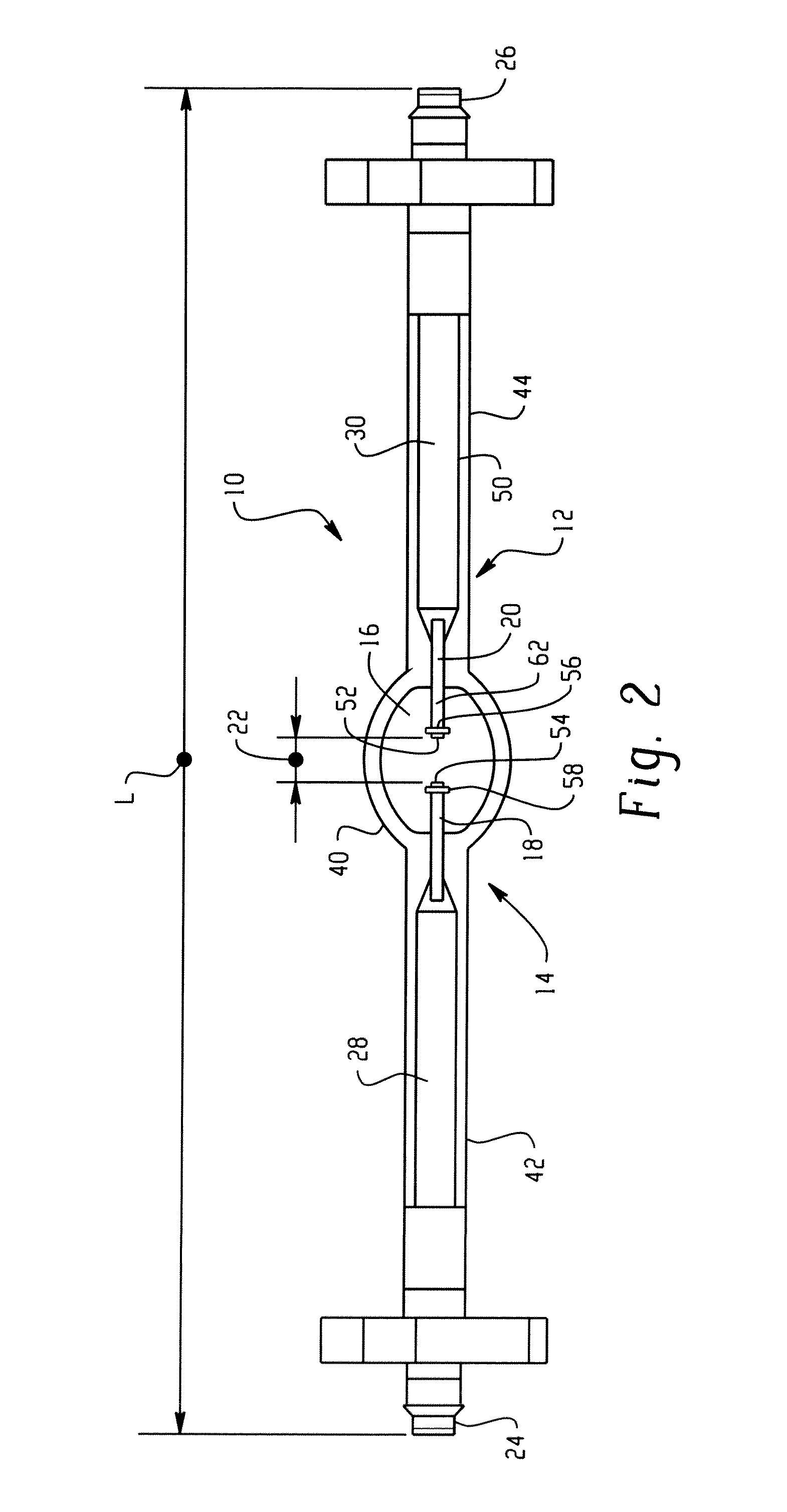
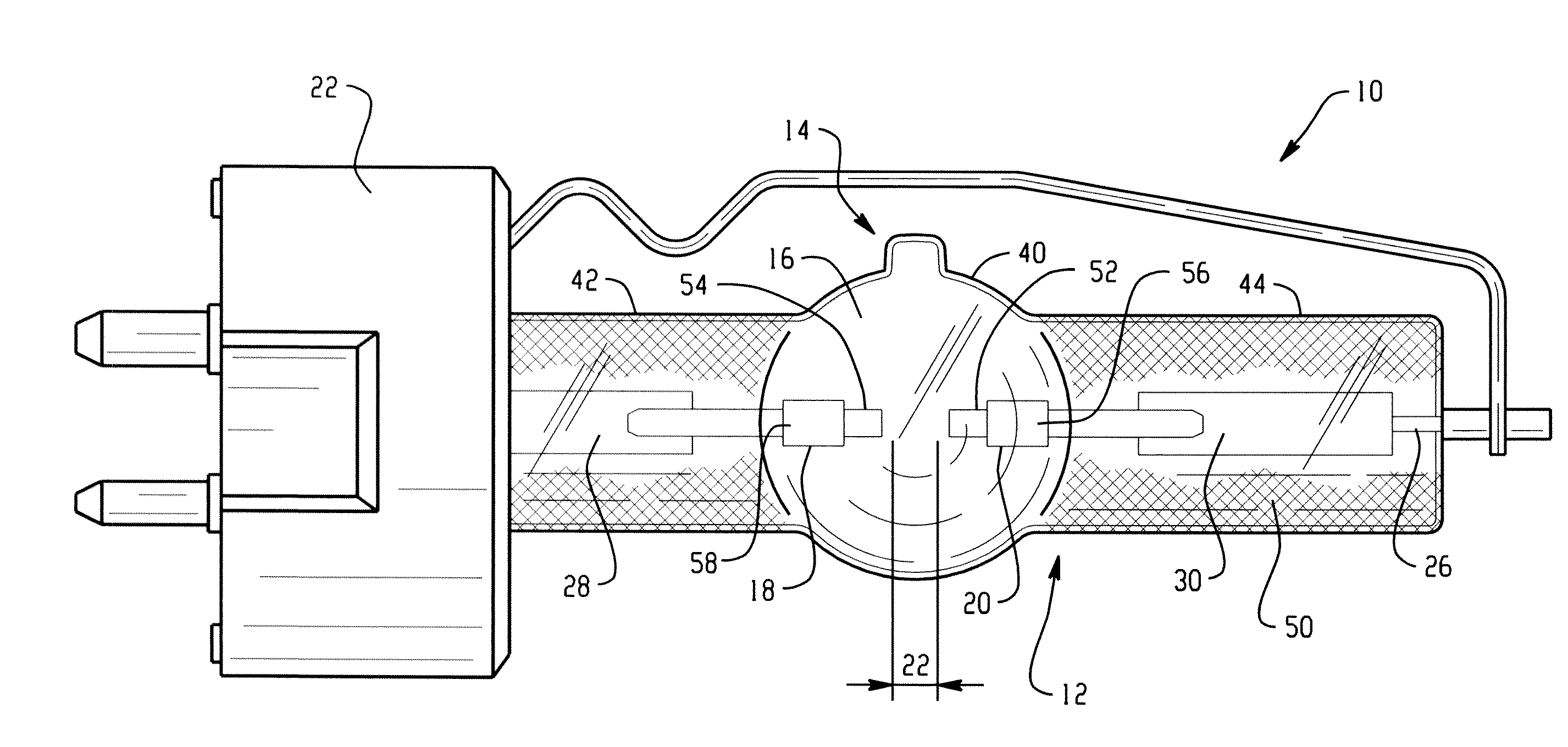
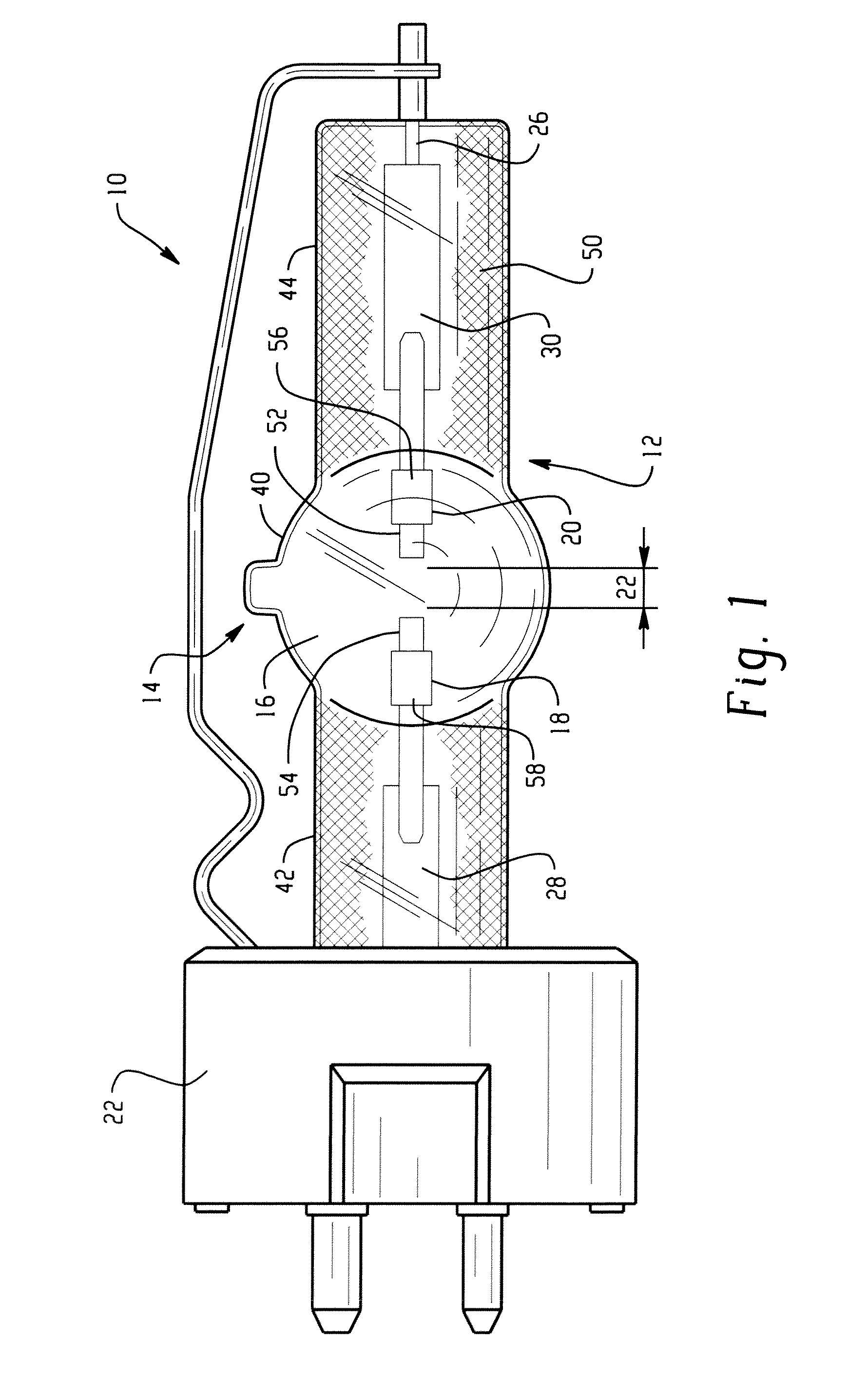
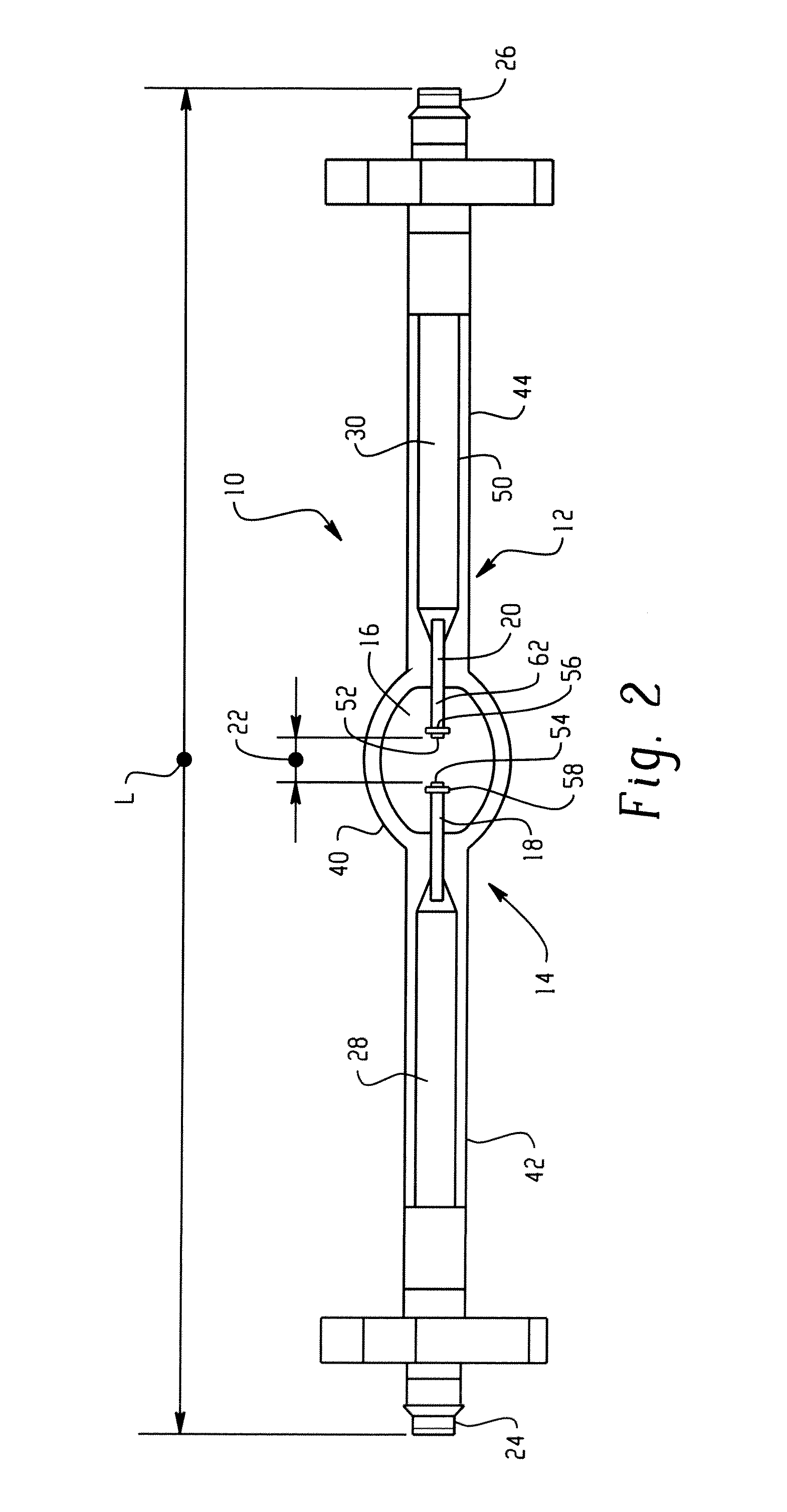
Lamp unit and infrared night-vision system
InactiveUS20040021420A1Simple designReduce power consumptionClosed circuit television systemsSolid cathode detailsNight visionThallium
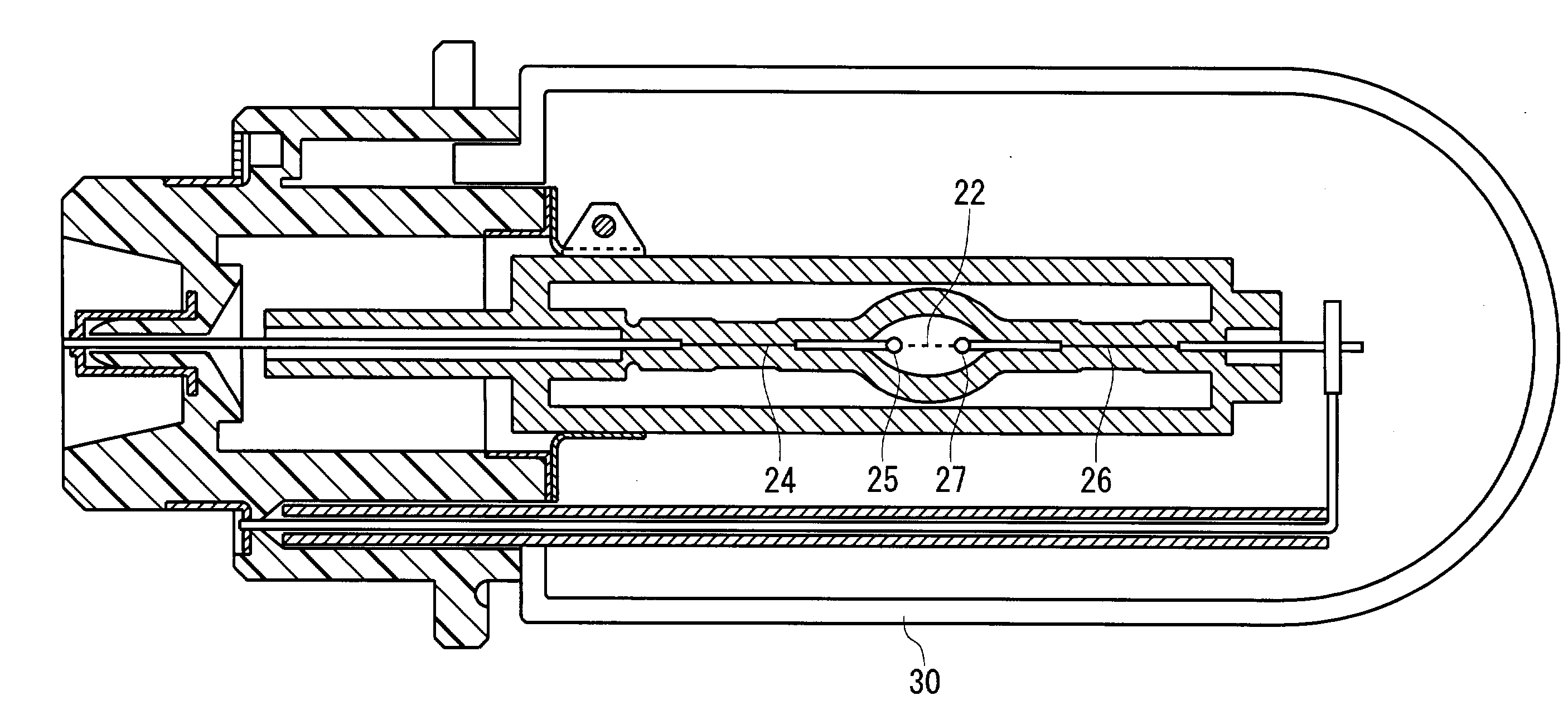
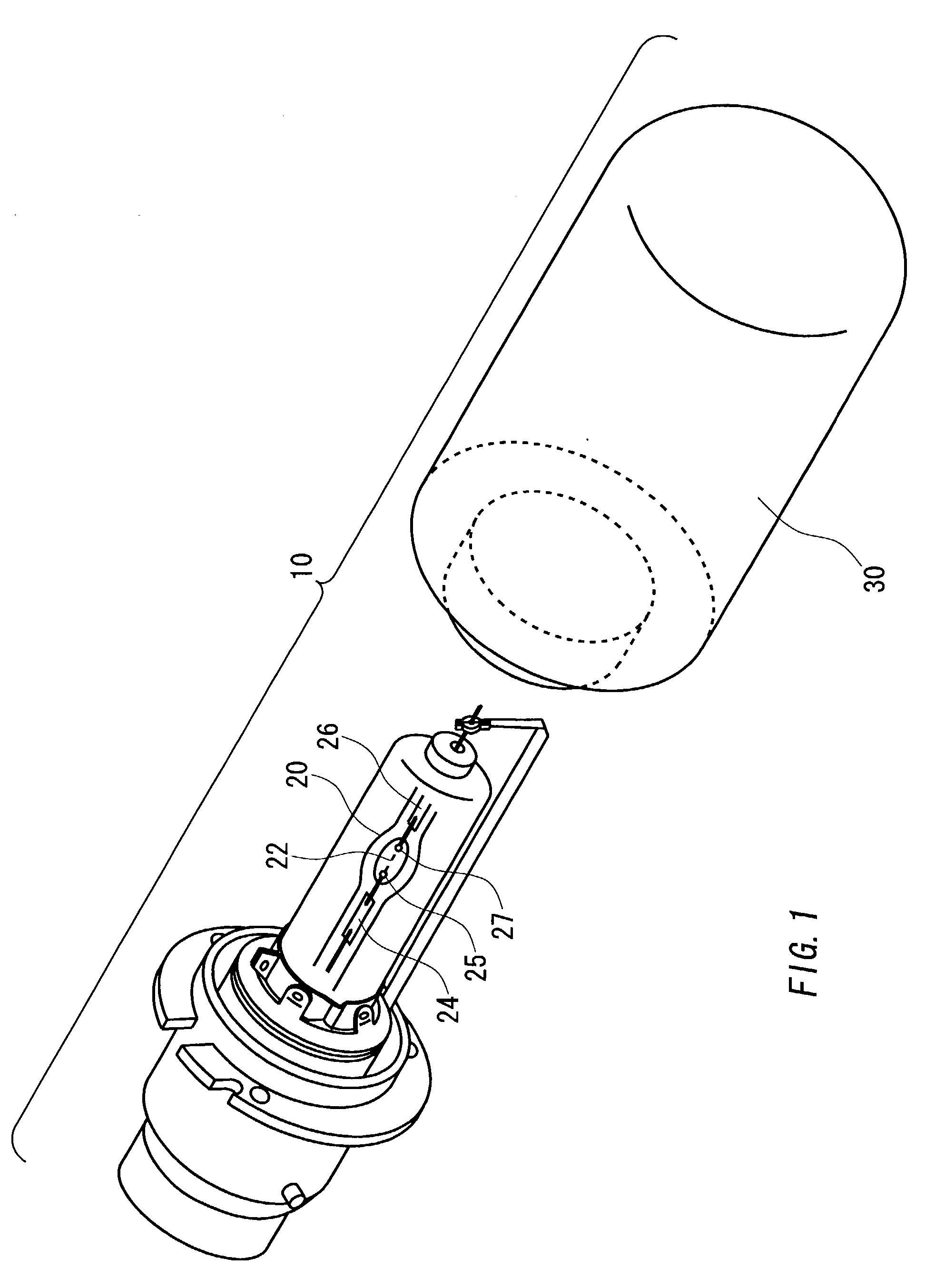
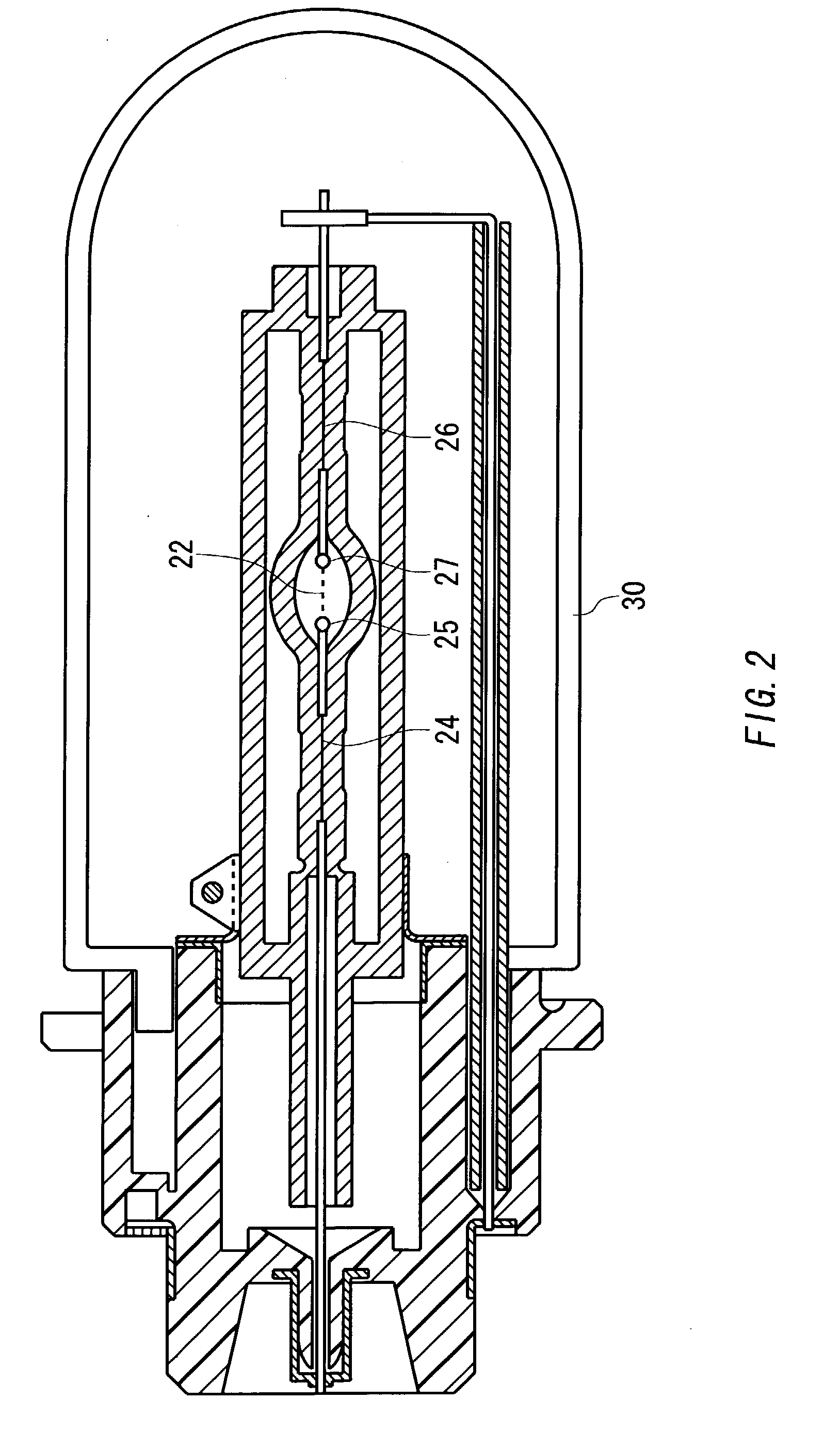
A lamp unit for emitting near-infrared light by electric discharge, has: a hollow discharge tube; a cesium halide enclosed in the hollow of the discharge tube; a near-infrared penetration filter covering around the discharge tube. The cesium halide may include at least one of cesium iodide and cesium bromide. Also, an indium halide and thallium halide may further be enclosed in the hollow discharge tube.
Owner:KOITO MFG CO LTD
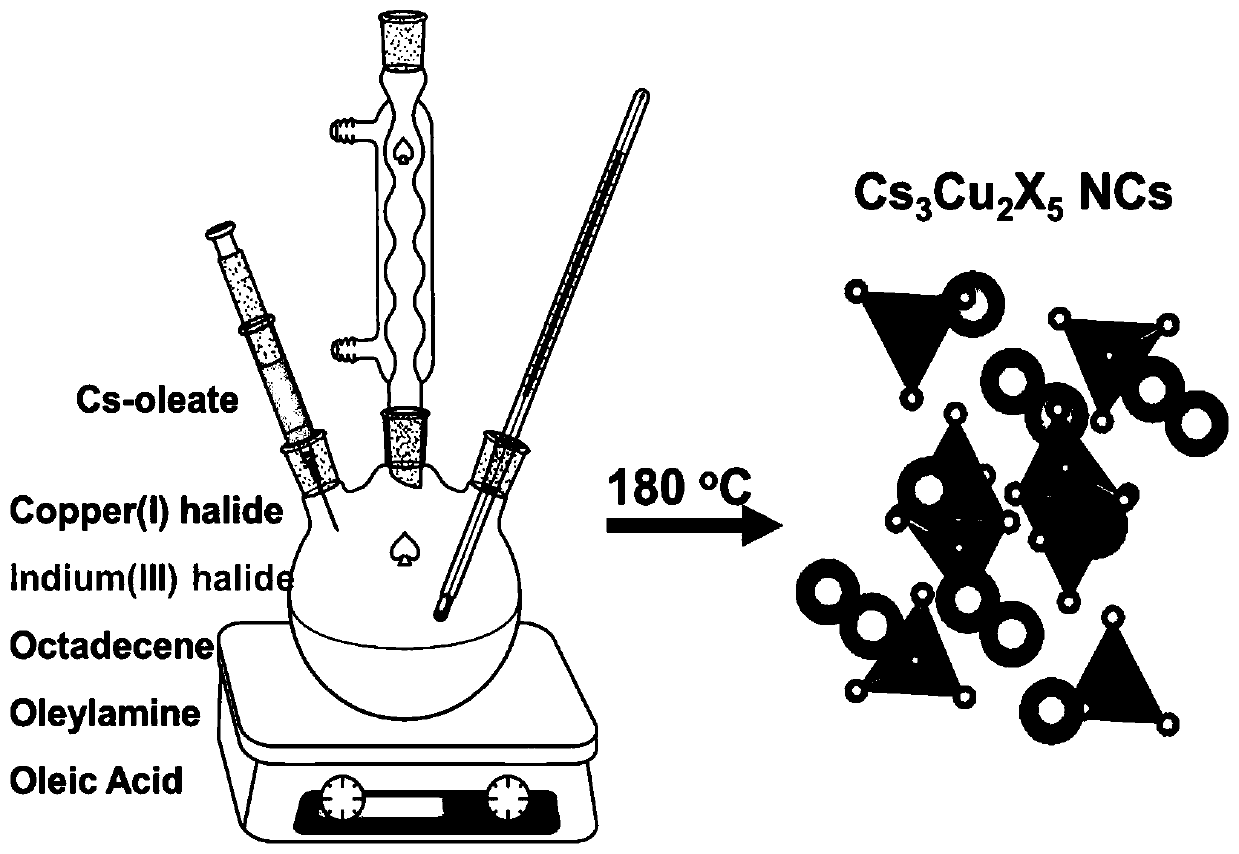
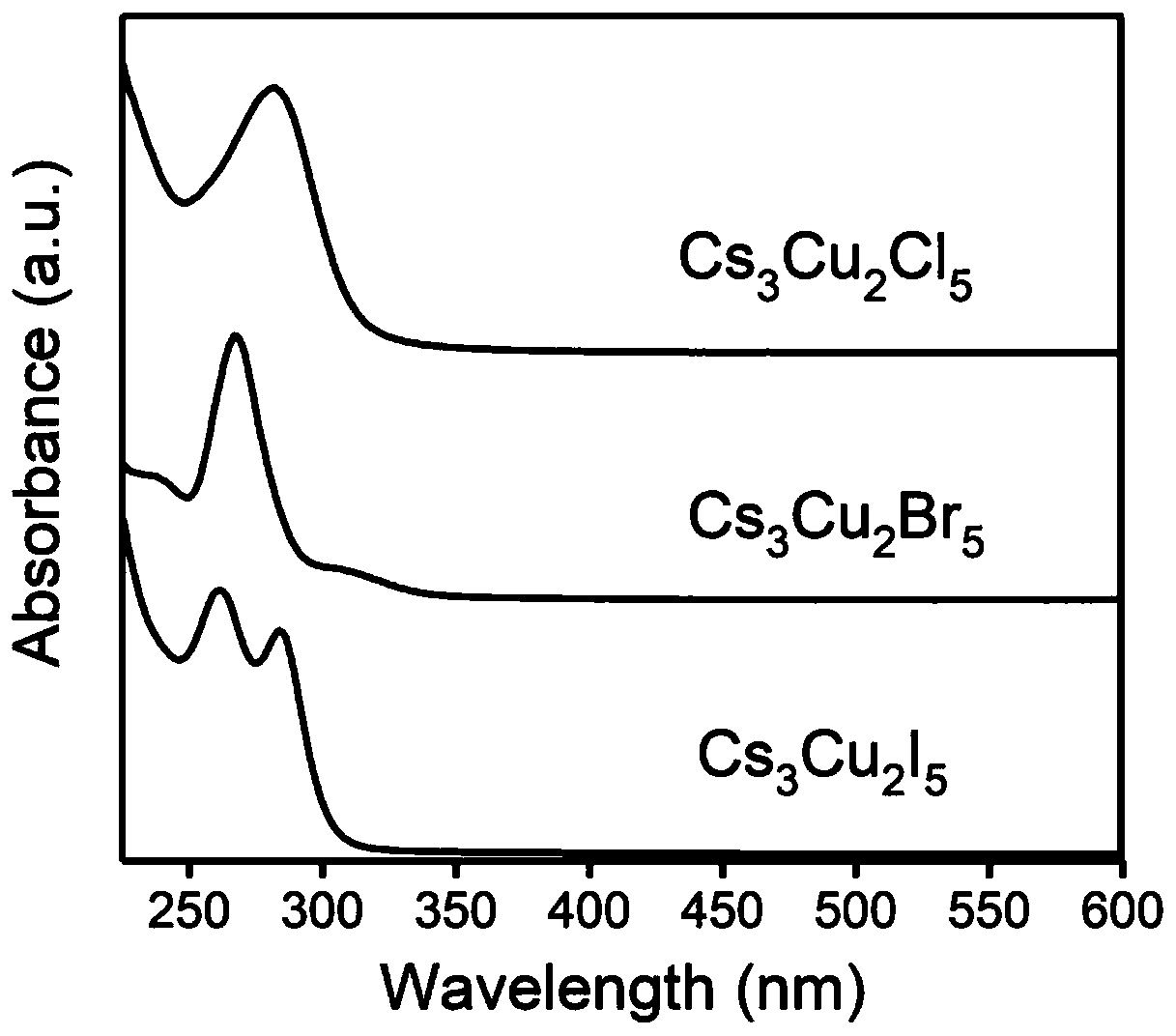
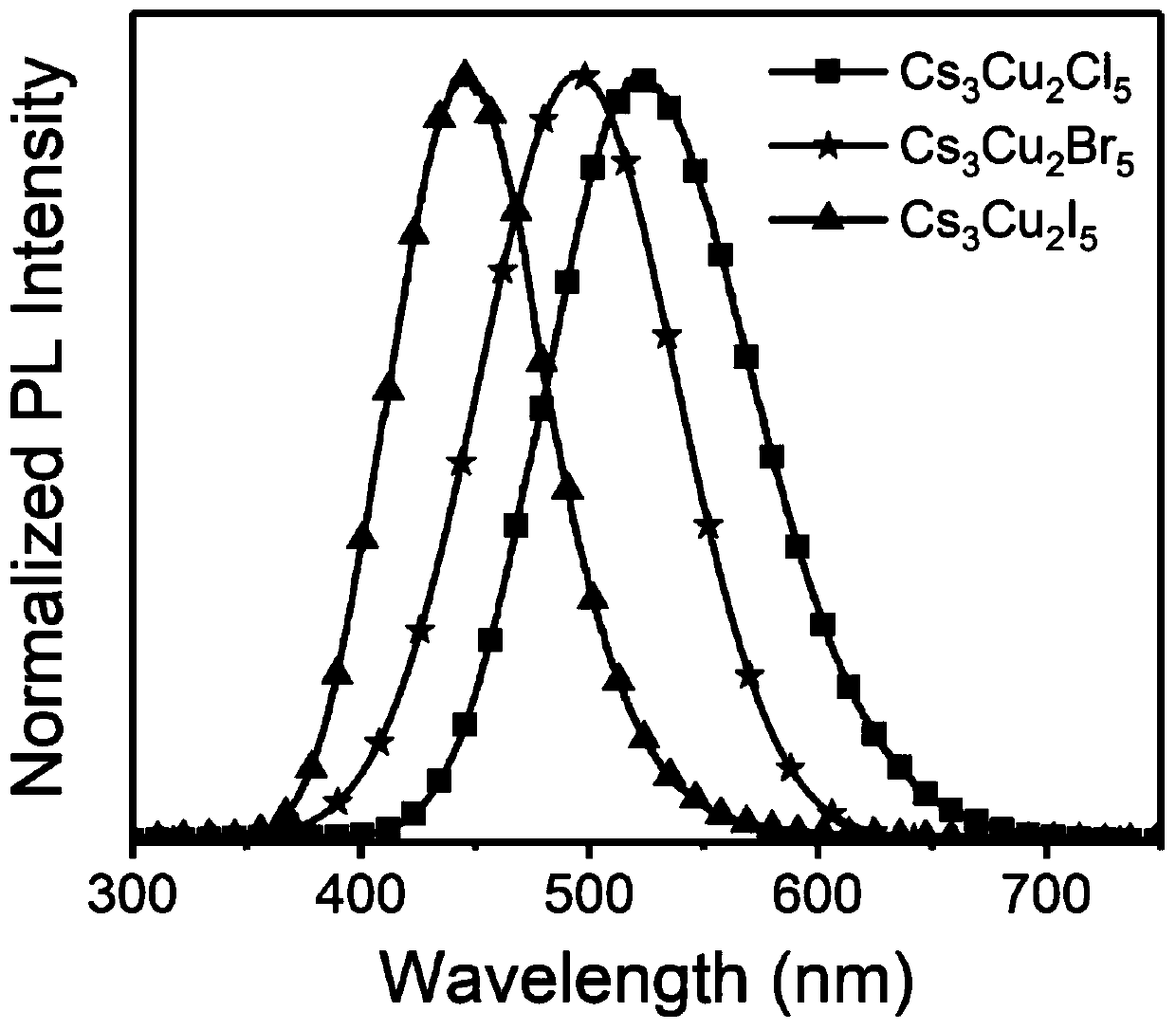
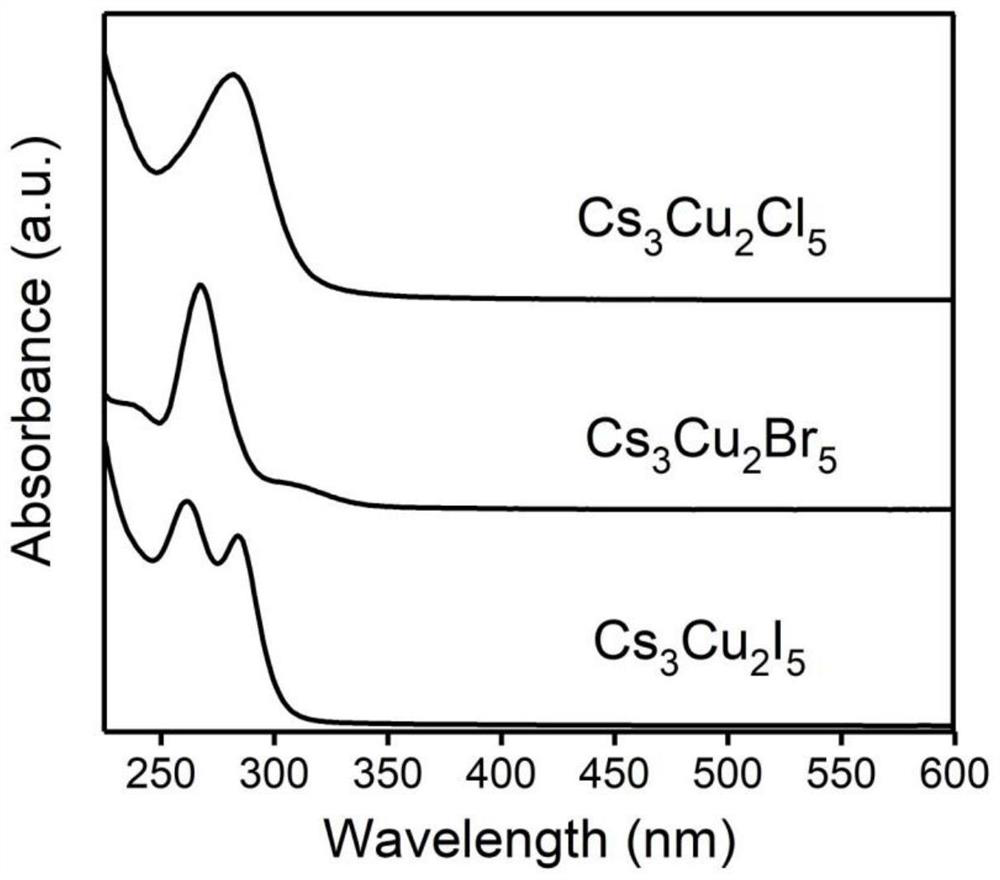
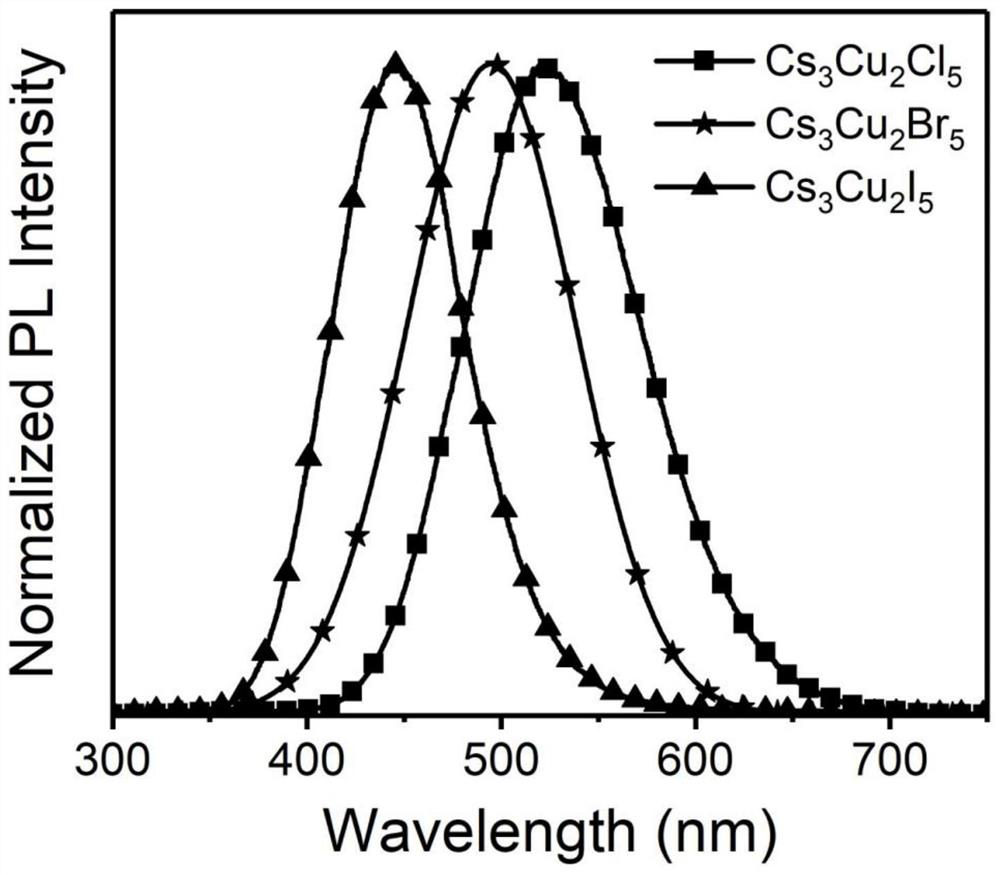
Preparation method and product of Cs<3>Cu<2>X<5> (X=Cl, Br, I) nanocrystalline
ActiveCN111348674AHigh Luminous Quantum EfficiencyStrong exciton-phonon couplingCopper compoundsLuminescent compositionsHalogenNanocrystal
The invention belongs to the technical field of perovskite-like nanocrystals. The invention discloses a preparation method of Cs<3>Cu<2>X<5> nanocrystalline and a product. The preparation method comprises the steps that indium halide InX3 or zinc halide ZnX2 is additionally introduced into a precursor solution for preparing Cs<3>Cu<2>X<5> nanocrystalline through a thermal injection method, preparing the Cs<3>Cu<2>X<5> nanocrystalline through a thermal injection method, wherein X is selected from Cl, Br and I. According to the preparation method, a key precursor for regulating and controlling the growth of the nanocrystalline in the preparation method of the nanocrystalline is improved; indium halide or zinc halide is used as an additive in a precursor, and a copper element is used as a substitute of a lead element, so that the preparation of the lead-free metal halide light-emitting nanocrystal is completed, the preparation process is easy to implement, the cost is low, and meanwhile,the defects of the lead-containing halogen perovskite nanocrystal can be overcome.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Apparatus for high temperature hydrolysis of water reactive halosilanes and halides and process for making same
InactiveUS20100061912A1Better for disposalLow costChlorine/hydrogen-chlorideHydrogen separationDistillationHydrolysis
A process for high temperature hydrolysis of halosilanes and halides with the steps of: providing a bed of fluidized particulate material heated to at least 300° C., injecting steam and an excess of reactants into the reactor, removing solid waste from a bottom outlet, removing the effluent gases through a solids removal device such as a cyclone, condensing and separating some of the unreacted waste from the effluent gas in a distillation column and sending the effluent gases containing hydrogen and hydrogen chloride to a compressor. In a preferred embodiment the reactants contain at least one water reactive halide, selected from the group halosilane, organohalosilane, aluminum halide, titanium halide, boron halide, manganese halide, copper halide, iron halide, chromium halide, nickel halide, indium halide, gallium halide and phosphorus halide and where the halide content is selected from chlorine, bromine and iodine.
Owner:LORD LTD LP
Method for preparing tetrahedral light-emitting indium phosphide/zinc sulfide core-casing quantum dots
ActiveCN107312534ALow priceStable chemical structureLuminescent compositionsChemical structureQuantum yield
The invention discloses a method for preparing tetrahedral light-emitting indium phosphide / zinc sulfide core-casing quantum dots. The method comprises the following steps: preparing high-boiling point tris(dialiphatic amido) phosphine, preparing InP quantum dot cores, preparing InP / ZnS core-casing quantum dots and separating the InP / ZnS core-casing quantum dots. Through the use of the high-boiling point tris(dialiphatic amido) phosphine as a phosphorus source, the price is low, the chemical structure is stable, the reaction is mild and safe, and gas is not produced. All reaction precursors are common chemical reagents and low in toxin and safe to store and use. The luminescent color of the InP / ZnS core-casing quantum dots can be precisely controlled simultaneously by the reaction temperature, the ratio of indium halide to the tris(dialiphatic amido) phosphine, the type of the halide and the reaction time, and high fluorescence quantum yield can be ensured.
Owner:南京紫同纳米科技有限公司
Metal-ion battery and method for preparing the same
ActiveCN107394271AIncrease the number of discharge voltage platformsIncrease power generationCell electrodesFinal product manufactureLanthanumCobalt
A metal-ion battery and a method for preparing the same are provided. The metal-ion battery includes a positive electrode, a separator, a negative electrode, and an electrolyte. The positive electrode is separated from the negative electrode via the separator, and the electrolyte is disposed between the positive electrode and the negative electrode. In particular, the electrolyte includes an ionic liquid, an aluminum halide, and a metal halide, wherein the metal halide is silver halide, copper halide, cobalt halide, ferric halide, zinc halide, indium halide, cadmium halide, nickel halide, tin halide, chromium halide, lanthanum halide, yttrium halide, titanium halide, manganese halide, molybdenum halide, or a combination thereof.
Owner:IND TECH RES INST
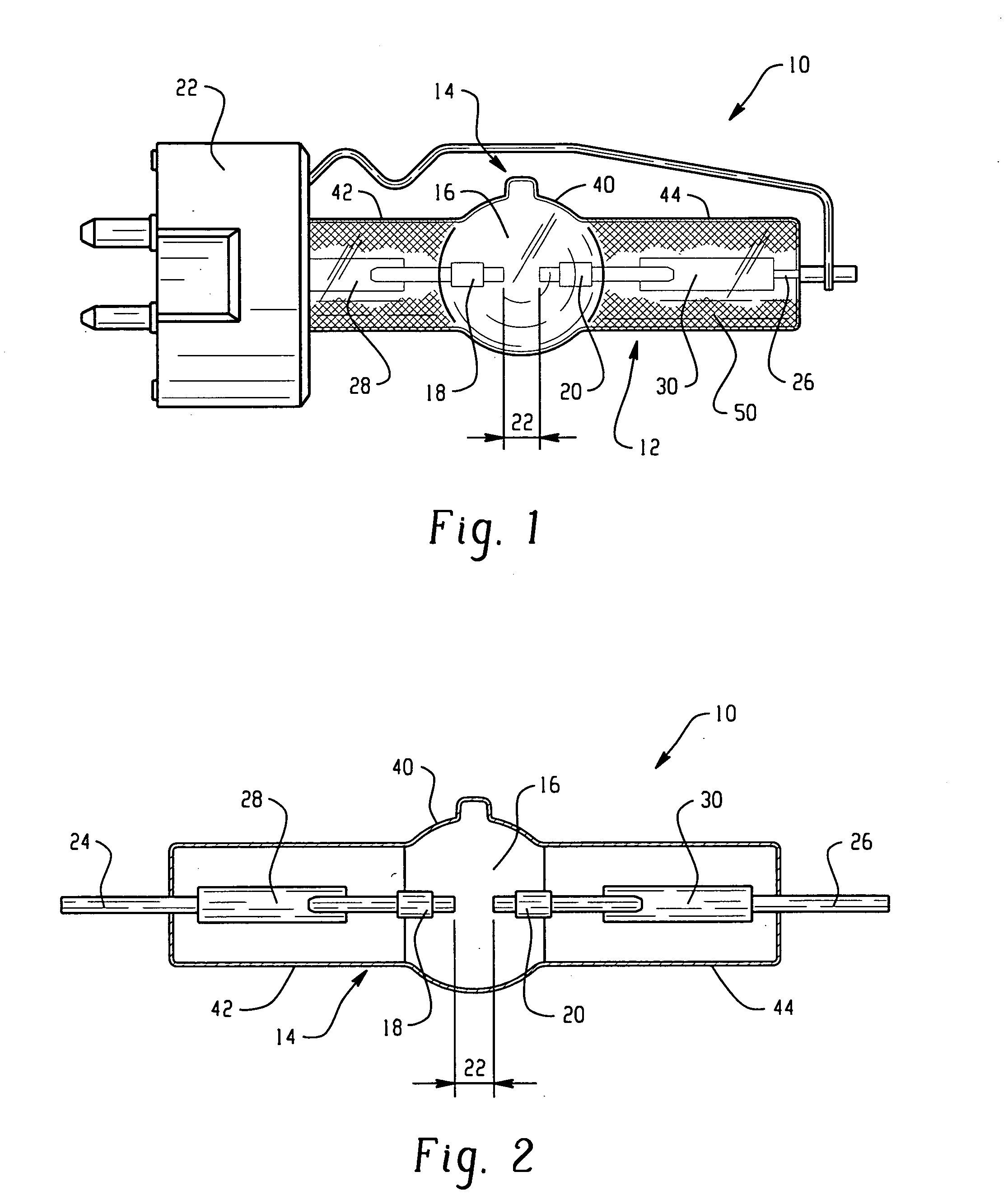
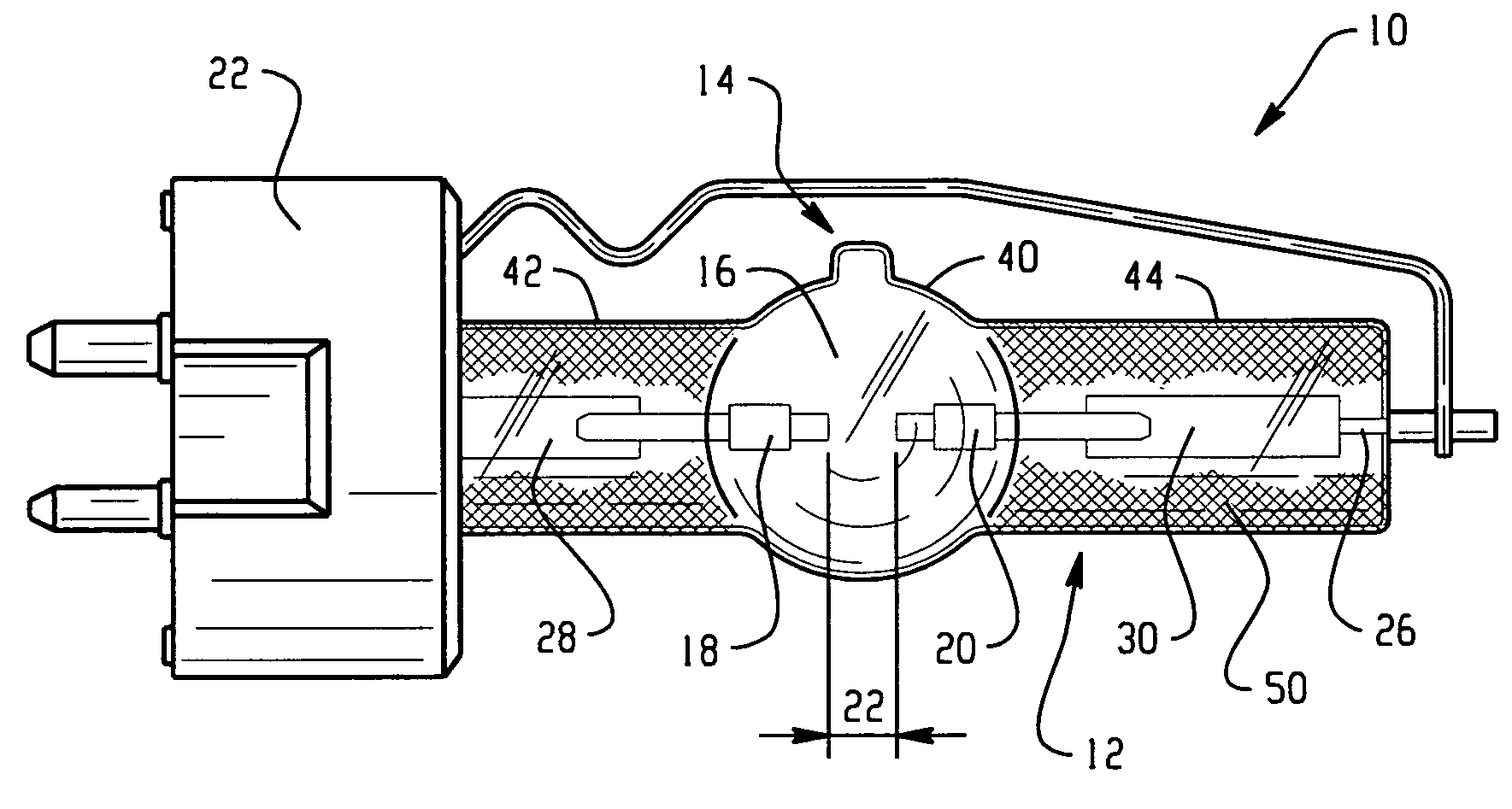
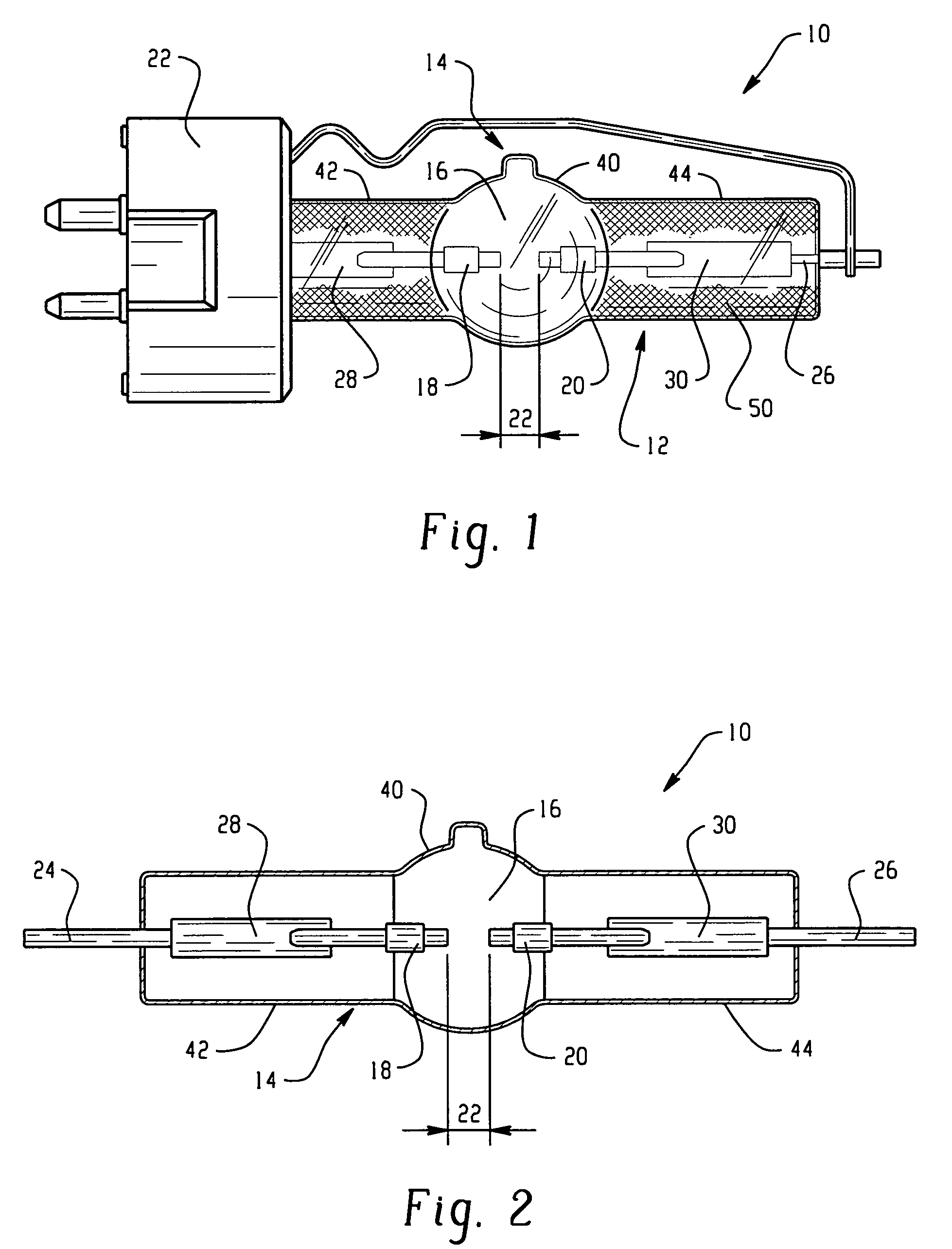
Discharge lamp with high color temperature
A lamp includes a discharge sustaining fill which includes cesium halide, one of indium halide and thallium halide, optionally gadolinium halide and a rare earth halide component selected from dysprosium halide, holmium halide, thulium halide, and neodymium halide. In operation without a jacket, the lamp may have a color temperature of from 7,000K to 14,000K and a color rendering index of at least 70 when operated at an arc wall loading in excess of about 2 W / mm2.
Owner:KOTO ELECTRIC +1
Phosphor Blend and Lamp Containing Same
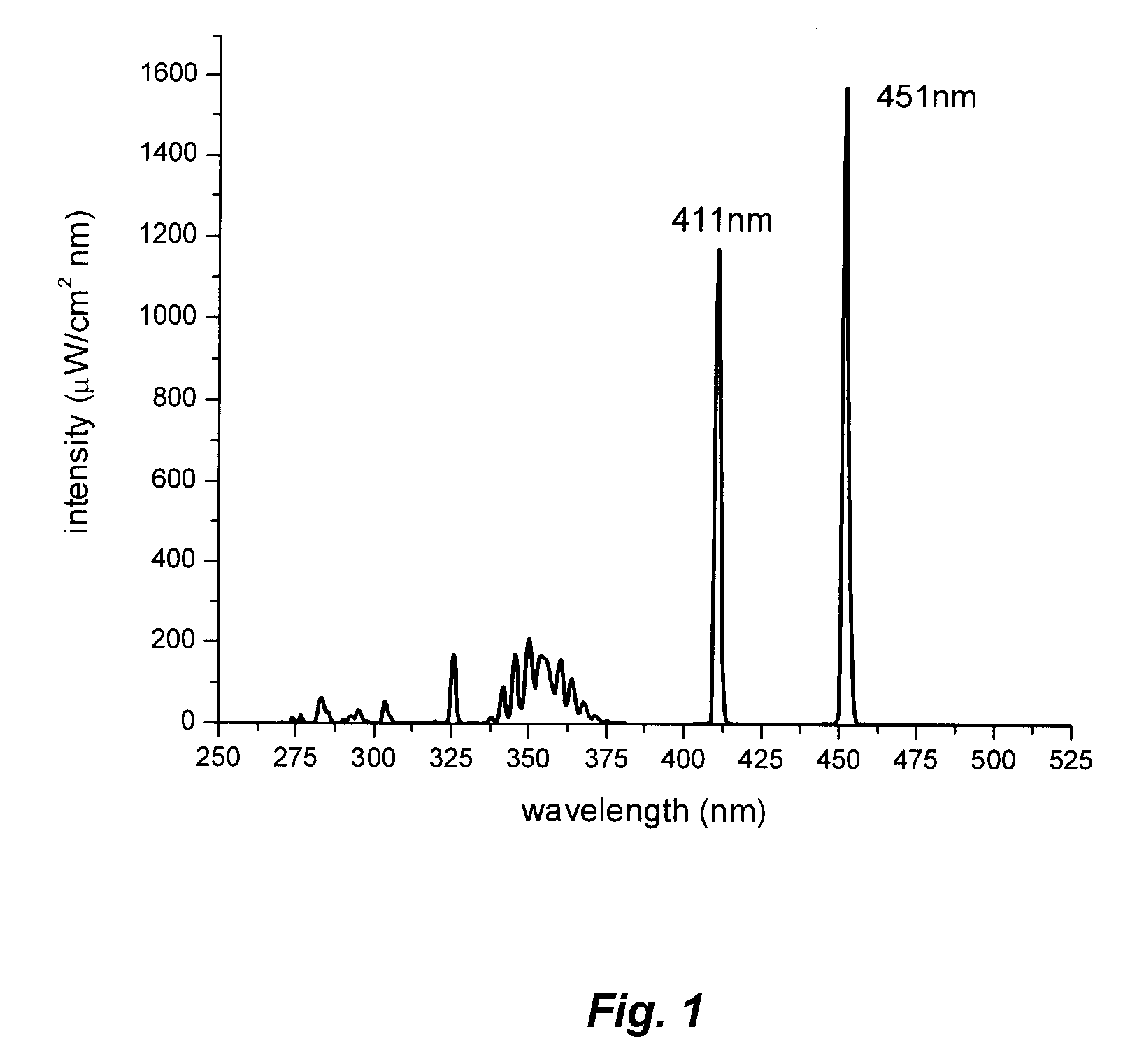
InactiveUS20070267960A1Improve launch performanceGood efficacy valueDischarge tube luminescnet screensLamp detailsPhotochemistryBlue emitting
Owner:OSRAM SYLVANIA INC
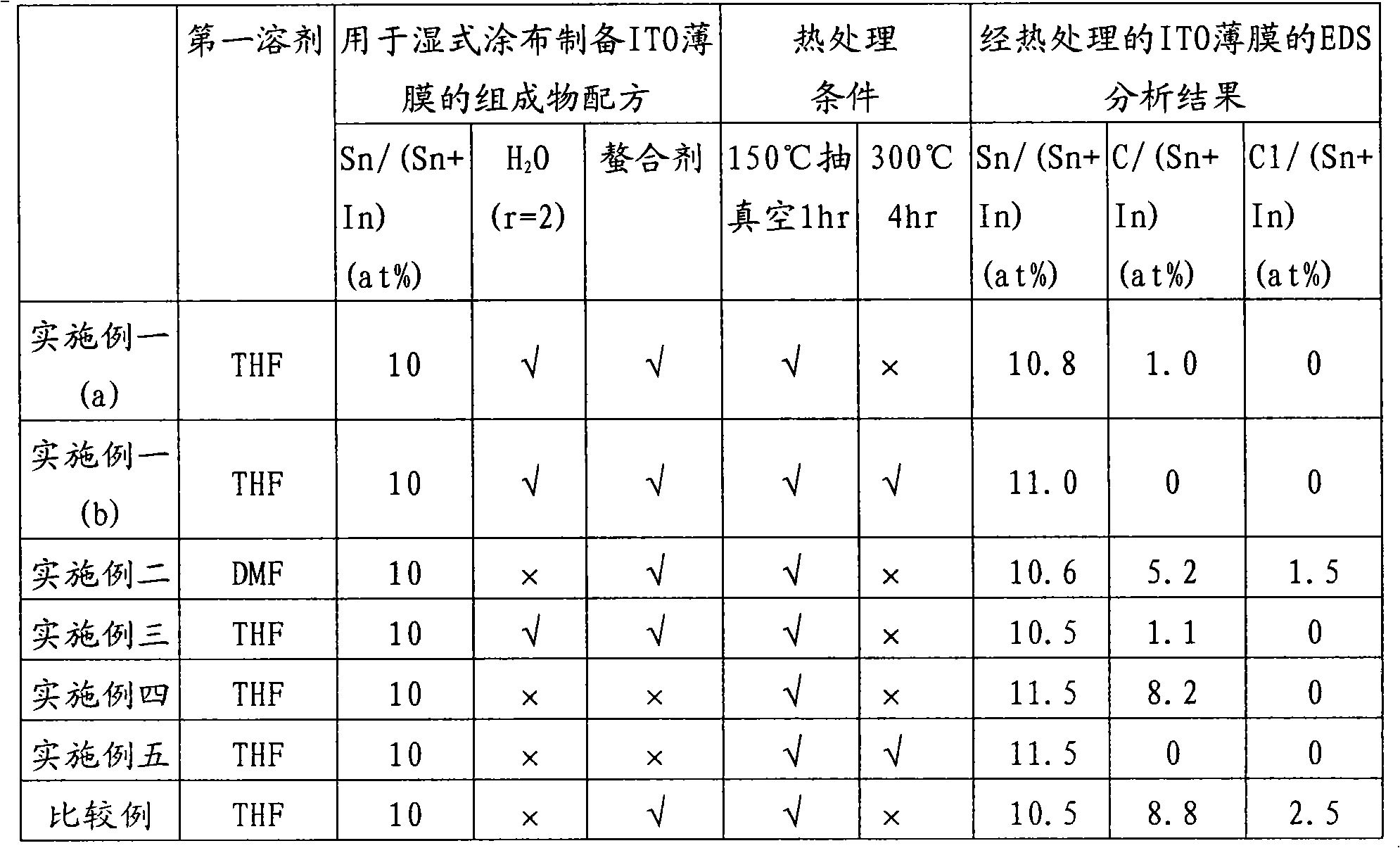
Method for preparing composition containing indium alcoholate and tin alcoholate and composition containing indium alcoholate and tin alcoholate prepared thereby
The invention discloses a method for preparing a composition containing indium alcoholate and tin alcoholate and the composition containing indium alcoholate and tin alcoholate prepared thereby. The method comprises the following steps: a, reacting anhydrous indium halide and anhydrous tin halide dissolved in a first solvent with an alcohol having 3 to 20 carbon atoms at a first reaction temperature between 40 DEG C below zero and 60 DEG C to obtain first solution containing an indium alcoholate precursor and a tin alcoholate precursor; and b, stepwise dripping alkali catalyst solution containing an alkali catalyst and a dilute solvent into the first solution, continuing reaction at a second reaction temperature between 40 DEG C below zero and 60 DEG C and precipitating a salt precipitate, and removing the salt precipitate to obtain the composition containing the indium alcoholate and the tin alcoholate. The first solvent does not contain hydroxyl and can dissolve the anhydrous indiumhalide, anhydrous tin halide, the alkali contact and the alcohol, and the dilute solvent may be the first solvent or the alcohol.
Owner:NAT KAOHSIUNG UNIV OF SCI & TECH
Discharge lamp with high color temperature
Owner:KOTO ELECTRIC +1
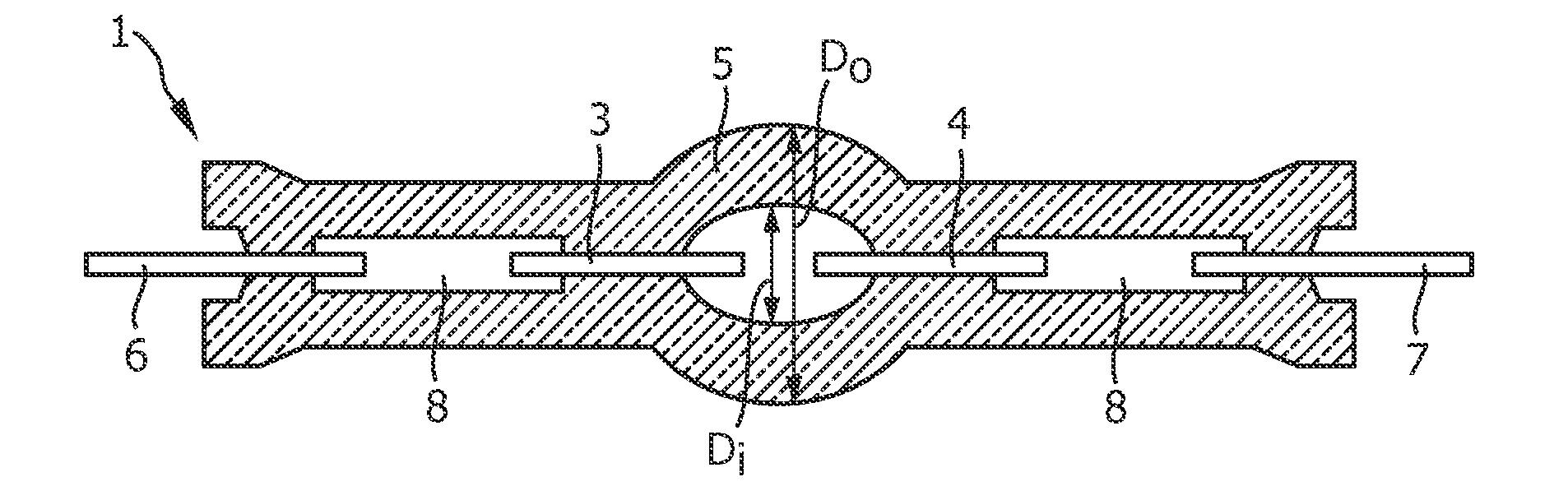
Mercury-free high intensity gas-discharge lamp
InactiveUS20130038207A1Improved emitter functionGood curative effectSolid cathode detailsGas discharge lamp detailsGas-discharge lampThallium
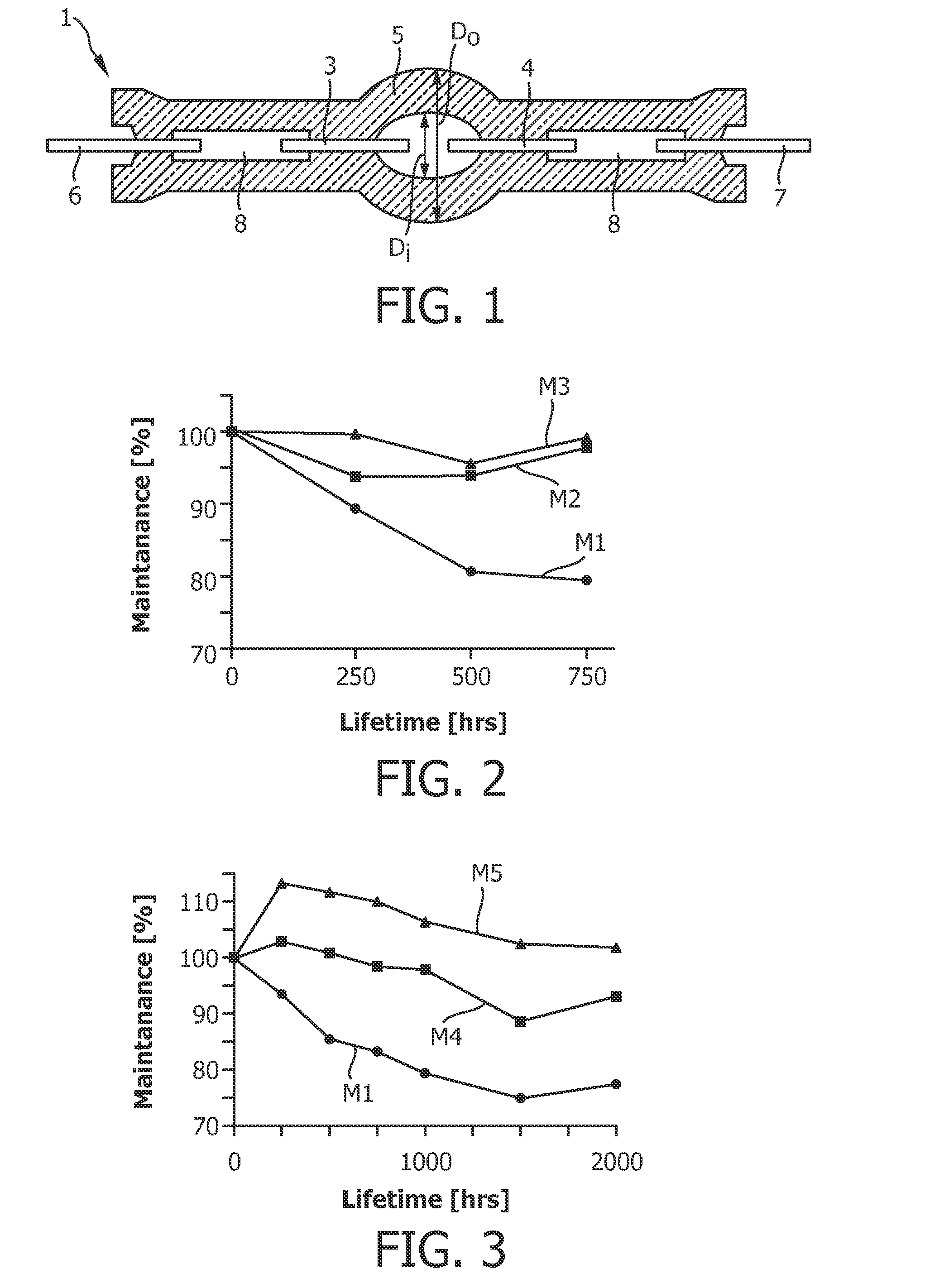
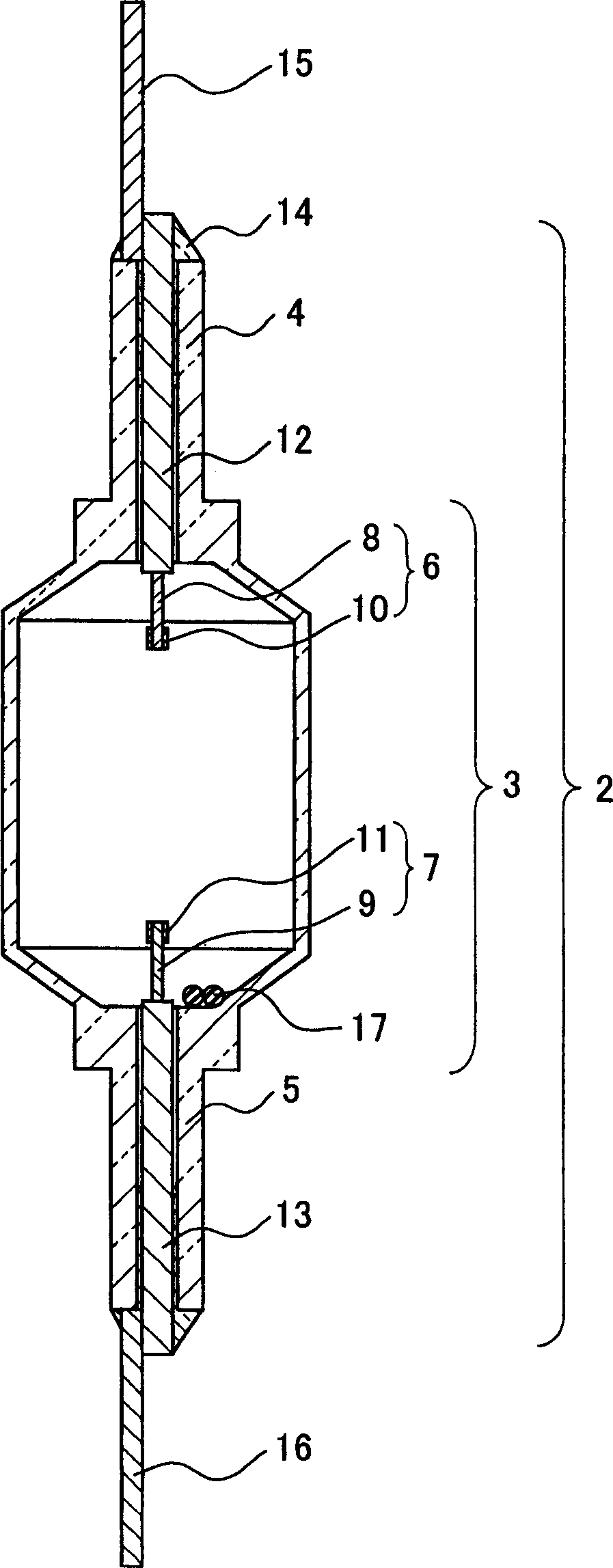
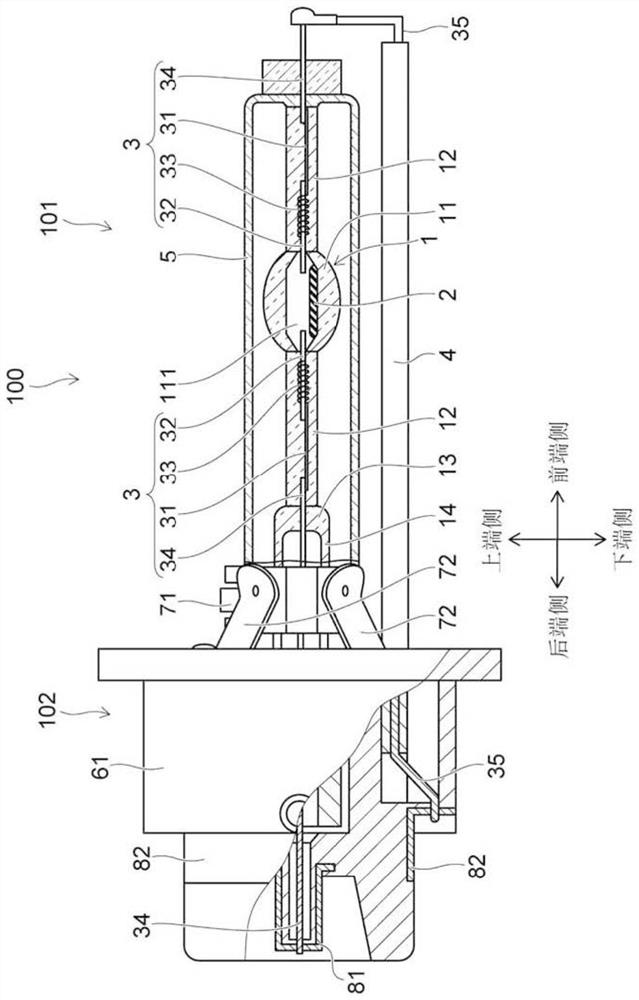
The invention describes a mercury-free high-intensity gas-discharge lamp (1) comprising a discharge vessel (5) enclosing a fill gas in a discharge chamber (2) and comprising a pair of electrodes (3, 4) extending into the discharge chamber (2), for which lamp (1) the fill gas is derived from a salt fill introduced into the discharge chamber (2) prior to sealing, which salt fill is free of scandium and includes a halide composition comprising a sodium halide to a proportion of at least 65 wt % and at most 97.2 wt %,a thallium halide to a proportion of at least 2 wt % and at most 25 wt %, and an indium halide to a proportion of at least 0.5 wt % and at most 25 wt %. Eliminating the highly reactive scandium from the fill gas significantly improves lumen maintenance.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Low-temperature aluminum soft brazing solder paste and preparing method thereof
ActiveCN110202295AEfficient removalAchieve strong bindingWelding/cutting media/materialsSoldering mediaSurface oxidationSolvent
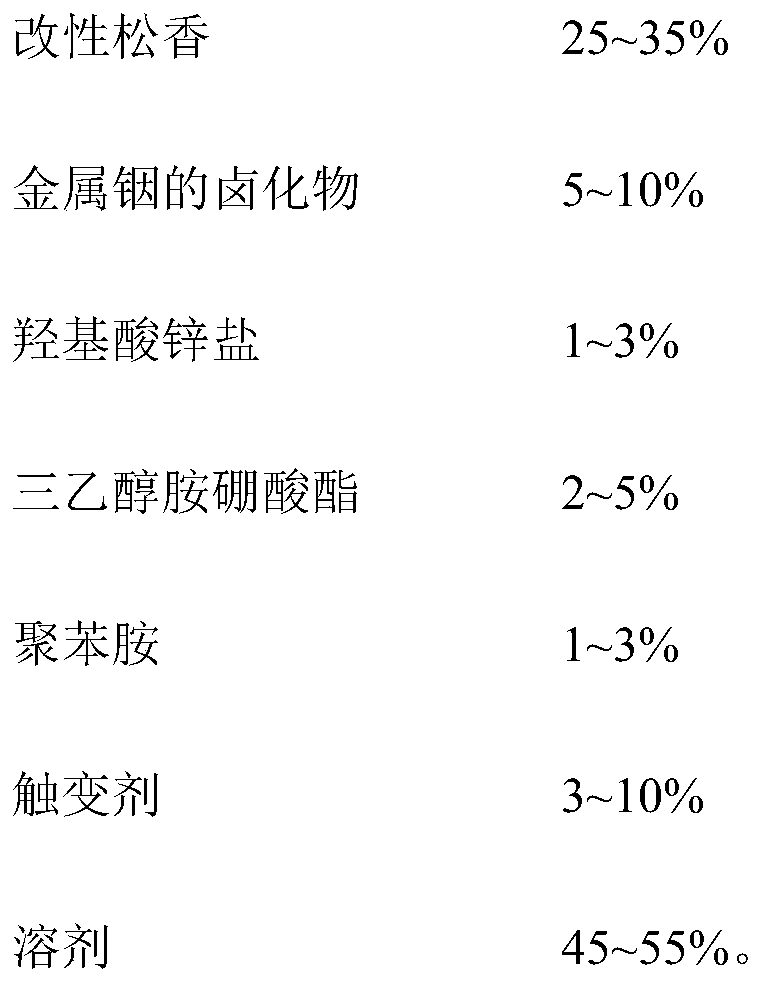
The invention discloses low-temperature aluminum soft brazing solder paste and a preparing method thereof. The low-temperature aluminum soft brazing solder paste comprises following components including, by mass percent, 80%-90% of brazing filler metal alloy powder and 10%-20% of scaling powder. The scaling powder comprises following components including, by mass percent, 25%-35% of modified rosin, 5%-10% of metal indium halide, 1%-3% of oxyacid zinc salt, 2%-5% of triethanolamine borate, 1%-3% of polyaniline, 3%-10% of a thixotropic agent and 45%-55% of a solvent. According to the low-temperature aluminum soft brazing solder paste, an aluminum surface oxidation film can be effectively removed, strong bonding of brazing filler metal and aluminum base metal is achieved, a formed aluminum soft brazing connector is firm, good electrochemistry corrosion resistance is achieved, the storage performance of the solder paste is good, and dryness and coarseness are avoided.
Owner:浙江强力控股有限公司
Metal halide lamp
InactiveCN1407596AExtend your lifeSuppresses reduction in beam maintenance rateElectric discharge tubesDischarge tube main electrodesCeriumLuminous flux
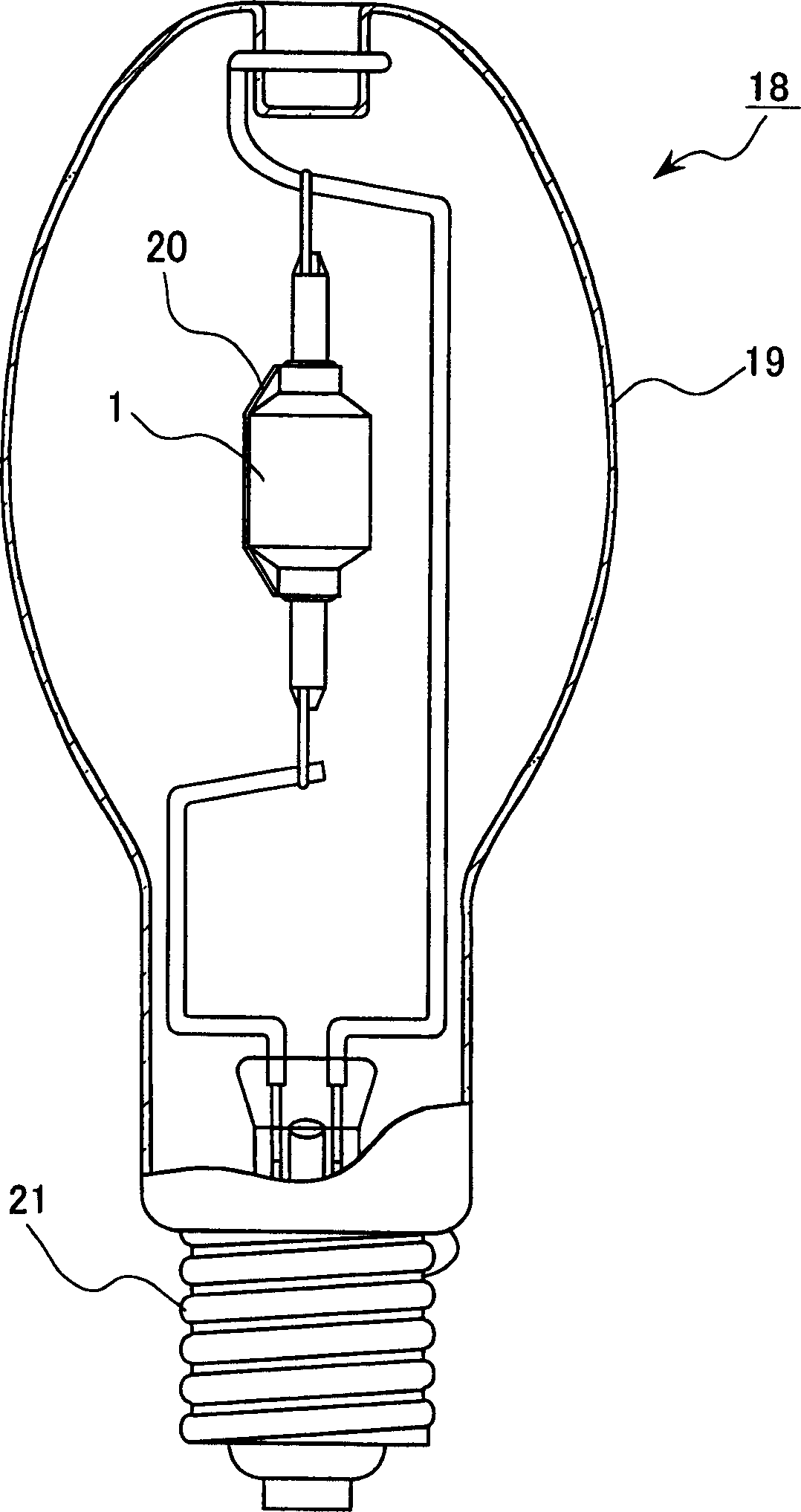
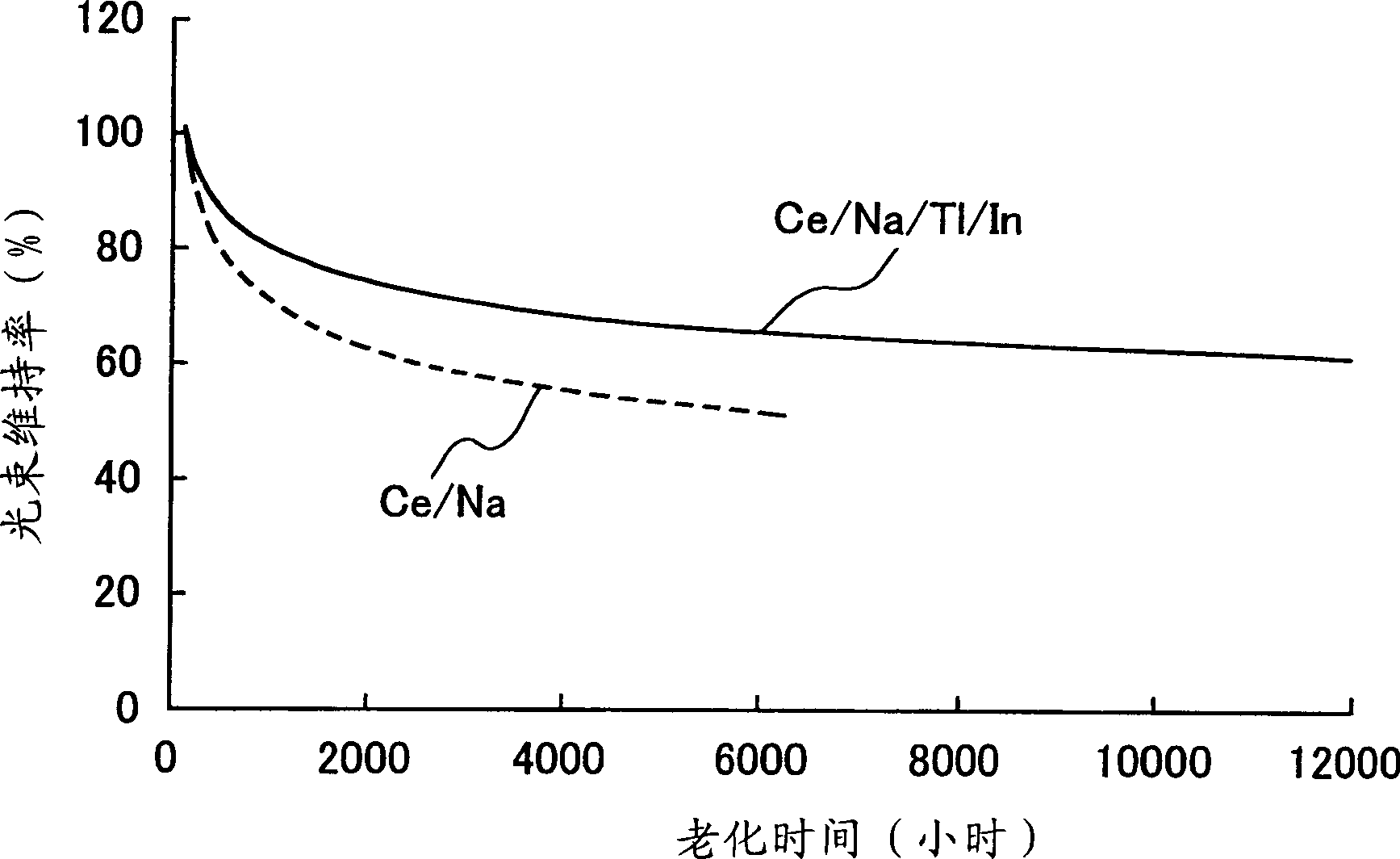
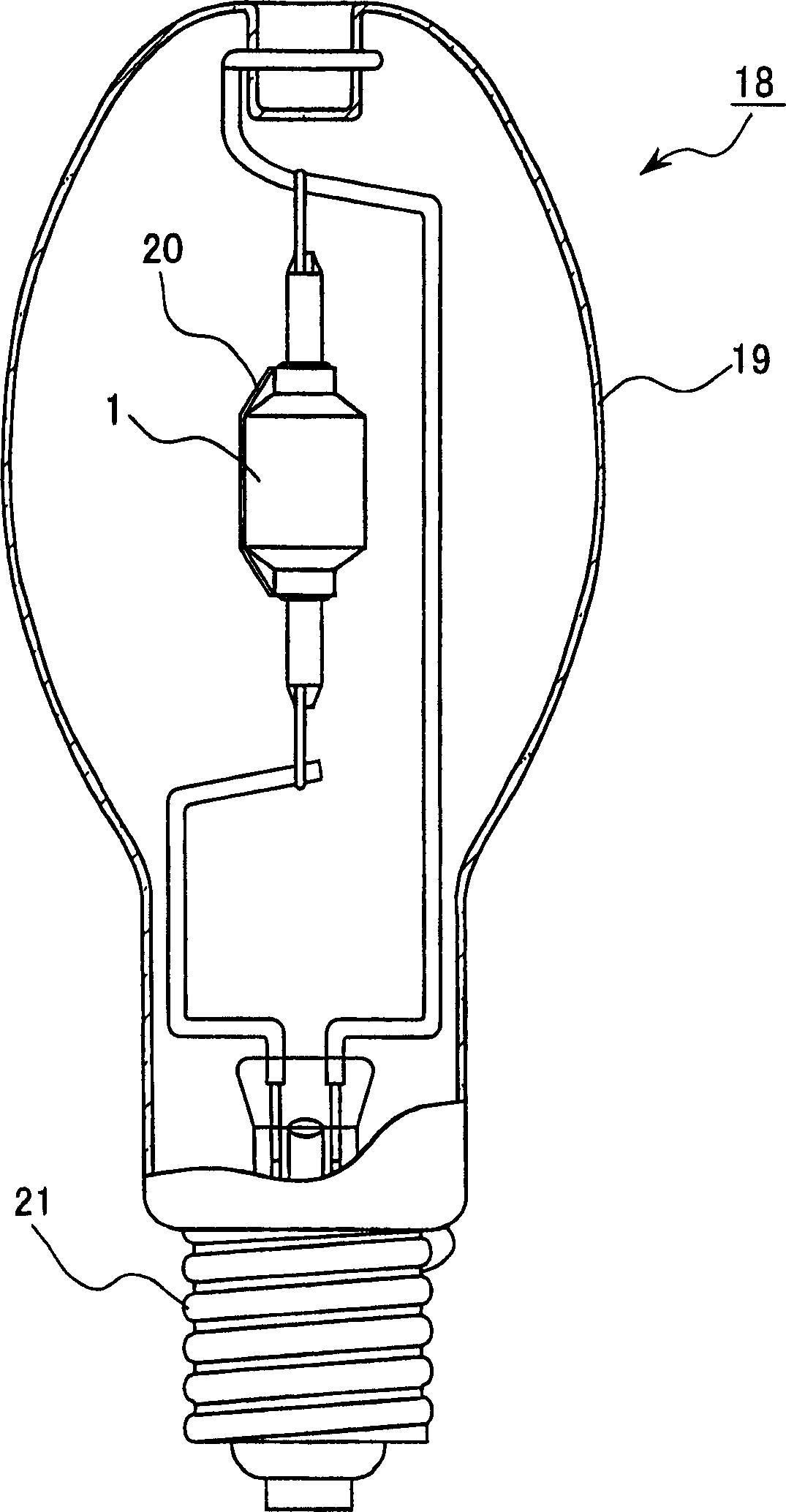
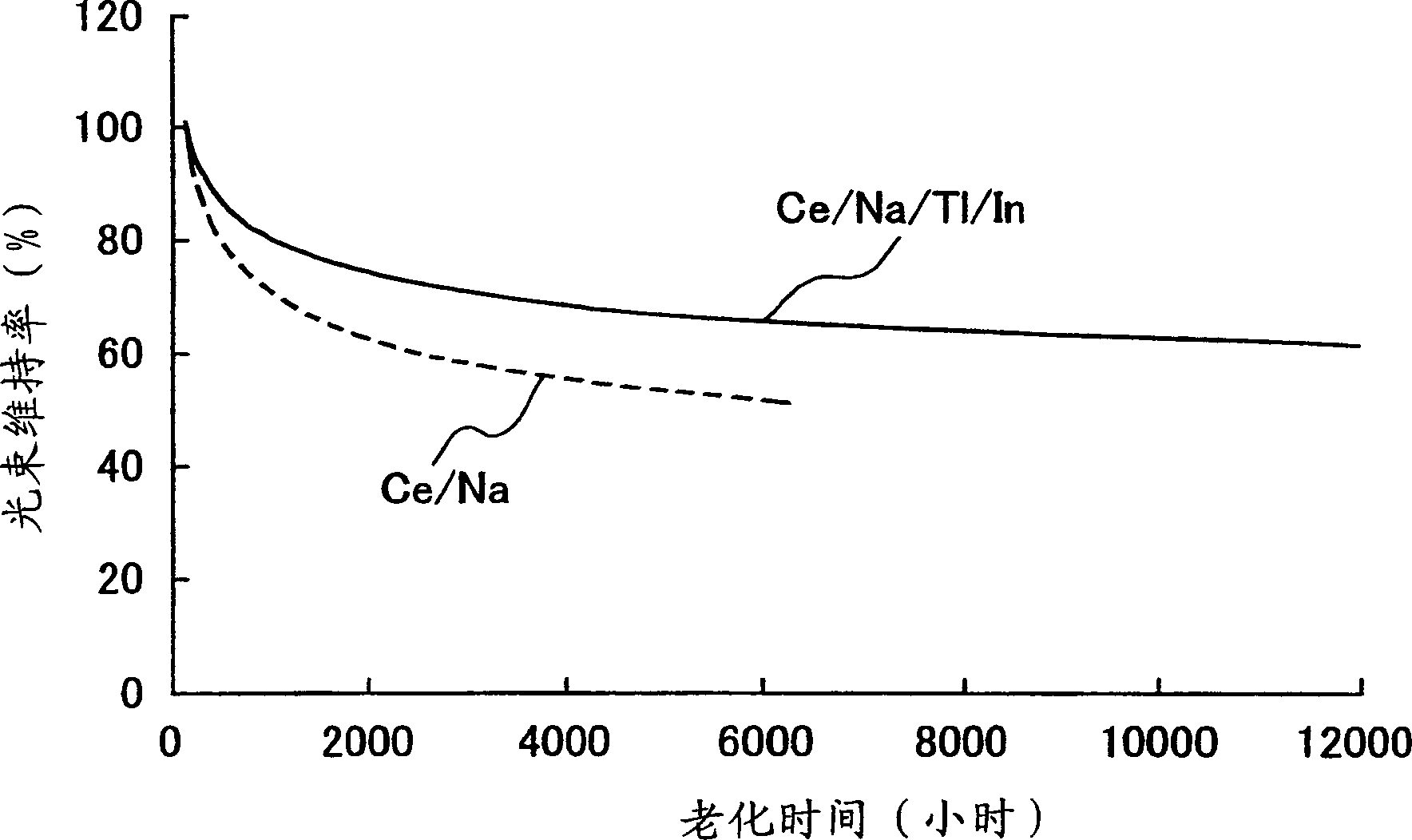
Provided is a metal halide lamp, comprising a luminous tube (3) whose outer shell is made of an oxide-based translucent ceramic material. In the luminous tube (3), cerium halides and sodium halides are combined and sealed as luminous substances. compounds, halides of thallium and halides of indium. With respect to all metal halides, it is preferable that the halide of cerium is more than 20wt% and less than 69.0wt%, the halide of sodium is more than 30wt% and less than 79.0wt%, and the total amount of halide of thallium and halide of indium is 1.0 More than wt% and less than 20 wt%. As a result, the discharge arc is broadened, the bending toward the wall of the luminous tube is suppressed, and the lamp efficiency is improved. Even if it is used for a long time, the reduction of the beam maintenance rate can be suppressed, and the hue of the luminous color can be compensated.
Owner:PANASONIC CORP
Lead-free indium-based double perovskite material and preparation method and application thereof
ActiveCN112358870AImprove stabilityHigh purityEnergy efficient lightingPhotovoltaic energy generationPhysical chemistryDouble perovskites
The invention relates to the technical field of perovskite materials, and provides a lead-free indium-based double perovskite material and a preparation method and application thereof. The preparationmethod of the lead-free indium-based double perovskite material comprises the following steps: providing a first solution containing a first halogenated metal salt and a second solution containing asecond halogenated metal salt, providing a third solution containing indium halide, and adjusting the pH value of the third solution to be less than 4.0, mixing the first solution, the second solutionand the third solution for reaction to obtain a first mixed solution, and separating and purifying the first mixed solution to obtain the lead-free indium-based double perovskite material. The preparation method is simple and flexible to operate, mild and controllable in preparation condition, capable of achieving zero emission of waste, free of pollution, excellent in environmental protection performance and suitable for large-scale stable production of the lead-free indium-based double perovskite material which is non-toxic, high in stability and high in purity.
Owner:SHENZHEN UNIV
High intensity discharge lamp
InactiveUS7893619B2Improved performance and luminous efficiencyElectroluminescent light sourcesHigh-pressure discharge lampsHolmiumColor rendering index
A lamp includes a discharge sustaining fill which includes mercury halide, cesium halide, optionally one of indium halide and thallium halide, and a rare earth halide component selected from dysprosium halide, holmium halide, and thulium halide. In operation without a jacket, the lamp may have a color temperature of about 5300K to 6000K, a color rendering index of at least about 92 and an efficacy of at least about 85 LPW.
Owner:GENERAL ELECTRIC CO
Synthesis of gallium and indium alkoxides
InactiveUS7585992B2Organic compound preparationGroup 8/9/10/18 element organic compoundsAlkaline earth metalPotassium
A method of synthesising gallium or indium alkoxide from the corresponding gallium or indium halide, the method comprising the step of reacting the gallium or indium metal halide with the alkoxide of an alkali earth metal, to produce the desired metal alkoxide essentially free of chlorine contamination. The method provides a composition comprising a solution of gallium or indium alkoxide of high purity, the composition having a chloride content of less than 30 ppm, a barium or strontium content of less than 30 ppm, and a sodium or potassium content of less than 30 ppm, without requiring any additional steps.
Owner:MULTIVALENT
High intensity discharge lamp
InactiveUS20100019675A1Improved performance and luminous efficiencyElectroluminescent light sourcesHigh-pressure discharge lampsHolmiumColor rendering index
A lamp includes a discharge sustaining fill which includes mercury halide, cesium halide, optionally one of indium halide and thallium halide, and a rare earth halide component selected from dysprosium halide, holmium halide, and thulium halide. In operation without a jacket, the lamp may have a color temperature of about 5300K to 6000K, a color rendering index of at least about 92 and an efficacy of at least about 85 LPW.
Owner:GENERAL ELECTRIC CO
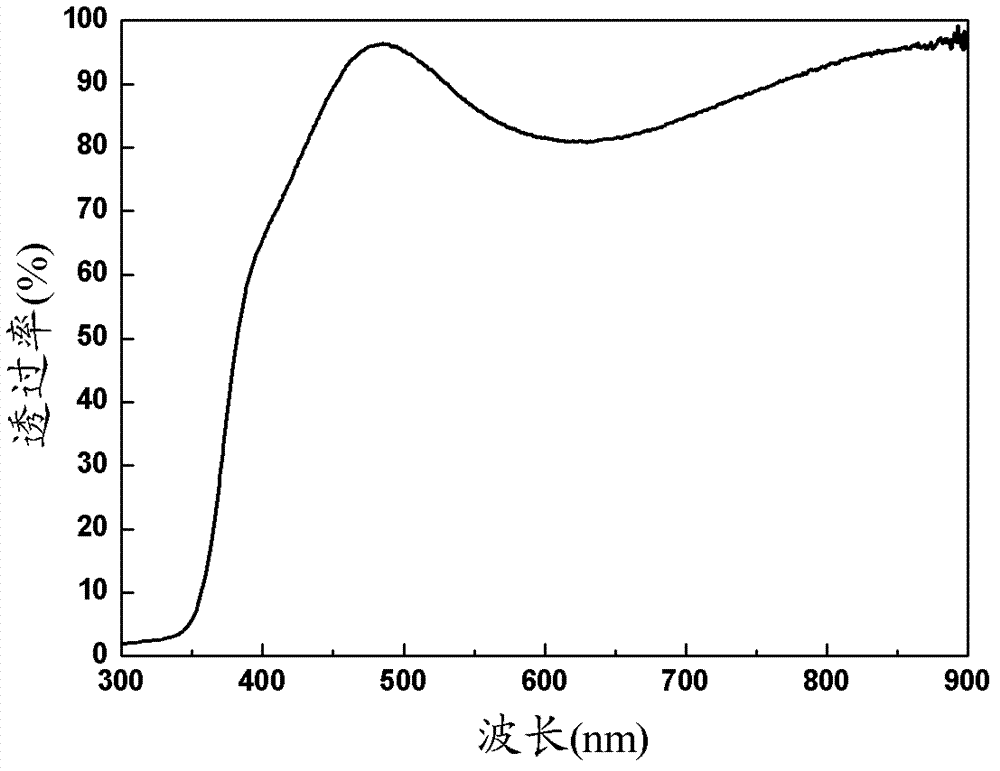
ITO-indium halide bilayer conductive film and preparation method thereof
InactiveCN103243296AImprove conductivityImprove work functionElectrical apparatusElectroluminescent light sourcesHalogenWork function
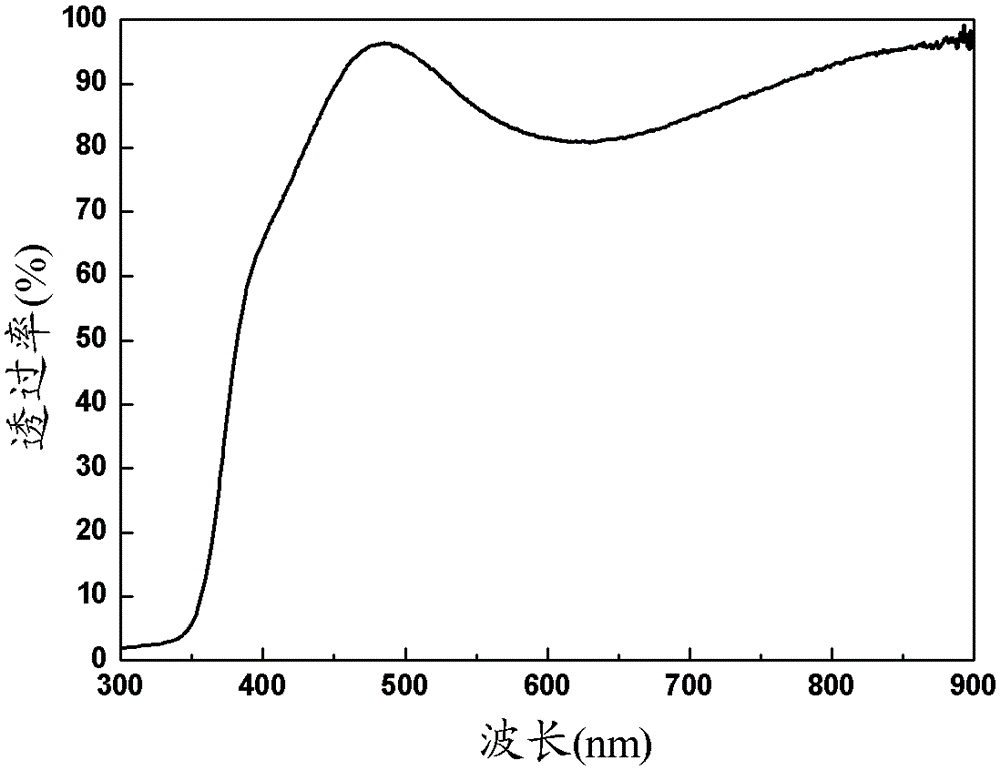
The invention belongs to the field of conductive films and discloses an ITO-indium halide bilayer conductive film and a preparation method thereof. The bilayer conductive film includes an ITO layer and an indium halide layer, wherein the ITO layer has a content of 80-97 wt% of In2O3 and 3-20% of SnO2, and the halogen in the indium halide is one selected from the group consisting of F, Cl or Br. The present invention uses a magnetron sputtering apparatus to prepare the ITO-indium halide bilayer conductive film. The conductive film has a visible light transmittance of 85-90% in the wavelength range of 450-790 nm, a square resistance range of 20-90 omega / square, and a surface work function of 5.5-6.1 eV.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
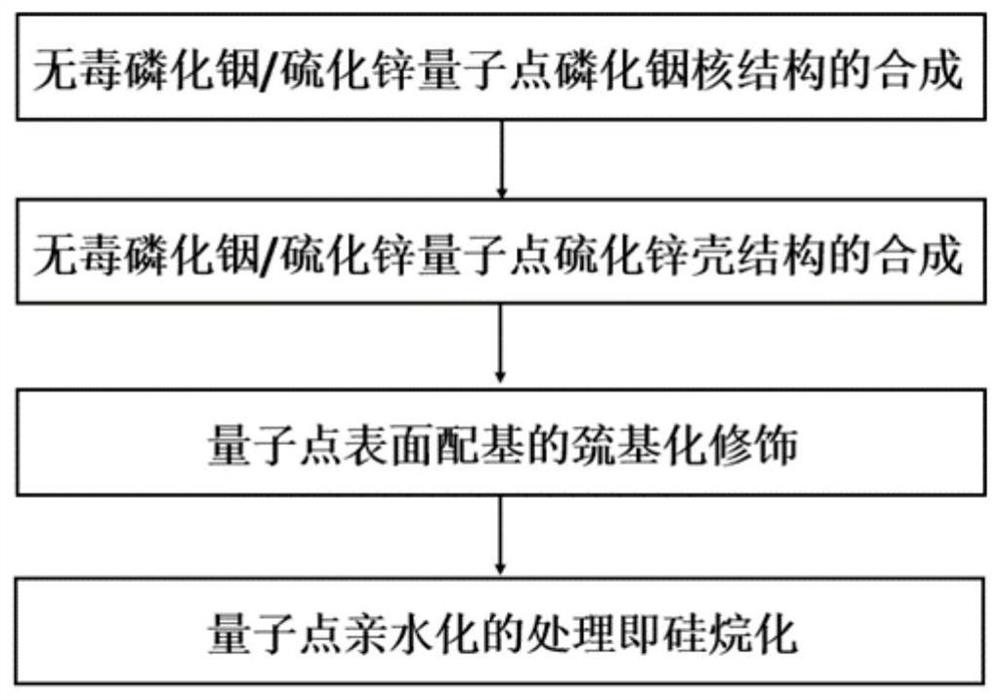
Non-toxic quantum dot with controllable particle size and preparation method thereof
InactiveCN112625672AAvoid it happening againReduce manufacturing costMaterial nanotechnologyNanoopticsToxic gasZinc sulphide
The invention discloses a non-toxic quantum dot with a controllable particle size and a preparation method thereof. The preparation method of the non-toxic quantum dot comprises the following steps: preparing a core structure and a shell structure; wherein the core structure is indium phosphide, the donor of indium is indium halide, and the donor of phosphorus is tri(diethylamino)phosphorus; the shell structure is zinc sulfide, the donor of zinc is zinc halide, and the donor of sulfur is trioctylsulfur. Tris(diethylamino)phosphorus is adopted, so that generation of toxic gas is avoided, and the production cost is reduced. And secondly, the method can effectively control the wavelength and the particle size of the quantum dots, controls the particle size of the QDs by adopting the composition and the proportion of different halogen elements, changes the instability and the determinacy of controlling the particle size of the QDs by controlling the synthesis time in the conventional QDs synthesis method, and improves the controllability of the method.
Owner:CHINA AGRI UNIV
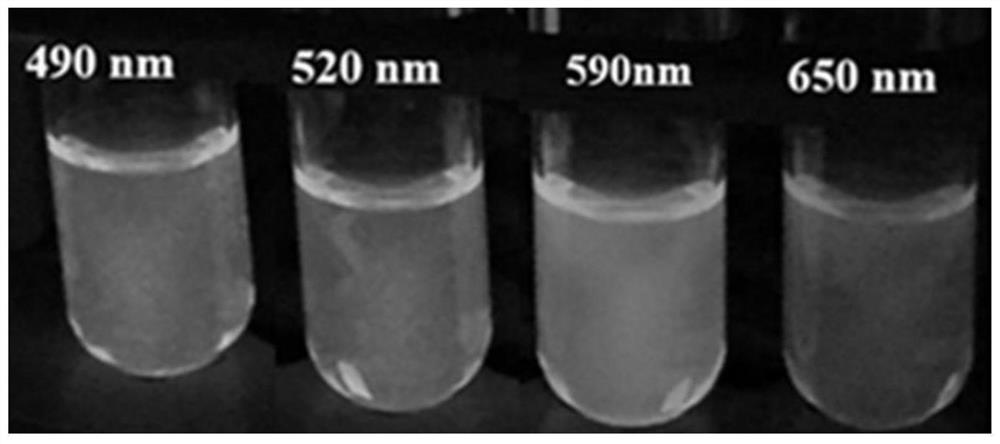
Cadmium-free quantum dots and preparation method thereof
PendingCN113637469AGood lookingImprove luminous efficiencyMaterial nanotechnologyNanoopticsFluorescencePhysical chemistry
The invention relates to the technical field of quantum dot synthesis, in particular to cadmium-free quantum dots and a preparation method thereof. The method comprises the steps of: mixing a phosphorus precursor, an indium aliphatate precursor and a non-coordination solvent at a first temperature, and heating to the second temperature for a reaction to obtain indium phosphide quantum dot cores; and dropwise adding an indium halide precursor into the reaction system of the indium phosphide quantum dot cores, and carrying out surface treatment on the indium phosphide quantum dot cores to obtain cadmium-free quantum dots. According to the method disclosed by the invention, the surface defects of the cadmium-free quantum dots can be reduced, the morphology, luminous efficiency and stability of the indium phosphide quantum dots are remarkably improved, and the cadmium-free indium phosphide quantum dots prepared simply at low cost are relatively narrow in half-peak width and high in fluorescence yield.
Owner:浙江臻纳科技有限公司
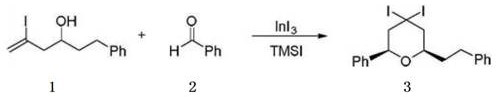
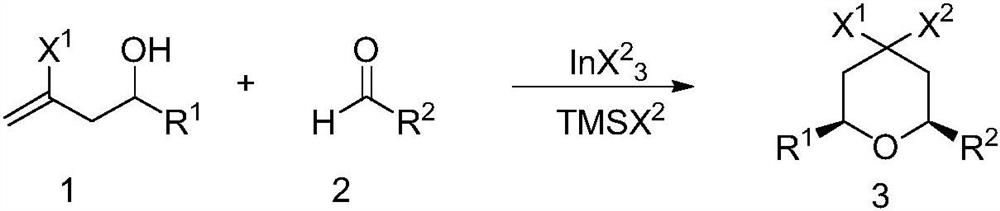
A kind of preparation method of 4,4-dihalogenated tetrahydropyran
Owner:QUZHOU UNIV
Metal halide lamp
A metal halide lamp has an arc tube including an envelope as an arc tube container made of an oxide-based translucent ceramic material, and the arc tube is filled with luminescent materials comprising at least a cerium halide, a sodium halide, a thallium halide and an indium halide. An amount of the cerium halide is in a range from 20 wt % to 69.0 wt %, an amount of the sodium halide is in a range from 30 wt % to 79.0 wt %, and a total amount of the thallium halide and the indium halide is in a range from 1.0 wt % to 20 wt % with respect to the entire metal halides. Accordingly, the arc discharge is spread, bending of the arc discharge toward the arc tube wall is suppressed, and thus, the metal halide lamp has improved luminescent efficiency, where lowering of the flux maintenance factor is suppressed even after a long-time use, and hues of the luminescent colors are corrected.
Owner:PANASONIC CORP
ito-indium halide double-layer conductive film and preparation method thereof
InactiveCN103243296BSimple preparation processEasy to controlElectrical apparatusElectroluminescent light sourcesHalogenWork function
The invention belongs to the field of conductive films and discloses an ITO-indium halide bilayer conductive film and a preparation method thereof. The bilayer conductive film includes an ITO layer and an indium halide layer, wherein the ITO layer has a content of 80-97 wt% of In2O3 and 3-20% of SnO2, and the halogen in the indium halide is one selected from the group consisting of F, Cl or Br. The present invention uses a magnetron sputtering apparatus to prepare the ITO-indium halide bilayer conductive film. The conductive film has a visible light transmittance of 85-90% in the wavelength range of 450-790 nm, a square resistance range of 20-90 omega / square, and a surface work function of 5.5-6.1 eV.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
discharge lamp
ActiveCN108807134BInhibit tube voltage riseImprove luminous efficiencyGas discharge lamp detailsMetal halidesElectric power
Owner:TOSHIBA LIGHTING & TECH CORP
Method for preparing composition containing indium alcoholate and tin alcoholate and composition containing indium alcoholate and tin alcoholate prepared thereby
The invention discloses a method for preparing a composition containing indium alcoholate and tin alcoholate and the composition containing indium alcoholate and tin alcoholate prepared thereby. The method comprises the following steps: a, reacting anhydrous indium halide and anhydrous tin halide dissolved in a first solvent with an alcohol having 3 to 20 carbon atoms at a first reaction temperature between 40 DEG C below zero and 60 DEG C to obtain first solution containing an indium alcoholate precursor and a tin alcoholate precursor; and b, stepwise dripping alkali catalyst solution containing an alkali catalyst and a dilute solvent into the first solution, continuing reaction at a second reaction temperature between 40 DEG C below zero and 60 DEG C and precipitating a salt precipitate, and removing the salt precipitate to obtain the composition containing the indium alcoholate and the tin alcoholate. The first solvent does not contain hydroxyl and can dissolve the anhydrous indiumhalide, anhydrous tin halide, the alkali contact and the alcohol, and the dilute solvent may be the first solvent or the alcohol.
Owner:NAT KAOHSIUNG UNIV OF SCI & TECH
Mercury-free high intensity gas-discharge lamp
InactiveCN102859643AImprove lumen maintenanceReduce crystallizationGas discharge lampsThalliumHigh intensity
The invention describes a mercury-free high-intensity gas-discharge lamp (1) comprising a discharge vessel (5) enclosing a fill gas in a discharge chamber (2) and comprising a pair of electrodes (3, 4) extending into the discharge chamber (2), for which lamp (1) the fill gas is derived from a salt fill introduced into the discharge chamber (2) prior to sealing, which salt fill is free of scandium and includes a halide composition comprising a sodium halide to a proportion of at least 65 wt% and at most 97.2 wt%,a thallium halide to a proportion of at least 2 wt% and at most 25 wt%, and an indium halide to a proportion of at least 0.5 wt% and at most 25 wt%.
Owner:KONINK PHILIPS ELECTRONICS NV
Method for preparing regular tetrahedral luminescent indium phosphide/zinc sulfide core-shell quantum dots
ActiveCN107312534BLow priceStable chemical structureLuminescent compositionsQuantum yieldChemical structure
Owner:南京紫同纳米科技有限公司
Lead-free indium-based double perovskite material, preparation method and application thereof
ActiveCN112358870BImprove stabilityHigh purityEnergy efficient lightingLuminescent compositionsPhysical chemistryDouble perovskites
The present application relates to the technical field of perovskite materials, and provides a lead-free indium-based double perovskite material and a preparation method and application thereof. Wherein, the preparation method of lead-free indium-based double perovskite material includes the following steps: providing a first solution containing a first halide metal salt and a second solution containing a second halide metal salt, and providing a third solution containing indium halide , and adjust the pH of the third solution to <4.0; mix the first solution, the second solution and the third solution for reaction to obtain a first mixed solution, and separate and purify the first mixed solution to obtain the lead-free indium-based double perovskite material. The preparation method has simple and flexible operation, mild and controllable preparation conditions, zero waste discharge, no pollution, excellent environmental performance, and is suitable for large-scale production of lead-free indium-based double perovskite materials with non-toxic, high stability and high purity. Scale and stable production.
Owner:SHENZHEN UNIV
cs 3 cu 2 x 5 (x=cl, br, i) preparation method and product of nanocrystal
ActiveCN111348674BLow costExperiment operation is simpleCopper compoundsLuminescent compositionsHalogenPhysical chemistry
The invention belongs to the technical field of perovskite-like nanocrystals and discloses a Cs 3 Cu 2 x 5 Preparation method and product of nanocrystal, wherein the preparation method is to prepare Cs by injecting into heat 3 Cu 2 x 5 Indium halide InX is additionally introduced into the precursor solution of nanocrystals 3 or zinc halide ZnX 2 , Cs was prepared by the process of preparing nanocrystals by thermal injection 3 Cu 2 x 5 Nanocrystal, wherein X is selected from Cl, Br, I. The present invention improves the key precursor for regulating the growth of nanocrystals in the preparation method of nanocrystals, uses indium halide or zinc halide as an additive in the precursor, and cooperates with copper element as a substitute for lead element to complete the lead-free metal halide The preparation of bioluminescent nanocrystals, the preparation process of the present invention is easy to realize, and the cost is low, and at the same time, it can also solve the defects of lead-containing halogen perovskite nanocrystals.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Ceramic xenon discharge lamp filled with lanthanide halide
InactiveCN107887252AImprove and maintain light effectExtended service lifeGas discharge lamp detailsLanthanideAlloy
The invention relates to the technical field of xenon discharge lamps and in particular to a ceramic xenon discharge lamp filled with a lanthanide halide. The ceramic xenon discharge lamp comprises asealed housing, a ceramic arc tube disposed in the sealed housing. A filling space and an electrode are arranged inside the ceramic arc tube. The filling space is filled therein with fillers includinga xenon gas, a metal halide, and nano silicon. By weight, the metal halide accounts for 60% to 70% of the fillers, and the metal halide Includes 80% to 90% of sodium halide, 2% to 6% of lanthanide halide, 0 to 1% of indium halide, 1% to 1.5% of thallium halide, 0.5% to 1.5% of scandium halide, and 1% to 5% of thorium halide. The electrode is tungsten or tungsten-bismuth alloy with a thorium-plated surface. The light efficiency of the ceramic xenon discharge lamp can be improved and maintained and the service life of the ceramic xenon discharge lamp is prolonged.
Owner:余雪强
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com