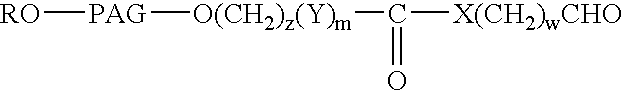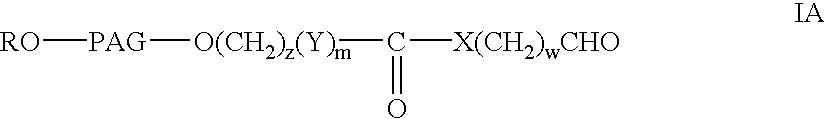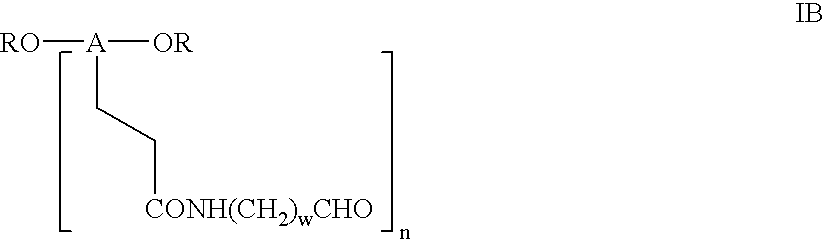Patents
Literature
34results about How to "Time-consume and expensive" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
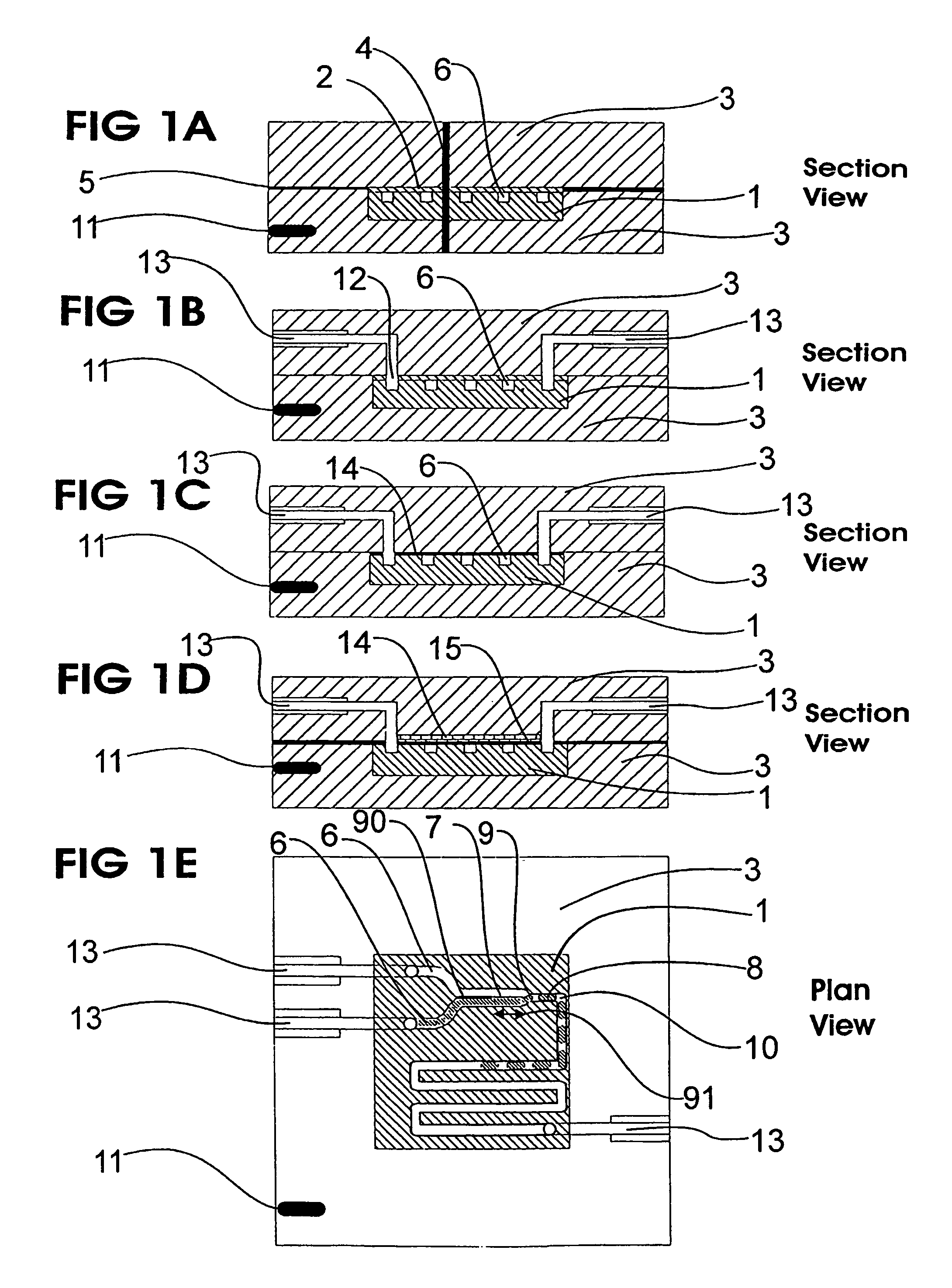
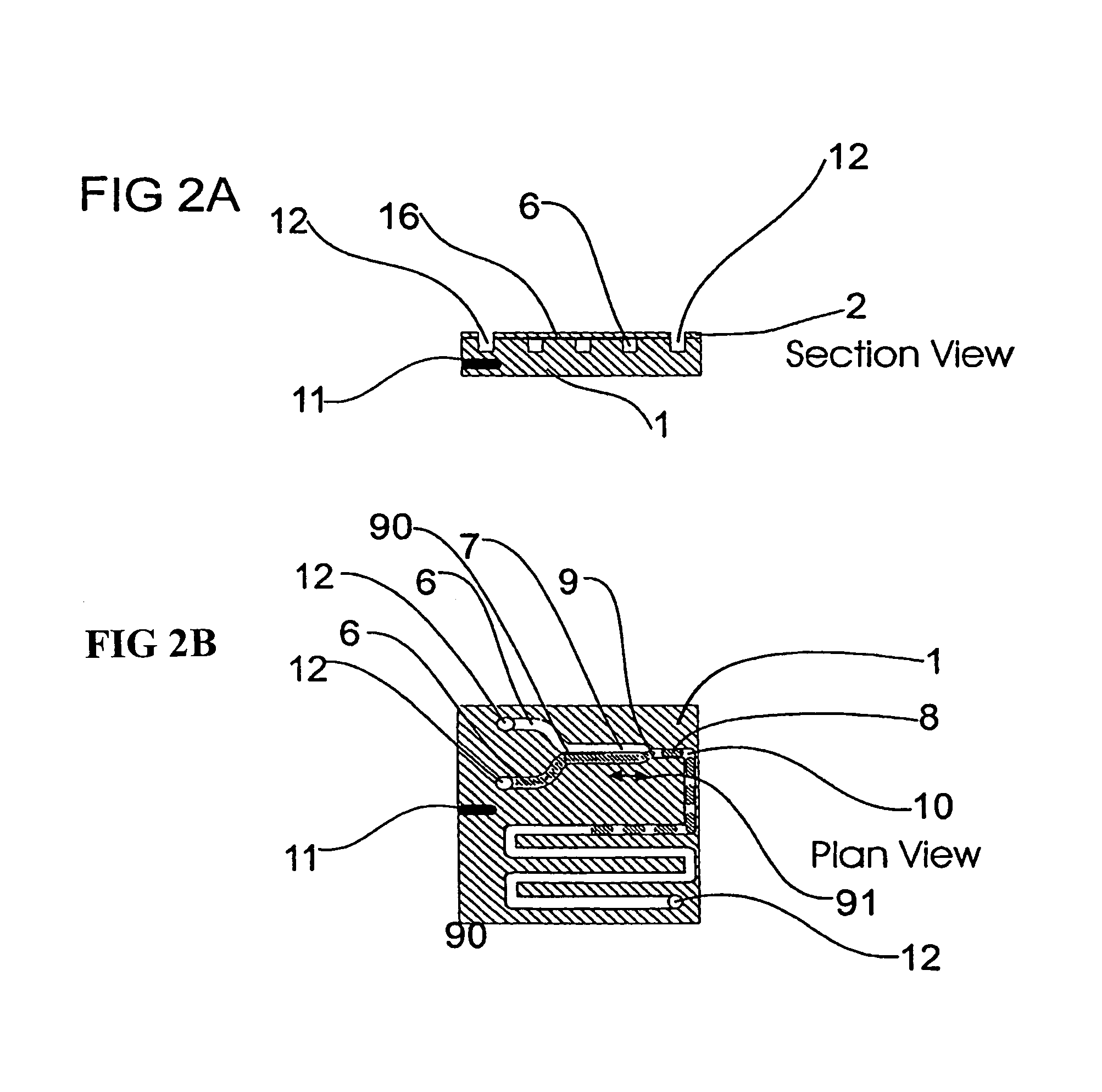
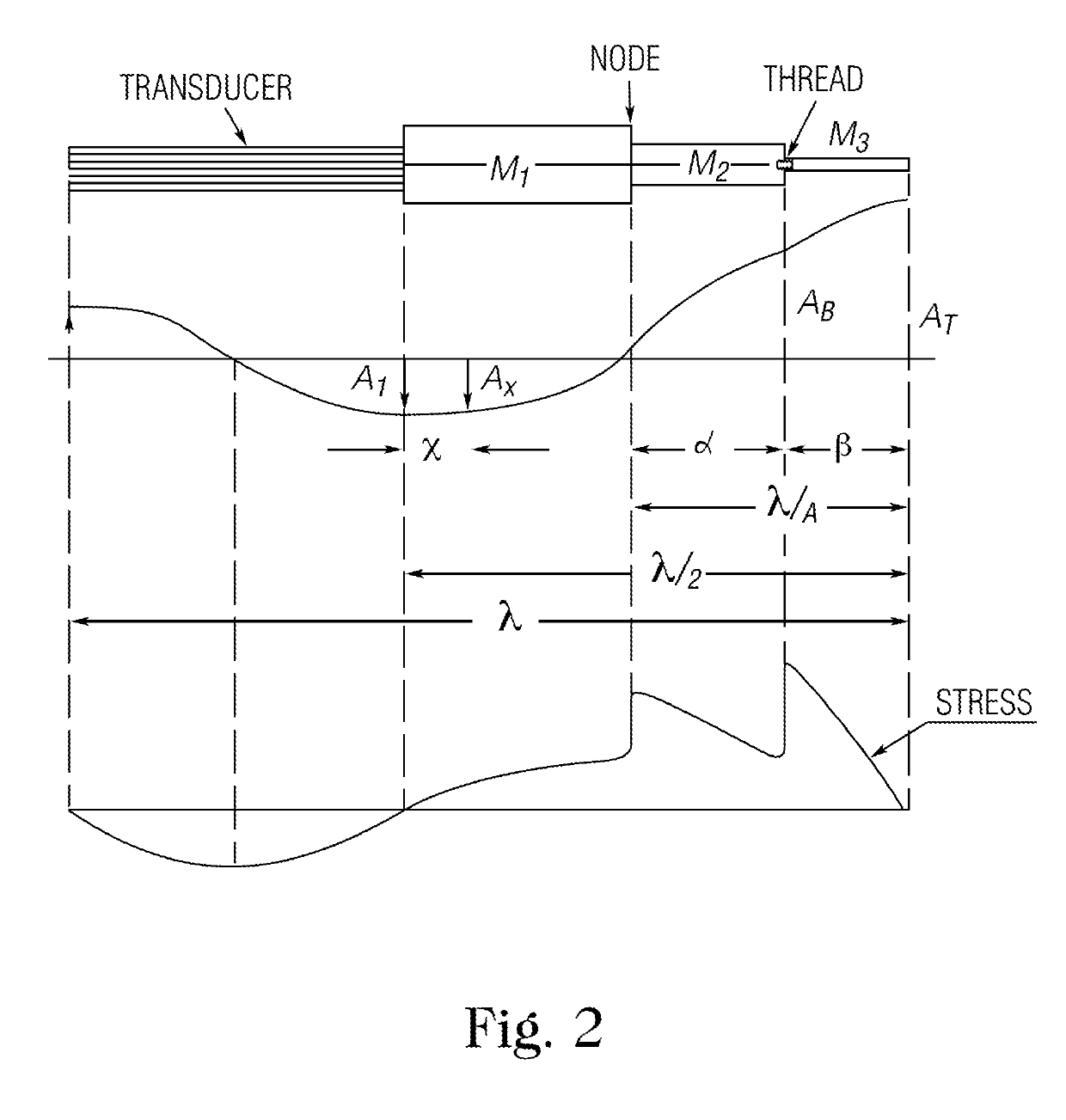
Microfluidic device and methods for construction and application
InactiveUS20060108012A1Considerable precisionEasy to controlMaterial nanotechnologyCircuit elementsEngineeringTwo fluid
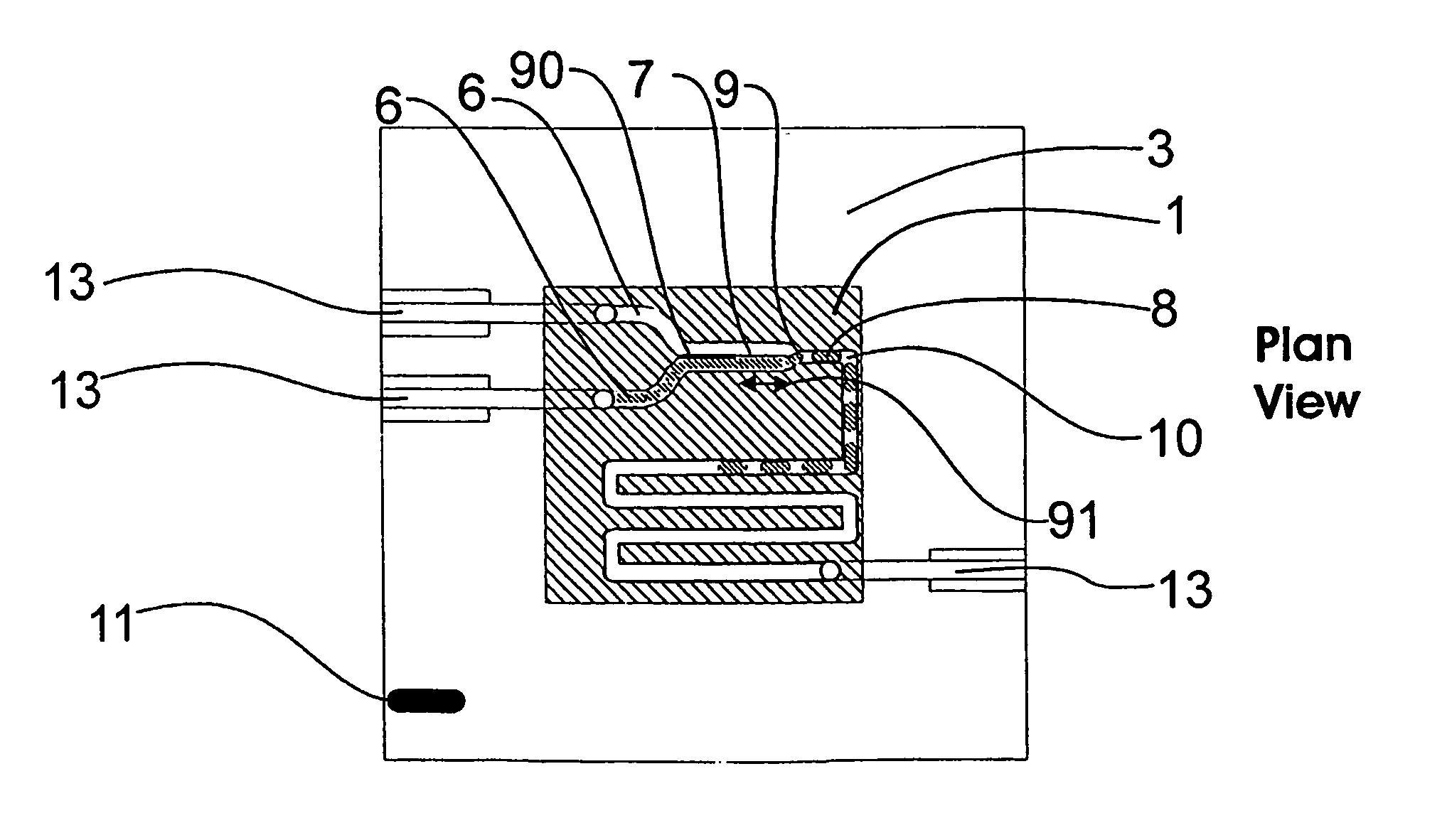
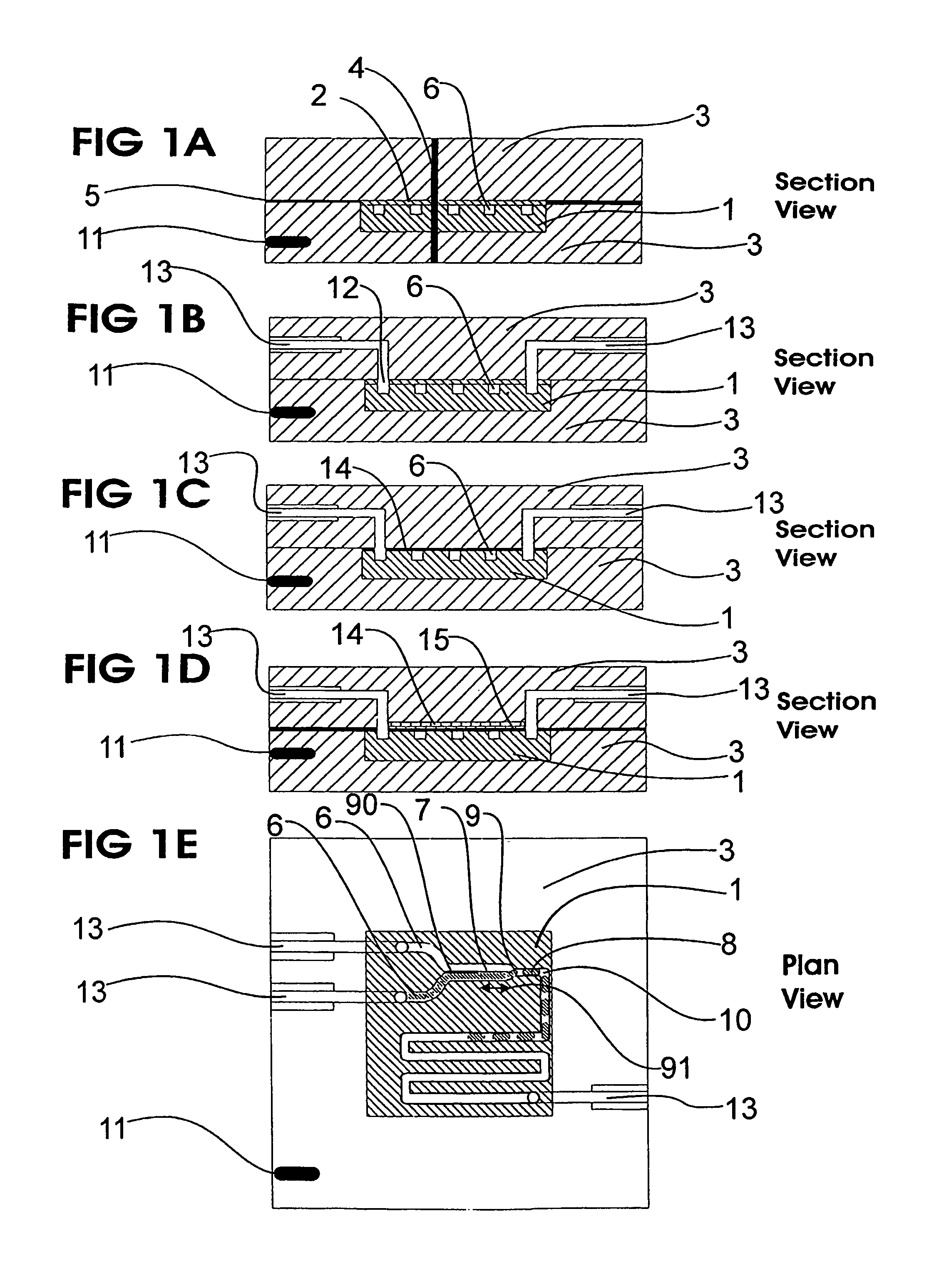
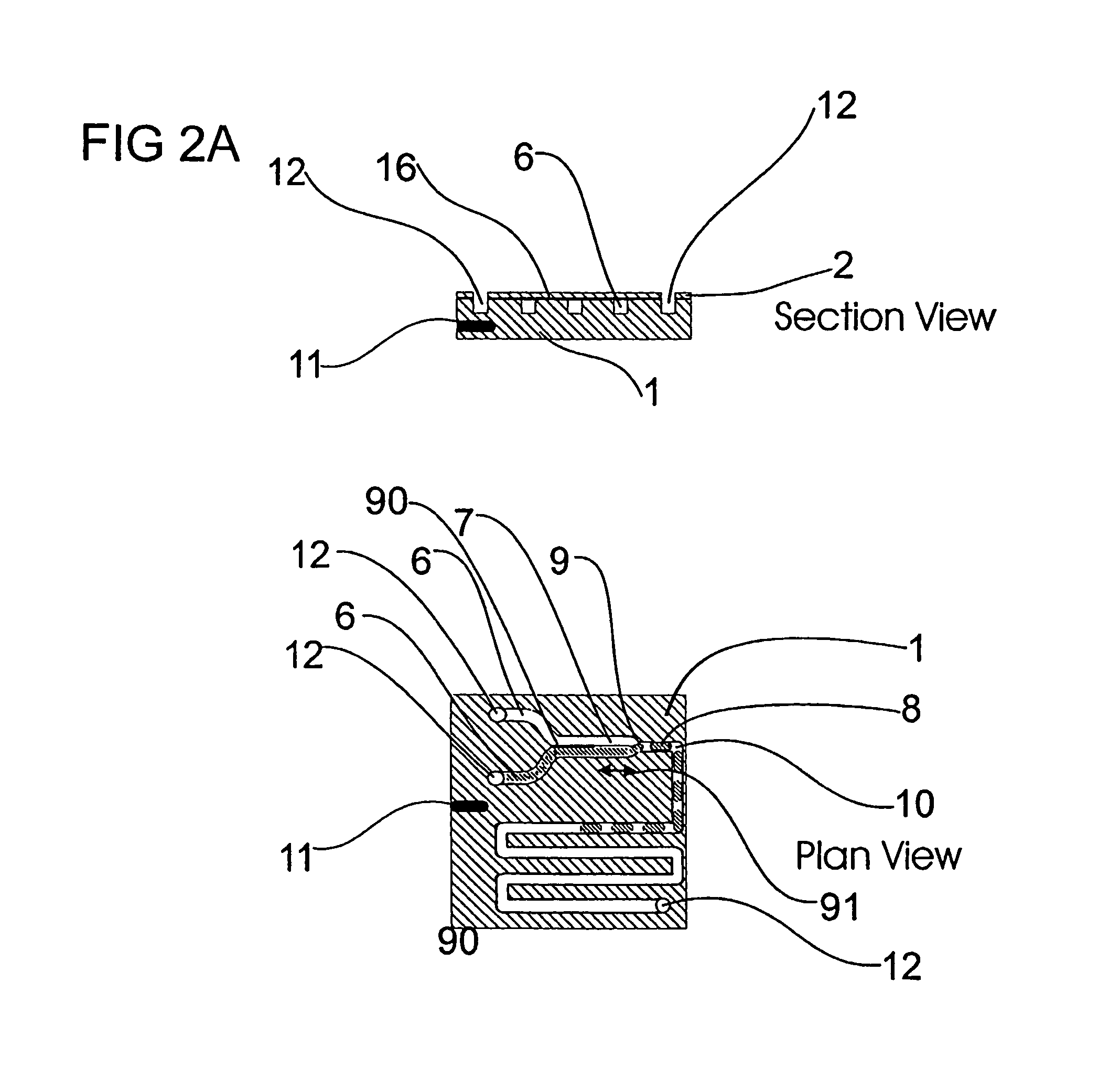
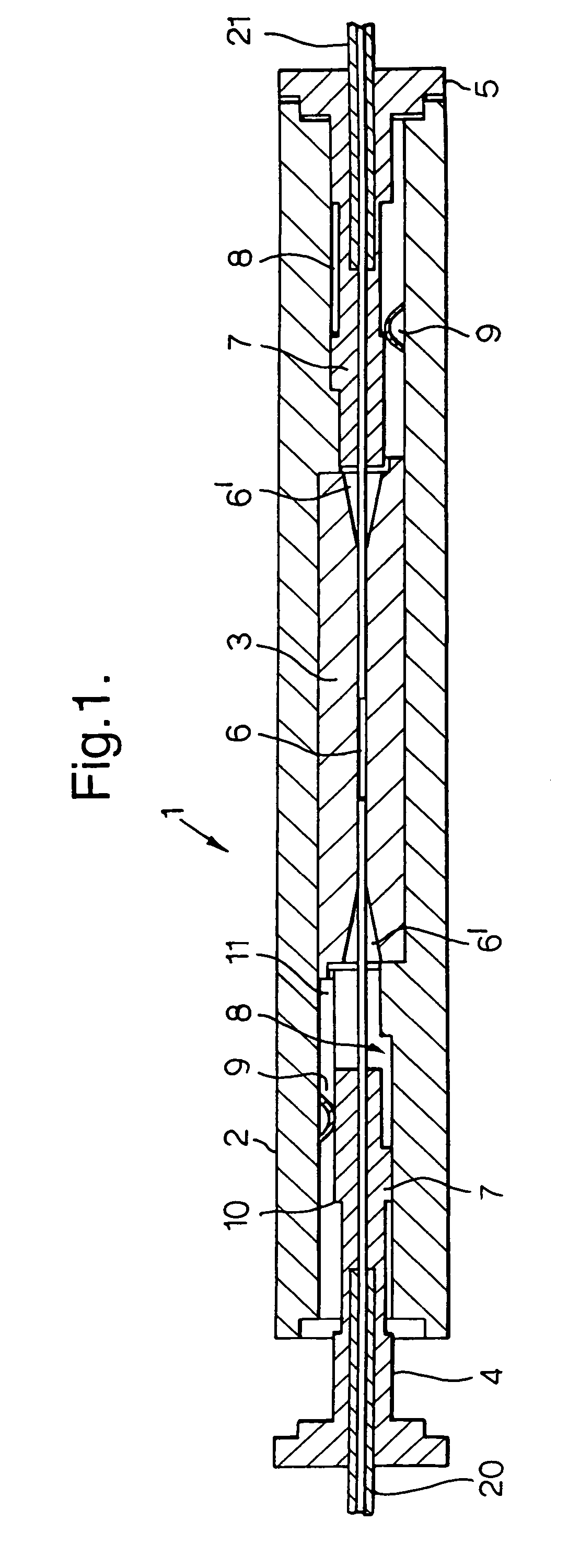
A microfluidic device comprises first and second inlet passages (13) for respective immiscible fluids, these inlet passages merging into a third passage (8) along which the two fluids flow under parallel laminar flow conditions, the third passage being formed with a constriction or other discontinuity (9) causing the two fluids to form into a flow of alternate segments.
Owner:Q CHIP
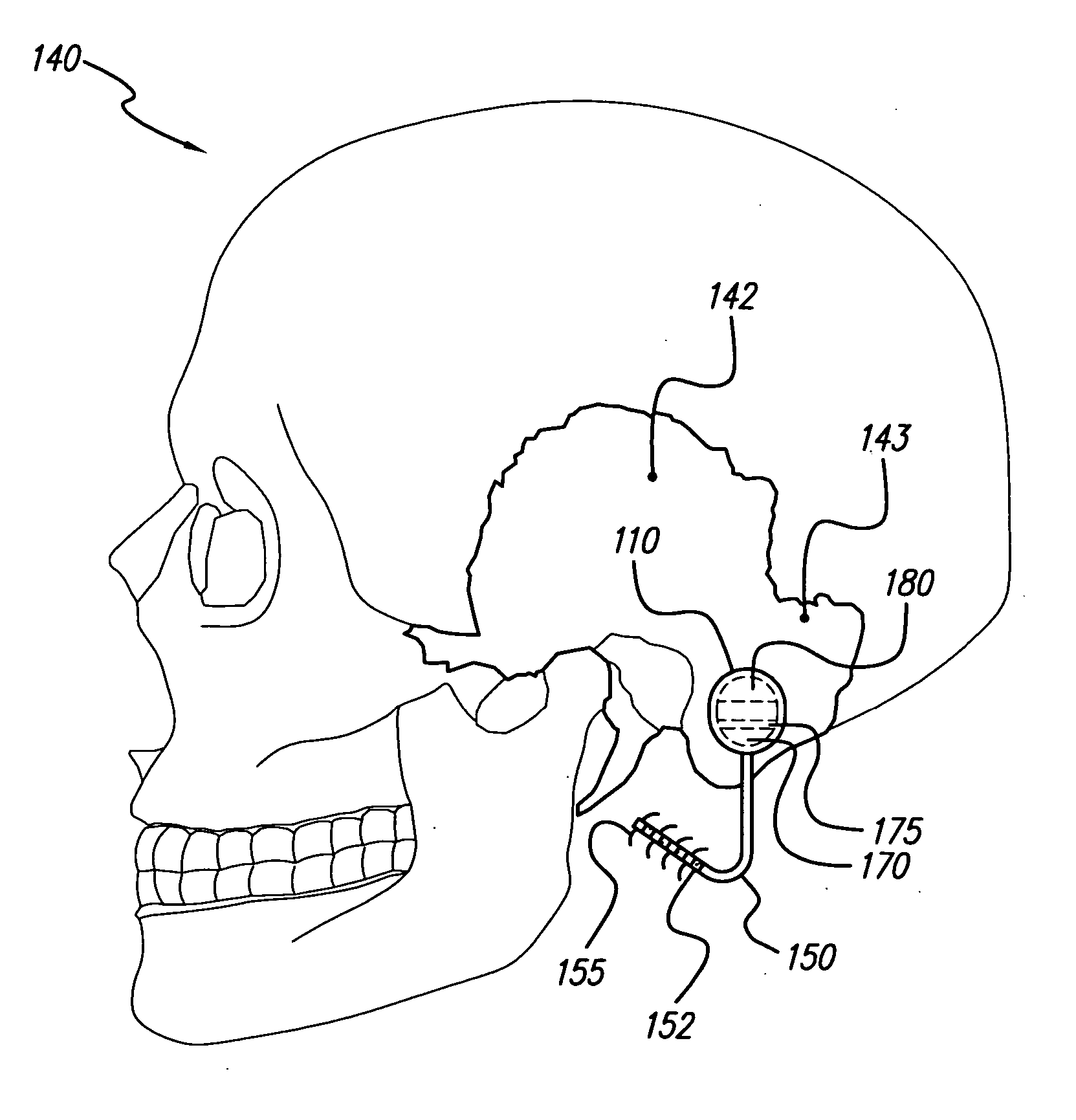
Skull-mounted electrical stimulation system
InactiveUS20050102006A1Substantial therapeutic benefitSignificant comprehensive benefitsElectrotherapyMedical devicesElectricityStimulation Parameter
Systems and methods for applying electrical stimulation to the brain to treat headaches and neuralgia use at least one implantable system control unit (SCU), specifically an implantable signal / pulse generator (IPG) with one or more electrodes. The IPG is implanted in the skull and communicates with at least one external appliance, such as a Behind-the-Ear (BTE) unit. In a preferred embodiment, the system is capable of open- and closed-loop operation. In closed-loop operation, at least one SCU includes a sensor, and the sensed condition is used to adjust stimulation parameters.
Owner:BOSTON SCI NEUROMODULATION CORP
Integrated circuits protected against reverse engineering and method for fabricating the same using vias without metal terminations
InactiveUS6791191B2Accurately determineTime-consume and expensiveSemiconductor/solid-state device detailsSolid-state devicesEngineeringMetal
A device adapted to protect integrated circuits from reverse engineering comprising a part looking like a via connecting two metal layers, but in fact attached only to one metal layer and spaced from the other. Having such "trick" via would force a reverse engineer to think there is a connection where there is none. A method for fabricating such device.
Owner:HRL LAB
Line testing method and apparatus therefor
InactiveUS6373923B1Minimizes problemTime-consume and expensiveSupervisory/monitoring/testing arrangementsSubstation equipmentData transmissionHigh frequency
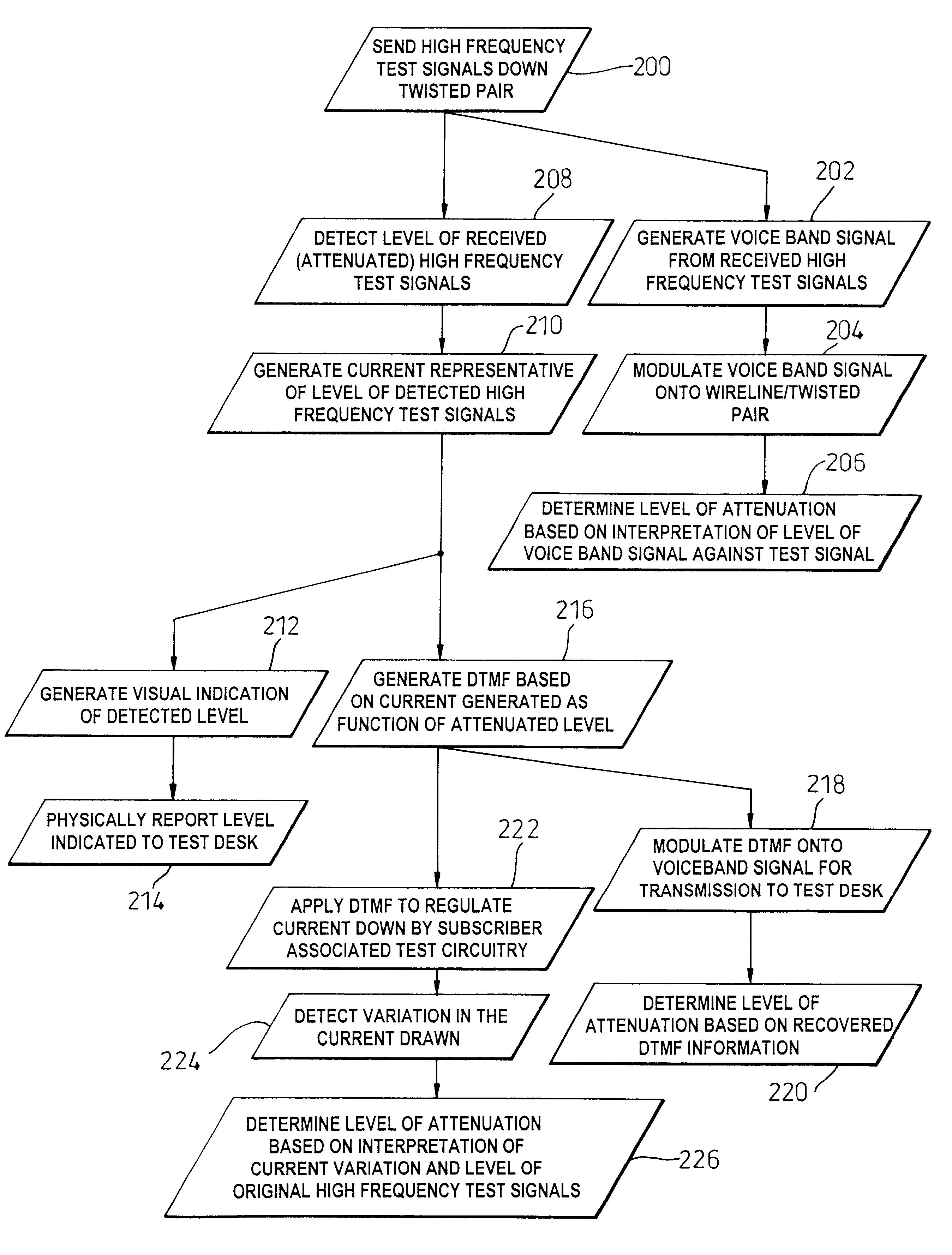
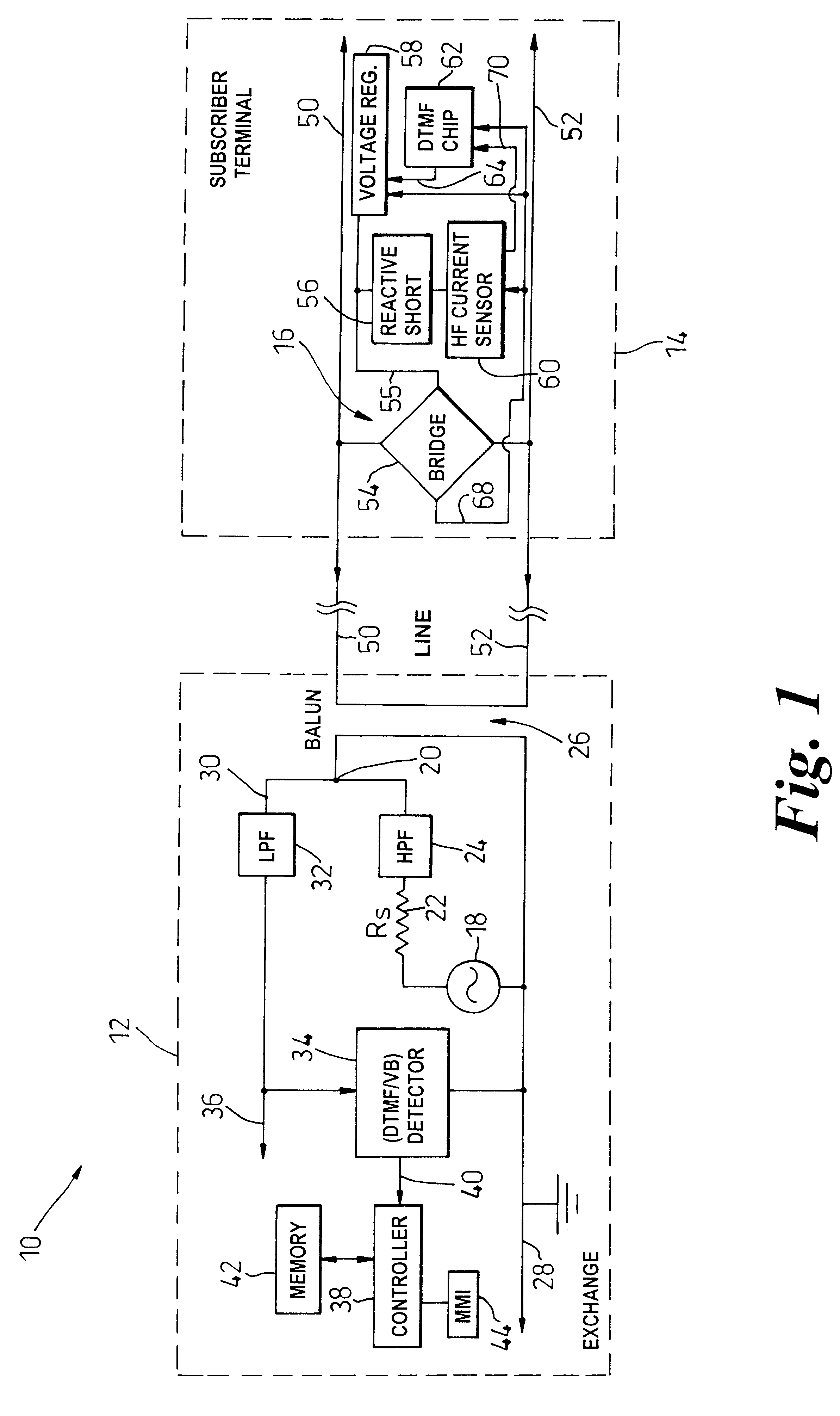
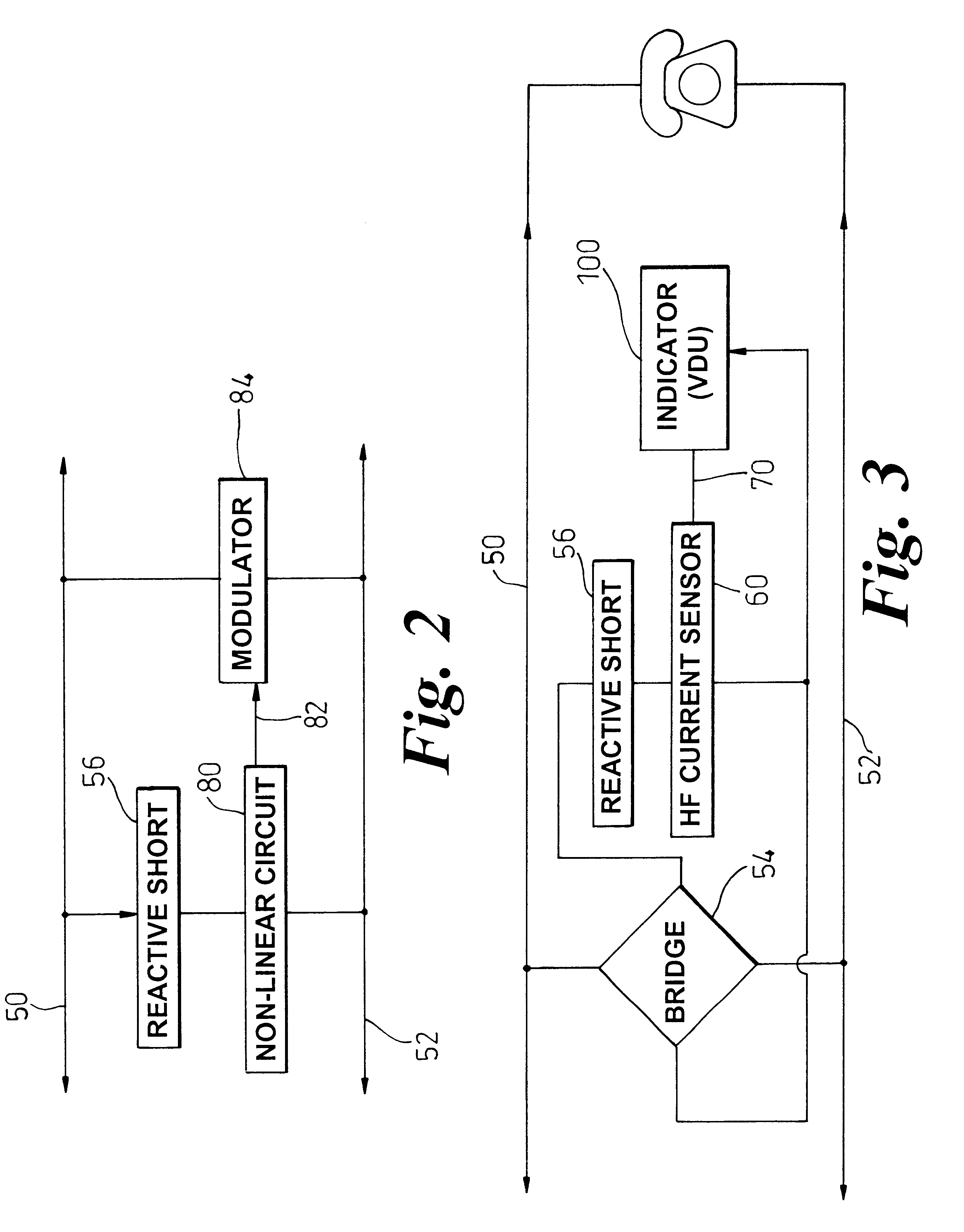
To facilitate the use of relatively high frequency data transmission services over a wireline communication resource originally deployed to support low frequency voice band services, an assessment of the wireline communication resource (50-52) is based on attenuation of high frequency test signals, of known original level, that are generated (18) injected into the wireline communication resource from a test point (12). A detector (60) at a potential point of service, e.g. at a customers' premises (14), detects an attenuated level of the test signals and generates a control signal (70, 82), such as a drive current, indicative of the attenuation caused by the wireline communication resource (50-52). The control signal (70, 82) is then indirectly communicated back to the test point (12) in a coded form, and preferably within a voice band transmission. The coded form may be realized as modulation of a power supply provided to the customers' premises by the wireline communication resource, or could be achieved by up-link frequency mixing. In its most basic form, the control signal causes fluctuation on a visual level indicator at the customers' premises and therefore relies on a customer providing a verbal or toned response to the test point as to the level of the visual indicator. Effective attenuation at high frequencies can then be assessed based upon analysis of information coded into the voice band signal and its direct relationship with the original level of the injected high frequency signals.
Owner:RPX CLEARINGHOUSE
Method and apparatus for splicing optical fibres
InactiveUS7014372B2Reduce adverse effectsTime-consume and expensiveCoupling light guidesFiberEngineering
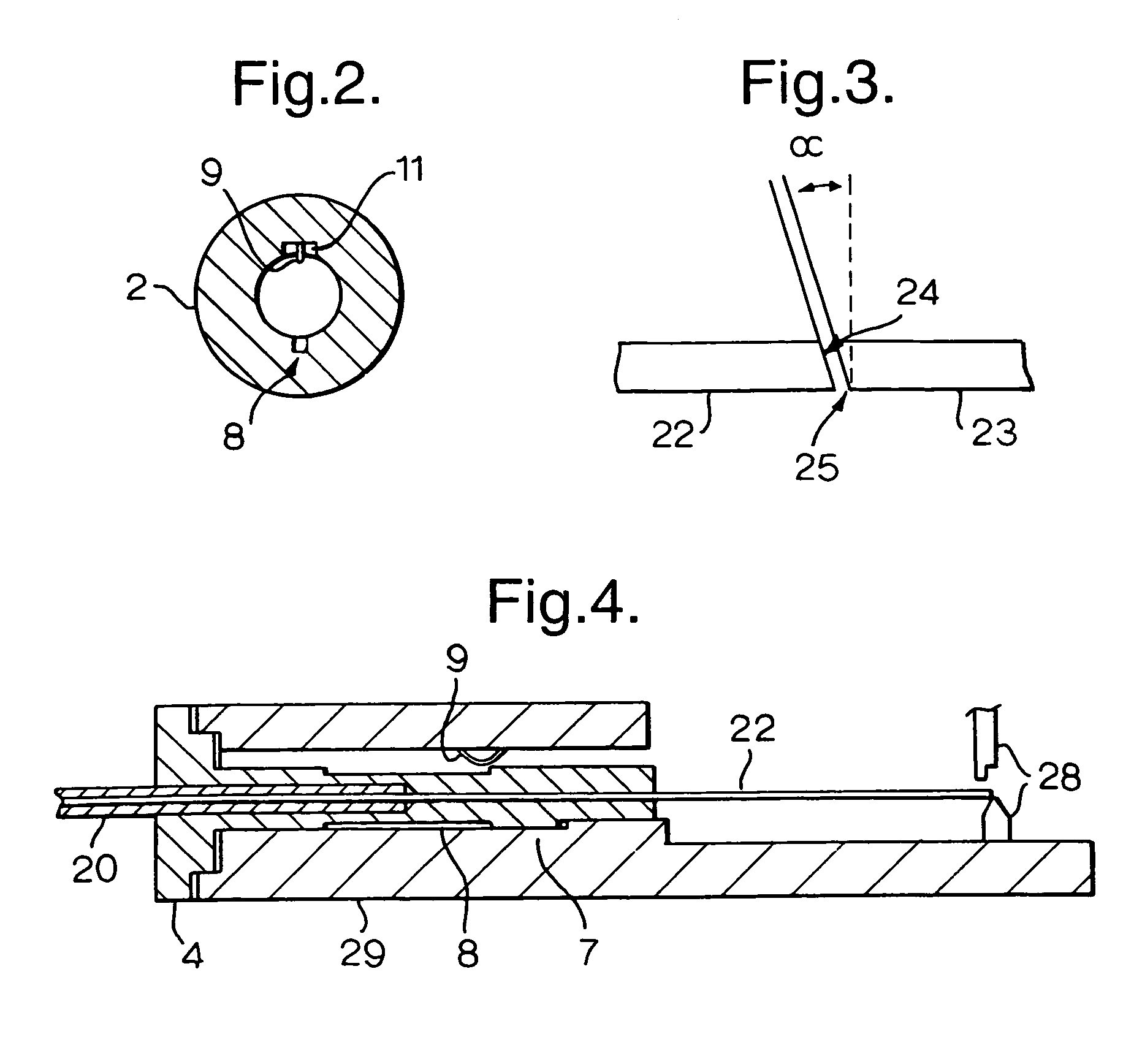
A method of splicing optical fibers includes affixing a first fiber to a first keying element having a particular radial orientation, inserting the keying element in a support which receives the keying element only in a specific radial orientation, cleaving the fiber affixed to the inserted keying element at a predetermined angle (α) relative to the support to form an angled fiber end face, removing the keying element from the support, inserting the keying element into a splicing body which receives the keying element only in a specific radial orientation such that the angled fiber end face has a predictable radial orientation with respect to the splice body, and repeating the above operations for a second fiber and a second keying element, whereby the first angled fiber end face and the second angled fiber end face abut in a substantially parallel orientation.
Owner:TYCO ELECTRONICS RAYCHEM BVBA
Multi-chip stack and method of fabrication utilizing self-aligning electrical contact array
InactiveUS6858941B2Efficient use ofHigh I/O densitySemiconductor/solid-state device detailsSolid-state devicesHigh bandwidthInterconnection
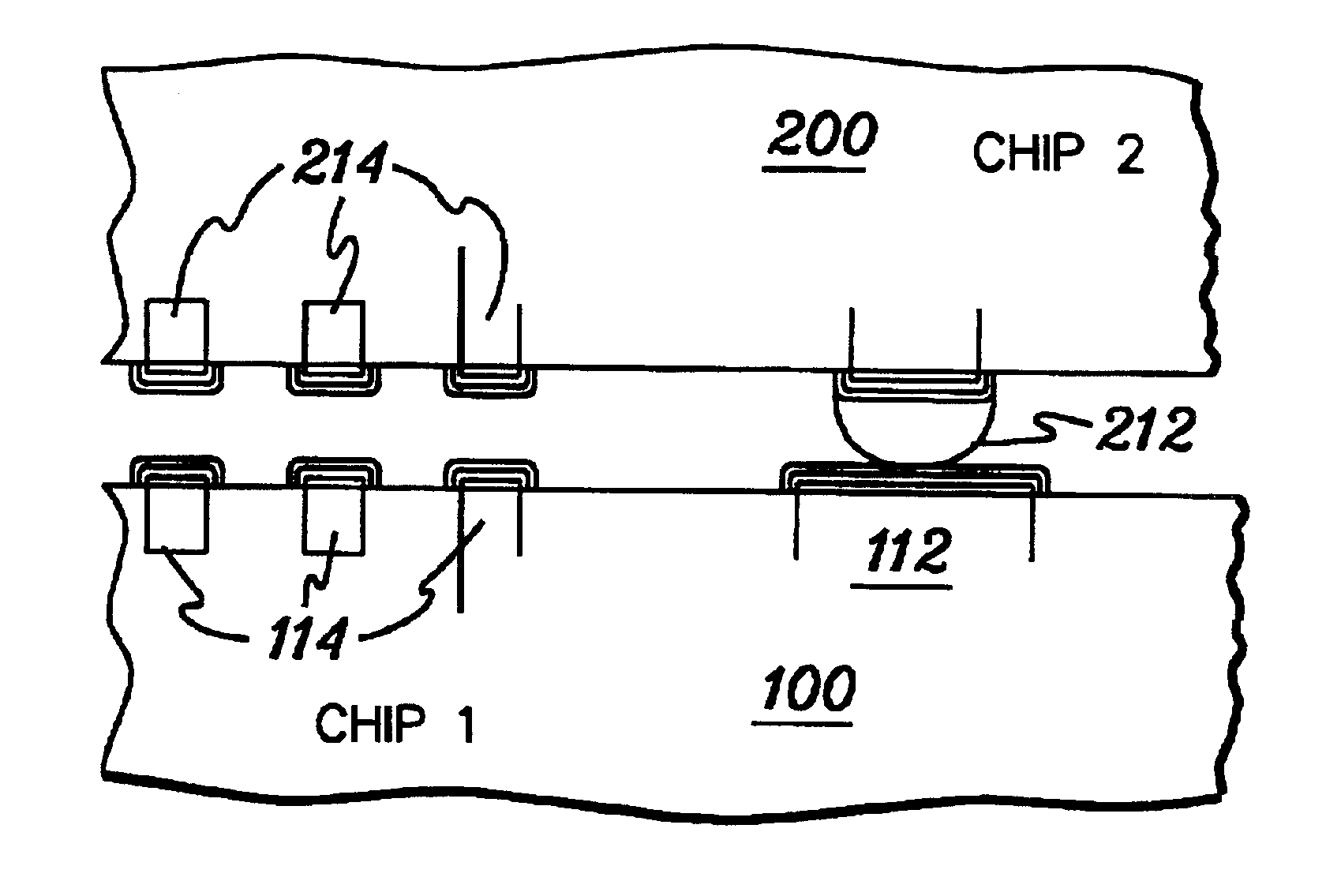
A multi-chip stack structure and method of fabrication are provided utilizing self-aligning electrical contact arrays. Two or more arrays of interconnection contacts are provided, with one array being a rough aligned contact array, and a second array being a high bandwidth contact array. The rough aligned contact array has larger contacts and at least a portion thereof which melts at a substantially lower temperature than the melting temperature of the contacts of the high bandwidth contact array. By positioning two integrated circuit chips in opposing relation with the arrays mechanically aligned therebetween, and applying heat to melt the contacts of the rough aligned array, the two chips will rotate to align the respective contacts of the high bandwidth contact arrays, thereby achieving improved connection reliability between the structures.
Owner:IBM CORP
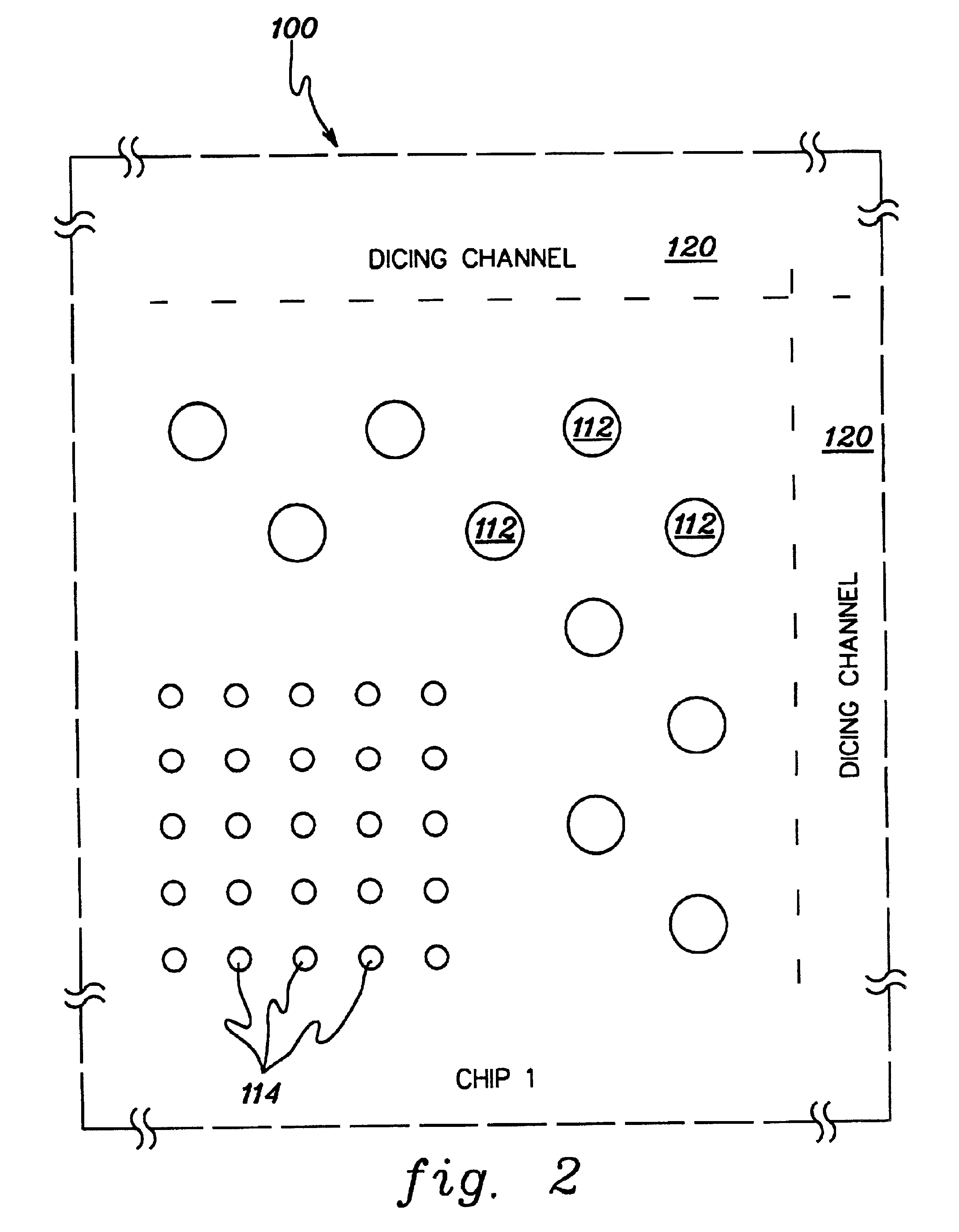
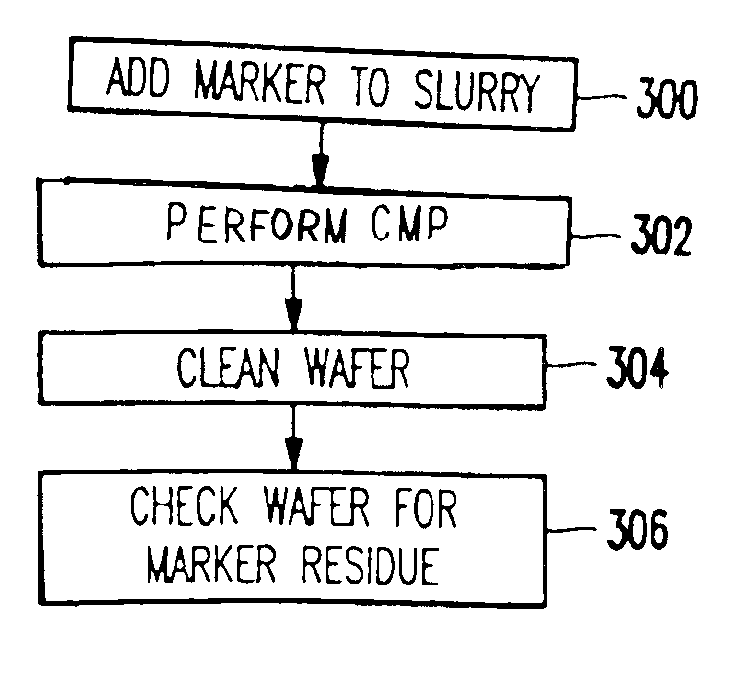
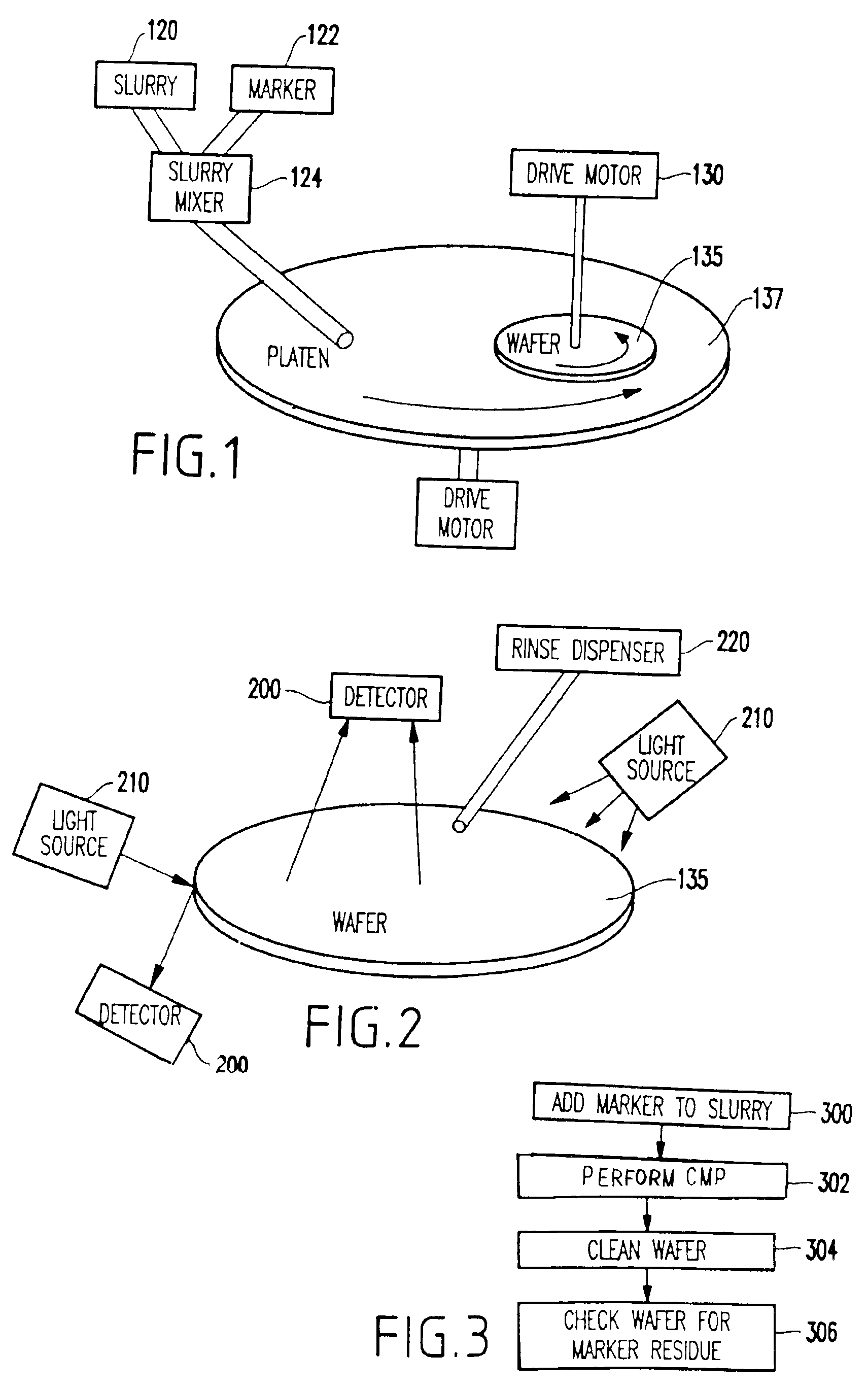
CMP slurry additive for foreign matter detection
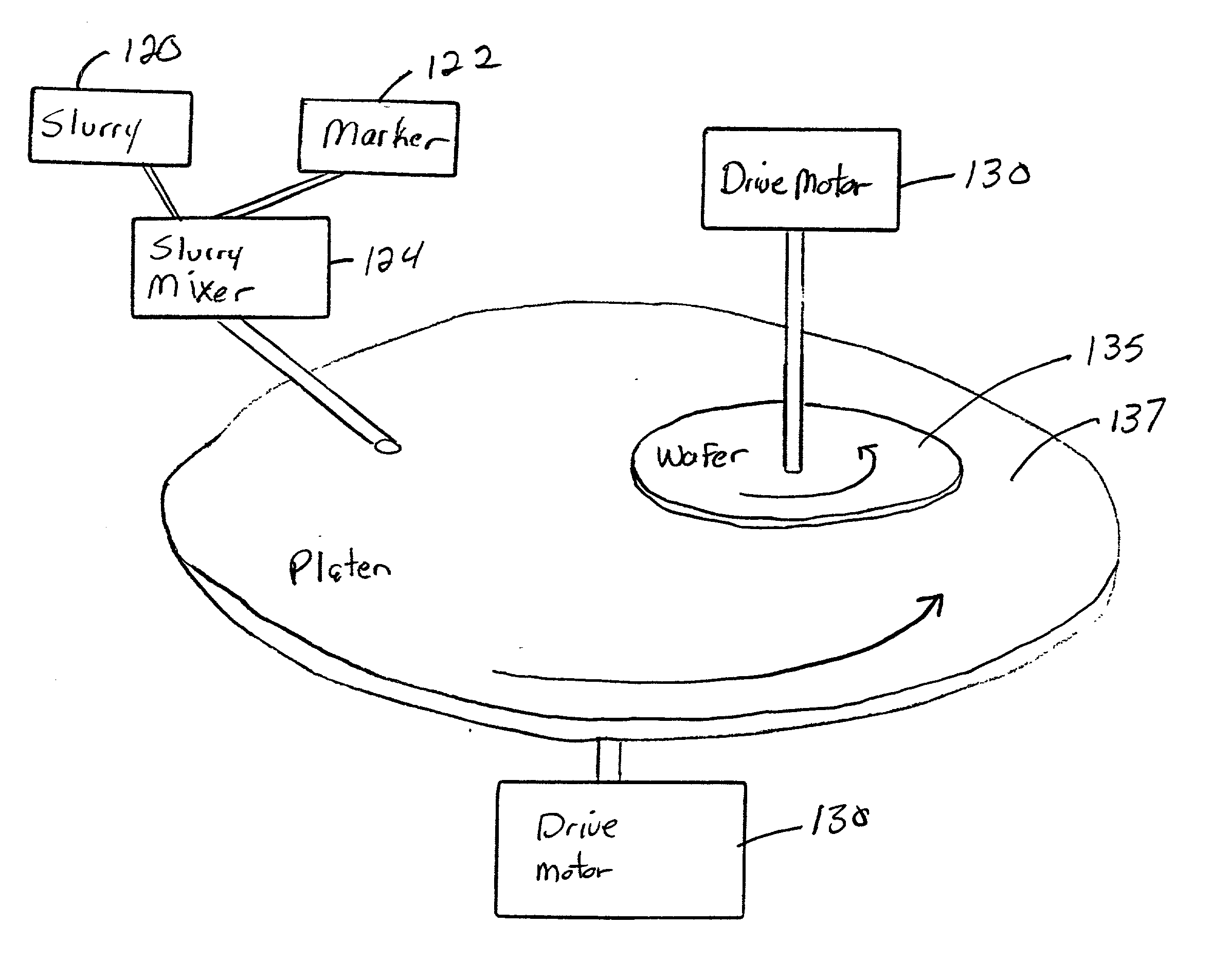
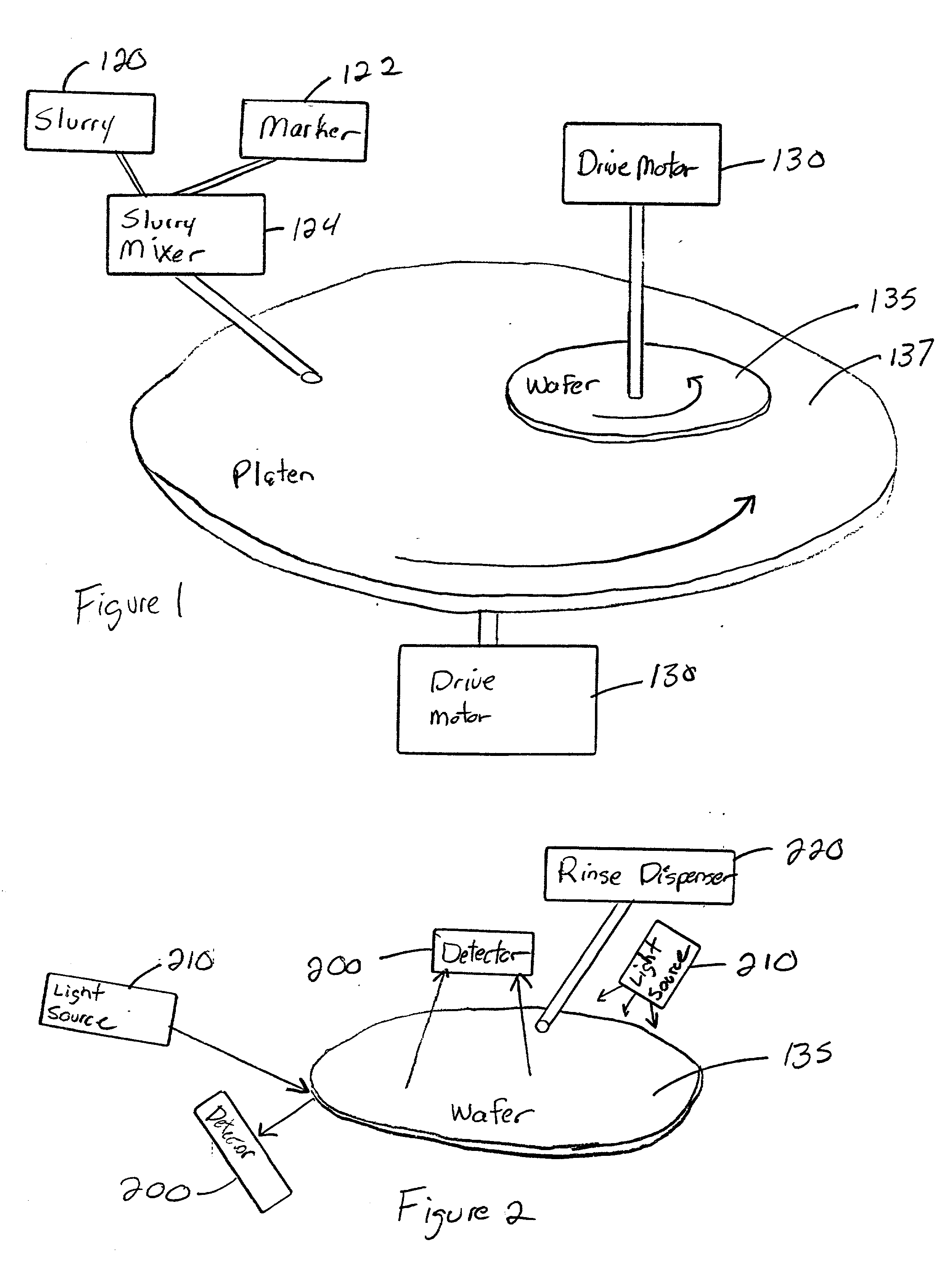
InactiveUS20030139048A1Avoid pollutionTime-consume and expensiveElectrostatic cleaningSemiconductor/solid-state device manufacturingForeign matterSlurry
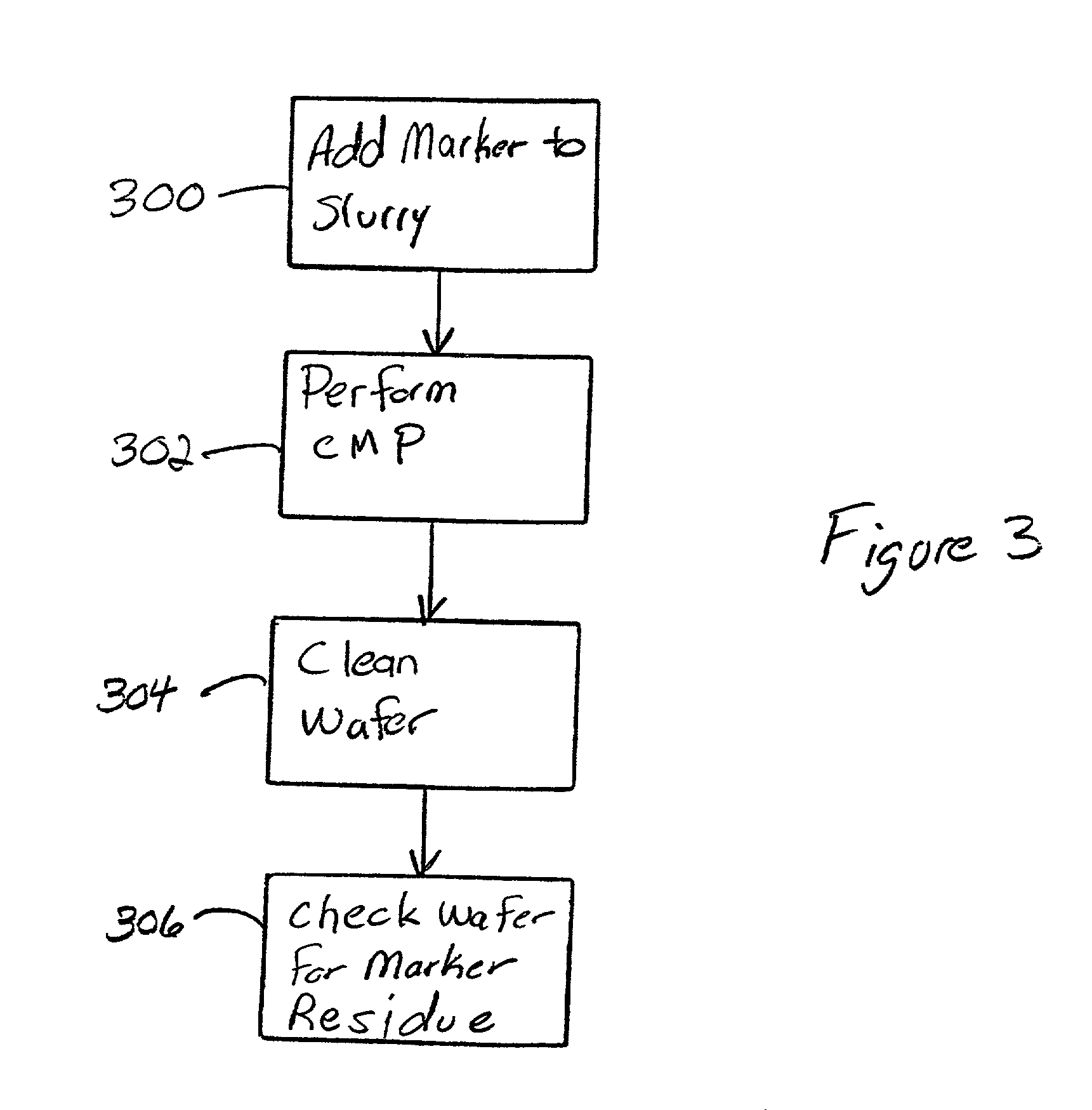
A method and structure polishes and cleans silicon wafers by mixing a marker with a slurry to form a slurry mixture, performs chemical mechanical polishing on a silicon wafer using the slurry mixture, rinses the slurry mixture from the silicon wafer, checks the silicon wafer for marker residue, and repeats the rinsing process if the checking process detects the marker residue on the wafer.
Owner:IBM CORP
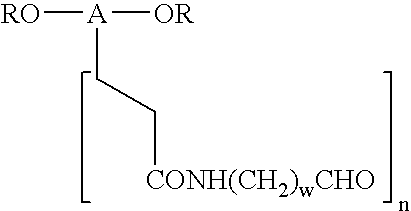
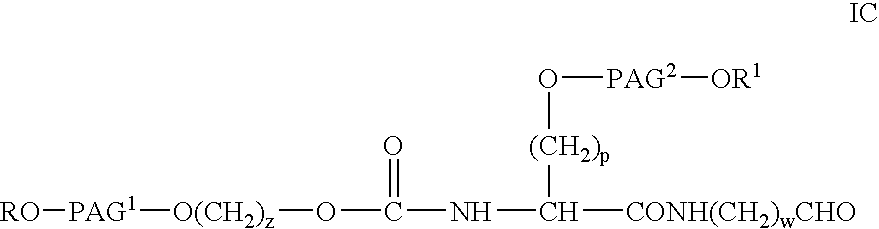
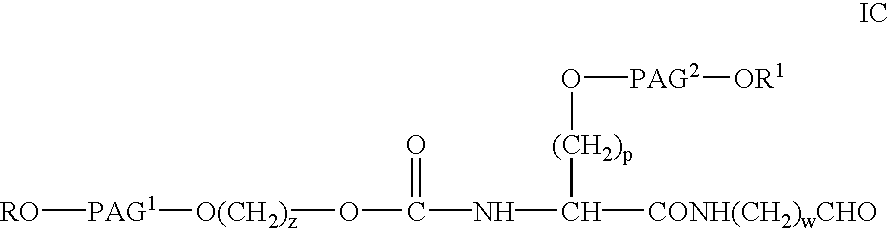
Novel monofunctional polyethylene glycol aldehydes
InactiveUS20040147687A1Procedure is time-consume and expensiveSignificant biological activityImmunoglobulinsPharmaceutical non-active ingredientsActive proteinImmunogenicity
The present invention provides novel monofunctional polyethylene glycol aldehydes for the pegylation of therapeutically active proteins. The pegylated protein conjugates that are produced, retain a substantial portion of their therapeutic activity and are less immunogenic than the protein from which the conjugate is derived. New syntheses for preparing such aldehydes are described.
Owner:SUN BIO INC
Rotary mixing device in molded packaging
ActiveUS7513678B2Readily printing and inking materialEliminate needOther chemical processesRotary stirring mixersShell moldingEngineering
Owner:VENUS DONALD W
Novel monofunctional polyethylene glycol aldehydes
InactiveUS20030153694A1Procedure is time-consume and expensiveSignificant biological activityUrea derivatives preparationCarbamic acid derivatives preparationActive proteinImmunogenicity
Novel monofunctional polyethylene glycol aldehyde for pegylating therapeutically active proteins to produce pegylated protein conjugates which retain a substantial portion of their therapeutic activity and are less immunogenic than the protein from which the conjugate is derived and a new synthesis for preparing such aldehydes.
Owner:SUN BIO INC
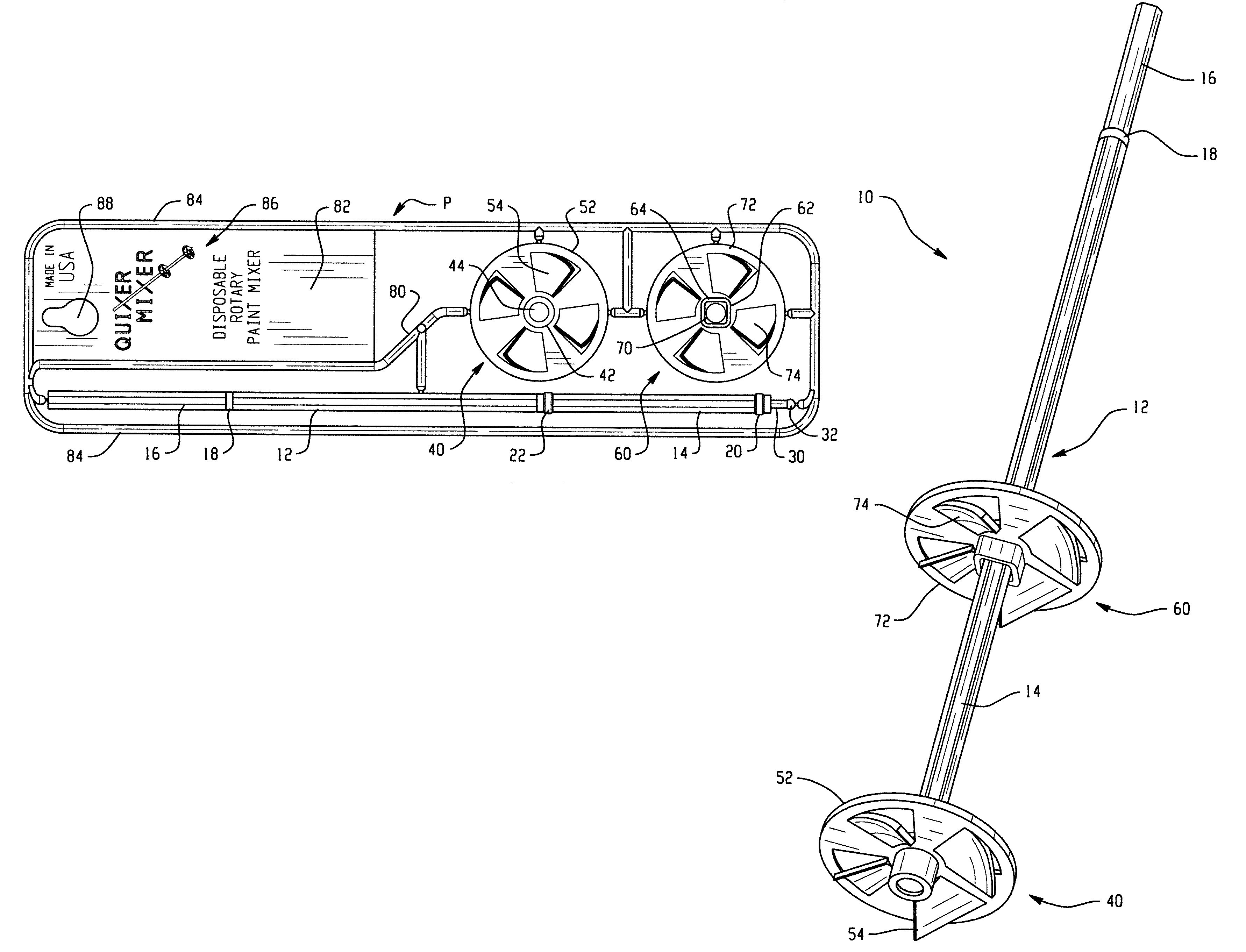
Rotary mixing device
ActiveUS20050007869A1Readily printing and inking materialEliminate needOther chemical processesTransportation and packagingLiquid polymerEngineering
The present disclosure relates to a rotary mixing device that is easy to produce, assemble, use and dispose of. The device comprises a shaft, and at least one mixing member. The shaft and mixing member are formed of the same composite material and are produced by molding the components in essentially a single plane. Moreover, the runners that provide the liquid polymer material to form the components of the rotary mixing device also serve as holding elements. Consequently, a one-piece, single shot molded unit is produced containing the components and the holding elements.
Owner:VENUS DONALD W
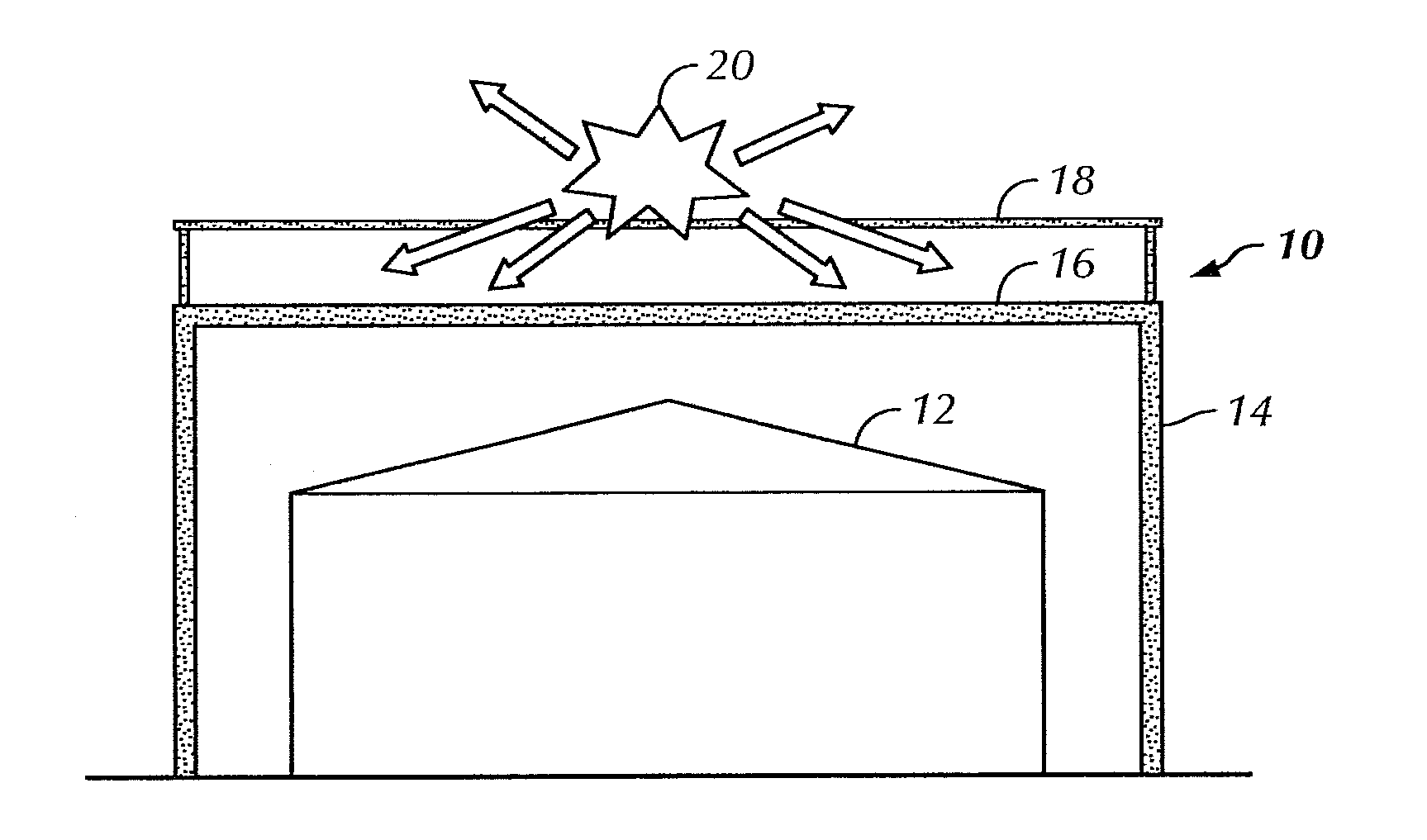
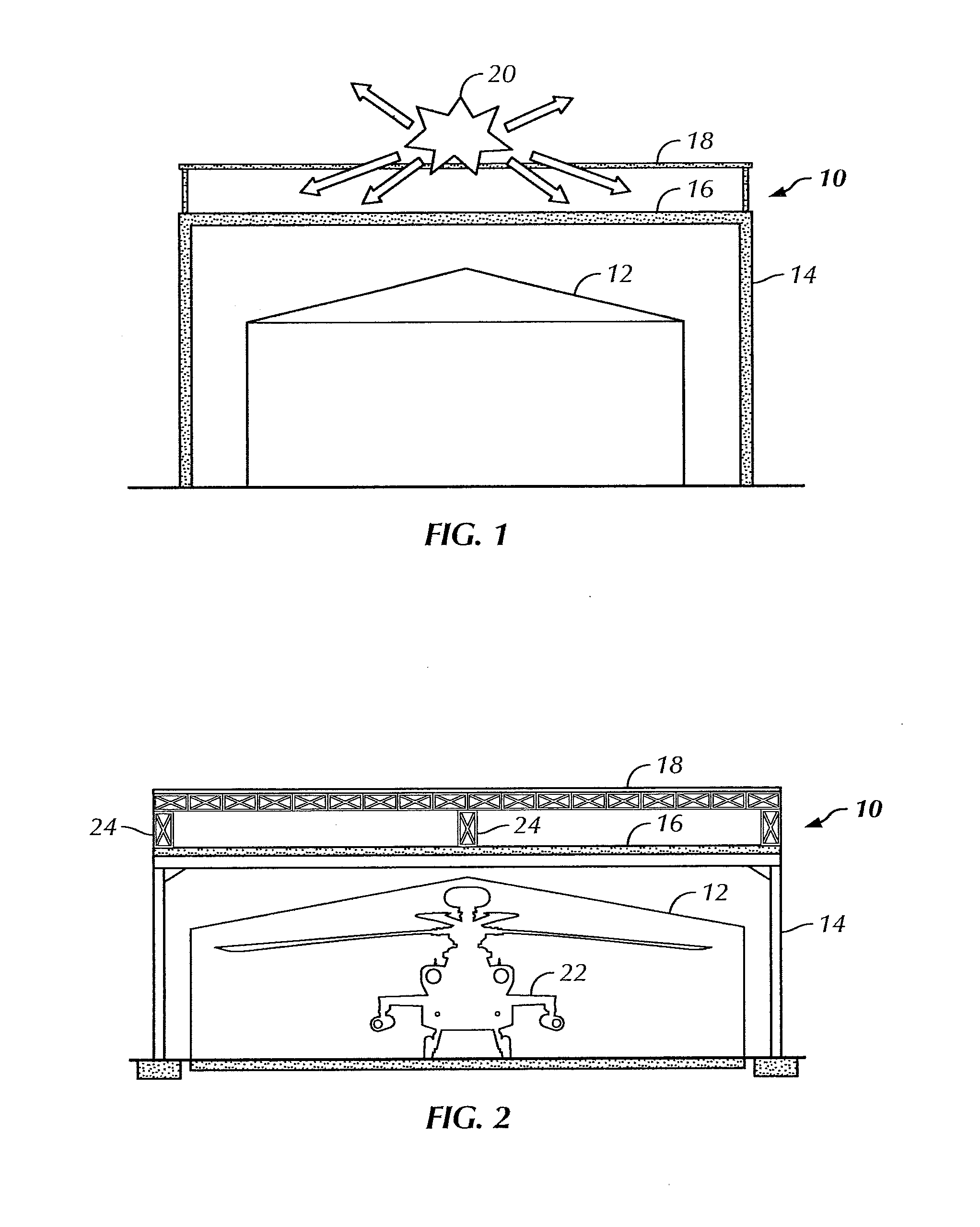
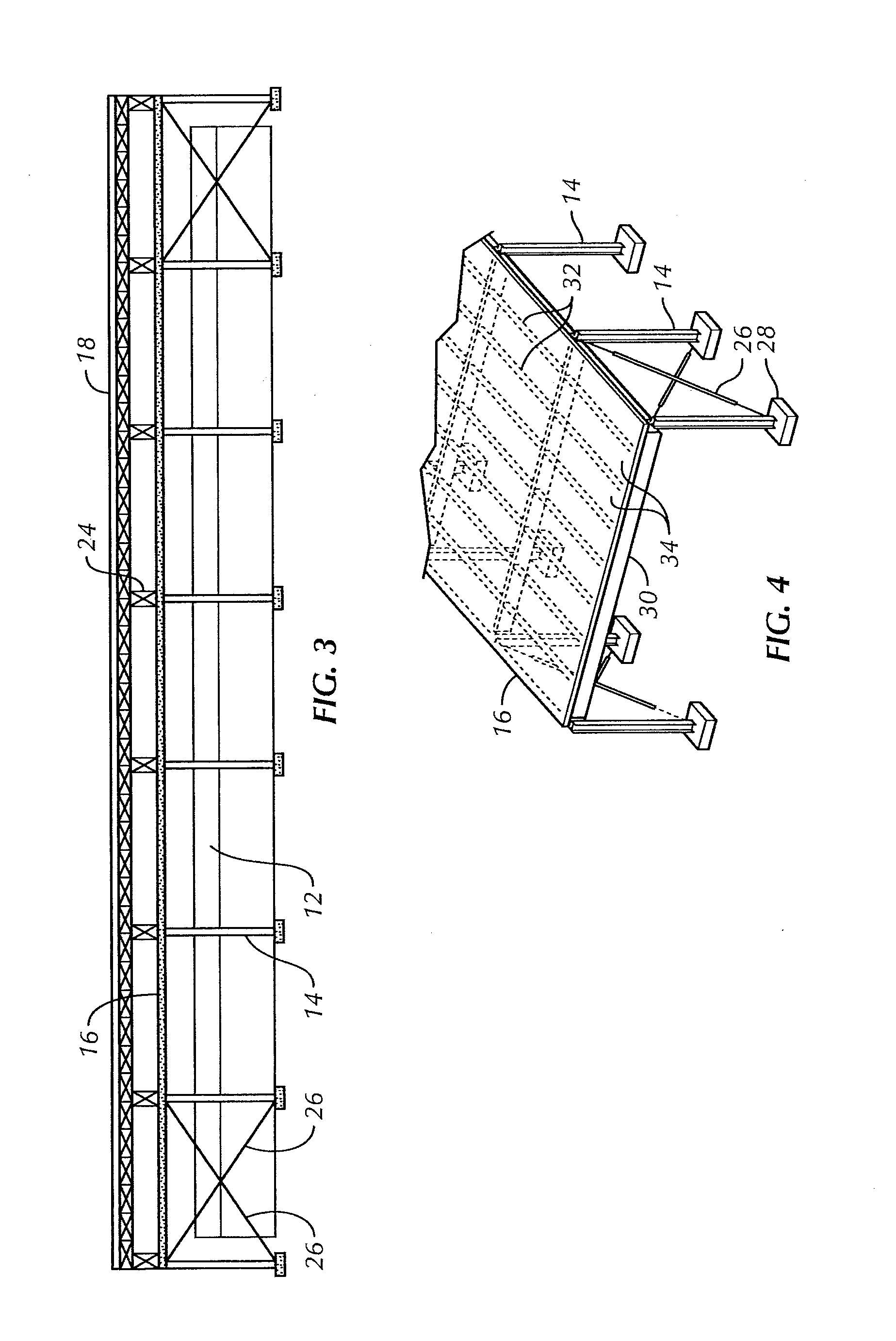
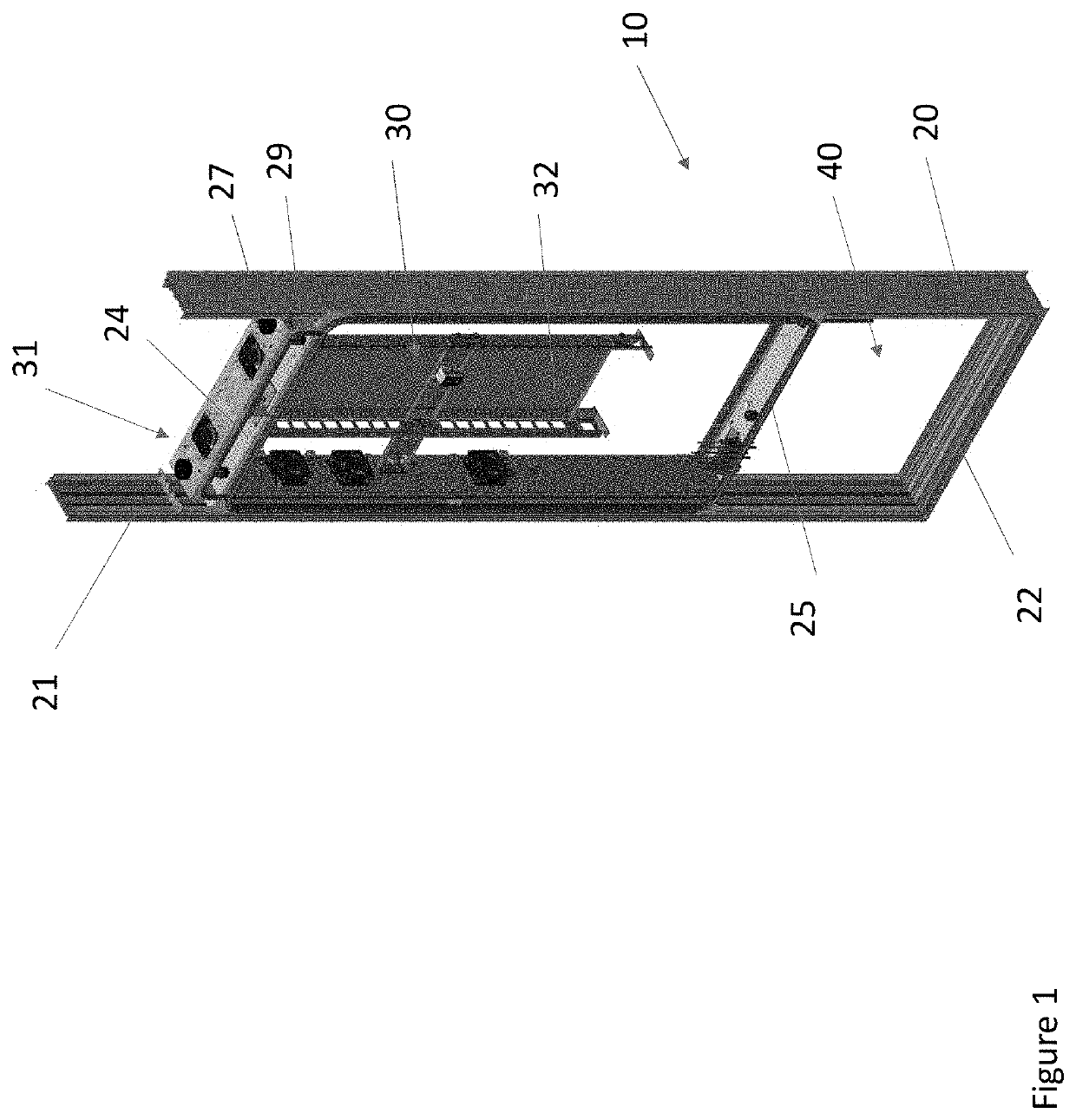
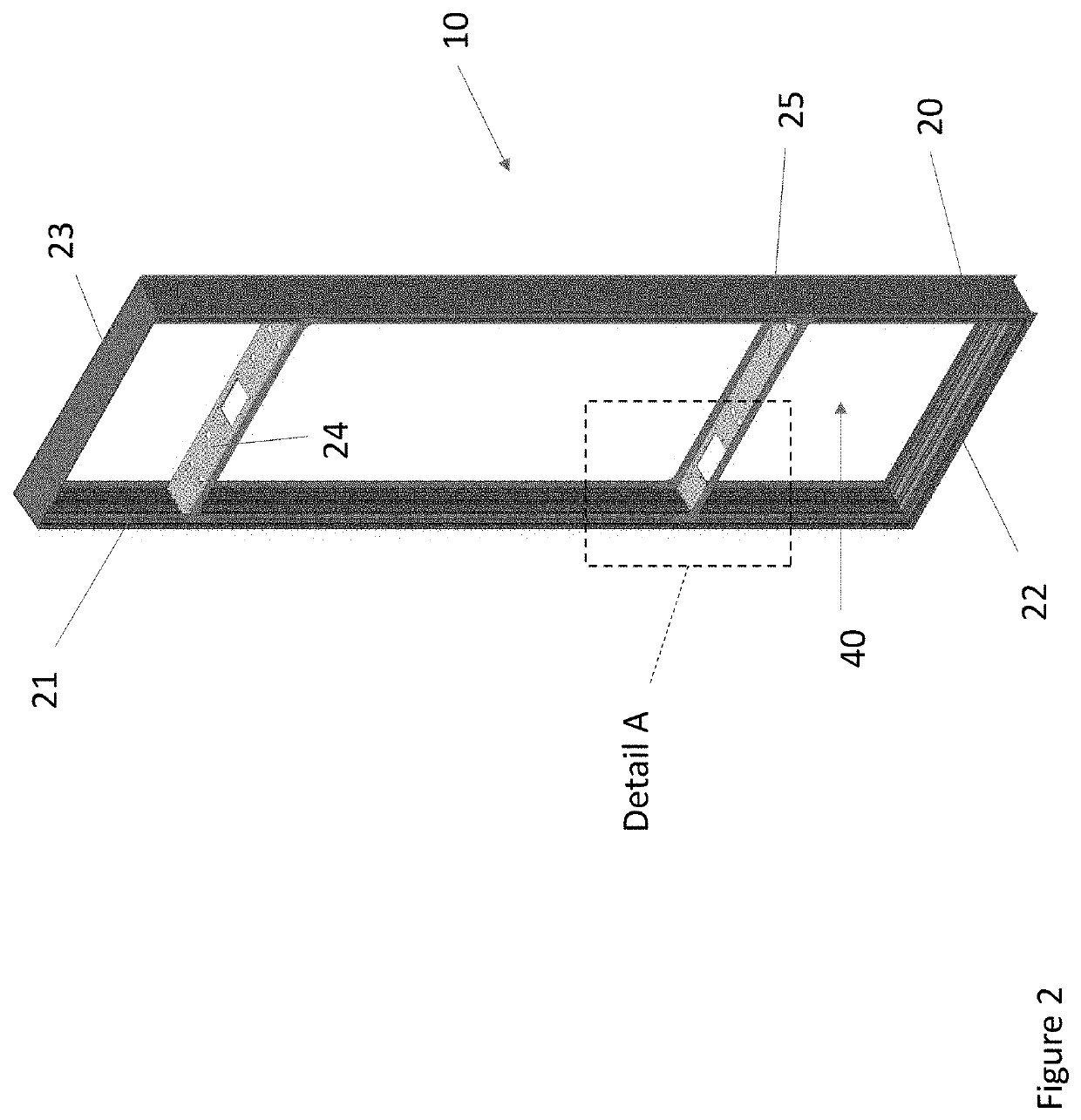
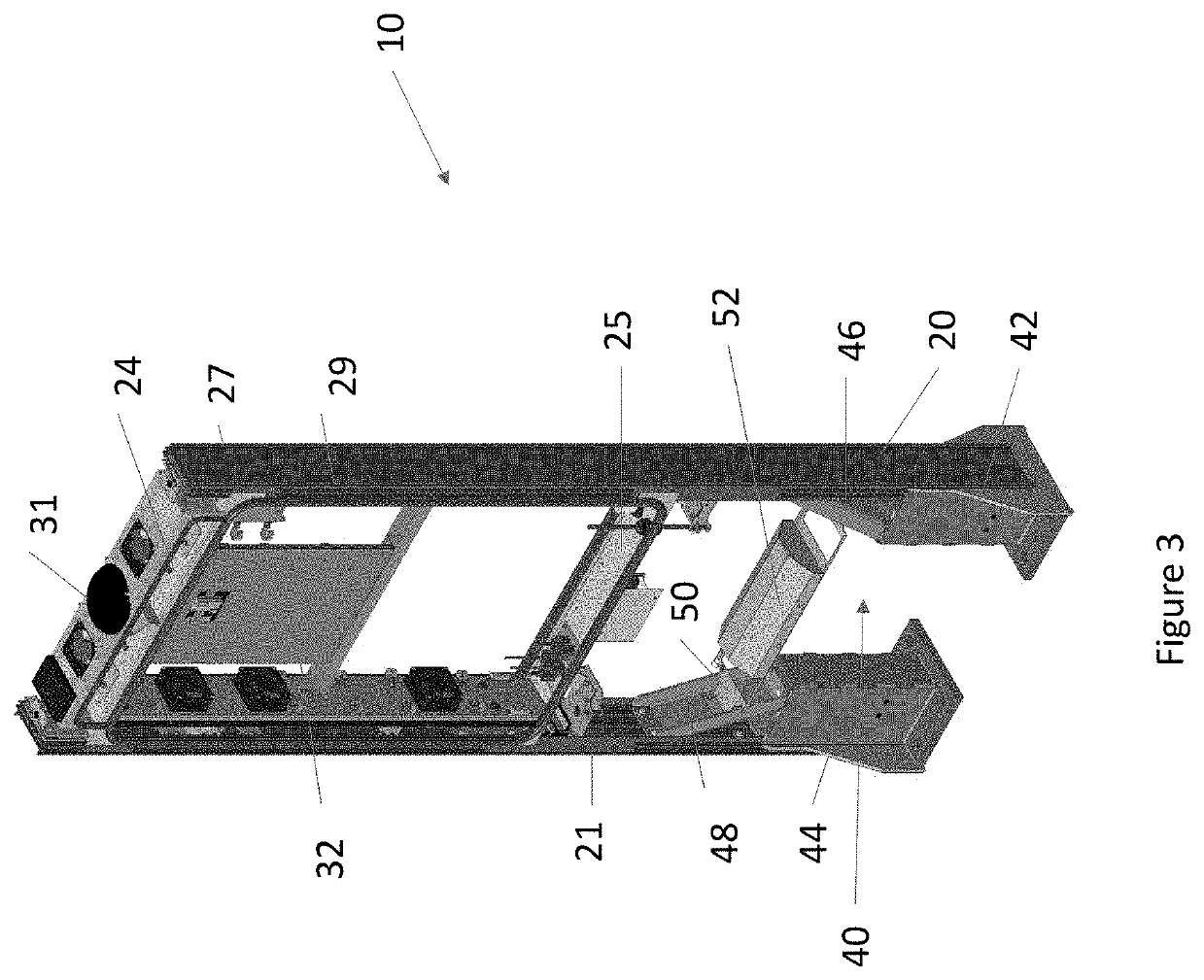
Overhead protection system
InactiveUS20110226166A1Easy to buildFast constructionBuilding repairsTowersBraced frameFacilities design
A protective shelter is disclosed. The shelter may be sized to protect an existing, unhardened structure, and its contents. Or, the shelter may be sized to protect an individual or other objects of similar size. In either case, the shelter provides protection from blasts from explosive shells, rockets, and the like. The shelter has a support frame that supports a blast cover that is positioned above the object or objects to be protected. A burster screen is positioned above the blast cover and may be supported by the support frame. The burster screen serves to detonate incoming ordinance before the ordinance reaches the protected object or objects. The blast cover is strong enough to withstand the resulting shock from the detonated ordinance, and thus prevents damage to the protected object or objects located below the protective shelter. This invention may be used to retrofit existing, unhardened structures in areas where additional protection is needed. It also may be used as part of the design of new facilities, and offers the option to later remove the hardened, protective part of the structure if the threat level changes for the better. In the smaller, personnel-protection embodiment, the invention may be air dropped to remote locations and assembled by personnel in the field.
Owner:RECON INT FZE
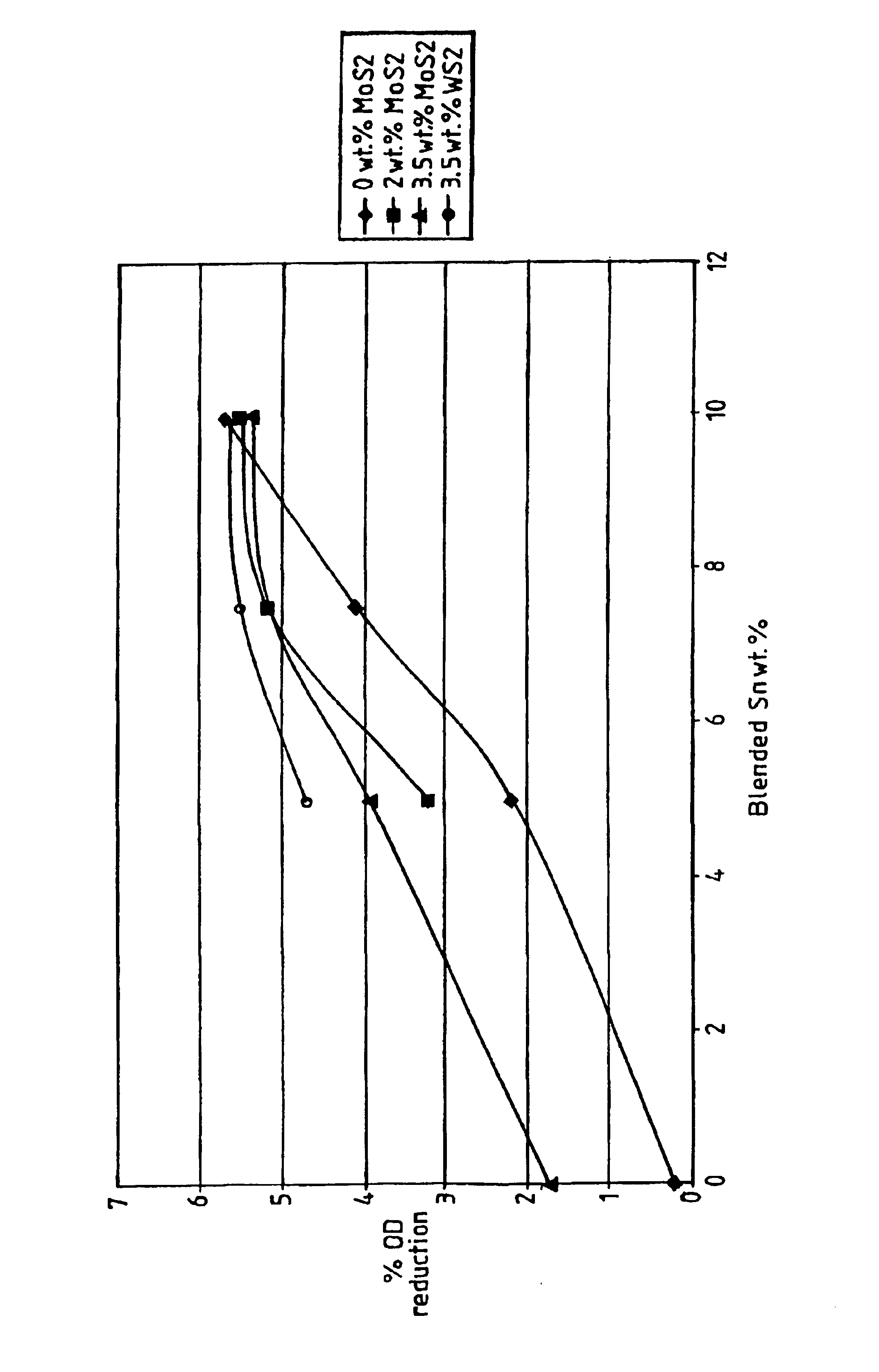
Sintered cobalt-based alloys
InactiveUS6958084B2Improve performanceConsiderable mechanical strengthBlade accessoriesShaftsNickelLubricant
A sintered material and a method for the production thereof is described. The material comprises an alloy selected from one of the groups having a composition comprising in weight %: either Cr 5-30 / Mo 0-15 / Ni 0-25 / W 0-15 / C 0-5 / Si 0-5 / Fe 0-5 / Mn 0-5 / others 10 max / Co balance, or Cr 10-20 / Mo 0-15 / Co 0-20 / W 0-5 / Fe 0-20 / Al 0-5 / Ti 0-5 / others 15 max / Ni balance; said alloy having incorporated therein from 3-15 weight % of Sn; and optionally from 1-6 weight % of a solid lubricant material.
Owner:FEDERAL MOGUL SINTERED PROD LTD
Methods for identifying drug pharmacology and toxicology
InactiveUS20060275816A1Significant utilityTime consuming and expensiveMicrobiological testing/measurementGene expressionCell based
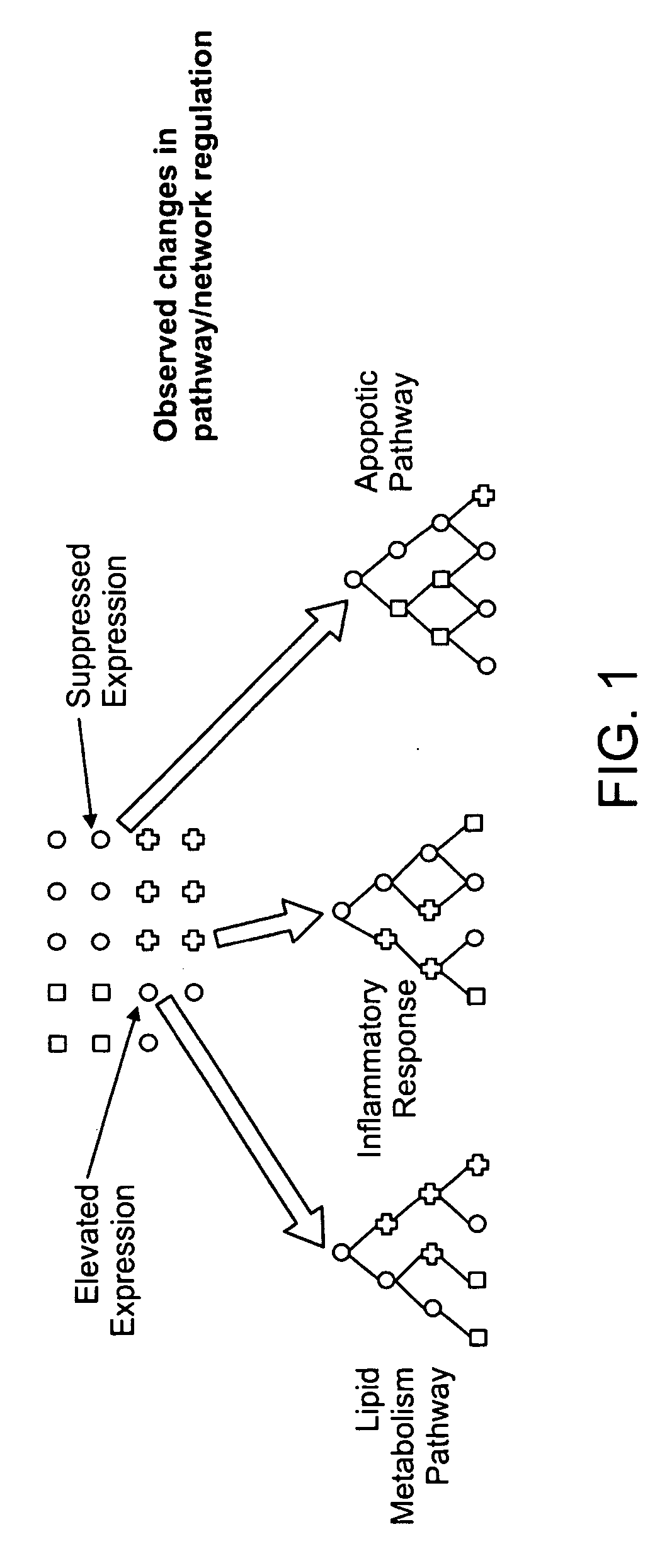
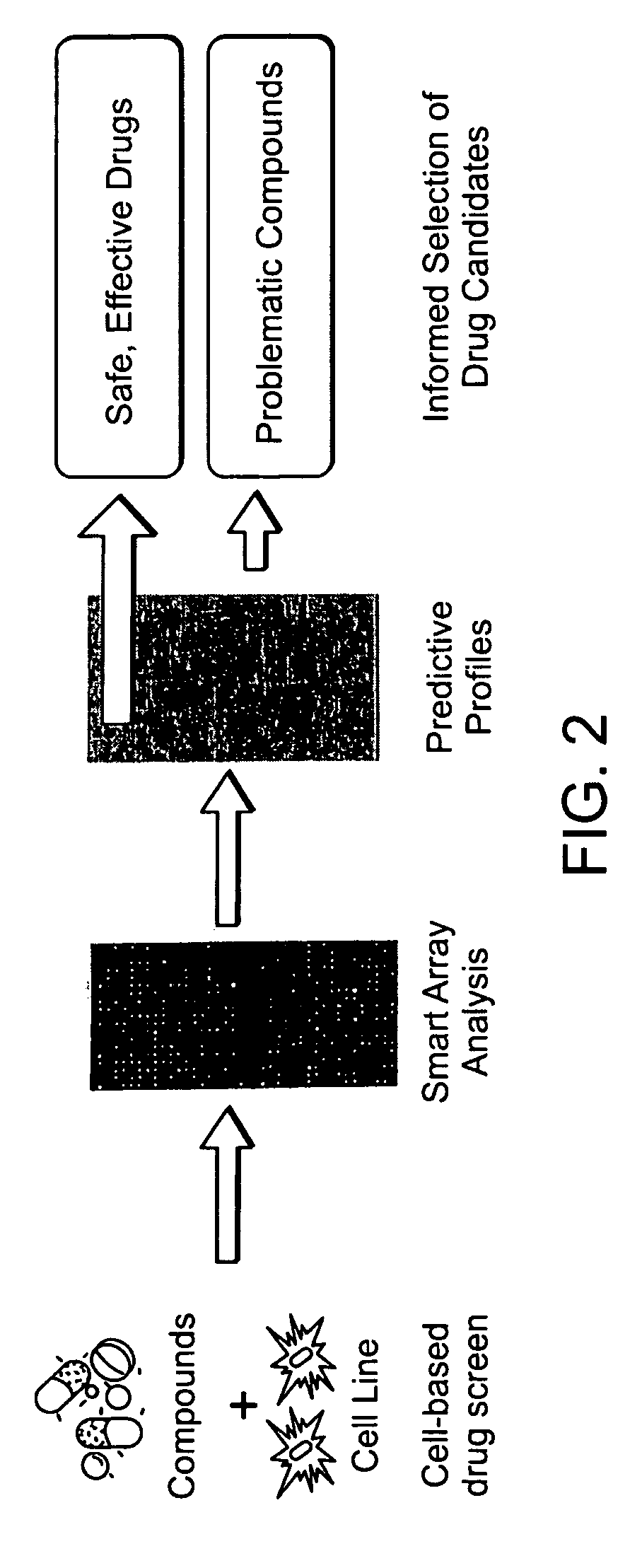
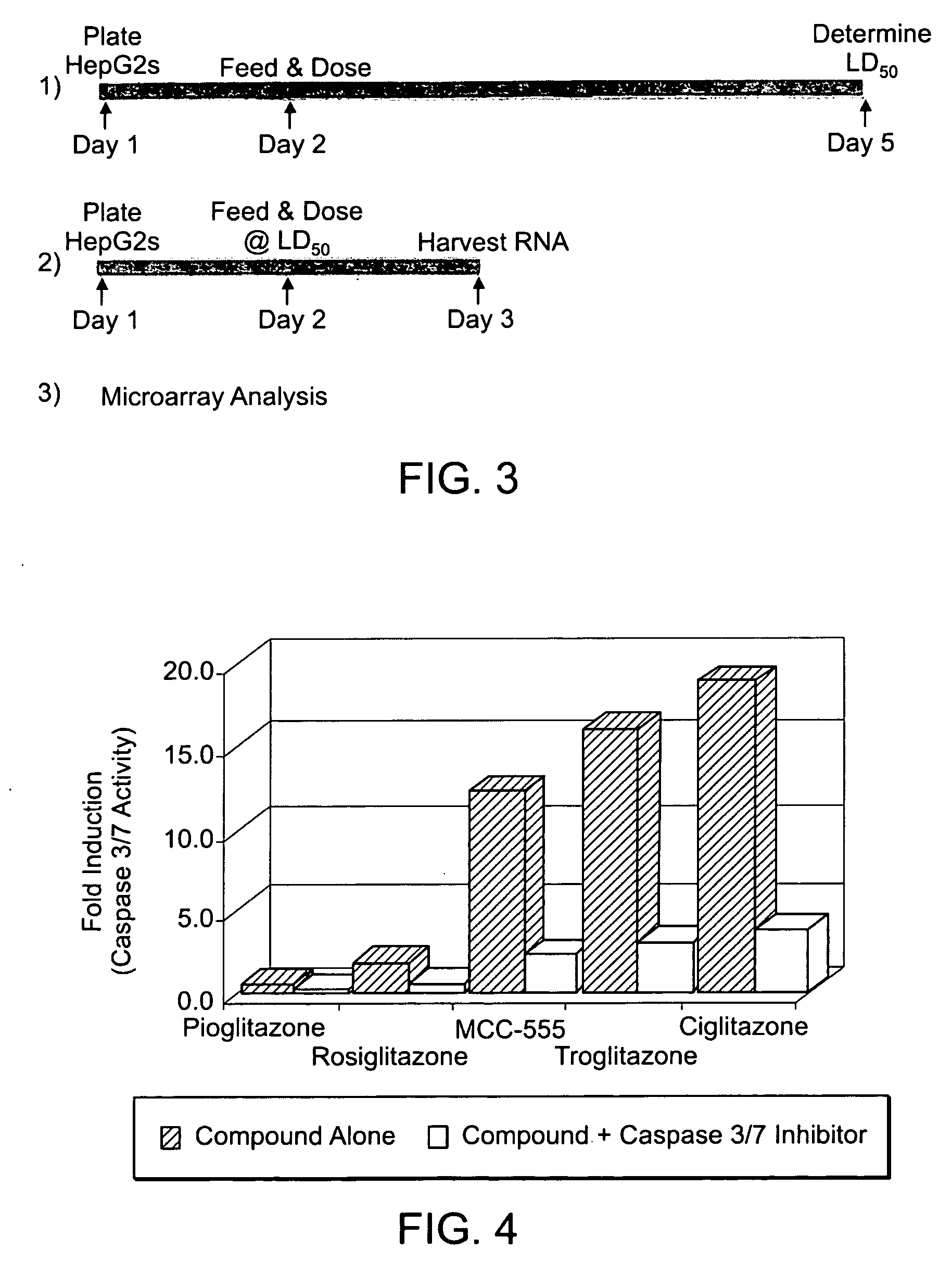
The invention combines a microarray and cell-based screening strategy that enables rapid identification of possible mechanisms underpinning the pharmacology and toxicology of drug candidates. The methods of the invention identified unique properties relating to apoptosis and the anti-inflammatory response elicited by several peroxisome proliferator activated receptor gamma (PPARγ) ligands. The methods illustrate, for example, that PPARγ ligands that are safe and effective drugs (e.g., Actos, Avandia) either do not induce apoptosis or only modestly induce apoptosis. Conversely, PPARγ ligands that have failed clinical development (e.g., Ciglitazone; Day, C., Diabet. Med., 16: 179-192 (1999)) or that have been withdrawn from the market (e.g., Troglitazone (Rezulin)) due to hepatotoxicity are potent inducers of apoptosis. The methods of the invention also illustrate that suppression of gene expression and protein expression for several pro-inflammatory factors by some PPARγ ligands occurs as a consequence of apoptotic induction (i.e., apoptosis produces an anti-inflammatory response). The invention also provides biomarkers for cellular pathways and methods for stratifying patient groups according to their biomarker expression as well as biomarkers that discriminate safe and effective drugs from compounds that have acute toxicities. These biomarkers provide novel insights into the mechanism of action and toxicity for test compounds, including cell death, anti-inflammatory activity, hepatotoxicity, and carcinogenicity. The methods are highly scalable and have broad application from discovery to the clinic, including compound prioritization, predictive pharmacology and toxicology; mechanism of action studies; and prognostic and diagnostic biomarker discovery.
Owner:RIBONOMICS
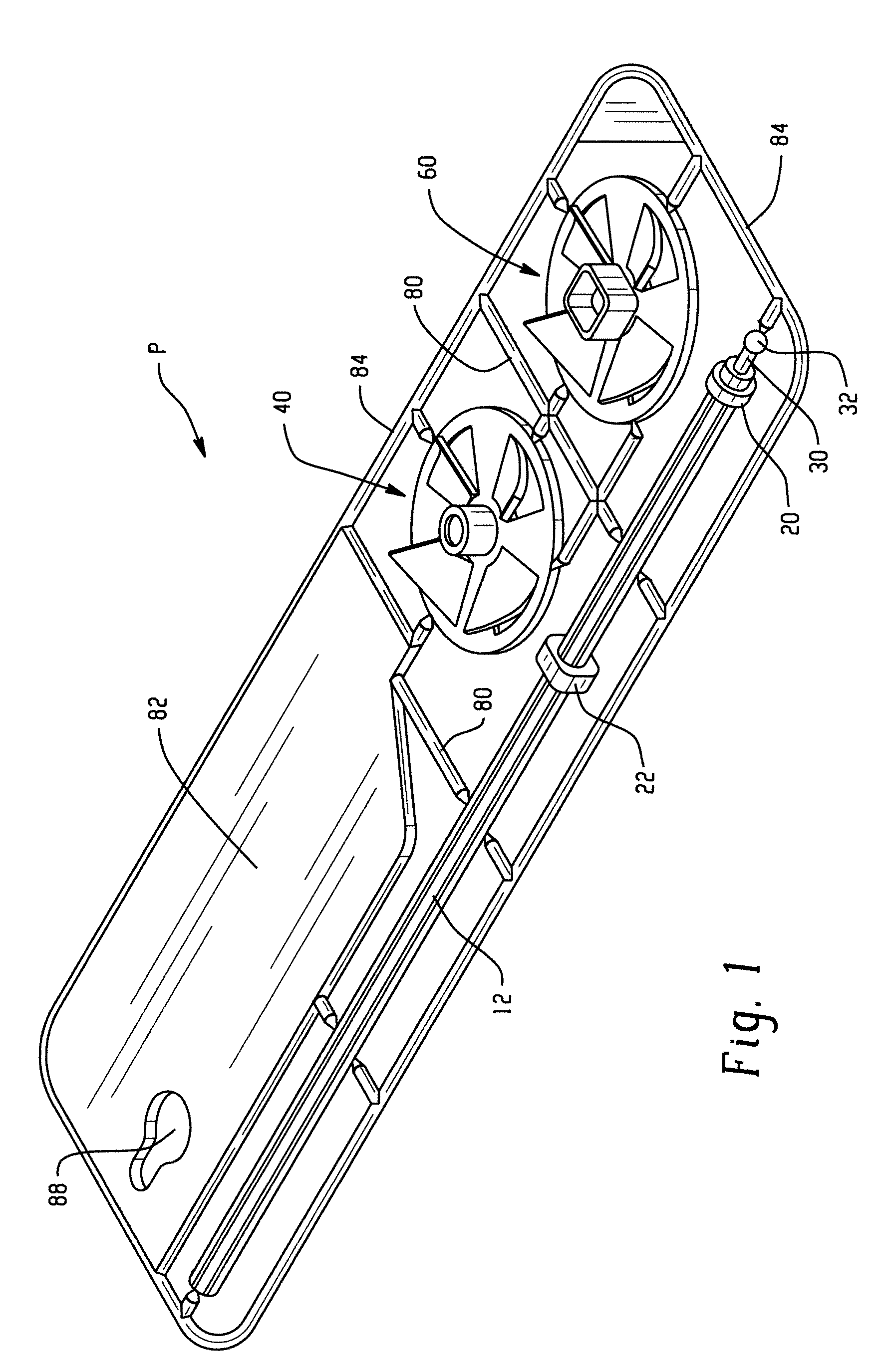
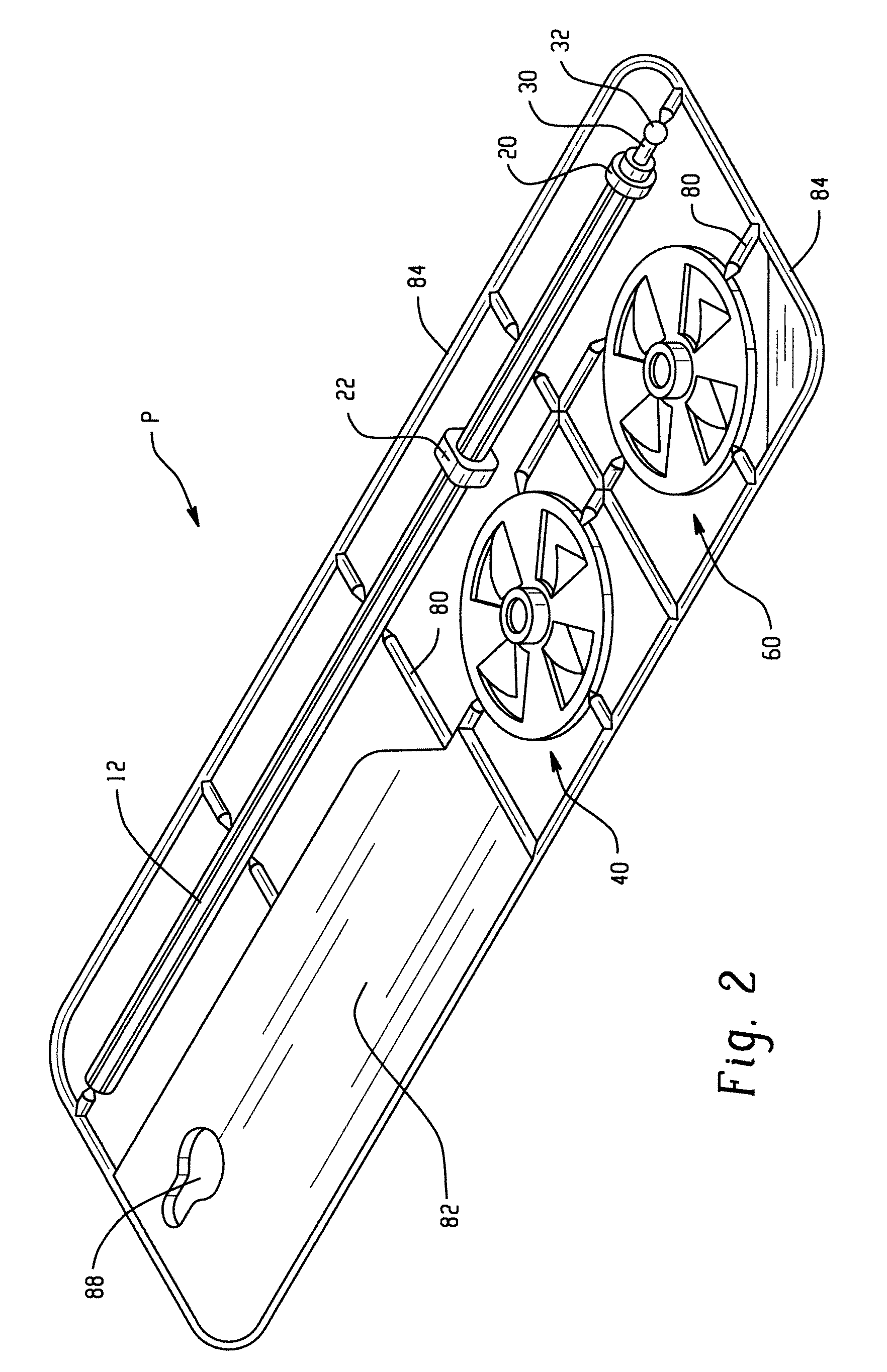
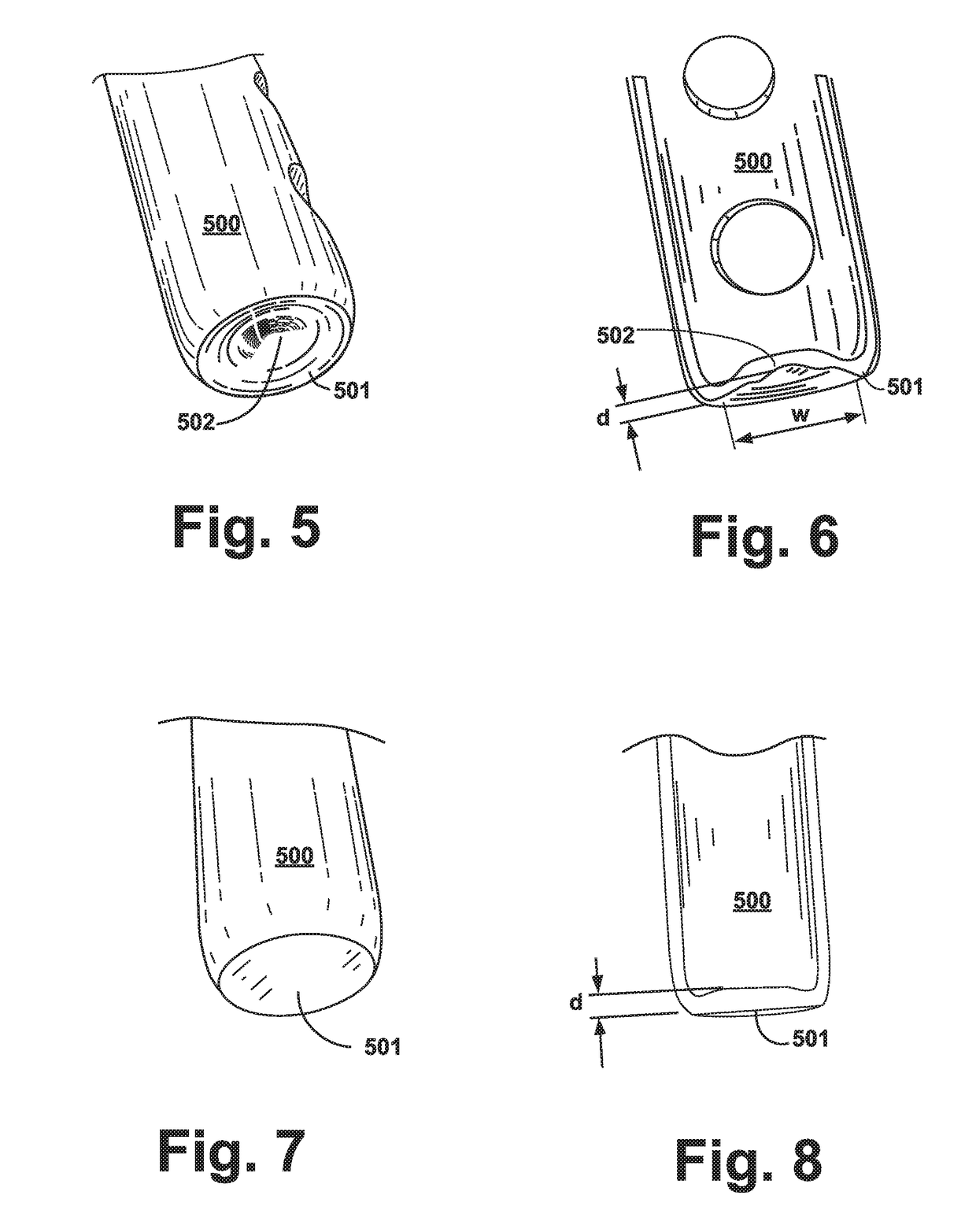
Microfluidic device and methods for construction and application
InactiveUS7802591B2Easy to produceTime-consume and expensiveMaterial nanotechnologyServomotor componentsEngineeringConstriction
A microfluidic device comprises first and second inlet passages (13) for respective immiscible fluids, these inlet passages merging into a third passage (8) along which the two fluids flow under parallel laminar flow conditions, the third passage being formed with a constriction or other discontinuity (9) causing the two fluids to form into a flow of alternate segments.
Owner:Q CHIP
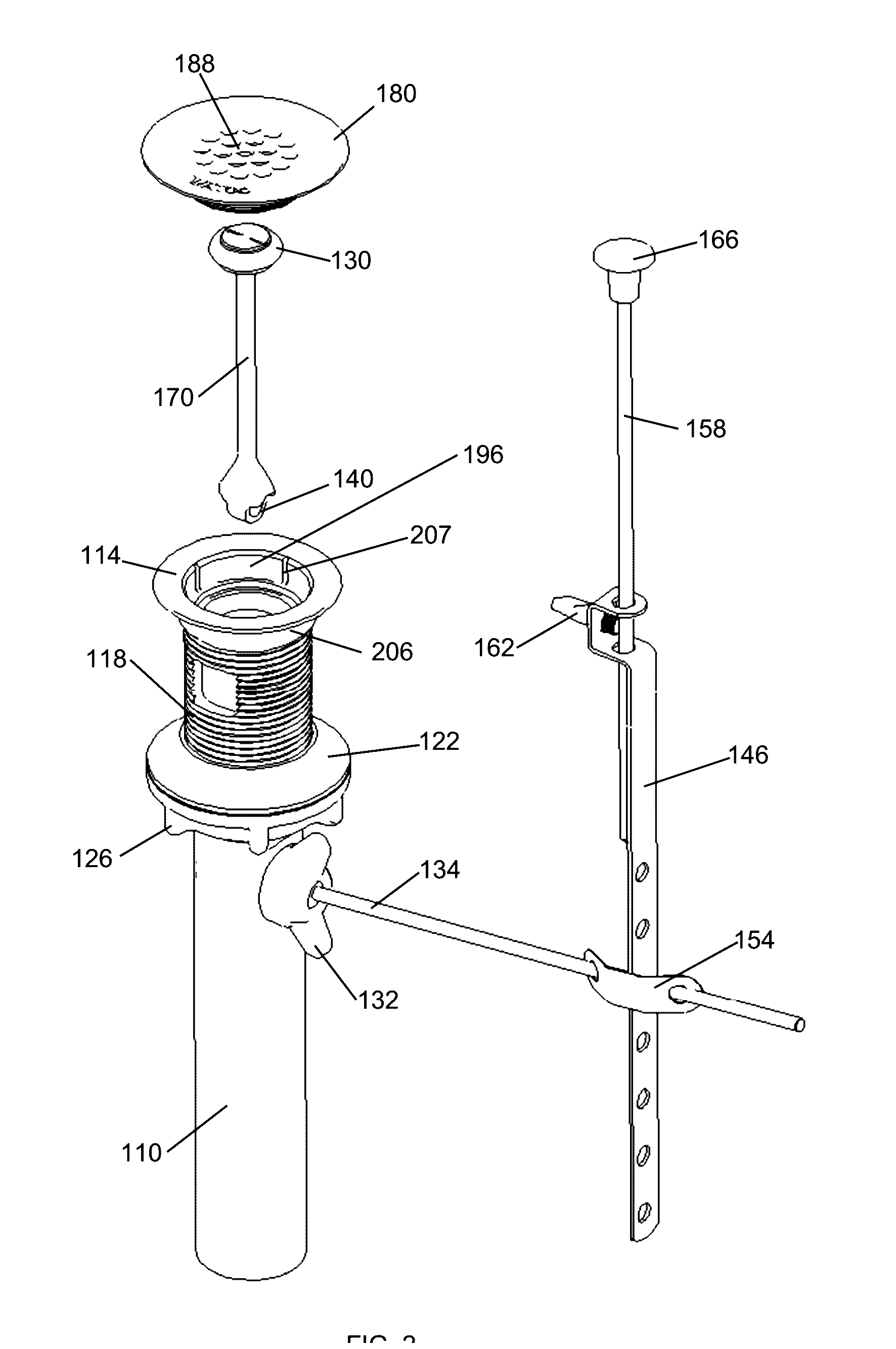
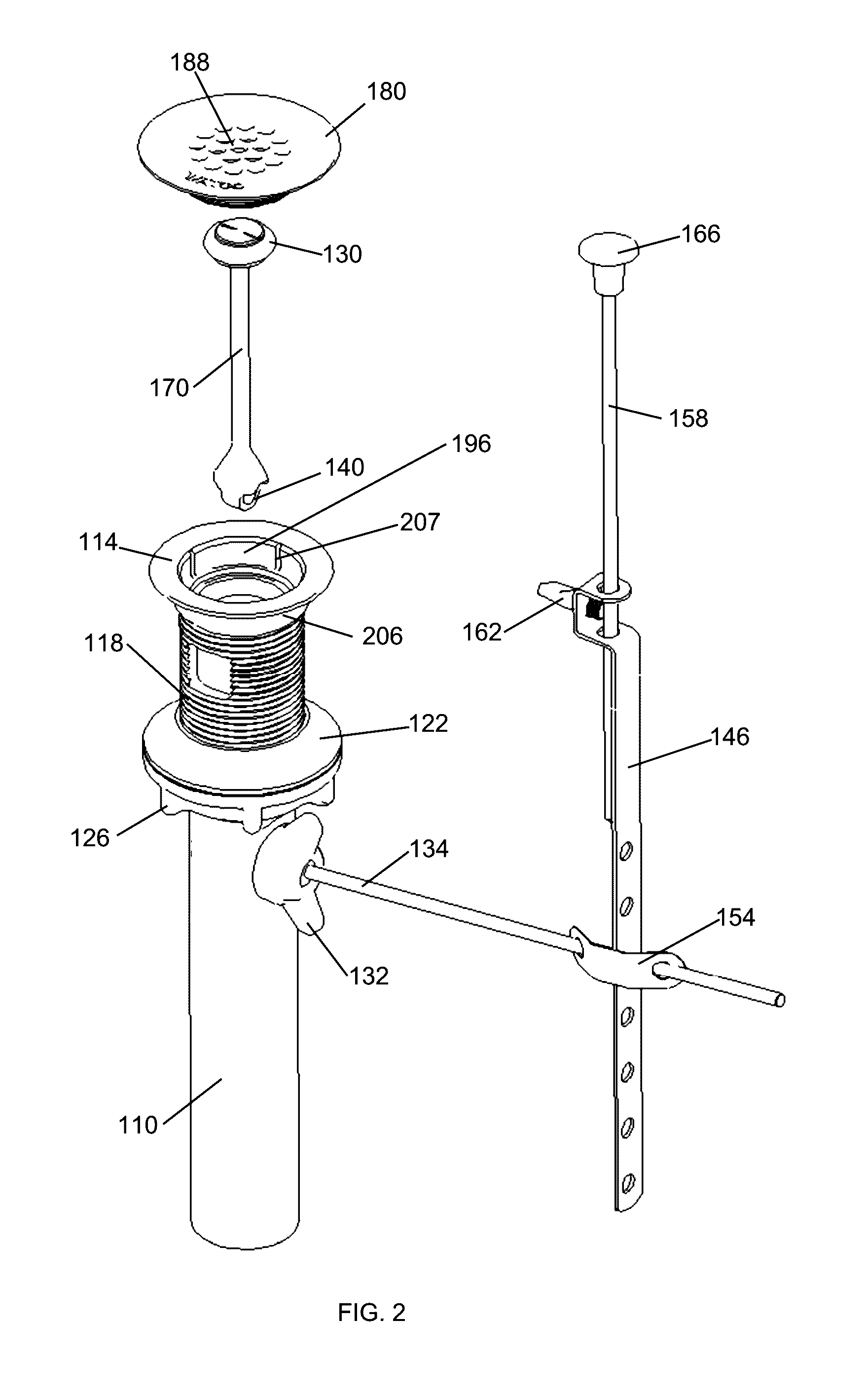
Wastewater drain stopper system
ActiveUS20160356028A1Reduce manufacturing costHighly efficient to useDomestic plumbingWastewaterFlange
A wastewater drain assembly is provided that includes a selectively openable drain stopper. The wastewater drain assembly is interconnected to a fluid basin wherein a flange, which is interconnected to wastewater plumbing, is situated within the sink. The flange receives an insert that has a portion that conceals the flange.
Owner:WCM IND INC
Integrated circuits protected against reverse engineering and method for fabricating the same using vias without metal terminations
InactiveUS20020096777A1Difficult to determineTime-consume and expensiveSemiconductor/solid-state device detailsSolid-state devicesIntegrated circuitEngineering
A device adapted to protect integrated circuits from reverse engineering comprising a part looking like a via connecting two metal layers, but in fact attached only to one metal layer and spaced from the other. Having such "trick" via would force a reverse engineer to think there is a connection where there is none. A method for fabricating such device.
Owner:HRL LAB
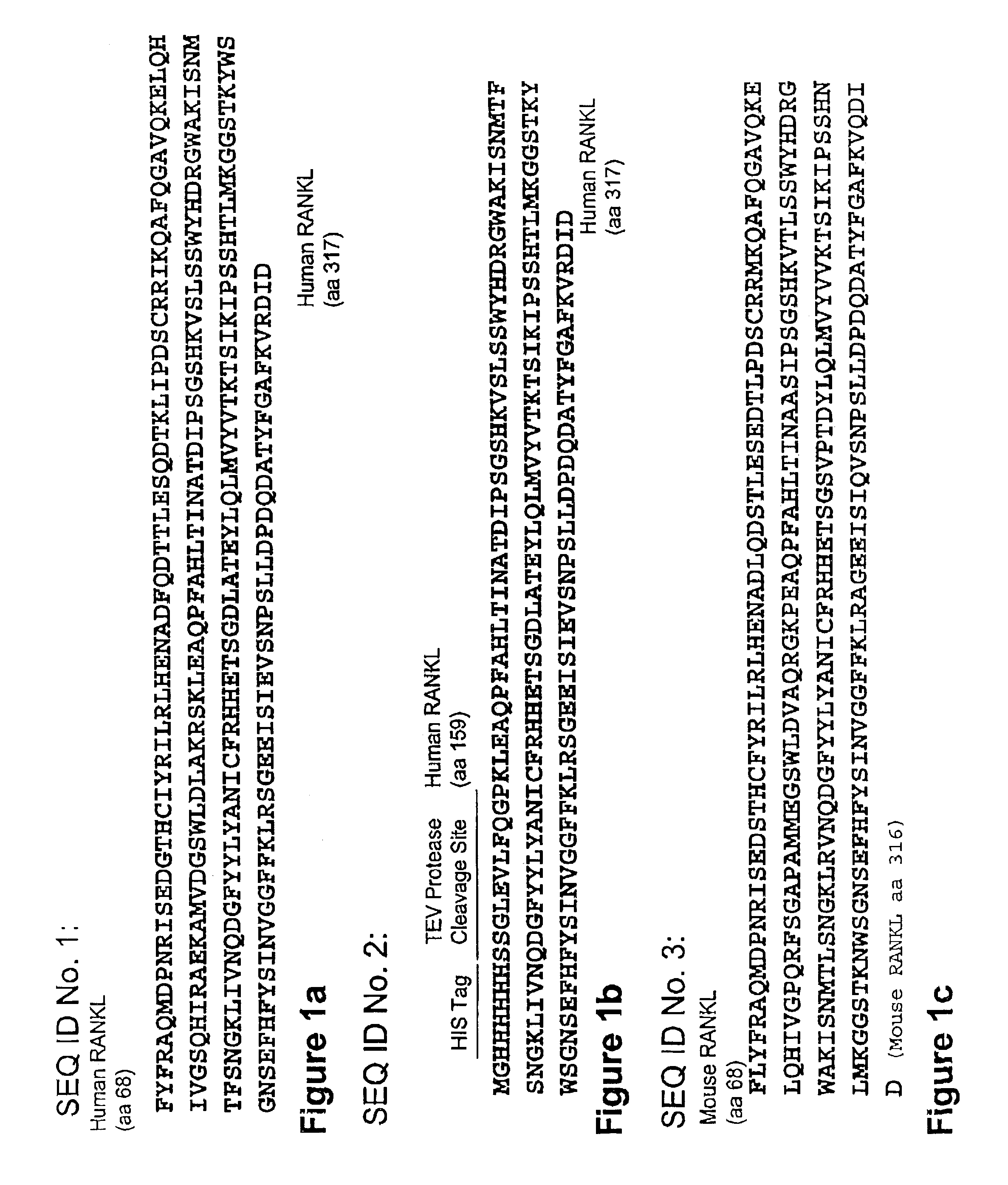
Variants of RANKL protein
InactiveUS7399829B2Efficient and cost-effective productionEfficient and cost-effective and manufacturingAntibacterial agentsFungiRANKL ProteinEscherichia coli
Owner:XENCOR
Tube end sealing method
In a method for closing open ends of tubes, an open tube end is closed using spin-tubing methods known in the art. Following the tube end closing, a concavity is formed in the tube end in a tube end forming machine. The tube end is brazed with the tube end facing upright, so that the braze alloy pools in the concavity, strengthening the tube end.
Owner:SHOALS TUBULAR PROD
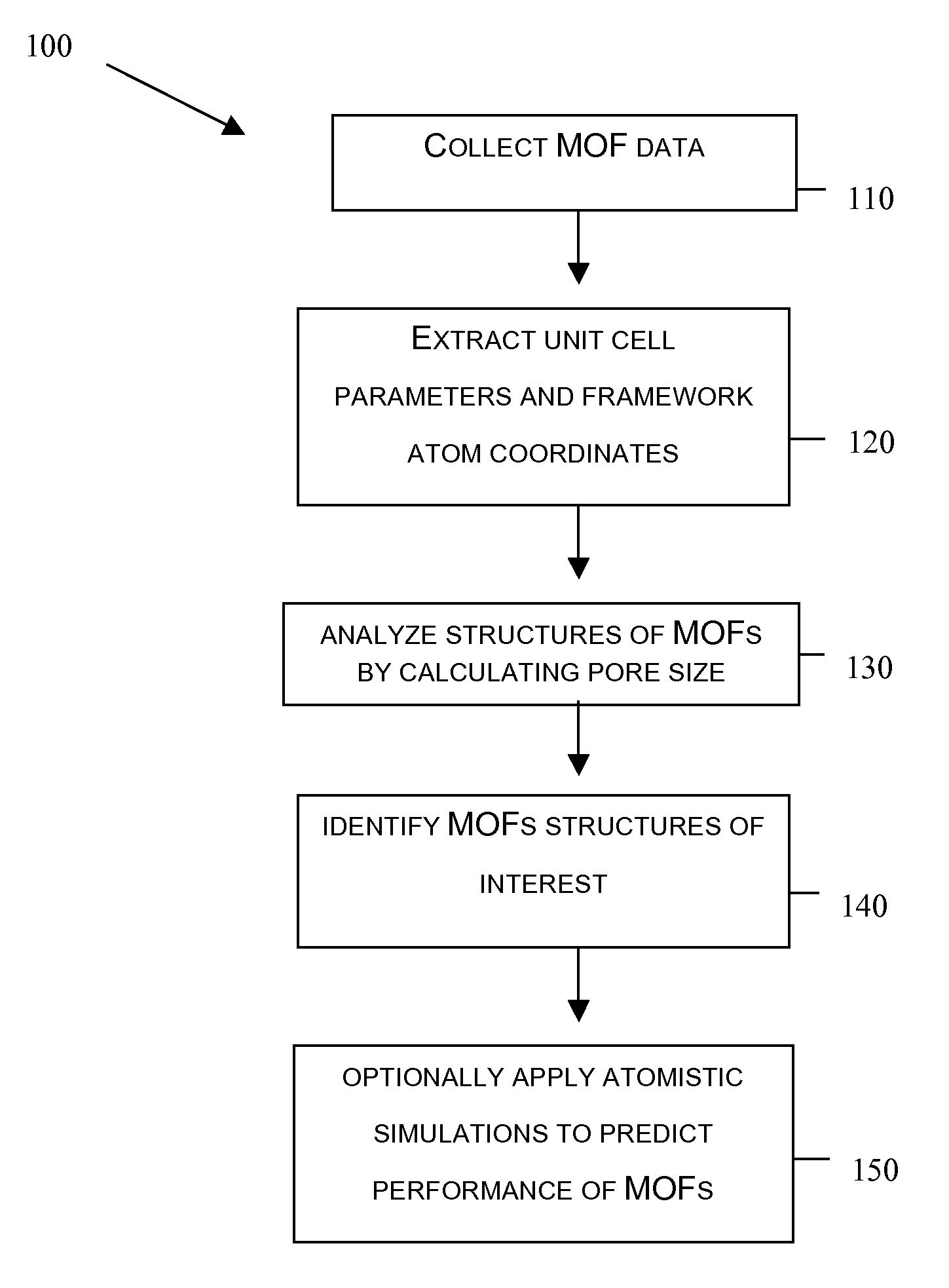
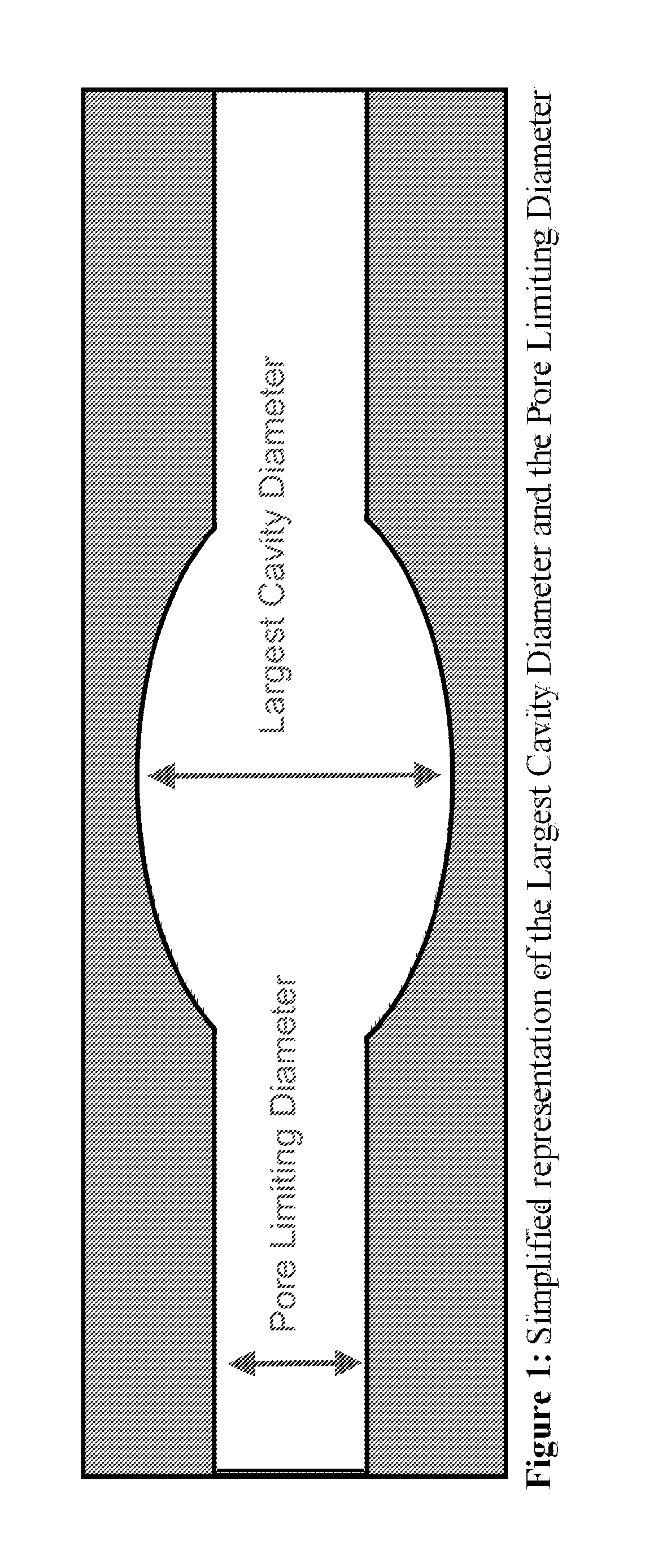
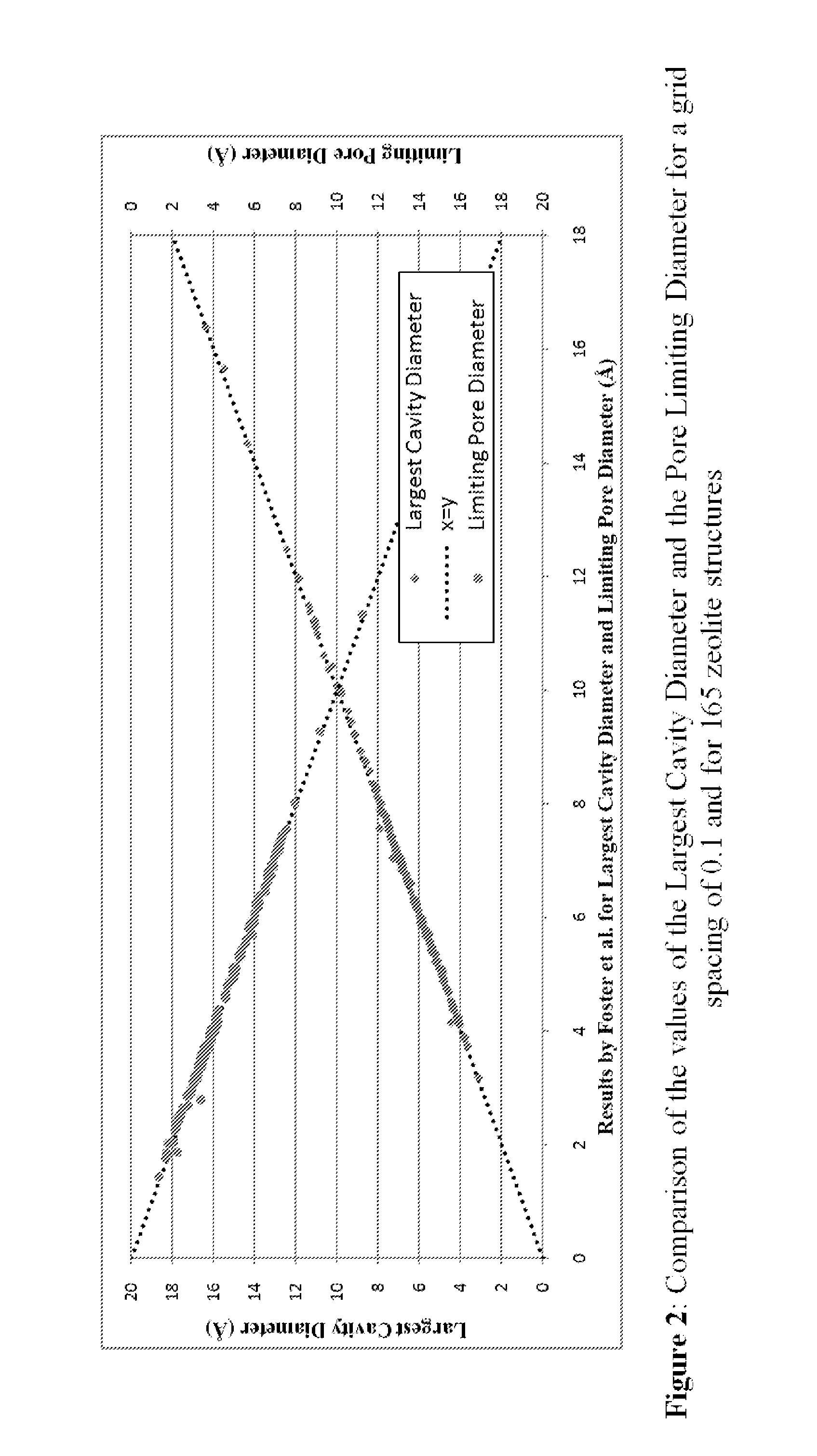
Screening metal organic framework materials
ActiveUS20110320176A1Time-consume and expensiveSimple methodChemical property predictionCopper organic compoundsMetal-organic frameworkMembrane configuration
This invention relates to a method for characterizing the pores of reticulated framework structures and using these characteristics to predict the actual performance characteristics of the reticulated framework structures as membranes for gas separation, and other purposes.
Owner:GEORGIA TECH RES CORP
Housing assembly for an integrated display unit
ActiveUS20200037456A1Reduce complexityLow costCasings with display/control unitsCasings/cabinets/drawers detailsDisplay deviceStructural engineering
A housing assembly for an integrated display unit is provided. The display unit display unit comprises an open loop pathway for ambient air, a closed loop pathway for circulating gas, and at least one electronic display. A first and second horizontal member extend between a vertically extending first and second side member and are secured within a first channel and a second channel. The first and second side members form exterior side surfaces of the display unit to define, in part, the closed loop pathway. The first horizontal member forms an upper surface of the display unit and the second horizontal member forms a lower surface of the display unit to further define, in part, the closed loop pathway.
Owner:MFG RESOURCES INT INC
CMP slurry additive for foreign matter detection
InactiveUS6857434B2Avoid pollutionTime-consume and expensiveElectrostatic cleaningSemiconductor/solid-state device manufacturingForeign matterSlurry
A method and structure polishes and cleans silicon wafers by mixing a marker with a slurry to form a slurry mixture, performs chemical mechanical polishing on a silicon wafer using the slurry mixture, rinses the slurry mixture from the silicon wafer, checks the silicon wafer for marker residue, and repeats the rinsing process if the checking process detects the marker residue on the wafer.
Owner:INT BUSINESS MASCH CORP
Novel monofunctional polyethylene glycol aldehydes
InactiveUS20040122164A1Procedure is time-consume and expensiveSignificant biological activityImmunoglobulinsPharmaceutical non-active ingredientsActive proteinImmunogenicity
The present invention provides novel monofunctional polyethylene glycol aldehydes for the pegylation of therapeutically active proteins. The pegylated protein conjugates that are produced, retain a substantial portion of their therapeutic activity and are less immunogenic than the protein from which the conjugate is derived. New syntheses for preparing such aldehydes are described.
Owner:SUN BIO INC
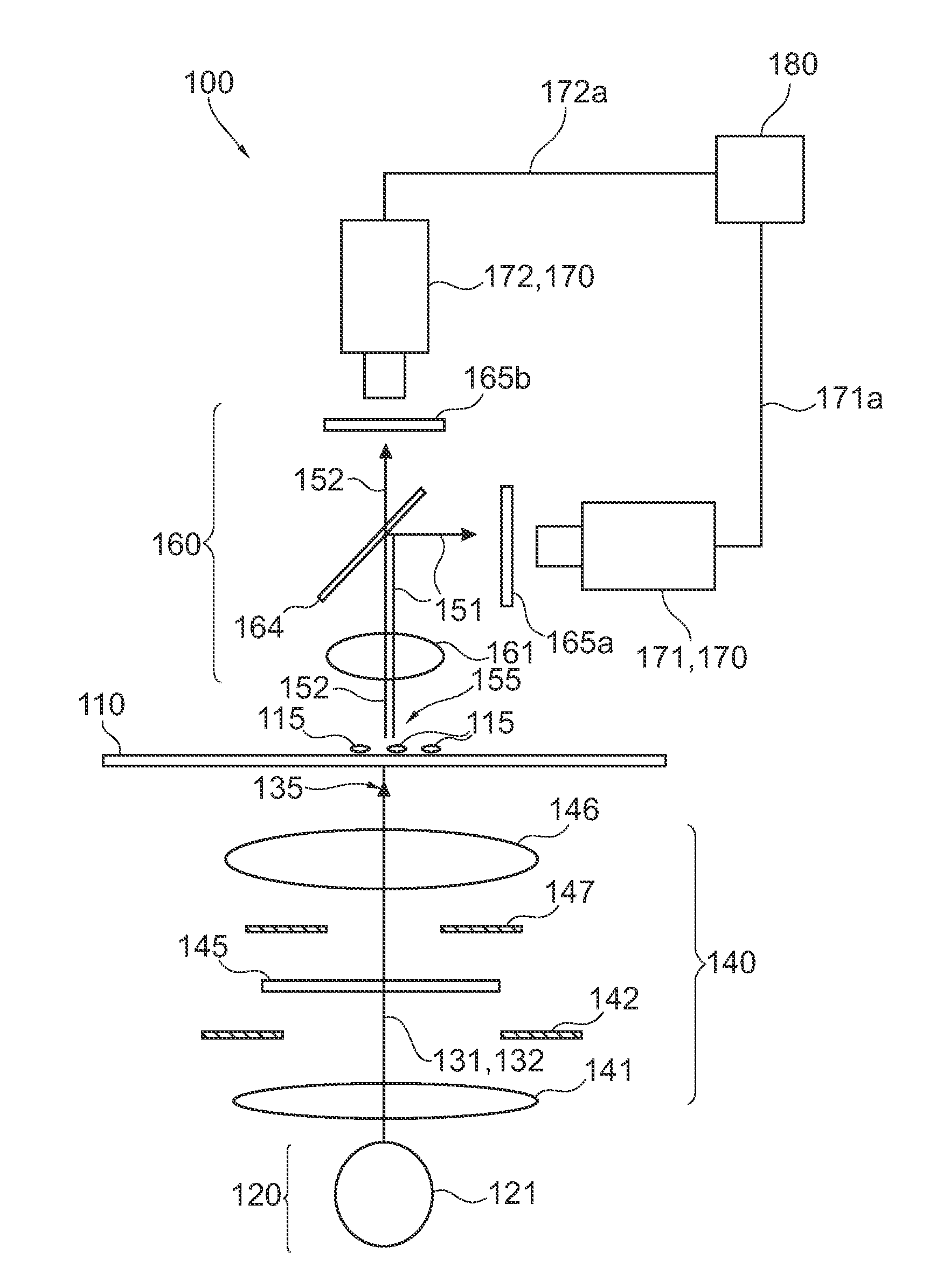
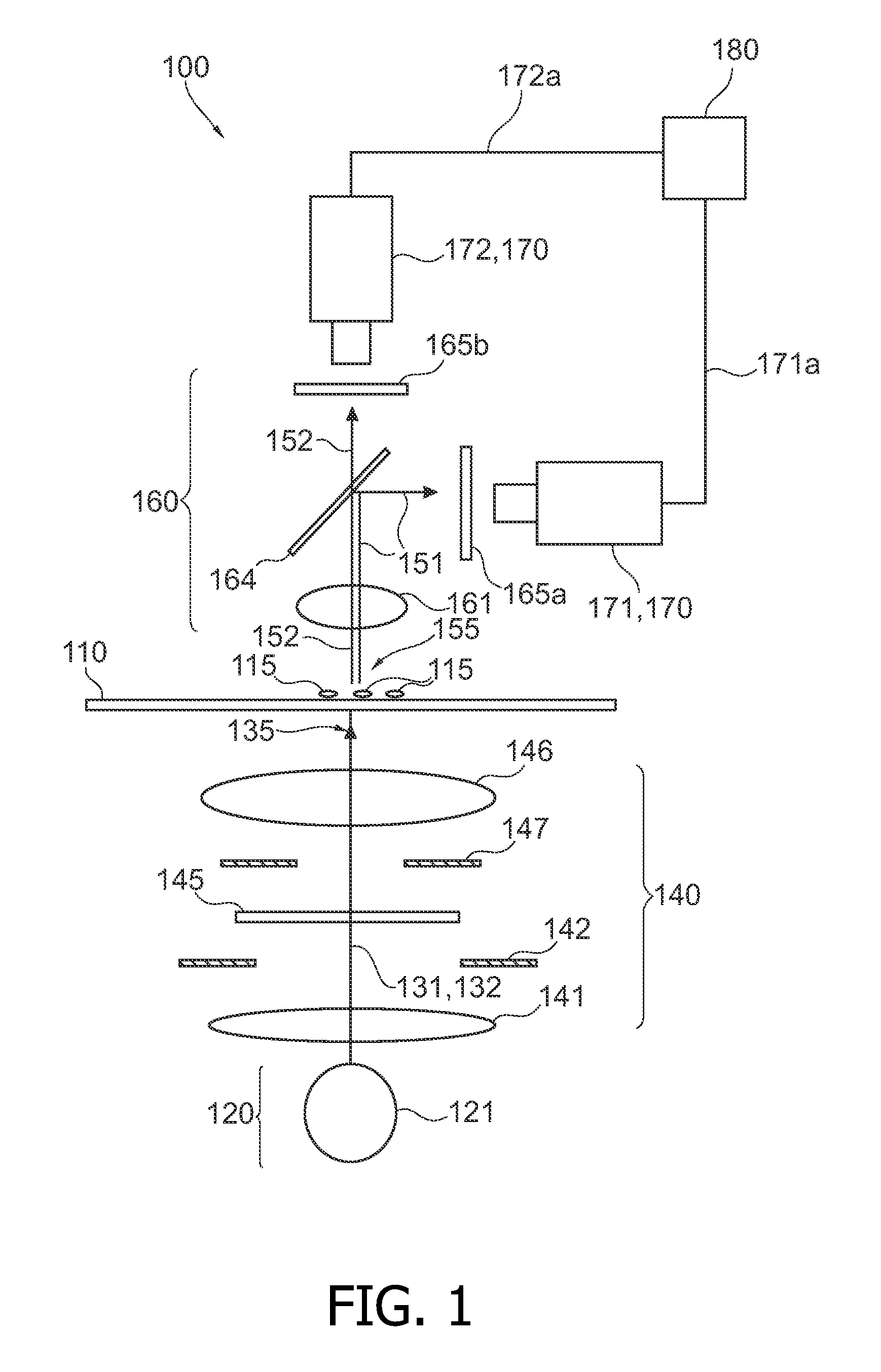
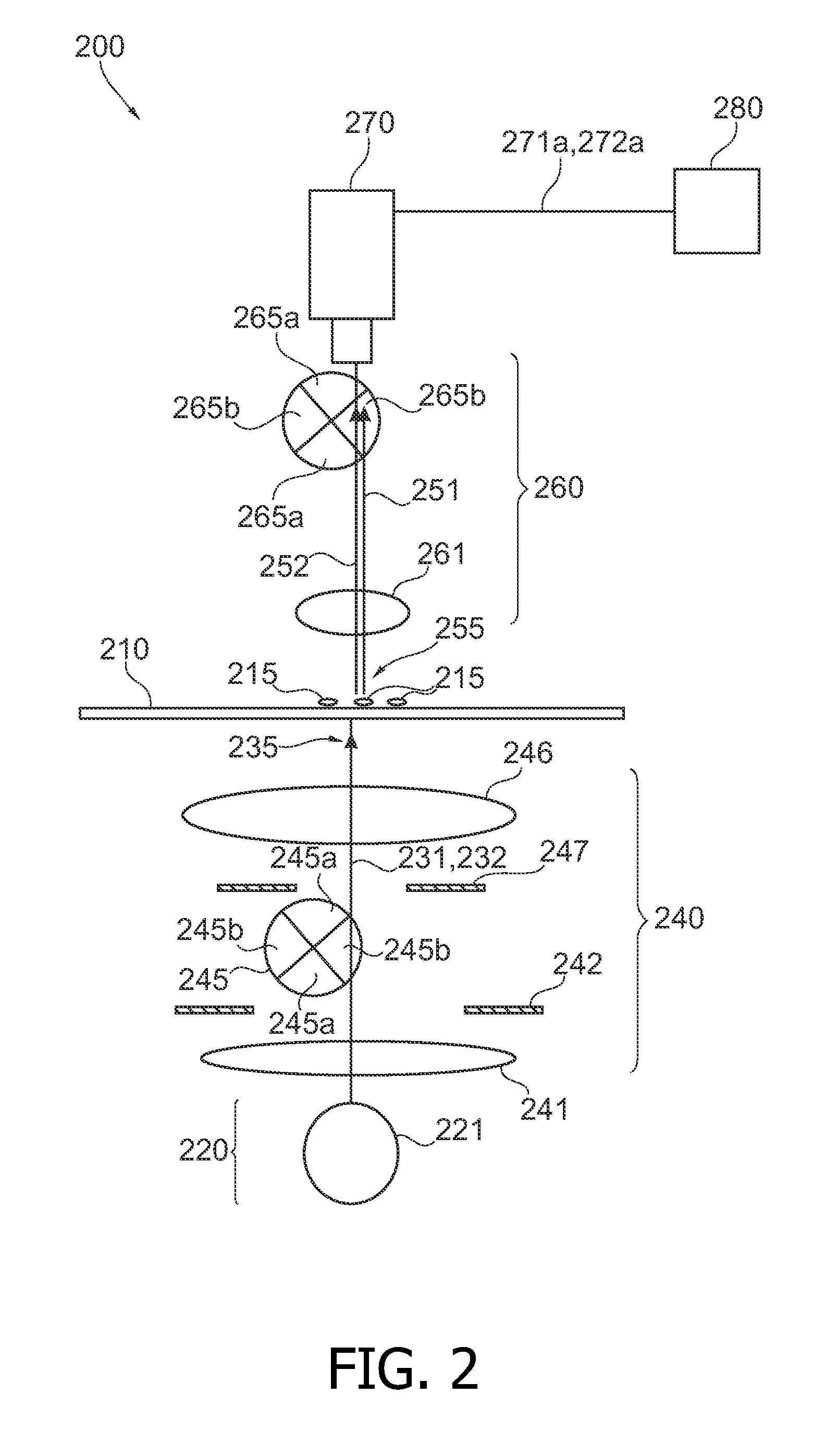
Analyzing biological cell material based on different interactions between illumination light and cell components
InactiveUS20100105022A1Improve diagnostic accuracyStrong change of optical propertyBioreactor/fermenter combinationsBiological substance pretreatmentsBiological cellDNA
It is described a device (100) for analyzing biological cell material (115). The device (100) comprises a light source arrangement (120), which is adapted for directing a first (131) and a second illumination light (132) towards the cell material (115), wherein the first (131) and the second (132) illumination light comprises a first and a second spectral radiation component, respectively. The device (100) further comprises a detector arrangement (170), which is adapted for receiving a first measurement light (151) based on a first interaction of the first illumination light (131) with the cell material (115) and a second measurement light (152) based on a second interaction of the second illumination light (152) with the cell material (115). Further, the device (100) comprises an evaluation unit (180), which is coupled the detector arrangement (170) and which is adapted to evaluate a first signal (171a) and a second signal (171b) being indicative for the first (151) and the second measurement light (151), respectively. The device (100) may be used for accomplishing ultraviolet DNA image cytometryin combination with autofluorescence measurements of NAD(P)H.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Single piece connecting member and work tip for surgical hand piece
Owner:SURGICAL DESIGN
Method for etching 3D structures in a semiconductor substrate, including surface preparation
InactiveUS20100216308A1Inhibition formationEliminate the causeSemiconductor/solid-state device manufacturingSemiconductorDry etching
A method is provided for producing 3D structures in a semiconductor substrate using Deep Reactive Ion Etching (DRIE), comprising at least the steps of: providing a substrate, and then grinding the backside of the substrate in order to achieve a thinned substrate, wherein extrusions and native oxides are left after said grinding step, and then performing a surface treatment selected from the group consisting of a wet etching step and a dry etching step in order to remove at least said native oxides and extrusions on the surface of said backside of the substrate which are causes for the grass formation during subsequent etching, and then performing deep reactive ion etching in order to achieve 3D vias.
Owner:INTERUNIVERSITAIR MICRO ELECTRONICS CENT (IMEC VZW)
A lifting assembly and method
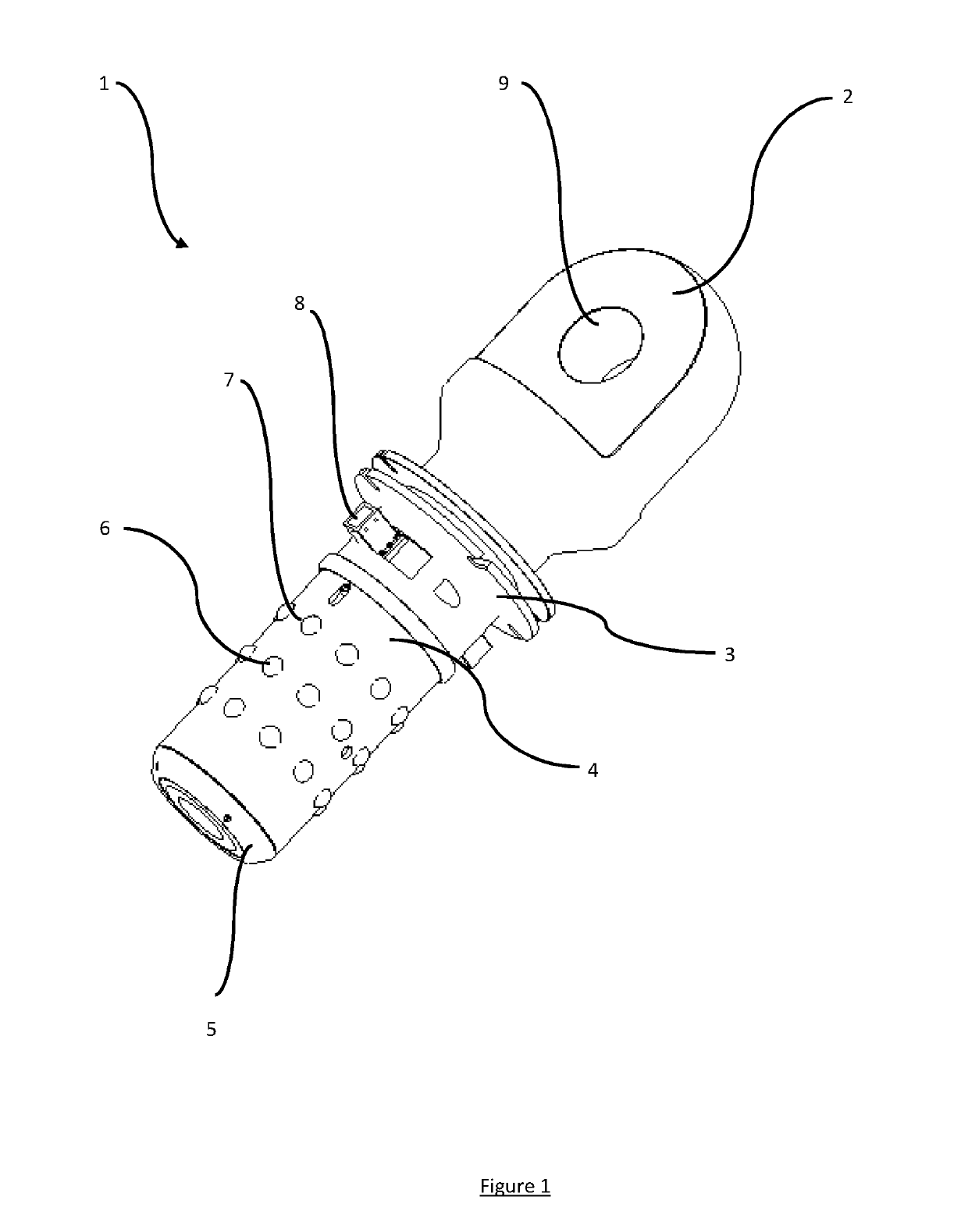
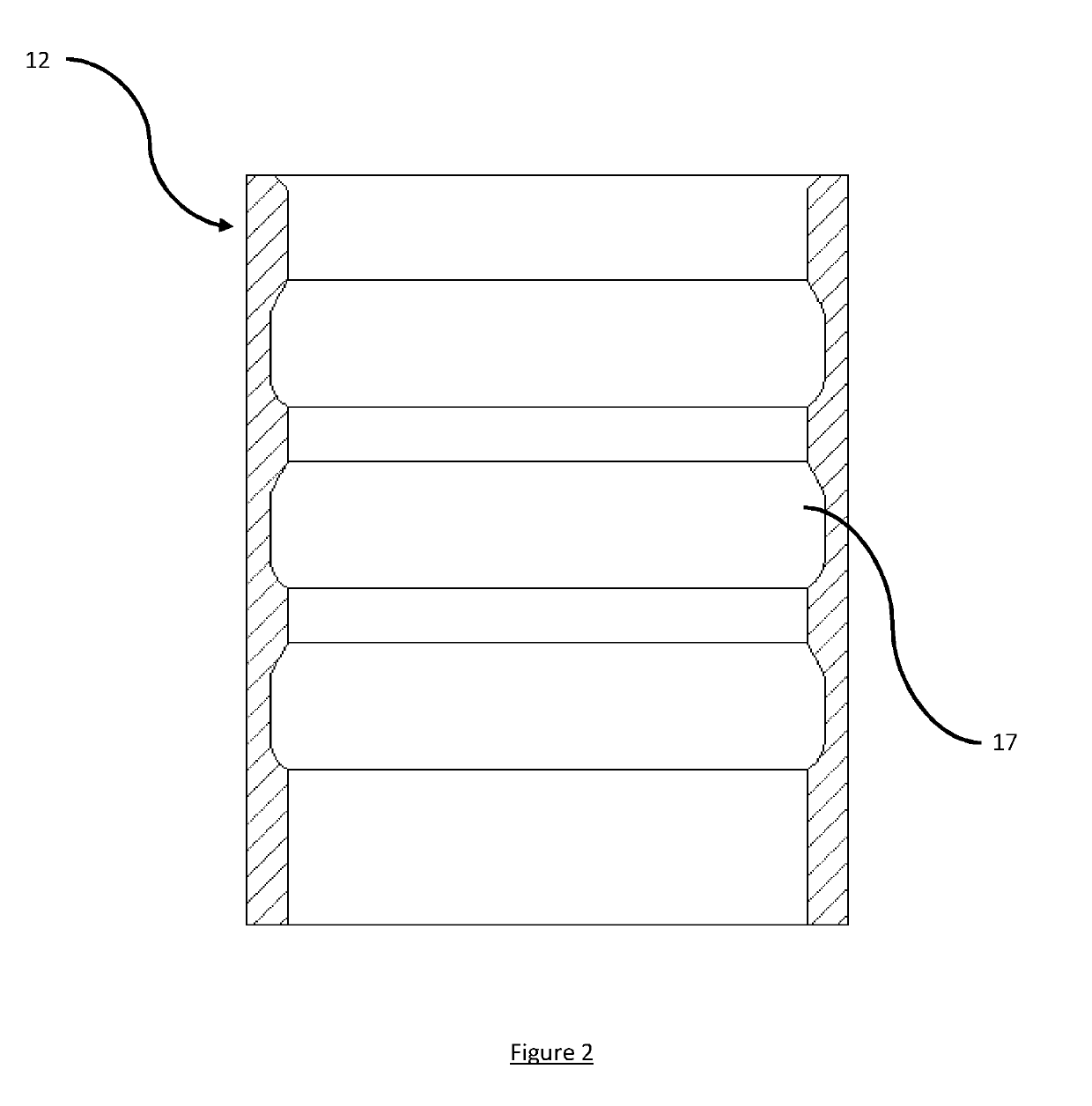
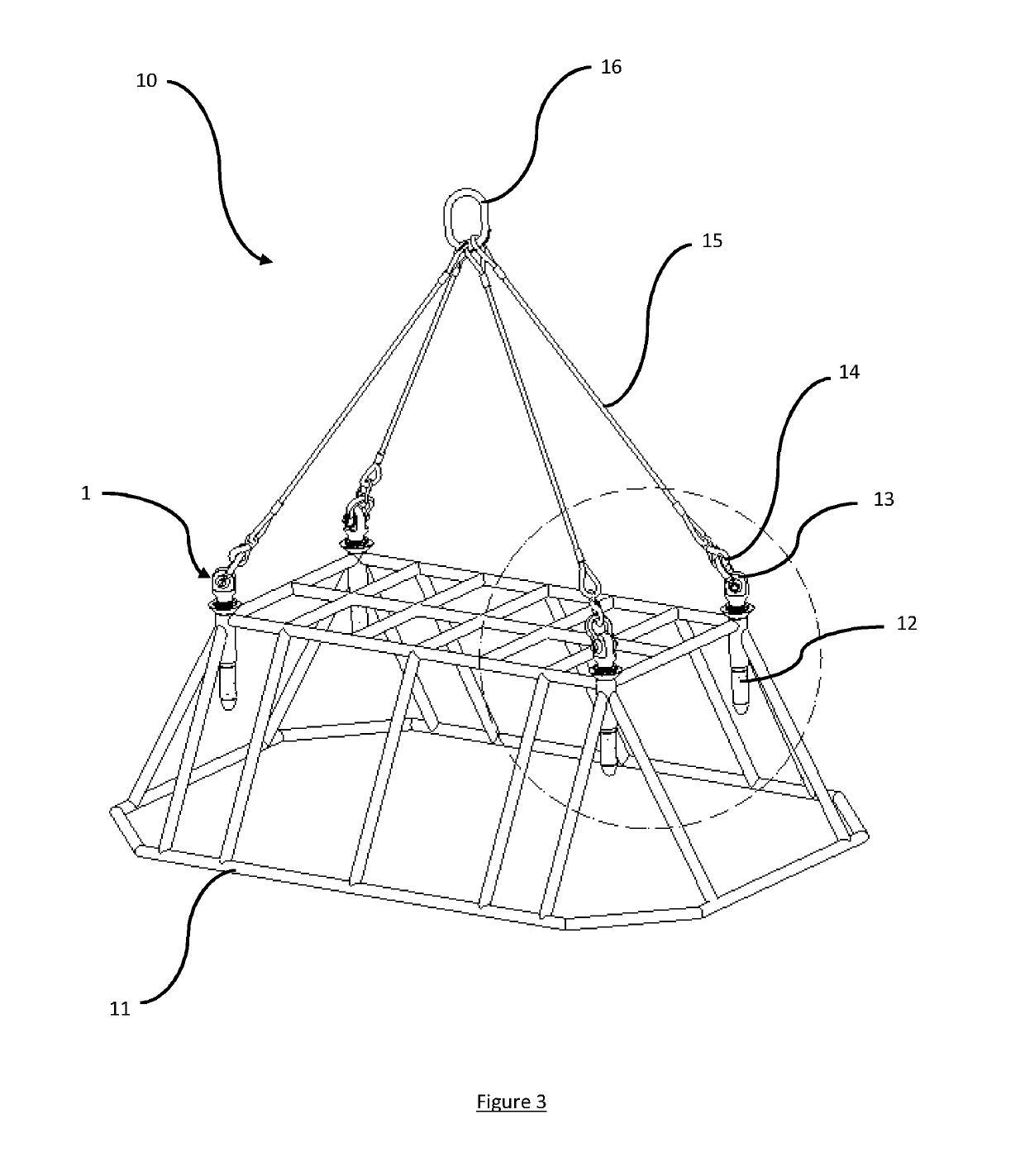
ActiveUS20190202670A1Function increaseHigh strengthLoad-engaging elementsMechanical engineeringEngineering
A lifting assembly (10) having a structure (11) with three or more receptacles and a two or more connectors (1). The structure comprises one or more receptacles (12). Each receptacle comprises one or more circumferential grooves (17) in its inside surface. The connector comprises a male element (4) for insertion into a receptacle, an attachment point (2), and one or more locking elements (6) provided on the outside surface of the male element for engaging with the or each groove in the inside surface of the or each receptacle so as to rotatably mount the attachment point relative to the structure. The connectors may be rotationally positioned within the receptacles to permit different lifting configurations to be used.
Owner:BALLTEC
Electricians wire labelling system
InactiveUS20170025046A1Risk minimizationPrevents inadvertent duplicationStampsInsulated cablesDistribution systemLabelling
The present invention includes a system for marking a plurality of wires connected to a 3 phase electric distribution system that includes a plurality of preprinted self-adhesive labels for use on electrical wires wherein each of the labels in the plurality of labels bears one of first, second and third unique indicia and a preprinted unique integer number.
Owner:BUTTS JEREMY
Housing assembly for an integrated display unit
ActiveUS10602626B2Time-consume and expensiveIncreased complexityCasings with display/control unitsCasings/cabinets/drawers detailsDisplay deviceStructural engineering
A housing assembly for an integrated display unit is provided. The display unit display unit comprises an open loop pathway for ambient air, a closed loop pathway for circulating gas, and at least one electronic display. A first and second horizontal member extend between a vertically extending first and second side member and are secured within a first channel and a second channel. The first and second side members form exterior side surfaces of the display unit to define, in part, the closed loop pathway. The first horizontal member forms an upper surface of the display unit and the second horizontal member forms a lower surface of the display unit to further define, in part, the closed loop pathway.
Owner:MFG RESOURCES INT INC
Artificial surfing reef for affecting surface waves
The present invention is an artificial surfing reef for modifying a body of water's bathymetry to create surfing waves. The artificial surfing reef is a shaped structure made up of many sized and shaped Expanded Polystyrene Geo Foam blocks. As swell moves toward the shore, its bottom is resisted by the leading edge of the Expanded Polystyrene Geo Foam artificial surfing reef. As the swell transverses over the Expanded Polystyrene Geo Foam Artificial reef, the wave topples over and forms a plunging surfing wave. An earth anchor, connected to a cable, linked to a round shaped anchor disc, is attached to each Expanded Polystyrene Geo Foam block for maintaining the foam artificial reef at a desired location submersed underwater. A plurality of Expanded Polystyrene Geo Foam blocks may be attached to one another and configured so as to define a surf reef, which enhances the suitability of waves for surfing.
Owner:BENNETT WALTER JUDSON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com