Patents
Literature
41 results about "High-dose radiation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
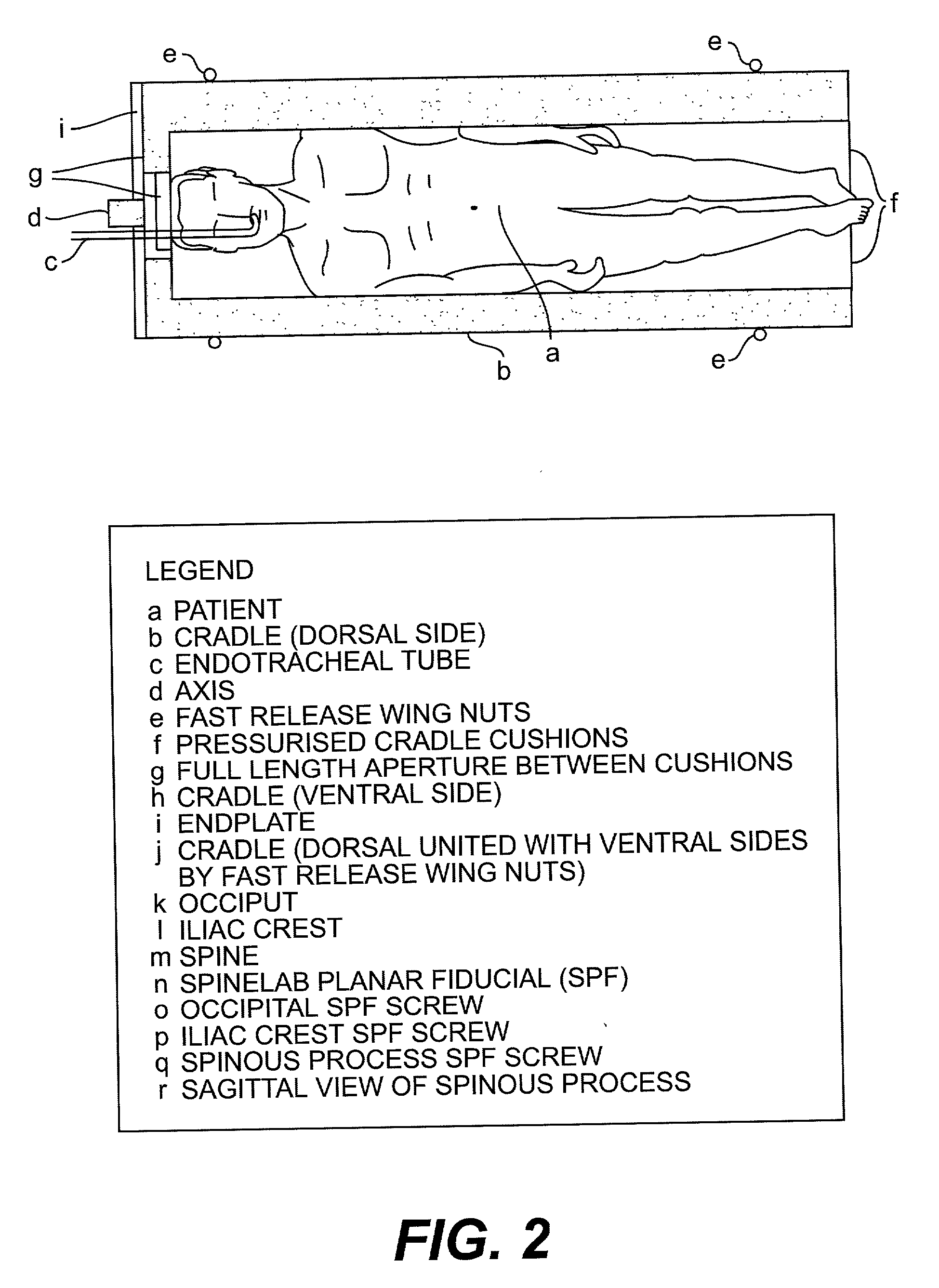
High-dose rate (HDR) brachytherapy is a type of internal radiation therapy that delivers radiation from implants placed close to, or inside, the tumor(s) in the body.
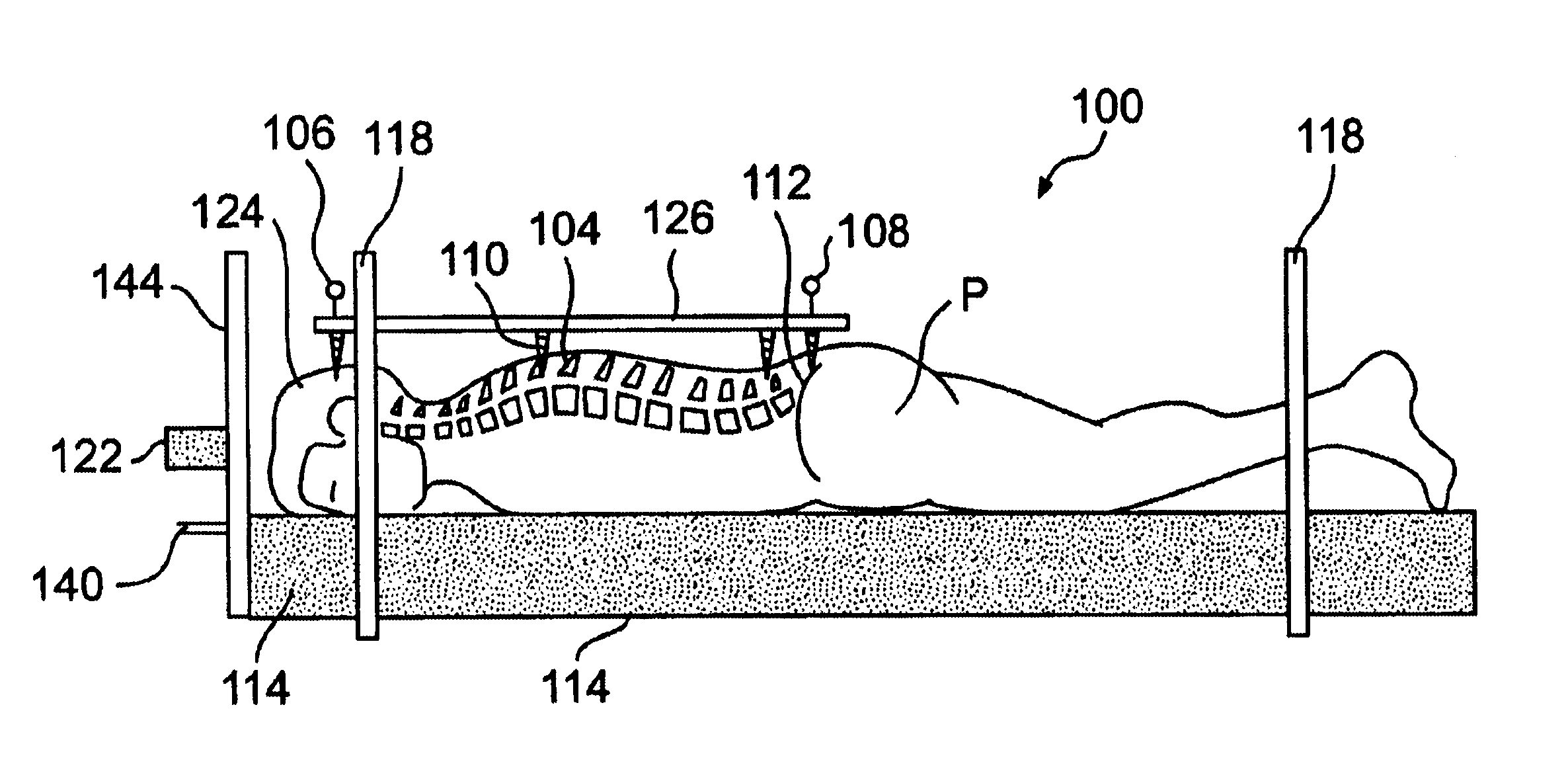
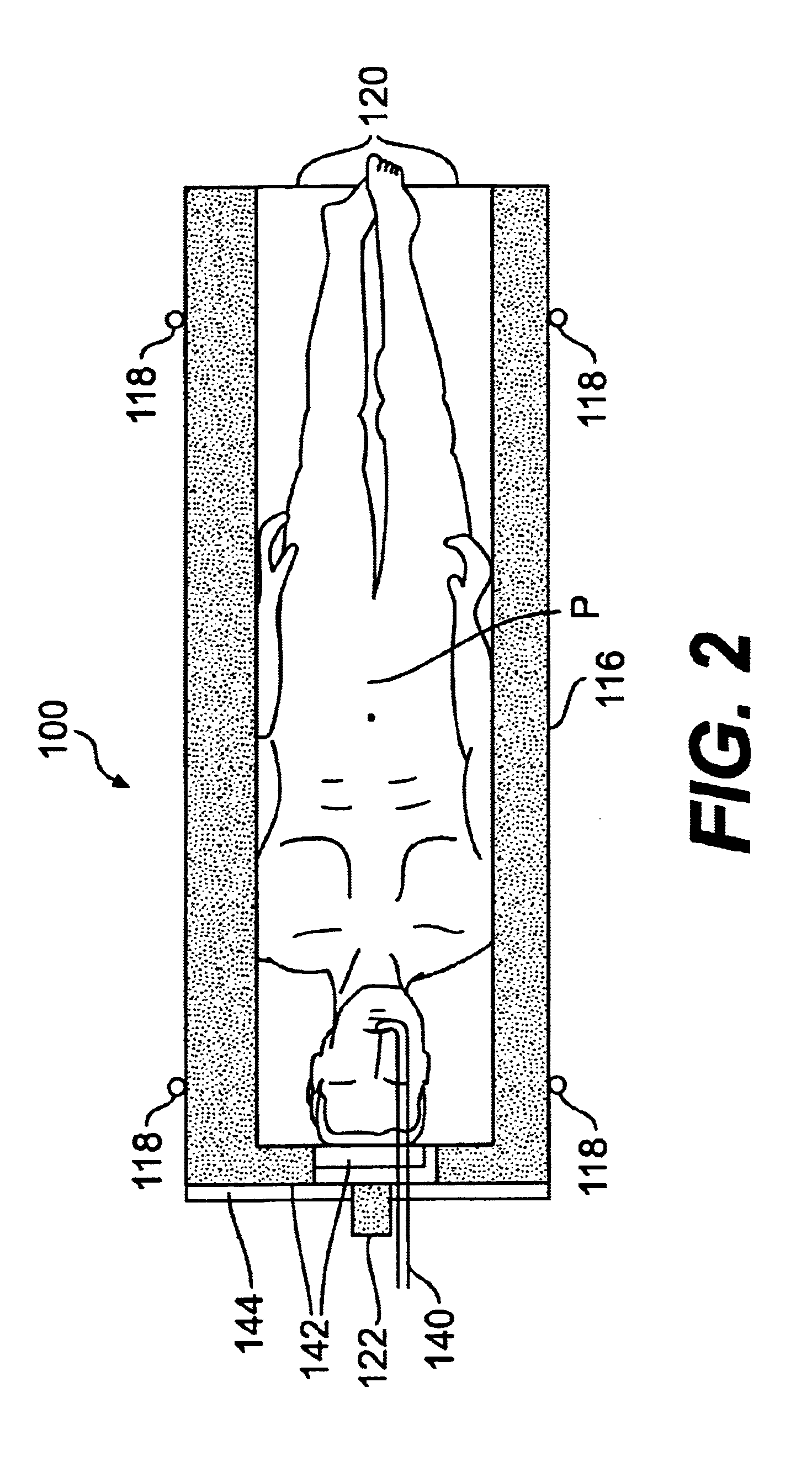
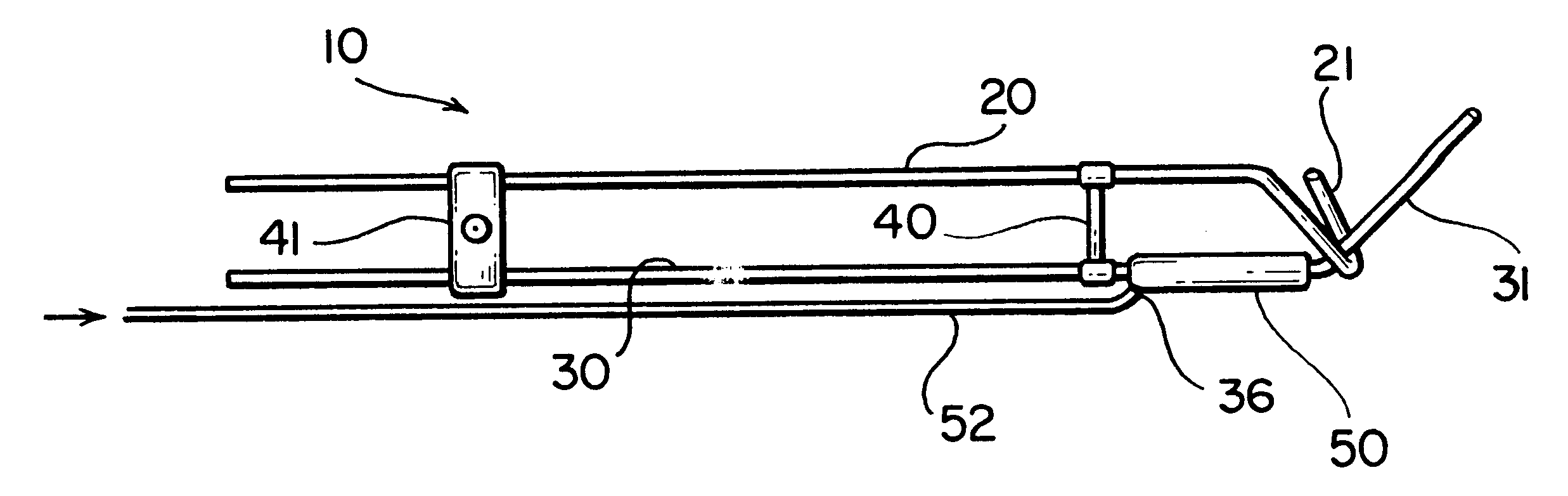
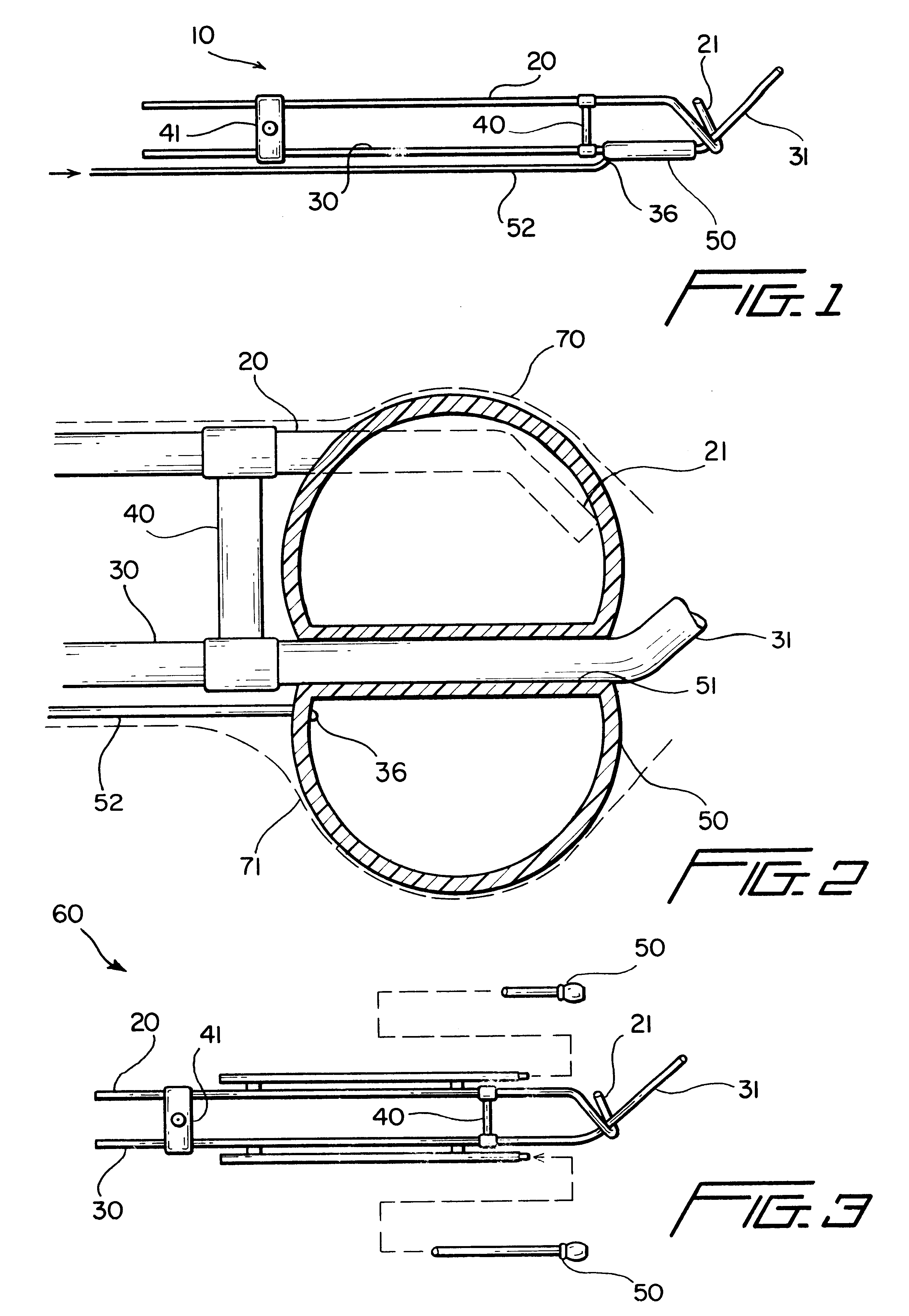
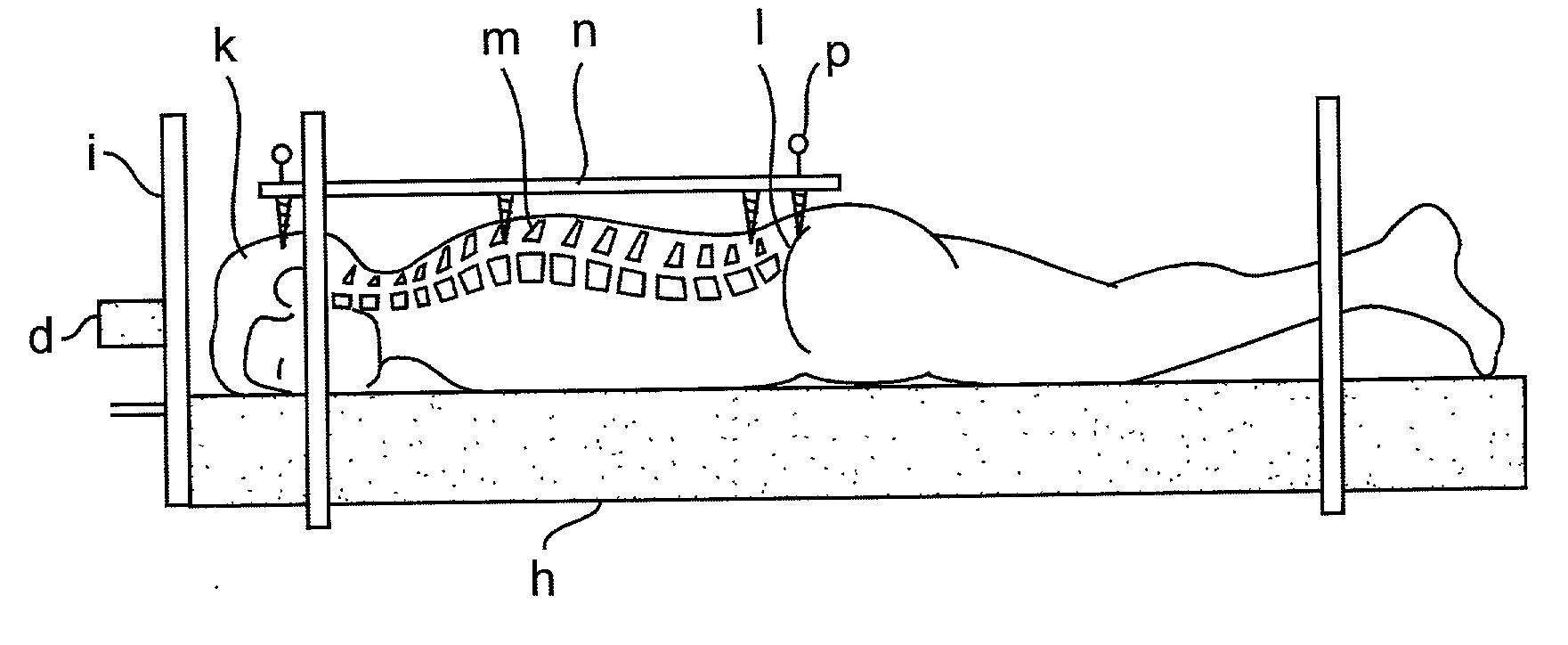
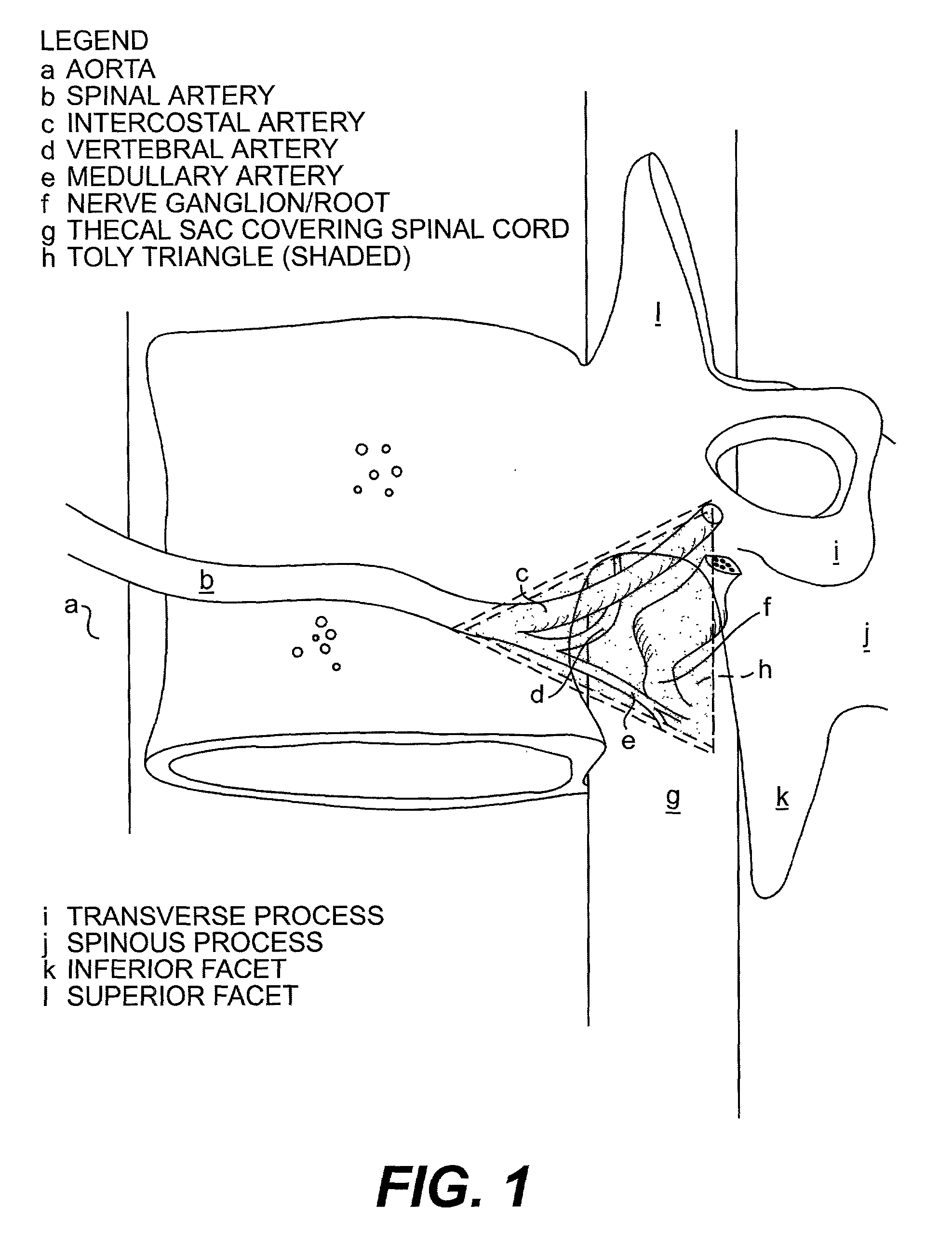
Radiosurgery methods that utilize stereotactic methods to precisely deliver high dosages of radiation especially to the spine
InactiveUS6665555B2Reduce decreaseAvoiding and minimizing deliveryRadiation diagnostic clinical applicationsSurgeryRadiosurgeryStereotaxis
Owner:GEORGETOWN UNIV
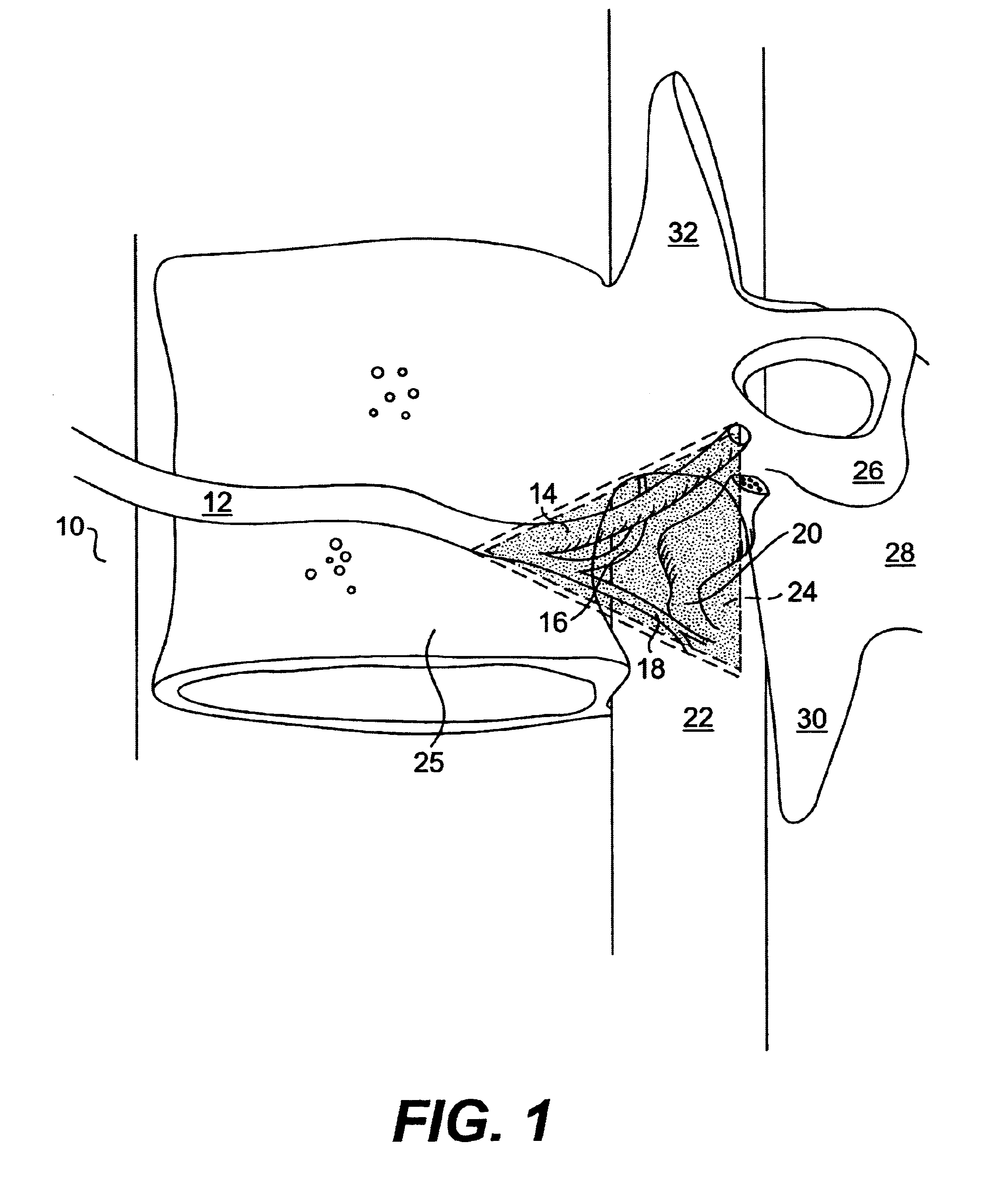
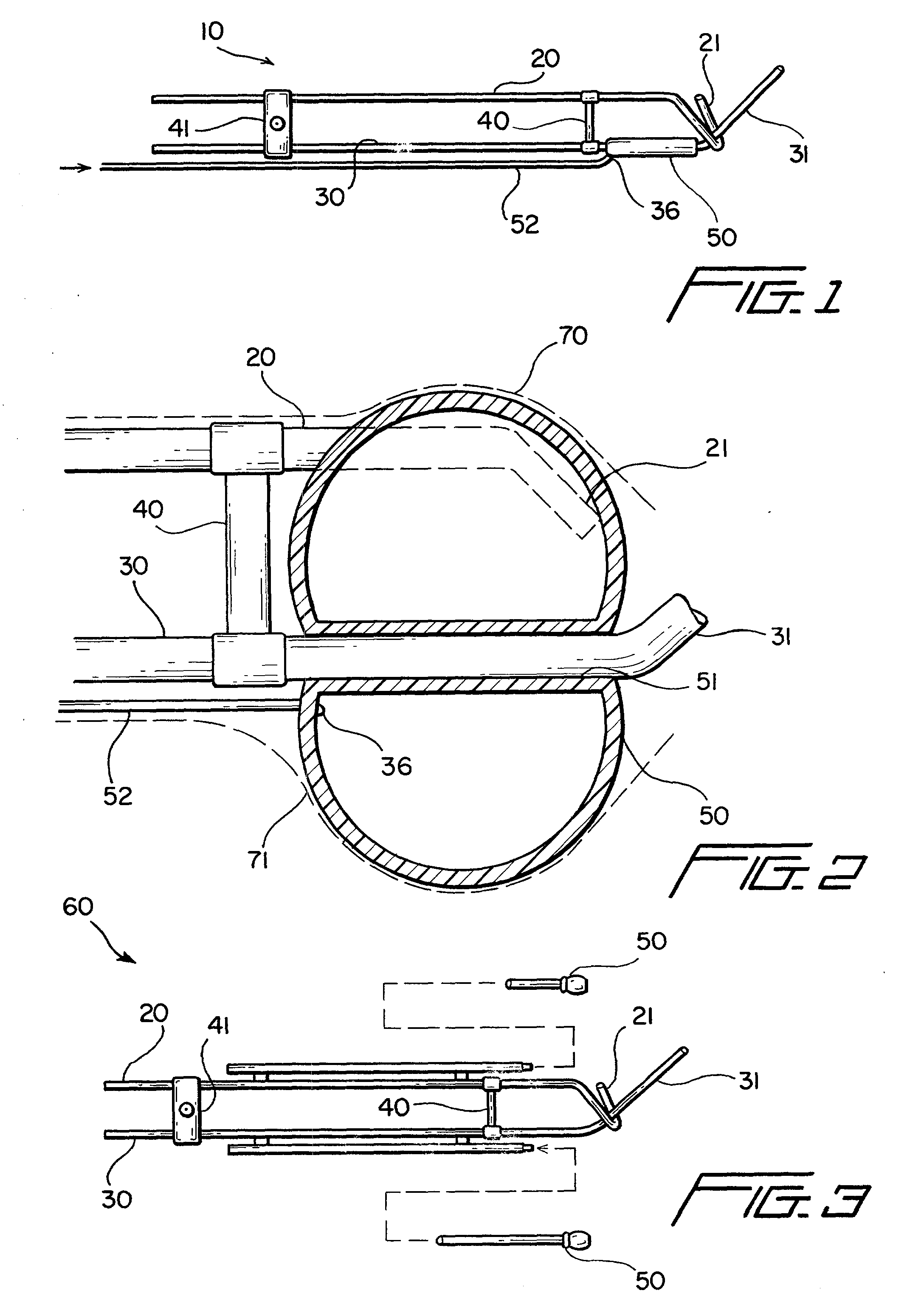
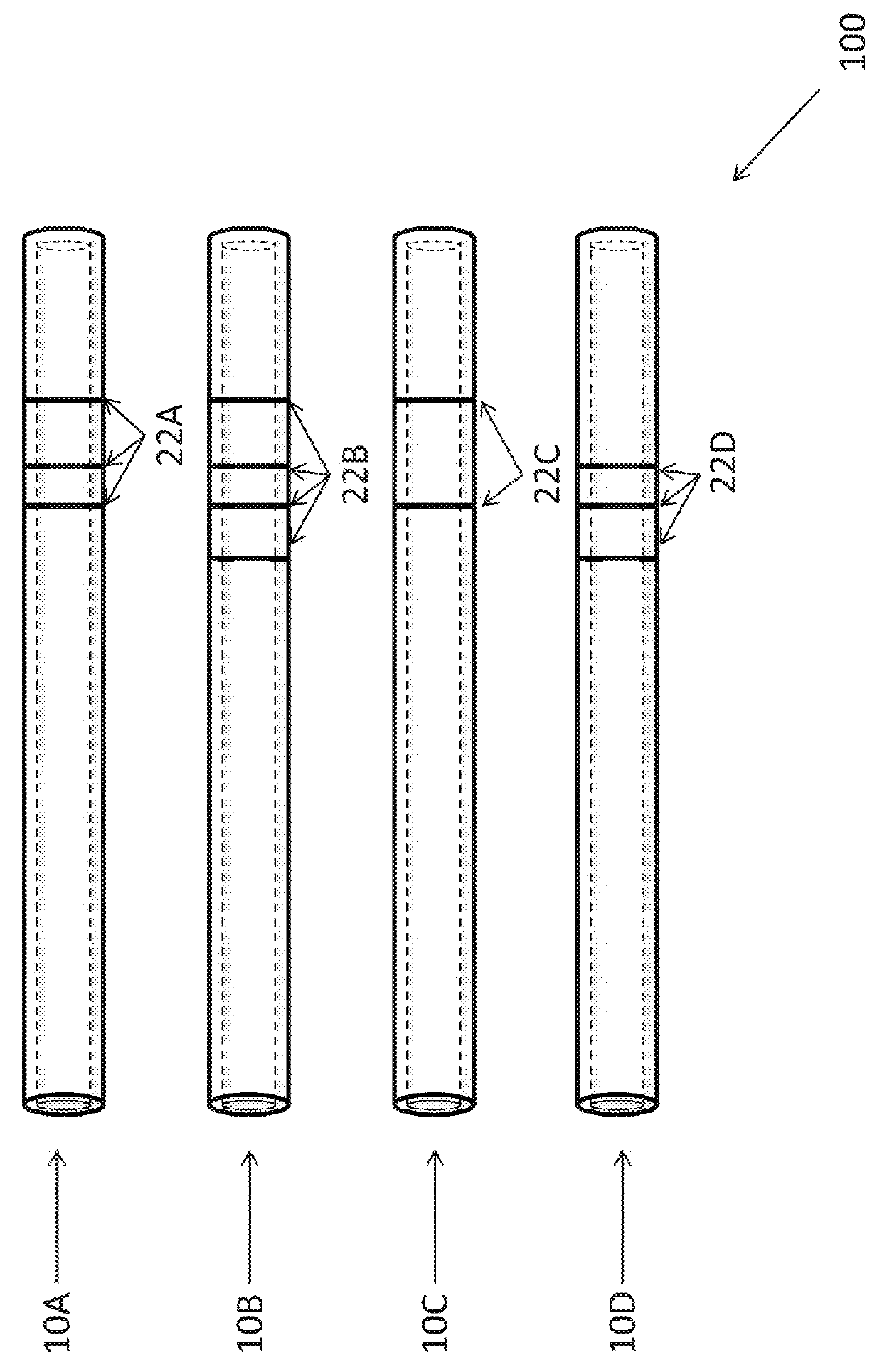
Cervical applicator for high dose radiation brachytherapy
InactiveUS20030153803A1Highly efficaciousImprove efficiencyMedical devicesX-ray/gamma-ray/particle-irradiation therapyBrachytherapyVaginal canal
A modified Fletcher-Suit tandem tube applicator includes a balloon which can be inflated to both positionally secure the applicator within the vaginal canal and to distend the confronting vaginal wall thereby increasing the distance of such tissue from the radioactive source contained in the tandem tube of applicator and correspondingly reducing radiation damage to nearby tissues and organs such as the rectum and bladder.
Owner:PAXTON EQUITIES
Prostatic hormonal implants treatment of prostate cancer
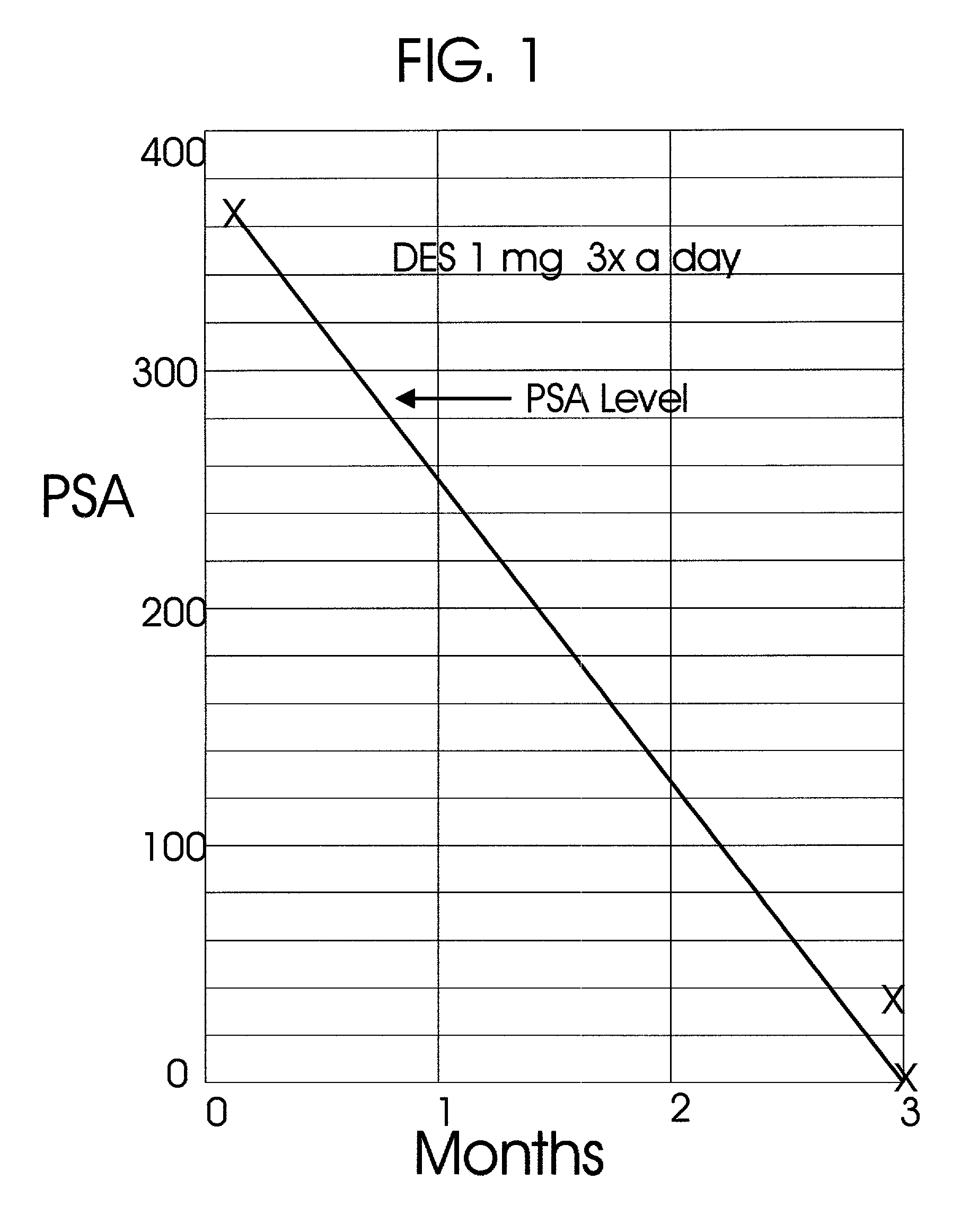
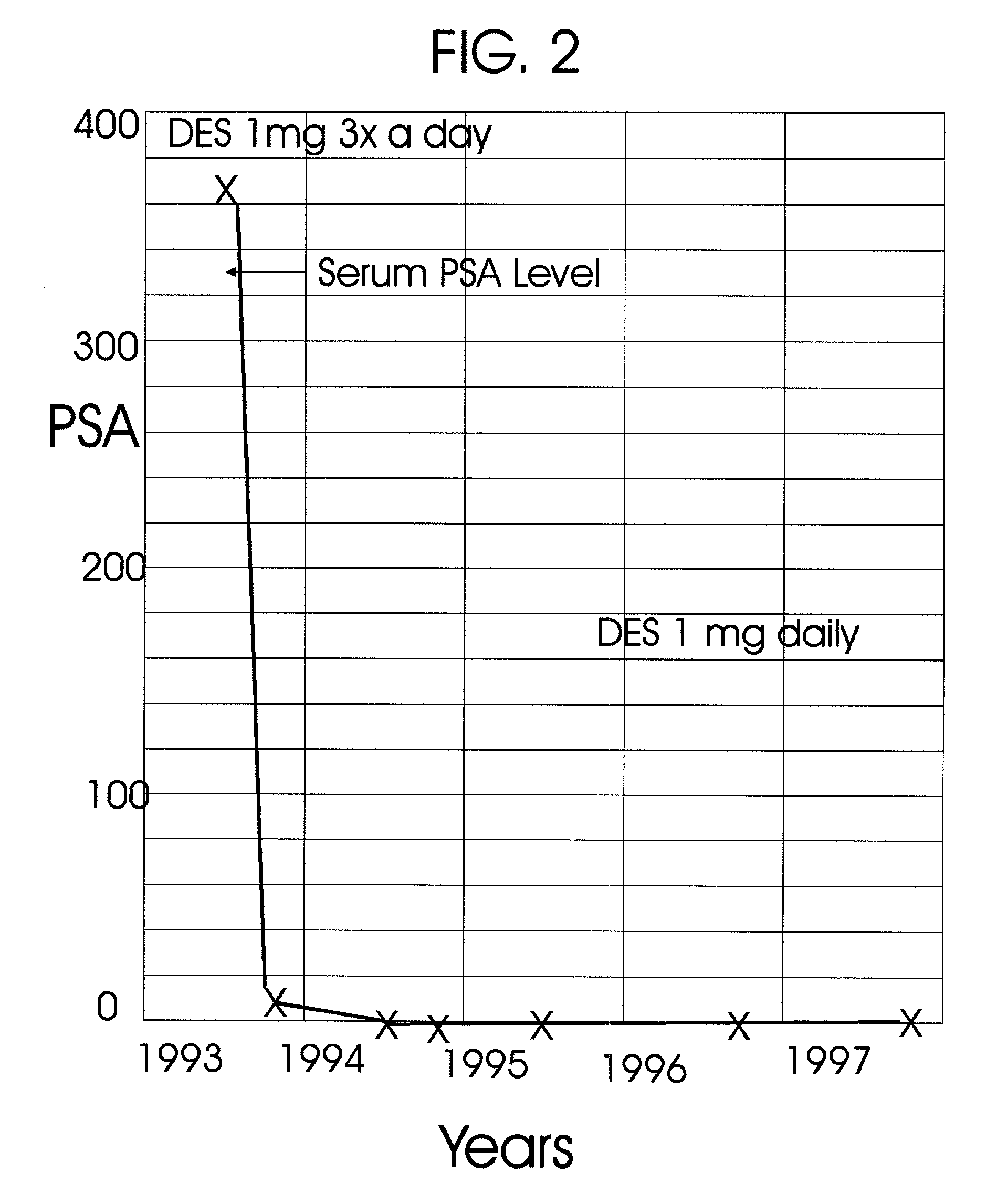
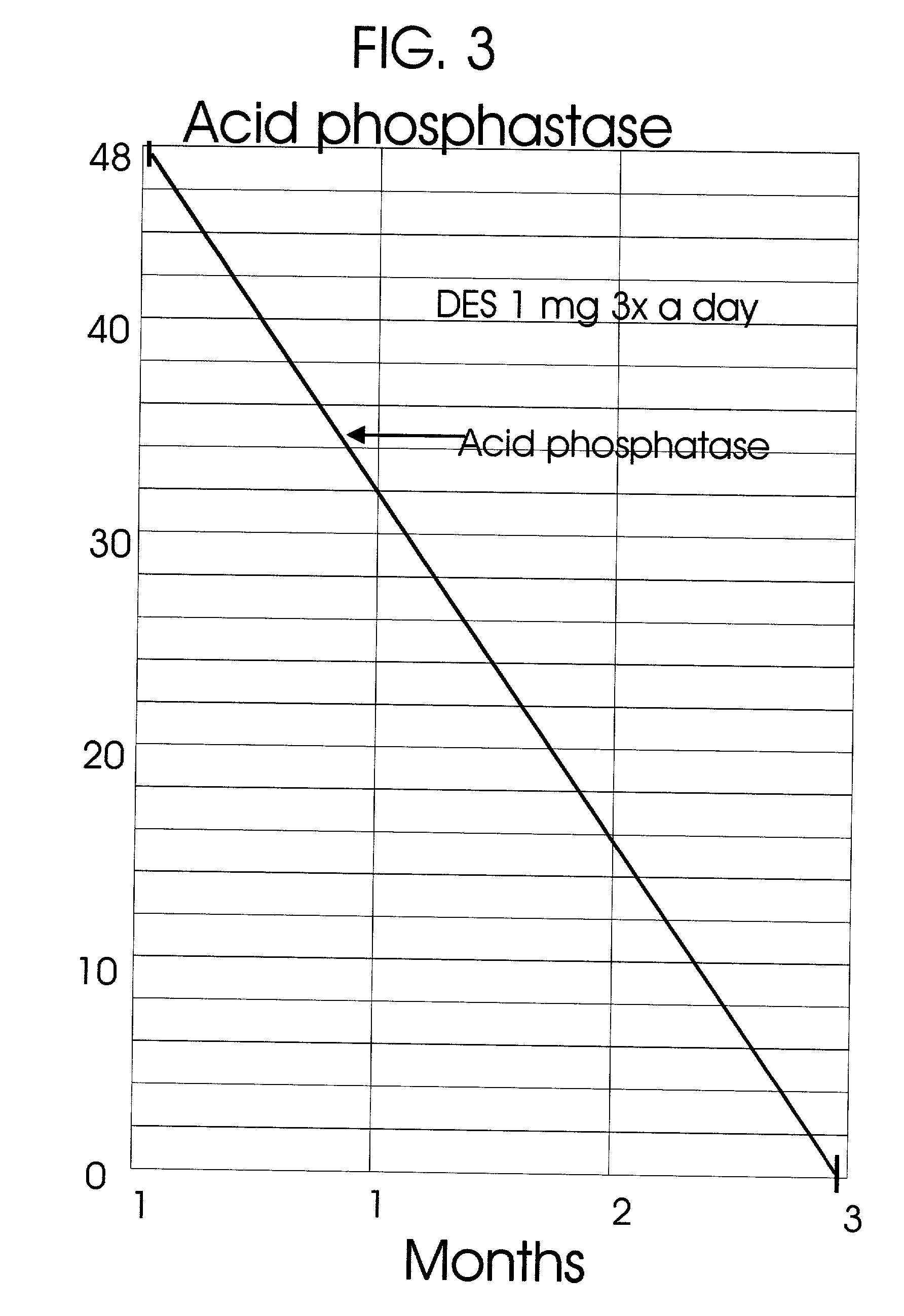
An improved method and products for the primary hormonal treatment of early stage, low and intermediate risk prostate cancers by prostatic implants of androgen suppressive drugs formulated as fused with a lipoid carrier or encapsulated in microcapsules or in Silastic capsules is provided. Such prostatic implants renders a constant slow-release of their contents to the prostate for extended periods by biodegradation and diffusion. It facilitates higher prostatic and lower systemic concentrations of androgen suppressive hormones. Because of their high prostatic and lower systemic concentrations, tumor control is much improved and the their systemic toxicity is minimized. Tumor control after such primary hormonal implant treatment is followed by clinical examinations and the biochemical tumor control is followed by periodic estimations of serum levels of PSA and acid phosphatase. More complex and expensive surgery or radiation therapy for this group of good prognostic early stage prostate cancer is reserved for those patients failing to this primary hormonal treatment. It will preserve potency more than by surgery or radiation therapy. Furthermore, it would reduce the cost of treatment for early stage prostate cancer significantly. Androgen suppressive hormonal implants to the prostate before, during or after lower dose conventional radiation therapy would also facilitate equal or better cure rates of localized prostate cancer as compared to the more complex and toxic higher dose radiation therapy.
Owner:SAHADEVAN VELAYUDHAN
Cervical applicator for high dose radiation brachytherapy
InactiveUS6699171B2Reduce deliveryMinimizing patient discomfortMedical devicesX-ray/gamma-ray/particle-irradiation therapyVaginal wallBrachytherapy
A modified Fletcher-Suit tandem tube applicator includes a balloon which can be inflated to both positionally secure the applicator within the vaginal canal and to distend the confronting vaginal wall thereby increasing the distance of such tissue from the radioactive source contained in the tandem tube of applicator and correspondingly reducing radiation damage to nearby tissues and organs such as the rectum and bladder.
Owner:PAXTON EQUITIES
Novel radiosurgery methods that utilize stereotactic methods to precisely deliver high dosages of radiation especially to the spine
InactiveUS20020032378A1Reduce decreaseAvoiding and minimizing deliveryRadiation diagnostic clinical applicationsSurgeryRadiosurgeryStereotaxis
A system for delivery of high dosage of radiation to a targeted spinal area is provided. This is accomplished by a system which provides for precise immobilization and positioning of the treated spinal area during dose planning and treatment via stereotactic radiosurgery. Advantages of the system include convenience to the patient, enhanced efficacy, and reduced risk of radiotoxicity to non-target tissues.
Owner:GEORGETOWN UNIV
Device and method for high dose per pulse radiotherapy with real time imaging
ActiveUS10603514B2Effective treatmentOptimize treatment planLight therapyX/gamma/cosmic radiation measurmentRadiation pulseHigh doses
A radiotherapy system comprising at least one pulsed radiation source, at least one imaging system, a control system, and a synchronization system is disclosed. The pulsed radiation source deposits high dose radiation pulses to a target region inside the patient; simultaneously the imaging system is used to monitor the target region, synchronized by the synchronization system. The dose per radiation pulse is high enough to deposit, within few pulses, 1 Gy at a depth of at least 1 cm in water. At each irradiation time step, the pulsed radiation source delivers short pulses of radiation (<1 ms) and the imaging system performs a snapshot of the position, and eventually the shape, of the target region during the irradiation time, with a time resolution better than 200 ms. Being both the pulsed radiation source and imaging system synchronized by the synchronization system with less than 200 ms jitter, this system allows for very precise reconstruction of the map of the dose deposited into the target region.
Owner:FYZIKALNI USTAV AV CR V V I
Device and Methods for Broadbeam and Microbeam Chemo-Radiosurgery Combined with Its Tumor Exosome Apheresis
InactiveUS20170368373A1Breathing protectionOther blood circulation devicesImmune complex depositionAtm kinase
Conventional single fraction 20-Gy broadbeam photonbeam or protonbeam chemo-radiosurgery does not sterilize EMT-MET cancer stem cell radiodurans but single fraction 100 to 10,000 Gy microbeam radiosurgery sterilizes them. Device and methods for microbeam chemo-radiosurgery including 250 MeV wakefield electronbeam is disclosed.Surgery, chemotherapy and broadbeam and microbeam radiosurgery releases billions of abscopal metastasis causing, tumor specific plasma soluble proteins, cell membranes, apoptotic bodies, DNA and RNAs, exosomes like telomere-telomerase, ATM-ATM kinase and others. They and adaptive resistance to chemo-radiosurgery, paraneoplastic and non-paraneoplastic diseases causing immune complexes are removed by pulse flow combined continuous flow ultracentrifugation apheresis and immune affinity chromatography. Chemotherapy and high dose radiation exposed tumor cells and their exosomes are made sensitive to telomerase inhibiting and apoptosis inducing and least toxic epigallocatechin and to heparin bound receptors. They convert triple negative breast tumors into receptor positive tumors which open new avenues for treating most aggressive breast cancers.
Owner:SAHADEVAN VELAYUDHAN
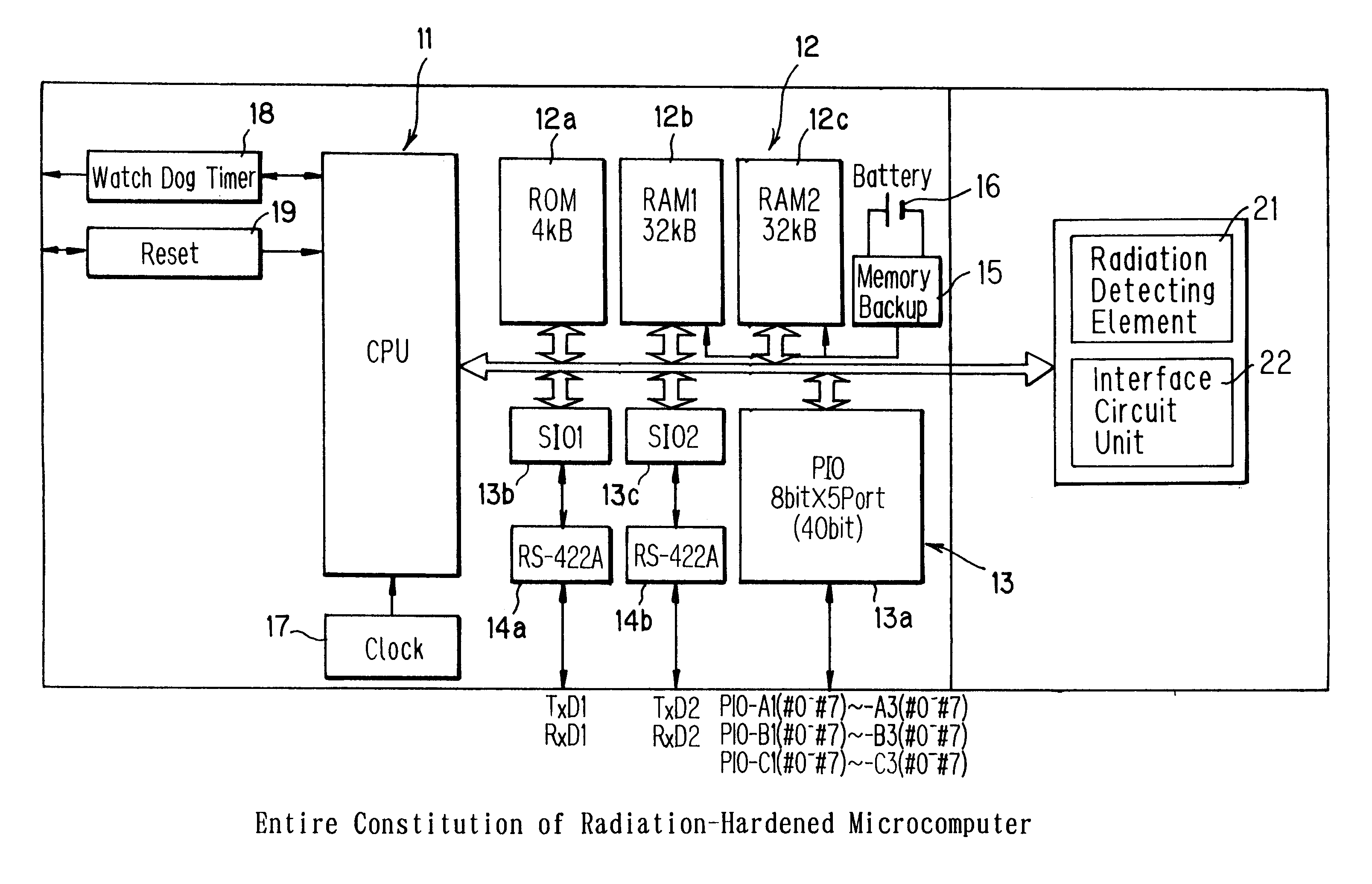
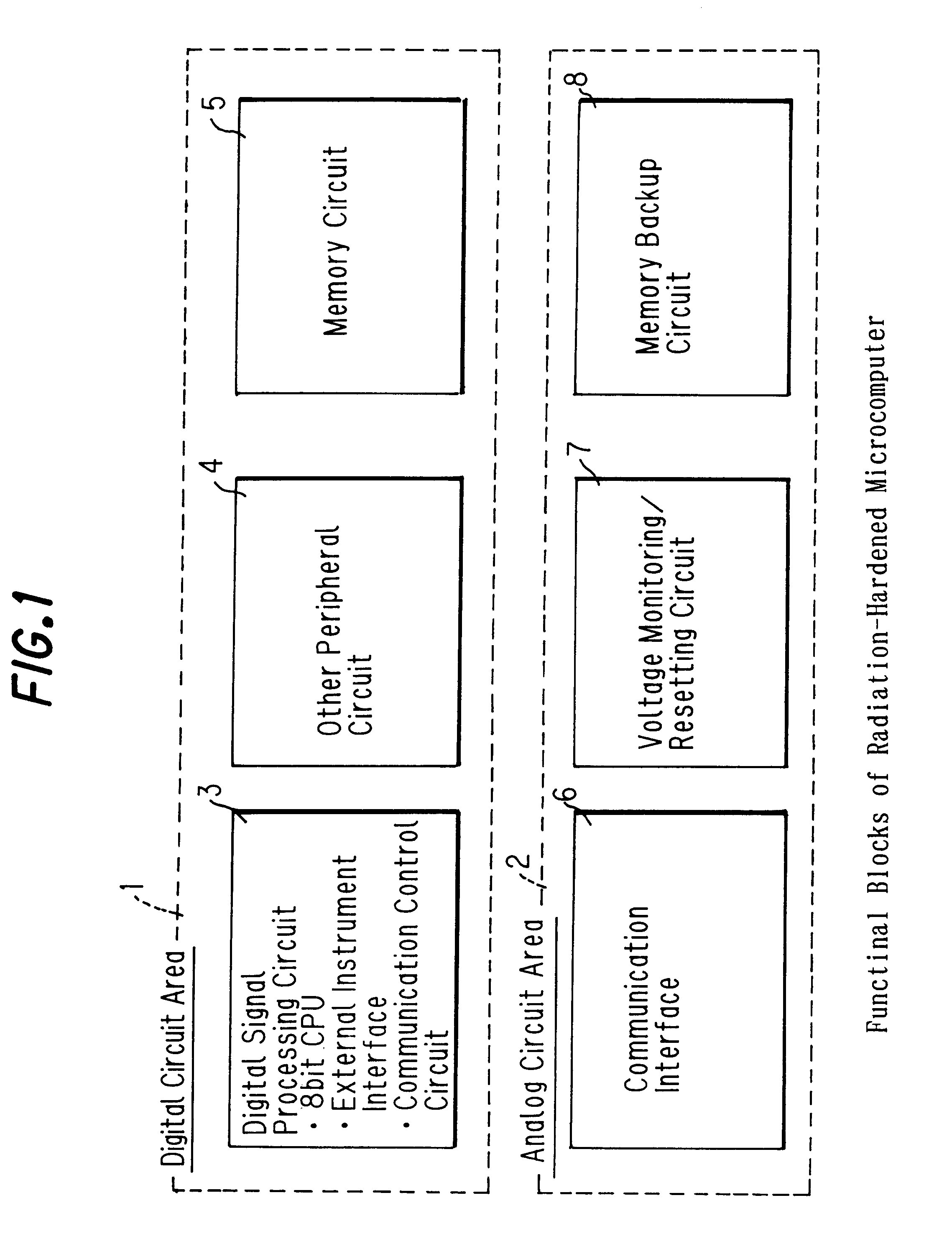
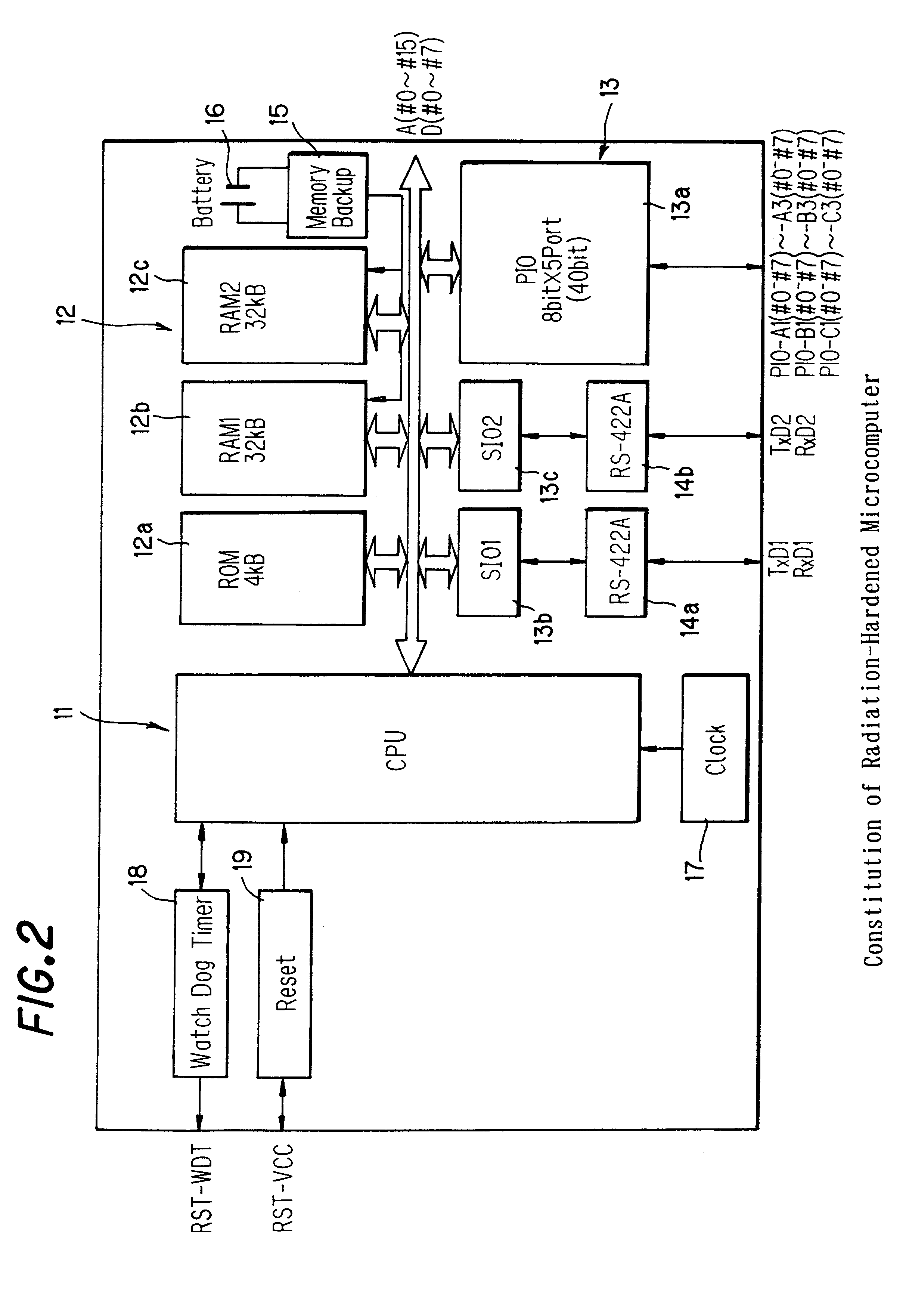
Microcomputer and its access speed control method
A microcomputer usable for a long period of time even when disposed in a high dose radiation-exposed environment, and its access speed control method are provided. According to the microcomputer and the method, the total dose of radiation that the microcomputer receives is determined on the basis of detection signals from a radiation detecting element. Based on the determined total dose of radiation, and table data preset by tests and stored into a memory unit, a CPU controls an access speed. Moreover, the CPU, and the memory unit and a circuit interface unit that access the CPU are integrated on a single chip (ASIC) These units on the same chip are deteriorated and changed in the same direction, without fail, on exposure to radiation.
Owner:MITSUBISHI HEAVY IND LTD
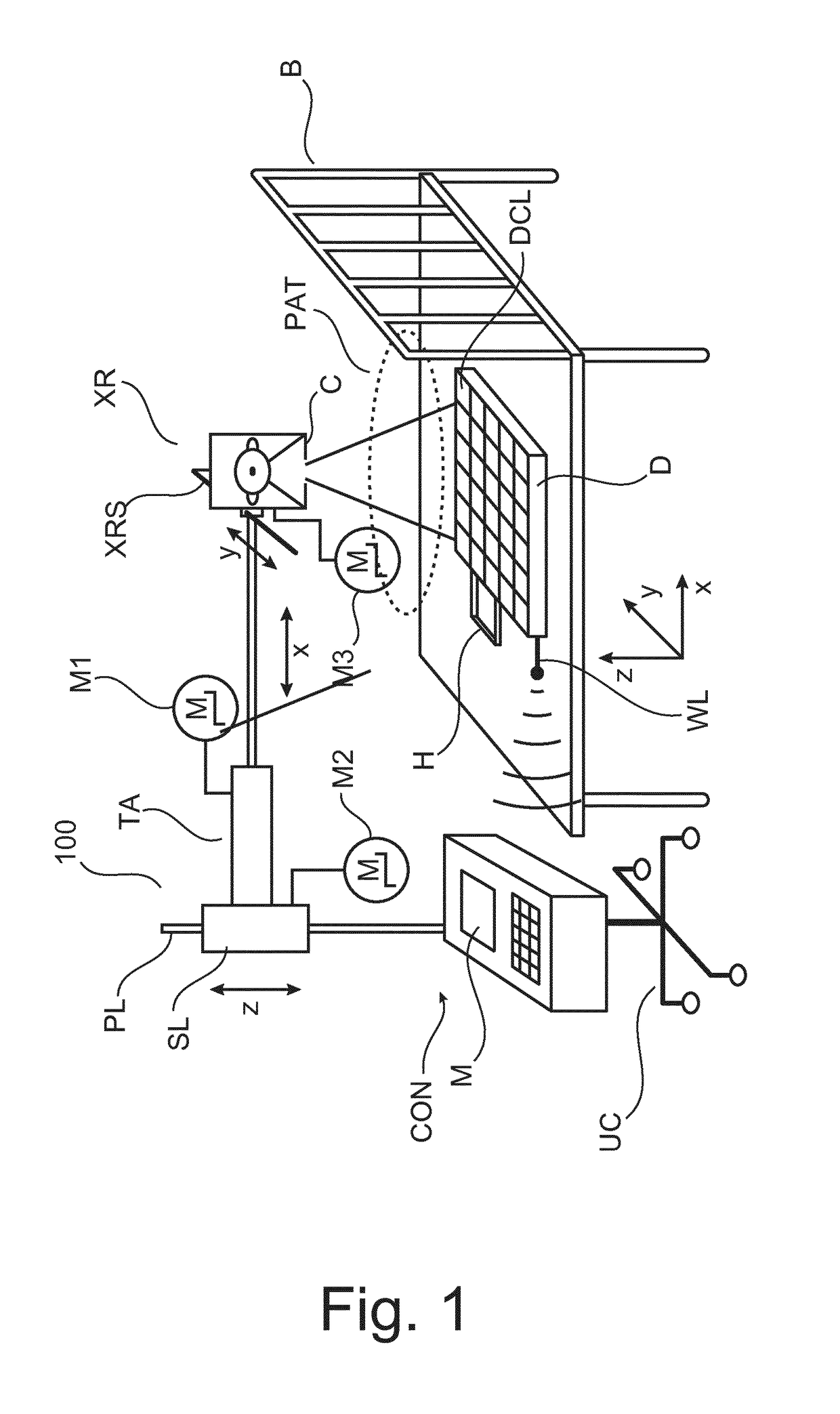
System and Method for Improved High Dose Radiation Therapy Treatment Planning
The present invention includes a catheter for providing contrast under magnetic resonance imaging (MRI). The catheter includes an elongated tubular member having a proximal end, a distal end and lumen. The catheter further includes a solution of saline and at least one contrast agent, where the solution is sealed within at least a portion of the catheter lumen.
Owner:RGT UNIV OF CALIFORNIA
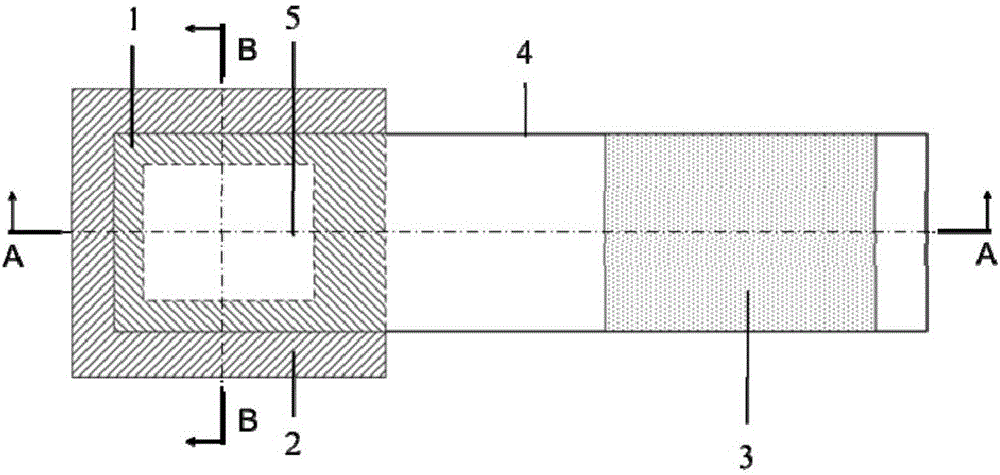
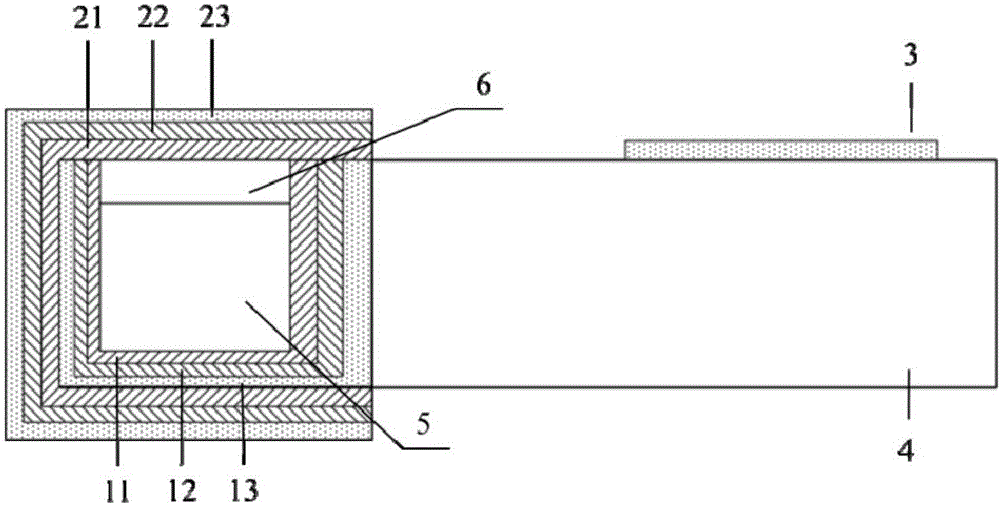
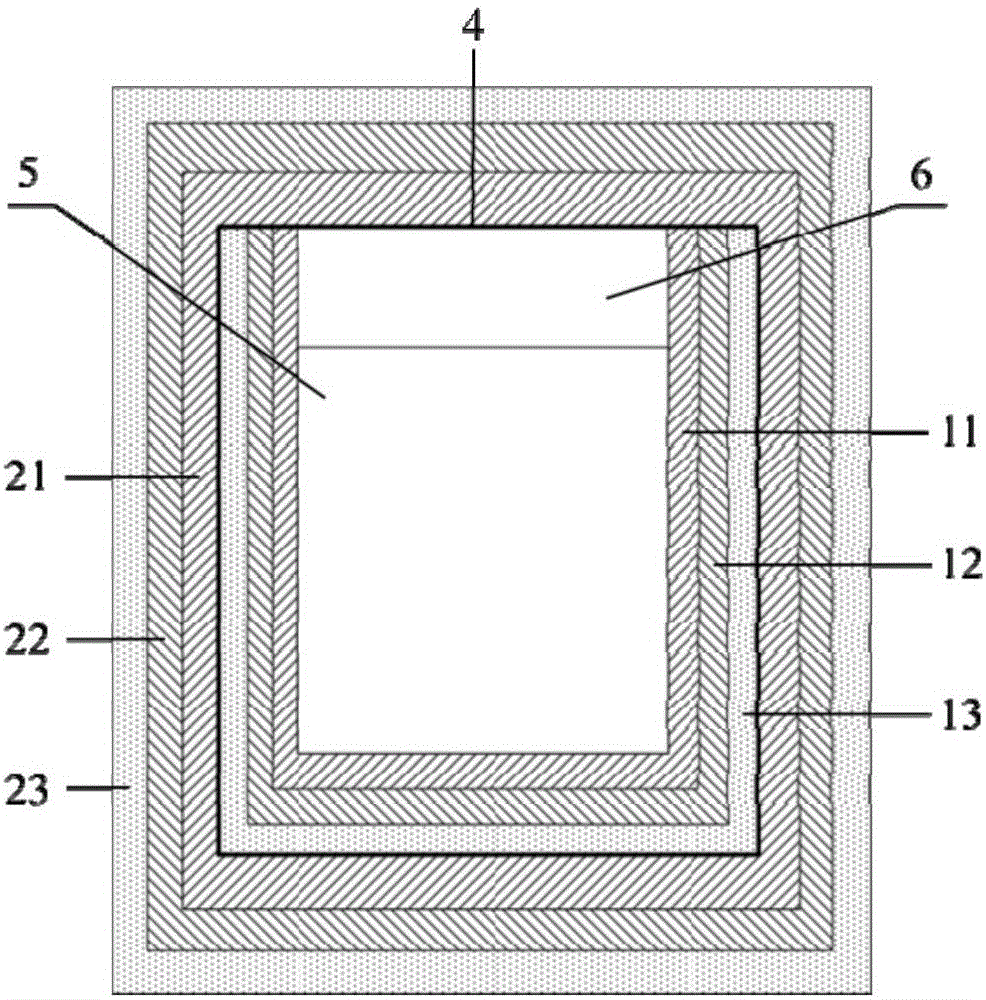
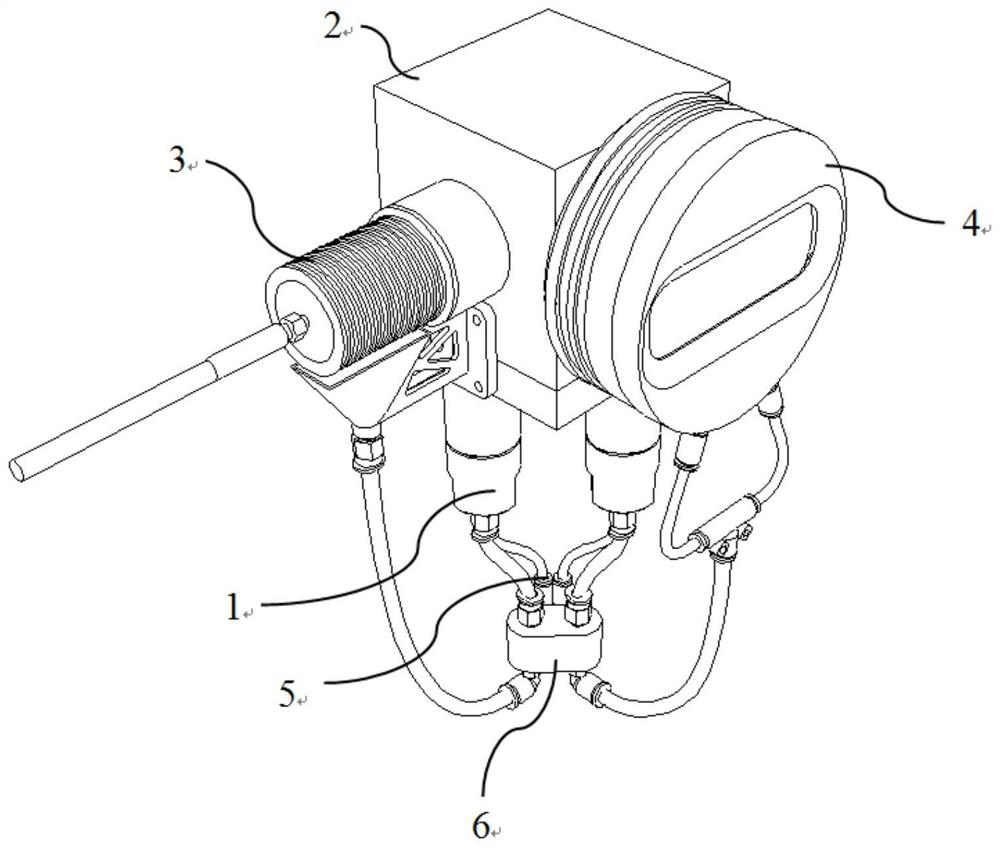
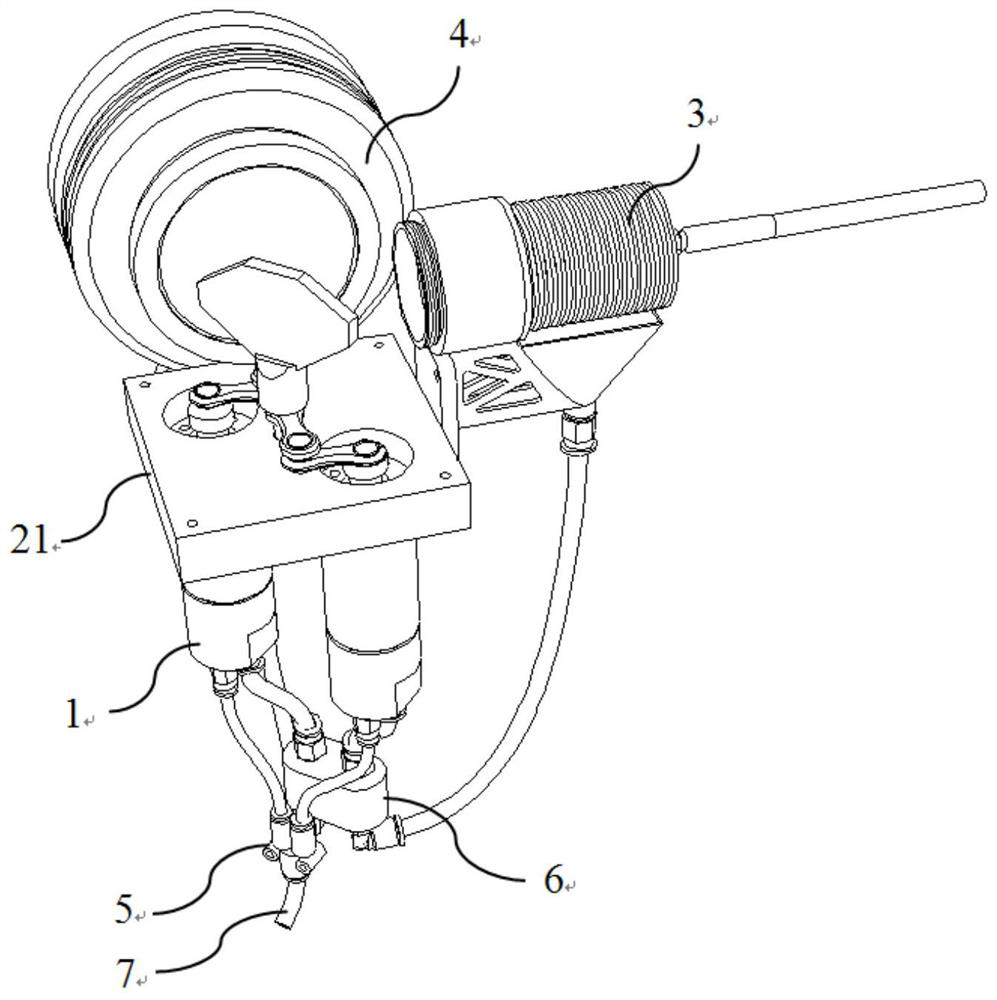
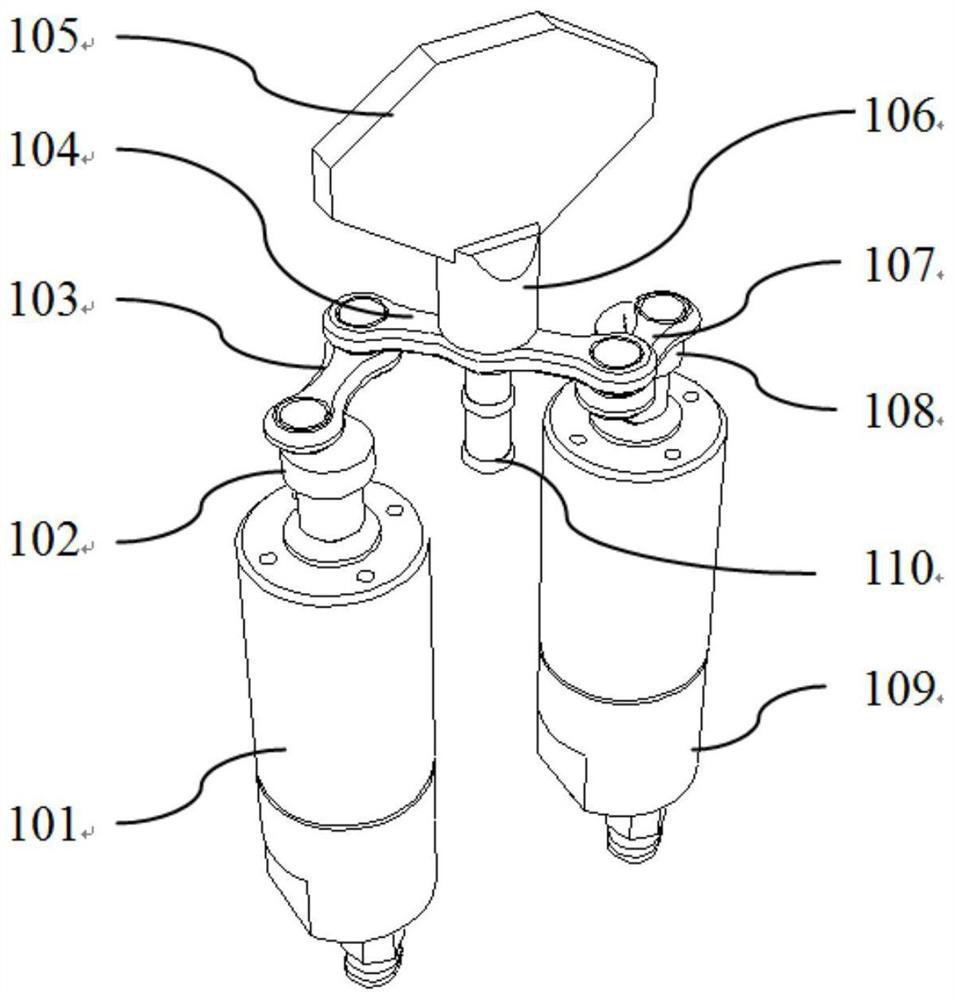
Movable box type combined shielding system
ActiveCN106782728ARealize the shielding effectAchieve protectionPortable shielded containersEngineeringRadioactive source
The invention discloses a movable box type combined shielding system which comprises a container, an in-box radiation shield, an out-box radiation shield and a photon shielding plate. A radioactive source generating device is arranged in the container; the space between the radioactive source generating device and the inner wall of the container is fixedly filled with the in-box radiation shield; the outer wall of the container is detachably covered with the out-box radiation shield; the photon shielding plate is arranged on the outer wall of the container in a sliding mode and used for sliding to an active area when the out-box radiation shielding body is detached to shield active area rays. According to the movable box type combined shielding system, the radioactive source generating device can run and be transported in the container; when the radioactive source generating device runs, the in-box radiation shield and the out-box radiation shield can be combined to shield high-dose radiation generated when the radioactive source generating device runs; when a container body is transported, the in-box radiation shield and the container body are detached, and the photon shielding plate moves to the active area to shield rays generated by neutron activation of a container active area structure material.
Owner:中科瑞华原子能源技术有限公司
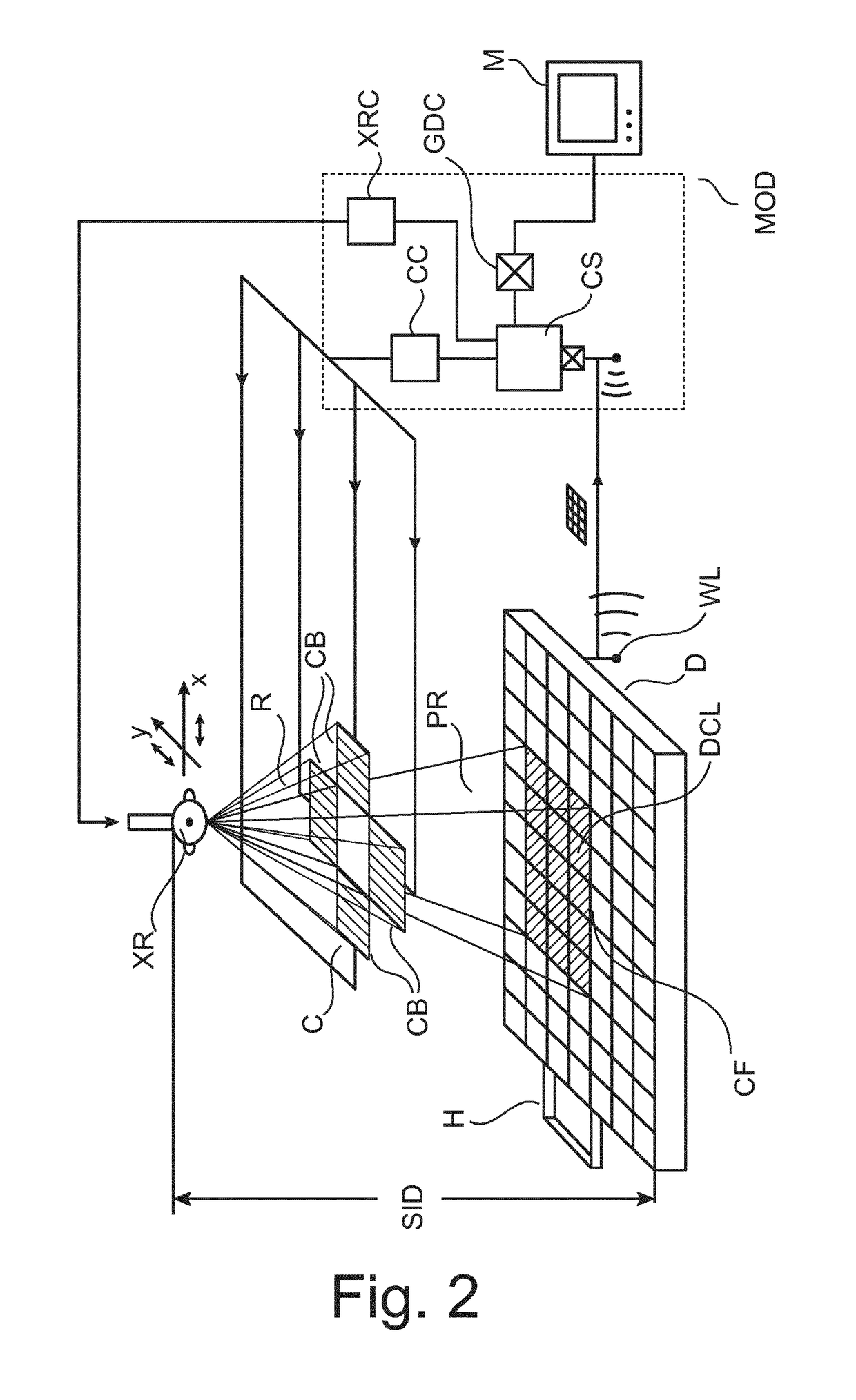
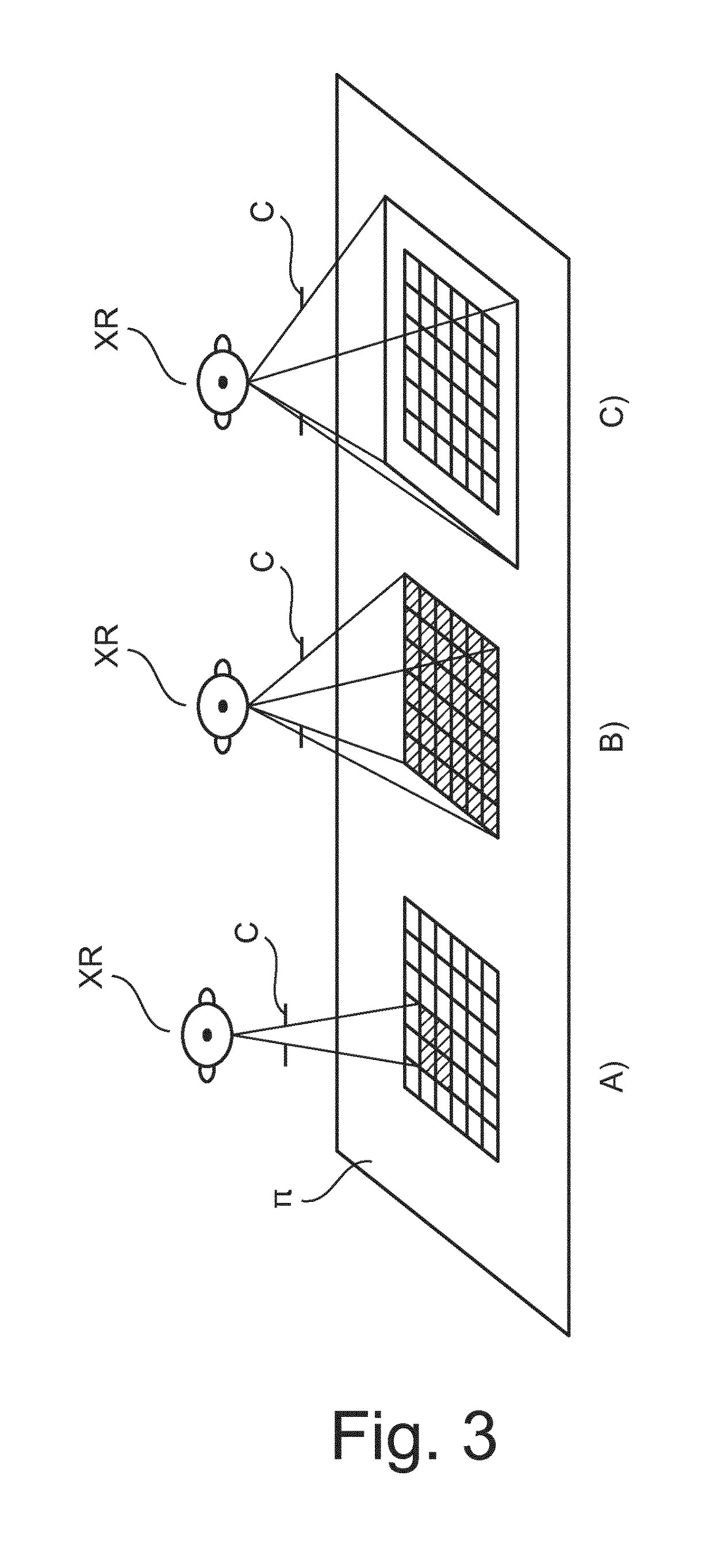
X-ray collimator size and position adjustment based on pre-shot
ActiveUS10136864B2Accurately determineIncrease doseTomographyDiaphragms for radiation diagnosticsX-rayHigh dosage
An X-ray apparatus for image acquisition and a related method. The apparatus comprises a field-of-view corrector (CS) configured to receive a scout image (SI) acquired by the imager with a tentative collimator setting in a pre-shot imaging phase where said imager operates with a low dosage radiation cone causing the detector to register the scout image. The low dosage cone has, in the detector's image plane, a first cross section smaller than the total area of the detector surface. The field-of-view corrector (CS) uses said scout image to establish field-of-view correction information for a subsequent imaging phase where the imager is to operate with a high dosage radiation cone, the high dosage higher than the low dosage.
Owner:KONINKLJIJKE PHILIPS NV
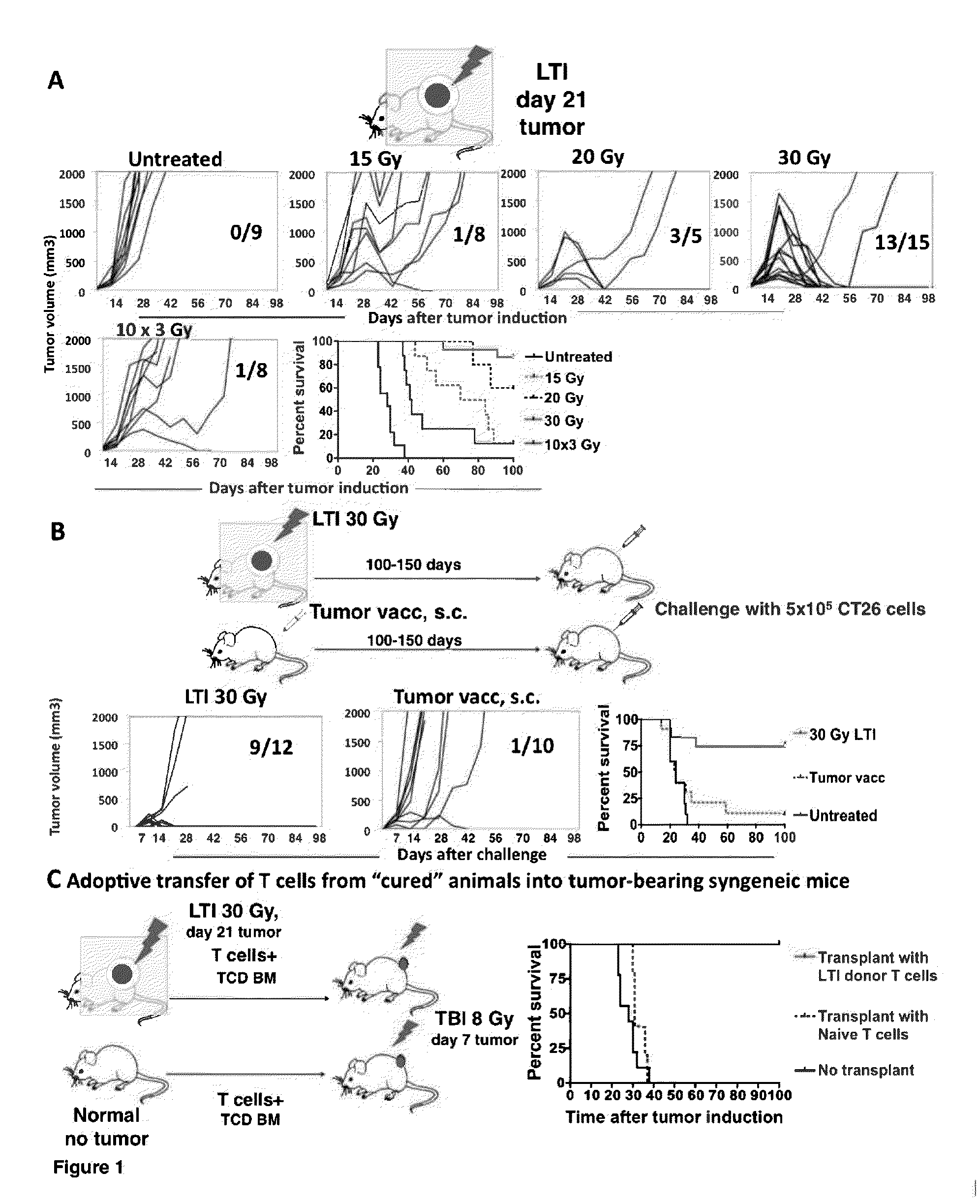
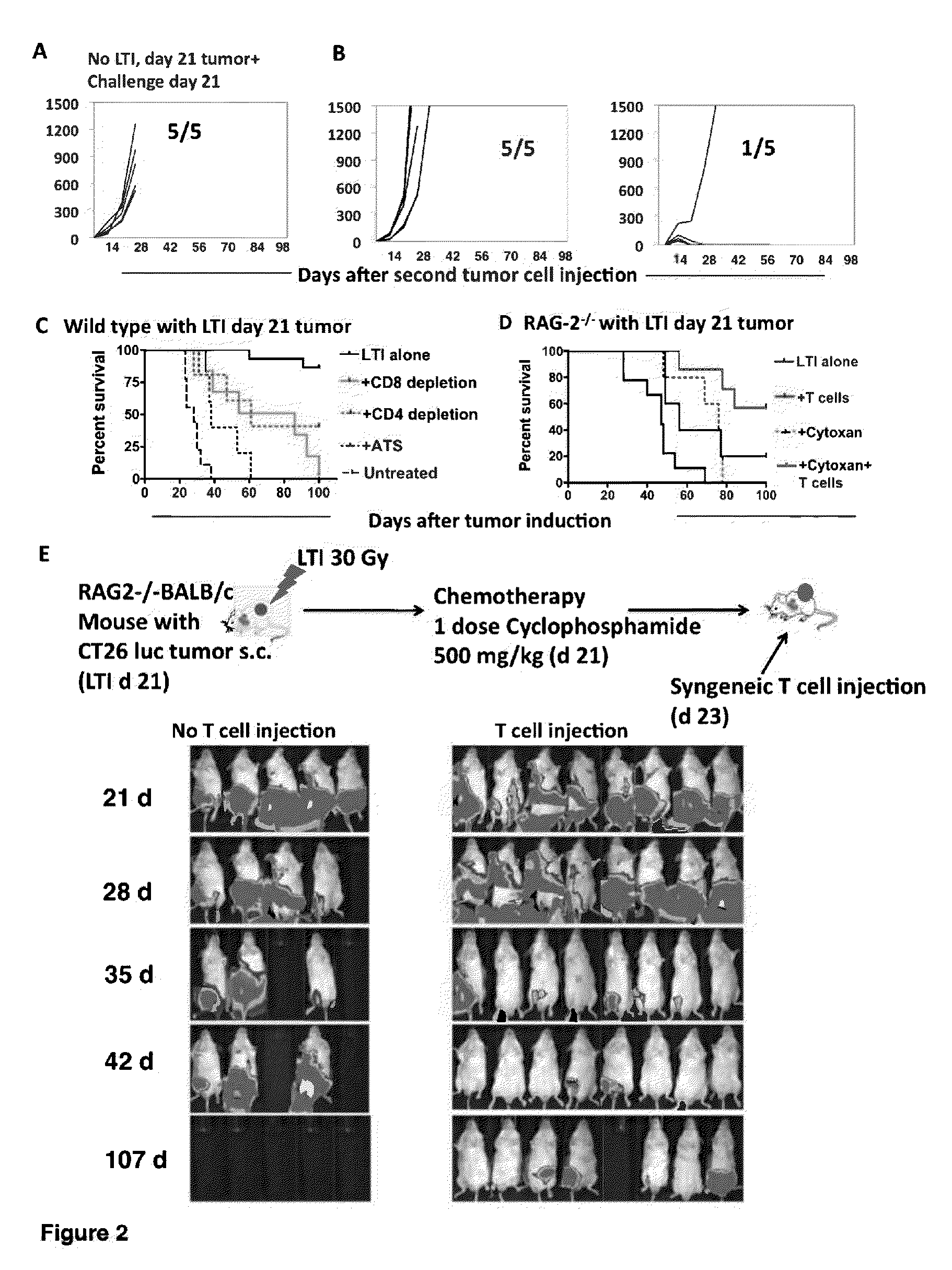
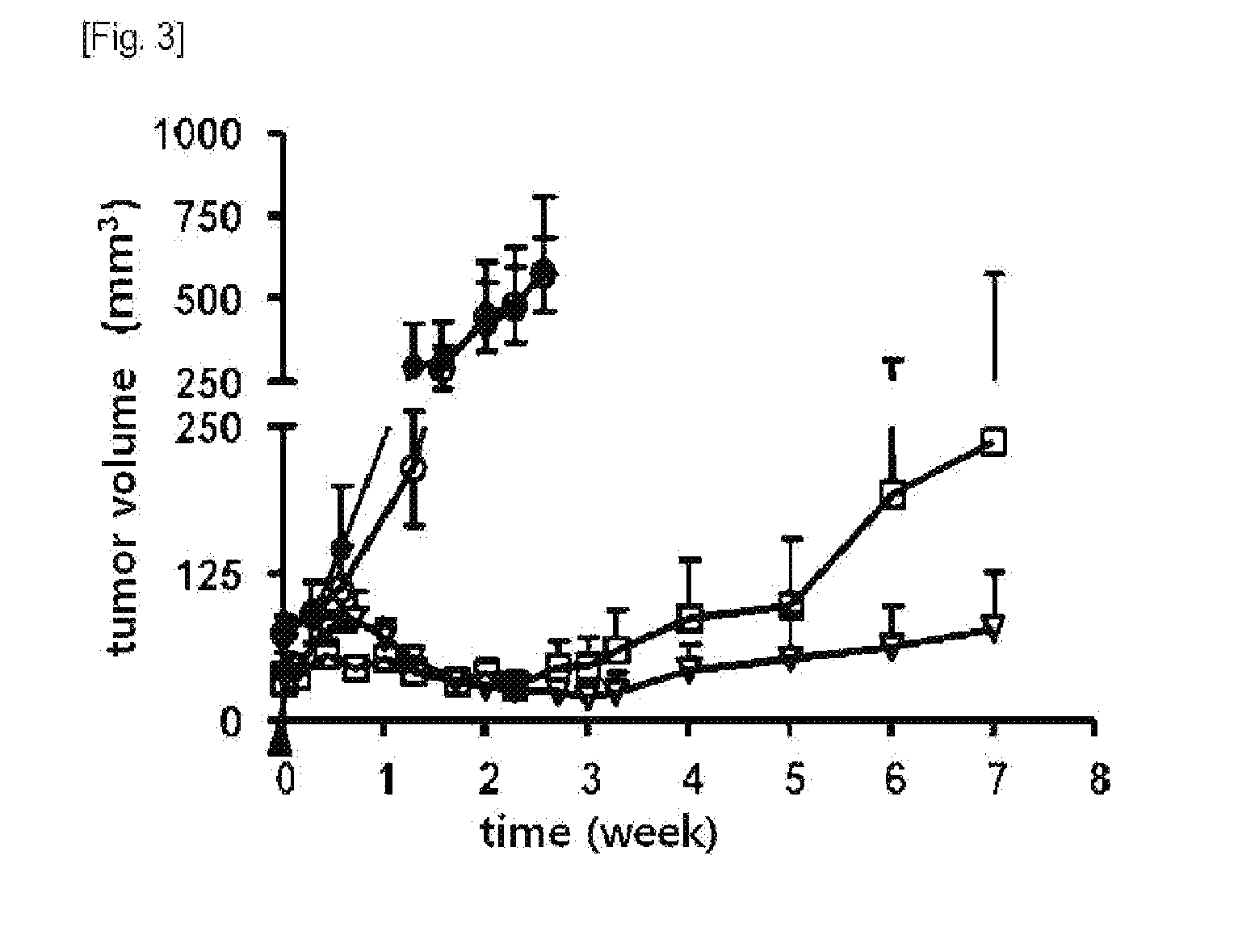
Anti-tumor T cell immunity induced by high dose radiation
ActiveUS9114157B2Growth inhibitionMammal material medical ingredientsBlood/immune system cellsTumor responseCancer therapy
Cancer treatment is provided, by irradiating an individual with a localized, high single dose or short course of doses at a primary tumor site; collecting T cells from the individual after a period of time sufficient activation of an anti-tumor response; treating the individual with an effective dose of dose of chemotherapy; and reintroducing the T cell population back to the individual.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
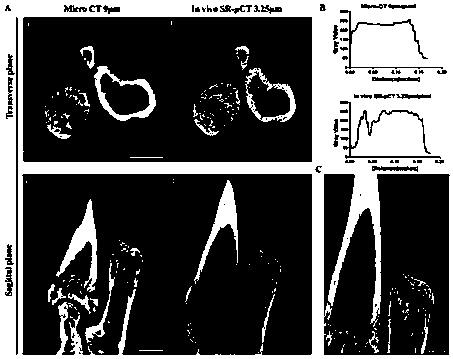
Synchrotron radiation X-ray microscopic CT suitable for imaging of living small animal limbs and fixing device
ActiveCN110353720AProtected SurvivalAvoid the effects of high-dose radiation exposurePatient positioning for diagnosticsComputerised tomographsSmall animalX-ray
The invention discloses a synchrotron radiation X-ray microscopic CT suitable for imaging of living small animal limbs and a fixing device. The fixing device comprises an objective table for an anesthetized small animal to be placed, a column fixed to the objective table and a lead cover which covers the objective table and seals the upper part and periphery of the objective table, a synchrotron radiation light source comprises the fixing device, the objective table of the fixing device is fixed to a rotating sample table of the synchrotron radiation X-ray microscopic CT, and moreover, the axis of a sleeve on the objective table is coaxially arranged with the rotating axis of the rotating sample table. The fixing device of the synchrotron radiation X-ray microscopic CT can be used for integrally and locally fixing the living small animal, and meanwhile, the influence of high-dose radiation on the trunk part outside the joints of the limbs can be avoided; the survival of the living small animal can be protected to the greatest extent, and long-term animal experiments can be conveniently carried out.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Rectal cancer radiotherapy resistance cell model and construction method therefor
InactiveCN106367391AThe number of exposures is lessScreening process is fastTumor/cancer cellsX-rayCell strain
A construction method of a cell model is disclosed, by which a rectal cancer radiotherapy resistant cell strain is obtained in a manner of gradiently increasing radiation dose of X-ray, wherein the cell strain is sourced from an SW480 cell and the resistance of the resistant cell under high radiation dose and low radiation dose are both higher than those of the parent SW480 cell strain.
Owner:李懿
Method for removing high-concentration dimethyl phthalate through radiation
InactiveCN102219280ATo achieve the purpose of degradationStrong penetrating powerWater/sewage treatment by irradiationHigh concentrationHigh energy
The invention discloses a method for removing high-concentration dimethyl phthalate through radiation. In the method, the dimethyl phthalate at the concentration of 300 to 5,000 mg / L is subjected to high-dose radiation treatment by high-energy rays; and then solid substances are separated and removed from the solution. By the method, the removal rate of the dimethyl phthalate is over 95 percent; radiation degradation products can be removed directly; chemical aids are not required; and energy is saved.
Owner:NANJING UNIV
Device and method for high dose per pulse radiotherapy with real time imaging
ActiveUS20190329071A1Optimize treatment planEffective treatmentLight therapyX/gamma/cosmic radiation measurmentHigh dosesRadiation pulse
A radiotherapy system comprising at least one pulsed radiation source, at least one imaging system, a control system, and a synchronization system is disclosed. The pulsed radiation source deposits high dose radiation pulses to a target region inside the patient; simultaneously the imaging system is used to monitor the target region, synchronized by the synchronization system. The dose per radiation pulse is high enough to deposit, within few pulses, 1 Gy at a depth of at least 1 cm in water. At each irradiation time step, the pulsed radiation source delivers short pulses of radiation (<1 ms) and the imaging system performs a snapshot of the position, and eventually the shape, of the target region during the irradiation time, with a time resolution better than 200 ms. Being both the pulsed radiation source and imaging system synchronized by the synchronization system with less than 200 ms jitter, this system allows for very precise reconstruction of the map of the dose deposited into the target region.
Owner:FYZIKALNI USTAV AV CR V V I
Production method for boron-10 element used for neutron detector
InactiveCN105137473ALarge specific surface areaEnabling Miniaturized ApplicationsNeutron radiation measurementDiffusionNitrogen
The invention provides a production method for a boron-10 element used for a neutron detector, relates to a production method for an element acting as a neutron detector cathode, and mainly aims at solving problems that existing neutron detectors are high in conductive substrate mass and limited in the number of the captured neutrons. According to the method, a graphite vinyl transparent film is adopted to act as a conductive substrate, boron powder is unformly sprayed on the graphite vinyl transparent conductive film by adopting an electrostatic spraying method and protected by high-purity nitrogen and heated for 2-10h under temperature of 50-150 DEG C, boron powder enters the film through thermal diffusion and entering depth is 0.5-1 micron; and thickness of a boron coating layer is 1-3 microns. Advantages are that the production method is applied to a high-dose radiation area.
Owner:时天成
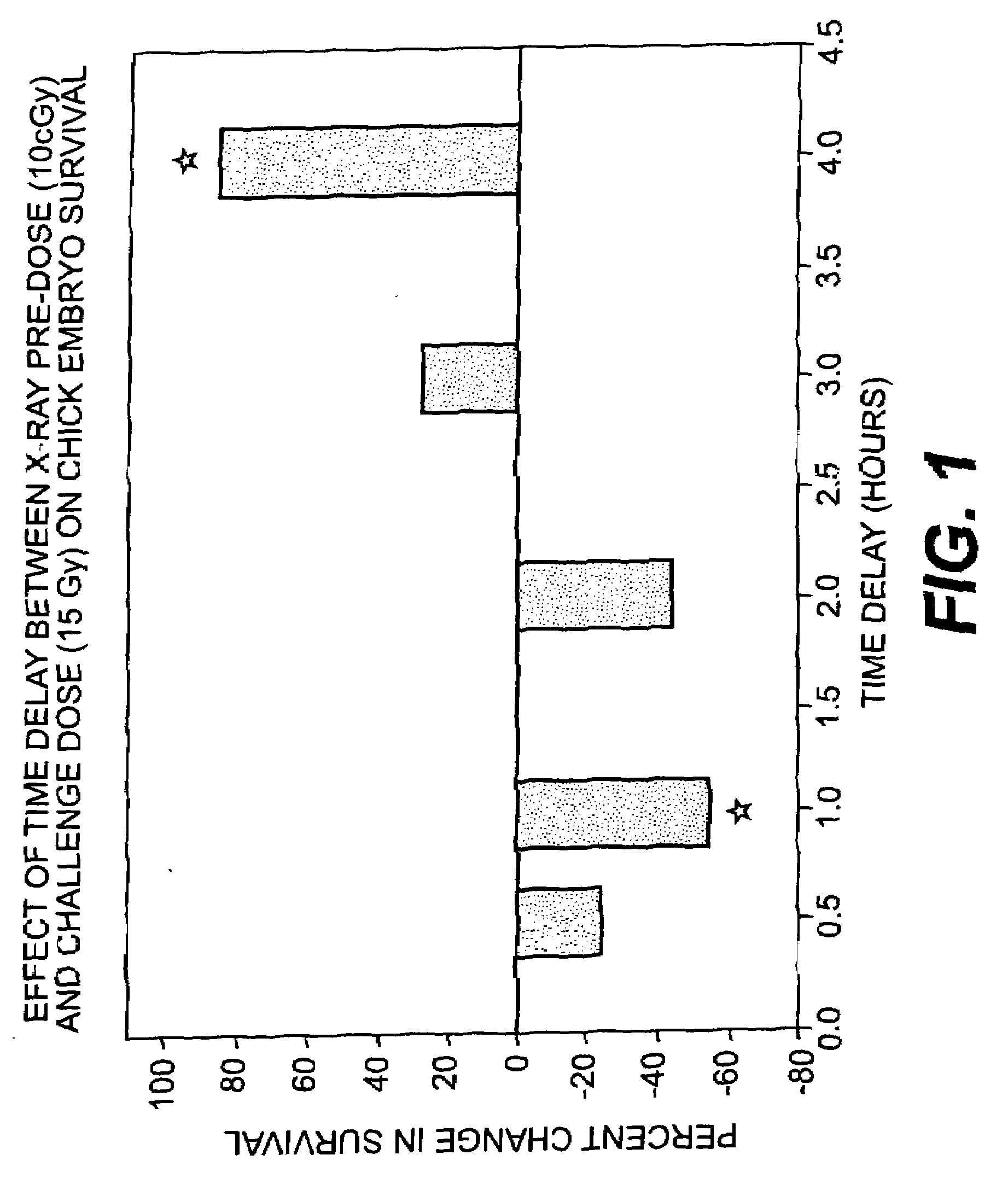
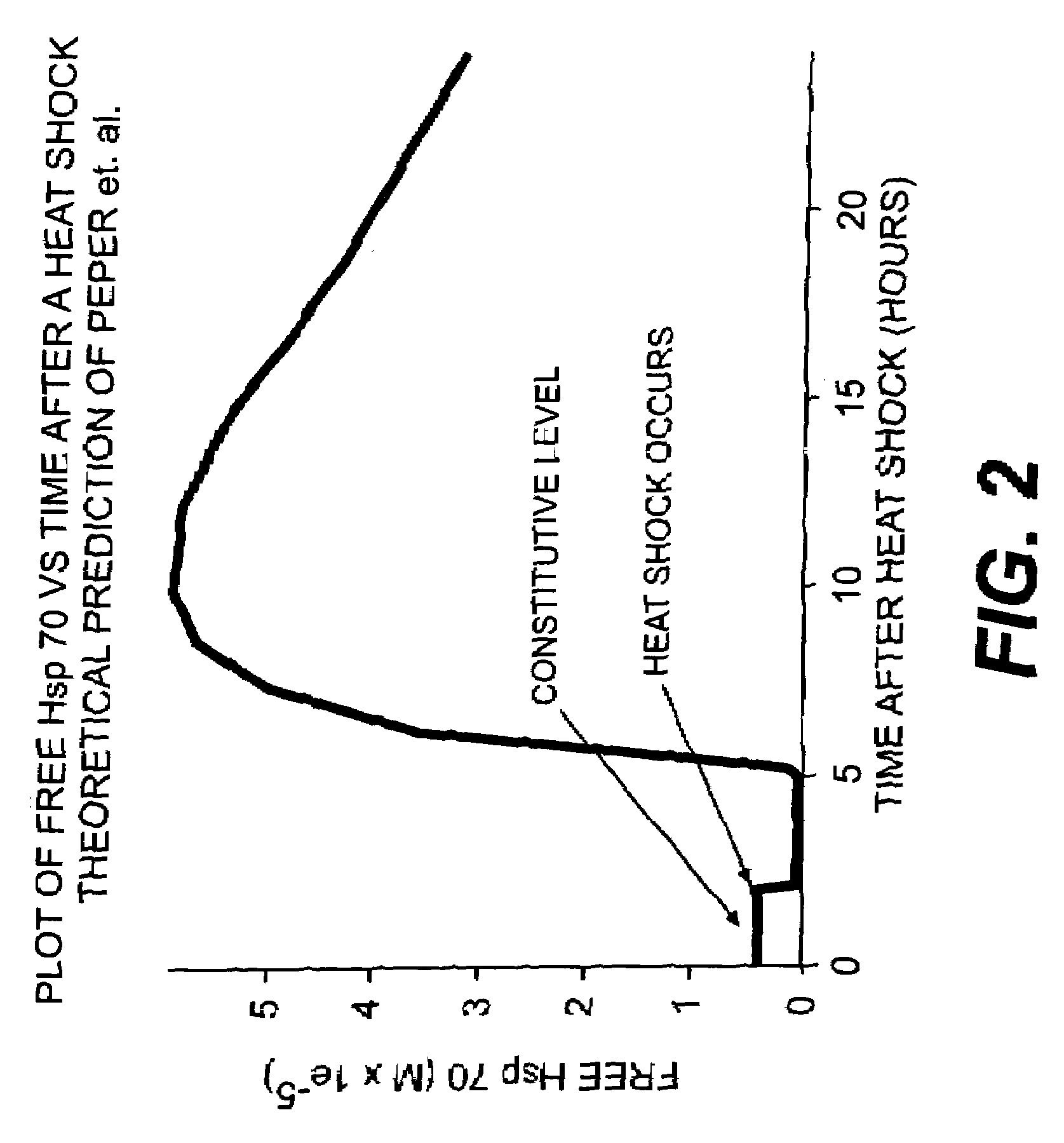
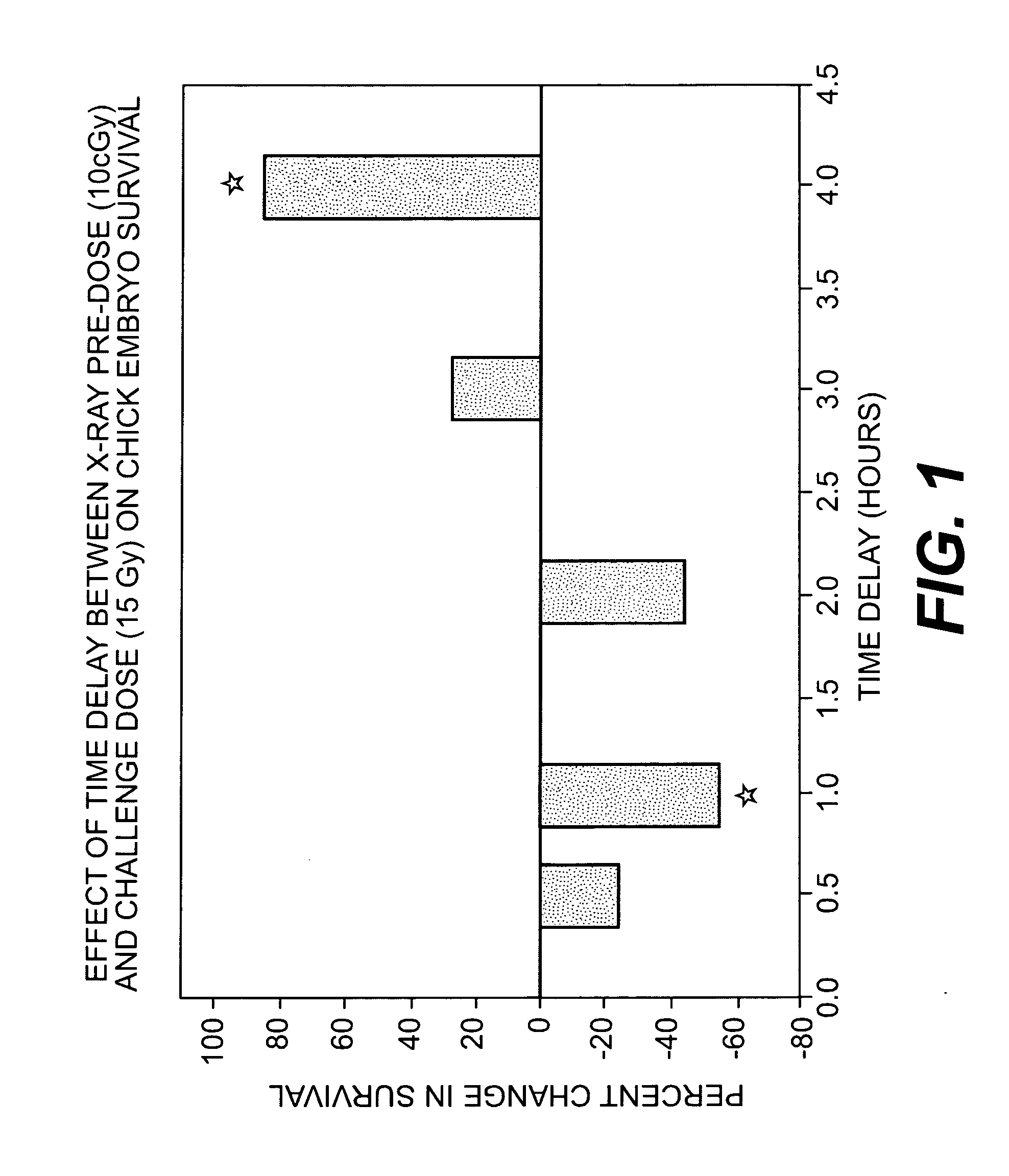
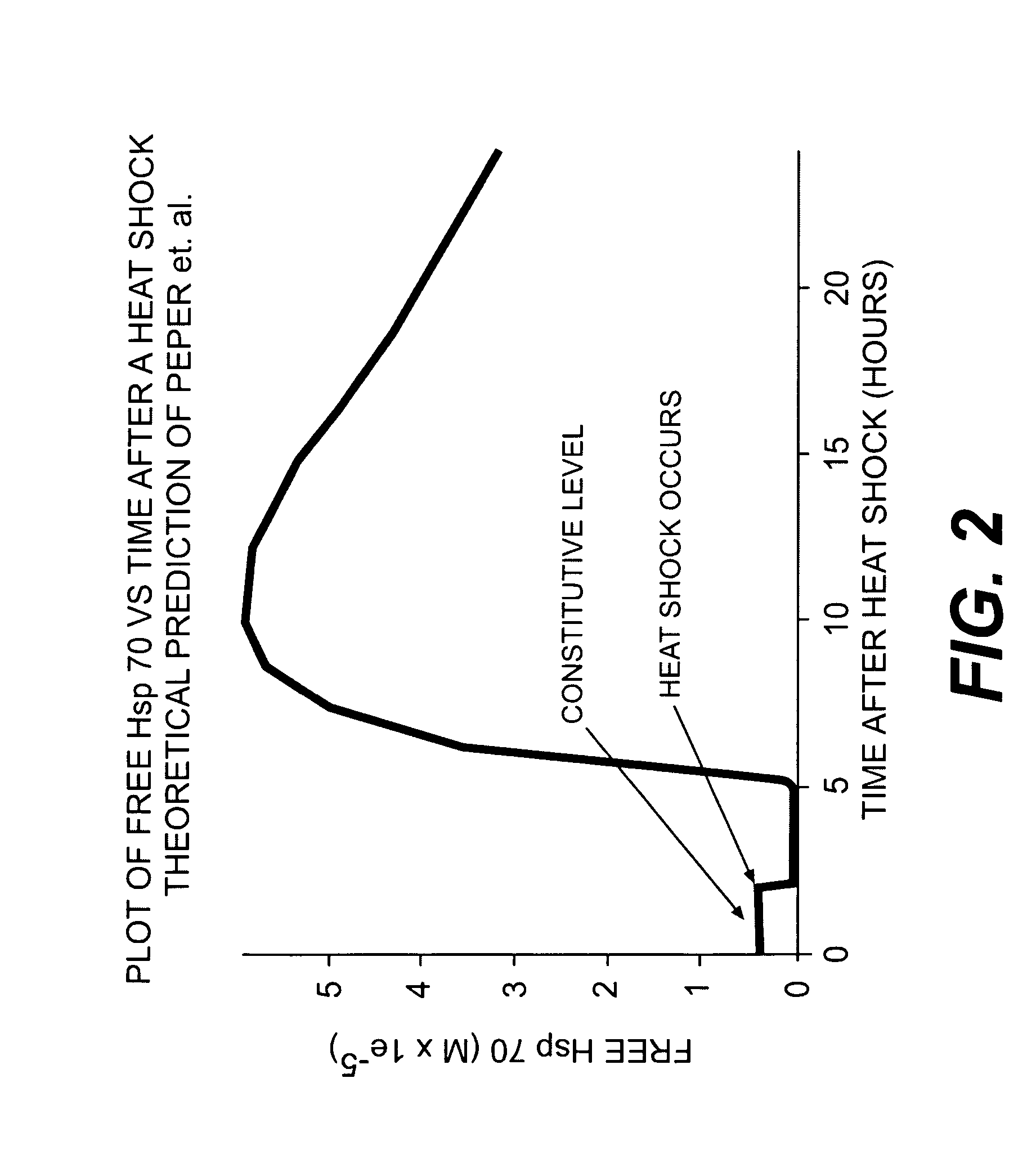
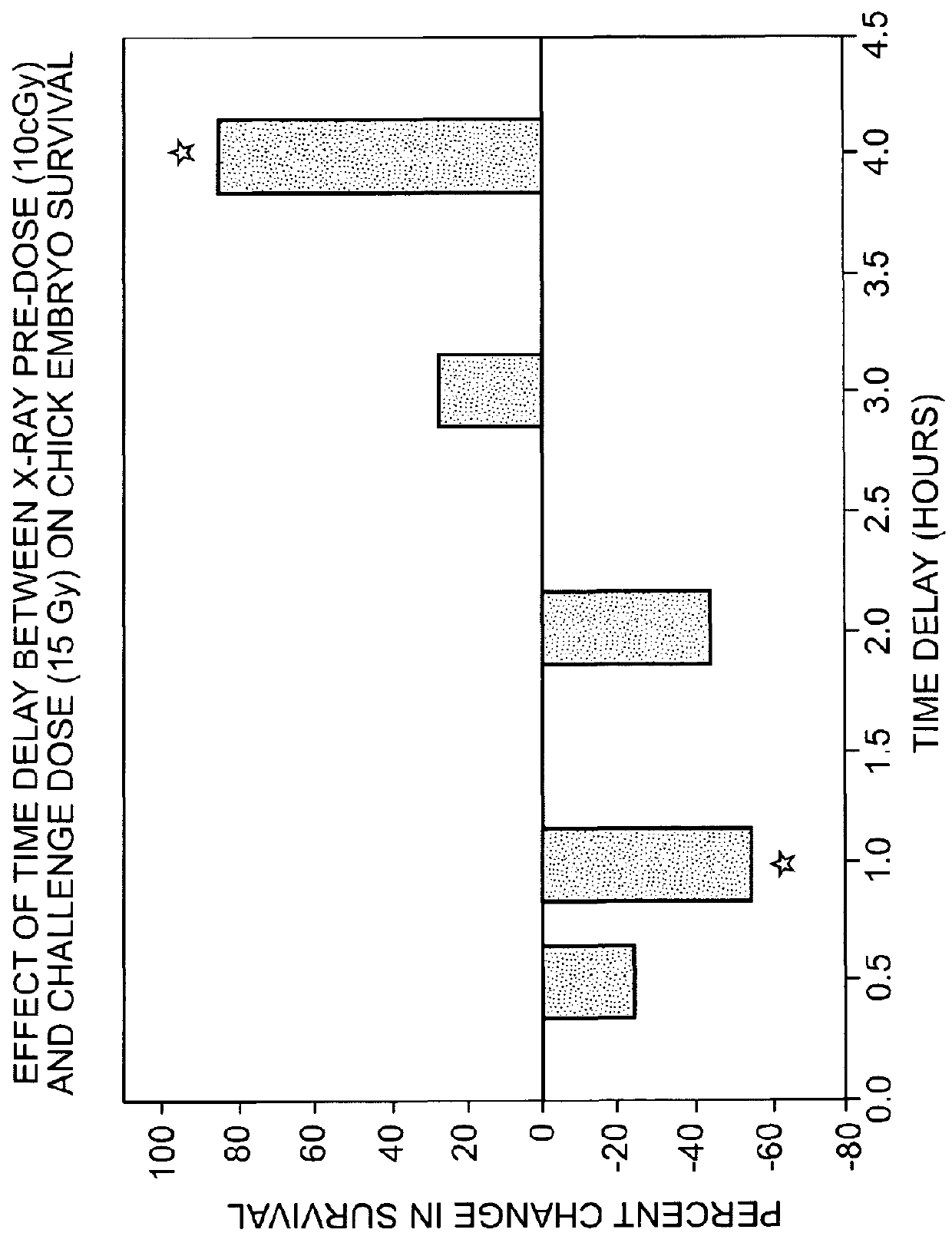
Use of weak stressors to enhance the effectiveness of ionizing radiation and other treatments of disease
Enhancing the effectiveness of therapeutic ionizing radiation and other treatments of disease in which cells are to be destroyed or modified, by subjecting cells in need thereof to low-dose radiation to increase the sensitivity of the cells to subsequent subjection with a lethal dose of high dose radiation (HDR), a chemotherapeutic agent, or other type of therapeutic treatment.
Owner:CATHOLIC UNIV OF AMERICA
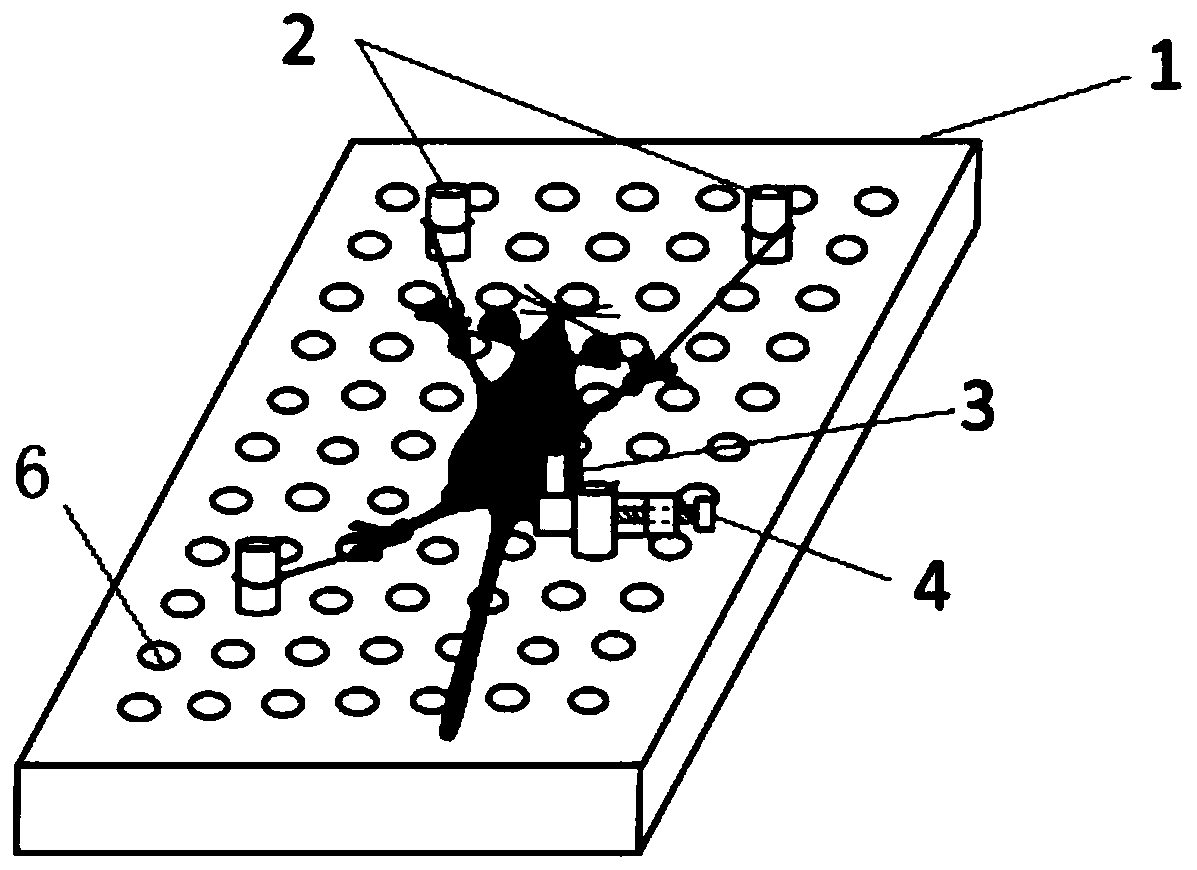
Remote control type large-batch sample point source irradiation frame and irradiation laboratory
ActiveCN111558406ASimple structureReduce weightBioreactor/fermenter combinationsBiological substance pretreatmentsRemote controlRadiation field
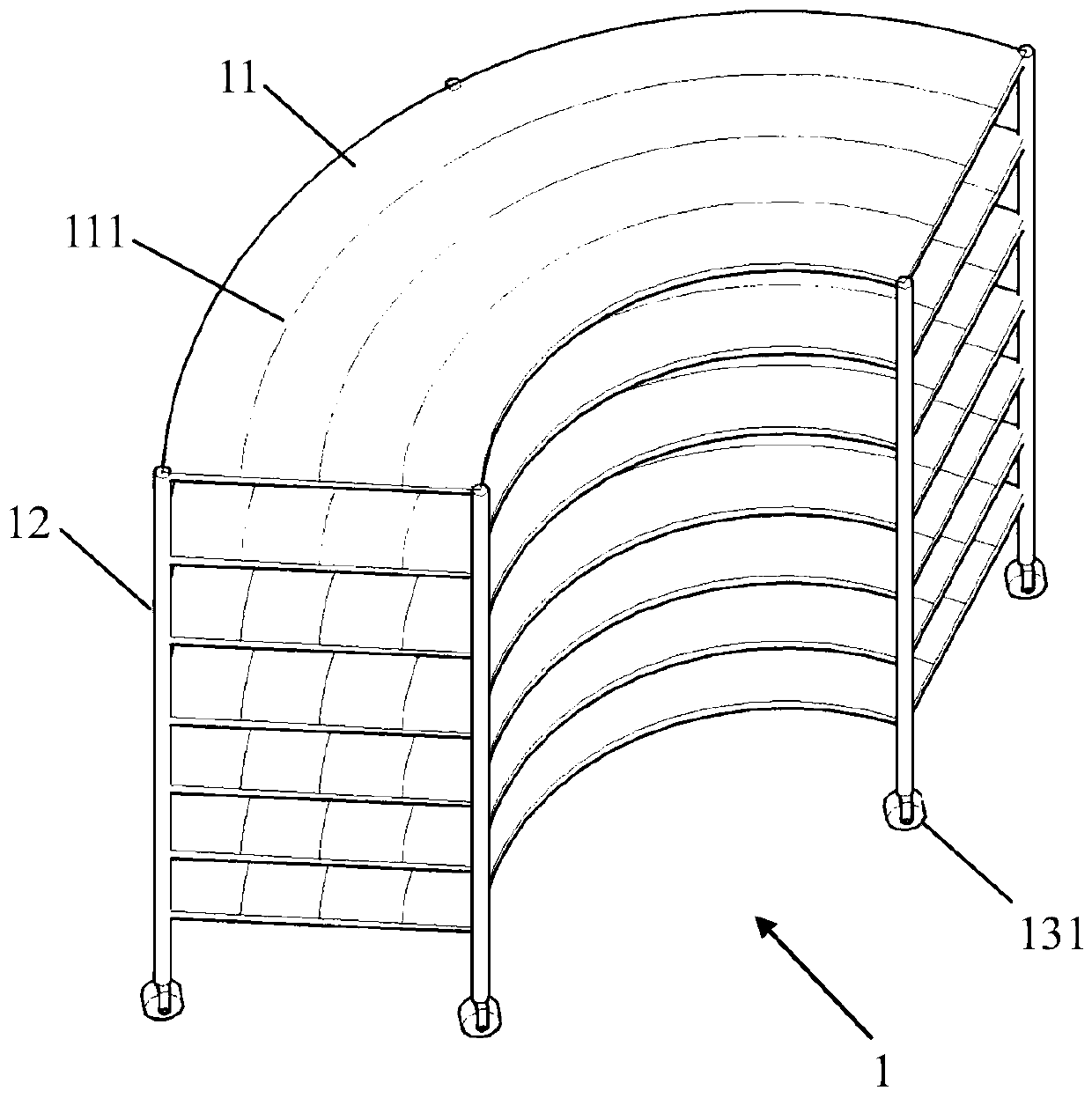
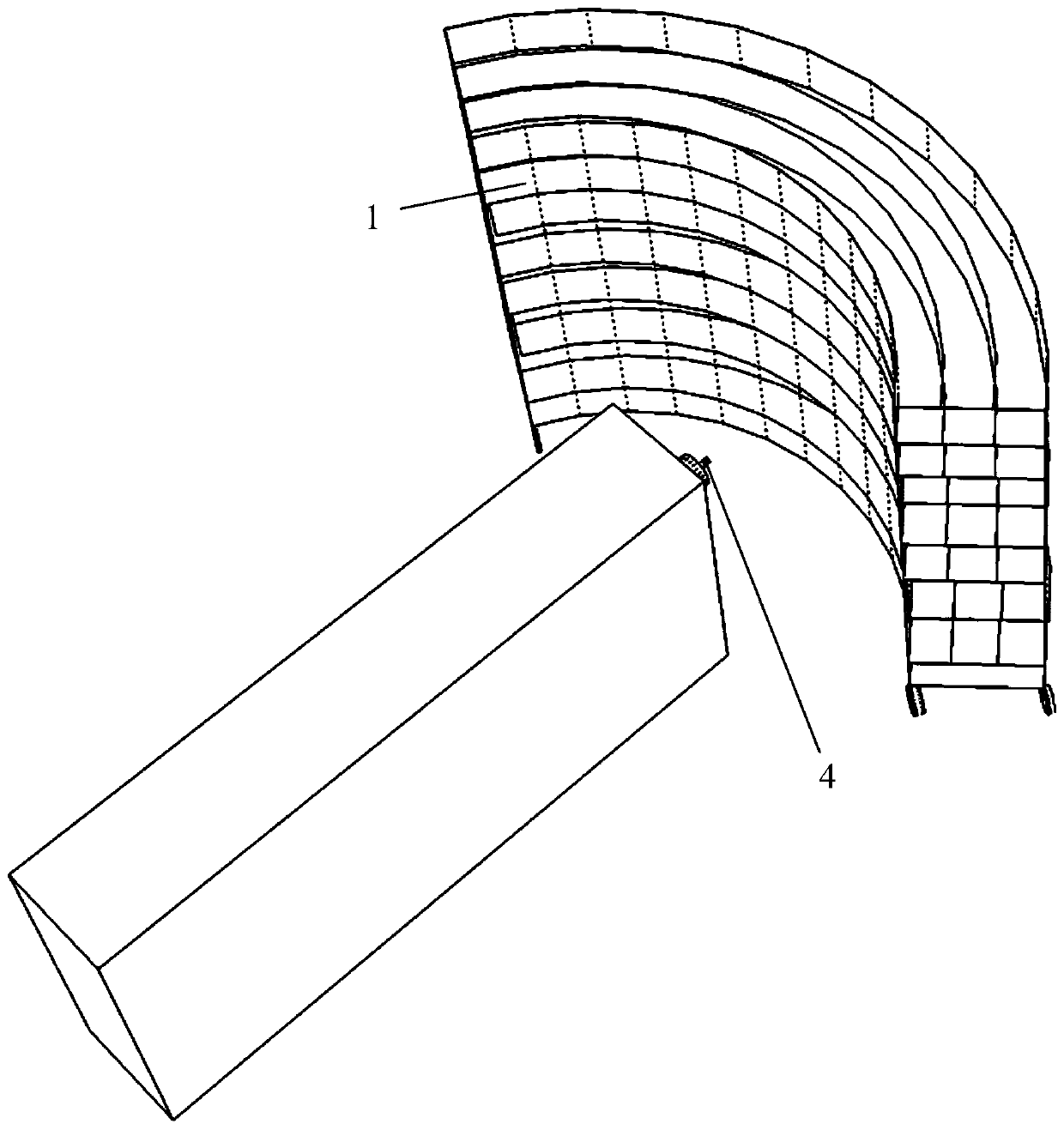
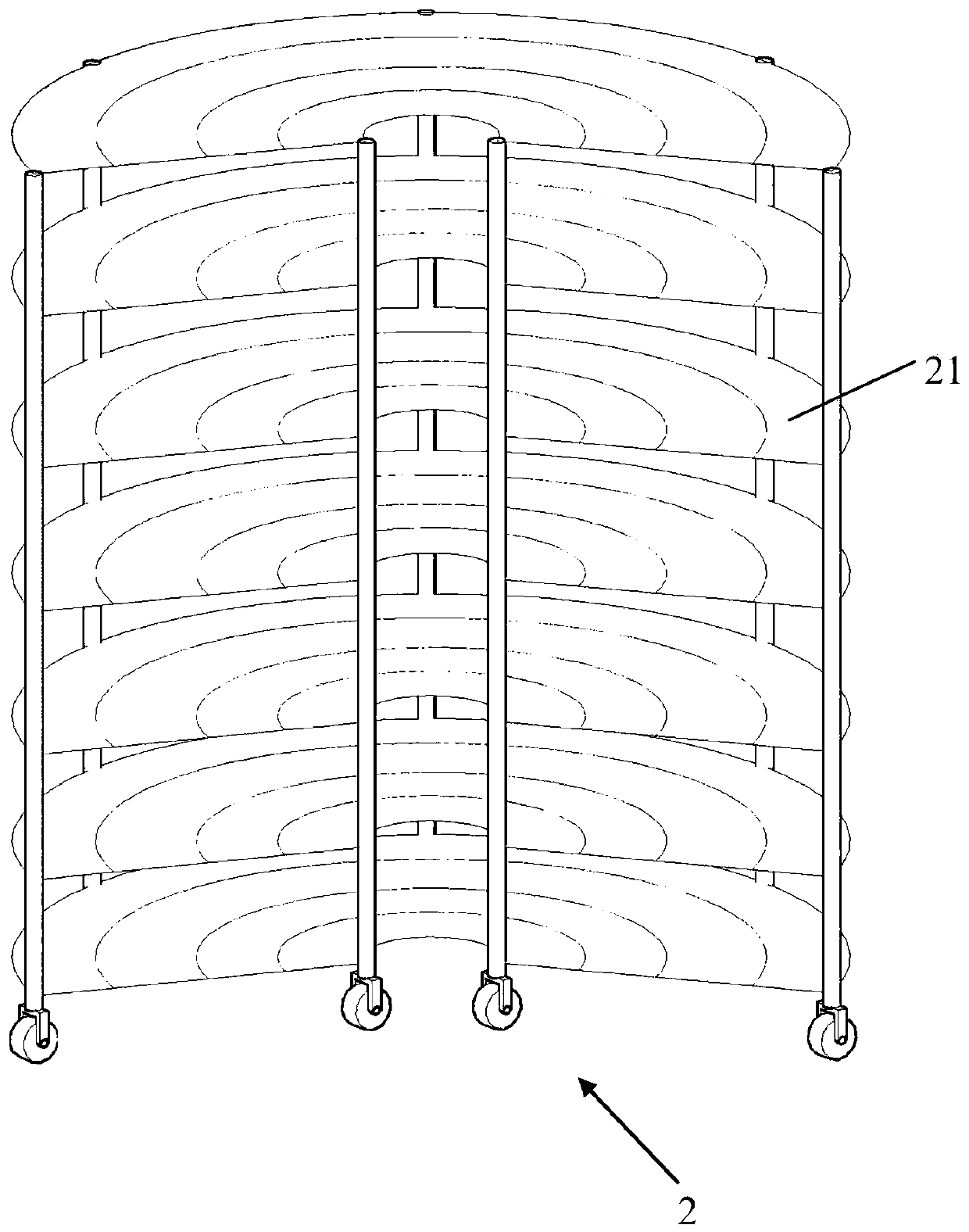
The invention relates to a remote control type large-batch sample point source irradiation frame, which comprises a plurality of fan-shaped supporting surfaces which are horizontally arranged at intervals relative to the vertical axis and are each provided with a plurality of concentric arc line marking scales, a plurality of supporting rods which are vertically supported and connected around theplurality of fan-shaped supporting surfaces, and a remote control movement mechanism which comprises a plurality of wheels, a motor, a carrying wireless receiving and transmitting module and a remotecontrol device. According to the large-batch sample point source radiation frame provided by the invention, precious space of a radiation field can be utilized to the greatest extent, and the time forplacing and withdrawing samples is saved, so that large-batch high-dose radiation of a point radiation source can be realized, an irradiated object can safely and quickly leave the radiation source after being irradiated, the problems urgently required to be solved in the type of experiment are solved, the experiment efficiency is effectively improved, the experiment is more scientific and reasonable, and the data result is more accurate.
Owner:THE NAVAL MEDICAL UNIV OF PLA
Colored-leaf forest seed mutagenesis method based on high-voltage electrostatic field
InactiveCN109548648ASimple methodShorten the breeding cycleSeed and root treatmentCultivating equipmentsMutation breedingHigh pressure
The invention discloses a colored-leaf forest seed mutagenesis method based on a high-voltage electrostatic field. The method includes: utilizing physical mutagenesis performance of the high-voltage electrostatic field to perform high-dosage radiation on colored-leaf forest seeds and specifically includes: selecting seeds, treating through the high-voltage electrostatic field, soaking the seeds toaccelerate germination, recording germination rate, sowing, managing colored-leaf forest seed cultivation records, and transplanting for cultivation. The method is simple and easy in operation; a high-voltage electrostatic power source is simple in structure and low in cost. The colored-leaf forest seeds obtained by the method can have leaf color phenotype mutagenesis rate of 0.5-1%, a new technical scheme is provided for forest mutation breeding, and superiority of the method is about to emerge as high-voltage electrostatic field technology becomes mature.
Owner:神舟绿鹏农业科技有限公司
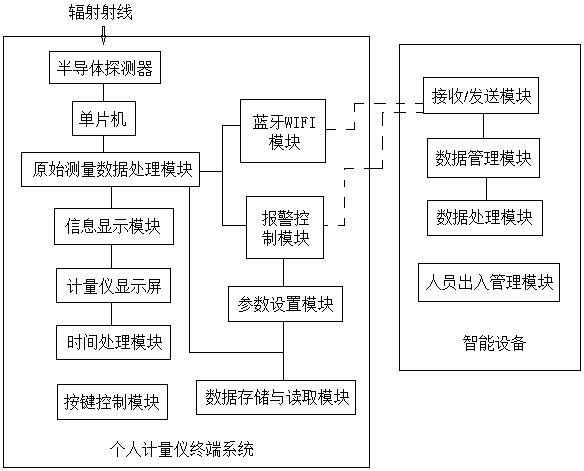
Real-time wireless detection system of personal measuring instrument
PendingCN108318906AReal-time monitoring of cumulative radiation doseReal-time monitoring of dose rateDosimetersMeasuring instrumentEngineering
The present invention provides a real-time wireless detection system of a personal measuring instrument. The real-time wireless detection system of the personal measuring instrument comprises a plurality of personal measuring instrument terminal systems and an intelligent device terminal system. The personal measuring instrument terminal systems are in communication connection with the intelligentdevice terminal system, and the personal measuring instrument terminal systems are distributed in a radiation area. The real-time wireless detection system of the personal measuring instrument can monitor a radiation dosage and a dosage rate in the radiation area in real time, can monitor the radiation accumulated dosage and the dosage rate of workers in real time and can timely emit alarm to allow related workers to get away from the radiation area so as to effectively protect the workers' health. For managers, the real-time wireless detection system of the personal measuring instrument is employed to guide workers on scene to avoid high-dosage radiation damaging and provide accurate data for comprehensively grasping dosage protection and radiation treatment of the workers.
Owner:四川云雾山峰商贸有限公司
Use of weak stressors to enhance the effectiveness of ionizing radiation and other treatments of disease
Enhancing the effectiveness of therapeutic ionizing radiation and other treatments of disease in which cells are to be destroyed or modified, by subjecting cells in need thereof to low-dose radiation to increase the sensitivity of the cells to subsequent subjection with a lethal dose of high dose radiation (HDR), a chemotherapeutic agent, or other type of therapeutic treatment.
Owner:CATHOLIC UNIV OF AMERICA
Composition for improving radiotherapy for cancer
InactiveUS20110250292A1Improve the effect of radiotherapyEnhancing radiotherapyBiocideInorganic active ingredientsSide effectArsenic oxide
Disclosed is a composition for improving radiotherapy for cancer, containing tetra-arsenic oxide (As4O6) as an active ingredient. The disclosed composition for improving radiotherapy for cancer improves the efficiency of radiotherapy, and thus reduces side effects caused by the high-dose radiation. The disclosed composition uses tetra-arsenic oxide, the safety of which is proven, from among conventional arsenic compound derivatives, to prevent side effects caused by arsenic trioxide, taxol, cisplatin, fluorouracil, leuprolide, or the like, which are known as conventional radiosensitizers.
Owner:CHONJISAN
Radiation-resistant laser cleaning irradiation tool based on pneumatic rotary motor, cleaning method thereof and laser cleaning machine
PendingCN112934854ALong transmission distanceSmooth swingCleaning processes and apparatusRadiation resistantCrank
The invention discloses a radiation-resistant laser cleaning irradiation tool based on a pneumatic rotary motor, a cleaning method thereof and a laser cleaning machine. The radiation-resistant laser cleaning irradiation tool based on the pneumatic rotary motor comprises a pneumatic galvanometer component, a collimator component, a plane field lens component and a shell component for sealing a laser light path, wherein the pneumatic galvanometer component comprises a pneumatic rotating motor, a crank connecting rod, a rocker and a galvanometer, wherein the crank connecting rod is driven by the pneumatic rotating motor, the rocker is driven by the crank connecting rod to swing, and the galvanometer is fixed to a rocker swing shaft; and a reflecting surface of the galvanometer is respectively aligned with a collimating mirror of the collimator component and a plane field lens of the plane field lens component so as to reflect a laser beam. The radiation-resistant laser cleaning irradiation tool based on the pneumatic rotary motor has the advantages that the galvanometer swings stably, and the tool is suitable for special environments such as a high-dose radiation environment, a strong magnetic environment and a strong electromagnetic interference environment.
Owner:RES INST OF PHYSICAL & CHEM ENG OF NUCLEAR IND
Use of weak stressors to enhance the effectiveness of ionizing radiation and other treatments of disease
Enhancing the effectiveness of therapeutic ionizing radiation and other treatments of disease in which cells are to be destroyed or modified, by subjecting cells in need thereof to low-dose radiation to increase the sensitivity of the cells to subsequent subjection with a lethal dose of high dose radiation (HDR), a chemotherapeutic agent, or other type of therapeutic treatment.
Owner:CATHOLIC UNIV OF AMERICA
A movable box-type combined shielding system
ActiveCN106782728BRealize the shielding effectAchieve protectionPortable shielded containersEngineeringRadioactive source
Owner:中科瑞华原子能源技术有限公司
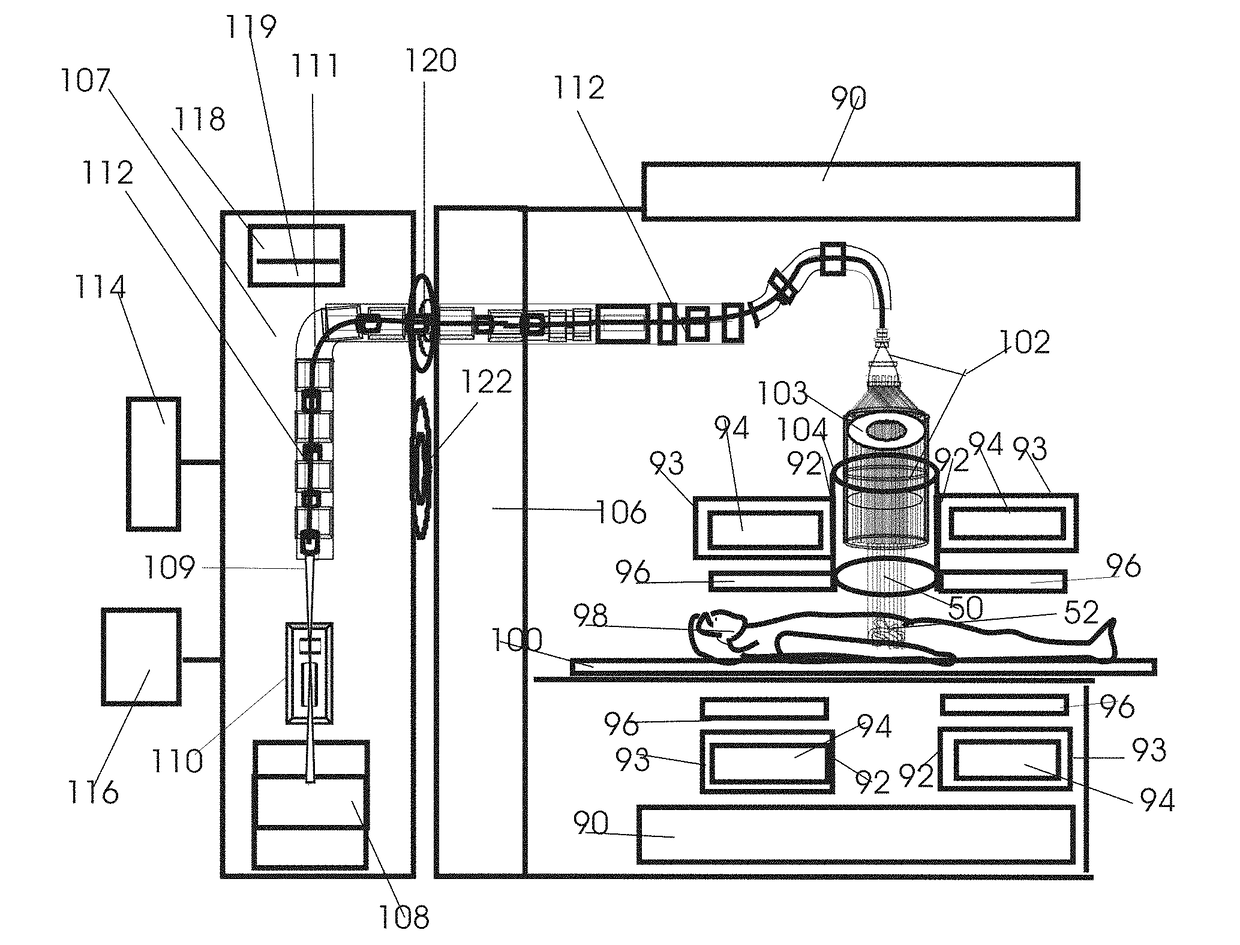
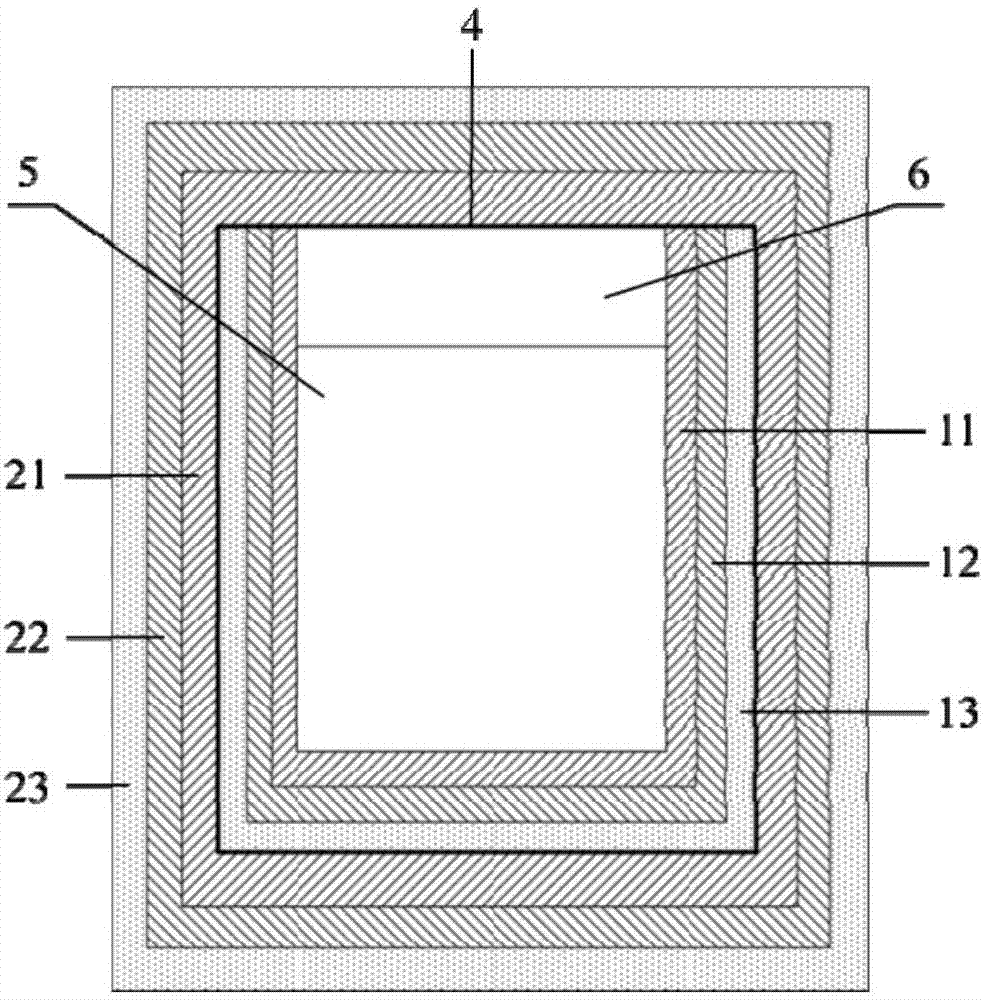
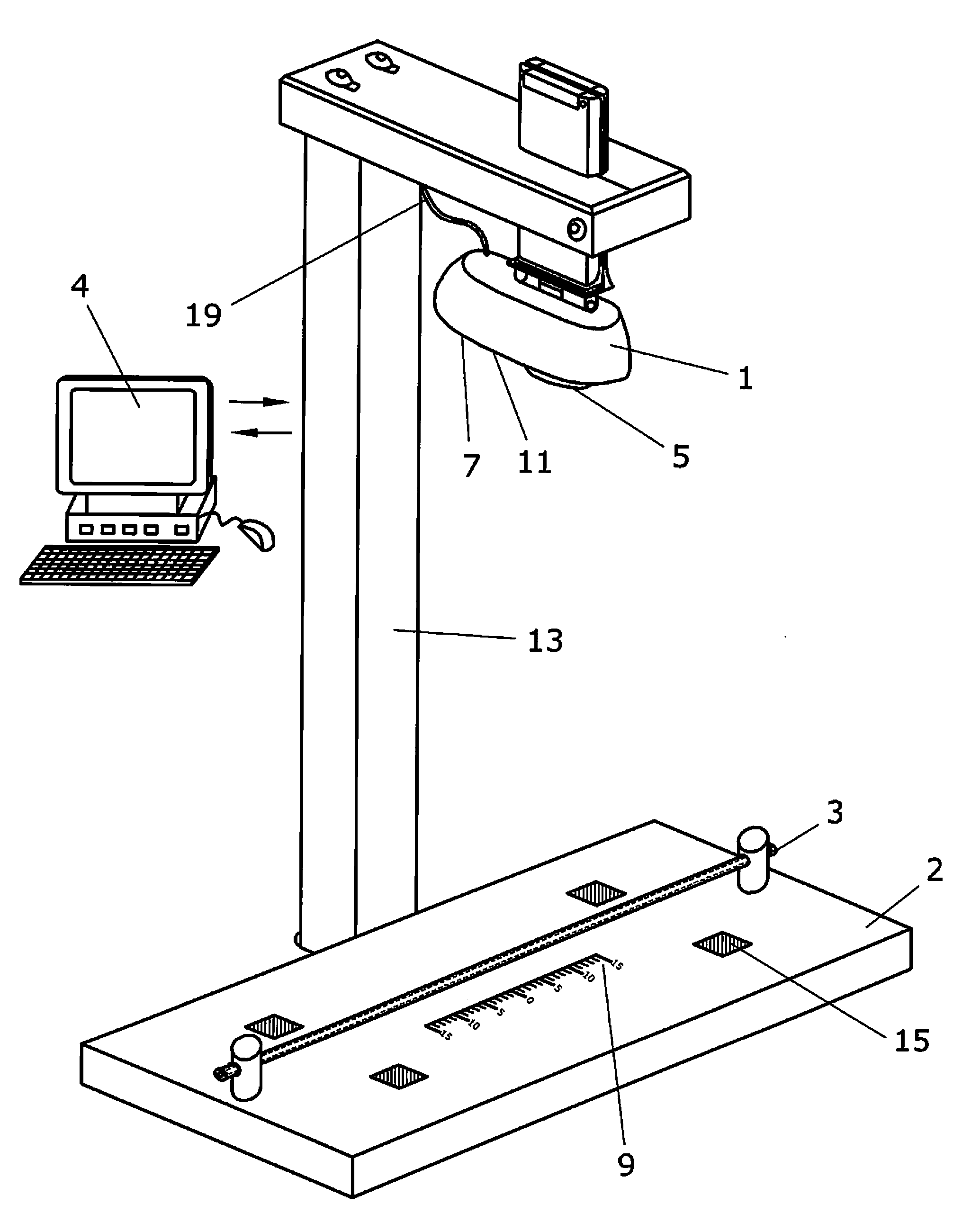
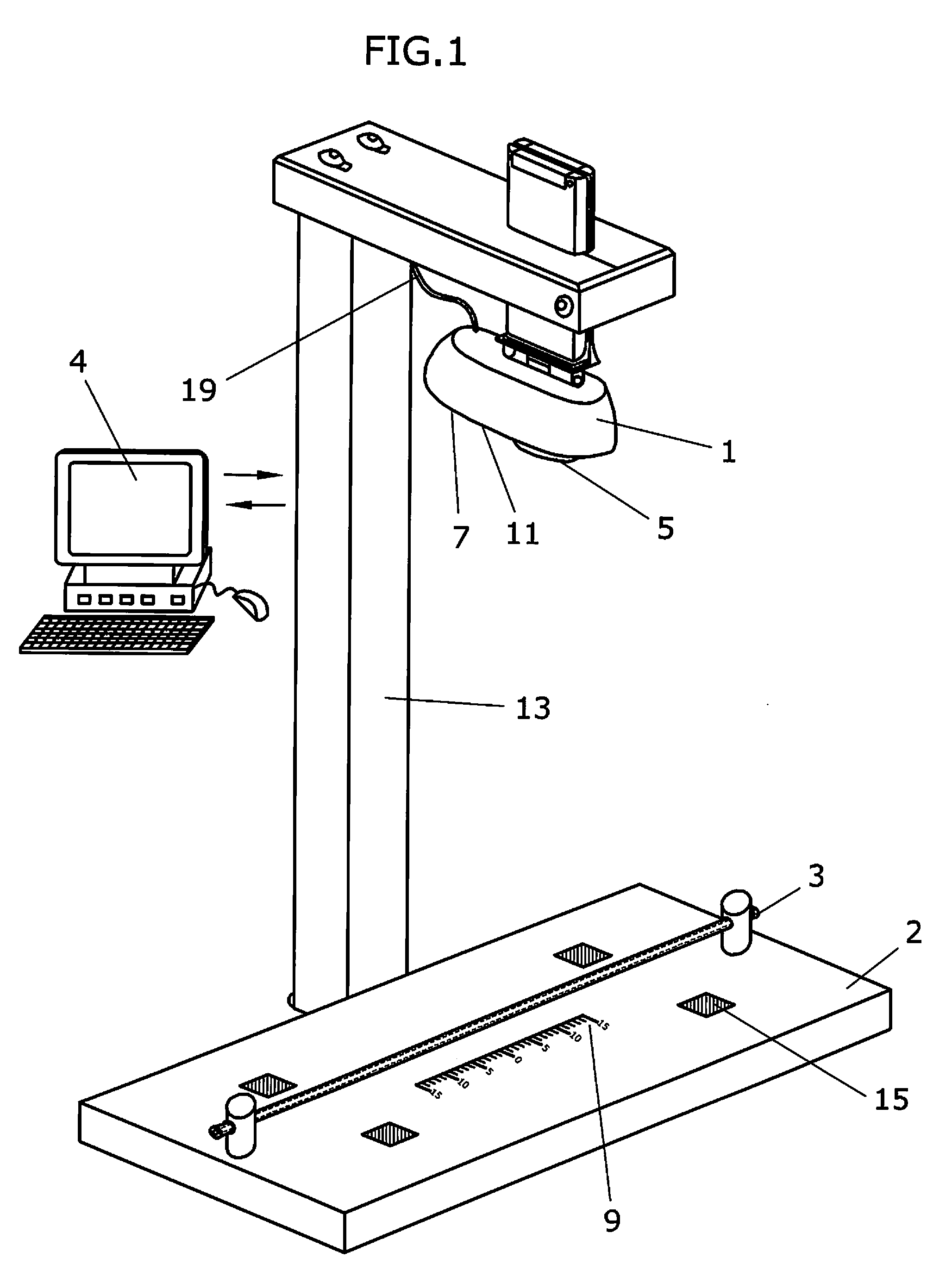
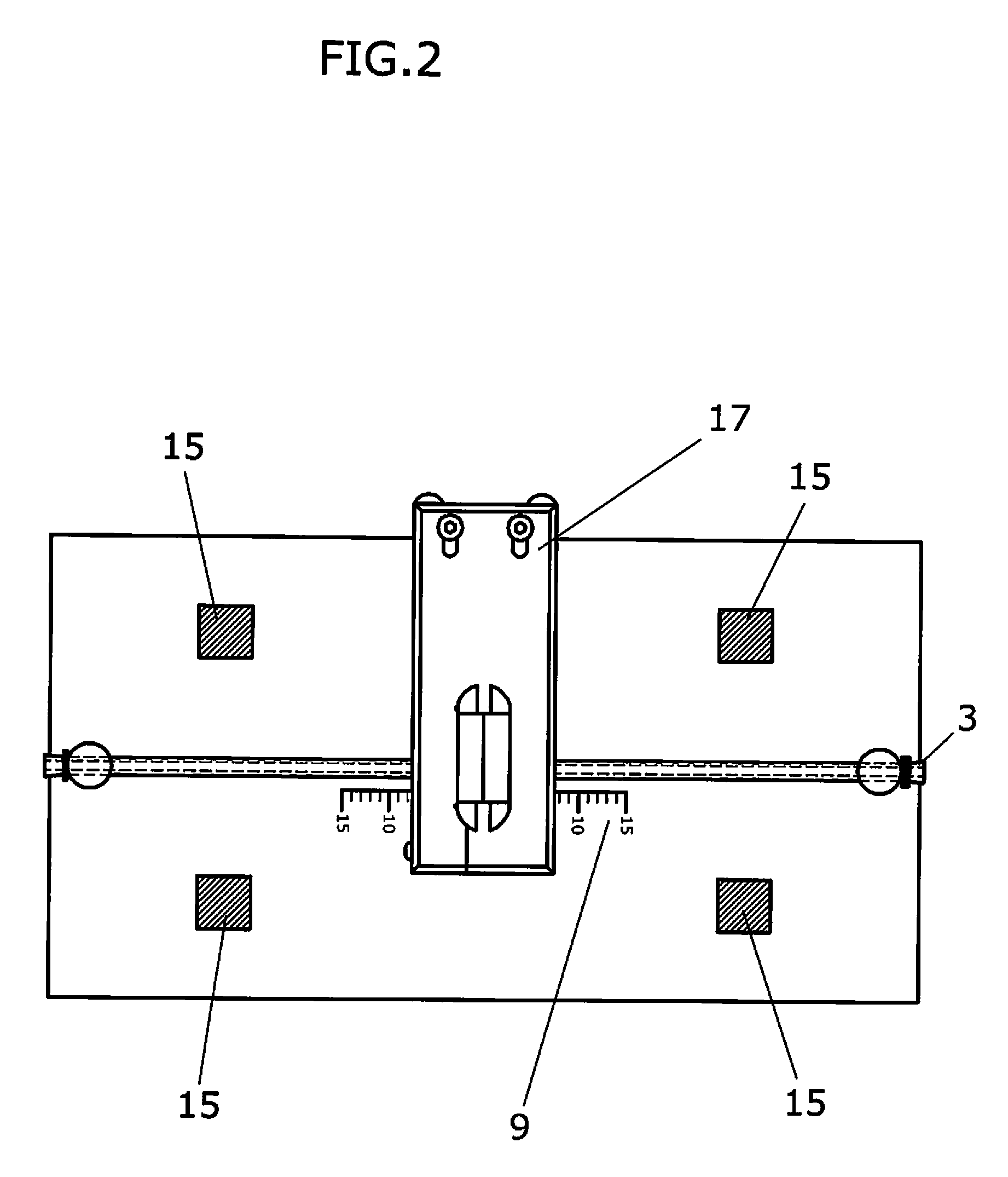
Apparatus and method to visually view high-dose-radiation apparatus used to verify quality assurance
An apparatus for testing a high-dose-rate afterloader machine comprising an image capturing device that used to capture a plurality of still images or a real-time video, wherein said image capturing device further comprises, a zoom lens, a microphone and a light, a measurement ruler that is located on a base plate; a plurality of calibration points located on the said base plate, a source insert that connects to said high-dose-rate afterloader machine to one end, wherein said source insert allows entry of a radioactive pellet and a source wire into one end, and an adjustable shaft that is connected to said base plate and connected to said image capturing device.
Owner:BEST CURE FOUND
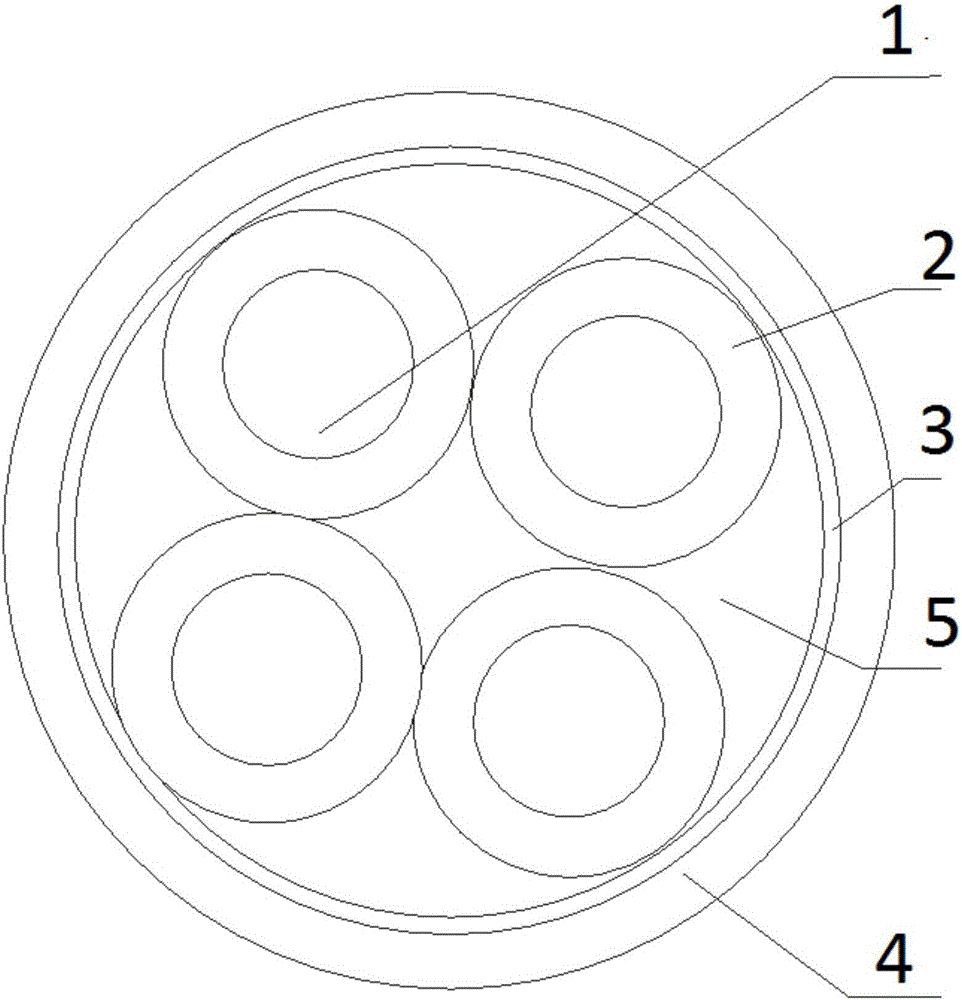
A high radiation dose resistant cable and its manufacturing method
ActiveCN105390201BImprove mechanical propertiesInsulated cablesAsbestosRadiation resistantElectrical conductor
A high radiation dosage resistant cable is disclosed. The cable comprises a plurality of conductors, an insulating layer, a wrapping layer and a sheath layer from inner to outer; the external of each conductor is wrapped with the insulating layer; the insulating layer is formed by an insulating band wrapping the external of the conductor; the conductors wrapped with the insulating layer are twisted to form the cable; the insulating band is a radiation resistant insulating band; the external of the cable core is wrapped with the wrapping layer; the space between the conductors wrapped with the insulating layer and the wrapping layer is filled with stuffing; the wrapping layer is formed by cable-making wrapping belts in a tight wrapping manner; and the sheath is an embossing copper sheath. The invention discloses the high radiation dosage resistant cable and a manufacturing method therefor. The manufacturing method comprises the steps of 1) manufacturing the conductors; 2) manufacturing the insulating layer; 3) making the cable, and 4) embossing the copper sheath. The cable is quite high in the radiation resistance, and can be safely used in the high-radiation environment of nuclear power stations.
Owner:JIANGSUSNGSHANG CABLE GROUP
Use of weak stressors to enhance the effectiveness of ionizing radiation and other treatments of disease
Enhancing the effectiveness of therapeutic ionizing radiation and other treatments of disease in which cells are to be destroyed or modified, by subjecting cells in need thereof to low-dose radiation to increase the sensitivity of the cells to subsequent subjection with a lethal dose of high dose radiation (HDR), a chemotherapeutic agent, or other type of therapeutic treatment.
Owner:CATHOLIC UNIV OF AMERICA
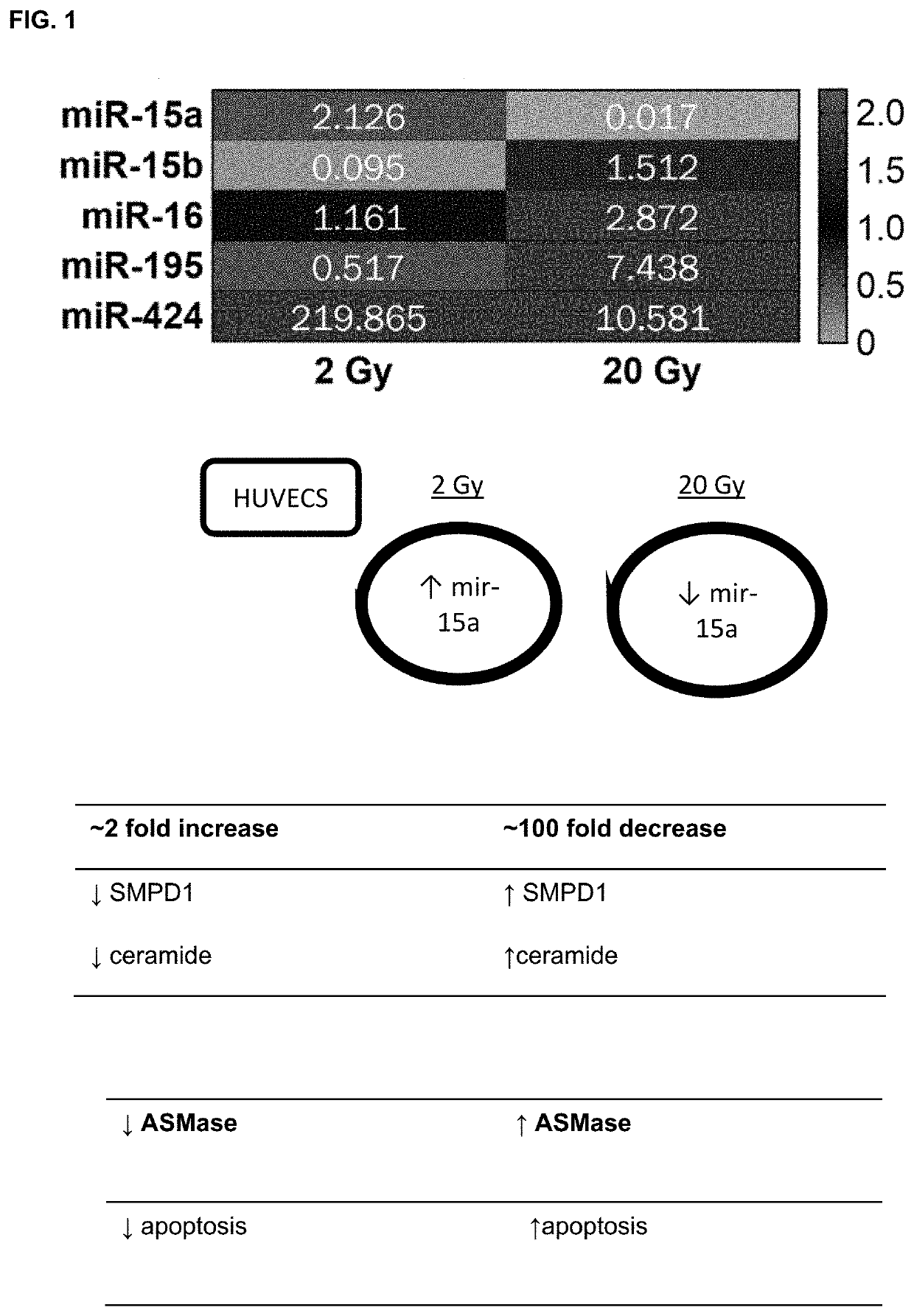
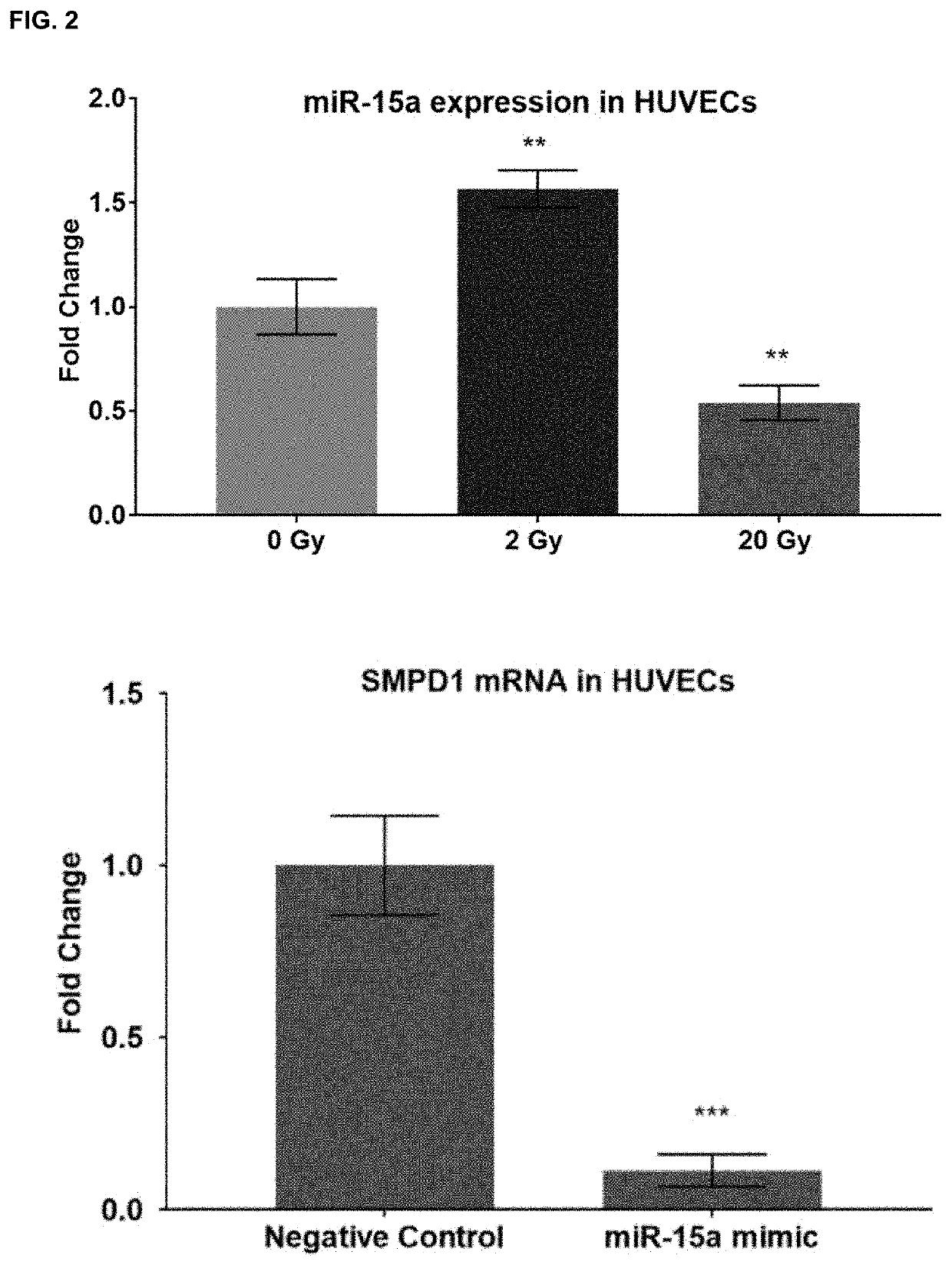
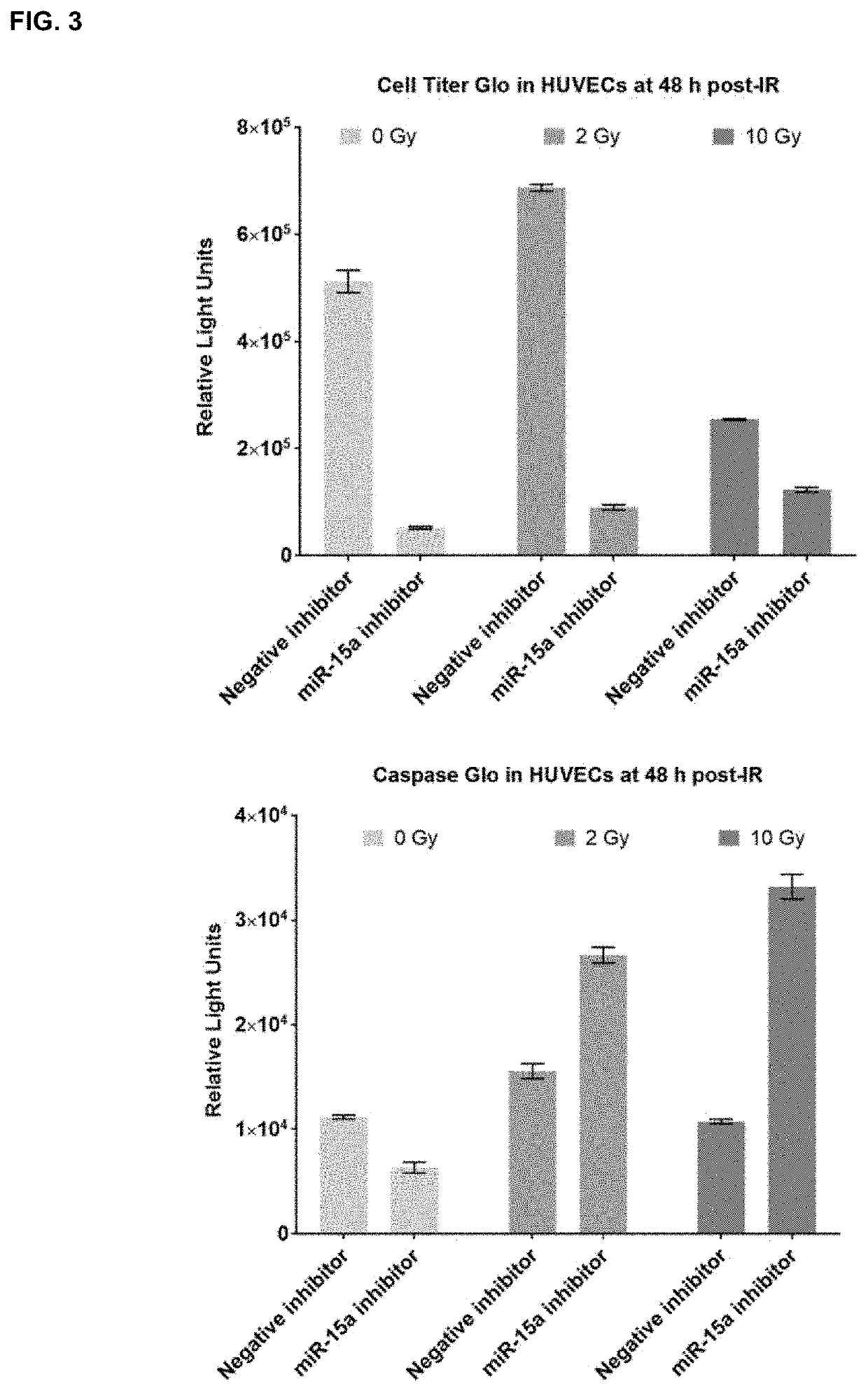
Microrna inhibitors as anti-cancer therapeutics
ActiveUS10941403B2Good curative effectAccelerated deathOrganic active ingredientsAntineoplastic agentsMicroRNABiochemistry
Owner:OREGON HEALTH & SCI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com