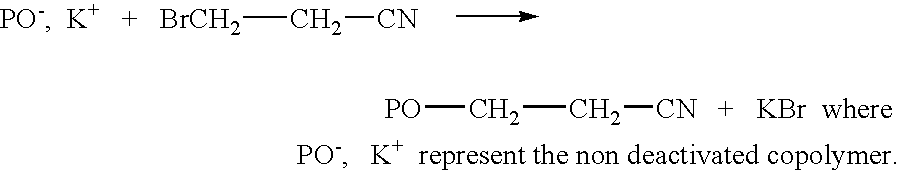
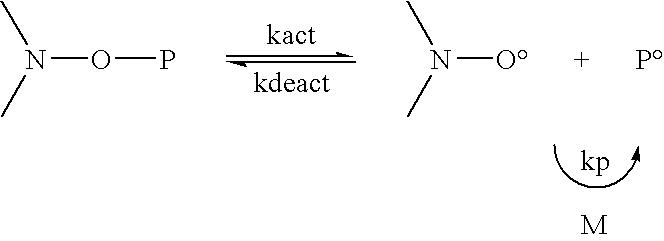
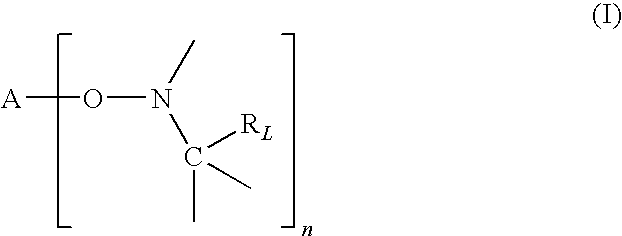
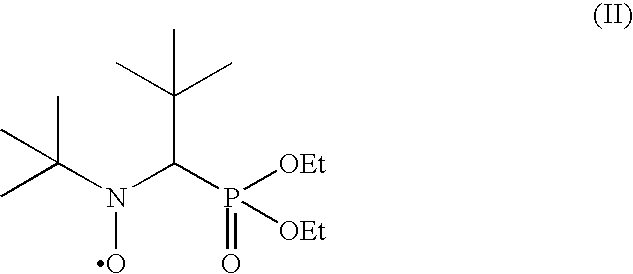
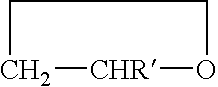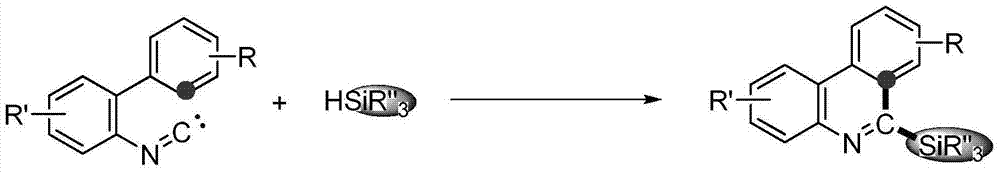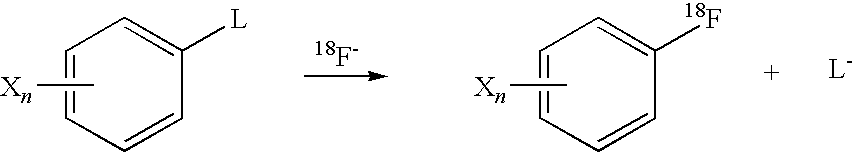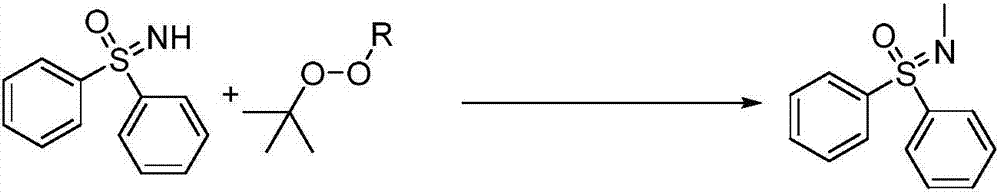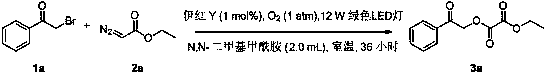Patents
Literature
30 results about "Free Radical Process" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Any subcellular metabolic process in which at least one of the participants has one or more electrons that singly occupy an electron orbital. Free radical processes may promote or prevent damage to tissues in which they arise.
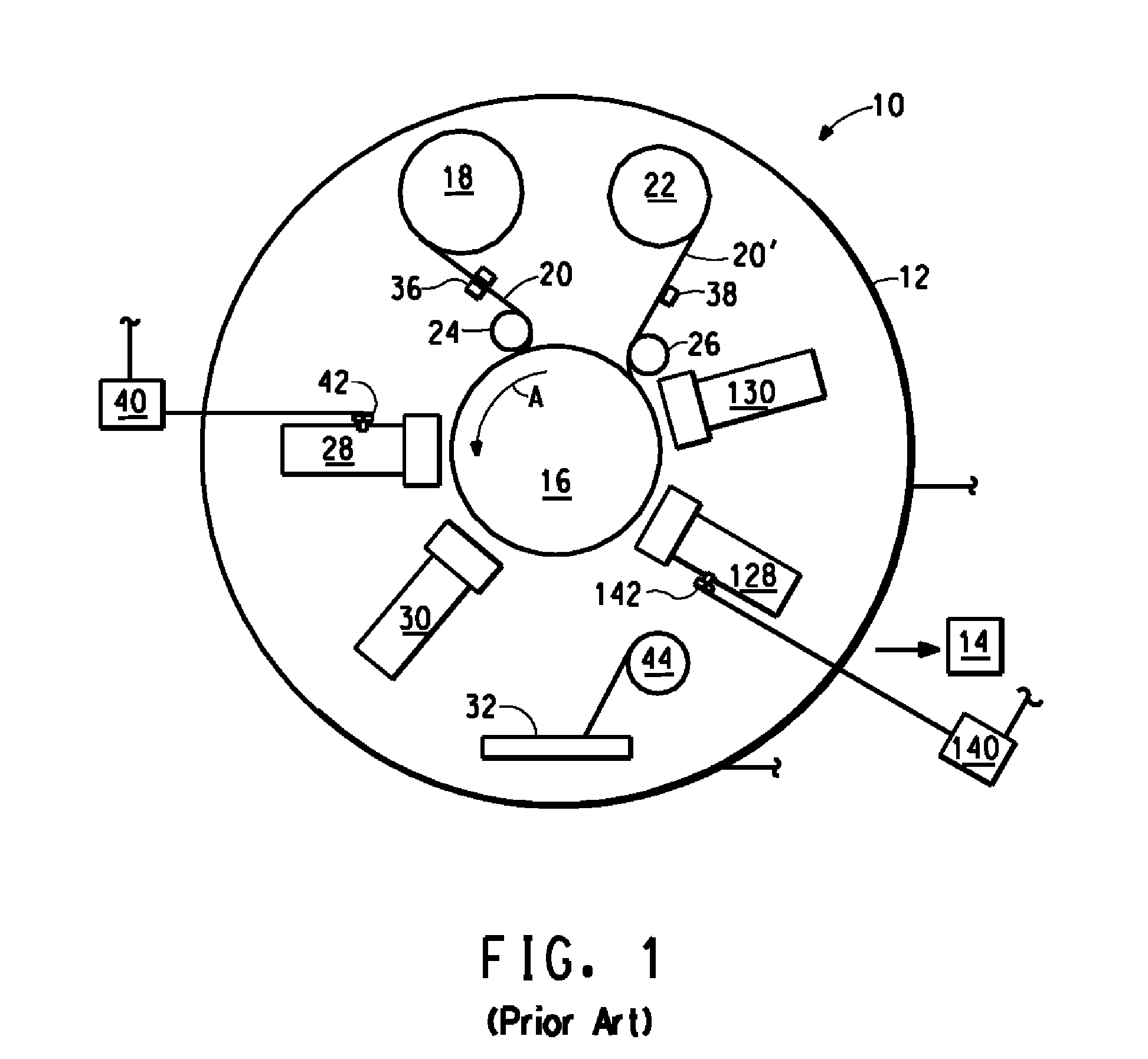
Processes for the production of chlorinated and/or fluorinated propenes and higher alkenes
InactiveUS8581012B2Improve efficiencyLow costPreparation by halogen replacementHalogenated hydrocarbon separation/purificationAlkaneGas phase
The present invention provides continuous, gas phase, free radical processes for the production of chlorinated and / or fluorinated propenes or higher alkenes from the reaction of chlorinated and / or fluorinated alkanes and chlorinated and / or fluorinated alkenes, wherein wherein at least a portion of any intermediate boiler by-products generated by the process are removed from the process.
Owner:BLUE CUBE IP
Method for preparing temperature-sensitive polyvinylidene fluoride intelligent membrane material and its product
This invention relates to a method for prparing temperature-sensitive polyvinylidene flourine intelligent membrane. The method is: NIPA as grafted monomer, PVDF as macromelocular initiator, cuprous chloride as catalyst,4,4 demethyl 2,2 bipyridine as ligand, using atom transfer free radical process, preparation of temperature-sensitive polyvinylidene flourine intelligent membrane comprising: in reaction kettle, adding 7-13% PVDF(WT%) and solvent N-methyl-2-pyrrolidone,heating to 50 deg. C to make it solve completely; then adding grafted monomer NIPA, its mol ratio with cuprous chloride is 2000:1-2500:1,filling nitrogen gas 30mins. at stirring, and separately adding 0.20-025g ligand 4,4-dimethyl 2,2-bipyridine and 0.04-0.06g catalyst cupous chloride, stirring and heating to 80-120deg.C,constant reaction 19-25 hrs; then washing reaction product using pure water to obtain light brown solid, filtering and 80 C degree drying to obtain finish product.
Owner:TIANJIN POLYTECHNIC UNIV
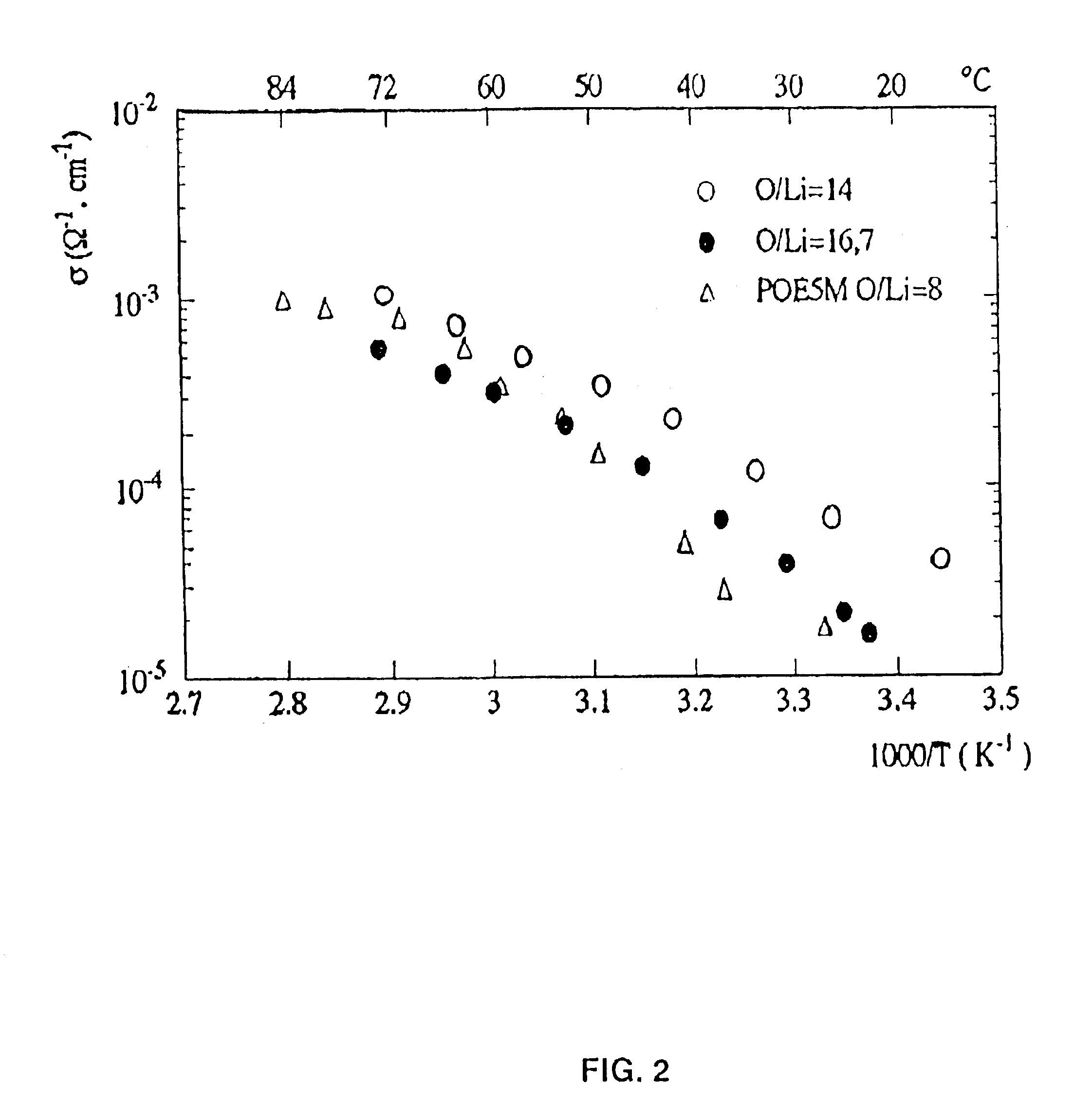
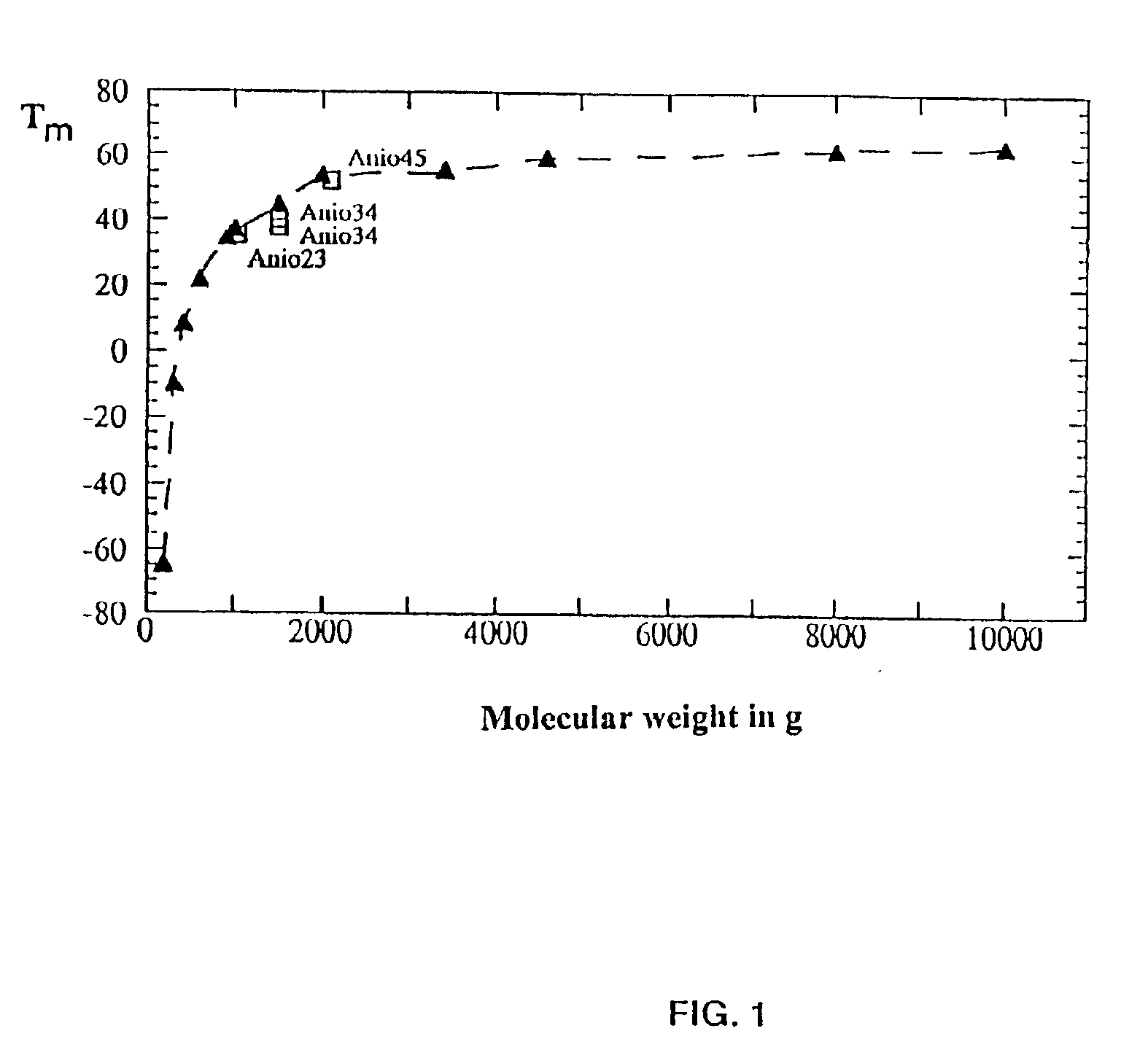
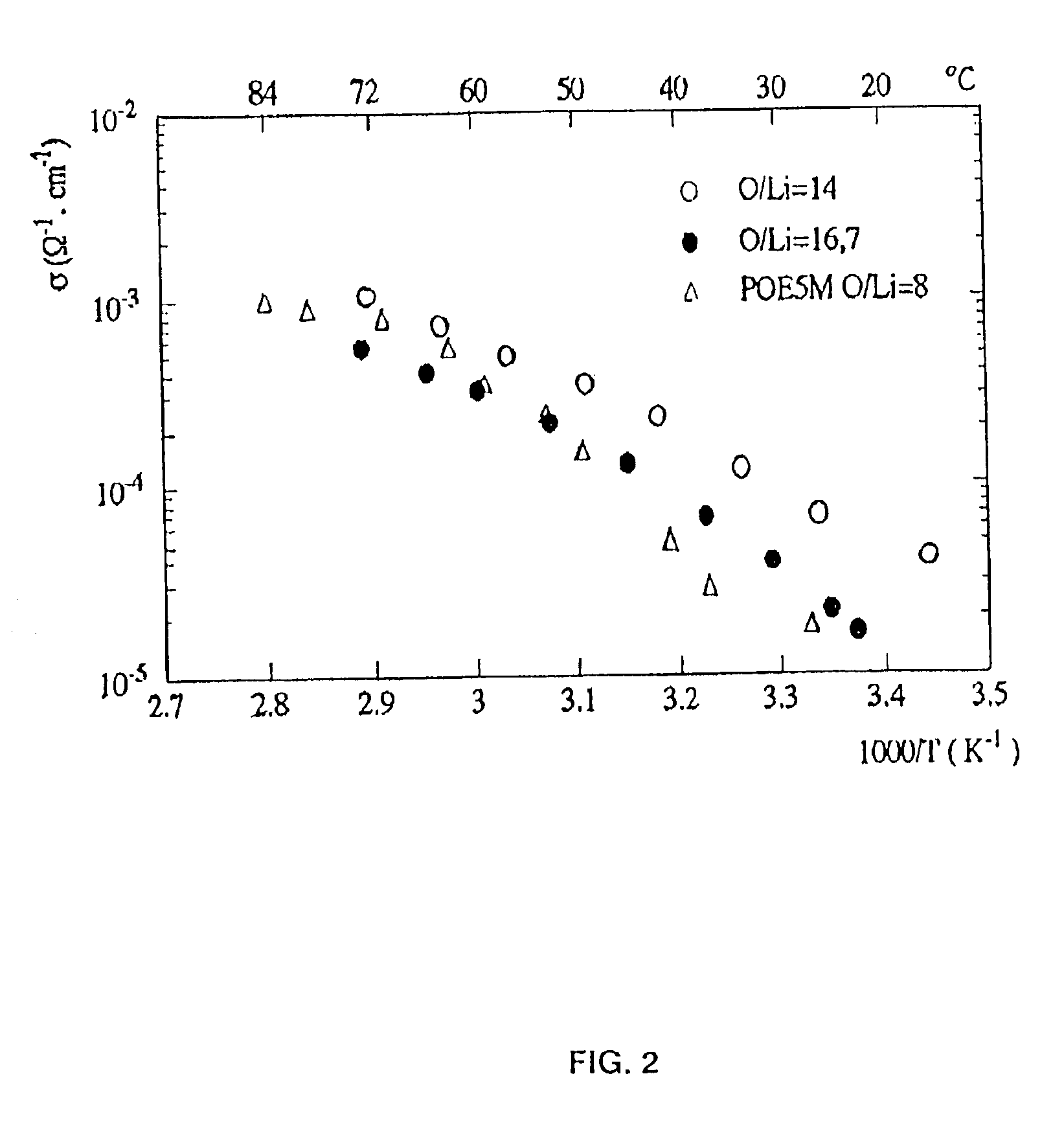
Copolymer of ethylene oxide and at least one substituted oxirane carrying a cross-linkable function, process for preparation thereof and use thereof for producing materials with ionic conduction
InactiveUS6903174B2High reaction yieldLess impuritiesConductive materialSolid electrolyte cellsLithiumEthylene oxide
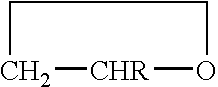
The invention concerns a copolymer of ethylene oxide and at least one substituted oxirane carrying a cross-linkable function. The copolymer comprises ethylene oxide, —O—CH2—CHR— units in which R is a substituent containing a reactive function which is cross-linkable by free radical process, R may be different from one unit to the other, and possibly —O—CH2—CHR′— units in which R′ is a substituent containing no reactive function which is cross-linkable by means of a free radical process, R′ may be different from one unit to the other. It is characterized by an excellent polymolecularity index I=Mw / Mn and a statistical distribution of the different monomer units. The copolymer is prepared by an anionic copolymerization process. The copolymer is useful for preparing a solid electrolyte having good mechanical properties, a good cationic conductivity and a good chemical compatibility with the electrodes of a generator operating with alkali metals such as lithium and sodium.
Owner:BATHIUM CANADA
Processes for the production of chlorinated and/or fluorinated propenes and higher alkenes
InactiveUS20110087055A1Improve efficiencyLow costPreparation by halogen replacementHalogenated hydrocarbon separation/purificationGas phaseFree Radical Process
The present invention provides continuous, gas phase, free radical processes for the production of chlorinated and / or fluorinated propenes or higher alkenes from the reaction of chlorinated and / or fluorinated alkanes and chlorinated and / or fluorinated alkenes, wherein wherein at least a portion of any intermediate boiler by-products generated by the process are removed from the process
Owner:BLUE CUBE IP
Copolymer of ethylene oxide and at least one substituted oxirane carrying a cross-linkable function, process for preparation thereof, and use thereof for producing ionically conductive materials
InactiveUS6855788B2High reaction yieldLess impuritiesCell electrodesConductive materialChemical compatibilityConductive materials
Owner:BATHIUM CANADA
Acrylic polymer low temperature flow modifiers in bio-derived fuels
ActiveUS20100175310A1Good shear stabilityReduce pointsLiquid carbonaceous fuelsBio-feedstockEthylene HomopolymersFree Radical Process
Owner:ARKEMA INC
Acrylic block copolymer low temperature flow modifiers in lubricating oils
ActiveUS7906468B2Excellent at modifying the low temperature flow behaviorReduce pointsLiquid carbonaceous fuelsAdditivesFree Radical ProcessPour point
The present invention relates to acrylic block copolymers synthesized by a controlled free-radical process, and their use as low temperature flow modifiers in oil-based compositions. They are especially useful in modifying the low temperature flow behavior in lubricating oils. The acrylic copolymers are especially useful as pour point depressants in lubricating oil.
Owner:ARKEMA INC
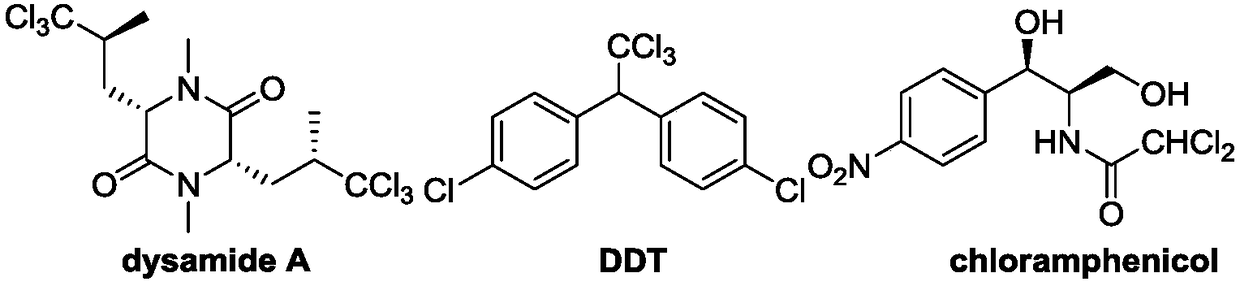
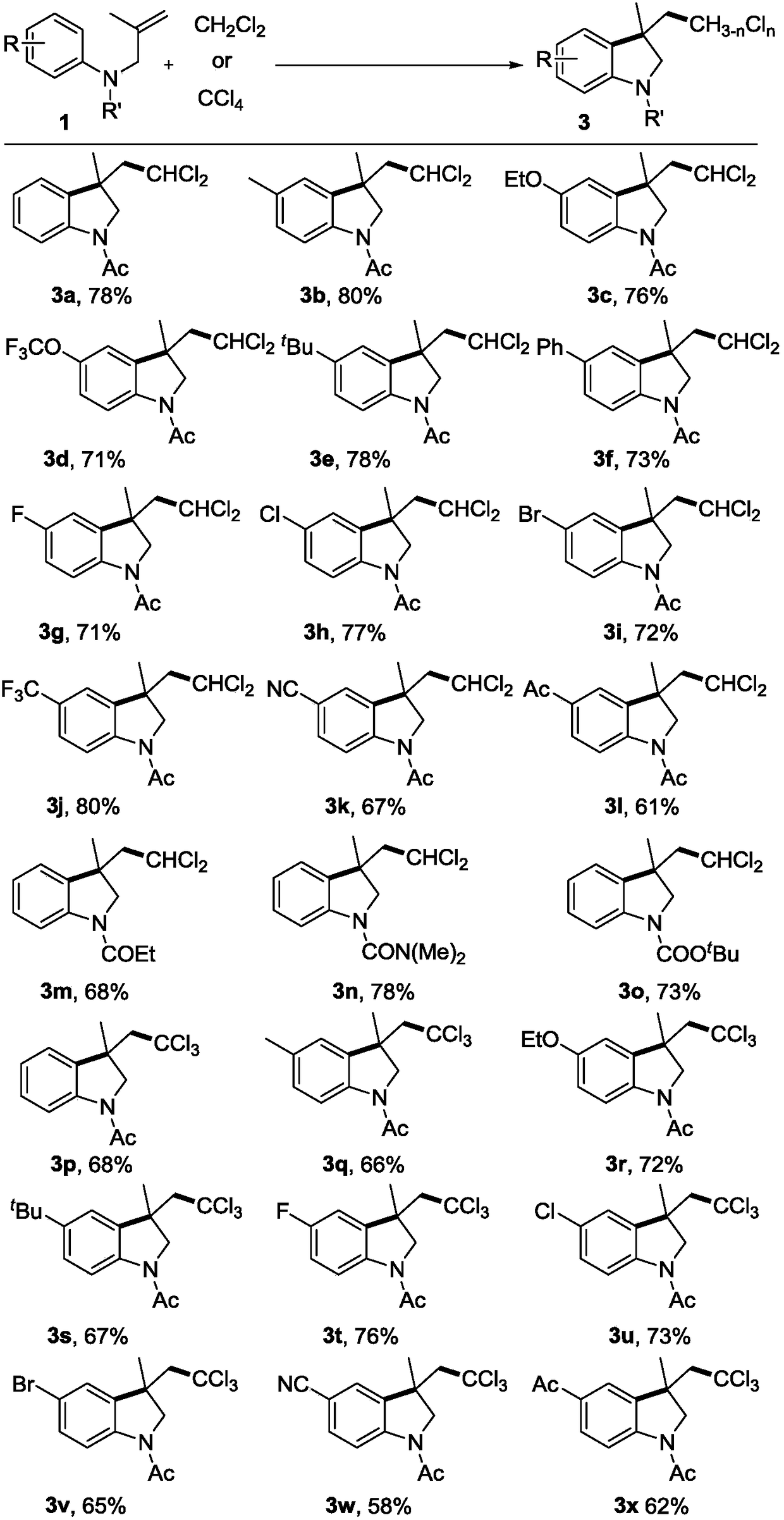
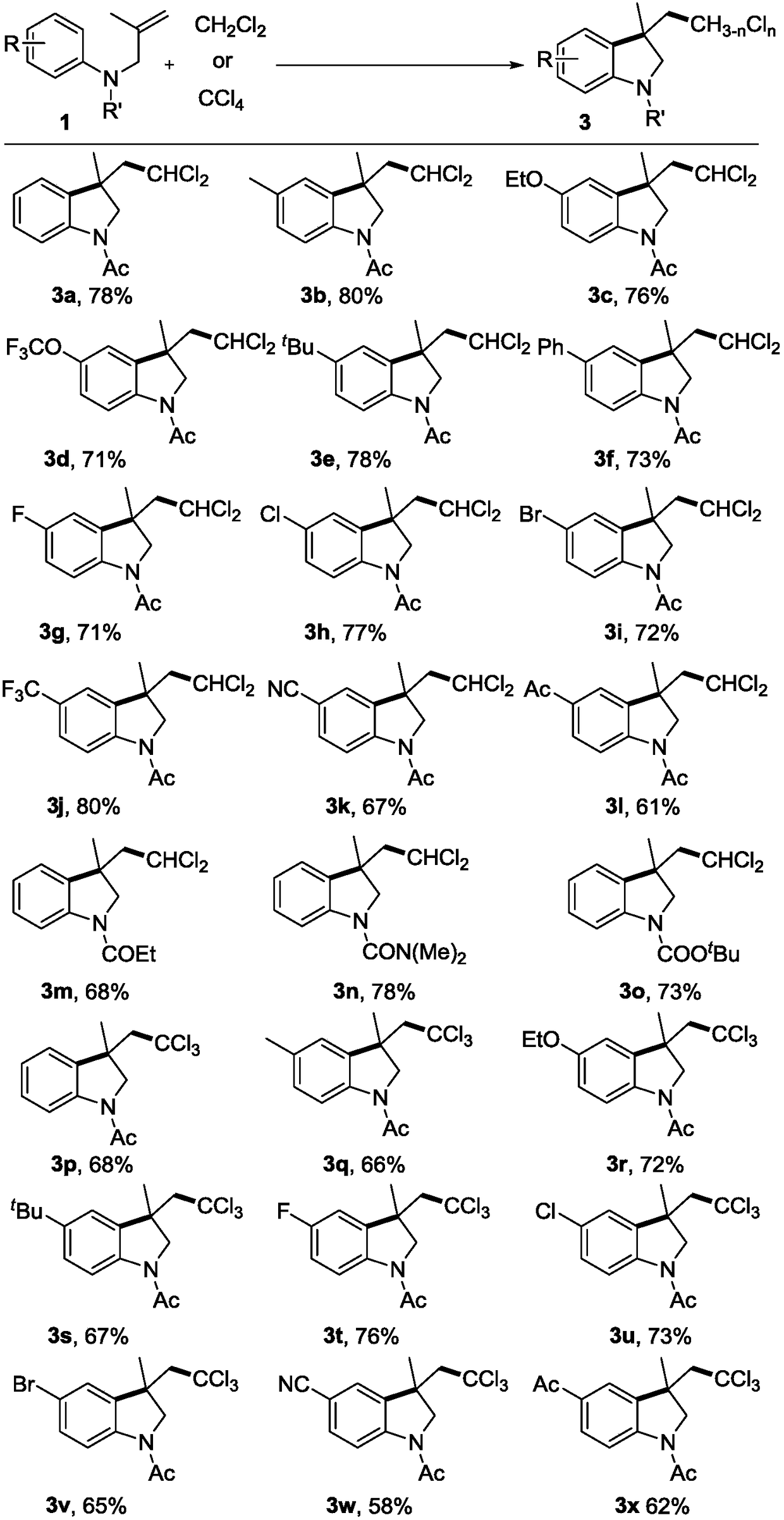
Polychloromethyl-substituted indoline compound and synthetic method and application thereof
ActiveCN108947886AEasy to handleAchieve synthesisAntipyreticOrganic chemistry methodsDi-tert-butyl peroxideMethyl group
The invention relates to a polychloromethyl-substituted indoline compound and a synthetic method and an application thereof, and specifically relates to the synthetic method of a 3-dichloromethyl and3-trichloromethyl-substituted indoline compound, which relates to the field of medicines, organic chemicals and fine chemicals. According to the method, N-(2-methylallyl)-acylarylamine compounds are utilized as raw materials, dichloromethane and carbon tetrachloride are utilized as sources of dichloromethyl and trichloromethyl, and a 3-dichloromethyl or 3-trichloromethyl-substituted N-acyl indoline compound is synthesized under catalysis of peroxide. Specific reaction conditions are that: tert-butyl hydroperoxide, tert-butyl peroxybenzoate, lauric aldehyde peroxide, di-tert-butyl peroxide, andbenzoyl peroxide are utilized as catalysts and free radical initiators under heating and stirring conditions. A reaction does not require participation of transition metal, an addition cyclization process of a free radical process is undergone, and post-treatment is simple, which is an effective way to synthesize the 3-dichloromethyl or 3-trichloromethyl-substituted N-acyl indoline compound.
Owner:JIANGSU UNIV OF TECH
Method for producing metalized fibrous composite sheet with olefin coating
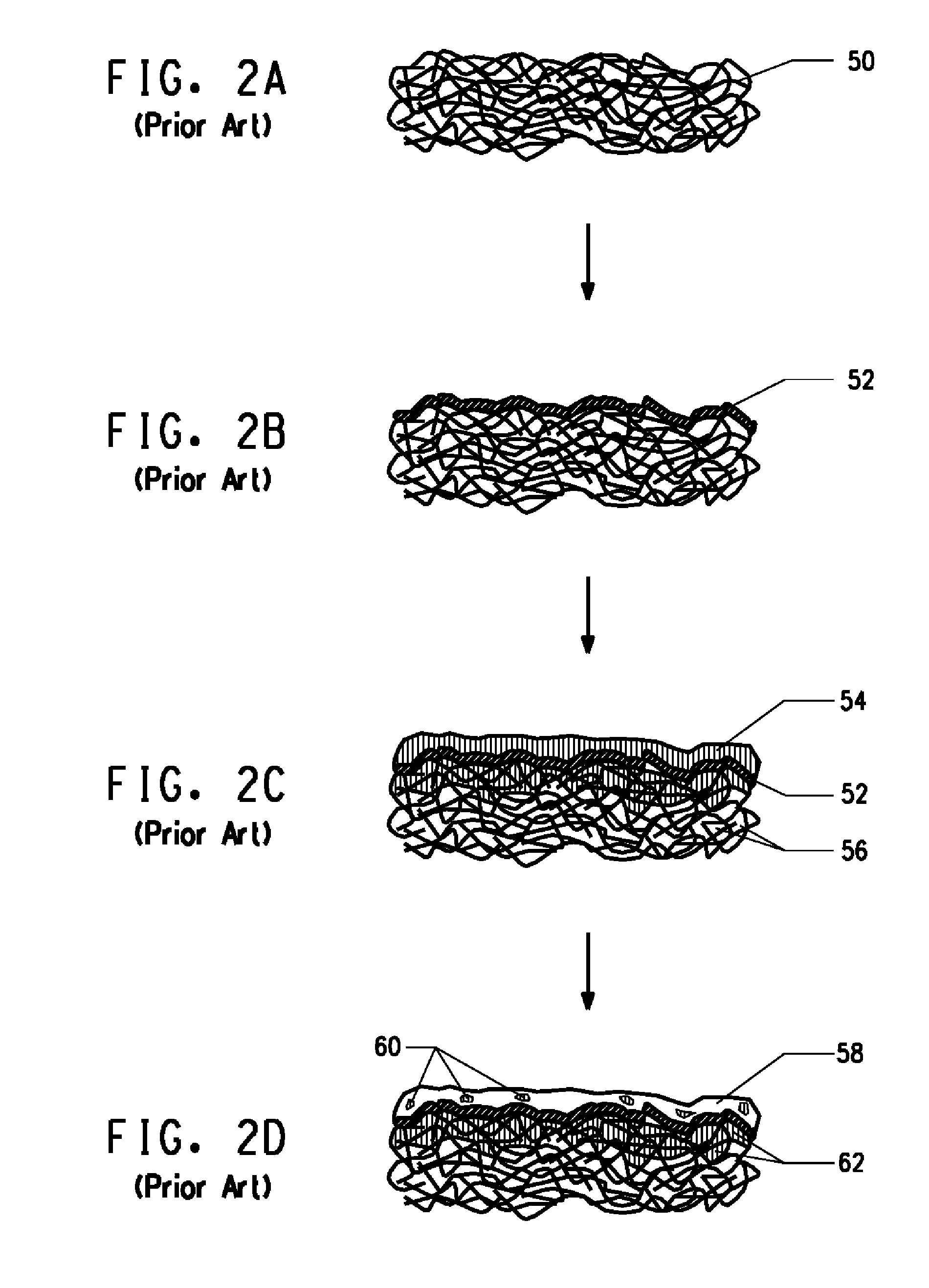
A composite sheet is manufactured by depositing a multi-layer coating on the outer surface of a substrate, the coating comprising a metal layer and an outer polymeric layer formed from a precursor comprising a composition capable of being polymerized and / or cross-linked by free-radical processes. After the precursor is applied, the composite sheet is exposed to beam radiation and ozone, which both promote conversion of the precursor. The function of the cured polymeric layer includes protecting the metal layer from corrosion. The use of both beam radiation and ozone promotes substantially full conversion and curing of the precursor, even in portions of the substrate that are geometrically shadowed from incident beam radiation.
Owner:EI DU PONT DE NEMOURS & CO
Copolymer of ethylene oxide and at least one substituted oxirane carrying a cross-linkable function, process for preparation thereof and use thereof for producing materials with ionic conduction
InactiveUS20040038132A1Easy to foreseeConductive materialSolid electrolyte cellsLithiumEthylene oxide
The invention concerns a copolymer of ethylene oxide and at least one substituted oxirane carrying a cross-linkable function. The copolymer comprises ethylene oxide, -O-CH2-CHR- units in which R is a substituent containing a reactive function which is cross-linkable by free radical process, R may be different from one unit to the other, and possibly -O-CH2-CHR'- units in which R' is a substituent containing no reactive function which is cross-linkable by means of a free radical process, R' may be different from one unit to the other. It is characterized by an excellent polymolecularity index I=Mp / Mn and a statistical distribution of the different monomer units. The copolymer is prepared by an anionic copolymerization process. The copolymer is useful for preparing a solid electrolyte having good mechanical properties, a good cationic conductivity and a good chemical compatibility with the electrodes of a generator operating with alkali metals such as lithium and sodium.
Owner:BATHIUM CANADA
Acrylic polymer low temperature flow modifiers in bio-derived fuels
ActiveUS8236069B2Excellent at modifying the low temperature flow behavior in bio-derived fuelsReduce pointsLiquid carbonaceous fuelsBio-feedstockEthylene HomopolymersFree Radical Process
Owner:ARKEMA INC
Application of catalyst taking waste adsorbent after adsorption-desorption as raw material in treatment of high-salt organic wastewater by activating persulfate
ActiveCN112340830ASolve problems that cannot be effectively handledSolve environmental problemsPhysical/chemical process catalystsWater contaminantsPtru catalystSorbent
The invention relates to an application of a catalyst taking a waste adsorbent after adsorption and desorption as a raw material in treatment of high-salt organic wastewater by activating persulfate.The method comprises the following steps: mixing a waste adsorbent which is desorbed after adsorbing heavy metal ions for multiple times with a nitrogen source, pyrolyzing under an oxygen-free condition to obtain a waste adsorbent-based catalyst, introducing the waste adsorbent-based catalyst into high-salt organic wastewater, and adding persulfate for activating sulfate to generate non-free radicals. Persistent organic pollutants in high-salt organic wastewater are efficiently degraded through non-free radicals, the problem that an adsorbent for adsorbing saturated heavy metal ions cannot beeffectively treated is solved, a new way is provided for degrading pollutants through a non-free radical process of activated persulfate, operation is easy, cost is low, and the method is suitable forindustrial production; meanwhile, the environmental problem can be effectively solved, and waste can be utilized.
Owner:SHANDONG UNIV
Method for preparing ester by catalyzing halogenated aromatic and carbonyl source through non-transition metal under visible light
InactiveCN108129308AEasy to recycleEasy to purifyPreparation by carbon monoxide or formate reactionPhenanthrolineProtein carbonyl
The invention discloses a method for preparing ester by catalyzing a halogenated aromatic and a carbonyl source through non-transition metal under visible light. The method is characterized by takinga complex formed by potassium tert-butoxide derivatives and 1,10-phenanthroline derivatives as a photocatalyst, taking carbon monoxide as the carbonyl source, taking the halogenated aromatic and derivatives thereof as reaction substrates, and generating ester through a free radical process under light illumination. Through the method, a metal-free organic complex is used for replacing the originalnoble metal catalyst; a visible light excitation mode is used for replacing the conventional heating manner; the carbonylation reaction of the halogenated aromatic is environmentally, economically and efficiently achieved; the synthesis conditions are mild; the raw materials are easily available and environmentally friendly; the solar energy is efficiently utilized; the method has obvious economic and social benefits.
Owner:FUZHOU UNIV
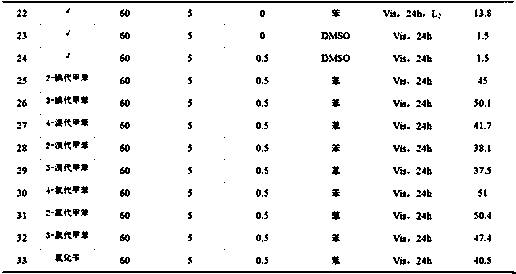
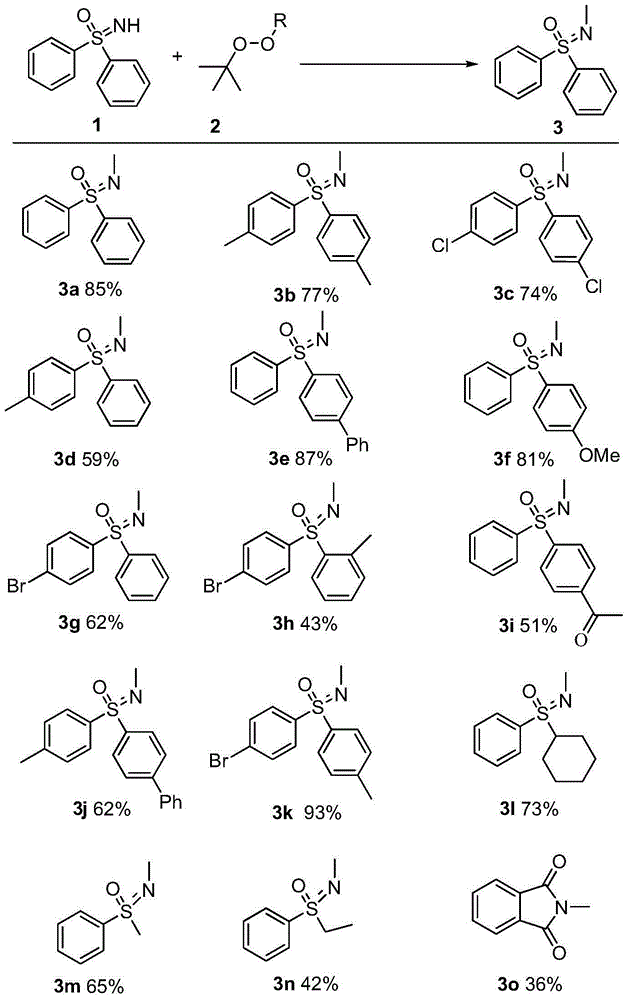
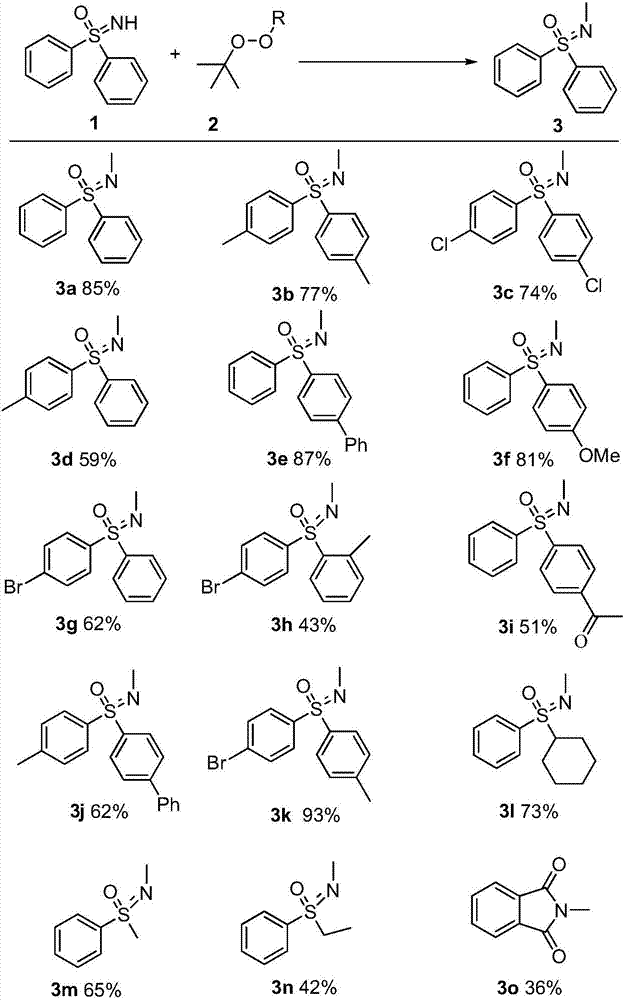
Synthetic method of novel N-methylated sulfoximine derivative
A simple, clean and high-effective synthetic method of a novel N-methylated sulfoximine derivative is provided in the invention. The invention relates to the fields of pesticides, organic chemical engineering and fine chemical engineering. The method essentially is an organic synthesis reaction that a methyl free radical, which is generated from peroxides, and a sulfoximine compound are subjected to free radical coupling to form new C-N bonds. The method includes the step of carrying out a reaction to obtain the N-methylated sulfoximine derivative in a common organic solvent under a heating condition from the raw materials including diphenyl sulfoximine and a methylating reagent (peroxides) under the catalysis by copper. The method is 2-20 h in reaction time under a heating and stirring condition. The molar ratio of the raw materials is that the diphenyl sulfoximine to the methylating reagent is 1:0.5-3.0. The raw materials are reacted under the heating condition and then reaction products are subjected to simple after treatments to obtain the N-methylated sulfoximine derivative at a yield being 36-87%. The invention develops a series of N-methylating methods based on a free radical process to prepare a series of N-methylated sulfoximine derivatives. The method is simple in operation and after treatments, and is simple and practical in synthesis of these compounds.
Owner:CHANGZHOU UNIV
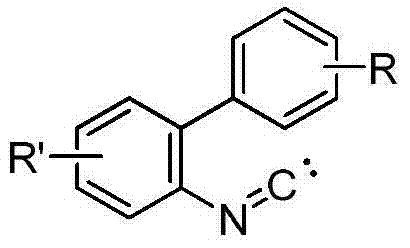
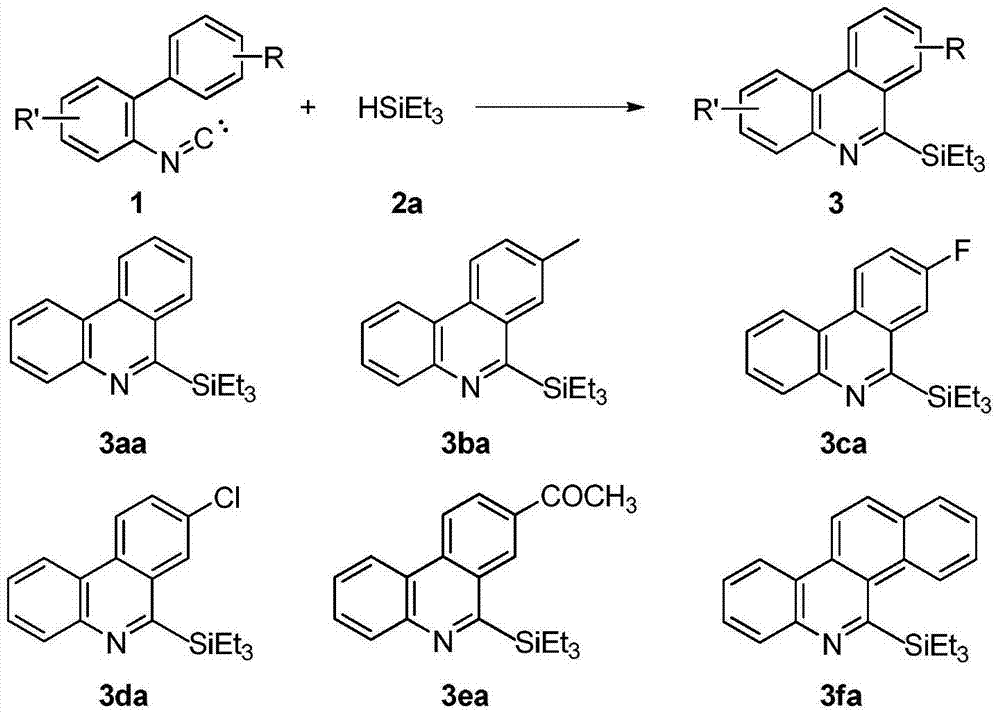
Method for synthesizing phenanthridine silane derivative
InactiveCN103936781AEasy to handleEasy to operateGroup 4/14 element organic compoundsSilanesCatalytic oxidation
The invention discloses a method for synthesizing a phenanthridine silane derivative, and relates to the fields of organic chemical engineering and fine chemical engineering. A small amount of organic solvent is added into 2-aryl aromatic isonitrile and silane under catalytic oxidation action of an oxidizing agent and a free radical initiator, and the mixture is subjected to cyclization reaction under the room temperature or heating condition to obtain the phenanthridine silane derivative. By using the method provided by the invention, the reaction time is 4-12 hours under the heating and stirring condition; the raw materials are reacted between the room temperature and 120 DEG C and are subjected to simple after-treatment so as to obtain the corresponding phenanthridine silane derivative with high yield of 68-93 percent. A novel aryl sp<2> silicon carbon bond formation method based on a free radical process is developed, two steps of cyclizing the free radicals and forming a silicon carbon bond are carried out to obtain a series of phenanthridine silane derivatives; therefore, the method is easy to carry out and is convenient for after-treatment.
Owner:CHANGZHOU UNIV
Processes for the production of chlorinated and/or fluorinated propenes and higher alkenes
InactiveUS20140031595A1Improve efficiencyLow costPreparation by hydrogen halide split-offPreparation by halogen replacementAlkaneGas phase
The present invention provides continuous, gas phase, free radical processes for the production of chlorinated and / or fluorinated propenes or higher alkenes from the reaction of chlorinated and / or fluorinated alkanes and chlorinated and / or fluorinated alkenes, wherein wherein at least a portion of any intermediate boiler by-products generated by the process are removed from the process
Owner:BLUE CUBE IP
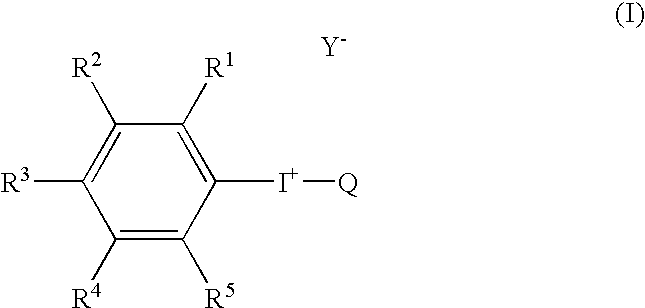
Radical trap in fluoridation of iodonium salt
Decomposition of iodonium salts by a free radical process has been identified as a significant factor in the observed yield variability of fluoridation reactions using said iodonium salts. Accordingly, the inclusion of a free radical trap in the reaction mixture blocks the radical chain decomposition pathway for iodonium salts such that only the reaction leading to fluoridation can occur and the yield of aryl fluoride becomes high and reproducible. The reaction may also be carried out on solid phase. In both the solution and the solid phase the preferred method of the present invention is radiofluoridation.
Owner:GE HEALTHCARE LTD
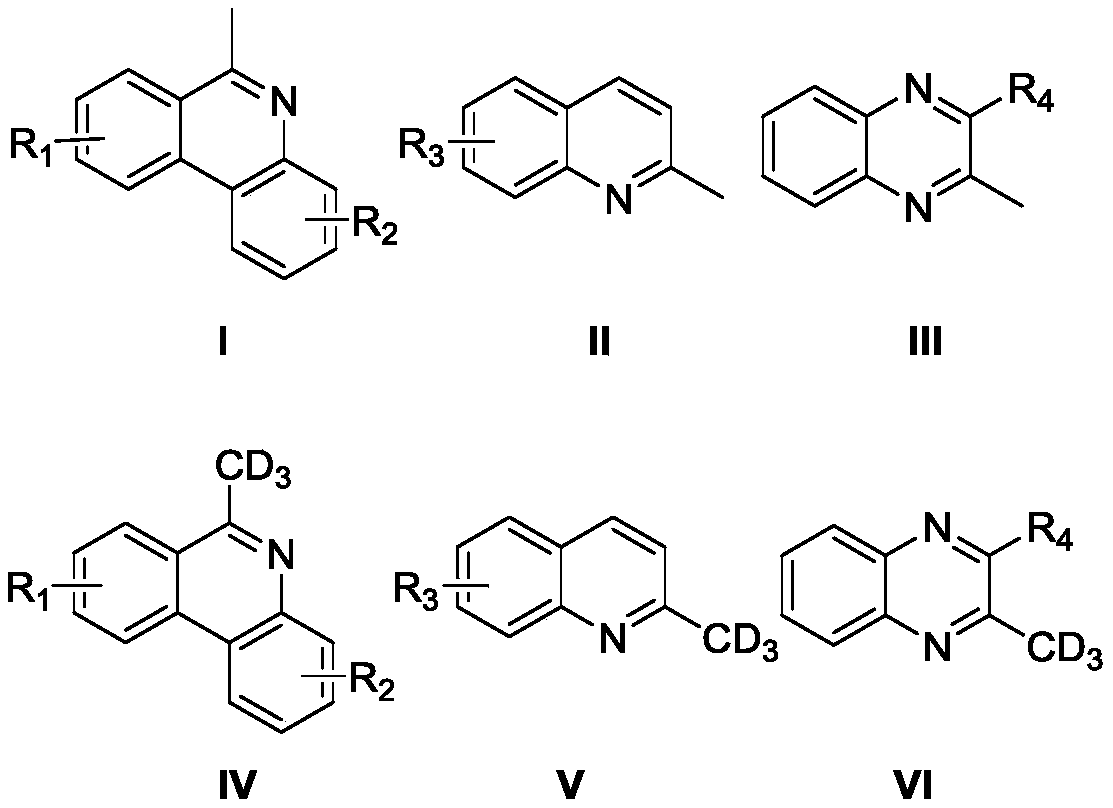
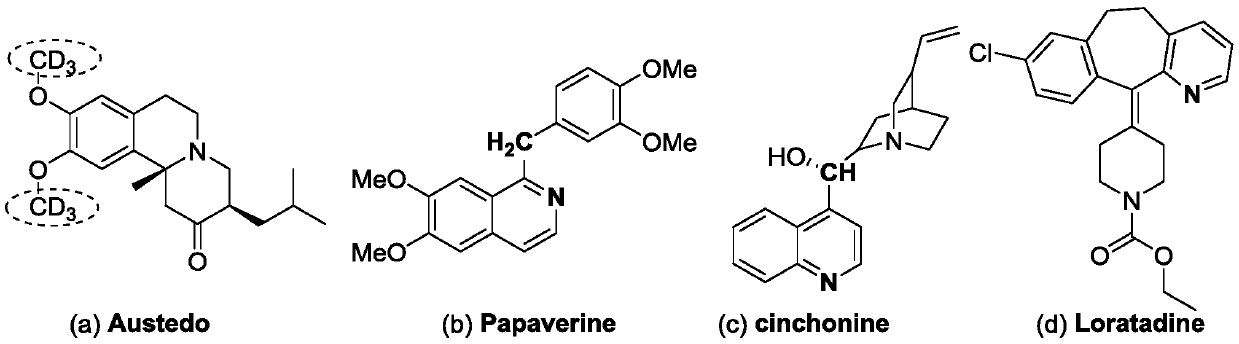
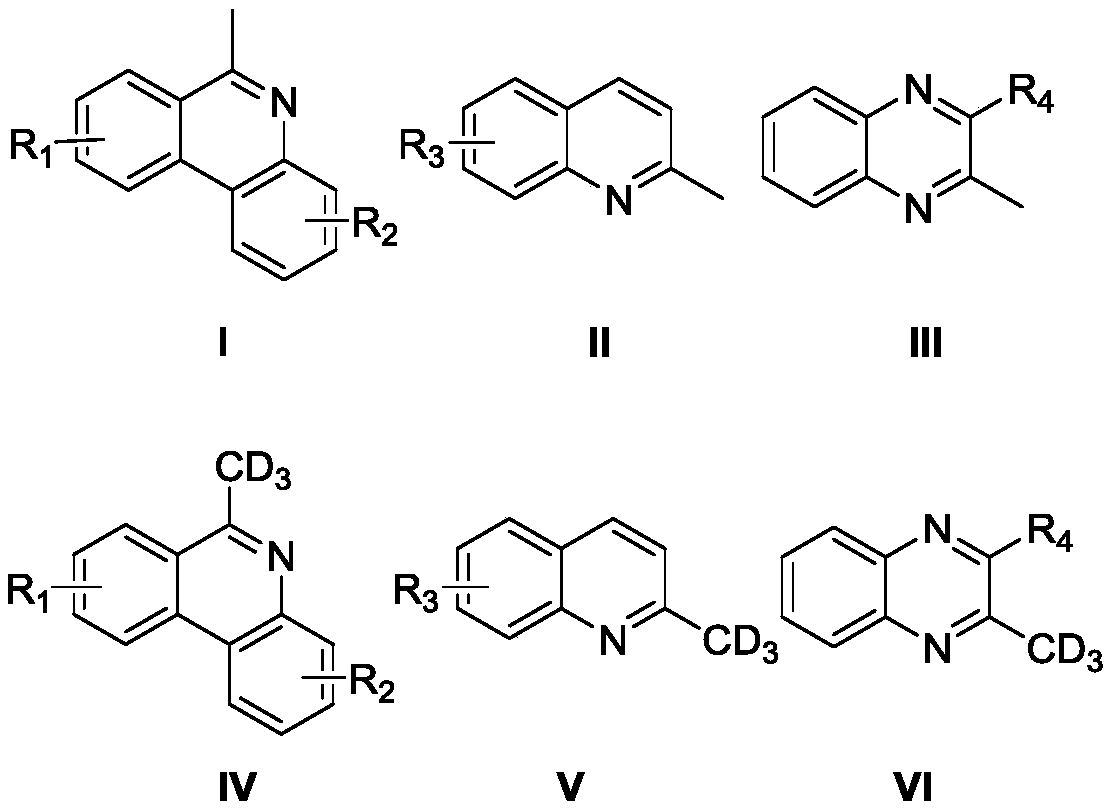
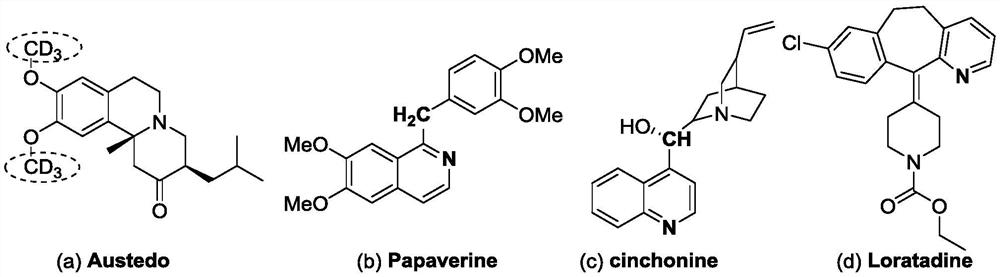
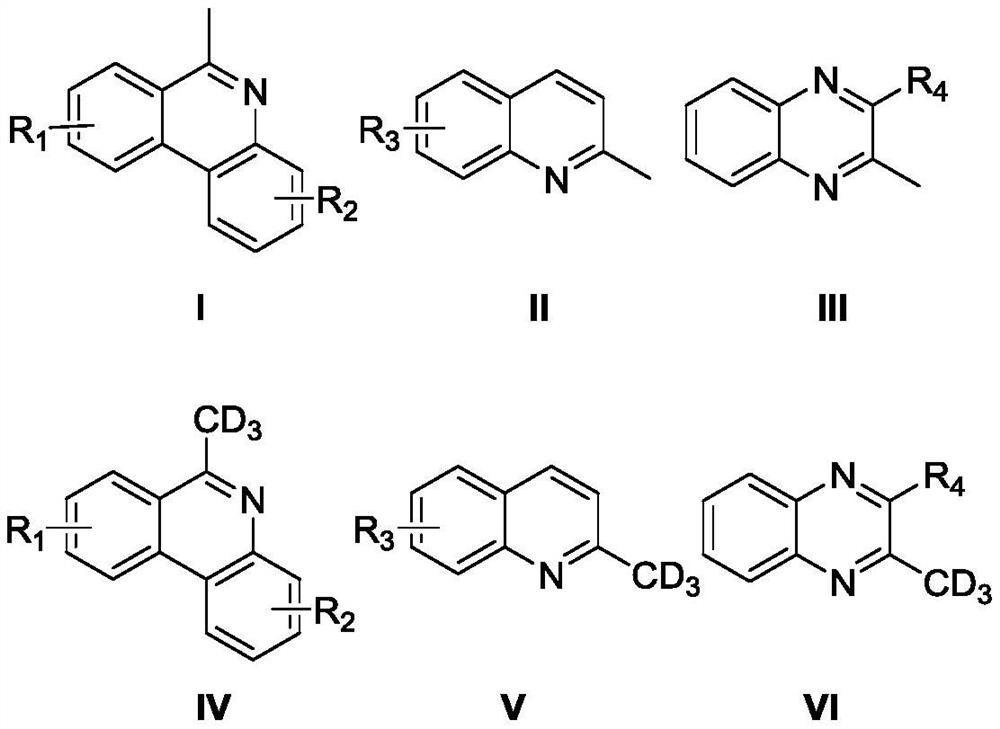
High-selectivity deuteration method of 2-methyl azacyclo compound
ActiveCN110563649AReduce consumptionUniversalIsotope introduction to heterocyclic compoundsShielding gasHigh selectivity
The invention discloses a high-selectivity deuteration method of a 2-methyl azacyclo compound. The method is carried out according to the following steps: adding a 2-methyl azacyclo compound shown asa formula I, a formula II or a formula III, an oxidant and an additive into a dry Schlenk reaction tube; and in the presence of a protective gas, adding deuterium water and an organic solvent into thereaction tube, performing a stirring reaction at 50-100 DEG C for 2-12 hours to obtain a reaction solution, and performing post-treatment on the reaction solution to respectively obtain a deuteratednitrogen-containing heterocycle shown in a formula IV, a formula V or a formula VI. The method provided by the invention is based on a free radical process, is efficient, can synthesize methyl-d3 substituted nitrogen-containing heterocyclic compounds which are difficult to prepare by conventional methods, and is high in deuteration rate of reaction; the method is carried out under a neutral condition and has low requirements on equipment; a catalytic amount of an oxidant is used, and additives are cheap and easily available; reaction conditions are mild, and energy consumption is reduced; theyield is high, the substrate universality is strong, the operation is simple and convenient, and the like.
Owner:ZHEJIANG UNIV OF TECH
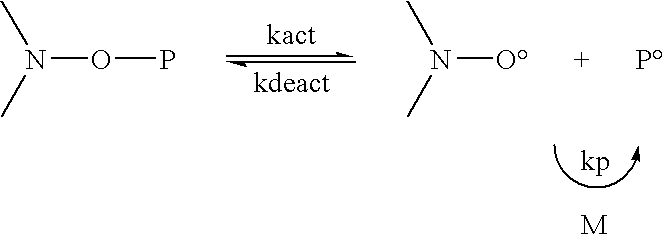
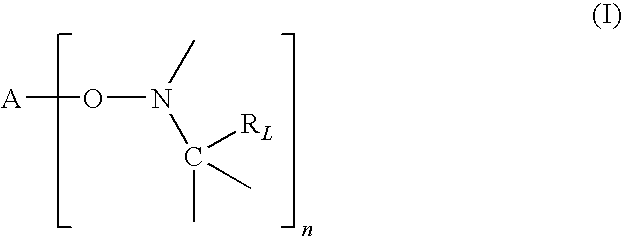
Free radical process for making low molecular weight compounds useful for making high octane fuels
PendingUS20210269378A1Boost octaneImprove production yieldOrganic compound preparationHydroxy compound preparationPolymer scienceOrganic compound
The present invention relates to free radical reaction methods in which low molecular weight, C2 to C6, unsaturated organic compounds such as ethylene and / or propylene are reacted with low molecular weight, C1 to C15, preferably C1 to C10 saturated organic compounds to form low molecular weight, linear or branched C3 to C24, preferably C3 to C 12 organic compounds. The present invention is based at least in part upon the concept of carrying out the free radical reaction in the presence of a typically low concentrations of the unsaturated reactant(s) in the reaction zone(s). By doing this, chain transfer mechanisms are more favored while chain extension mechanisms are less favored. In some embodiments, principles of the present invention are helpful to create conditions under which chain transfer to form more stable, secondary or tertiary branched radicals is favored over olefin addition via chain extension.
Owner:KEEN PROCESS TECH LLC
A kind of method for preparing oxalate
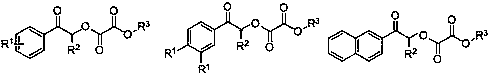
ActiveCN108863777BToxicReaction economyOrganic compound preparationCarboxylic acid esters preparationPtru catalystOrganic dye
The invention discloses a method for preparing oxalate: using diazo compounds and α-Br ketones as reaction substrates, and using O 2 As oxygen source and oxidizing agent, visible light is used as energy source, organic dye is used as photocatalyst, and oxalate is obtained through free radical process in organic solvent. The method used in the present invention has the following characteristics: the reaction is more environmentally friendly and economical, the substrate has wider applicability, the later functional group is easier, the reaction condition is mild, it can be carried out in the air, the amount of photocatalyst is less, and the post-treatment is simple. At the same time, the reactants, photocatalysts and other raw materials used in the present invention are cheap and easy to obtain, the reaction composition is reasonable, no ligand is needed, the atom economy is high, the reaction steps are few, and a higher yield can be obtained with only one step of reaction, which is in line with contemporary green The requirements and direction of chemistry and sustainable development are suitable for the synthesis of asymmetrically substituted oxalates that are difficult to synthesize by traditional methods.
Owner:SUZHOU UNIV
Synthetic method of novel n-methylated sulfoximine derivatives
ActiveCN105130863BReduce usageShort reaction timeOrganic chemistryMethylating AgentOrganic synthesis
A simple, clean and efficient method for preparing N-methylated sulfoximine derivatives is invented, which relates to the fields of pesticides, organic chemicals and fine chemicals. The method is essentially an organic synthesis reaction in which methyl radicals generated from peroxides are coupled with sulfoximine compounds to form new carbon-nitrogen bonds. The raw materials used are diphenyl sulfoximine and methylating reagent (peroxide) under the catalysis of copper, in common organic solvents, under heating conditions, react to obtain N-methylated sulfoximine derivatives thing. Using the method proposed by the present invention under heating and stirring conditions, the reaction time is 2-20 hours. The molar ratio of the raw materials is diphenylsulfoximine: methylating reagent = 1:0.5-3.0, reacting under heating conditions, and then undergoing simple post-treatment, the corresponding N-methyl can be obtained in a higher yield sulfoximine derivatives with a yield of 36‑87%. The present invention develops a novel N-methylation method based on a free radical process to obtain a series of N-methyl sulfoximine derivatives, which is simple in operation and convenient in post-processing. It is a simple and practical method for synthesizing such compounds.
Owner:CHANGZHOU UNIV
Preparation method of oxalate
ActiveCN108863777APrefabRaw materials are highly toxicOrganic compound preparationCarboxylic acid esters preparationEnvironmental resistanceOrganic dye
The invention discloses a preparation method of oxalate. The preparation method comprises the following steps of using a diazo compound and alpha-Br ketone compound as reaction primers, using O2 (oxygen) as an oxygen source and an oxidant, using visible light as an energy source, using organic dye as a photocatalyst, and performing the free radical process in an organic solvent, so as to obtain the oxalate. The preparation method has the advantages that the reaction is more green, environment-friendly and economic; the generality of the primer is broad, the later functionalization is easier, the reaction conditions are moderate, and the reaction can be performed in air; the usage amount of the photocatalyst is small, and the post-treatment is simple and convenient; the used raw materials of reaction matter, photocatalyst and the like are low in prices and are easy to obtain, the reaction composition is reasonable, the ligand is not required, the atom economy is high, and the number ofreaction steps is fewer; the higher yield rate can be obtained by one-step reaction, the current green chemical and sustained development requirements and orientation are met, and the preparation method is suitable for synthesizing the asymmetric substituted oxalate which is difficult to synthesize by the traditional method.
Owner:SUZHOU UNIV
Substrate handling equipment and heat shields
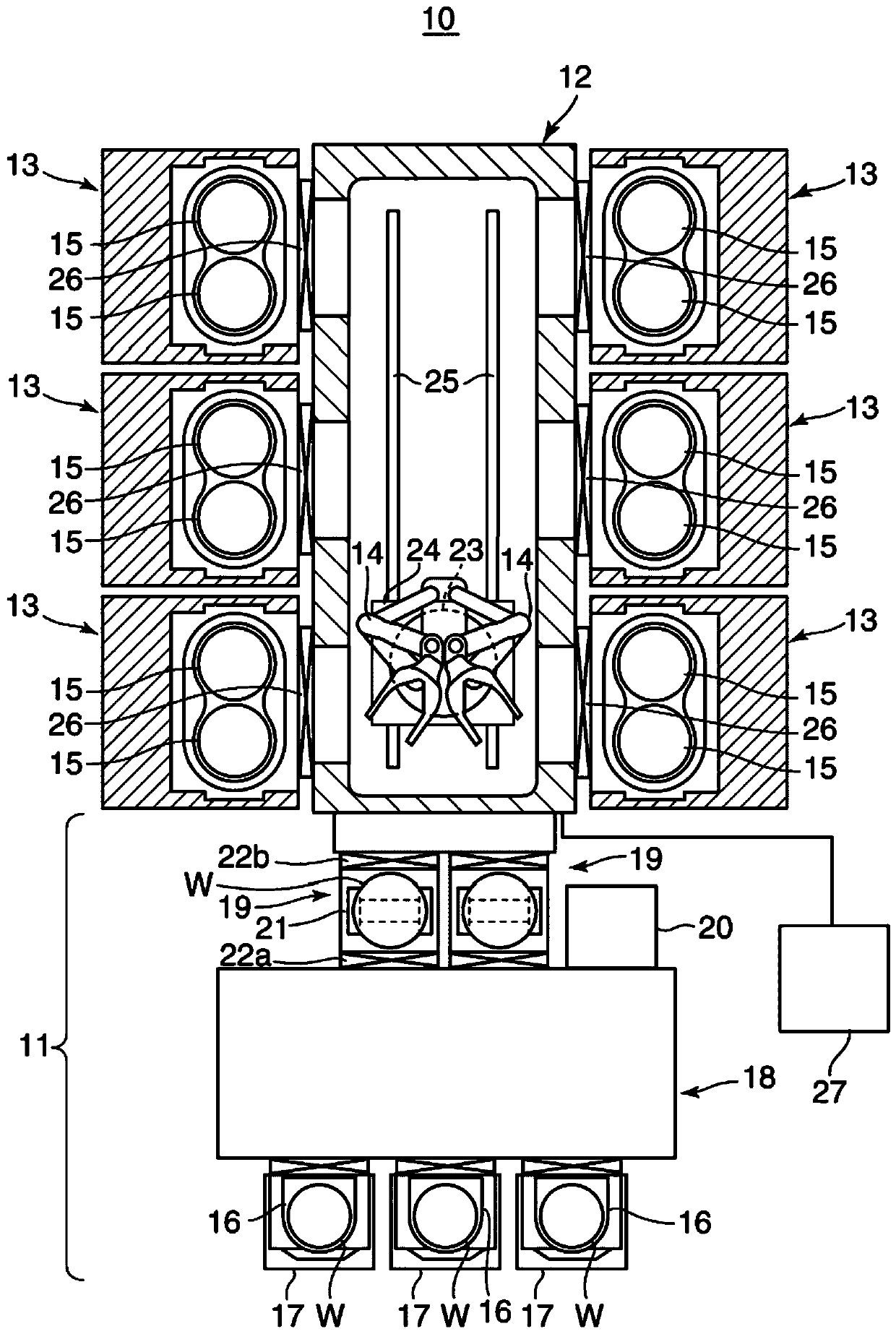
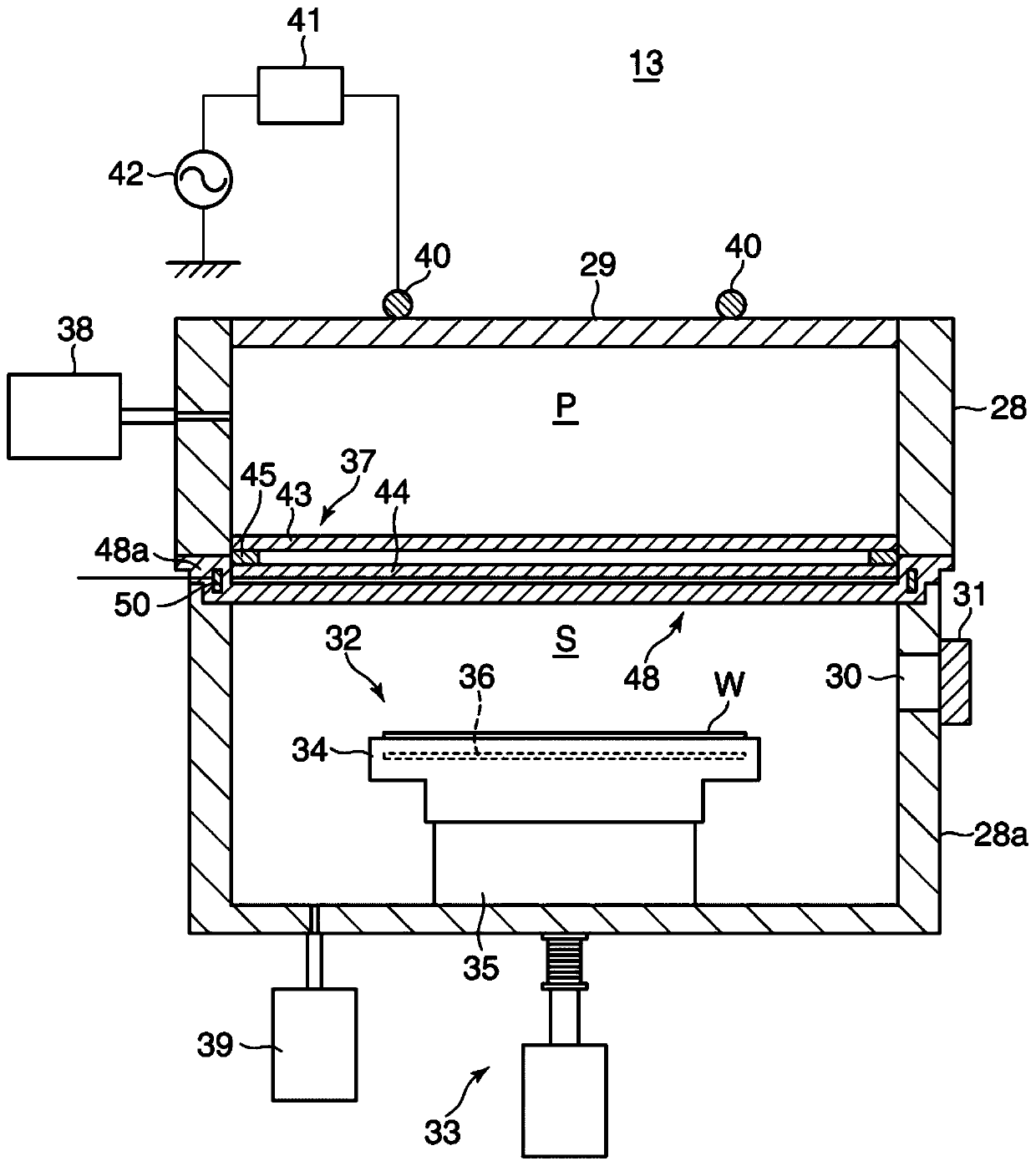
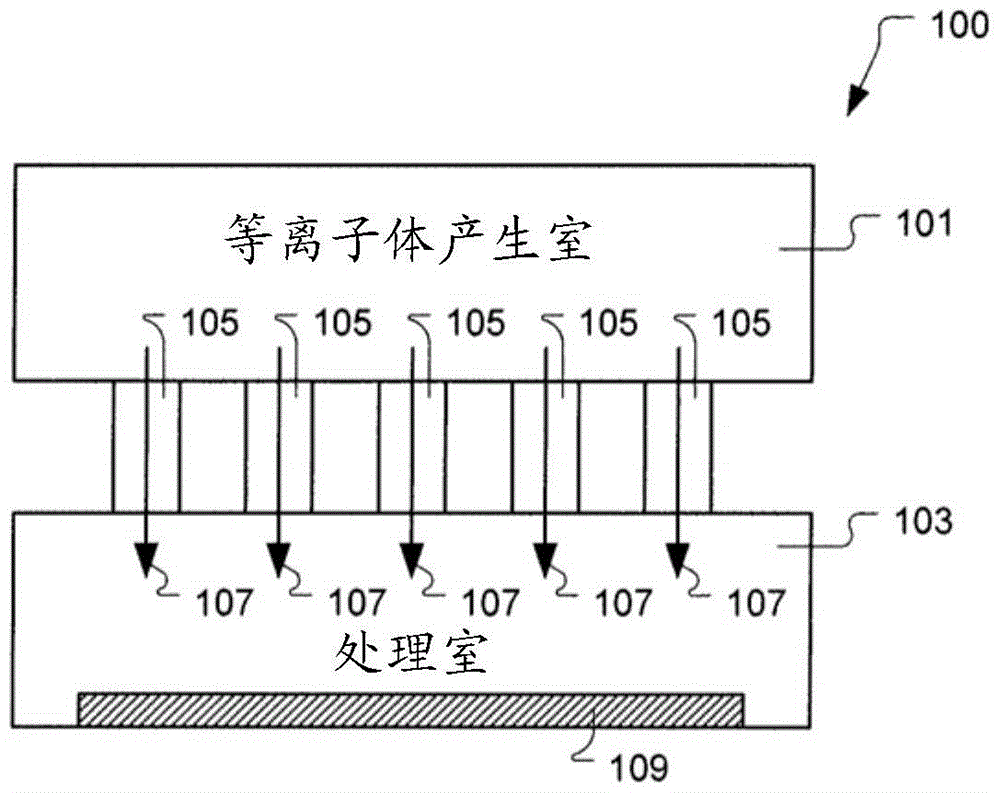
ActiveCN108122727BRadiation suppressionPrevents skewed distributionElectric discharge tubesSemiconductor/solid-state device manufacturingProcess moduleComputer module
The invention provides a substrate processing device and a heat shield. The substrate processing apparatus can uniformly perform processing using radicals on a substrate even when processing is repeated. The process module (13) has: a processing container (28) for accommodating the wafer (W); (W); a heat shield (48), which is configured between the partition plate (37) and the wafer (W), and the partition plate (37) is selectively placed in the plasma generation space (P) Radicals in the generated plasma permeate toward the wafer (W), and the heat shield (48) is arranged in a manner facing the wafer (W). )connect.
Owner:TOKYO ELECTRON LTD
Method for producing metalized fibrous composite sheet with olefin coating
A composite sheet is manufactured by depositing a multi-layer coating on the outer surface of a substrate, the coating comprising a metal layer and an outer polymeric layer formed from a precursor comprising a composition capable of being polymerized and / or cross-linked by free-radical processes. After the precursor is applied, the composite sheet is exposed to beam radiation and ozone, which both promote conversion of the precursor. The function of the cured polymeric layer includes protecting the metal layer from corrosion. The use of both beam radiation and ozone promotes substantially full conversion and curing of the precursor, even in portions of the substrate that are geometrically shadowed from incident beam radiation.
Owner:EI DU PONT DE NEMOURS & CO
Method for preparing temperature-sensitive polyvinylidene fluoride intelligent membrane material and its product
This invention relates to a method for prparing temperature-sensitive polyvinylidene flourine intelligent membrane. The method is: NIPA as grafted monomer, PVDF as macromelocular initiator, cuprous chloride as catalyst,4,4 demethyl 2,2 bipyridine as ligand, using atom transfer free radical process, preparation of temperature-sensitive polyvinylidene flourine intelligent membrane comprising: in reaction kettle, adding 7-13% PVDF(WT%) and solvent N-methyl-2-pyrrolidone,heating to 50 deg. C to make it solve completely; then adding grafted monomer NIPA, its mol ratio with cuprous chloride is 2000:1-2500:1,filling nitrogen gas 30mins. at stirring, and separately adding 0.20-025g ligand 4,4-dimethyl 2,2-bipyridine and 0.04-0.06g catalyst cupous chloride, stirring and heating to 80-120deg.C,constant reaction 19-25 hrs; then washing reaction product using pure water to obtain light brown solid, filtering and 80 C degree drying to obtain finish product.
Owner:TIANJIN POLYTECHNIC UNIV
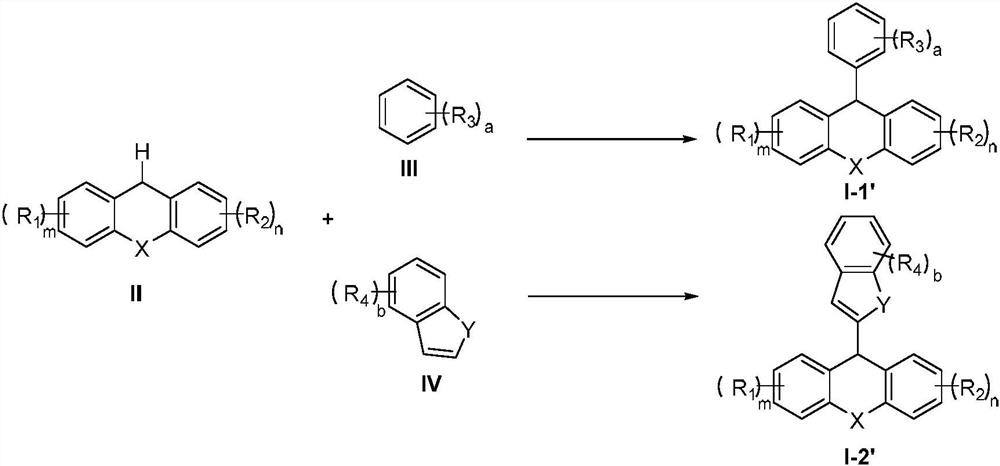
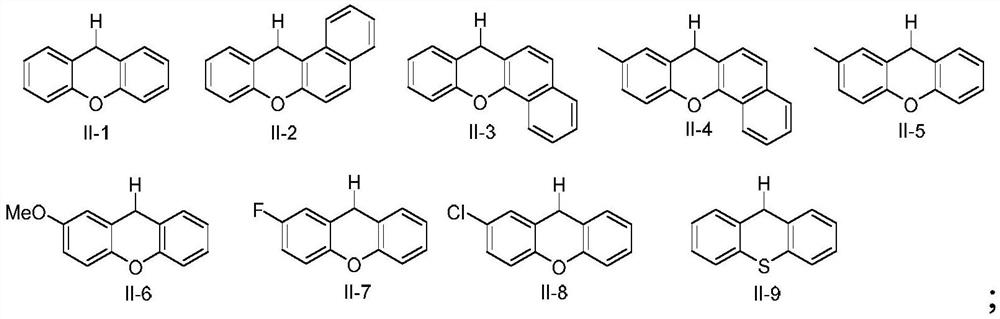
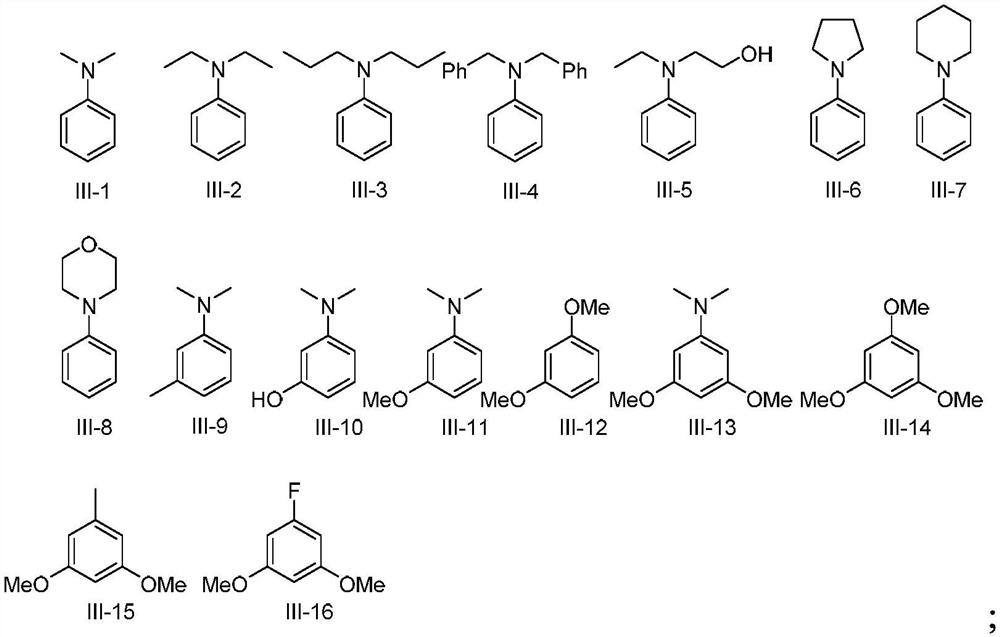
Preparation method of 9-aryl xanthene compounds
The invention discloses a novel preparation method of 9-aryl xanthene compounds, xanthene compounds are subjected to C (sp3)-H arylation reaction with aromatic hydrocarbon in a free radical process to prepare a series of 9-aryl xanthene compounds. Compared with the prior art, the method has the remarkable advantages that traditional severe reaction conditions such as a transition metal catalyst, an oxidizing agent and high temperature are not needed, and the method has the advantages of mild reaction conditions, wide substrate application range, good atom economy and convenient reaction operation.
Owner:NANCHANG HANGKONG UNIVERSITY
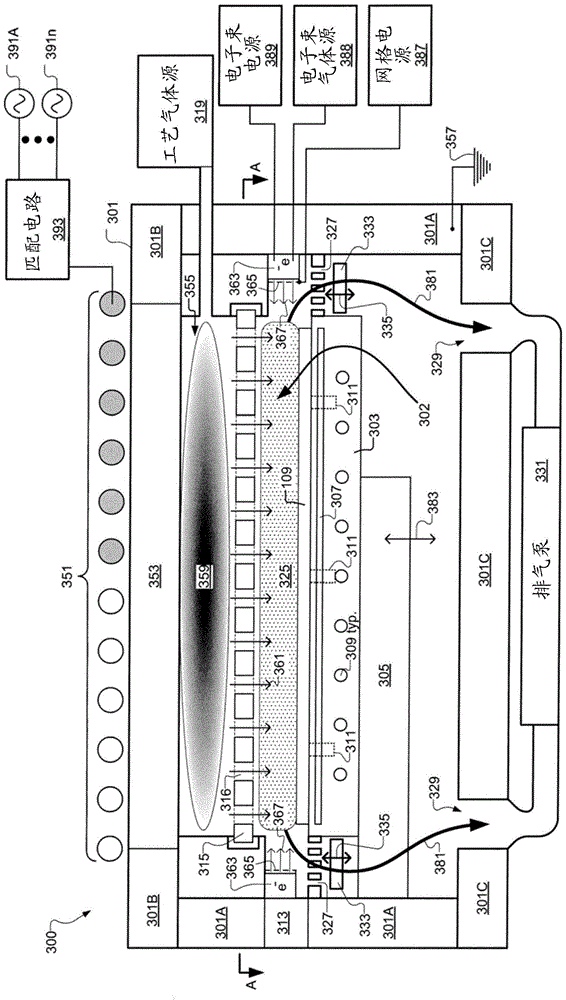
Electron Beam Enhanced Decoupling Sources for Semiconductor Processing
A semiconductor substrate processing system includes a processing chamber and a substrate support defined to support a substrate in the processing chamber. The system also includes a plasma chamber defined separate from the processing chamber. The plasma chamber is defined to generate plasma. The system also includes a plurality of fluid transfer passages fluidly connecting the plasma chamber and the processing chamber. The plurality of fluid delivery pathways are defined to supply reactive components of the plasma from the plasma chamber to the processing chamber. The system further includes an electron injection device for injecting electrons into the processing chamber to control the energy distribution of the electrons within the processing chamber to thereby control the ion-to-radical density ratio within the processing chamber. In an embodiment, an electron beam source is defined to deliver an electron beam through the process chamber above and across the substrate support.
Owner:LAM RES CORP
Application of Catalyst Using Waste Adsorbent After Adsorption-Desorption as Raw Materials in Activated Persulfate Treatment of High Salt Organic Wastewater
ActiveCN112340830BEfficient Value UtilizationAvoid secondary pollutionPhysical/chemical process catalystsWater contaminantsPtru catalystSorbent
The invention relates to the application of a catalyst using the waste adsorbent after adsorption-analysis as a raw material in the treatment of high-salt organic wastewater by activating persulfate. In the present invention, the discarded adsorbent after repeated adsorption of heavy metal ions-desorption is mixed with a nitrogen source, and the discarded adsorbent-based catalyst is obtained after pyrolysis under anaerobic conditions, and the discarded adsorbent-based catalyst is introduced into high-salt organic wastewater, and added Sulfate is used to activate sulfate to generate non-free radicals, and efficiently degrade persistent organic pollutants in high-salt organic wastewater through non-free radicals. Sulfate provides a new way to degrade pollutants through a non-free radical process, which is simple to operate and low in cost, and can effectively help solve environmental problems and can be used for waste.
Owner:SHANDONG UNIV
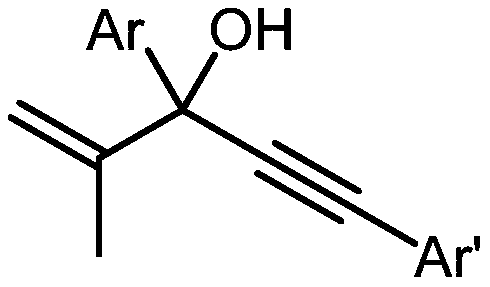
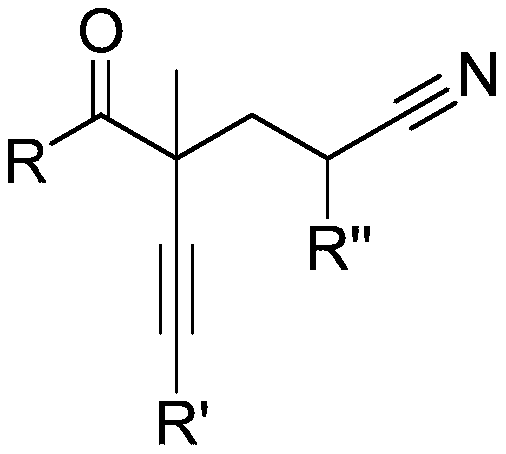
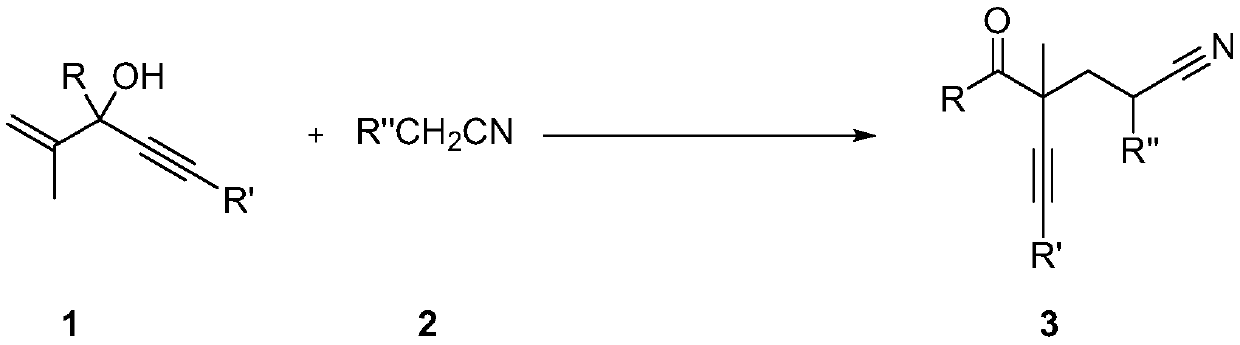
Method for preparing alpha-alkynyl gamma-cyano functionalized ketones from allyl alcohol
ActiveCN110950777AWide applicabilityHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationPropanolAryl
The invention belongs to the technical field of bifunctionalization reaction in a free radical process, and particularly relates to a method for preparing alpha-alkynyl gamma-cyano functionalized ketones from allyl alcohol; the bifunctionalization reaction of alpha-aryl alpha-alkynyl allyl alcohol and alkyl nitriles is researched, and a series of alpha-alkynyl gamma-cyano functionalized ketones are prepared. The method comprises the specific process steps: adding raw material allyl alcohol, an initiator and a nitrile solvent into a dehydrated and deoxidized Schlenk tube in proportion, puttingthe Schlenk tube into an oil bath pan at the temperature of 120 DEG C, and stirring for 12 hours; and finally, carrying out silica gel column chromatography to obtain the alpha-alkynyl gamma-cyano functionalized ketone products. The invention relates to the formation of alkyl nitrile free radicals, followed by intermolecular addition of the alkyl nitrile free radicals and olefins and migration of1,2-alkynyl, and a series of alpha-alkynyl gamma-cyano functionalized ketones are obtained.
Owner:CHANGZHOU UNIV
A highly selective deuteration method for 2-methyl nitrogen heterocyclic compounds
ActiveCN110563649BReduce consumptionUniversalIsotope introduction to heterocyclic compoundsOrganic solventCombinatorial chemistry
The invention discloses a highly selective deuteration method for 2-methyl nitrogen heterocyclic compounds. The method is carried out as follows: the 2-methyl nitrogen-containing compound shown in formula I, formula II or formula III Heterocyclic compounds, oxidants, and additives are added to a dry Schlenk reaction tube. Under protective gas conditions, deuterium water and organic solvents are added to the above reaction tube, and the reaction is stirred at 50-100°C for 2-12 hours to obtain the reaction The solution is post-treated to obtain deuterated nitrogen-containing heterocyclic rings shown in formula IV, formula V or formula VI respectively. The method of the present invention is based on a free radical process, is efficient, and can synthesize methyl-d3 substituted nitrogen-containing heterocyclic compounds that are difficult to prepare by existing methods, and the deuteration rate of the reaction is high; it is carried out under neutral conditions, and requires equipment. low; using a catalytic amount of oxidant, the additives are cheap and easy to obtain; the reaction conditions are relatively mild, energy consumption is saved; the yield is high, the substrate universality is strong, and the operation is simple and so on.
Owner:ZHEJIANG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com