Patents
Literature
82 results about "ORDER PRIMATES" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Order Primates is part of the clade Euarchontoglires, which is nested within the clade Eutheria of Class Mammalia.Recent molecular genetic research on primates, colugos, and treeshrews has shown that the two species of colugos are more closely related to primates than to treeshrews, even though treeshrews were at one time considered primates.
Methods and materials for the growth of primate-derived primordial stem cells in feeder-free culture
Methods and materials for culturing primate-derived primordial stem cells are described. In one embodiment, a cell culture medium for growing primate-derived primordial stem cells in a substantially undifferentiated state is provided which includes a low osmotic pressure, low endotoxin basic medium that is effective to support the growth of primate-derived primordial stem cells. The basic medium is combined with a nutrient serum effective to support the growth of primate-derived primordial stem cells and a substrate selected from the group consisting of feeder cells and an extracellular matrix component derived from feeder cells. The medium further includes non-essential amino acids, an anti-oxidant, and a first growth factor selected from the group consisting of nucleosides and a pyruvate salt.
Owner:ASTERIAS BIOTHERAPEUTICS INC
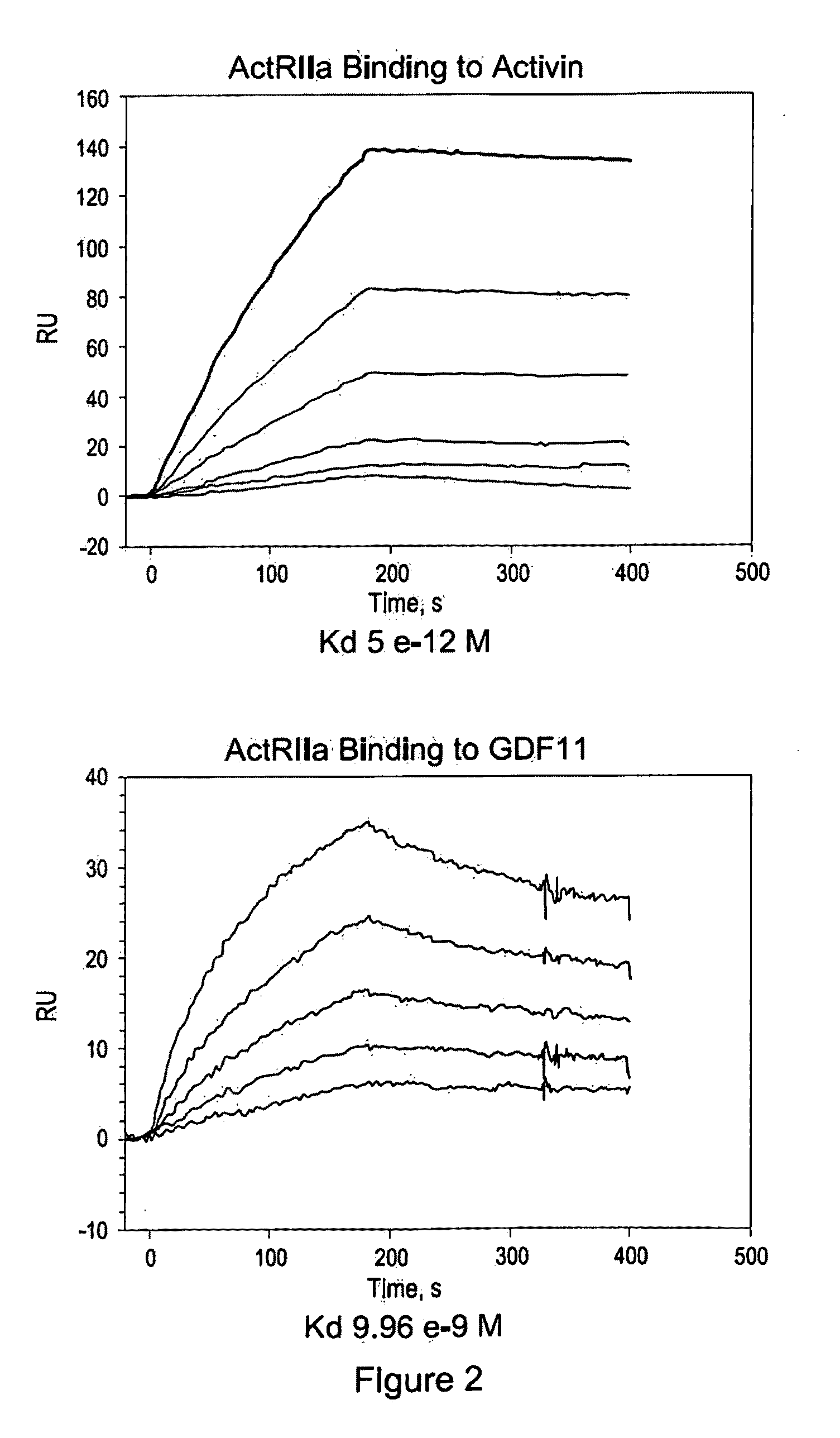
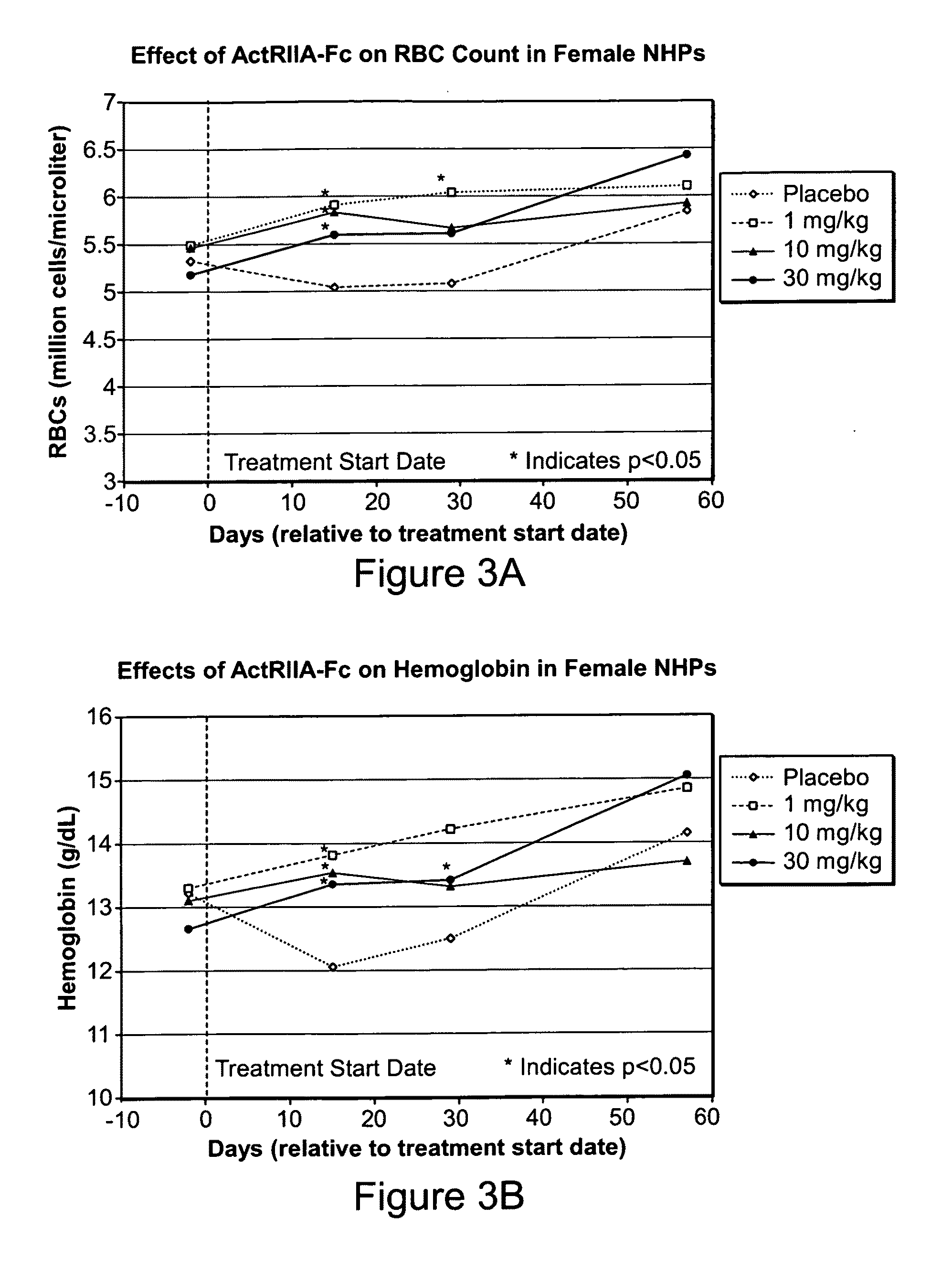
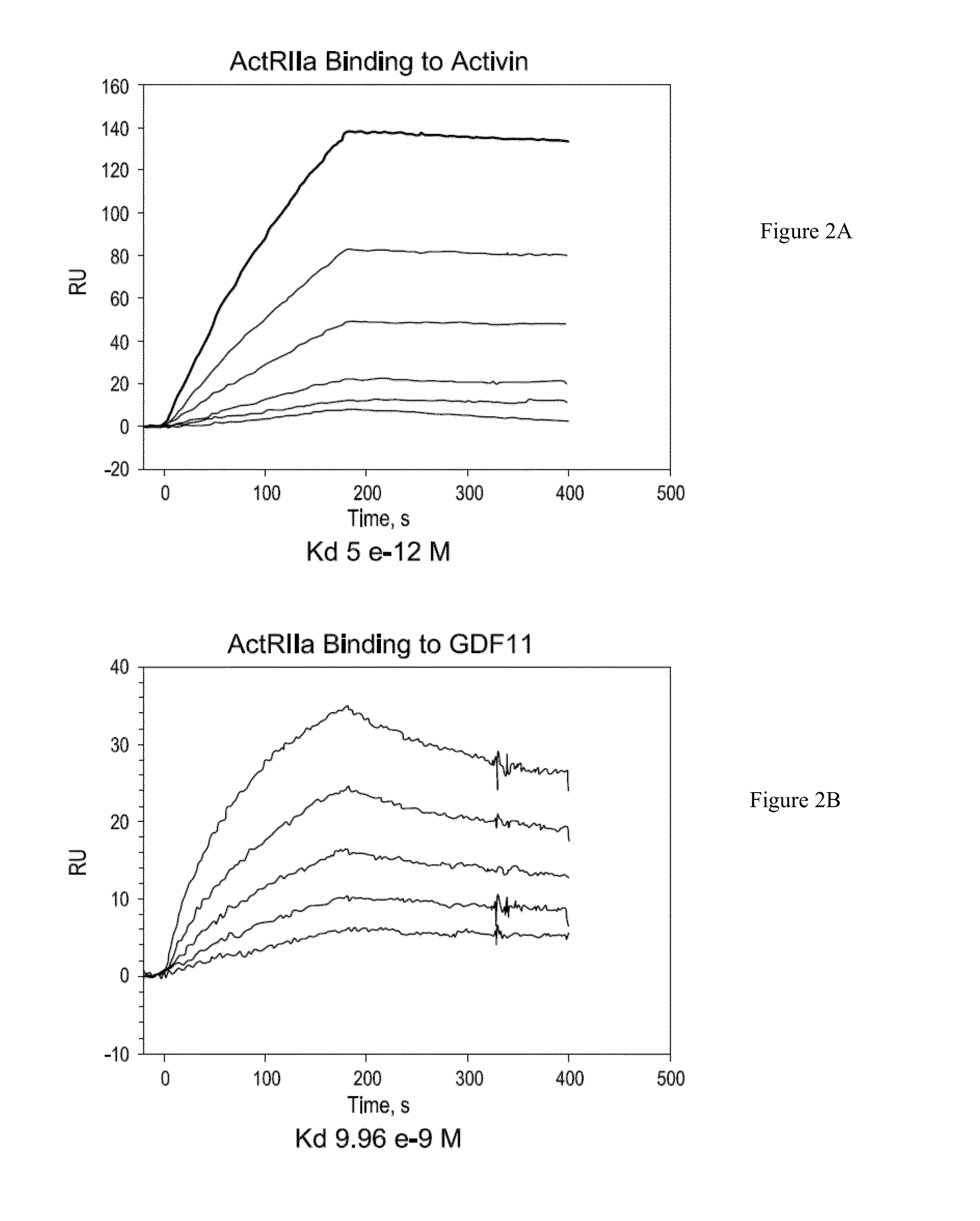
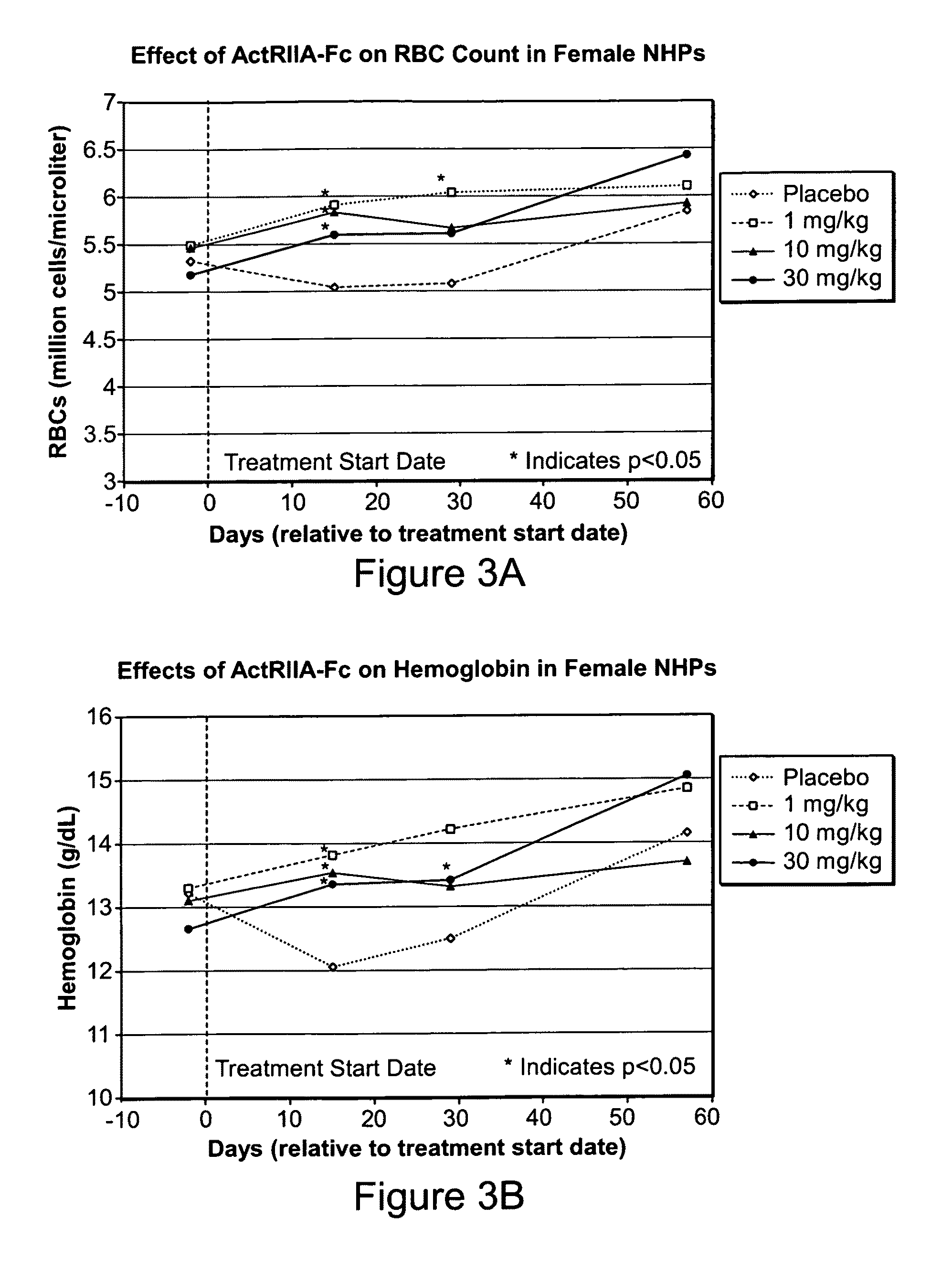
Use of GDF traps to increase red blood cell levels
ActiveUS20100068215A1Improve pharmacokineticsImprove purification effectPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed Cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
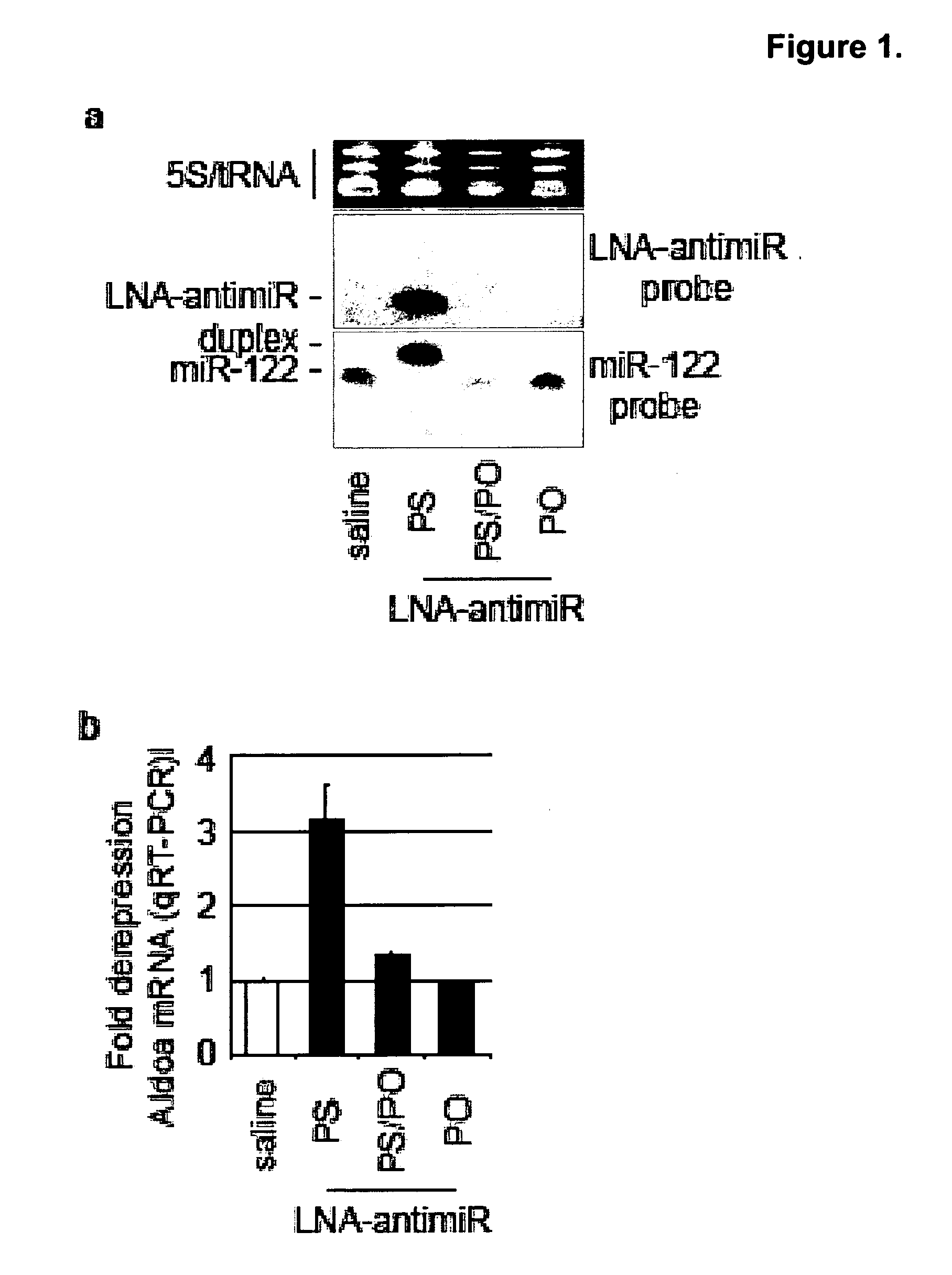
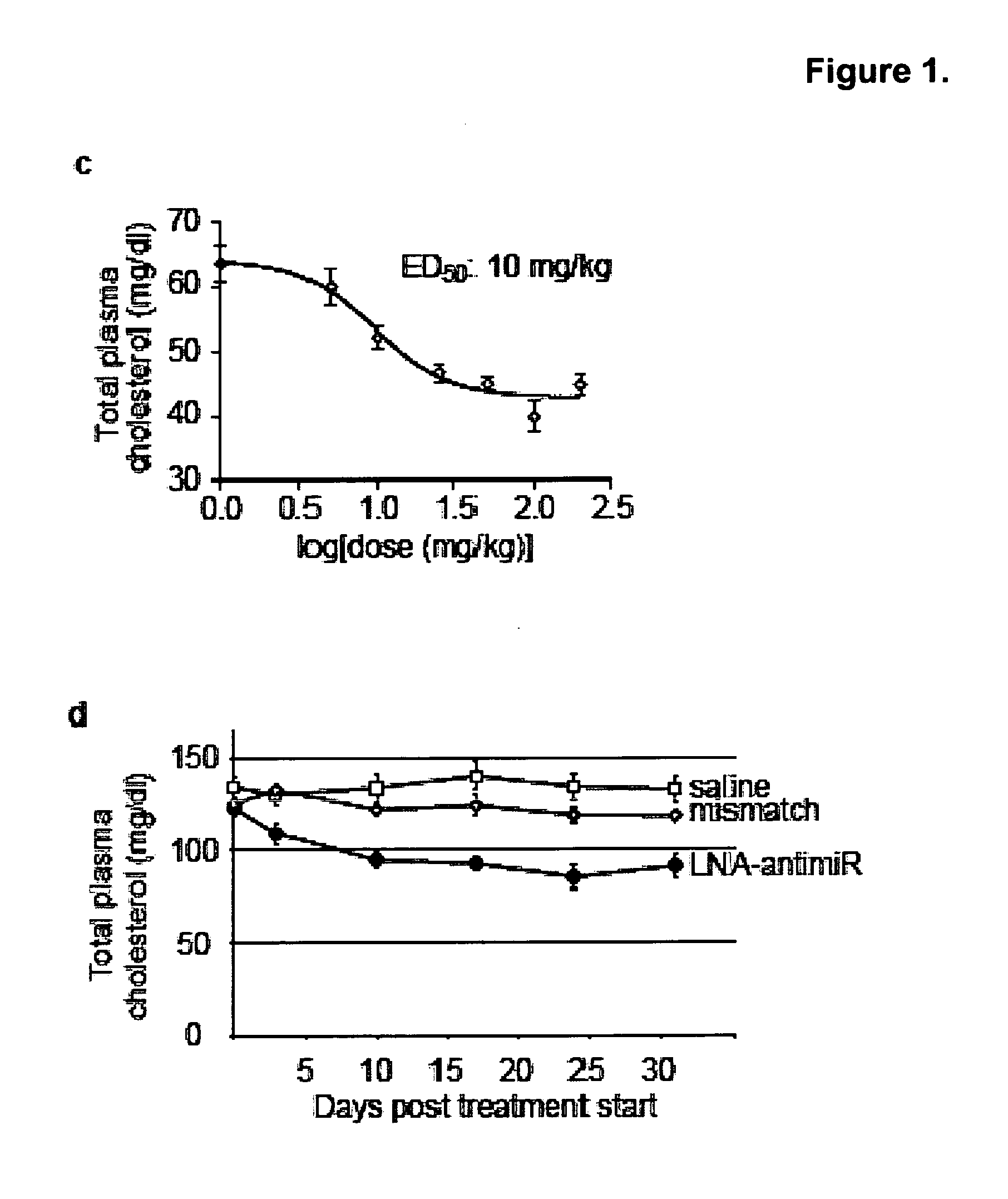
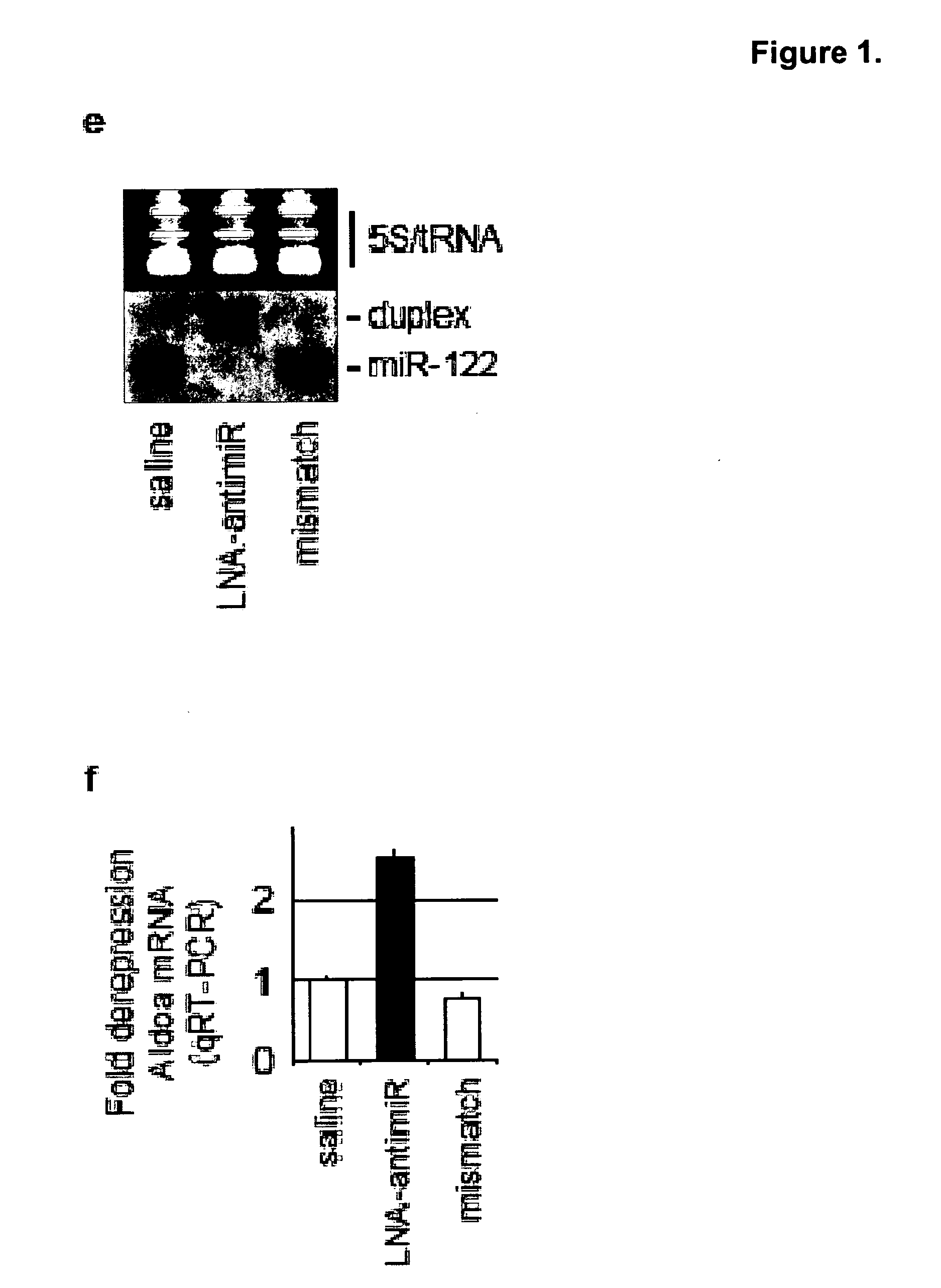
Pharmaceutical compositions for treatment of microRNA related diseases
InactiveUS20090298916A1Inhibitory activityOrganic active ingredientsMetabolism disorderDiseasePrimate
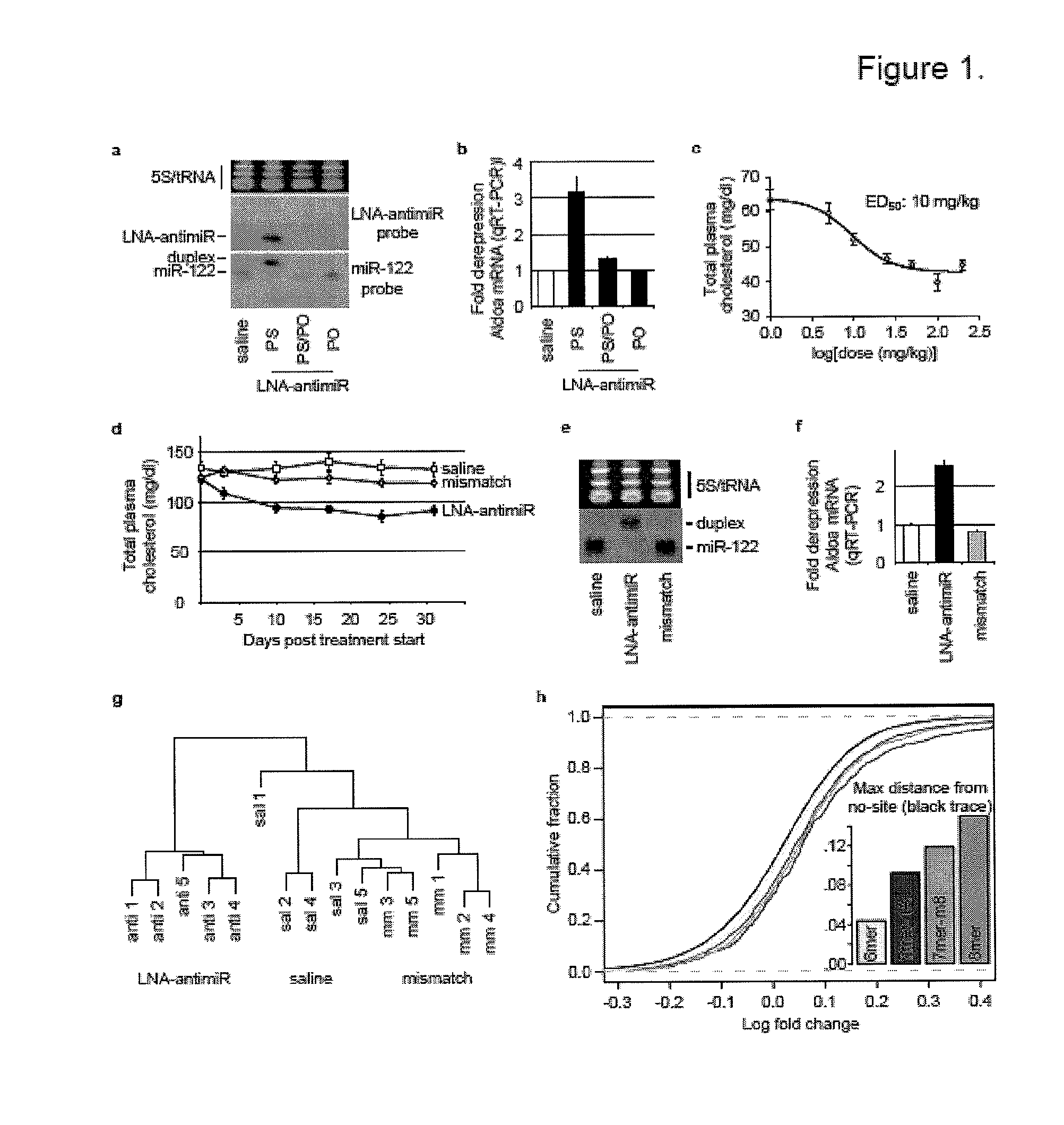
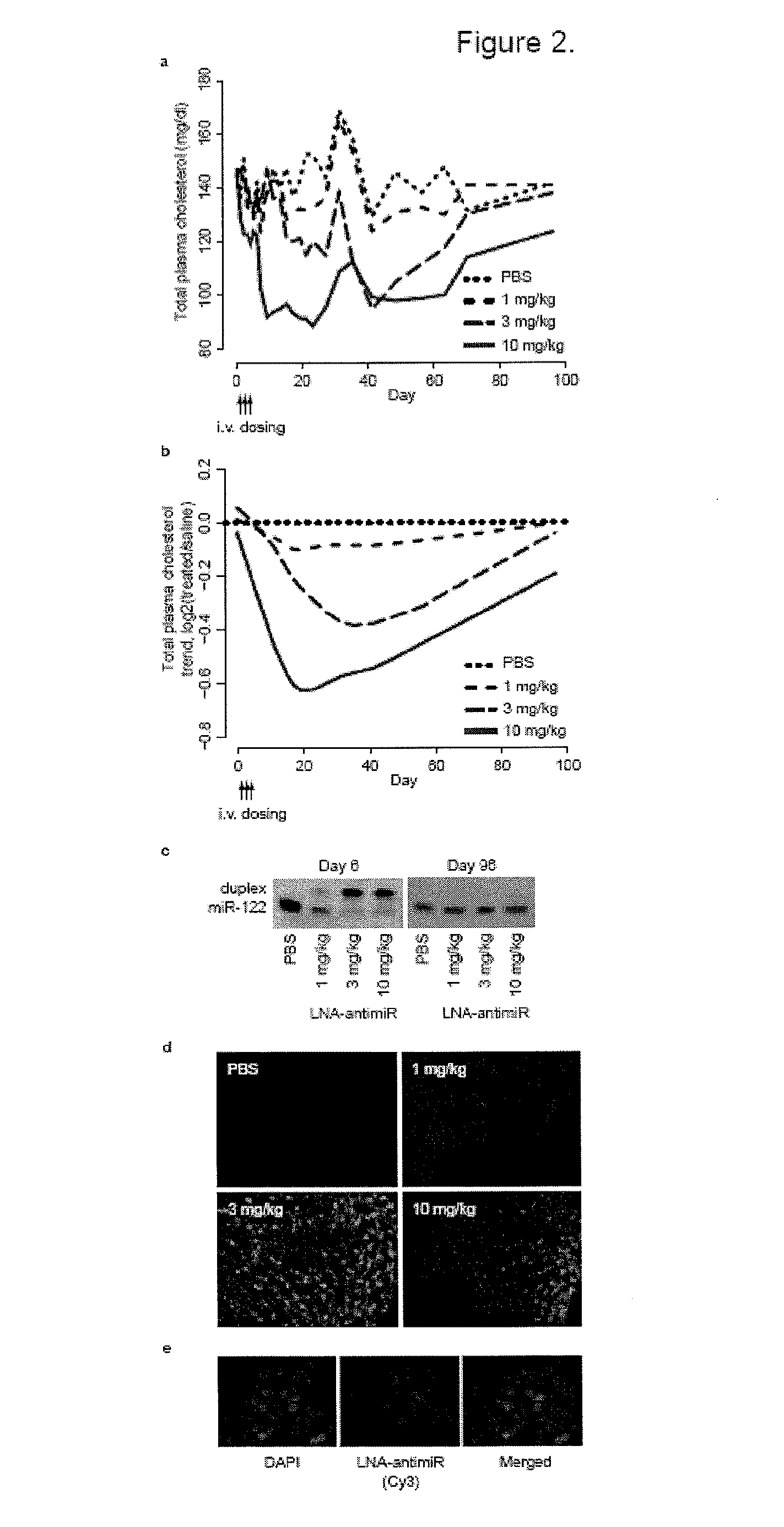
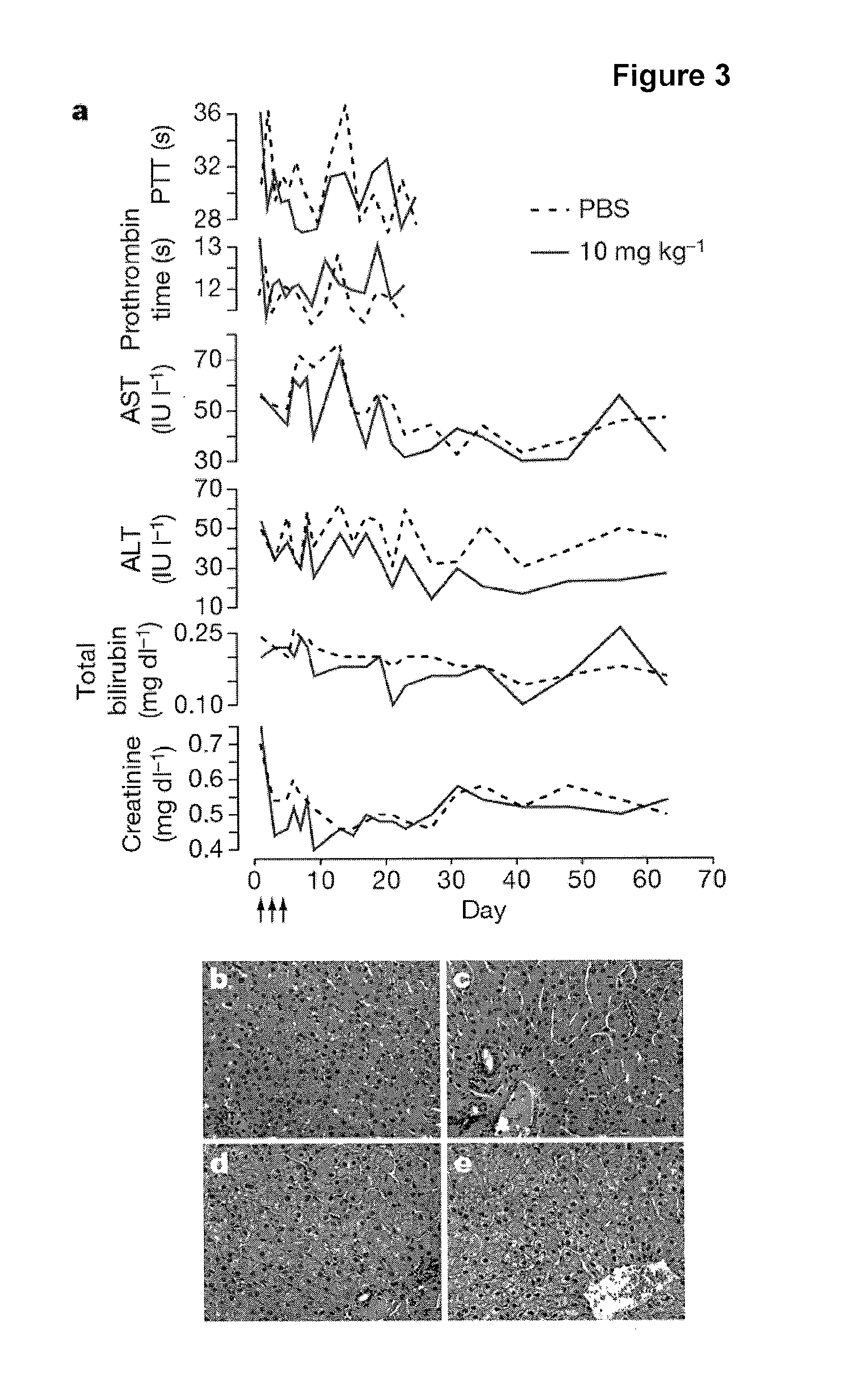
The present invention provides compositions and methods of treatment of diseases that are sensitive to drugs that downregulate the function of microRNA's, mRNA, non-coding RNA, or viral genomes. In particular, it has been discovered that a very long term effect of an anti microRNA oligonucleotide may be obtained when administered to a primate. Therefore, the present invention relate to pharmaceutical compositions and methods for treatment of primates, including humans wherein the compositions are administered with a long time interval.
Owner:ROCHE INNOVATION CENT COPENHAGEN
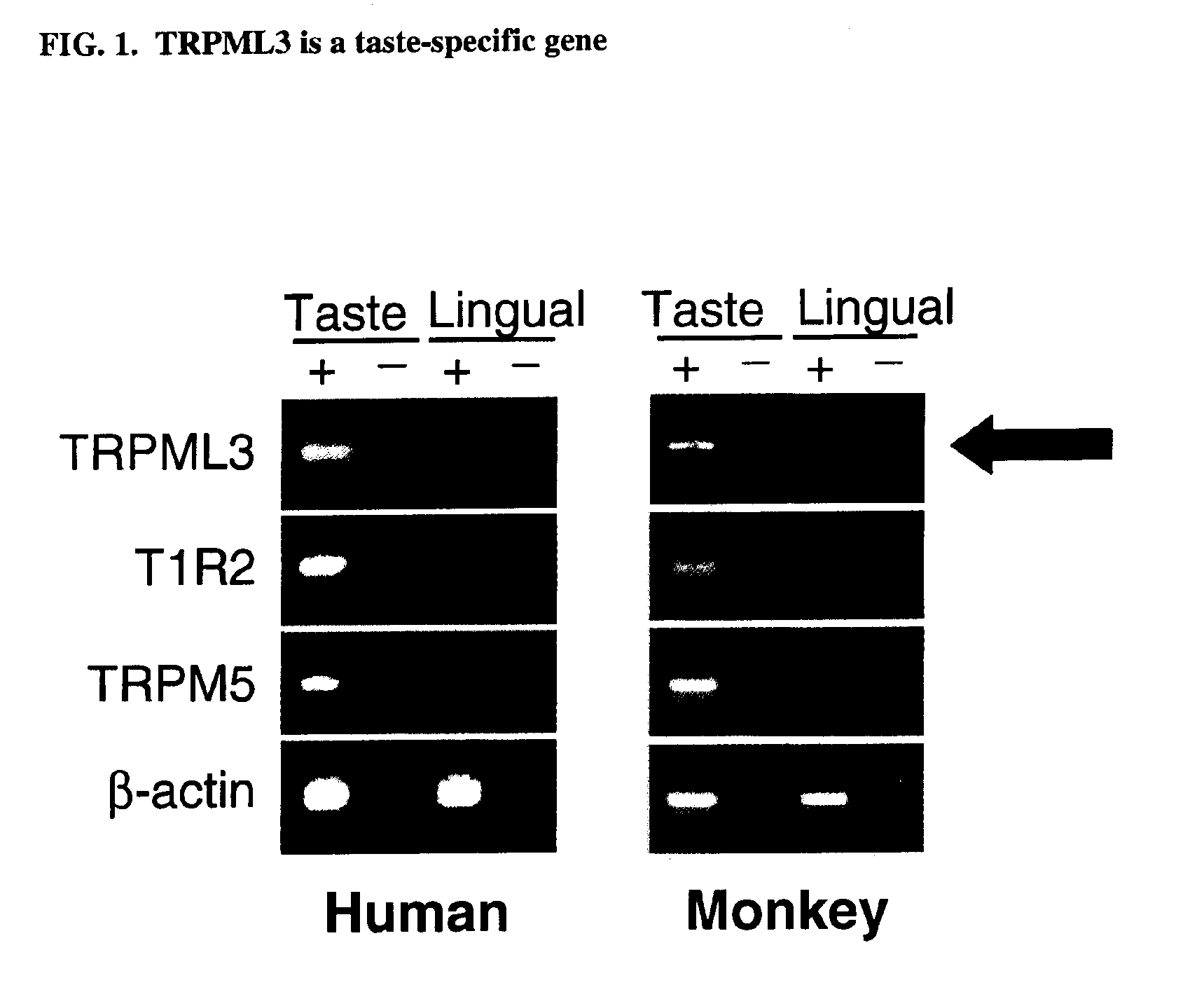
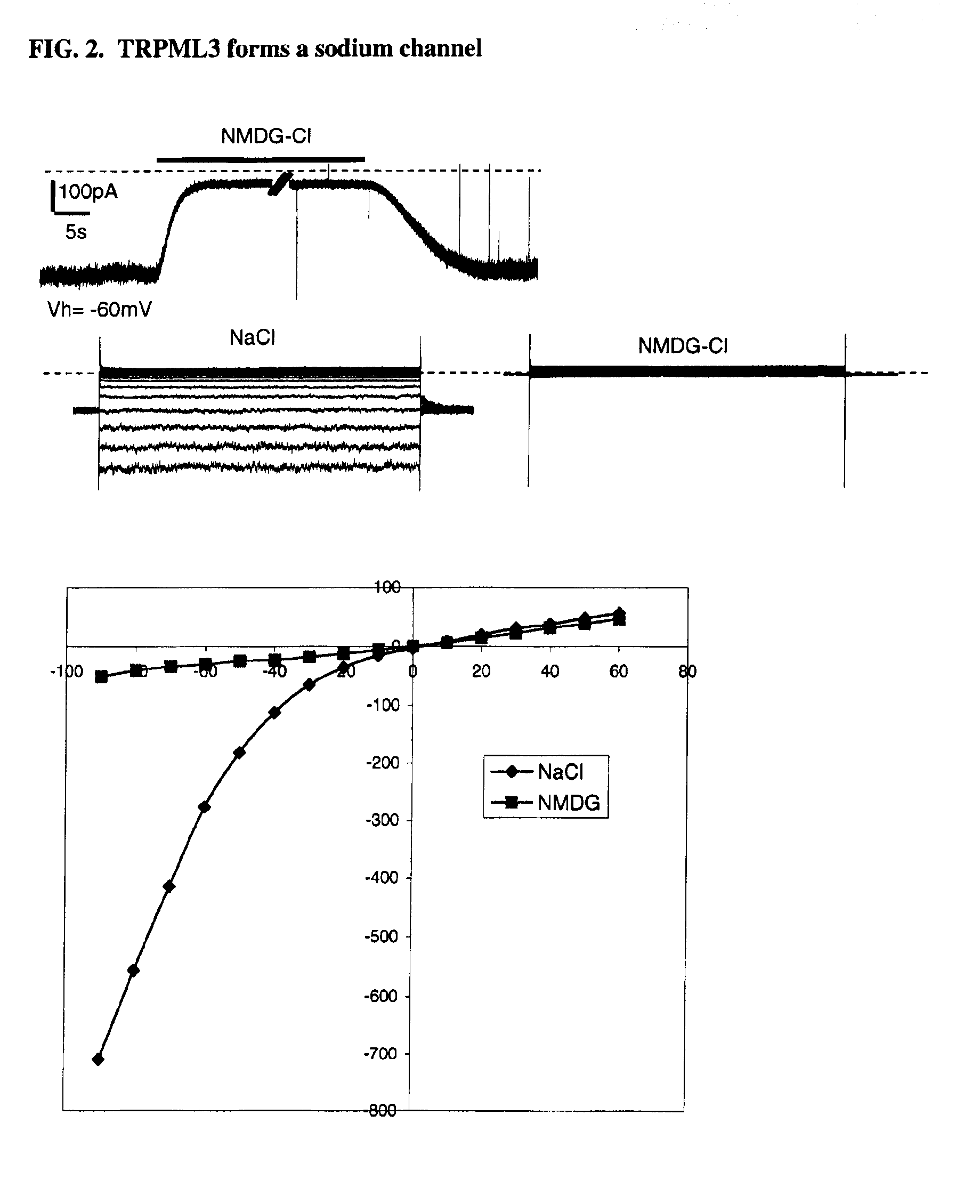
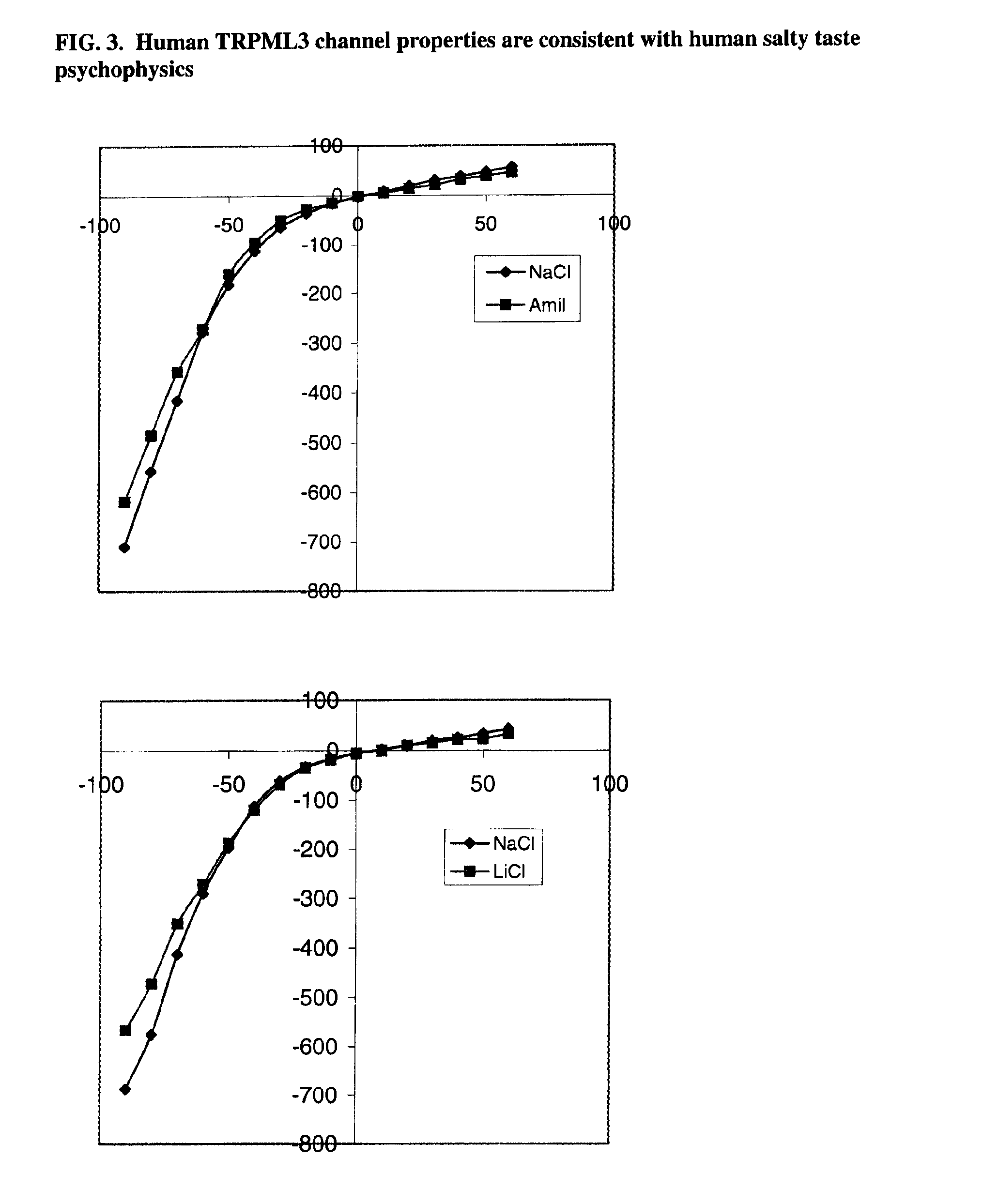
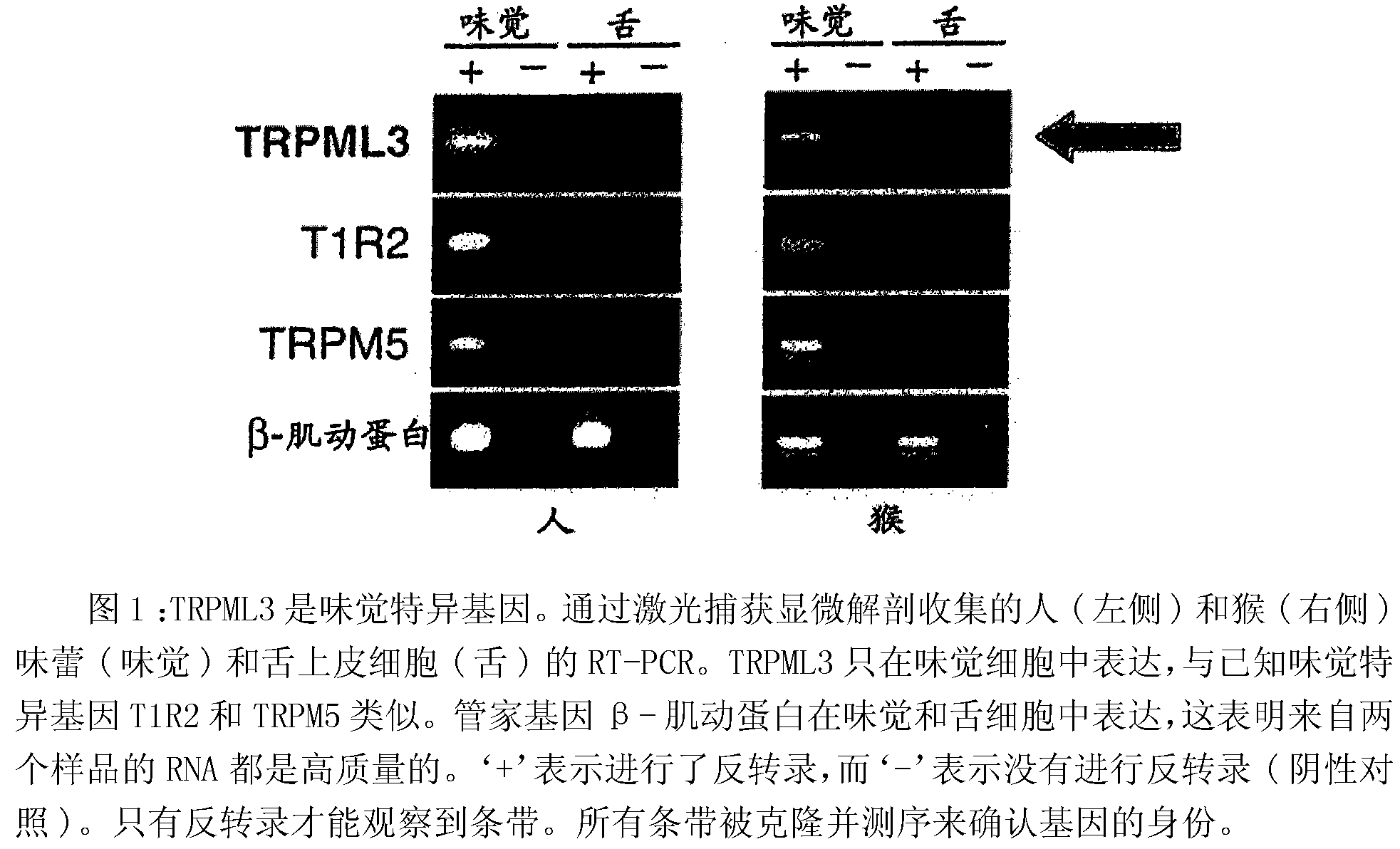
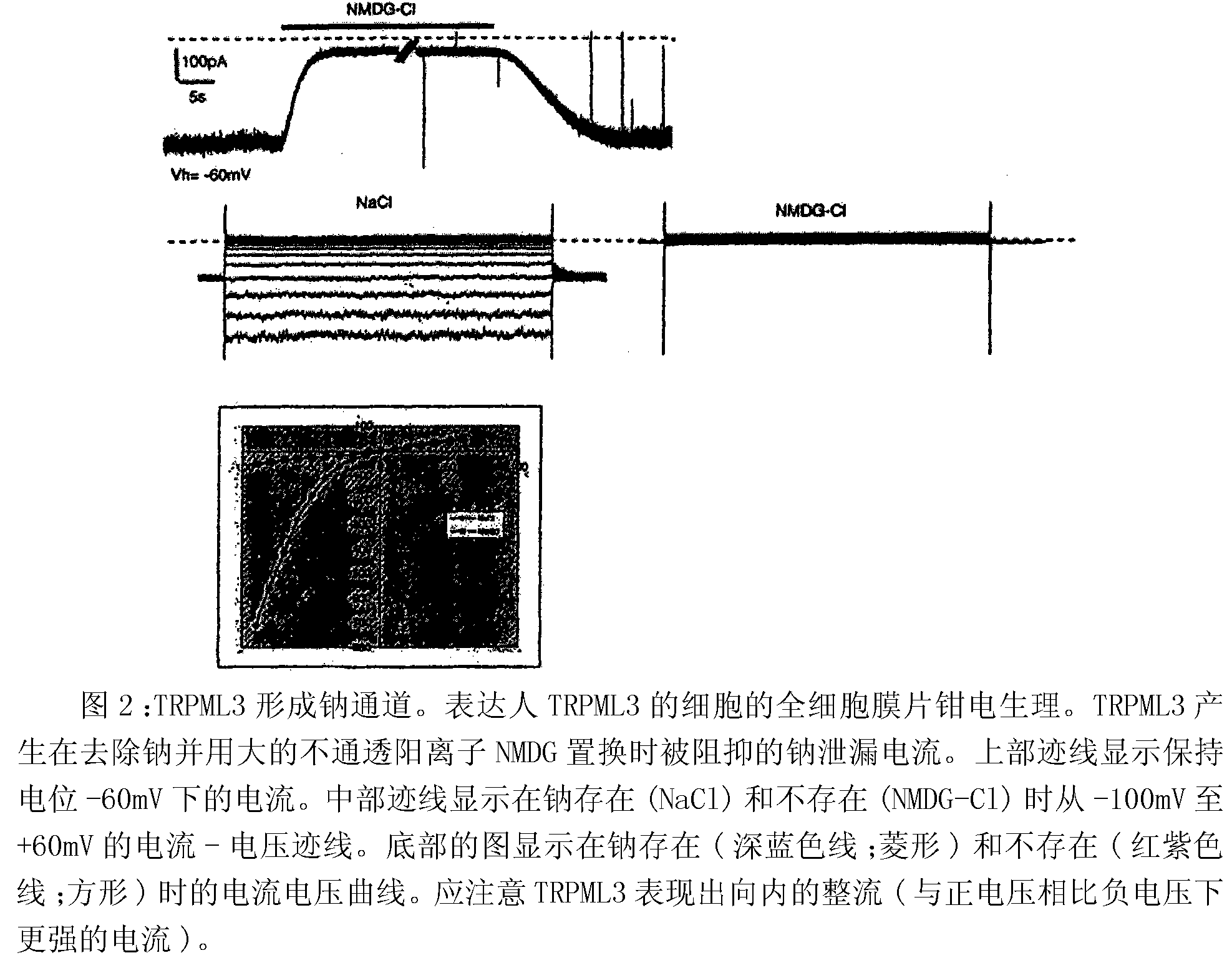
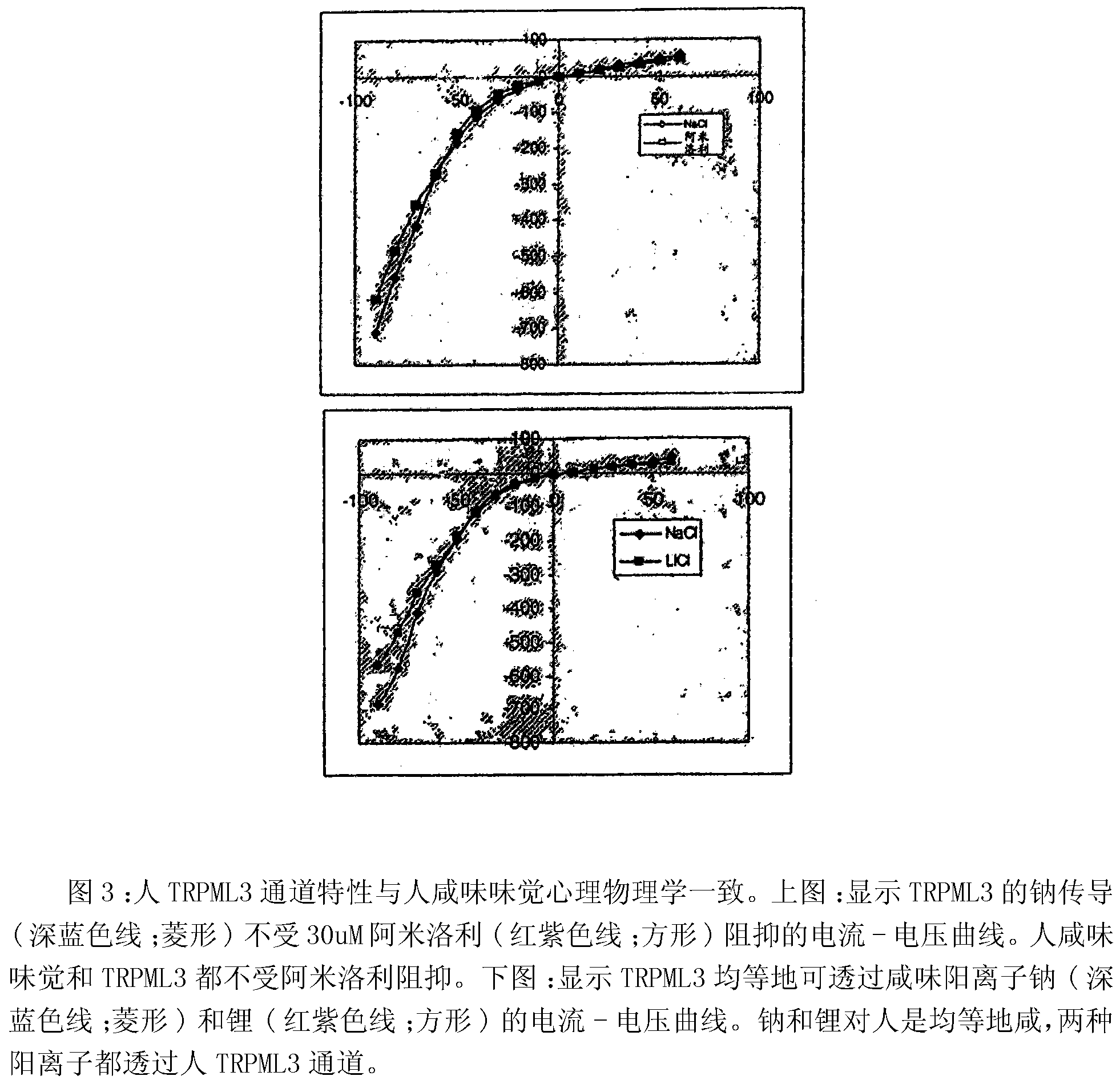
Identification of TRPML3 (MCOLN3) as a salty taste receptor and use in assays for identifying taste (salty) modulators and/or therapeutics that modulate sodium transport, absorption or excretion and/or aldosterone, and/or vasopressin production or release
InactiveUS20090210953A1Reduce volumeIncrease pressureCompound screeningApoptosis detectionSalty taste perceptionReceptor
The present invention relates to the elucidation that TRPML3 is involved in salty taste perception in primates including humans and likely other mammals and based thereon high-throughput mammalian and medium-throughput oocyte-based electrophysiological assays for identifying human TRPML3 modulators, preferably TRPML3 enhancers. Compounds that modulate TRPML3 function in the assay are expected to affect salty taste in humans. The inventive electrophysiological assays, such as the two-electrode voltage-clamp technique, facilitate the identification of compounds which specifically modulate human TRPML3. The assays of the invention provide a robust screen useful to detect compounds that facilitate (enhance) or inhibit TRPML3 function. Compounds that enhance or block TRPML3 channel activity should thereby modulate salty taste. In addition, these compounds may be used to regulate sodium excretion, urinary output and other biological functions relating to sodium levels and TRPML3 related functions.
Owner:SENOMYX INC
Antagonists of activin-actriia and uses for increasing red blood cell levels
ActiveUS20100028331A1Easy to produceReduce expressionPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed Cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Antagonists of activin-actriia and uses for increasing red blood cell levels
ActiveUS8895016B2Reduce expressionIncrease red blood cell and hemoglobin levelPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed blood cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Pharmaceutical Compositions for Treatment of MicroRNA Related Diseases
The present invention provides compositions and methods of treatment of diseases that are sensitive to drugs that downregulate the function of microRNA's, mRNA, non-coding RNA, or viral genomes. In particular, it has been discovered that a very long term effect of an anti microRNA oligonucleotide may be obtained when administered to a primate. Therefore, the present invention relate to pharmaceutical compositions and methods for treatment of primates, including humans wherein the compositions are administered with a long time interval.
Owner:ROCHE INNOVATION CENT COPENHAGEN
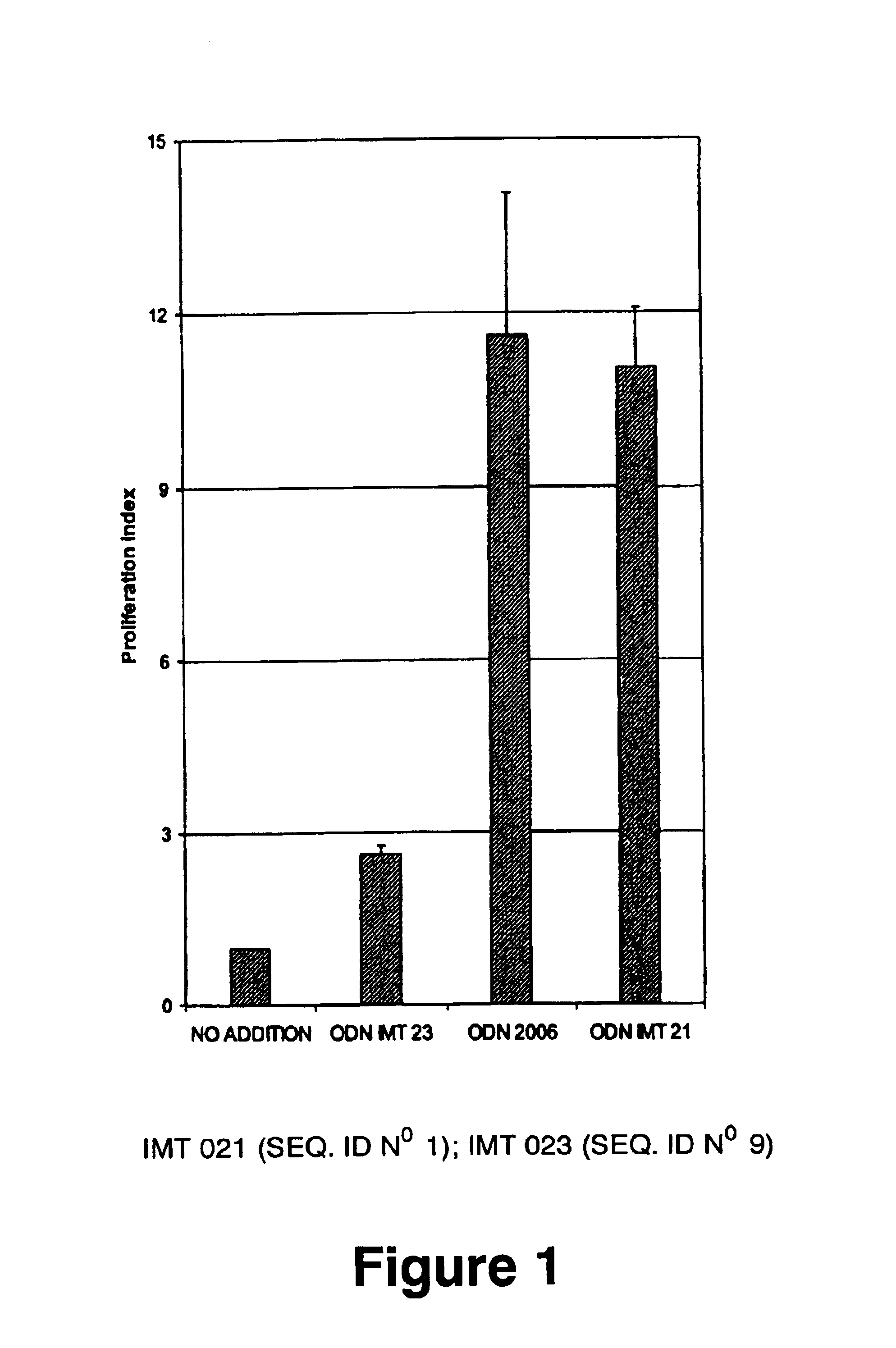
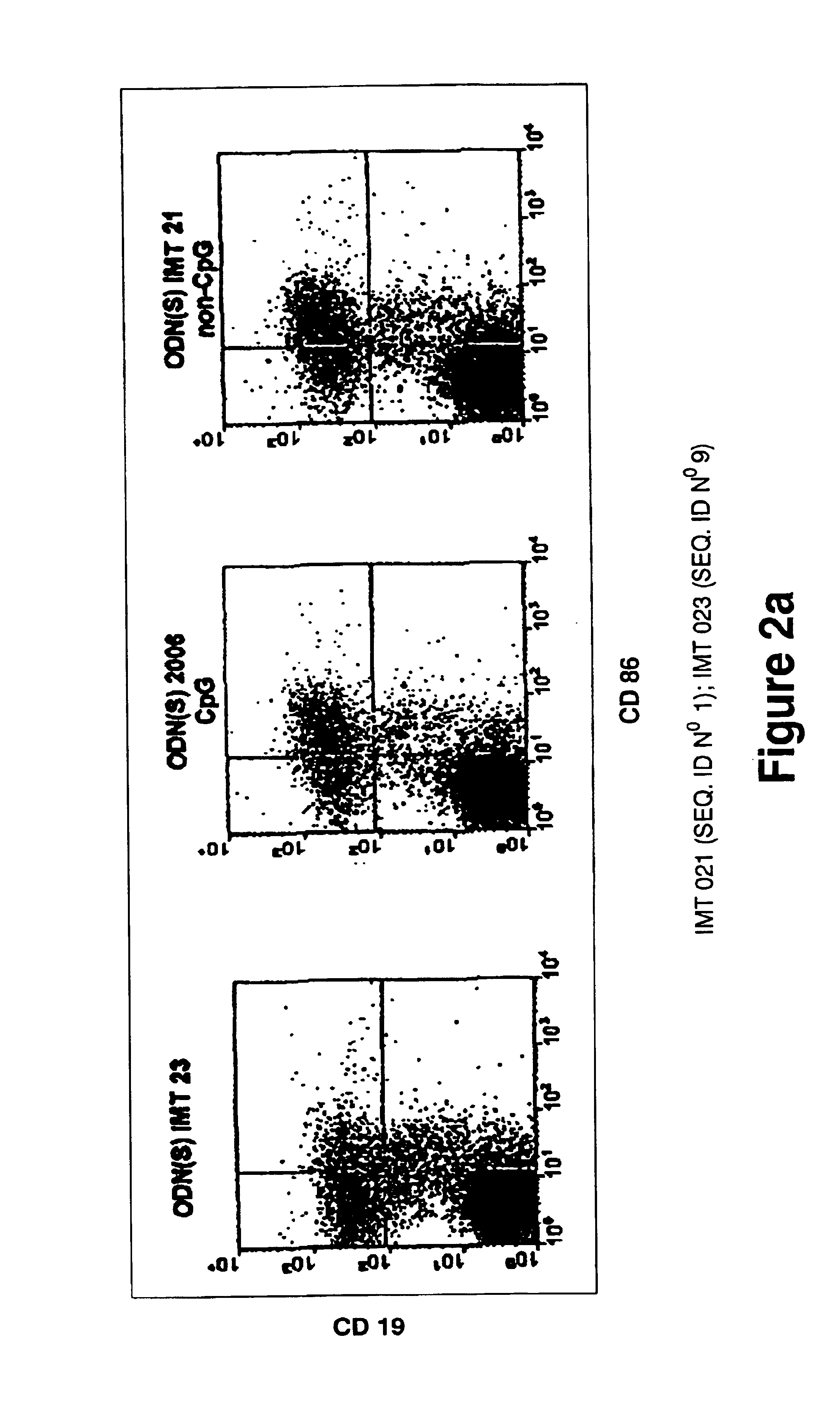
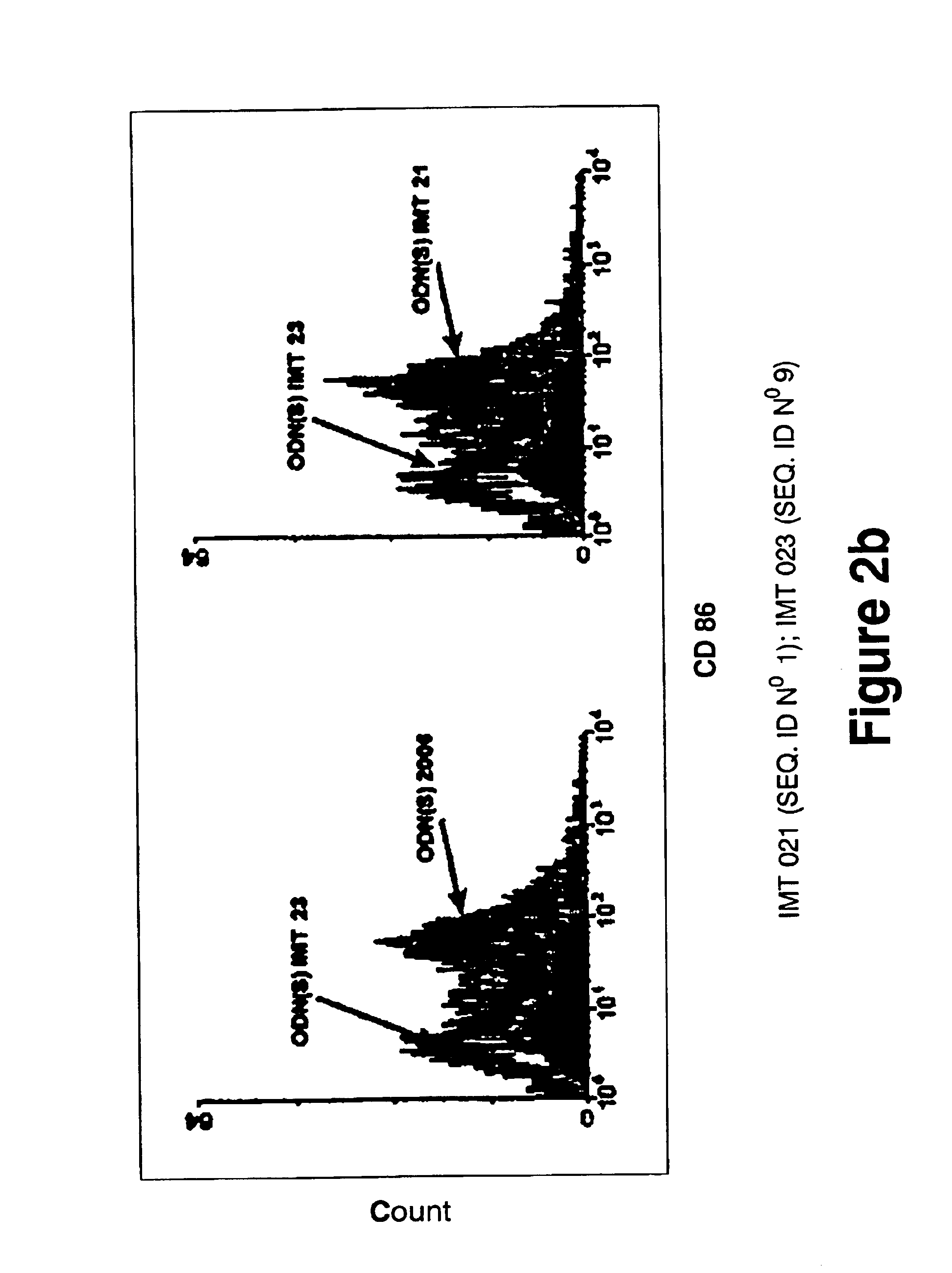
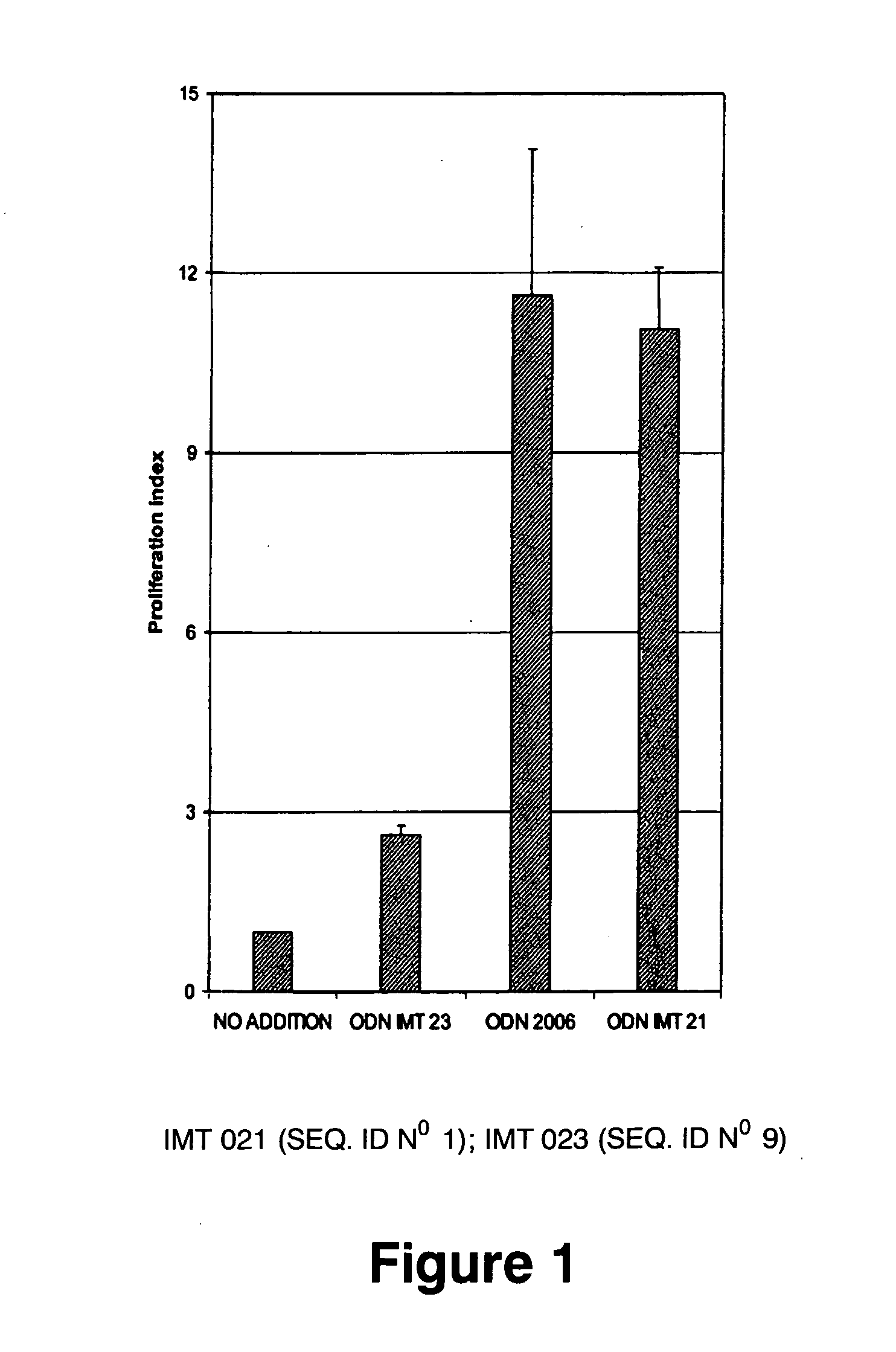
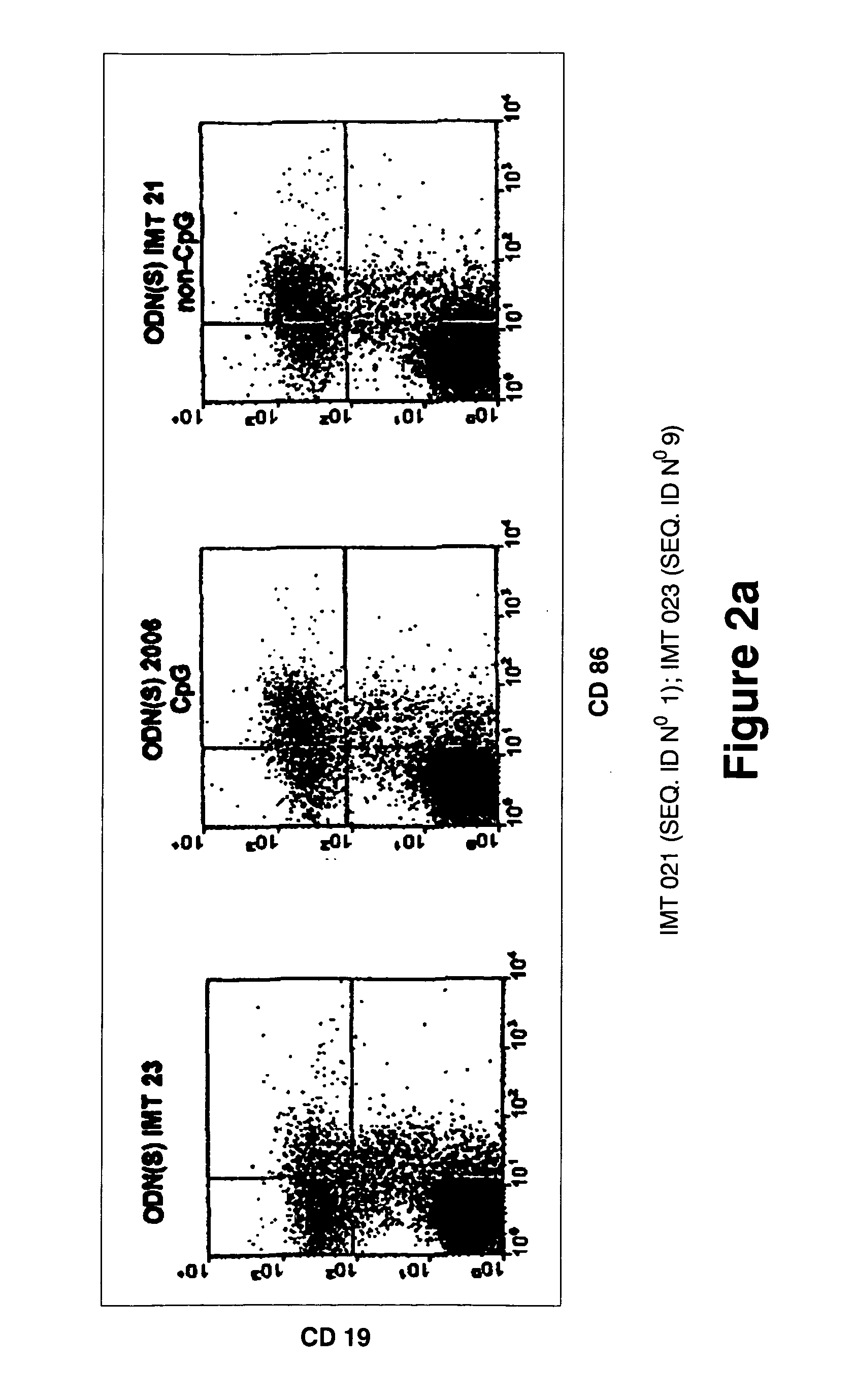
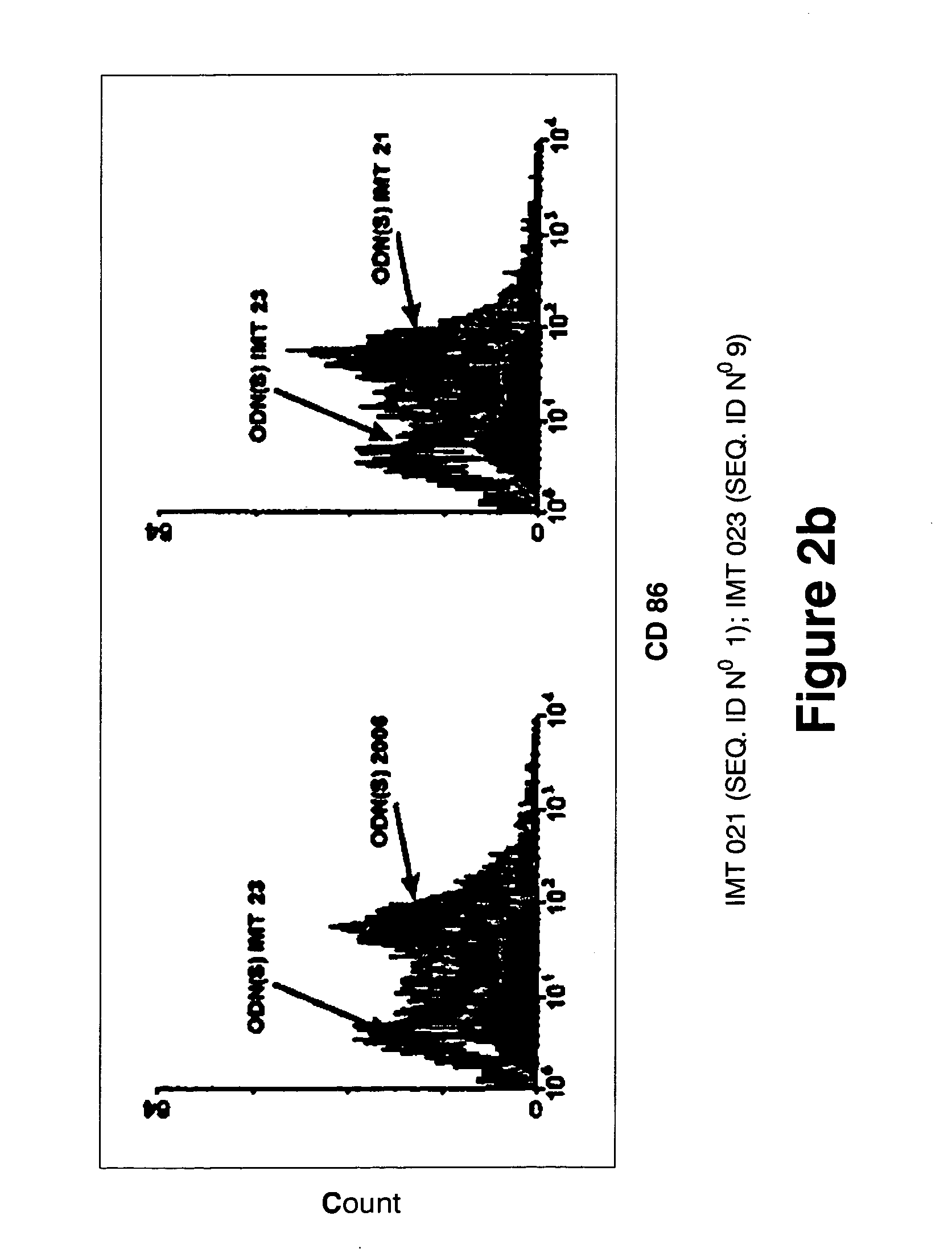
Immunostimulatory oligonucleotides and uses thereof
Oligonucleotides containing the non-palindromic sequence motif:X1X2X3X4X5X6X7X8,wherein X1 is C,T,G or A (preferably T or C); wherein X2 is C,T,G or A; wherein X7 is C,T,G or A (preferably G); at least three, and preferably all, of X3, X4, X5, X6 and X8 are T; and with the proviso that, in the motif, a C does not precede a G (in other terms, the nucleic acid motif does not consist of a CpG oligonucleotide), that modulate the immune response of animals of the order Primate, including humans, are disclosed. This immune modulation is characterized by stimulation of proliferation, differentiation, cytokine production and antibody production on B-cells and cell differentiation on plasmacytoid dendritic cells.
Owner:DAVID HORN LLC
Gynogenetic or androgenetic production of pluripotent cells and cell lines, and use thereof to produce differentiated cells and tissues
InactiveUS20030129745A1Rejection is prevented and reducedEliminate, orMammal material medical ingredientsSkeletal/connective tissue cellsPrimateEmbryo
Methods for obtaining pluripotent (embryonic stem) cells from parthenogenetic embryos, especially primates, are provided. These cells are useful for producing differentiated cells, tissues and organs, especially human and non-human primate cells, tissues and organs.
Owner:ROBL JAMES M +2
Microcarriers for Stem Cell Culture
InactiveUS20110143433A1Stable and long-term culturingIncrease differentiationCulture processArtificial cell constructsMatrigelORDER PRIMATES
We disclose a particle comprising a matrix coated thereon and having a positive charge, the particle being of a size to allow aggregation of primate or human stem cells attached thereto. The particle may comprise a substantially elongate, cylindrical or rod shaped particle having a longest dimension of between 50 μm and 400 μm, such as about 200 μm. It may have a cross sectional dimension of between 20 μm and 30 μm. The particle may comprise a substantially compact or spherical shaped particle having a size of between about 20 μm and about 120 μm, for example about 65 μm. We also disclose a method of propagating primate or human stem cells, the method comprising: providing first and second primate or human stem cells attached to first and second respective particles, allowing the first primate or human stem cell to contact the second primate or human stem cell to form an aggregate of cells and culturing the aggregate to propagate the primate or human stem cells for at least one passage. A method of propagating human embryonic stem cells (hESCs) in long term suspension culture using microcarriers coated in Matrigel or hyaluronic acid is also disclosed. We also disclose a method for differentiating stem cells.
Owner:AGENCY FOR SCI TECH & RES
Products and methods for enhanced transgene expression and processing
InactiveUS20120231449A1Increase volumeHigh activityAnimal cellsVectorsADAMTS ProteinsSecreted substance
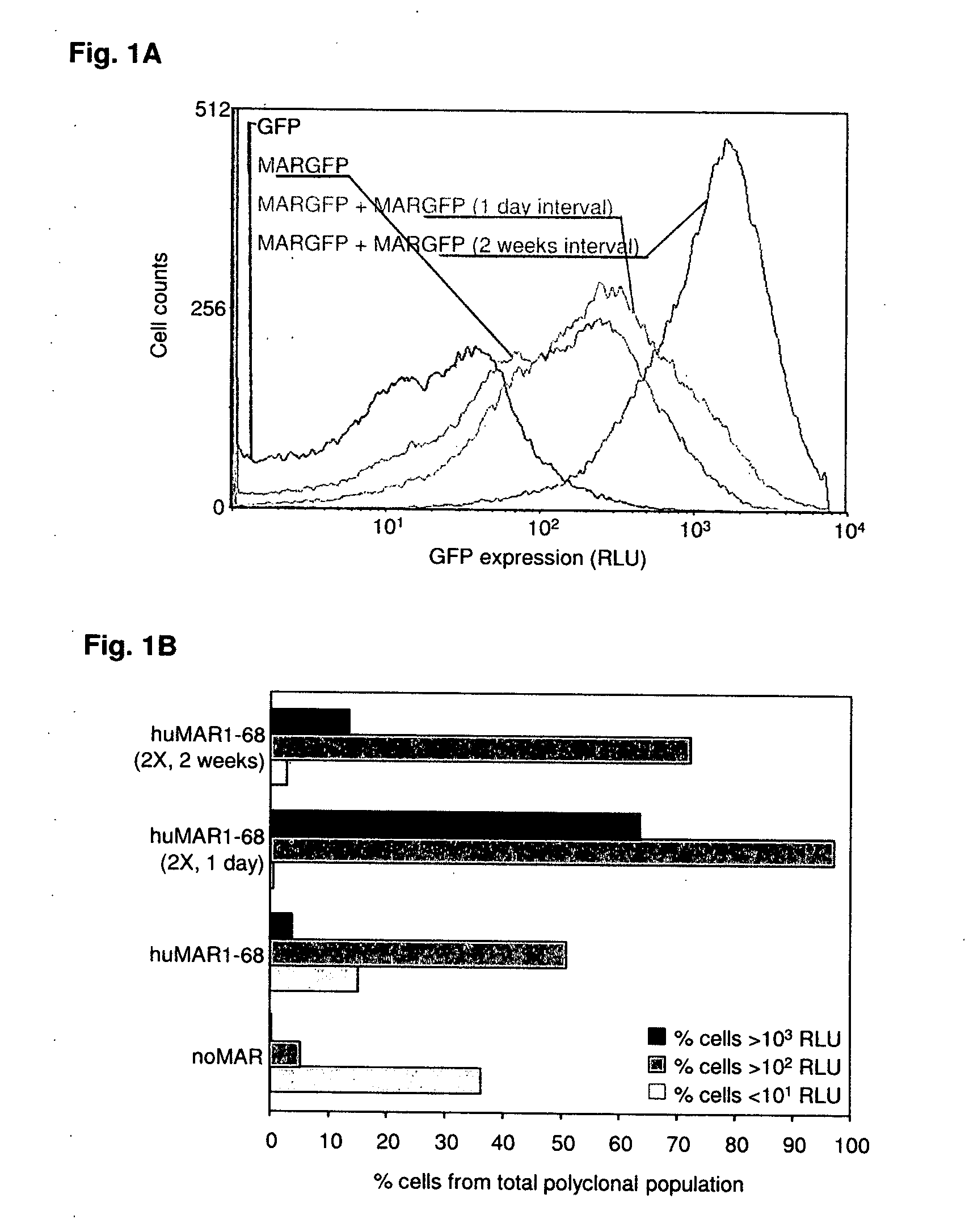
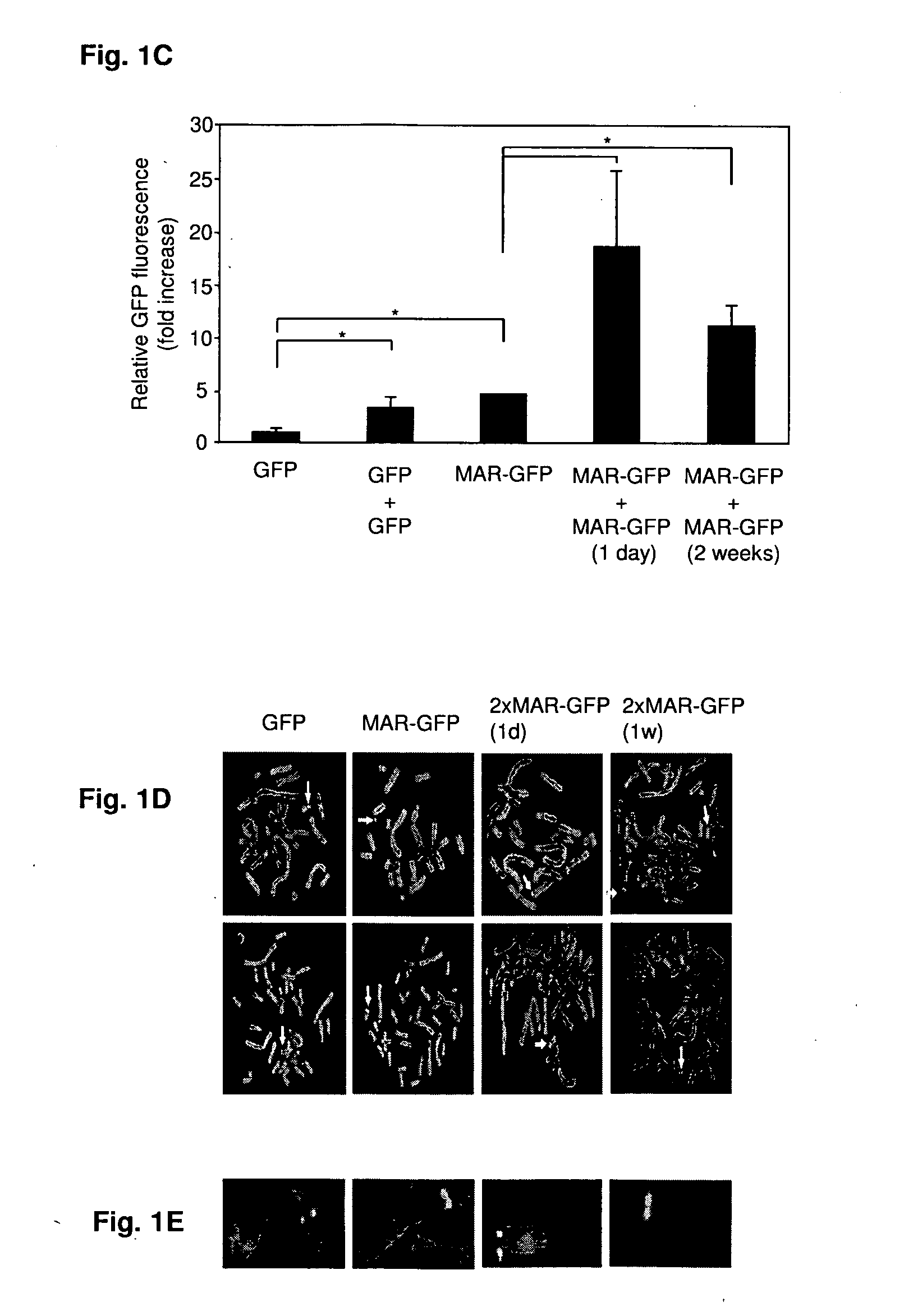
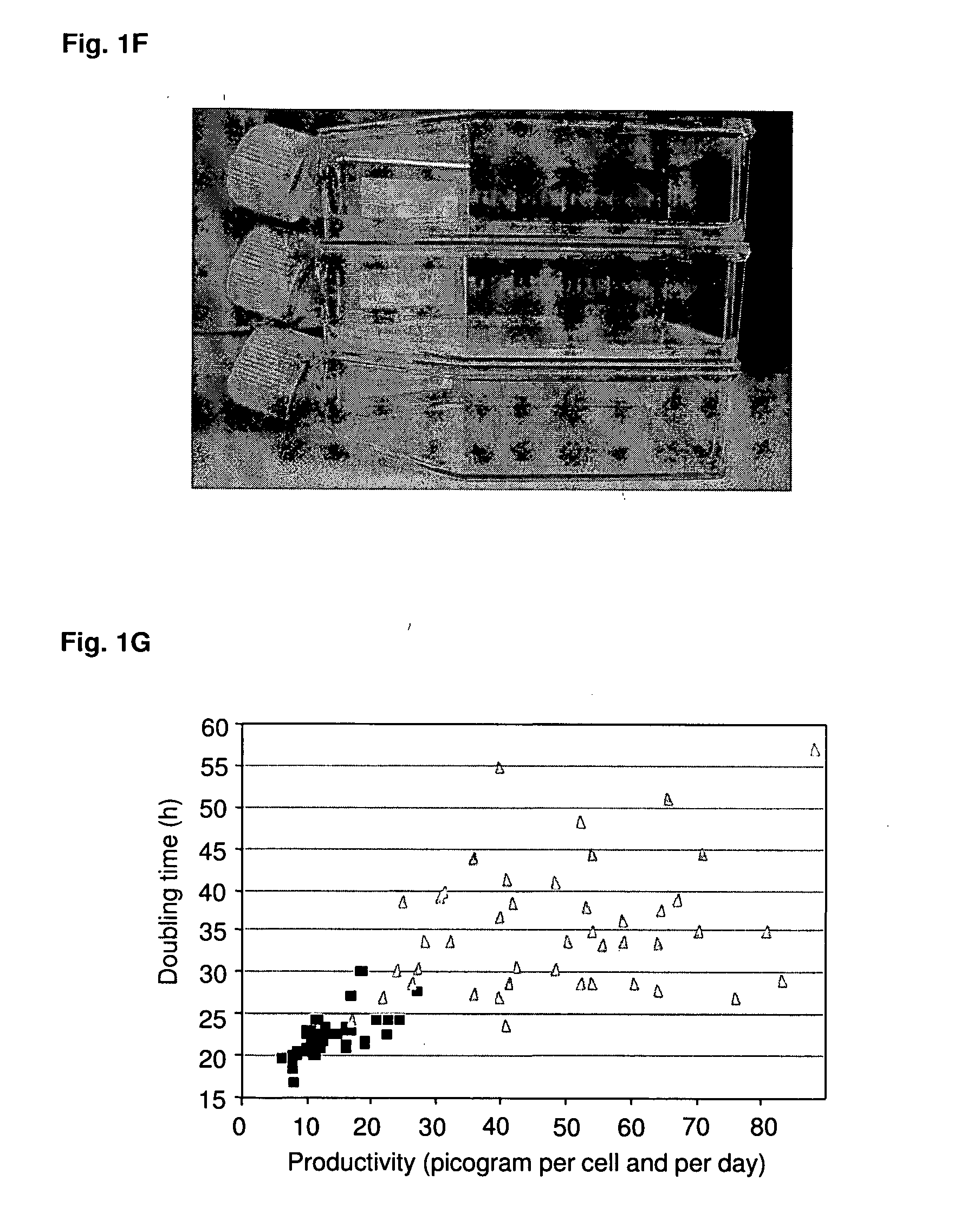
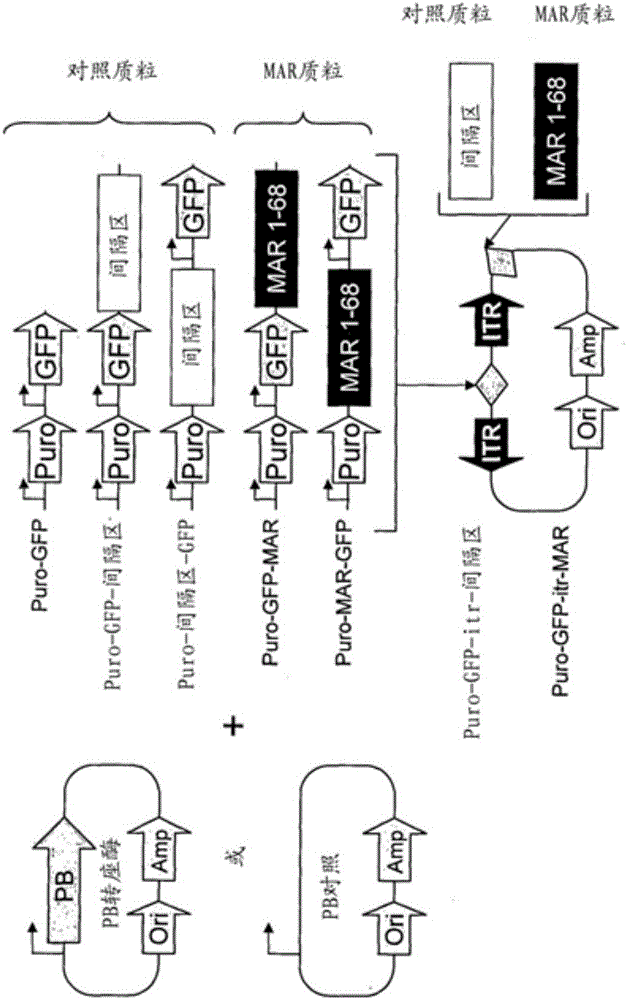
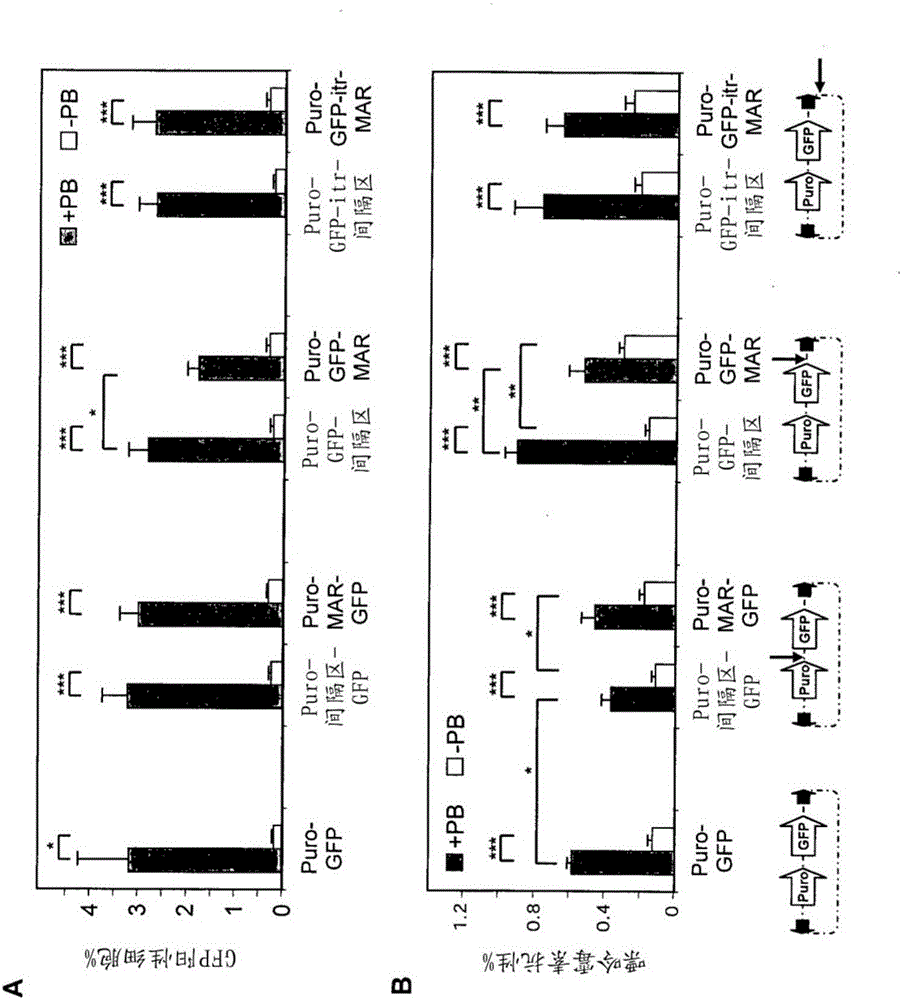
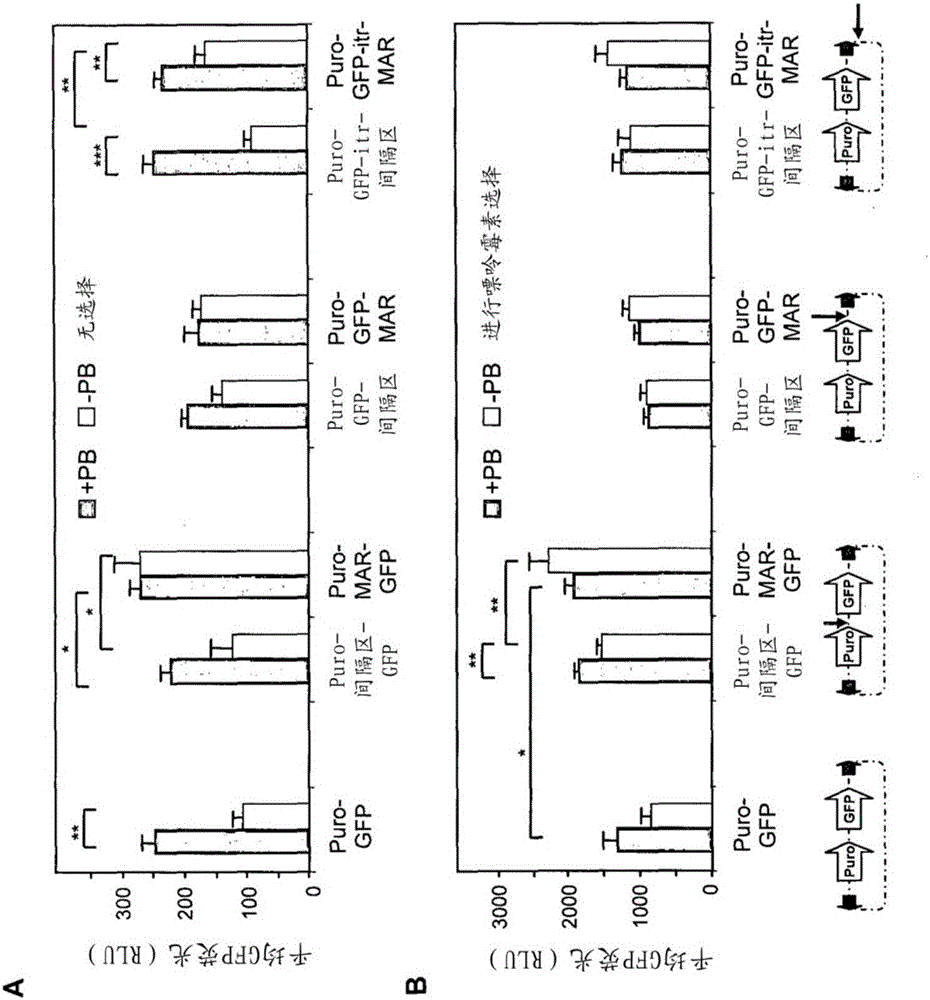
Disclosed are methods and eukaryotic host cells for transgene expression. The cells may be treated and / or modified to increase homologous recombination (HR), decrease non homologous end joining (NHEJ) and / or to enhance a HR / NHEJ ratio in said cell. Such cells can be transfected with vectors comprising the transgene, which advantageously integrates into the genome of the cell to form a concatemeric structure which may comprise more than 200 transgene copies. Certain expression enhancing elements such as MARs are advantageously provided to further enhance and / or facilitate transgene expression. Disclosed is also a recombinant eukaryotic host cell, in particular a non-primate host cell, comprising a transgenic sequence encoding a protein and / or a RNA, in particular a primate protein and / or RNA, involved in translocation across the ER membrane and / or secretion across the cytoplasmic membrane.
Owner:SELEXIS SA
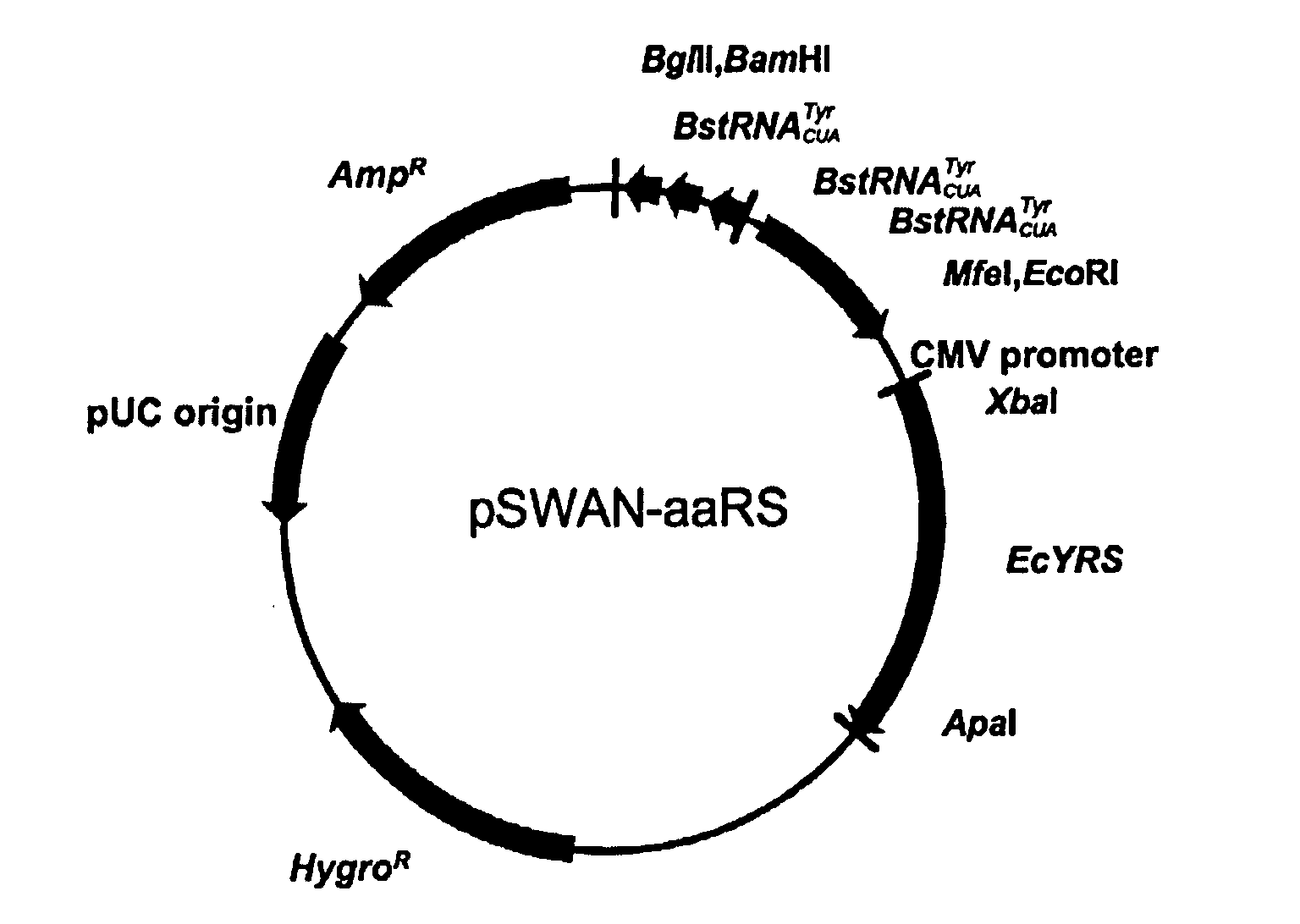
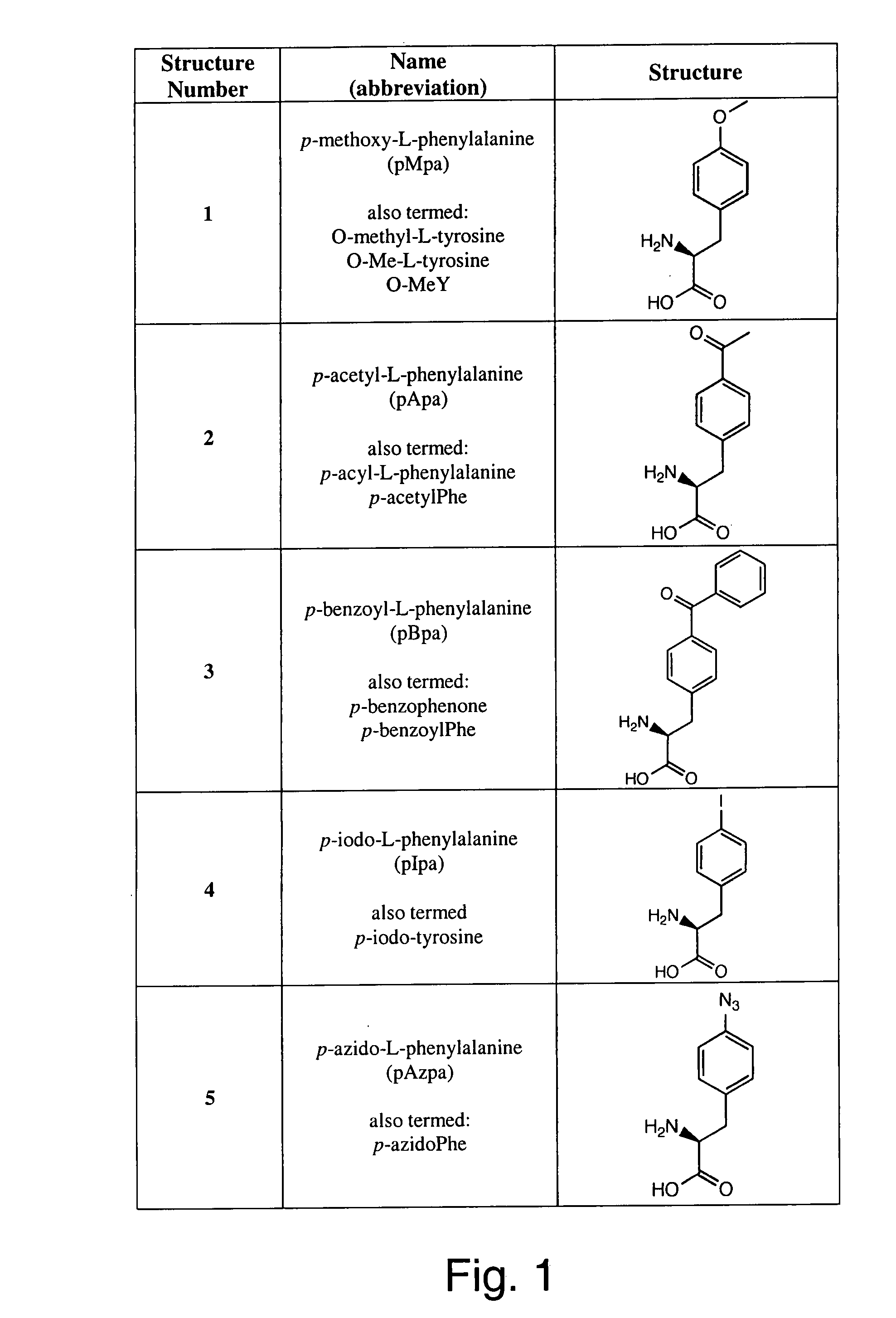
Genetic incorporation of unnatural amino acids into proteins in mammalian cells
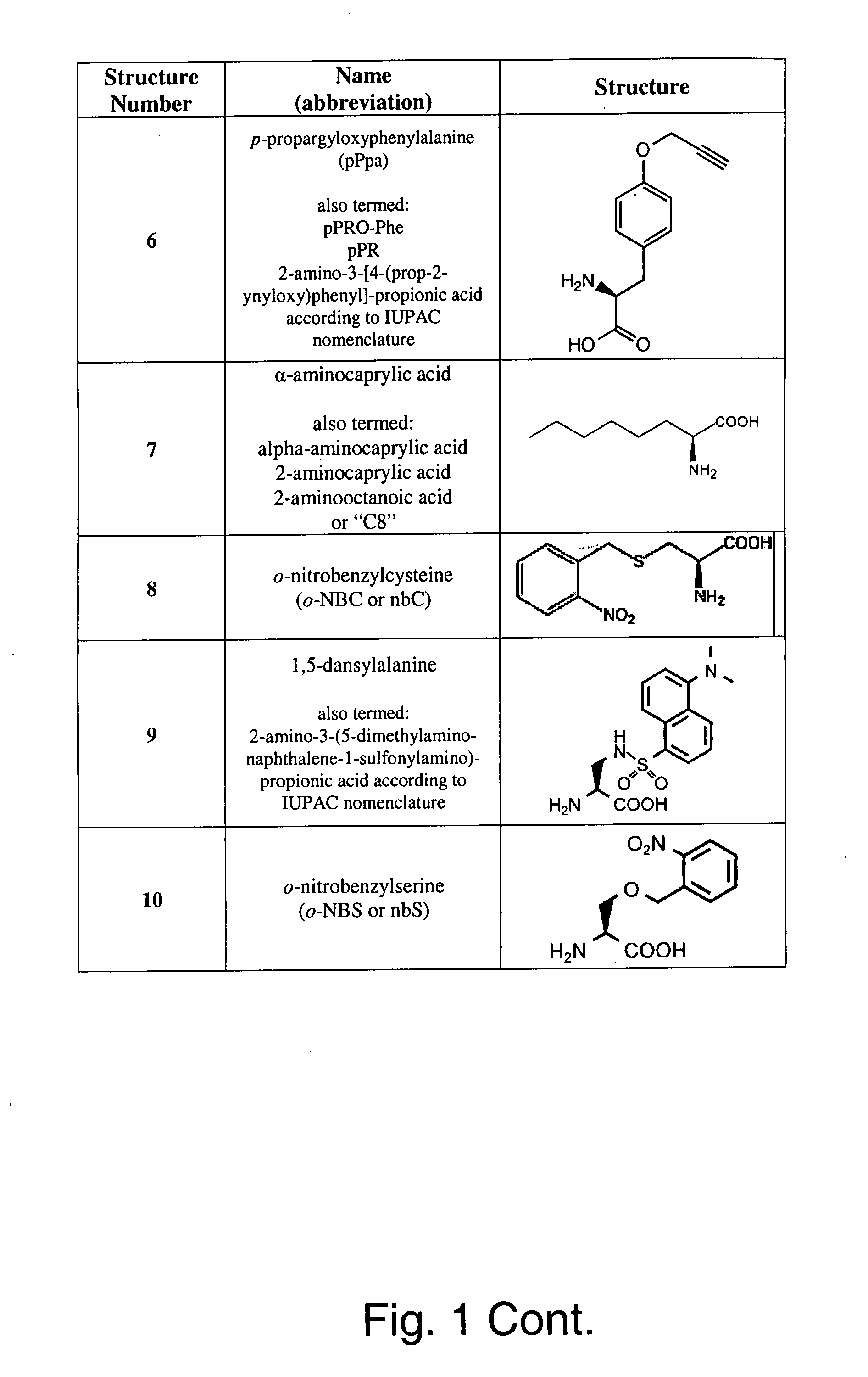
ActiveUS20100021963A1High translational fidelityConvenient introductionVertebrate cellsPeptidesADAMTS ProteinsCell system
Owner:THE SCRIPPS RES INST
Enhanced transgene expression and processing
Disclosed are constructs and methods for expressing DNAs of interest in particular in non- primate eukaryotic host cells that display advantages with regard quantity and quality of expression including high stability of expression and, if appropriate, transport of the expression product out of the cell.
Owner:SELEXIS SA
Polyherbal compositions and methods for treating viral infections
The present invention relates to pharmaceutical or veterinary or nutritional compositions of polyherbal extracts useful as anti-viral or immune-supporting agents. Particularly, the present invention of polyherbal composition comprises of extracts of Withania somnifera, Mangifera indica and purified Shilajit. This cost effective immune-supporting agent is ideal for use during the maintenance phase of the treatment, following an initial viral load reduction phase in which it is used as an adjuvant to conventional anti-viral drug therapy. The anti-viral and immune-supporting composition of this invention can perhaps be the sole basis of treatment where affordability is an issue. Additionally, this composition is used for the treatment, prevention or management of immune-supporting system in primates in need, especially humans.
Owner:INDIAN HERBS RES & SUPPLY +1
Polyherbal compositions and methods for treating viral infections
The present invention relates to pharmaceutical or veterinary or nutritional compositions of polyherbal extracts useful as anti-viral or immune-supporting agents. Particularly, the present invention of polyherbal composition comprises of extracts of Withania somnifera, Mangifera indica and purified Shilajit. This cost effective immune-supporting agent is ideal for use during the maintenance phase of the treatment, following an initial viral load reduction phase in which it is used as an adjuvant to conventional anti-viral drug therapy. The anti-viral and immune-supporting composition of this invention can perhaps be the sole basis of treatment where affordability is an issue. Additionally, this composition is used for the treatment, prevention or management of immune-supporting system in primates in need, especially humans.
Owner:INDIAN HERBS RES & SUPPLY +1
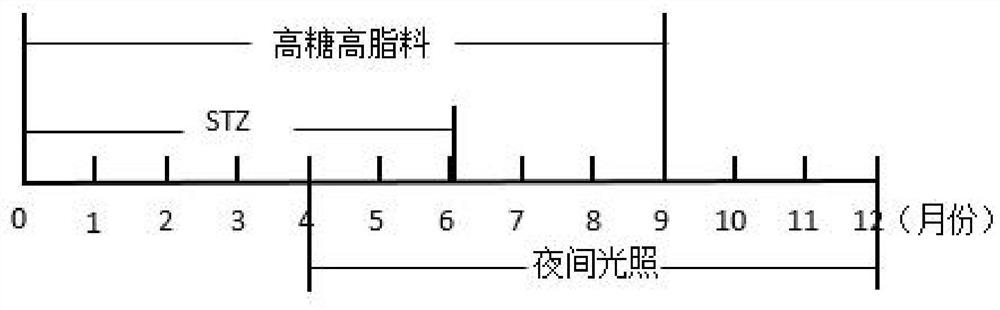
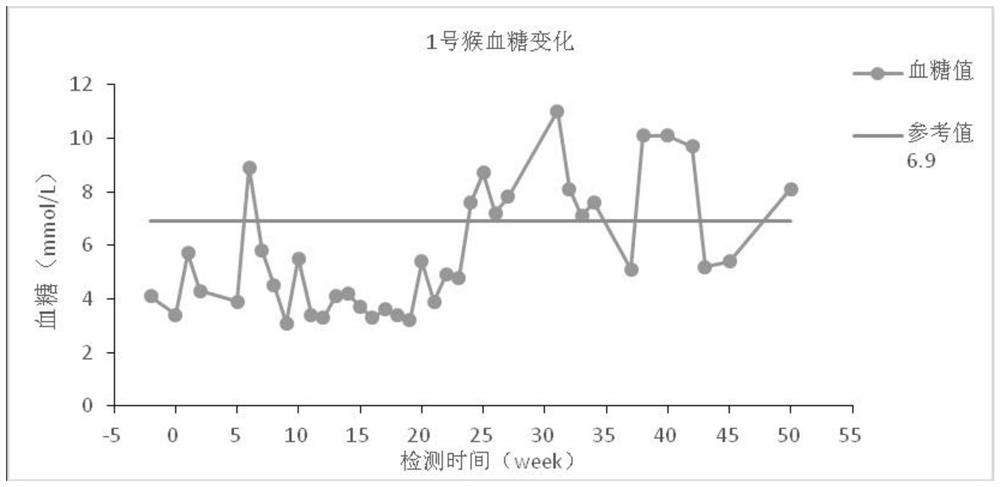
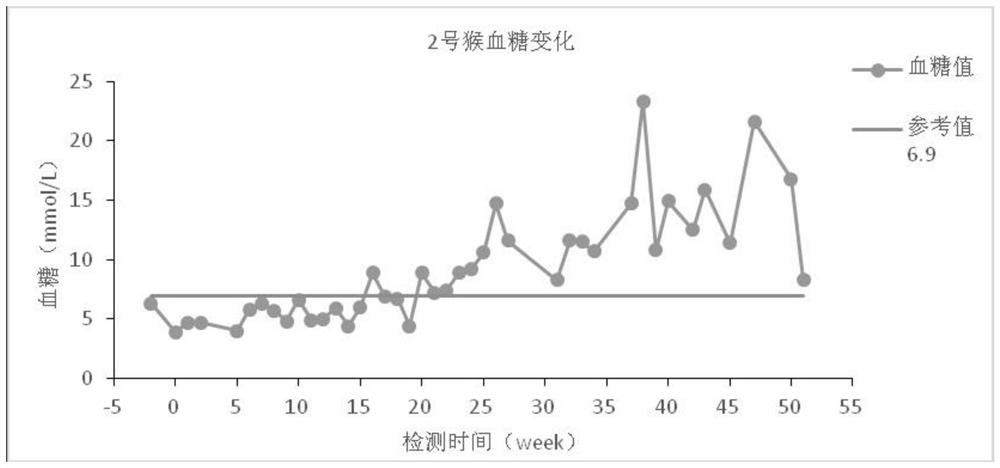
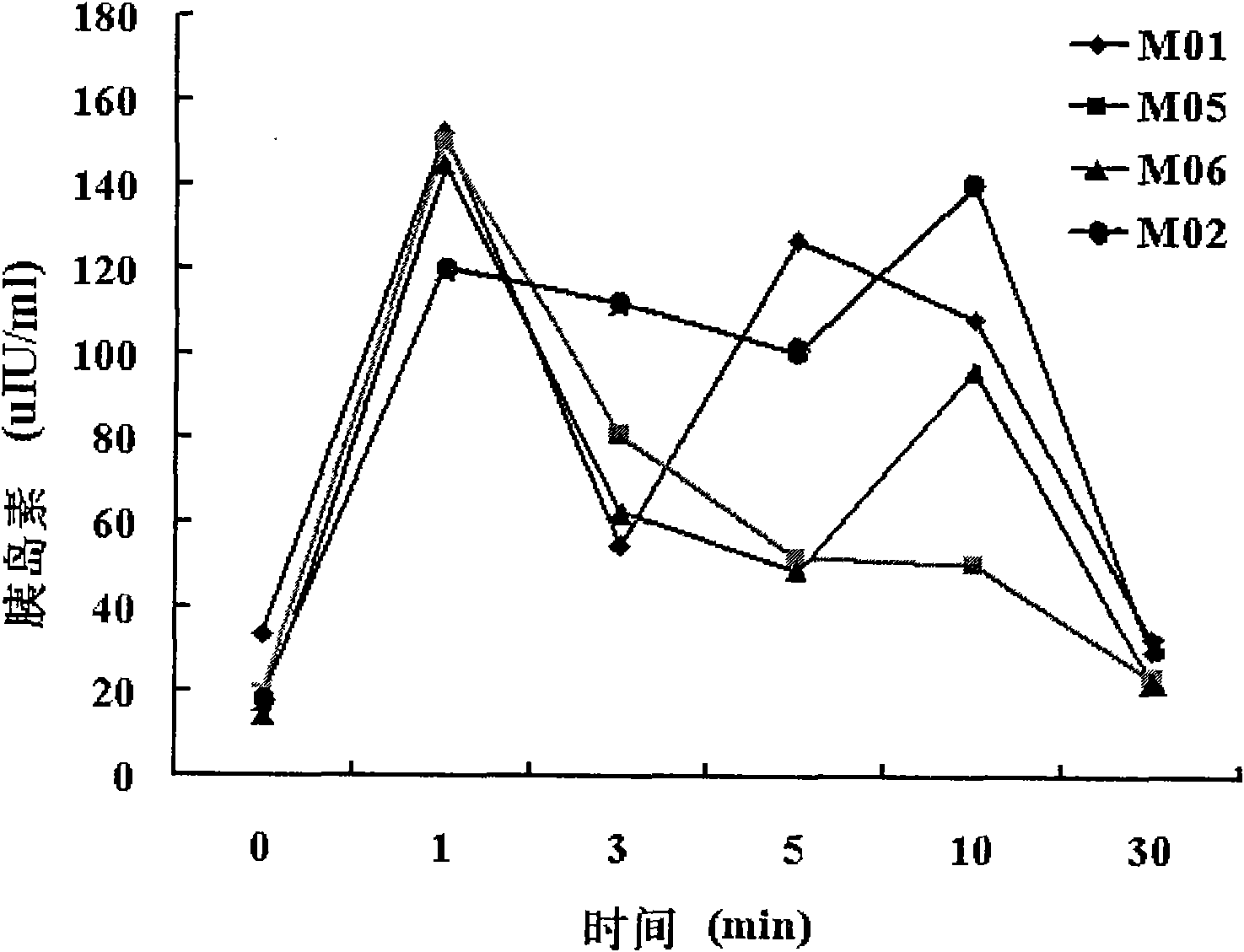
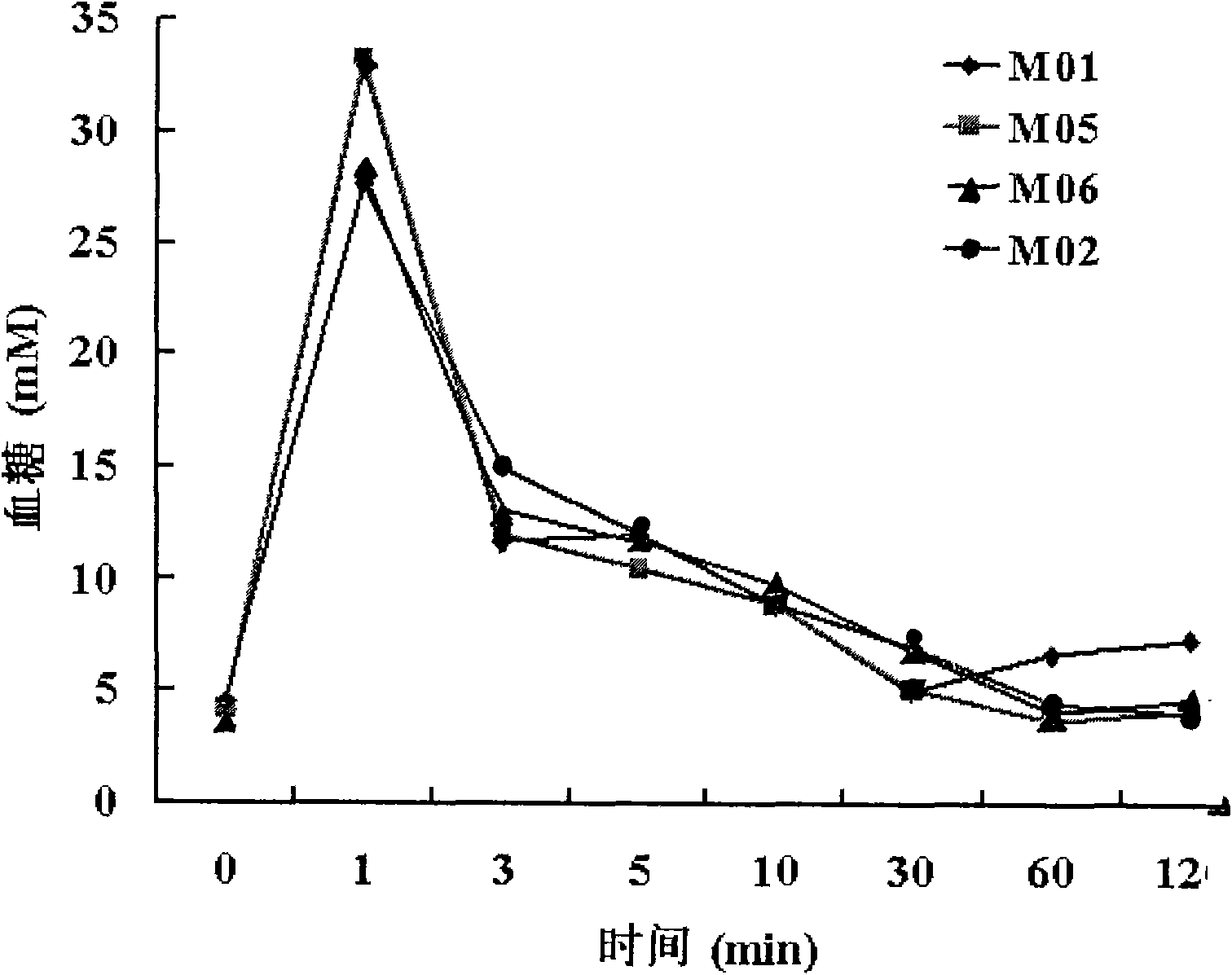
Diabetes primate model construction method
ActiveCN112425561ADisturb the rhythm of lifeLow toxicityCompounds screening/testingOrganic active ingredientsAnimal scienceORDER PRIMATES
The invention provides a diabetes primate model construction method. The method comprises the following steps of injecting streptozotocin into cynomolgus monkeys according to a small-dose progressiveincrease method, feeding the cynomolgus monkeys with high-sugar and high-fat feed at the same time, and after injecting the streptozotocin into the cynomolgus monkeys and feeding the cynomolgus monkeys with the high-sugar and high-fat feed for a period of time, increasing night illumination to induce the cynomolgus monkeys to become diabetes models. The diabetic monkey model is successfully established by adopting the method for 1.5-6 months, the model has the core characteristics of insulin resistance and hyperglycemia, the duration of hyperglycemia is long, and the hyperglycemia is not reduced after more than 200 days. The animal model established by the invention can be used in animal experiments with longer duration, and can be sold as a mature animal model to generate economic benefits.
Owner:广东蓝岛生物技术有限公司
Method for preparing diabetic animal model
InactiveCN101637405AClear physiological backgroundImprove economyOrganic active ingredientsDiagnosticsPancreas partPancreatic structure
The invention relates to a method for preparing an animal model of an insulin-dependent diabetes. In the method, the pancreas part of a primate is excised and a method of streptozotocin induction is used together to establish a rhesus monkey diabetes model, wherein the using method and the dosage of streptozotocin are as follows: the streptozotocin is injected in vein in a ratio of 15 to 20 mg / kgeach time. The method establishes the rhesus monkey diabetes model by combining the excision of the pancreas with the method of streptozotocin (STZ) induction, and carries out observation and comparison of physical indexes of the model. The insulin treatment scheme is economical, safe and feasible.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
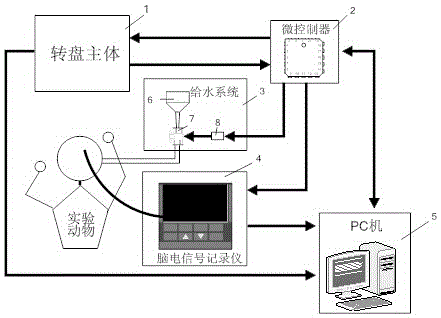
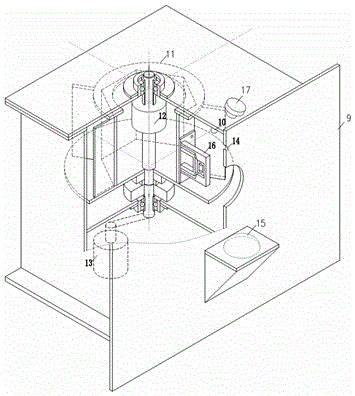
Automatic grabbing training and recording device for non-human primates
ActiveCN105028232AEasy to operateImprove training efficiencyTaming and training devicesMicrocontrollerORDER PRIMATES
The invention provides an automatic grabbing training and recording device for non-human primates for solving the technical problem how to enable trained primates to rapidly grasp various specified grabbing actions. The device comprises a rotating disc body, a microcontroller, a water feed control system, a brain electrical signal recorder and a PC. The device is easy to operate and high in training efficiency. The training mode is adjustable as needed, and the training paradigm of different grabbing masks can be achieved; the gestures are accurately detected, the specified experimental paradigm is conveniently designed, data precision of follow-up analysis is improved, and later analysis and arrangement are convenient. According to the automatic grabbing training and recording device, specified gesture grabbing training of non-human primates can be achieved, the grabbing training of the non-human primates can be rapidly and efficiently achieved through the painless automatic training process, manpower resource consumption can be greatly reduced, and the automatic grabbing training and recording device is applicable to research and development of rehabilitation therapy instruments in the field of brain-computer interfaces.
Owner:ZHEJIANG UNIV
Immunostimulatory oligonucleotides and uses thereof
Oligonucleotides containing the non-palindromic sequence motif: X1X2X3X4X5X6X7X8, wherein X1 is C,T,G or A (preferably T or C); wherein X2 is C,T,G or A; wherein X7 is C,T,G or A (preferably G); at least three, and preferably all, of X3, X4, X5, X6 and X8 are T; and with the proviso that, in the motif, a C does not precede a G (in other terms, the nucleic acid motif does not consist of a CpG oligonucleotide), that modulate the immune response of animals of the order Primate, including humans, are disclosed. This immune modulation is characterized by stimulation of proliferation, differentiation, cytokine production and antibody production on B-cells and cell differentiation on plasmacytoid dendritic cells.
Owner:DAVID HORN LLC
Identification of TRPML3 (MCOLN3) as a salty taste receptor and use in assays for identifying taste (salty) modulators and/or therapeutics that modulate sodium transport, absorption or excretion and/or aldosterone and/or vasopressin production or release
The present invention relates to high-throughput mammalian and medium-throughput oocyte-based electrophysiological assays for identifying human TRPML3 modulators, preferably TRPML3 enhancers. Compounds that modulate TRPML3 function in the assay are expected to affect salty taste in humans. The inventive electrophysiological assays, such as the two-electrode voltage-clamp technique, facilitate the identification of compounds which specifically modulate human TRPML3. The assays of the invention provide a robust screen useful to detect compounds that facilitate (enhance) or inhibit TRPML3 function. Compounds that enhance or block TRPML3 channel activity should thereby modulate salty taste. In addition, these compounds may be used to regulate sodium excretion, urinary output and other biological functions relating to sodium levels. This invention relates to the elucidation that TRPML3 is involved in salty taste perception in primates including humans and likely other mammals (given the significance of sodium and other ions to physiological functions and conditions this phenotype is likely strongly conserved in different animals). The TRPML3 gene also modulates one or more of sodium metabolism, sodium excretion, blood pressure, fluid retention, cardiac function and urinary functions such as urine production and excretion. The inventors have identified TRPML3 as encoding a salty taste receptor in primates and humans (and likely other mammals) based on gene expression assays which have determined that TRPML3 is expressed specifically in taste bud cells and not in lingual epithelial cells, similar assays that have determined that TRPML3 is specifically expressed or enriched in the top half of taste bud cells in a subset of taste cells which do not express TRPM5 or PKD2L1, prior reports that document the expression of TRPML3 in other sensory organs such as the ear (therefore further substantiating the role of TRPML3 as a 'professional' sensory gene).
Owner:SENOMYX INC
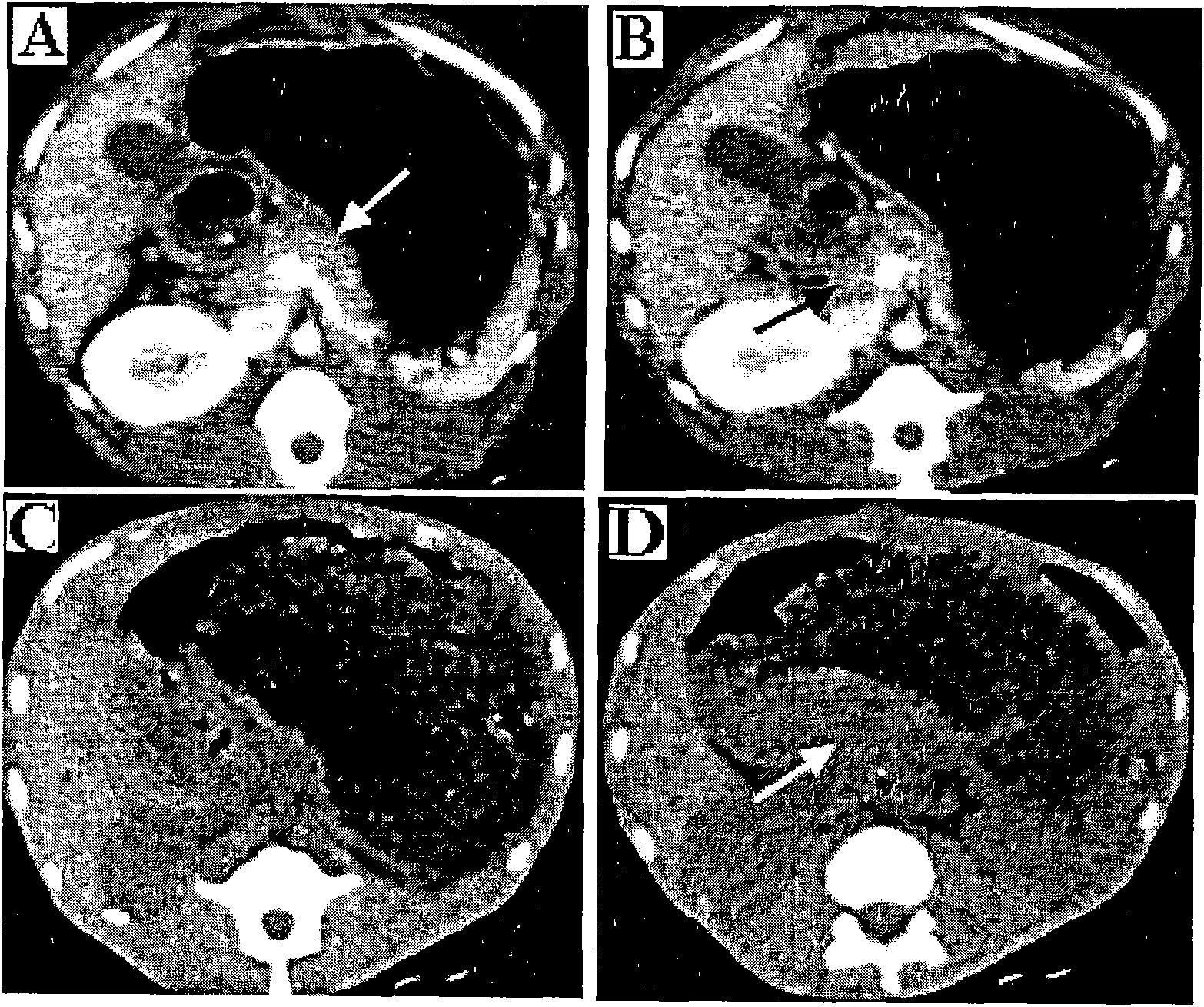
Establishment and application of rhesus monkey pulmonary fibrosis model
ActiveCN105126075AEasy to operateEasy to repeatSaccharide peptide ingredientsRespiratory disorderRodent modelMedicine
The invention discloses a method for preparing an animal pulmonary fibrosis model. According to the method, bleomycin is applied to a primate, the application dose of the bleomycin is 7 mg / Kg for each time, and application is performed once for each week and totally performed twice. The invention further provides a rhesus monkey pulmonary fibrosis model prepared with the method and an application of the rhesus monkey pulmonary fibrosis model. The rhesus monkey pulmonary fibrosis model is established successfully, an operation process is simple and easy to repeat, and the model can be used for new biotechnological drug evaluation which cannot be finished by a rodent model and has bright clinical application prospects.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Construction method of primate miRNA-122 knockout model, primate liver cancer model and application thereof
A construction method of a primate liver cancer model comprises the following steps: a) inserting gRNA sequence for specifically targeting miR122 into an appropriate vector to construct a template vector for in vitro transcription of miR122; b) inserting cas9 gene sequence into an appropriate vector to construct a template vector for in vitro transcription of cas9 gene; c) carrying out PCR amplification by using the vector in the Step a) so as to obtain a gRNA temperate for in vitro transcription of miR122; d) linearizing the template vector in the Step b) by the use of restriction enzyme so as to obtain a template for in vitro transcription of cas9 gene; e) carrying out in vitro transcription by using products in the Step c) and Step d) as templates so as to obtain gRNA for specifically targeting miR122 and mRNA of cas9 gene; f) injecting the gRNA and mRNA in the Step e) into fertilized egg cell of the animal so as to generate stable miR122-knockout animal offspring.
Owner:PEKING UNIV
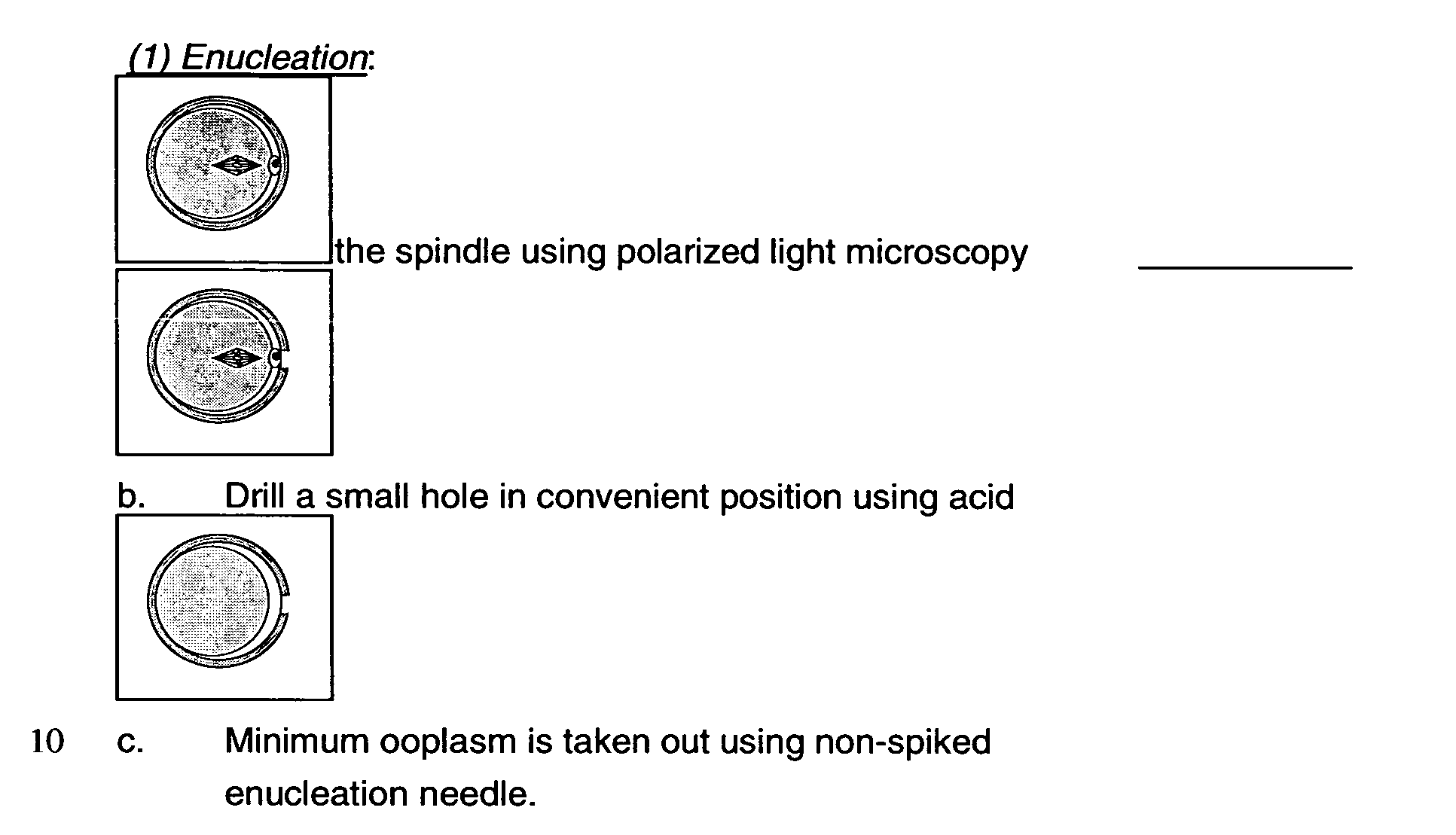
Method of enucleation and oocyte activation in somatic cell nuclear transfer in primates
InactiveUS20050063962A1Improve efficiencyImprove efficiency and successBiocideGenetic material ingredientsCompound (substance)Somatic cell nuclear transfer
The present invention relates to a method for cell enucleation, comprising removal of the spindle body from a cell in metaphase with a minimal amount of cytoplasm from the cell, said method comprising using polarized light microscopy for visualization of the spindle body during cell enucleation. The present invention also relates to a method for production of an activated reconstructed vertebrate cell, said method comprising: a) culturing a reconstructed vertebrate cell for about 1.5 to about 3 hours after being reconstructed; b) applying at least one electrical pulse to the reconstructed cell; c) culturing the reconstructed cell for about a further 2 hours; and d) treating the reconstructed cell with a chemical activator. The methods of the invention find application in the preparation of totipotent or pluripotent cells of substantially identical genotype, as well as the cloning of vertebrates.
Owner:NAT UNIV OF SINGAPORE
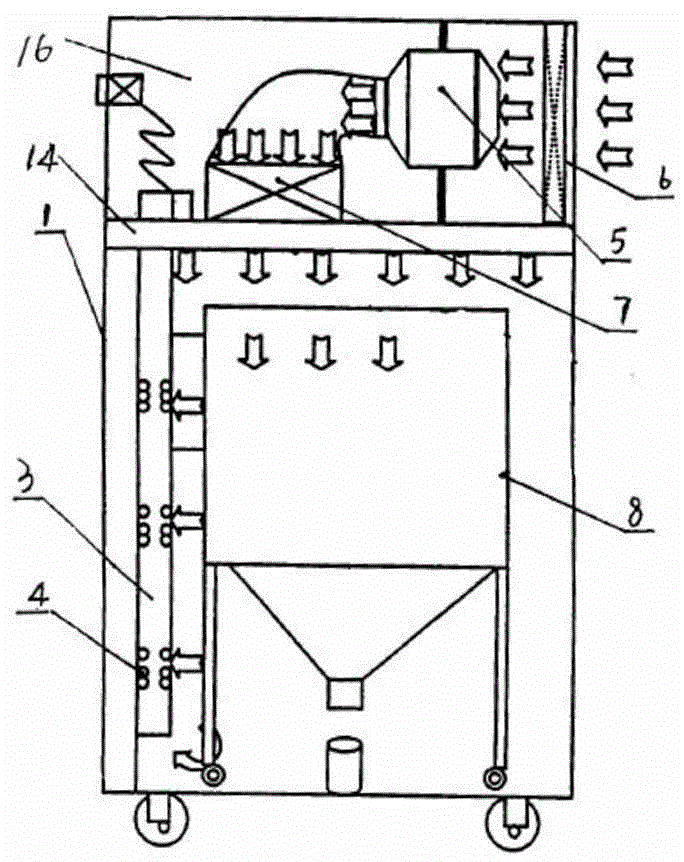
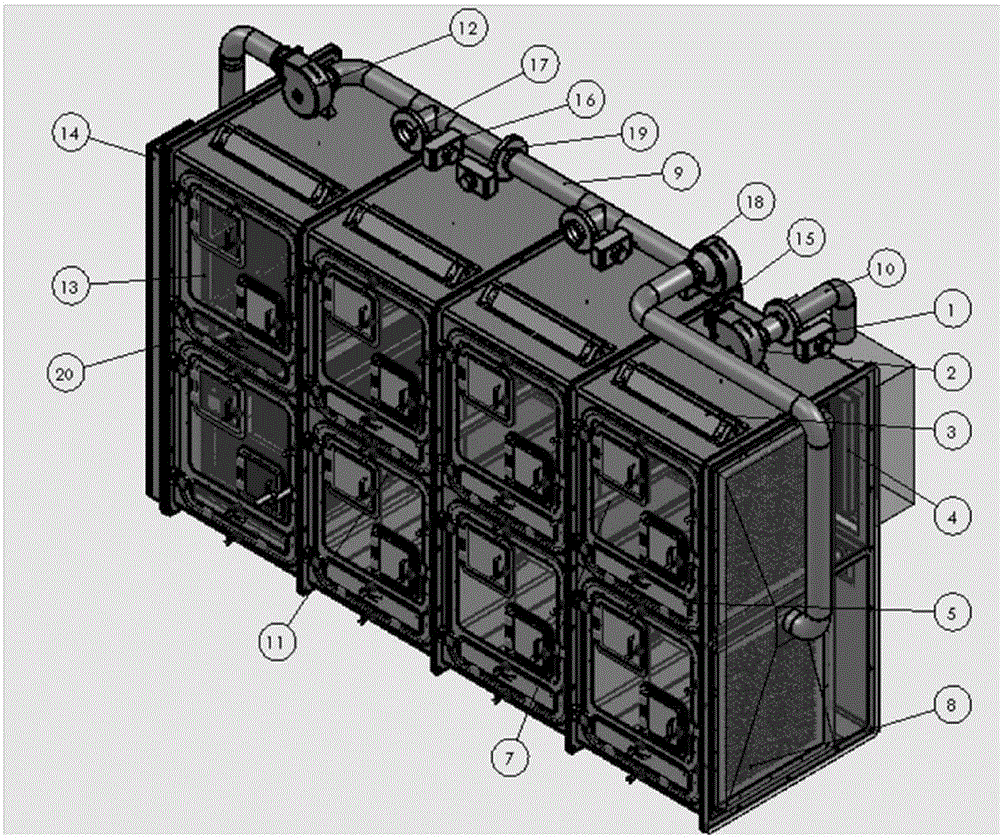
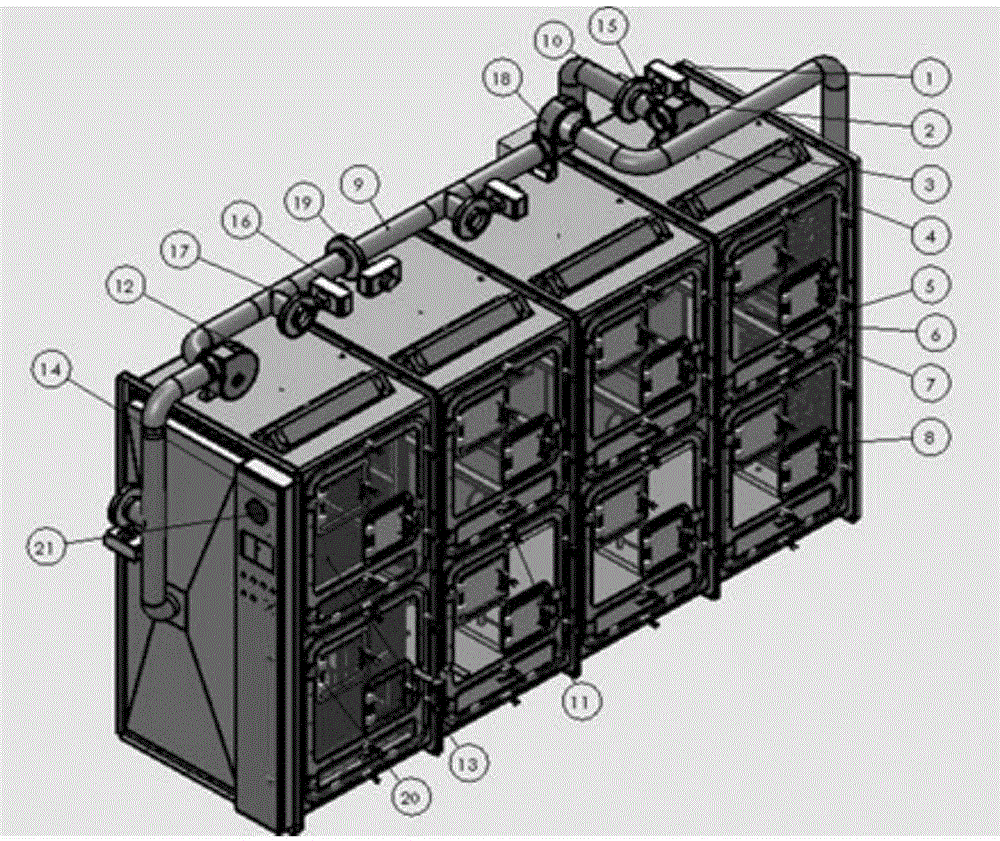
Method and device for establishment of Mycobacterium tuberculosis naturally infected non-human primate model
InactiveCN104127444ABacteria material medical ingredientsAnimal housingLatent tuberculosisMycobacterium w
The invention provides a method for establishment of a Mycobacterium tuberculosis naturally infected non-human primate model. According to the invention, a healthy non-human primate and a non-human primate artificially infected with tuberculosis and suffering cough are raised in an air flow self-circulation combined type negative pressure isolator so as to infect the healthy non-human primate with tuberculosis. Establishment of the natural infection model helps to reveal conditional factors (like HIV) activating latent tuberculosis infection and related mechanisms. The non-human primate model with more clinical significance is provided for research, development and evaluation of anti-tuberculosis vaccines and drugs.
Owner:WUHAN UNIV
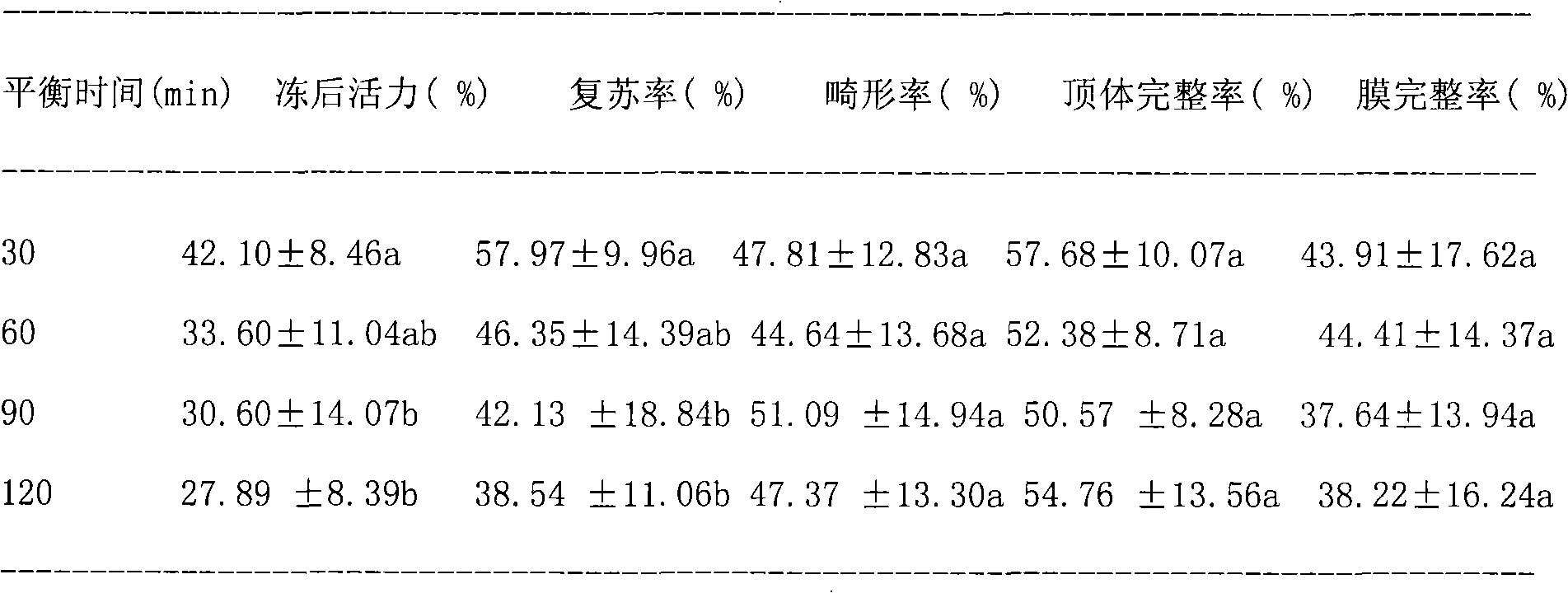
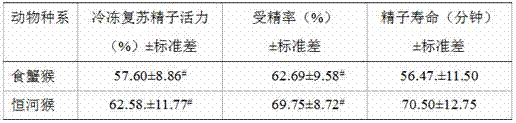
Method for freezing semen of Macaca fascicularis
InactiveCN101810163AGood geneticsExcellent reproductive efficiencyDead animal preservationMotilityBiology
The invention discloses a method for freezing the semen of a Macaca fascicularis. In the method, fresh semen activity of a semen sample is required to be more than or equal to 0.7. The freezing method comprises the following operating steps of: 1) freezing diluent prescription and diluent preparation for the semen of the Macaca fascicularis; 2) semen dilution; 3) semen package; 4) semen balance; 5) semen freezing; and 6) frozen semen unfreezing. The method has the following advantages that: by using the technology of the invention, in the processes of freezing and unfreezing the semen of the Macaca fascicularis, the motility rate and the recovery rate of the semen after freezing and unfreezing respectively reach 45 percent and 62 percent, so that the reproductive efficiency of good male Macaca fascicularis can be brought into full play, genetic gene of good Macaca fascicularis can be conserved, and referential experience is provided for the research on semen cryopreservation of other rare or endangered primates at the same time.
Owner:GUANGXI UNIV
Development of therapeutics for the treatment of endotoxin-meidated diseases
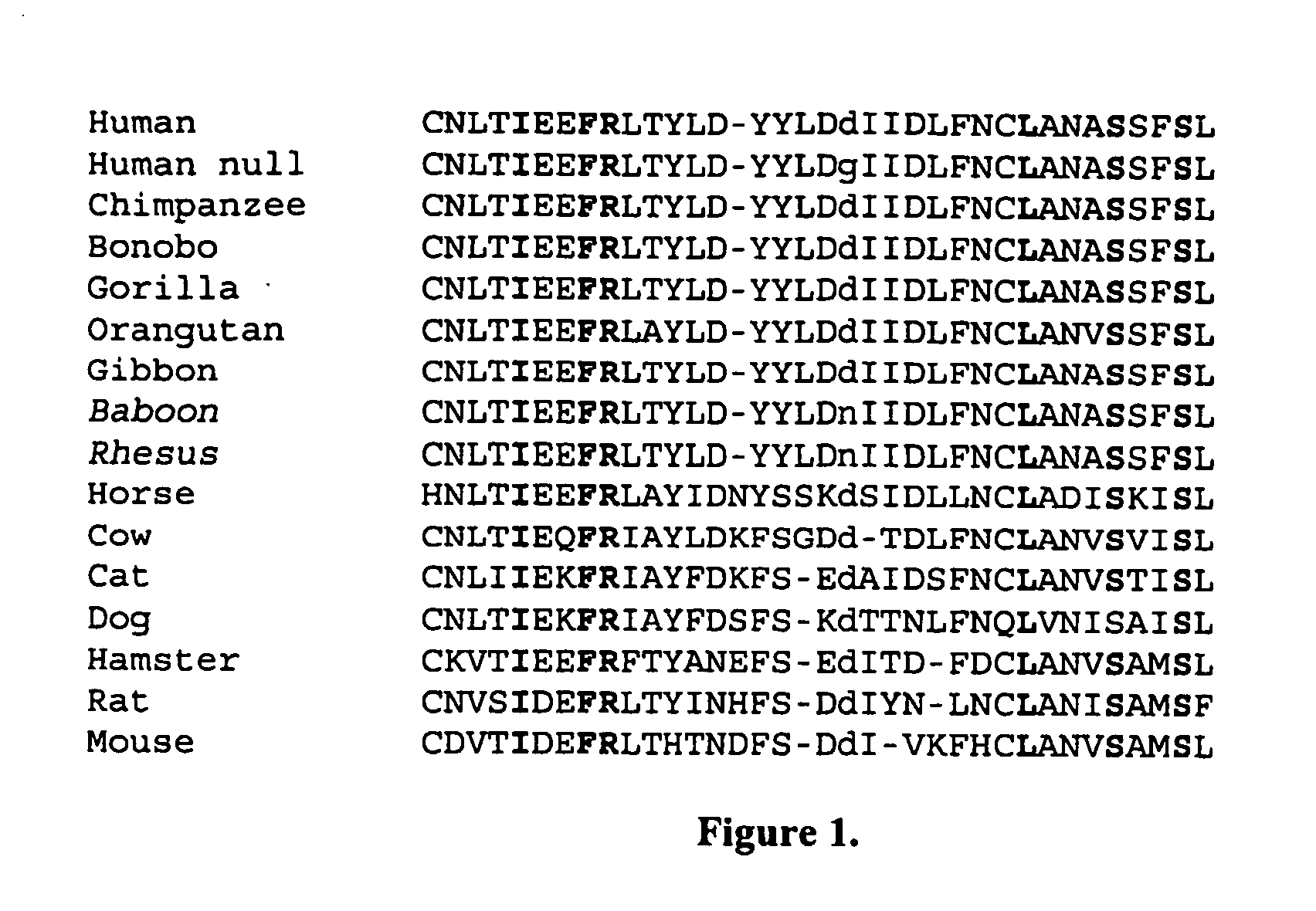
InactiveUS20060014150A1Improve severityReduce the impactAntibacterial agentsBiocidePrimateBiological activation
The subject invention comprises a method for identifying an evolutionarily meaningful nucleotide change in a primate's TLR4 polynucleotide. It further comprises methods for identifying agents that interact with the corresponding evolutionarily meaningful amino acid change so as to modulate the function of the TLR4 polypeptide, thereby attenuating activation of the NF-kB pathway. Such agents are useful in mitigating the LPS mediated response and in the treatment of sepsis, severe sepsis and septic shock.
Owner:EVOLUTIONARY GENOMICS LLC
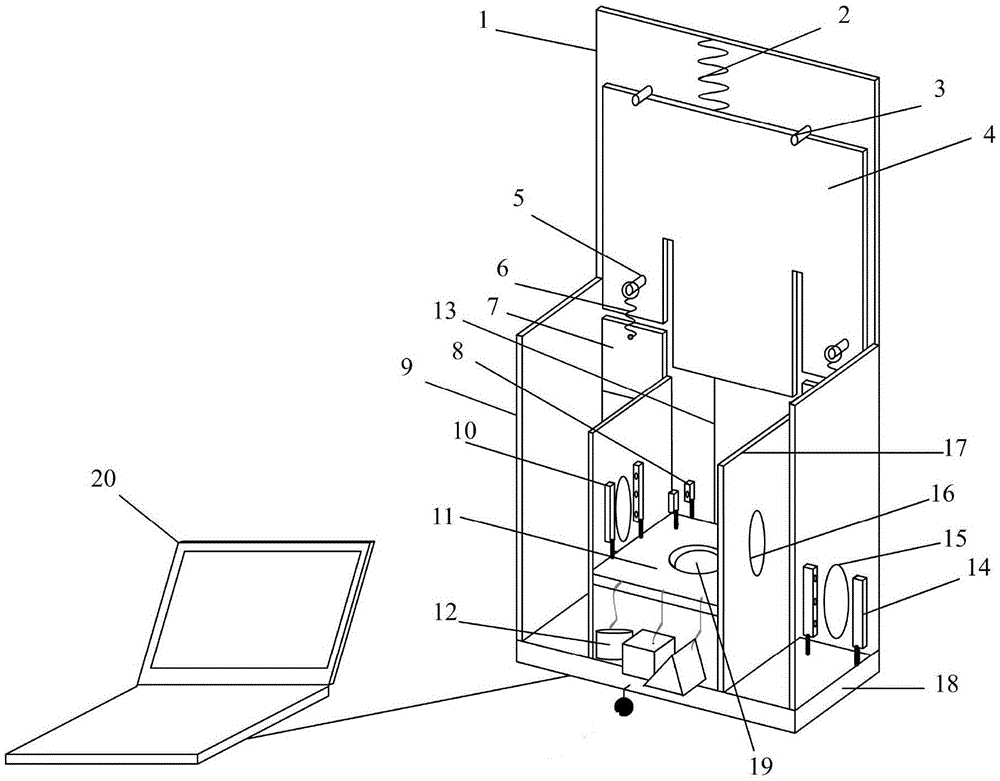
Method for testing memory, judgment and execution capabilities of primate
The present invention discloses a method for testing memory, judgment and execution capabilities of a primate. The method comprises the following steps that: a testing device is disposed before a primate, the testing device is connected to a monitoring computer (20), and monitoring software is started; and the monitoring computer (20) automatically records the result tested every time and stores the test results in corresponding storage paths as data files. The present invention also discloses a structure of the testing device. By adoption of the testing device, the primate is liable to understand test schemes, and domestication success rate is high; behaviors such as reaction capability and arm and finger agility of the primate can be monitored conveniently, and automation requirements of data acquisition can be satisfied; and the method has the advantages that accuracy of experimental data is high, reaction speed is fast, and jamming is prevented.
Owner:广西南宁灵康赛诺科生物科技有限公司
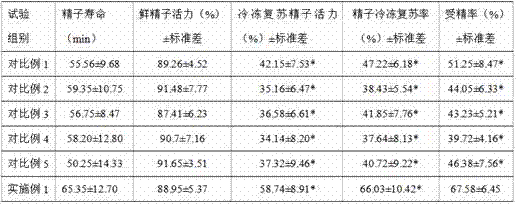
Refrigeration preservation and resuscitation method of sperm of non-human primates
ActiveCN106962323AHigh activityImprove fertilization rateDead animal preservationGerm cellsNon human primateFertility
The invention discloses a refrigeration preservation and resuscitation method of sperm of non-human primates. According to the refrigeration preservation and resuscitation method of sperm of non-human primates, in refrigeration preservation, sperm is subjected to liquefaction at normal temperature for 30min, and monkey sperm is diluted with HTF culture solution, wherein the volume ratio of the monkey sperm to the HTF culture solution is controlled to be 1:9; glycerin is added based on the volume of the monkey sperm until the volume final concentration of glycerin is 10%; an obtained mixture is subjected to subpackage in 1.5ml cryopreservation tubes, is stored at 4 DEG C for 30min, is stored for 30min in suspension above liquid nitrogen, and then is stored in liquid nitrogen; in resuscitation, the cryopreservation tubes stored in liquid nitrogen are shaken slightly in water bath at 37 DEG C for 1 to 3min until complete thawing is realized. Compared with the prior art, the survival rate and the fertility rate of the refrigeration preservation and resuscitation method are both increased; the refrigeration preservation and resuscitation method is effective, is rapid, and is convenient, and can be used for sperm refrigeration preservation and genetic resource conservation of other non-human primates.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
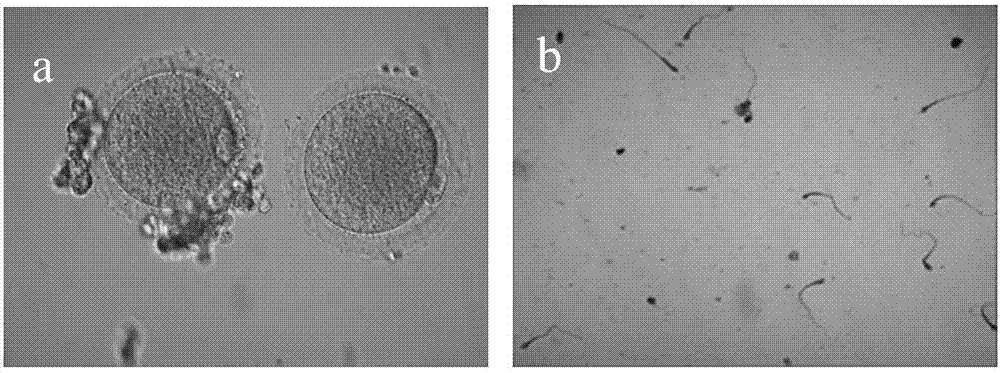
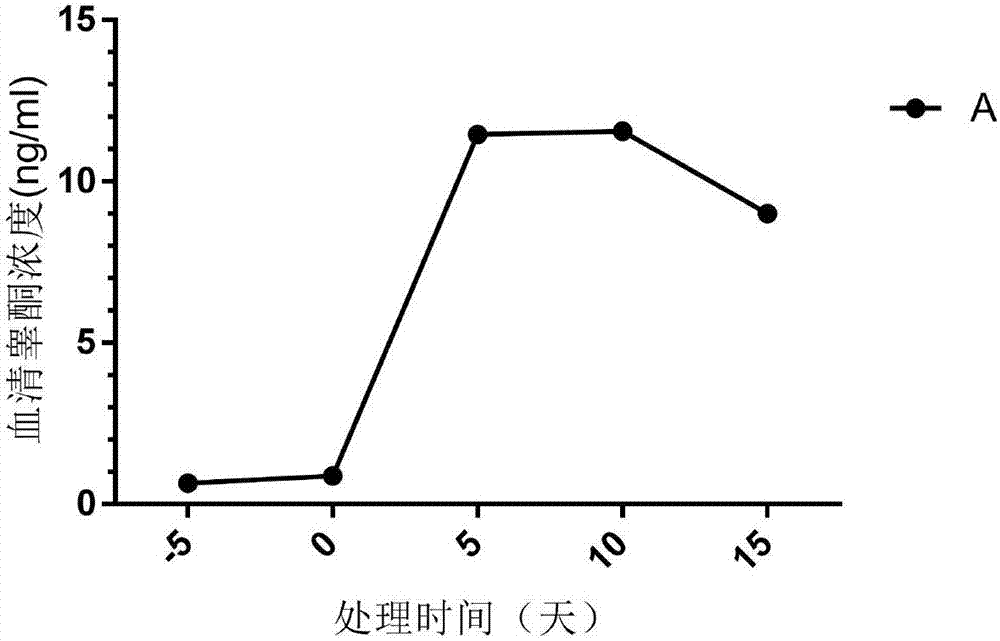
A method of inducing young primates to obtain gonad gametes and applications thereof
A method of inducing young primates to obtain gonad gametes is disclosed. The method includes administering a female young primate with gonadotropin-releasing hormone or an analogue thereof, performing combined administration of gonadotropin, and inducing follicular development to obtain mature oocytes that are female gametes; administering a male young primate with gonadotropin, performing combined administration of gonadotropin-releasing hormone or an analogue thereof, and inducing testis spermatogenesis to obtain sperms that are female gametes; and subjecting the obtained female gametes and male gametes to fertilization, and allowing fertilized eggs to develop until blastulas or a filial generation. A hormone combination is adopted by the method to promote induction of young primate sexual prematurity, thus obtaining the gonad gametes in advance, significantly shortening the generation period of primates, and accelerating offspring breeding. The method can achieve large-scale expanding propagation of the young primates, shorten the generation period, reduce the cost, and provide experience references for germplasm preservation of precious and endangered animal species and rapid animal propagation through gene editing.
Owner:广州高新区生物研究和实验发展有限公司
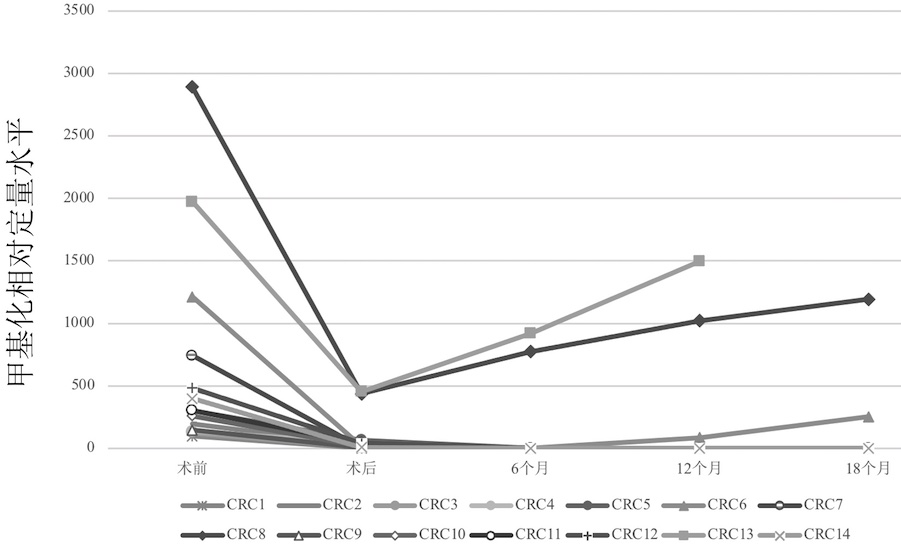
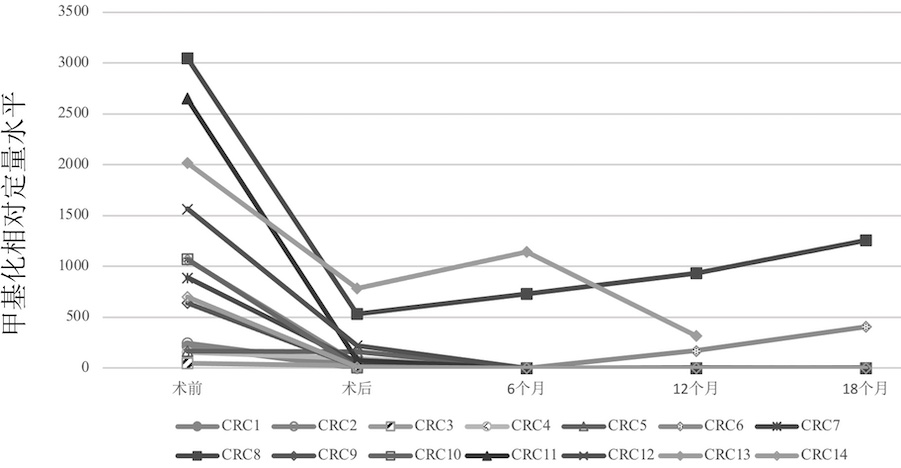
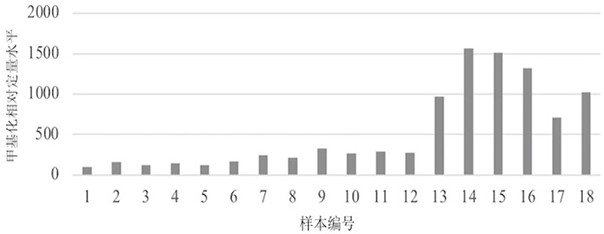
Primate animal DNA methylation relative quantification kit
The invention relates to the field of molecular biology, in particular to a primate animal DNA methylation relative quantification kit. The kit comprises: a) a juvenile zebrafish DNA or zebrafish DNA without CpG site, and b) a DNA quantitative detection reagent which at least comprises a reagent for quantitative detection of a), wherein the juvenile zebrafish is zebrafish from fertilized egg formation to development within 120 hours. According to the kit, accurate quantification of DNA methylation of primates can be realized, and the problem that endogenous internal reference is prone to instability or genome DNA pollution caused by own factors or sample collection reasons is solved.
Owner:JIANGSU MICRODIAG BIOMEDICINE TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com