Patents
Literature
42 results about "Immunohis tochemistry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
New plasma membrane biomarkers preferentially expressed in pancreatic beta cells useful in imaging or targeting beta cells
ActiveUS20100322850A1Evaluate effectivenessEarly detectionDisease diagnosisDiagnostic recording/measuringDiseaseDendritic cell
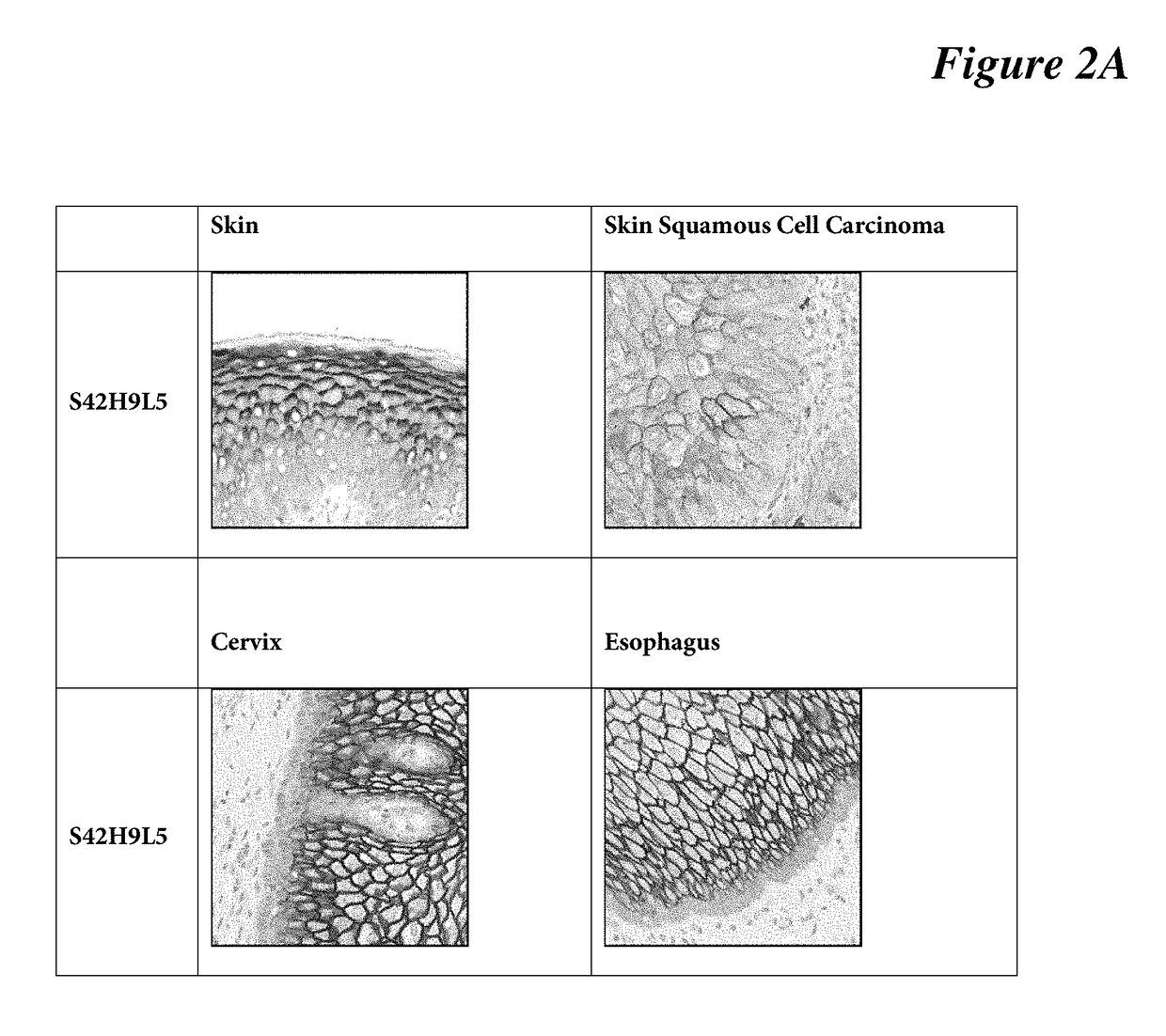
The present invention is directed to the identification of a biomarker specifically located in the plasma membrane of pancreatic beta cells. It was selected by a Systems Biology approach on Massively Parallel Signal Sequencing datasets obtained in human islets and Affymetrix microarray datasets on human islets, purified rat primary beta and non beta cells and insulinoma cells. Based on a set of specific features the biomarker is a unique candidate for imaging and targeting strategies to study the pancreatic beta cell mass in health and disease (T1 D, T2D, pancreatic cancers, obesity, islet transplantation, beta cell regeneration). The five specific features of the selected biomarkers are: 1) Preferentially expressed in pancreatic islets as compared to surrounding tissues; 2) Higher expression in pancreatic beta cells than in pancreatic alpha cells or than in other islet non-beta cells; 3) Expression levels in pancreatic beta cells are higher or comparable to glucokinase which is an enzyme specifically expressed in the pancreatic beta cell; 4) Located in the membrane and as such targetable with antibodies, peptides or small molecules which allows imaging, targeting and immunohistochemistry; and 5) Expression is not induced during the process of inflammation of the beta cell mass and the protein is not enriched in T-cells and dendritic cells or in other cells participating in the inflammation process.
Owner:UNIV LIBRE DE BRUXELIES +2
Specific combined polypeptide for lung cancer, preparation and uses thereof
InactiveCN101492505AEasy to detectReliable detectionImmunoglobulins against animals/humansAntibody ingredientsTumor targetBacterial virus
The invention discloses a polypeptide that can be specifically combined with lung cancer cells and a preparation method and application thereof. The polypeptide specifically combined with lung cancer cells shows peptide library reducing screening by a bacterial virus so as to obtain bacterial virus clone specifically with lung cancer; the bacterial virus clone having stronger affinity with lung cancer is identified by Elisa and an immunohistochemical method; and sequencing is sent so as to obtain a 12 polypeptide, and the amino acid sequence is shown as SEQ ID NO: 1. The 12 polypeptide screened by the invention not only has the advantages of traditional antibody molecule but also has small molecular weight, strong penetrating power and easy arriving to tumor tissue; the 12 polypeptide has good tumor targeting effect and more sensibility of tumor diagnosis, and can be used for preparing a kit for tumor diagnosis and drug for curing tumors.
Owner:GUANGDONG PHARMA UNIV
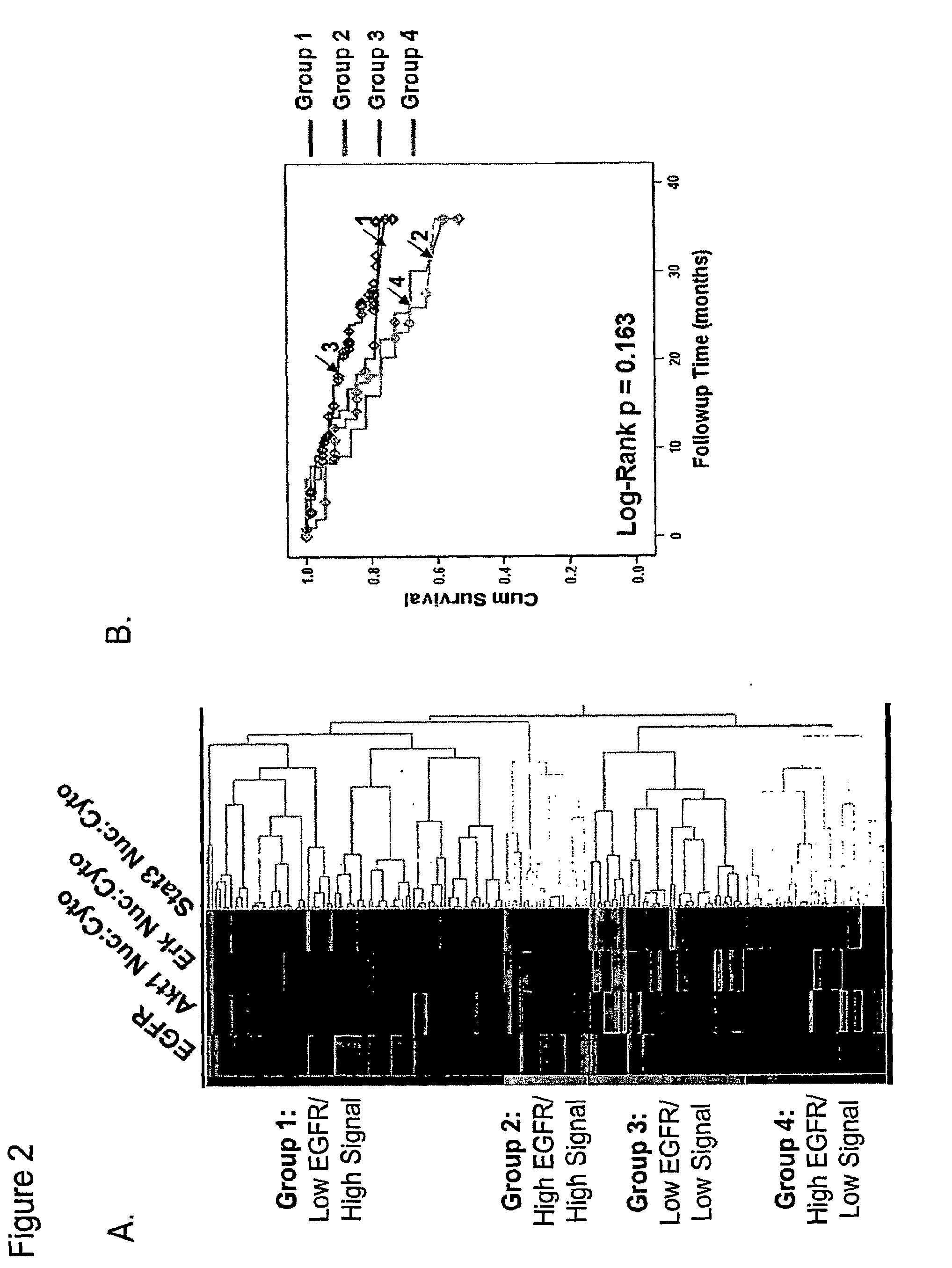
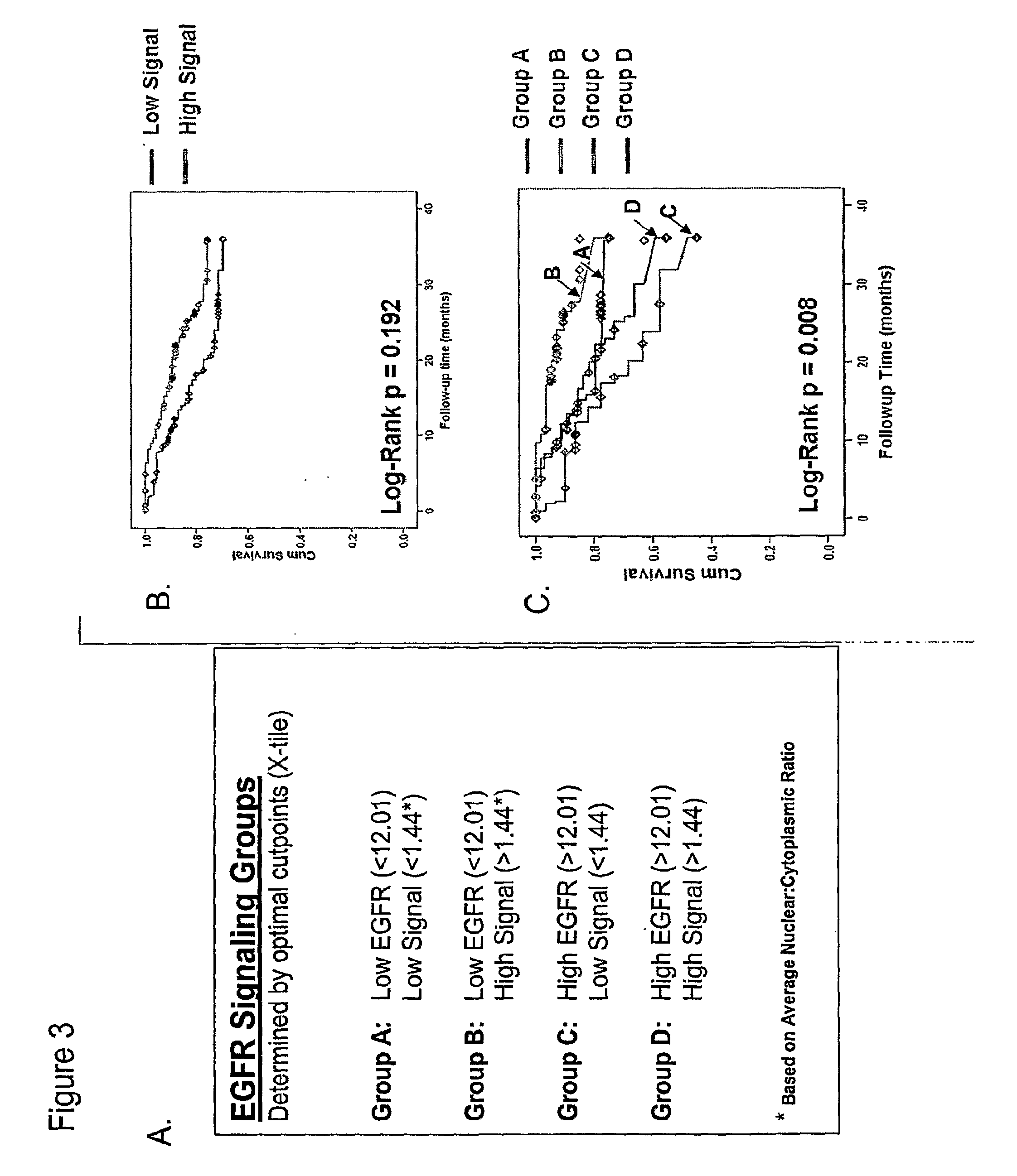
Methods for determining signal transduction activity in tumors
ActiveUS20100062452A1Peptide/protein ingredientsMicrobiological testing/measurementImmunohistochemistry techniqueBiology
The method of the invention pertains to determining the signal transduction activity in a tissue section by immunohistochemistry techniques. The expression level of the receptor of interest is determined as well as the expression levels of one or more effector molecules of the receptor signal transduction pathway. Furthermore a combined ratio of expression levels of effector molecules in subcellular compartments with the receptor expression was found to have prognostic significance.
Owner:NOVARTIS AG +1
Antibodies, compositions, and immunohistochemistry methods for detecting c4.4a
ActiveUS20180031566A1Preparing sample for investigationImmunoglobulins against cell receptors/antigens/surface-determinantsWilms' tumorAntibody
Antibodies, compositions, systems, and methods for detecting C4.4a, for example immunohistochemistry methods for detecting C4.4a using a C4.4a antibody. The antibody may be obtained by immunizing a host with a C4.4a protein such as a peptide downstream of the signal peptide. The antibodies may be adapted to detect the uPAR-like domain 1 and uPAR-like domain 2. Also featured are methods for diagnosing C4.4a-associated tumors using C4.4a antibodies disclosed herein.
Owner:VENTANA MEDICAL SYST INC
Application of multi-functional PAP pen in cell climbing immunohistochemical detection
ActiveCN104359741AImprove comparabilityImprove reliabilityPreparing sample for investigationBiological testingStainingPetri dish
The invention discloses an application of a multi-functional PAP pen in cell climbing immunohistochemical detection. The application comprises the following steps: inoculating cells in a glass slide-type culture dish for enabling cells to grow on the glass slide-type culture dish; after cell culture, conducting immunohistochemical dyeing identification. The glass slide-type culture dish is provided with a culture dish body and a culture dish cover, wherein the culture dish body comprises a single-pore chamber model and a porous chamber model; the area of each pore chamber is greater than that of the glass slide; the bottom of the culture dish body can be directly used as the glass slide; a rectangular streak line groove, the size of which is corresponding to that of the glass slide, is formed in the outer side surfaces of the bottom of the culture dish body; the bottom of the culture dish body can be pulled out of the rectangular streak line groove under the action of external force. The application is suitable for various immunohistochemical dyeing experiments on a glass slice, can remarkably reduce antibodies and the reagent dosage, avoids liquid flow and diffusion in dyeing, improves the operation speed, and is particularly suitable for large-scale large-sample and multi-group immunohistochemical dyeing in experimental study of cell growing on the glass slide-type culture dish or cell smearing.
Owner:绍兴橡树企业管理咨询合伙企业(有限合伙)
Anti-HPV16 E6 protein monoclonal antibody and application thereof
The invention discloses an anti-HPV16 E6 protein monoclonal antibody, which is characterized by having heavy chain variable regions with the amino acid sequences shown as SEQ ID NO:4, SEQ ID NO:6 andSEQ ID NO:8 and light chain variable regions with the amino acid sequences shown as SEQ ID NO:10, SEQ ID NO:12 and SEQ ID NO:14. The immunoblot experiments show that the antibody can be used for specifically recognizing the recombinant HPV16 E6 protein and cervical cancer tissues expressing HPV16 E6 protein. The antibody can be used for detecting the expression level of the HPV16 E6 protein in thecells in artificial or automatic modes through immunohistochemical staining (IHC), enzyme-linked immunosorbent assay (ELISA) or western blot manners; the HPV16 viruses can be specifically diagnosed;the novel measure is provided for detecting HPV16 viruses on the protein level; the antibody can be used or the study of human reproductive system infectious diseases and tumors, and can also be usedfor the study of relevant malignant tumor such as esophagus cancer, breast cancer and anal carcinoma.
Owner:BEIJING SOLARBIO TECH CO LTD
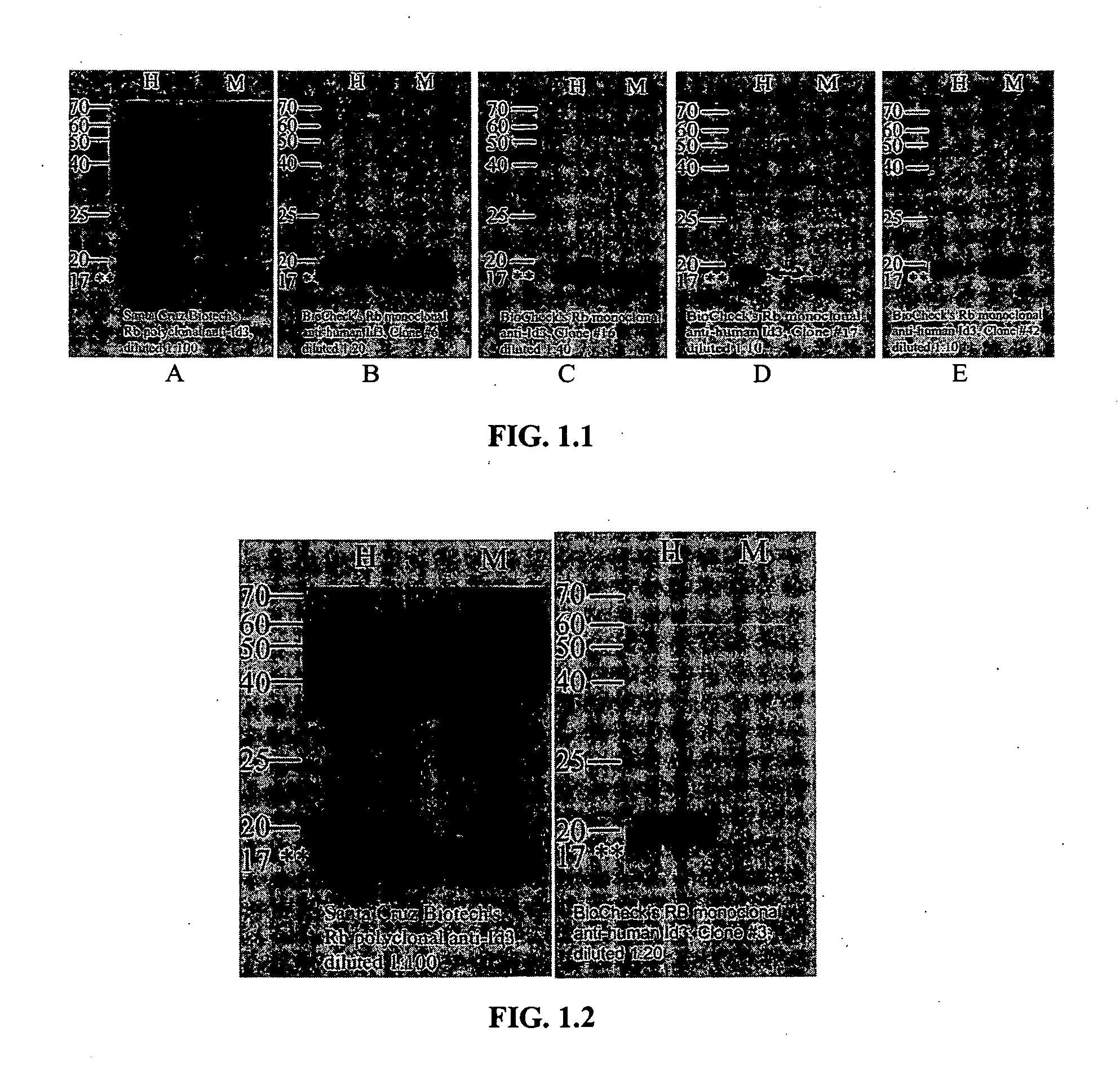
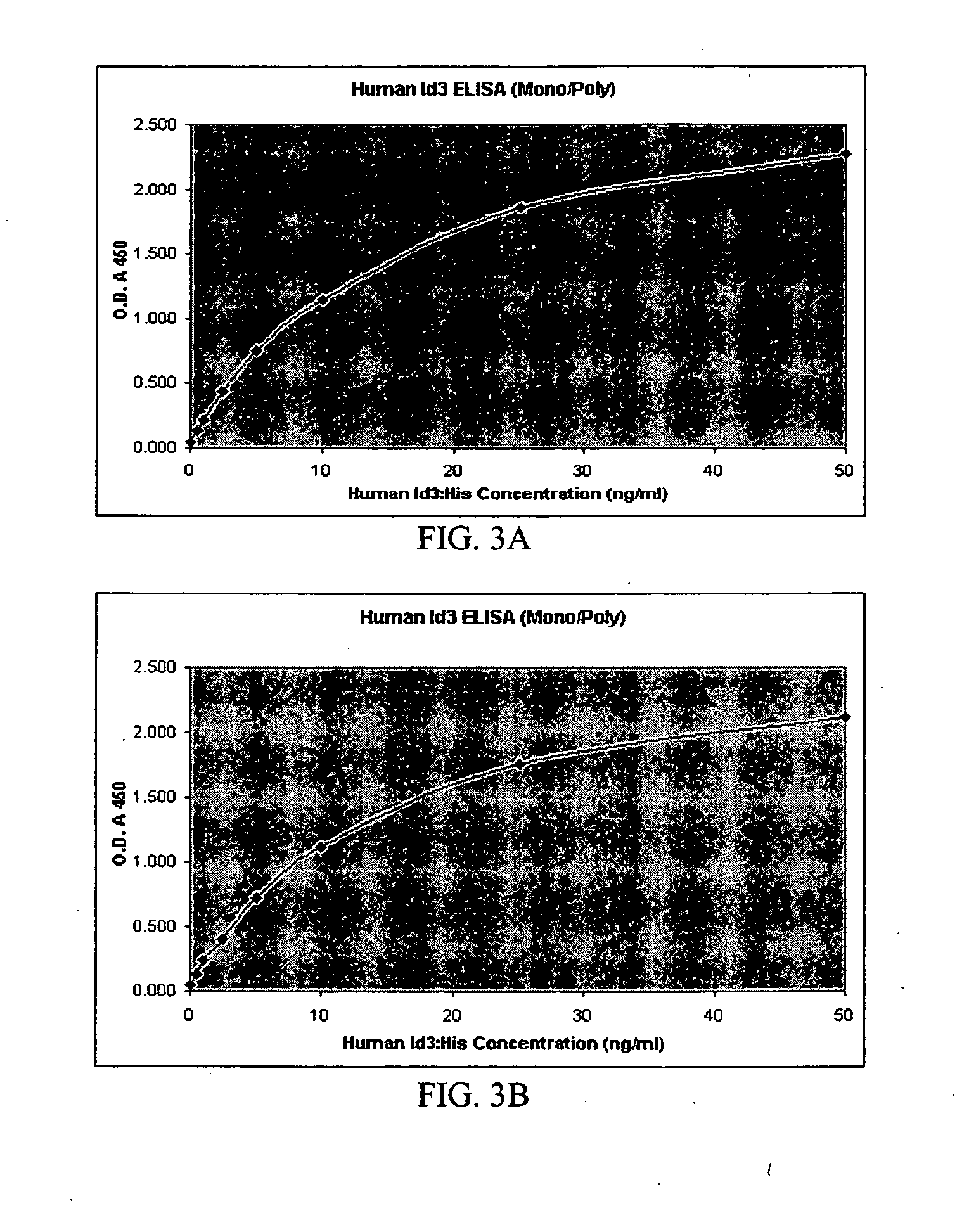
Rabbit monoclonal antibodies against mouse/human Id3 proteins
InactiveUS20070178531A1SensitiveImmunoglobulins against animals/humansBiological material analysisStainingSpecific detection
The present invention relates to a rabbit monoclonal antibody that binds to human Id3 protein and / or mouse Id3 protein with high specificity and high affinity. The antibody has a binding constant, measured with respect to human Id3 protein or mouse Id3, of greater than 1×108 / molar. The antibody has no substantial cross-reactivity to other family Id proteins such as Id1, Id2, or Id4, or other endogenous proteins present in the cells that express Id3 protein. The specificity and high affinity of the rabbit monoclonal antibodies of the present invention allows sensitive and specific detection and / or quantitation of human or mouse Id3 protein in biological samples. The antibodies are useful in immunochemical-based assays such as ELISA, western blot, and immunohistochemical staining.
Owner:BIOCHECK +1
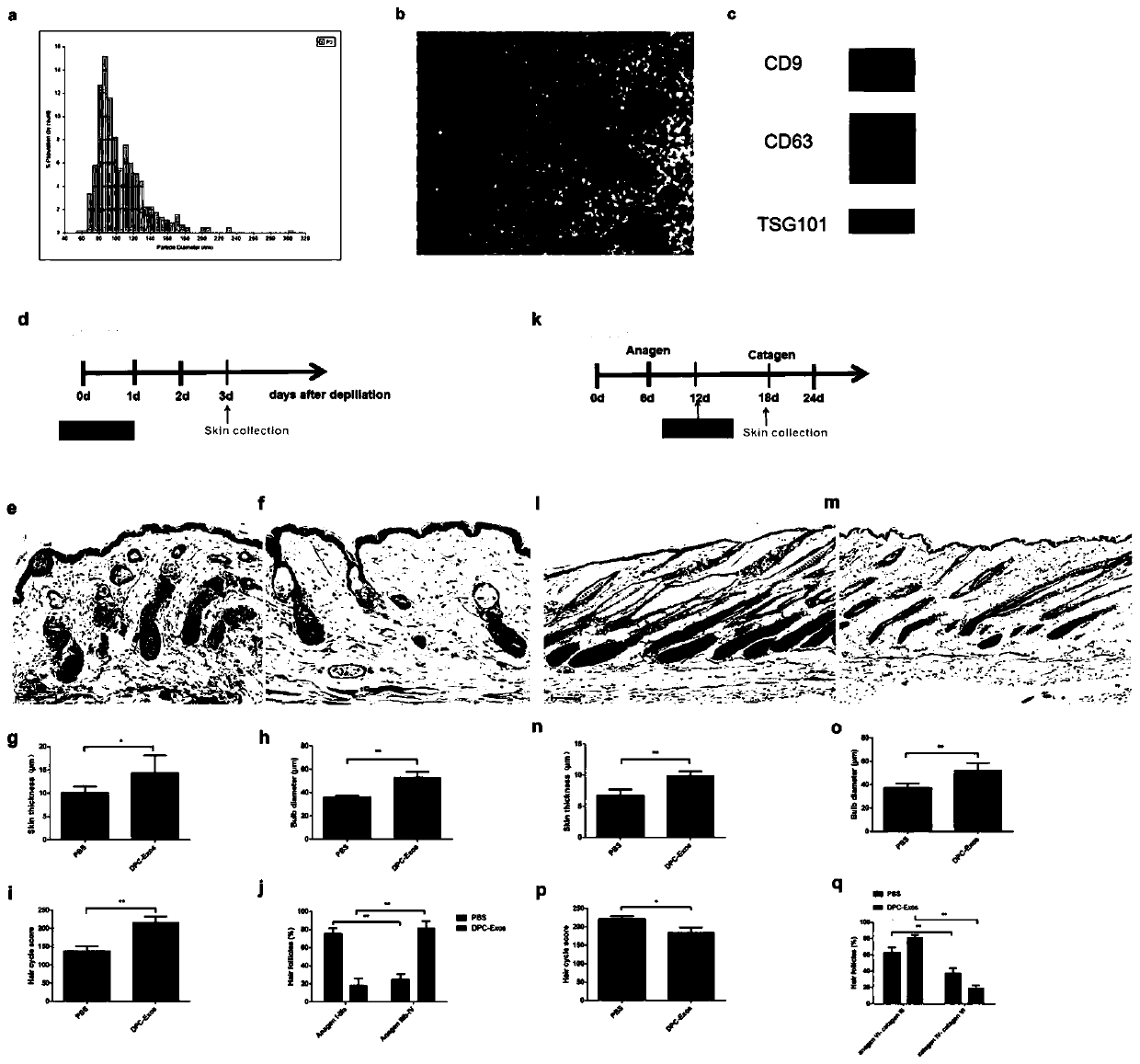
Method for extracting exosome derived from human hair follicle dermal papilla cells
PendingCN109852578AHelp regulate growthHelp regulate developmentMicrobiological testing/measurementArtificial cell constructsGrowth phaseCD63
The invention belongs to the technical field of cell secretion extraction, and discloses a method for extracting exosome derived from human hair follicle dermal papilla cells (DPC-Exos). The method isas below: identifying DPC-Exos by controlled resistance pulse sensing (TRPS) analysis, electron microscopy and Western Blot; and adding the DPC-Exos to a culture medium of human hair follicle outer root sheath cells (ORSCs) cultured in vitro. The DPC-Exos are identified to be about 105 nm in diameter and expressing tumor susceptibility gene (TSG) 101, differentiation cluster (CD) 9 and CD63; DPC-Exos promote proliferation and migration of human ORSCs, and mRNA and protein levels of beta-catenin and Sonichedgehog (Shh) in ORSCs are detected to be up-regulated after treatment; transdermal injection of DPC-Exos accelerates an HF growth phase of mice and delays the occurrence of regression. Immunohistochemical analysis shows that the expression levels of beta-catenin and Shh are up-regulatedin the hair follicles of an experimental group. DPC-Exos help regulate the growth and growth cycle of the HF and provide a new potential for the treatment of clinical hair loss.
Owner:ZHEJIANG UNIV
Recombinant lentiviral vector containing ubiquitin-specific protease gene USP39-shRNA (short hairpin ribonucleic acid) and application thereof
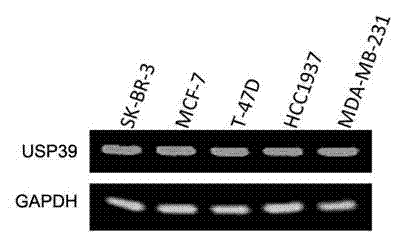
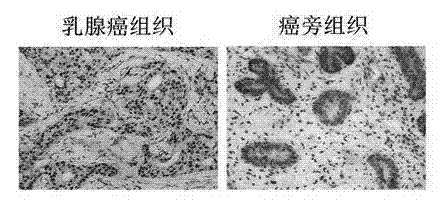
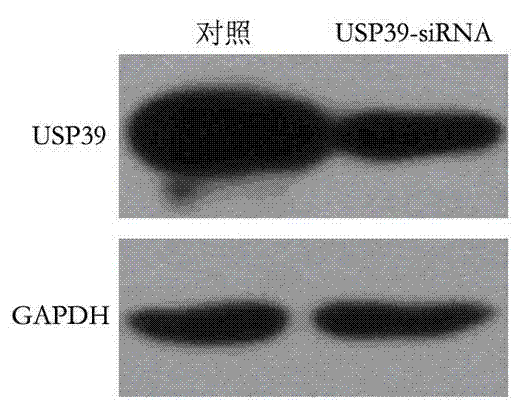
The invention provides a recombinant lentiviral vector containing ubiquitin-specific protease gene USP39-shRNA (short hairpin ribonucleic acid) and application thereof. The test proves that expression of USP39 in breast cancer tissue is obviously higher than that in normal breast tissue; the immunohistochemical detection confirms that the expression of USP39 in the breast cancer tissue is obviously higher than that in normal breast tissue; the immunohistochemical detection confirms that expression of USP39 protein in the breast cancer tissue is obviously higher than that in para-carcinoma tissue; siRNA (small interfering ribonucleic acid) is designed in a breast cancer cell system to interfere the expression of the USP39; it is found that proliferation of breast cancer cell after reducing the USP39 is obviously restrained; the cell apoptosis ratio is obviously improved; the cell ratio at the G1 stage is increased; the cell ratio at the S sage is reduced; and the clonality of the cell is obviously reduced. The test proves that the USP39 has an important role in facilitation of development of the breast cancer; and theoretical and experimental basis is provided for preparation of a drug for preventing and treating the breast cancer by the lentiviral vector containing USP39-shRNA.
Owner:HOSPITAL ATTACHED TO QINGDAO UNIV
Antibodies, compositions, and immunohistochemistry methods for detecting C4.4a
ActiveUS10948493B2Preparing sample for investigationImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesImmunohis tochemistry
Antibodies, compositions, systems, and methods for detecting C4.4a, for example immunohistochemistry methods for detecting C4.4a using a C4.4a antibody. The antibody may be obtained by immunizing a host with a C4.4a protein such as a peptide downstream of the signal peptide. The antibodies may be adapted to detect the uPAR-like domain 1 and uPAR-like domain 2. Also featured are methods for diagnosing C4.4a-associated tumors using C4.4a antibodies disclosed herein.
Owner:VENTANA MEDICAL SYST INC
Methods and systems for quantitative immunohistochemistry
ActiveCN110337588AWeather/light/corrosion resistanceMicrobiological testing/measurementAbzymeProtein target
Methods and systems are provided for quantitative immunohistochemistry (IHC) of a target protein molecule including a secreted target protein molecule. The method comprises introducing to the sample:a primary antibody specific for the target protein molecule; a secondary antibody conjugated to a secondary antibody enzyme, the secondary antibody is specific for the primary antibody; a tyramide conjugated with a tyramide hapten, wherein the secondary antibody enzyme catalyzes deposition of the tyramide hapten onto the sample; a tertiary antibody conjugated with a tertiary antibody enzyme, the tertiary antibody is specific for the tyramide hapten; and a chromogen, wherein the tertiary antibody enzyme catalyzes a reaction with the chromogen to make the chromogen visible. The chromogen is visible as a punctate dot using microscopy.
Owner:VENTANA MEDICAL SYST INC
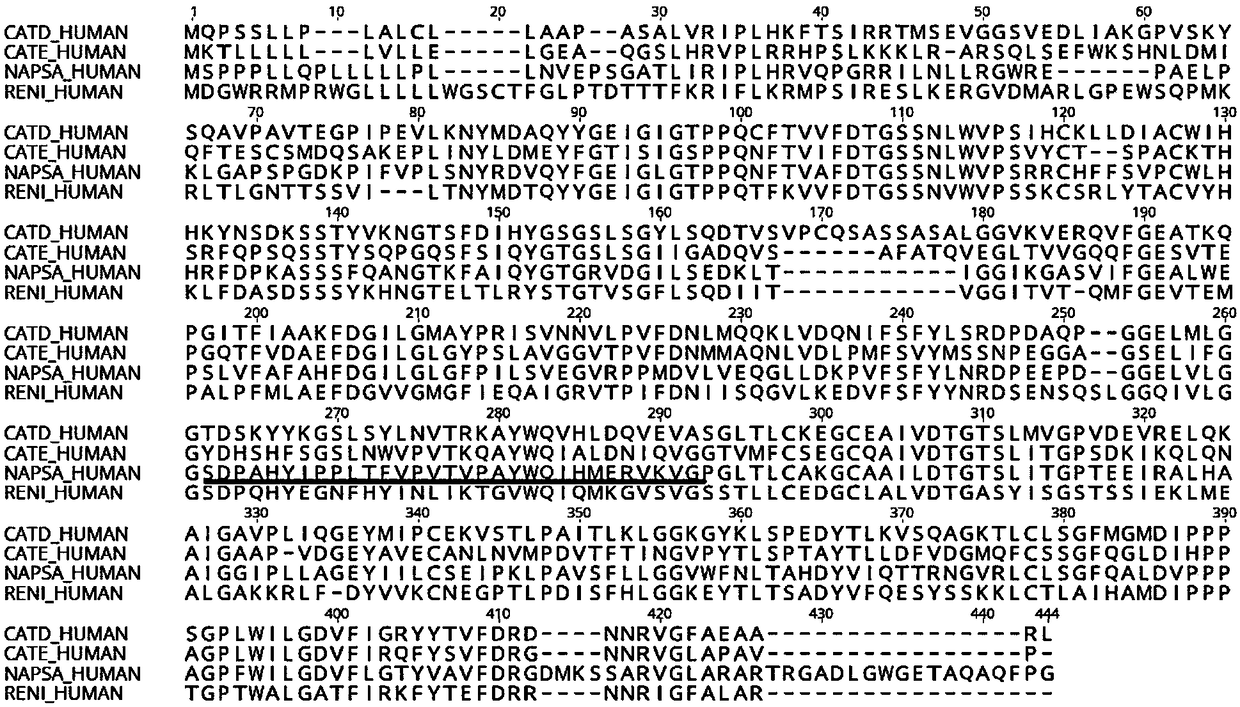
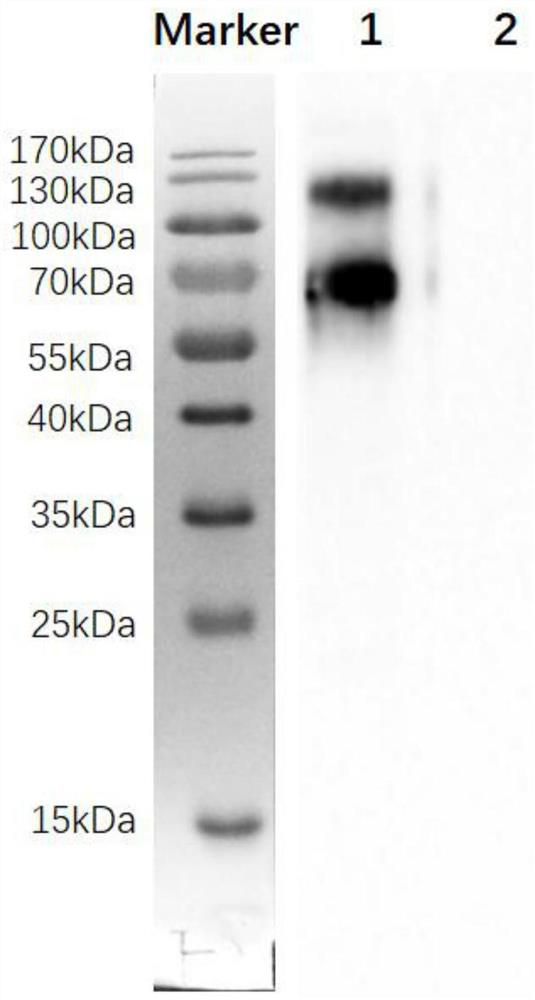
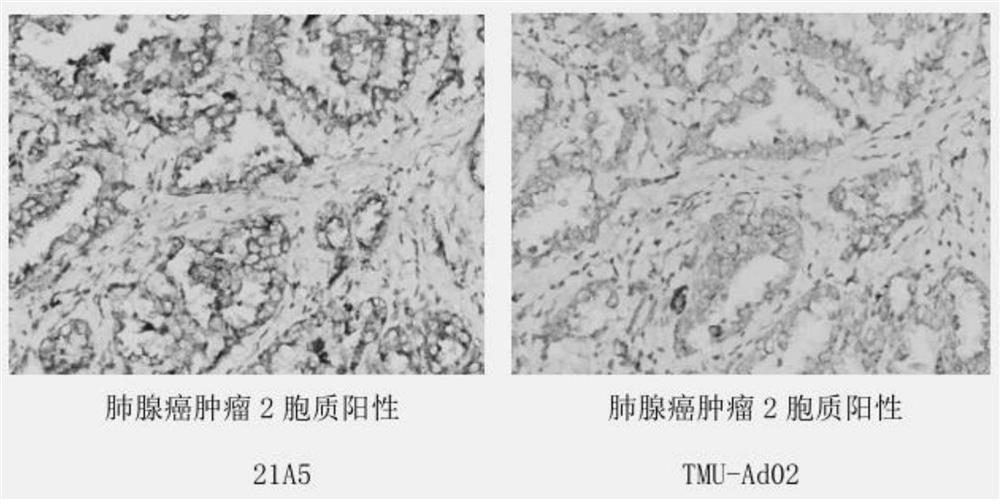
Monoclonal antibody against Napsin A protein, cell strain, preparation method and application
ActiveCN108467433AStrong characteristicIncreased sensitivityMicroorganism based processesTissue cultureChemical synthesisImmunogen
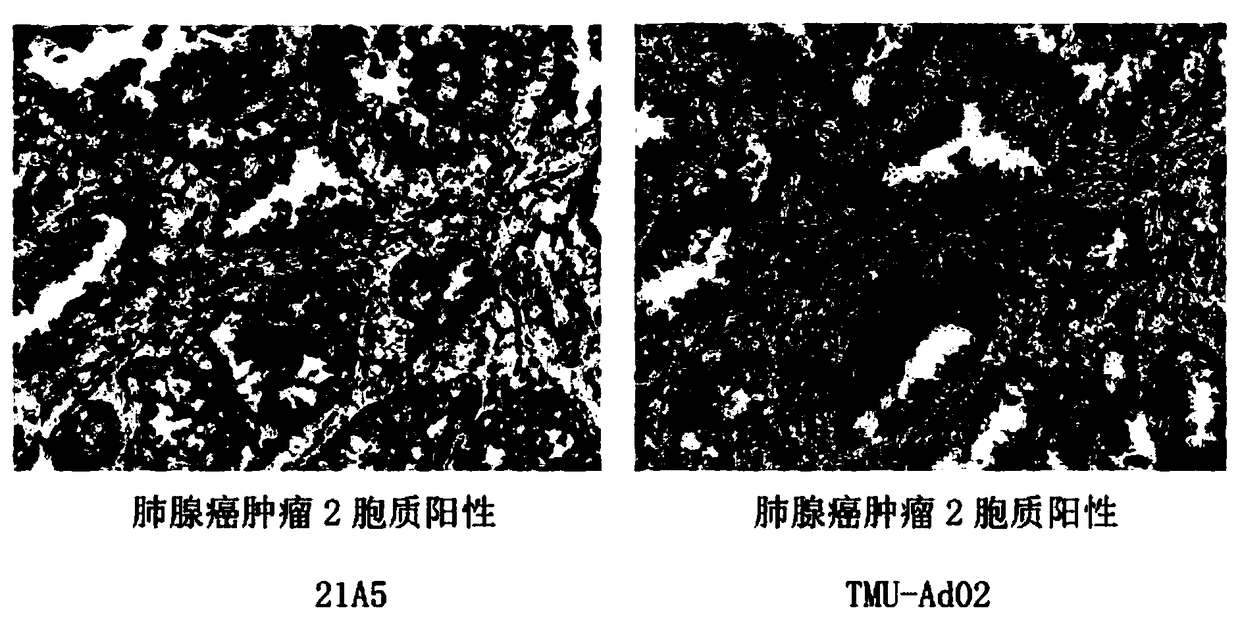
The invention relates to a novel monoclonal antibody of pepsin-like aspartic protease (Napsin A) and a preparation method of the hybridoma cell strain 21A5, and the monoclonal antibody is used for tumor tumorigenesis and prognosis evaluation. The antigen for preparing the antibody is a preferred antigen peptide, and after being chemically synthesized, the antigen is coupled with the KLH carrier protein into an immunogen to immunize mice, and finally the obtained antibody belongs to the Ig2b subtype, and the sequence of the variable region of the antibody is obtained through gene cloning. Basedon the immunohistochemical detection of a plurality of tumor tissues, the finally obtained antibody can be used for detection of tumorigenesis, and can be used for immunological diagnosis of these tumors.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
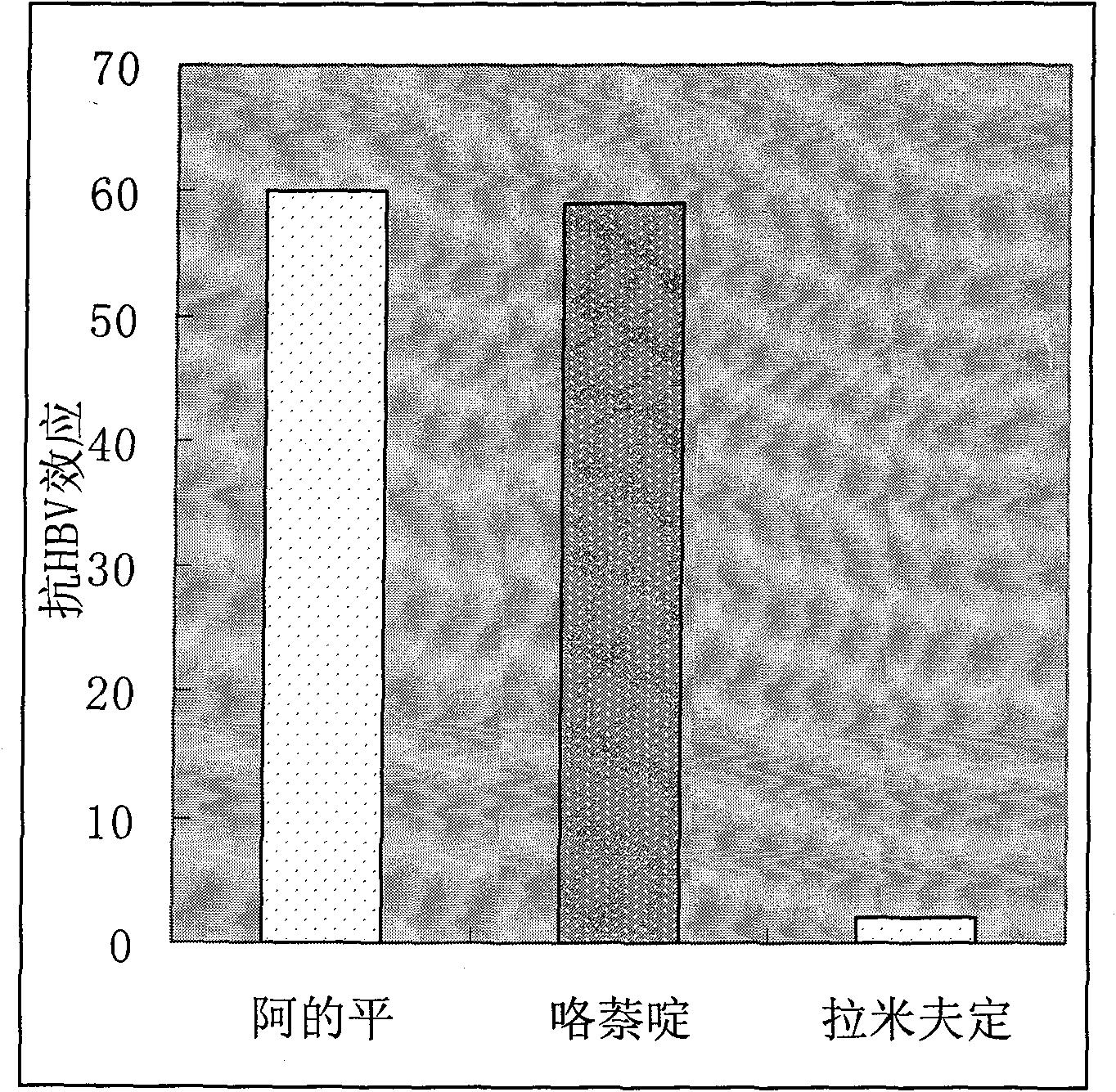
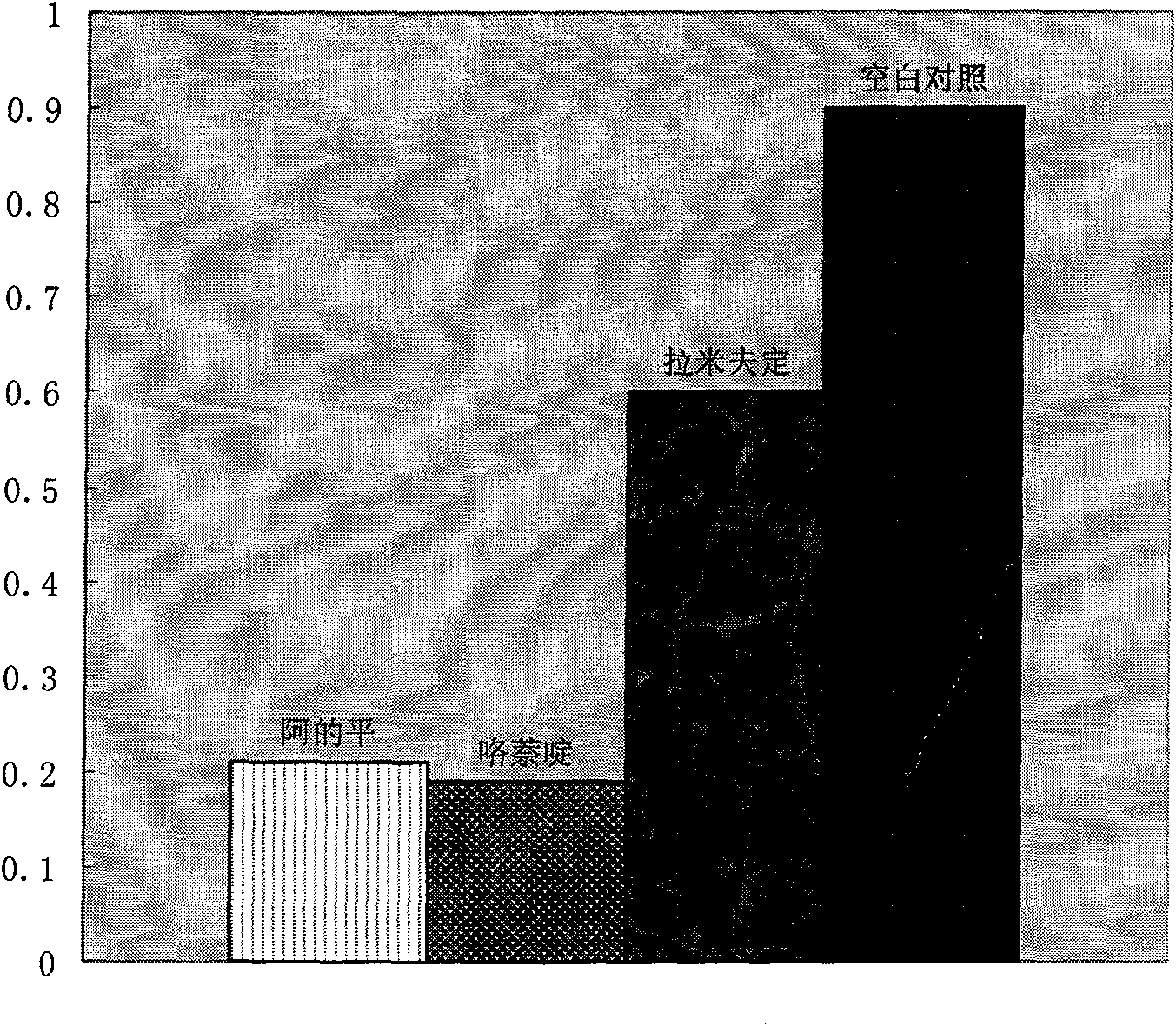
Therapeutical effect of atabrine and substitutes thereof on hepatitis B
InactiveCN101856355ALower titerInhibition killOrganic active ingredientsAntiviralsAntigenNorthern blot
Owner:蔡荣 +3
Application of FLT3LG protein to preparation of lung adenocarcinoma postoperation and prognosis evaluation reagents or kits
ActiveCN110836974AShort survival timeEarly relapseBiological material analysisBiological testingDiseaseOncology
The invention belongs to the technical field of biomedicine, and particularly discloses an application of an FLT3LG protein to preparation of lung adenocarcinoma postoperation and prognosis evaluationreagents or kits. According to the application, the FLT3LG protein is taken as a marker to detect lung adenocarcinoma tissue specimens through an immunohistochemical method and judge the prognosis ofthe lung adenocarcinoma patients; the FLT3LG protein has high expression in the lung adenocarcinoma and has low expression in para-carcinoma tissues; the expression level of the FLT3LG protein in thelung adenocarcinoma is related to the survival of the patients; and the high-expression patients are bad in prognosis and short in survival. The expression level of the FLT3LG protein is not only related to the total survival of the lung adenocarcinoma patients, but also related to the disease-free survival of the patients, and the patients, the FLT3LG protein of which has high expression, have recurrence or metastasis earlier; and serving as a lung adenocarcinoma postoperation and prognosis marker, the FLT3LG protein has a wide application prospect and a relatively large social benefit.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
Method for selecting patients for treatment with an EGFR inhibitor
The present invention relates to cancer diagnostics and therapies and to the detection of alterations in cancer cells that are diagnostic, prognostic or predictive. In particular, the present invention provides a method for detecting and analyzing whether a patient suffering from a cancer is responsive to the treatment with an EGFR inhibitor. In the method, a tissue section from a cancer sample is subjected to assays based on immunohistochemistry and enzymatic metallography.
Owner:CARPEN OLLI +5
Preparation method of polyclonal antibody capable of marking fish germline stem cells
ActiveCN113173988AFill in the application gapSerum immunoglobulinsImmunoglobulins against animals/humansBiotechnologyIMMUNE FLUORESCENCE
The invention relates to the field of molecular biology, in particular to a preparation method of a polyclonal antibody capable of marking fish germline stem cells. The antibody is prepared from zebra fish Nanos2 protein as an immunogen immune animal, the prepared antibody can specifically recognize the specific molecular marker protein Nanos2 of germline stem cells, can be used for immunohistochemistry and immunofluorescence detection, specifically marks and detects germline stem cells in zebra fish testis and ovaries, and fills the blank of the application of detecting the fish germline stem cells by using the antibody specificity.
Owner:INST OF AQUATIC LIFE ACAD SINICA
Folding dyeing frame, horizontal incubation box and using method
InactiveCN111521470AImprove processing efficiencyQuick switchPreparing sample for investigationFlat carrier supportsStainingStructural engineering
The invention provides a folding dyeing frame, a horizontal incubation box and a using method. The folding dyeing frame comprises a plurality of clamping plates, wherein the plurality of clamping plates are folded in a zigzag shape, a foldable first connecting piece is arranged between every two adjacent clamping plates, empty grooves used for containing slides are formed in the clamping plates, transverse ribs used for supporting the slides are arranged at the rear ends of the empty grooves at intervals, blocking piece sets used for clamping the slides are arranged at the front ends and the rear ends of the empty grooves, and each blocking piece set comprises two baffles which are located on the two sides of the lower end of the corresponding empty groove respectively. According to the invention, the rapid switching of horizontal incubation and vertical repair washing processes in the immunohistochemistry experiment process is realized, the time is saved, the operation is convenient,and the sample treatment efficiency is improved.
Owner:河南卓旭企业管理咨询有限公司
Application of procyanidin SA hydrogel in preparation of medicine for treating tendon ectopic calcification
InactiveCN110946855ANo acuteNo subacute toxicityOrganic active ingredientsAerosol deliveryDiseaseEosin
The invention discloses an application of procyanidin in regulation and control of stem cell functions in a pathological microenvironment of tendon diseases, and provides a preparation method of procyanidin SA hydrogel and an application of the procyanidin SA hydrogel in treatment of tendon ectopic calcification. Based on the application, the invention proves that: the addition of the procyanidininto tendon stem / progenitor cells does not influence cell proliferation, and can effectively inhibit the calcium deposition of the tendon stem / progenitor cells and the subsequent potential for treating tendon diseases. Through Micro CT, hematoxylin-eosin staining, crocus sativus-solid green staining, collagen II and osteocalcin (OCN) immunohistochemical staining, the situation of the procyanidin SA hydrogel for treating tendon ectopic calcification is detected. The invention finds and proves that the procyanidin SA hydrogel can effectively inhibit tendon ectopic calcification. Meanwhile, the administration route of sustained-release administration can reduce corresponding side effects generated by systemic administration while improving the local drug concentration, and has a clinical application prospect.
Owner:ZHEJIANG UNIV
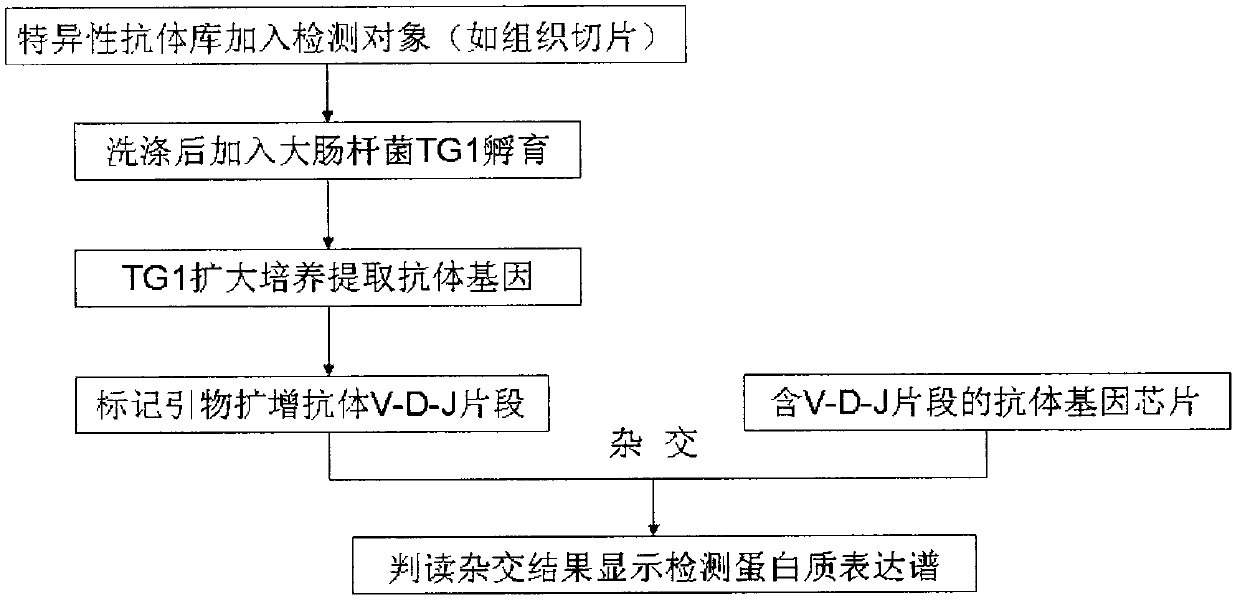
Development and application of antibody gene chip
InactiveCN103376321AEnable Personalized TreatmentSolve the problem of in situ detection of large amounts of protein expressionMicrobiological testing/measurementMaterial analysisTumor-Related ProteinDisease
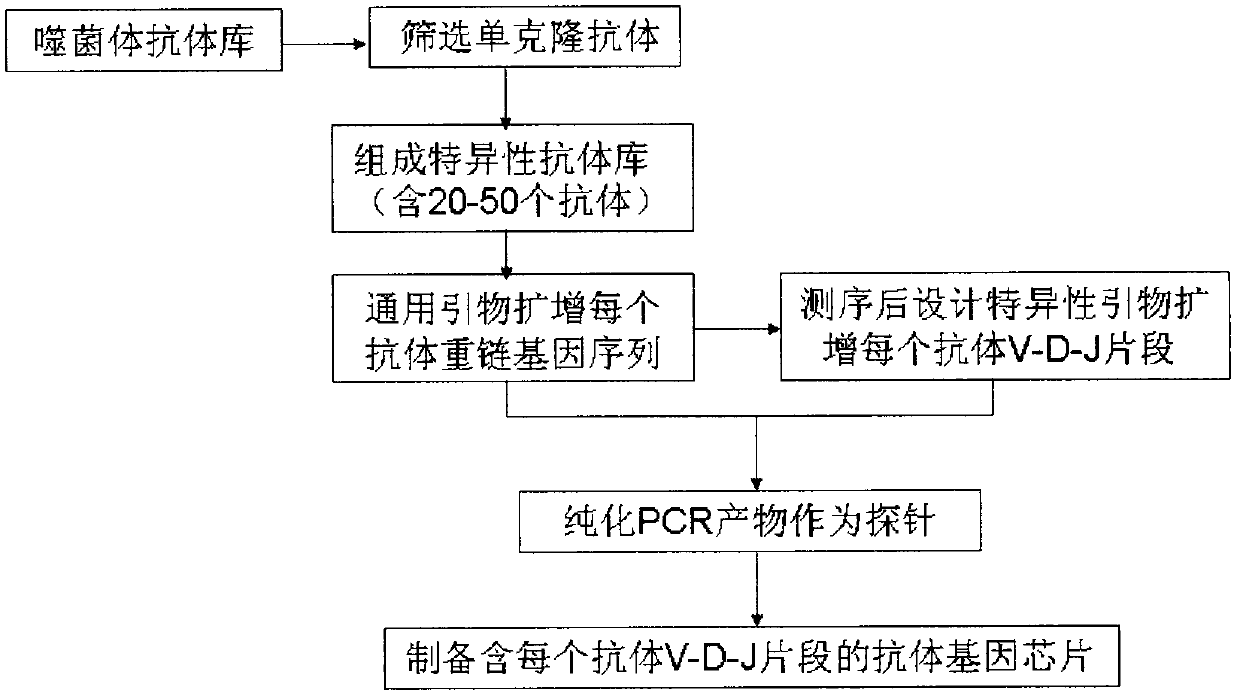
The invention relates to a technical invention which can be used for detecting a protein expression profile of a tissue slice. According to the technical invention, after a plurality of different antibodies are simultaneously bonded with a plurality of proteins to be detected of a tumor tissue, an identification V-D-J sequence subjected to PCR (Polymerase Chain Reaction) amplification is hybridized with an antibody gene chip, and the protein expression profile of the tumor tissue is displayed through a hybridization result; and certain protein in-situ expression statuses are selectively displayed through immunohistochemistry. The technical invention can be used for clinically detecting individual tumor related protein expression profiles of patients, solving the problem that a large number of protein expressions are subjected to in-situ detection for tissue slices, and directly applying a proteome research result to a clinical diagnosis process, thereby being an important premise for realizing individual treatment of diseases.
Owner:朱有凯
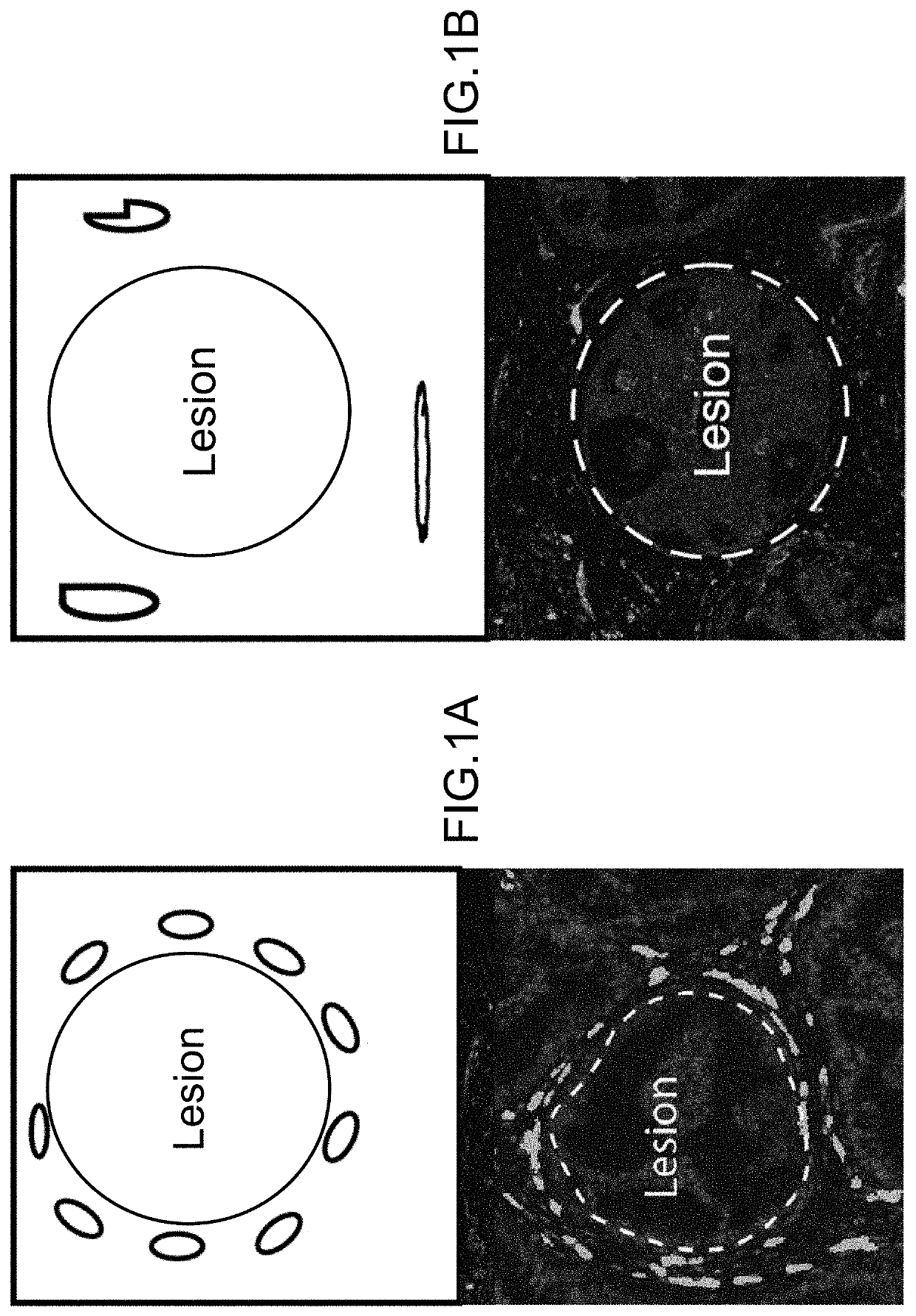
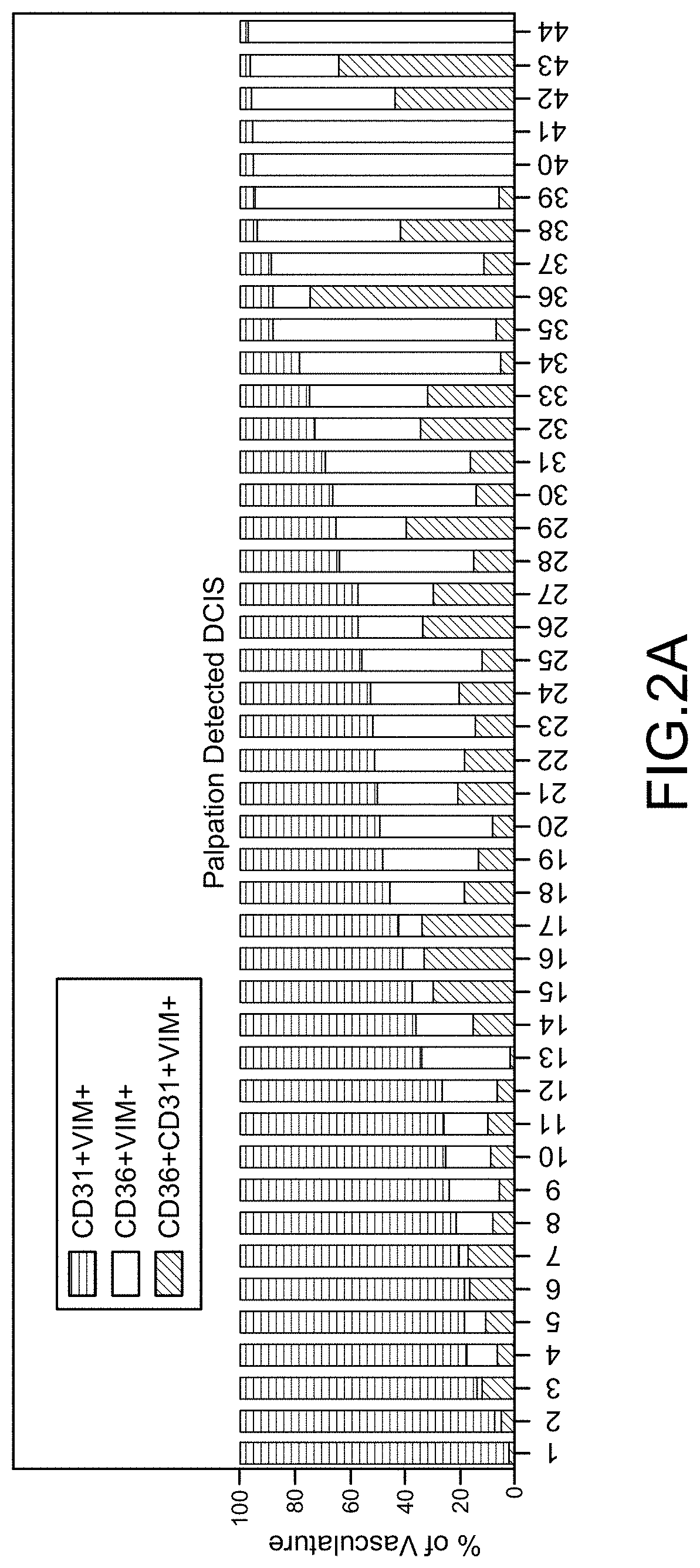
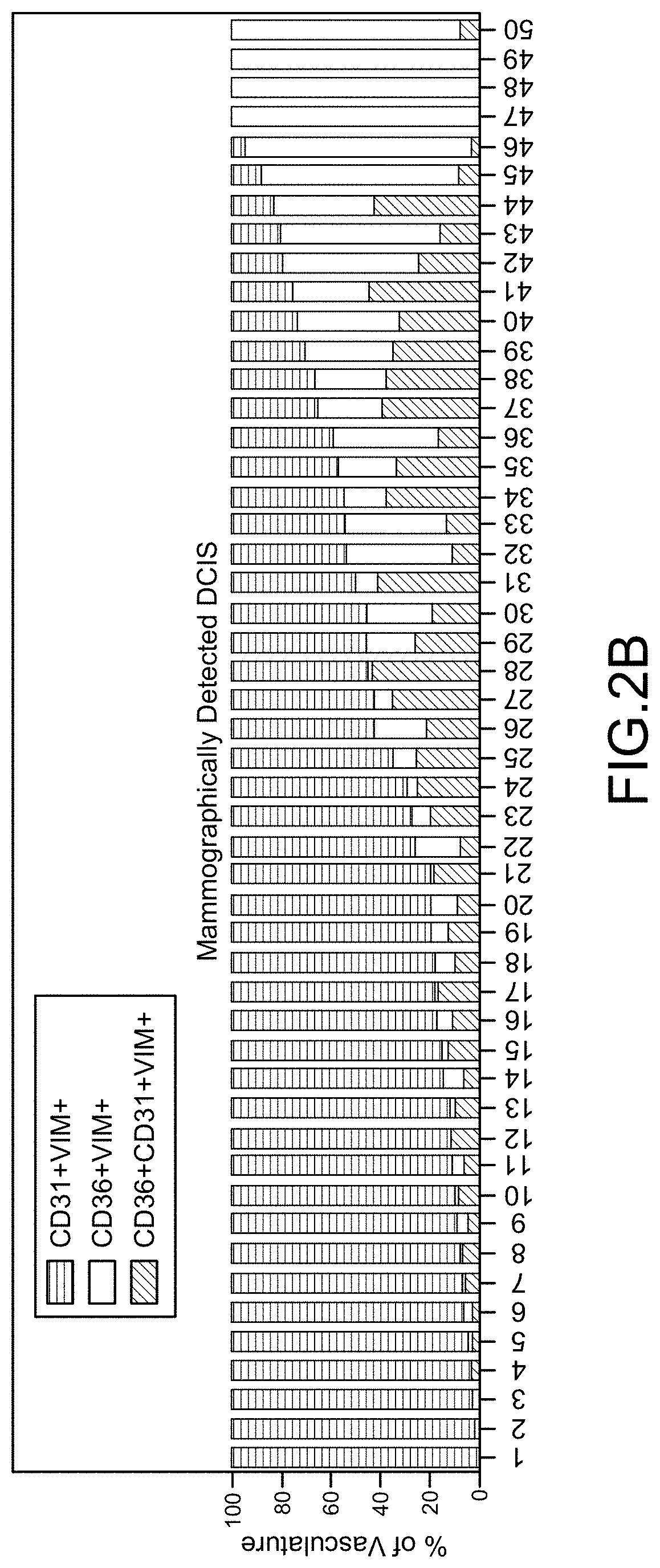
Cancer Progression Risk Assessment by Microvascular Phenotype
PendingUS20220107321A1Increased riskReduce riskDisease diagnosisBiological testingInfiltrating cancerCancer type
Repression of CD36 vasculature in stromal tissues surrounding a pre-cancerous lesion provides is indicative of increased risk of subsequent progression to invasive cancer. Methods of assessing progression risk by measuring the abundance of CD36 vasculature in stromal tissues surrounding a precancerous lesion are provided, useful for various cancer types, including for assessing the risk of DCIS progression to breast cancer. Immunohistochemical analysis methods and diagnostic thresholds are provided, as well as associated methods of treatment and diagnostic kits.
Owner:RGT UNIV OF CALIFORNIA
Descriptive measurements and quantification of staining artifacts for in situ hybridization
ActiveUS10614284B2Analysis is limitedAvoid inclusionsPreparing sample for investigationAcquiring/recognising microscopic objectsHistocytochemistryBiology
Immunohistochemistry (IHC) and in situ hybridization (ISH) have the aim of detecting, localizing and quantifying certain analytes for various diagnostic purposes. The quality of the stains which are analyzed may deviate for various reasons. Therefore, the present invention provides a method and system for assessing the stain quality and for establishing objective criteria for assessing the stain quality for application in the fields of in-situ hybridization and immunohistochemistry. In one possible embodiment, the invention comprises the steps of unmixing multi-spectral image data of a tissue specimen to obtain analyte intensity images, each analyte intensity image comprising signals from a single stain, computing metrics based on the analyte intensity images, wherein the metrics are uniformity, distribution and / or dispersion of pixel intensity values in the analyte intensity images and assessing a stain quality of a slide by comparing the computed metrics to pre-determined cutoff values regarding uniformity, distribution and / or dispersion of pixel intensity, wherein the stain quality of the slide is assessed as acceptable if the computed metrics meet or exceed the pre-determined cutoff values, and wherein the stain quality of the slide is assessed as unacceptable if the computed metrics do not meet the pre-determined cutoff values. In order to establish objective criteria for assessing stain quality, in one possible embodiment, the method and system includes the step of deriving cut-off values for uniformity, distribution and / or dispersion of pixel intensity by combining the computed metrics based on the analyte intensity images with pre-established data quantifying the stain quality.
Owner:VENTANA MEDICAL SYST INC
Anti-napsin A protein monoclonal antibody and its cell line, preparation method and application
ActiveCN108467433BStrong characteristicIncreased sensitivityMicroorganism based processesTissue cultureChemical synthesisAntiendomysial antibodies
The invention relates to a new pepsin-like aspartic acid protease (Napsin A) monoclonal antibody, a preparation method of the hybridoma cell line 21A5, and the application of the monoclonal antibody in tumor occurrence and prognosis evaluation. The antigen for preparing the antibody is a preferred antigenic peptide, which is chemically synthesized and coupled with KLH carrier protein as an immunogen to immunize mice. The finally obtained antibody belongs to IgG2b subtype, and the sequence encoding its variable region is cloned by gene way to get. The immunohistochemical detection of the finally obtained antibody on a variety of tumor tissues found that the antibody can well identify the occurrence of tumors and can be used for the immunological diagnosis of these tumors.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
Pathological immunohistochemistry wet box
PendingCN111505270AImprove efficiencyQuality improvementBiological testingAnatomyStructural engineering
The invention provides a pathological immunohistochemistry wet box. The box comprises a box body, wherein the box body is hollow and is provided with an upward opening; a plurality of connecting grooves are formed in the front side wall of the box body; the plurality of connecting grooves are arranged at equal intervals in the left-right direction; the connecting groove is provided with an upwardopening; a plurality of supporting parts are arranged on the inner side of the rear side wall of the box body; the supporting part is arranged corresponding to the connecting groove. The box also comprises a slide, wherein the front end of the slide is connected to the supporting part; the rear end of the slide penetrates through the connecting groove and extends to the outer side of the box body;a pressing block is further arranged on the connecting groove; the pressing block is matched with the connecting groove; protruding sliding blocks are arranged on the left side and the right side ofthe pressing block respectively. Sliding grooves corresponding to the sliding blocks are formed in the left side wall and the right side wall of the connecting groove respectively, the sliding blocksare in sliding fit with the sliding grooves in the vertical direction, the cover body covers an opening in the upper end of the box body and is provided with a plurality of sets of liquid adding holes, each set of liquid adding holes corresponds to one slide, and a liquid outlet communicated with the interior of the box body is formed in the bottom of the box body.
Owner:江西省肿瘤医院
Automated tumour-stroma interface zone detection for Anti-tumour response assessment by immunogradient indicators
PendingUS20220138955A1Extraction of informationImprove statistics performanceImage enhancementImage analysisDiseaseAnalysis data
We present a new method to automatically sample the tumour / stroma interface zone (IZ) from microscopy image analysis data. It first delineates the tumour edge using a set of explicit rules in grid-subsampled tissue areas; then the IZ of controlled width is sampled and ranked by the distance from the edge to compute TIL density profiles across the IZ. From this data, a set of novel Immunogradient indicators are computed to reflect TIL “gravitation” towards the tumour. We applied the method on CD8 immunohistochemistry images of surgically excised breast and colorectal cancers to predict overall patient survival. In both patient cohorts, we found strong and independent prognostic value of the Immunogradient indicators, outperforming methods currently available. We conclude that data-driven, automated, human operator-independent IZ sampling enables precise spatial immune response measurement in the tumour / host interaction frontline for prediction of disease and therapy outcomes.
Owner:VILNIUS UNIV
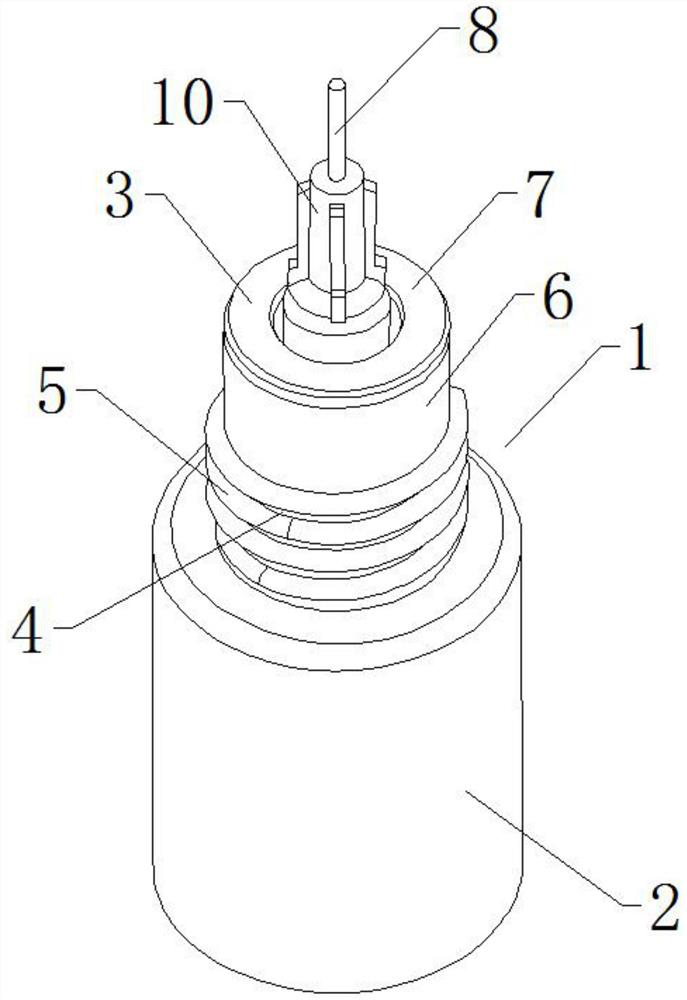
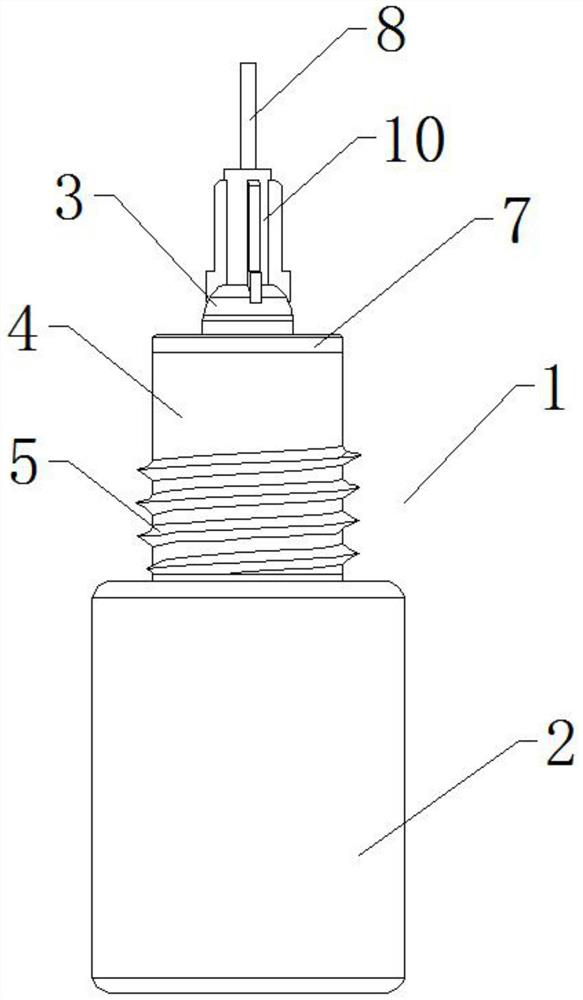
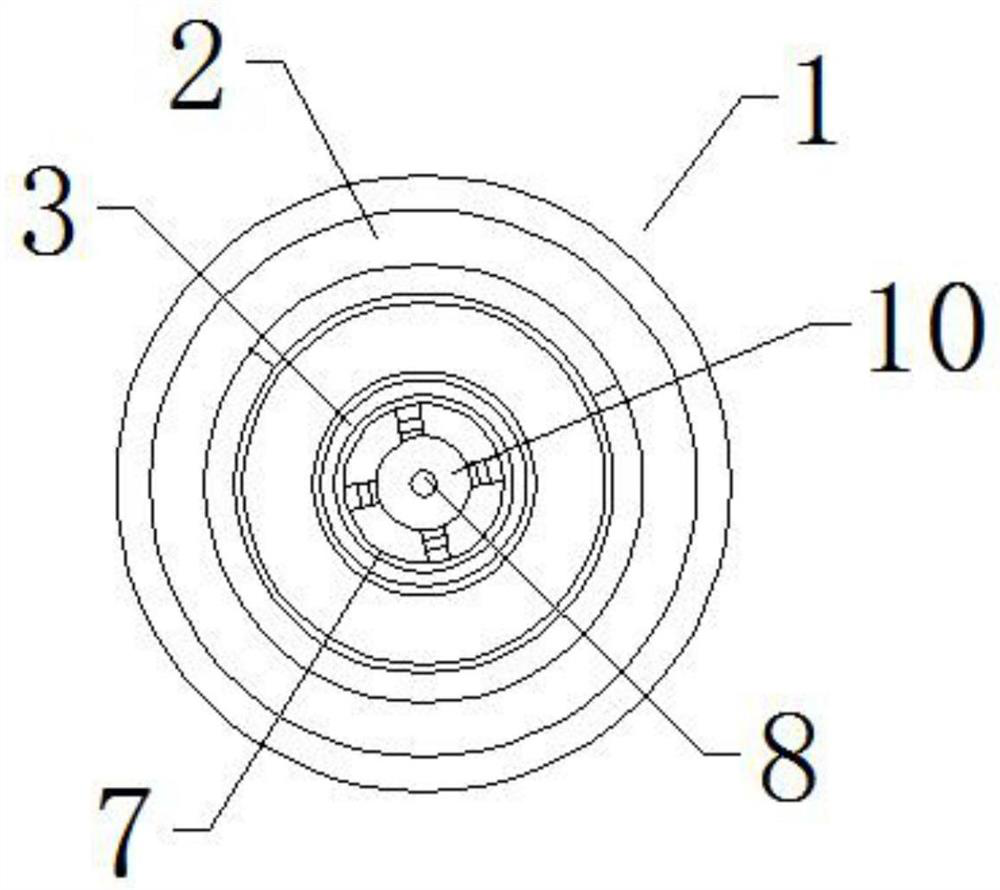
Reagent bottle capable of controlling dropping liquid quality
InactiveCN112536073ALow costEliminate cleaningReagent containersPreparing sample for investigationStainingImmunohis tochemistry
The invention relates to the technical field of immunohistochemical staining, in particular to a reagent bottle capable of controlling dropping liquid quality, which comprises a reagent bottle body, the reagent bottle body comprises a reagent bottle lower bottle body and a reagent bottle upper bottle body, the lower side of the reagent bottle body is provided with the reagent bottle lower bottle body, and the upper side of the reagent bottle lower bottle body is provided with a reagent bottle opening; the outer wall of the reagent bottle opening is provided with a connecting external thread, the upper side of the reagent bottle opening is provided with a bottle opening mounting end, the upper side of the reagent bottle body is provided with the reagent bottle upper body, and the reagent bottle upper bottle body comprises a needle head connecting seat and a needle head body; and the needle head connecting base is arranged at the position, corresponding to a bottle opening installation end, of the lower side of the reagent bottle upper bottle body, and a limiting installation plug is installed at the bottom of the needle head connecting base. On the whole, the device can greatly reduce the implementation complexity and cost, completely eliminate cleaning and switching operation during switching of different kinds of liquid and improve the working efficiency. Operation time is greatly shortened.
Owner:INNOVEL INTELLIGENT TECH CO LTD
cancer treatment
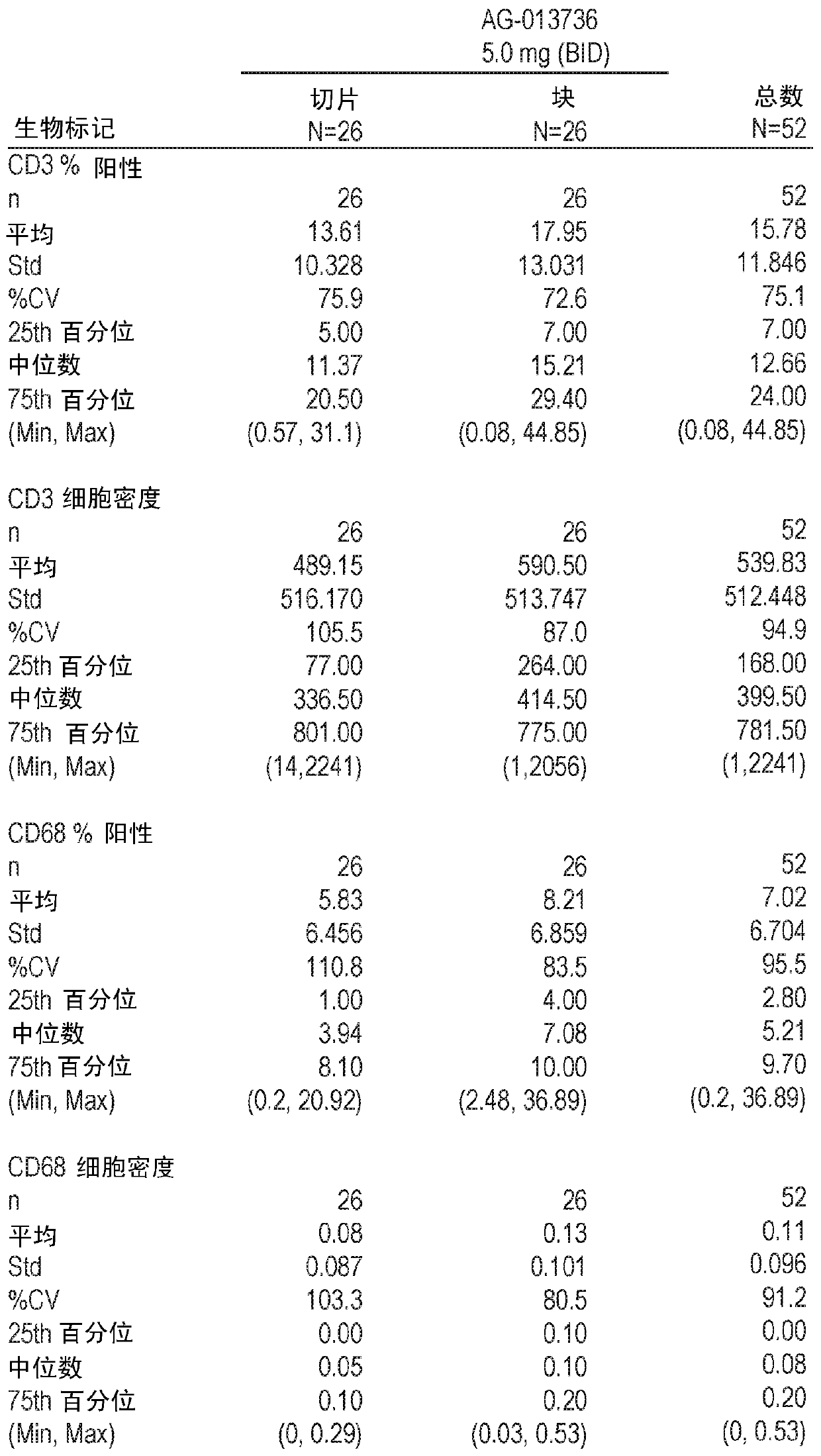
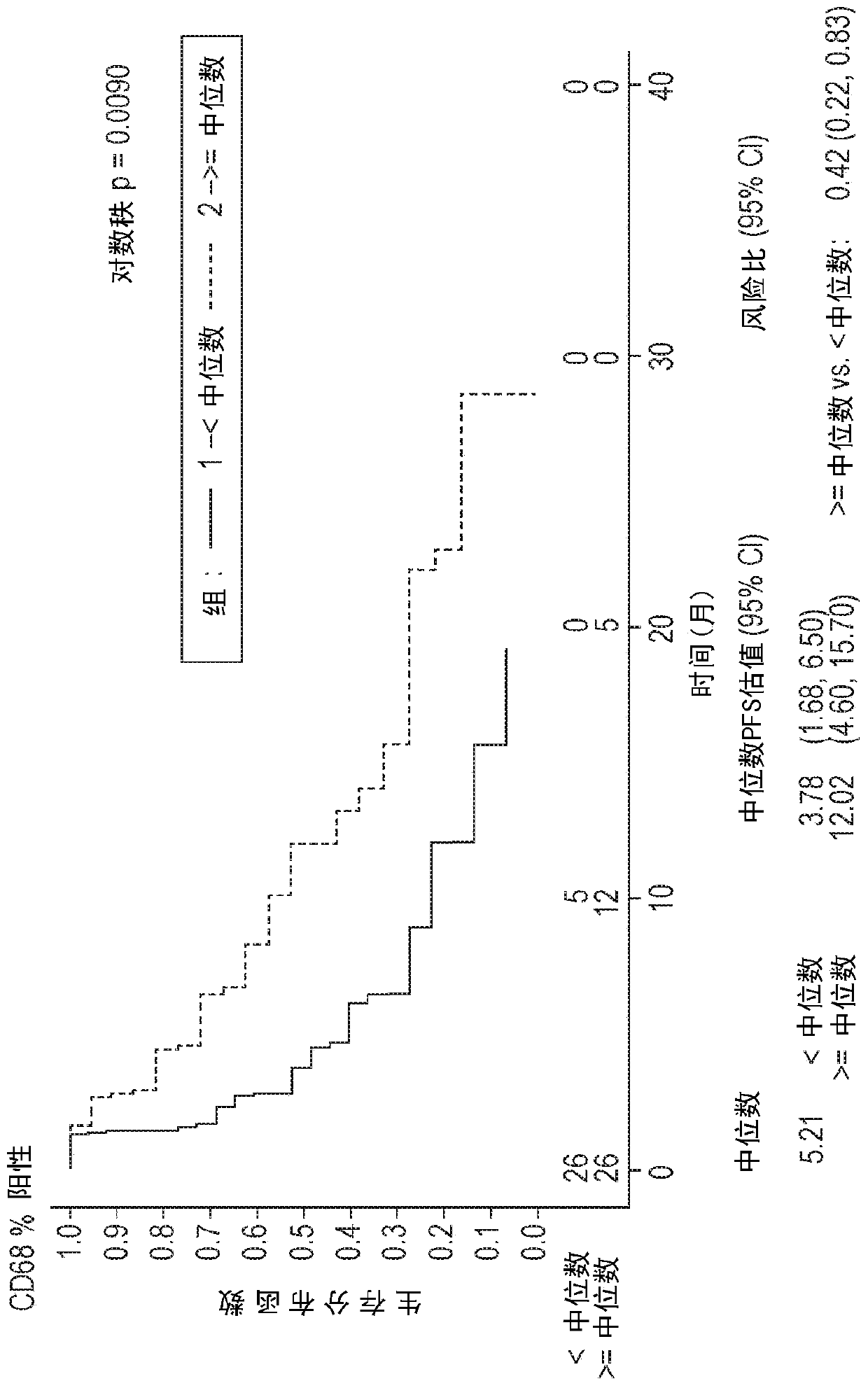
The invention discloses a diagnostic method for predicting whether human tumors are sensitive to axitinib treatment and a method for treating human tumors. These methods are based on measuring the expression level of the CD68 polypeptide in a tumor-derived tissue sample. CD68 expression levels can be measured using immunohistochemistry, wherein the percentage of CD68 positive cells in the tumor and the density of CD68 positive cells can be determined.
Owner:PFIZER INC
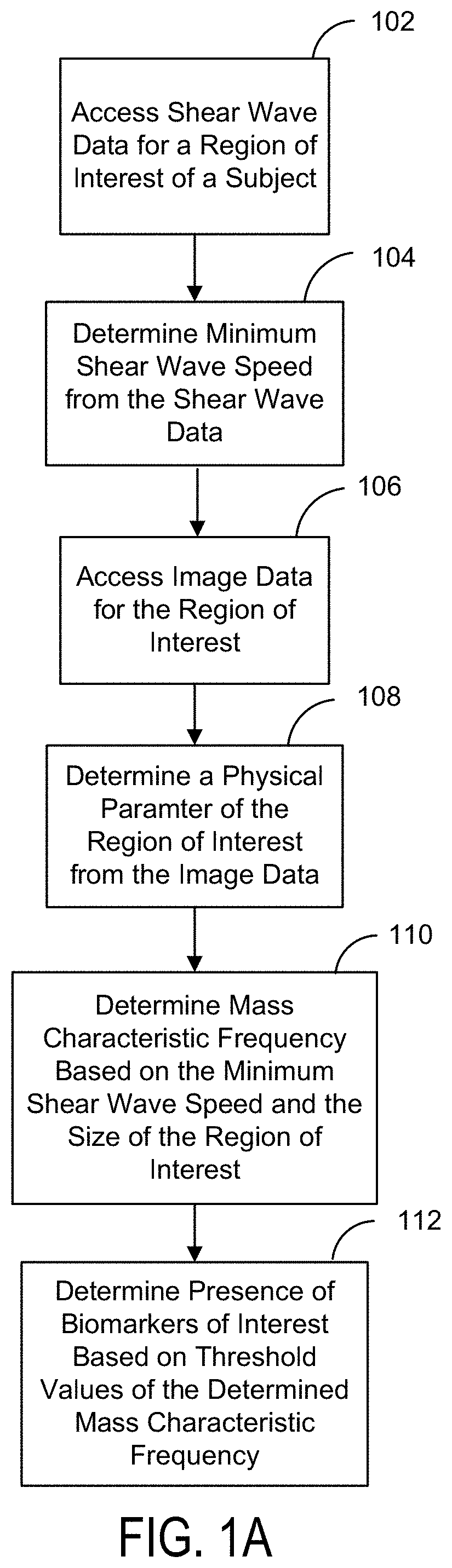
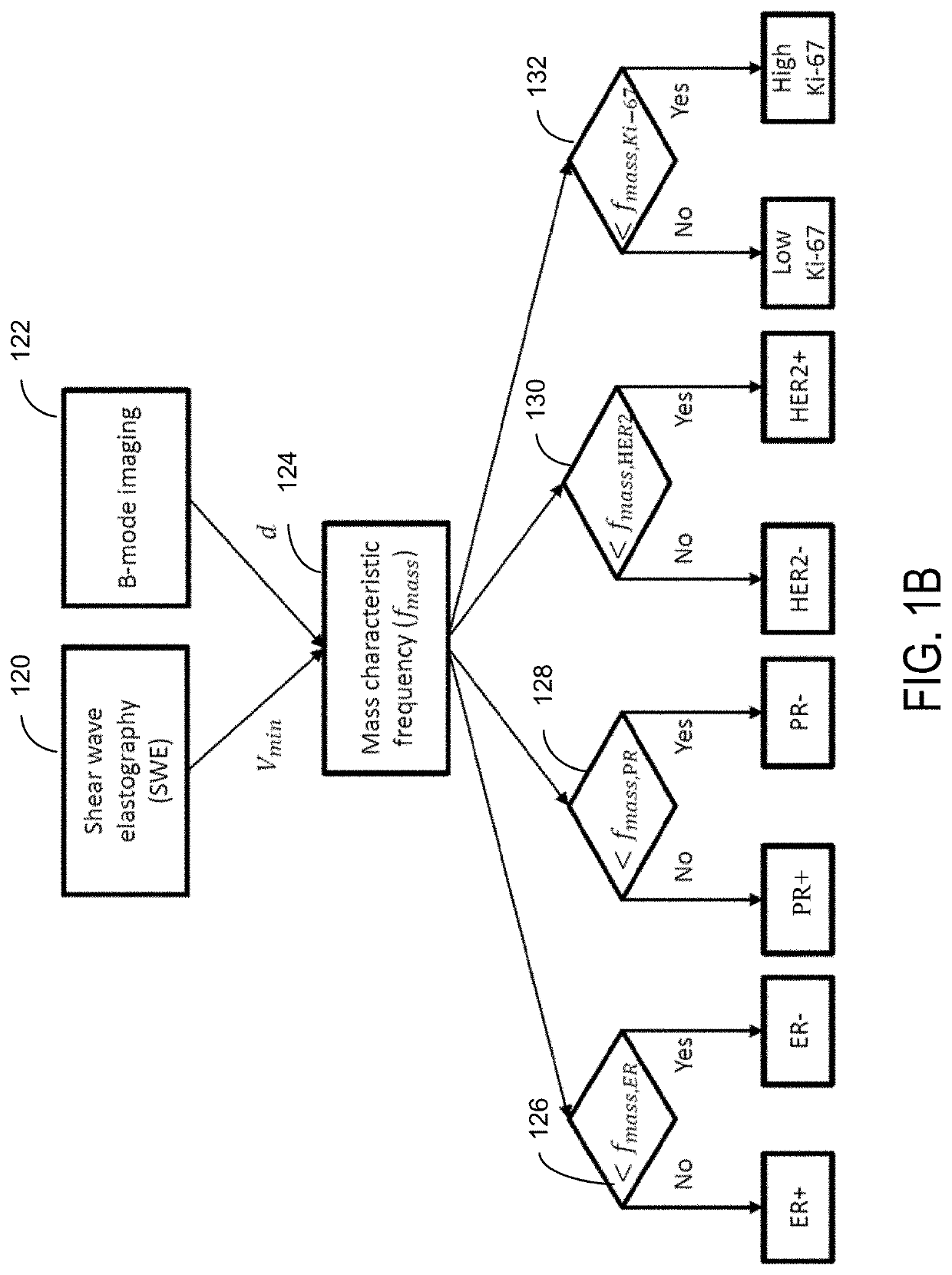
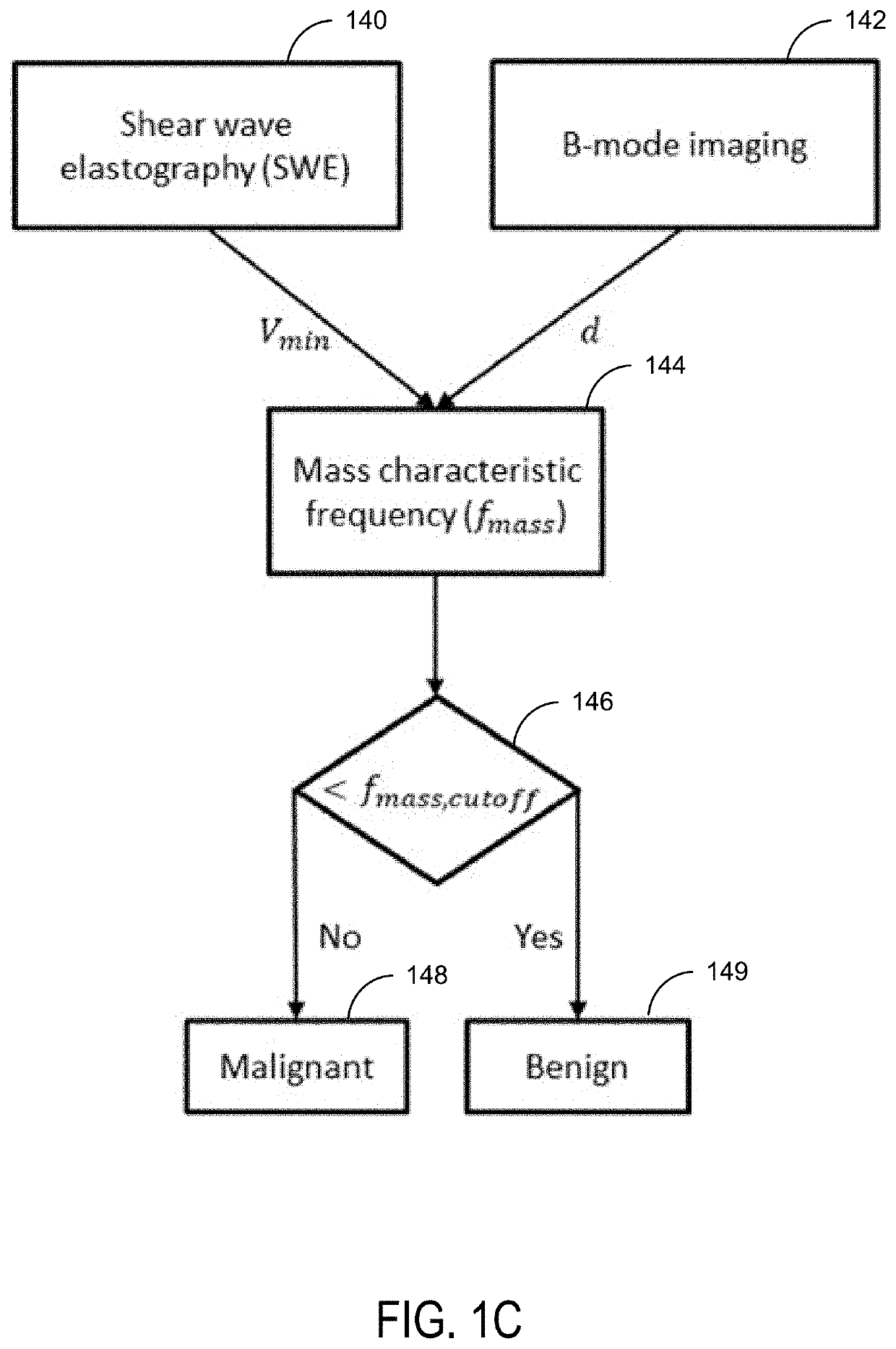
Ultrasound Systems and Methods Using Mass Characteristic Frequency
PendingUS20220142613A1Organ movement/changes detectionInfrasonic diagnosticsBreast cancer classificationMalignancy
Ultrasound systems and methods are provided using mass characterization frequency methods that provide for predicting benign or malignant lesions, a response to treatment, tumor grading, and / or the expressions of immunohistochemical biomarkers, which are currently used for breast cancer classification and hormone therapy determination. The systems and methods are based on the shear wave parameter, mass characteristic frequency. The status of malignancy, treatment response, grade, and / or each immunohistochemical biomarker may be determined based on a corresponding mass characteristic frequency threshold.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
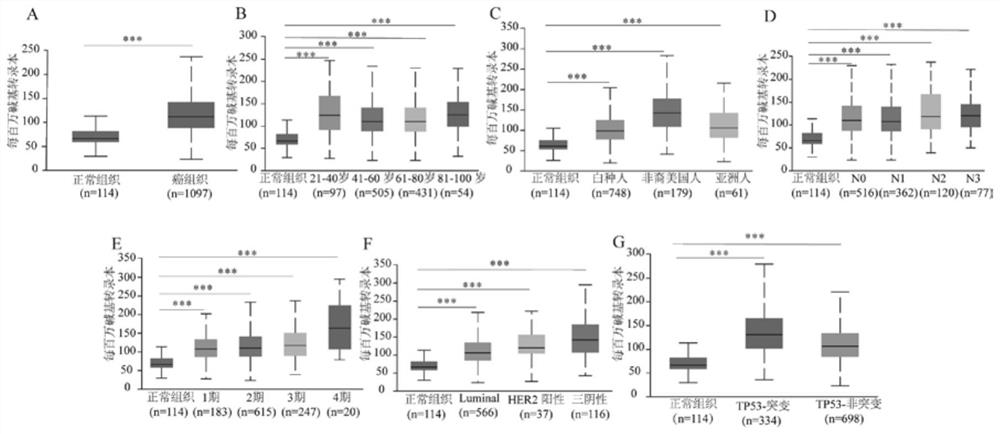
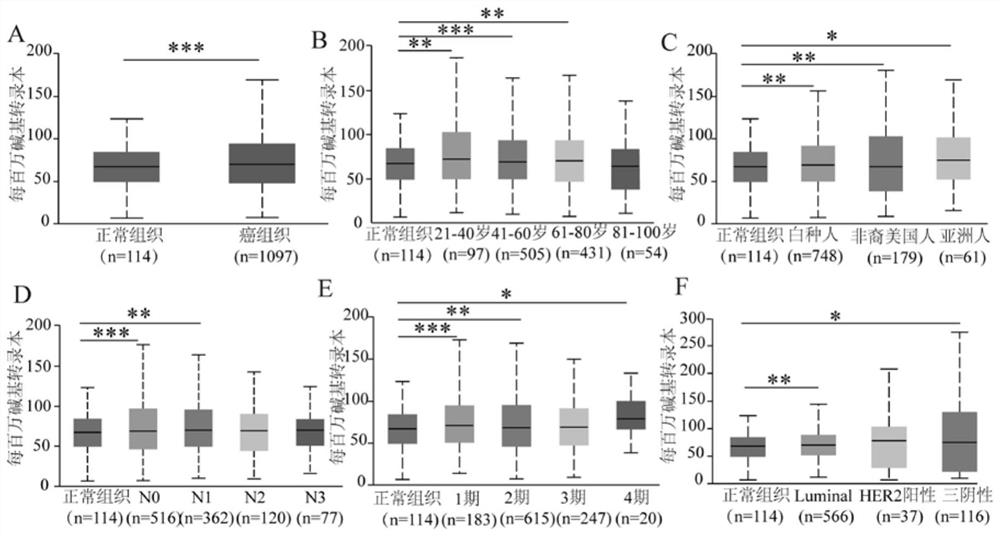
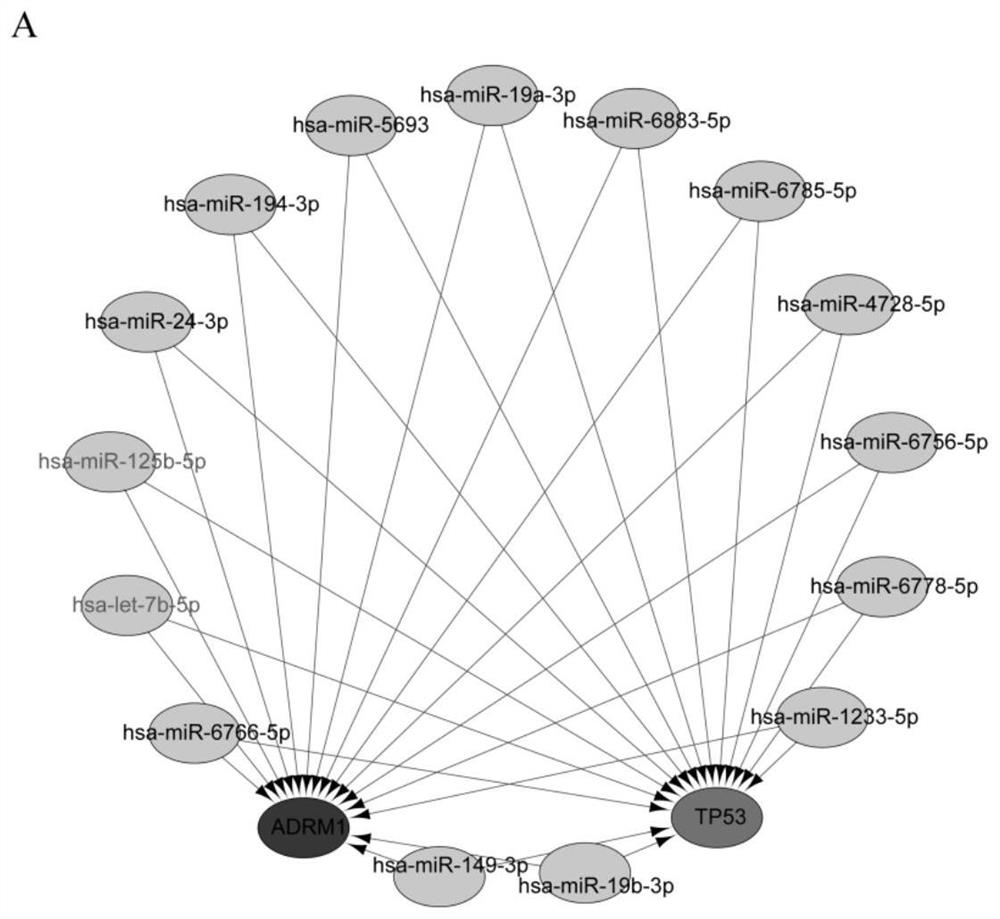
Breast cancer diagnosis marker and application thereof
PendingCN114034866AImprove accuracyBiological material analysisDatabase analysisImmunohis tochemistry
The invention discloses a breast cancer diagnosis marker and application thereof. Expression of ADRM1 protein in breast cancer tissues is detected through an immunohistochemical method, and the result shows that ADRM1 is remarkably up-regulated in the cancer tissues of breast cancer patients and is remarkably related to the tumor size. Meanwhile, through UALCAN database analysis, it is found that the expression level of ADRM1 in TP53 mutant and non-mutant breast cancer is significantly higher than that in normal breast cancer, it is indicated that the expression of ADRM1 is closely related to the expression of TP53 in breast cancer, the expression of ADRM1 and TP53 protein in breast cancer patient tissue can be jointly detected through ADRM1 protein and TP53 protein, and the accuracy rate is high.
Owner:HUZHOU CENT HOSPITAL
A kind of anti-hpv16 E6 protein monoclonal antibody and application thereof
The invention discloses a monoclonal antibody against HPV16 E6 protein. The monoclonal antibody of the present invention is characterized by having an amino acid sequence such as the heavy chain variable region shown in SEQ ID NO: 4, SEQ ID NO: 6 and SEQ ID NO: 8, having an amino acid sequence such as SEQ ID NO: 10, SEQ ID NO: The light chain variable region shown in ID NO: 12 and SEQ ID NO: 14. Western blot experiments showed that the antibody could specifically recognize recombinant HPV16 E6 protein and cervical cancer tissues expressing HPV16 E6 protein. The antibody can be used to detect the expression level of HPV16 E6 protein in cells by immunohistochemical staining (IHC), enzyme-linked immunosorbent assay (ELISA) or western blot (Western Blot) manually or automatically, and can specifically diagnose HPV16 This provides a new method for the detection of HPV16 virus at the protein level, and can be used in the research of human reproductive system infectious diseases and tumors, as well as related malignant tumors such as esophageal cancer, breast cancer, and anal cancer.
Owner:BEIJING SOLARBIO TECH CO LTD
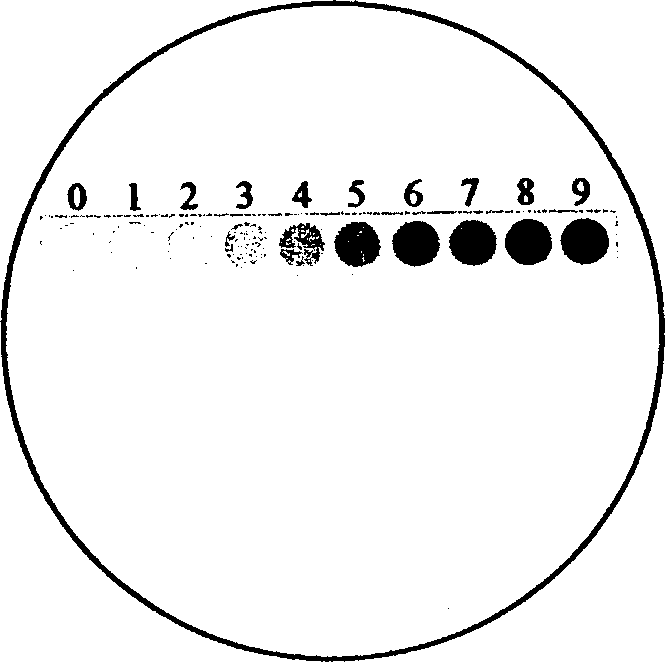
Immune tissue chemistry micro colourimeter and its making method
InactiveCN1138144CEasy to manufactureEasy to placeRadiation pyrometryBiological testingEyepieceEye lens
An immunohistochemical microchromatometer has a transparent sheet as base body, on which there is a transparent chromatic band from light colour (tan) to dark colour (tawny), which are graduted. Said microchromatometer can be put in eye lens of microscopic to provide a colour reference to judge the immunohistochemical stain result. It can be made up by photography, copy and colour print.
Owner:申洪
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com