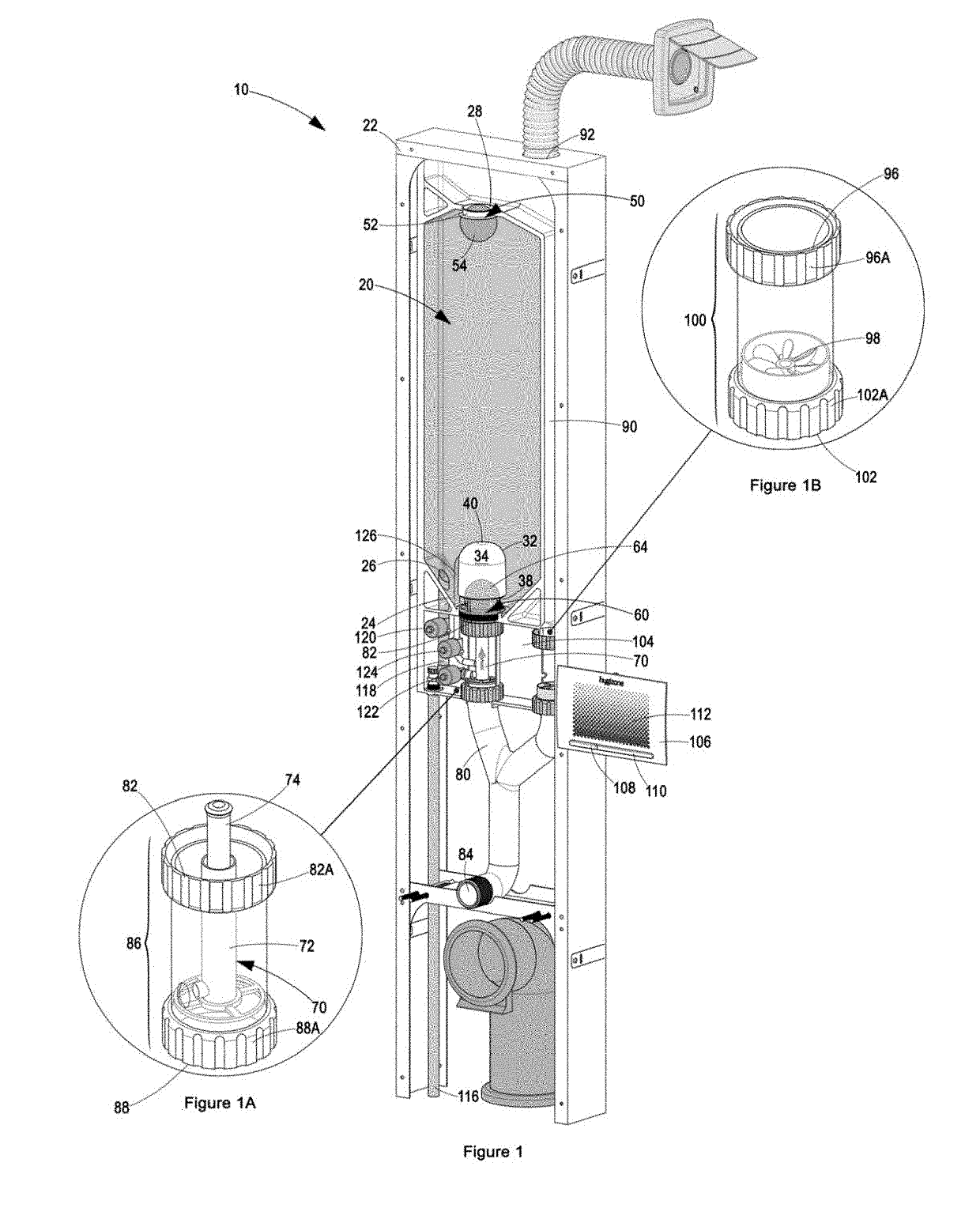
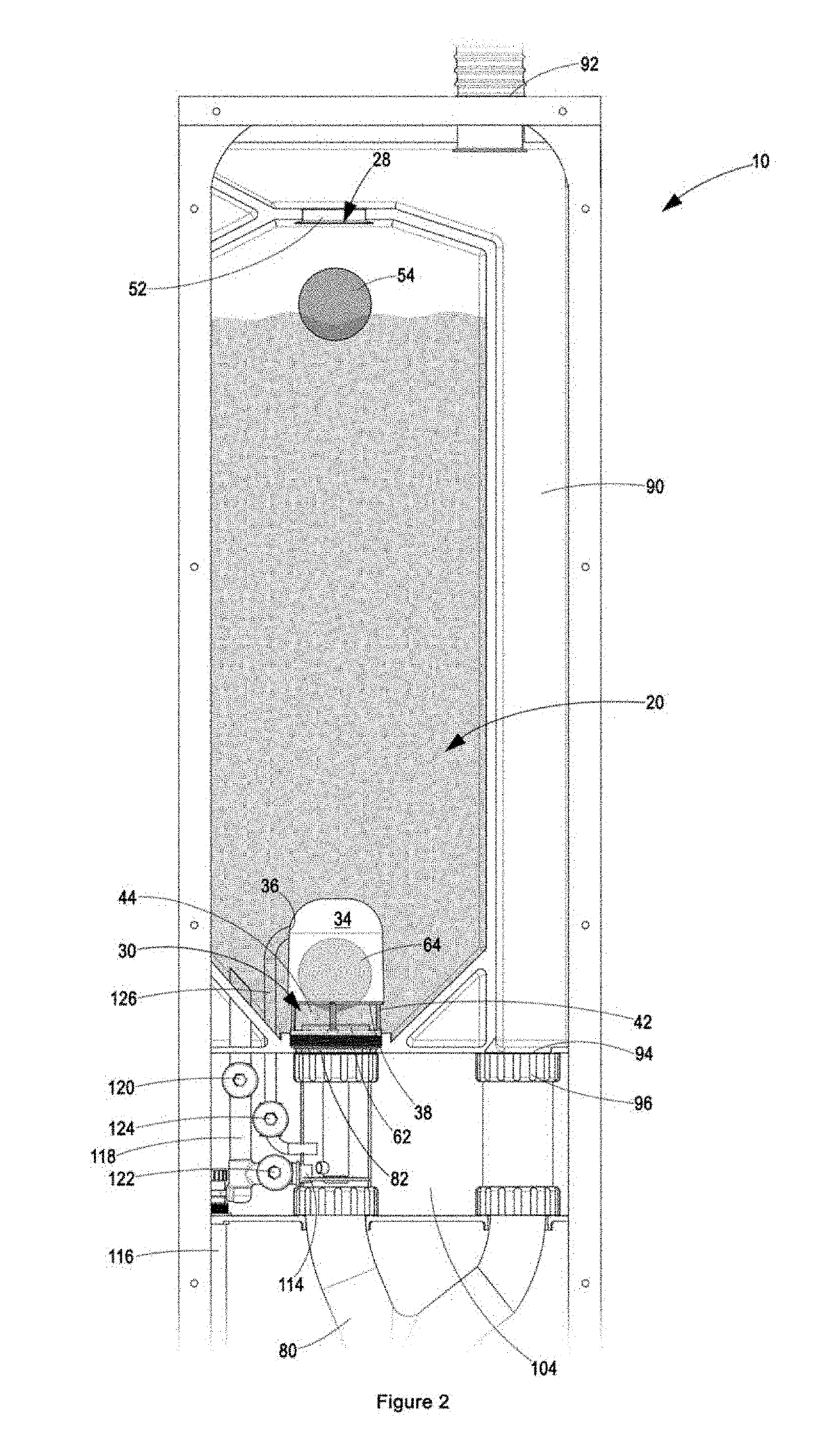
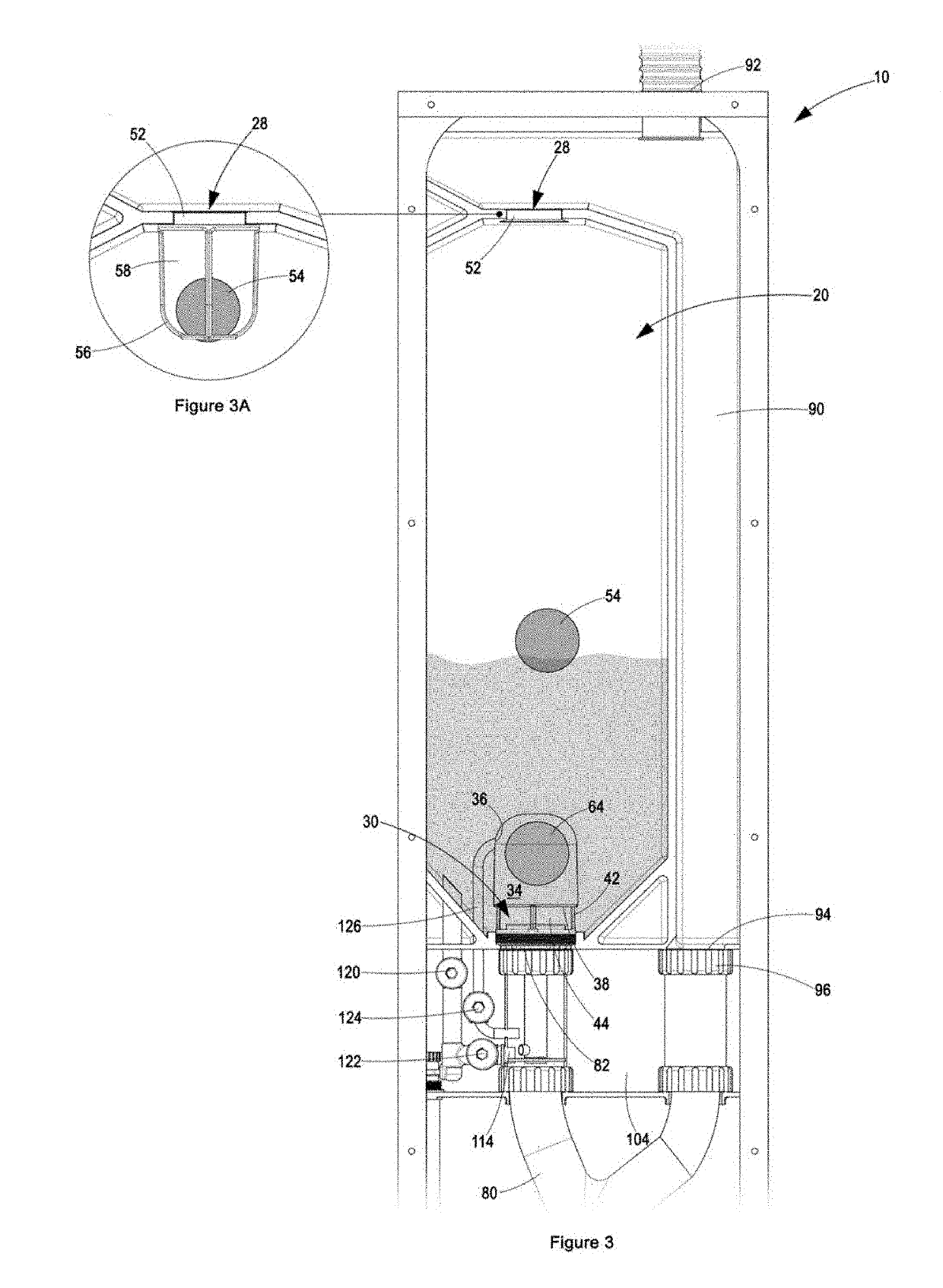
Patents
Literature
30 results about "Saline flush" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
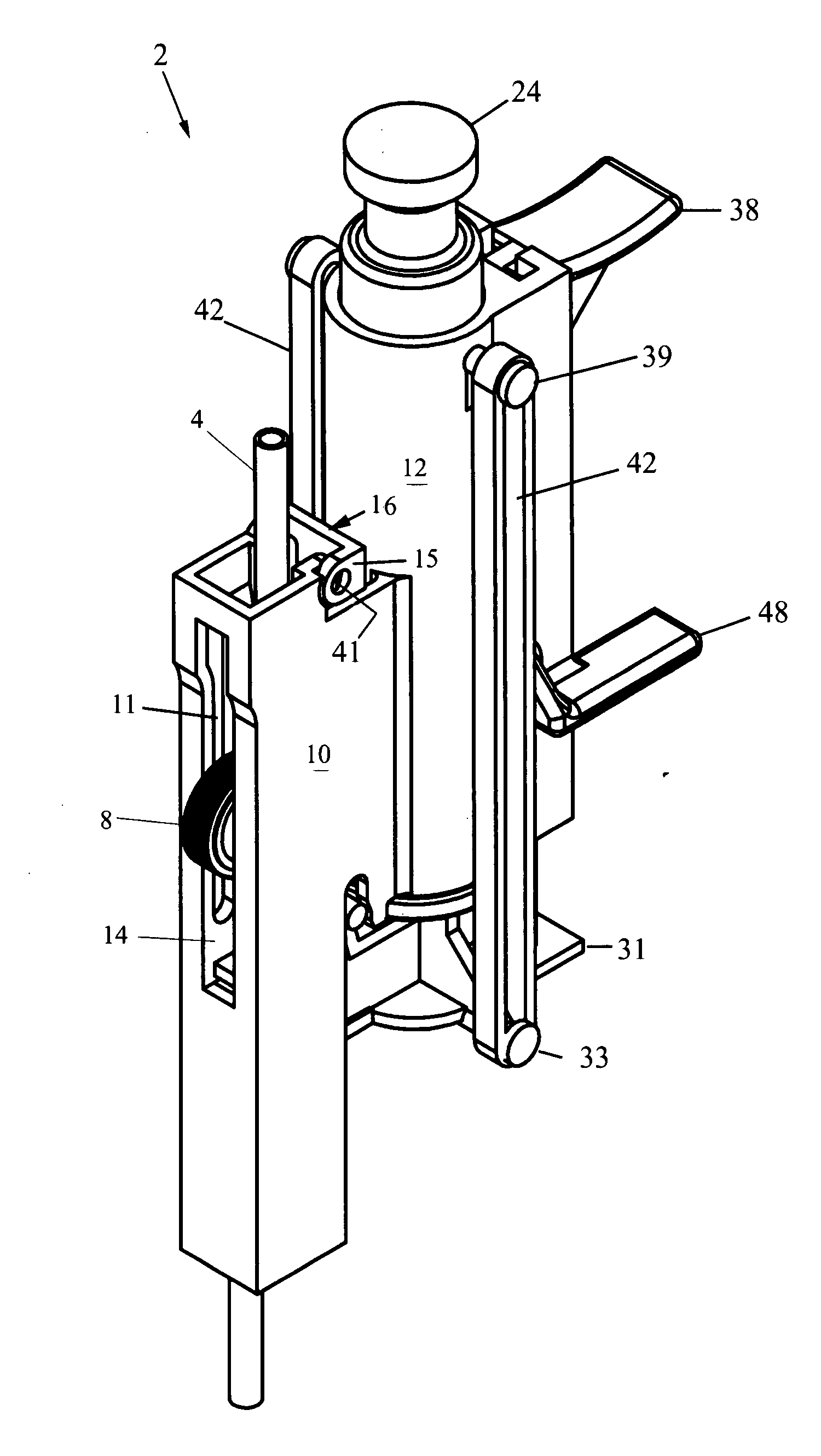
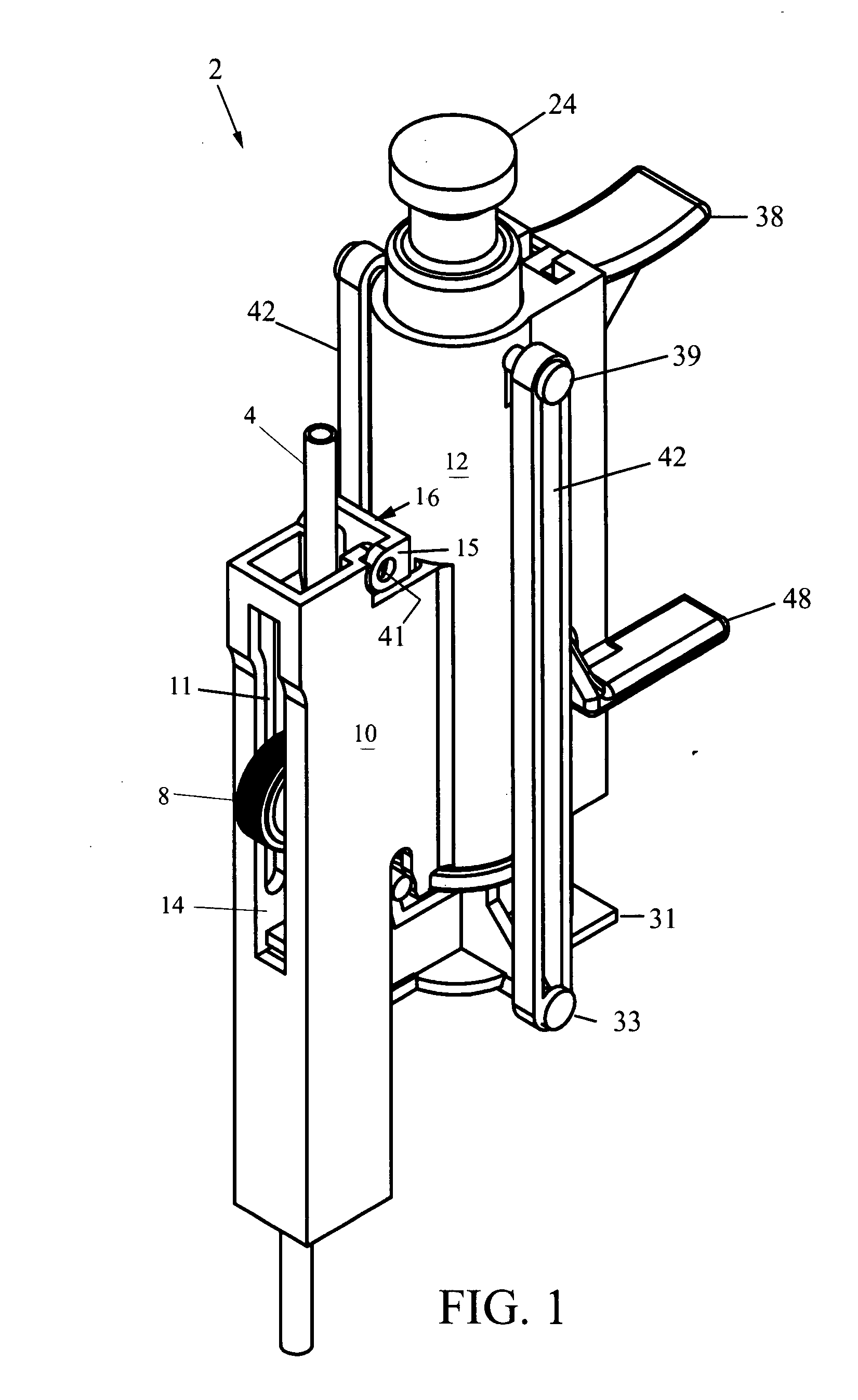
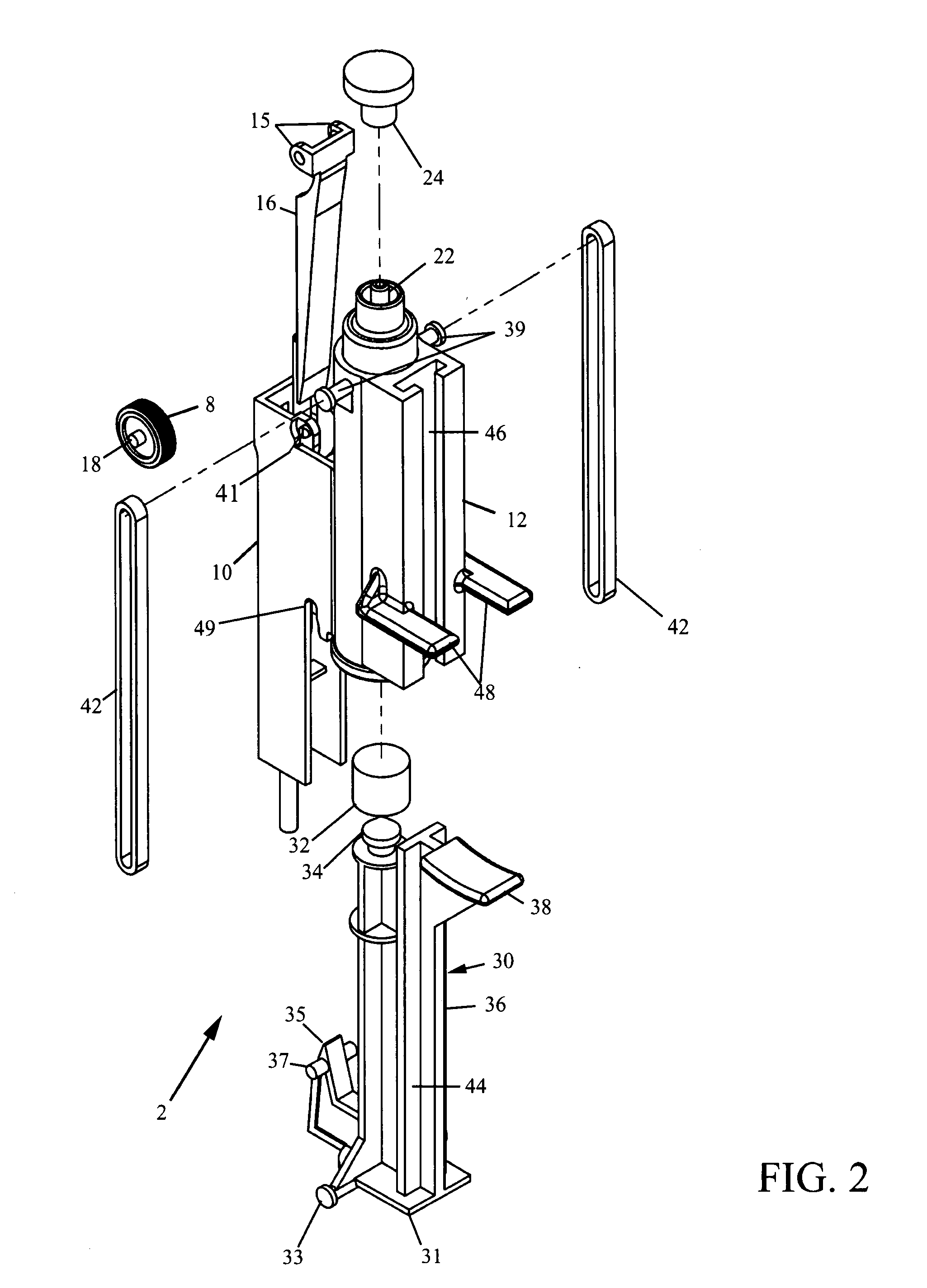
A saline flush is the method of clearing intravenous lines (IVs), Central Lines or Arterial Lines of any medicine or other perishable liquids to keep the lines (tubes) and entry area clean and sterile. Typically in flushing an intravenous cannula, a 5ml syringe of saline is emptied into the medication port of the cannula's connecting hub after insertion of the cannula. Blood left in the cannula or hub can lead to clots forming and blocking the cannula. Flushing is required before a drip is connected to ensure that the IV is still patent.
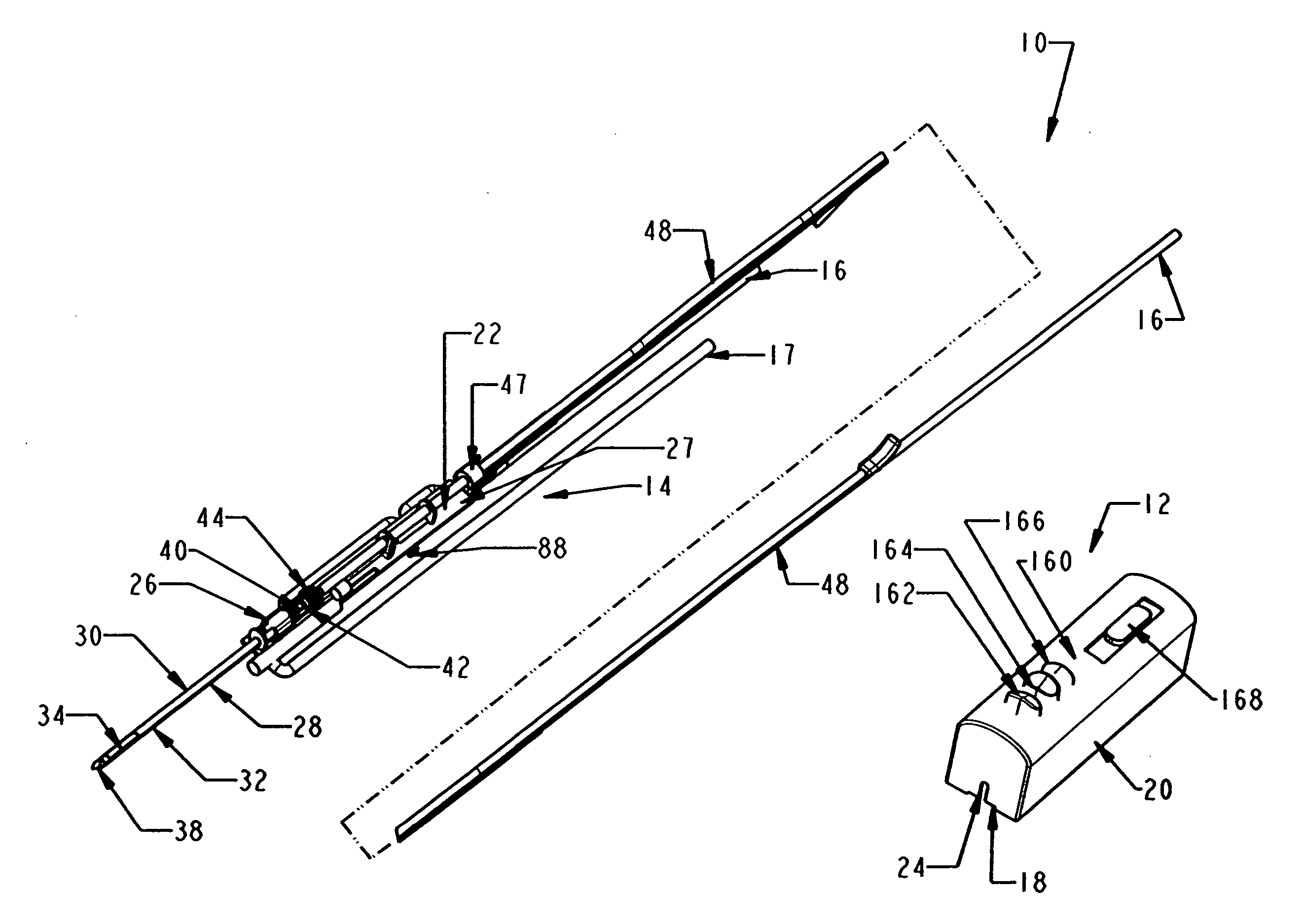
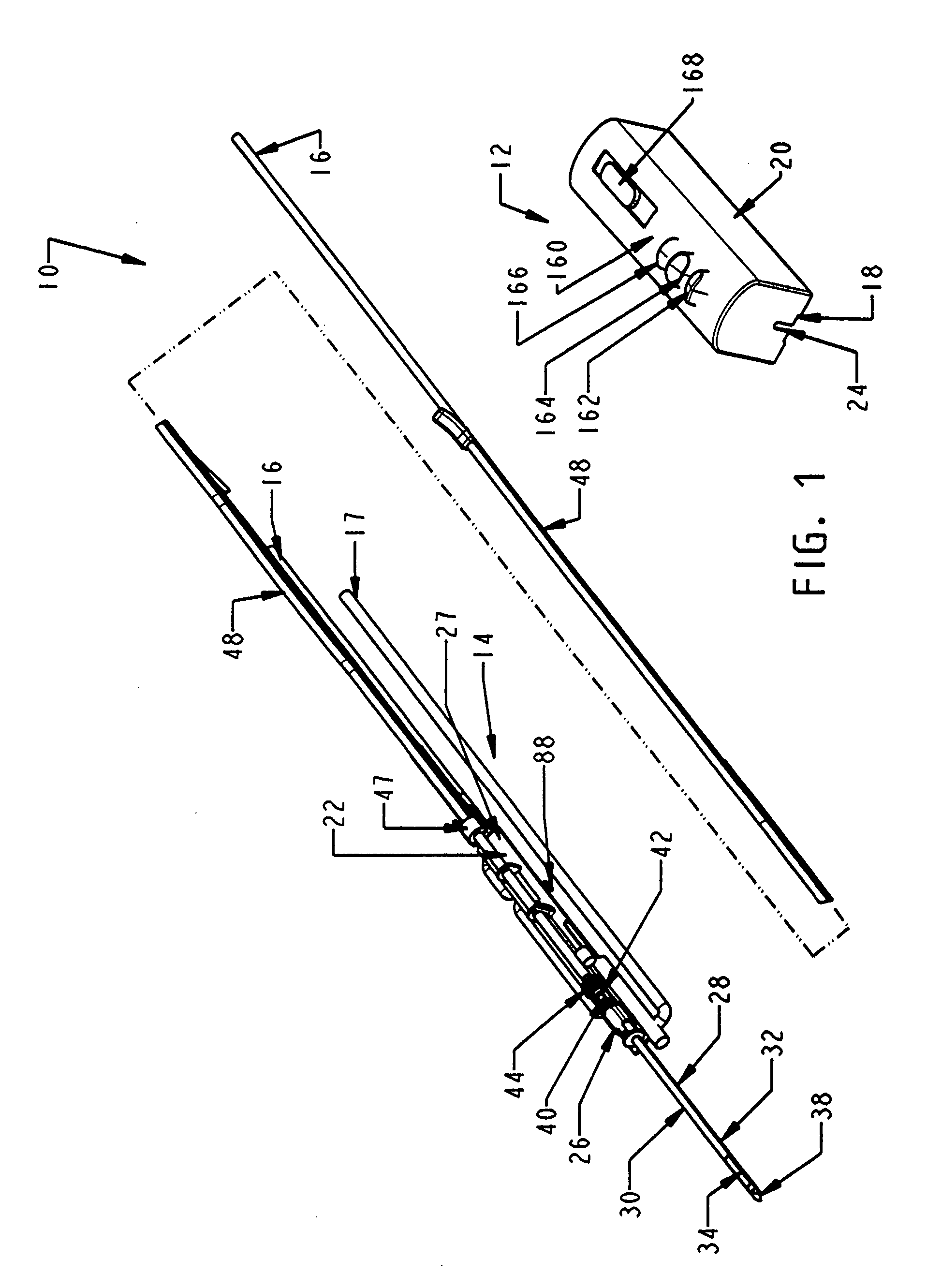
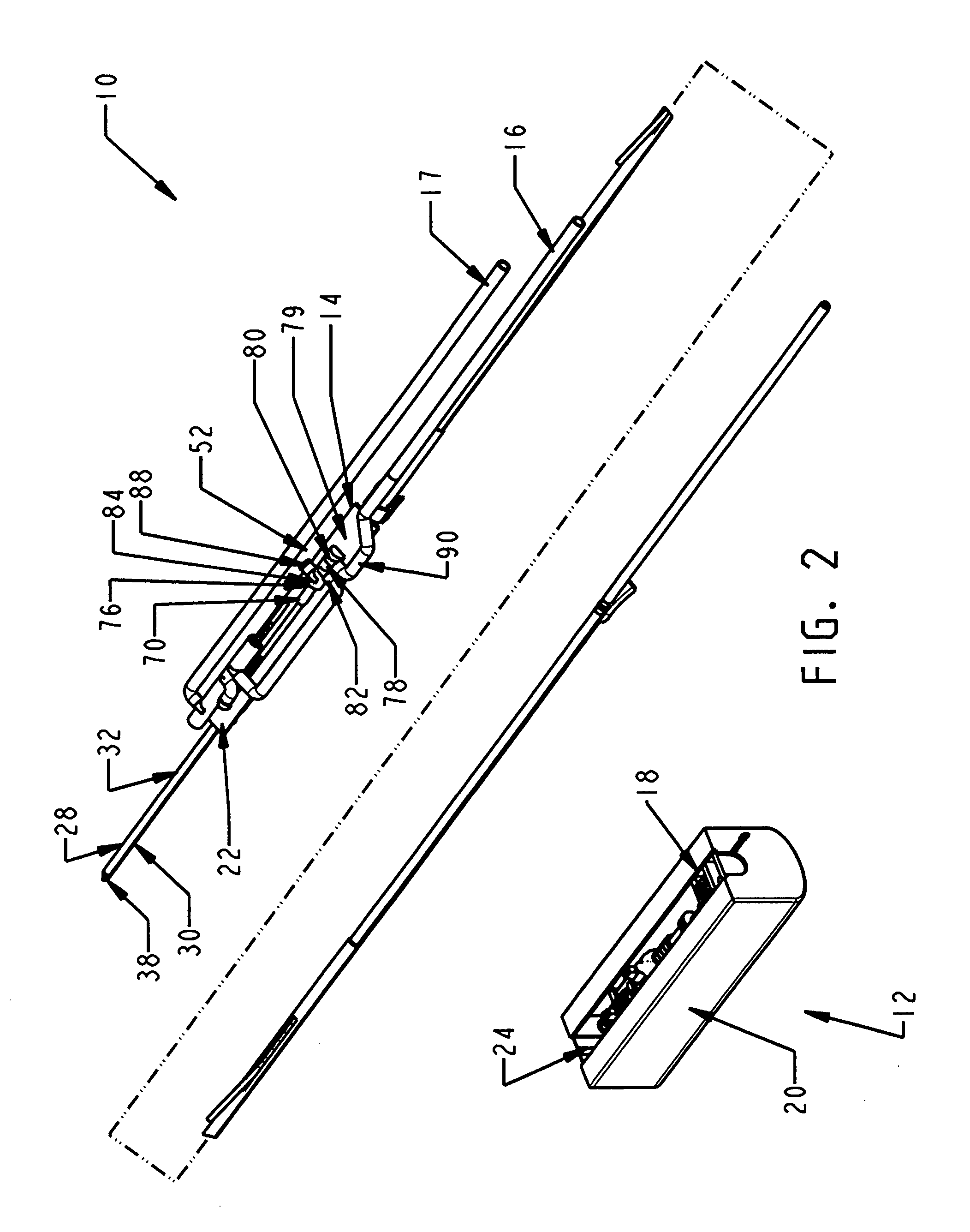
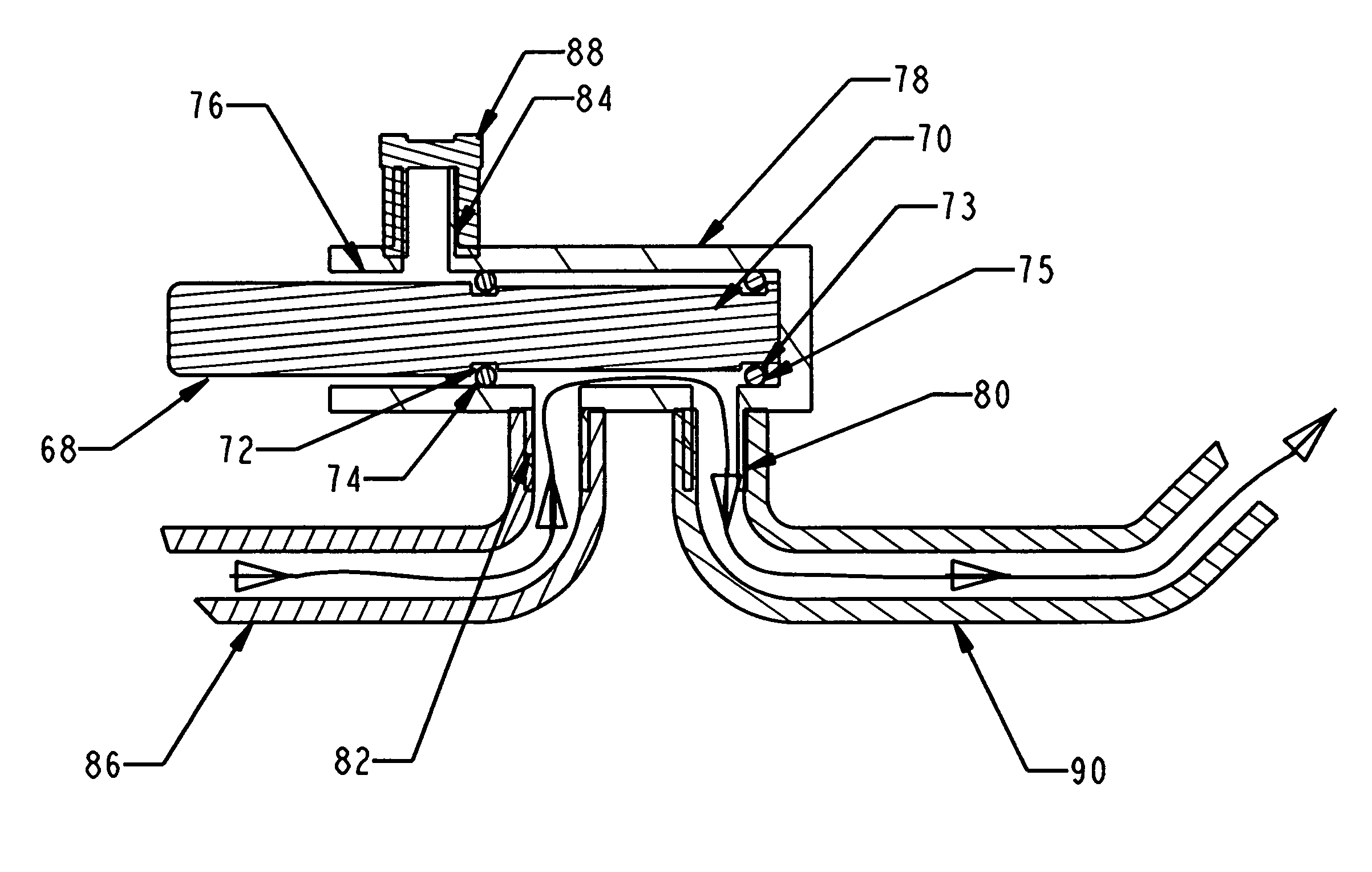
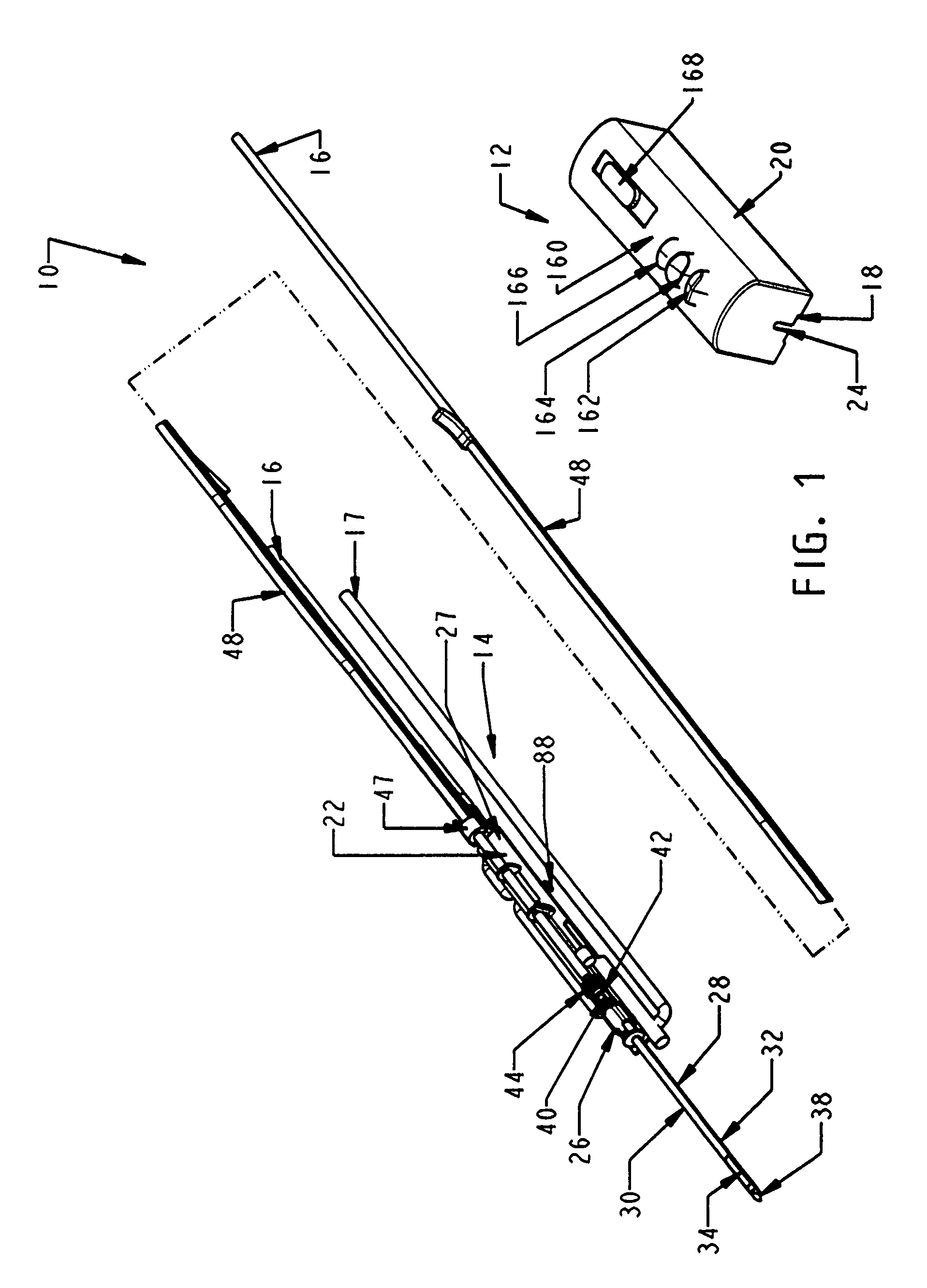
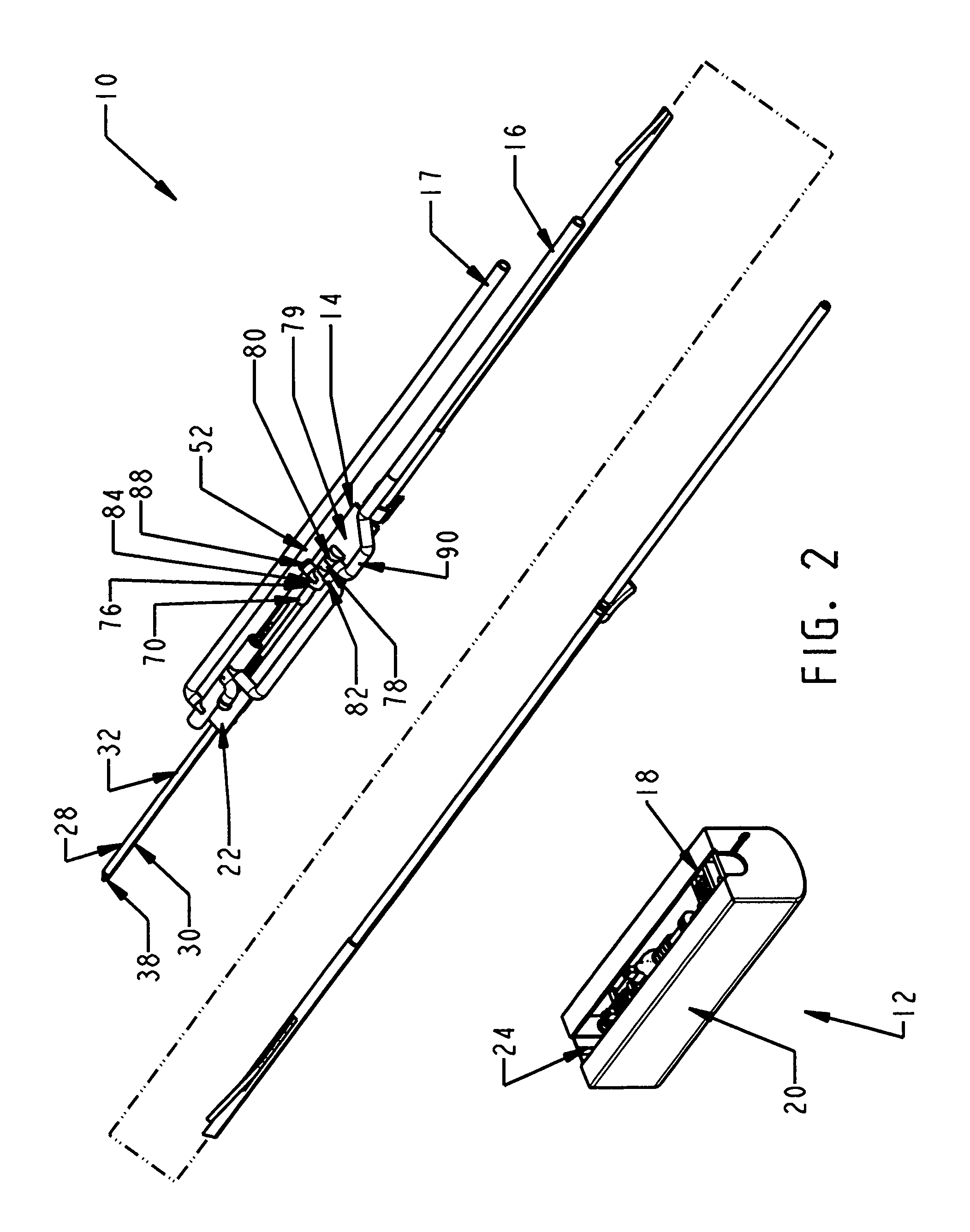
Biopsy device with replaceable probe incorporating static vacuum source dual valve sample stacking retrieval and saline flush
InactiveUS20070179401A1Small sizeInexpensively incorporatedSurgeryVaccination/ovulation diagnosticsSaline flushUltrasonic imaging
A biopsy device and method are provided for obtaining a tissue sample, such as a breast tissue biopsy sample. The biopsy device includes a disposable probe assembly with an outer cannula having a distal piercing tip, a cutter lumen, and a cutter tube that rotates and translates past a side aperture in the outer cannula to sever a tissue sample. The biopsy device also includes a reusable hand piece with an integral motor and power source to make a convenient, untethered control for use with ultrasonic imaging. The reusable hand piece incorporates a probe oscillation mode to assist when inserting the distal piercing tip into tissue. A saline valve positioned by the reusable hand piece communicates a saline supply through the probe assembly to perform saline flush of the cutter tube and outer cannula.
Owner:DEVICOR MEDICAL PROD
Biopsy device with replaceable probe incorporating static vacuum source dual valve sample stacking retrieval and saline flush
InactiveUS7662109B2Small sizeInexpensively incorporatedSurgeryVaccination/ovulation diagnosticsSaline flushUltrasonic imaging
A biopsy device and method are provided for obtaining a tissue sample, such as a breast tissue biopsy sample. The biopsy device includes a disposable probe assembly with an outer cannula having a distal piercing tip, a cutter lumen, and a cutter tube that rotates and translates past a side aperture in the outer cannula to sever a tissue sample. The biopsy device also includes a reusable hand piece with an integral motor and power source to make a convenient, untethered control for use with ultrasonic imaging. The reusable hand piece incorporates a probe oscillation mode to assist when inserting the distal piercing tip into tissue. A saline valve positioned by the reusable hand piece communicates a saline supply through the probe assembly to perform saline flush of the cutter tube and outer cannula.
Owner:DEVICOR MEDICAL PROD
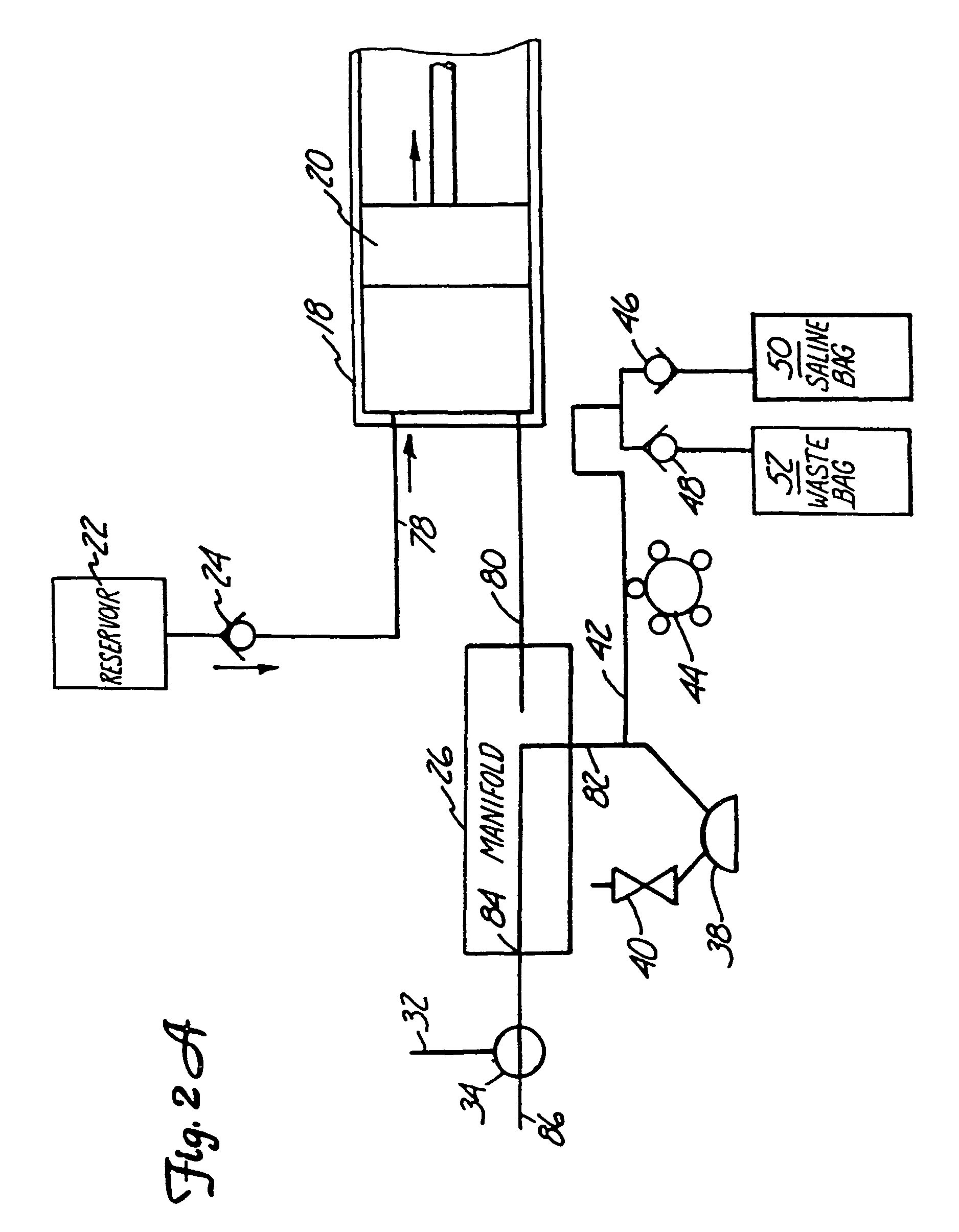
Angiographic injector and injection method
An angiographic injector system includes a manifold and valve which selectively connects either a syringe pump or a low pressure system to a catheter which is inserted into a patient. The valve is normally biased to a state which connects the low pressure system to the catheter for pressure monitoring, saline flushing, or aspirating functions. When an injection is to be made, the valve automatically switches so that the low pressure system is disconnected and not exposed to high pressure, while the syringe pump is connected through the manifold to the catheter.
Owner:INVASATEC
Devices for collecting blood and administering medical fluids
InactiveUS20050027233A1Preventing vacuum-induced collapseFacilitating active flow of bloodSamplingInfusion syringesBLOOD FILLEDBlood collection
Novel devices which can be used to both collect blood samples from and administer medical fluids to a patient on a repeated and continual basis using one rather than multiple needle insertions. The devices are capable of removing blood from one of the patient's veins using the intrinsic venous pressure of the blood and capillary action of the device, thereby preventing vacuum-induced collapse of the vein. The device typically includes a main tubing segment confluently connected to a cannula for insertion in the patient's vein. A syringe port and a volumeter for collecting blood branch separately from the main tubing segment. The device is used to collect blood by attaching an empty blood collection syringe to the syringe port, inserting the cannula in the patient's vein, allowing passive flow of blood from the main tubing segment into the volumeter under intrinsic venous blood pressure and capillary action, and then facilitating active flow of blood from the volumeter into the blood collection syringe by extending the syringe plunger. The blood-filled syringe may be replaced by additional empty blood collection syringes and the procedure repeated, as needed, depending on the quantity of blood to be obtained. The device may be used to administer medical fluids to the patient by first removing the residual blood from the main tubing segment and volumeter, flushing the main tubing segment with sterile normal saline and administering the fluids to the patient through the main tubing segment from a medical fluid syringe or catheter attached to the syringe port.
Owner:ONE STICK LLC
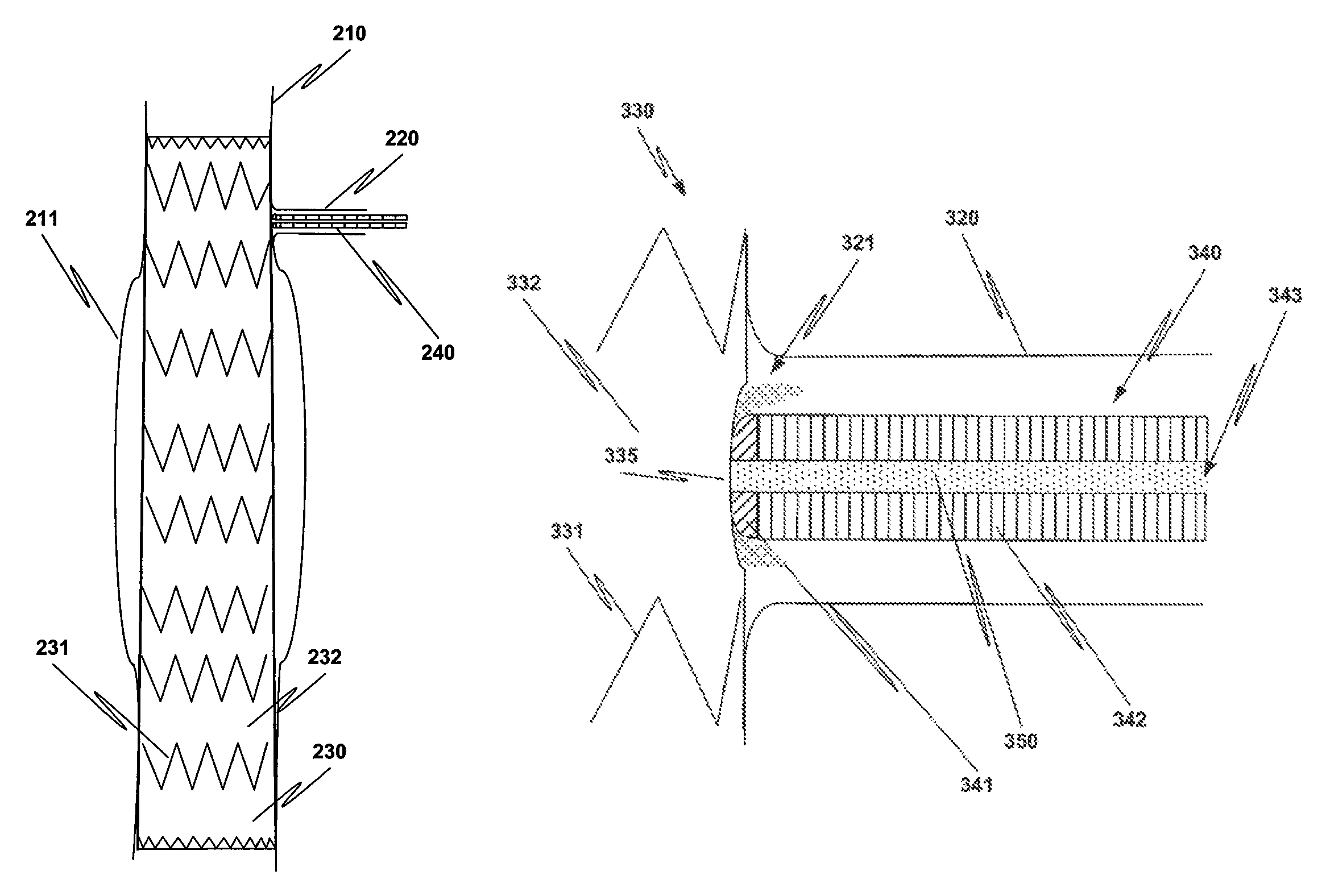
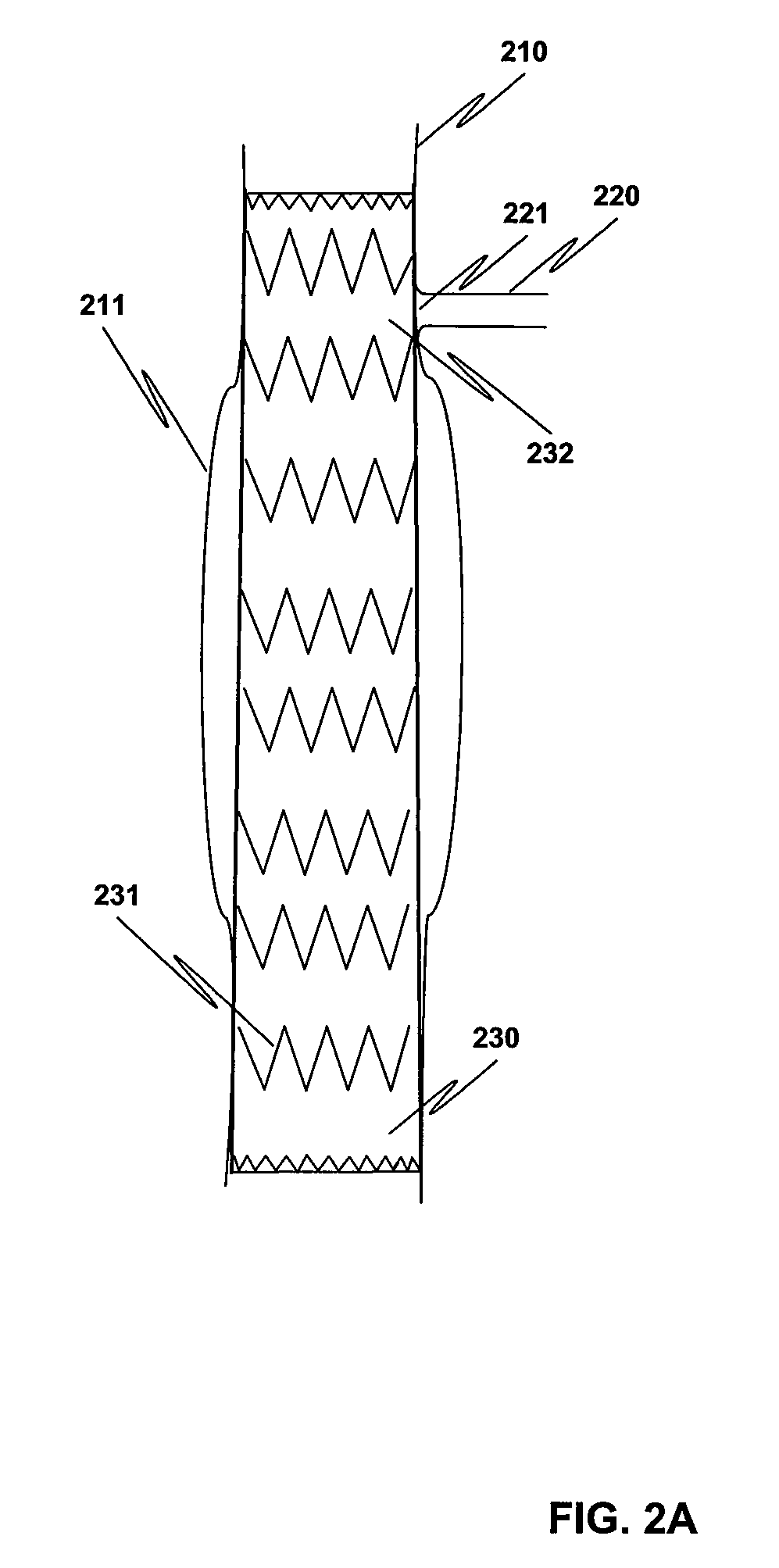
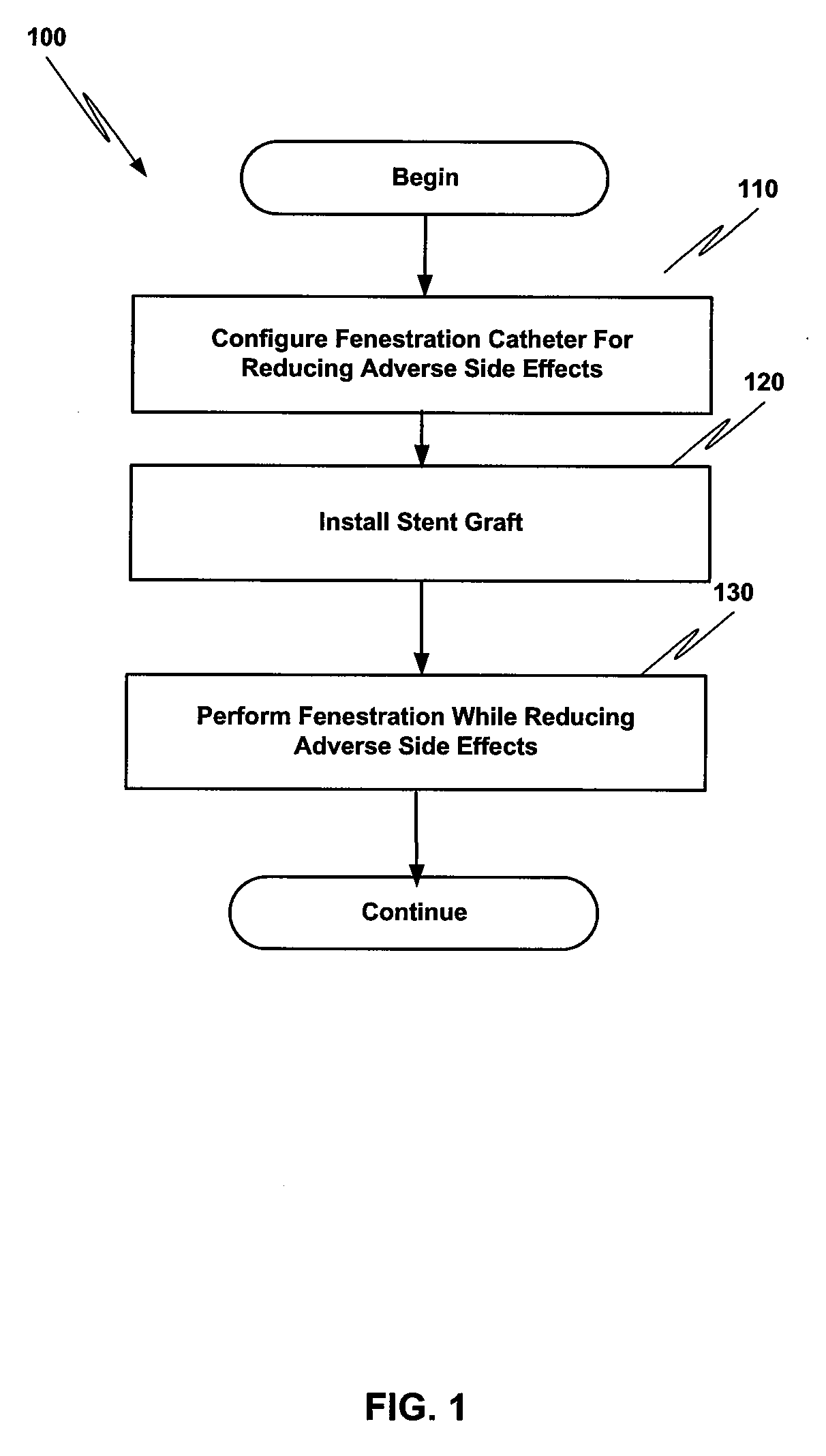
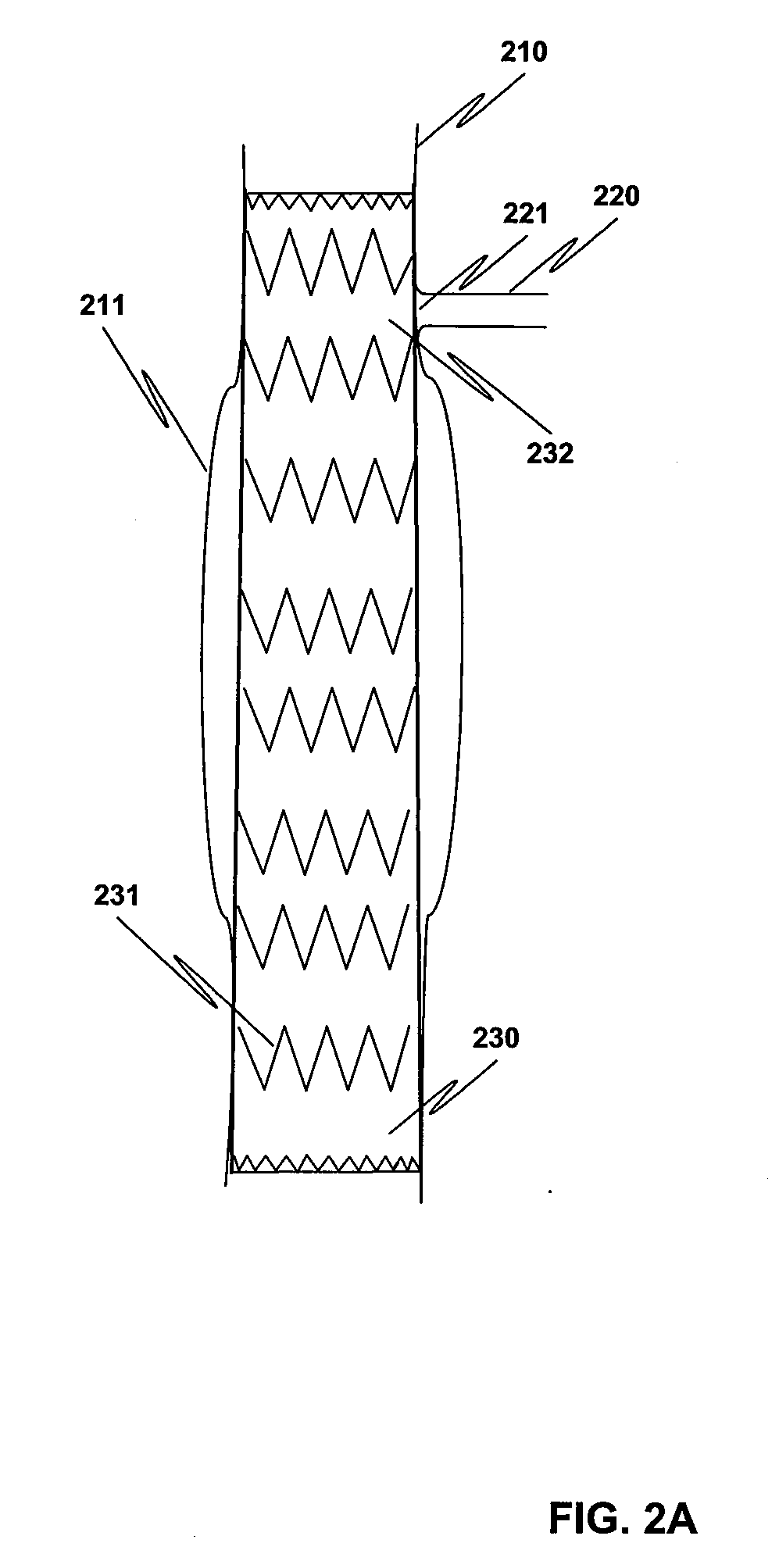
Method and structure for ameliorating side-effects of performing in situ fenestration using a plasma RF catheter
ActiveUS8197475B2Reduce adverse side effectsEliminate side effectsStentsLigamentsSide effectInsertion stent
When a main stent-graft is placed in a vessel of a patient and a branch vessel is blocked by the main stent-graft, a RF plasma catheter is used to cut out a portion of the graft cloth of the main-stent graft adjacent to an ostium of the branch vessel to be perfused. To ameliorate possible adverse effects associated with the use of the RF plasma catheter, e.g., creation of coagulum, (desiccated, coagulated blood) or perhaps a cut stent strut, a special process using saline flushing, a novel RF plasma catheter with an insulated tip, or a combination of the two is used.
Owner:MEDTRONIC VASCULAR INC
Double chamber syringe
InactiveUS20070208295A1Reduce riskReduce human contactInfusion syringesMedical devicesSaline flushSurgery
Double chamber syringe is designed with double tasks. This kind of syringe has ability to deliver amount of medication followed by delivering the certain amount of salin flush fluid or second prescribed medication. At this moment, Those tasks are performed by two separate syringes. The advantages of using double chamber syringe are reducing the risk of infection, cost and the time spending for administration of medication. The double chamber syringe is presented in three different types.
Owner:OLOODMIYAZDI MOHAMMADALI
Method for cleaning reverse osmosis membrane element
InactiveCN106512744AEfficient removalImprove efficiencySemi-permeable membranesSaline flushReverse osmosis
The invention discloses a method for cleaning a reverse osmosis membrane element. The method comprises the following steps: soaking the reverse osmosis membrane element in a non-oxidative bactericide; carrying out conventional closed-cycle cleaning on the soaked reverse osmosis membrane element with a detergent; flushing the cleaned reverse osmosis membrane element with de-mineralized water; preparing an alkaline cleaning solution by taking de-mineralized water as a solvent; carrying out conventional closed-cycle cleaning on the flushed reverse osmosis membrane element with the prepared alkaline cleaning solution; cleaning the cleaned reverse osmosis membrane element with de-mineralized water, thereby completing the cleaning of the reverse osmosis membrane element. According to the method, the hydrochloric acid added detergent and the special prepared alkaline cleaning solution are adopted to carry out cleaning, so that organic matters, colloids and residual bacteria can be effectively removed, the damage to the membrane element caused by chemical agents is reduced, the pollution to the membrane element caused the bacteria is avoided, the efficiency of use of the membrane element is increased, and the service life of the membrane element is prolonged.
Owner:HEFEI XINDA MEMBRANE TECH
Angiographic injector and injection method
An angiographic injector system includes a manifold and valve which selectively connects either a syringe pump or a low pressure system to a catheter which is inserted into a patient. The valve is normally biased to a state which connects the low pressure system to the catheter for pressure monitoring, saline flushing, or aspirating functions. When an injection is to be made, the valve automatically switches so that the low pressure system is disconnected and not exposed to high pressure, while the syringe pump is connected through the manifold to the catheter.
Owner:INVASATEC
Reverse osmosis membrane cleaning method
InactiveCN107261853AEfficient removalImprove efficiencySemi-permeable membranesSaline waterSaline flush
The invention discloses a reverse osmosis membrane cleaning method. The method includes: placing a reverse osmosis membrane into a soak solution for soaking; subjected to the soaked reverse osmosis membrane to regular closed cycle cleaning with a cleaner; flushing the cleaned reverse osmosis membrane with desalted water; configuring an alkaline cleaner by taking the desalt water as a solvent; subjected to the flushed reverse osmosis membrane to regular closed cycle cleaning with the alkaline cleaner; cleaning the cleaned reverse osmosis membrane with the desalted water to complete cleaning of the reverse osmosis membrane. The reverse osmosis membrane is cleaned with the cleaner added with hydrochloric acid and the specially configured alkaline cleaner, organic matter, gel and residual bacteria can be effectively removed, damage of chemical agents to membrane components is reduced, pollution the membrane components by the bacteria is avoided, use efficiency of the membrane components is improved, and service life of the membrane components is prolonged.
Owner:HEFEI XINDA MEMBRANE TECH
Medical device extraction tool
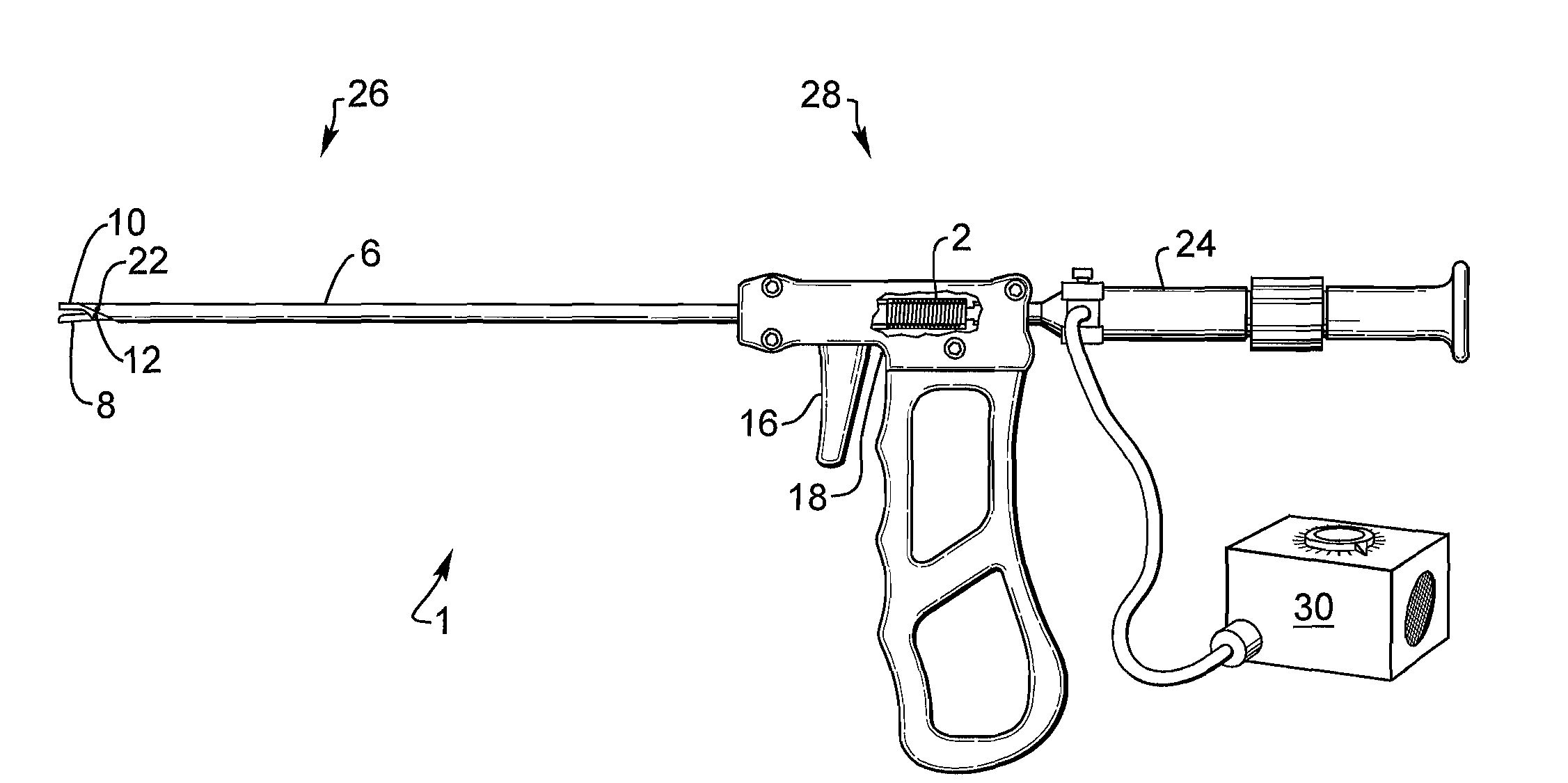
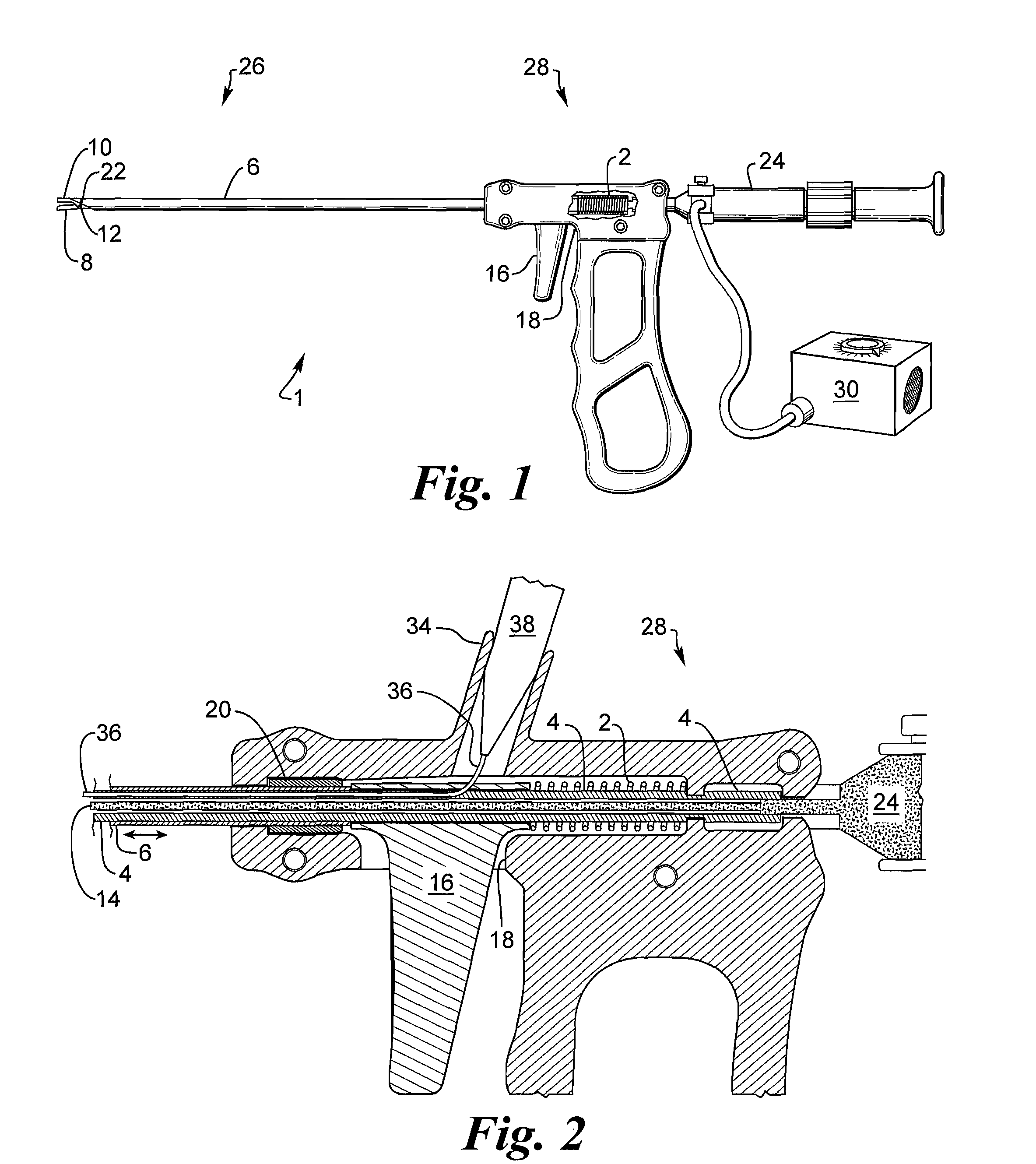
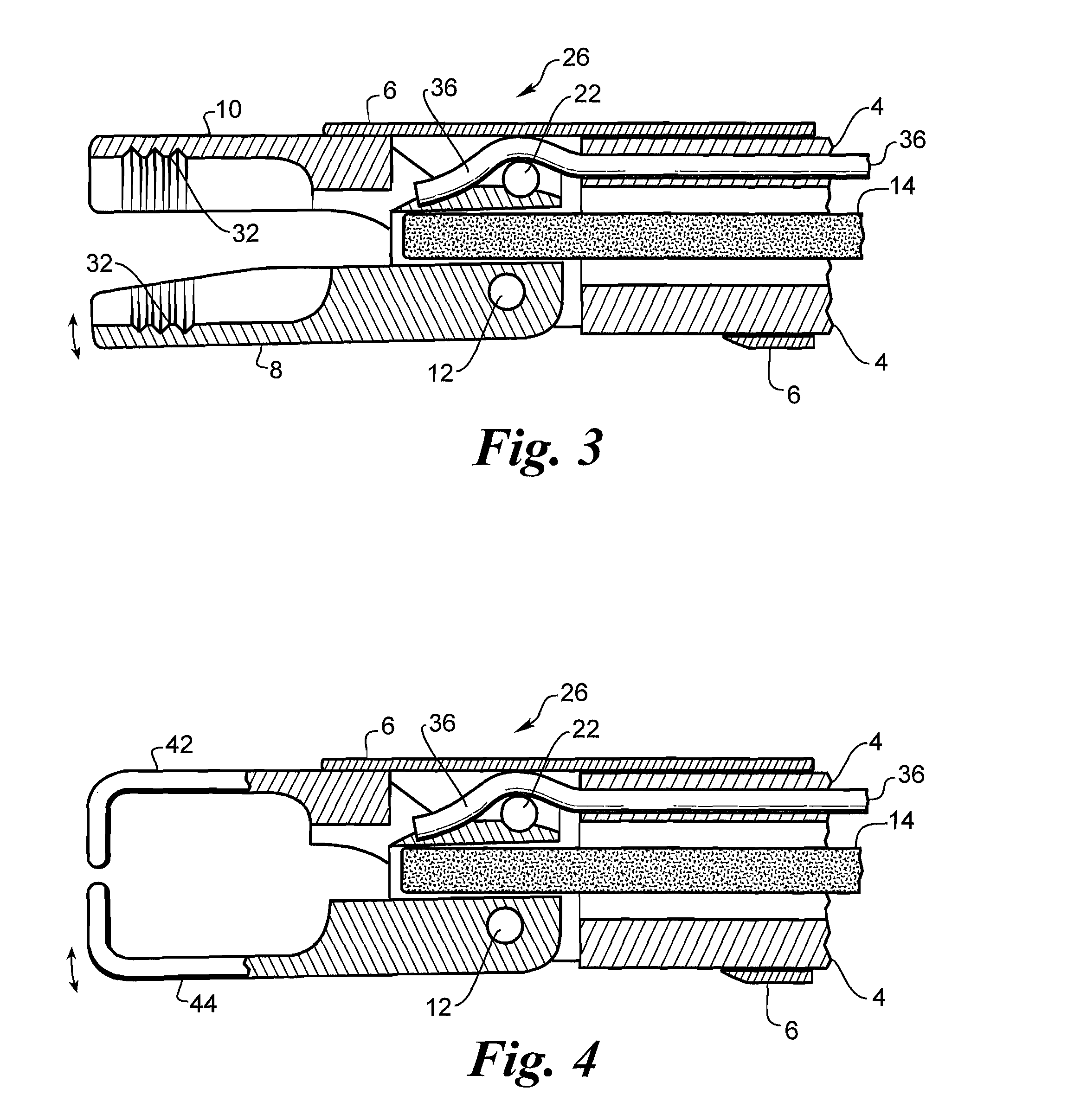
A medical instrument, particularly a surgical instrument with a displaceable push / pull activation tube arranged on the proximal end of a hand manipulator, that also contains an inner tube for retaining a fixed jaw, for activating remote tool parts on the distal end, in which a force-limiting device limits the transmission of force to the remote tool parts from the hand manipulator via an extendable coil spring. The medical instrument optionally features a fiber scope to enable optical viewing to locate the implanted device for removal as well as a saline flush capability. The instrument has jaws that are cylindrical in shape to facilitate grasping an implanted cylindrical device.
Owner:ALFRED E MANN FOUND FOR SCI RES
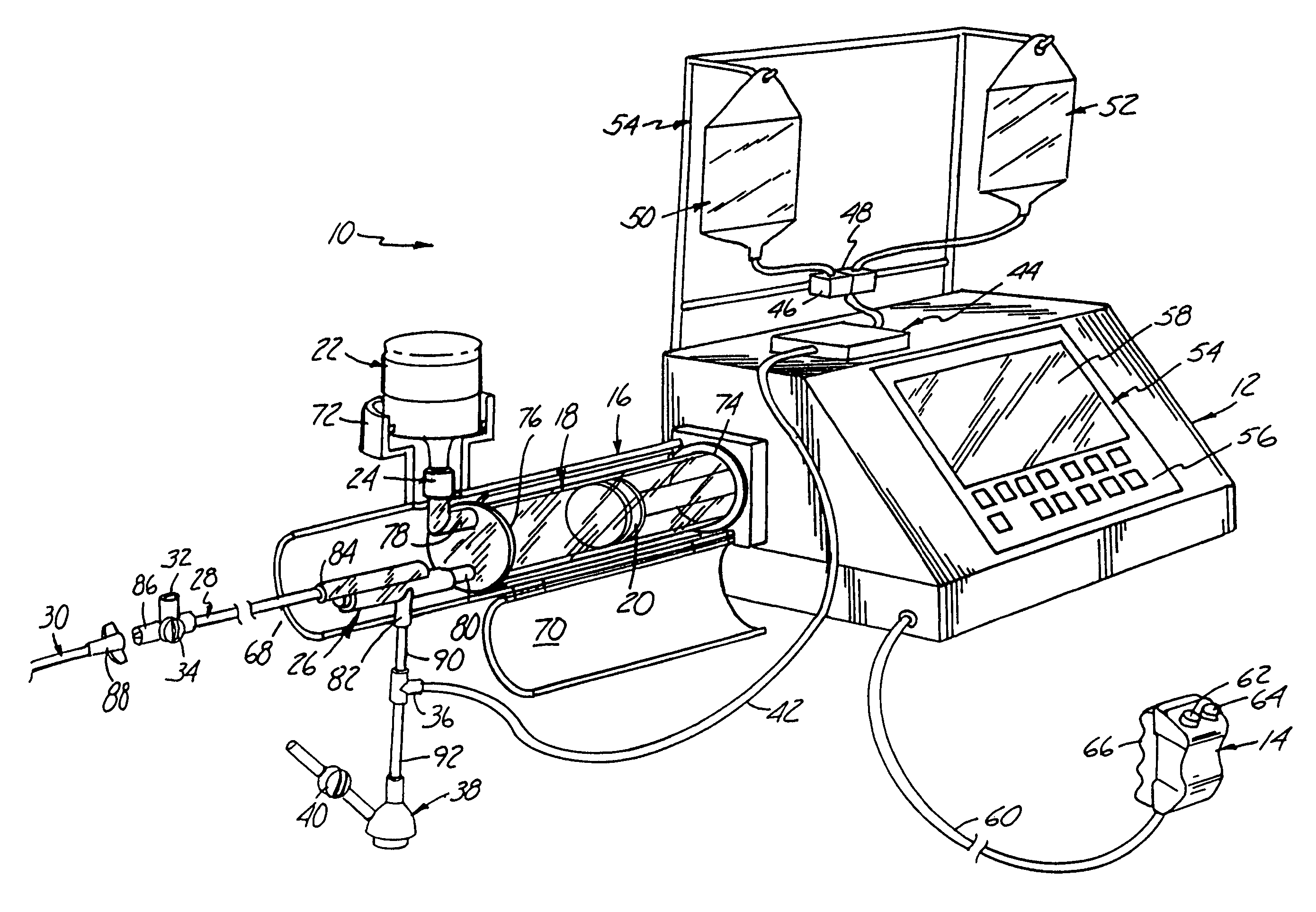
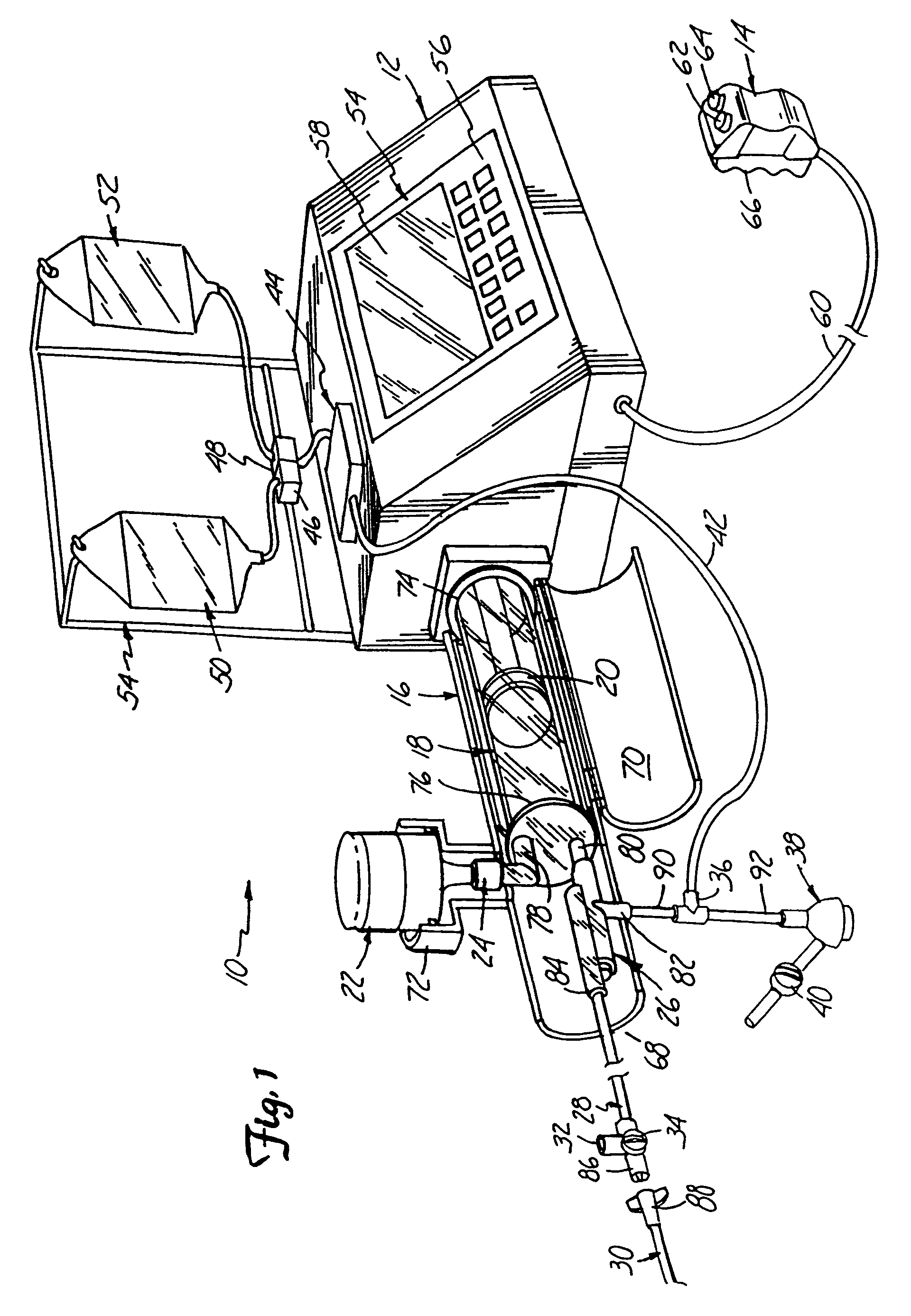
IV regulator with integral flushing mechanism
InactiveUS20080091150A1Safety managementSimple processInfusion devicesMedical devicesSaline flushSpecific volume
The present invention describes IV regulators that allow interruption of normal controlled-flow for a safe and convenient bolus flush, either for a specific period of time or for a specific volume of saline flush. Two time-controlled IV regulators are shown, one employing a pneumatic cylinder counter-opposed by elastic band(s) for administering a bolus flush, and one employing a compressible lever and torsion spring counter-opposed by a rotary damper. In both cases the mechanisms disengage the roller-clamp from the IV tube for a predetermined amount of time to fully open fluid flow there through for a bolus flush before return to the regulated-flow position. The volume-controlled flow regulator employs two internal bladders and allows one bladder to fill while the other bladder drains (and vice versa) by flipping a slider paddle-type toggle, thus enabling the administration of a set volume of flush fluid.
Owner:MURPHY TODD +3
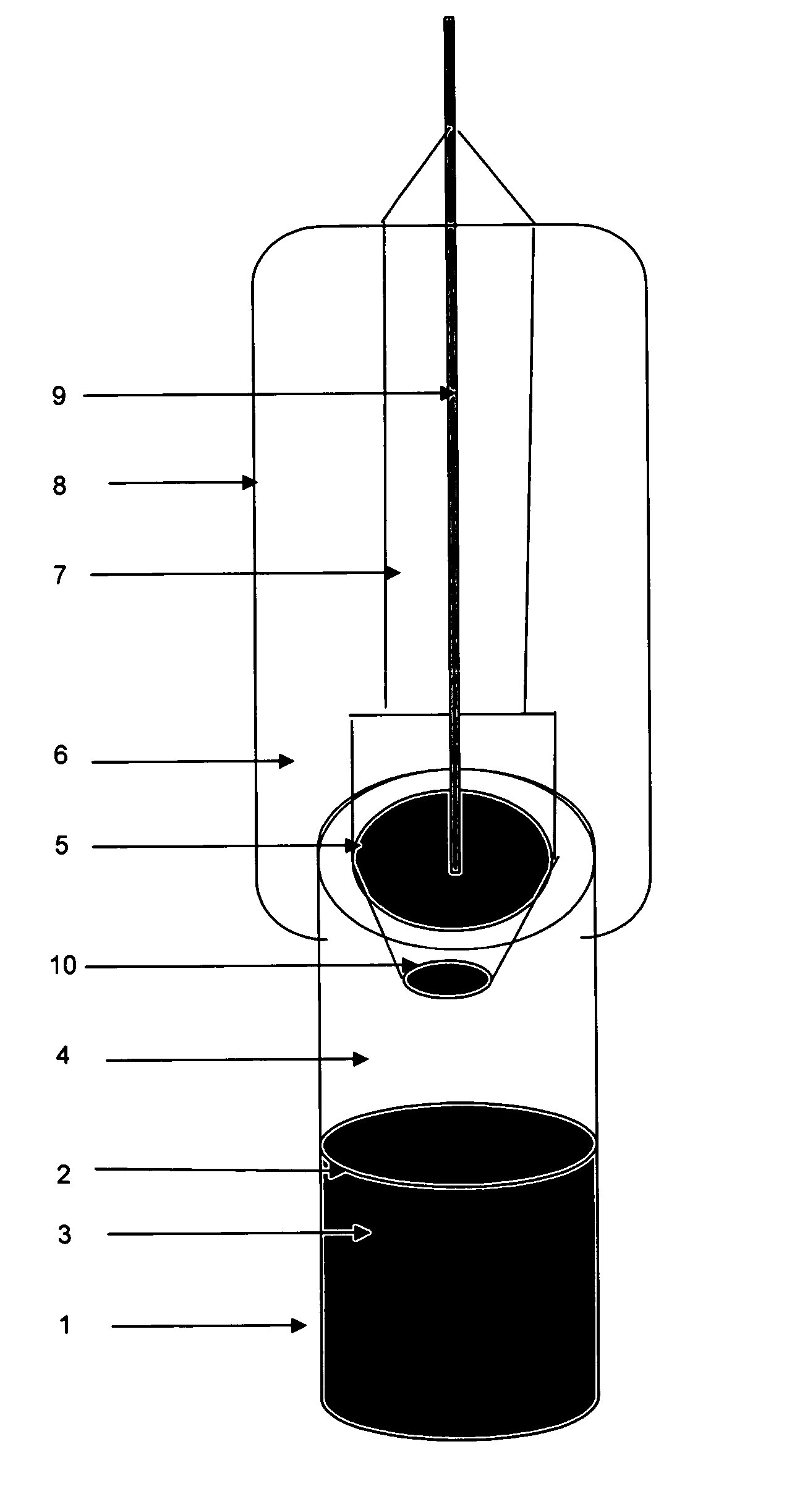
Infusion apparatus allowing convenient tube flushing
PendingCN106730115AShorten the timeSimple processMedical devicesFlow monitorsMedical equipmentSaline flush
An infusion apparatus allowing convenient tube flushing belongs to medical equipment and is characterized in that a venous infusion needle (1), a miniature filter (2), a drop speed adjuster (3), a Murphy's dropper (4) and an infusion puncture tube (5) are hermetically and sequentially connected through an infusion tube, an infusion intake tube (6) is arranged on the lateral side of the infusion puncture tube (5), an infusion control valve (10) is arranged on the infusion tube between the Murphy's dropper (4) and the infusion puncture tube (5), the upper portion of the Murphy's dropper (4) is provided with a flushing puncture tube (7) through an infusion tube, the lateral side of the flushing puncture tube (7) is provided with a flushing intake tube (8), and a flushing control valve (9) is arranged on the infusion tube between the Murphy's dropper (4) and the flushing puncture tube (7). The infusion apparatus has the advantages that switching the puncture tubes between a saline flushing bottle and an infusion bottle is not required, time is saved, the procedure is simplified, work efficiency is improved, and medical risk is relieved.
Owner:宋霞
IV regulator with integral flushing mechanism
InactiveUS7976515B2Safe and convenientSafety managementInfusion devicesMedical devicesSaline flushNormal control
Owner:MURPHY TODD +3
Method and Structure for Ameliorating Side-Effects of Performing in Situ Fenestration Using a Plasma RF Catheter
ActiveUS20090234348A1Reduce adverse side effectsEliminate side effectsStentsSurgical instruments for heatingSide effectInsertion stent
When a main stent-graft is placed in a vessel of a patient and a branch vessel is blocked by the main stent-graft, a RF plasma catheter is used to cut out a portion of the graft cloth of the main-stent graft adjacent to an ostium of the branch vessel to be perfused. To ameliorate possible adverse effects associated with the use of the RF plasma catheter, e.g., creation of coagulum, (desiccated, coagulated blood) or perhaps a cut stent strut, a special process using saline flushing, a novel RF plasma catheter with an insulated tip, or a combination of the two is used.
Owner:MEDTRONIC VASCULAR INC
Method for detecting volatile phenol content of water
InactiveCN104374728AThe analysis result is accurateShorten distillation timeMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsVolatile phenolsDistillation
The invention discloses a method for detecting volatile phenol content of water and belongs to the field of environment sample detection methods. The method comprises the following steps of taking a water sample, adding a methyl orange indicator into the water sample, adjusting the water sample to orange red or red color solution by a phosphoric acid solution, pouring a copper sulphate solution into the water sample, carrying out heating distillation, collecting the distillate by a volumetric flask with a sodium hydroxide solution, washing the wall of a condenser tube by desalinized water, adding a washing liquid into the same volumetric flask, carrying out dilution by desalinized water to obtain a solution having a desired volume, pouring the distillate into a colorimetric pipe, adjusting the pH of the solution to less than 7 by a hydrochloric acid solution, determining distillate absorbance by the obtained acidic solution as a reference solution and calculating phenol content corresponding to the absorbance according to a standard curve regression equation. The method has a phenol recovery rate more than 94.0%, lowest detection limit of 0.1mg / L, standard deviation of 0.05 and a variation coefficient of 3%, and has the characteristics of high accuracy, high repeatability, operation simpleness and low detection limit.
Owner:SHAANXI HUALU CHEM ENVIRONMENTAL PROTECTION
Microbial limit detection method for membrane used for multi-layer coextrusion infusion
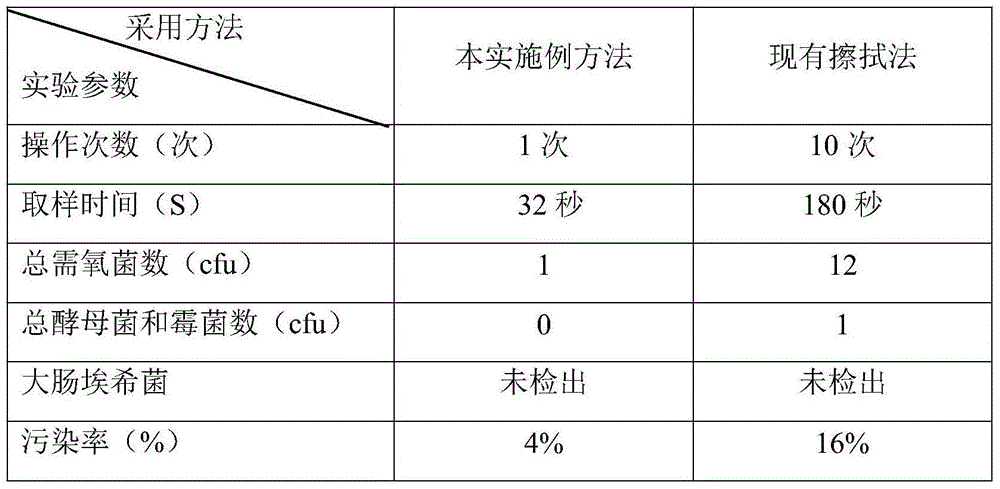
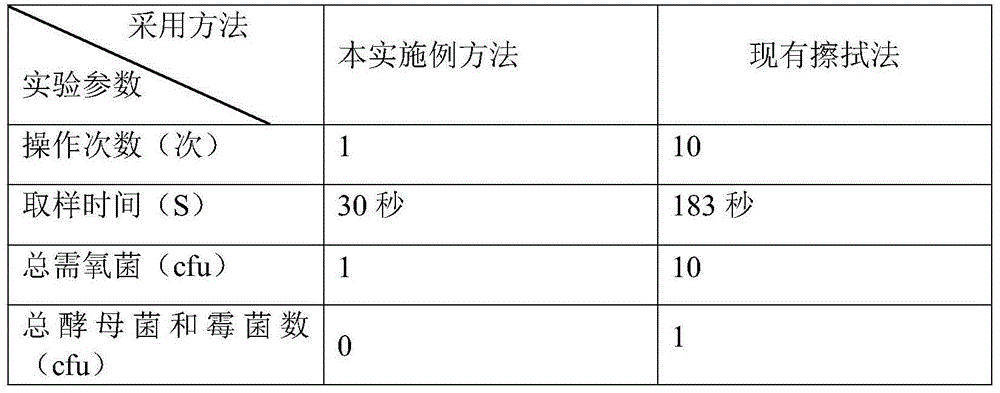
InactiveCN104878071ARaise quality standardsAvoid secondary pollutionMicrobiological testing/measurementMicroorganism based processesBottleDigestion
The invention relates to a microbial limit detection method for a membrane used for multi-layer coextrusion infusion. The method includes the steps of taking out the membrane used for multi-layer coextrusion infusion under the aseptic environment, flushing the outer layer face of the membrane used for multi-layer coextrusion infusion through a stroke-physiological saline solution at least three times, taking a membrane material with the area of 50 cm<2> in the middle, cutting the taken membrane material with the area of 50 cm<2> into pieces through a pair of aseptic scissors, placing the cut membrane material in a conical aseptic bottle, adding 100 ml of aseptic sodium chloride peptone buffer solutions with the pH value of 7.0, shaking the mixture for 1 minute, standing still for 10 minutes, preparing a test solution, taking 10 ml of the test solution and filtering the test solution through a membrane filter after a filter membrane of the membrane filter is wetted through 10 ml of the aseptic sodium chloride peptone buffer solutions with the pH value of 7.0, taking out the filter membrane, attaching the filter membrane to a culture medium with the bacterium face of the filter membrane facing upwards, taking 10 ml of the test solution, inoculating the test solution to 100 ml of soybean casein digestion meat juice, placing the culture medium where the filter membrane is attached and the soybean casein digestion meat juice where inoculation is conducted in an incubator, and observing the result. By means of the established microbial limit detection method, the quality standard of the membrane used for multi-layer coextrusion infusion is improved, and gaps in microbial limit detection are filled in.
Owner:QINGDAO HUAREN PHARMA PACKAGING MATERIAL SCI & TECH
Fat injection applied to injection cosmetology and preparation method
InactiveCN108671268AIncrease concentrationHigh activityPharmaceutical delivery mechanismTissue regenerationHigh concentrationVitamin C
The invention provides fat injection applied to injection cosmetology and a preparation method. The preparation method comprises the following steps: firstly, extracting autologous inner thigh fat; freezing and grinding the autologous inner thigh fat; washing under the nitrogen environment; adding water and an emulsifier to obtain emulsion; then adding nanoscale silicon dioxide aerogel and a demulsifier into the emulsion; evenly dispersing and centrifugally separating the mixture to obtain adipose-derived stem cells; finally, matching the adipose-derived stem cells with vitamin C, collagen andhyaluronic acid to prepare the fat injection applied to injection cosmetology. According to the method, the fat is emulsified and dispersed into silicon dioxide aerogel with nano pores, and the adipose-derived stem cells are preserved in the silicon dioxide aerogel after demulsification; thus, the concentration and the survival rate of the adipose-derived stem cells are improved; when being applied to injection cosmetology, the high-concentration adipose-derived stem cells can promote autologous fat growth and have obvious treating effects on the plastic and aesthetic surgery aspects of rhytidectomy, lip enhancement, chin augmentation, rhinoplasty, nasolabial grooves and the like.
Owner:CHENDU NEW KELI CHEM SCI CO LTD
Device for Thorough Delivery of Intravenous Medicine
InactiveUS20190290837A1Take advantage ofPharmaceutical delivery mechanismMedical devicesSaline flushIntravenous drug
A device for the thorough delivery of liquid medicine in an intravenous (IV) delivery system. The device consists of a negative pressure chamber filled with saline solution that is used to flush all of the residual liquid medicine from a secondary liquid medicine delivery tube. The negative pressure chamber sits below the drip chamber attached to the secondary medicine bag and is attached to the secondary medicine delivery tube in such a way that when the liquid medicine from the delivery tube has flowed below the negative pressure chamber, the negative pressure chamber releases the stored saline to flush the liquid medicine from the secondary liquid medicine delivery tube.
Owner:WARTA AARON
A Cistern
ActiveUS20190264433A1Reduce offensive smellReduce airborne diseaseFlushing devicesDomestic plumbingFlushing timeSaline flush
This invention relates to a cistem (1010): More specifically, the invention relates primarily to concealed cisterns for toilets having integrated, and preferably adjustable, dual-flushing and odour extraction capability. The cistem (1010) includes a primary chamber (1020) for storing a volume of liquid, a liquid inlet (1026) for the primary chamber (1020) of the cistem (1010) with liquid, an air outlet (1028) for exhausting air from the primary chamber (1020) of the cistem (1010) operatively during filling; and a liquid outlet (30) through which liquid operatively stowed in the primary chamber (1020) of the cistem is dischargeable. The cistem (1010) further includes a fill valve for controlling liquid supply into the primary chamber from the liquid inlet (1026) and a flush valve (60) for controlling flow through the liquid outlet (30). The flush valve (60) comprises of a flush valve seat and a flush plug (1064) being moveable relative to the flush valve seat between an open position, wherein the flush plug (1064) is displaced from the flush valve seat thereby to open the liquid outlet (30) and enable the liquid in the primary chamber (1020) to discharge there through during flushing, and a closed position, wherein the flush plug (1064) is seated on the flush valve seat thereby to close the liquid outlet (30) and prevent the passage of liquid there through. The cistern (1010) also includes at least one actuator (1070) for actuating displacement of the flush plug (1064) from the closed position to the open position, and a controller (1123) for controlling one or more of a group of flushing conditions including: (i) flush type, wherein the cistem (1010) is adjustable between a single flush, dual-flush, on-demand flush or a combination of such flush type conditions; and (ii) flush time, wherein the time of the displacement of the flush plug (1064) by the actuator (1070) for each flush type condition is adjustable.
Owner:ROSS KENT DYLAN HUGH
Production method of gecko wine
InactiveCN105524809ARemove fishy smellKeep active ingredientsAlcoholic beverage preparationSaline waterSaline flush
The invention discloses a production method of gecko wine. The production method comprises the following steps: (1) taking a live gecko, washing by adopting saline water, slightly hitting the head of the live gecko till death, removing the head, the feet, the scales and the internal organs, completely washing away the blood and slime, soaking by adopting sodium bicarbonate solution, fishing up, and washing till the gecko is clean; (2) soaking the gecko by adopting fishiness-removing water for 4-6 h, airing the water on the surface of the gecko, and carrying out sterilization; (3) sealing the sterilized gecko, placing the sealed gecko into a freezing chamber for freezing, taking out the gecko, removing the sealing device, and placing the gecko into a low-temperature drying machine for drying for 21-30 h; and (4) soaking the gecko by adopting soy sauce flavor type baijiu as base liquor for 2-24 months. According to the production method, the water inside the body of the gecko is removed through the low-temperature drying manner, so that the fishy smell volatilizes along with the sublimation of the water, and meanwhile, the effective components inside the body of the gecko can be maintained to the greatest extent, so that the integral quality of the product can be improved.
Owner:李小婷
Device for sutureless repair of an injured nerve
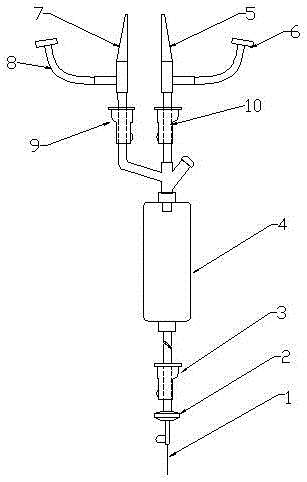
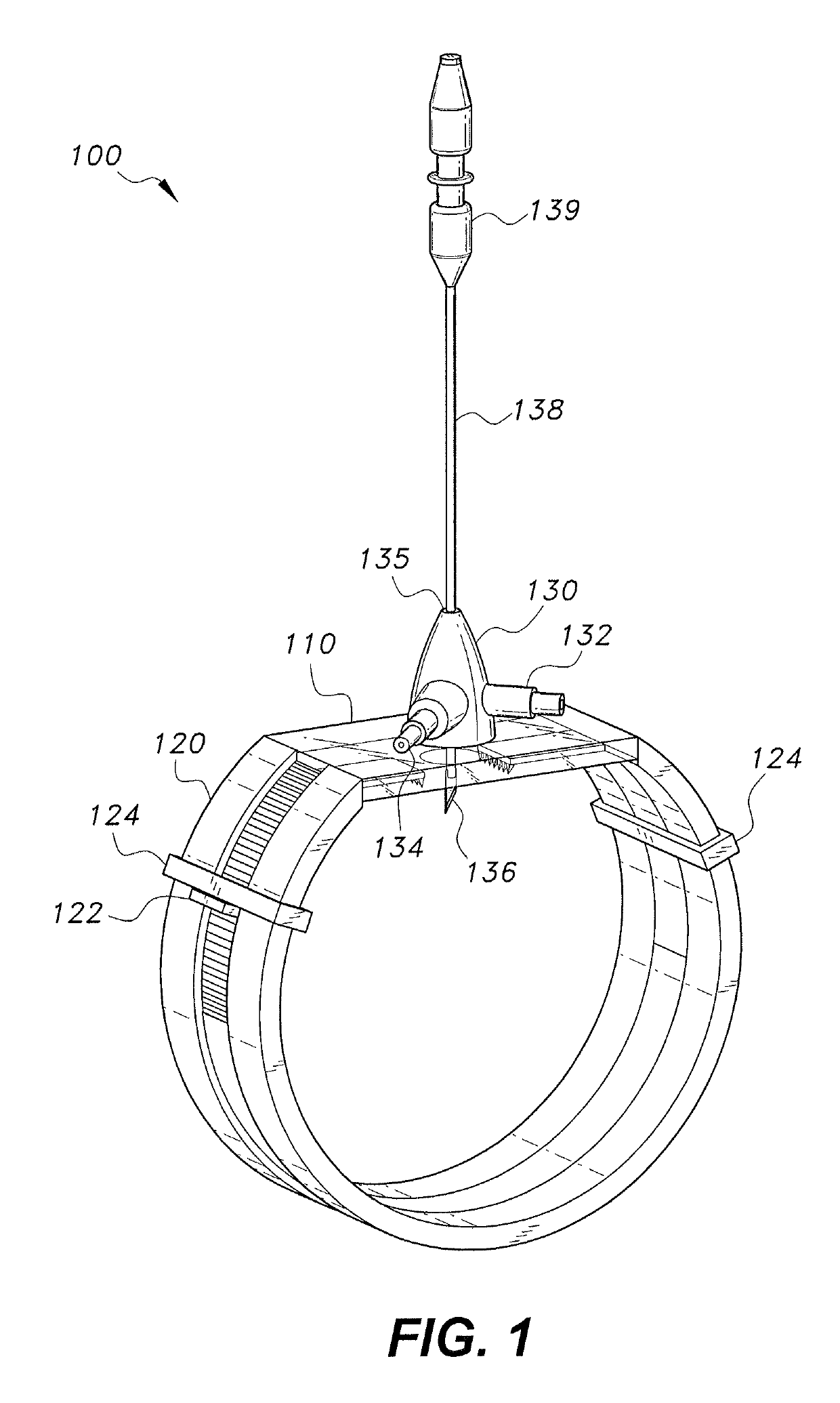
The device for sutureless repair of an injured (severed) nerve includes a securement band connected by a transparent membrane to form a loop. The band includes two opposing approximation claws that extend into the region of the transparent membrane. An aperture in the transparent membrane is covered by an enclosure having an inlet nozzle and an outlet nozzle. An elongate member having a blade on its bottom end extends through an aperture in the top of the enclosure. The band is strapped around the patient's limb with the transparent membrane adhesively secured over the incision, the severed nerve ends are irrigated with saline and air is evacuated in the process. The blade incises the severed ends of the nerve to expose fresh nerve tissue under vacuum, and the severed ends are approximated. The device is left in place for the severed nerve ends to reunite.
Owner:KING SAUD UNIVERSITY
Automatic angiography injection system
PendingCN109010978AReduce radiation intakePrecise and consistent injectionIntravenous devicesSafety monitoringPower unit
Owner:安吉特(天津)科技有限公司
Preparation method of medical porcine or bovine pleura biological dressing
InactiveCN102085386AWide variety of sourcesEasy to implementAbsorbent padsBandagesBiological dressingSaline flush
The invention discloses a preparation method of a medical porcine or bovine pleura biological dressing, which comprises the following steps: (1) selecting healthy adult swine or cattle, slaughtering, and integrally taking down the parietal pleura; (2) removing the incidental fat from the pleura; (3) soaking to disinfect the pleura having the fat removed in a disinfection liquid, and rinsing out with sterile normal saline; and (4) under sterile working conditions, putting the disinfected and rinsed pleura into a medical plastic bag, adding a proper amount of skin storage liquid into the bag, and refrigerating for later use, wherein the product is preferably used within one week. The preparation method disclosed by the invention has a simple process, and raw materials can be easily acquiredfrom wide-range sources, so that the preparation method can be easily completed by grass-roots medical treatment units. The prepared pleura dressing is low in cost, is relatively stable in size specification and flexibility and has high transparency, thereby providing convenience for observing the change of a wound surface; the prepared pleura dressing has soft texture and good elasticity and randomness, has less reject reaction on the wound surface and can not cause the occupation problem; and the prepared pleura dressing can relieve the pain of the burning wound surface, reduce the exudation of the wound surface, protect the wound surface, promote the healing of the wound surface and lower the scar hyperplasia degree.
Owner:HBIS COMPANY LIMITED HANDAN BRANCH COMPANY
Combined micro-pump control device
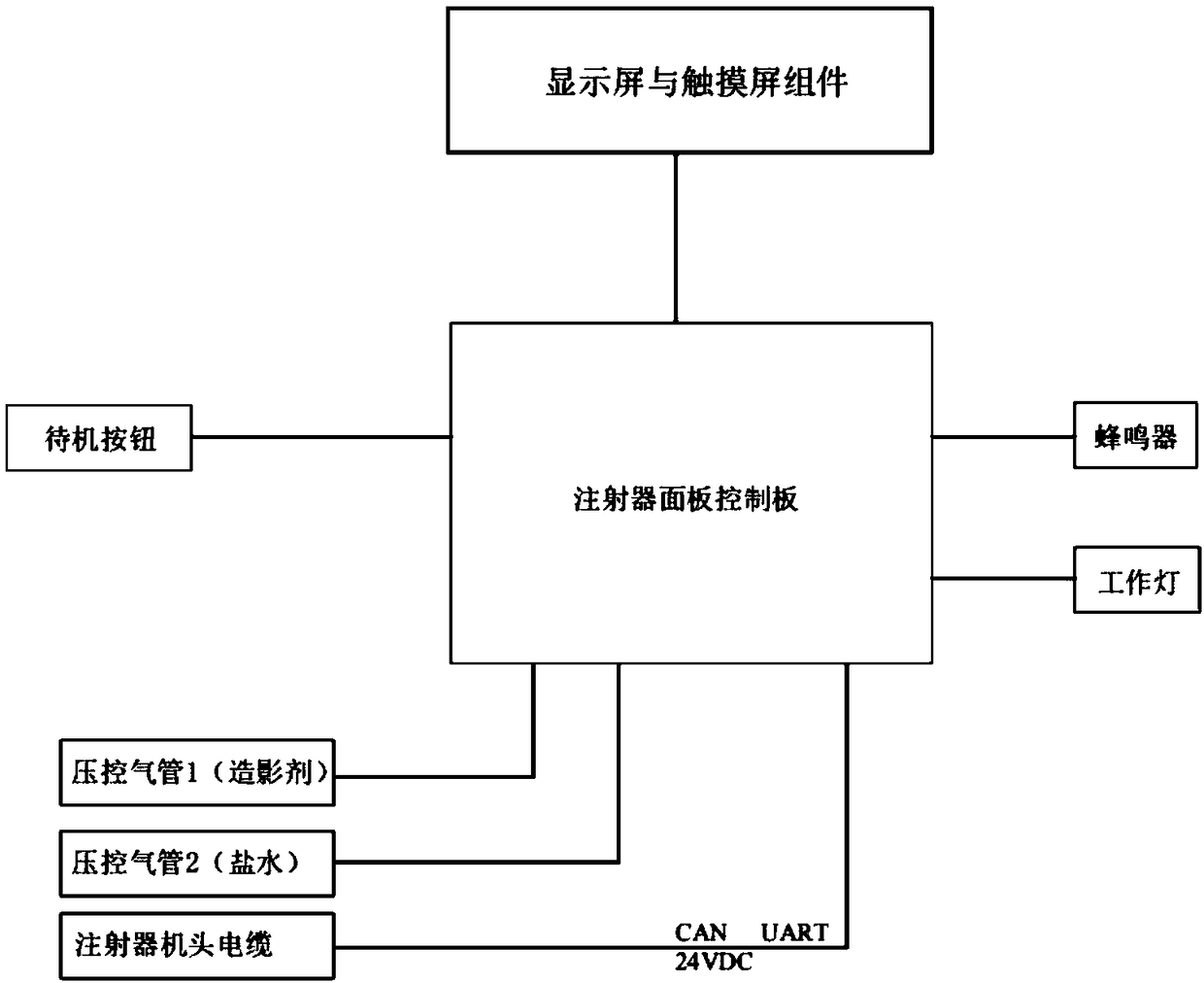
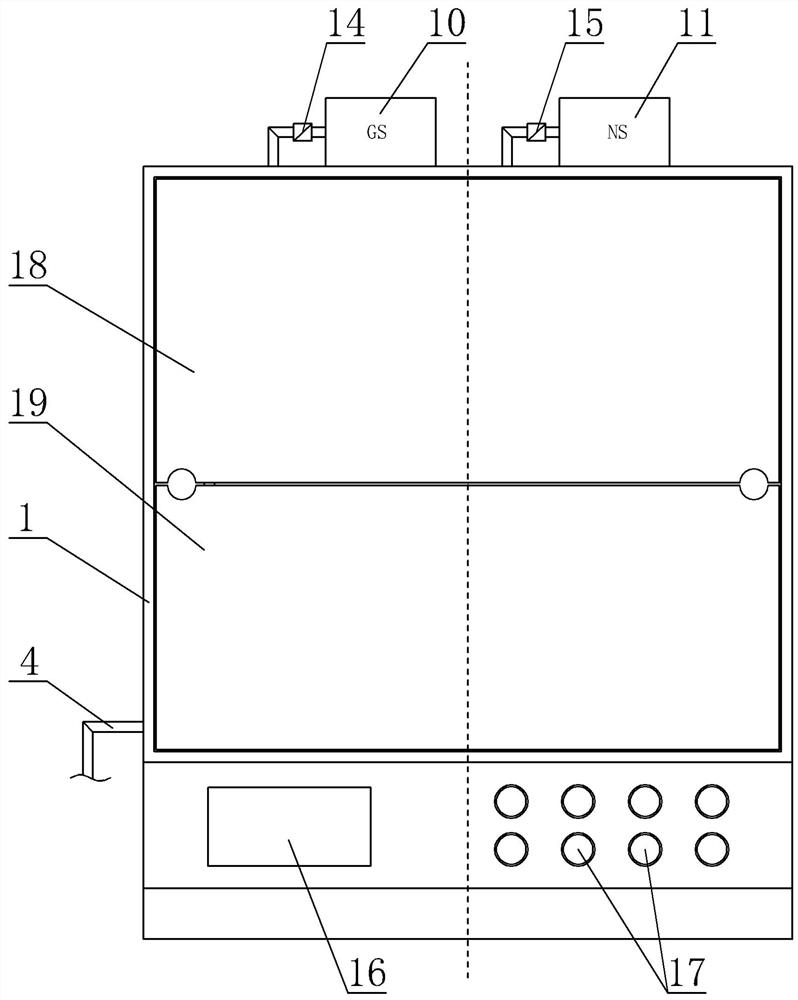
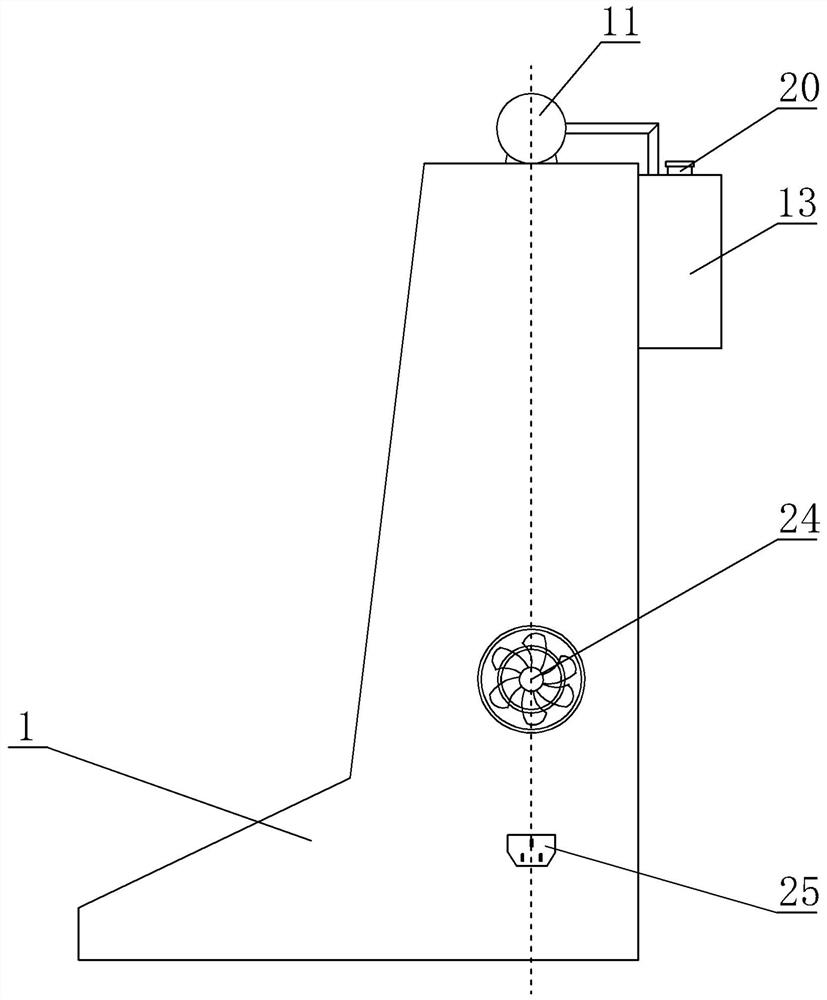
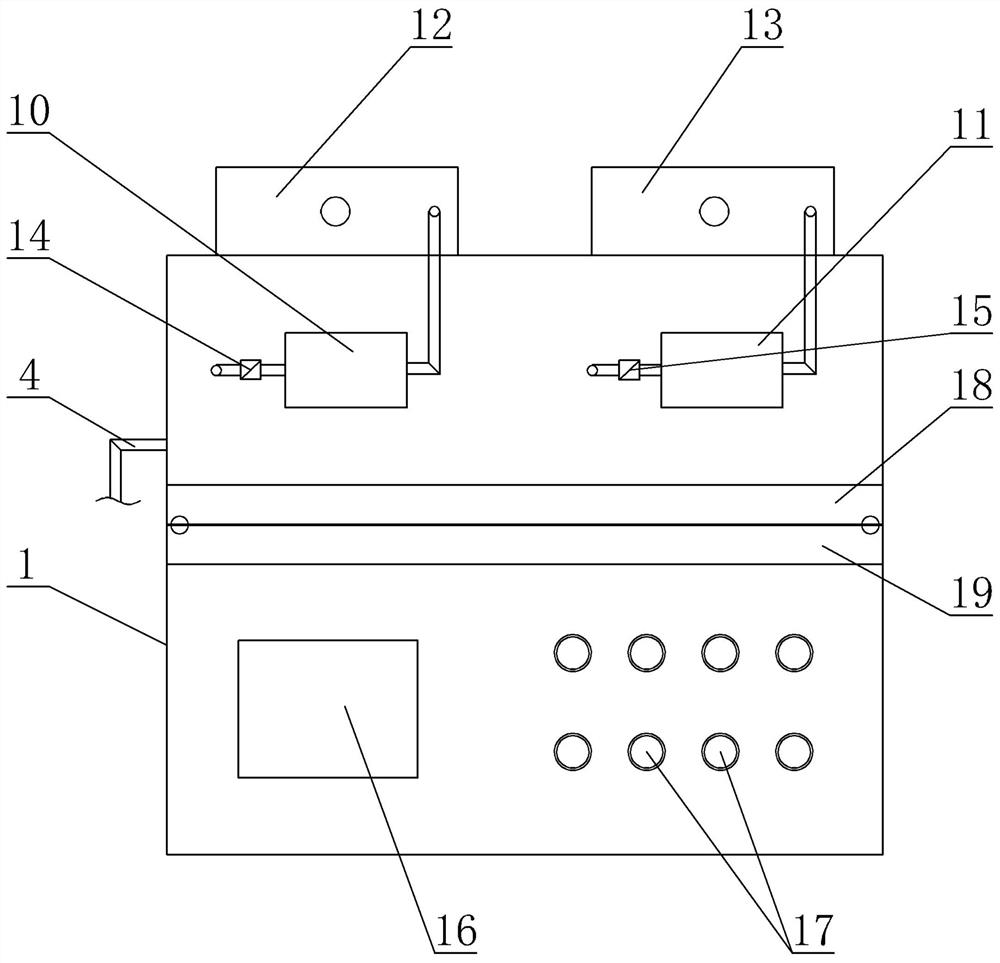
ActiveCN113975531AAvoid mixingSolve the disadvantages of not being able to equip multiple drugs at the same timeMedical devicesPressure infusionSaline flushPharmaceutical drug
A combined micro-pump control device comprises a shell, an injection main tube, a plurality of micro-injection devices, a saline flushing pump, a sweet water flushing pump and a circuit part, a micro-pump bin and a circuit bin are arranged in the shell, and each micro-injection device is composed of a micro-pump-in device, an injector, an injection branch tube and an injection electric control valve. The injectors are clamped on the micro pump-in devices, one ends of the injection branch tubes are communicated with the injection main tube, the other ends of the injection branch tubes are communicated with the liquid outlet of the injector, and liquid outlets of the saline water flushing pump and the sweet water flushing pump are communicated with the injection main tube through pipelines. According to the combined micro-pump control device, multiple times of micro injection of a patient can be completed by simultaneously preparing multiple (such as four) medicines at a time, the injection main tube can be flushed, the injection speed, pipeline flushing and the injection frequency and injection time of each medicine can be braked and controlled, and the defect that an existing micro-injection device cannot be provided with multiple kinds of medicine at the same time is overcome.
Owner:SHANDONG UNIV QILU HOSPITAL
A self-paving tree pit flushing system in saline-alkali land
InactiveCN105493675BRealize the function of self-laying pipelineReduce labor intensitySoil lifting machinesSaline flushEngineering
Owner:WEIFANG YOURONG IND
A method for controlling the volatile components of vacuum freeze-dried shallots
ActiveCN104255905BEasy to freezeEasy to storeFruits/vegetable preservation by heatingFruits/vegetable preservation using sugarsMicrowaveFreeze-drying
The invention discloses a method for controlling volatile components of shallots in vacuum freeze-drying, and belongs to the technical field of vegetable food processing. The main processing process is as follows: first select the green onion raw material, wash, remove the stalk, remove the clothes, cut, rinse, soak in microwave to kill enzymes, drain, vacuum freeze-dry, cool, and pack. The invention adopts techniques such as washing with salt water, impregnating trehalose, and inactivating enzymes with microwaves, so as not to change the quality of vacuum freeze-dried shallot products, and at the same time, it is more conducive to the preservation of allyl sulfide and volatile capsaicin.
Owner:JIANGSU NATURAL FOOD
Preparation method of medical porcine or bovine pleura biological dressing
InactiveCN102085386BWide variety of sourcesEasy to implementAbsorbent padsBandagesBiological dressingSaline flush
Owner:HBIS COMPANY LIMITED HANDAN BRANCH COMPANY
Daily health-care method capable of clearing lung and suppressing cough, and technical specification
InactiveCN107050551ANon-irritatingFlushing strength can be adjusted freelyCannulasEnemata/irrigatorsNasal cavitySaline flush
The invention relates to a daily health-care method capable of clearing lung and suppressing cough, and a technical specification, and belongs to the field of health care. A trachea flusher is adopted and consists of a flow diversion hose 1, a hose joint 2, an infusion device 3, a liquid storage box 4 and an electric heating thermostat 5, wherein the flow diversion hose 1 is connected with the hose joint 2, and the hose joint 2 is connected with the infusion device 3. The trachea flusher is characterized in that the flow division hose comprises three kinds with inner diameter and outer diameter phi (1.5*3mm, 2*4mm and 3*5mm) and with the length being 30 cm; the infusion device comprises an injector, an airbag and an electric pump; an operator stretches the flow diversion hose from a nasal cavity of a user to a trachea and performs flushing by using pure saline with the concentration of 3 percent, 2 percent and 1 percent separately according to the cough severity degree of the users, the use amount of the flushing liquid at each time is 300 to 500 ml, flushing is conducted for 3 to 5 times per day, each kind of flushing liquid is used for 3 to 7 days according to the condition, the trachea is flushed with pure water for 2 to 3 times per day after the cough of the user suffering from chronic obstructive pulmonary disease disappears, long-term use is persisted, cleanness of the trachea is maintained, and cold and cough reoccurrence can be prevented effectively.
Owner:张宗箭
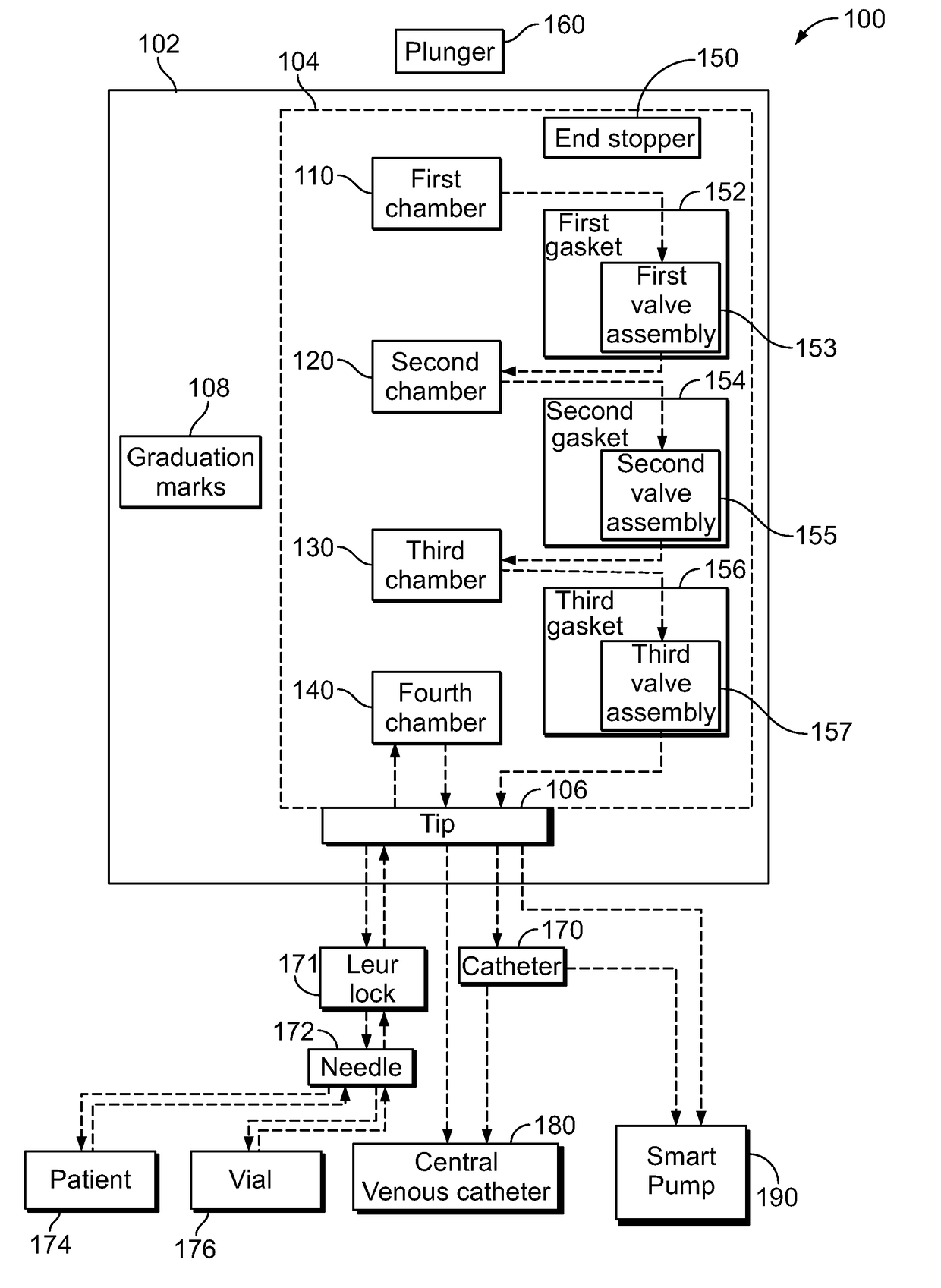
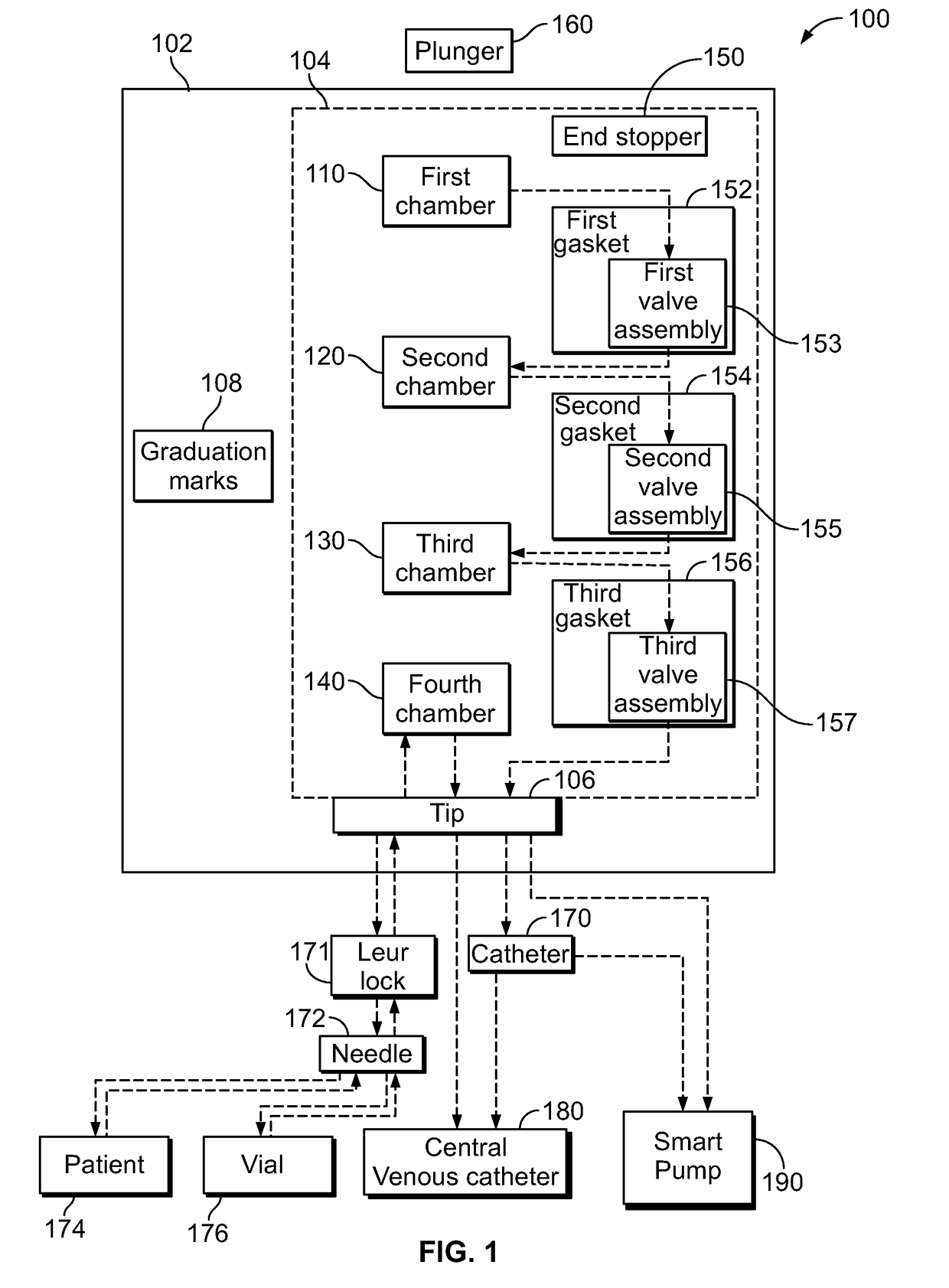
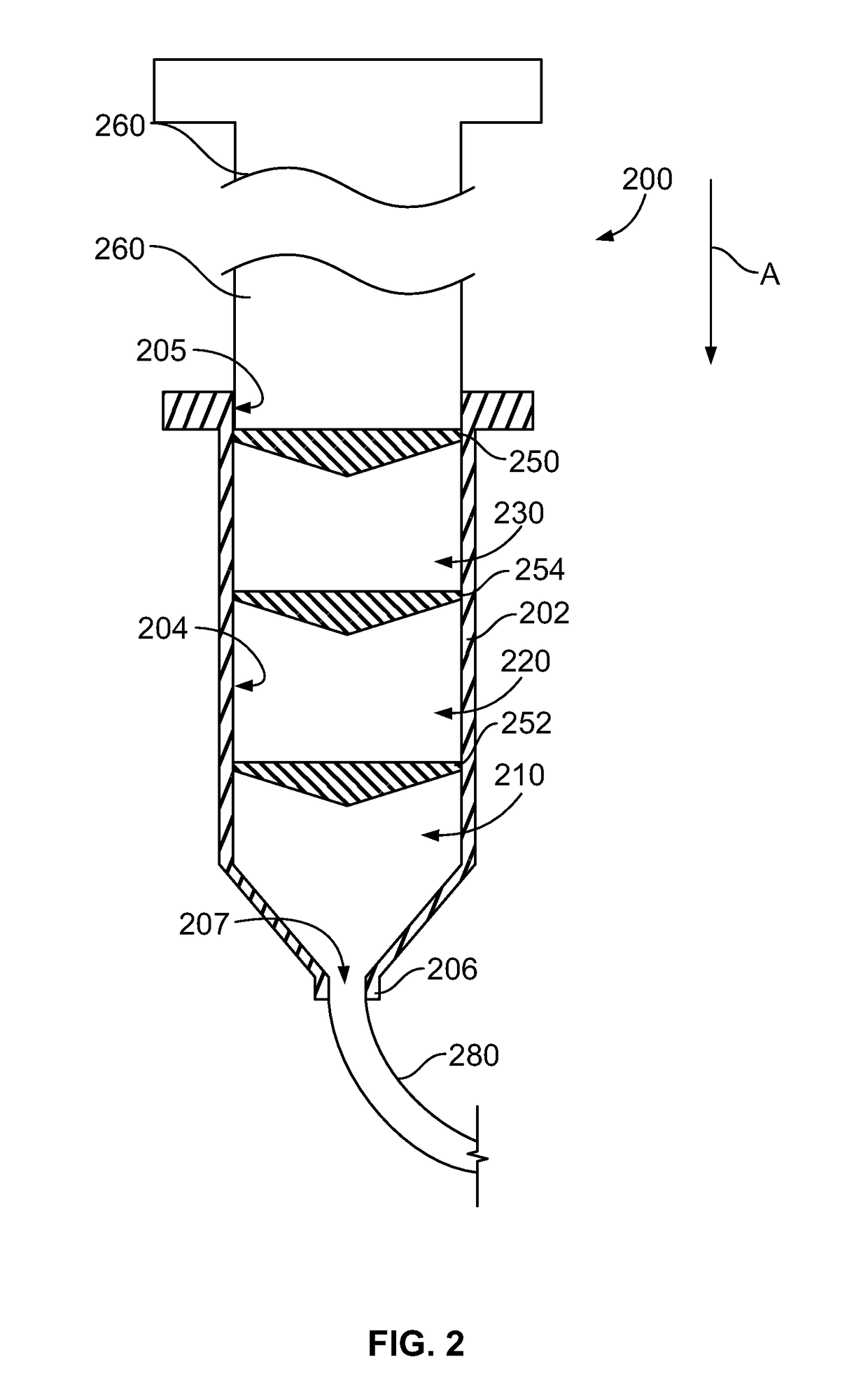
Multi-Chamber Syringes And Methods For Using The Same
A multi-chamber syringe for delivering a plurality of fluids to a central venous catheter includes a barrel and a plunger. The barrel includes a plurality of chambers, and a first one of the plurality of chambers has a predefined volume of a saline flush stored therein. Actuation of the plunger causes the predefined volume of the saline flush to be dispensed from the barrel into the central venous catheter.
Owner:ARRENDONDO OLIVIA +7
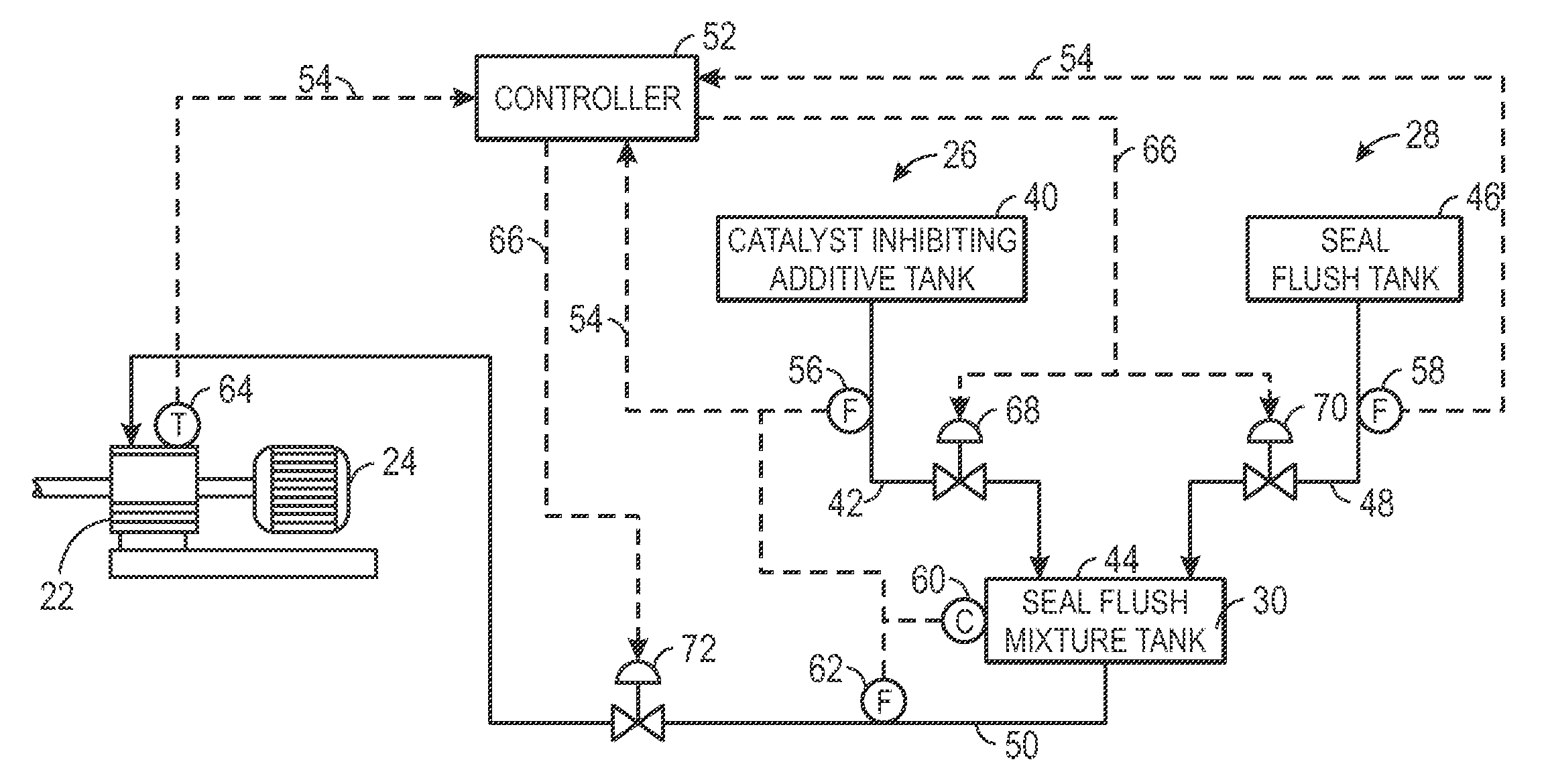
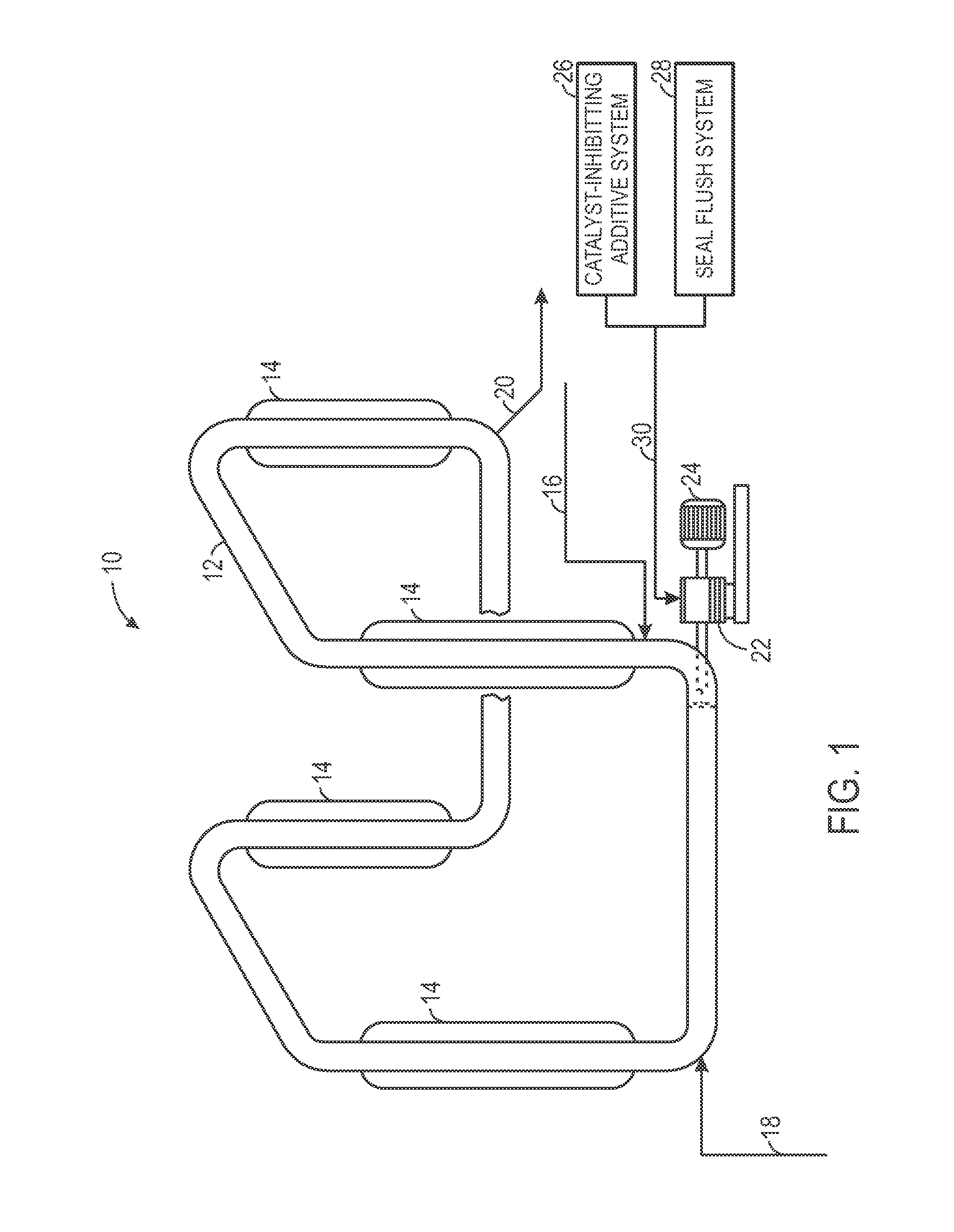
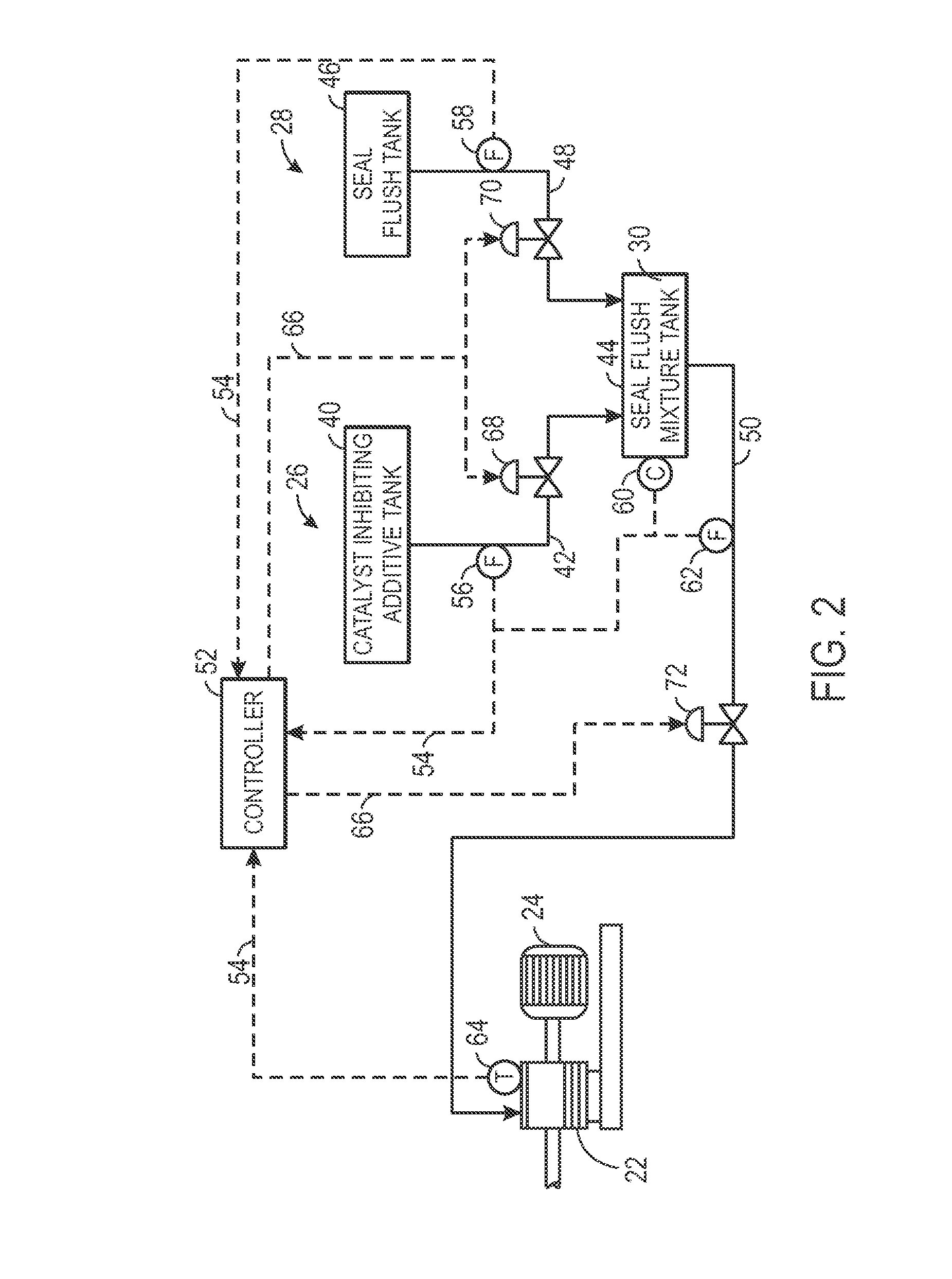
System and method for seal flush
Techniques are provided for seal flush systems. A system may include a reactor circulation pump configured to circulate a slurry through a polymerization reactor. The slurry may include an olefin monomer, a catalyst, and a diluent. The system may also include a catalyst-inhibiting additive system configured to supply a catalyst-inhibiting additive to a seal of the reactor circulation pump and a seal flush system configured to generate a seal flush mixture and supply the seal flush mixture to the seal of the reactor circulation pump.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com