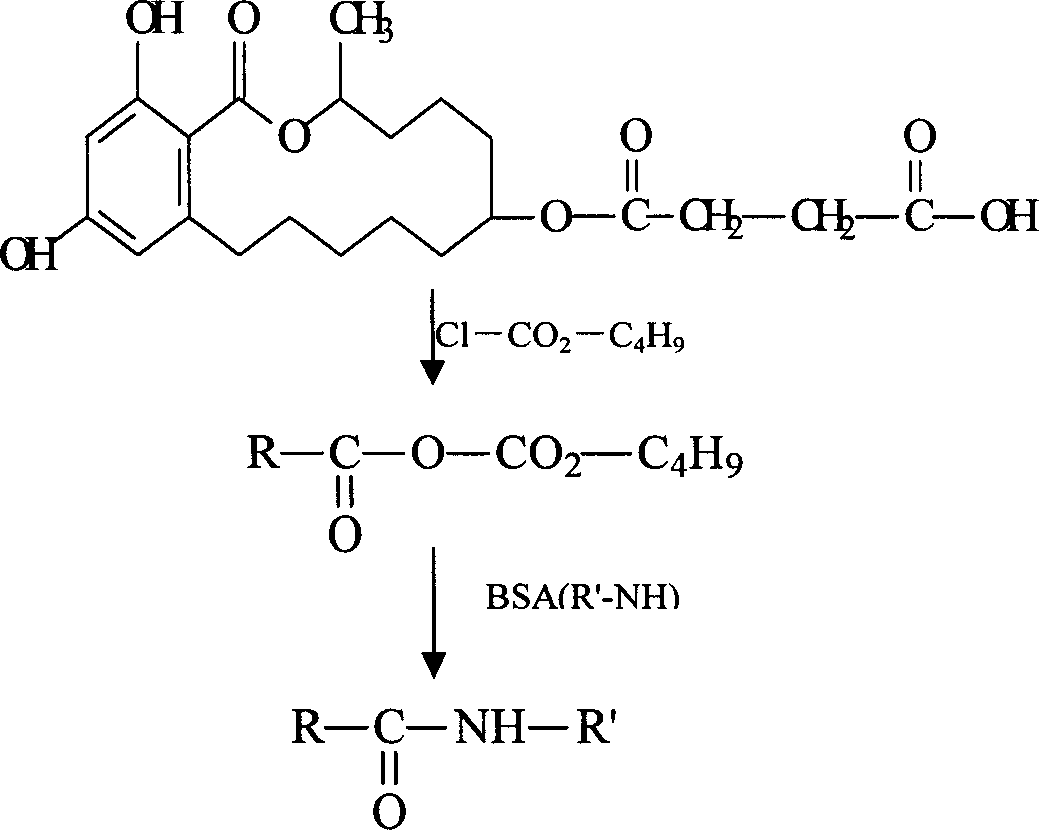
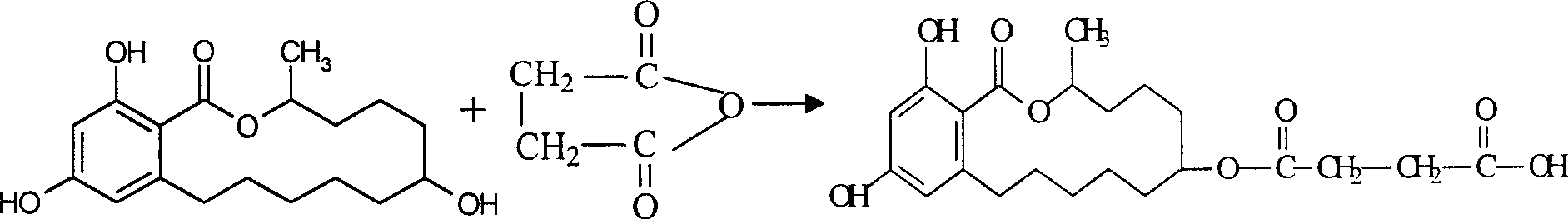
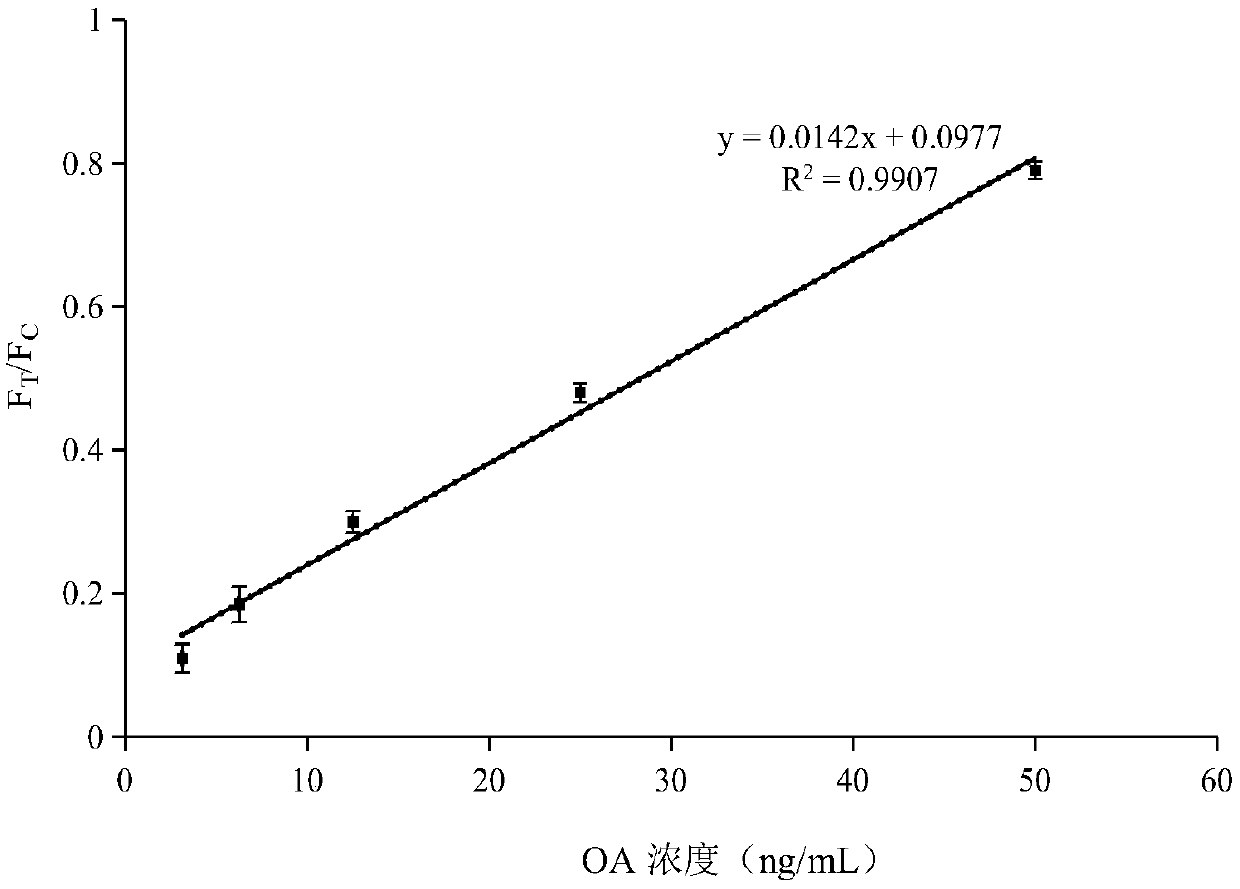
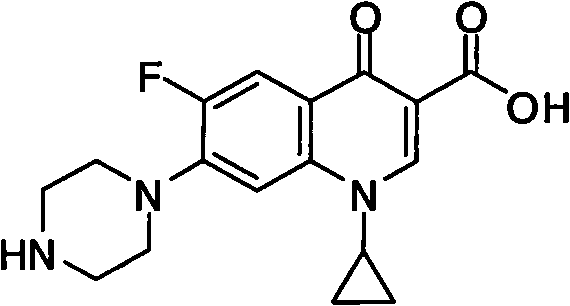
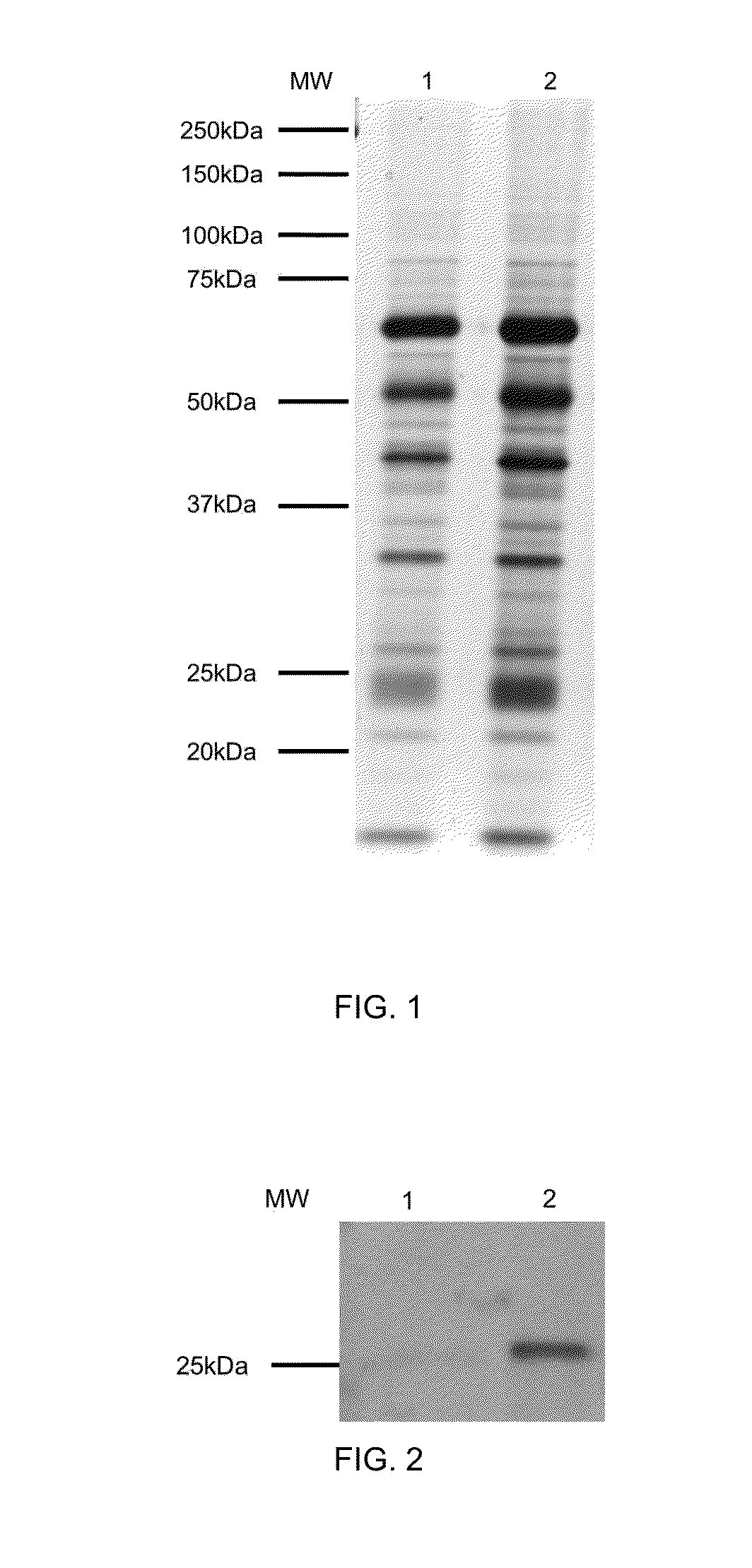
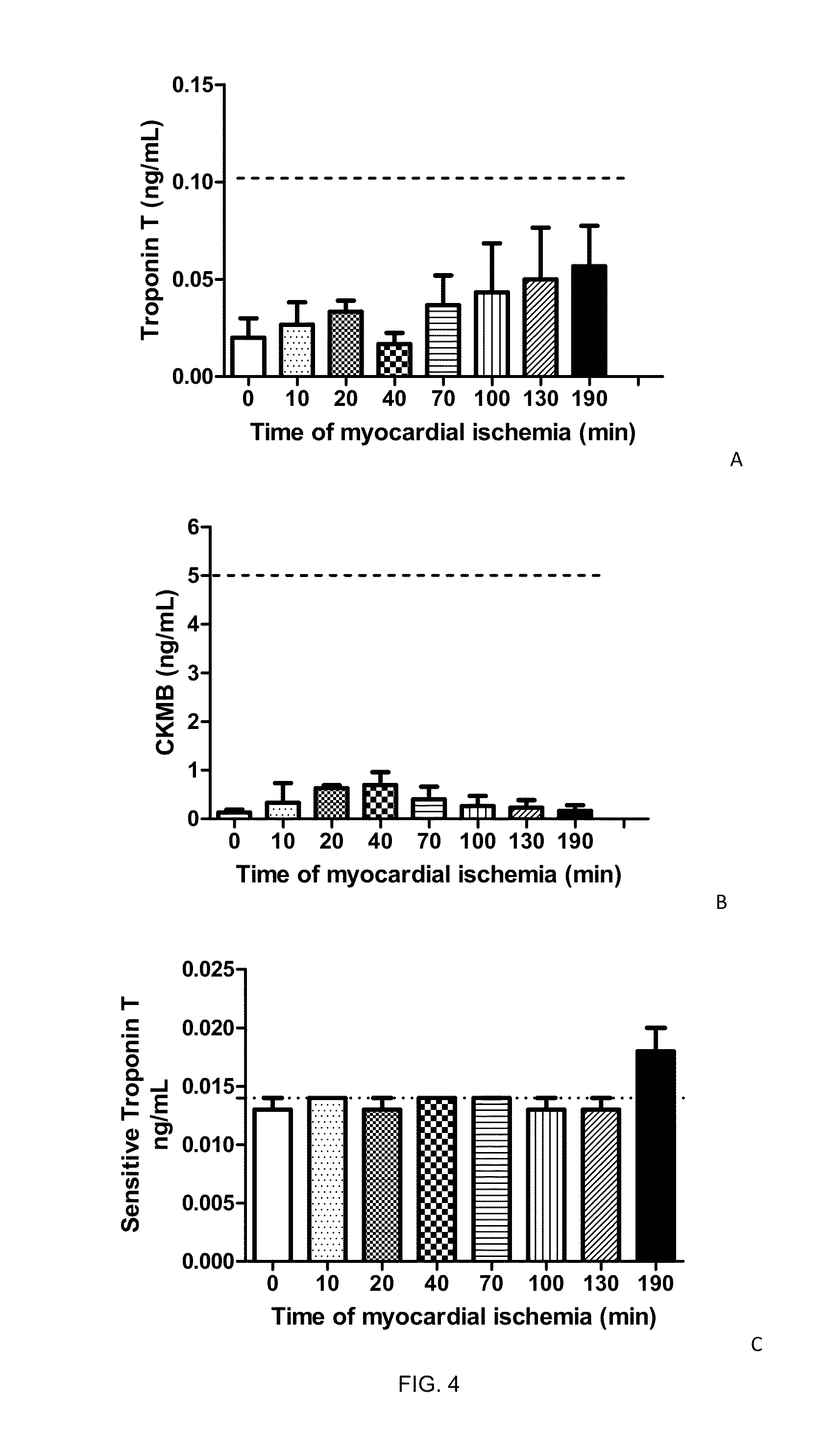
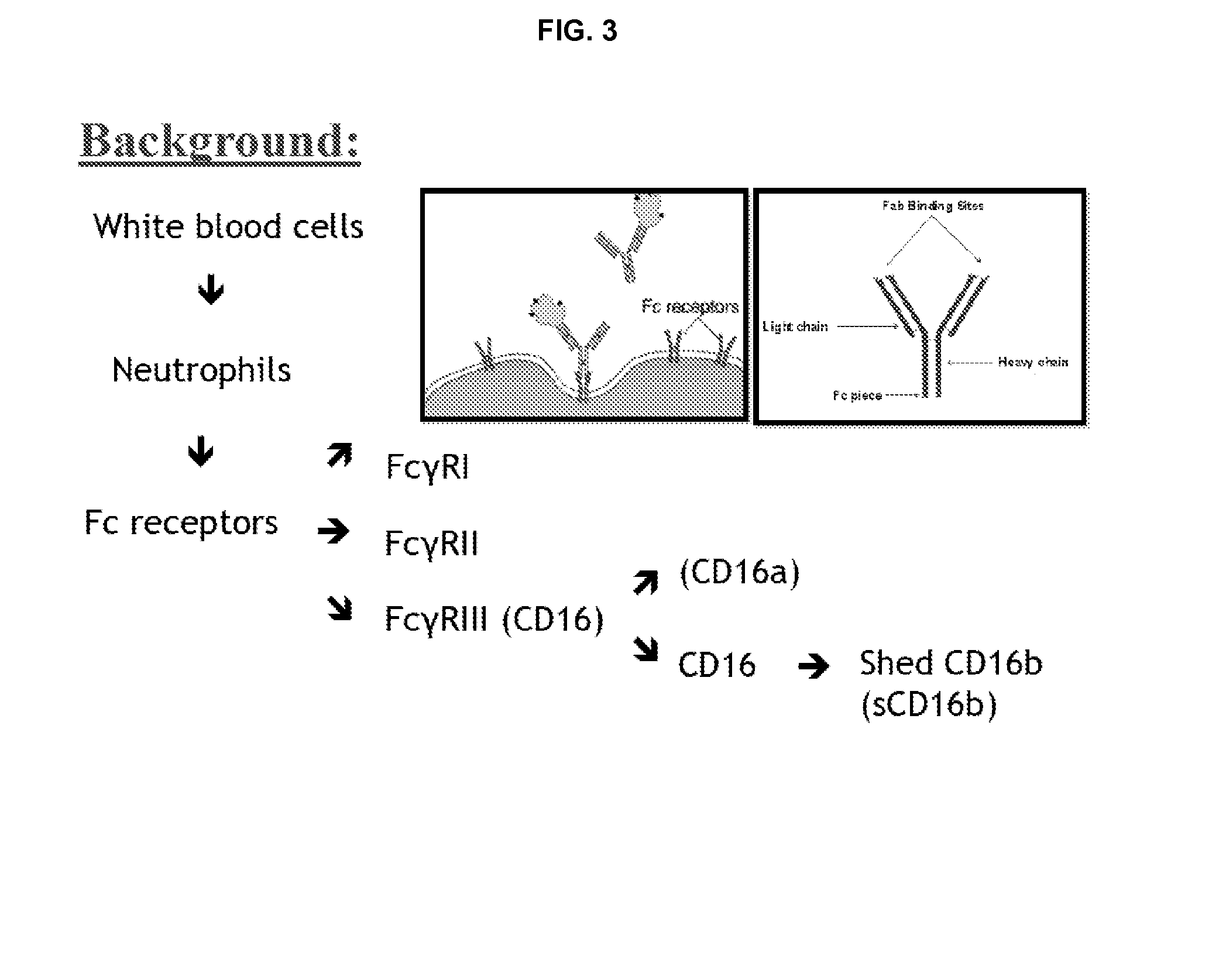
Patents
Literature
54 results about "Immunoassay technique" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of immunoassay : a technique or test used to detect the presence or quantity of a substance (such as a protein) based on its capacity to act as an antigen
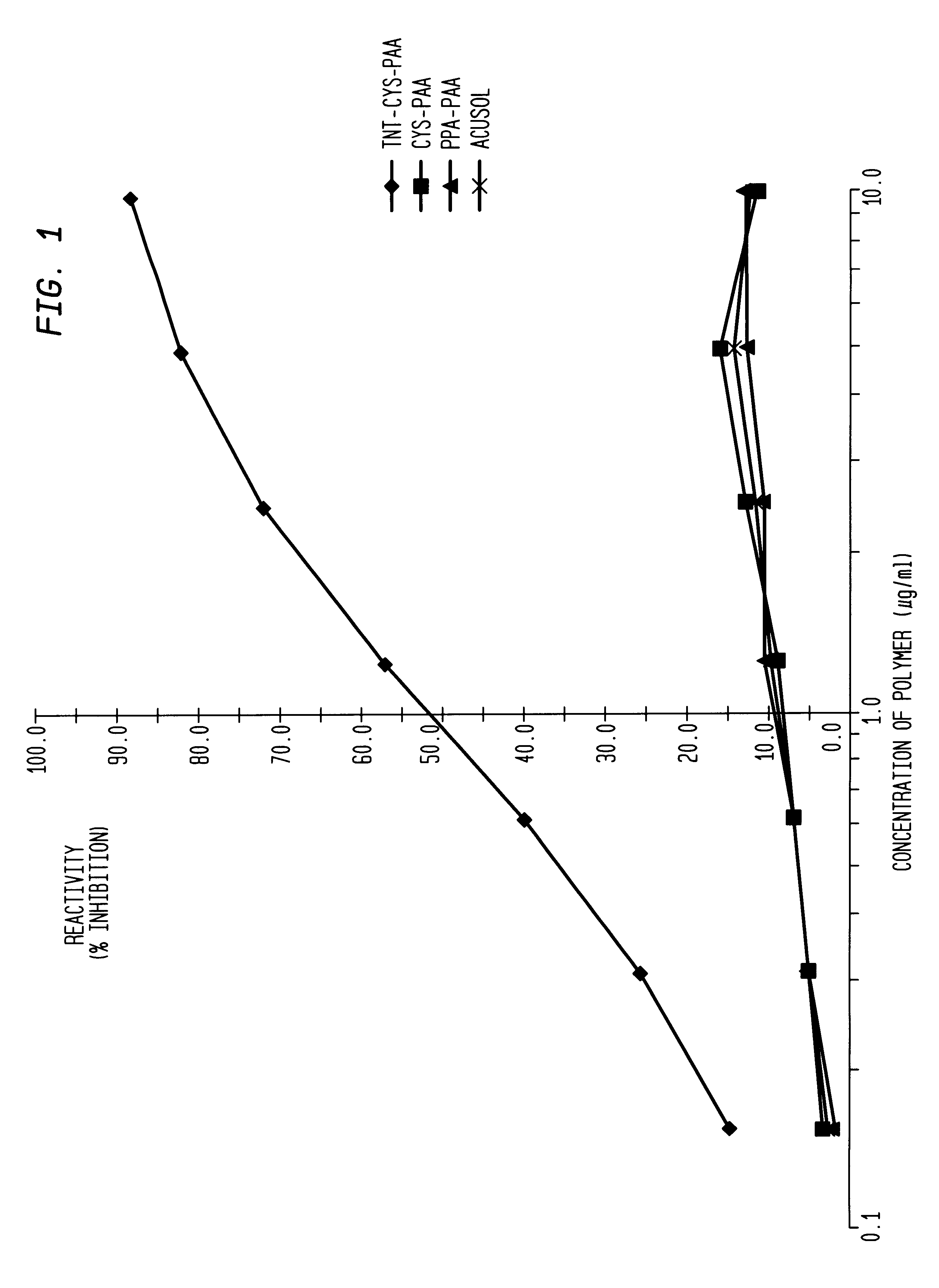
Method for identifying and quantifying polymers utilizing immunoassay techniques
Disclosed is a method for quantitatively identifying polymers in an aqueous system using immunoassay techniques, wherein at least a portion of the polymers contain a detectable terminus. This is particularly useful in water treatment systems. Also disclosed are new hybridoma cell lines which express MAbs which specifically recognize such a detectable terminus.
Owner:ROHM & HAAS CO
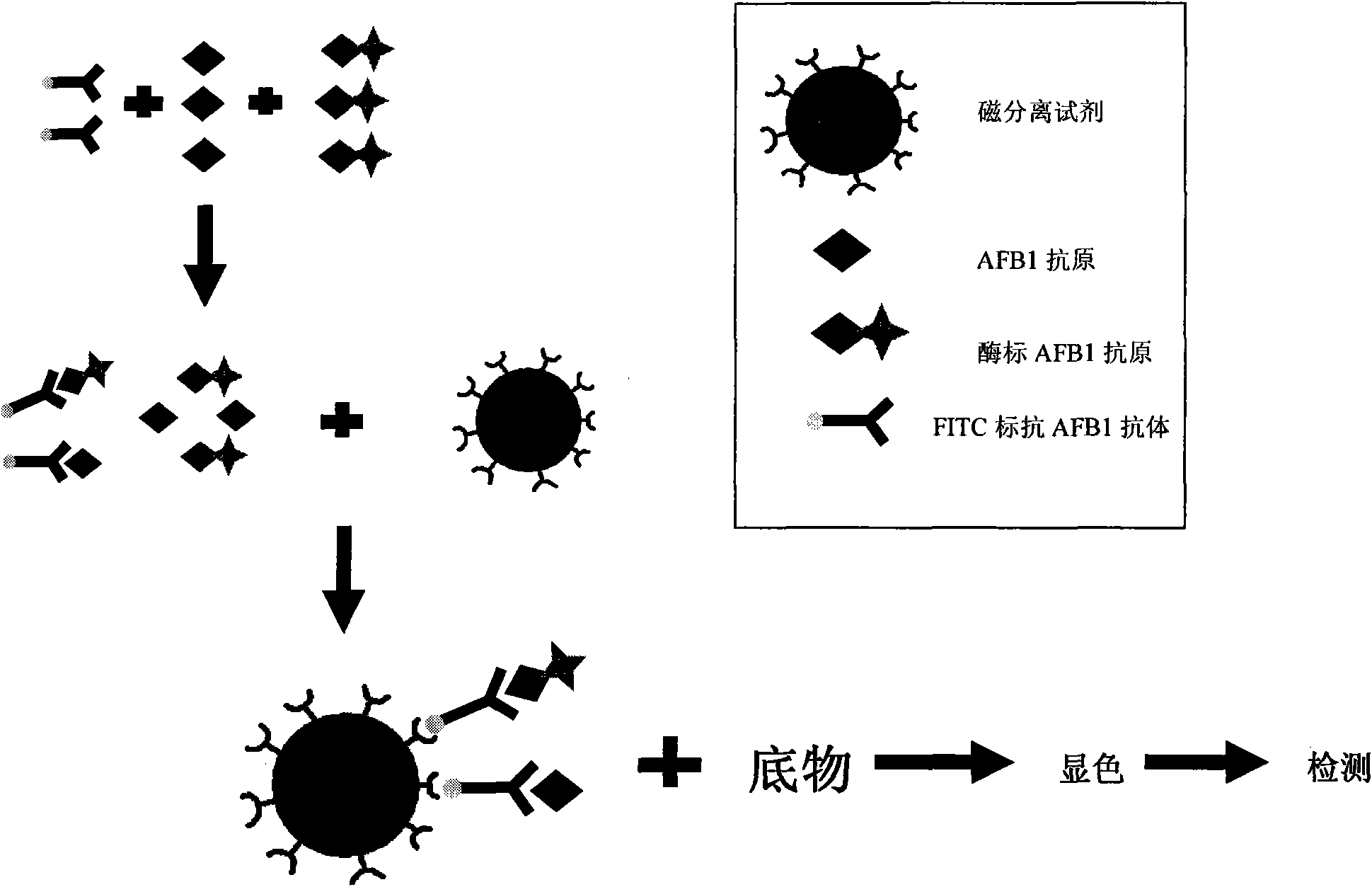
Aflatoxin B1 magnetic particle separation enzyme-linked immunoassay
InactiveCN101661036ASimple processing methodHigh detection sensitivityColor/spectral properties measurementsFluorescence/phosphorescenceSorbentFluorescein isothiocyanate
The invention provides an aflatoxin B1 (AFB1) magnetic separation enzyme-linked immunity quantitative detection method, belonging to the field of food safety immunoassay technique. The method adopts the immuno-detection principle of competition law; and AFB1 is connected with biological enzyme to prepare enzyme-labeled antigen reagent, anti-fluorescein isothiocyanate (FITC) antibody is absorbed onthe surfaces of magnetic particles to prepare magnetic separation reagent, and the FITC is connected with the AFB1 antibody to prepare anti-reagent. In a sample, the AFB1 competes with the enzyme-labeled AFB1 and is combined with a small amount of FITC-labeled anti-AFB1 antibody, so that antigen-antibody complex can be formed. After the magnetic separation reagent is added, the complex is caughtonto the surfaces of the magnetic particles by the anti-FITC antibody connected on the surfaces of the magnetic particles. After being washed, the product is finally added with substrate and detected.The method has the advantages that (1) the magnetic particles are used for replacing the traditional enzyme-labeled plate to be taken as a solid-phase carrier, so that immunoreaction is carried out under the approximate liquid phase condition; and the reaction is more complete and rapid, and has the characteristics of high specificity and good repeatability compared with the traditional enzyme-linked immuno sorbent assay (ELISA); furthermore, (2) by adopting one-step competition law principle, the time used for detection is short.
Owner:北京倍爱康生物技术有限公司
Fluorescent latex granular immune chromatography by time resolution
InactiveCN1818653AFast flowResolving directly coated antibodiesFluorescence/phosphorescenceAntigenFluorescence
A microparticle immune chromatography of time-resolution fluorescence emulsion includes preparing biotinylation antibody and preparing immune time-resolution fluorescence microparticles, using Fusion 5 film to prepare avidin solid phase film detection line and quality control line by utilizing avidin to envelop said film, finally using double-antibody sandwich method to detect antigen quickly.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Immunoassay system and immunoassay method
InactiveUS20050202572A1InterferenceAvoid interferenceMicrobiological testing/measurementMaterial analysis by optical meansMagnetizationAntigen-antibody reactions
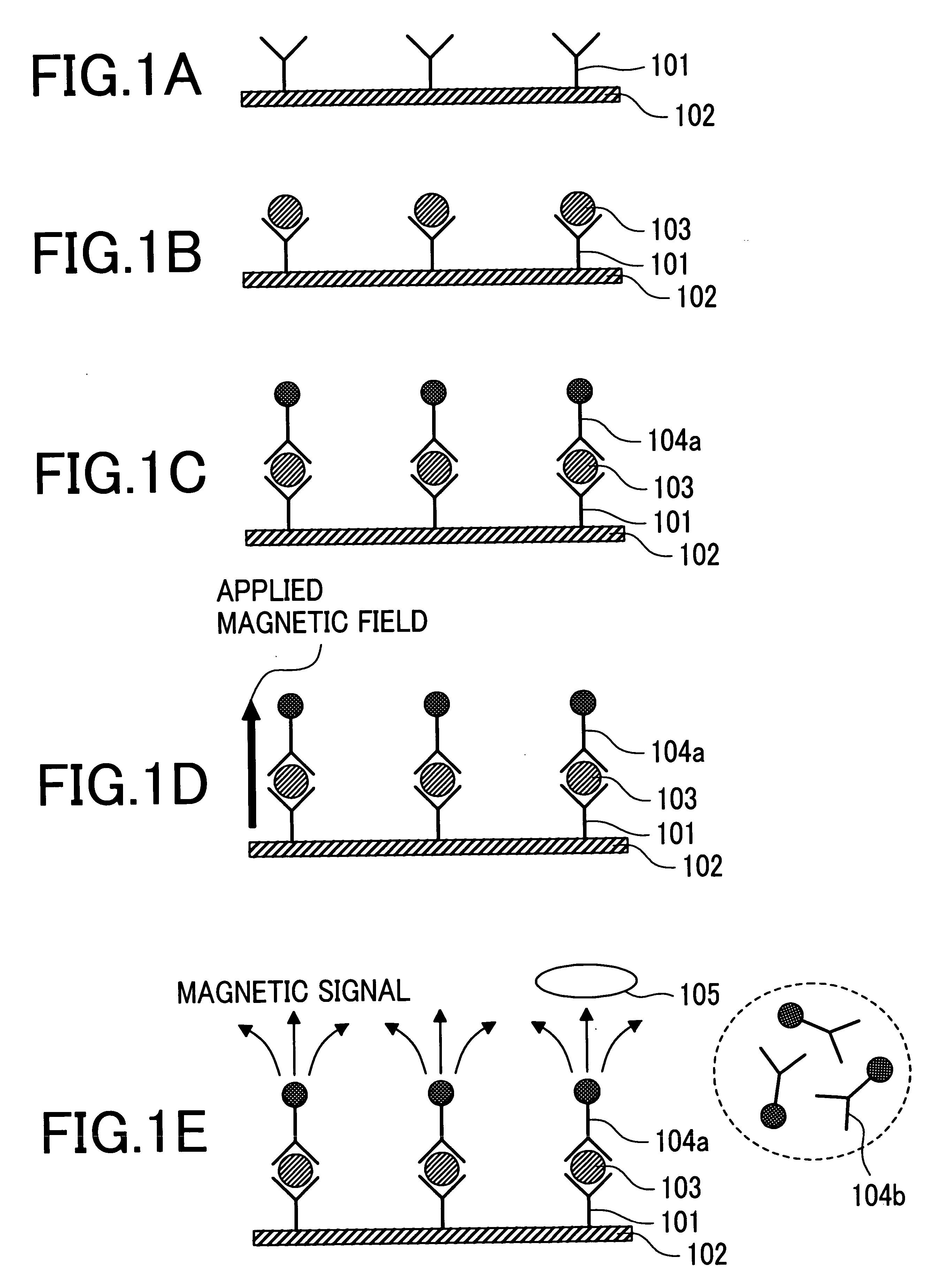
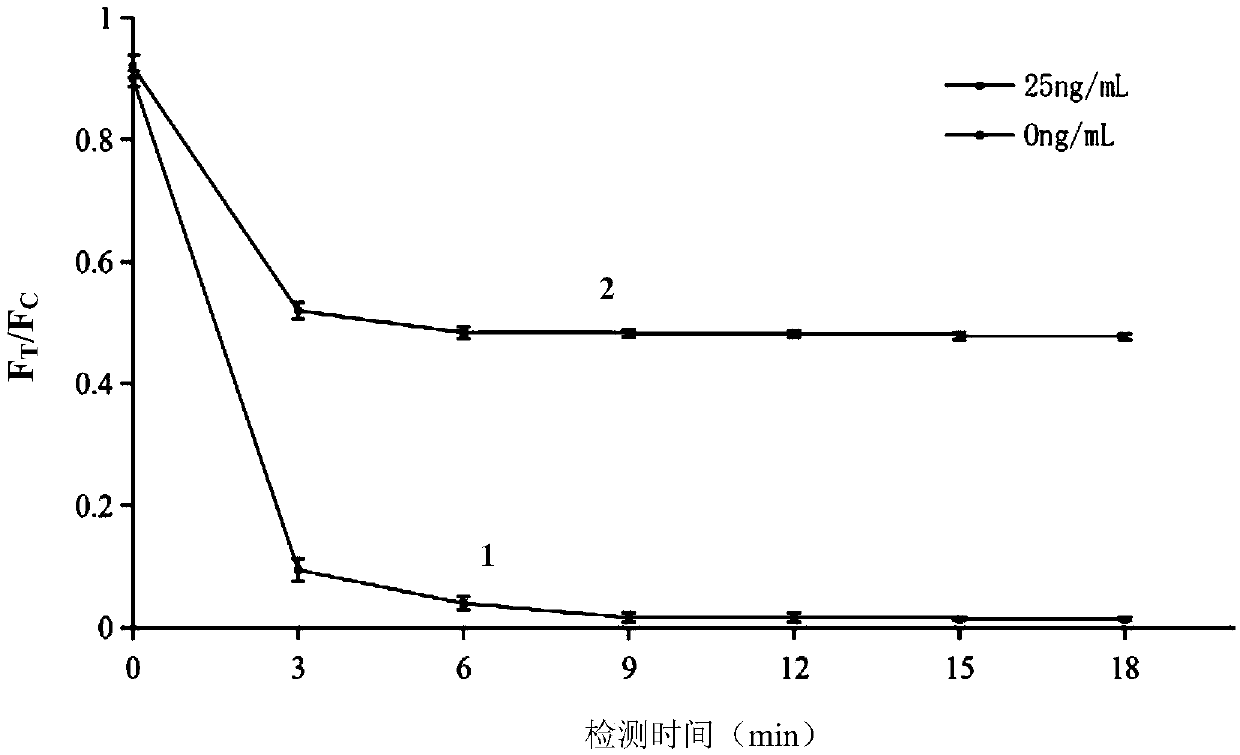
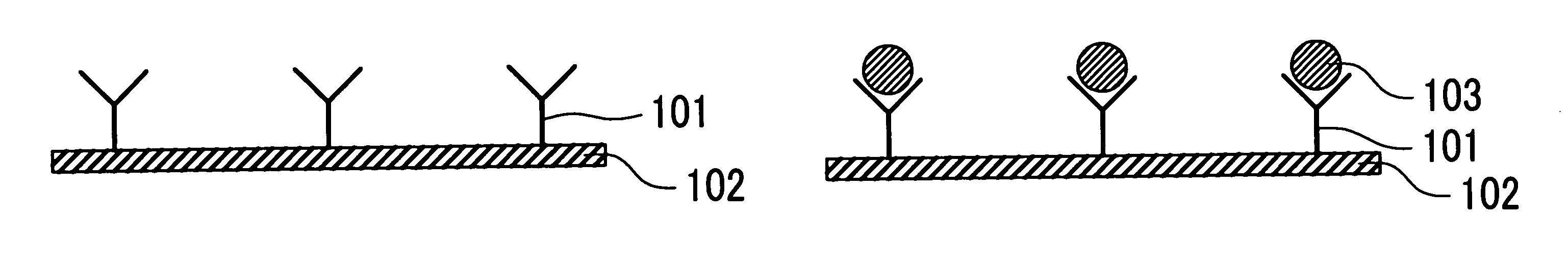
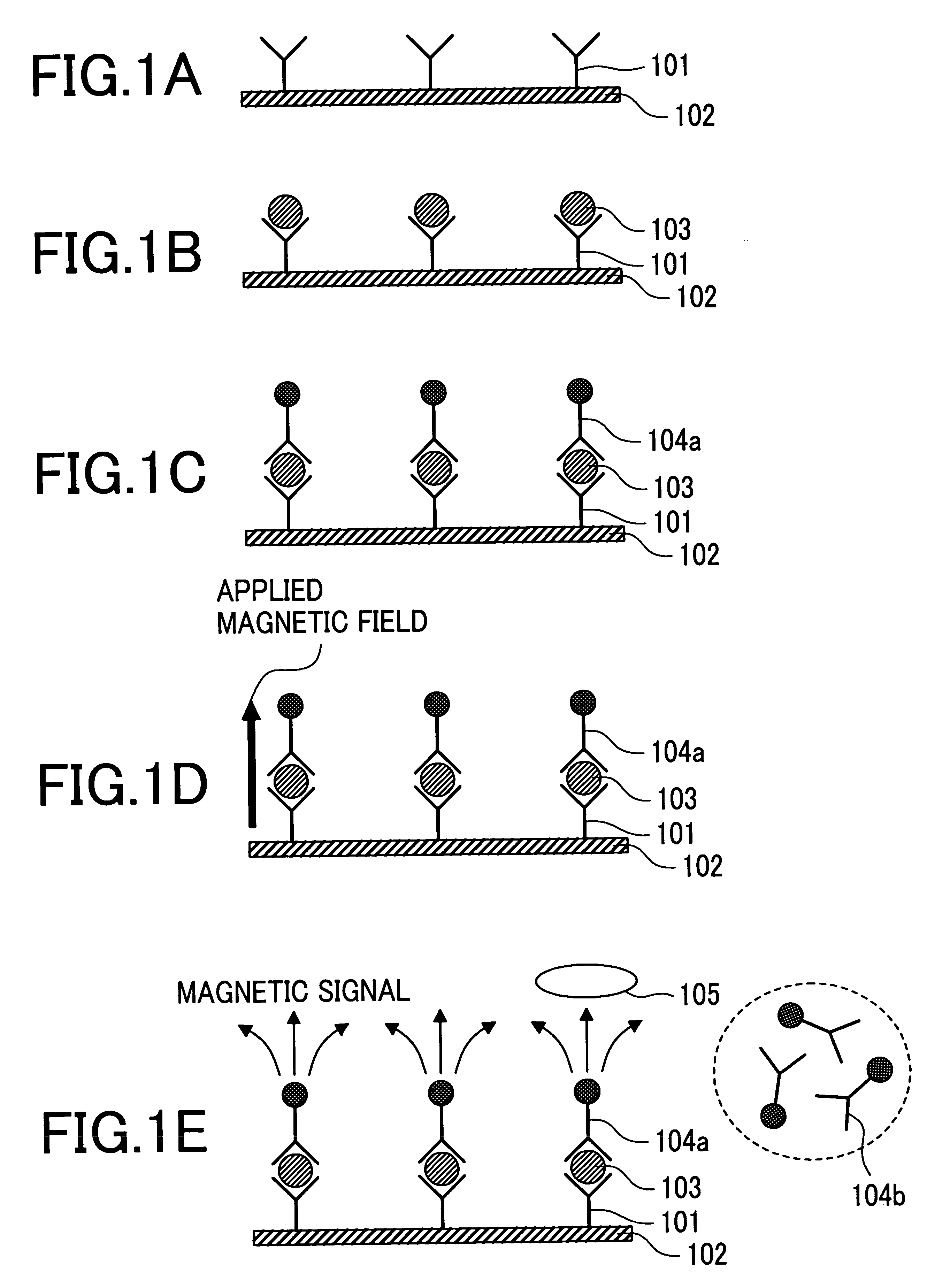
The present invention provides an immunoassay technique which enables efficient detection of antigen-antibody reaction with high sensitivity by a magnetic method using magnetic particles and a SQUID magnetic sensor or sensors. A system based on the technique includes a disk-shaped sample holder which holds on a circle a plurality of sample containers for accommodating marked samples, resulting from marking of samples with magnetic particles by antigen-antibody reaction; rotating means for rotating the holder around its central shaft; magnetizing means for magnetizing the marked samples outside a magnetic shield; and a magnetic sensor for detecting, within the magnetic shield, magnetic fields generated from the marked samples which have been magnetized. By rotation of the holder, areas fixing and holding different ones of the sample containers are successively inserted into the magnetic shield, and the magnetization of the marked samples accommodated in first ones of the sample containers and the detection of magnetic fields generated from the marked samples accommodated in second ones of the sample containers are executed in parallel.
Owner:HITACHI LTD
Method and apparatus for the detection of pathogens, parasites, and toxins
InactiveUS7931788B1Fast and sensitive and inexpensive and portable immunoassayFaster and cheap methodImmobilised enzymesBioreactor/fermenter combinationsPathogenic microorganismConductive materials
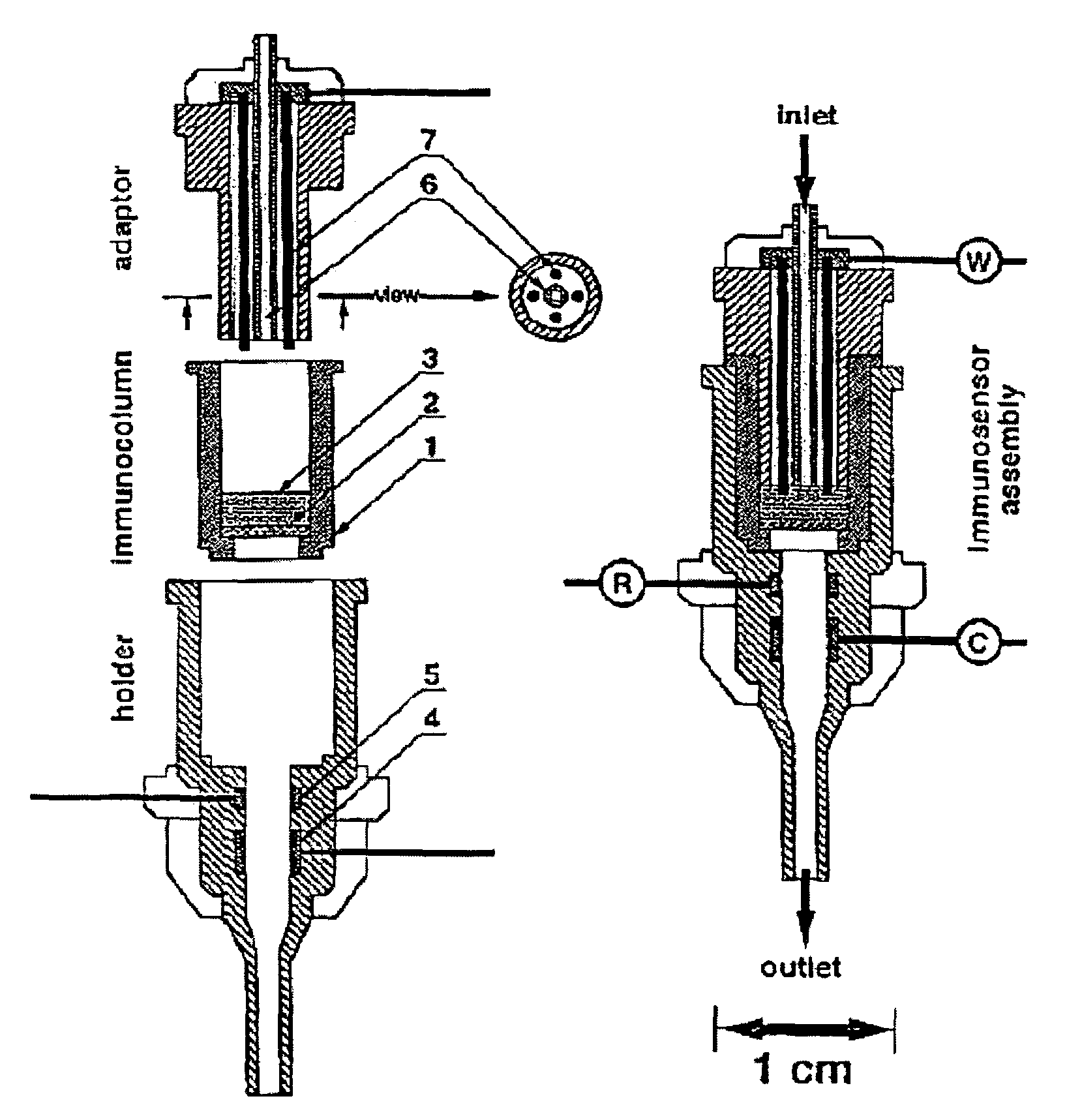
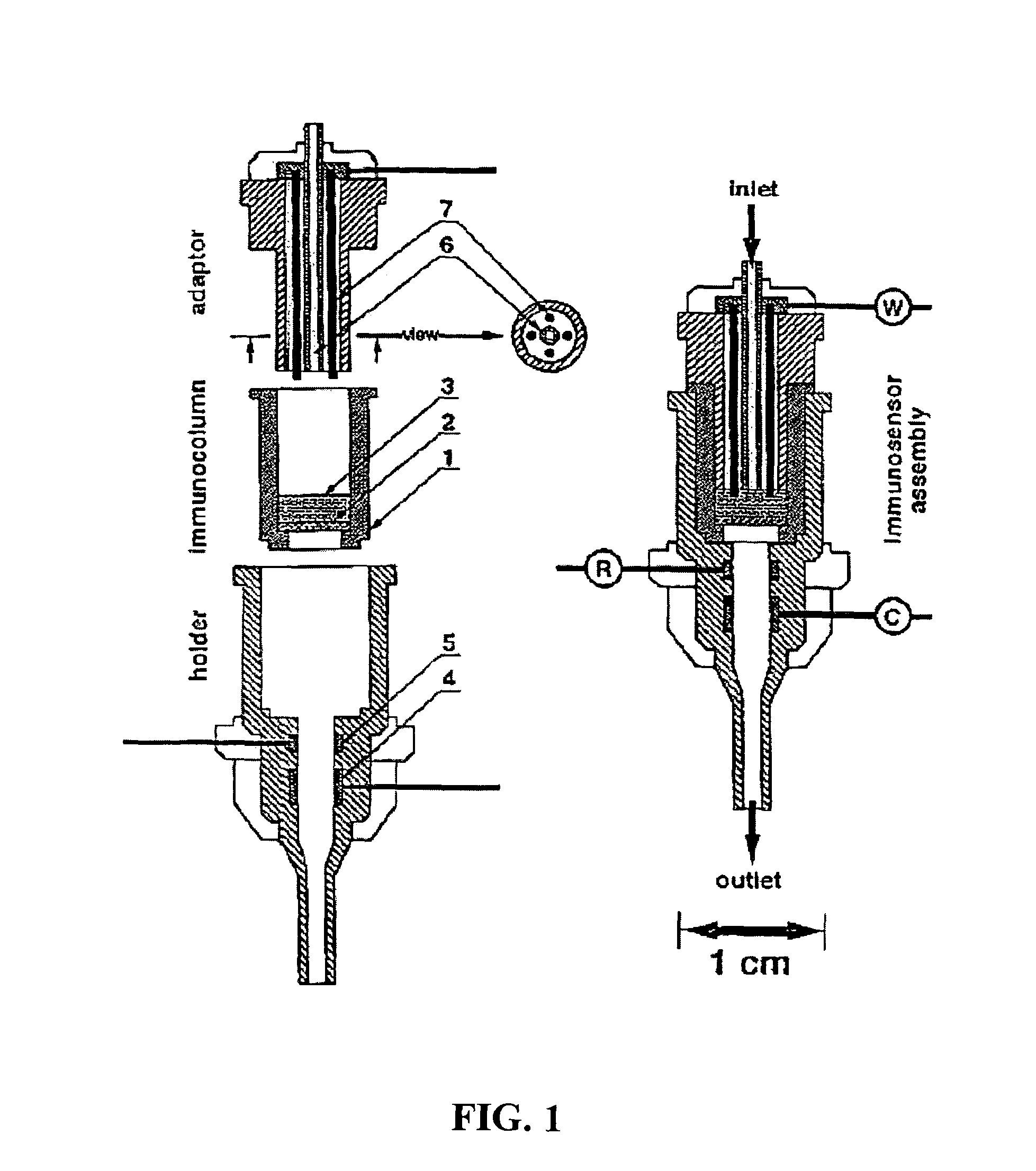
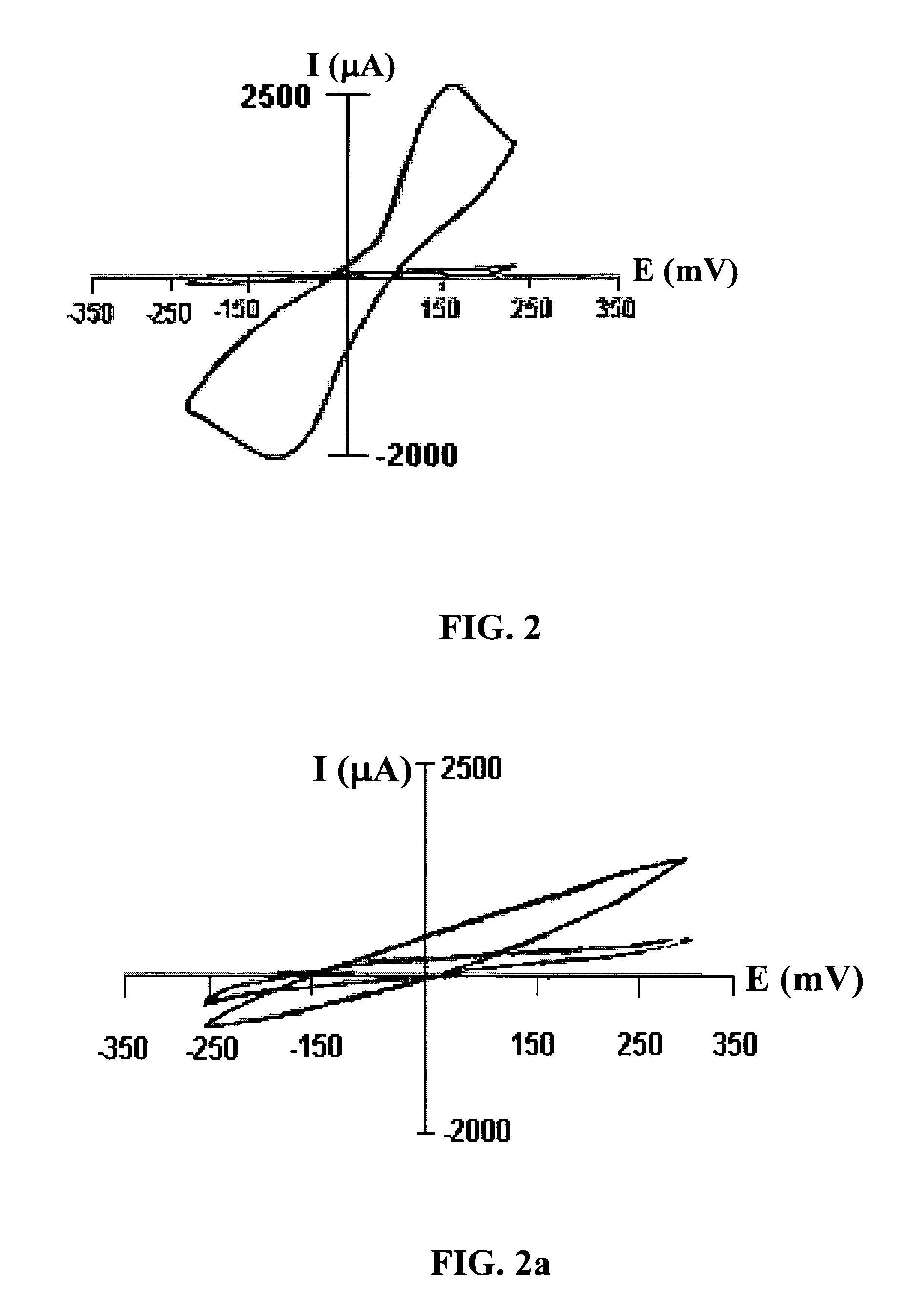
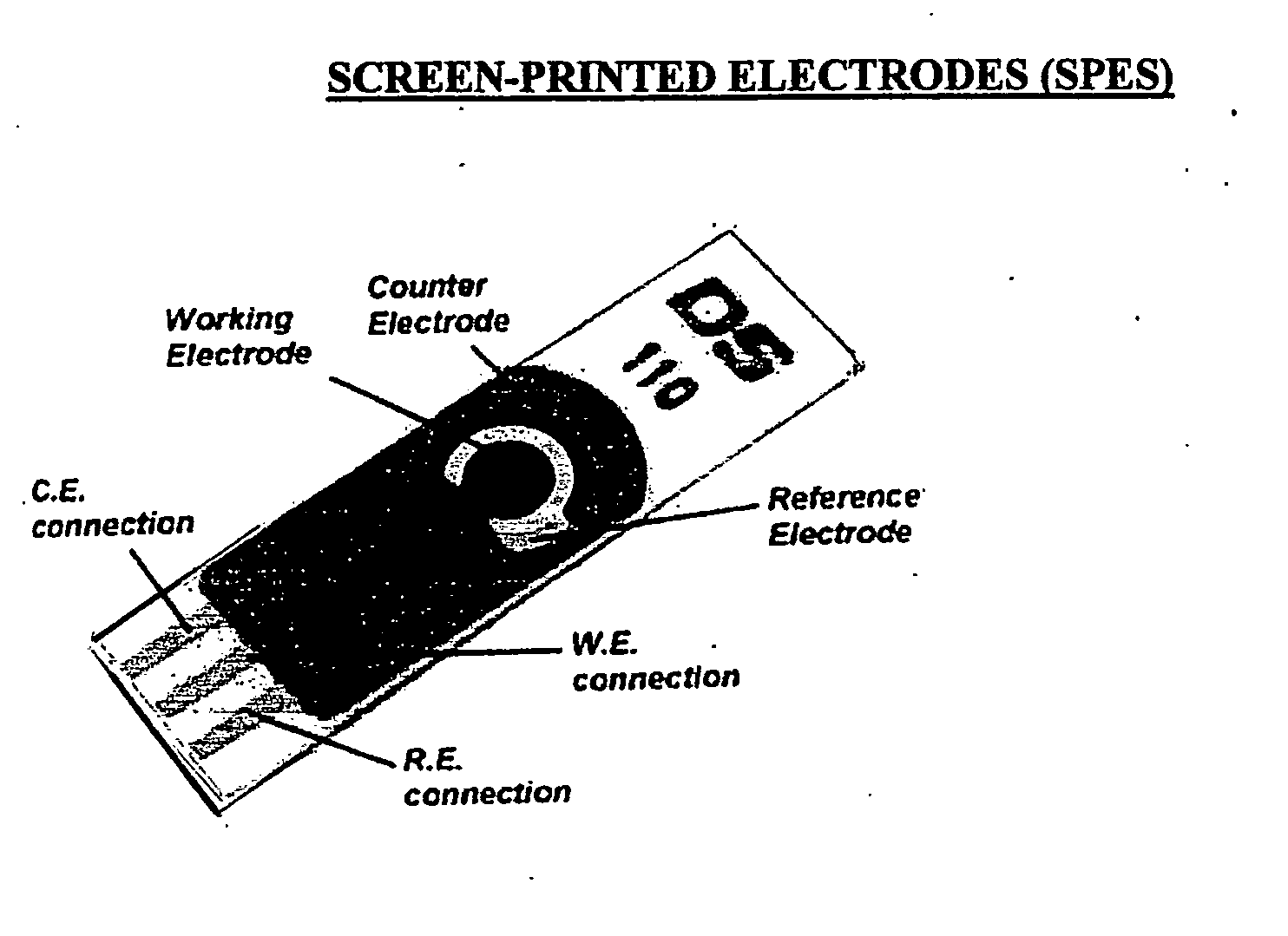
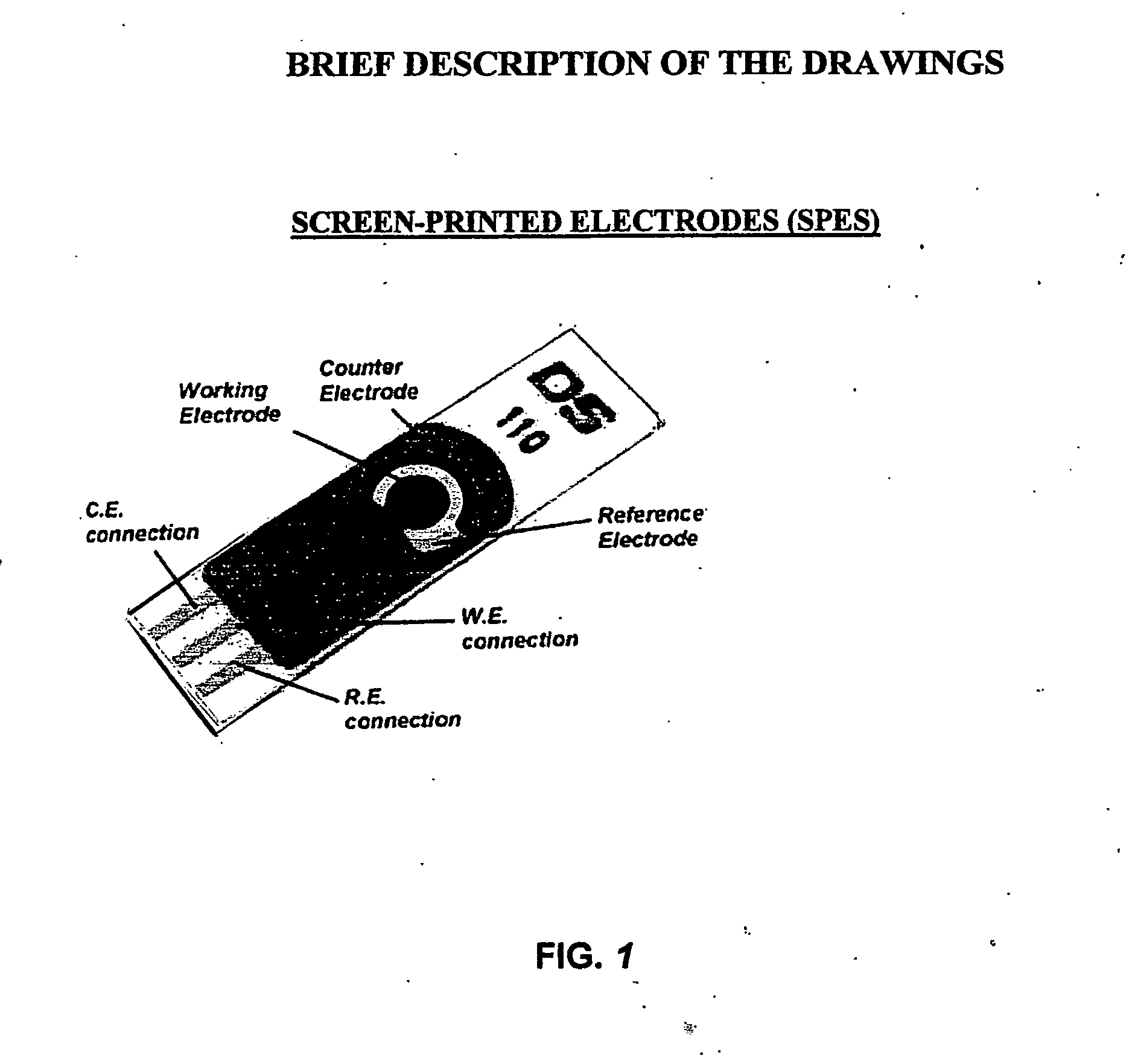
The present invention is directed to a method and apparatus for an immunoassay technique that uses amperometric measurements to rapidly analyze different pathogenic microorganisms, including bacteria, viruses, toxins, and parasites. In accordance with one aspect of the present invention, at least one conductive membrane is used to provide support for antibody immobilization and serve as a working electrode; it could also be independent rather than the working electrode. This conductive membrane or powder can be fabricated of a conductive material or can be a nonconductive material over which a conductive material is coated. In either case, the proposed technique is adaptable for use with different materials so as to form a membrane having a pore size that is suited to the particular application. Another aspect of the present invention relates to a compact and simple disposable element that can be easily disposed of after use. In still another aspect of the present invention, the immunoassay can be automated using microprocessor control so as to reduce the amount of human intervention in sample analysis.
Owner:WILKINS EBTISAM S
Method of purifying abamectin kind medicine and its immune affinity chromatographic column
InactiveCN1830547AHigh selectivityEasy to handleComponent separationOther chemical processesAbamectinMonoclonal antibody
A method for purifying abamectin medicines by the special immunoaffinity chromatographic column carrying immunoaffinity adsorbent is disclosed. Said adsorbent is composed of solid carrier and its coupled abamectin polyclonal or monoclonal antibody. It features that the chromatography is used to measure the contents of abamectin, having high correctness.
Owner:CHINA AGRI UNIV
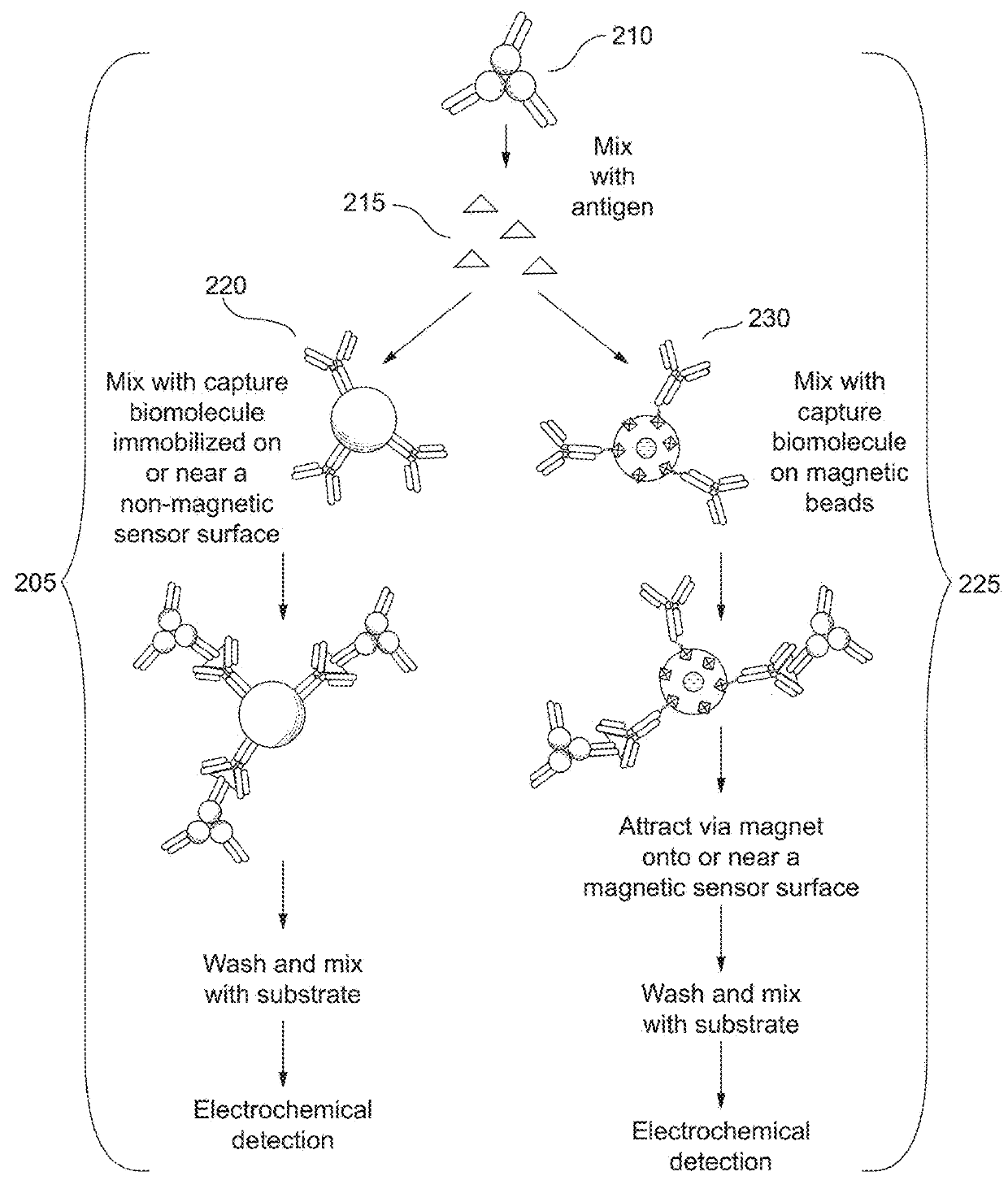
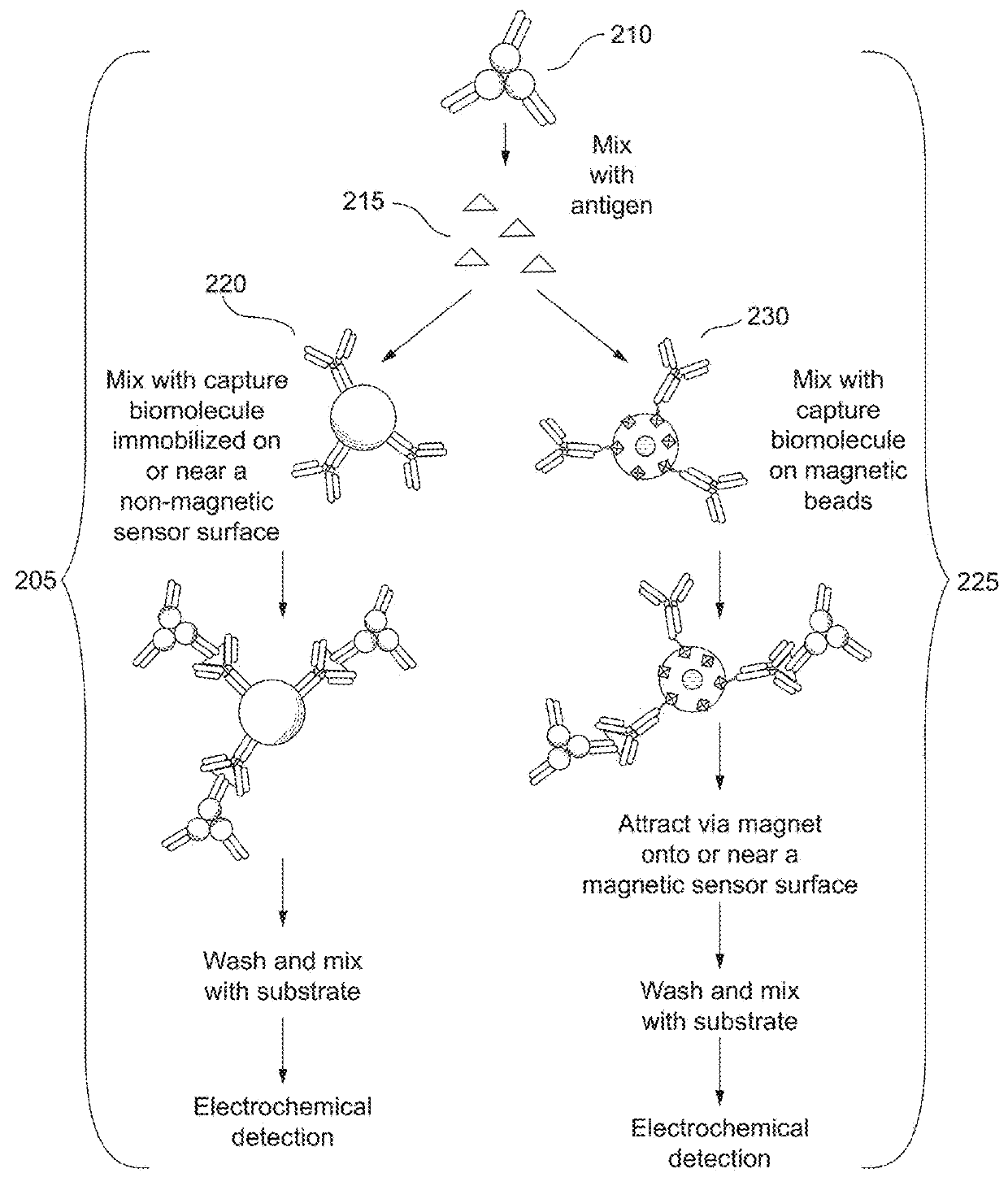
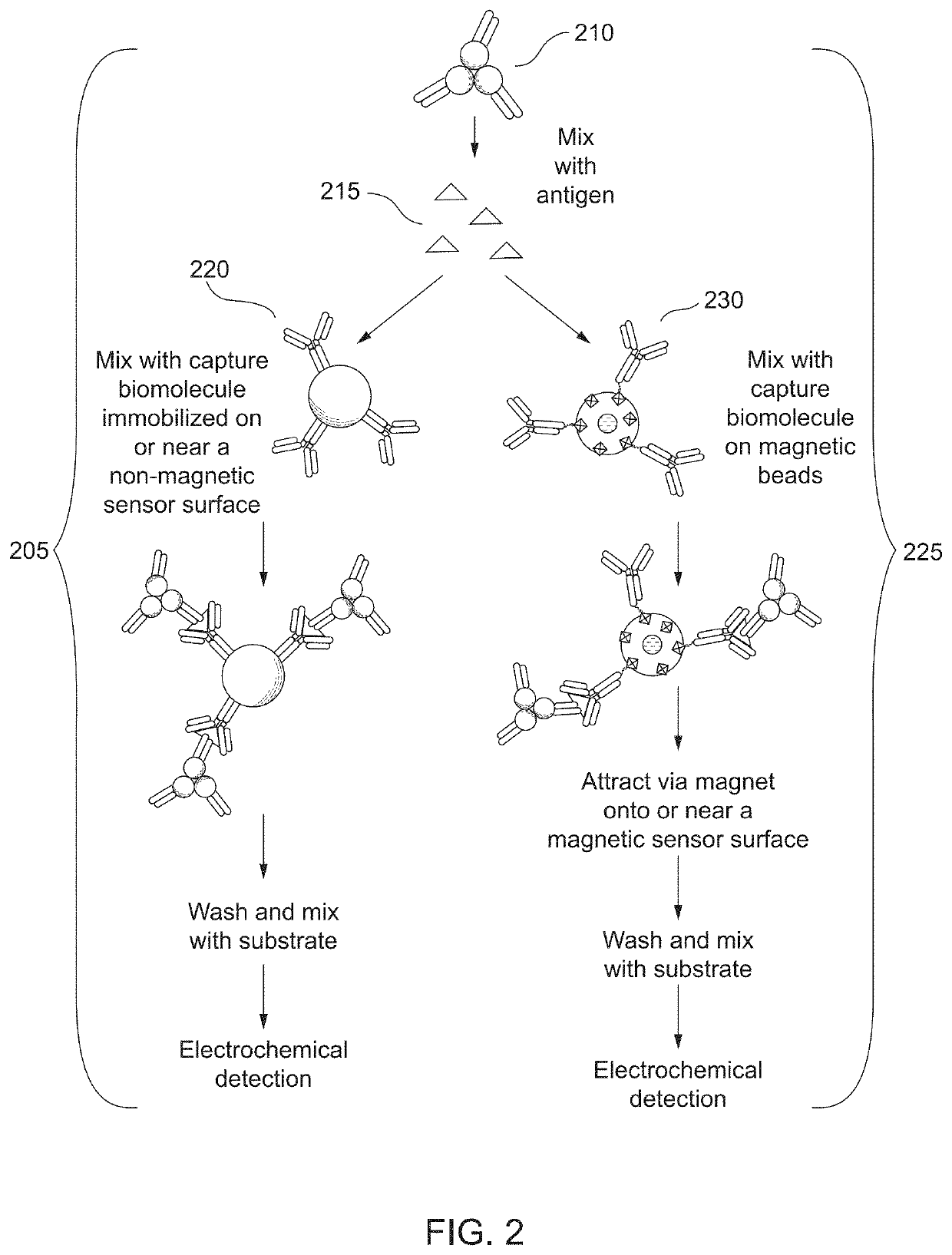
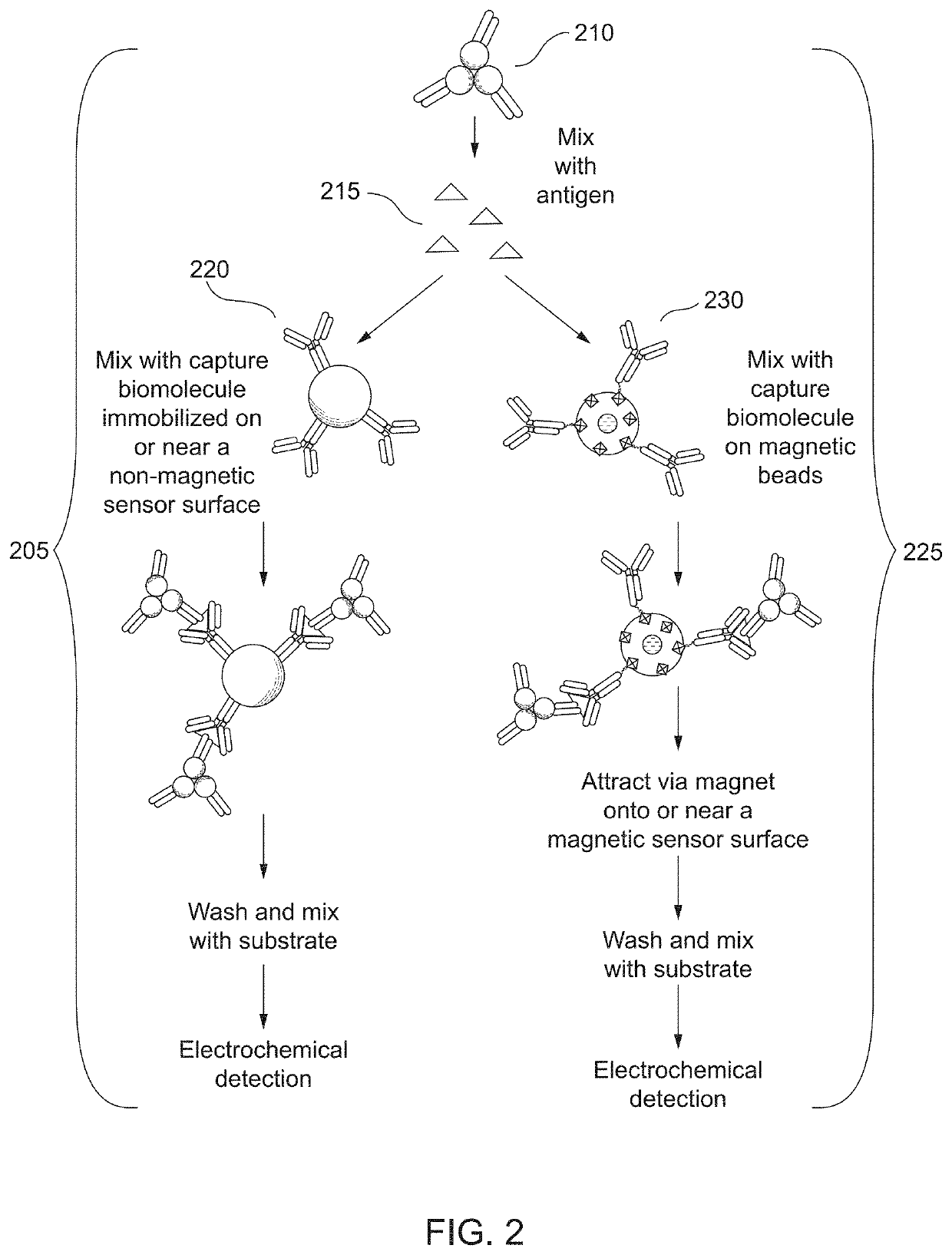
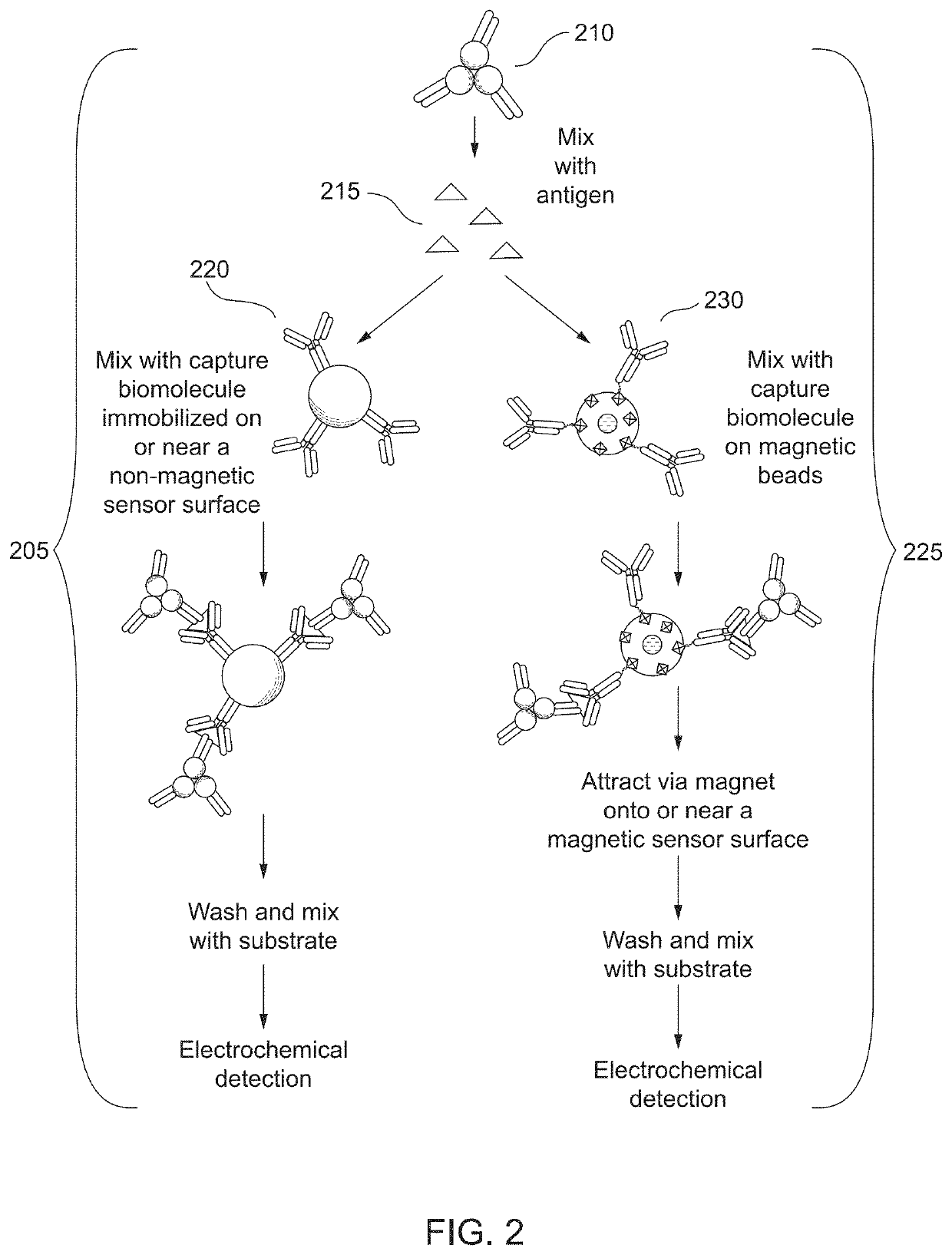
Combined immunoassay and magnetic immunoassay systems and devices for extended range of sensitivity
ActiveUS20180164303A1Material analysis by electric/magnetic meansLaboratory glasswaresImmobilized AntibodiesAnalyte
The present invention relates to systems that utilize a combination of immunoassay and magnetic immunoassay techniques to detect an analyte within an extended range of specified concentrations. In particular, a device is provided for detecting an analyte in a biological sample. The device includes a first electrochemical sensor positioned on a substrate. The first electrochemical sensor includes an immobilized layer of antibody configured to bind to the analyte. The device further includes a second electrochemical sensor positioned adjacent to the first electrochemical sensor on the substrate, and a magnetic material that generates a magnetic field aligned with respect to the second electrochemical sensor. The magnetic field captures magnetic beads that have an immobilized layer of antibody configured to bind to the analyte, and concentrates the magnetic beads on or near a surface of the second electrochemical sensor.
Owner:ABBOTT POINT CARE
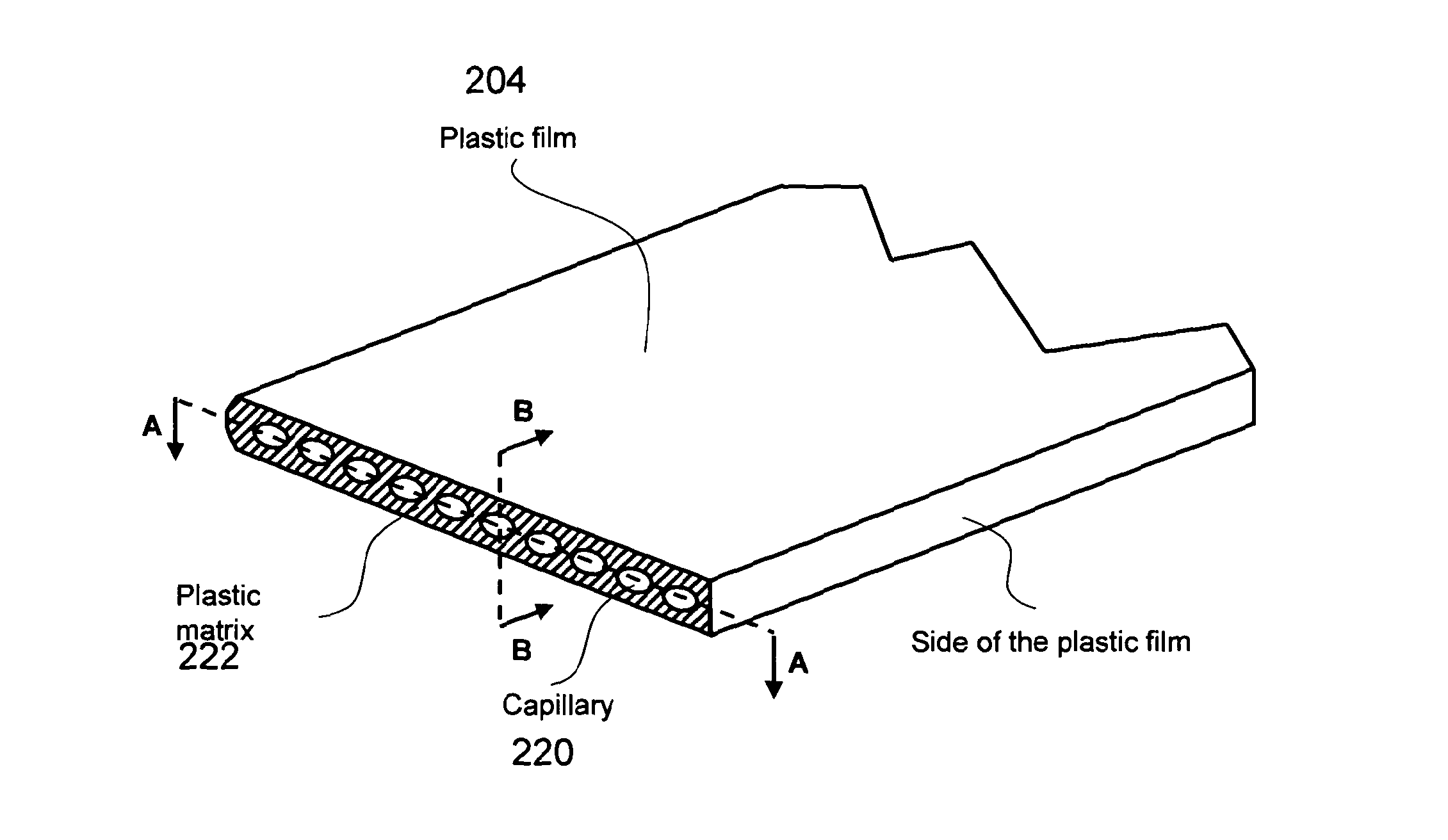
Immunoassays, methods for carrying out immunoassays, immunoassay kits and method for manufacturing immunoassay kits
ActiveUS20130011913A1Increase contactMeasurable differenceBioreactor/fermenter combinationsLiquid surface applicatorsImmunoassay techniqueChemistry
The invention relates to immunoassays, methods for carrying out immunoassays, immunoassay kits and methods for manufacturing immunoassay kits. In particular, the invention has relevance to capillary (especially microcapillary) immunoassay technology.
Owner:CAMBRIDGE ENTERPRISE LTD
Development of a novel assay for mgmt (methyl guanine methyl transferase)
InactiveUS20070264672A1Shorten the timeLower potentialBioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceRepair enzyme
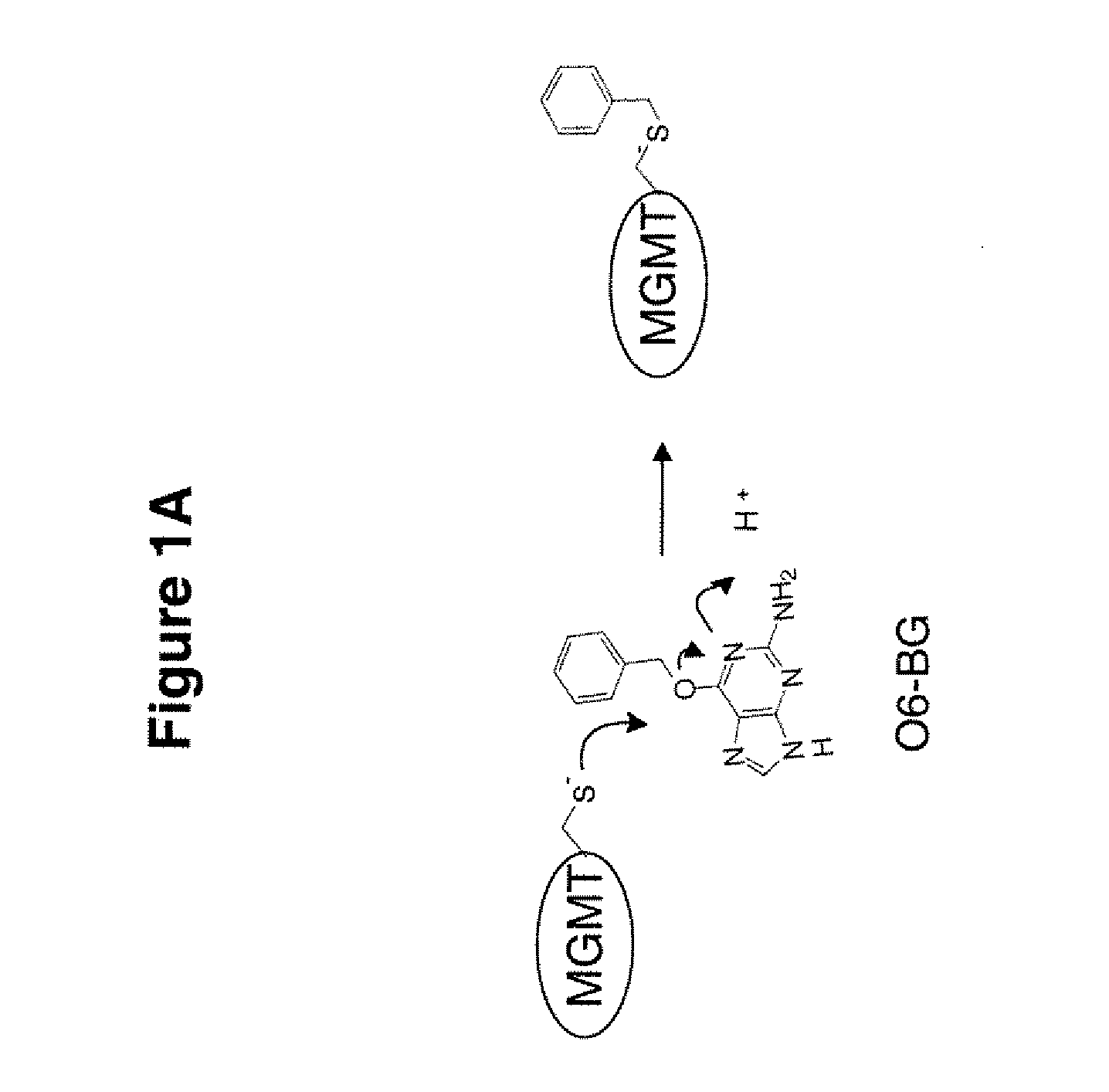
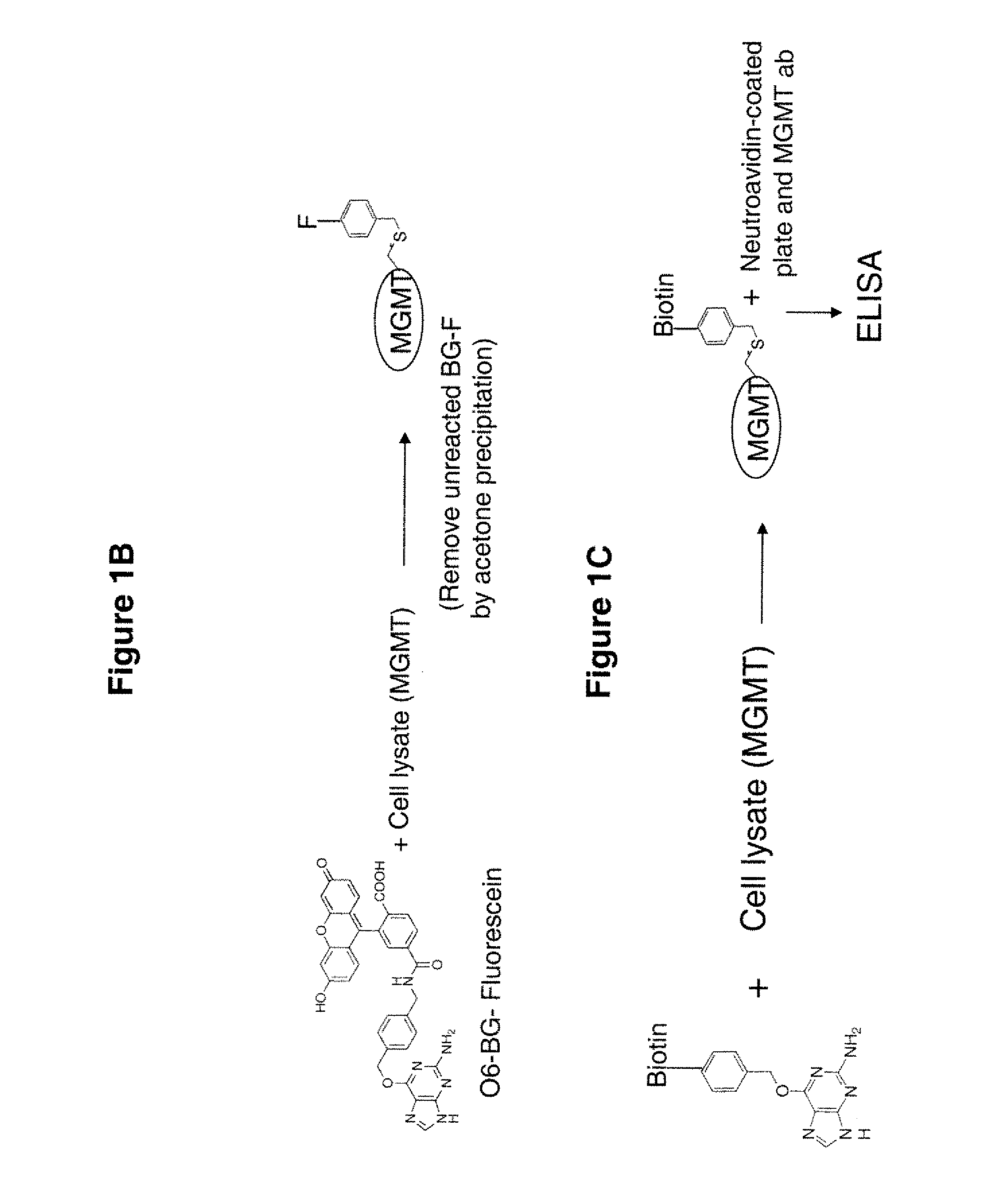
The present invention provides improved methods for assessing the level of MGMT activity in a variety of biological preparations. MGMT, a DNA repair enzyme, can reduce the chemotherapeutic efficacy of alkylating agents by repairing the damage that alkylating agents do to tumor cell DNA. The methods of the present invention can be used, inter alia, to measure MGMT levels and to thereby predict the clinical response to alkylating agents. The present invention includes three preferred assays for assessment of MGMT activity: (1) the immunoassay technique, (2) the labeled O6—BG technique, and (3) the fluorescence polarization technique. Kits useful for the performance of such assays are also provided.
Owner:SCHERING CORP
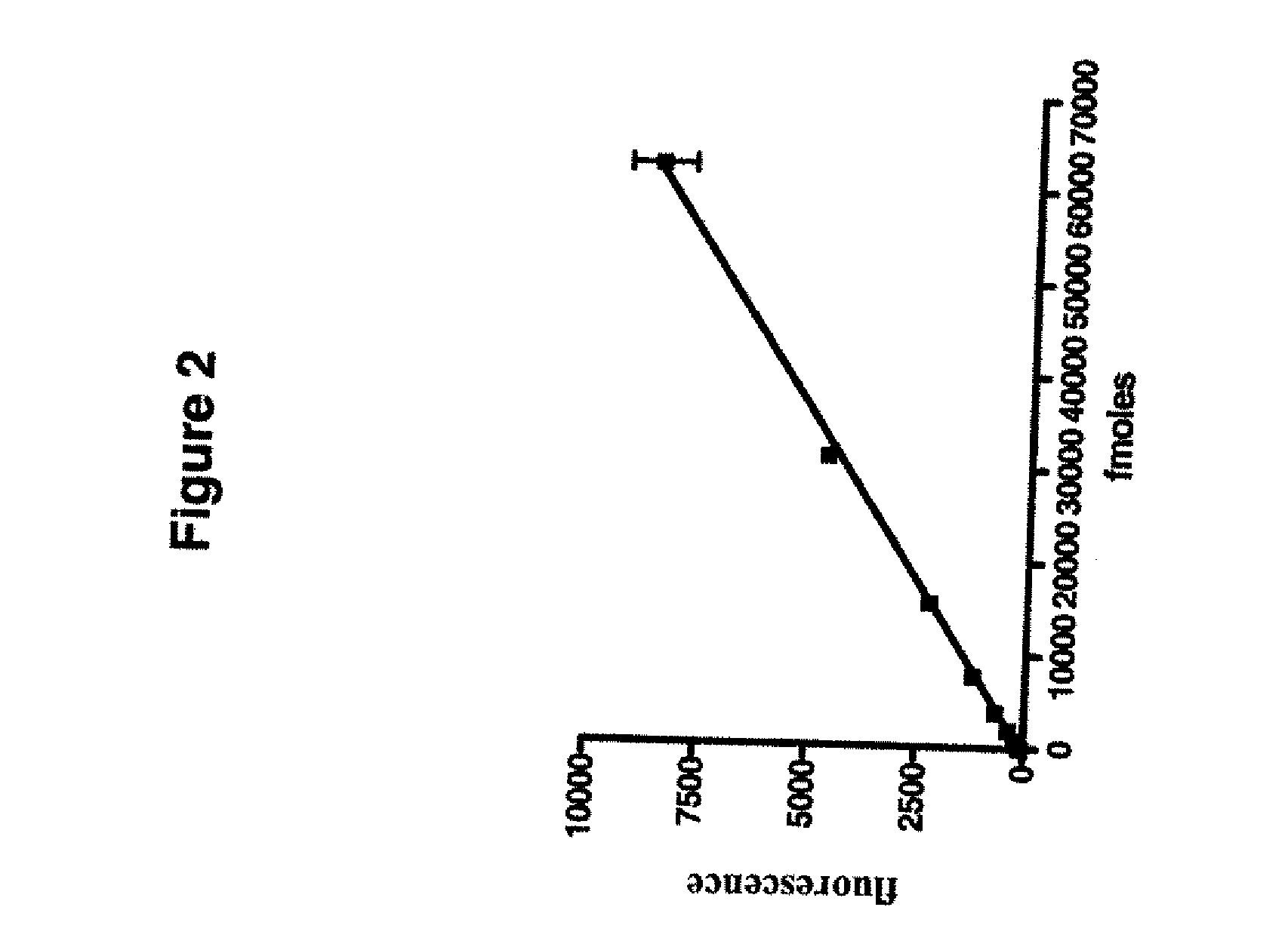
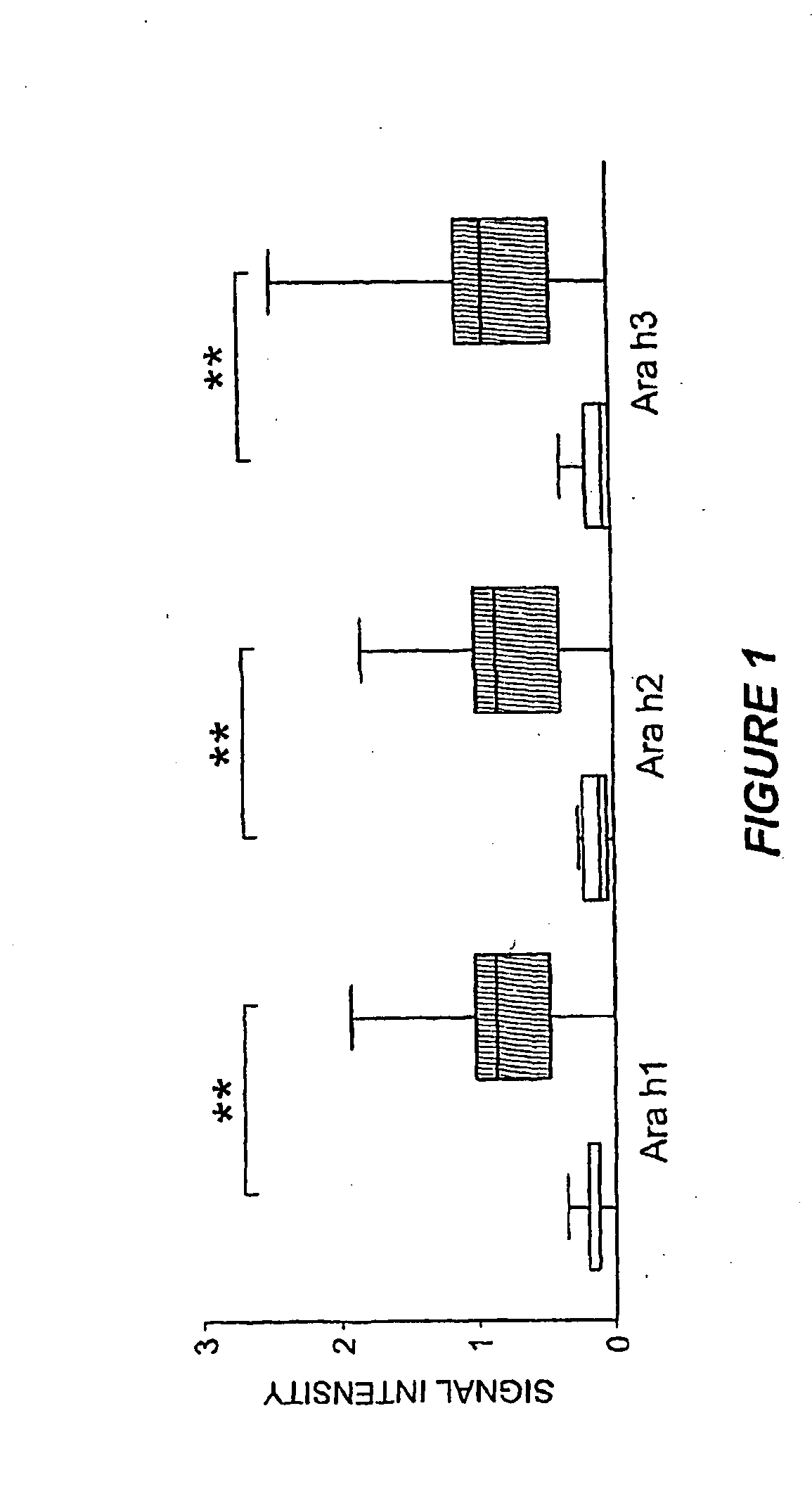
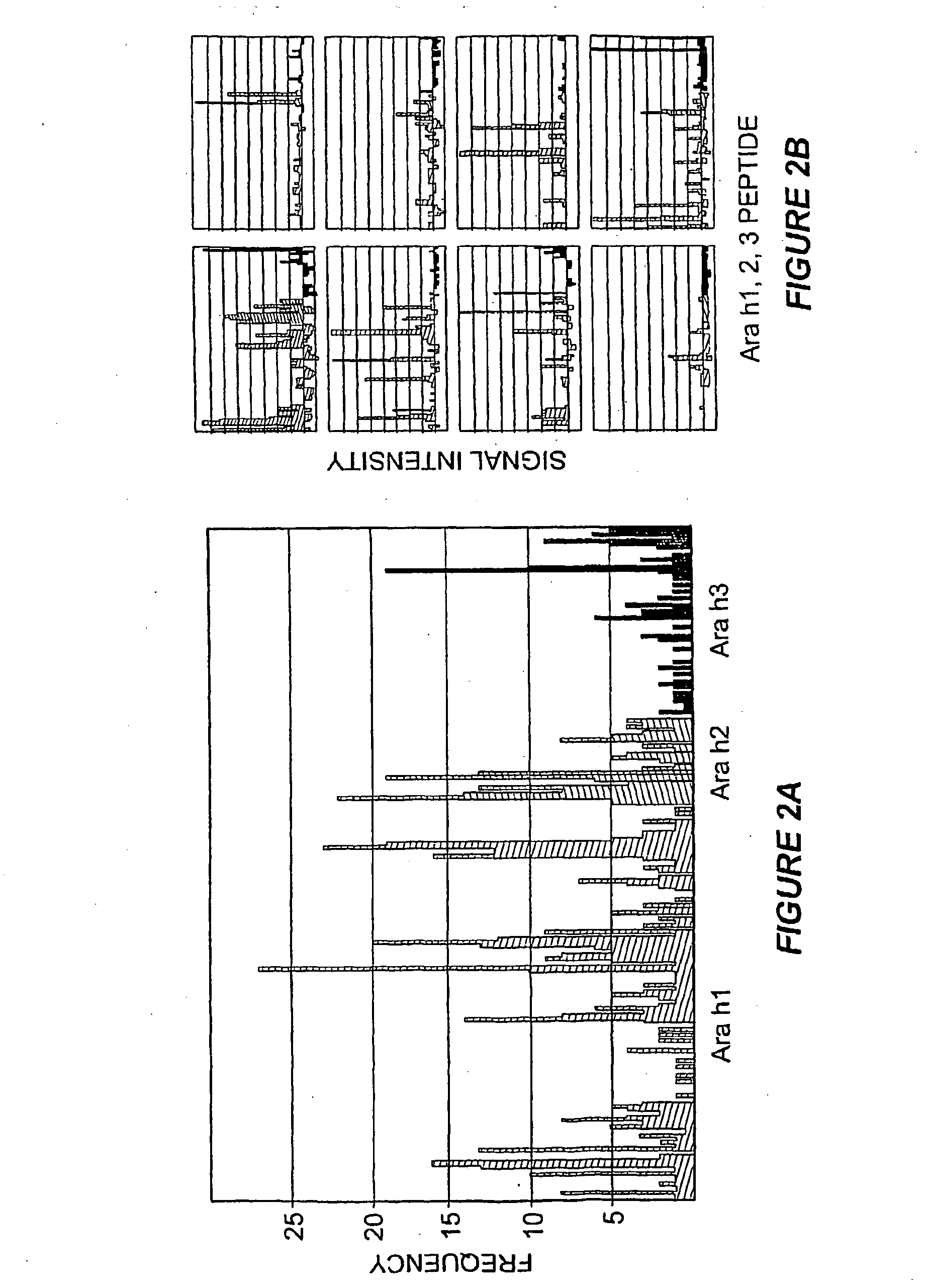
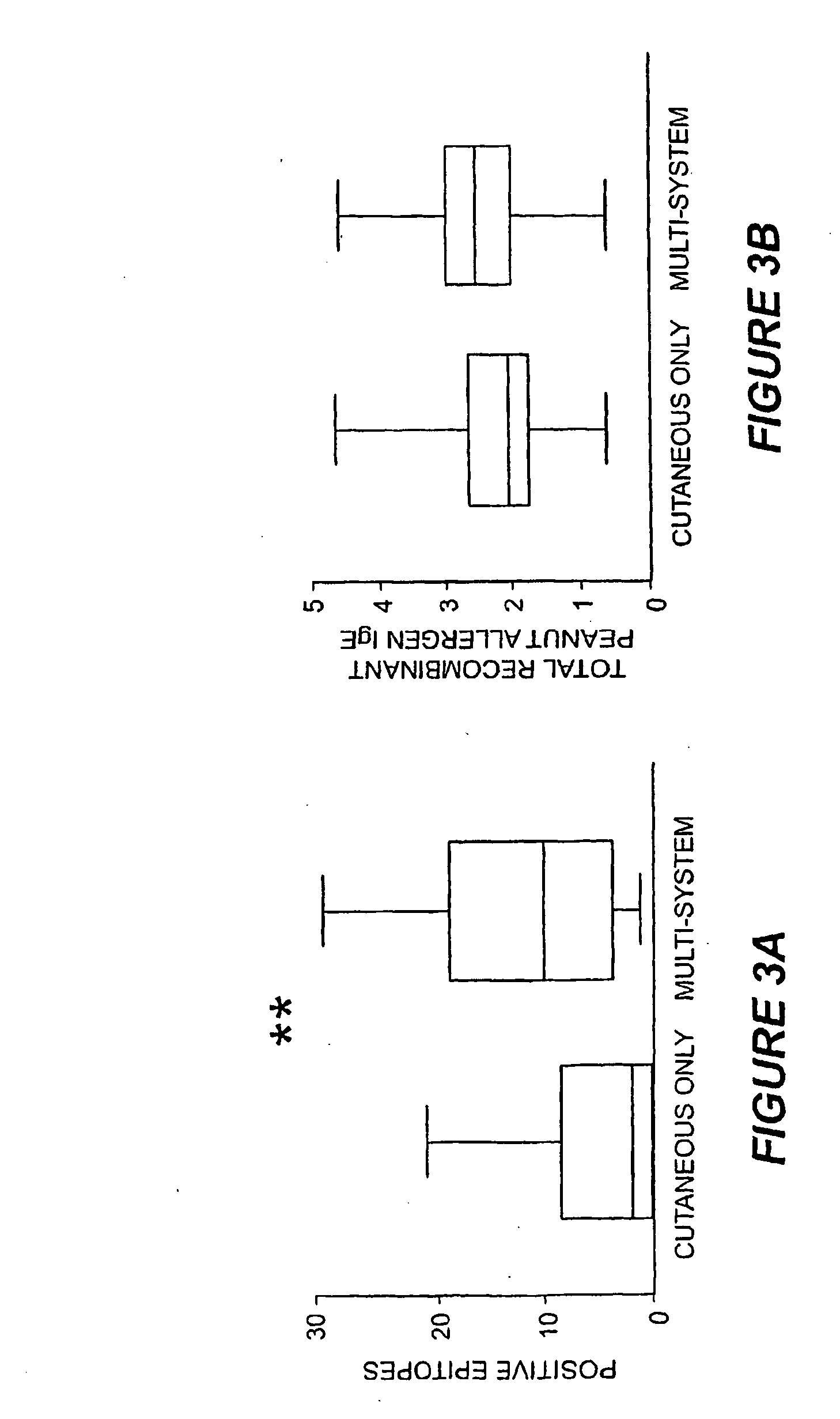
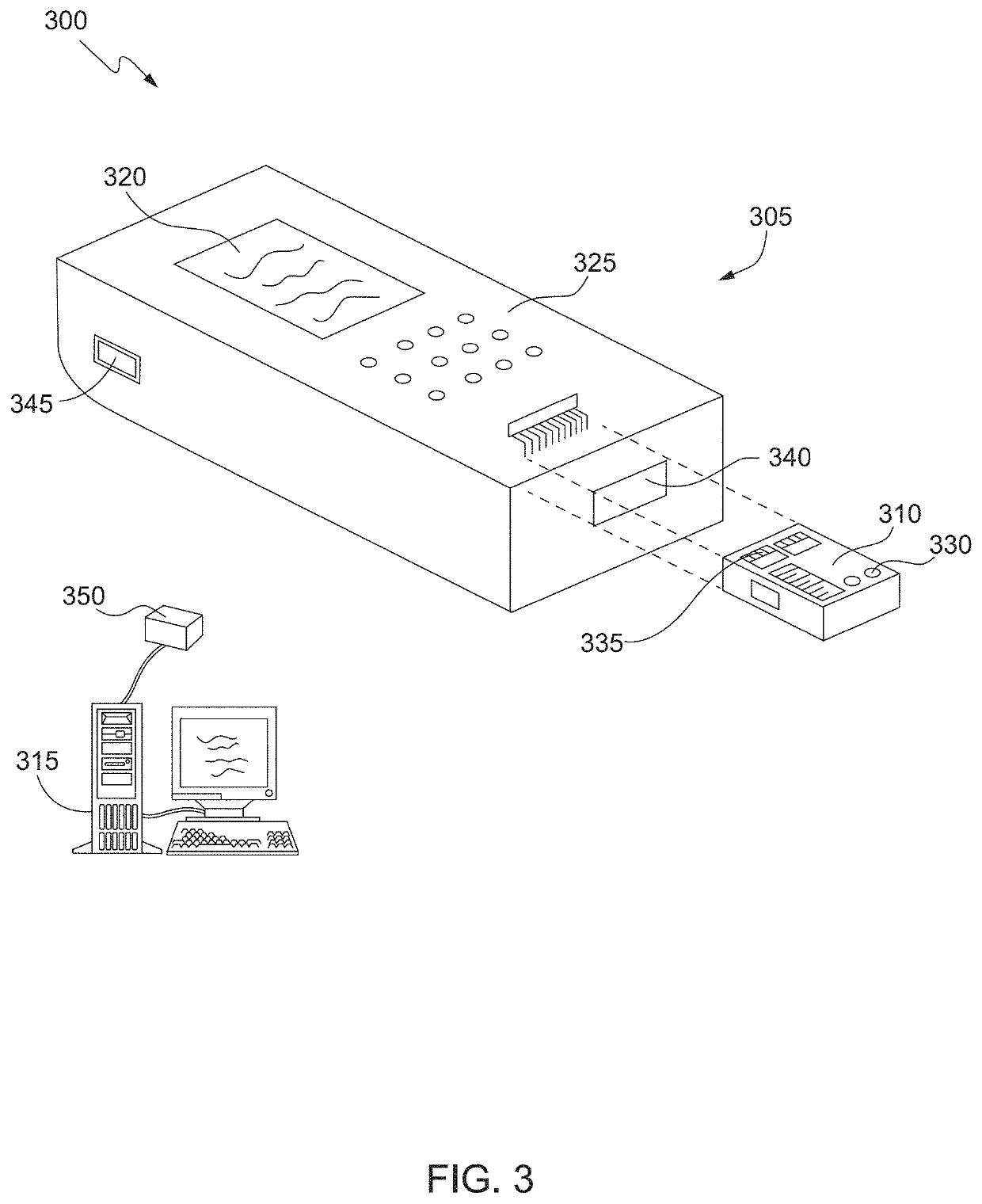
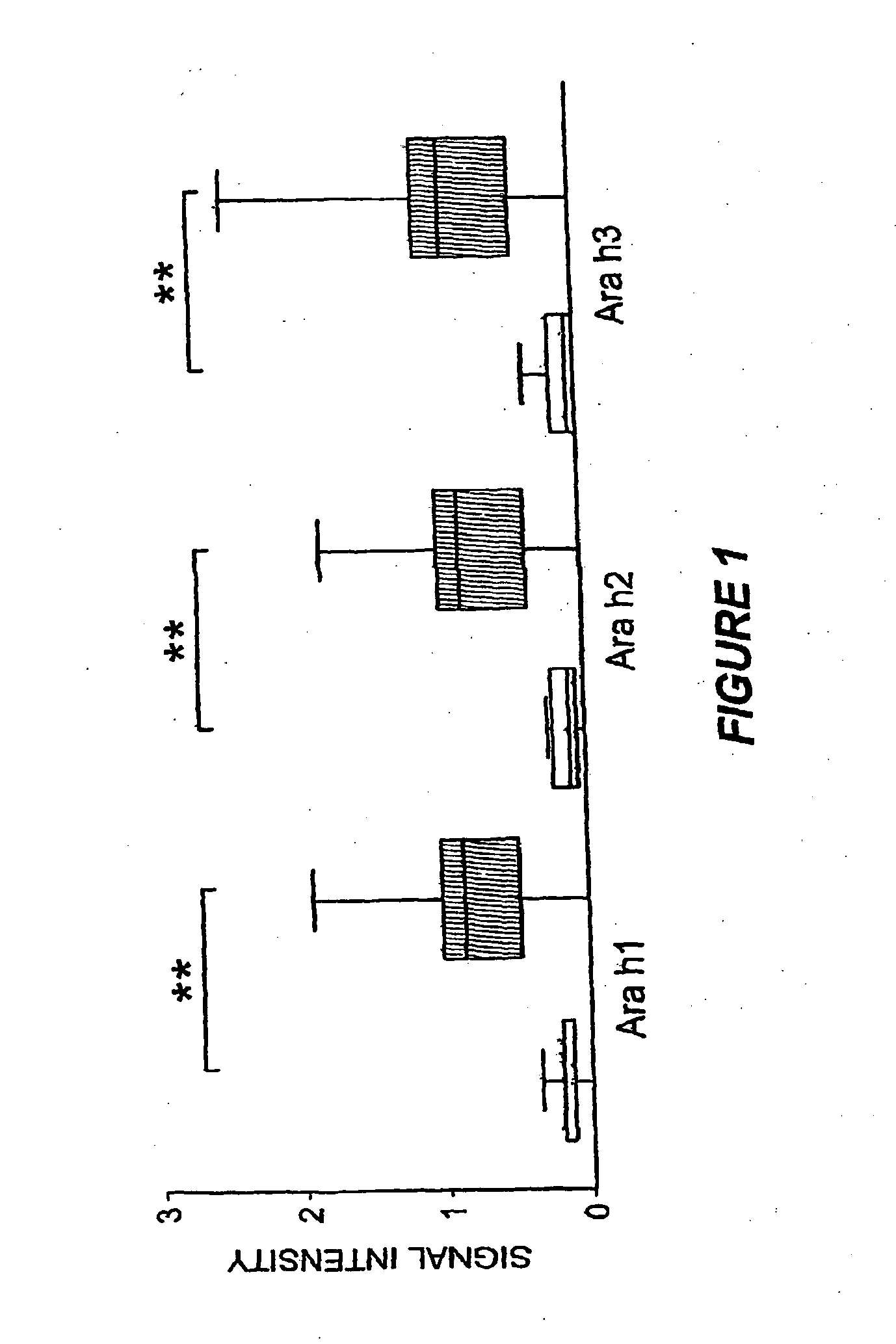
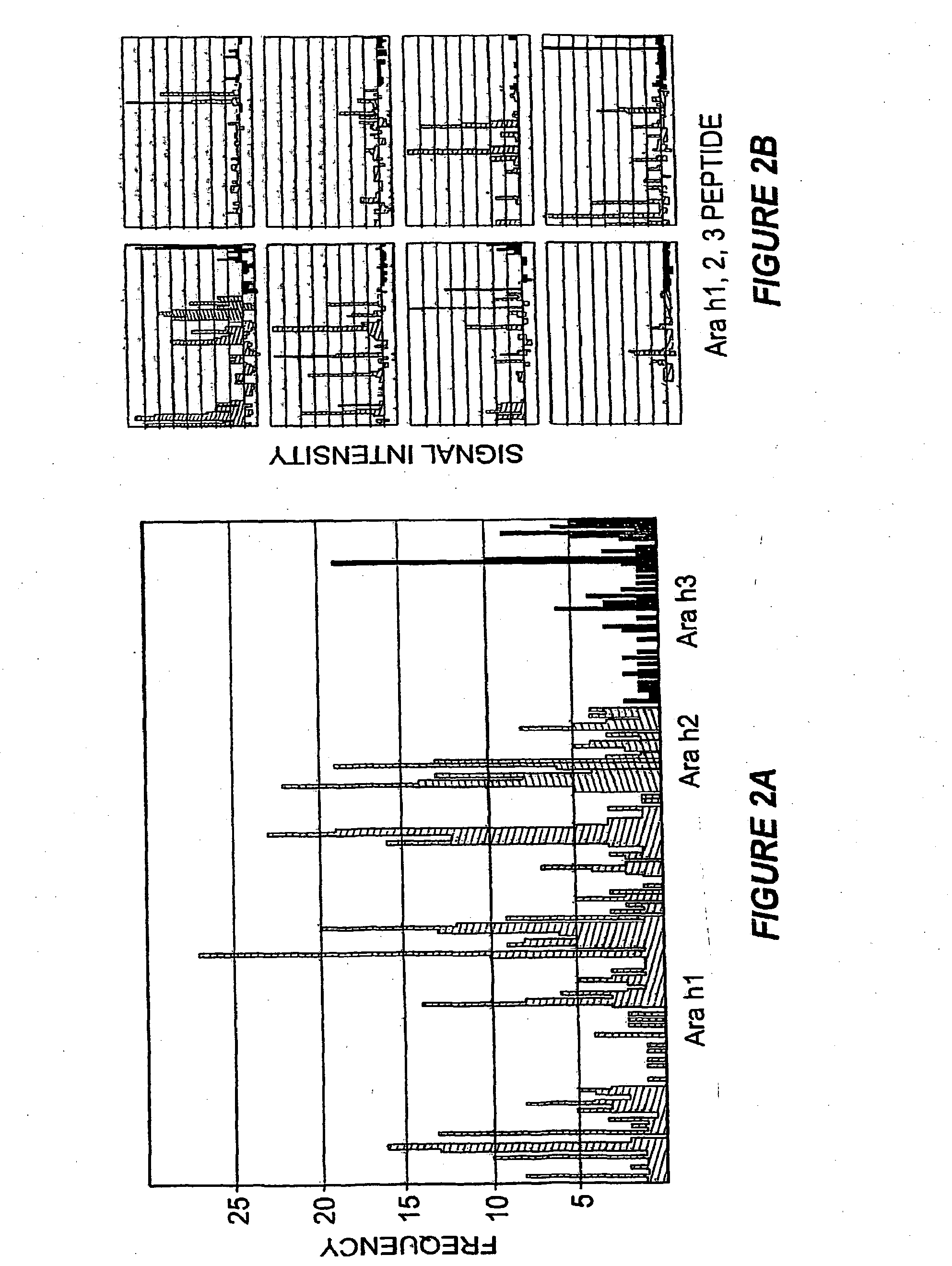
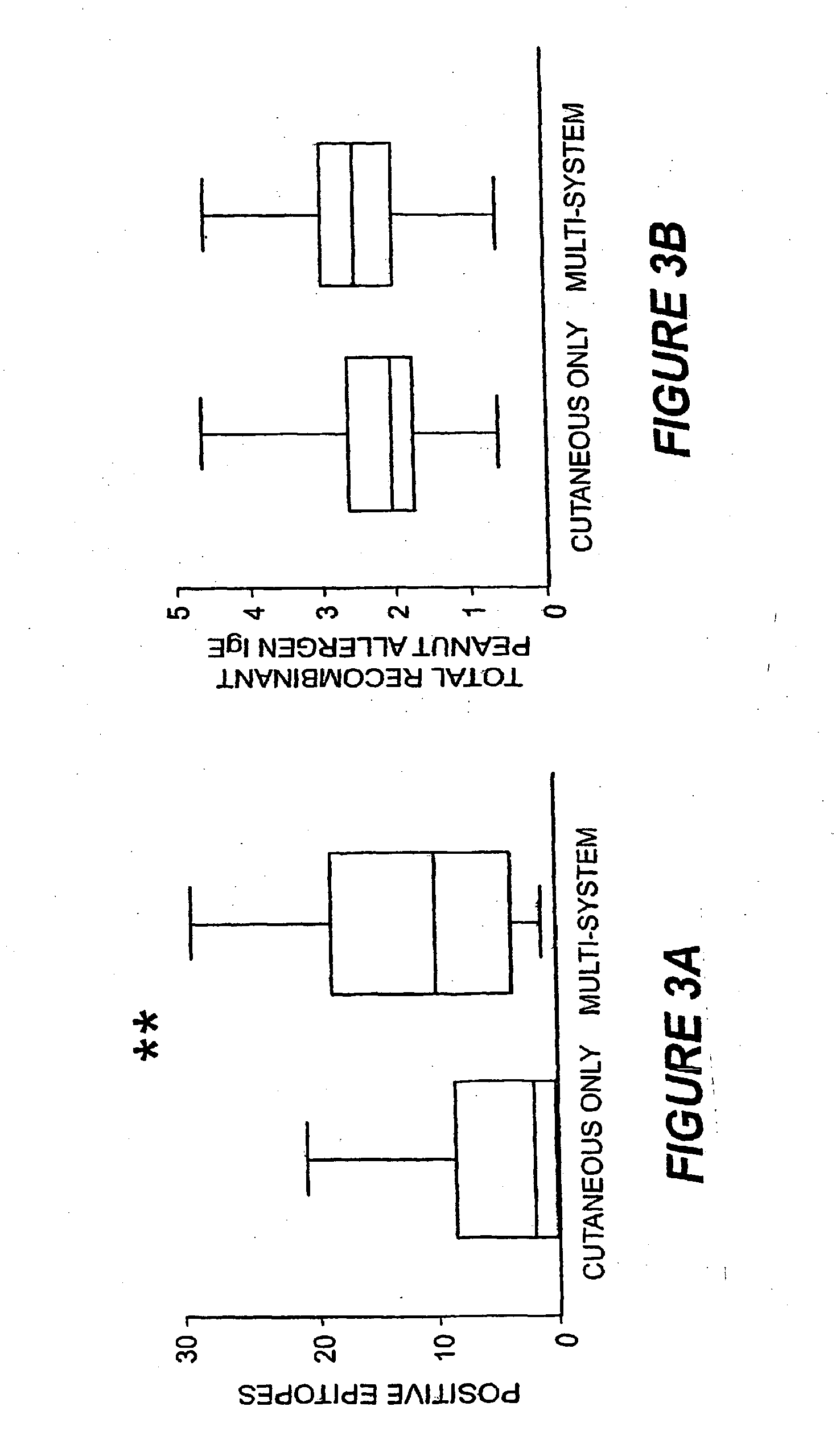
Methods of determining allergen response using microarray immunoassay techniques
The present invention is directed to materials and methods that may be used in diagnosing and / or characterizing allergies. More specifically, the specification describes methods and compositions for making and using a plurality of peptides having allergen epitopes that may be used in immunoassays e.g., microarray-based immunoassays to predict the severity of an allergic response.
Owner:NIH DEITR
Chip for a pathogens, parasites, toxins and desired chemical compounds detection
ActiveUS20140332409A1Fast and sensitive and inexpensive and portable immunoassayFaster and cheap methodImmobilised enzymesBioreactor/fermenter combinationsImmunosorbentsSorbent
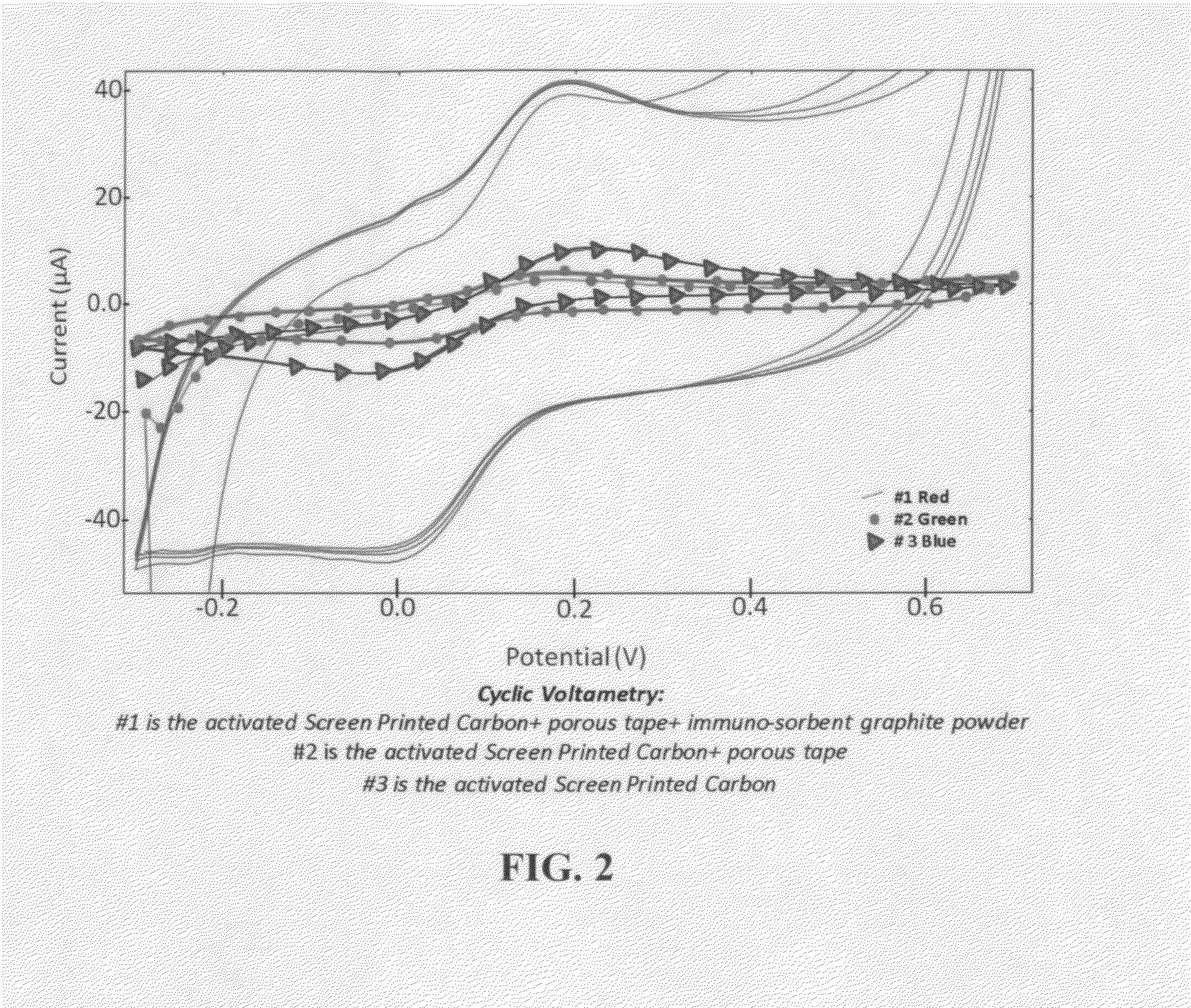
The present invention is directed to a method and apparatus for an immunoassay technique that uses amperometric measurements to rapidly analyze different pathogenic microorganisms, including bacteria, viruses, toxins, and parasites and chemical compounds using a disposable element. In accordance with one aspect of the present invention, at least one conductive immunosorbent is used to provide support for antibody immobilization and placed on the top of the working electrode; it could also be used by itself as a working electrode. This immunosorbent or powder can be fabricated of conductive material or nonconductive material over which a conductive material is coated. A membrane cover of the working electrode forms a fluidic chamber having a pore size that is suited to the particular application to insure no contact between the working electrode and counter or silver electrode. The immunoassay can be automated using microprocessor control to reduce the amount of human intervention.
Owner:WILKINS EBTISAM
Method of purifying alpha corn gibberellol and its metabolic substance and immune affinity chromatographic column
A method for purifying alpha-zearalol and / or beta-zearalol and / or zearalone and / or alpha-zearalenol by the special immunoaffinity chromatographic column carrying immunoaffinity adsorbent is disclosed. Said adsorbent is composed of solid carrier and its coupled alpha-zearalol monoclonal antibody. It features that the chromatography is used to measure the contents of alpha-zearalol and its metabolites, having high correctness.
Owner:CHINA AGRI UNIV
Artificial antigen and specific antibody of veterinary drug penicillin G degradation product benzylpenicilloic thiazole acid
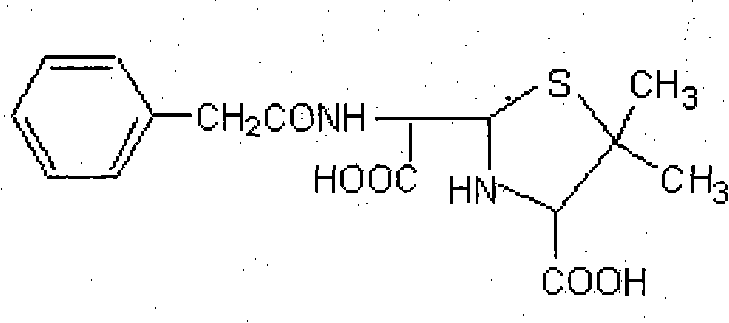
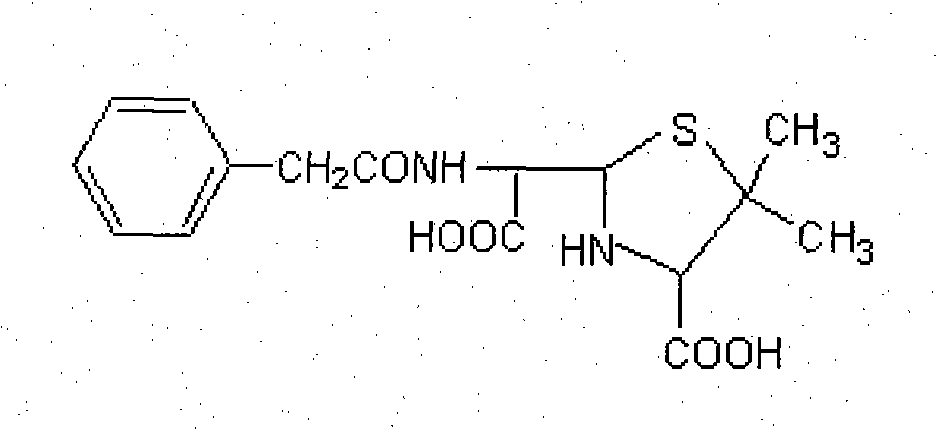
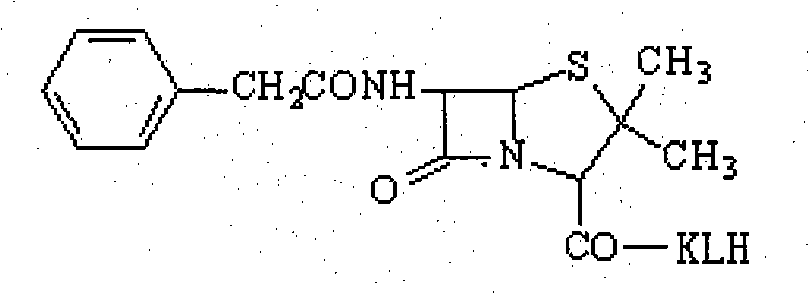
InactiveCN102040661AHigh similarityThe characteristic structure remains intactPeptide preparation methodsDepsipeptidesImmune profilingVeterinary Drugs
An artificial antigen and an antibody of a veterinary drug penicillin G degradation product benzylpenicilloic thiazole acid and preparation method thereof. The invention relates to the preparation of a hapten, an artificial antigen and an antibody of benzylpenicilloic thiazole acid having a structure of (2S, 5R, 6R)-3,3-dimethyl-6-(2-pheylacetamino)-7-oxo-4-thia-1-azabicyclo[3,2,0]heptane-2-formic acid and their application in the establishment of immunoassay. The invention solves the problem that traditional physical and chemical analysis methods are complicated, high in cost and slow in analysis, and provides a simple, quick, sensitive and accurate immunoassay technique. According to the invention, (2S, 5R, 6R)-3,3-dimethyl-6-(2-pheylacetamino)-7-oxo-4-thia-1-azabicyclo[3,2,0]heptane-2-formic acid is adopted as a hapten, and the haptens are linked with KLH and HRP respectively to synthesize artificial antigens and enzyme-labeled antigens. The antibody is prepared by animal immunization, blood drawing, antiserum separation, and purification of artificial antigen. The antibody is stable and has good specificity and sensitivity; the synthetic method is simple; the invention is applicable to the rapid immunoassay of veterinary drug penicillin G degradation product benzylpenicilloic thiazole acid, and has a good application prospect.
Owner:TIANJIN UNIV OF SCI & TECH
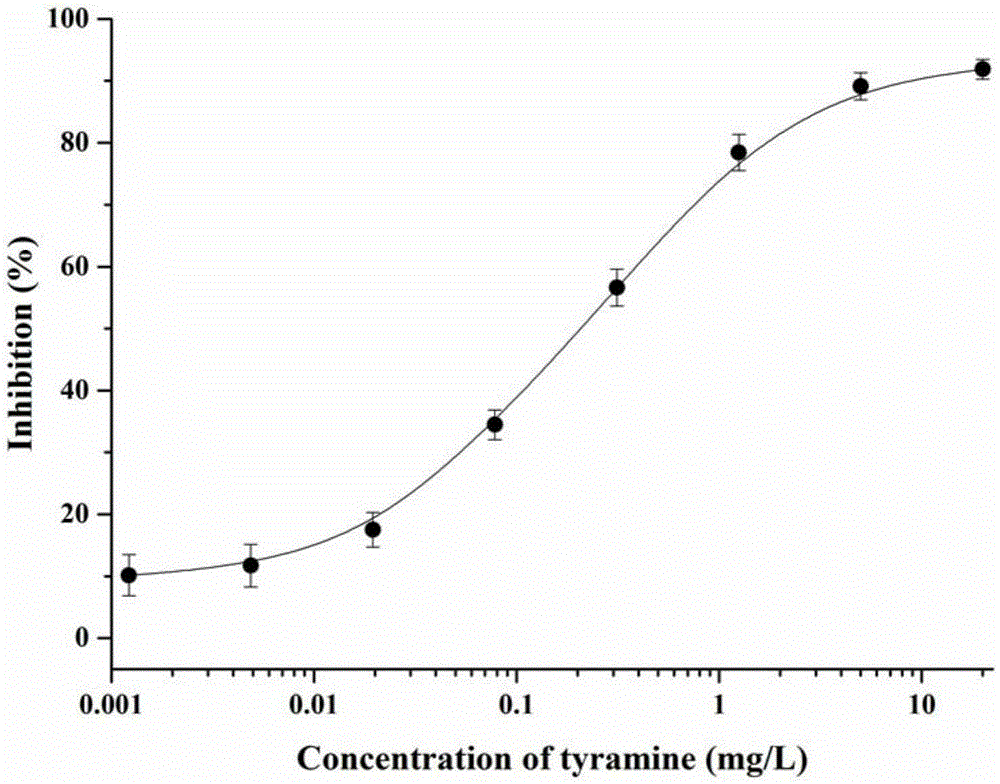
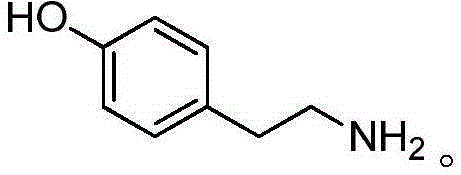
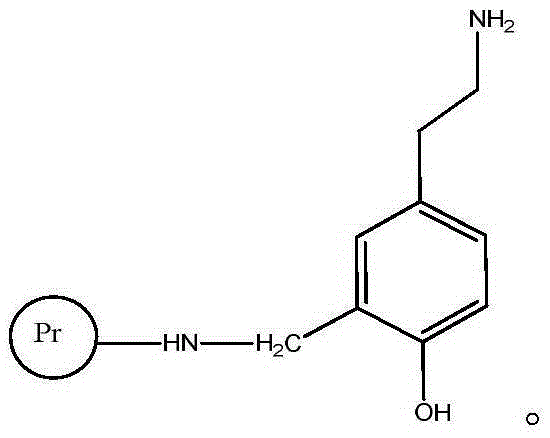
Tyramine artificial antigen and antibody, and preparation methods and application thereof
InactiveCN105399639AHigh sensitivityStrong specificityOvalbuminSerum albuminBovine serum albuminAnalysis method
The invention provides a tyramine artificial antigen and antibody, and preparation methods and an application thereof, and relates to preparation of the tyramine artificial antigen and antibody and establishment of an immunoassay method. The problems that a traditional physical and chemical analysis method is tedious and complex, relatively high in cost and slow in analysis speed are overcome, and a simple, rapid, sensitive and accurate immunoassay technique is provided. With adopting of a formaldehyde method and a glutaraldehyde method, tyramine is coupled respectively with cationized bovine serum albumin and ovalbumin, and the tyramine artificial antigen and a tyramine coated antigen are successfully prepared. The artificial antigen is subjected to animal immunization, blood acquirement and separation purification to obtain the specific antibody. A detection method established by using the antibody and used for detection of tyramine in food has good specificity and sensitivity, and the detection limit can be stabilized to 0.02 mg / L; the detection method is low in cost, simple to operate, and suitable for rapid detection of tyramine in the food.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method of purifying albuterol and/or clenbuterol and immune affinity chromatographic column
InactiveCN1830546AHigh selectivityKeep strongOther chemical processesBiological testingSalbutamolMonoclonal antibody
A method for purifying salbutamol and / or clenbuterol by the special immunoaffinity chromatographic column carrying immunoaffinity adsorbent is disclosed. Said adsorbent is composed of solid carrier and its coupled salbutamol polyclonal or monoclonal antibody. It features that the chromatography is used to measure the contents of salbutamol and clenbuterol, having high correctness.
Owner:CHINA AGRI UNIV
Fluorescent quenching test paper for detecting field okadaic acid as well as a preparation method and application of the fluorescent quenching test paper
ActiveCN109655435AStrong specificityNo cross reactionFluorescence/phosphorescenceFluorescent quenchingSoftware
The invention belongs to the field of immunoassay technical methods, and particularly relates to fluorescence quenching test paper for detecting field okadaic acid as well as a preparation method andapplication of the fluorescence quenching test paper. The fluorescence quenching test paper comprises a sample pad, a combination pad, a nitrocellulose membrane, a water absorption pad and a PVC bottom plate. An OA monoclonal antibody marked by colloidal gold particles is sprayed on the combination pad, detection lines on the nitrocellulose membrane are fluorescent microspheres and OA-BSA mixed solution, and quality control lines are fluorescent microspheres. The fluorescence quenching test paper is high in specificity and free of cross reaction, the sensitivity of the fluorescence quenching test paper is 1.56 ppb and is improved by 9.6 times compared with that of colloidal gold test paper with the same parameters, the detection limit of the fluorescence quenching test paper is 3.12-50 ppb, and the detection time is as short as 9 min. The obtained fluorescence quenching test paper has very high stability on interference of shellfish tissue, can achieve quantification of OA toxin by matching with a simple fluorescence immunochromatographic camera and data analysis software, result judgment is visual, and cause misjudgment of non-professionals can be provided. The fluorescence quenching test paper has bright development and application prospects.
Owner:SHENZHEN CENT FOR DISEASE CONTROL & PREVENTION +1
Immunoassay system and immunoassay method
InactiveUS7993581B2Efficient detectionHigh sensitivityMicrobiological testing/measurementPreparing sample for investigationMagnetizationAntigen-antibody reactions
Owner:HITACHI LTD
Combined immunoassay and magnetic immunoassay methods for extended range of sensitivity
The present invention relates to methods that utilize a combination of immunoassay and magnetic immunoassay techniques to detect an analyte within an extended range of specified concentrations. In particular, a method includes forming, in a biological sample, a first complex of signal antibodies and analyte, and a second complex of the first complex and capture antibodies immobilized on magnetic beads, and contacting a first immunosensor with the biological sample to form a third complex localized on or near a surface of the first immunosensor. The first immunosensor includes an immobilized layer of capture antibodies configured to bind to the analyte, and the third complex includes the first complex bound to the immobilized layer of capture antibodies. The method further includes contacting a magnetic field localized around a second immunosensor with the biological sample such that the second complex is localized on or near a surface of the second immunosensor.
Owner:ABBOTT POINT CARE
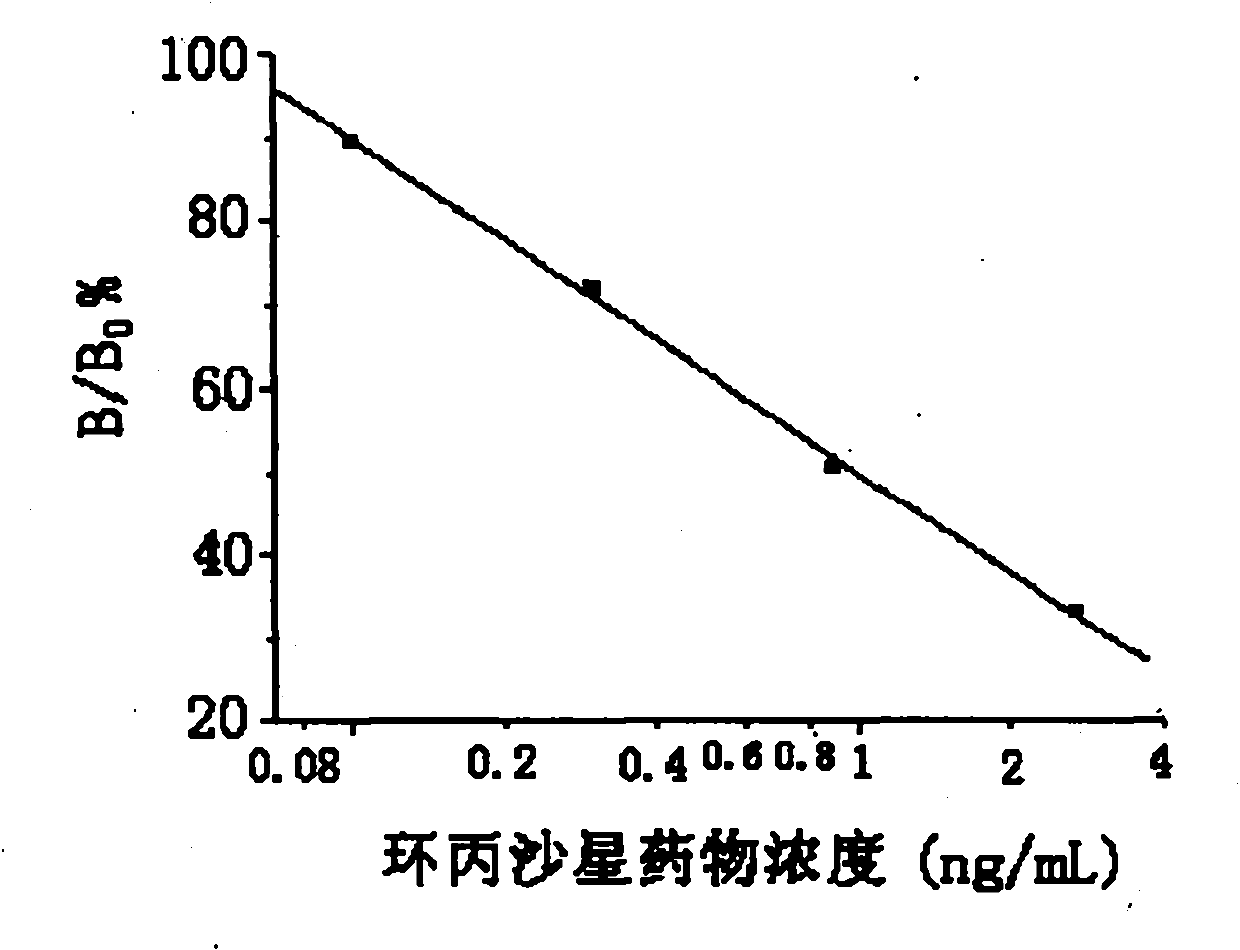
Time resolution immunoassay test kit and method of ciprofloxacin residual
InactiveCN101995468AGood effectLow costSerum immunoglobulinsFluorescence/phosphorescenceAntigenLanthanide
The invention provides a time resolution immunoassay test kit and method of ciprofloxacin residual. The test kit of the invention is coated with an elisa plate of a ciprofloxacin antigen, a lanthanide-labeled goat anti-rabbit or goat anti-mouse antibody, a ciprofloxacin antibody and the like. The invention also discloses the method for testing the ciprofloxacin residual by utilizing the test kit. The test kit for testing the ciprofloxacin provided by the invention has the advantages that an indirect competition time resolution immunoassay technique is adopted, the sensitivity is high, the stability is good, the operation step and the reaction time are greatly simplified, errors caused by the complex operation are reduced, and the cost is lowered, thereby being extremely suitable for screening a large number of samples, and having important realistic significance.
Owner:SOUTH CHINA AGRI UNIV
Electrochemiluminescence detection method for tumor necrosis factor alpha and kit of electrochemiluminescence detection method
InactiveCN109164090AHigh detection sensitivityAvoid interferenceChemiluminescene/bioluminescenceMaterial electrochemical variablesQuantum yieldSmall sample
The invention discloses an electrochemiluminescence detection method for a tumor necrosis factor alpha and a kit of the electrochemiluminescence detection method. According to the method, the tumor necrosis factor alpha is adopted as a detection target, a high quantum yield gold nano cluster electrochemiluminescence technique and an immunoassay technique are organically combined, a high quantum yield gold nano cluster electrochemiluminescence probe is prepared by using a reduction method, a manganese dioxide nano material is adopted as an electrochemiluminescence quenching agent, an electrochemiluminescence signal is recovered through a redox reaction between ascorbic acid generated from enzyme-linked immunosorbent assay and manganese dioxide, and the method is a high-performance electrochemiluminescence tumor necrosis factor alpha detection method based on the high quantum yield gold nano cluster probe. The method has a linear range of 0.06-31pg / mL for tumor necrosis factor alpha detection, and has a detection limit of 36fg / mL. The method has the characteristics of rapidness, accuracy, high sensitivity, good selectivity, good stability, small sample use amount and the like, and has relatively good clinical application prospects.
Owner:FUJIAN MEDICAL UNIV
Extended range immunoassay devices with immunosensor and magnetic immunosensor
The present invention relates to systems and methods that utilize a combination of immunoassay and magnetic immunoassay techniques to detect an analyte within an extended range of specified concentrations. In particular, a device includes a housing, a heterogeneous surface capture immunosensor within the housing and configured to generate a first signal indicative of the concentration of the analyte in an upper concentration range, and a homogeneous magnetic bead capture immunosensor within the housing and configured to generate a second signal indicative of the concentration of the analyte in a lower concentration range.
Owner:ABBOTT POINT CARE
Immunoaffinity stir bar for adsorbing diethylstilbestrol drug, and preparation method and application thereof
InactiveCN104698179AEfficient separationEfficient ConcentrationMaterial analysisDiethylstilbestrolImmunologic Technique
The invention discloses an immunoaffinity stir bar for adsorbing diethylstilbestrol drug, and a preparation method and an application thereof. The immunoaffinity stir bar for adsorbing diethylstilbestrol drug is composed of solid phase carrier and diethylstilbestrol murine monoclonal antibody coupled with the same, wherein the ratio of the coupling length of the solid phase carrier and coupled diethylstilbestrol DES-6B4 murine monoclonal antibody to the weight thereof is 1 cm: 2 mg. Through combining with a chromatography, an extracting method can effectively separate and condense the ingredients to be detected in a sample, the toluylene compound content detection is facilitated, the disadvantages of less information amount, low quantification precision, low physico-chemical method selectivity and the like due to using the simplex immunoassay to directly detect the sample are overcome, and the analysis mechanism complementarity between the immunological technique and conventional physical and chemical technology is reflected.
Owner:TIANJIN AGRICULTURE COLLEGE
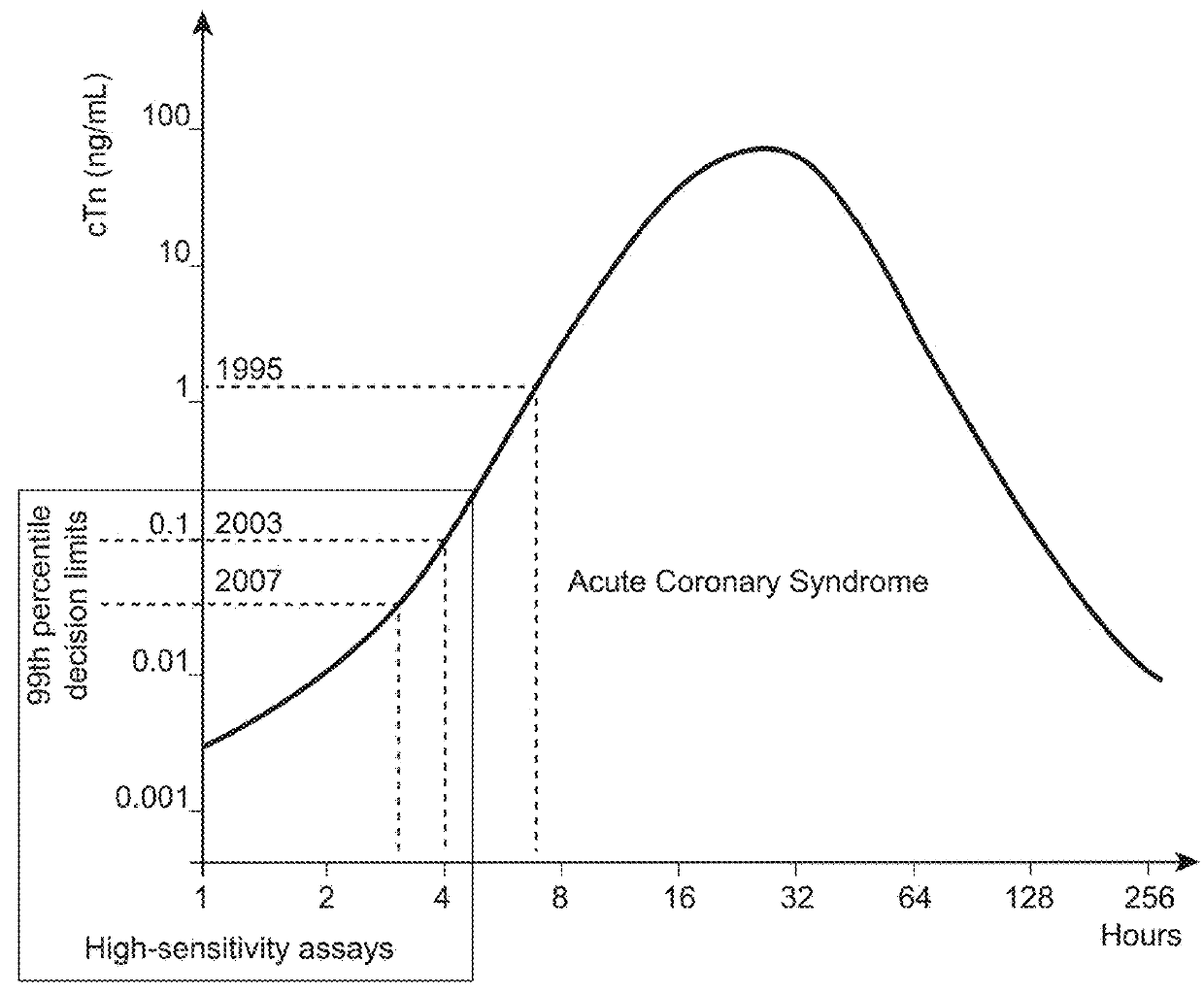
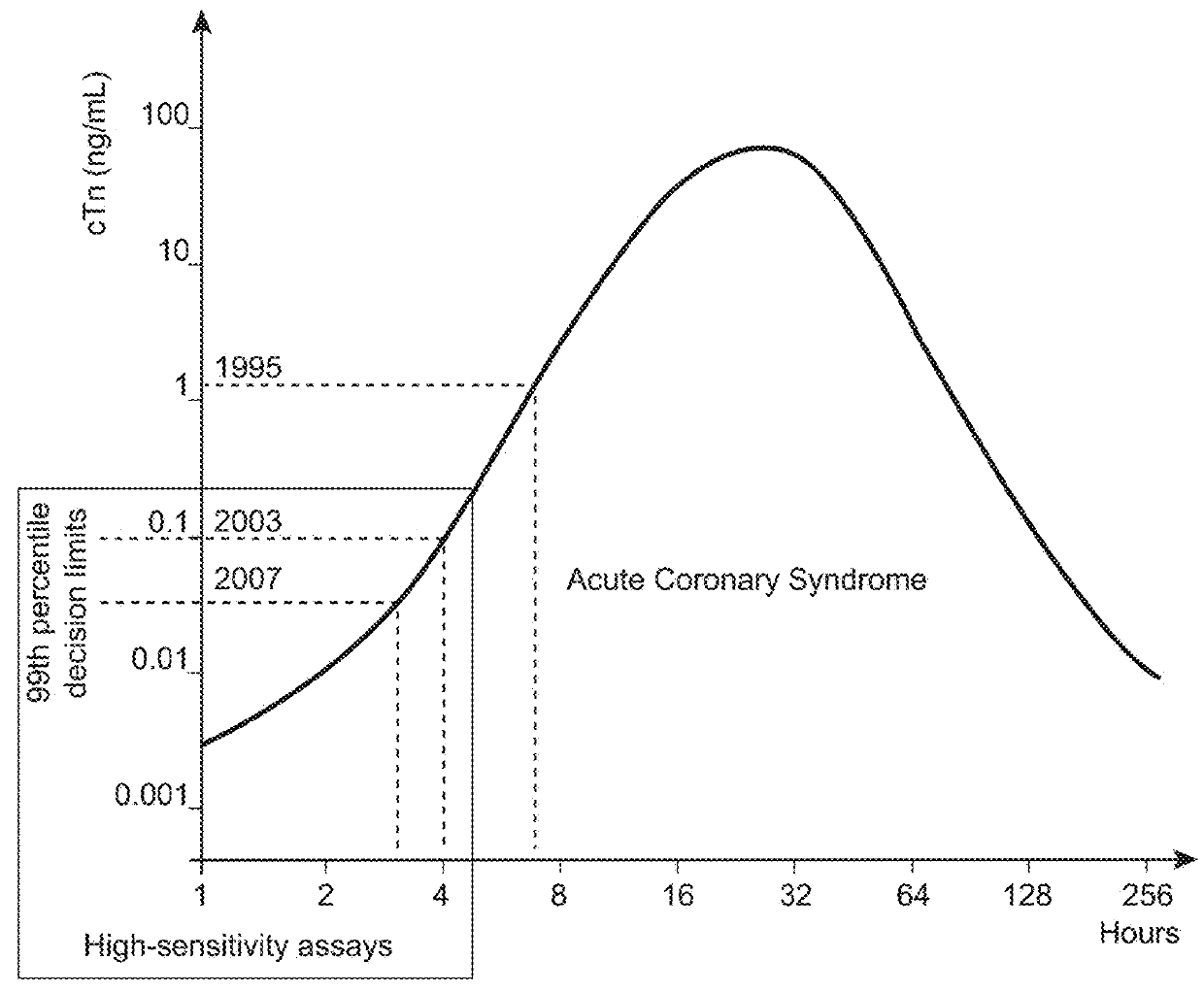
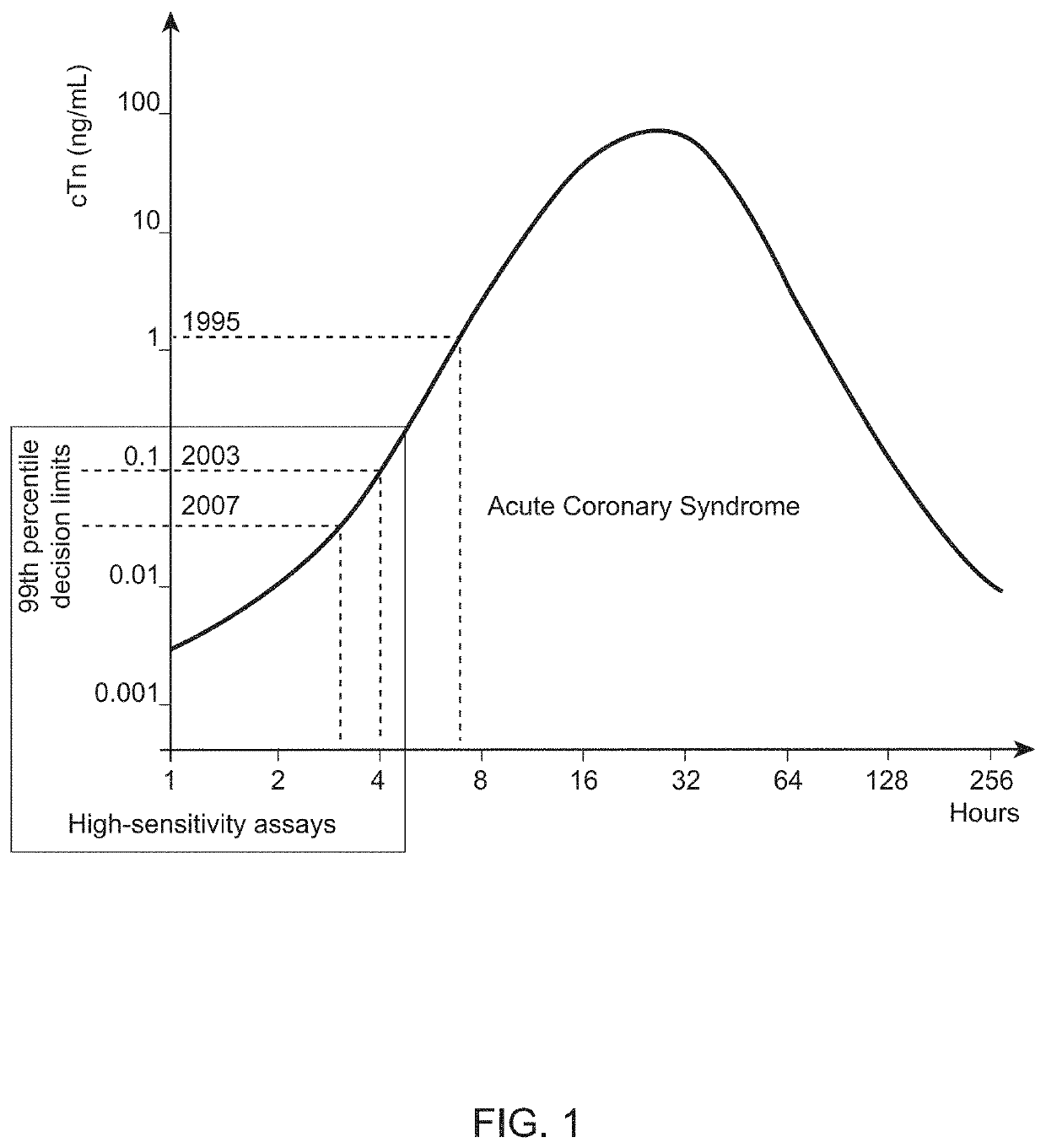
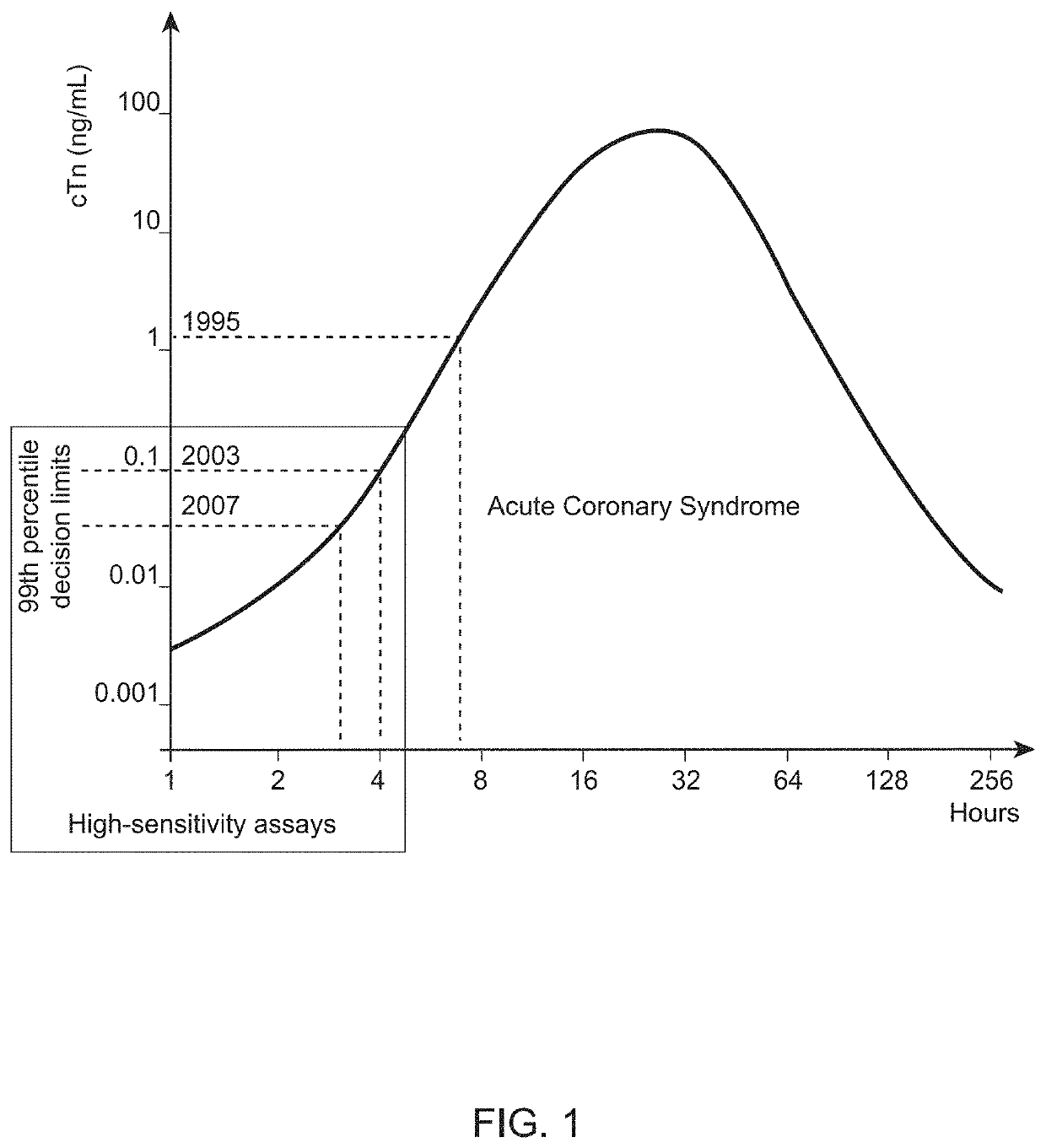
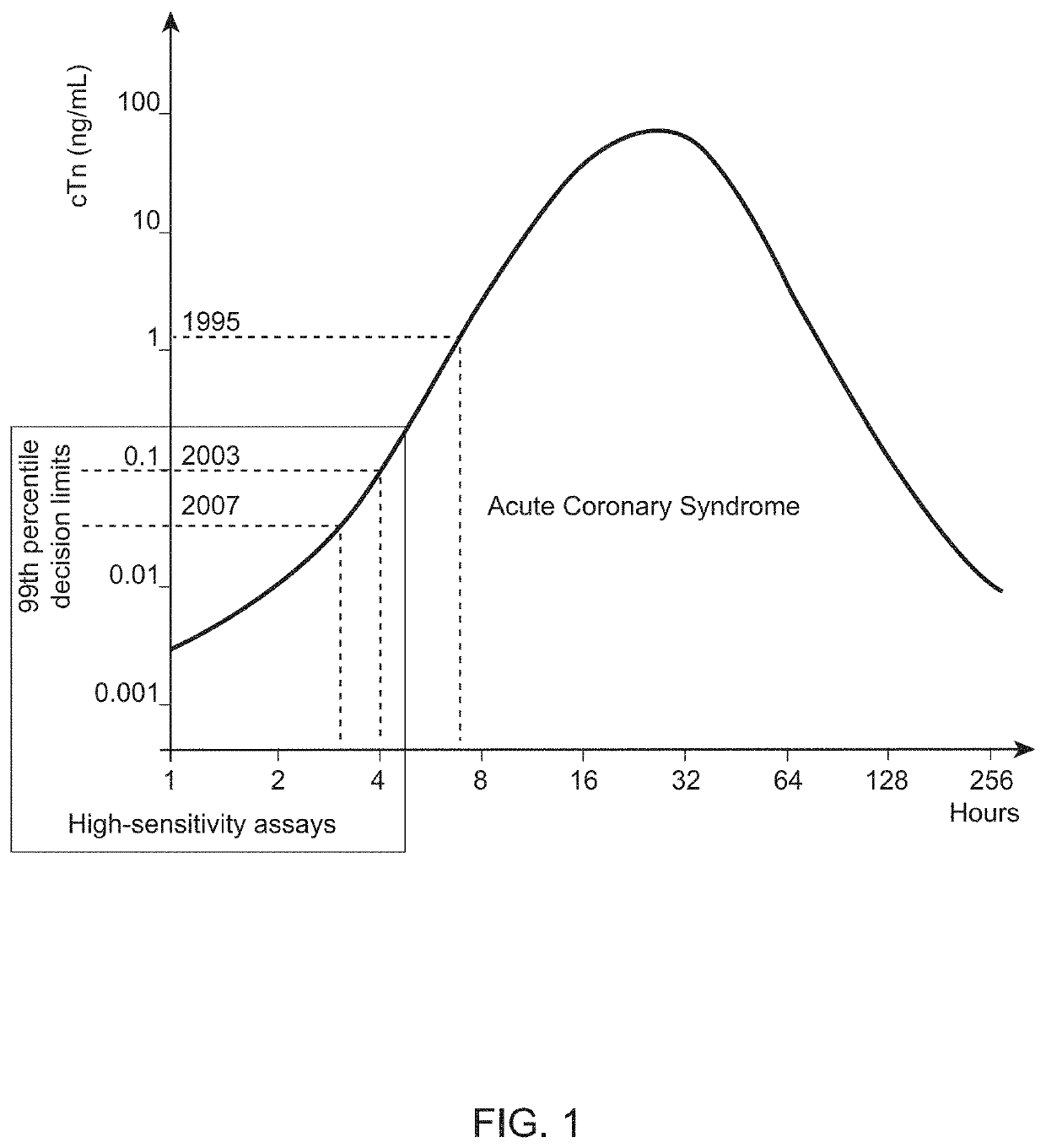
Nitrated cardiac troponin i as a biomarker of cardiac ischemia
ActiveUS20130330744A1Peptide/protein ingredientsMicrobiological testing/measurementTreatment managementTandem mass spectrometry
The present invention relates to the identification of a novel biomarker for cardiac ischemia: nitrated cardiac troponin I. The present invention also provides methods for the identification and use of a nitrated cardiac troponin as a biomarker for the diagnosis, prognosis and treatment management of myocardial ischemia, with and without necrosis of heart muscle.Diagnosis and prognosis is conducted by determining the amount of nitrated cardiac troponin I in serum samples of subjects and the ratio of nitrated cardiac troponin I to non-nitrated cardiac troponin I in serum samples of subjects. This biomarker can be detected by immunoassay techniques and tandem mass spectrometry. The present invention further relates to peptides, antibodies, compositions, methods, techniques, tests and kits for the identification and quantification of nitrated cardiac troponin I in samples of subjects.
Owner:FLEURY +2
Method for extracting salinomycin compound from animal sample and special immune affinity sorbent thereof
InactiveCN101433825AFacilitates residue analysisHigh selectivityOrganic chemistryOther chemical processesAntigenSorbent
The invention discloses a method for extracting salinomycin compounds from an animal sample and a special immunoaffinity absorbent thereof. The immunoaffinity absorbent for extracting the salinomycin compounds from the animal sample consists of a solid phase carrier and a salinomycin monoclonal antibody which is coupled with the solid phase carrier; the salinomycin monoclonal antibody is an antibody obtained by taking a conjugate of salinomycin semiantigen and carrier protein as immunogen; and the salinomycin compounds are salinomycin or methylsalinomycin. The immunoaffinity absorbent and a chromatographic column use the high specific salinomycin monoclonal antibody, have high selectivity, ensure the reliability of detection effect, greatly simplify the pretreatment processes of samples at the same time, are particularly applicable to the pretreatment of a trace amount of salinomycin and methylsalinomycin in muscle and liver, and improve the analysis quality. A detection method of the invention can efficiently detect the content of the salinomycin and the methylsalinomycin, and remedy the disadvantages that the direct assay of the samples by a single immunoassay technology has less information amount, poor quantification accuracy, or low selectivity of physical and chemical methods and so on, so the immunoaffinity absorbent, the chromatographic column, a reagent kit and the methods for extracting and detecting the salinomycin compounds in the animal sample are suitable to be promoted and applied.
Owner:CHINA AGRI UNIV
Methods for assessing the immune system in a patient
InactiveUS20130130286A1Efficient identificationDisease diagnosisBiological testingImmunological diseasesImmunoassay technique
Methods of determining the onset or susceptibility of an immunological disease are provided herein. Also provided are immunoassay techniques for carrying out such methods.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
High throughput screening method for anti-influenza virus inhibitor with PAC-PB1N complex as target
InactiveCN103185790AAntiviralsColor/spectral properties measurementsHigh-Throughput Screening MethodsNatural product
The invention provides a screening method for an anti-influenza virus material, and an application thereof, and more specifically provides a method for screening the anti-influenza virus material from natural product extracts based on an enzyme-linked immunoassay technique. The screening method can screen and obtain effective anti-influenza virus material from a great amount of the natural product extracts efficiently, conveniently and accurately, and can be used for preparing drugs capable of treating and / or preventing the influenza virus. The invention also relates to a high throughput screening method for an anti-influenza virus inhibitor with PAC-PB1N complex as a target.
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE
Combined immunoassay and magnetic immunoassay systems and devices for extended range of sensitivity
ActiveUS10908154B2Material analysis by electric/magnetic meansLaboratory glasswaresImmobilized AntibodiesAnalyte
The present invention relates to systems that utilize a combination of immunoassay and magnetic immunoassay techniques to detect an analyte within an extended range of specified concentrations. In particular, a device is provided for detecting an analyte in a biological sample. The device includes a first electrochemical sensor positioned on a substrate. The first electrochemical sensor includes an immobilized layer of antibody configured to bind to the analyte. The device further includes a second electrochemical sensor positioned adjacent to the first electrochemical sensor on the substrate, and a magnetic material that generates a magnetic field aligned with respect to the second electrochemical sensor. The magnetic field captures magnetic beads that have an immobilized layer of antibody configured to bind to the analyte, and concentrates the magnetic beads on or near a surface of the second electrochemical sensor.
Owner:ABBOTT POINT CARE
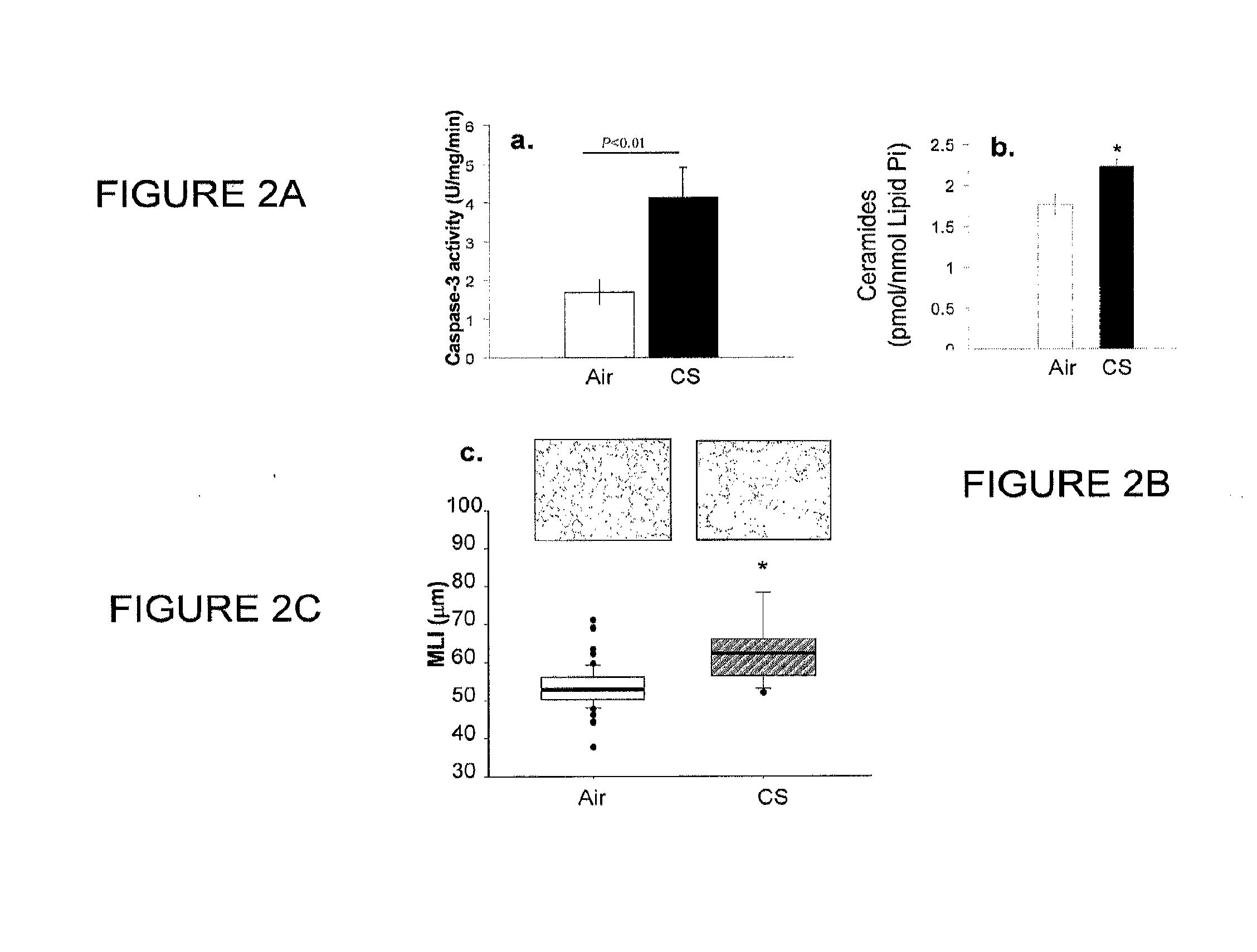
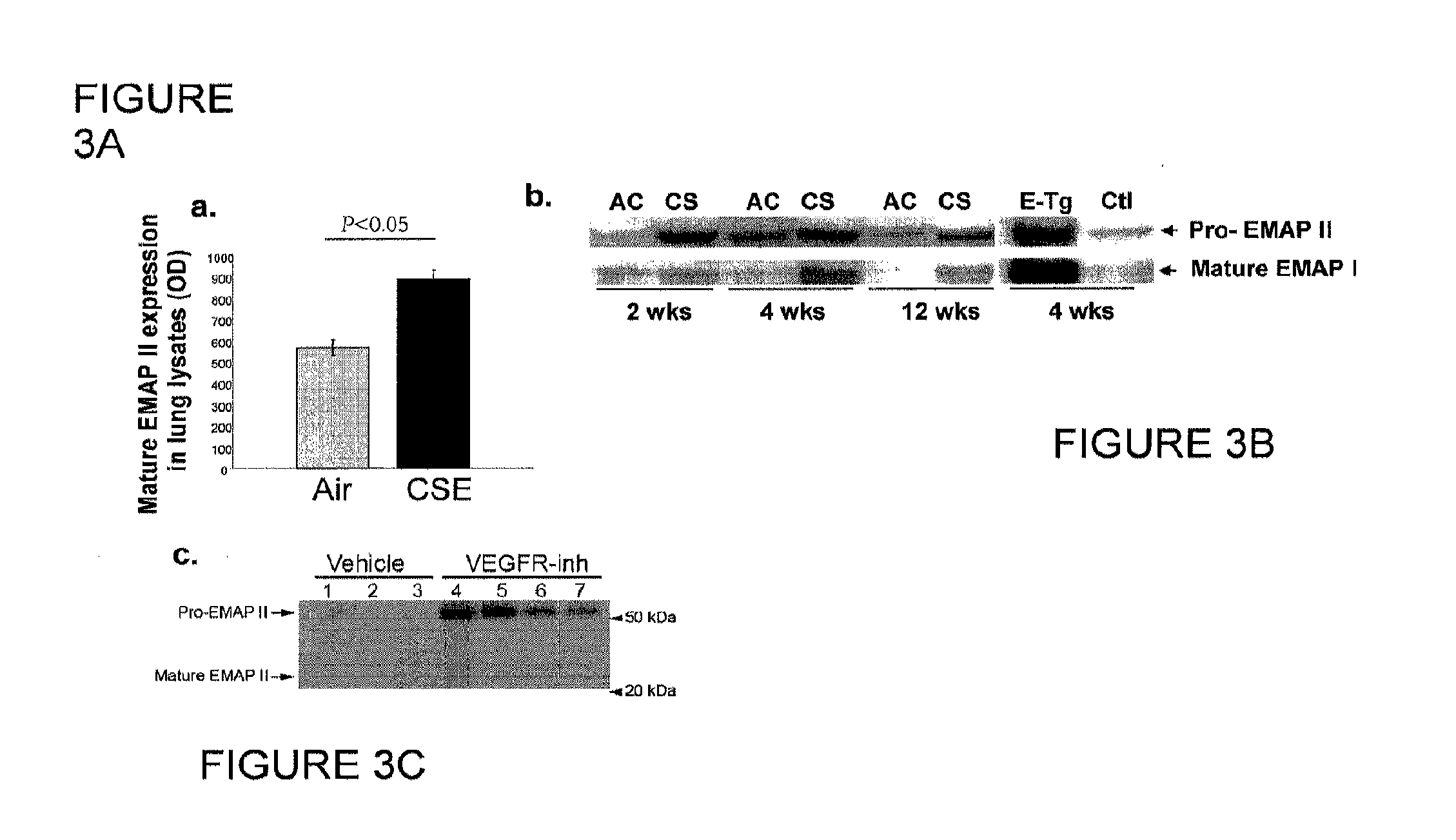
Method for diagnosing and treating emphysema
Owner:INDIANA UNIV RES & TECH CORP
Crossover analytical systems and methods using an immunosensor and magnetic immunosensor
The present invention relates to systems and methods that utilize a combination of immunoassay and magnetic immunoassay techniques to detect an analyte within an extended range of specified concentrations. In particular, a method includes determining a first concentration of an analyte at a first immunosensor from a reaction of a signal agent with a first complex of signal antibodies, the analyte, and capture antibodies immobilized on a surface of the first immunosensor, determining a second concentration of the analyte at a second immunosensor from a reaction of the signal agent with a second complex of the signal antibodies, the analyte, and capture antibodies immobilized on magnetic beads that are localized on or near a surface of the second immunosensor via a magnetic field, determining a weighted average of the first concentration and the second concentration, and comparing the weighted average to a predetermined crossover concentration point or zone.
Owner:ABBOTT POINT CARE
Methods Of Determining Allergen Response Using Microarray Immunoassay Techniques
The present invention is directed to materials and methods that may be used in diagnosing and / or characterizing allergies. More specifically, the specification describes methods and compositions for making and using a plurality of peptides having allergen epitopes that may be used in immunoassays e.g., microarray based immunoassays to predict the severity of an allergic response.
Owner:MT SINAI SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com