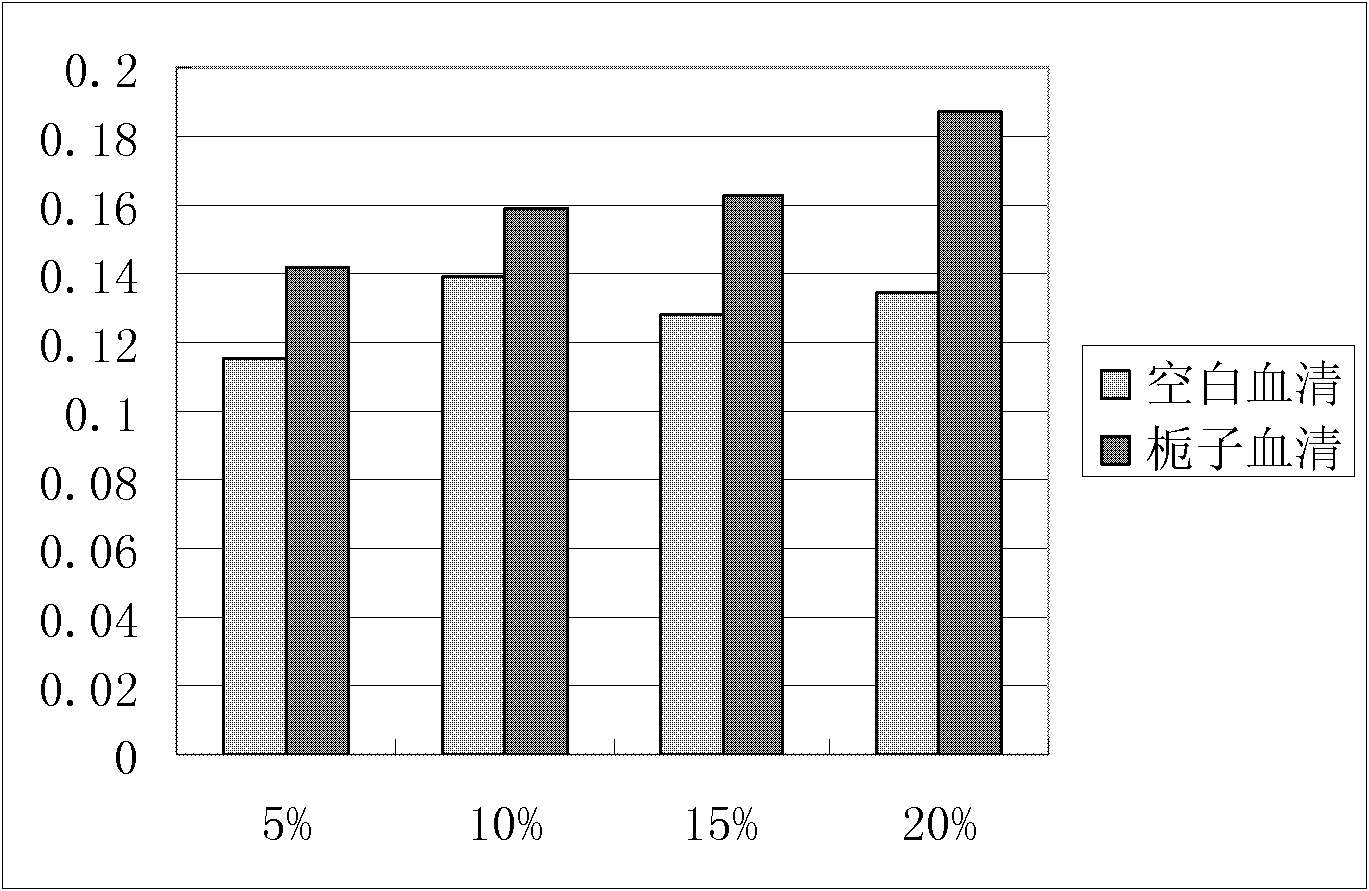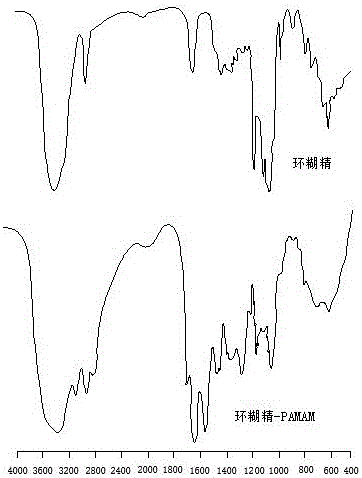Patents
Literature
37 results about "Percutaneous transluminal coronary angioplasty" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Percutaneous transluminal coronary angioplasty (PTCA) is a minimally invasive procedure to open up blocked coronary arteries, allowing blood to circulate unobstructed to the heart muscle.
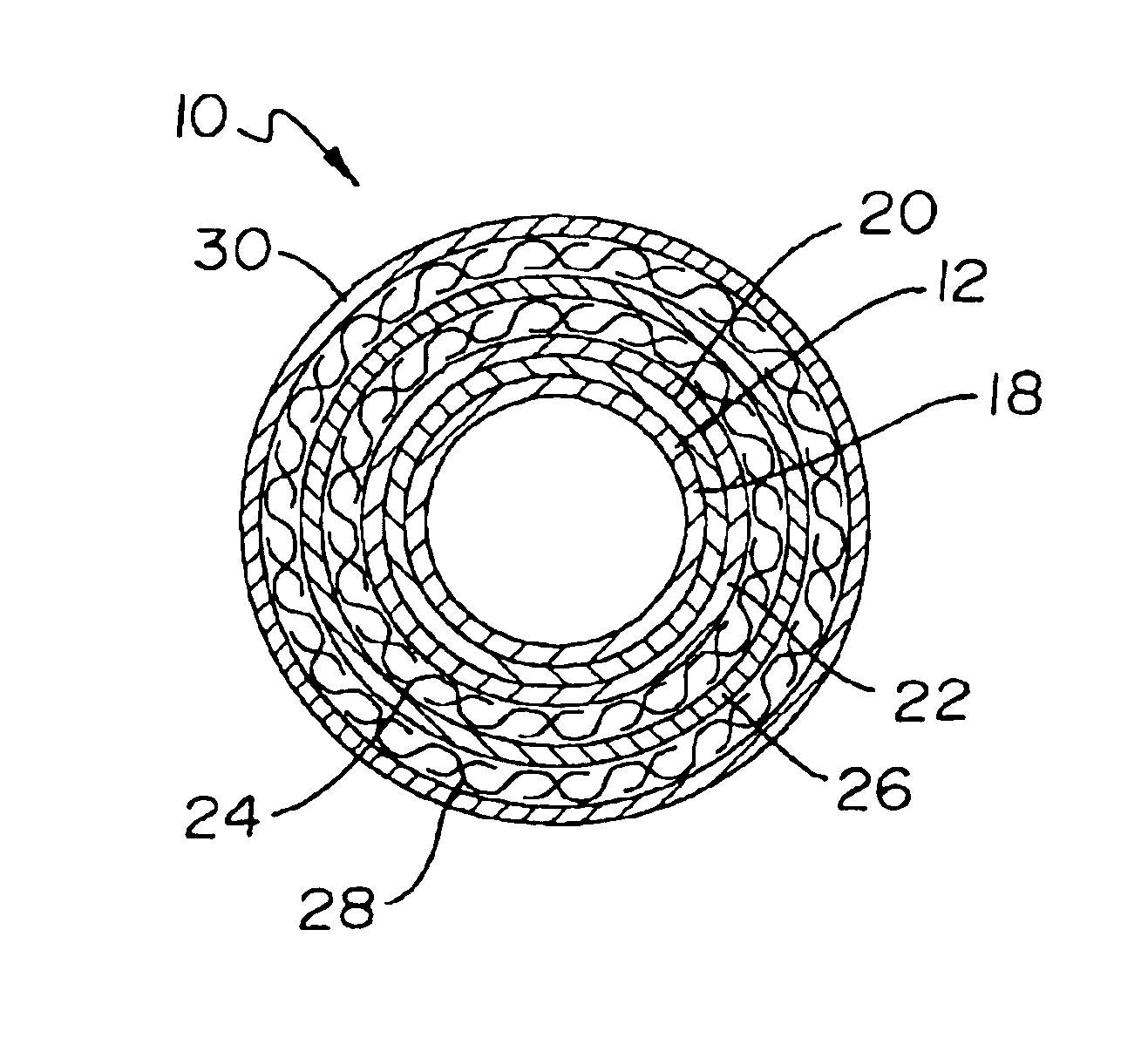
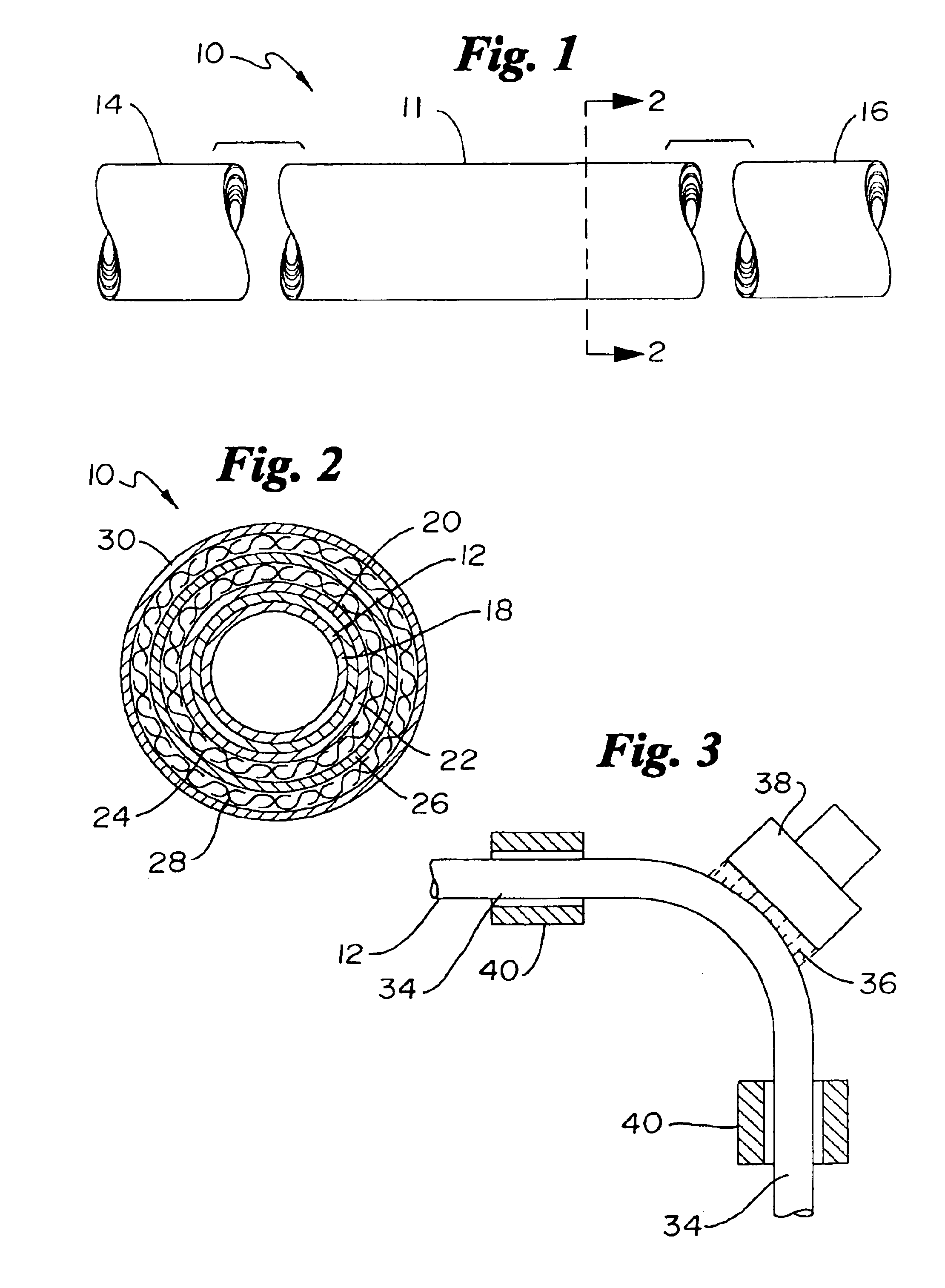
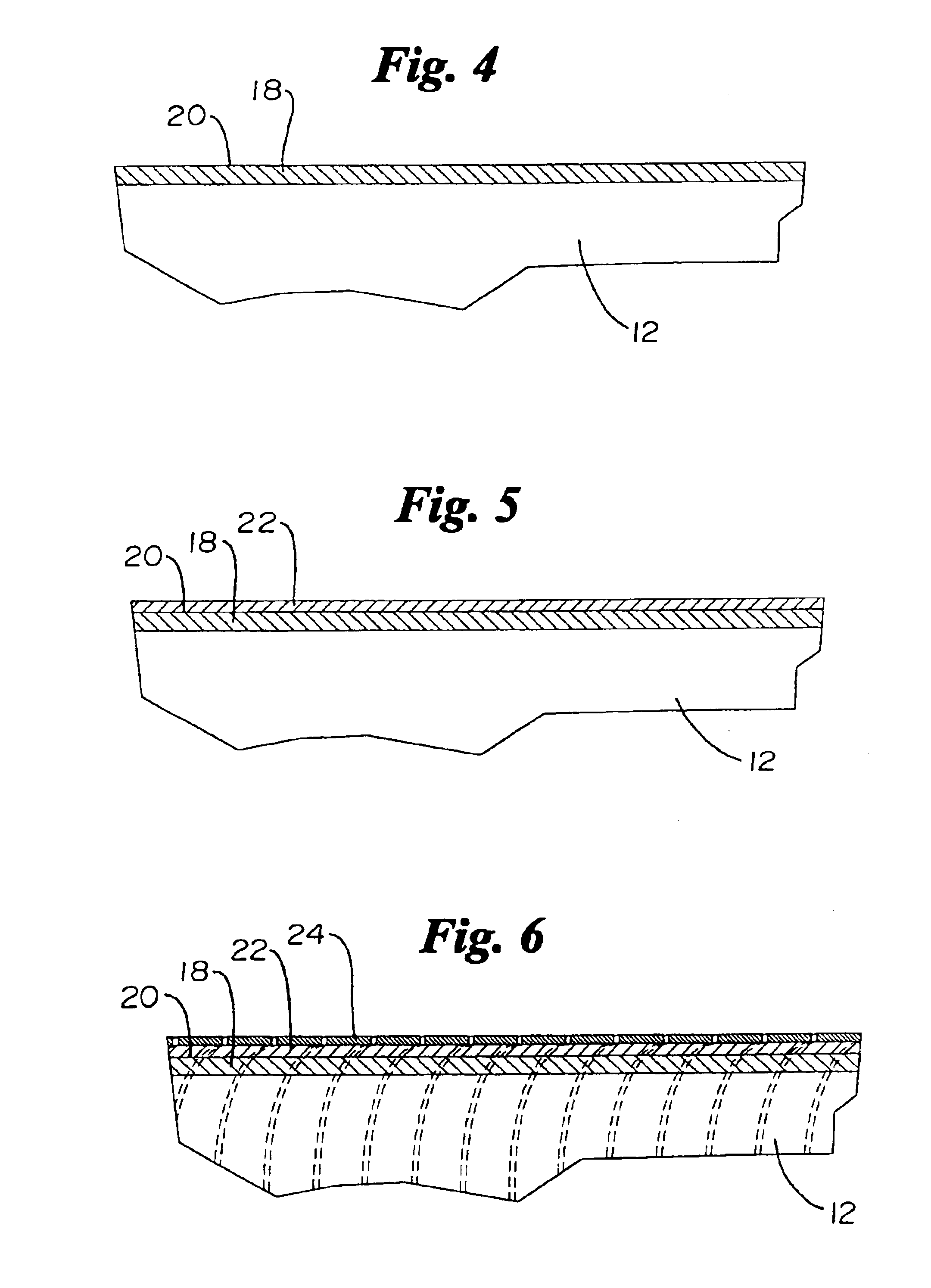
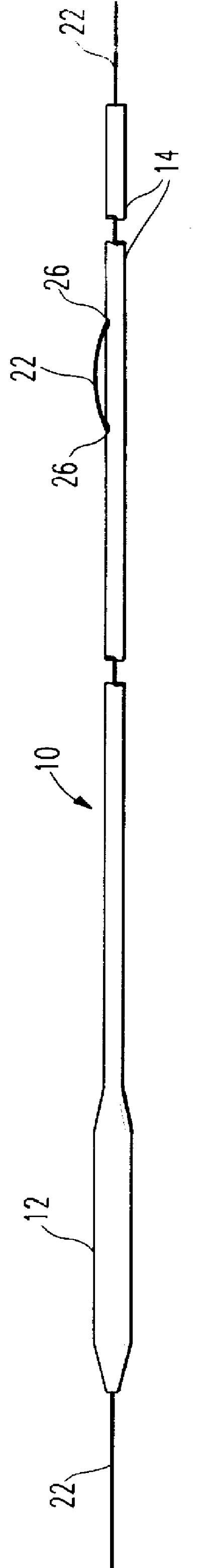
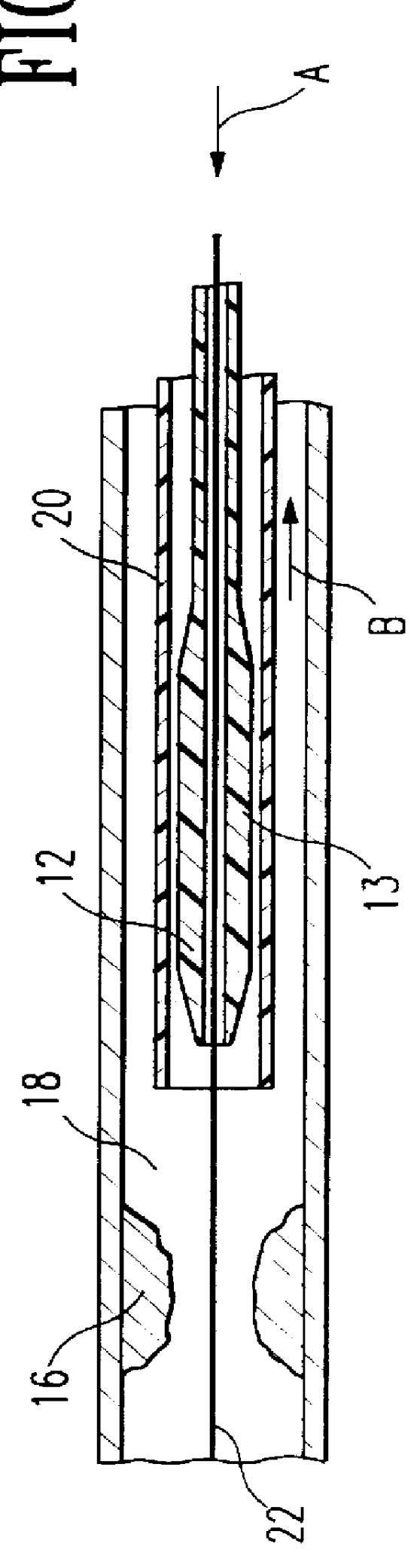
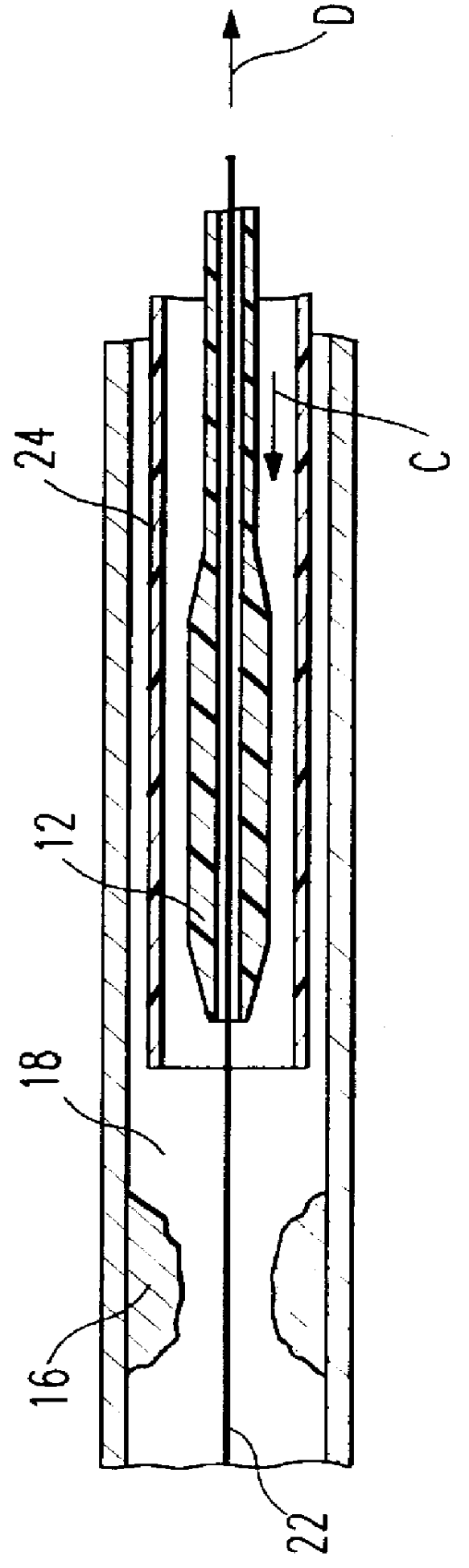
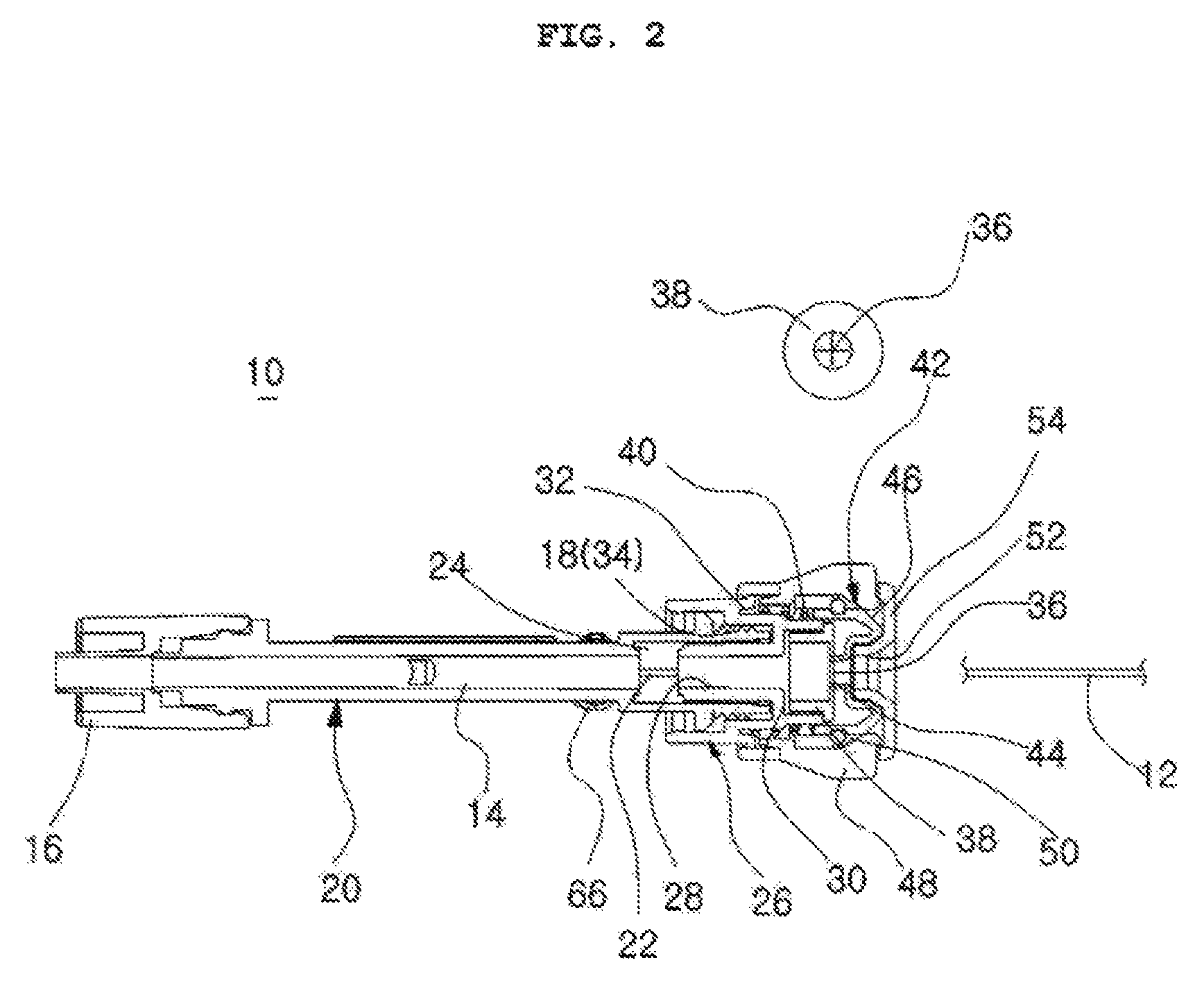
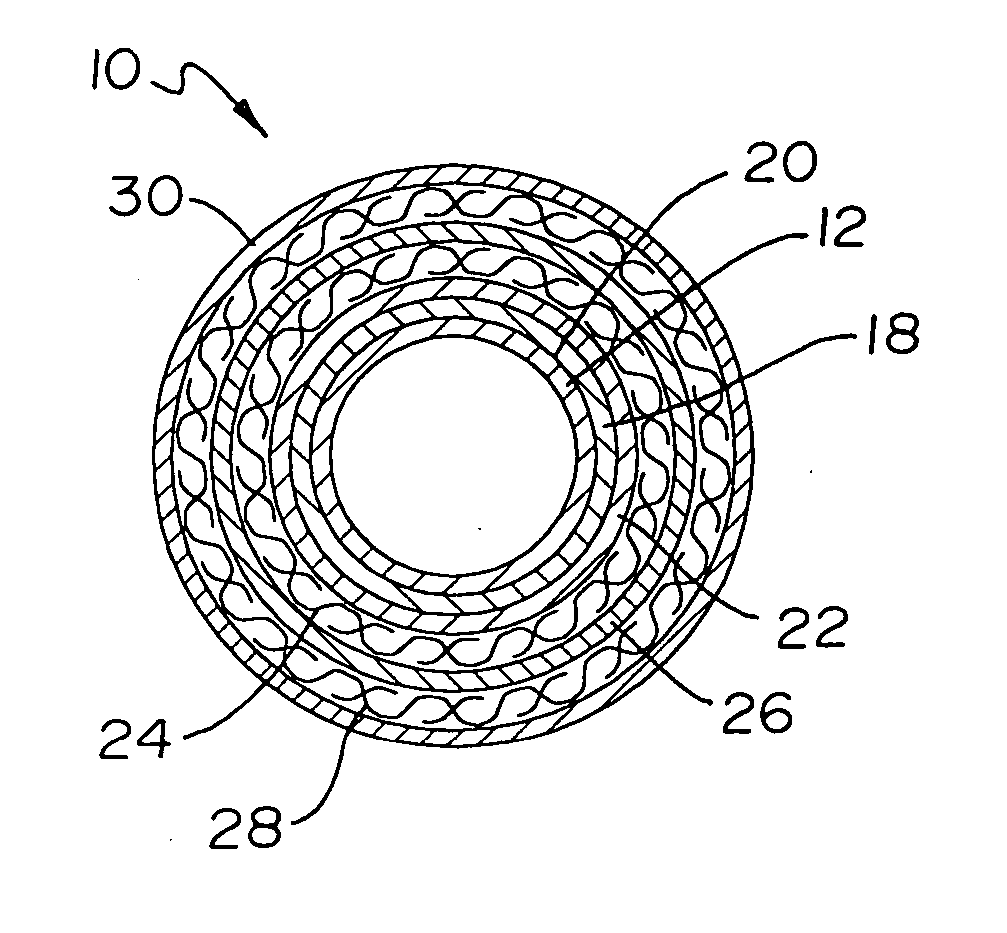
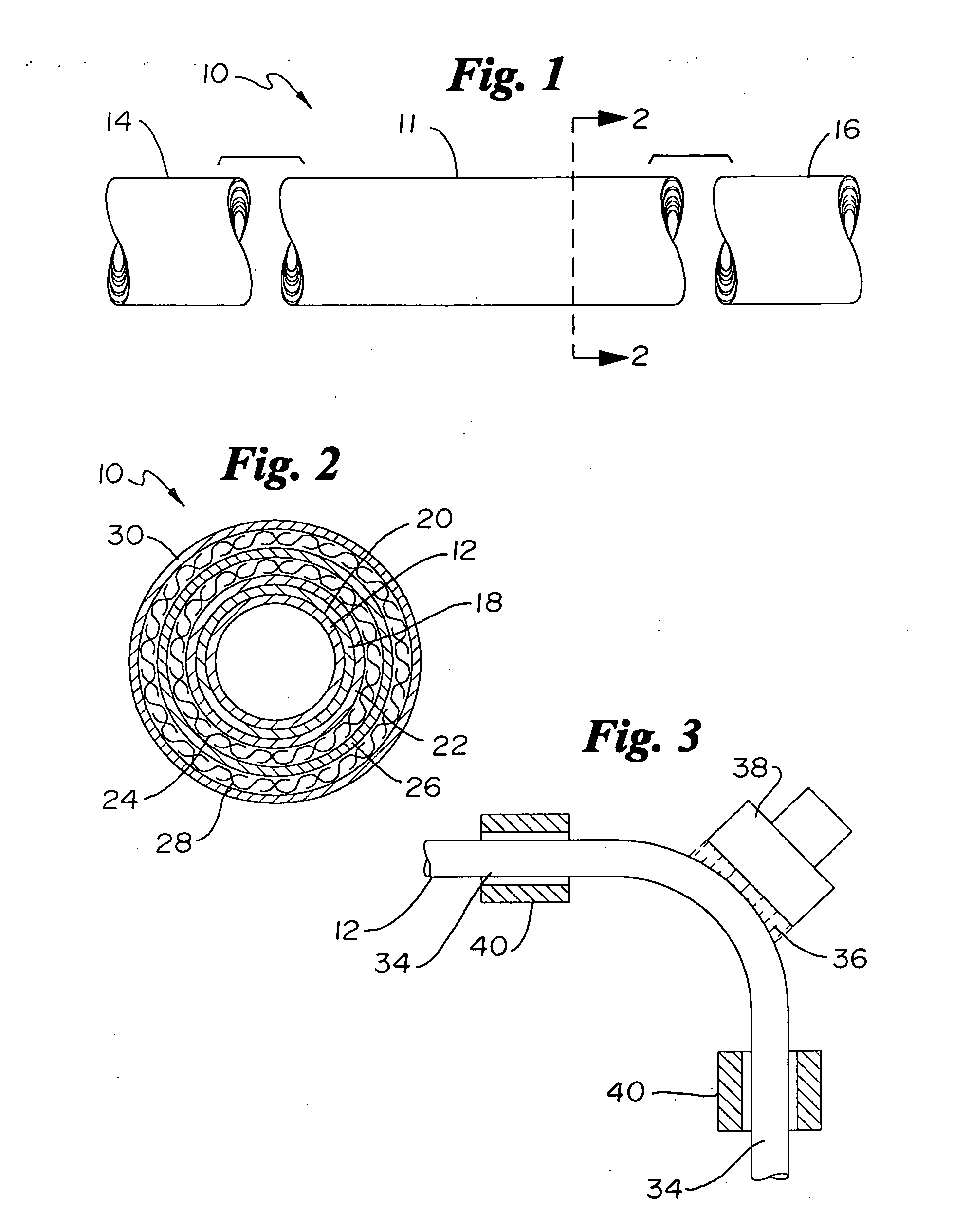
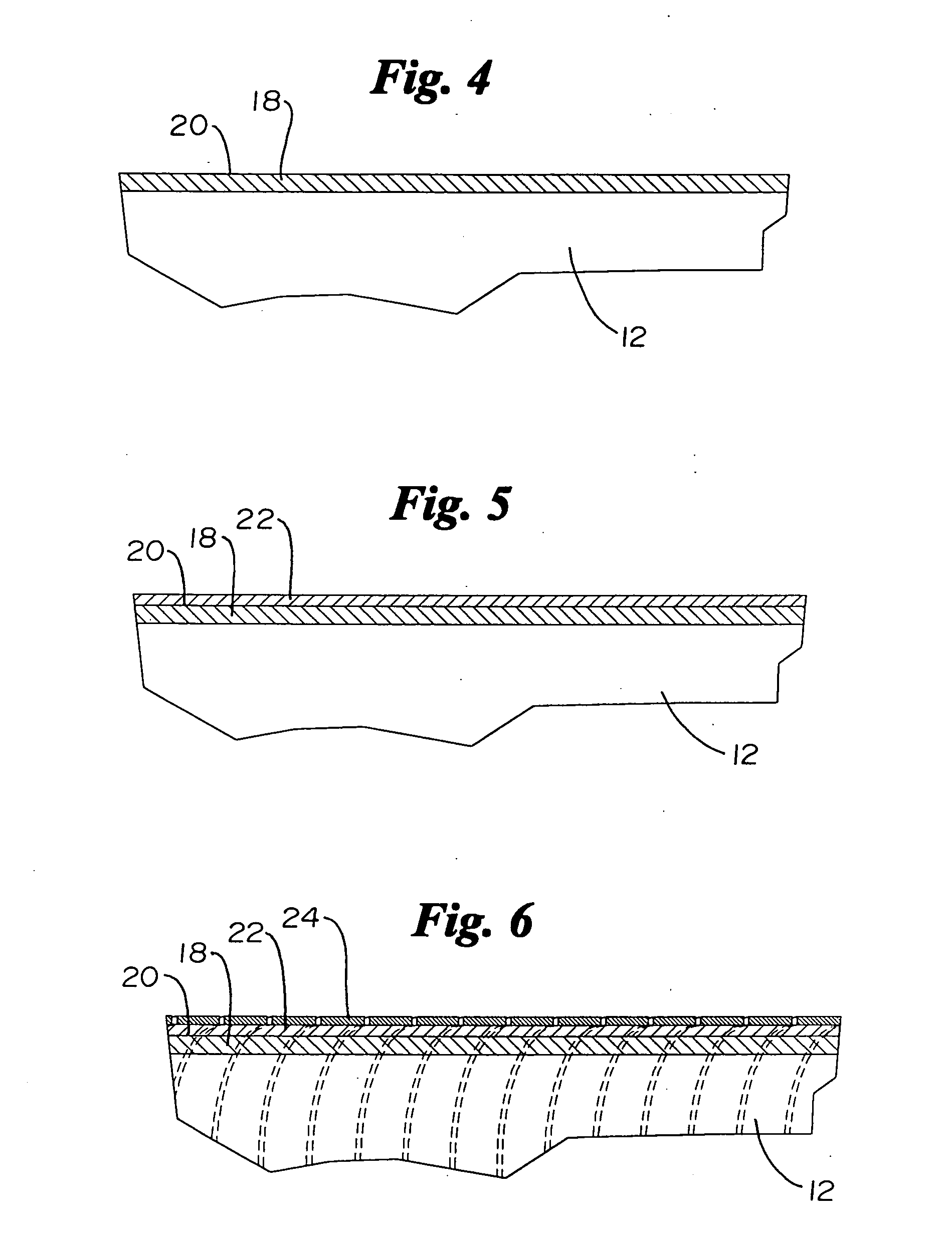
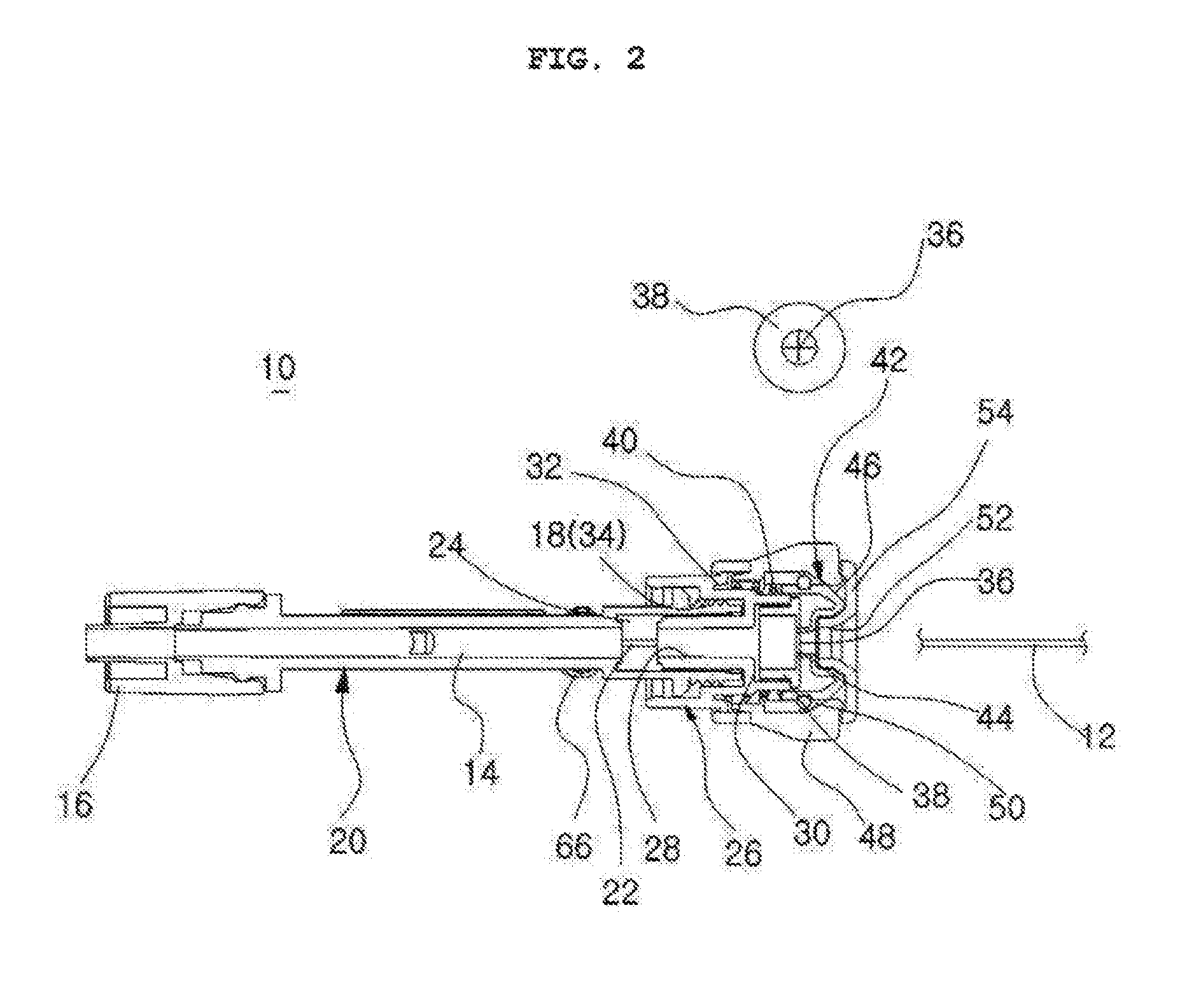
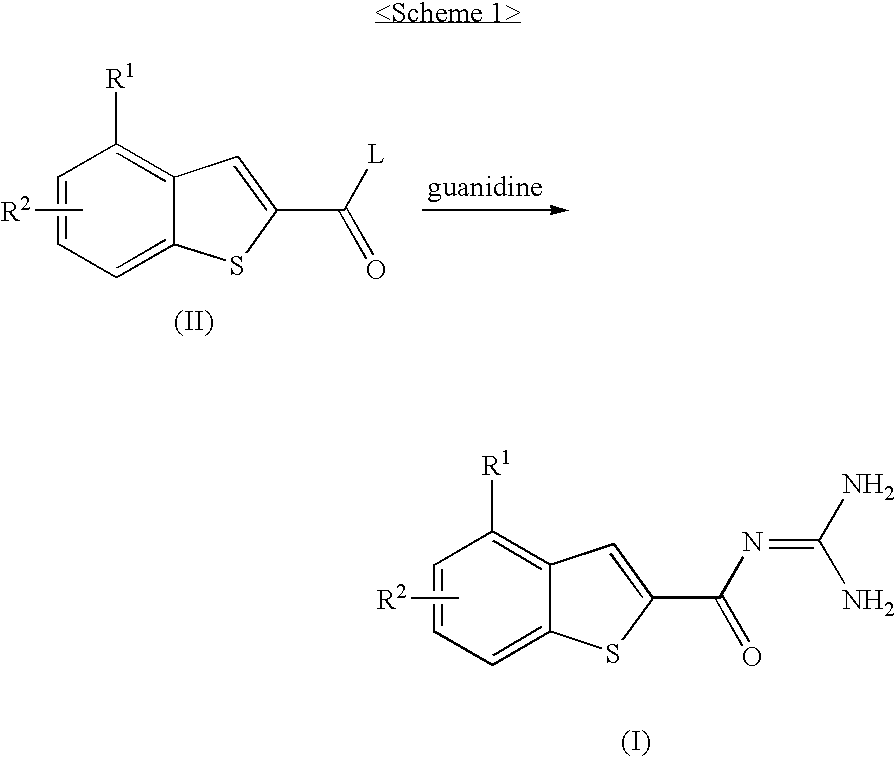
Catheter incorporating a curable polymer layer to control flexibility and method of manufacture
The present invention relates generally to catheters for performing medical procedures including percutaneous transluminal coronary angioplasty. More particularly, the present invention relates to guide catheters, diagnostic catheters and balloon catheters with an improved shaft design. In a preferred embodiment, the present invention includes a catheter shaft comprising an elongate support member having an outer surface, the elongate support member preferably defining a lubricious liner; a first layer disposed over the lubricious liner, a second layer disposed over the first layer, a third layer disposed over the second layer, a fourth layer disposed over the third layer, and a fifth layer disposed over the fourth layer. In preferred embodiments, the first and third layers comprise an ultraviolet-curable epoxy which is cured to desired degrees at select axial locations on the shaft to provide desired stiffness.
Owner:BOSTON SCI SCIMED INC
Catheters with enhanced exchange capability
An exchange catheter facilitates the replacement of an existing guiding catheter with a larger guiding catheter during catheterization procedures such as percutaneous transluminal coronary angioplasty without disturbing the position of a guidewire. The exchange catheter has a distal portion through which a guidewire is slidable. The outer diameter of the distal portion is slightly less than the inner diameter of the existing guiding catheter. A femoral sheath is slidable over the proximal portion of the exchange catheter so as to facilitate femoral sheath exchange.
Owner:ABBOTT CARDIOVASCULAR
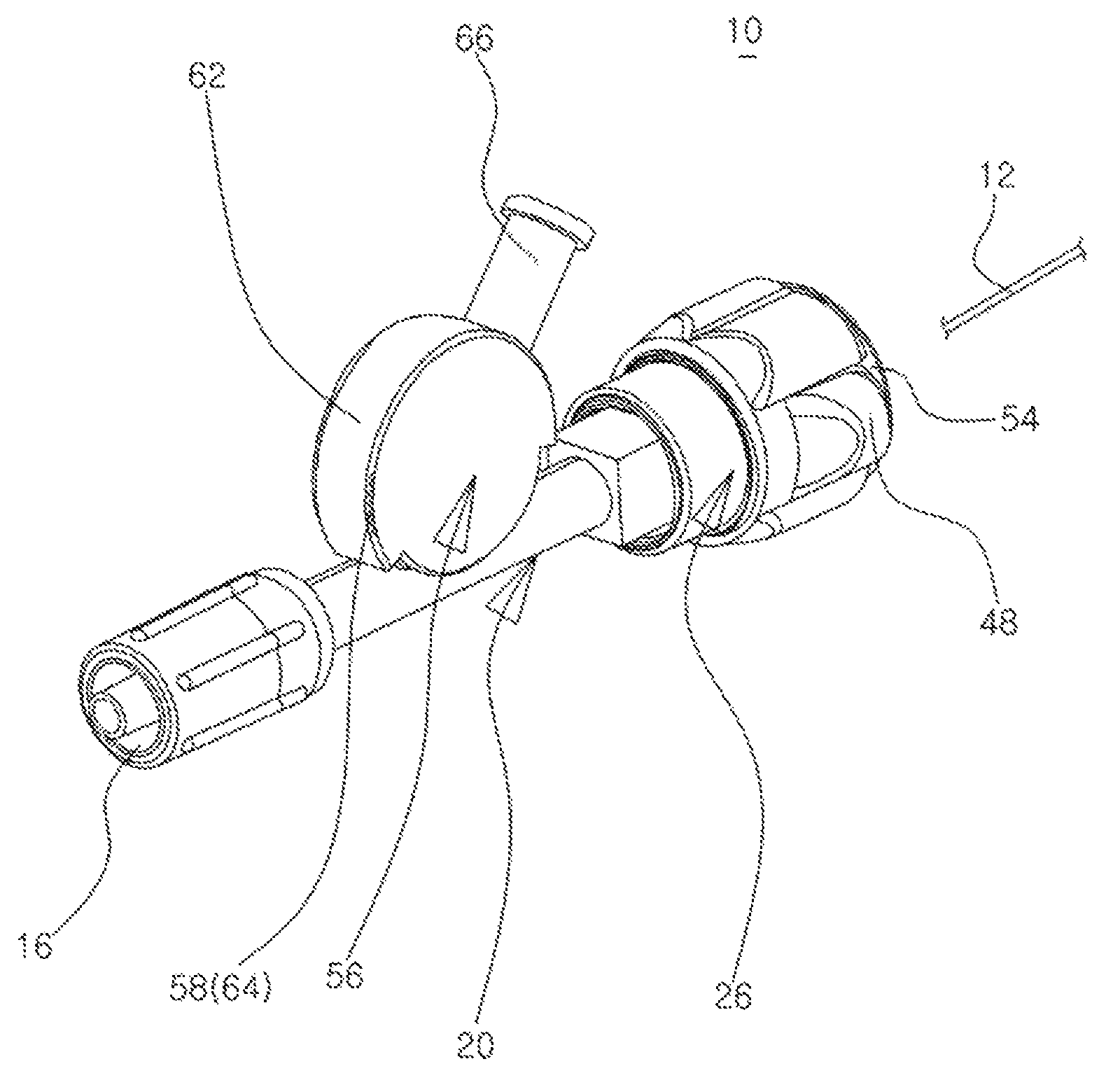
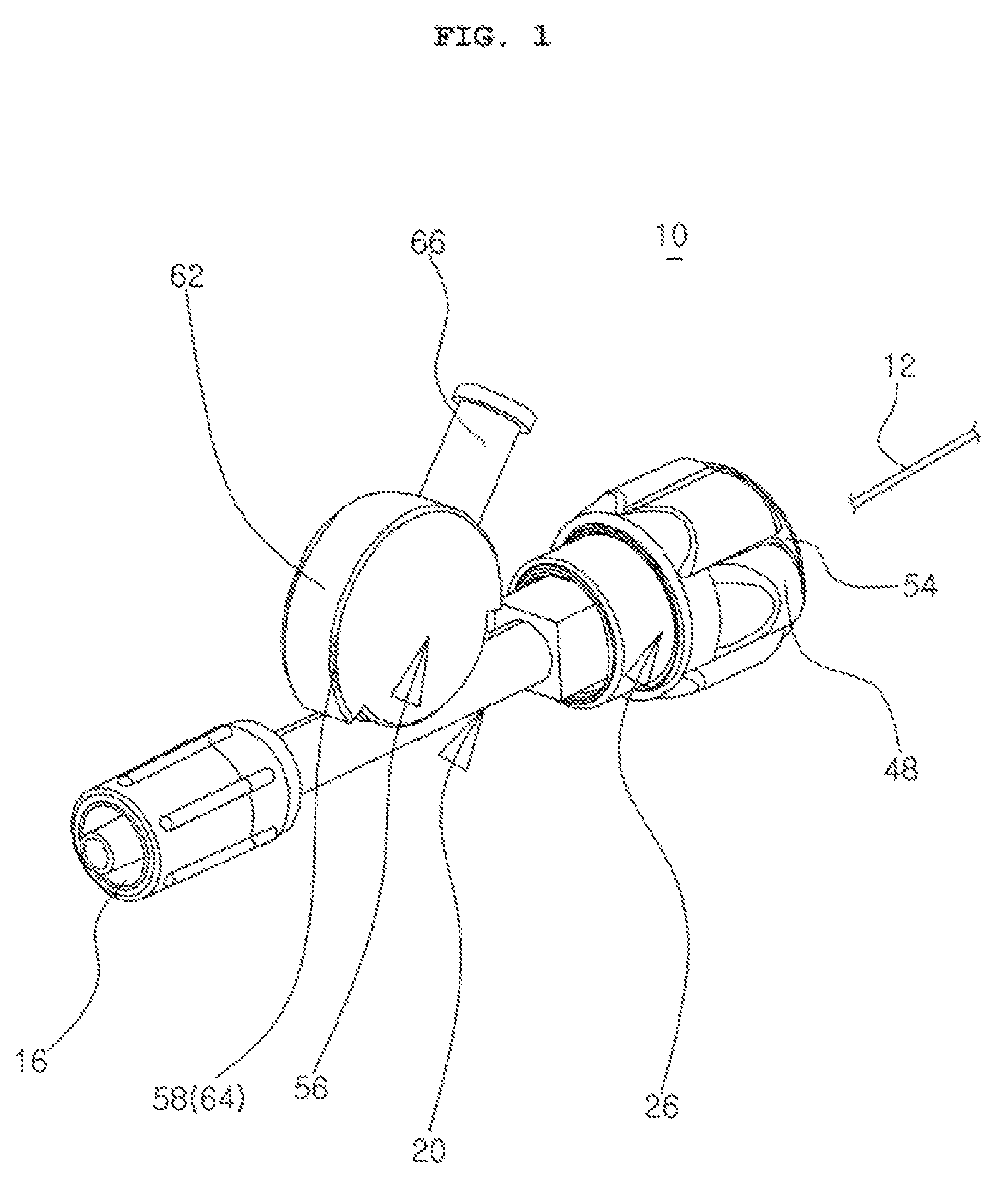
Hemostasis valve device
The present invention relates to a hemostasis valve device which allows a wire or a catheter to be inserted into the left or right coronary artery via the femoral artery or an arm artery when a Cardiac Catheterization or Percutaneous Transluminal Coronary Angioplasty operation is performed, wherein two independent sealing members are opened and closed by press and release actions of push buttons coupled to a body and the rotation of a fastening tube, respectively, so that the leakage of blood or the inflow of outside air is simply and effectively blocked during the operation, and a drug influx tube for allowing a medicine such as a thrombolitic drug to flow into a patient during the operation pivots and is adjusted in a stepwise manner within a certain range of angles according to body conditions or movements of the patient.
Owner:HUBIOMED INC
Methods for reducing morality associated with acute myocardial infarction
InactiveUS7361339B2Reduce mortalityImmunoglobulins against blood coagulation factorsOrganic active ingredientsMortality rateAntibody
Methods of reducing mortality in myocardial infarction patients receiving a stent in connection with percutaneous transluminal coronary angioplasty include administering an anti-inflammatory compound to the patient. In one embodiment, the anti-inflammatory compound is an antibody to a complement component.
Owner:ALEXION PHARMA INC
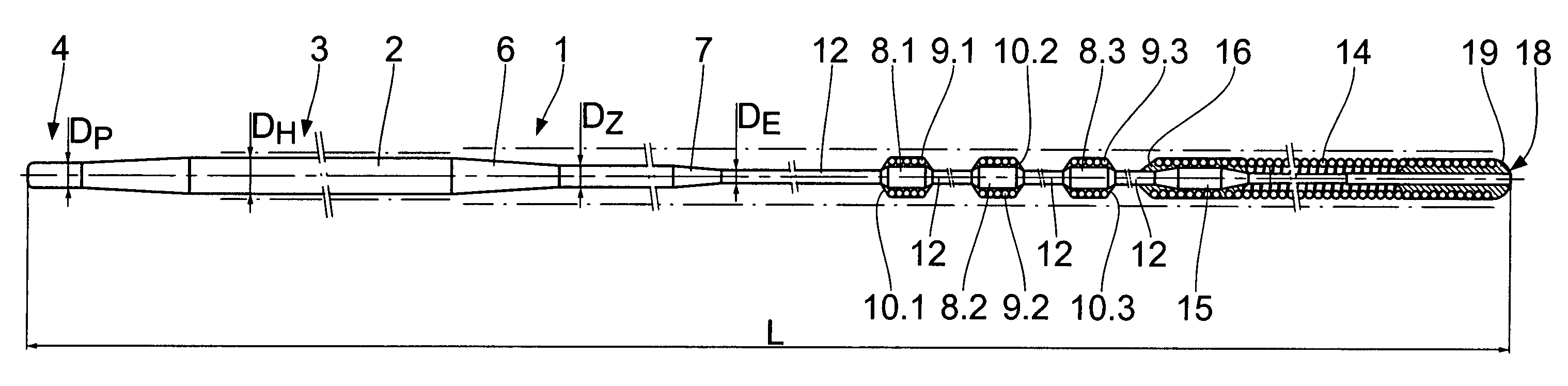
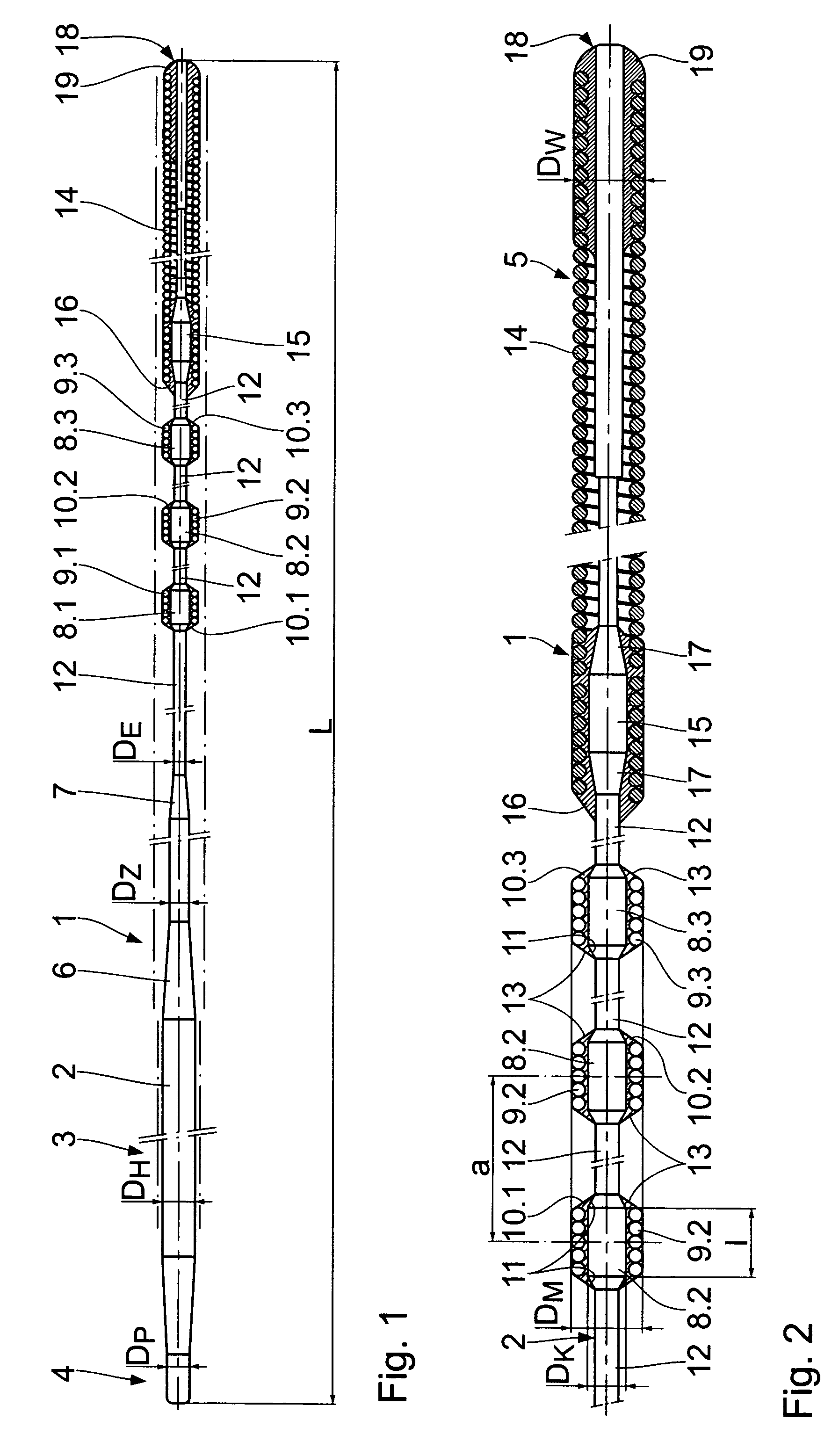
Catheter guide wire especially for percutaneous transluminal coronary angioplasty
A catheter guide wire, especially for percutaneous transluminal coronary angioplasty, comprises an elongated wire shaft of a flexible material having a proximal and a distal end and disposed on the wire shaft at least one radiopaque, sleeve-like marker adjacent to the distal end. The at least one marker is disposed on a core section of the wire shaft, said core section being widened in its diameter relative to the diameter of the adjoining shaft sections.
Owner:BIOTRONIK AG
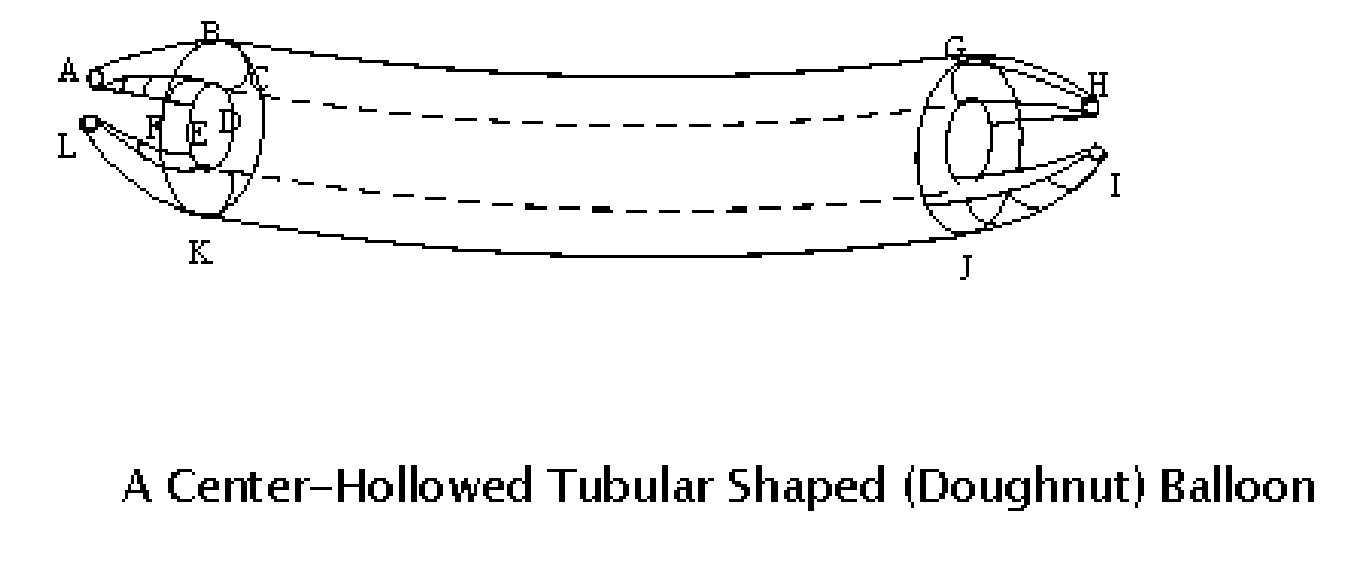
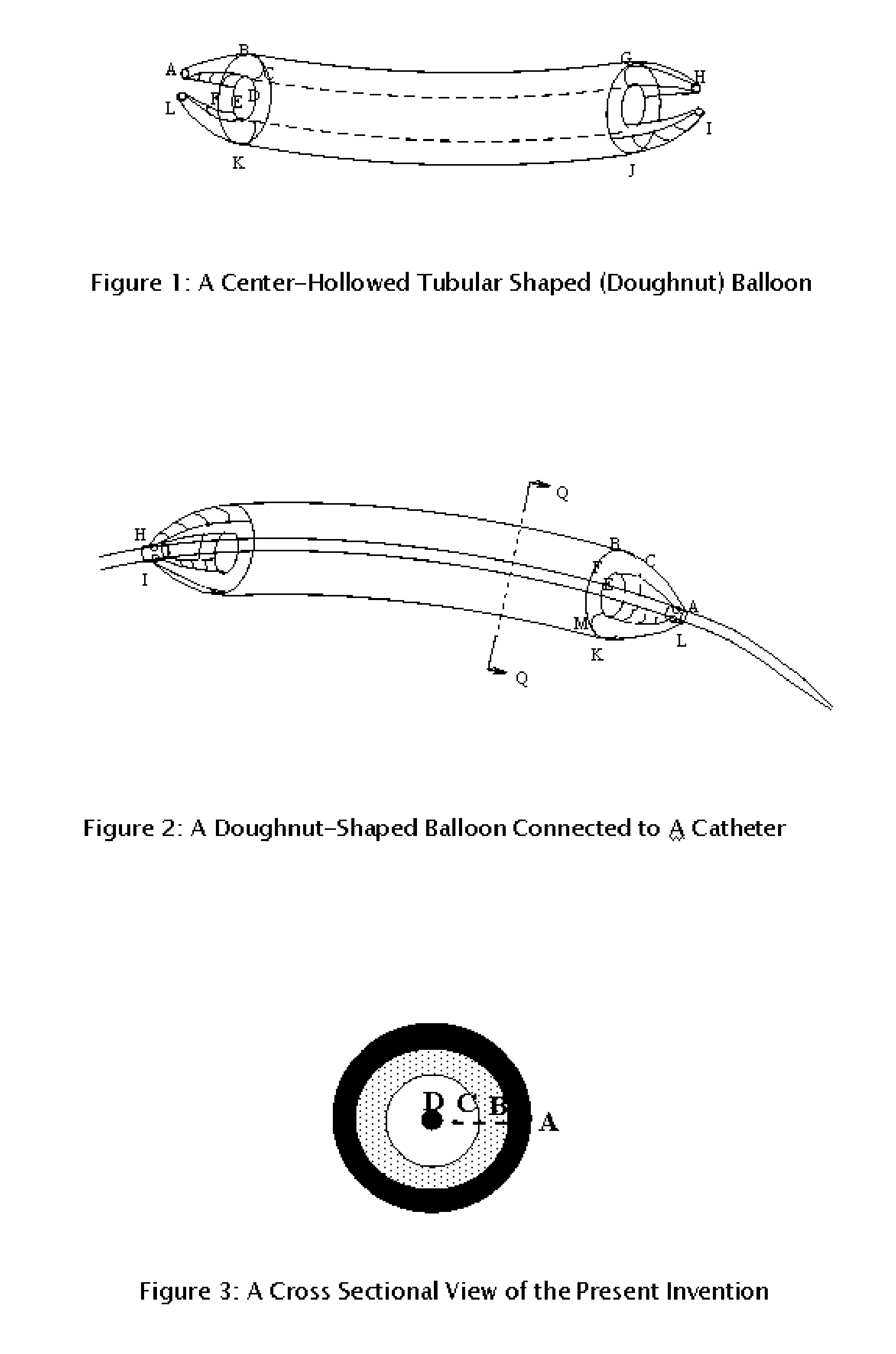
Center_hollowed_tubular_shaped balloon for
The present invention is directed to a procedure and a special balloon used in balloon angioplasty and stent installation in percutaneous transluminal coronary angioplasty (PTCA), which employs a heart catheter with a balloon at its distal end. After being inflated, the balloon is in doughnut shape with a hole in the center; its length is made to fit the length of the stent to be inflated. During an angioplasty procedure, the balloon is pushed forward into the stenosis and inflated with a device to dilate the blockage. The doughnut_shaped balloon provides a pass way for blood to flow through the coronary artery vessel, which does not cause angina akin to a heart attack resulted from traditional tube_shaped balloons used in the PTCA procedure that completely block blood flow after being fully inflated during balloon angioplasty and stent installation.
Owner:WANG WILLIAM TIANYUAN +1
Medicament for therapeutic treatment of vascular disease
A medicament for prophylactic and / or therapeutic treatment of a vascular disease such as vascular restenosis and / or reocclusion after percutaneous transluminal coronary angioplasty using an intravascular stent, which comprises as an active ingredient a substance selected from the group consisting of retinoids and agents for controlling actions of retinoids such as 4-[5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbamoyl]benzoic acid or a salt thereof, or 4-[[[(3,5-bis-(trimethylsilyl)phenyl]carbonyl]amino]benzoic acid or a salt thereof, wherein said substance has substantially no antiproliferative action on vascular endothelial cells, but substantially has antiproliferative action on vascular smooth muscle cells.
Owner:RES FOUND ITSUU LAB
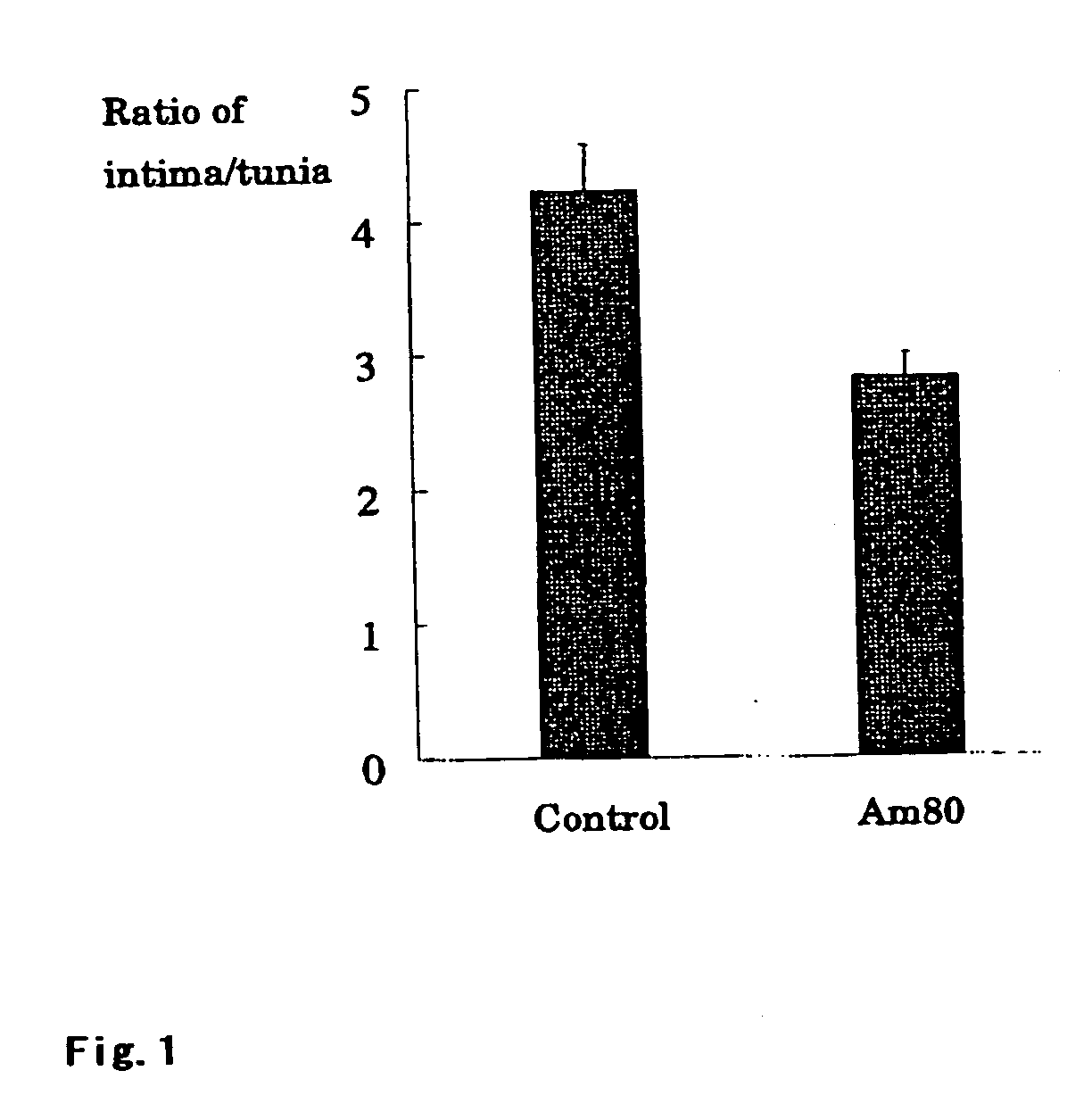
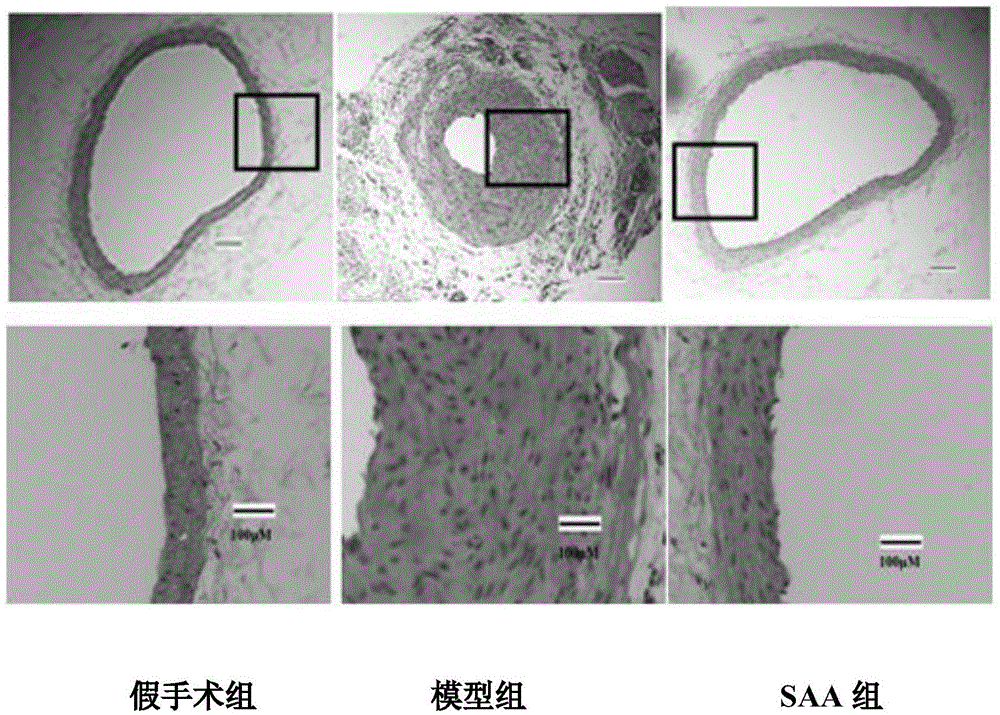
Application of salvianolic acid A in preparation of medicine for resisting tunica intima thickening, post-angioplasty restenosis and/or in-stent restenosis
InactiveCN105434417AReduce the degree of intimal hyperplasiaPrevent proliferationOrganic active ingredientsCardiovascular disorderBalloon injuryPercent Diameter Stenosis
The invention discloses application of salvianolic acid A in preparation of medicine for resisting tunica intima thickening, post-angioplasty restenosis and / or in-stent restenosis. Experimental research finds that salvianolic acid A can restrain proliferation of umbilical cord artery blood vessel smooth muscle cells of a person and reduce the tunica intima thickening rate and the blood vessel lumen restenosis rate of a tunica intima thickening animal model after balloon injury, has the remarkable effects of resisting tunica intima thickening and post-angioplasty restenosis and / or in-stent restenosis, can be used for preparing the medicine for restraining tunica intima thickening, post-angioplasty restenosis and / or in-stent restenosis and is particularly used for preparing medicine for preventing post-percutaneous coronary angioplasty restenosis and in-stent restenosis or used for preparing a drug eluting stent. In this way, efficient, safe and economical prevention and treatment medicine and an efficient, safe and economical solution are provided for prevention and treatment of post-angioplasty, particularly post-percutaneous coronary angioplasty restenosis and in-stent restenosis.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Catheter incorporating a curable polymer layer to control flexibility and method of manufacture
InactiveUS20050283135A1Altered stiffnessIncrease stiffnessLaminationLamination apparatusEpoxyMedicine
The present invention relates generally to catheters for performing medical procedures including percutaneous transluminal coronary angioplasty. More particularly, the present invention relates to guide catheters, diagnostic catheters and balloon catheters with an improved shaft design. In a preferred embodiment, the present invention includes a catheter shaft comprising an elongate support member having an outer surface, the elongate support member preferably defining a lubricious liner; a first layer disposed over the lubricious liner, a second layer disposed over the first layer, a third layer disposed over the second layer, a fourth layer disposed over the third layer, and a fifth layer disposed over the fourth layer. In preferred embodiments, the first and third layers comprise an ultraviolet-curable epoxy which is cured to desired degrees at select axial locations on the shaft to provide desired stiffness.
Owner:BOSTON SCI SCIMED INC
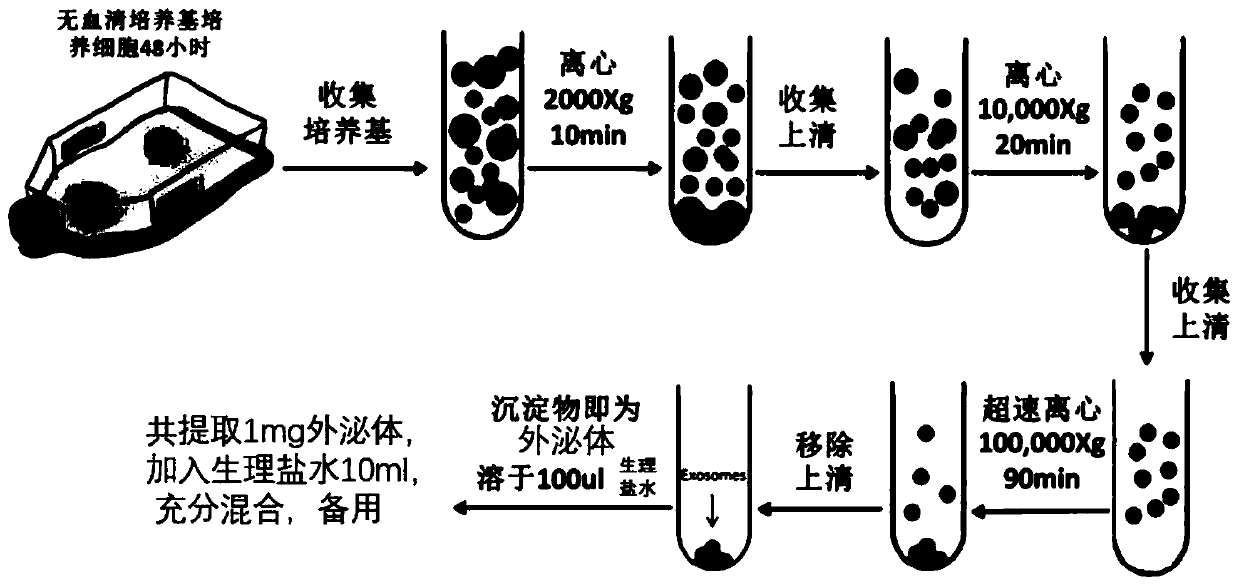
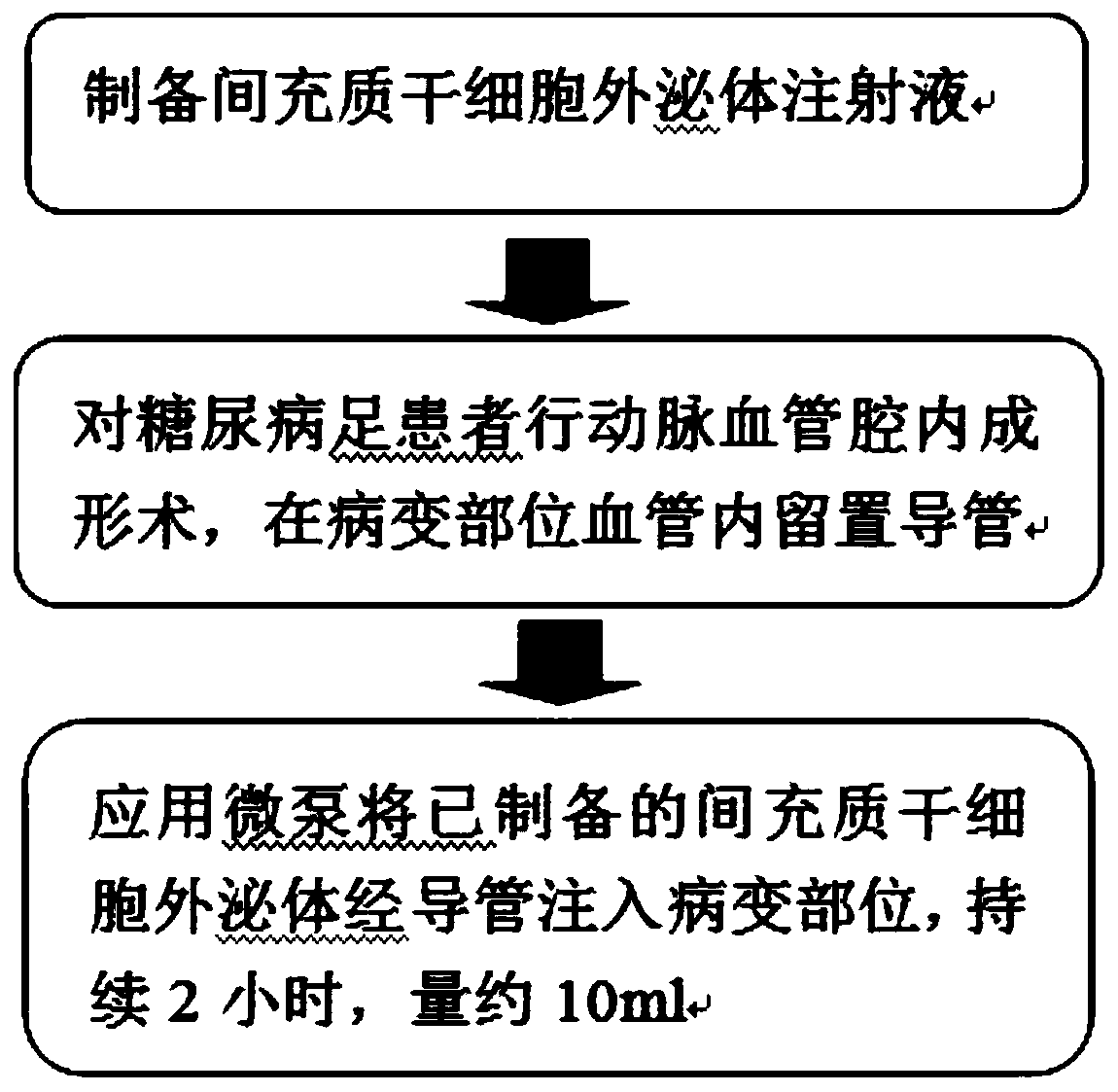
Preparation method of stem cell exosomes and novel application thereof
InactiveCN110193027APromote generationImprove microcirculationCell dissociation methodsMetabolism disorderCapillary networkPercent Diameter Stenosis
The invention relates to the field of biological preparations, in particular to a preparation method of stem cell exosomes and a novel application thereof. The method comprises the steps of extractionof mesenchymal stem cell exosomes, percutaneous transluminal coronary angioplasty, and stem cell exosome transplantation, and can be used for treating diabetic foot. The novel application of exosomescan accurately and effectively improve the microcirculation of diabetic foot, promotes angiogenesis, reconstructs capillary network, reduces occlusion of blood vessel restenosis, reduces amputation rate, and provides survival rate.
Owner:广州市番禺区中心医院
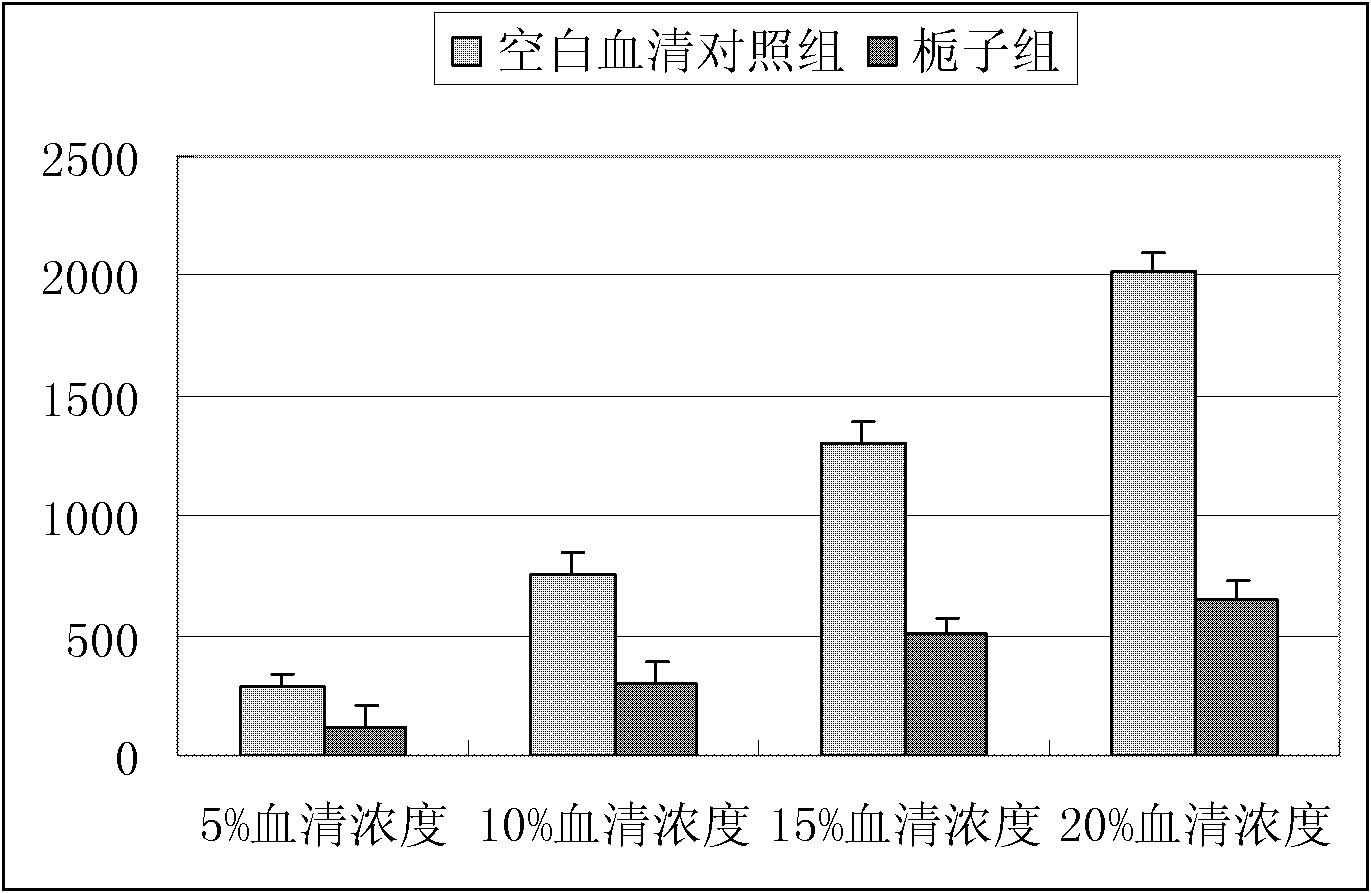
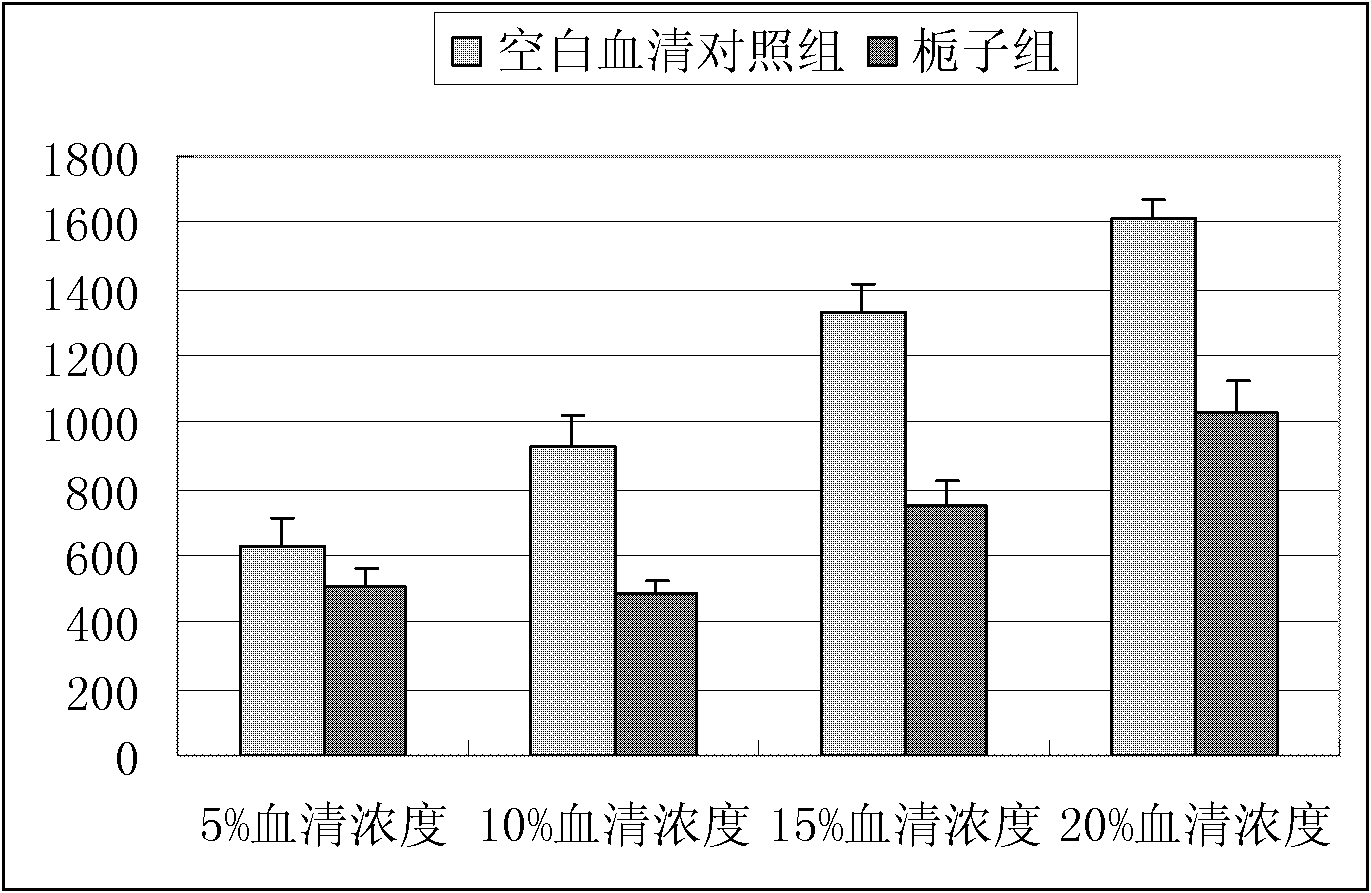
Novel applications of cape jasmine extract product in preparation of drugs
InactiveCN102716230ALower LDH levelsCardiovascular disorderPlant ingredientsAcute myocardial ischaemiaCoronary heart disease
Through experiments, the invention proves applications of a cape jasmine and a cape jasmine extract product in the preparation of drugs for treating myocardial infarction, coronary heart disease, myocardial ischemia and cardiac arrhythmia, especially to an application in treatment of acute myocardial ischemia or myocardial ischemia of coronary heart disease caused by restenosis after percutaneous transluminal coronary angioplasty (PTCA). In addition, it is proved through experiments that the above various kinds of applications are accomplished by protection of cardiac muscle and cardiac muscle cell from damage or resistance against inflammatory lesion and oxidative damage of cardiac muscle cell. And an effective dose of cape jasmine and the cape jasmine extract product in a unit preparation is obtained by detection.
Owner:BEIJING ZHONGYAN TONGRENTANG CHINESE MEDICINE R & D
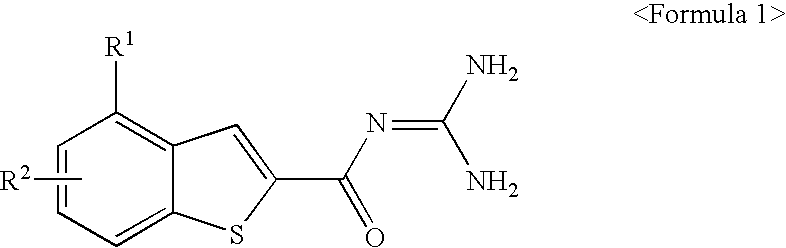
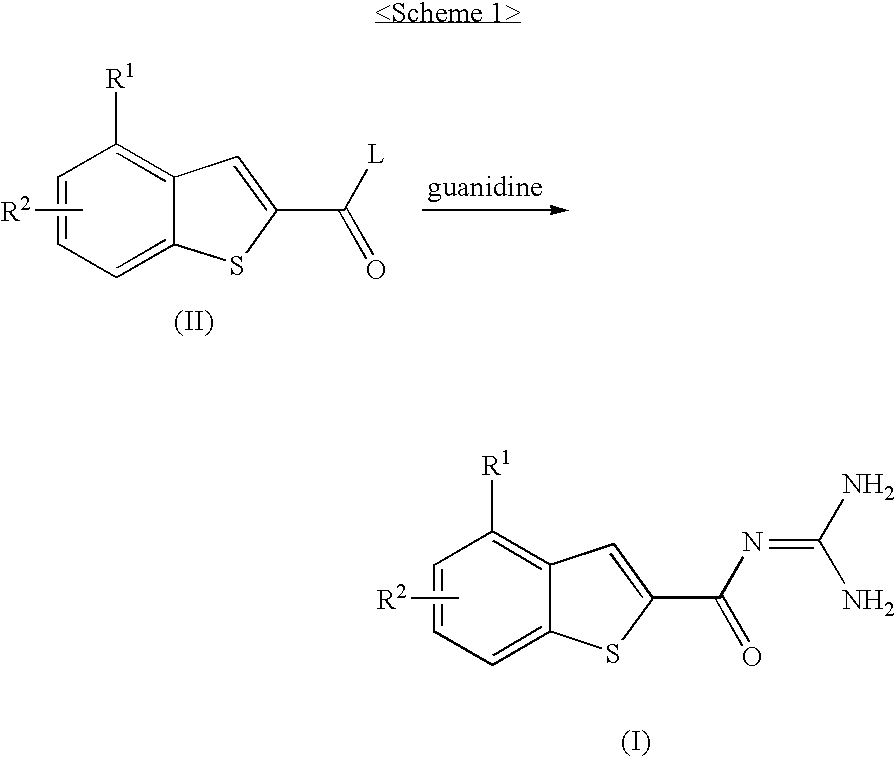
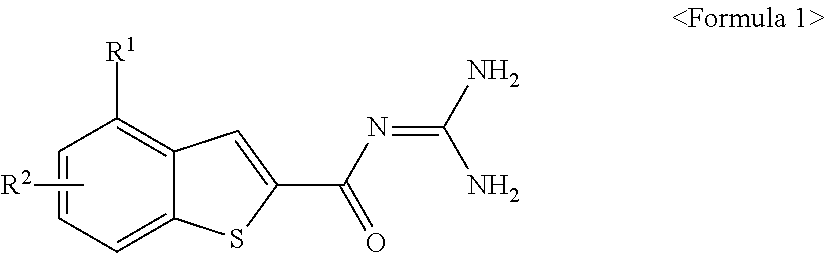
Benzothiophen-2-carbonylguanidine derivatives, preparation thereof, and pharmaceutical composition containing the same
ActiveUS20100004466A1Inhibitory activityPromote functional recoveryBiocideNervous disorderIschemic heartApoptosis
The present invention is related to benzothiophen-2-carbonylguanidine derivatives, a preparation method thereof, and pharmaceutical compositions containing the same. The derivatives have potent inhibitory effect on the sodium / hydrogen exchanger NHE-I, improve the functional recovery of ischemia / reperfusion-induced heart injury in isolated ischemic heart models, and significantly reduce the myocardiac infarct size in in vivo ischemic animal models, thereby showing excellent cardioprotective effects. Also, the derivatives are protective of both neuronal cells and the brain as proven by their protective effects on neuronal cells from necrosis and apoptosis and by their ability to significantly reduce cerebral infarct sizes in in vivo ischemic brain models. The derivatives can be effectively used for the prevention and treatment of ischemic heart diseases such as myocardiac infarction, arrhythmia, angina pectoris and the like, and cerebrovascular diseases such as cerebral stroke and be used as cardioprotective agents to the patients undergoing reperfusion therapy including chemicals such as thrombolytic agents, or surgery such as coronary artery bypass and percutaneous transluminal coronary angioplasty.
Owner:KOREA RES INST OF CHEM TECH
A balloon dilatation catheter
ActiveCN102258825ANot easy to slide and shiftAvoid insufficient frictionStentsSurgeryBalloon dilatation catheterInterventional treatment
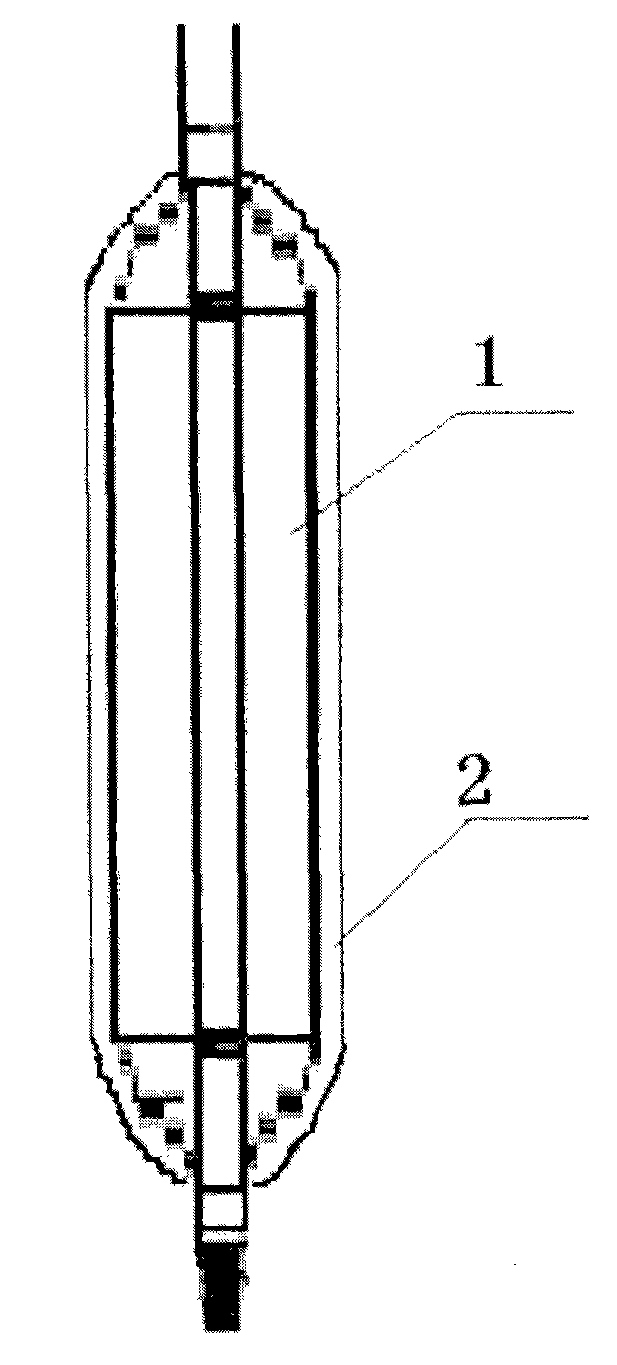
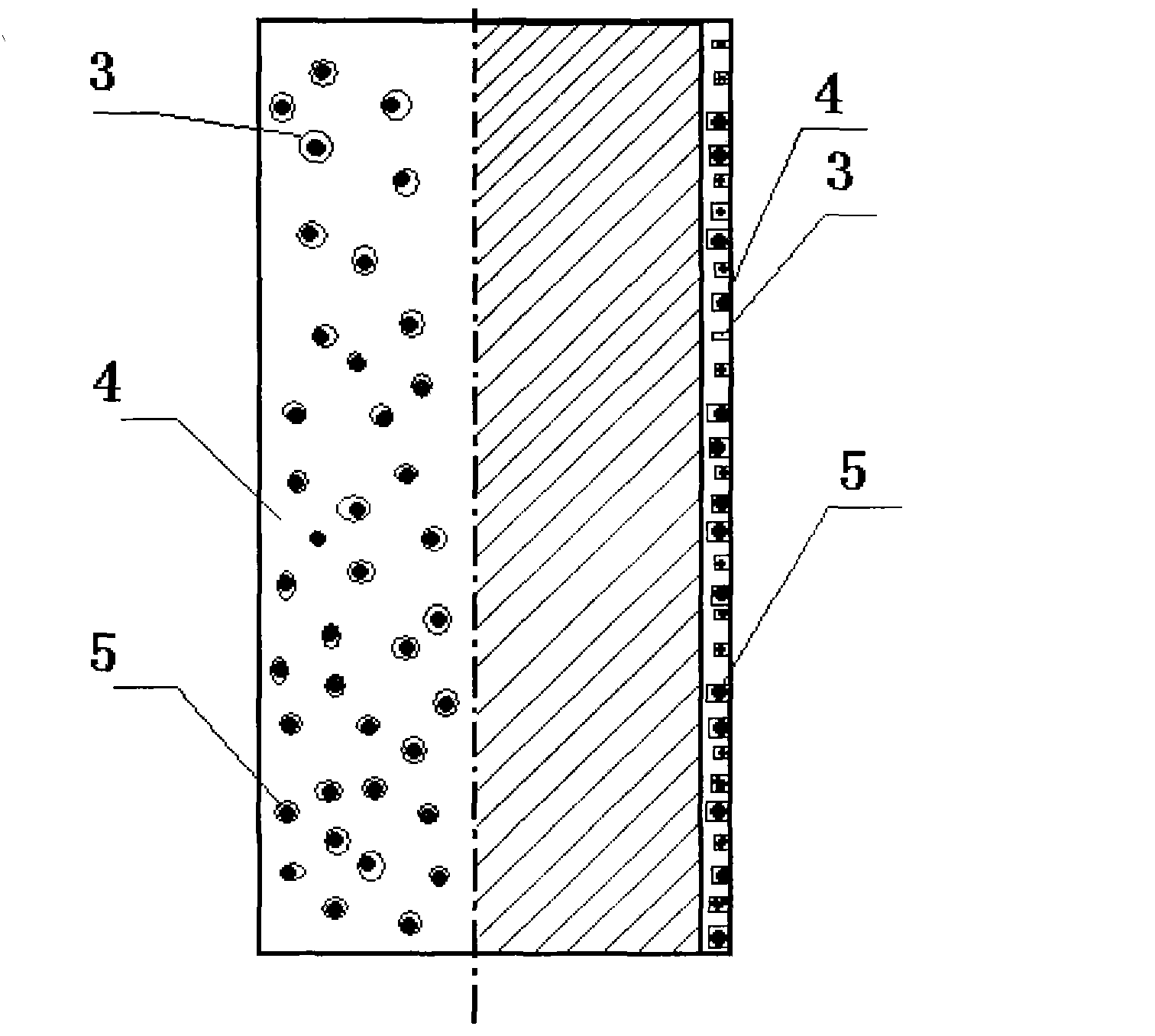
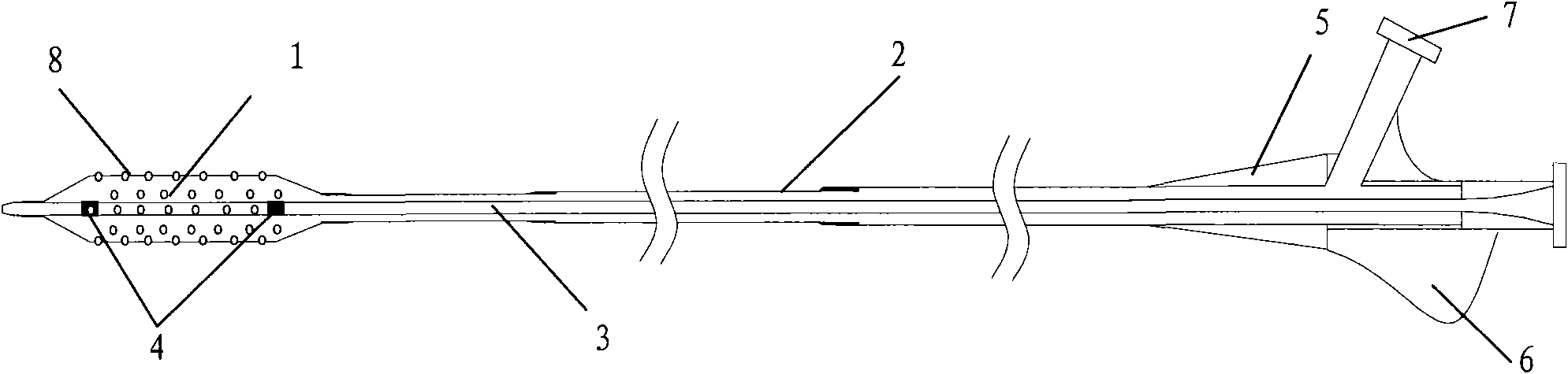
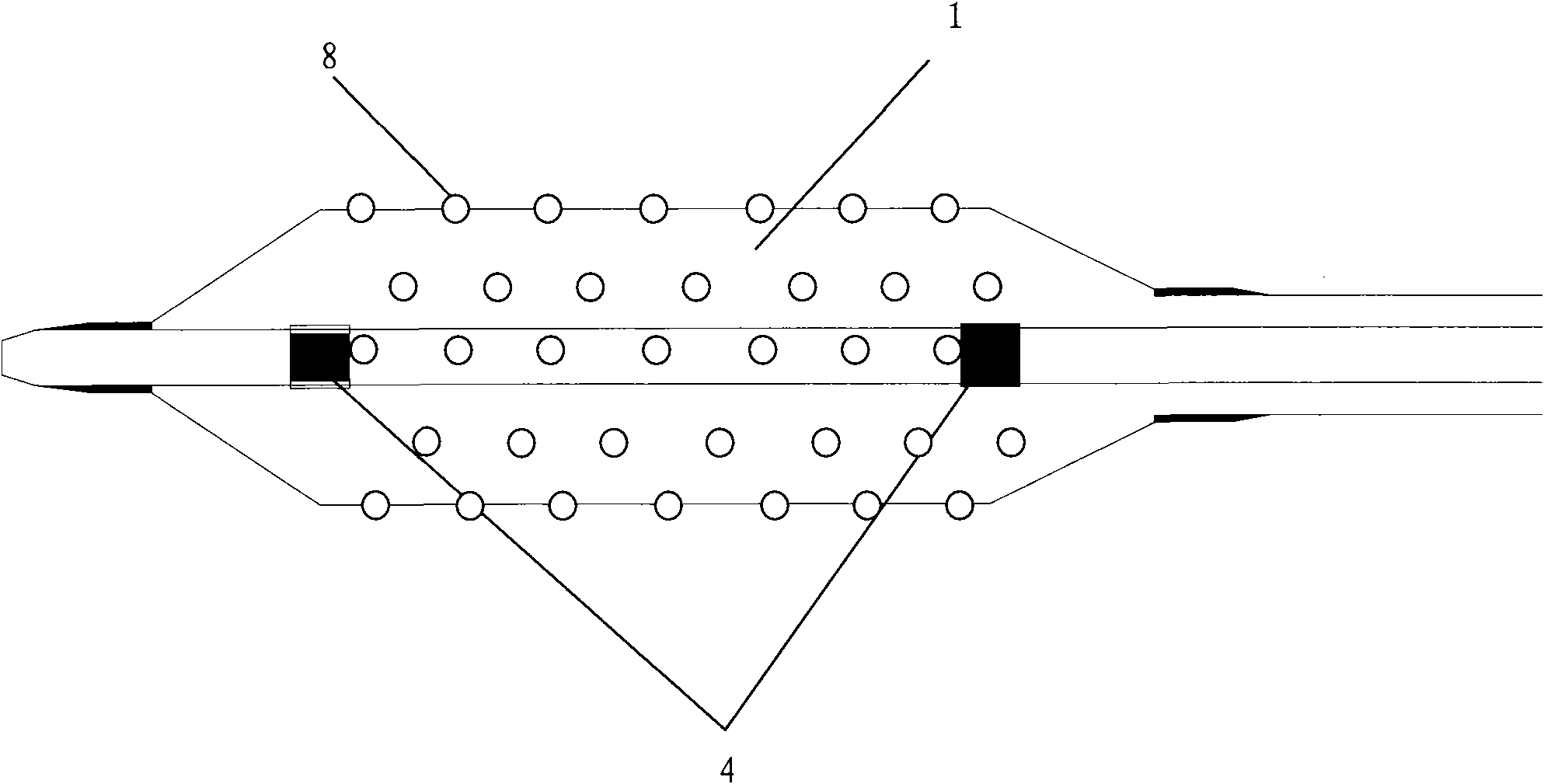
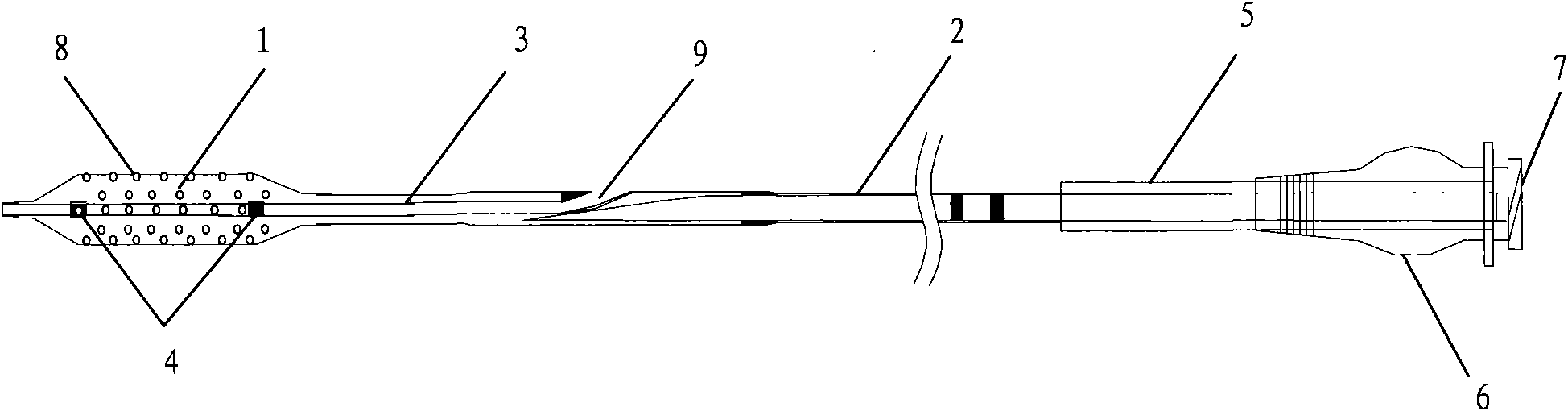
The invention discloses a percutaneous transluminal coronary angioplasty (PTCA) balloon catheter, which comprises a push rod, a balloon, an internal tube, a developing mark, a stress diffusion tube and a connecting device, wherein a plurality of bumps are arranged on the outside surface of the balloon. The outside surface of the balloon of the PTCA balloon catheter provided by the embodiment of the invention has a plurality of bumps, so that the bumps can tightly adhered onto the blood vessel walls of blood vessels at lesions in an interventional treatment operation to provide enough friction to prevent the balloon from sliding and displacing in or after expansion; and thus, the operation is safe and stable.
Owner:SHANGHAI VASOLUTIONS MEDTECH CO LTD
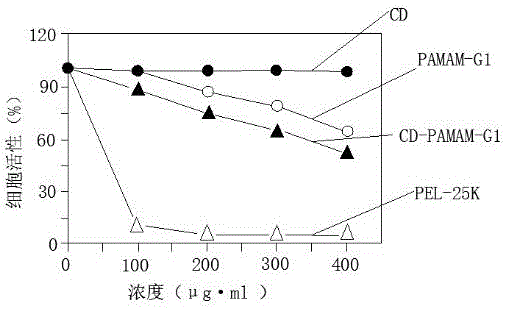
Polyamide-amine (PAMAM)-based cyclodextrin functionalized derivative carrier system and application thereof
InactiveCN105694050APrevent proliferationEfficient repairOrganic active ingredientsMacromolecular non-active ingredientsEthylenediamineChemical structure
The invention discloses a cyclodextrin functionalized derivative carrier system based on polyamide-amine and its application. The carrier system is made of cyclodextrin functionalized with polyamide-amine. The chemical The structure is as follows: Among them, cyclodextrin is β-cyclodextrin or its derivatives, γ-cyclodextrin or its derivatives, PAMAM is the core of ethylenediamine, and the end is aminated polyamide-amine (G0-G4), m =2-6; the carrier system has the function of simultaneously loading tanshinone IIA drugs, and can jointly load drugs and genes; the carrier system can be applied to in vitro gene cell transfection and treatment of arterial restenosis; the present invention is aimed at According to the characteristics of endothelial injury, a new type of carrier system is proposed, which can simultaneously load tanshinone drugs and genes, which can effectively repair the damaged endothelium, inhibit the proliferation of tissue cells, and achieve the effect of synergistic treatment. It has a good application prospect in prevention and treatment.
Owner:GUANGDONG PHARMA UNIV
Method for treating vascular disease
A medicament for prophylactic and / or therapeutic treatment of a vascular disease such as vascular restenosis and / or reocclusion after percutaneous transluminal coronary angioplasty using an intravascular stent, which comprises as an active ingredient a substance selected from the group consisting of retinoids and agents for controlling actions of retinoids such as 4-[5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbamoyl]benzoic acid or a salt thereof, or 4-[[[3,5-bis-(trimethylsilyl)phenyl]carbonyl]amino]benzoic acid or a salt thereof, wherein said substance has substantially no antiproliferative action on vascular endothelial cells, but substantially has antiproliferative action on vascular smooth muscle cells.
Owner:RES FOUND ITSUU LAB
Arsenic trioxide controllable-releasing balloon and preparing method thereof
The invention discloses an arsenic trioxide controllable-releasing drug balloon. The quick water soluble performance of arsenic trioxide is used. The arsenic trioxide and a slow-releasing layer are coated on the surface of the balloon. Even minitype concave holes which restrain releasing of the arsenic trioxide are formed on the slow-releasing layer. The size of the concave holes is adjusted, so that the concave holes are expanded to form micro holes in the process of expanding of the balloon, the facts that the arsenic trioxide is quickly released on a vascular wall and is evenly gathered on the surface of the vascular wall are achieved, quick phagocytosis of a cell is used, proliferation of the cell is avoided, and accordingly the effect that restenosis is avoided is achieved, the balloon can be used for expansion of a percutaneous transluminal coronary angioplasty (PTCA) operation and combination with the PTCA operation and bare stents, and the influence on restenosis caused by drug stent lay-coated materials is lowered.
Owner:BEIJING AMSINO MEDICAL
Hemostasis valve device
InactiveUS20140194831A1Improve convenienceInfusion syringesSurgical needlesThrombolytic drugFemoral artery
The present invention relates to a hemostasis valve device which allows a wire or a catheter to be inserted into the left or right coronary artery via the femoral artery or an arm artery when a Cardiac Catheterization or Percutaneous Transluminal Coronary Angioplasty operation is performed, wherein two independent sealing members are opened and closed by press and release actions of push buttons coupled to a body and the rotation of a fastening tube, respectively, so that the leakage or blood or the inflow of outside air is simply and effectively blocked during the operation, and a drug influx tube for allowing a medicine such as a thrombolytic drug to flow into a patient during the operation pivots and is adjusted in a stepwise manner within a certain range of angles according to body conditions or movements of the patient.
Owner:HUBIOMED INC
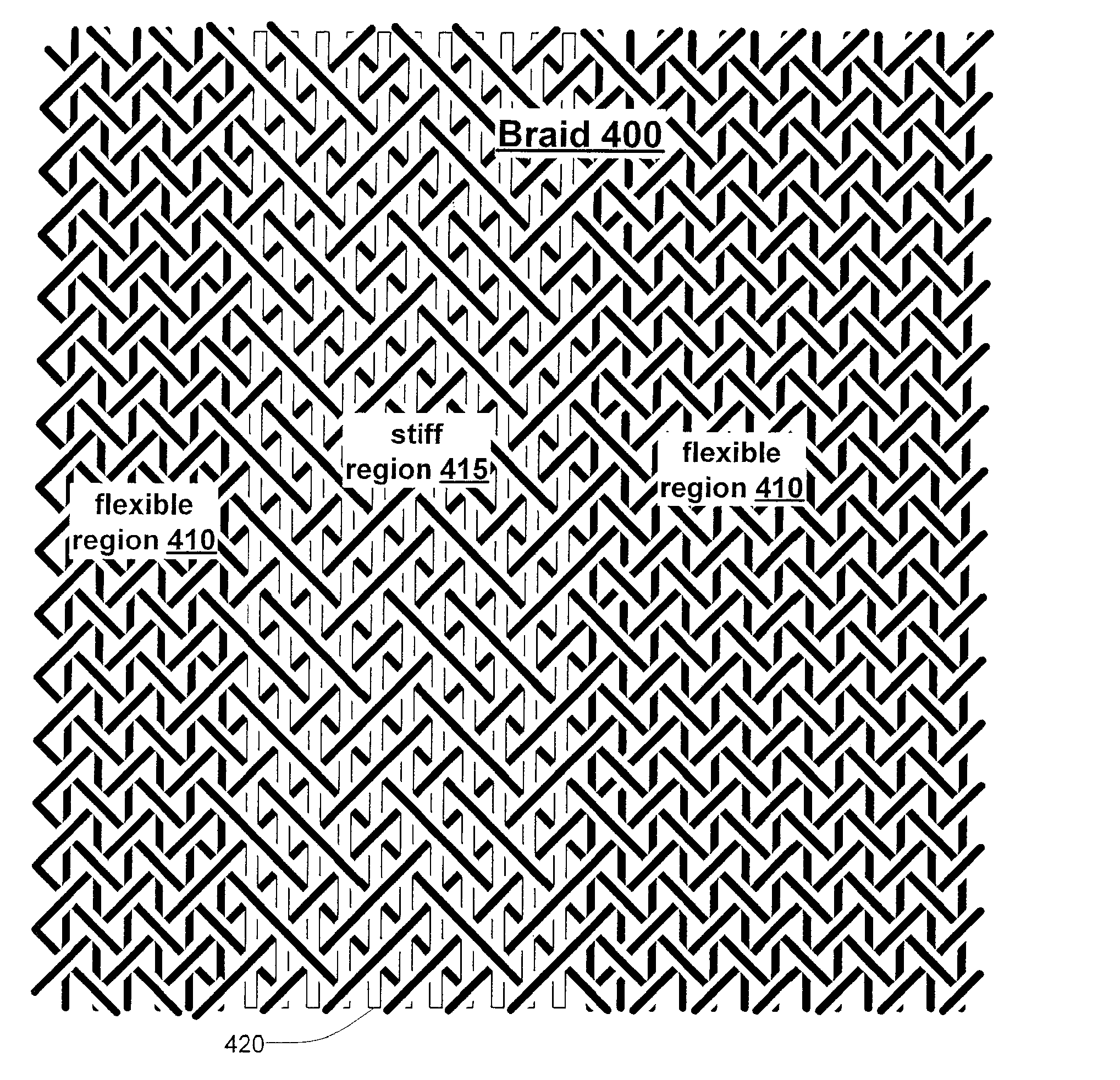
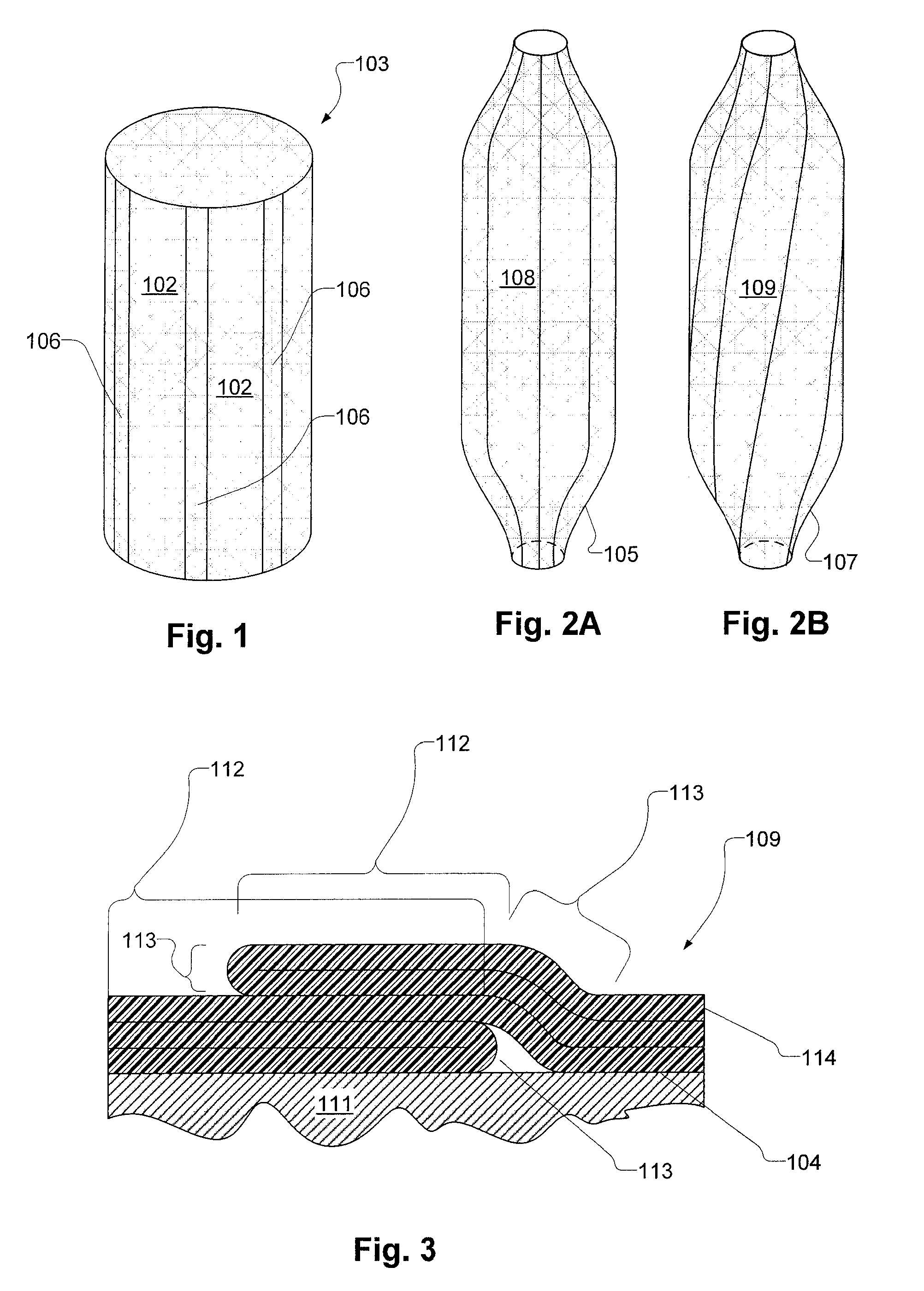
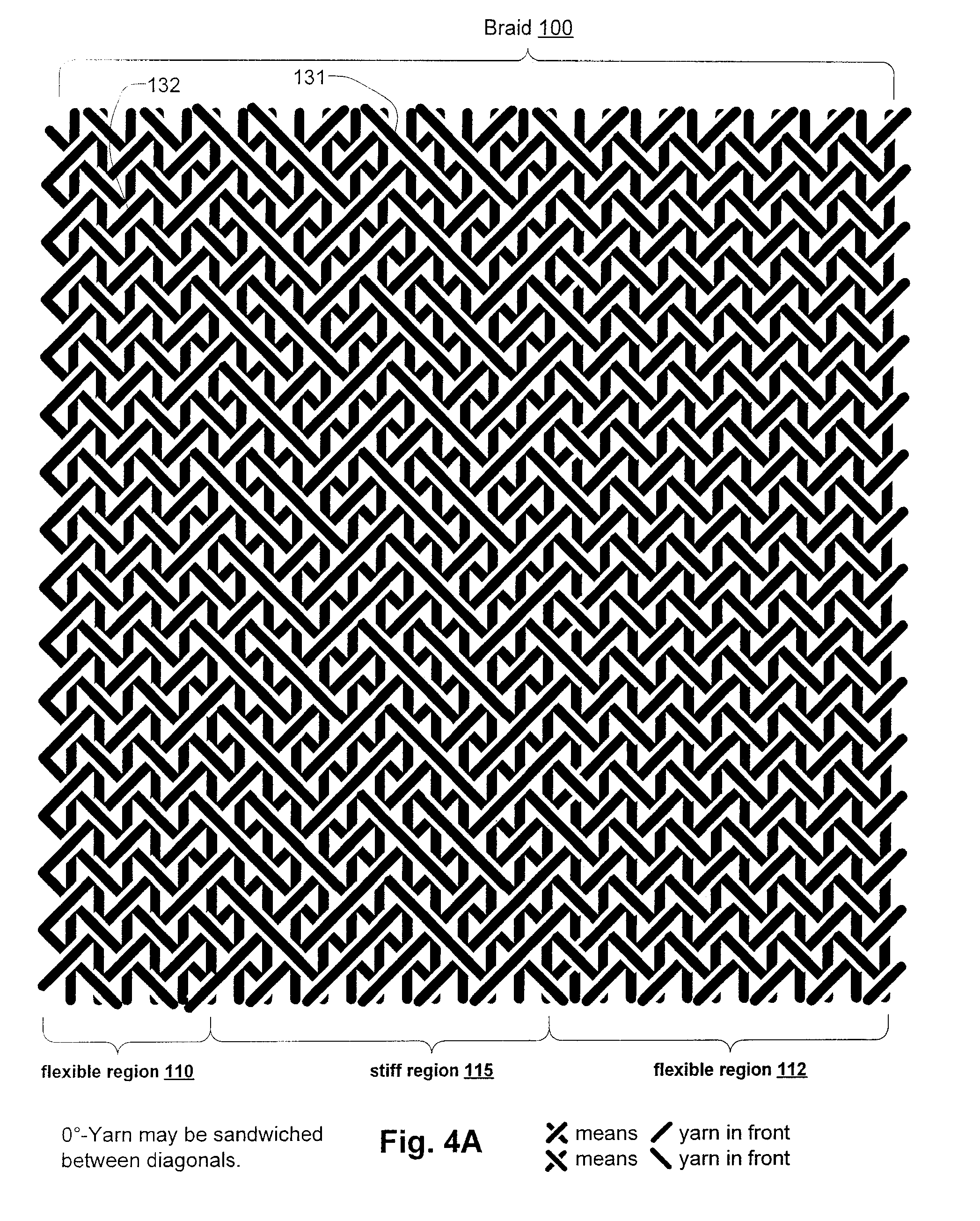
Inflatable Structure With Braided Layer
A balloon for medical treatments such as percutaneous transluminal coronary angioplasty (PTCA), delivery of a vascular stents or stent grafts, employs reinforcement materials that are patterned so as to promote consistent folding of the balloon. Also disclosed are methods and apparatus for biocidal treatment using a balloon, including balloons with fiber fabric reinforcements.
Owner:CR BARD INC
Thrombolytic balloon catheter device
ActiveCN109821139AReduced through outer diameterReduce thrombus sheddingBalloon catheterMedical devicesThrombusIntracoronary thrombus
The invention provides a rapid exchange catheter device which can be used for thrombolysis in intracoronary thrombosis and percutaneous transluminal coronary angioplasty (PTCA). The device comprises aguide wire tube, an inner tube, an outer tube and a balloon. The middle distal section of the guide wire tube, the inner tube and the outer tube are coaxially overlapped from inside to outside; the proximal section of the guide wire tube penetrates through the wall of the inner tube and the wall of the outer tube to form a guide wire outlet, and the distal end of the guide wire tube is freely opened in the inner cavity of the inner tube; the inner cavity of the inner tube is a communicated perfusion cavity, the proximal end of the inner tube is provided with a medicine injection port, and thedistal section of the inner tube is provided with a perfusion side hole and a guide wire inlet which are opened in multiple directions; the cavity gap between the outer tube and the inner tube is a balloon pressurization and decompression cavity, the proximal end is provided with a balloon pressurization and decompression hole, and the distal end is communicated with the balloon cavity. Accordingto the invention, the functions of the thrombolytic catheter and the balloon catheter are integrated into one catheter, so that two operations of thrombolysis and PTCA in coronary artery thrombosis can be quickly completed, the thrombotic load can be reduced, the thromboembolism can be prevented, the operation time can be shortened, and the X-ray exposure to doctors and patients can be reduced.
Owner:钟文
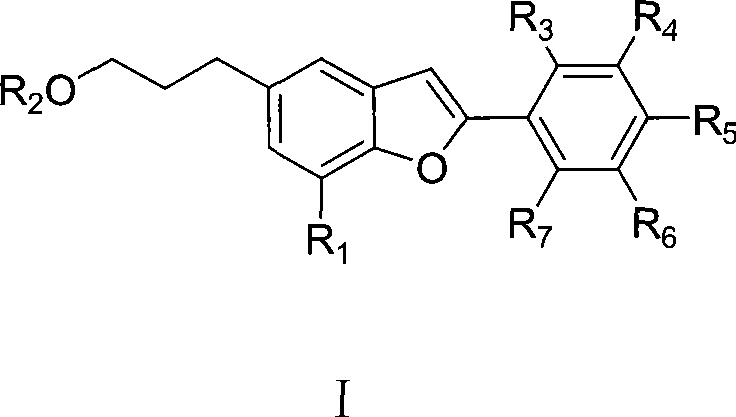
Egonol type benzofuran and novel use in pharmacy of glycosides thereof
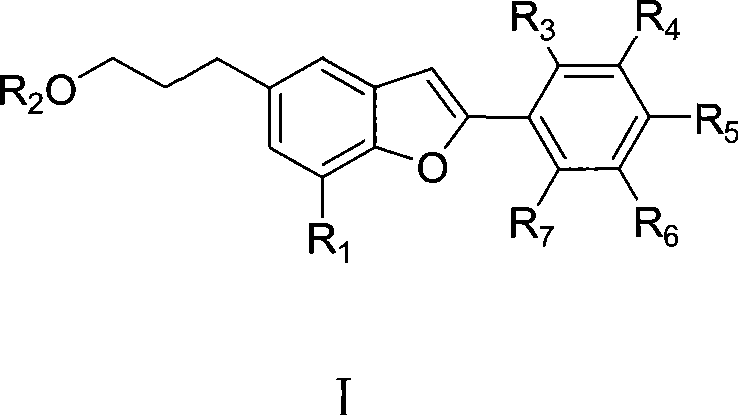
The invention discloses the application of egonol-type benzofuran and glycosides thereof in the preparation of an ACAT (Acyl-CoA:Cholesterol Acyltransferase) inhibitor, provides the application of egonol-type benzofuran as shown in general formula I and glycosides thereof in the preparation of the ACAT inhibitor, and further provides the drug combination of the ACAT inhibitor, which contains egonol-type benzofuran and glycosides thereof with the active component being shown in the general formula I. The egonol-type benzofuran and the glycosides thereof can be prepared into various forms of drug, such as tablets, capsules and the like, because of the significant effect of the ACAT inhibitor thereof. The invention is suitable for preventing and treating diseases, such as angina, myocardial infarction, cerebral infarction, Alzheimer's disease, acute coronary syndromes (ACS), percutaneous transluminal coronary angioplasty (PTCA) or post-stenting coronary restenosis; and the invention, as a therapeutic agent for regulating the sebaceous gland function and inhibiting the excessive production of sebum, is also suitable for curing diseases, such as oily skin, acne, seborrhea, acne-like lesion caused by corticosteroid and the like.
Owner:CHENGDU DIAO JIUHONG PHARMA FACTORY
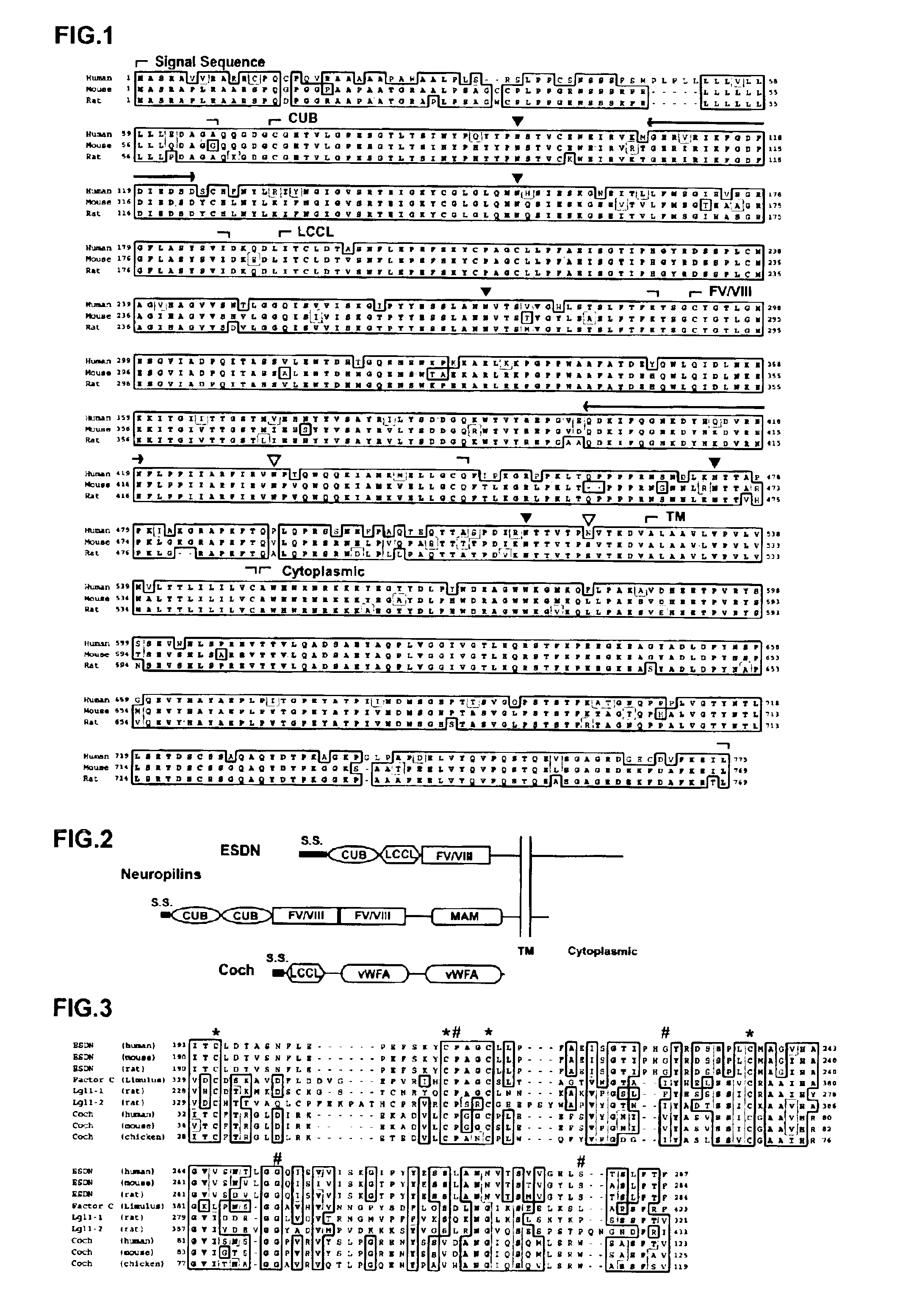
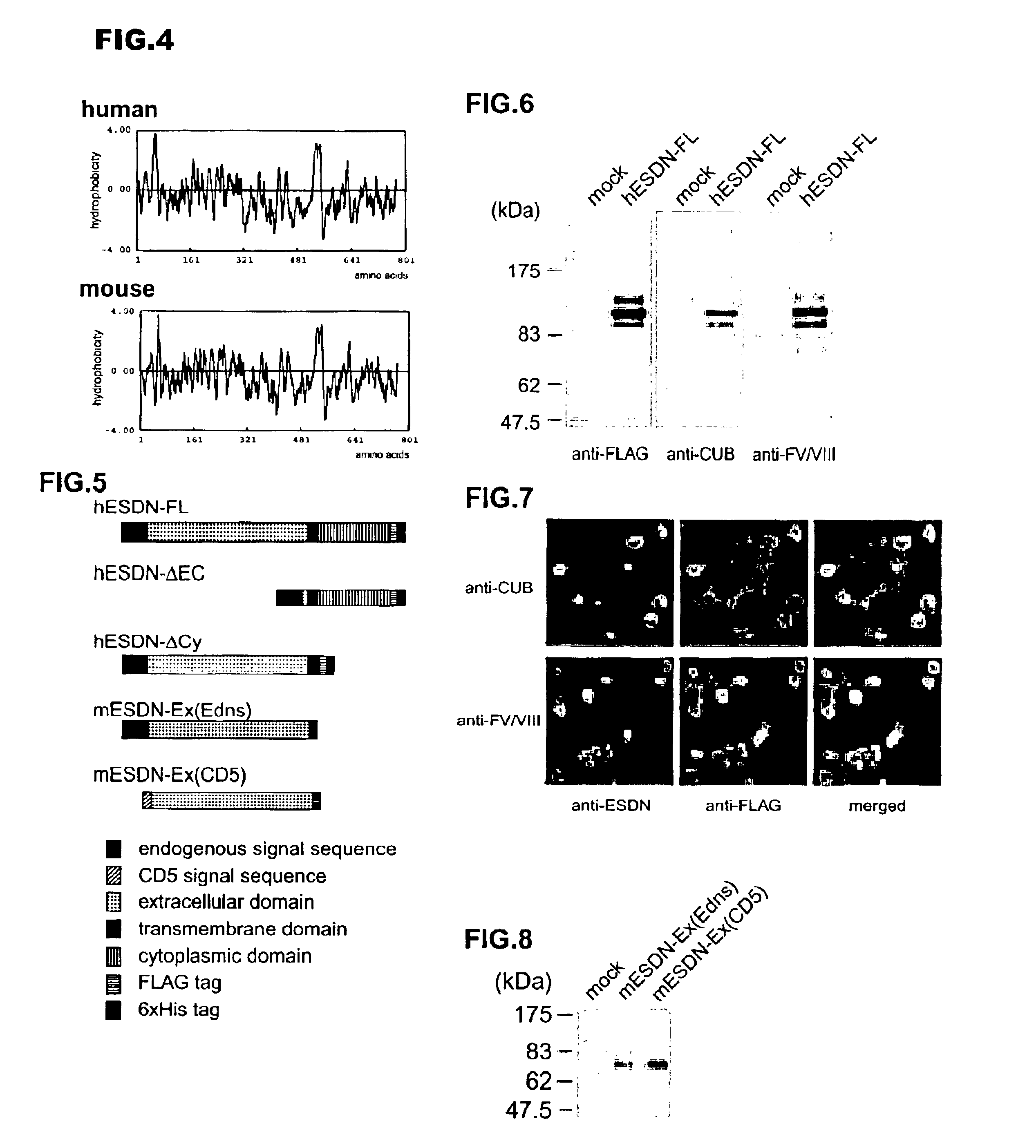
Polypeptide ESDN, polynucleotides encoding the polypeptide, and utility of the polypeptide
InactiveUS6900031B2The process is simple and effectiveImmunoglobulins against blood coagulation factorsFungiNucleotidePercent Diameter Stenosis
The invention discloses a useful and novel factor (polypeptide) which plays an important role for morbid vascular smooth muscle in restenosis after percutaneous transluminal coronany angioplasty (PTCA) and arterial sclerosis in the field of cardiovascular system.
Owner:ONO PHARMA CO LTD
Establishment method of diarrhea-type irritable bowel syndrome compound animal model
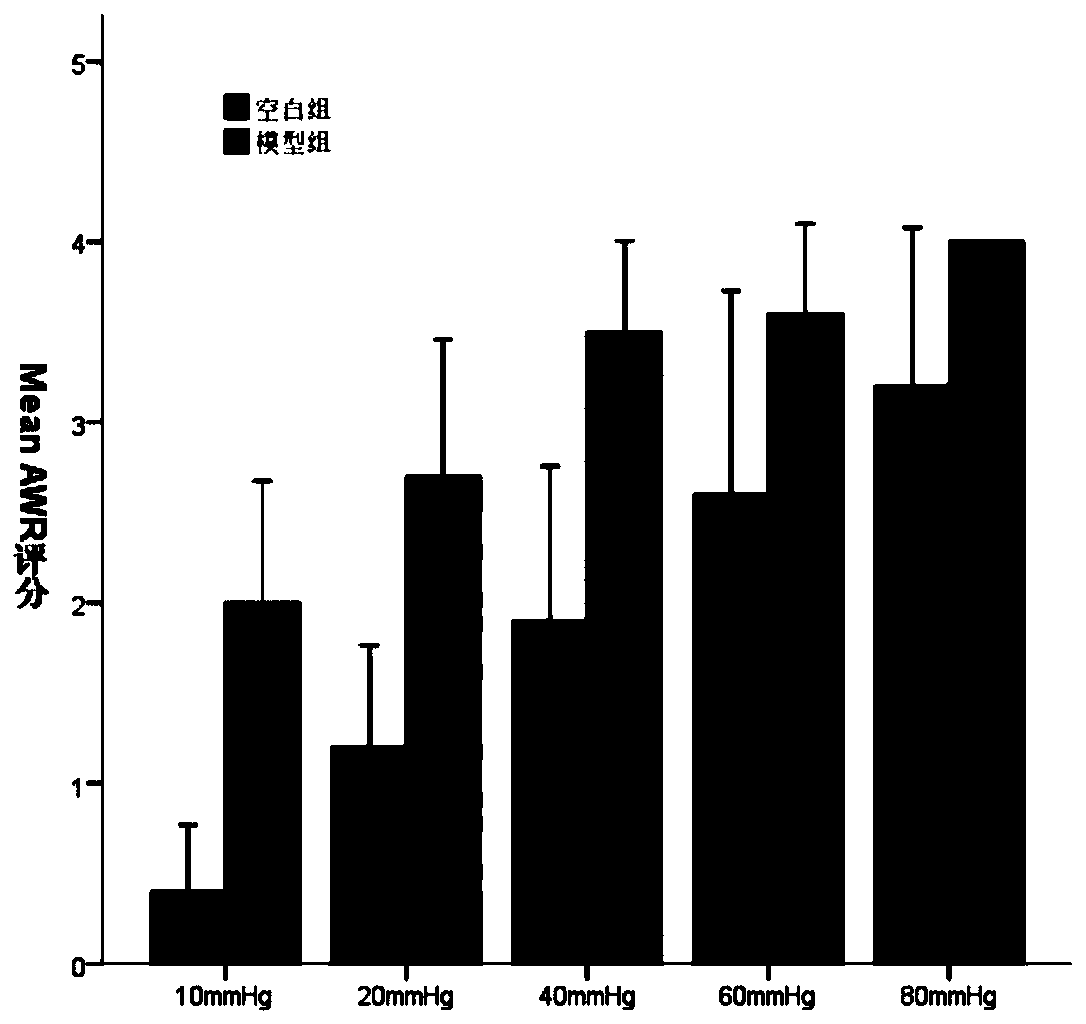
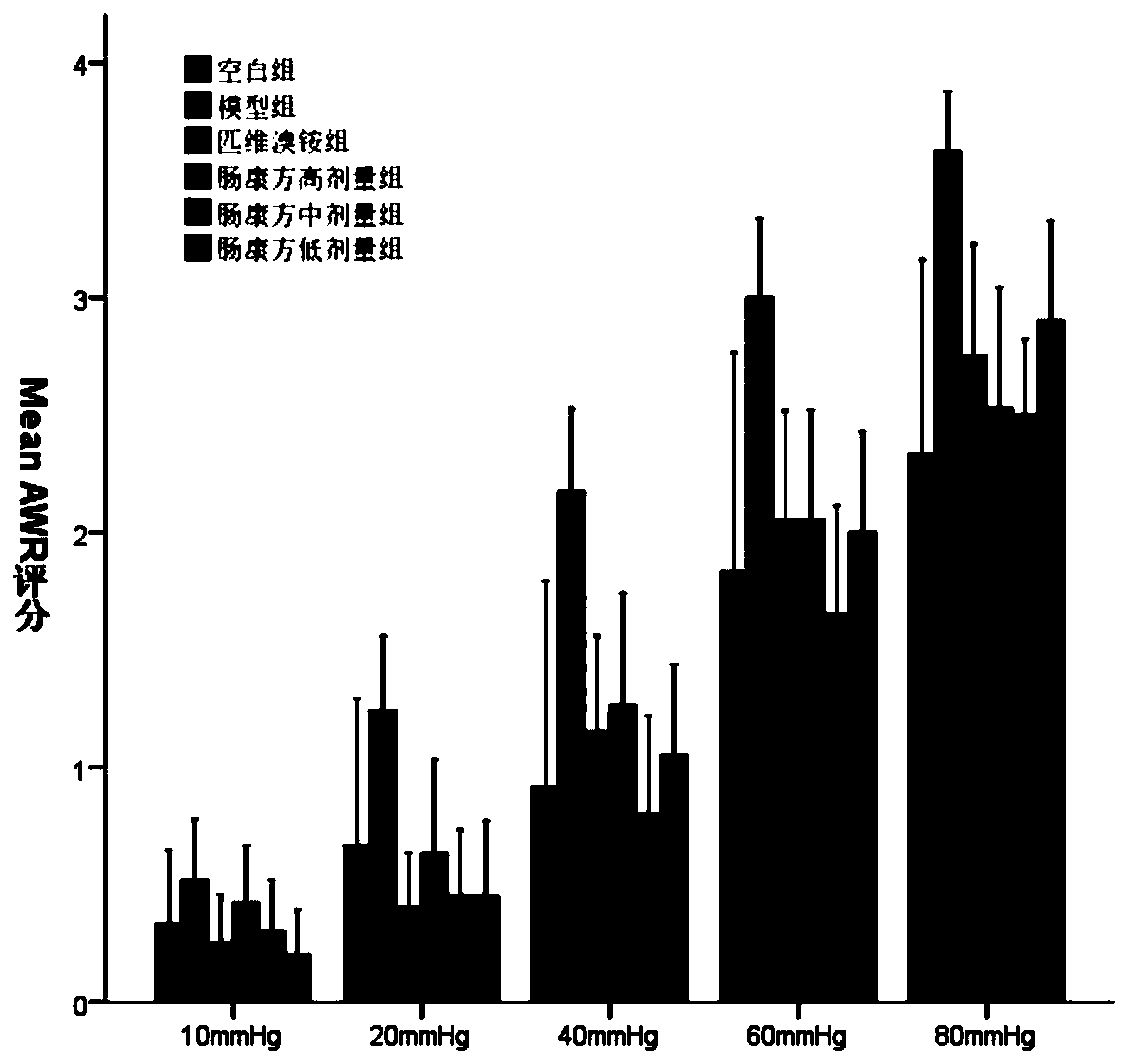
PendingCN110178794AConvenient experimentGood experimental responseAnimal husbandryMedicineAbdominal wall
The invention discloses an establishment method of a diarrhea-type irritable bowel syndrome compound animal model. The establishment method specifically comprises the steps of repurchasing Sprague-Dawley pregnant rats for predelivery, and recording the delivery date. The method comprises the procedures of 1, colorectum stimulation in the neonatal stage, wherein colorectum stimulation is separatelyconducted in the 8 th day, the 10 th day and the 12 th day after the birth of modeling groups of young rats, a percutaneous transluminal coronary angioplasty balloon is inserted in the colorectum viathe anus, after the balloon completely enters the colorectum, gas is injected into the balloon, so that the pressure in the balloon is 60 mmHg and is kept for 1 minute, stimulation is conducted onceevery day and conducted three times in total, after stimulation, the young rats and mother rats are fed together to the 22 nd day and then weaned, cage allocation is conducted on the 30 th day, then normal feeding is conducted, 60 days after the birth, abdominal wall withdrawal reflex examination is conduced, intestinal-tract high-sensitivity rats are screened out, and the next step of modelling is conducted; 2, gavage with folium sennae juice, wherein folium sennae is put into boiled water which is 5 times the volume of the folium sennae to be soaked for one night, after filtering, a filtrateis taken, and then concentrated until the concentration is 0.45 g / mL, the 0.45 g / mL folium sennae juice is fed for gavage of the screened-out 60-day intestinal tract high-sensitivity rats, 2 mL of the juice is fed to each rat once every day, gavage is continuously conducted for 2 weeks, and then the IBS-D rat model is established.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
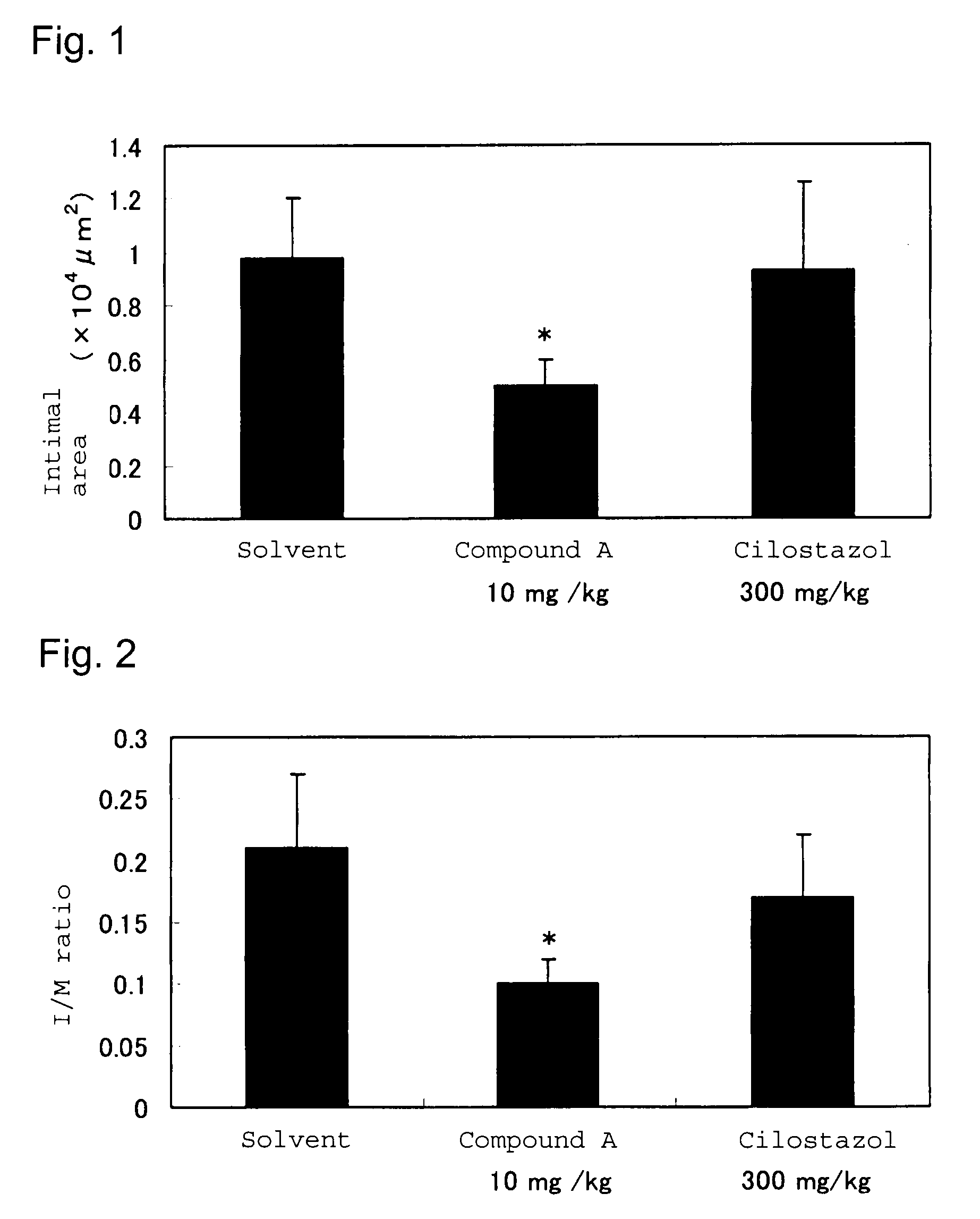
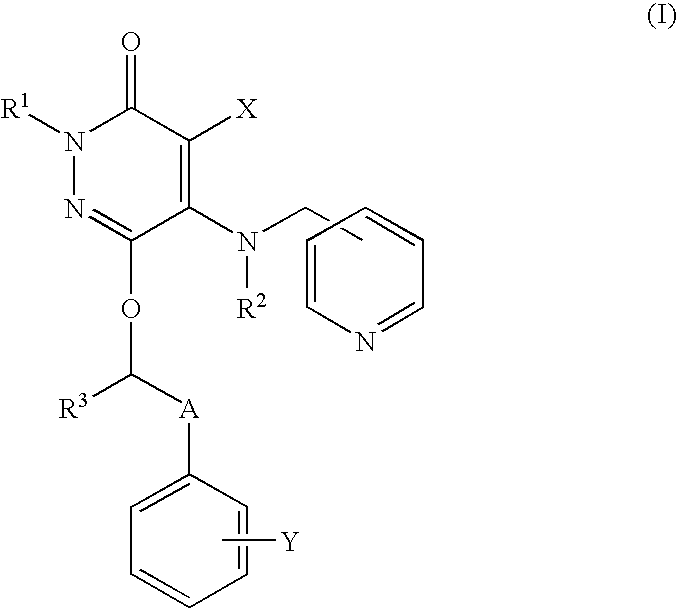
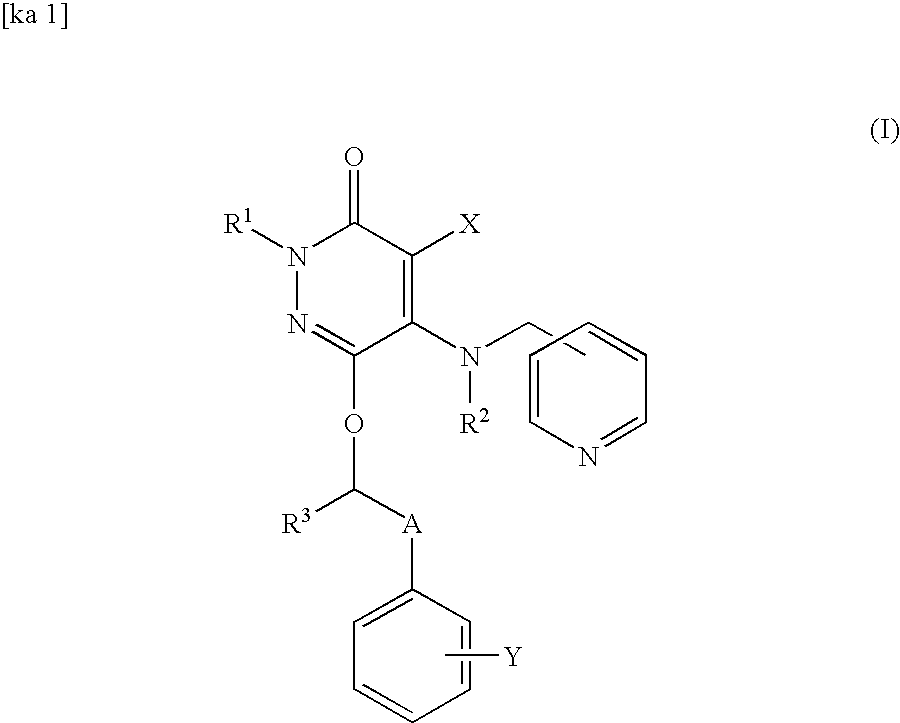
Drug for inhibiting vascular intimal hyperplasia
To provide an intimal hyperplasia inhibitor useful for prevention of restenosis after percutaneous transluminal coronary angioplasty (PTCA) or vascular stent placement or treatment of its progress.An intimal hyperplasia inhibitor containing a 3(2H)-pyridazinone compound represented by the formula (I):[wherein each of R1, R2 and R3 is independently a hydrogen atom or a C1-6 alkyl group, X is a halogen atom, cyano or a hydrogen atom, Y is a halogen atom, trifluoromethyl or a hydrogen atom, and A is a C1-8 alkylene which may be substituted with a hydroxyl group] or a pharmacologically acceptable salt thereof.
Owner:NISSAN CHEM IND LTD +1
Tissue Factor Production Inhibitor
InactiveUS20080255111A1Inhibit productionReduce thrombosisBiocideOrganic chemistryAbnormal tissue growthPercent Diameter Stenosis
A medicament which has an activity of inhibiting production of tissue factor and comprises an LXR ligand as an active ingredient; and a medicament for treatment and / or prophylaxis of vascular restenosis following angioplasty, endarterectomy, percutaneous transluminal coronary angioplasty (PTCA) or stent implantation, or treatment and / or prophylaxis of blood coagulation diseases, diseases induced by platelet aggregation including stable or unstable angina pectoris, cardiovascular and cerebrovascular diseases including thromboembolism formation diseases accompanying diabetes, rethrombosis following thrombolysis, cerebral ischemic attack, infarction, stroke, ischemia-derived dementia, peripheral artery disease, thromboembolism formation diseases during use of an aorta-coronary artery bypass, glomerulosclerosis, renal embolism, tumor or cancer metastasis, which comprises an LXR ligand as an active ingredient.
Owner:DAIICHI SANKYO CO LTD
Inflatable structure with braided layer
A balloon for medical treatments such as percutaneous transluminal coronary angioplasty (PTCA), delivery of a vascular stents or stent grafts, employs reinforcement materials that are patterned so as to promote consistent folding of the balloon. The reinforcements may be fiber fabric reinforcements and the balloon may be used for biocidal treatment.
Owner:CR BARD INC
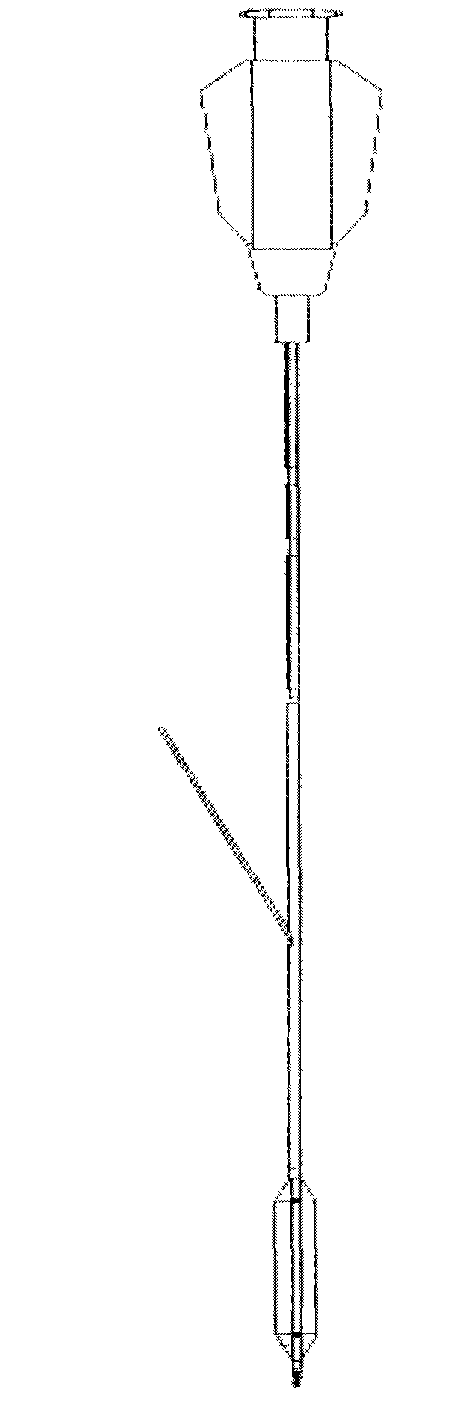
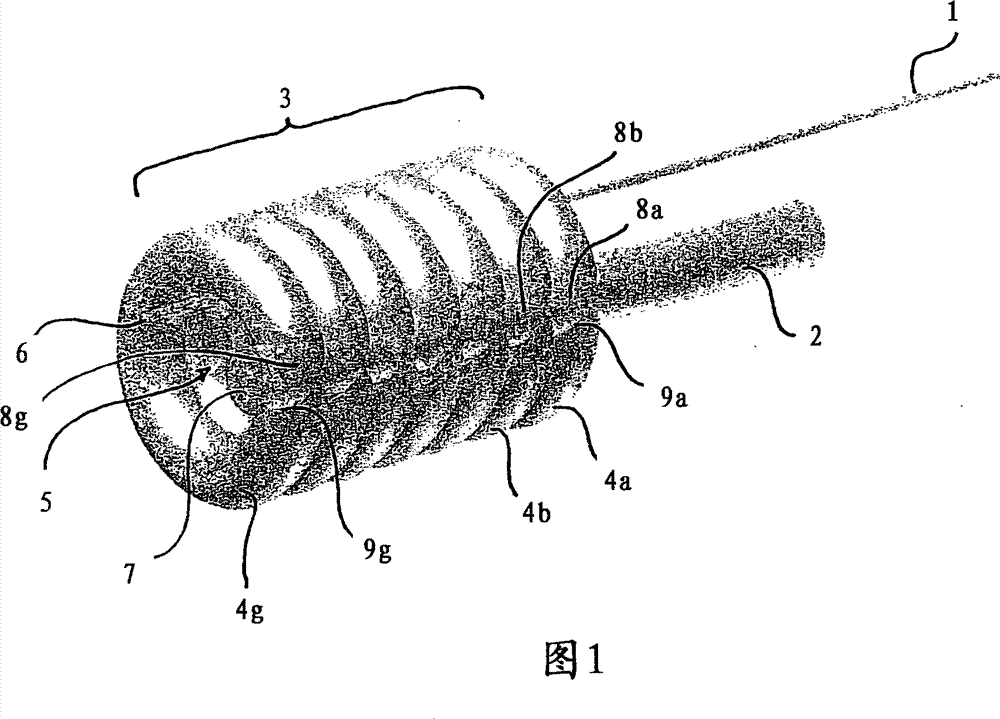
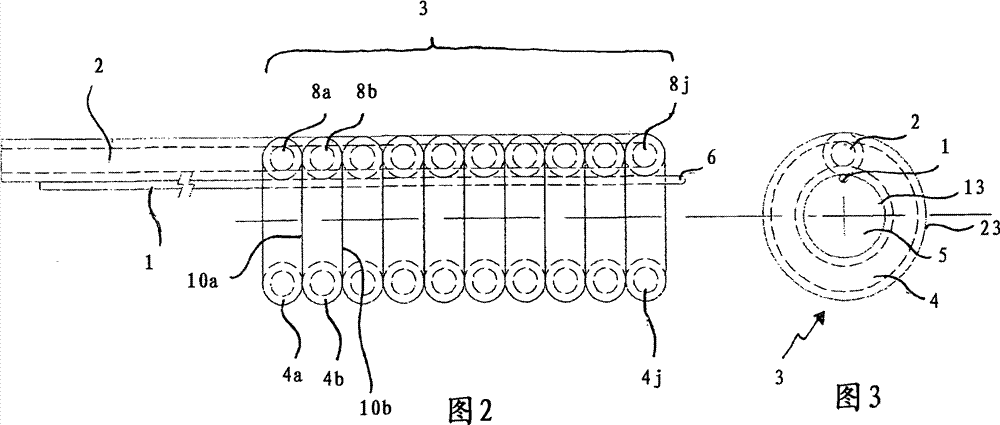
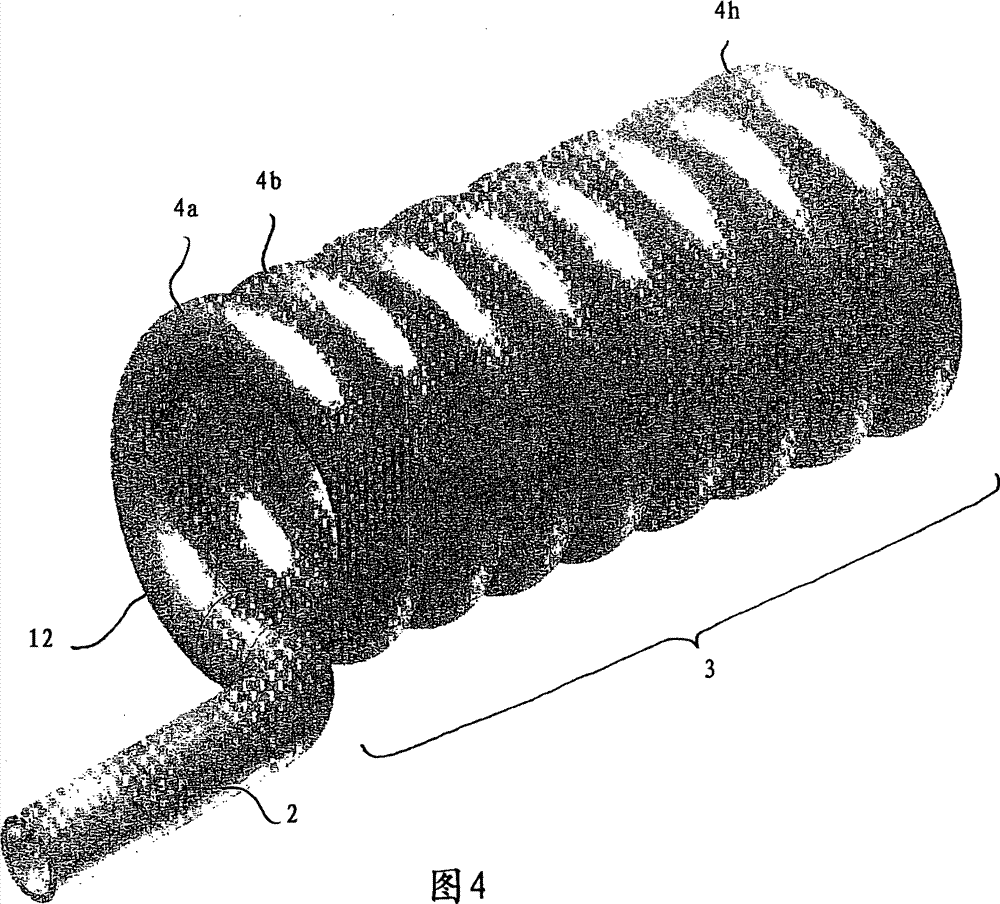
Balloon dilatation catheter
InactiveCN101400400BProlong the action timeBalloon catheterBlood vesselsBalloon dilatation catheterCatheter
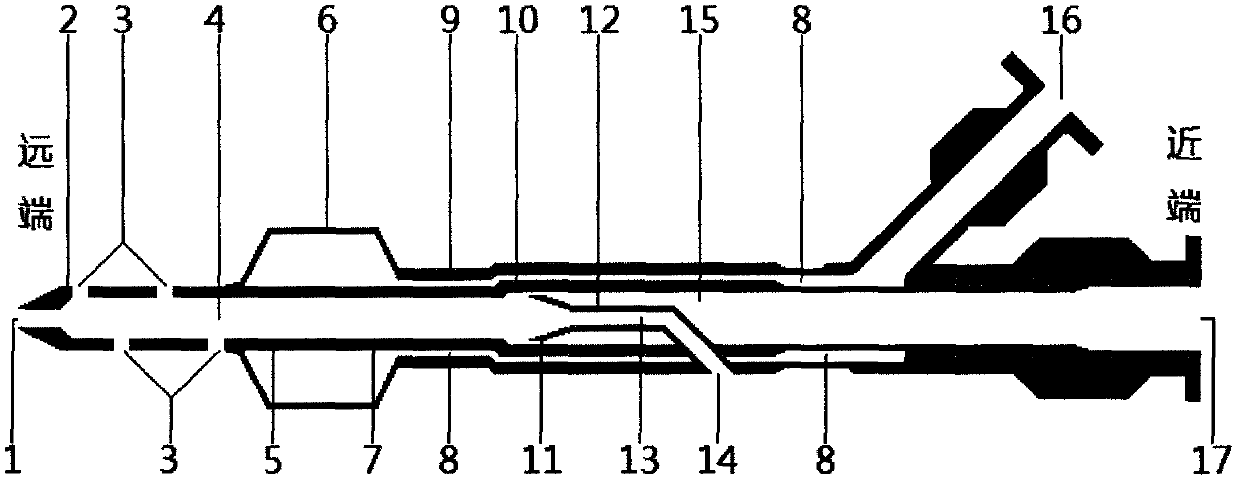
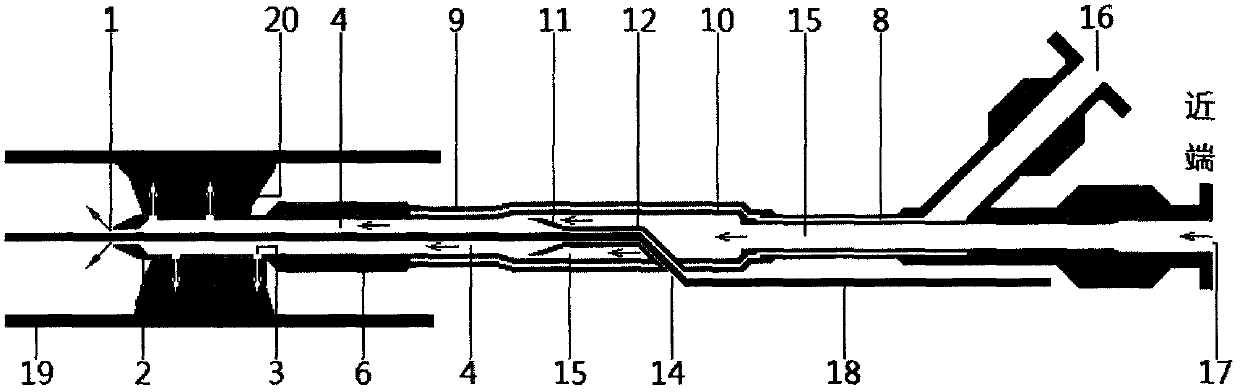
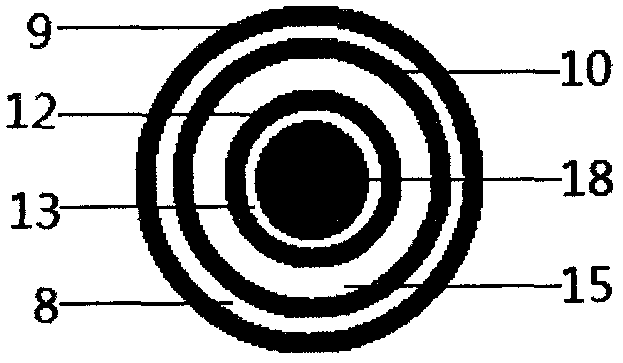
The invention relates to a balloon dilatation catheter, in particular for percutaneous transluminal coronary angioplasty (PTCA), with a guide wire (1), with a supply tube (2) which extends parallel to the guide wire (1) and is used for inflating a dilatation balloon (3), and with the dilatation balloon (3) arranged in the distal area of the supply tube (2), said dilatation balloon (3) being of tubular design with a wall made up of a multiplicity of mutually contiguous tube segments (4), of which at least one is attached to the supply tube (2).
Owner:QUALIMED INNOVATIVE MEDIZINPROD
Benzothiophen-2-carbonylguanidine derivatives, preparation thereof, and pharmaceutical composition containing the same
ActiveUS8143307B2Inhibitory activityPromote functional recoveryBiocideNervous disorderIschemic heartApoptosis
The present invention is related to benzothiophen-2-carbonylguanidine derivatives, a preparation method thereof, and pharmaceutical compositions containing the same. The derivatives have potent inhibitory effect on the sodium / hydrogen exchanger NHE-I, improve the functional recovery of ischemia / reperfusion-induced heart injury in isolated ischemic heart models, and significantly reduce the myocardiac infarct size in in vivo ischemic animal models, thereby showing excellent cardioprotective effects. Also, the derivatives are protective of both neuronal cells and the brain as proven by their protective effects on neuronal cells from necrosis and apoptosis and by their ability to significantly reduce cerebral infarct sizes in in vivo ischemic brain models. The derivatives can be effectively used for the prevention and treatment of ischemic heart diseases such as myocardiac infarction, arrhythmia, angina pectoris and the like, and cerebrovascular diseases such as cerebral stroke and be used as cardioprotective agents to the patients undergoing reperfusion therapy including chemicals such as thrombolytic agents, or surgery such as coronary artery bypass and percutaneous transluminal coronary angioplasty.
Owner:KOREA RES INST OF CHEM TECH
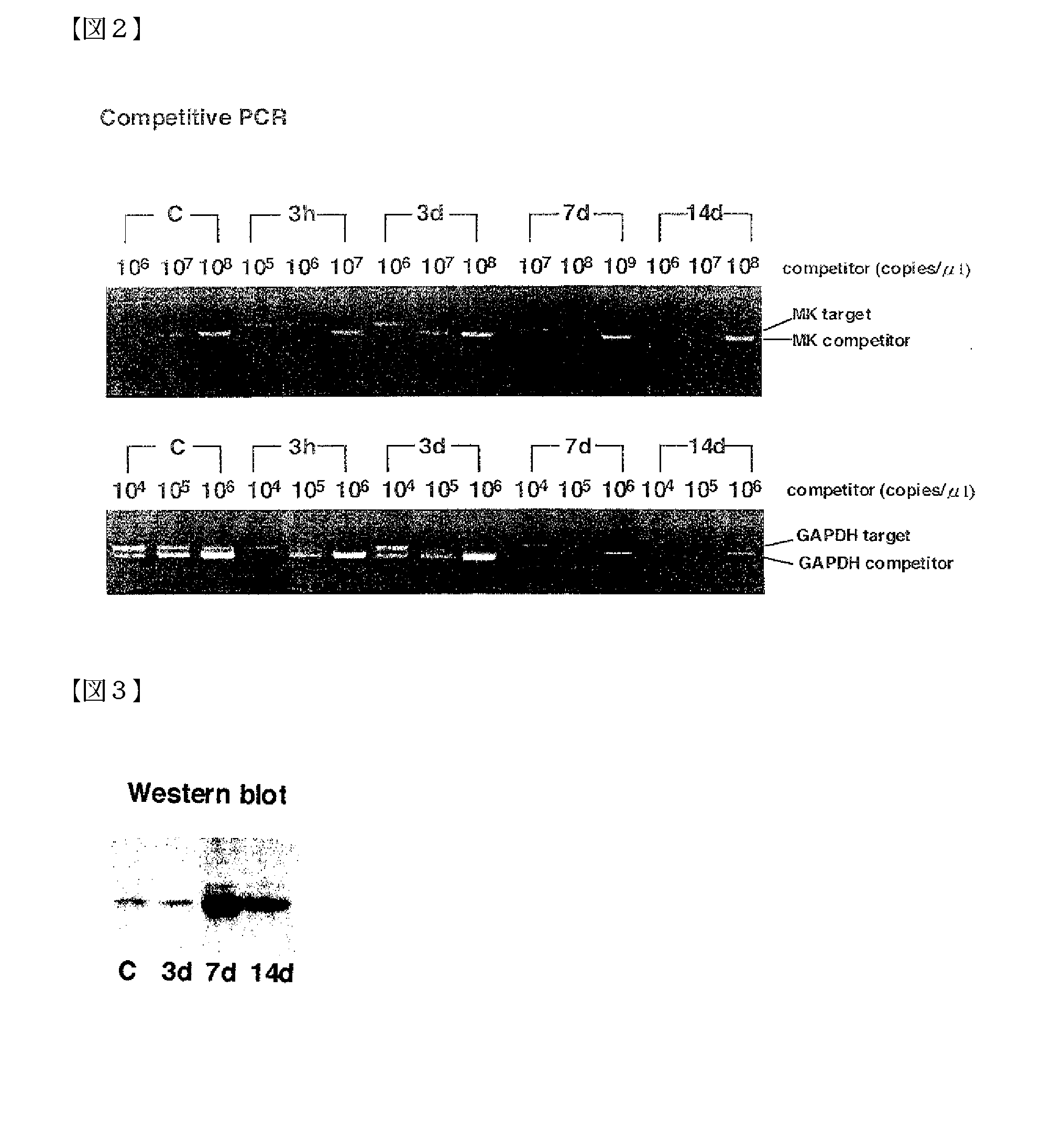
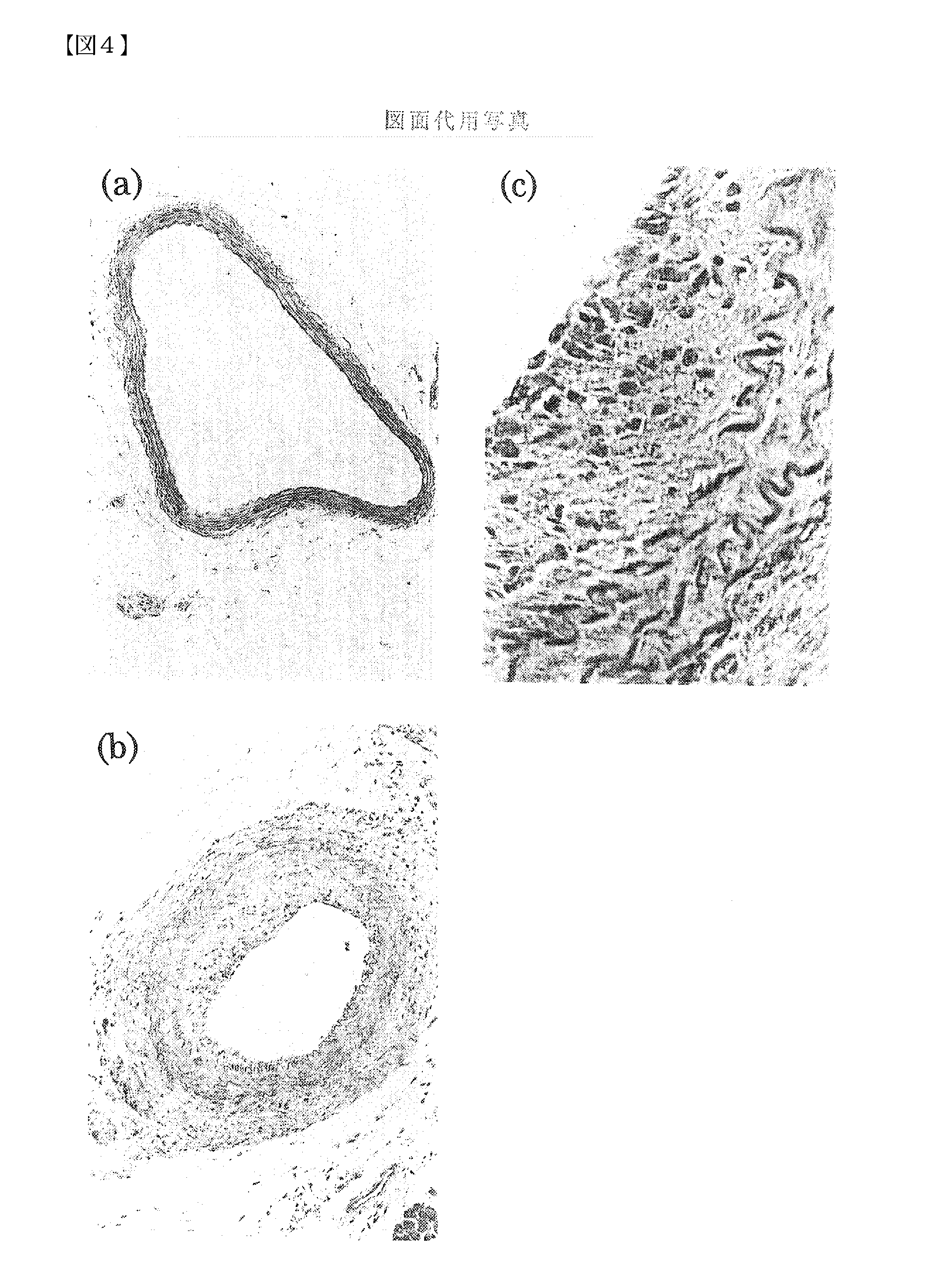
Pharmaceutical Compositions for the Prevention and Treatment of Atherosclerosis and Restenosis After PTCA
InactiveUS20080107659A1Increased neointima sizeHigh expressionImmunoglobulins against growth factorsAntibody ingredientsBlood Vessel TissueAdditive ingredient
The present invention provides a pharmaceutical composition for the prevention or treatment of angiostenosis, comprising a compound inhibiting the function of midkine (MK) in blood vessel tissues as an effective ingredient. The present invention is useful for the prevention or treatment of angiostenosis attributed to arteriosclerosis or restenosis after percutaneous transluminal coronary angioplasty (PTCA). As compounds inhibiting the function of MK, antisense oligonucleotides that bind to a segment of a single-stranded mRNA transcribed from the MK gene to inhibit the synthesis of MK protein in cells, antibodies against the MK protein, and such can be used.
Owner:MEDICAL THERAPIES
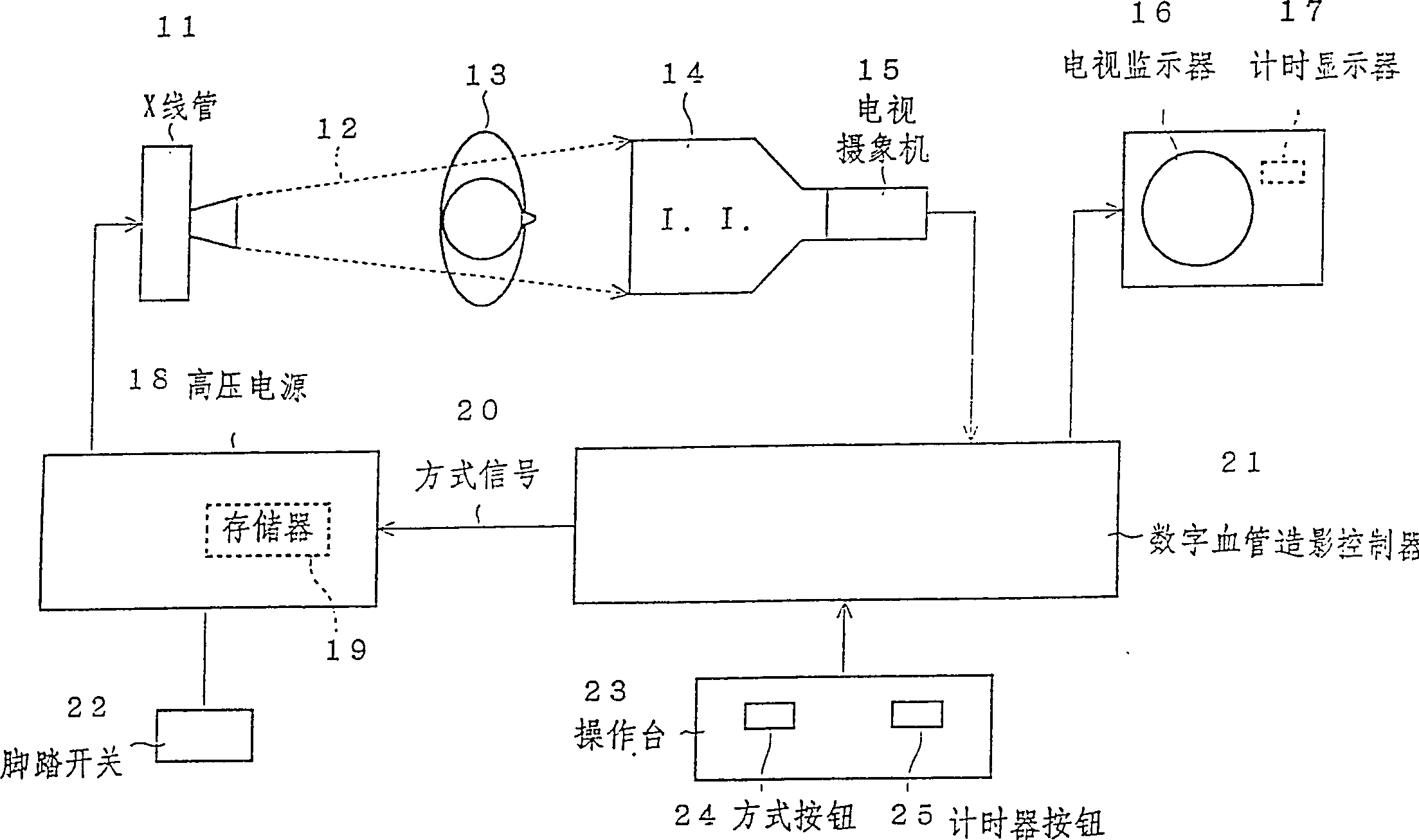
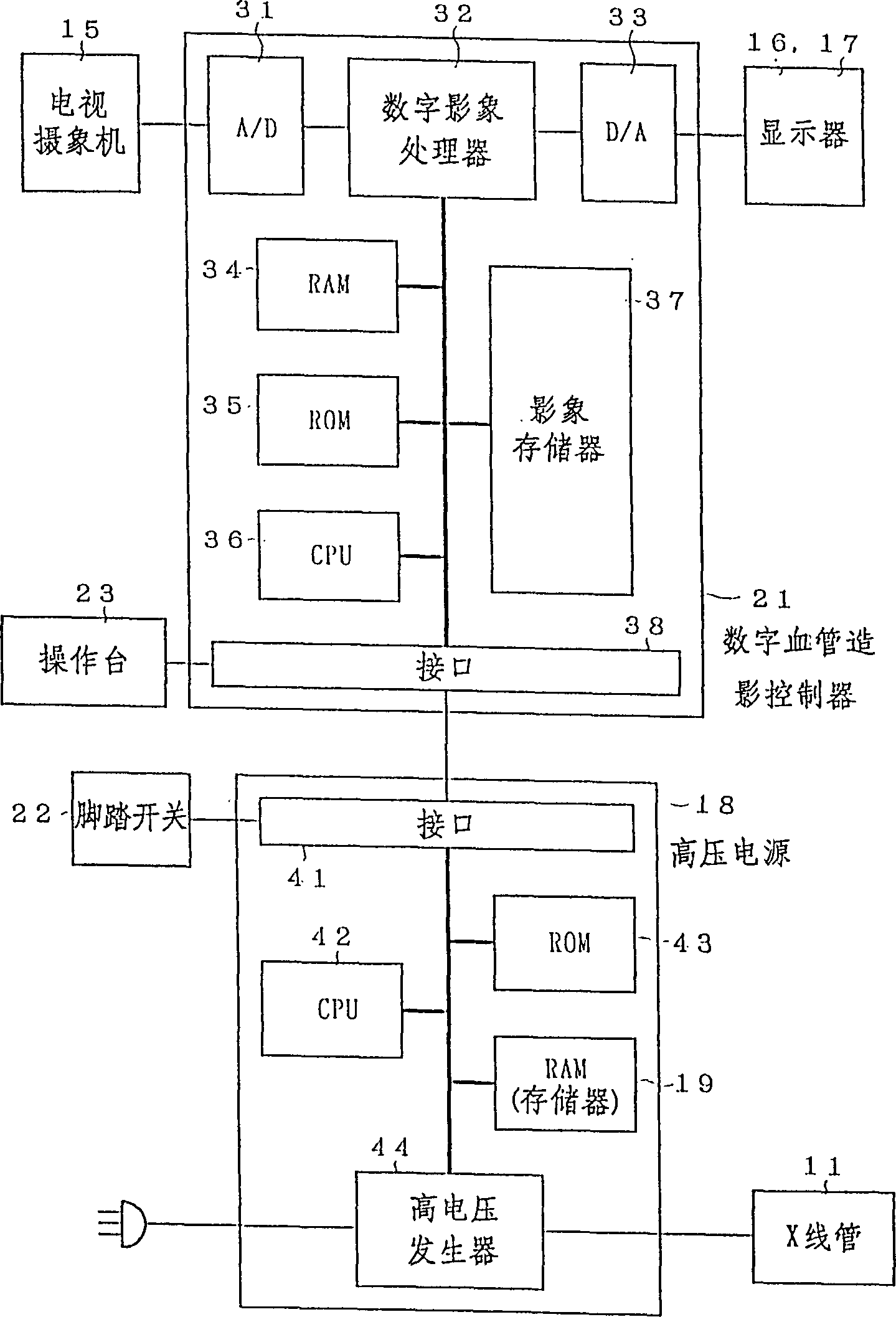
Fluoroscopic imaging system
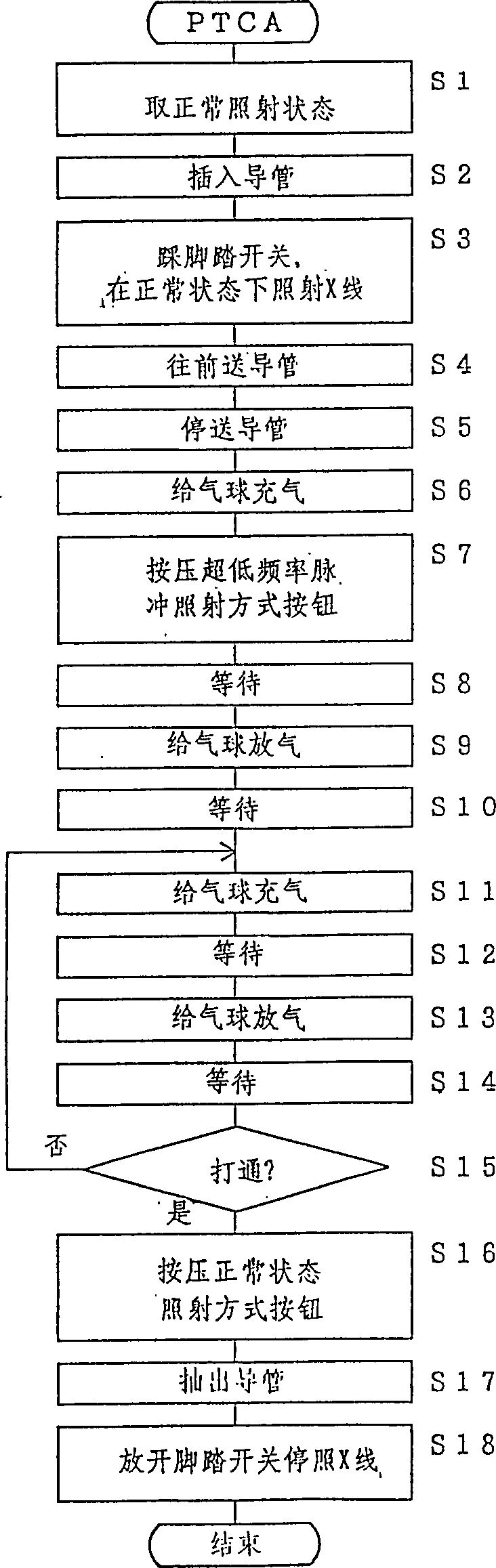
In an interventional radiography (IVR), for example in a percutaneous transluminal coronary angioplasty (PTCA), when the IVR includes a period in which the movement of the object is slow, a normal X-ray radiating mode (a continuous X-ray radiation or a normal rate pulse radiation of about 15-30 pulses per second) is changed to a very slow rate pulse X-ray radiation of about 1-5 pulses per second to decrease the X-ray dose of the patient. Since the movement is slow, the very slow rate pulse radiation can still trace the movement adequately. Further, the quality of the X-ray image is improved by increasing the tube current in the very slow rate pulse radiation because less heat is generated in the X-ray tube.
Owner:SHIMADZU SEISAKUSHO CO LTD
Balloon catheter
ActiveCN102258825BNot easy to slide and shiftAvoid insufficient frictionStentsSurgeryBalloon dilatation catheterInterventional treatment
Owner:SHANGHAI VASOLUTIONS MEDTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com