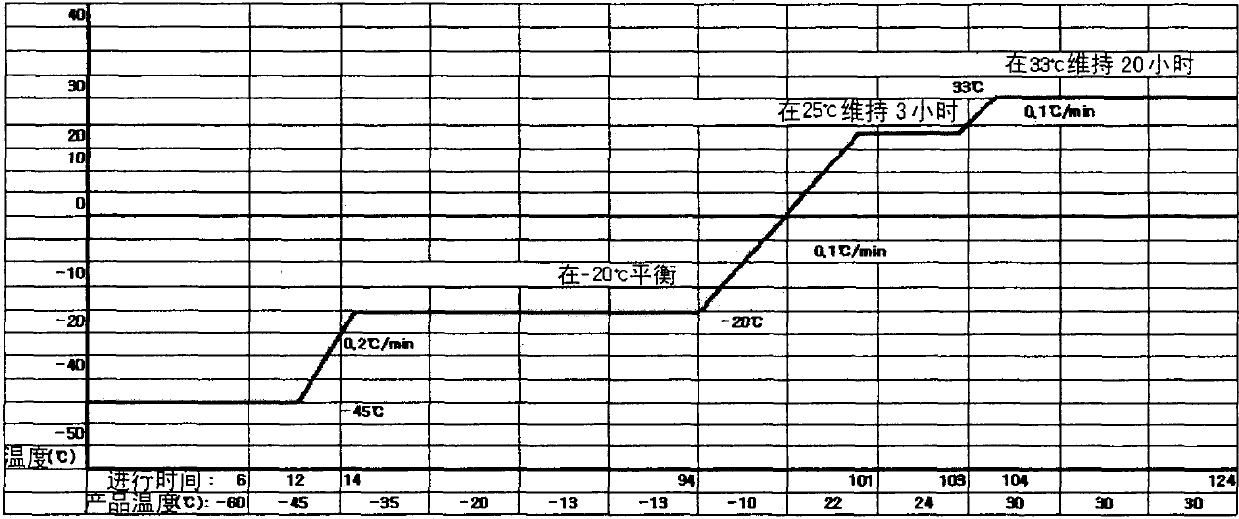
Patents
Literature
47 results about "Blood clotting factors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
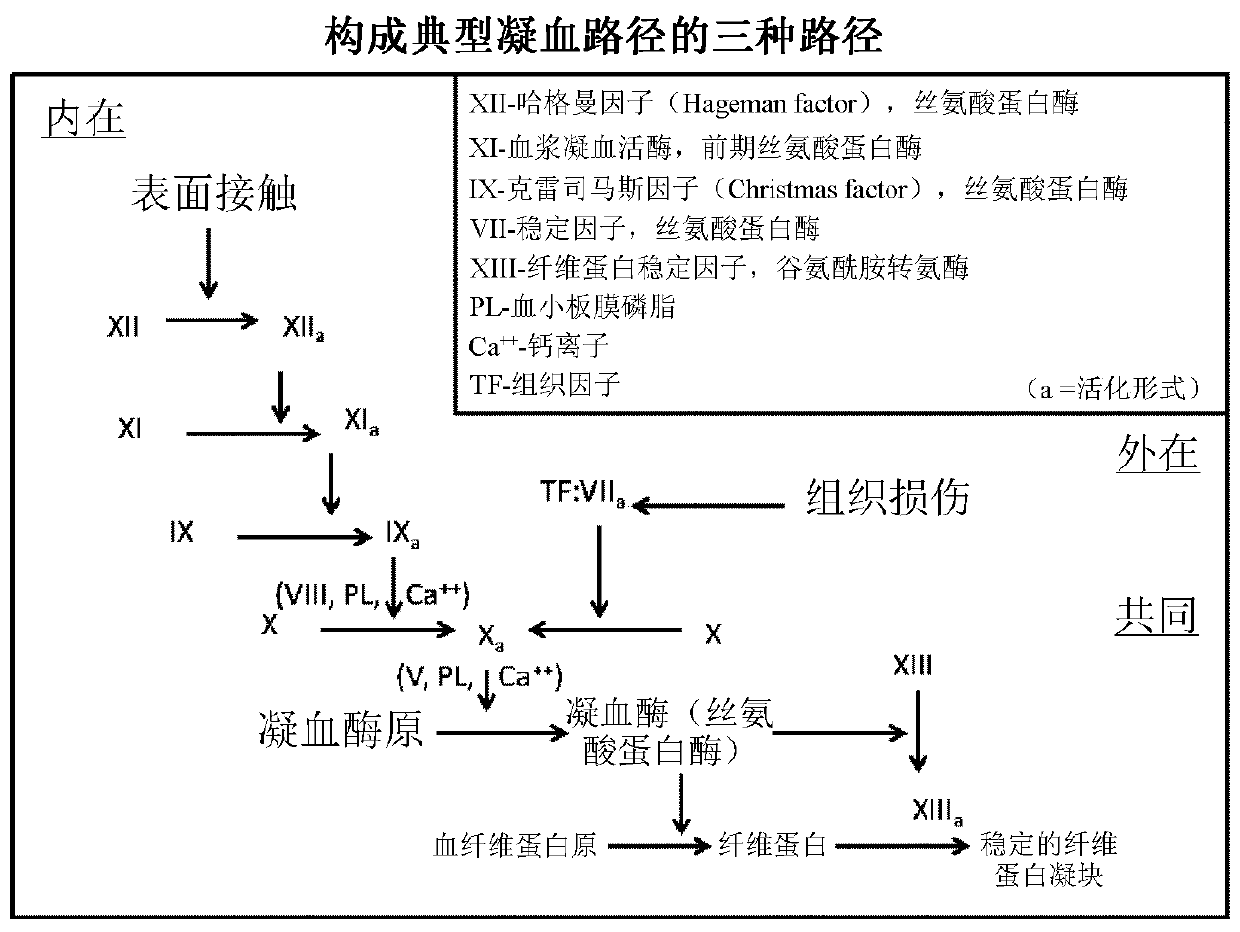
Clotting factors are proteins in the blood that control bleeding. Many different clotting factors work together in a series of chemical reactions to stop bleeding. This is called the clotting process. Problems with factor VIII and factor IX are known as hemophilia A and B, respectively.
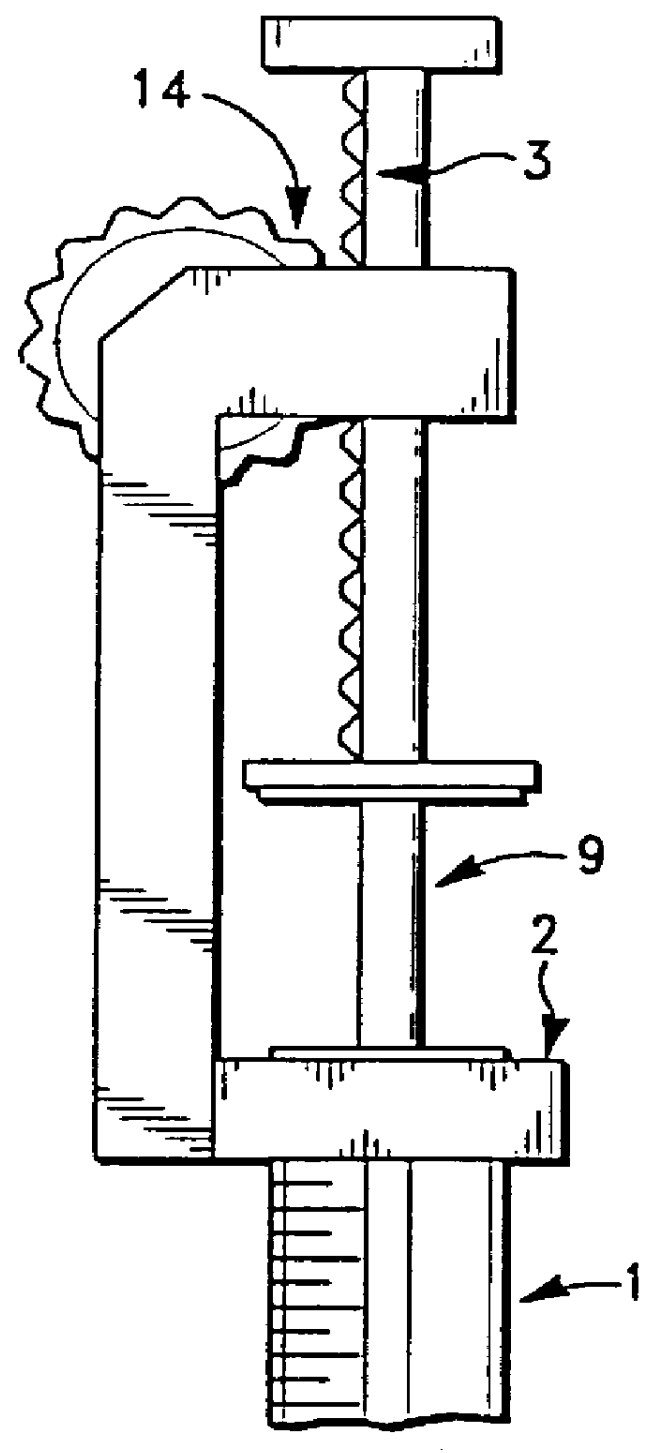
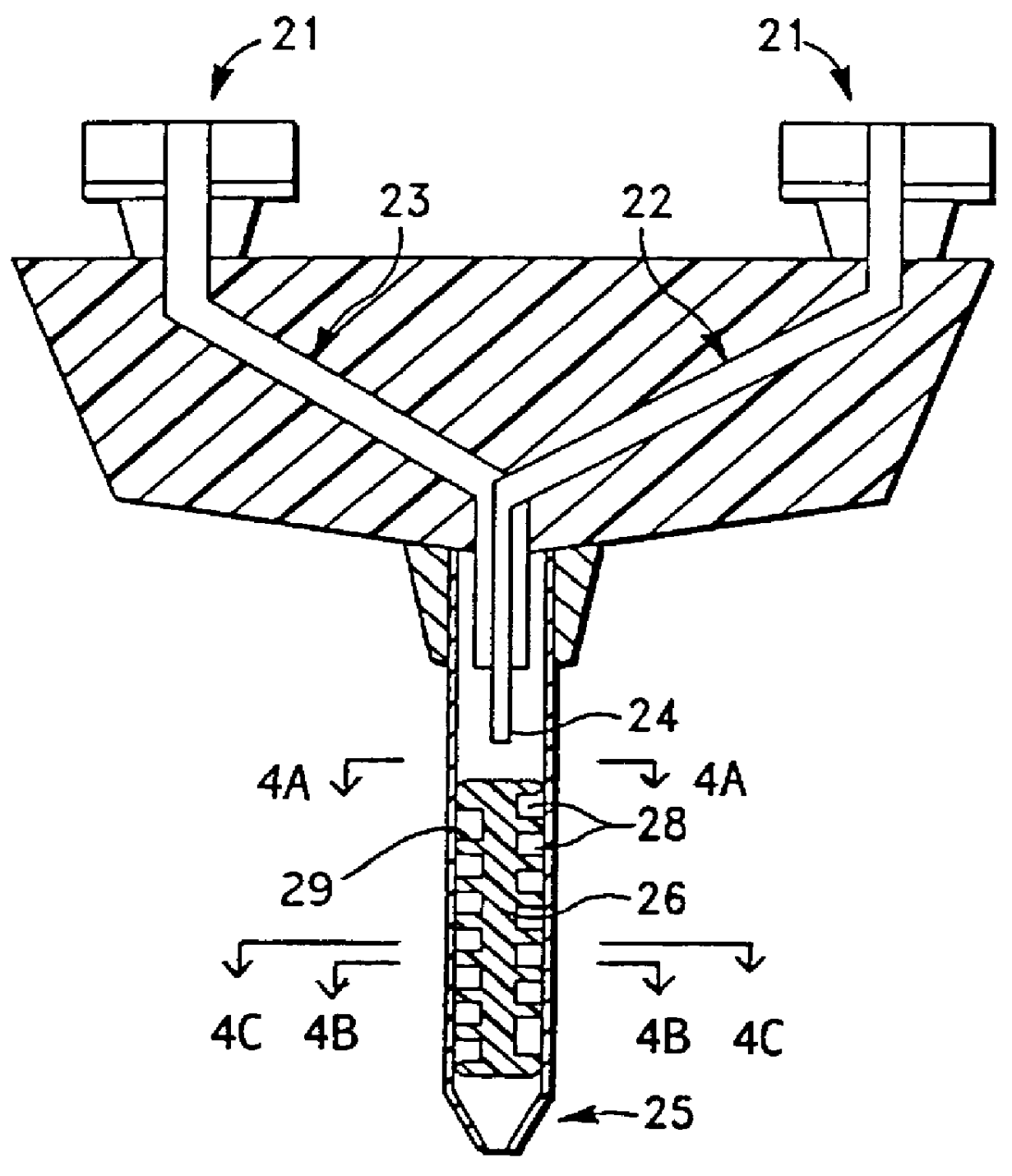
Apparatus for applying tissue sealant
InactiveUS6132396AEasy to fillEasy to assembleLiquid surface applicatorsSurgeryTissue sealantGear wheel
A device and method for applying a fibrinogen-based tissue sealant to seamlessly connect human or animal tissues or organ parts, to seal wounds, stop bleeding and the like by mixing fibrin or fibrinogen with blood clot-promoting coagulation factors are disclosed. The device includes two cylindrical compartments for separately containing the separate fluid components of the sealant preparation, which are simultaneously displaced from the respective compartments by plungers commonly depressable with the same effective strokes. The plungers may be depressed directly or by a common mechanism (e.g., rack and pinion) for accurately controlling the rate of dispensing fluid. The cylindrical compartments are of the same or different cross-sectional area and are arranged either concentrically or side-by-side. The device further includes structure for merging the two fluid components within an outer sleeve housing an inner needle. The sleeve and needle contain conduits for the flow of the two fluid sealant components as they are expressed from the respective compartments. Also disclosed are a convenient device for filling the two compartments, structure for mixing the fluid components, and for atomizing the effluent sealant fluid stream (i.e., spraying).
Owner:PLASMASEAL
Lentiviral vectors featuring liver specific transcriptional enhancer and methods of using same
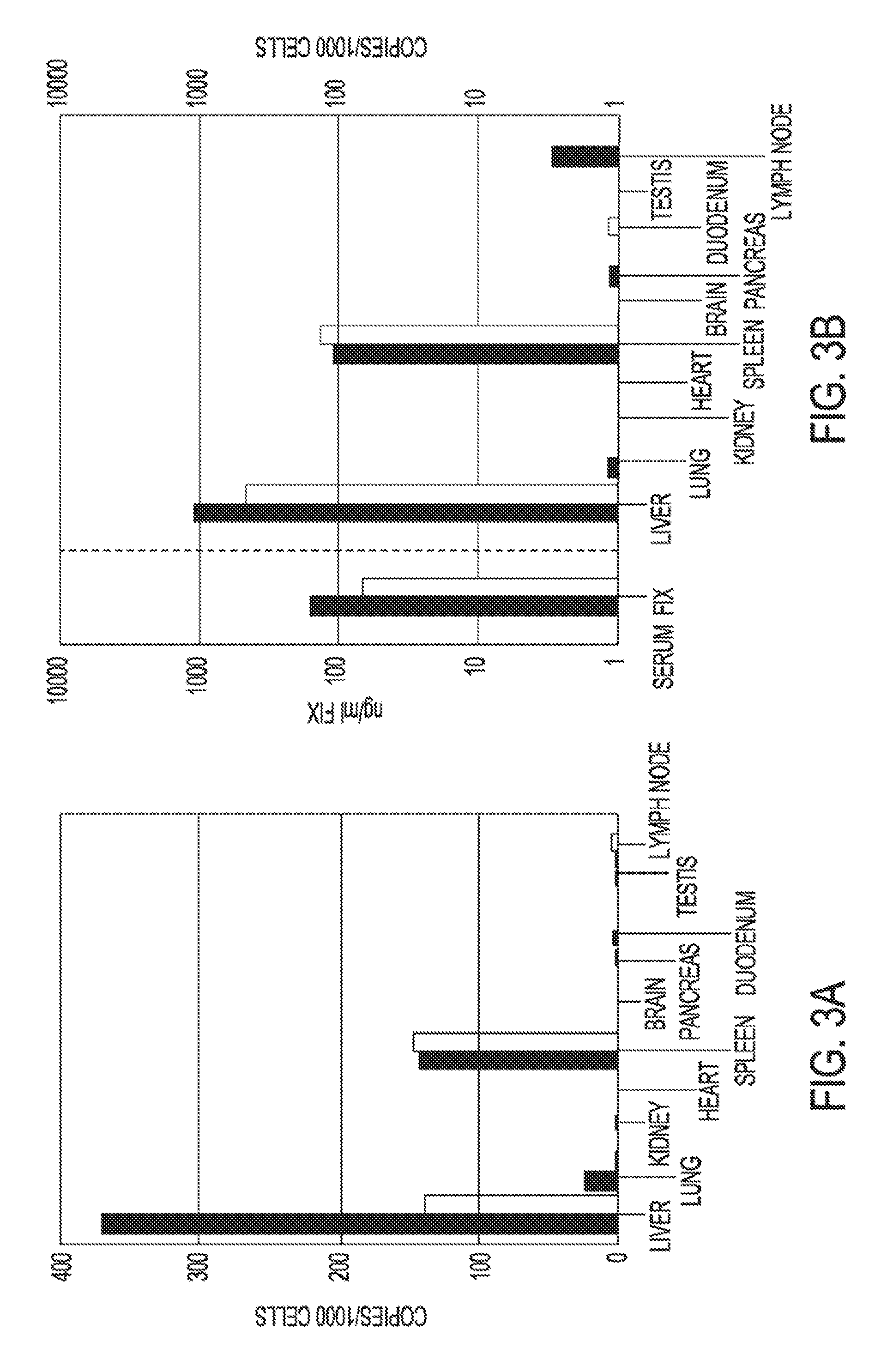
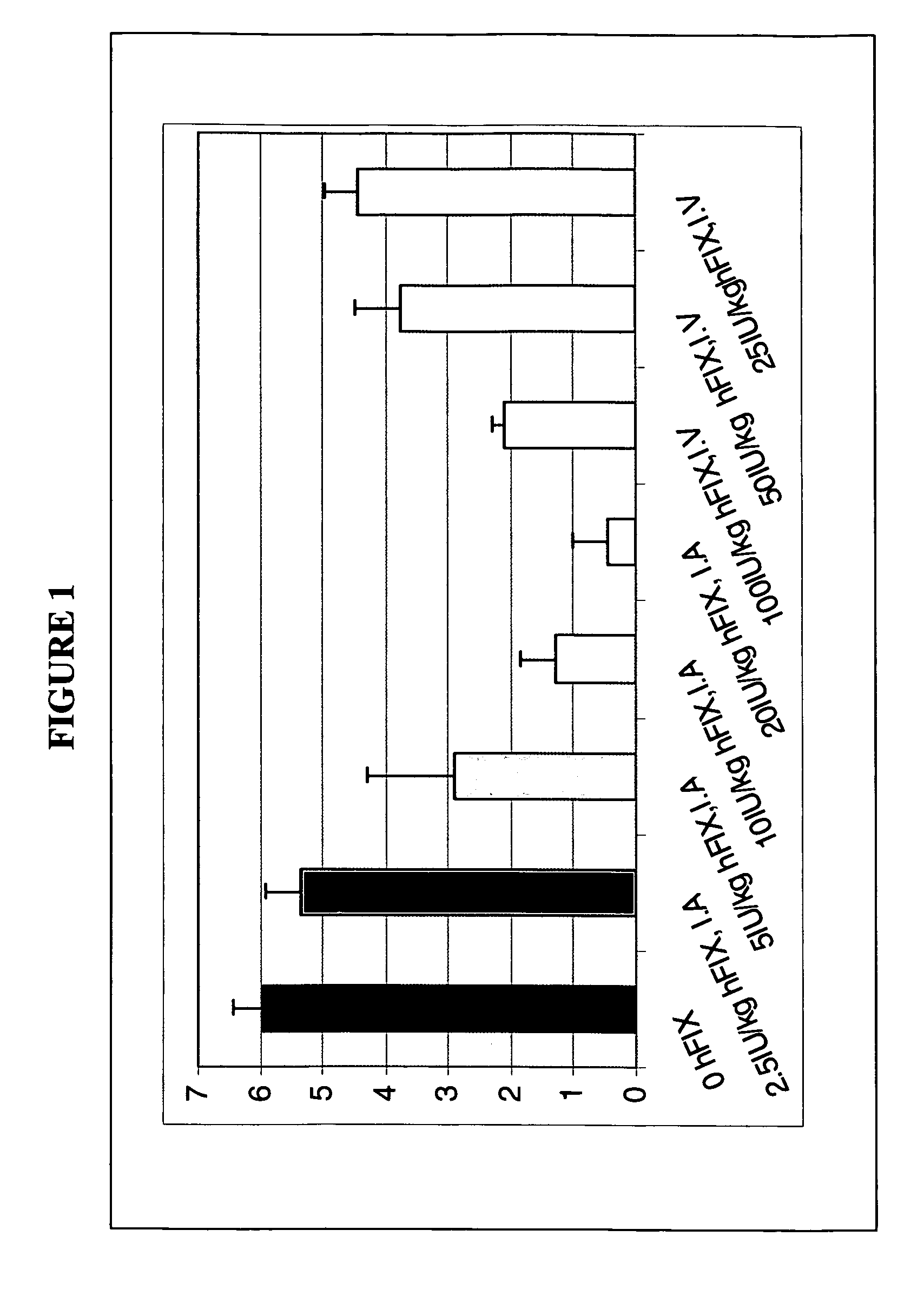
Recombinant lentiviruses and transfer vectors for transgene delivery as well as methods for gene therapy using such vectors are disclosed. The invention provides a third generation lentiviral packaging system and a set of vectors for producing recombinant lentiviruses, as well as novel tissue specific enhancer and promoter elements useful for optimizing liver specific transgene delivery. The transgene is preferably a blood clotting factor such as human factor IX (hFIX) or human factor VIII (hFVIII) and can be used for treatment of hemophilia.
Owner:MILTENYI BIOTEC B V & CO KG
Heterocyclic compounds regulating clotting
InactiveUS6180625B1Inhibition formationPreventing initiationOrganic active ingredientsOrganic chemistryFactor VIIaFactor VII
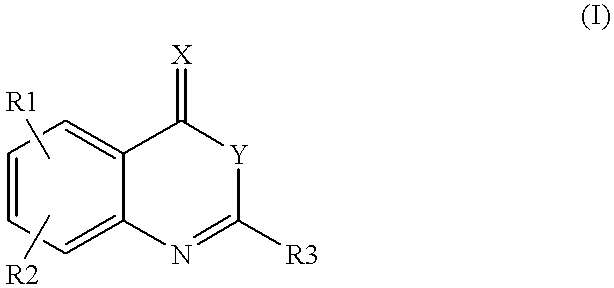
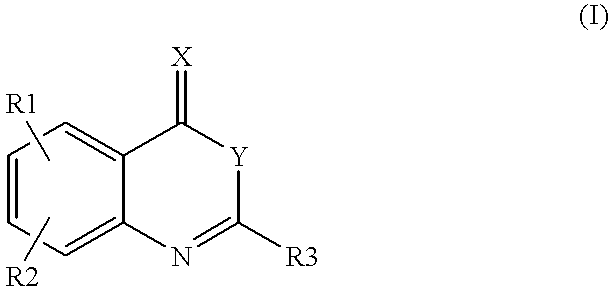
Compounds of formula (I)as factor VII-tissue factor inhibitors as well as novel benzoxazin derivatives are disclosed, wherein R1, R2, R3, X and Y are as defined in the specification. These compounds, and pharmaceutically acceptable salts thereof, have been shown to be inhibitors of factor VIIa-tissue factor activity and have anticoagulant properties. These compounds are useful for treating deficiencies of blood clotting factors or the effects of inhibitors to blood clotting factors. Methods for inhibiting clotting activity are also disclosed.
Owner:NOVO NORDISK AS
Hemophilia treatment by inhalation of coagulation factors
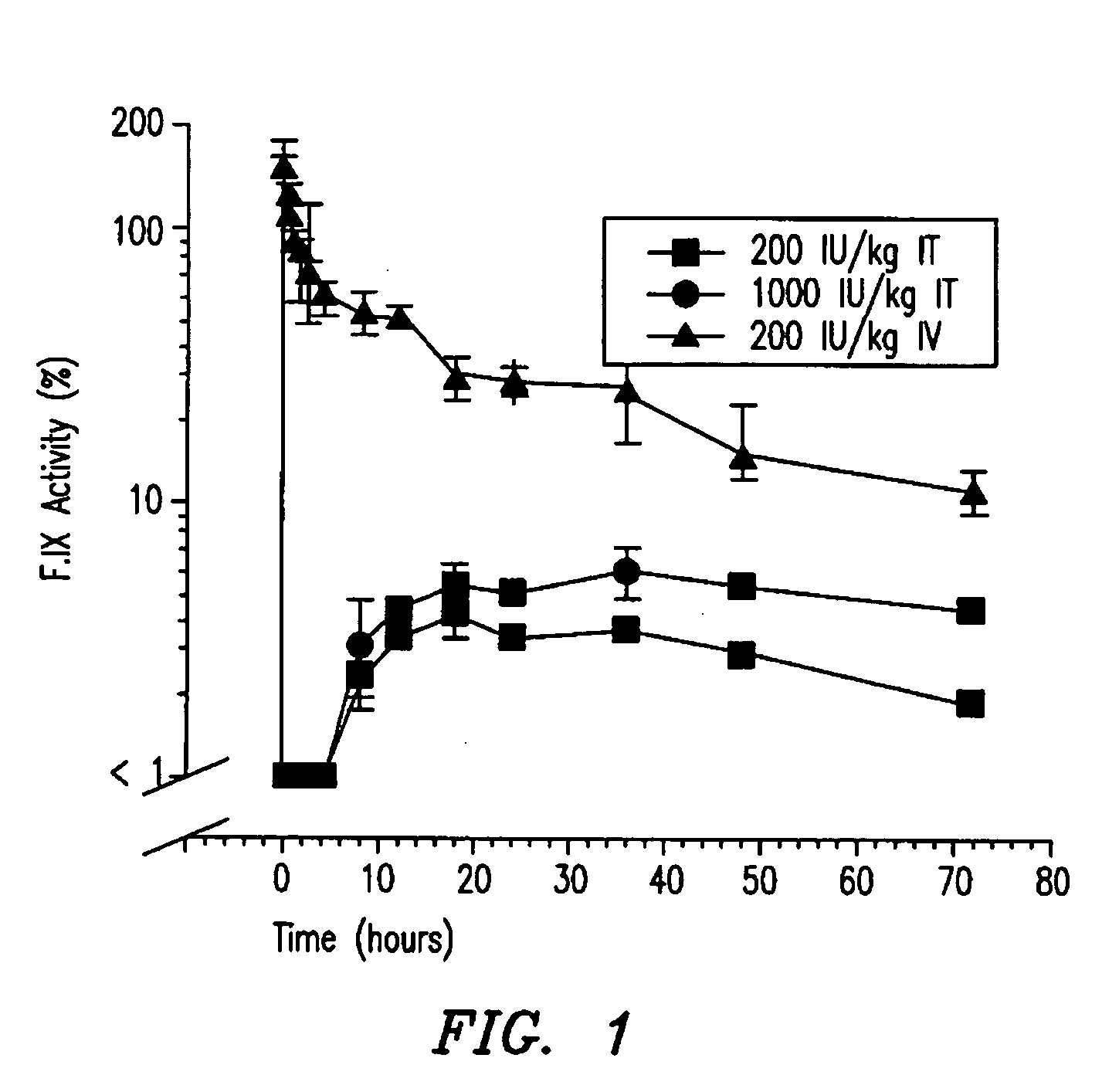
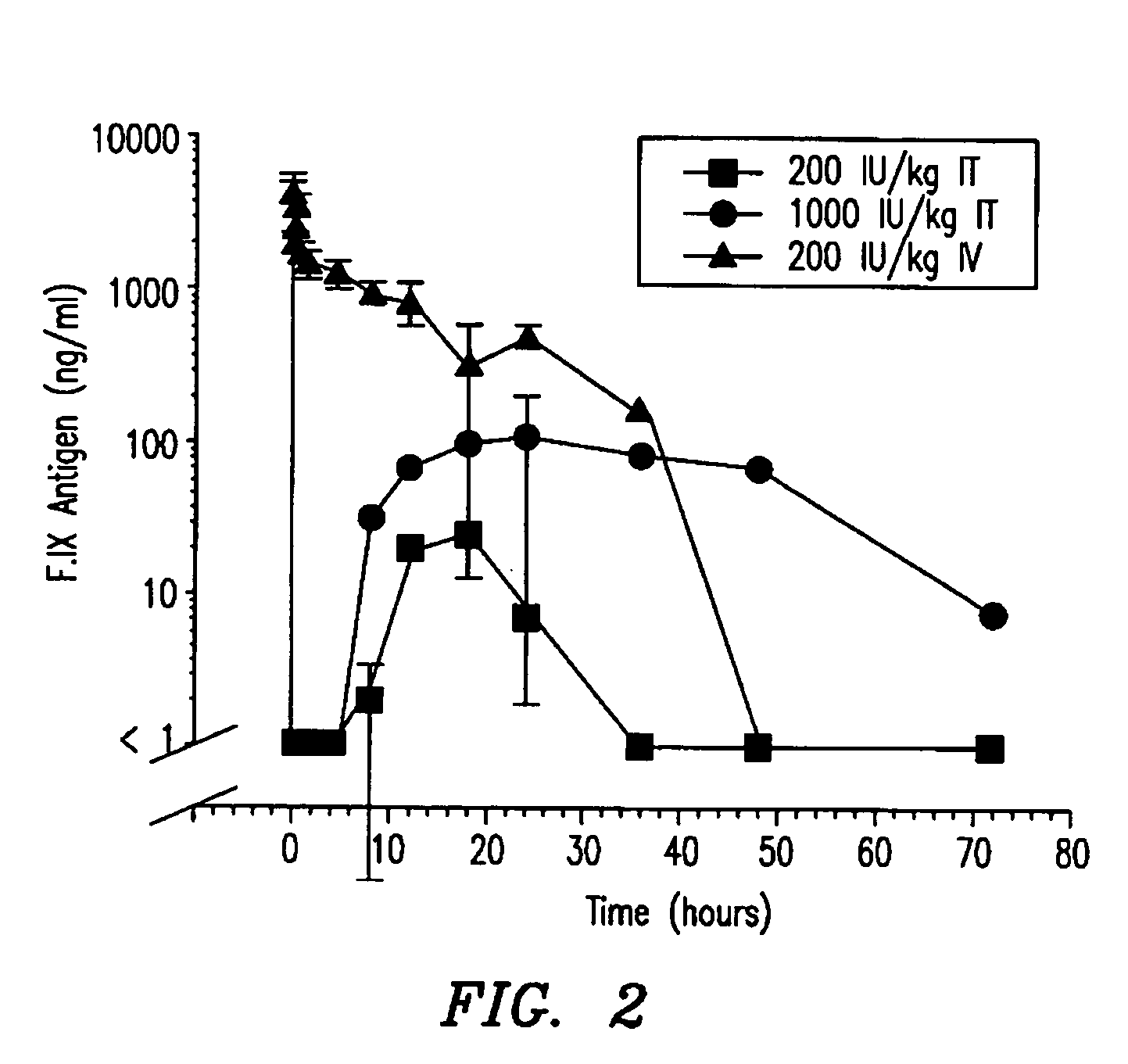
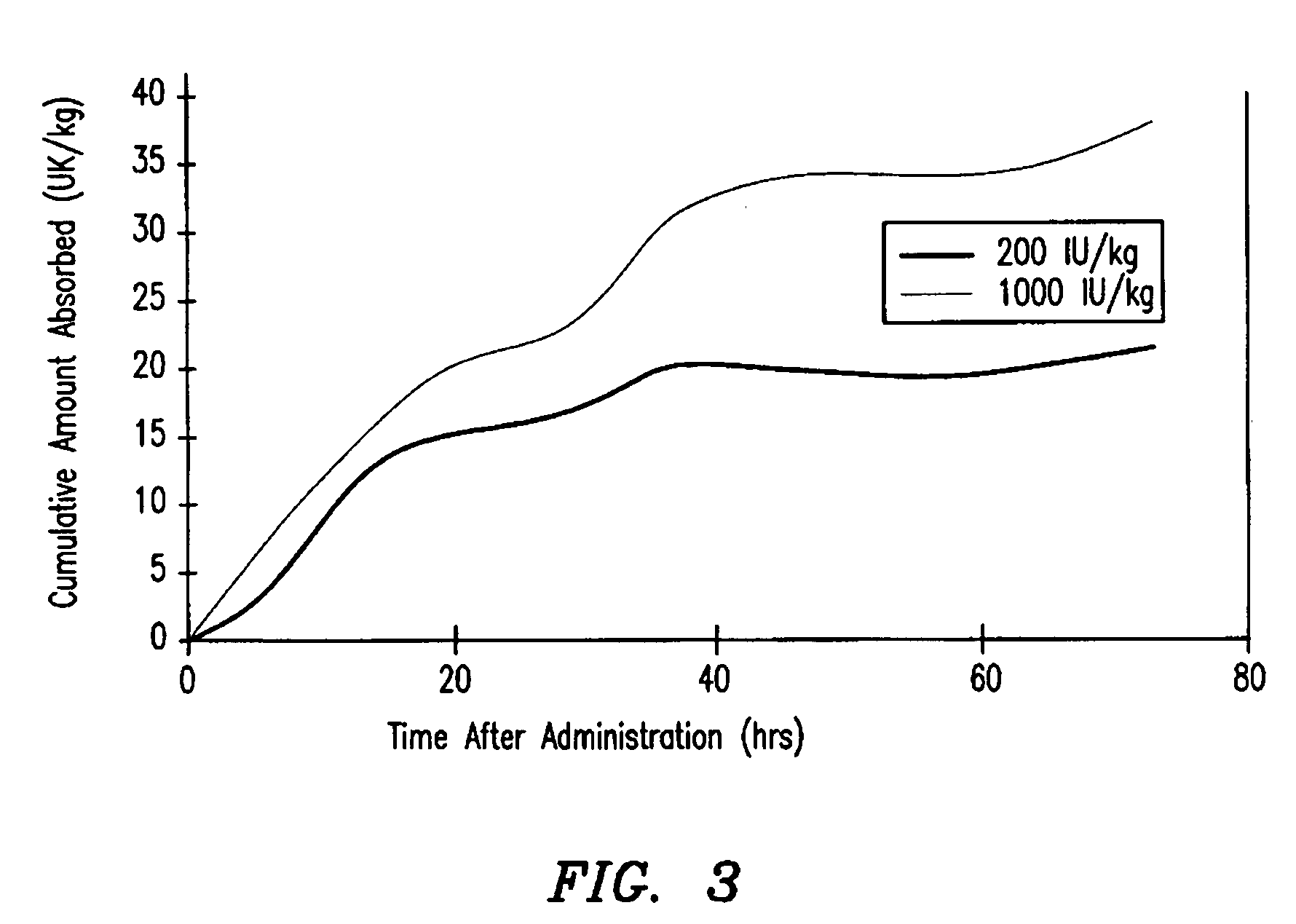
Hemophilia treatment by the inhalation of coagulation factors. Dry powder Factor IX is aerosolized to a mass median aerodynamic diameter of 4 μm or less, with at least 90% monomer content, at least 80% activity level, and 10% water or less. The aerosol is slowly, and deeply inhaled into the lung, and followed by a maximal exhale.
Owner:WYETH LLC +1
Human coagulation factor VII variants
InactiveUS6905683B2Increased tissue factor-independent activityPeptide/protein ingredientsMammal material medical ingredientsProteinase activityCoagulation system
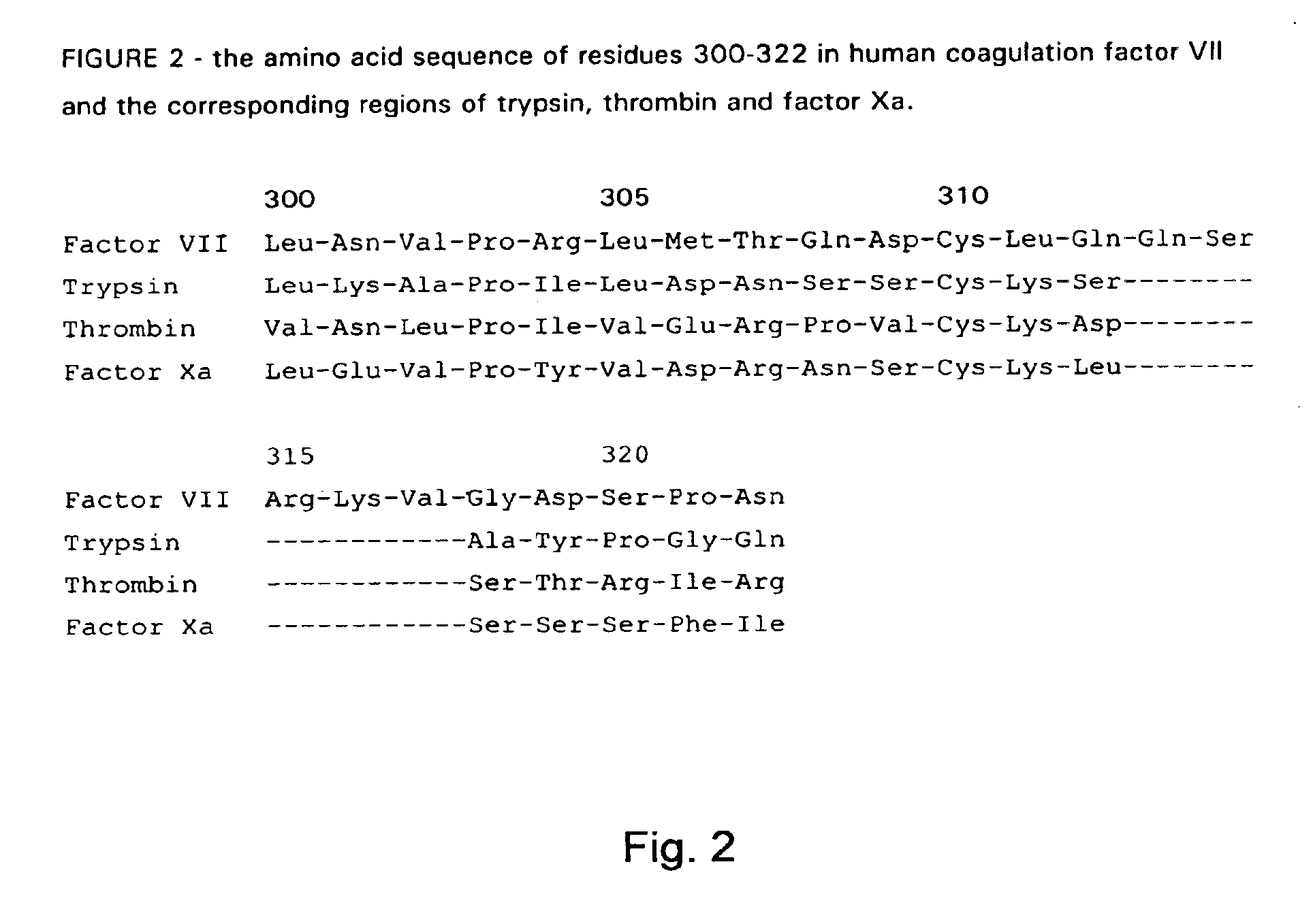
The invention concerns novel coagulation factor VII variants, wherein the Leu residue in position 305 or the Phe residue in position 374 of SEQ ID NO 1 has been replaced by another amino acid residue which can be encoded by nucleic acid constructs and, optionally, wherein at least one other amino acid residue in the remaining positions in the protease domain has been replaced by another amino acid residue which can be encoded by nucleic acid constructs;with the proviso that the variant is not FVII(Ala305).The invention further concerns nucleic acids encoding the Factor VII variants; vectors and cells comprising the nucleic acid; methods for producing the variants; pharmaceutical compositions comprising a Factor VII variant wherein the Leu residue in position 305 or the Phe residue in position 374 of SEQ ID NO 1 has been replaced by another amino acid residue which can be encoded by nucleic acid constructs and, optionally, wherein at least one other amino acid residue in the remaining positions in the protease domain has been replaced by another amino acid residue which can be encoded by nucleic acid constructs; use of the variants for producing a medicament for treatment or prophylaxis of bleeding disorders or enhancement of the coagulation system; and methods of treatment.
Owner:NOVO NORDISK AS
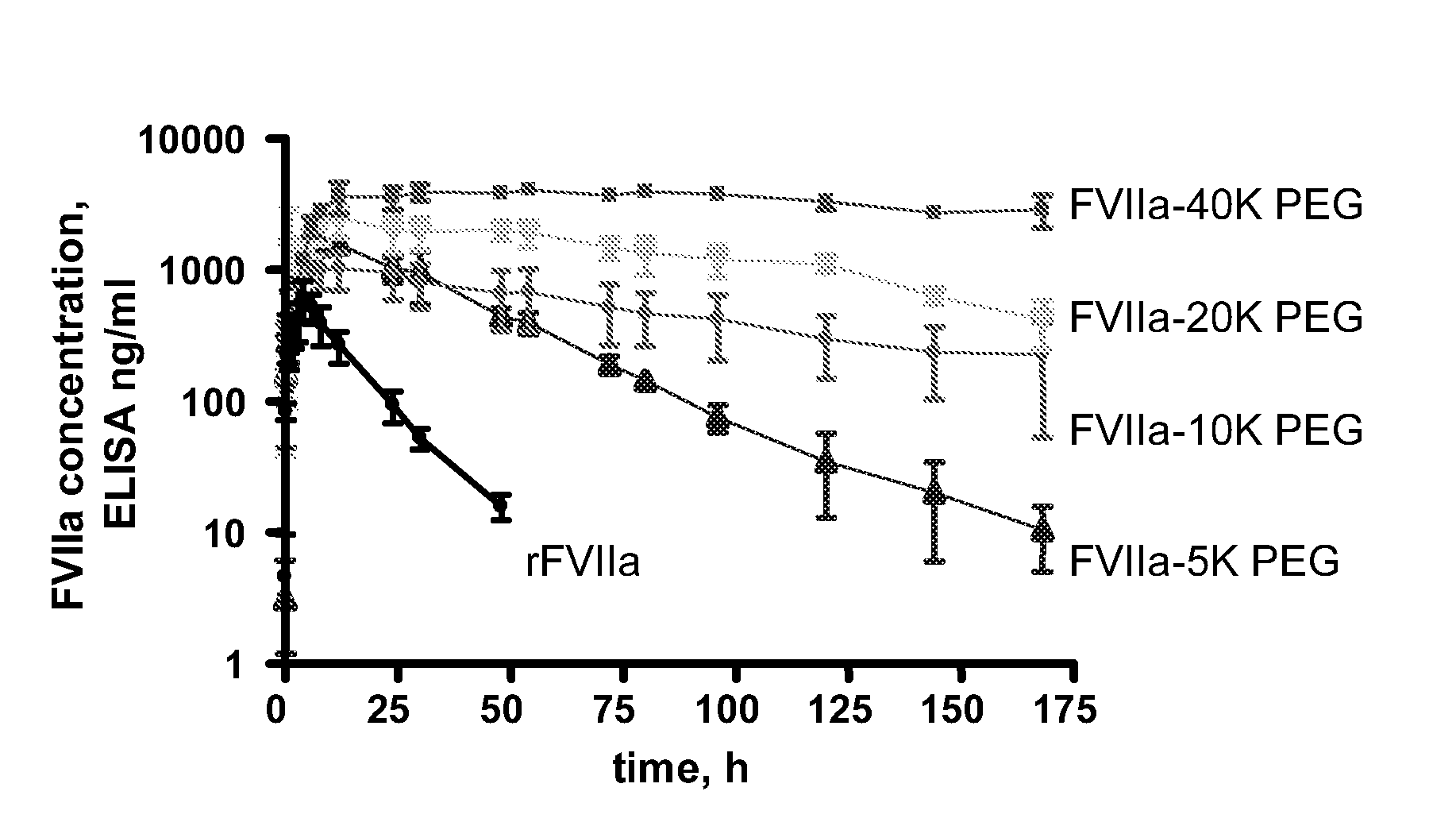
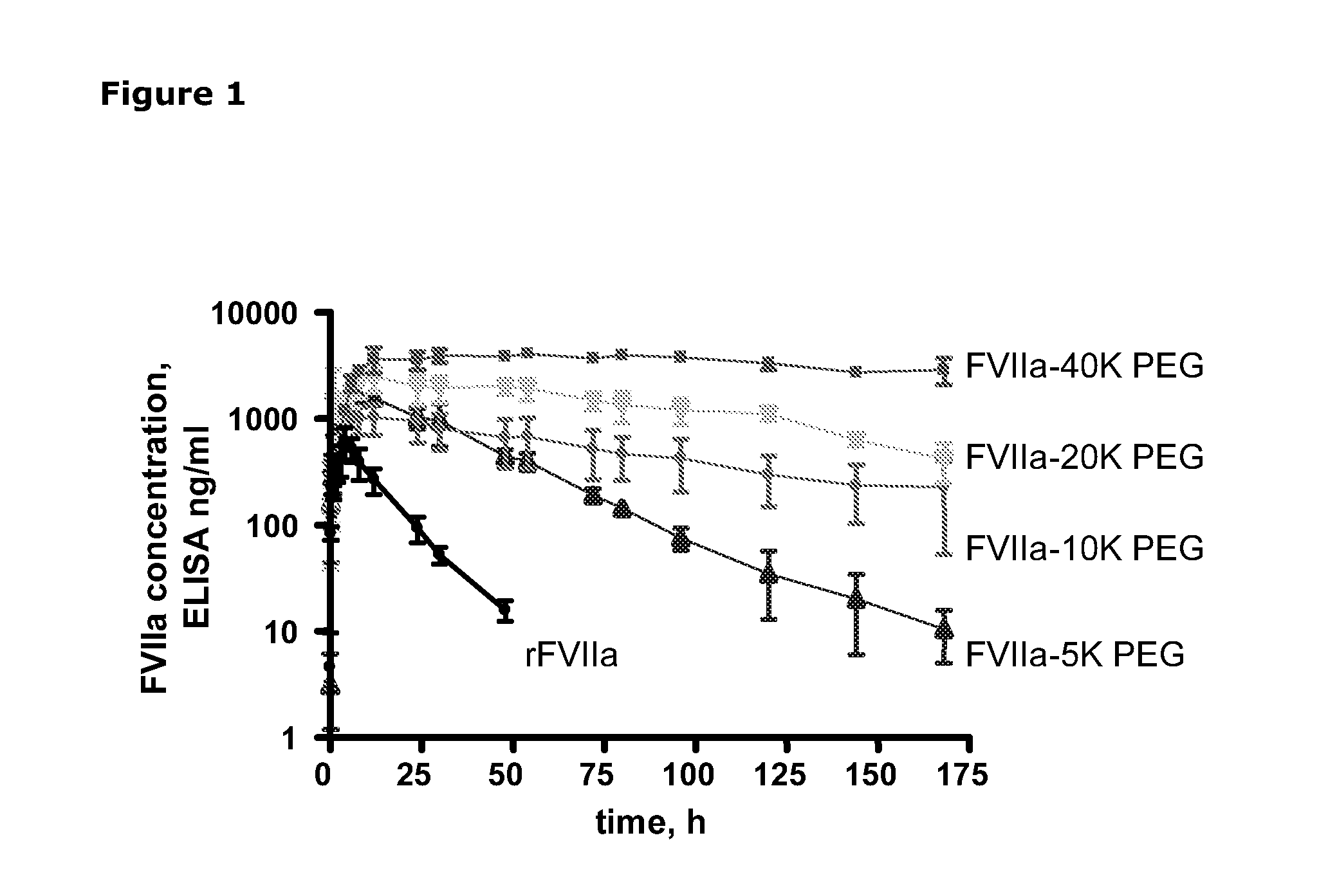
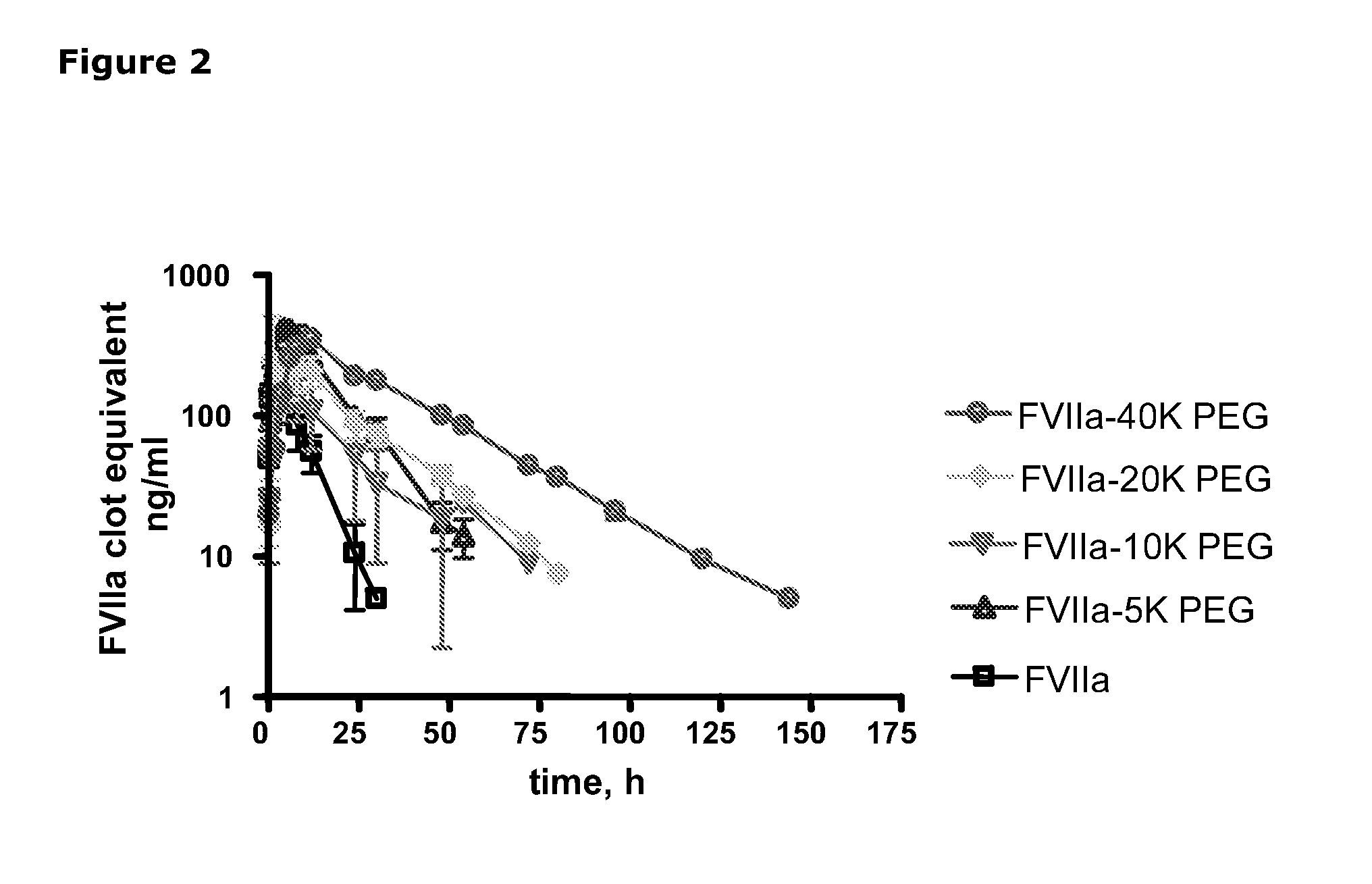
SUBCUTANEOUS ADMINISTRATION OF COAGULATION FACTOR VIIa-RELATED POLYPEPTIDES
InactiveUS20100143326A1Preventing and treating bleedingPeptide/protein ingredientsBlood disorderDrugCoagulation factor VIIa
The invention relates to the use of a Factor VIIa-related polypeptide for the manufacture of a medicament for treatment of a condition affectable by Factor VIIa, in particular a bleeding episode, said medicament being for subcataneous or intramuscular administration.
Owner:NOVO NORDISK AS
Hemostatic gel suppository
InactiveCN101524545AImprove induction abilityImprove deformationOrganic active ingredientsSuppositories deliveryTissue fluidHemostatics
The invention relates to a hemostatic gel suppository, which is characterized in that the hemostatic gel suppository is compounded by sodium carboxymethyl cellulose and other gels. The suppository has shape of hollow thin shell. The hemostatic gel suppository has the advantages that the hemostatic gel suppository has stronger induction function for the aggregation of platelet, can accelerate the deformation, adhesion, aggregation and release of the platelet so as to accelerate human physiological hemostasis, can activate blood clotting factors XII at a bleeding part to accelerate blood clotting, quickly absorbs water in blood and tissue fluid to form a gel closed blood capillary end face so as to function as mechanical blockage and stanch, can be firmly adhered to a wound surface to mechanically press the wound surface so as to stanch, does not adhere to the skin, has the function of inhibiting bacteria growth, and is accepted more easily by a patient.
Owner:大连永兴生物医药孵化器有限公司
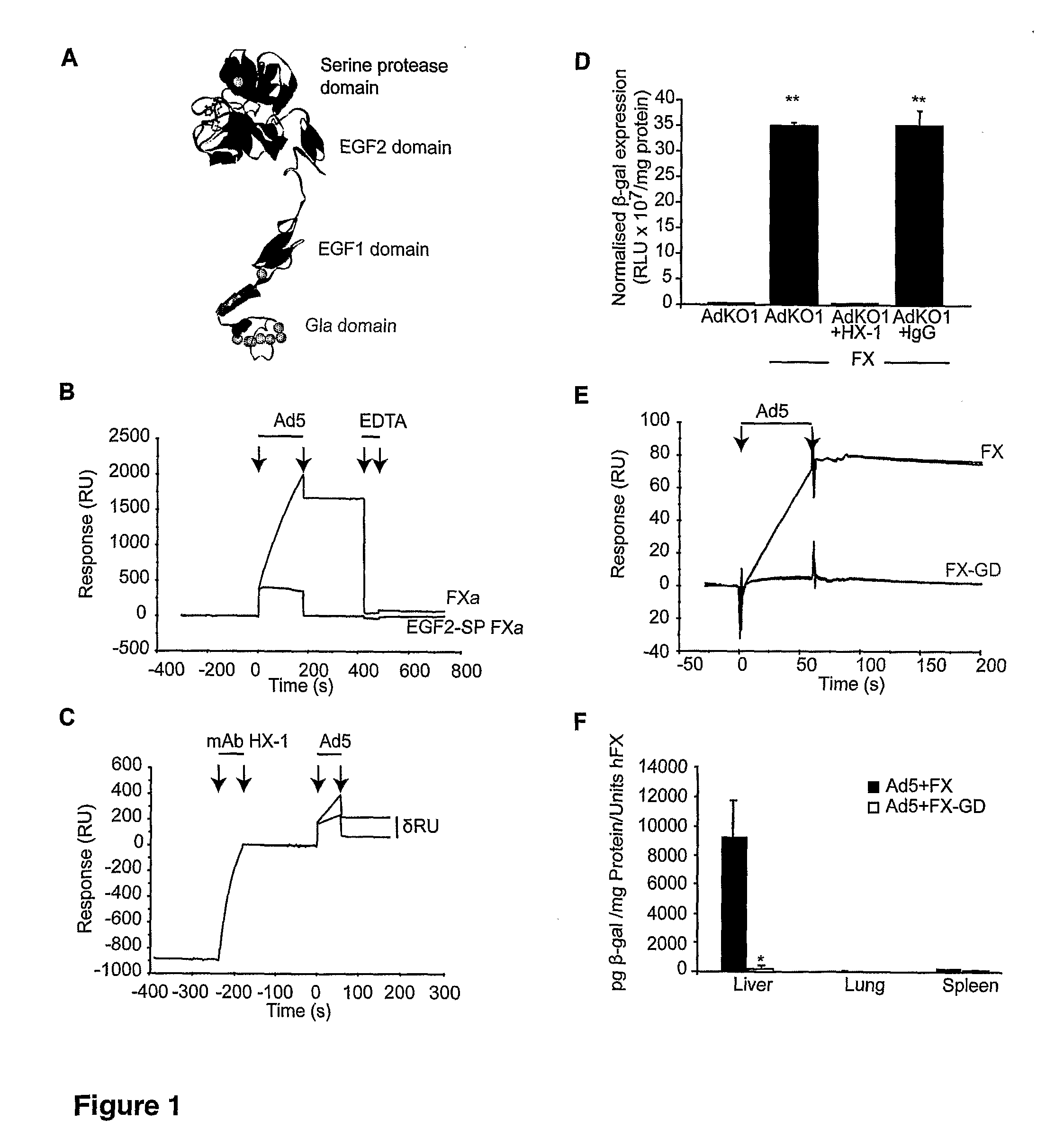
Modulation of Adenoviral Tropism
InactiveUS20110104788A1Increase opportunitiesHelp studyCompound screeningApoptosis detectionProviding materialTissues types
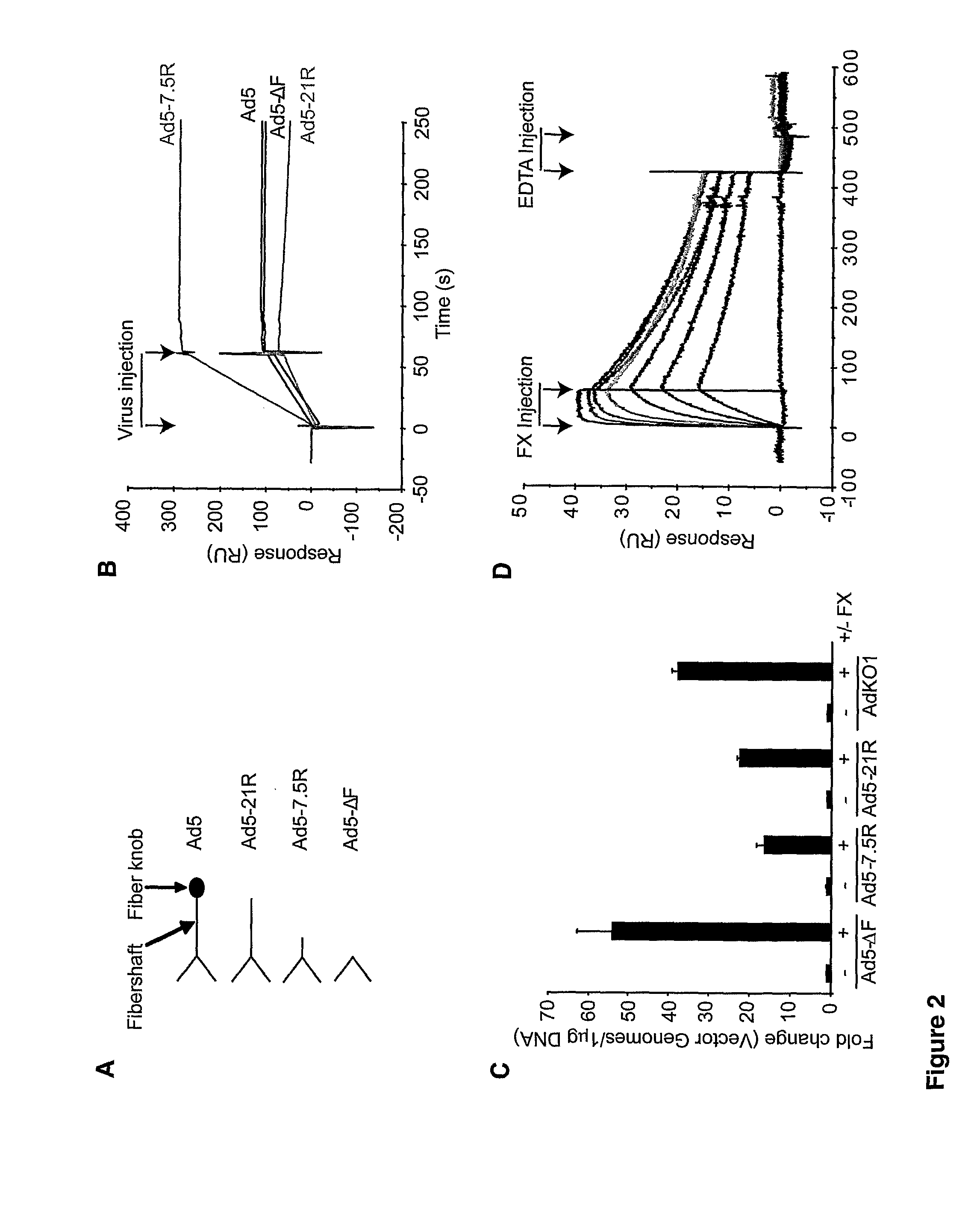
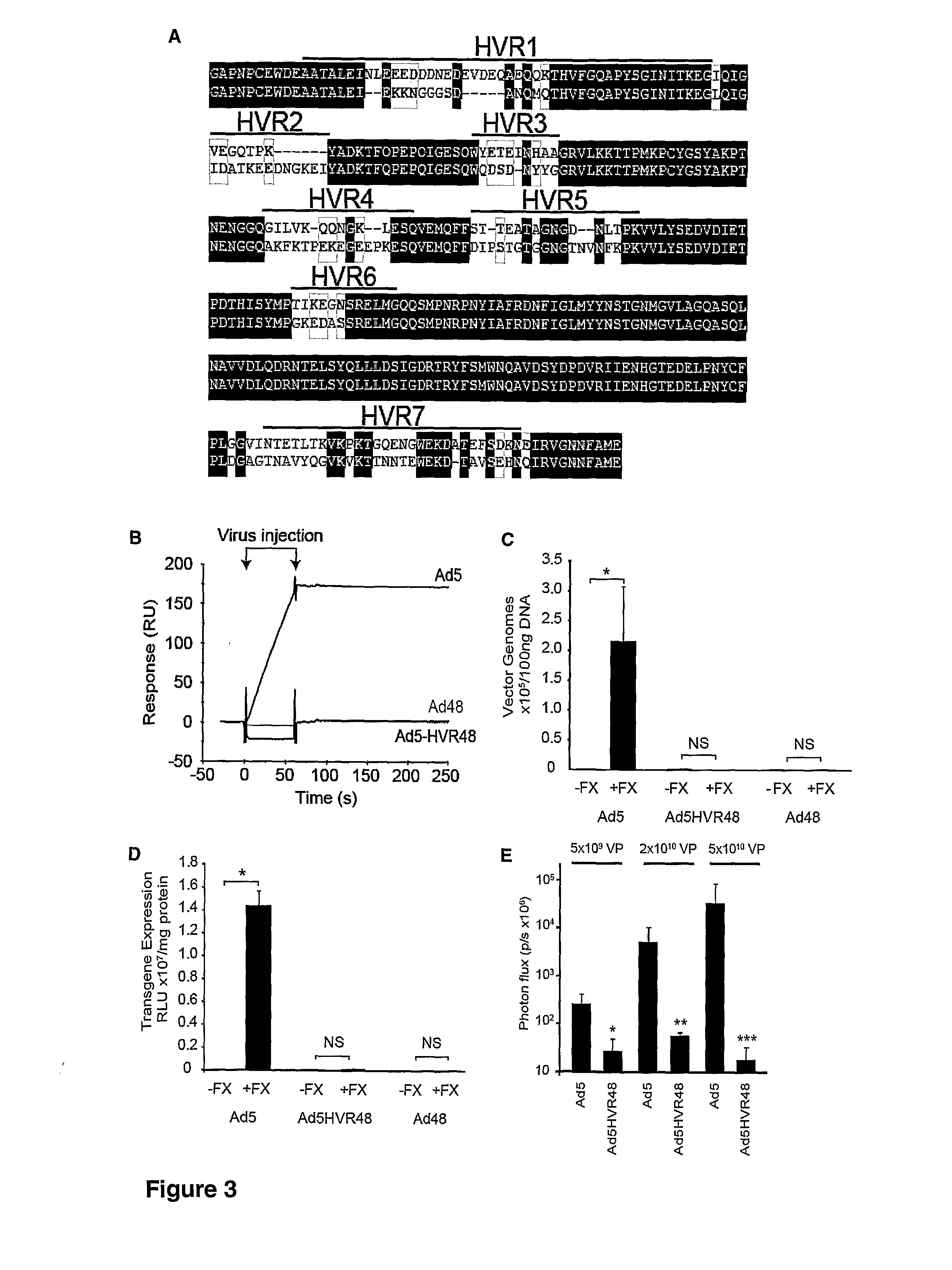
The invention provides materials and methods for modulating adenoviral tropism for hepatocytes and other cell types such as splenocytes. It relates to the findings that hypervariable regions (HVRs) of the viral hexon protein interact with the Gla domain of the blood clotting factor FX as part of the infective process in vivo. The invention provides means to disrupt the interaction between hexon and FX, thus reducing infection of hepatocytes and splenocytes, as well as use of targeting agents comprising the Gla domain or a fragment thereof to direct adenoviral vectors to desired target cell or tissue types.
Owner:BAKER ANDREW +2
Methods and compositions for intra-articular coagulation proteins
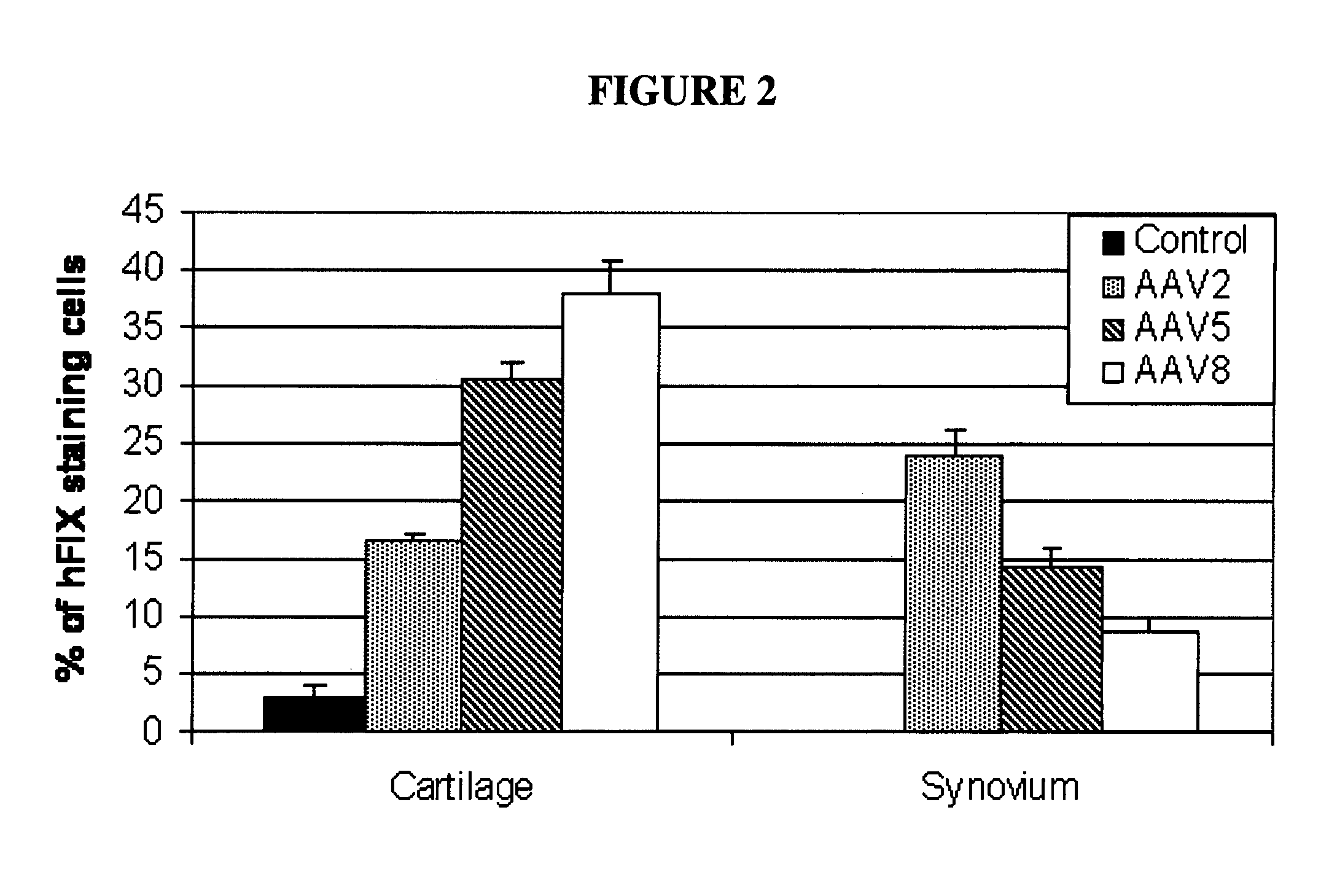
InactiveUS20100137211A1Reducing bleeding-associated joint damageProtecting from further bleedingOrganic active ingredientsPeptide/protein ingredientsJoint damageCoagulation protein
The present invention provides methods and compositions for treating blood clotting factor disorders and / or reducing bleeding-associated joint damage by treatments delivered to the joint in a subject.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Lupus anticoagulant (LA) screening and determining reagent kit (freezing method)
The invention discloses a reagent for screening and determining lupus anticoagulant (LA) in blood plasma by a dilution viper venom test method. The LA screening and determining reagent comprises viper venom blood clotting factor X activating agents, rapeseed phosphatide and calcium ions. The rapeseed phosphatide replaces the animal cephalin in the prior art, the proper addition quantity is low, and the rapeseed phosphatide has higher specificity and sensitivity for LA. The LA screening and determining reagent has the advantages of good specificity, high sensitivity, high stability and high detection feasibility degree.
Owner:SHANGHAI SUNBIO TECH
Compositions and methods for treating excessive bleeding
InactiveUS20100047352A1Augment and accelerate natural clotting processesExtended shelf lifePowder deliveryBiocideSilica particleExcessive Bleeding
The inventive material is a unique family of externally used wound sealants based upon a binding agent of reactive submicron silica particles that, when hydrated, agglomerate in the form of a supramolecular cross-linked network serving as the structural framework facilitating clot formation. A thrombolytic cascade accelerant can be provided, optionally with additional clotting factors, to further accelerate the clotting process.
Owner:HEMO NANOSCI
Pharmaceutical preparation of recombinant factor VIII lyophilized without albumin as a stabilizer
Disclosed is a lyophilized preparation of recombinant factor VIII used as a therapeutic preparation of hemophilia A. The lyophilized preparation of recombinant factor VIII is prepared by performing lyophilization using a mixture comprising 6 to 100 mM of L-arginine, 3.5 to 50 mM of L-isoleucine, and 10 to 100 mM of L-glutamic acid as a stabilizer for stabilizing the recombinant factor VIII which exhibits an unstable activity during lyophilization, rather than using human blood derived albumin.
Owner:GREEN CROSS CORP THE
Preparation method of blood coagulation factor IX quality control product
InactiveCN104181313AEasy to detectImprove uniformityPreparing sample for investigationBiological testingFreeze-dryingBlood plasma
The invention relates to a preparation method of a clinical blood coagulation inspection preparation and particularly relates to a preparation method of a blood coagulation factor IX quality control product. The preparation method comprises the following steps: carrying out affinity chromatography on the mixed blood plasma of multiple persons by using an anti-human blood coagulation factor IX monoclonal antibody immunoaffinity chromatography column, removing a blood coagulation factor IX in the mixed blood plasma of multiple persons to obtain a blood plasma in shortage of the blood coagulation factor IX; mixing the mixed blood plasma of multiple persons with the blood plasma in shortage of the blood coagulation factor IX according to a certain proportion to prepare a blood coagulation factor IX quality control product with the content of the blood coagulation factor IX at different concentration levels, adding a freeze-drying protective additive, carrying out sub-packaging, and carrying out freeze drying so as to obtain the blood coagulation factor IX quality control product. According to the blood coagulation factor IX quality control product prepared by the preparation method, the uniformity, the stability and the stability of freeze-dried aquatic product subjected to re-melting are good, and the quality control product can replace an imported product to be used for quality control on detection of blood coagulation factor IX, so that the reduction of the detection cost is facilitated and the capability of detecting the blood coagulation factor IX in China can be promoted.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
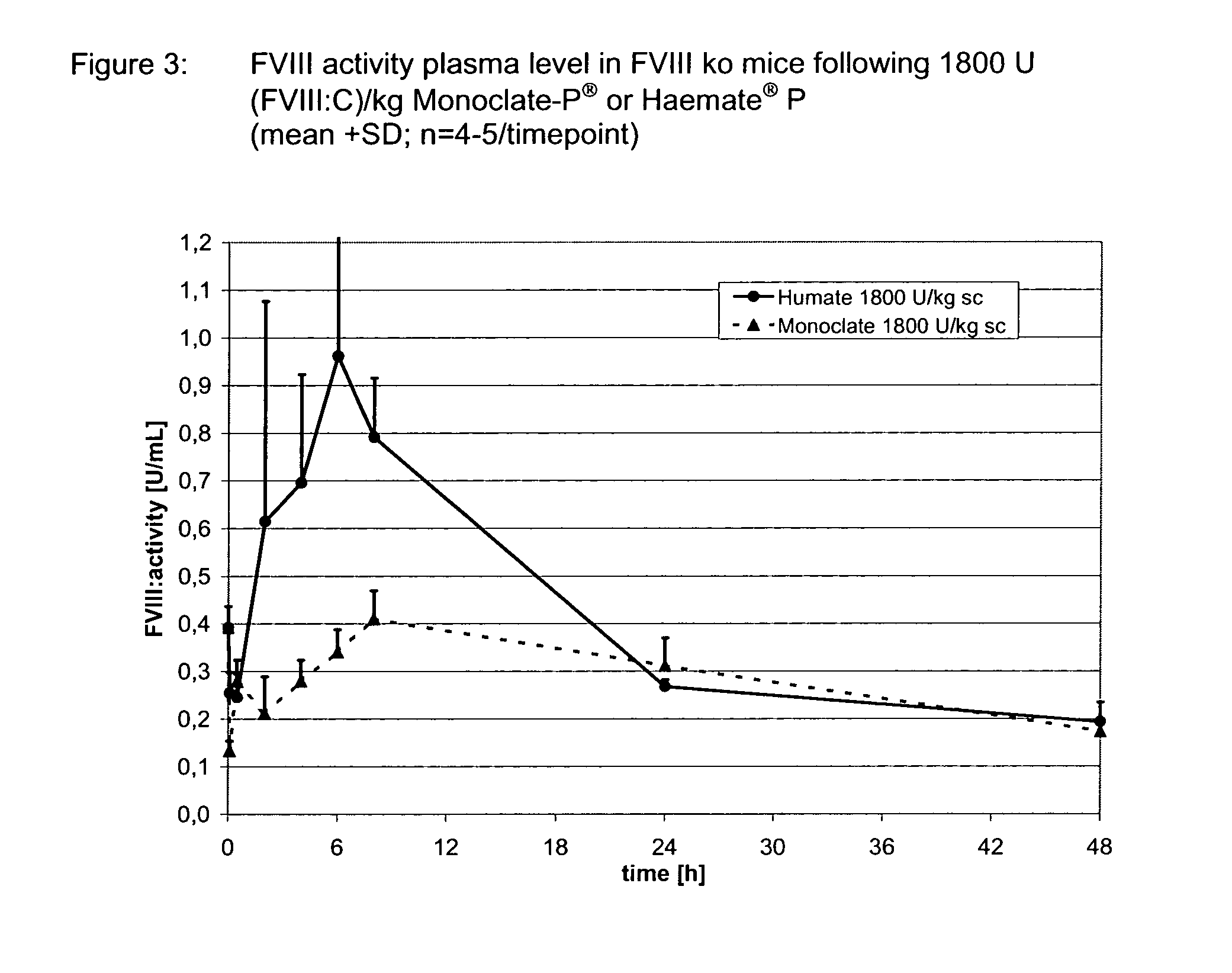
Use of VWF stabilized FVIII preparations and of VWF preparations without FVIII for extravascular administration in the therapy and prophylactic treatment of bleeding disorders
The present invention relates to the use of von Willebrand Factor (VWF) preparations or of a VWF preparation in combination with coagulation Factor VIII (FVIII) for extravascular administration in the therapy and prophylactic treatment of bleeding disorders.
Owner:CSL BEHRING GMBH
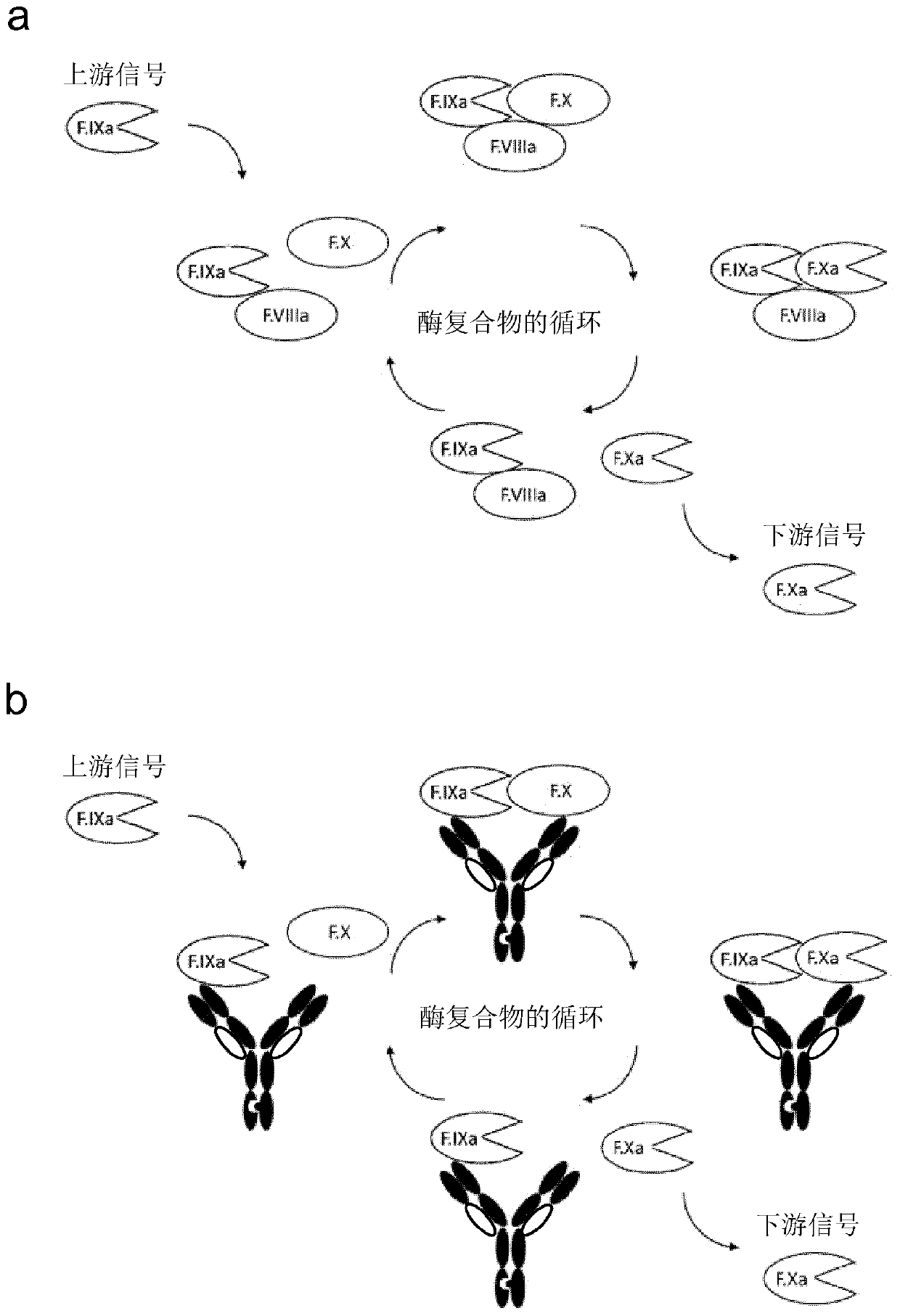
Bispecific antibodies for factor ix and factor x
PendingCN110753704AReduce dosing frequencyEasy for subcutaneous injectionImmunoglobulins against blood coagulation factorsHybrid immunoglobulinsAntiendomysial antibodiesBispecific antibody
Bispecific antigen binding molecules (e.g., antibodies) that bind blood clotting factors, factor IXa (FIXa) and factor X (FX), and enhance the FIXa-catalysed activation of FX to FXa. Use of the bispecific antigen binding molecules is to control bleeding, by replacing natural cofactor FVIIIa which is deficient in patients with haemophilia A.
Owner:KIMAB LTD
Antithrombotic agents
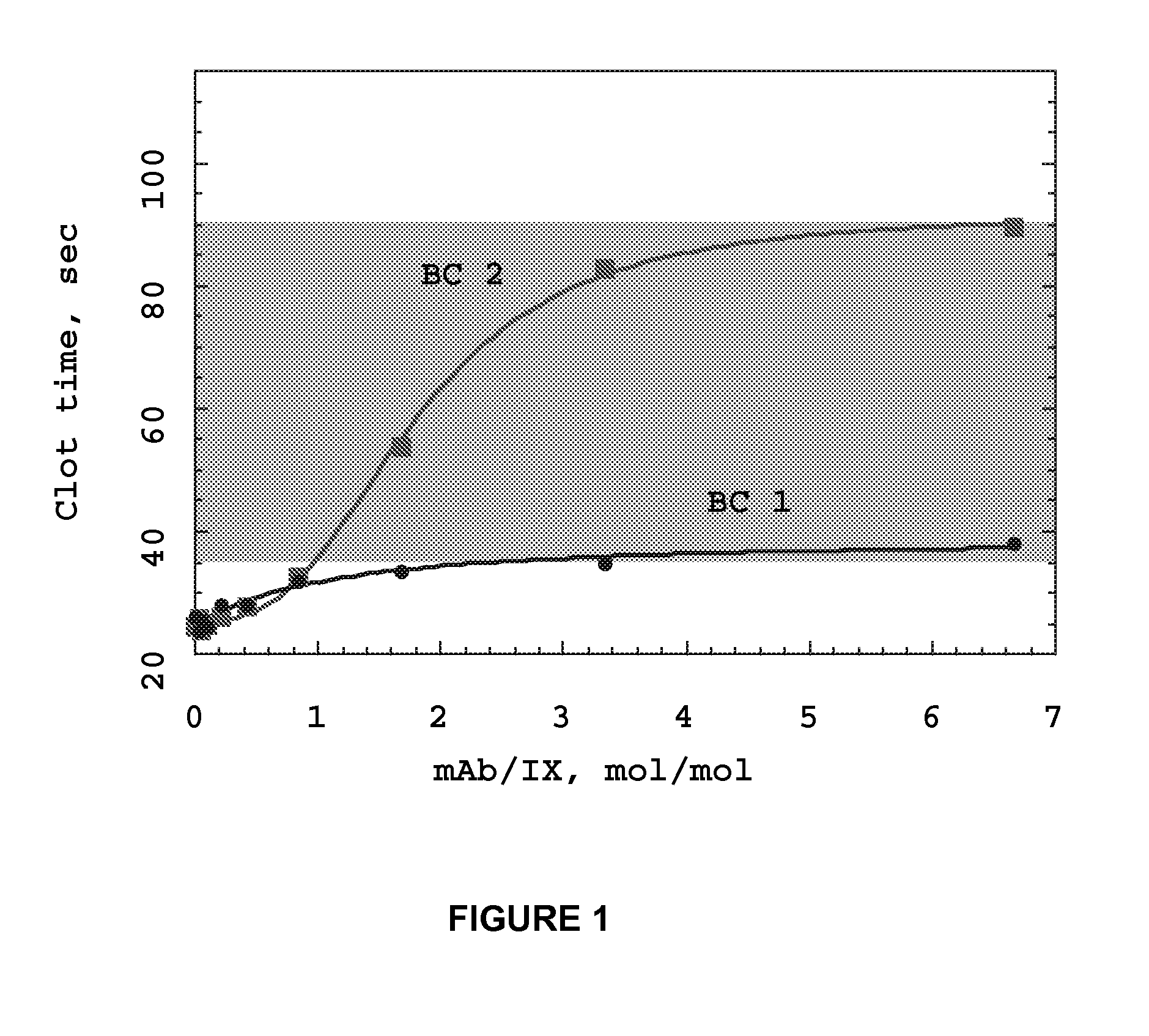
InactiveUS20070190059A1Reduce required dosePreventing thromboembolic strokeImmunoglobulins against blood coagulation factorsAntibody ingredientsAntithrombotic AgentMonoclonal antibody
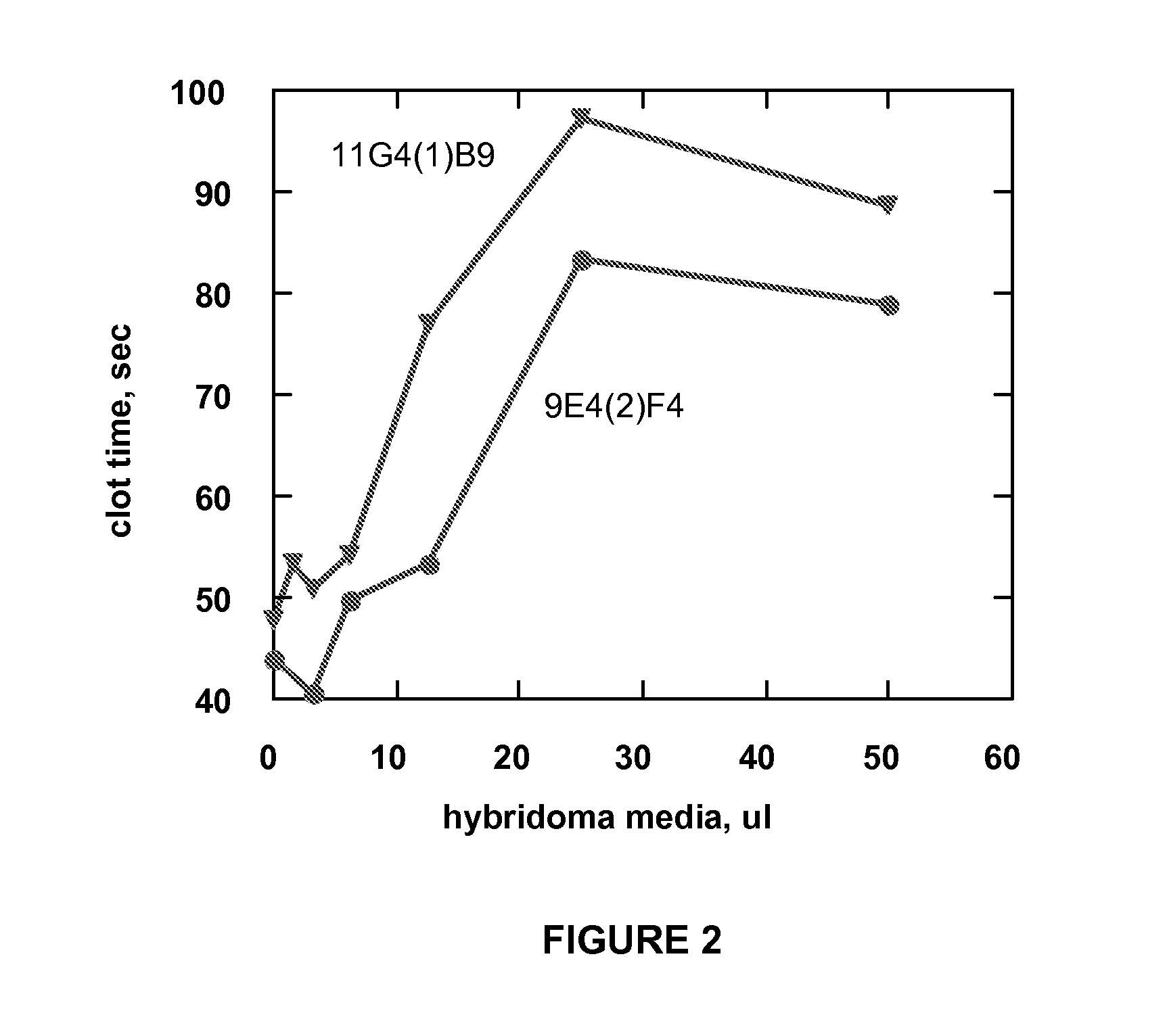
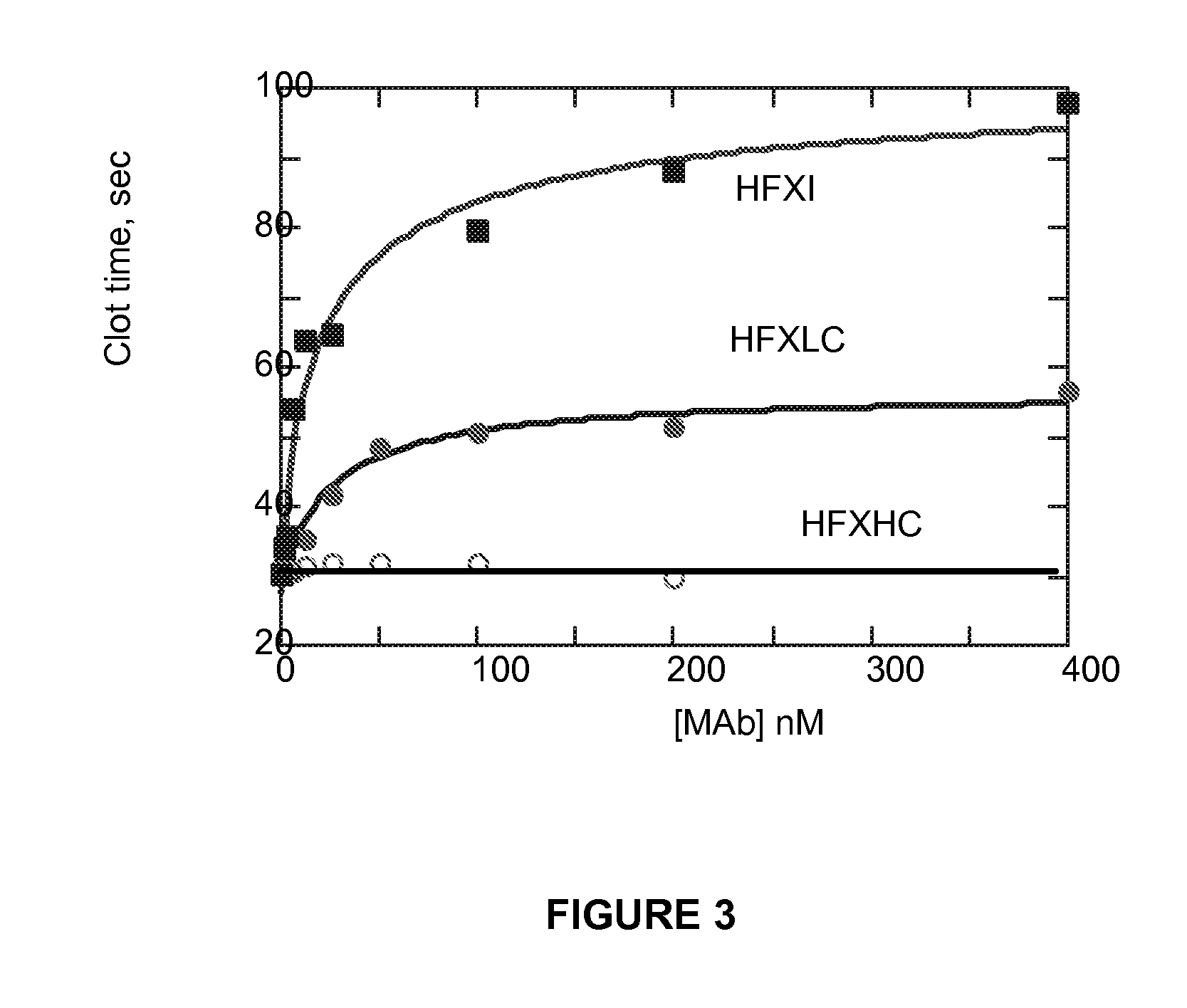
Monoclonal antibodies directed against coagulation factor IX and their use in inhibiting thrombosis are disclosed.
Owner:UNIVERSITY OF VERMONT
Detection method for functions of platelets
ActiveCN106885895AReliable test resultsReduce testing costsBiological testingTesting medicinal preparationsPlatelet inhibitorsComputer science
The invention discloses a detection method for functions of platelets. The detection method is used for analyzing and evaluating the functions of the platelets and comprises platelet detection as follows: (1) carrying out platelet activated detection; (2) carrying out blood clotting factor activated detection; (3) carrying out platelet inhibited detection. The detection method is applicable to the platelet function determination of a thromboelastography by a clotting method, and a platelet function detection result is obtained through separately adding obtained platelet detection values in a detected blood sample and carrying out further calculation. According to the method, by adopting a platelet inhibitor, a detection value serves as a background control parameter in platelet function analysis, and thus, the detection result is more reliable. Compared with the former detection methods, the method has the advantages that expensive hemocoagulase is not required to be used, so that the detection cost is reduced, and the method is applicable to clinical popularization.
Owner:ZIRCON BIOTECH CO LTD
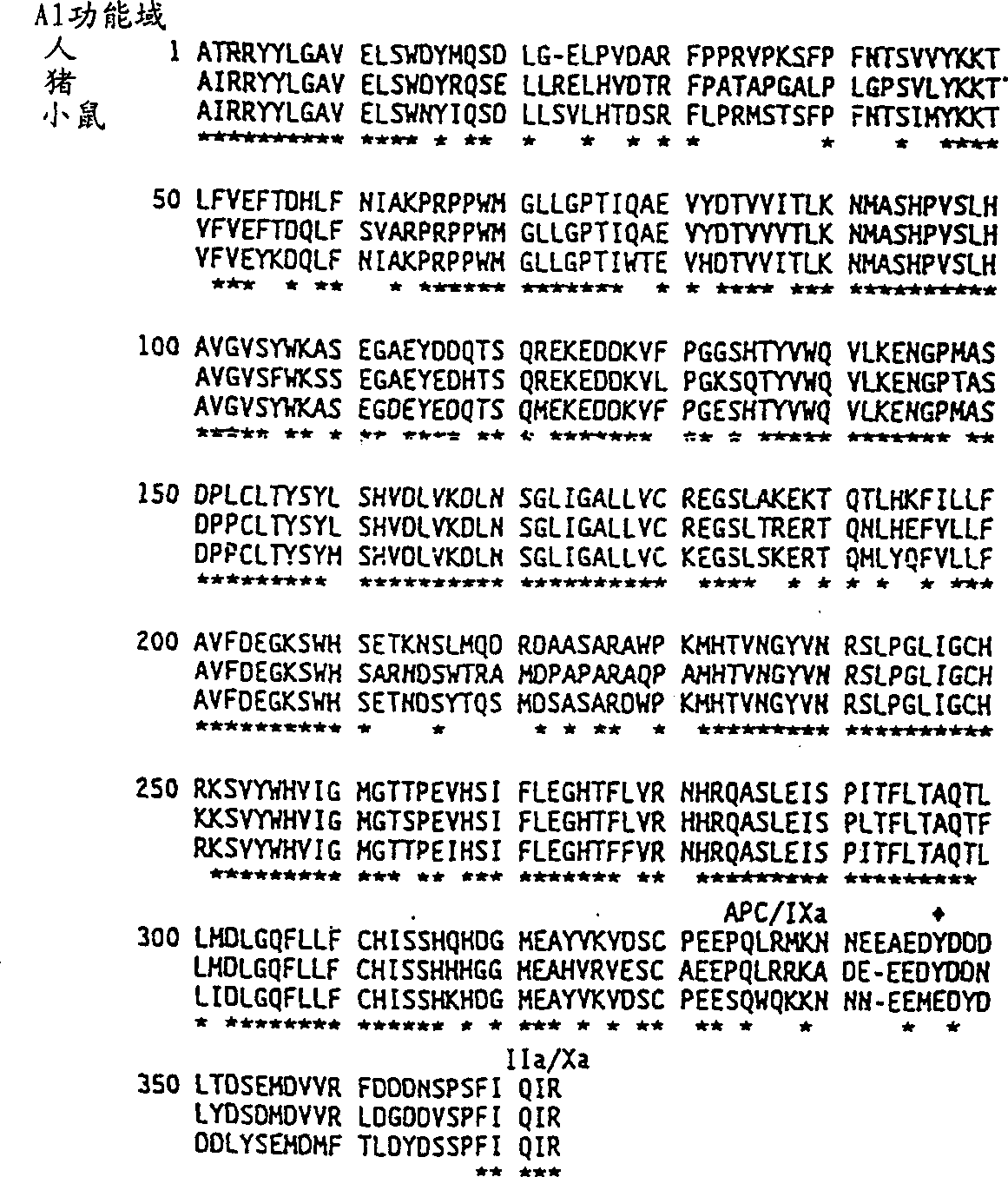
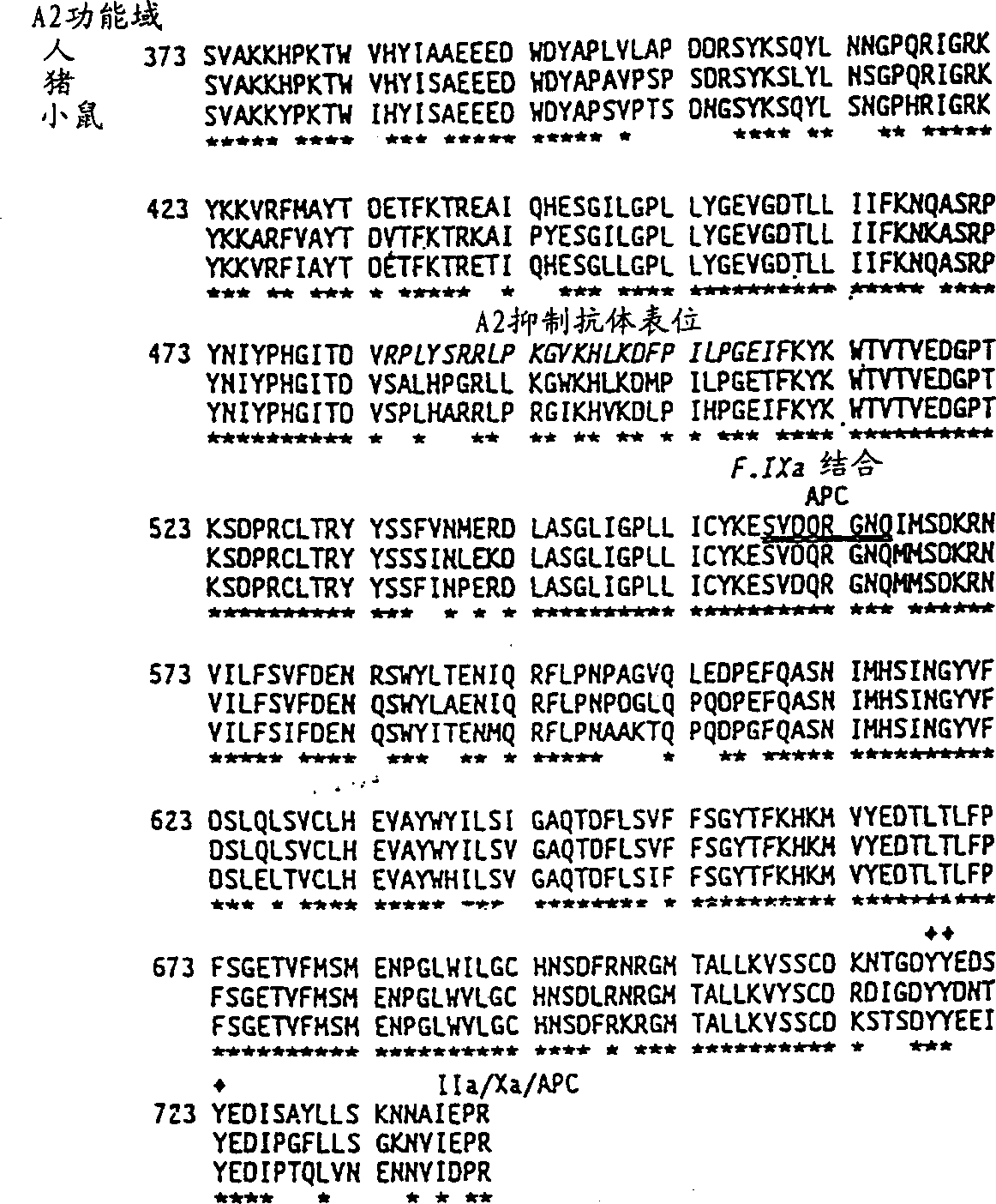
Modified factor VIII
Specific amino acid loci of human factor VIII interact with inhibitory antibodies of hemophilia patients who have developed such antibodies after being treated with factor VIII. Modified factor VIII is disclosed in which the amino acid sequence is changed by a substitution at one or more of the specific loci. The modified factor VIII is not inhibited by inhibitory antibodies against the A2 or C2 domain epitopes. The modified factor VIII is useful for hemophiliacs, either to avoid or prevent the action of inhibitory antibodies.
Owner:EMORY UNIVERSITY
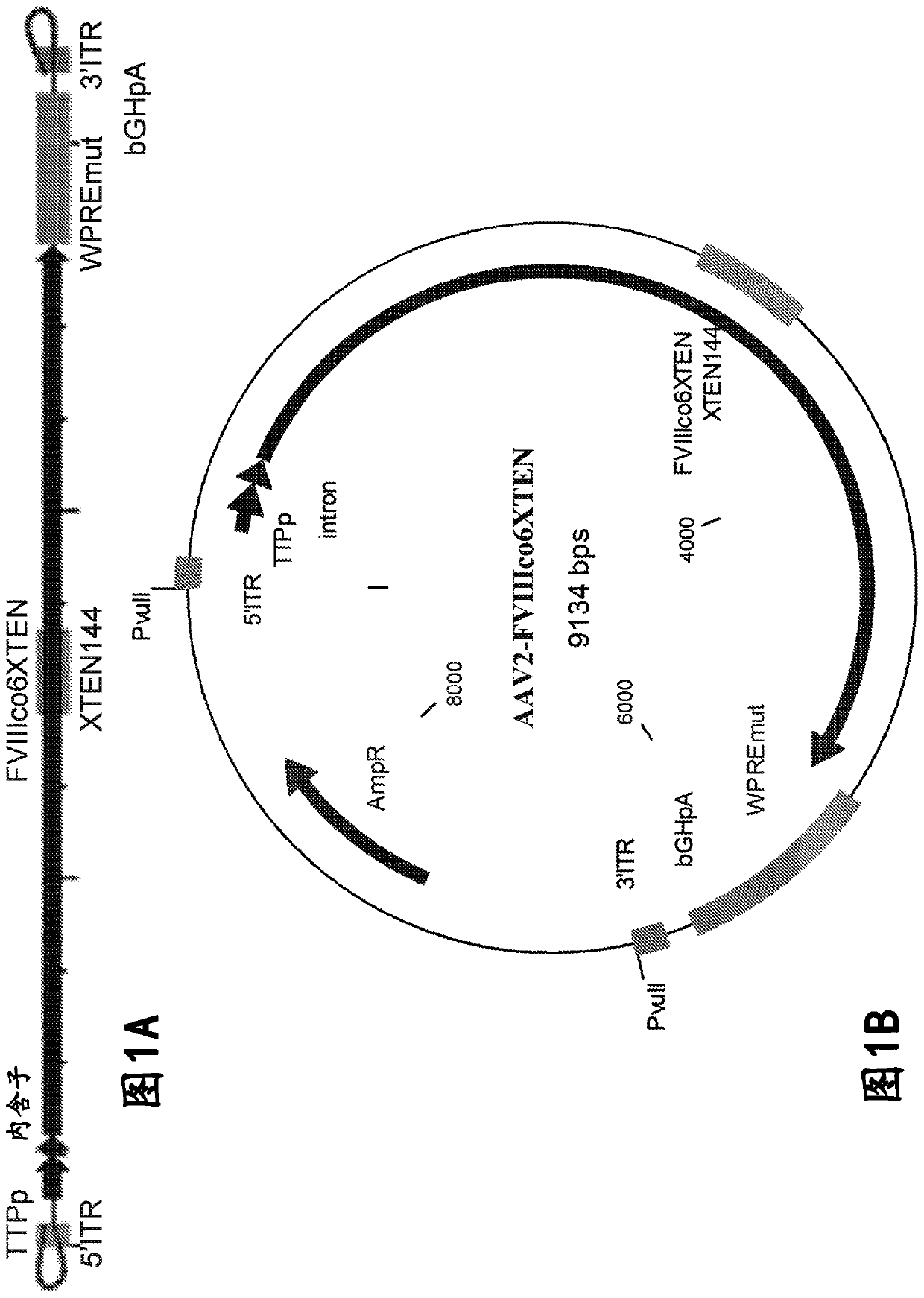
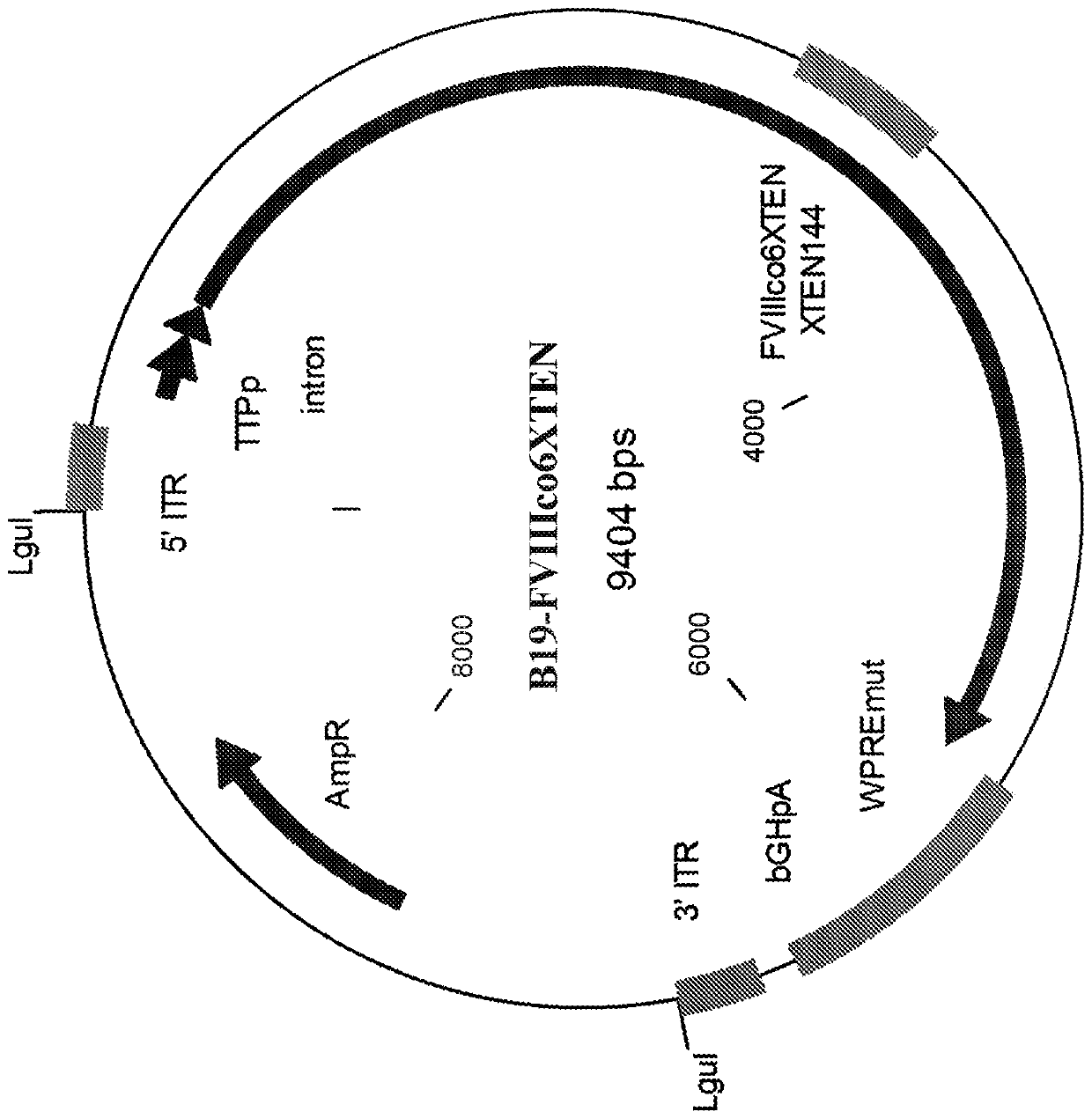
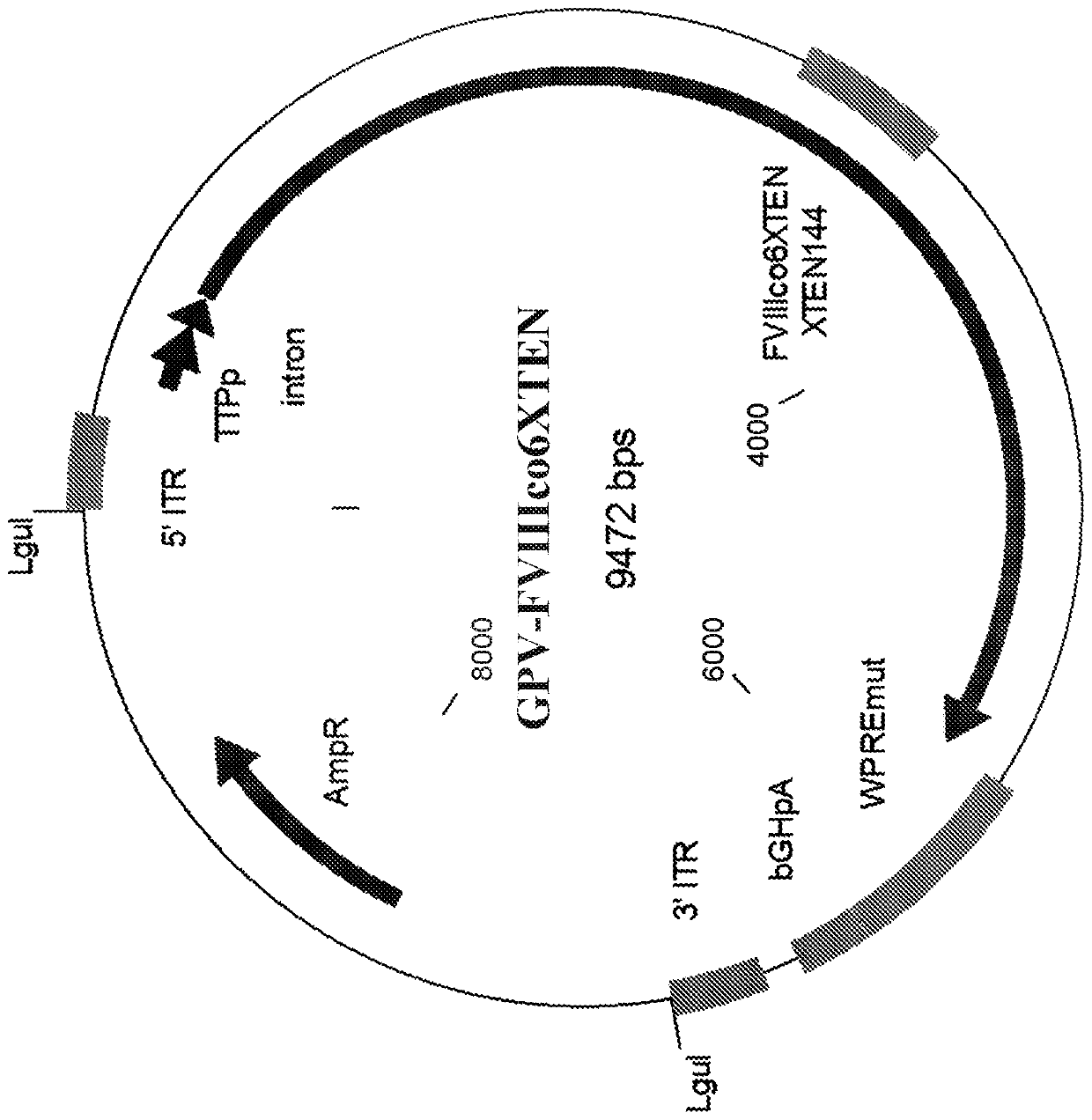
Nucleic acid molecules and uses thereof
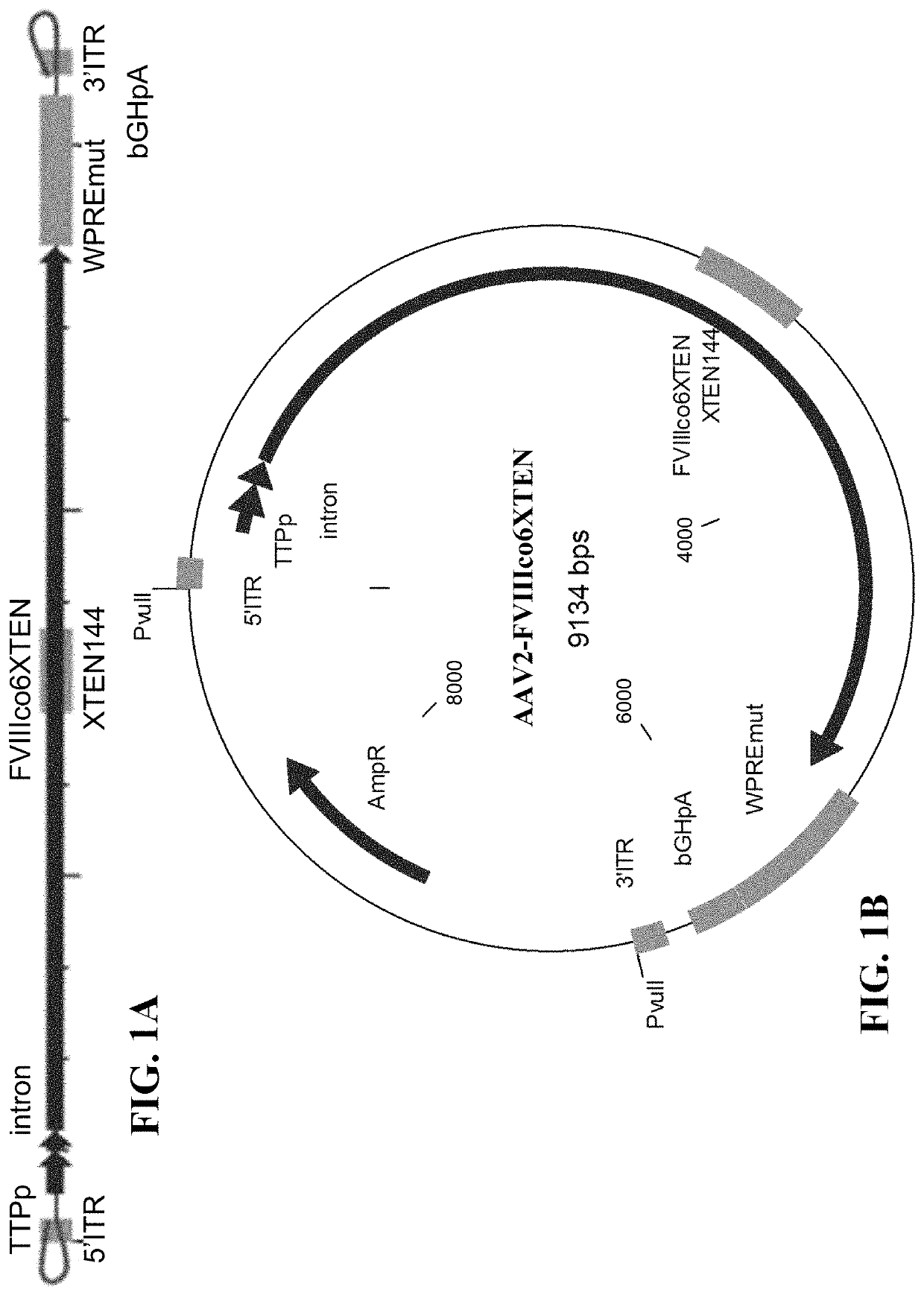
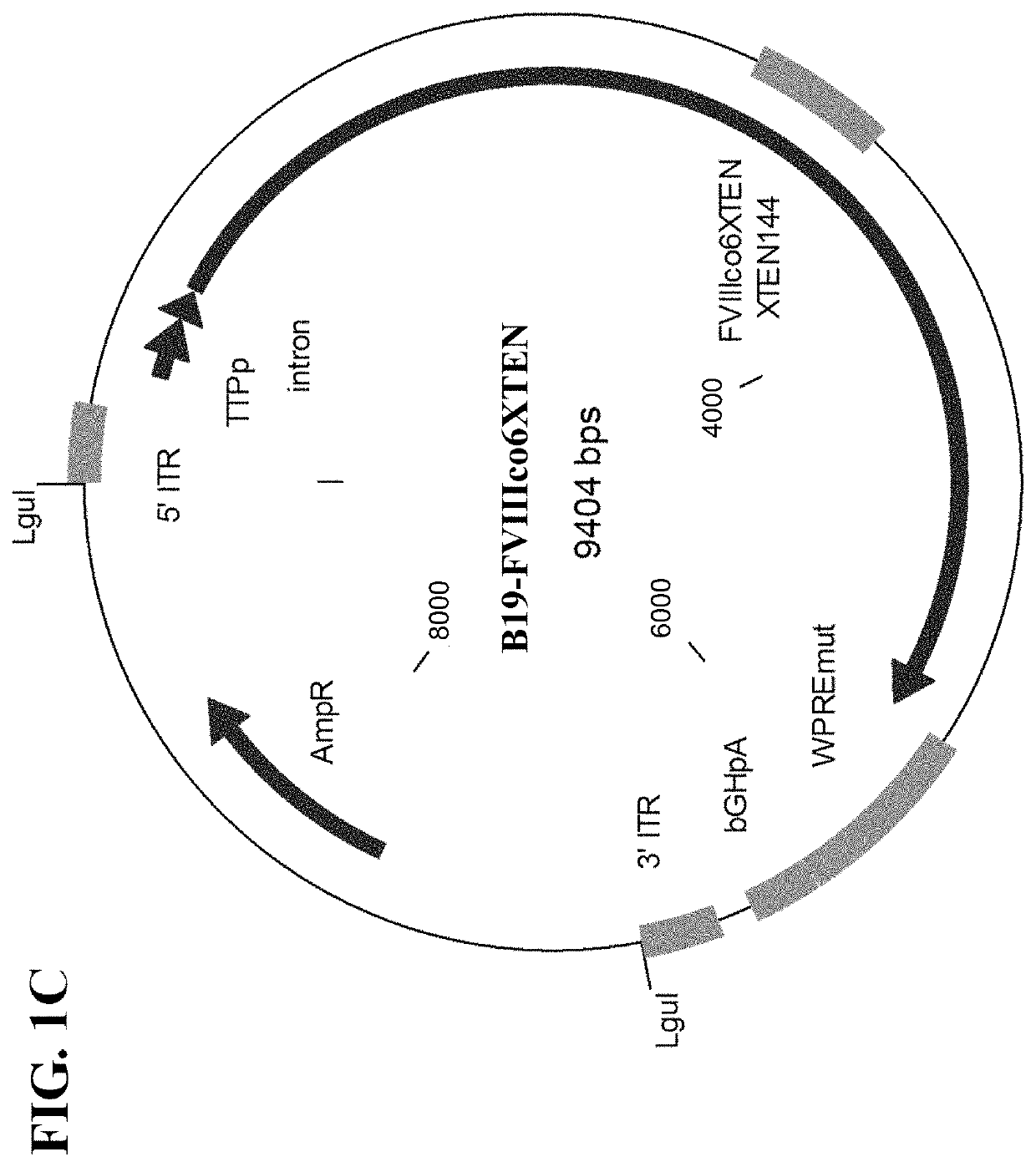
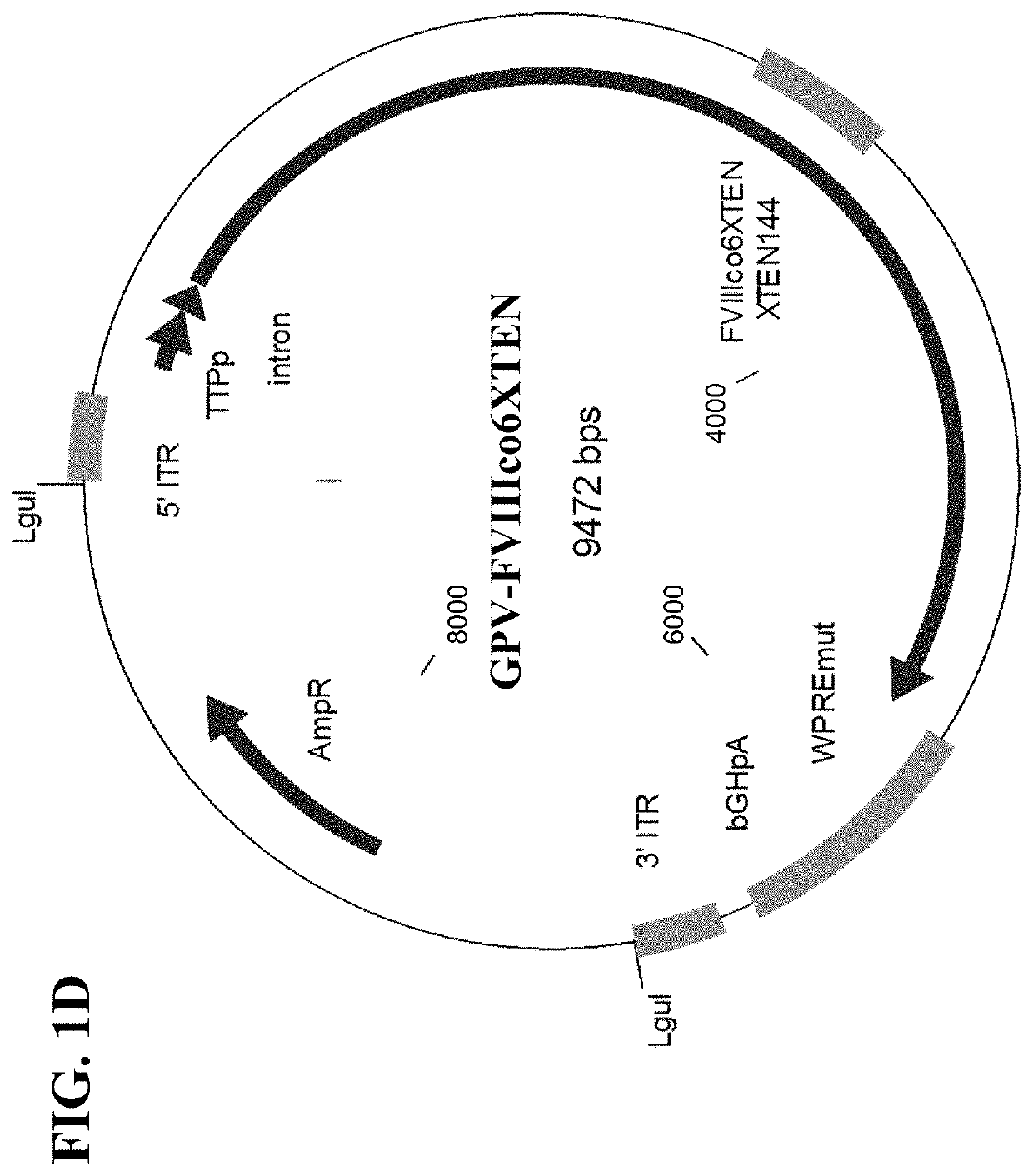
The present disclosure provides nucleic acid molecules comprising a first inverted terminal repeat (ITR), a second ITR, and a genetic cassette encoding a miRNA and / or a therapeutic protein. In certainembodiments, the therapeutic protein comprises a clotting factor, e.g., a FVIII polypeptide, a FIX polypeptide, or a fragment thereof. In some embodiments, the first ITR and / or the second ITR is an ITR of a non-adeno-associated virus (AAV). The present disclosure also provides methods of treating bleeding disorders such as hemophilia comprising administering to the subject the nucleic acid molecule or a polypeptide encoded thereby.
Owner:比奥维拉迪维治疗股份有限公司
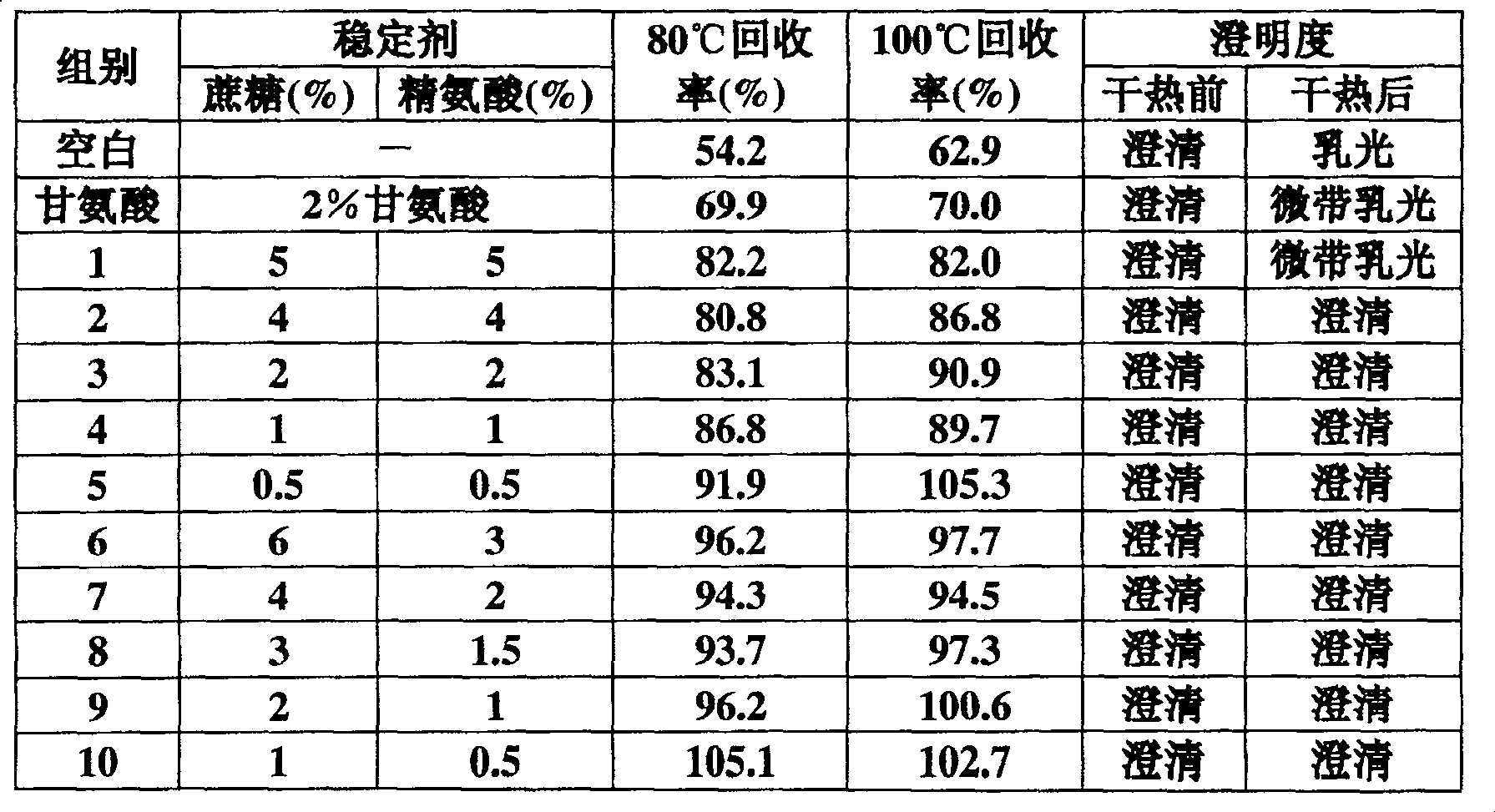
Dry heat processing stabilizer for prothrombin complex or factor v a IX preparation
InactiveCN100482272CLittle loss of activityAvoid damagePeptide/protein ingredientsPharmaceutical non-active ingredientsZymogenArginine
The invention relates to a stabilizing agent for preventing blood clotting factor reactive loss for prothrombin composite or blood clotting factor IX preparation during virus animatum eradication by dry heat, wherein the stabilizing agent is sucrose or / and arginine or its salt, it also can contain one or more of the conventional glycine, NaCl, citric acid trisodium and hamocura.
Owner:SHANGHAI XINXING MEDICINE
Polynucleotides encoding coagulation factor viii
PendingUS20200268666A1Improve clotting rateReduce frequencyFactor VIIPeptide/protein ingredientsMetaboliteIn vivo
The invention relates to mRNA therapy for the treatment of Hemophilia A. mRNAs for use in the invention, when administered in vivo, encode Factor VIII, isoforms thereof, functional fragments thereof, and fusion proteins comprising Factor VIII. mRNAs of the invention are preferably encapsulated in lipid nanoparticles (LNPs) to effect efficient delivery to cells and / or tissues in subjects, when administered thereto. mRNA therapies of the invention increase and / or restore deficient levels of Factor VIII expression and / or activity in subjects. mRNA therapies of the invention further decrease levels of toxic metabolites associated with deficient Factor VII I activity in subjects.
Owner:MODERNATX INC +1
Anticoagulant polypeptide and application thereof
The invention relates to anticoagulant polypeptide and an application thereof. The amino acid sequence of the polypeptide is shown as SEQ ID NO.2. Anticoagulation is an endogenous blood coagulation resisting pathway, and the endogenous blood coagulation pathway is a blood coagulation effect participated or mediated by a blood coagulation factor XIa (FXIa). The invention also relates to a nucleotide sequence for encoding the anticoagulant polypeptide, and the nucleotide sequence is shown as SEQ ID NO.1. The invention also relates to an application of the anticoagulant polypeptide.
Owner:HUBEI UNIVERSITY OF MEDICINE
Methods of using a bispecific antibody that recognizes coagulation factor IX and/or activated coagulation factor IX and coagulation factor X and/or activated coagulation factor X
ActiveUS11352438B2Reduce morbidityImmunoglobulins against blood coagulation factorsAntibody ingredientsDosing regimenAntigen
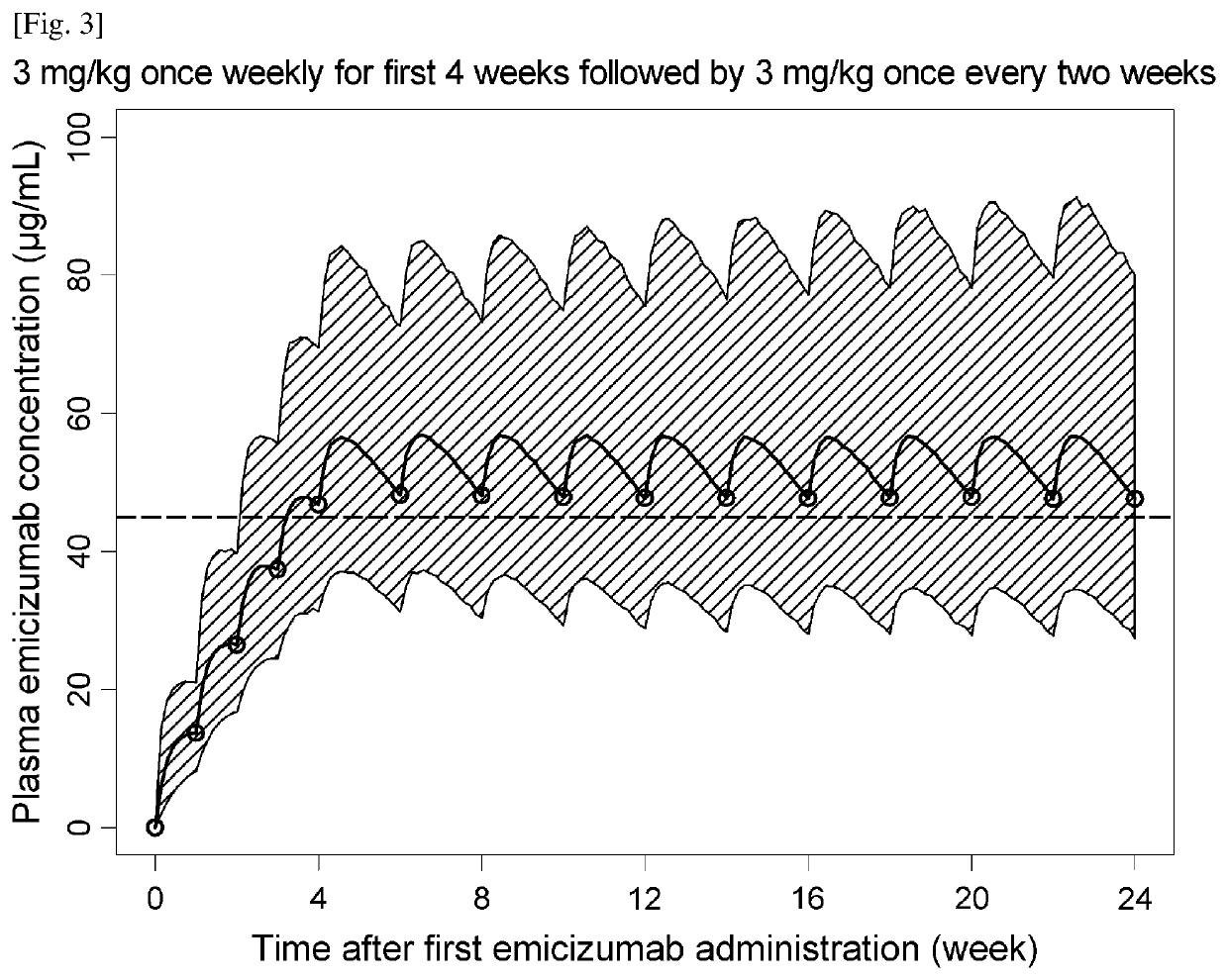
An objective of the present invention is to provide an effective pharmaceutical composition or a dosage regimen for preventing and / or treating bleeding, a disease accompanying bleeding, or a disease caused by bleeding. The inventors discovered that by administering a pharmaceutical composition comprising a bispecific antigen-binding molecule that recognizes (a) blood coagulation factor IX and / or activated blood coagulation factor IX and (b) blood coagulation factor X and / or activated blood coagulation factor X according to a given dosage regimen, bleeding, a disease accompanying bleeding, or a disease caused by bleeding can be prevented and / or treated more effectively.
Owner:F HOFFMANN LA ROCHE & CO AG +1
De-immunized factor viii molecule and pharmaceutical compositions comprising the same
The present invention relates to the field of therapeutic proteins, in particular, to recombinant coagulation factors. It provides a recombinant Factor VIII (FVIII) protein comprising specific point mutations at defined positions, which serve to reduce the immunogenicity of said FVIII protein, wherein the Factor VIII protein substantially retains its coagulant activity. The invention further provides nucleic acids encoding said de-immunized protein, cell lines and methods of recombinant preparation as well as pharmaceutical compositions comprising the recombinant FVIII of the invention, whichare advantageous for use in treatment of patients with Hemophilia A, particularly those who have not yet been treated with a FVIII product. Additionally, it can be a safe alternative for previously treated patients and even for patients who have developed an immune-response to FVIII, e.g., for immune-tolerance-induction therapy (ITI / ITT) or rescue ITI. The invention also provides an assay for determining immunogenicity of a protein.
Owner:BIOTEST SERUM INST GMBH
Nucleic acid molecules and uses thereof
The present disclosure provides nucleic acid molecules comprising a first inverted terminal repeat (ITR), a second ITR, and a genetic cassette encoding a miRNA and / or a therapeutic protein. In certain embodiments, the therapeutic protein comprises a clotting factor, e.g., a FVIII polypeptide, a FIX polypeptide, or a fragment thereof. In some embodiments, the first ITR and / or the second ITR is an ITR of a non-adeno-associated virus (AAV). The present disclosure also provides methods of treating bleeding disorders such as hemophilia comprising administering to the subject the nucleic acid molecule or a polypeptide encoded thereby.
Owner:BIOVERATIV THERAPEUTICS INC
Method of providing anesthesia
InactiveUS20070191430A1Preserve integrityInduce “damping” of turbulence and mixing in bloodBiocideEther/acetal active ingredientsEtiologyWhole body
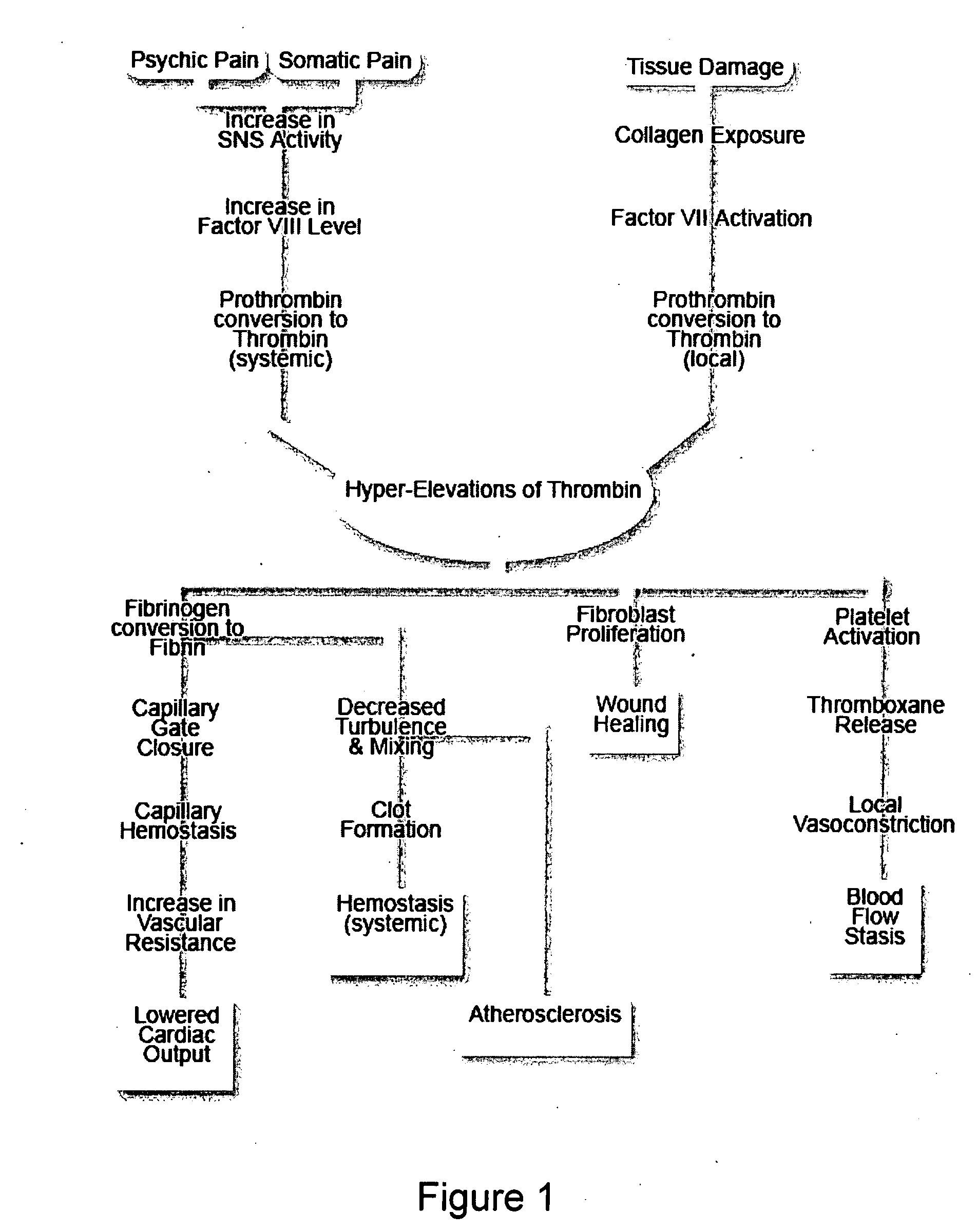
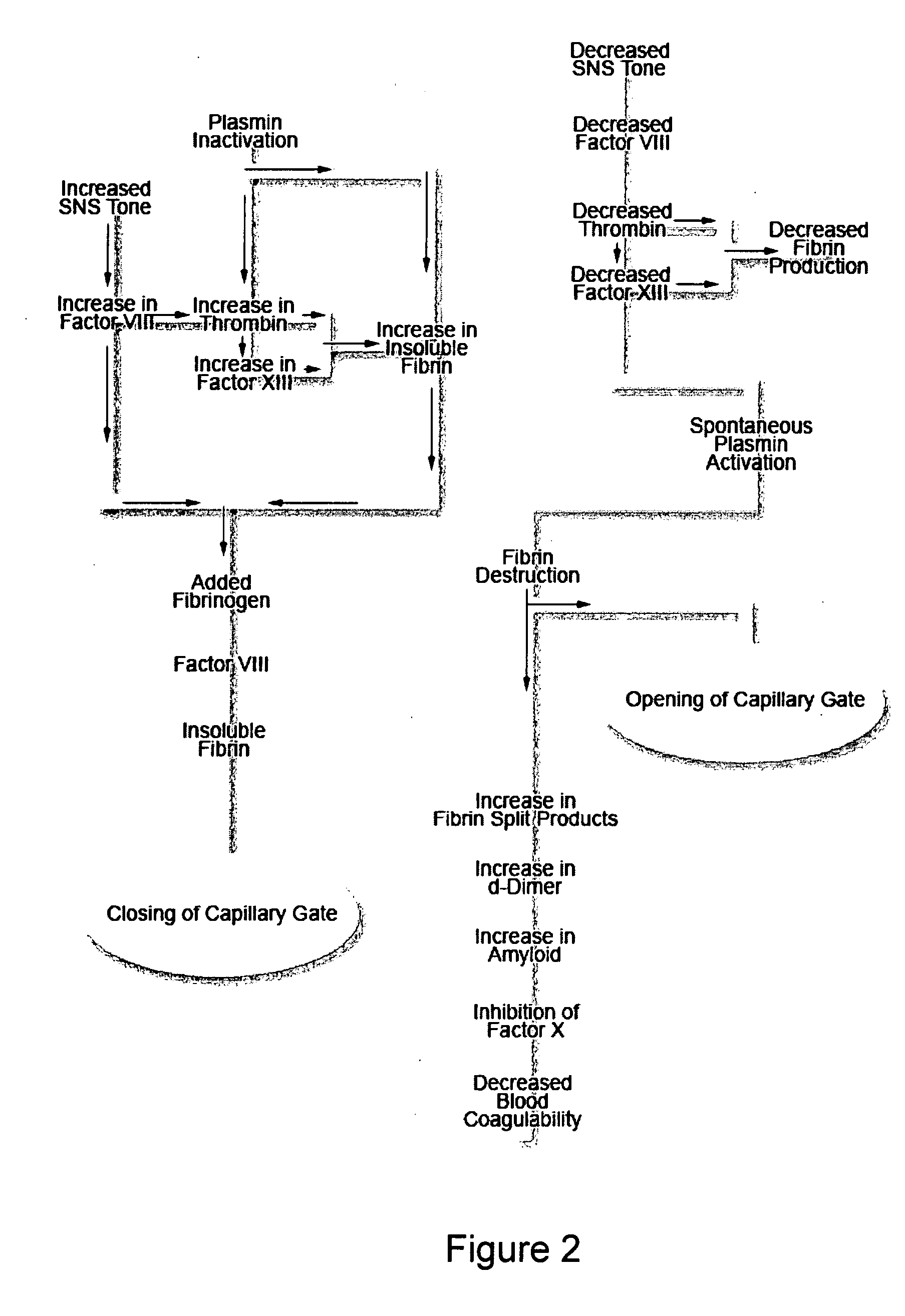
A theory has been presented that provides a simplified explanation of a cohesive mechanism of embryological development, hemostasis, coagulation, wound repair and tissue maintenance that operates continuously in the animal body to oppose the effects of stress. The theory endeavors to fit all known facts, and is based on the hypothesis that coagulation Factors VII and VIII are respectively local and systemic stress agents that regulate thrombin activity and synergize each other's effects. Stress Theory may explain the etiologies of several hitherto mysterious disease syndromes, and the stress mechanism and may play a more pervasive role in disease than is generally appreciated. The theory offers fresh avenues for research and clinical strategy.
Owner:COLEMAN LEWIS S
Urine blood coagulation factor IX and application of polypeptide fragment thereof in burns
PendingCN114113640AEasy to storeMicrobiological testing/measurementMaterial analysis by electric/magnetic meansURINE BLOODSevere burn
The invention provides application of a urine coagulation factor IX and a polypeptide fragment of the urine coagulation factor IX, and particularly relates to application of the urine coagulation factor IX and the polypeptide fragment of the urine coagulation factor IX in preparation of preparations for burn diagnosis, differential diagnosis, burn area and degree evaluation, treatment effect evaluation, monitoring, prognosis evaluation, mechanism research and the like. Burns are common important wounds in daily life, about 5000-100000 people in every one million people are burnt every year, according to statistics of World Health Organization, more than 300,000 people die from burn patients globally every year, and the treatment survival rate of serious burns is still at a lower level. Researches prove that compared with healthy people (a normal control group), the expression of the urine blood coagulation factor IX and the polypeptide fragment thereof in burn patients is increased, and the content of the urine blood coagulation factor IX and the polypeptide fragment thereof is gradually increased along with the aggravation of the burn degree. The method can be used for various purposes of application detection of burn patients. The advantages of noninvasive acquisition, large-scale repeated sampling and convenient preservation of a urine sample are exerted, and the urine blood coagulation factor IX and the polypeptide fragment thereof are detected by utilizing the urine sample.
Owner:张曼
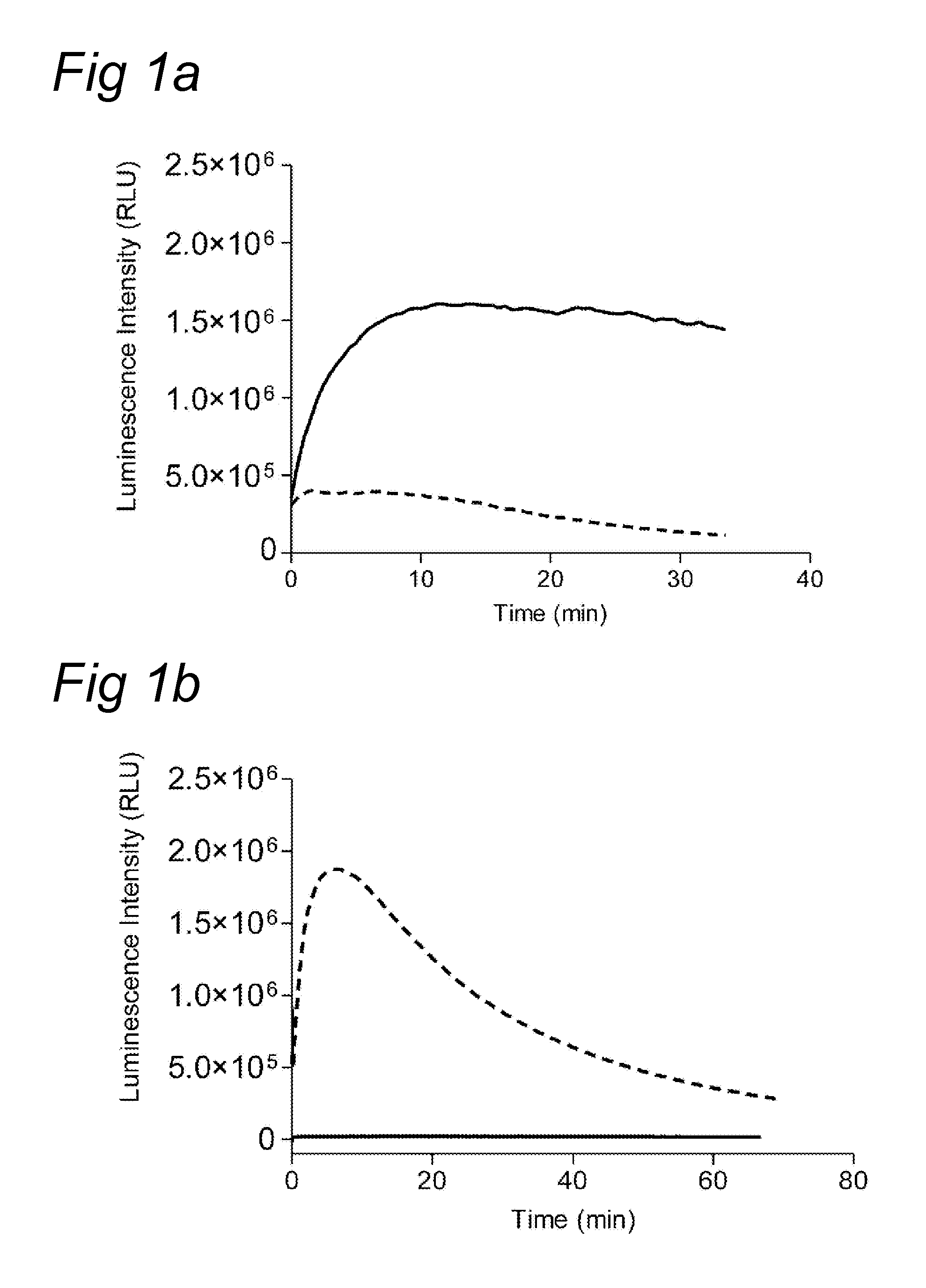
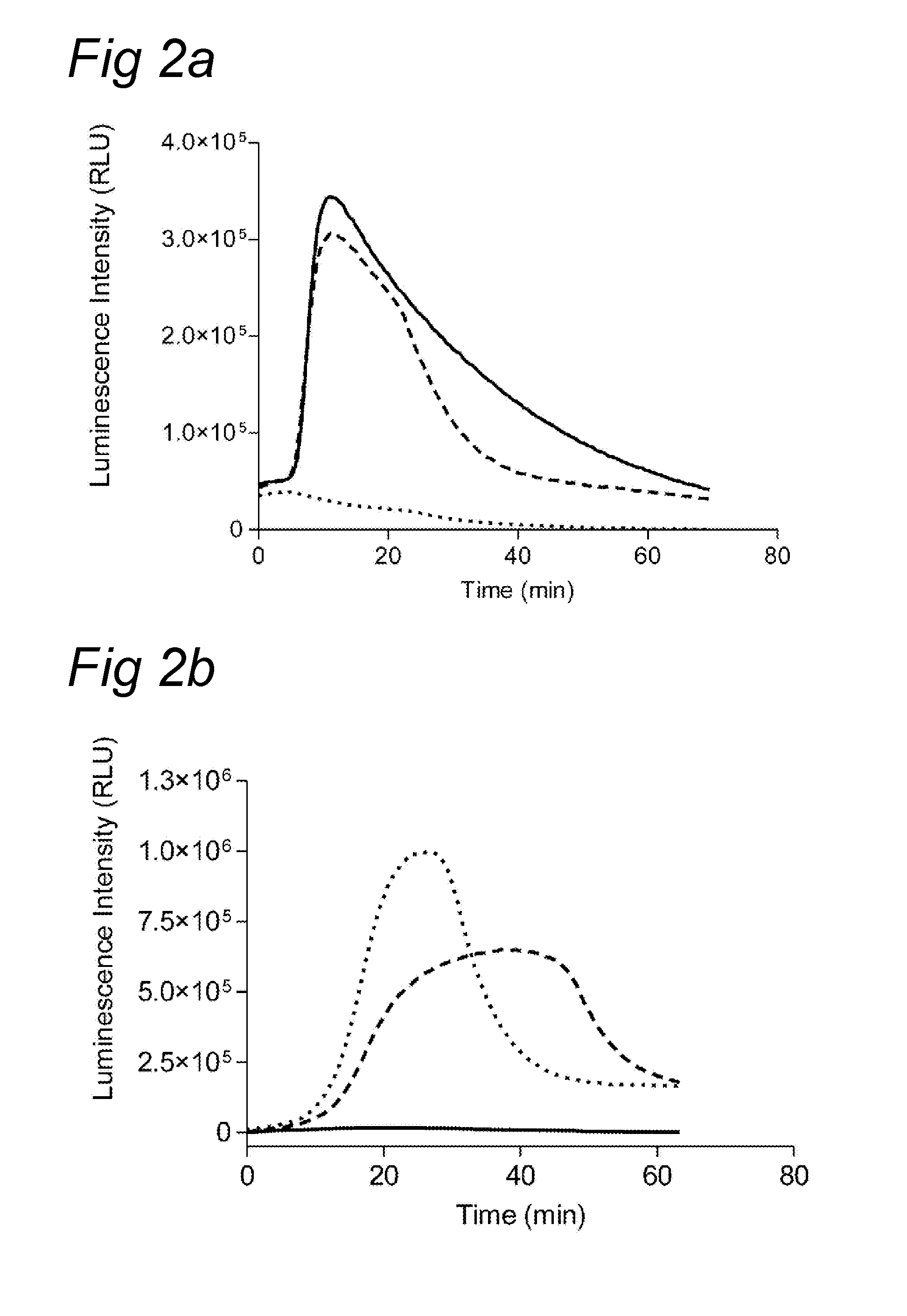
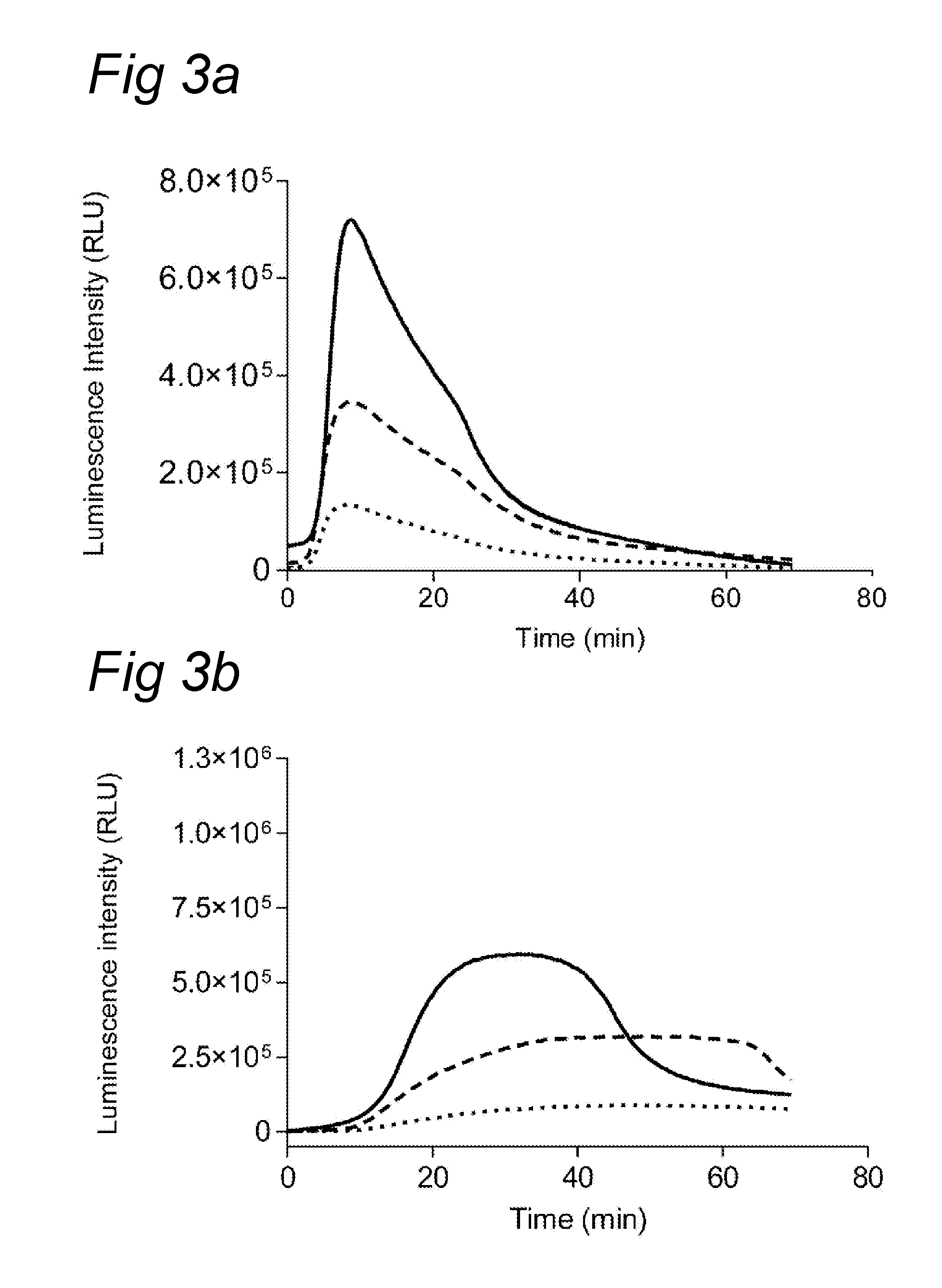
Chemiluminescence-based haemostasis assay
ActiveUS20130337480A1Peptide/protein ingredientsMicrobiological testing/measurementPlasminTest sample
The present invention relates to a method for in vitro determining generation of a haemostatis factor such as thrombin and / or plasmin in a test sample using a chemiluminescent substrate specific for said blood clotting factor. Upon cleavage of the substrate, a luminescent signal is generated via aminoluceferin with the aid of a luciferase. The invention also relates to a kit for in vitro determining generation of a haemostasis factor in a test sample, and to novel chemiluminescent substrates for the determination of thrombin and plasmin.
Owner:STICHTING KATHOLIEKE UNIV +1
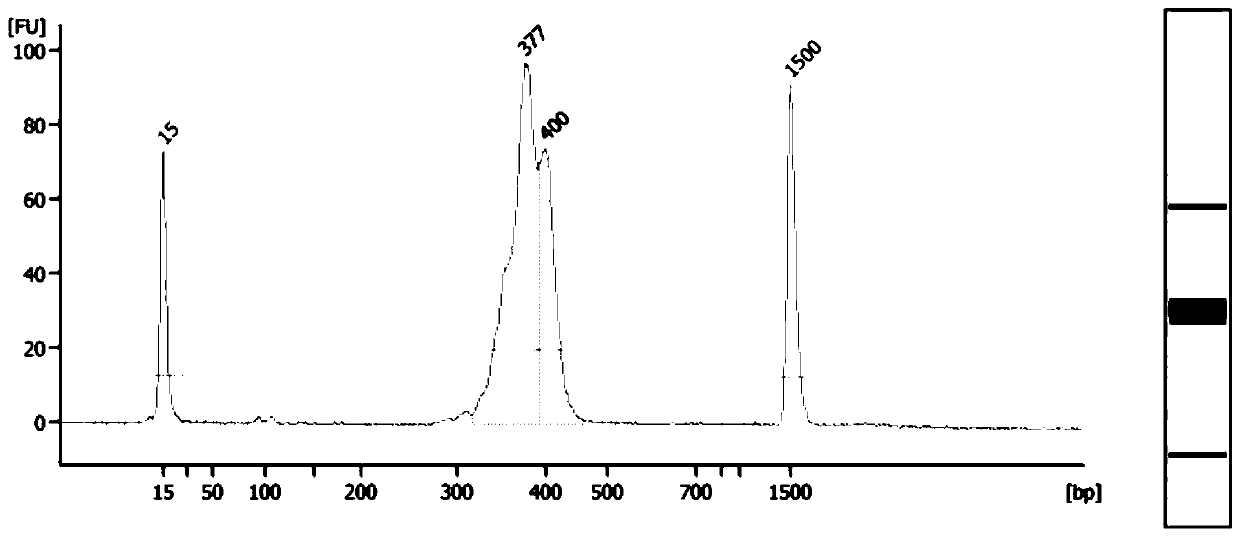
Primer combinations, methods and kits for constructing multiple hemophilia targeting libraries based on high-throughput sequencing
ActiveCN109207573BSave time and costEasy to operateMicrobiological testing/measurementLibrary creationDiseaseBase J
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Novel chemiluminescent substrates for factor xa
PendingCN113195463AMicrobiological testing/measurementBiological material analysisBiochemistryAnticoagulation Agents
The present invention relates to chemiluminescent substrates of formulas (I-3) and (I-4) for blood clotting enzyme Factor Xa. The substrates are particularly useful for assaying coagulation factors and for quantifying an anticoagulant in a sample.
Owner:ENZYRE BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com