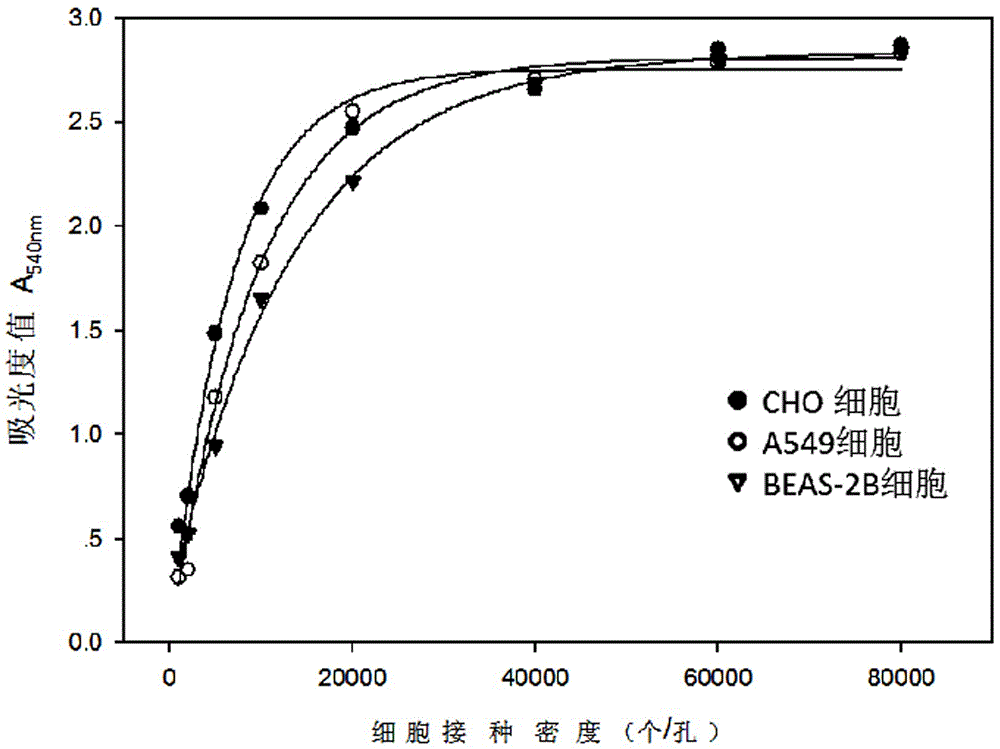
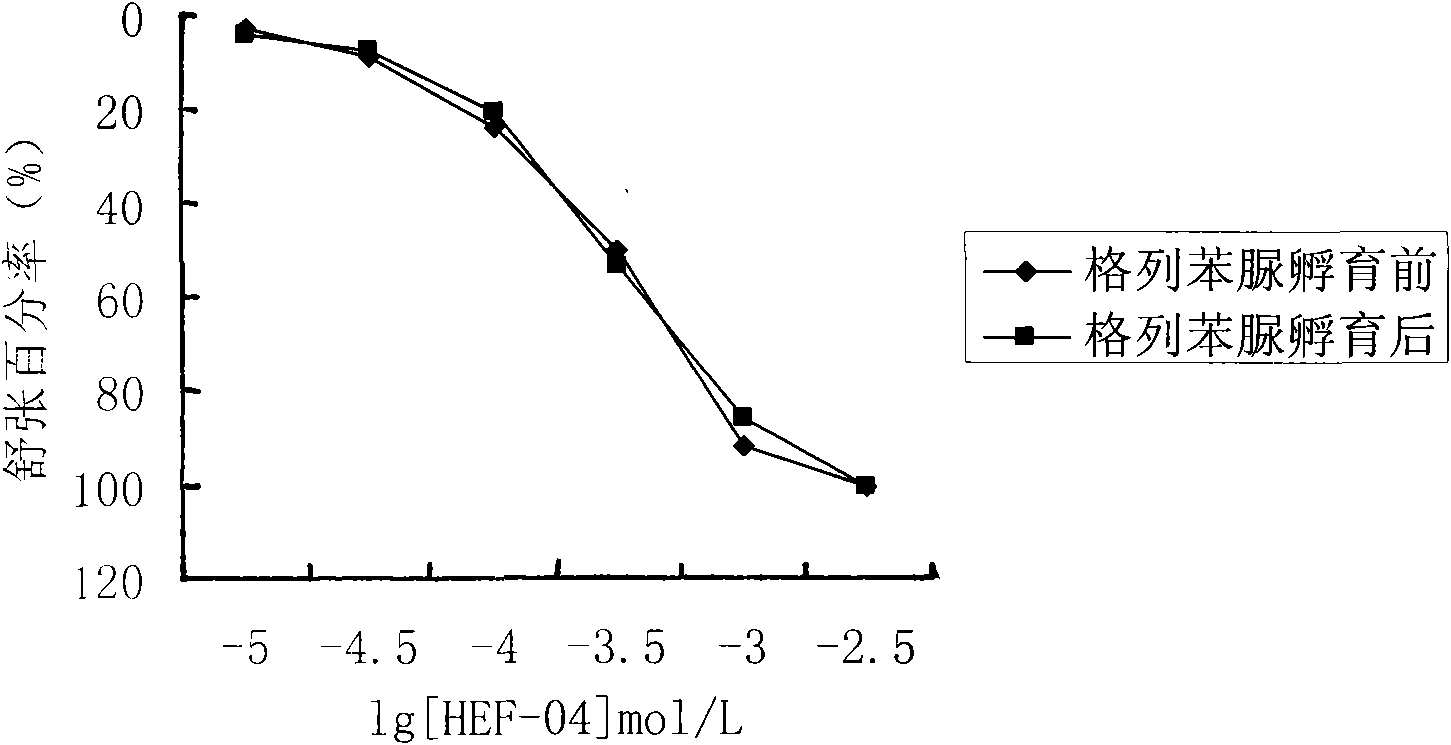Patents
Literature
35 results about "Dose-effect curve" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dose-effect curve (dose-response curve) a graphic representation of the effect caused by an agent (such as a drug or radiation) plotted against the dose, showing the relationship of the effect to changes in the dose.
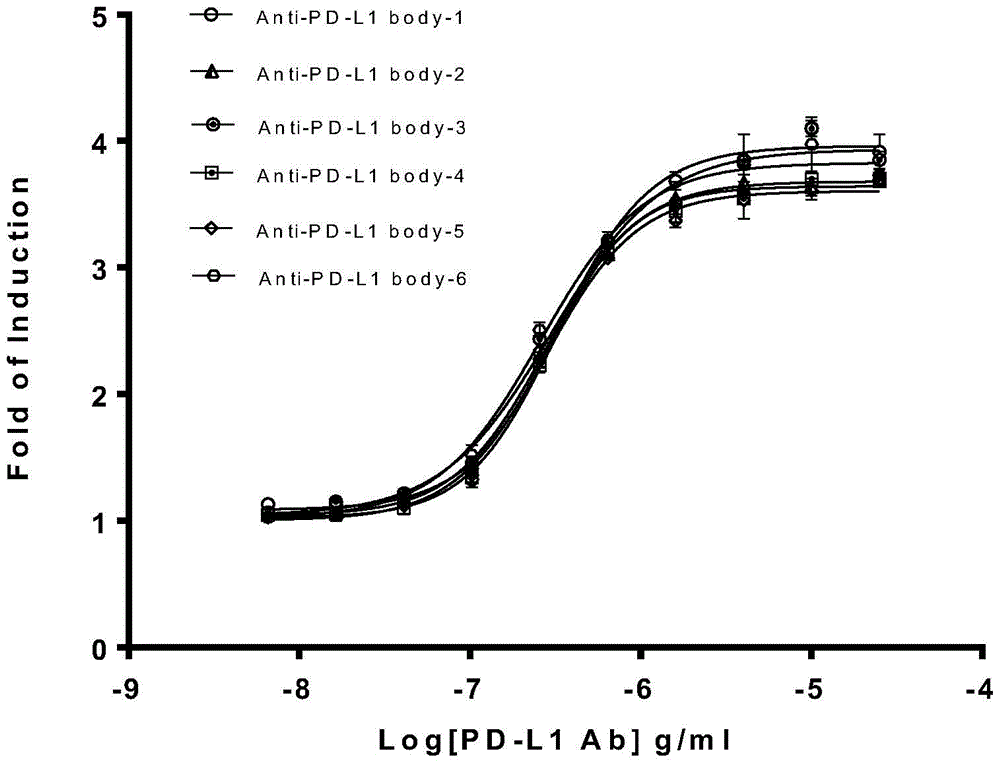
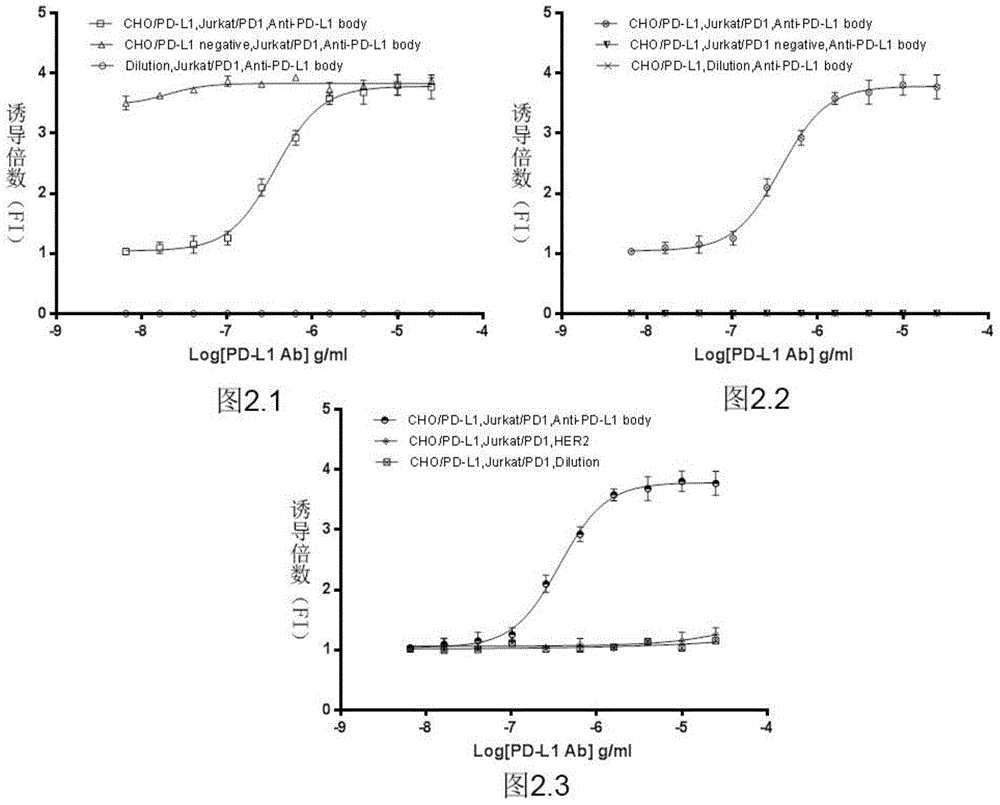
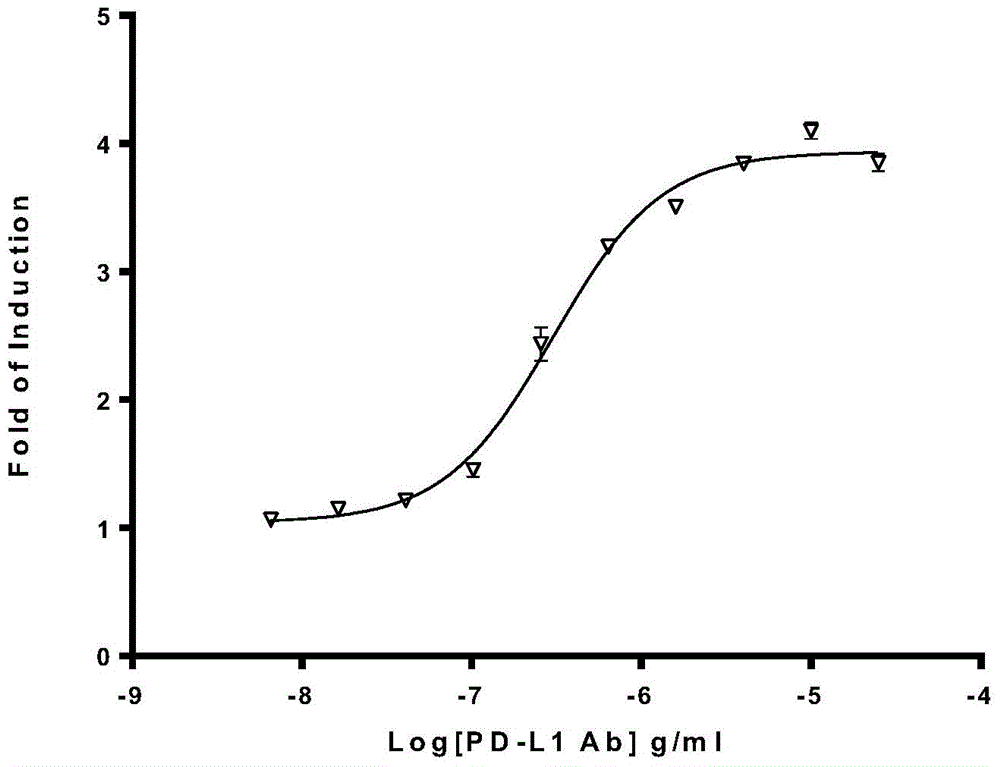
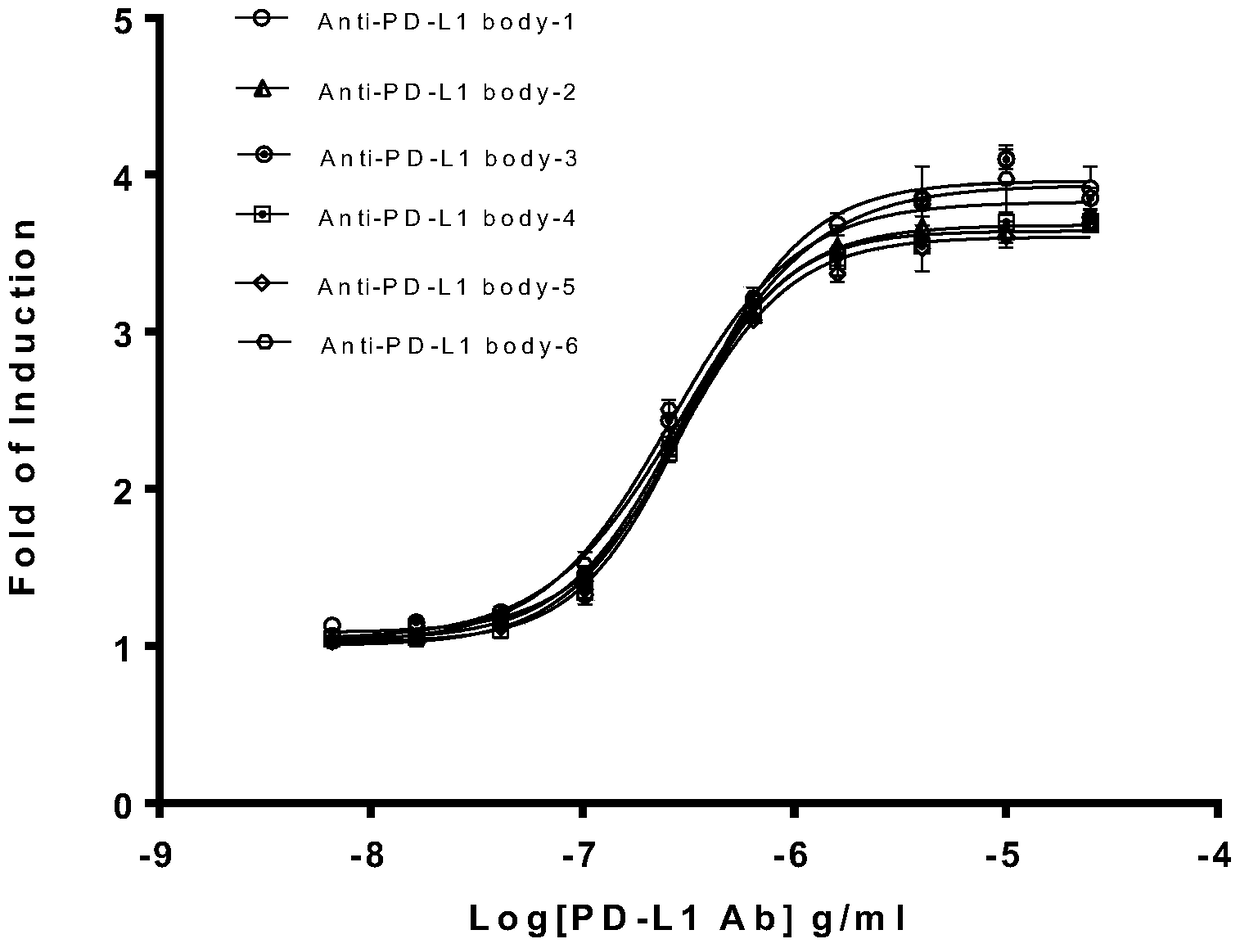
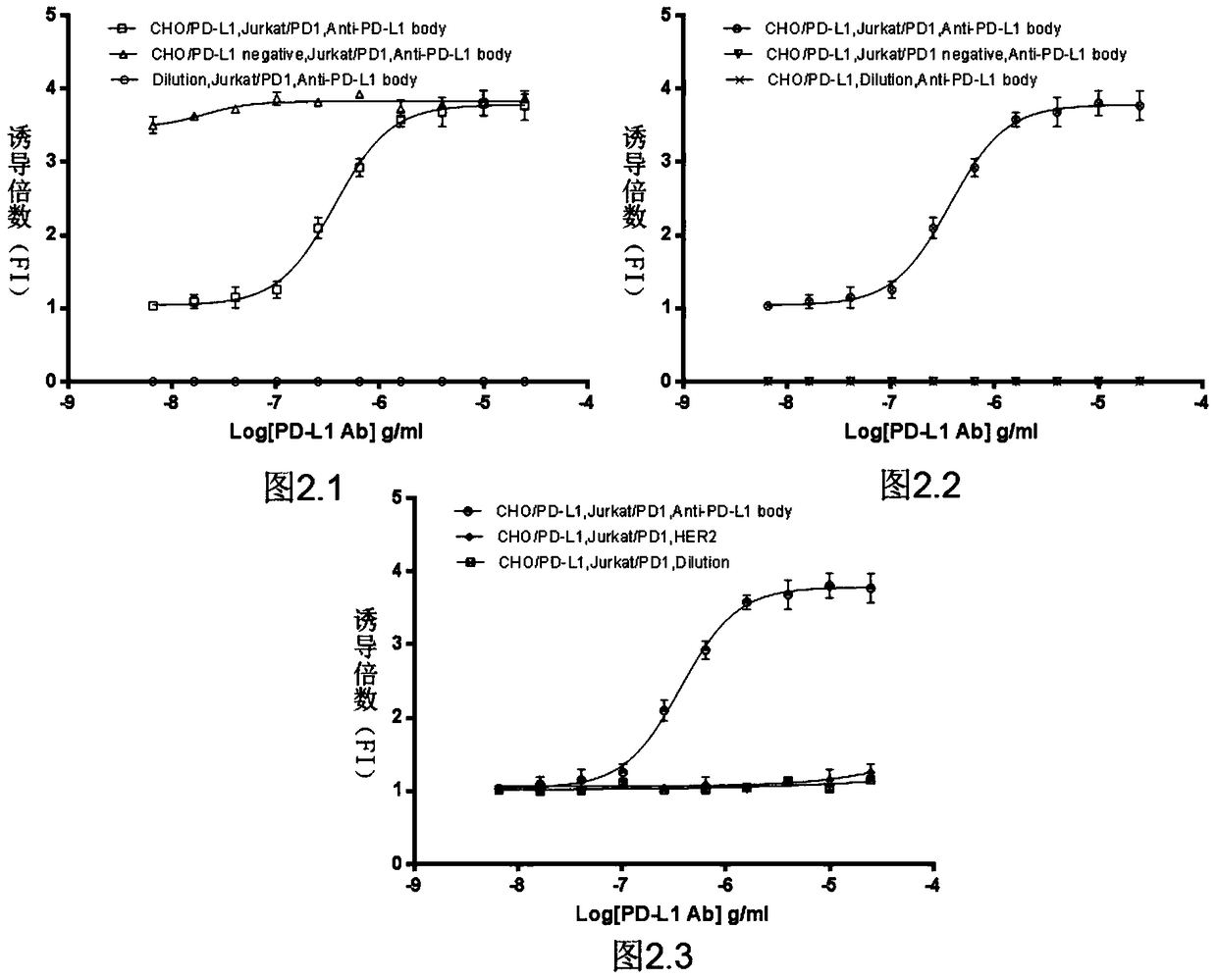
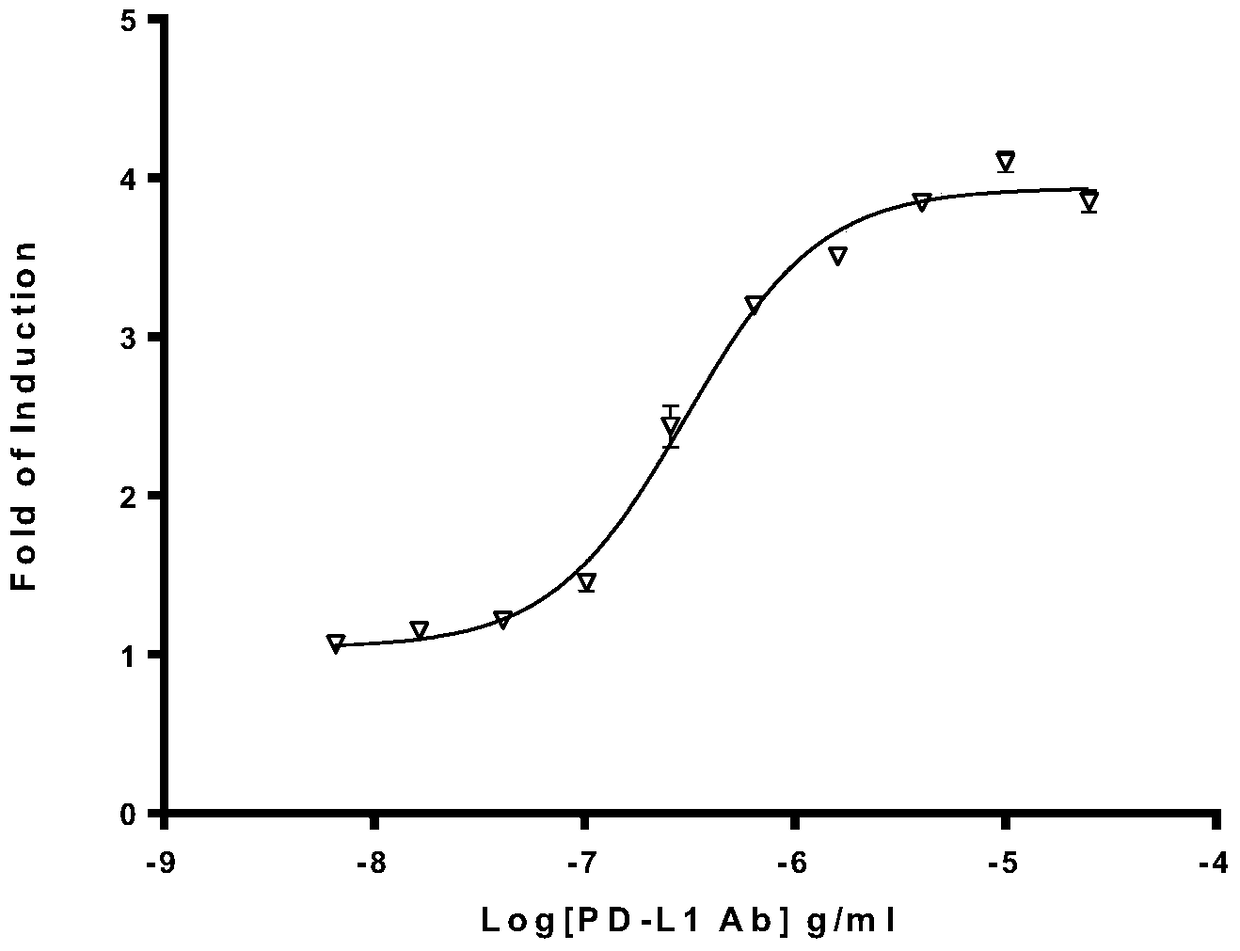
Biological activity determination method for anti-PD-L1 monoclonal antibody
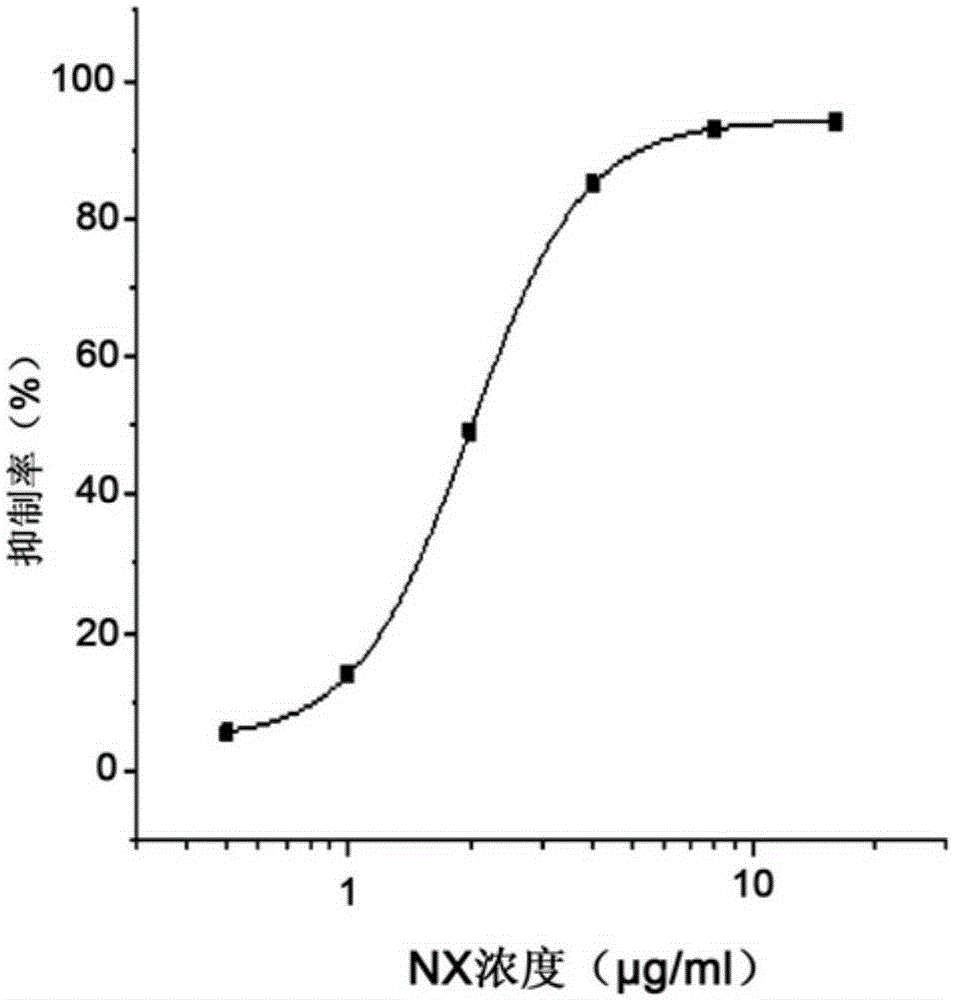
The invention relates to the field of activity detection of biological medicine, in particular to a biological activity determination method for an anti-PD-L1 monoclonal antibody. According to the method, a PD1 / PD-L1 interruption detection system is used, binding of PD1 and PD-L1 protein is interrupted when the anti-PD-L1 monoclonal antibody is bound with PD-L1 expressed by target cells, a transcription factor NFAT is activated through signal transduction, expression of luciferase is started, the expression quantity of luciferase is positively correlated to the biological activity of the anti-PD-L1 monoclonal antibody, the chemiluminescence value of the anti-PD-L1 monoclonal antibody is determined after a cell lysis solution and a luciferase substrate are added, a dose-effect curve is drawn, and accordingly the biological activity of the anti-PD-L1 monoclonal antibody is determined. The method is easy to implement, strong in specificity and high in durability and has great significance on quality control and clinical application of the anti-PD-L1 monoclonal antibody.
Owner:兆科(广州)肿瘤药物有限公司
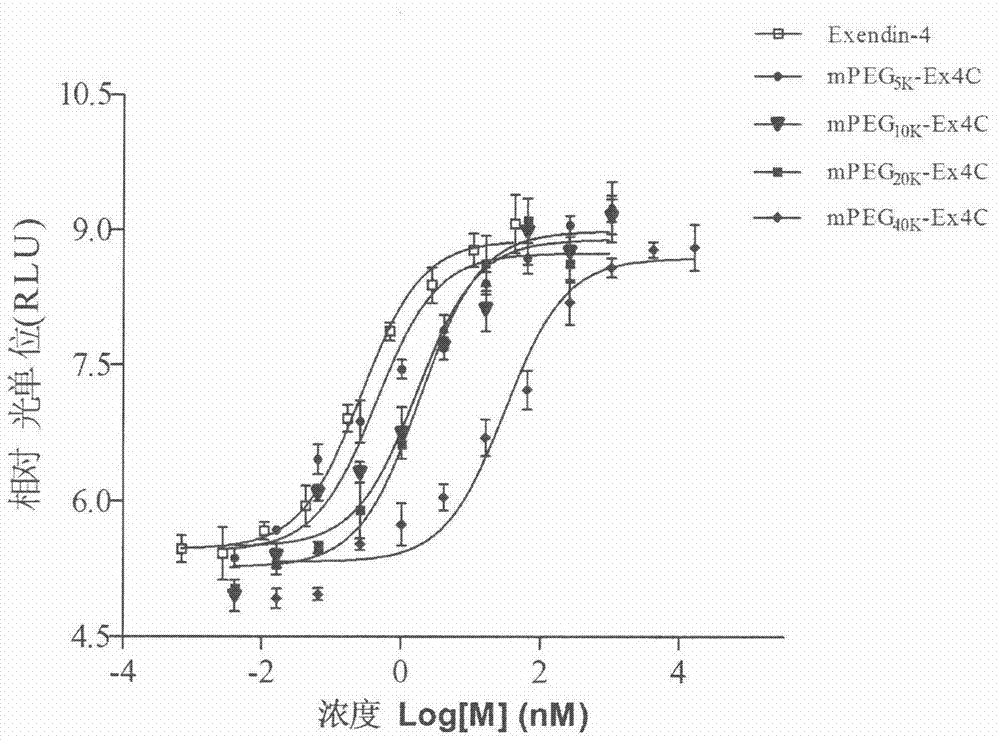
Method for determining receptor affinity of GLP-1 receptor agonist
InactiveCN104846061AQuick evaluationQuick filterMicrobiological testing/measurementCytosolResponse element
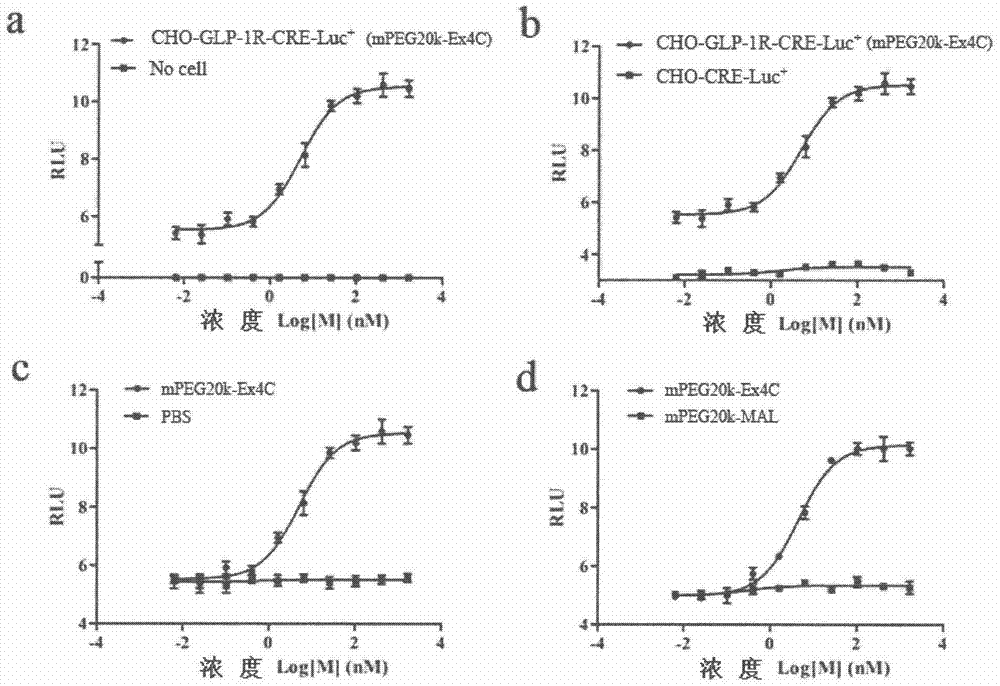
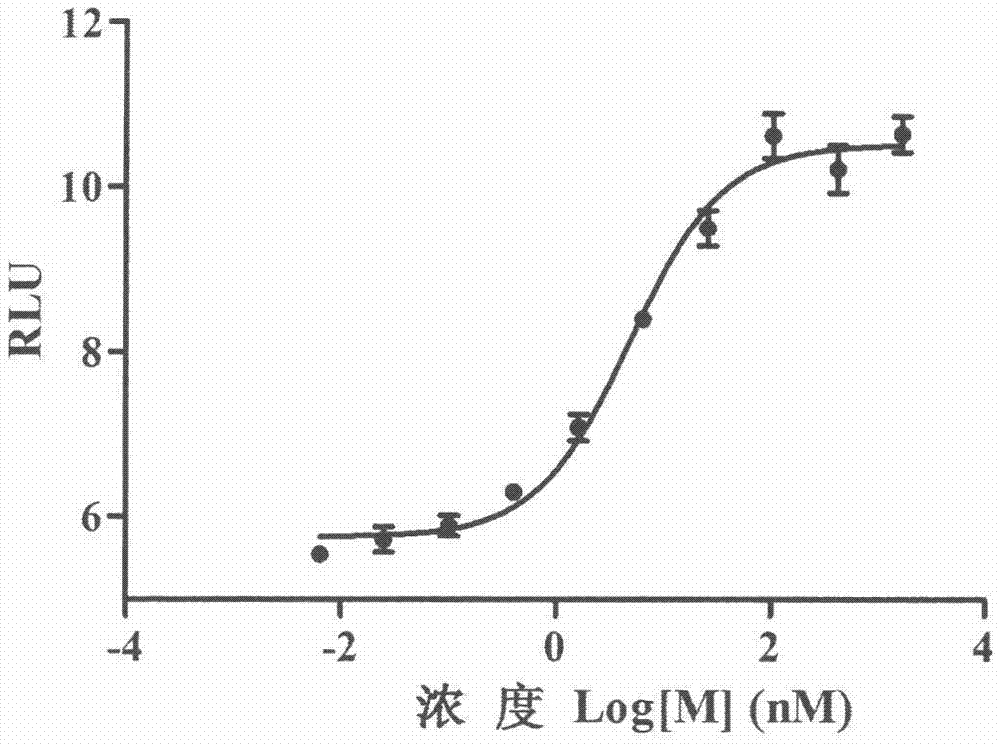
The invention provides a method for determining the receptor affinity of a GLP-1 receptor agonist. The method is based on a tool cell line, namely CHO-GLP-1R-CRE-Luc<+>, when a sample to be determined is combined with GLP-1 receptor on the surface of a cell, the receptor-mediated signal cascade reaction can be activated, cAMP-response element (CRE) is activated specifically, the expression of the luciferase reporter gene is promoted, the quantity of luciferase in cytosol is detected for drawing and fitting to obtain a dose-effect curve of acting of the sample and the GLP-1 receptor; and the half effective concentration (EC50) is calculated. By inspecting and optimizing all influence factors in the determining process, the method for determining the receptor affinity of the GLP-1 receptor agonist is finally established. The method has the advantages of being high in specificity, precision and accuracy, good in durability and convenient to operate, and the like. The method can be used for determining the receptor affinity of the GLP-1 receptor agonist, and thus such type of drug can be fast evaluated and screened.
Owner:CHINA PHARM UNIV
Detection method of anti-tumor activity of dendrobium huoshanense refined polysaccharide
InactiveCN108635512AGrowth inhibitionIn vitro anti-tumor effect is obviousCompounds screening/testingOrganic active ingredientsDendrobium huoshanenseIn vivo
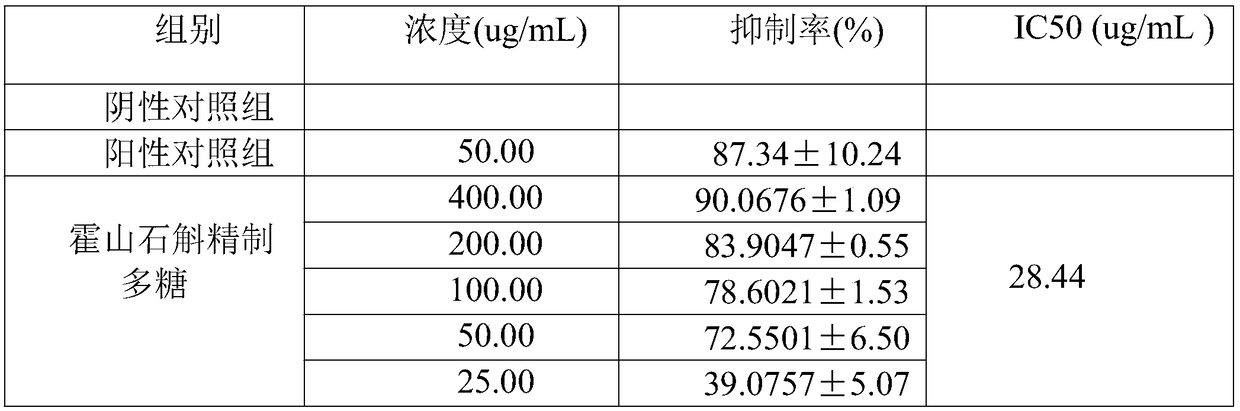
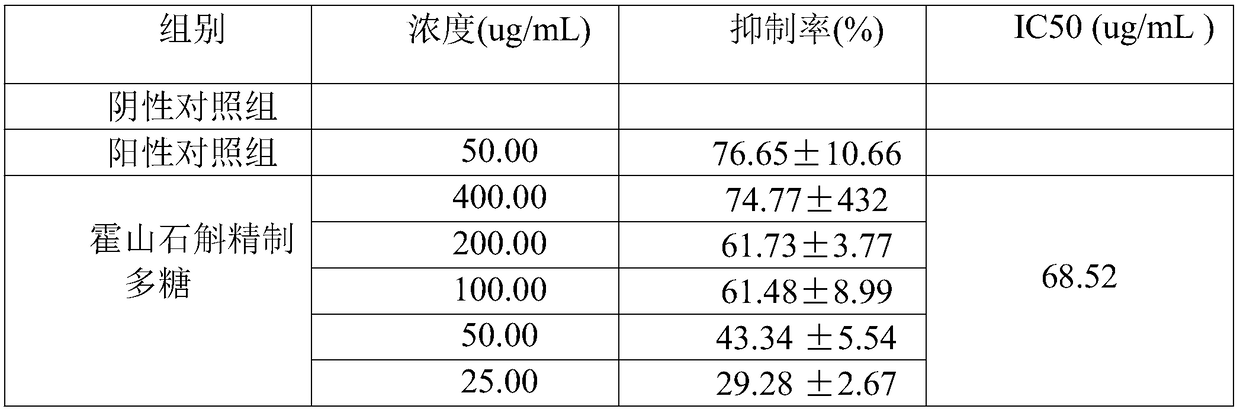
The invention discloses a detection method of anti-tumor activity of dendrobium huoshanense refined polysaccharide. The detection method comprises an in-vitro anti-tumor detection method and an in-vivo anti-tumor activity detection method; the inhibition rate of the dendrobium huoshanense refined polysaccharide on tumor cells is calculated through the in-vitro anti-tumor detection method; a dose-effect curve is drawn according to different concentration inhibition rates, and the half inhibitory concentration IC50 of the tumor cells is calculated; the in-vivo growth inhibition effect of the dendrobium huoshanense refined polysaccharide on a C57BL / 6J mouse in-vivo transplantable mouse lung cancer LLC tumor is detected through the in-vivo anti-tumor activity detection method; an in-vitro experiment shows that the dendrobium huoshanense refined polysaccharide has an obvious dose effect on an in-vitro anti-tumor effect; an in-vivo activity experiment shows that the dendrobium huoshanense refined polysaccharide has good anti-tumor activity and a relatively good dose-effect relation; the growth of a mouse lung cancer transplantable tumor can be remarkably inhibited and the dendrobium huoshanense refined polysaccharide has an obvious in-vivo anti-tumor effect; two experiment methods are combined so that the detection of the anti-tumor activity of the dendrobium huoshanense refined polysaccharide is more accurate.
Owner:HUOSHAN COUNTY TIANXIA ZEYU BIOLOGICAL TECHDEV
Method for mutagenesis breeding neuter protease high yield bacterial strain by hypophrenia N+ion injection technology
InactiveCN101182513AImprove enzyme production capacityReduce manufacturing costBacteriaMutant preparationNeutral proteinaseBacterial strain
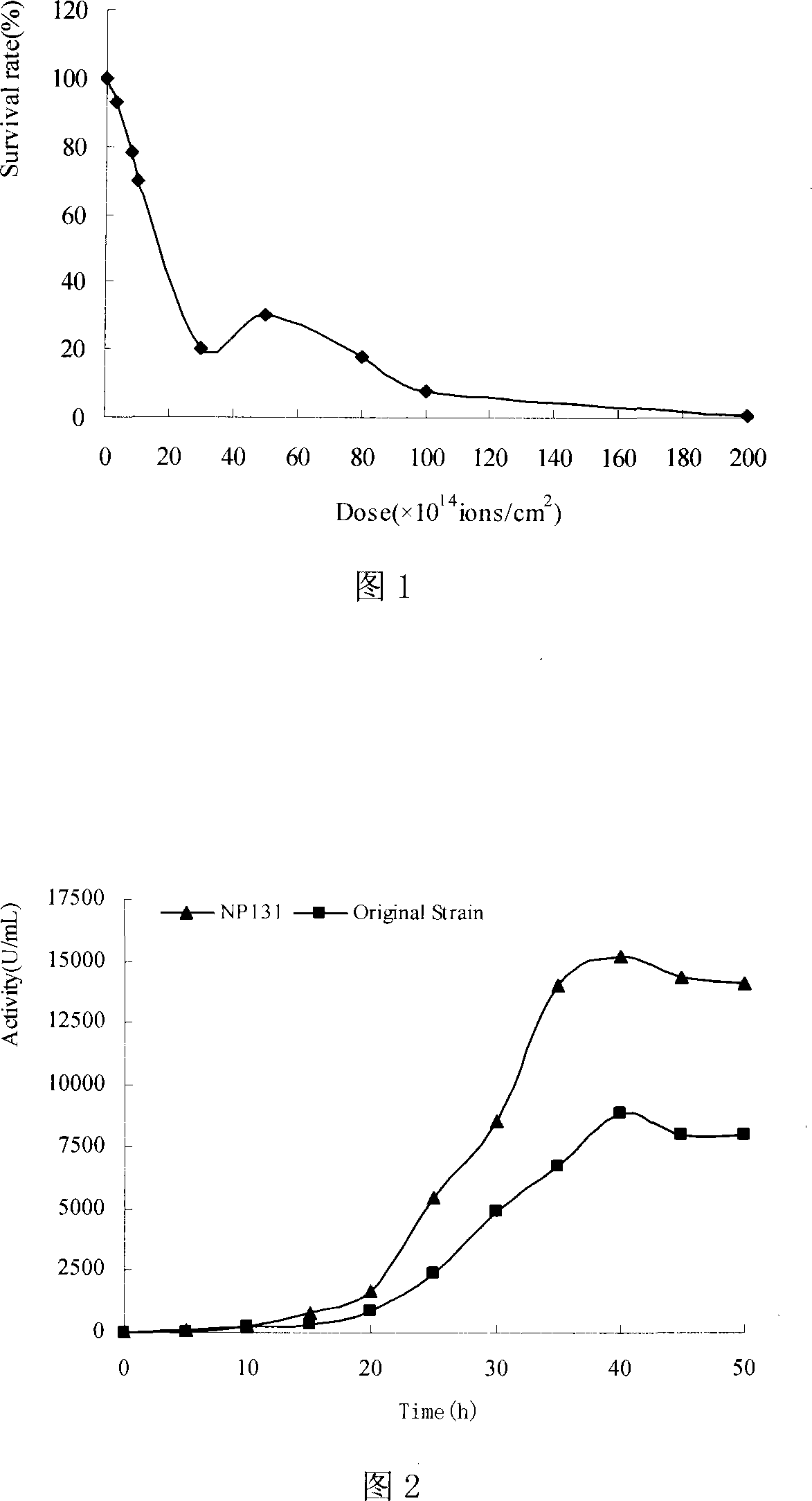
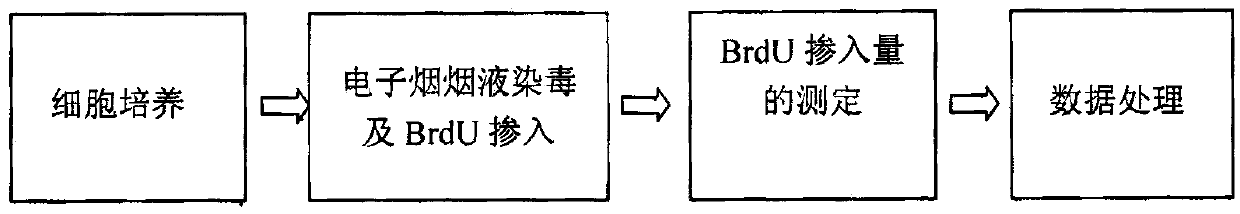
The invention relates to an inducing and breeding method of high yield neutral proteinase strain by using low energy N <+> ion injection technology. The steps are that (1) the initial strain is screened; (2) the N <+> ion is injected for the induced mutation; (3) the high yield strain is screened; (4) the N <+> ion is injected for the induced mutation; (5) the high yield neutral proteinase strain is determined. The invention uses the N <+> ion injection technology for the induced mutation of Bacillus Subtilis AS1.398 of neutral proteinase; after the injection of different dosage of N <+> ion, the survival rate of the strain takes a typical saddle shape dose-effect curve; a high yield mutant strain with good genetic stability can be screened at the best injection dosage of 50*10 <14> ions / cm <2>; the shaking flask activity of the neutral proteinase is about 8230U / mL, which is improved by 81.3 percent. The ion injection technology can be applied into the mutation and selection of high yield neutral proteinase strain; the invention has a higher mutation rate and a wider mutation spectrum for the microorganism; the invention has good mutation effects, which is an ideal breeding method for the microorganism.
Owner:TIANJIN UNIV OF SCI & TECH
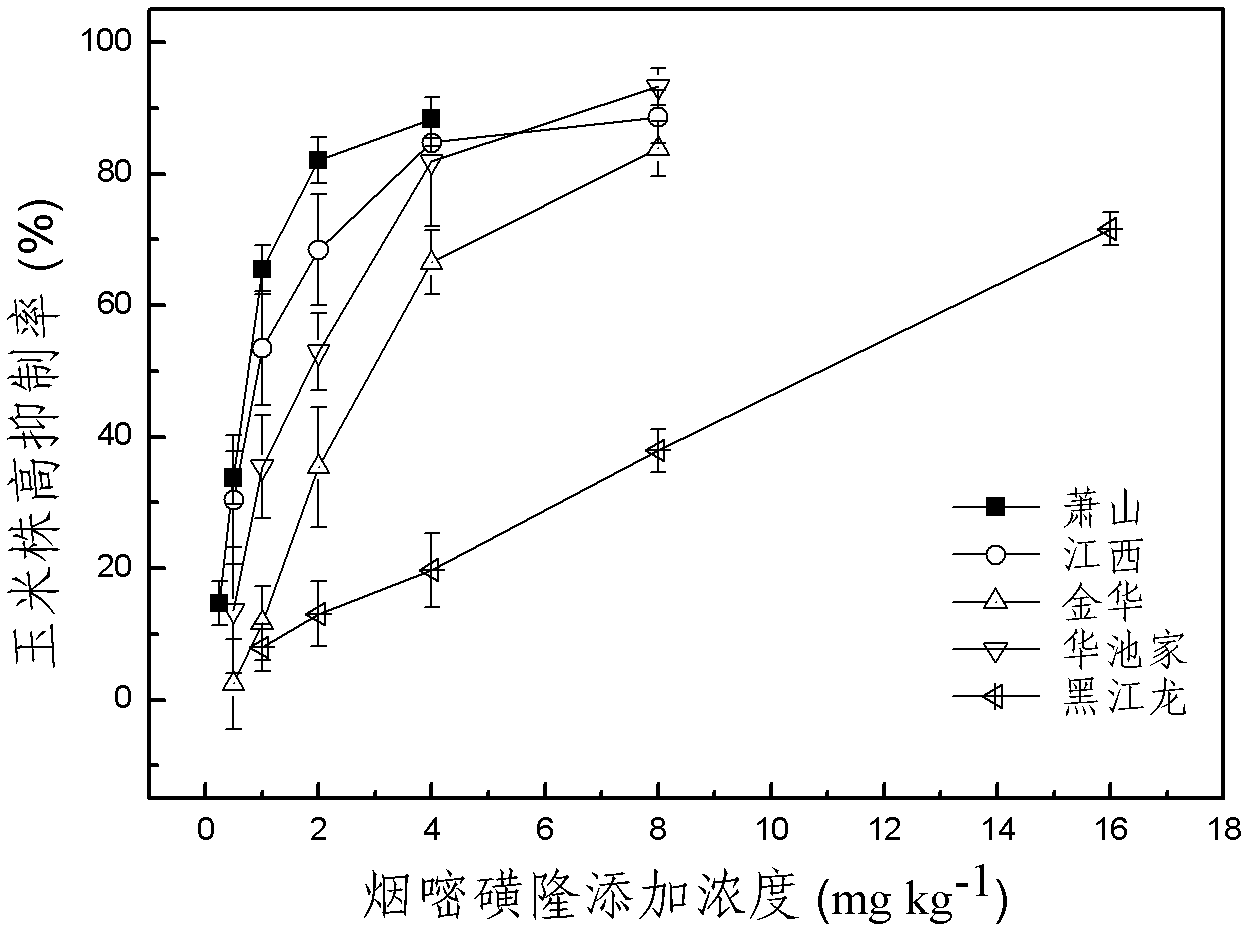
Method for detecting pesticide residue pollution of corn
InactiveCN106501413ARapid Prediction of PhytotoxicityEasy to operateComponent separationPhytotoxicityPesticide residue
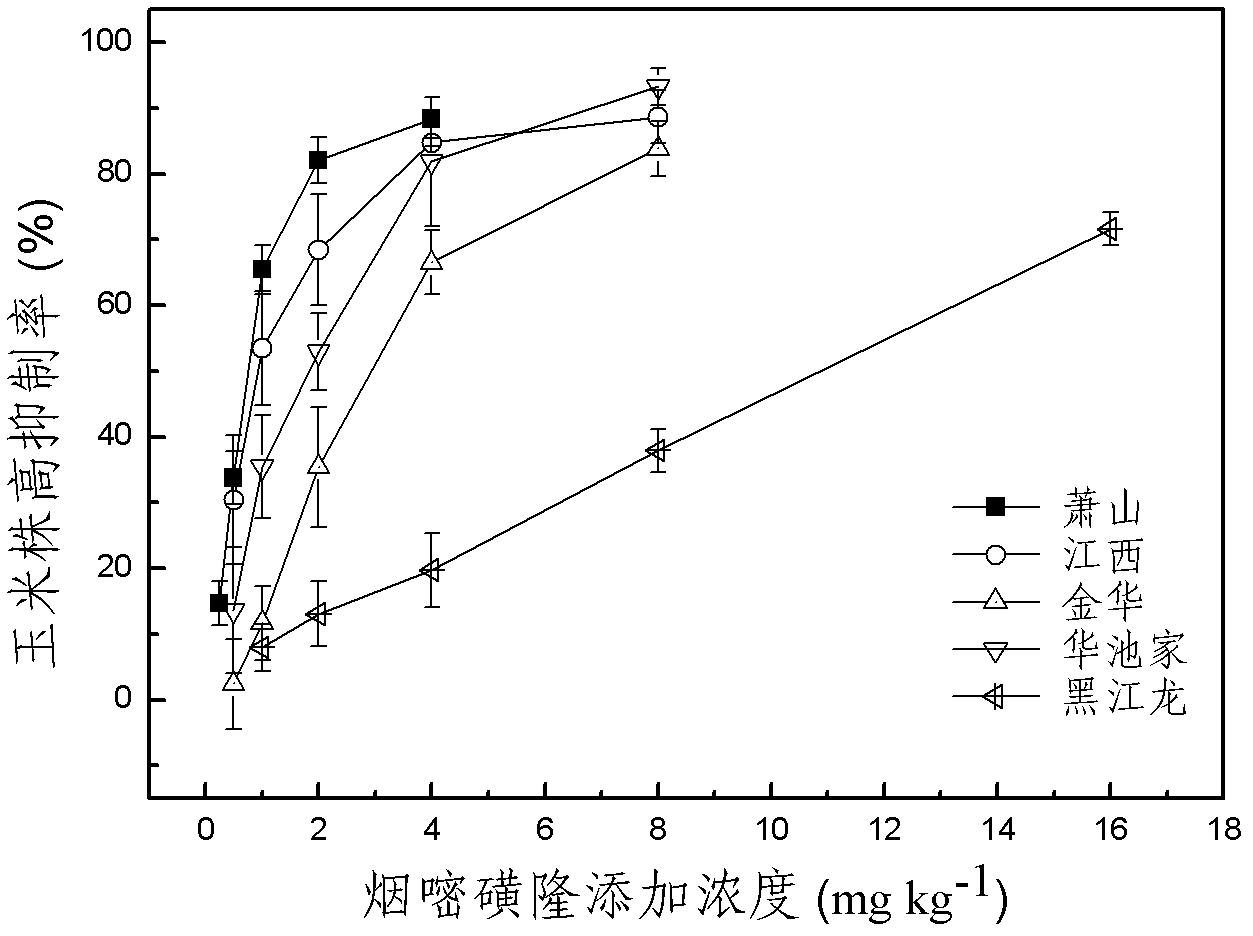
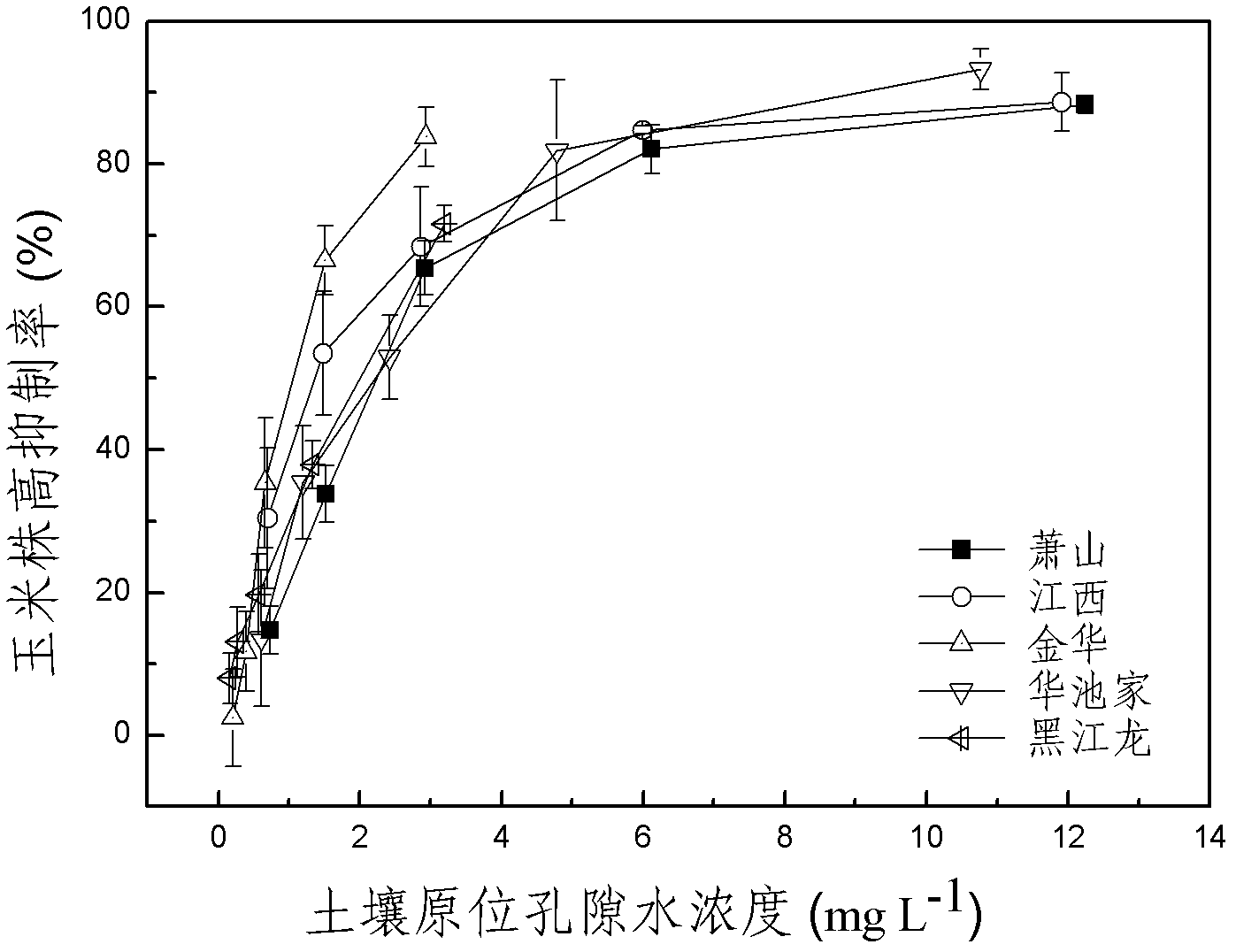
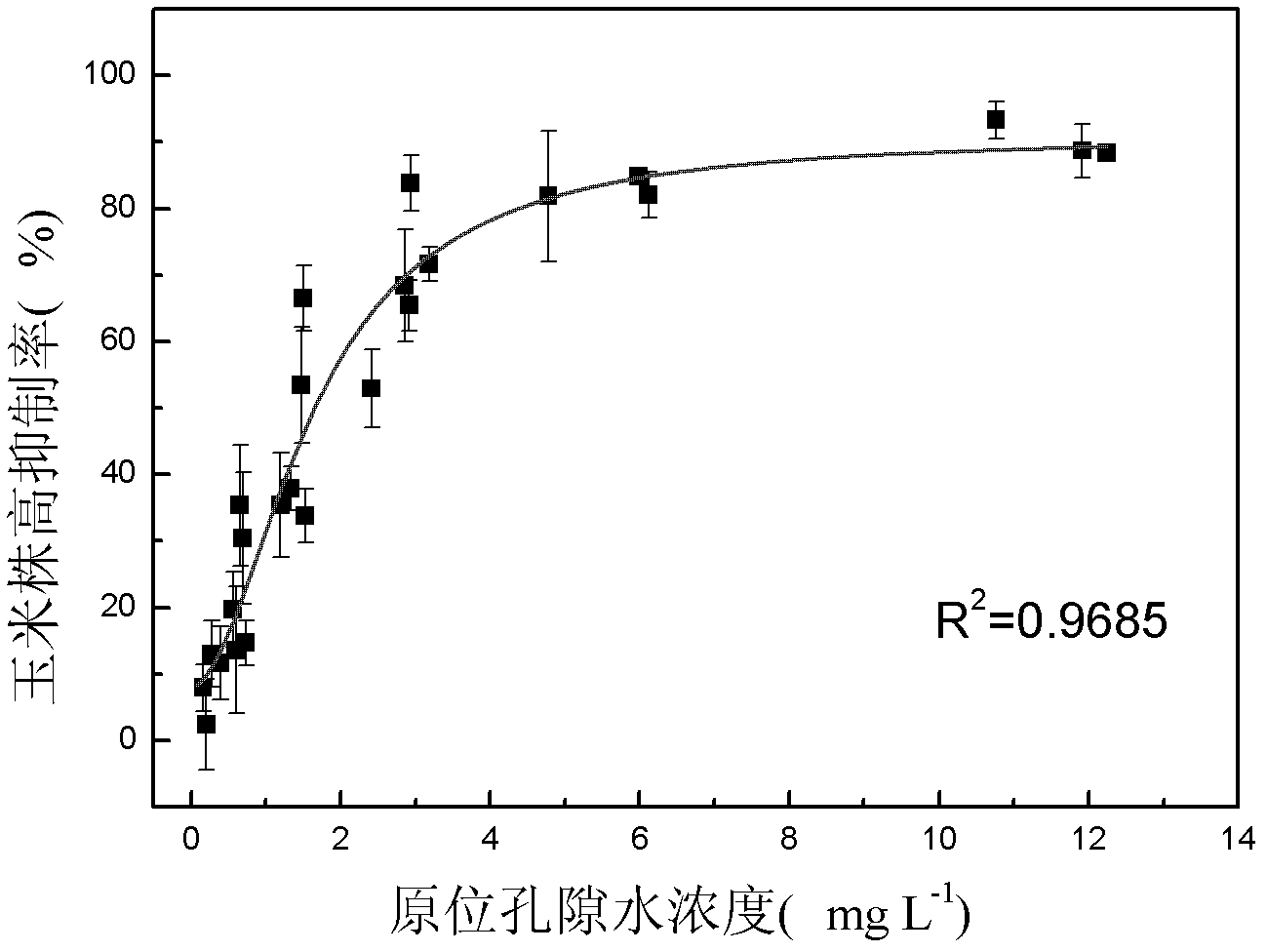
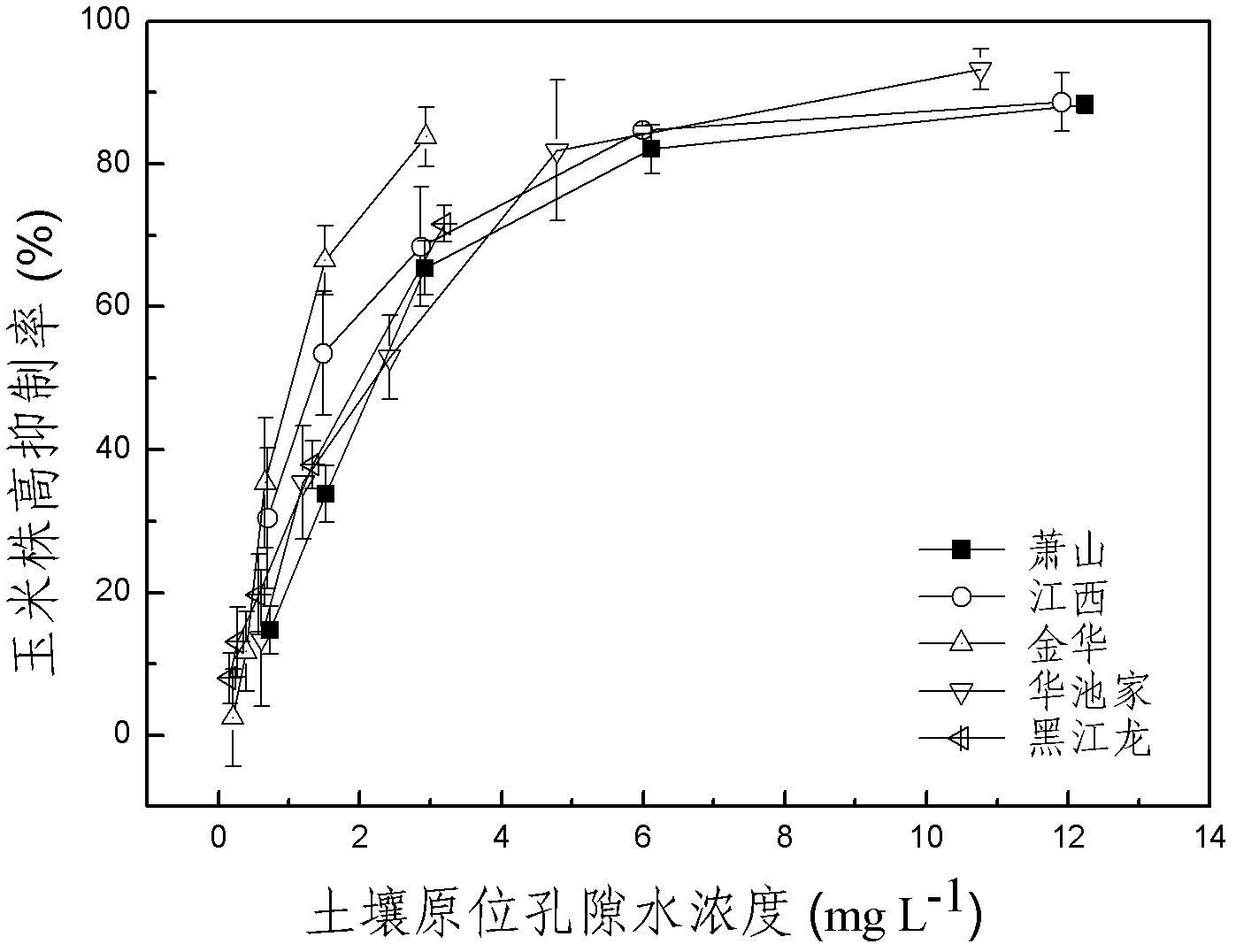
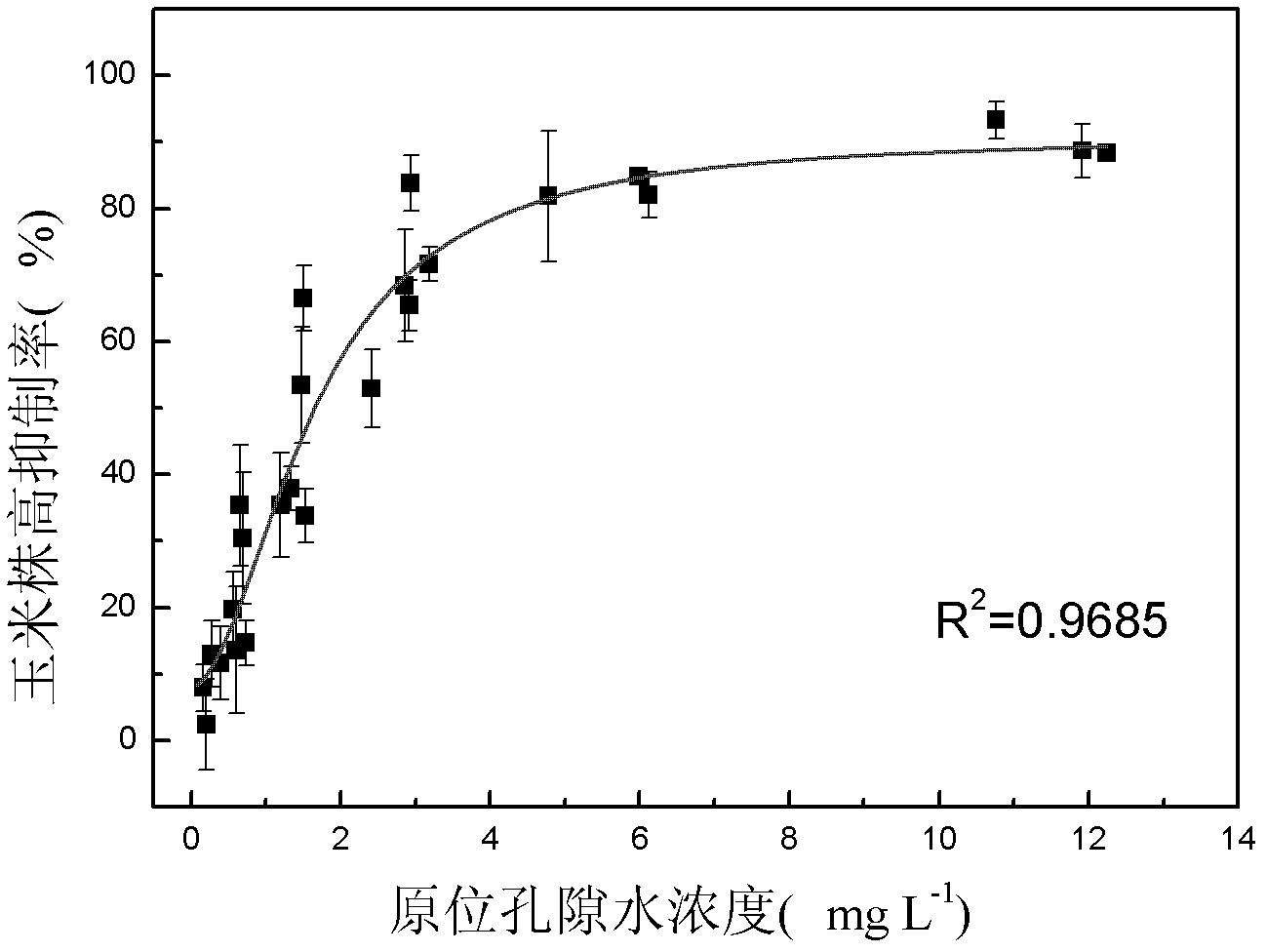
The invention discloses a method for detecting pesticide residue pollution of corn. The method comprises the following steps: spraying a group of nicosulfuron solutions with gradient concentration into a group of test soil, and sowing corn seeds into the test soil after spraying; one day after spraying, collecting in-situ porous water of the soil, and testing the concentration of nicosulfuron in the in-situ porous water; performing culture for multiple days, and testing the height of corn plants; finally performing fitting so as to obtain a dosage effect curve equation of the height of the corn plants and the concentration of the nicosulfuron in the in-situ porous water, and the concentration IC50 of the nicosulfuron in the in-situ porous water when the corn plant height inhibition rate is 50%; testing the concentration of the nicosulfuron in the in-situ porous water of the soil, and predicting the plant height inhibition rate of the corn planted in the soil according to the dosage effect curve equation. The method is simple and rapid to operate, and the plant toxicity of the nicosulfuron in the soil to be tested can be rapidly predicted according to the dosage effect curve equation of the nicosulfuron in the soil and the concentration of the nicosulfuron in the in-situ porous water of the soil to be tested.
Owner:GUANGXI UNIV
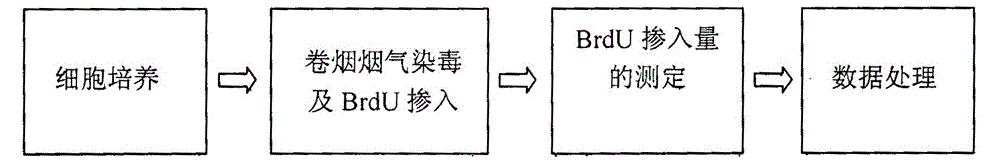
Electronic cigarette smoke solution cell proliferation toxicity evaluation method
ActiveCN103808917ASimple and fast operationThe result is stableColor/spectral properties measurementsBiological testingCell membranePermeation
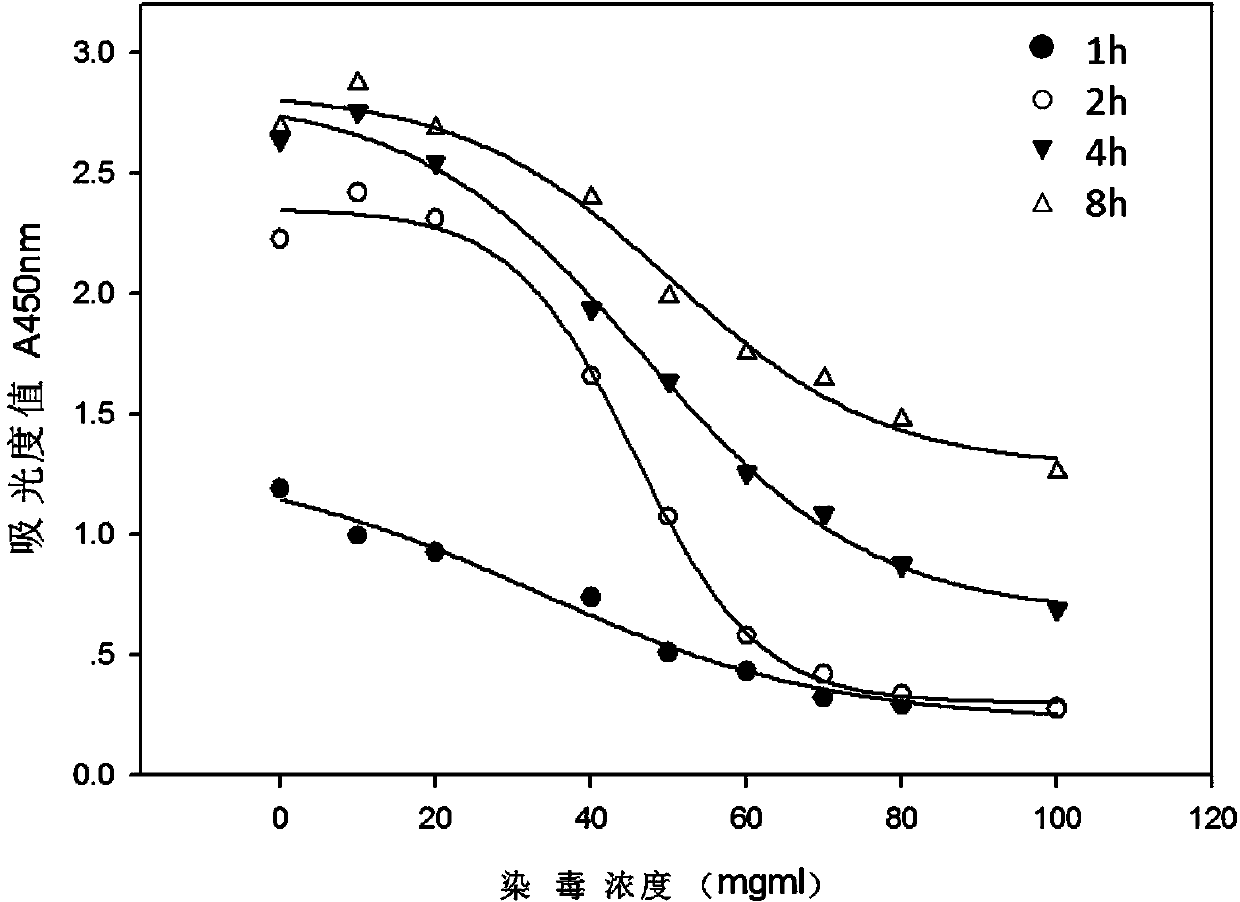
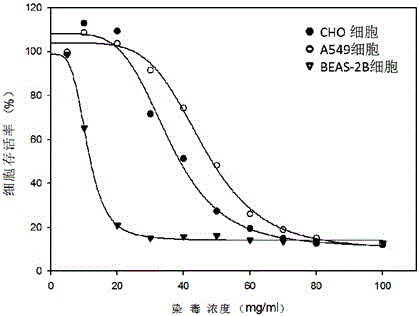
The invention relates to an electronic cigarette smoke solution cell proliferation toxicity evaluation method. The method is characterized by comprising the following steps: (1) preparing an electronic cigarette smoke solution; (2) carrying out cell inoculation; (3) contaminating the electronic cigarette smoke solution and doping BrdU (Bromodeoxyuridine); assaying the doped amount of BrdU; (5) resulting and analyzing. Compared with the prior art, the method has the following characteristics that aiming at the characteristic that most of electronic cigarette smoke solution samples are high in density, a method that an electronic cigarette contaminated solution is prepared by adopting mass weighing is determined; by a cell applicability validation step, the method is applicable to a variety of adherent culture cells and can be used for inspecting the cell toxicity to different cell lines of the lung caused by the electronic cigarette smoke solution; the sensitivity of detection is improved through optimizing the doping time of the BrdU and the concentration of cell membrane permeation liquid; the best contaminating concentration is determined through optimizing the contaminating concentration of the electronic cigarette smoke solution, so as to obtain the optimal dose-effect curve. In addition, the method further has the advantages of quickness, simplicity and convenience in operation, high sensitivity and stable results.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Method for detecting genotoxic agent in water by utilization of Pseudorasbora parva
InactiveCN105510543AFully assess health risksComprehensive assessment of health risksMaterial analysis by optical meansTesting waterPositive controlToxicant
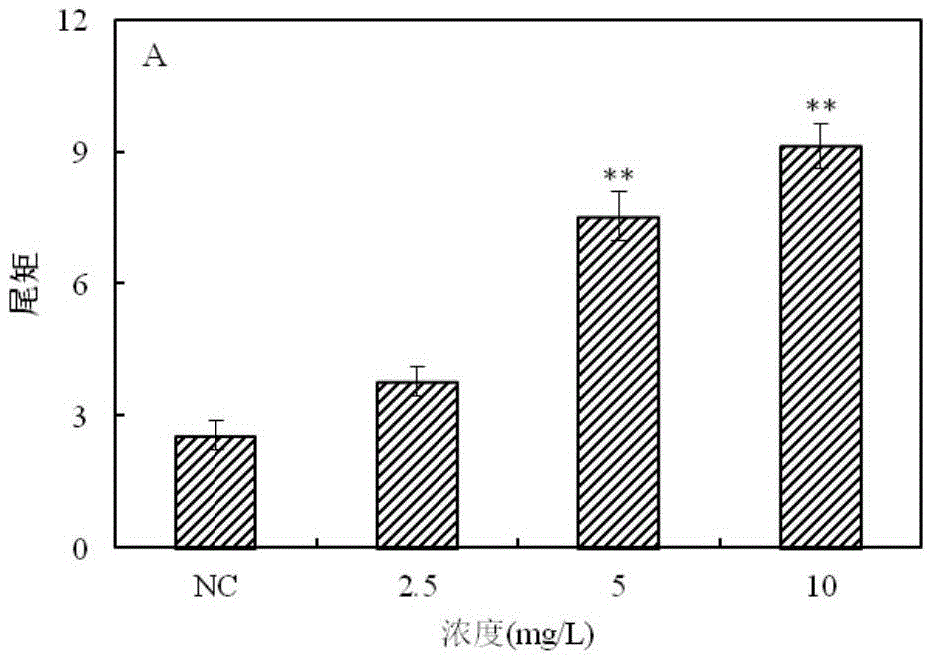
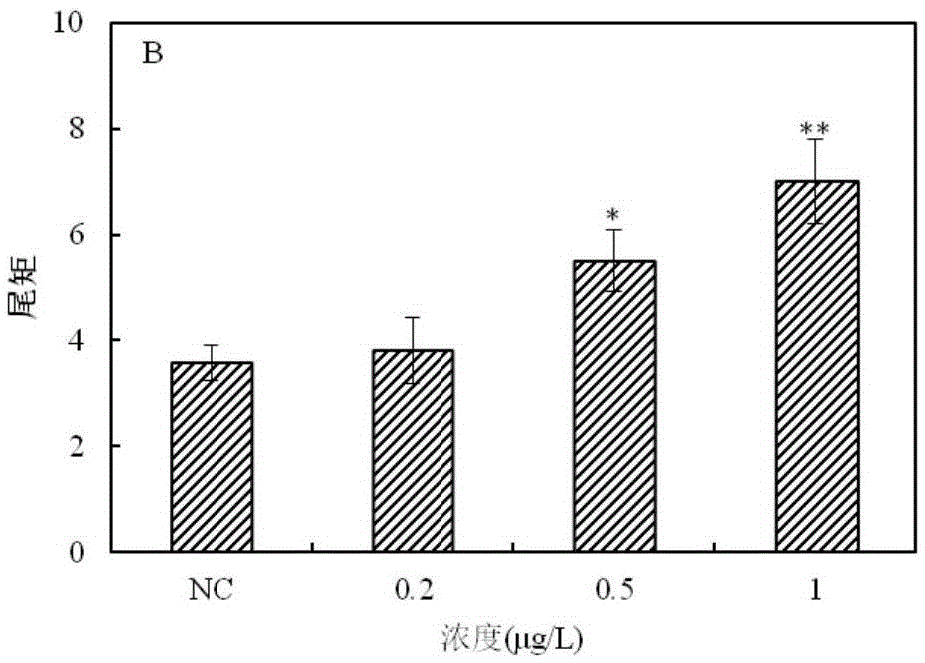
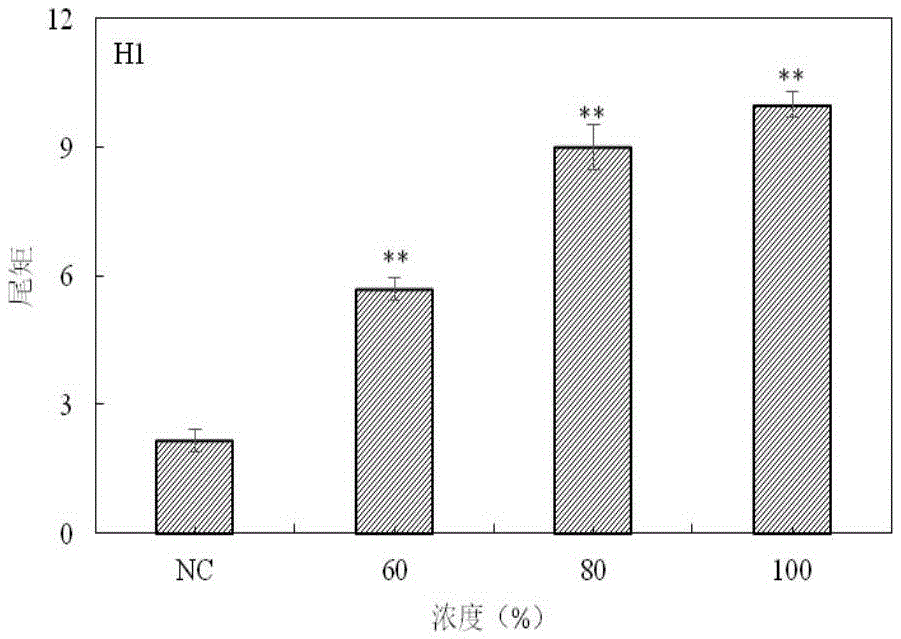
The invention provides a method for detecting a genotoxic agent by the utilization of Pseudorasbora parva and belongs to the field of chemicals safety evaluation and environmental monitoring. The method comprises the following steps: (1) processing Pseudorasbora parva by exposure treatment by the use of a water sample to be tested; and (2) carrying out single cell gel electrophoresis assay by using hepatic cells of Pseudorasbora parva processed after exposure treatment, detecting and counting cell tail length, DNA content and tail moment, and drawing a dose-effect curve. Through comparison between the result and a blank control and a positive control and through the dose-effect relationship, genetic toxicity of the test sample is determined. The detection method of the invention can sensitively and rapidly determine genetic toxicity of chemicals. The invention provides a China native species-based detection method for chemicals genetic toxicity evaluation and environmental genetic toxicity monitoring.
Owner:DALIAN UNIV OF TECH
Neutral red absorption assaying method for evaluating cell toxicity of electronic cigarette smoke solution
ActiveCN103808681AOptimize the exposure concentrationHigh detection throughputColor/spectral properties measurementsElectronic cigaretteElectron
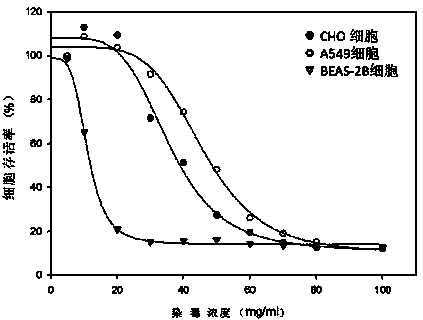
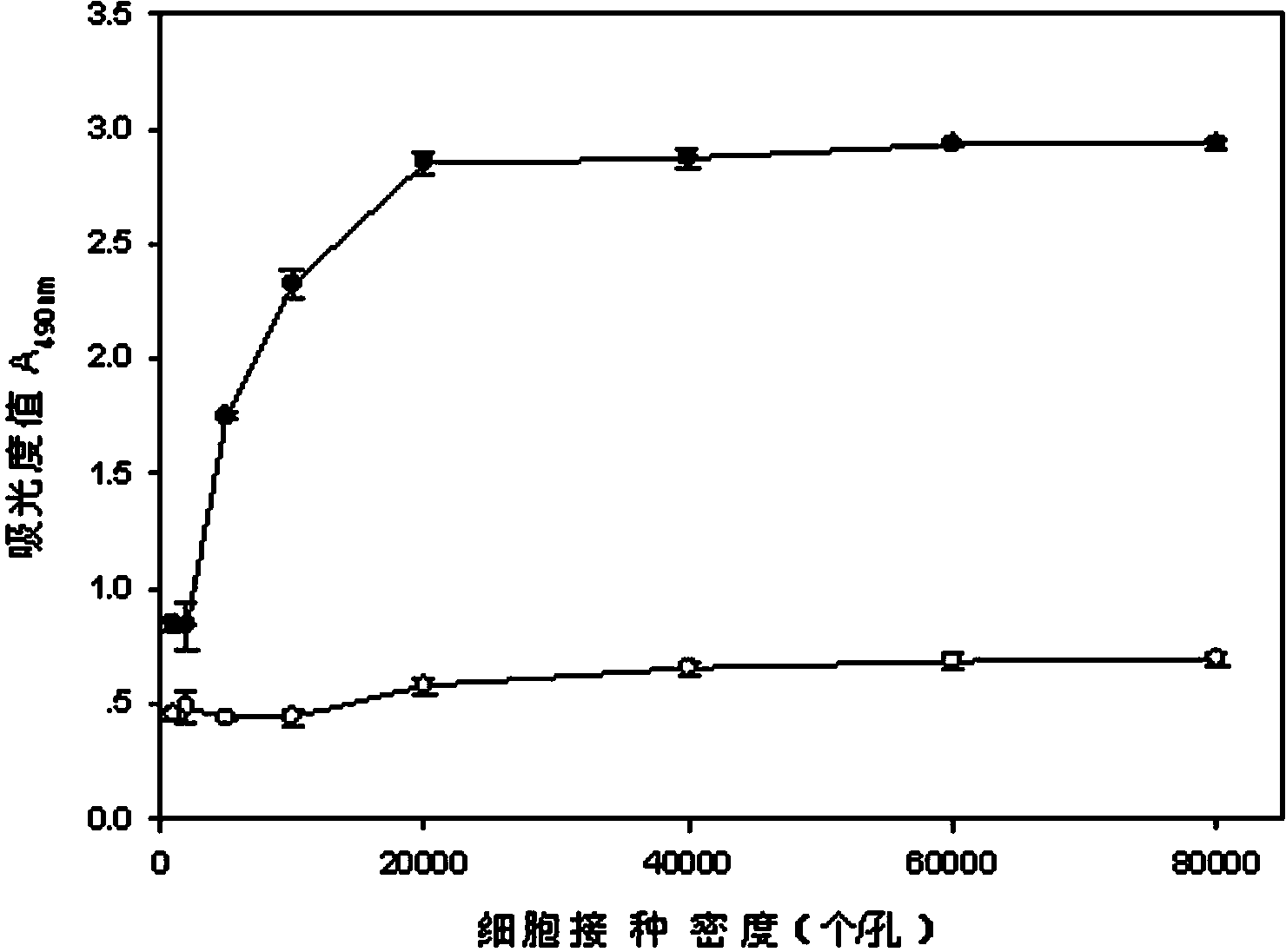
The invention relates to a neutral red absorption assaying method for evaluating the cell toxicity of an electronic cigarette smoke solution. The method is characterized by comprising the following steps: (1) preparing an experiment reagent; (2) carrying out cell inoculation; (3) contaminating the electronic cigarette smoke solution; (4) dyeing with neutral red; (5) resulting and analyzing. According to the method, aiming at the characteristic that most of electronic cigarette smoke solution samples are high in density, a method that an electronic cigarette contaminated solution is prepared by adopting mass weighing is determined; by a cell inoculation density optimizing step, cells of a cell line BEAS-2B, which are most applicable to the toxicity evaluation of the electronic cigarette smoke solution under the optimal inoculation density, are screened; the best contaminating concentration is determined through optimizing the contaminating concentration of the electronic cigarette smoke solution, so as to obtain the optimal dose-effect curve. The assaying method disclosed by the invention further has the advantages of quickness, simplicity, convenience and high sensitivity.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Lactic dehydrogenase determination-based cigarette smoke cell toxicity evaluation method
InactiveCN103820524AExtended reaction timeOptimal dose-response curveMicrobiological testing/measurementParticulatesLactate dehydrogenase
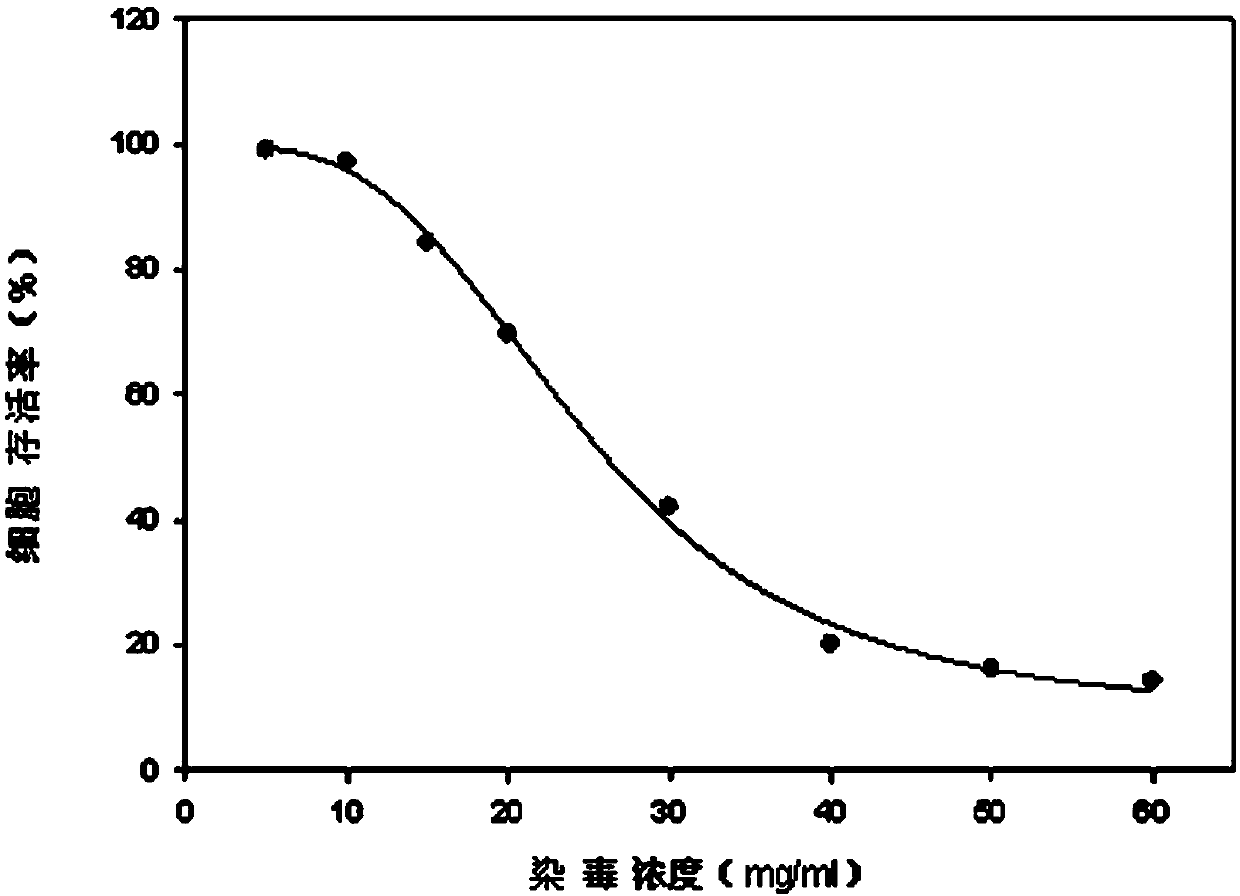
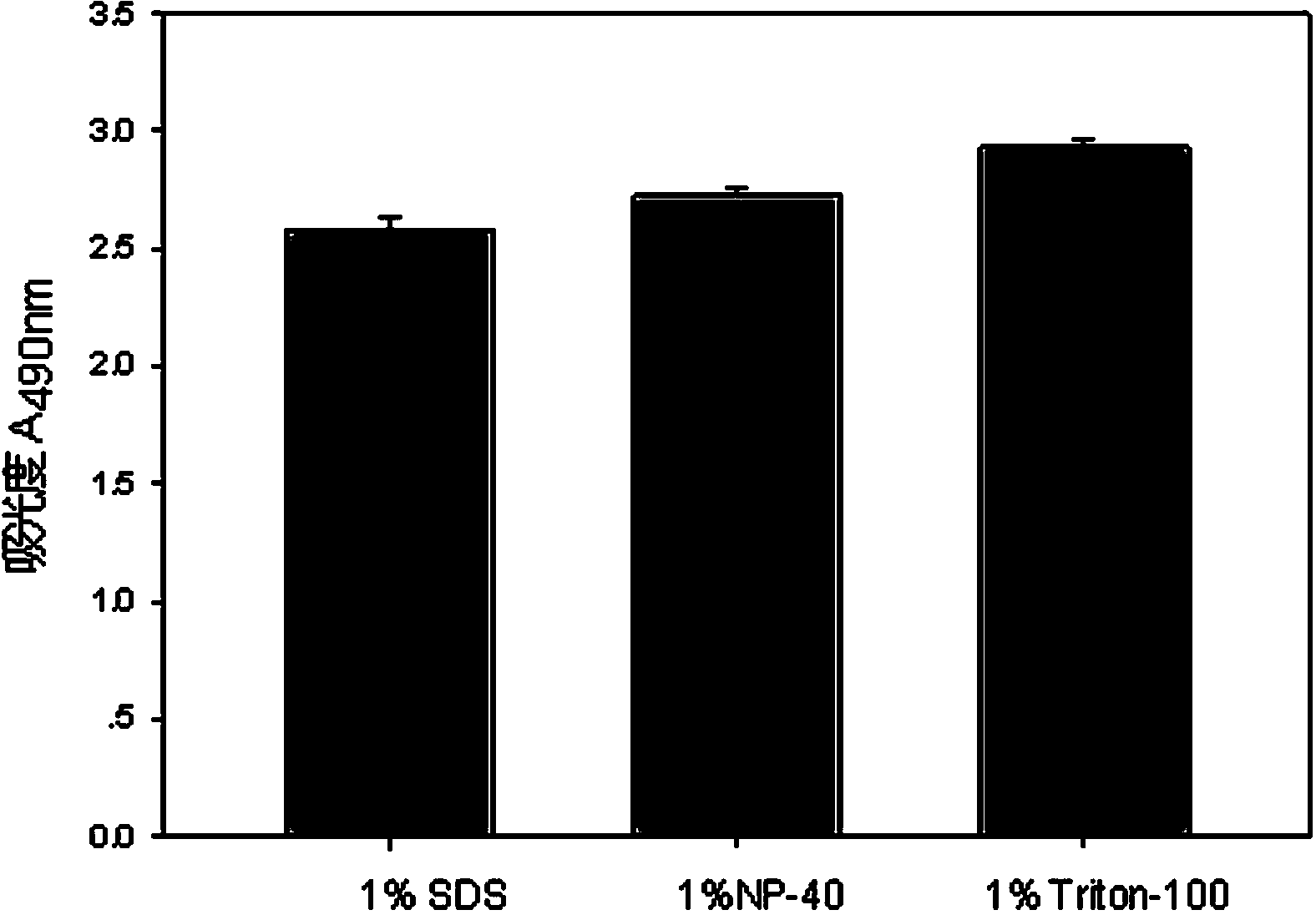
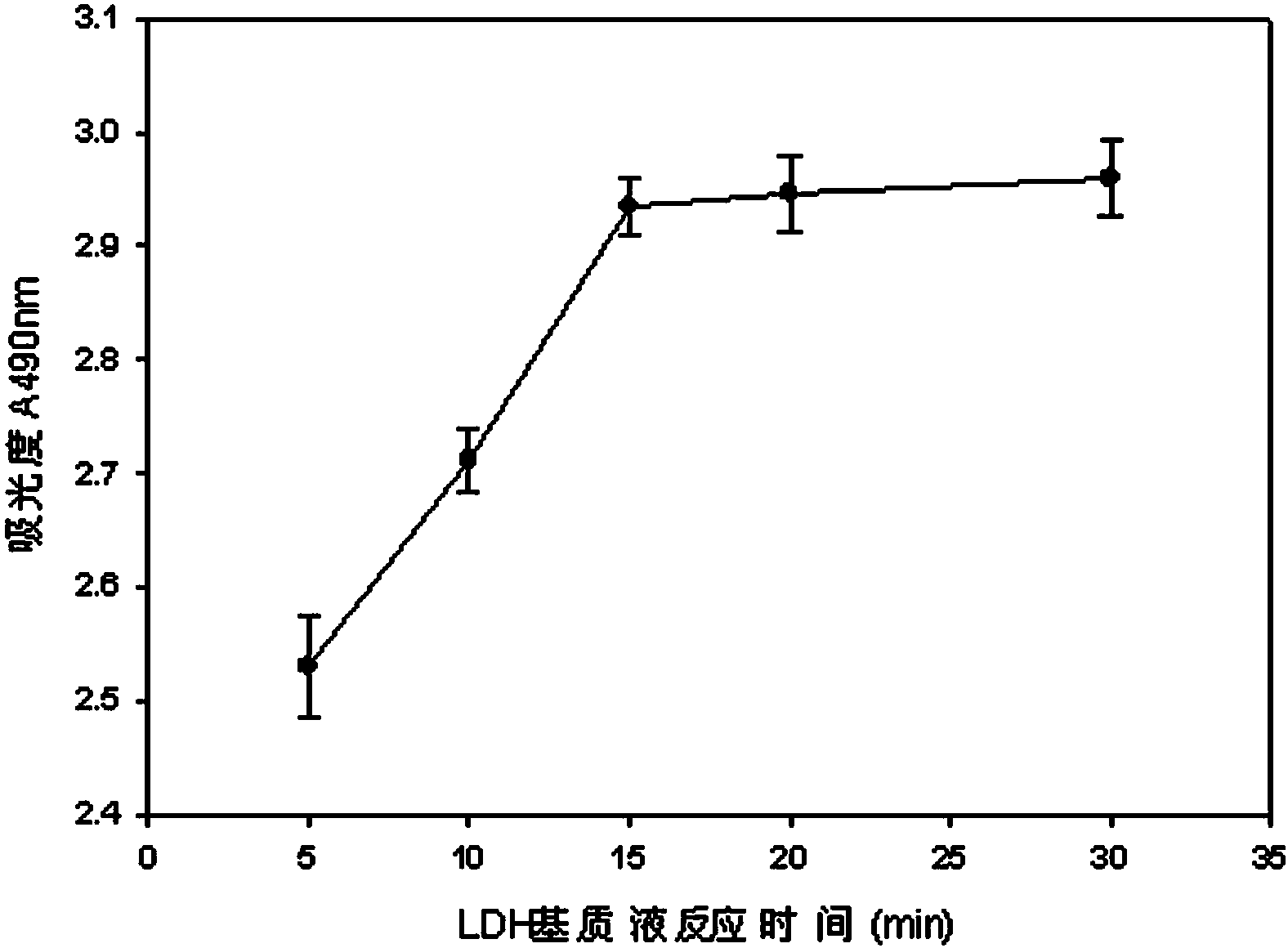
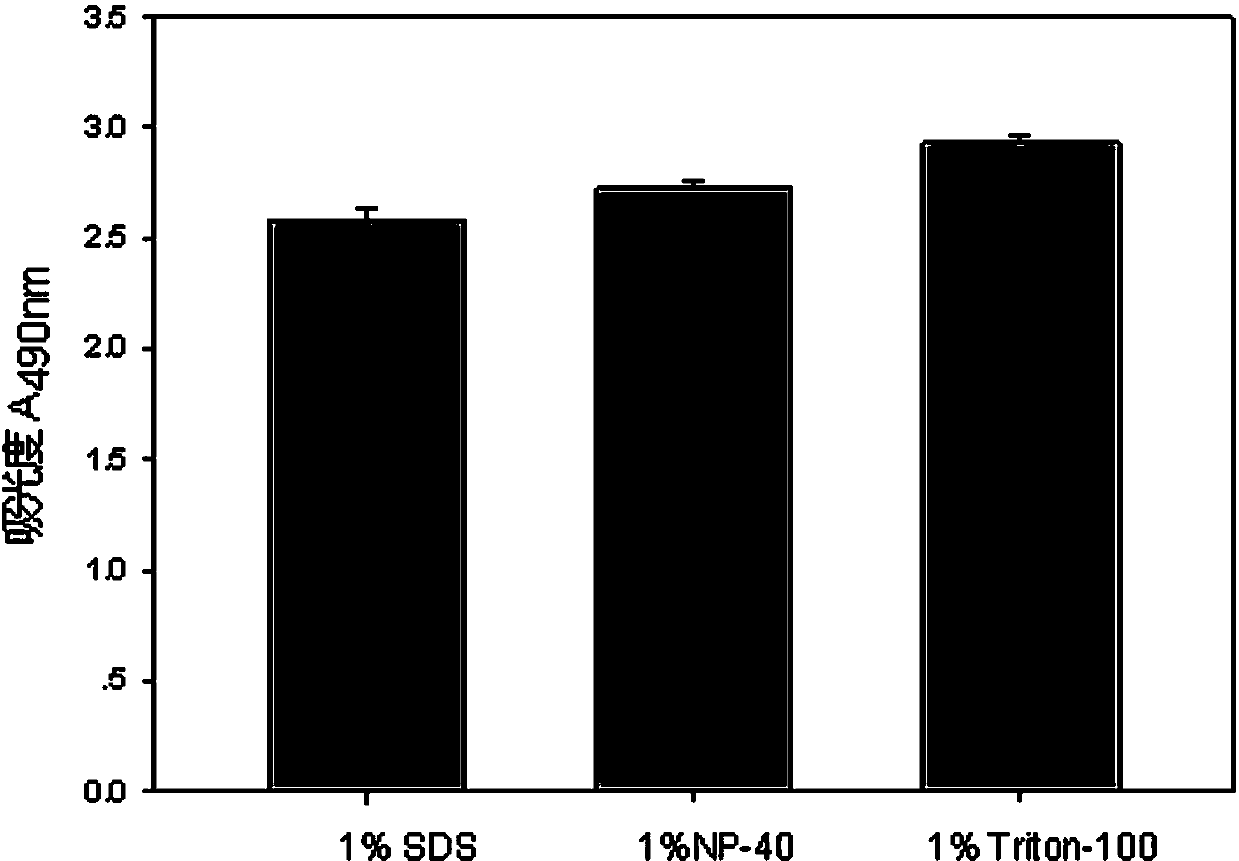
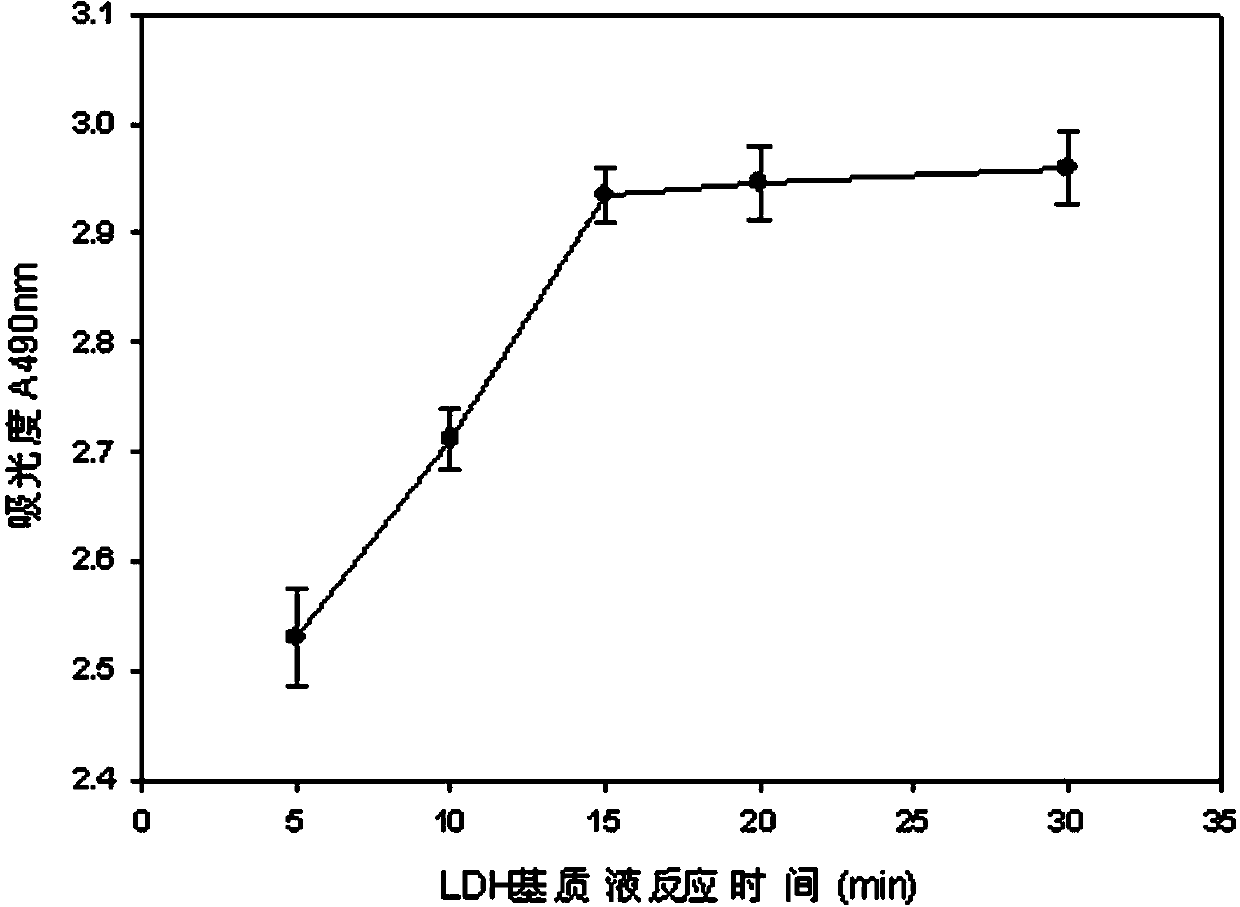
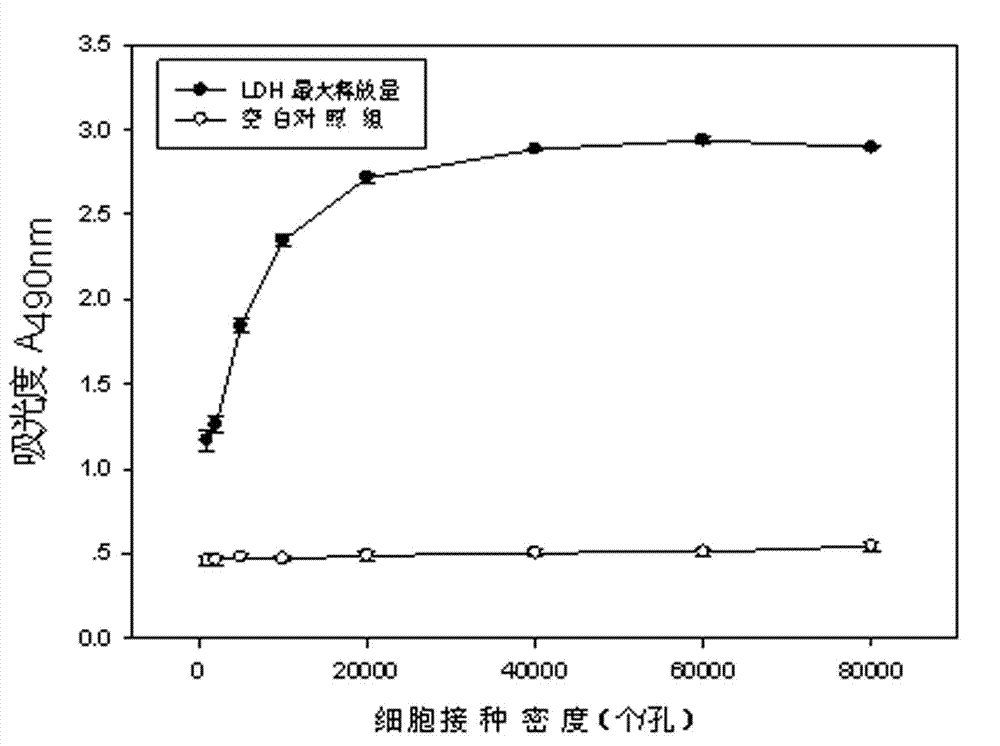
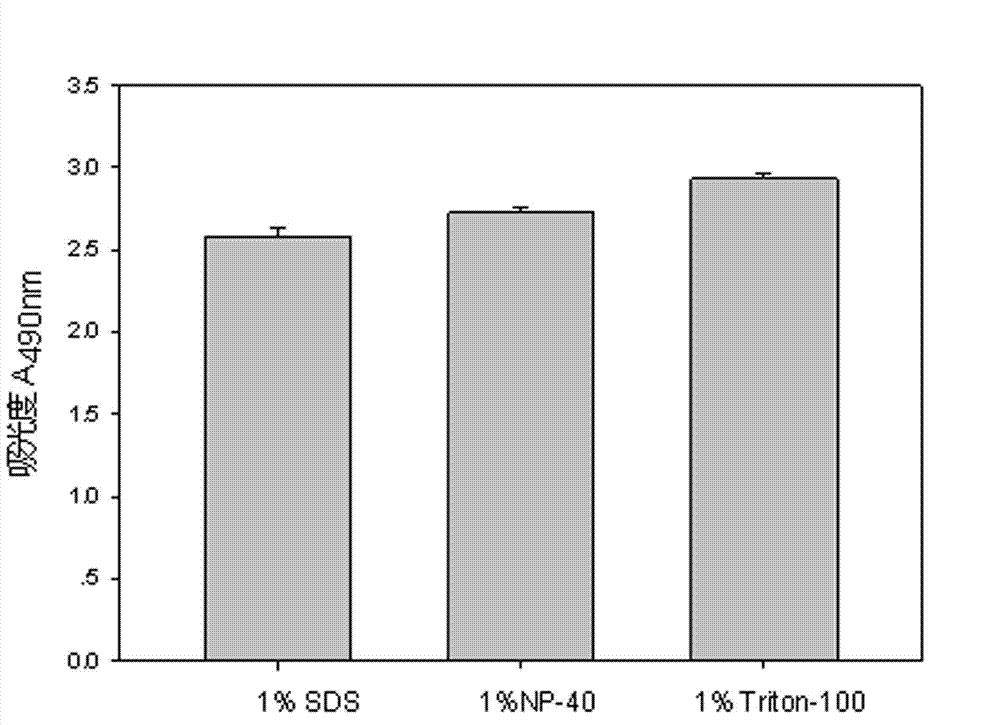
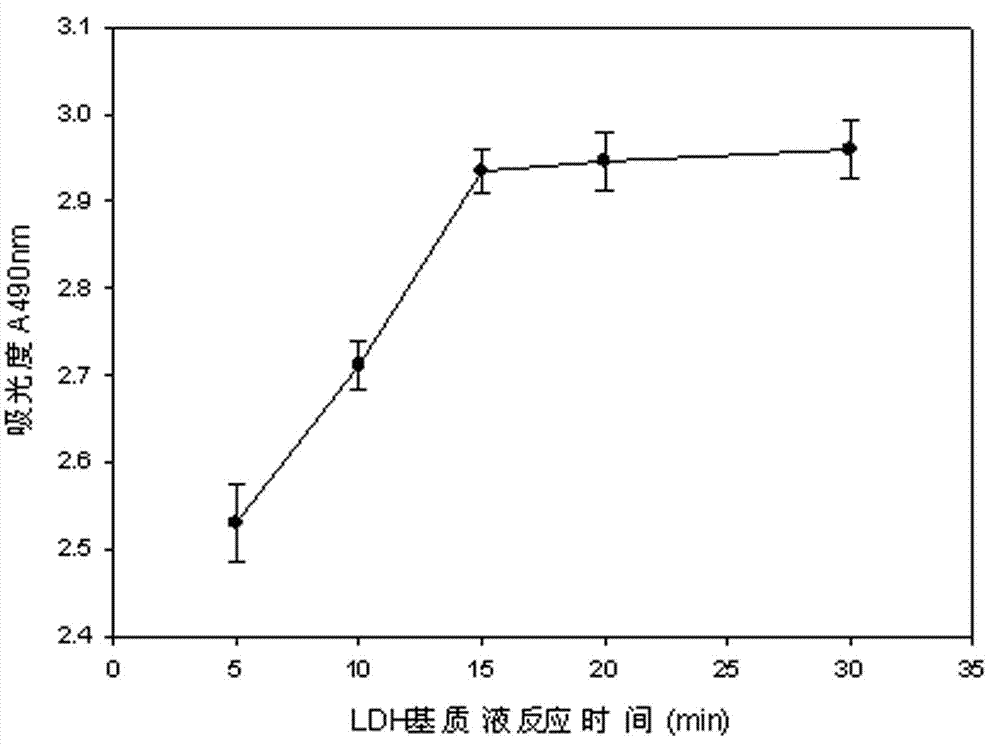
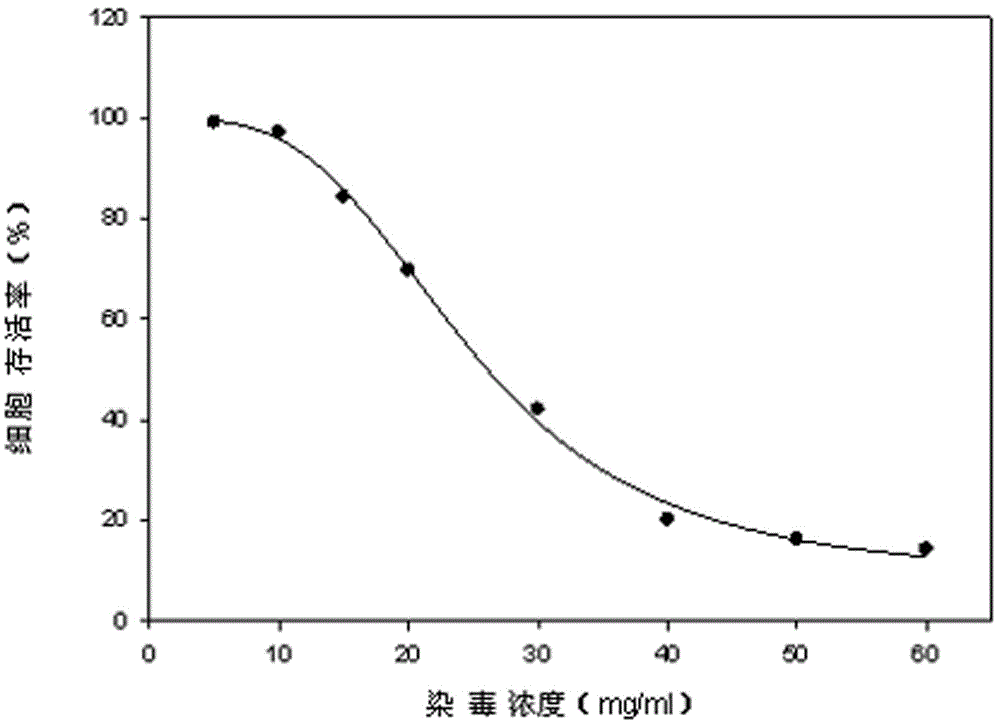
The invention discloses a lactic dehydrogenase determination-based cigarette smoke cell toxicity evaluation method, comprising the following steps: 1) preparation of an experiment reagent; 2) cell inoculation; 3) cigarette smoke contamination; 4) lactate dehydrogenase (LDH) determination; 5) result and analysis. The cell toxicity of a smoke particulate matter and a smoke gas phase matter can be respectively inspected by preparation of a smoke particulate matter extracting solution and a smoke gas phase matter absorption liquid; the lactic dehydrogenase determination-based cigarette smoke cell toxicity evaluation method is applicable to a plurality of adherent culture cells, and can be used for inspecting the cell toxicity of the cigarette smoke on different cell lines. The detection sensitivity is improved by selection of cell lysis buffer and optimization of reaction time of LDH matrix liquid. The optimal contamination concentration is determined by optimizing the cigarette smoke contamination concentration, so as to obtain an optimal dose effect curve. The damage degree to a cell membrane caused by the smoke particulate matter and the smoke gas phase matter of the cigarette can be reflected by determination of the LDH, and the cell toxicity mechanism of the cigarette smoke is announced. In addition, the evaluation method also has the advantages of being rapid, simple and convenient to operate, high in sensitivity, and stable in result.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Processing method and processing device for efficacy of combined medicine
ActiveCN105224799ARealize detectionSolve problems that cannot be accurately detectedChemical property predictionMathematical modelsMedicineToxicology studies
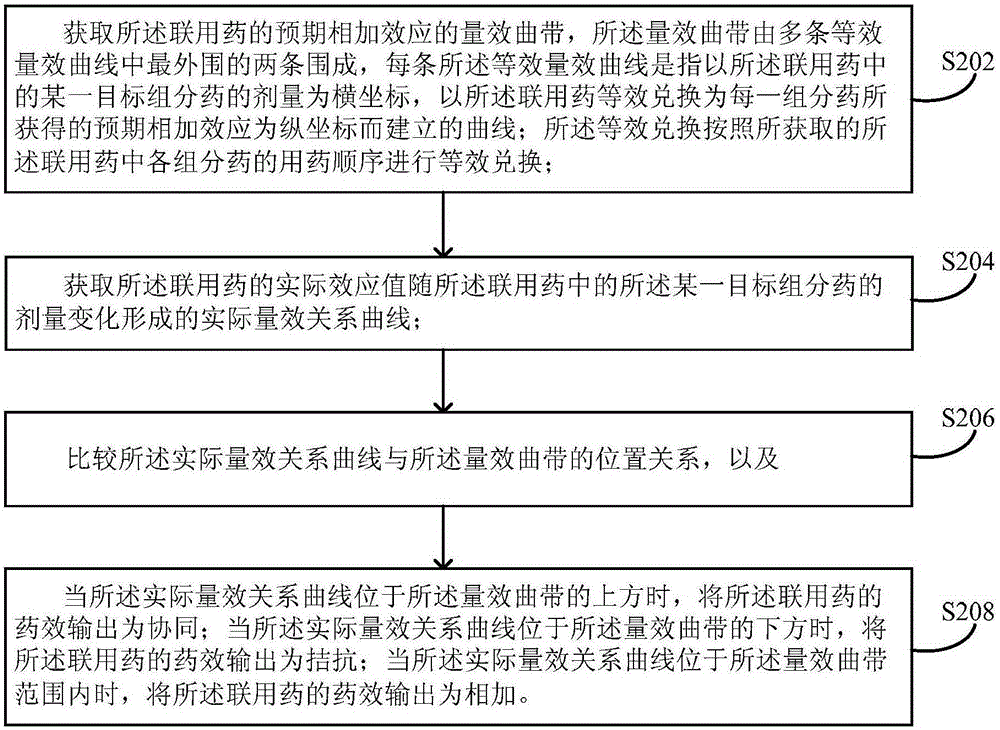
The invention provides a processing method and a processing device for efficacy of combined medicine. The processing method comprises: obtaining a dose-effect curved band of expected additive effect of combined medicine; obtaining an actual dose-effect relationship curve formed by variation of actual effect value of the combined medicine with variation of dosage of a certain target component medicine in the combined medicine; comparing position relationships of the actual dose-effect relationship curve and the dose-effect curved band, and when the actual dose-effect relationship curve is above the dose-effect curved band, efficacy output of the combined medicine being synergy; when the actual dose-effect relationship curve is below the dose-effect curved band, efficacy output of the combined medicine being antagonism; and when the actual dose-effect relationship curve is in the dose-effect curved band range, the efficacy output of the combined medicine being addition. The processing method solves a problem in the prior art that efficacy cannot be accurately detected when multiple medicines are used in a combined manner. The method can be widely applied in research and development of compound medicines, toxicologic study, efficacy and safety evaluation of the combined medicine, and environmental evaluation.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Subcloned cell strain TF-1-A2, preparation method thereof and application
ActiveCN102660505AEasy to trainGood passage stabilityMicrobiological testing/measurementBlood/immune system cellsSignal-to-noise ratio (imaging)Cell growth
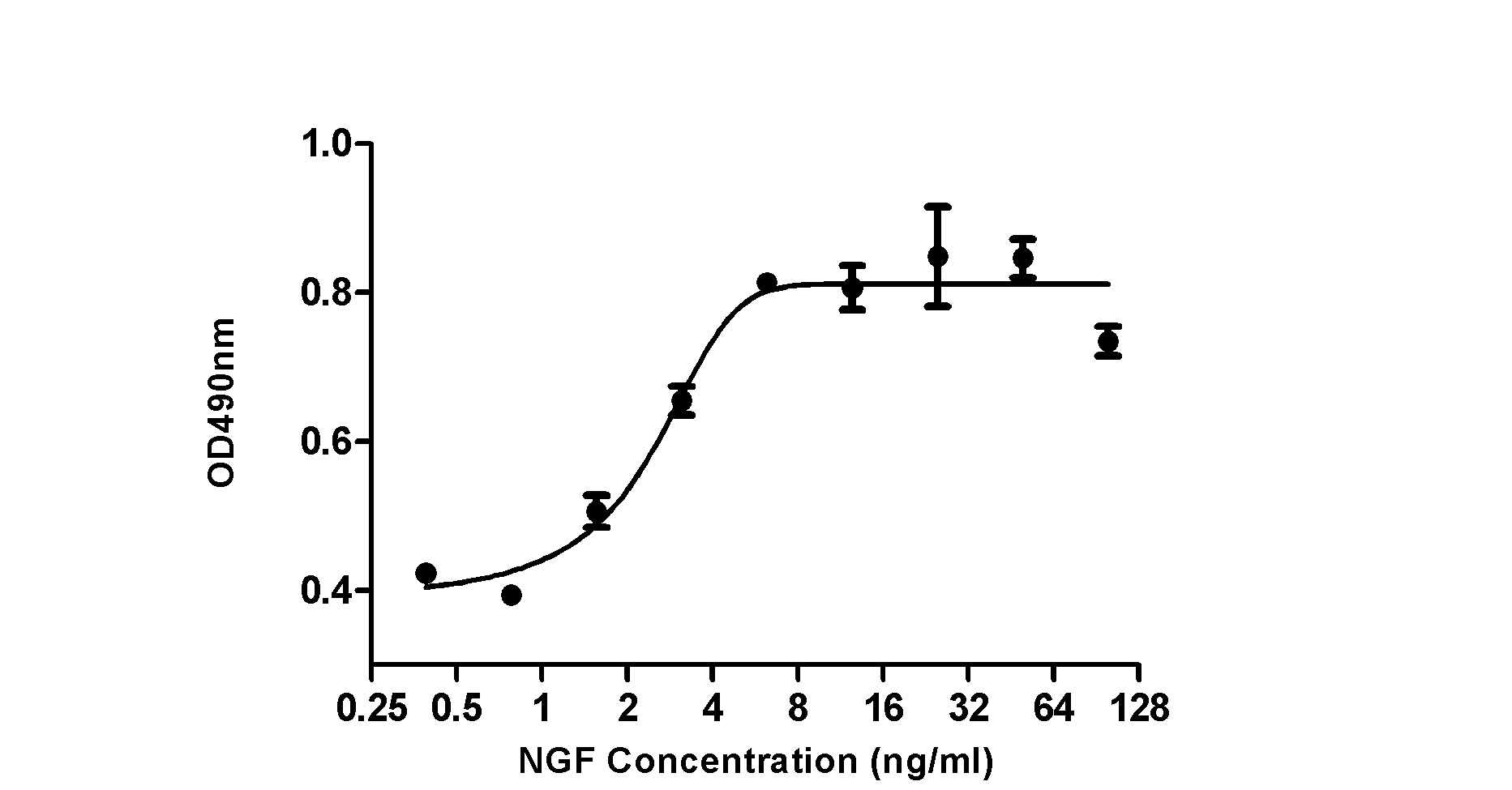
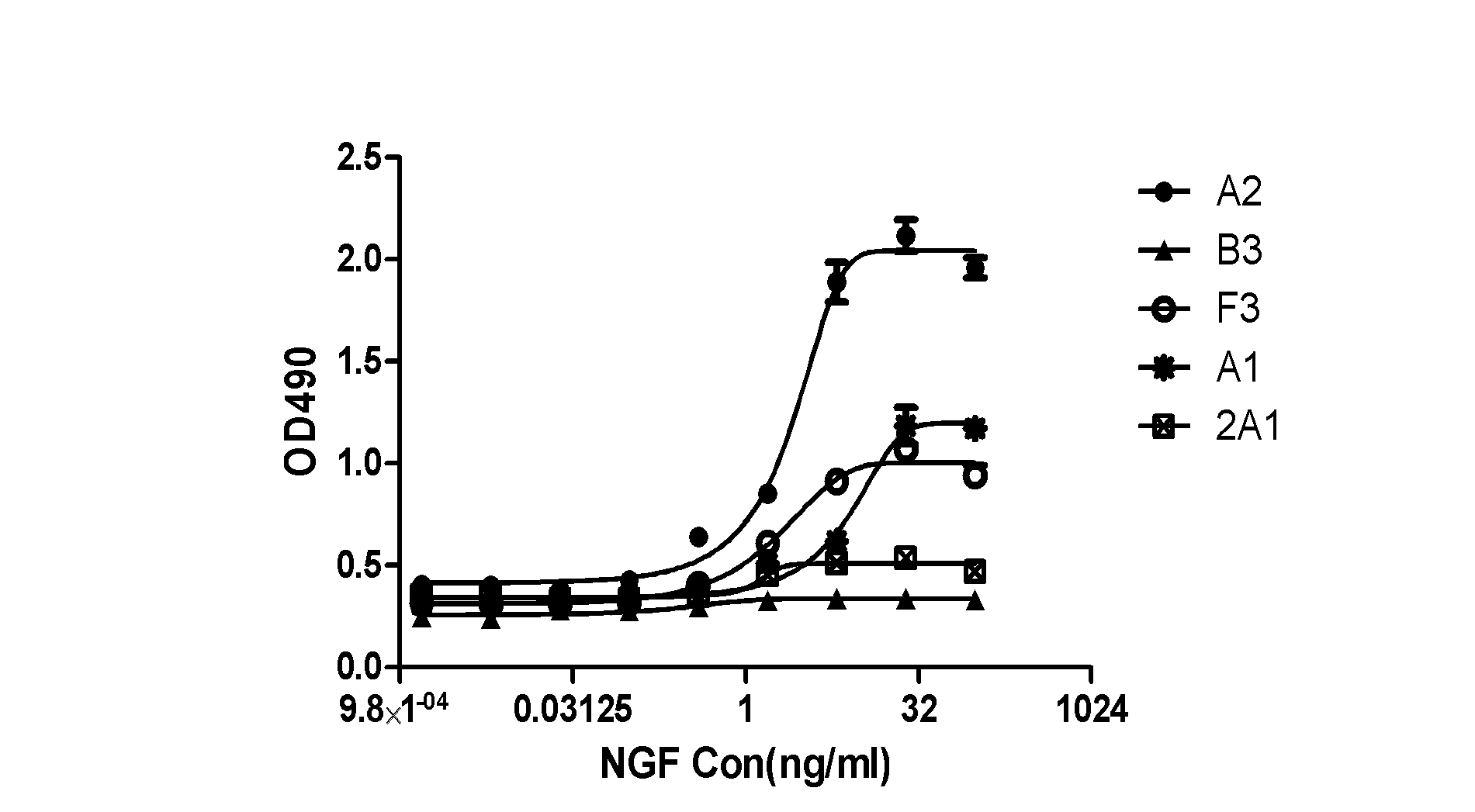
The invention relates to 'a subcloned cell strain TF-1-A2, a preparation method thereof and application' and belongs to the technical field of biology. The preparation method of the stable subcloned cell strain TF-1-A2 includes the steps: domesticating GM-CSF (granulocyte-macrophage colony-stimulating factor) dependent TF-1 cells into NGF (nerve growth factor) dependent strains; cloning the induced NGF dependent TF-1 cells by the aid of methylcellulose semisolid media; picking monoclonal cells from the semisolid media and enlarging and cultivating the monoclonal cells; and preparing a dose-effect curve of each monoclonal cell strain to the NGF and performing screening to obtain the subcloned cell strain TF-1-A2. The subcloned cell strain TF-1-A2 obtained by the preparation method is highly sensitive to the NGF and has fine reactivity and continuous inheritance stability. A TF-1 cell growth method established by the aid of the subcloned cell strain TF-1-A2 is used for externally and quantitatively measuring biological activity of the nerve growth factor, and is high in signal-to-noise ratio, fine in repeatability and superior to an existing method.
Owner:北京福睿君安科技有限公司
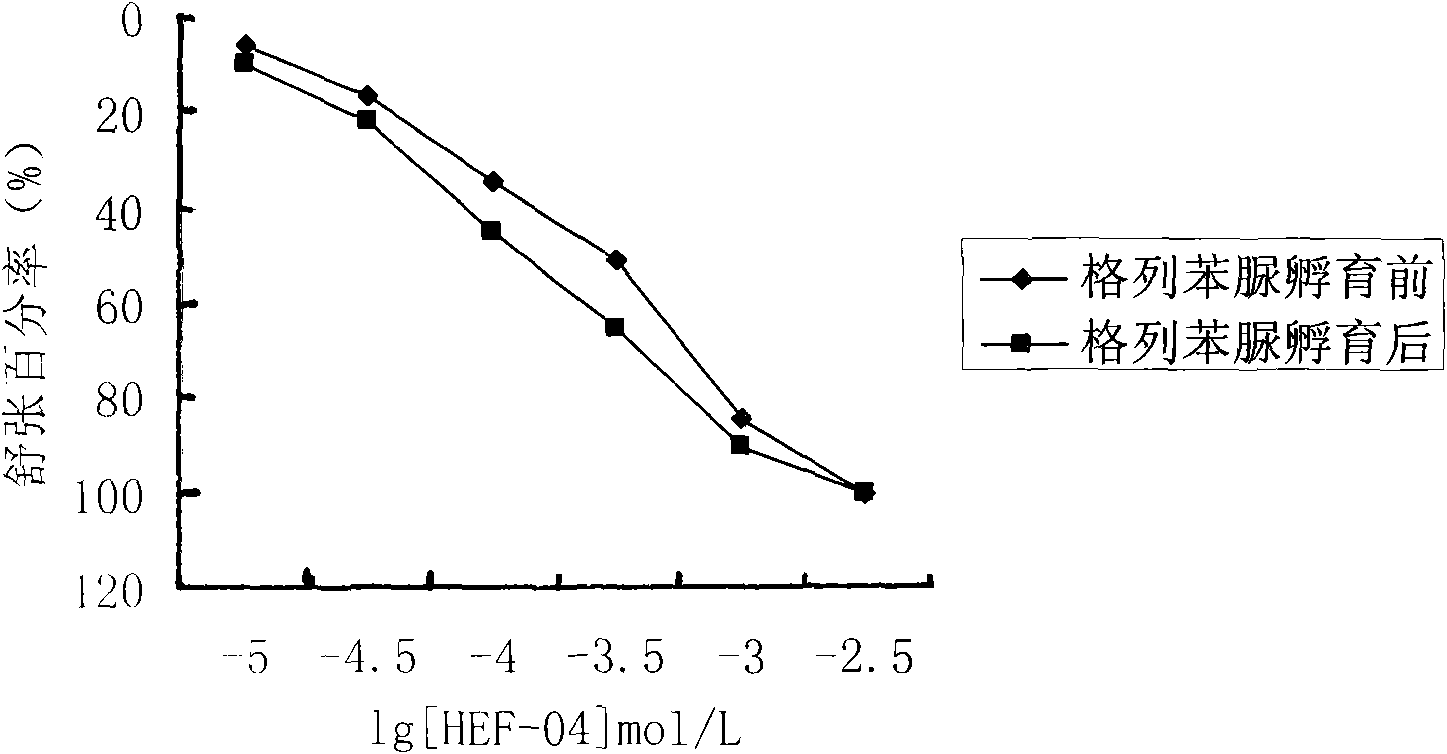
Application of chromane compound HEF-04 in relaxing blood vessel smooth muscle
InactiveCN101618041AReduce tensionOrganic active ingredientsUrinary disorderVascular endotheliumPotassium
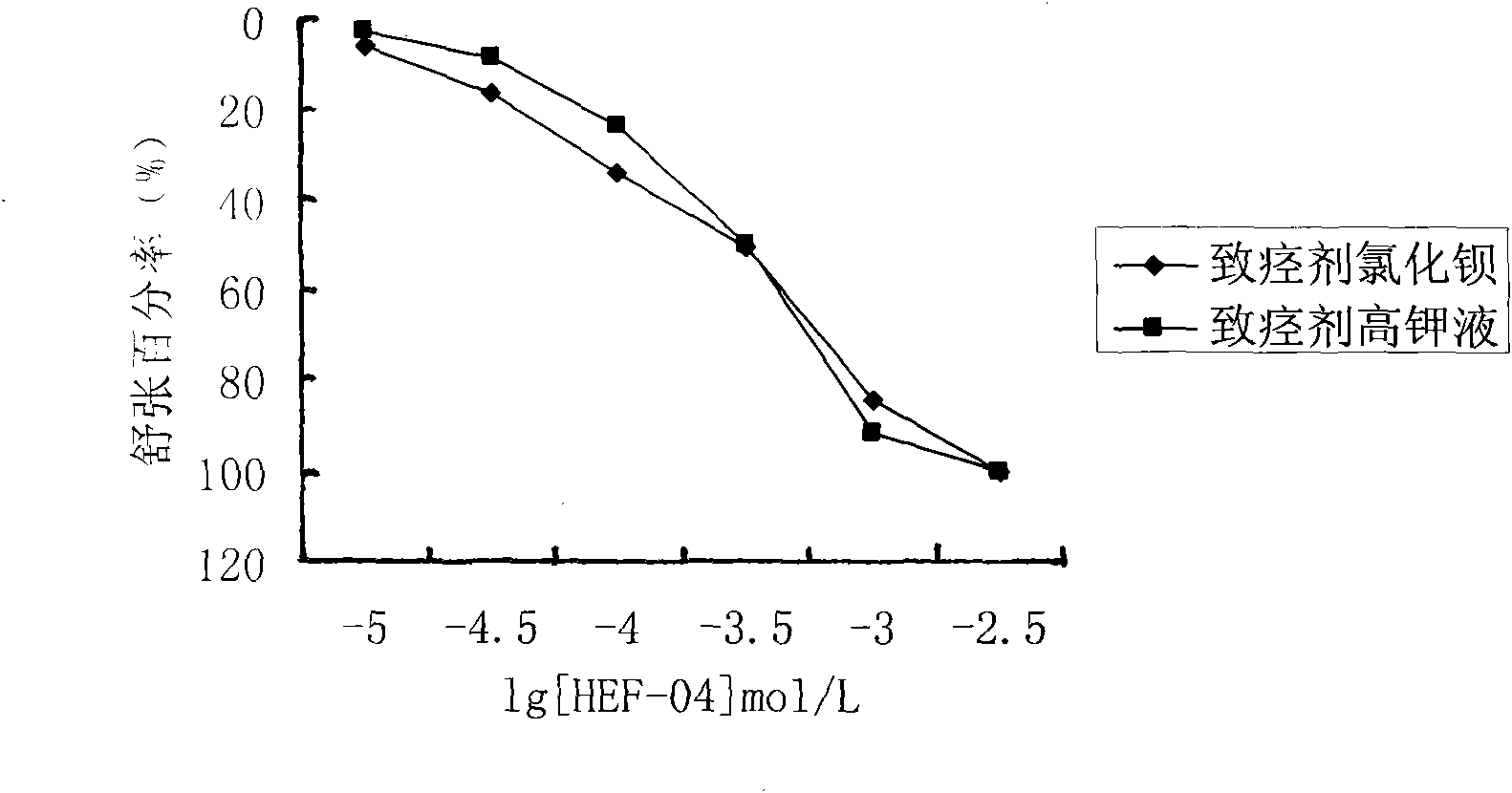
The invention relates to the technical field of medicine and provides an application of chromane compound HEF-04 in relaxing blood vessel smooth muscle. In animal in vitro experiment, HEF-04 can relax rabbit in vitro blood vessel smooth muscle shrunk by barium chloride and potassium chloride; the vasodilation effect of chromane compound HEF-04 is not inhibited by the incubation of methylene blue, on the contrary, the blood vessel dose-effect curve shifts left due to higher level of potassium in spasm-induced agent; EC50 value has significant difference before and after the incubation. Documents report that the existence of NO and PGI2 can weaken the generation of EDHF; after NO and PGI2 are inhibited, the effect of endothelium-dependent vascular relaxation caused by EDHF is more prominent, showing that the vascular relaxation effect of HEF-04 is possibly related to the increase of EDHF releasing or caused by the interactions of vascular endothelium relaxing factors.
Owner:SHENYANG PHARMA UNIVERSITY
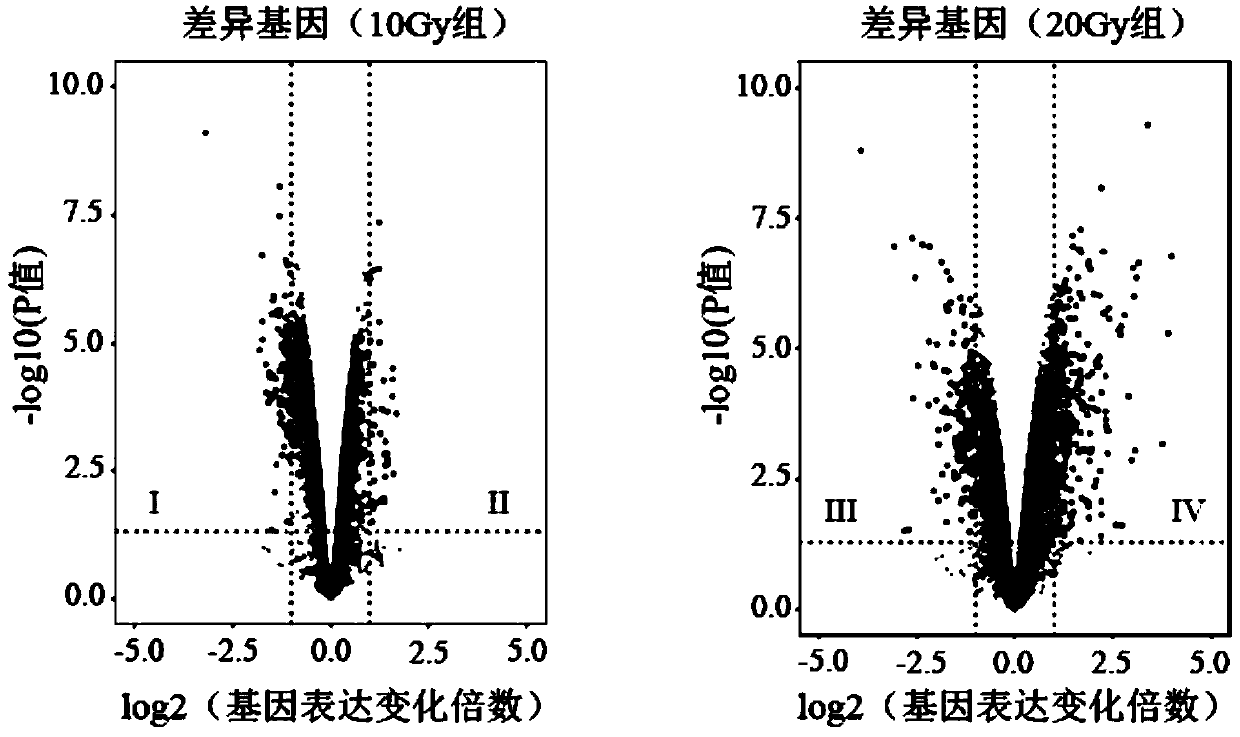
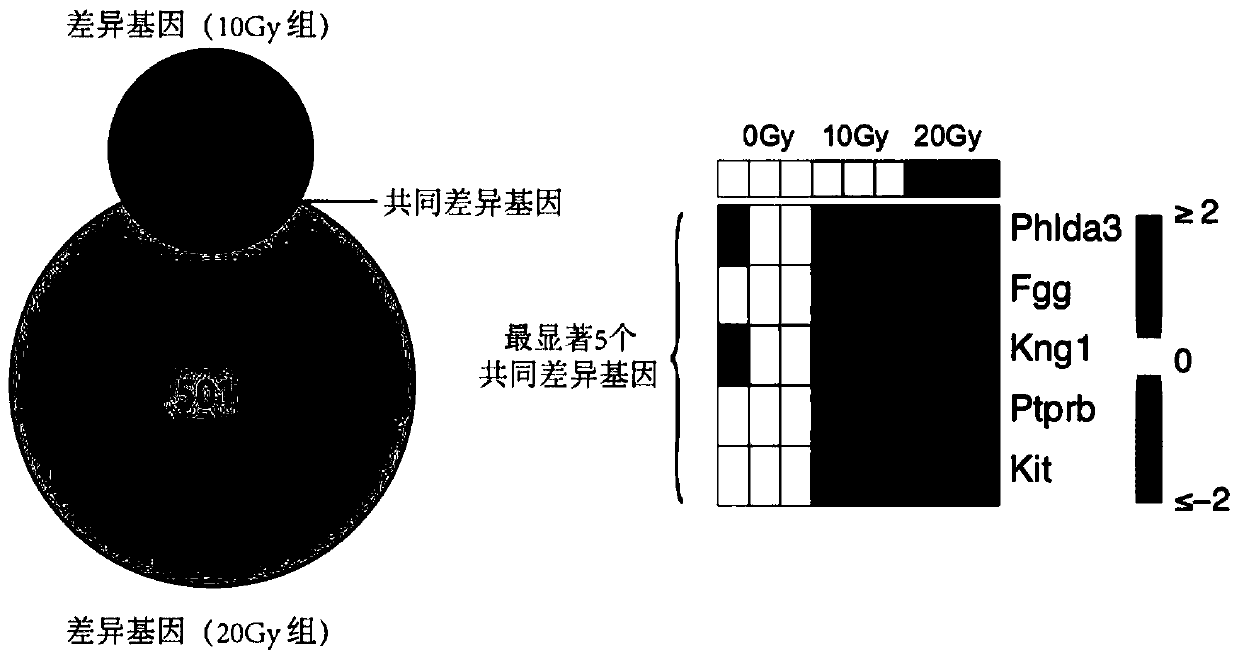
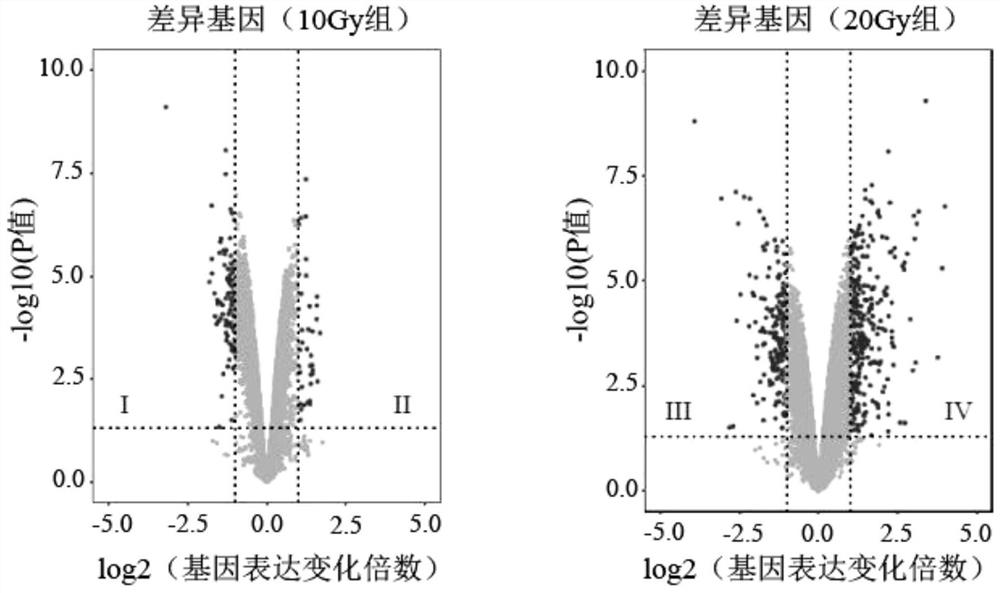
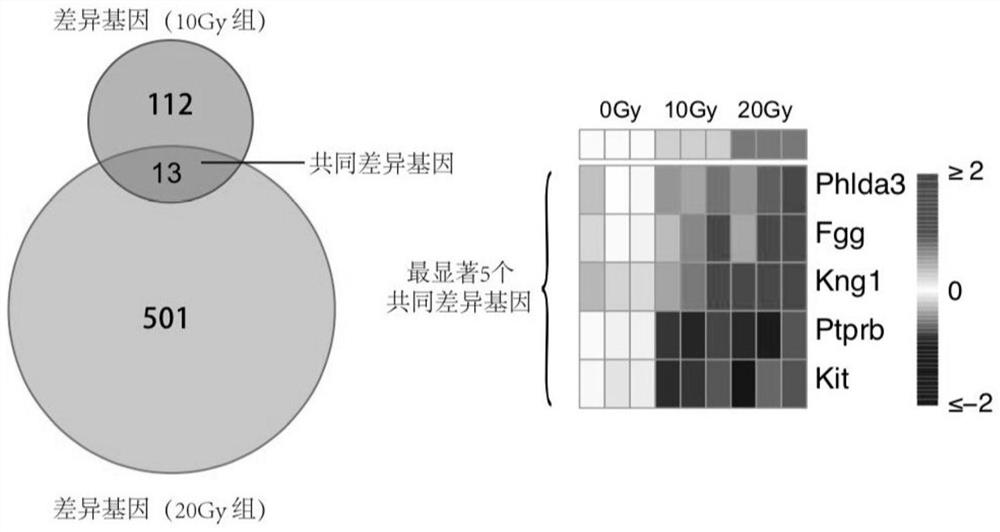
Radiosensitive gene marker and application thereof in X-ray radiation dose monitoring
ActiveCN109913543ASensitiveMonitoring Exposure DoseMicrobiological testing/measurementDNA/RNA fragmentationNuclear radiationX-ray
The invention provides a radiosensitive gene marker and application thereof in X-ray radiation dose monitoring. The radiosensitive gene marker comprises three up-regulation gene markers and two down-regulation gene markers; the up-regulation gene markers include Phlda3, Fgg and Kng1; the down-regulation gene markers include Ptprb and Kti. A dose-effect curve constructed based on the expression level of the radiosensitive gene marker helps determine the range of radiation dose effectively, so that the level of X-ray radiation dose can be monitored effectively to accurately prompt a reference dose of X-ray radiation based on biological effect in order to estimate nuclear radiation damage.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Method for testing toxicity of residual nicosulfuron on corn
ActiveCN102680594ARapid Prediction of PhytotoxicityEasy to operateComponent separationTesting toxicityPhytotoxicity
The invention discloses a method for testing the toxicity of residual nicosulfuron on corn. A group of nicosulfuron solutions, the concentrations of which are gradiently distributed, are sprayed in a group of tested soils, and corn seeds are sowed on the sprayed tested soils; on the first day after spraying, the in-situ pore water of all the soils is collected, and the nicosulfuron concentrationsin the in-situ pore waters are determined; after a plurality of days of culture, the plant heights of corn are determined; finally, a dose-effect curve equation for the plant heights and the nicosulfuron concentrations in the in-situ pore water and the nicosulfuron concentrations IC50 in the in-situ pore water at the corn plant height inhabitation rate of 50 percent are obtained by fitting; the nicosulfuron concentrations in the in-situ pore waters of the soils to be tested are determined, and according to the dose-effect curve equation, the plant height inhabitation rate of the soils plantedwith corn is predicted. The method is easy and rapid to operate, and can rapidly predict the phytotoxicity of nicosulfuron in soil to be tested according to the dose-effect curve equation of the nicosulfuron in the soil and the concentration of the in-situ pore water in the soil to be tested.
Owner:ZHEJIANG UNIV
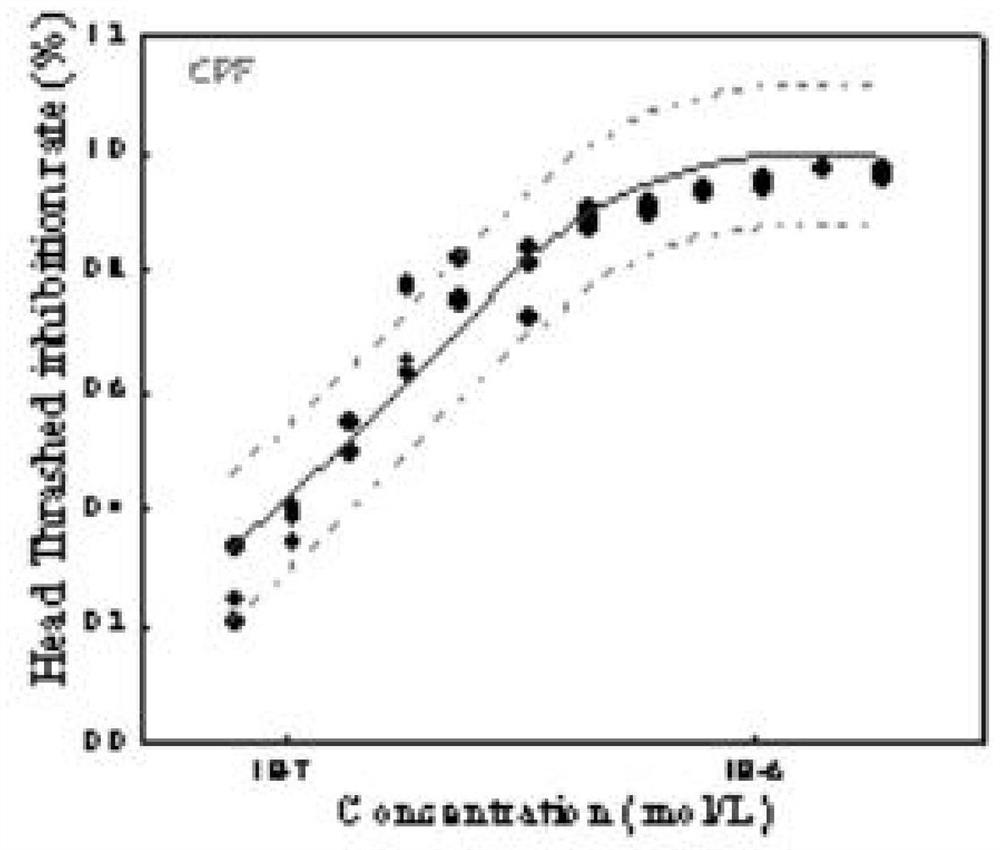
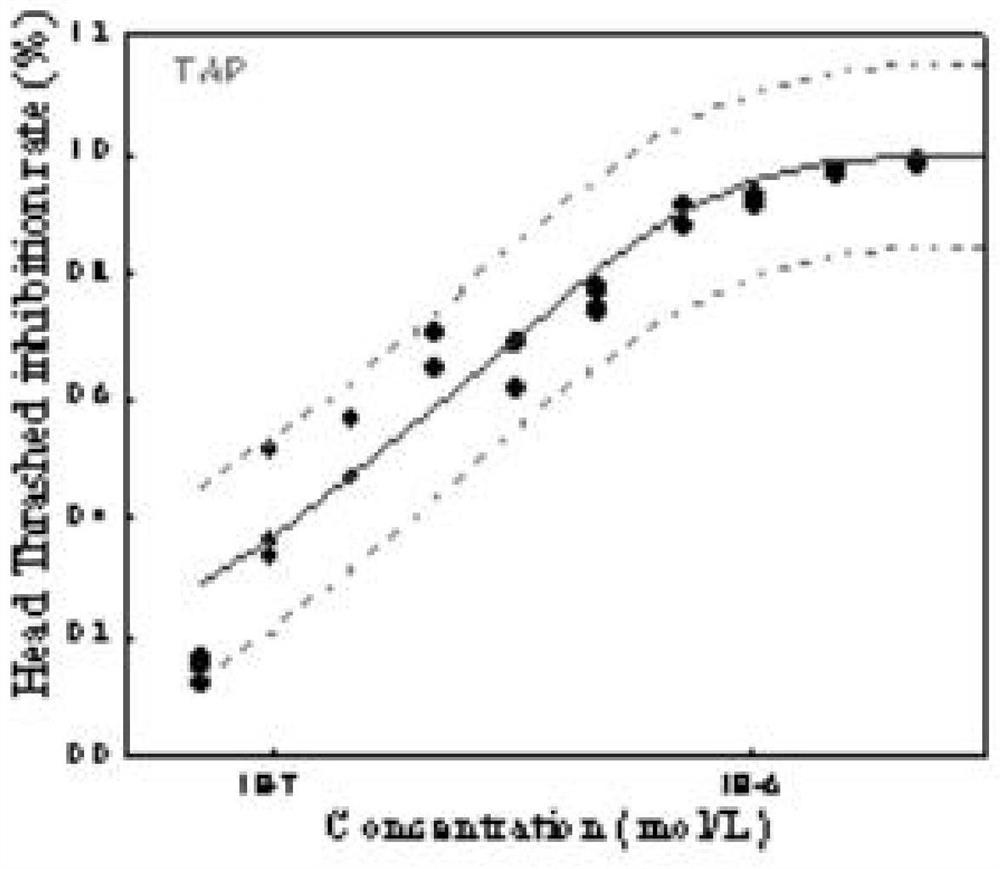
Method for analyzing caenorhabditis elegans head swing inhibition rate of pollutants
The invention discloses a method for analyzing the caenorhabditis elegans head swing inhibition rate of pollutants. A plurality of concentration gradients are set to perform high-flux toxicity exposure on caenorhabditis elegans respectively, toxicity data of a plurality of concentration points are acquired by setting a plurality of parallel groups and repeatedly measuring the toxicity of the samepollutant for multiple times, and a dose-effect curve of a target pollutant is fitted. The method mainly comprises the following steps: preparing a caenorhabditis elegans growth medium; preparing an escherichia coli OP50 culture medium; obtaining a synchronized caenorhabditis elegans stock solution; obtaining environmental pollutant sample solutions with different concentration gradients; carryingout a caenorhabditis elegans toxicity exposure experiment and acquiring toxicity data; and fitting data. According to the method, the neurotoxicity of the environmental pollutants to caenorhabditis elegans can be accurately calculated, and a more scientific theoretical support is provided for evaluating the safety of the environmental pollutants to an ecological system and human health.
Owner:TONGJI UNIV
Detection technology and method for meat containing animal-derived components
InactiveCN106148509AReduce distractionsEasy to handle directlyMicrobiological testing/measurementDNA/RNA fragmentationFluorescencePcr ctpp
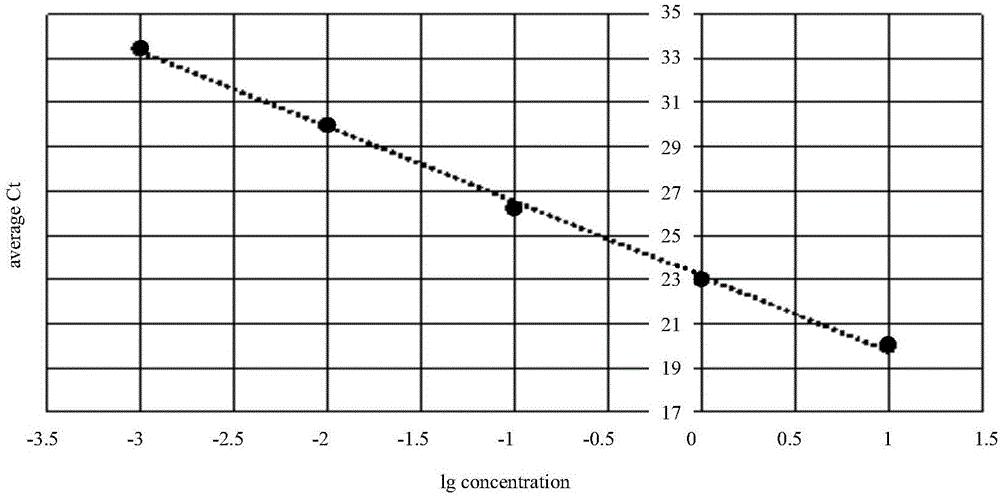
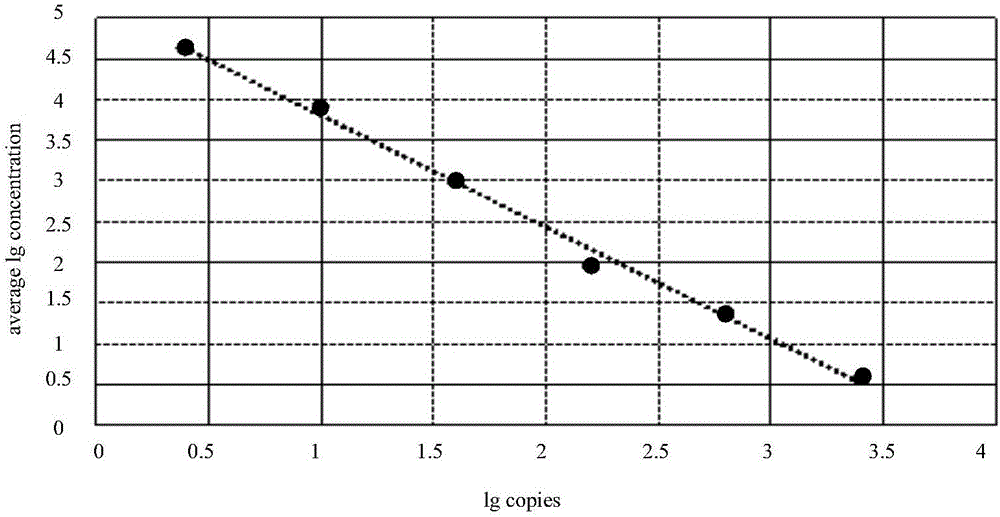
The invention provides a detection technology and method for meat containing animal-derived components. The detection technology and method is based on 3D digital PCR (polymerase chain reaction) technology and fluorescent quantitative PCR technology and comprises the following steps: 1) DNA of a to-be-detected sample is extracted, and a PCR amplification template is obtained; 2) a PCR system is prepared, and 3D digital PCR and fluorescent quantitative PCR amplification are performed, wherein the PCR system comprises the PCR amplification template, a universal primer pair, a fluorescent probe and the like and adopts a dose-effect curve of a pure animal-derived sample standard as the standard curve; 3) the copy number and the percentage composition of the animal-derived components in the to-be-detected sample are calculated according to fluorescence signals produced through the 3D digital PCR. According to the method, a PCR liquid is subjected to smear processing with the 3D digital PCR technology, so that interference by background DNA and a substrate is reduced greatly, and the sensitivity can be as low as one copy; meanwhile, by means of the standard curve, direct processing of follow-up samples is facilitated, and operation steps are simplified greatly.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Method for detecting dose of ionizing radiation on human peripheral blood lymphocytes
InactiveCN103805682AGood linear relationshipGood repeatabilityMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceGene expression level
The present invention relates to a method for detecting a dose of ionizing radiation on human peripheral blood lymphocytes. The method mainly comprises: designing primers and a Taqman-MGB probe according to a lymphocyte pig3 gene expression sequence, and constructing a recombinant vector containing the lymphocyte pig3 gene expression sequence as a standard substance; adopting the 10-fold serial diluted standard substance to carry out real-time fluorescence PCR, and drawing an absolute quantification standard curve; carrying out quantitative determination on the expression levels of the pig3 gene of lymphocytes with different culture times after ionizing radiations with different doses to obtain a dose-effect fitting curve of the radiation dose and the pig3 gene expression level; and carrying out quantitative determination on the expression level of the pig3 gene of lymphocytes with the radiation dose to be detected, and calculating the dose of the ionizing radiation on the lymphocytes requiring detection. According to the present invention, the dose of ionizing radiation on human peripheral blood lymphocytes can be rapidly and quantitatively detected so as to meet requirements of simpleness, rapid quantitation and high throughput, and the advantages can be provided when the large-scale radiation accident occurs.
Owner:NAT INST FOR RADIOLOGICAL PROTECTION & NUCLEAR SAFETY CHINESE CENT FOR DISEASE CONTROL & PREVENTION
Electronic cigarette smoke solution cell toxicity evaluation method based on lactate dehydrogenase (LDH) assaying
ActiveCN103808906AHigh detection sensitivityHigh sensitivityColor/spectral properties measurementsLactate dehydrogenaseMatrix solution
The invention relates to an electronic cigarette smoke solution cell toxicity evaluation method based on lactate dehydrogenase (LDH) assaying. The method comprises the following steps: (1) preparing an experiment reagent; (2) carrying out cell inoculation; (3) contaminating an electronic cigarette smoke solution; (4) assaying LDH; (5) resulting and analyzing. According to the method, aiming at the characteristic that most of electronic cigarette smoke solution samples are high in density, a method that an electronic cigarette contaminated solution is prepared by adopting mass weighing is determined; the sensitivity of detection is improved through optimizing cell inoculating density, selecting a cell lysing solution and optimizing the reaction time of an LDH matrix solution; the best contaminating concentration is determined through optimizing the contaminating concentration of the electronic cigarette smoke solution, so as to obtain the optimal dose-effect curve; through assaying LDH, the degree of damage to cell membranes caused by the electronic cigarette smoke solution can be reflected, so that the cell toxicity mechanism of electronic cigarette is disclosed. In addition, the method further has the advantages of quickness, simplicity and convenience in operation, high sensitivity and stable results.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
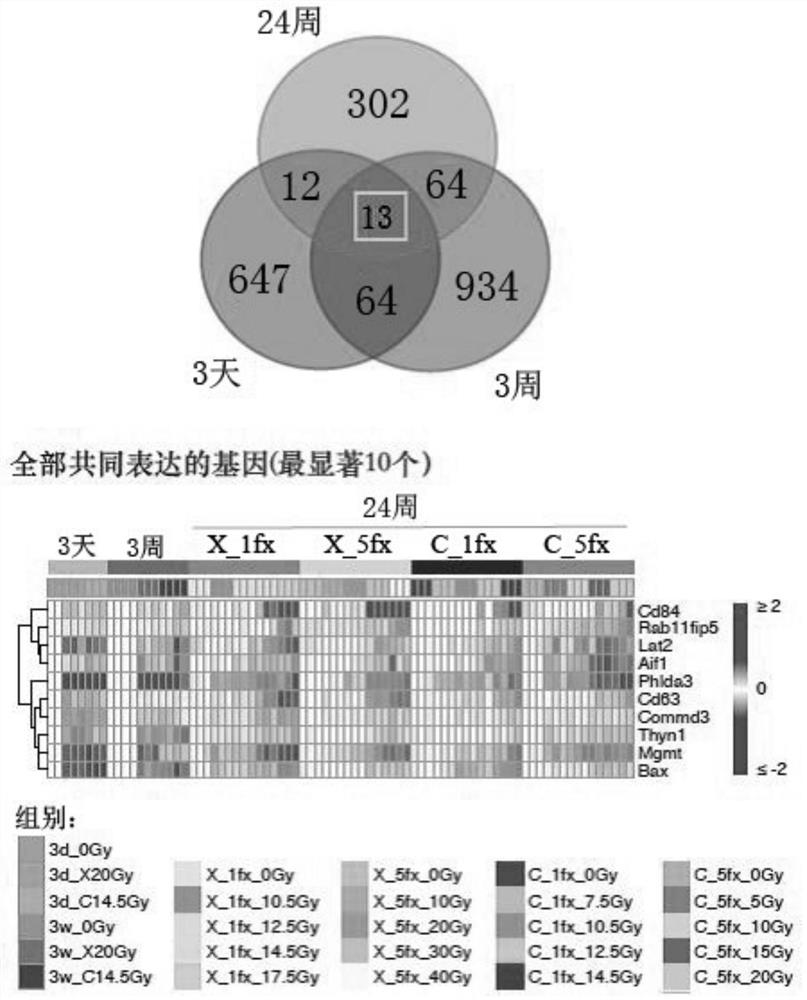
Radiation-sensitive gene markers and their application in different types of radiation dose monitoring
ActiveCN109913542BSensitiveEffectively monitor the level of radiation doseMicrobiological testing/measurementDNA/RNA fragmentationNuclear radiationRadiation exposure
The invention provides a radiation-sensitive gene marker and its application in different types of radiation dose monitoring. The radiosensitive gene markers include Mgmt, Bax, Thyn1 and Phlda3. The dose-response curve constructed based on the expression level of the radiation-sensitive gene marker can effectively determine the range of the radiation dose, so as to effectively monitor the dose of different types of radiation exposure, and then accurately indicate the different types of radiation exposure based on biological effects. Reference dose for nuclear radiation damage assessment.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Radiation Sensitive Gene Markers and Its Application in X-ray Radiation Dose Monitoring
ActiveCN109913543BSensitiveMonitoring Exposure DoseMicrobiological testing/measurementDNA/RNA fragmentationNuclear radiationDownregulated gene
The invention provides a radiation-sensitive gene marker and its application in X-ray radiation dose monitoring. The radiosensitive gene markers include three up-regulated gene markers and two down-regulated gene markers; the up-regulated gene markers include Phlda3, Fgg and Kng1; the down-regulated gene markers include Ptprb and Kti. The dose-response curve constructed based on the expression level of the radiation-sensitive gene marker can effectively determine the range of radiation dose, thereby effectively monitoring the level of X-ray exposure dose, and then accurately prompting the reference dose of X-ray exposure based on biological effects , for nuclear radiation damage assessment.
Owner:ACADEMY OF MILITARY MEDICAL SCI
A method for evaluating proliferation toxicity of cigarette smoke based on bromodeoxyuridine incorporation
ActiveCN103808919BHigh detection sensitivityHigh sensitivityColor/spectral properties measurementsBiological testingGas phaseCell membrane
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Estimation Method of γ-ray Irradiation Dose Based on Transcription Factor IIIA
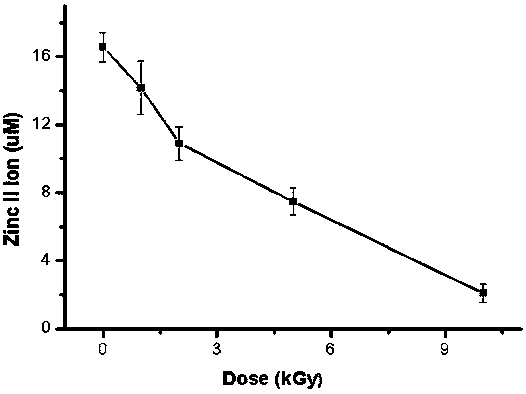
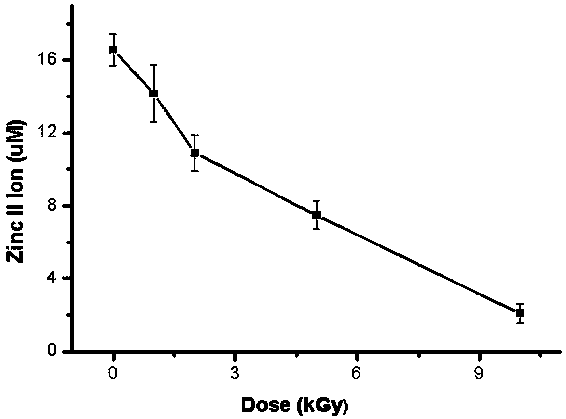
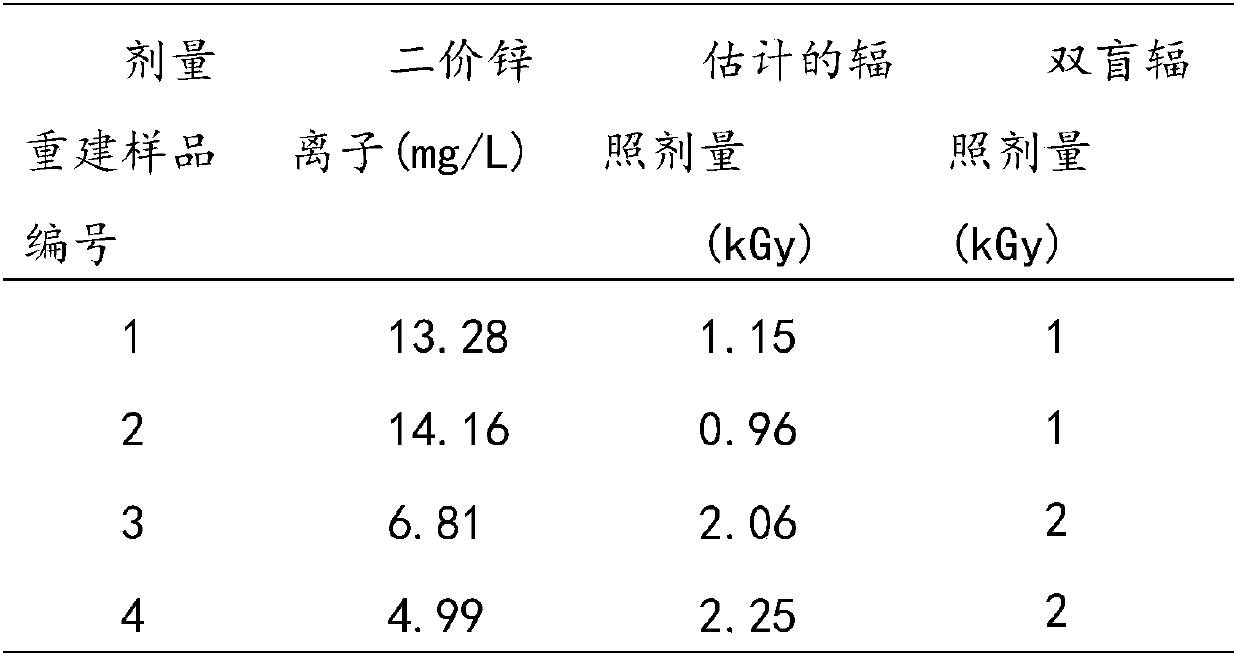
InactiveCN106918833BExpand the scope of detectionExtended dose-response rangeChemical dosimetersDose estimationColorimetry
The embodiment of the invention discloses a [Gamma] ray irradiation dose estimation method based on a transcription factor IIIA, and relates to the nucleonic field. The irradiation dose is scaled through the changing of the transcription factor IIIA divalent zinc ion concentration. The [Gamma] ray irradiation dose estimation method based on the transcription factor IIIA employs the chemical colorimetry to detect the transcription factor IIIA divalent zinc ion concentration of different doses [Gamma] ray irradiation, and a dose-effect curve between the transcription factor IIIA divalent zinc ion concentration and the irradiation dose is established. According to the established dose-effect curve, a double blind method is employed to estimate the [Gamma] ray irradiation dose, the dose estimation range is wide and the operation is simple.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
A method for evaluating the cytotoxicity of e-cigarette liquid based on lactate dehydrogenase assay
ActiveCN103808906BHigh detection sensitivityHigh sensitivityColor/spectral properties measurementsLactate dehydrogenaseMatrix solution
The invention relates to an electronic cigarette smoke solution cell toxicity evaluation method based on lactate dehydrogenase (LDH) assaying. The method comprises the following steps: (1) preparing an experiment reagent; (2) carrying out cell inoculation; (3) contaminating an electronic cigarette smoke solution; (4) assaying LDH; (5) resulting and analyzing. According to the method, aiming at the characteristic that most of electronic cigarette smoke solution samples are high in density, a method that an electronic cigarette contaminated solution is prepared by adopting mass weighing is determined; the sensitivity of detection is improved through optimizing cell inoculating density, selecting a cell lysing solution and optimizing the reaction time of an LDH matrix solution; the best contaminating concentration is determined through optimizing the contaminating concentration of the electronic cigarette smoke solution, so as to obtain the optimal dose-effect curve; through assaying LDH, the degree of damage to cell membranes caused by the electronic cigarette smoke solution can be reflected, so that the cell toxicity mechanism of electronic cigarette is disclosed. In addition, the method further has the advantages of quickness, simplicity and convenience in operation, high sensitivity and stable results.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
A method for determining the dose-effect of traditional Chinese medicine compound prescription based on variable importance projection analysis
ActiveCN105181917BReasonable and accurate analysis resultsGood understanding of analysis resultsComplex mathematical operationsTesting medicinal preparationsAdditive ingredientMedicine
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Method for testing toxicity of residual nicosulfuron on corn
ActiveCN102680594BRapid Prediction of PhytotoxicityEasy to operateComponent separationSoil sciencePhytotoxicity
Owner:ZHEJIANG UNIV
Personal care product acute and chronic dose-effect relationship characterization method
InactiveCN111796086AAvoid the disadvantages of only studying the toxicity of active ingredientsReduce mistakesBiological testingProcess engineeringPersonal Care Product
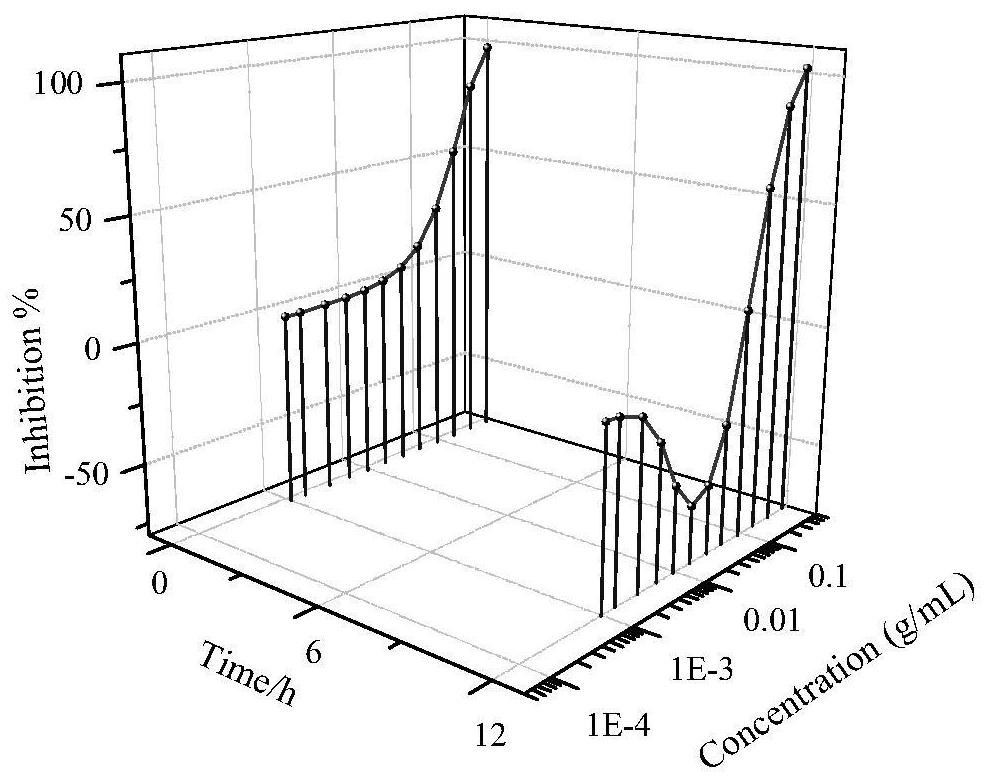
The invention belongs to the field of environmental science and engineering, and discloses a personal care product acute and chronic dose-effect relationship characterization method. The method mainlycomprises the steps of obtaining a dilution factor microplate test; determining S-shaped and J-shaped dose-effect relationship dilution factors; carrying out dose-effect curve fitting; and drawing athree-dimensional dose-effect curve graph. The method disclosed by the invention is beneficial to researching the dose-effect relationship of commodity type actual environmental pollutants with unknown components and unknown concentrations.
Owner:TONGJI UNIV
A test method for e-cigarette liquid external cytotoxicity wst-1
ActiveCN103834716BHigh detection sensitivityHigh sensitivityMicrobiological testing/measurementColor/spectral properties measurementsVolumetric Mass DensityBiology
The invention discloses a WST-1 method for testing in-vitro cytotoxicity of tobacco juice of an electronic cigarette. The method is characterized by comprising the following steps: (1) preparing a laboratory reagent; (2) culturing cells; (3) inoculating cells; (4) contaminating the tobacco juice of the electronic cigarette; (5) dyeing through WST-1; and (6) obtaining a result and analyzing. Compared with the prior art, the method has the characteristics that a method for preparing an electronic cigarette contamination solution by adopting weighing is determined aiming at the characteristic of high density of most electronic cigarette tobacco juice samples, the sensitivity of detection is improved by optimizing the reaction time of the WST-1 dye liquor through an optimization step of a cell inoculation density, an optimal contamination concentration is determined by optimizing an electronic cigarette tobacco juice contamination concentration so that an optimal dosage effect curve can be obtained, and a test step of washing cells a plurality of times is omitted by virtue of WST-1 dyeing; the testing method also has the advantages of faster and simpler operation, higher sensitivity and more stable result.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Caenorhabditis elegans dyskinesia-based microplate toxicity analysis method
PendingCN113834910AEasy to operateAnalysis results are safe and reliableAccessory food factorsMaterial analysisStatistical analysisNonlinear curve fitting
The invention discloses a caenorhabditis elegans dyskinesia-based microplate toxicity analysis method, which is established by taking caenorhabditis elegans as a model organism and introducing a 24-pore plate as a video shooting carrier on the basis of a caenorhabditis elegans lethal microplate toxicity analysis method. The method is characterized by comprising the following steps of: performing pollutant toxicity exposure on caenorhabditis elegans by setting a plurality of concentration gradients in a 96-pore plate by adopting a high-throughput method, then transferring the caenorhabditis elegans into the 24-pore plate to perform caenorhabditis elegans movement behavior disorder video shooting, performing statistical analysis on the video by adopting WormLab software to obtain toxicity data, and finally, carrying out nonlinear curve fitting on the data by adopting a least square method so as to obtain a dose-effect curve of a pollutant to the caenorhabditis elegans dyskinesia. The caenorhabditis elegans dyskinesia-based microplate toxicity analysis method disclosed by the invention can be used for rapidly and accurately acquiring the toxicity data of the pollutant to the caenorhabditis elegans dyskinesia, and is an improvement and an expansion on a traditional caenorhabditis elegans exercise behavior toxicity determination method.
Owner:TONGJI UNIV
Neutral red absorption assay method for e-cigarette liquid cytotoxicity evaluation
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
A kind of biological activity assay method of anti-PD-L1 monoclonal antibody
ActiveCN105717296BQuick evaluationQuick filterMicrobiological testing/measurementMaterial analysisAnti-PD-L1 Monoclonal AntibodyPD-L1
Owner:兆科(广州)肿瘤药物有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com