Patents
Literature
102results about How to "Ensure reproducibility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
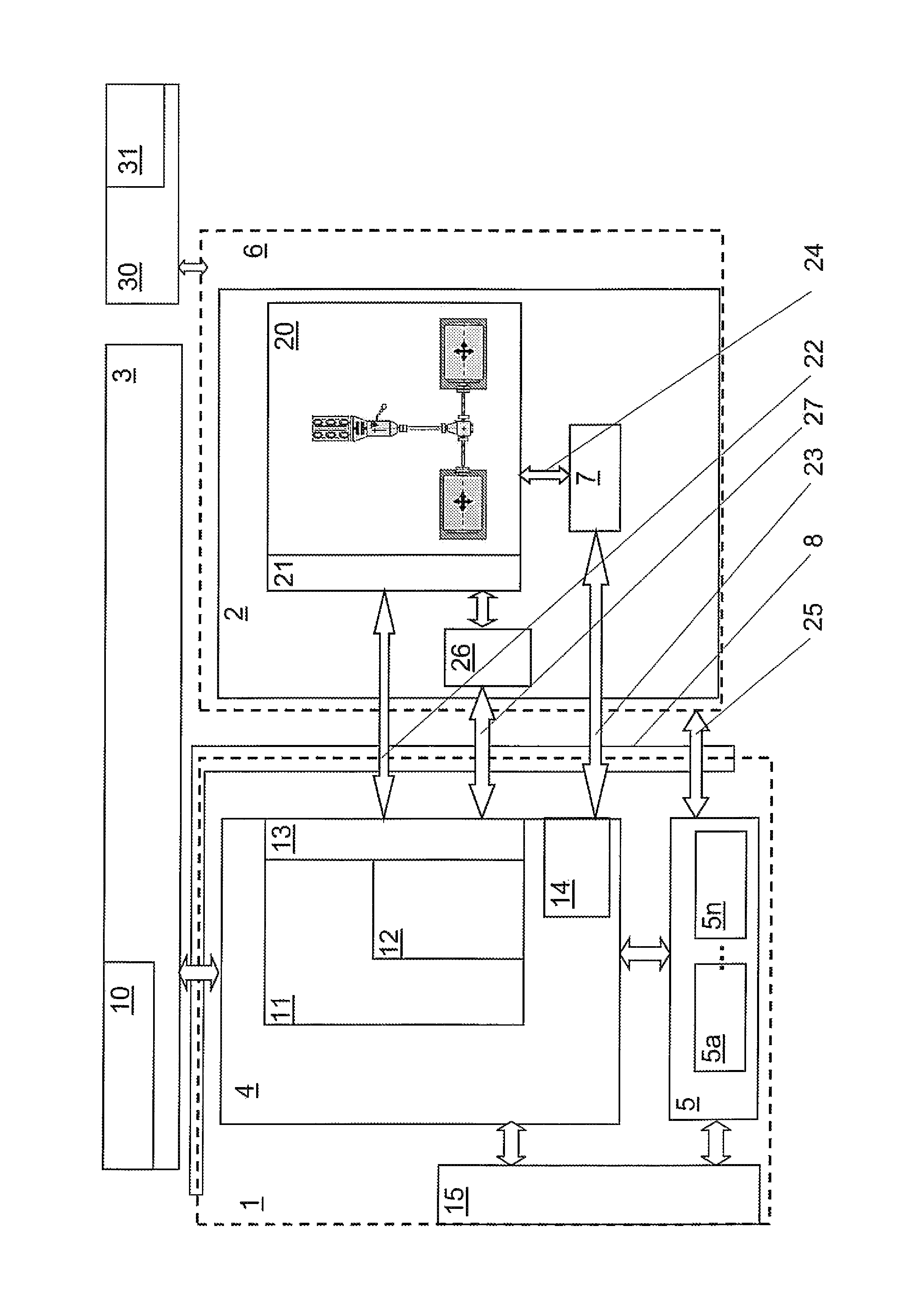
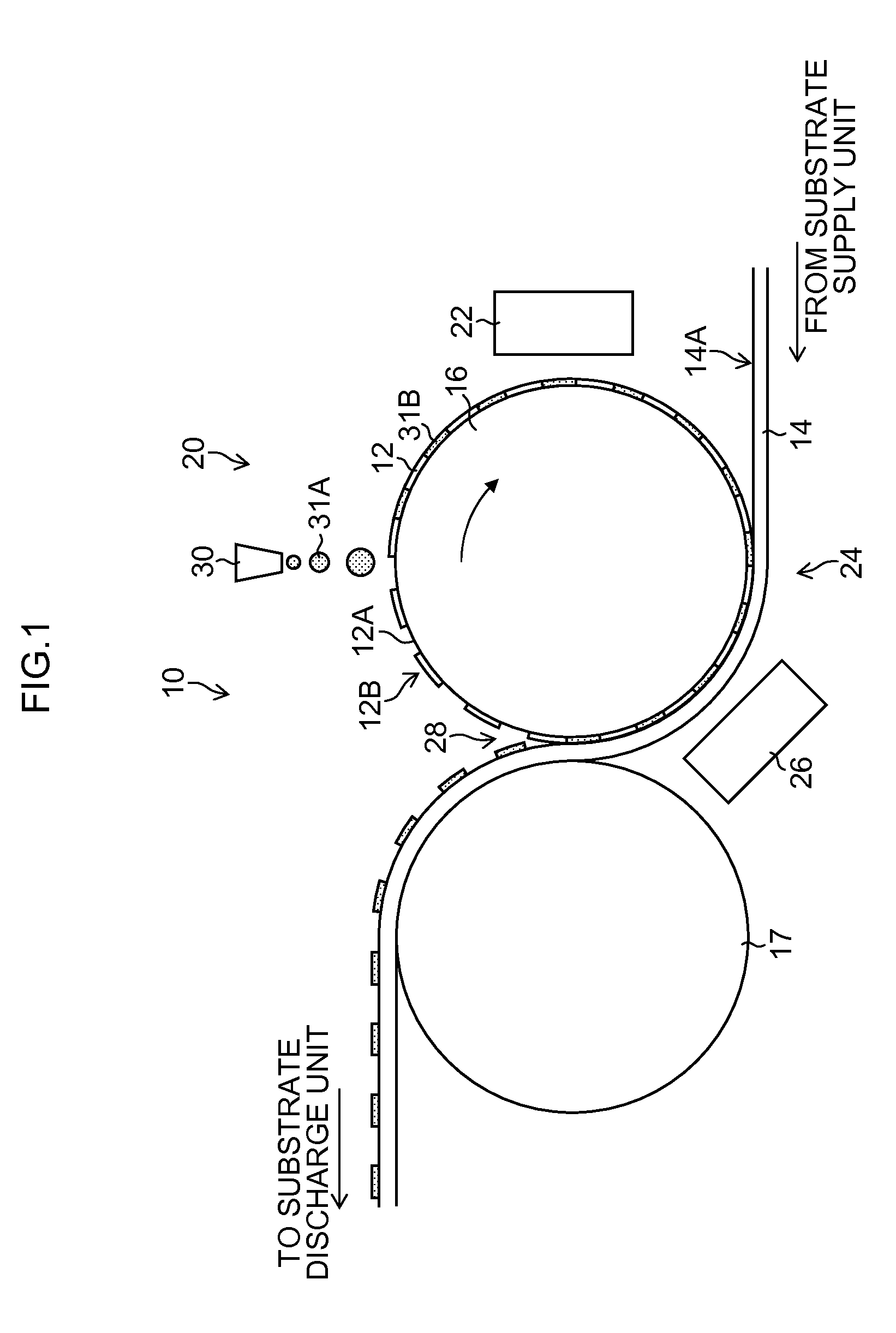
Plasma processing apparatus, plasma processing method, and computer readable storage medium
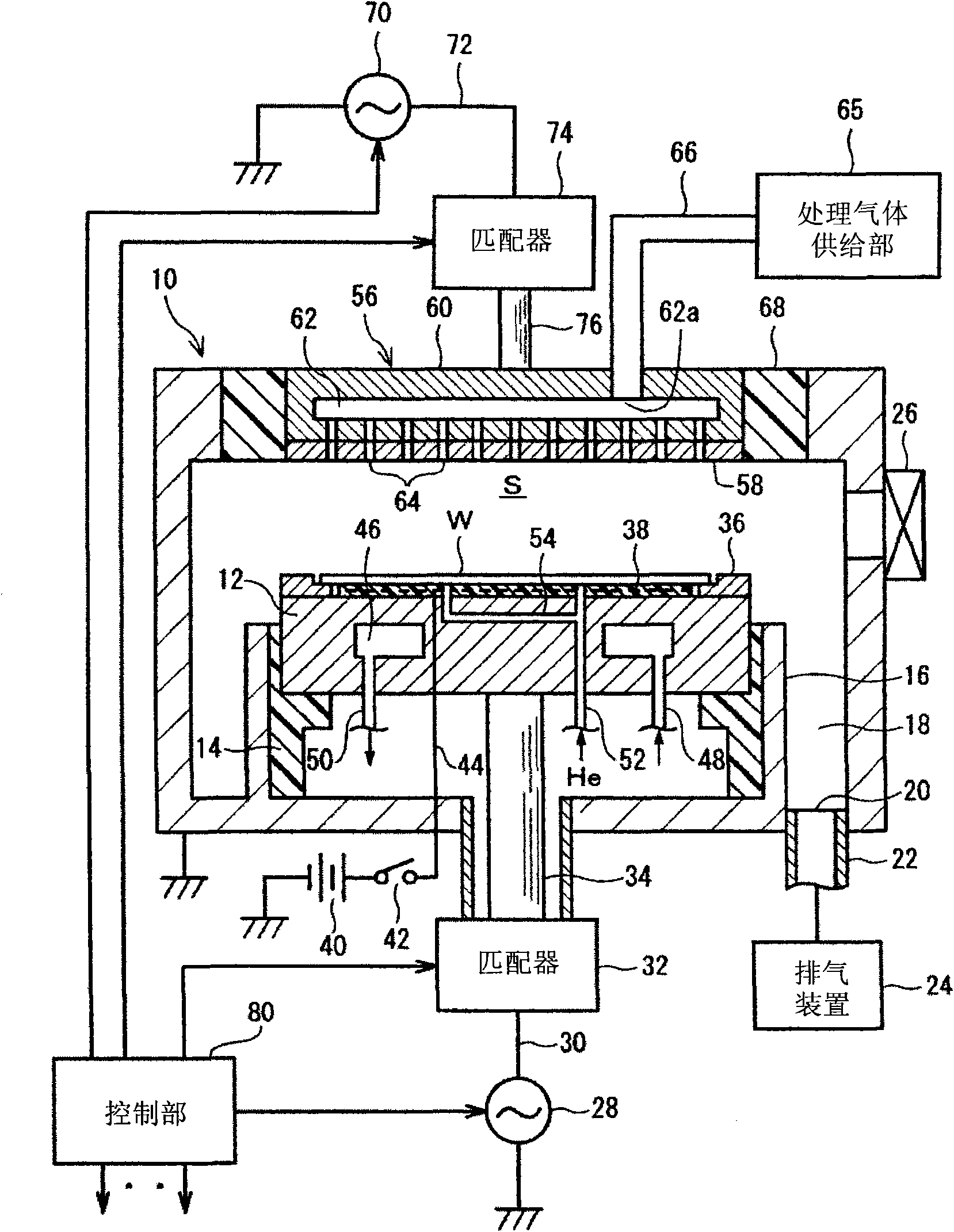
ActiveCN101552187AReduce impedance variationGuaranteed security protectionElectric discharge tubesSemiconductor/solid-state device manufacturingPlasma impedanceHigh frequency power
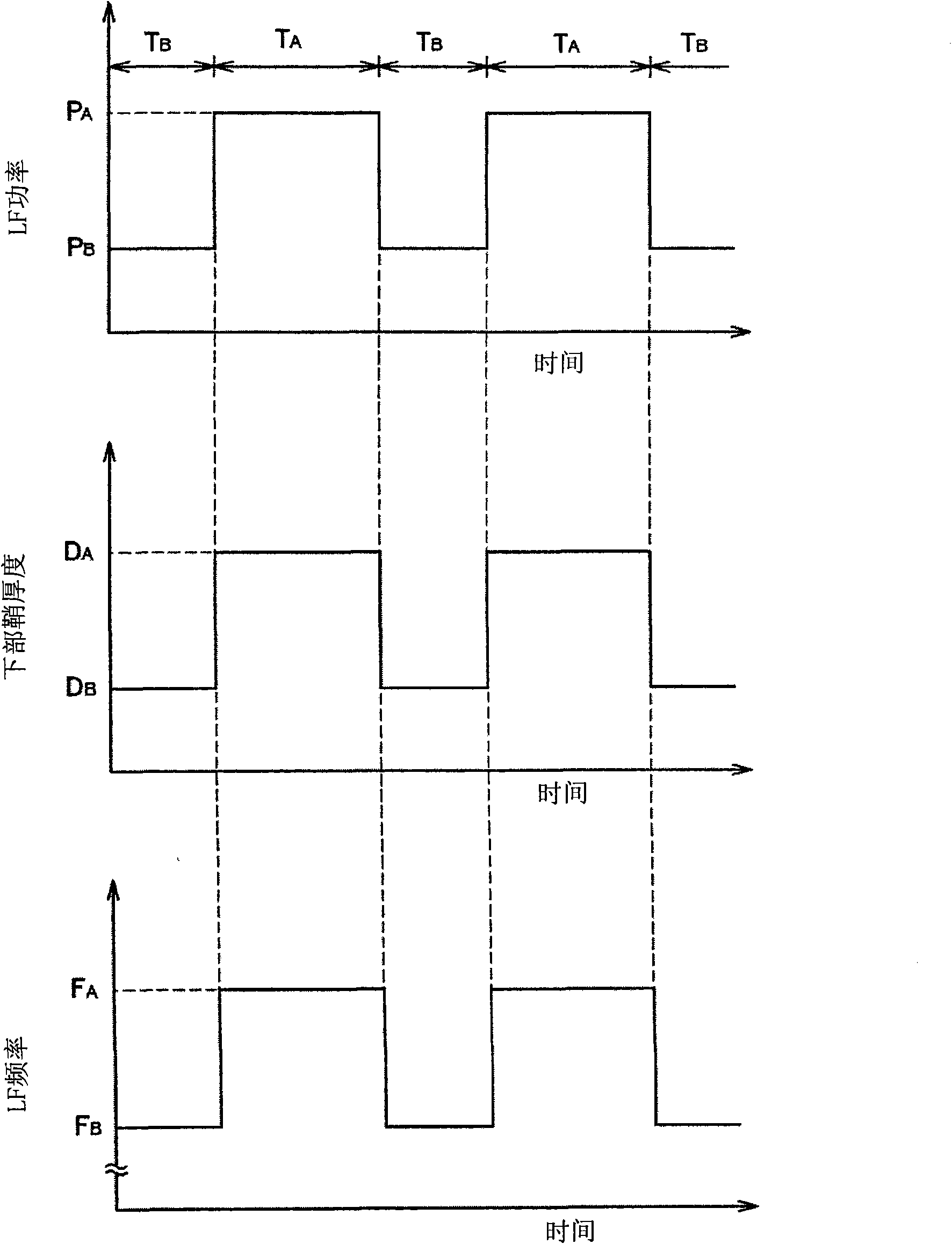
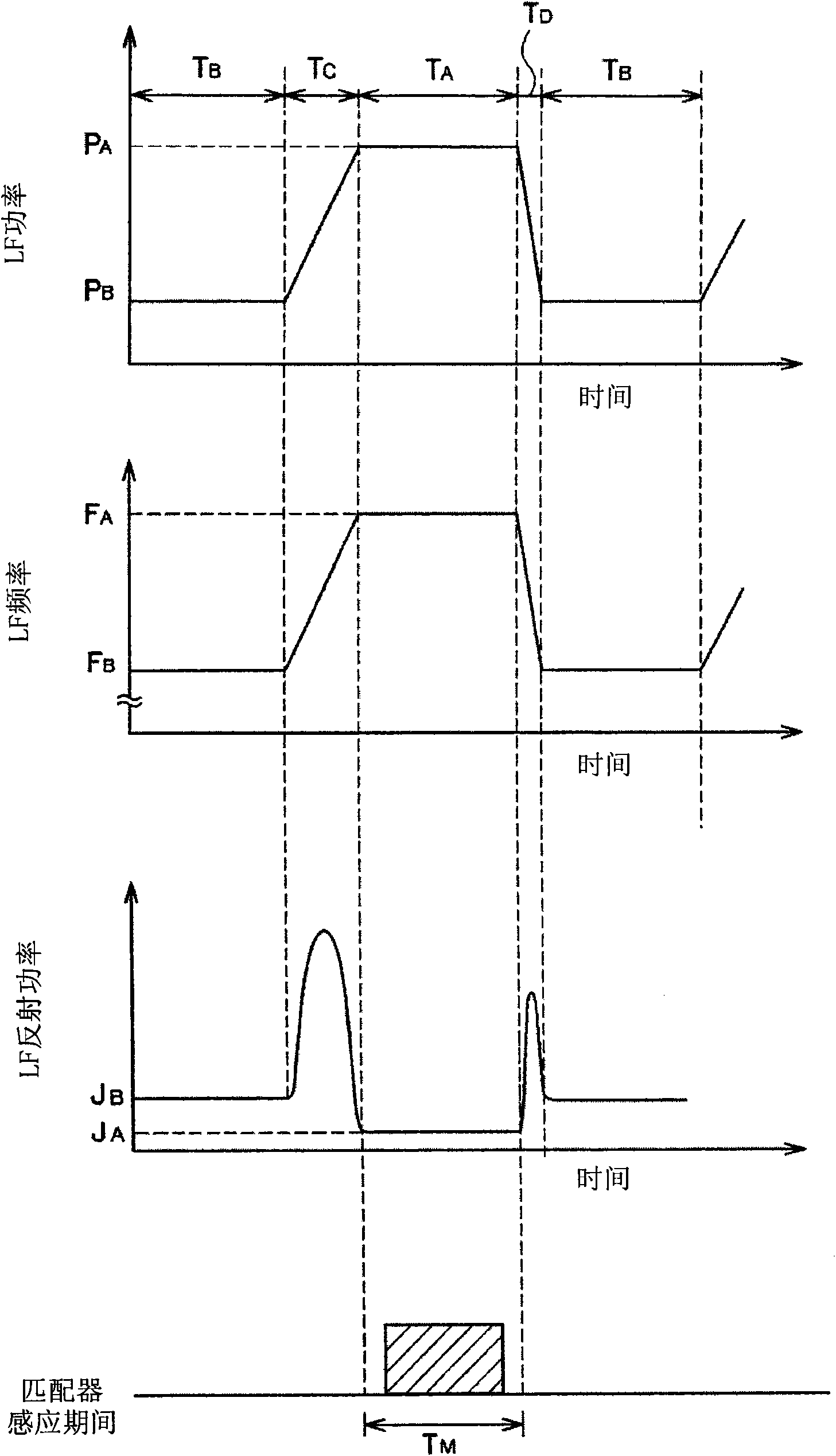
The invention provides a plasma processing device and a plasma processing method. In the manner of periodically modulating the power of the high frequency used in the plasma processing, variation of plasma impedance and reflection towards the high frequency power supply are reduced as much as possible, so as to ensure the stability and reproducibility of the plasma and the safety of the high frequency power supply. In the plasma processing device, frequency modulation is carried out on the power of the high frequency (LF) for controlling bias voltage by the characteristics corresponding to the processing, and pulse modulation is synchronously carried out on the frequency (LF frequency) thereof together with the pulse modulation of the LF power, namely, the LF frequency and the LF power have the following synchronous relationship in a period: in the time interval of the period T when the LF power is maintained as a set value P of H level, the LF frequency is also maintained as the set value F of H level, and in the time interval of the period when the LF power is maintained as a set value P of L level, the LF frequency is also maintained as the set value F of L level.
Owner:TOKYO ELECTRON LTD
Method of preparing clubbed nano-cellulose
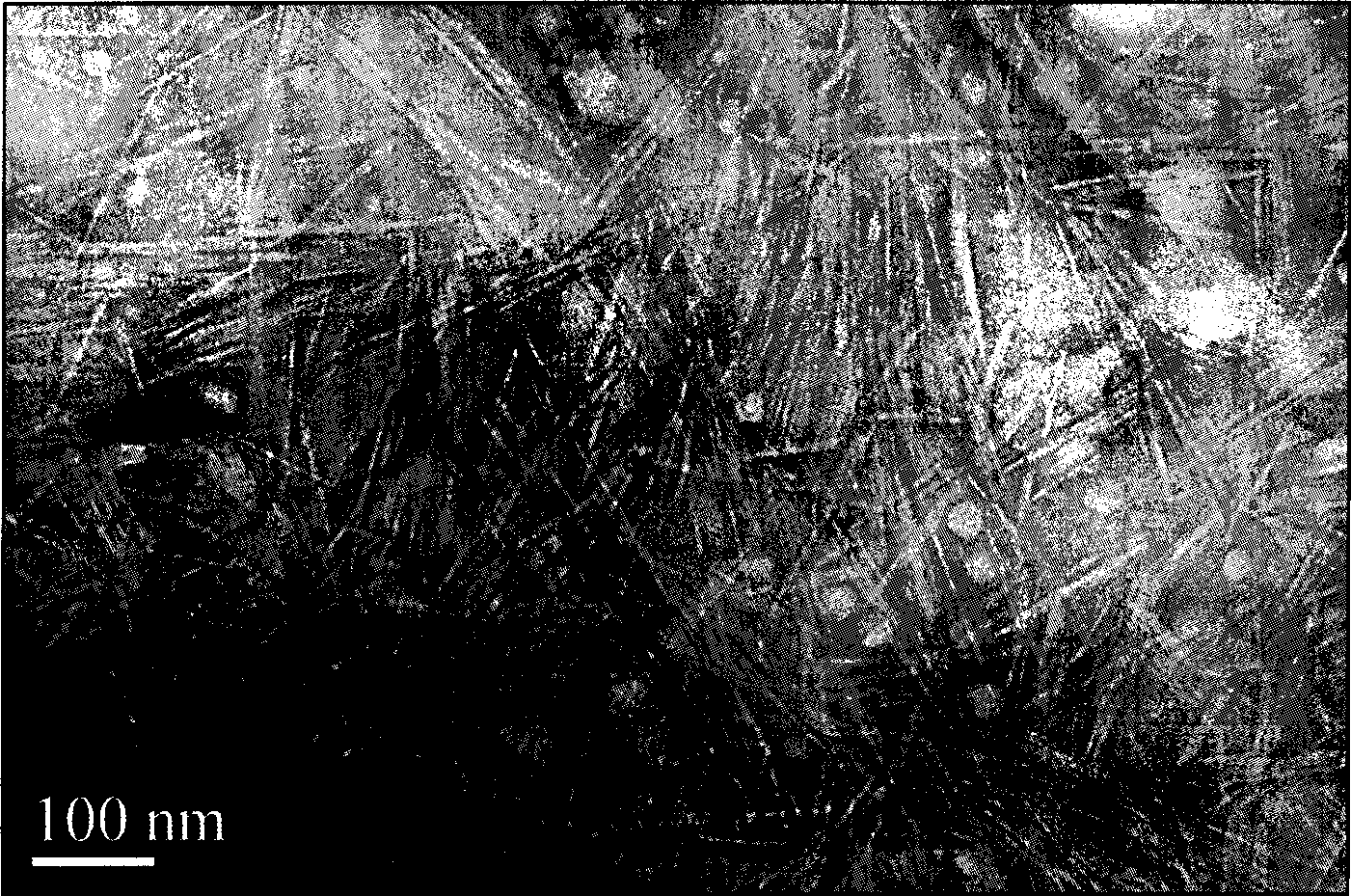
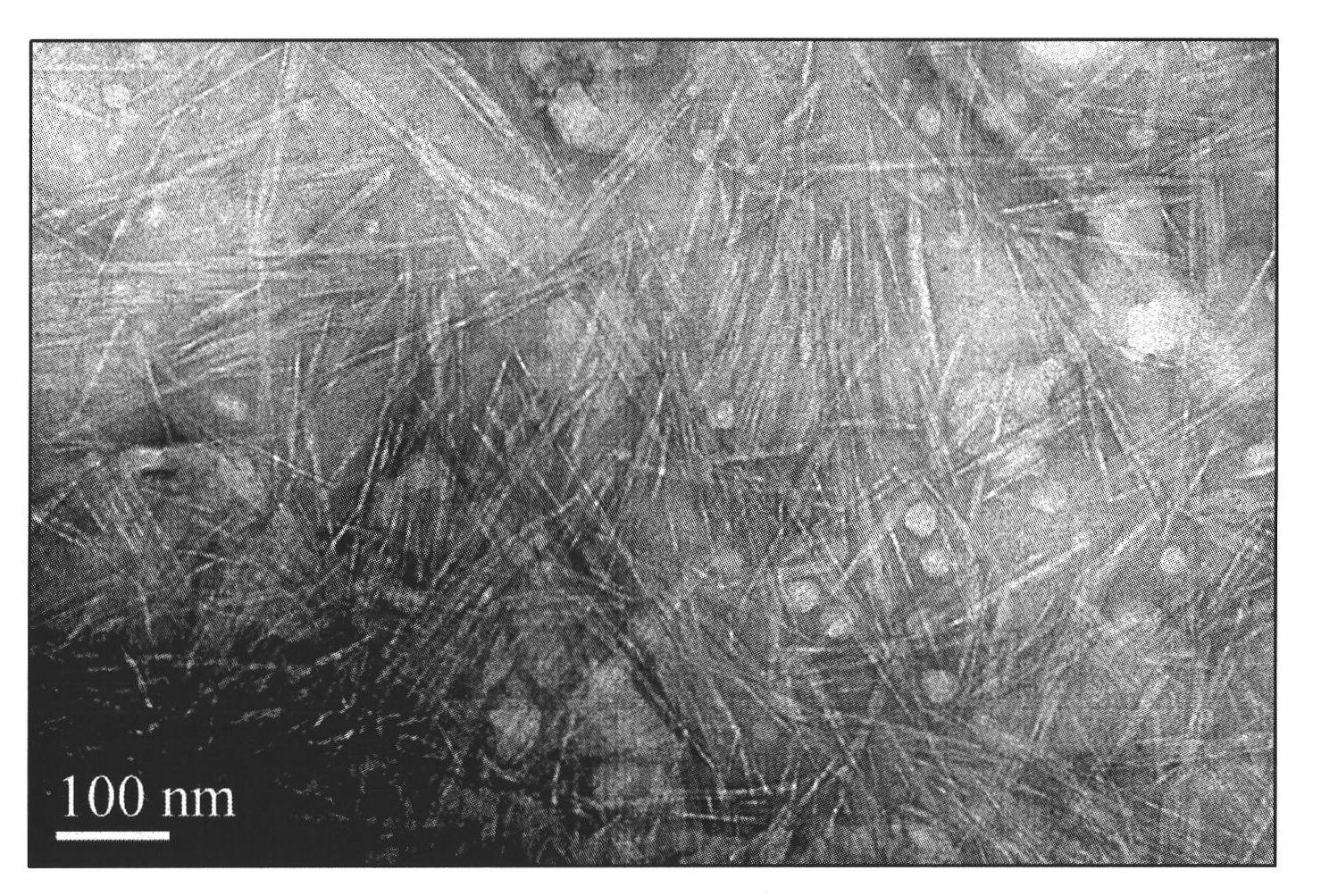
The invention discloses a method for preparing nano-rod cellulose, which comprises the following steps: dispersing fiber raw material into sulphuric acid water solution of which mass concentration is 50 to 65 percent, hydrolyzing the fiber raw material by single-mode microwave radiation for 5 to 60 minutes at a temperature of between 20 and 50 DEG C and at the microwave radiation power of 10 to 100 watts, then adding distilled water into hydrolysis solution and diluting the hydrolysis solution to have 10 times of volume, and obtaining stable nano-rod cellulose suspension through post treatment processes such as centrifugal separation, dialysis, filter, ultrasonic dispersion and the like of the diluent, so as to obtain the nano-rod cellulose of which width is about 10 nanometers and length is about 200 to 300 nanometers.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
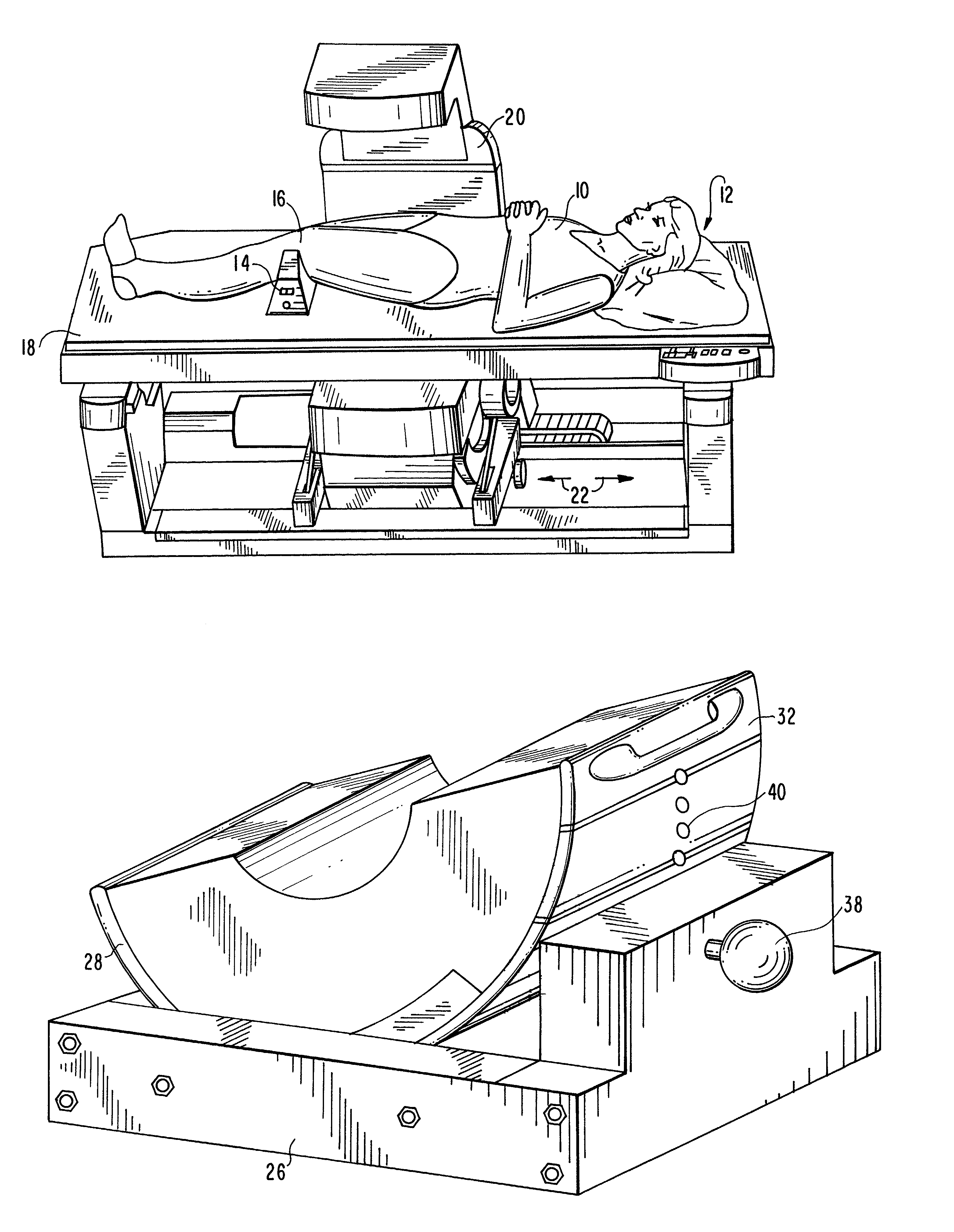
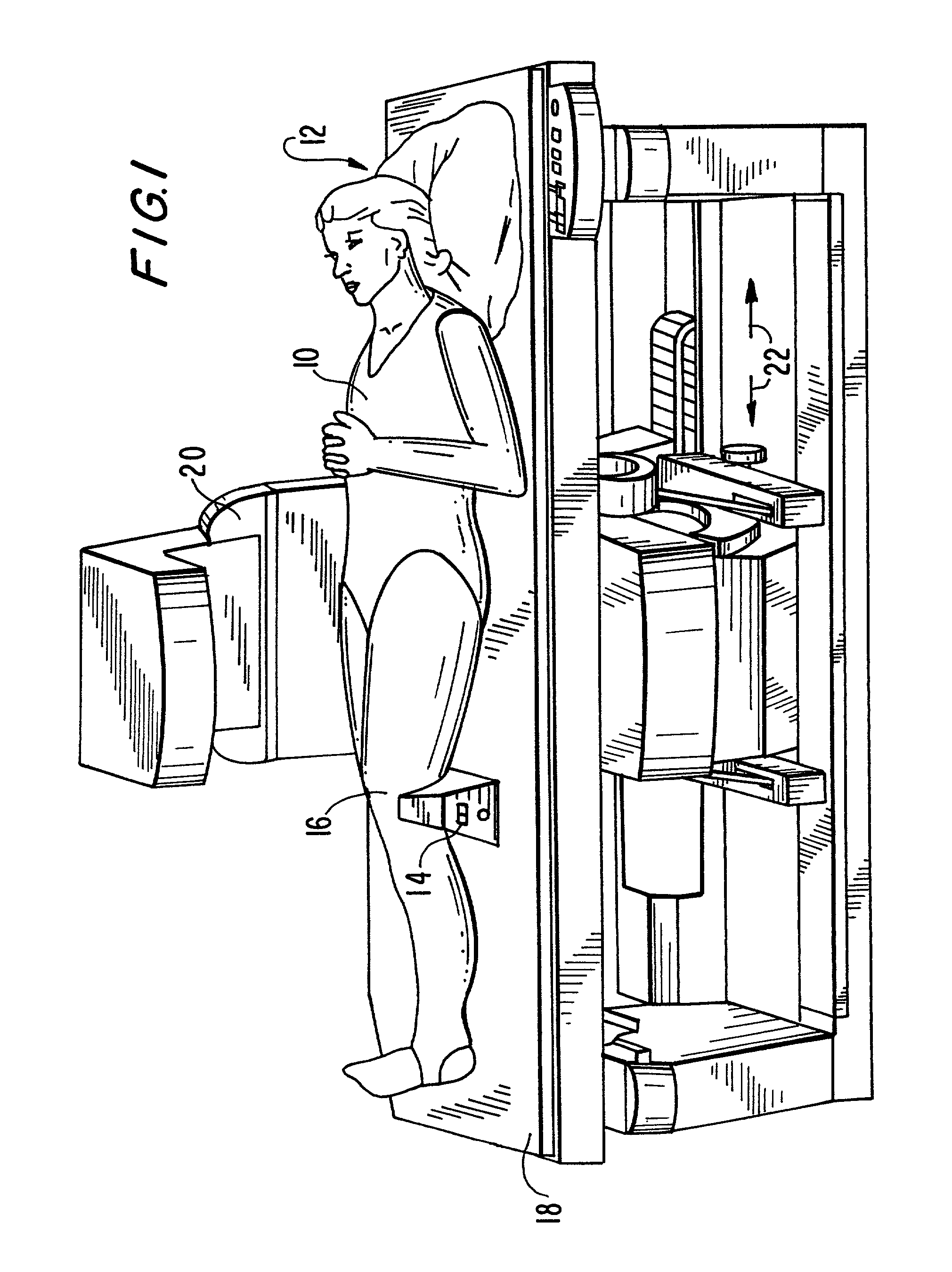
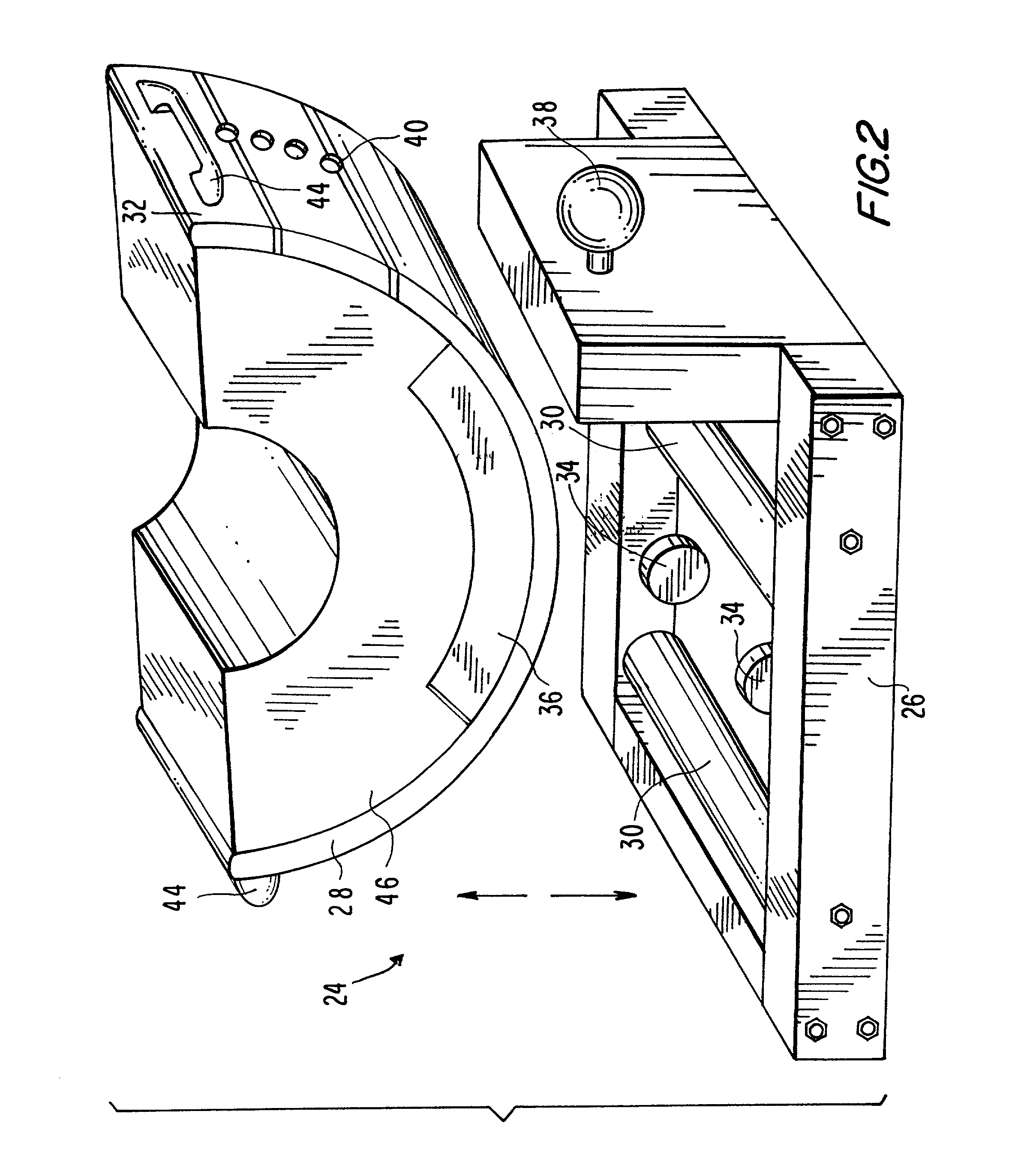
Device for the calibration and standardization of hip rotation
InactiveUS7103930B1Calibrating and standardizing hip rotationCalibrating and standardizing and movementDiagnostic recording/measuringSensorsHip rotationBone density
The present invention concerns a device for the calibration and standardization of the rotation and movement of a patient's hip. More particularly the present invention relates to a device for the calibration and standardization of the rotation and movement of the hip for use in connection with improving the reproducibility and accuracy of a diagnostic procedure such as, for example, bone density measurement using dual x-ray absorptiometry (DXA).
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Bismuth molybdate-based catalysts, method of preparing thereof and method of preparing 1,3-butadiene using thereof
ActiveUS8003840B2High activitySimple constituents and synthesis routesHydrocarbon by hydrogenationDistillation purification/separationDehydrogenationImpurity
This invention relates to a bismuth molybdate catalyst, a preparation method thereof, and a method of preparing 1,3-butadiene using the same, and to a bismuth molybdate catalyst, a preparation method thereof, and a method of preparing 1,3-butadiene using the same, in which 1,3-butadiene can be prepared through oxidative dehydrogenation directly using a C4 mixture including n-butene and n-butane as a reactant in the presence of a mixed-phase bismuth molybdate catalyst including α-bismuth molybdate (Bi2Mo3On) and γ-bismuth molybdate (Bi2MoO6). According to this invention, the C4 raffinate, containing many impurities, is used as a reactant, without an additional n-butane separation process, thus obtaining 1,3-butadiene at high yield. Unlike complicated multicomponent-based metal oxides, the catalyst of the invention has simple constituents and synthesis routes, and can be easily formed through physical mixing, and thus is very advantageous in assuring reproducibility and can be directly applied to commercial processes.
Owner:SK GEO CENTRIC CO LTD +1
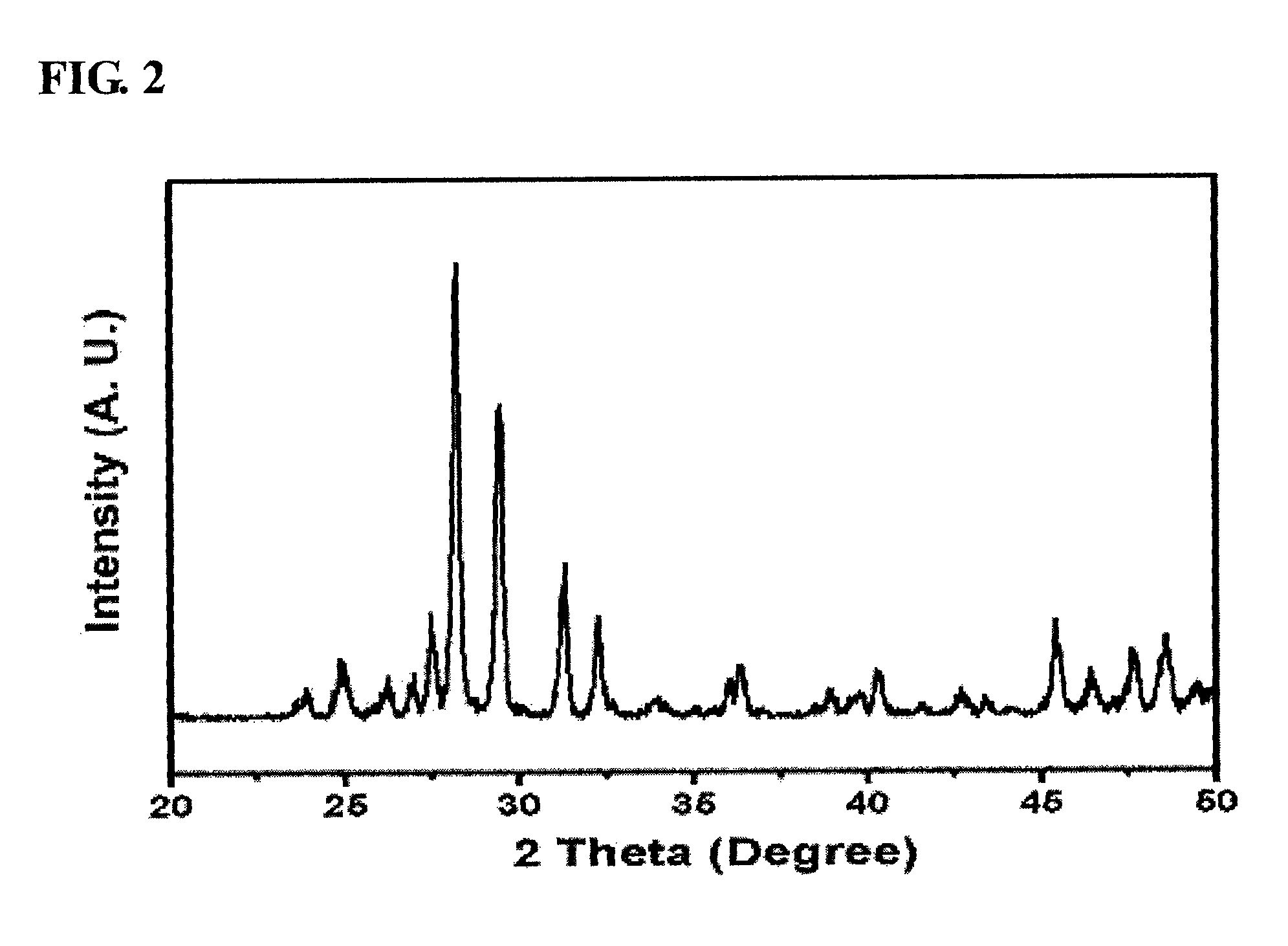
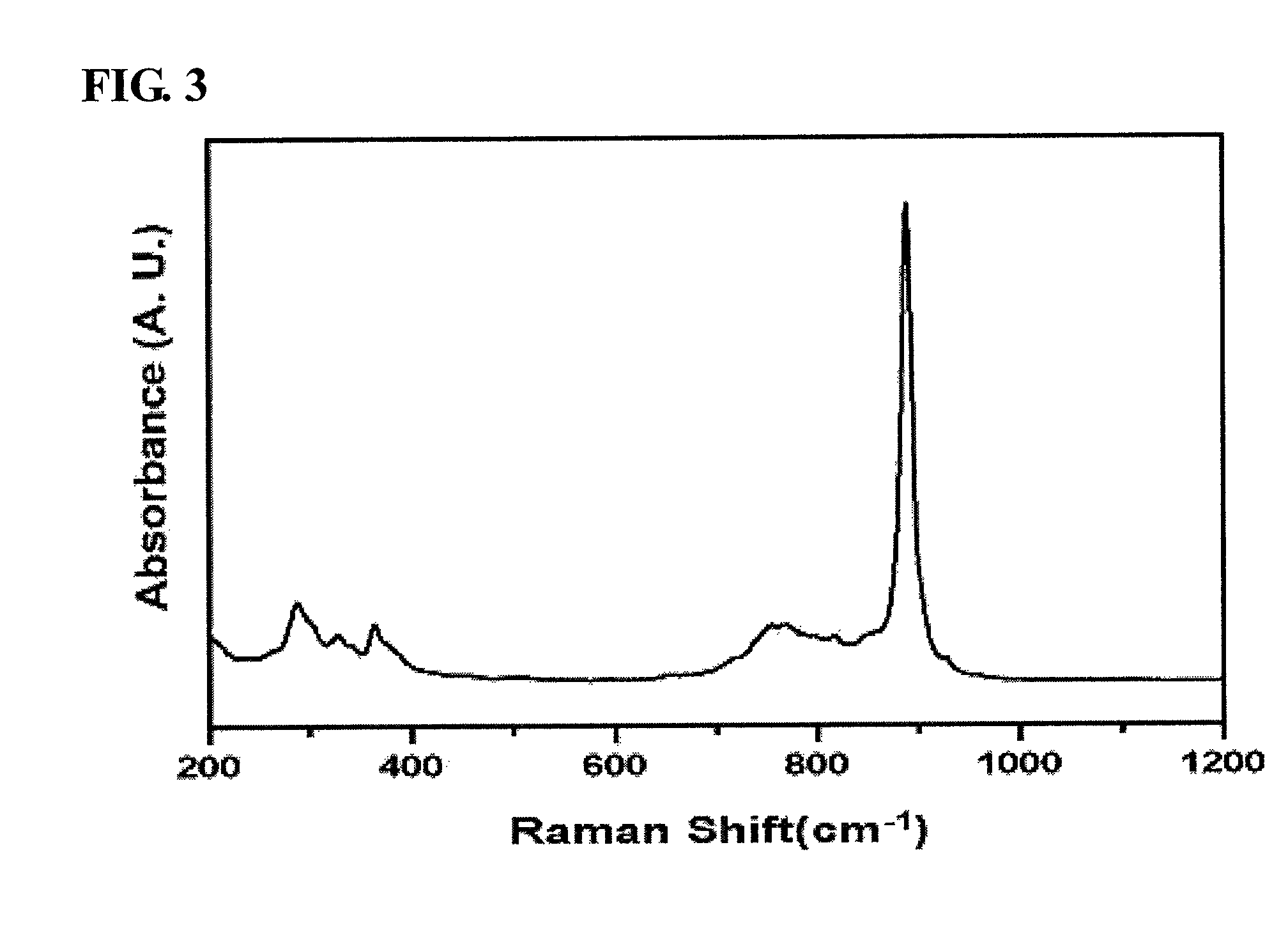
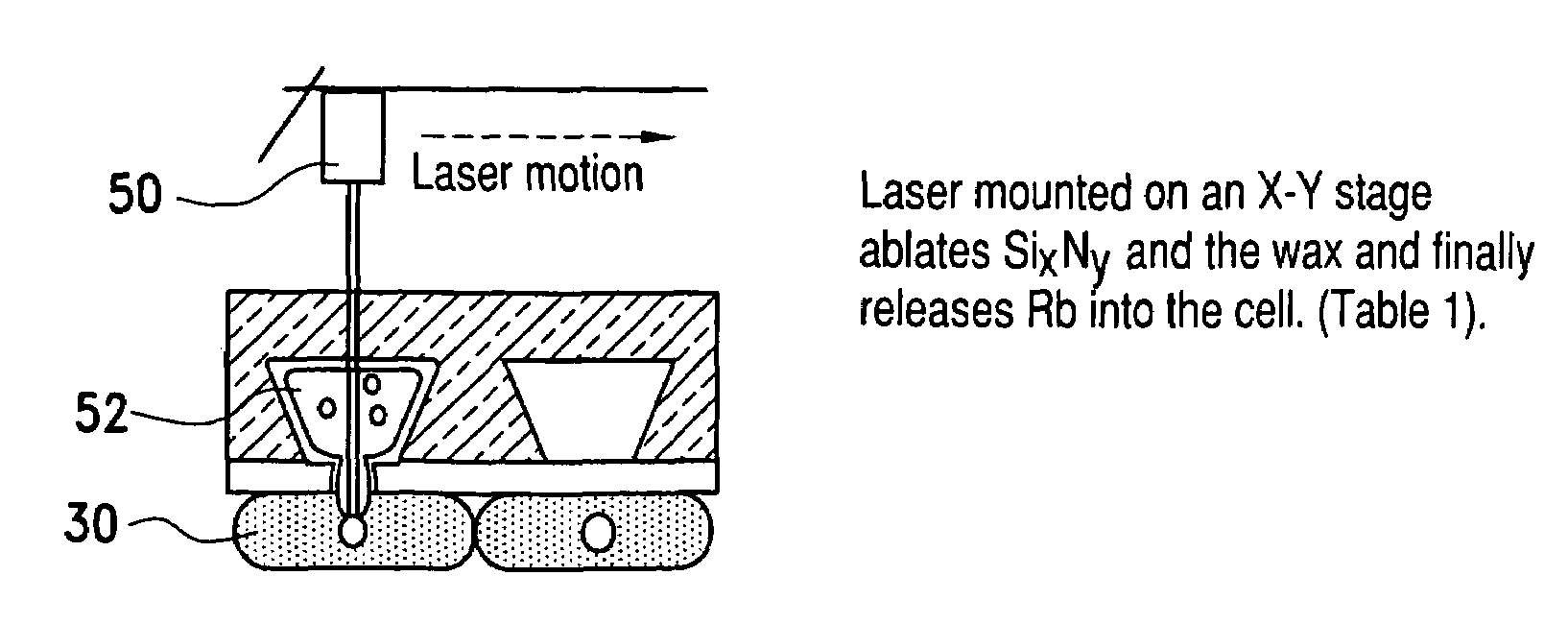
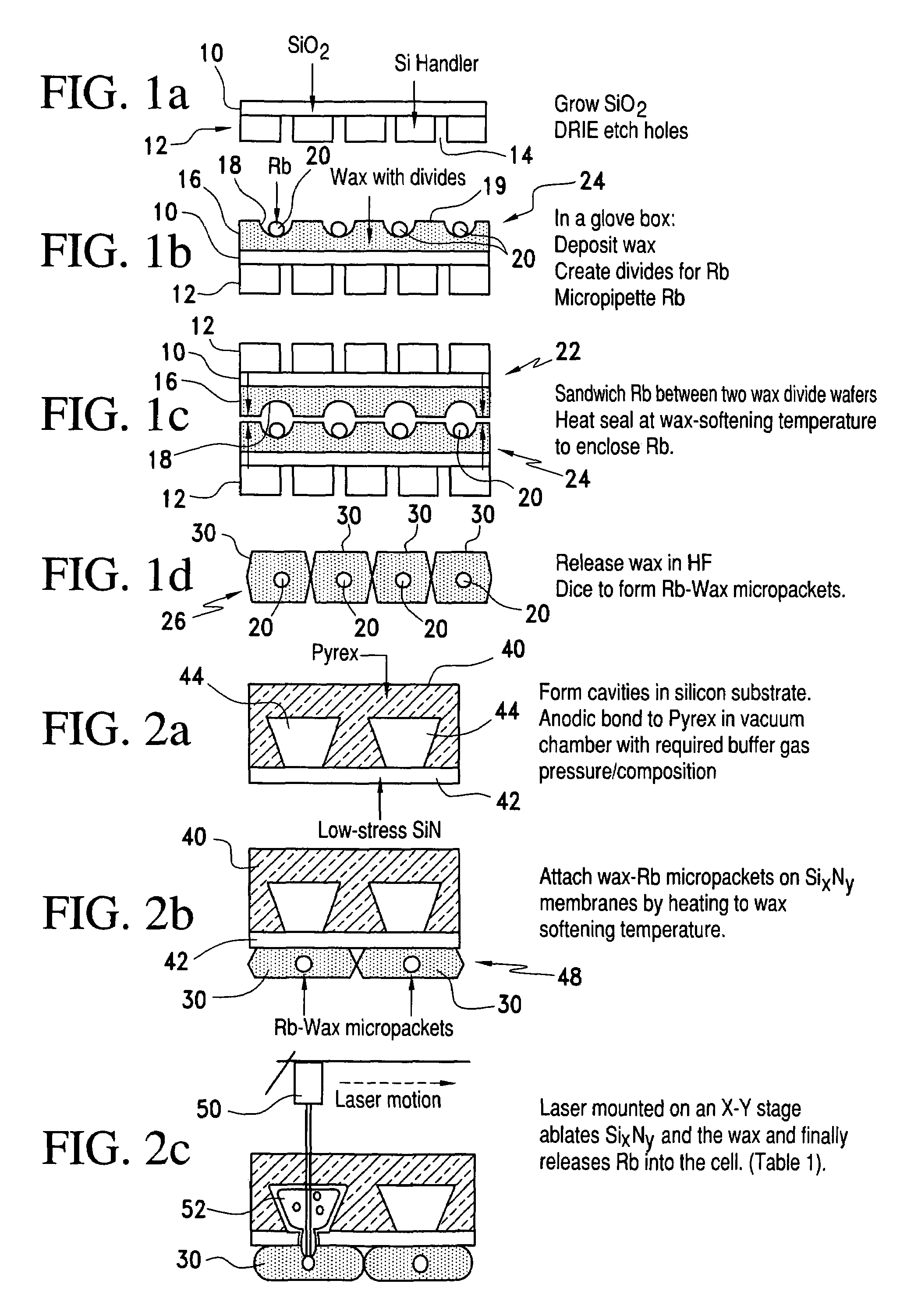
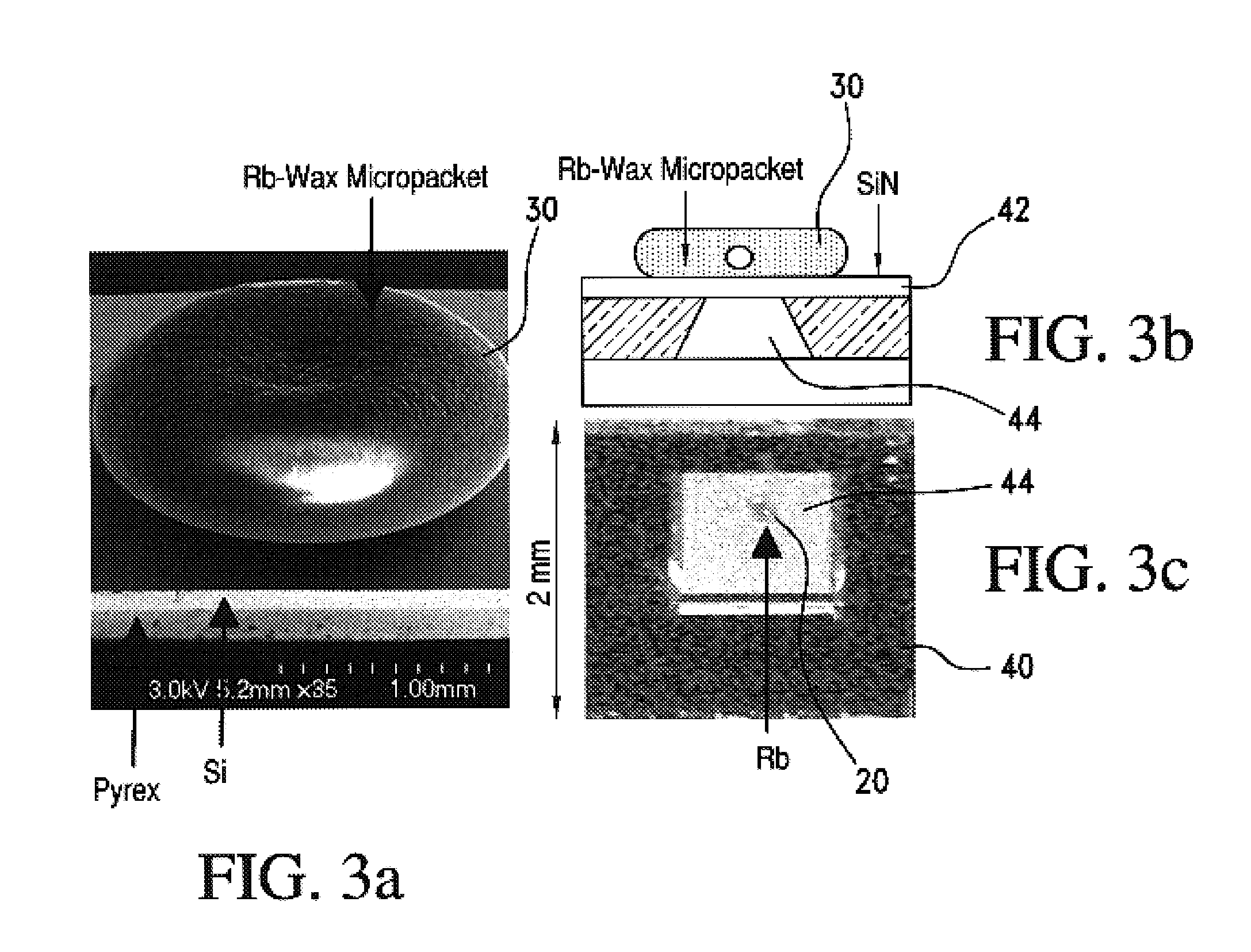
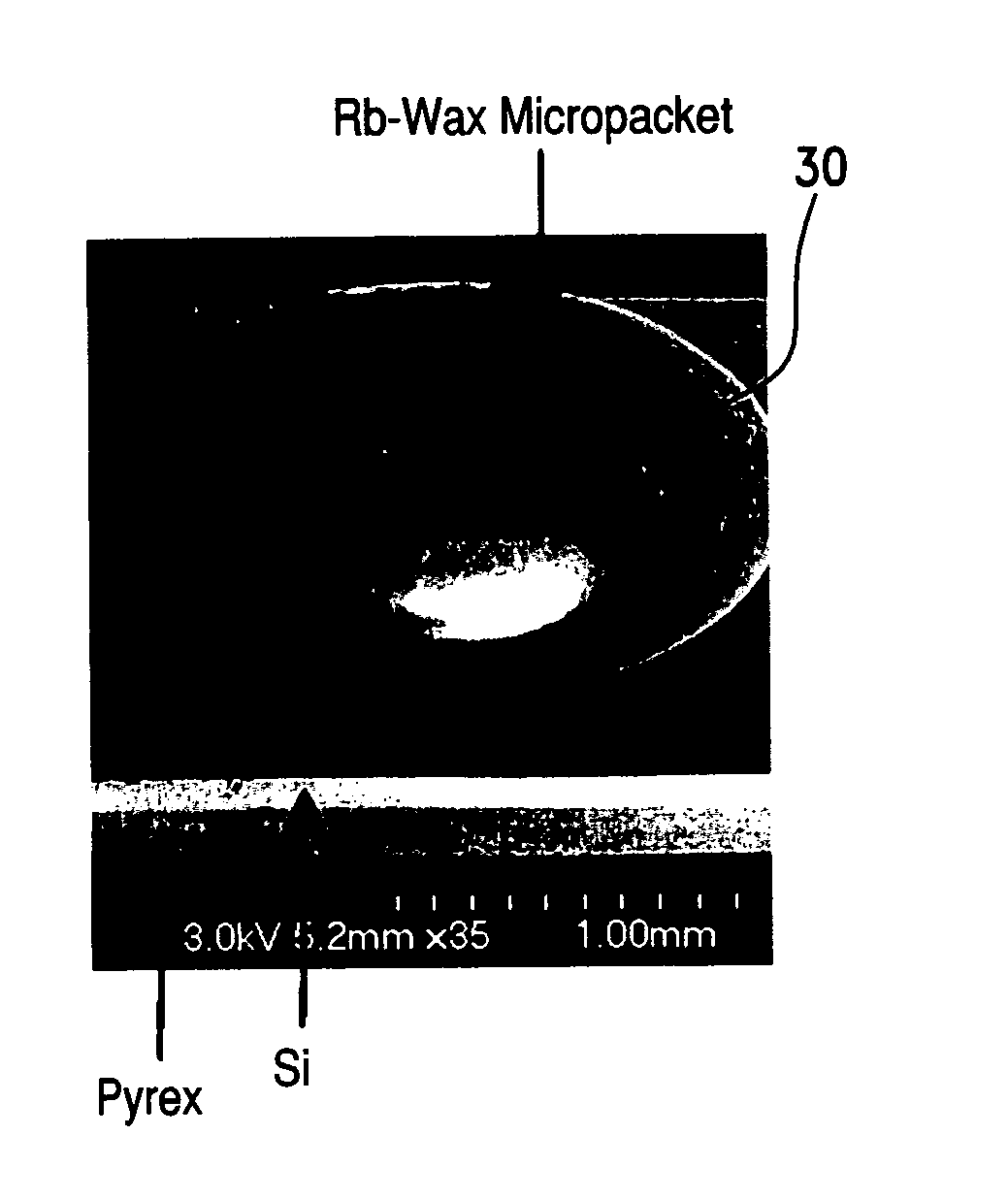
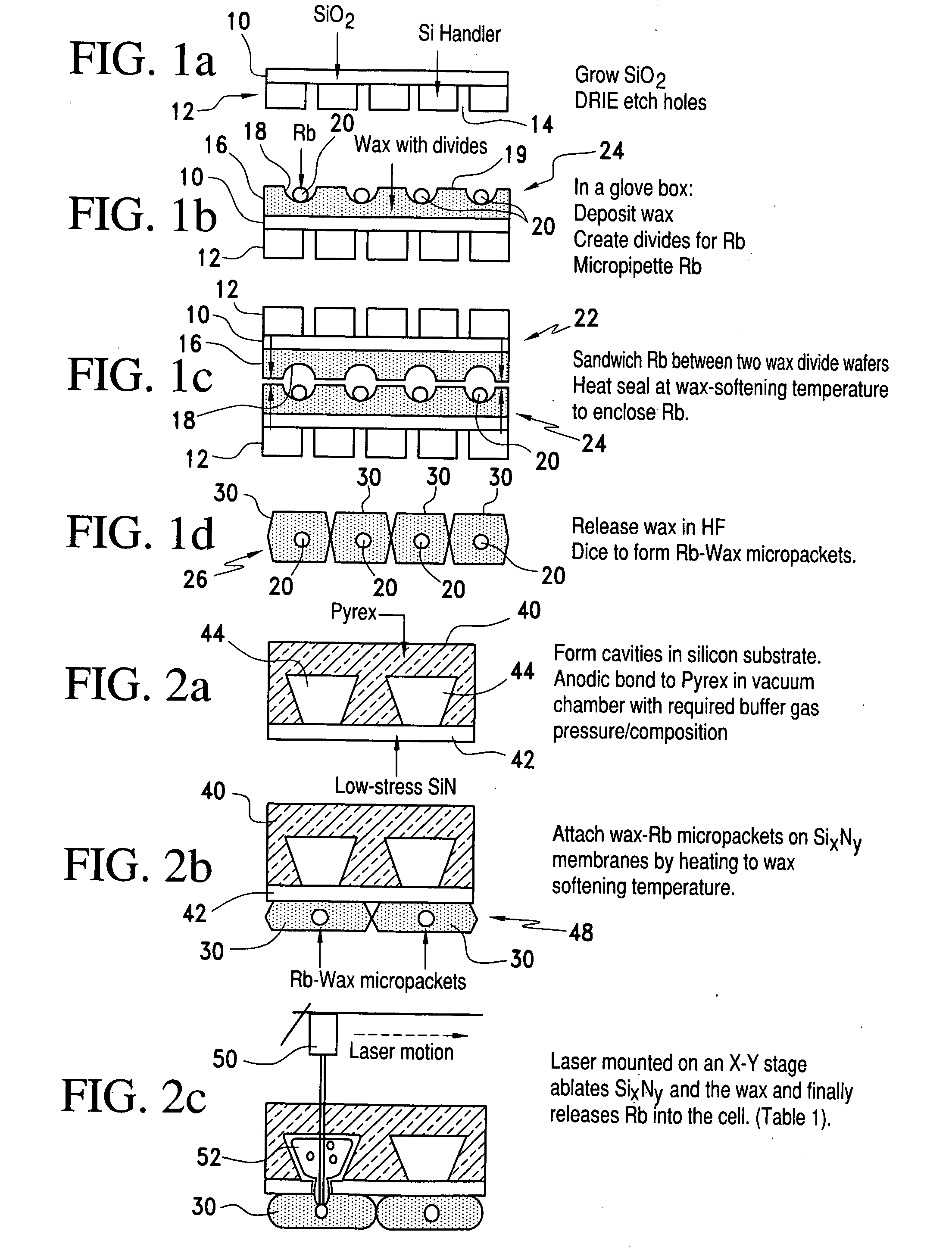
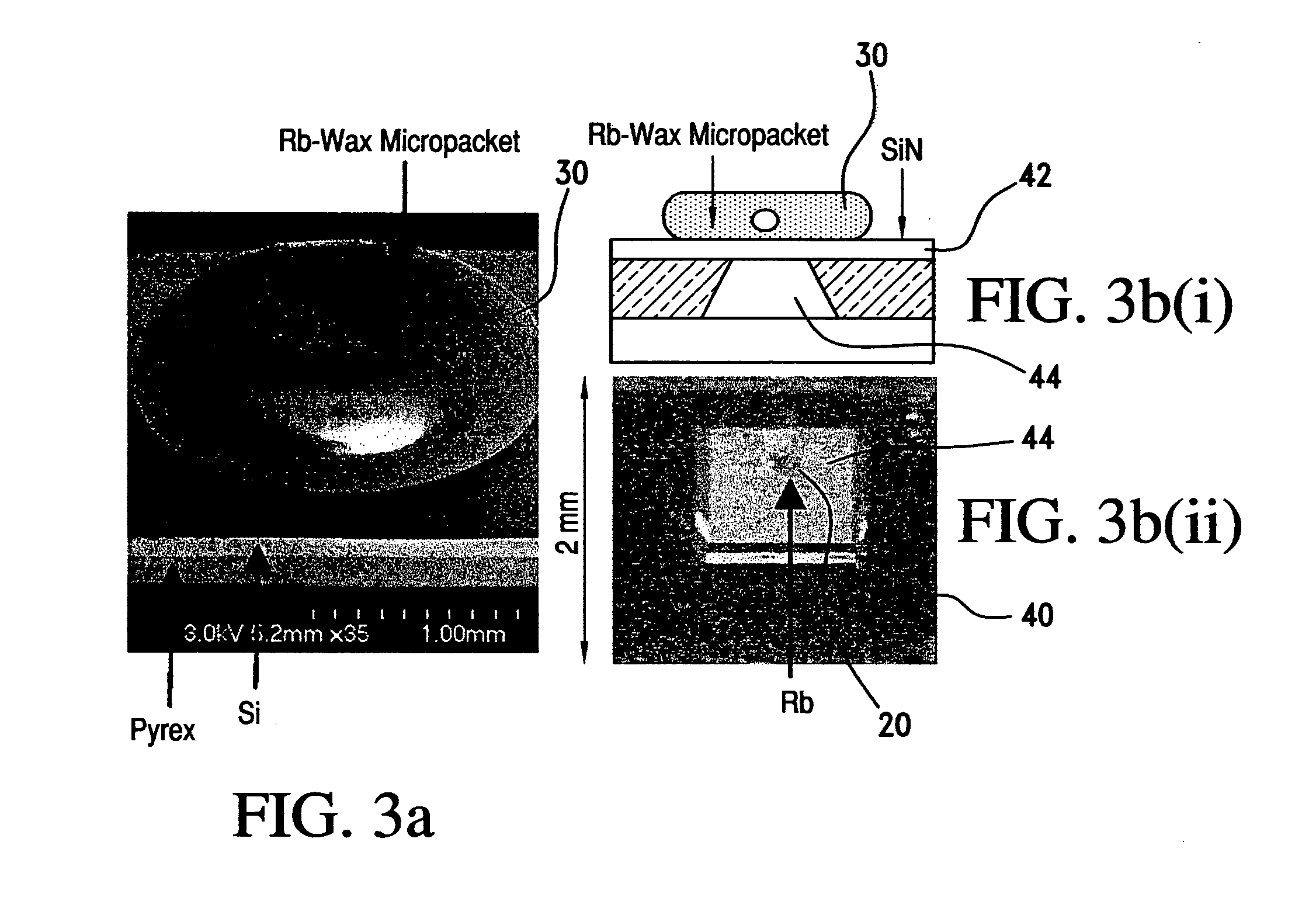
Alkali metal-wax micropackets for alkali metal handling
ActiveUS7666485B2Inexpensive and flexible manufacturingEasy to operateEnvelopes/bags making machineryApparatus using atomic clocksWaxPhysical chemistry
A method of making alkali-metal vapor cells by first forming microscale-wax micropackets with alkali metals inside allows fabrication of vapor cells at low cost and in a batch fabricated manner. Alkali metals are enclosed in a chemically inert wax to preform alkali metal-wax micropackets, keeping the alkali metals from reacting with the ambient surroundings during the vapor cell fabrication. This enables the deposition of precise amounts of pure alkali metal inside the vapor cells. Laser ablation of the alkali metal-wax micropackets provides a simple and effective way of releasing the enclosed metal. The method reduces the cost of making chip-scale atomic clocks and allows shipping of alkali vapor packets without contamination issues, thereby creating a technology for alkali-metal vendors to provide small packets of alkali metals.
Owner:CORNELL UNIVERSITY
Alkali metal-wax micropackets for alkali metal handling
ActiveUS20070034809A1Fast and effectiveIncreasing coherence lifetimeEnvelopes/bags making machineryApparatus using atomic clocksWaxAlkali metal
A method of making alkali-metal vapor cells by first forming microscale-wax micropackets with alkali metals inside allows fabrication of vapor cells at low cost and in a batch fabricated manner. Alkali metals are enclosed in a chemically inert wax to preform alkali metal-wax micropackets, keeping the alkali metals from reacting with the ambient surroundings during the vapor cell fabrication. This enables the deposition of precise amounts of pure alkali metal inside the vapor cells. Laser ablation of the alkali metal-wax micropackets provides a simple and effective way of releasing the enclosed metal. The method reduces the cost of making chip-scale atomic clocks and allows shipping of alkali vapor packets without contamination issues, thereby creating a technology for alkali-metal vendors to provide small packets of alkali metals.
Owner:CORNELL UNIVERSITY
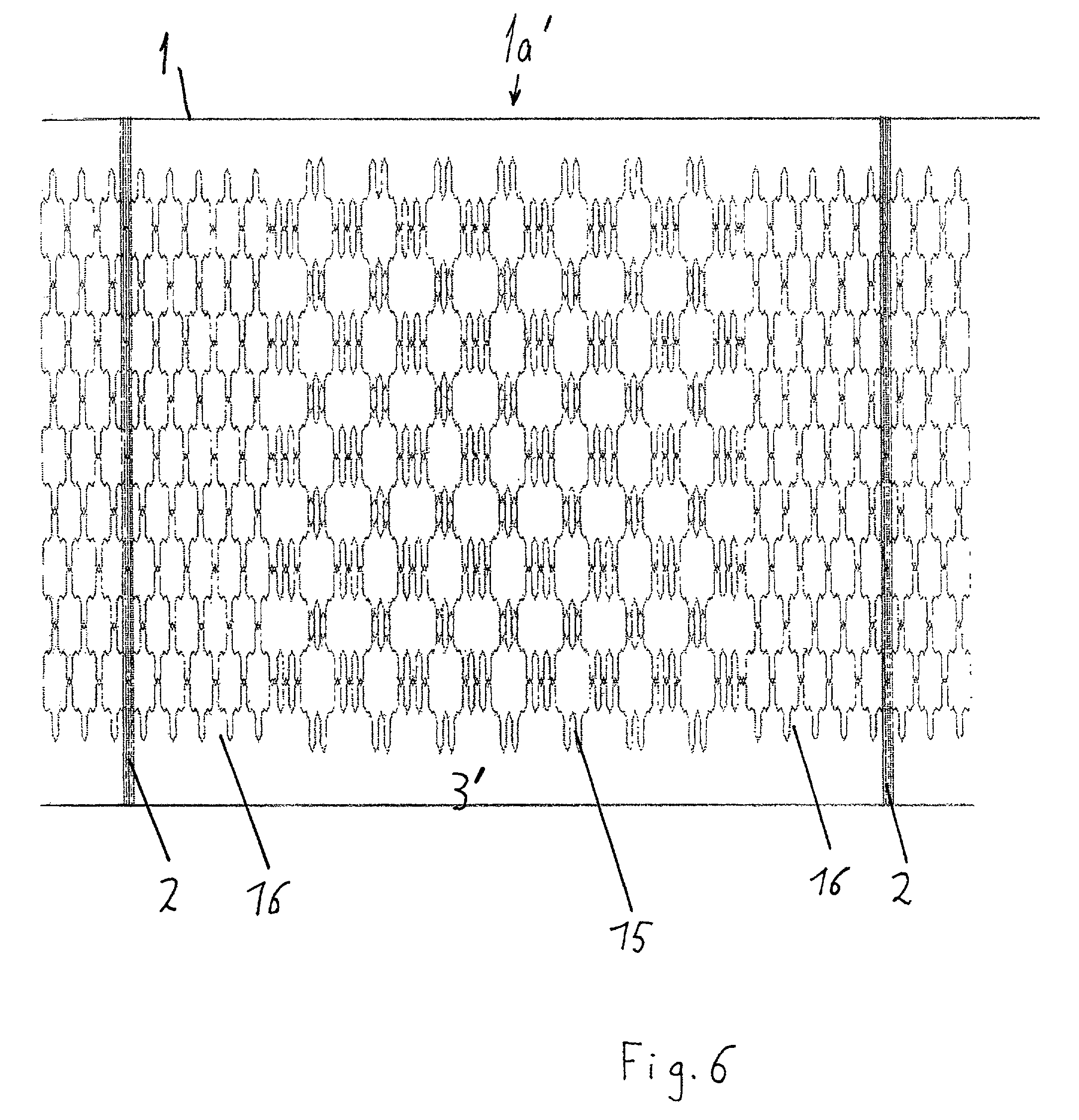
Knitted two-dimensional heating element
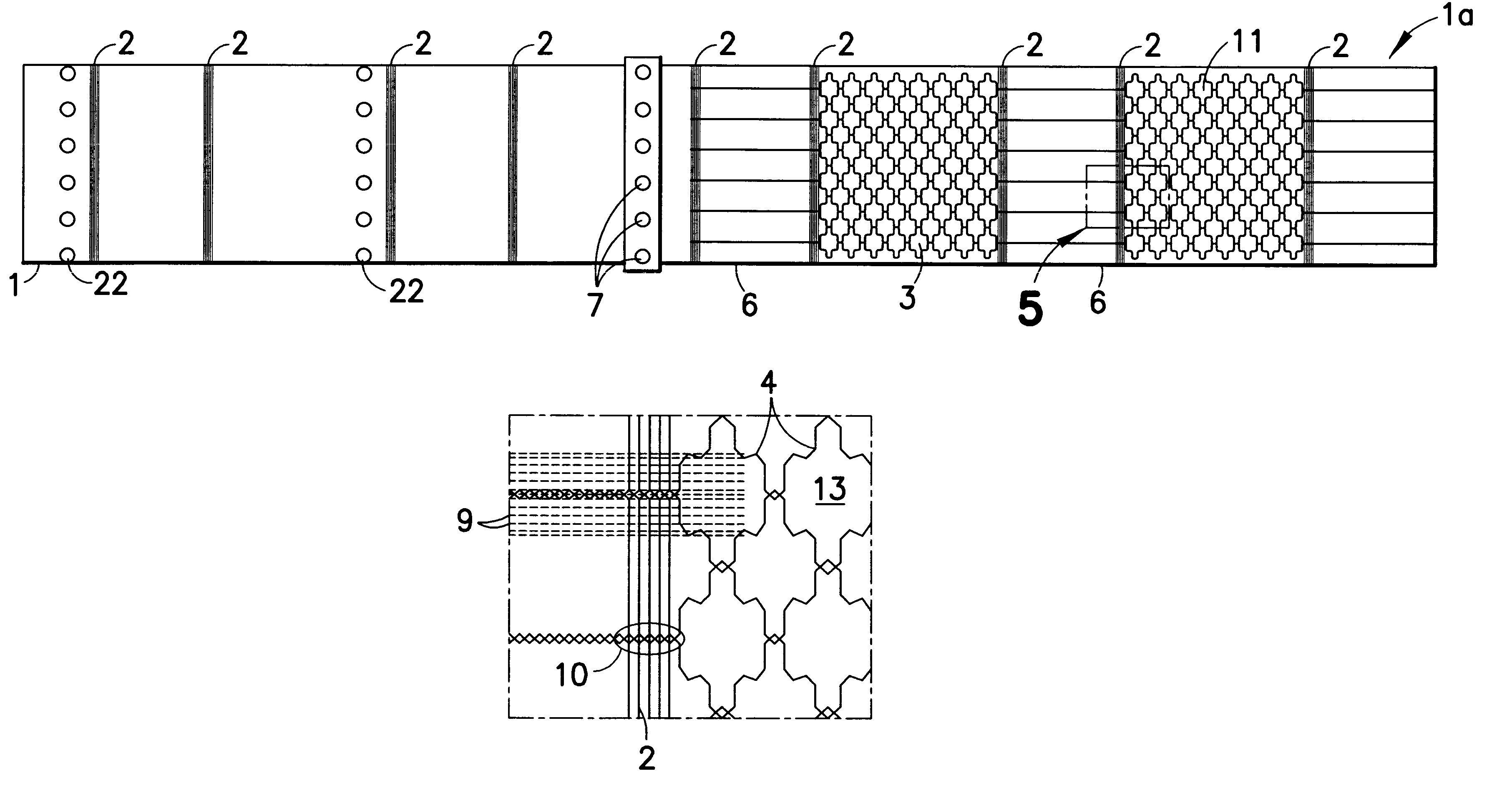
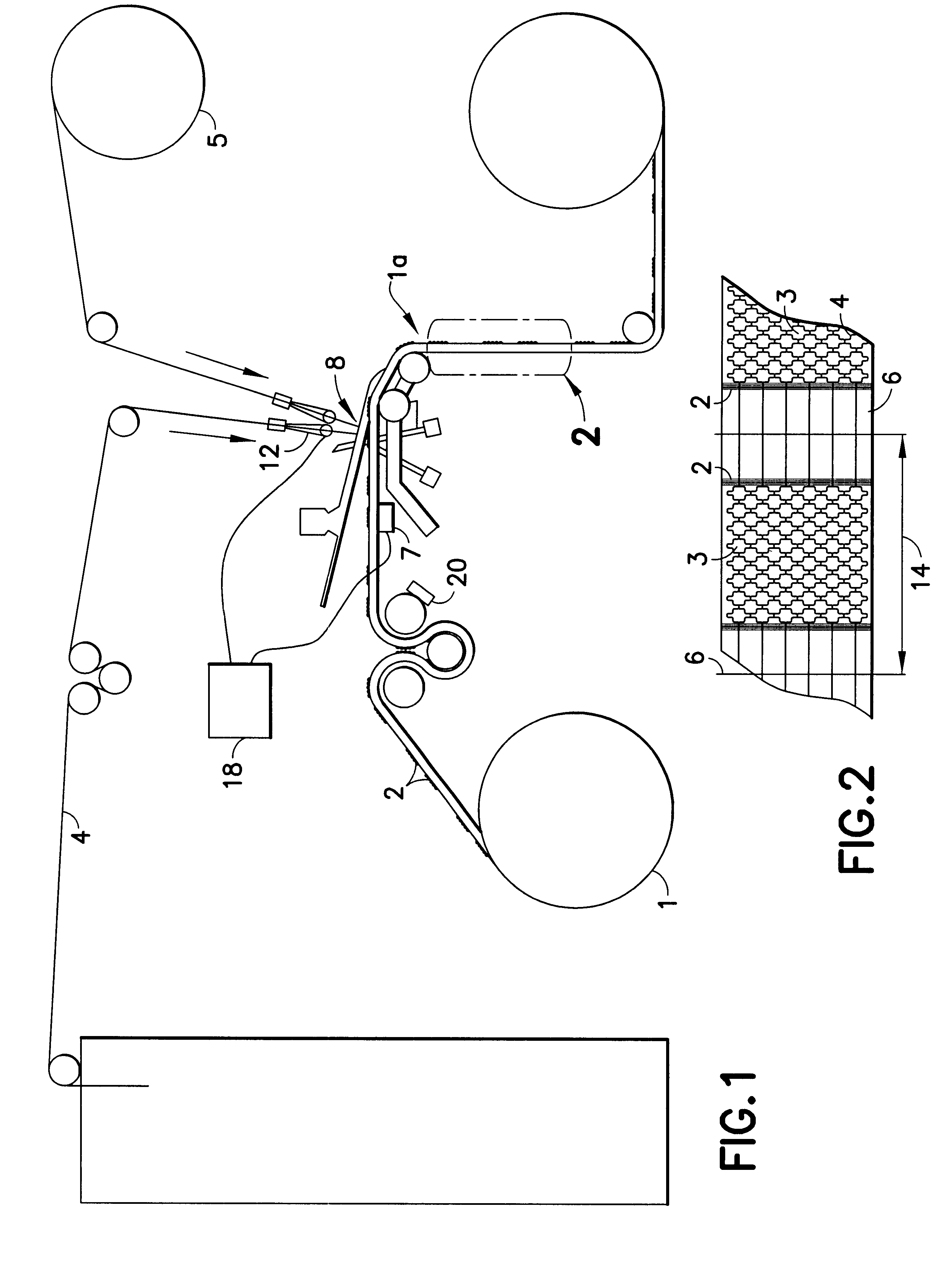
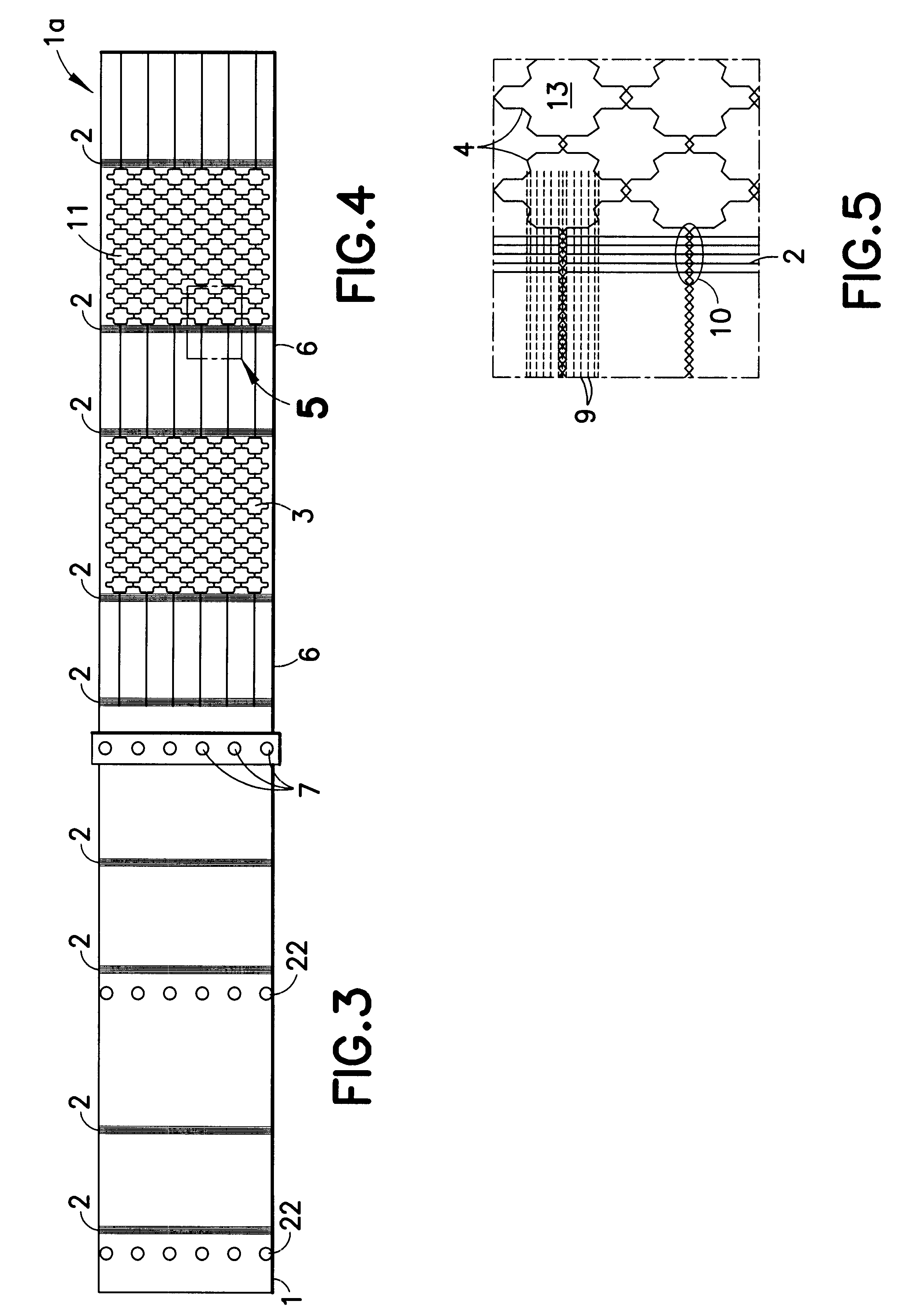
InactiveUS6531687B2Ensure reproducibilityControl freedomSeat heating/ventillating devicesWarp knittingElectrical conductorEngineering
A two-dimensional heating element, especially for seat heaters in the motor vehicle sector, having a textile base material and contact conductors and heating conductors which are electrically conductive and touch one another. The contact conductors delimit heating areas. The heating conductors are laid effectively in the direction of stitch wales and orthogonal to the stitch wales in such a way that there are at least two zones functioning at least as a main heating area and at least as a secondary heating area with different heating capacities.
Owner:I G BAUERHIN GMBH
High temperature high pressure acoustic emission electrochemistry simulation experiment apparatus capable of loading stress
InactiveCN103226091AConvenient researchAffect accuracyMaterial analysis using acoustic emission techniquesWeather/light/corrosion resistanceAcoustic emissionEngineering
The invention provides a high temperature high pressure acoustic emission electrochemistry simulation experiment apparatus capable of loading stress. The apparatus comprises: a pressure kettle, wherein the pressure kettle is provided with an inner cavity, and a specimen is arranged inside the inner cavity; stretching test systems installed on both ends of the specimen, wherein the tension test system stretches the specimen, tests and obtains tension and a strain signal of the specimen; a solution circulation inflation system connected with the inner cavity of the pressure kettle and provided for providing a solution required by an experiment for the pressure kettle; acoustic emission probes arranged on both ends of the specimen and provided for testing an acoustic signal of the specimen; an electrochemistry test system connected with the specimen and the pressure kettle, and provided for applying polarization current on the specimen to carry out polarization, testing and obtaining an electrochemistry signal; and a data collector for collecting the simultaneously measured strain signal, the electrochemistry signal and the acoustic emission signal. With the present invention, controllable pressurizing, controllable heating and controllable inflation can be performed, and an actual working condition of the on-site material is well simulated.
Owner:BC P INC CHINA NAT PETROLEUM CORP +1
Polishing pad and chemical mechanical polishing apparatus comprising the same
InactiveUS20080090503A1Improve uniformityEnsure reproducibilityPolishing machinesRevolution surface grinding machinesCompound (substance)Engineering
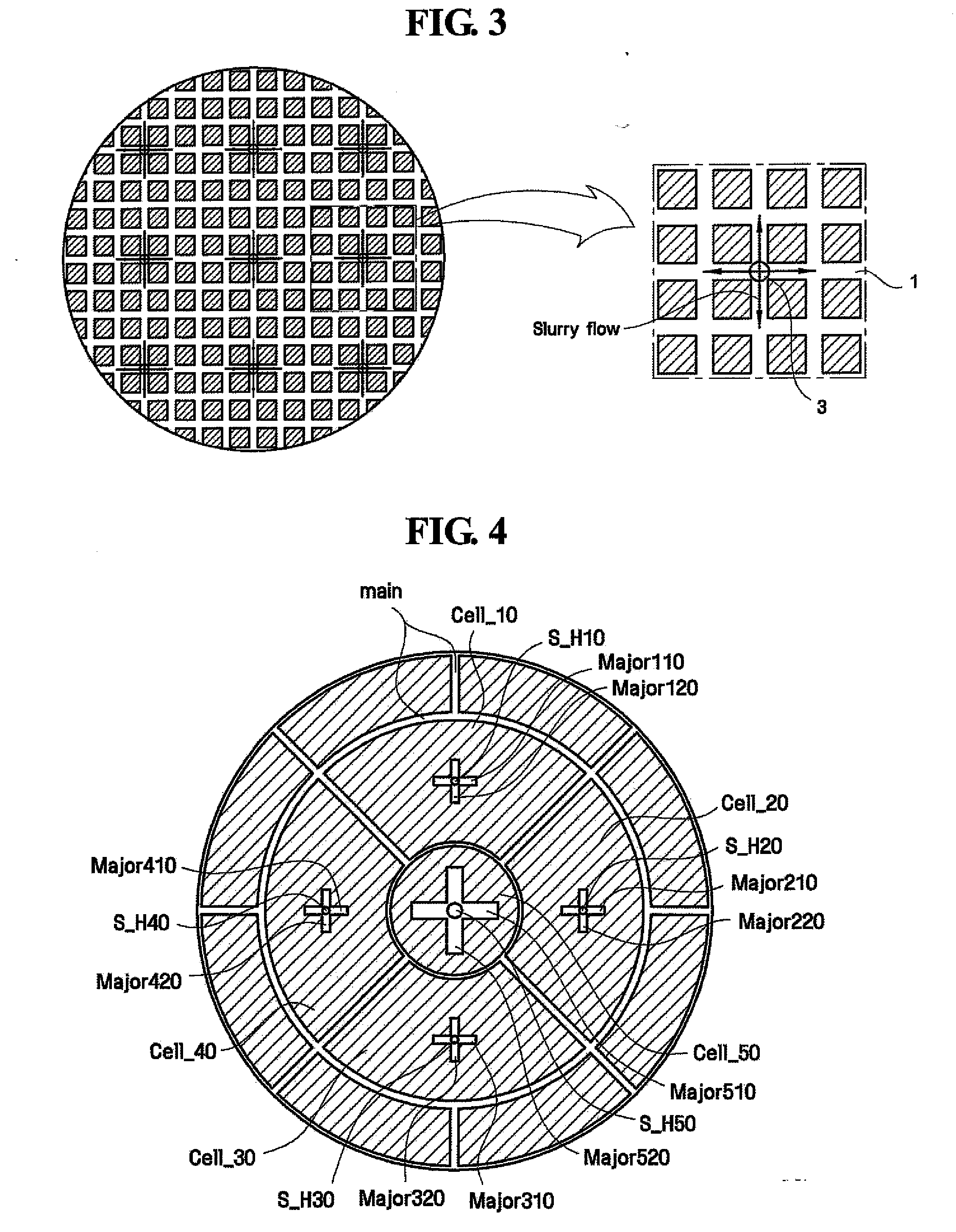
A polishing pad for use in chemically mechanically polishing a semiconductor substrate enhances the uniformity of the rate at which material is removed from the surface of the semiconductor substrate, thereby ensuring the reproducibility of the chemical mechanical polishing process. The polishing pad has main grooves that divide an upper portion of the pad into a plurality of cells. At least one of the cells includes a land portion and a grooved portion substantially enclosed by the land portion. A respective slurry hole extends through the pad to the grooved portion such that slurry supplied through the slurry hole feeds into the grooved portion but is impeded by the land portion from flowing outwardly of the cell.
Owner:SAMSUNG ELECTRONICS CO LTD
Fixator used for gas sampling
ActiveCN104864237AEasy to fixAvoid manpower requirementsWithdrawing sample devicesStands/trestlesAdhesiveEngineering
The invention discloses a fixator used for gas sampling. The fixator comprises a support, fixing discs used in cooperation with the support and a fixing device; the fixing device is located at the top of the support, supported by the support and used for fixing sampling equipment, suckers are arranged at the bottom of the support, adhesive is arranged on one face of each fixing disc and used for adhering to the ground, and the suckers are adsorbed on the fixing discs through negative pressure. The auxiliary fixing device special for the gas sampling equipment is provided, the fixing discs used in cooperation with the support can be fixed to any surface through the adhesive, and therefore the fixator can be fixed to different surfaces conveniently. By using the fixator, the manual requirement for handheld sampling of an operator is omitted, and safety is improved; the influence on detection data by manual operation in the manual handheld sampling process is reduced, and the stability and reproducibility of the detection data are ensured.
Owner:临沂鑫润建铜业有限公司
Knitted two-dimensional heating element
InactiveUS20010027973A1Ensure reproducibilityControl freedomHeater elementsWarp knittingElectrical conductorElectrically conductive
A two-dimensional heating element, especially for seat heaters in the motor vehicle sector, having a textile base material and contact conductors and heating conductors which are electrically conductive and touch one another. The contact conductors delimit heating areas. The heating conductors are laid effectively in the direction of stitch wales and orthogonal to the stitch wales in such a way that there are at least two zones functioning at least as a main heating area and at least as a secondary heating area with different heating capacities.
Owner:I G BAUERHIN GMBH
Method for machine-cleaning and machine-disinfecting objects
InactiveCN103052408AClean thoroughlyAchieve resultsLavatory sanitoryHeatDental instrumentsBiomedical engineering
The invention relates to a method for machine-cleaning and machine-disinfecting objects, in particular medical and / or dental instruments and / or work equipment.
Owner:洛塔尔·赛格尔
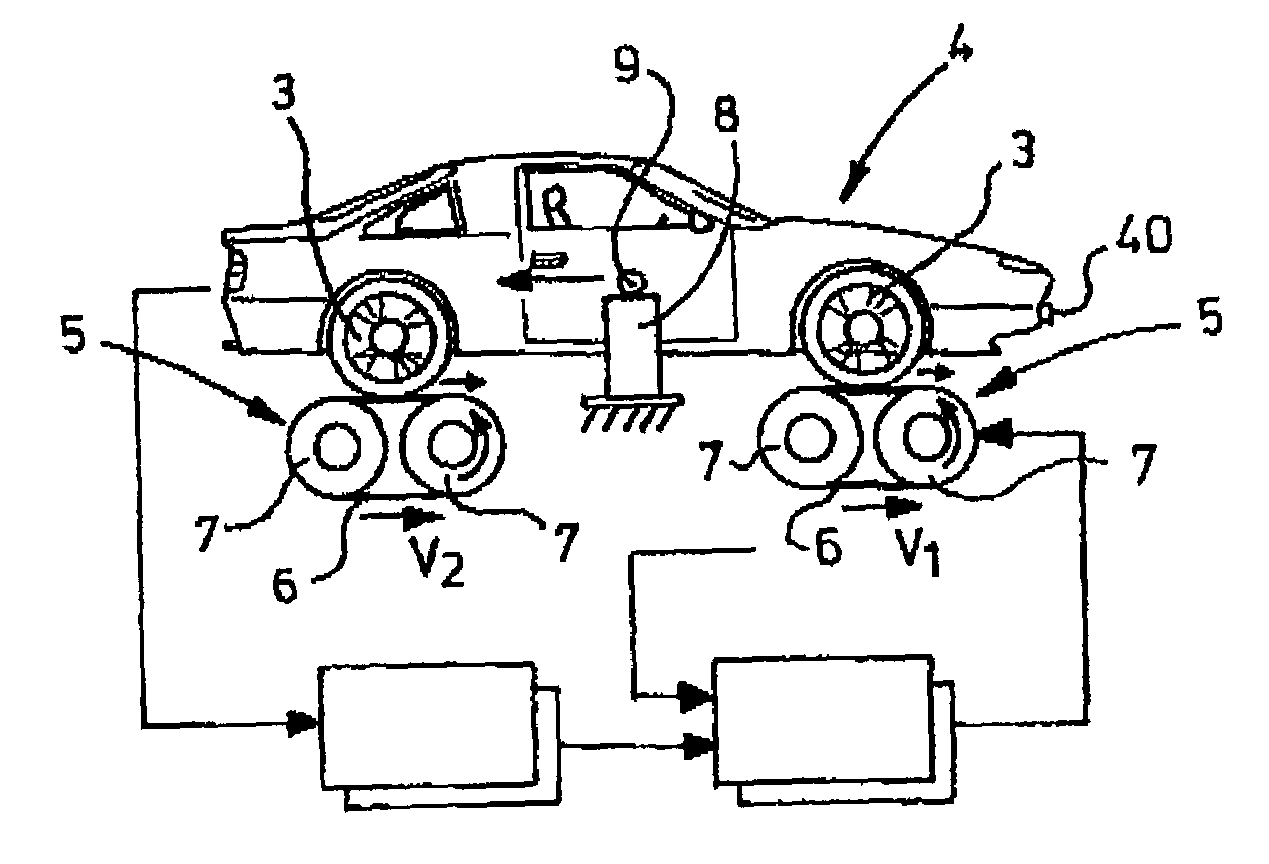
System for performing tests on intelligent road vehicles
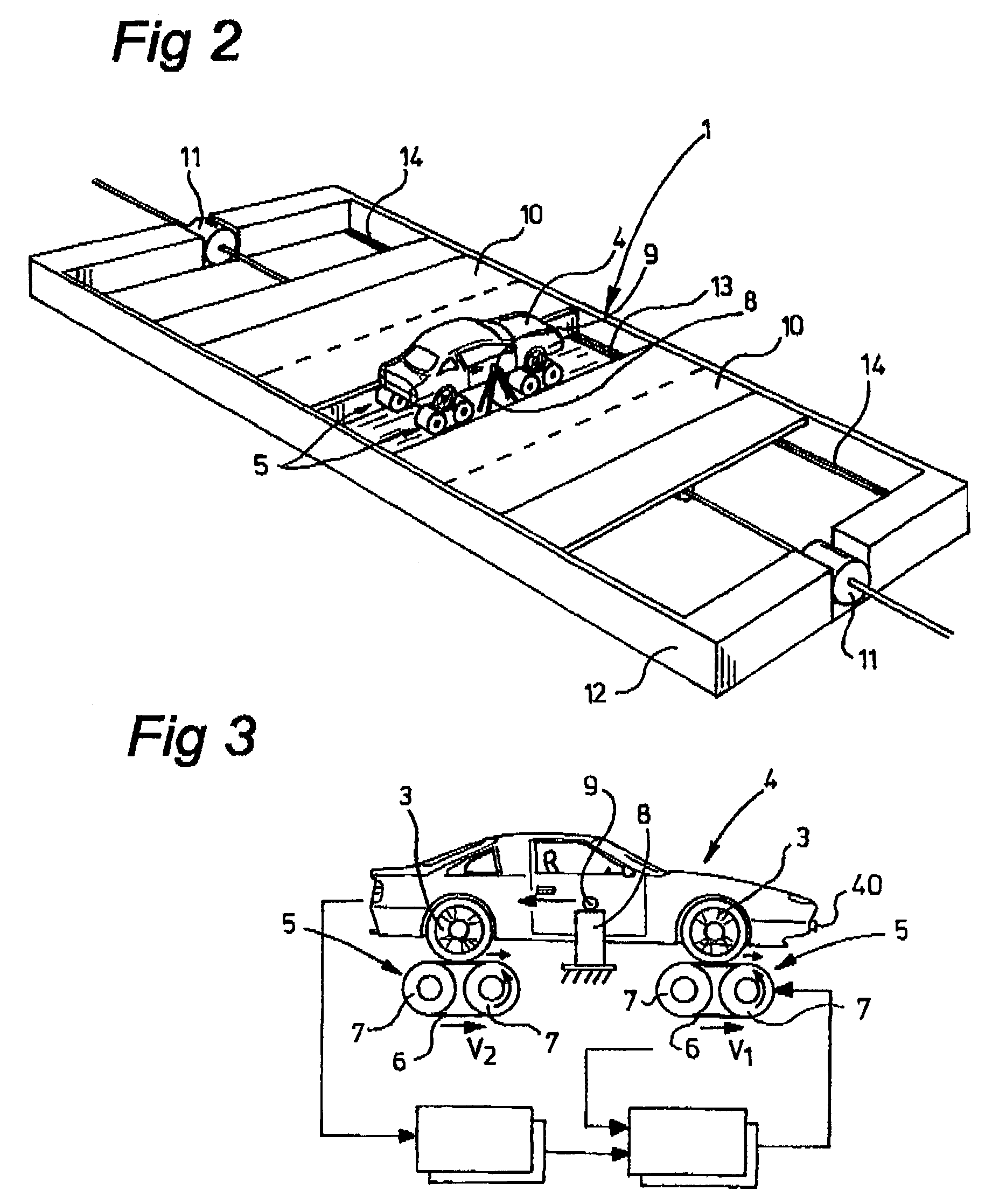
InactiveUS7013704B2Increase speedTested reliably and realisticallyVehicle testingWave based measurement systemsRelative motionTest rig
A system for carrying out research on a drivable vehicle or a component thereof, includes: a test stand on which the vehicle or the component can be positioned, which vehicle or which component is provided with at least one sensor for receiving signals from the environment of the vehicle or of the component, at least one object which is situated in the vicinity of the test stand, elements for generating a relative movement between the test stand and the at least one object, and a control computer for coordinating the relative movement between the test stand and the object.
Owner:NEDERLANDSE ORG VOOR TOEGEPAST-NATUURWETENSCHAPPELIJK ONDERZOEK (TNO)
Ultrasonic probe and ultrasonic imaging device
InactiveCN1964670AImprove picture qualityAvoid issues that depend on inspectorsOrgan movement/changes detectionSurgeryUltrasonic imagingImaging quality
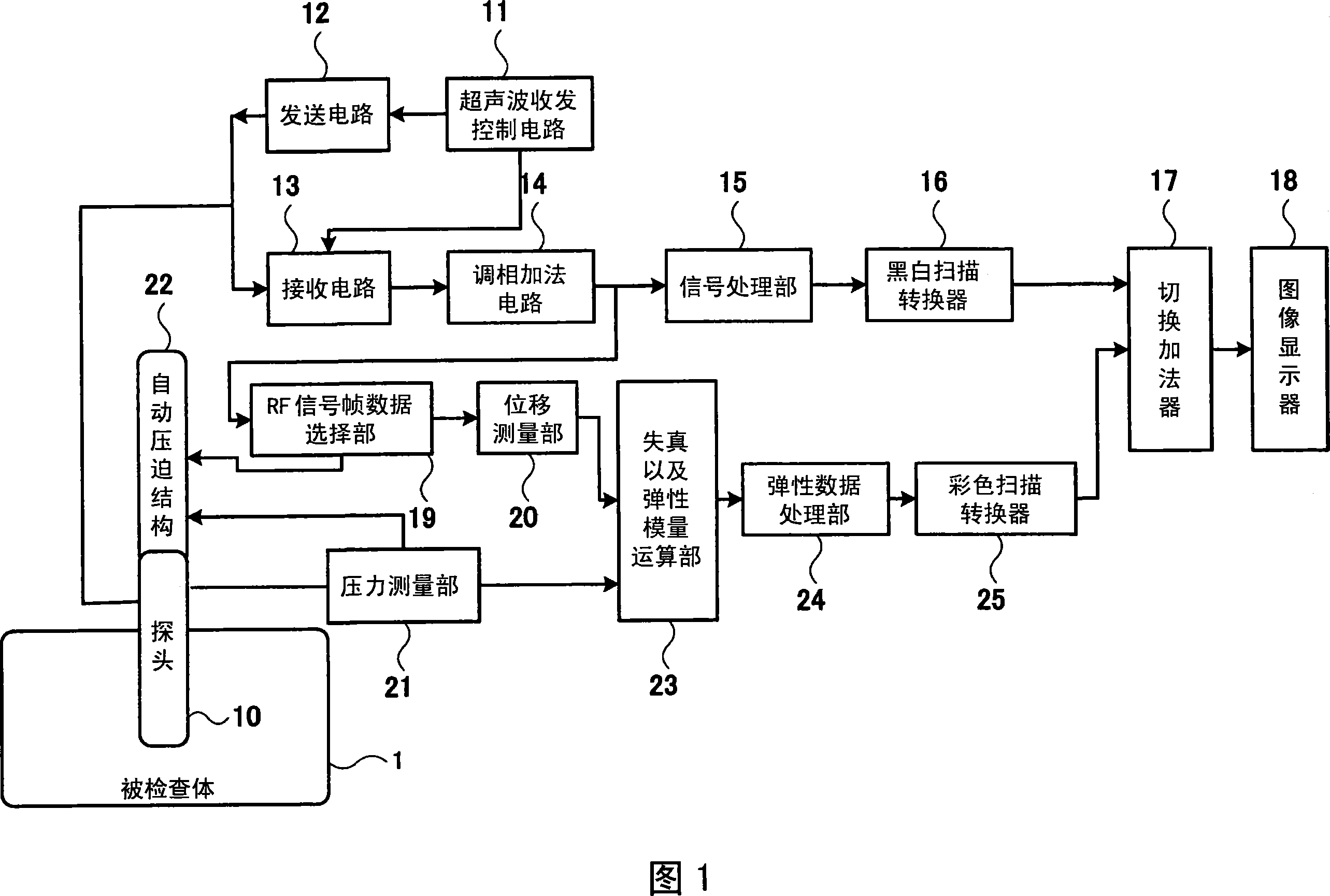
An ultrasonic probe that is pressed to a subject to obtain an elasticity image. The probe has a contact surface used for contact with the subject and is orthogonal to the direction of the pressing and has an automatic pressing means or a manual pressing means. The automatic pressing means is adapted for moving the contact surface in a pressing direction to press a to-be-imaged portion of the subject at a predetermined pressing force, and manual pressing means presses the portion by a manual force. Using the ultrasonic prove having the automatic pressing means or the manual pressing means enables a pressing stage to be automatically or manually moved in a vertical fixed direction at a desired speed, and as a result elasticity image data with high image quality can be obtained at a desired time. Further, repeatability of pressing operation can be retained, which enables a problem of the quality of an elasticity image being dependent on an inspector to be eliminated.
Owner:HITACHI MEDICAL CORP
Redundant backlight for liquid crystal displays
InactiveUS20120147293A1Costly and time-consumeContinuous operationElectrical apparatusStatic indicating devicesLiquid-crystal displayEngineering
A redundant LED backlight and method for controlling the same. At least two plurality of LEDs are used to create a backlight where any individual set of LEDs may be driven at power level P to provide the desired overall luminance for the backlight. If two plurality of LEDs are used, then the LEDs may be driven simultaneously at one-half P during normal operations until a failure has been detected in one of the plurality of LEDs. In alternate embodiments, four plurality of LEDs may be used and driven simultaneously at one-fourth P during normal operations until a failure has been detected in one of the plurality of LEDs. The LEDs may be in edge-lit, direct-lit, or any combination of these two orientations. Upon failures, only one plurality of LEDs may be used to provide operation of the LED backlight and any associated LCD device.
Owner:AMERICAN PANEL CORP INC
Device and method for the simulation of a development system
InactiveUS8825460B2Ensure reproducibilitySimple reproducibilityGeometric CADVehicle testingEngineeringProcess simulation
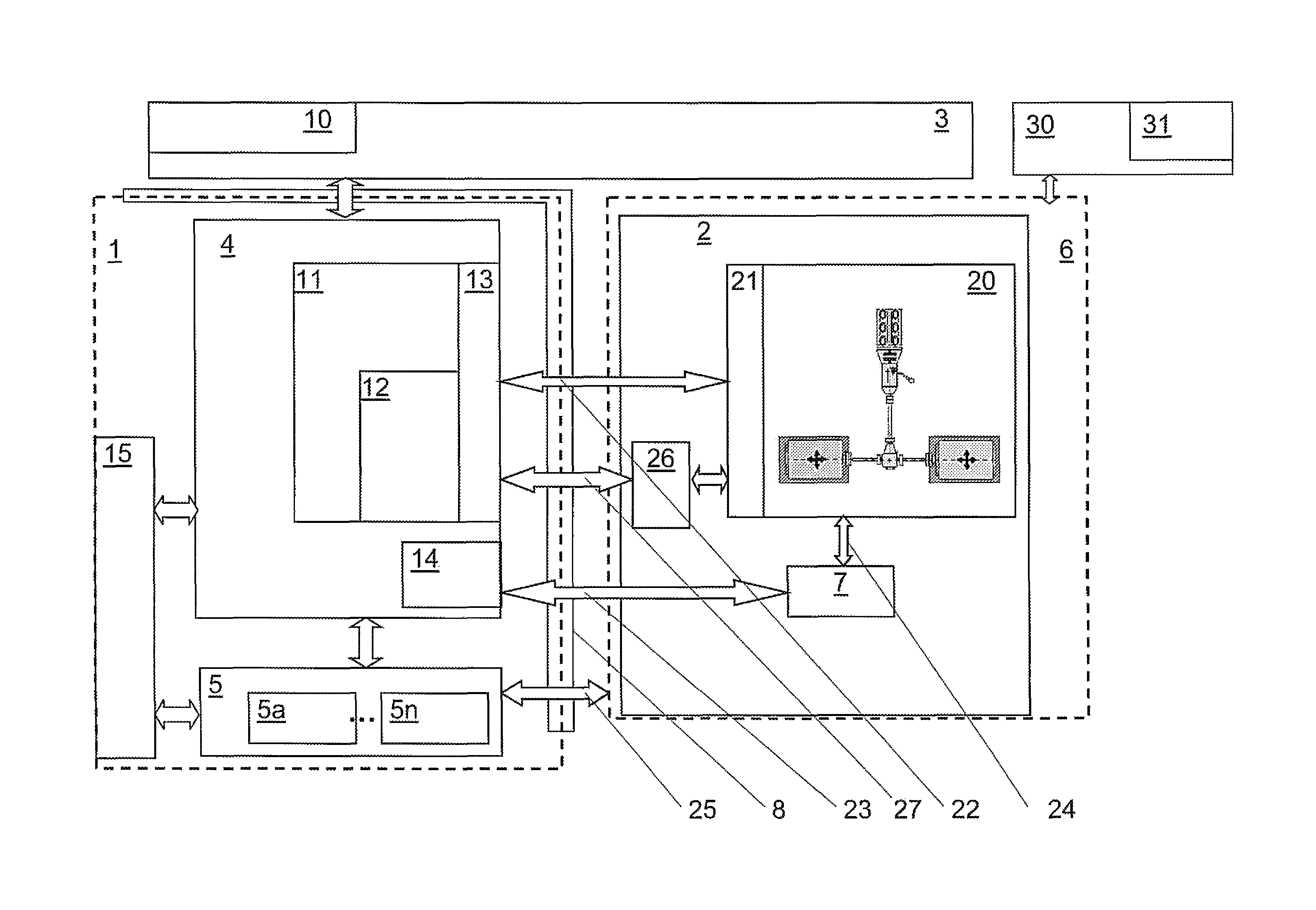
In order to be able to simulate a development system of a complex development environment, such as a test bench environment for motor, drive train, transmission, vehicle component, or vehicle development, utilizing an automation device and development tools in a continuous and reproducible manner, device models (7) generating development data (23) run in a simulation device (6), wherein the device models (7) at least partially process simulation data (24) from a test model (20), and a number of real development tools (5) are connected to an automation device (4) and / or to the simulation device (6) via real interfaces, and development tools (5) process the development data (23).
Owner:AVL LIST GMBH
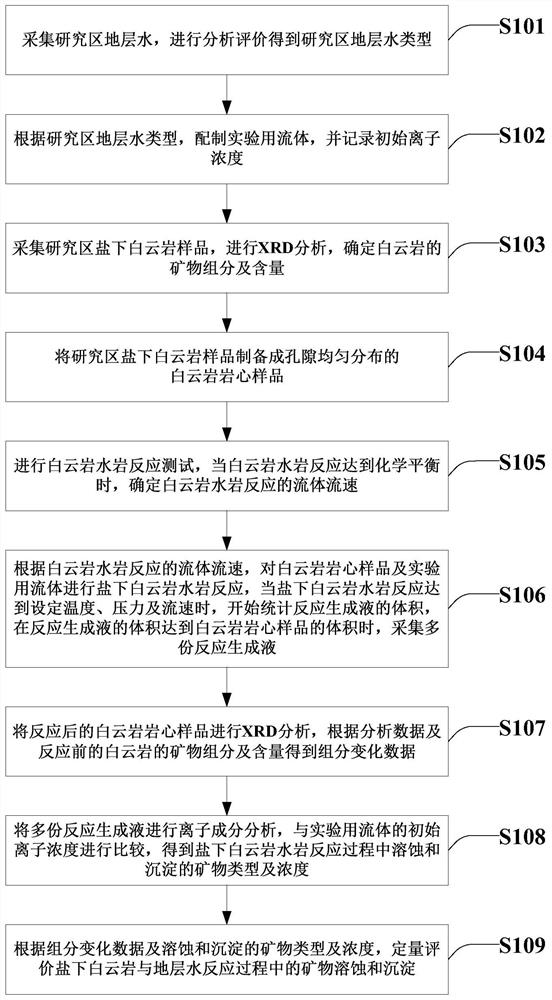
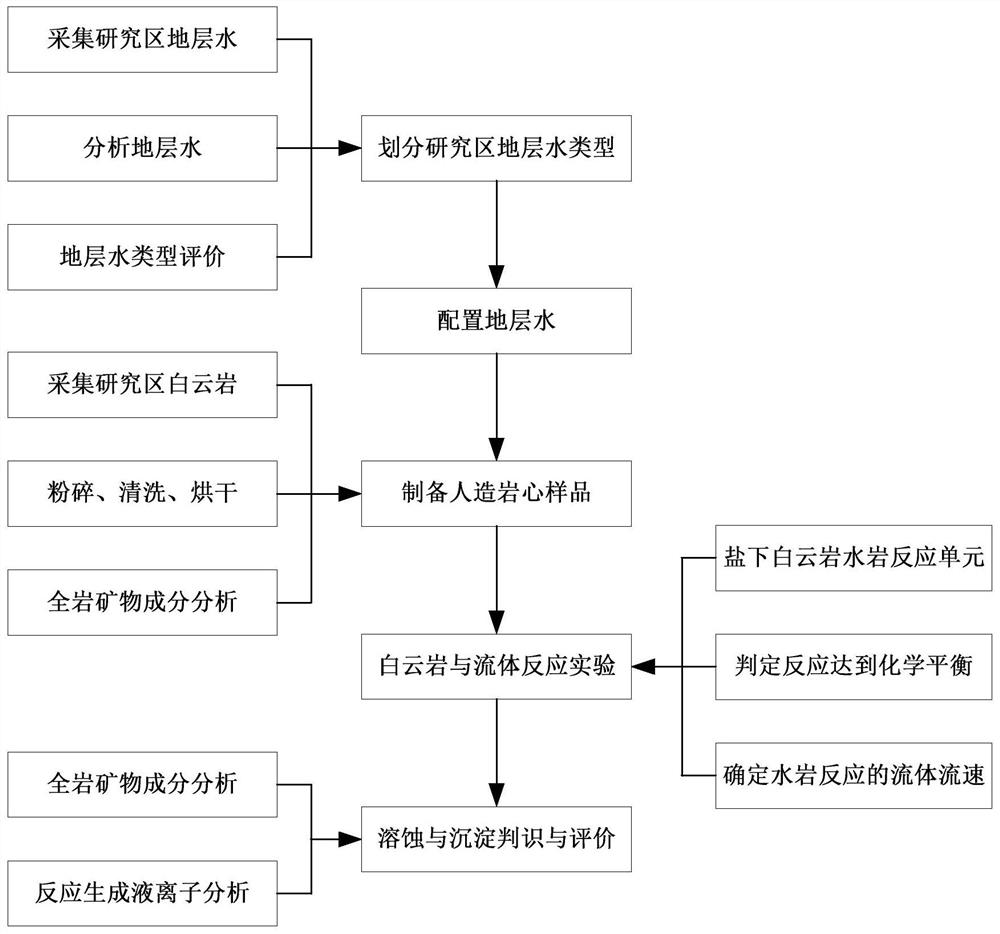
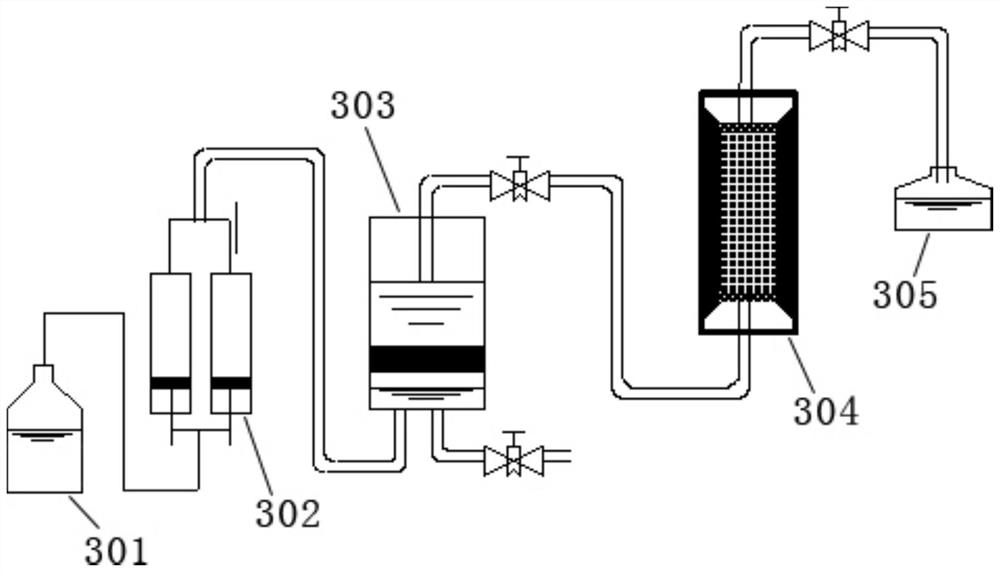
Simulation and evaluation method and system for under-salt dolomite water-rock reaction
ActiveCN111855715AClear controlImprove efficiencyEarth material testingMaterial analysis using radiation diffractionDolostoneSoil science
The invention provides a simulation and evaluation method and system for an under-salt dolomite water-rock reaction. The method comprises analyzing stratum water of a research area; stratum water classification is carried out by using a Sulin method; formulating experimental fluids, preparation of dolomite core sample, dolomite water rock reaction test is performed; the dolomite water-rock reaction is judged to reach chemical equilibrium; the method comprises the following steps: determining the fluid flow rate of the dolomite water rock reaction, carrying out the under-salt dolomite water rock reaction by using the prepared experimental fluid and a dolomite rock core sample, analyzing and calculating the changes of mineral components, corrosion and precipitation, and quantitatively evaluating the influence of the under-salt dolomite formation water on the corrosion and precipitation of the dolomite. According to the method, the quantitative evaluation of the reaction between the dolomite and the corresponding formation water under the geological background of the subsalt dolomite reservoir can be realized; the specific influence of stratum water attributes in the dolomite water-rock reaction process can be analyzed, control factors beneficial to formation and storage of under-salt dolomite pores are defined, and an analysis basis is provided for large-scale and efficient under-salt dolomite reservoir distribution and prediction.
Owner:PETROCHINA CO LTD
Metal wiring of a semiconductor device and method of forming the same
InactiveUS20090065940A1Reduce processing difficultyComplete fillingSemiconductor/solid-state device detailsSolid-state devicesInsulation layerEngineering
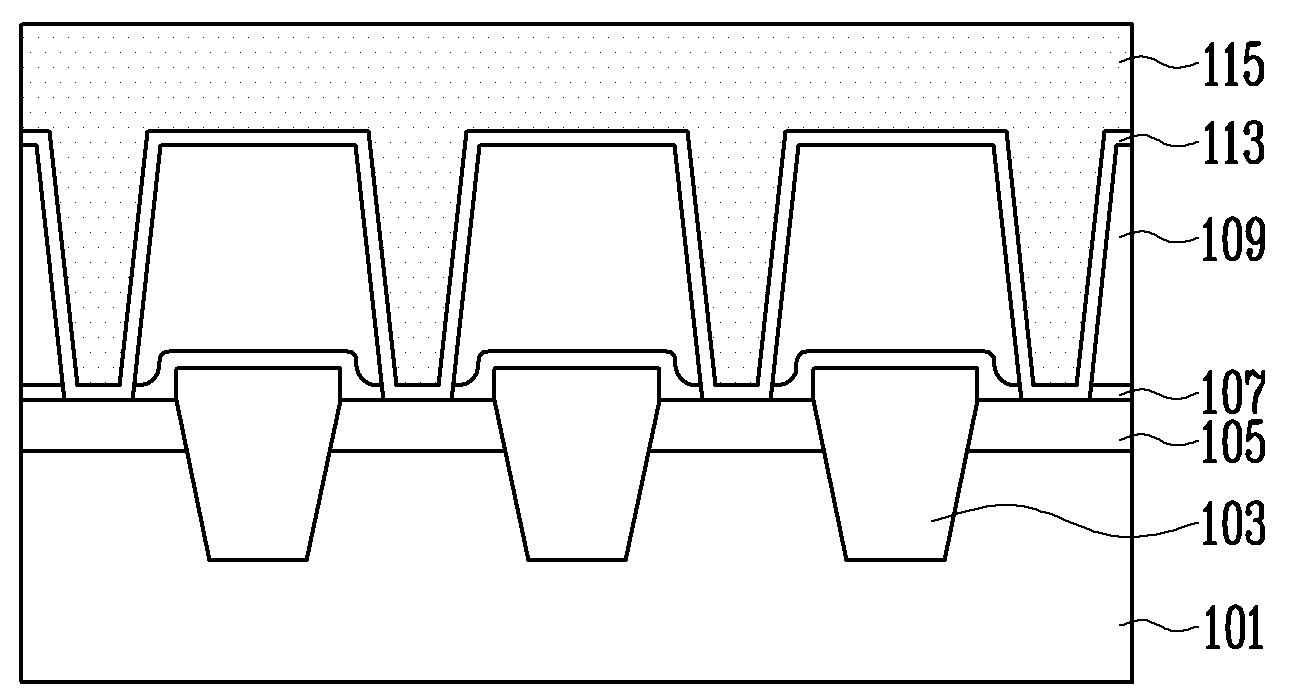
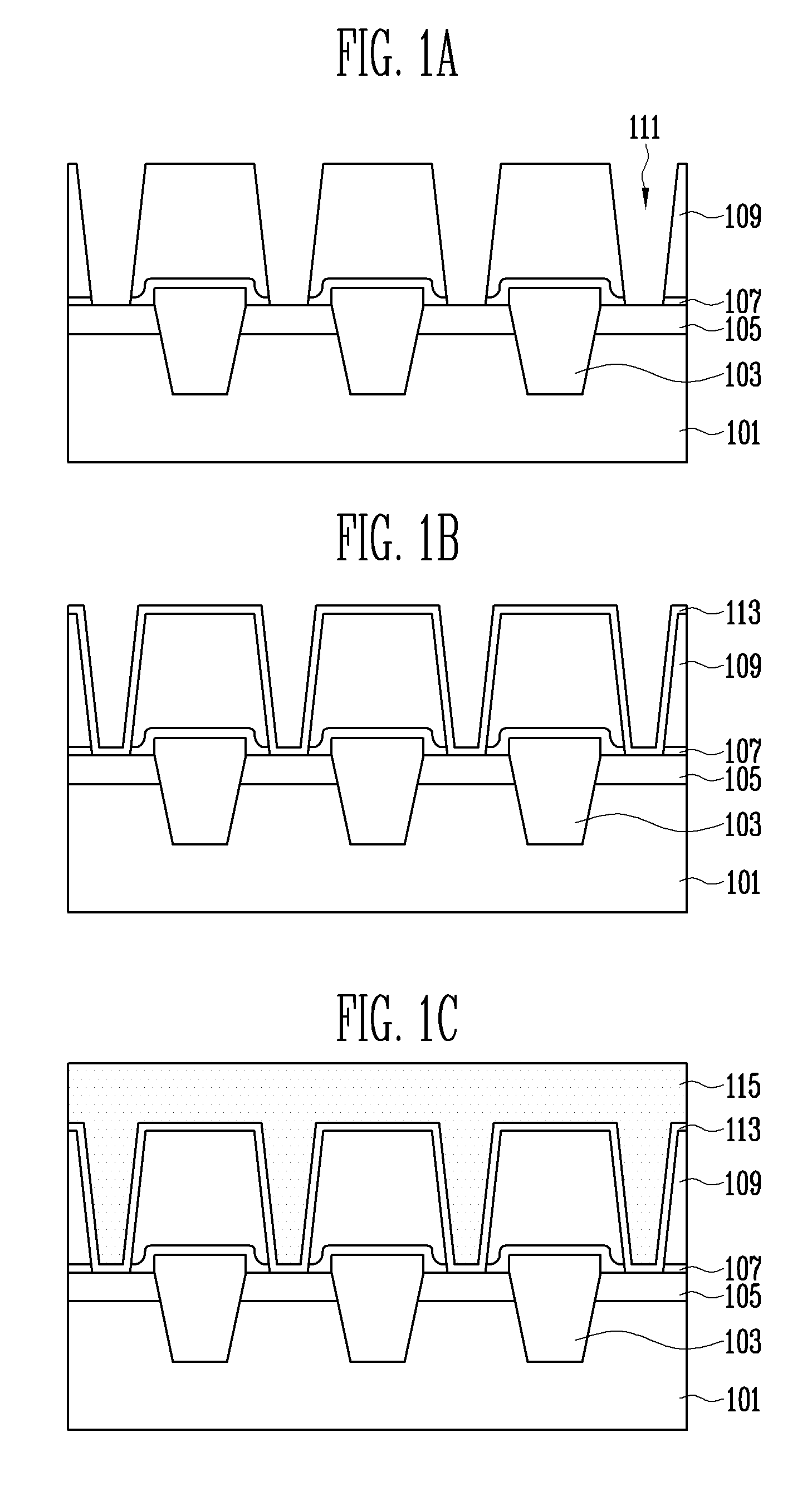
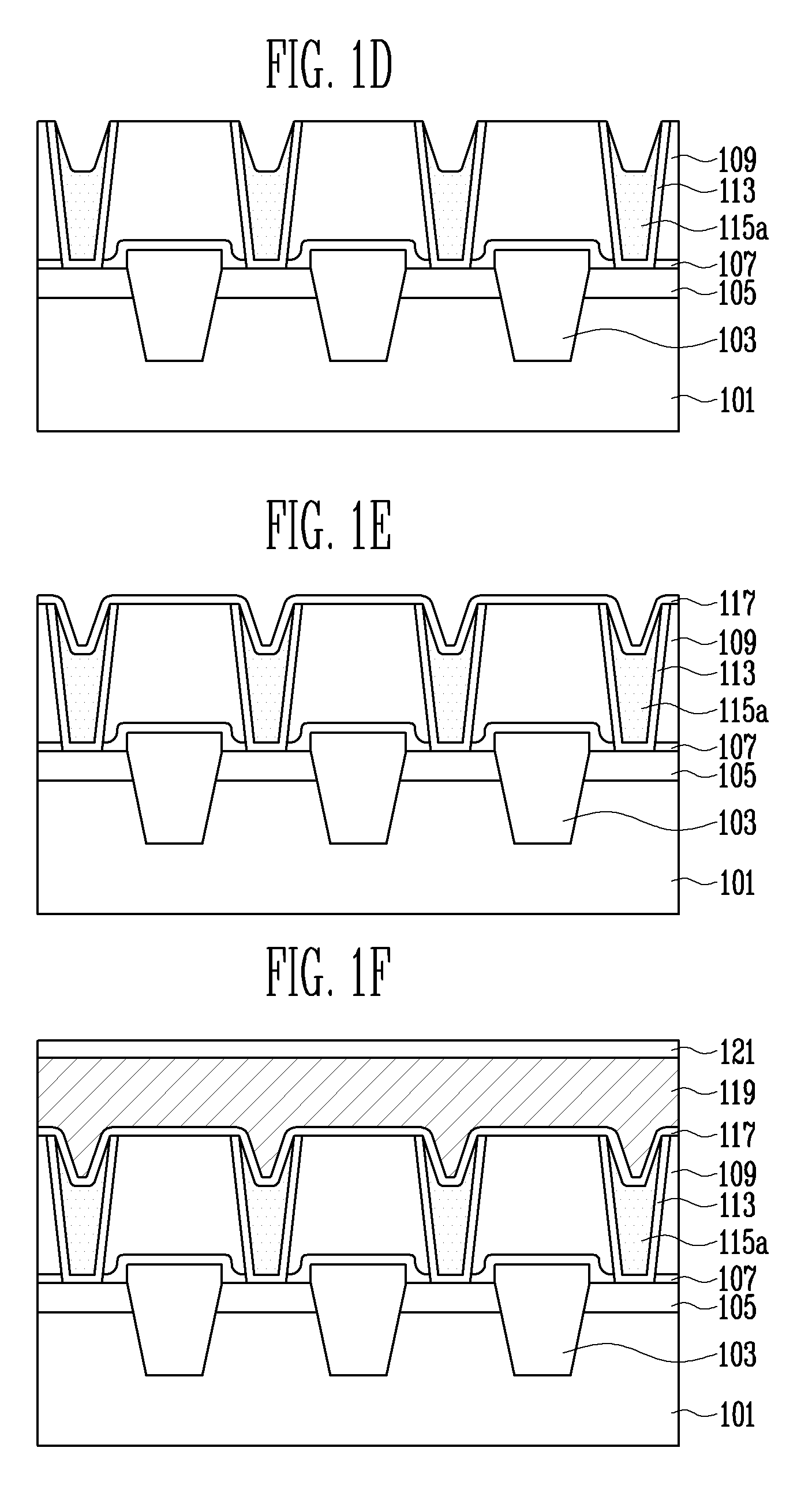
According to a method of forming a metal wiring of a semiconductor device, a contact plug is formed at height lower than the contact hole, which is formed on an interlayer insulation layer, and then a metal wiring is formed over the contact plug and interlayer insulation layer to completely fill inside of the contact hole, decreasing process difficulty, ensuring reproducibility, and improving electrical property.
Owner:SK HYNIX INC
In situ tailoring of material properties in 3D printed electronics
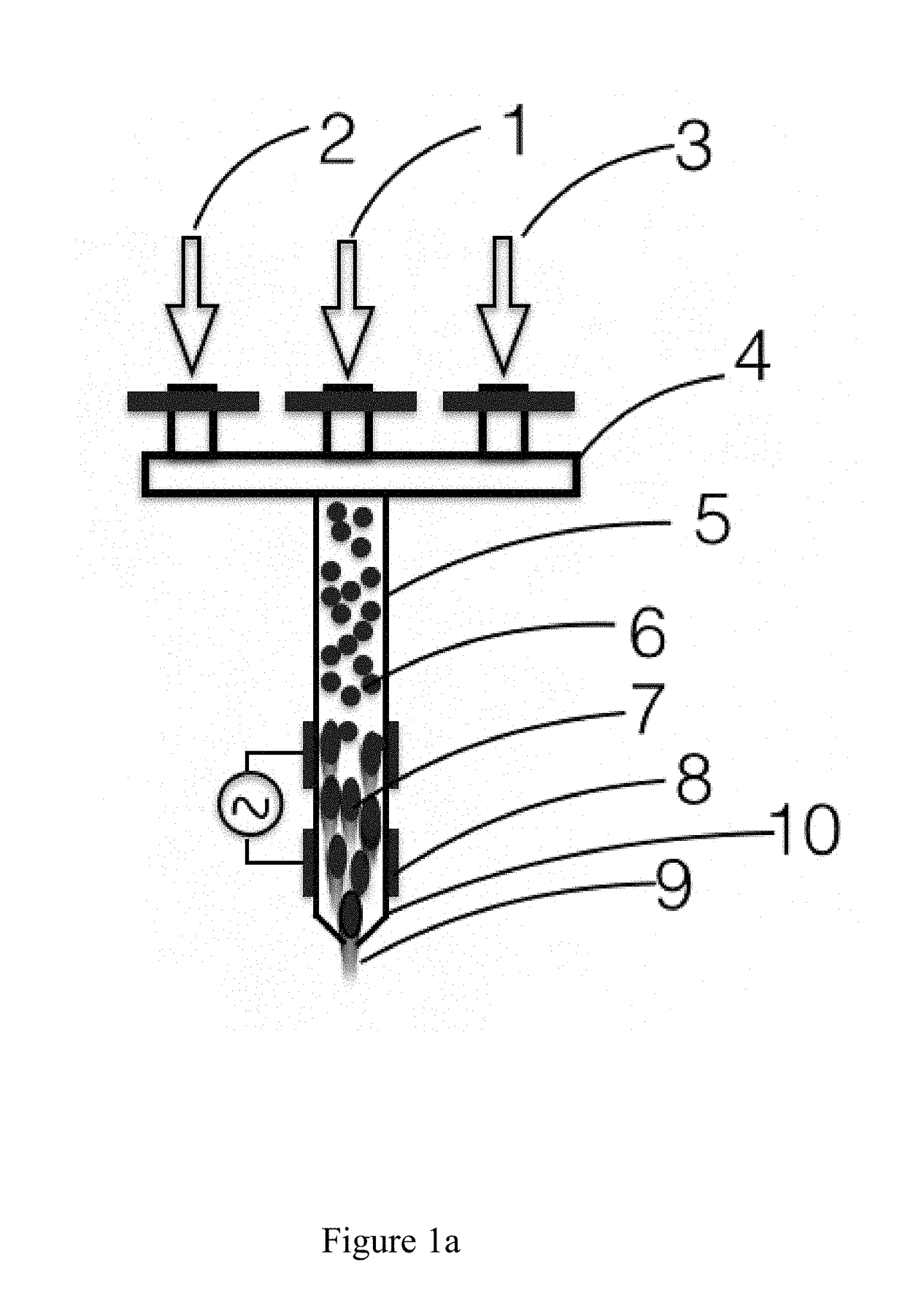
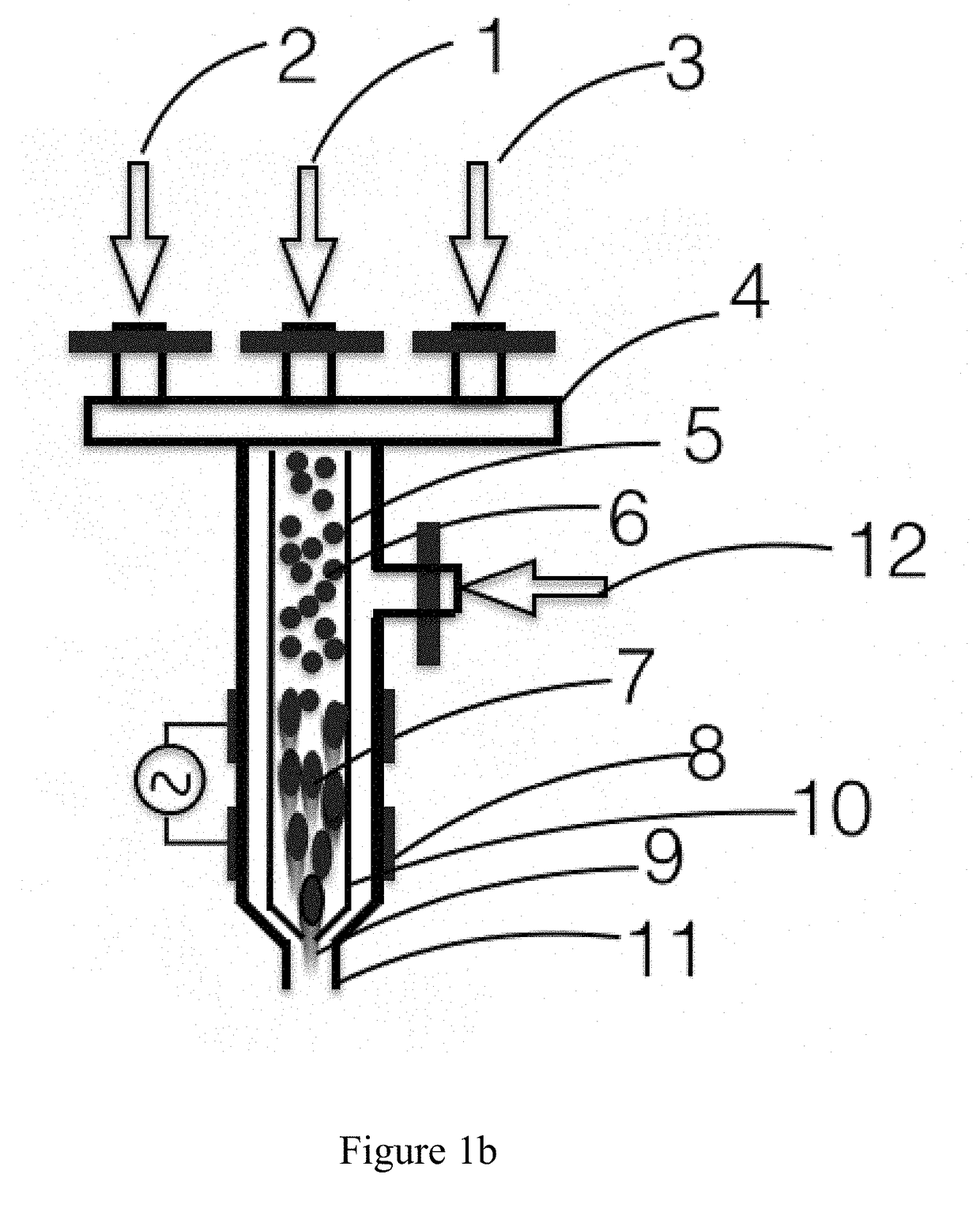
ActiveUS20170298516A1Improve adhesionEnsure reproducibilityAdditive manufacturing apparatusChemical vapor deposition coatingPlasma jetDielectric
Systems and methods for highly reproducible and focused plasma jet printing and patterning of materials using appropriate ink containing aerosol through nozzles with narrow orifice and tubes with controlled dielectric constant connected to high voltage power supply, in the presence of electric field and plasma, that enables morphological and / or bulk chemical modification and / or surface chemical modification of the material in the aerosol and / or the substrate prior to printing, during printing and post printing.
Owner:UNIVERSITIES SPACE RES ASSOC
Content determination method for scutellarin and scutellarein in sculellaria barbata medicinal material and formula granule of sculellaria barbata medicinal material
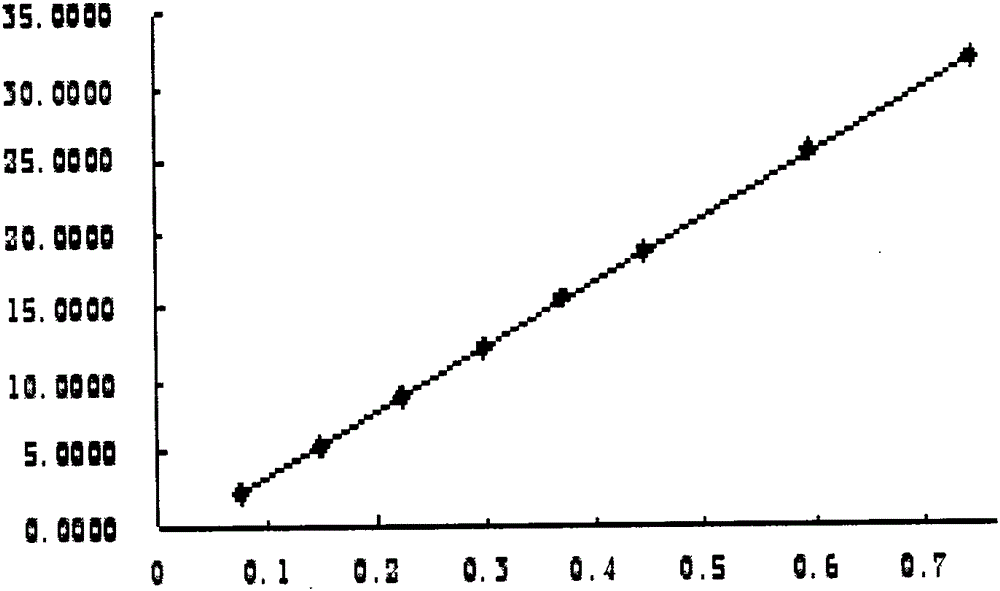
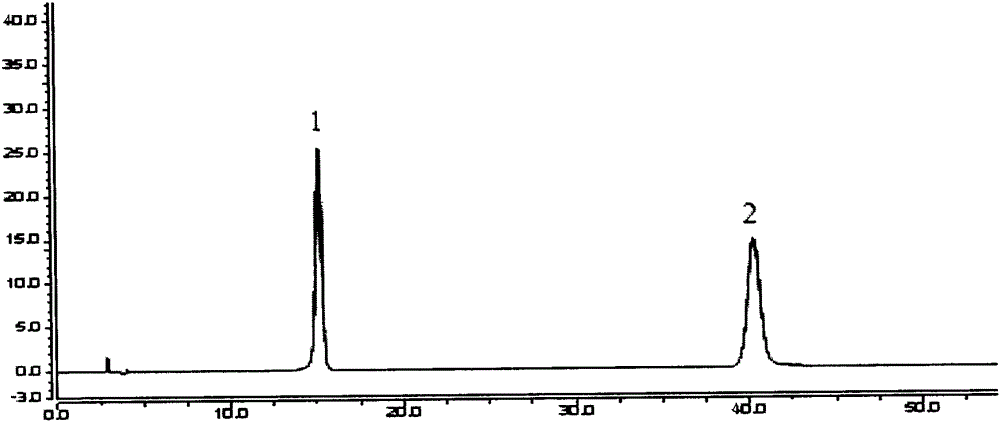
ActiveCN105353053AGood peak separationEfficient separationComponent separationScutellareinPhosphoric acid
The invention relates to a content determination method for scutellarin and scutellarein in sculellaria barbata medicinal material and formula granule of sculellaria barbata medicinal material. The content determination method is characterized in that common isocratic elution is adopted, acetonitrile-methyl alcohol-0.1 percent phosphoric acid of which the volume ratio is 12.5:(15 to 16.2):(71.3 to 72.5) is taken as a mobile phase, and the contents of the scutellarin and the scutellarein in the sculellaria barbata medicinal material and the formula granule of the sculellaria barbata medicinal material are simultaneously determined at a wavelength of 340+ / -2nm. The method is simple, convenient, fast, and easy to popularize and master; the separations of wave crests on three different chromatographic columns are good. Compared with a method of recording under a sculellaria barbata medicinal material item of the first part of the pharmacopeia, the prominent improvement is that a sample treatment process is simplified, the time and cost are saved, the determination is advanced from single component determination of the scutellarin to the simultaneous determination of the two components, the quality controllability is improved, and the defects that impurity peaks exist and the content results are inaccurate are overcome.
Owner:HEBEI UNIV OF CHINESE MEDICINE +1
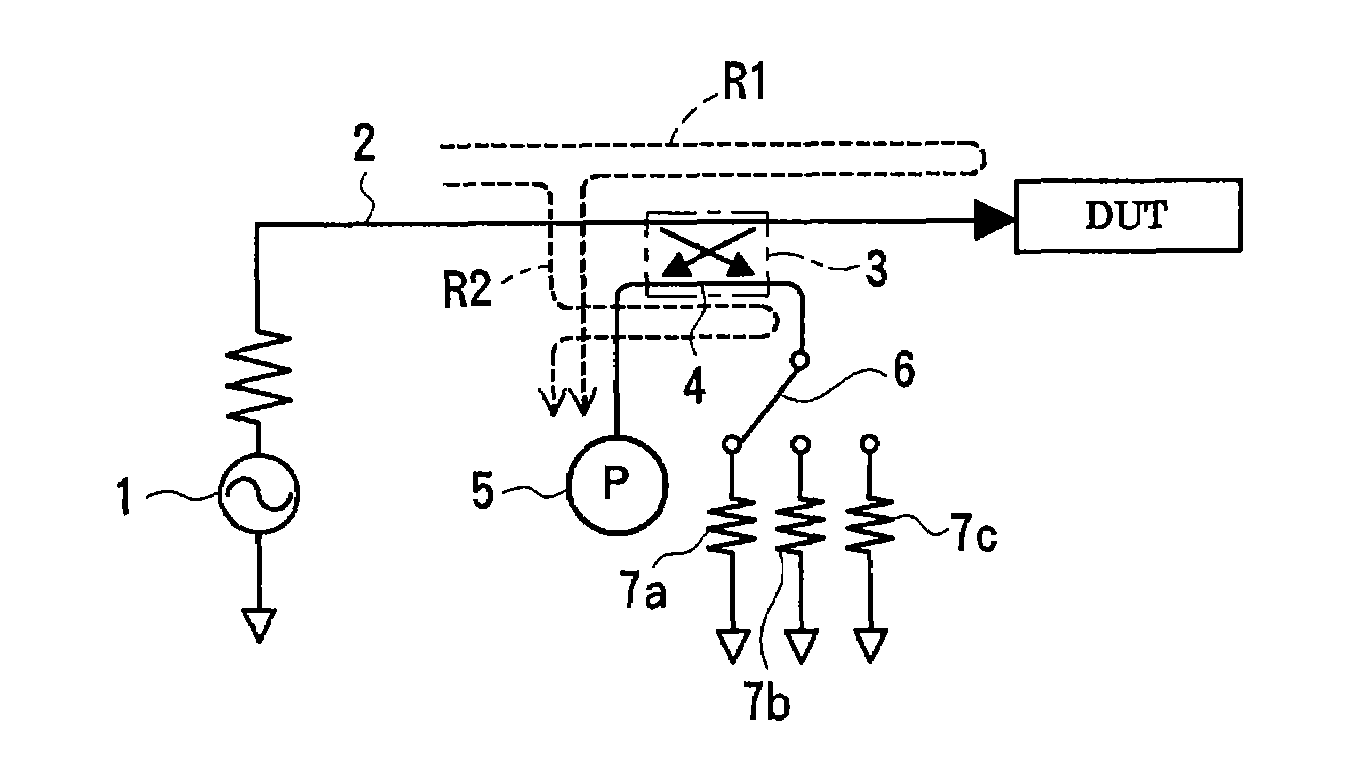
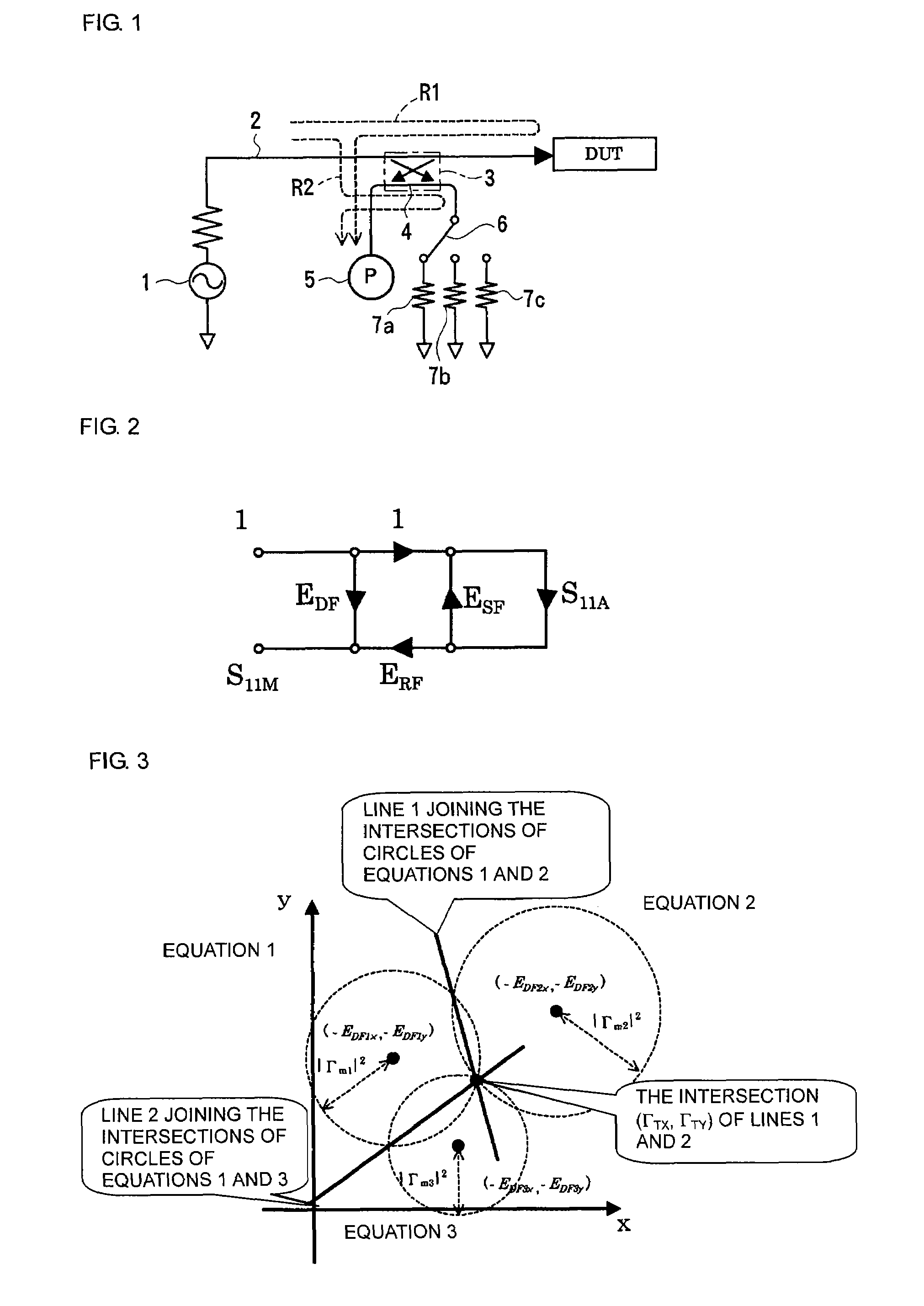
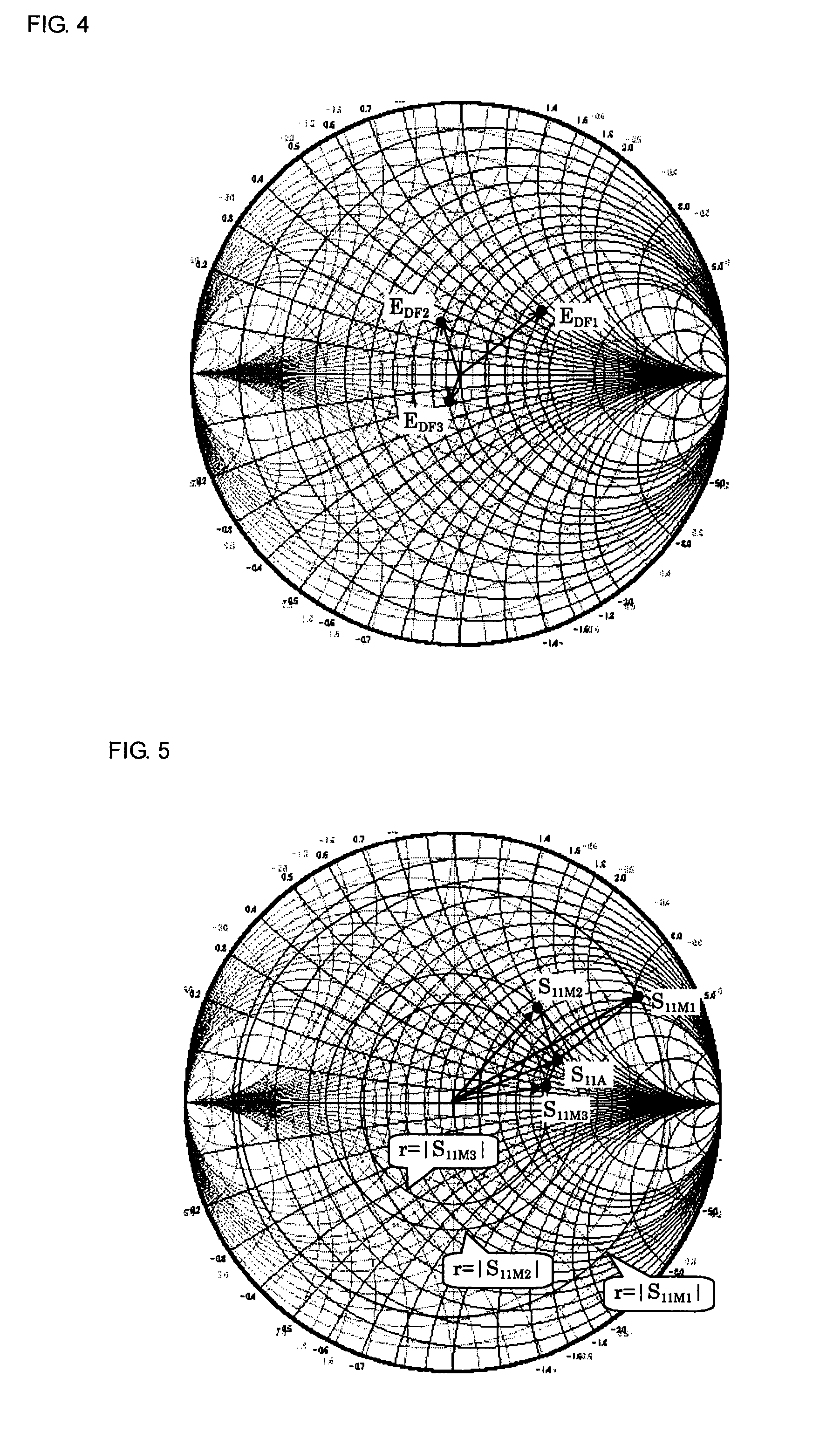
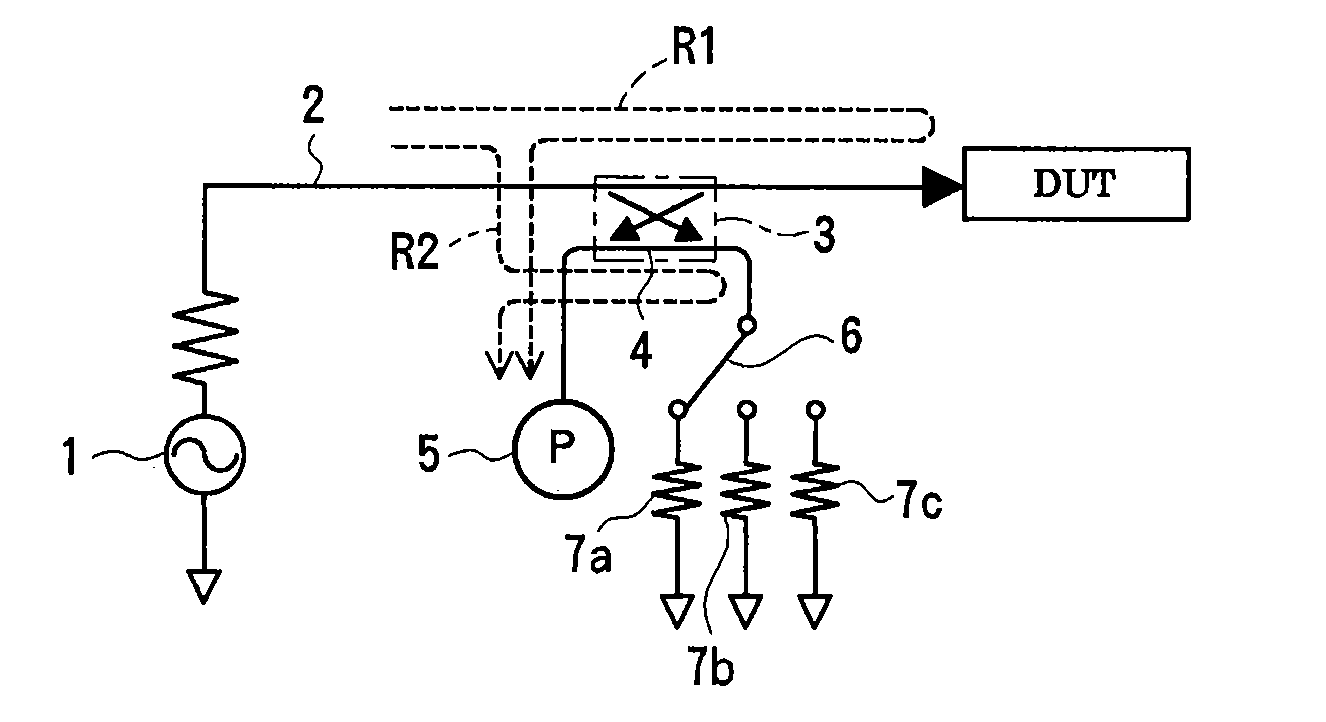
Method and apparatus for measuring scattering coefficient of device under test
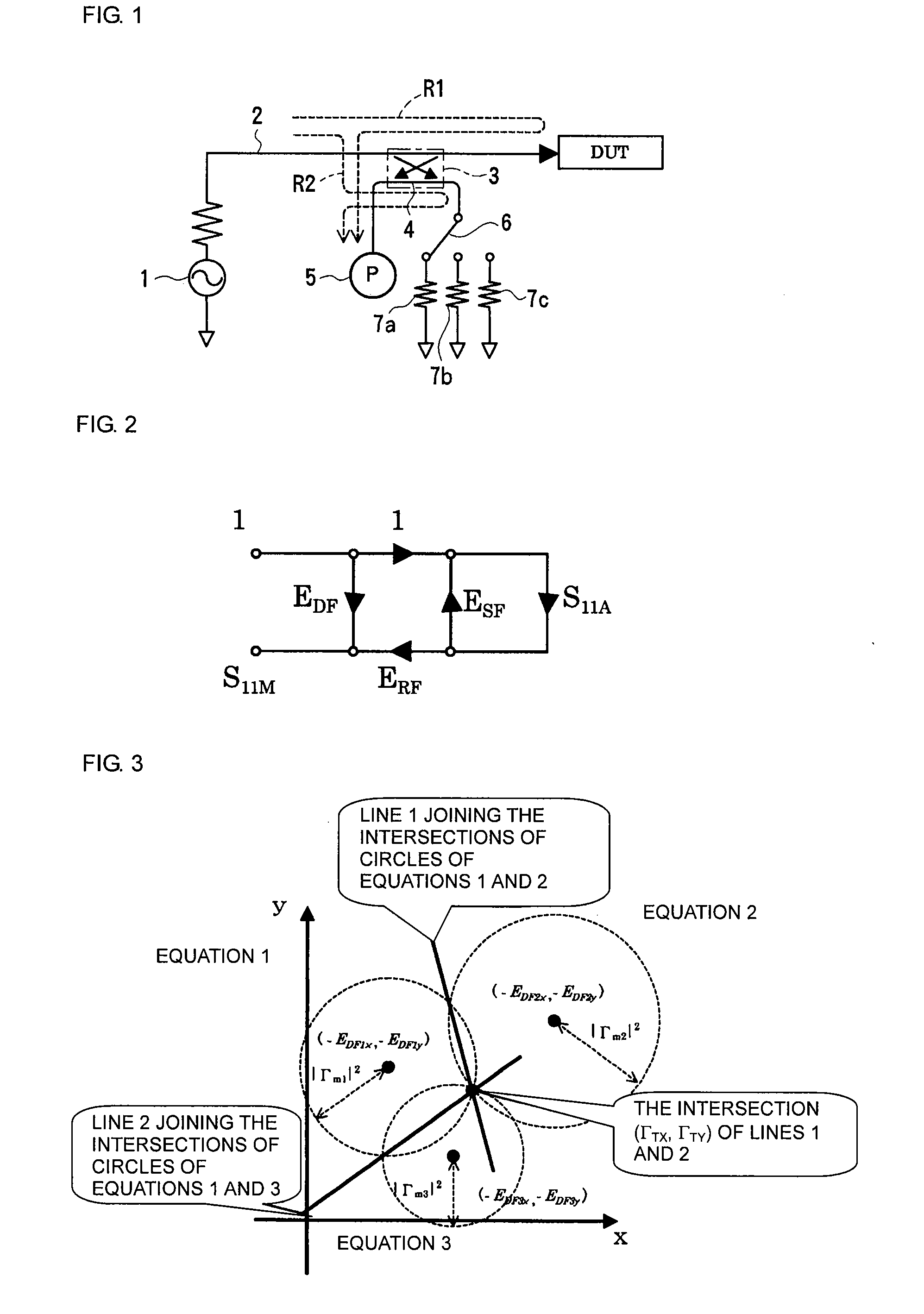
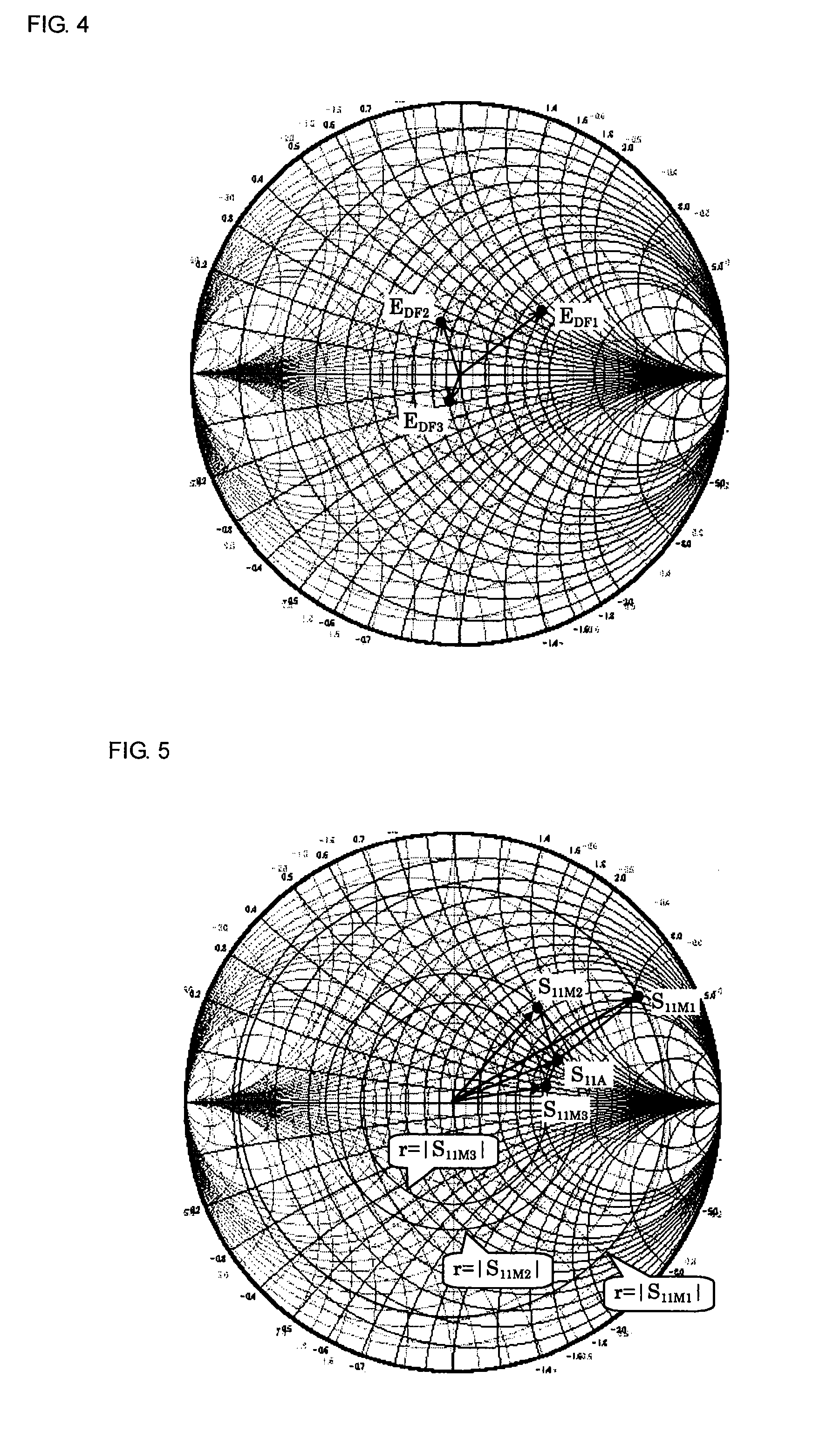
A measuring method and measurement system that includes a signal source that applies a signal to a device under test, a scalar measuring instrument that measures a reflected wave reflected from the device under test or a transmitted wave transmitted through the device under test as a scalar value, and a superimposing signal system that superimposes three different vector signals whose relation values are specified in advance on the reflected wave or the transmitted wave of the device under test. The three vector signals are superimposed on the reflected wave or the transmitted wave of the device under test, and the superimposed signals are each measured as a scalar value by the electric-power measuring instrument. The three measured scalar values are converted into a single vector value using the specified relation values of the three vector signals, thereby obtaining a transmission coefficient of the device under test.
Owner:MURATA MFG CO LTD
Bismuth molybdate-based catalysts, method of preparing thereof and method of preparing 1,3-butadiene using thereof
ActiveUS20090088594A1High activitySimple constituents and synthesis routesHydrocarbon by hydrogenationDistillation purification/separationDehydrogenationRaffinate
This invention relates to a bismuth molybdate catalyst, a preparation method thereof, and a method of preparing 1,3-butadiene using the same, and to a bismuth molybdate catalyst, a preparation method thereof, and a method of preparing 1,3-butadiene using the same, in which 1,3-butadiene can be prepared through oxidative dehydrogenation directly using a C4 mixture including n-butene and n-butane as a reactant in the presence of a mixed-phase bismuth molybdate catalyst including α-bismuth molybdate (Bi2Mo3On) and γ-bismuth molybdate (Bi2MoO6). According to this invention, the C4 raffinate, containing many impurities, is used as a reactant, without an additional n-butane separation process, thus obtaining 1,3-butadiene at high yield. Unlike complicated multicomponent-based metal oxides, the catalyst of the invention has simple constituents and synthesis routes, and can be easily formed through physical mixing, and thus is very advantageous in assuring reproducibility and can be directly applied to commercial processes.
Owner:SK GEO CENTRIC CO LTD +1
Method and Apparatus for Measuring Scattering Coefficient of Device Under Test
ActiveUS20080211515A1Low purityReduce in quantityResistance/reactance/impedenceMeasurement deviceMeasuring instrument
A measuring method and measuring apparatus for vector-measuring a scattering coefficient of a device under test substantially using a scalar measuring instrument while enabling a reduction in the size of the measuring instrument and the cost. The measurement system includes a signal source that applies a signal to a device under test, a scalar measuring instrument that measures a reflected wave reflected from the device under test or a transmitted wave transmitted through the device under test as a scalar value, and a superimposing signal system that superimposes three different vector signals whose relation values are specified in advance on the reflected wave or the transmitted wave of the device under test. The three vector signals are superimposed on the reflected wave or the transmitted wave of the device under test, and the superimposed signals are each measured as a scalar value by the electric-power measuring instrument. The three measured scalar values are converted into a single vector value using the specified relation values of the three vector signals, thereby obtaining a transmission coefficient of the device under test.
Owner:MURATA MFG CO LTD
Method of preparing clubbed nano-cellulose
The invention discloses a method for preparing nano-rod cellulose, which comprises the following steps: dispersing fiber raw material into sulphuric acid water solution of which mass concentration is 50 to 65 percent, hydrolyzing the fiber raw material by single-mode microwave radiation for 5 to 60 minutes at a temperature of between 20 and 50 DEG C and at the microwave radiation power of 10 to 100 watts, then adding distilled water into hydrolysis solution and diluting the hydrolysis solution to have 10 times of volume, and obtaining stable nano-rod cellulose suspension through post treatment processes such as centrifugal separation, dialysis, filter, ultrasonic dispersion and the like of the diluent, so as to obtain the nano-rod cellulose of which width is about 10 nanometers and lengthis about 200 to 300 nanometers.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
System and apparatus for measuring displacements in electro-active materials
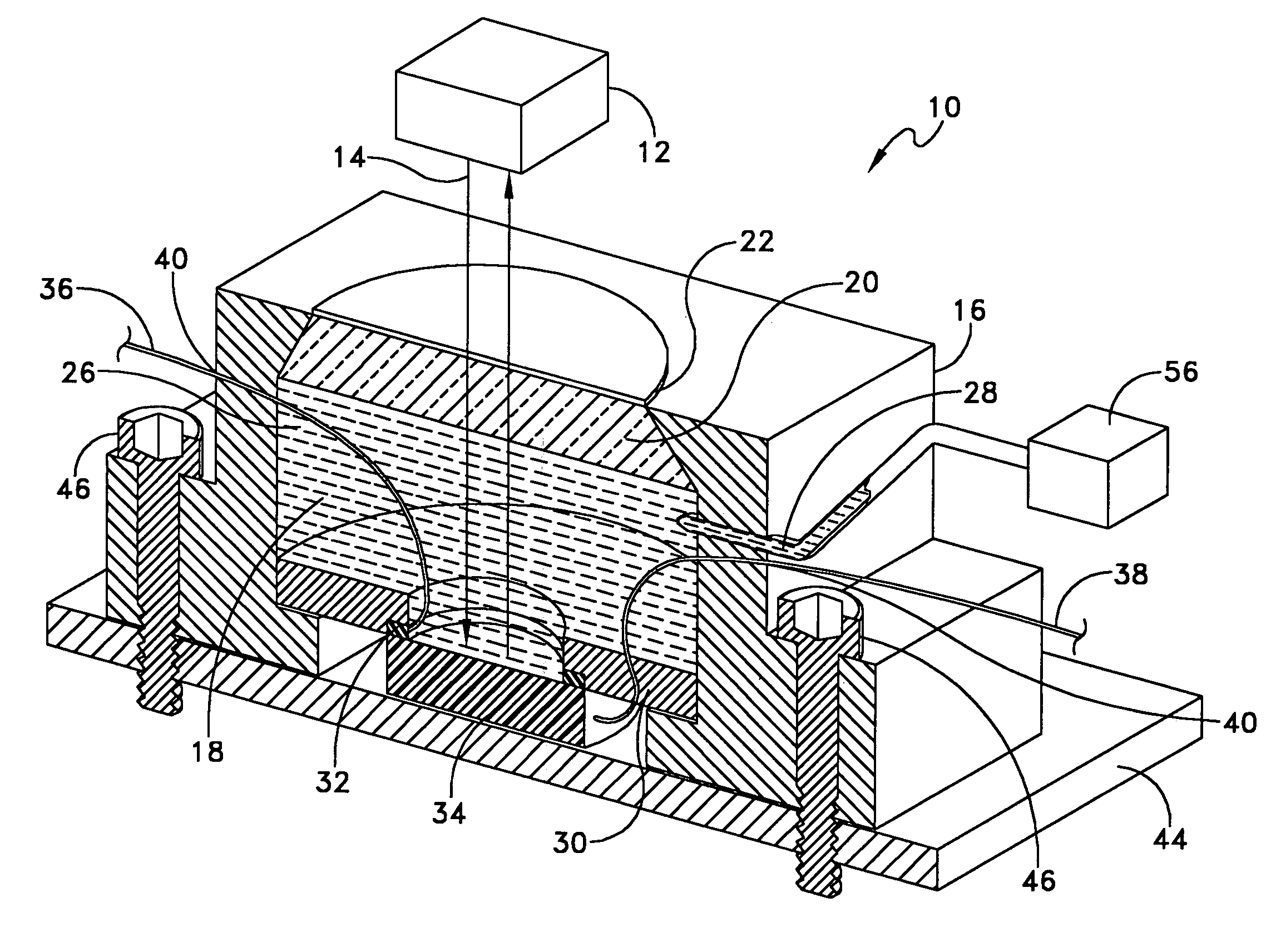
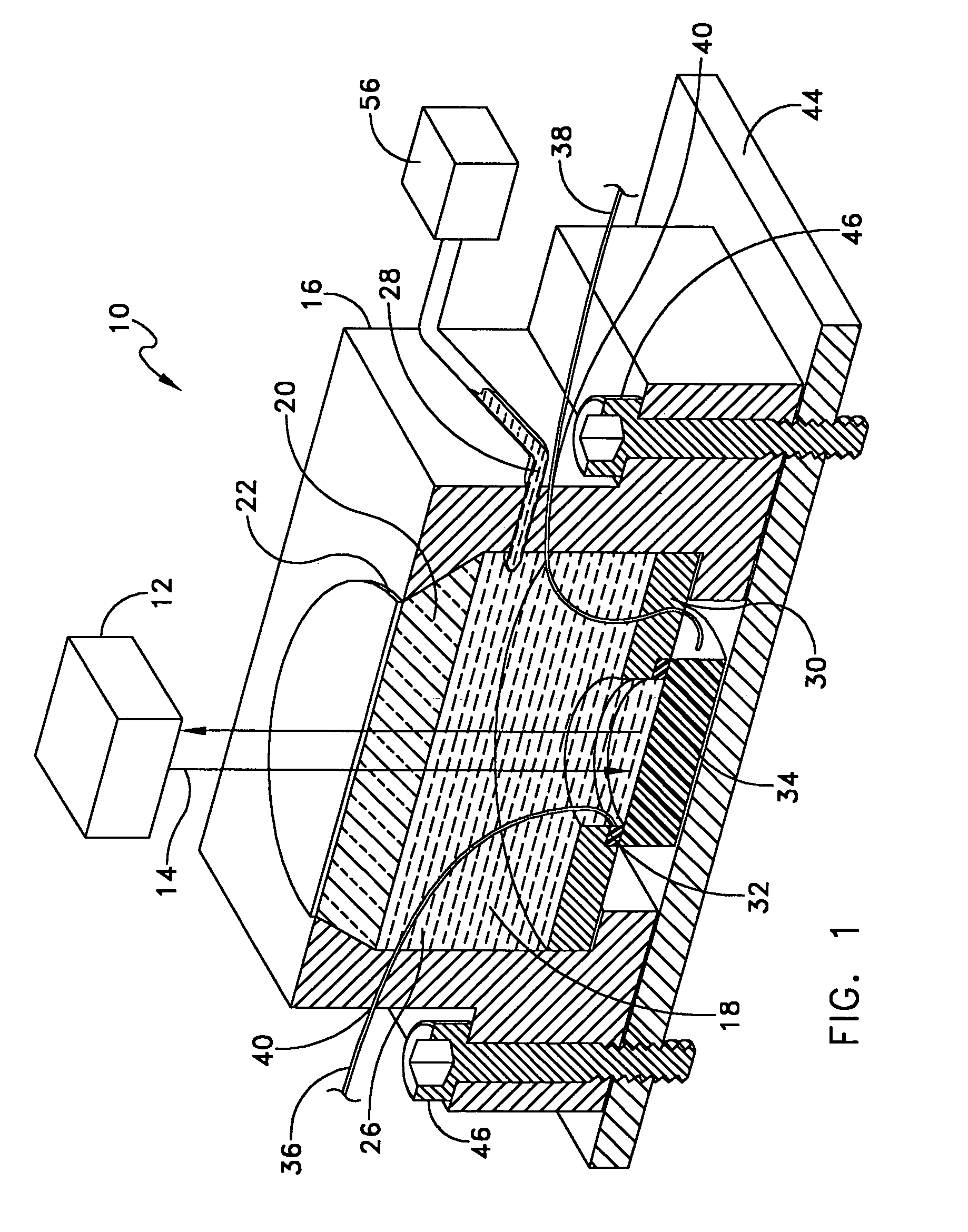
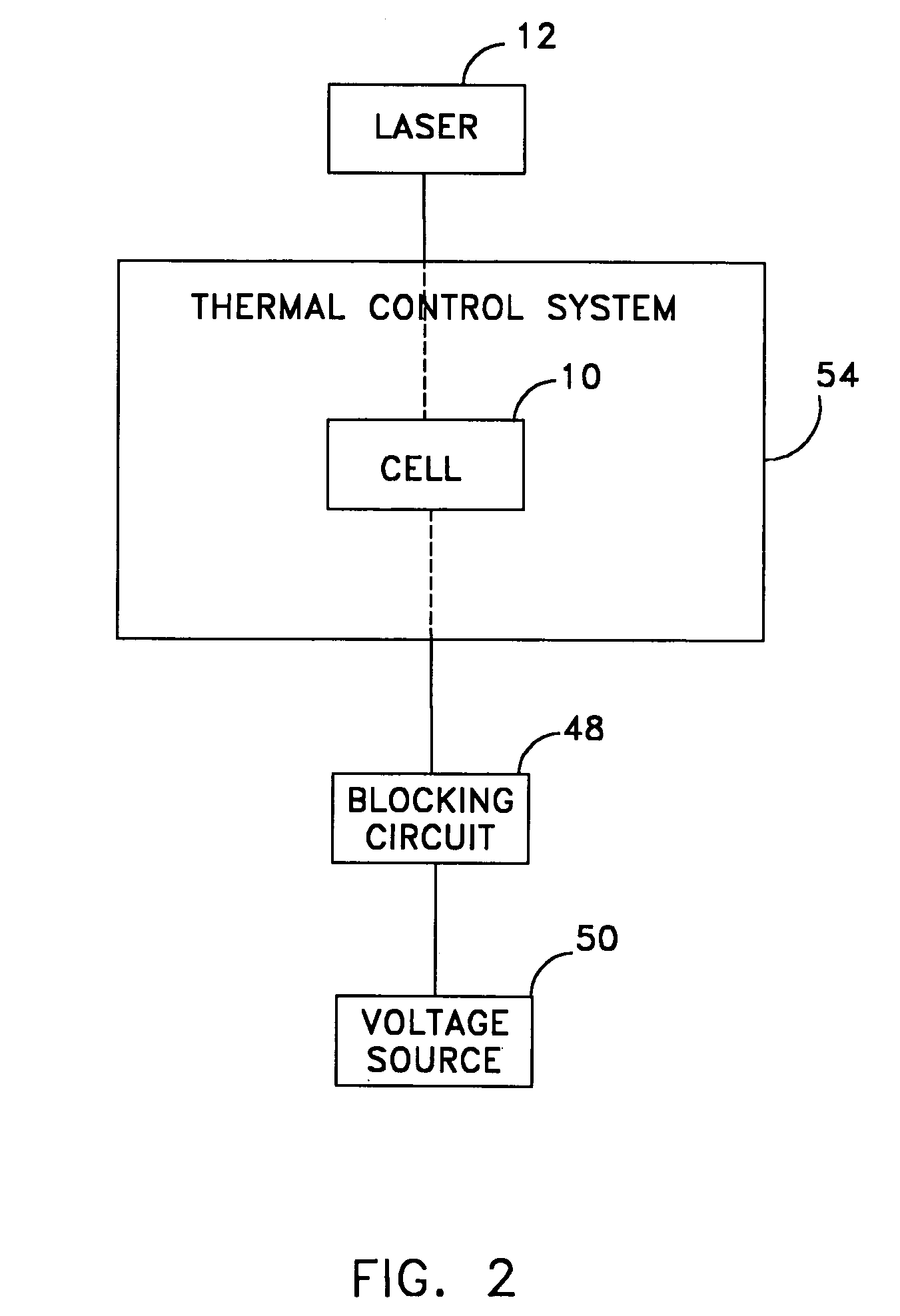
InactiveUS7236252B1Ensure reproducibilityTesting/calibration apparatusForce measurementThermal control systemUniaxial pressure
A device designed to apply uniaxial pressure to the surface of an electro-active material while simultaneously applying a current to the material under controlled temperature conditions and then measuring the displacement of the material by means of a laser interferometer. The device involves a housing with a chamber in which a sample of material is secured. The chamber has an aperture with a quartz window that allows the laser beam from the interferometer to pass. The sample is connected to electrodes and the chamber is filled with dielectric oil that applies the uniaxial pressure to one side of the sample. The device is placed onto a thermal control system. When the appropriate thermal and pressure conditions are established, current is applied to the sample and the interferometer measures the displacement.
Owner:THE US SEC OFTHE NAVY
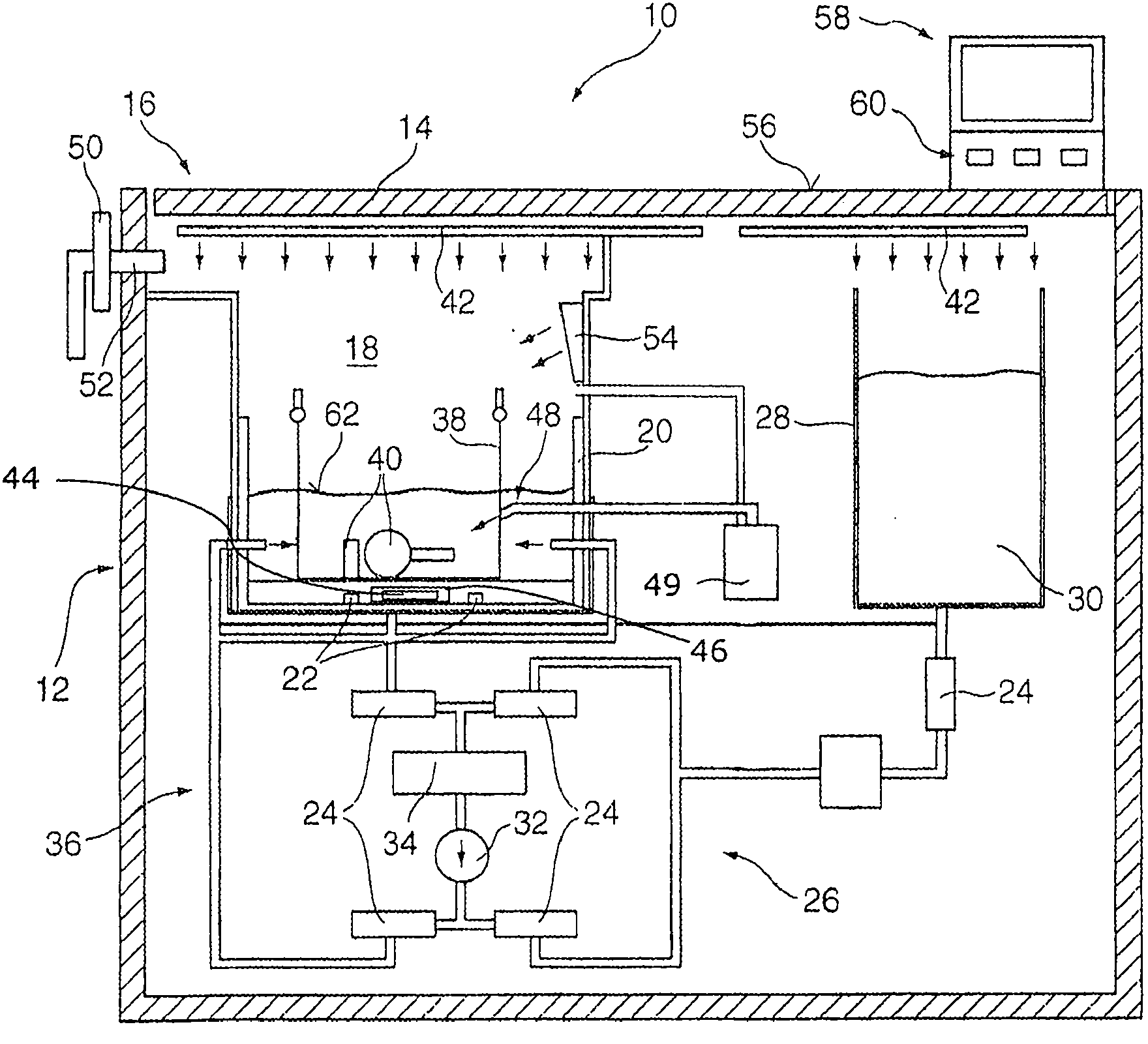
Heat treatment method and heat treatment apparatus
InactiveUS20090325393A1Prevent oxidationAvoid it happening againDomestic stoves or rangesLighting and heating apparatusAcetic anhydrideMaterials science
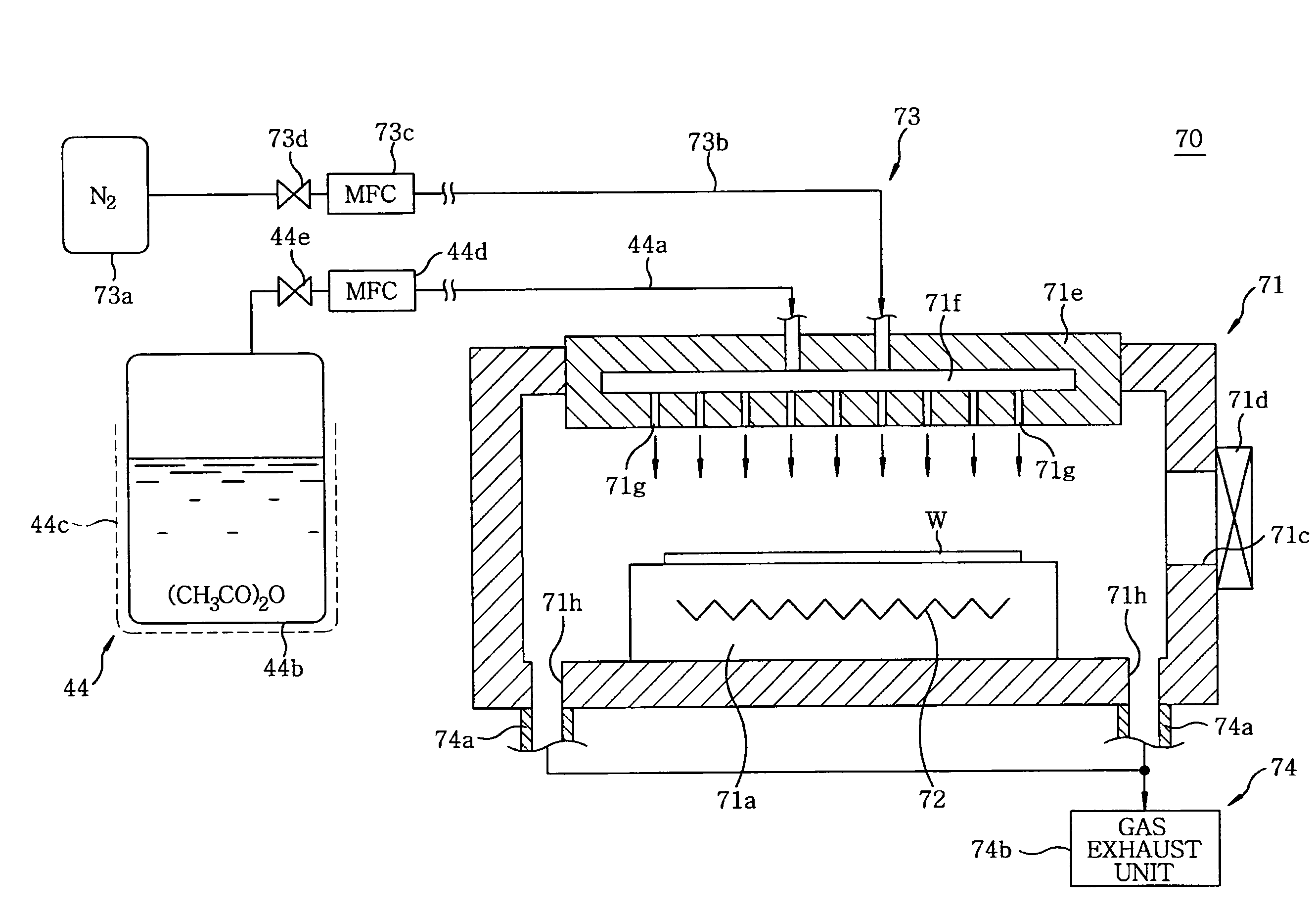
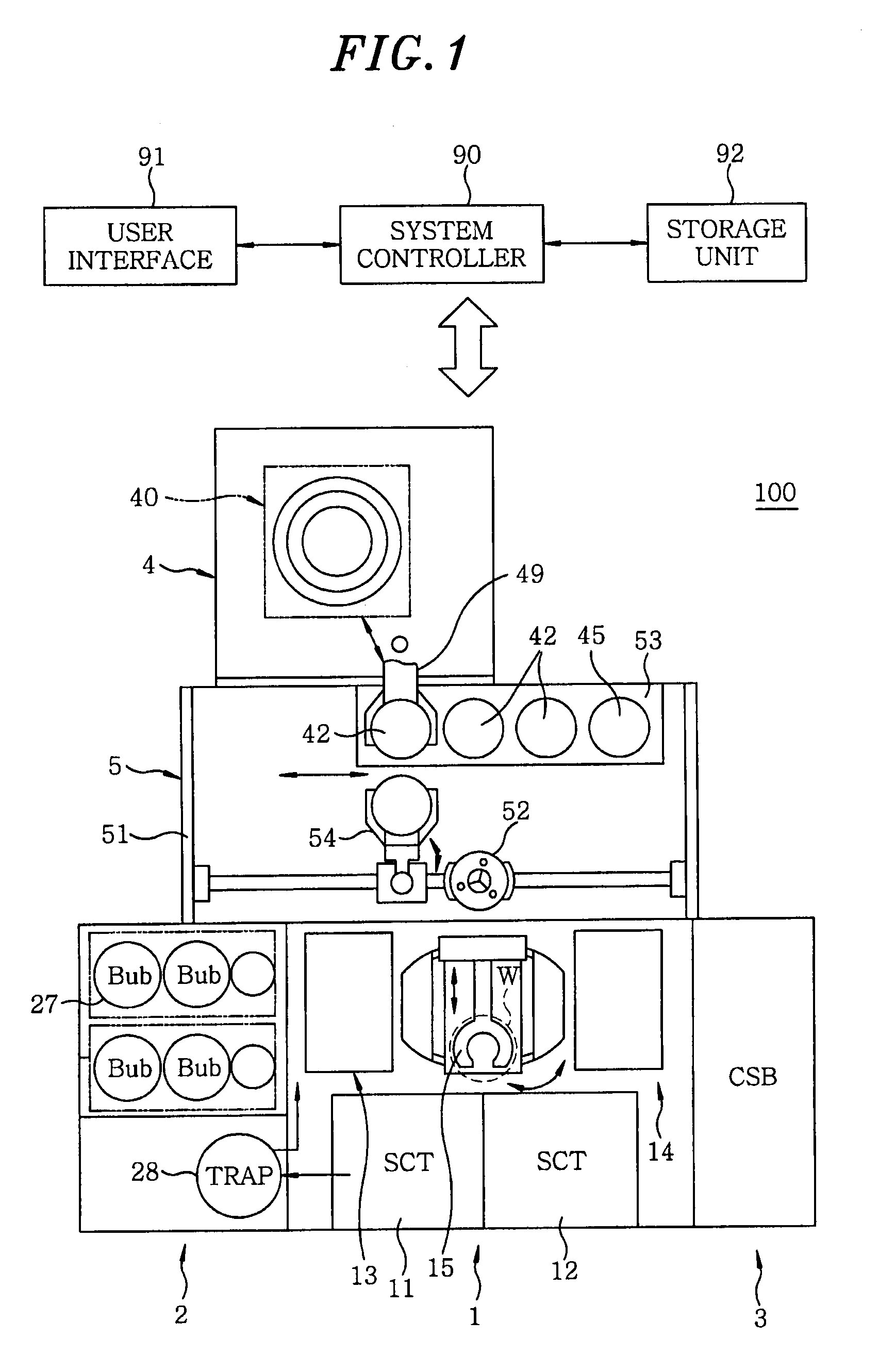
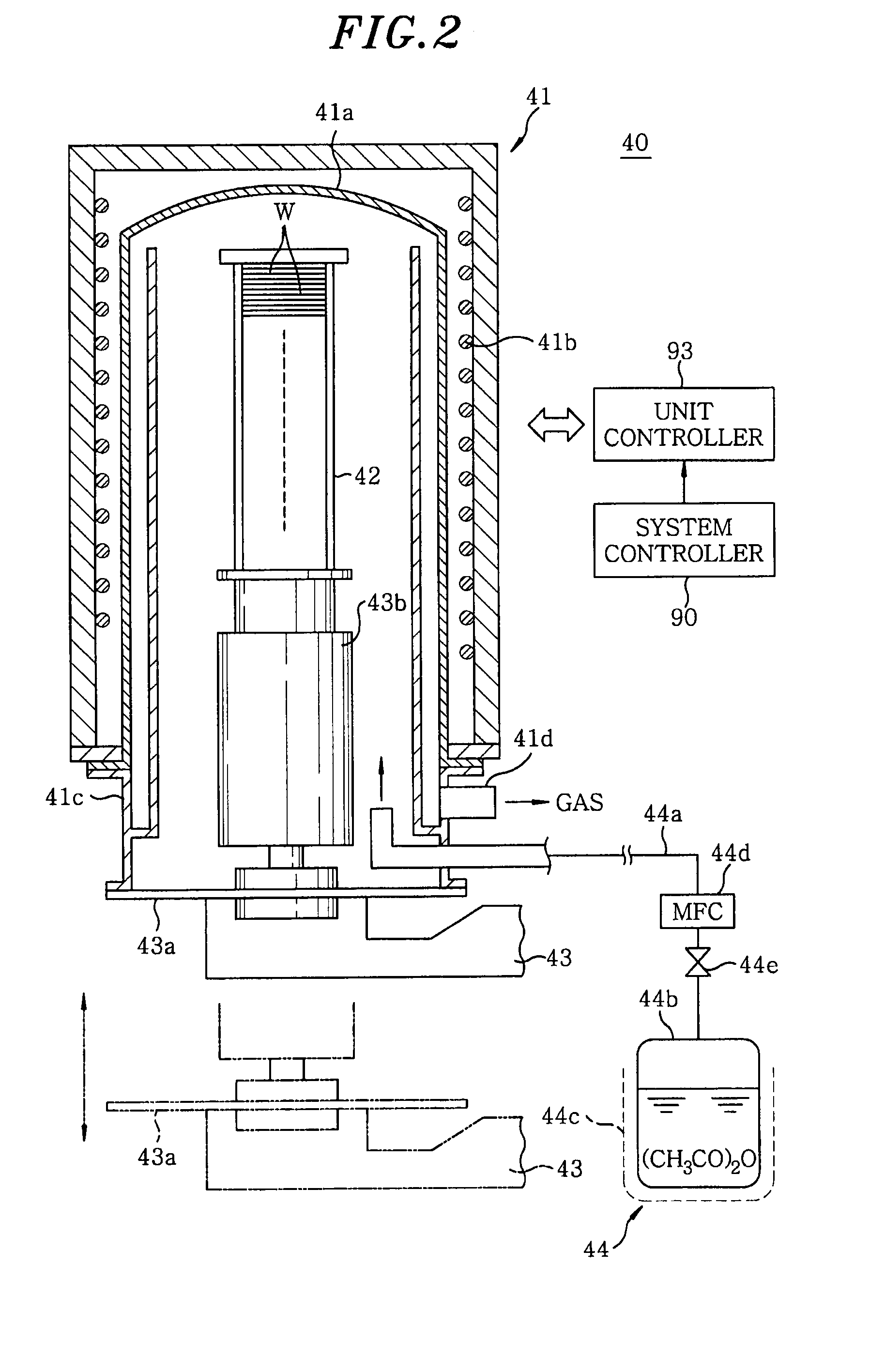
Disclosed is a heat treatment method including a step of placing a wafer W provided with a low-k film and a metal layer in a heat treatment furnace 41, a step of supplying gaseous acetic anhydride into the heat treatment furnace 41, while controlling the flow rate using a mass flow controller 44d, and a step of heating the wafer W in the heat treatment furnace 41 supplied with gaseous acetic anhydride by using a heater 41b provided in the heat treatment furnace 41.
Owner:TOKYO ELECTRON LTD
Printing apparatus and printing method
ActiveUS20160001577A1Reduce fluidityPreventing scummingCylinder pressesRotary intaglio printing pressEngineeringElectrical and Electronics engineering
A printing apparatus includes: a printing plate having a recessed portion formed therein shaped corresponding to a pattern to be formed on a substrate; an inking unit including an inkjet head that performs an inking process of ejecting a liquid into the recessed portion, the liquid having particles as a material of the pattern dispersed therein; a laminating unit that performs a laminating process of laminating the substrate on a surface having the recessed portion formed therein after the inking process; a post-drying unit that performs a drying process on the liquid inside the recessed portion in a state in which the substrate is laminated on the surface having the recessed portion formed therein, thereby to reduce the fluidity of the liquid; and a peeling unit that peels off the substrate from the printing plate after the drying process by the post-drying unit.
Owner:FUJIFILM CORP
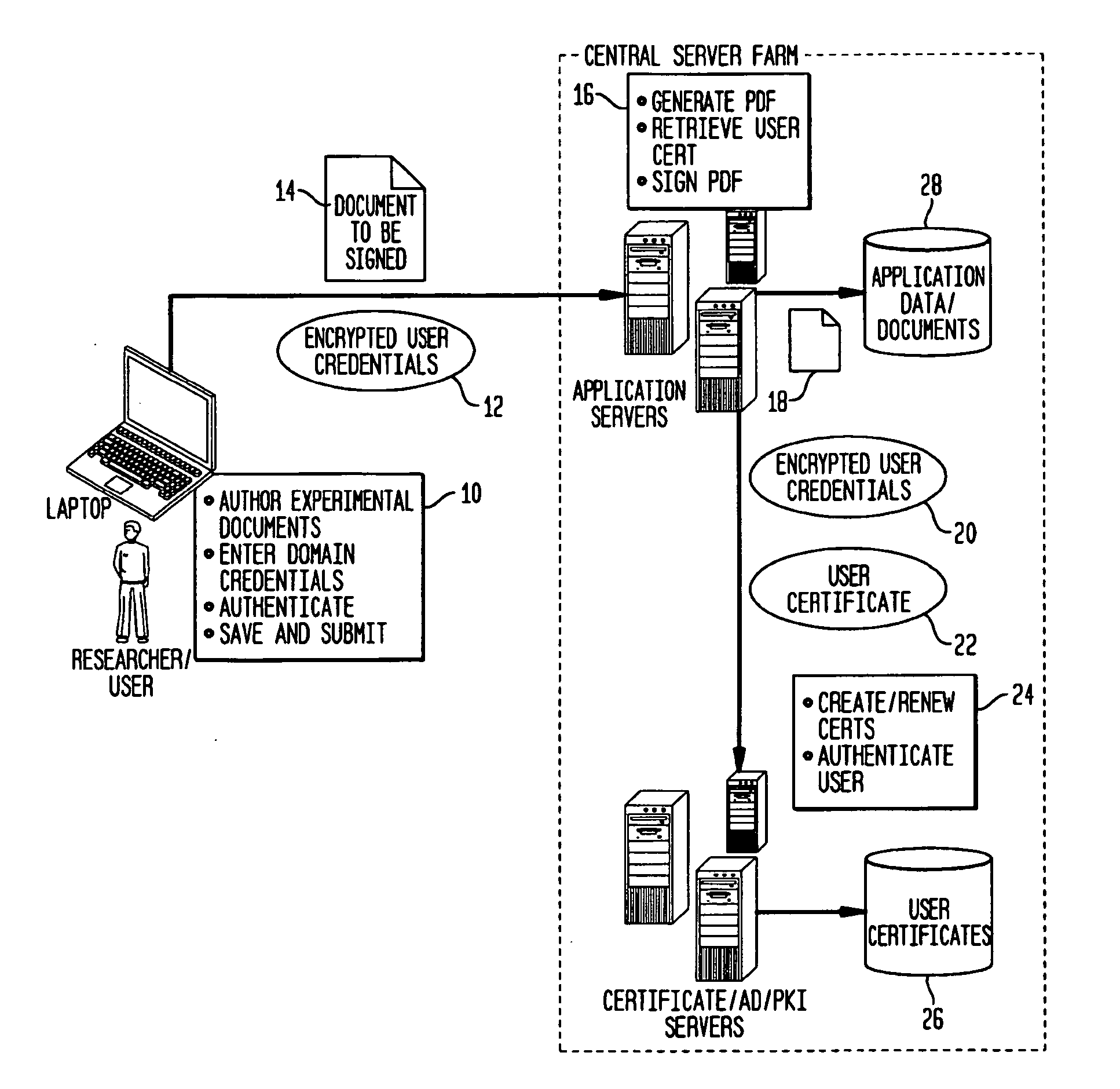
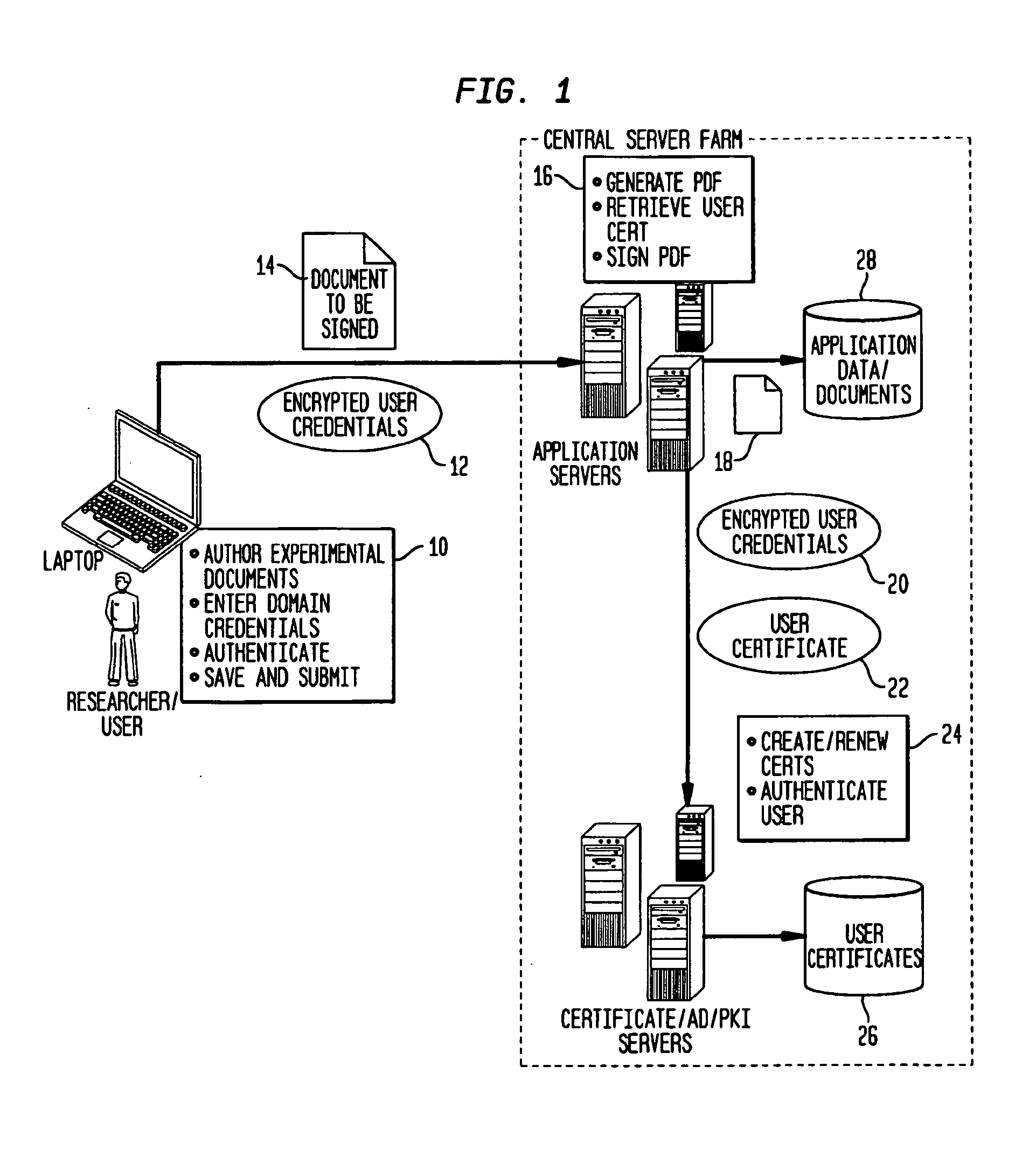
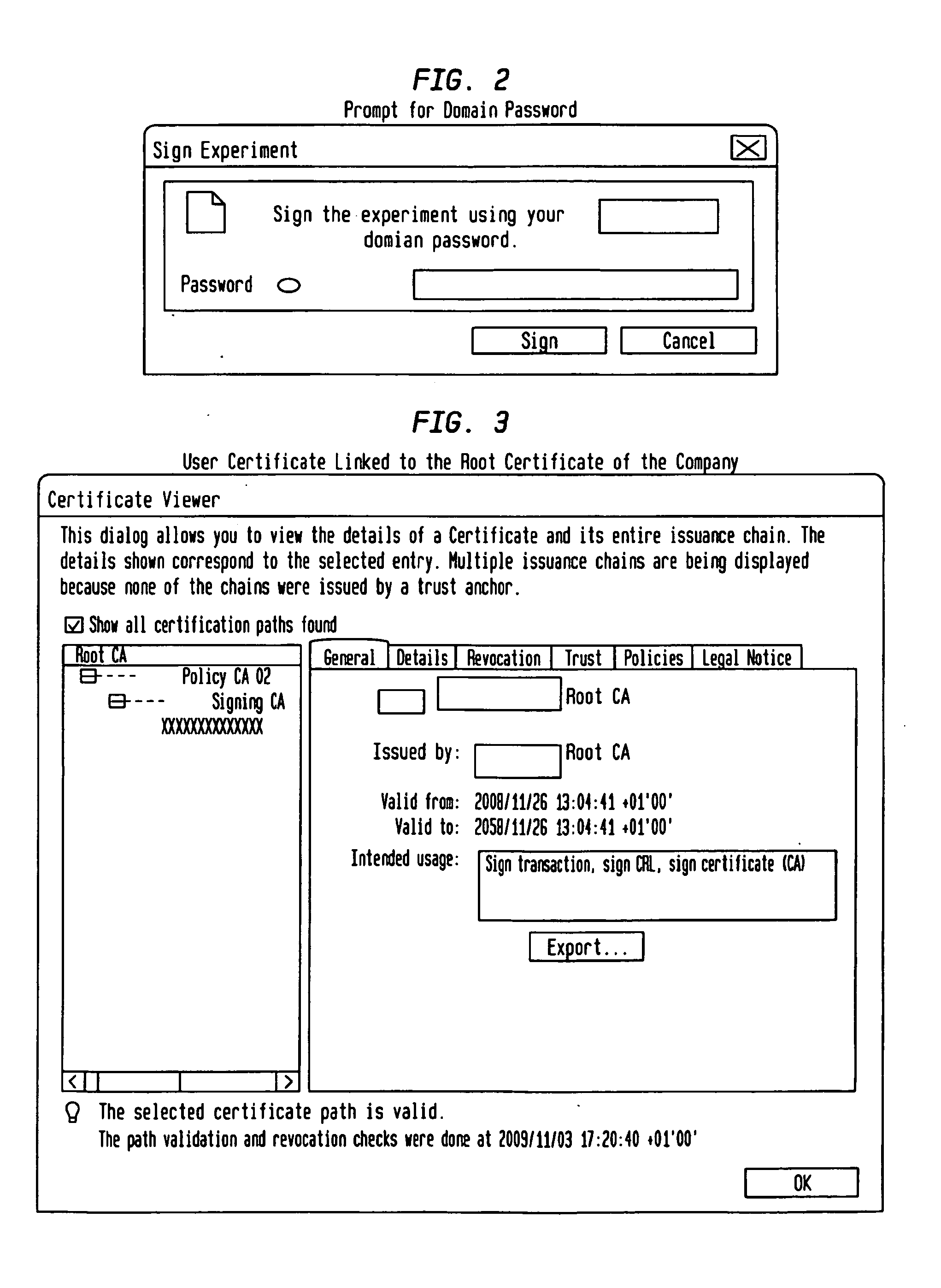
Fully Electronic Notebook (ELN) System And Method
InactiveUS20130160102A1Eliminate riskReduce the risk of fraudDigital data processing detailsDigital data protectionData validationData integrity
A system, for record keeping in scientific, industrial, and commercial applications where records are used to document inventions and discoveries, such as in a research laboratory. Such systems are referred to in the applicable field as Electronic Laboratory Notebooks (ELNs). The system deploys data validation and signature validation modules to ensure data integrity and satisfy legal requirements for signature and witnessing documents in a completely paperless environment.
Owner:NOVOZYMES AS
Fluid product dispenser holder opener
ActiveCN101128236AEnsure accuracy andEnsure reproducibilityLiquid flow controllersMedical devicesEngineeringMechanical engineering
Opener (40), designed to cut or pierce a single dose holder (21) such as a blister on a strip or disc, has a mechanism (41) that cuts the wall (23) of the product holder so that the cut portion does not obstruct the opening (25) formed in the holder. It comprises two piercing ends (42, 43) separated by a gap, each end being formed by a portion of a hollow cylinder with a cutting edge that allows an air flow to penetrate the holder.
Owner:APTAR FRANCE SAS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com