Patents
Literature
66results about How to "Reduce the impact of failure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
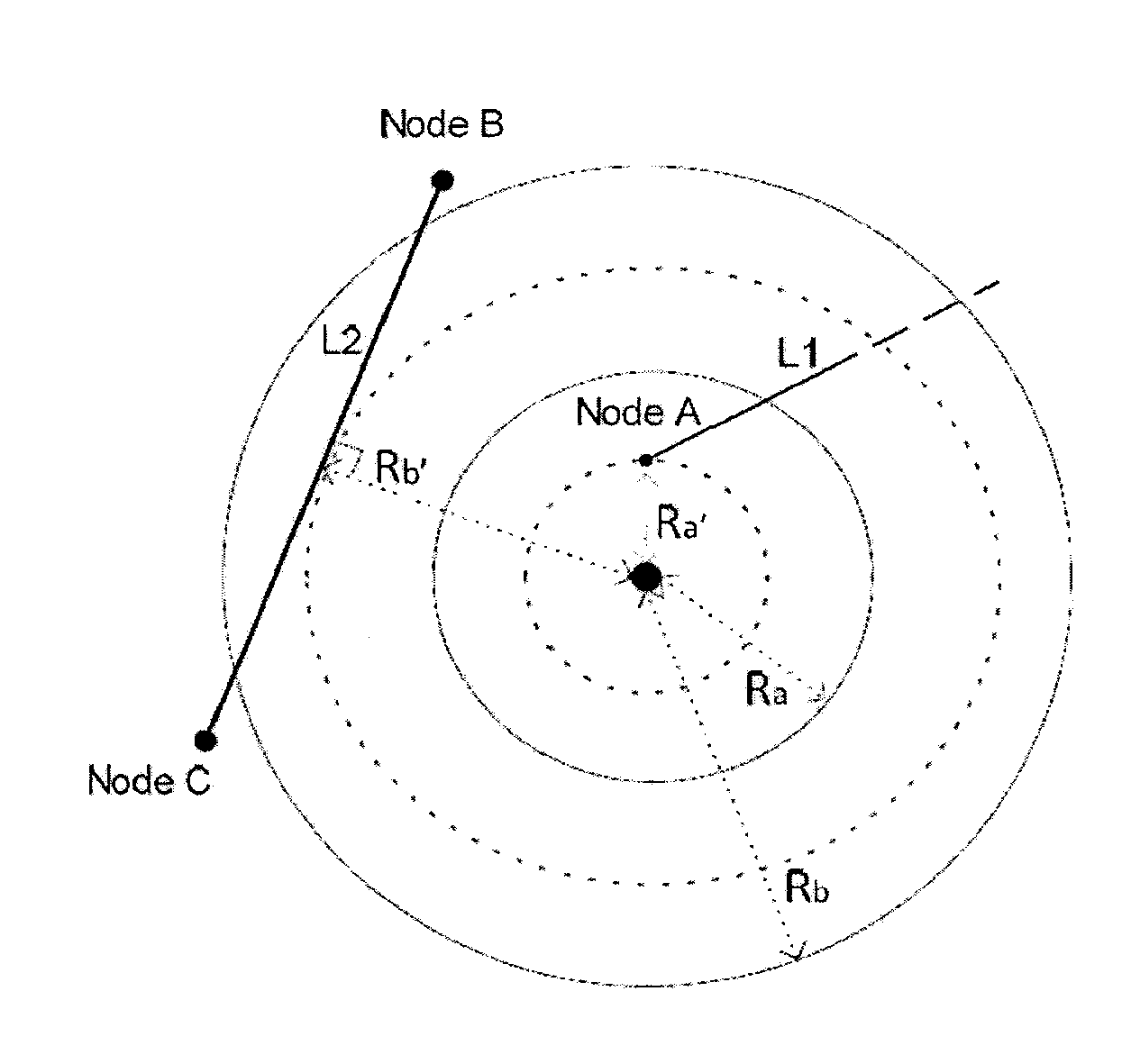
Proactive controller for failure resiliency in communication networks
ActiveUS20150195190A1Reduce the impact of failureHardware monitoringReliability/availability analysisTraffic capacityReal-time data
Network devices and systems relating to prevention of large-scale network failure and determining a probability of a large-scale network failure for a network. The network devices may control rerouting of network traffic from failed network paths to preventative paths with probabilities of failure below determined thresholds. The network devices monitor and process real time data for dynamic and proactive rerouting when large-scale network failure occurs or is likely to occur.
Owner:UNIV OF ONTARION INST OF TECH
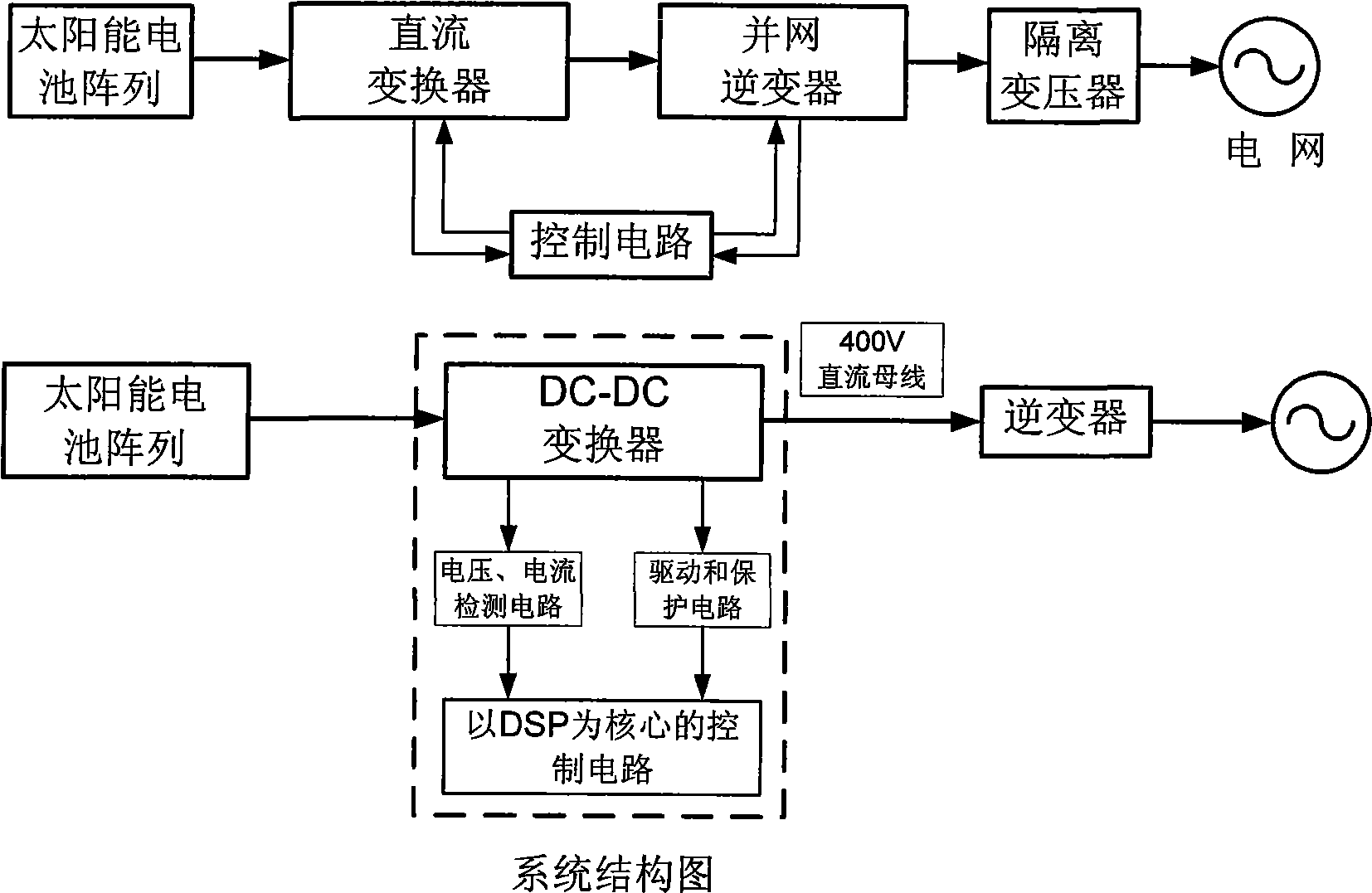
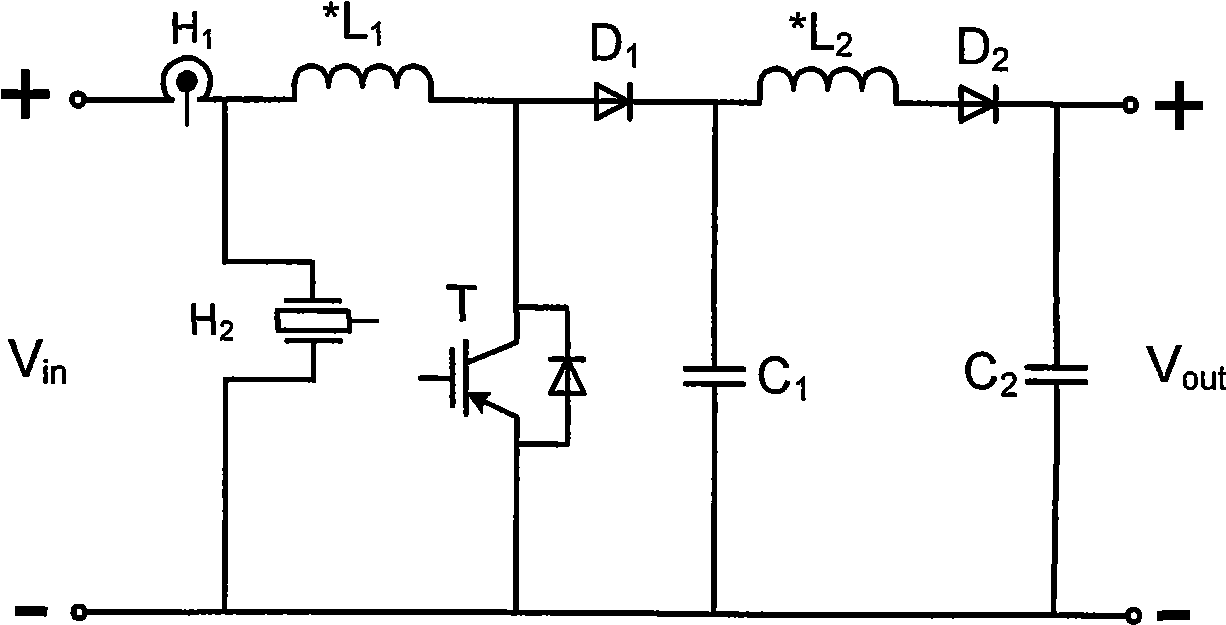
Photovoltaic grid connection power generation system based on DC converter and working method thereof
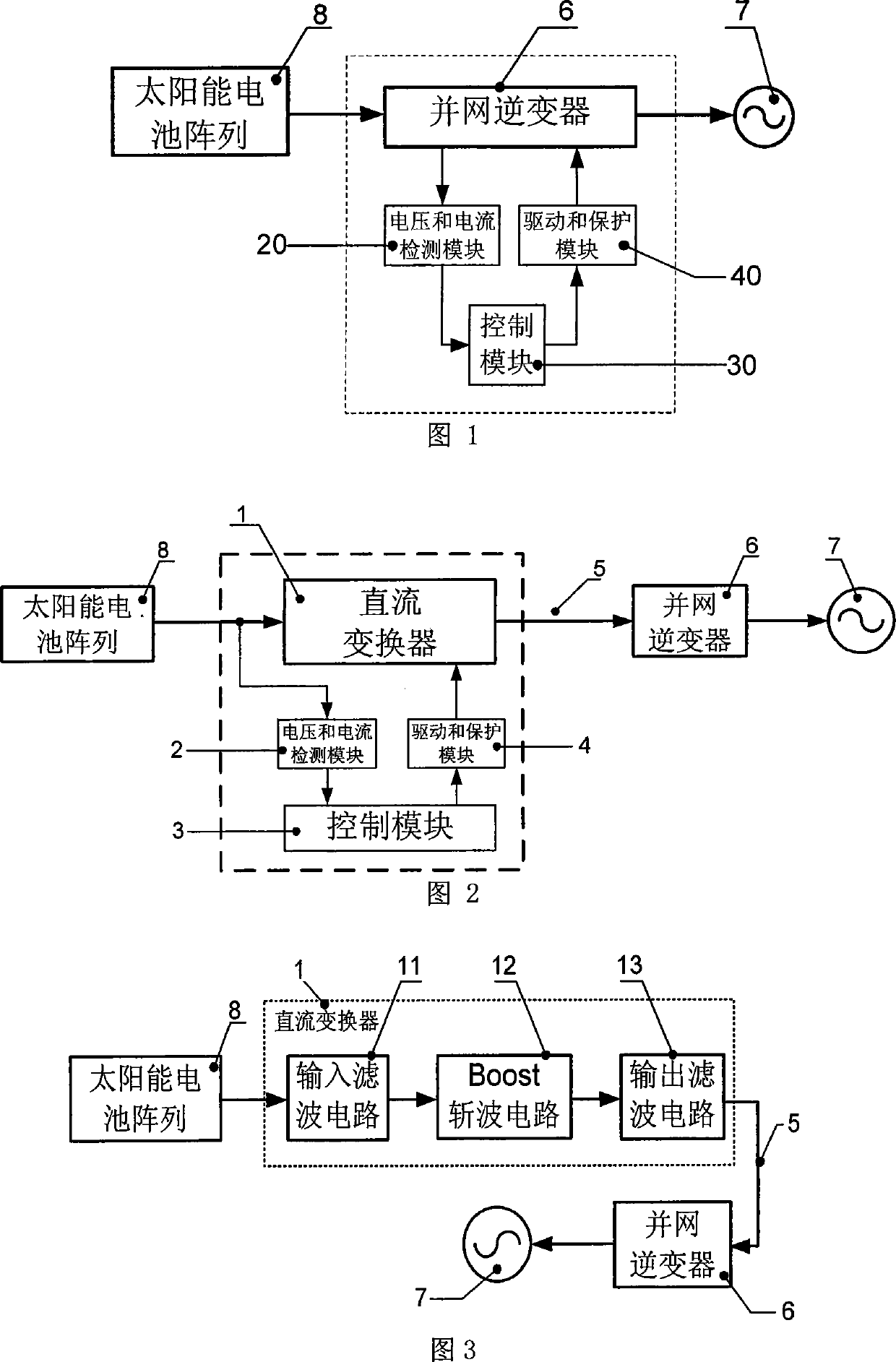
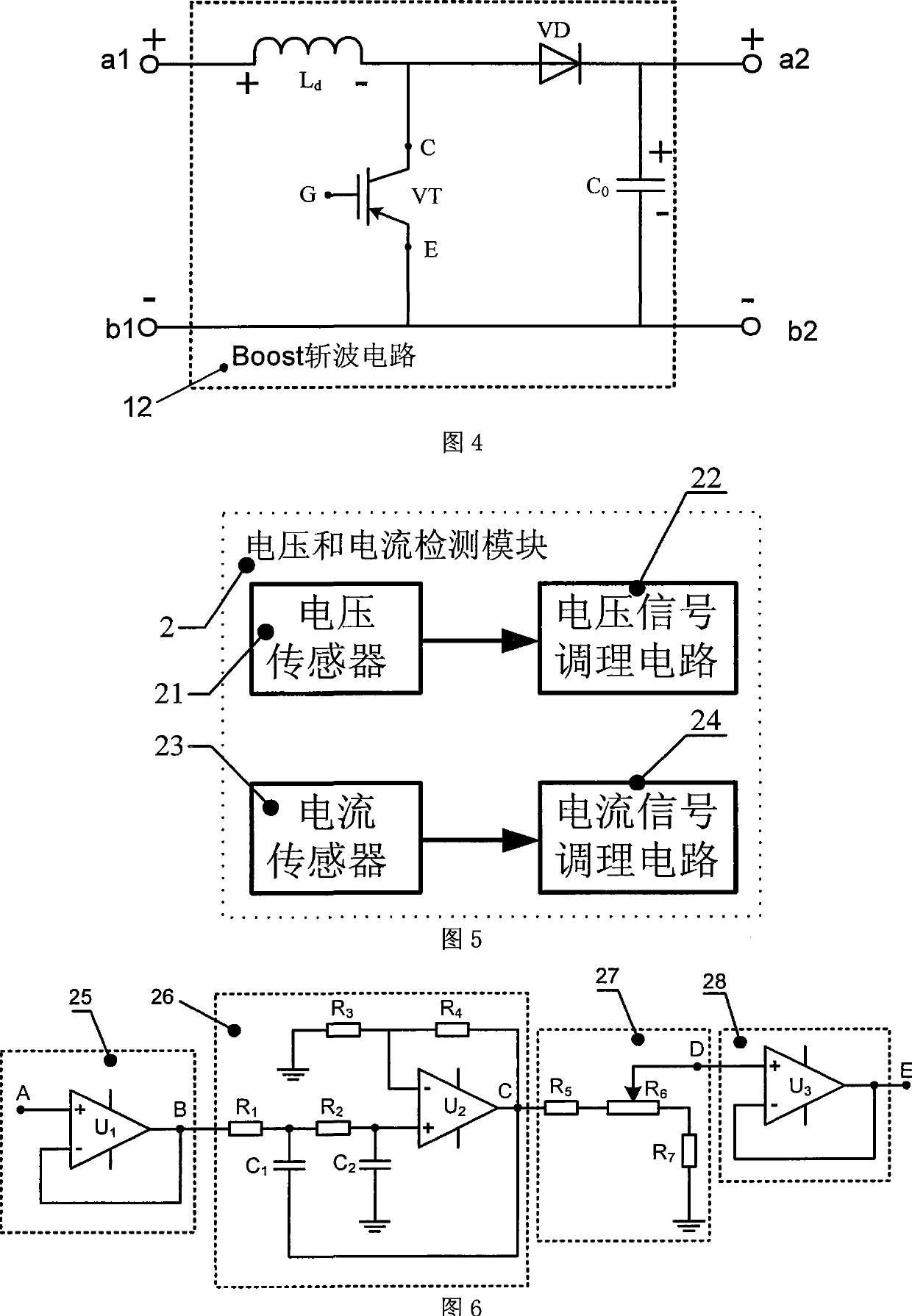
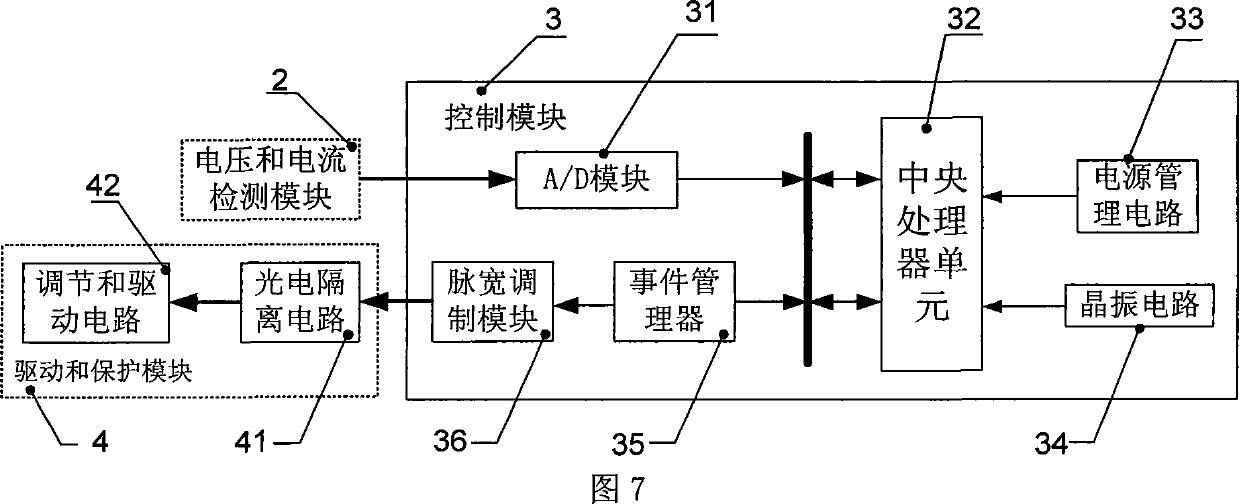
InactiveCN101499666ADifficult to achieveLow costSingle network parallel feeding arrangementsPhotovoltaic energy generationDc converterGrid connection
The invention provides a grid-connected photovoltaic power generating system based on a novel direct current converter and a working method of the grid-connected photovoltaic power generating system. The grid-connected photovoltaic power generating system is characterized in that the grid-connected photovoltaic generating power system consists of a direct current converter, a grid-connected inverter, an isolation transformer and a control circuit; the working method comprises the steps as follows: 1. collecting signal; 2. MPPT module data processing; 3 applying an MPPT arithmetic; 4 inverter module data processing; 5. applying a grid-connected arithmetic; and 6. applying SPWM arithmetic. The grid-connected photovoltaic power generating system and the working method thereof have the advantages of small technical realization difficulty, obviously reducing the cost, flexible installation, convenient maintenance, high voltage and current detection precision, advanced control arithmetic, quick running speed of control chip, the system being capable of obtaining excellent tracking precision and stability, combining the hardware device with the software programming of a digital signal processor, simple design, low cost and easy realization of the hardware device, brief and understandable software programming arithmetic.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
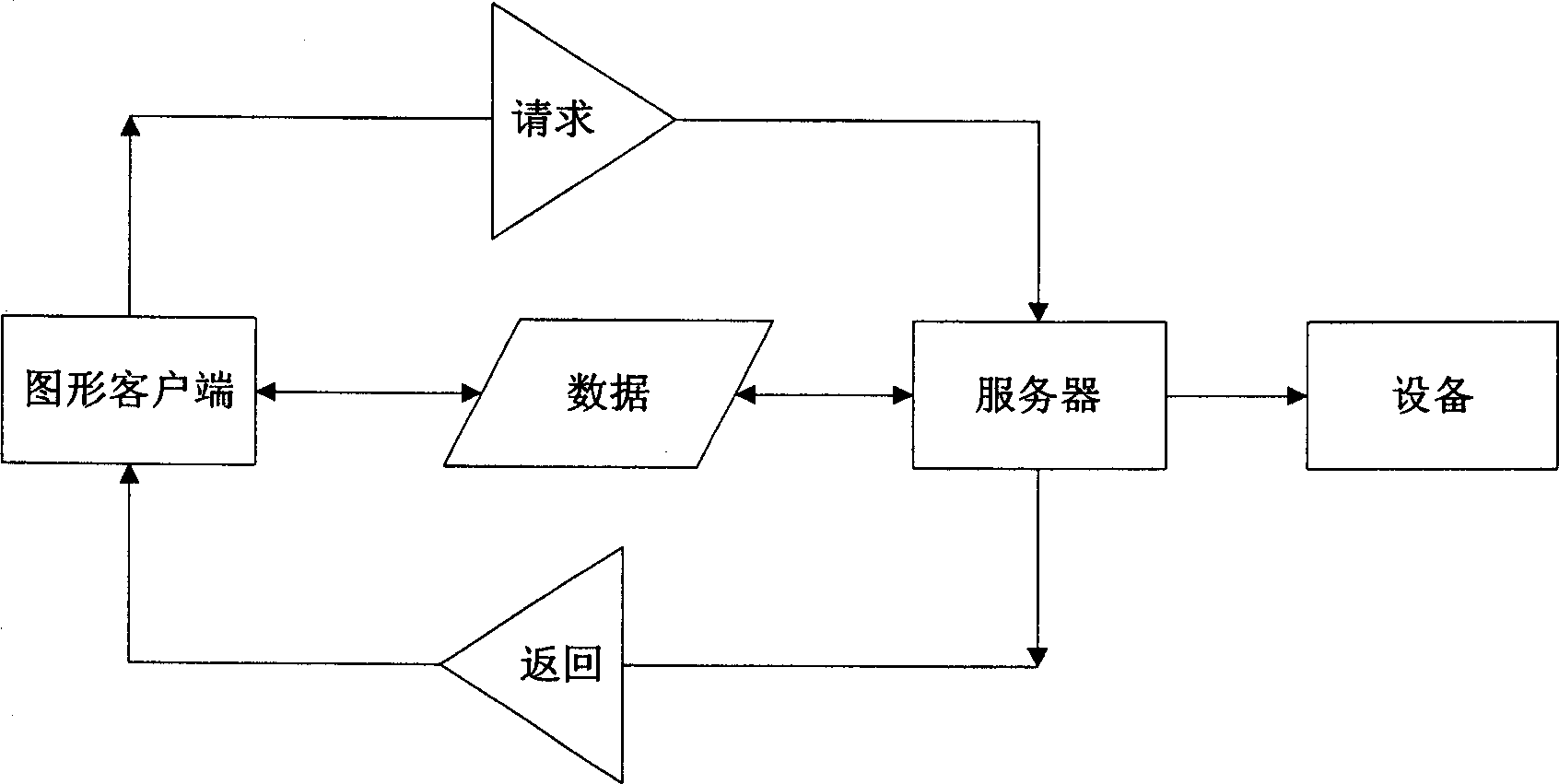
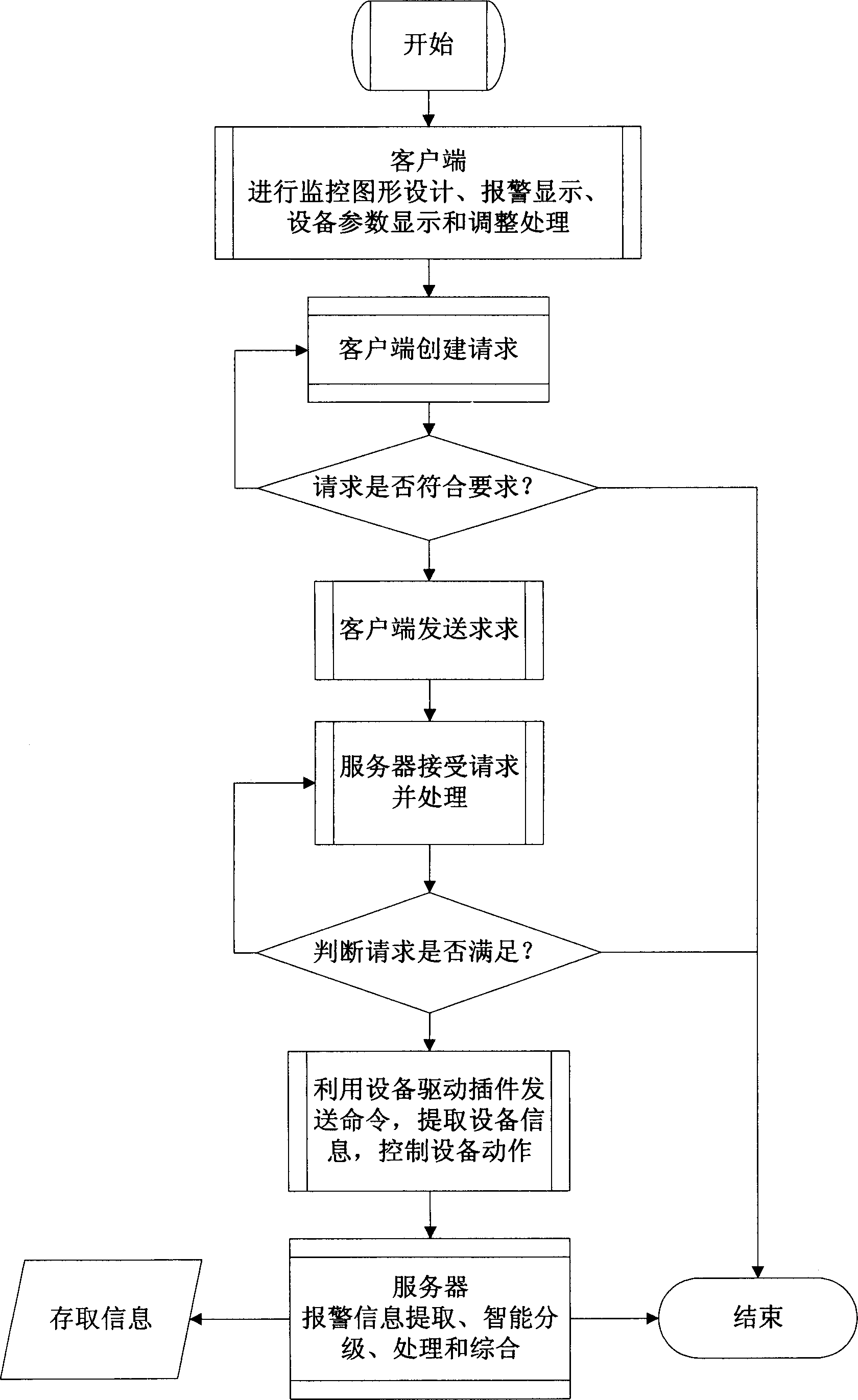
Alarm processing system and method
InactiveCN1870750AImprove alarm visibilityReduce the impact of failureClosed circuit television systemsAlarmsProcess systemsInformation delivery
This invention relates to an alarm process system and a method, in which, the system includes an information combined process server, a data exchange device and a pattern customer end, the method includes: the information combined process server picks up and records the operation information of devices then tansmits the information to the data exchange device, which transfers the operation information to the pattern customer end to crry out mode combination of sound, light and pattern and display alarm information based on the alarm levels.
Owner:BEIJING FOUNDER ELECTRONICS CO LTD +1
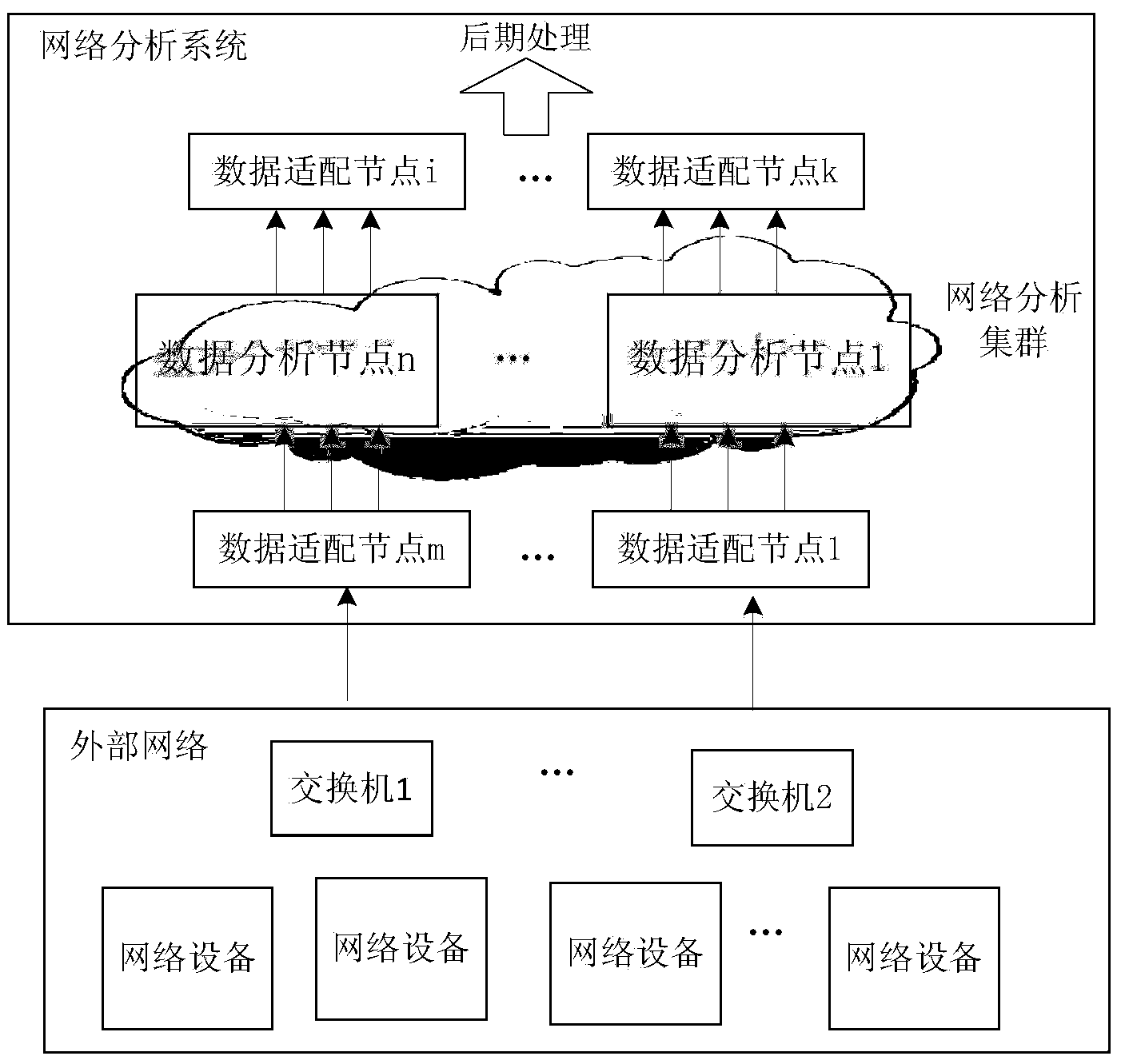
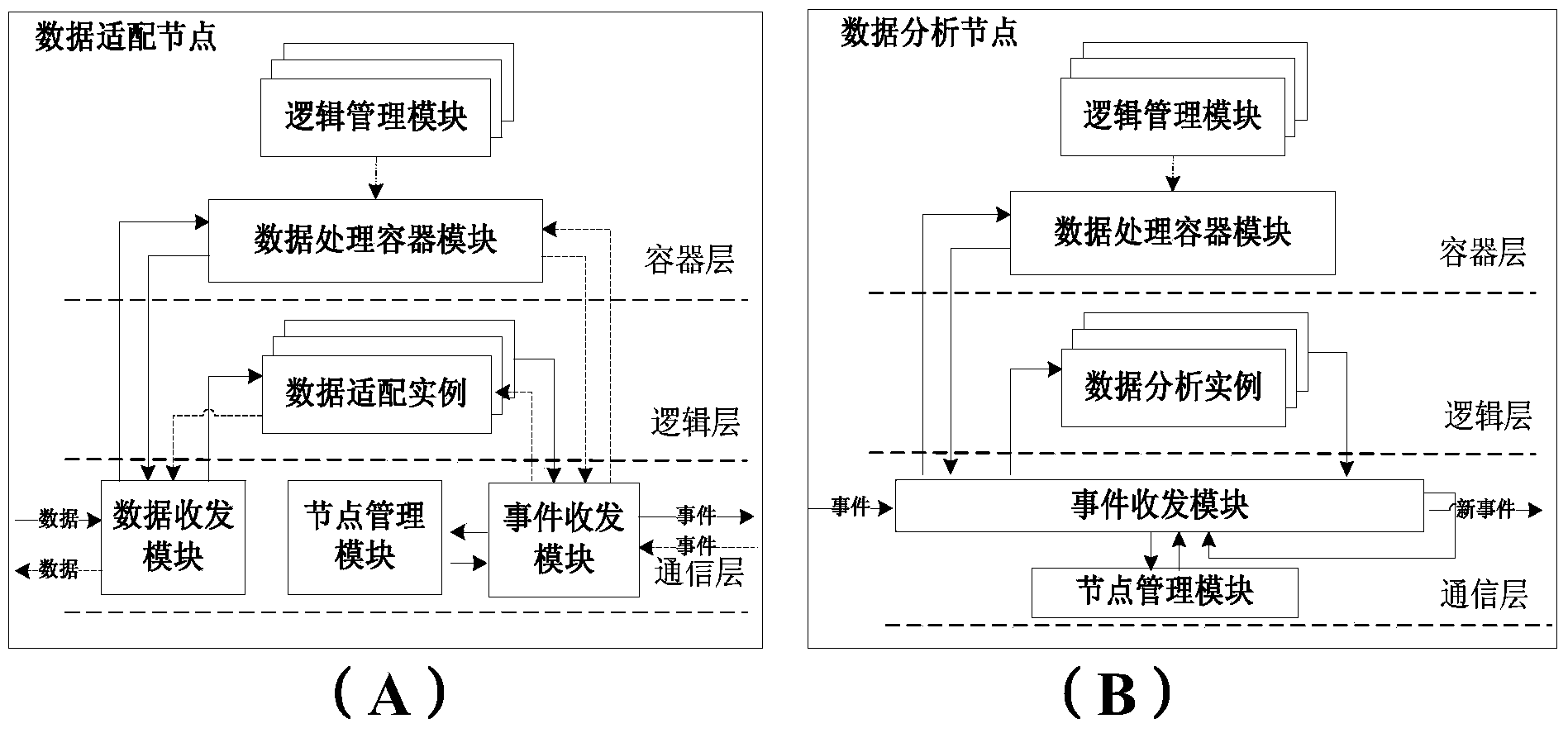
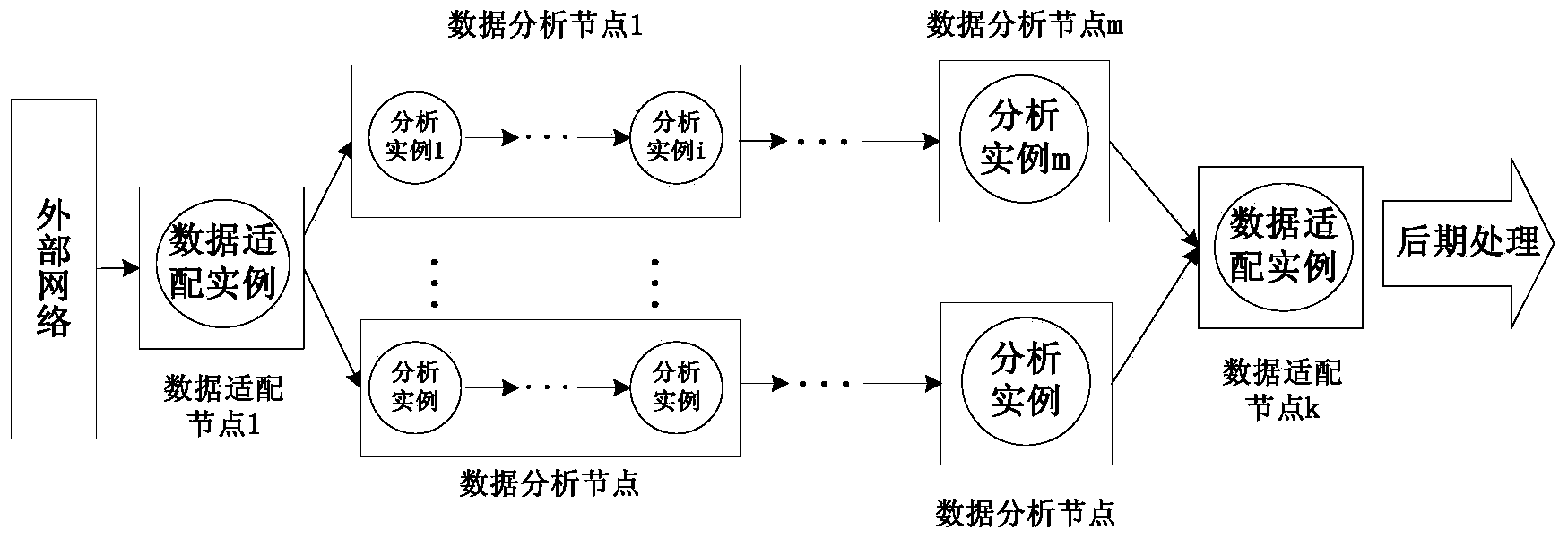
Network analytic system and method supporting real-time mass data processing
ActiveCN103560943AEasy to handleReduce business capability requirementsData switching networksReal time analysisNetwork analytics
Provided are a network analytic system and method based on the mass data real-time processing technology. The system comprises a plurality of data adaptive nodes and data analytic clusters which are composed of a plurality of data analytic nodes and arranged in a network in a distribution mode, wherein the data analytic nodes support a P2P networking mode and a load balancing mechanism, so that the data analytic clusters have a telescopic function; the flow line type analytic processing process is completed among the data analytic nodes through an incident mechanism. According to the network analytic system and method supporting the real-time mass data processing, mass network data including operations of network fault monitoring, statistics, trouble shooting, diagnosis and the like can be analyzed and processed in real time, the network data can be analyzed in a fine mode, distribution type dynamic expansion is supported to expand system functions, and users can expand demands of themselves according to the analytic types to be detected in a self-defined manner. Furthermore, a distribution type structure is utilized by the system without excessively depending on single hardware performance, and the system can complete processing of complex logics of network data analysis and the like better. The system and method support multiple types of processing logics and lower the requirements for professional qualification of developers.
Owner:BEIJING UNIV OF POSTS & TELECOMM
Electric power communication equipment alarm information processing method and device
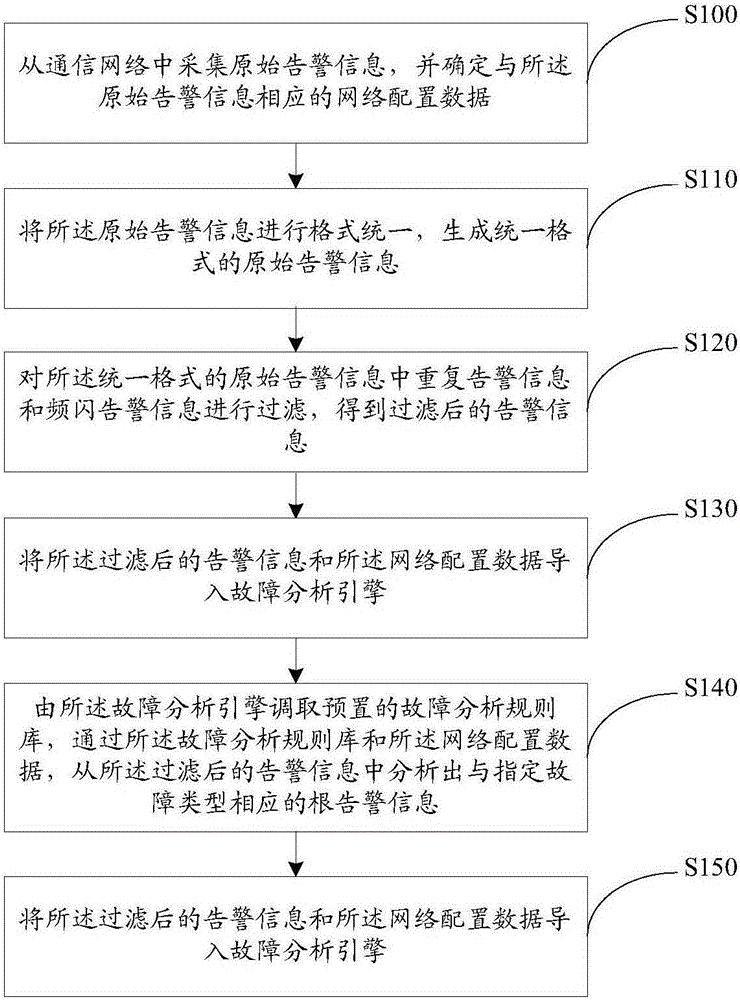
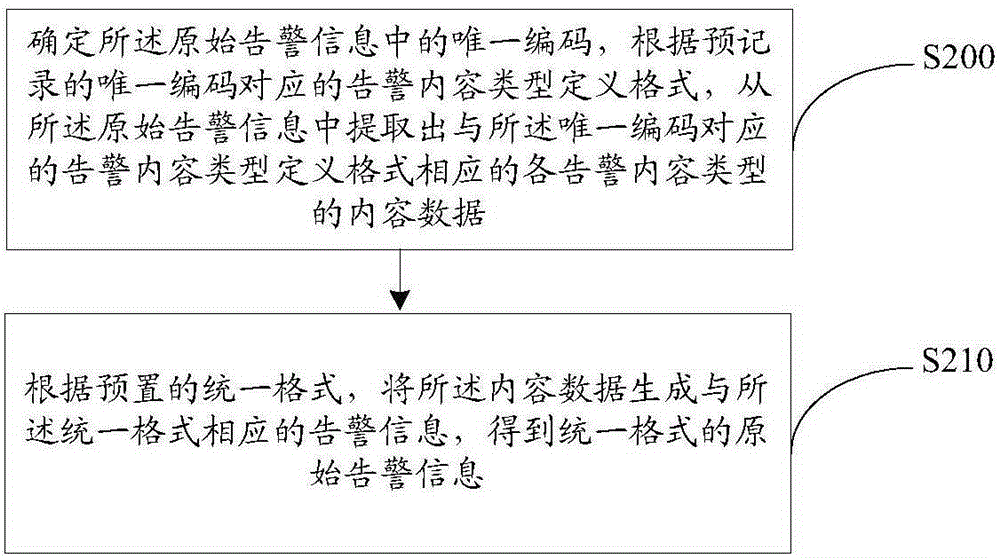
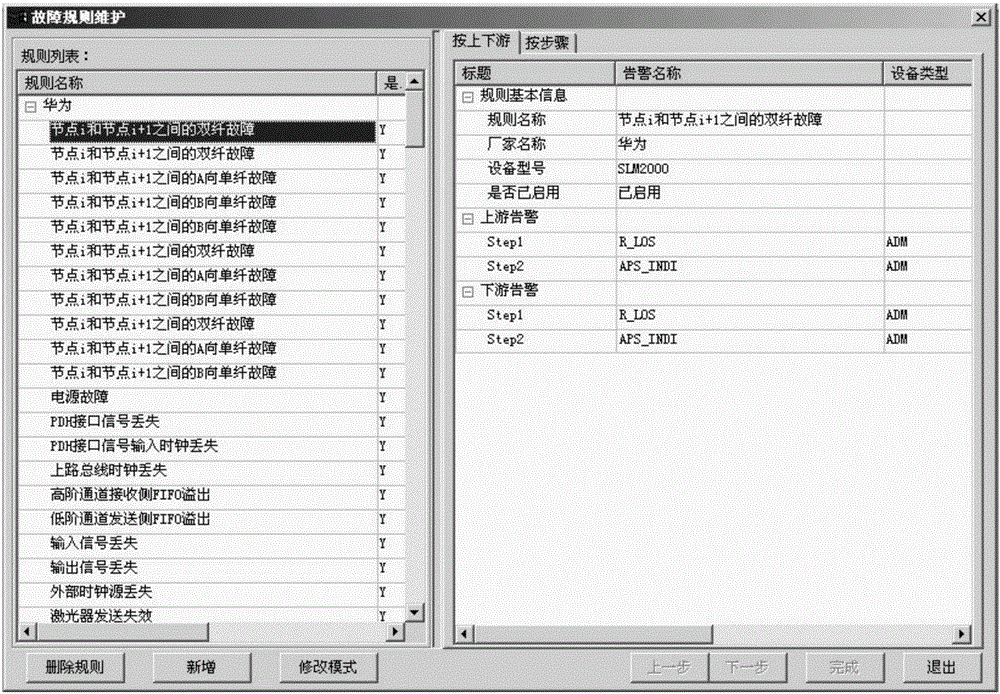
InactiveCN105207822AShorten the timeTimely processingData switching networksInformation processingFault analysis
The invention provides an electric power communication equipment alarm information processing method and device. The method includes the steps that original alarm information is acquired from a communication network, and network configuration data corresponding to the original alarm information are determined; format unification is conducted on the original alarm information for forming the original alarm information of the unified format; repeated alarm information and stroboscopic alarm information in the original alarm information of the unified format are filtered; the filtered alarm information and network configuration data are imported into a fault analysis engine; the fault analysis engine calls a predetermined fault analysis rule base, root alarm information corresponding to the designated fault type is analyzed out from the filtered alarm information through the fault analysis rule base and the network configuration data; the root alarm information and the designated fault type are displayed. By means of the electric power communication equipment alarm information processing method and device, the root alarm information corresponding to the designated fault type can be analyzed out from a great number of alarms, so that maintenance personnel can be liberated from the great amount of alarm information.
Owner:INFORMATION & TELECOMM COMPANY SICHUAN ELECTRIC POWER +1
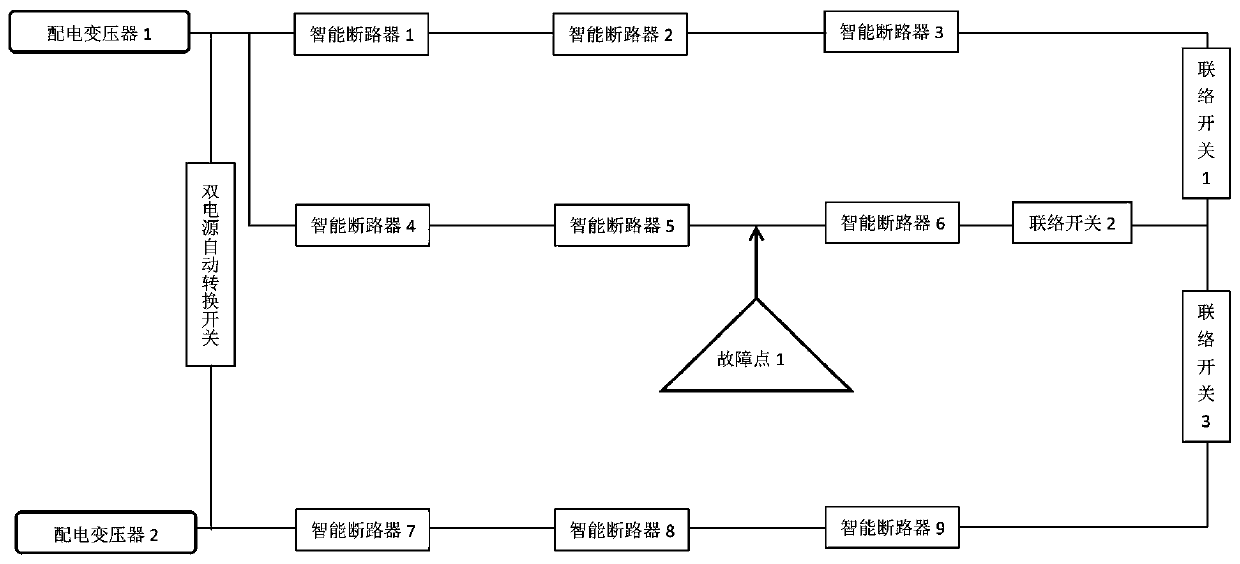
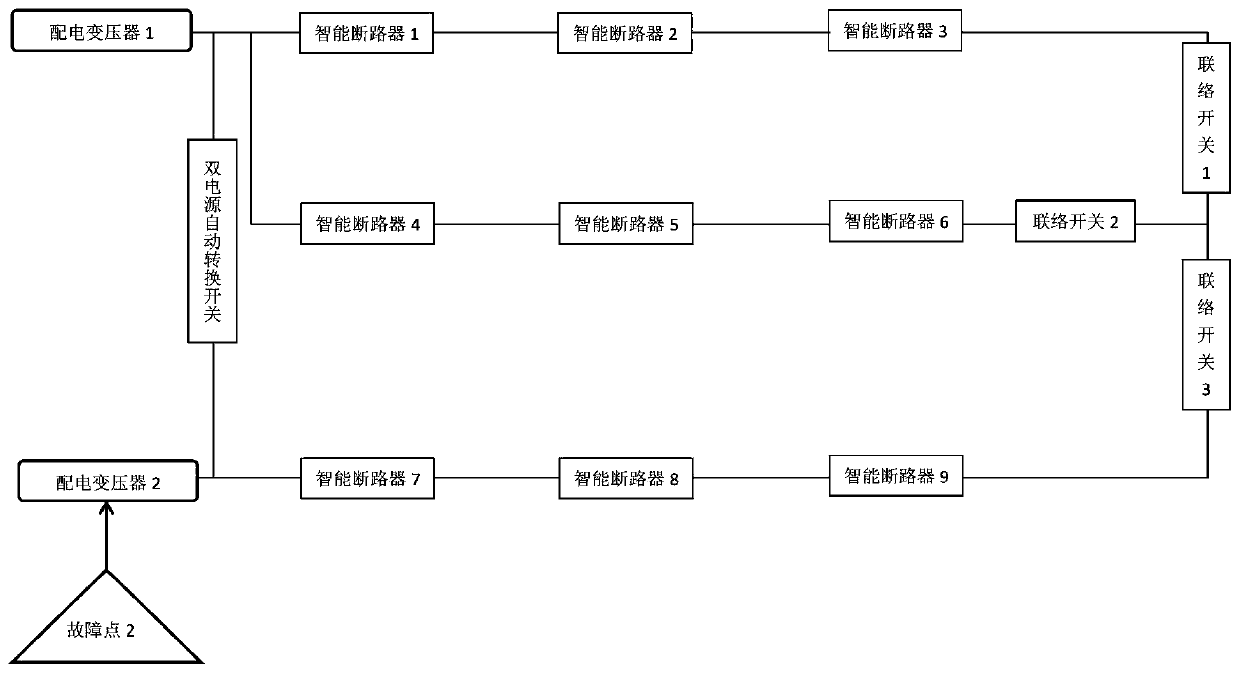
Low-voltage transformer area line fault positioning system based on intelligent circuit breakers
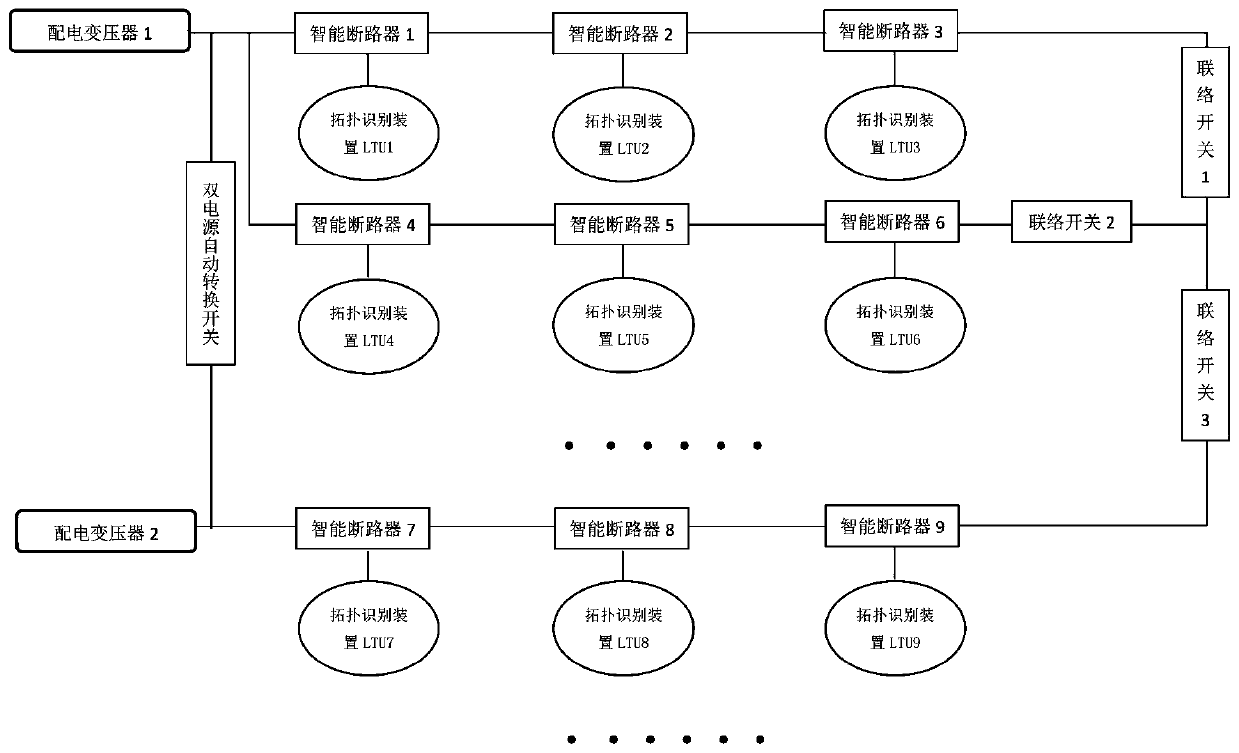
InactiveCN110703042AImprove failure response speedReduce the impact of failureEmergency protective circuit arrangementsFault location by conductor typesElectrical malfunctionDistribution transformer
The invention provides a low-voltage transformer area line fault positioning system based on intelligent circuit breakers. The system comprises a low-voltage transformer area distribution network anda monitoring center, wherein the intelligent circuit breakers have selectivity when encountering an electrical fault, and can freely set the tripping time or select different tripping times; the intelligent circuit breakers are used for setting the current-level circuit breaker close to the fault point to be tripped and opened preferentially when a line fault occurs between the two levels of intelligent circuit breakers, and sending the fault information to the monitoring center; and the monitoring center is used for acquiring the fault point information through an intelligent distribution transformer terminal TTU, sending an instruction to control the upper-level intelligent circuit breaker and the lower-level intelligent circuit breaker of the fault point to be opened, controlling an interconnection switch to be connected at the same time, supplying power to a fault-free area, and isolating the fault area. According to the invention, fault positioning of a distribution line of a public transformer area of the national power grid can be realized, the fault points of the transformer area are isolated, power supply is recovered outside the isolation area, the fault response speed isimproved, the fault influence is reduced to the minimum, the equipment is reasonably put into operation, and the equipment loss is reduced.
Owner:中电科安科技股份有限公司 +1
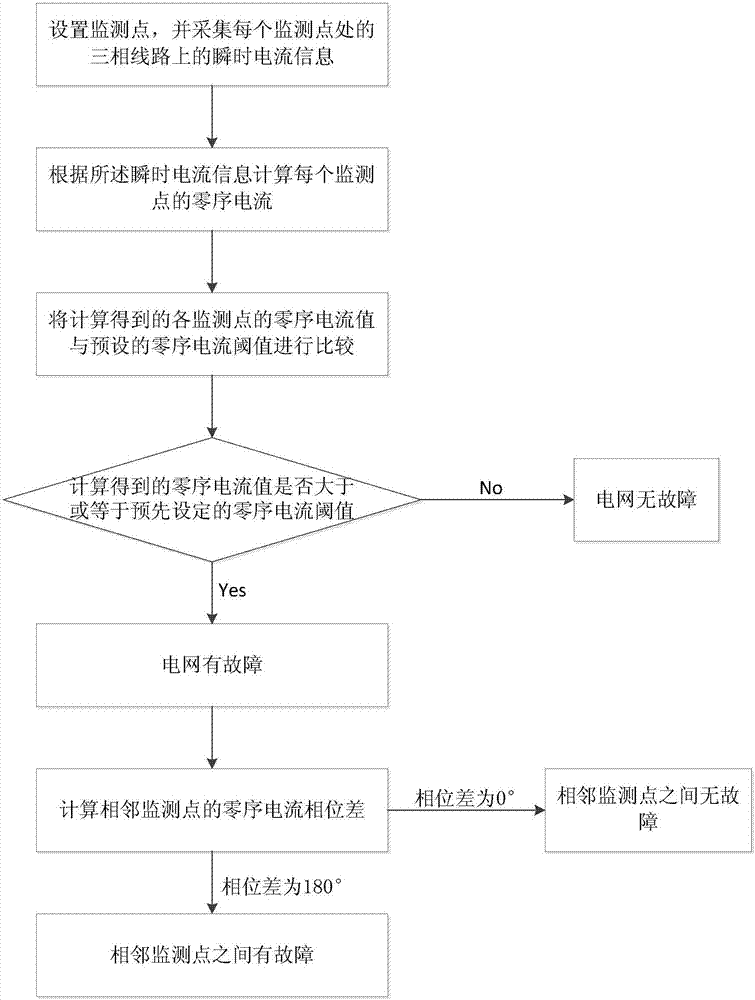
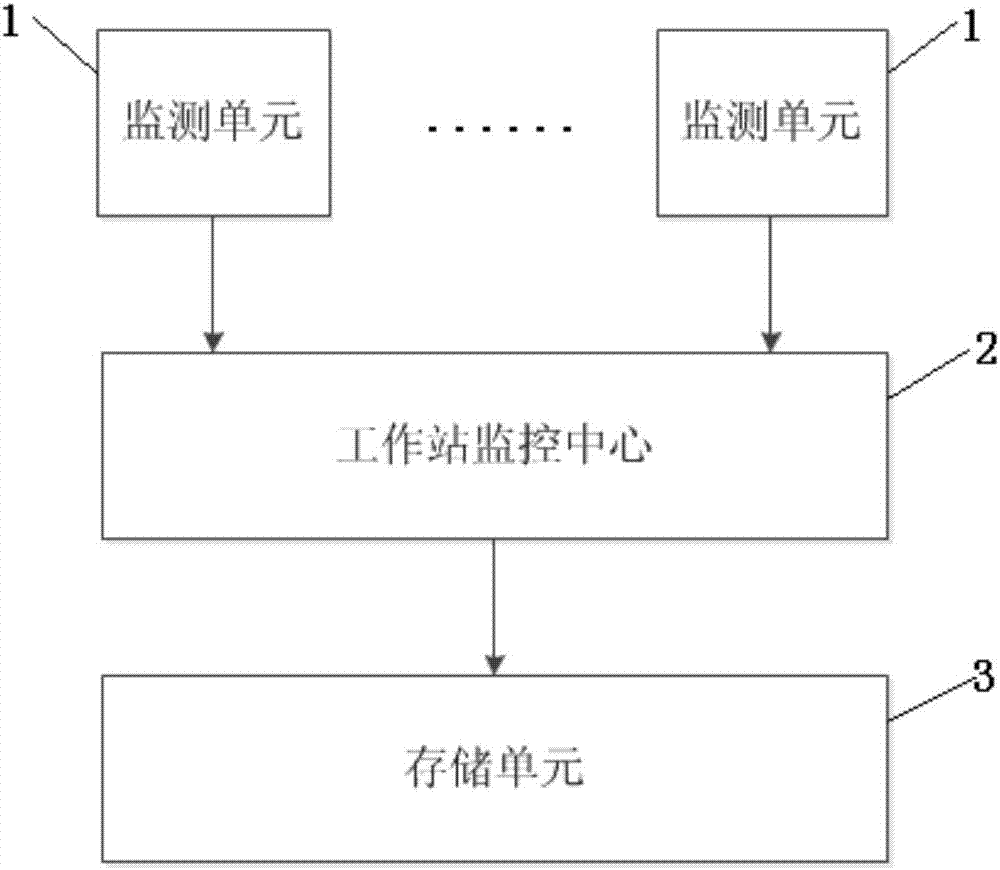
Power grid fault location method and system
InactiveCN107167709AReduce patrolShorten the timeFault location by conductor typesPower gridEngineering
The present invention discloses a power grid fault location method and system. The method comprises the following steps: setting a monitoring point, and collecting the instant current information on a three-phase line at each monitoring point; calculating the zero sequence current of each monitoring point; determining whether a power grid has faults or not according to a zero sequence current value; and determining the fault position according to the zero sequence current phase difference of adjacent monitoring points. The system comprises monitoring units arranged at intervals along the three-phase line of the power grid and a work station monitoring center connected with the monitoring units. Compared to the prior art, the power grid fault location method and system can perform fault location in time and accurately perform feedback of the power grid fault points without further manual checking so as to greatly reduce the maintenance cost, improve the power grid fault maintenance efficiency and be worth of being generalized.
Owner:JILIN UNIV
Database SQL index dynamic optimization method
InactiveCN108170775AImprove execution efficiencyReduce failure rateSpecial data processing applicationsExecution planProgram planning
The invention discloses a database SQL index dynamic optimization method. The method comprises the steps that S1, by configuring timed tasks, SQL resource usage is collected at fixed time, and a resource base line is established; S2, for collected SQL resource usage, an execution plan and indexes of predicate conditions are analyzed; and S3, analyzed data is tracked, and if an optimization threshold is reached, optimization of the corresponding indexes is performed. According to the database SQL index dynamic optimization method, comprehensive monitoring and analysis are performed on SQL usage, and analysis is performed according to the predicate conditions to realize optimization, so that SQL execution efficiency is greatly improved; an SQL performance problem is systematically intervenedand optimized at the initial stage, so that the fault occurrence probability and the fault influence are lowered; and all SQL predicate index analysis is automatically completed without the need forhuman intervention, so that human dependency and misoperation are lowered.
Owner:上海新炬网络技术有限公司
Clinical medicine laboratory instrument management system and standardized management method therefor
InactiveCN108550393ATimely updateDoes not consume spaceMedical equipmentManagement processQuality control
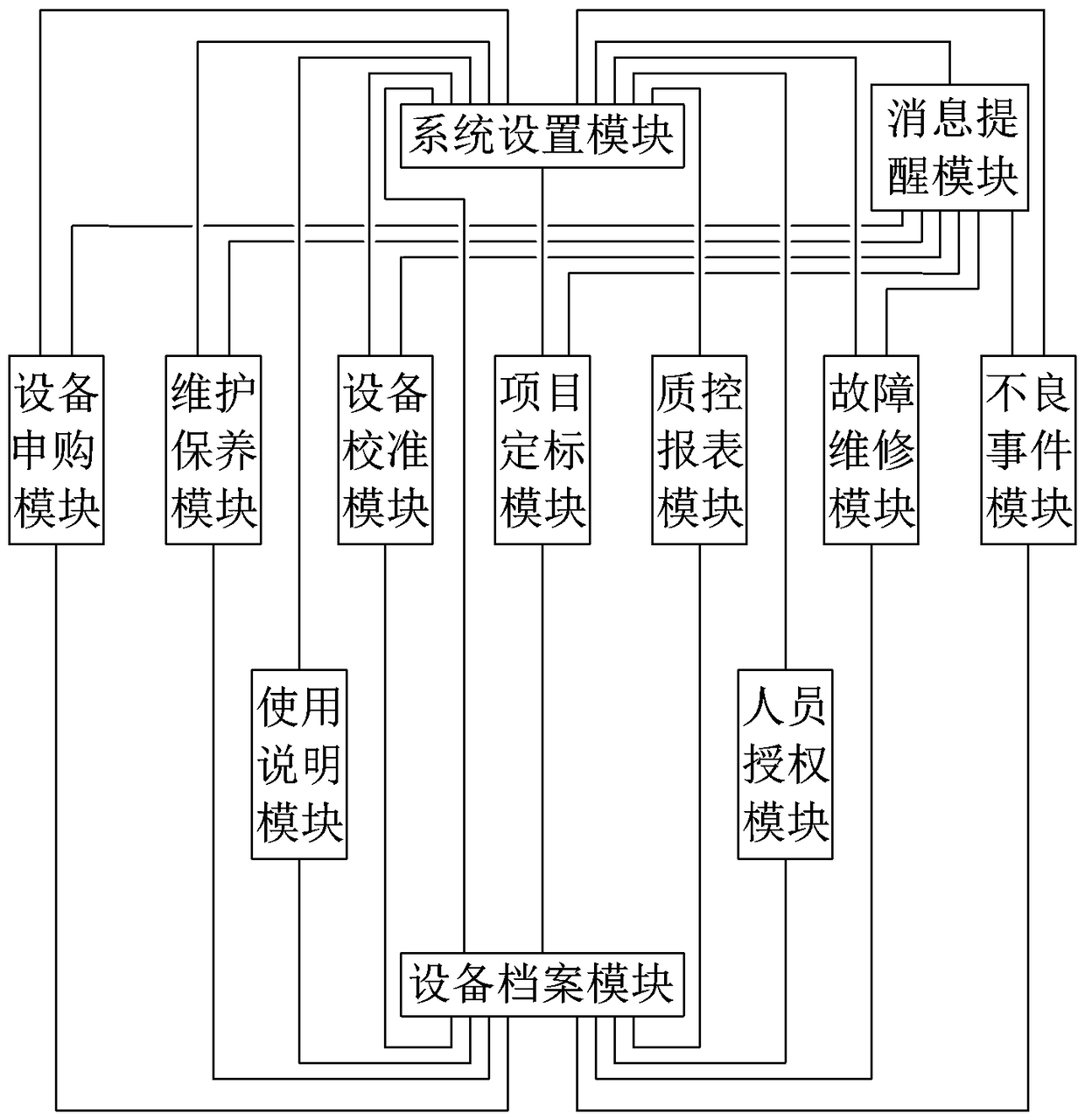
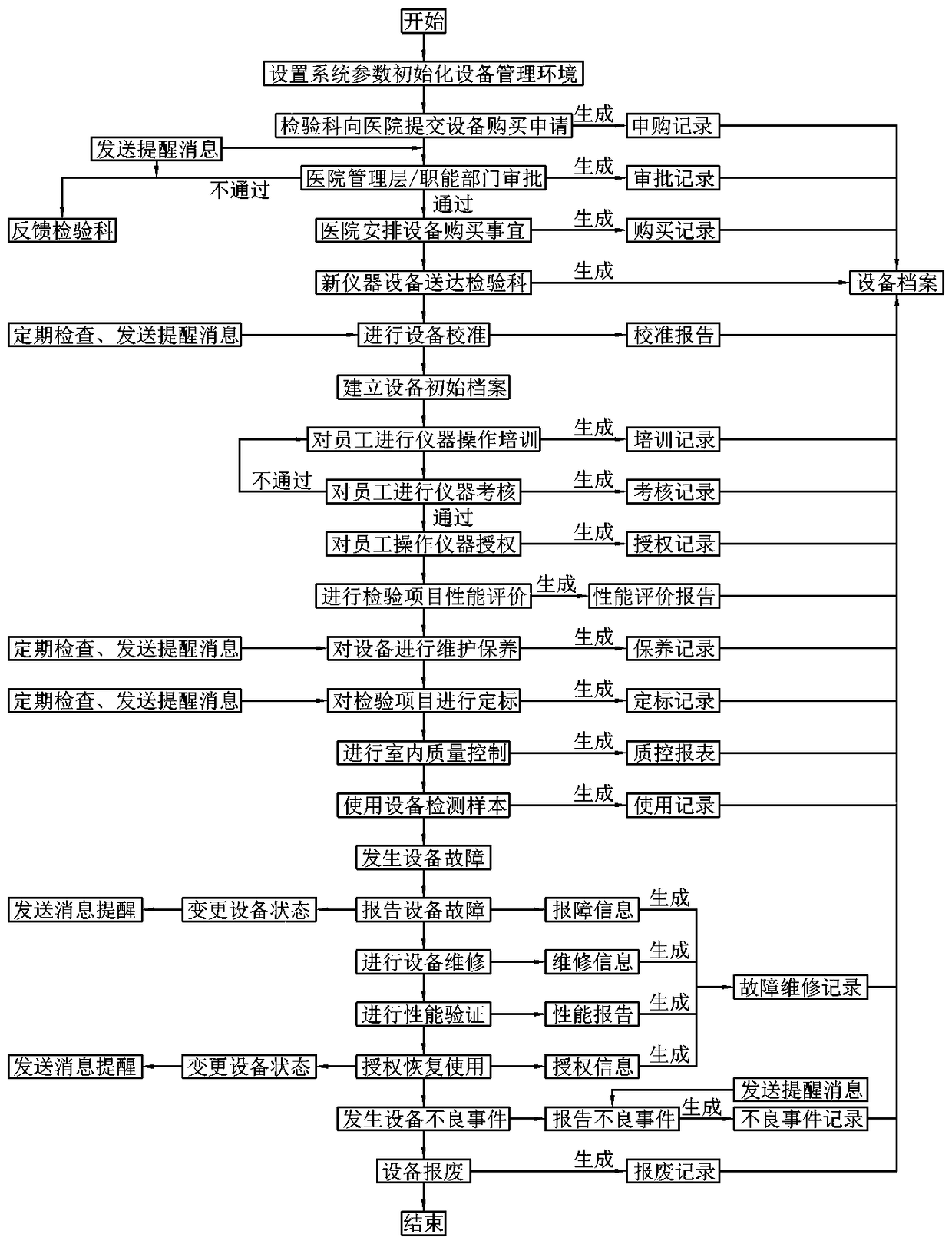
The invention discloses a clinical medicine laboratory instrument management system and a standardized management method therefor. The management system comprises a system setting module, a device purchase application module, a device file module, an instruction module, a personnel authorization module, a maintenance module, a device calibration module, a project calibration module, a quality control report module, a fault maintenance module, an adverse event module, and a message reminding module. The method provided by the invention can achieve the setting of the accounts, roles and authorities of device administrative departments and users, the setting of the device purchase application and adverse event reports according to the management systems of hospitals and departments, and achieves the building of device management environments. Therefore, the method achieves a series of record and management paths: device purchase application and filing, instructions, personnel authorization, maintenance, device calibration, performance evaluation, calibration and quality control, fault management and adverse event report, and achieves the convenient and efficient comprehensive management of the instruments in a clinical laboratory.
Owner:王伟佳
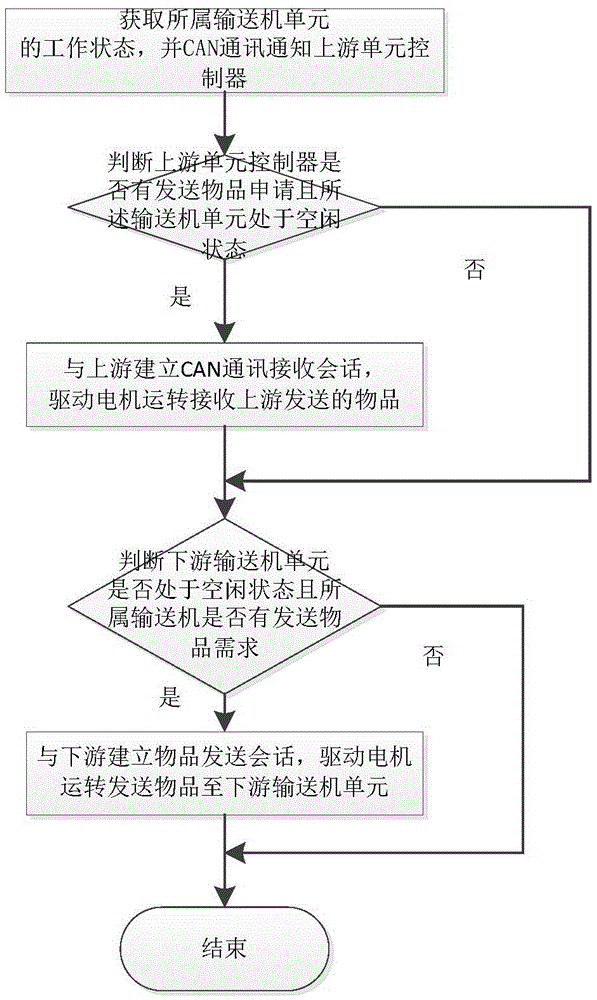
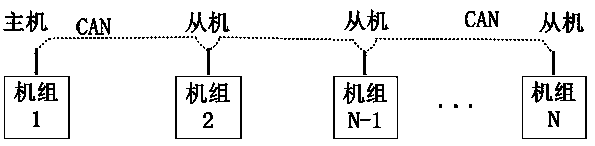
Distributed control system and control method for conveyer
ActiveCN105607626AEasy to modularizeSave costsProgramme controlElectric testing/monitoringData informationData interface
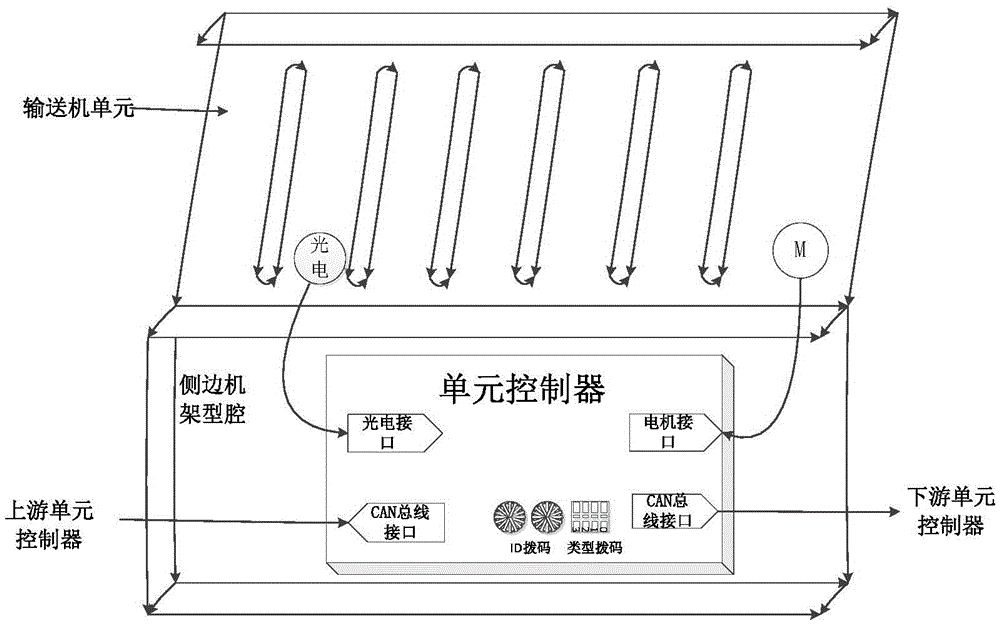
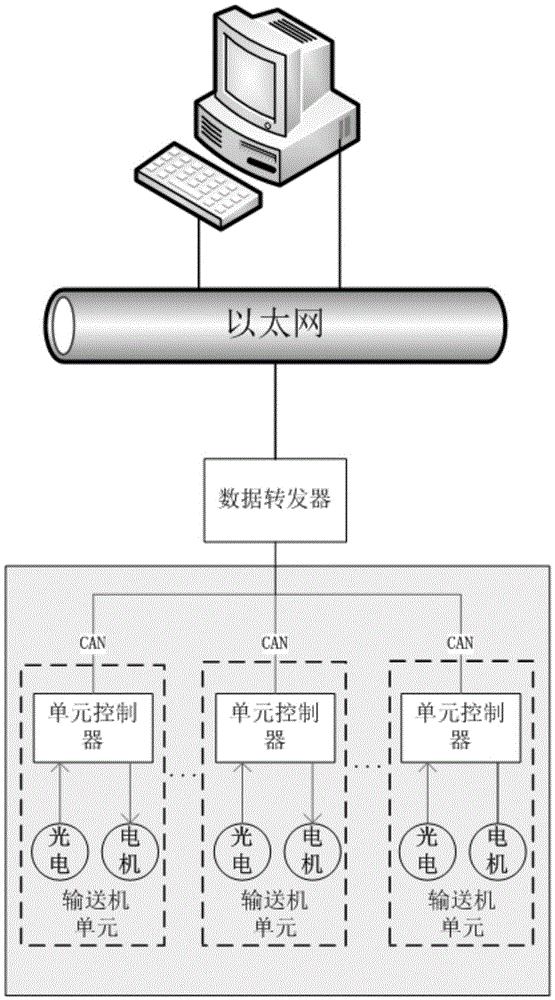
The invention discloses a distributed control system and control method for a conveyer. The distributed control system for a conveyer includes a data interface terminal, a data transponder and cell controllers, wherein the data interface terminal monitors the state of the conveyer and logic control of conveying service; the data transponder is connected with the data interface terminal and the cell controllers through Ethernet and CAN network respectively, and perform Ethernet-CAN format conversion for the received data information; the cell controllers are arranged on conveyer cells; each cell controller is provided with an photoelectric interface, a motor interface and a CAN bus interface; the upstream and downstream cell controllers are connected, and the related conveying state can be acquired through the CAN network; and the conveying logic is executed and conveying of the articles supported on the conveyer cells are controlled. The distributed control system and control method for a conveyer utilize distributed control, arrange the cell controllers, the sensors, the motor and the conveyer cells together, and utilize complete distributed arrangement, thus greatly saving the cable cost and the wiring cost, and being convenient for maintenance and overhauling.
Owner:JIANGSU HUAZHANG LOGISTICS TECH CO LTD
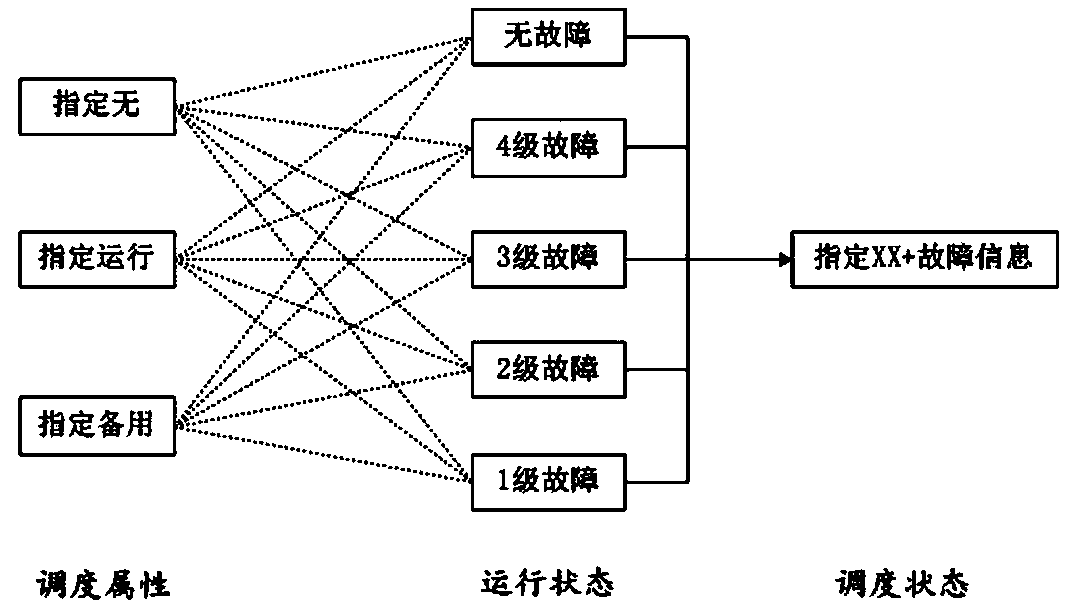
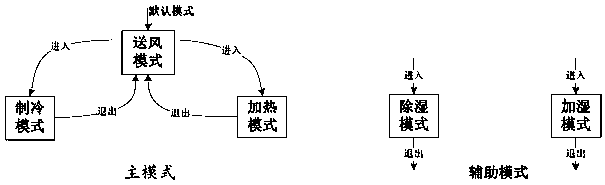
Air conditioning grouping system capable of improving reliability and grouping control method
InactiveCN109883006AUniform temperature changeAvoid race runsMechanical apparatusSpace heating and ventilation safety systemsOperation modeGroup operation
The invention discloses an air conditioning grouping system capable of improving reliability and a grouping control method. The grouping control method of an air conditioning comprises the steps thatthe operation status of units in a group is obtained by the group control end at intervals and is processed to obtain operation modes of the group; dispatching units and a dispatching order thereof are determined by the group control end according to dispatching attributes and the operation status of the units, and an issuing order is formulated according to a certain temperature order or a humidity order and the different operation modes in the dispatching order; and the group control end issues mode commands to the dispatching units for executing according to the issuing order. According tothe air conditioning grouping system capable of improving the reliability and the grouping control method, the competitive operation between slaves can be avoided, the temperature changes can furtherbe quickly responded, and the safety and the reliability of group operation are improved.
Owner:GREE ELECTRIC APPLIANCES INC
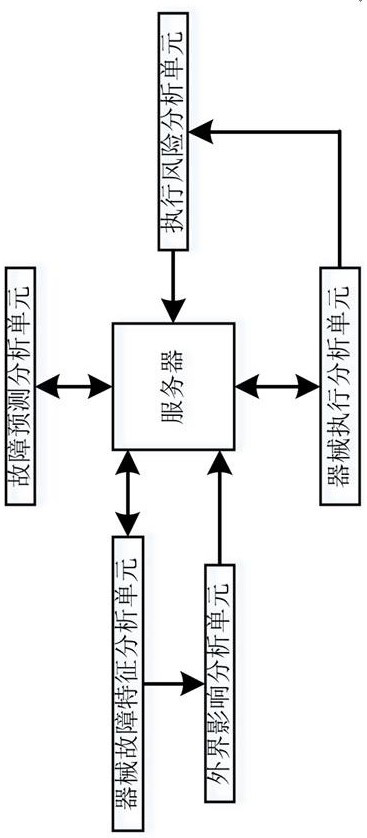
Big data-based laparoscopic surgical instrument operation fault prediction system
ActiveCN114628016AEasy to useImprove fault maintenance efficiencyDiagnosticsSurgeryPrediction systemComputer science
The invention discloses a big data-based laparoscopic surgical instrument operation fault prediction system, relates to the technical field of operation fault prediction, and solves the technical problems of less fault prediction basis and low corresponding prediction accuracy during fault prediction of existing laparoscopic surgical instruments. According to the method, the occurrence risk of high influence characteristics in the execution process of the analysis object is judged, so that an accurate basis is provided for fault prediction of the analysis object, the prediction accuracy is high, the timeliness of fault discovery is enhanced, the fault influence is reduced to the minimum, and the working efficiency of the analysis object is indirectly improved; according to the method, the execution of the corresponding analysis object is subjected to risk analysis, and whether the specific execution of the analysis object affects the instrument fault is judged, so that the accuracy of fault prediction of the analysis object is improved, and meanwhile, the monitoring strength of the execution process is increased, so that the fault risk in the operation process of the analysis object is effectively avoided; and the working efficiency of analyzing the object is improved.
Owner:THE AFFILIATED HOSPITAL OF SOUTHWEST MEDICAL UNIV
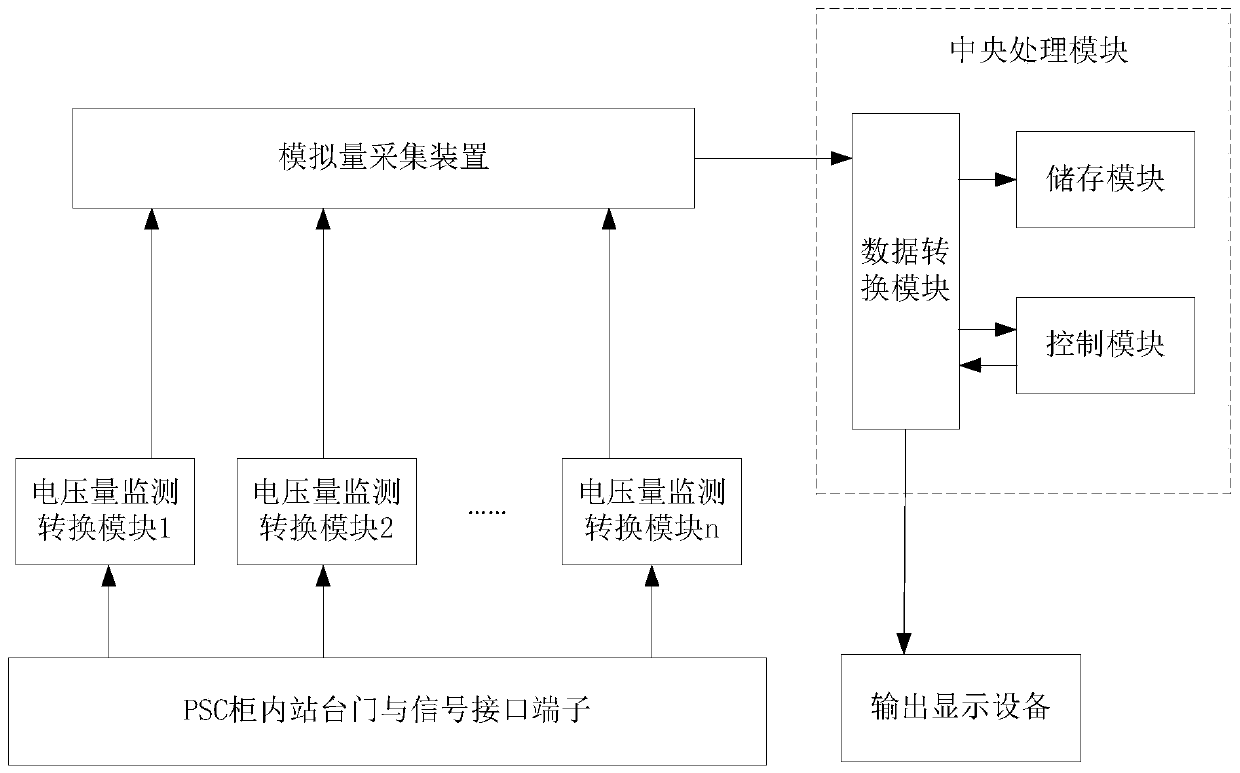
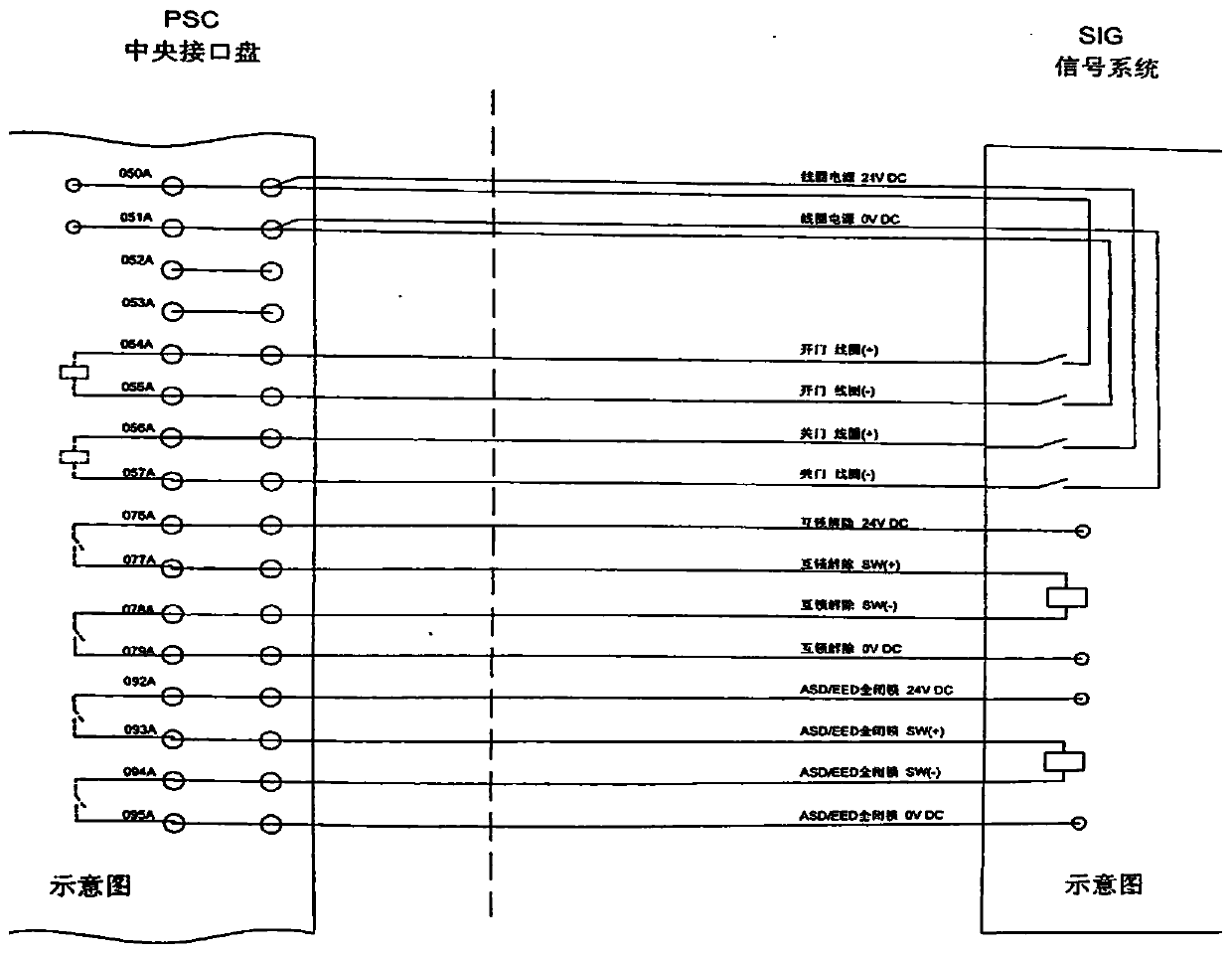
Platform door and signal interface state monitoring device
PendingCN111007780AQuick Repair HandlingReduce repair processing timeProgramme controlComputer controlDevice MonitorEmbedded system
The invention, which relates to the technical field of electrician preparation and application, provides a platform door and signal interface state monitoring device. The device comprises a voltage quantity monitoring conversion module, an analog quantity acquisition device, a central processing module and output display equipment. The input terminal of the voltage quantity monitoring conversion module is connected with interfaces of platform door side and signal side equipment, and the output terminal of the voltage quantity monitoring conversion module is connected with the input terminal ofthe analog quantity acquisition device; the analog quantity acquisition device is connected with the central processing module; and the output terminal of the central processing module is connected with the input terminal of output display equipment. The device monitors and analyzes the state signals of the platform door and the signal interface in real time, so that when a fault occurs, the fault reason and the fault position can be visually displayed at the first time; and thus the maintenance personnel can conveniently and quickly maintain and process the fault, so that the troubleshootingprocessing time is greatly shortened.
Owner:OPERATION BRANCH OF SHENYANG METRO CO LTD
Load balance operation and management method for distributed acquisition systems
InactiveCN104954455AAvoid overloadingIncreased resilience to unexpected failuresTransmissionReal-time computingData acquisition
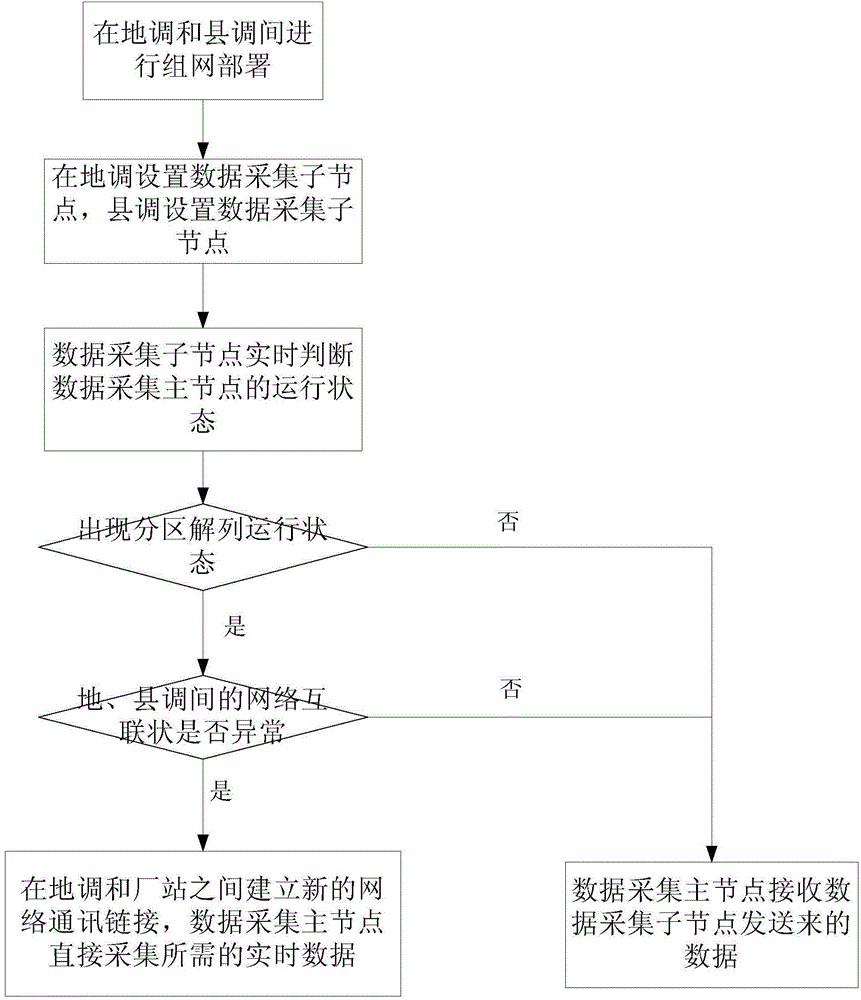
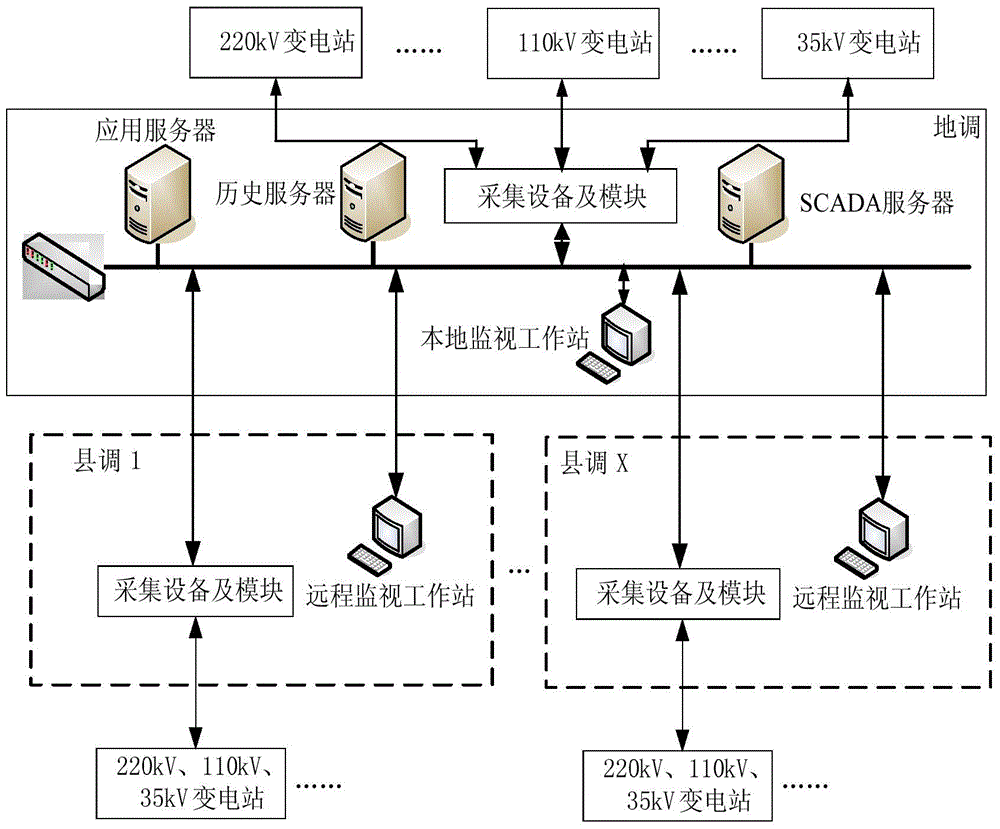
The invention discloses a load balance operation and management method for distributed acquisition systems. The method includes the steps of S1, performing networking deployment for distributed data acquisition between regional dispatch and county dispatches; S2, setting a data acquisition master node for the regional dispatch, and setting a data acquisition slave node for each county dispatch, with each data acquisition slave node used for acquiring data of local substations; S3, allowing the data acquisition master node of the regional dispatch to acquire in real time the data acquired by the county dispatches, allowing the county dispatches to acquire in real time the operation state of the regional dispatch, and judging whether partitioning and splitting operation state occurs according to the operation state of the regional dispatch; S4, if yes, judging networking condition between the regional dispatch and the county dispatches to process the data of the county dispatches. The method has the advantages that acquisition tasks can be evenly distributed to the different data acquisition nodes, excess load on the single data acquisition node is avoided, and the county dispatches can independently run in a short time to minimize the fault influence in case of occurrence of the partitioning and splitting operation state.
Owner:STATE GRID CORP OF CHINA +4
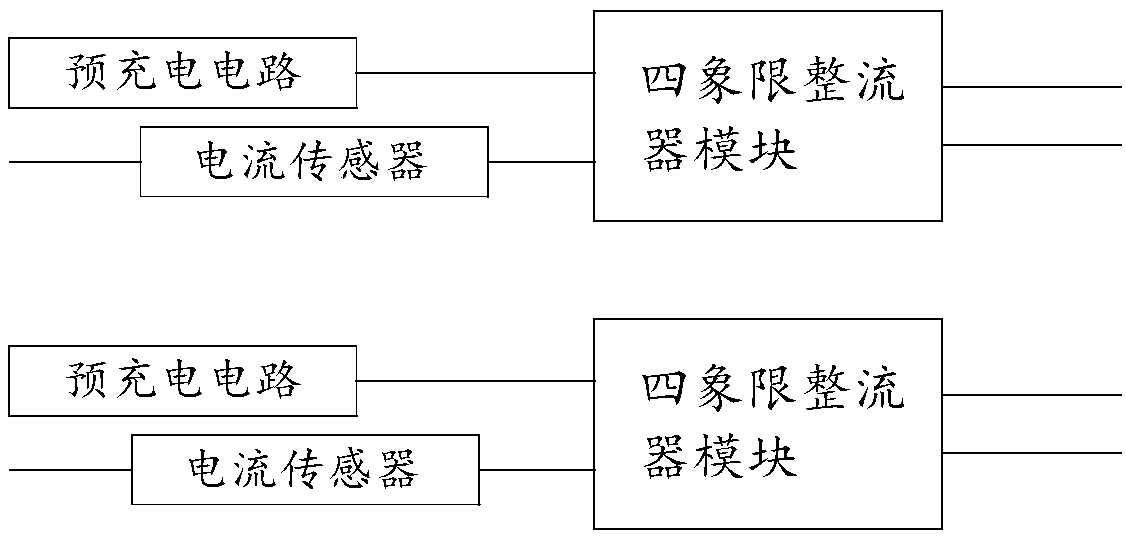
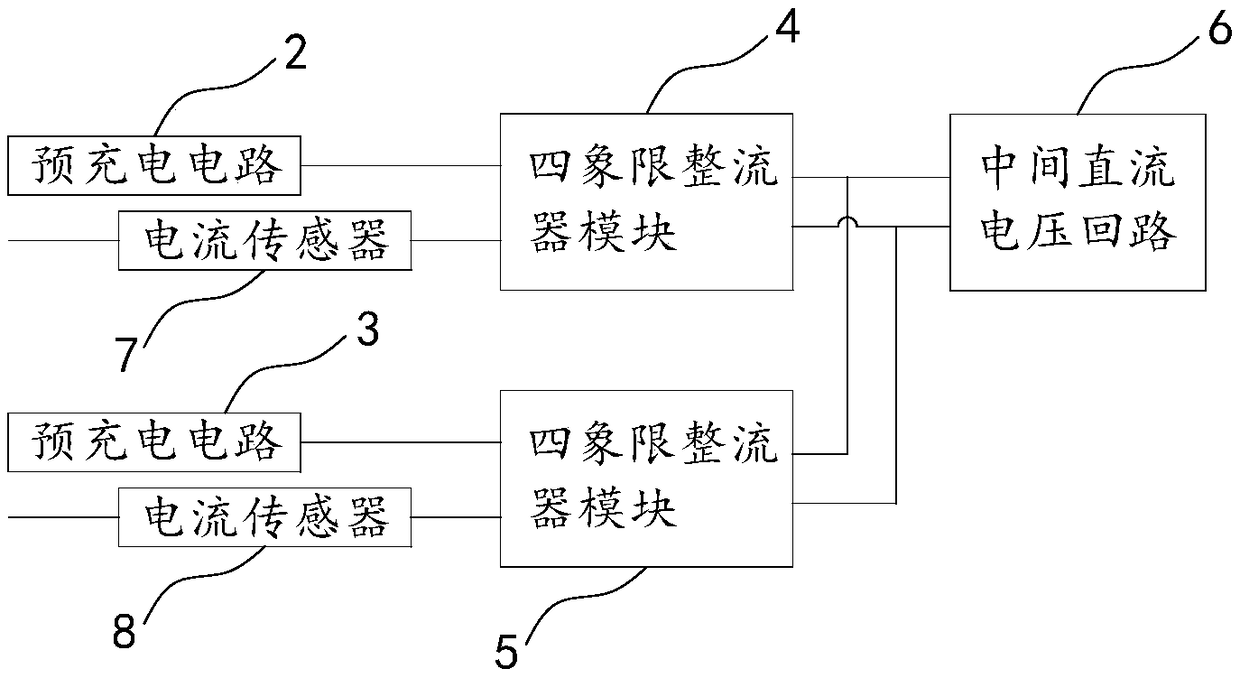
Pre-charge loop of traction converter and control method
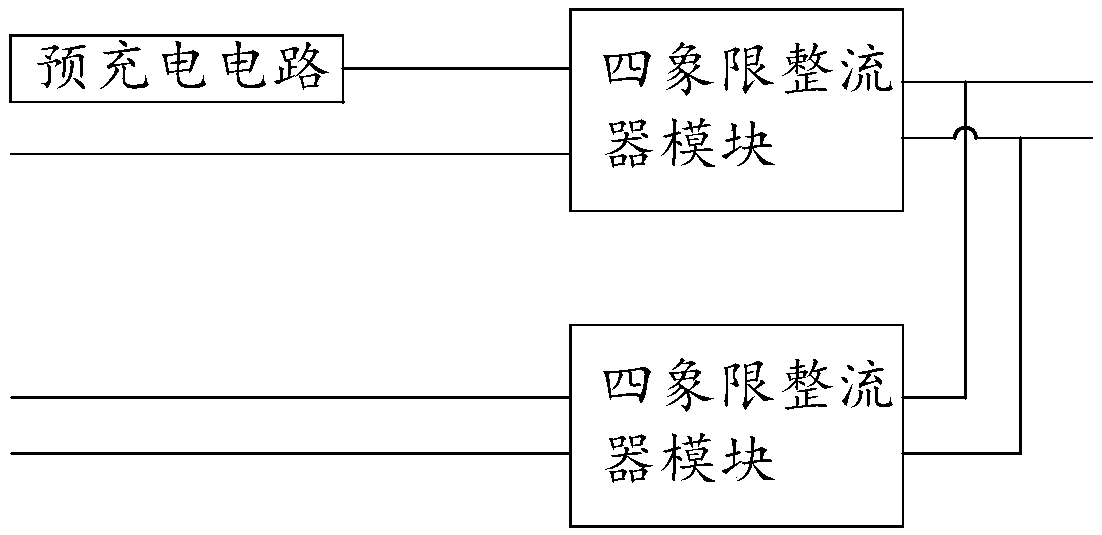
InactiveCN108631571AIncreased power availabilityRobustConversion with intermediate conversion to dcCapacitanceEngineering
The invention relates to a pre-charge loop of a traction converter and a control method. The pre-charge loop comprises a first heavy pre-charge circuit and a second heavy pre-charge circuit which arerespectively connected with the secondary side windings of a traction transformer, a first heavy four-quadrant rectifier module connected in series with the first heavy pre-charge circuit and a secondheavy four-quadrant rectifier module connected in series with the second heavy pre-charge circuit, wherein the output end of the first heavy four-quadrant rectifier module is connected with a first middle direct-current supporting capacitor FC11 at the input end of a middle direct-current voltage loop, and the output end of the second four-quadrant rectifying module is connected with a second middle direct-current supporting capacitor FC12 at the input end of the middle direct-current voltage loop. The invention is provided with double pre-charge circuits. Two four-quadrant rectifier modulesshare a bus of the middle direct-current voltage loop, so that the traction converter is provided with a hardware condition of single four-quadrant operation. The power availability of the traction converter is improved, and the robustness is high.
Owner:CRRC QINGDAO SIFANG ROLLING STOCK RES INST
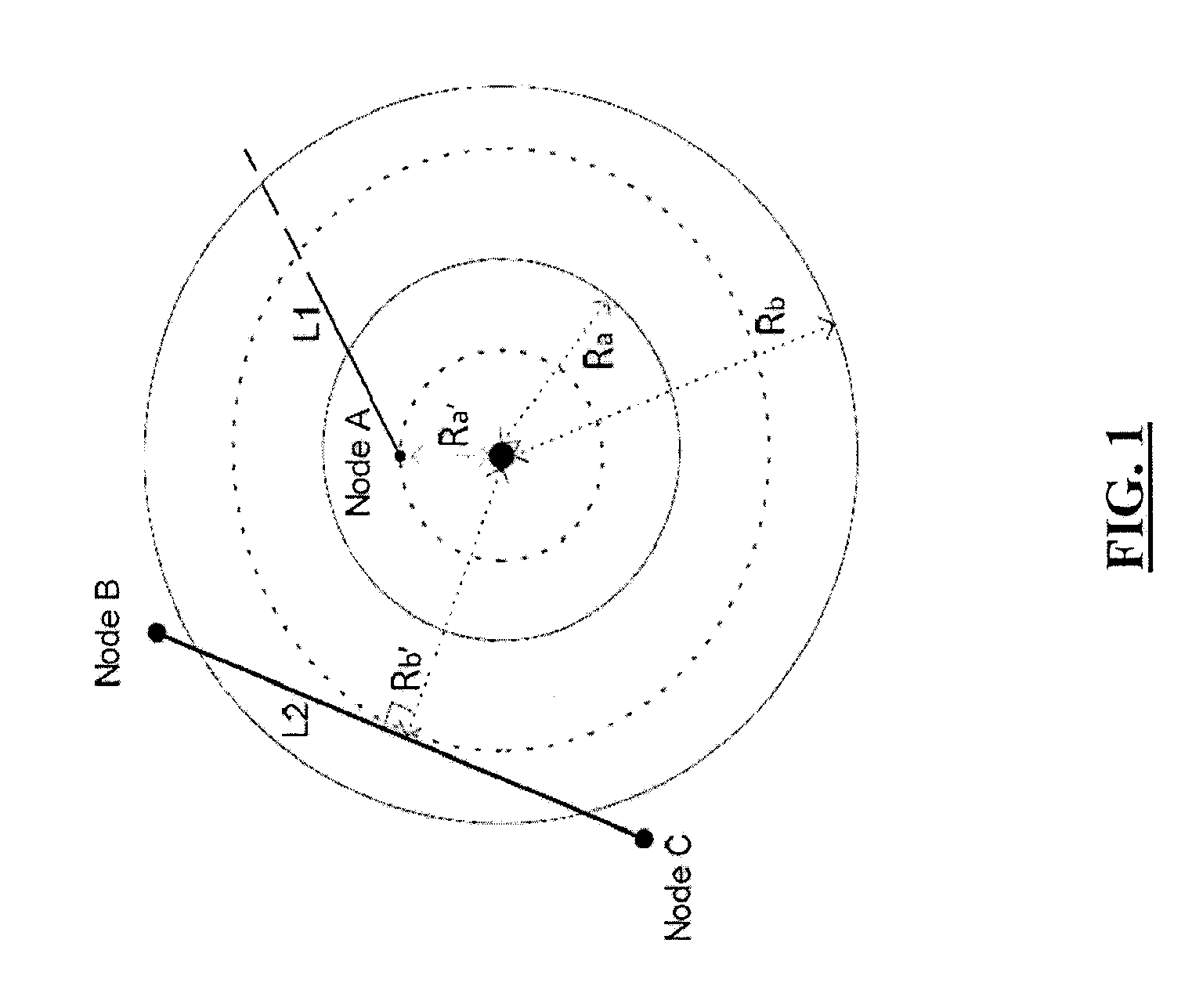
Proactive controller for failure resiliency in communication networks
ActiveUS9590892B2Reduce the impact of failureHardware monitoringReliability/availability analysisTraffic capacityReal-time data
Network devices and systems relating to prevention of large-scale network failure and determining a probability of a large-scale network failure for a network. The network devices may control rerouting of network traffic from failed network paths to preventative paths with probabilities of failure below determined thresholds. The network devices monitor and process real time data for dynamic and proactive rerouting when large-scale network failure occurs or is likely to occur.
Owner:UNIV OF ONTARION INST OF TECH
Spacecraft control system on-orbit stable operation capability construction method
ActiveCN111913469ASolve problems such as abnormal system postureImprove reliabilityProgramme controlElectric testing/monitoringSatelliteSpace vehicle control
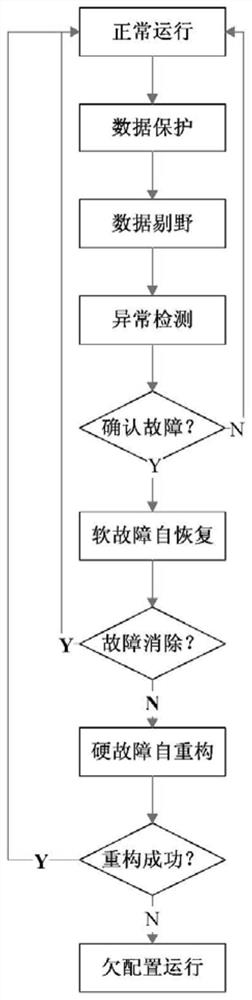
The invention relates to a spacecraft control system on-orbit stable operation capability construction method which can be used for control system ground development and on-orbit flight full life cycle, and the design method is widely applied to spacecrafts such as high, medium and low orbit satellites, airships, space stations and deep space detectors to improve the robustness of the spacecraft full life cycle. According to the spacecraft control system on-orbit stable operation capability construction method, six capabilities including the data protection capability, the data culling capability, the anomaly detection capability, the soft fault self-recovery capability, the hard fault self-reconstruction capability and the under-configuration operation capability are taken as supports. Inthe scheme design and the technical design of the control system, the attitude and the working mode of the spacecraft can be kept in a stable and continuous state according to the method.
Owner:BEIJING INST OF CONTROL ENG
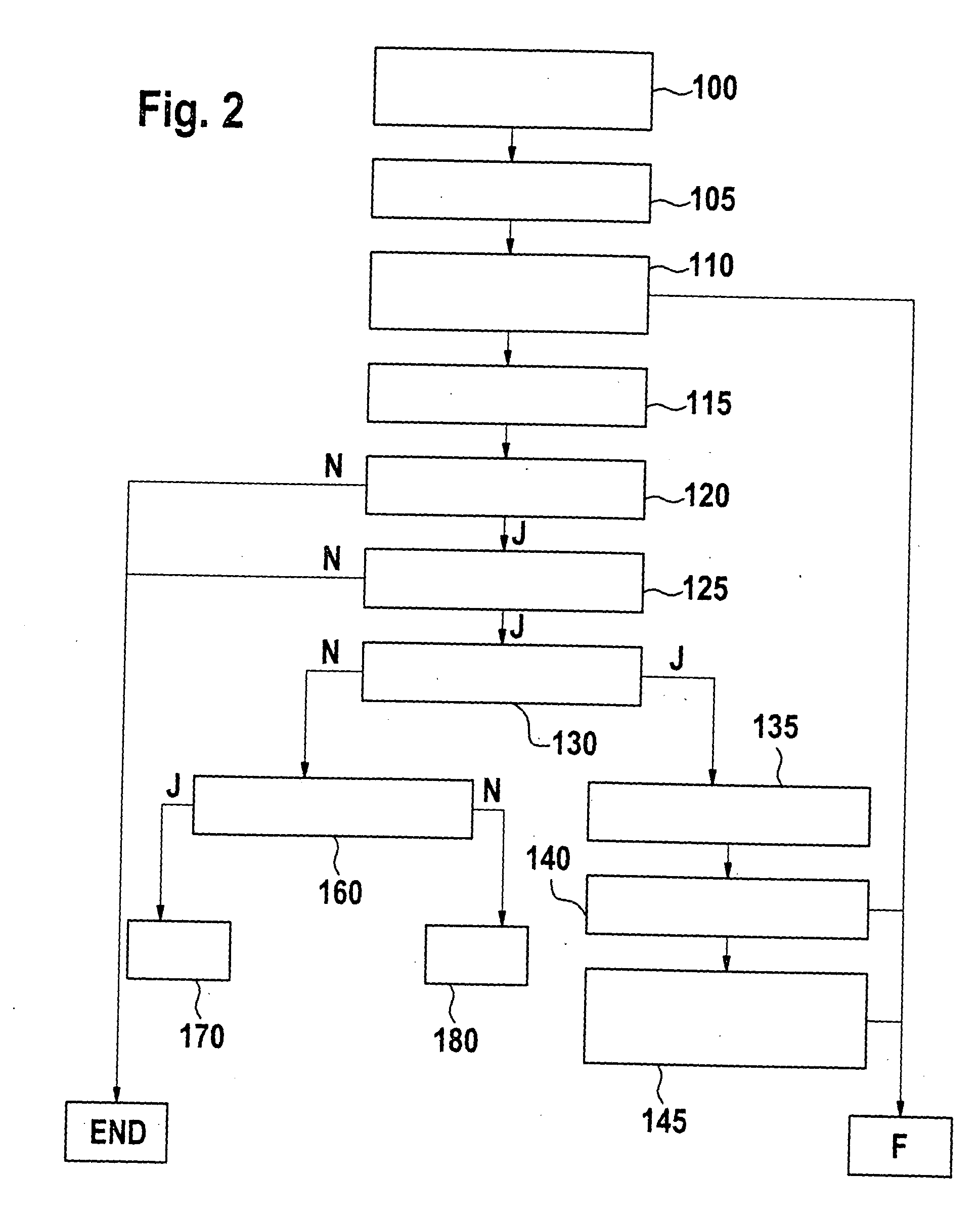
Method and device for monitoring a brake system
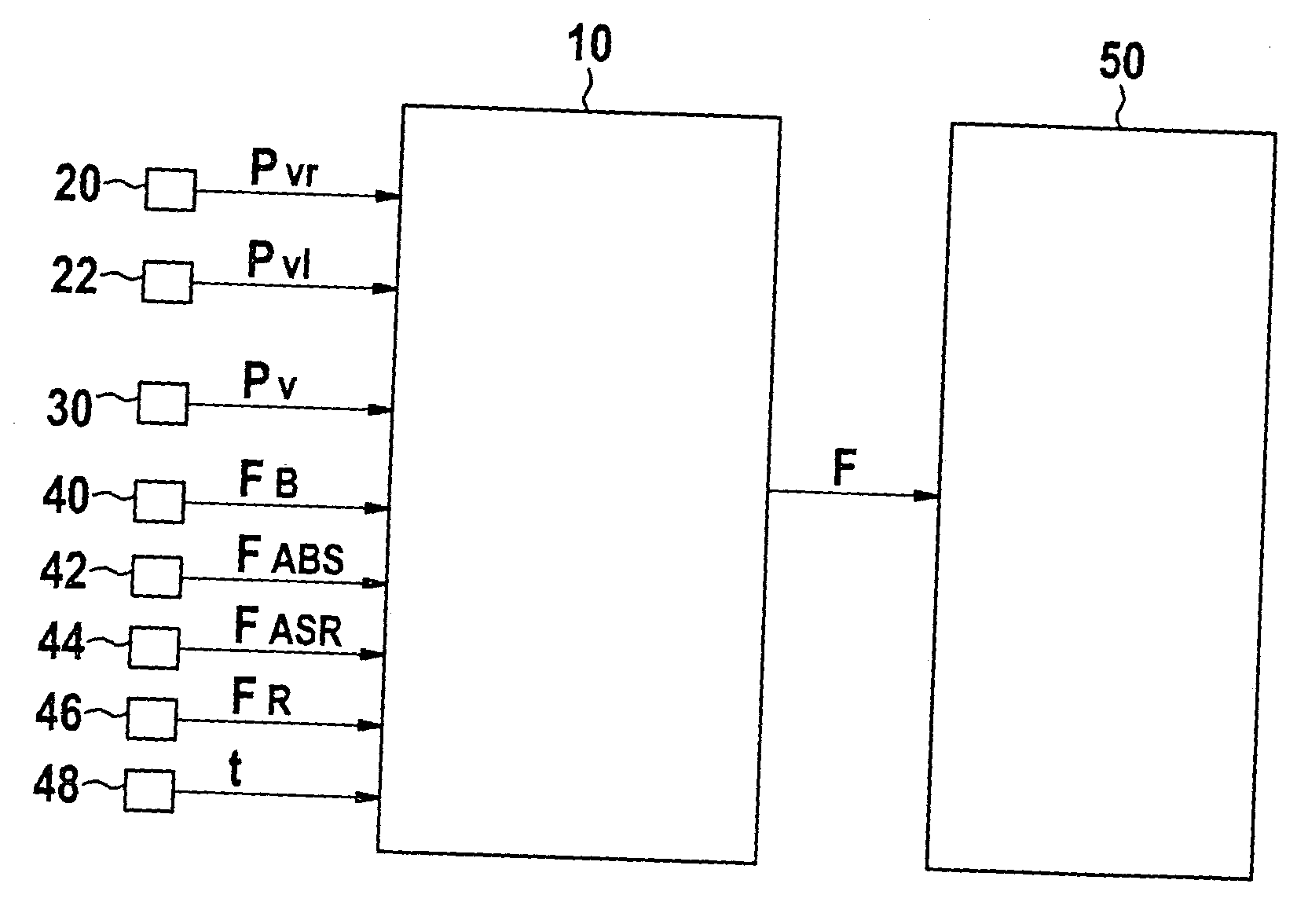
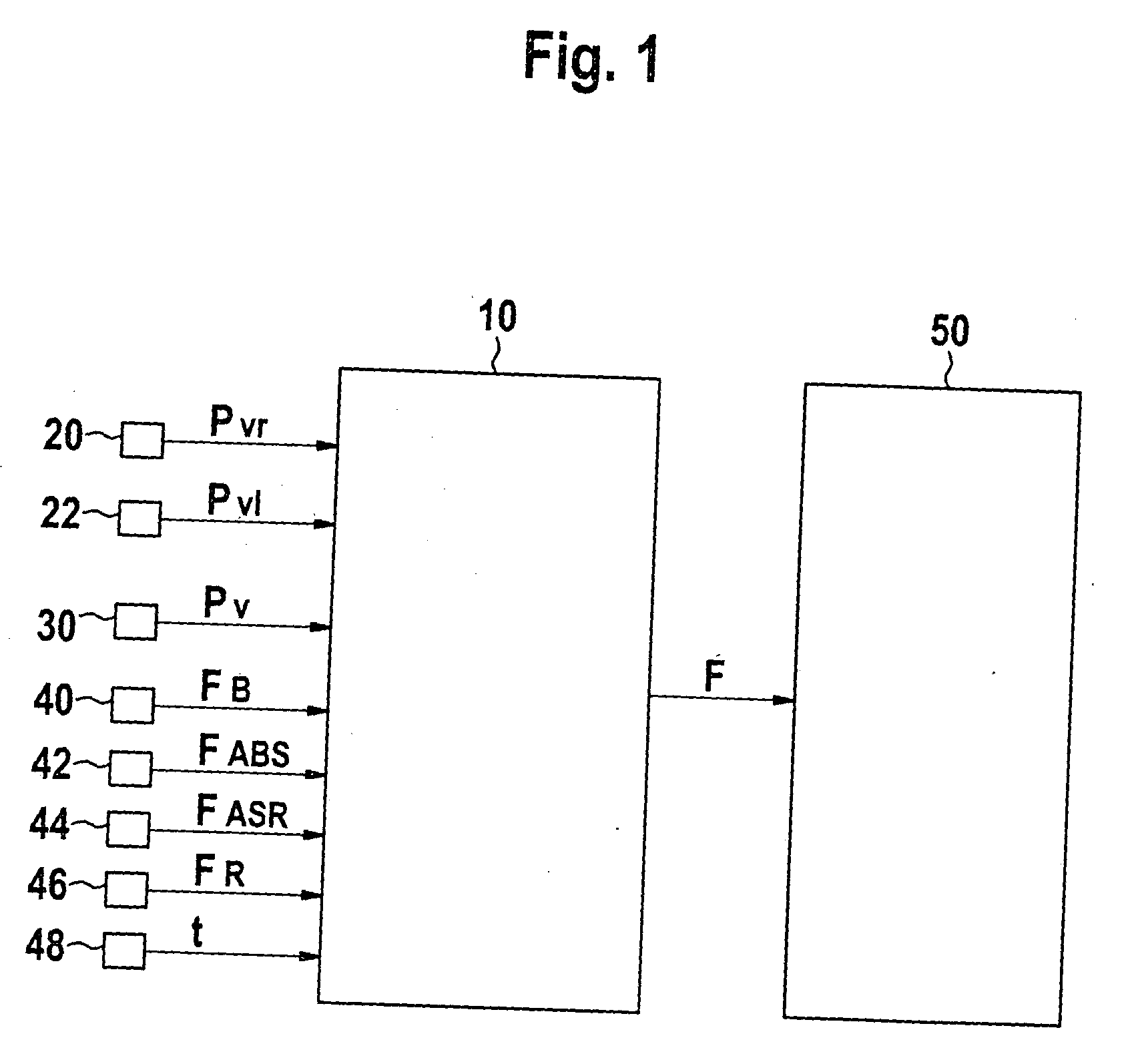
InactiveUS20050253452A1Reduce the impact of failureAffecting its functionAnalogue computers for trafficBrake control systemsBrake pressurePressure sensor
In a method and a device for monitoring a brake system, e.g., a wheel pressure sensor suite of a brake system of a motor vehicle, the fault detection is implemented on the basis of a differential threshold being exceeded by a signal that is representative for the difference of the brake pressures at the individual wheel brakes of a wheel axle. In the process, the differential threshold is set as a function of the averaged rate of increase of the individual pressures at the wheel brakes. Fault detection is carried out on the basis of a model, which takes the instantaneous operating state of the brake system into account.
Owner:ROBERT BOSCH GMBH
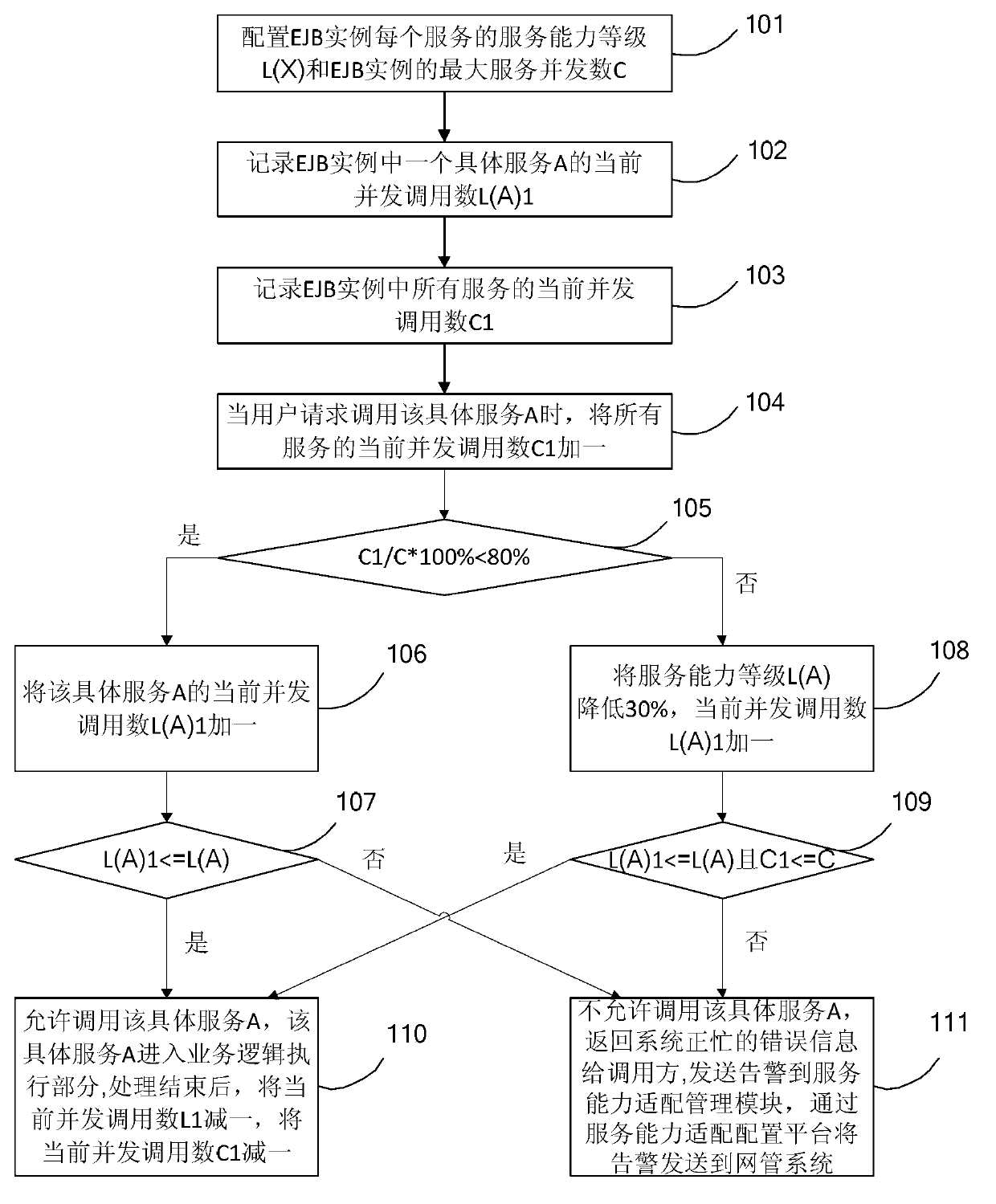
EJB service overload protection method and system thereof
ActiveCN103873509ARealize overload protection functionPrevent calls from hangingTransmissionOperating systemBusiness logic
The invention discloses an EJB service overload protection method and a system thereof. the method comprises the following steps: service capability level L(X) of each service in an EJB instance and the maximum service concurrency C of the EJB instance are configured, wherein X indicates a specific service; a current concurrence call number L(A)1 of a specific service A in the EJB instance is recorded; a current concurrence call number C1 of all services in the EJB instance is recorded; and when requesting to call the specific service A, whether calling the specific service A is allowed and whether the specific service A enters a business logic execution part are judged according to the service capability level L(X) of each service in the EJB instance, the maximum service concurrency C of the EJB instance, the current concurrence call number L(A)1 of the specific service A and the current concurrence call number C1 of all services. By the technical scheme, service overload protection capability can be realized so as to prevent single or more service failures from leading to the whole system failure, reduce failure influence surface and shorten system interrupt time.
Owner:CHINA MOBILE GROUP ZHEJIANG
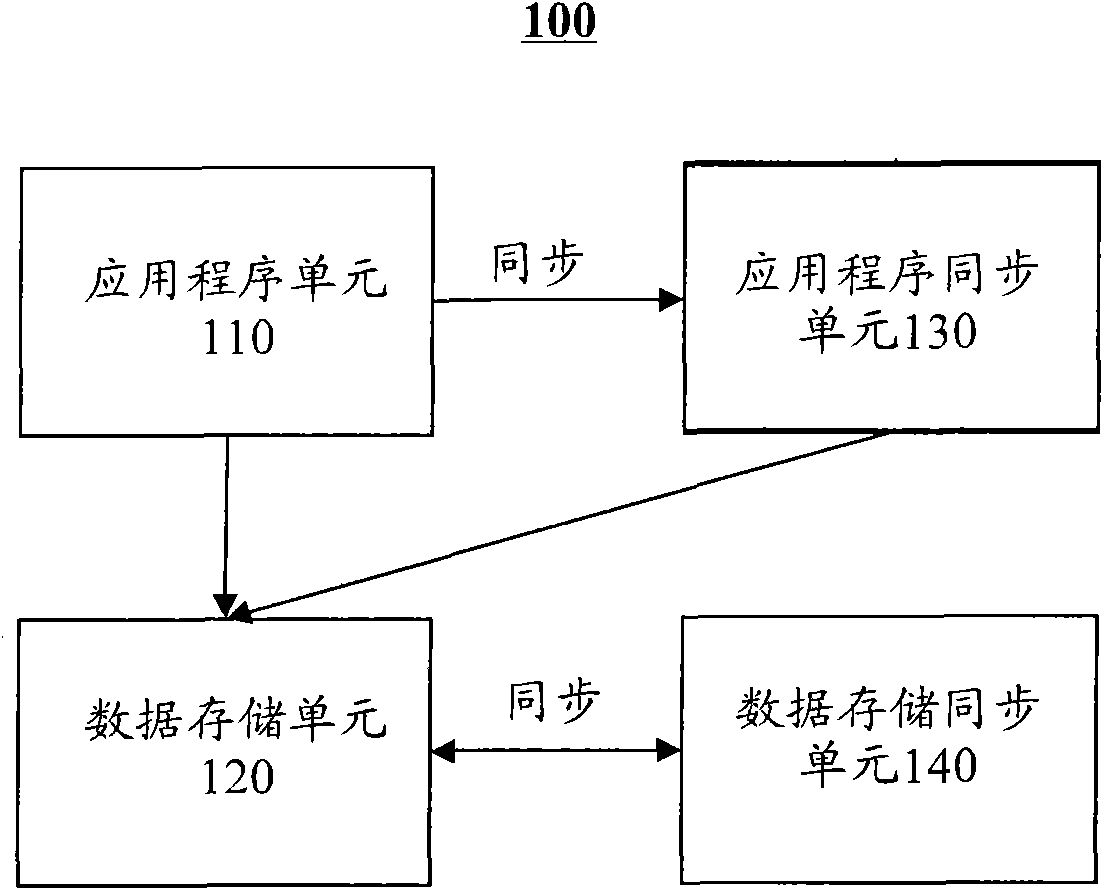
Integration equipment and method for improving availability of information system
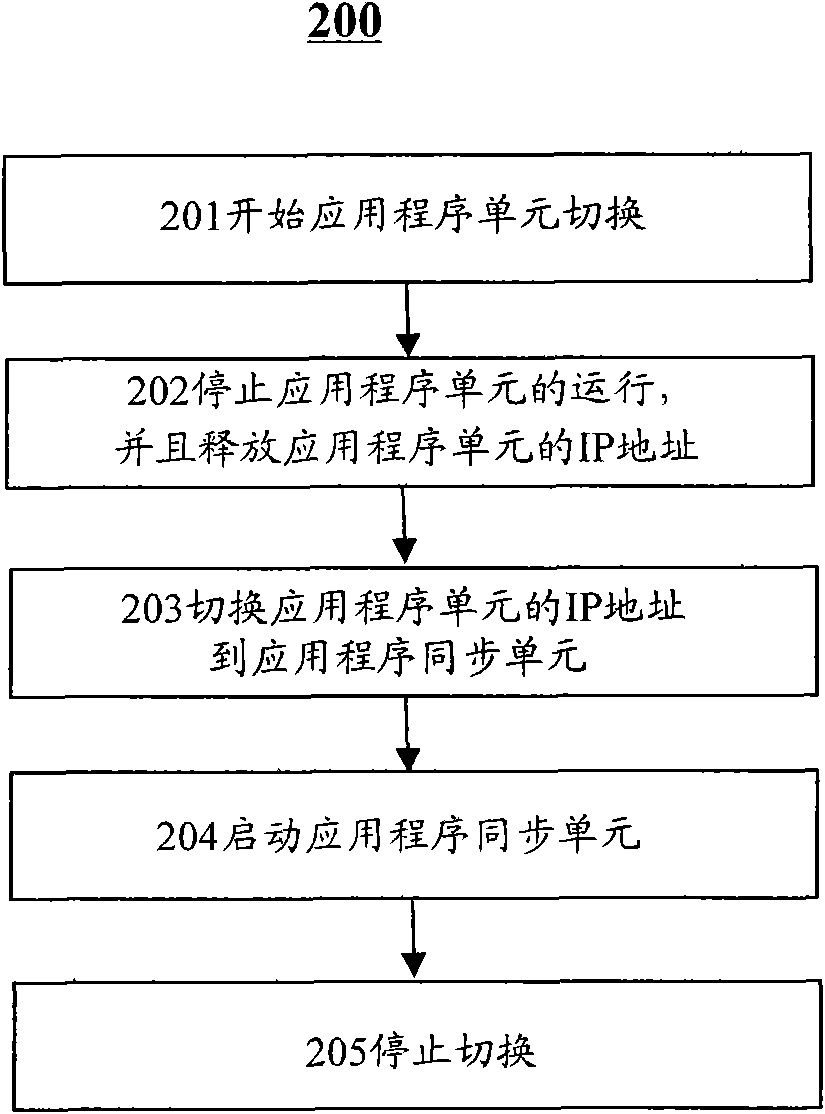
InactiveCN102314381AReduce the impact of production and operationImprove usabilityRedundant hardware error correctionProgram planningHigh availability
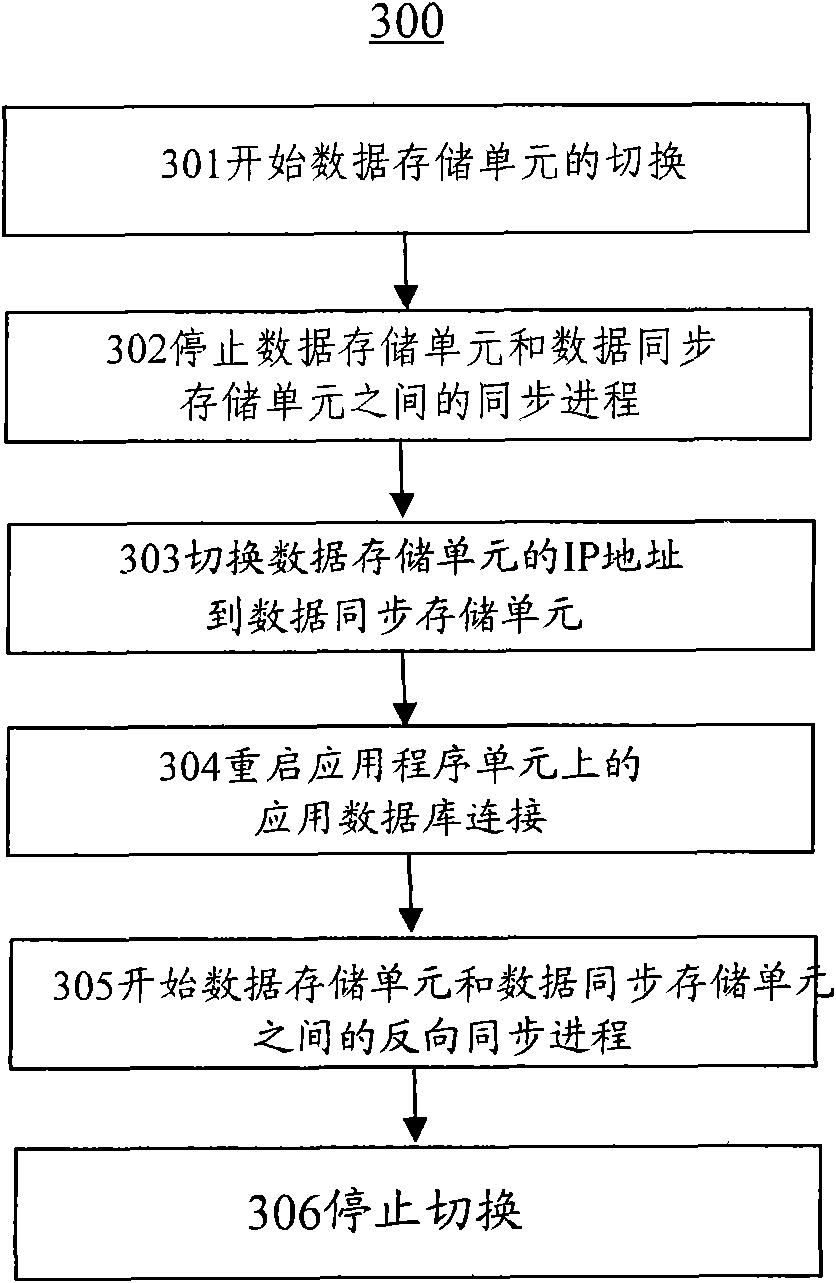
The invention discloses integration equipment and an integration method for improving the availability of an information system. The integration equipment comprises an application program unit 110, a data storage unit, an application program synchronization unit subjected to synchronous mirroring with the application program unit, and a data storage synchronization unit, wherein the application program synchronization unit is switched through a server script to continuously serve when an abnormal condition occurs; the data storage synchronization unit is kept synchronous with the data storage unit by a database replication technology; meanwhile, two disk arrays are established for redundancy. Therefore, the possibility that system operation is influenced by a system fault is greatly reduced, the high availability of the system is improved, scheduled outage times and time are reduced, and the influence of outage caused by the fault on the production and management of a client is reduced.
Owner:SHANGHAI BAOSIGHT SOFTWARE CO LTD
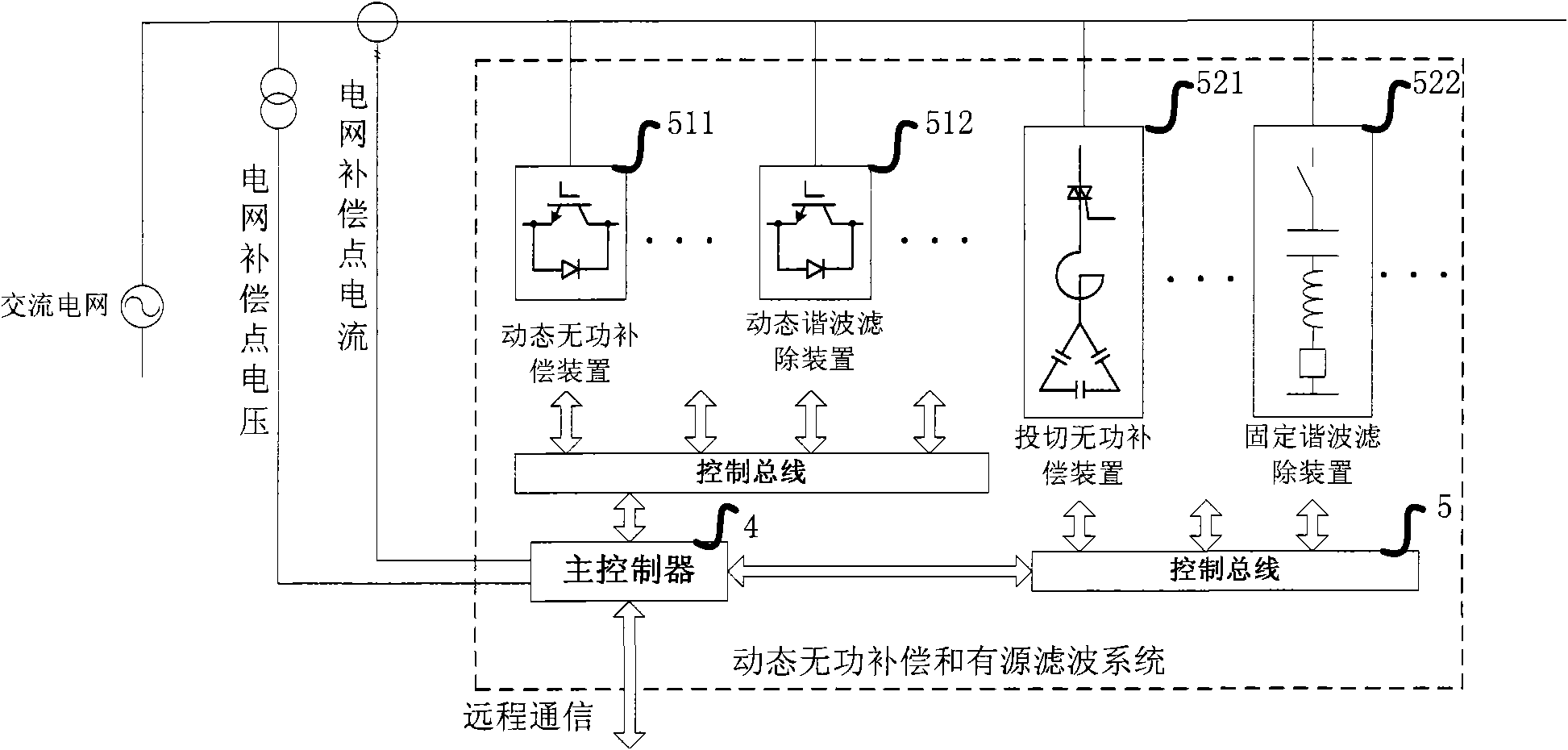
Dynamic reactive compensation and active filtration system and control method thereof
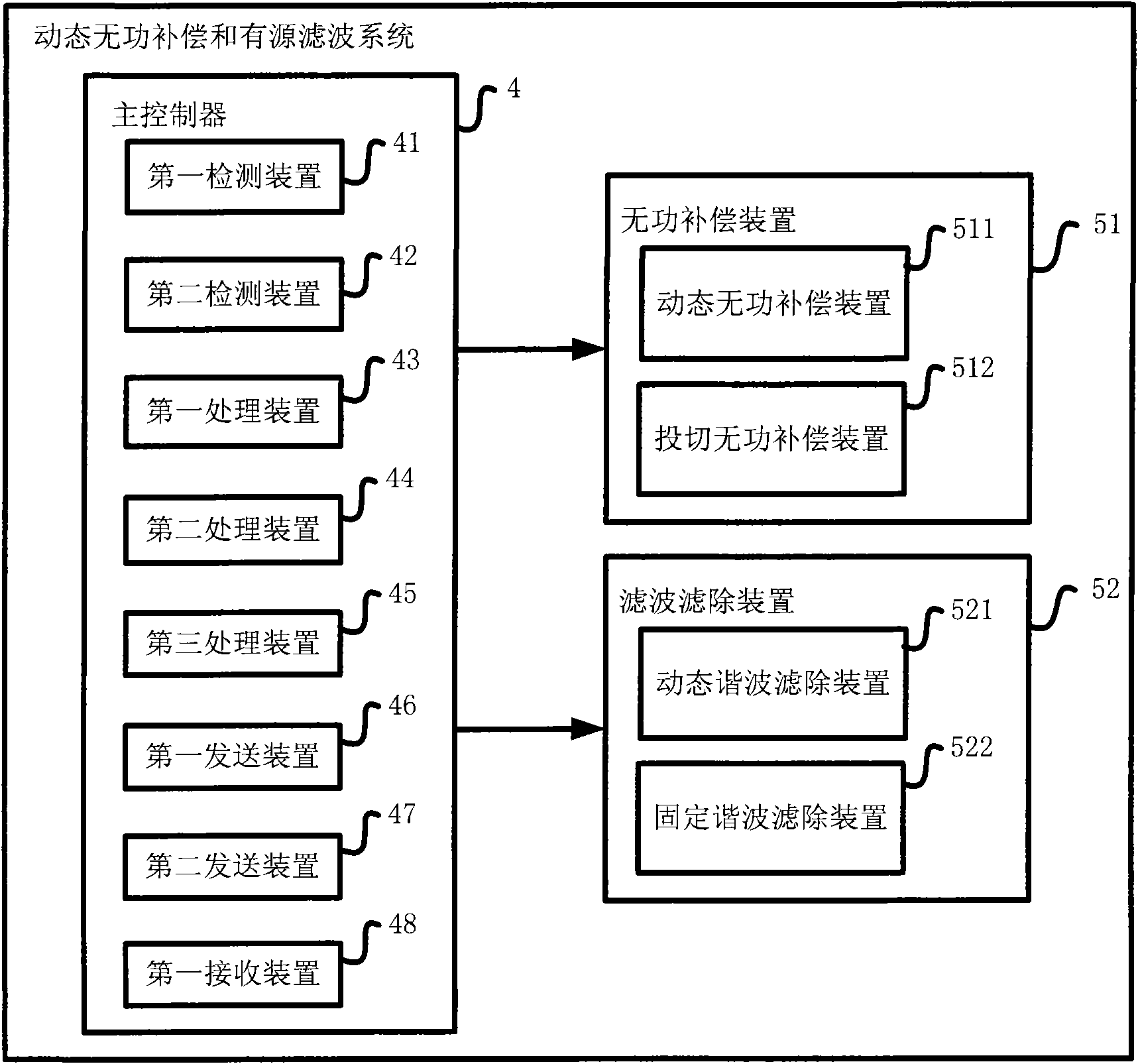
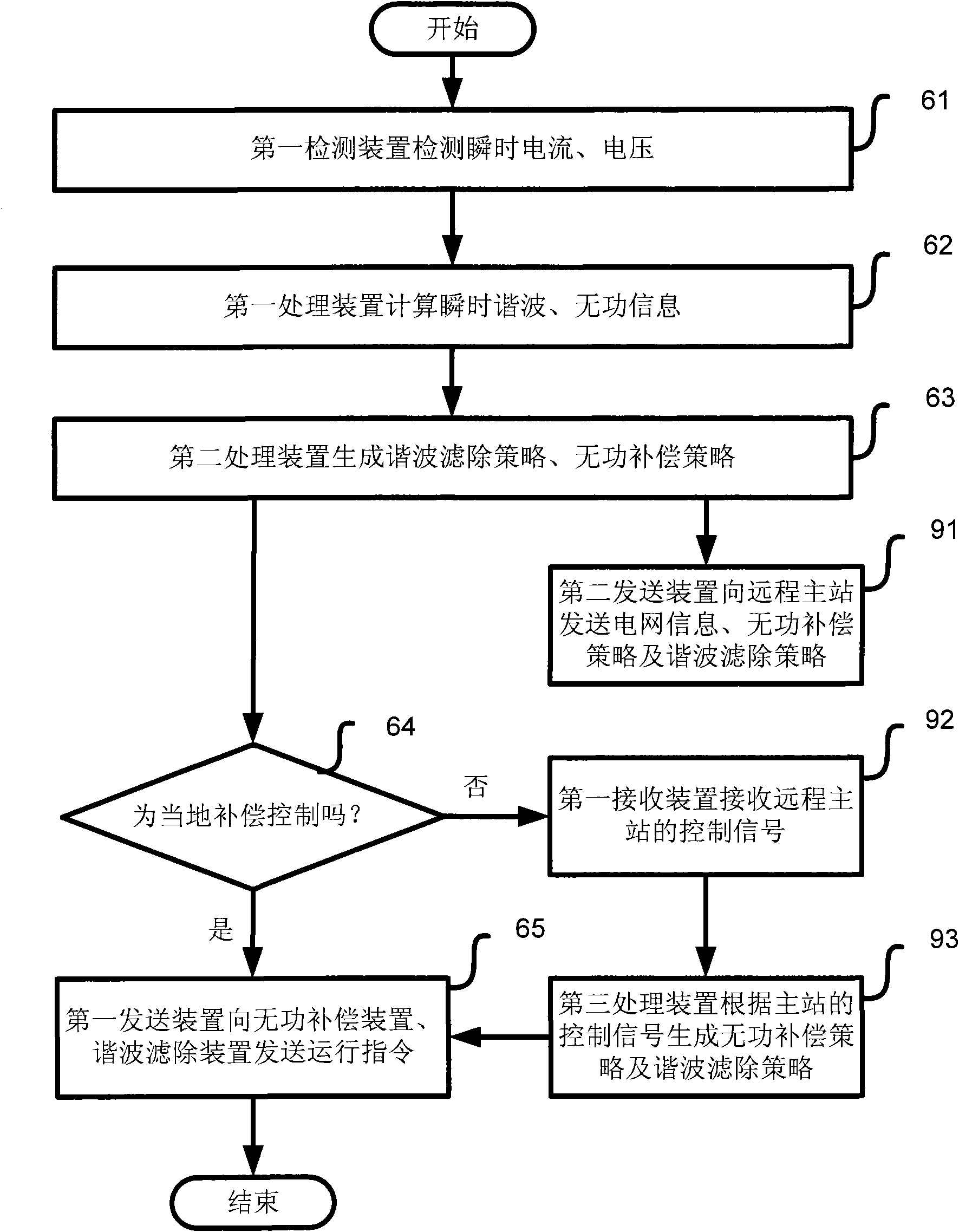
InactiveCN102377185AMeet actual needsLow costActive power filteringReactive power adjustment/elimination/compensationFiltrationSeries compensation
The invention provides a dynamic reactive compensation and active filtration system, which comprises a main controller, a dynamic reactive compensation device, a switching reactive compensation device, a dynamic harmonic wave filtering device and a fixed harmonic wave filtering device. The invention also provides a corresponding control method. In the invention, various parallel compensation units are combined, an efficient and reasonable switching strategy is used for coordinating the work of each unit so as to meet the actual needs to the maximum extent. The system disclosed by the invention has the functions of quick, smooth and large-capacity reactive compensation and harmonic wave filtration, simultaneously the capacity configuration is reasonable and flexible, the device cost is low, the protection reliability is high, the operation is intelligent, and optimum combination of the effect, the intelligence, the cost and the stability is realized, so the invention can be widely applied in the fields of reactive compensation and harmonic wave filtration of a power grid.
Owner:周玉姝
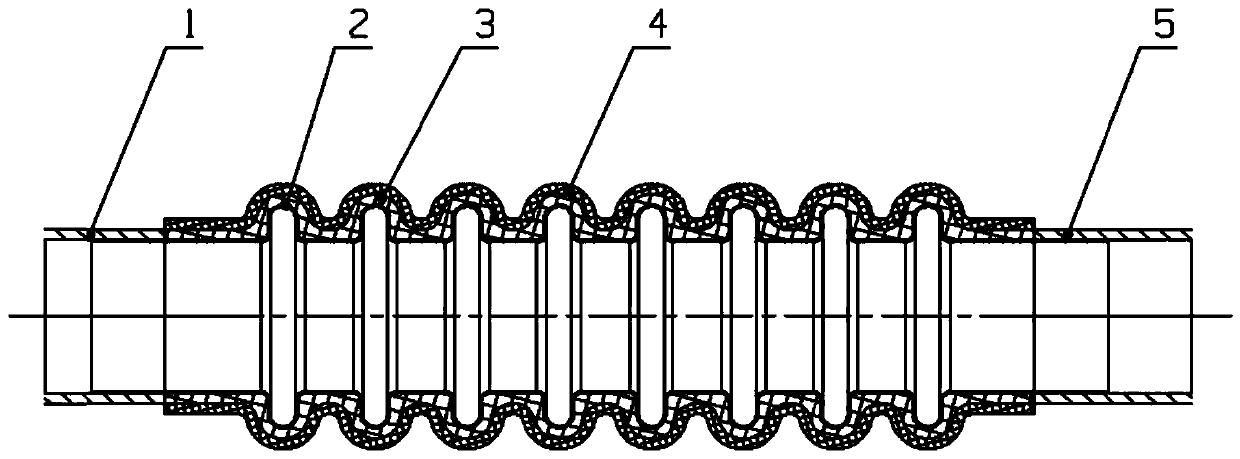
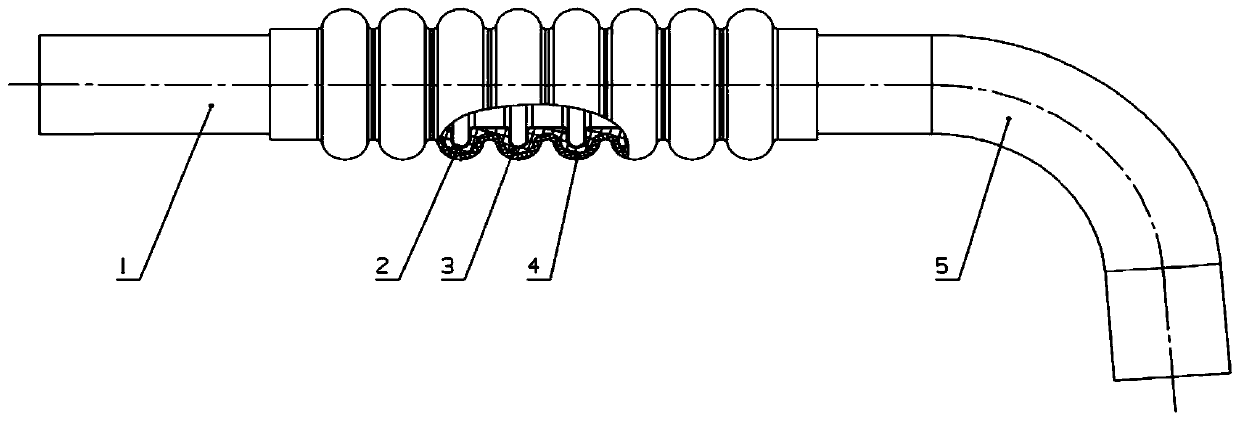
Complex structure corrugated pipe
PendingCN111473166ASolve the shortcomings of easy crackingReduce vibrationCorrosion preventionPipe protection against corrosion/incrustationCompound structureMechanical engineering
The invention discloses a complex structure corrugated pipe. The complex structure corrugated pipe comprises a metal corrugated pipe, a rubber inner layer and a rubber outer layer, wherein the metal corrugated pipe is formed by machining corrugations on a thin-wall steel pipe; the rubber inner layer is wrapped on the outer wall of the metal corrugated pipe; and the rubber outer layer is wrapped onthe outer wall of the rubber inner layer. The rubber layers of the complex structure corrugated pipe are closely combined with the corrugated pipe. Damping is high. Vibration of the corrugated pipe is reduced. Vibration parameters of the corrugated pipe are reduced. The anti-fatigue-cracking service life of the corrugated pipe is prolonged. The defect that the corrugated pipe cracks easily is overcome. Besides, when having cracks, the complex structure corrugated pipe can continuously achieve the normal delivery function with the aid of the sealing effect of the rubber layers and the secondary protective effect, prevent serious secondary faults that leakage of the substances such as oil, air and water delivered in the corrugated pipe causes fires, the performance is reduced and machines and equipment cannot operate, gain time for subsequent use and maintenance and minimize the fault influence.
Owner:广西玉柴船电动力有限公司
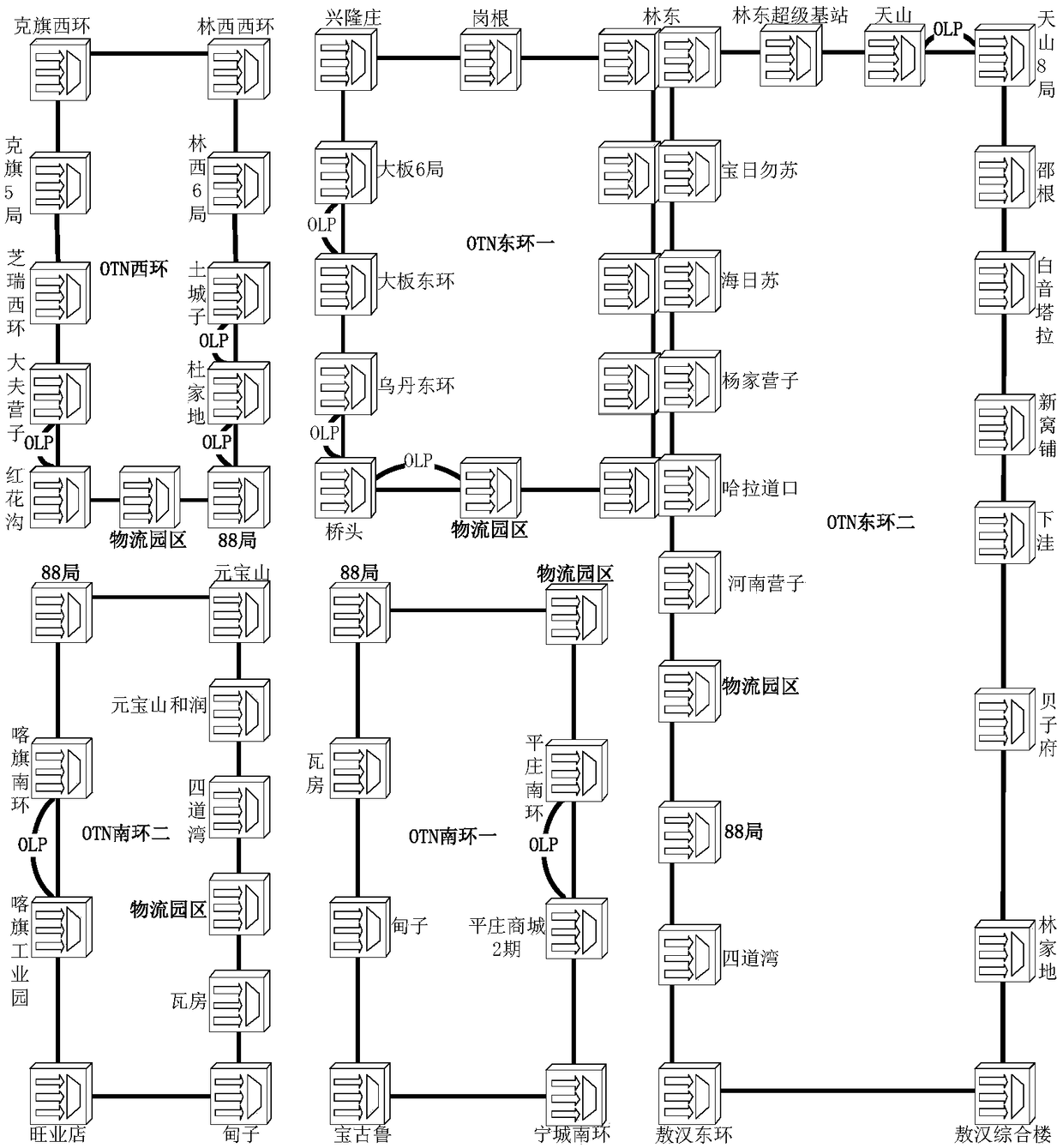
Networking method of metro backbone optical transmission network (OTN)
InactiveCN108270648AAvoid mismatchEasy accessLoop networksMetropolitian area networksStructure of Management InformationRouting algorithm
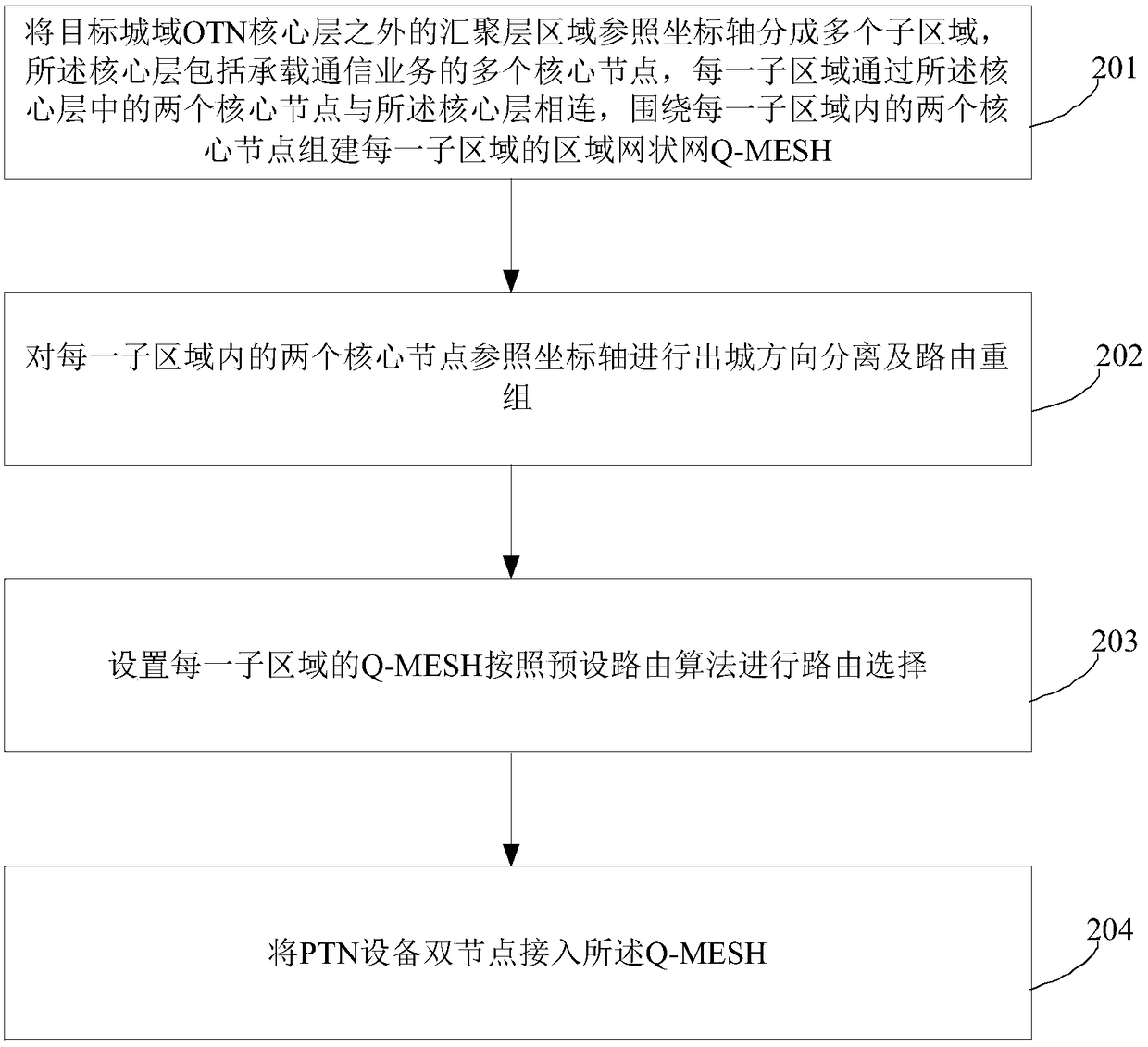
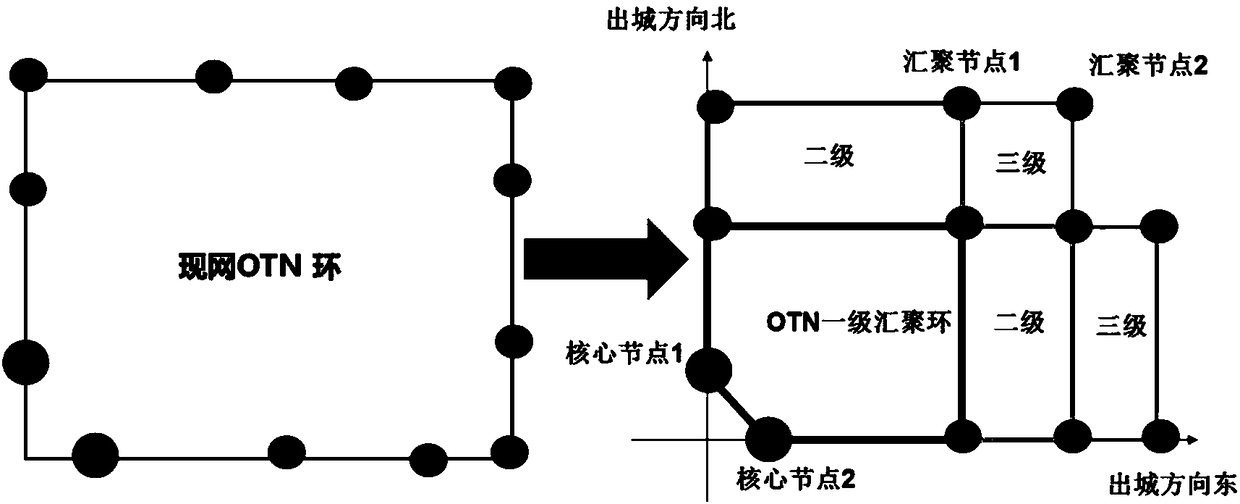
The invention provides a networking method of a metro backbone optical transmission network (OTN). The method comprises the steps of dividing a convergence layer area outside a core layer of the target metro OTN into a plurality of sub-areas according to a coordinate axis, wherein the core layer comprises a plurality of core nodes which bear a communication service, each sub-area is connected witha core layer through two core nodes, establishing the Q-MESH of each sub-area around the two core nodes; performing city-leaving-direction separation and route regrouping on the two core nodes in each sub-area according to the coordinate axis; setting for making the Q-MESH of each sub-area perform route selection according to a preset route algorithm; and accessing the Q-MESH by double nodes of PTN equipment. The networking method can realize networking structure matching between OTN and PTN and furthermore realizes advantages of realizing quick accessing and capacity expansion of the PTN service, reducing capacity expansion cost, preventing a potential hazard of concealed same route, reducing fault influence and improving network safety.
Owner:中国移动通信集团内蒙古有限公司 +1
Method for predicting service life of yaw bearing of wind turbine generator
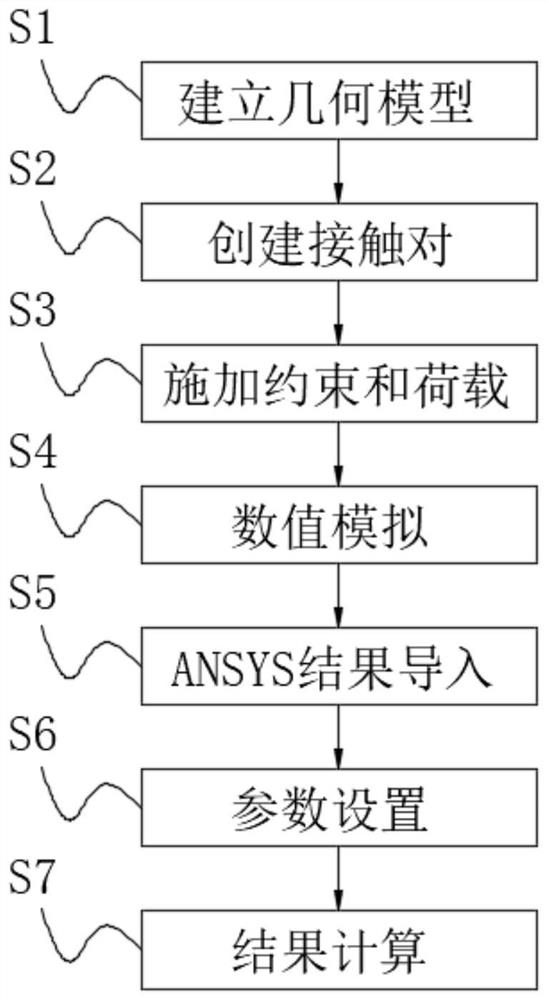
PendingCN114021288AEasy maintenanceReduce the impact of failureGeometric CADDesign optimisation/simulationModeling softwareGeometric modeling
A method for predicting the service life of a yaw bearing of a wind turbine generator comprises the following steps: establishing a geometric model, creating a contact pair, applying constraint and load, performing numerical simulation, importing an ANSYS result, setting parameters, calculating the result, establishing the geometric model when the bearing clearance is 0 by using three-dimensional modeling software, inputting the parameters of a model material, setting the unit size, and performing grid division on the model by adopting a sweeping mode. According to the method for predicting the service life of the yaw bearing of the wind turbine generator, the contact stress of the bearing of the wind turbine generator is analyzed and calculated by using ANSYS software through a fatigue life numerical value simulation method, so that the service life of the bearing can be predicted, the prediction accuracy is improved, a worker can conveniently maintain the wind turbine generator, and the influence caused by wind turbine generator faults is reduced.
Owner:XIAN THERMAL POWER RES INST CO LTD
Intelligent optical cable monitoring method and device, computer equipment and storage medium
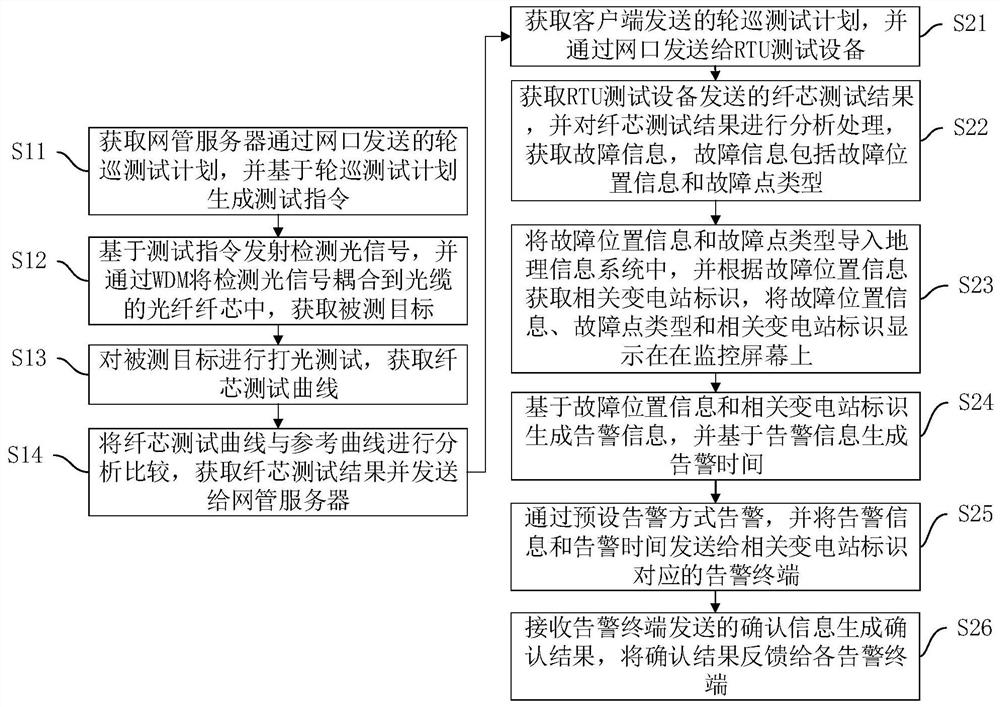
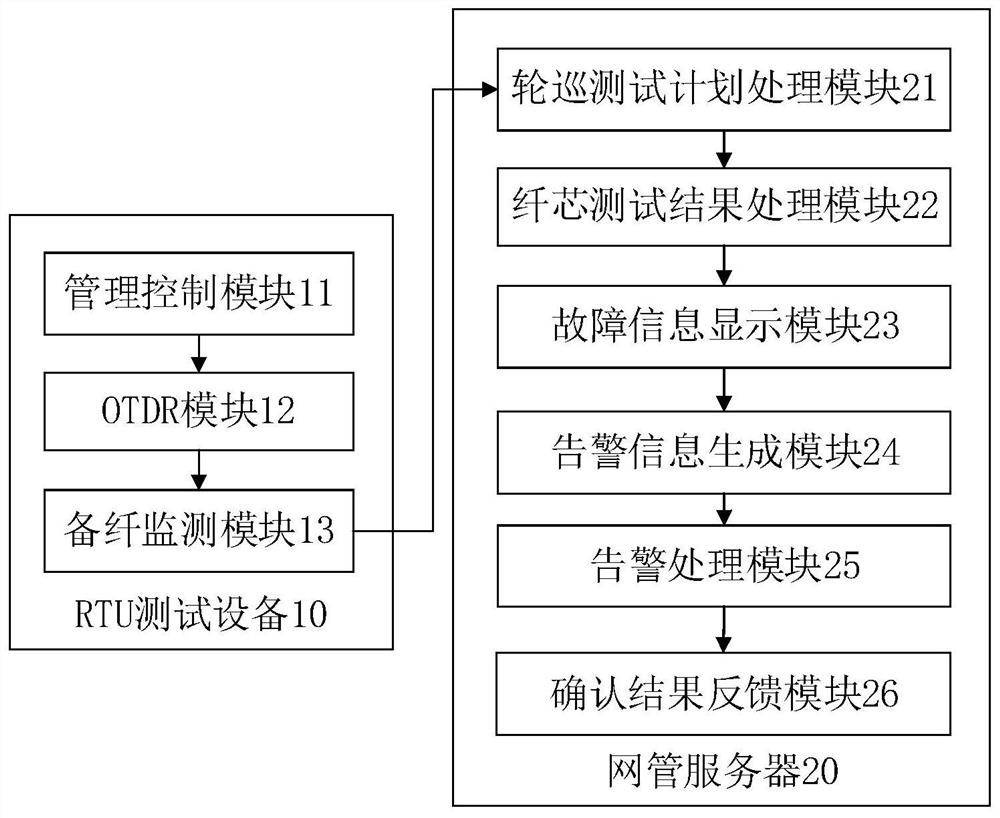
ActiveCN111884710AEnable proactive maintenanceImprove maintenance efficiencyElectromagnetic transmissionNetwork managementLight signal
The invention discloses an intelligent optical cable monitoring method and device, computer equipment and a storage medium, and the method comprises the steps that RTU test equipment obtains a pollingtest plan transmitted by a network management server through a network port, generates a test instruction based on the polling test plan, so as to transmit a detection optical signal to obtain a tested target, carries out the lighting test of the tested target, obtains a fiber core test curve so as to obtain a fiber core test result and sends the fiber core test result to the network management server; the network management server obtains a polling test plan sent by the client, sends the polling test plan to the RTU test device through the network port, then obtains a fiber core test resultsent by the RTU test device, analyzes and processes the fiber core test result, sends the analyzed and processed result to the geographic information system, and displays the analyzed and processed result on the monitoring screen, so that workers can conveniently and visually know the information in time and then give an alarm in a preset alarm mode, active monitoring and active maintenance of theoptical cable are achieved, the maintenance efficiency of the optical cable is improved, and the influence of optical cable faults is reduced.
Owner:GUANGAN POWER SUPPLY COMPANY STATE GRID SICHUANELECTRIC POWER
Intelligent dense medium separation system and working method thereof
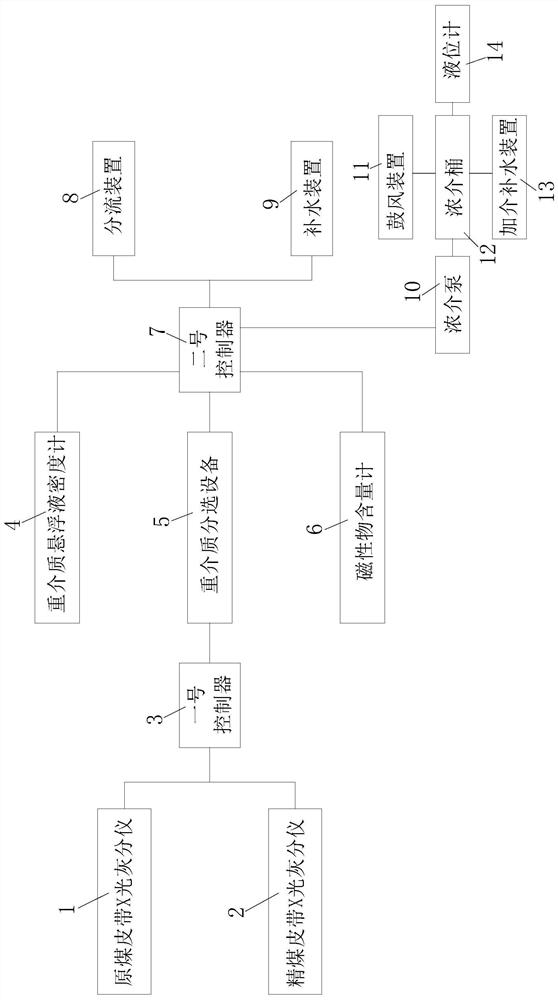
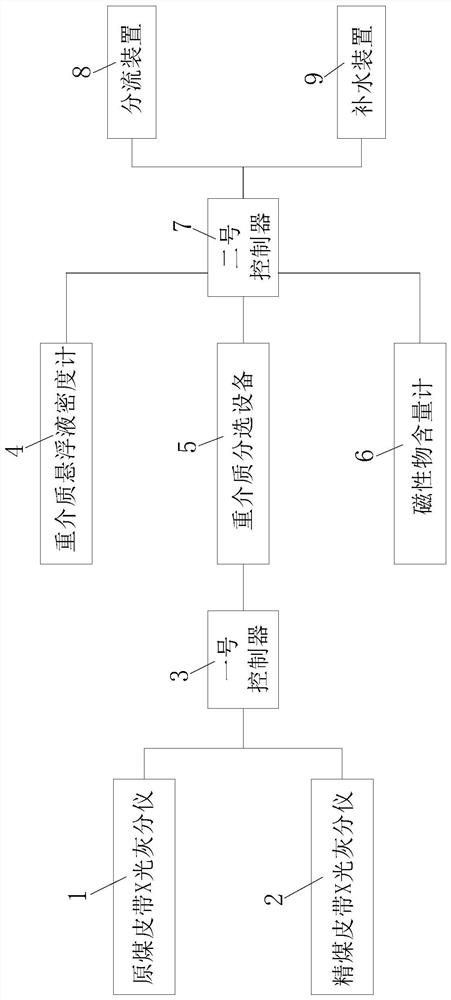
InactiveCN113210119AEasy to adjustImplement automatic additionWet separationThermodynamicsProcess engineering
The invention relates to an intelligent dense medium separation system and a working method thereof, and belongs to the technical field of coal washing. The system comprises a raw coal belt X-ray ash content testing system, a clean coal belt X-ray ash content testing system, a first controller, a dense medium suspension densimeter, dense medium separation equipment, a magnetic substance content meter, a second controller, a flow dividing device and a water supplementing device, wherein the raw coal belt X-ray ash content testing system and the clean coal belt X-ray ash content testing system are both connected with the first controller, the first controller is connected with the dense medium suspension densimeter, the dense medium separation equipment and the magnetic substance content meter, the dense medium suspension densimeter, the dense medium separation equipment and the magnetic substance content meter are all connected with the second controller, and the second controller is connected with the flow dividing device and the water supplementing device.
Owner:HUADIAN ELECTRIC POWER SCI INST CO LTD
Product of foaming-type polyurethane shock pad and manufacturing method thereof
The invention provides a product of a foaming-type polyurethane elastic shock pad and a manufacturing method of the product. The method is characterized in that a polyether glycol mixture ( material A for short) is used as a main material, and prepolymer slurry based on diphenylmethane diisocyanate (MDI) ( material B for short) is used as a curing agent and water is used as a foaming agent. The method comprises the steps of: adding material B and water into material A, preheating materials A and B, uniformly mixing materials A and B under the conditions of a vacuum environment and high-speed stirring, and casting to obtain the final product. The prepared product has a change rate of static stiffness of less than or equal to 20% after a fatigue test for 4000000 times and a ratio of dynamic stiffness to static stiffness of less than or equal to 1.3, and the product has an obvious damping effect, good resistance to wear and oil, and excellent anti-aging property. The whole manufacturing process is high in automation degree and safe, and the product has stable quality.
Owner:ZHUZHOU TIMES NEW MATERIALS TECH
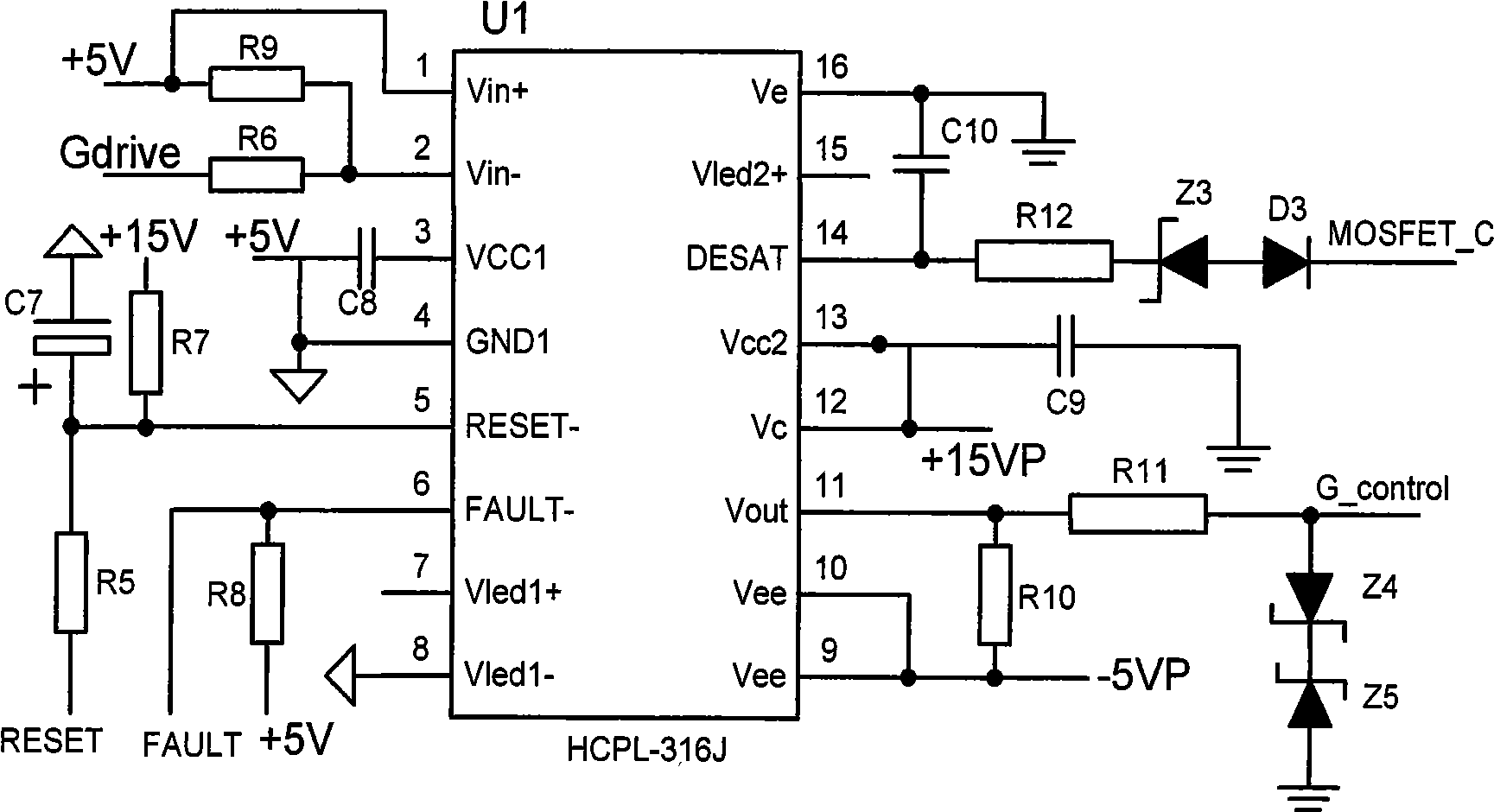
Maximum power tracking controller for photovoltaic power generation based on digital signal processor
InactiveCN101227090BLow costReduce difficultyDc-dc conversionDc source parallel operationDigital signal processingGrid-tie inverter
The invention discloses a photovoltaic power-generating maximal power tracking controlling device based on a digital signal processing device, which comprises a direct current converter, a voltage and electric current detecting module, a controlling module and a driving and protecting module, wherein the input end of the direct current converter is connected with the output end of a solar batteryarray, the output end is connected with the input end of a synchronization inverter through a direct current bus bar, the synchronization inverter is connected with an electric network, the input endof the voltage and electric current detecting module is connected with the output end of the voltage and electric current detecting module, the output end of the voltage and electric current detecting module is connected with the input end of the controlling module, the output end the controlling module is connected with the input end of the driving and protecting module, and the output end of the driving and protecting module is connected with a grid electrode G of an isolated-gate bipolar field effect transistor of the direct current converter. The device has the beneficial effects of beingeasy to popularize and use as the cost is low, reducing the difficulty of the system controlling realization, and increasing controlling effect. The installation of the invention is flexible, maintenance is convenient, and the influence of a single branch fault can be reduced in the maximum limit.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
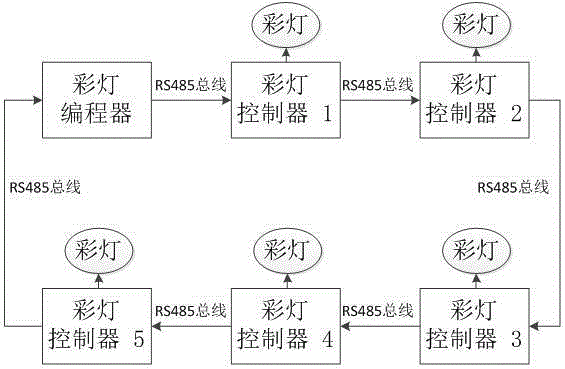
High-reliability colored lamp system control method
InactiveCN105188234AImprove reliabilitySimple structureElectric light circuit arrangementEnergy saving control techniquesEngineeringBrightness perception
The invention discloses a high-reliability colored lamp system control method, and mainly relates to the field of illumination control. In the high-reliability colored lamp system control method, a colored lamp controller and a colored lamp programmer are adopted for finishing the method; the colored lamp programmer is used for sending light control data; the colored lamp controller is used for receiving, processing and forwarding the light control data, and controlling the brightness, the color and the turning-on time of colored lamps according to the light control data. According to the high-reliability colored lamp system control method disclosed by the invention, multiple colored lamp controllers can work cooperatively, thus achieving uniform control on the brightness, the color and the turning-on time of the colored lamps; furthermore, in a working process, the colored lamp programmer can detect a system control state in real time; when a fault occurs, a colored lamp system enters a preset colored lamp display state so as to reduce the influence caused by the fault.
Owner:樊星宇
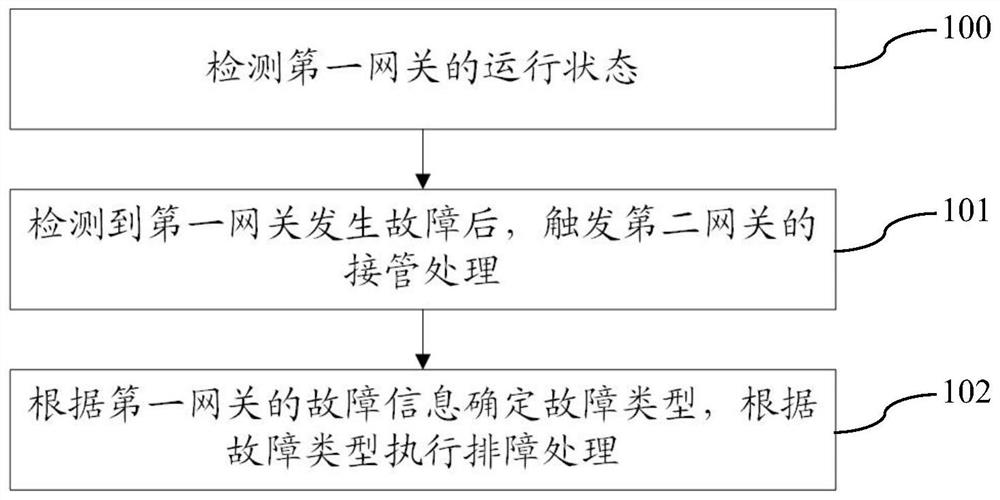
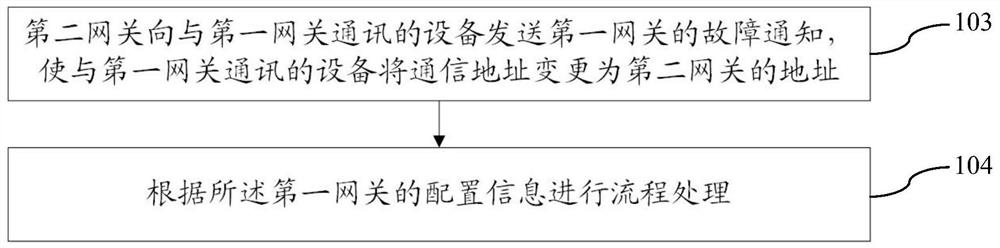
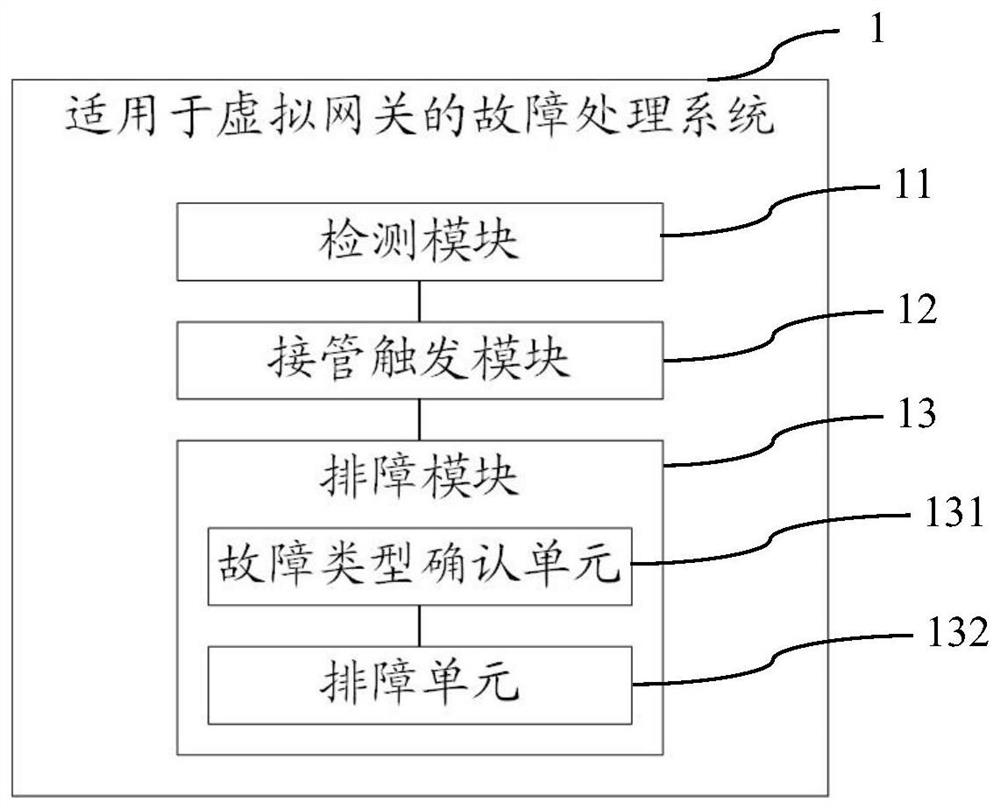
Fault processing method, system and device suitable for virtual gateway and storage medium
ActiveCN112003748AAutomate processingReduce human interventionCharacter and pattern recognitionData switching networksComputer networkDependability
The invention provides a fault processing method, system and device suitable for a virtual gateway, and a storage medium. The fault processing method comprises the steps of: detecting an operation state of a first gateway; after detecting that the first gateway has a fault, triggering takeover processing of a second gateway to enable the second gateway to take over a process of the first gateway;and determining a fault type according to the fault information of the first gateway, and executing troubleshooting processing according to the fault type. According to the fault processing method, the system and the device, when one gateway fails, the other gateway can be switched to ensure that the service is not influenced, and meanwhile, the fault is subjected to troubleshooting processing through using the fault type, so that the automatic processing of the fault is realized, the human intervention is reduced, and the reliability is improved.
Owner:CHINA CONSTRUCTION BANK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com