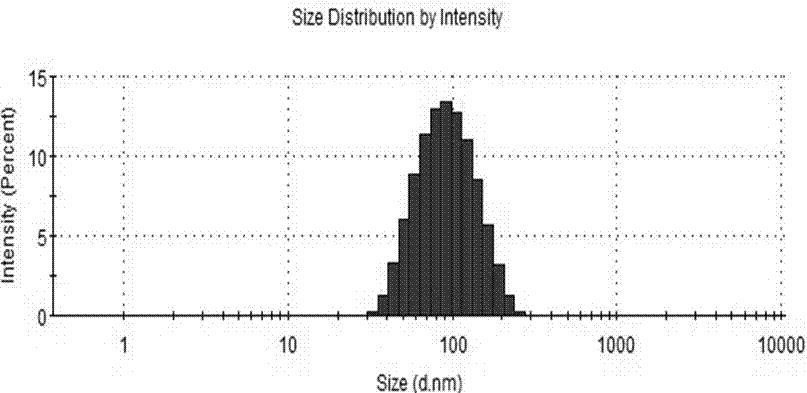
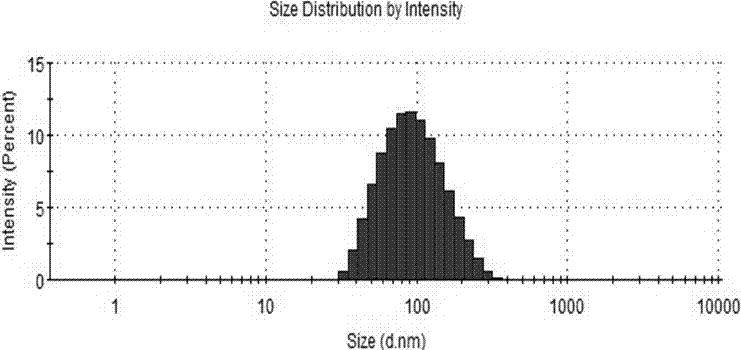
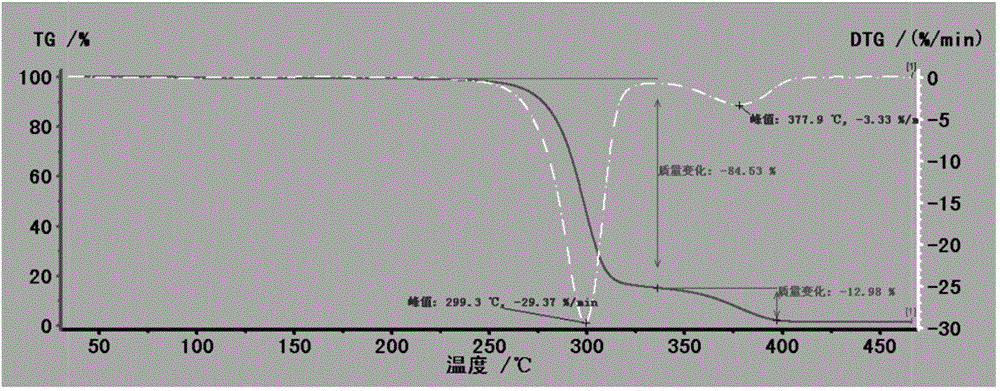
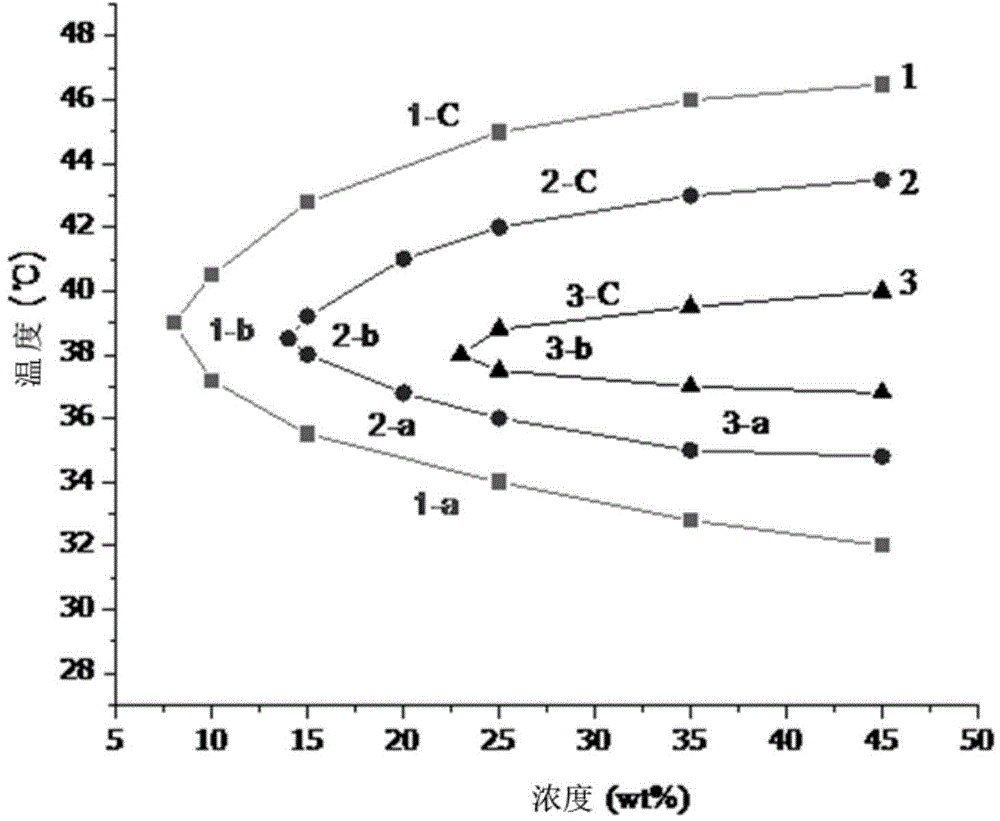
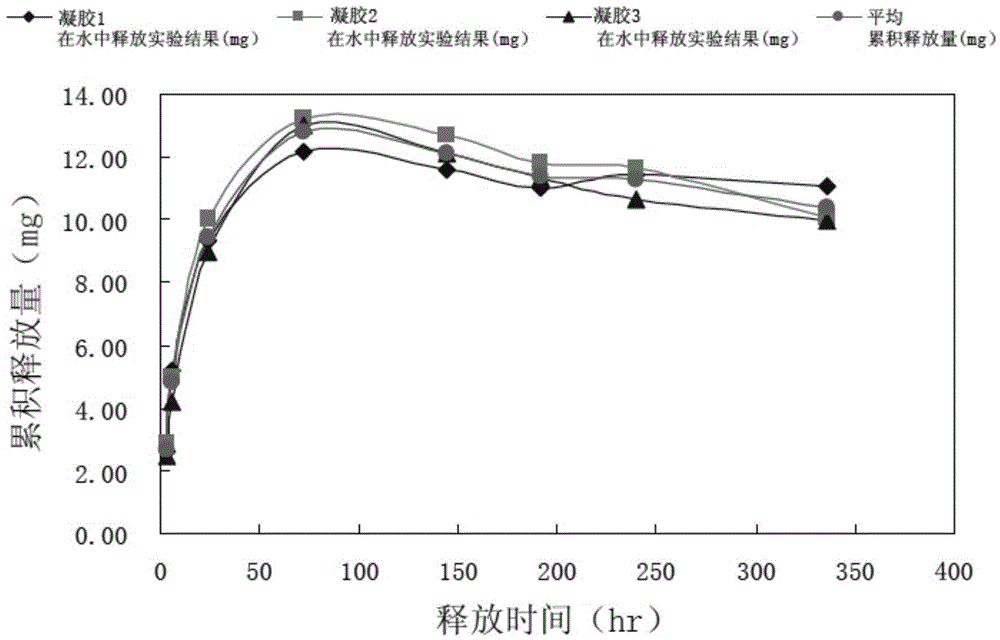
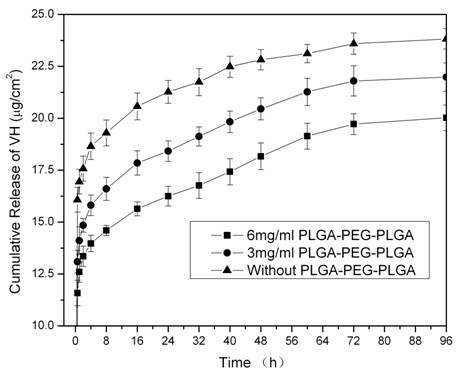
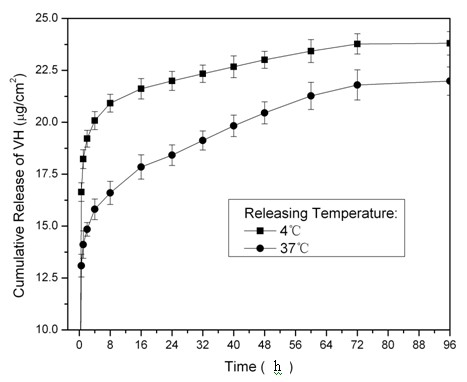
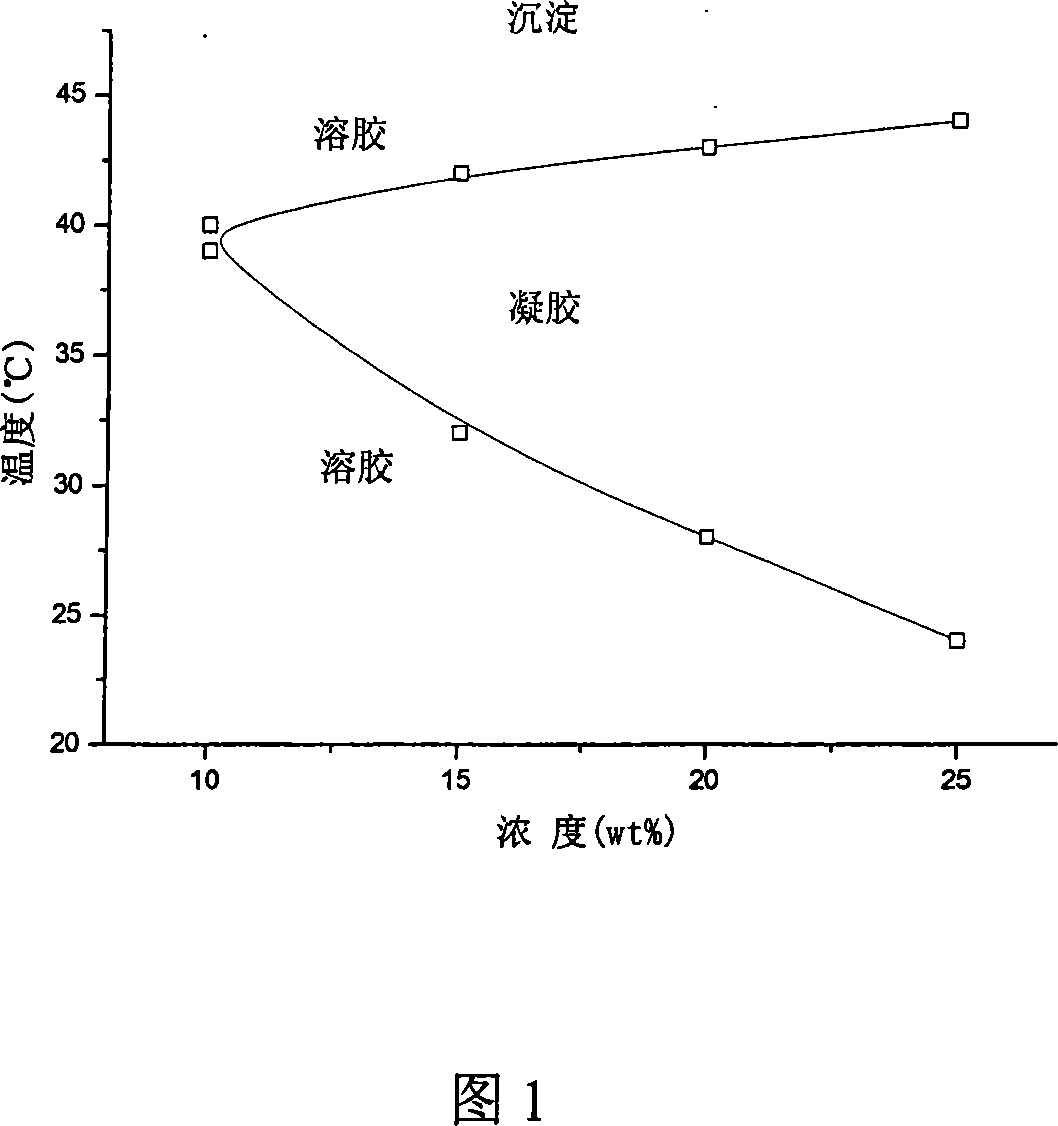
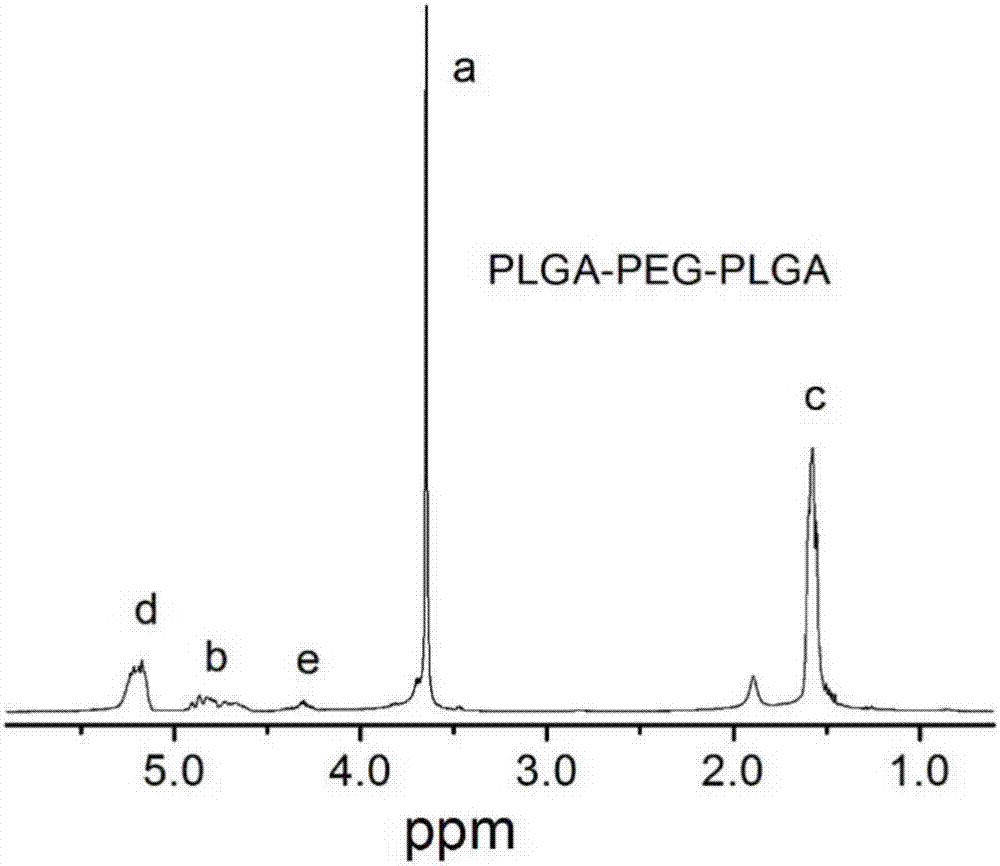
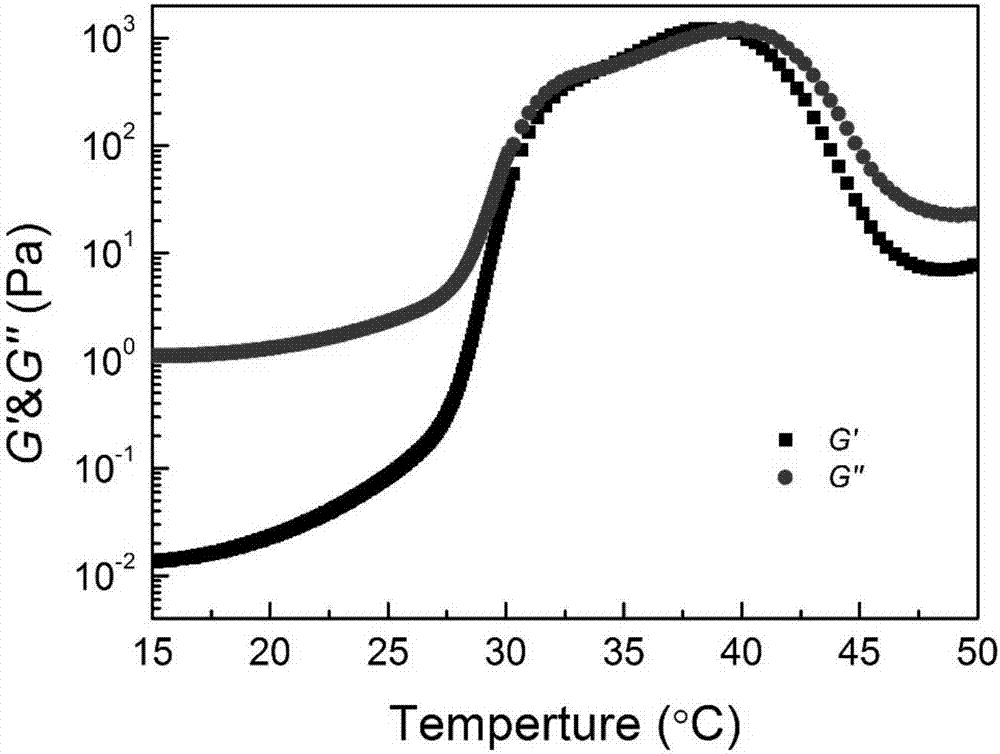
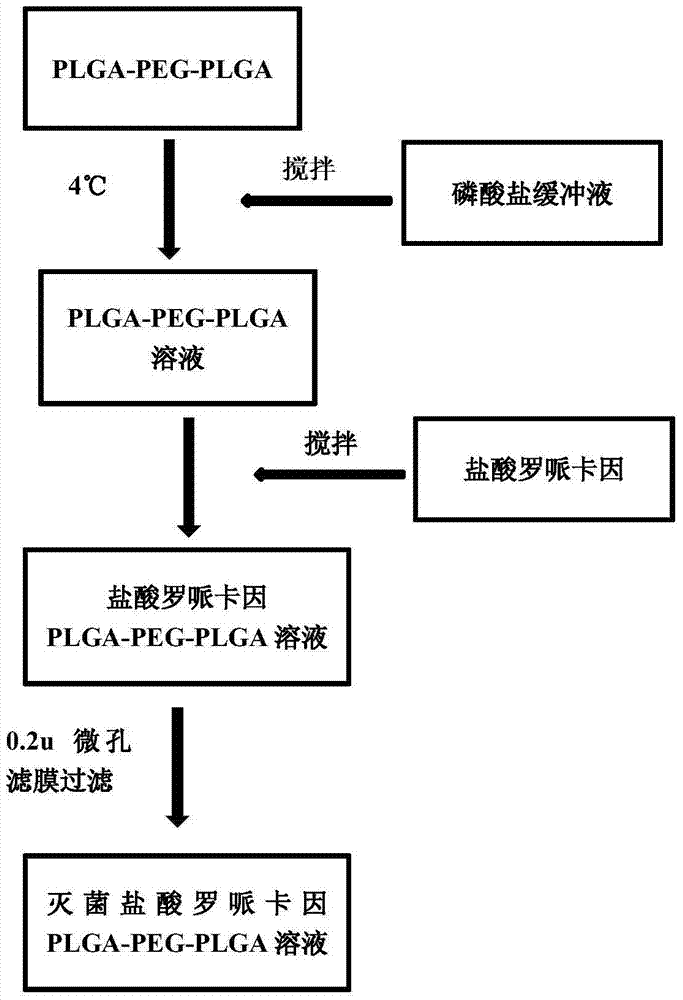
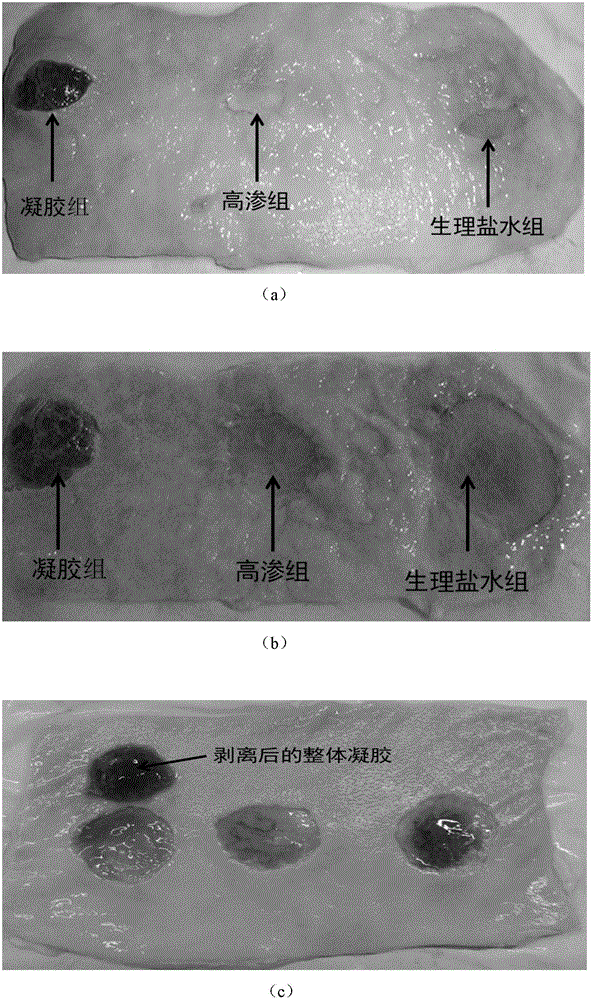
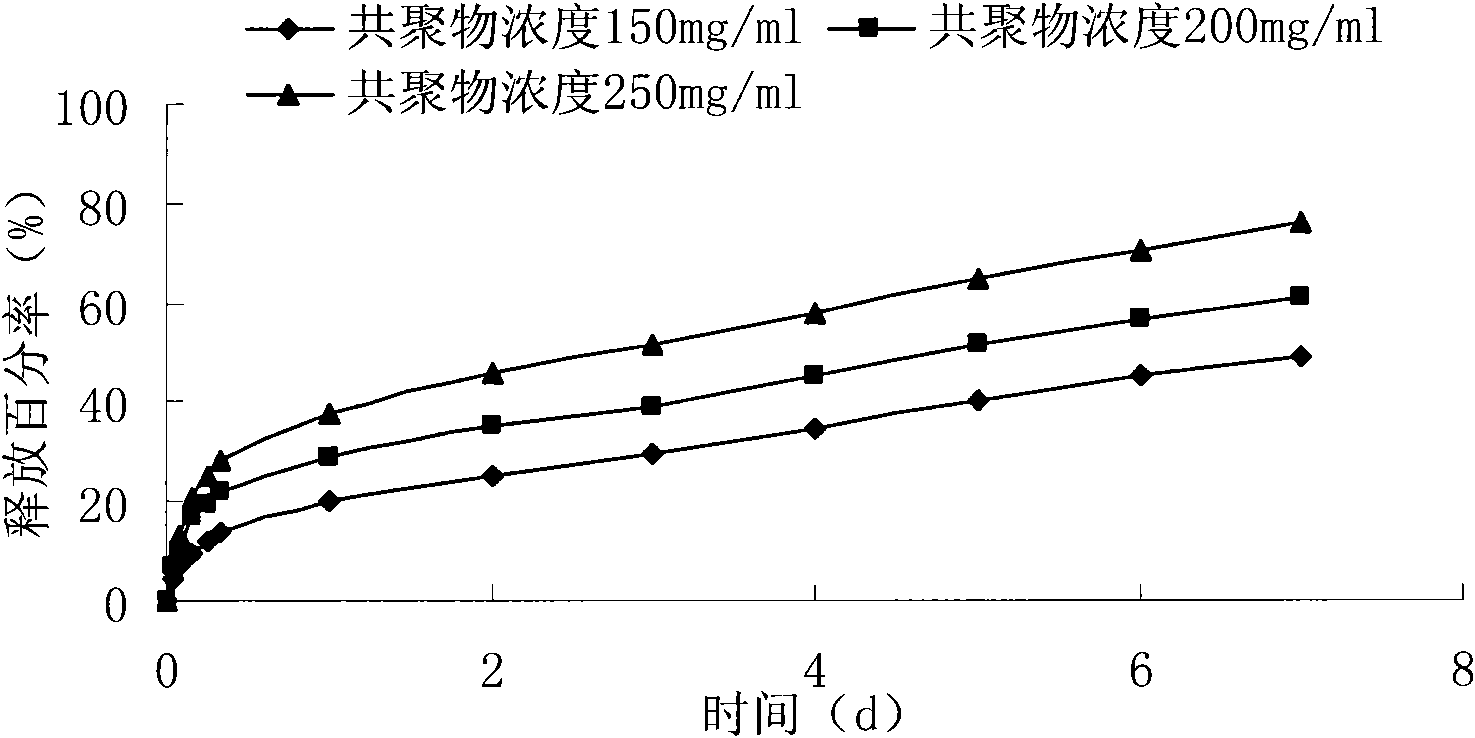
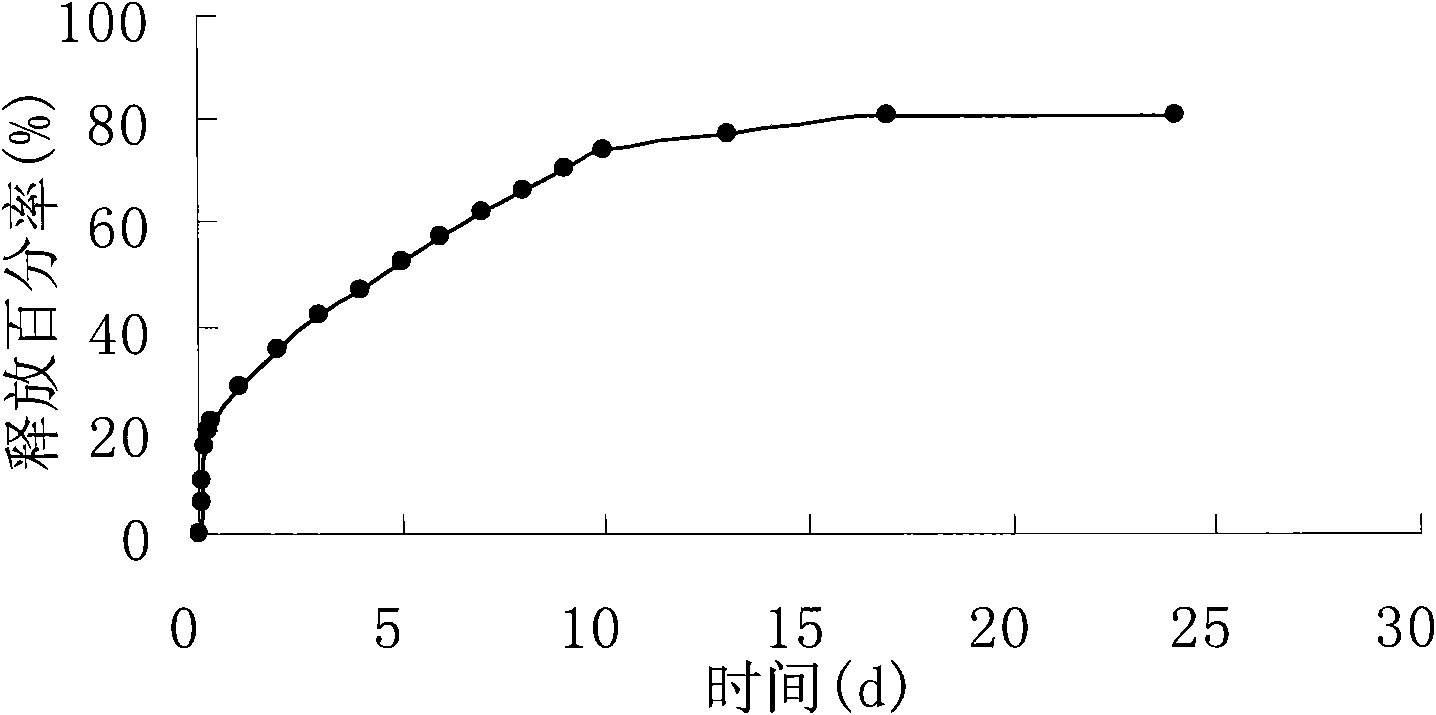
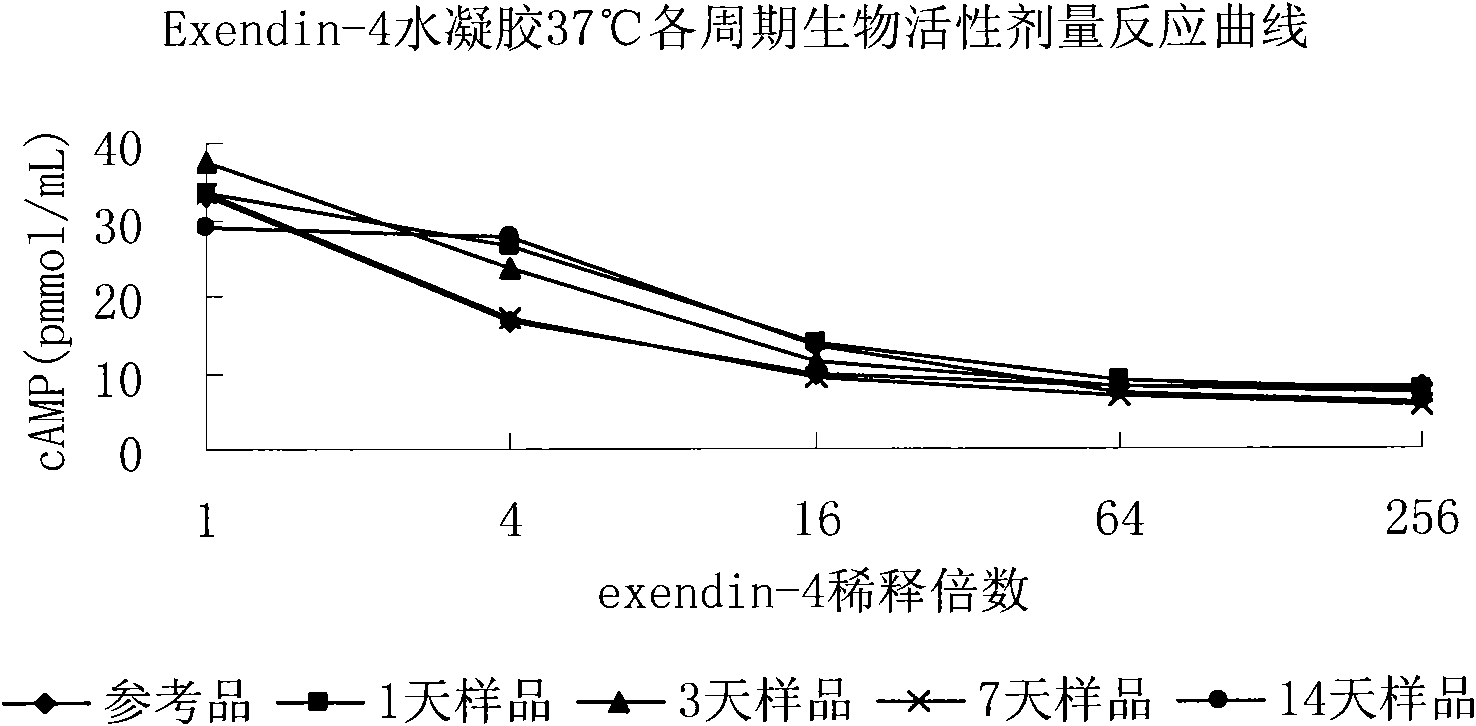
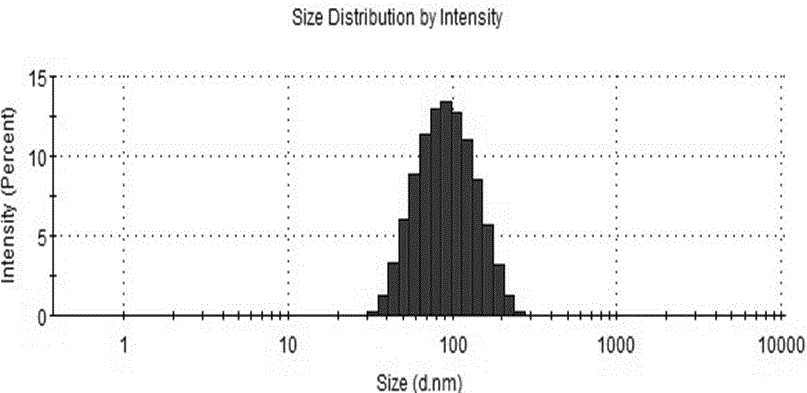
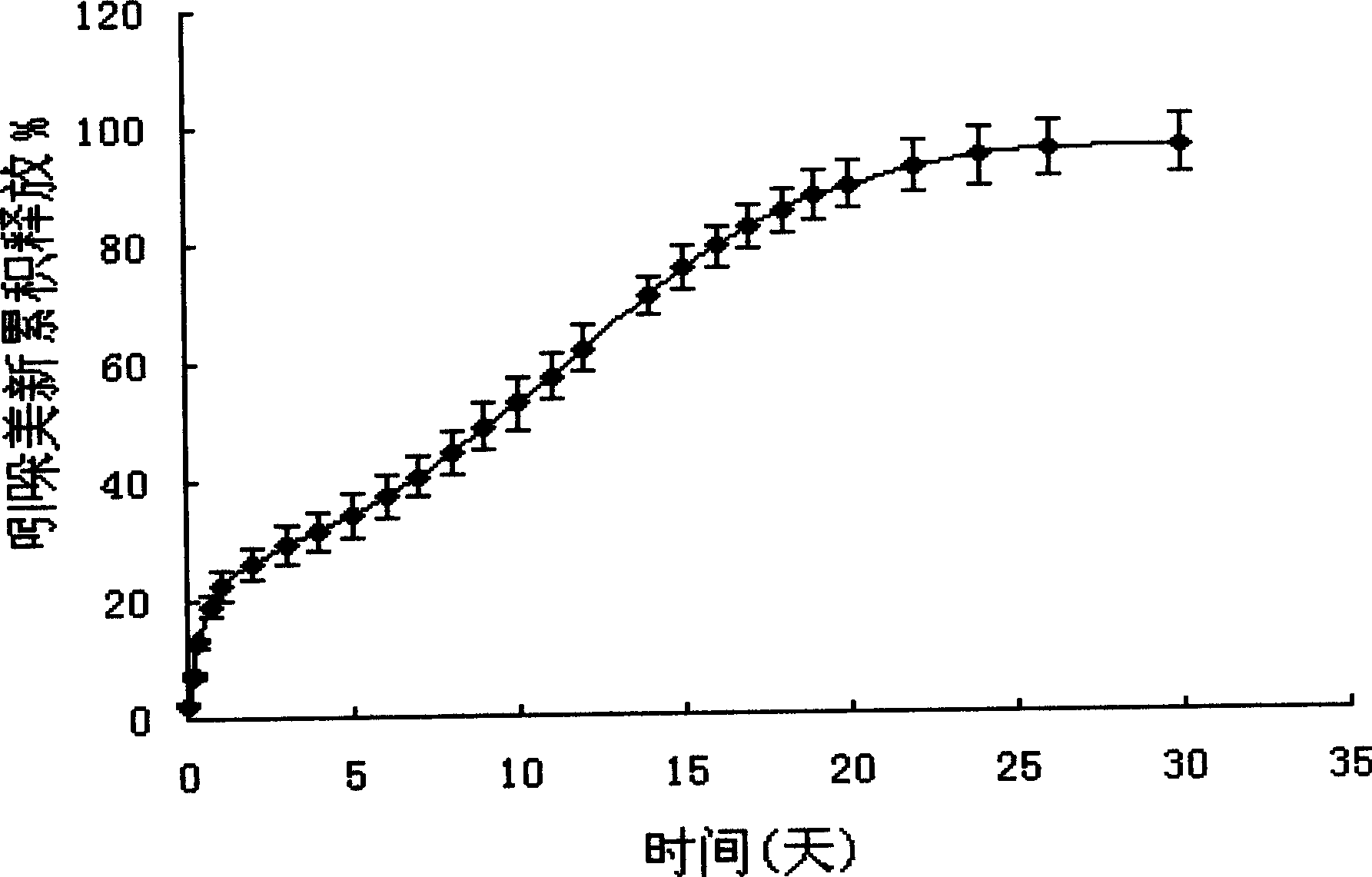
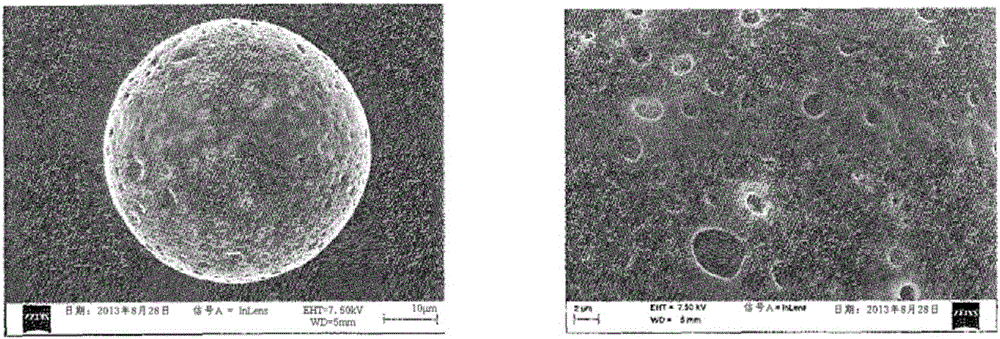
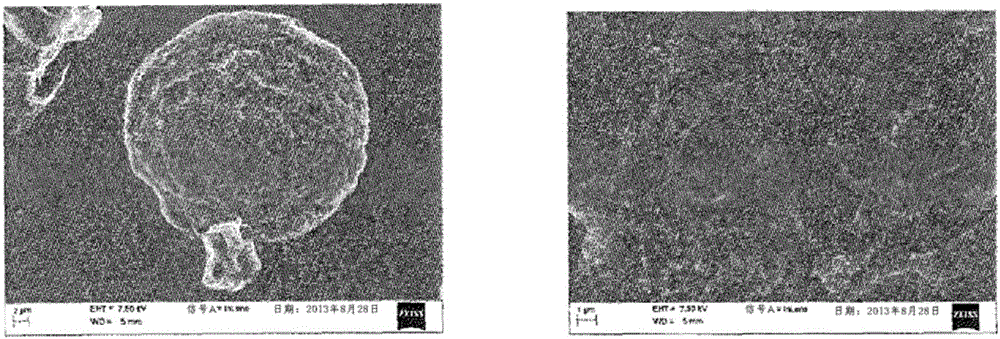
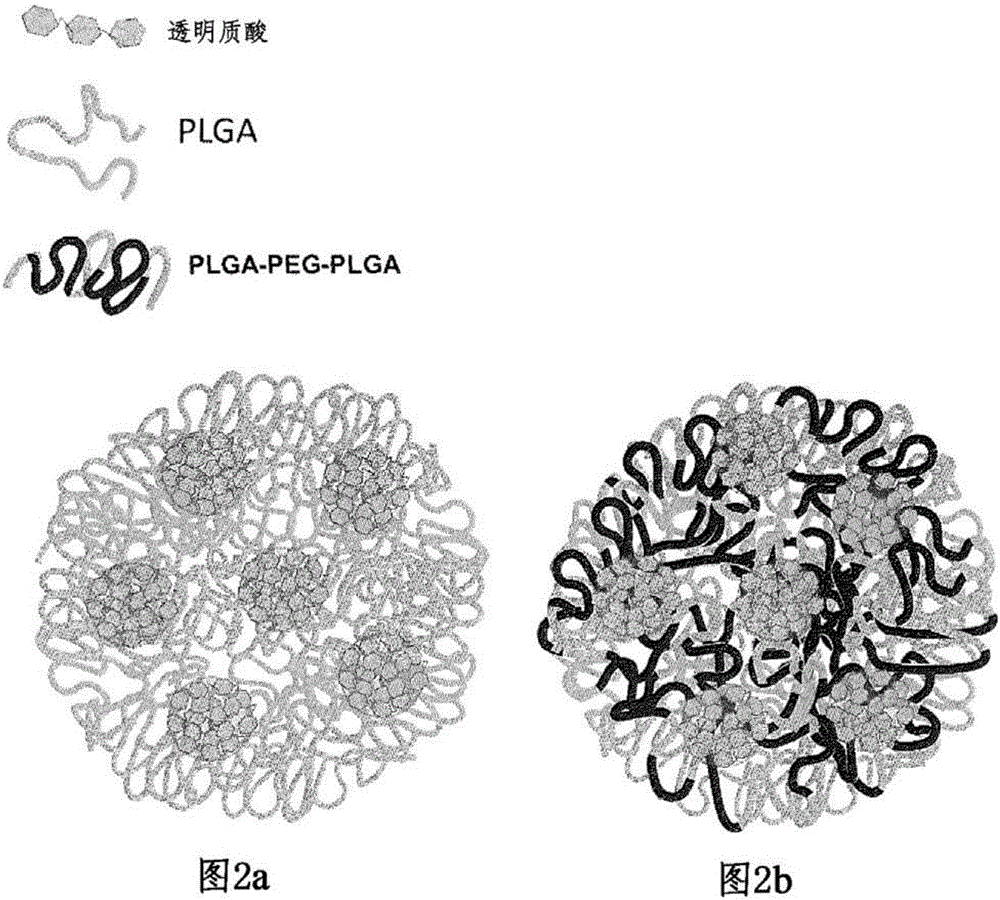
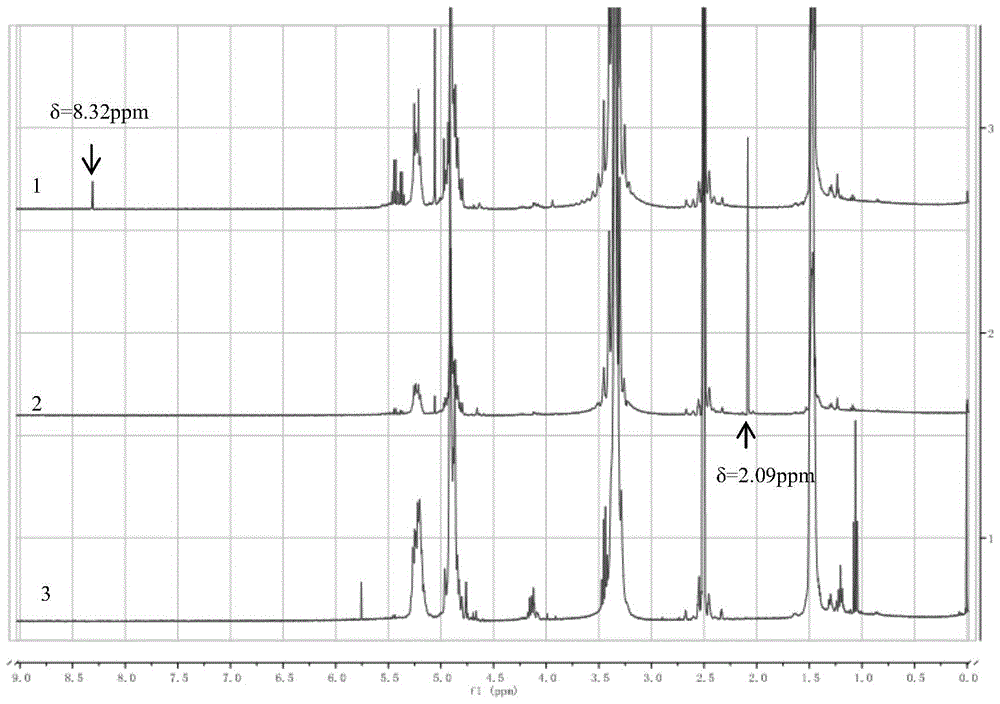
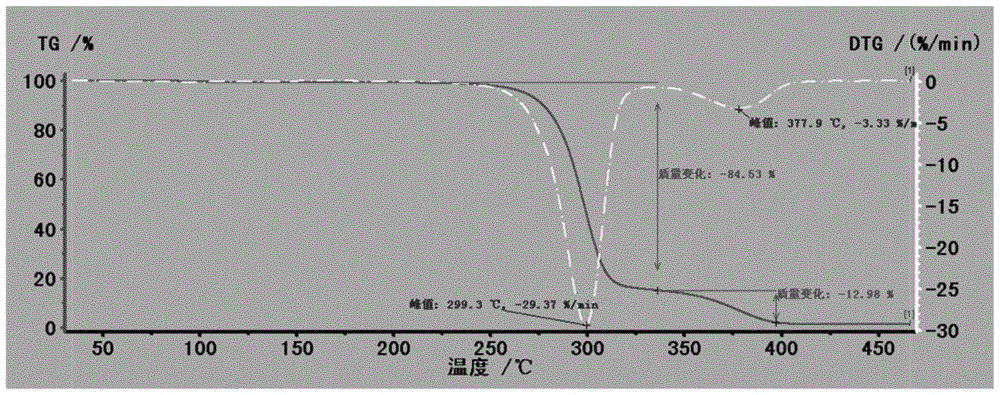
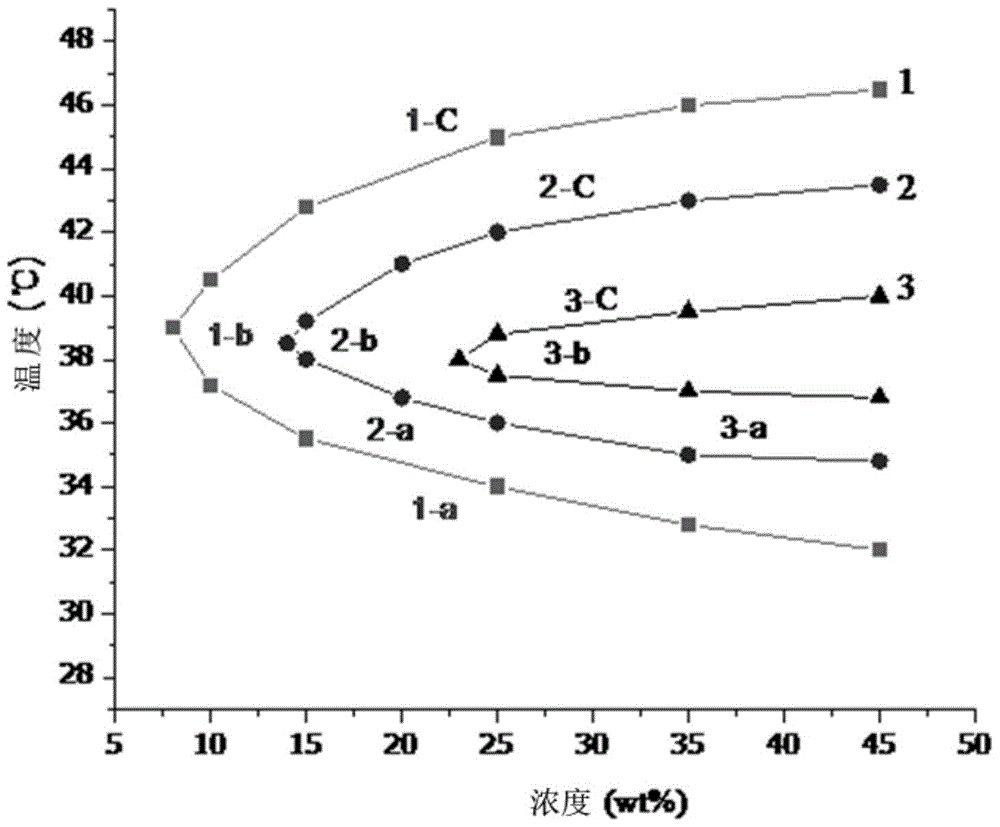
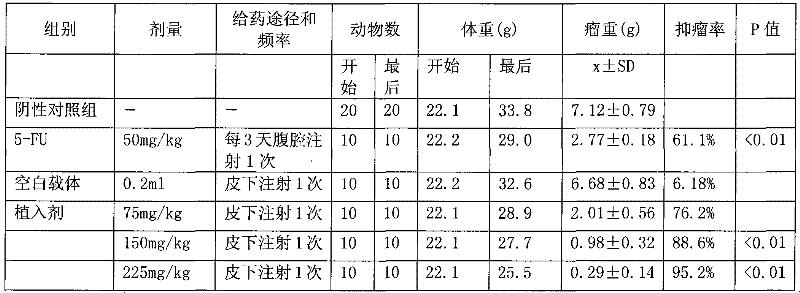
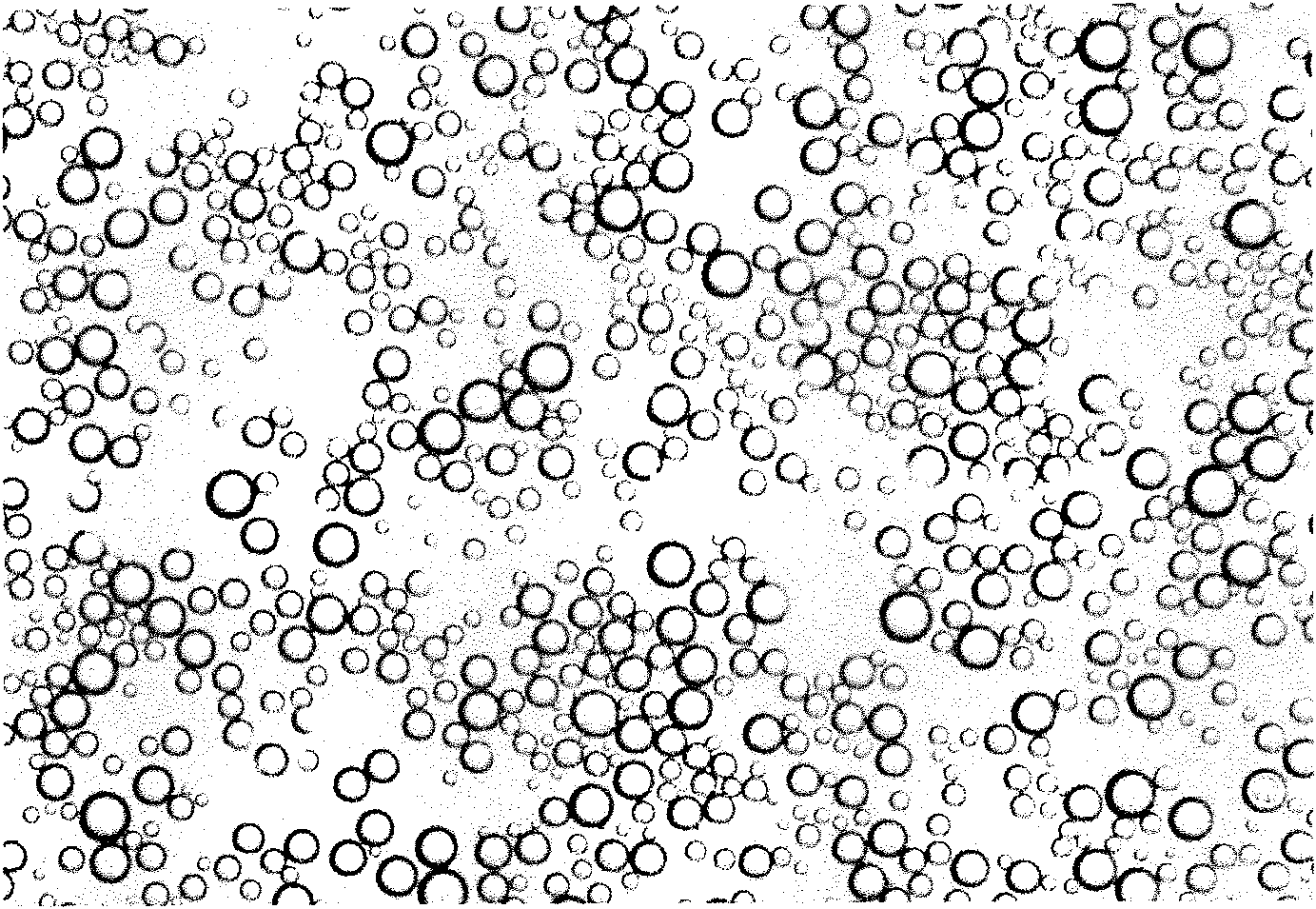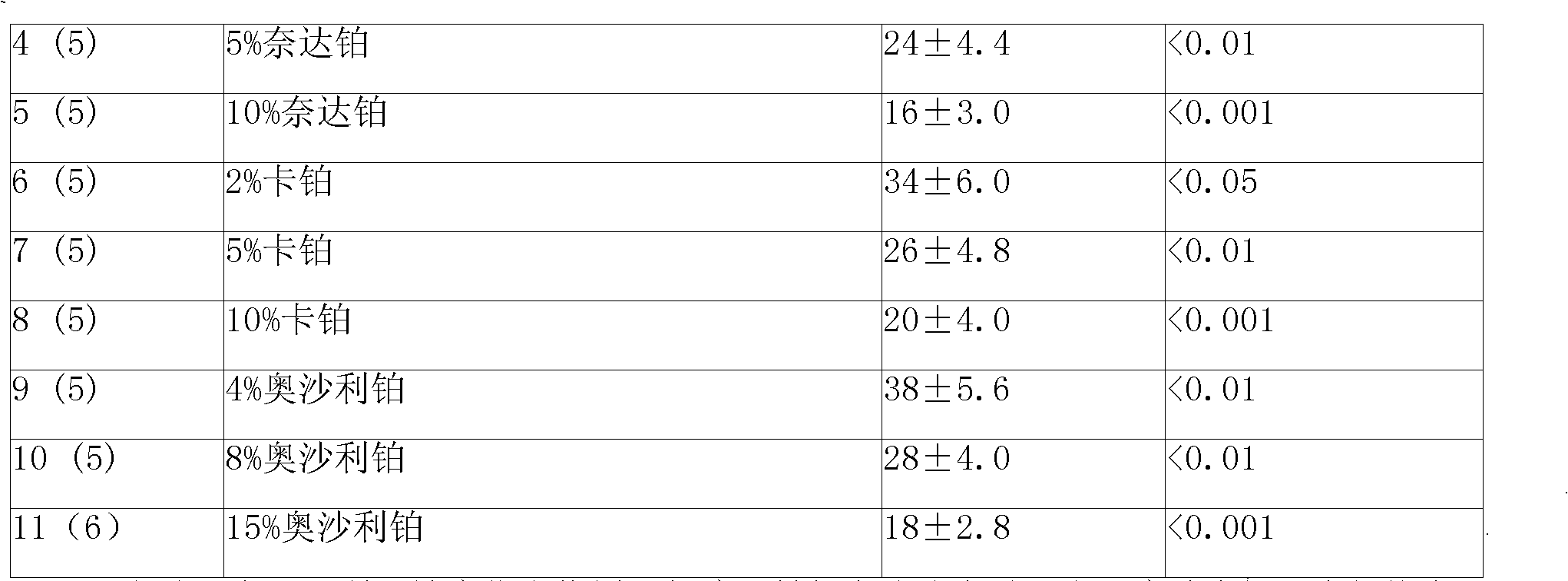Patents
Literature
35 results about "Plga peg plga" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Temp-sensitive, slow-releasing gel used for local injection, and its prepn. method
InactiveCN1861041AImprove stabilityEasy to administerAntipyreticAnalgesicsInjection sitePEG-PLGA-PEG
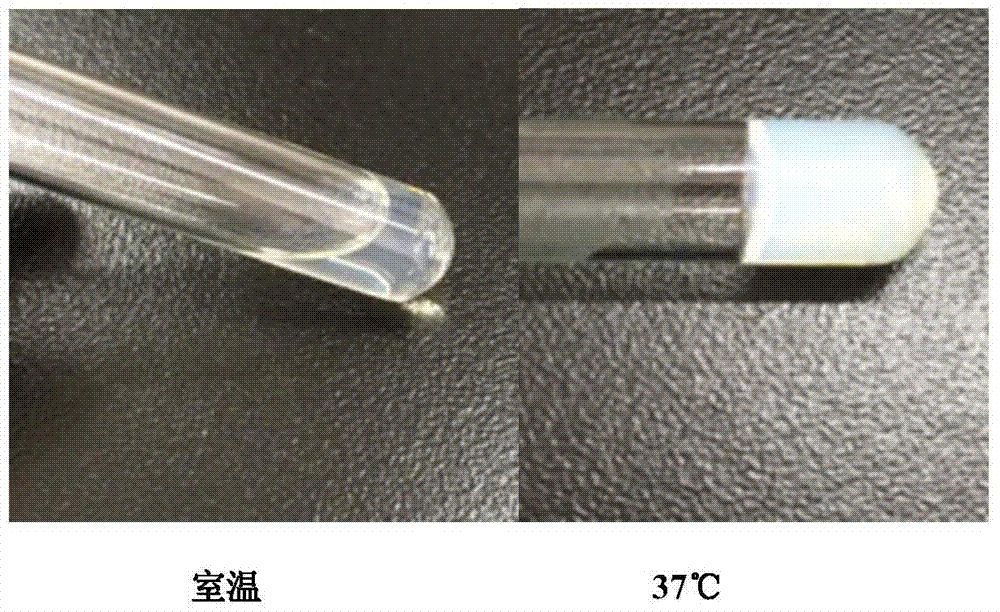
A temp-sensitive slow-release gel for local injection features that when its temp is lower than that of human body, it exists in liquid state and after it is injected into human body, it becomes gel for slowly releasing its medicine component. Its preparing process includes such steps preparing the temp-sensitive polymer as carrier from PLGA-PEG-PLGA block copolymer, PEG-PLGA-PEG block copolymer and poloxamer, dissolving it in water, and dissolving or dispersing the medicine in said aqueous solution.
Owner:SHENYANG PHARMA UNIVERSITY
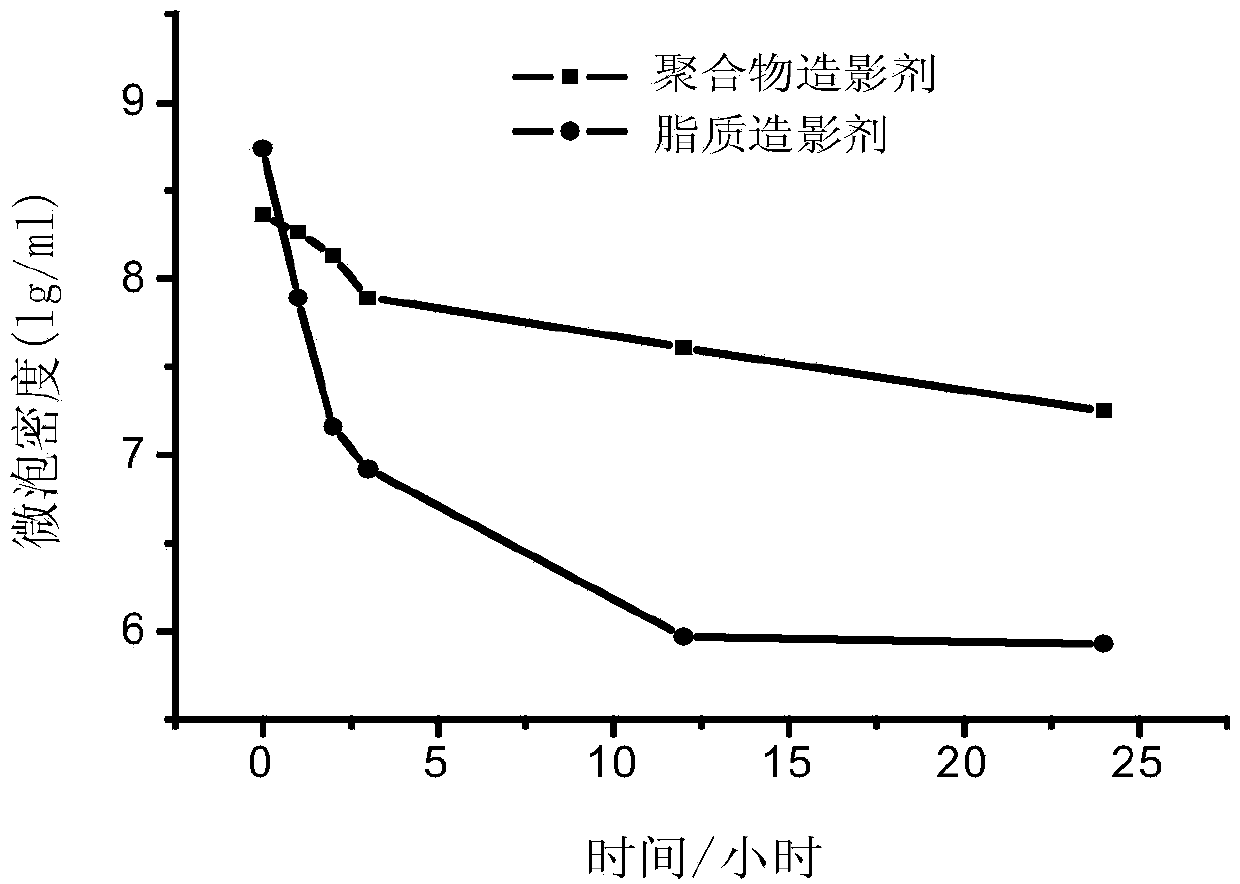
Novel PLGA-PEG-PLGA multipolymer microbubble ultrasound contrast agent and preparation method thereof
InactiveCN101574530AMeet the basic requirementsGood backscattering propertiesEchographic/ultrasound-imaging preparationsUltrasound contrast mediaPlga peg plga
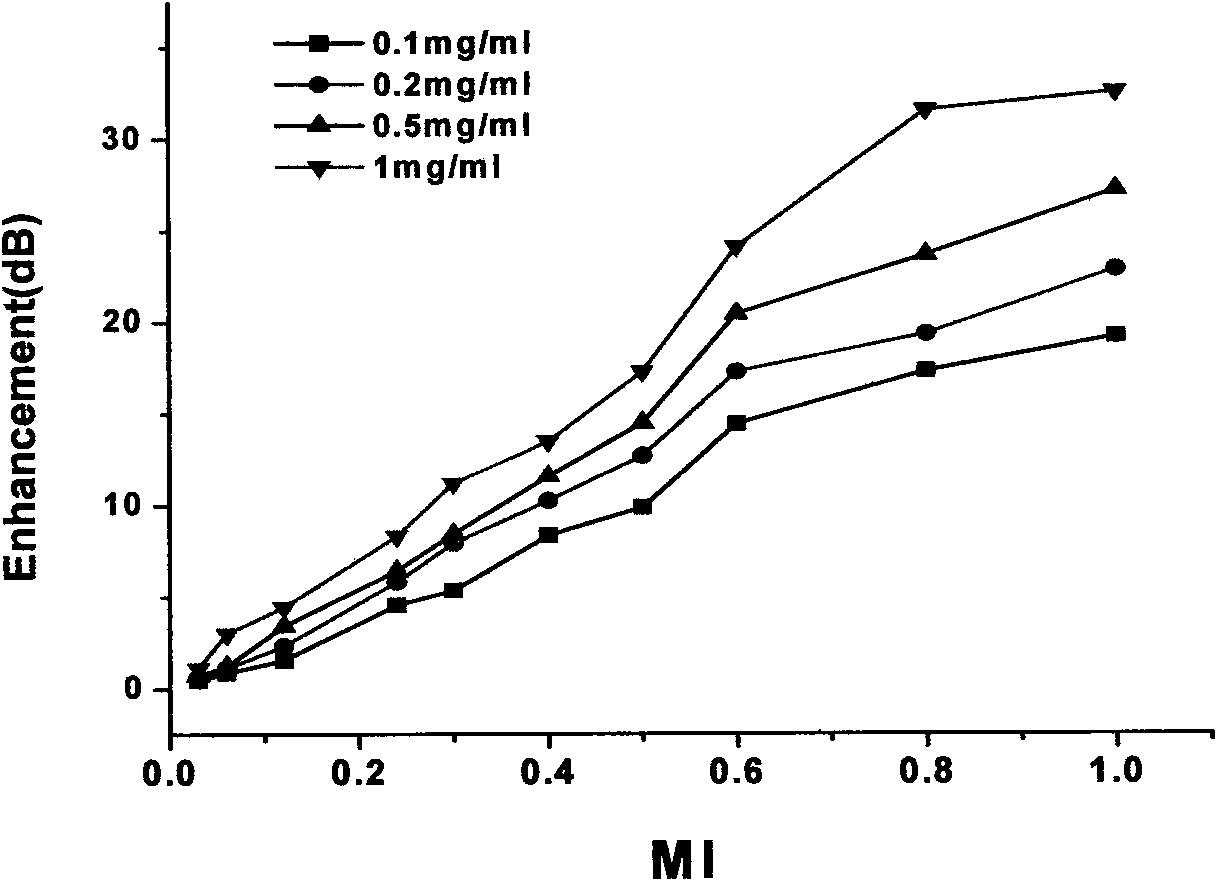
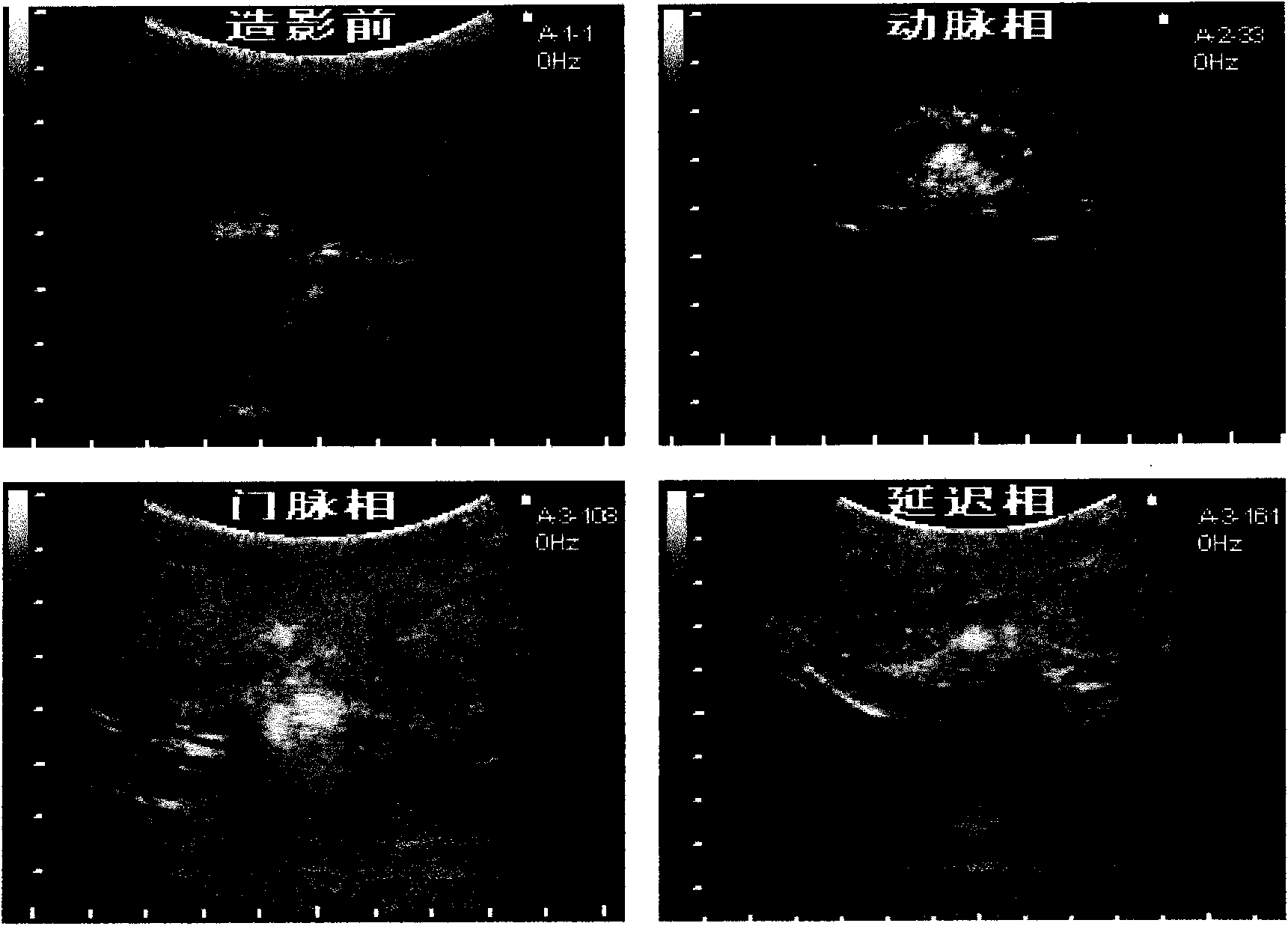
The invention discloses a novel PLGA-PEG-PLGA multipolymer microbubble ultrasound contrast agent and preparation method thereof. The microbubble ultrasound contrast agent is provided with an outer housing prepared from the polymer material PLGA-PEG-PLGA, the components of the inner core of gas include perfluoropropane, decafluorobutane or sulphur hexafluoride, and the like, and the molecular weight of the PLGA-PEG-PLGA multipolymer ranges from 2000 to 20000 dal. The preparation of the agent adopts the double emulsion method, and after being frozen and dried, gas molecules are led into microbubbles. The multipolymer microbubble contrast agent has favorable backscattering performance, shows the enhanced effect of ultrasonic contrast during in vivo and in vitro experiments, is safe and nontoxic, and meets the requirement of the ultrasound contrast agent.
Owner:FUJIAN MEDICAL UNIV UNION HOSPITAL
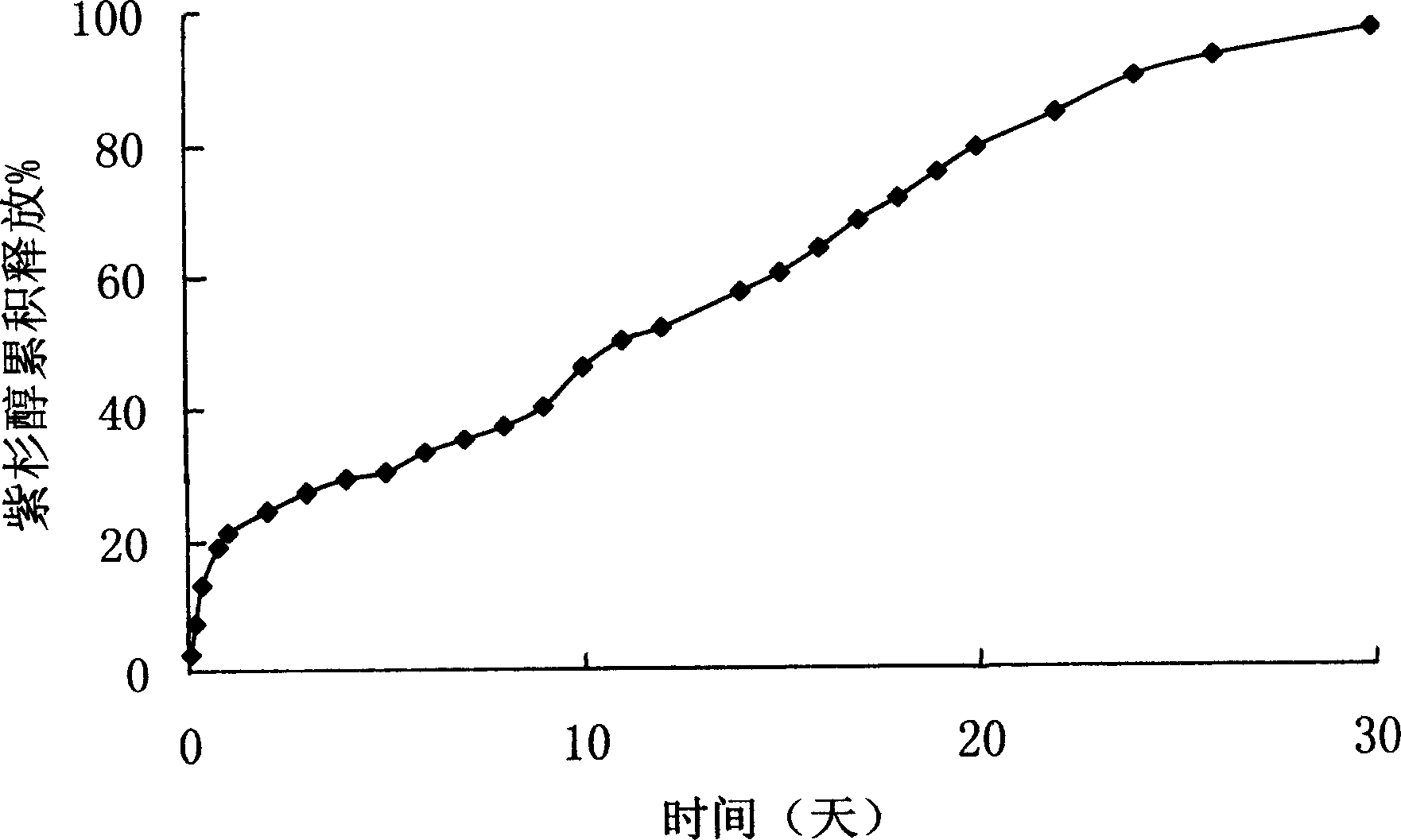
Anticancer sustained-release gel injection containing taxone medicine
InactiveCN101283976APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTreatment effectLactide
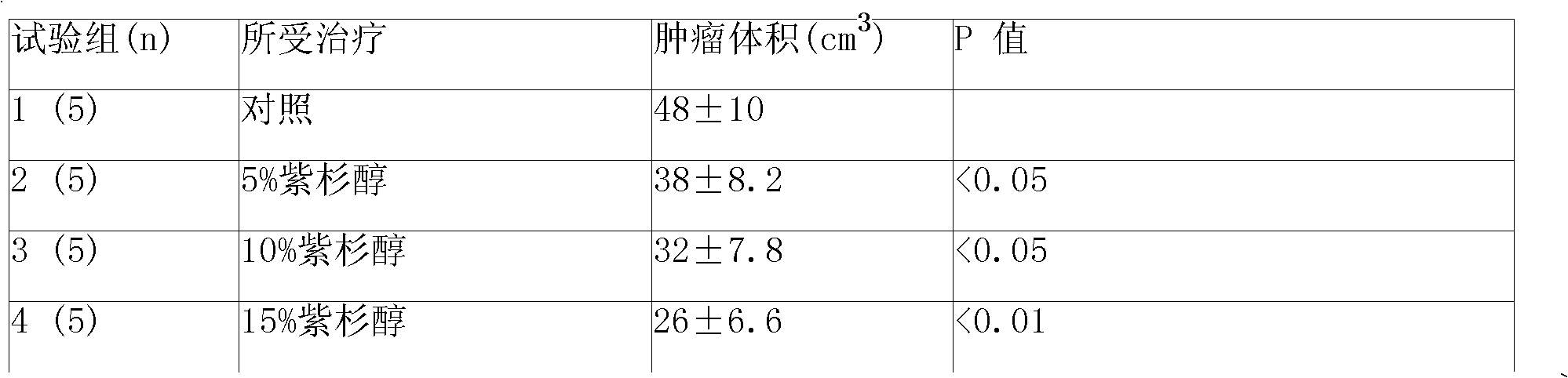
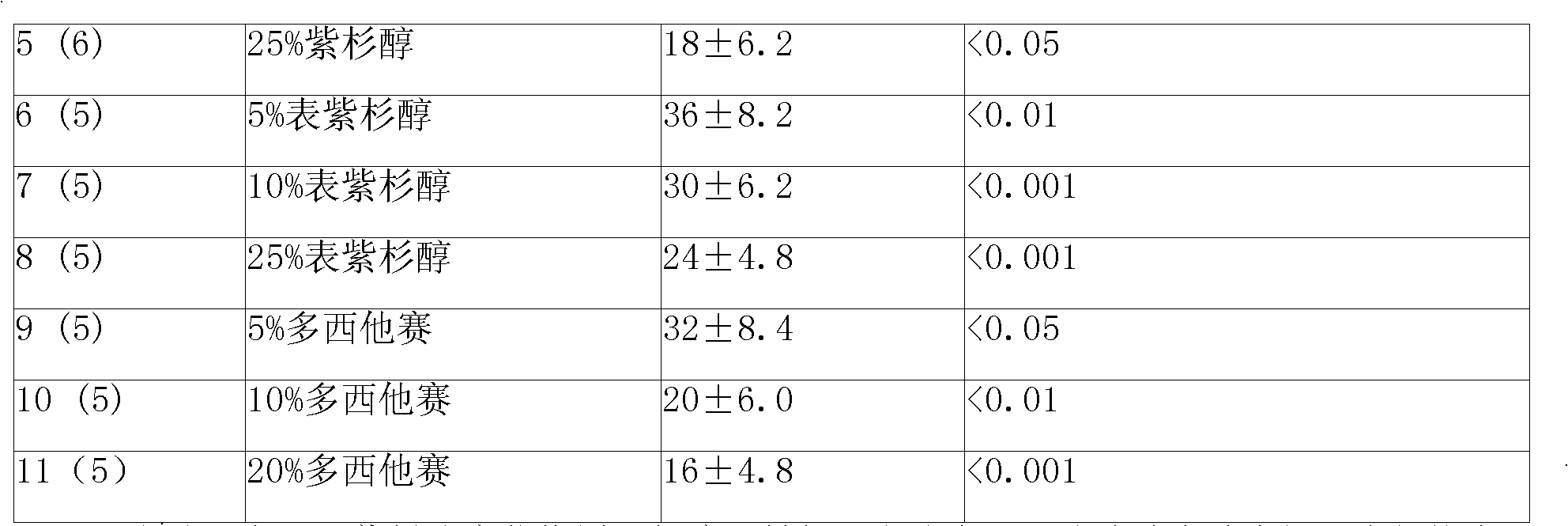
A sustained-release gel injection comprises sustained-release microspheres containing taxanes drug, amphiphilic block copolymer, solvent and release regulator, wherein the mixture of the amphiphilic block copolymer and the solvent has a temperature sensitive gelatinization characteristic, and can automatically become non-flowing degradable water insoluble gel, which can locally and slowly release drug at tumor foci for several weeks to several months, after injection into body. The preparation can be injected into or around a tumor for treating solid tumors at different stages. The preparation has the effects of controlling residual tumor cells relapsed after operation and tumors that can not be excised via operation, controlling complications at late stage of tumors, and enhancing treatment effect of chemotherapy and radiotherapy (particularly radioactive particles). The taxanes are selected from docetaxel, taxol, epitaxol, hydroxyl taxol and deacetyltaxol; the amphiphilic block copolymer is PLGA-PEG-PLGA copolymer, wherein the PEG has molecular weight of 1200-1600, accounting for 20 wt% of the amphiphilic block copolymer; and in the glycolide-lactide copolymer, the mol ratio of glycolide and lactide is 6:1.
Owner:济南基福医药科技有限公司
Stable gel containing bromelain
ActiveCN103637978AEasy to useFix stability issuesAntibacterial agentsPeptide/protein ingredientsBULK ACTIVE INGREDIENTActive ingredient
The invention relates to stable gel containing bromelain. The stable gel contains bromelain as an active ingredient, and a pharmaceutical acceptable carrier PLGA-PEG-PLGA, and has the characteristics of being good in stability, convenient to use and quick to act, simultaneously has bacteriostatic action, is applied to protein denaturation repair, inflammation diminishing, pain relieving, and antibiotic effect improvement, and belongs to the technical field of medicines.
Owner:NKD PHARMA CO LTD
Temperature-sensitive nasal spray gel for treating rhinitis
InactiveCN105031615AReduce the risk of infectionPeptide/protein ingredientsAerosol deliveryNasal cavityAdhesive
The invention belongs to the field of medicine, and relates to temperature-sensitive nasal spray gel for rhinitis treatment and daily protection of the nasal cavity. The temperature-sensitive nasal spray gel contains poloxamer 407, a PLGA-PEG-PLGA (poly(lactic-co-glycolic acid-polyethylene glycol-poly(lactic-co-glycolic acid) three-block polymer, gulcomannan, an epidermal growth factor and water, wherein poloxamer 407 is taken as a temperature-sensitive material, and the using amount of poloxamer 407 is 15 to 25 percent; the PLGA-PEG-PLGA three-block polymer is taken as a phase change temperature regulator, and the using amount of the PLGA-PEG-PLGA three-block polymer is less than 2 percent; the PLGA-PEG-PLGA three-block polymer is the phase change temperature regulator; gulcomannan with a molecular weight of 1 to 3 millions is taken as a mucous adhesive, and the using amount of gulcomannan is 0.2 to 1 percent; the epidermal growth factor is taken as a mucous healing agent, and the using amount of the epidermal growth factor is less than 0.1 percent.
Owner:SUZHOU JIOU BIOTECH CO LTD
Polyester carbonic ester anhydride 3D printing bio-ink, and 3D printing method
ActiveCN107400412ALarge organizational structureThick tissue structureAdditive manufacturing apparatusInksBiological cellPolyester
The invention discloses a polyester carbonic ester anhydride 3D printing bio-ink, and a 3D printing method. The polyester carbonic ester anhydride 3D printing bio-ink is composed of a gel ink and a support ink; the gel ink is composed of a hydrogel and biological cells; the hydrogel contains a PLGA-PEG-PLGA triblock copolymer with temperature responsiveness; the support ink is a polyester carbonic ester anhydride copolymer (P(LLA-TMC-SA)copolymer) with surface degradation characteristics. The 3D printing bio-ink possesses thermosensitivity and surface degradable performance, and multicomponent gradient printing and coaxial printing are adopted based on requirements of 3D biologically printed scaffold in vivo degradation structure controllable maintenance so as to obtain models which contain biological cells and are supported by scaffolds with surface controllable degradability, nutrient and metabolism channels with porous structures are obtained, and it is beneficial for obtaining of larger and thicker tissue structures via 3D printing.
Owner:HANGZHOU MEDZONE BIO-TECH CO LTD
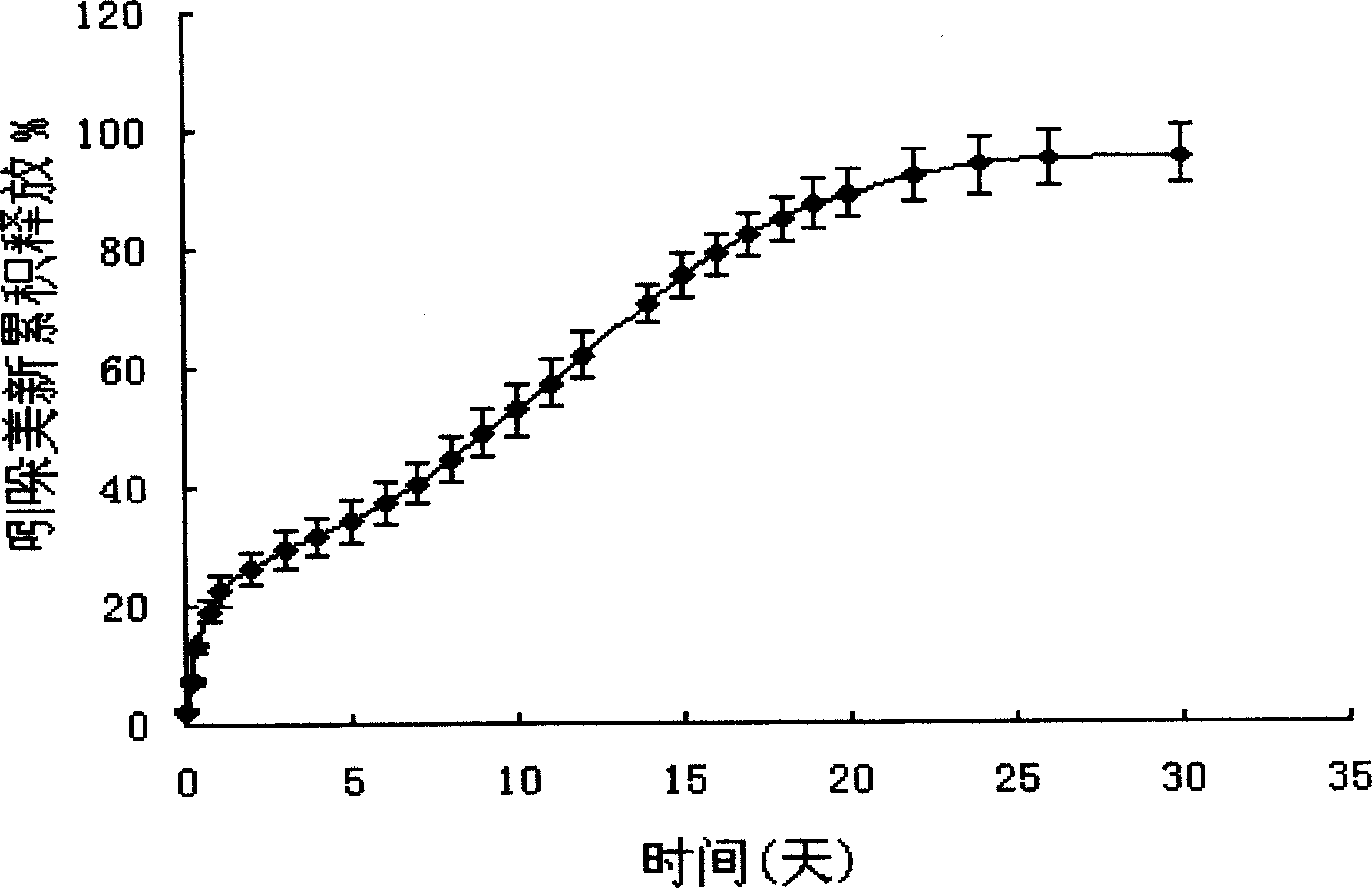
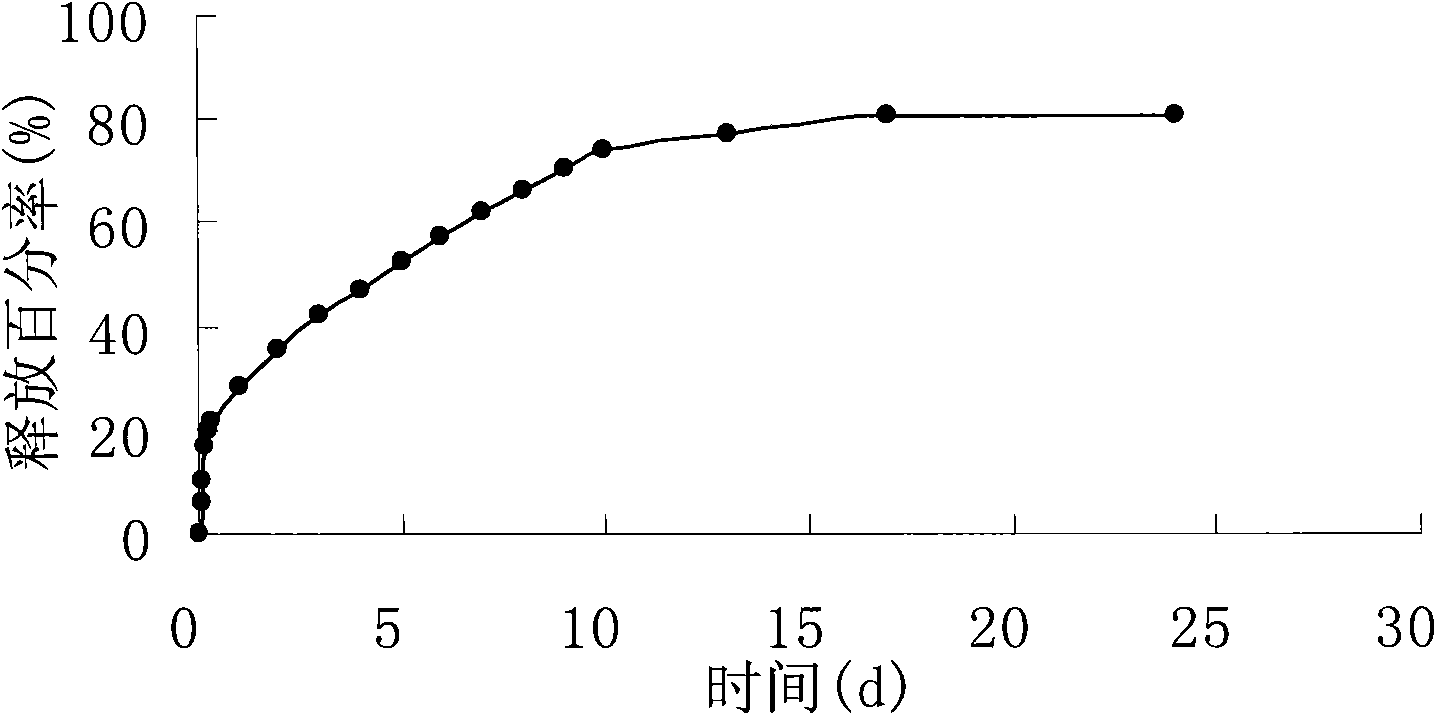
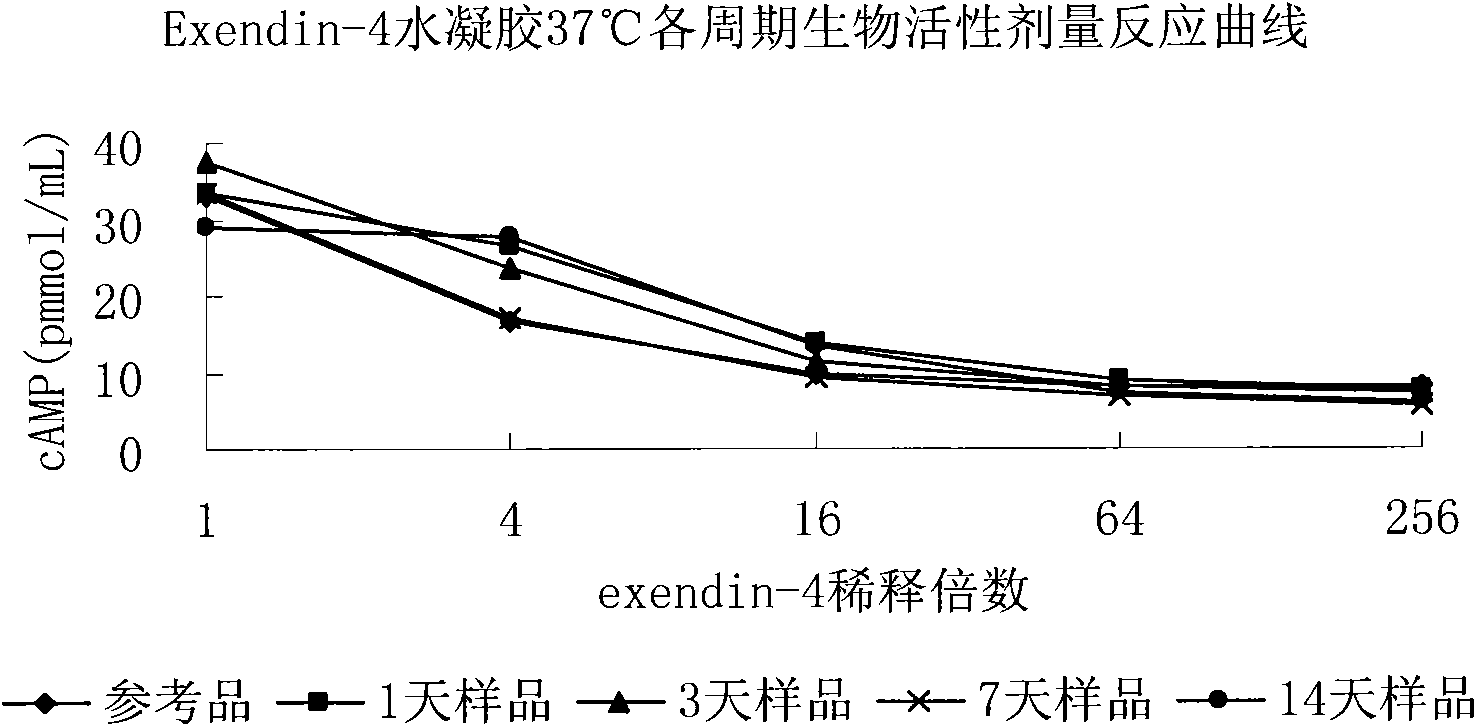
Temperature-sensitive hydrogel containing exendin-4 and injection thereof
ActiveCN102370611AImprove complianceGood drug releasePeptide/protein ingredientsMetabolism disorderPolyesterLarge dose
The invention relates to a temperature-sensitive hydrogel containing exendin-4, comprising exendin-4 as an effective ingredient and an amphiphilic copolymer as a medicine slow release carrier, wherein, the amphiphilic copolymer comprises polyester and polyethylene glycol, preferred PLGA-PEG-PLGA. The temperature-sensitive hydrogel is a liquid at room temperature, and is a solid water insoluble gel at the temperature of 37 DEG C, can be used for treating diabetes, and has good drug release property, environmental protection, little irritation, convenient application, and obvious effect. Compared with preparations with a large dose of exendin-4, the hydrogel disclosed herein has the advantages of in vivo formation of gel, easy operation, and continuous release property, and improves bioavailability, reduces administration times, and enhances patient compliance.
Owner:BEIJING GENETECH PHARML
Perfluorooctylbromide coated block polymer ultrasound microbubble contrast agent and preparation method thereof
InactiveCN103432601AUniform particle size distributionImprove stabilityEchographic/ultrasound-imaging preparationsImage diagnosisUltrasonic imaging
The invention discloses a perfluorooctylbromide coated block polymer ultrasound microbubble contrast agent and a preparation method thereof. The perfluorooctylbromide coated block polymer ultrasound microbubble contrast agent refers to a perfluorooctylbromide coated mPEG-PLLA, PLGA-PEG-PLGA or mPEG-PCL block polymer ultrasound microbubble contrast agent. A preparation method of the agent comprises the steps of preparing an mPEG-PLLA, PLGA-PEG-PLGA or mPEG-PCL block polymer solution, adding perfluorooctylbromide perfluoropentane, perfluorohexane or perfluoropropane to the solution, and then preparing the ultrasound microbubble contrast agent by an electric internal cutting homogenizing, ultrasonic emulsifying or mechanical oscillating method. The perfluorooctylbromide coated block polymer ultrasound microbubble contrast agent provided by the invention is applied to preparation of an ultrasonic imaging diagnosis contrast agent. The high polymer material used by the perfluorooctylbromide coated block polymer ultrasound microbubble contrast agent is safe and nontoxic, and is obviously cheaper than synthetic phospholipids; the microbubble preparation process is simple and convenient; the novel perfluorooctylbromide coated block polymer ultrasound microbubble contrast agent has good potential prospect in clinical application.
Owner:FUJIAN MEDICAL UNIV UNION HOSPITAL
Anticancer sustained-release gel injection containing stines medicine
The invention relates to an anticancer sustained release gel injection with stine drugs, comprising sustained release microspheres with stine drugs, a amphiphilic block copolymer, solvent and releasing moderator; wherein, the mixture of the amphiphilic block copolymer and the solvent possesses sensitive gelation property; after in vivo injection, the injection can be transformed into nonflowing, biodegradable gel insoluble in water; the insoluble gel can release the contained drugs in local tumor for weeks, even months. Intra-tumor injection or local injection can be used to treat different tumors and unresectable tumors, control late complications and the postoperative residual tumor cell recurrent, reinforce the effect of radiotherapy and chemotherapy and the effect of radiotherapy particles; nimustine and carmustine and other stines; the amphiphilic block copolymer is a PLGA-PEG-PLGA copolymer with the molecular weight of PEG 1200-1600 accounting for 20% of the amphiphilic block copolymer weight; in the poly lactide coglycolide copolymer, the molar ratio of glycolide and lactide is 6:1.
Owner:济南基福医药科技有限公司
Surface-degradable three-dimensional (3D) printing bio-ink and 3D printing method
The invention relates to surface-degradable three-dimensional (3D) printing bio-ink and a 3D printing method. The 3D printing bio-ink consists of gel ink and support ink, wherein the gel ink is prepared from hydrogel and biological cells, wherein the hydrogel contains a poly(lactide-co-glycolide)-polyethylene glycol-poly(lactide-co-glycolide) (PLGA-PEG-PLGA) triblock copolymer with temperature response; the support ink is a poly(trimethylene carbonate)-poly(L-lactide) (PTMC-PLLA) copolymer having the surface degradation characteristic. By utilizing the temperature-sensitive and surface-degradable properties of the 3D printing bio-ink and aiming at the requirements of structural controllability and maintenance in an in vivo degradation process of a 3D bio-printing support, a double-layer printing structure similar to a coaxial tube is constructed by using a multi-component material gradient printing technology; after the 3D printing bio-ink and the 3D printing method are adopted, a model which contains biological cells and is supported by a controllably surface-degradable support can be obtained by printing and molding at a time, nutritional and metabolic channels of a porous structure can be also obtained, and a bigger and thicker structure can be promoted to be obtained by means of 3D printing.
Owner:HANGZHOU MEDZONE BIO-TECH CO LTD
Temperature sensitive hydrogel, and preparation method and application thereof
ActiveCN104031361APharmaceutical non-active ingredientsIn-vivo testing preparationsMass ratioLactide
The invention discloses a temperature sensitive hydrogel. The temperature sensitive hydrogel is a PLGA-PEG-PLGA triblock copolymer, wherein a mass ratio of lactide to glycolide in the above PLGA copolymer is 2-4:1, and a ratio of the total mass of lactide and glycolide to PEG is 1-2:1. The invention also discloses a preparation method of the temperature sensitive hydrogel, and an application of the temperature sensitive hydrogel in endoscopic submucosal dissection. The temperature sensitive hydrogel can increase the mucosal protrusion height and prevent the diffusion of a musical lower layer as a marker carrier in the endoscopic submucosal dissection, so the cutting completeness in the endoscopic submucosal dissection is ensured, the perforating and bleeding risks are reduced, the operation safety and efficiency are improved, and the pains of patients are mitigated.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Temperature-sensitive gel containing ropivacaine and used for long-acting injection and preparation method of temperature-sensitive gel
ActiveCN104606129AEasy to administerSimple preparation processAntipyreticAerosol deliveryLactidePh regulation
The invention discloses temperature-sensitive gel containing ropivacaine and used for long-acting injection and a preparation method of the temperature-sensitive gel. The gel comprises a main drug ropivacaine, ropivacaine hydrochloride or ropivacaine mesylate, a PLGA (poly (lactic-co-glycolic acid)) -PEG (polyethylene glycol)-PLGA copolymer as well as water, normal saline or pH regulation solution for injection, wherein the mole ratio of lactide to glycolide is (2-3): 1, and the PEG molecular weight of the PLGA-PEG-PLGA copolymer is 1,000. The preparation method comprises the following steps: the copolymer is sufficiently swelled in a solvent firstly, then the main drug is added, and sterilization is performed through a filter membrane. The temperature-sensitive gel facilitates drug delivery and can gel rapidly at human body temperature so as to play a slow release role, smaller than or equal to 35%-45% of the drug is released in vitro after 12 h, larger than or equal to 65%-75% of the drug is released after 48 h, larger than or equal to 80% of the drug is released after 72 h, and the design requirement for postoperative continuous analgesia for 48 h through single injection of a local anesthesia drug is met. Pharmacodynamics study shows that a temperature-sensitive gel group containing ropivacaine can prolong the efficacy maintaining time remarkably and continuously play 48 h analgesic effect.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
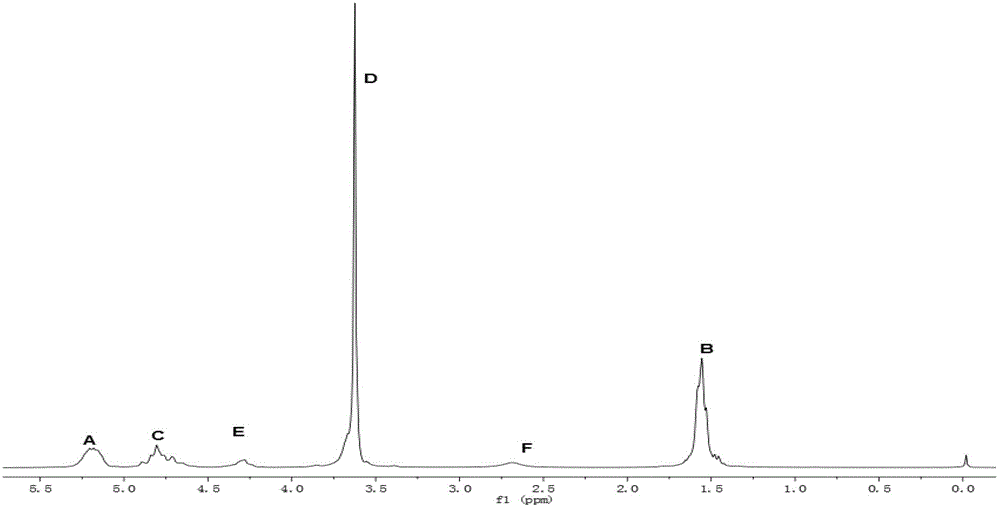
Poly lactic acid-glycolic acid (PLGA)-polyethylene glycol (PEG)-PLGA triblock copolymer and preparation method thereof
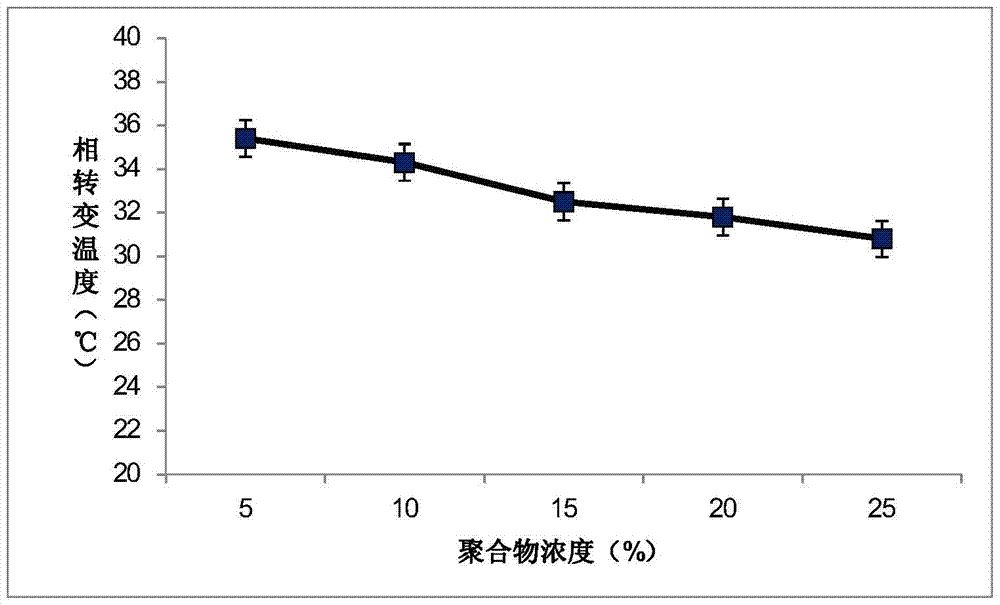
The invention relates to processing of macromolecular materials, and in particular relates to a poly lactic acid-glycolic acid (PLGA)-polyethylene glycol (PEG)-PLGA triblock copolymer and a preparation method thereof. According to the PLGA-PEG-PLGA triblock copolymer provided by the invention, the number-average molecular weight of the PLGA is more than 2,000; and the number-average molecular weight of the PEG is 2 to 5 times that of the PLGA. Compared with the traditional PLGA-PEG-PLGA which is matched and polymerized by PLGA and PEG with Mn less than 2,000, the aqueous solution of the PLGA-PEG-PLGA triblock copolymer provided by the invention has a wider solution-gel conversion area.
Owner:厦门瑞瓷医疗器械有限公司
Medicine-loaded nanoparticle vector and temperature-sensitive gel compounding system and preparation method thereof
InactiveCN106512019AReduce inflammationImproves damage repair processAerosol deliveryOintment deliveryDendrimerCartilage cells
The invention discloses a medicine-loaded nanoparticle vector and temperature-sensitive gel compounding system and a preparation method thereof. The preparation method includes the following steps that nanoparticle vectors are prepared; medicine molecules are connected with amino groups or hydroxyl groups at the ends of polyamidoamine dendrimers in a covalent bonding mode, and a medicine-loaded polyamidoamine dendrimer polymer is prepared; temperature-sensitive gel is prepared, the temperature-sensitive gel is a PLGA-PEG-PLGA copolymer, PLGA is a lactide-glycolide copolymer, and PEG is a polyethylene glycol copolymer; a copolymer solution is prepared by putting the PLGA-PEG-PLGA copolymer into a solvent; and under the condition of stirring, the medicine-loaded polyamidoamine dendrimer polymer is added into the copolymer solution, stirring is continued, and the medicine molecule-loaded nanoparticle vector and temperature-sensitive gel compounding system is obtained. The medicine-loaded nanoparticle vector and temperature-sensitive gel compounding system has a dual slow release function, and can stimulate synovial membrane mesenchymal cells to develop into cartilage cells, relieve the inflammation of a cartilage injured part, and promote the injury repair process of articular cartilages.
Owner:SUZHOU UNIV
Anticancer sustained-release gel injection containing platinum compound
InactiveCN101283978APharmaceutical delivery mechanismPharmaceutical non-active ingredientsPicoplatinSolvent
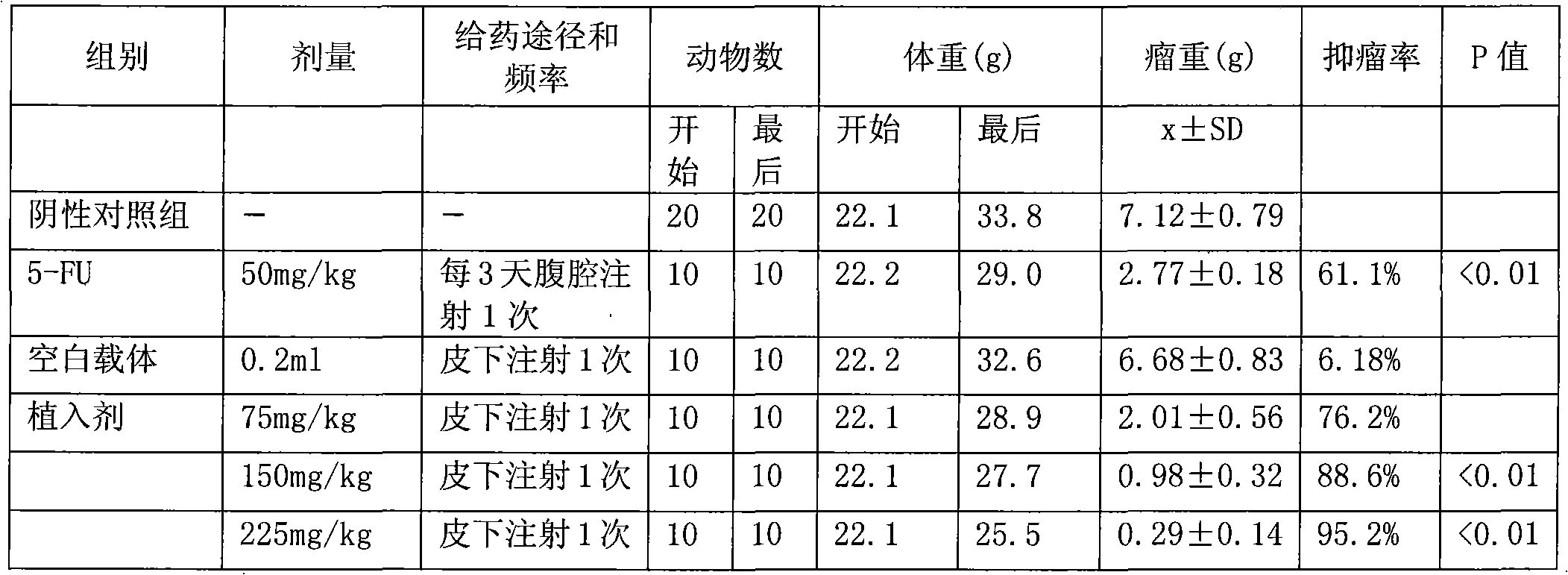
An anticancer sustained-release gel injection sustained-release microspheres containing platinum-based compound, amphiphilic block copolymer, solvent and release regulator, the mixture of amphiphilic block copolymer and solvent has a temperature sensitive gelatinization characteristic, and can automatically become non-flowing degradable water insoluble gel, which can locally and slowly release drug at tumor foci for several weeks to several months, after injection into body. The adjuvant in the sustained-release microspheres is poly(lactic acid)-glycollic acid copolymer; and the amphiphilic block copolymer is PLGA-PEG-PLGA copolymer. The preparation can be injected into artery, inside a tumor or around a tumor with remarkably reduced systemic toxicity of drug, and can be used for solid tumors at different stages. The platinum-based compound is selected from nedaplatin, carboplatin, cisplatin, enloplatin, ormaplatin, sulfato-1,2-diaminocyclohezane-platinum (SHP), picoplatin, cyclopentylamine platium, zeniplatin, spiroplatin, lobaplatin, iproplatin, oxaliplatin, cycloplatin, and dexormaplatin. The preparation can be used with radioactive particles and can enhance chemotherapy effect.
Owner:济南基福医药科技有限公司
2-methoxy estradiol injectable hydrogel implant
InactiveCN101953774AEffectively maintain the effective concentration of the target siteFormulation ScienceOrganic active ingredientsPharmaceutical delivery mechanismMedicineTherapeutic effect
The invention relates to a 2-methoxy estradiol injectable hydrogel implant which can effectively solve the problems of maintaining the effective concentration of the target part of 2-methoxy estradiol and enhancing the treatment effect. The invention has the technical scheme that a temperature sensitive type PLGA-PEG-PLGA segmented copolymer having temperature sensitive hydrogel properties is adopted to be mixed with water to prepare dissolution swelling solution; the solution is mixed with the 2-methoxy estradiol to prepare an injection; the temperature sensitive type PLGA-PEG-PLGA segmented copolymer is uniformly mixed with the water to be kept stewing at 0 to 25 DEG C; the temperature sensitive type PLGA-PEG-PLGA segmented copolymer is fully dissolved and swelled into the solution; and the 2-methoxy estradiol is added to be uniformly mixed with solution at 0 to 25 DEG C on the use day or one day before use. The invention has scientific formula, easy production, low cost and good injection effect, can effectively maintain the effective concentration of the target part of the 2-methoxy estradiol and enhance the treatment effect is enhanced and is the innovation on the path of cancer treatment drug forms and drug administration.
Owner:ZHENGZHOU UNIV
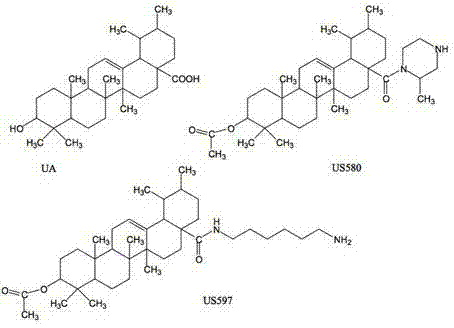
Medicine carrying micelle capable of increasing bioavailability of ursolic acid and structural modifier thereof
InactiveCN103877022AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsPolyethylene glycolUrsolic acid
The invention relates to the fields of biological functional materials and nanotechnologies and particularly relates to a novel amphiphilic polymer nano medicine carrying micelle which is proper in particle size distribution range, stable in encapsulation rate and medicine carrying rate, high in biological safety and capable of increasing bioavailability of ursolic acid and a structural modifier thereof. The technical scheme of the invention provides a medicine carrying micelle capable of increasing bioavailability of ursolic acid and a structural modifier thereof. The medicine carrying micelle is characterized in that the preparation method comprises the following steps of 1, synthesizing PLGA-PEG-PLGA (poly(lactic-co-glycolic acid)-polyethylene glycol-poly(lactic-co-glycolic acid)) by using a ring opening polymerization method; 2, simultaneously dissolving an insoluble drug and a triblock copolymer into an organic solvent to prepare an oil phase; 3, dropwise adding the oil phase into a water phase stirred at a high speed; 4, stirring until the oil phase is completely volatilized, and filtering a membrane in the solution to obtain a medicine carrying nanoparticle contained ultrapure water solution; freezing and drying to obtain the medicine carrying micelles.
Owner:FUZHOU UNIV
Application of PLGA-PEG-PLGA (poly(lactic acid-glycolic acid)-polyethylene glycol-poly(lactic acid-glycolic acid)) triblock copolymer in medicine loading and slow-release medicine, and preparation method of PLGA-PEG-PLGA triblock copolymer
ActiveCN104856943AIncrease awarenessBroaden applicationAerosol deliveryDigestive systemGlycolic acidPolyethylene glycol
The invention discloses an application of a PLGA-PEG-PLGA (poly(lactic acid-glycolic acid)-polyethylene glycol-poly(lactic acid-glycolic acid)) triblock copolymer in medicine loading and a slow-release medicine, and a preparation method of the PLGA-PEG-PLGA triblock copolymer. The PLGA-PEG-PLGA triblock copolymer is especially suitable for treatment of periodontitis. A water solution of the PLGA-PEG-PLGA triblock copolymer is subjected to solution-gel phase transformation along with the temperature increment; the phase transformation temperature is 35-39 DEG C; the medicine-loaded water solution of the PLGA-PEG-PLGA triblock copolymer becomes medicine-loaded gel after being injected into a human body; and the medicine-loaded gel is capable of releasing the medicine within 6-8 days. The PLGA-PEG-PLGA triblock copolymer water solution is suitable for application as a medicine carrier and a slow-release medicine.
Owner:厦门瑞瓷医疗器械有限公司
PLGA-PEG-PLGA inlaid mineralized collagen coating and its preparation method
InactiveCN102423927AImproved sustained release behaviorEfficient carryingElectrolytic organic material coatingMetal layered productsAntibiotic YHYDROSOL
The invention discloses a PLGA (poly(lactic-co-glycolic acid))-PEG (poly(ethylene glycol))-PLGA inlaid mineralized collagen coating and its preparation method. The method of the invention conducts electrochemical deposition, adjusts deposition parameters, constructs a mineralized collagen coating with a porous structure on a metal implant surface, and inlays a temperature sensitive polymer PLGA-PEG-PLGA into the structure, so that the coating becomes an excellent drug slow release system and can be used for controlled release of biotic factors, antibiotics and other drugs in vivo. PLGA-PEG-PLGA can make sol-gel conversion with temperature variation. On account of the characteristic, biotic factors and antibiotics, etc. are loaded under a sol state of PLGA-PEG-PLGA, under the gel state of which the drugs can be released, thus optimizing the slow release behavior of the coating. The coating prepared by the method of the invention can make use of mineralized collagen to transmit biological signals and the temperature sensitive polymer to control drug release effectively, so that the coating can has high biological responsiveness as well as a good drug slow release function simultaneously, thus boasting wide application prospects in hard bone tissue repair field.
Owner:ZHEJIANG UNIV
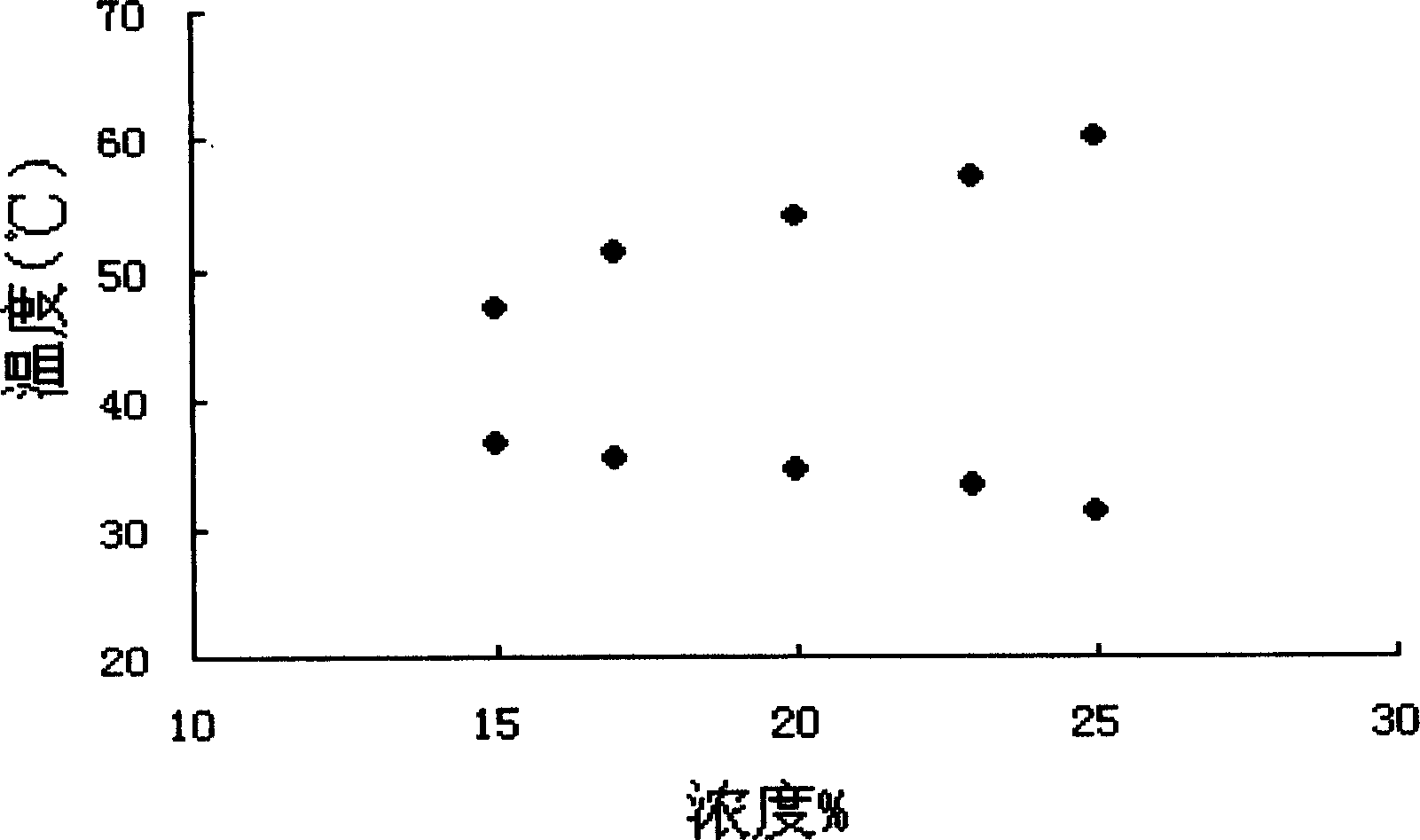
Method for preparing temperature-sensitive three-segmented copolymer
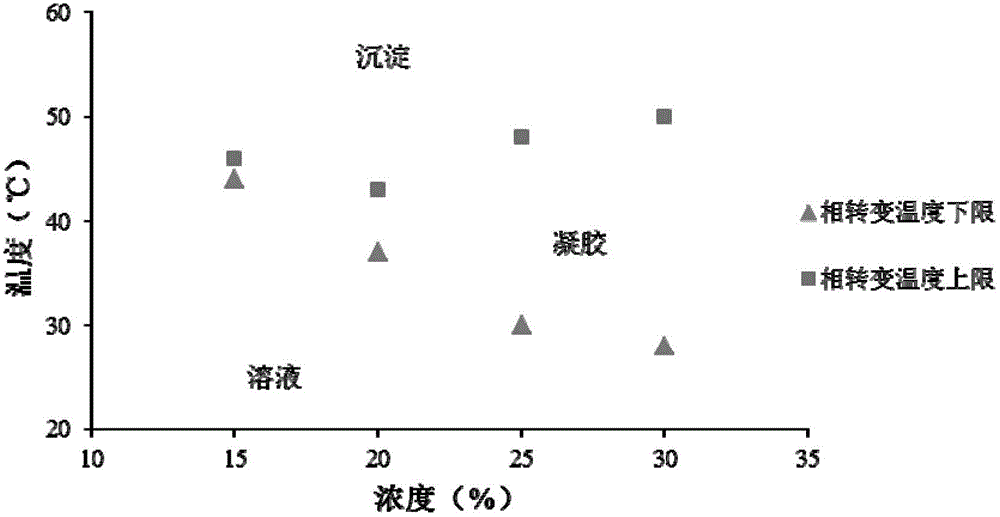
The present invention relates to a preparation method of temperature-sensitive triblock polymer (PLGA-PEG-PLGA), which pertains to the field of high polymer medical materials and medical technology. The copolymer makes use of an open-loop copolymerization method, takes the polyethylene glycol (PEG), D, L-lactide, glycolic as the raw materials and is prepared by the polymerization with the involvement of an initiator. The gel-sol phase transition temperature (40 DEG C to 44 DEG C) can meet the requirements on the clinical application of local temperature-oriented drug target, which can not only be higher than the human physiological temperature, but also be in the affordable scope of the human body at the same time. The polymer is the pharmaceutical material, which can be mixed with the drug to prepare the microcapsules, micro-particles, nanoparticles and other preparations, so the present invention has potential application in a local temperature-oriented targeting delivery system.
Owner:CHONGQING UNIV
In-vivo in-situ drug-loading hydrogel carrier as well as preparation method and applications thereof
InactiveCN106860912AEasy to integrateImprove nonunionTissue regenerationProsthesisLactidePolyethylene glycol
The invention relates to the technical field of medicinal preparations, and in particular relates to an in-vivo in-situ drug-loading hydrogel carrier as well as a preparation method and applications of the in-vivo in-situ drug-loading hydrogel carrier. For the in-vivo in-situ drug-loading hydrogel carrier PLGA-PEG-PLGA, the hydrogel carrier is formed by polymerizing lactide LA, polyethylene glycol PEG and glycolide GA, wherein the number average molecular weight ratio of PLGA to PEG is (1.5-3):1, and the molar ratio of LA to GA is (3-5):1. For the in-vivo in-situ drug-loading hydrogel carrier provided by the invention, by embedding bone growth promoting factors, when natural marrow fills a porous artificial bone scaffold, the release of important signal factors during the fracture repairing process can be simulated, and thus the in-vivo in-situ drug-loading hydrogel carrier is well applied to the fields of improving spinal fusion, bone ununion, bone defect repair, and the like.
Owner:SECOND AFFILIATED HOSPITAL SECOND MILITARY MEDICAL UNIV
Thermosensitive gel for ropivacaine long-acting injection and preparation method thereof
ActiveCN104606129BEasy to administerSimple preparation processAntipyreticAerosol deliveryLactideSingle injection
The invention discloses a temperature-sensitive gel for ropivacaine long-acting injection and a preparation method thereof. The gel comprises main ingredients ropivacaine, ropivacaine hydrochloride or ropivacaine mesylate; Glycolide molar ratio 2-3:1, PLGA-PEG-PLGA copolymer with PEG molecular weight of 1000, PEG accounts for 18-30% of the total mass of the copolymer; water for injection, normal saline or pH adjustment solution. The preparation method is to fully swell the copolymer in a solvent, add the main drug, and sterilize through a filter membrane. The temperature-sensitive gel is convenient for administration, gels rapidly at body temperature and exerts a slow-release effect. The drug release in vitro is ≤35-45%, 48 hours ≥65%-75%, and 72 hours ≥80%, which is in line with local anesthetics. After a single injection of continuous analgesia 48h design requirements. Pharmacodynamic studies have shown that the ropivacaine temperature-sensitive gel group can significantly prolong the maintenance time of the drug effect, and can continuously exert an analgesic effect for 48 hours.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
Thermosensitive hydrogel and preparation method and application thereof
ActiveCN104031361BPharmaceutical non-active ingredientsIn-vivo testing preparationsLactideMaterial Perforation
The invention discloses a temperature-sensitive hydrogel, which is a PLGA-PEG-PLGA triblock copolymer, wherein the mass ratio of lactide to glycolide in the PLGA copolymer is 2-4:1, the The mass ratio of the sum of lactide and glycolide to the mass of PEG is 1-2:1. The invention also discloses a preparation method of the thermosensitive hydrogel and its application in endoscopic submucosal dissection. The temperature-sensitive hydrogel of the present invention, when used as a marker carrier in endoscopic submucosal dissection, can increase the height of the mucosal bulge and prevent the diffusion of markers in the submucosa, thereby ensuring complete cutting during endoscopic submucosal dissection , reduce the risk of perforation and bleeding, improve the safety and efficiency of surgery, and reduce the pain of patients.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Temperature-sensitive hydrogel containing exendin-4 and injection thereof
ActiveCN102370611BImprove complianceGood drug releasePeptide/protein ingredientsMetabolism disorderPolyesterPatient compliance
Owner:BEIJING GENETECH PHARML
A multi-cytokines sequential slow-release microsphere-hydrogel composite system and its preparation method
InactiveCN104622795BImprove repair effectPeptide/protein ingredientsAerosol deliveryMicrosphereCytokine
Owner:HANGZHOU CITY XIAOSHAN DISTRICT TRADITIONAL CHINESE MEDICAL HOSPITAL
A drug-loaded micelle that improves the bioavailability of ursolic acid and its structural modifications
InactiveCN103877022BImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsUrsolic acidPolyethylene glycol
The invention relates to the fields of biological functional materials and nanotechnologies and particularly relates to a novel amphiphilic polymer nano medicine carrying micelle which is proper in particle size distribution range, stable in encapsulation rate and medicine carrying rate, high in biological safety and capable of increasing bioavailability of ursolic acid and a structural modifier thereof. The technical scheme of the invention provides a medicine carrying micelle capable of increasing bioavailability of ursolic acid and a structural modifier thereof. The medicine carrying micelle is characterized in that the preparation method comprises the following steps of 1, synthesizing PLGA-PEG-PLGA (poly(lactic-co-glycolic acid)-polyethylene glycol-poly(lactic-co-glycolic acid)) by using a ring opening polymerization method; 2, simultaneously dissolving an insoluble drug and a triblock copolymer into an organic solvent to prepare an oil phase; 3, dropwise adding the oil phase into a water phase stirred at a high speed; 4, stirring until the oil phase is completely volatilized, and filtering a membrane in the solution to obtain a medicine carrying nanoparticle contained ultrapure water solution; freezing and drying to obtain the medicine carrying micelles.
Owner:FUZHOU UNIV
Temp-sensitive, slow-releasing gel used for local injection, and its preparation method
InactiveCN1861041BImprove stabilityGood sustained release effectAntipyreticAnalgesicsInjection sitePEG-PLGA-PEG
A temp-sensitive slow-release gel for local injection features that when its temp is lower than that of human body, it exists in liquid state and after it is injected into human body, it becomes gel for slowly releasing its medicine component. Its preparing process includes such steps preparing the temp-sensitive polymer as carrier from PLGA-PEG-PLGA block copolymer, PEG-PLGA-PEG block copolymer and poloxamer, dissolving it in water, and dissolving or dispersing the medicine in said aqueous solution.
Owner:SHENYANG PHARMA UNIVERSITY
Continuous release compositions made from hyaluronic acid, and therapeutic applications of same
The present invention concerns polymer particles made from poly(lactic-co-glycolic acid) (PLGA) polymer, poly(lactic-co-glycolic acid)-polyethylene glycol-poly(lactic-co-glycolic acid) (PLGA-PEG-PLGA) copolymer, or the mixture of same, combined with hyaluronic acid molecules or hyaluronic acid salts, and the method for preparing same. The present invention also concerns injectable pharmaceutical or cosmetic compositions comprising such polymer particles, the method for preparing such compositions, and the use thereof for medical purposes, in particular for the prevention and / or treatment of musculoskeletal diseases, diseases and traumatic conditions of the skin, oral disorders, vaginal mucosa dryness and urinary infections or cystitis, dryness of the eye membrane and eye infections, obesity, and the use of same to combat ageing of the skin and / or for repairing the dermal tissue (mesotherapy). More particularly, the compositions of the invention can be intended to supplement joint fluids, and in particular synovial fluid, restoring the physiological and rheological conditions of pathological joints, for example arthritic joints, or indeed for treating and repairing the epidermis by remodelling, hydrating and protecting the skin.
Owner:伊伯萨制药有限公司
A plga-peg-plga triblock copolymer and preparation method thereof
ActiveCN104892912BLarge-scale transformation characteristicsHigh temperature sensitivityPolymer scienceGlycolic acid
The invention relates to processing of macromolecular materials, and in particular relates to a poly lactic acid-glycolic acid (PLGA)-polyethylene glycol (PEG)-PLGA triblock copolymer and a preparation method thereof. According to the PLGA-PEG-PLGA triblock copolymer provided by the invention, the number-average molecular weight of the PLGA is more than 2,000; and the number-average molecular weight of the PEG is 2 to 5 times that of the PLGA. Compared with the traditional PLGA-PEG-PLGA which is matched and polymerized by PLGA and PEG with Mn less than 2,000, the aqueous solution of the PLGA-PEG-PLGA triblock copolymer provided by the invention has a wider solution-gel conversion area.
Owner:厦门瑞瓷医疗器械有限公司
2-methoxy estradiol injectable hydrogel implant
InactiveCN101953774BEffectively maintain the effective concentration of the target siteFormulation ScienceOrganic active ingredientsPharmaceutical delivery mechanismMedicineTherapeutic effect
The invention relates to a 2-methoxy estradiol injectable hydrogel implant which can effectively solve the problems of maintaining the effective concentration of the target part of 2-methoxy estradiol and enhancing the treatment effect. The invention has the technical scheme that a temperature sensitive type PLGA-PEG-PLGA segmented copolymer having temperature sensitive hydrogel properties is adopted to be mixed with water to prepare dissolution swelling solution; the solution is mixed with the 2-methoxy estradiol to prepare an injection; the temperature sensitive type PLGA-PEG-PLGA segmentedcopolymer is uniformly mixed with the water to be kept stewing at 0 to 25 DEG C; the temperature sensitive type PLGA-PEG-PLGA segmented copolymer is fully dissolved and swelled into the solution; andthe 2-methoxy estradiol is added to be uniformly mixed with solution at 0 to 25 DEG C on the use day or one day before use. The invention has scientific formula, easy production, low cost and good injection effect, can effectively maintain the effective concentration of the target part of the 2-methoxy estradiol and enhance the treatment effect is enhanced and is the innovation on the path of cancer treatment drug forms and drug administration.
Owner:ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com