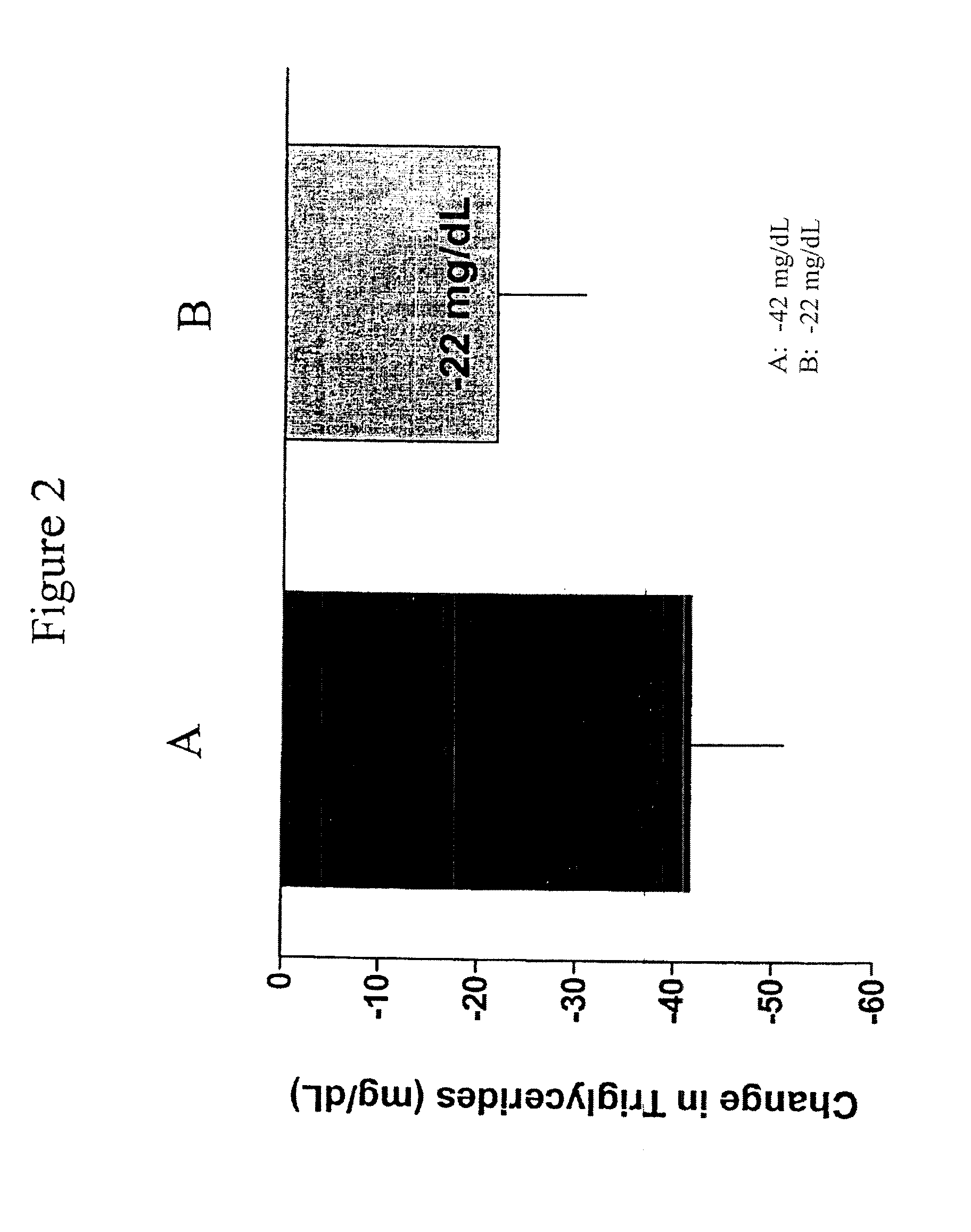
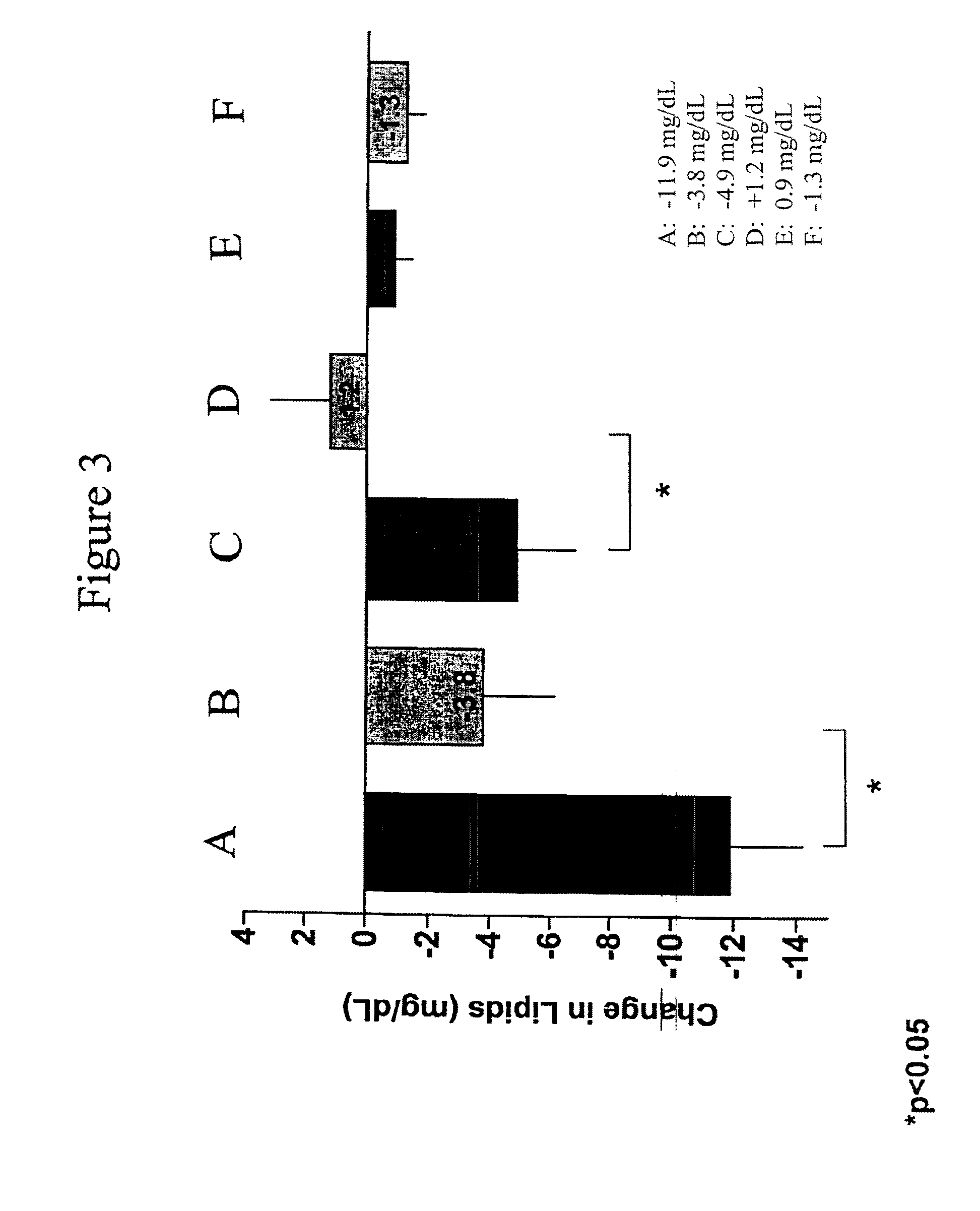
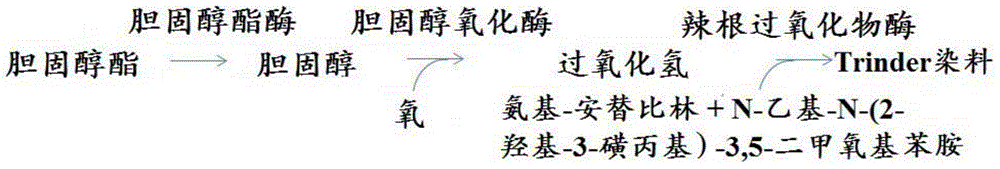
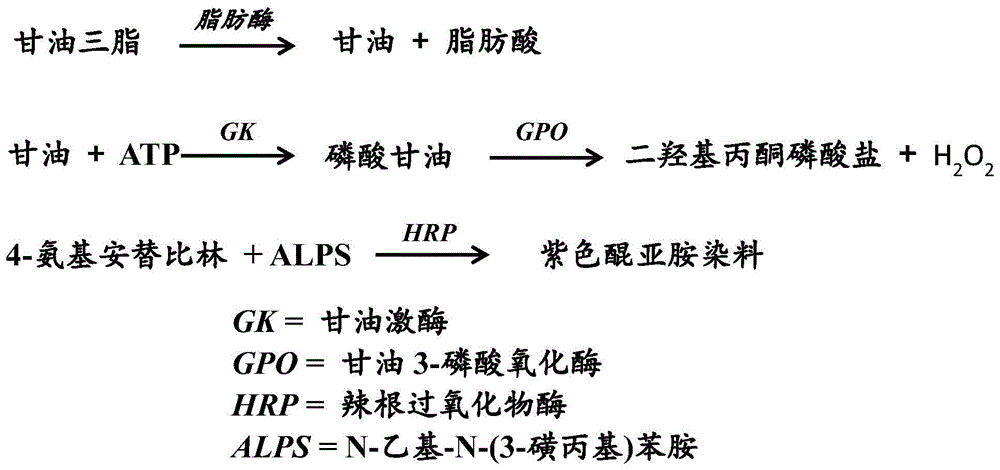
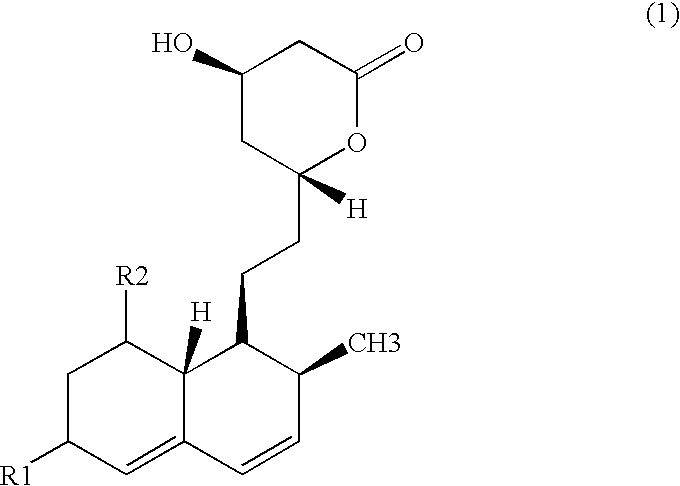
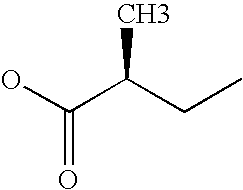
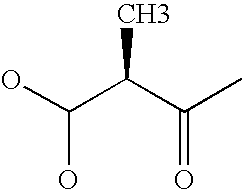
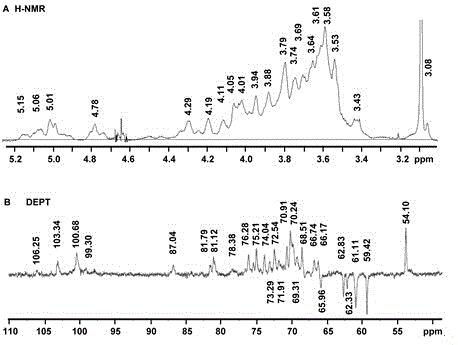
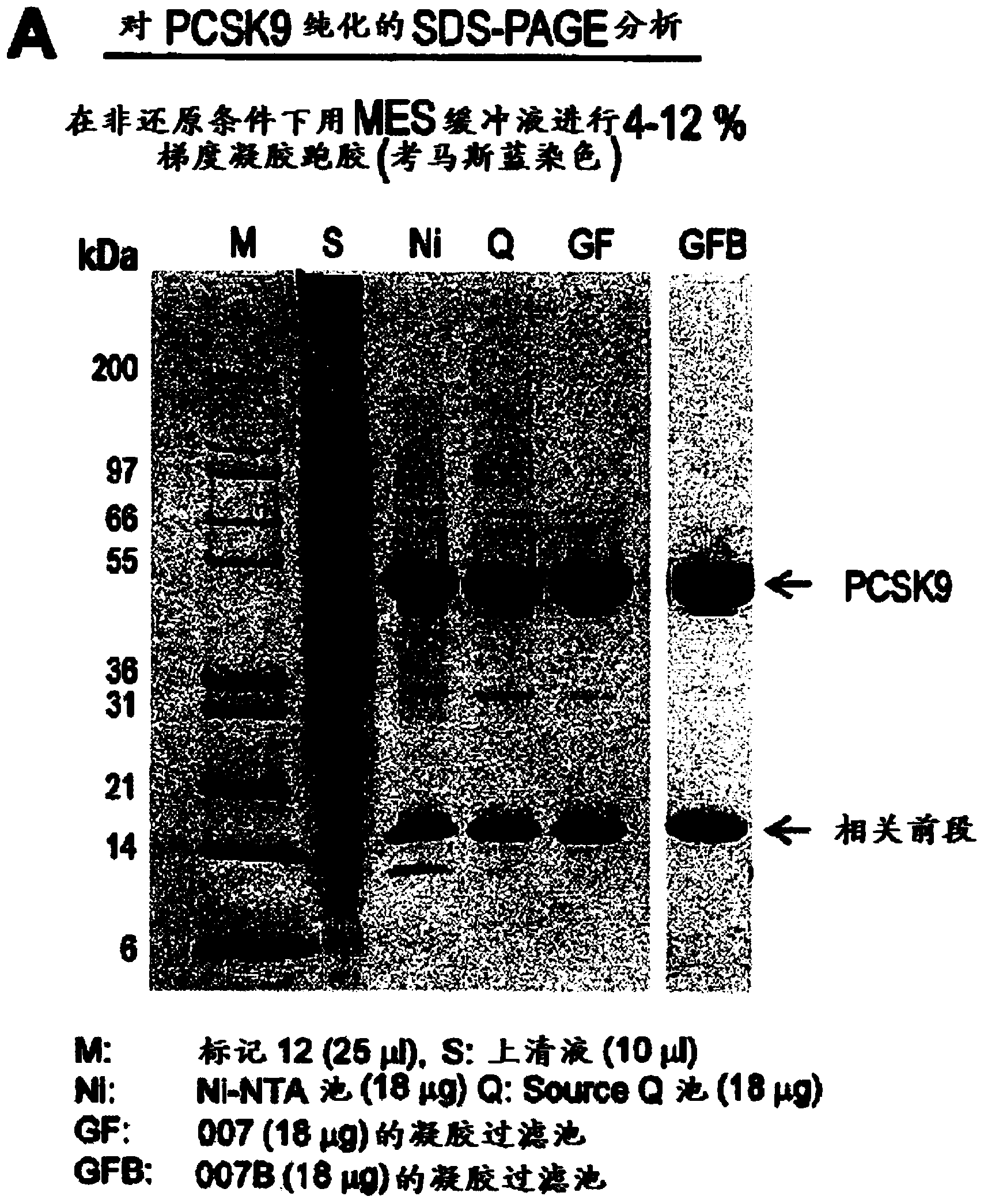
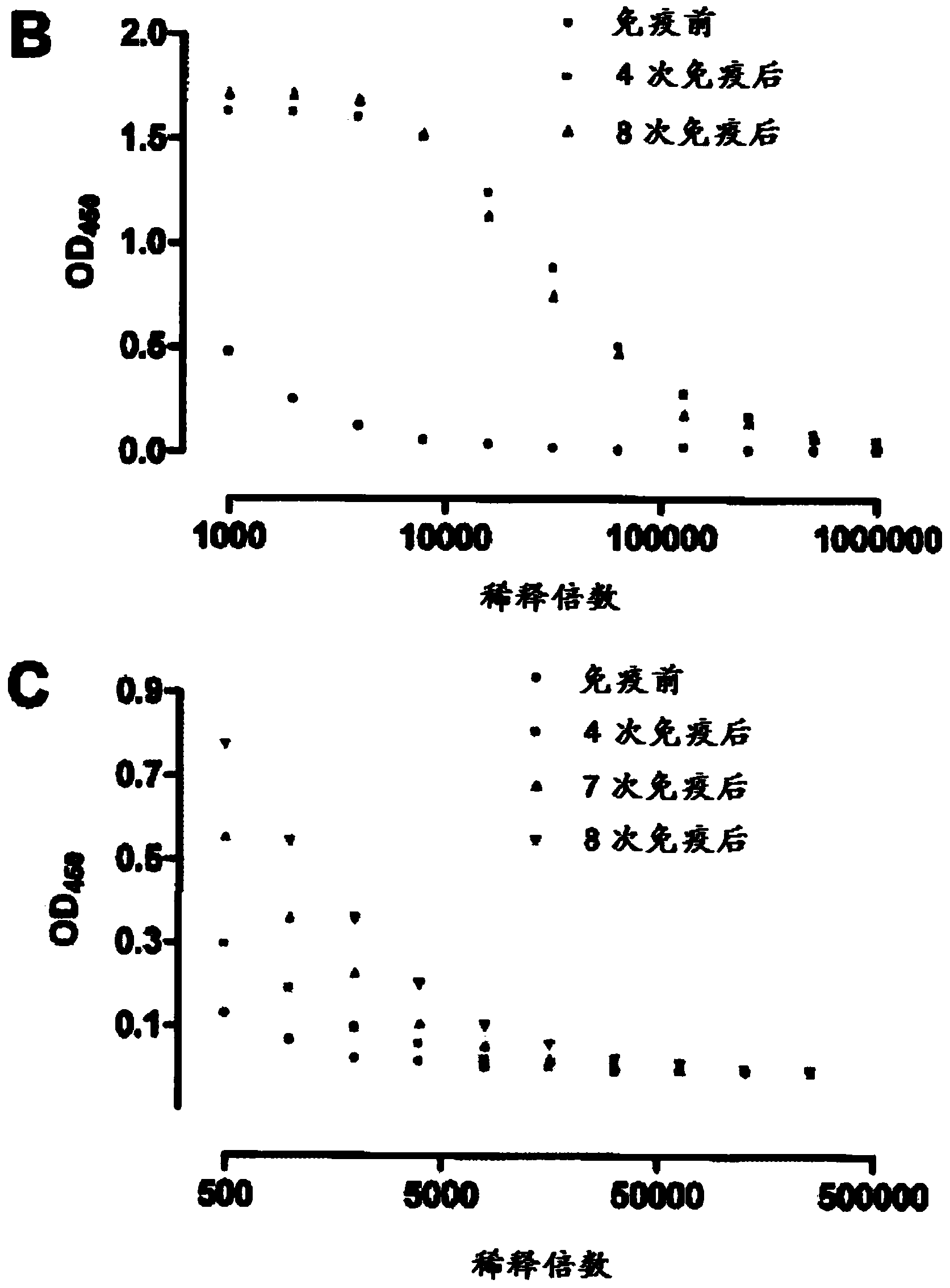
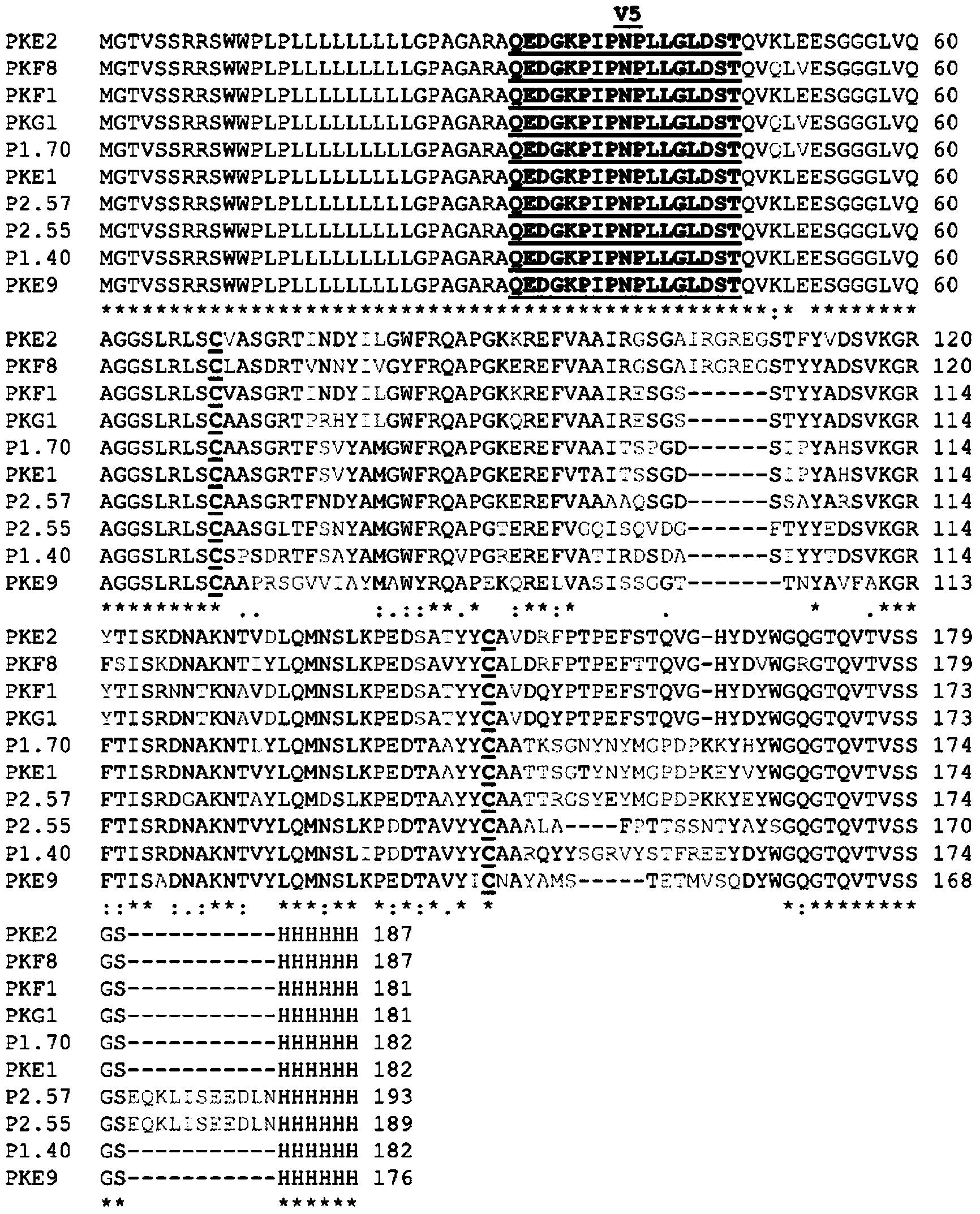
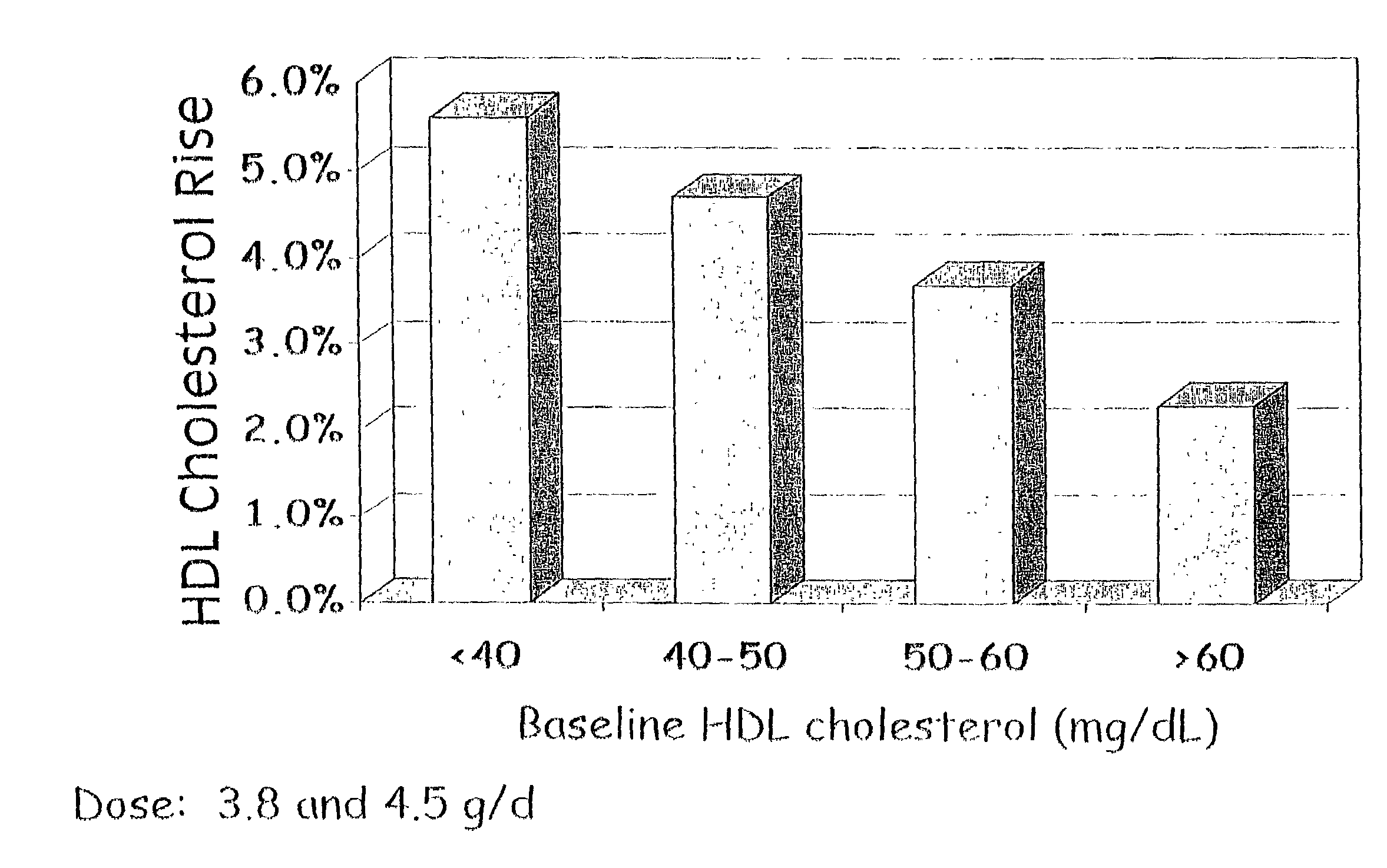
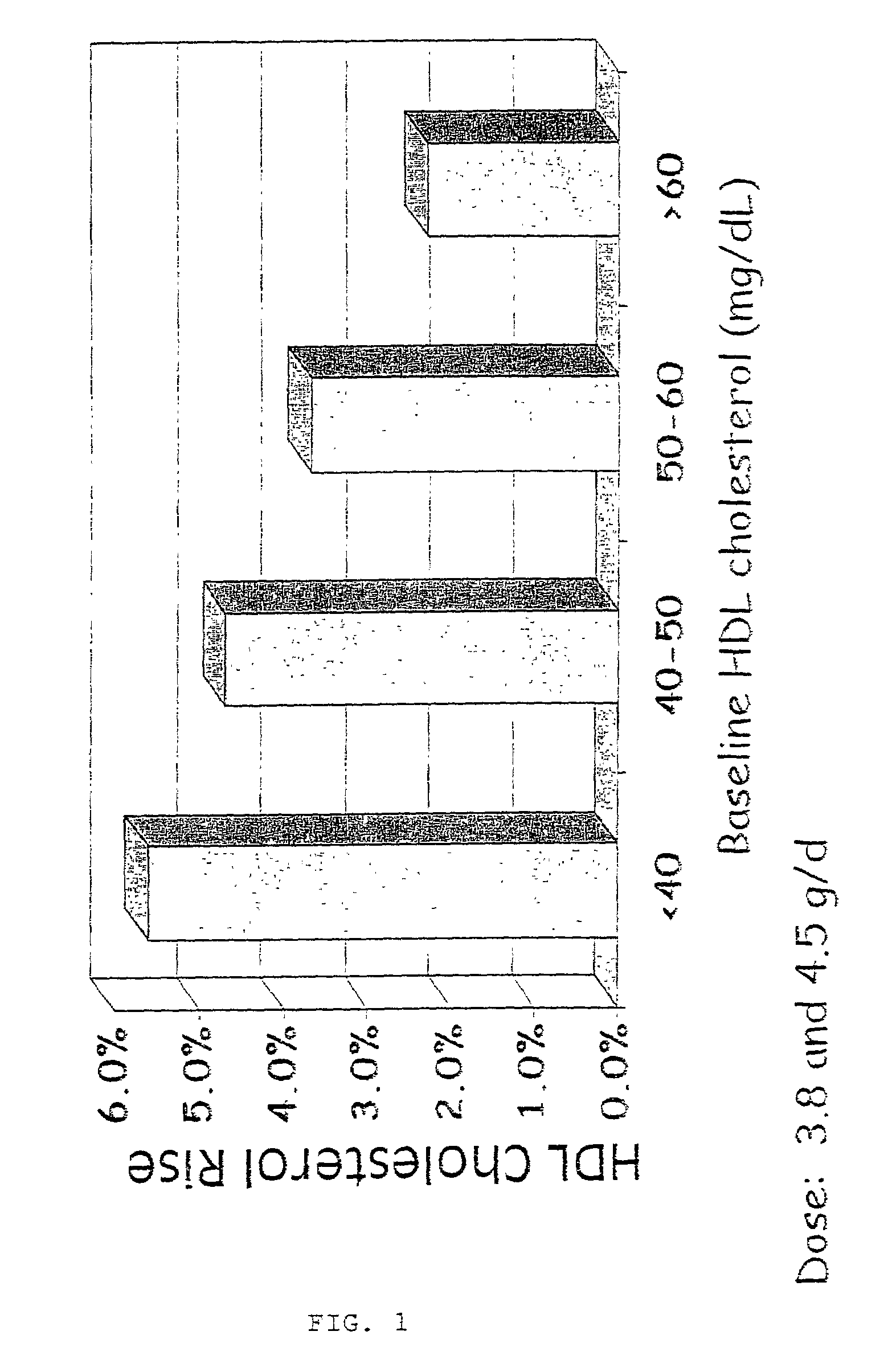
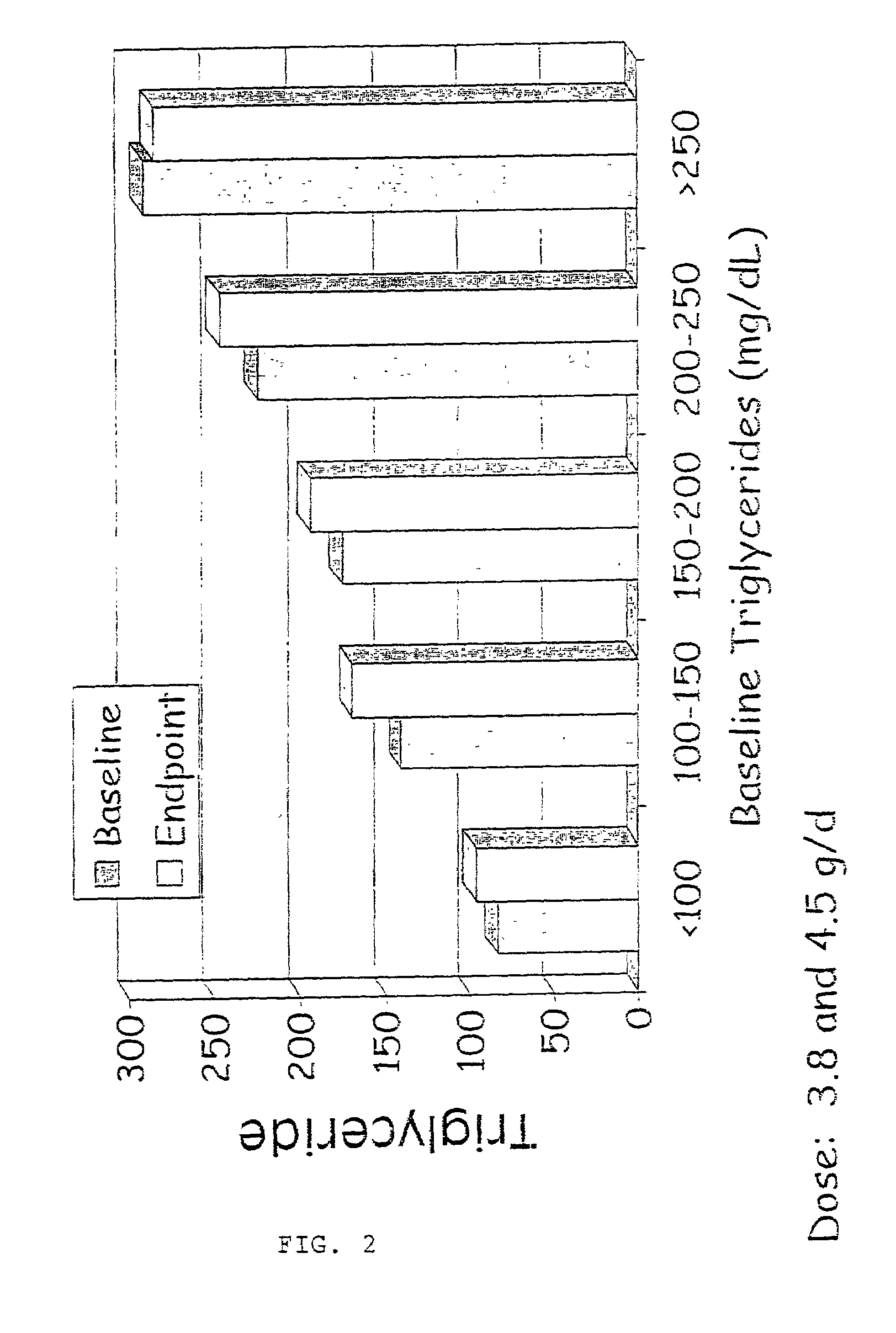
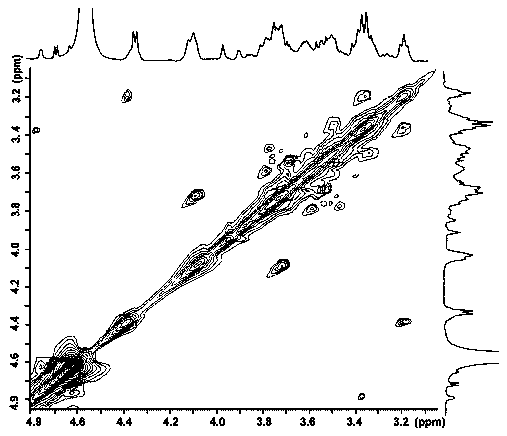
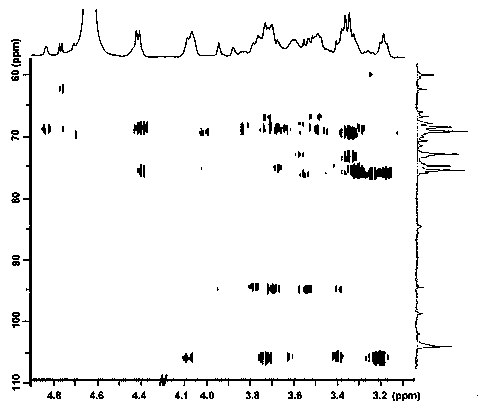
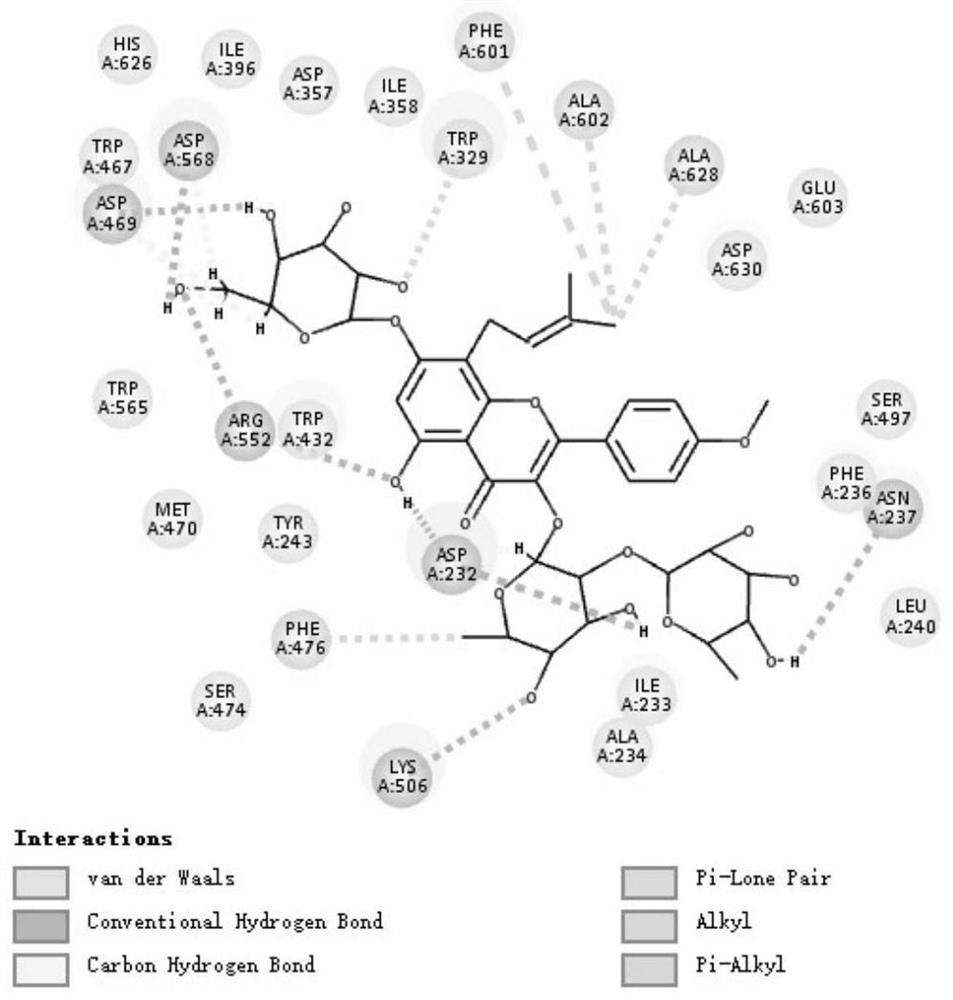
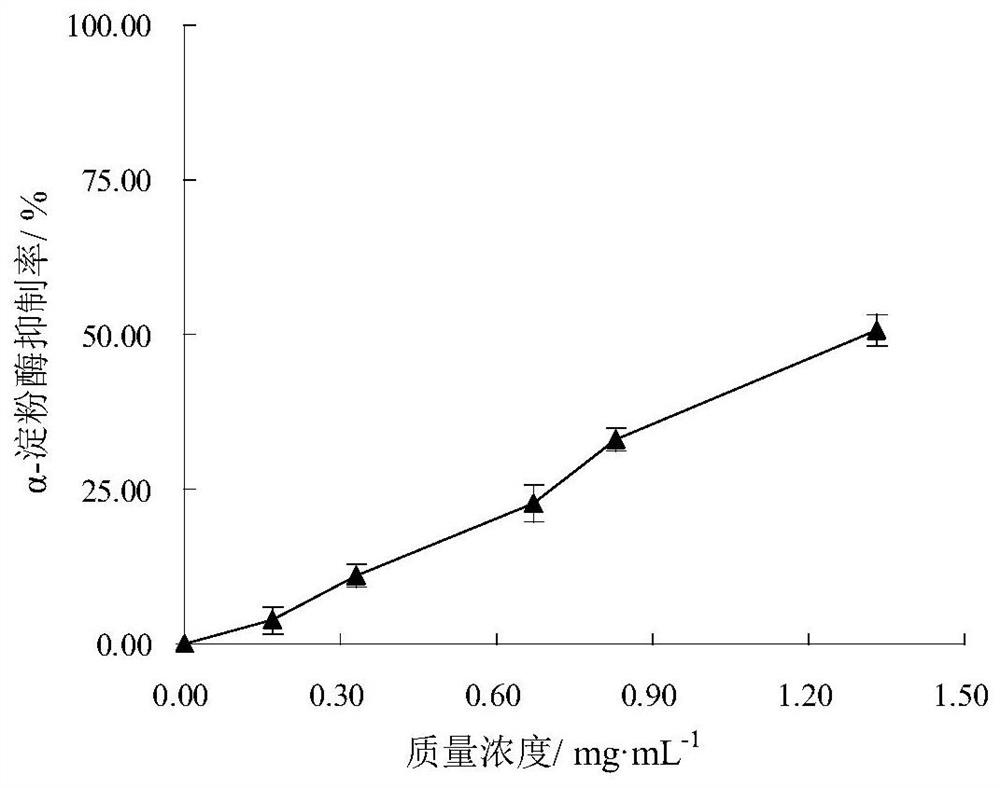
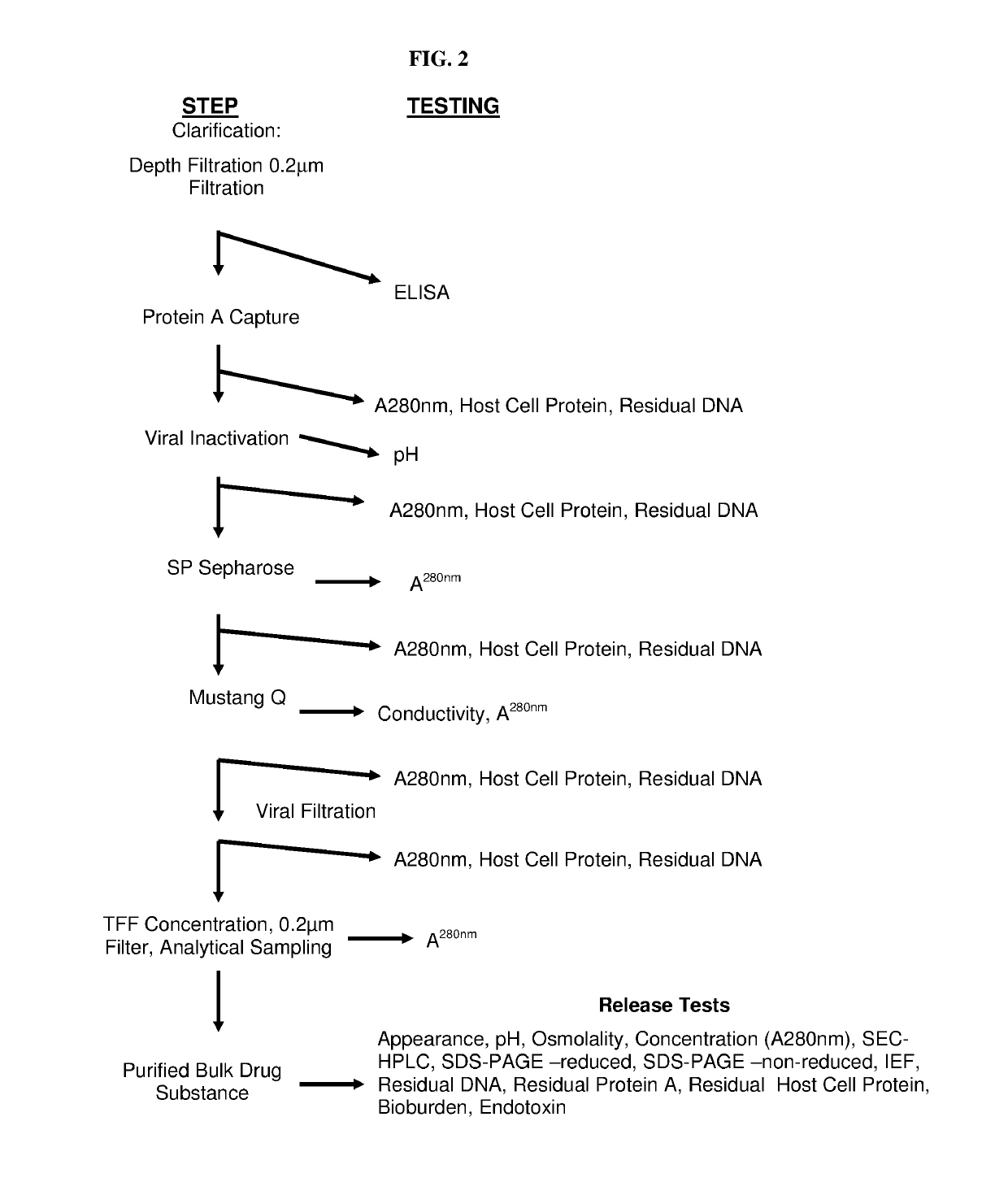
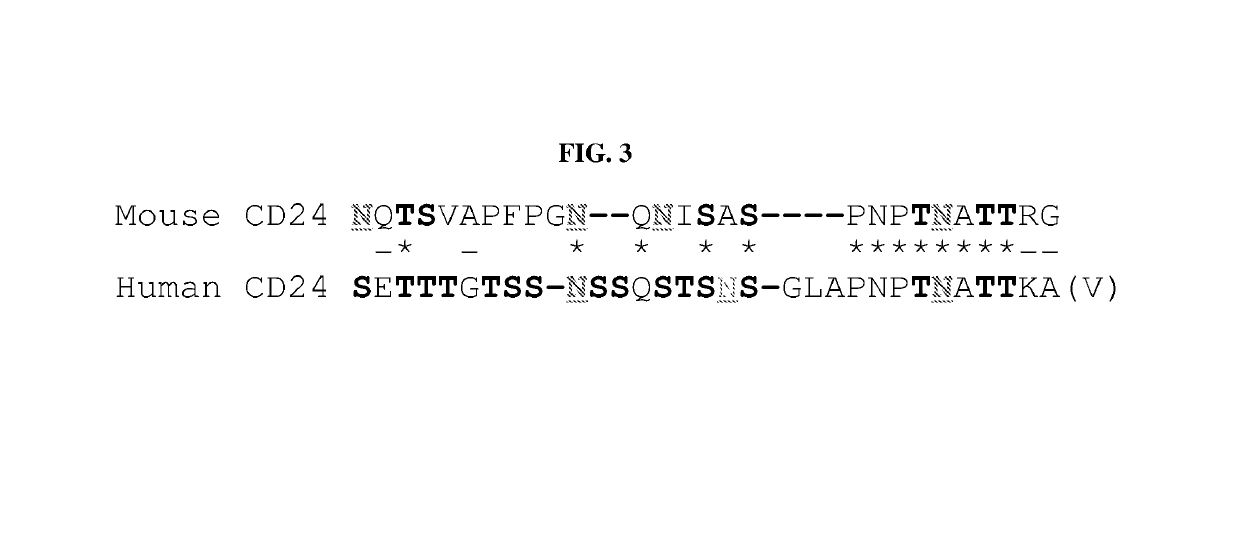
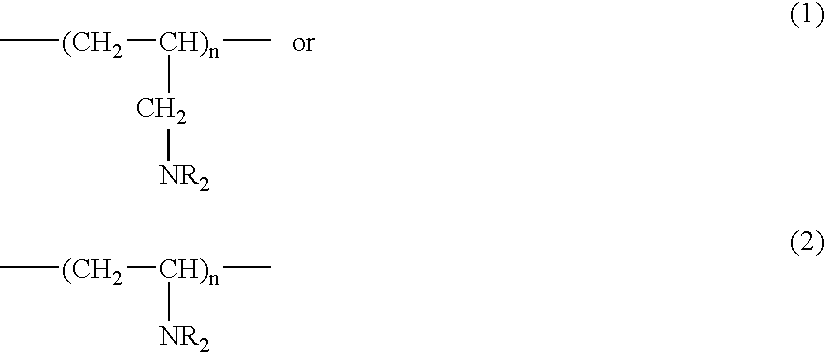Patents
Literature
50 results about "Low density lipoprotein cholesterol level" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nutritional supplements
InactiveUS20050032757A1Reducing detectable measureReduce detectable measureBiocideFood ingredientsNutrition supplementationLow-density lipoprotein
The invention provides compositions containing one or more sterol compounds and one or more fatty acid compounds. The invention also provides methods for reducing CVD risk factors such as LDL cholesterol levels.
Owner:MELALEUCA INC
Exendins to lower cholesterol and triglycerides
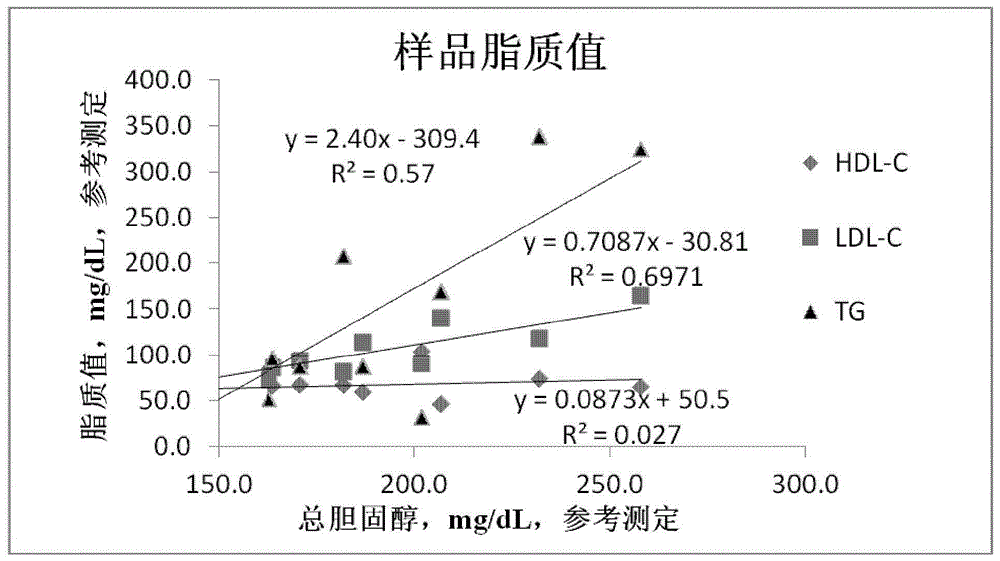
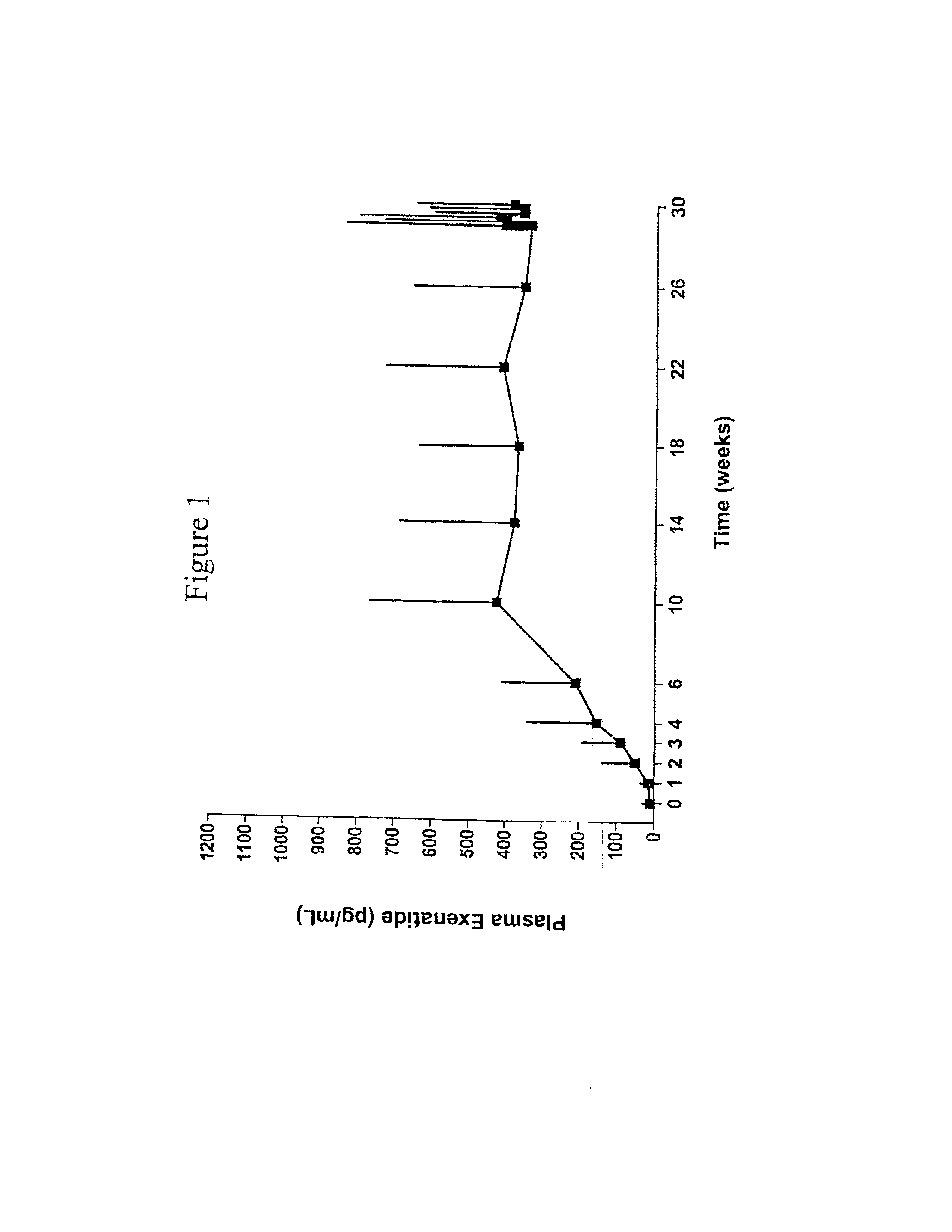
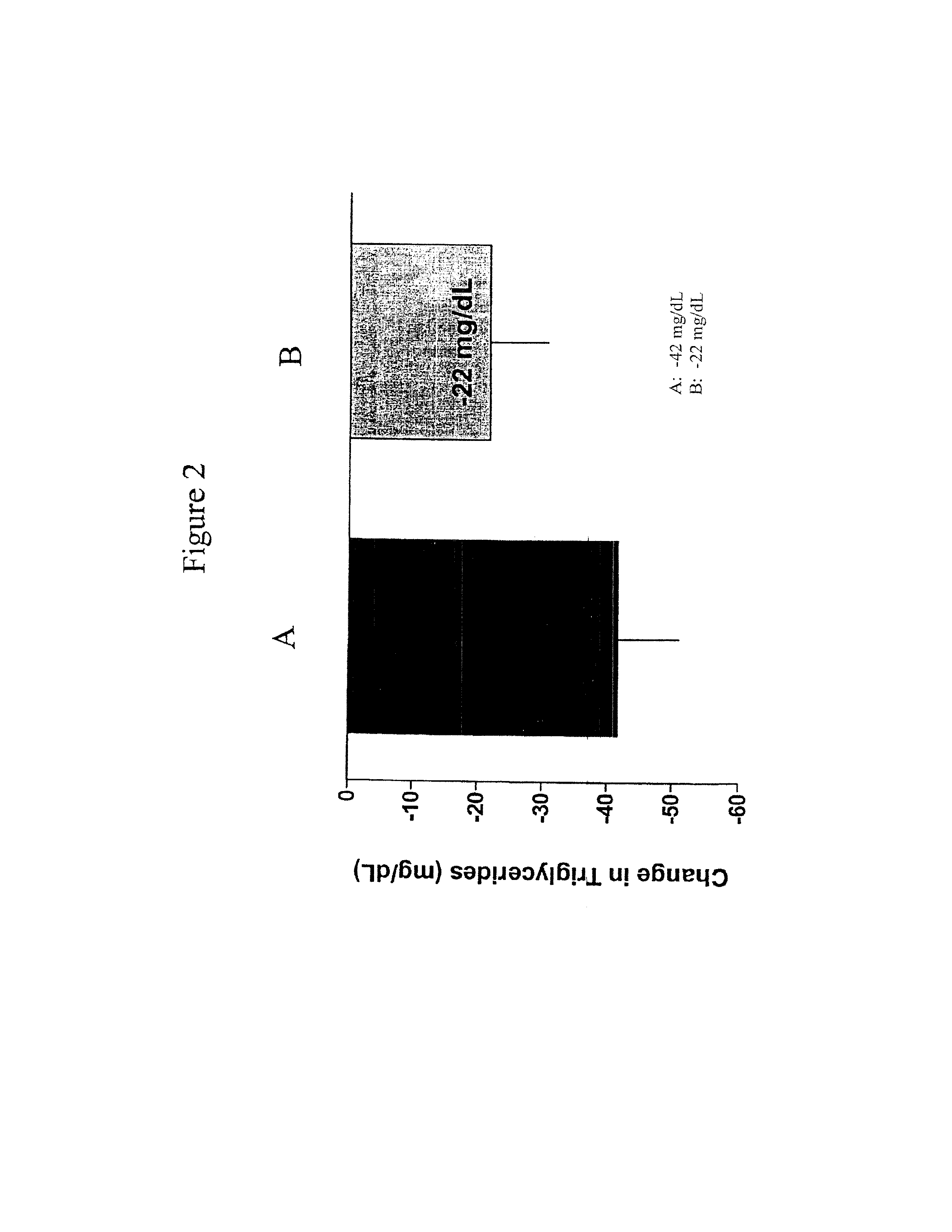
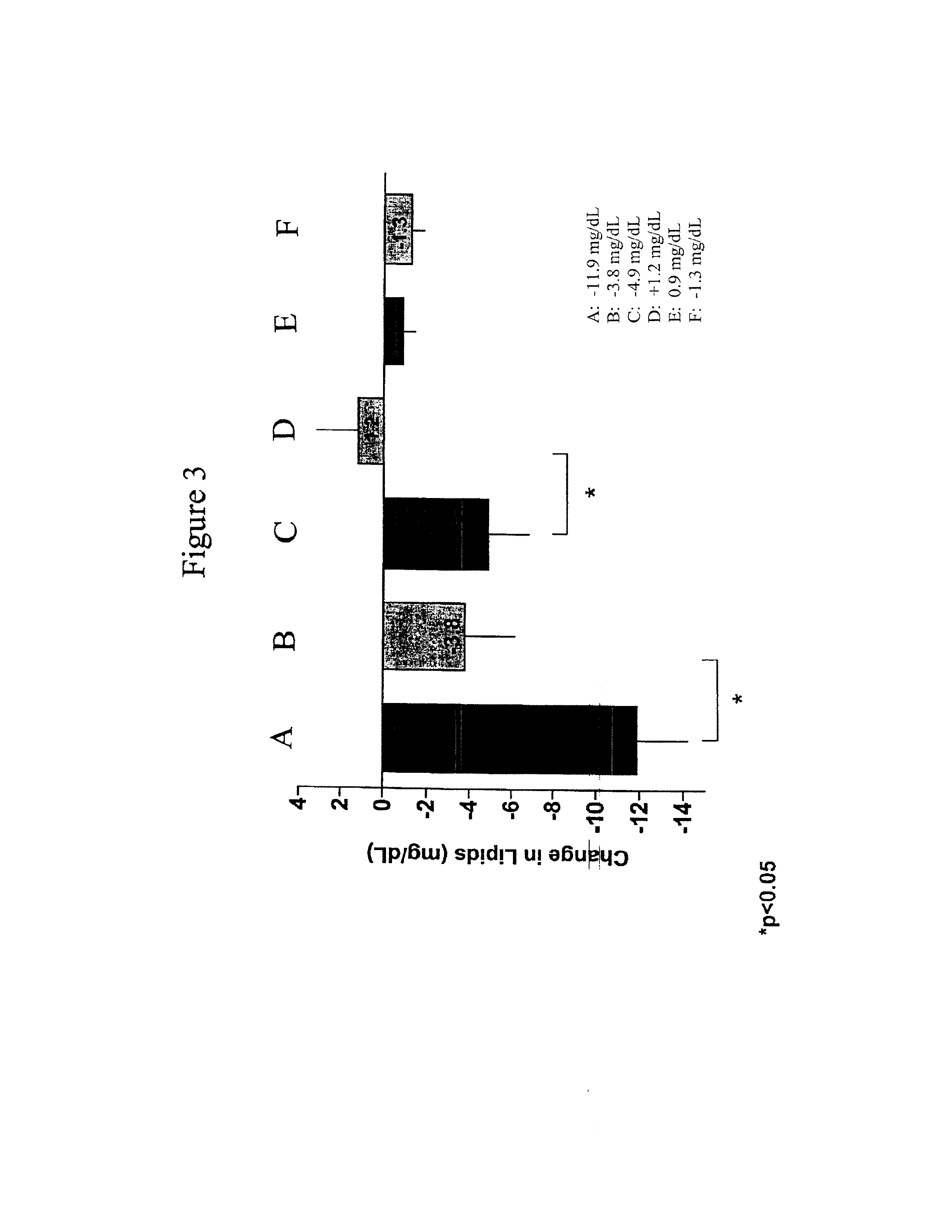
InactiveUS20110263496A1Lower cholesterol levelsStable statePeptide/protein ingredientsMetabolism disorderDyslipidemiaSucrose
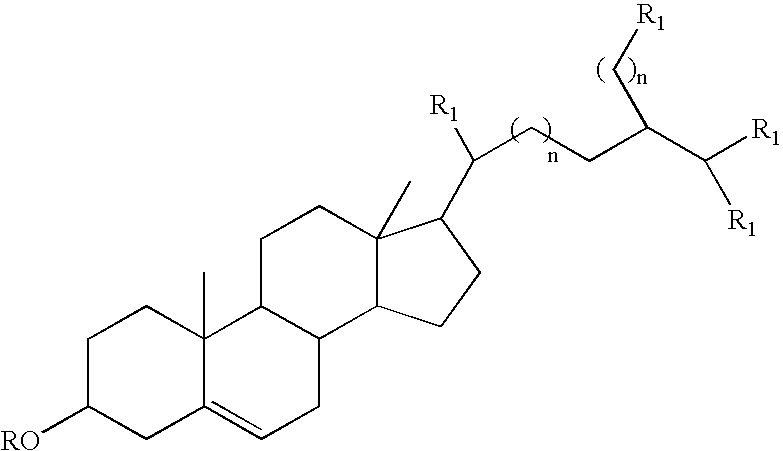
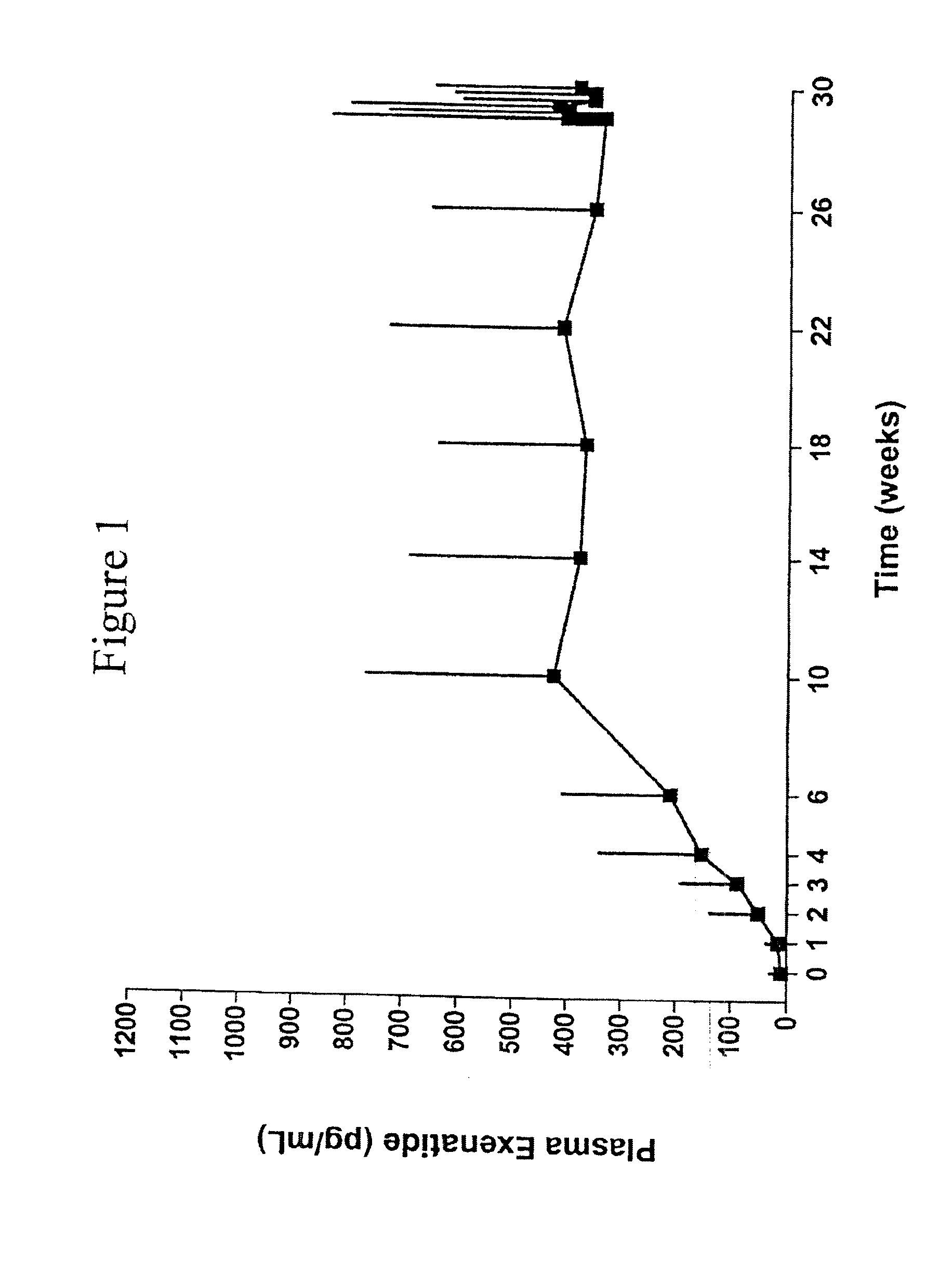
Provided herein are pharmaceutical formulations containing exendins, exendin agonists, or exendin analog agonists that are administered at therapeutic plasma concentration levels over a sustained period of time to lower total cholesterol levels; to lower LDL-cholesterol levels; to lower triglyceride levels; to treat dyslipidemia; to treat and slow the progression of atherosclerosis; and to treat, prevent, and reduce the risk of heart attacks and strokes in patients. In the pharmaceutical formulations and methods of the invention, the exendin may be exendin-4, an exendin-4 agonist, or an exendin-4 analog agonist. The pharmaceutical formulations may be polymer-based pharmaceutical formulations that may be administered once weekly. An exemplary pharmaceutical formulation comprises 5% (w / w) of exenatide, about 2% (w / w) of sucrose, and about 93% (w / w) of a poly(lactide-co-glycolide) polymer, wherein the poly(lactide-co-glycolide) polymer is in the form of microshperes encapsulating the exenatide.
Owner:ASTRAZENECA PHARMA LP
Rapid, low-sample-volume cholesterol and triglyceride assays
ActiveCN104995310ALow variabilityFast and cheap to measureMicrobiological testing/measurementBiological material analysisVery low-density lipoproteinLow density lipoprotein cholesterol
Reagents, assays, methods, kits, devices, and systems for rapid measurement of cholesterol and cholesterol sub-fractions from a blood sample are provided. Total cholesterol, low density lipoprotein cholesterol, and high density lipoprotein cholesterol can be measured in a single assay using kinetic measurements, under conditions in which cholesterol sub-species are converted to a detectable product at distinct rates. The detectable product is measured at different times after assay initiation. A lipase, cholesterol esterase, cholesterol oxidase and a peroxidase may be used together to produce colored product in amounts directly proportional to the quantity of cholesterol converted. Methods for calculating very-low density lipoprotein cholesterol levels by further including triglyceride measurements are disclosed. Assays may be performed in a single reaction mixture, allowing more accurate and precise cholesterol determinations, including ratios of cholesterol sub-fractions to total cholesterol, at less expense, than would be expected by performing several different assays in different reaction mixtures.
Owner:THERANOS IP CO LLC
Exendins To Lower Cholesterol And Triglycerides
InactiveUS20130172250A1Stable stateReduce riskPeptide/protein ingredientsMetabolism disorderDyslipidemiaSucrose
Provided herein are pharmaceutical formulations containing exendins, exendin agonists, or exendin analog agonists that are administered at therapeutic plasma concentration levels over a sustained period of time to lower total cholesterol levels; to lower LDL-cholesterol levels; to lower triglyceride levels; to treat dyslipidemia; to treat and slow the progression of atherosclerosis; and to treat, prevent, and reduce the risk of heart attacks and strokes in patients. In the pharmaceutical formulations and methods of the invention, the exendin may be exendin-4, an exendin-4 agonist, or an exendin-4 analog agonist. The pharmaceutical formulations may be polymer-based pharmaceutical formulations that may be administered once weekly. An exemplary pharmaceutical formulation comprises 5% (w / w) of exenatide, about 2% (w / w) of sucrose, and about 93% (w / w) of a poly(lactide-co-glycolide) polymer, wherein the poly(lactide-co-glycolide) polymer is in the form of microshperes encapsulating the exenatide.
Owner:ASTRAZENECA PHARMA LP
Food product suitable for reducing low density lipoprotein cholesterol levels
InactiveUS6849281B2Good health effectInhibit coloringBiocideEdible oils/fats ingredientsLow density lipoprotein cholesterolGram
A food product suitable for reducing low density lipoprotein cholesterol levels comprising an amount of soy protein of at least 5 grams per average serving and at least 5 mg / kg statins is described. Preferably the food product comprises a fermented soy ingredient.
Owner:UNILEVER BESTFOODS NORTH AMERICA DIV OF CONOPCO
Cordyceps militaris sporocarp heteropolysaccharide and application thereof
ActiveCN105585639AReduce savingsAnti-atherosclerotic effectMetabolism disorderCardiovascular disorderArcus aortaeFreeze-drying
The invention discloses a preparation method and application of cordyceps militaris sporocarp heteropolysaccharide. The preparation method mainly comprises the following steps that cordyceps militaris sporocarp is subjected to drying, pulverization, ethanol degreasing and hot water ultrasonic extraction, and an extracting solution is subjected to concentration, alcohol precipitation, deproteinization, column chromatography, dialysis and freeze-drying, so that the cordyceps militaris sporocarp heteropolysaccharide TY258 is obtained. The cordyceps militaris polysaccharide has the efficacy of lowering lipid and resisting to atherosclerosis and can remarkably lower the cholesterol level, the triglyceride level and the low density lipoprotein level of high-fat induced atherosclerosis model mice (mice with apolipoprotein and low density lipoprotein receptors knocked out) and lipid accumulation of the livers and arcus aortae at the concentration of 25 mg / kg, lift the high density lipoprotein cholesterol level of the mice and improve activity of superoxide dismutase and lipoprotein esterolysis enzyme. The cordyceps militaris sporocarp polysaccharide can also remarkably lower the contents of plasma triglyceride, cholesterol, malonaldehyde and oxidized low-density lipoprotein of high-fat induced guinea pigs and rats.
Owner:TAISHAN MEDICAL UNIV
Products and methods using soy peptides to lower total and LDL cholesterol levels
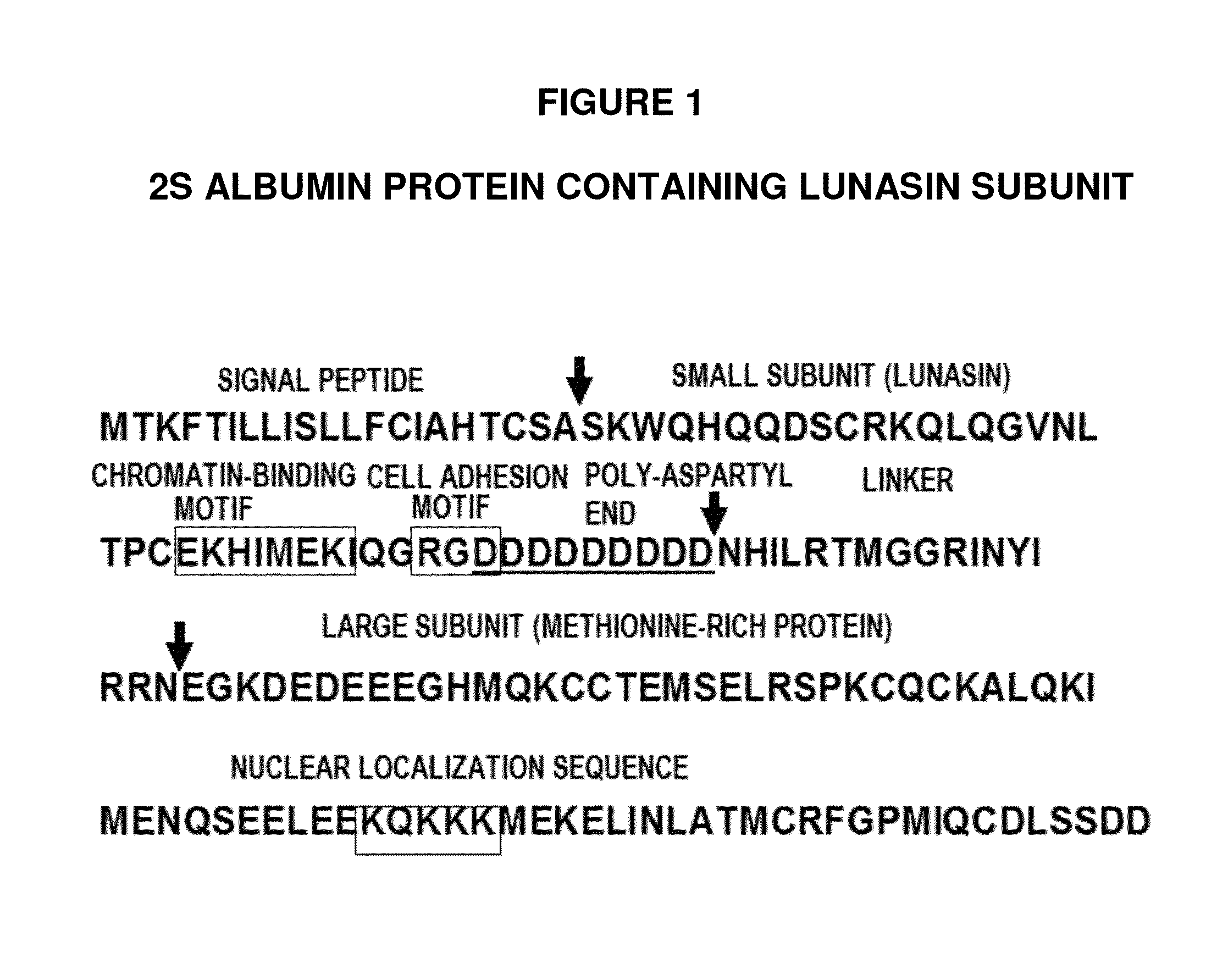
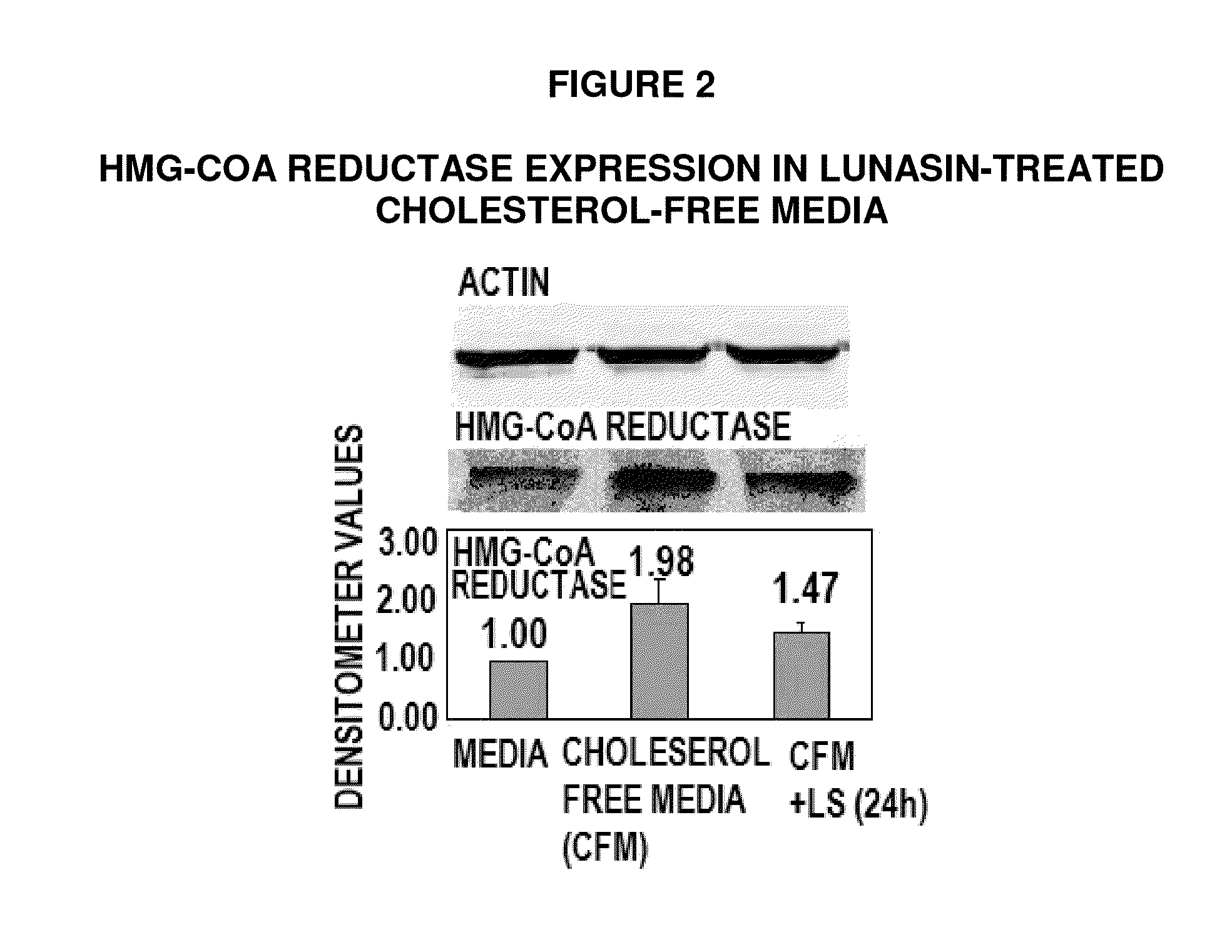
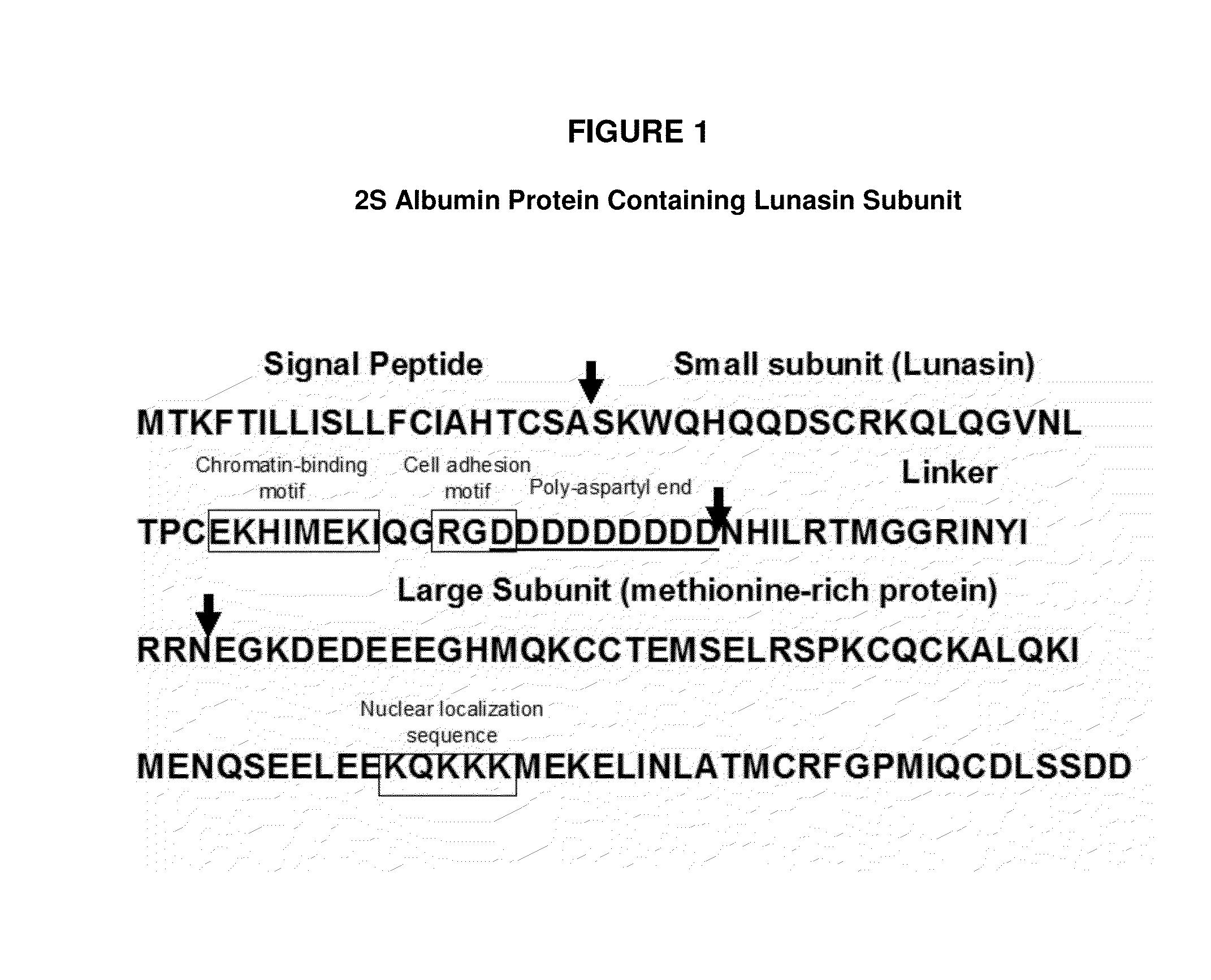
ActiveUS8598111B2Lower cholesterol levelsLower Level RequirementsPeptide/protein ingredientsMetabolism disorderLipid formationLunasin
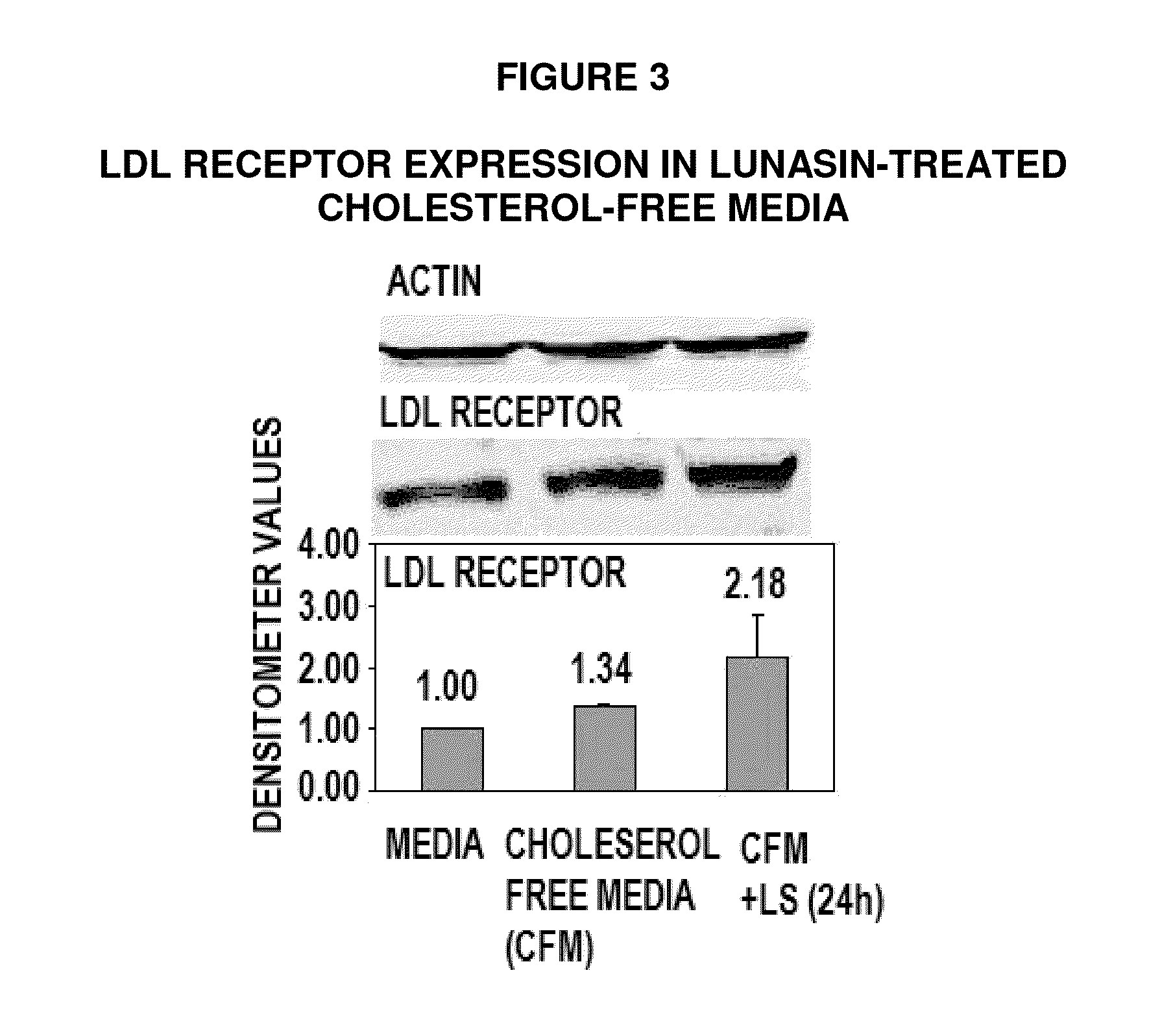
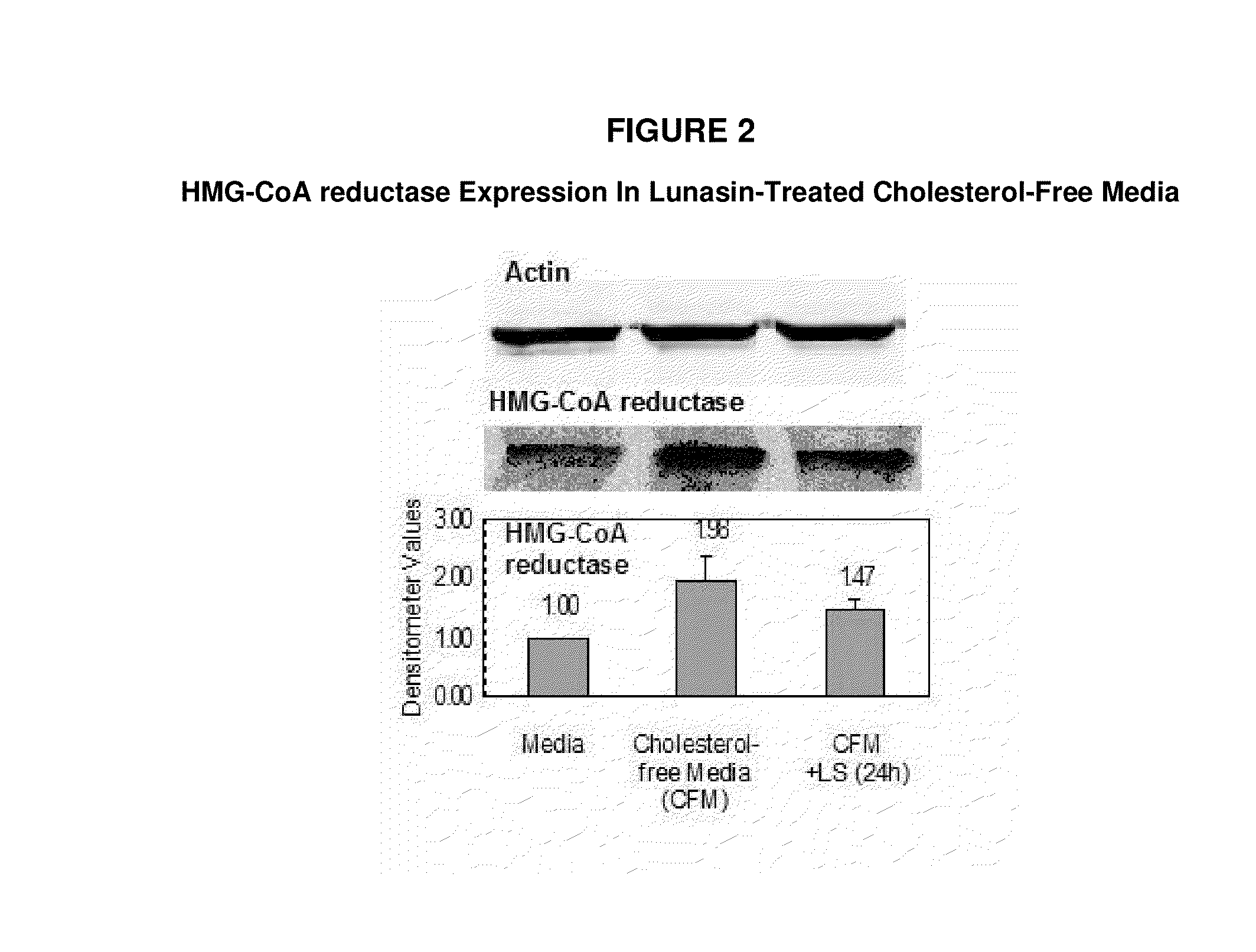
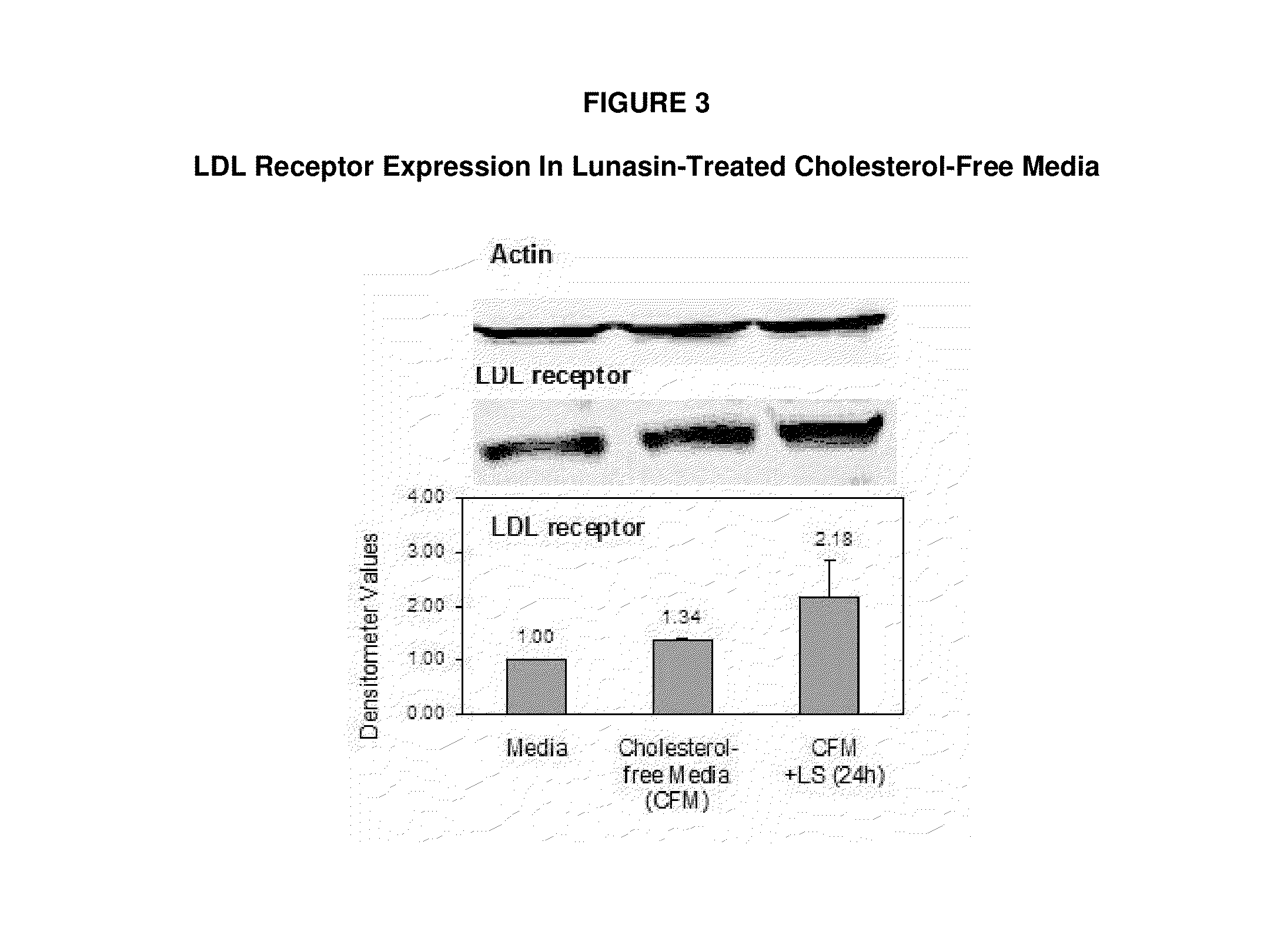
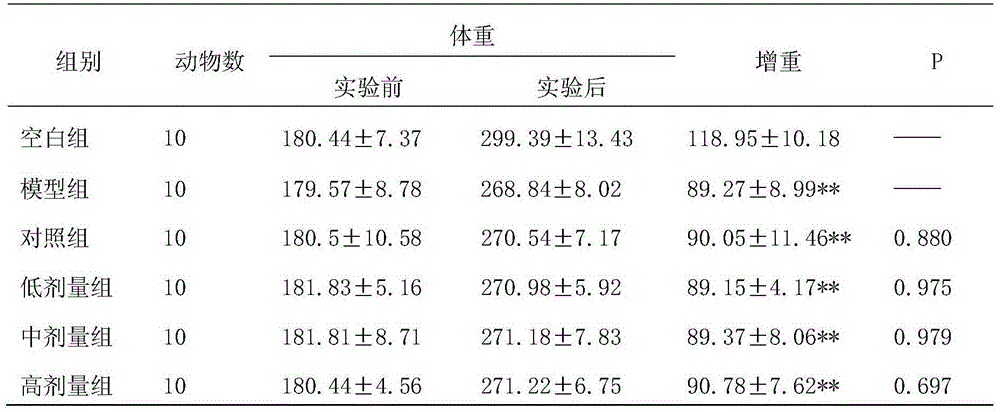
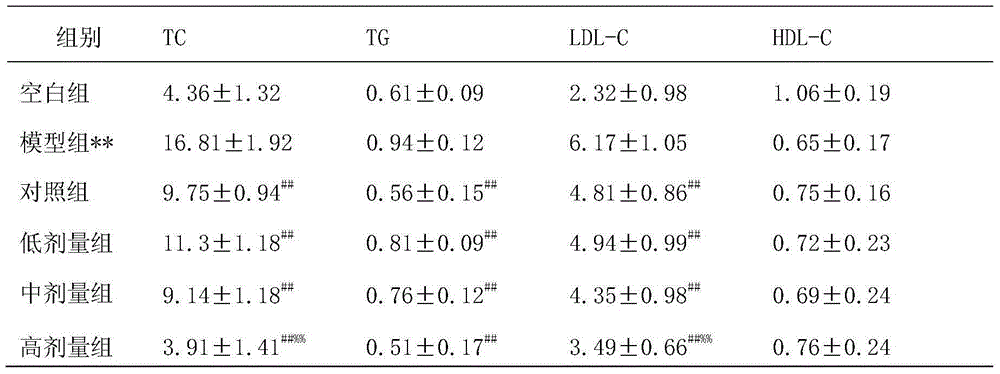
Controlled studies demonstrate that products and related methods using soy related peptides lower total and LDL cholesterol levels in individuals. In one exemplary embodiment of the present disclosure, a product containing an effective amount of lunasin peptides that lowers cholesterol levels in an individual that consumes the lunasin peptides is provided. In another exemplary embodiment of the present disclosure, a composition containing an effective amount of lunasin peptides or lunasin peptide derivatives and one or more enzyme inhibitors is provided. In a related exemplary embodiment of the present disclosure, a method for lowering or reducing cholesterol levels in an individual is provided where a product containing an effective amount of lunasin peptides to an individual is provided and a claim that the product lowers or reduces cholesterol, total cholesterol, LDL cholesterol or lipid levels in an individual that consumes the composition is made.
Owner:S L TECH +1
Application of pigeon pea ketonic acid A in terms of preparation of medicines for accompanied diseases of diabetes mellitus and hyperlipidaemia
ActiveCN102670576ALower cholesterol levelsNormal blood sugarOrganic active ingredientsMetabolism disorderSerum triglyceride levelsKetonic acids
The invention discloses application of pigeon pea ketonic acid A in terms of preparation of medicines for accompanied diseases of diabetes mellitus or hyperlipidaemia. Animal experiment data show that the pigeon pea ketonic acid A can remarkably reduce the serum triglyceride level, the total cholesterol level and the low-density lipoprotein cholesterol level of SD (Sprague-Dawley) rats with type-2 diabetes mellitus, and can treat or improve the hyperlipidaemia as an accompanied disease of the diabetes mellitus; and pathological research results prove that the pigeon pea ketonic acid A can reduce spotty necrosis of hepatocytes, can treat or improve liver injury as an accompanied disease of the diabetes mellitus, reverses injury to tissues of pancreas inlets of the rats due to glucoses, and has protection and repair effects on pancreas. The pigeon pea ketonic acid A can remarkably reduce the serum triglyceride level and the total cholesterol level of Zucker rats with congenital obesity, and is proved to be capable of treating or improving the hyperlipidaemia.
Owner:GUANGZHOU YUNZHONG BIOTECH
Methods of treating Syndrome X with aliphatic polyamines
InactiveUS20080085872A1Lower Level RequirementsBiocideMetabolism disorderHMG-CoA reductaseCross-link
Owner:BURKE STEVEN K +1
Composition Comprising Lactobacillus Plantarum 2830 (ECGC 13110402)
ActiveUS20180318364A1Enhanced cholesterol reduction and controlImprove levelPowder deliveryBacteria material medical ingredientsMutant strainLactobacillus plantarum
The present invention relates to compositions comprising Lactobacillus plantarum 2830 (ECGC 13110402), or mutant strain or strains thereof, for use in the treatment or prevention of hypercholesterolaemia, and in particular reducing the total cholesterol (TC) and low density lipoprotein cholesterol (LDL-C) levels, in an individual. Specific dosage regimes and methods of production are also claimed and described.
Owner:PROBIOTIX HEALTH LTD
Single domain antibodies as inhibitors of PCSK9
InactiveCN104169304AMetabolism disorderBiological material analysisLow density lipoprotein cholesterolSingle-domain antibody
Antibodies (e.g., sdAbs) binding to PCSK9 are described. Nucleic acids encoding such Abs, host cells expressing such Abs and pharmaceutical composition comprising same are described. The use of these PCSK9-binding Abs for lowering low-density lipoprotein-cholesterol (LDL-C) levels and for the treatment of cardiovascular disorders, is also described.
Owner:ADAERATA +1
Methods of treating Syndrome X with aliphatic polyamines
InactiveUS7261880B2Slow onsetSymptoms improvedMetabolism disorderAntinoxious agentsHMG-CoA reductaseCross-link
The invention relates to a method for treating Syndrome X, or inhibiting the onset of symptoms of Syndrome X in a patient, and includes administering a therapeutically effective amount of a salt of at least one alkylated and cross-linked polymer, or a copolymer thereof, the polymer salt formed as a product of the reaction of one or more polymers, or salts and copolymers thereof, having a repeat unit that is essentially:where n is a positive integer and each R, independently, is H or a C1–C8 alkyl group; at least one aliphatic alkylating agent; and a cross-linking agent. Long term administration of the cross-linked polyamine salts of the invention increases HDL levels and decreases LDL levels in patients. The invention also provides for administration of the polymer salt colesevelam, in combination with an HMG-CoA reductase inhibitor; the combined administration is effective in further lowering serum total-cholesterol and LDL-cholesterol levels beyond that achieved by either agent alone.
Owner:GENZYME CORP
Cordyceps militaris glucan and preparation method and application thereof
ActiveCN105504085AClear structureEasy to prepareOrganic active ingredientsMetabolism disorderFreeze-dryingHigh fat
The invention relates to a Cordyceps militaris fruiting body glucan and a preparation method and application thereof. The preparation method provided by the invention mainly comprises the following steps: baking and crushing Cordyceps militaris fruiting bodies, carrying out ethanol degreasing, carrying out hot-water ultrasonic extraction, and subjecting an extract to concentration, alcohol precipitation, deproteinization, column chromatography, dialysis and freeze drying in succession, thereby obtaining the Cordyceps militaris fruiting body glucan TY517. The Cordyceps militaris polysaccharide provided by the invention has a lipid-lowering effect, and the Cordyceps militaris polysaccharide with the concentration of 25mg / kg can be used for remarkably lowering the cholesterol, triglyceride and low-density lipoprotein cholesterol level of high-fat-induced laboratory animal models and increasing the high-density lipoprotein cholesterol level.
Owner:TAISHAN MEDICAL UNIV
Food composition with effects of assisting blood fat reduction, weight loss and liver protection, and preparation method, preparation and application thereof
ActiveCN110075216AReduce absorptionReduce heatMetabolism disorderDigestive systemBacteroidesLow density lipoprotein cholesterol
The invention relates to a food composition with the effects of assisting blood fat reduction, weight loss and liver protection. The food composition is prepared from the following raw materials in parts by weight: 20-25 parts of fructus crataegi, 20-25 parts of lotus leaf, 10-16 parts of radix puerariae, 10-18 parts of green tea, 4-6 parts of fructus lycii, 4-8 parts of poria cocos, 2-4 parts offructus hippophae, 1-3 parts of celery powder, 1-3 parts of folium mori, 1-2 parts of konjac powder, 1 to 3 parts of fructus chaenomelis lagenariae, and 1 to 2 parts of rhizoma polygonati. The prepared food composition can significantly reduce the body weight, fat / body ratio, food utilization rate and blood triglyceride, total cholesterol and low density lipoprotein cholesterol levels in a high fat model rat in the dosage range of each component, also can reduce the liver index, alanine aminotransferase and alkaline phosphatase levels in the high-fat model rat, can significantly increase the relative abundance of bacteroides and decrease the relative abundance of firmicutes, and has the clear effects of assisting blood fat reduction, weight loss, liver protection and intestinal flora improvement.
Owner:中国医科大学
Food product comprising soy protein and statins
InactiveCN1491085AGood health effectOrganic active ingredientsMilk preparationLow density lipoprotein cholesterolAdditive ingredient
Owner:UNILEVER NV
Peotopanaxadiol ginsenoside derivative and preparation method and application thereof
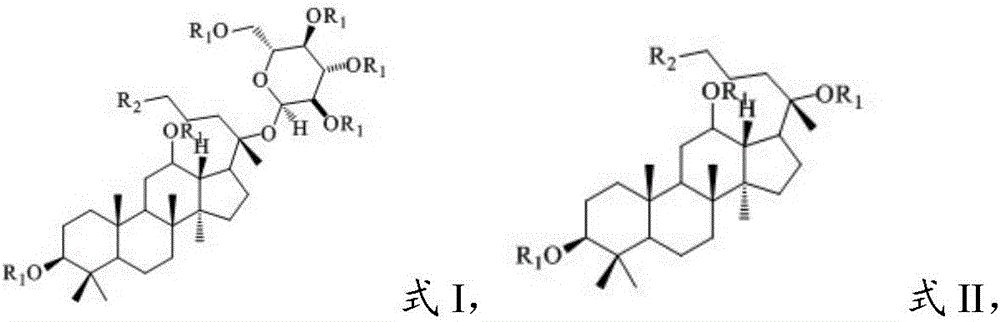
ActiveCN106632570ALow cytotoxicityLow toxicitySugar derivativesGlycoside steroidsA lipoproteinPlaque area
The invention provides a peotopanaxadiol ginsenoside derivative with a structure shown as formula I or a formula II and provides application of the peotopanaxadiol ginsenoside derivative to preparation of drugs for preventing and curing atherosclerosis. The peotopanaxadiol ginsenoside derivative has the advantages that the peotopanaxadiol ginsenoside derivative is low in cytotoxicity and capable of decreasing the percentage of the apoE- / -mouse atherosclerotic plaque area, reducing medium-low density lipoprotein cholesterol level in mouse serum effectively and raising high-density lipoprotein cholesterol level effectively; the peotopanaxadiol ginsenoside derivative is capable of reducing partial TNF-alpha level of apoE- / -mouse arteries, thereby having a good anti-inflammatory effect; the peotopanaxadiol ginsenoside derivative is capable of reducing RAW264.7 cell-derived foam cell forming degree under a dosage of 30 micron remarkably. The invention further provides a preparation method of the peotopanaxadiol ginsenoside derivative. The preparation method is simple and convenient to implement and high in yield.
Owner:ARMY MEDICAL UNIV +1
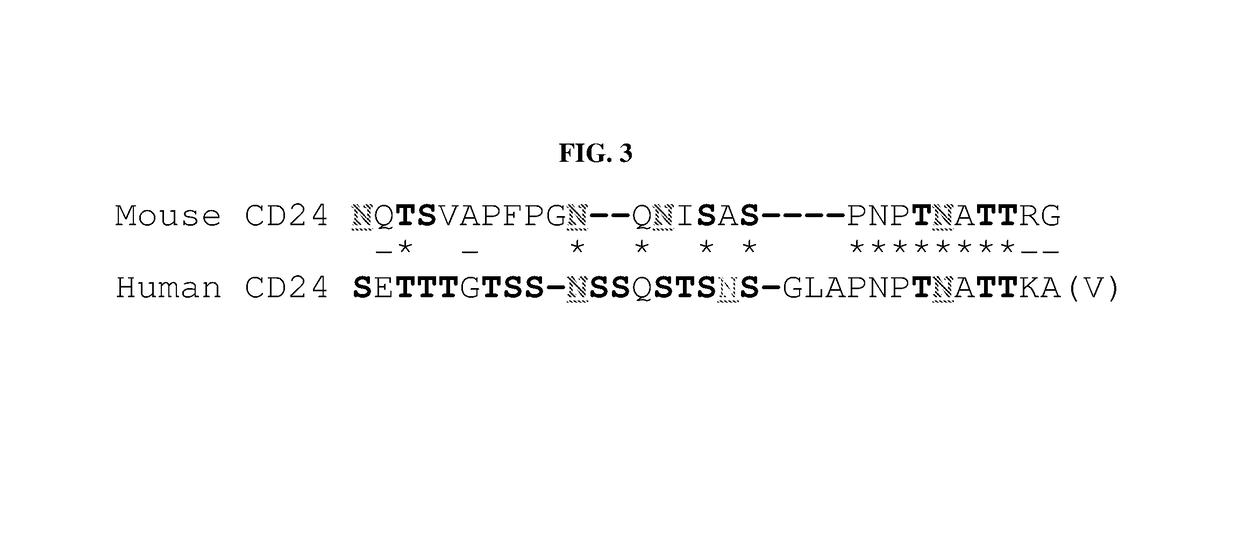
Use of cd24 for lowering low-density lipoprotein cholesterol levels
InactiveUS20180110828A1Reduction of ldl c levelReduce riskPeptide/protein ingredientsAntibody mimetics/scaffoldsLow density lipoprotein cholesterolCD24
The present invention relates to the use of a CD24 protein for lowering low-density lipoprotein cholesterol levels, treating and preventing atherosclerosis, and for reducing risk of cardiovascular disease.
Owner:ONCOIMMUNE INC
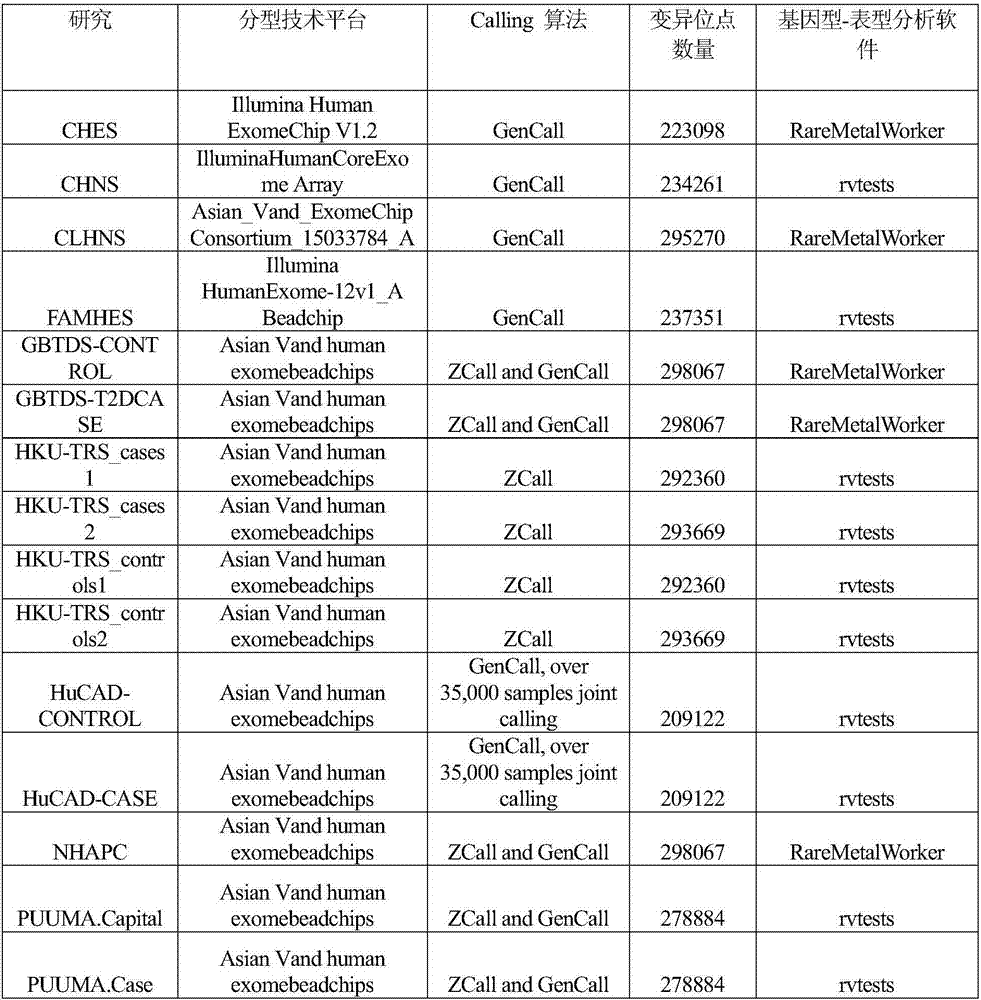
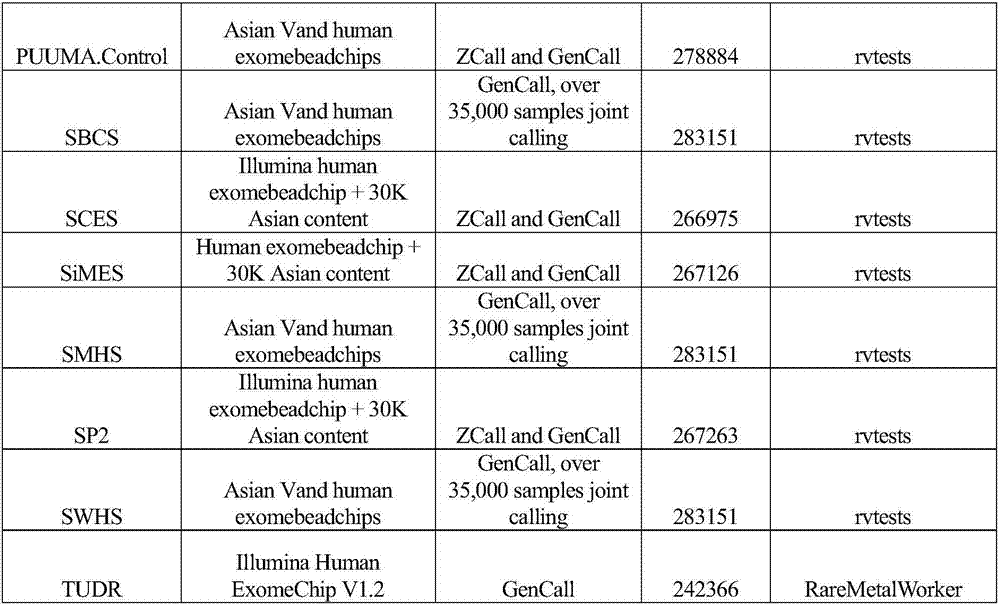
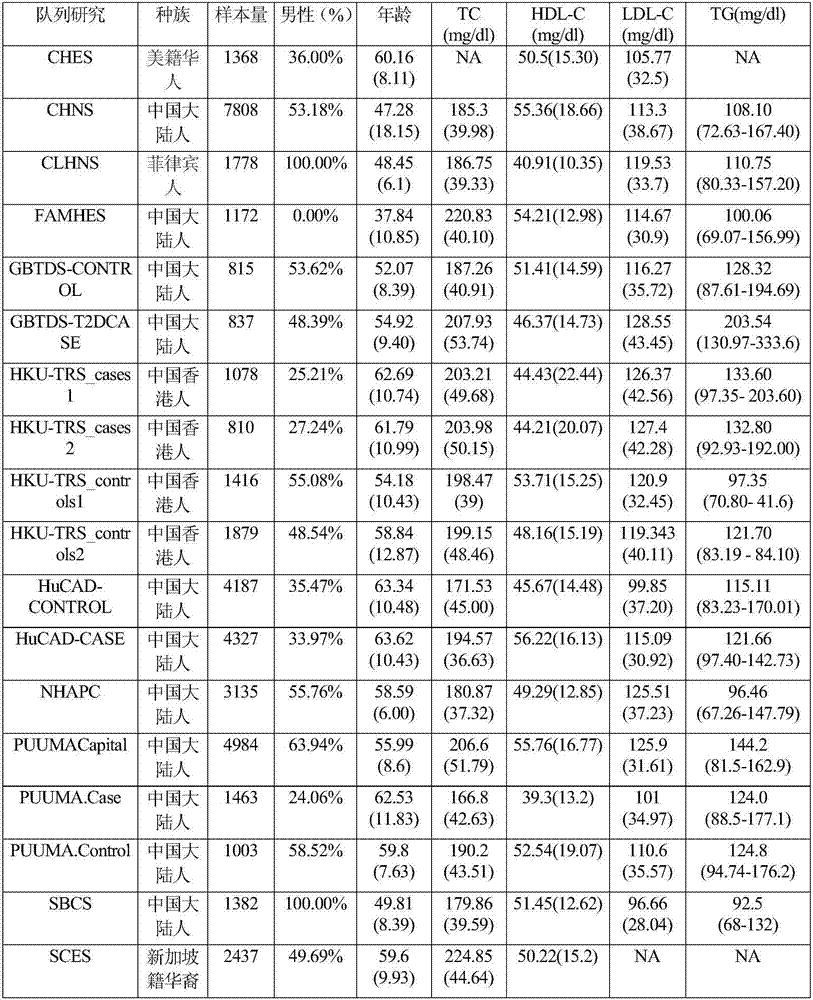
Gene functional genetic variation correlated to LDL-C (Low-density Lipoproteincholesterol) level and related application thereof
ActiveCN107502667AHelps prevent hyperlipidemiaAids in diagnosisMicrobiological testing/measurementDisease diagnosisCoronary heart diseaseCvd risk
The invention provides gene functional genetic variation correlated to LDL-C (Low-density Lipoproteincholesterol) level and related application thereof. Specifically, the invention provides application of reagent materials and / or instrument equipment for detecting the following protein amino acid sites and / or corresponding gene loci in samples from a to-be-tested individual in preparation of a detection system used for evaluating a blood lipid level and / or abnormal blood lipid onset risk. The protein amino acid sites include EVI5 gene rs117711462, APOB gene rs376825639 and / or PKD1L3 gene rs17358402 heritable variations causing EVI5 protein 354th amino acid coding change, APOB gene 3768th amino acid coding change and / or PKD1L3 gene 1572nd amino acid change. The to-be-tested individual with EVI5Arg354Cys and / or PKD1L3Arg1572His variation has high high-density lipoproteincholesterol level and / or high abnormal blood lipid onset risk. The to-be-tested individual with APOBIle3768Thr variation has low high-density lipoproteincholesterol level, low abnormal blood lipid onset risk and / or low coronary heart disease incidence risk.
Owner:FUWAI HOSPITAL CHINESE ACAD OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE +2
Method for measuring low-density lipoprotein (LDL) cholesterol
ActiveUS8304204B2Simple configurationSimple structureBioreactor/fermenter combinationsBiological substance pretreatmentsHigh-density lipoproteinTotal cholesterol
A method for measuring LDL cholesterol in a sample using a test piece is provided which involves a step of measuring the total cholesterol in the sample; a step of measuring the non-LDL cholesterol in the sample; and a step of subtracting the non-LDL cholesterol value from the total-cholesterol value to obtain the LDL cholesterol level.
Owner:ARKRAY INC
Drug Application of Epimedin C in Treating Diabetic Liver Injury
ActiveCN111494360BEnhanced inhibitory effectAlleviate lipid metabolism disordersOrganic active ingredientsMetabolism disorderSerum glutamate pyruvate transaminaseA lipoprotein
The invention discloses the drug application of epimedin C for treating diabetic liver injury, and belongs to the technical field of biomedicine. The present invention discloses for the first time that epimedin C can increase the level of high-density lipoprotein cholesterol in the liver of diabetic model animals, reduce the level of low-density lipoprotein cholesterol in the liver, alleviate the problem of lipid metabolism disorder, reduce liver index, and enhance superoxide dismutase in the liver , Glutathione peroxidase activity, reduce malondialdehyde content, improve liver oxidative stress state, maintain normal shape and structure of liver cells and tissues, reduce liver alanine aminotransferase and aspartate aminotransferase activity levels, and promote liver function repair, Therefore, it can be used to prepare medicines for treating diabetic liver damage. Epimedin C prepares a medicine for treating diabetic liver damage and uses it in the form of pharmaceutical preparations, including buccal tablets, coated tablets and hard capsules. The invention provides a basis for the treatment of diabetic liver damage, and also provides ideas for the deep development and utilization of epimedium resources.
Owner:陕西新修本草健康产业有限公司
Use of CD24 for lowering low-density lipoprotein cholesterol levels
InactiveUS10369197B2Reduction of ldl c levelReduce riskHybrid immunoglobulinsCell receptors/surface-antigens/surface-determinantsA lipoproteinCD24
The present invention relates to the use of a CD24 protein for lowering low-density lipoprotein cholesterol levels, treating and preventing atherosclerosis, and for reducing risk of cardiovascular disease.
Owner:ONCOIMMUNE INC
Proprotein convertase subtilisin kexin type 9 (PCSK9) allosteric binding ligands to modulate serum low density lipoprotein (LDL) levels
ActiveUS10287317B2Low level modulationIncreased and reduced level and levelPeptidesSerum low density lipoproteinLiver Dysfunctions
This invention is related to the field of hypercholesterolemia. In particular, the invention provides compositions and methods to modulate circulating levels of low density lipoproteins by altering the conformation of the protein PCSK9 using synthetic ligands and / or synthetic ligand derivative sequences of 3-8 amino acids ranging between 350-2,000 Da. Altering the conformation of PCSK9 affects the interaction between PCSK9 and an endogenous low density lipoprotein receptor, and can lead to reduced or increased levels of circulating LDL-cholesterol. High LDL-cholesterol levels are associated with increased risk for heart disease. Low LDL-cholesterol levels may be problematic in other conditions, such as liver dysfunction; thus, there is also utility for ligands which can raise LDL levels.
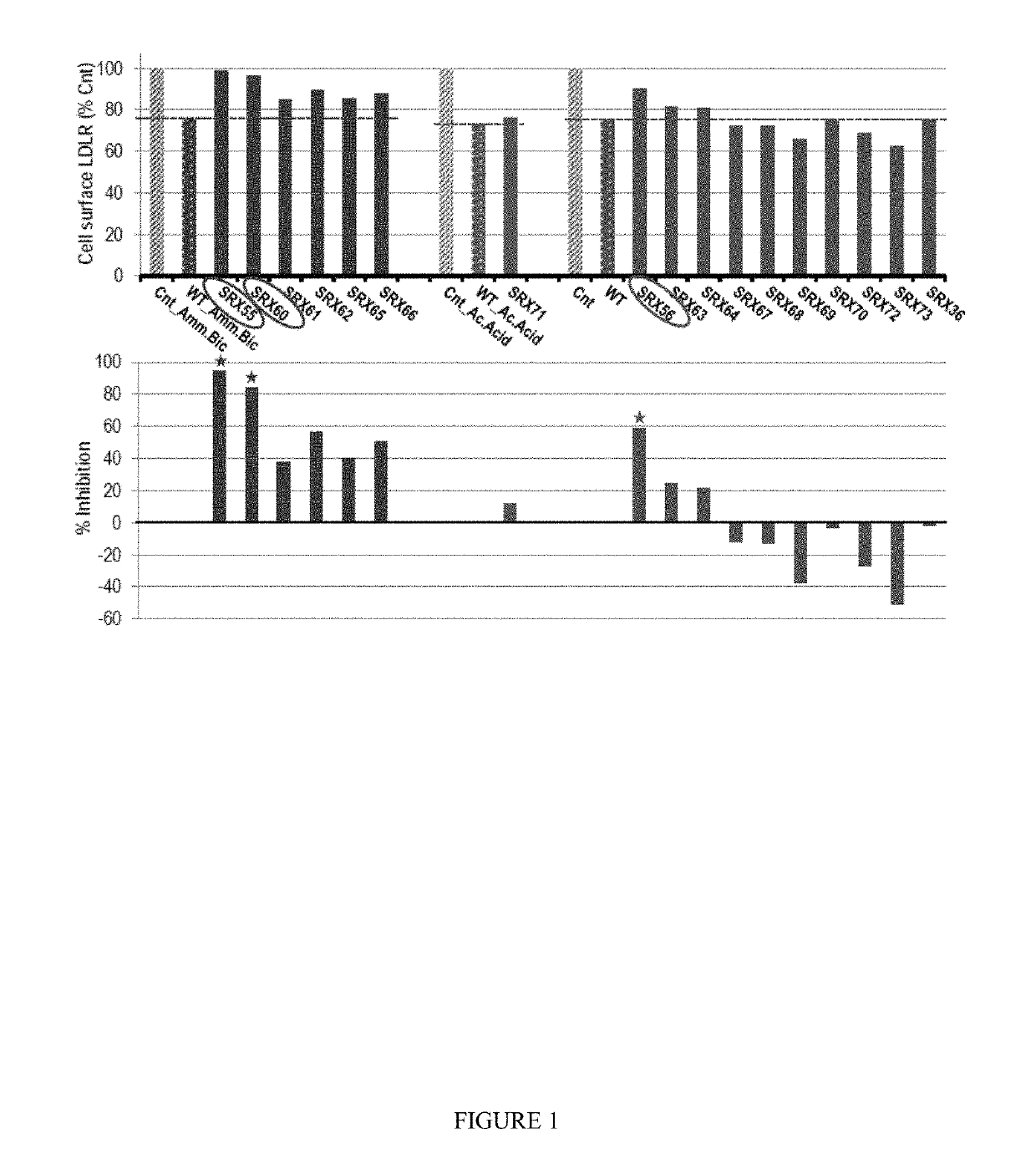
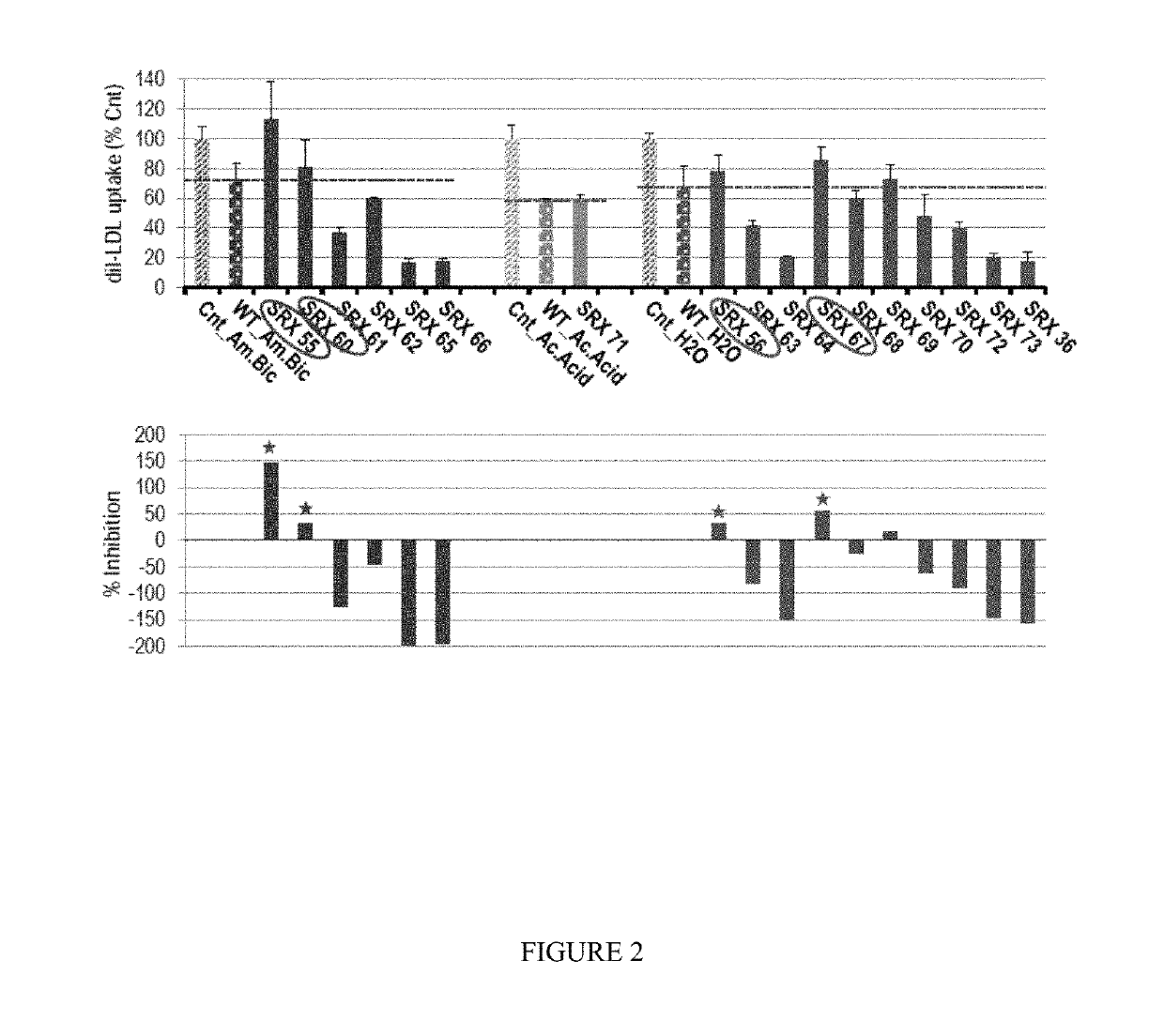
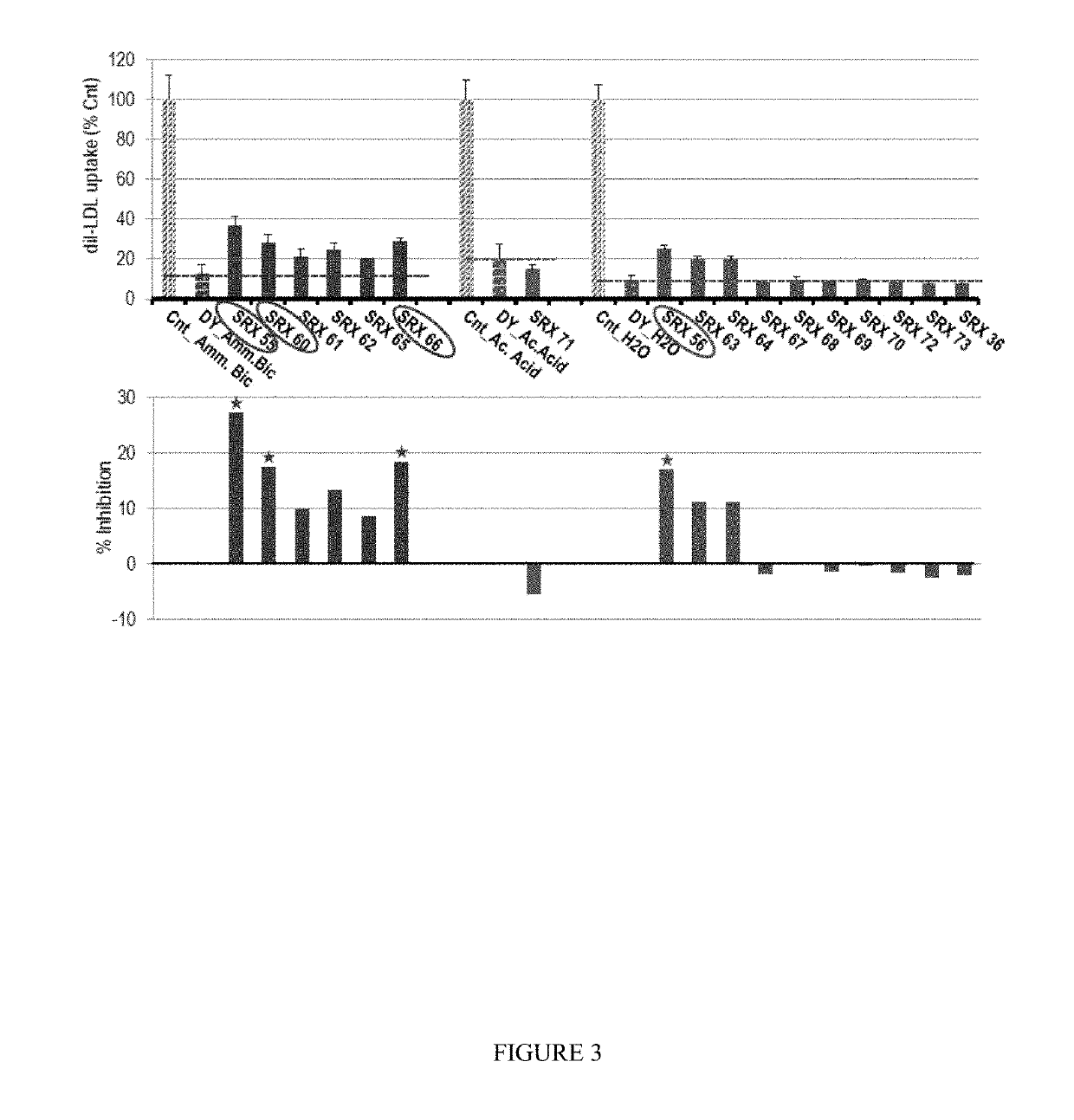
Owner:SRX CARDIO
Products and methods using soy peptides to lower total and ldl cholesterol levels
ActiveUS20140057846A1Lower cholesterol levelsLower Level RequirementsMetabolism disorderPharmaceutical delivery mechanismLunasinLower total cholesterol
Controlled studies demonstrate that products and related methods using soy related peptides lower total and LDL cholesterol levels in individuals. In one exemplary embodiment of the present disclosure, a product containing an effective amount of lunasin peptides that lowers cholesterol levels in an individual that consumes the lunasin peptides is provided. In another exemplary embodiment of the present disclosure, a composition containing an effective amount of lunasin peptides or lunasin peptide derivatives and one or more enzyme inhibitors is provided. In a related exemplary embodiment of the present disclosure, a method for lowering or reducing cholesterol levels in an individual is provided where a product containing an effective amount of lunasin peptides to an individual is provided and a claim that the product lowers or reduces cholesterol, total cholesterol, LDL cholesterol or lipid levels in an individual that consumes the composition is made.
Owner:S L TECH
Applications of eleven-component golden pills in preparation of medicine treating hyperlipemia
ActiveCN105213539ASignificant improvementGood treatment effectMetabolism disorderPlant ingredientsLow density lipoprotein cholesterolSecondary hyperlipidemia
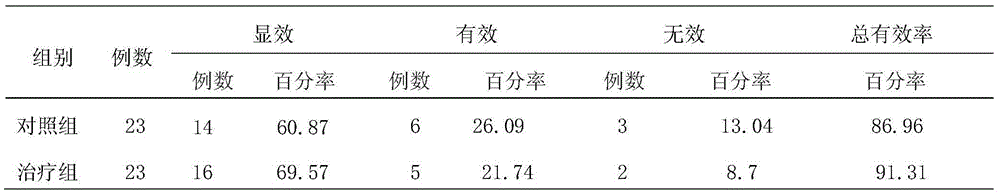
The invention discloses applications of eleven-component golden pills in preparation of a medicine treating hyperlipemia. Animal experiments prove that eleven-component golden pills have substantial treatment effects on experiment mouse hyperlipidemia models, cholesterol, triglyceride and low-density lipoprotein cholesterol levels in blood plasma can be lowered substantially (P is less than 0.01), and lowering effects on part of blood lipid indexes in a high-dosage group is even better than effects of a control medicine. Clinic experiments show that the total clinic effective rate of a treatment group is 91.31%, blood lipid indexes of TC, TG, LDL-C and the like are lowered substantially, HDL-C is raised substantially, and the treatment group is better than the control group in improvement of TC (P is less than 0.05), which show that there is no difference between the eleven-component golden pills and a conventional lipid lowering medicine simvastatin tablets in improvement effects on blood lipid indexes of TG, LDL-C, HDL-C and the like, but the eleven-component golden pills are better than simvastatin tablets in improvement of TC.
Owner:SHANDONG JINHE DRUG RES DEV
Phenylpiperazine proprotein convertase subtilisin/kexin type 9 (PCSK9) modulators and their use
ActiveUS10568882B2Reduced and increased level of LDL-cholesterolHigh LDL-cholesterol levelOrganic active ingredientsMetabolism disorderPhenylpiperazineLiver Dysfunctions
This invention is related to the field of PCSK9 biology and the composition and methods of use of small organic compounds as ligands for modulation of PCSK9 biological activity. In particular, the invention provides compositions of small organic compounds that modulate circulating levels of low density lipoproteins by altering the conformation of the protein PCSK9. Binding these small organic compound ligands to PCSK9 alters the conformation of the protein, modifying the interaction between PCSK9 and an endogenous low density lipoprotein receptor, and can lead to reduced or increased levels of circulating LDL-cholesterol. High LDL-cholesterol levels are associated with increased risk for heart disease. Low LDL-cholesterol levels may be problematic in other conditions, such as liver dysfunction; thus, there is also utility for small organic compound ligands that can raise LDL levels.
Owner:SRX CARDIO
Functional genetic variation of genes related to ldl-c level and related applications
ActiveCN107502667BHelps prevent hyperlipidemiaMicrobiological testing/measurementDisease diagnosisDyslipidemiaA lipoprotein
The present invention provides functional genetic variation of genes related to LDL-C levels and related applications; specifically, the present invention provides detection of the following protein amino acid sites and / or their corresponding gene sites in a sample from an individual to be tested The application of reagent materials and / or instruments and equipment in the preparation of a detection system for evaluating blood lipid levels, dyslipidemia and / or risk of coronary heart disease: EV15 gene rs117711462, APOB gene rs376825639 and / or PKD1L3 gene rs17358402 respectively cause EVI5 protein Amino acid 354, amino acid 3768 of APOB protein, and / or amino acid 1572 of PKD1L3 protein code change. Individuals with EVI5Arg354Cys and / or PKD1L3Arg1572His mutations have higher total cholesterol and / or low-density lipoprotein cholesterol levels and / or higher risk of dyslipidemia. Individuals with APOBIle3768Thr mutation have lower low-density lipoprotein cholesterol level, lower risk of dyslipidemia and / or lower risk of coronary heart disease.
Owner:FUWAI HOSPITAL CHINESE ACAD OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE +2
Application of pigeon pea ketonic acid A in terms of preparation of medicines for accompanied diseases of diabetes mellitus and hyperlipidaemia
ActiveCN102670576BLower cholesterol levelsNormal blood sugarOrganic active ingredientsMetabolism disorderDiseaseKetonic acids
Application of the pigeon pea ketonic acid A and a pharmaceutically acceptable derivative thereof in preparation of a medicine for a diabetes-accompanying disease or hyperlipidemia as well as a functional health product. The diabetes-accompanying disease is hyperlipidemia, liver damage, kidney damage, or pancreas damage accompanying diabetes.
Owner:GUANGZHOU YUNZHONG BIOTECH
Diaphragma juglandis phenol extract and application thereof in preparation of medicine for preventing abnormal glucose and lipid metabolism
PendingCN114366765ASuppresses serum total triglyceridesReduce damageMetabolism disorderAnimal feeding stuffA lipoproteinFeed additive
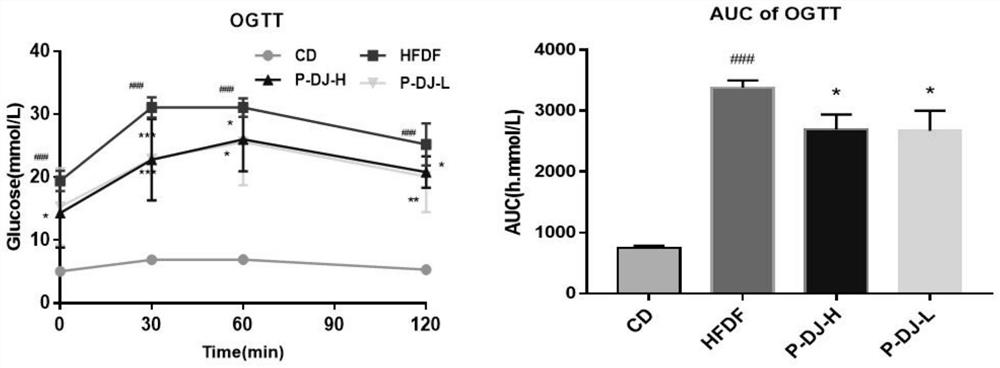
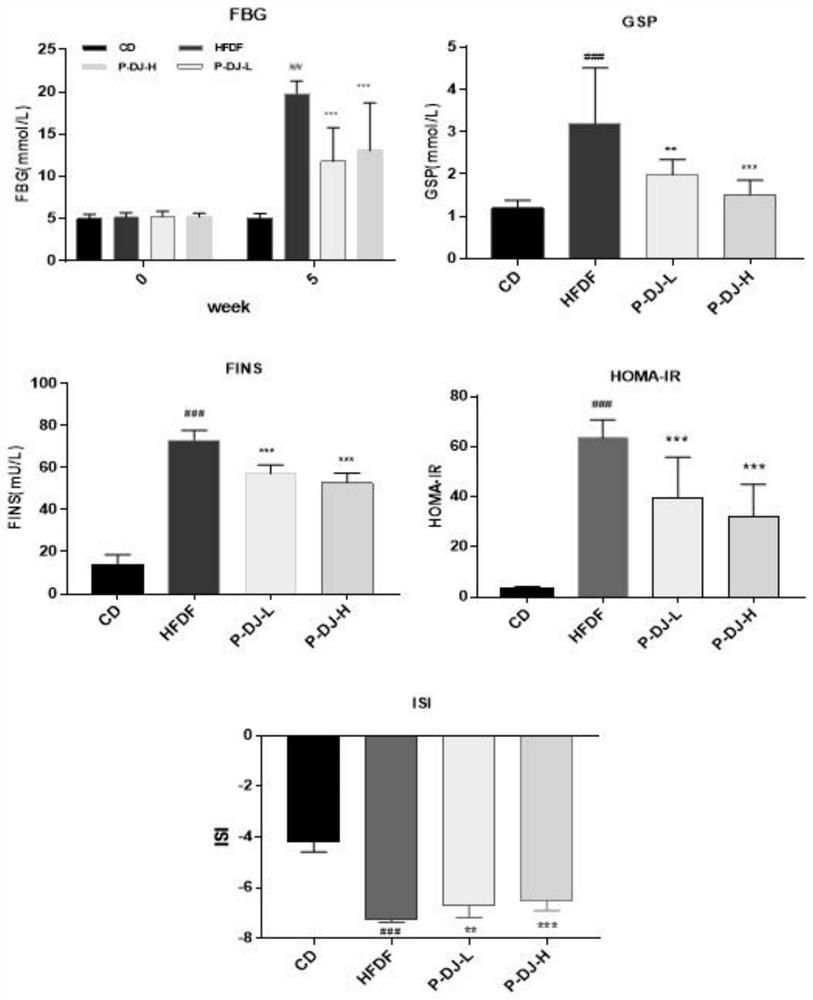
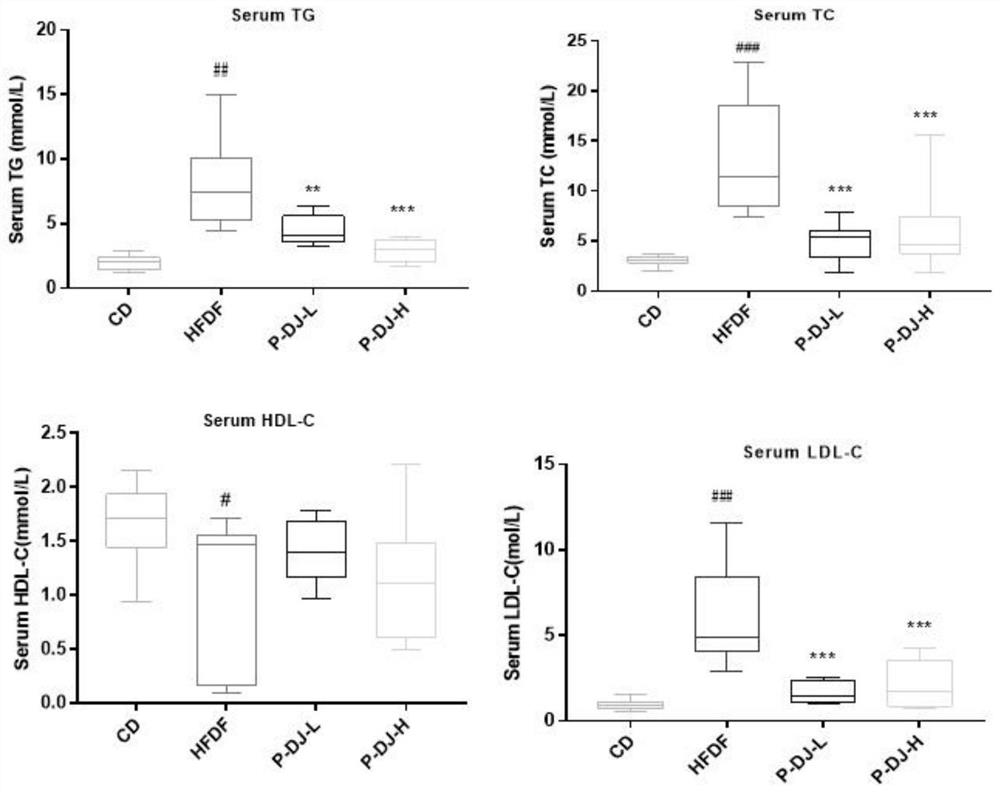
The invention discloses a walnut diaphragma juglandis phenolic extract, which is characterized in that the content of flavone in the phenolic extract is 64.5-66.1%, the walnut diaphragma juglandis phenolic extract has the effect of preventing abnormal glucose and lipid metabolism, and the phenolic extract is obtained by pretreatment, extraction, separation, concentration, drying and determination. The walnut diaphragma juglandis phenol low-dose extract and the walnut diaphragma juglandis phenol high-dose extract can remarkably inhibit impairment of oral glucose tolerance of a model animal with abnormal glucose and lipid metabolism, remarkably reduce the damage degree of OGTT, remarkably inhibit increase of AUC, glycated serum protein GSP, fasting blood glucose FBG, fasting insulin FINS, insulin resistance index HOMA-IR and insulin sensitivity index ISI, and can be used for preparing the medicine for treating the abnormal glucose and lipid metabolism. The rise of serum total triglyceride TG, total cholesterol TC and low-density lipoprotein cholesterol LDL-C level and the reduction of the low-density lipoprotein cholesterol HDL-C level of a model animal with abnormal glucose and lipid metabolism are obviously inhibited. The walnut diaphragma juglandis phenol extract is high in flavone content and can be used as a food or feed additive and a human or animal medicine raw material.
Owner:YUNNAN UNIV OF TRADITIONAL CHINESE MEDICINE
Composition and methods of use of tetrahydroisoquinoline small molecules to bind and modulate pcsk9 protein activity
ActiveUS20200207718A1Reduced and increased level of LDL-cholesterolHigh LDL-cholesterol levelOrganic chemistryMetabolism disorderA lipoproteinReceptor
This invention is related to the field of PCSK9 biology and the composition and methods of use of small organic compounds as ligands for modulation of PCSK9 biological activity. In particular, the invention provides compositions of small organic compounds that modulate circulating levels of low density lipoproteins by altering the conformation of the protein PCSK9. Binding these small organic compound ligands to PCSK9 alters the conformation of the protein, modifying the interaction between PCSK9 and an endogenous low density lipoprotein receptor, and can lead to reduced or increased levels of circulating LDL-cholesterol. High LDL-cholesterol levels are associated with increased risk for heart disease. Low LDL-cholesterol levels may be problematic in other conditions, such as liver dysfunction; thus, there is also utility for small organic compound ligands that can raise LDL levels.
Owner:SRX CARDIO
Methods and compositions for treating arteriosclerotic vascular diseases
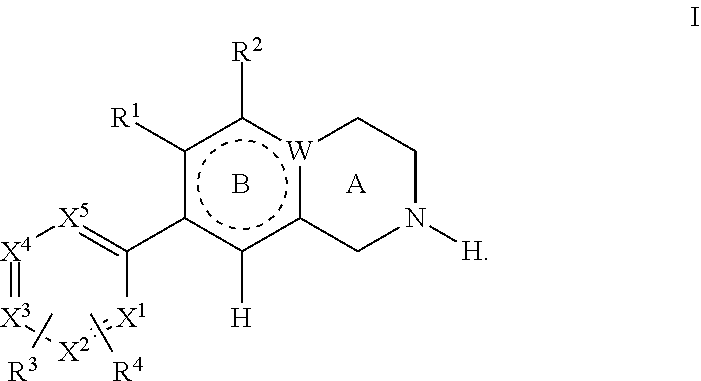
ActiveUS10905656B2Organic active ingredientsOrganic chemistryLow density lipoprotein cholesterolCyclohexenone
The present invention provides methods and compositions for treating arteriosclerotic vascular diseases by cyclohexenone compounds. In some embodiments, the compound in the methods inhibits PDGF-stimulated smooth muscle cell proliferation or migration. In some embodiments, the atherosclerosis is associated with coronary artery disease, aneurysm, arteriosclerosis, myocardial infarction, embolism, stroke, thrombosis, angina, vascular plaque inflammation, vascular plaque rupture, Kawasaki disease, calcification or inflammation. In some embodiments, the compound lowers low-density lipoprotein (LDL) cholesterol in the subject. In some embodiments, the compound maintains a normal low-density lipoprotein (LDL) cholesterol level in the subject.
Owner:GOLDEN BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com