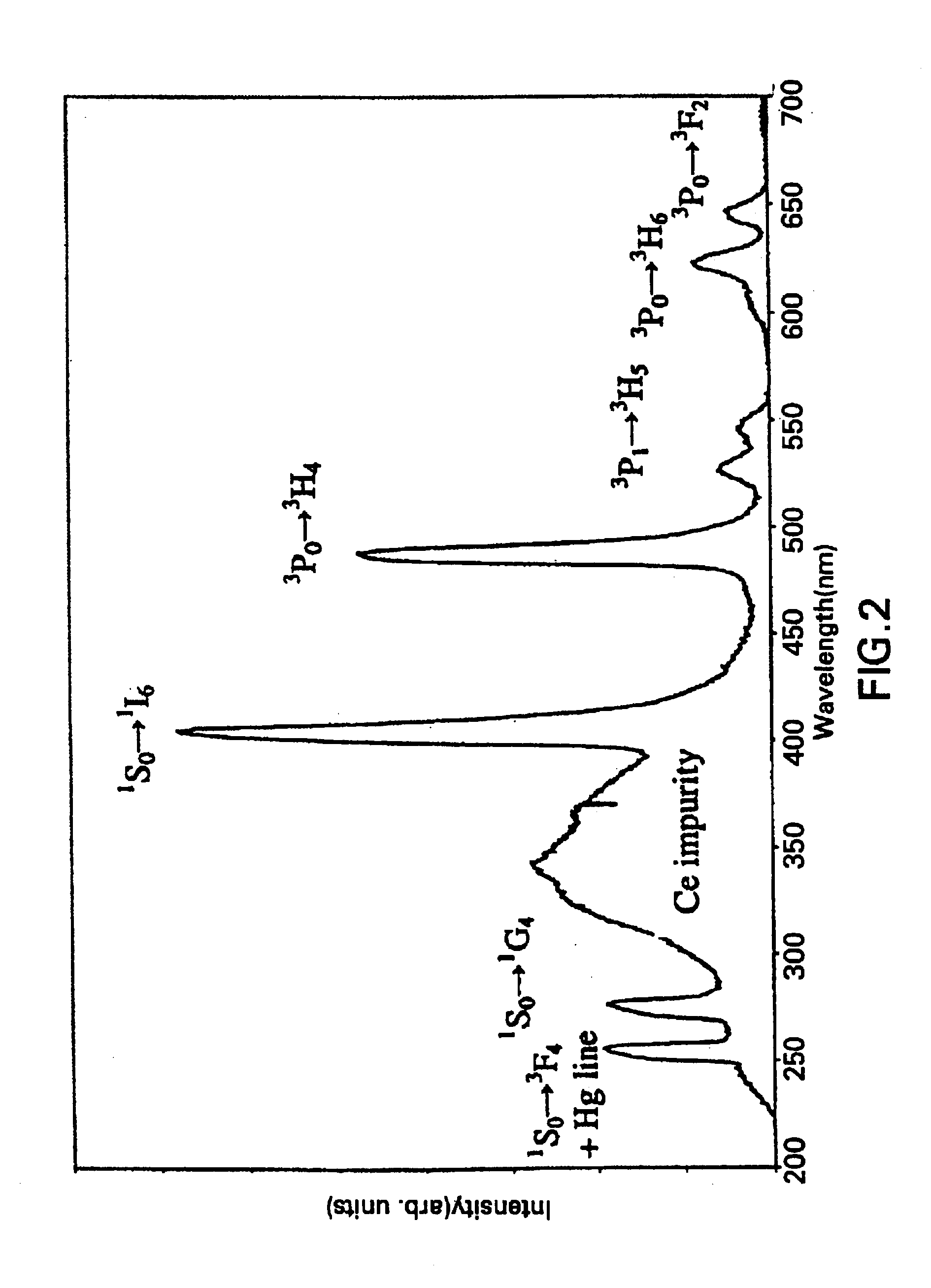
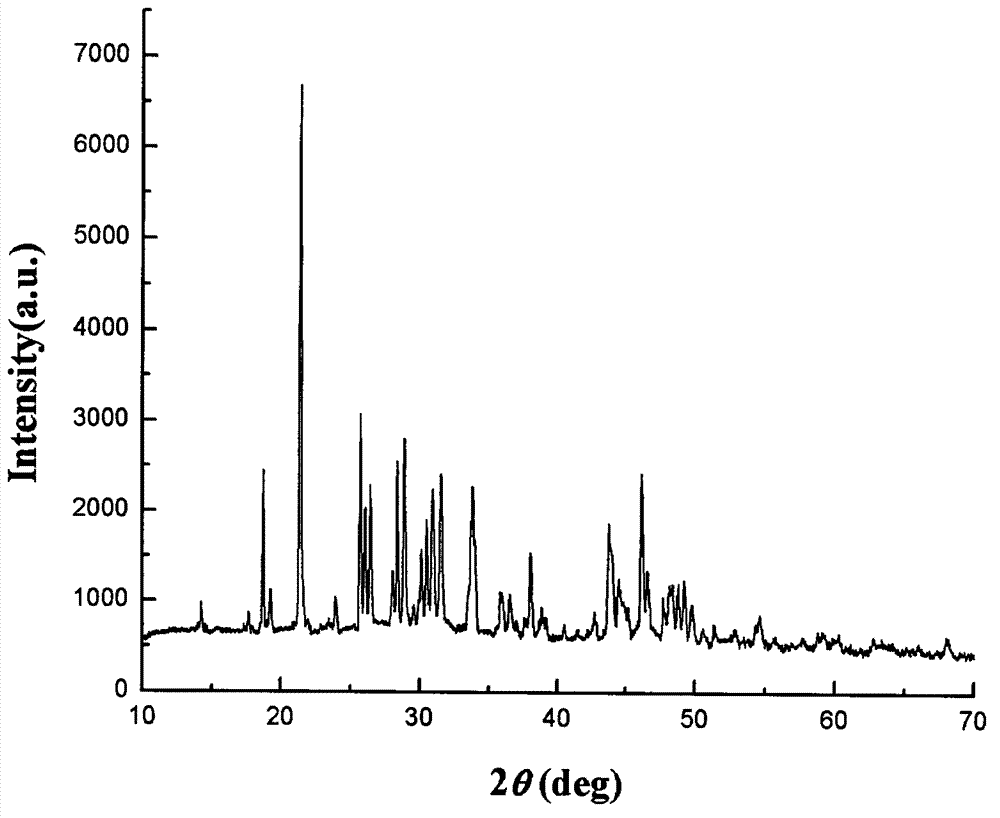
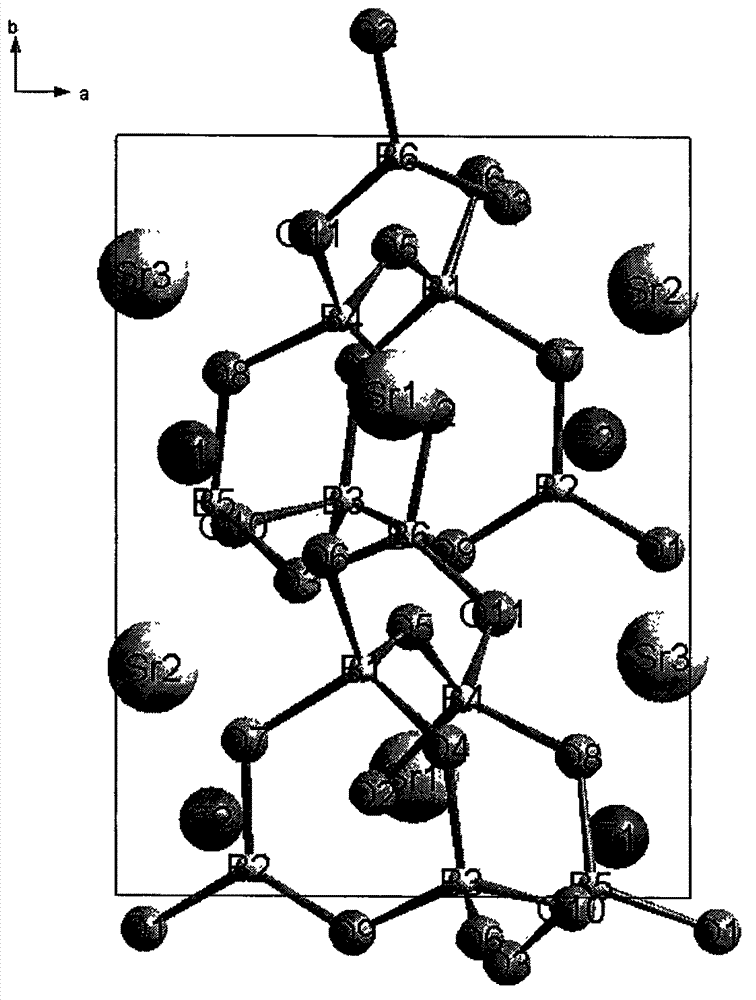
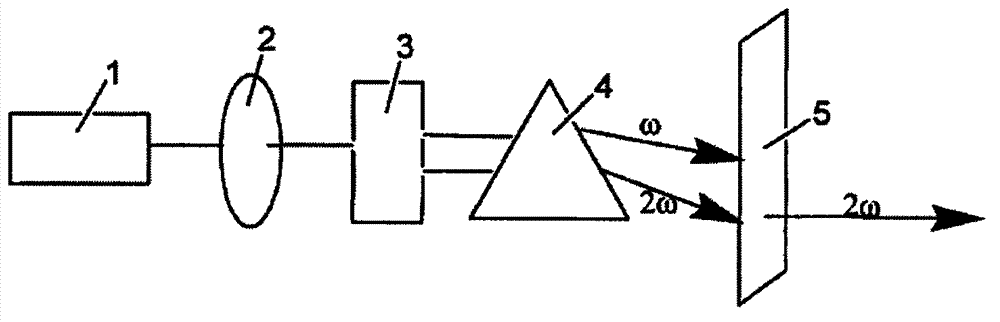
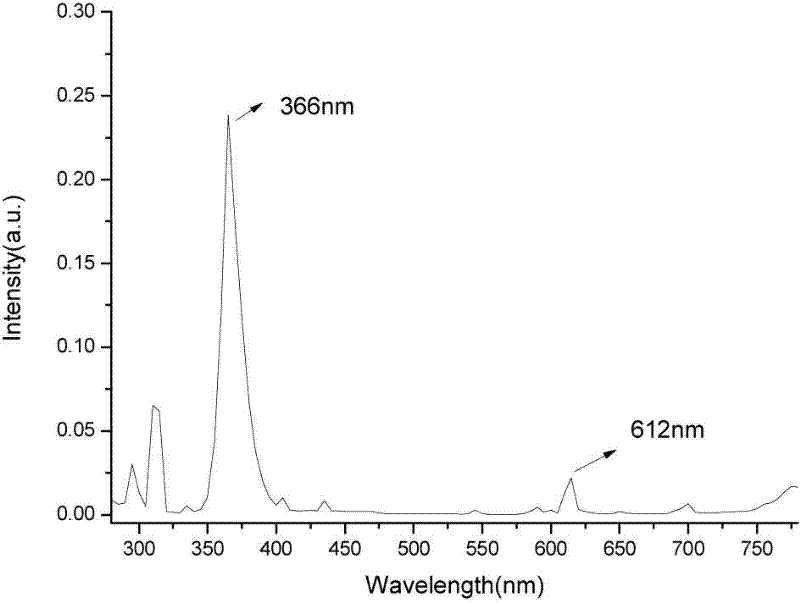
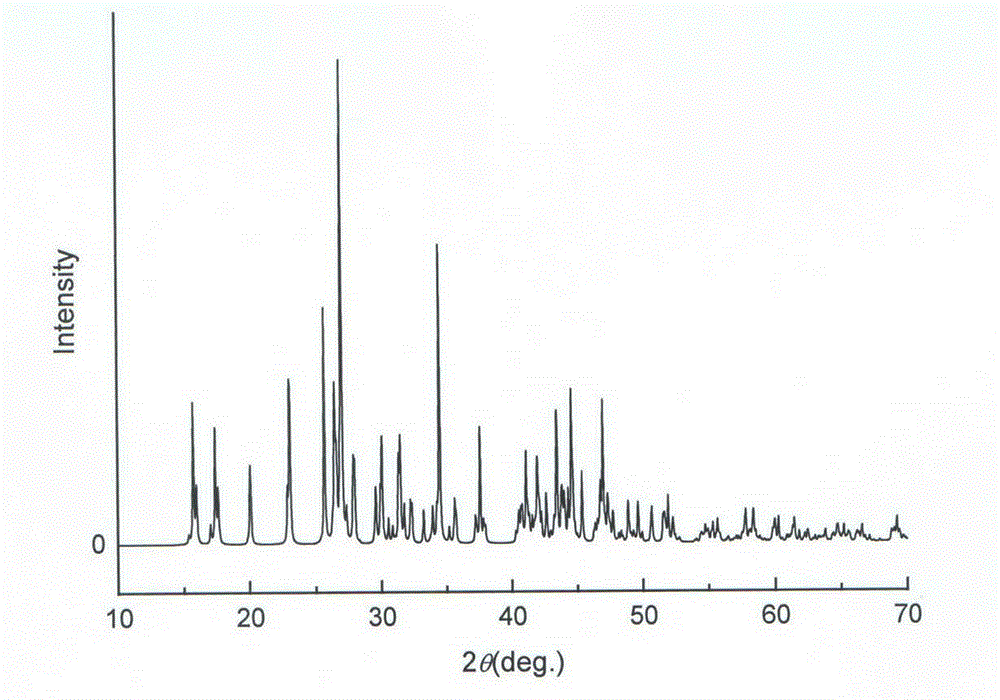
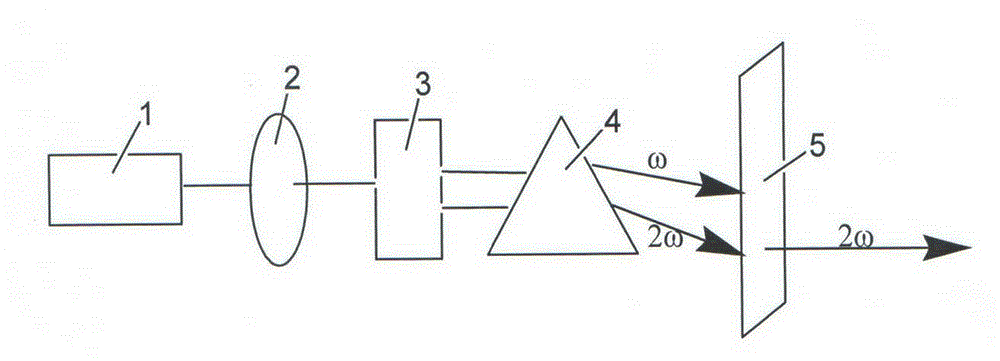
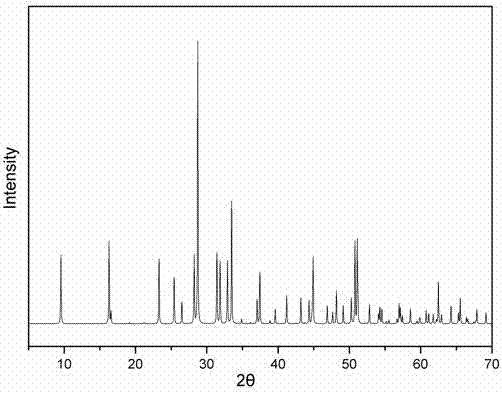
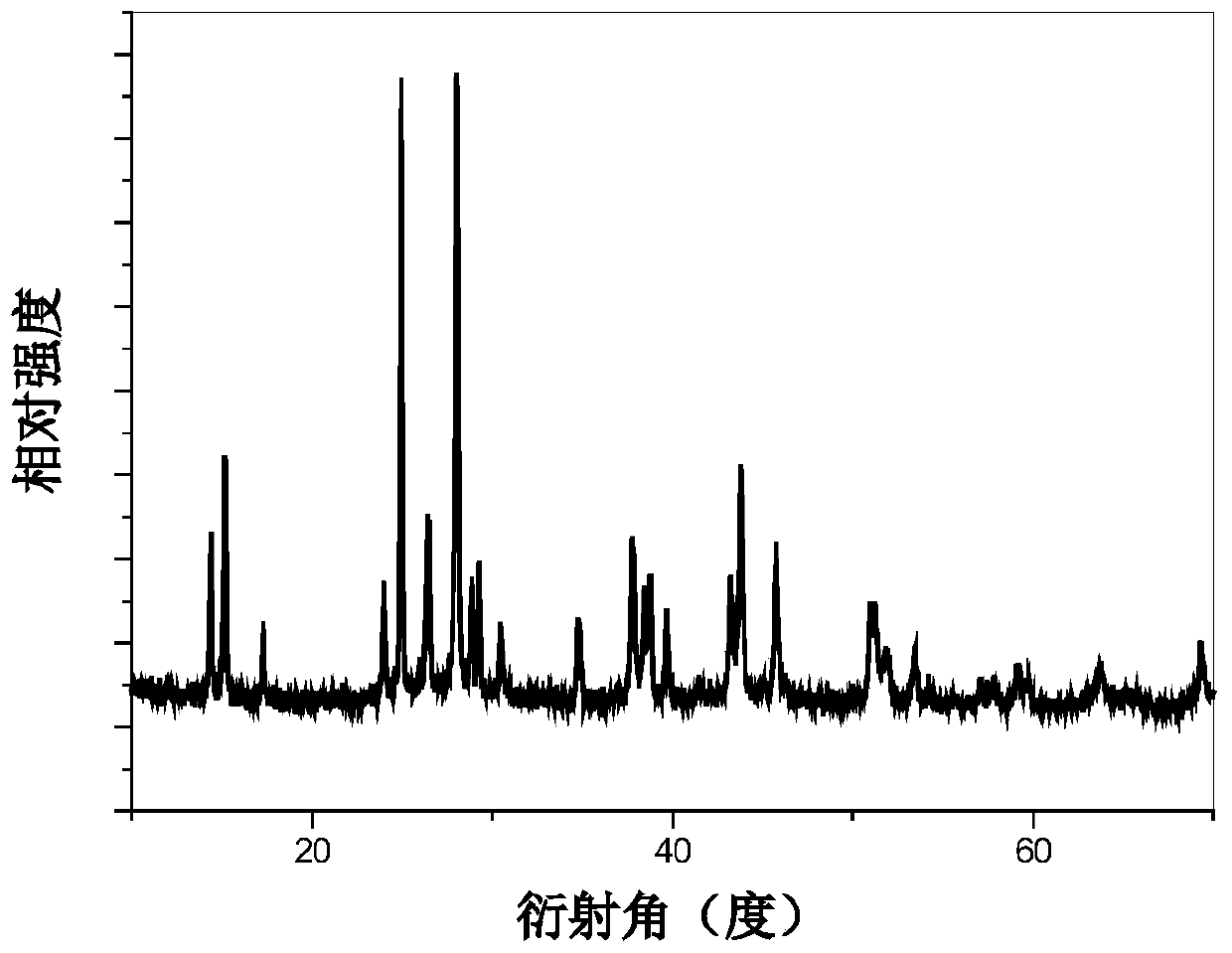
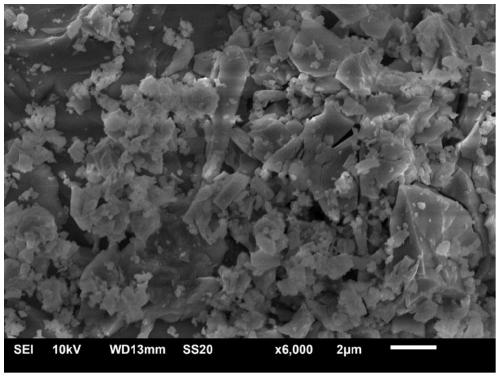
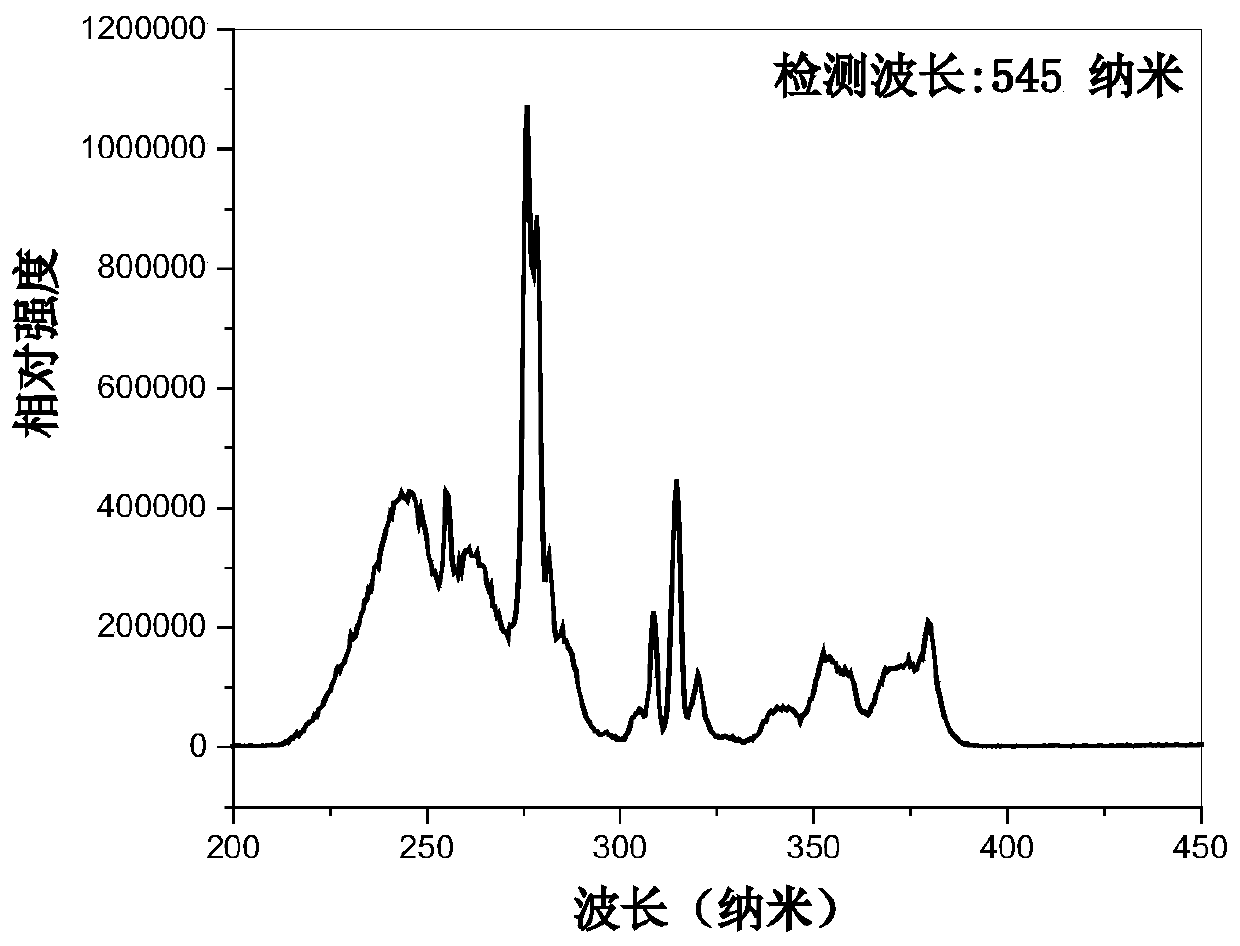
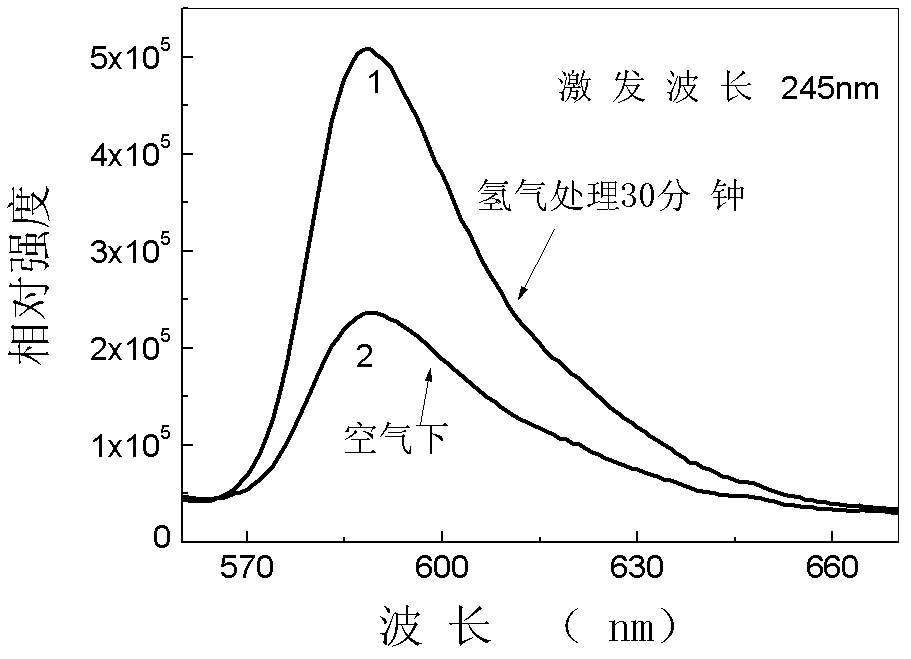
Patents
Literature
50 results about "Strontium borate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Large size potassium strontium borate nonlinear optical crystal, preparation and use thereof
InactiveCN101514492AOptical processing accuracy without special requirementsPolycrystalline material growthFrom frozen solutionsNonlinear optical crystalSpace group
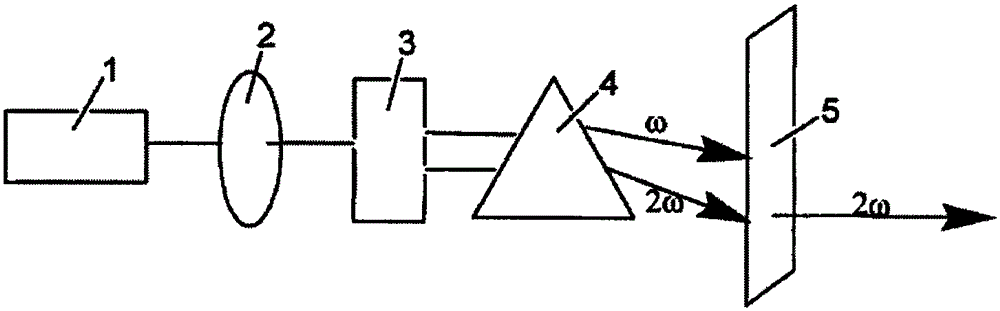
The invention relates to a large size potassium strontium borate nonlinear optical crystal, the preparation and use thereof. The formula of the crystal is: KSr4B3O9, which belongs to rhombic system, the space group is Pna2(1), and the molecular weight is 566.01, and the crystal size is 10-60mmx10-60mmx10-60mm. The preparation contains the following steps of: evenly mixing the potassium strontium borate compound with a fusing assistant, heating, maintaining the constant temperature, cooling to the saturation temperature and obtaining a mixture solution, placing a seed crystal into the mixture solution, lowering the temperature to the saturation temperature to obtain the required crystal, and subsequently extracting the crystal from the liquid level, cooling to room temperature, and finally obtaining the large size potassium strontium borate nonlinear optical crystal. The nonlinear optical effect of the crystal is approximately the same as the KDP, the transparent optical band is 220nm-3000nm. The crystal is simple in operation, low in cost, large in crystal size, short in growth period, less in coating, high in laster damage threshold, good in mechanical property, firm, stable in physicochemical properties, uneasy to deliquescence, convenient for processing and storage, or the like. Accordingly, the nonlinear optical crystal of the invention can be abroadly applied in nonlinear optical devices such as frequency doubler, optical parametric oscillator or the like.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Quantum-splitting oxide-based phosphors and method of producing the same
InactiveUS6613248B2Energy efficiencyDischarge tube luminescnet screensLamp detailsAluminateCalcium magnesium
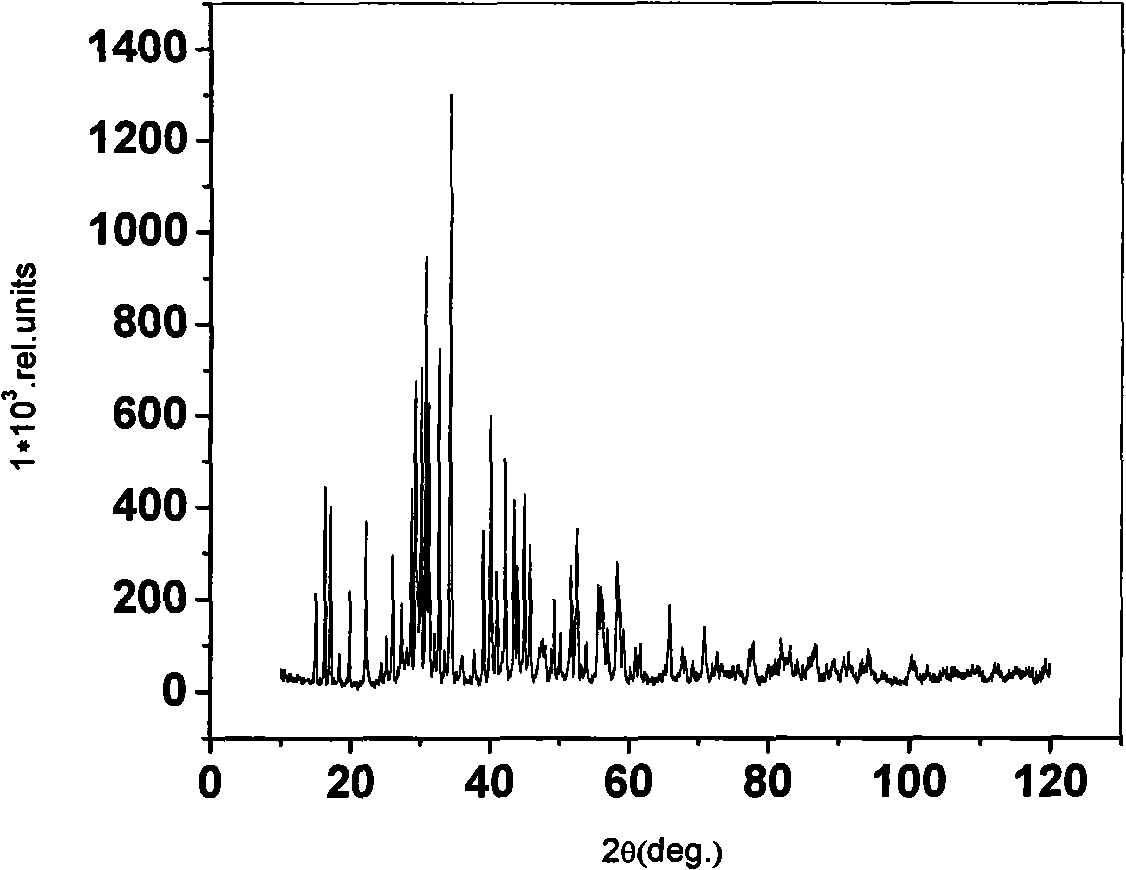
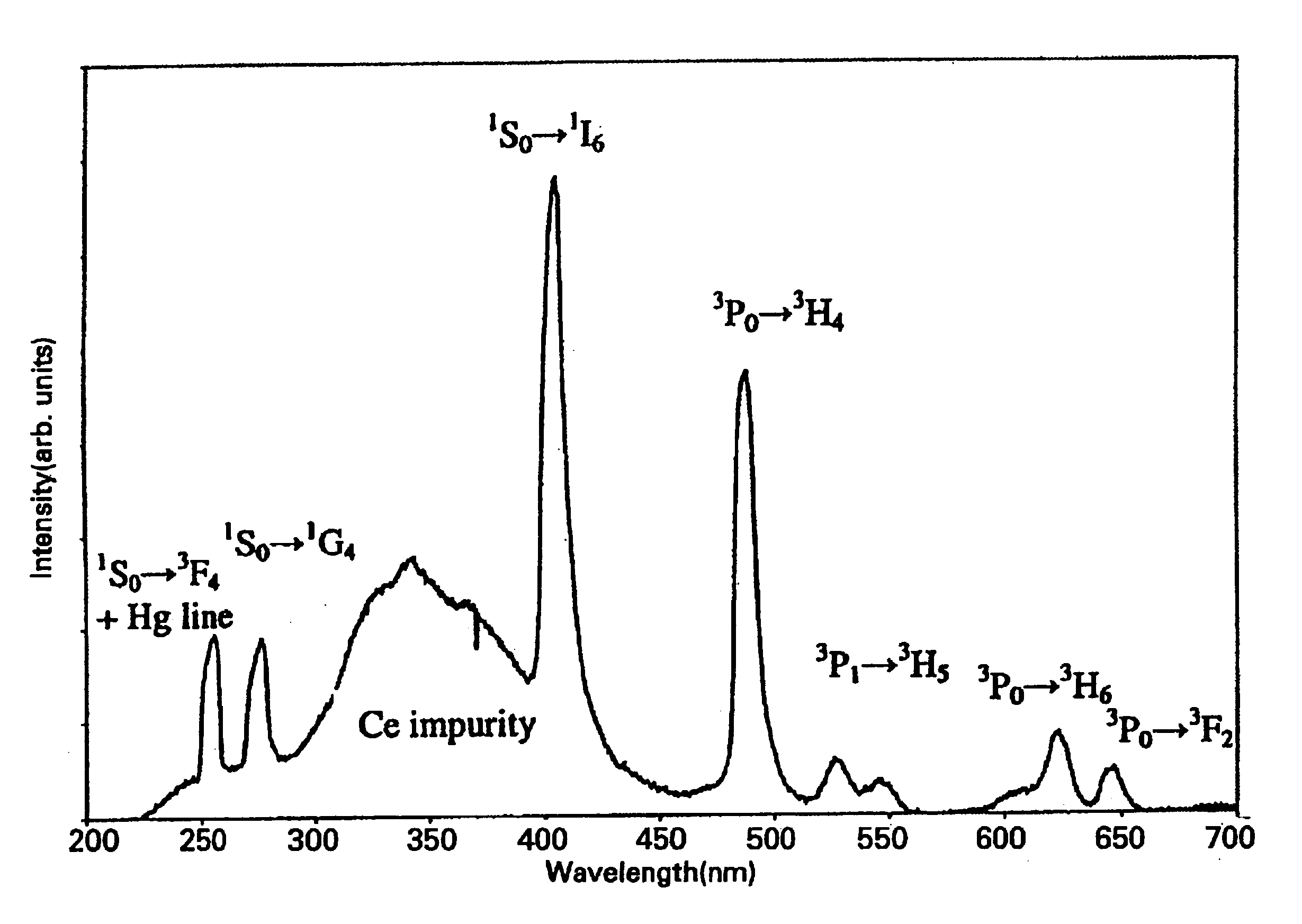
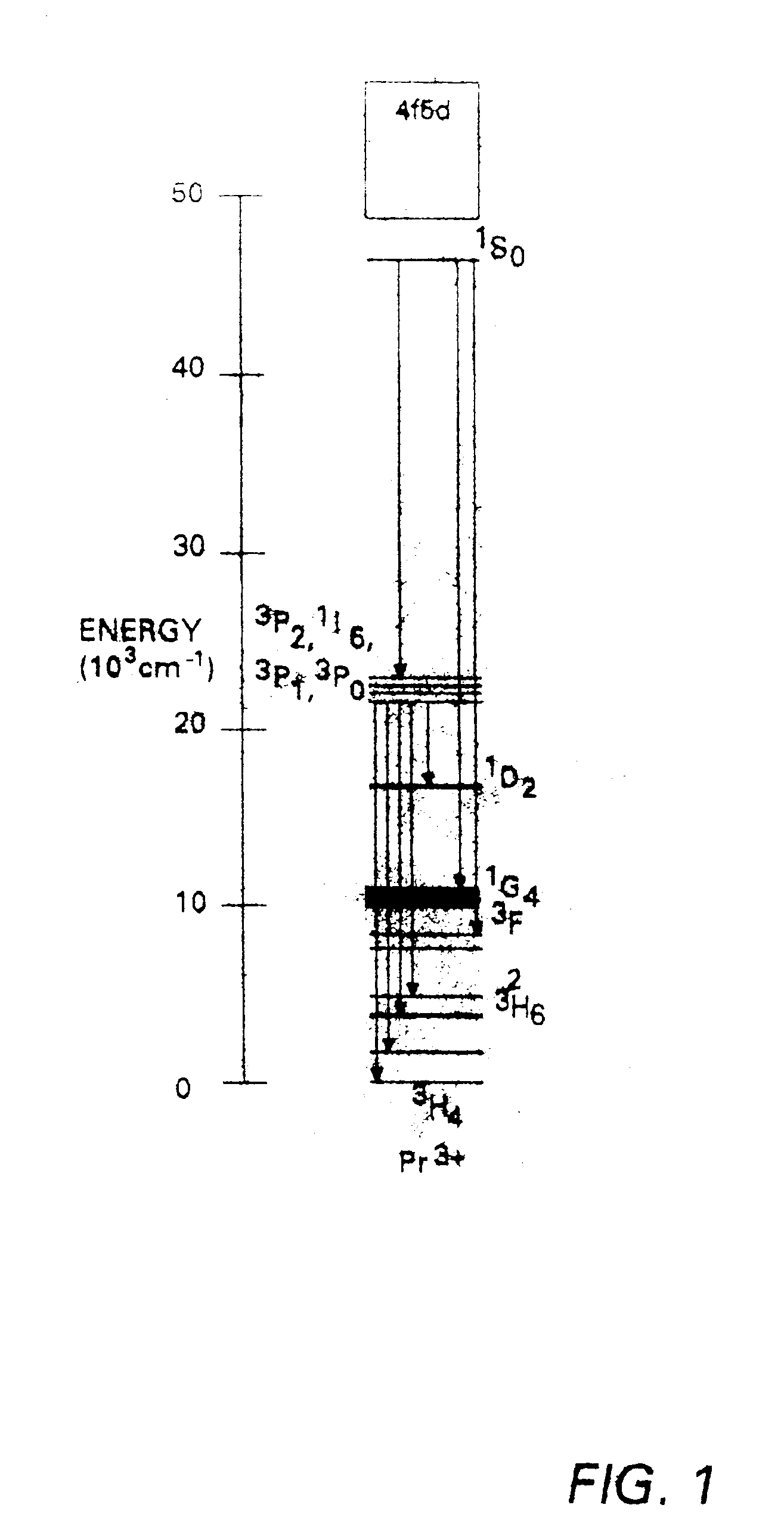
Strontium, calcium, strontium calcium, strontium calcium magnesium, calcium magnesium aluminates, and strontium borates activated with Pr3+ exhibit characteristics of quantum-splitting phosphors under VUV excitation. A large emission peak at about 405 nm under VUV excitation is used conveniently to identify quantum-splitting phosphors. Improvements may be achieved with addition of fluorides or boric acid as a flux during the preparation of the phosphors. It is also possible to predict improvement in quantum efficiency by observing the ratio of emission intensities at about 480 nm and about 610 nm.
Owner:GENERAL ELECTRIC CO
Preparation method of strontium fluoroborate nonlinear optical crystals, and applications of strontium fluoroborate nonlinear optical crystals
ActiveCN103590106APolycrystalline material growthFrom melt solutionsNonlinear optical crystalFlux growth
The invention relates to a preparation method of strontium fluoroborate nonlinear optical crystals, and applications of the strontium fluoroborate nonlinear optical crystals. Compound synthesis is realized via solid phase reaction, and crystal growth is realized via flux growth method. The preparation method is simple in operation, and low in cost. The obtained strontium fluoroborate nonlinear optical crystals will not deliquesce in the air, possesses excellent mechanical properties and stable physico-chemical properties, is unbreakable, is convenient for processing, and is suitable for preparation of nonlinear optical devices. Frequency-doubled effect of the strontium fluoroborate nonlinear optical crystals obtained by the preparation method is three times of that of KDP, and the strontium fluoroborate nonlinear optical crystals can be widely used in nonlinear optical devices such as double frequency converters and optical parametric oscillators.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Novel high-efficiency strontium borate-like photocatalyst
ActiveCN101879443AHydrocarbon from carbon oxidesMetal/metal-oxides/metal-hydroxide catalystsPtru catalystStrontium borate
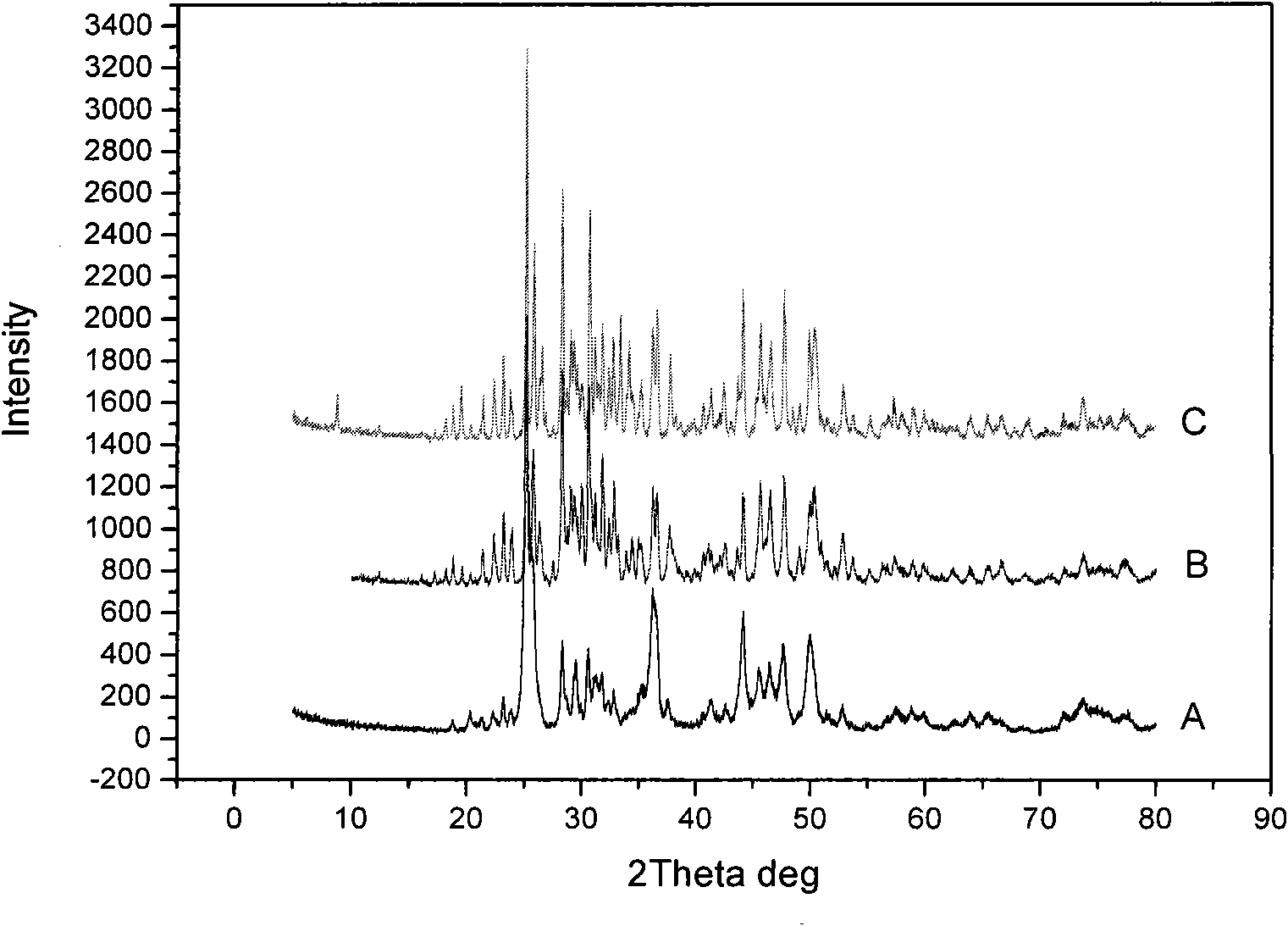
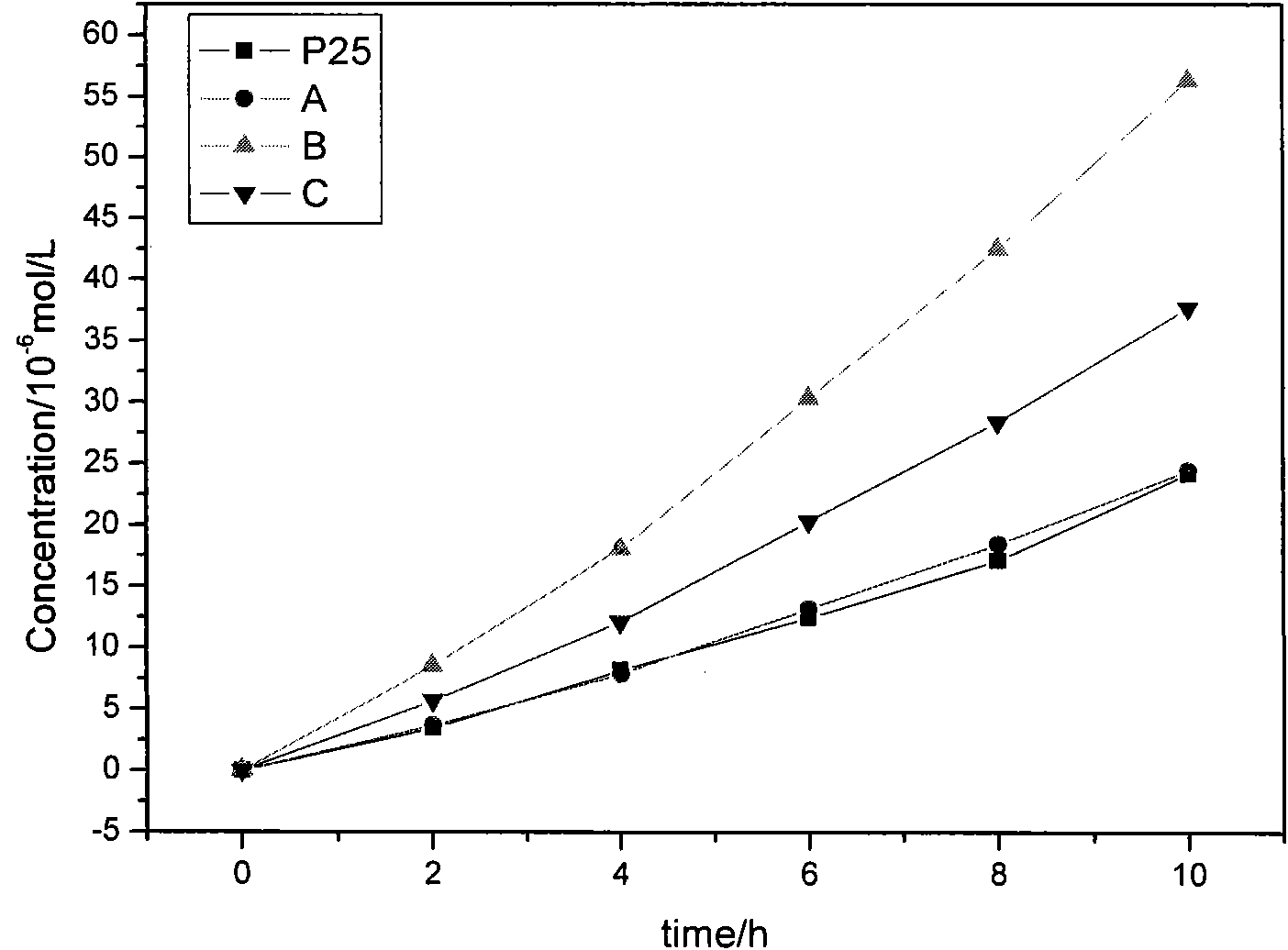
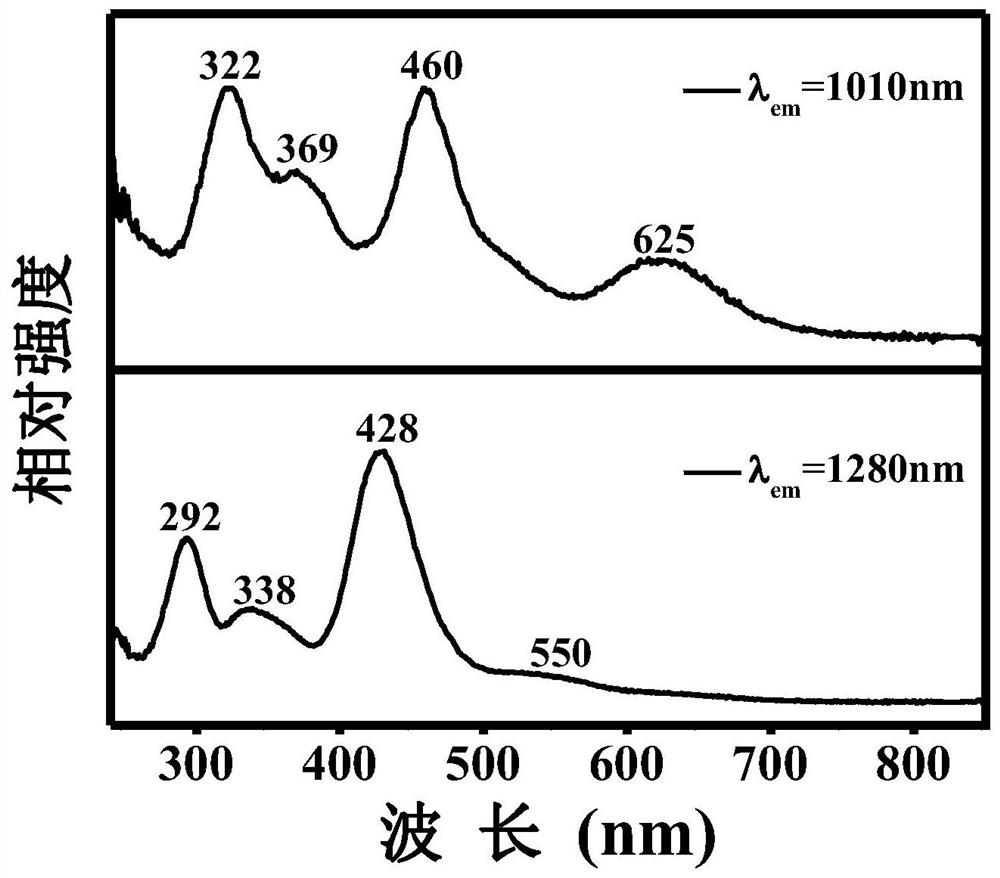
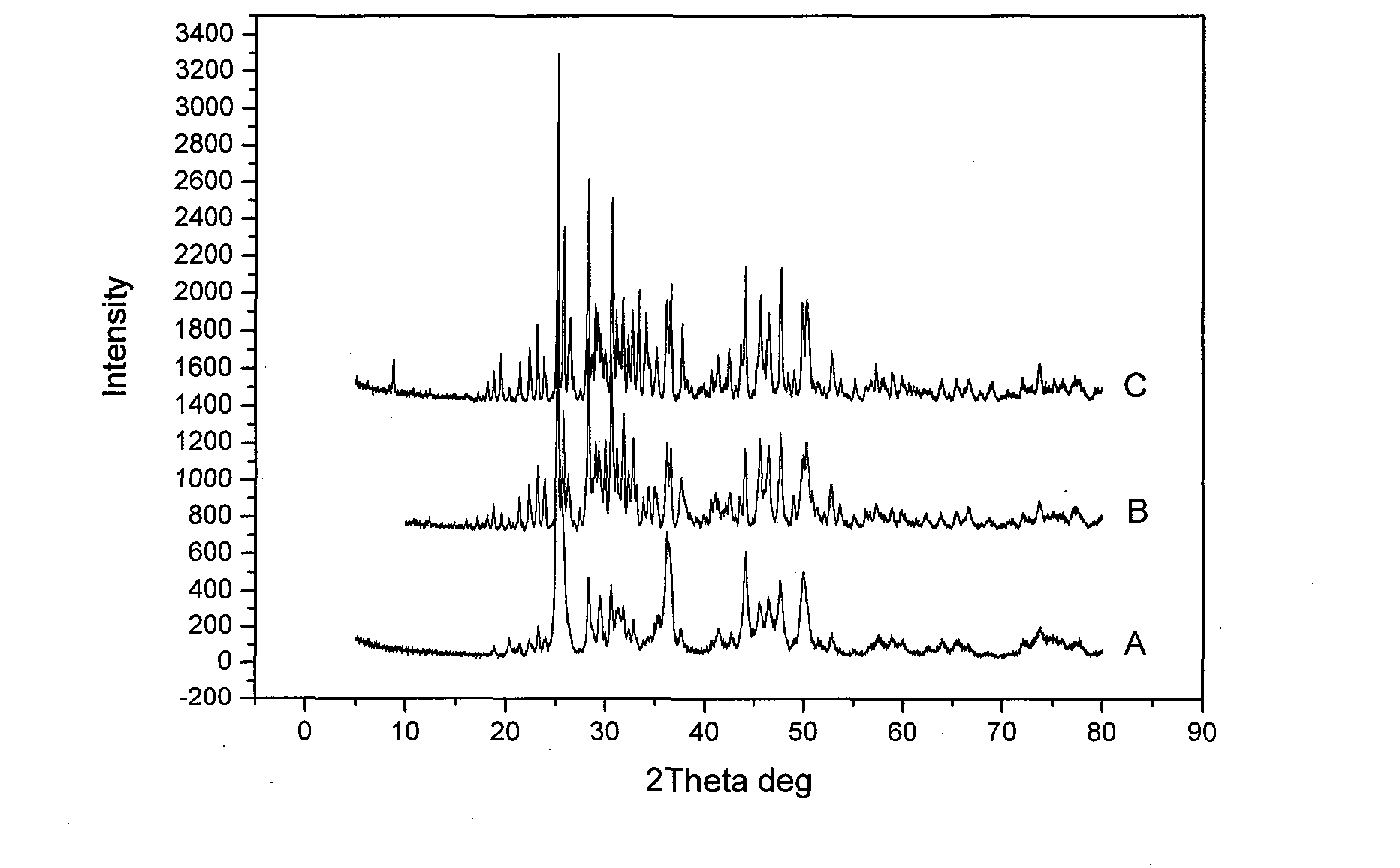
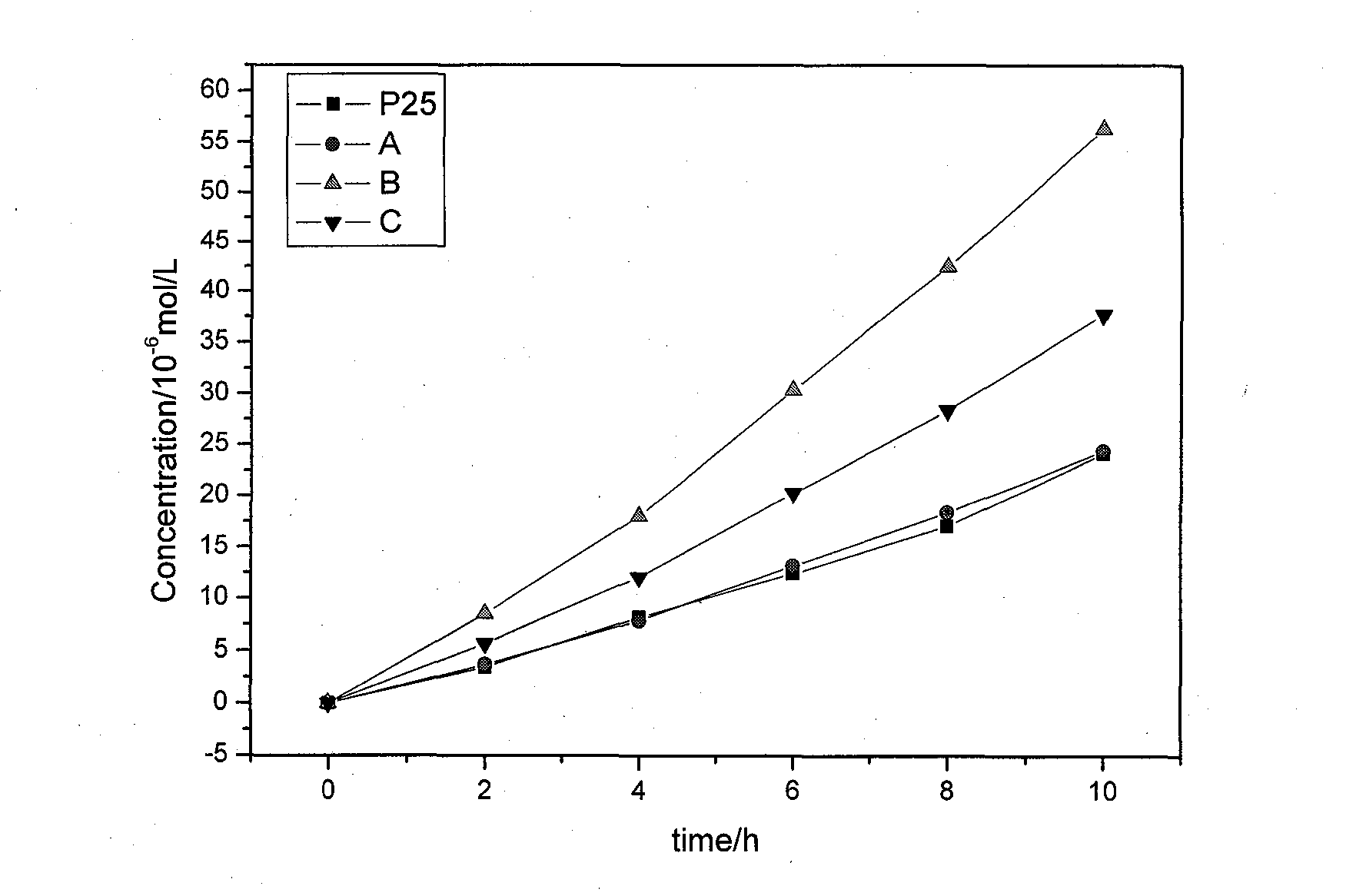
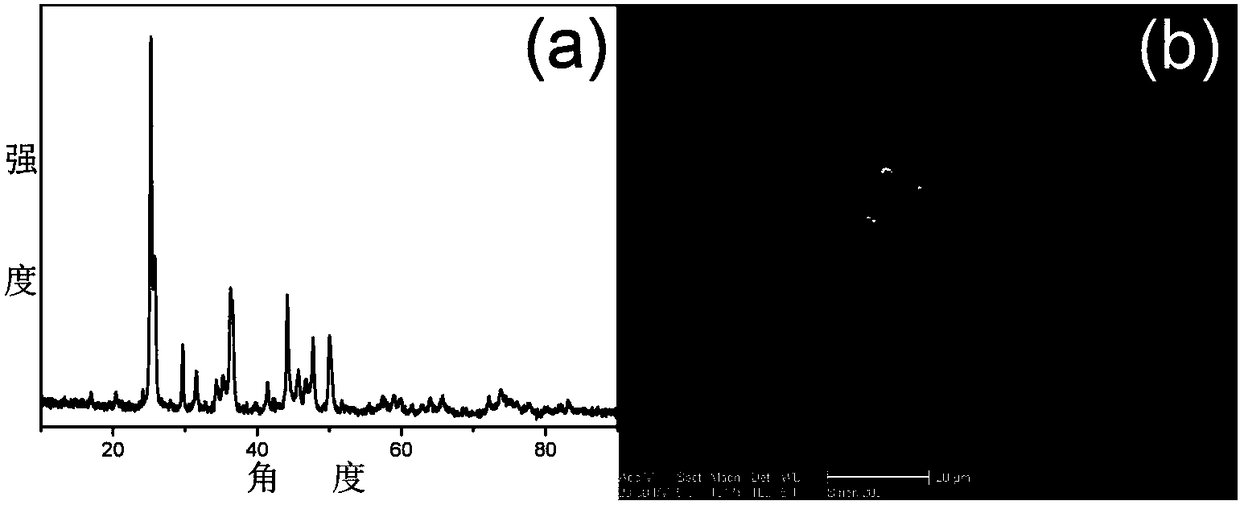
The invention discloses a high-efficiency strontium borate-like photocatalyst and a preparation method thereof and aims to prepare methane by reducing carbon dioxide and water through photocatalysis technology. In the photocatalyst, strontium salt, a boron-containing compound, citric acid or citrate and alcohols are used as precursors. The photocatalysis efficiency of reducing the carbon dioxide and water to prepare the methane of the high-activity strontium borate-like photocatalyst prepared by a sol-gel method is superior to that of a TiO2 photocatalyst (P25, which is known as the photocatalyst with higher photocatalysis efficiency at present). The novel high-efficiency strontium borate-like photocatalyst has the advantages of simple preparation process, easy operation, low requirements on equipment and the like and has wide application prospect and development potential in the fields of emission reduction of carbon dioxide, recycling of energy, environmental science and the like.
Owner:NANKAI UNIV
Method for preparing samarium-doped strontium borate under high temperature and high pressure by using precursor
The invention discloses a method for preparing samarium-doped strontium borate by taking analytically pure diboron trioxide, strontium carbonate and samarium oxalate decahydrate as raw materials and combining precursor preparation under normal pressure and high pressure and a high temperature and high pressure solid phase reaction. The method comprises the following steps: pressing mixture powderto be cylindrical by using a tablet press; respectively pressing zirconia powder and sodium oxalate powder into a pair of wafers by using the tablet press; staking and filling the prepared wafers intoa platinum tube to be sealed in a sandwich form, completing high-pressure assembling, and carrying out the high temperature and high pressure reaction by a large cubic press, thereby obtaining the samarium-doped strontium borate sample. The technical problems in the prior art that synthesis conditions are difficultly controlled in the process of taking strontium carbonate, boric acid and samariumsesquioxide as raw materials and synthesizing the samarium-doped strontium borate by using a high-temperature solid-phase reaction method, a hydrothermal synthesis method and the like and the synthesized samarium-doped strontium borate is low in purity, poor in crystallinity, low in granularity, small in samarium doping amount and low in luminous efficiency and the like are solved.
Owner:INST OF GEOCHEM CHINESE ACADEMY OF SCI
Granules and method for producing same, production method for molten glass, and production method for glass article
ActiveCN103547541AReduce contentHigh strengthCharging furnaceGlass shaping apparatusAlkali freeAlkali metal oxide
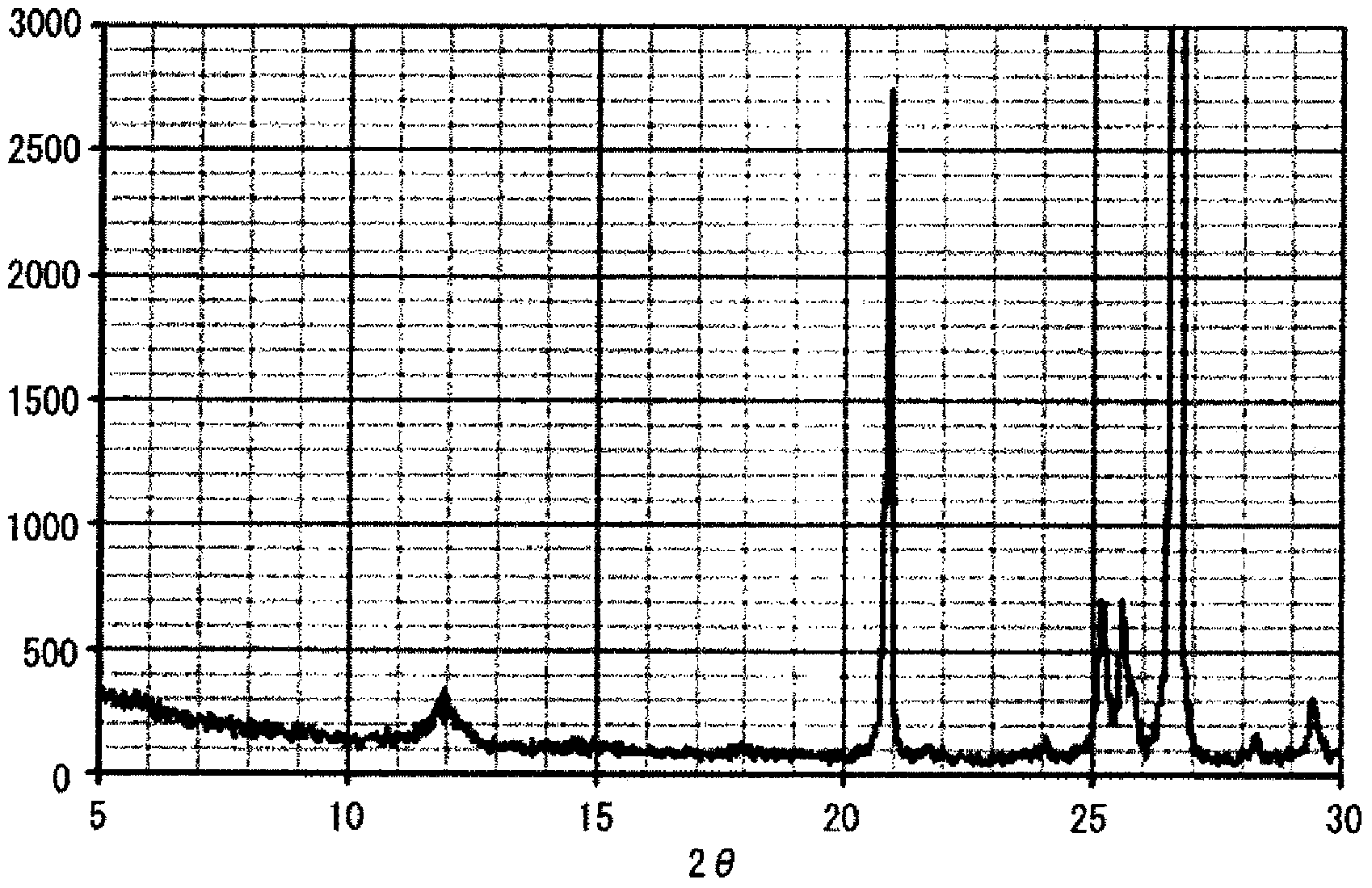
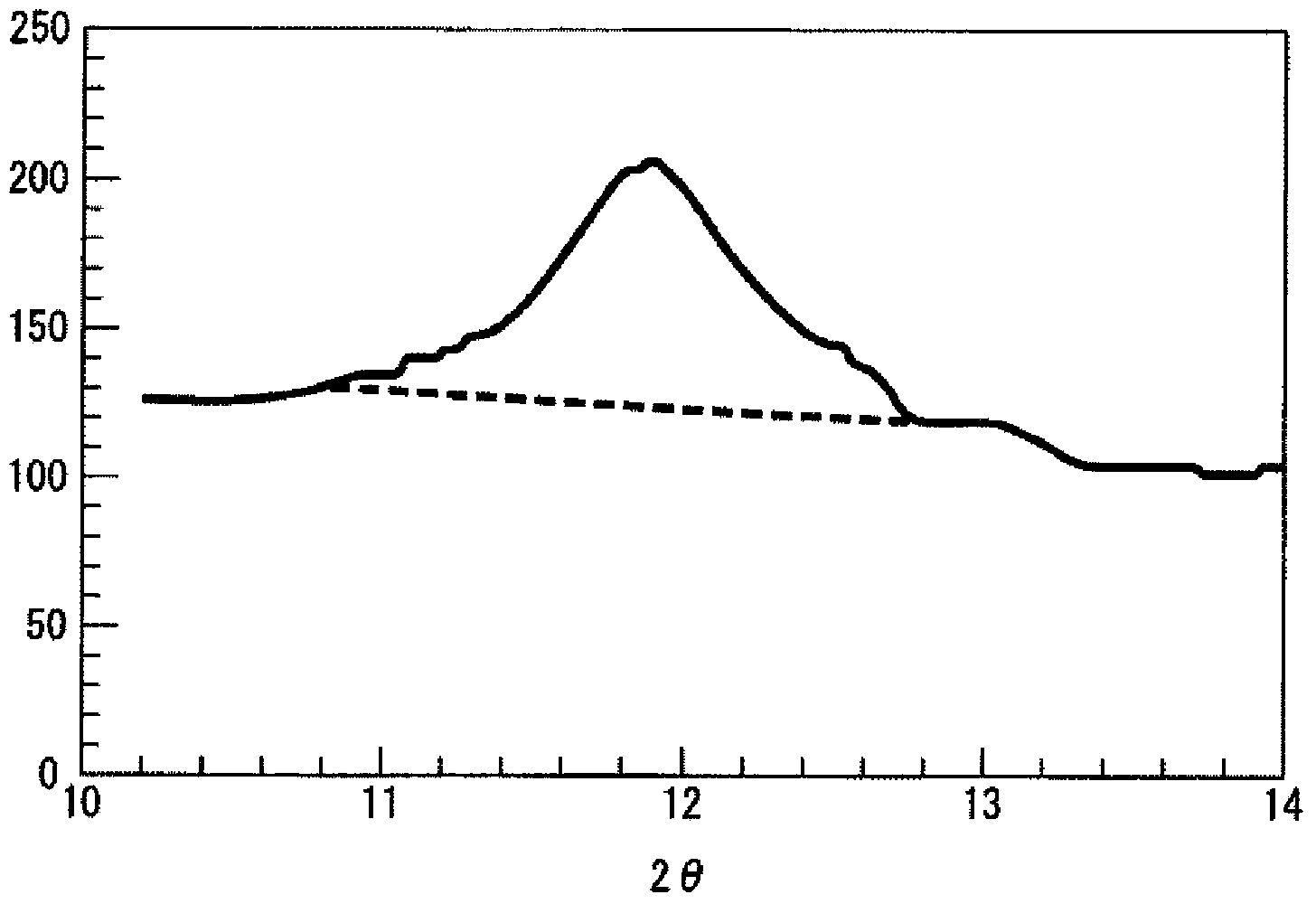
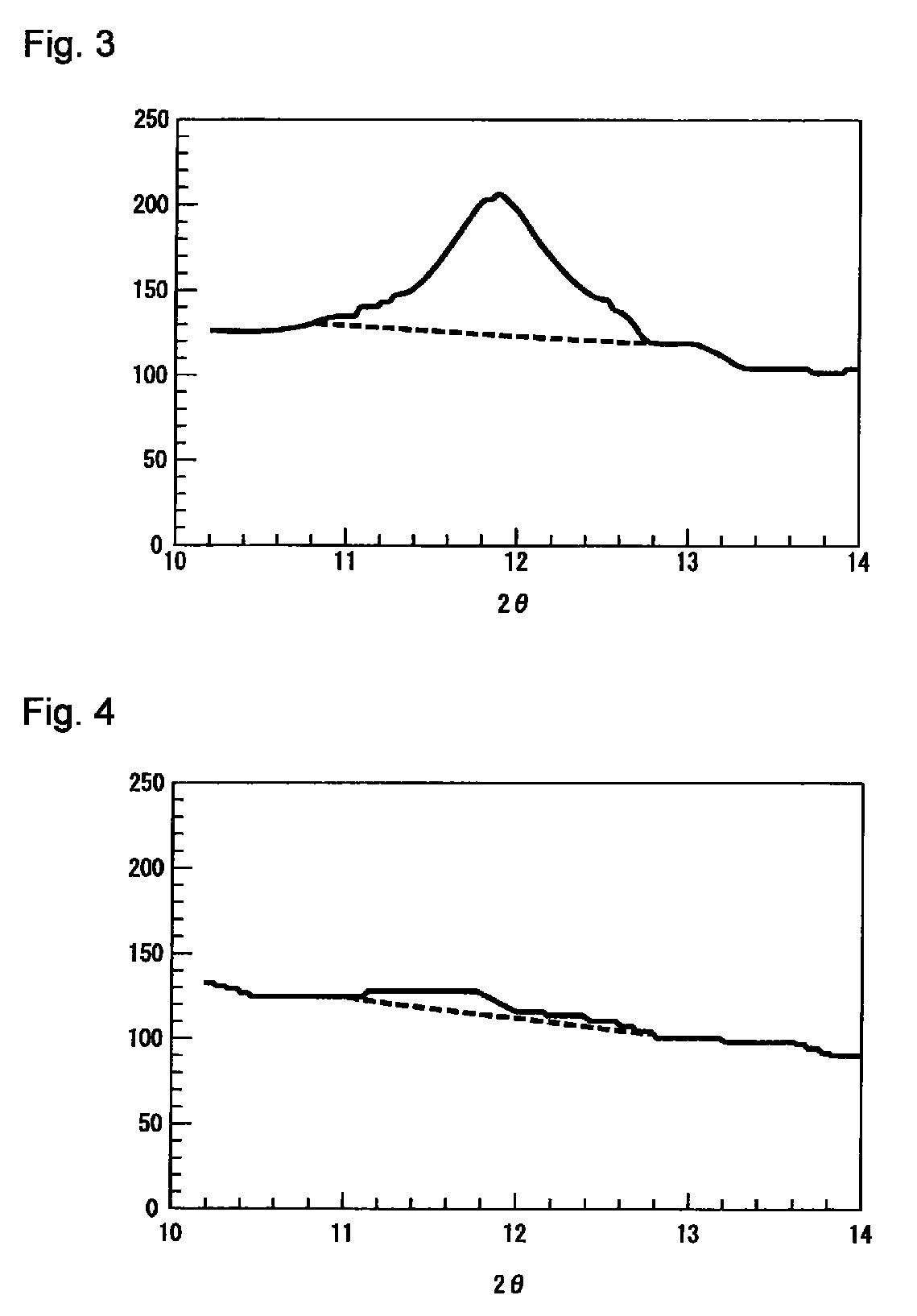
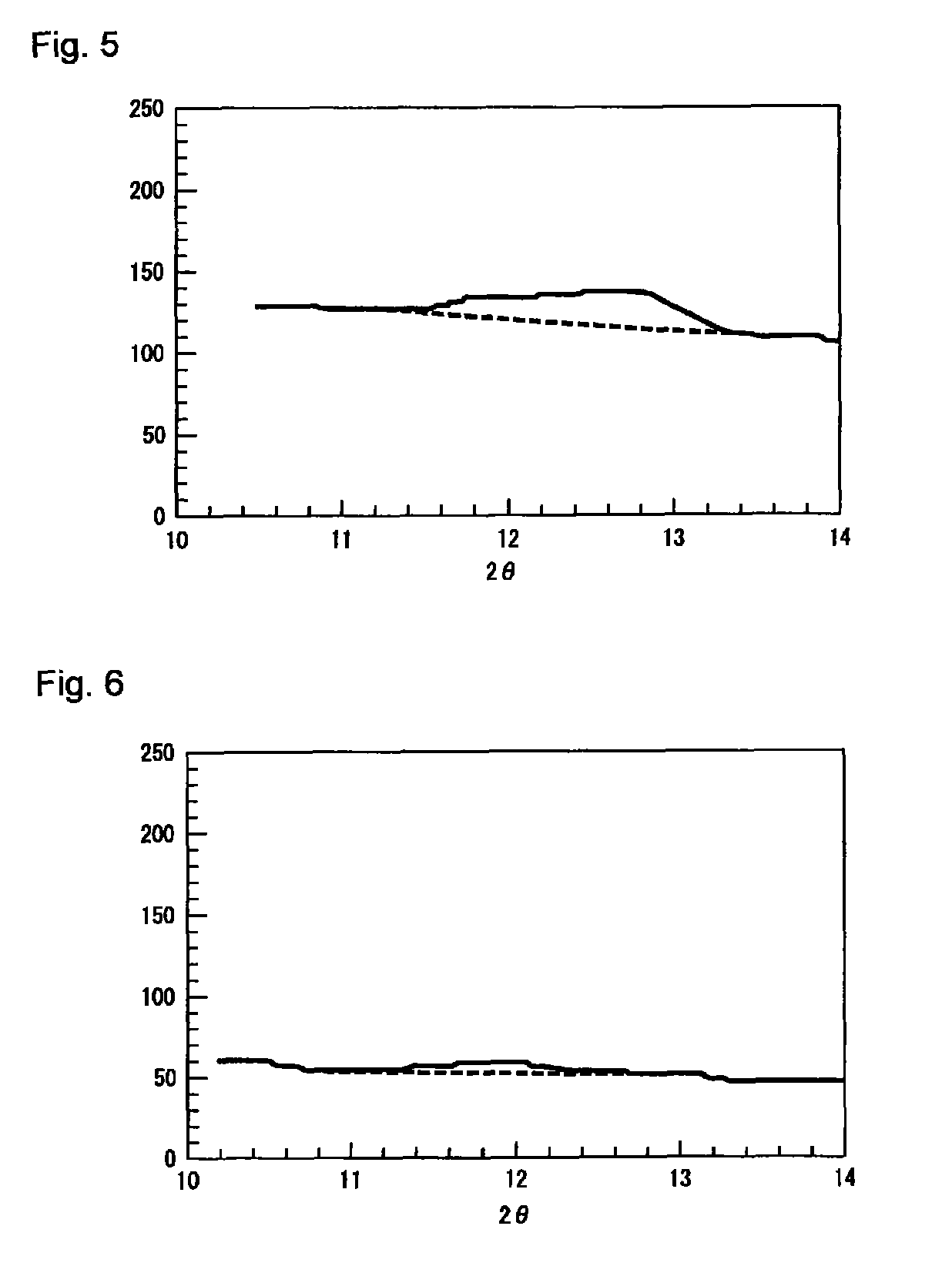
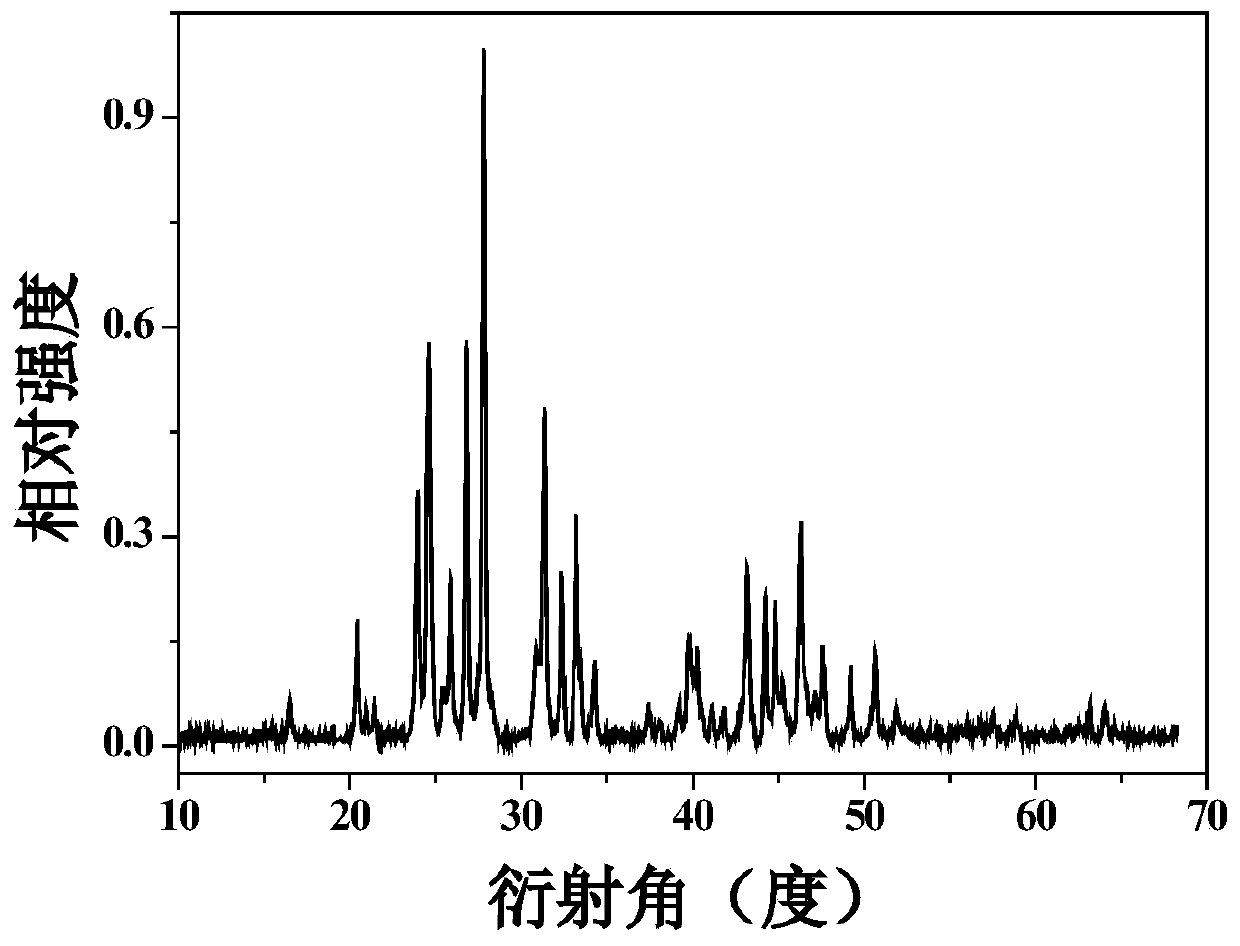
The present invention relates to granules, which are a glass raw material mixture for the production of alkali-free glass containing substantially no alkali metal oxides, wherein the glass structure of glass obtained from the granules is, in mol% on an oxide basis, 60-75 mol% SiO2, 5-15 mol% Al2O3, 1-9 mol% B2O3, 0-15 mol% MgO, 0-20 mol% CaO, 0-12 mol% SrO, 0-21 mol% BaO, and more than 0 mol% for the total of CaO, SrO and BaO; and for which in an X-ray diffraction spectrum obtained by means of a CuKa X-ray powder diffraction, when the diffraction peak area of quartz (100) in which 2Theta is 19.85-21.71 degrees is taken as 1, the total of the relative value of the diffraction peak area of strontium borate hydrate in which 2Theta is 10.81-13.01 degrees, the relative value of the diffraction peak area of calcium borate hydrate in which 2Theta is 11.11-13.49 degrees, and the relative value of the diffraction peak area of barium borate hydrate in which 2Theta is in the range of 10.91-13.27 degrees, is at least 0.005. The present invention also relates to a production method for the granules, a method for producing molten glass using the granules, and a method for producing a glass article using the production method for molten glass.
Owner:AGC INC
Erbium ytterbium boracic acid gadolinium strontium doped laser crystal and preparation method and usage thereof
A erbium- and ytterbium-intermingled gadolinium-strontium borate laser crystal and its preparing method and its use, relate to artificial lens field. Er3+ / Yb3+:Sr3Gd(BO3)3 crystal having high quality and bigger size is produced by the pulling method at 1354 DEG C, at the crystal rotational speed of 5-20 turns / minute, under the drawing speed of 0.5-2 mm / hour. The crystal belongs to trigonal system, space group of which is R3, and the refractive index is 1.73. The crystal is a novel Erbium- and ytterbium-intermingled laser crystal, which is suitable to be pumped by laser diode(LD), accordingly the infrared laser output of 1.5 mum wavelength can be reduced.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Granules and method for their production, method for producing molten glass, and method for producing glass product
ActiveUS9216922B2High strengthAvoid distractionCharging furnaceNon-linear opticsAlkali freeAlkali metal oxide
Granules of a glass raw material mixture, for producing alkali-free glass containing substantially no alkali metal oxides, such that the glass composition of glass obtained from the granules comprises, as represented by mol % based on oxides, 60-75 mol % of SiO2, 5-15 mol % of Al2O3, 1-9 mol % of B2O3, 0-15 mol % of MgO, 0-20 mol % of CaO, 0-12 mol % of SrO and 0-21 mol % of BaO, provided that the total of CaO, SrO and BaO is more than 0, and in an X-ray diffraction spectrum of the granules obtained by means of a CuKα ray, when the diffraction peak area of quartz (100) in a range of 2θ being 19.85-21.71 degrees is taken as 1, the total of the relative values of the diffraction peak areas of strontium borate hydrate, calcium borate hydrate and barium borate hydrate, is at least 0.005.
Owner:AGC INC
Nonlinear optical crystal material Sr3Y3BiB4O15 and preparation method and application thereof
InactiveCN102071464AEnhance nonlinear effectsModerate hardnessPolycrystalline material growthFrom melt solutionsNonlinear optical crystalFlux growth
The invention discloses a nonlinear optical crystal material Sr3Y3BiB4O15 and a preparation method and application thereof. The crystal material is bismuth yttrium strontium borate shown as the chemical formula of Sr3Y3BiB4O15, belongs to a hexagonal system, and has the space group of P63 and the unit cell parameters of a=10.6975(13)A and c=6.7222(12)A. A crystal structure comprises BO3 radicals which are arranged in accordant direction and a BiO3 radical with lone pair electrons which are arranged in accordant direction, so that nonlinear effect is 3 to 5 times that of KH2PO4. A crystal has moderate hardness, is free from being deliquesced or cleaved, and is easy to cut, polish and store. The crystal grows by a flux growth method. The preparation method comprises the following steps of: mixing bismuth yttrium strontium borate and a fluxing agent; heating the mixture to obtain solution; stirring; preserving heat; cooling until the temperature is 0.5 to 3 DEG C above a saturation point; adding crystal seeds; controlling an appropriate temperature lowering speed; when the crystal grows to a certain degree, extracting the crystal out of a liquid surface; and slowly lowering the temperature to room temperature so as to obtain Sr3Y3BiB4O15 monocrystal. The crystal can serve as a frequency doubling crystal in an optical parametric oscillator and a harmonic generator. Moreover, the crystal comprises a yttrium element, so that other rare earth ions can be doped so as to obtain a self-frequency-doubling laser crystal.
Owner:NORTHWEST UNIV
Lithium strontium borate non-linear optical crystal as well as preparation method and application thereof
ActiveCN103436961APolycrystalline material growthBy pulling from meltNonlinear optical crystalCrystal system
The invention discloses a lithium strontium borate non-linear optical crystal, wherein the molecular formula of lithium strontium borate is LiSrB9O15; the crystal belongs to trigonal system; the space group is R3c; the crystal cell parameters are as follows: a is equal to 10.6096(2)Angstrom, b is equal to 10.6096(2)Angstrom, c is equal to 17.5423(9)Angstrom, Alpha and Beta are equal to 90 degrees, Gamma is equal to 120 degrees and Z is equal to 6; the light-transmitting wave band of the crystal is 190-3000 nm; the non-linear optical effect is about 2.5 times of KDP; a Q-switched Nd:YAG laser (1064 nm) is used as a light source and can generate laser radiation with the wavelength of 532 nm; the crystal is not deliquescent, can reach centimetre level in size and is easy to cut, polish and preserve. The crystal can be used for manufacturing non-linear optical devices, such as double frequency generators, upper frequency converters, lower frequency converters or optical parameter oscillators.
Owner:XINJIANG ZIJING OPTICAL ELECTRICAL TECH
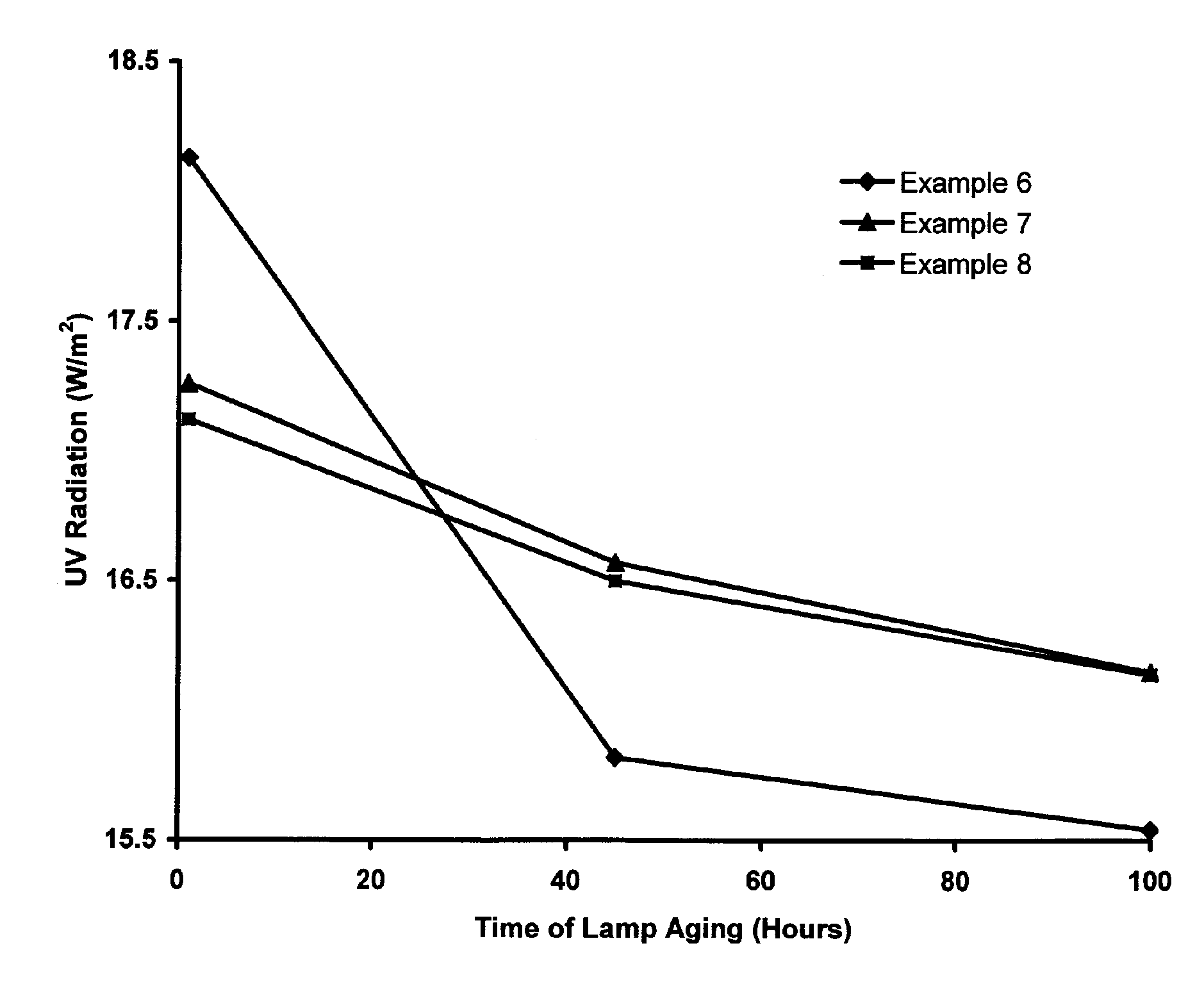
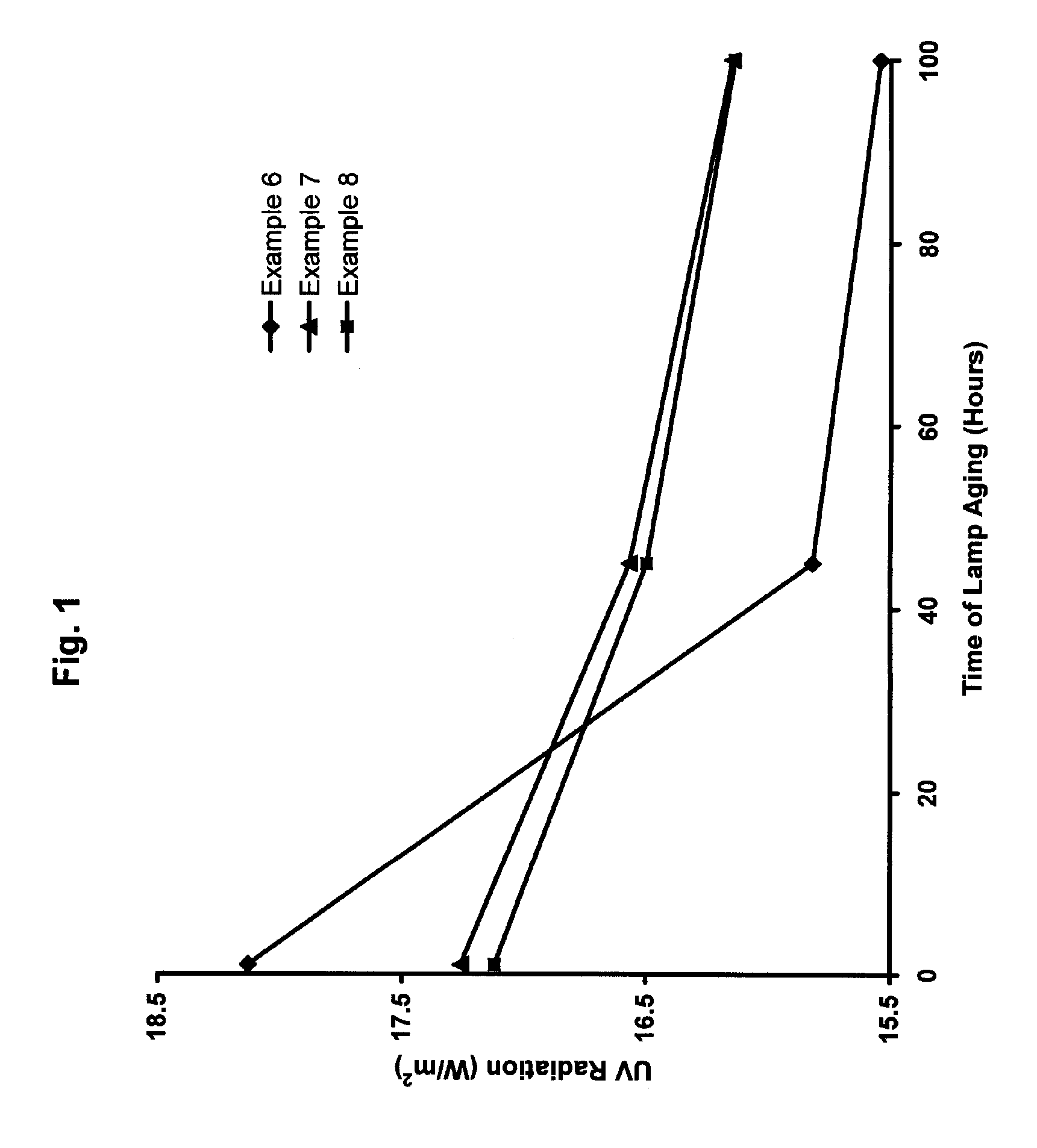
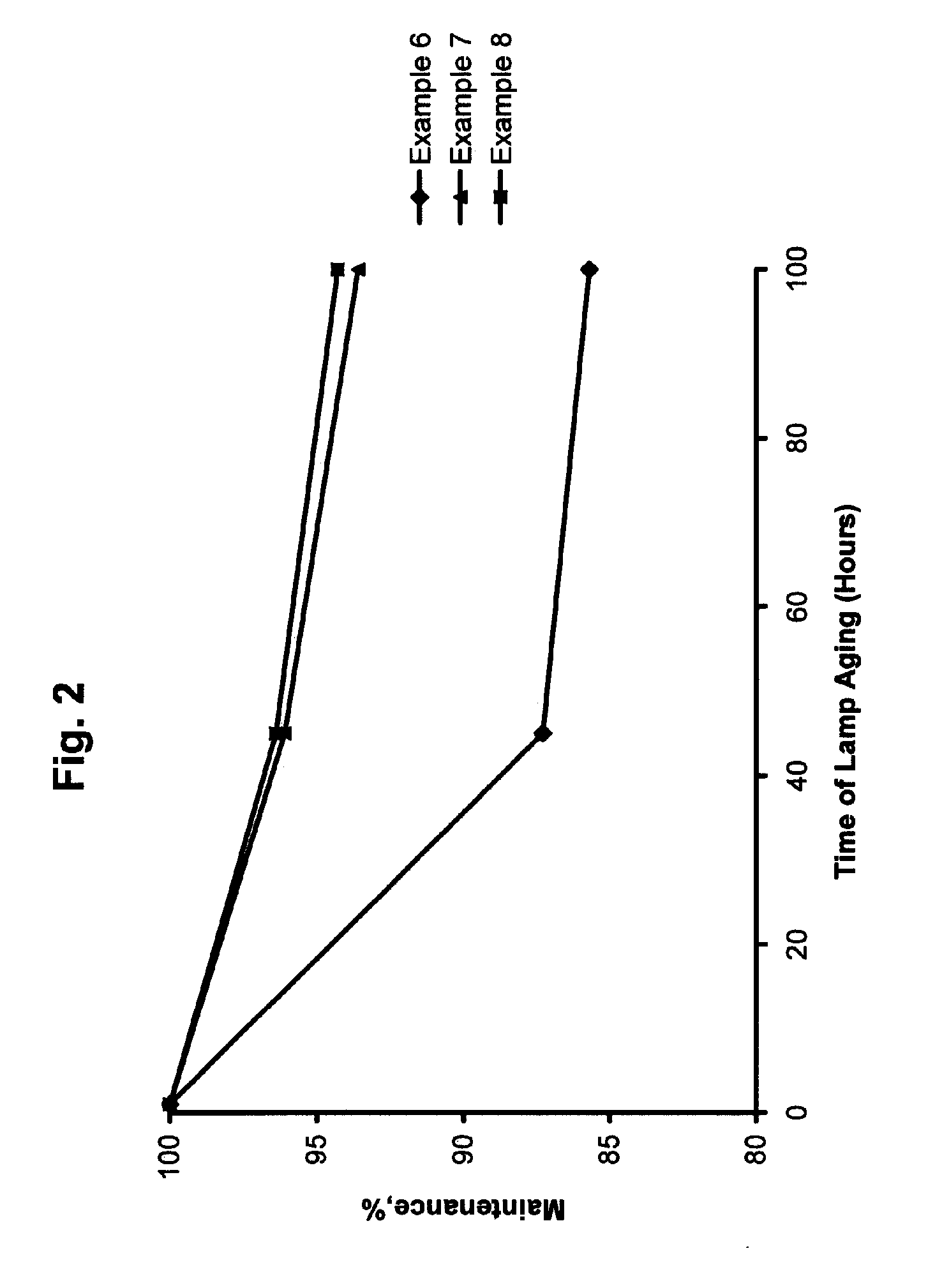
UV-Emitting Strontium Borate Phosphor with Improved Holdover Stability
ActiveUS20060237689A1Adversely affectingLiquid surface applicatorsSynthetic resin layered productsSurface layerTanning lamp
A UV-emitting phosphor comprising SrB4O7:Eu phosphor particles that have been treated to yield a surface layer containing from greater than 0 to about 25 atomic percent aluminum. The holdover stability of the treated SrB4O7:Eu phosphor improves the 100-hour maintenance in a fluorescent tanning lamp to the extent that the treated phosphor may be subject to a holdover period of more than 25 days without a significant change in its 100-hour maintenance.
Owner:LEDVANCE LLC
Europium doped lanthanum-strontium triborate based green fluorescent powder and preparation method thereof
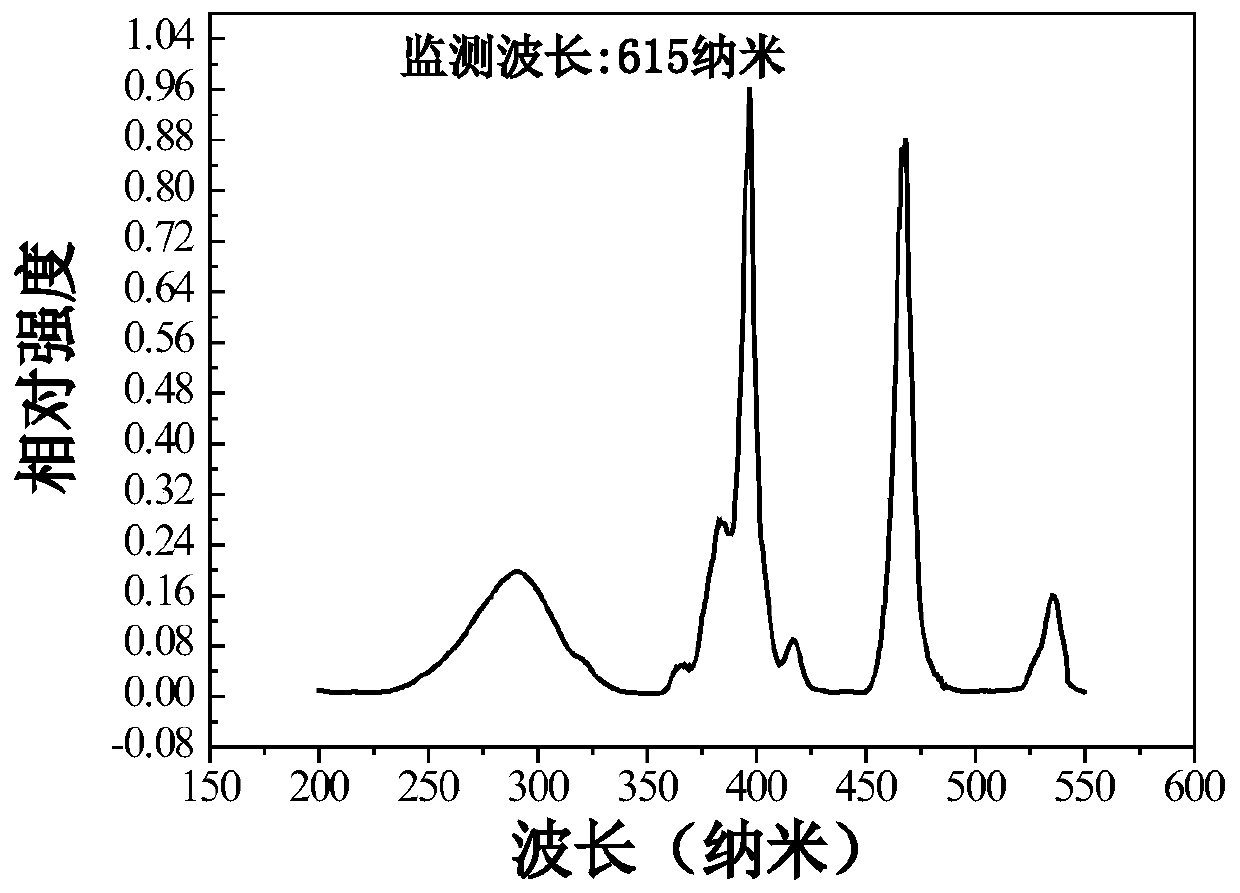
InactiveCN107033900ALow costImprove stabilityLuminescent compositionsAir atmosphereLanthanum strontium manganite
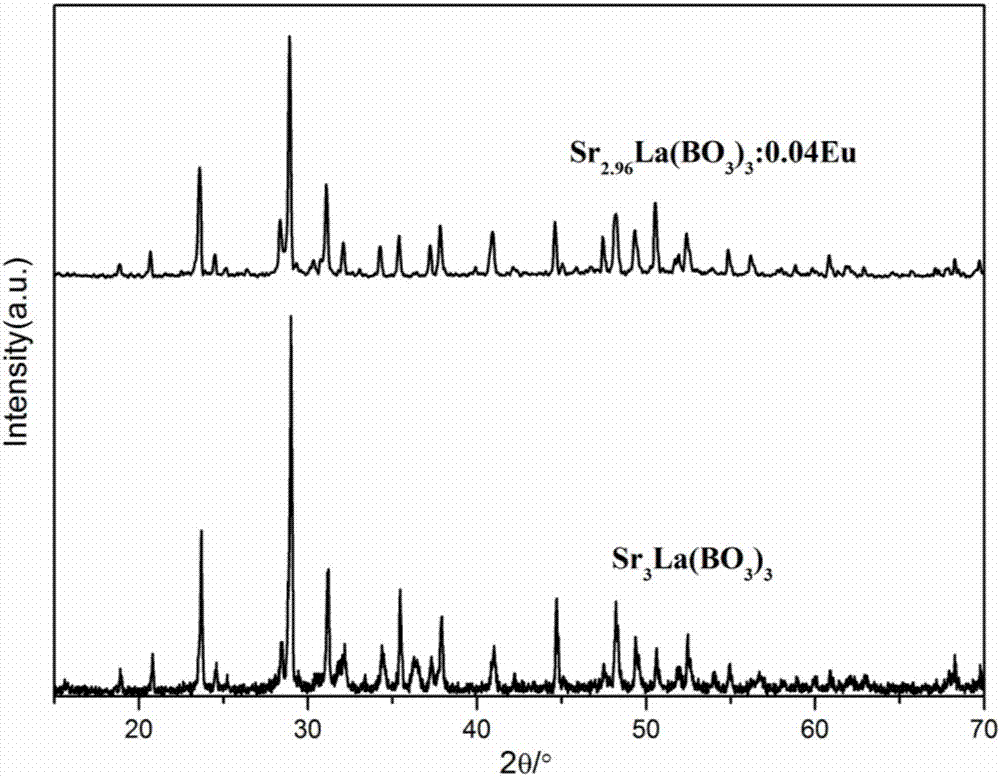
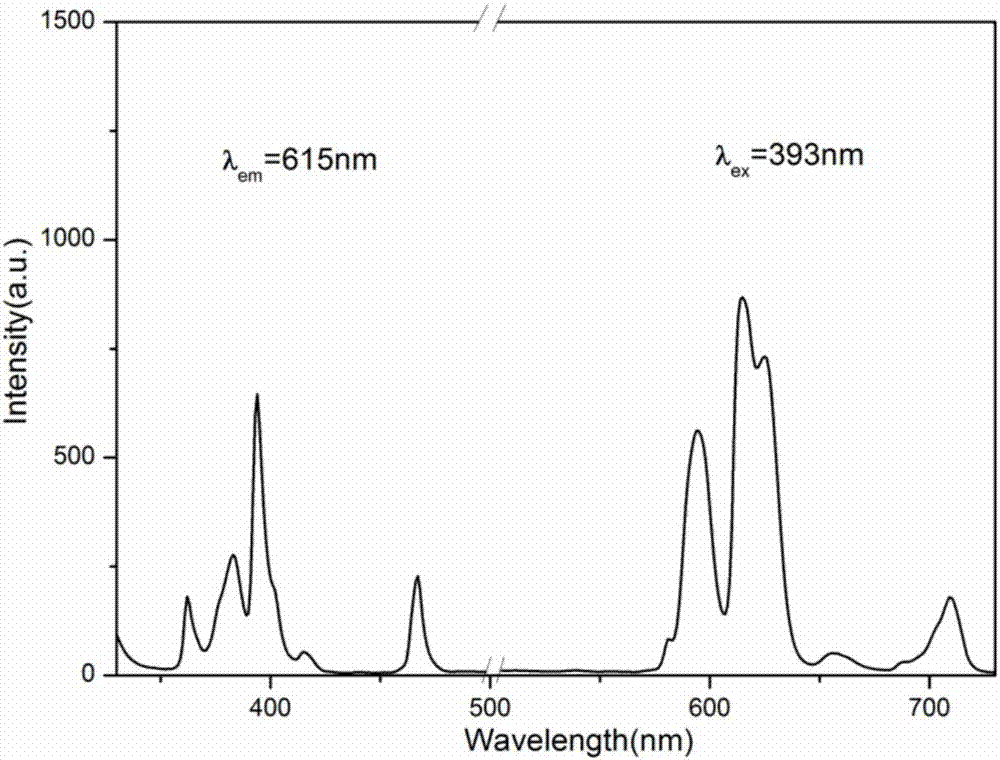
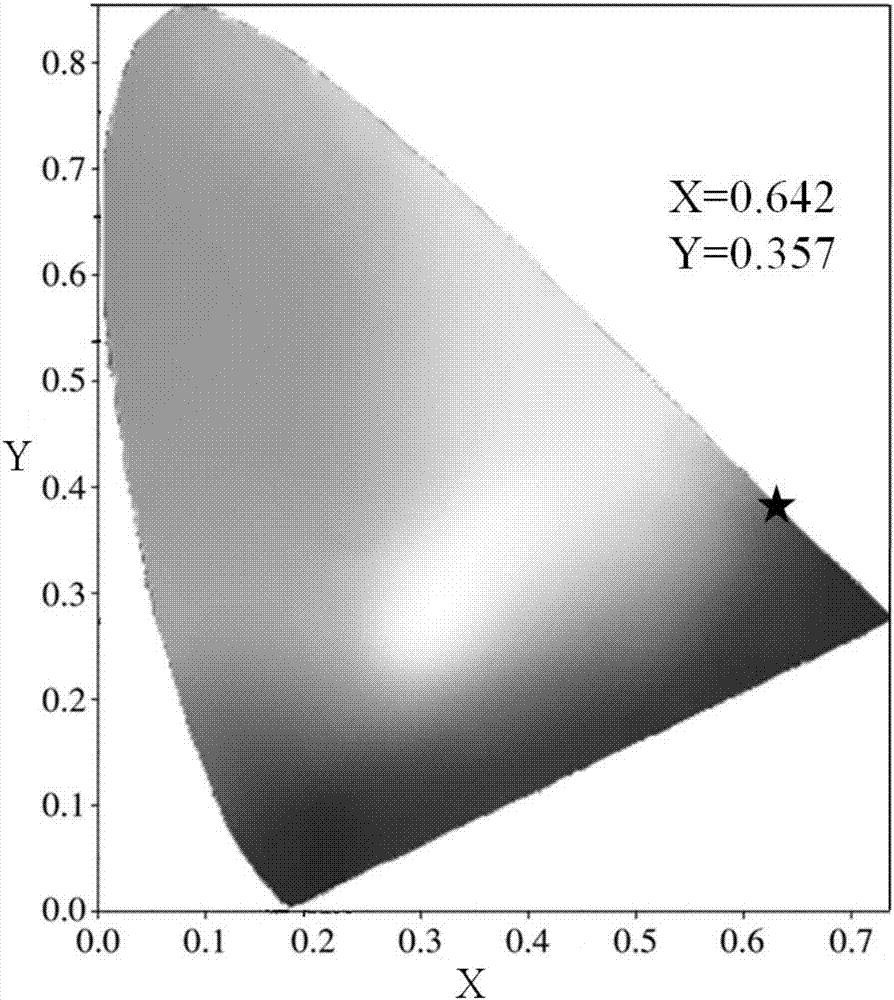
The invention discloses a europium-doped lanthanum strontium triborate-based green fluorescent powder, which uses Sr3La(BO3)3 as a matrix, and has a general chemical formula of Sr3-xLa(BO3)3:xEu, wherein 0.02≤x≤0.20, through Doped with activated ions Eu3+ to prepare europium-doped lanthanum strontium triborate-based green light emitting phosphors. Under near-ultraviolet and blue light excitation, red light with a main peak at around 615nm is obtained, which is suitable for near-ultraviolet or blue light excitation, and the emission peaks are located at 594nm respectively. , 615nm, 625nm, 709nm, the color coordinates are located in the red light region, the chemical properties are stable, the luminous performance is good, the luminous intensity is high, and the color rendering is good. It is a green phosphor with excellent luminous performance; the invention also discloses its preparation Method: SrCO3, La2O3, H3BO3 and Eu2O3 are mixed and ground, calcined in the air atmosphere, kept warm, cooled with the furnace and then ground, and finally europium-doped lanthanum strontium triborate-based green phosphor is obtained, which is prepared based on solid-phase synthesis method , strong operability, simple heating process, simple method, good reproducibility, and short preparation period.
Owner:SHAANXI UNIV OF SCI & TECH
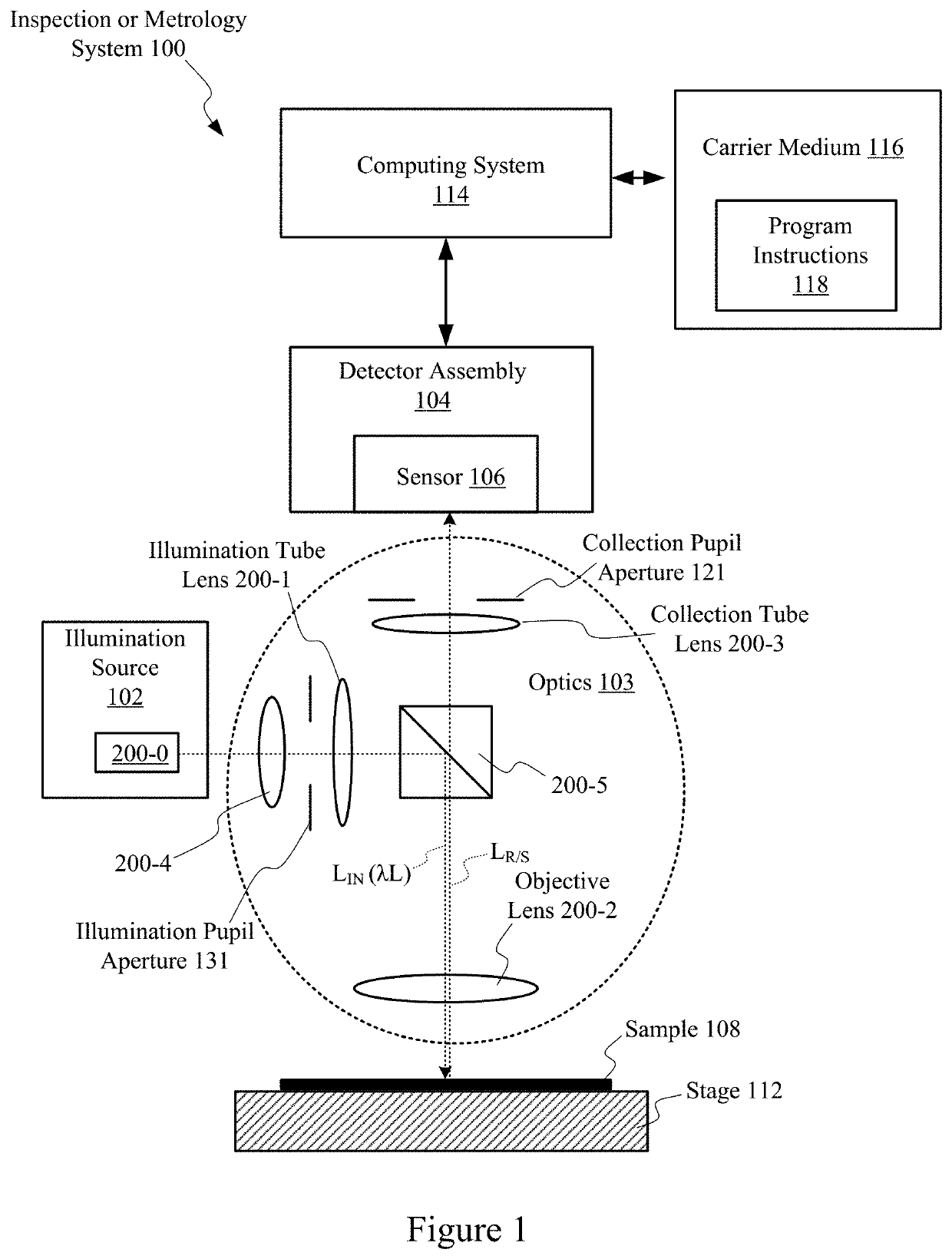
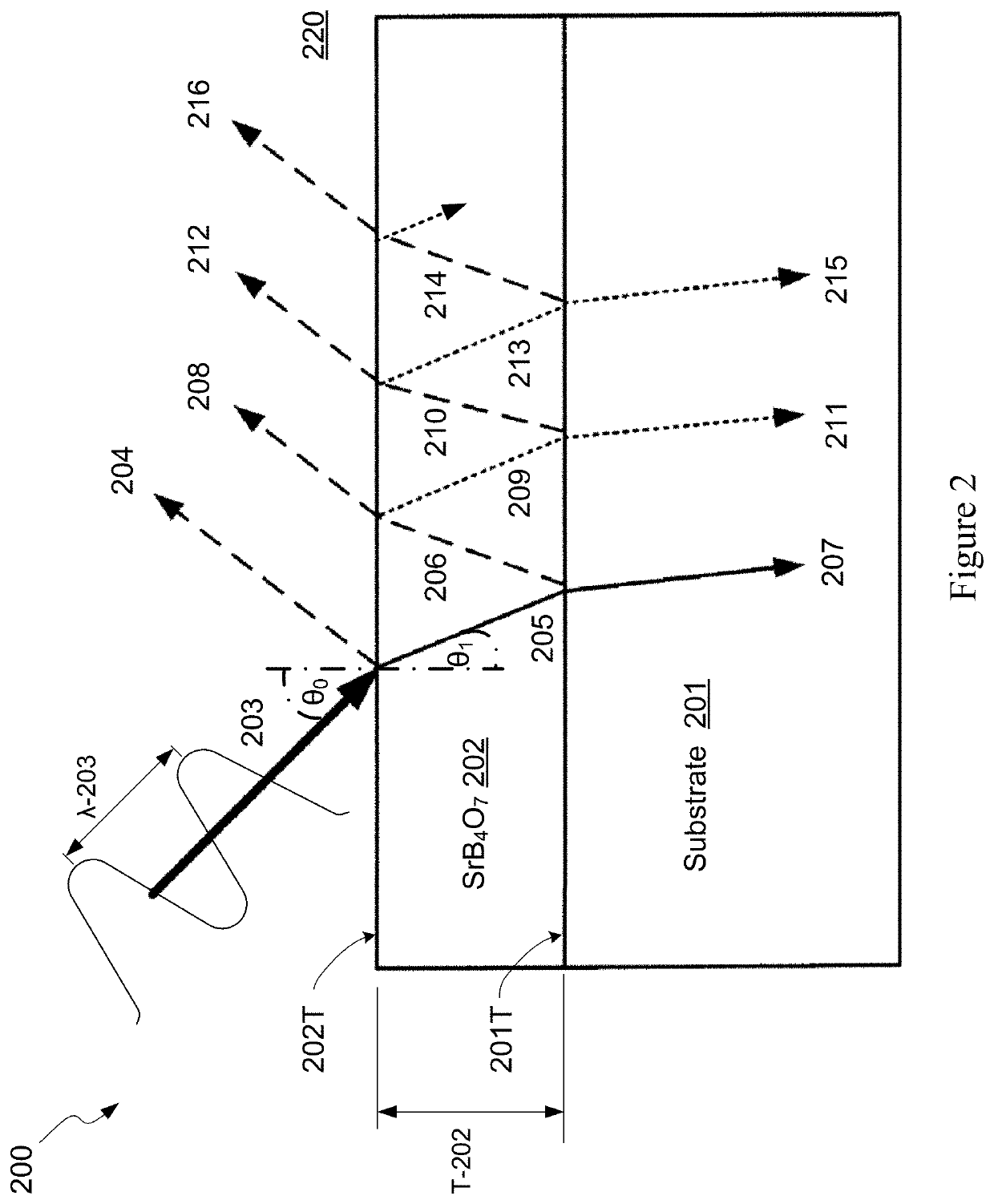
Strontium tetraborate as optical coating material
ActiveUS10921261B2Longer uninterrupted operating periodHigh optical damage thresholdSemiconductor/solid-state device testing/measurementVacuum evaporation coatingRefractive indexMaterials science
Strontium tetraborate is used as an optical coating material for optical components utilized in semiconductor inspection and metrology systems to take advantage of its high refractive indices, high optical damage threshold and high microhardness in comparison to conventional optical materials. At least one layer of strontium tetraborate is formed on the light receiving surface of an optical component's substrate such that its thickness serves to increase or decrease the reflectance of the optical component. One or multiple additional coating layers may be placed on top of or below the strontium tetraborate layer, with the additional coating layers consisting of conventional optical materials. The thicknesses of the additional layers may be selected to achieve a desired reflectance of the optical component at specific wavelengths. The coated optical component is used in an illumination source or optical system utilized in a semiconductor inspection system, a metrology system or a lithography system.
Owner:KLA CORP
Preparation method for SrB6O10/5H2O:Eu<3> luminous material
InactiveCN103074056AEasy to prepareLow reaction temperatureLuminescent compositionsReaction temperatureCommercial Sources
The invention relates to a preparation method for a nano-structure strontium borate SrB6O10 / 5H2O:Eu<3> luminous material. According to the invention, the strontium borate is uniform in shape and adopts a nano structure assembled by nanosheets, and the thickness of each of the nanosheet is 100 nm; and analytic reagents SrOH2 / 8H2O, Na2B4O7 / 10H2O, H3BO3 and EuNO3, which are purchased through commercial sources, are taken as main raw materials, and a simple water solution reaction method is adopted to prepare the material under the assistance of additives at the temperature of 50-80 DEG C. The method is simple and low in reaction temperature. The strontium borate SrB6O10 / 5H2O:Eu<3> luminous material is high in color purity and in rare earth ion quenching concentration, and strong in Eu<3> stability compared with an anhydrous strontium borate substrate luminescent material SrB6O10:Eu<3>, and can be applied to the fields of development displaying, light sources and the like.
Owner:SHAANXI NORMAL UNIV
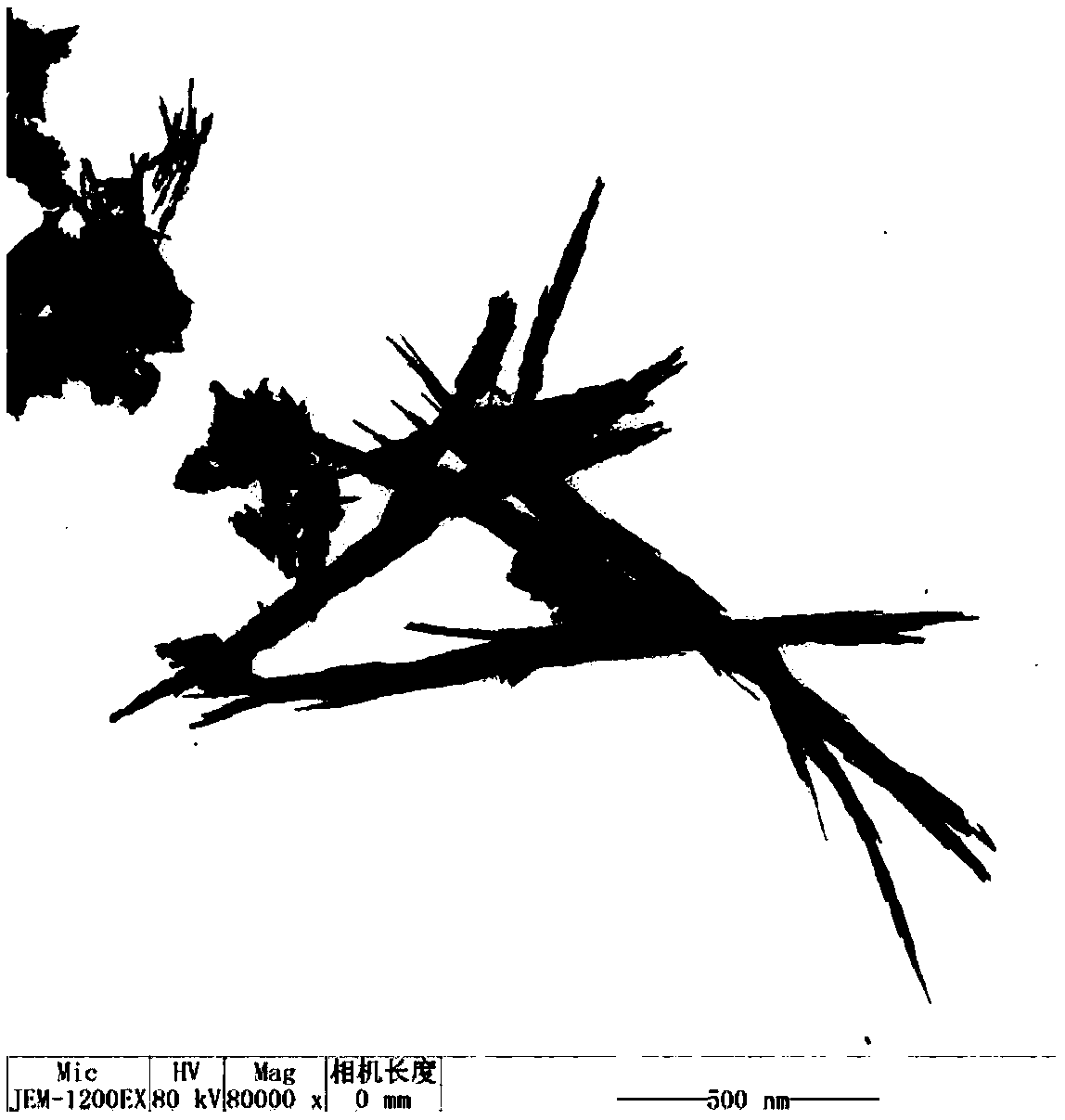
A kind of preparation method of strontium chloroborate whisker
InactiveCN102296350ARich varietyGood microscopic appearancePolycrystalline material growthFrom melt solutionsStrontium borateWhiskers
The invention relates to a method for preparing strontium chloroborate whiskers. The method comprises the following steps: (1) uniformly mixing a strontium-containing raw material, a boron-containing raw material and a flux, and then grinding to a particle size of less than 60 mesh to obtain a mixed raw material; (2) ) placing the mixed raw materials in a high-temperature furnace, raising the temperature to 650-800° C. at a heating rate of 5-10° C. / min, keeping the temperature for 6-15 hours, and cooling naturally to obtain a sintered product; (3) sintering the The product is put into deionized water, soaked for 2 to 15 hours, filtered and washed to obtain a white solid powder of the crude product; (4) drying the solid powder of the crude product to constant weight to obtain white strontium chloroborate crystals beard products. The invention has simple process and low cost, and the obtained strontium chloroborate whisker product has good microscopic appearance, high product purity and high yield.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Europium-lithium excited strontium borate ultraviolet fluorescent powder and preparation method thereof
ActiveCN102344803AHigh luminous intensityTo promote metabolismLuminescent compositionsLuminous intensityChemical composition
The invention discloses a preparation of europium-lithium excited strontium borate ultraviolet fluorescent powder. The chemical composition of the fluorescent powder is SrBxO7:Euy,Liz; the peak wavelength of a luminescent spectrum under the excitation of ultraviolet rays is about 370 nm; and the full width at half maximum of a band is 17-19 nm. Luminous intensity of the fluorescent powder is about 10% higher than that of strontium-europium tetraborate fluorescent powder. After the fluorescent powder which is prepared by the method disclosed by the invention is fired at a temperature of 600 DEG C for 30 min, luminous intensity thereof is hardly attenuated. The preparation method of the europium-lithium excited strontium borate ultraviolet fluorescent powder disclosed by the invention is relatively simple in process and reduces the production cost.
Owner:JIANGMEN KANHOO IND
Compound mono-boric dihydroxyl strontium decaborate monohydrate nonlinear optical crystal and preparation method and use thereof
InactiveCN104562207AEasy to makeShorten the growth cyclePolycrystalline material growthNon-linear opticsNonlinear optical crystalStrontium
The invention relates to a compound mono-boric dihydroxyl strontium decaborate monohydrate nonlinear optical crystal and a preparation method and use thereof. The compound mono-boric dihydroxyl strontium decaborate monohydrate nonlinear optical crystal has a chemical formula of Sr2(B5O8(OH))2B(OH]3.H2O, has the molecular weight of 653.21, belongs to a monoclinic crystal system, has the space group of P21 and has the crystal cell parameters that a is equal to 6.7003(2) angstroms, b is equal to 20.7996(3) angstroms, c is equal to 6.6013(3) angstroms, beta is equal to 119.2503(3) degrees, and V is equal to 802.53(3) angstroms<3>. The compound nonlinear optical crystal is obtained through a programmed cooling or natural cooling method by adopting a hydrothermal method; the powder frequency-doubled effect of the crystal is about twice that of KDP(KH2PO4), and the ultraviolet cutoff edge of the crystal is smaller than 190nm, so that the crystal can serve as a deep-ultraviolet nonlinear optical crystal. A growth process of the crystal has the advantages of simplicity in operation, low cost, low raw material toxicity, short growth cycle, stable physicochemical properties and the like, and the crystal is extensively applied to nonlinear optical devices, such as frequency doubling generators, up frequency converters, down frequency converters or optical parametric oscillators.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Nonlinear optical crystal material Sr3Y3BiB4O15 and preparation method and application thereof
InactiveCN102071464BEnhance nonlinear effectsModerate hardnessPolycrystalline material growthFrom melt solutionsNonlinear optical crystalFlux growth
The invention discloses a nonlinear optical crystal material Sr3Y3BiB4O15 and a preparation method and application thereof. The crystal material is bismuth yttrium strontium borate shown as the chemical formula of Sr3Y3BiB4O15, belongs to a hexagonal system, and has the space group of P63 and the unit cell parameters of a=10.6975(13)A and c=6.7222(12)A. A crystal structure comprises BO3 radicals which are arranged in accordant direction and a BiO3 radical with lone pair electrons which are arranged in accordant direction, so that nonlinear effect is 3 to 5 times that of KH2PO4. A crystal has moderate hardness, is free from being deliquesced or cleaved, and is easy to cut, polish and store. The crystal grows by a flux growth method. The preparation method comprises the following steps of: mixing bismuth yttrium strontium borate and a fluxing agent; heating the mixture to obtain solution; stirring; preserving heat; cooling until the temperature is 0.5 to 3 DEG C above a saturation point; adding crystal seeds; controlling an appropriate temperature lowering speed; when the crystal grows to a certain degree, extracting the crystal out of a liquid surface; and slowly lowering the temperature to room temperature so as to obtain Sr3Y3BiB4O15 monocrystal. The crystal can serve as a frequency doubling crystal in an optical parametric oscillator and a harmonic generator. Moreover, the crystal comprises a yttrium element, so that other rare earth ions can be doped so as to obtain a self-frequency-doubling laser crystal.
Owner:NORTHWEST UNIV
Preparation method and application of strontium fluoroborate nonlinear optical crystal
ActiveCN103590106BPolycrystalline material growthFrom melt solutionsNonlinear optical crystalFlux growth
The invention relates to a preparation method of strontium fluoroborate nonlinear optical crystals, and applications of the strontium fluoroborate nonlinear optical crystals. Compound synthesis is realized via solid phase reaction, and crystal growth is realized via flux growth method. The preparation method is simple in operation, and low in cost. The obtained strontium fluoroborate nonlinear optical crystals will not deliquesce in the air, possesses excellent mechanical properties and stable physico-chemical properties, is unbreakable, is convenient for processing, and is suitable for preparation of nonlinear optical devices. Frequency-doubled effect of the strontium fluoroborate nonlinear optical crystals obtained by the preparation method is three times of that of KDP, and the strontium fluoroborate nonlinear optical crystals can be widely used in nonlinear optical devices such as double frequency converters and optical parametric oscillators.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Eu<3+>-activated barium strontium fluoborate red fluorescent powder, preparation and application thereof
ActiveCN109957403AUniform particlesStrong excitation efficiencyLuminescent compositionsSemiconductor devicesLuminous intensityDisplay device
The invention discloses Eu<3+>-activated barium strontium fluoborate red fluorescent powder as well as preparation and application thereof. The general chemical formula of the fluorescent powder is Ba<3-x>Eu<x>Sr2B4O10F2, wherein x is the substitution molar ratio of Eu<3+> to Ba<2+>, and x is greater than or equal to 0.005 and less than or equal to 0.35. The method comprises the following steps: (1) preparing fluorine-free barium strontium borate precursor powder Ba<2-x>EuxSr2B4O10 by using a chemical sol-gel method; and (2) adding barium fluoride and ammonium fluoride into the prepared fluorine-free barium strontium borate precursor powder, fully mixing all the components, tabletting the mixture, and then preparing the Eu<3+>-activated barium strontium fluoborate red luminescent fluorescent powder Ba<3-x>Eu<x>Sr2B4O10F2 through solid-phase sintering. The red fluorescent powder is in a pure phase and uniform in particle, can emit red light with the central wavelength of 615 nanometersunder excitation of near ultraviolet light 395 nanometers, and is high in luminous intensity and good in thermal stability; therefore, the red fluorescent powder can be used for preparing an illumination or display device taking near ultraviolet light as an excitation light source.
Owner:XUZHOU NORMAL UNIVERSITY
Tb<3+>-activated barium strontium fluoborate green fluorescent powder, preparation and application thereof
ActiveCN109957397AUniform particlesStrong excitation efficiencyLuminescent compositionsSemiconductor devicesLuminous intensityDisplay device
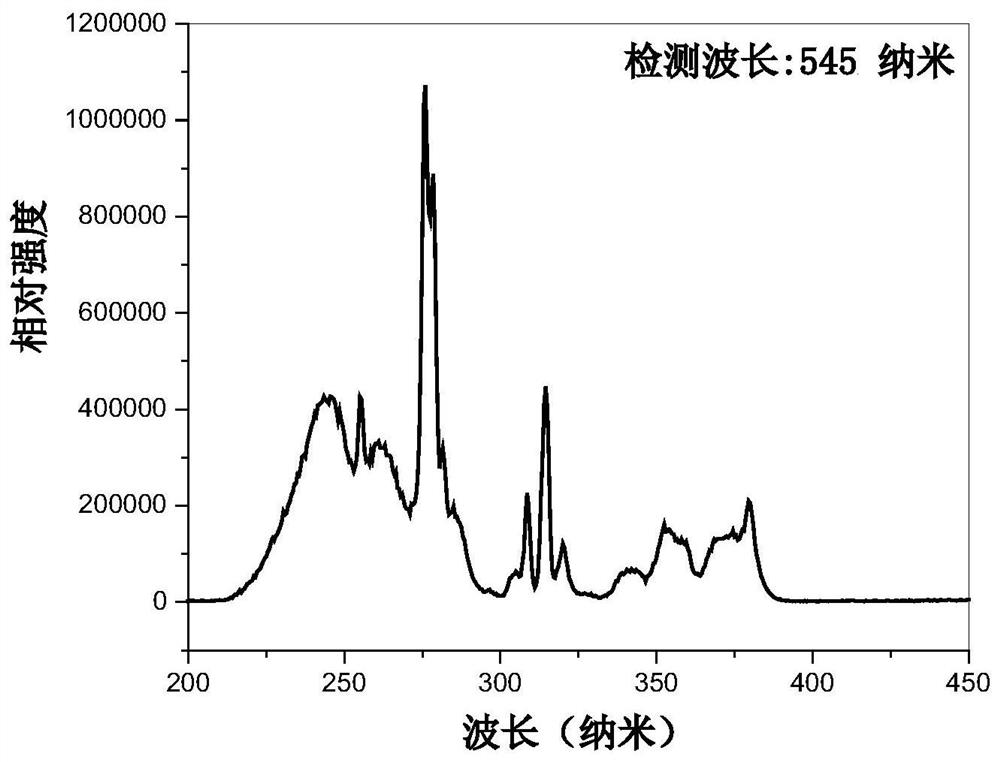
The invention discloses Tb<3+>-activated activated barium strontium fluoborate green fluorescent powder as well as preparation and application thereof. The general chemical formula of the fluorescentpowder is Ba<3-x>Tb<x>Sr2B4O10F2, wherein x is the substitution molar ratio of Tb<3+> to Ba<2+>, and x is greater than or equal to 0.005 and less than or equal to 0.35. The method comprises the following steps: (1) preparing fluorine-free barium strontium borate precursor powder Ba<2-x>TbxSr2B4O10 by using a chemical sol-gel method; and (2) adding barium fluoride and ammonium fluoride into the prepagreen fluorine-free barium strontium borate precursor powder, fully mixing all the components, tabletting the mixture, and then preparing the Tb<3+>-activated barium strontium fluoborate green luminescent fluorescent powder Ba<3-x>Tb<x>Sr2B4O10F2 through solid-phase sintering. The green fluorescent powder is in a pure phase and uniform in particle, can emit green light with the central wavelength of 545 nanometers under excitation of near ultraviolet light 276 nanometers, and is high in luminous intensity and good in thermal stability; therefore, the green fluorescent powder can be used forpreparing an illumination or display device taking near ultraviolet light as an excitation light source.
Owner:XUZHOU NORMAL UNIVERSITY
Divalent-bismuth-ion-doped strontium borate fluorescent material and preparation method thereof
The invention discloses a divalent-bismuth-ion-doped strontium borate fluorescent material and a preparation method thereof. The preparation method comprises the following steps of: respectively weighing compound raw materials containing strontium, boron and bismuth according to a molecular formula: Sr1-xB4O7:xBi or Sr1-xB6O10:xBi, wherein x is not less than 0.001 and not more than 0.05; evenly grinding and mixing the raw materials, then, preburning, and controlling the temperature at 300-500 DEG C; after preburning, evenly grinding and mixing again, carrying out high-temperature burning twice, and controlling the temperature at 600-900 DEG C; and putting a sample in a reducing atmosphere at the temperature of 600-900 DEG C, and carrying out reaction for 15 min to 10 hours. The luminous intensity of the fluorescent powder prepared by the preparation method disclosed by the invention is over 2 times or 140 times of that of a bismuth-doped sample with the same concentration, which is synthesized in an air atmosphere, and the color-rendering performance of a white LED (Light Emitting Diode) is hopeful to improve.
Owner:SOUTH CHINA UNIV OF TECH
A method for preparing samarium-doped strontium borate using precursor under high temperature and high pressure
The invention discloses a method for preparing samarium-doped strontium borate by taking analytically pure diboron trioxide, strontium carbonate and samarium oxalate decahydrate as raw materials and combining precursor preparation under normal pressure and high pressure and a high temperature and high pressure solid phase reaction. The method comprises the following steps: pressing mixture powderto be cylindrical by using a tablet press; respectively pressing zirconia powder and sodium oxalate powder into a pair of wafers by using the tablet press; staking and filling the prepared wafers intoa platinum tube to be sealed in a sandwich form, completing high-pressure assembling, and carrying out the high temperature and high pressure reaction by a large cubic press, thereby obtaining the samarium-doped strontium borate sample. The technical problems in the prior art that synthesis conditions are difficultly controlled in the process of taking strontium carbonate, boric acid and samariumsesquioxide as raw materials and synthesizing the samarium-doped strontium borate by using a high-temperature solid-phase reaction method, a hydrothermal synthesis method and the like and the synthesized samarium-doped strontium borate is low in purity, poor in crystallinity, low in granularity, small in samarium doping amount and low in luminous efficiency and the like are solved.
Owner:INST OF GEOCHEM CHINESE ACADEMY OF SCI
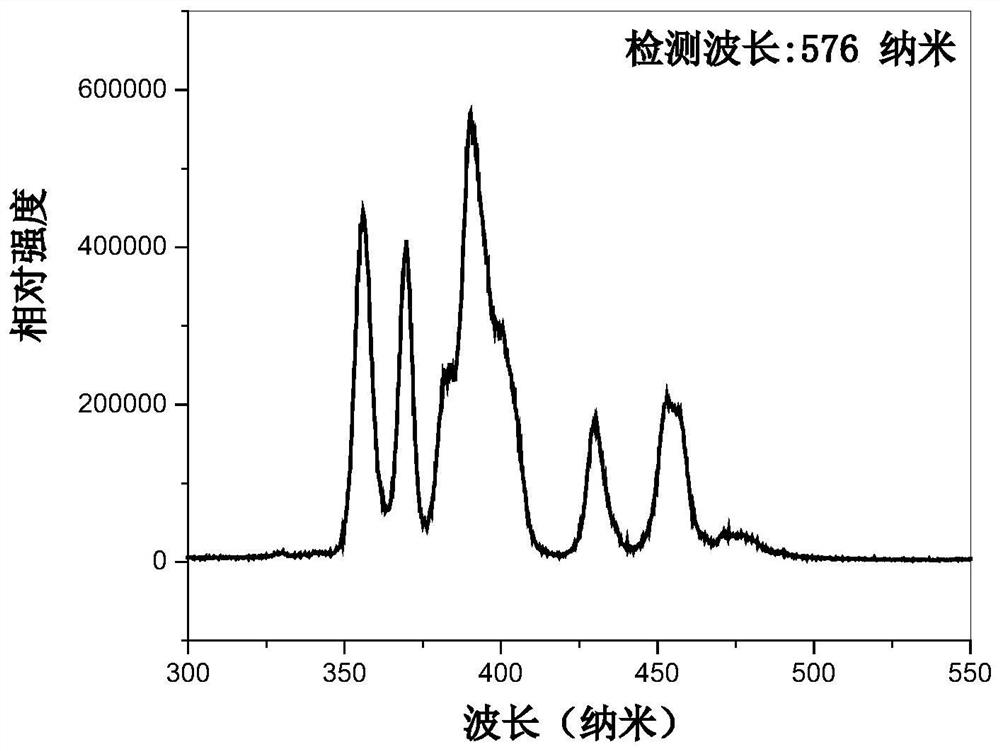
a kind of dy 3+ Activated strontium barium fluoroborate yellow phosphor and its preparation and application
ActiveCN109988567BUniform particlesForbidden Warp BreakLuminescent compositionsSemiconductor devicesUltraviolet lightsFluoroboric acid
The invention discloses a Dy 3+ Activation of strontium barium fluoroborate yellow fluorescent powder and its preparation and application, the general chemical formula of the fluorescent powder is Ba 3‑x Dy x Sr 2 B 4 o 10 f 2 , x is Dy 3+ Replace Ba 2+ The molar ratio of 0.005≤x≤0.35; the preparation steps are: (1) using the chemical sol-gel method to prepare the fluorine-free strontium barium borate precursor powder Ba 2‑ x Dy x Sr 2 B 4 o 10 (2) In the prepared fluorine-free strontium barium borate precursor powder, add barium fluoride and ammonium fluoride, fully mix, and mix the mixture, use solid-state sintering method to prepare, obtain a Dy 3+ Activate strontium fluoroborate barium yellow luminescent phosphor Ba 3‑ x Dy x Sr 2 B 4 o 10 f 2 . The yellow fluorescent powder prepared by the present invention is a pure phase with uniform particles, and emits yellow light with a center wavelength of 576 nm under the excitation of near-ultraviolet light at 390 nm. It has high luminous intensity and good thermal stability, and can be used to prepare near-ultraviolet light as an excitation light source. lighting or display devices.
Owner:XUZHOU NORMAL UNIVERSITY
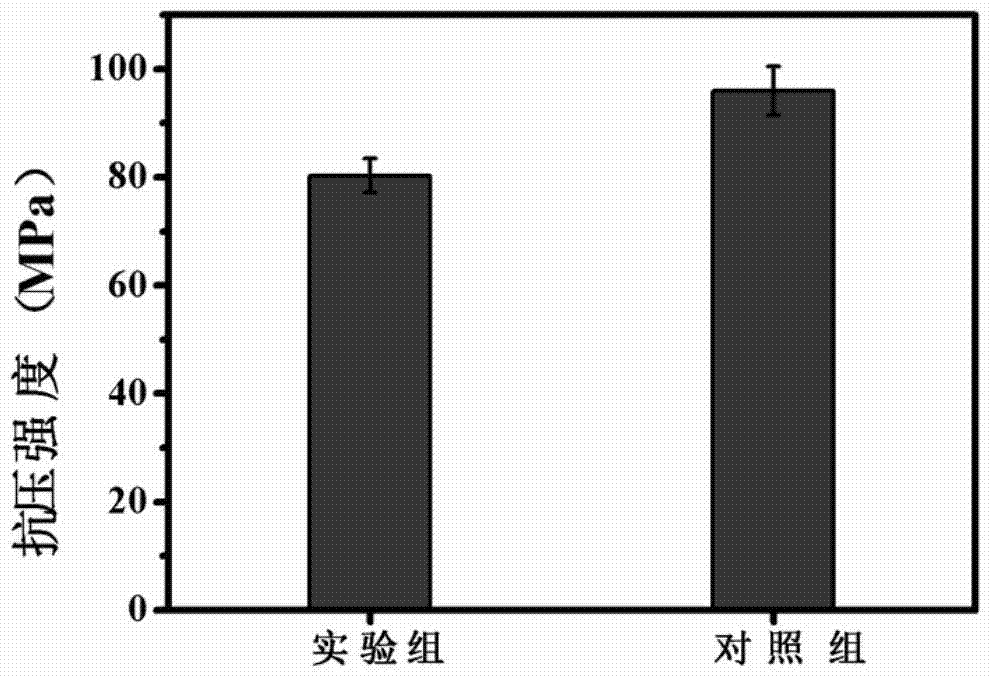
Preparation method and application of composite bone cement precursor, strontium borate bioglass/polymethyl methacrylate composite bone cement
ActiveCN104922731BGood injectabilityImprove biological activityProsthesisPolymethyl methacrylateBone cement
The invention discloses a polymethyl methacrylate (PMMA) compound bone cement precursor which comprises solid phase powder and solidification liquid, wherein the solid phase powder comprises strontium borate bioglass, polymethyl methacrylate powder and a polymerization initiator, the solidification liquid comprises methyl methacrylate and a polymerization activator, and the mass of the solid phase powder is 50 to 80 percent of the mass sum of the solid phase powder and the solidification liquid. The invention also provides a preparation method of polymethyl methacrylate compound bone cement, the compound bone cement combines the performance of the strontium borate bioglass and pure PMMA bone cement, has excellent biological activity, biological degradability, biological compatibility as well as mechanical strength matched with bones, so as to achieve a good effect on prompting bone reconstruction; the invention also provides an application of the strontium borate bioglass / polymethyl methacrylate compound bone cement.
Owner:深圳市中科海世御生物科技有限公司
A method of hydrothermally preparing samarium borate nanowires
The invention provides a simple preparation method of a SmBO3 nanowire. The method is mianly characterized by preparing the nanowire SmBO3 by use of borax and Sm(NO3)3 as raw materials and hydrothermal process under the condition of adding a sodium oleate modifier. The main principle is as follows: an anionic surfactant sodium oleate can form a certain coordination with the Sm<3+> ion in the Sm(NO3)3 solution in the hydrothermal process, thereby forming the nano samarium oxide; under the protection effect of the sodium oleate and along the prolonging of the reaction time, the samarium oxide is reacted with the borate in the solution to generate the SmBO3 nanowire. The method provided by the invention is characterized in that: the obtained SmBO3 nanowire has multiple strong absorption peaks in the infrared area, particularly, the absorption at the 1064cm<-1> wavelength is the strongest, and can be used as a wave-absorbing material.
Owner:QINGDAO UNIV OF SCI & TECH
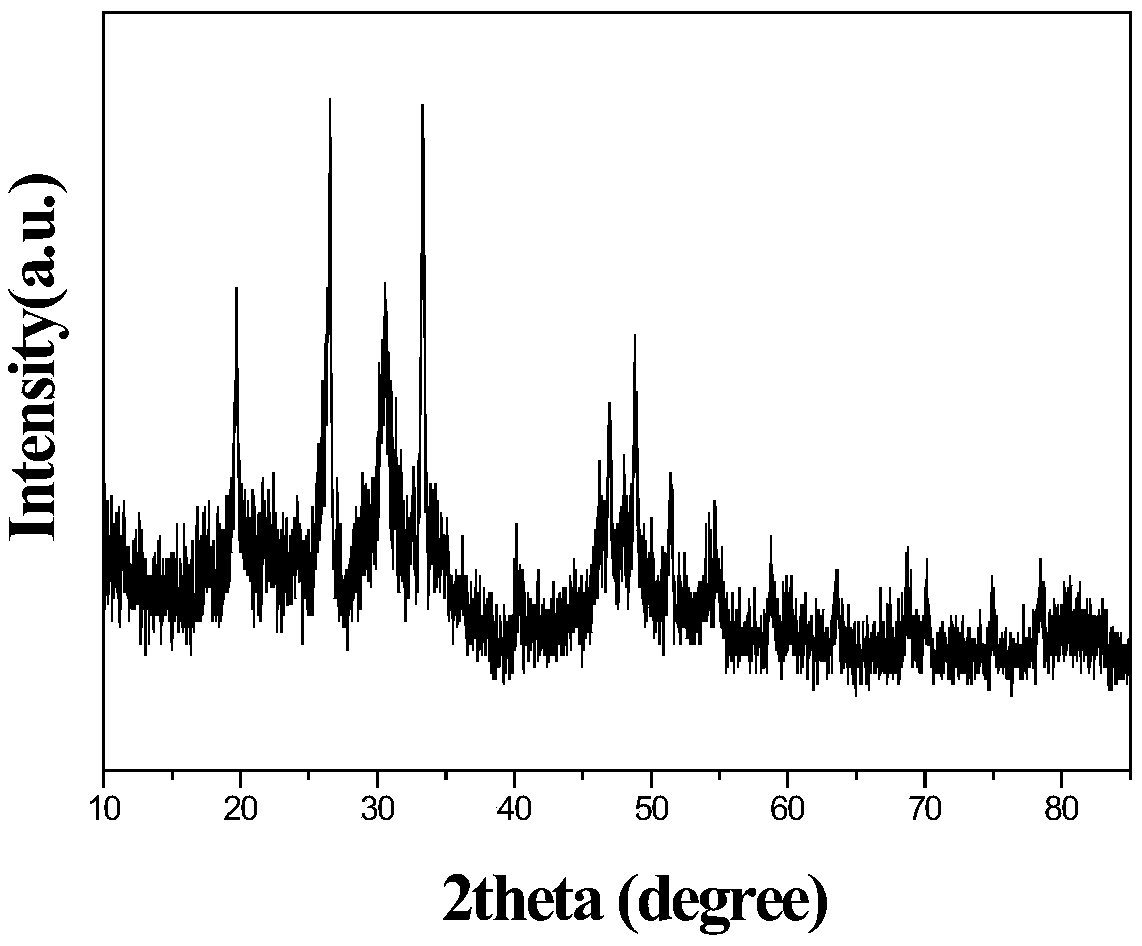
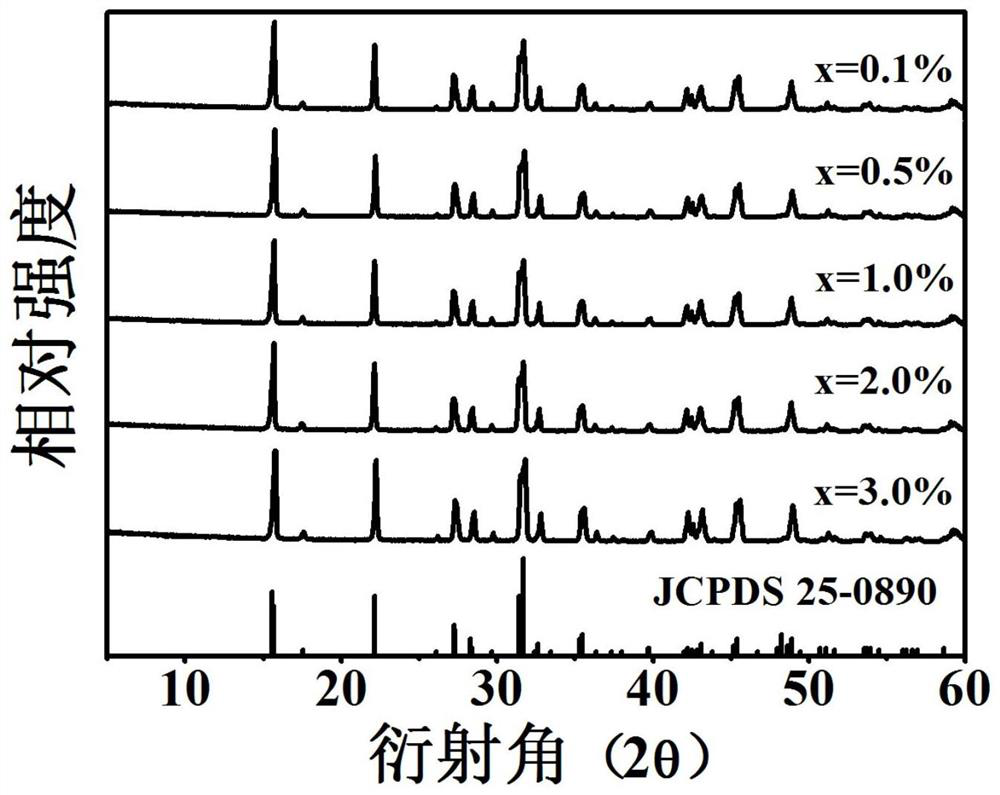
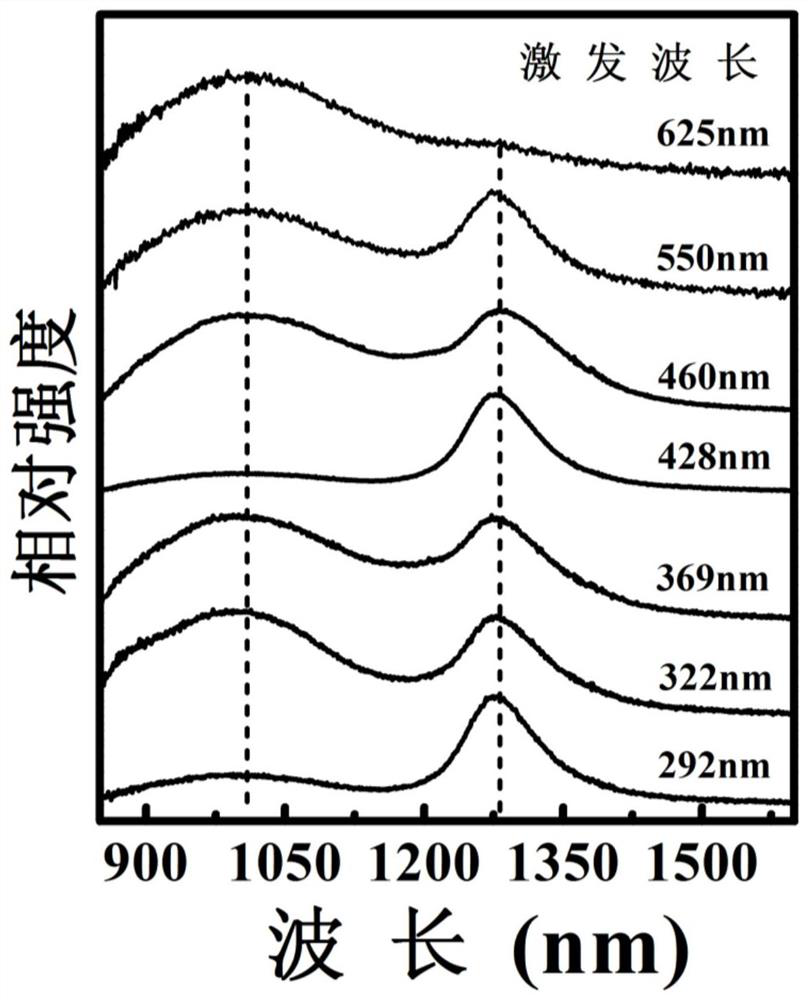
A near-infrared luminescent bismuth-doped strontium chloropentaborate crystal and its preparation method
InactiveCN110093156BGood choiceExcitation bandwidthLuminescent compositionsUltra-widebandBoronic acid
The invention discloses a near-infrared luminescent bismuth doped strontium chloro-pentaborate crystal and a preparation method thereof. The general formula of the crystal is Sr2(1-x)B5O9Cl : 2xBi, 0.1% <= x <= 3.0%. respectively weighing strontium containing, boron containing, chlorine containing and bismuth containing compounds as raw materials; preforming pre-burning at 623-823 K for 3-6 hoursafter the raw materials are uniformly ground, performing cooling to room temperature, and performing grinding and well mixing; and performing calcining at 1,073-1,173 K for 4-6 hours, performing reaction for 15 min - 10 hours under a reducing atmosphere of 1,073-1,173 K on an obtained sample after performing cooling to the room temperature, and performing grinding to obtain the crystal after performing cooling to the room temperature. The near-infrared fluorescence full width at half maximum is greater than 200 nm and wider than the luminescence of rare earth ions, and has longer fluorescencelifetime, so that the crystal can be taken as a laser gain medium and applied to the fields of ultra-wideband wavelength tunable novel laser sources or ultrashort pulse lasers.
Owner:SOUTH CHINA UNIV OF TECH
Novel high-efficiency strontium borate-like photocatalyst
ActiveCN101879443BHydrocarbon from carbon oxidesMetal/metal-oxides/metal-hydroxide catalystsCITRATE ESTERAlcohol
The invention discloses a high-efficiency strontium borate-like photocatalyst and a preparation method thereof and aims to prepare methane by reducing carbon dioxide and water through photocatalysis technology. In the photocatalyst, strontium salt, a boron-containing compound, citric acid or citrate and alcohols are used as precursors. The photocatalysis efficiency of reducing the carbon dioxide and water to prepare the methane of the high-activity strontium borate-like photocatalyst prepared by a sol-gel method is superior to that of a TiO2 photocatalyst (P25, which is known as the photocatalyst with higher photocatalysis efficiency at present). The novel high-efficiency strontium borate-like photocatalyst has the advantages of simple preparation process, easy operation, low requirements on equipment and the like and has wide application prospect and development potential in the fields of emission reduction of carbon dioxide, recycling of energy, environmental science and the like.
Owner:NANKAI UNIV
Strontium borate microparticles and preparation method thereof
The invention discloses strontium borate microparticles and a preparation method thereof. The borate microparticles are prepared from strontium, boron and oxygen with the atomic ratio of 1:2:4, and the particle sizes range from 0.5 micrometer to 20 micrometers. The method comprises the steps that a sodium borate aqueous solution or a strontium salt aqueous solution are dropwise added into the strontium salt aqueous solution or the sodium borate aqueous solution which is under stirring, and a suspension is obtained; solid-liquid separation and washing and drying treatment are conducted on the suspension in sequence, and amorphous phase strontium borate particles are obtained; sodium hydroxide or sodium carbonate is added into an amorphous phase strontium borate particle aqueous solution at first to obtain a mixed solution, the mixed solution is placed at the temperature ranging from 150 DEG C to 280 DEG C, closed reacting is conducted for at least 1 h, and a reaction solution is obtained; the reaction solution is subjected to solid-liquid separation and washing and drying treatment in sequence, and then a target product is obtained. The strontium borate microparticles can be used as matrix materials of fluorescent powder for LED and are widely applied to the field of electroluminescence.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
A tb3+ activated barium strontium fluoroborate green phosphor and its preparation and application
ActiveCN109957397BUniform particlesStrong excitation efficiencyLuminescent compositionsSemiconductor devicesUltraviolet lightsFluoroboric acid
The invention discloses a Tb 3+ Activated barium strontium fluoroborate green fluorescent powder and its preparation and application, the general chemical formula of the fluorescent powder is Ba 3‑x Tb x Sr 2 B 4 o 10 f 2 , where x is Tb 3+ Replace Ba 2+ The molar ratio of 0.005≤x≤0.35; the preparation steps are: (1) using the chemical sol-gel method to prepare the fluorine-free strontium barium borate precursor powder Ba 2‑x Tb x Sr 2 B 4 o 10 (2) In the prepared fluorine-free strontium barium borate precursor powder, add barium fluoride and ammonium fluoride, fully mix, and mix the mixture, use solid-state sintering method to prepare, obtain a kind of Tb 3+ Activation of barium strontium fluoroborate green luminescent phosphor Ba 3‑x Tb x Sr 2 B 4 o 10 f 2 . The green fluorescent powder prepared by the present invention is a pure phase with uniform particles, and emits green light with a center wavelength of 545 nanometers under the excitation of near-ultraviolet light at 276 nanometers. lighting or display devices.
Owner:XUZHOU NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com