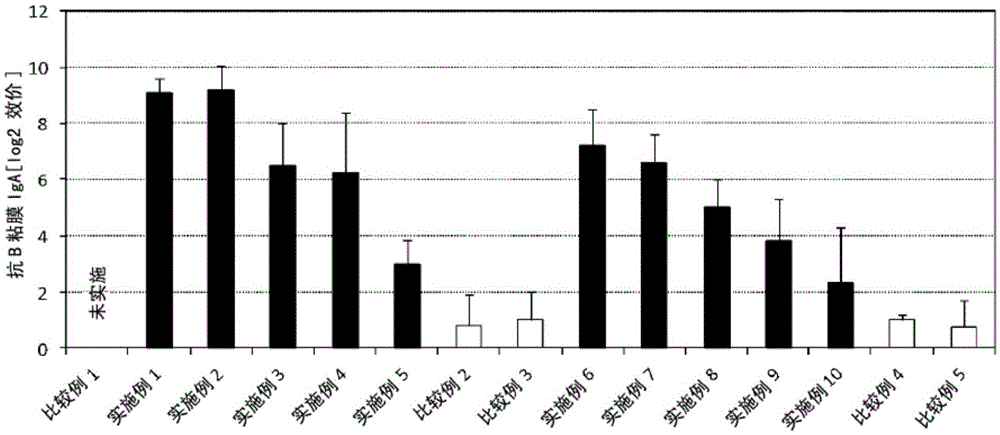
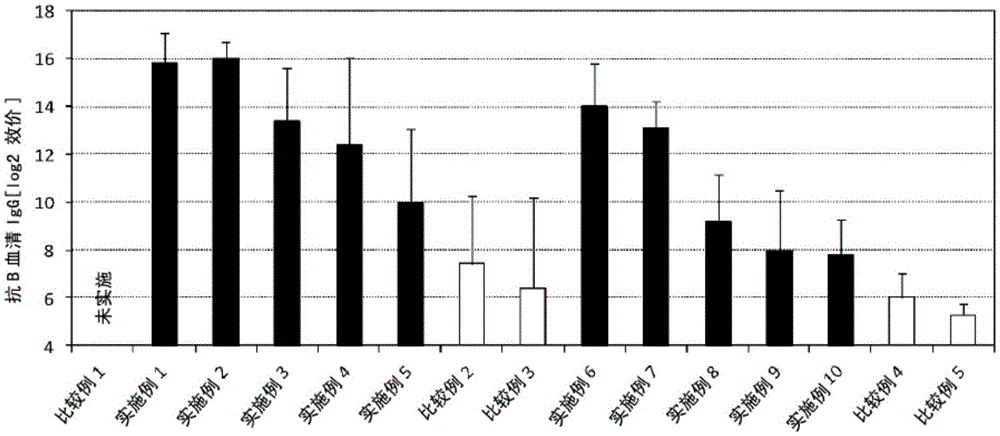
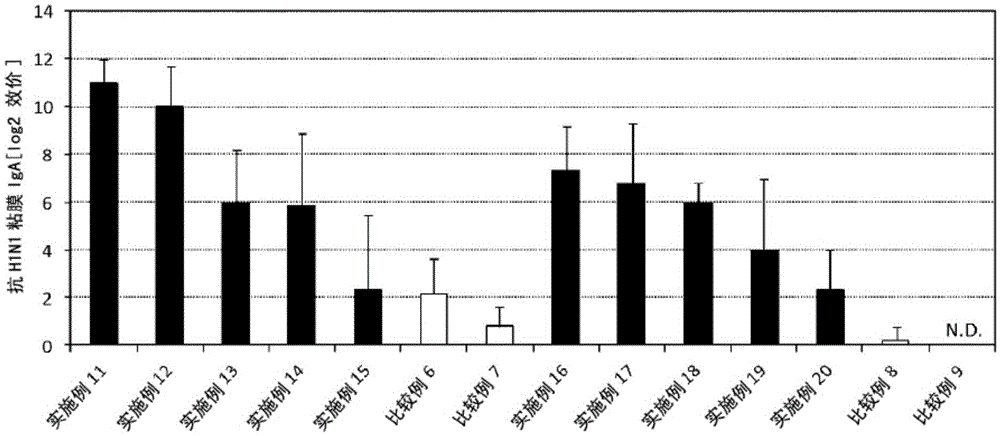
Patents
Literature
31 results about "Genus Serratia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
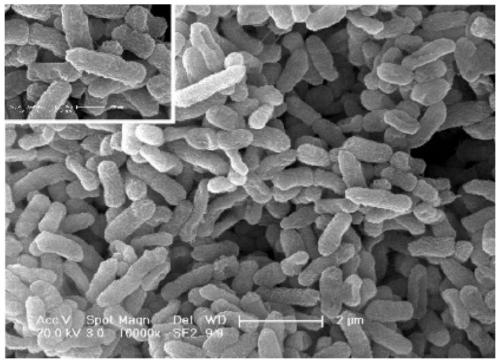
Serratia is a genus of Gram-negative, facultatively anaerobic, rod-shaped bacteria of the Enterobacteriaceae family. The most common and pathogenic of the species in the genus, S. marcescens, is normally the only pathogen and usually causes nosocomial infections.
Method for ecologically restoring polluted water by applying compound microorganism technology
InactiveCN103723837APromote growthIncrease concentrationBiological water/sewage treatmentNitrifying bacteriaTotal nitrogen
The invention discloses a method for ecologically restoring polluted water by applying a compound microorganism technology. A compound microorganism thallus concentrated solution consists of the following 10 thalluses in parts by weight: 5 to 15 parts serratia, 5 to 15 parts of photosynthetic bacteria, 5 to 15 parts of yeast cell, 5 to 15 parts of denitrifying bacteria, 5 to 15 parts of lactic acid bacteria, 5 to 15 parts of actinomycetes, 5 to 15 parts of BD bacteria, 5 to 15 parts of nitrogen-fixing bacteria, 5 to 15 parts of nitrifying bacteria and 5 to 15 parts of bacillus subtilis, wherein the total bacterial count in the compound microorganism thallus concentrated solution is not less than 109 pic / ml. According to the invention, the treating process is environment-friendly, healthful and safe, undesirable odor such as hydrogen sulfide and ammonia nitrogen is not easy to generate, the transparency of the restored water is more than 1.5 m, and the removal ratio on algae, total nitrogen, total phosphorus and COD is 95-99%, 95-99% , 90-95% and 90-95% respectively.
Owner:刘军亮
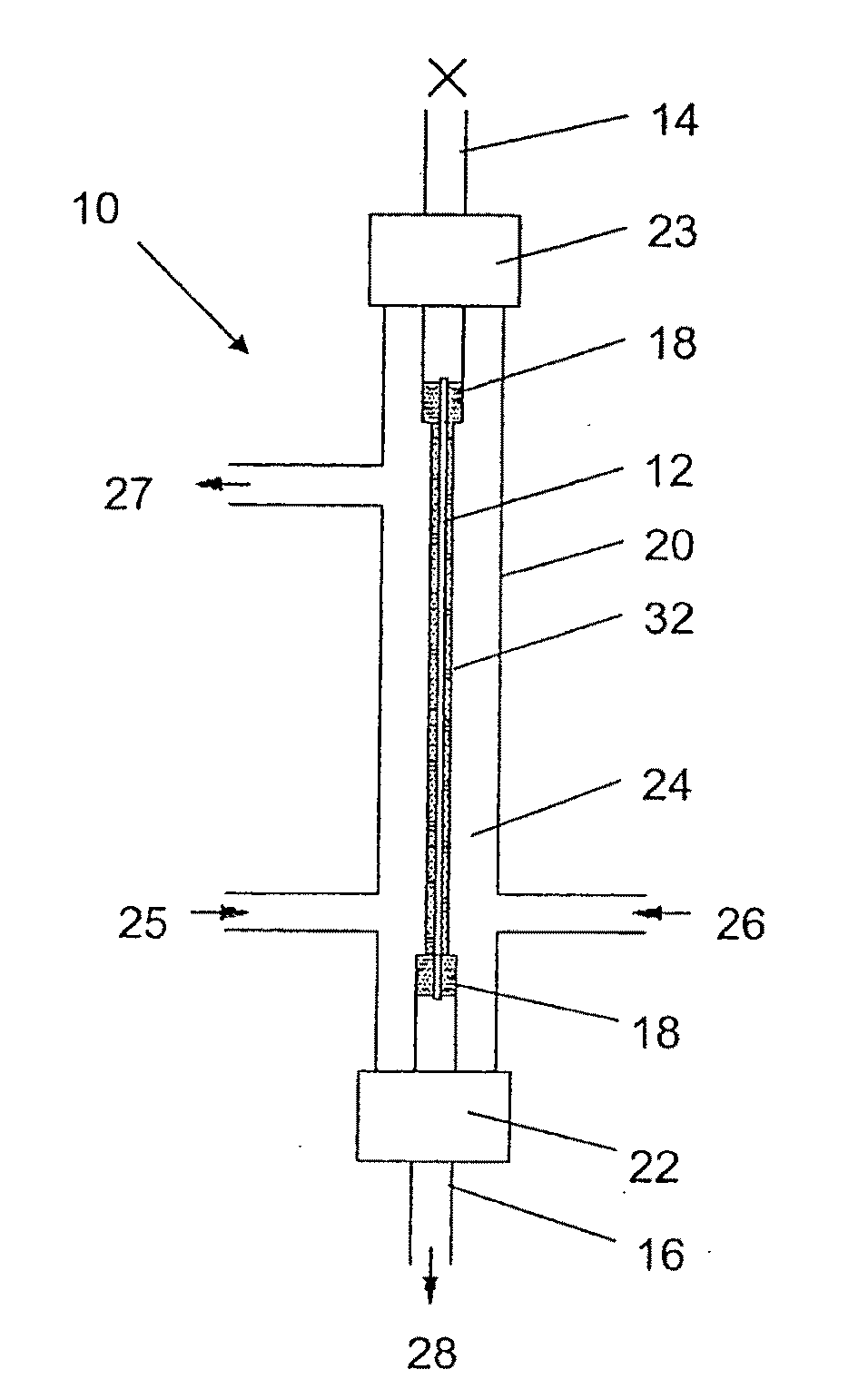
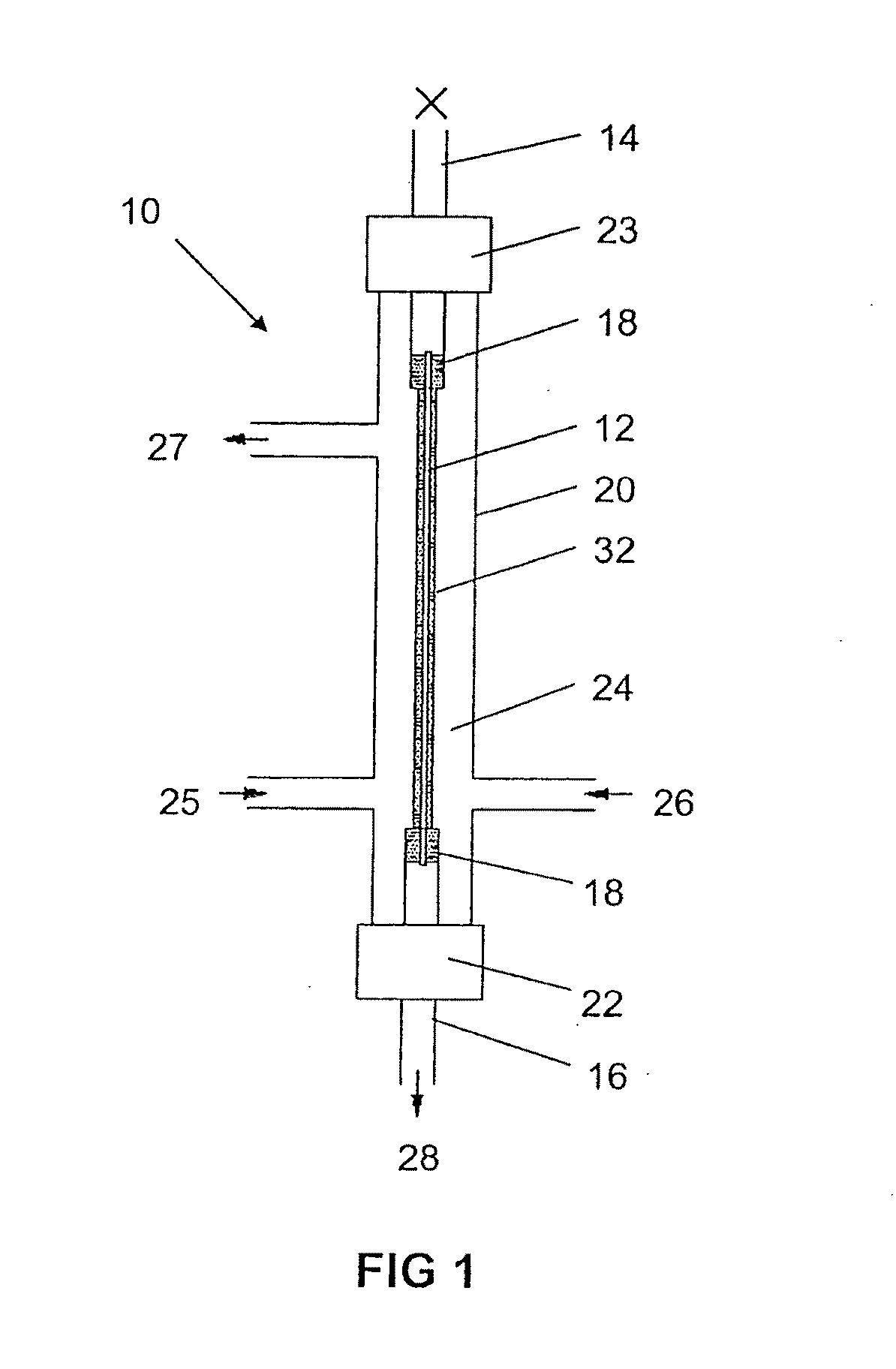
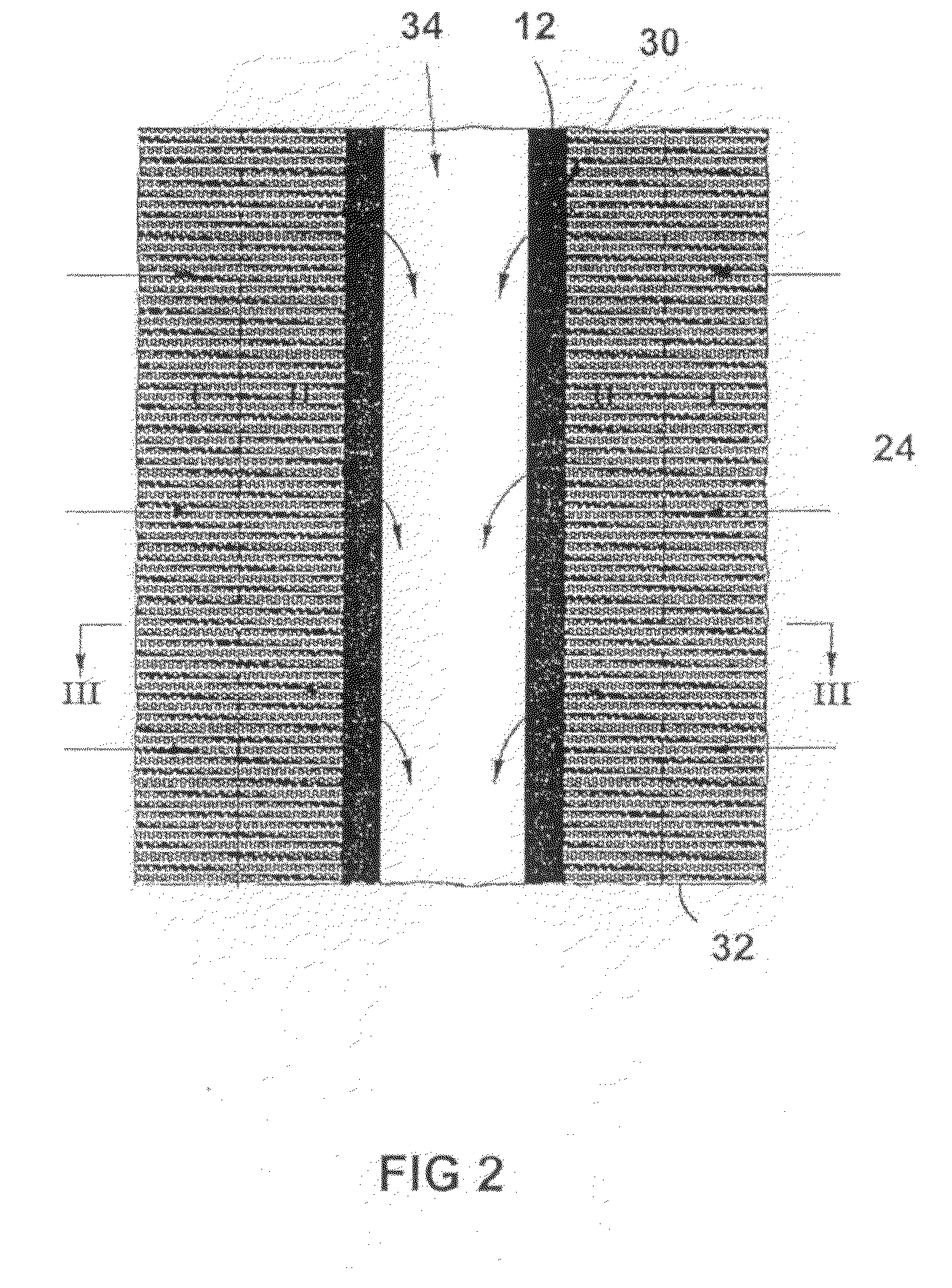
Production of secondary metabolites using capillary membranes
InactiveUS20100159555A1Increase productivityImprove stabilityFermentationMicroorganism fixing/supporting apparatusPorous substrateEscherichia coli
Owner:SYNEXA LIFE SCI (PTY) LTD
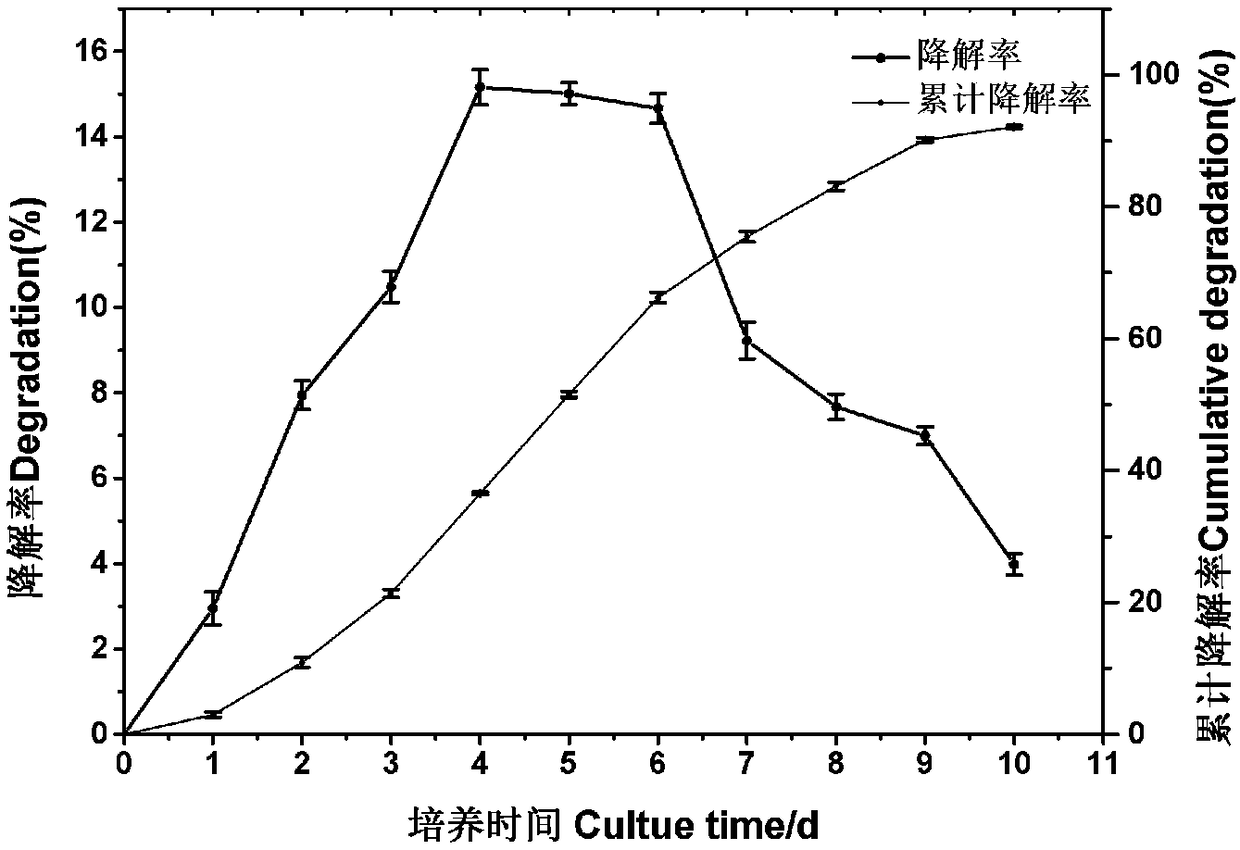
Serratia marcescens strain and application thereof to production of ligninolytic enzyme and degradation of lignin
InactiveCN108504599AEfficient degradationIncrease enzyme activityBacteriaMicroorganism based processesSerratia speciesLigninolytic enzymes
The invention provides a serratia marcescens strain and application thereof to production of a ligninolytic enzyme and degradation of lignin and belongs to the technical field of lignin degradation. The serratia marcescens strain SM01 (Serratia marcescens) has a preservation number of CGMCC (China General Microbiological Culture Collection Center) NO. 15175. The serratia marcescens strain providedby the invention is applied to the production of the ligninolytic enzyme. The serratia marcescens strain provided by the invention is applied to the degradation of the lignin. The serratia marcescensstrain has an efficient lignin degradation capability and the accumulated degradation rate of the lignin reaches 92 percent or more.
Owner:FUJIAN AGRI & FORESTRY UNIV
Antibodies against flagellin and uses thereof
InactiveUS20090191208A1Avoid loweringIncrease intakeSnake antigen ingredientsImmunoglobulins against bacteriaEscherichia coliCitrobacter
The present invention provides a novel class of monoclonal antibodies which have a high affinity, broad spectrum neutralizing reactivity to flagellin from various Gram-negative bacteria including, but not limited to, E. coli, Salmonella, Serratia, Proteus, Enterobacter, Citrobacter, Campylobacter and Pseudomonas. The present invention further provides methods of treating inflammatory bowel disease (IBD) and methods of treating enterobacterial infections using anti-flagellin antibodies in humans, other animals and birds.
Owner:INOTECK PHARMA CORP
Serratia liquefaciens A13 as well as fermentation culture method and application thereof
The invention discloses Serratia liquefaciens A13 with a preservation number: CGMCC No.9490. The invention discloses a fermentation culture method for the Serratia liquefaciens A13. The fermentation culture method comprises the following steps: selecting YPG or NYBD as a culture medium, and a triangular flask with liquid filling amount of 125mL / 500mL, wherein an initial pH value of the culture medium is 7.0, a culture temperature is 40 DEG C and harvest time is 22-26 hours. The Serratia liquefaciens A13 or bacterial liquid obtained by culture by virtue of the method has a bacteriostatic purpose of inhibiting Aeromonas veronii, can be used for preparing a misgurnus anguillicaudatus breeding drug or can be used as a bacteriostatic drug to be add into misgurnus anguillicaudatus breeding feed.
Owner:HUAIHAI INST OF TECH
Injectable vaccine composition
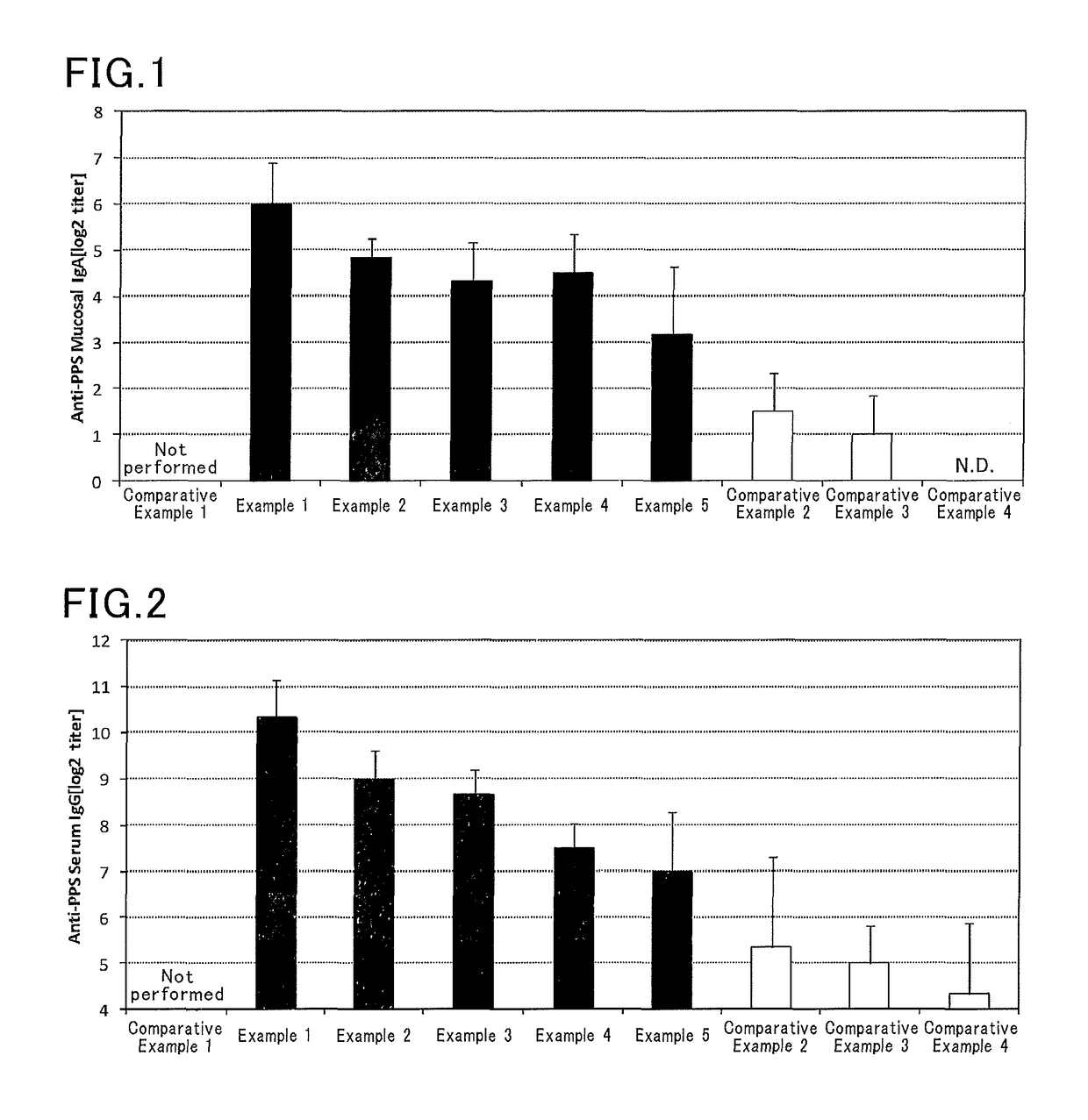
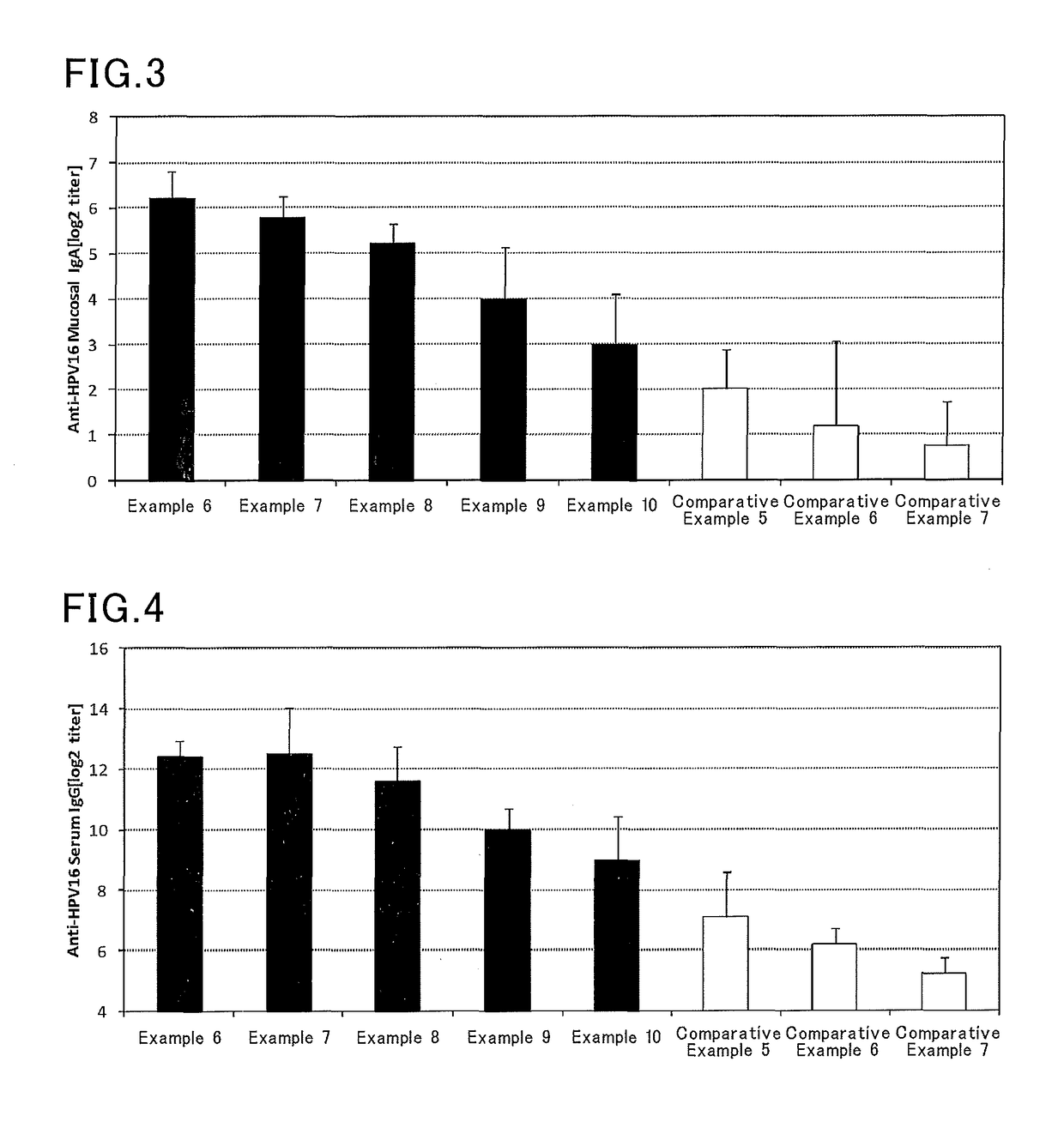
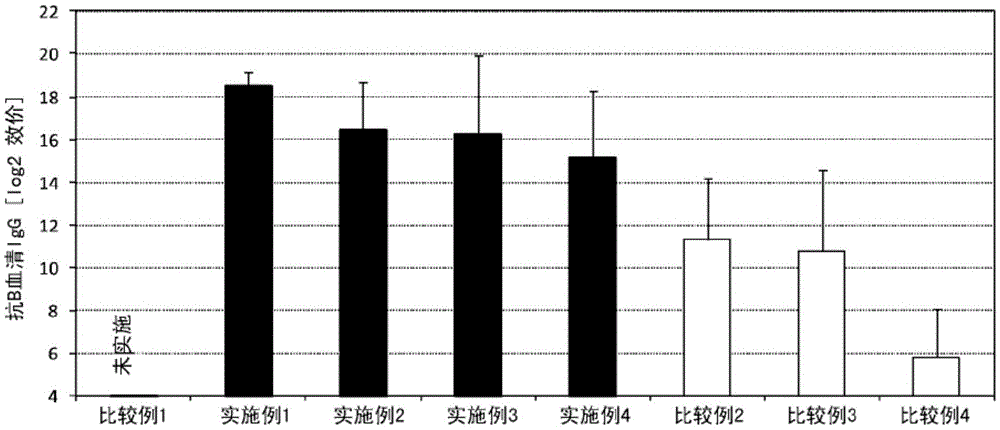
InactiveUS9962439B2Induce systemic immune responsePharmaceutical delivery mechanismAntiviralsBacteroidesAntigen
The present invention aims at providing an injectable vaccine composition that is safe, useful as a prophylactic or therapeutic agent for cancers or infectious diseases, and capable of inducing the systemic immune response safely and effectively. The present invention is an injectable vaccine composition to be administered by injection to a human being or an animal, containing: at least one antigen, and as an adjuvant, a lipopolysaccharide derived from at least one gram negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof.
Owner:NITTO DENKO CORP
Mucosal vaccine composition
ActiveUS20160193327A1Safe and effectiveSsRNA viruses negative-senseAntibacterial agentsMucosal Immune ResponsesXanthomonas
The present invention aims at providing a mucosal vaccine composition that can be administered to an intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, or rectal mucous membrane, that is useful as a prophylactic or therapeutic agent for infectious diseases or cancers, and is capable of safely and effectively inducing the systemic immune response and mucosal immune response. The present invention provides a mucosal vaccine composition to be administered to at least one mucous membrane selected from the group consisting of a human or animal intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, and rectal mucous membrane, containing: at least one antigen; and as an adjuvant, a lipopolysaccharide derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof.
Owner:NITTO DENKO CORP
Injectable vaccine composition
InactiveUS20160287697A1Effectively induce systemic immune responseInduce systemic immune responsePharmaceutical delivery mechanismAntiviralsAntigenBacteroides
The present invention aims at providing an injectable vaccine composition that is safe, useful as a prophylactic or therapeutic agent for cancers or infectious diseases, and capable of inducing the systemic immune response safely and effectively. The present invention is an injectable vaccine composition to be administered by injection to a human being or an animal, containing: at least one antigen, and as an adjuvant, a lipopolysaccharide derived from at least one gram negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof.
Owner:NITTO DENKO CORP
Detection, identification and differentiation of Serratia species using the spacer region
InactiveUS20060099618A1Rapid and reliable hybridizationRapid and reliable detectionSugar derivativesMicrobiological testing/measurementSpecific detectionIts region
The present invention relates to new nucleic acid sequences derived from the ITS (Internal Transcribed Spacer) region, between the 16S and 23S rRNA genes, to be used for the specific detection and / or identification of Serratia species, in particular of Serratia marcescens, Serratia ficaria and / or Serratia fonticola, in a biological sample. The present invention relates also to a method for the specific detection and / or identification of Serratia species, in particular Serratia marcescens, Serratia ficaria and / or Serratia fonticola, using said new nucleic acid sequences derived from the ITS region. It relates also to nucleic acid primers to be used for the amplification of said spacer region of Serratia species in a sample.
Owner:INNOGENETICS NV +1
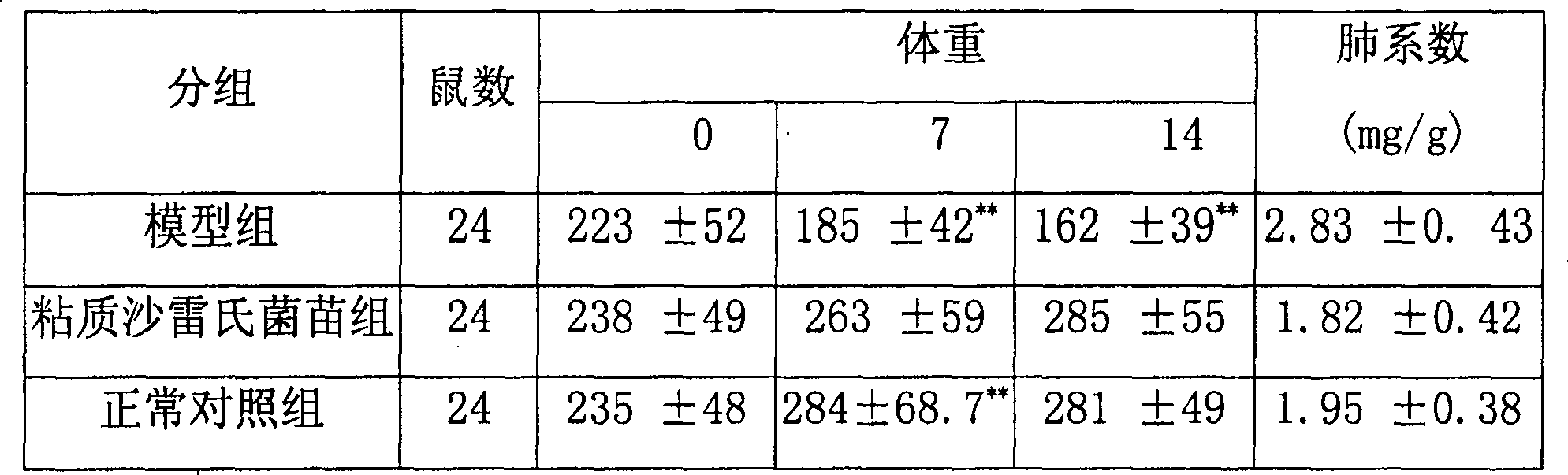
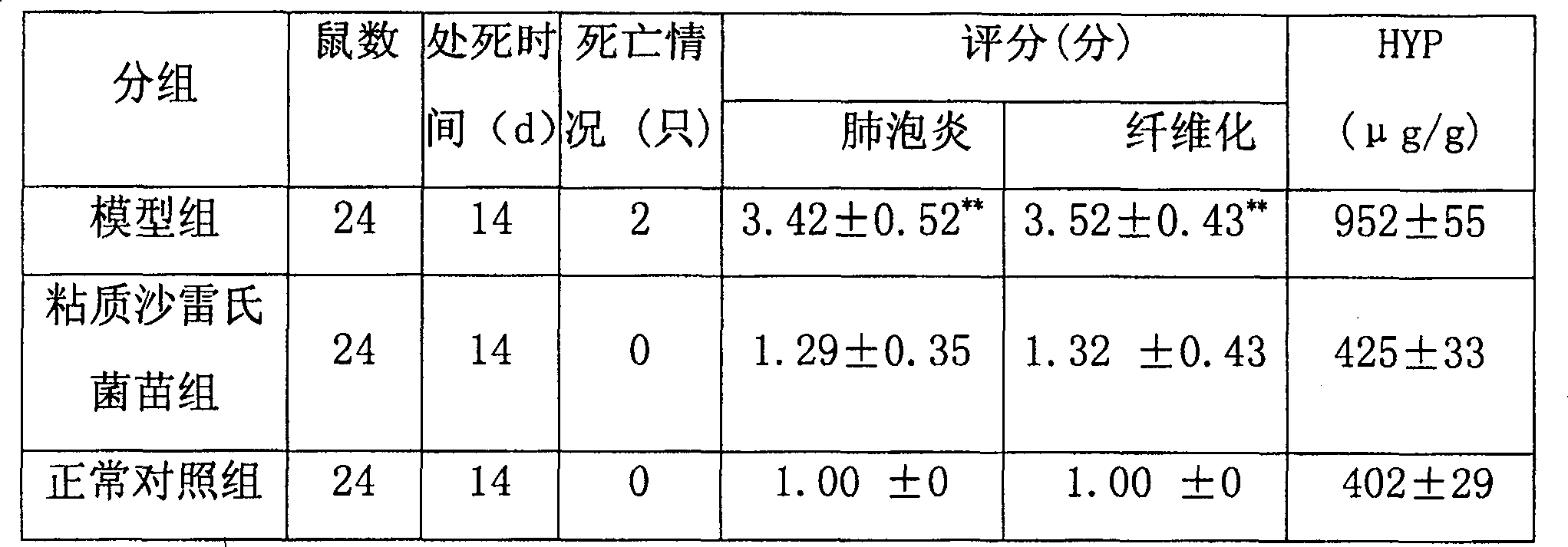
Use of serratia marcesens bacterial vaccine for preparing drug for treating lung fibrosis
The present invention discloses an application of serratia marcescens vaccine in preparation of medicine for curing pulmonary fibrosis. The animal tests show that said serratia marcescens vaccine has obvious action for curing pulmonary fibrosis. As compared with hormone said serratia marcescens has incomparable safety.
Owner:NAVY MEDICINE RES INST OF PLA
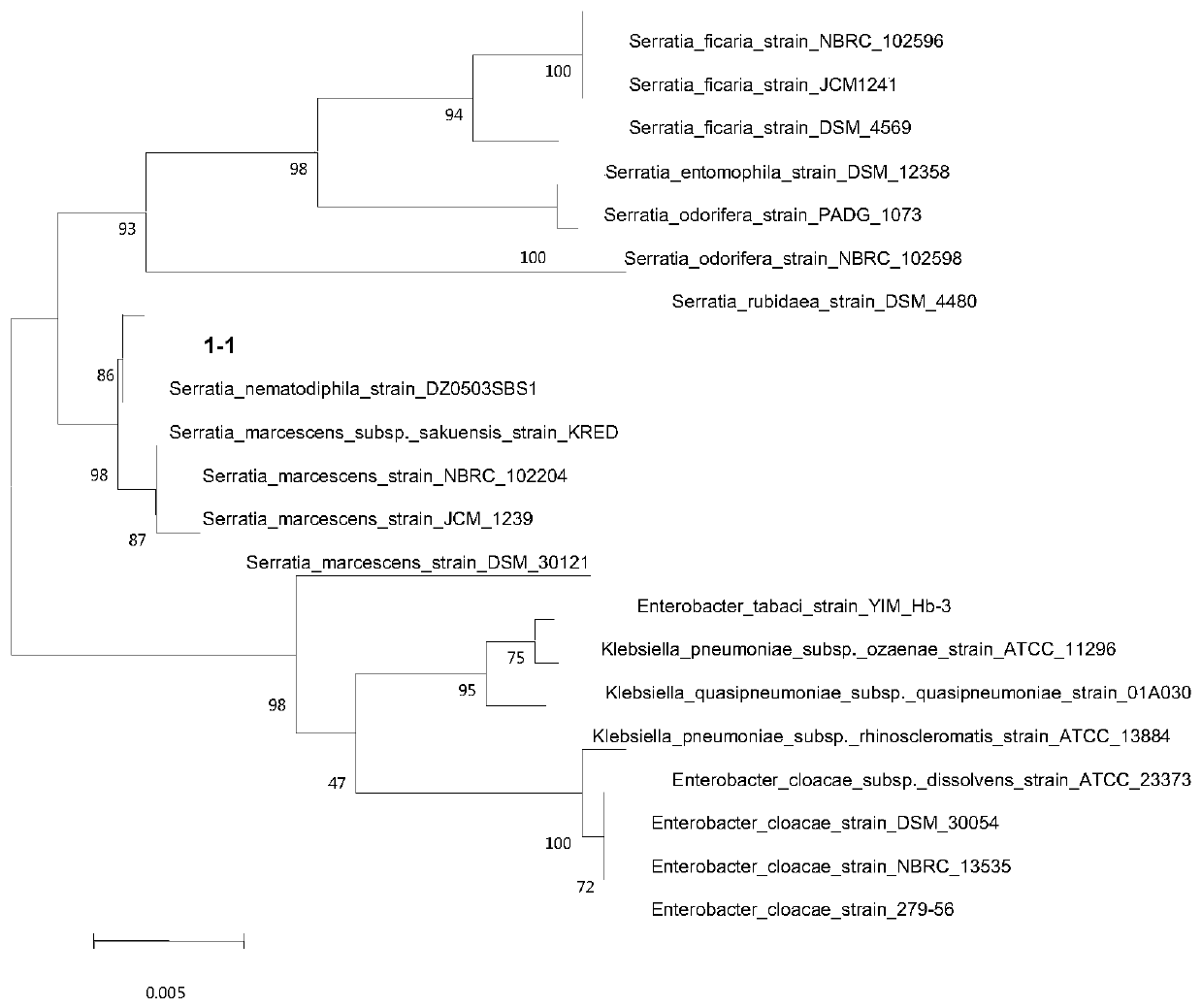
Serratia nematodiphila and acquisition method and application thereof
ActiveCN110317756APromote degradationStable degradationBacteriaWater contaminantsEnvironmental remediationSerratia nematodiphila
The invention discloses a serratia nematodiphila and an acquisition method and application thereof, and belongs to the technical field of bioengineering. The serratia nematodiphila is named as serratia nematophila DH-S01 and deposited in the China center for type culture collection on January 14, 2019, and the preservation number is CCTCCM2019038. The screened serratia nematophila DH-S01 has a strong degradation ability to estradiol. When the serratia nematici DH-S01 is cultured in a medium with estradiol as the sole carbon source for 7 days, the estradiol with the concentration of 100 mg / L can be rapidly degraded, and the degradation rate is 99% or above. Therefore, the serratia nematici DH-S01 can degrade estradiol stably and efficiently, and can be applied to the biodegradation and environmental remediation of estradiol in an environment.
Owner:NORTHEAST NORMAL UNIVERSITY
Immobilized Serratia lipase, preparation method and application for catalyzing and splitting diltiazem chiral precursor
The invention discloses an immobilized Serratia lipase, a preparation method and application for catalyzing and splitting diltiazem chiral precursor. The immobilized lipase can catalyze and split the diltiazem chiral precursor-racemic p-methoxyphenyl glycidic acid methyl ester ((plus or minus)-MPGM). The invention uses chitosan as immobilized carrier of Serratia lipase, uses glutaradehyde as crosslinker and binds the Serratia lipase on the immobilized carrier in a covalent manner. The immobilized lipase is applied to reaction of enzymatic hydrolysis and splitting of the (plus or minus)-MPGM, and can be repeatedly used for a plurality of times, thus showing good operation stability and practical application feasibility.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Polyester resin for low gloss powder paint
ActiveCN110079563AGood lookingReduce glossMicroorganism based processesPowdery paintsSucroseCelsius Degree
The invention discloses polyester resin for low gloss powder paint, which is prepared as follows: a Serratia marcescens strain is applied to the modification of saturated polyester resin. The modification steps are as follows: (1) the Serratia serrata strain is activated for later use; (2) a thallus buffer solution is prepared, wherein the formula is as follows (in mass percentage): 2 to 3% of trehalose, 1.2 to 1.5% of white carbon black, 5 to 8% of sucrose, 1% of glycerol, 1% of polyethylene glycol 200, and the balance of water; (3) bacterial liquid is prepared, specifically, the activated bacterial suspension is dissolved into the thallus buffer solution to adjust the bacterial concentration to 10<7> cfu / ml; (4) the bacterial solution is evenly sprayed to resin powder, wherein the mass ratio of the bacterial liquid to the resin powder is 1 to (50 to 60), and the temperature is maintained at 40 DEG C for bio-fermentation modification for 8 to 10 hours; (5) drying and inactivation areperformed at 80 Celsius degree.
Owner:中山市创华化工实业有限公司
Preparation method of whole cell suspension of streptococcus pyogenes and serratia marcescens
InactiveCN102232972ASignificant effectPromote sheddingBacteria material medical ingredientsSolution deliveryStreptococcus pyogenesBacteroides
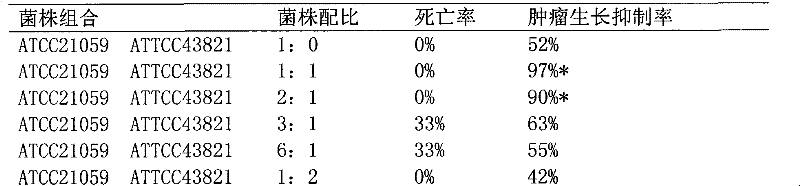
The invention discloses a preparation method of a whole cell suspension of streptococcus pyogenes and serratia marcesens, comprising the following steps of: respectively culturing streptococcus pyogenes and serratia marcesens to obtain whole cell culture solutions, then mixing the two bacteria according to the ratio of 1:1 or 2:1 as per the bacterial number, and after heat inactivation, diluting by a phenol normal saline of 1-3g / l containing phenol or resorcinol to obtain the whole cell suspension. In the whole cell suspension of streptococcus pyogenes and serratia marcesens prepared by the invention, the total number of bacteria of the streptococcus pyogenes and the serratia marcesens is 100000-3000000 per milliliter, and the whole cell suspension of streptococcus pyogenes and serratia marcesens can be used for treating tumor by the routes of administration such as intratumor injection, intracavitary injection or intramuscular injection.
Owner:SHANDONG UNIV
Nasal mucosal vaccine composition
InactiveUS10071155B2Safely and effectively induce humoral immunitySsRNA viruses negative-senseAntibacterial agentsMucosal Immune ResponsesInfectious illness
The present invention provides a nasal mucosal vaccine composition which is safe, useful as a preventive or therapeutic agent for infectious diseases or cancers, and capable of inducing systemic immune responses and mucosal immune responses effectively. The present invention provides a nasal mucosal vaccine composition to be administered to a human or animal nasal mucous membrane, the nasal mucosal vaccine composition containing at least one antigen excluding antigens derived from influenza viruses; and as an adjuvant, a lipopolysaccharide derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof.
Owner:NITTO DENKO CORP
Method for the purification and stabilisation of enzyme gluconate dehydrogenase (gadh, ec 1.1.99.3), enzyme gluconate dehydrogenase (gadh, ec 1.1.99.3), and the use of enzyme gluconate dehydrogenase (gadh, ec 1.1.99.3)
InactiveUS20130260407A1Microbiological testing/measurementBiological material analysisK pneumoniaeEscherichia coli
Process of purification and stabilization of the enzyme Gluconate Dehydrogenase (GADH, EC 1.1.99.3) either recombinant or not, from several microorganisms such as: Pseudomonas aeruginosa, Pseudomonas fluoresoens, Gluconobacter oxydans, Gluconobacter industrius, Serratia maroescens Kiebsielia pneumoniae and Eschericia coli and its use as an element of biological recognition in biosensors for the determination of gluconic acid in samples of interest.Bro-catalytic biosensor with electrochemical transduction free of interfering chemicals thanks to the high selectivity of the enzyme obtained by the specified method and stabilized with the optimized chemical agents
Owner:BIOLAN MICROBIOSENSORES
Injectable vaccine composition
InactiveCN105530953AInduce systemic immune responsePharmaceutical delivery mechanismAntiviralsAntigenEnterobacter
The purpose of the present invention is to provide an injectable vaccine composition that is safe and useful as a prophylactic or therapeutic agent for cancer or infectious diseases and can safely and effectively induce a systemic immune response. An injectable vaccine composition that is to be administered by injection to human or animals, characterized by comprising at least one kind of antigen and a lipopolysaccharide or a salt thereof as an immunostimulator, said lipopolysaccharide being derived from at least one kind of gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas and Enterobacter.
Owner:NITTO DENKO CORP
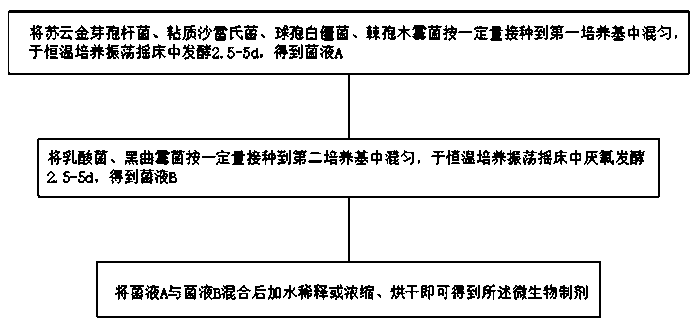
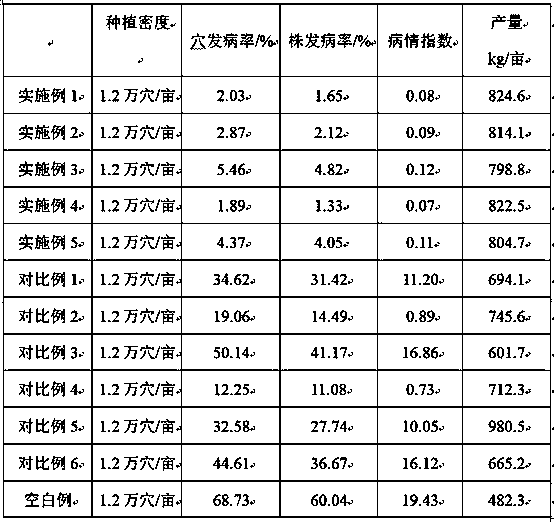
Microbial preparation and organic fertilizer for prevention and treatment of rice banded sclerotial blight
ActiveCN110484464AActivate defense systemIncrease resistanceBiocideFungiAureobasidium sp.Comparative test
The invention provides a microbial preparation and organic fertilizer for prevention and treatment of rice banded sclerotial blight, and relates to the technical field of fertilizer. The microbial preparation includes bacillius thuringiensis, serratia marcesces, beauveria bassiana, trichoderma asperellum, lactic acid bacteria and aspergillus niger. The microbial preparation has good prevention andtreatment effect on rice banded sclerotial blight, and no matter the incidence rate of holes or the incidence rate of strains of experimental fields with the microbial preparation are 10% or less, and through comparative tests, bacillius thuringiensis, serratia marcesces, beauveria bassiana, trichoderma asperellum, lactic acid bacteria and aspergillus niger in the microbial preparation are indicated to have good synergistic effect on each other.
Owner:南京宁粮环保集团有限公司
Mucosal vaccine composition
InactiveCN105555308AImmune inductionSsRNA viruses negative-senseSsRNA viruses positive-senseMucosal Immune ResponsesGenus Serratia
The purpose of the present invention is to provide a vaccine composition which is safe and effective as a preventive medicine or a treatment medicine for infections and / or cancer, can effectively induce a systemic immune response and a mucosal immune response, and which is capable of being administered to the oral mucosa, ocular mucosa, ear mucosa, genital mucosa, pharyngeal mucosa, respiratory mucosa, bronchial mucosa, pulmonary mucosa, gastric mucosa, intestinal mucosa, or rectal mucosa. The present invention is a mucosal vaccine composition administered to at least one type of mucous membrane of a person or animal, the mucous membrane being selected from a group comprising the oral mucosa, ocular mucosa, ear mucosa, genital mucosa, pharyngeal mucosa, respiratory mucosa, bronchial mucosa, pulmonary mucosa, gastric mucosa, intestinal mucosa, and rectal mucosa. The mucosal vaccine composition is characterized by containing at least one type of antigen, and, as an immunostimulant agent, a lipopolysaccharide or a salt thereof derived from at least one type of a gram-negative bacterium selected from a group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter. Moreover, the mucosal vaccine composition is characterized in that the mass ratio of the abovementioned immunostimulant agent and antigen (the total mass of the immunostimulant agent / the total mass of the antigen) is between 0.002 and 500.
Owner:NITTO DENKO CORP
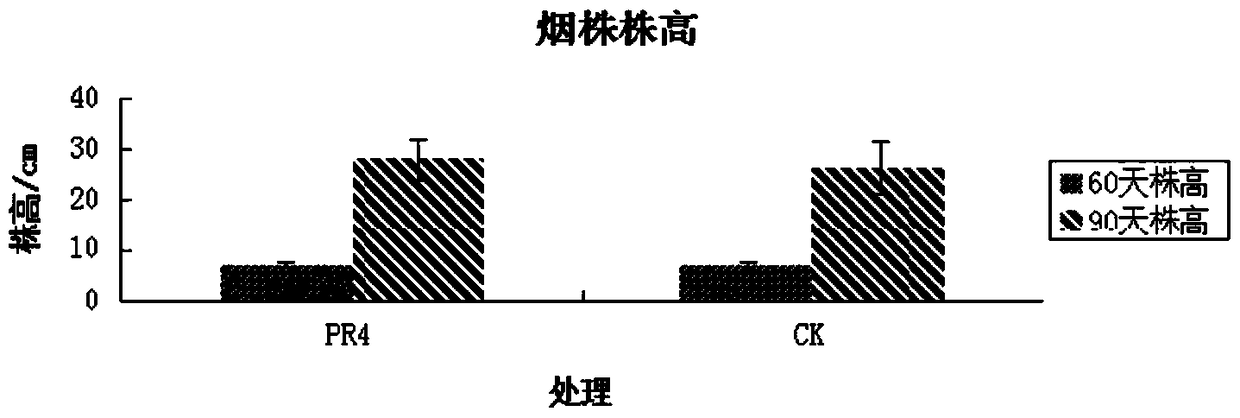
A strain of Serratia nematophila and its application
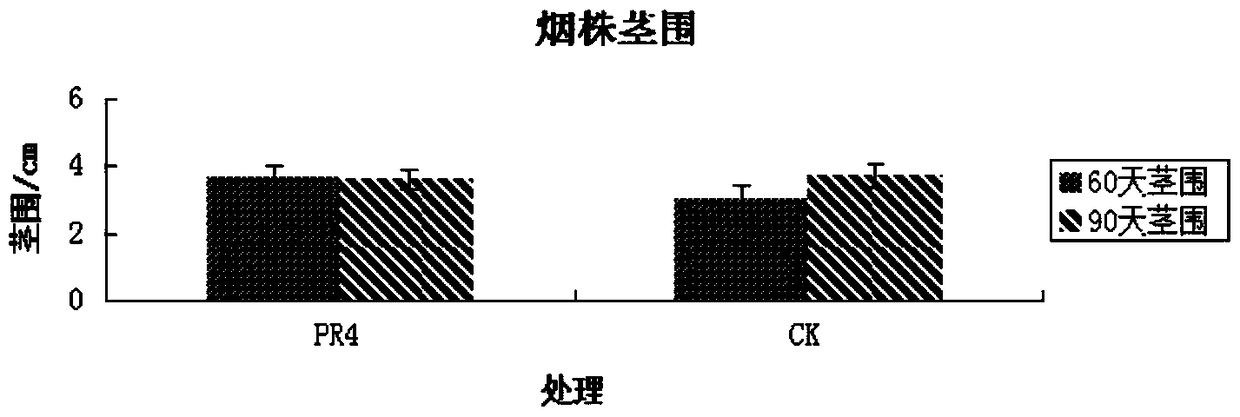
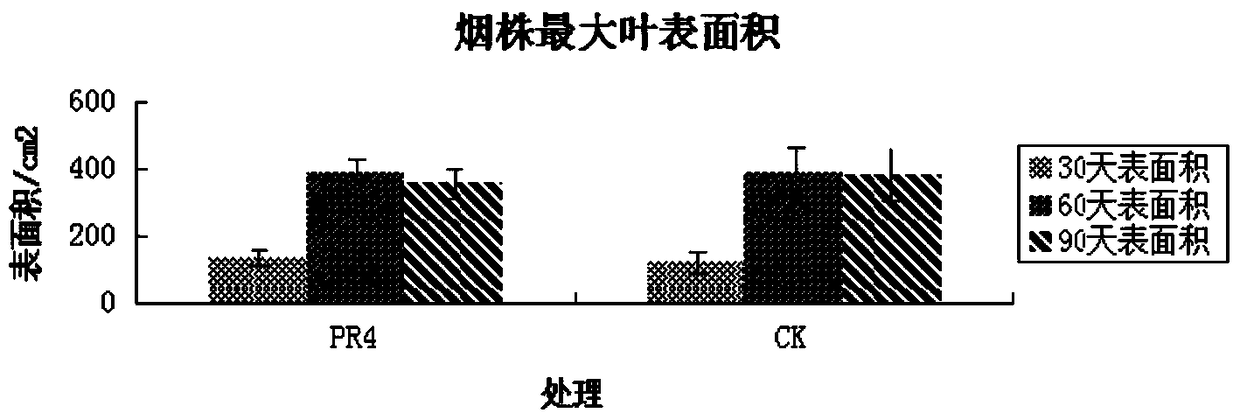
The invention discloses a strain of Serratia nematophila and its application. The strain is classified as Serratia nematodiphila PR4, depository unit: General Microbiology Center (CGMCC), China Committee for Microbial Culture Collection, address: No. 3, Yard No. 1, Beichen West Road, Chaoyang District, Beijing, preserved Date: August 28, 2014, deposit number: CGMCC No.9614. The bacterial strain of the invention has good degradation effect on protein. The enzyme activity of the protease produced by the strain was determined to be 3.03IU by the Folinfen method. The strain has a good phosphorus-dissolving effect measured by the molybdenum blue colorimetric method. It indicated that the PR4 strain had potential application value in promoting the growth of tobacco.
Owner:CHINA TOBACCO HUNAN INDAL CORP
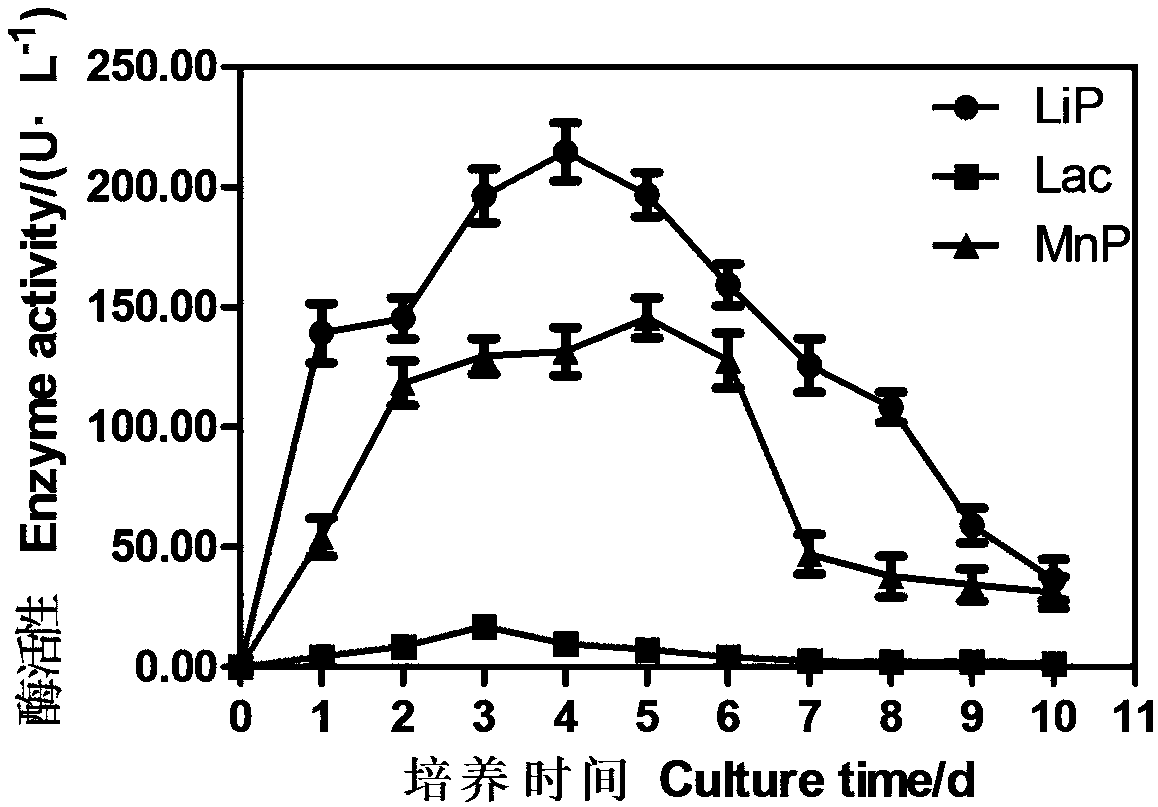
Microorganism of serratia family, isolation method and the preparation method of lignin lyases using this
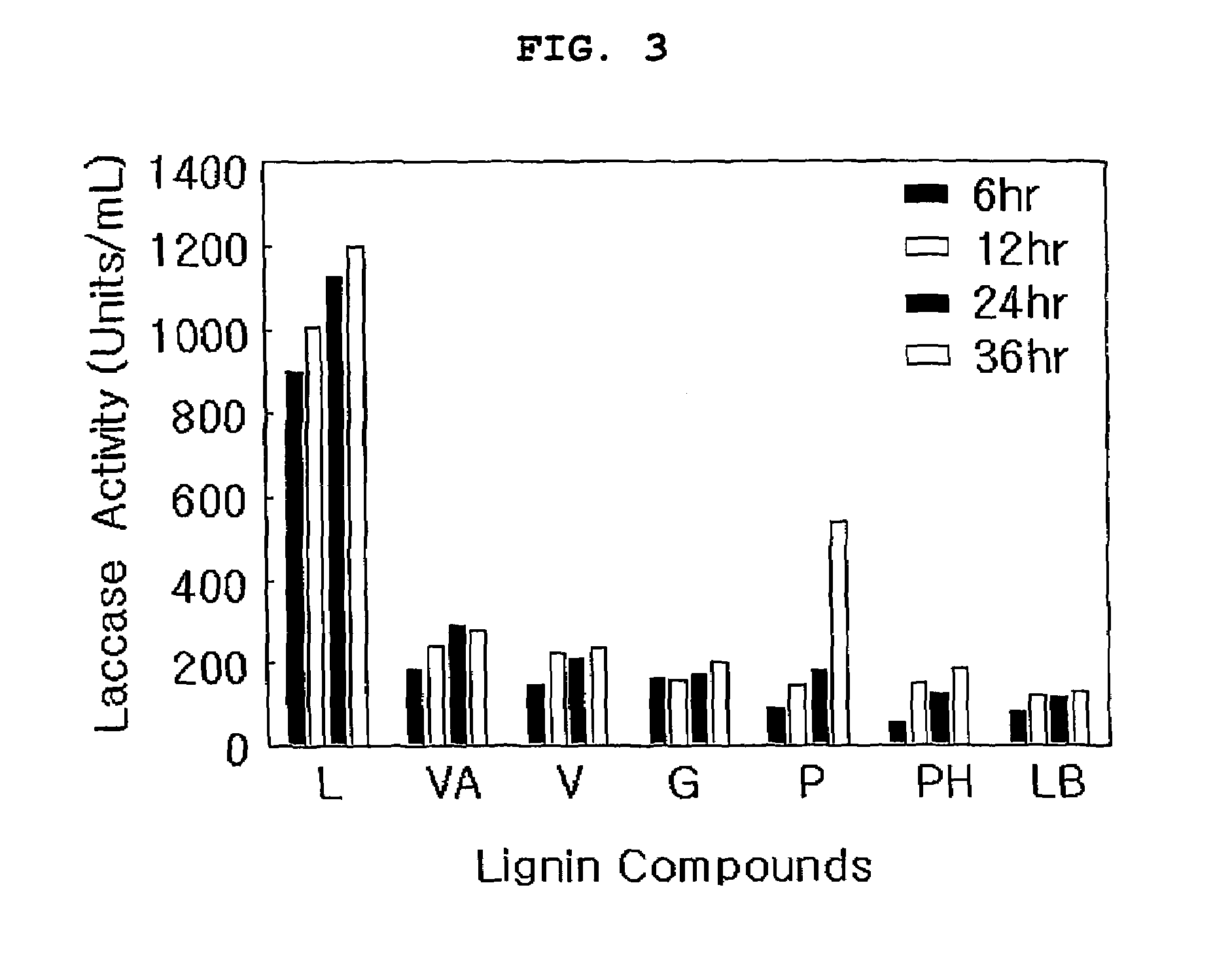
The present invention relates to a novel microbe belonging to Serratia family, its isolation method and the preparation method using it. More particularly, the present invention relates to a microorganism of Serratia family producing laccase, lignin peroxidase and Mn-dependent-peroxidase which are the kinds of lignin lyases, and a method for separating the microbe from insect gut, as well as a method for preparing laccase, lignin peroxidase and Mn-dependent-peroxidase. The microorganism of the present invention can be used not only in the treatment of industrial sewage and harmful environmental substance but also in oil degradation, in biological bleaching of pulp since it can produce lignin lyases. More over, the microbe can also be used effectively in industry of preparing fuel, forage and other chemicals through lignin degradation.
Owner:KOREA RES INST OF BIOSCI & BIOTECH +1
Use of serratia marcesens bacterial vaccine for preparing drug for treating lung fibrosis
The present invention discloses an application of serratia marcescens vaccine in preparation of medicine for curing pulmonary fibrosis. The animal tests show that said serratia marcescens vaccine has obvious action for curing pulmonary fibrosis. As compared with hormone said serratia marcescens has incomparable safety.
Owner:NAVY MEDICINE RES INST OF PLA
Mucosal vaccine composition
InactiveCN105530958AImmune inductionSsRNA viruses negative-senseAntibacterial agentsMucosal Immune ResponsesGenus Serratia
The purpose of the present invention is to provide a mucosal vaccine composition that is effective and safe as a prophylactic or therapeutic drug for infections and cancer, which effectively induces a systemic immune response and mucosal immune response, and which can be administered to the oral mucosa, ocular mucosa, ear mucosa, genital mucosa, pharyngeal mucosa, respiratory mucosa, bronchial mucosa, pulmonary mucosa, gastric mucosa, intestinal mucosa, or rectal mucosa. The present invention is a mucosal vaccine composition administered to at least one mucous membrane of a person or animal, said mucous membrane being selected from the group consisting of oral mucosa, ocular mucosa, ear mucosa, genital mucosa, pharyngeal mucosa, respiratory mucosa, bronchial mucosa, pulmonary mucosa, gastric mucosa, intestinal mucosa, and rectal mucosa. The mucosal vaccine composition is characterized by including at least one type of antigen, and, as an immunostimulant, a lipopolysaccharide or a salt thereof derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter.
Owner:NITTO DENKO CORP
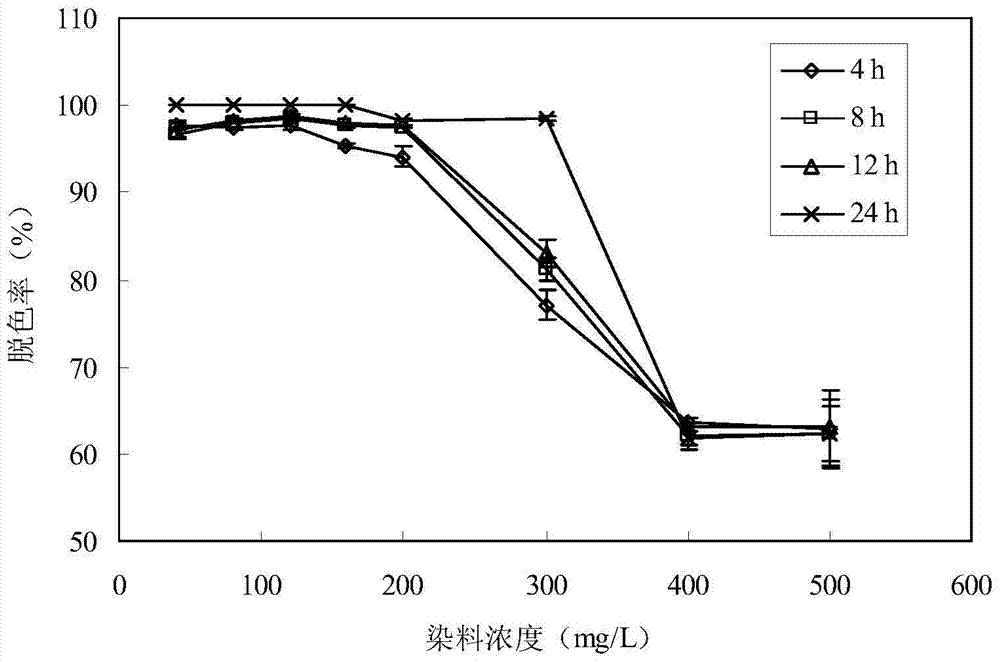
A strain of Serratia for degrading triphenylmethane dye and method for degrading triphenylmethane dye
ActiveCN104974951BImprove degradation efficiencyImprove environmental adaptabilityBacteriaWater contaminantsTriphenylmethaneBacteria
The invention discloses a Serratia strain degrading triphenylmethane dye and a method for degrading triphenylmethane dye, the strain is named Serratia sp. WKD, the preservation number is CCTCC NO: M2015178, and the preservation date As of March 29, 2015, it was deposited in the China Center for Type Culture Collection. The Serratia bacteria that degrades triphenylmethane dyes of the invention has strong environmental adaptability, has high degradation efficiency for triphenylmethane dyes malachite green and crystal violet, and has significantly better decolorization efficiency than reported strains, and has extremely high application prospects. It is broad and can solve the practical problem that the water body polluted by triphenylmethane dyes is difficult to control. Compared with the traditional physical and chemical treatment methods with high cost, low efficiency and easy to produce secondary pollution, the Serratia degraded triphenylmethane dye screened by the present invention is an environment-friendly treatment method, with low treatment cost and high efficiency, and has the advantages of low cost and high efficiency. With a certain broad spectrum, it is expected to truly realize the processing requirements of "low consumption".
Owner:WENZHOU VOCATIONAL COLLEGE OF SCI & TECH
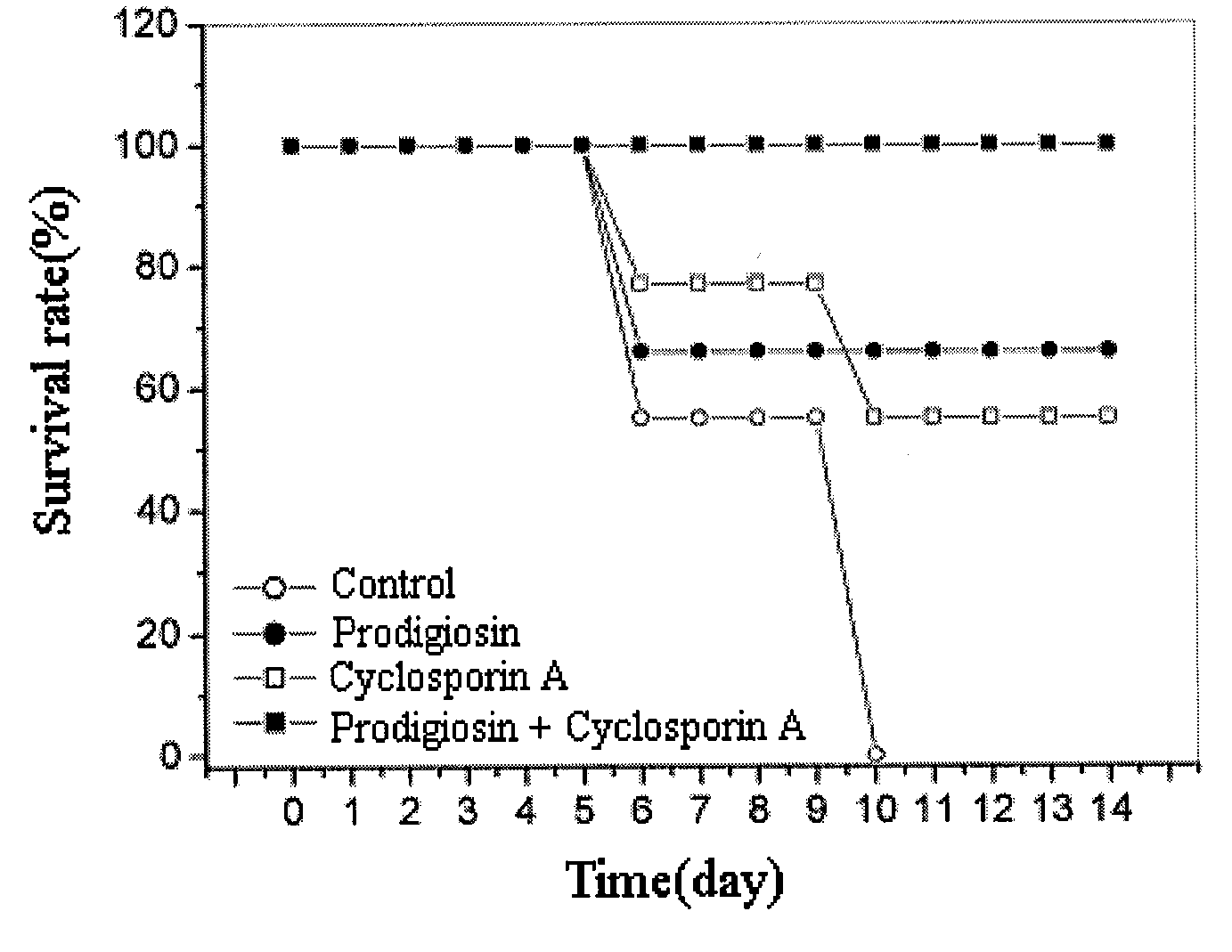
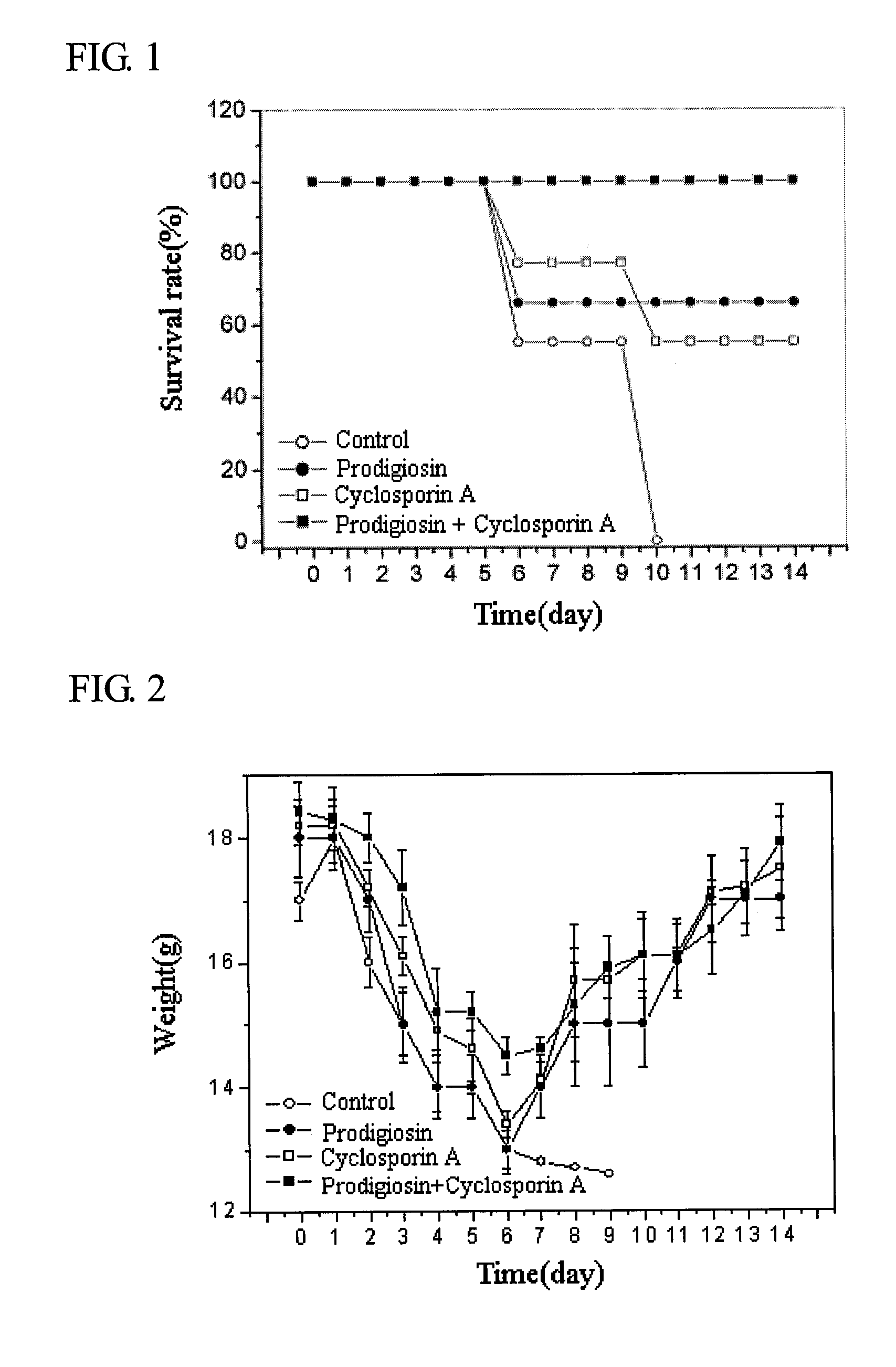
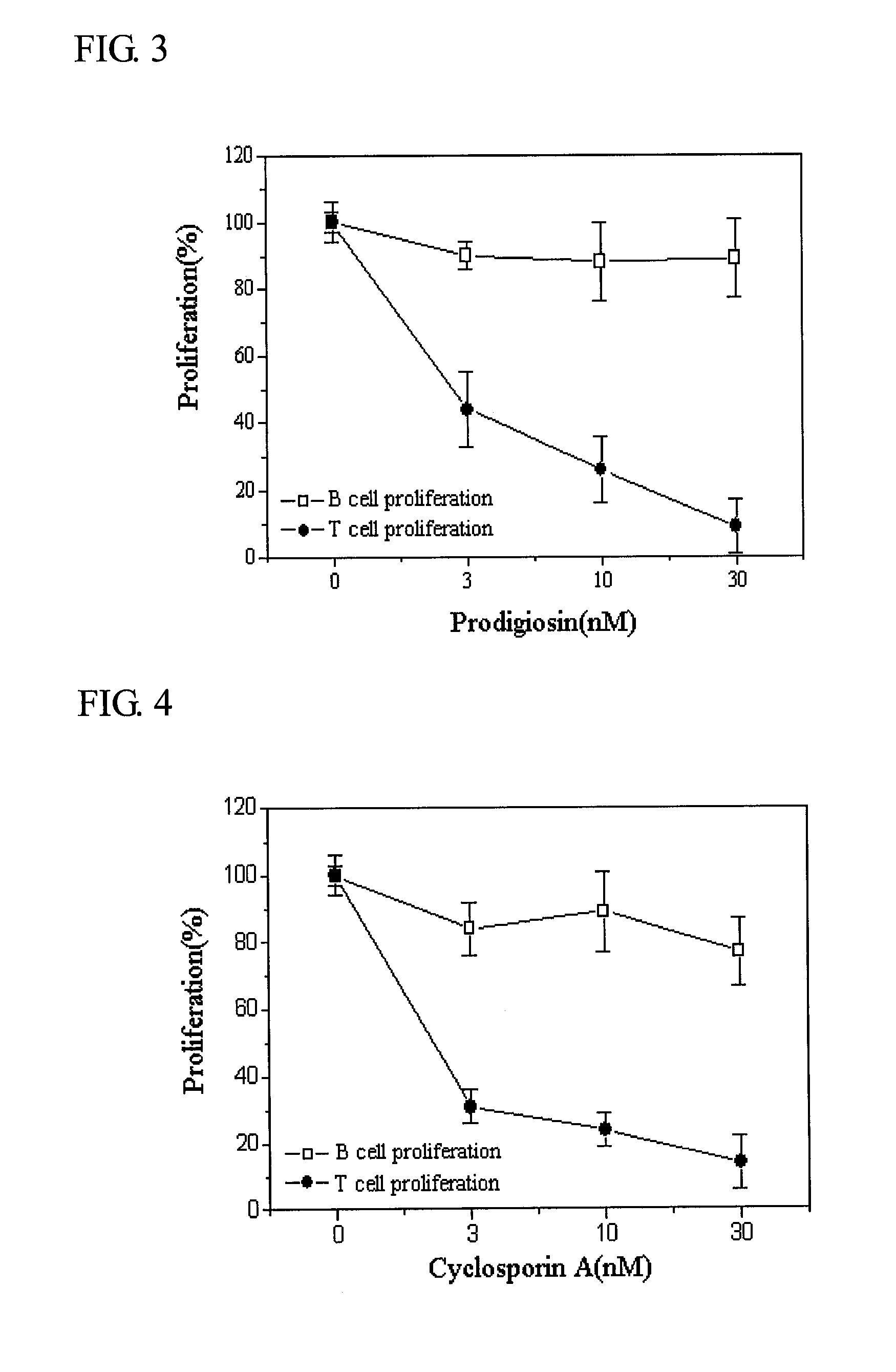
Compound Comprising Prodigiosin From Serratia Macescence B-1231 Kctc 0386Bp for Prevention and Treatment of Acute Graft-Versus-Host Disease
InactiveUS20090048328A1Suppressing proliferation of T-cellsGood effectBiocideAnimal repellantsMode of actionMarcescence
This invention relates to a composition for the prevention and treatment of acute graft-versus-host disease comprising prodigiosin isolated from Serratia marcescence B-1231 KCTC 0386BP, as an effective ingredient. The prodigiosin is immunosup-pressive by selectively suppressing the proliferation of T-cells through the suppression of the expression of IL-2 receptors, which is needed for the activation of T-cells. Prodigiosin can be used either alone or in conjunction with cyclosporin A for greater effect, since the two substances have different mode of actions. These make prodigiosin effective for the prevention and treatment of acute graft-versus-host disease.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
A strain of Serratia with rich phosphorus, organic phosphorus degradation and inhibition of plant pathogenic fungi
ActiveCN106119168BHas the ability to inhibit pathogenic fungiPromote degradationBacteriaMicroorganism based processesOrganophosphorus pesticidesShort terms
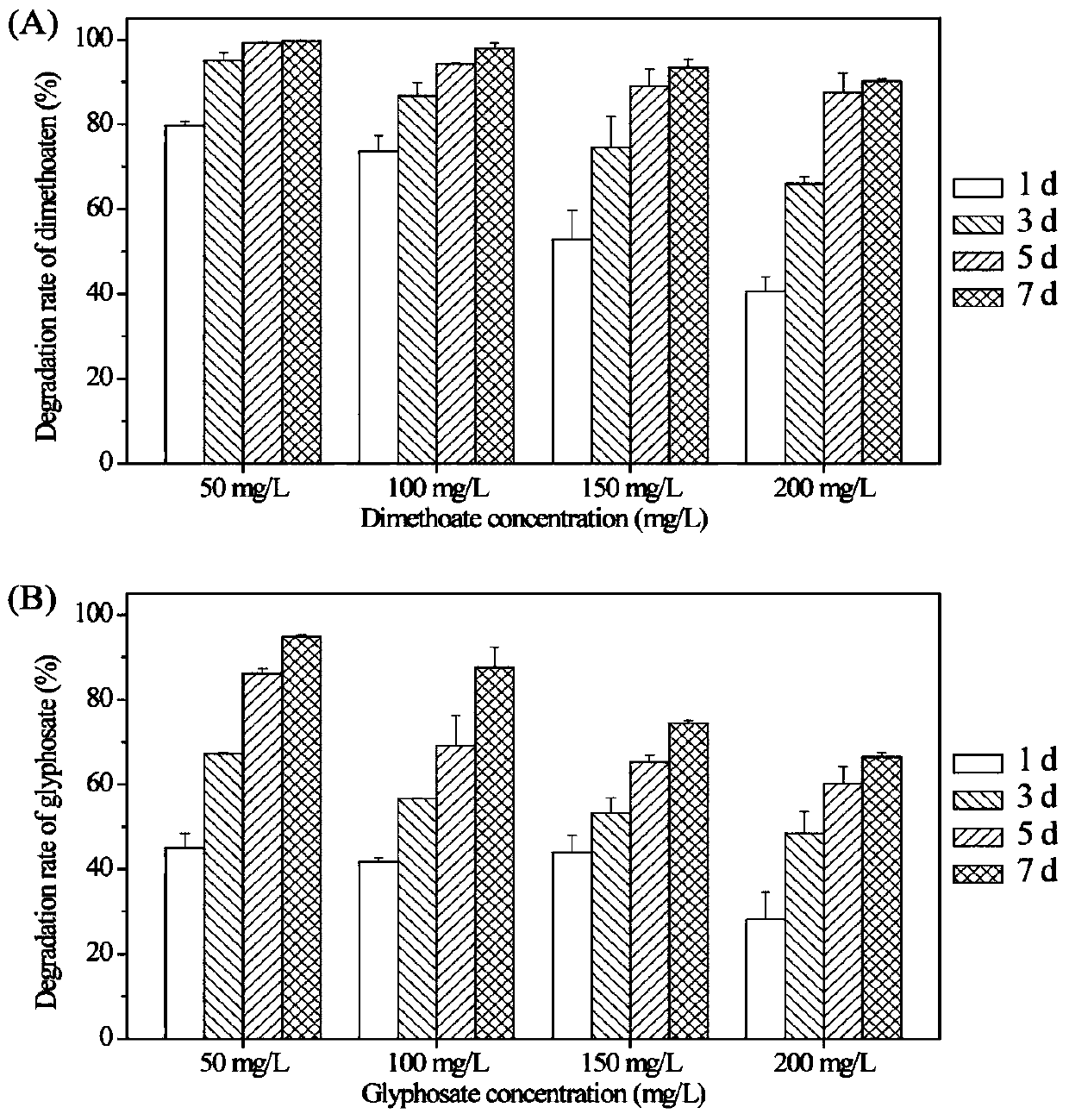
The invention discloses a serratia strain which is rich in phosphorus, degrades organic phosphorus and inhibits plant pathogenic fungi, and belongs to the technical field of microorganisms. the strain Serratia sp.MEW06 is preserved in the China Type Culture Collection Center, and the preservation number is CCTCC NO.M 2015779. The Serratia bacterium of the present invention has a short-term and high-efficiency phosphorus-enriching ability, which can be used to prepare biological fertilizers, and also has the effect on pathogenic fungi of crops. Disease suppression, can be applied to microbial control, at the same time, the bacterial strain has a good degradation effect on organophosphorus pesticide glyphosate and dimethoate, can effectively remove organophosphorus pesticide pollution, Serratia of the present invention has a wide range of applications scope.
Owner:沃达农业科技股份有限公司 +2
Vaccine pharmaceutical composition for transdermal administration
InactiveUS10420837B2Improve the quality of lifeImprove complianceBacterial antigen ingredientsViral antigen ingredientsAntigenInfectious illness
The present invention provides a vaccine pharmaceutical composition for transdermal administration which is safe, usable as a prophylactic or therapeutic agent for cancer or infectious diseases, and capable of safely and effectively inducing a systemic immune response. It can be administered to a human or animal skin, the vaccine pharmaceutical composition including: a lipopolysaccharide or its salt, derived from at least one gram-negative bacterium such as Serratia, Lelercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, as an immunostimulant; and at least one antigen, the vaccine pharmaceutical composition having a mass ratio between the immunostimulant and the antigen, the total mass of the immunostimulant / the total mass of the antigen, of 0.002 to 500.
Owner:NITTO DENKO CORP
Detection, identification and differentiation of serratia species using the spacer region
InactiveUS20090191558A1Rapid and reliable detectionRapid and reliable identificationSugar derivativesMicrobiological testing/measurementSpecific detectionIts region
The present invention relates to new nucleic acid sequences derived from the ITS (Internal Transcribed Spacer) region, between the 16S and 23S rRNA genes, to be used for the specific detection and / or identification of Serratia species, in particular of Serratia marcescens, Serratia ficaria and / or Serratia fonticola, in a biological sample.The present invention relates also to a method for the specific detection and / or identification of Serratia species, in particular Serratia marcescens, Serratia ficaria and / or Serratia fonticola, using said new nucleic acid sequences derived from the ITS region.It relates also to nucleic acid primers to be used for the amplification of said spacer region of Serratia species in a sample.
Owner:INNOGENETICS NV +1
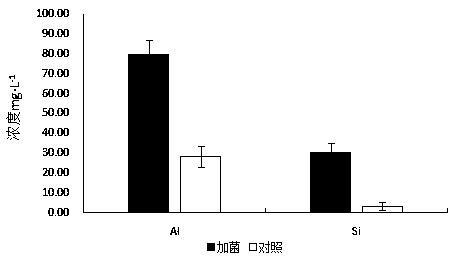
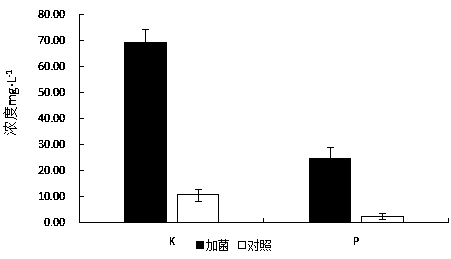
A kind of high-efficiency erosion bacterium Serratia liquefaction nlx-15 of silicate rock and its application
ActiveCN105861384BImprove erosion abilityFacilitated releaseBiocidePlant growth regulatorsStrong solutionsDissolution
The invention discloses a silicate rock high-efficiency corrosion bacterium Serratia liquefaction NLX-15 and application thereof. The efficient erosion bacteria of silicate rock, its taxonomic name is Serratia liquefactionus Serratia liquefaciens NLX‑15 has been deposited in the China Center for Type Culture Collection, accession number CCTCC NO: M2016239, preservation date: May 3, 2016; preservation address: Wuhan University, Wuhan, China. Serratia liquefaction NLX‑15, an efficient rock erosion bacterial strain screened out by the present invention, has a strong erosion ability to silicate rocks, can promote the release of major ions such as Ca and Al in rocks, and accelerate the erosion of limestone into soil; at the same time It also has a strong potential to decompose K and P; at the same time, it can promote the growth of plants after spraying, and has a good application and development prospect.
Owner:NANJING FORESTRY UNIV
A strain of Serratia marcescens resistant to X. citrus
A strain of Serratia marcescens (Serratia marcescens) T861 resistant to canker sores of citrus was preserved in the China Center for Type Culture Collection on April 10, 2015, with the preservation number CCTCC? NO: M2015222. The fermented liquid of the bacterial strain T861 of the present invention has inhibitory effect on X. citrus bacteria, and the in vitro inhibition rate of the aseptic fermented liquid on X. citrus bacteria is more than 86.7% as measured by the acupuncture method.
Owner:HUNAN AGRICULTURAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com