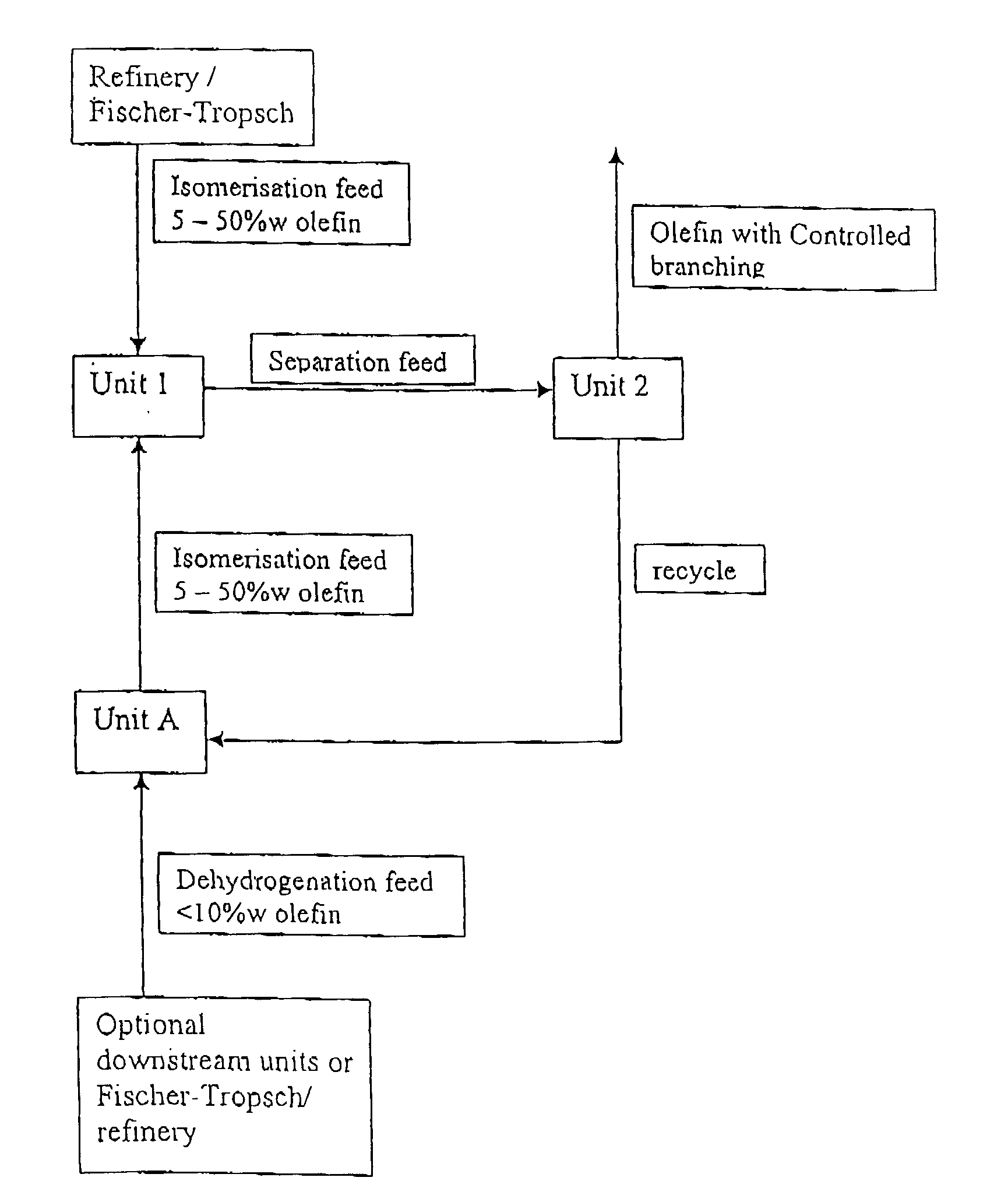
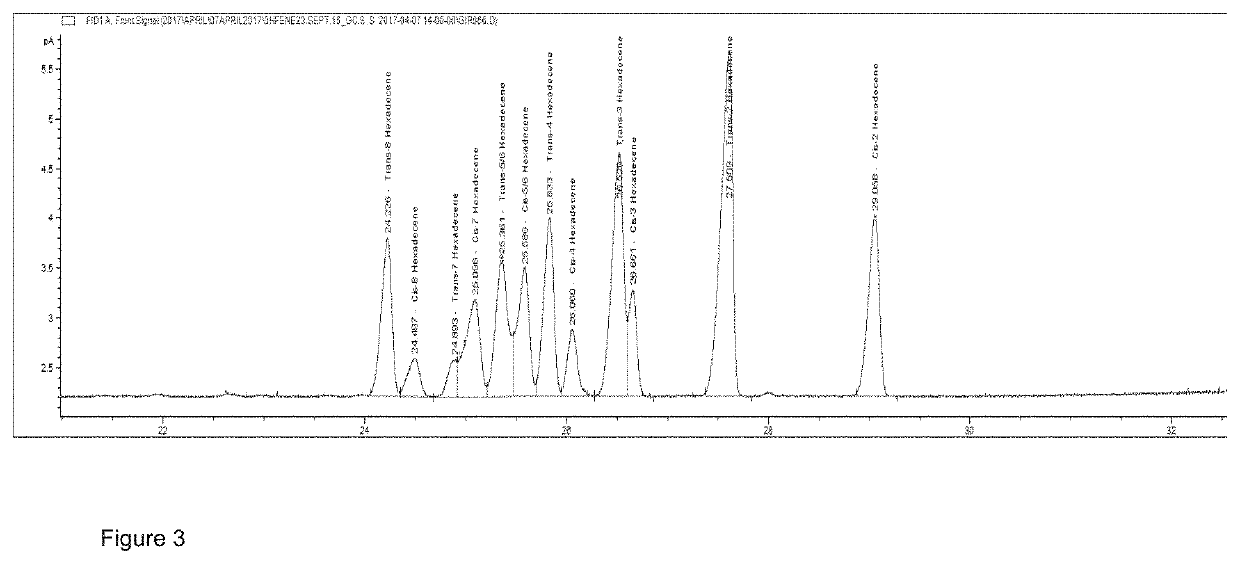
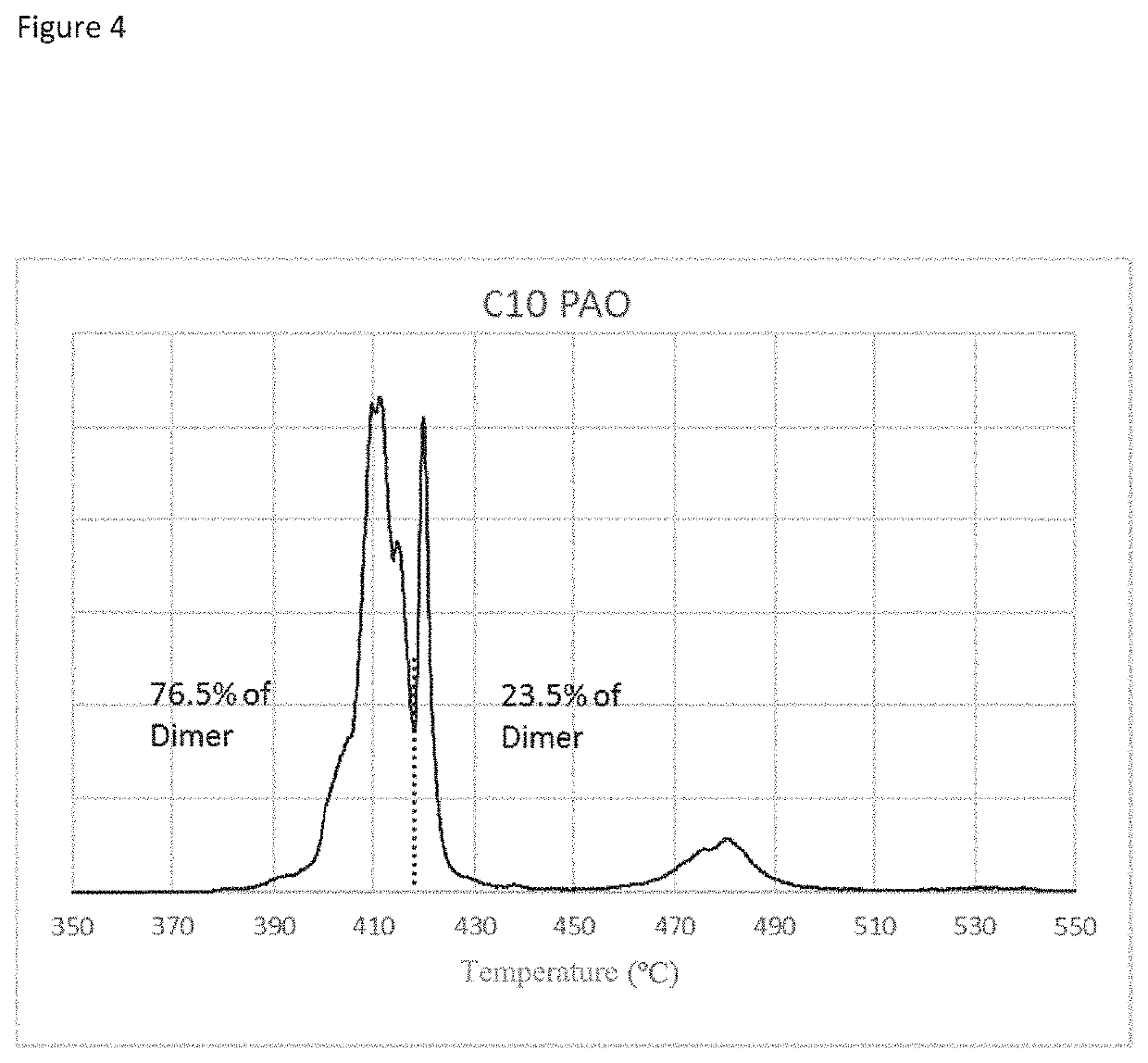
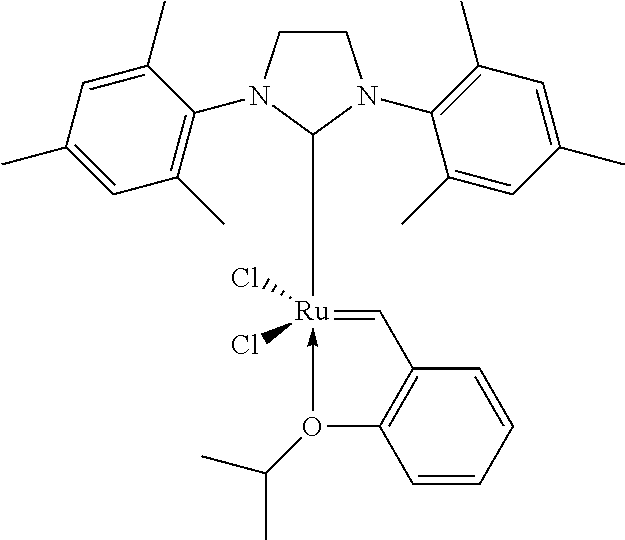
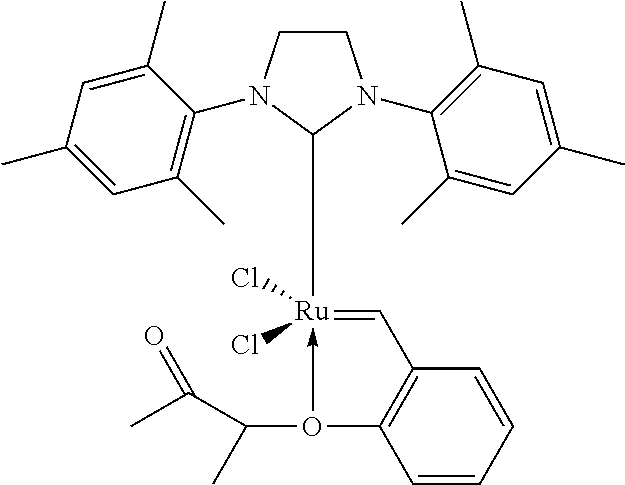
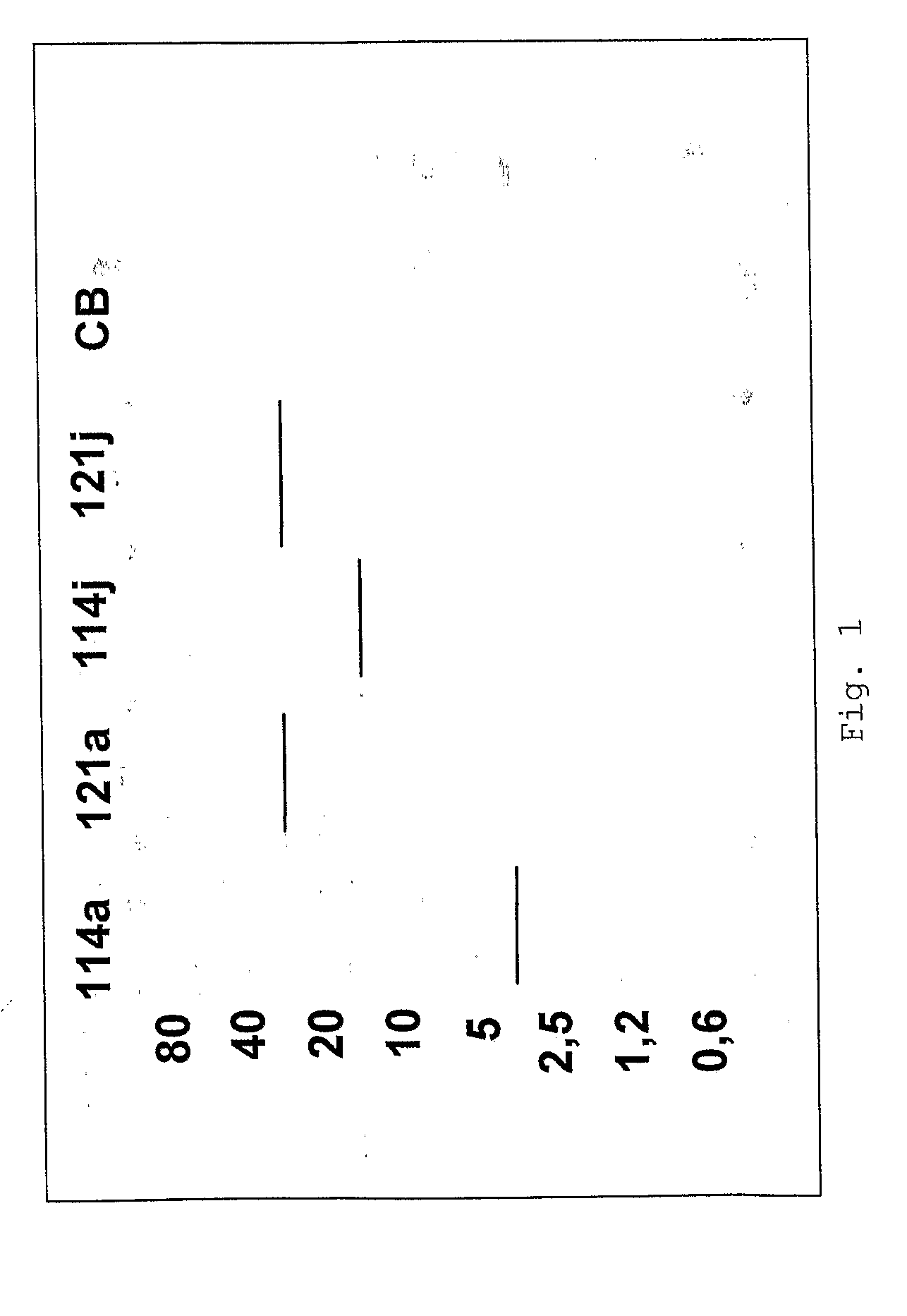
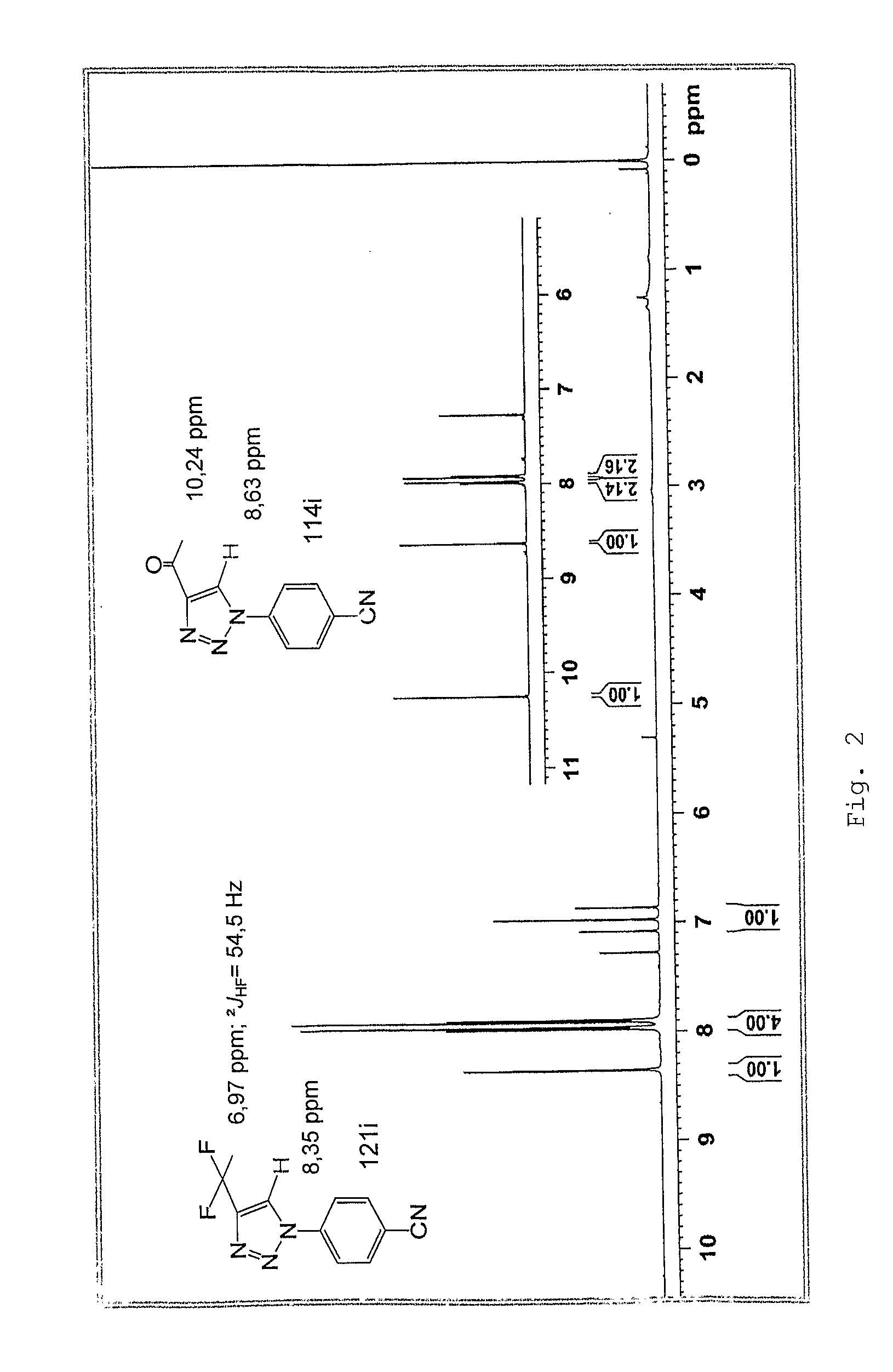
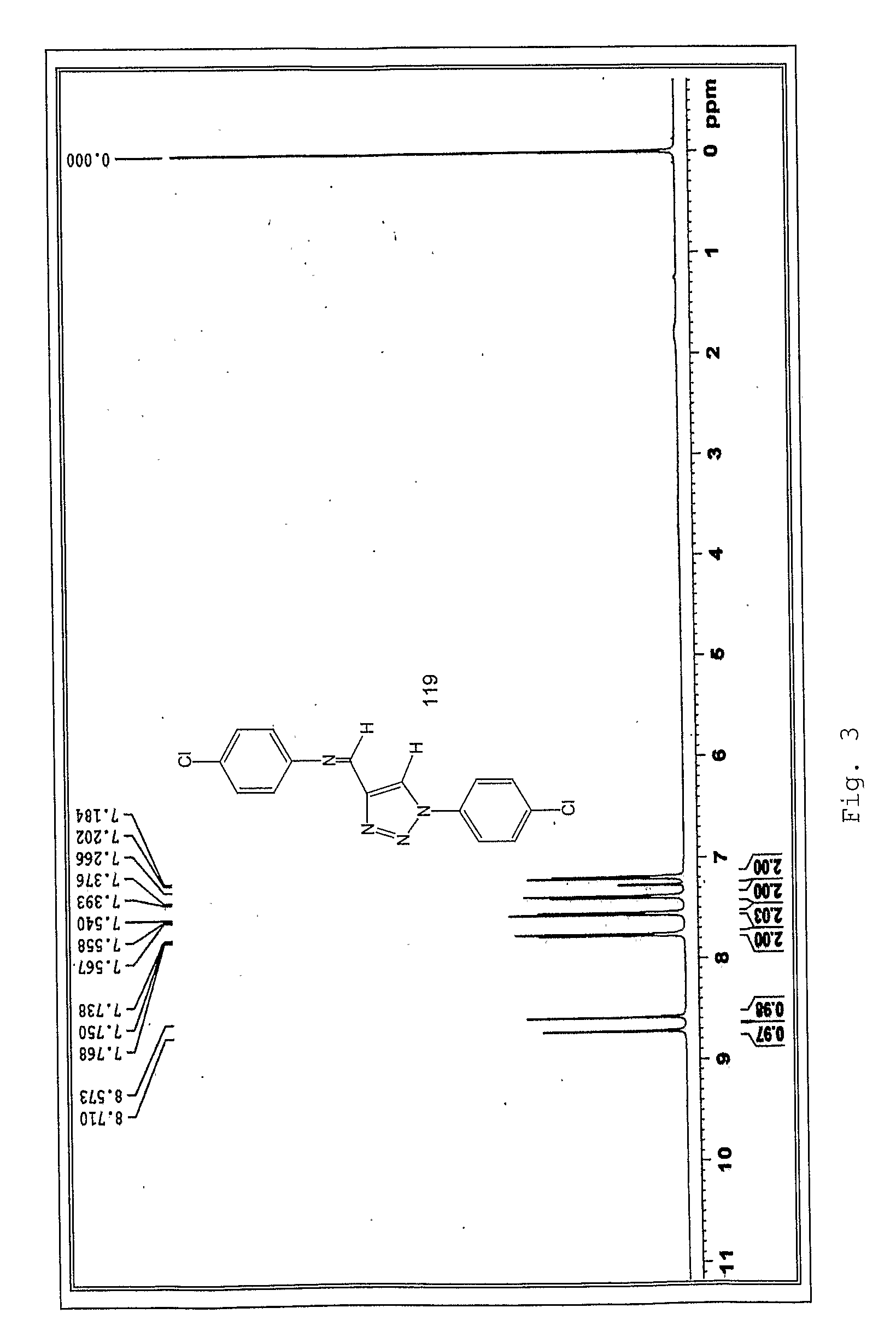
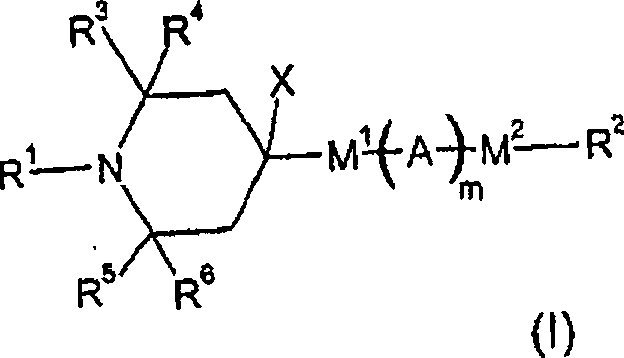
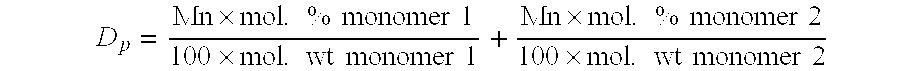Patents
Literature
38 results about "Branched chain alkene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
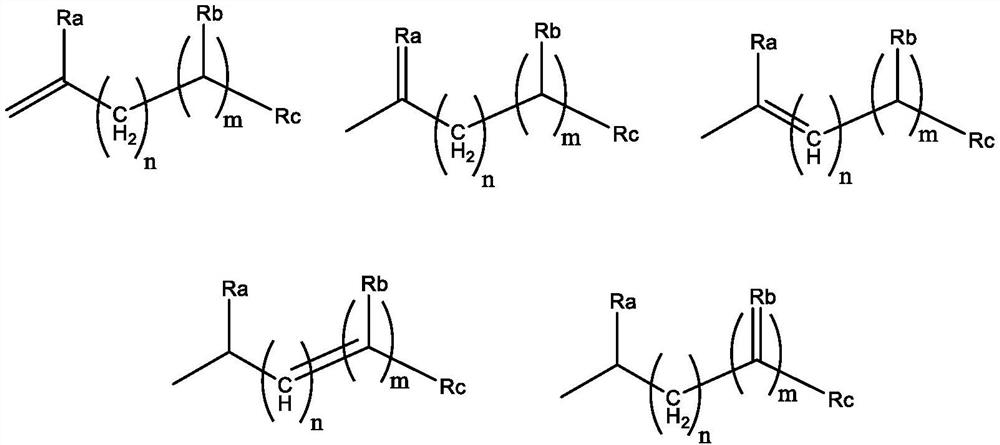
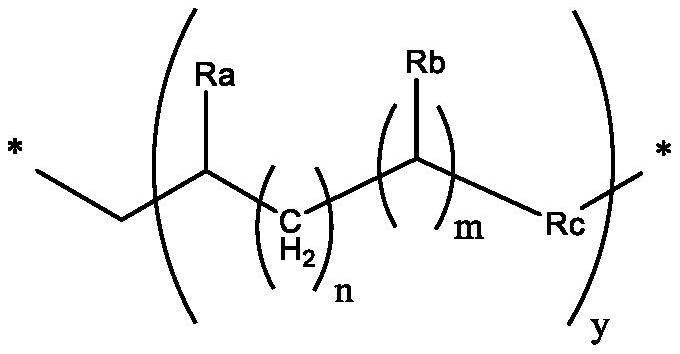
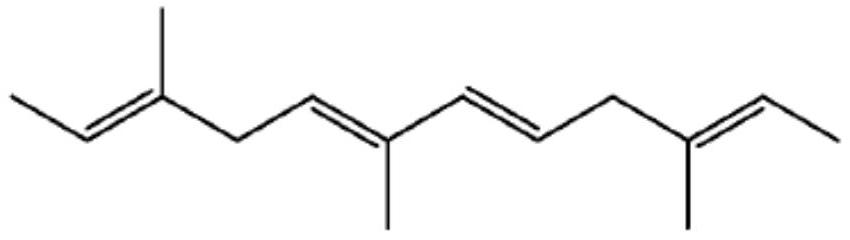
Branched olefinic macromonomer, olefin graft copolymer, and olefin resin composition
The invention relates to olefin branched macromonomers, olefin graft copolymers and olefin resin compositions having the advantage of good compatibility with polyolefin resins and good moldability and workability. The olefin branched macromonomer satisfies the following (a) and (b):[0001](a) its weight-average molecular weight (Mw) measured through gel permeation chromatography (GPC) falls between 400 and 200000;[0002](b) its vinyl content is at least 70 mol % of all the unsaturated groups in the macromonomer.
Owner:IDEMITSU KOSAN CO LTD
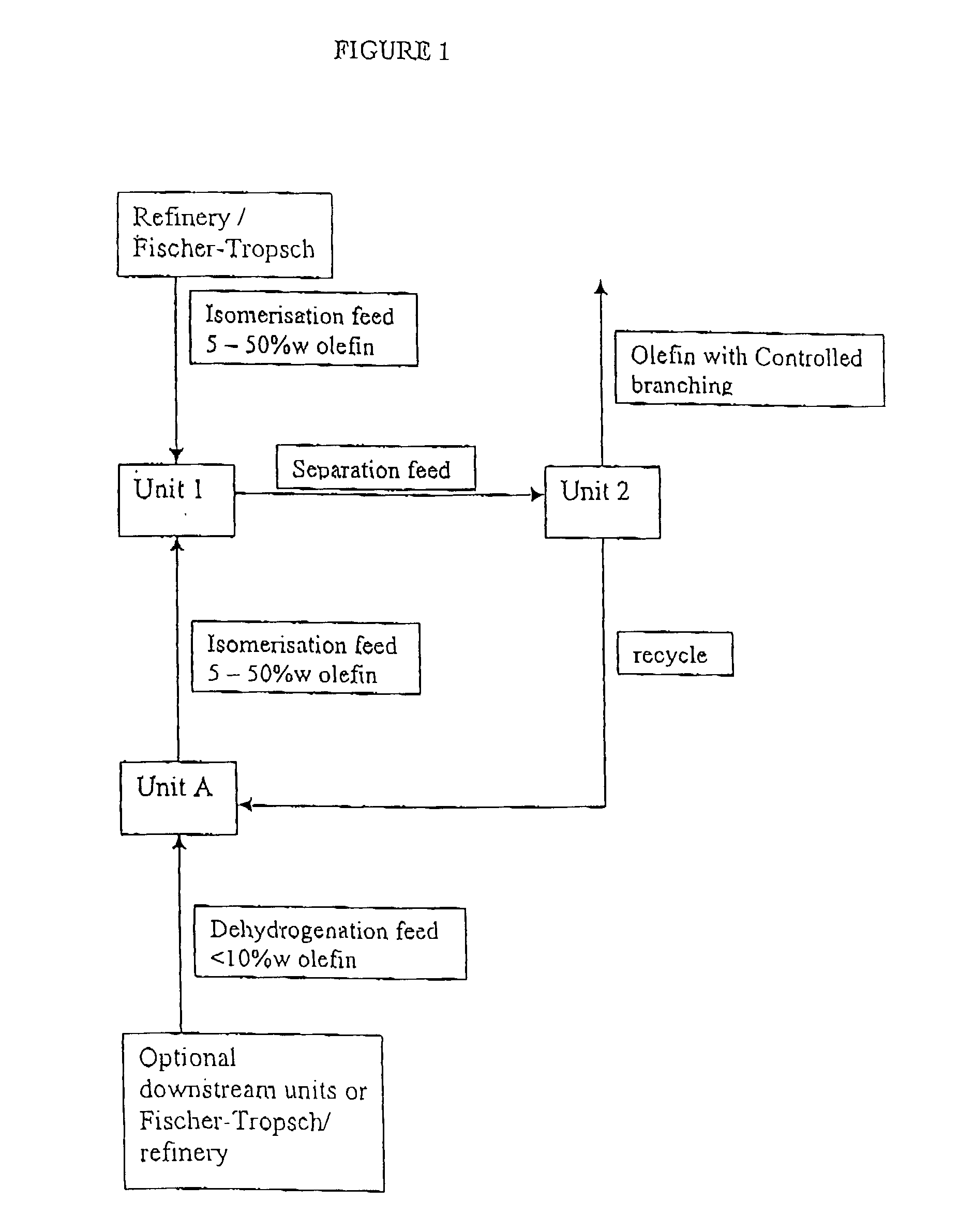
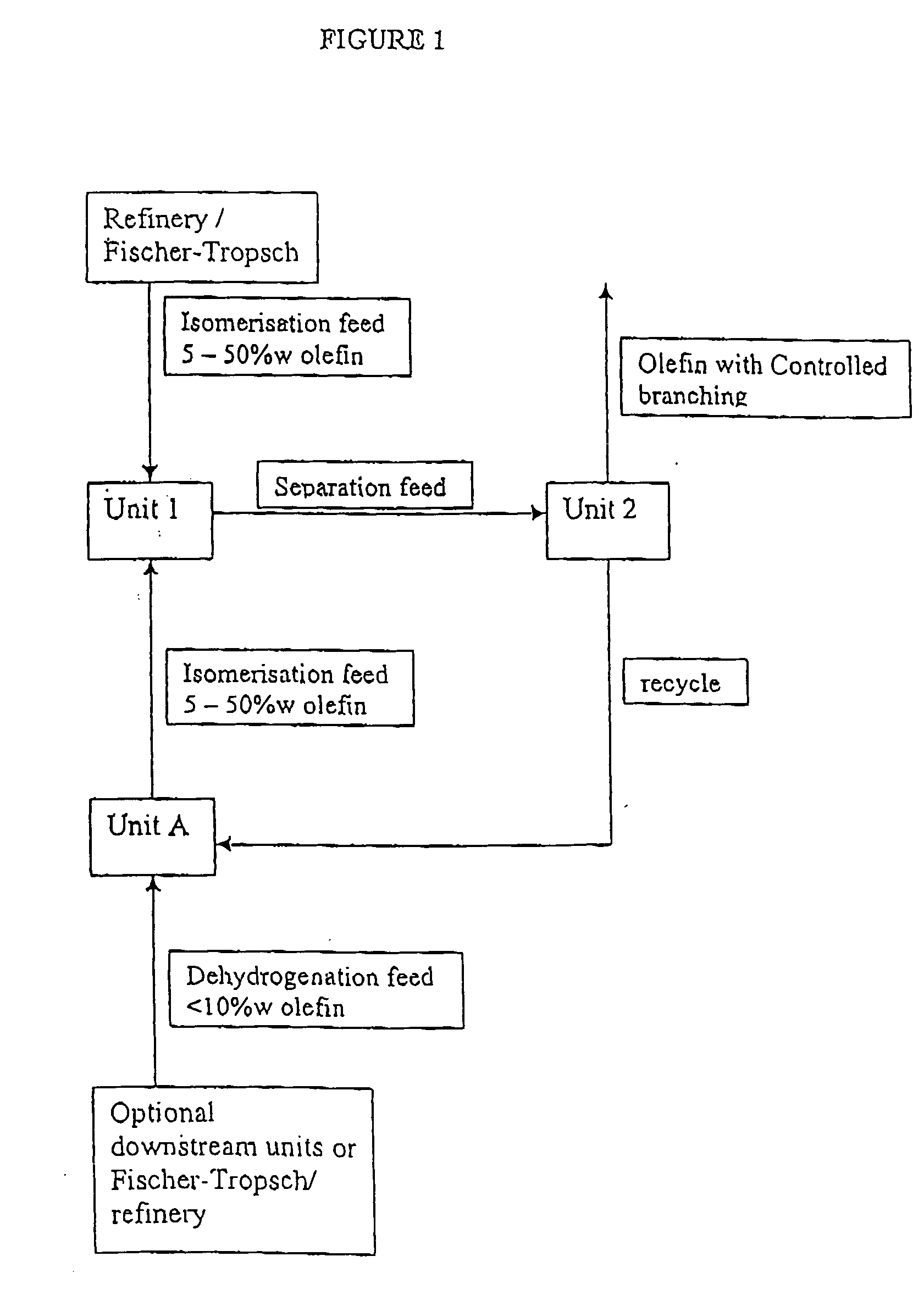
Process for producing branched olefins from linear olefin/paraffin feed
InactiveUS7157613B2Good choiceEasy to separateHydrocarbon by isomerisationOrganic chemistry methodsAlkanePolymer science
Owner:SHELL OIL CO
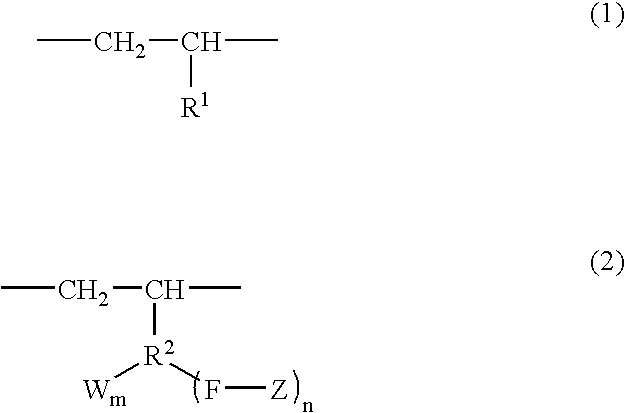
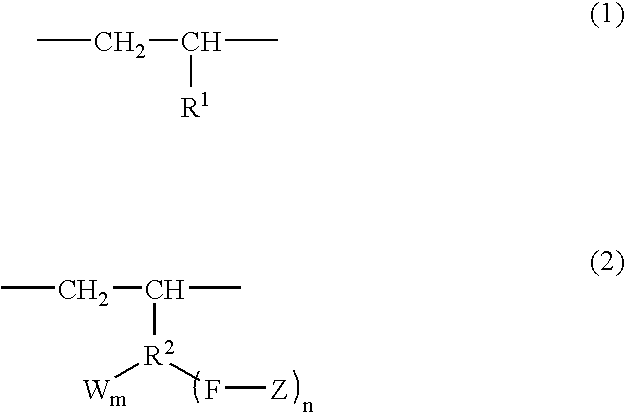
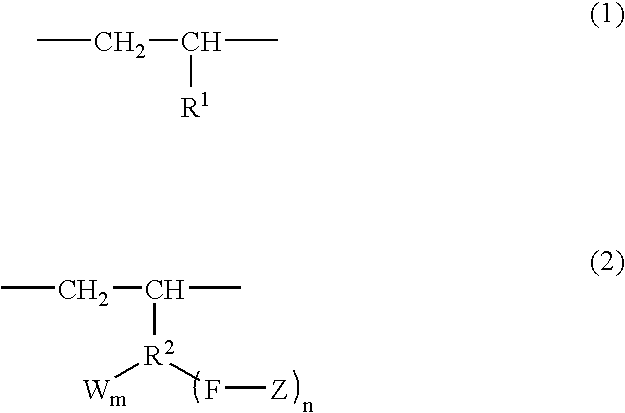
Branched olefin copolymer, process for producing the same, and use thereof
ActiveUS20040110903A1Improve elongationExtend the rest timeCoatingsGraft polymer adhesivesDouble bondOxygen atom
A branched olefin copolymer demonstrating excellent performance in various uses, comprising a building block (A) represented by formula (1) below and a building block (B) represented by formula (2) below, obtained by e.g. a method which involves sequentially conducting ( step 1) a step of synthesizing -group-containing olefin copolymer (Q) containing at least one functional group (G) selected from a hydroxyl group, a carboxylic acid group, an ester group, an amino group, an epoxy group, a silanol group and an acid anhydride group, (step 2) a step of converting the functional group (G) into a group having an ability to initiate radical polymerization, and (Step 3) a step of installing a polymer segment (Z) by radical polymerization of a monomer essentially comprising a carbon-carbon double bond-containing compound (R) containing at least one selected from an oxygen atom, a nitrogen atom, a halogen atom and an aryl group; wherein the formula (1), R<1 >represents a hydrogen atom and a C1-18 linear or branched aliphatic hydrocarbon group; in the formula (2), R<2 >represents a C1-18 linear or branched aliphatic or aromatic hydrocarbon group; F represents a heteroatom or a heteroatom-containing linking group; Z represents a polymer segment containing at least one selected from an oxygen atom, a nitrogen atom, a halogen atom and an aryl group and having a molecular-weight distribution of 1.0 to 3.0; W represents a group selected from an alcoholic hydroxyl group, a phenolic hydroxyl group, a carboxylic acid group, a carboxylate group, an acid anhydride group, an amino group, an epoxy group, a siloxy group and a mercapto group; n is an integer of 1 to 3 and m is 0, 1 or 2 provided that when n is 2 or 3, Z may be the same or different to each other, and when m is 2, W may be the same or different to each other; and W may be bound to the same or different atom of R<1 >to form a cyclic structure.
Owner:MITSUI CHEM INC
Method of making 2-thiols
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
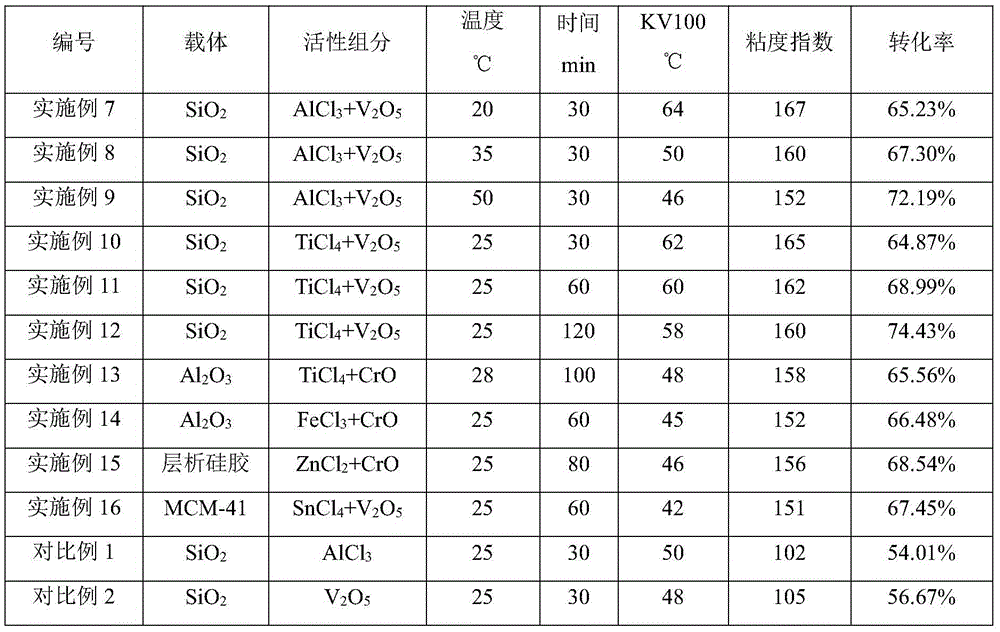
Method for preparing poly-alpha-olefin synthetic oil with high viscosity index
ActiveCN106883899AIncrease profitLow costCatalystsHydrocarbon preparation catalystsDistillationEconomic benefits
The invention relates to a method for preparing poly-alpha-olefin synthetic oil with a high viscosity index. The method comprises the following steps: adding an oligomerization catalyst into raw materials, and carrying out a reaction for 30 to 120 minutes at the temperature of 20 DEG C to 50 DEG C; and separating the catalyst, a reaction product and an unreacted alpha-olefin monomer, and subjecting the reaction product to distillation and hydrogenation, thereby obtaining the poly-alpha-olefin synthetic oil which has the kinematic viscosity of greater than 40cSt and the viscosity index of greater than 150 at the temperature of 100 DEG C. According to the method, PAO with the high viscosity index is polymerized by taking cheap branched-chain alpha-olefin as a synthesis raw material instead of expensive 1-decene. By taking the branched-chain alpha-olefin as the synthesis raw material, byproducts can be utilized so as to increase utilization ratio, and the PAO with the high viscosity index is polymerized by using self-produced decene instead of the expensive 1-decene, so that the cost is reduced, and the economic benefit is increased.
Owner:PETROCHINA CO LTD
Additives and lubricating oil compositions containing same
ActiveUS20100173811A1Improving piston cleanlinessAmino preparation from aminesAdditivesOligomerChemical composition
Para-alkylated substituted diphenylamines are made by catalytically alkylating diphenylamine with a branched-chain alkene, such as propene, oligomer mixture in which the oligomer present in the greatest percentage has 15-24 carbon atoms. The alkylated diphenylamines are useful crankcase lubricant additives such as for reducing piston deposits and engine sludge.
Owner:INFINEUM INT LTD
Base oils and methods of making the same
ActiveUS20200216772A1Liquid hydrocarbon mixtures productionTreatment with hydrotreatment processesPolymer sciencePtru catalyst
Aspects of the present disclosure relate to a process for producing synthetic hydrocarbon base oils having advantageous properties for formulation of engine oils, and the base oils obtained by such processes, involving the production of branched alkenes from the oligomerization of C14-C18 olefins. According to one embodiment, the base oils are obtained by first forming a mixture of two or more olefins ranging from C14-C18, where one of the olefins is an alpha olefin and the other has an average double bond position between 1.5-5.0, and oligomerizing this mixture in the presence of a catalyst to form one or more branched alkenes, hydrogenating the branched alkenes, and fractionating to form base oils. According to one aspect, advantageous properties can be obtained by controlling one or more of the degree of branching, branch length, branching positions, selection of the C14-C18 olefins, and catalytic isomerization, during or after the oligomerization process.
Owner:NOVVI
Synthesis of a branched unsaturated compound by means of cross metathesis
ActiveUS20150376108A1Fatty acid chemical modificationCarboxylic acid nitrile preparationBranched chain alkeneCleansing Agents
The invention relates to a method for the synthesis of a branched unsaturated fatty compound, said method comprising the metathesis, in the presence of a metathesis catalyst, of a linear unsaturated fatty compound and a branched olefin. The branched unsaturated fatty compound is used in particular for the production of at least one of the following products: dielectric fluids, specialty surfactants, emulsifiers, frictions agents, antistatic additives, antifogging additives, mould release agents, pigment dispersants, high-performance lubricants, waxes and wax emulsifiers, polymer conversion additives, PVC stabilising agents, inks, resins, paints, varnishes, solvents, lipsticks, creams for the skin, deodorants, particularly stick deodorants, hair dyes, shampoos and other liquid soaps, shaving foam, laundry detergents, cleaning agents, fabric softeners, and mixtures of same.
Owner:ARKEMA FRANCE SA
Method for preparing polyethylene catalyst
ActiveCN106928381AParticles in good shapeImprove the complex preparation process and difficult matching problemsBulk chemical productionMicrosphereSlurry
The invention discloses a method for preparing a polyethylene catalyst. The method comprises the following steps that 1, in the presence of inert gas nitrogen, organic oxide and an alkoxy magnesium compound are subjected to a haptoreaction to form a homogeneous system; 2, a granular metallocene compound is added into the homogeneous system, stirring is carried out, and uniform slurry is formed; 3, the slurry is spray-dried, and microsphere particles are obtained; 4, the microsphere particles and a titanium active component are subjected to a haptoreaction, and the solid catalyst is obtained. Ethylene is adopted as a unique monomer, different active components in the catalyst act at the same time, and the polyethylene product with a high branch degree is prepared. It is avoided that when a branched polyethylene product is produced, expensive short-branch alpha olefin is used, and the product production cost is reduced. The problems that in the prior art, multiple compounded catalysts are complex in preparation process, and matching performance is hard to achieve are solved.
Owner:谢炳
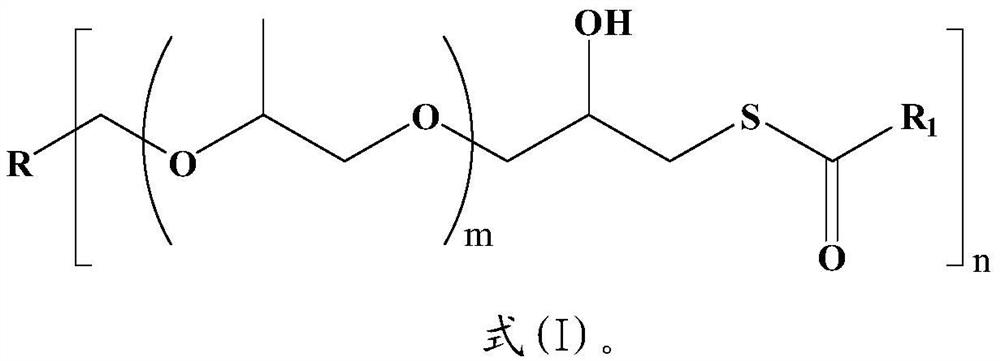
Latent macromolecular polymercaptan curing agent as well as preparation method and application thereof
The invention relates to the field of novel curing agents, in particular to a synthesis method and application of a latent macromolecular polymercaptan curing agent. The structure of the compound is shown as a formula (I), m and n are respectively independent integers from 1 to 8, and R1 is C1-C6 straight-chain or branched-chain olefin. The synthesis method comprises the following steps of: (1) under the catalytic action of a Lewis acid catalyst, reacting polyether polyol with epoxy chloropropane to prepare chlorinated polyether; (2) performing ring closure on the generated chlorinated polyether in an alkaline solution to form a polyether polybasic epoxy compound; and (3) reacting the polyether polyol epoxy compound with thiocarboxylic acid to obtain the latent epoxy curing agent shown inthe formula (I), and mixing the latent epoxy curing agent with epoxy resin to form a polymerizable composition which is stable at low temperature and cured at high temperature. The compound shown in the formula (I) has the characteristics of good storage stability, no odor, and low curing temperature when being used as a latent curing agent, and has a wide application prospect in the fields of thermosetting coatings, adhesives and the like.
Owner:SHANDONG EFIRM BIOCHEMISTRY & ENVIRONMENTAL PROTECTION CO LTD
Pyrolysis reactions in the presence of an alkene
Described herein are methods for producing branched alkanes and branched alkenes from the pyrolysis of radical precursors. The branched alkanes and branched alkenes have numerous applications as fuels, platform chemicals, and solvents.
Owner:福吉碳氢化合物公司
Processes for forming alkylated aryl phosphite compositions from complex hydrocarbon streams
Processes for alkylating hydroxyaryl compounds by reacting a hydroxyaryl with at least one olefin of a complex hydrocarbon stream. The complex hydrocarbon stream preferably comprises a fraction of a cracked hydrocarbon feed stream or the reaction products of a dehydrogenation of a paraffinic feedstock. The olefin of the complex hydrocarbon stream is preferably a branched olefin, e.g., isobutylene or isoamylene. The alkylated compositions are suitable for forming liquid phosphite compositions.
Owner:SI GRP USA USAA LLC
Azole compounds used as tuberculostatic and leishmanicide agents
This invention refers to new 1,2,3-triazole and imidazole compounds included in the families of compounds represented by general formula VIII.where: X is an atom of “C” or “N;” where X is “N” the radicals do triazole ring are represented by: R1=COR2, CSR3, CN(R4)R5 or CF2R6; R2=H, NHNH2, alkyl, aryl substituted or not, OH, NR7R8 or OR9; R3=alkyl or aryl substituted or not; R4=H, OH, alkyl or aryl substituted or not; R5=R6=R7=R8=R9=R10=H, alkyl or aryl substituted or not; where X is “C” the radicals do imidazolic ring are represented by: R1=COR2; R2=NHNH2, OH, OR3, or NR4R5; R3=alkyl or aryl substituted or not; =R5H, alkyl or aryl substituted or not; R10=NHR6 or NR6R7; R6=R7=COR8; R8=aryl substituted or not; while radical Rn can be located in any one or in more than one of the carbon atoms of the aromatic ring, and these radicals can be equal or different, represented by hydrogen, alkylic groups with 1 or more carbon atoms in a linear or branched chain alkenes or alkynes, hydroxyl, hydroxyalkyl or oxygenated functions in acyclic or cyclic systems forming an heterocyclic ring, free or substituted amines, thioalkyl, donators and / or removing groupings of electrons or halogens, thus “n” can vary from 1 to 5. This invention also refers to a pharmaceutical composition comprising, as active principle, at least one of the azole compounds represented by the general formula VIII, to the use of such compositions and to method of treatment or inhibition de tuberculosis and leishmaniasis.
Owner:BOECHAT NUBIA +5
Liquid crystal mixture
Liquid crystal mixtures comprising one or more compounds of formula (I), wherein R 1 is H or a straight chain or branched chain alkyl group of 1 to 20 carbon atoms or a straight chain or branched chain alkenyl group of 2 to 20 carbon atoms, wherein in both cases, optionally one -CH 2 -group replaced by cyclohexa-1,4-diyl, or 1 or 2 -CH 2 -group, if not adjacent to nitrogen (N), optionally replaced by -O-, or by -C(=O)-, -Si(CH 3 ) 2 - replace; and / or one or more H on the alkyl or alkenyl is optionally replaced by F or CH 3 alternative, R 2 Represents a) H or F; b) straight-chain or branched-chain alkyl of 1 to 20 carbon atoms or straight-chain or branched alkenyl of 2 to 20 carbon atoms, wherein in both cases, 1 or 2 -CH 2 - optionally replaced by -O-, -C(=O)O-, -Si(CH 3 ) 2 - Replacement, and / or one or more H on the alkyl or alkenyl group is replaced by F or CH 3 Replacement; c) group (a), wherein independently of the meanings of formula (I), R 3 , R 4 , R 5 , R 6 independently of each other is an alkyl group of 1-8 carbon atoms, M 1 , M 2 Each independently represents a single bond, -OC(=O), -C(=O)O-, -OCH 2 -, -NH-, A is a) a straight-chain or branched alkane-α, ω-diyl group of 1 to 20 carbon atoms or a straight-chain or branched alkene-α, ω of 2 to 20 carbon atoms - Diyl group, if not with M 1 / 2 Adjacent, optional 1 or 2 non-adjacent -CH 2 -may be replaced by -O-; b) a group -C(=Y)-, wherein Y is CH-Z, and Z is benzene-1,4-diyl optionally substituted by 1 to 3 halogen atoms, An alkyl or alkoxy group of 1 to 4 carbon atoms, with the proviso that M 1 and M 2 is -C(=O)O- and -OC(=O)-; c) the group -CHY, wherein Y is CH 2 -Z, and Z is benzene-1,4-diyl optionally substituted by 1 to 3 halogen atoms, an alkyl or alkoxy group of 1 to 4 carbon atoms, with the proviso that M 1 and M 2 Be -C(=O)O- and -OC(=O)-; d) group (b), wherein p, q are 0, 1 or 2, p+q≥1; M 3 is a single bond or -OC(=O)-, -C(=O)O-, -OCH 2 -, -CH 2 O-, -C≡C-, -CH 2 CH 2 -, -CH 2 CH 2 CH 2 CH 2 -, groups (c) and (d) independently of each other are benzene-1,4-diyl optionally substituted by 1, 2 or 3 F, or optionally substituted by 1 CN, CH 3 Or cyclohexa-1,4-diyl substituted by F, or pyrimidine-2,5-diyl optionally substituted by 1 F, pyridine-2,5-diyl optionally substituted by 1 F , or naphthalene-2,6-diyl optionally substituted by 1, 2 or 3 Fs, or 1,2,3,4-tetrahydronaphthalene-2,6-diyl (the aromatic ring is optionally substituted by 1, 2 or 3 F), or decalin-2,6-diyl, or indane-2,5(6)-diyl, or fluorene-2,7-diyl, Or phenanthrene-2,7-diyl, or 9,10-dihydrophenanthrene-2,7-diyl, or (1,3,4)-thiadiazole-2,5-diyl, or (1, 3) Thiazole-2,5-diyl, or (1,3) thiazole-2,4-diyl, or thiophene-2,4-diyl, or thiophene-2,5-diyl, or (1, 3) Dioxane-2,5-diyl, or piperidine-1,4-diyl, or piperazine-1,4-diyl; X is H, OH, a straight chain of 1 to 20 carbon atoms Or branched chain alkyl or alkoxy, of which 1 or 2 -CH 2 - can be replaced by -O-, -C(=O)O-, -Si(CH 3 ) 2 - Replacement, optionally one or more hydrogens are replaced by F or CH 3 Substitution; m is 0 or 1, X and M 1 -(A) m -M 2 -R 2 Together they can constitute a) a 4-16-membered ring optionally substituted by an alkyl group of 1-15 carbon atoms, b) a combination of two directly connected or spiro-connected 4-16-membered rings independently connected to each other, optionally substituted by Alkyl substitution of 1 to 15 carbon atoms, regardless of which of the three cases, the rings can be independently of each other carbocycles or carbocycles containing boron, nitrogen, oxygen or sulfur heteroatoms.
Owner:MERCK PATENT GMBH +1
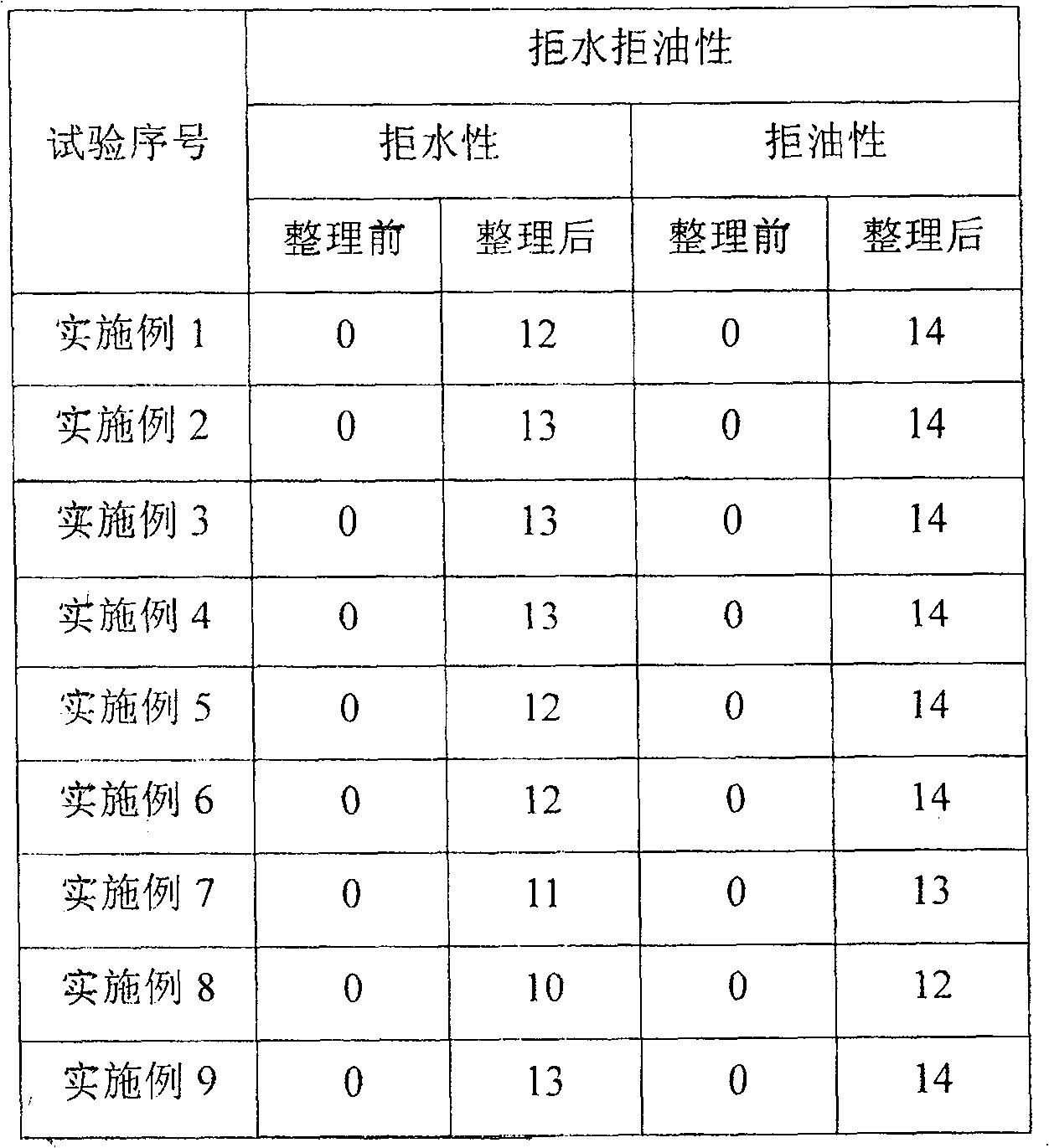
Phosphate water, oil-repellent containing fluoroolefin and its preparation method and uses
There is provided a phosphas agent with alkenes base that contains fluorine. The agent can resist aqua and oils, the preparation and the application of the agent is also disclosed in the invention. The general formula of the phosphas is [RfQ]m[OH]n[Y+O-]kP=O. While in the formula, Rf stands for perfluoro side chain alkenes base; Q stands for divalent organic group; Y+ stands for (N+HR1R2R3) or base metal iron, of which R1, R2, R3 stand for H atom or -CH2CH2OH (m= integer from 1-3, n=integer from 0-2, k=integer form 0-2, m+n+k=3). The invention can assign the base material excellent quality of resisting aqua and oils. It has no special restriction to the base materials but it's preferable to use base materials which have irregular fiber tissues such as paper, leather and wood.
Owner:SHANDONG DONGYUE POLYMER MATERIAL
Olefin oligomerization using delaminated zeolite supports as catalyst
ActiveUS20160122258A1Elimination of mass transferHigh selectivityMolecular sieve catalystsMolecular sieve catalystPolymer sciencePtru catalyst
Provided is an improved process for olefin oligomerization allowing one to realize superior selectivity. The process comprises contacting a hydrocarbon feed comprised of straight and branched chain olefins under oligomerization conditions with a catalyst comprising delaminated SSZ-70. The delaminated SSZ-70 offers a zeolite layer with a single unit cell of thickness in one dimension, allowing for elimination of mass transfer in comparison with regular SSZ-70. The result is superior selectivity.
Owner:CHEVROU USA INC
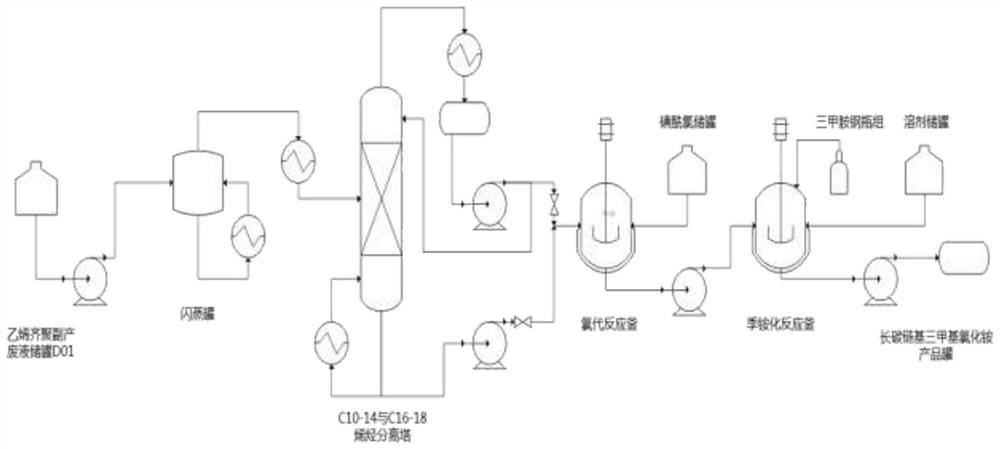
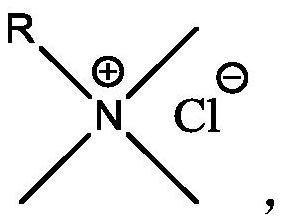
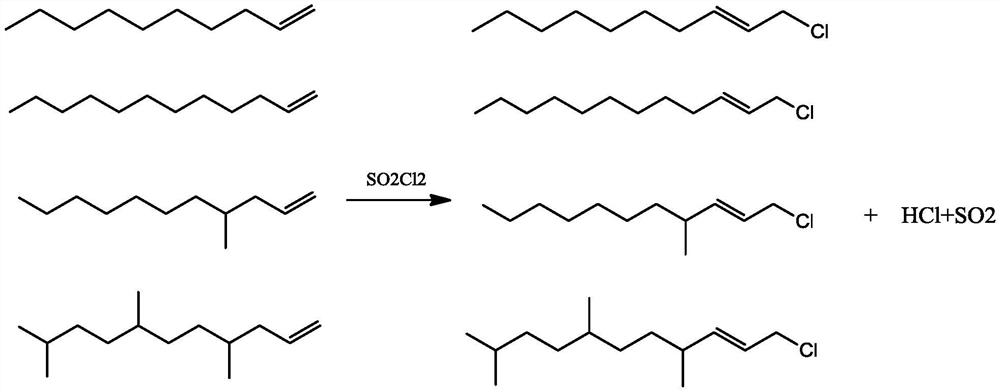
Synthesis method of low-cost long-carbon-chain cationic quaternary ammonium salt
PendingCN113501760AMild reaction conditionsShort reaction timeOrganic compound preparationAmino compound preparationSulfonyl chloridePtru catalyst
The invention relates to a synthesis method of low-cost long-carbon-chain cationic quaternary ammonium salt, wherein the synthesis method comprises the steps: (1) adding C10-C18 straight-chain or branched-chain olefin into a chlorination reaction kettle, carrying out nitrogen displacement, heating under a stirring condition, dropwise adding sulfonyl chloride, and carrying out constant-temperature reaction to obtain corresponding alkene chloride; (2) adding alkene chloride, a catalyst and a solvent into a quaternization reaction kettle, carrying out nitrogen displacement, heating to a certain temperature while stirring, introducing trimethylamine gas, carrying out a constant-temperature reaction, and cooling to obtain a trimethyl ammonium chloride cationic quaternary ammonium salt crude product; and (3) filtering the cationic quaternary ammonium salt crude product to remove the catalyst, carrying out rotary evaporation to remove the solvent to obtain a colloidal solid, washing, and filtering to finally obtain a white solid product. Compared with an existing cationic quaternary ammonium salt production technology, the method has the advantages that the synthetic raw materials are rich in source and low in price, the process is simple and continuous, and meanwhile, the problem that the utilization efficiency of a byproduct C10-18 olefin of a POE device is low is solved.
Owner:WANHUA CHEM GRP CO LTD
Method for separating non-linear olefins from an olefin feed by reactive distillation
ActiveUS20210371360A1Low costLimit lossDistillation regulation/controlChemical industryButenePolymer science
The present invention relates to a process for treating, by reactive distillation, an olefinic feedstock comprising linear olefins containing n carbon atoms, and branched olefins, the branched olefins comprising tertiary branched olefins, for example a mixture of n-butenes and of tertiary branched olefins comprising isobutene, so as to produce an olefinic effluent with a mass content of tertiary branched olefin of less than or equal to 3% by weight and a heavy hydrocarbon effluent, said process comprising the feeding of a reactive distillation section with said olefinic feedstock and with an alcohol feedstock comprising a primary alcohol, said reactive distillation section comprising a column composed at least of an upper reflux zone into which is introduced said alcohol feedstock, comprising, for example, ethanol, an intermediate reaction zone comprising at least 6 reactive doublets, and a lower fractionation zone at the level of which said section is fed with said olefinic feedstock, said reactive distillation section being operated at a relative pressure of between 0.3 and 0.5 MPa, a column head temperature of between 40° C. and 60° C., with a reflux ratio of between 1.8 and 2.2.
Owner:INST FR DU PETROLE +1
Additives and lubricating oil compositions containing same
Para-alkylated substituted diphenylamines are made by catalytically alkylating diphenylamine with a branched-chain alkene, such as propene, oligomer mixture in which the oligomer present in the greatest percentage has 15-24 carbon atoms. The alkylated diphenylamines are useful crankcase lubricant additives such as for reducing piston deposits and engine sludge.
Owner:INFINEUM INT LTD
Copolymer solution as well as preparation method and application thereof
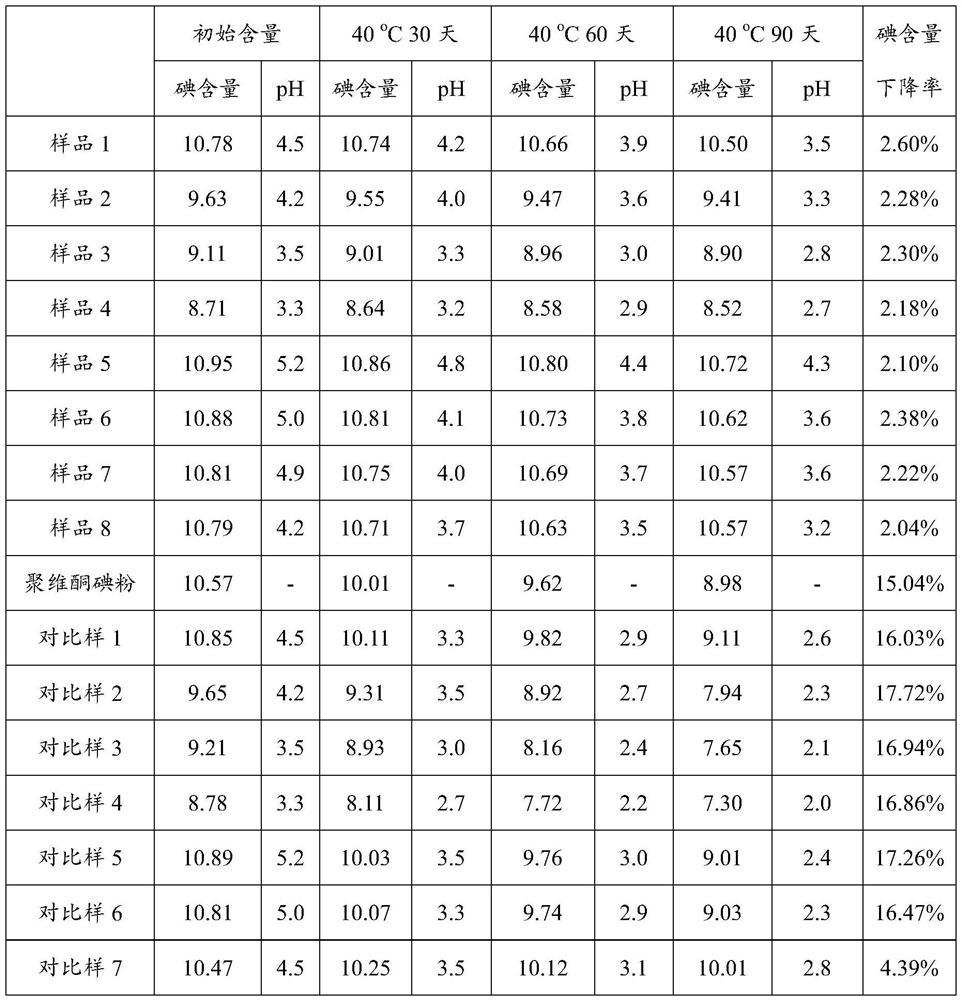
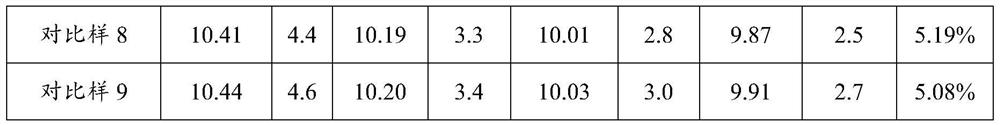
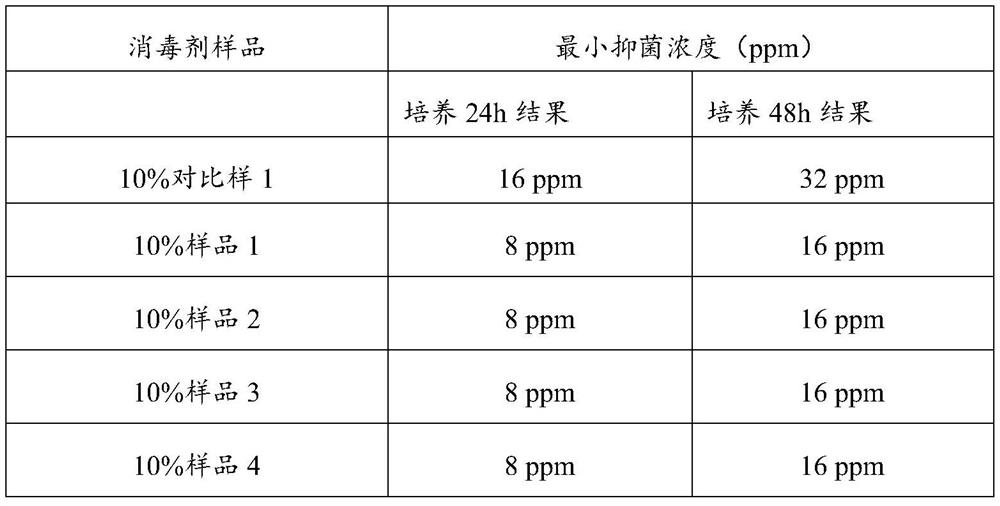
PendingCN114306377AReduce consistencyReduce riskAntisepticsPharmaceutical delivery mechanismPolymer sciencePyrrolidinones
The invention provides a copolymer solution as well as a preparation method and application thereof. The copolymer solution comprises 15%-30% of a vinyl pyrrolidone-enol copolymer solution, 10%-15% of iodine, 25%-35% of a solvent, 0.5%-3% of a cosolvent, 0.75%-1.5% of a pH regulator, 0.5%-5% of a dispersant and 0%-0.3% of a stabilizer. Wherein the vinyl pyrrolidone-enol copolymer solution is a reaction liquid system which is obtained by taking vinyl pyrrolidone and C4-20 straight-chain or branched-chain alpha-olefin acetate as reactants and carrying out acidizing treatment on the basis of completing a polymerization reaction. The vinyl pyrrolidone-enol copolymer solution is used for replacing polyvinylpyrrolidone, so that the problem of complexing stability with iodine is solved, the storage time of iodine content is prolonged, the pH value of a solution system is not continuously reduced in the storage process, and the consistency and agglomeration risk of the solution are reduced; furthermore, iodine can be completely and stably complexed with the copolymer for a long time, so that the purpose of prolonging the validity period of the disinfection effect is achieved.
Owner:成都科宏达科技有限公司 +1
Method for separating non-linear olefins from an olefin feed by reactive distillation
The present invention relates to a process for treating, by reactive distillation, an olefinic feedstock comprising linear olefins containing n carbon atoms, and branched olefins, the branched olefins comprising tertiary branched olefins, for example a mixture of n-butenes and of tertiary branched olefins comprising isobutene, so as to produce an olefinic effluent with a mass content of tertiary branched olefin of less than or equal to 3% by weight and a heavy hydrocarbon effluent, said process comprising the feeding of a reactive distillation section with said olefinic feedstock and with an alcohol feedstock comprising a primary alcohol, said reactive distillation section comprising a column composed at least of an upper reflux zone into which is introduced said alcohol feedstock, comprising, for example, ethanol, an intermediate reaction zone comprising at least 6 reactive doublets, and a lower fractionation zone at the level of which said section is fed with said olefinic feedstock, said reactive distillation section being operated at a relative pressure of between 0.3 and 0.5 MPa, a column head temperature of between 40° C. and 60° C., with a reflux ratio of between 1.8 and 2.2.
Owner:INST FR DU PETROLE +1
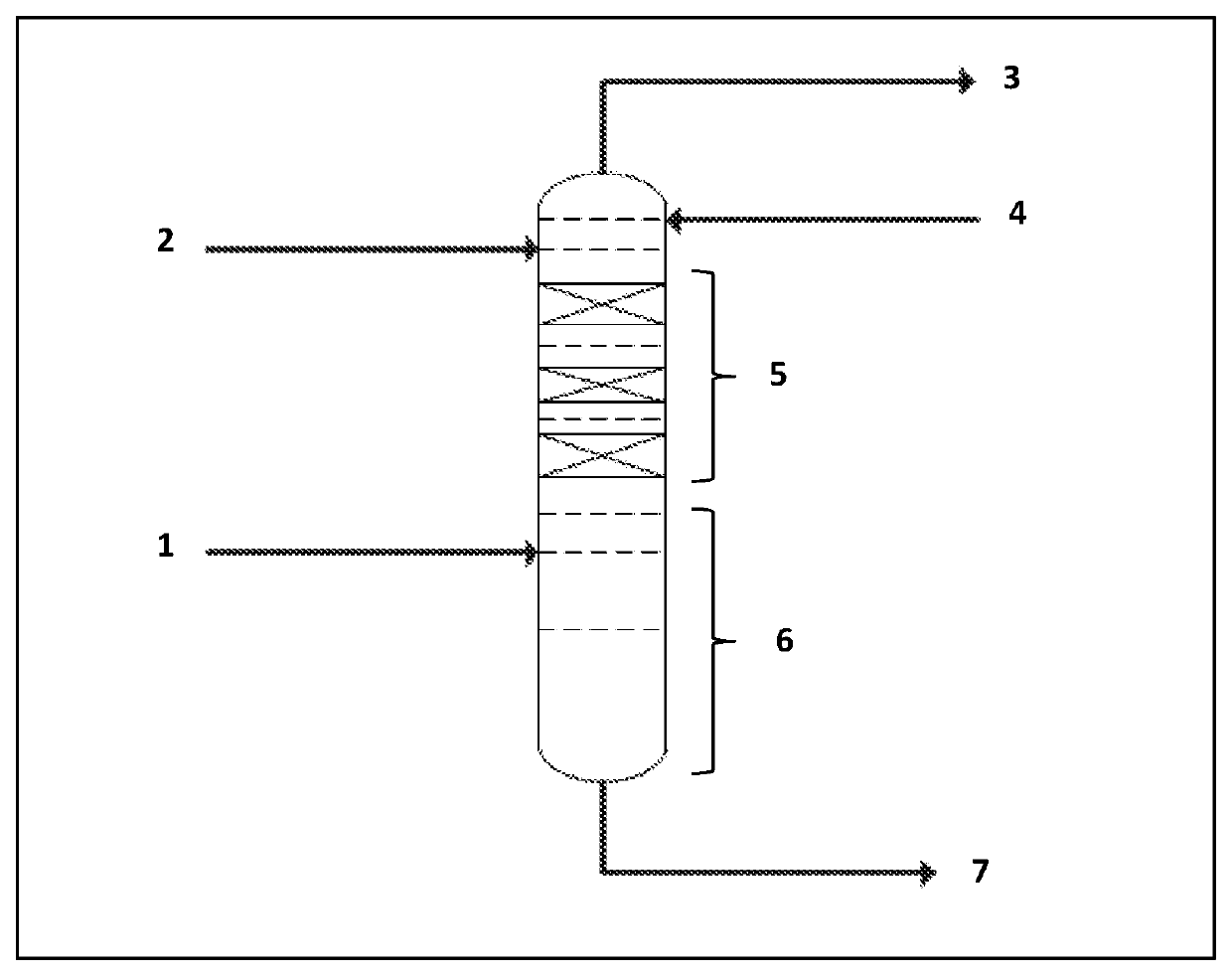
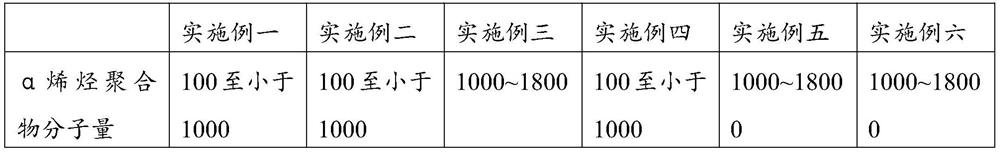
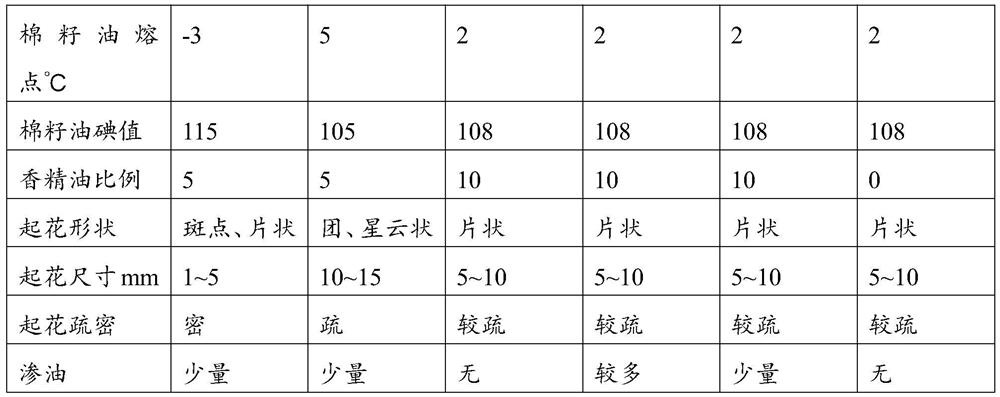
A kind of cottonseed blossom oil and blossom candle
ActiveCN107011999BSimple processImprove blooming effectCandle ingredientsParaffin waxBranched chain alkene
The invention provides cottonseed figure oil and a figure candle. The cottonseed figure oil is based on discovery of new applications of cottonseed oil, a melting point of the cottonseed oil ranges from -5 DEG C to 5 DEG C, and an iodine value of the cottonseed oil ranges from 105 to 120. The cottonseed figure oil comprises the cottonseed oil, essential oil, mineral oil and a branch chain alpha olefin copolymer, and the weight percentage of the cottonseed oil to the figure oil is 1%-100%. The cottonseed figure oil for the figure candle has excellent figure effect, can be made into various snowflake shapes such as points, flakes, clusters and nebulas and is applicable to candles of various raw material systems including paraffin base candles, plant wax base candles, animal fat base candles and mixed base candles. The cottonseed figure oil is convenient to use, a process for manufacturing the figure candle is simple, and cost is saved.
Owner:QINGDAO KINGKING APPL CHEM CO LTD
Phosphate water, oil-repellent containing fluoroolefin and its preparation method and uses
There is provided a phosphas agent with alkenes base that contains fluorine. The agent can resist aqua and oils, the preparation and the application of the agent is also disclosed in the invention. The general formula of the phosphas is [RfQ]m[OH]n[Y+O-]kP=O. While in the formula, Rf stands for perfluoro side chain alkenes base; Q stands for divalent organic group; Y+ stands for (N+HR1R2R3) or base metal iron, of which R1, R2, R3 stand for H atom or -CH2CH2OH (m= integer from 1-3, n=integer from 0-2, k=integer form 0-2, m+n+k=3). The invention can assign the base material excellent quality of resisting aqua and oils. It has no special restriction to the base materials but it's preferable to use base materials which have irregular fiber tissues such as paper, leather and wood.
Owner:SHANDONG DONGYUE POLYMER MATERIAL
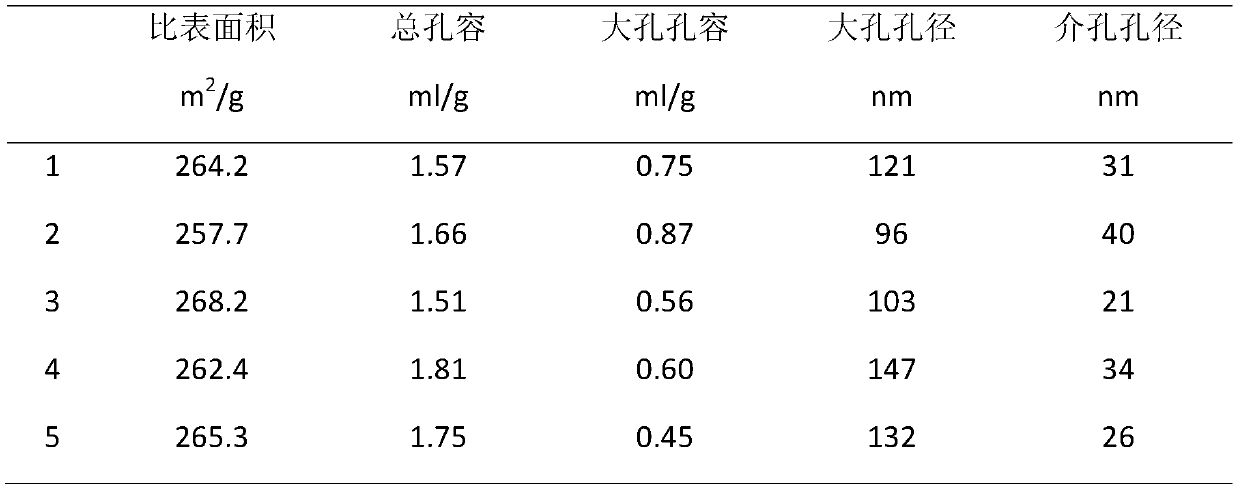
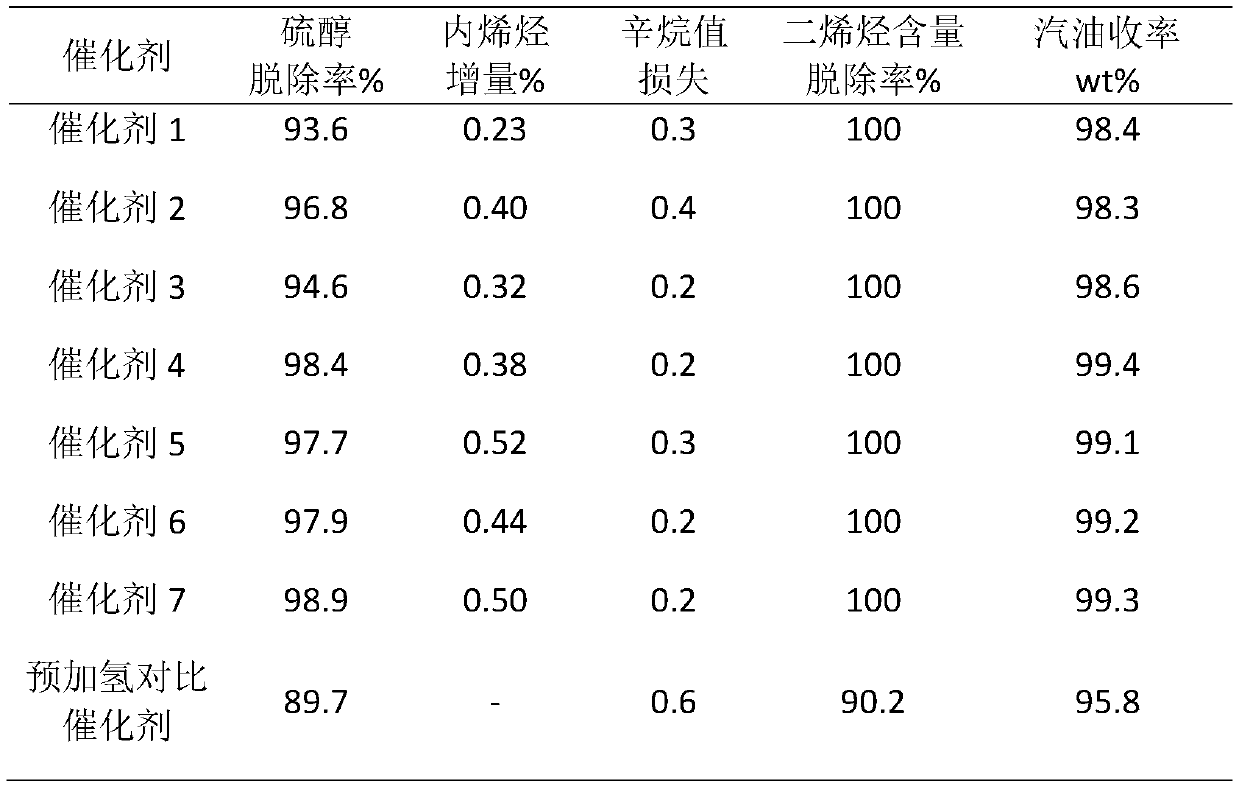
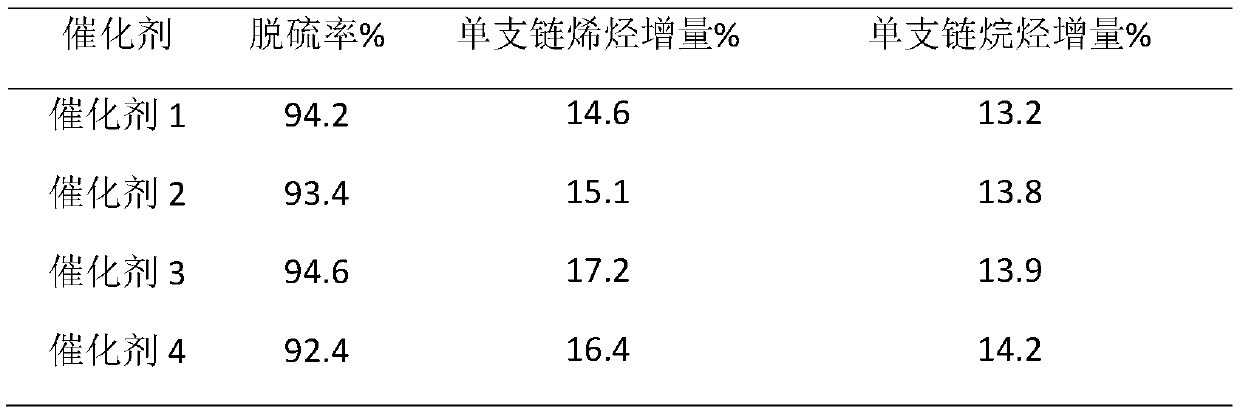
A kind of fcc gasoline cleaning method
ActiveCN109082303BKeep octaneRealize ultra-deep desulfurizationMolecular sieve catalystsTreatment with hydrotreatment processesPtru catalystIsomerization
Owner:FUZHOU UNIV
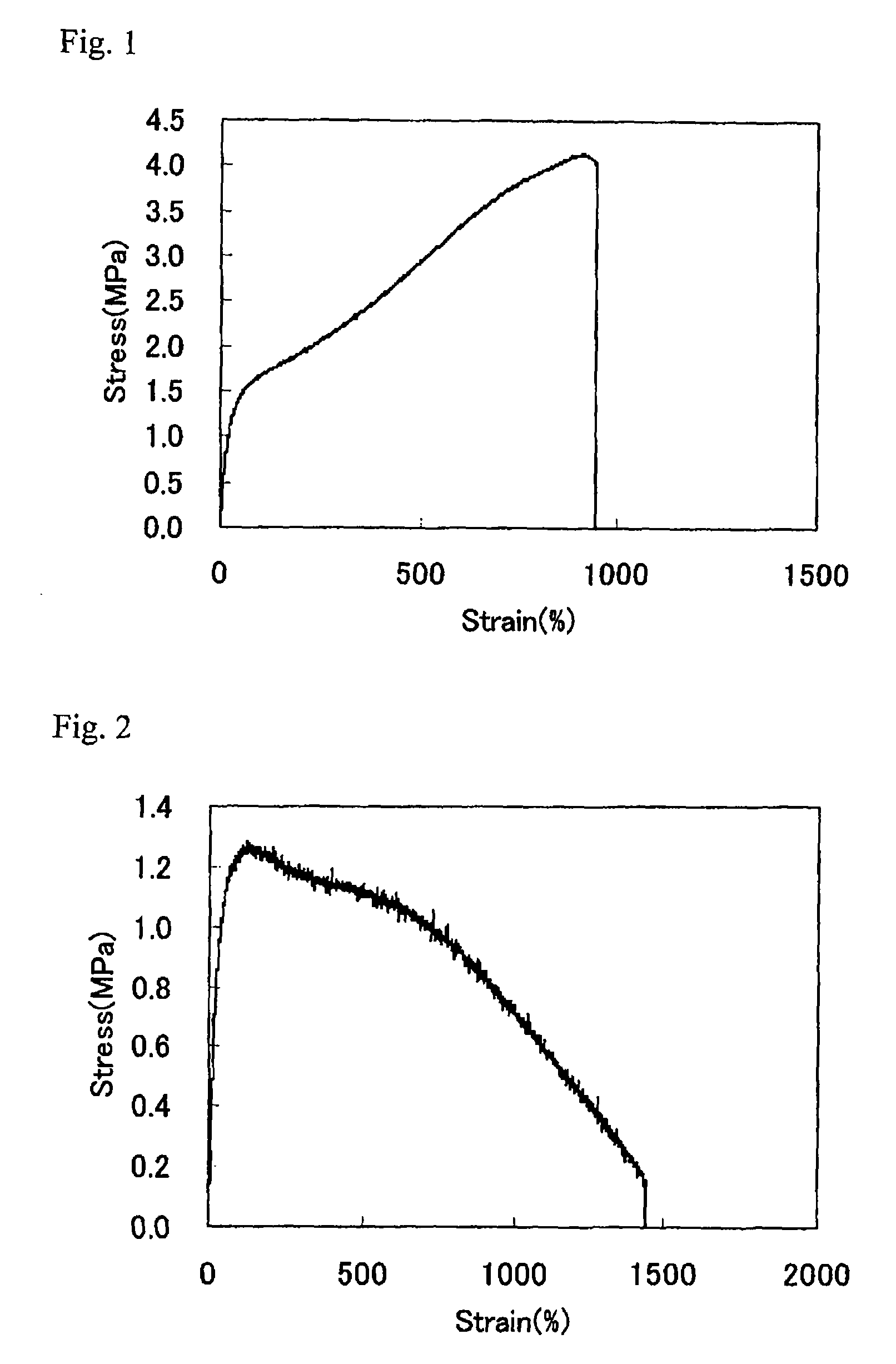
Thermoplastic elastomer composition and process for manufacturing the same
The present invention relates to a thermoplastic elastomer composition which comprises a branched olefin copolymer comprising: a main chain of an ethylene / α-olefin copolymer; and a side chain derived from a crystalline propylene polymer containing a vinyl group at one end, wherein the content of α-olefin in the main chain is 70 mol % or less, the composition has an elongation at break of 500% or higher, and has an elastic recovery rate of 70% or more when recovering from an extension to 300% elongation, and a process for manufacturing the same.
Owner:CORNELL UNIVERSITY +1
A kind of preparation method of polyethylene catalyst
ActiveCN106928381BParticles in good shapeImprove the problem of continuous polymerization difficultiesBulk chemical productionPtru catalystMicrosphere
Owner:谢炳
Catalytic cracking gasoline hydro-upgrading method
ActiveCN114075453AHydrodesulfurization - high isomerization activityMolecular sieve catalystsCatalyst activation/preparationPtru catalystHydrodesulfurization
The invention relates to a catalytic cracking gasoline hydro-upgrading method, which comprises the following steps: firstly, full-fraction catalytic cracking gasoline passes through a pre-hydrogenation reactor to remove alkadiene, mercaptan and thioether under the action of a pre-hydrogenation catalyst, then a pre-hydrogenation product is subjected to selective hydro-desulfurization under the action of hydro-desulfurization and isomerization catalysts, and meanwhile, straight-chain olefin is isomerized into single-branched olefin or single-branched alkane, so that clean gasoline with ultra-low sulfur content is obtained; and the pre-hydrogenation catalyst takes one or more of amorphous silica-alumina, alumina, a Y molecular sieve and ZSM-5 as a carrier, and is impregnated with one or more of active components of cobalt, molybdenum and nickel. The catalytic cracking gasoline hydro-upgrading method is used for producing clean gasoline with ultra-low sulfur and low octane number loss.
Owner:PETROCHINA CO LTD +1
Method for increasing propylene yield and gasoline yield through high-selectivity catalytic cracking
ActiveCN113735676AHydrocarbon by isomerisationMolecular sieve catalystsBranched chain alkeneFuel oil
Owner:CHINA PETROLEUM & CHEM CORP +1
Process for producing branched olefins from linear olefin/paraffin feed
InactiveUS20070021628A1Good choiceSmall sizeHydrocarbon by isomerisationOxygen-containing compound preparationIsomerizationParaffin oils
Owner:SHELL OIL CO
Polymeric surfactant compounds
The present disclosure relates to a lubricant composition of an oil of lubricating viscosity, a polymeric surfactant compound that is an acylated polymer having a number average molecular weight of from about 500 to about 50,000 wherein the polymer comprises a branched olefin having from 8 to 30 carbon atoms. The acylated polymer may be further reacted with an amine or alcohol to form an amide, imide, ester, or a combination thereof. The present disclosure further relates to lubricant compositions exhibiting good dispersibility and viscosity properties.
Owner:THE LUBRIZOL CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com