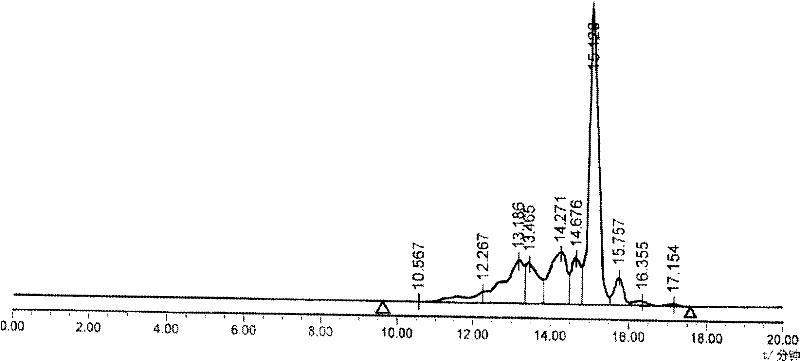
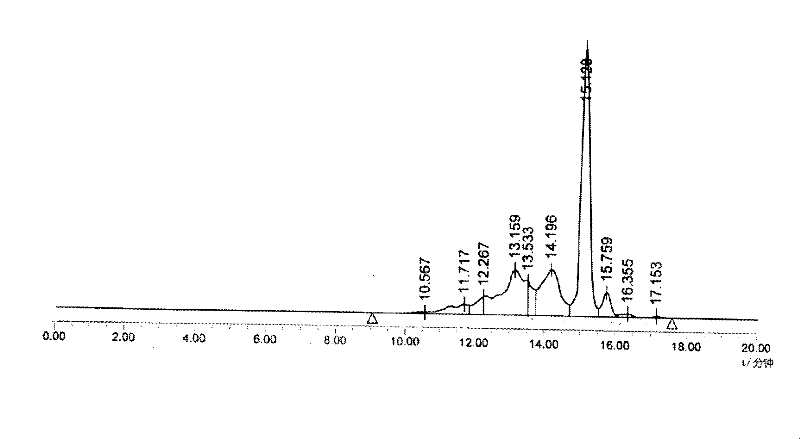
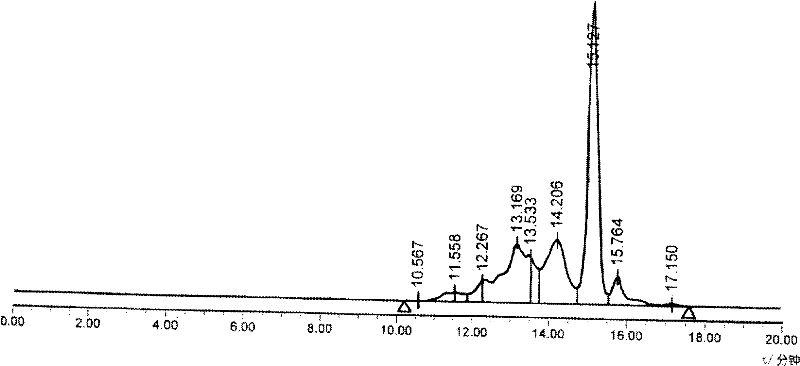
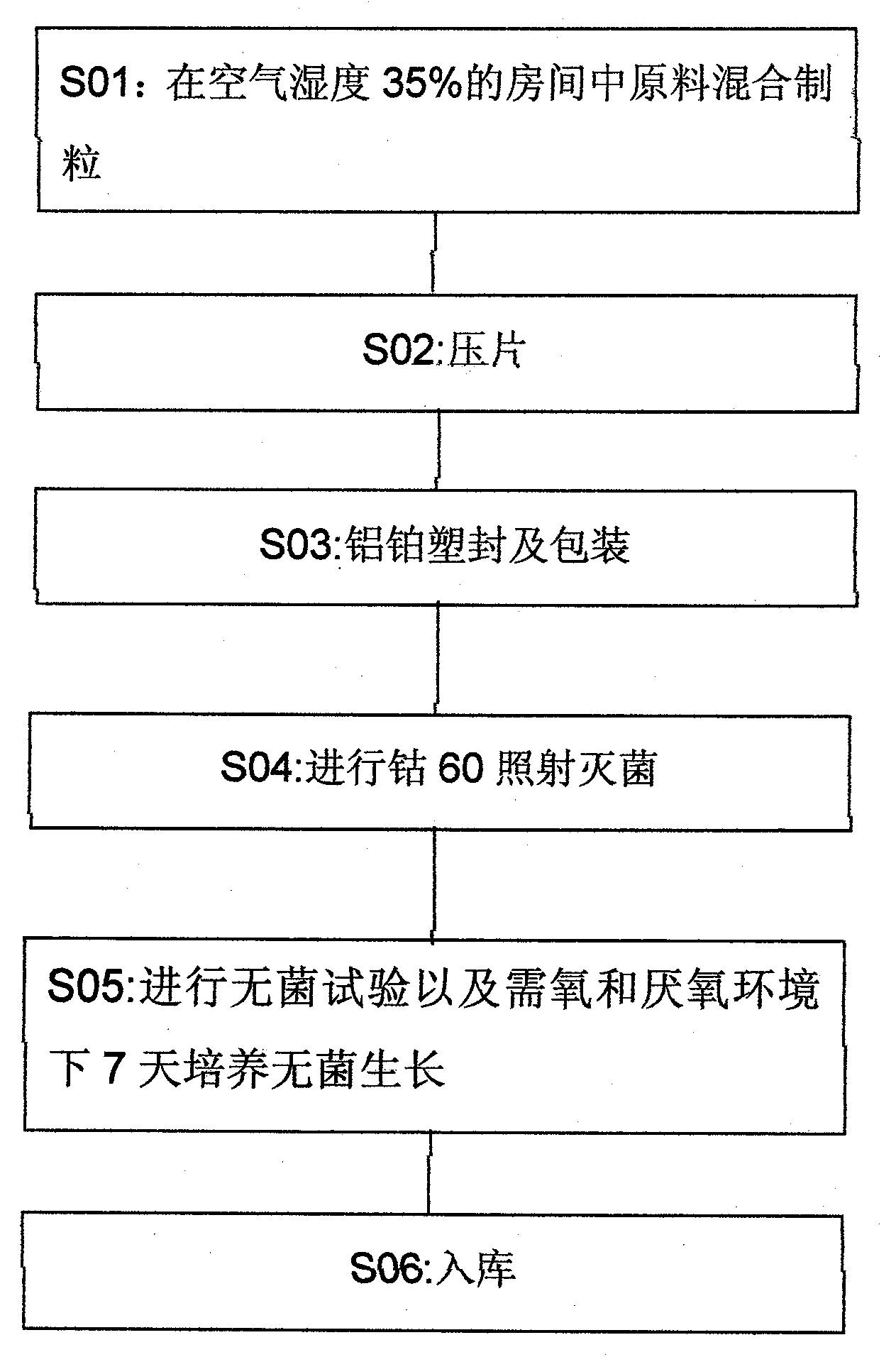
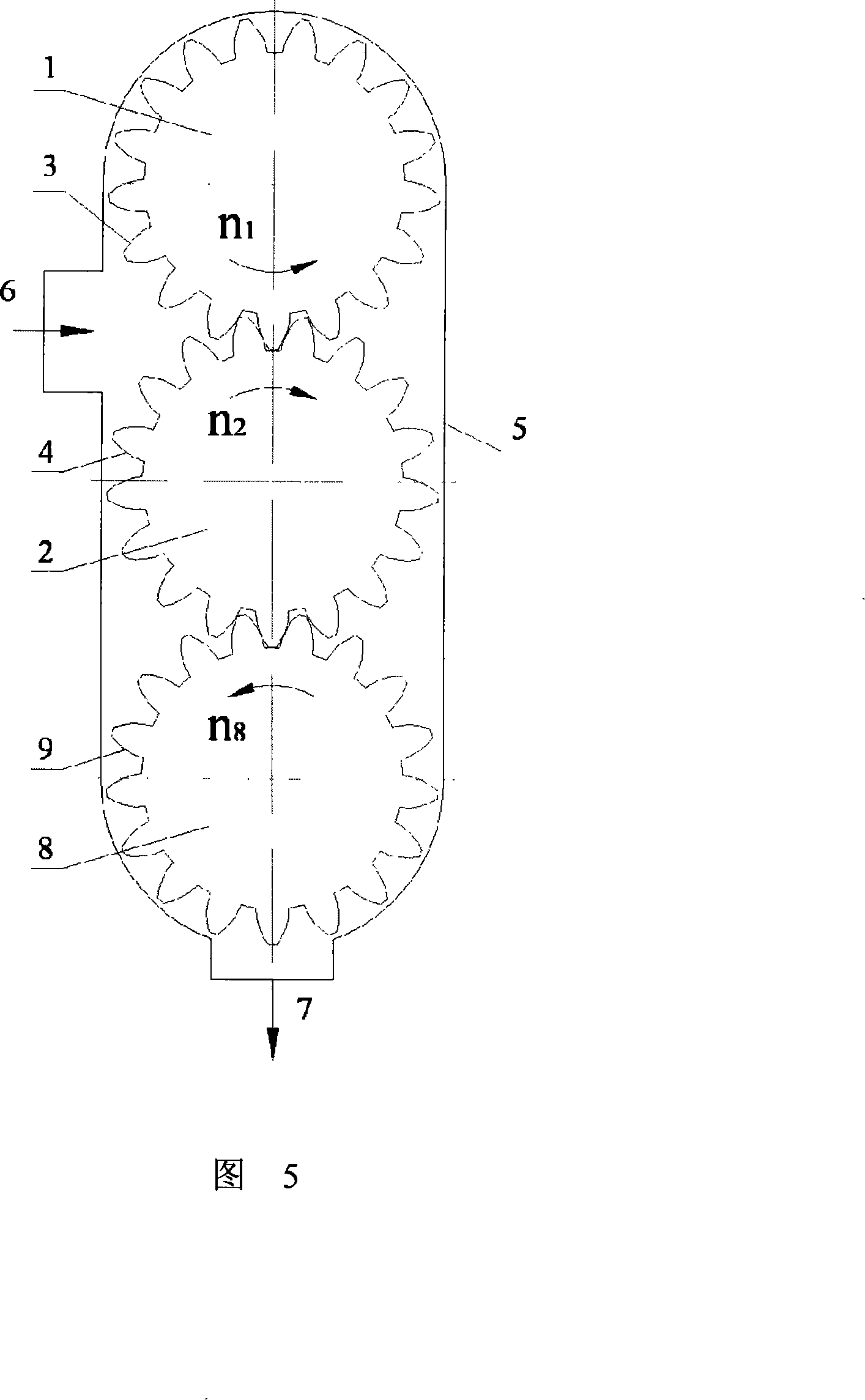Patents
Literature
33 results about "Drugs industry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing polyvinylidene chloride and acrylic ester copolymerization latex
The invention provides a preparation method of copolymerization latex of polyvinylidene chloride and acrylate, which comprises that reacts raw materials polyvinylidene chloride monomer, second monomer, functional monomer, composite emulsifier, pH adjuster, initiator, seed latex, and water in inertia gas, 0.08-0.12MPa and 50-60DEG C, collects polyvinylidene chloride and acrylate copolymerization latex from reaction product. The inventive PVDC latex is white stably, without changing into yellow after coating, with high stability. The latex can be directly coated on surface of base material as BOPP, BOPET and PVC hard sheet, while the coated or coated and dried film has barrier property, damp proofness, fragrance protectiveness, chemical drug resistance, and fireproof or the like. The film is transparent for long time, without changing into yellow. The inventive product can be used in one-time environment-friend dinnerware and inner layer of paper package, as tobacco, food and drug.
Owner:SHANGHAI CHLOR ALKALI CHEM
Resonance driven changes in chain molecule structure
InactiveUS6060293AEfficient inductionPeptide preparation methodsElectrical/wave energy microorganism treatmentChemical industryDisease
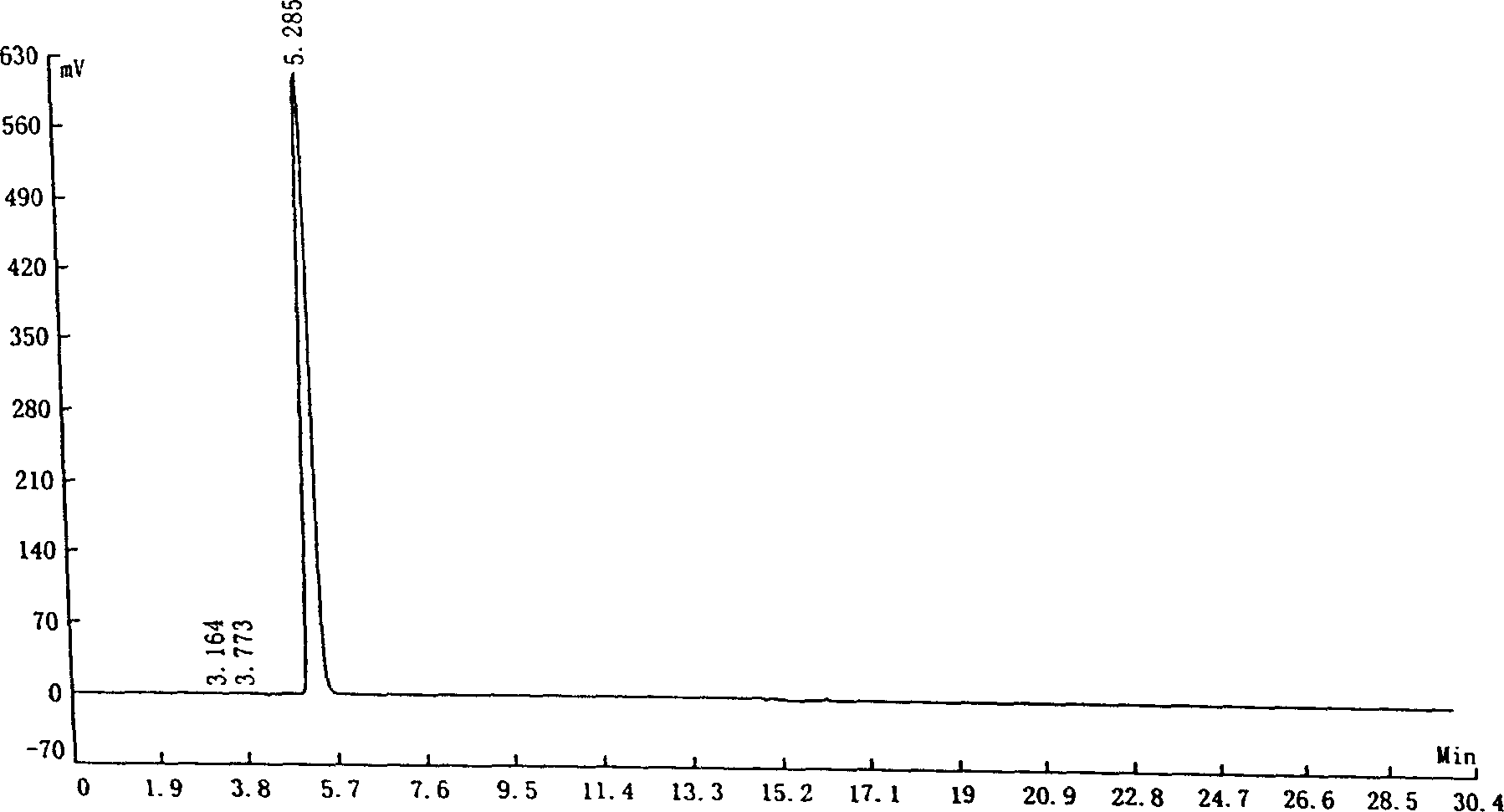
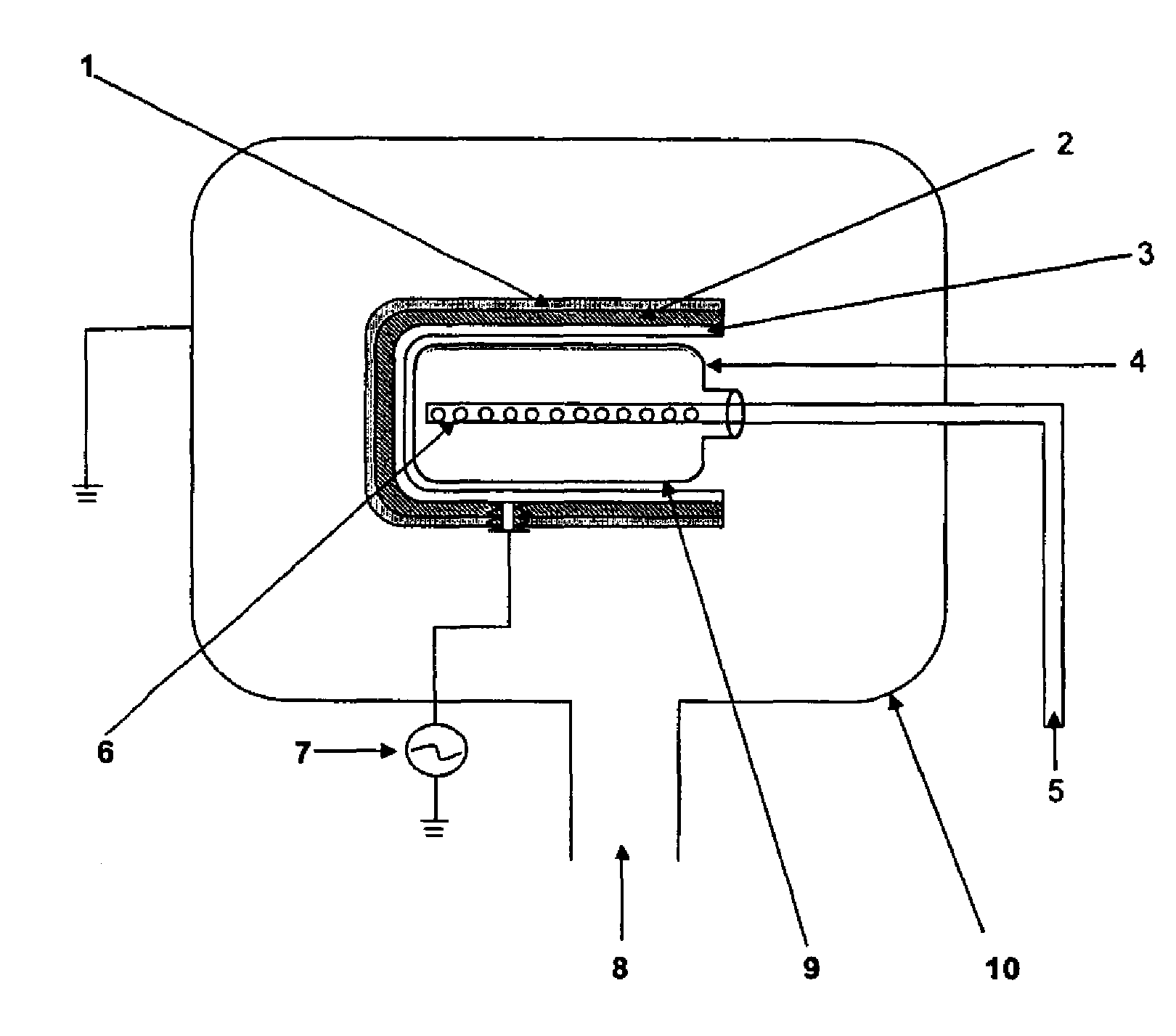
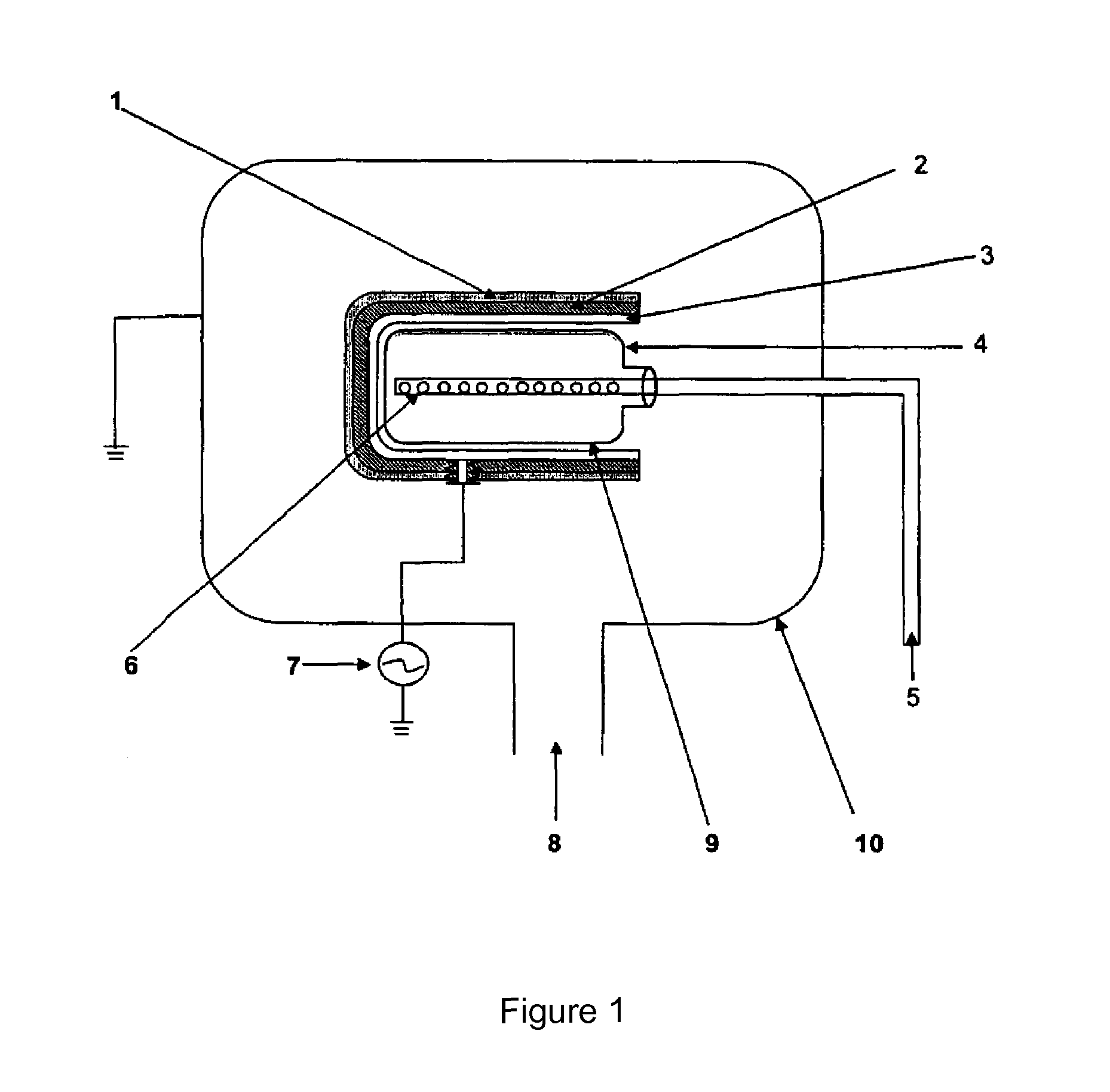
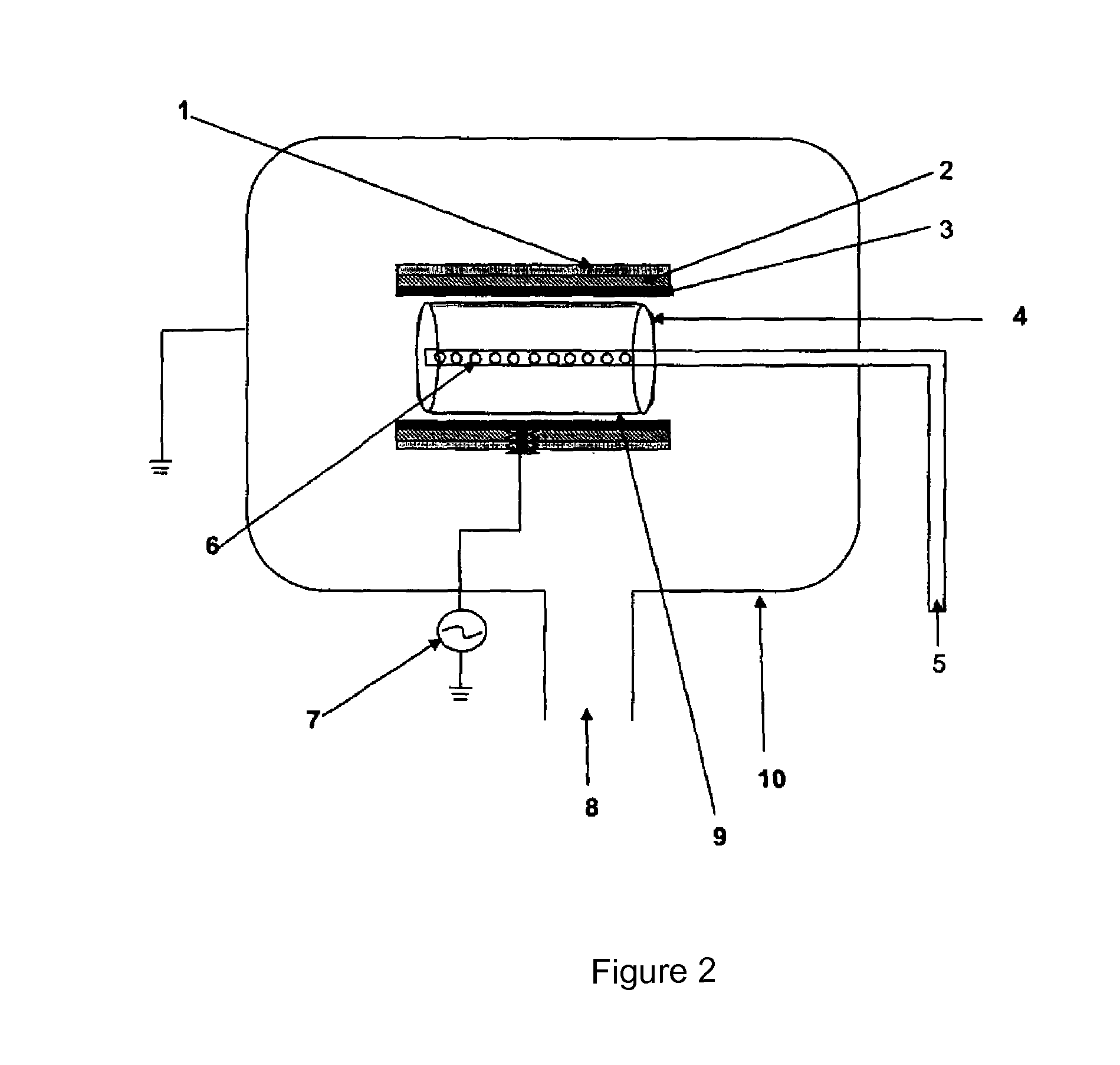
PCT No. PCT / DK96 / 00158 Sec. 371 Date Nov. 26, 1997 Sec. 102(e) Date Nov. 26, 1997 PCT Filed Apr. 1, 1996 PCT Pub. No. WO96 / 30394 PCT Pub. Date Oct. 3, 1996The invention relates to the technical application of electromagnetic radiation such as microwaves and radiowaves and application of ultra sound to chain molecules. In particular, the present invention relates to the utilization of topological excitations such as wring, twist and torsional modes, e.g., for generating structure, such as in folding, refolding or renaturation, and denaturation or unfolding of peptides, polypeptides, proteins, and enzymes; for generating changes in molecular affinity; for stimulating drug receptor interactions; and for changing molecular communication, is described. The technique is based on a new understanding of the underlying physical phenomenon and can also be applied to other chain molecules and biologically active biomolecules and tailored polymers such as glucoproteins, antibodies, genomic chain molecules such as DNA and RNA as well as PNA, carbohydrates, and synthetic and natural organic polymers. The invention is especially applicable for solving problems related to inclusion bodies and aggregation when using recombinant DNA and protein engineering techniques. Furthermore, the invention can be utilized in therapeutic treatment and in development and production of pharmaceuticals. The area of applicability ranges from biotechnological industry, food industry, drug industry, pharmacological industry, chemical industry, and concerns, e.g., the treatment of conditions and diseases related to influenza, hepatitis, polio, malaria, borrelia, diabetes, Alzheimer's disease, Creutzfeldt Jakob disease, other prion related diseases, multiple sclerosis, cataract, heart diseases, cancer, and aging.
Owner:PROKYON
Process to Deposit Diamond Like Carbon as Surface of a Shaped Object
InactiveUS20120045592A1Accurate frequencyMinimize and completely avoid damageElectric discharge tubesLinings/internal coatingsDiamond-like carbonCompanion animal
A plasma based deposition process to deposit thin film on the inner surfaces of the shaped objects such as plastic or metallic object like bottles, hollow tubes etc. at room temperature has been developed. In present invention uniform hydrogenated amorphous carbon (also called Diamond-Like Carbon, DLC) films on inner surfaces of plastic bottles is successfully deposited. Applications of such product include entire food and drug industries. There is a huge demand of polyethylene terephthalate (PET) or polyethylene naphthalate (PEN)) bottles, meant for the storage of potable water, carbonated soft drinks, wines, medicines etc. However, the higher cost prohibits their wide, spread use. The cheaper alternative is to use plastic bottles inside coated with chemically inert material such as Diamond-Like Carbon (DLC) will be commercially viable. Inventor process can be scaled up for mass production. This process can also be used for coating on inner surface of metallic cane or tube with a carbide forming interlayer (like hydrogenated amorphous silicon) to get the DLC films with better adhesion to inner surface of metals.
Owner:COUNCIL OF SCI & IND RES
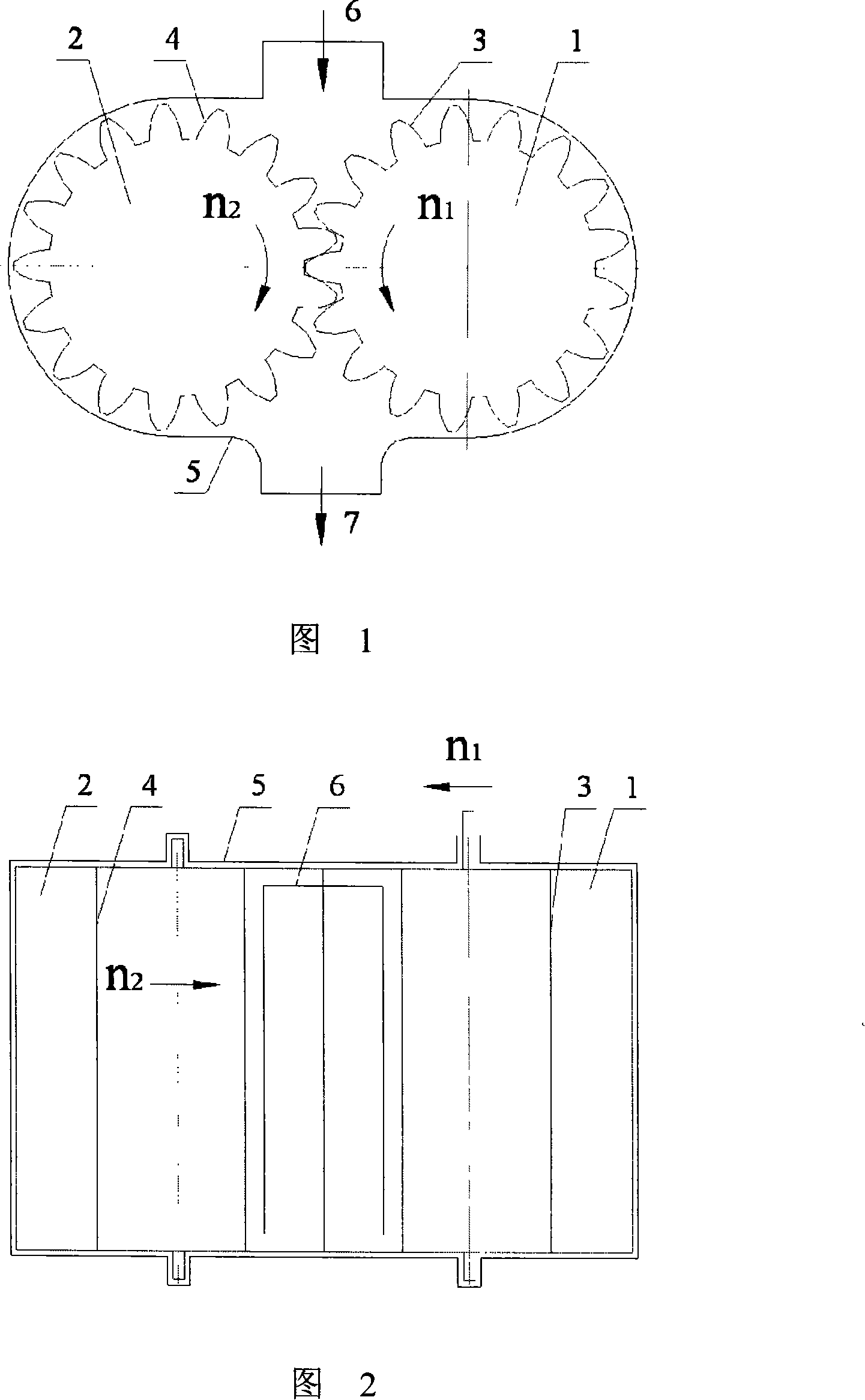
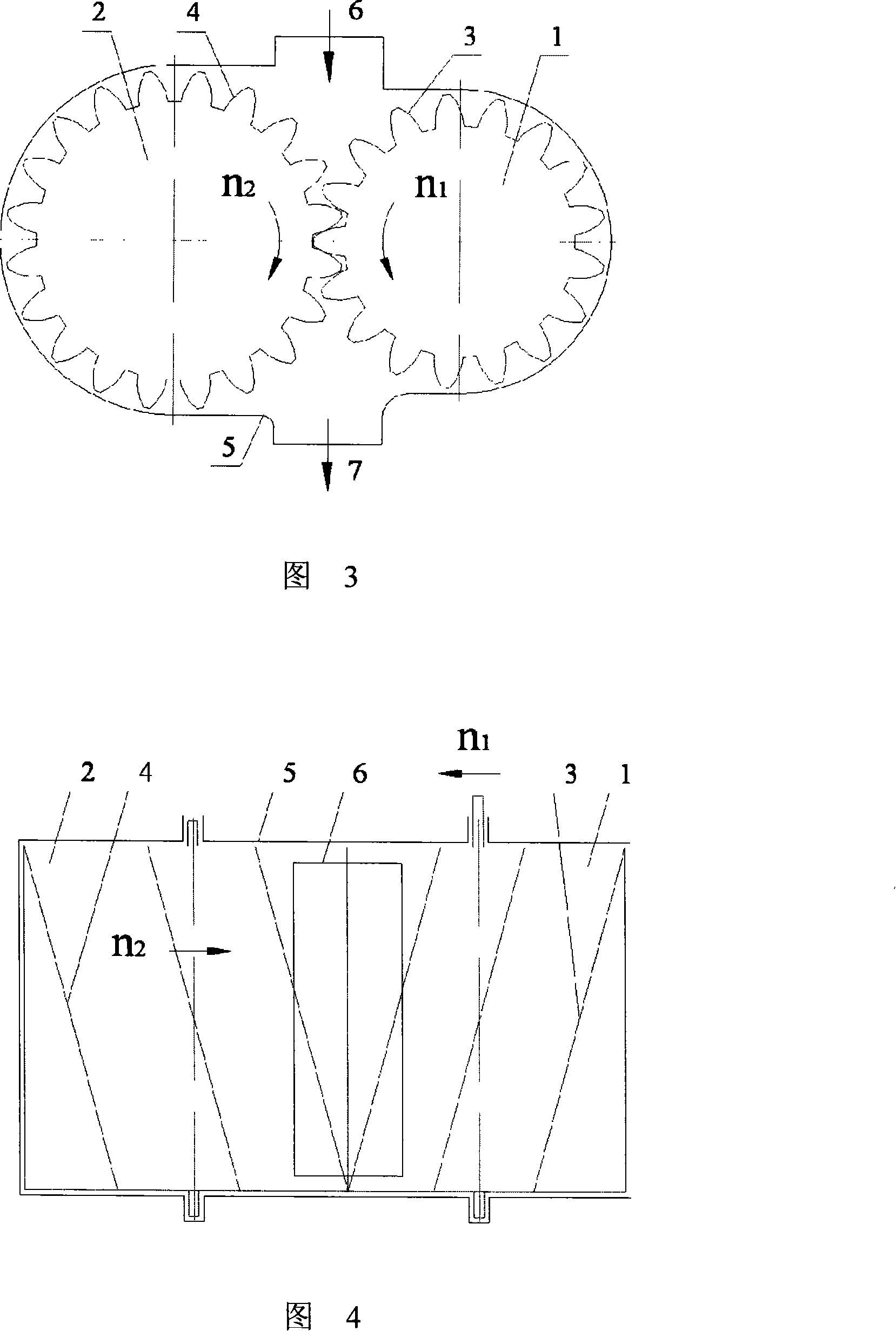
Micro grinding gear mill
The invention discloses a micro-grinding gear grinder, belonging to micro-grinding machinery. The technical scheme includes that a grinding roller (1) and a conjugate tooth profile (3) uniformly distributed on the surface of an outer cylinder of the roller compose a gear grinding roller, and a grinding roller (2) and a conjugate tooth profile (4) uniformly distributed on the surface of the outer cylinder of the roller compose one another gear grinding roller. Two gear grinding rollers with parallel axes and definite central distances are placed in a grinding body (5), each gear grinding roller is in toothed rotation around each axial cord relative to the grinding body (5) via fixed velocity ratio, and a feed inlet (6) and a feed outlet (7) are respectively arranged on the grinding body (5), forming the micro-grinding gear grinder. The working principle of the invention is that the material is disintegrated through the squeezing, grinding and shearing of the two tooth surfaces of the rollers, the invention can be adaptable to micro-grinding of powder or liquid material of the fields of chemical industry, building material, drug industry and the like.
Owner:XINGTAI POLYTECHNIC COLLEGE
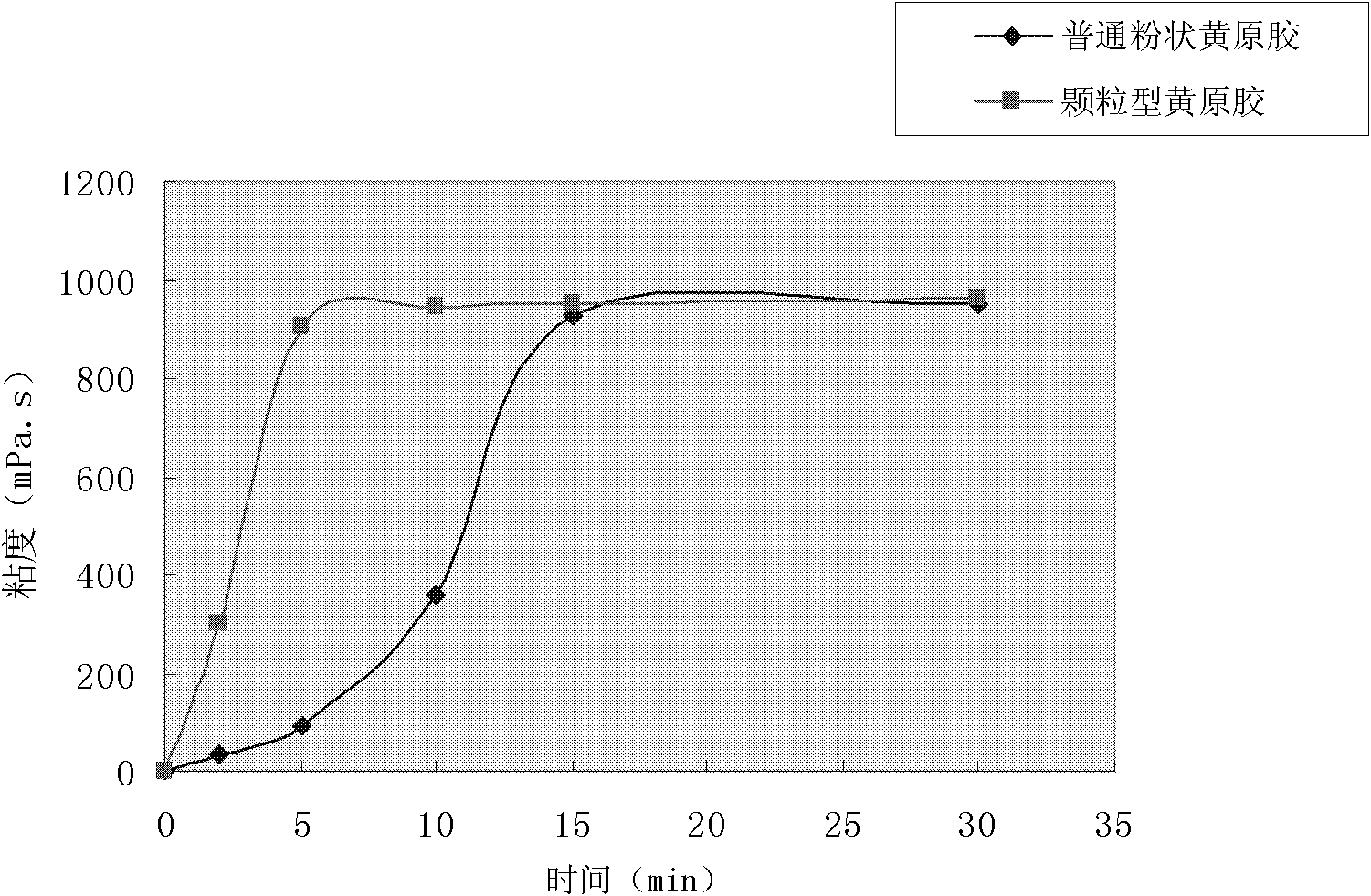
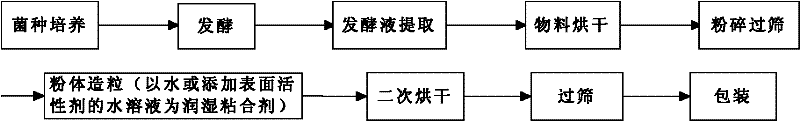
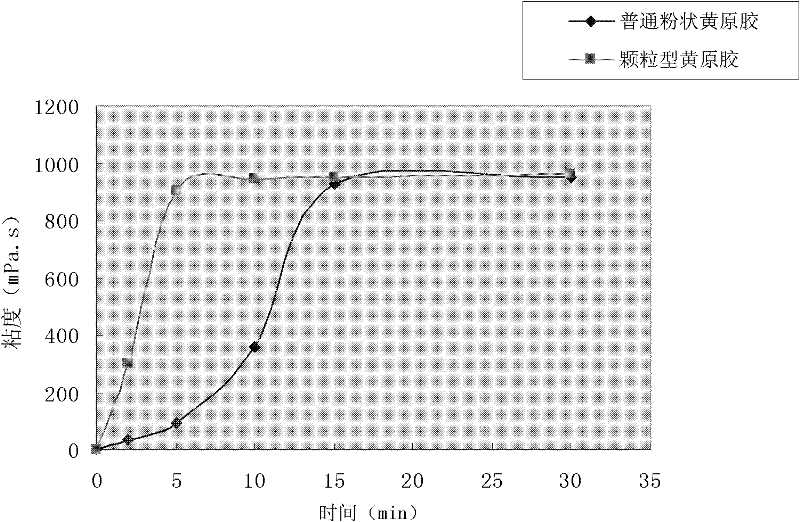
Preparation method for granular type xanthan gum
ActiveCN102219912AImprove dispersion hydration speedSolve the technical problems of agglomerationPharmaceutical non-active ingredientsFood preparationPorosityD-Glucose
The invention relates to a preparation method for granular type xanthan gum, belonging to the field of preparing glucose residue-containing compound. The preparation method is characterized by taking xanthan gum powder as starting raw materials, adopting a boiling granulation drying device to prepare the granular type xanthan gum, and comprises the following steps of 1.discharging of xanthan gum powder; 2. introduction of hot air; 3. spraying of atomized water; 4. boiling granulation; 5. secondary drying; 6. screening; and 7. quality inspection, and finally packaging for storage after qualification. The invention provides a preparation method for granular type xanthan gum with large specific area and high porosity. The prepared granular type xanthan gum substitutes powder products selling on the market, and solves the problems that the powder xanthan gum in the prior art is slowly dissolved, and blocked during dissolving process and has dust pollution. The preparation method is applicable for granulation and processing of unpackaged powder after crushing and screening during production process of xanthan gum powder production devices, as well as granulation and processing xanthan gum powder used in market sauce seasoning, soup bases, drinks, food, drug industry and petroleum industry.
Owner:ORDOS ZHONGXUAN BIOCHEM
Gold nanometer particle grain size control method based on glutathione
Disclosed is a method for controlling the grain diameter of gold nanometer particles with glutathion, belonging to the field of nanometer technology. The specific steps are as following: a. mixing the citric acid trisodium solution with glutathion solution; b. heating separately the solution prepared by step-a and chlorauric acid solution, then mixing; c. heating the solution to boiling to make the reaction complete after the solution prepared by step-b off-color, then cooling the liquid to prepare gold nanometer particle sol solution. The method is characterized in that: it is simple and the efficiency is high, the particle dimension is easy to adjust, and the creature compatibility is good, and the prepared nanometer particles has a good dispersibility and a uniform grain diameter which can be controlled by a range of 8-40nm. The gold nanometer particles can apply in the field of DNA detection, biology, drug industry, and so on.
Owner:SHANGHAI JIAO TONG UNIV
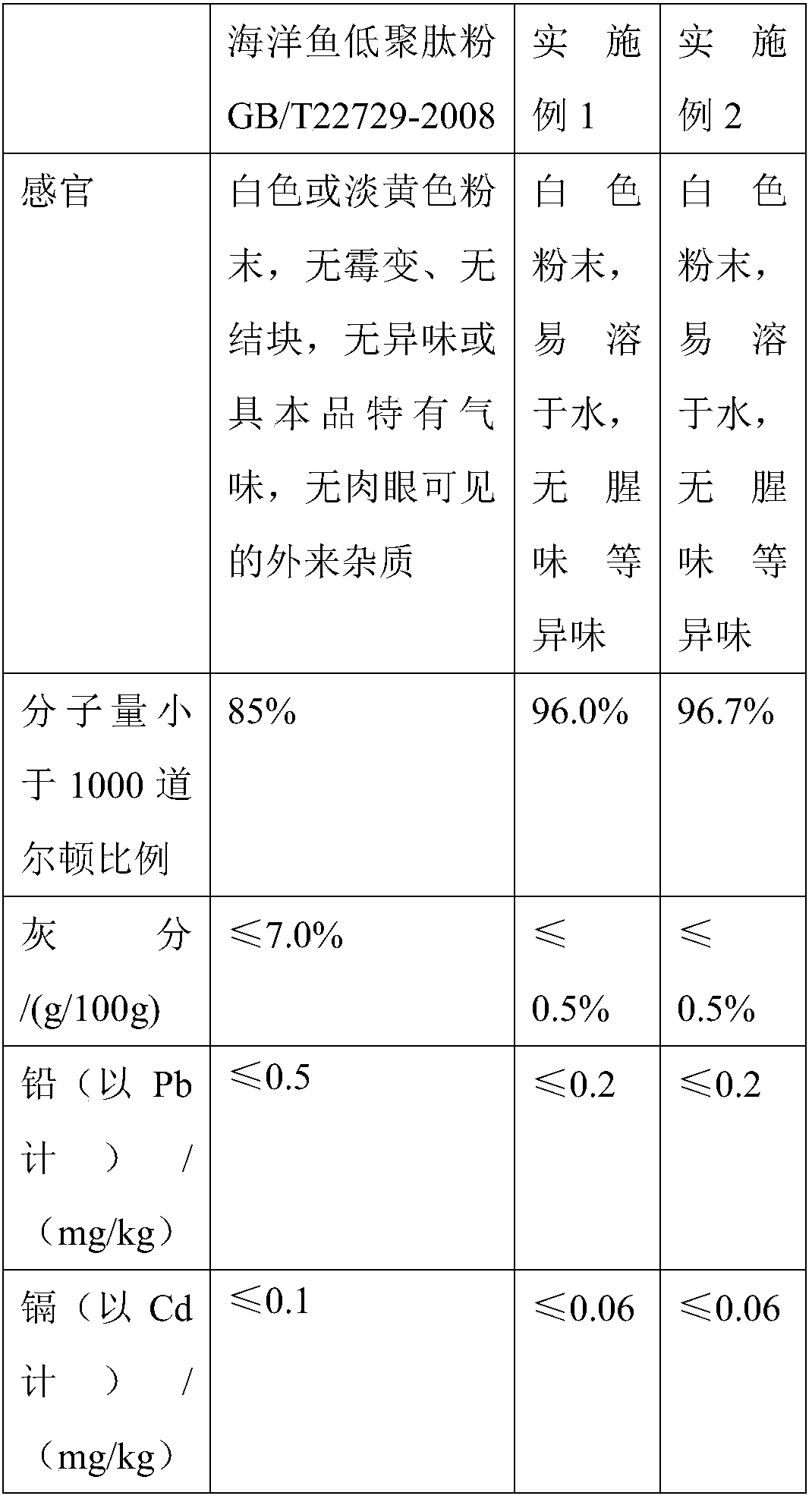
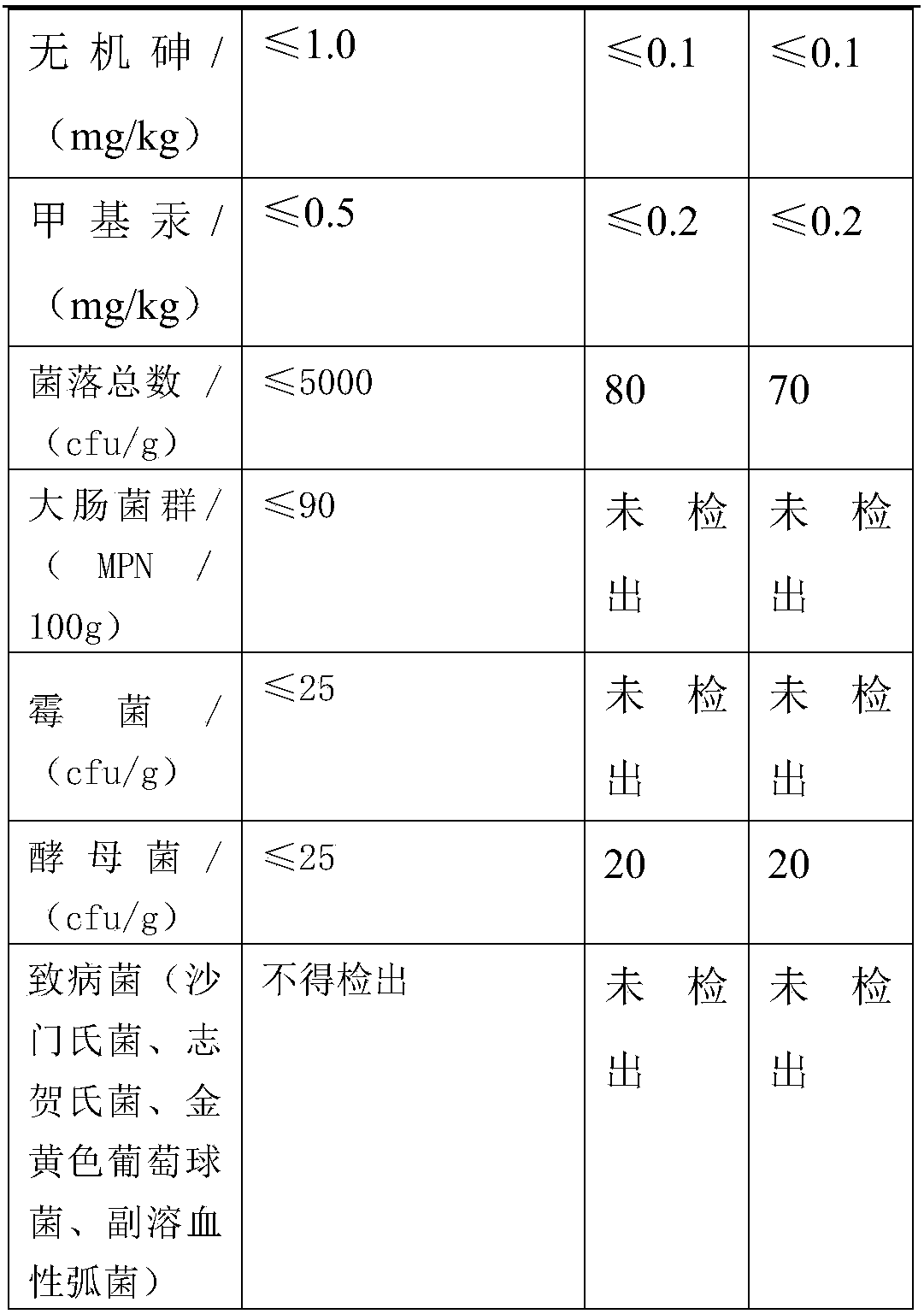
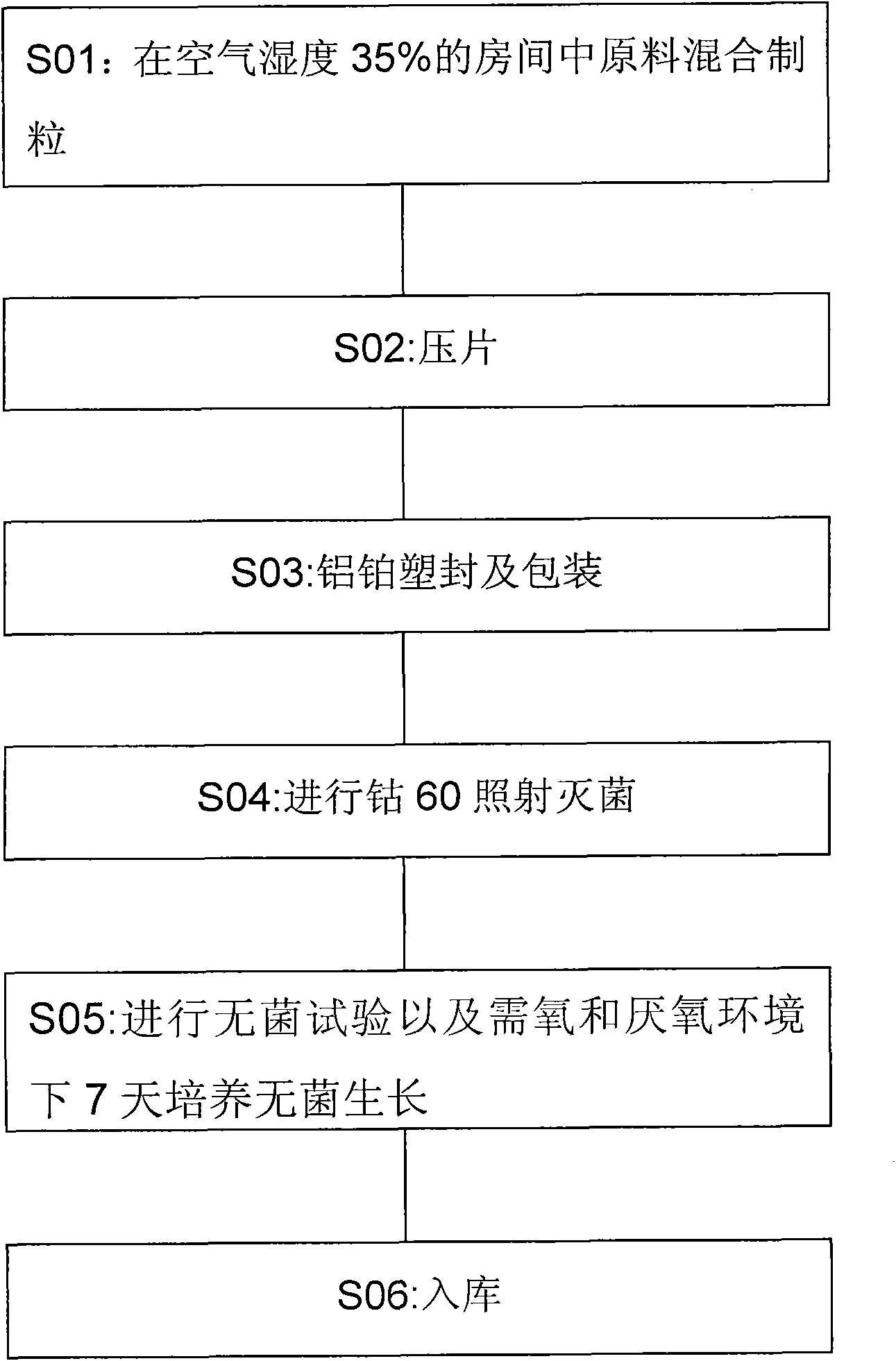
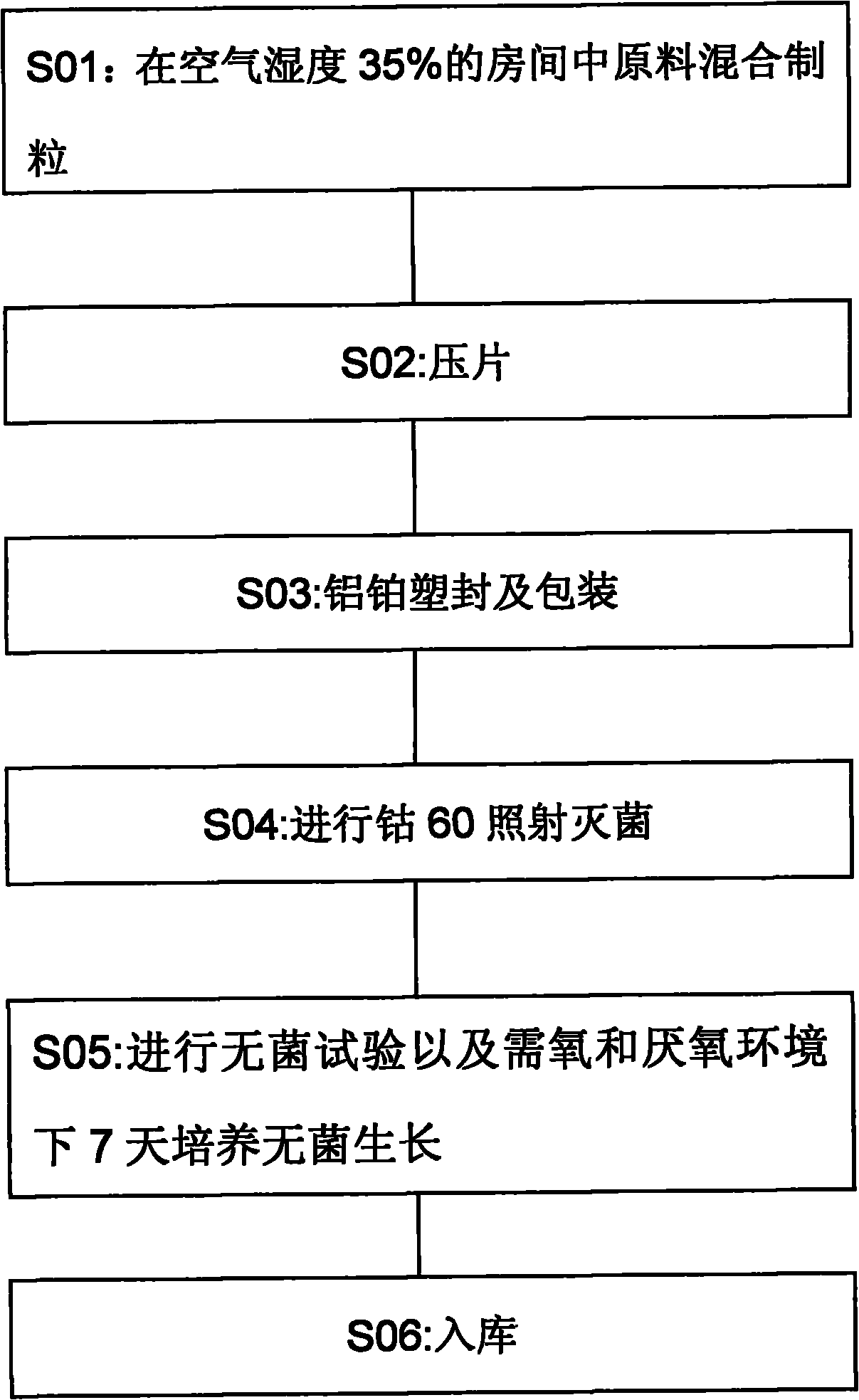
Preparation method of oligopeptide powder of marine-fish collagen and application thereof
InactiveCN108103131AEvenly dispersedImprove solubilityPeptide preparation methodsFermentationMarine fishWine industry
The invention provides a preparation method of oligopeptide powder of marine-fish collagen and application thereof. The preparation method comprises the following steps: adopting marine-fish skin as araw material, and adopting a method of mixing acidolysis and enzymolysis to prepare the oligopeptide powder with the oligopeptide content being higher than 96%. The oligopeptide powder can be widelyapplied to the fields of functional food and medicine industries such as the wine industry, the beverage industry, the seasoning industry, the health-care industry, the oral liquid, tablets and capsules.
Owner:北京姿美堂生物技术股份有限公司
Intestinal enriched tablet culture medium and process method thereof
InactiveCN102212604AWide detection rangeGood effect of bacteria enrichmentMicrobiological testing/measurementAgainst vector-borne diseasesHigh concentrationEscherichia coli
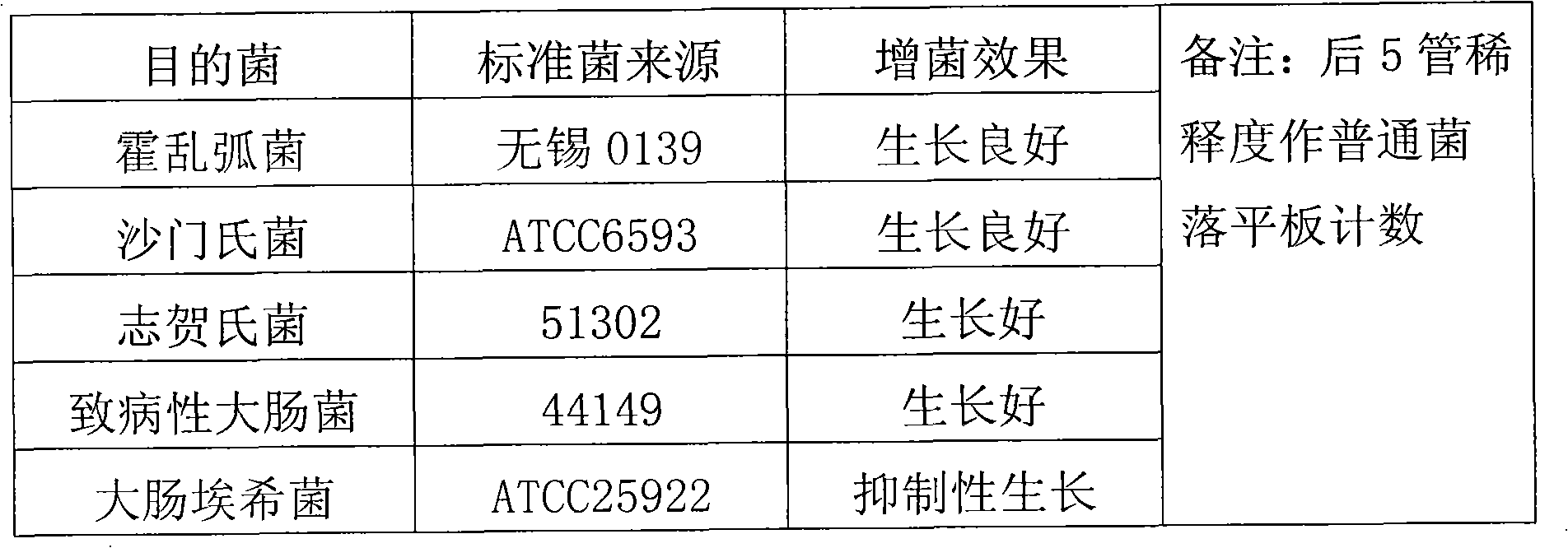
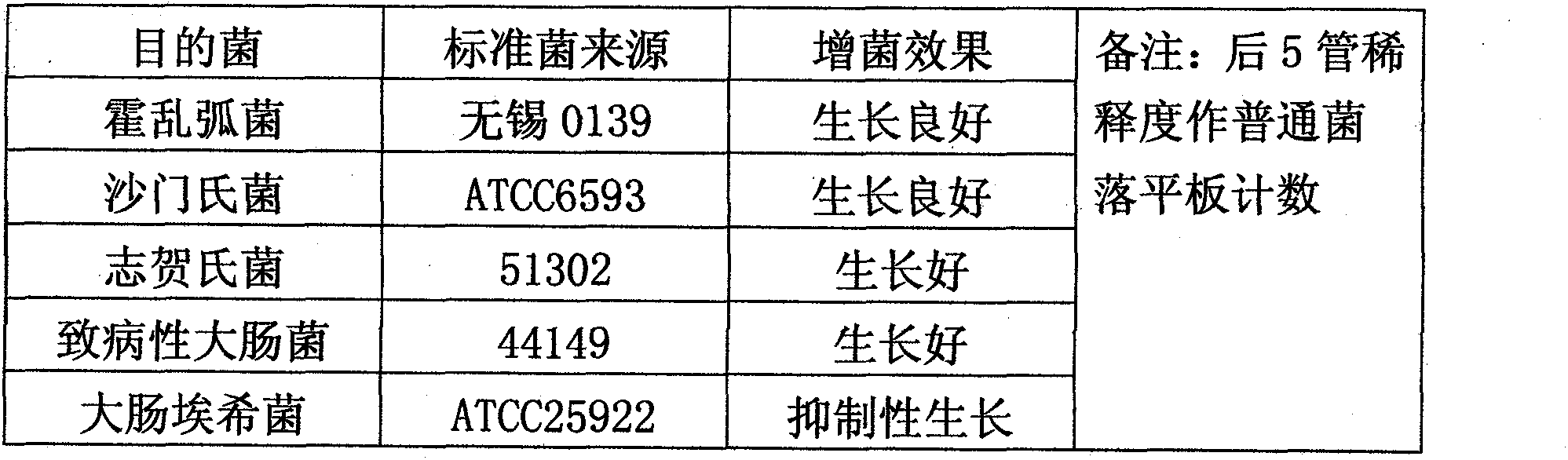
The invention discloses an intestinal enriched tablet culture medium. An enriched tablet of the intestinal enriched tablet culture medium mainly comprises peptone, trypsin, beef powder, bovine bile salt, sodium deoxycholate, a special matrix, an additive, sodium citrate, sodium thiosulfate, brilliant green and the like, wherein the high-concentration bovine bile salt and sodium deoxycholate in the culture medium can inhibit the growth of gram-positive bacteria in excrement, have certain synergistic inhibitory effects on Escherichia coli and have good enriching effects on cholera bacteria, salmonella and shigella. The invention also provides a process method of the intestinal enriched tablet culture medium. The intestinal enriched tablet culture medium has the advantages of wide detection range, good stability and high sensitivity, can realize energy conservation and emission reduction, and is a low-carbon product with great popularization prospects. At present, the technology has passed various tests and detections of the State Food and Drug Administration and has been approved for production, and the detection results and data of the technology have become new standards in the drug industry.
Owner:WUXI HUSNG BIOLOGICAL REAGENT
Process for Determining the particle size distribution of an aerosol and apparatus for carrying out such a process
InactiveUS20060246010A1Less-time-consuming and costlyPowder deliveryInvestigating moving fluids/granular solidsCascade impactorDrug industry
The present invention provides, for the first time, a process that meets the needs of the drug industry for measuring the particle size of nebulised aerosols simultaneously or one after another by the laser diffraction method and the cascade impactor method which is known in the art. In this way it is possible to bring the reliability of the results of the laser diffraction process according to the invention into conformity with that of the cascade impactor, and thereby obtain a process which combines the advantages of the rapid laser diffraction process with the accuracy of the otherwise time-consuming cascade impactor method. In addition to the process, apparatus for carrying out the process are also disclosed.
Owner:BOEHRINGER INGELHEIM INT GMBH
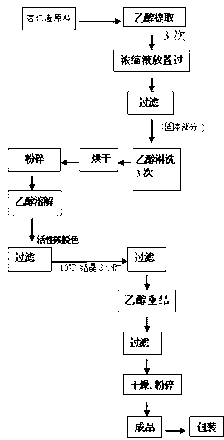
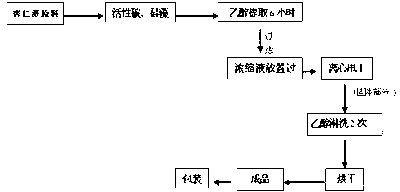
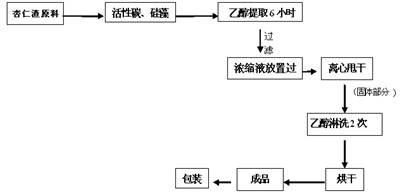
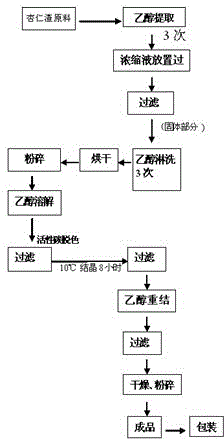
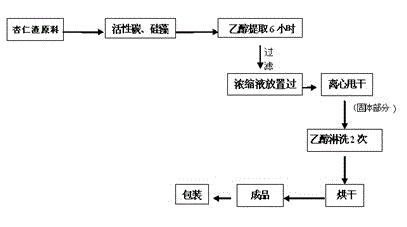
Method for separating amygdalin from almond oil residue
ActiveCN102702282AGood colorGood lookingSugar derivativesSugar derivatives preparationActivated carbonEnvironmental engineering
The invention discloses a method for separating amygdalin from almond oil residue by one-step solvent extraction, which sequentially comprises the following steps that 0.4-0.6 time of activated carbon and 0.4-0.6 time of diatomite are added into the total almond oil residue, and are fully mixed uniformly with the almond oil residue; the raw material is put into an extraction device, is heated with 5 times of ethanol, extracted, filtered, crystallized, and then put into a clean container for standing still overnight; and the obtained material is centrifugally dripped and eluted with the ethanol, a product is directly put into a blast drying oven to be dried for 24 hours, and then the amygdalin is obtained. The product has good color and appearance and high quality and purity, the operation is simple and rapid, the investment of equipment is greatly reduced, the yield of the product is more than 78%, the purity of the product is more than 99.0% and maximumly achieves 99.9%, the recovery rate of various solvents can reach more than 92%, and the BOD (Biochemical Oxygen Demand) of waste water is lower than 100, which is suitable for industrial production; and the method is widely used in the industrials of health care products and medicines.
Owner:西安天丰生物科技股份有限公司
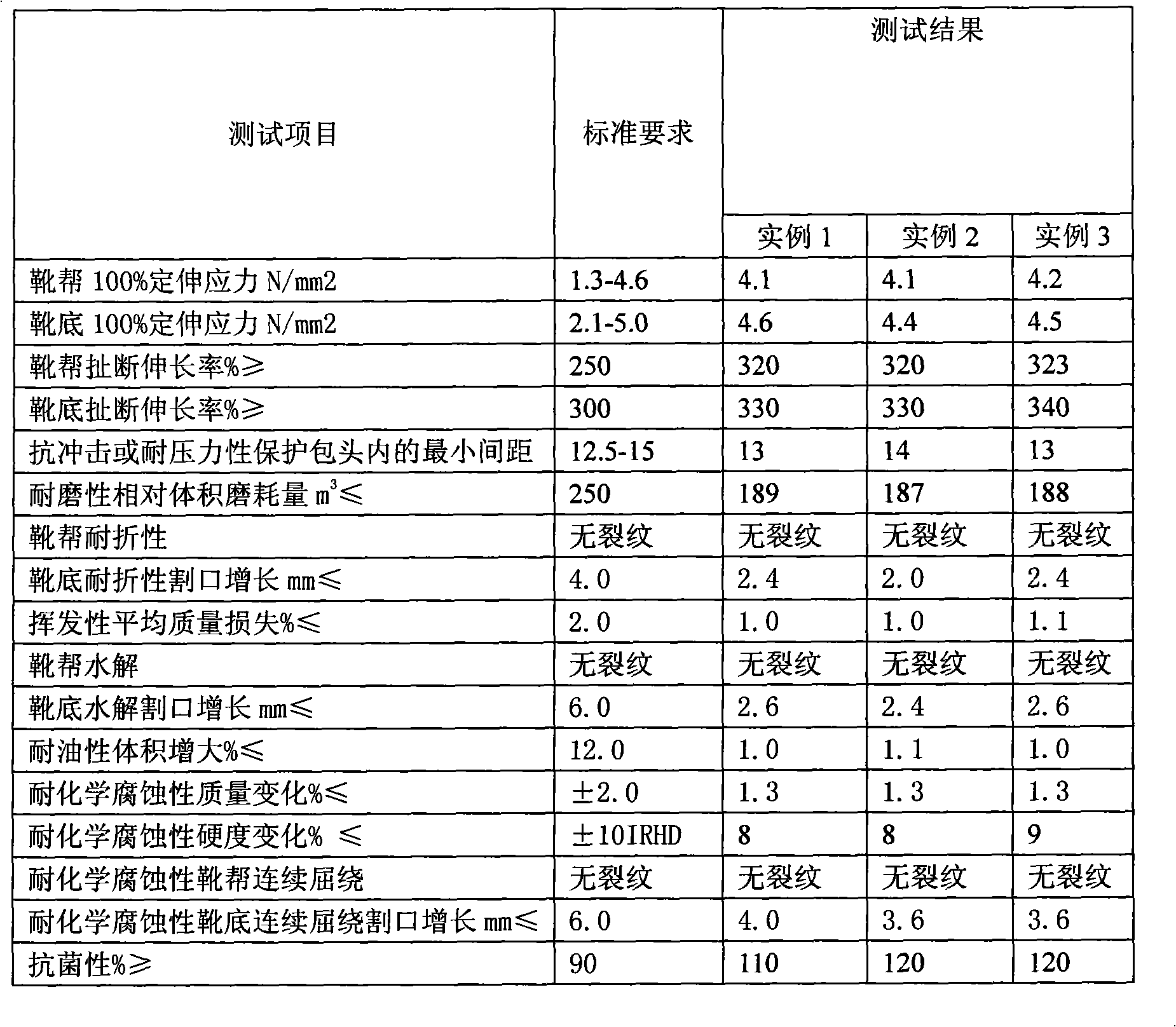
Food and drug industry protective boots and boots producing method and boots technical criterion
The invention discloses safety shoes for food and medicinal industries and the production method and the technical standard of the shoes. The sides and the soles of the shoes respectively contain PVC-S-1000 51.0%, 51.2%; DOP 30.6%, 26.1%; polyester plasticizer GLOBINEX-2340-S 15.3%, 19.1%; compound rare earth stabilizer 2.1%, 2.5%; and inorganic silver antibacterial agent 1.0%, 1.2%. The safety shoes are manufactured by following steps: weighting materials of the sides and the soles of the shoes according to the weight percentage, granulating by a granulator to obtain plastic particles, respectively adding the plastic particles into an injection molding machine, and molding twice to obtain the safety shoes. The safety shoes produced by the method meet a certain technical standard, have resistance to chemical corrosion, animal and vegetal oil, impact and bacteria, and obviate hazardness existing in the food and the medicinal industries.
Owner:朱国侯
Process for determining the particle size distribution of an aerosol and apparatus for carrying out such a process
InactiveUS20050238588A1Less-time-consuming and costlyPowder deliveryMedical devicesCascade impactorDrug industry
The present invention provides, for the first time, a process that meets the needs of the drug industry for measuring the particle size of nebulised aerosols simultaneously or one after another by the laser diffraction method and the cascade impactor method which is known in the art. In this way it is possible to bring the reliability of the results of the laser diffraction process according to the invention into conformity with that of the cascade impactor, and thereby obtain a process which combines the advantages of the rapid laser diffraction process with the accuracy of the otherwise time-consuming cascade impactor method. In addition to the process, apparatus for carrying out the process are also disclosed.
Owner:BOEHRINGER INGELHEIM INT GMBH
Indole-3-formic acid purification process
InactiveCN1807412ASuitable for purity requirementsSuitable for high efficiency requirementsOrganic chemistryInorganic saltsOrganic solvent
The invention discloses a purifying process of indole-3-aminic acid, this process can accomplish by procedures as follows: 1)adding methyl ketones organic solvent into alkali metal salt solution of indole-3-aminic acid which is made by indole-3-formaldehyde; 2)adding reduction inorganic salt into alkali metal salt solution of indole-3-aminic acid which is made by indole-3-formaldehyde to reduce it; 3)acidizing the products of 1)and 2)by inorganic acid; 4)conductting recrystallization of the products uupwords to have high-purity indole-3-aminic acid. This invention is simple to operate, easy to find the material and low in cost. It is propitious to industrial production and adapt to the request of chemical industry, drug industry. ect.
Owner:北京成宇化工有限公司 +1
Process to deposit diamond like carbon as surface of a shaped object
InactiveUS9260781B2Minimize and completely avoid damageSuitable for mass productionElectric discharge tubesLinings/internal coatingsDiamond-like carbonCompanion animal
A plasma based deposition process to deposit thin film on the inner surfaces of the shaped objects such as plastic or metallic object like bottles, hollow tubes etc. at room temperature has been developed. In present invention uniform hydrogenated amorphous carbon (also called Diamond-Like Carbon, DLC) films on inner surfaces of plastic bottles is successfully deposited. Applications of such product include entire food and drug industries. There is a huge demand of polyethylene terephthalate (PET) or polyethylene naphthalate (PEN)) bottles, meant for the storage of potable water, carbonated soft drinks, wines, medicines etc. However, the higher cost prohibits their wide, spread use. The cheaper alternative is to use plastic bottles inside coated with chemically inert material such as Diamond-Like Carbon (DLC) will be commercially viable. Inventor process can be scaled up for mass production. This process can also be used for coating on inner surface of metallic cane or tube with a carbide forming interlayer (like hydrogenated amorphous silicon) to get the DLC films with better adhesion to inner surface of metals.
Owner:COUNCIL OF SCI & IND RES
Preparation method for granular type xanthan gum
ActiveCN102219912BLarge specific surface areaGood dispersionPharmaceutical non-active ingredientsFood preparationProcess engineeringXanthan gum
The invention relates to a preparation method for granular type xanthan gum, belonging to the field of preparing glucose residue-containing compound. The preparation method is characterized by taking xanthan gum powder as starting raw materials, adopting a boiling granulation drying device to prepare the granular type xanthan gum, and comprises the following steps of 1.discharging of xanthan gum powder; 2. introduction of hot air; 3. spraying of atomized water; 4. boiling granulation; 5. secondary drying; 6. screening; and 7. quality inspection, and finally packaging for storage after qualification. The invention provides a preparation method for granular type xanthan gum with large specific area and high porosity. The prepared granular type xanthan gum substitutes powder products sellingon the market, and solves the problems that the powder xanthan gum in the prior art is slowly dissolved, and blocked during dissolving process and has dust pollution. The preparation method is applicable for granulation and processing of unpackaged powder after crushing and screening during production process of xanthan gum powder production devices, as well as granulation and processing xanthan gum powder used in market sauce seasoning, soup bases, drinks, food, drug industry and petroleum industry.
Owner:ORDOS ZHONGXUAN BIOCHEM
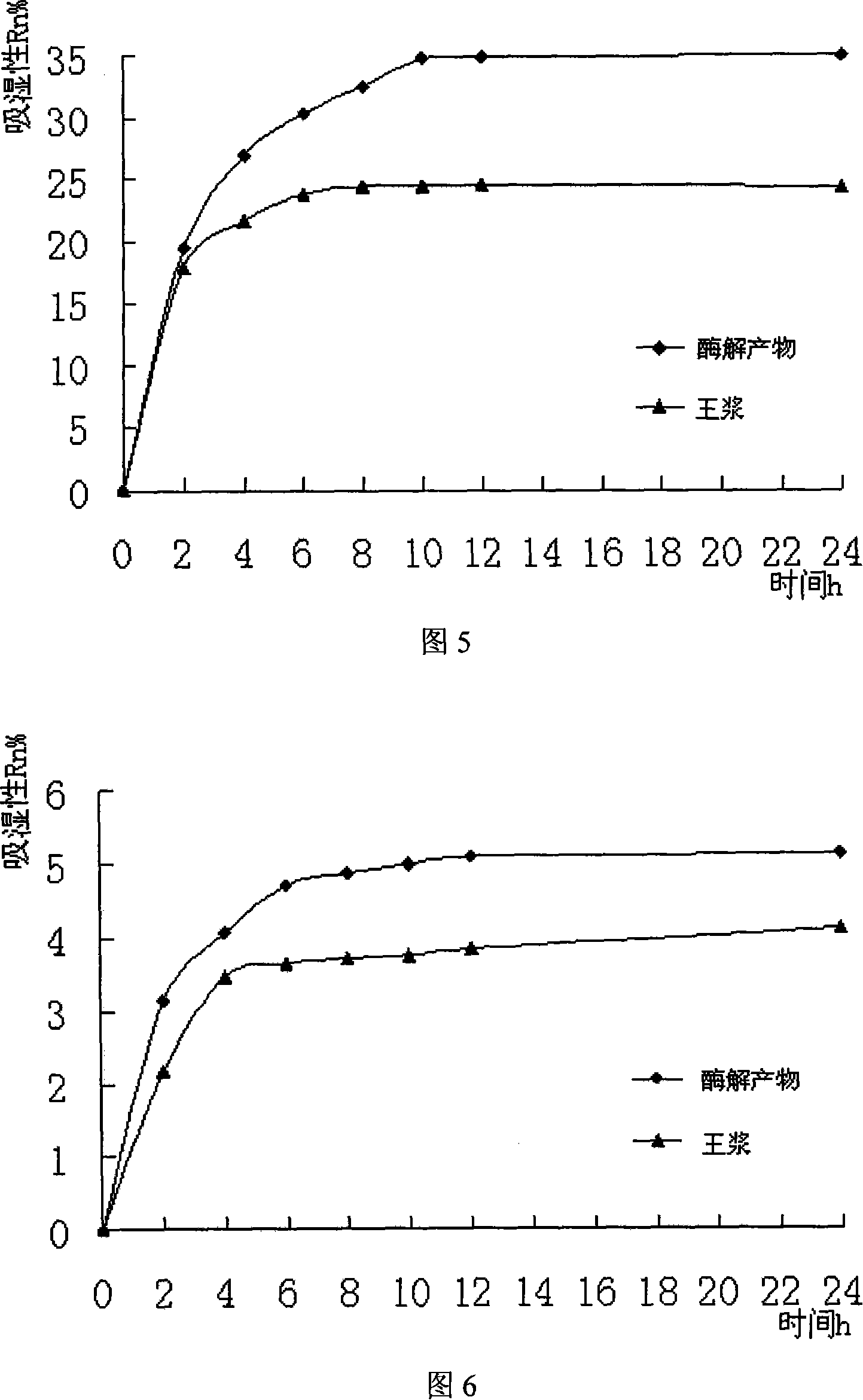
Royal jelly enzymolysis product with moisture-absorbing moisture-keeping function
InactiveCN101129410AIncrease added valueGood moisture absorptionCosmetic preparationsAnthropod material medical ingredientsBee productsDrug industry
The invention provides a bee milk enzymolysis product with hygroscopic and humectant property, which enzymolyzes bee milk with parenzyme in the constant temperature and freezes and dries the product after filtering. The bee milk of the invention improves the bound water ability of the product, increases more hygroscopic and humectant property of the product than the raw material by enzymolysising and increasing amino and carboxy group or the like hydrophilic group. The invention explores the bee milk product deeply, which improves the added value of the bee product, develops the application range, has the good hygroscopic and humectant property, the nutrient function, can reinforces the effect by inside and outside association, and can apply to drug industry, food and cosmetics field as the nutrient extender and humectant.
Owner:ZHEJIANG UNIV
Indole-3-formic acid purification process
The invention discloses a purifying process of indole-3-aminic acid, this process can accomplish by procedures as follows: 1)adding methyl ketones organic solvent into alkali metal salt solution of indole-3-aminic acid which is made by indole-3-formaldehyde; 2)adding reduction inorganic salt into alkali metal salt solution of indole-3-aminic acid which is made by indole-3-formaldehyde to reduce it; 3)acidizing the products of 1)and 2)by inorganic acid; 4)conductting recrystallization of the products uupwords to have high-purity indole-3-aminic acid. This invention is simple to operate, easy to find the material and low in cost. It is propitious to industrial production and adapt to the request of chemical industry, drug industry. ect.
Owner:北京成宇化工有限公司 +1
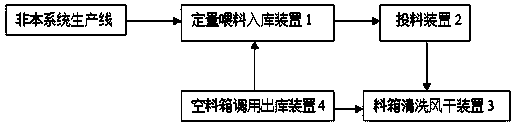
Storage-type full-automatic feeding and charging conveying system
InactiveCN108516328AAddress the degree of automationSolve efficiency problemsPharmaceutical product form changeConveyor partsControl systemChain type
The invention relates to the technical field of material conveying equipment, in particular to a storage-type full-automatic feeding and charging conveying system. The storage-type full-automatic feeding and charging conveying system comprises a quantitative feeding warehouse-in device, a charging device, a material box cleaning and air-drying device and an empty material box calling warehouse-outdevice. Chain-type conveying belts are arranged among the four devices and provided with a rotating charging shuttle vehicle for material conveying. The quantitative feeding warehouse-in device, thecharging device, the material box cleaning and air-drying device, the empty material box calling warehouse-out device and the rotating charging shuttle vehicle are connected with an external control system. The problems of low automation degree, low efficiency and poor effect in the production process in current food and drug industries can be solved.
Owner:云南迦南飞奇科技有限公司
Stomachic digestion tablet
InactiveCN105233101AEasy to prepareHigh extraction rateHydroxy compound active ingredientsDigestive systemGizzardPharmaceutical industry
The invention discloses a stomachic digestion tablet which comprises, by weight parts, 10-16 parts of red ginseng, 8-12 parts of lily, 10-16 parts of lotus leaf, 10-20 parts of malt, 10-20 parts of hawthorn, 8-12 parts of radix glycyrrhizae, 8-10 parts of chicken's gizzard-membrane, 12-18 parts of medicated leaven, 10-16 parts of xylitol, 10-14 parts of honey, 30-50 parts of starch and 50-70 parts of water. The stomachic digestion tablet is high in product extraction rate, can reduce production cost and can remove food retention, invigorate the spleen and the stomach, harmonize the stomach and soothe the liver. The stomachic digestion tablet belongs to the health pharmaceutical industry and has wide development prospects.
Owner:刘书元
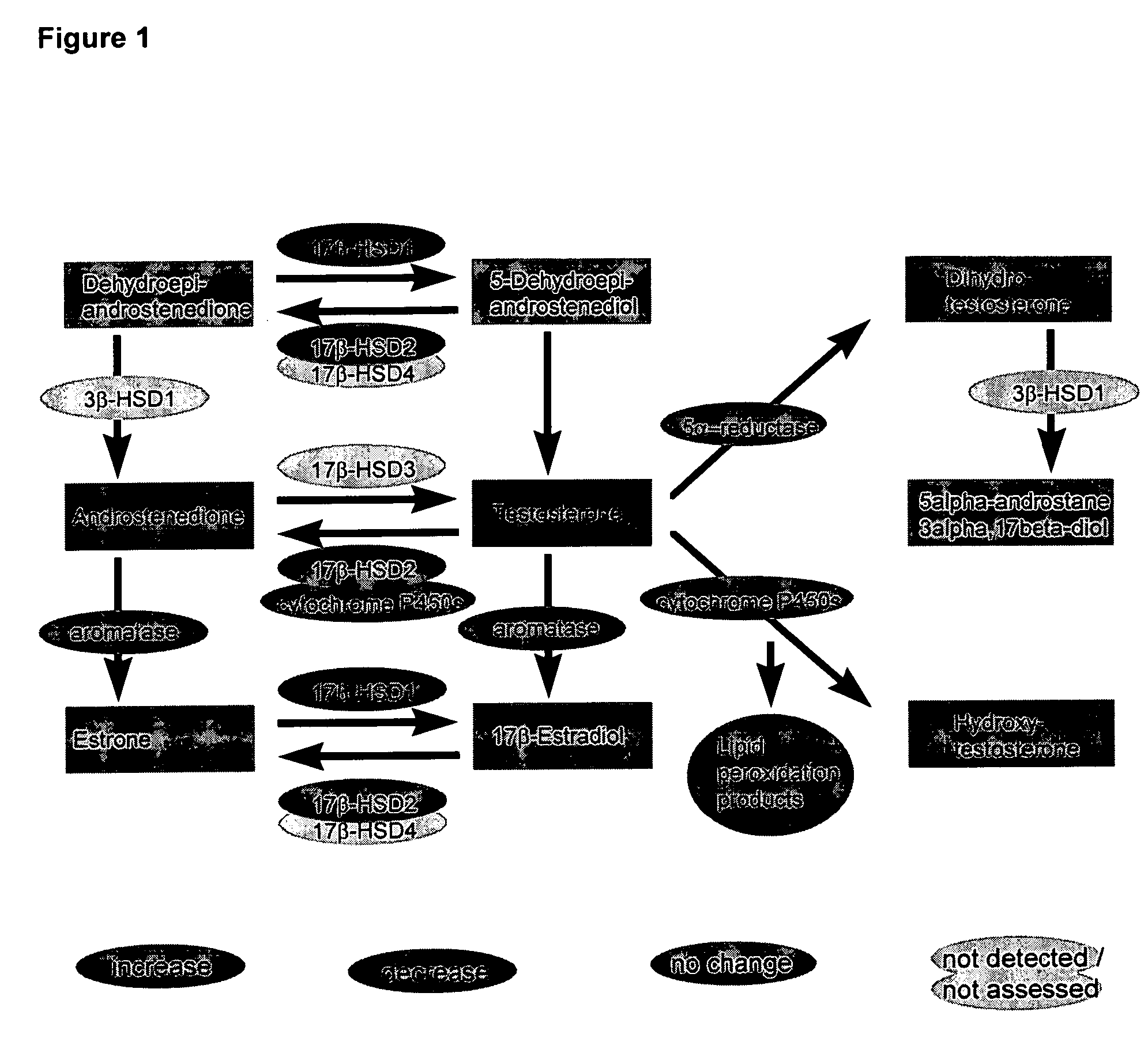
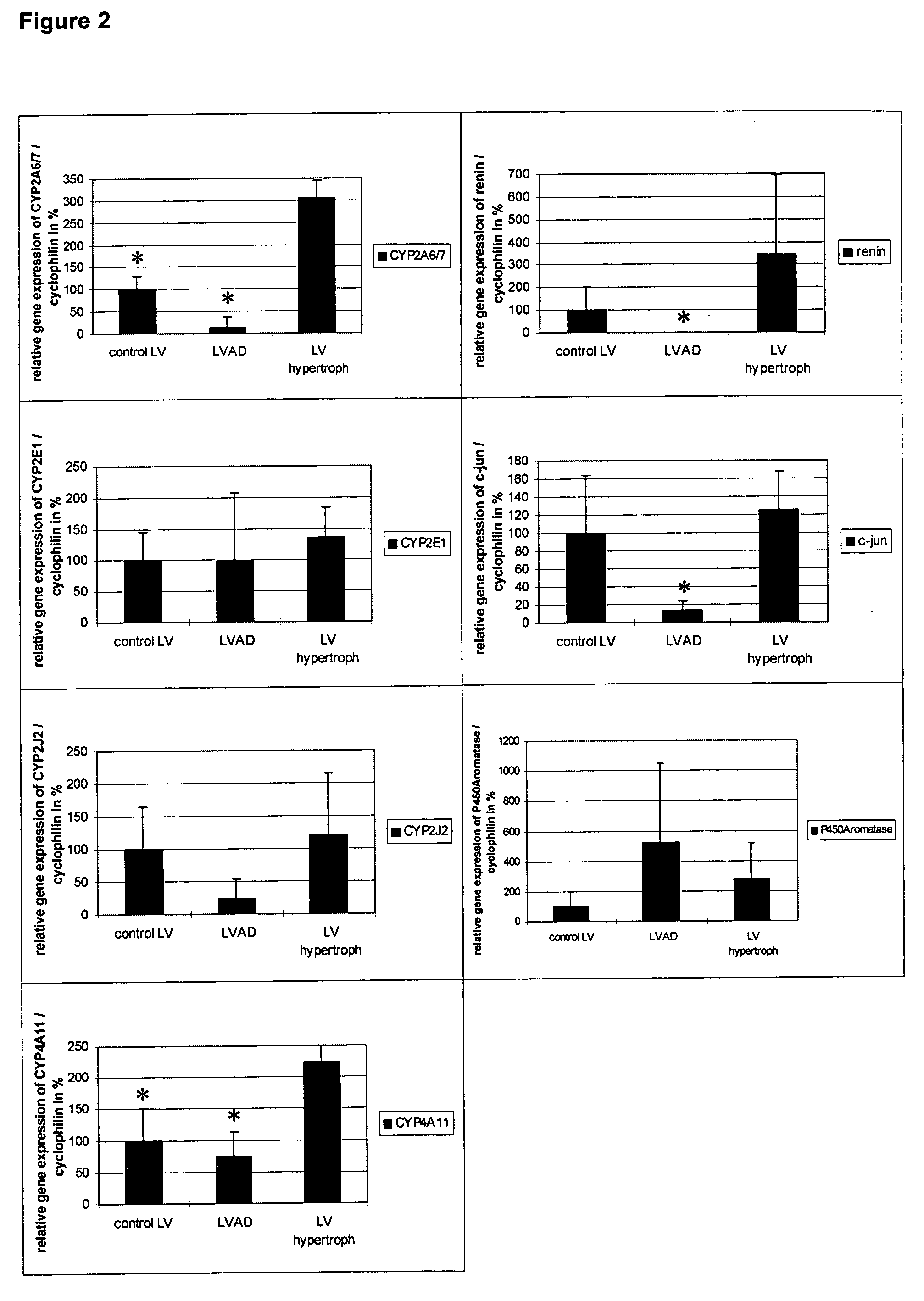
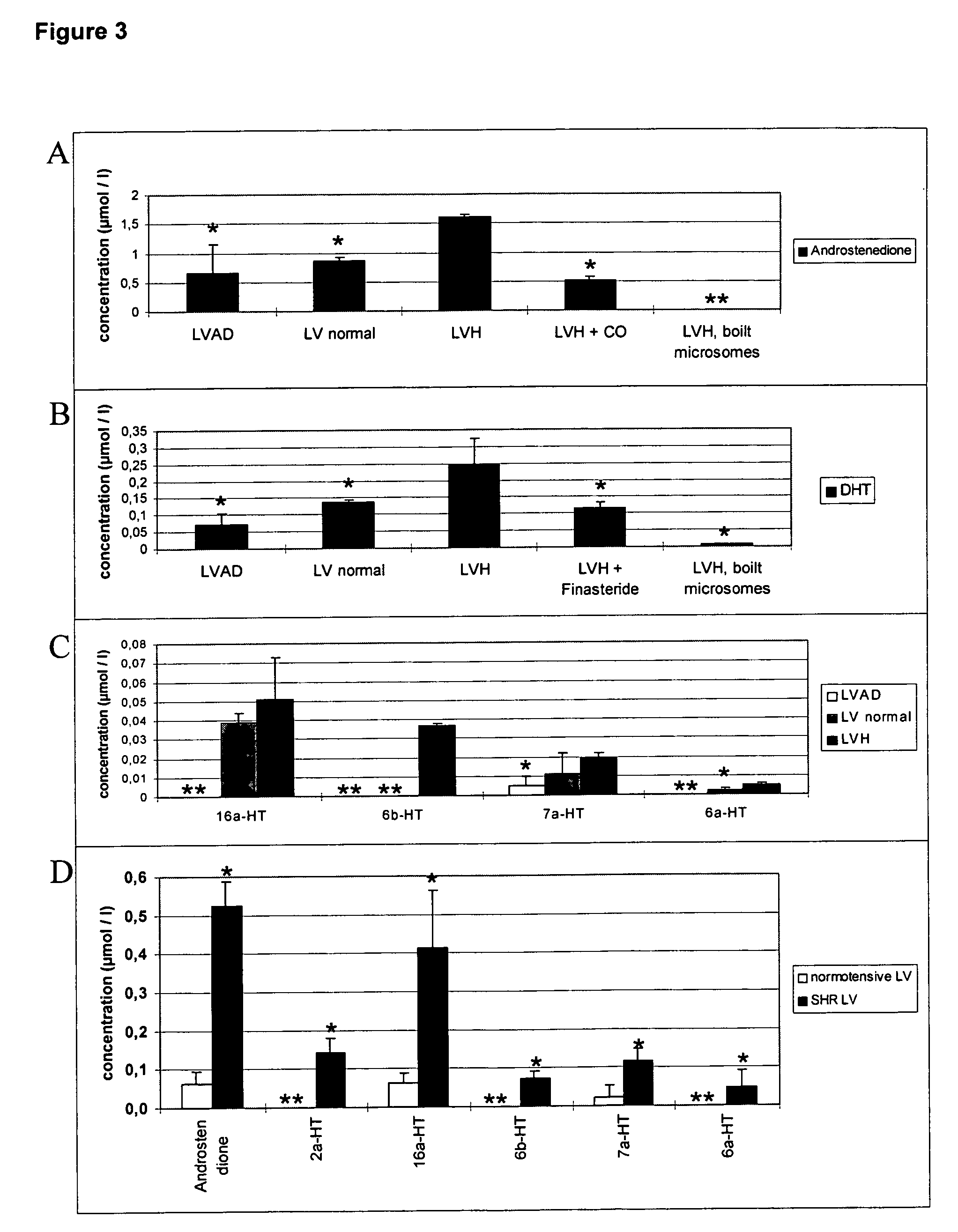
Methods and agents for treating cardiovascular diseases
This invention relates to a method and an agent for treating cardiovascular diseases, especially cardiac hypertrophy, wherein said method and agent consist in increasing testosterone concentration in pathological tissues to normal levels or inhibiting and / or eliminating metabolites from testosterone metabolism. The testosterone concentration in pathological tissues can be increased to normal levels by administering at least one substance from the following groups: testosterone; substances with effects similar to those of testosterone; testosterone mimetics; substances that enhance testosterone synthesis; substances that inhibit testosterone metabolism. Metabolites from testosterone metabolism can be inhibited and / or eliminated by administering at least one substance from the following groups: substances that bind to the androgen receptor, causing the receptor levels to be regulated and thus normalized; substances that bind to the androgen receptor, regulating the androgen receptor-mediated gene expression by inhibiting it, as is observed in cardiac hypertrophy. Areas of application are medicine and pharmaceuticals industry.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Soluble L-calcium lactate preparation and preparing method
InactiveCN1206985CImprove solubilityGood absorption rateMetabolism disorderAnhydride/acid/halide active ingredientsSolubilityFood sector
Owner:HUAHONG BIOLOGICAL PROD SHANGHAI
Tablets with functions of strengthening stomach and promoting digestion
InactiveCN105213895AEasy to prepareHigh extraction rateDigestive systemBird material medical ingredientsGizzardPotato starch
The invention discloses tablets with functions of strengthening the stomach and promoting digestion. The tablets comprise raw materials in parts by weight as follows: 12-16 parts of roots of straight ladybell, 40-50 parts of yams, 15-25 parts of hawthorn fruits, 10-12 parts honey, 10-12 parts of peanuts, 10-12 parts of chicken's gizzard membranes, 14-16 parts of xylitol, 16-18 parts of sweet potato starch, 15-25 parts of malt, 8-10 parts of figs, 10-12 parts of oat and 50-60 parts of water. The preparation method is simple, the product extraction rate is high, the production cost can be reduced, and the tablets can disperse accumulations, transform stagnation, strengthen the spleen, promote appetite, harmonize the stomach and sooth the liver, belong to the industry of health and medicines and have broad development prospect.
Owner:QINGDAO JUNENG PIPELINE EQUIP CO LTD
Medicine traceability management system
InactiveCN112487320ACommerceSpecial data processing applicationsBusiness enterpriseSoftware engineering
The invention discloses a medicine traceability management system which comprises: a medicine production system, an enterprise node, an identification analysis secondary node and a medicine information query system. According to the system, enterprise node connection identification analysis secondary nodes are built in a medicine enterprise, a production system of the medicine enterprise is in butt joint with the enterprise nodes, codes of minimum medicine packages are registered to the identification analysis secondary nodes through the enterprise nodes, and one medicine corresponds to one code; and meanwhile, drug traceability management is developed to help each link of a drug industry chain to understand each piece of information of the drug, such as raw material information, production time, delivery time, logistics information, instructions of drug use and the like.
Owner:苏州协同创新智能制造装备有限公司
Continuous vacuum fluidization freeze drying device
PendingCN111102820AFully dryKeep dryDrying solid materials without heatDrying machines with progressive movementsMedical equipmentFreeze-drying
The invention relates to the technical field of material freeze drying devices and discloses a continuous vacuum fluidization freeze drying device. The continuous vacuum fluidization freeze drying device comprises a vacuum chamber. One end of the vacuum chamber is connected with a vacuum chamber maintenance and overhaul end cover. The upper end of the vacuum chamber communicates with a feed port mechanism. The position, close to the lower end, of the chamber wall of the vacuum chamber communicates with a discharge port mechanism. The vacuum chamber communicates with a vacuum port. Unpowered material tray conveying constant-temperature table boards are mounted in the vacuum chamber. The unpowered material tray conveying constant-temperature table boards include the multiple layers of constant-temperature table boards vertically distributed in the horizontal direction. Each layer of constant-temperature table board comprises a plurality of constant-temperature table board modules. The continuous vacuum fluidization freeze drying device can well meet the requirements for continuity, batches and accuracy of the powder drying process in the food and drug industries, especially the pharmaceutical industry, and GMP mass production of medical equipment can be achieved. Meanwhile, the problems that materials are prone to caking and the drying effect of the materials is affected are solved.
Owner:SUZHOU DEEPCOLD REFRIGERATION TECH CO LTD
Royal jelly enzymolysis product with moisture-absorbing moisture-keeping function
InactiveCN101129410BIncrease added valueGood moisture absorptionCosmetic preparationsAnthropod material medical ingredientsBee productsMoisture
Owner:ZHEJIANG UNIV
Intestinal enriched tablet culture medium and process method thereof
InactiveCN102212604BWide detection rangeGood effect of bacteria enrichmentMicrobiological testing/measurementAgainst vector-borne diseasesEscherichia coliHigh concentration
The invention discloses an intestinal enriched tablet culture medium. An enriched tablet of the intestinal enriched tablet culture medium mainly comprises peptone, trypsin, beef powder, bovine bile salt, sodium deoxycholate, a special matrix, an additive, sodium citrate, sodium thiosulfate, brilliant green and the like, wherein the high-concentration bovine bile salt and sodium deoxycholate in the culture medium can inhibit the growth of gram-positive bacteria in excrement, have certain synergistic inhibitory effects on Escherichia coli and have good enriching effects on cholera bacteria, salmonella and shigella. The invention also provides a process method of the intestinal enriched tablet culture medium. The intestinal enriched tablet culture medium has the advantages of wide detection range, good stability and high sensitivity, can realize energy conservation and emission reduction, and is a low-carbon product with great popularization prospects. At present, the technology has passed various tests and detections of the State Food and Drug Administration and has been approved for production, and the detection results and data of the technology have become new standards in the drug industry.
Owner:WUXI HUSNG BIOLOGICAL REAGENT
A kind of preparation method of loquat cough syrup
ActiveCN108567943BQuality improvementGood curative effectDispersion deliveryHydroxy compound active ingredientsBiotechnologySucrose
The present invention relates to the field of medicine production, and specifically refers to a preparation method of loquat cough syrup, which specifically includes the following steps: (1) drying and pulverizing raw materials such as loquat leaves, poppy shells, basilica, baipei, morus alba, and platycodon grandiflorum, Mix, add water and refrigerate; (2) take out the product after refrigerating, add a surfactant, ultrasonically obtain the extract, filter, heat and concentrate the filtrate and store it at room temperature for later use; (3) take sucrose and add water, After heating and mixing evenly, add citric acid and sodium benzoate to obtain a sucrose mixed solution; (4) dissolve menthol and essence in ethanol to obtain an ethanol solution; (5) mix the extract, sucrose mixed solution and ethanol solution, Stir, stand still, filter, add water to the full amount, and mix well to obtain the target product. The invention achieves antibacterial and solubilizing effects by increasing the concentration of flavonoids and controlling the concentration of surfactants, and optimizes the quality and curative effect of the syrup; in addition, flavonoids are the original substances in the syrup, and rhamnolipids are also used in the food and drug industry , neither will have any adverse effect on the syrup.
Owner:GUIZHOU MAQIKA PHARMA
Data acquisition and recording method and system for purchase-sales-stock management system
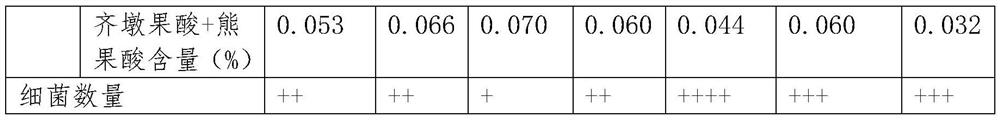
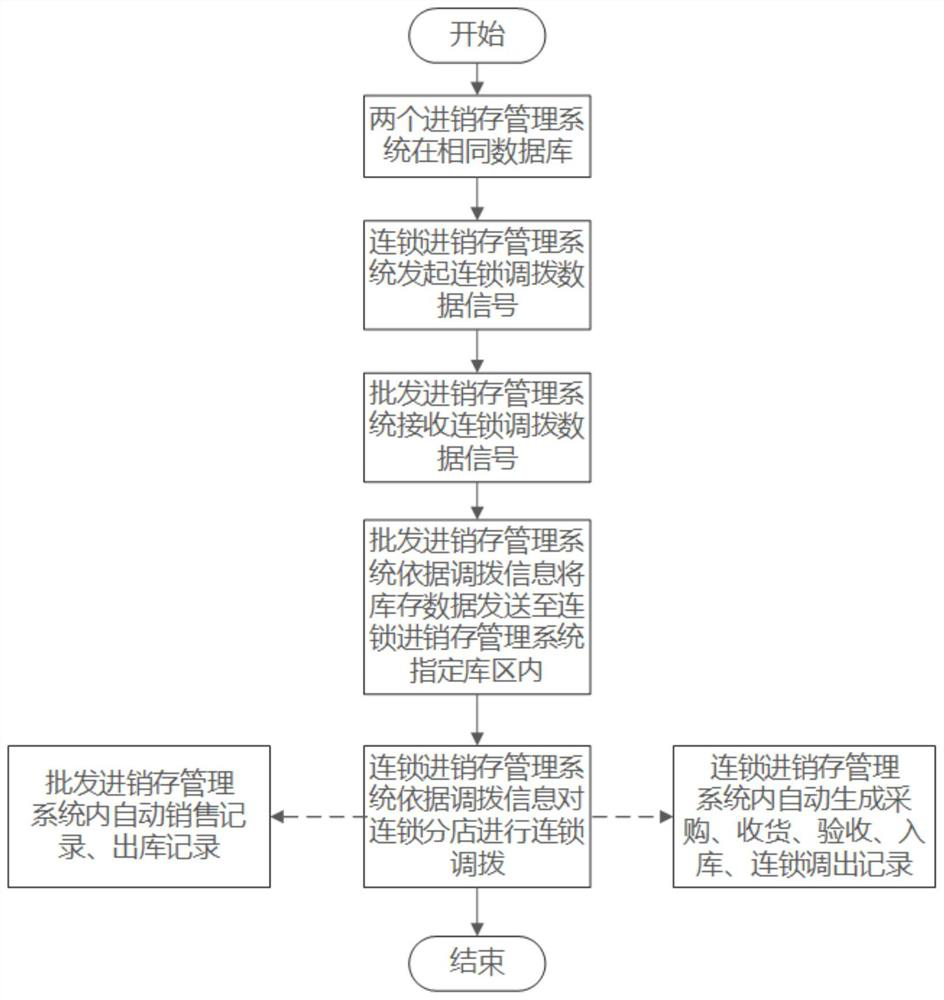
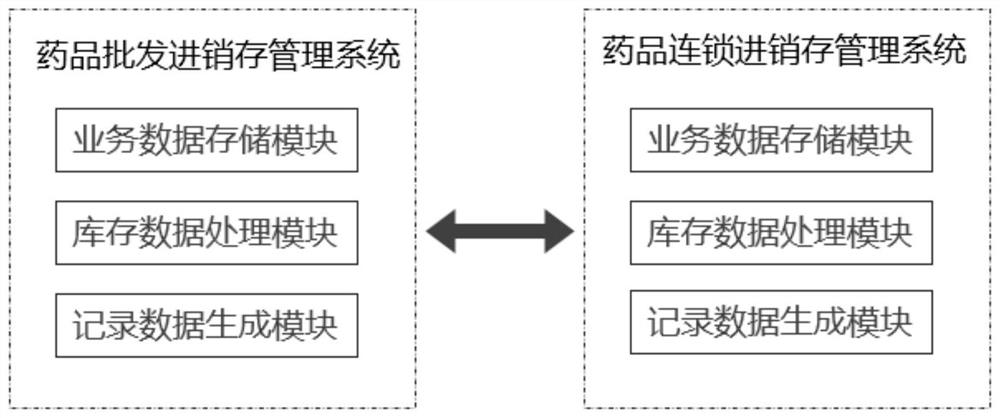
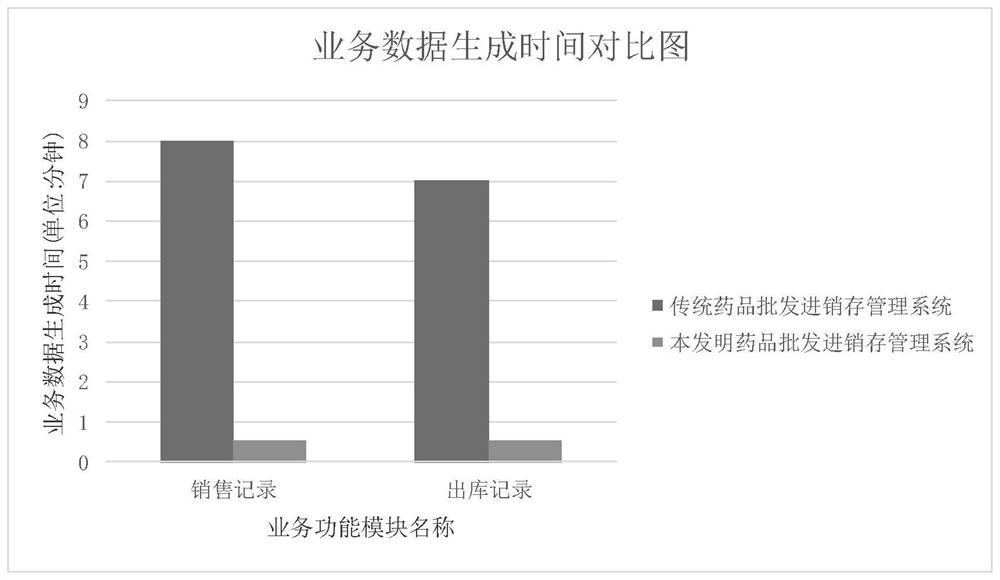
PendingCN112446667AImprove work efficiencySave time and costLogisticsPharmaceutical industryData acquisition
The invention belongs to the field of wholesale enterprise purchase-sales-stock management systems and chain purchase-sales-inventory management systems in the pharmaceutical industry, and discloses adata acquisition and recording method and system for a purchase-sales-stock management system, and the method comprises the steps: the pharmaceutical purchase-sale-stock management system and a pharmaceutical chain headquarters purchase-sales-stock management system are placed in the same database; the drug chain headquarters purchase-sales-stock management system allocates commodities to the chain store, initiates a chain allocation task and sends an allocation signal to the drug purchase-sales-stock management system; the drug wholesale purchase-sale-stock management system acquires an allocation data signal of the drug chain headquarters purchase-sale-stock management system; through continuous calculation of background data of the drug wholesale purchase-sales-stock management systemand the drug chain headquarters purchase-sales-stock management system, inventory sharing and cross-system chain allocation can be realized. And each system automatically generates a related quality record, and manual tedious operation software is not needed, so that the working efficiency of personnel is greatly improved, and the time cost is saved.
Owner:吉林省裕林信息科技有限公司
A kind of metal complexing method for separating and purifying sagebrush flavonoids
InactiveCN104739914BBreak through the technical bottleneck of industrial separation and purificationPromote development and utilizationCosmetic preparationsToilet preparationsEthylenediamineGrass mouse
Owner:FUJIAN AGRI & FORESTRY UNIV
Method for separating amygdalin from almond oil residue
ActiveCN102702282BGood colorGood lookingSugar derivativesSugar derivatives preparationActivated carbonEnvironmental engineering
The invention discloses a method for separating amygdalin from almond oil residue by one-step solvent extraction, which sequentially comprises the following steps that 0.4-0.6 time of activated carbon and 0.4-0.6 time of diatomite are added into the total almond oil residue, and are fully mixed uniformly with the almond oil residue; the raw material is put into an extraction device, is heated with 5 times of ethanol, extracted, filtered, crystallized, and then put into a clean container for standing still overnight; and the obtained material is centrifugally dripped and eluted with the ethanol, a product is directly put into a blast drying oven to be dried for 24 hours, and then the amygdalin is obtained. The product has good color and appearance and high quality and purity, the operation is simple and rapid, the investment of equipment is greatly reduced, the yield of the product is more than 78%, the purity of the product is more than 99.0% and maximumly achieves 99.9%, the recovery rate of various solvents can reach more than 92%, and the BOD (Biochemical Oxygen Demand) of waste water is lower than 100, which is suitable for industrial production; and the method is widely used in the industrials of health care products and medicines.
Owner:西安天丰生物科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com