Patents
Literature
62 results about "Prothrombase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In the clotting of blood, also known as: Stuart factor, Stuart-Prower factor, prothrombase, and prothrombinase. Its active form, factor Xa (EC 3.4.21.6), is formed from factor X by limited proteolysis and assists in the conversion of prothrombin to thrombin.
A starch composite hemostatic dressing of mesoporous silica microspheres
InactiveCN106806931AHas a hemostatic effectThe hemostatic effect is achievedAbsorbent padsBandagesWound dressingMicrosphere
The invention relates to a starch composite hemostatic dressing of mesoporous silica microspheres, which adopts a sol-gel method and uses a three-block copolymer pluronic as a template agent to synthesize mesoporous silica microspheres with a pore diameter of about 5 nm. It is compounded with starch to prepare a mesoporous silicon oxide microsphere / starch composite hemostatic dressing, and the hemostatic performance of the composite material is observed through animal experiments. The mesoporous silica microsphere / starch composite material not only has strong water absorption performance, but also has obvious in vitro coagulation performance, which can significantly shorten the partial thromboplastin time and prothrombin time. The mesoporous silica microsphere / starch composite material can prevent the bleeding of the rabbit's back skin and liver injury and shorten the bleeding time, and has obvious hemostatic effect.
Owner:TIANJIN YIYAO SCI & TECH
Preparation process of human prothrombin compound
ActiveCN101974070AImprove securitySimplify production stepsPeptide preparation methodsPharmacyVirus inactivation
The invention relates to a preparation process of a human prothrombin compound, belonging to the field of biological pharmacy. The preparation process comprises the following steps of: absorbing blood plasma, washing, eluting and clarifying by filtration; inactivating viruses by using the S / D (Solvent / Detergent) method; purifying by absorption; subpackaging; freeze-drying; and inactivating viruses by the dry heat method. The human prothrombin compound is directly absorbed from blood plasma by using gel, only the gel chromatography technology is used in the entire extraction process, so that the production steps are simplified, the pollution of various factors on the production process of the product is reduced, meanwhile, the yield of the product is increased by 25-30%. In addition, the S / D method is used for removing lipid-enveloped viruses and the dry heat method is used for removing non lipid-enveloped viruses in the production process, and the safety of clinic medication is obviously increased through the two virus inactivation steps.
Owner:华润博雅生物制药集团股份有限公司
Method and apparatus for determining anticoagulant therapy factors
InactiveUS20110224292A1BiocideAnalysis by subjecting material to chemical reactionAnti coagulationCoagulation reagent
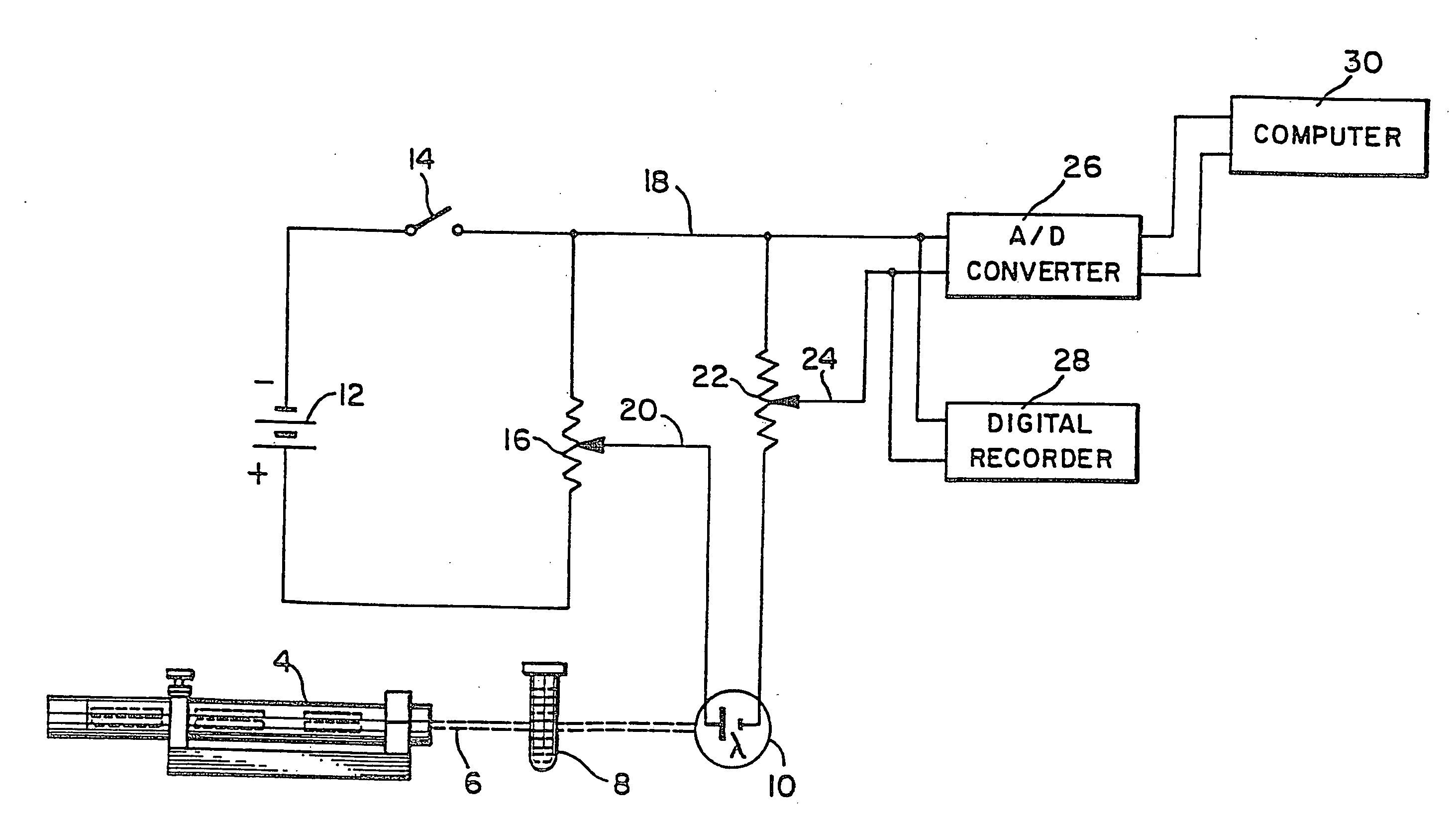
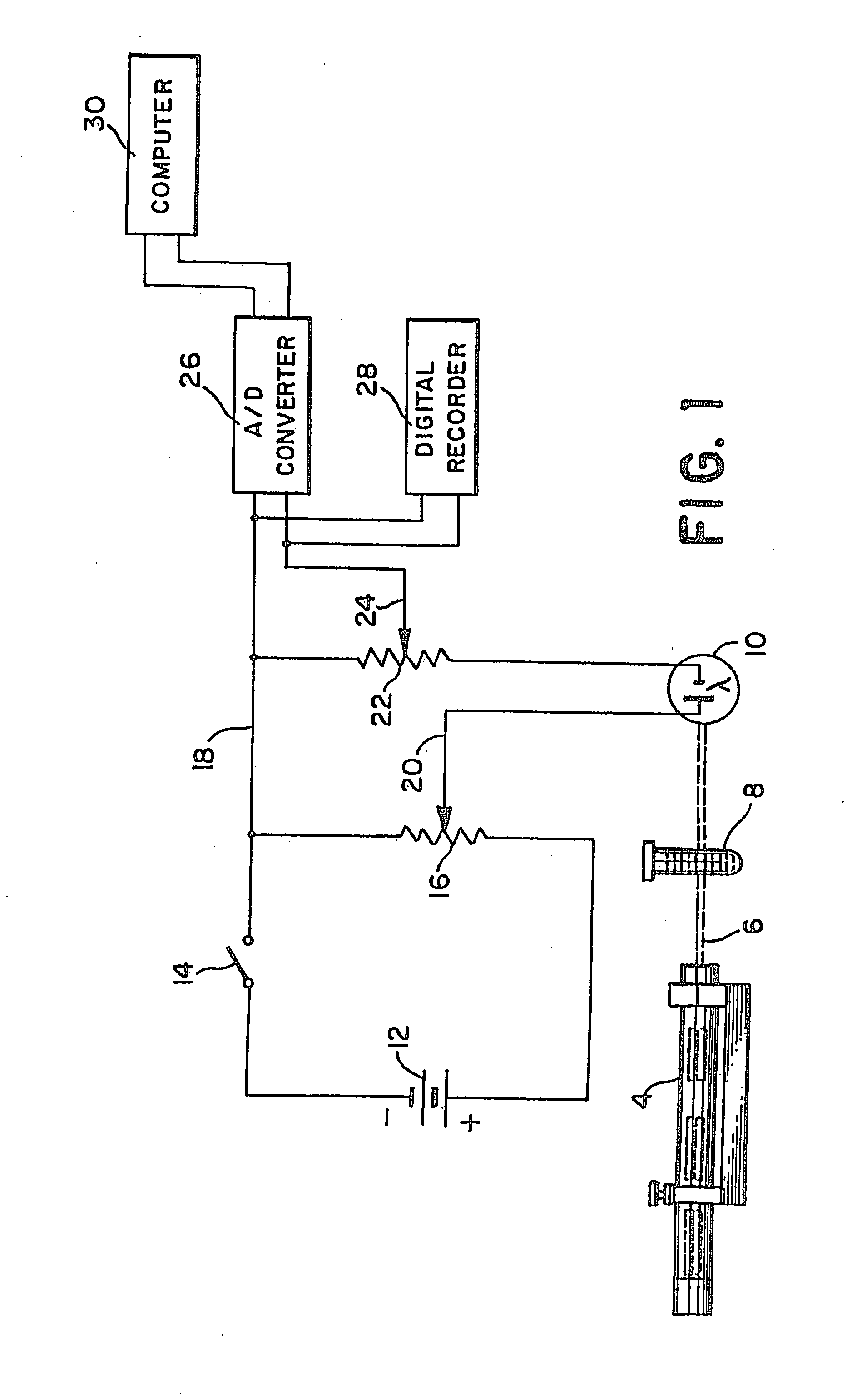
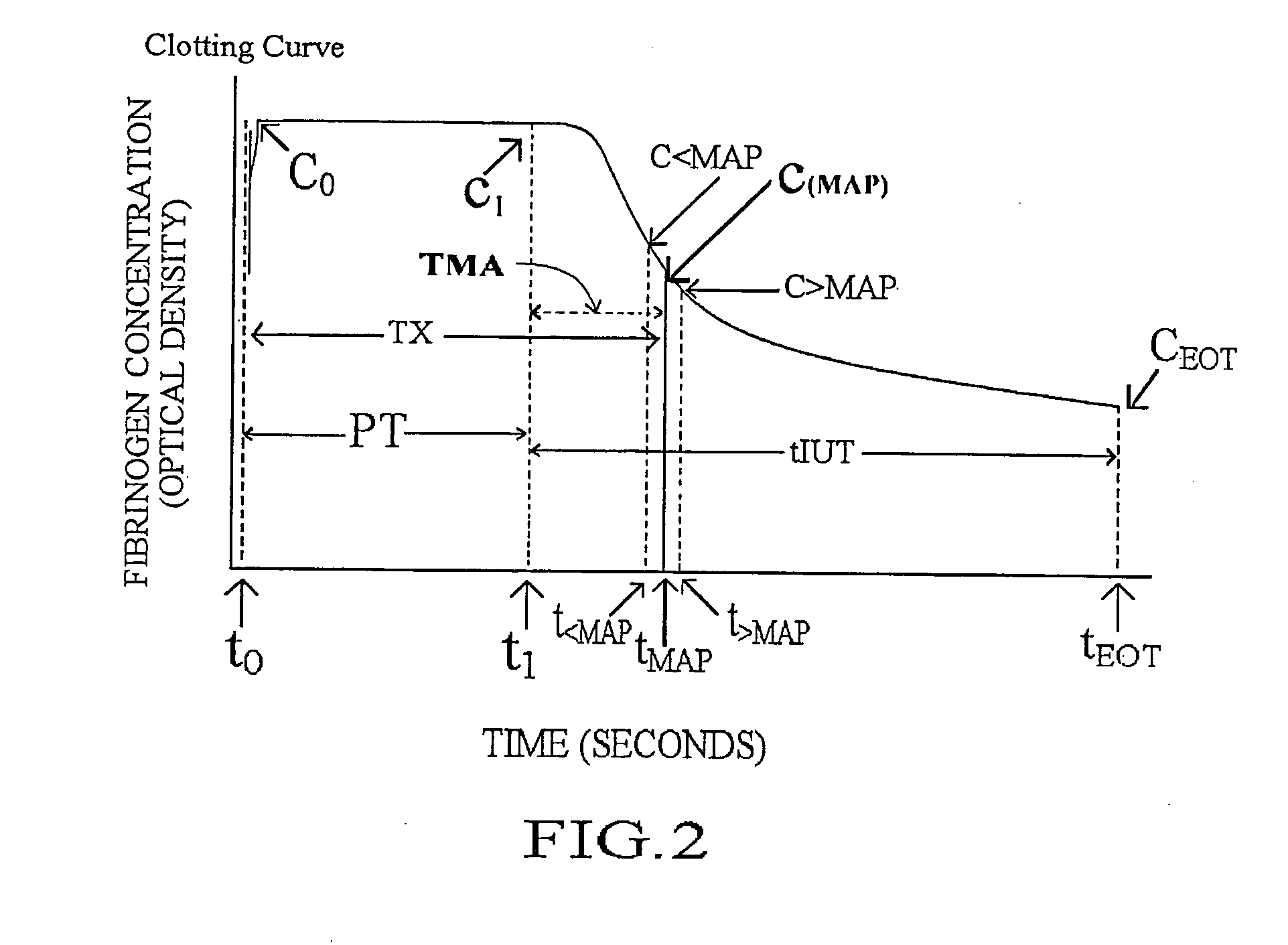
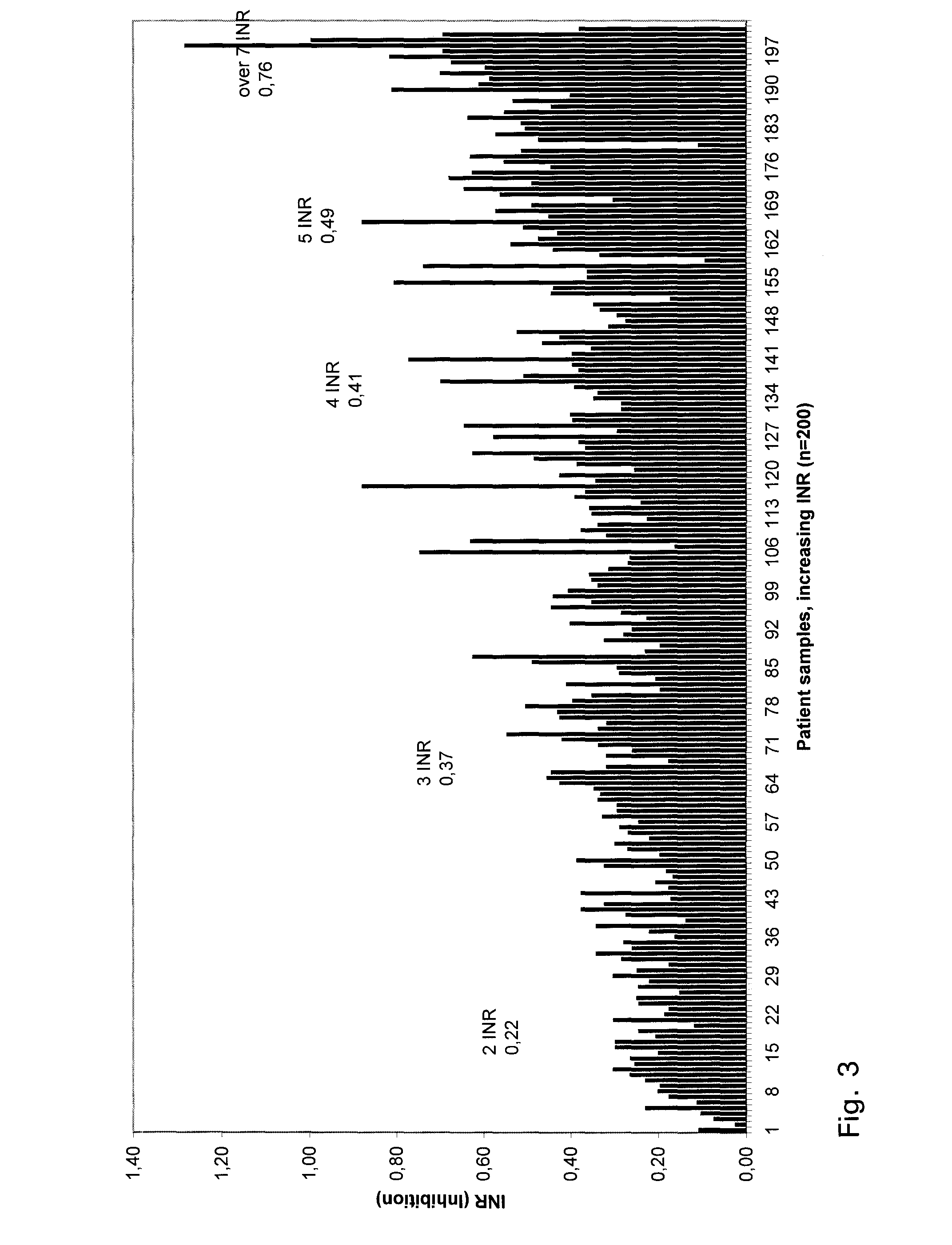
Methods and apparatus are disclosed for determining new anticoagulant therapy factors for monitoring oral anticoagulant therapy to help prevent excessive bleeding or deleterious blood clots that might otherwise occur before, during or after surgery. The inventive methods and apparatus provide an International Normalization Ratio (INR) based on a coagulation reaction with a blood sample of a living being. Embodiments include methods and apparatus for determining an anticoagulant therapy factor without requiring use of a mean normal prothrombin time determination or an ISI, and may be carried out with the patient sample and a coagulation reagent, where the coagulation reagent may be selected from a number of coagulation reagents. One embodiment provides an INRs value which is determined from a prothrombin time (PT or T1) of a patient blood sample and a theoretical end of test time (TEOT), where a theoretical clotting area is used to determine the INRs value according to the expression, INRs=T1*TEOT*MUL, where MUL is a multiplier that takes into account pixel parity and sampling times. The INRs may be used to determine a course of treatment for a patient or other living being without regard to the specific coagulation regent used to generate the coagulation data (e.g., time and optical activity values).
Owner:WADA
Process for preparing thrombase
The present invention provides process of extracting thrombase from animal blood or human blood. The process includes adsorbing thrombin from plasma with chromatographic medium, eluting the chromatographic column, activating thrombin to obtain thrombase solution, ultrafiltering to concentrate and finally freeze drying to obtain the thrombase product. The process is one normal temperature process, environment friendly, short in production period, high in thrombase extracting rate of 50000 IU / L, simple in operation, and easy in industrial realization.
Owner:黄耀江
Method for detecting whole-blood sample coagulation item using magnetic-bead method
InactiveCN1936580AEasy to masterAccurate reflectionBiological testingMagnetic beadBlood coagulations
One kind blood samples detection method using magnetic beads, completed with the following steps: (1) Get fresh clinical samples; (2)Put these specimen on coagulation analyzer, use magnetic beads and special adjustment reagents aiming at the whole blood specimens, measure four coagulations: Activated partial thromboplastin time (APTT), Prothrombin time (PT), Prothrombin time (TT), Fibrinogen (FIB). The advantages of this invention are: 1.The method is simple, time-saving and easy to technical staff. 2. Reduce costs and save centrifuges and other equipment, reduce errors; 3. It is efficient and suitable for the battlefield, natural disasters and remote mountainous areas, medical teams. Particularly, it's suitable for hemorrhagic diseases and natural disasters (such as hemophilia, etc.). 4. Save specimen volume. 5. Reflect the level of specimens of blood coagulation preciously and accurately.
Owner:MEIDE TAIPINGYANG PRECISION INSTR MFG
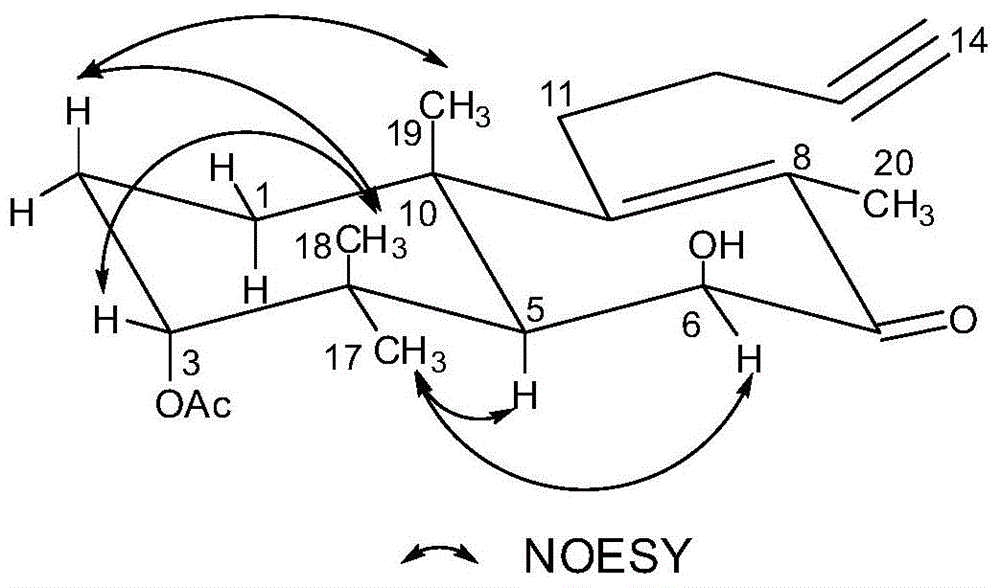
Terpenoid and preparation method and application thereof
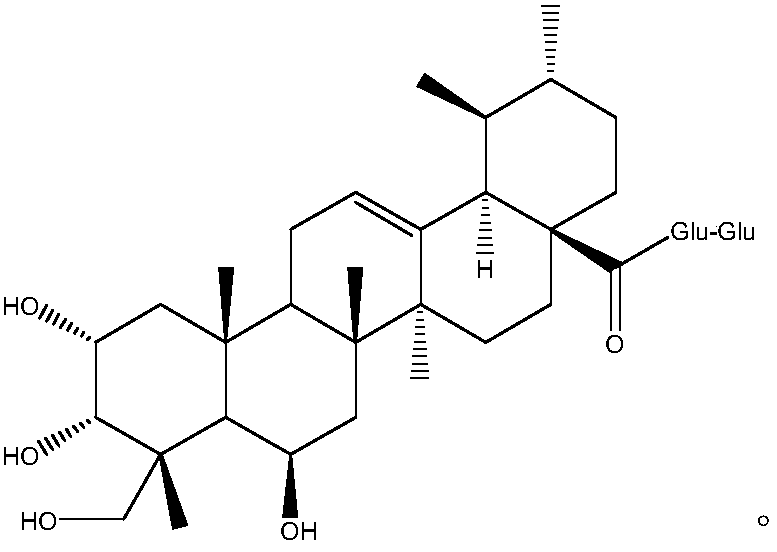
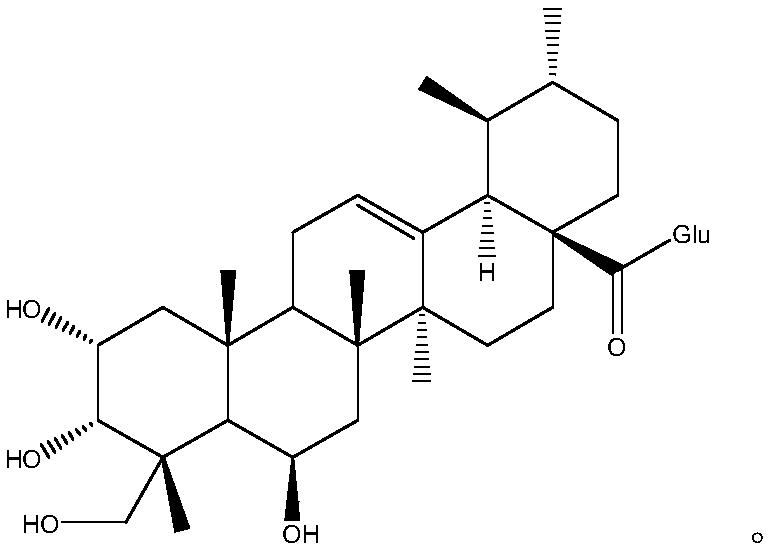
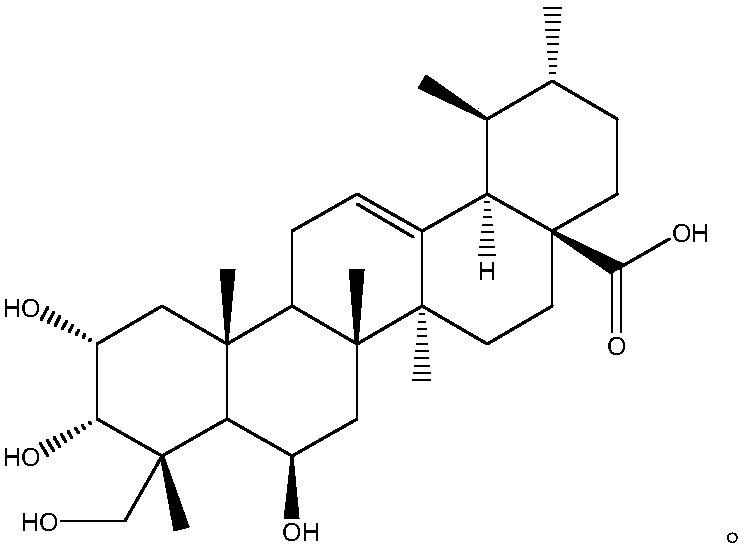
ActiveCN103553919AAvoid smallImprove coagulation functionCarboxylic acid esters separation/purificationEster active ingredientsCoagulation functionEnzyme
The invention provides a terpenoid shown by the formula I or pharmaceutically acceptable salt, ester or hydrate thereof. The invention also provides a preparation and application of the terpenoid. In the invention, a carbon-drop diterpene new compound separated from leonurus is carbon-drop labdane diterpene obtained from the nature for the first time. The compound can obviously shorten the PT (prothrombin time), APTT (activated partial thromboplastin time) and TT (thrombin time) in vitro and obviously increase the amount of FIB (fibrinogen) in blood, and realizes a certain effect on enhancing the coagulation function, thereby providing a new option for developing a novel natural coagulation drug.
Owner:CHENGDU FIRST PHARMACEDTICAL CO LTD
Separating and purifying method for thrombase
The thrombase separating and purifying process includes: adding sodium citrate solution as anticoagulant into animal blood as material; centrifugally separating to obtain plasma; freezing, defreezing and re-centrifuging to obtain supernatant; adding BaCl2 into supernatant, centrifuging and collecting precipitate; adding EDTA solution into precipitate to desorb, and centrifuging to eliminate precipitate and obtain coarse thrombin solution; adding CaCl2 solution to the coarse thrombin solution to make the coarse thrombin solution reach a concentration of 0.1 mol / L, chromatographic separation in DEAE-cellulose anionic exchange column to obtain pure thrombase product; and freeze drying to obtain the thrombase product. The present invention has high thrombase yield of 0.4 % and high thrombase purity, specific activity of 1500 U / mg protein.
Owner:SHAANXI UNIV OF SCI & TECH
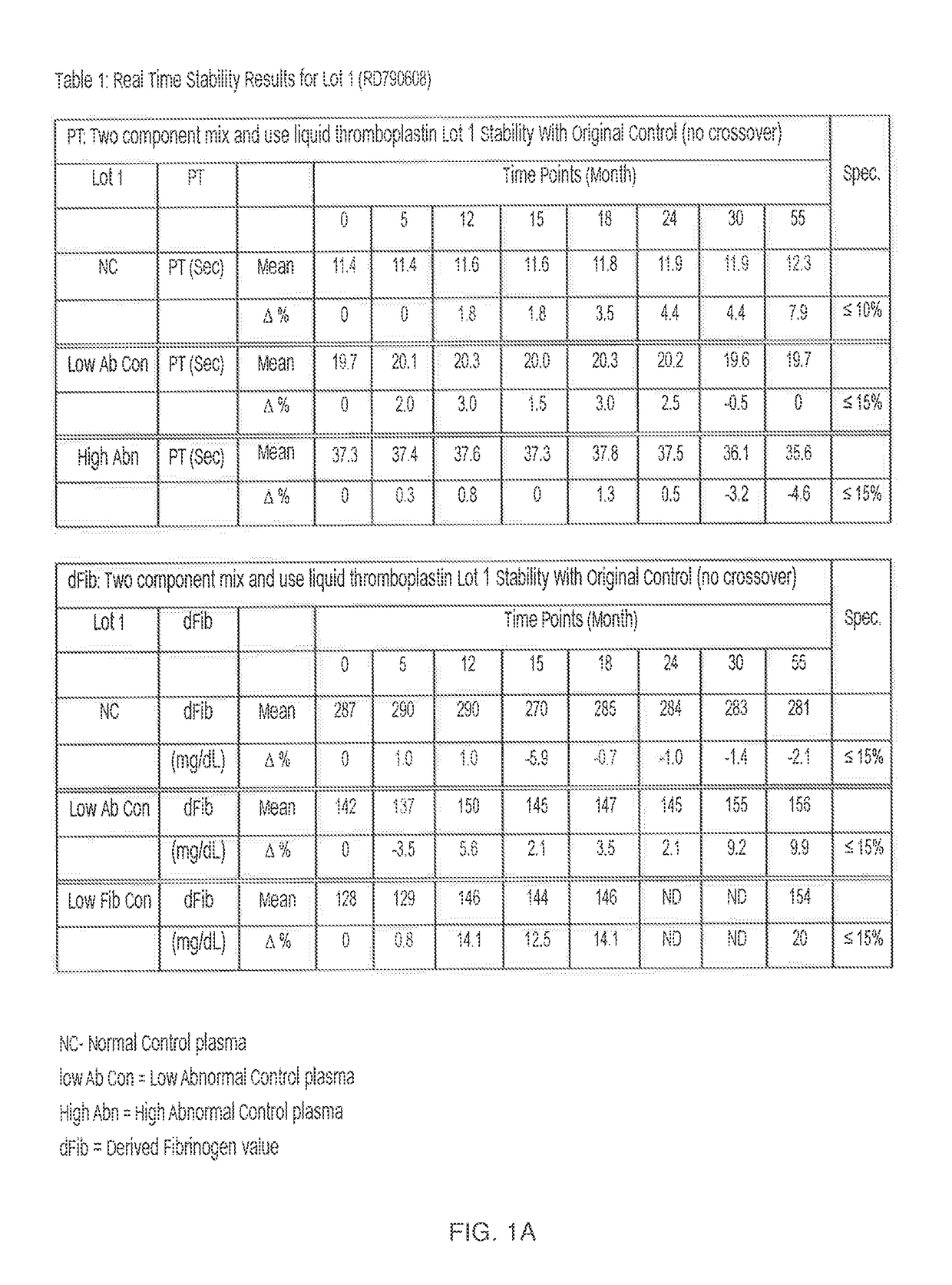
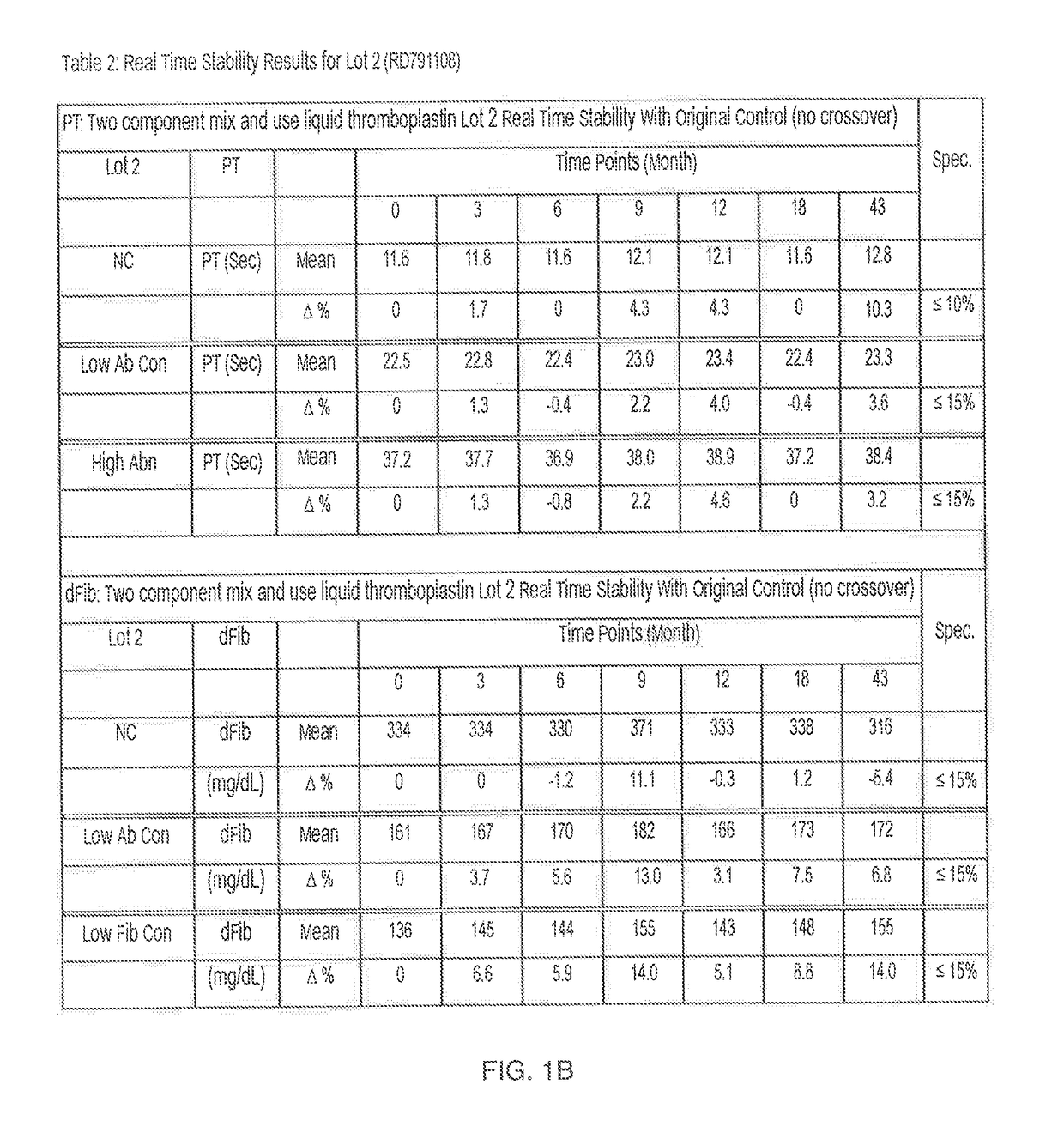
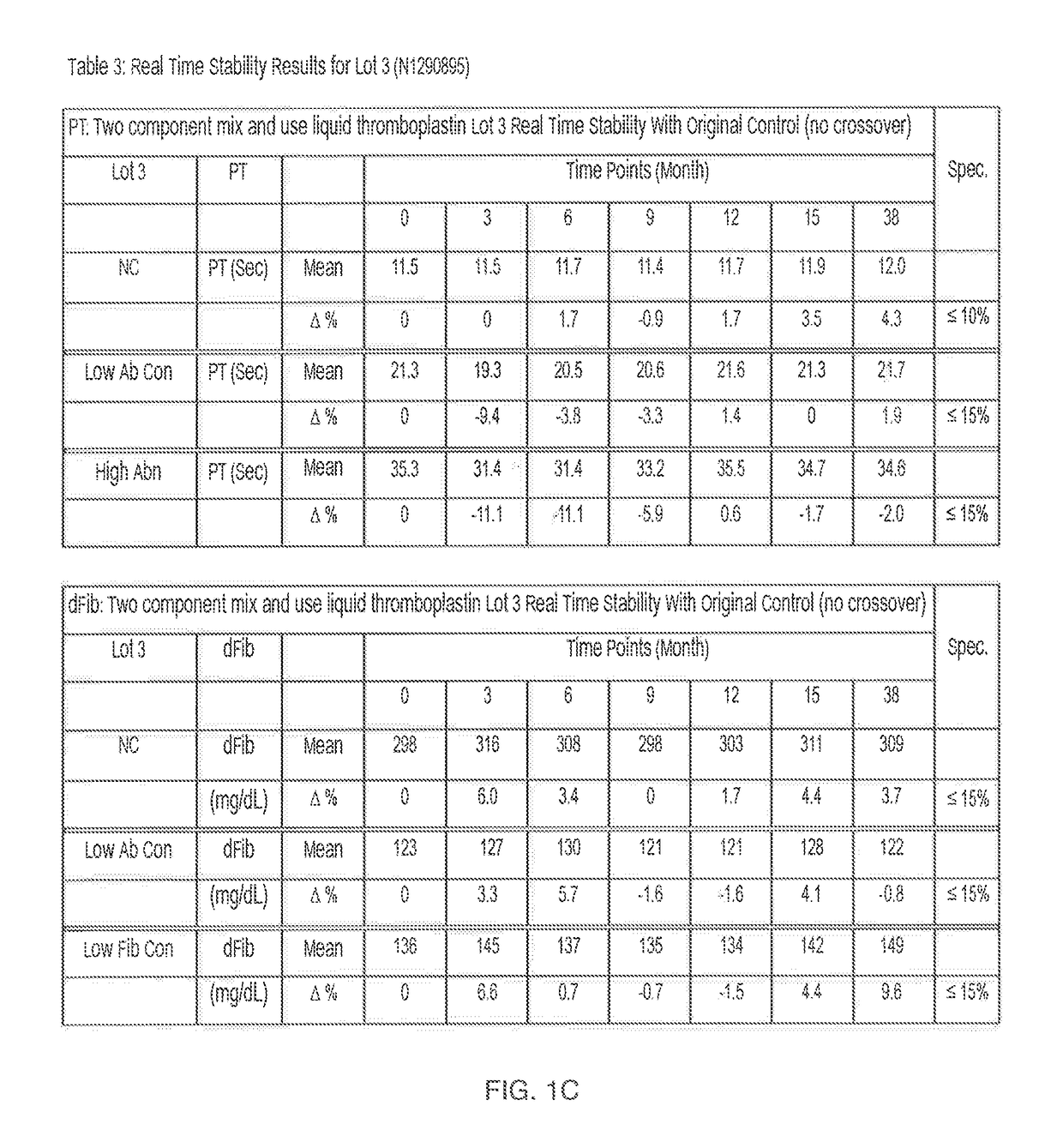
Two Component "Mix and Use" Liquid Thromboplastin Reagent, Methods of Making, and Methods of Use Thereof
ActiveUS20170234895A1Extended shelf lifeBiological material analysisBiological testingProthrombin time assayReagent
What is described is a kit for preparing a liquid thromboplastin reagent for a prothrombin time assay. The kit simplifies and minimizes reagent preparation time and is stable for 2-5 years.
Owner:INSTR LAB
Electrochemical detection chip and electrochemical sensor, as well as preparation methods and application thereof
PendingCN109507260AEasy to makeHigh detection sensitivityMaterial analysis by electric/magnetic meansReaction layerElectricity
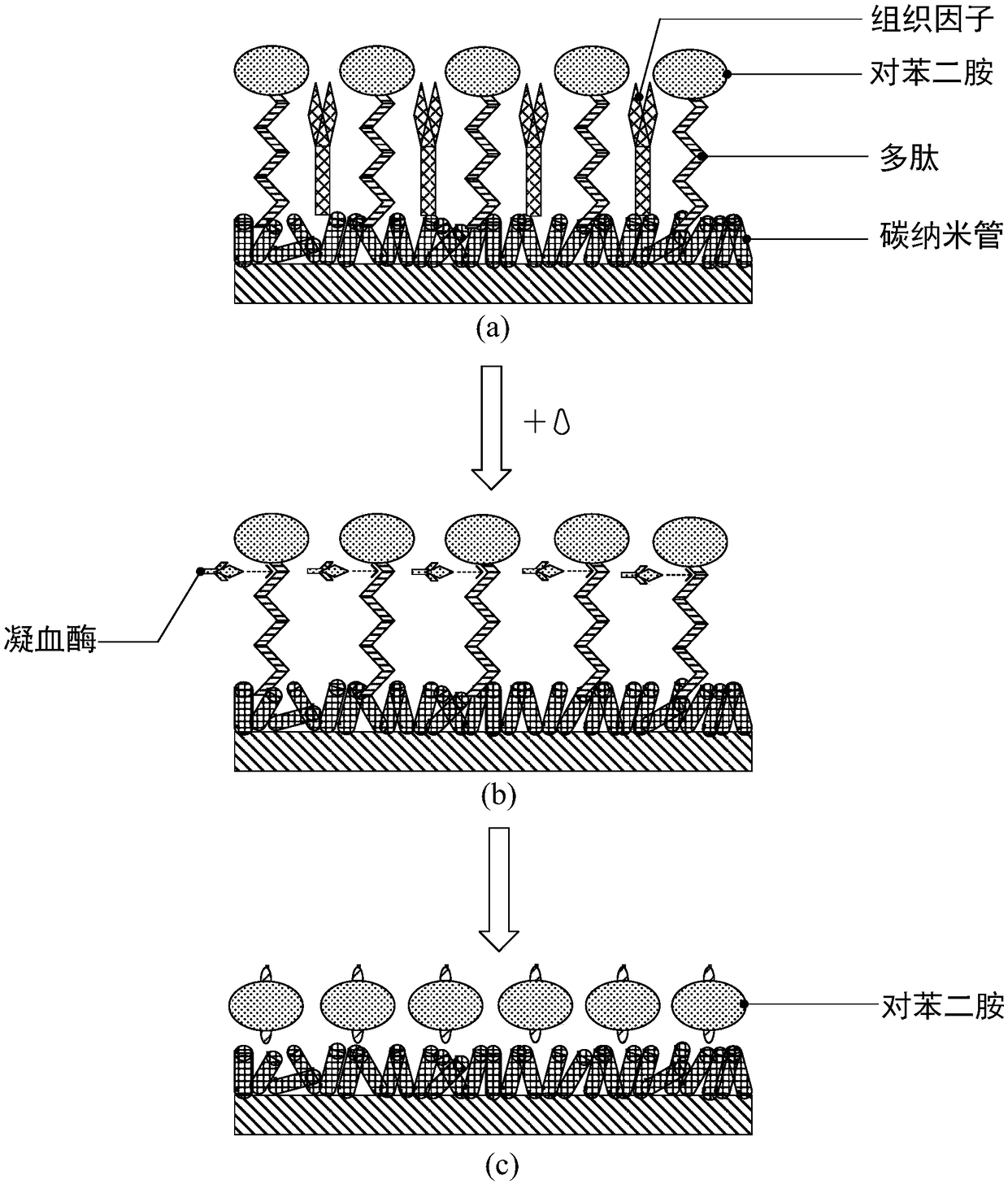
The invention discloses an electrochemical detection chip. The electrochemical detection chip comprises an electrode layer, wherein a working electrode of the electrode layer comprises a conducting layer, a nanomaterial layer and a blood clotting reaction layer which are sequentially laminated, wherein the blood clotting reaction layer is used for producing an electrical signal for detecting prothrombin time; the nanomaterial layer is used for transferring and amplifying the electrical signal. By using high conductivity, large specific area, good biocompatibility and the like of the nanomaterial layer, amplification and enhancement of the electrical signal in the detection process of the prothrombin time are realized so as to improve the sensitivity of chip detection and shorten the detection time. The invention discloses an electrochemical sensor. The electrochemical sensor comprises the electrochemical detection chip. The invention discloses a preparation method of the electrochemical sensor. The preparation method is suitable for preparing the electrochemical sensor with high sensitivity and accurate detection results.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
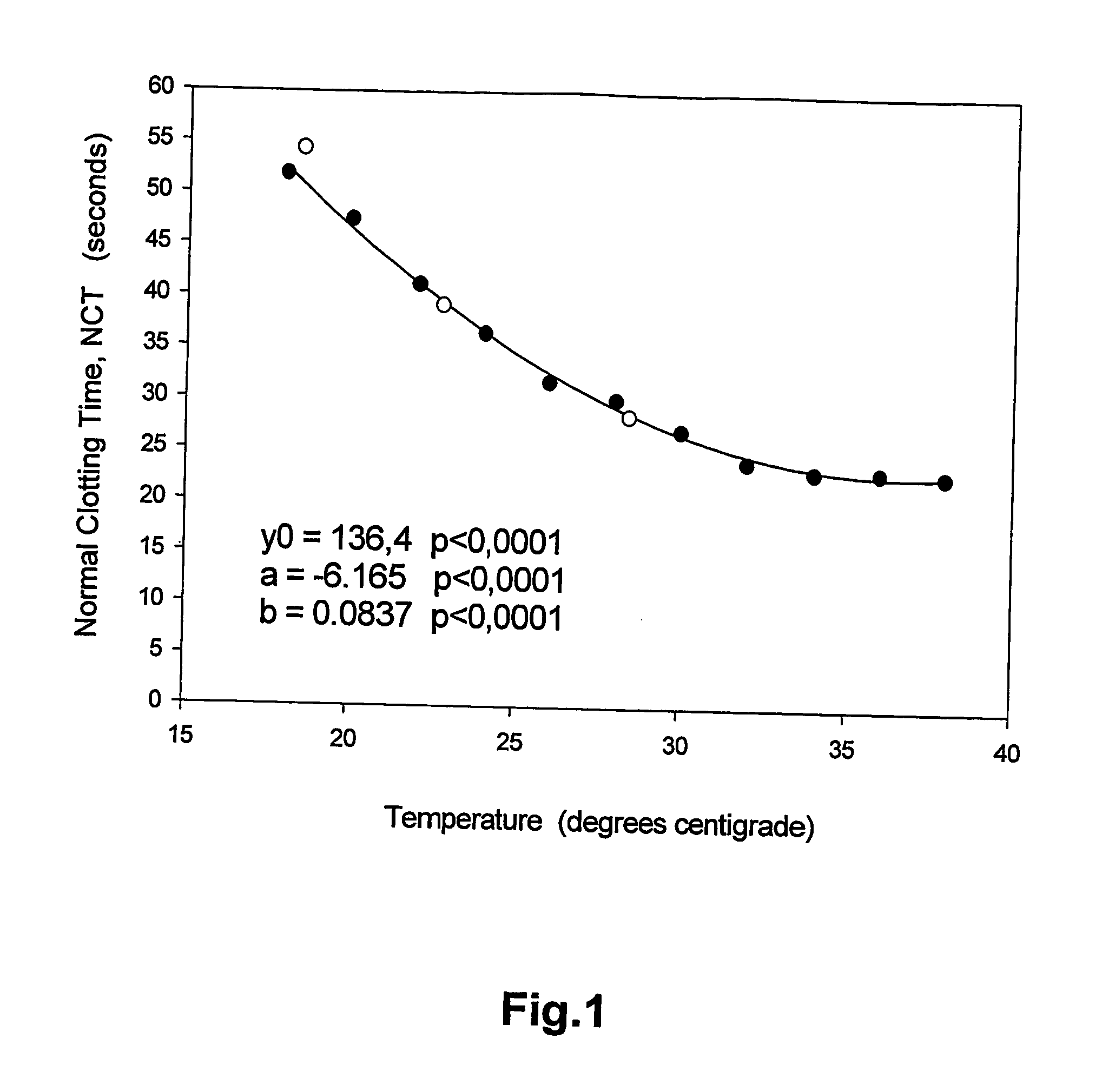
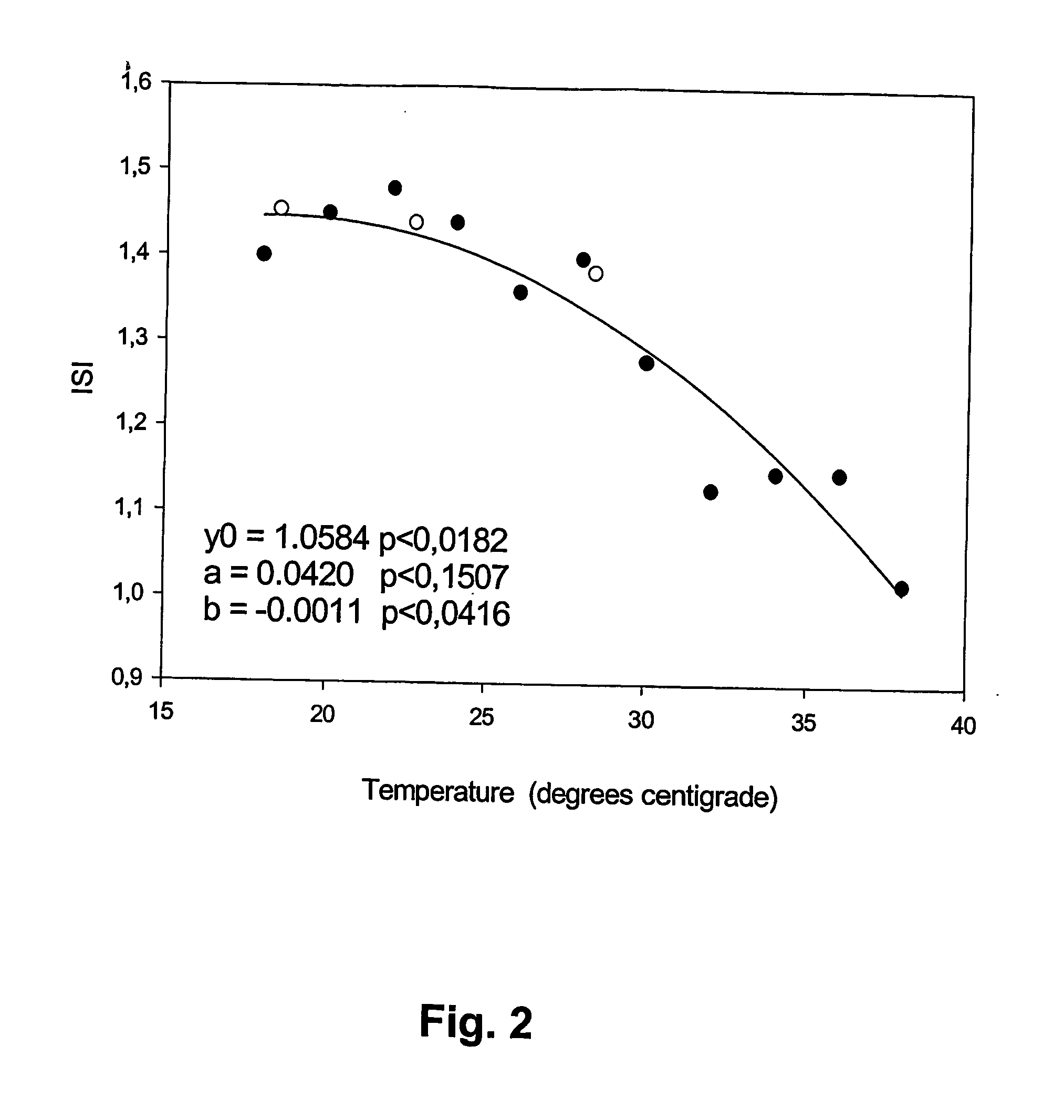
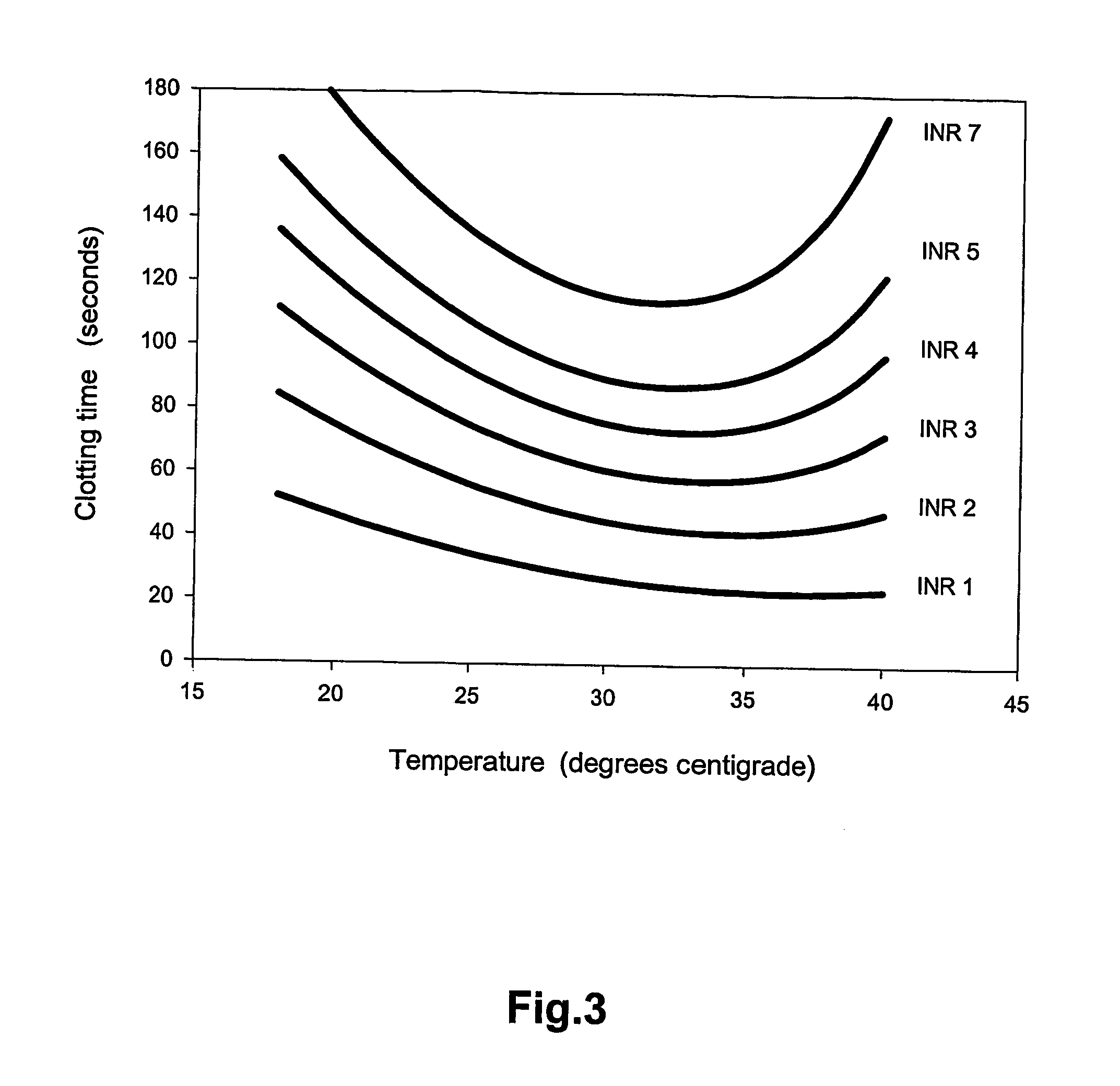
Coagulation tests at ambient temperature
InactiveUS20060281140A1Enabling detectionAbolish needMicrobiological testing/measurementBiological testingBlood plasmaPathology
A method of determining prothrombin time (PT) in a whole blood, anti-coagulated blood, blood plasma or anti-coagulated blood plasma sample at an ambient temperature in the range of 15° C. to 45° C. is described. The method is performed with liquid reagents and the PT, preferably expressed as International Normalized Ratio (INR), is calculated based on said temperature and the clotting time (CT). A test kit is also described for analysis of PT which comprises temperature recoding means, and one or several separate sealed vessels containing reagents, optionally in lyophilized form for reconstitution prior to use, for clotting one or more defined volumes of whole blood, anti-coagulated blood, blood plasma or anti-coagulated blood plasma sample, and optionally time registration means and volume determining means.
Owner:ZAFENA
Preparation method for prothrombin time determination reagent
The invention relates to a preparation method for prothrombin time determination reagent (PT reagent), comprising the following steps. Cow or sheep tissue factor with the purity of more than 90% is prepared by the genetic engineering method, phospholipid is dispersed in phosphate buffer solution containing surfactant, and then the tissue factor and the phospholipid are mixed and sufficiently combined, and finally, C18 resin is adopted to adsorb and separate the surfactant, and the PT reagent product is obtained after freeze-drying. The preparation method is stable in technique and strong in operability, and further, the obtained PT reagent has better stability, sensitiveness and fewer impurities, and the quality can be easily ensured.
Owner:苏州良辰生物医药科技有限公司
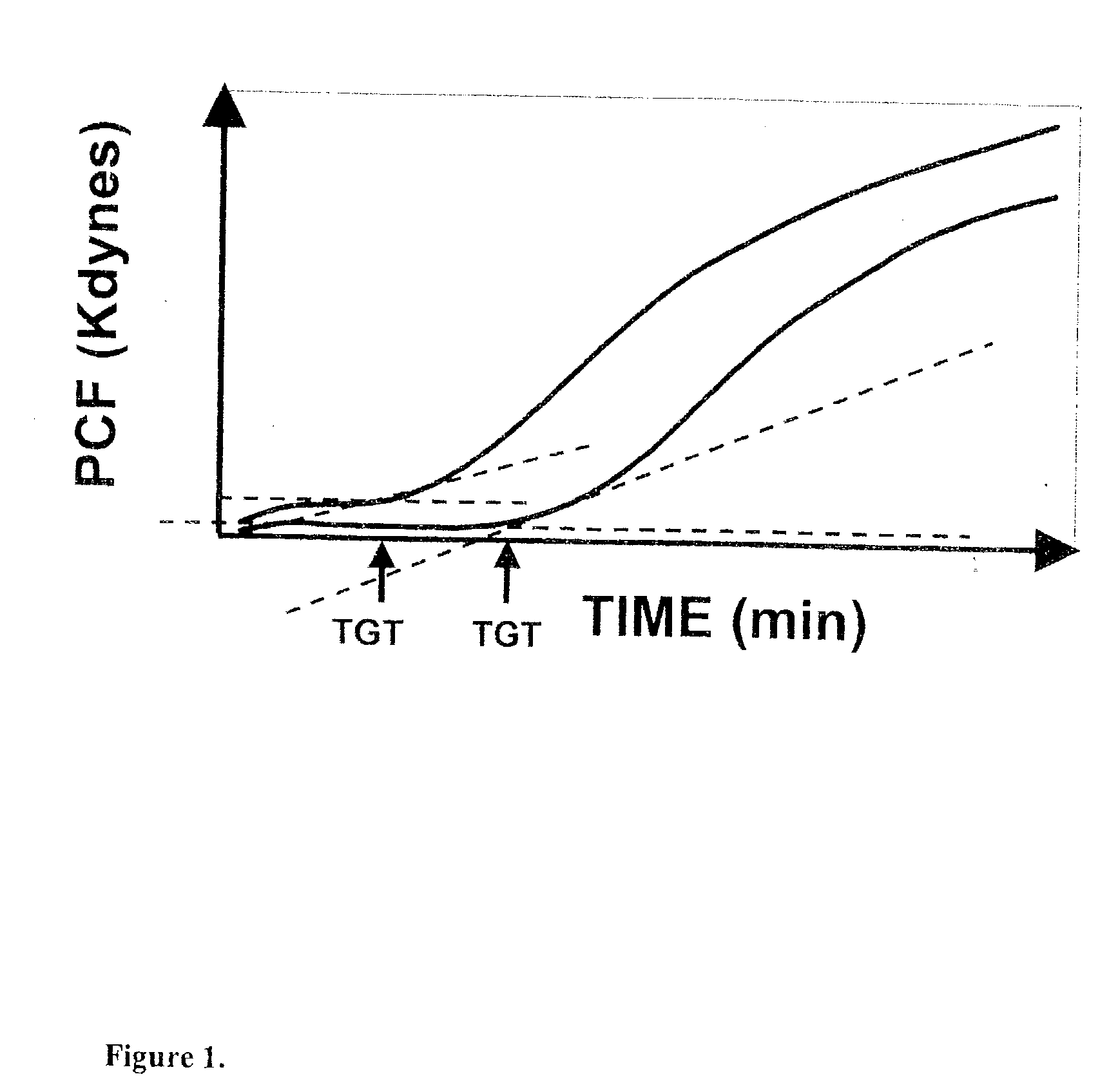
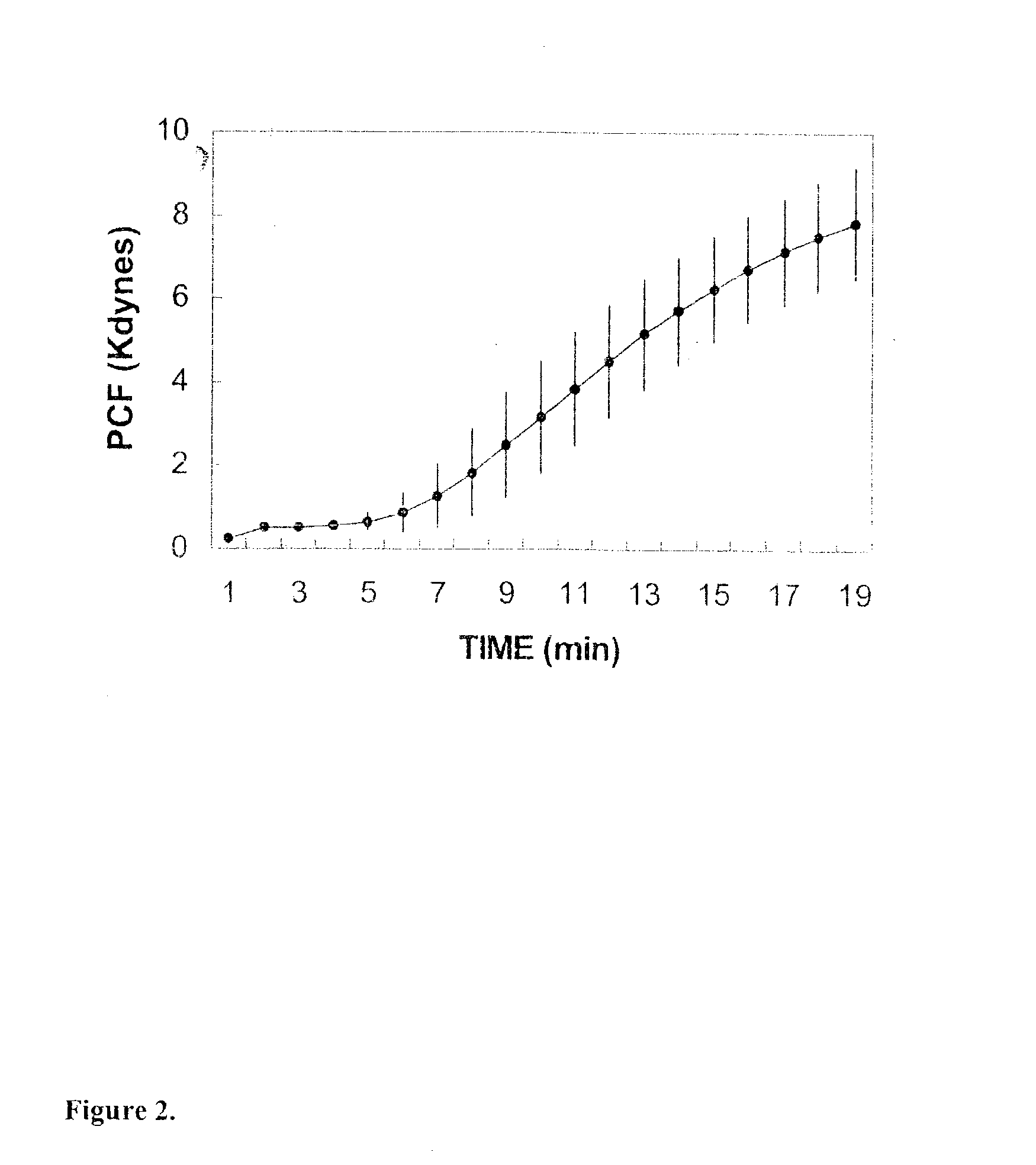
Onset of force development as a marker of thrombin generation
InactiveUS20030199428A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseCoronary artery disease
Platelet contractile force (PCF) is used as a surrogate marker of thrombin generation. PCF generation occurs concomitant with the burst of prothrombin fragment F 1+2 release. The time between assay start and PCF onset is identified as the thrombin generation time (TGT), and is used in assessing risk of bleeding, in diagnosing various disorders, and in monitoring the effects of pharmaceutical and other treatments. TGT is prolonged in clotting factor deficiencies and in the presence of direct and indirect thrombin inhibitors. TGT shortens to normal with clotting factor replacement and shortens with administration of rVIIa. TGT is short in thrombophilic states such as coronary artery disease, diabetes and thromboangiitis obliterans and prolongs toward normal with oral and intravenous anticoagulants.
Owner:HEMODYNE
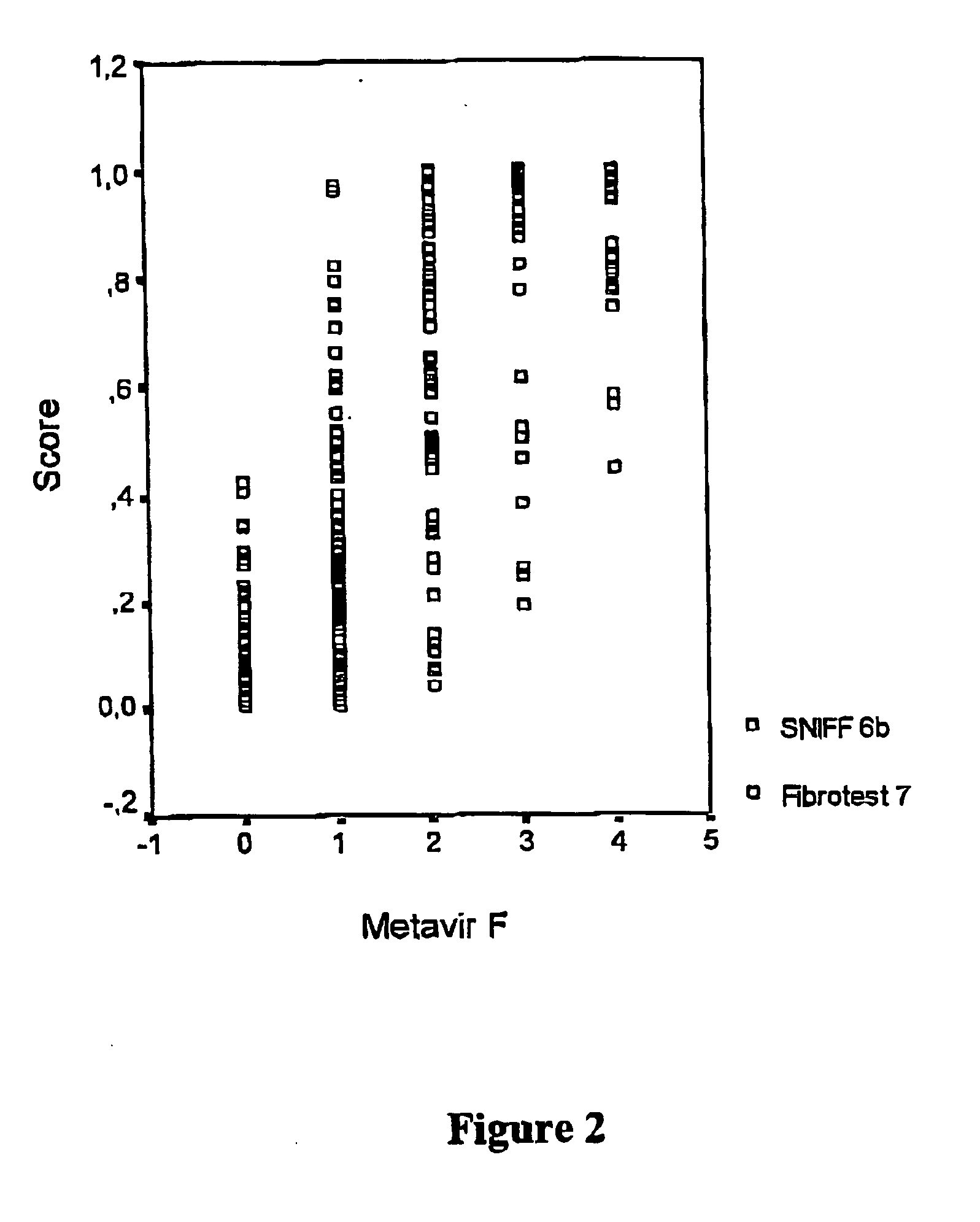
Method for diagnosing liver fibrosis
InactiveUS20070178443A1Improve reliabilityMicrobiological testing/measurementDisease diagnosisDiseaseTissue inhibitor of metalloproteinase
The invention concerns a method for the detection of the presence and / or the severity of a liver disease in a patient comprising measuring in an isolated sample TIMP-1 (Tissue Inhibitor of Metalloproteinase I), A2M (a-2-macroglobulin), PLT (number of blood plateletes, PI (prothrombin index), optionally at least one additional parameter selected from the group consisting of urea and GGT (γ-glutamyltranspeptidase) and optionally measuring at least one additional biochemical or clinical parameter and diagnosing the presence and / or severity of a liver disease based on the presence or measured levels of these parameters. The method can be used for monitoring therapeutic treatment of liver fibrosis and staging of liver fibrosis.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
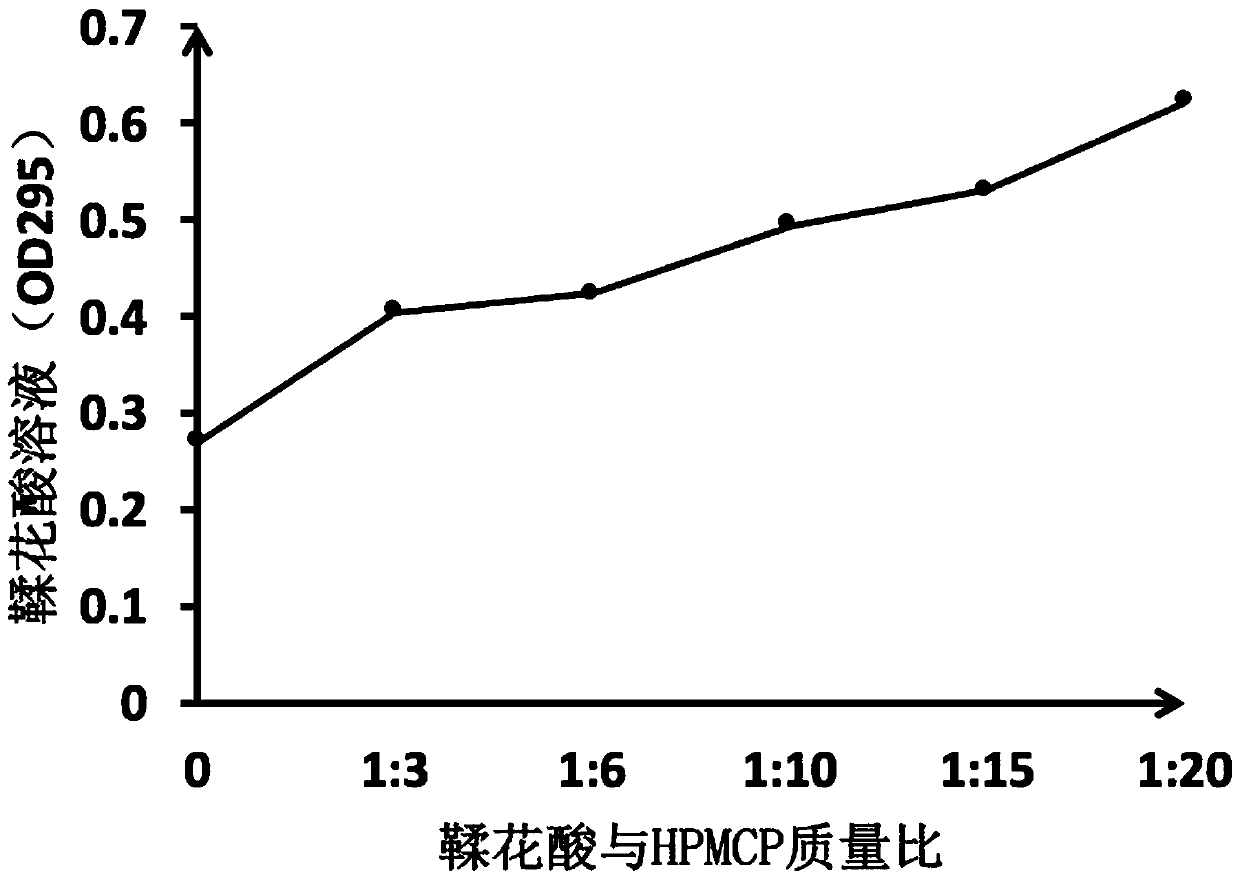
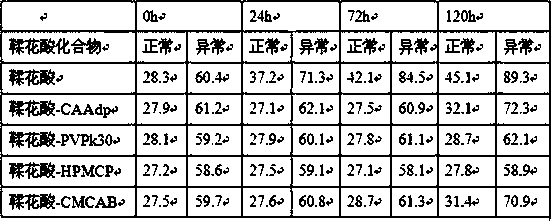
Novel ellagic acid compound and method for preparing activated partial thromboplastin time measuring reagent through ellagic acid compound
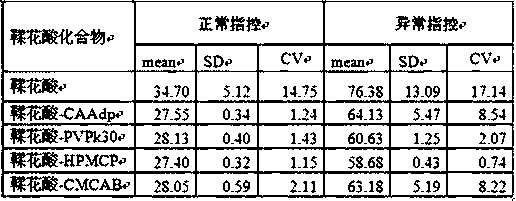
The invention belongs to the technical field of blood coagulation reagent preparation, and provides a novel ellagic acid compound aiming at the characteristic that ellagic acid is difficult to dissolve in water. The novel ellagic acid compound is prepared from the ellagic acid and a solid dispersant which are mixed according to a certain proportion. The solubility of the ellagic acid is improved,the problem that the ellagic acid is prone to being precipitated is solved, and the stability of the ellagic acid in products is guaranteed. The invention also provides a method for preparing an activated partial thromboplastin time measuring reagent through the ellagic acid compound. The reagent is prepared from liposomes, the ellagic acid compound, metal ions, a stabilizer and a preservative according to a certain proportion. According to the prepared reagent, through the ellagic acid solid compound, the problem that the ellagic acid is prone to being precipitated is solved, components of the liposomes are optimized, the inter assay variation between the activated partial prothrombin reagent products is lowered, the stability of the products is good, and the quality of the activated partial prothrombin reagent is guaranteed.
Owner:太原博奥特生物技术有限公司
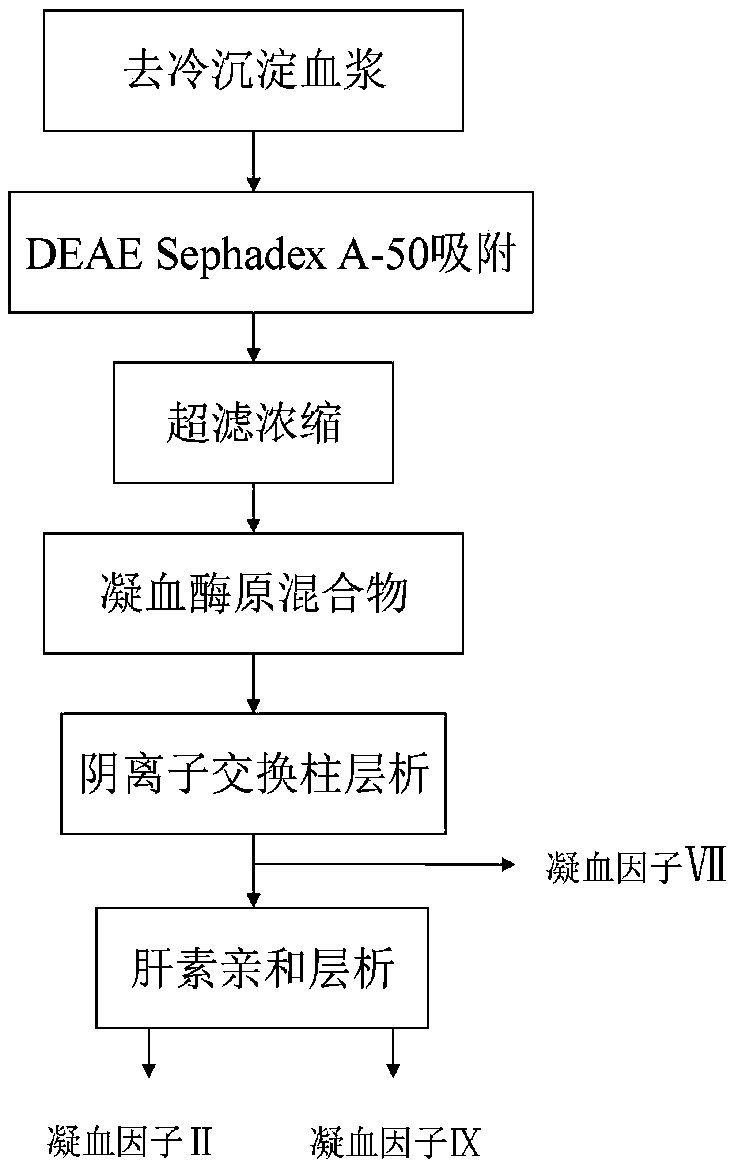
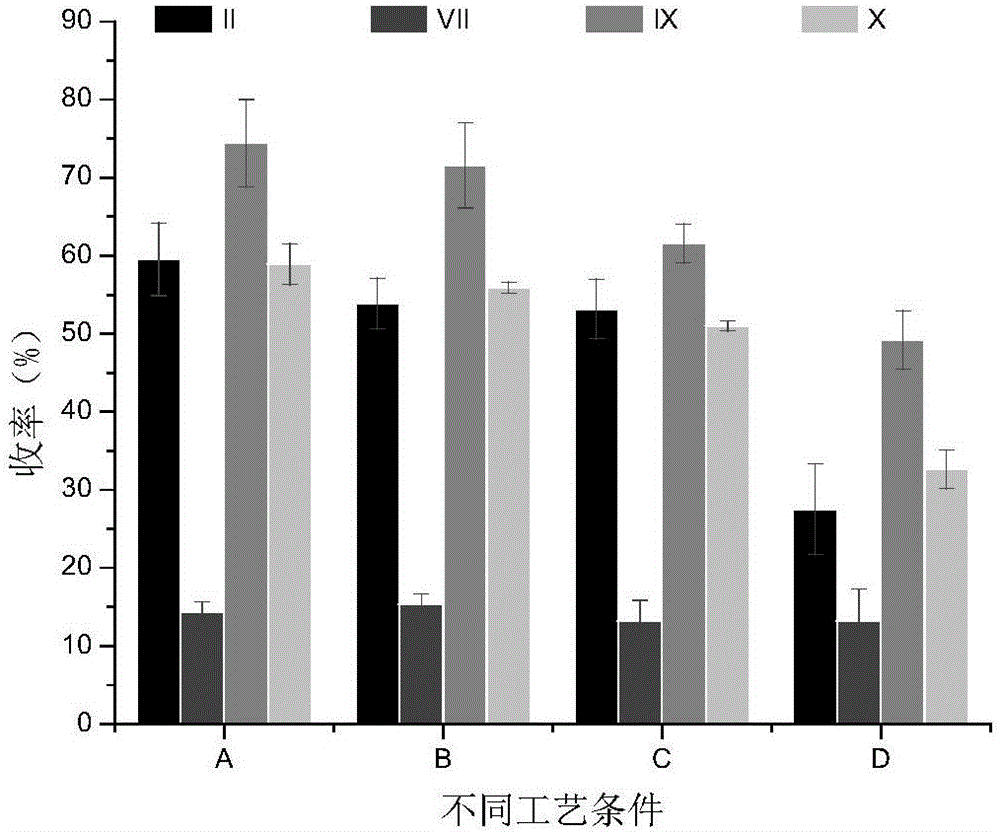
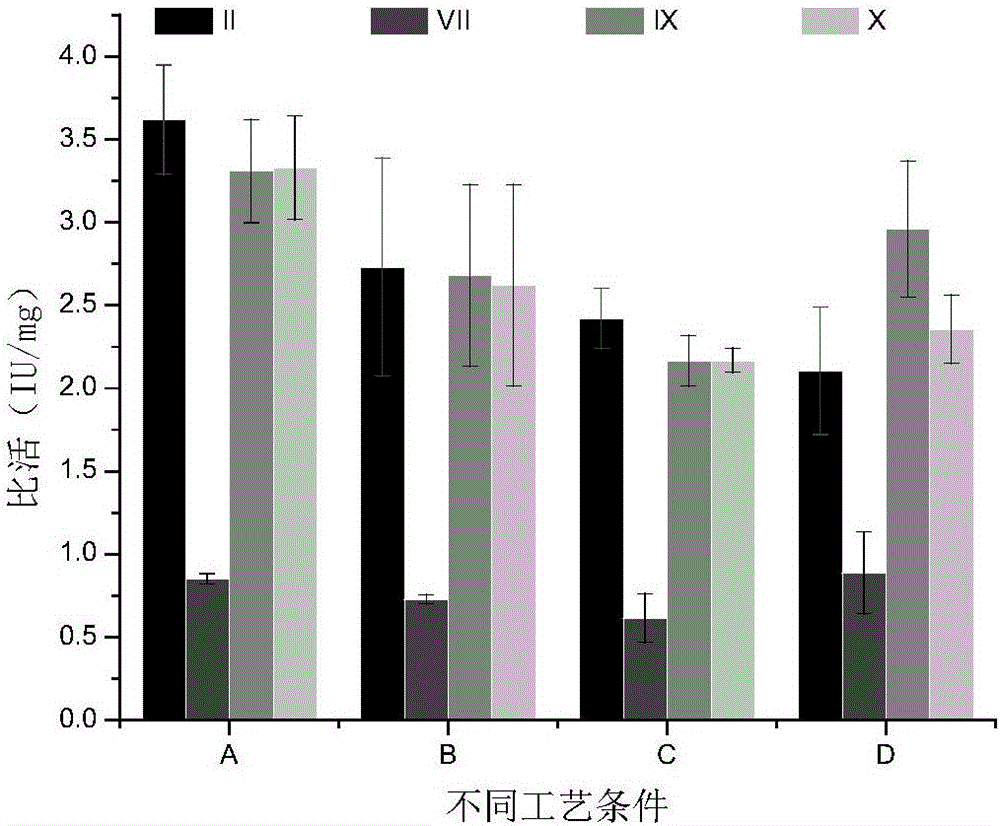
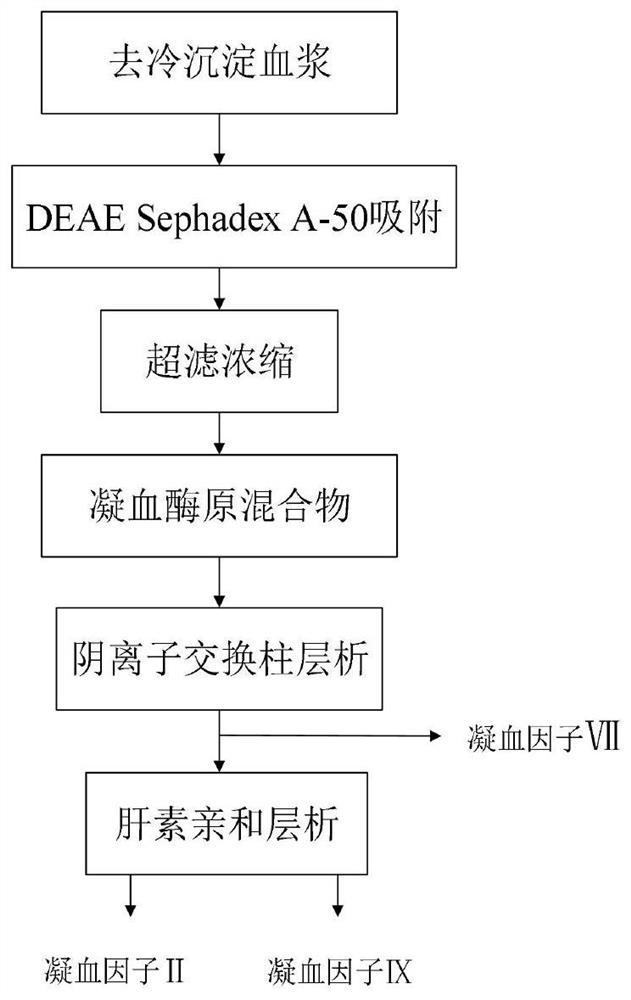
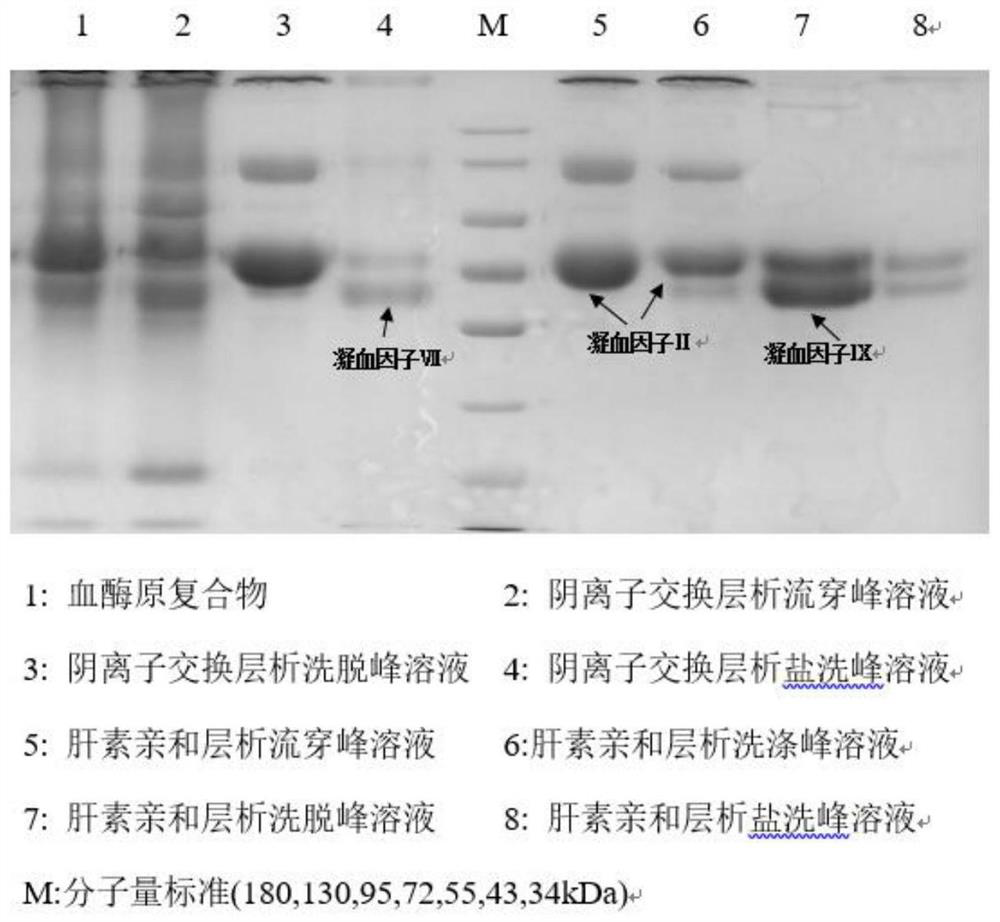
Method for simultaneously separating and purifying coagulation factors IX, X and VII from human plasma
ActiveCN109651502AAchieve separationEasy to operateFactor VIIPeptide preparation methodsUltrafiltrationAnion-exchange chromatography
The invention discloses a method for simultaneously separating and purifying coagulation factors IX, X and VII from human plasma, which comprises the following steps: performing centrifugal impurity removal, gel adsorption and ultrafiltration concentration to prepare a prothrombin compound; separating the coagulation factors VII and a mixed solution containing IX and II through an anion exchange resin column; separating the coagulation factor II and IX from the mixed solution containing IX and II by affinity chromatography. According to the method, by combining anion exchange chromatography with heparin affinity chromatography, the separation and preparation of three coagulation factors II, VII and IX at the same time can be achieved; the method has the advantages of high raw material utilization, simple operation and short time consumption; meanwhile, by detecting the electric signals in the chromatography process, the corresponding coagulation factors are accurately collected, the purity of the coagulation factors is effectively improved, and the economic benefit is improved.
Owner:HUALAN BIOLOGICAL ENG INC +2
Process for purifying prothrombin compound
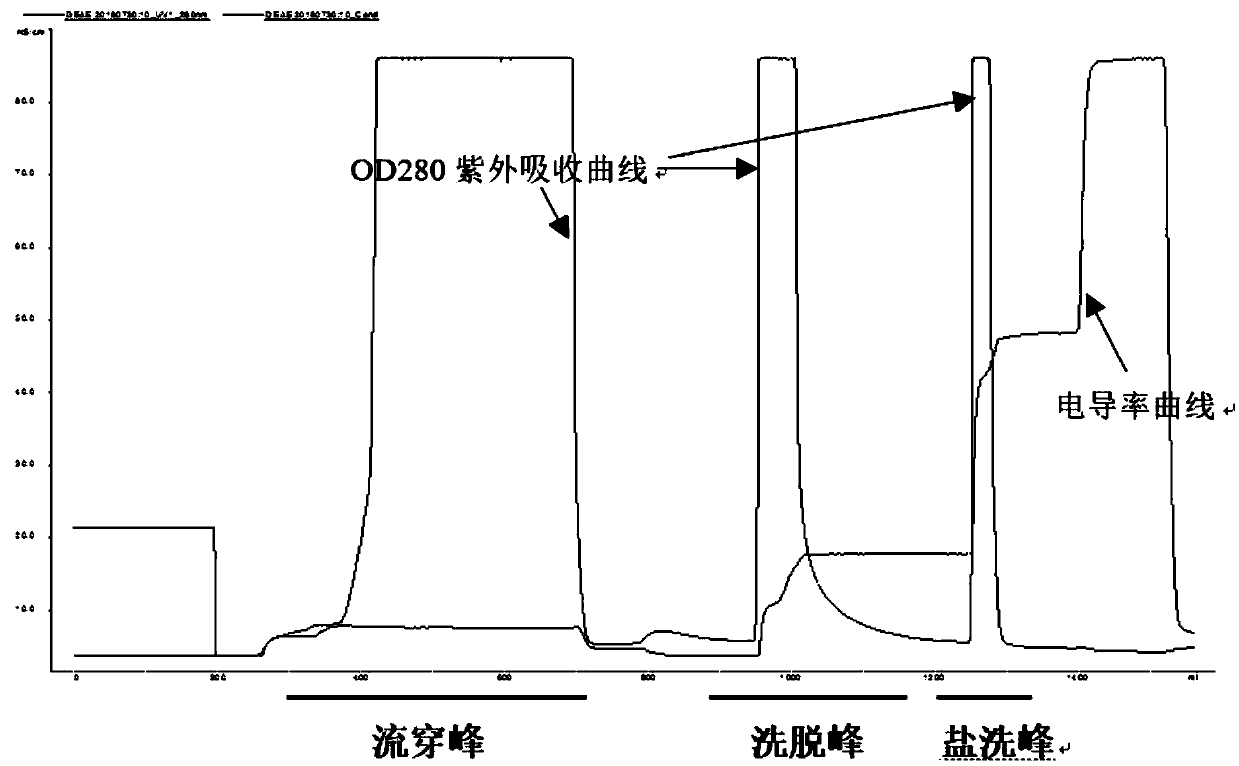
ActiveCN106497903AHigh purityImprove balanceFibrinogenPeptide preparation methodsDEAE SephadexCentrifugation
The invention provides a process for purifying a prothrombin compound. The process comprises the following steps: subjecting qualified detected plasma of a healthy person to centrifugation for removal of sediments so as to a raw plasma material; subjecting the raw plasma material to adsorption with DEAE-Sephadex A-50 gel and carrying out washing and elution so as to obtain crude extract of the prothrombin compound; and subjecting the crude extract of the prothrombin compound to further adsorption by using Capto DEAE gel column chromatography and carrying out washing and elution so as to obtain the liquid prothrombin compound. According to the invention, the prothrombin compound is prepared through two-step operations via the DEAE-Sephadex A-50 gel and Capto DEAE gel; and under the designed process conditions, the prepared prothrombin compound has a purity far higher than the purity of a prothrombin compound product prepared by using a traditional process, and blood coagulation factors II, IX and X are good in equalization.
Owner:DAAN PHARMA +1
Method for determining prothrombin time
InactiveUS7767459B2Microbiological testing/measurementDiagnostic recording/measuringTest samplePhysical chemistry
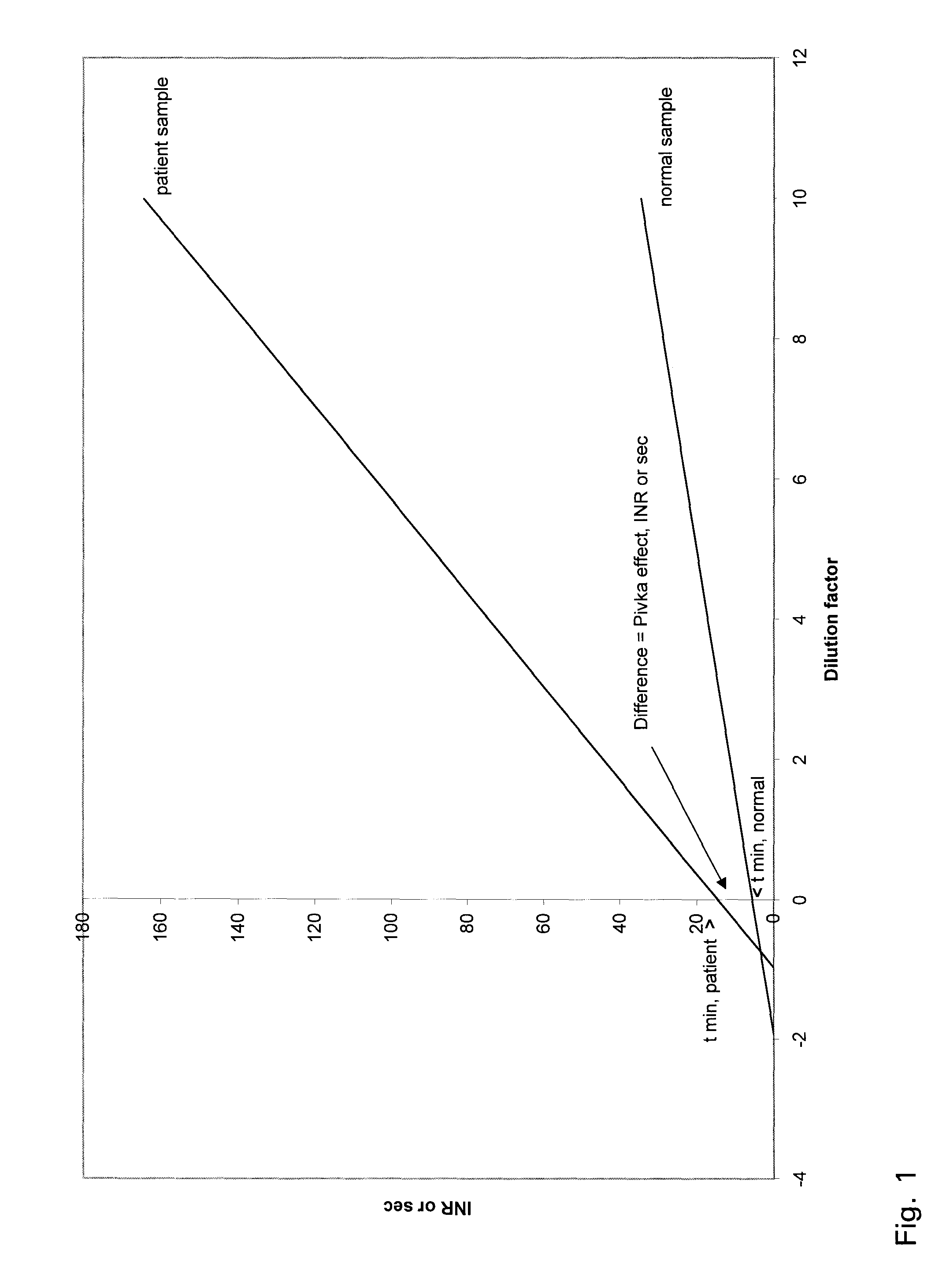
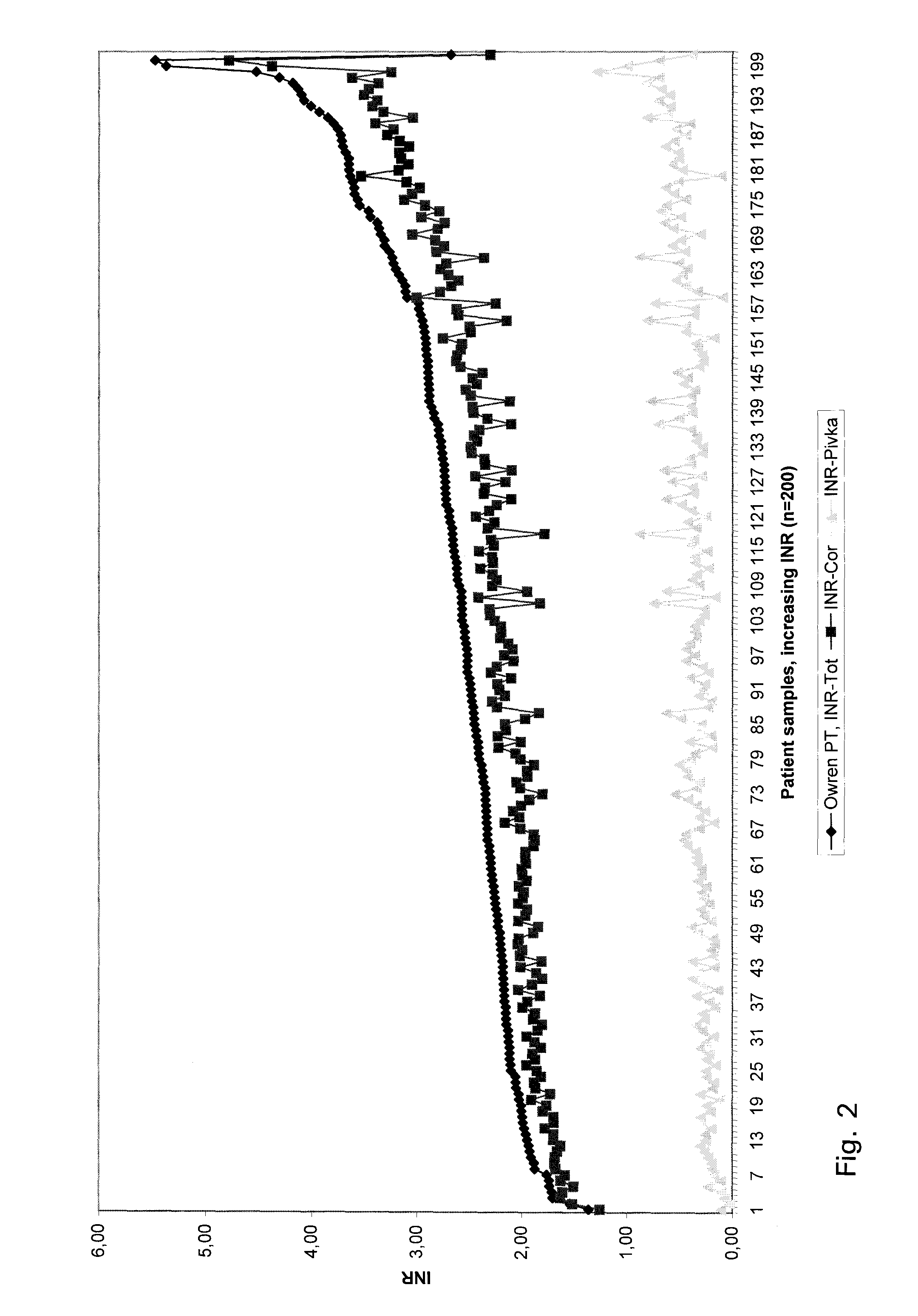
A method for determining prothrombin time of a plasma or whole blood sample includes measuring prothrombin time for at least two different dilutions for a test sample to determine tmin or INRmin. The prothrombin time for at least two different dilutions for normal plasma is measured to determine tmin or INRmin values for normal plasma. Next, tPivka or INRPivka values are calculated by subtracting the value for normal plasma from the value for the test sample. The Pivka corrected prothrombin time for the test sample is calculated by subtracting tPivka or INRPivka from the prothrombin time measured for the test sample.
Owner:HORSTI JUHA
Method for establishing liver cancer diagnosis model based on liver cancer triple detection
PendingCN112331333AEasy to getReduce mistakesMedical data miningMedical automated diagnosisAlpha-fetoproteinOncology
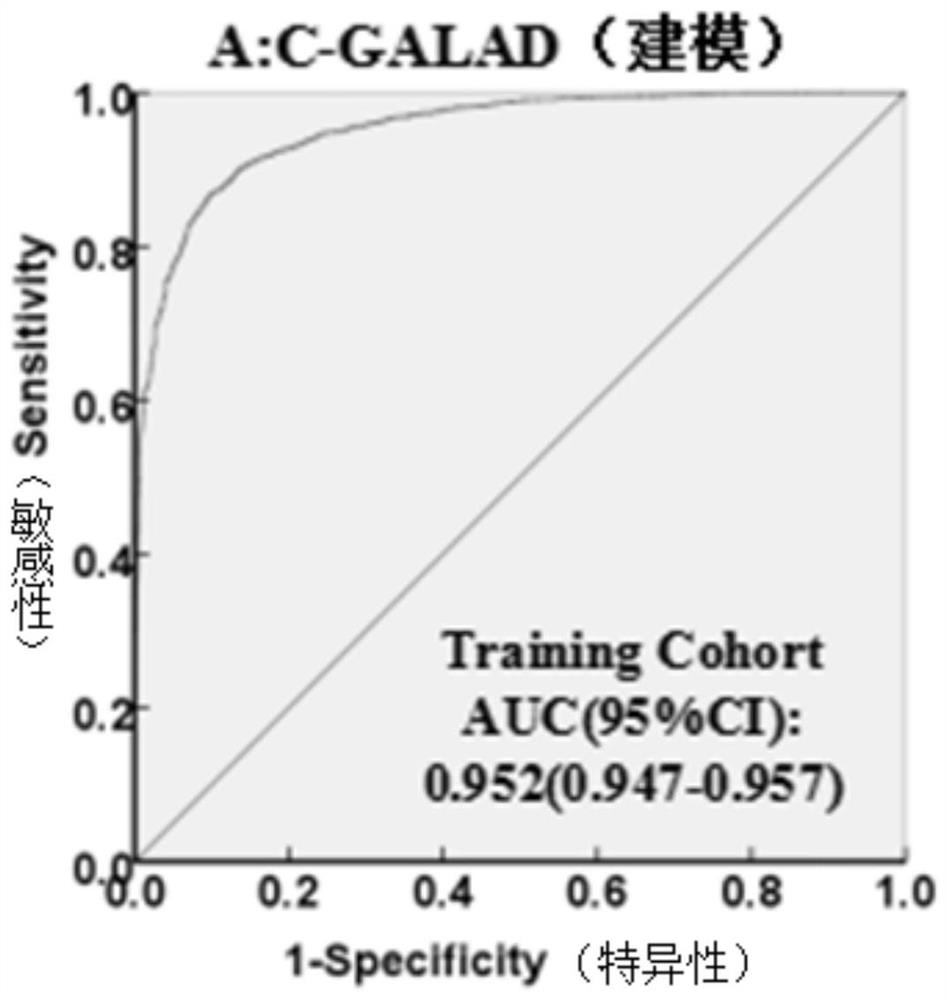
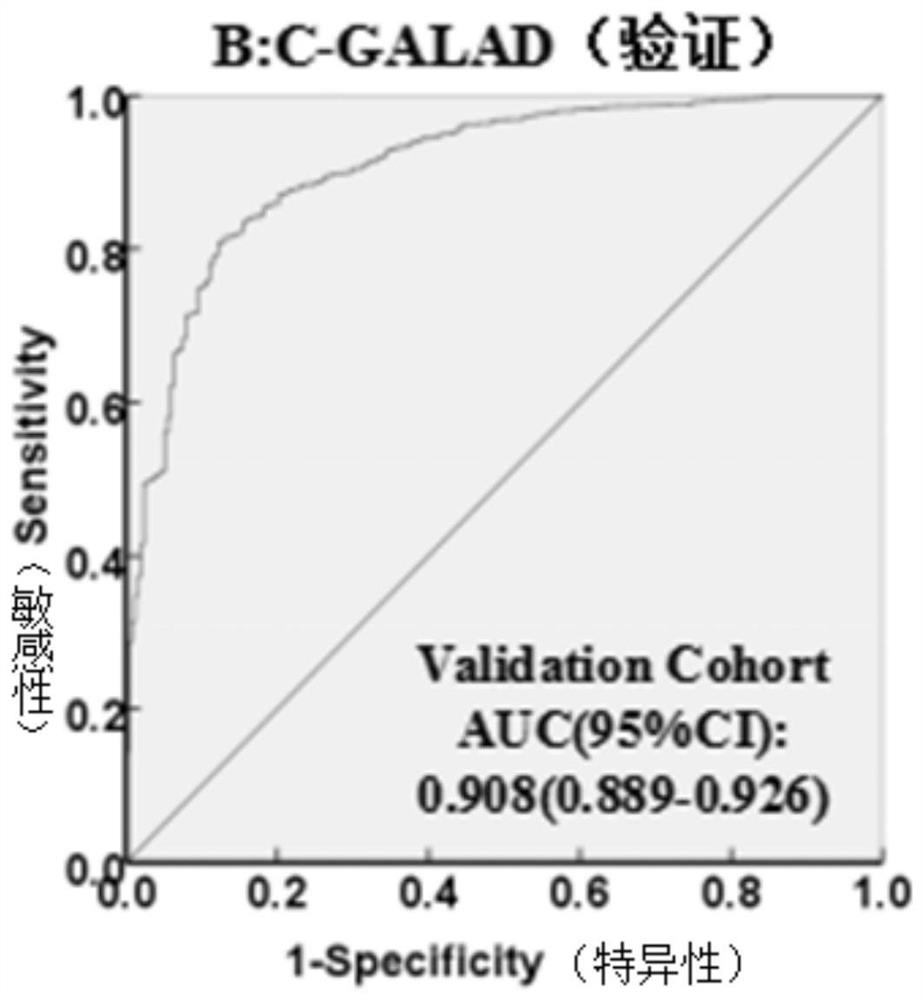
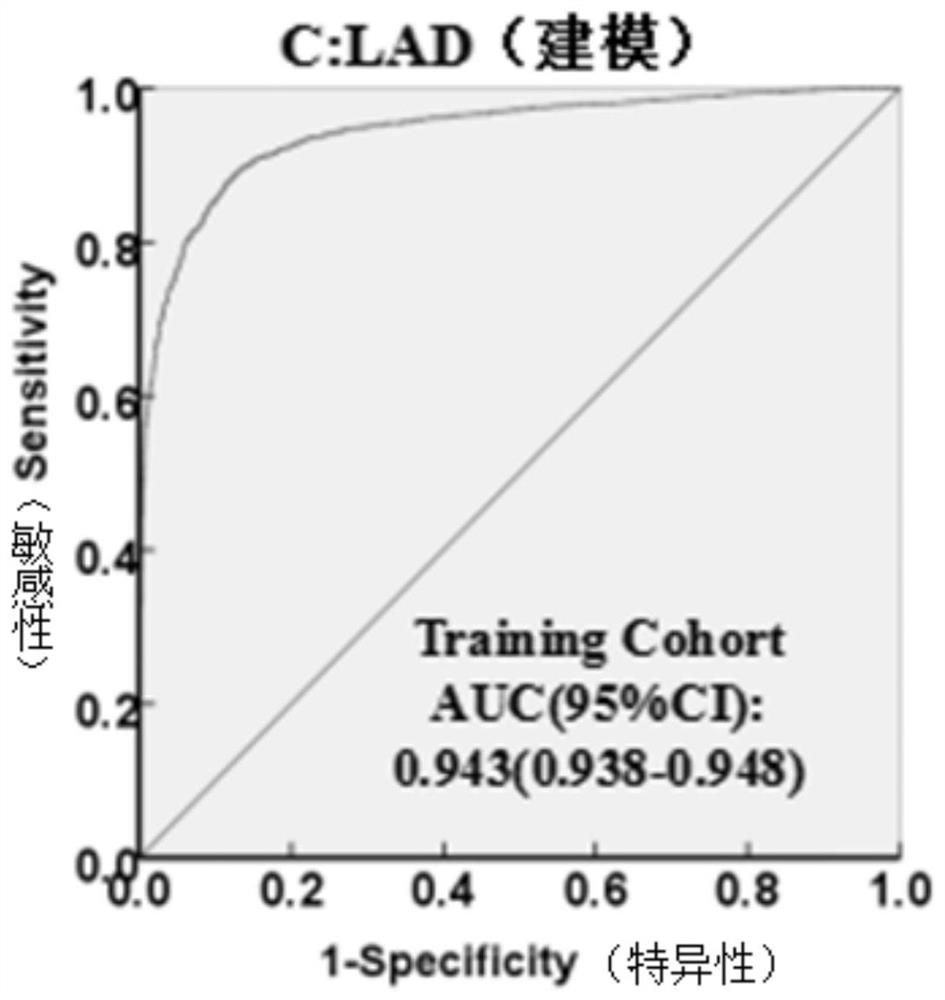
The invention discloses a method for establishing a liver cancer diagnosis model based on liver cancer triple detection. The method comprises the following steps of: 1, establishing a primary hepatocellular carcinoma database of primary hepatocellular carcinoma clinical characterization and laboratory data, and collecting laboratory indexes of a patient; and 2, arranging laboratory indexes, and establishing a multi-factor Logistic regression model to obtain a liver cancer diagnosis model. The laboratory indexes of the liver cancer diagnosis model CGALAD comprise the basic information gender and age of a patient, and liver cancer serological marker alpha fetoprotein heteroplasmon, alpha fetoprotein and abnormal prothrombin. Laboratory indexes of the liver cancer diagnosis model LAD compriseliver cancer serological marker alpha fetoprotein heteroplasmon, alpha fetoprotein and abnormal prothrombin. Parameters of the two liver cancer diagnosis models are easy to obtain, the parameters areon the same inspection subdisciplinary detection platform, a single report unit is few, influence factors are few, so that the clinical applicability and feasibility of the method are high.
Owner:高春芳
Oral caring composition and application thereof in inhibiting gum bleeding
InactiveCN110870870AResolve the bleedingAvoid bleedingCosmetic preparationsOrganic active ingredientsBleeding gumPlatelet aggregation ratio
The invention discloses an oral caring composition which comprises asiatic centella triterpenoids and an orally acceptable carrier, wherein the triterpenoids comprise one of or a combination of more of asiaticoside, asiaticoside degradation products, hydroxy asiaticoside and hydroxyl asiaticoside degradation products. The invention further discloses application of the asiatic centella triterpenoids in inhibiting gum bleeding. Four blood coagulation indexes of the oral caring composition disclosed by the invention meet standards that the prothrombin time (PT) is less than or equal to 14.5 seconds, the thrombin time (TT) is less than or equal to 42.5 seconds, the activated part thromboplastin time (APTT) is less than or equal to 27.0 seconds, and the platelet aggregation rate is greater thanor equal to 53.5. The problem of gum bleeding can be effectively solved.
Owner:HAWLEY & HAZEL BVI
Coagulation and fibrinolytic cascades modulator
InactiveCN101184775APeptide/protein ingredientsMicrobiological testing/measurementFactor iiPolyphosphate
The invention provides a thromboplastin reagent comprising: tissue factor, a phospholipid and a polyphosphate that acts as a blocker of tissue factor pathway inhibitor (TFPI). Also provided are a composition for promoting clotting comprising polyphosphate, and a reagent for a clotting assay also comprising polyphosphate together with an activator of clotting. Methods for stopping or slowing wound bleeding and fibrinolysis using compositions comprising polyphosphate are also disclosed.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Fibrin glue without fibrinogen and biosealant compositions and methods
InactiveUS20010016204A1Stop the flowPrevents unwanted and premature clot formationCosmetic preparationsSurgical adhesivesFibrin glueClot formation
The invention is a fibrin glue that avoids the use of fibrinogen and thus eliminates the need for premixing and premature clot formation. The fibrin glue of the invention comprises thrombin, thromboplastin and calcium and may have clotting Factors, VII, IX and X, and the like. The invention also comprises a biosealant for use with the fibrin glue without fibrinogen or for use alone. The biosealant is a two component mixture of gelatin / resorcinol and glyoxal / glutaraldehyde / 4-(p-maleimidophenyl) butyric acid. The two components are mixed on use.
Owner:WORTHAM LEON
Bacterium resisting anticoagulant ventriculoperitoneal branch pipe silicone pipe body and preparation method and application thereof
InactiveCN110694118APermanent antibacterialPermanent anticoagulant abilityCatheterVentriculoperitoneal shuntingPtru catalyst
The invention relates to a bacterium resisting anticoagulant ventriculoperitoneal branch pipe silicone pipe body and a preparation method and application thereof. The branch pipe silicone pipe body isprepared from an A component and a B component in the weight ratio of 1:(0.7-1.3), wherein the A component comprises the following components in parts by weight of 100-120 parts of vinyl-containing dimethyl silicone, 0.05-10 parts of barium sulfate, 0.05-15 parts of an antibacterial agent, 0.05-15 parts of an anticoagulant and 0.5-15 parts of a dispersing agent, and the B component comprises thefollowing components in parts by weight of 70-130 parts of active hydrogen and platinum gold catalyst containing dimethyl silicone. Through extruding, curing and shaping, the bacterium resisting anticoagulant ventriculoperitoneal branch pipe silicone pipe body is obtained. The material has permanent bacterium resistance, has good bacterium resistance and long recalcification time, time for activating part of blood coagulation live enzymes, and prothrombin time.
Owner:JIANGSU UNIV OF TECH
Early diagnosis kit based on combined detection of four items of liver cancer and application
PendingCN114414802AHigh sensitivityImprove featuresBiological material analysisMagnetic beadImmuno detection
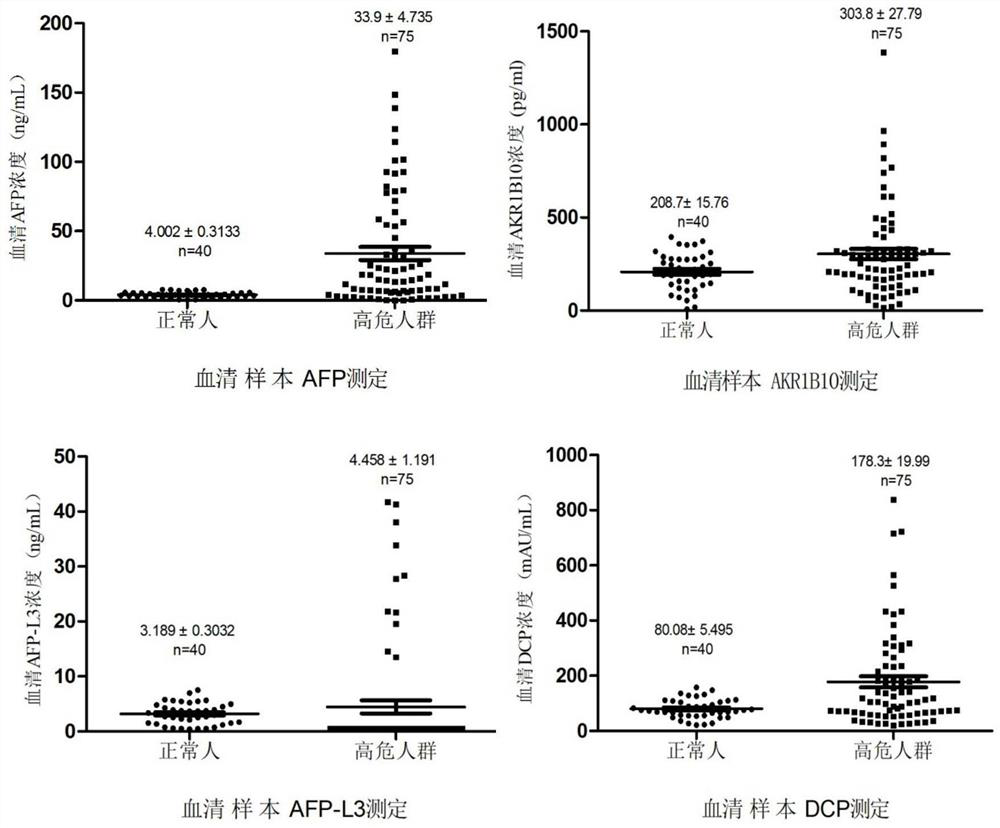
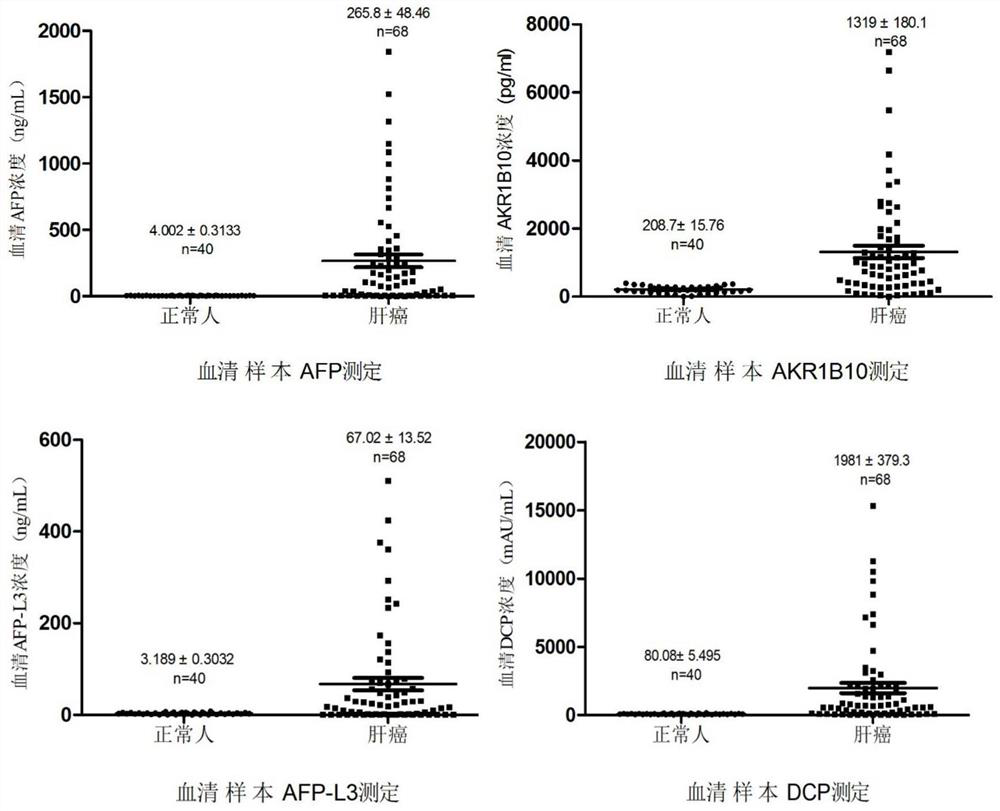
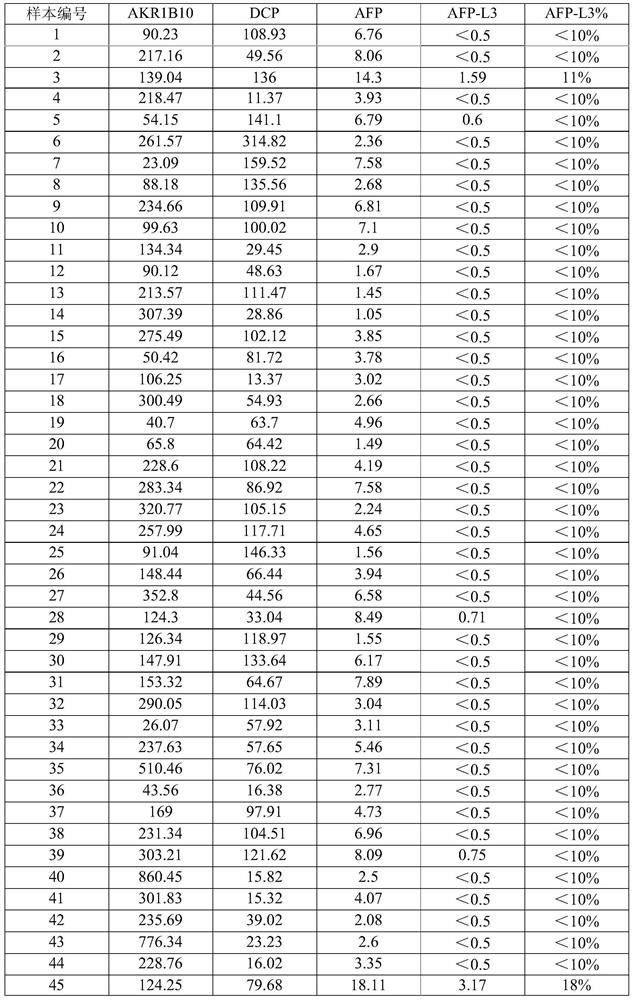
The invention belongs to the technical field of immunodetection, and discloses a combined detection kit based on four liver cancer tumor markers: aldoketoreductase 1B10 (AKR1B10), abnormal prothrombin (DCP), alpha fetoprotein (AFP) and alpha fetoprotein variant (AFP-L3). The kit comprises: streptavidin magnetic beads; labeling AKR1B10, DCP (dicumyl peroxide) and AFP antibodies with biotin; labeling AKR1B10, DCP and AFP antibodies with acridinium ester; an LCA magnetic bead, a cleaning solution and an eluent; and AKR1B10, DCP and AFP calibration products and quality control products. By simultaneously detecting the contents of four tumor markers in serum of a subject, the risk of potential development of liver cancer of the subject is analyzed and judged. According to the invention, the sensitivity and specificity of early diagnosis of liver cancer are improved by combined detection of four tumor markers, and a more reliable basis is provided for clinical diagnosis of liver cancer.
Owner:湖南莱拓福生物科技有限公司
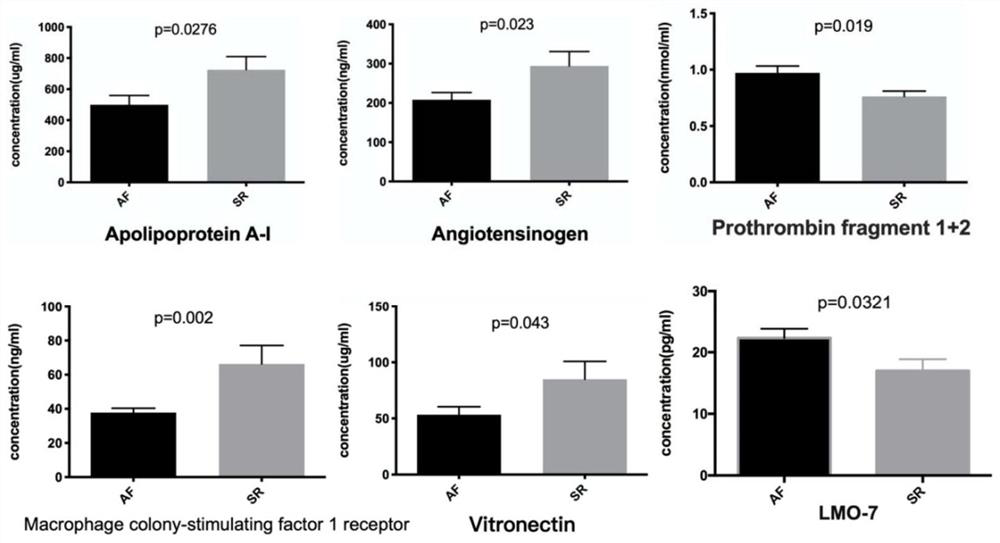
Multi-protein combined biomarker, application and heart valvular disease complicated with atrial fibrillation diagnostic kit
The invention provides a multi-protein combined biomarker, application and a heart valvular disease complicated with atrial fibrillation diagnostic kit. The multi-protein combined biomarker is composed of an apolipoprotein A1 protein antibody, an angiotensin antigen antibody, an LIM structural domain protein 7, a macrophage colony stimulating factor receptor 1, a vitronectin antibody and a prothrombin fragment F1 + 2 antibody. Modern biological technology and bioinformatics analysis find that combined application of the six proteins has the application of predicting valvular heart disease complicated with atrial fibrillation, can be applied to preparation of targeted drugs for early intervention of related diseases, and can also be applied to preparation of a heart valvular disease complicated with atrial fibrillation screening kit. According to the kit, modeling is carried out after the concentrations of the six proteins are measured, and good evaluation efficiency and potential hugeapplication value are achieved on whether atrial fibrillation happens to a patient suffering from the heart valvular disease or not.
Owner:TEDA INT CARDIOVASCULAR HOSPITAL
System and method for testing likelihood that subject suffers from liver cancer
The invention discloses a system and a method for testing the possibility that a subject suffers from liver cancer. The system comprises a data acquisition module for acquiring data of gender, age, abnormal prothrombin (DCP) and alpha fetoprotein (AFP) of a subject; and the data processing module is used for processing the data acquired in the data acquisition module, including respectively taking logarithmic functions for the DCP data and the AFP data, and further respectively setting weights for the gender, the age and the DCP and the AFP after taking the logarithmic functions, thereby calculating an evaluation value P (Y). According to the system and the method, the problem that prediction of the liver cancer by a single index cannot meet clinical requirements is solved. In addition, by means of the system, the sensitivity and specificity of liver cancer screening can be remarkably improved.
Owner:PEKING UNIV
Improved clotting composition
ActiveUS20170304407A1High percentage homologyImprove stabilityPeptide/protein ingredientsMicrobiological testing/measurementAnalyteMedicine
The present invention relates to improved clotting compositions for producing high quality blood serum samples for analyte testing, such as for pathology testing and other biological assays. In particular, the present invention relates to the use of prothrombin activators in combination with stabilizing agents such as colloids for producing high quality blood serum samples. The present invention also relates to associated methods for preparing clotting compositions, tubes, kits and methods of diagnosis, prognosis and monitoring for responsiveness to therapy.
Owner:Q SERA
Prothrombin time detection test strip and preparation method thereof
PendingCN111175357AImprove accuracyQuick to useMaterial electrochemical variablesRed blood cellTest sample
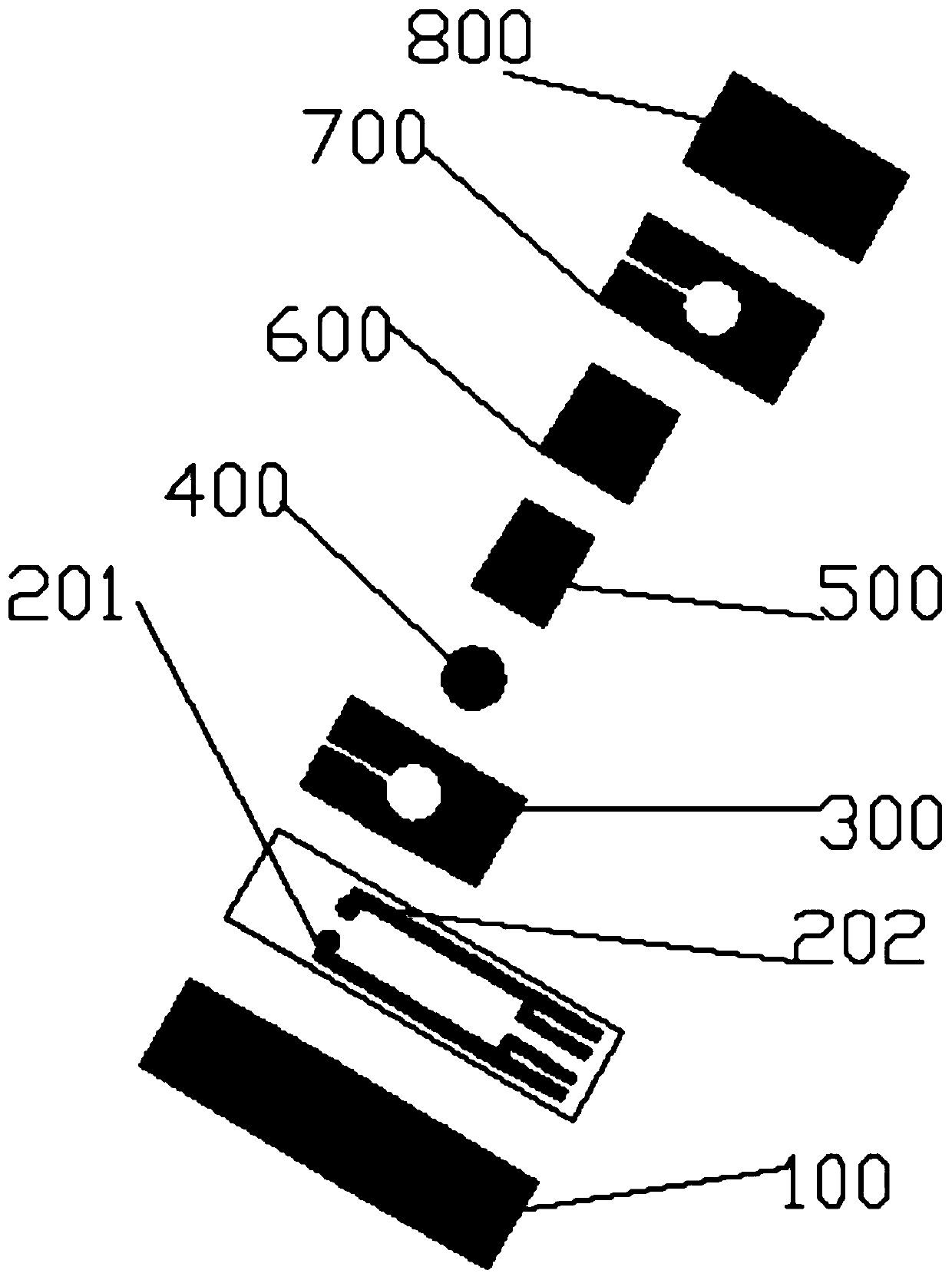
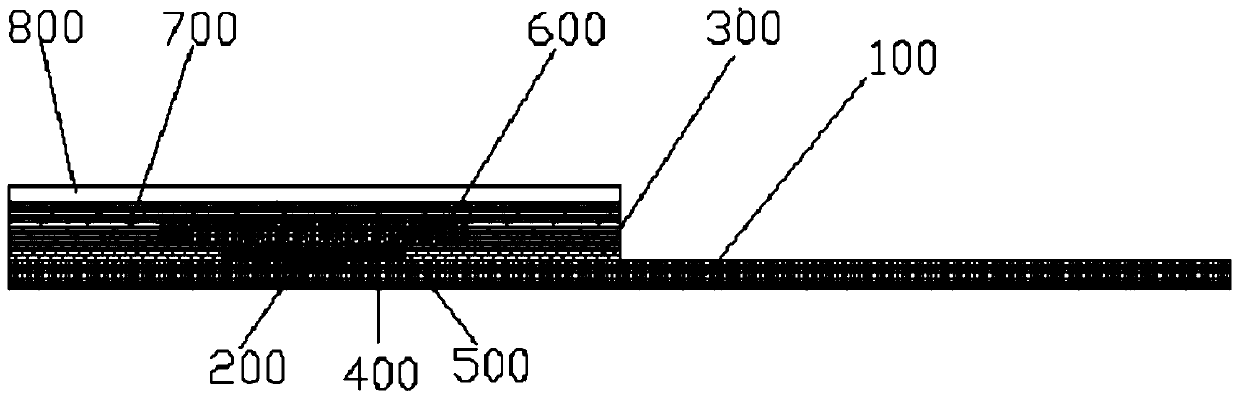
The invention discloses a prothrombin time detection test strip and a preparation method thereof. The test strip comprises a hydrophilic layer, a diffusion layer arranged below the hydrophilic layer,a first bonding layer arranged between the hydrophilic layer and the diffusion layer, a red blood cell separation layer arranged below the diffusion layer, a reagent layer arranged below the red bloodcell separation layer, an electrode layer arranged below the reagent layer, a substrate arranged below the electrode layer, and a second bonding layer which fixes the reagent layer and is located between the first bonding layer and the substrate. The detection test strip is advantaged in that a single-hole single-reagent method is adopted to test the prothrombin time, the test strip can be suitable for testing samples with different hematocrit, is matched with a dry-type multifunctional analyzer for use, and is rapid in detection, and the obtained detection result has high accuracy.
Owner:杭州联晟生物科技有限公司
Rapid hemostat suitable for war wound massive hemorrhage
PendingCN111904520ANon-toxicNo exclusionSurgical adhesivesPharmaceutical delivery mechanismGreat Blood VesselHemostat
The invention discloses a rapid hemostat suitable for war wound massive hemorrhage, and relates to the technical field of medical instruments; the rapid hemostat comprises an injection mechanism and ahemostatic material, wherein the injection mechanism comprises an injection cylinder, a piston, an injection cylinder front cover and a percussion assembly; the hemostatic material is composed of barium sulfate powder with a diameter of less than 2 [mu] m, chitosan particles with a high deacetylation degree (greater than 90%) and a diameter of 2 mm, and prothrombin powder. The chitosan particlesin the hemostatic material rapidly expand to press a bleeding point, prothrombin is combined to promote fibrinogen to be converted into fibrin solidified blood to achieve the purpose of rapid hemostasis; and the chitosan particles are filled with barium sulfate powder which can be developed under X-ray radiography, so that identification and thorough taking of rear hospitals are facilitated. The rapid hemostat has the advantages of being simple, safe, economical and practical, is suitable for bleeding which is not prone to compression control and is caused by trauma of a body joint area and ajunction area and damage to large blood vessels, and is exact in hemostasis effect and high in hemostasis speed.
Owner:海军军医大学第三附属医院上海东方肝胆外科医院
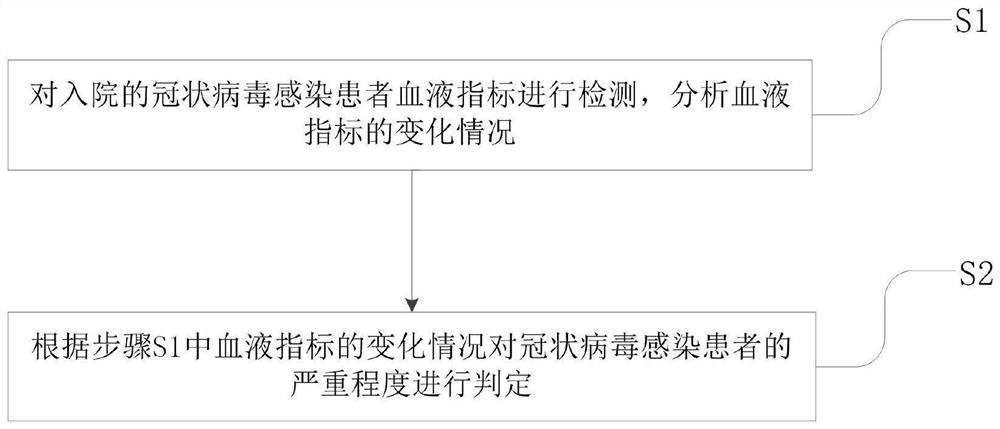
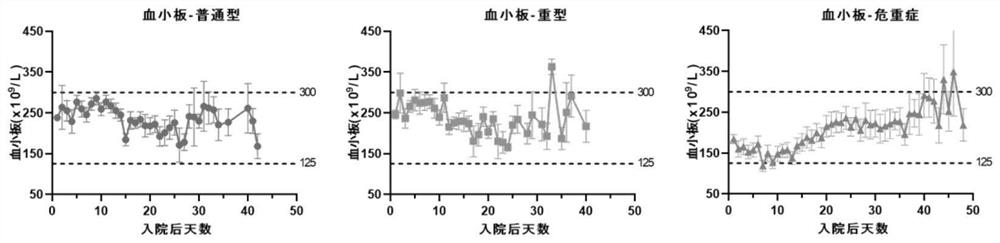
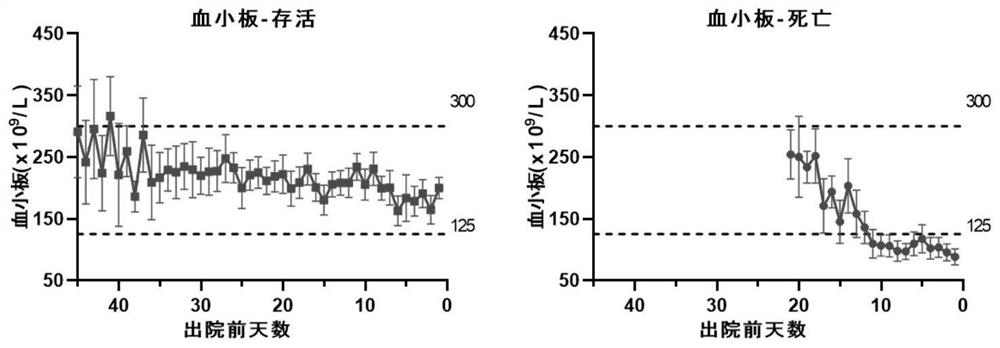
Application of blood index for detecting severity of patient infected with coronavirus
InactiveCN111681768AJudgment realizedMedical data miningMedical automated diagnosisA lipoproteinCholesterol total
The invention specifically discloses application of a blood index for detecting the severity of a patient infected with coronavirus, comprising the following specific steps of: S1, detecting blood indexes of the hospitalized coronavirus-infected patients, and analyzing the change condition of the blood indexes; and S2, judging the severity of the patient infected with the coronavirus according tothe change condition of the blood indexes in the step S1. The blood indexes in the invention include at least one of platelet content, blood glucose content, total cholesterol content, high density lipoprotein content, low density lipoprotein content, prothrombin time, D-dimer content, and DIC score. Therefore, the severity of the coronavirus-infected patient can be accurately judged by detectingand analyzing the change condition of the blood indexes. The invention has the characteristics of timeliness, accuracy and high efficiency, and the problem that the treatment of the coronavirus-infected patient by using a clinical phenotype has a certain hysteresis phenomenon in the prior art is effectively solved.
Owner:HUNAN YUANPIN CELL TECH CO LTD
A method for simultaneously separating and purifying blood coagulation factors ix, x and ⅶ from human plasma
ActiveCN109651502BAchieve separationEasy to operateFactor VIIPeptide preparation methodsUltrafiltrationAnion-exchange chromatography
The invention discloses a method for simultaneously separating and purifying coagulation factors IX, X and VII from human plasma, which comprises the following steps: performing centrifugal impurity removal, gel adsorption and ultrafiltration concentration to prepare a prothrombin compound; separating the coagulation factors VII and a mixed solution containing IX and II through an anion exchange resin column; separating the coagulation factor II and IX from the mixed solution containing IX and II by affinity chromatography. According to the method, by combining anion exchange chromatography with heparin affinity chromatography, the separation and preparation of three coagulation factors II, VII and IX at the same time can be achieved; the method has the advantages of high raw material utilization, simple operation and short time consumption; meanwhile, by detecting the electric signals in the chromatography process, the corresponding coagulation factors are accurately collected, the purity of the coagulation factors is effectively improved, and the economic benefit is improved.
Owner:HUALAN BIOLOGICAL ENG INC +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com