Patents
Literature
30 results about "Virus genetics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Heredity, especially the mechanisms of hereditary transmission and the variation of inherited characteristics among a virus or viruses; the genetic constitution of viruses.
Newcastle disease virus heat resistant live vaccine vector system and application thereof
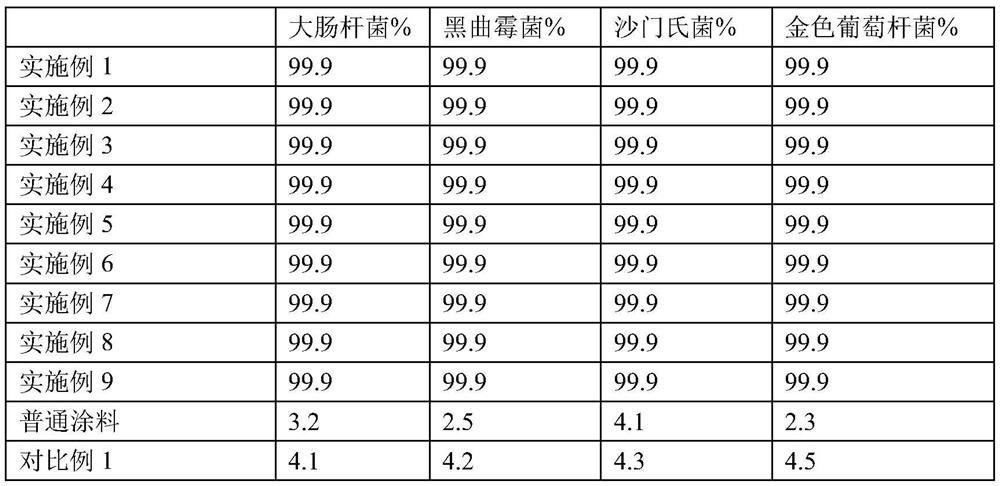
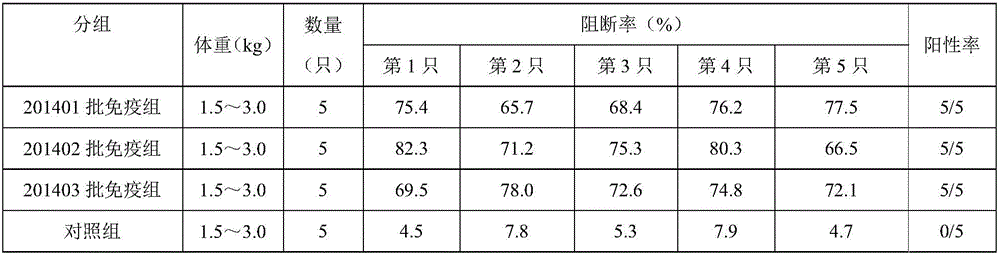
ActiveCN104059942AHeat resistantHigh heat resistanceSsRNA viruses negative-senseMicroorganism based processesDiseasePBR322
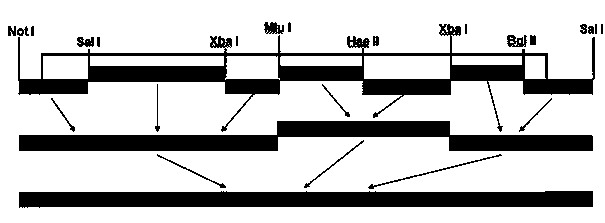
The invention belongs to the field of virus genetic operation, and in particular to a Newcastle disease virus (NDV) heat resistant live vaccine vector system and application thereof. The Newcastle disease virus (NDV) heat resistant live vaccine vector system comprises a) a transcription plasmid, b) three auxiliary plasmids and c) host cells. The transcription plasmid is obtained by cloning genomic full-length cDNA of a NDV heat resistant vaccine strain to pBR322 vector; and the three auxiliary plasmids are obtained by cloning nucleoprotein, phosphoprotein and large polymerase protein gene of the NDV heat resistant vaccine strain to pcDNA3.1 vector. The artificial recombinant Newcastle disease virus has the characteristic of heat resistance, and the Newcastle disease virus (NDV) heat resistant live vaccine vector system is established for the first time. The artificial recombinant Newcastle disease virus has great application prospect in the aspects of research and development of multiple (multivalent) heat resistant genetic engineering live vaccines of the NDV, avian influenza and other major diseases of poultry, research on virus heat-resistant mechanism, and the like.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
High-purity chito-oligosaccharide controlled release fertilizer based on separation techniques and preparing method thereof
InactiveCN104926534APromote growthActivation of defense responsesFertilizer mixturesBiotechnologyCoated urea
The invention discloses a high-purity chito-oligosaccharide controlled release fertilizer and relates to the technical field of agricultural fertilizers. The high-purity chito-oligosaccharide controlled release fertilize is made from 4% of chito-oligosaccharide coated urea, 6% of another chito-oligosaccharide coated urea, urea, ammonium sulfate, calcium superphosphate, calcium magnesium phosphate, potassium sulfate, humic acid, and EDTA (ethylene diamine tetraacetic acid) chelated trace elements. The high-purity chito-oligosaccharide controlled release fertilizer has unique action on growth of crops, has the effects such as activating crop cells and promoting growth, can activate defensive reaction in plants, start anti-virus genetic expression and induce disease resistance in the crops, has the function of immunizing and killing various fungi, germs and viruses to decrease disease and pest damage to the crops, also can effectively increase the quantity and activity of soil microorganisms and improve the soil environment, is a multifunction novel fertilizer green, pollution free and soil improving, and is worthy of wide application.
Owner:山东卫康生物医药科技有限公司
Complete virus gene engineering vaccine of aftosa and its preparation method
InactiveCN1602962ANot contagiousLow costSsRNA viruses positive-senseAntibody mimetics/scaffoldsNucleic Acid ProbesViral Vaccine
The invention discloses a kind of foot-and-mouth disease genetic engineering vaccine and its the preparing method.Its structure expression is: Entire virus genetic engineering vaccine: Phage T4-Soc-Hoc-FMDV-P1; Asian unit genetic engineering vaccine: Phage T4-Soc-Hoc-FMDV-F3.The preparing method is: Takes O blood serum foot-and-mouth disease virus poisonous, extracts total gene group RNA; From blood serum poisonous DNA measure foreword to synthesizes two DNA nucleic acid probe;takes the probe as the directing thing, carries on Rt-pcr chain type DNA expand increase response, obtains the viral granule P1gene;gene recombinate the P1gene and the T4bacteriophage material particle, pRH-Soc, obtains T4bacteriophage conformity material particle pRH-Soc-P1;homology reorganizes pRH-Soc-P1with the T4bacteriophage carrier phage T4- í¸ Soc & í¸ Hoc, then enters in the backwoods coli E.coli.CR63host mycelium, obtains this invention genetic engineering vaccine phage T4-Soc-FMDV-P1.compares this invention with the deactivation entire viral vaccine, it does not have to fight fire, maintains the entire viral antigenicity but not have the infection.may inject, also may take orally, convenient, quick, the immunity effect is good.
Owner:任兆钧
Method for preparing gosling plague virus-like granules with escherichia coli system
ActiveCN106754981AGood reactogenicityVirus peptidesInactivation/attenuationEscherichia coliDigestion
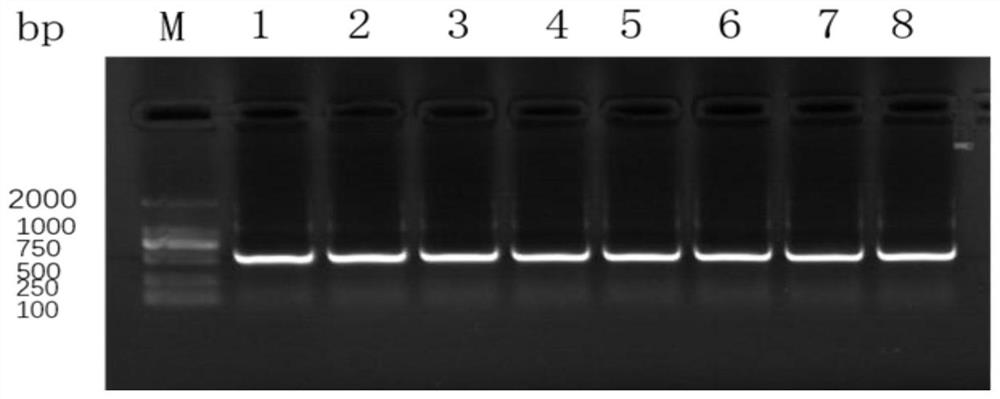
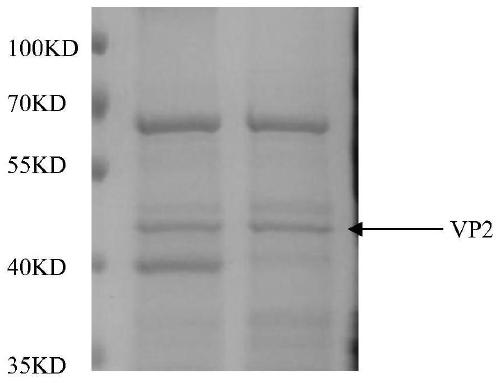
The invention relates to a method for preparing gosling plague virus-like granules with an escherichia coli system for soluble expression of gosling plague virus VP2 protein. The method for soluble expression of gosling plague virus VP2 protein comprises the following steps: performing codon optimization on a gosling plague virus VP2 gene, performing site-specific mutagenesis, namely, mutating a codon AGA into CGC and mutating GGA into GGT, cloning to a pET-Sumo vector, establishing a recombinant expression vector pET-Sumo-VP2, transforming the pET-Sumo-VP2 into a prokaryotic expression bacterium, and inducing with IPTG (isopropyl beta-D-1-Thiogalactopyranoside) at 37 DEG C so as to obtain soluble recombinant VP2 recombinant protein; and performing digestion on the recombinant protein with a ULP enzyme, and purifying with a Ni column, thereby obtaining purified VP2 protein. Electron microscope results show that the gosling plague virus-like granules can be prepared from VP2 protein after digestion, and moreover, the purified VP2 protein has good reactogenicity and can be applied to preparation of subunit vaccines of gosling plague virus genetic engineering.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Ankara vaccinia virus genetic engineering vaccine for pig replication and respiration complex
InactiveCN101209351AImprove immunityHigh protein expressionGenetic material ingredientsAntiviralsBALB/cCowpox virus
The invention discloses a porcine reproductive and respiratory syndrome-Ankara vaccinia virus genetic engineering vaccine, the preparation method is that: 1. the PCR amplification of GP5 gene is carried out from the natural porcine reproductive and respiratory syndrome virus; 2. the porcine preferred codon is used for replacing the codon in the natural GP5 gene, so as to carry out GP5 gene optimization and the synthesis of the optimized sequence GP5A; 3. the epitope A in the optimized GP5A gene is replaced by the epitope B of the GP5 gene, so as to obtain the GP5-DB gene; 4. the GP5 and GP5-DB genes are respectively cloned to the expression carrier JN-2; 5. the constructed JN-2-GP5 and the JN-2-GP5-DB plasmid are recombined with Ankara vaccinia virus, so as to obtain the porcine reproductive and respiratory syndrome GP5 and GP5-DB gene Ankara vaccinia virus recombinant vaccine. Experiments confirm that the invention has excellent immune protection in BALB / c mice and pigs.
Owner:WUCHANG SHIPBUILDING IND
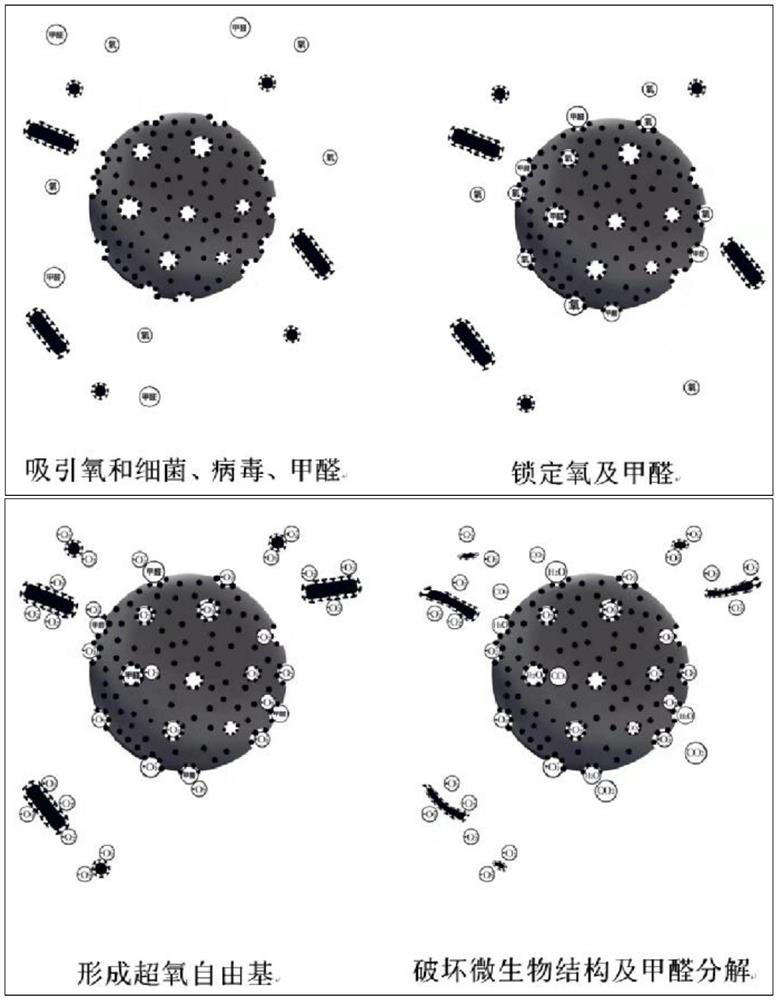
Monatomic antibacterial antiviral formaldehyde-removing coating additive suitable for interior wall coating and preparation method of monatomic antibacterial antiviral formaldehyde-removing coating additive
ActiveCN113652110APowerfulSave raw materialsAntifouling/underwater paintsPaints with biocidesBiotechnologyCell membrane
The invention relates to the technical field of interior wall coating fillers, in particular to a monatomic antibacterial antiviral formaldehyde-removing coating additive suitable for an interior wall coating and a preparation method of the monatomic antibacterial antiviral formaldehyde-removing coating additive. The monatomic antibacterial antiviral formaldehyde-removing coating additive suitable for the interior wall coating is composed of a carrier and transition metal, wherein the carrier is a complex of special fillers for various coatings; and the transition metal is uniformly distributed on the surfaces of the carrier and pore channels in a monatomic form. The coating additive has the advantages of excellent stability and compatibility, does not destroy the structure of the coating after being added into the coating, has rich monatomic active sites, can efficiently activate oxygen molecules in space, generate active oxygen species, oxidize cell membranes of bacteria, proteins and virus genetic materials and directly destroy a biological structure, and enables the coating to have antibacterial and antiviral effects; and meanwhile, the coating additive also has a rich specific surface area, can adsorb and lock formaldehyde small molecules, and is decomposed into carbon dioxide and water by oxidation seeds, so the coating is allowed to have an aldehyde removal effect.
Owner:LINKWAY TECH CO LTD
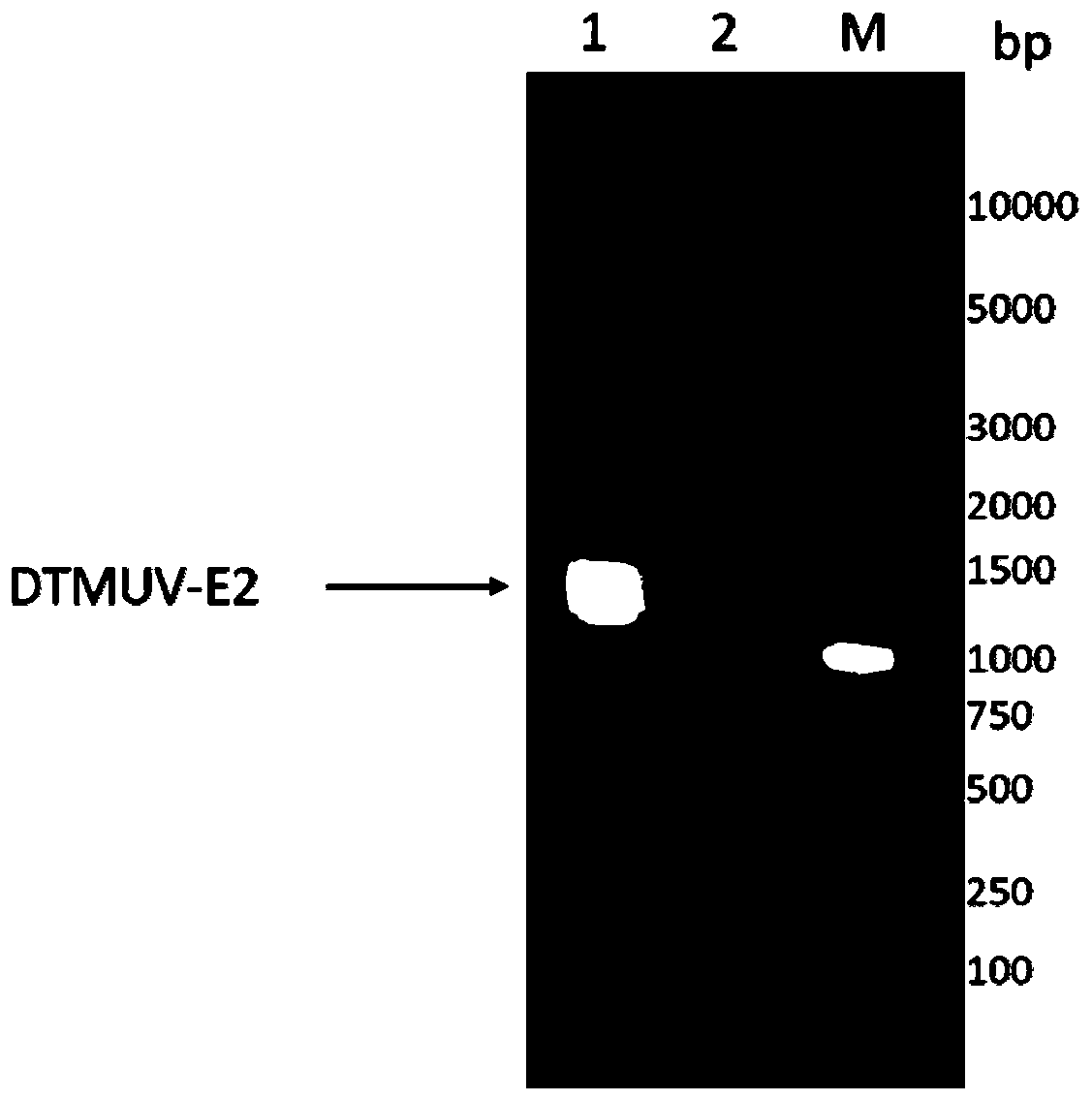
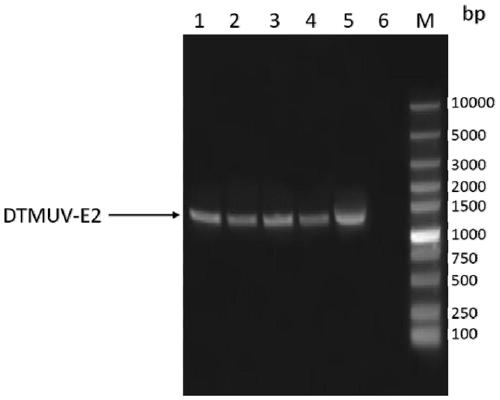
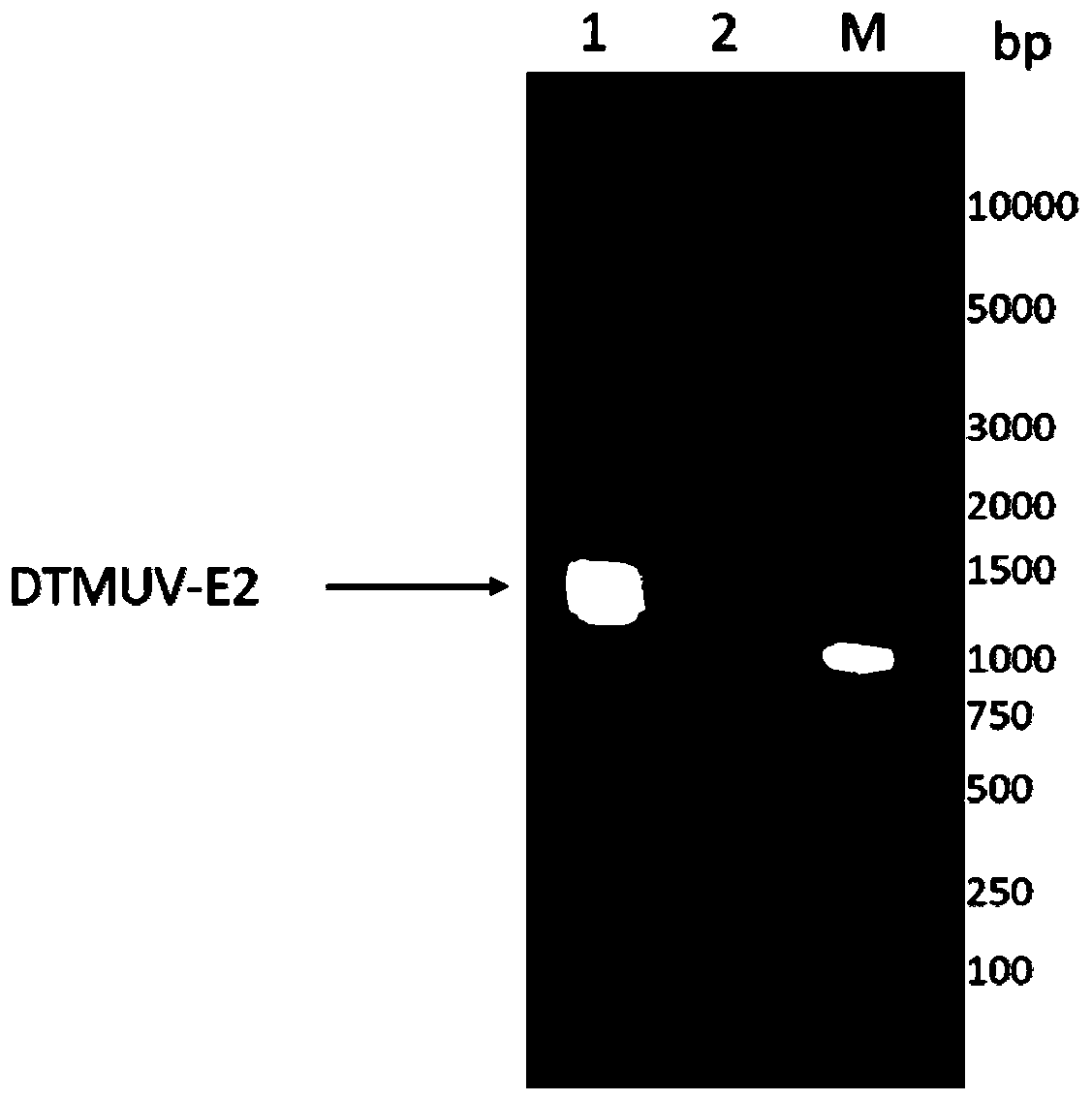
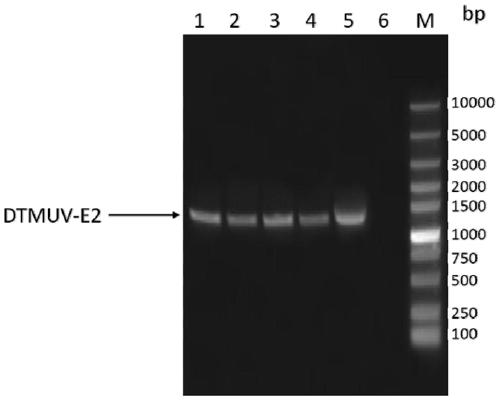
Duck tembusu virus genetic engineering subunit vaccine and preparation method and application thereof
ActiveCN110237244AOutstanding advantagesOutstanding effectSsRNA viruses positive-senseViral antigen ingredientsNucleotideVaccine Production
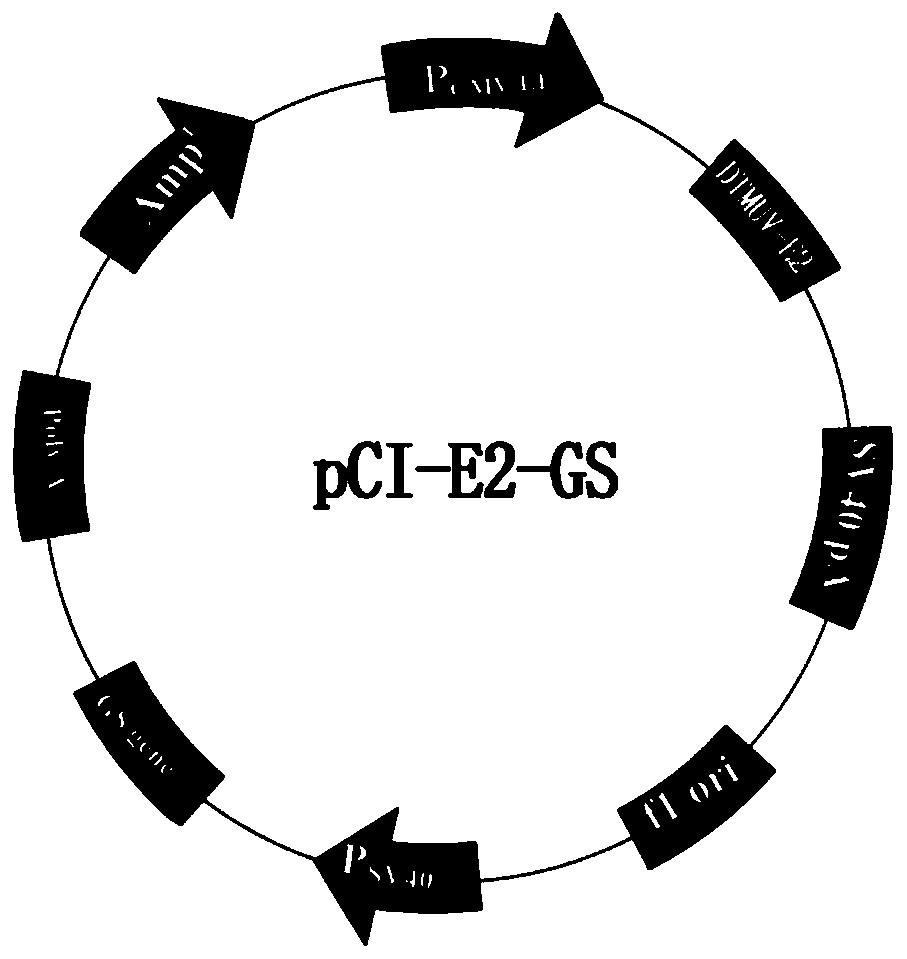
The present invention provides a duck tambosu virus genetic engineering subunit vaccine and a preparation method and an application thereof. A nucleic acid molecule having a sequence shown in SEQ ID NO:1 or a nucleic acid molecule identical to 95% or more of the nucleotide sequence shown in the SEQ ID NO:1 encodes a duck tambosu virus E2 protein, an immune composition comprising the duck tambosu virus E2 protein can be used to prepare the duck tambosu virus genetic engineering subunit vaccine, antigenicity, immunogenicity and functions of the vaccine are similar to natural proteins, and the vaccine is relatively high in expression level, strong in the immunogenicity, free of pathogenicity to ducks, and can also be prepared by large-scale serum-free suspension culture in a bioreactor, which greatly reduces a production cost of the vaccine.
Owner:苏州世诺生物技术有限公司
Coronal virus genetic engineering protein and use thereof
InactiveCN1990502AImprove expression efficiencyNon-pathogenicDepsipeptidesViruses/bacteriophagesEngineering proteinVirus genetics
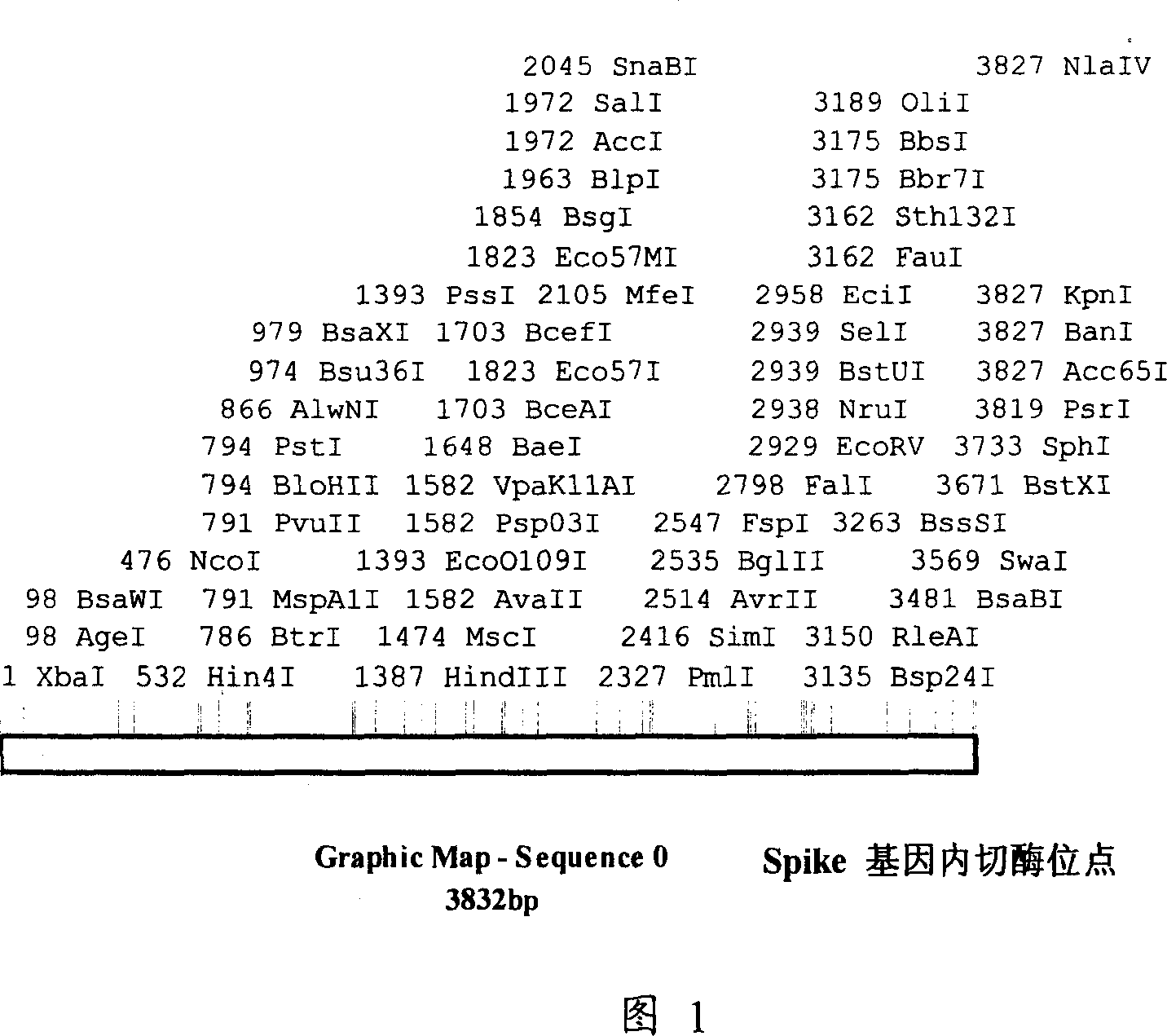
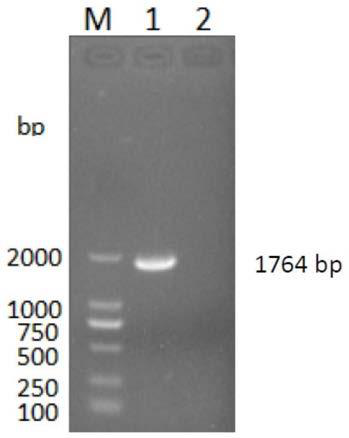
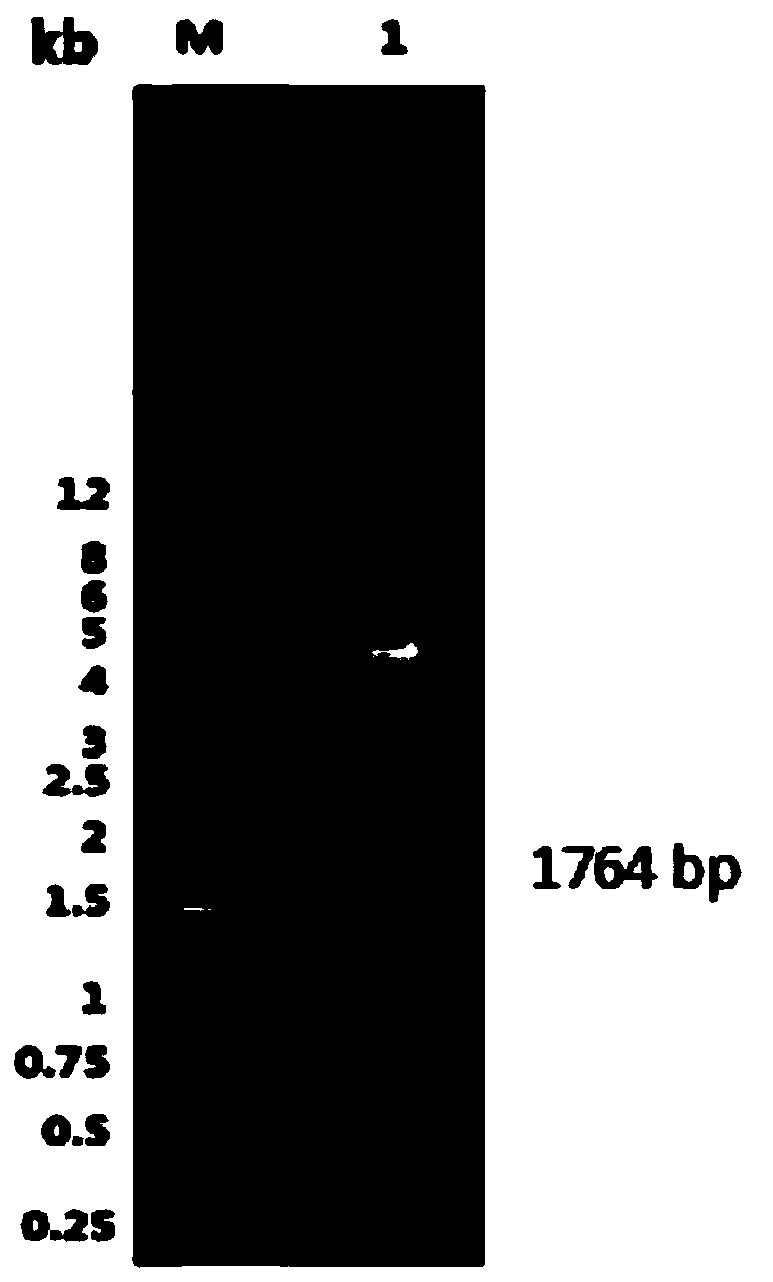
The invention discloses a genetic engineering protein of coronavirus and its application. That is genetic engineering protein FSPA relevant to S protein of coronavirus SARS- CoV and nucleotide sequences amnio sequence and code said FSPA. The invention also discloses a recombinant insect virus strain containing SARS- CoV Spike- recombinant autographa californica nucleopolyhe-drovirus AcNPV- FSPA, CCTCC No.V200513, inserts into the expression box of SARS- CoV Spike. The inventiton also discloses the application of FSPA in checking the coronavirus SARS- CoV, which is the pathogen for acute respiratory syndrome.
Owner:WUHAN UNIV
Antibody testing method for potency of CFS (classical swine fever) genetic engineering subunit vaccine
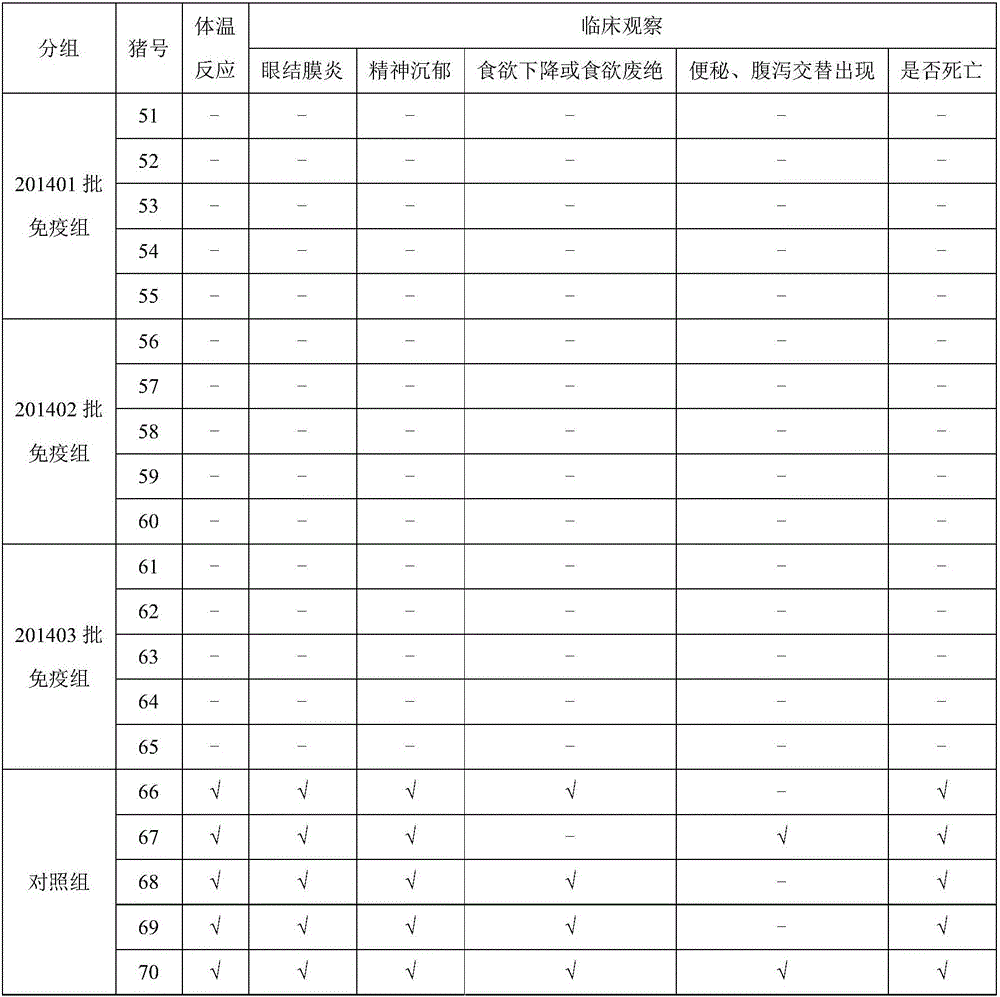
ActiveCN106771186AReduce inspection costsShort inspection periodBiological material analysisBiological testingVirus geneticsBlood sampling
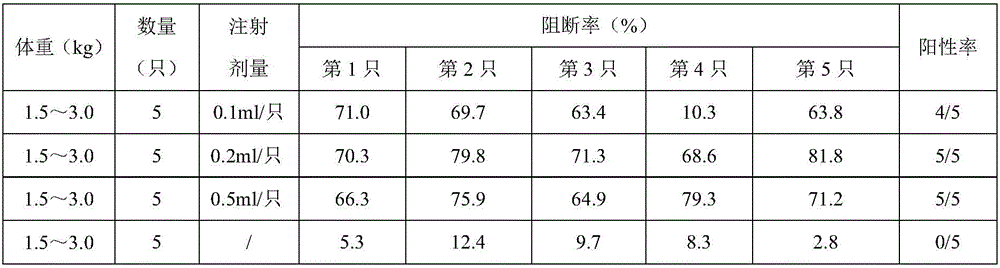
The invention provides a method for testing potency of a CFS (classical swine fever) virus genetic engineering subunit vaccine. The method comprises steps as follows: healthy susceptible rabbits are taken as test objects and divided into a control group and immunization groups; the CFS virus genetic engineering subunit vaccine is injected into rabbits of the immunization groups while no substances are injected to rabbits of the control group; after 21 days of vaccine injection, the rabbits of the immunization groups and the control group are subjected to blood sampling from hearts, heart blood is centrifuged, then liquid supernatant is taken, a CFS ELSIA (enzyme linked immunosorbent assay) antibody is detected, and when at least 4 / 5 of the antibody is positive, the test for the CFS virus genetic engineering subunit vaccine is qualified. The method for testing the potency of the CFS virus genetic engineering subunit vaccine can replace a potency testing method adopting swine for immunity challenge; the test animals, namely, the rabbits, are easily available, so that the testing cost is reduced, the testing period is short, CFS live virus infection is not involved, and biological safety is high.
Owner:YEBIO BIOENG OF QINGDAO
Feline herpes virus antibody sequence, tetrapeptide chain molecule and immunoglobulin molecule
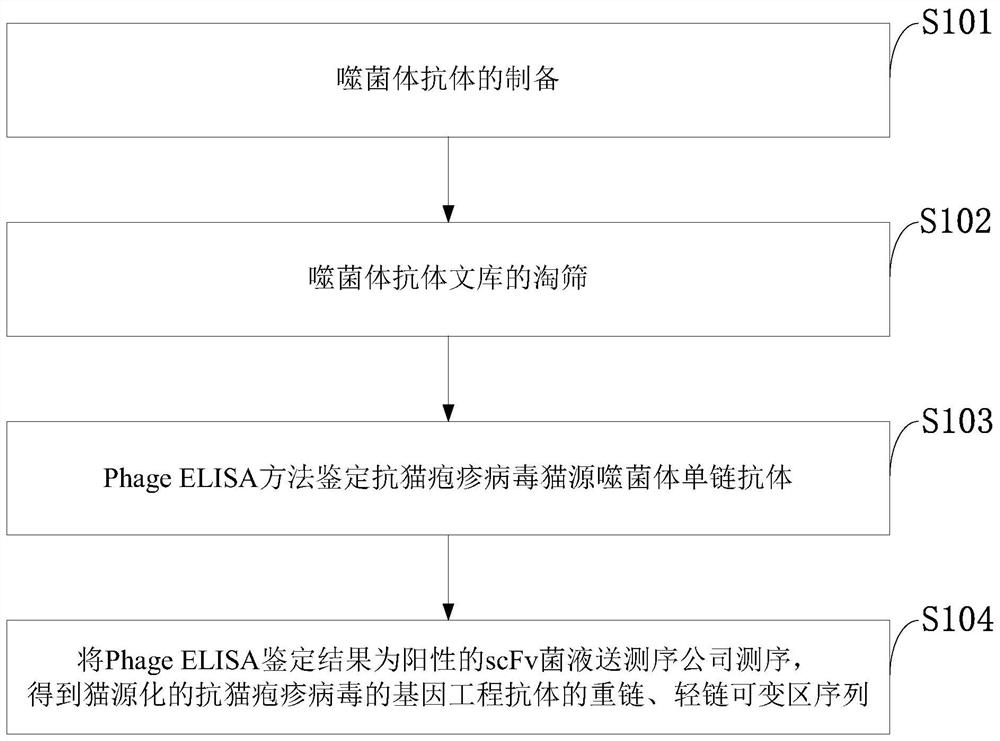
ActiveCN111848785AHomogenizationBiological material analysisImmunoglobulins against virusesPhage antibodiesViral antibody
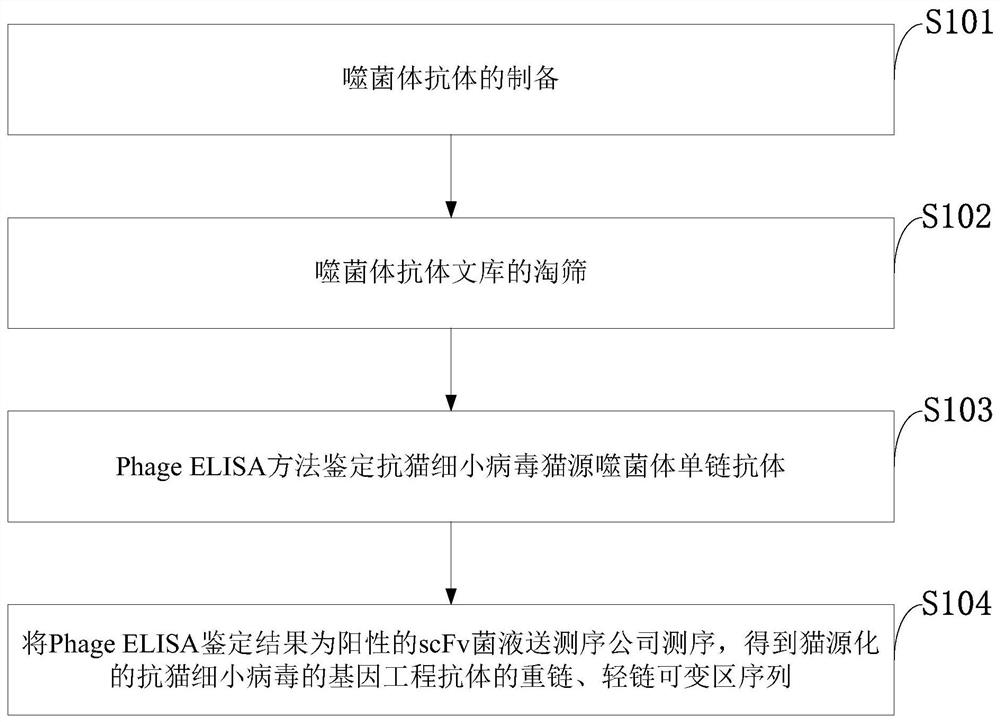
The invention belongs to the technical field of virus antibodies, and discloses a feline herpes virus antibody sequence, a tetrapeptide chain molecule, an immunoglobulin molecule and an application thereof, and the sequences are a heavy chain variable region amino acid sequence SEQ ID NO: 1 and a nucleotide sequence SEQ ID NO: 3; and the amino acid sequence of the light chain variable region is SEQ ID NO: 2 and the nucleotide sequence is SEQ ID NO: 4. The sequence screening method comprises the following steps: preparing a bacteriophage antibody; screening the phage antibody library; identifying an anti-feline herpes virus feline phage single-chain antibody by using a PhageELISA method; sending the scFv bacterial liquid with a positive Phage ELISA identification result to a sequencing company for sequencing to obtain variable region sequences of a heavy chain and a light chain of the feline-derived anti-feline herpes virus genetic engineering antibody. The invention provides support for constructing a feline-derived anti-feline herpes virus genetic engineering antibody with high affinity and low immunogenicity. The product has important significance for promoting the development ofcat-derived antibody drugs.
Owner:青岛博隆基因工程有限公司
Avian bursal disease virus genetic engineering vaccine as well as preparation method and application thereof
InactiveCN111548394AStrong antigen immunityHigh antigen immunityViral antigen ingredientsVirus peptidesProtective antigenBaculovirus expression
The invention provides an avian bursal disease virus genetic engineering vaccine as well as a preparation method and application thereof. The avian bursal disease virus genetic engineering vaccine isprepared by expressing avian bursal disease protective antigen VP2 protein through an insect cell-baculovirus expression system to form avian bursal disease virus-like particles on spatial conformation, adding an adjuvant, and emulsifying. The vaccine provided by the invention is simple in preparation method, capable of preparing a large amount of avian bursal disease virus antigen protein, shortin time consumption, high in expression quantity and beneficial to large-scale production, and the obtained genetic engineering vaccine is good in immune effect and capable of effectively preventing avian bursal disease virus infection.
Owner:乾元浩生物股份有限公司
Maize auxin transport gene ZmABCB15 as well as application thereof in resisting rough dwarf virus
ActiveCN110616225AReduced proliferation rateIncrease resistancePlant peptidesFermentationGermplasmVirus genetics
The invention discloses application of a maize auxin transport gene ZmABCB15 as well as application thereof in resisting rough dwarf virus and aims to solve the technical problem that maize germplasmresources in the prior art cannot meet the current maize production requirement. The maize auxin transport gene ZmABCB15 and transport protein thereof are obtained by differential gene expression analysis, and the auxin transport gene ZmABCB15 can be applied to resistance of plants to rough dwarf virus and breeding of anti-rough dwarf virus plants. The invention confirms for the first time that the expression level of the maize auxin transport gene ZmABCB15 is in positive correlation with resistance of maize to the rough dwarf virus, and the ZmABCB15 gene is over-expressed in maize to improveresistance of maize to the rough dwarf virus; and the study of anti-rough dwarf virus genetic mechanism and disease-resistant molecular breeding of maize.
Owner:HENAN ACAD OF AGRI SCI
Construction method and application of vector vaccine for resisting infectious spleen and kidney necrosis viruses
InactiveCN113476597ANon-pathogenicHigh biosecurityViral antigen ingredientsVirus peptidesRenal necrosisDisease
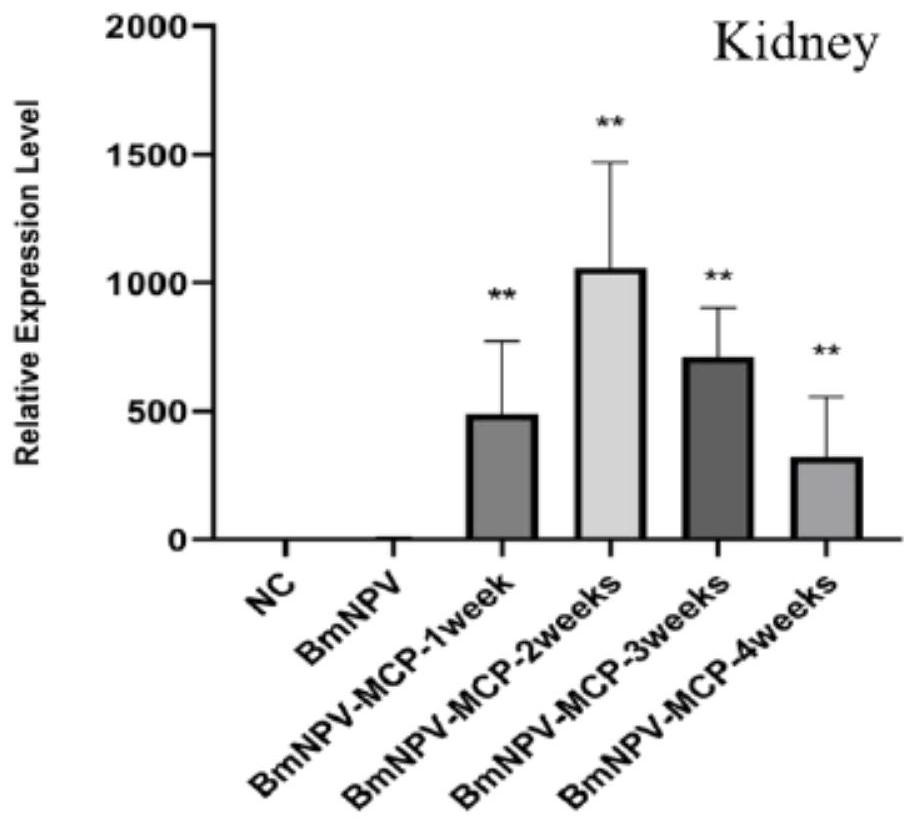
The invention discloses a construction method and application of a vector vaccine for resisting infectious spleen and kidney necrosis viruses, and belongs to the technical field of virus genetic engineering. The vector vaccine comprises an antigen protein MCP expressed by a bombyx mori nuclear polyhedrosis virus and shown as SEQ ID NO: 10, and the antigen protein is encoded by a cmv-mcp expression cassette shown as SEQ ID NO: 1. According to the construction method, the mcp expression cassette cmv-mcp controlled by a cytomegalovirus promoter cmv is cloned to a transfer plasmid to construct a recombinant plasmid, then competent cells are transformed to obtain Bacmid-MCP, the Bacmid-MCP DNA transfects bombyx mori culture cells to obtain BmNPV-MCP, then the BmNPV-MCP is inoculated to larvae or primary pupae of bombyx mori at the age of 4-5, and homogenate is carried out after the disease is attacked to obtain the vector vaccine. The vaccine is used for immunizing siniperca chuatsi / weever through an injection, oral administration or soaking method, and the occurrence of infectious spleen and kidney necrosis diseases can be reduced.
Owner:苏州培恩特生物科技有限公司
Gene sequence modification method based on codon synonymous mutation of codon and its application in vaccine preparation
InactiveCN110305880AIncreased probability of synthesis terminationHigh genetic stabilitySsRNA viruses positive-senseViral antigen ingredientsVirulent characteristicsVirus genetics
The invention discloses a gene sequence modification method based on codon synonymous mutation of codon and its application in vaccine preparation. The method is transformed into TTA, TTG, TCA or TCGby base mutation of a synonymous codon in a sequence. in the process of gene duplication, only one base needs to be mutated to form a stop codon (TAA, TGA, TAG), and the probability of termination ofpeptide chain synthesis by gene mutation is greatly increased, and the species or virus genetic stability thereby can be greatly increased. In the use of an attenuated live vaccine for viruses, safetyis the main problem, and the risk of virulence reversion of the attenuated live vaccine is always existed. In the design of the attenuated live vaccine, synonymous mutation of the codon of the viralgene can improve the fidelity of a vaccine sequence, and the risk of virulence reversion of vaccine poisons in the body is reduced, so that the safety of the attenuated live vaccine is increased.
Owner:安子琛
Carrier pigeon Newcastle disease virus genetic engineering modified attenuated strain as well as preparation method and application thereof
PendingCN114774373AWeak toxicityImprove securitySsRNA viruses negative-senseViral antigen ingredientsF proteinTGE VACCINE
The invention discloses a carrier pigeon Newcastle disease virus genetic engineering modified attenuated strain as well as a preparation method and application of the carrier pigeon Newcastle disease virus genetic engineering modified attenuated strain. The method specifically comprises the following steps: carrying out site-directed mutagenesis on amino acids of three cleavage sites of F protein of a carrier pigeon Newcastle disease virus strain (PNDV-YQ strain) by using a reverse genetic manipulation technology, constructing and obtaining a full-length cDNA plasmid pOK-YQ of a low-toxicity gene in a segmented cloning manner, respectively cloning ORF genes of NP, P and L of the PNDV-YQ strain to an eukaryotic expression vector pCIneo, and carrying out expression by using a recombinant vector pCIneo, according to the invention, helper plasmids pCI-NP, pCI-P and pCI-L are constructed and obtained. After a BSR-T7 cell is co-transfected by the full-length plasmid and a helper plasmid, a recombinant attenuated virus, namely the pigeon Newcastle disease virus (PNDV-aYQ strain), is rescued. The strain of virus is a low-pathogenicity or non-pathogenicity strain, and has good proliferation characteristics and stable hereditary characteristics. Compared with the existing newcastle disease vaccine, the inactivated vaccine prepared from the virus strain has the advantages of low side reaction, early immune production period and high protection rate.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES +1
Porcine parvovirus recombinant pseudorabies virus genetic engineering live vector vaccine and preparation method thereof
InactiveCN102266557AImprove the situationProduction technology safetyAntiviralsAntibody medical ingredientsVector vaccineEngineered genetic
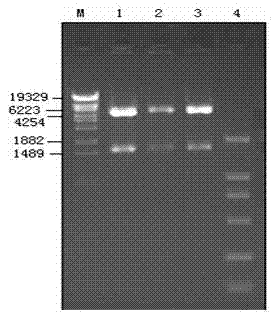
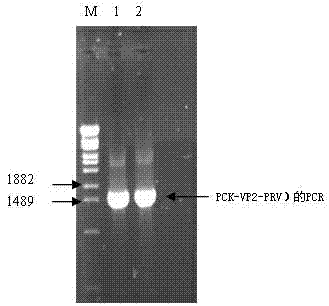
The invention relates to a porcine parvovirus recombinant pseudorabies virus genetically engineered live vector vaccine, the main material of which is composed of porcine parvovirus VP2 gene, porcine pseudorabies virus vector and VERO cells; the vaccine is composed of porcine parvovirus VP2 gene and porcine pseudorabies virus The rabies virus vector is recombined and then obtained by virus screening. The specific method is as follows: first, using the porcine parvovirus NADL-2 strain genome as a template, PCR amplifies the porcine parvovirus VP2 gene; then clones the VP2 gene into the porcine pseudorabies virus vector PCK to obtain the PCK-VP2 plasmid; - VP2 plasmid and pseudorabies virus PRV genome were co-transfected into VERO cells; finally, porcine parvovirus-porcine pseudorabies recombinant virus PCK-VP2-PRV was obtained through screening. Experiments have proved that it has good immune protection in piglets.
Owner:河南亚卫动物药业有限公司
Feline panleukopenia virus antibody sequence, tetrapeptide chain molecule, globulin molecule and application
InactiveCN111909259AHomogenizationHigh affinityBiological material analysisImmunoglobulins against virusesFeline panleukopeniaFeline parvovirus
The invention belongs to the technical field of virus antibodies, and discloses a feline panleukopenia virus antibody sequence, a tetrapeptide chain molecule, an immunoglobulin molecule and application, wherein the feline panleukopenia virus antibody sequence is a heavy chain variable region amino acid sequence SEQ ID NO: 1 and a nucleotide sequence SEQ ID NO: 3; and a light chain variable regionamino acid sequence SEQ ID NO: 2 and a nucleotide sequence SEQ ID NO: 4. A sequence screening method comprises the following steps of preparing a bacteriophage antibody; screening a bacteriophage antibody library; identifying an anti-feline panleukopenia virus feline-derived bacteriophage single-chain antibody by using a PhageELISA method; sending a scFv bacterial liquid with a positive PhageELISAidentification result to a sequencing company for sequencing to obtain variable region sequences of a heavy chain and a light chain of the feline-derived anti-feline panleukopenia virus genetic engineering antibody. The invention provides support for the construction of feline-derived anti-feline panleukopenia virus genetically engineered antibodies with high affinity and low immunogenicity. Themethod has important significance for promoting the development of feline-derived antibody drugs.
Owner:青岛博隆基因工程有限公司
Canine distemper virus replication defect strain and establishing method thereof
The invention relates to rescuing and verifying of a canine distemper virus replication defect strain of a canine distemper virus. A system comprises a transcription plasmid, one or more auxiliary plasmids and a Vero-SLAM-M cell line, wherein the transcription plasmid pCI-CDV-SD16F can express the genome full-length cDNA sequence of the canine distemper virus prevalent strain SD16F, the plasmid pCI-CDV-SD16F-M subjected to fixed-point mutation is a recombinant plasmid not expressing the protein M, the auxiliary plasmids can express the nucleoprotein (NP), phosphoprotein (P) and large polymerase protein (L) of the canine distemper virus prevalent strain SD16F, and the Vero-SLAM-M cell line can stably express proteins SD16FM. Through the reverse genetic operation system, the recombinant replication defect canine distemper virus is successfully rescued. Through the research, the canine distemper virus prevalent replication defect strain creates convenient conditions for a novel canine distemper virus genetic engineering biological control preparation and provides an excellent technological platform for the canine distemper virus related basic research.
Owner:QINGDAO AGRI UNIV
Duck Tembusu virus genetically engineered subunit vaccine and its preparation method and application
ActiveCN110237244BNot pathogenicReduce manufacturing costSsRNA viruses positive-senseViral antigen ingredientsVaccine ProductionNucleotide
The present invention provides a duck tambosu virus genetic engineering subunit vaccine and a preparation method and an application thereof. A nucleic acid molecule having a sequence shown in SEQ ID NO:1 or a nucleic acid molecule identical to 95% or more of the nucleotide sequence shown in the SEQ ID NO:1 encodes a duck tambosu virus E2 protein, an immune composition comprising the duck tambosu virus E2 protein can be used to prepare the duck tambosu virus genetic engineering subunit vaccine, antigenicity, immunogenicity and functions of the vaccine are similar to natural proteins, and the vaccine is relatively high in expression level, strong in the immunogenicity, free of pathogenicity to ducks, and can also be prepared by large-scale serum-free suspension culture in a bioreactor, which greatly reduces a production cost of the vaccine.
Owner:苏州沃美生物有限公司
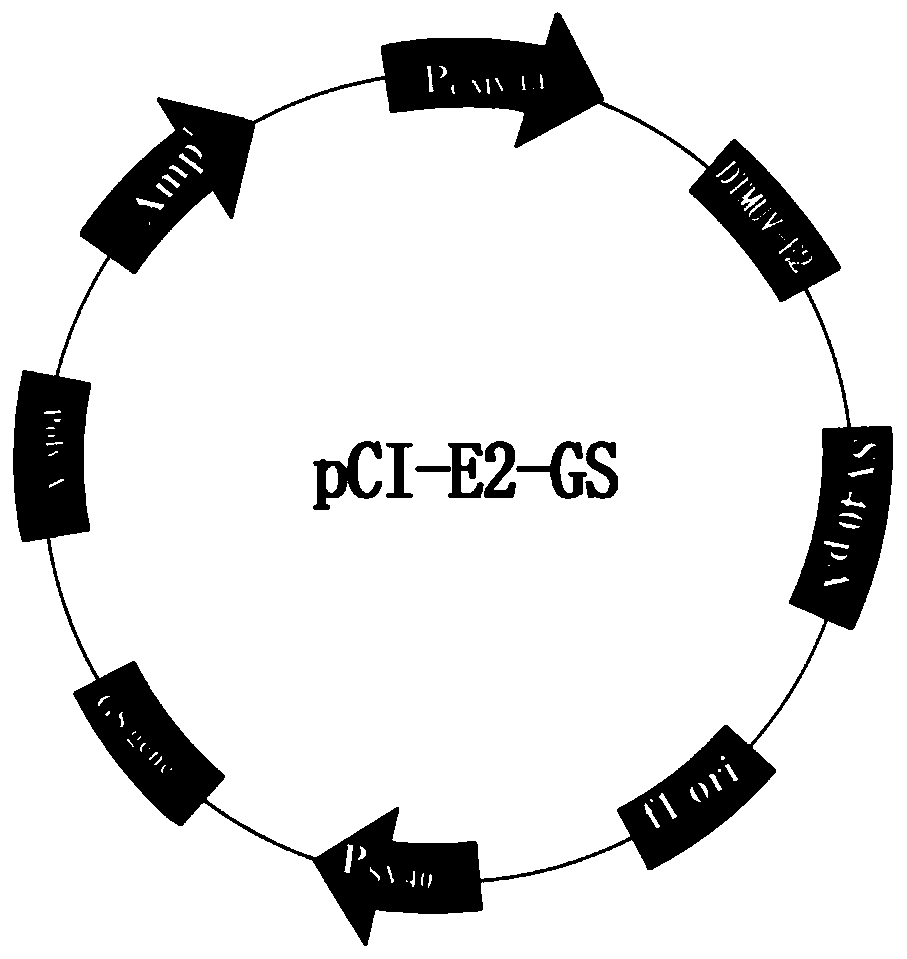
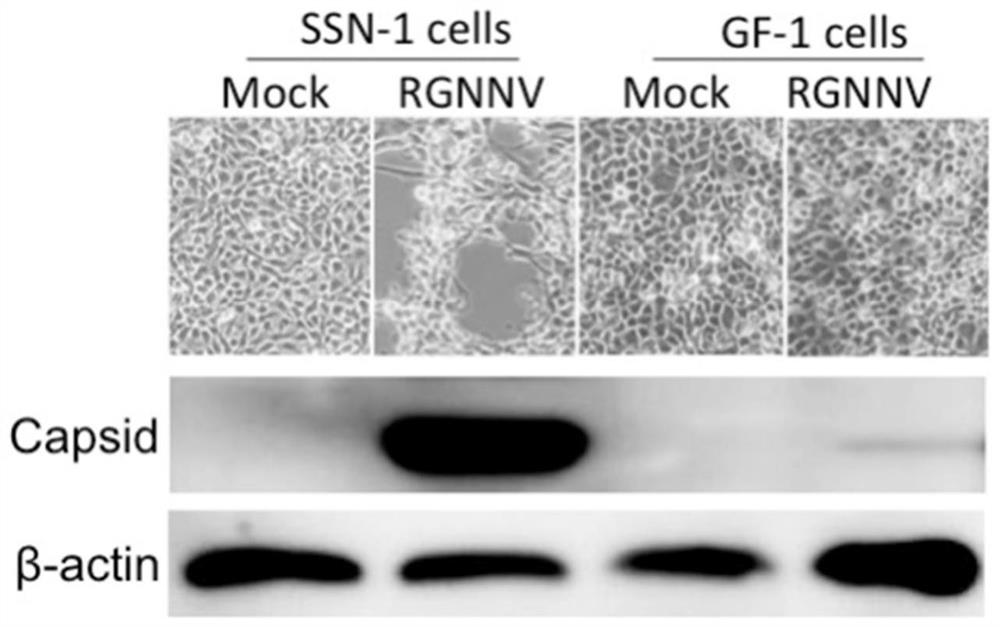
A method for constructing a reverse genetic system of red-spotted grouper neuronecrosis virus
ActiveCN111471710BEasy to saveOvercoming difficult problemsSsRNA viruses positive-senseMicroorganism based processesNecrovirusAquatic animal
The invention belongs to the technical field of aquatic animal virus genetic engineering, and in particular relates to a method for constructing a red-spotted grouper nervous necrosis virus (RGNNV) reverse genetics system. The present invention uses CMV as a promoter to construct a eukaryotic expression plasmid containing the RGNNV genome, and adopts a method of combining human embryonic kidney 293T cells with high transfection efficiency and striped snakehead cells (SSN‑1) that can efficiently replicate RGNNV To rescue RGNNV, it overcomes the problem of low transfection efficiency of fish cells.
Owner:HUAZHONG AGRI UNIV
Tobacco mosaic virus gene fragment, attenuated vaccine, preparation method and application thereof for efficient production of siRNA
ActiveCN110857438BEfficient off-targetReduce harmSsRNA viruses positive-senseBacteriaNicotiana tabacumTobacco mosaic virus
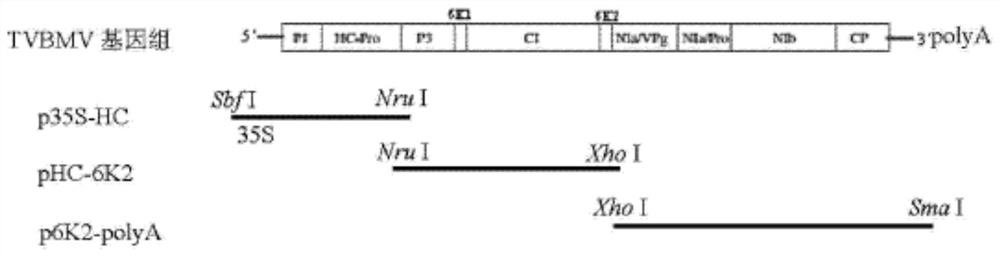
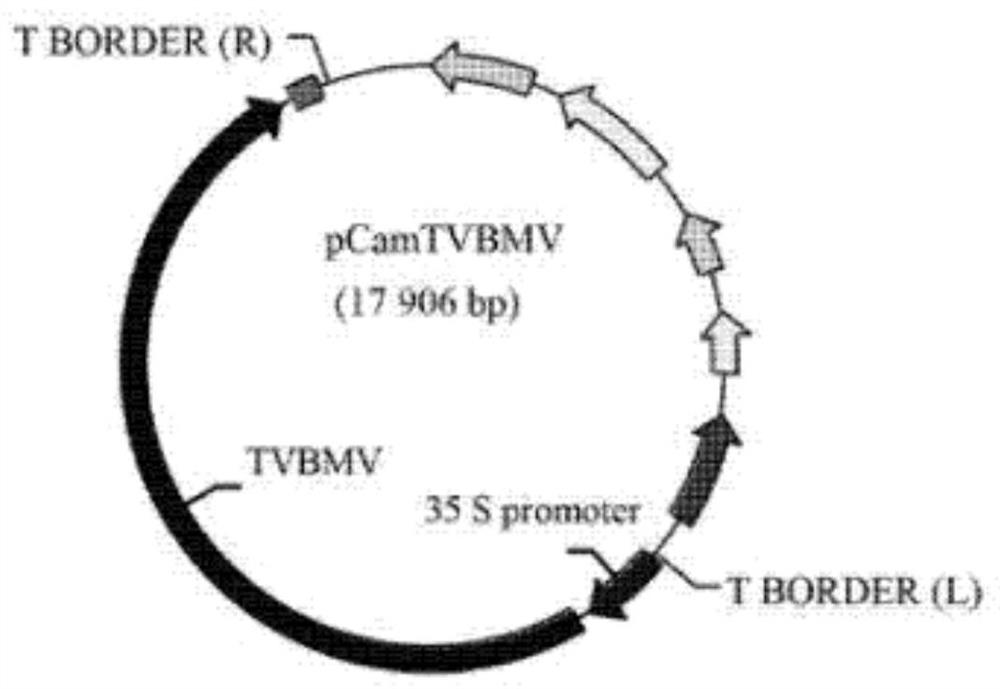
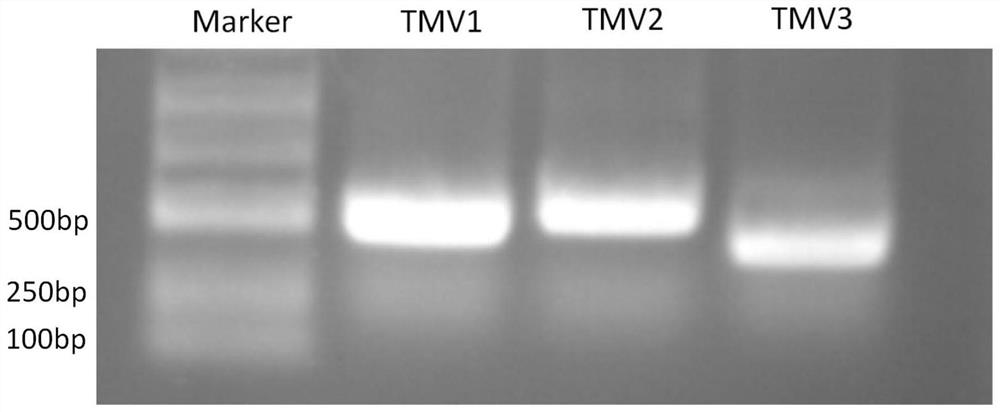
The invention relates to the field of plant anti-virus genetic engineering, and discloses a tobacco mosaic virus gene fragment for efficiently producing siRNA, an attenuated vaccine, a preparation method and an application thereof. The tobacco mosaic virus gene fragments that efficiently produce siRNA include at least one of the TMV1 fragment, the TMV2 fragment and the TMV3 fragment, and the nucleotide sequences of the TMV1 fragment, the TMV2 fragment and the TMV3 fragment are respectively Seq ID No.13, Seq ID No. 14. As shown in Seq ID No.15, the gene fragment can efficiently produce siRNA after being inoculated with parasitic plants. The attenuated vaccine is based on the attenuated TVBMV mutant, and the TVBMV attenuated mutant is embedded with an effective gene fragment that can induce cross-protection against tobacco mosaic virus, and the effective gene fragment includes a tobacco mosaic virus gene fragment that can produce siRNA. The anti-tobacco mosaic virus attenuated vaccine of the present invention has a stable effect, can play an effective cross-protection role, significantly reduces the damage of plants infected by strong tobacco mosaic virus strains, delays the onset of plants, and greatly reduces losses.
Owner:中国烟草总公司黑龙江省公司烟草科学研究所 +1
Pseudorabies virus genetically engineered gb recombinant attenuated vaccine strain and its application
ActiveCN106929485BImproving immunogenicityEasy to solveViral antigen ingredientsVirus peptidesRabiesVariant strain
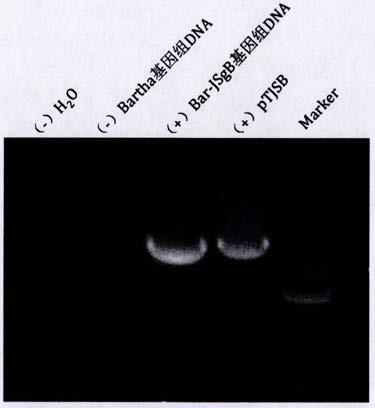
The invention discloses a pseudorabies virus gene engineering gB recombinant attenuated vaccine strain and application thereof. CRISPR / Cas9 is combined with techniques such as homologous recombination, and a genome of a pseudorabies virus vaccine strain Bartha-K61 is taken as a framework, and a recombinant pseudorabies virus strain Bar-JS-gB (BJB) is successfully acquired by replacing a gB gene of the Bartha-K61 with a gB gene of an epidemic pseudorabies virus variant JS-2012 in China. The strain BJB has stable heredity, and an immune mouse processed by an inactivated vaccine prepared by taking the strain BJB as a vaccine strain has relatively good capacity of effectively resisting attacking of a virulent strain JS-2012 than an immune mouse processed by an inactivated vaccine prepared from the Bartha-K61. Therefore, the strain BJB has relatively good immunogenicity, can be used for effectively controlling the prevalence of a PRV variant in China and can play an important role on control of pseudorabies.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Family of synthetic polynucleotide-binding peptides and uses thereof
The present invention provides novel synthetic peptides (including the TZIP peptide) as oncogenic and genetic modulators, including genetics of viruses, as well as methods of making and using the same. These peptides are useful for inhibiting the proliferation of cancer cells characterized as having amplified c-MYC genes. The invention provides methods for the therapeutic uses of the peptides in the treatment of various cancers including small cell lung carcinoma, prostate cancer, lymphoma, brain tumors, colon cancer, bladder cancer, AML, malignant melanoma, mesothelioma, and cancers of head and neck. The peptides are also useful in the treatment of and prevention of transmission of HIV and treatment of expanded nucleotide repeat diseases, including certain currently untreatable and debilitating diseases.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL
A method for preparing gosling plague virus-like particles using Escherichia coli system
ActiveCN106754981BGood reactogenicityVirus peptidesInactivation/attenuationEscherichia coliTGE VACCINE
The invention relates to a method for preparing gosling plague virus-like granules with an escherichia coli system for soluble expression of gosling plague virus VP2 protein. The method for soluble expression of gosling plague virus VP2 protein comprises the following steps: performing codon optimization on a gosling plague virus VP2 gene, performing site-specific mutagenesis, namely, mutating a codon AGA into CGC and mutating GGA into GGT, cloning to a pET-Sumo vector, establishing a recombinant expression vector pET-Sumo-VP2, transforming the pET-Sumo-VP2 into a prokaryotic expression bacterium, and inducing with IPTG (isopropyl beta-D-1-Thiogalactopyranoside) at 37 DEG C so as to obtain soluble recombinant VP2 recombinant protein; and performing digestion on the recombinant protein with a ULP enzyme, and purifying with a Ni column, thereby obtaining purified VP2 protein. Electron microscope results show that the gosling plague virus-like granules can be prepared from VP2 protein after digestion, and moreover, the purified VP2 protein has good reactogenicity and can be applied to preparation of subunit vaccines of gosling plague virus genetic engineering.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Coronal virus genetic engineering protein and use thereof
InactiveCN100540564CImprove expression efficiencyNon-pathogenicDepsipeptidesViruses/bacteriophagesGenetically engineeredPolyhedral virus
The invention discloses a coronavirus genetically engineered protein and its application. The genetically engineered protein FSPA related to the S protein of coronavirus SARS-CoV and the nucleotide sequence and amino acid sequence encoding the FSPA. A recombinant insect virus strain containing SARS-CoV Spike gene - recombinant Autographa californica nuclear polyhedrosis virus strain AcNPV-FSPA, CCTCC NO.V200513, the recombinant virus strain is inserted into the SARS-CoV Spike expression cassette. Application of genetically engineered protein FSPA in detecting the pathogen of severe acute respiratory syndrome—coronavirus SARS-CoV.
Owner:WUHAN UNIV
Construction method and application of recombinant prrs virus genetic engineering vaccine expressing classical swine fever virus e2 protein
ActiveCN104561092BImprove securityEffectively induce immune responseViral antigen ingredientsAntiviralsClassical swine fever virus CSFVHighly pathogenic
The invention provides a method for constructing recombinant plasmids of porcine reproductive and respiratory syndrome virus (PRRSV) capable of expressing classic swine fever virus (CSFV) E2 protein, and a genetically engineered vaccine constructed according to the recombinant plasmids. Based on a reverse genetic operating platform, pA-SM-E2 and pA-C-E2 recombinant plasmids are constructed according to E2 genes of CSFV Shimen strains and hog cholera virus strains C, and genes of highly pathogenic porcine reproductive and respiratory syndrome virus cell attenuated vaccine strains HuN4-F112. After MARC-145 cells are transfected, live viruses are successfully rescued, and a situation that recombinant viruses (vA-SM-E2 and vA-C-E2) and a parental virus vHuN4-F112 have similar virological characteristics is found. After the vA-SM-E2 is selected for carrying out an immunological experiment on porcine bodies, the vA-SM-E2 has good security to the porcine bodies, and the vA-SM-E2 after being immunized can induce the bodies to produce immune response so as to produce high-level PRRSVN protein and CSFV E2 protein based antibodies, therefore, the vA-SM-E2 is a candidate strain of the genetically engineered vaccine.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Fishes infectious spleen and kidney necrosis virus gene engineering vaccine and preparation method thereof
InactiveCN100566752CPromote growthSimple preparation processViral antigen ingredientsAntiviralsAntigenDisease
The present invention proposes a fish infectious spleen-kidney necrosis virus genetic engineering vaccine and a preparation method thereof. The genetic engineering vaccine proposed by the present invention contains two gene recombinant protein antigens protein-24 and protein-50, which are respectively screened from The two open reading frames ORF24 and ORF50 in the ISKNV genome were cloned, recombinant plasmids were prepared, and recombinant proteins were expressed. The vaccine is a recombinant protein vaccine that can inhibit the infection of infectious spleen and kidney necrosis virus to fish, so that aquatic fish can grow healthily, reduce the loss of aquaculture in terms of disease, increase aquatic production and efficiency, and avoid DNA vaccines The existing disadvantages.
Owner:SUN YAT SEN UNIV
Antibody testing method for efficacy of a classical swine fever genetically engineered subunit vaccine
ActiveCN106771186BReduce inspection costsShort inspection periodBiological material analysisBiological testingGenetic engineeringOrganism
The invention provides a method for testing potency of a CFS (classical swine fever) virus genetic engineering subunit vaccine. The method comprises steps as follows: healthy susceptible rabbits are taken as test objects and divided into a control group and immunization groups; the CFS virus genetic engineering subunit vaccine is injected into rabbits of the immunization groups while no substances are injected to rabbits of the control group; after 21 days of vaccine injection, the rabbits of the immunization groups and the control group are subjected to blood sampling from hearts, heart blood is centrifuged, then liquid supernatant is taken, a CFS ELSIA (enzyme linked immunosorbent assay) antibody is detected, and when at least 4 / 5 of the antibody is positive, the test for the CFS virus genetic engineering subunit vaccine is qualified. The method for testing the potency of the CFS virus genetic engineering subunit vaccine can replace a potency testing method adopting swine for immunity challenge; the test animals, namely, the rabbits, are easily available, so that the testing cost is reduced, the testing period is short, CFS live virus infection is not involved, and biological safety is high.
Owner:YEBIO BIOENG OF QINGDAO
Ankara vaccinia virus genetic engineering vaccine for pig replication and respiration complex
InactiveCN101209351BImprove immunityHigh protein expressionGenetic material ingredientsAntiviralsBALB/cEpitope
The invention discloses a porcine reproductive and respiratory syndrome-Ankara vaccinia virus genetic engineering vaccine, the preparation method is that: 1. the PCR amplification of GP5 gene is carried out from the natural porcine reproductive and respiratory syndrome virus; 2. the porcine preferred codon is used for replacing the codon in the natural GP5 gene, so as to carry out GP5 gene optimization and the synthesis of the optimized sequence GP5A; 3. the epitope A in the optimized GP5A gene is replaced by the epitope B of the GP5 gene, so as to obtain the GP5-DB gene; 4. the GP5 and GP5-DB genes are respectively cloned to the expression carrier JN-2; 5. the constructed JN-2-GP5 and the JN-2-GP5-DB plasmid are recombined with Ankara vaccinia virus, so as to obtain the porcine reproductive and respiratory syndrome GP5 and GP5-DB gene Ankara vaccinia virus recombinant vaccine. Experiments confirm that the invention has excellent immune protection in BALB / c mice and pigs.
Owner:WUCHANG SHIPBUILDING IND
Ivermectin preparation as well as preparation method and application thereof
ActiveCN113995845ADefense against invading infectionsFacilitate targeted deliveryOrganic active ingredientsCell dissociation methodsIntracellularGenetic Materials
The invention discloses an ivermectin preparation as well as a preparation method and an application thereof. The ivermectin preparation is prepared by taking an exosome as a drug carrier to load ivermectin. According to the invention, the exosome generated by ACE2 positive human cells is used as a carrier, so that a virus taking ACE2 as a binding target can be bound with the carrier, on one hand, the binding degree of the virus and the human cells can be partially closed, and the virulence of the virus is reduced, and on the other hand, after the virus is bound with the ACE2 positive exosome preparation, when infection occurs, Ivermectin can enter cells together with virus genetic materials to prevent virus replication in target cells. According to the invention, infection of viruses such as SARS-CoV-2 and the like on ACE2 positive cells can be effectively inhibited.
Owner:SOUTHEAST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com