Patents
Literature
94 results about "Solid oxygen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Solid oxygen forms at normal atmospheric pressure at a temperature below 54.36 K (−218.79 °C, −361.82 °F). Solid oxygen O₂, like liquid oxygen, is a clear substance with a light sky-blue color caused by absorption in the red part of the visible light spectrum.
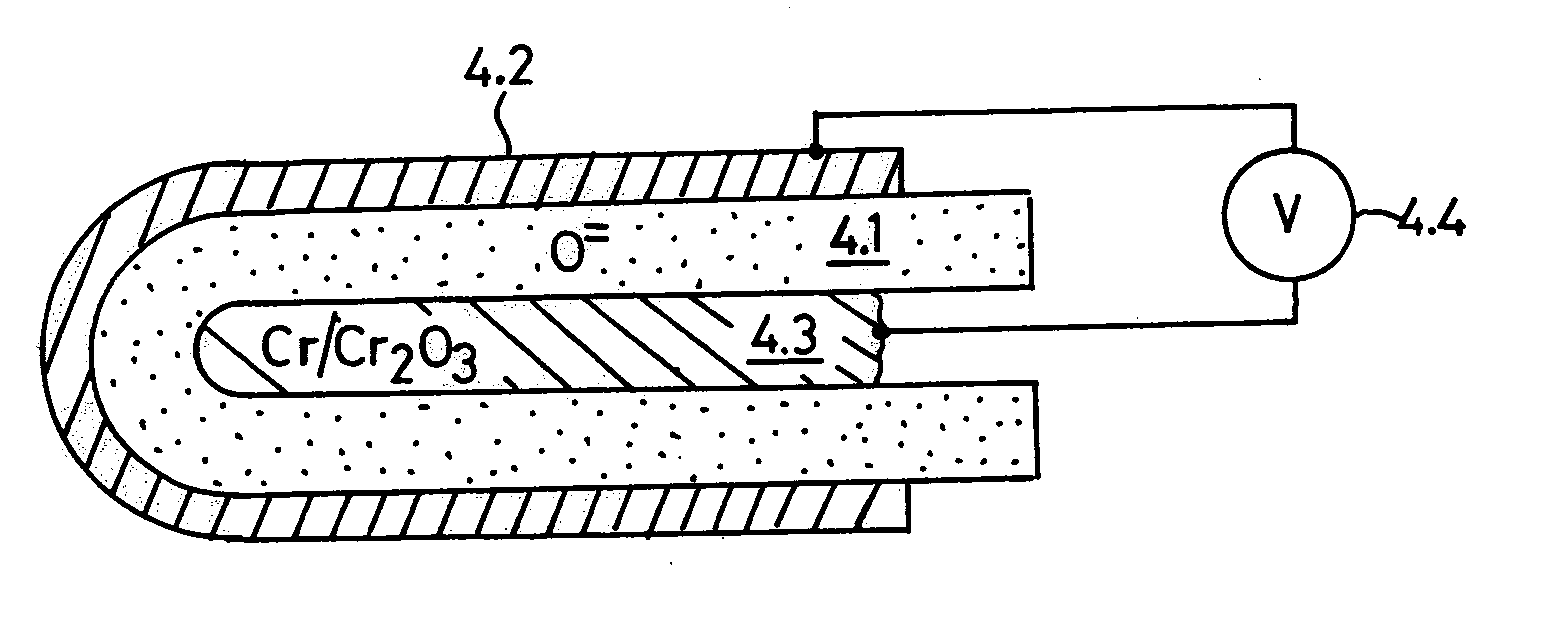
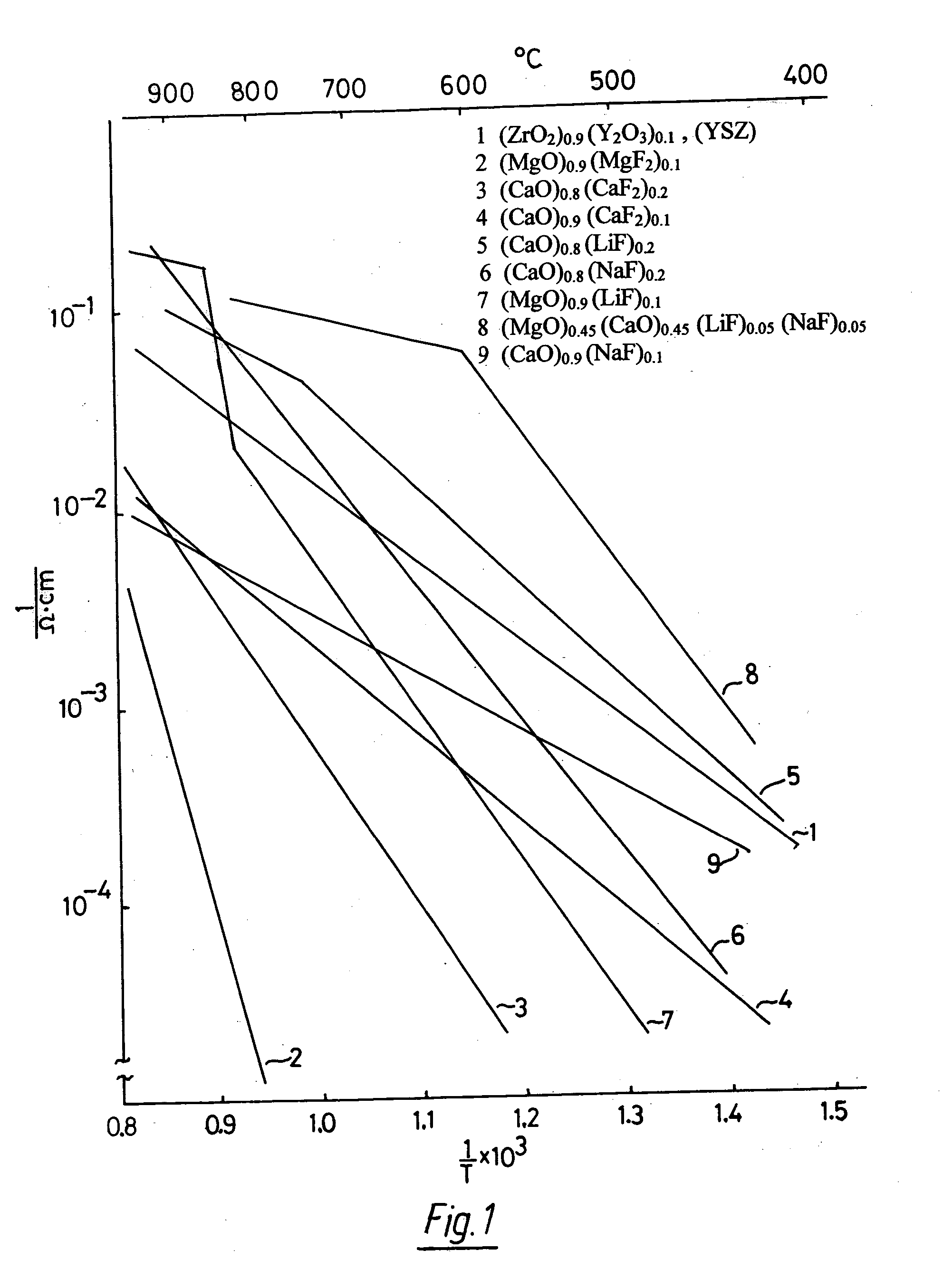
Oxygen ion conductors for electrochemical cells
InactiveUS20070054170A1Stable oxygen concentrationImprove electrode stabilityCell electrodesFinal product manufactureElectrical conductorAlkaline earth metal
In solid oxygen ion conducting electrolytes for electrochemical cells based on magnesium oxide and calcium oxide, obtained by the addition of metal fluorides selected from elements in the groups of alkali metals and earth alkali metals to the host oxides of magnesium and calcium, conductivity values are obtained, which are comparable with those of stabilized zirconia, but the magnesium oxide and calcium oxide based oxygen ion conducting electrolytes have a superior thermodynamic stability and, therefore, can operate at much lower oxygen concentrations in comparison with other oxygen ion conducting electrolytes and without becoming noticeably electronically conductive.
Owner:ISENBERG ARNOLD O
Novel multifunctional compound bio-fertilizer and preparation method thereof
The invention relates to a novel multifunctional compound bio-fertilizer which is prepared from the following components: humic acid, fermented feces of livestock and poultry, turf, amino acid raw powder, bacillus megatherium, bacillus laterosporus, bacillus mucilaginosus, chelating state trace elements, bentonite, zeolite, hydrotalcite, bactericide, and a sodium percarbonate type solid oxygen producer. The compound bio-fertilizer provided by the invention can ensure no loss of inorganic trace elements, and is helpful for plant growth.
Owner:赤峰蒙鼎生物科技有限公司 +1
Solid oxygen scavenger composition and process for producing the same
ActiveUS20090159846A1Increase in sizeReduce air permeabilityPigmenting treatmentHydrogenScavengerShell molding
A molded oxygen absorbent composition is composed of a molded product of an oxygen absorbent composition which contains an oxygen absorbing substance, water or moisture, and a swelling component capable of being swelled with water or moisture. The molded oxygen absorbent composition is reduced in its size and excellent in oxygen absorbing property.
Owner:MITSUBISHI GAS CHEM CO INC
Method for directly preparing titanium and titanium alloy by titanium-containing waste residue
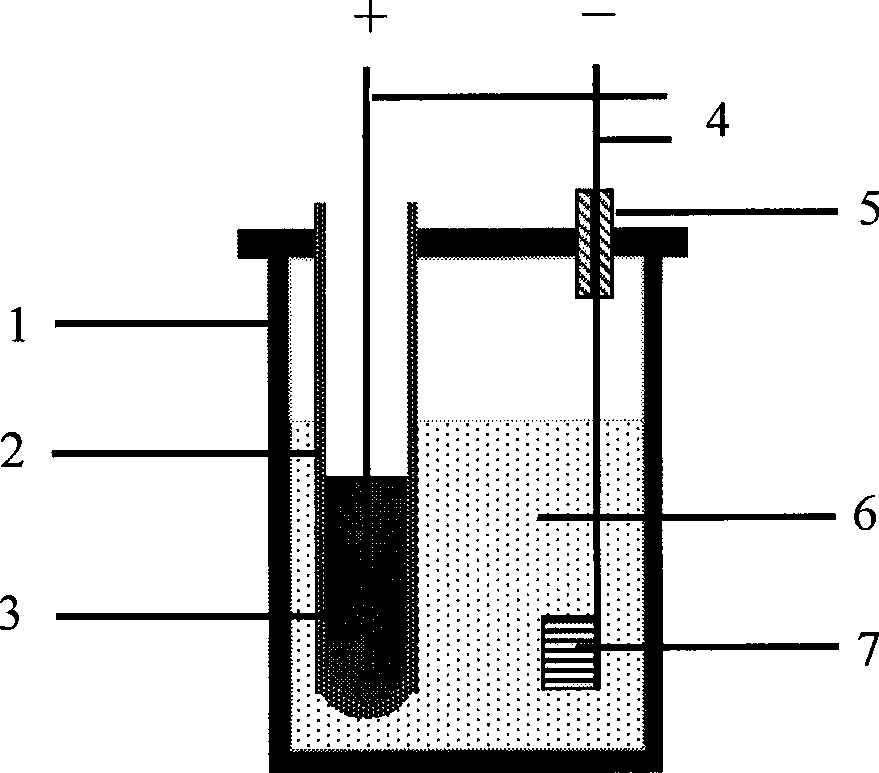
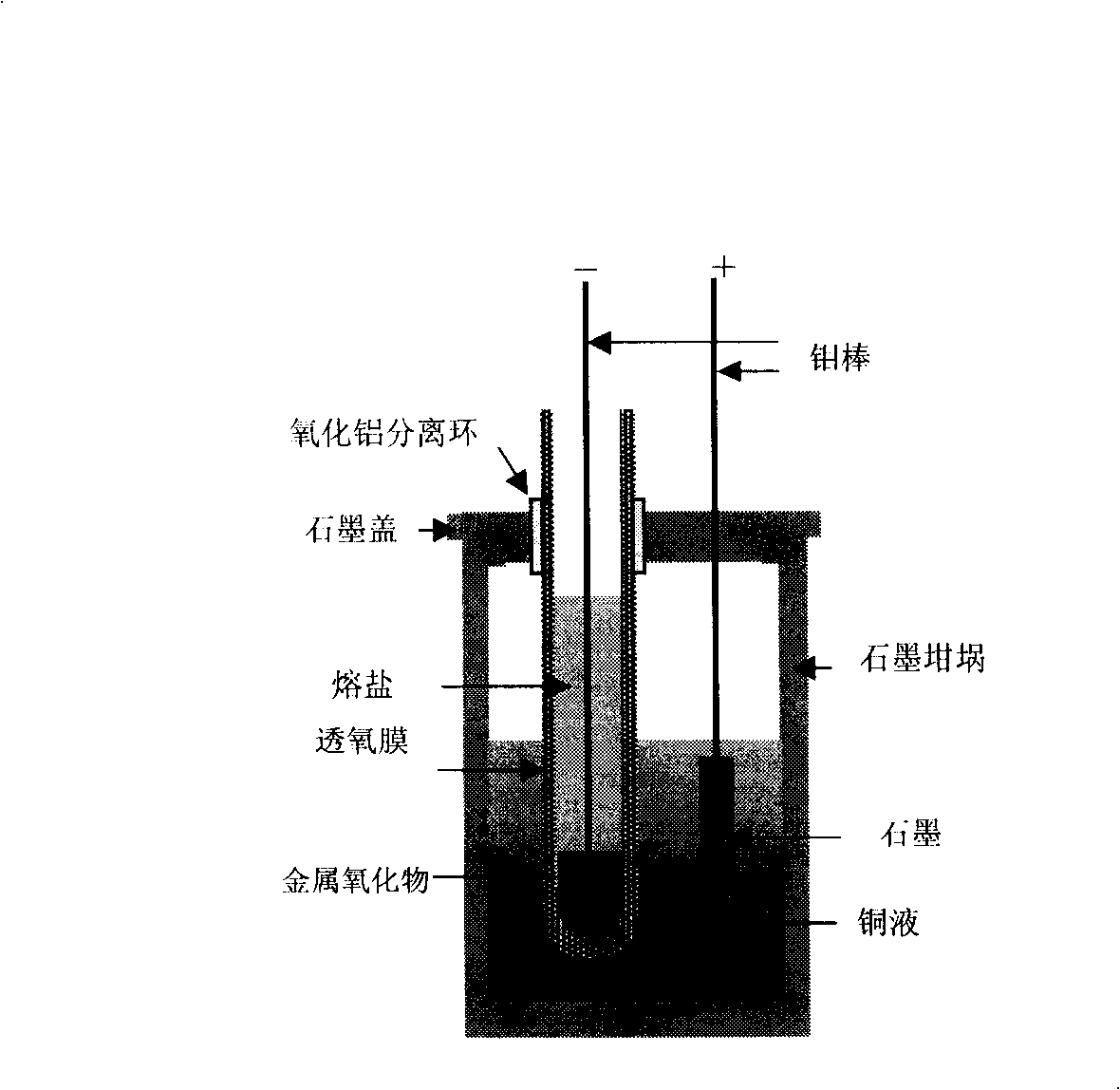
The invention relates to a method for directly preparing titanium and titanium alloy from titaniferous waste residues, and belongs to the technical field of electrochemical metallurgy. The method is characterized in that the titaniferous waste residues are made into an electrolysis electrode, and the electrolysis is performed in a crucible of an electrolytic cell by a solid oxygen permeable membrane in a specific molten salt electrolyte. The method comprises the following process steps: the titaniferous residues is processed by ball milling, then pressed and formed in a mould at 3-6MP, dried in the air, and then sinteredat the temperature of 1000-1100 DEG C for 2h to be made into an electrolysis cathode; molten salt is separated from an anode by an oxygen permeable membrane tube which only conducts oxyanion, the anode is liquid copper or copper alloy with saturated carbon powder, calcium chloride is taken as the molten salt electrolyte, the cathode and the anode are vertically arranged, the electrolytic temperature is 1000-1100 DEG C, the electrolytic voltage is 3.0-3.5V, the electrolysis time is 2-6h, and the cathodic products obtained by the electrolysis are metallic titanium and the titanium alloy. The method has the advantages of simple process, high electrolysis speed and high current efficiency.
Owner:SHANGHAI UNIV
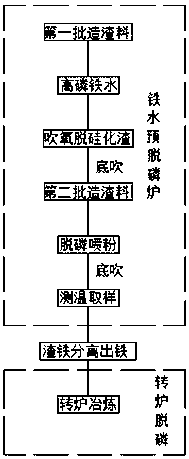
Double-smelting dephosphorization process for high-phosphorus molten iron
ActiveCN107723412AReduce the temperatureEasy to handleManufacturing convertersSteelmakingSmelting process
The invention discloses a double-smelting dephosphorization process for high-phosphorus molten iron. The double-smelting dephosphorization process comprises a pre-dephosphorization process and a converter smelting process. The pre-dephosphorization process comprises the steps that pre-dephosphorization is performed by the adoption of a pre-dephosphorization furnace; a desiliconization slagging material is added to the high-phosphorus molten iron, and oxygen injection is performed for desiliconization slagging; the desiliconization slagging material is put into the furnace after desiliconization slagging, a solid oxygen agent is put into the furnace after dephosphorization slagging, meanwhile dephosphorization agent injection is performed, and inert gas bottom-blowing is performed so that mixing can be strengthened; and the pre-dephosphorized molten iron passes iron-slag separation equipment and then enters the converter smelting process. The dephosphorization process has the beneficialeffects of being stable, low in temperature, large in treatment capacity and the like, and the problem about high-phosphorus ultrahigh-phosphorus molten iron dephosphorization is solved thoroughly. The double-smelting dephosphorization process is suitable for high-phosphorus molten iron and ultrahigh-phosphorus molten iron, and the dephosphorization rate of pre-dephosphorization can reach 64-71%.According to the process, the molten iron pre-dephosphorization furnace and converter double-smelting are adopted, the high-phosphorus ultrahigh-phosphorus molten iron smelting operation can be achieved, the converter steelmaking load is lowered greatly, steelmaking is more efficient, and the smoothness and stability of the production procedures are guaranteed.
Owner:唐钢国际工程技术有限公司
Solid oxygen scavenger composition and process for producing the same
ActiveUS20110172091A1Increase in sizeReduce air permeabilityPigmenting treatmentHydrogenScavengerMoisture
A molded oxygen absorbent composition and a process of producing the molded oxygen absorbent composition are disclosed. The molded oxygen absorbent composition is composed of a molded product of an oxygen absorbent composition which contains an oxygen absorbing substance, water or moisture, and a swelling component capable of being swelled with water or moisture. The product is formed by pressure molding the composition. The molded oxygen absorbent composition is reduced in its size and excellent in oxygen absorbing property.
Owner:MITSUBISHI GAS CHEM CO INC
Process for preparing titanium sponge from titanium oxide composite ore
The present invention relates to a method for preparing spongy titanium by using titanium oxide composite ore, and said method includes the following steps: firstly, liquating the composite ore raw material containing titanium dioxide is a selected molten-salt system under the condition of 1000-1100 deg.C, then inserting the graphite cathode and tubular solid oxygen permeable membrane whose internal surface is covered with a porous metal ceramic coating layer as anode into the above-mentioned mixed molten-salt in a heating container or crucible together, and introducing hydrogen gas in the high-temp. ceramic pipe of anode end to make electrolysis under the action of a certain voltage and current of D.C. power supply, and finally obtaining metal titanium on the cathode end.
Owner:SHANGHAI UNIV +1
Method for directly preparing titanium alloy by titanium concentrate powder
The invention relates to a method for directly preparing titanium alloy by titanium concentrate powder, and belongs to the technical field of the preparation process for metal titanium alloys. The method mainly comprises the following steps: taking Gossampinus titanium concentrate powder as a raw material to obtain a fine material of titanium concentrate through ball milling and screening; then performing tabletting and sintering; winding round tablets on a molybdenum rod by using a fine molybdenum wire to prepare a cathode, or sintering the ore concentrate powder to form circular sheets, putting the circular sheets into a small stainless steel crucible directly, immersing the integral small stainless steel crucible into molten salt in an electrolytic cell, leading the small stainless steel crucible out by using the molybdenum wire as the cathode; taking copper solution of saturated carbon dust in a solid oxygen-permeable membrane tube as an anode; taking a corundum crucible as a reaction vessel, and taking CaCl2 as a molten salt electrolyte; taking high-purity argon as protective gas; performing electrolysis at a temperature of 1,100 DEG C under the constant voltage of between 3.5 and 4.0 V for 2 to 6 hours, taking the electrolyzed cathode out, and washing and drying the electrolyzed cathode to prepare the titanium alloy. The method has the characteristics of short flow, high efficiency, no pollution, low cost and the like.
Owner:SHANGHAI UNIV
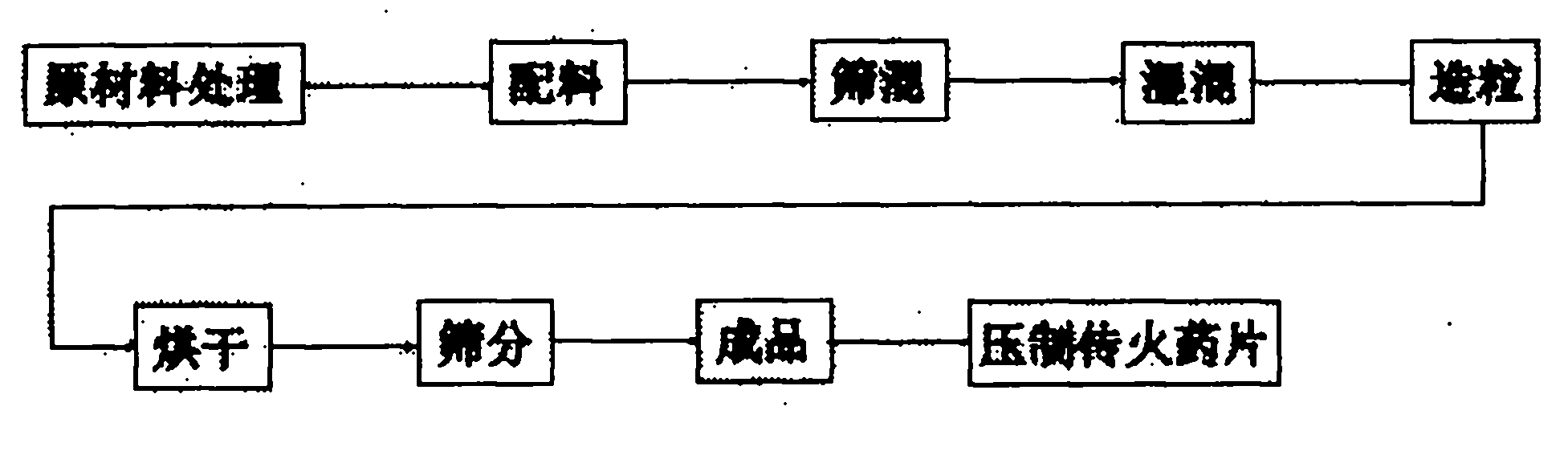
Ignition drug for lifesaving solid oxygen and preparation method thereof
ActiveCN103204755AReliable ignitionHigh energyExplosive working-up apparatusPerchlorate saltSodium chlorate
The invention provides an ignition drug for lifesaving solid oxygen and a preparation method thereof. The ignition drug is prepared from the following raw materials by mass percent by using a compacting technology: 32-44 % of sodium chlorate, 20-30 % of potassium perchlorate, 14-18 % of iron powder, 4-8 % of titanium powder, 5-12 % of cobalt chloride, 3-7 % of ferric oxide, 0.5-5 % of barium peroxide and 0.1-1.5 % of mica powder. The method solves problems of poor technology quality and low efficiency existing in prior art. According to the invention, electric igniter or ignition by mechanically knocking primer is employed, and thus energy is relatively high, impact force is large and solid-oxygen columns can be reliably ignited; residues do not flow after the ignition drug burns, thereby ensuring product safety; both high and low temperature performances satisfy corresponding product technology standards, remote automatic control is realized to start solid oxygen, and production quality and production efficiency of the ignition drug can be improved.
Owner:HUBEI INST OF AEROSPACE CHEMOTECHNOLOGY
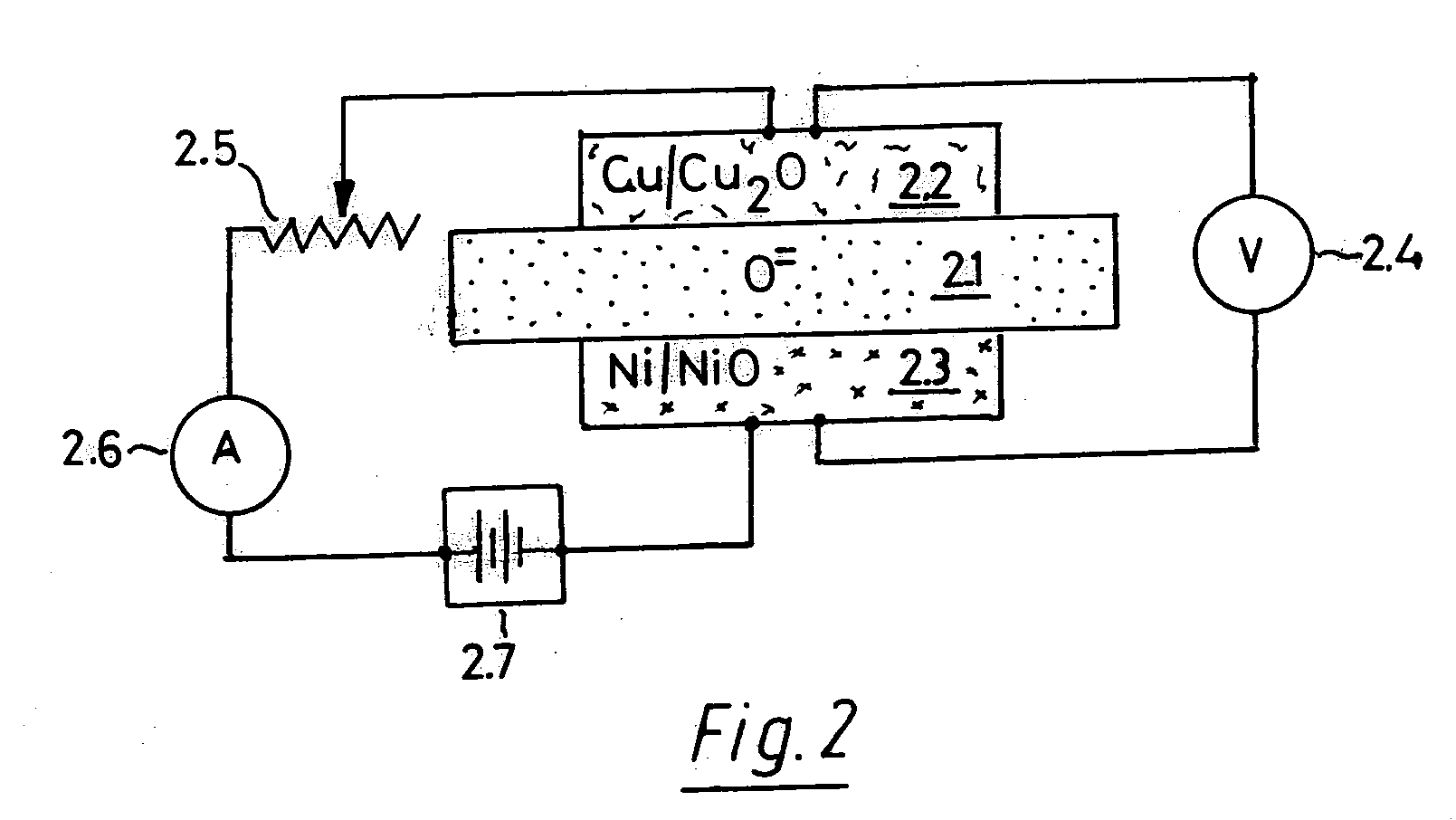
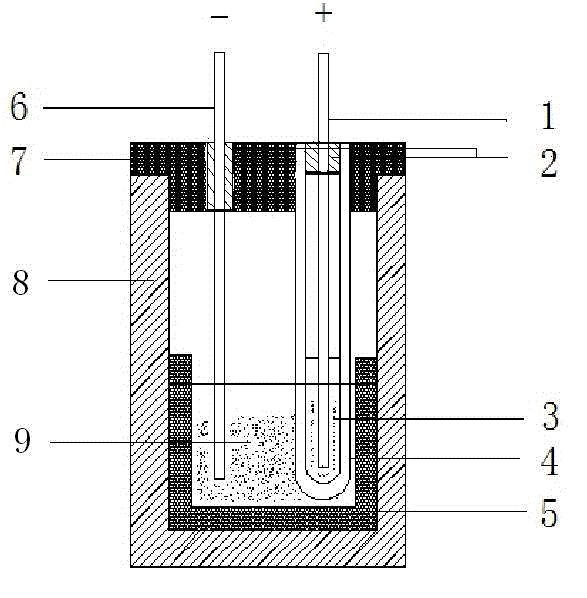
Oxygen chemical sensor for high-temperature and high-pressure hydrothermal system and preparation method of oxygen chemical sensor
ActiveCN105004777AImprove corrosion resistanceGood mechanical strength at high temperatureMaterial analysis by electric/magnetic meansOxygen sensorMetal sheet
The invention discloses an oxygen chemical sensor for a high-temperature and high-pressure hydrothermal system and a preparation method of the oxygen chemical sensor. The oxygen chemical sensor comprises a base, circular platform-shaped high-temperature-resistant insulating conical pads, a solid oxygen buffer agent, high-temperature-resistant insulating conical sleeves, circular platform-shaped solid electrolyte ceramic, circular platform-shaped high-temperature-resistant insulating ceramic, spongy inert metal layers, inert metal sheets and electrode leads. The oxygen chemical sensor, namely an oxygen sensor based on an electrochemical cell, is formed by combining all the components into a conical self-tightening mechanism, and can be utilized for in-situ direct measurement of the oxygen fugacity or the oxygen activity in the high-temperature (250 to 700 DEG C) and high-pressure (normal pressure to 100 MPa) hydrothermal system, so as to solve the problems that various conventional oxygen chemical sensors for a high-pressure hydrothermal system are low in operating temperature and operating pressure (the operating temperature and the operating pressure can hardly exceed 400 DEG C and 40 MPa simultaneously), low in response speed, and low in reliability when being applied to a complex concentrated water fluid sample.
Owner:INST OF GEOCHEM CHINESE ACADEMY OF SCI
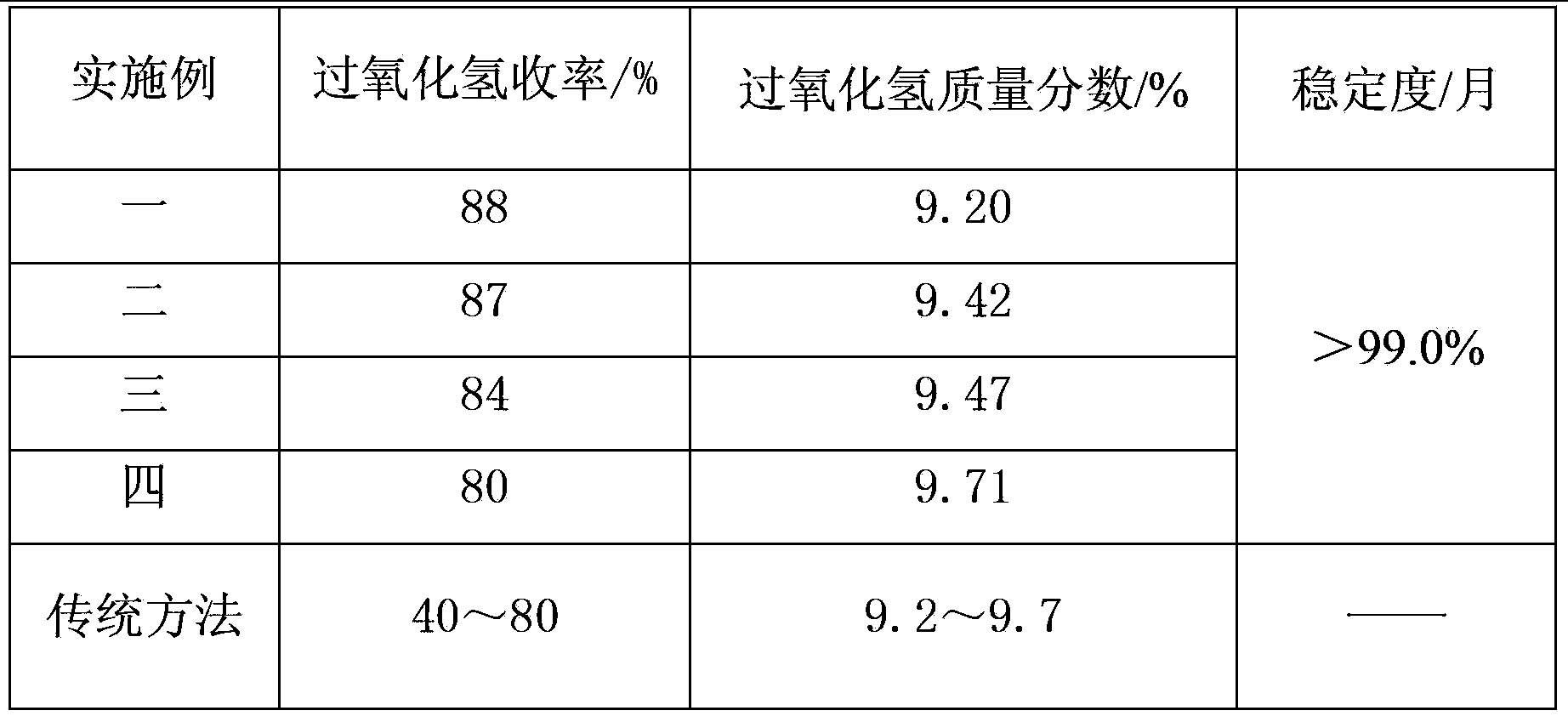
Solid hydrogen peroxide preparation technology
ActiveCN103351961AOvercome underutilizationOvercoming the problem of liquid waste dischargeNon-surface-active detergent compositionsBleaching apparatusBleachSodium sulfate
The invention relates to the technical field of preparation of solid oxygen-bearing bleaching agent, in particular to a solid hydrogen peroxide preparation technology. The preparation technology comprises the step of kneading sodium sulfate, hydrogen peroxide and salt in a kneader at a certain proportion to obtain the finished product. According to the invention, the ordinary 30% hydrogen peroxide can serve as a basic material, the problems that the preparation cost is higher, the mother liquor is not fully utilized and waste liquid is discharged in the prior art are solved, the yield of hydrogen peroxide in the obtained finished product is more than 80 percent, the process period is shorter, the cost is lower, no three-waste emission exists, and the solid hydrogen peroxide preparation technology is suitable for large-scale popularization and application.
Owner:CHANGZHOU XIAOGUO INFORMATION SERVICES
High-capacity all solid oxygen generator
ActiveCN104176709ASolve the problem of generating harmful impurity gasesGuaranteed oxygen contentOxygen preparationEngineeringSTI Outpatient
The invention discloses a high-capacity all solid oxygen generator which comprises a cylindrical shell and an upper cover, wherein the upper cover is installed on the cylindrical shell and is provided with an ignition starting assembly which is connected with an oxygen generating medicine block by virtue of a lead, the oxygen generating medicine block is arranged inside the cylindrical shell, and a filter assembly is arranged between the ignition starting assembly and the oxygen generating medicine block. The oxygen generating medicine block is prepared from the following components in parts by weight: 3 parts of ignition block, 23 parts of first oxygen generating medicine block, 25 parts of second oxygen generating medicine block, 25 parts of third oxygen generating medicine block and 25 parts of fourth oxygen generating medicine block. The generator disclosed by the invention has the beneficial effects that the problem that harmful impure gases are generated by high-temperature side reactions is solved, and the generator can provide sufficient and pure oxygen for personnel in a tight space in an emergency.
Owner:三亚光远新型材料有限公司
Preparation method of oxygen slow release preparation for repairing of contaminated site
InactiveCN104560051AExcellent oxygen release performanceOxygen release rate controlled releaseTreatment using aerobic processesOther chemical processesAlkaline earth metalPhosphate
The invention provides a preparation method of an oxygen slow release preparation for the repairing of a contaminated site, and belongs to the field of environmental protection technology. The adopted process comprises the following steps: firstly, mixing alkaline earth hyperoxide with dihydric phosphate and inorganic ammonium salt uniformly to obtain mixed solid particles; secondly, the mixed solid particles are coated with a long-chain tertiary amine ionic surface active agent and dried to obtain solid oxygen slow release preparation particles, so as to achieve the purpose that oxygen can be slowly released from the oxygen slow release preparation in soil and underground water; thirdly, the coated solid oxygen slow release preparation particles and permeable materials can be mixed uniformly, so that the permeability of an oxygen release material can be improved. With the adoption of the preparation method of the oxygen slow release preparation for repairing of the contaminated site, the prepared oxygen slow release preparation is especially suitable for in-situ pollution repairing of oil pollutants like benzenes, methyl tert-butyl ether and petroleum hydrocarbon; the oxygen slow release preparation can slowly release reactive oxygen, at the same time, load and slowly release nutrient substances needed by organisms, no pollution is caused to the environment, and the biological degradability is achieved.
Owner:ESD CHINA LTD
Method for preparing AB5 type hydrogen-storage alloy directly from metal oxide mixture
The invention relates to a method for direct preparation of AB5 type hydrogen storage alloys through metal oxide mixture, belonging to the solid oxygen permeable membrane electrolysis method alloy preparation technology technical field. The method mainly utilizes the solid oxygen permeable membrane technology and an electrochemical method to directly prepare the AB5 type hydrogen storage alloys through the oxide mixture of target alloys. The metal oxide mixture which forms the elemental constituent of the hydrogen storage alloys is pressed into a wafer which is used as a cathode, and graphite is used as an anode; the metal oxide mixture wafer is arranged inside a solid oxygen permeable membrane pipe; the metal oxide mixture wafer and the graphite which are used as the cathode and the anode are arranged inside a graphite crucible electrolytic cell which is stored with copper liquid, and then electrolysis is performed after galvanization and heating; the electrolytic voltage is 0 to 4.5 volts and the electrolytic current is 0 to 6.0 amperes; finally the AB5 type hydrogen storage alloys are obtained. The compositions of raw materials used by the AB5 type hydrogen storage alloys are as follows: La2O3 of 18.1+-0.46 weight percent, CeO2 of 8.54+-0.28 weight percent, Pr2O3 of 0.73+-0.18 weight percent , Nd2O3 of 2.46+-0.18 weight percent, MnO2 of 6.42+-0.24 weight percent, Al2O3 of 1.77+-0.30 weight percent , Co2O3 of 11.17+- 0.22 weight percent and residual NiO.
Owner:SHANGHAI UNIV
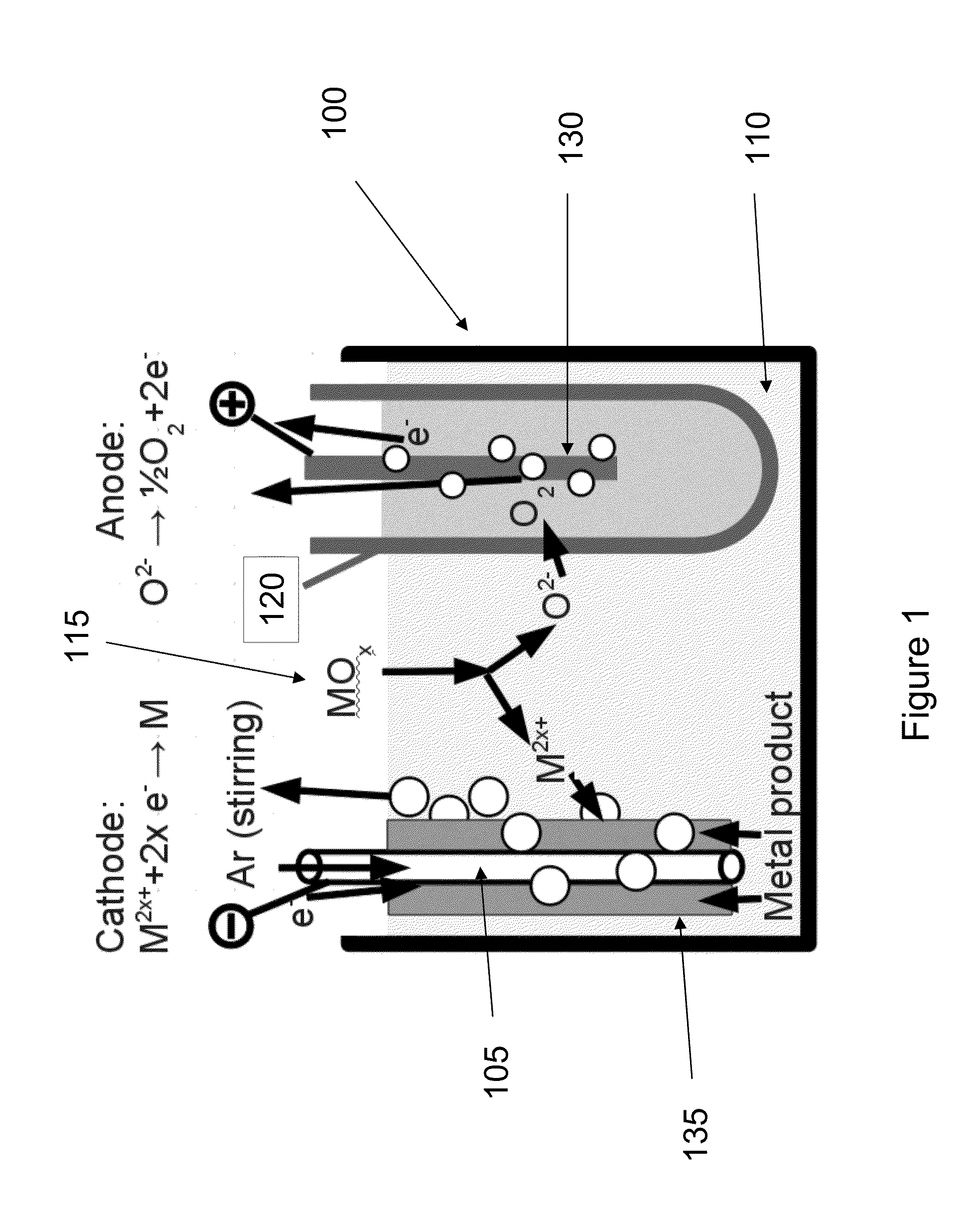
Liquid anodes and fuels for production of metals from their oxides by molten salt electrolysis with a solid electrolyte
ActiveUS20130186769A1Maintain structural integrityCellsPhotography auxillary processesElectrolysisMolten salt
In one aspect, the present invention is directed to liquid anodes and fuels for production of metals from their oxides. In one aspect, the invention relates apparatuses for producing a metal from a metal oxide comprising a cathode in electrical contact with an electrolyte, a liquid metal anode separated from the cathode and the electrolyte by a solid oxygen ion conducting membrane, a fuel inlet, and a power supply for establishing a potential between the cathode and the anode. In another aspect, the invention relates to methods for production of metals from their oxides comprising providing a cathode in electrical contact with a molten electrolyte, providing a liquid metal anode separated from the cathode and the molten electrolyte by a solid oxygen ion conducting membrane, providing a fuel inlet, delivering a gaseous fuel comprising hydrogen to the liquid metal anode via the fuel inlet, and establishing a potential between the cathode and the anode.
Owner:INFINIUM
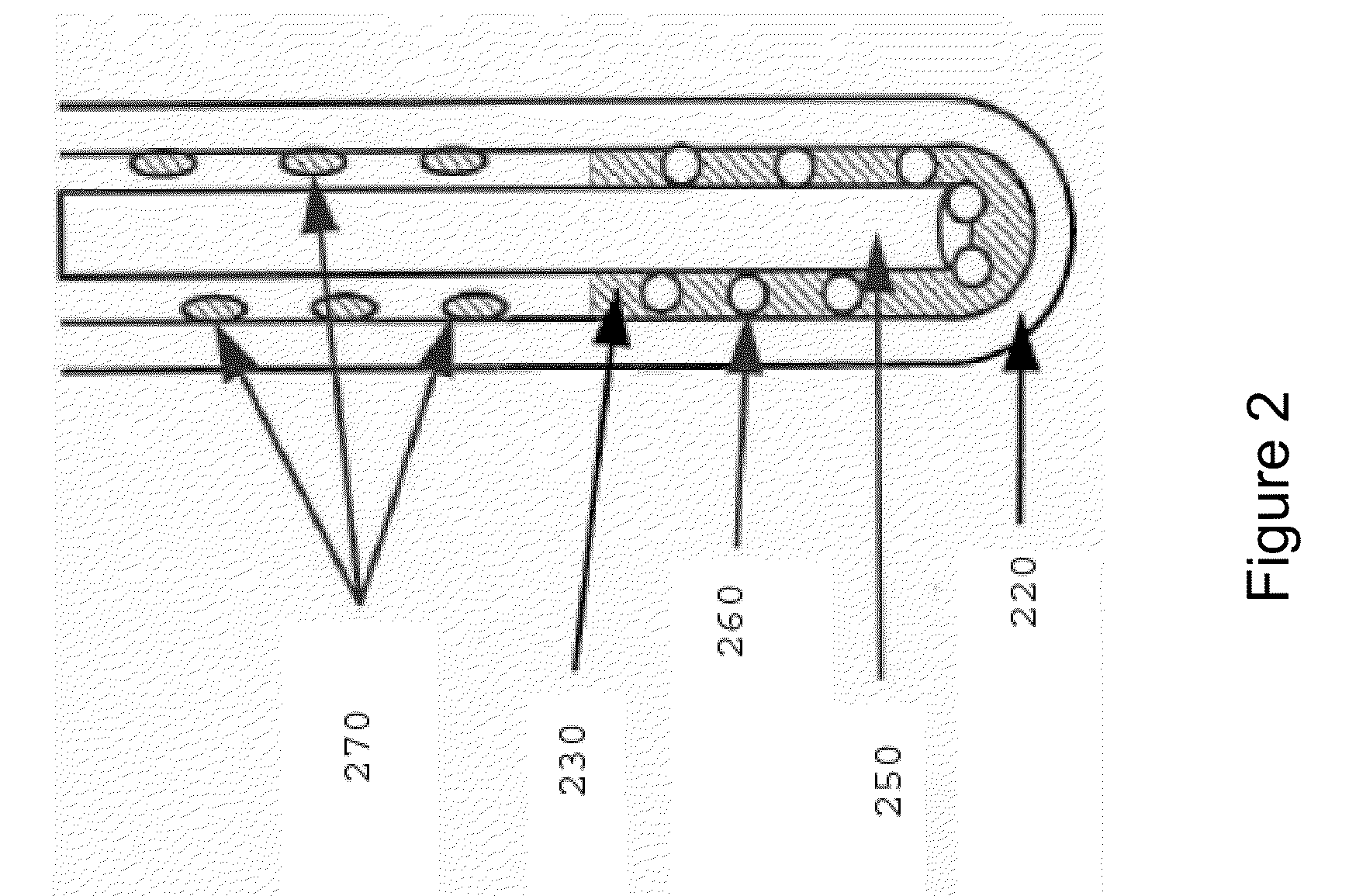
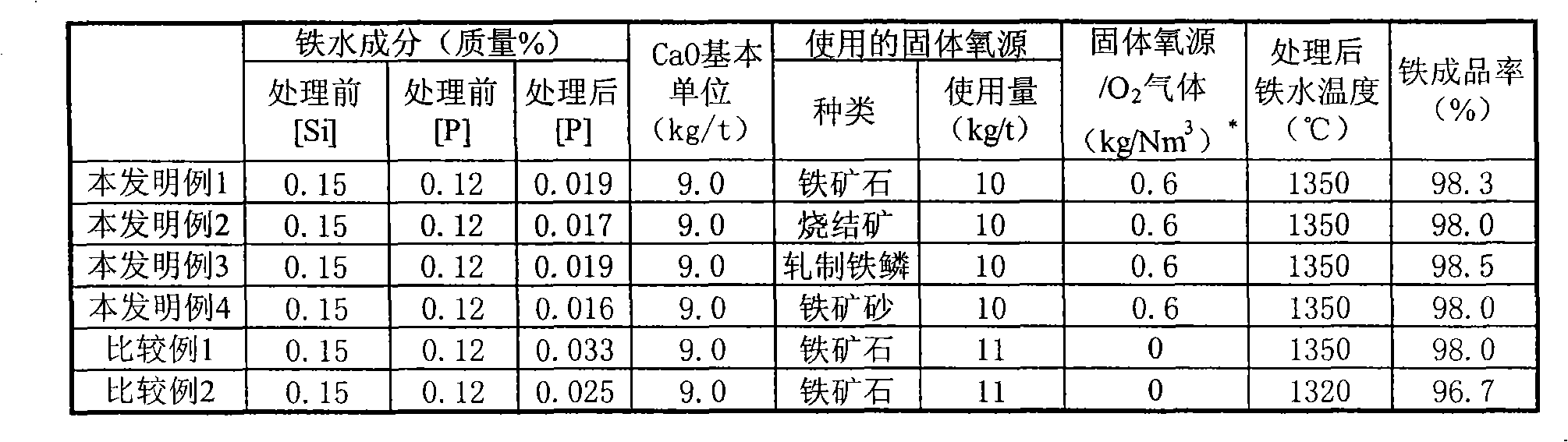
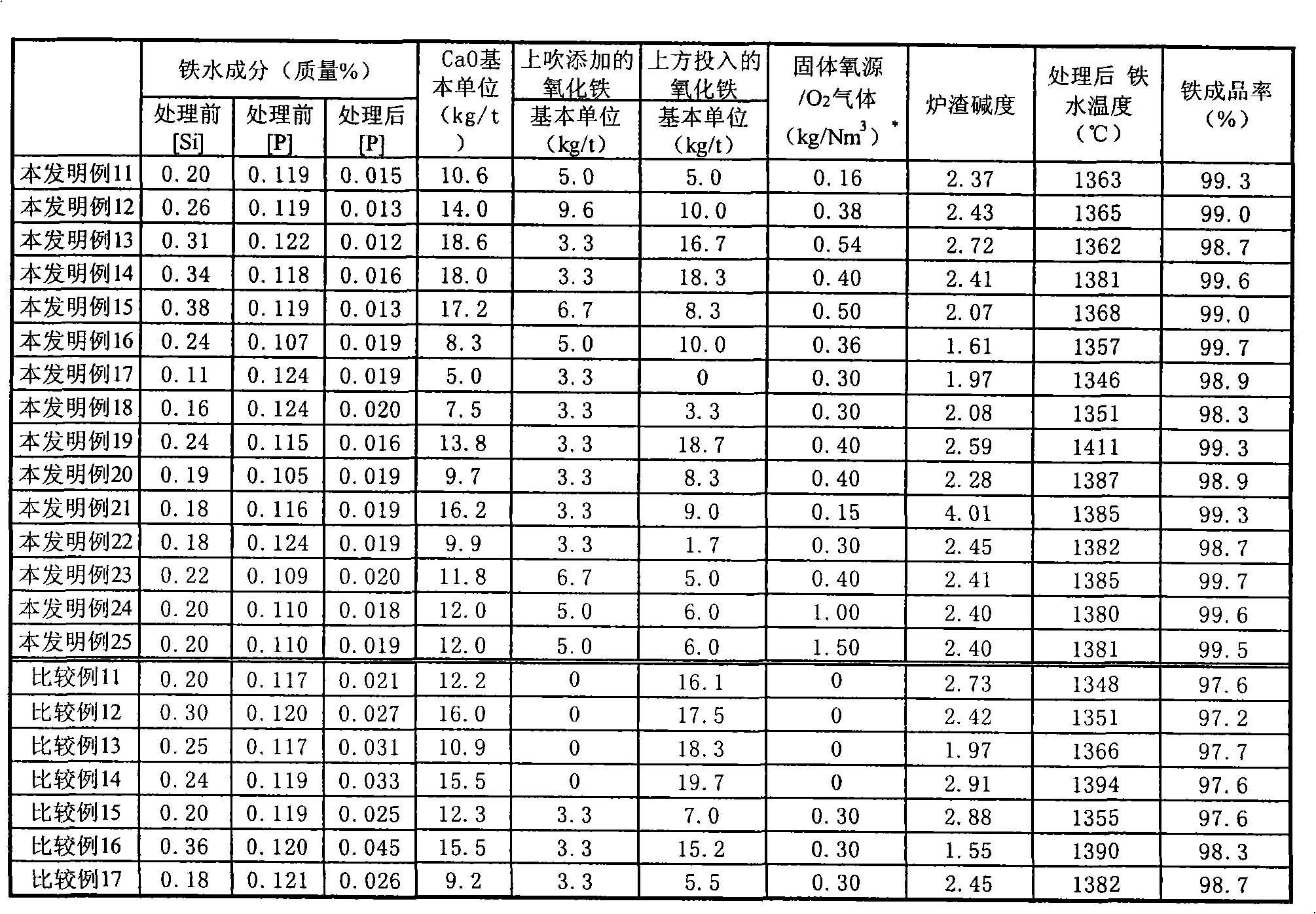
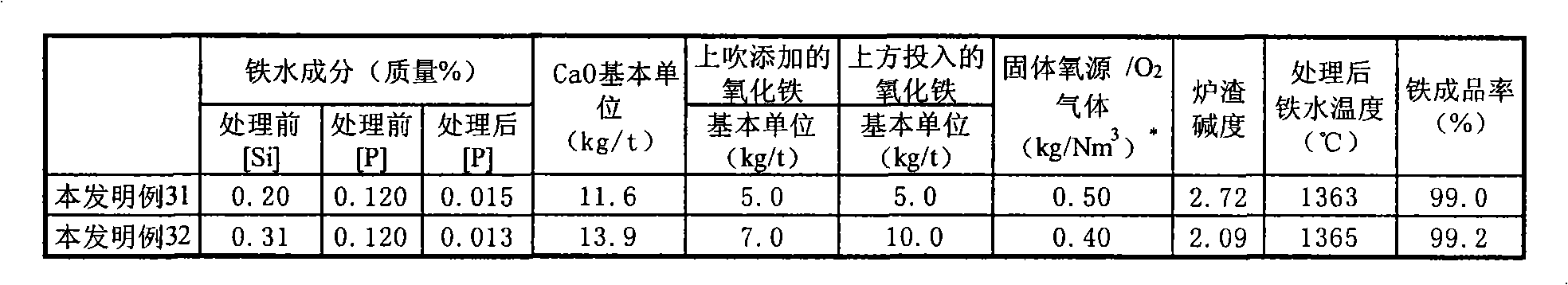
Method of hot metal dephosphorization treatment
A method of hot metal dephosphorization treatment, comprising adding a dephosphorization refining agent composed mainly of CaO and reducing the added dephosphorization refining agent composed mainly of CaO to slags to thereby accomplish dephosphorization of the hot metal, wherein a gaseous oxygen source is fed from one supply system to a surface of hot metal bath and a solid oxygen source is fed from another supply system to a surface of hot metal bath in the vicinity of the site where the gaseous oxygen source is fed by the use of carrier gas. Thus, without the use of any medium solvent containing fluorine, there can be accomplished dephosphorization treatment with dephosphorization efficiency and iron yield equal to or higher than those of the prior art.
Owner:JFE STEEL CORP
Method for increasing dissolved oxygen in gamma-polyglutamic acid fermenting liquid
ActiveCN106947724ANo tolerance requirementIncrease dissolved oxygenBacteriaMicroorganism based processesBacterial strainBiological activation
The invention belongs to the field of microorganism fermenting, which aims at providing a method for increasing dissolved oxygen in gamma-polyglutamic acid fermenting liquid with universality. The method adopts the technical scheme that the collection number of bacillus amyloliquefaciens JX-6 is CGMCC No.13715, and comprises the following steps of inoculating the bacteria strain into a sterilized oblique activation culture medium, and culturing, so as to obtain activated bacterial strain; inoculating the activated bacteria strain into a sterilized and cooled seed culture medium, and culturing, so as to obtain a seed liquid; inoculating the seed liquid into a fermenting culture medium, adding solid oxygen in the fermenting process, and culturing, so as to obtain the fermenting liquid. The method has the advantages that the universality is strong, the operation is simple, the cost is low, the secondary pollution is avoided, the resistance requirement on the bacteria strain is avoided, the method is suitable for large-scale production, and the application prospect is good.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Device and method for rapidly producing oxygen
The invention relates to a device and method for producing oxygen, in particular to a device and method for rapidly producing oxygen, aiming at the problems of the existing solid oxygen generator, such as complicated structure, too many used reagents and high cost, and the problem of an automatic oxygen generator that hydrogen peroxide is easily decomposed, hard to control and harmful to human bodies. The device for rapidly producing the oxygen comprises a container, an oxygen discharging pipe, a triggering pipe, a molecular sieve and oxygen producing reactants, wherein the oxygen producing reactants are put in the container; the molecular sieve is arranged at the opening of the container and forms a reacting cavity together with the container; the lower end of the triggering pipe is inserted into the oxygen producing reactants, and the upper end of the triggering pipe penetrates through the molecular sieve and is exposed out of the reacting cavity; the lower end of the oxygen discharging pipe penetrates through the molecular sieve to be inserted into the reacting cavity and is arranged above the oxygen producing reactants; and the oxygen producing reactants include sodium superoxide, white alum and silicon dioxide. The device and method are used for producing the oxygen.
Owner:HARBIN INST OF TECH
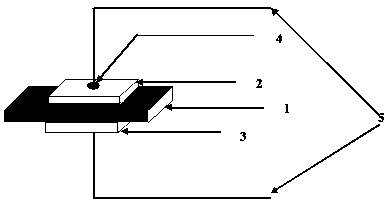
YSZ (yttria-stabilized-zirconia)-based HCs gas sensitive sensor based on NiO sensitive electrode and preparation method of YSZ (yttria-stabilized-zirconia) -based HCs gas sensitive sensor
ActiveCN104198547AImprove responsivenessHigh sensitivityMaterial electrochemical variablesScreen printingElectrical conductor
The invention discloses an YSZ (yttria-stabilized-zirconia)-based HCs gas sensitive sensor based on a NiO sensitive electrode and a preparation method of the YSZ (yttria-stabilized-zirconia)-based HCs gas sensitive sensor. YSZ solid electrolyte is prepared by adopting a tape casting technology, the NiO sensitive electrode and a Pt reference electrode are formed in a printing manner by adopting a thick-film screen printing technology, and an electrode lead is led out. A YSZ solid oxygen ion conductor material is used as the solid electrolyte of the HCs sensor, the flower-shaped nano-topography NiO is used as the sensitive electrode, no liquid phase component is used, the safety is high, the sensitivity is high, the selectivity is good, and the safety is good; moreover, the sensor is simple in structure, small in size and simple in preparation process. When the HCs sensor works, no external working voltage is needed, and the detection range of the HCs concentration is 0 to 500ppm.
Owner:宁波永林智控科技有限公司
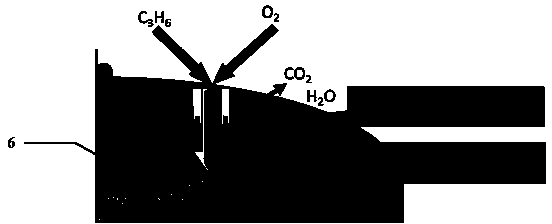
Selective Oxidation Agent of Hydrocarbons to Synthesis Gas Based on Separate Particles of O-Carrier and Hydrocarbon Activator
A solid material is presented for the partial oxidation of natural gas. The solid material includes a solid oxygen carrying agent and a hydrocarbon activation agent. The material precludes the need for gaseous oxygen for the partial oxidation and provides better control over the reaction.
Owner:UOP LLC
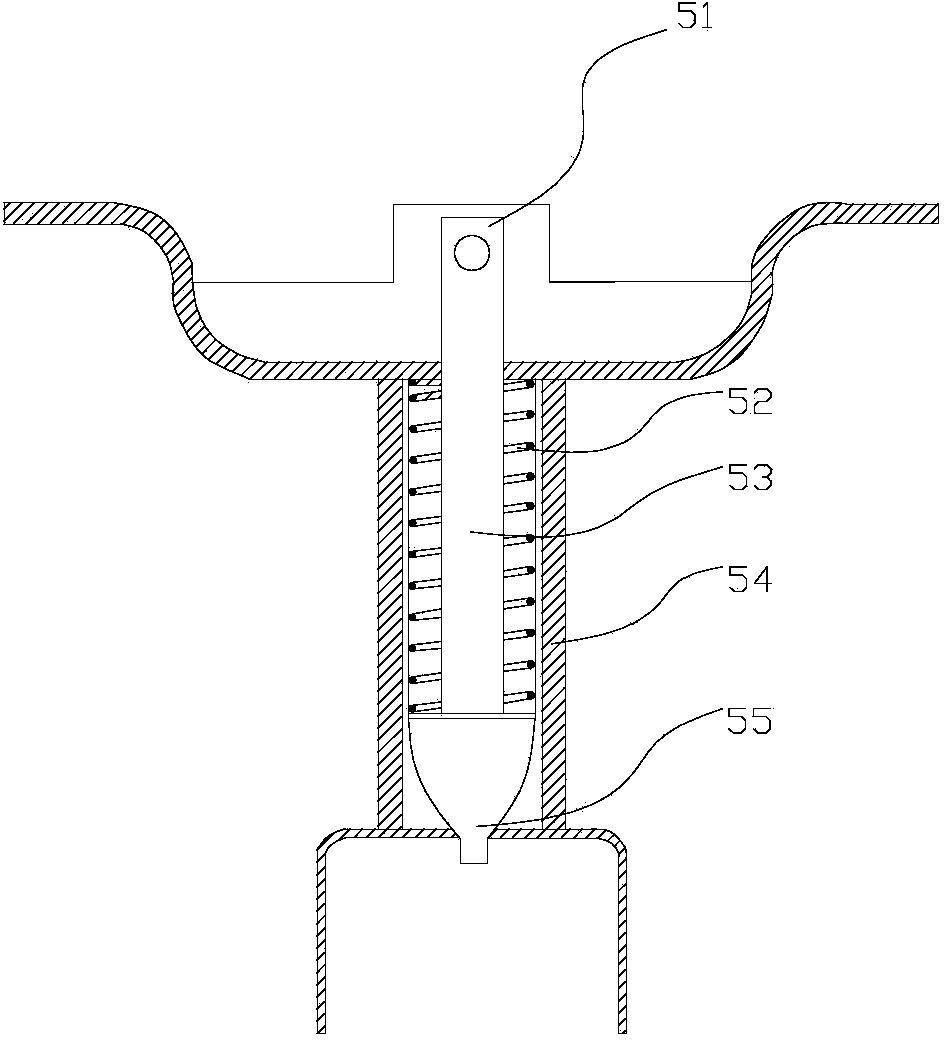
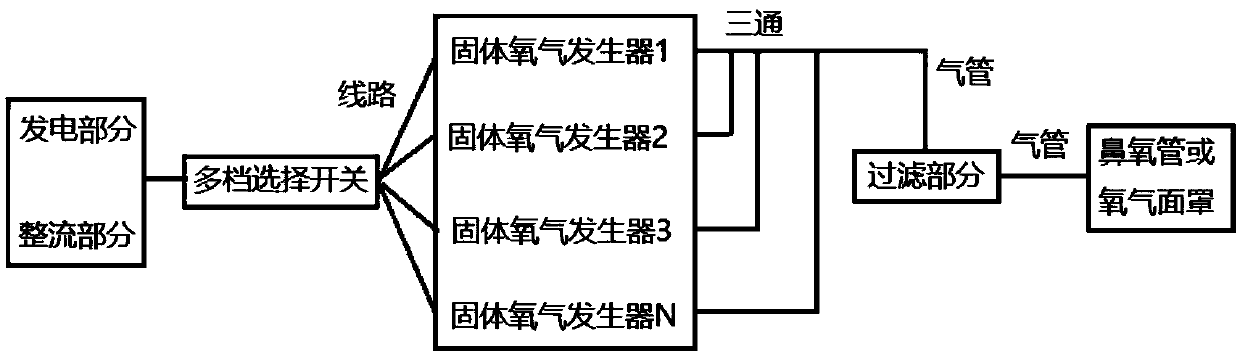
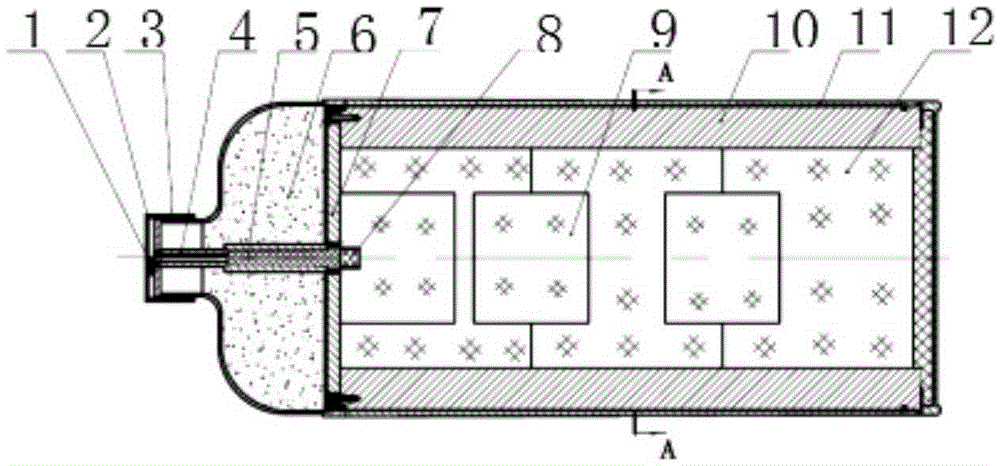
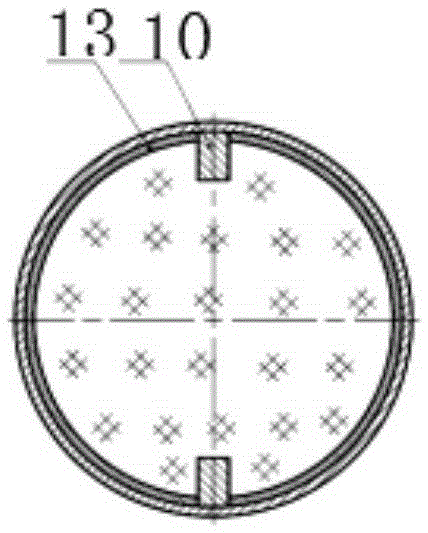
Passive replaceable solid oxygen generating device
InactiveCN109534294AAdapt to a wide temperature rangeImprove reliabilityOxygen preparationEngineeringExtreme temperature
The invention discloses a passive replaceable solid oxygen generating device which comprises a generating rectifying device, a multi-gear option switch, a plurality of solid oxygen generators, a filtering device and an oxygen use device, wherein the generating rectifying device comprises a generating device and a rectifying device; the generating device is connected with the rectifying device through a wire; the generating rectifying device is connected with the plurality of solid oxygen generators through the multi-gear option switch; the solid oxygen generators are connected with the filtering device through gas pipes; the filtering device is connected with the oxygen use device. The passive replaceable solid oxygen generating device can be used under the condition of extreme temperature; power supply need not be replaced; the device is small in volume, is simple for operation and is free from maintenance.
Owner:HUBEI INST OF AEROSPACE CHEMOTECHNOLOGY
Complex RH decarburization method
The invention relates to a complex RH decarburization method, which comprises the following steps: 1) decarburizing RH decarburization oxygen supply by adopting a mode of combining solid oxygen with blown high-pressure oxygen; 2) when the vacuum interior pressure reaches 20-25kPa, adding 0.1-3.0kg / tonnage steel containing Fe2O3 solid spheres; 3) controlling the vacuum pressure at 10-15kPa, adding 0.1-3.0kg / tonnage steel containing Fe2O3 solid spheres; 4) automatically vacuumizing; 5) reducing the oxygen blowing by a top lance; 6) promoting the gas flow at the oxygen blowing earlier stage; 7) stopping oxygen blowing when carbon in the steel reaches 100ppm below; 8) after the circulation is finished, breaking and carrying out. Compared with the prior art, the complex RH decarburization method has the beneficial effects that the fast, stable and safety decarburization is realized, and the method can be used for producing low-carbon special steel in which the content of molten steel carbon is distributed in 1000-2000ppm, and of finished carbon is distributed below 350ppm before RH decarburization.
Owner:ANGANG STEEL CO LTD
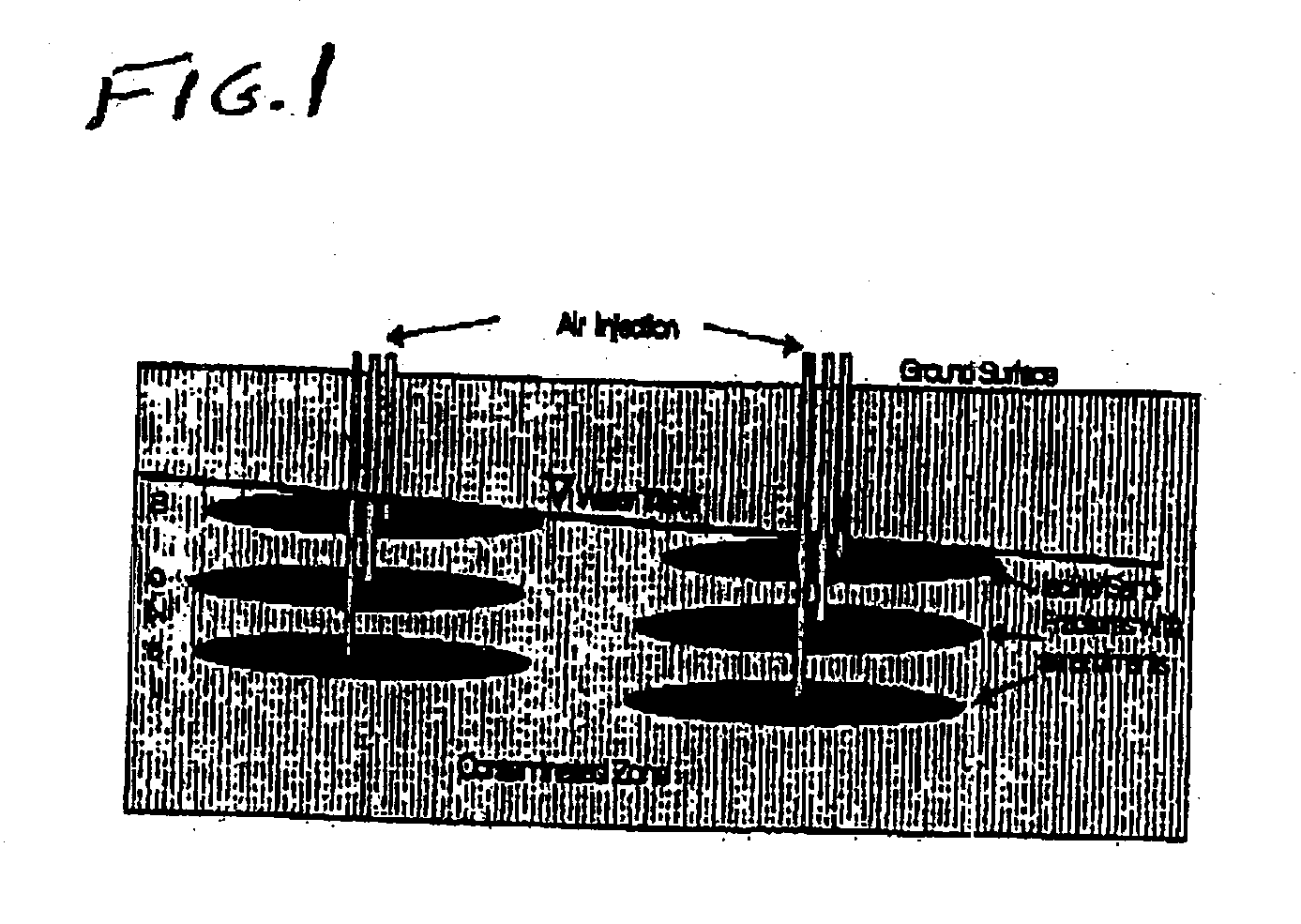
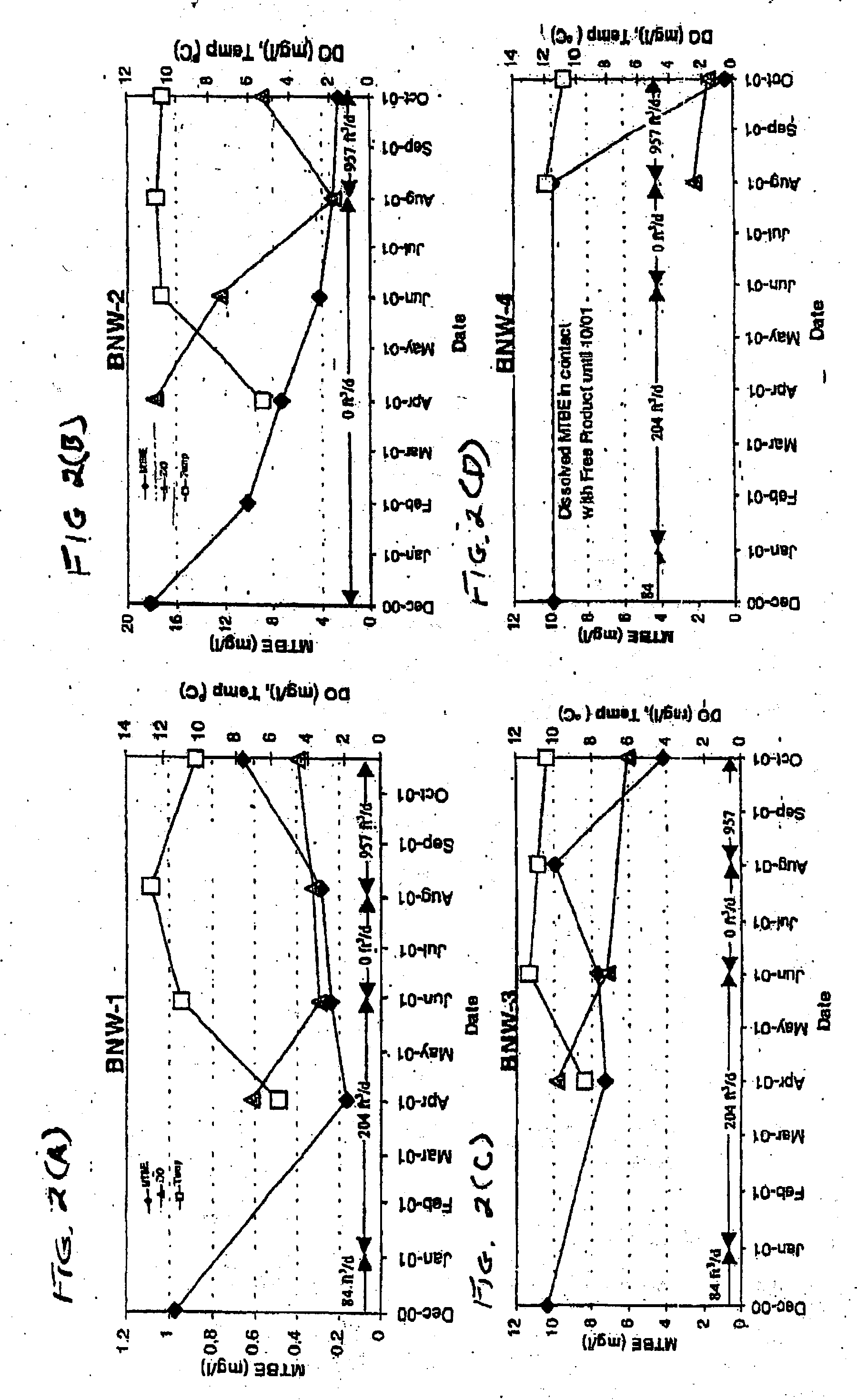
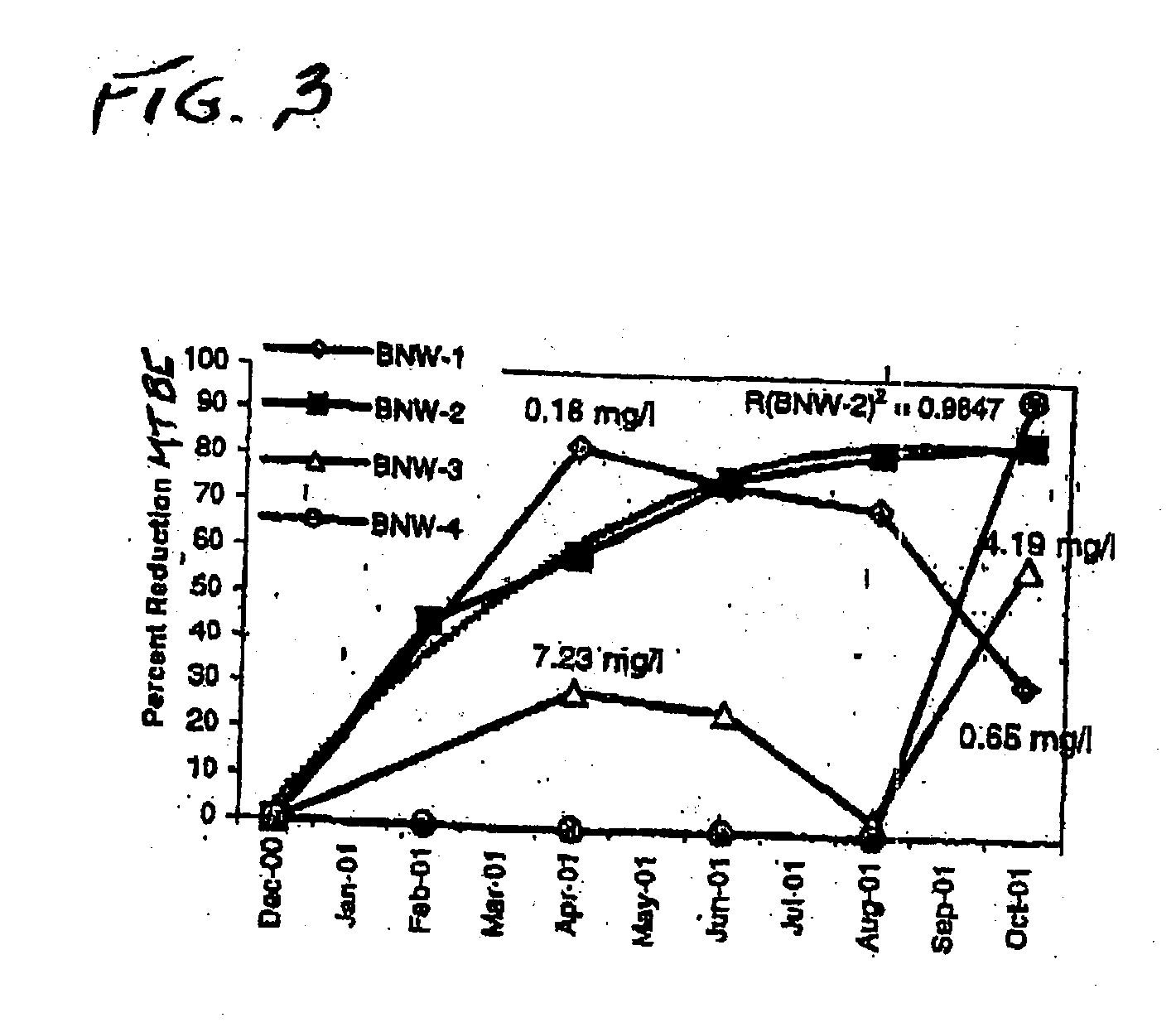
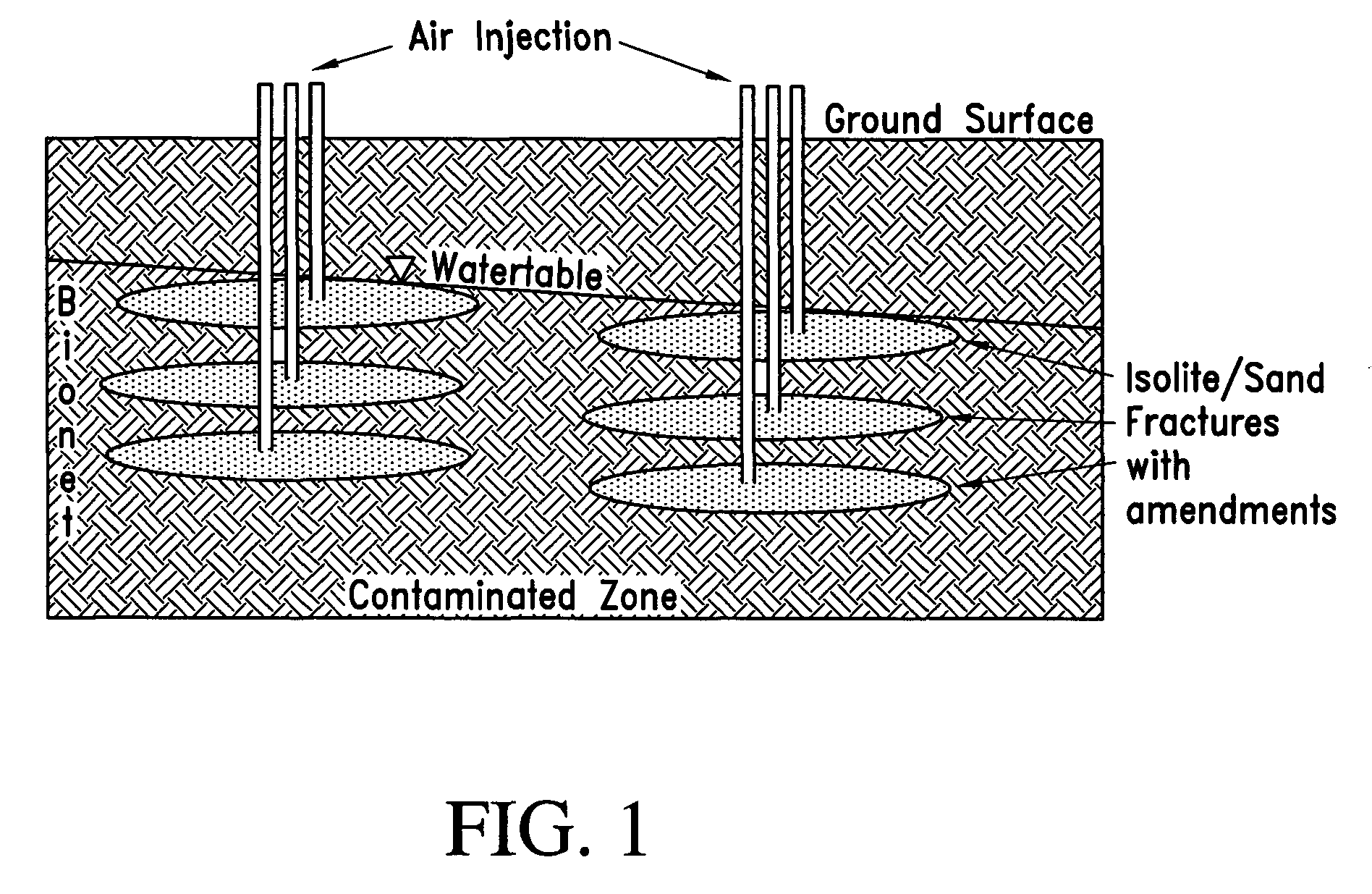
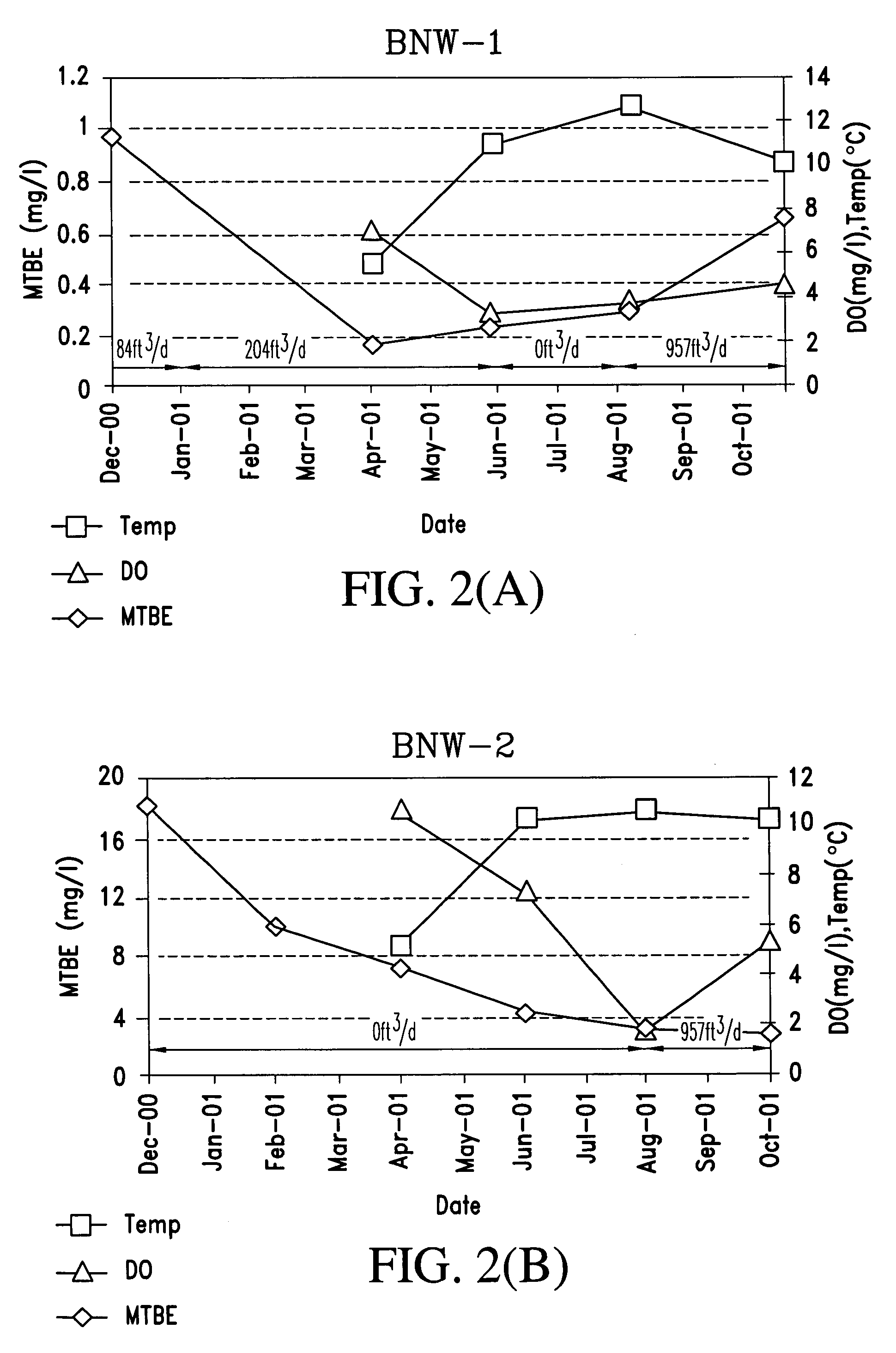
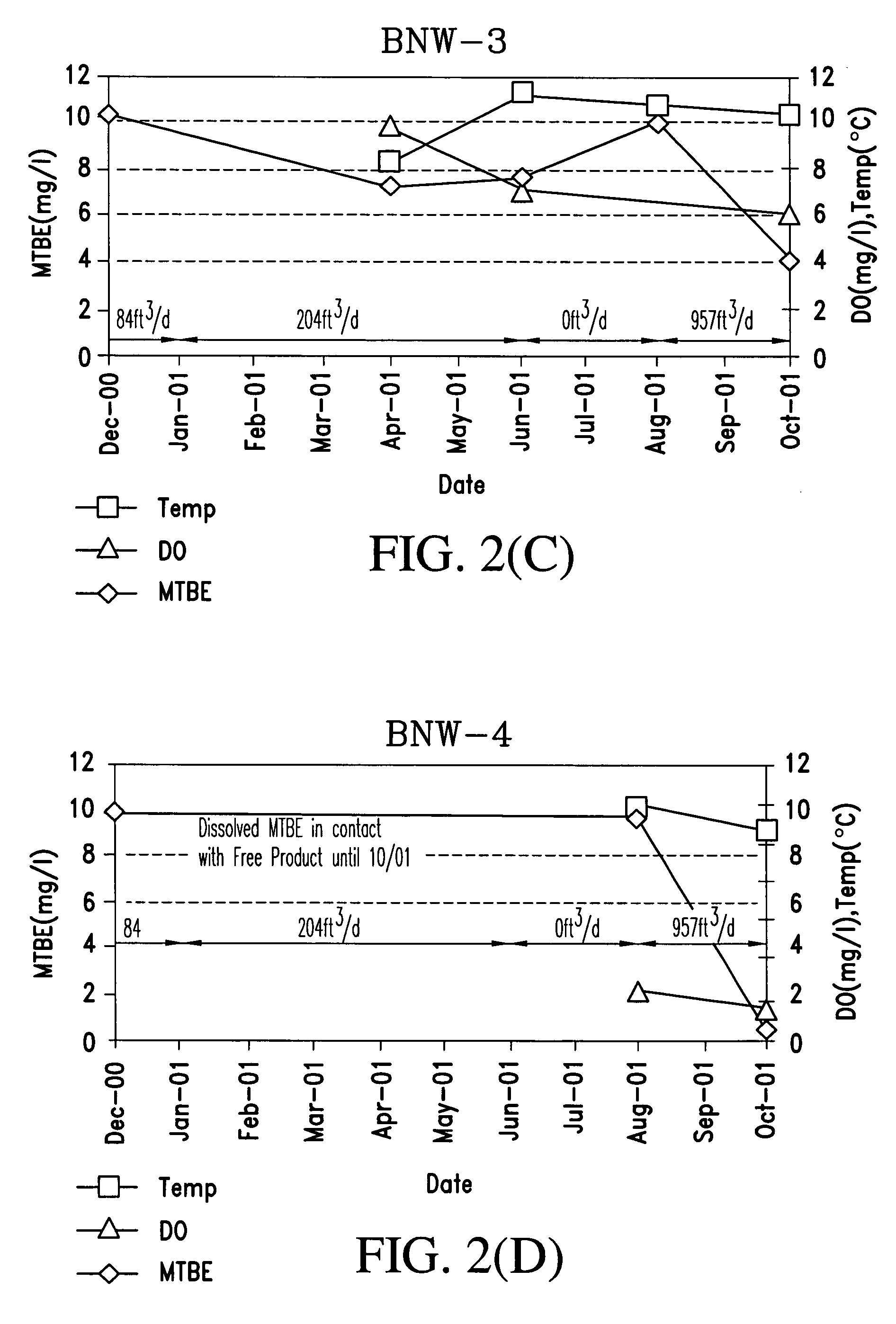
Process for the biodegradation of hydrocarbons and ethers in subsurface soil by introduction of a solid oxygen source by hydraulic fracturing
A bioremediation of subsurface soil formations contaminated with hazardous wastes is achieved by hydraulic fracturing of the subsurface soil formation with simultaneous introduction of sodium percarbonate coated with polyvinylidene chloride as a solid oxygen source (SOS) for establishing colonies of the biodegrading bacteria within the fractures of the soil formation.
Owner:PROTECTION AGENCY U S ENVIRONMENTAL +1
Starting mix of solid oxygen generator
InactiveCN101898924ARaise the ignition pointLow mechanical sensitivityExplosivesZirconium hydrideExplosive Agents
The invention discloses a starting mix of a solid oxygen generator, belonging to the technical field of explosives. The starting mix of the invention is mainly suitable for quickly igniting an oxygen generating grain to generate oxygen when the solid oxygen generator is impacted and ignited, and has the main technical characteristic of comprising the following components in percentage by weight: 40-70 of ferric oxide, 5-25 of zirconium powder, 10-30 of zirconium hydride, 1-15 of kieselguhr and 1-10 of shellac varnish, wherein the zirconium powder is substituted for magnesium powder, and the ferric oxide is substituted for barium nitrate and barium peroxide as oxidizers. The starting mix has the characteristics of moisture protection, water resistance, oxidation protection and the like, has good ignition property and safety, and can meet the requirement of long-term storage of products.
Owner:江南工业集团有限公司
Oxygen candle grain and preparation method thereof
InactiveCN107867675AMake sure it doesn't burn outLow calorific valueOxygen preparationSodium chlorateCandle
The invention provides an oxygen candle grain and a preparation method thereof. The oxygen candle grain comprises an inflammable drug layer formed by an inflammable drug, a grain inner core formed byan inner core drug as well as a grain outer ring formed by an outer ring drug, wherein the inflammable drug layer is located at the top end of the oxygen candle grain; the grain outer ring is of a cylindrical structure; the grain inner core is arranged in the cylindrical structure of the grain outer ring; both the grain inner core and the grain outer ring are located below the inflammable drug layer. The inner core drug contains a small quantity of metal powder fuel; the outer ring drug comprises sodium chlorate and a catalyst in a proper proportion as main components and contains no metal fuel. By means of the design, the outer ring drug is catalytically decomposed by heat transmitted by the inner core drug, so that heat emission of the oxygen candle is reduced greatly. The oxygen candlegrain is applicable to various specifications of solid oxygen generators and applies to the fields of marine vessels, aerospace, mines, self-rescue from various disasters and the like. By means of oxygen candle grain, the heat production quantity of the oxygen candle is small, oxygen evolution is stable, and the overall structure of the oxygen candle is stable.
Owner:HUBEI INST OF AEROSPACE CHEMOTECHNOLOGY
Process for the biodegradation of hydrocarbons and ethers in subsurface soil by introduction of a solid oxygen source by hydraulic fracturing
InactiveUS7252986B2Effectively degrade the contaminantSolid waste disposalContaminated soil reclamationSoil scienceEther
A bioremediation of subsurface soil formations contaminated with hazardous wastes is achieved by hydraulic fracturing of the subsurface soil formation with simultaneous introduction of sodium percarbonate coated with polyvinylidene chloride as a solid oxygen source (SOS) for establishing colonies of the biodegrading bacteria within the fractures of the soil formation.
Owner:PROTECTION AGENCY U S ENVIRONMENTAL +1
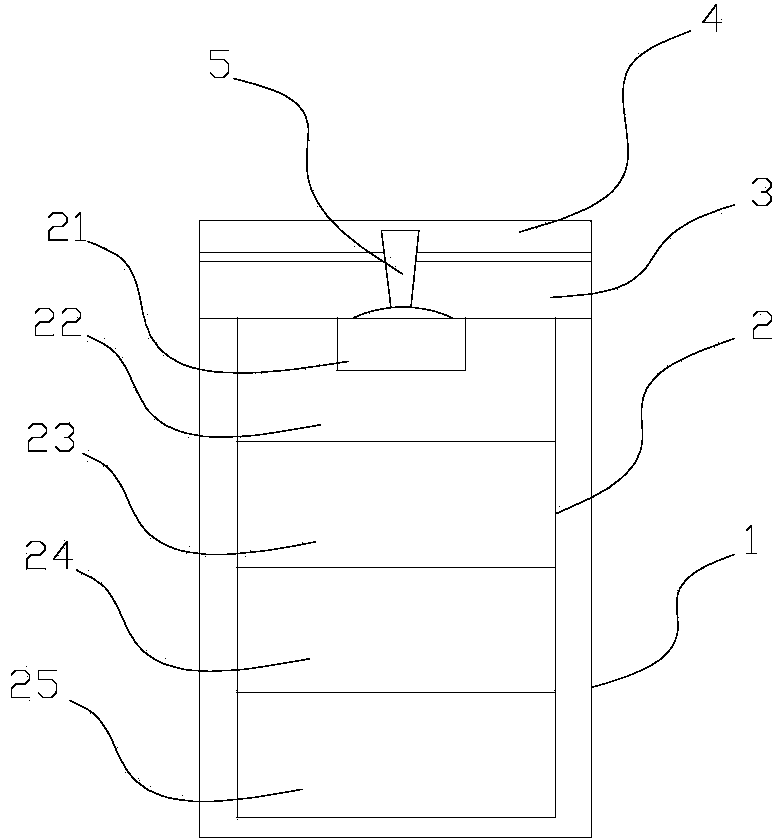
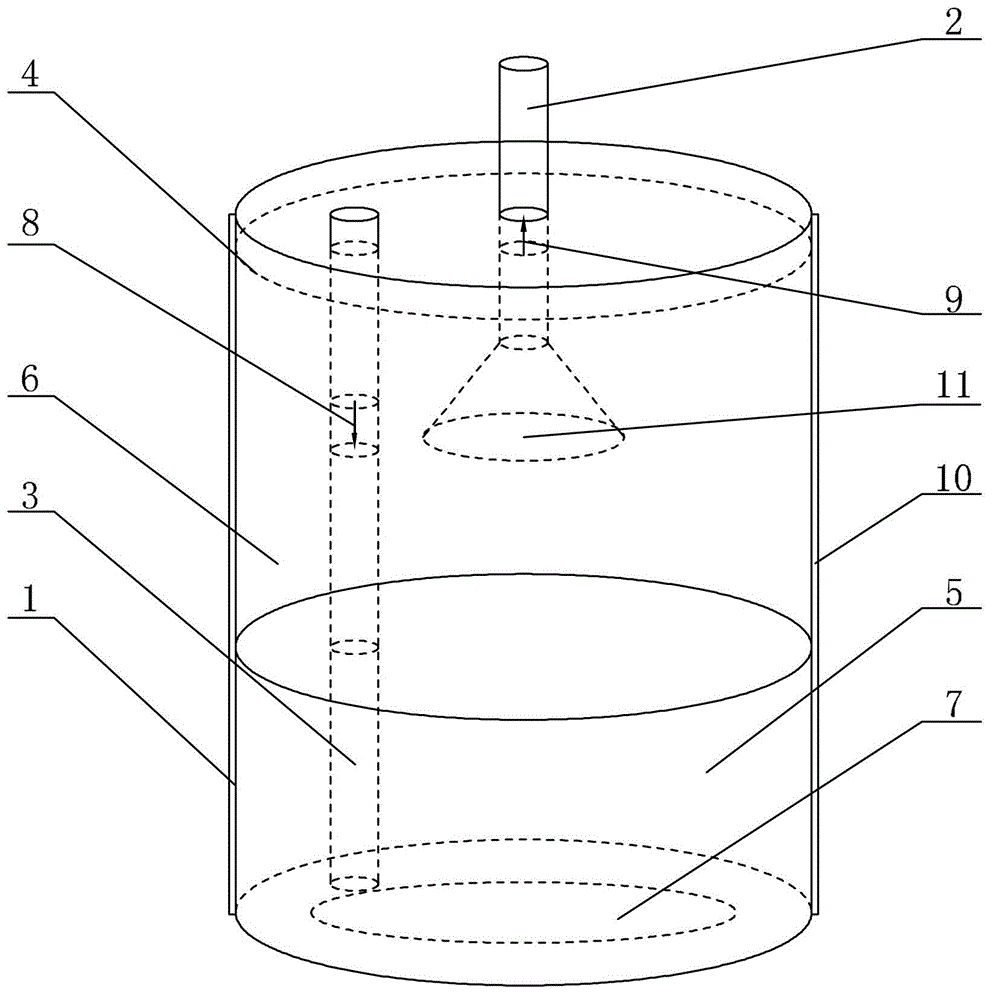
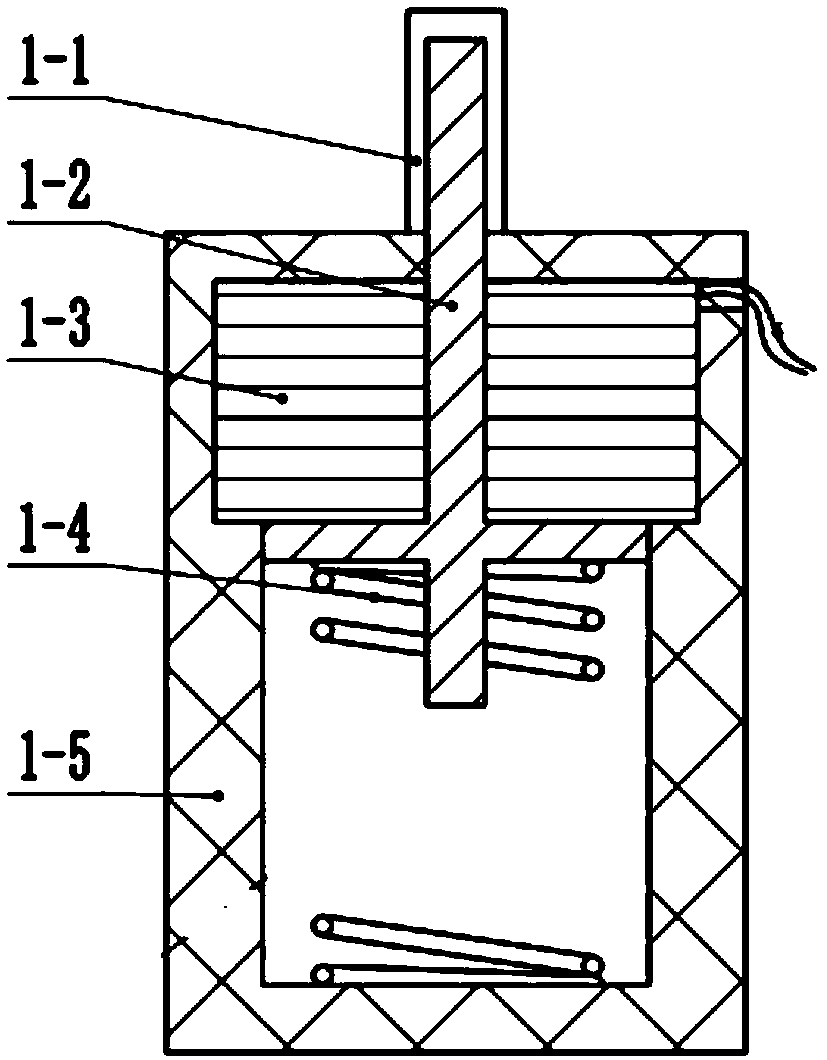
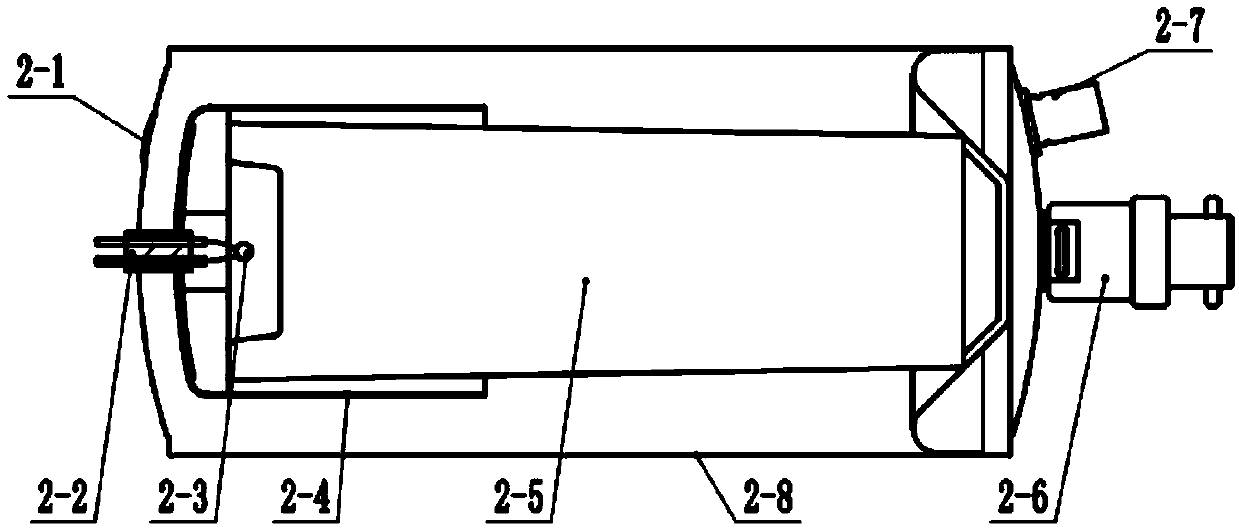
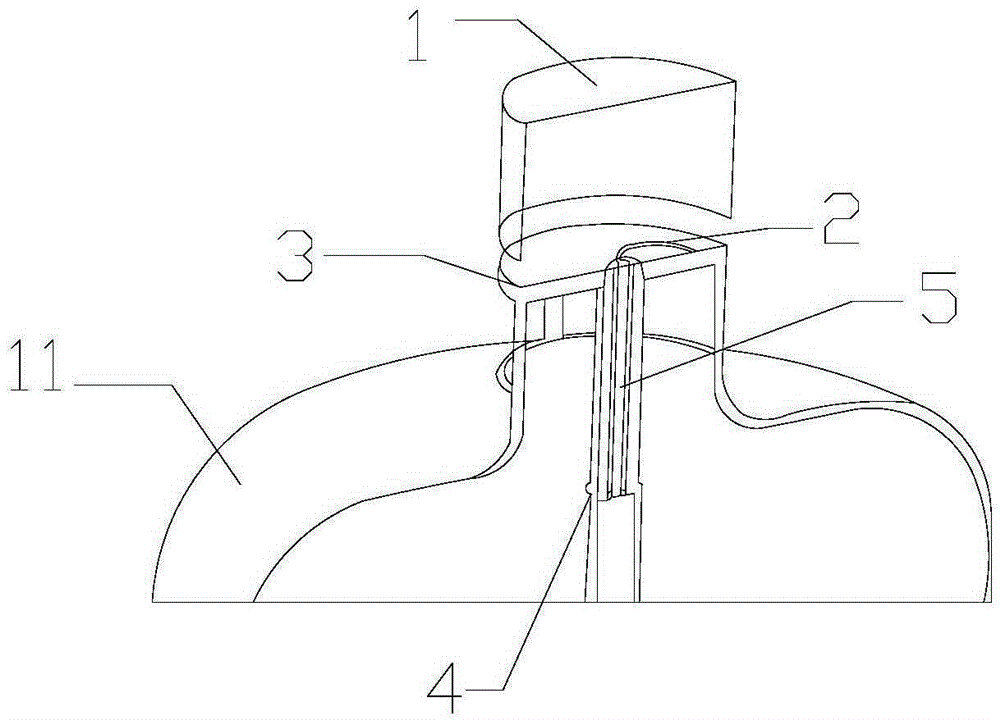
Solid oxygen generation device
The invention discloses a solid oxygen generation device. The solid oxygen generation device comprises a casing, an ignition assembly and an oxygen containing reactant, wherein the casing is provided with an oxygen outlet, and the oxygen containing reactant is arranged in casing; the ignition assembly is inserted into the casing from the oxygen outlet, one end of the ignition assembly extends outside the oxygen outlet, and the other end of the ignition assembly is connected with the oxygen containing reactant; a gas filtering layer is arranged between the oxygen containing reactant and the oxygen outlet; a thermal insulating protection structure is arranged on the surface of the inner wall of the casing. The solid oxygen generation device does not need external power during using, is convenient to carry and safe and reliable to use and can stably provide the flow larger than 100 L / min within 30s, the oxygen releasing amount is higher than 3,000 L, the oxygen purity is not lower than 99%, the lasting time is not shorter than 30 min, and accordingly, the purpose of emergency first aid is achieved.
Owner:三亚光远新型材料有限公司
Electrochemical deoxidization method for refining titanium and titanium alloy solution
The invention discloses an electrochemical deoxidization method for refining a titanium and titanium alloy solution, belonging to the technical field of metallurgical refining processes. The electrochemical deoxidization method is characterized in that an yttrium oxide crucible is used as a reaction vessel, a graphite rod is used as a cathode, and a carbon saturated copper solution in an yttrium oxide stabilized zirconium oxide pipe is used an anode, wherein the self-made yttrium oxide stabilized zirconium oxide pipe is used as a solid oxygen permeable membrane. The self-made yttrium oxide crucible is arranged in a graphite crucible with a cover, insulating corundum sleeves are added between the cover of the graphite crucible and the cathode and between the cover of the graphite crucible and the anode, and high-purity industrial argon gas is used as protective gas. A constant current is applied between the cathode and the anode through an external power supply, and O<2-> ions migrate with the electrolysis in the solution at high temperature, thus achieving the purpose of deoxidizing and purifying the metal solution.
Owner:SHANGHAI UNIV +1
Method for producing metallic titanium
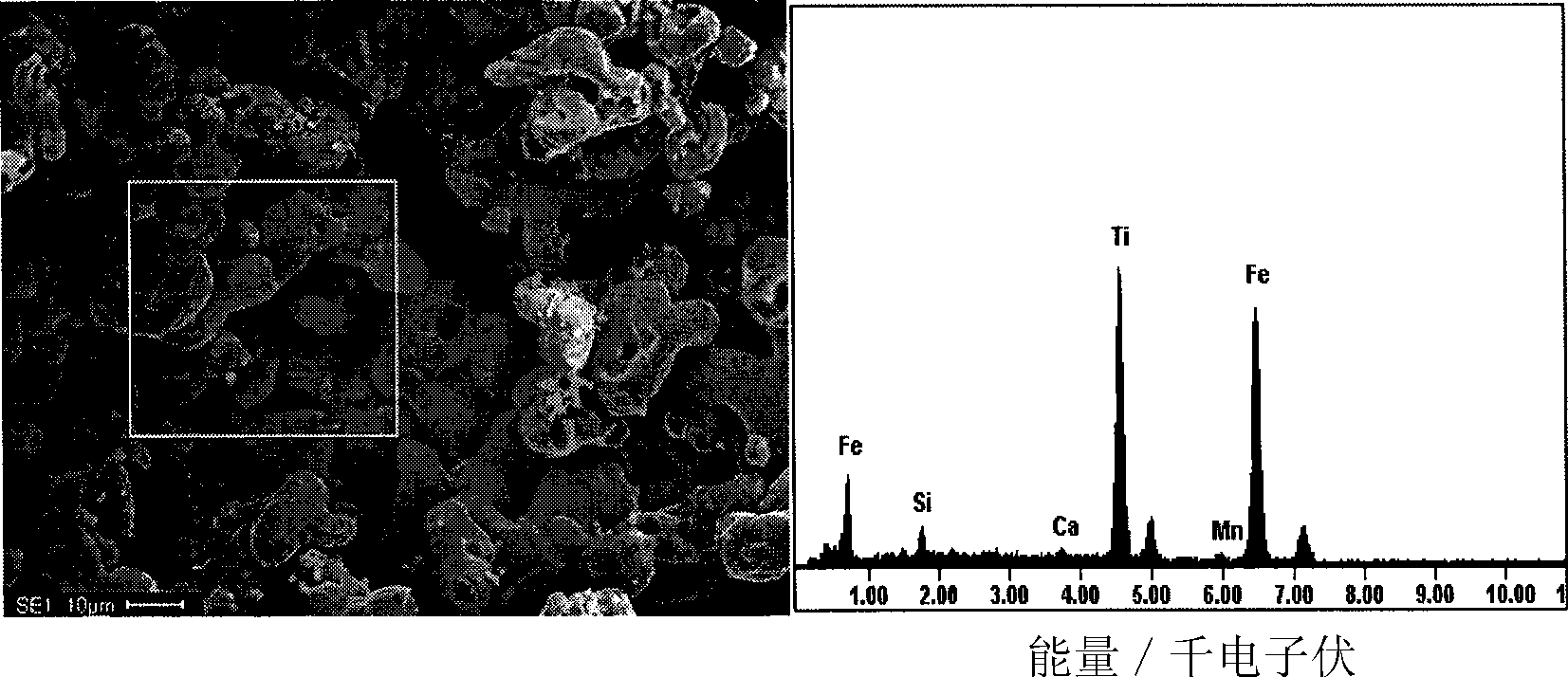
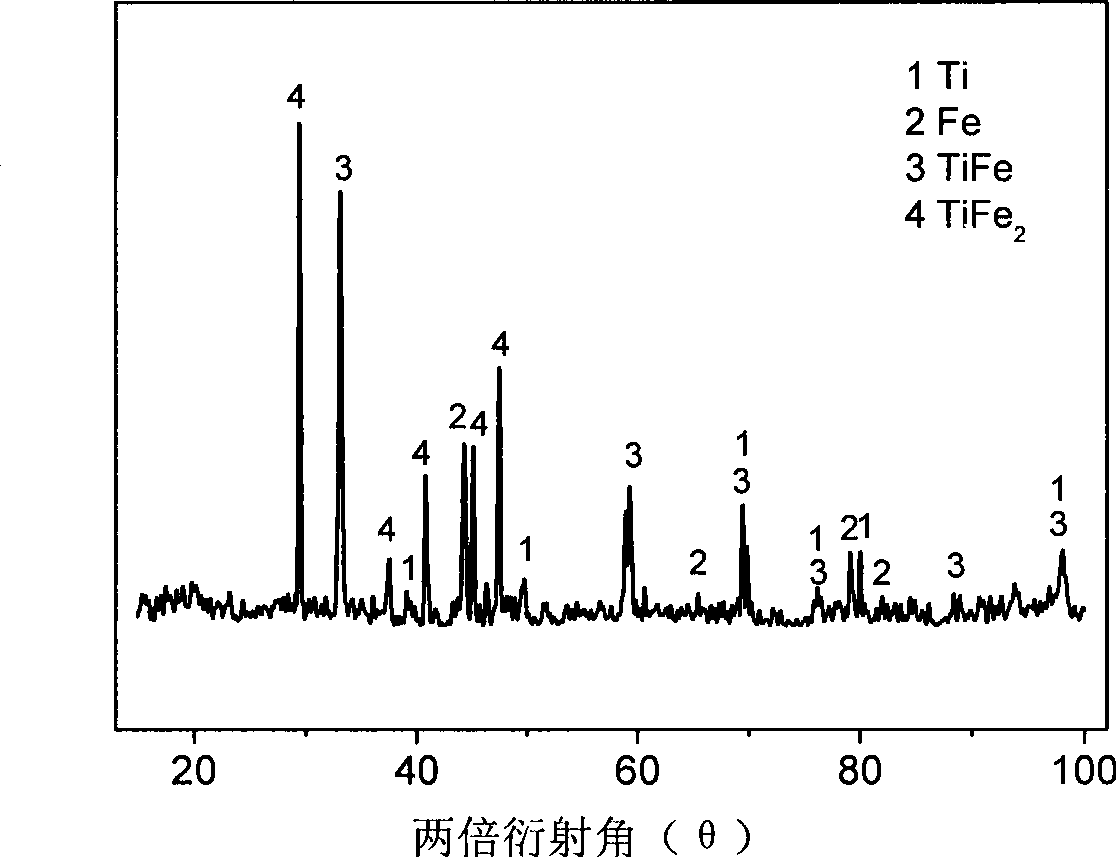
The invention provides a method for producing metallic titanium, particularly a method for preparing sponge metallic titanium through directly electrolyzing and reducing TiO2 by using a SOM (Solid Oxygen-permeation Membrane). The method for producing the sponge metallic titanium solves the problems of carbon pollution, low current efficiency and strict requirement on raw materials in an FFC (Flexible Flat Cable) method. The method adopted by the invention comprises the steps of exerting 3.2V voltage in an MgF2-CaF2 system of 1150 DEG C, and displaying the generation of the sponge metallic titanium through SEM (Scanning Electron Microscope) and XRD (X-Ray Diffraction) analysis after the electrolysis is carried out for two hours.
Owner:程思邈
Novel technology for producing medium/low carbon manganese iron by air three-times blowing method
InactiveCN103572057AGood production process controllabilityGood product quality controllabilityFurnace temperatureHigh carbon
The invention provides a novel technology for producing medium / low carbon manganese iron by an air three-times blowing method. The novel technology comprises the following steps: blowing primary air into a high carbon manganese iron flux, which is different from blowing oxygen by which high temperature evaporation manganese is generated and blowing components are damaged, and completely decarburizing through powerful dynamics stirring; blowing secondary air on a high carbon manganese iron flux liquid level so as to burn carbon monoxide generated by primary-air blowing for increasing heat, and adding more manganese ores so as to increase manganese oxide solid oxygen which facilitates decarburization and the recovery of manganese metal; blowing tertiary air and fuel gas into a furnace at the same time so as to provide external heat for increasing the furnace temperature, conditionally adding more manganese ores in an oxidation period and more reducing agent in a reduction period, conditionally adding a refinement period and meanwhile adding the manganese ores and the reducing agent so as to ensure the blowing technological operation and adjust product components for ensuring the quality.
Owner:王洪东
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com