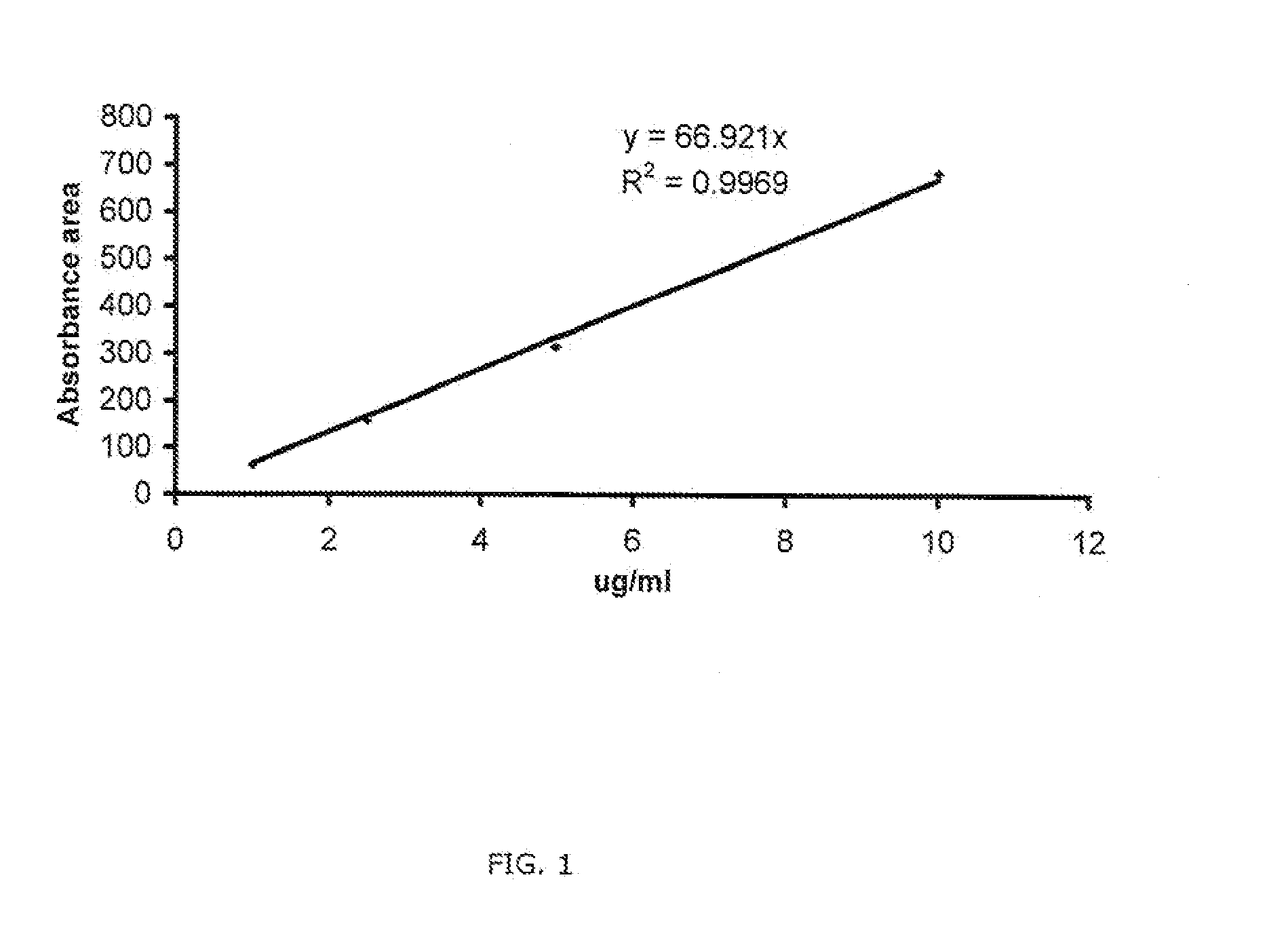
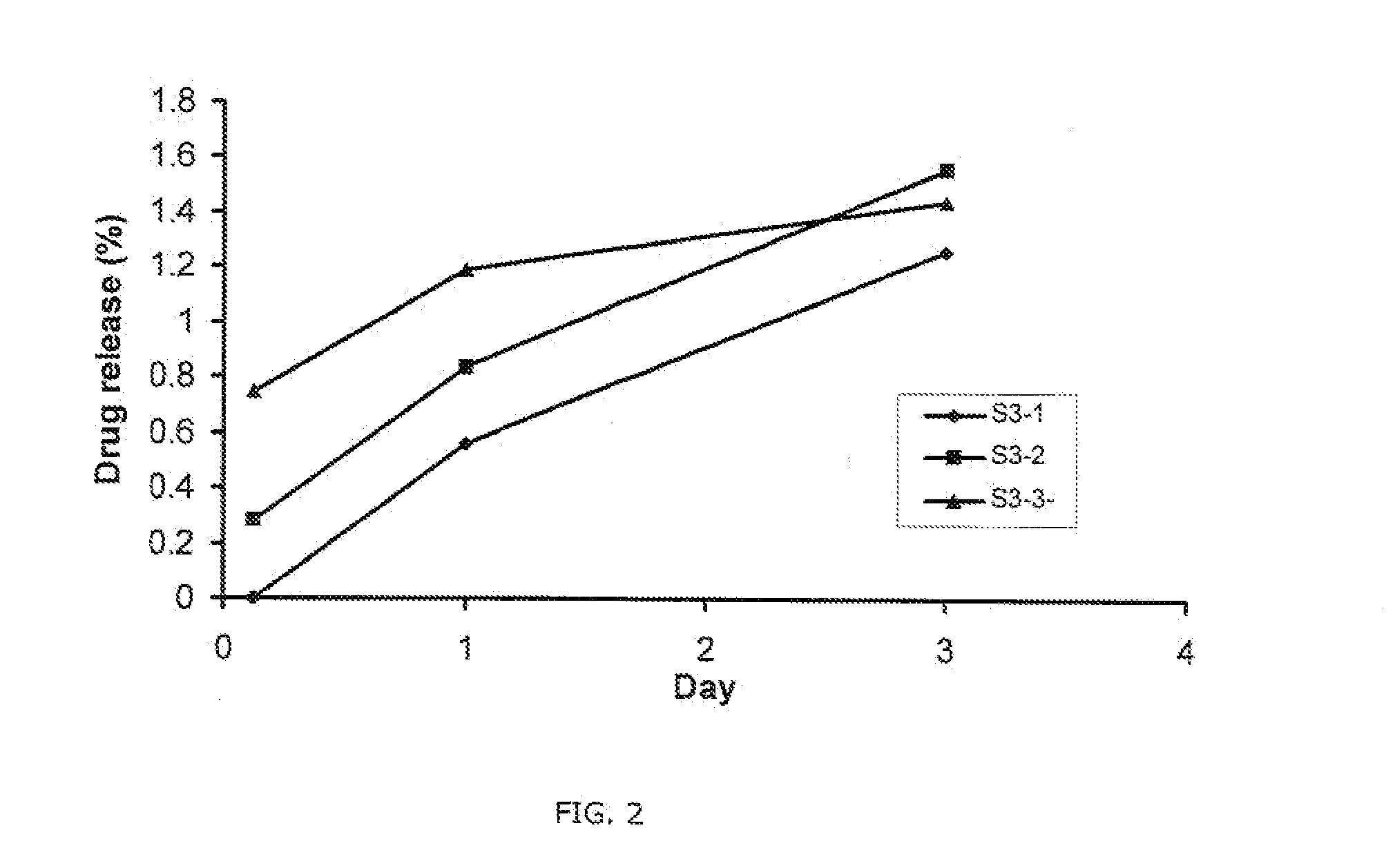
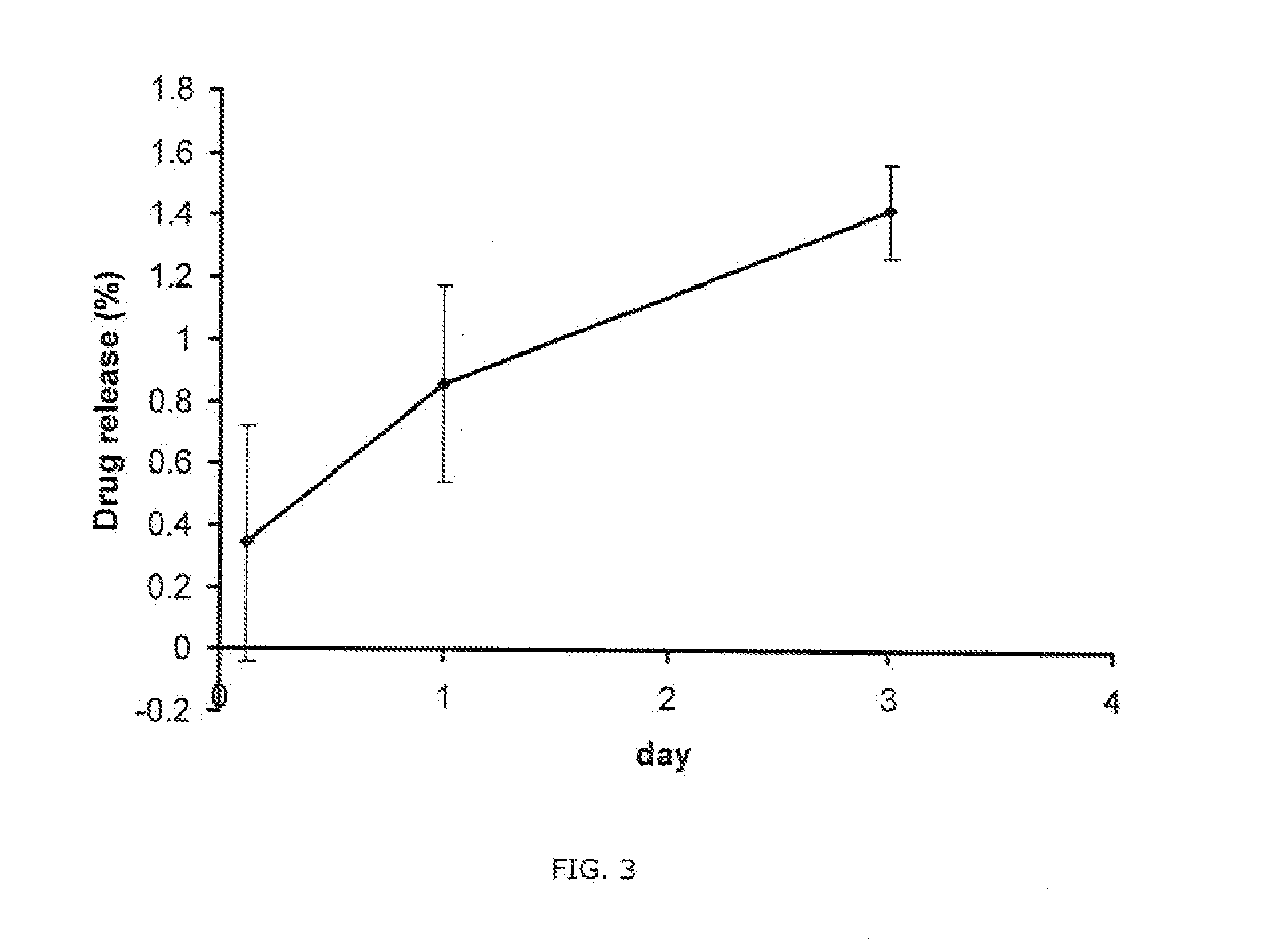
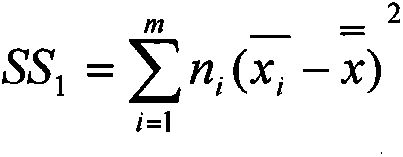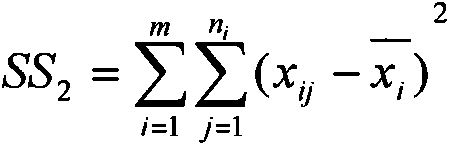Patents
Literature
31 results about "Gelatin matrix" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Gelatin Matrix, manufactured by Baxter Healthcare Corporation, consists of crosslinked gelatin granules and is provided sterile and nonpyrogenic in a - standard disposable syringe.
Non-adhesive elastic gelatin matrices
ActiveUS20060068013A1Minimize and inhibit infectionReduce chronic inflammationPowder deliveryAntipyreticCross-linkGelatin matrix
The present invention is a substantially non-adhesive elastic gelatin matrix. The matrix is both non-adhesive to wounds, tissues and organs and is also elastic such that it is flexible. The matrix is a lyophilized mixture of protein(s), polymer(s), cross-linking agent(s) and optional plasticizer(s). The invention also provides methods for making the non-adhesive elastic gelatin matrix.
Owner:COVALON TECH LTD
Processed dairy products
InactiveUS6503553B1Powder deliveryPeptide/protein ingredientsAdditive ingredientRefrigeration temperature
A range of products and methods are based on a creamy base of cream, artificial cream, thickened cream, cream cheese, mixtures thereof and mixtures with compatible incidental ingredients, the creamy base being aerated while cold and intimately mixed with a hot aqueous gelatin solution typically around 80° C. and providing 1-3% gelatin in the mixed product and the overrun in the aeration being typically in the range of 10-40%. Various further components are included or introduced with further processing selected from methods including heating the product with a further component to boiling point whereby de-aeration and enhanced shelf life is found in the resultant product. An alternative method is where flavor components are added prior to de-aeration. Another application is as a carrier for a pharmaceutical agent which is incorporated into the creamy base and protected with the gelatin matrix, a powder being formed ready for packaging in pharmaceutical doses. Another embodiment which does not require heating is incorporating a flavoring agent and food acid to obtain a pH of 3.0 to 4.2 an adding a large volume of water which has the effect of ensuring a low viscosity and the elimination of aeration whereby a pourable sauce-like product can be achieved at refrigeration temperatures.
Owner:AUSTRALIAN COOP FOODS
Particulate flavouring composition
The present invention relates to particulate compositions comprising controlled release particles wherein discrete elements of flavouring-containing fat are dispersed in a gelatine matrix, said particles containing: 0.1-40 wt%, preferably 5-30 wt% of flavouring; 10-70 wt%, preferably 20-50 wt% of gelatine; and 0.1-75 wt%, preferably 5-50 wt% of fat, the fat having a melting point of at least 35 DEG C; and said particles having a volume weighted average diameter of 50 - 1500 m. The particulate composition according to the present invention will release the flavouring comprised therein under specific conditions e.g. under the influence of shear forces, heat and / or moisture. The release rate upon consumption can be affected by varying the relative amounts of gelatine and fat and the gelling strength of the gelatine. These compositions are therefore particularly suitable for imparting longer lasting taste sensation to confectioneries, in particular chewing gume, and to toothpaste.
Owner:GIVAUDAN NEDERLAND SERVICES
Controlled Release Composition
The invention relates to the field of pharmacology. More specific, the invention relates to a controlled release composition.This invention is related to a controlled release composition comprising a cross-linked gelatin and at least one therapeutic protein wherein the ratio of the average mesh size (ξ) of the gelatin matrix and the average hydrodynamic radius (RH) of the therapeutic protein is smaller than 2, preferably smaller than 1.5.
Owner:FUJIFILM MFG EURO
Lyrate nightshade gelatin and its preparation method and use
InactiveCN1943676AFine and even textureImprove general conditionAntimycoticsPharmaceutical delivery mechanismGelatin matrixPreservative
This invention relates to the field of medical preparations, revealing a Lyrate nightshade gelatin and its preparation method and use. Performance and quality of the gelatin processed by the method of this invention is table. Compared with the common used external applied soft creams, the lyrate nightshade gelatin has many strong points: easy outspreading of its coated cloth, easy absorbed, fast action, without greasiness. Animal test results indicate that the gelatin can be used effectively to treat eczema and fungal skin disease. Toxin tests show that the gelatin is clinically safe. It can be safely and effectively externally used to treat chromic moist symptom, dermatistis, mycodermatitis. In the lyrate nightshade gelatin of this invention, the abstract from lyrate is 0.5-20%, gelatin matrix 1.5-30%, transdermal accelerant 0.05-8.0%, the moisture remaining agent 1.0-20%, the antiseptic 0.15-8.0% of the weight of the gelatin.
Owner:张干元 +1
Non-adhesive elastic gelatin matrices
The present invention is a substantially non-adhesive elastic gelatin matrix. The matrix is both non-adhesive to wounds, tissues and organs and is also elastic such that it is flexible. The matrix is a lyophilized mixture of protein(s), polymer(s), cross-linking agent(s) and optional plasticizer(s). The invention also provides methods for making the non-adhesive elastic gelatin matrix.
Owner:COVALON TECH LTD
Controlled release composition
The invention relates to the field of pharmacology. More specific, the invention relates to a controlled release composition.This invention is related to a controlled release composition comprising a cross-linked gelatin and at least one therapeutic protein wherein the ratio of the average mesh size (ξ) of the gelatin matrix and the average hydrodynamic radius (RH) of the therapeutic protein is smaller than 2, preferably smaller than 1.5.
Owner:FUJIFILM MFG EURO
Topical bisphosphonates for prevention of bone resorption
Bisphosphonates inhibit bone resorption associated with periodonatal or orthopedic surgery when applied topically to the bone. A novel formulation for topical application contains a gelatin matrix which is soaked in a solution containing a bone absorption inhibiting effective amount of a bisphosphonate or a pharmaceutically acceptable salt.
Owner:MERCK & CO INC
Chromium-containing gelatin standard substance and preparation method thereof
InactiveCN103558069AImprove uniformityImprove stabilityPreparing sample for investigationGelatin matrixWarm water
The invention discloses a chromium-containing gelatin standard substance and a preparation method thereof. The standard substance takes gelatin as a matrix, and chromium is added into the gelatin so that the chromium content of the standard substance is 10mg / kg. The chromium-containing gelatin standard substance and preparation method thereof disclosed by the invention have the following advantages and beneficial effects that: based on a characteristic that the gelatin is insoluble in clod water and soluble in warm water, trace of chromium is added into the gelatin matrix, so that the standard substance for detecting the chromium content of the gelatin is formed; the application of the standard substance facilitates the improvement of the matching degree of the standard substance and the sample matrix in the detection of the chromium content of the gelatin, thereby improving the accuracy and reliability of detection results. The preparation method of the standard substance is simple to operate and easy to promote, and the chromium content of the prepared gelatin standard substance is uniform.
Owner:NAT INST OF METROLOGY CHINA +1
Method for preparing graphene oxide based on gelatin, and application
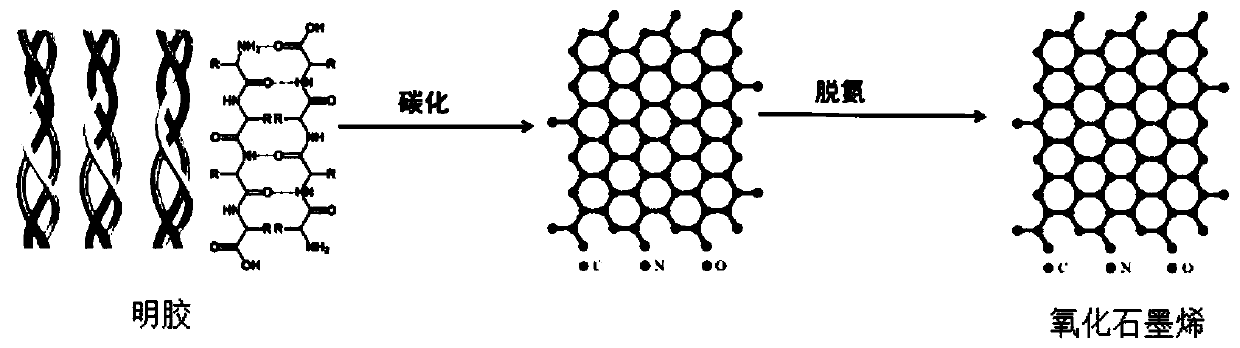
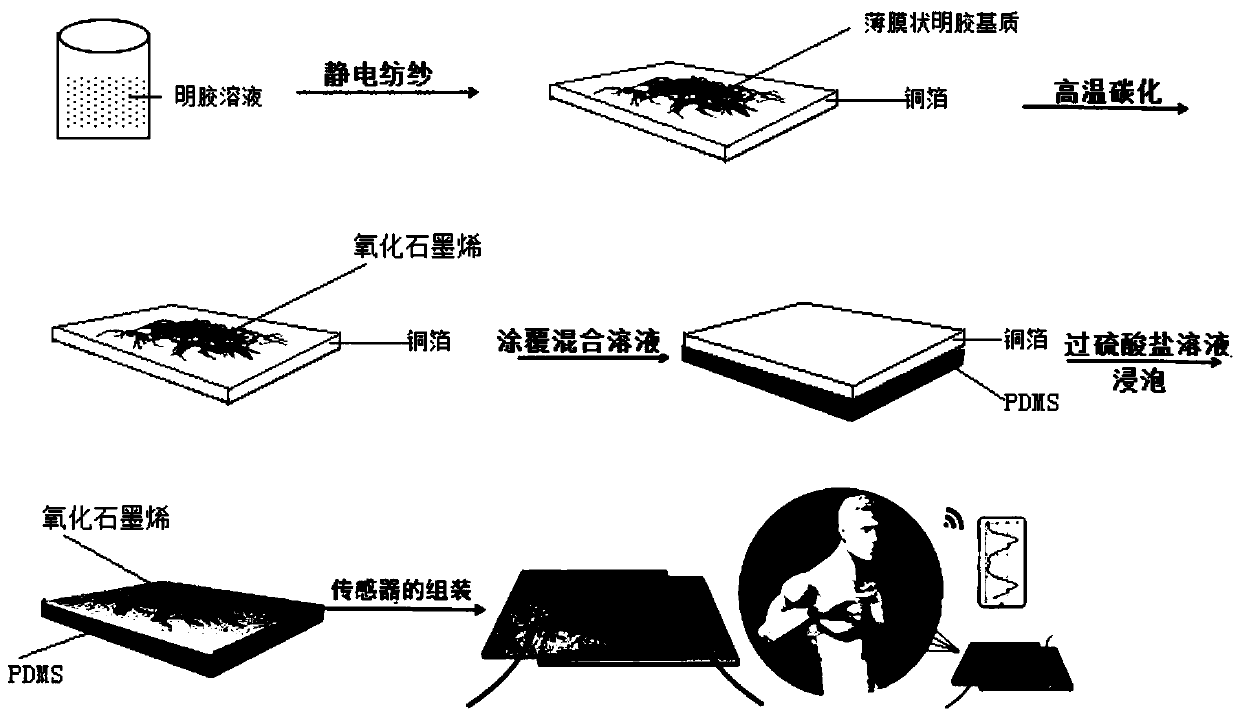
ActiveCN110357088AAbundant resourcesNo pollution in the processSubsonic/sonic/ultrasonic wave measurementCarbon compoundsCarbonizationSolvent
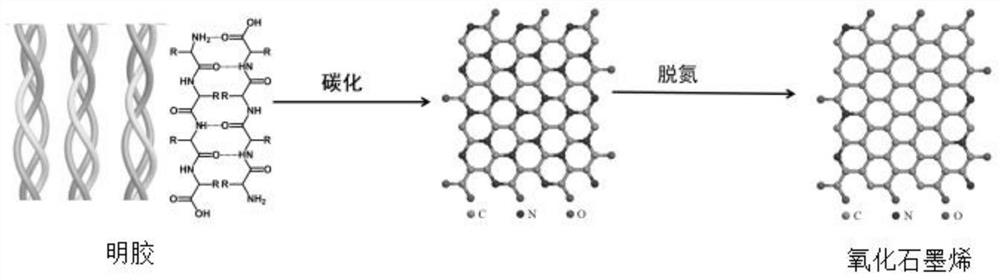
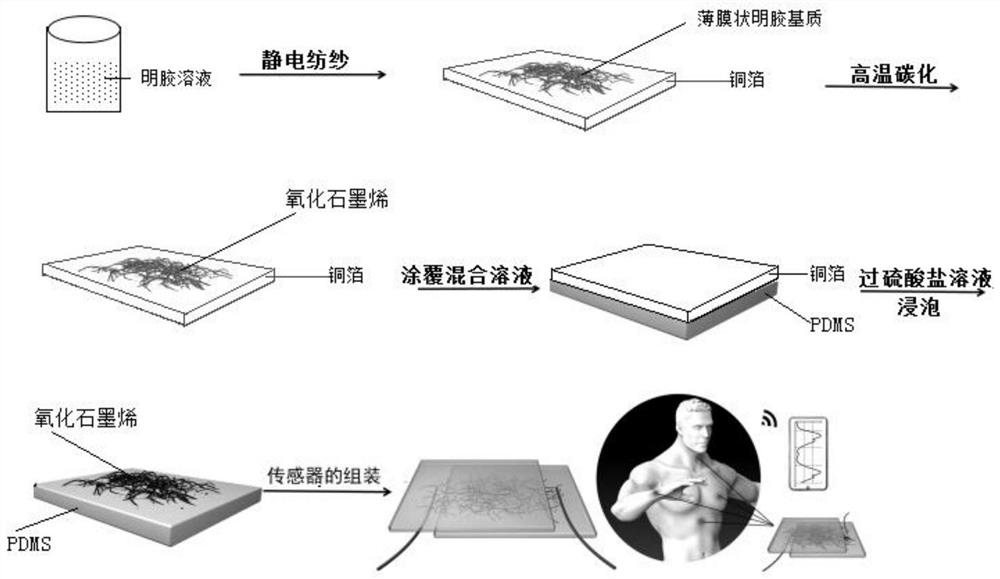
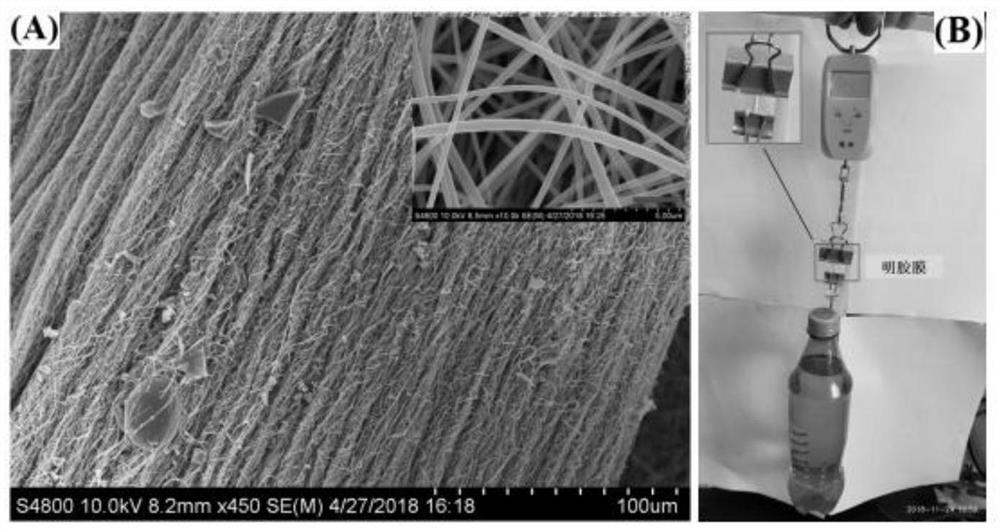
The invention relates to a method for preparing graphene oxide based on gelatin, and application thereof, and belongs to the technical field of graphene oxide. The technical problem that the method for preparing the graphene oxide in the prior art is unsafe, complex in process flow and high in cost is solved. The preparation method comprises the following steps: dissolving gelatin with solvent, and filtering to remove impurity to obtain a gelatin solution; volatizing the solvent in the gelatin solution to obtain a gelatin matrix in a fixed shape; and performing high-temperature carbonization treatment on the gelatin matrix in the fixed shape under the protection of the inert gas atmosphere protection, and cooling to room temperature to obtain graphene oxide. The preparation method takes biomass as a precursor to prepare the graphene oxide, the raw material is rich in resource, cheap and easy to obtain, pollution-free and renewable; and meanwhile, the method is safe and controllable inprocess, simple in flow and easy to operate.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Chewing gum containing a cross-linked gelatin matrix gum base
A chewing gum product containing a cross-linked gelatin matrix gum base and a bulking agent of erythritol, mannitol, or mixtures thereof. The cross-linked gelatin matrix can make up all or a portion of the gum base and still be comparable to that of a traditional elastomeric chewing gum product in its elasticity, cohesiveness, and feel in the mouth. The chewing gum of the present invention is also more environmentally friendly because it can be digestible and partially replace the need for petroleum-derived elastomers.
Owner:WM WRIGLEY JR CO
Method for preparing high-strength transparent chitin nanofiber gelatin composite membrane by mild method
PendingCN112523007AReduce degradationNatural structure intactFibre chemical featuresSynthetic cellulose fibresPolymer sciencePolymer chemistry
The invention provides a method for preparing a high-strength transparent chitin nanofiber gelatin composite membrane by a mild method, solves the technical challenges of chemical and mechanical treatment processes in the process of separating chitin fibers from chitin biological exoskeletons, and reduces the possibility of damage to the chitin fiber structure and performance reduction of the chitin fiber structure. The chitin nanofiber prepared by the mild method has a high specific surface area and a high length-diameter ratio. The chitin fiber can be better preserved by a mild treatment extraction mode, and the positive influence is generated on the performance of the mechanical film. Besides, different from a mixed casting method, the method has the advantages that the nano network structure of part of deacetylated chitin nanofibers is reserved in the gelatin matrix, and meanwhile, the higher transparency is still kept in a dry state. Good compatibility is shown through hydrogen bonds, and the mechanical property of the material is improved. The partially deacetylated chitin nanofiber gelatin nano composite material based on biocompatibility and biodegradable raw materials haspotential application prospects in biomedicine and food packaging industries.
Owner:NANJING FORESTRY UNIV
Method of culturing proliferative hepatocytes
The present invention relates to a method of culturing animal cells, preferably primary hepatocytes, comprising a first step of culturing the animal cells in non-adherent culture vessel, preferably alow or ultra-low attachment culture vessel, a second step of embedding the animal cells in a collagen matrix or in a gelatin matrix, and a third step of culturing the animal cells embedded in the collagen matrix or in the gelatin matrix, thereby obtaining 3D animal cell structures comprising proliferative animal cells, preferably spheroids comprising proliferative primary hepatocytes. The invention also relates to a spheroid comprising proliferative primary hepatocytes and the uses thereof for engineering an artificial liver model or an artificial liver organ, and for assessing in vitro the liver toxicity, genotoxicity and / or the effects of a drug or a compound.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Preparation method of degradable food fresh-keeping bag taking fish scale gelatin as matrix
InactiveCN111423679AMake up for some shortcomings of degradable plastic bagsHigh strengthFlexible coversWrappersCellulosePolyvinyl alcohol
The invention discloses a preparation method of a degradable food fresh-keeping bag taking fish scale gelatin as a matrix. The invention is prepared from the following components in parts by weight: 15 to 20 parts of fresh water scale gelatin, 25 to 35 parts of polyvinyl alcohol, 10 to 15 parts of calcium carbonate, 15 to 25 parts of lignocellulose aerogel, 5 to 10 parts of antioxidant, 10 to 15 parts of glycerinum and 5 to 10 parts of chitosan. Preparation of a solution includes mixing polyvinyl alcohol and deionized water in a mass ratio of 1: 1 to form a solution; then sequentially adding gelatin, lignocellulose aerogel, and calcium carbonate, and evenly mixing; carrying out ultrasonic treatment for 15 minutes, putting the mixed solution into a high-temperature steam oven at 110-120 DEGC, carrying out hot steam treatment, adding the antioxidant, glycerol and chitosan again, uniformly stirring, homogenizing for 2-3 times by using a microjet homogenizer (220-30MPa), finally putting the material into an extruder, and pressing to form a film. The degradation degree of the fish scale gelatin matrix food fresh-keeping bag within 50 days is greater than 30%. The invention can providea food plastic fresh-keeping bag with good degradability, antibiosis and antioxidation.
Owner:JIANGXI NORMAL UNIV
Preparation method of easily-degradable food preservative film with fish scale gelatin matrix
InactiveCN111349343AImprove high-value utilization valueReduce pollutionFlexible coversWrappersVitamin CWheat starch
The invention discloses an easily-degradable food preservative film with fish scale gelatin matrix. The invention belongs to the technical field of aquatic product processing. The preservative film isprepared from the following components in percentage by weight: 30%-40% of fresh water fish scale gelatin, 1%-5% of wheat starch, 0.01%-0.05% of vitamin C, 0.05%-0.1% of an essential oil, 1%-5% of 100u / ml glucoamylase, 1%-3% of a 5% magnesium chloride solution and the balance of deionized water. Fresh water fish scales are subjected to water washing, alkali washing, acid washing, hot water extraction and the like to prepare scale gelatin, and the scale gelatin is used as a main raw material to prepare the easily-degradable food preservative film with the scale gelatin matrix. According to a preparation method, wheat soluble starch, 100u / ml glucoamylase, and the 5% magnesium chloride solution are added, a degradation promoter is obtained thorugh mixing of the above three components, so that the propagation of soil aspergillus oryzae can be effectively promoted, and the soil aspergillus oryzae can accelerate the degradation rate of protein products, thereby obtaining the preservative film which is extremely easy to degrade.
Owner:JIANGXI NORMAL UNIV
Chewing article
InactiveCN100571784CReduce rubber propertiesGood physical propertiesPill deliveryMacromolecular non-active ingredientsGelatin matrixColloid
The invention relates to a gelatin matrix containing pharmaceutically active ingredients. In a preferred embodiment, hydrocolloids are added to the gelatin matrix to improve the physical properties, taste and mouthfeel of the gelatin matrix. A method of making a gelatin matrix comprising a hydrocolloid is also disclosed.
Owner:MCNEIL PPC INC
Chewing gum containing a cross-linked gelatin matrix gum base
A chewing gum product containing a cross-linked gelatin matrix gum base and a bulking agent of erythritol, mannitol, or mixtures thereof. The cross-linked gelatin matrix can make up all or a portion of the gum base and still be comparable to that of a traditional elastomeric chewing gum product in its elasticity, cohesiveness, and feel in the mouth. The chewing gum of the present invention is also more environmentally friendly because it can be digestible and partially replace the need for petroleum-derived elastomers.
Owner:WM WRIGLEY JR CO
A kind of agarose crystal gel matrix particles with super large pores and preparation method thereof
The invention relates to an agarose crystal gel matrix particle with extra large pores and a preparation method thereof. The agarose crystal gel matrix particle is characterized in that the super-pore agarose crystal gel matrix particle is a porous particle, and has the super pore of tens to hundreds of microns, the particle size of 2-5mm, the pore diameter of 5-200 micrometers and the porosity of 94-97 percent. The invention also provides a method for preparing the agarose crystal gel matrix particle by multi-micropipe reactor droplet forming and solvent crystallization hole forming. The agarose crystal gel matrix particle has good biocompatibility and the mutually communicated extra large pores, is high in porosity, excellent in permeability, and easy for realizing in-pore convective mass transfer of target objects, and therefore can serve as base materials of carriers for immobilization and biological separation media. The preparation method is simple, parallel amplification can be realized, large-scale industrial preparation is convenient, and the agarose crystal gel matrix particle with the extra large pores and the preparation method thereof have wide application prospect in the fields of biological separation, tissue engineering, immobilization of enzymes or cells, and the like.
Owner:ZHEJIANG UNIV OF TECH +1
Suppository for treating gynecopathy and its preparation technology
InactiveCN1224414CHighly effective anti-inflammatory effectGood purifying effect on the vaginaInorganic boron active ingredientsHydroxy compound active ingredientsDiseaseSide effect
The suppository for treating women's diseases is prepared with flavescent sophora root, sessile stemona root, cnidium fruit, agrimonia herb, alkanna, alum, boric acid, borneol, camphor and glycerine-gelatin matrix. It has the advantages of being convenient and safe, sanitary, high treating effect, no secondary and no toxic side effect. After the suppository with sanitary cotton strip is pushed into deep vagina with a disposable pushing device, the cotton strip meeting body fluid will expand to block medicine flow out and the cotton strip may be taken out easily together with inflammatory secretion. The vagina is cleaned while producing no contamination to clothing. The present invention is one Chinese medicine preparation without toxic side effect.
Owner:贵州长生药业有限责任公司
Prescription and preparation method of compound ornidazole suppository for treating vaginitis
InactiveCN105663125AExtract completelySimple preparation processOrganic active ingredientsSuppositories deliveryGelatin matrixMedicine
The invention relates to technical field of gynecology medical treatment, in particular to a prescription and preparation method of a compound ornidazole suppository for treating vaginitis.The compound ornidazole suppository is prepared by, by weight, 20-30 parts of ornidazole, 15-20 parts of clotrimazole and 365-375 parts of glycerin gelatin.The preparation method includes: evenly grinding the ornidazole, the clotrimazole and the glycerin gelatin, then adding into glycerin gelatin matrix melt by heat, pouring into a suppository die while the mixture is hot, and solidifying, smoothing and packaging to obtain the compound ornidazole suppository.The preparation method has the advantages that the influence of the matrix on content measuring can be eliminated, complete medicine extraction is achieved, the content measuring of the ornidazole and the clotrimazole is unaffected, and the absorbance of the ornidazole and the clotrimazole in a certain time is constant.The compound ornidazole suppository is simple in preparation process and stable in curative effect.The compound ornidazole suppository is sensitive and accurate, good in repeatability and easy to master when high performance liquid chromatography is used for measuring main components.
Owner:李达
Red pear fruit tree wound filling paste and preparation method thereof
InactiveCN105753574APromotes budding vitalityAvoid churnBiocideDead animal preservationGibberellinAmino acid
The invention disclose red pear fruit tree wound filling paste, and relates to the technical field of fruit tree planting. The red pear fruit tree wound filling paste is prepared from the following raw materials in parts by weight: 600-700 parts of montmorillonite, 20-30 parts of bentonite, 60-80 parts of gelatin matrix, 20-30 parts of modified starch, 2-4 parts of glucose, 3-5 parts of amino acid, 3-5 parts of nucleotide salts, 2-4 parts of manganese sulfate, 1-3 parts of ammonium molybdate, 1-3 parts of ferrous sulfate, 2-4 parts of magnesium stearate, 2-4 parts of zinc sulfate, 2-4 parts of a gibberellin agent, 2-4 parts of maltodextrin, 2-4 parts of magnesium stearate and 60-80 parts of traditional Chinese medicine extract. The red pair fruit tree wound filling paste has the beneficial effects that: the treatment agent can promote sprouting vital force of fruit trees, has good disease resistance, can implant outside nutrients into the fruit trees through the wound while preventing the nutrients from losing through the wound, can reduce the planting cost and can improve the economic benefits.
Owner:HEXIAN ZHUHAN MELON & FRUIT PLANTING FAMILY FARM
Microgel for delivering adipose-derived stem cells and preparation method thereof
PendingCN114276989AEvenly distributedAvoid damageBioreactor/fermenter combinationsAdditive manufacturing apparatusGelatin matrixBiocompatibility
The invention provides a preparation method of microgel for delivering adipose-derived stem cells, which comprises the following steps: completely dissolving methacryloyl gelatin and a photoinitiator phenyl-2, 4, 6-trimethylbenzoyl lithium phosphonate in phosphate buffer normal saline to obtain a gelatin matrix; taking adipose-derived stem cells transferred to the third generation or after the third generation in the logarithmic phase, putting the adipose-derived stem cells into the gelatin matrix, and enabling the adipose-derived stem cells to suspend in the gelatin matrix; and printing the gelatin matrix mixed with the adipose-derived stem cells by using DLP-3D printing equipment to obtain the microgel carrying the adipose-derived stem cells. According to the prepared microgel bearing the adipose-derived stem cells, the adipose-derived stem cells are evenly distributed in the microgel, the adipose-derived stem cells are basically spherical in the microgel, damage to the adipose-derived stem cells is small, and survival of the adipose-derived stem cells is facilitated. The biocompatibility is good, and the adipose-derived stem cells have no adverse reaction. The microgel contributes to in-vitro adipogenic differentiation of the adipose-derived stem cells, promotes proliferation of the adipose-derived stem cells, and can support in-vitro 3D culture of the adipose-derived stem cells.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
A kind of gelatin-based food packaging film and preparation method thereof
ActiveCN111073308BImprove securityGood physical and mechanical propertiesFlexible coversWrappersGlycerolCross linker
The invention discloses a preparation method of a gelatin-based food packaging film, which comprises the following process steps: 1) mixing and soaking 16-30 parts of gelatin and 100 parts of distilled water according to the mass parts of raw materials for 0.5-1.5 hours to obtain a gelatin matrix; 2. ) Add 15 to 25 parts of an aqueous solution of a titanium compound with a concentration of 3 to 10 g / L into the gelatin matrix, then add 15 to 25 parts of an aqueous solution of high-polymerization flavanols with a concentration of 0.5 to 2 g / L, and then add 3 to 6 parts of aqueous sodium hydroxide solution with a concentration of 0.1-1.2 mol / L and 3-6 parts of glycerin are cast into a film and dried to obtain a finished product. The present invention uses natural polyflavanol and high-safety titanium ion as a composite crosslinking agent, utilizes the principle that protein reactive sites increase after the coordination compound is formed by titanium ion and polyflavanol, and prepares high-safety Gelatin-based food packaging film with excellent physical and mechanical properties.
Owner:SHANTOU UNIV
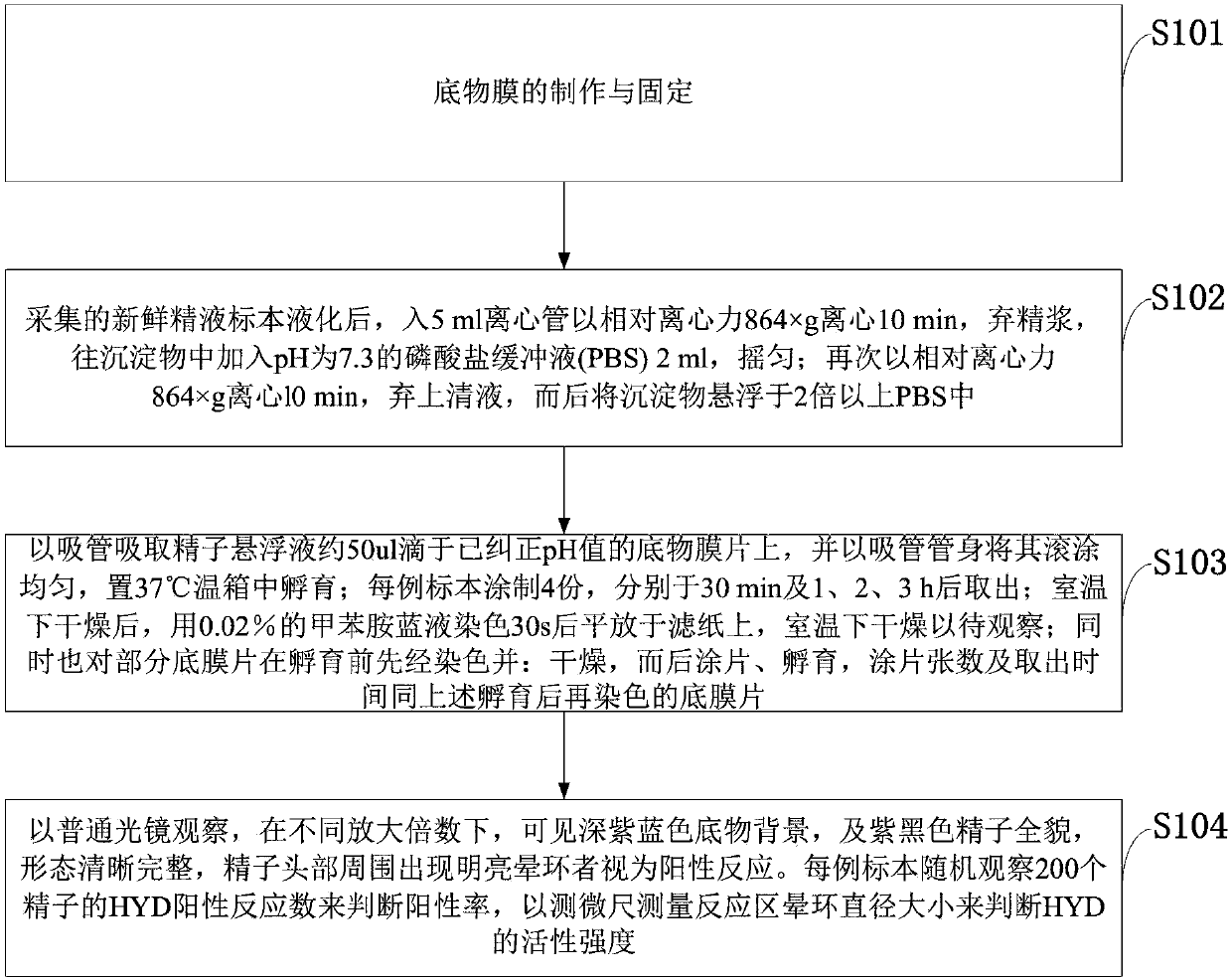
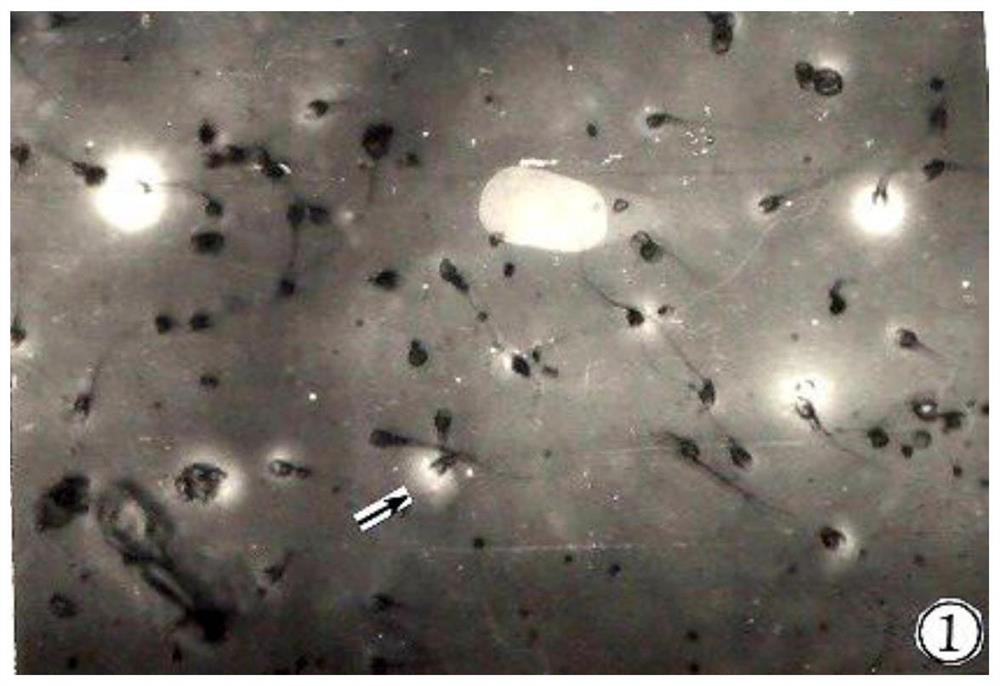
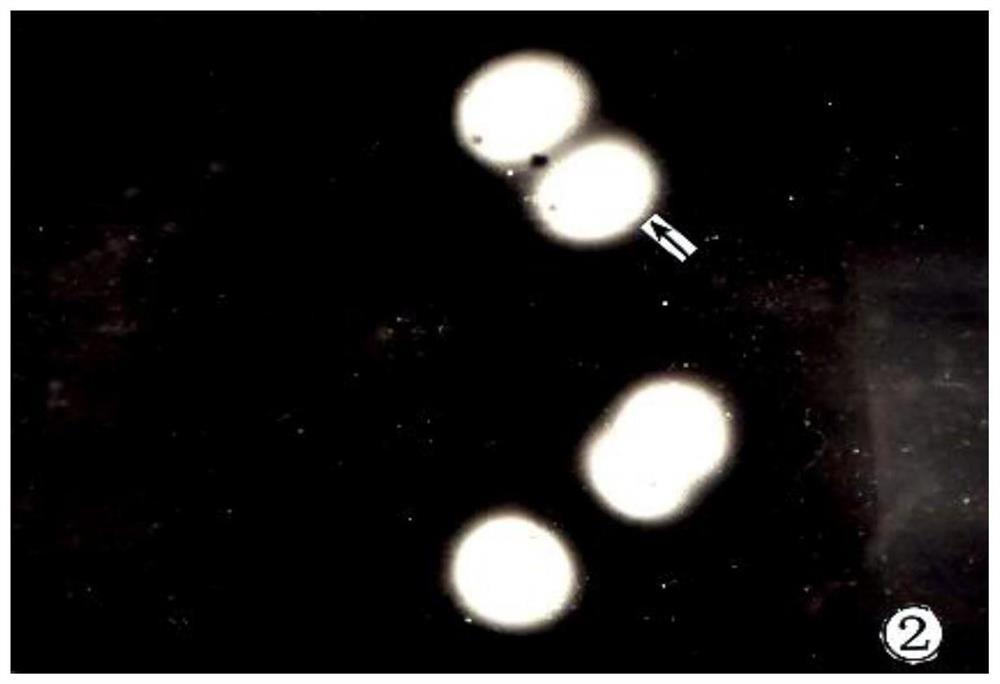
Manufacturing and applications of human sperm hyaluronidase kit
InactiveCN107807031APreparing sample for investigationMaterial analysis by optical meansDepolymerizationPhosphate
The invention belongs to the technical field of sperm hyaluronidase measurement, and discloses manufacturing and applications of a human sperm hyaluronidase kit. Sodium hyaluronate and a gelatin mixedsolution are used to prepare a sol-like matrix solution, then the sol-like matrix solution is painted and fixed to form a uniform base membrane for display activity of HYD; then sperms are incubatedon the ultrathin sodium hyaluronate-gelatin base membrane, centrifugation is performed, a phosphate buffer solution is used to wash the membrane inhibition factors to depolymerize sperm acrosome; thesodium hyaluronate-gelatin base membrane is released and dissolved, a pore area that surrounds the front part of sperms is formed; the sperms are observed by a light microscope, light penetrates the pore area, bright aureole appears on the periphery of the sperm heads; after dyeing, the enzyme reactions can be observed under a dark purplish blue background, and the HYD activity of each sperm can be estimated by measuring the diameter of each bright ring. The method is simple and the cost is low. The positive reaction rate of HYD can be calculated accurately. Stronger sperms are screened out for the test-tube baby technology, and the success rate is improved.
Owner:高晓勤 +1
Gelatin-based food packaging film and preparation method thereof
ActiveCN111073308AImprove securityGood physical and mechanical propertiesFlexible coversWrappersGlycerolEngineering
The invention discloses a preparation method of a gelatin-based food packaging film, which comprises the following steps: 1) mixing and soaking 16-30 parts by mass of gelatin and 100 parts by mass ofdistilled water for 0.5-1.5 hours to obtain a gelatin matrix; 2) adding 15-25 parts of a titanium compound aqueous solution with the concentration of 3-10g / L into the gelatin matrix; and adding 15-25parts of 0.5-2g / L high-polymerization-degree flavanol aqueous solution, adding 3-6 parts of 0.1-1.2 mol / L sodium hydroxide aqueous solution and 3-6 parts of glycerol, casting to form a film, and drying to obtain the finished product. According to the preparation method, natural polyflavanol and high-safety titanium ions are used as composite cross-linking agents, and the high-safety gelatin-basedfood packaging film with excellent physical and mechanical properties is prepared by utilizing a principle that protein reaction active sites are increased after the titanium ions and the polyflavanolform a coordination compound.
Owner:SHANTOU UNIV
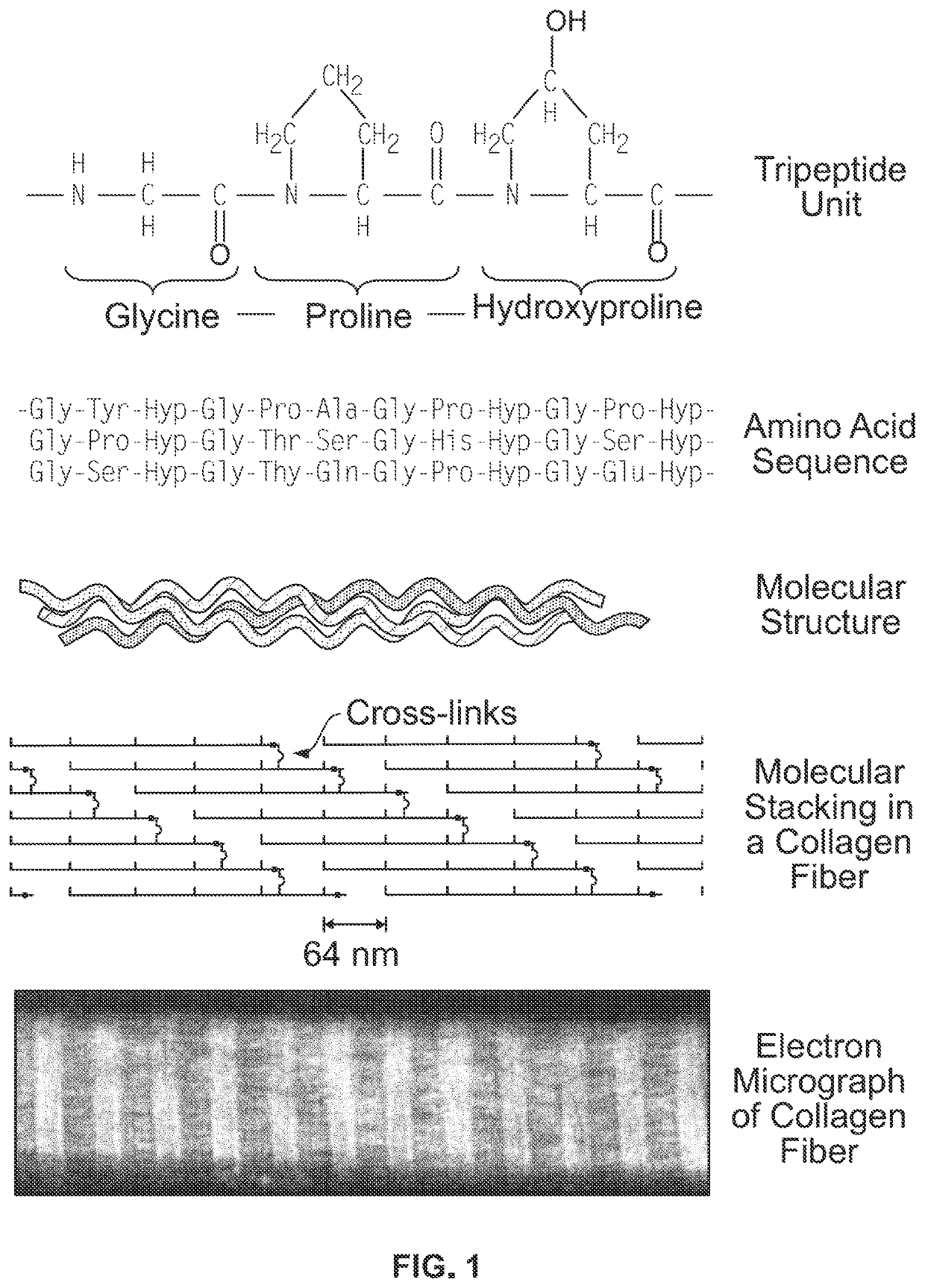
Material for bone implants and method of producing same
The present invention relates to a material for bone implants, comprising: a surface of oxidic ceramic materials, titanium or polyether ether ketone (PEEK) or other polymer or composite materials, a matrix of collagen or gelatin, which is covalently bound to said surface, and calcium phosphate embedded into said matrix. The present invention further relates to a method for producing the material according to the invention, to bone implants comprising the material according to the invention, and to its use as a bone implant material.
Owner:STIMOS GMBH
A kind of method and application of preparing graphene oxide based on gelatin
ActiveCN110357088BAbundant resourcesNo pollution in the processSubsonic/sonic/ultrasonic wave measurementCarbon compoundsGelatin matrixCarbonization
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
A kind of human sperm acrosome enzyme kit
ActiveCN107858398BObserve the reactionAccurate calculation of positive response rateMicrobiological testing/measurementStainingMatrix solution
The invention belongs to the technical field of sperm acrosinase measurement, and discloses a human sperm acrosinase kit. The gelatin mixture is made into a sol-like matrix liquid, which is coated and fixed into a uniform base film, and the acroinase (Acroin) is displayed. Activity; spermatozoa are incubated on an ultra-thin gelatin base membrane, centrifuged and washed with phosphate buffer to remove the membrane inhibitor sperm acrosome depolymerization, release and dissolve-gelatin matrix membrane, forming a pore area around the front of the spermatozoon; by Light microscope observation, the light penetrates the hole area, and a bright halo is seen around the sperm head. Against the background of dark purple blue after staining, the enzyme reaction is observed, and the diameter of each bright ring is measured to estimate a single sperm The strength of acrosome enzyme activity. The method of the invention is simple and low in cost, accurately calculates the positive reaction rate of the acrosome enzyme, screens out more active sperm for the test-tube baby technique, and improves the success rate.
Owner:高晓勤 +1
Gelatin standard substance containing chromium and preparation method thereof
InactiveCN103558069BImprove uniformityImprove stabilityPreparing sample for investigationGelatin matrixWarm water
The invention discloses a chromium-containing gelatin standard substance and a preparation method thereof. The standard substance takes gelatin as a matrix, and chromium is added into the gelatin so that the chromium content of the standard substance is 10mg / kg. The chromium-containing gelatin standard substance and preparation method thereof disclosed by the invention have the following advantages and beneficial effects that: based on a characteristic that the gelatin is insoluble in clod water and soluble in warm water, trace of chromium is added into the gelatin matrix, so that the standard substance for detecting the chromium content of the gelatin is formed; the application of the standard substance facilitates the improvement of the matching degree of the standard substance and the sample matrix in the detection of the chromium content of the gelatin, thereby improving the accuracy and reliability of detection results. The preparation method of the standard substance is simple to operate and easy to promote, and the chromium content of the prepared gelatin standard substance is uniform.
Owner:NAT INST OF METROLOGY CHINA +1
Compositions for radiotherapy and uses thereof
Provided herein are kits, compositions, and methods for treatment of a disease, disorder, or condition, such as a proliferative disease, disorder, or condition. One aspect provides a composition including a radioisotope, a gelatin matrix and bovine collagen or a thixotropic gel. Another aspect provides methods for treating a disease, disorder, or condition.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com