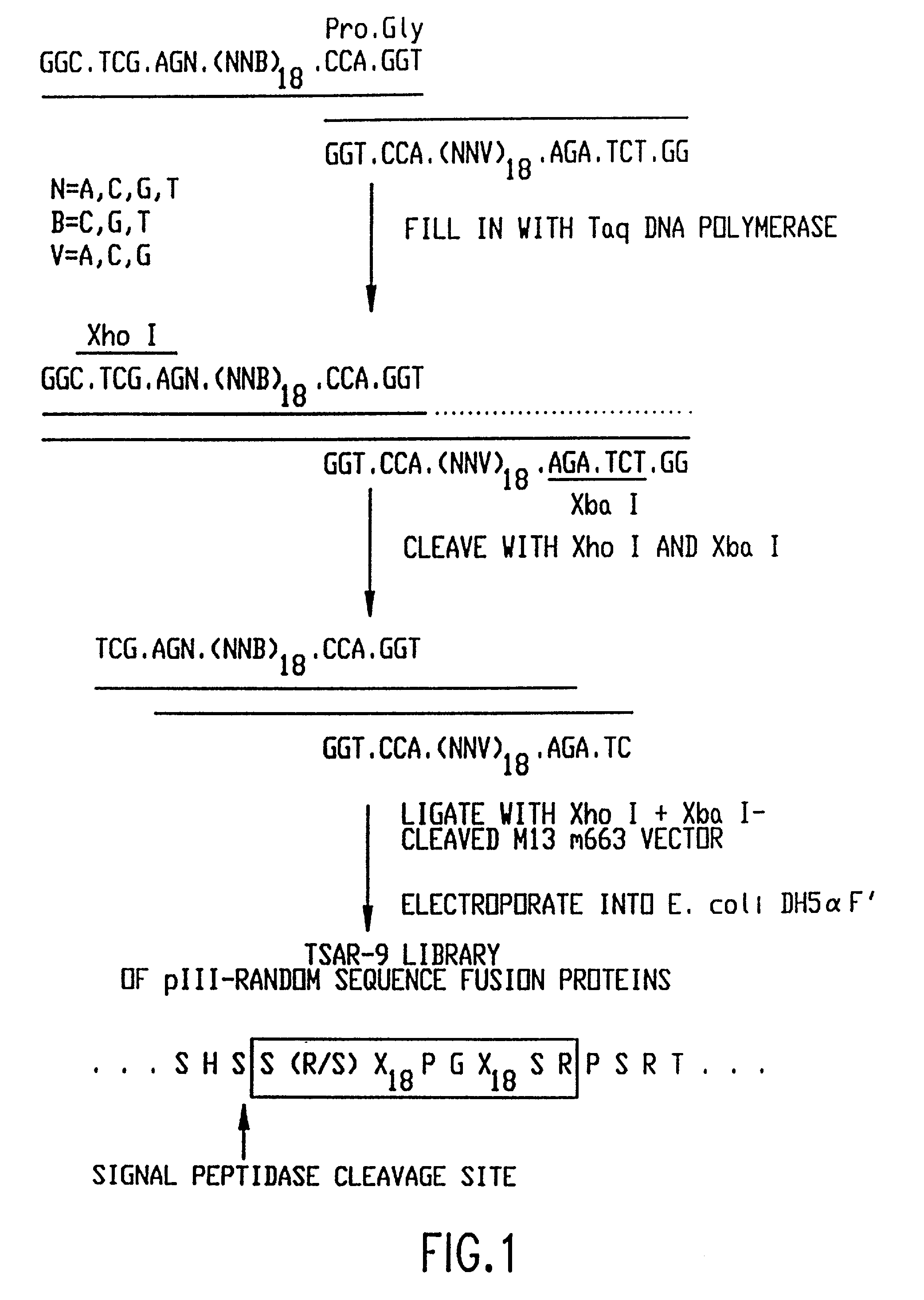
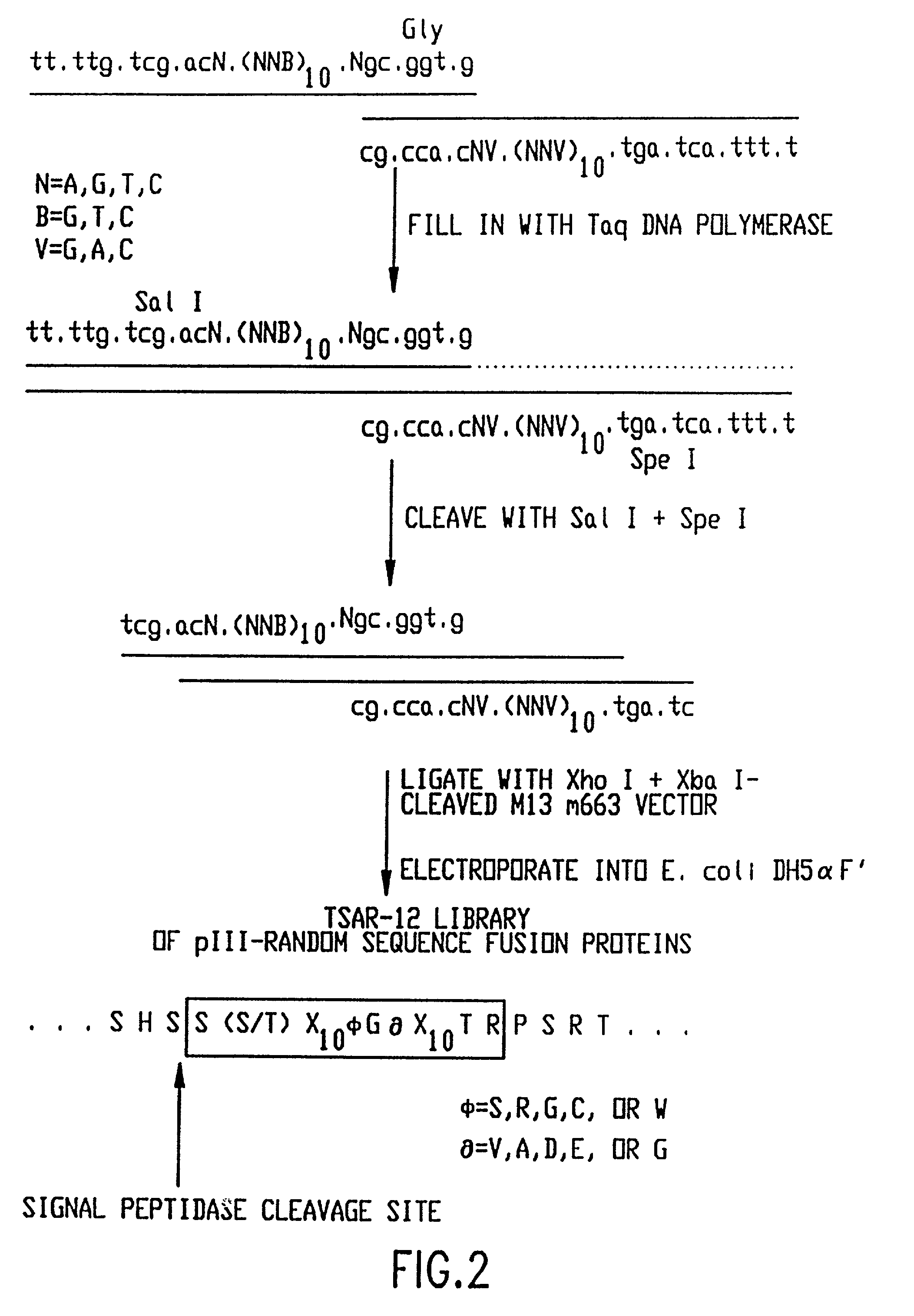
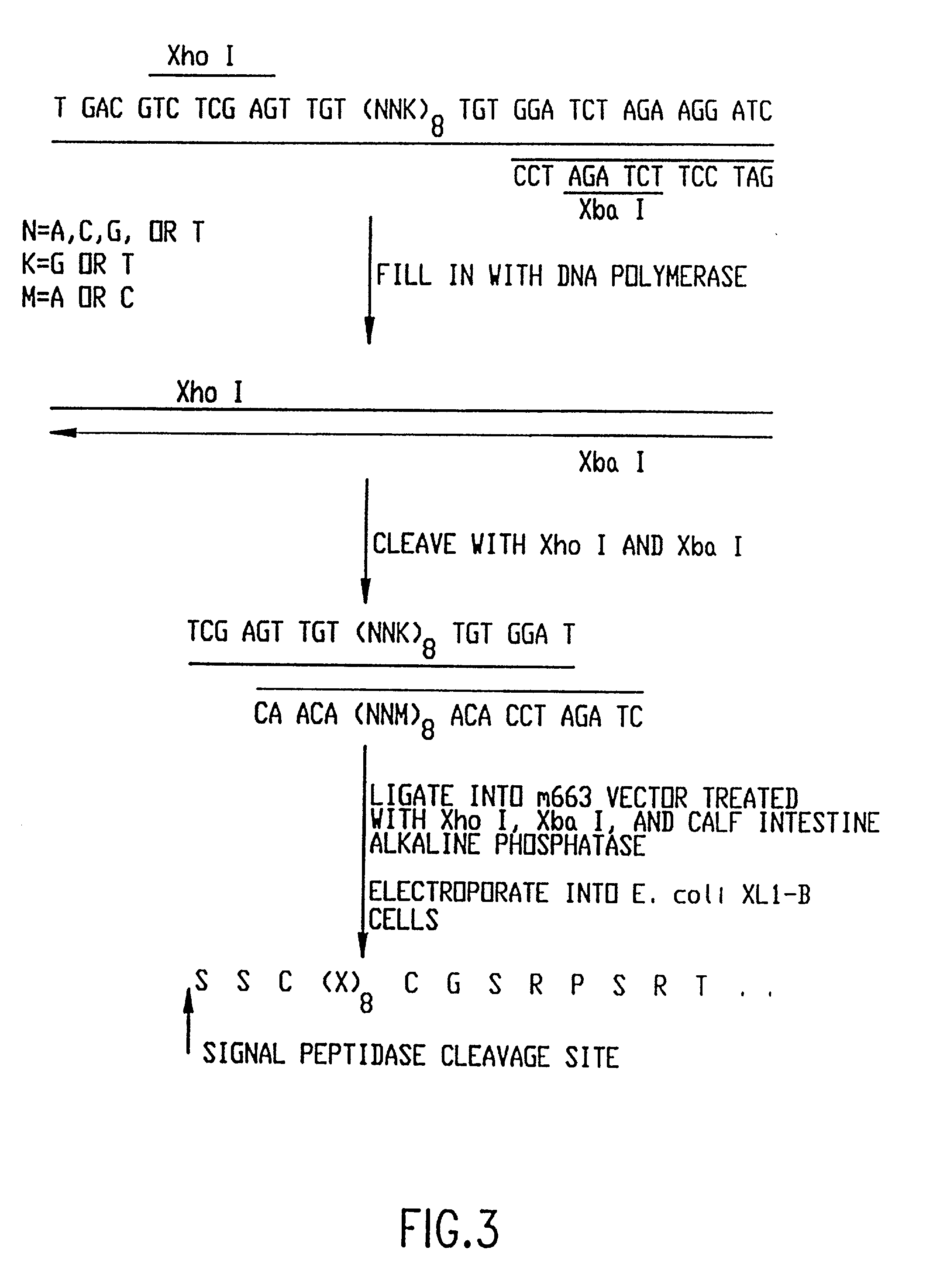
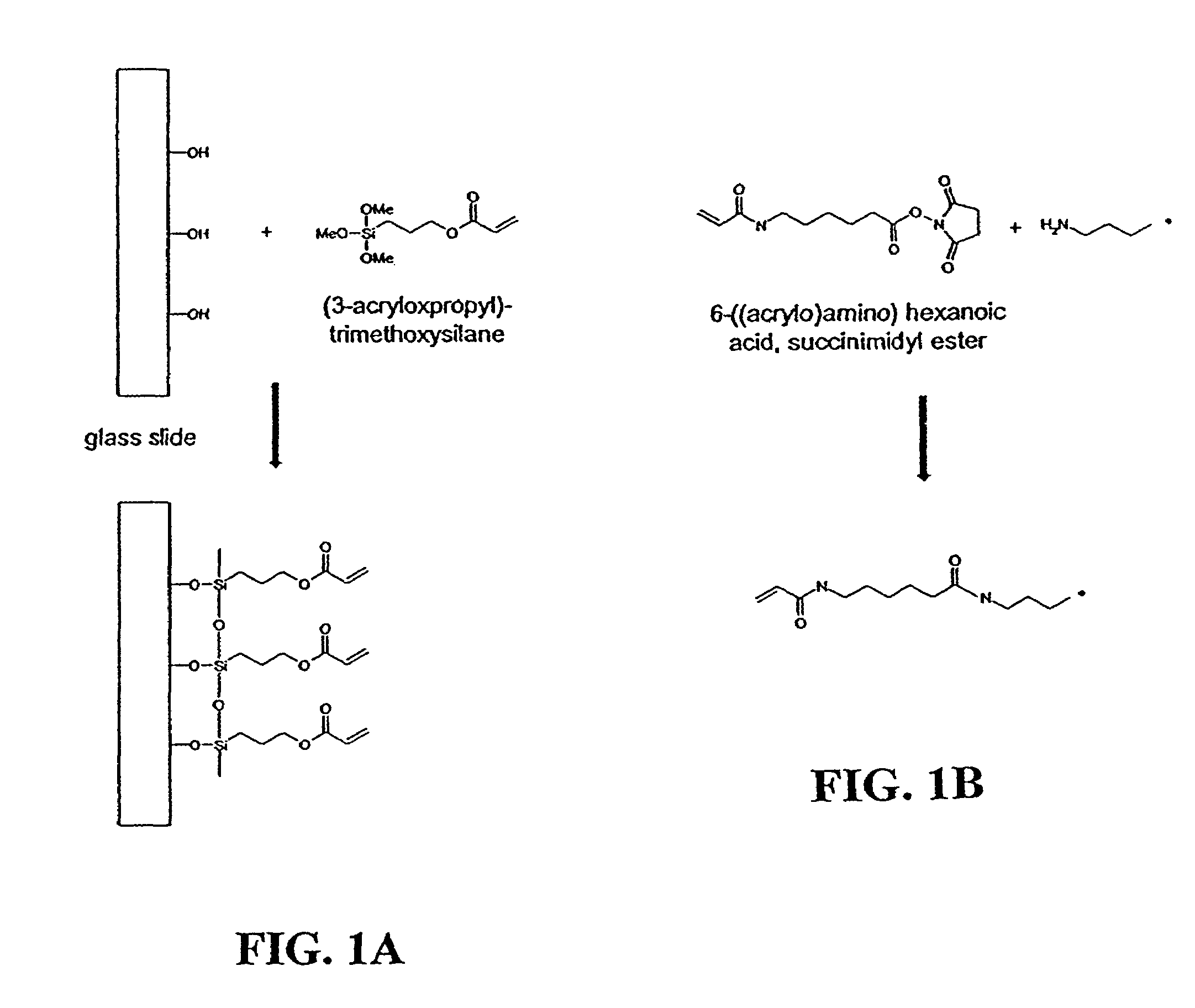
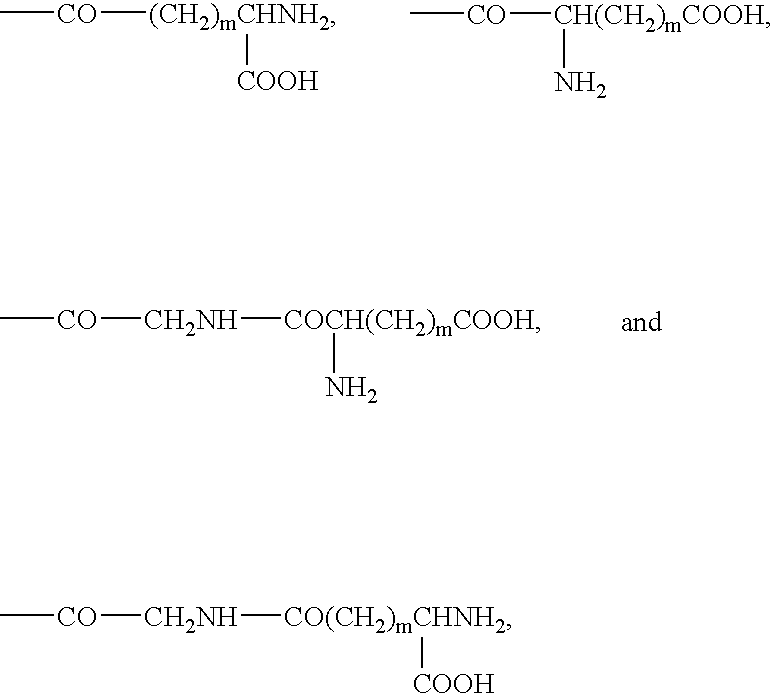Patents
Literature
40 results about "Glutathione Transferases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glutathione S Transferase (GST) is a vital enzyme, that is separate from glutathione (GSH), the master antioxidant (and the main subject of this website). GST and GSH work together in harmony to detoxify! Glutathione S Transferase (GST) is an enzyme that aids in detoxification.
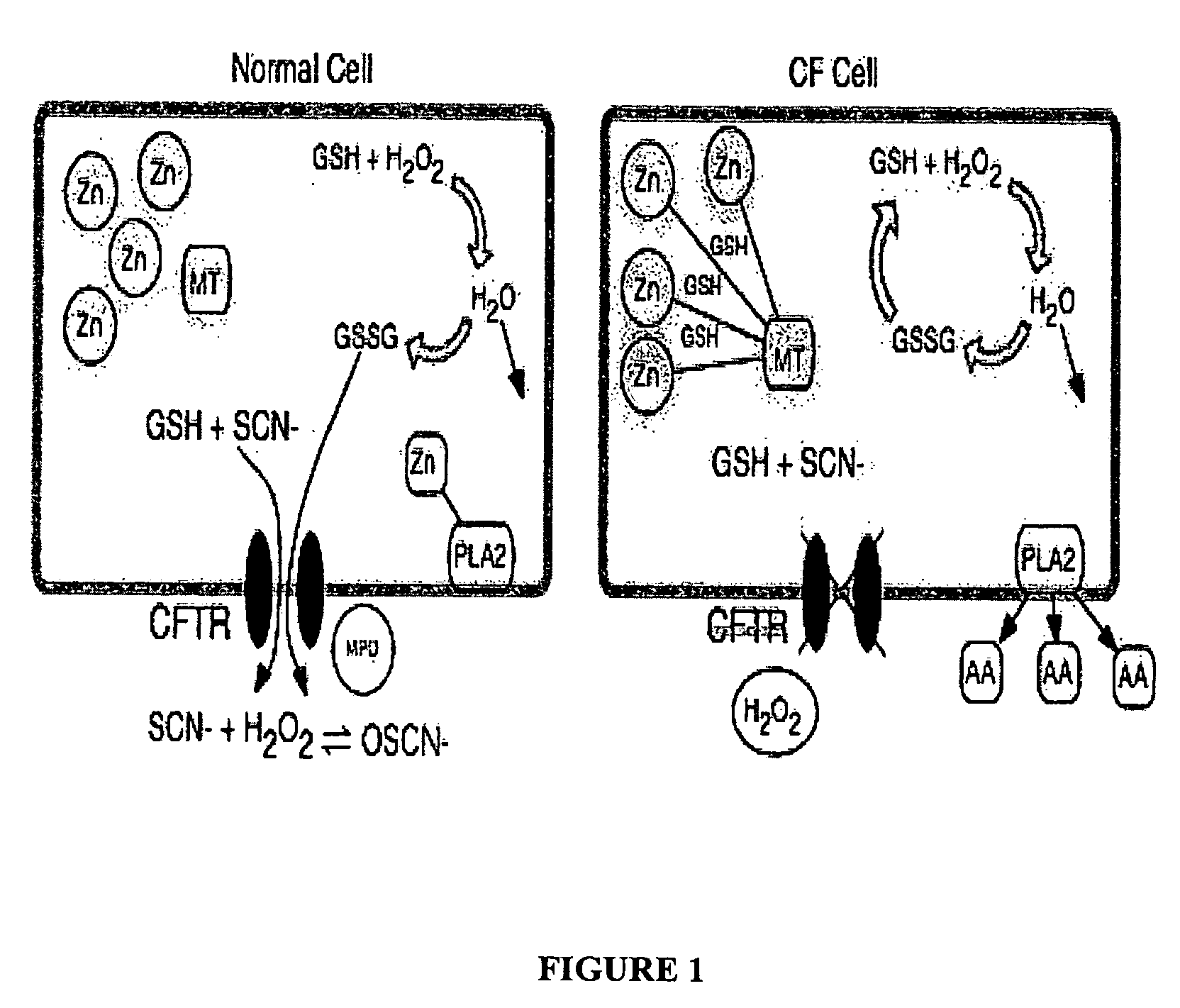
Methods and composition for the treatment of cystic fibrosis and related illnesses
InactiveUS20060258599A1Effective treatmentCorrect cellular GSH imbalancesBiocideCarbohydrate active ingredientsDiseaseGlutathione binding
The present invention relates to methods and compositions to treat subjects having cystic fibrosis. These compositions comprise the class of isothiocyanates. Isothiocyanates, absorbed by a cell are conjugated with glutathione GSH by glutathione-s-tranferase (GST). The conjugates are substrates of the multi-drug resistance associated (MRP) / multi-drug resistance (MDR) proteins. These proteins are functionally redundant to the cystic fibrosis transmembrane conductance regulator (CFTR), allowing for the substrate conjugates to be exported from the cell. The export of GSH conjugates restores intracellular and extracellular levels of GSH to normal levels. Normalizing both extracellular and intracellular GSH via the increased conjugation of isothiocyanates with GSH, and subsequent export, can significantly rectify numerous enzymatic processes and correct the pathologies that are typical of patients suffering from cystic fibrosis.
Owner:CHILDERS MELANIE
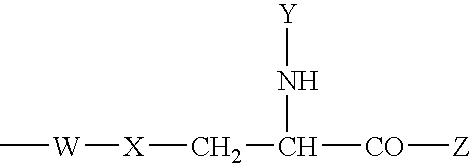
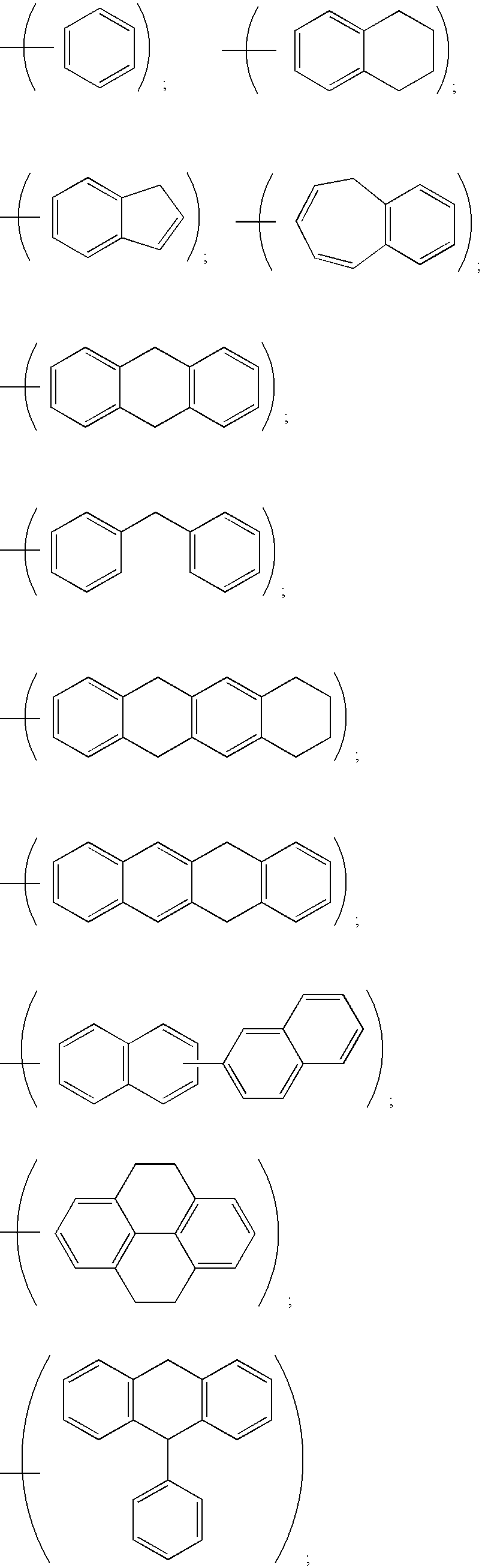
Bivalent inhibitors of Glutathione-S-Transferases
InactiveUS20050004038A1High affinityHigh selectivityBiocideTetrapeptide ingredientsCrystallographyIsozyme
Bivalent inhibitors having affinity for one or more dimeric GST isozymes are provided. The bivalent inhibitors comprise two ligand domains connected by a molecular linker, wherein the ligand domains have affinity for one or more monomers in the one or more dimeric GST isozymes. The ligand domains are separated by a distance ranging from about 5 to about 100 Å. The bivalent inhibitors of the invention demonstrate greatly improved affinity for GST isozymes. In a specific embodiment, the bivalent inhibitors of the invention further provide affinity for substantially one GST isozyme and for substantially one GST class. The bivalent inhibitors of the invention have numerous uses that include the treatment of drug-resistant cancer, malaria, and stimulation of hematopoiesis.
Owner:LYON ROBERT P +3
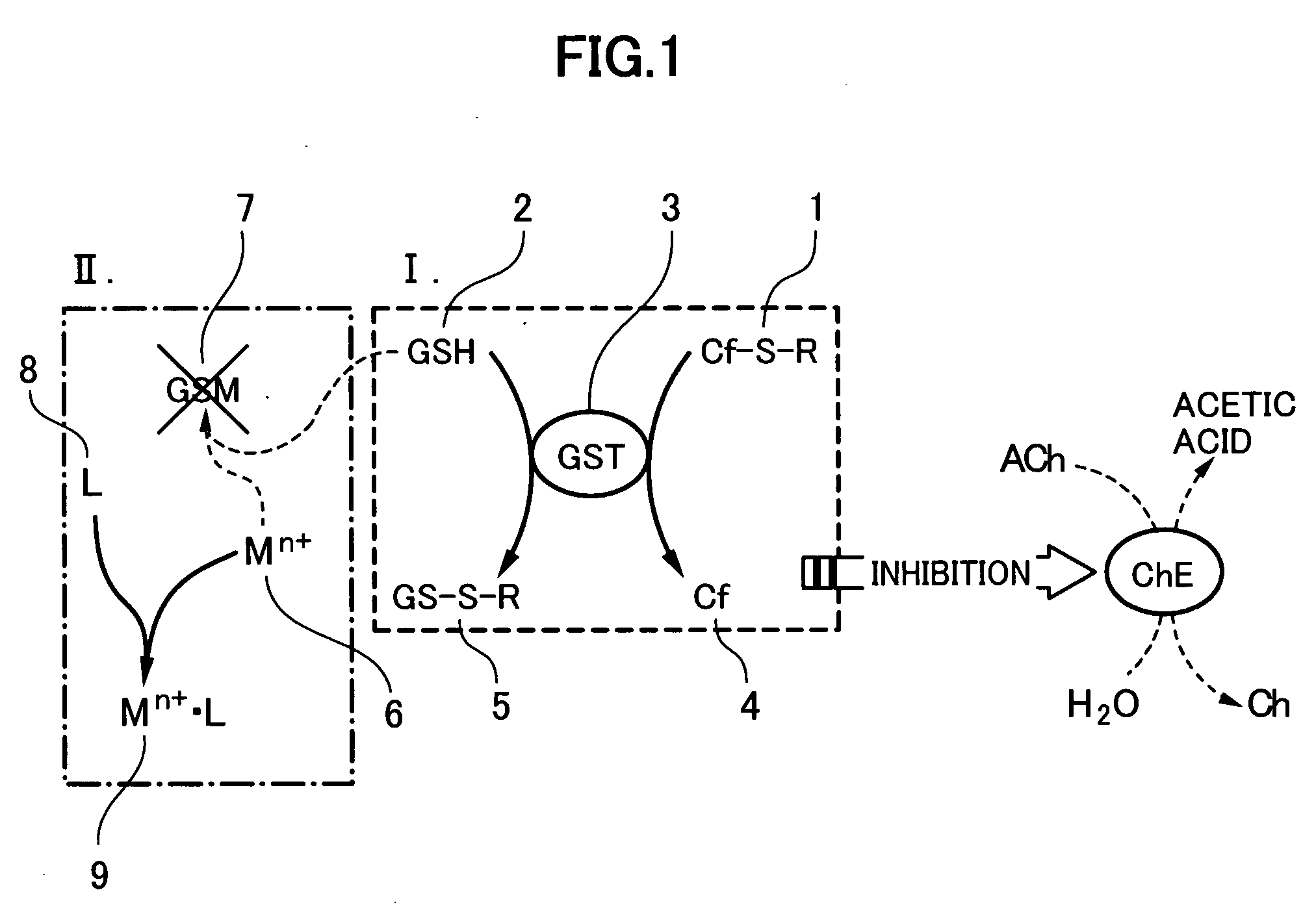
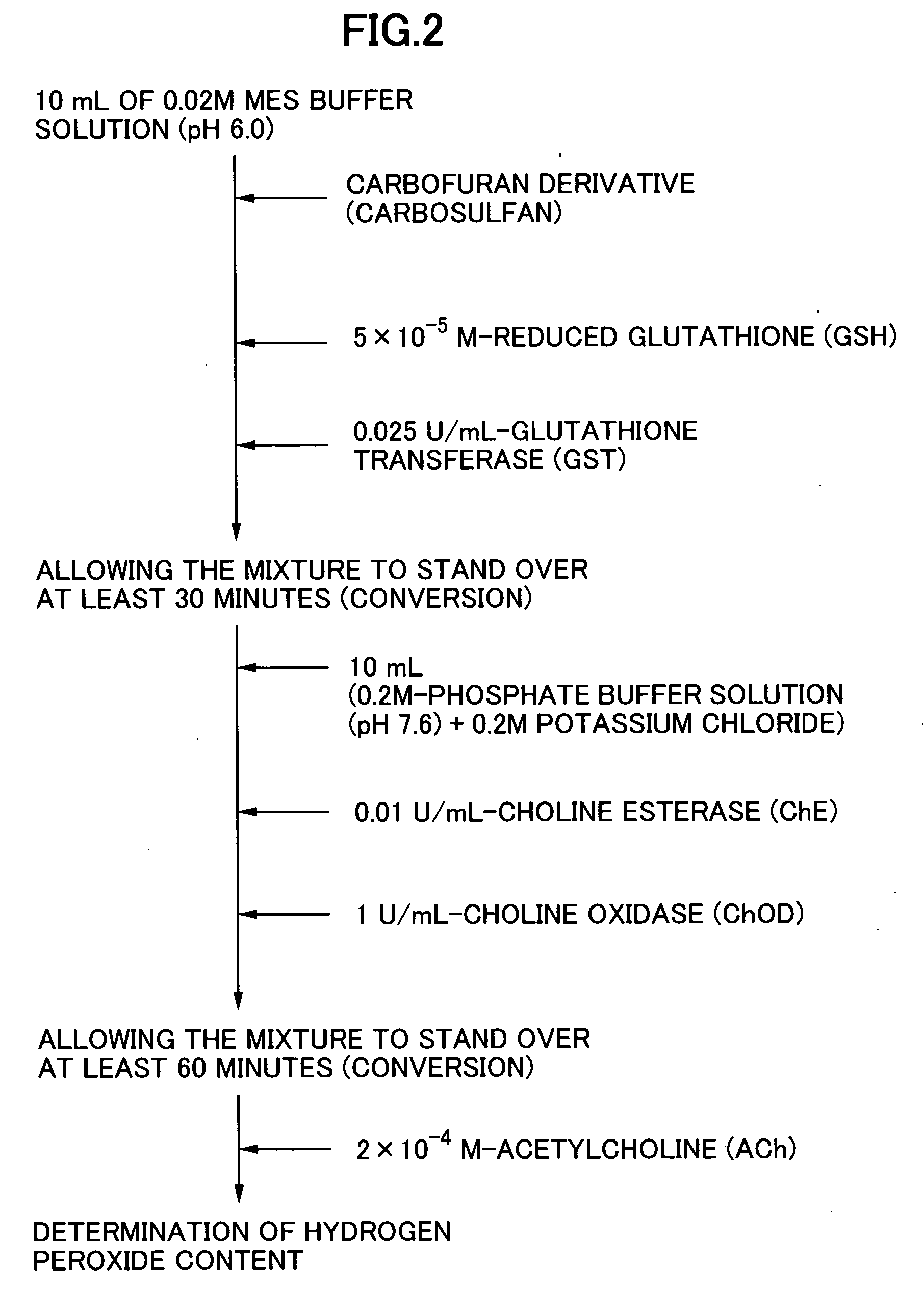
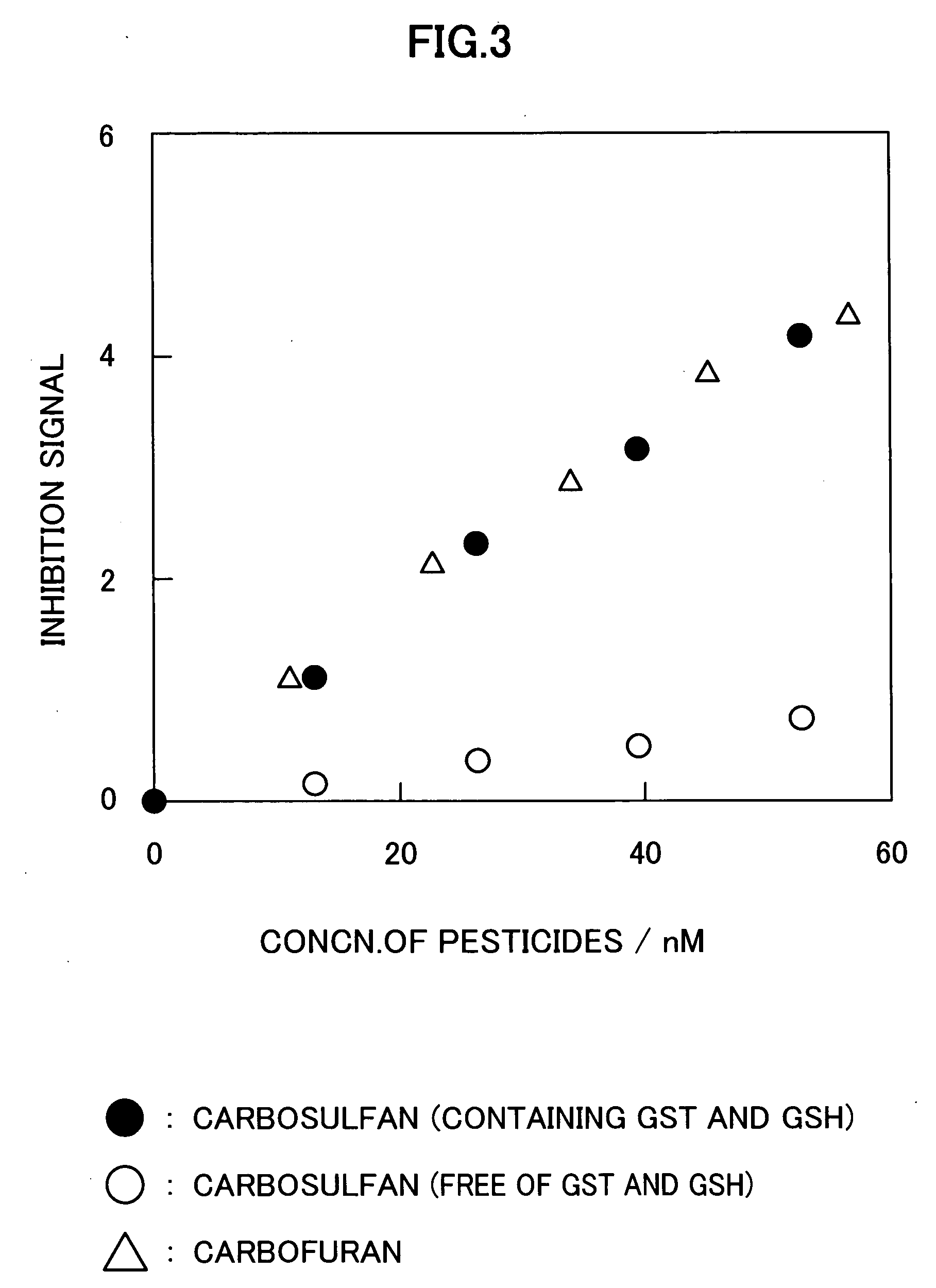
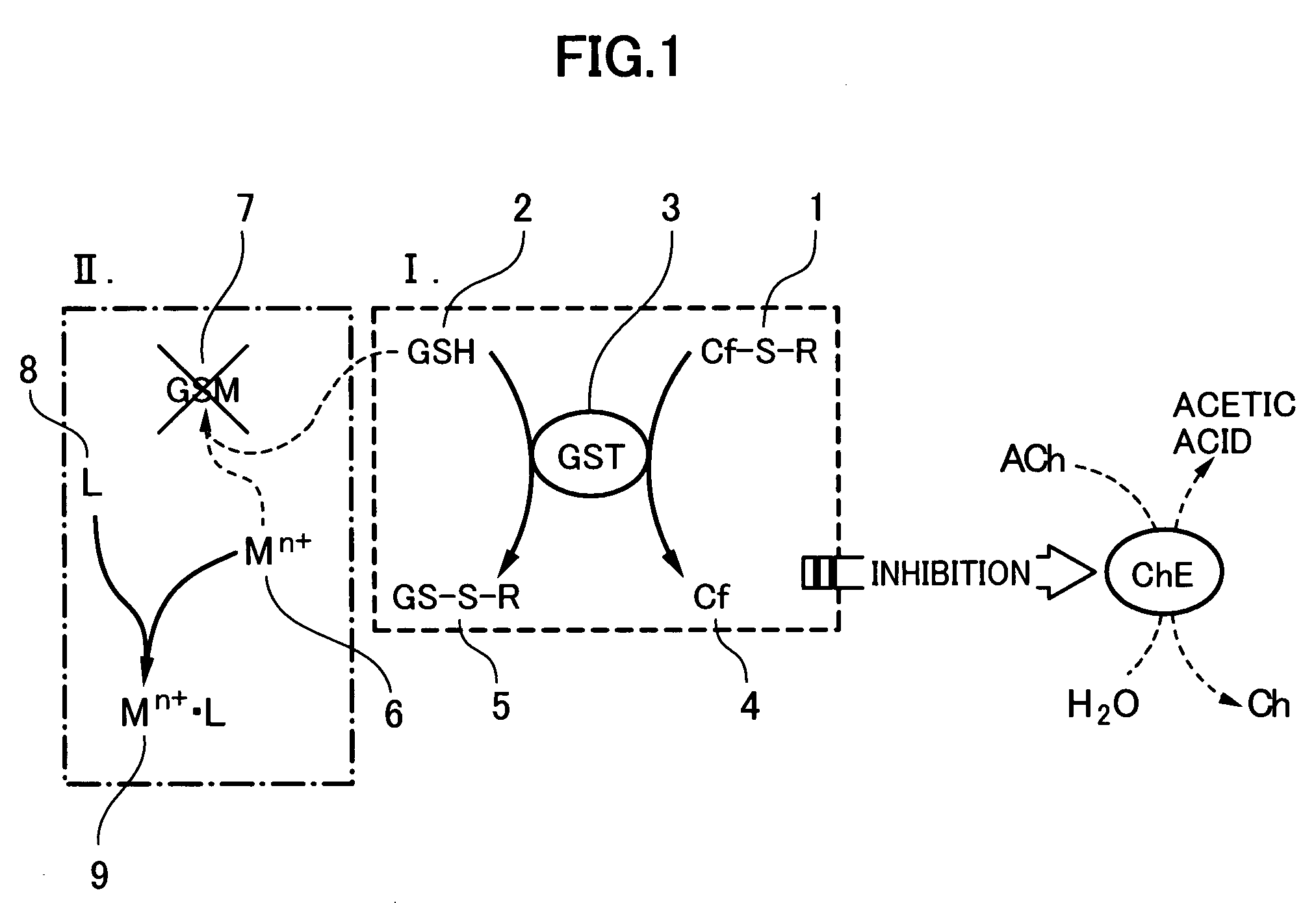
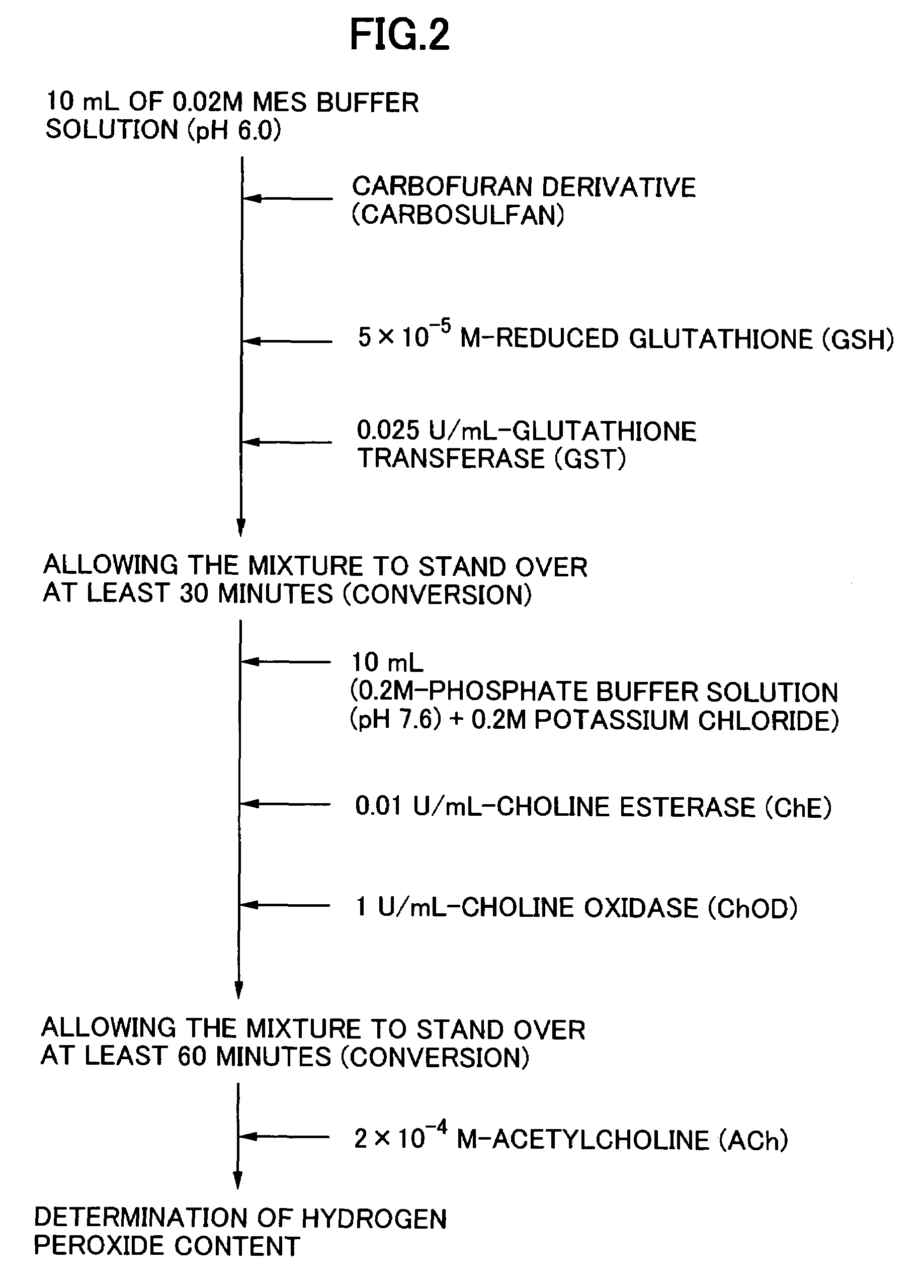
Method for analyzing residual agricultural chemical
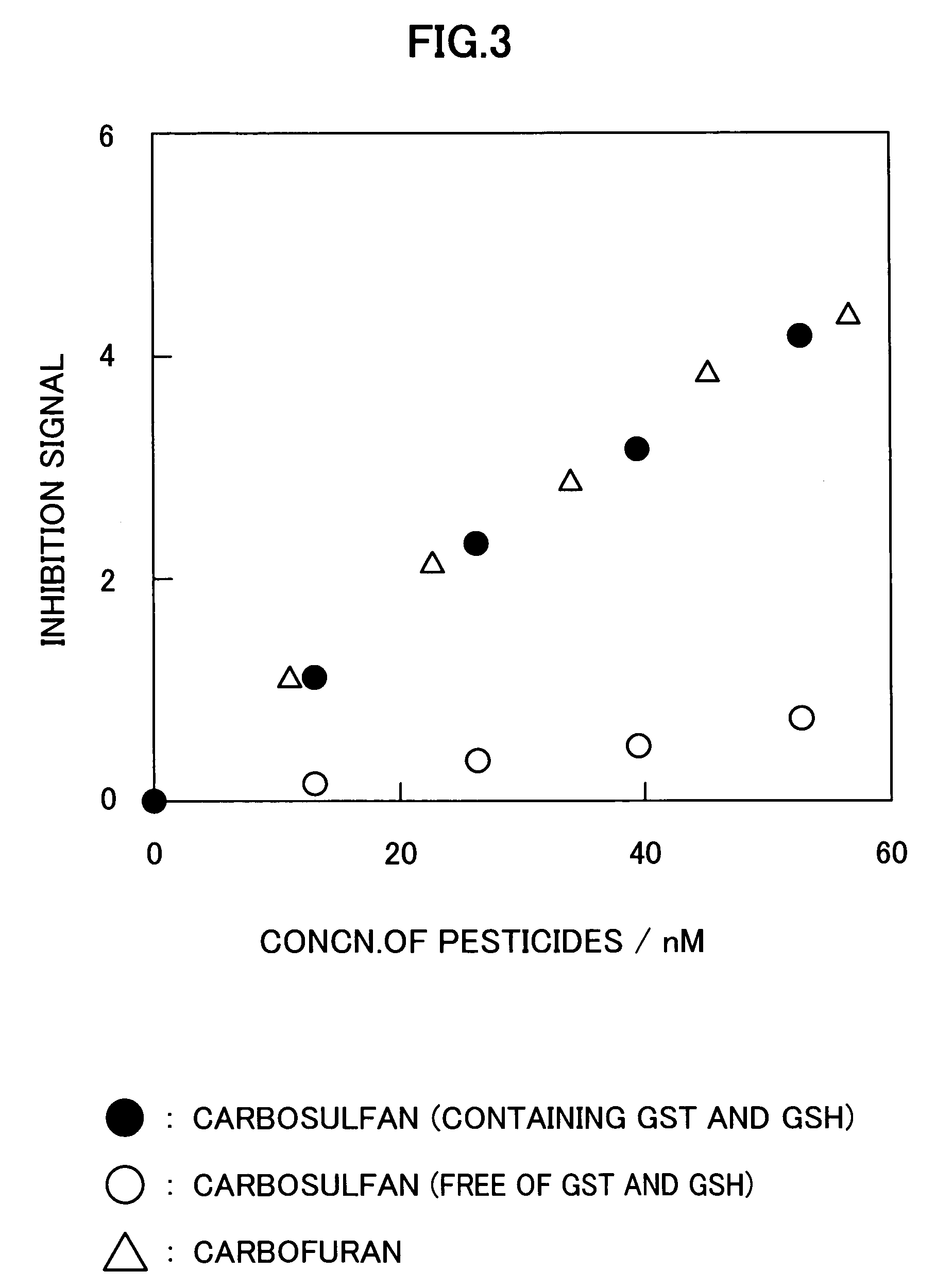
InactiveUS20050214887A1Improve accuracyHigh detection sensitivityAnalysis using chemical indicatorsMicrobiological testing/measurementCarbofuranMethomyl
The present invention relates to a method for analyzing residual agricultural chemicals which comprises the steps of acting a reduced glutathione as a reactive substrate and a glutathione transferase serving as a catalyst for the reaction on a carbofuran derivative or a methomyl derivative as a carbamate type agricultural chemical of a new series to thus derivatize the agricultural chemical into a substance having a high choline esterase-inhibitory activity; reacting the substances formed through the derivatization reaction with a choline esterase; and then detecting the presence of the agricultural chemical as the new series of carbamate type one included in a sample to be examined on the basis of the changes in the choline esterase activity thus detected. The method of the present invention may serve as a powerful tool for the detection of the residual agricultural chemicals in grains such as rice and the detection of the content of agricultural chemicals remaining in agricultural products such as vegetables and fruits.
Owner:SATAKE CORP +1
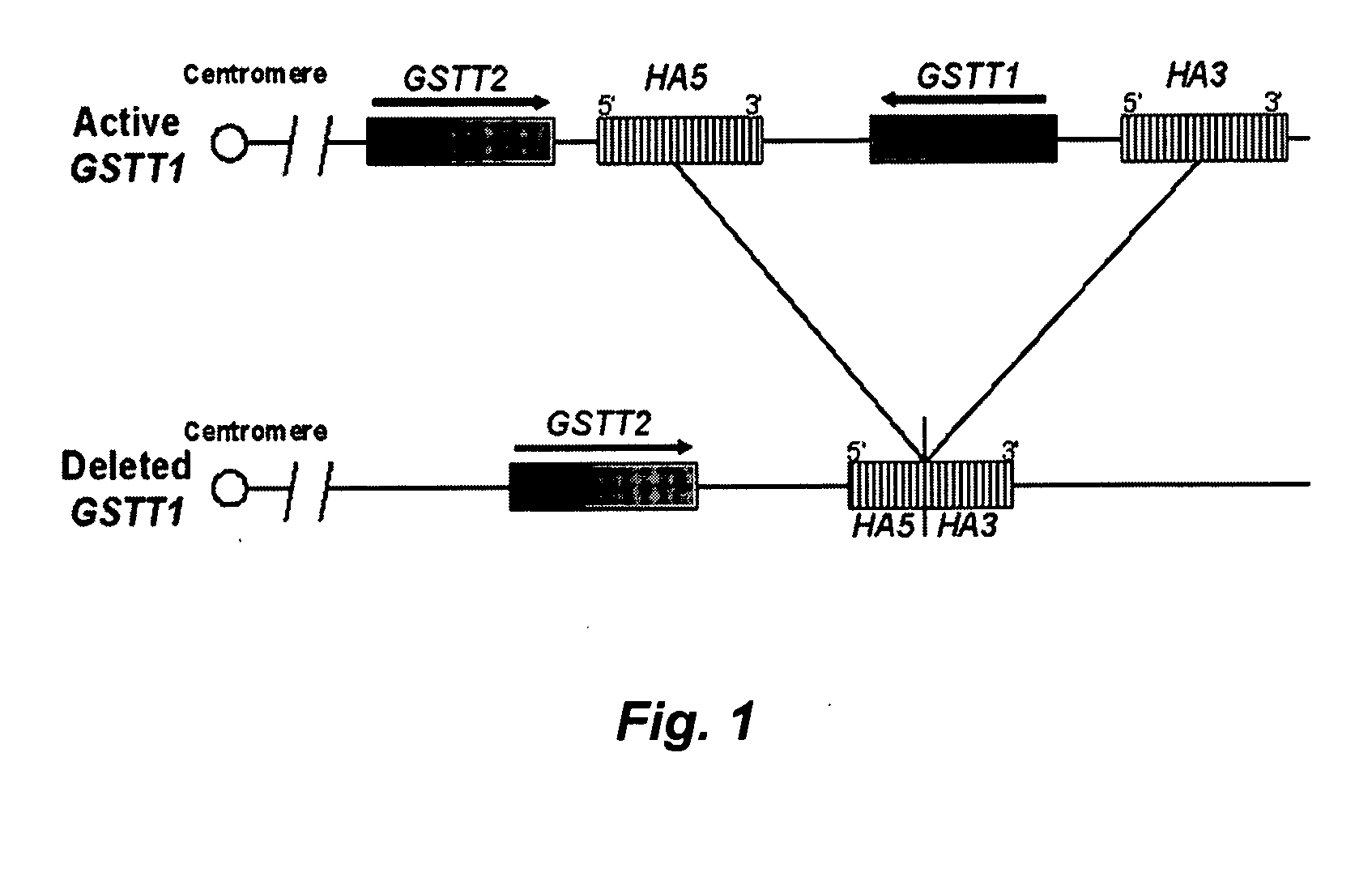
Methods for determining glutathione S-transferase theta-1 genotype
InactiveUS20060269925A1Avoids the lack of certaintyMicrobiological testing/measurementFermentationGenotypeGlutathione S-transferase
The invention relates to methods for determining GSTT1genotype, and the diagnostic and prognostic uses of these methods.
Owner:THE GENERAL HOSPITAL CORP
Genetic markers for predicting disease and treatment outcome
InactiveUS20070218487A1Microbiological testing/measurementThymidine Phosphorylase DeficiencyPlatinum
The present invention provides for a method for identifying patients that are suitably treated by a therapy, such as a therapy involving administration of a fluoropyrimidine drug and / or a platinum drug. The method includes determining the expression level of at least one gene selected from a phospholipase 2 (PLA2) gene, a thymidine phosphorylase (TP) gene, and a glutathione S-transferase P1 (GSTP-1) gene in suitable sample isolated from the patient. Overexpression of the gene or genes identifies the patient as not being suitable for the therapy.
Owner:UNIV OF SOUTHERN CALIFORNIA
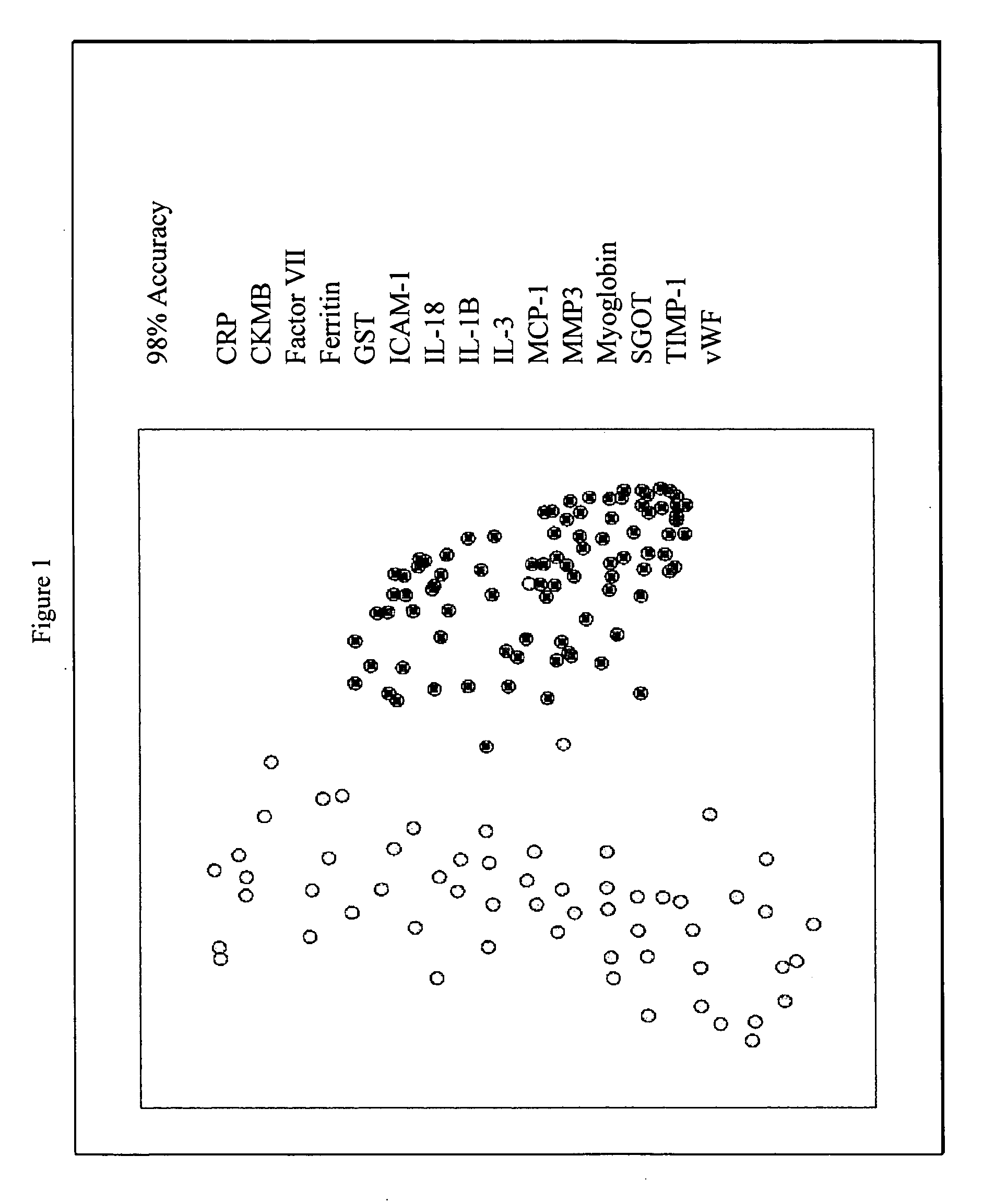
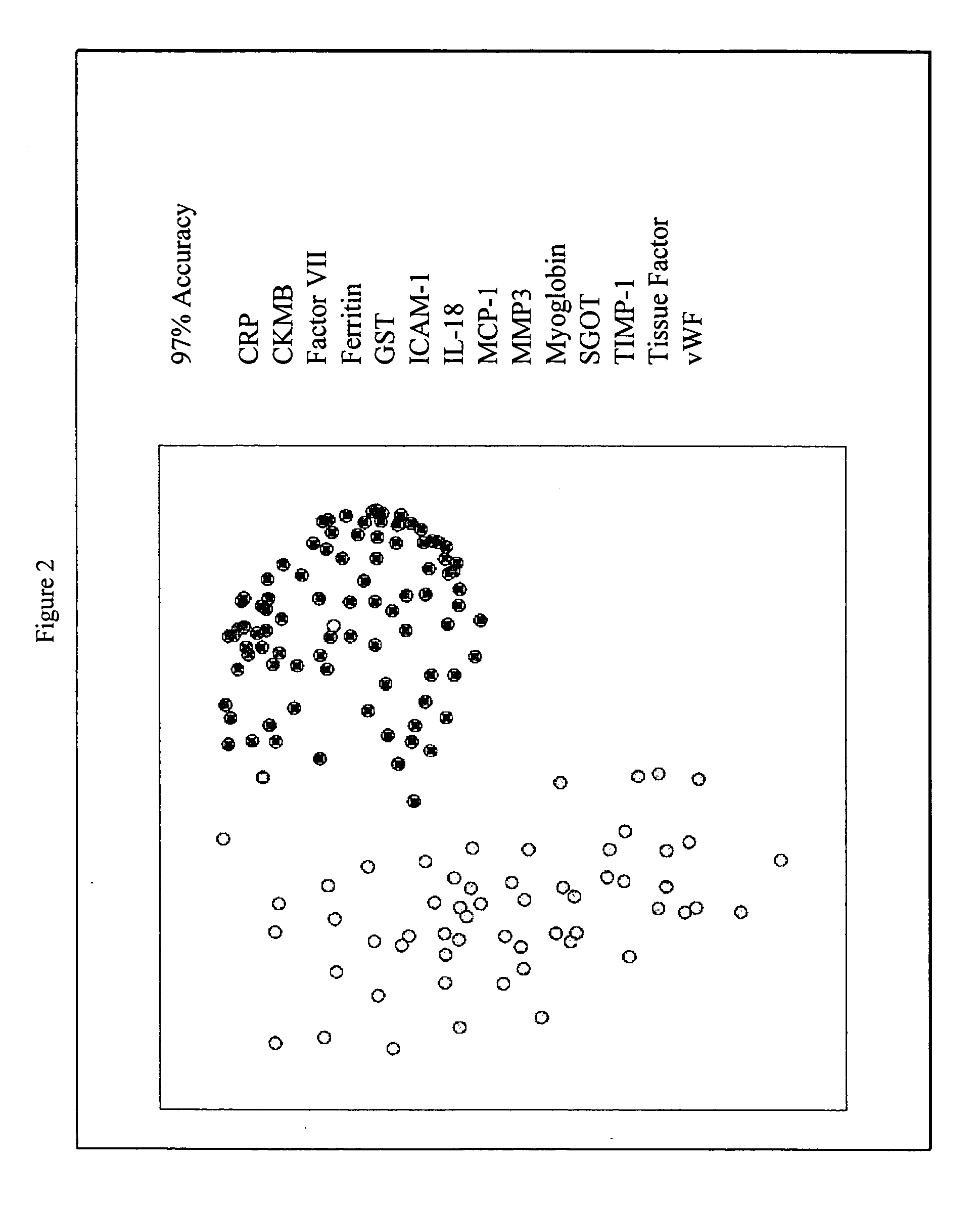
Methods and kits for the diagnosis of acute coronary syndrome
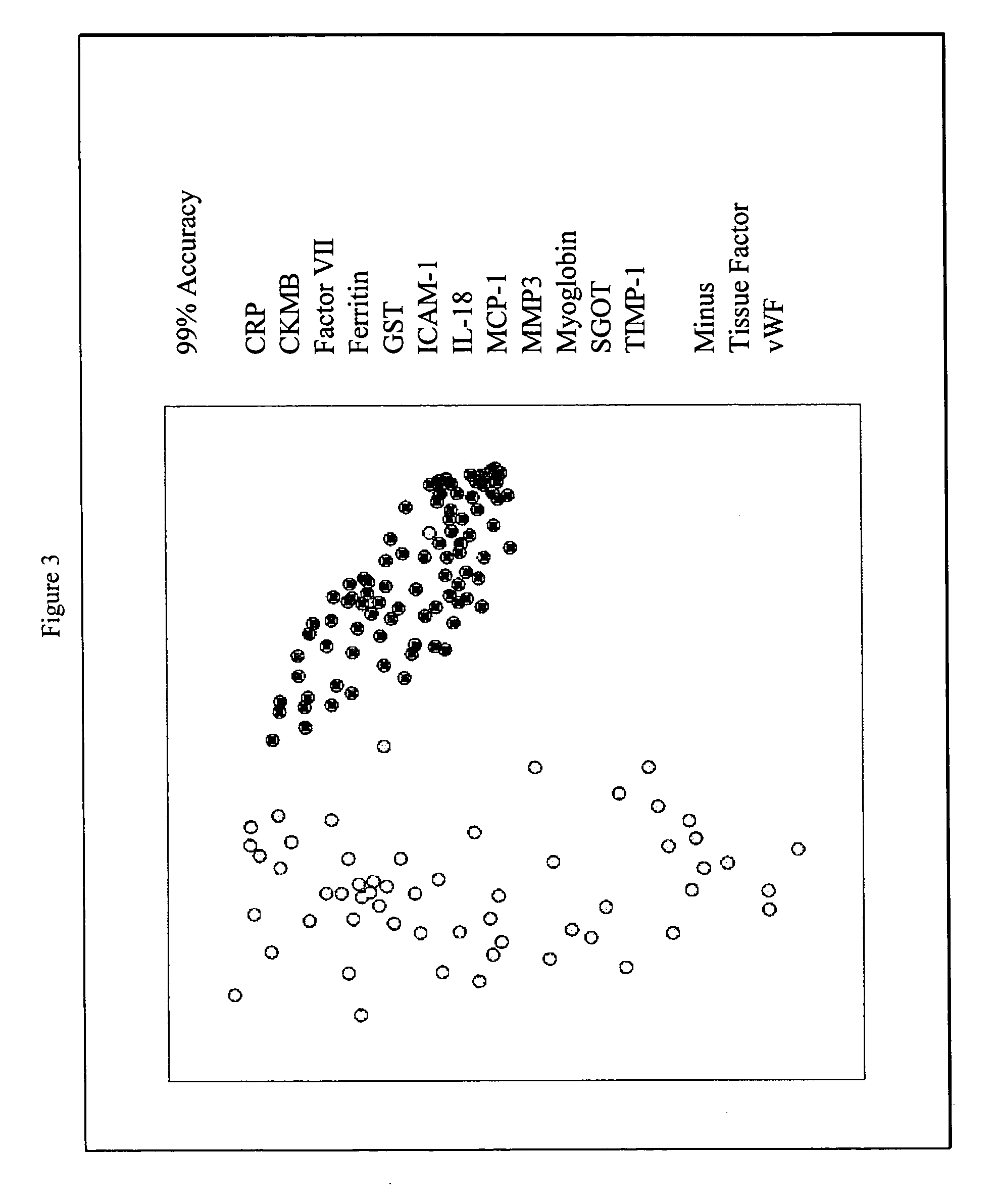
InactiveUS20070003981A1Quick checkAccurate diagnosisMicrobiological testing/measurementDisease diagnosisComplement 3Factor VII
Provided are methods for the detection and diagnosis of acute coronary syndrome or ACS. The methods are based on the discovery that abnormal levels of selected analytes in sample fluid, typically blood samples, of patients who are at risk are supportive of a diagnosis of ACS. At least two new biomarkers for ACS are thus disclosed, MMP-3 and SGOT. Altogether the concentrations of twelve analytes provide a sensitive and selective picture of the patient's condition, namely, whether the patient is suffering a heart attack. Other important biomarkers for ACS are described, including but not limited to IL-18, Factor VII, ICAM-1, Creatine Kinase-MB, MCP-1, Myoglobin, C Reactive Protein, von Willebrand Factor, TIMP-1, Ferritin, Glutathione S-Transferase, Prostate Specific Antigen (free), IL-3, Tissue Factor, alpha-Fetoprotein, Prostatic Acid Phosphatase, Stem Cell Factor, MIP-1-beta, Carcinoembryonic Antigen, IL-13, TNF-alpha, IgE, Fatty Acid Binding Protein, ENA-78, IL-1-beta, Brain-Derived Nerotrophic Factor, Apolipoprotein A1, Serum Amyloid P, Growth Hormone, Beta-2 microglobulin, Lipoprotein (a), MMP-9, Thyroid Stimulating hormone, alpha-2 Macroglobulin, Complement 3, IL-7, Leptin, and IL-6. Kits containing reagents to assist in the analysis of fluid samples are also described.
Owner:RULES BASED MEDICINE
Reagent box for enzyme linked immunosorbent assay of EB virus protease and its preparation
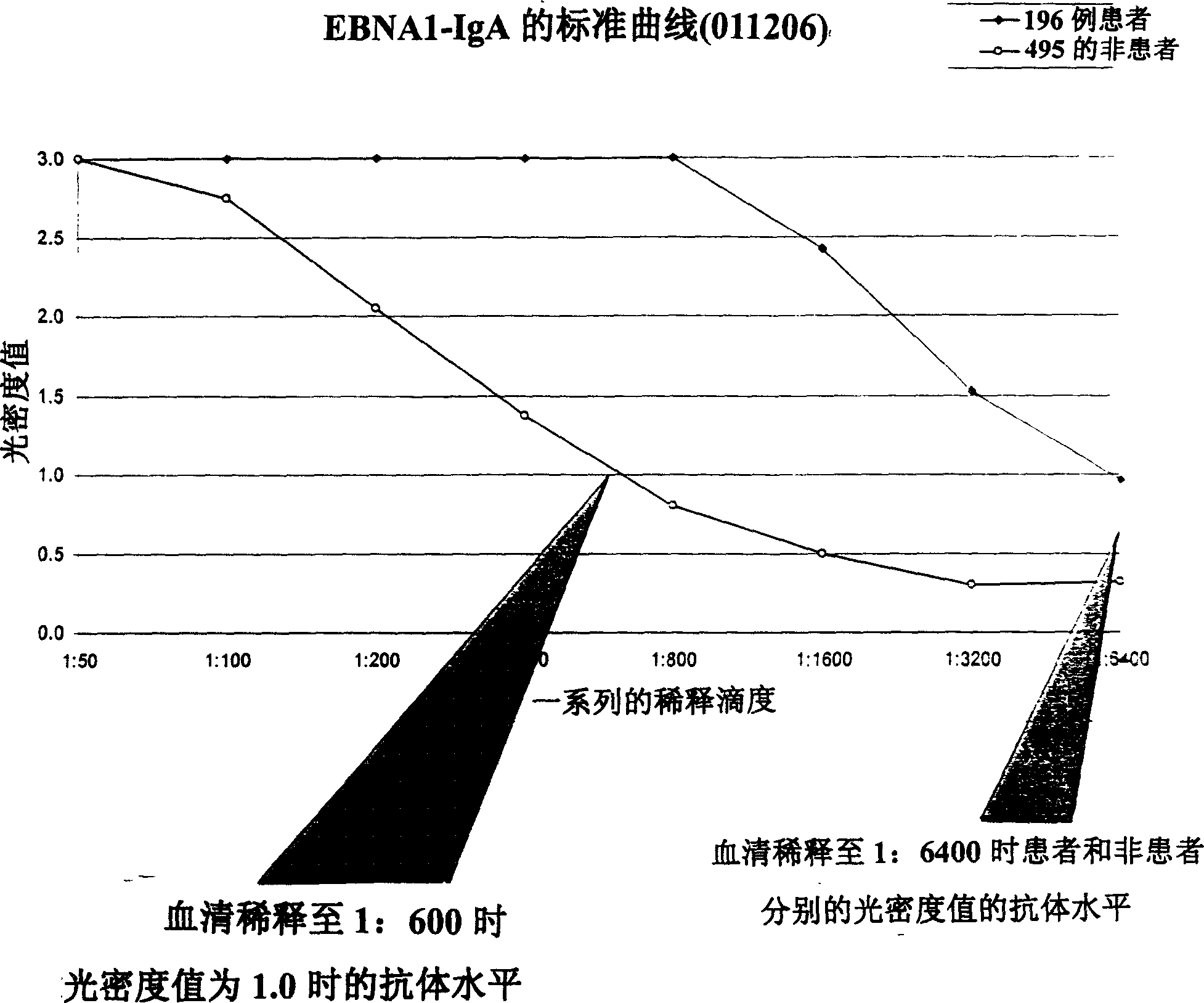
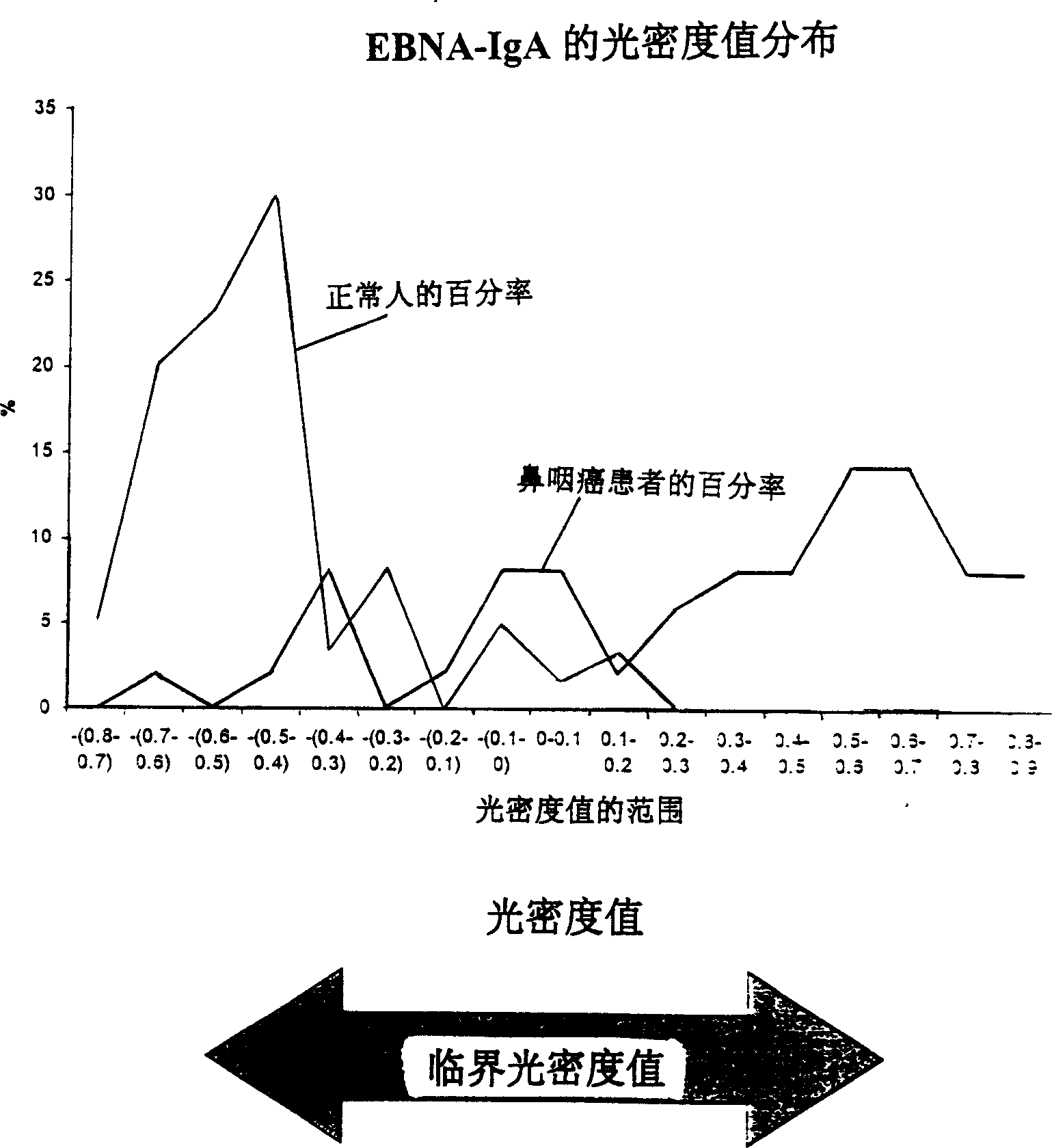
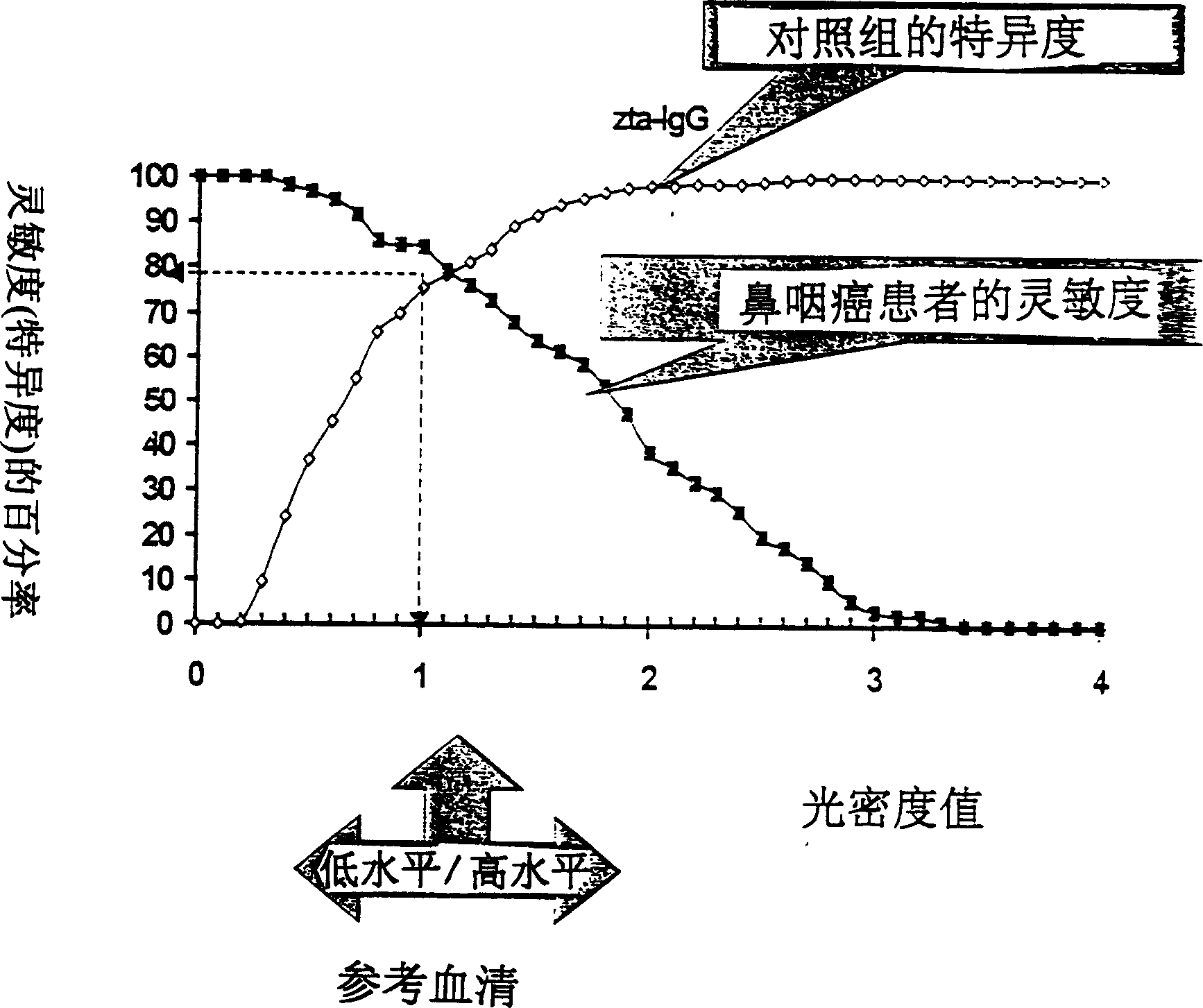
A method for preparing EB viral protein enzyme-linked immunosorbent diagnostic kit includes selectng EBNAl (BKRF1) prote, Zta (BZLF1) protein and VCA-p18 protin in EB viral protein for diagnosing nasopharyngeal carcinoma of serodiagnosis as target antigen for detecting antibody level in blood serum, using glutathione-transferase gene fusion system to carry on clone, presentation and purification of EB viral protein for creating diagnostic kit.
Owner:SINOCLONE LTD
Src SH3 binding peptides and methods of isolating and using same
InactiveUS20020091085A1Increase ratingsPeptide-nucleic acidsPeptide/protein ingredientsRandom Peptide LibraryBinding peptide
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Hydrogels for biomolecule analysis and corresponding method to analyze biomolecules
InactiveUS7588906B2Eliminate needSamples introduction/extractionMicrobiological testing/measurementPhosphorylationFluorescence
Owner:WISCONSIN ALUMNI RES FOUND
Method for detecting methylated CpG islands
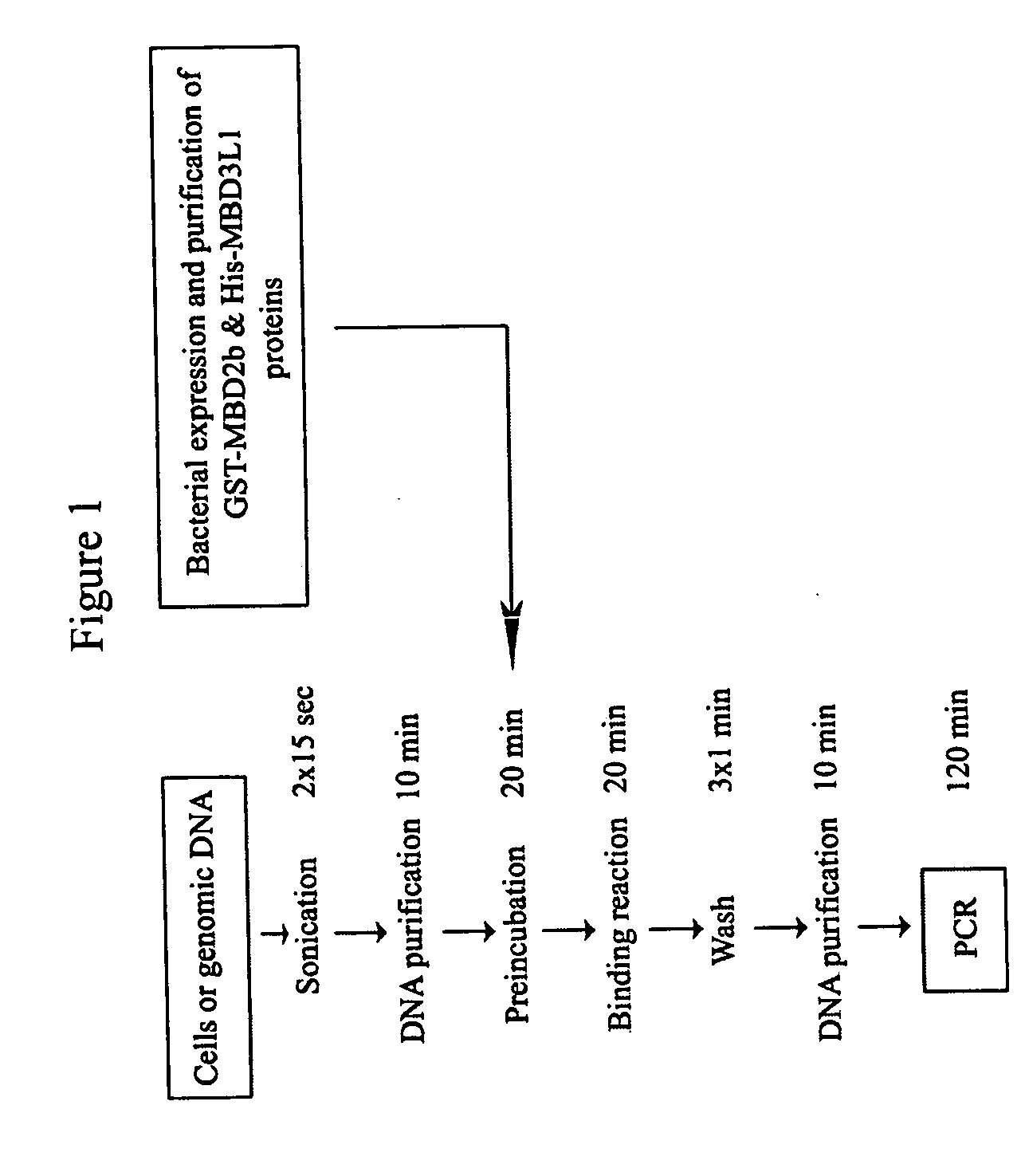
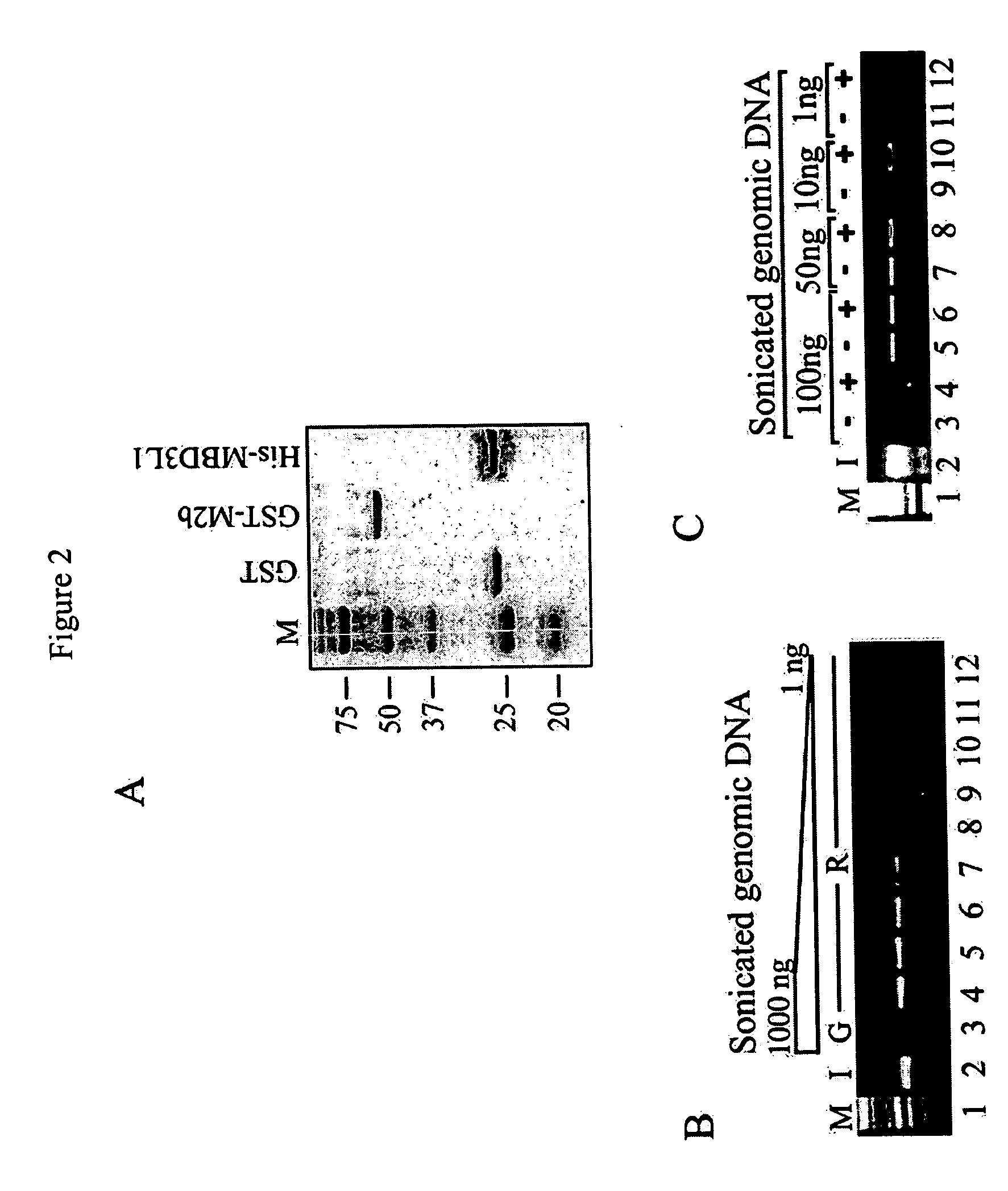
The present invention provides new and improved assay for detection of genomic methylated CpG islands. This new method is termed the methylated-CpG island recovery assay (MIRA). In accordance with one embodiment, MIRA comprises the steps of: (a) incubating genomic DNA fragments with a methylated CpG island binding protein in the presence of a binding partner for the binding protein to produce bound DNA containing methylated CpG islands, (b) isolating the bound DNA, and (c) detecting CpG island methylation by gene-specific amplification reactions. In accordance with a preferred embodiment, MIRA comprises the steps of: (a) incubating sonicated genomic DNA with a matrix containing a fusion protein of glutathione S-transferase (GST) and MBD2b (GST-MBD2b) in the presence of MBD3L1 to produce bound DNA containing methylated CpG islands, (b) eluting bound DNA from the matrix, and (c) detecting CpG island methylation by gene-specific amplification reactions.
Owner:CITY OF HOPE
Strawberry glutathione-transferase FaGST gene of, express protein thereof and application thereof
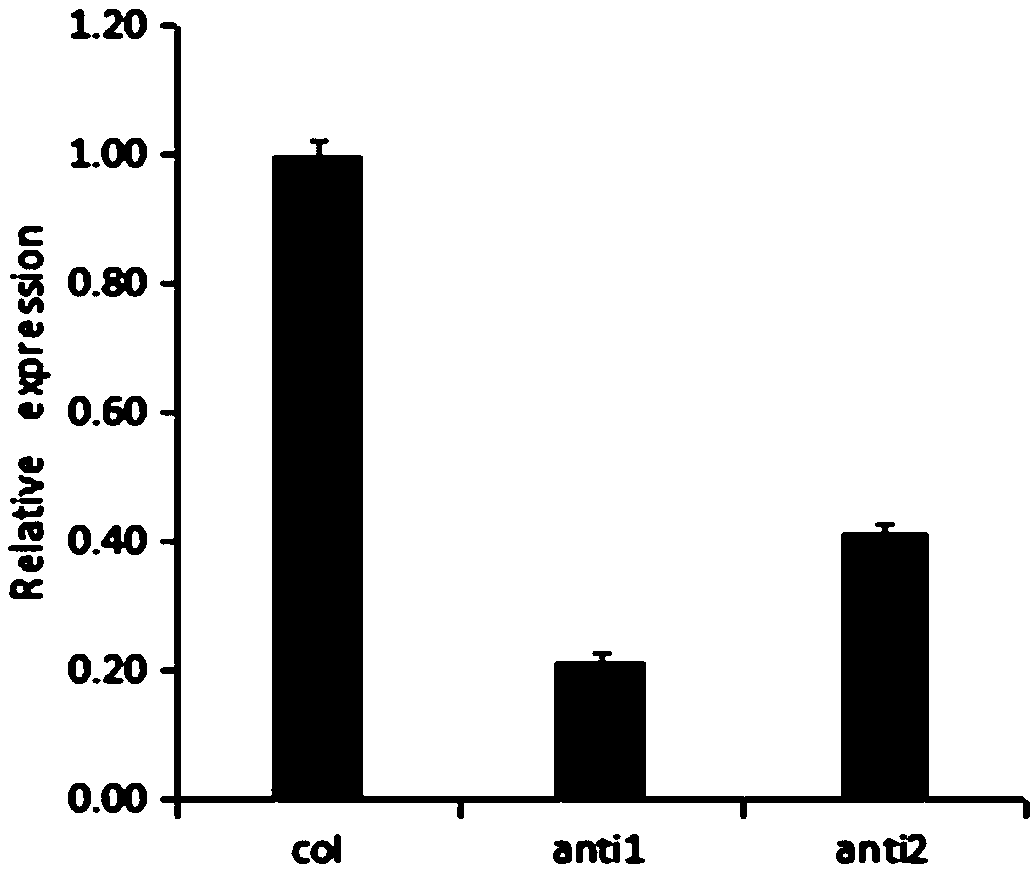
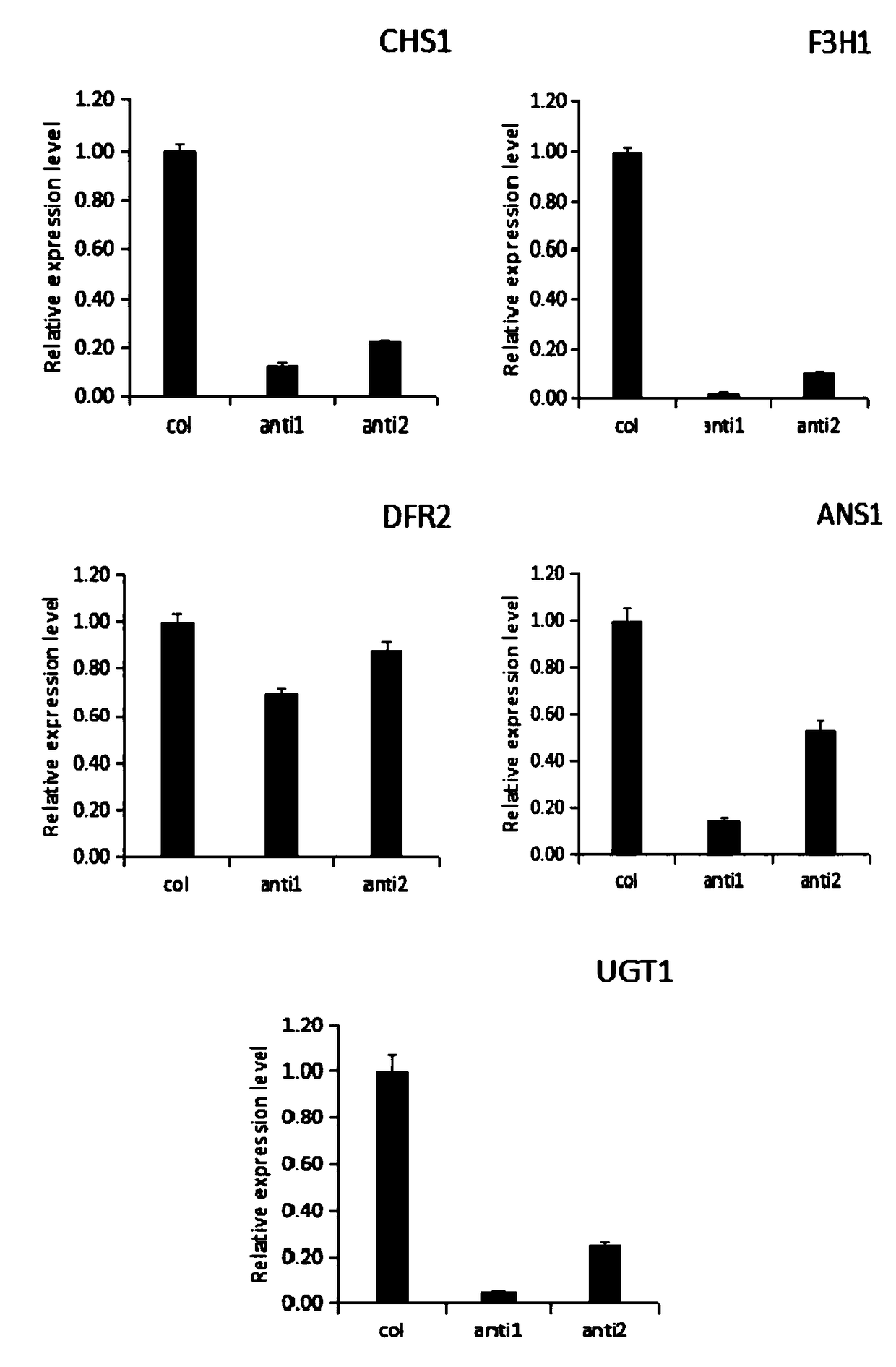
The invention discloses a strawberry glutathione-transferase FaGST gene, an express protein thereof and an application thereof, wherein the base sequence of the strawberry glutathione transferase FaGST gene is shown as SEQ ID NO. 1. The strawberry glutathione-transferase FaGST gene is cloned, and the FaGST1 is found to be related to the accumulation of anthocyanin in strawberry fruit through an infection, and after the gene is silenced, the expression amount is reduced, and the expression amount of main anthocyanin synthesis genes such as CHS1, F3H1, ANS1, UGT1 and the like are inhibited. Therefore, the FaGST gene will be widely used in anthocyanin synthesis.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Expression vector, host cell and method for producing fusion proteins
InactiveUS20050106671A1Avoid interferenceHigh expressionSugar derivativesTransferasesNucleotideSecretory protein
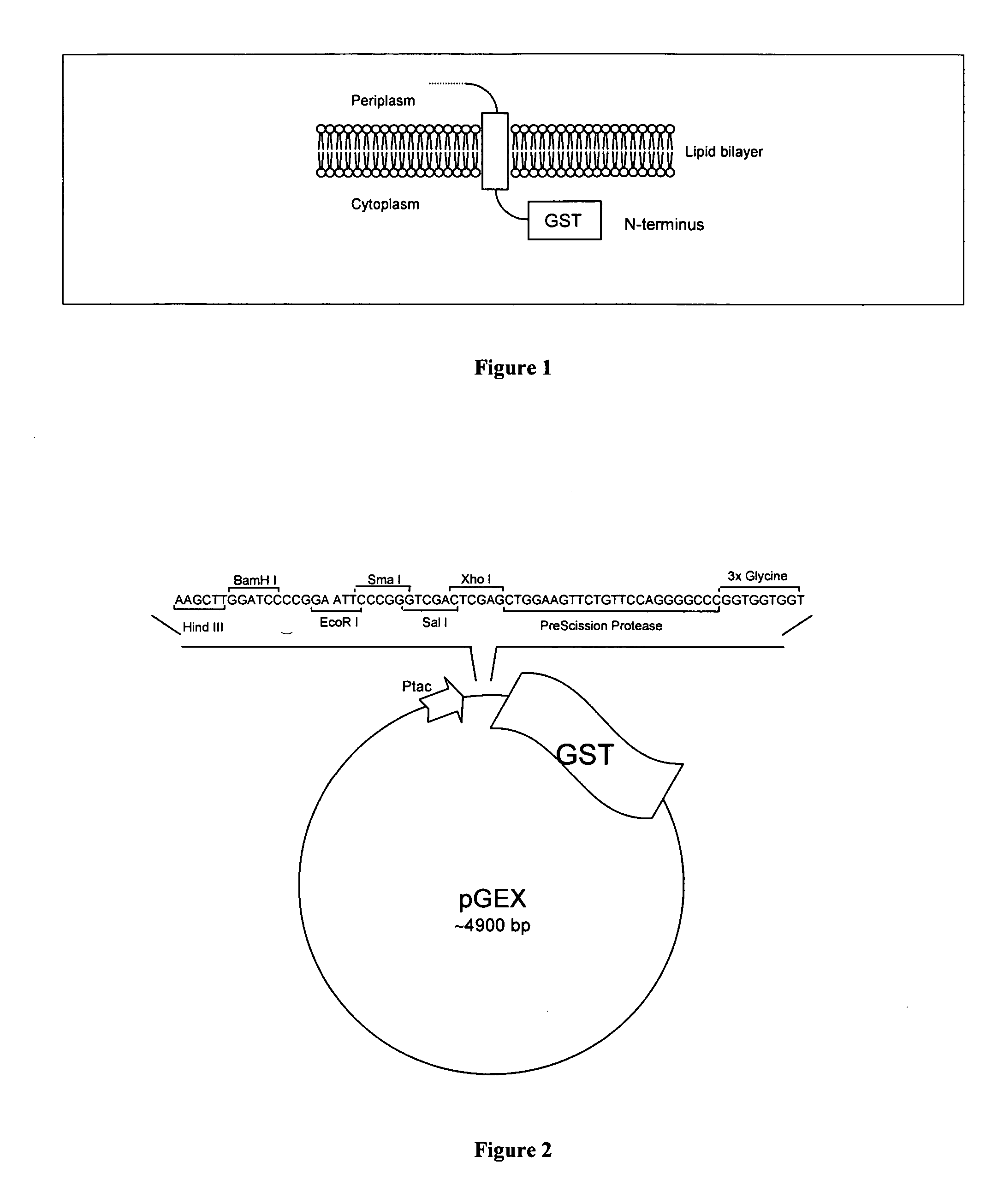
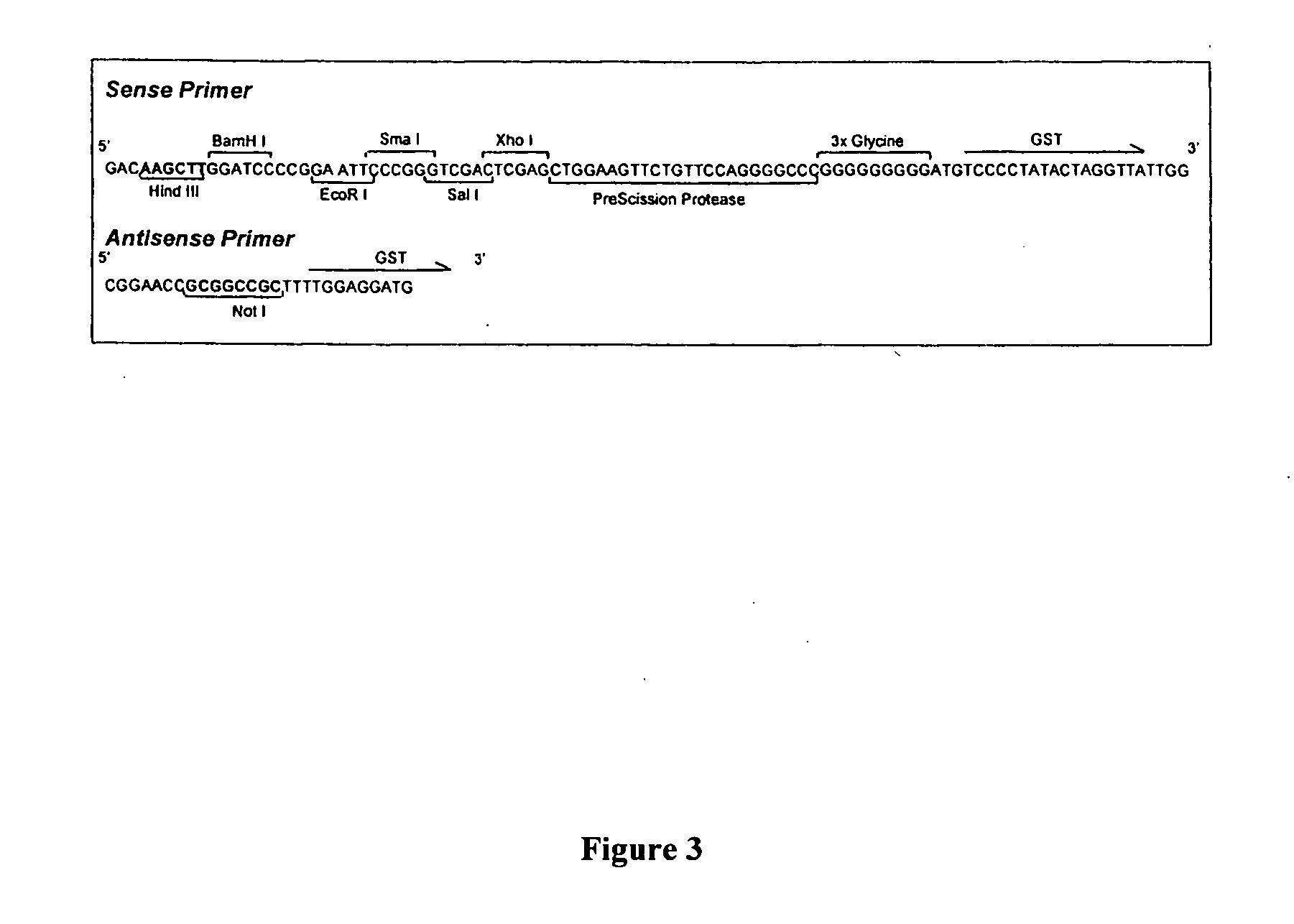
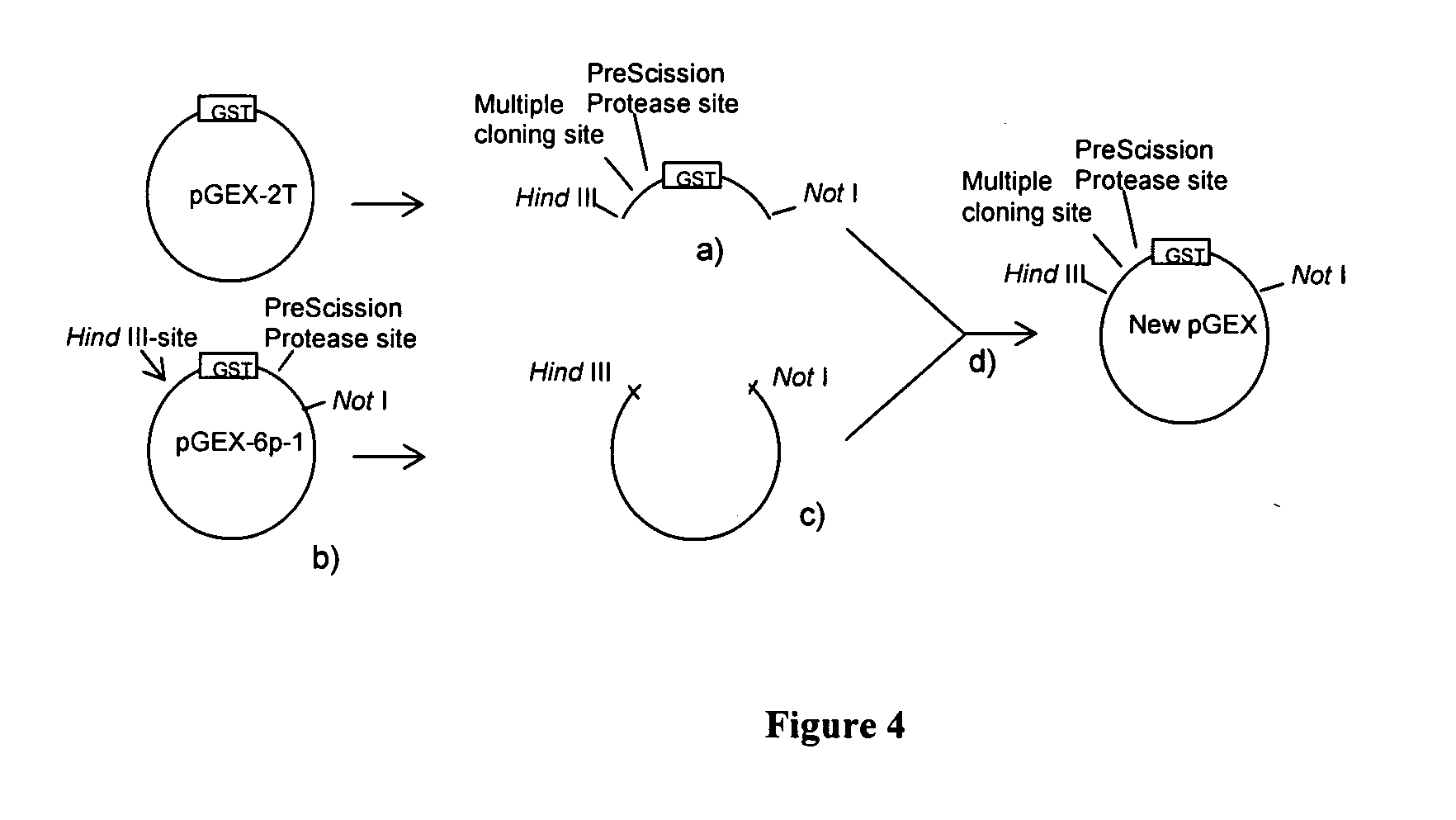
The present invention relates to an expression vector comprising, in 5′ to 3′ direction, a promoter, a multiple cloning site and a nucleotide sequence encoding glutathione-S-transferase (GST), for production of a fusion protein comprising a membrane protein, secretory protein or toxic protein or peptide, fused directly or indirectly with the N-terminal of GST. Preferably, the fusion protein comprises GST and a membrane protein or membrane localised peptide. The invention is especially suitable for membrane proteins having their C-terminals in the cytoplasm. The invention also relates to methods for producing such fusion proteins using host cells transformed with the expression vector in which a desired gene has been cloned.
Owner:BIRSE DARCY +1
Topical use of plant extract for skin detoxification
InactiveUS20090306219A1Maintain cellular redox homeostasisReduce hydrogen peroxideBiocideSulfur/selenium/tellurium active ingredientsPeroxideCollard Green
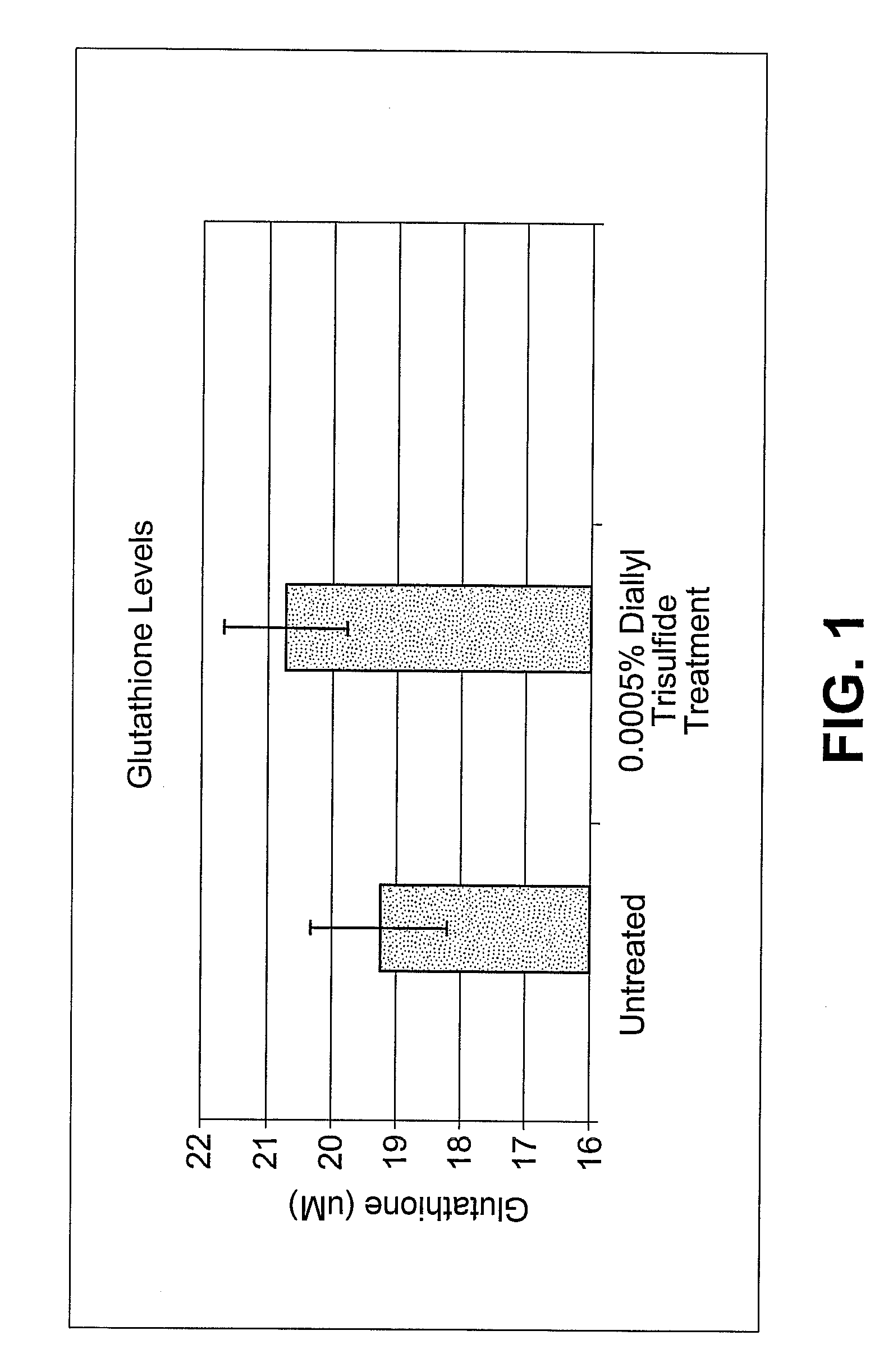
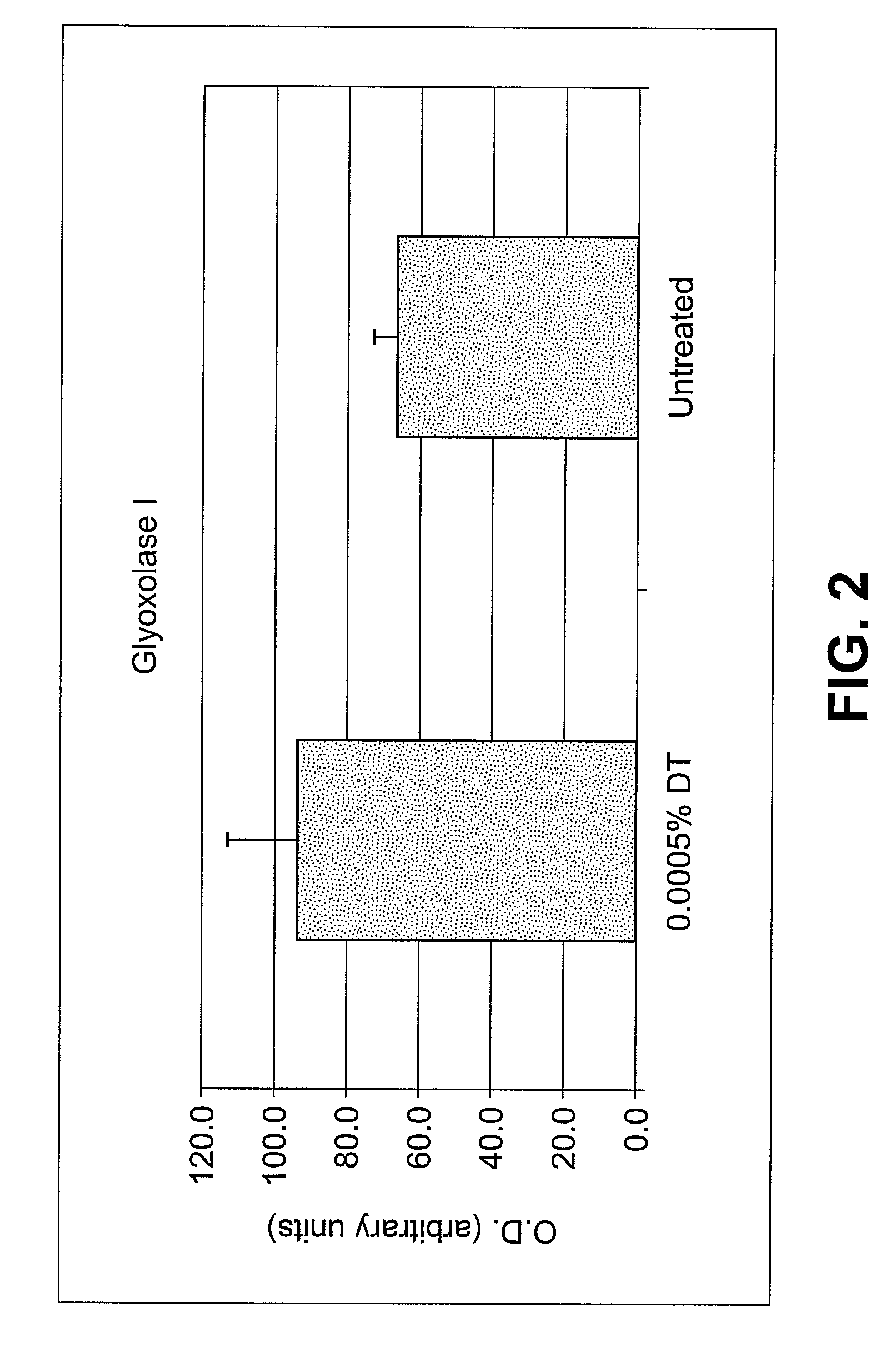
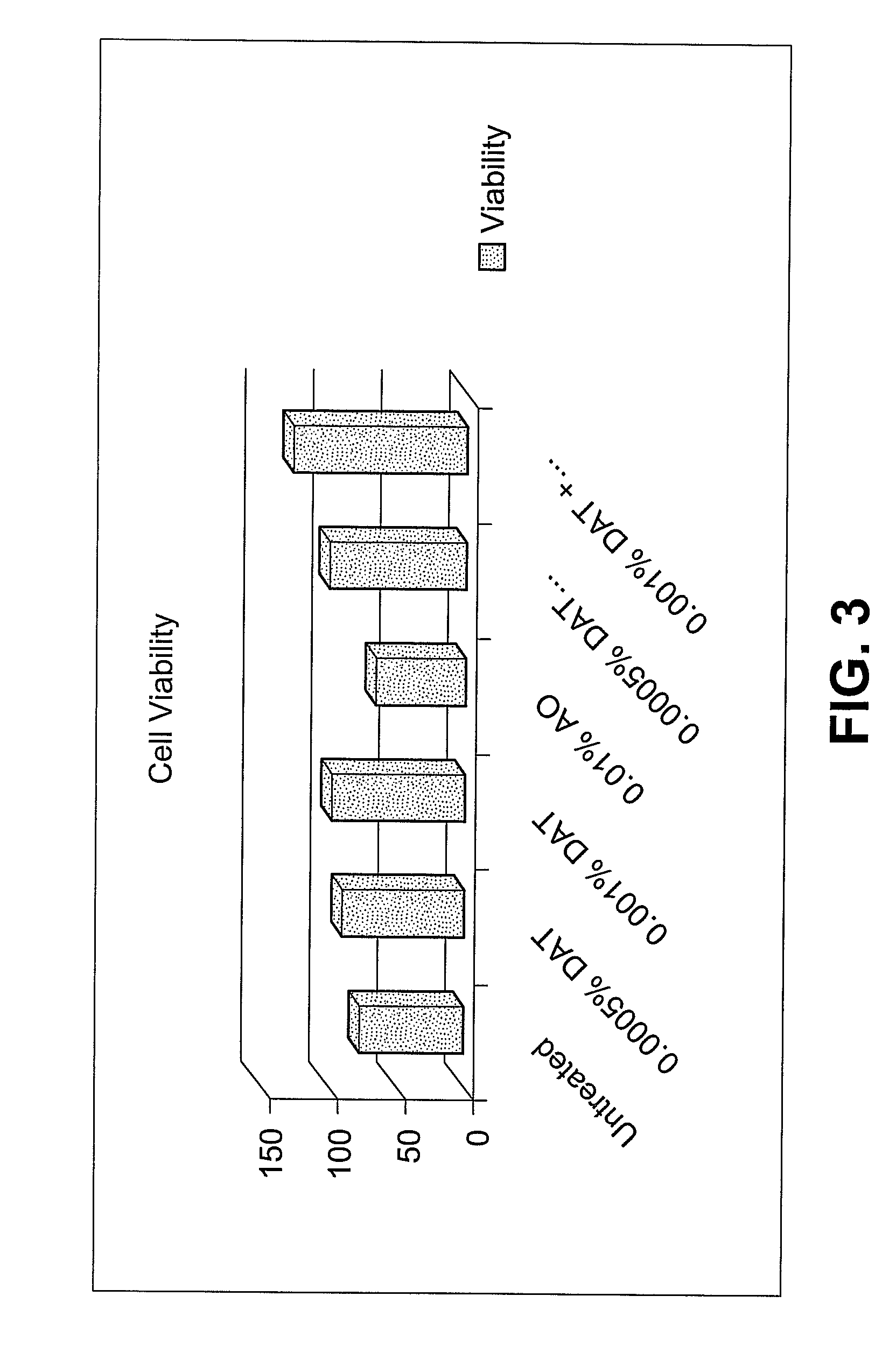
Specific plant extracts are applied topically to upregulate genes which code for proteins to prevent the build-up of and mitigate the activity of various harmful materials within the skin. These upregulated proteins include glutathione transferases, peroxiredoxins, oxidoreductases, glutaredoxins and glutathione syntheses. These proteins produce additional glutathione, glyoxalase I, maintain cellular redox homeostasis, reduce hydrogen peroxide and alkylhydroperoxides, and conjugate glutathione to a wide number of exogenous and endogenous hydrophobic electrophiles. Keratinocytes have been induced to significantly upregulate glutathione and glyoxalase I protein expression. Additionally, keratinocytes pre-treated with diallyl trisulfide have increased viability when exposed to ozone, UV radiation or cigarette smoke extract. The active material can be extracted from plants such as garlic, onions, shallots, leeks, chives, scallions, brussel sprouts, broccoli, cabbage, cauliflower, bok choy, kale, mustard, turnip or radish, may include any compound of the allyl sulfide family, and may be of synthetic origin.
Owner:BEILIS DEV
Gene sequence of glutathionetransferase of vinca rosea
A vinca rosea Glutathione S-transferase gene sequences, and it coding product amino acid sequence. It relates to a kind of new gene sequence, characterized in that: this gene is 929bp, at the point of 56bp there is the start coding triplet ATG, 718bp the ending coding triplet TGA, 910bp additional signal polyA. The gene sequence has not any inner gene, 663bp completely disclosed code-reading frame, code 220 amino acid. Nucleotide sequence and Capsicum Chinese gene GST1 has reached 67.7% homeotic source, and Euphorbia esula gene GST 57.7 homeotic source. This invention also provides a method to gain a full length of forced gene. First we enrich the anti-dry expressing gene in a moderate dry condition; then we establish full length genomic library of cDNA and detecting order, obtain the anti-dry related full length forcing gene in most short period. This anti-dry gene straightly links to eukaryotes expressing carrier pCMV plasmid, detected of the order by t3, t7 primer, filtered by eukaryotes expressing. This invention distills the full length gene of glucoside peptide transferase. This gene has a good anti-oxidate function, increases express in adverse circumstance forces in the process, the gene eliminates the free radical of oxidation. This gene colon expands the gene source of plant anti-dry, and provides a more rich and good usable gene for enhancing the anti-dry function and meliorating breed.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Seleniferous composition for producing antioxidase in vivo
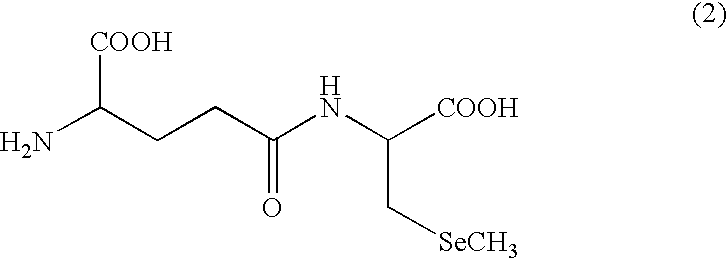
InactiveUS20060093684A1Avoid it happening againImproving antibody productionBiocideOrganic active ingredientsCysteine thiolateIn vivo
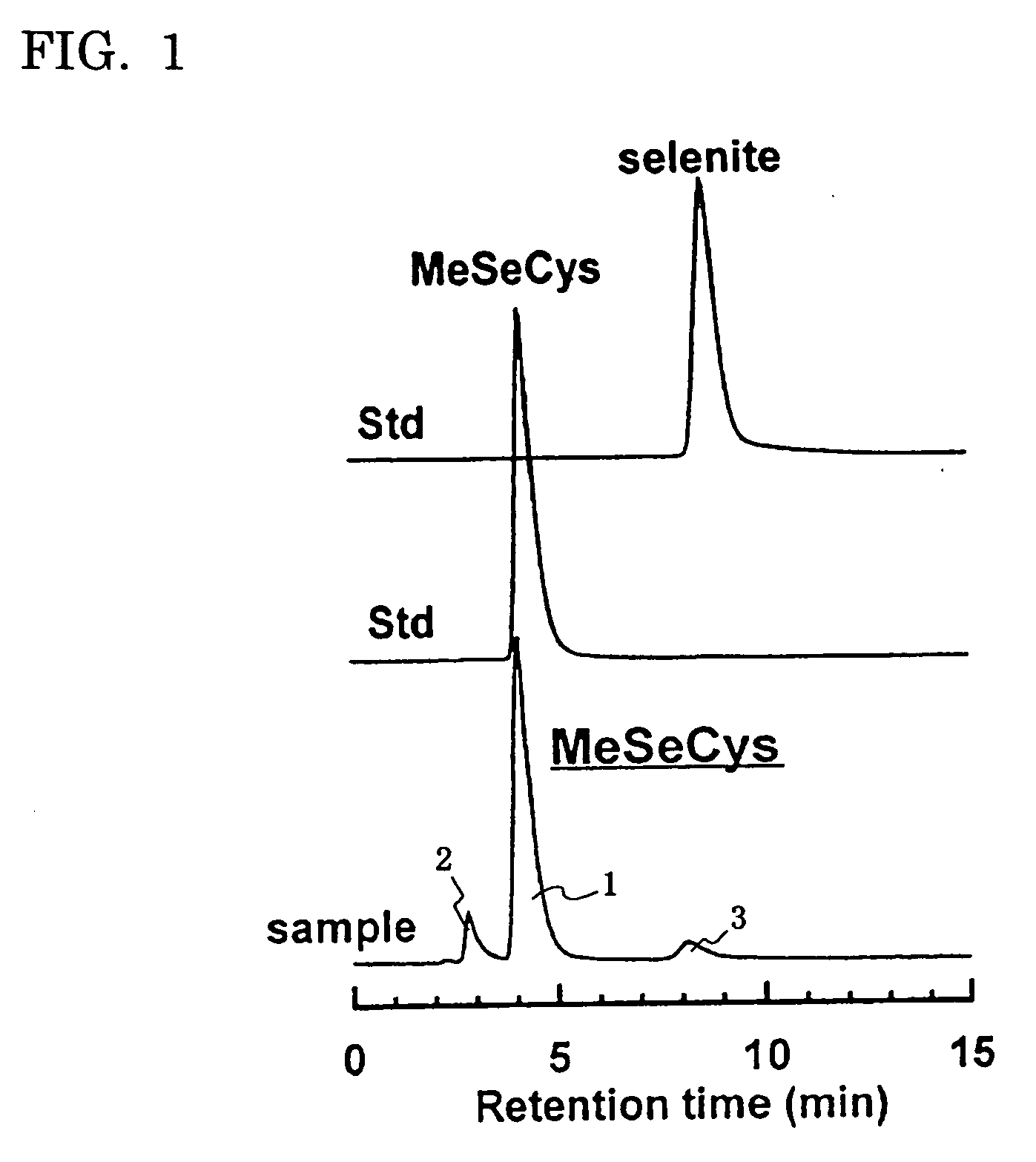
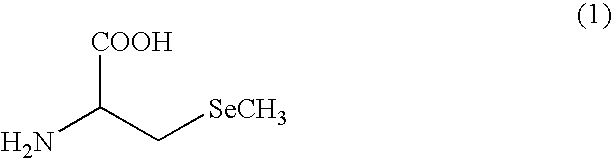
Seleniferous composition is provided which comprises Se-methylseleno-cysteine (MeSeCys: SeCH3—CH2—CHNH2—COOH) or derivative thereof. When human or animal body ingests the seleniferous composition, their living body naturally produces enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione-S-transferase (GST) for decomposing active oxygen to positively prevent and treat cancer.
Owner:TAKEDA KK +2
Application of ginsenoside Rd in aspect of controlling diamondback moths
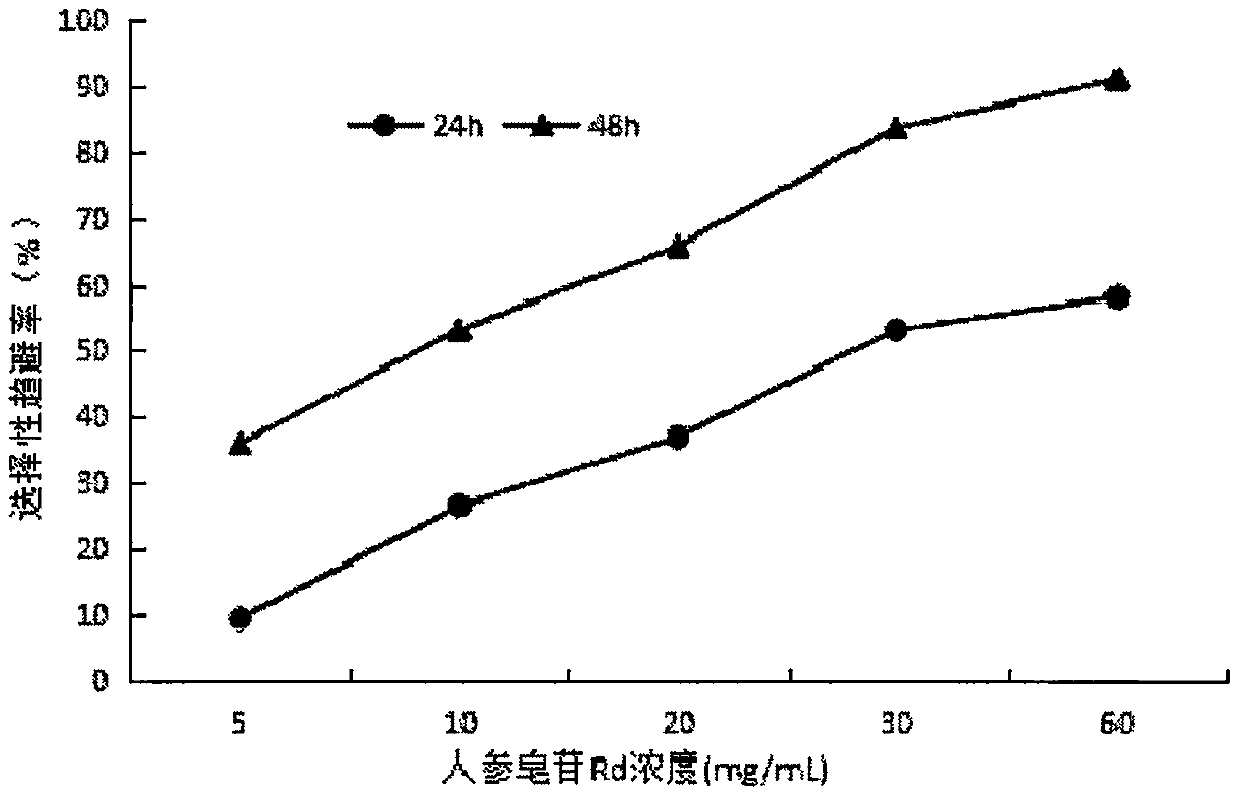
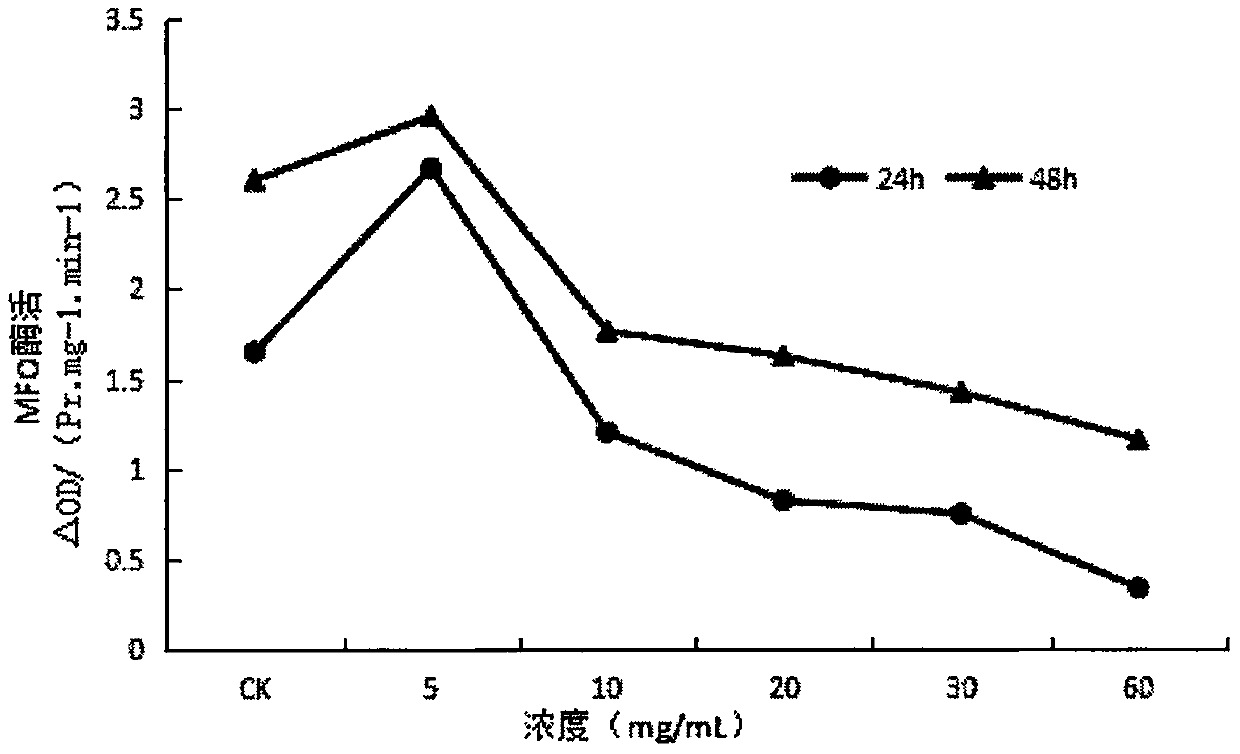
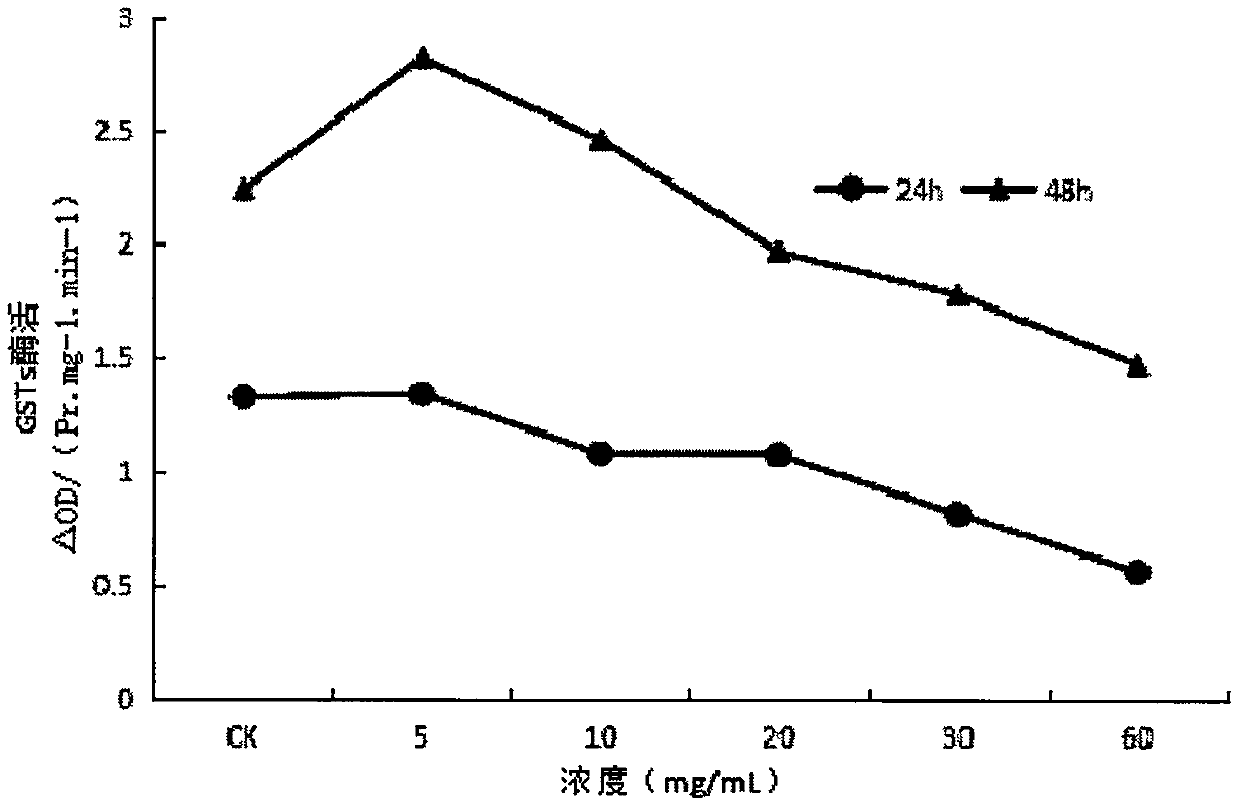
InactiveCN107668052AReduce enzyme activityEnhanced inhibitory effectBiocidePest repellentsNervous systemExperimental methods
The invention provides application of ginsenoside Rd in the aspect of controlling diamondback moths. A large number of experimental methods shows that the ginsenoside Rd has an avoidance function on feeding of second instar diamondback moths and has a certain inhibiting effect on the activity of detoxifying enzymes (mixed-functional oxidase, glutathione transferase and carboxylesterase) and a nervous system enzyme (acetylcholin esterase) in the second instar diamondback moths, and therefore, the ginsenoside Rd can be used for controlling the diamondback moths, and the theoretical foundation isprovided for development of a novel pesticide. When in application, the content of the ginsenoside Rd can be set as 10-60 mg / mL; preferably, when the concentration of the ginsenoside Rd is 60 mg / mL,the activity of a GSTs enzyme reaches the lowest after the diamondback moths are treated for 24 h, and the inhibiting effect is relatively strongest.
Owner:JILIN AGRI SCI & TECH COLLEGE
Detection method for food quality
InactiveCN104330404AQuick checkEasy to detectMaterial analysis by observing effect on chemical indicatorOrganic solventTest sample
The invention discloses a detection method for food quality. The detection method comprises the following steps of preparing a detection reagent, detection enzyme, contrast test sample liquid and a set control unit according to food to be detected; smashing the food to be detected, uniformly mixing the smashed food to be detected and a plant organic solvent, performing stirring and extraction twice under an ultrasonic extraction condition, then adding an adsorbent for centrifugal treatment, and recycling an extracting solution on the upper layer after centrifugal treatment is finished; performing microfiltration and nanofiltration in sequence on the solution, and removing the plant organic solvent in the extracting solution in a blowing manner; adding a chelating agent into the extracting solution without the solvent for mixing, adding reduced glutathione and glutathione transferase for catalytic mixing reaction, and after reaction is completed, adding a buffering solution to prepare a test sample solution to be detected. The food quality can be obtained by analyzing and processing a large quantity of test results; additives in food or pesticide in vegetables can be quickly and easily detected, and the detection sensitivity is high.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Gene product detection method of degradative graminearum toxin
InactiveCN102156192AStrong specificityAchieving control over metabolismBiological testingEscherichia coliTrichothecene
The invention discloses a gene product detection method of degradative graminearum toxin. A rabbit anti-GST(glutathione transferase)-Tri101 antibody acting as a primary antibody, and a goat anti-rabbit IgG-HRP (horse radish peroxidase) acting as a secondary antibody and an object to be tested containing the Tri101 protein carry out Western blot analysis. The trichothecene 3-O-acetyl invertase coded by Tri101 gene in the invention is efficiently expressed in colibacillus; a purified protein immune New Zealand white rabbit obtains an antibody with relatively good specificity, and can carry out immunoblotting reaction with Tri101 protein secreted and expressed in BL21 (DE3); and a new detection method is provided for trangenosis of Tri101 gene in wheat and corns; the detection cost is reduced; and a solid basis for further researching the biological functions of Tri101 protein is provided.
Owner:HENAN ACAD OF AGRI SCI
Leading stabilizing element for enhancing activity and expression of exogenous protein in bacteria
ActiveCN113461790AHigh expressionHigh activityMicrobiological testing/measurementDepsipeptidesTyphimurium strainInfectious Disorder
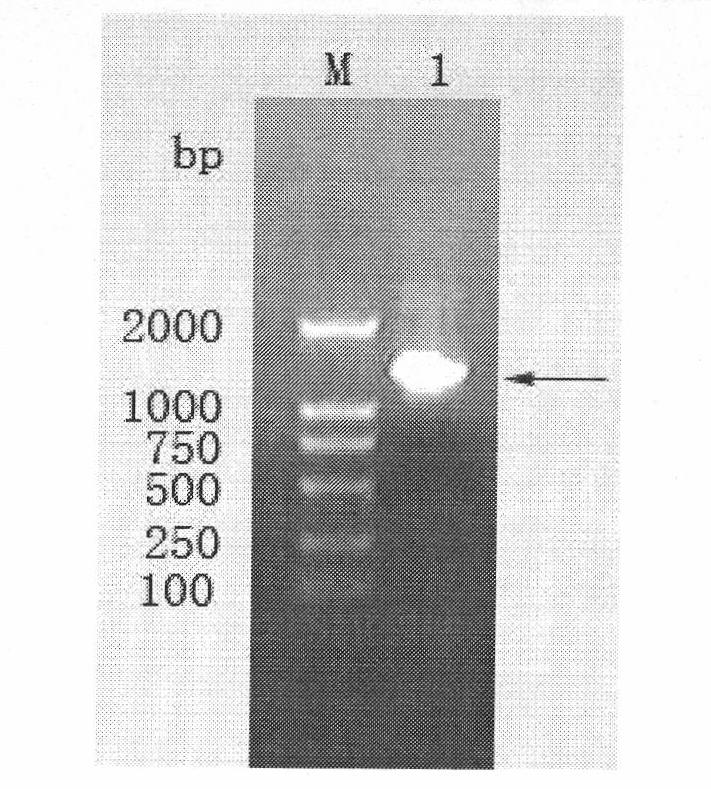
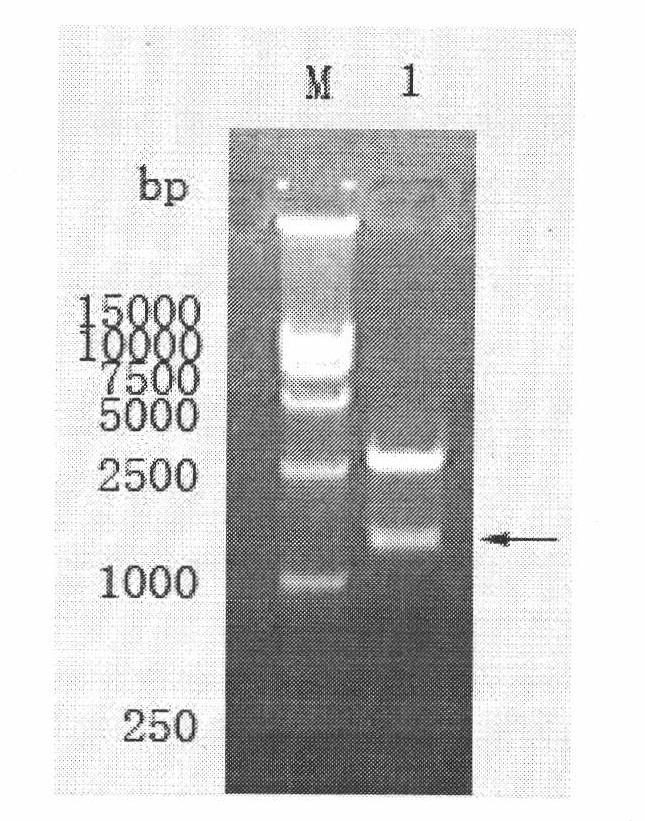
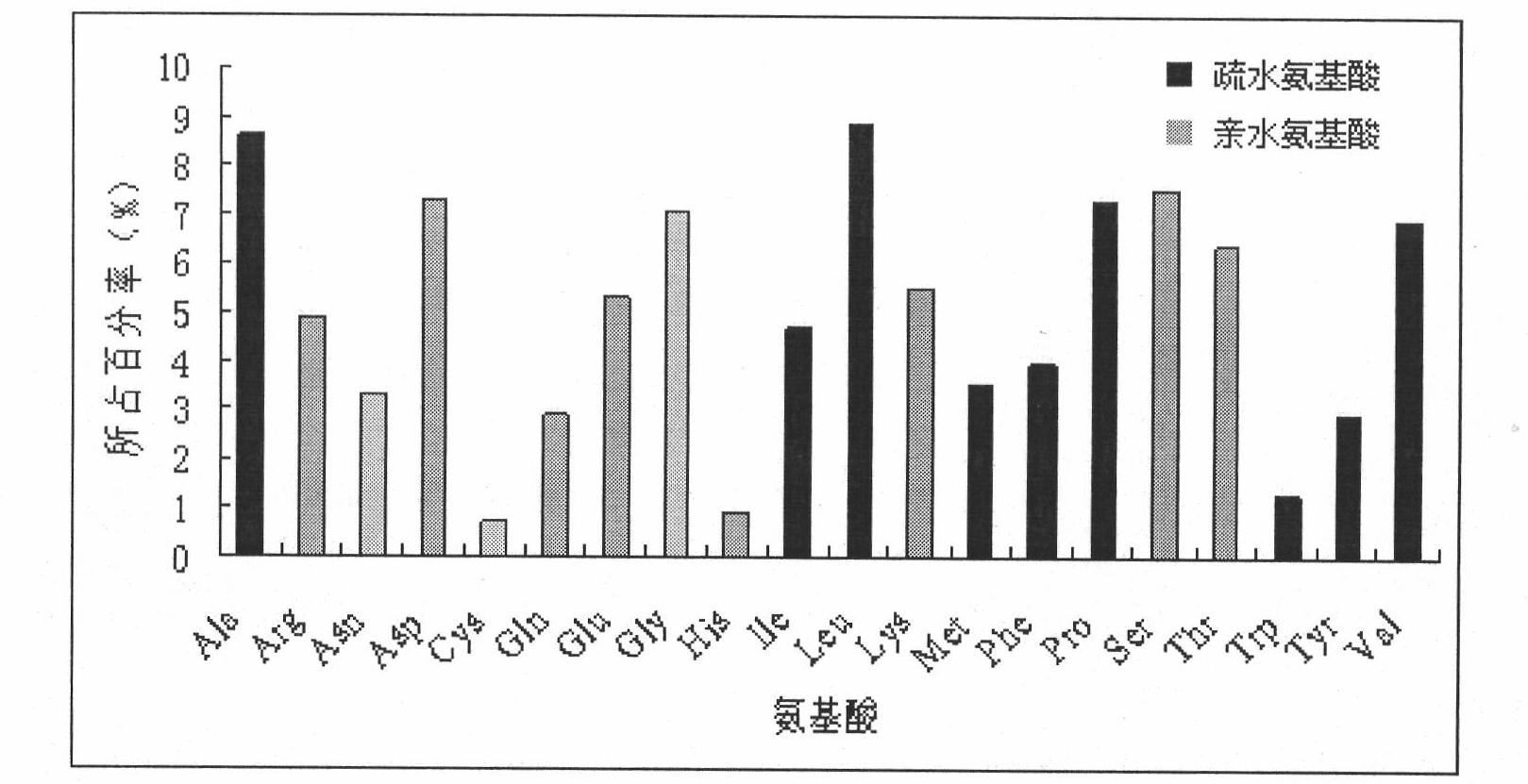
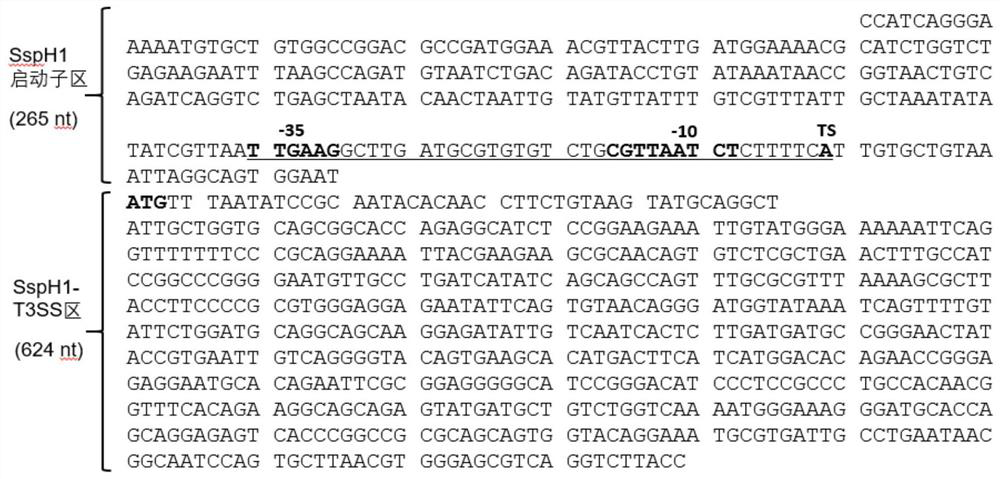
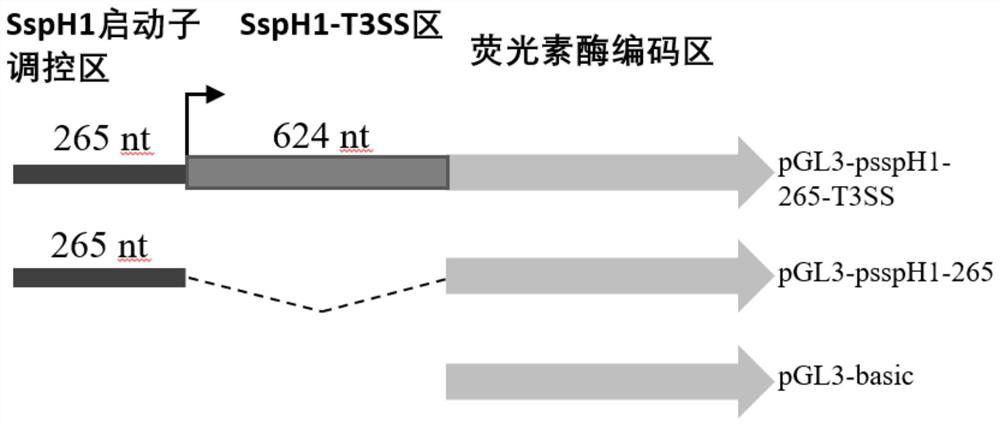
The invention belongs to the technical field of biology, and discloses a leading stabilizing element for enhancing activity and expression of exogenous protein in bacteria. The leading stabilizing element is 208 amino acid residue segments at the N end of the salmonella enteric subspecies chlamydia typhimurium strain RM13672SspH1 protein, the nucleotide sequence is shown in SEQ.ID. NO: 1, and the amino acid sequence is shown in SEQ.ID. NO: 2. the leading stabilizing element for enhancing activity and expression of the exogenous protein in the bacteria can be used as a tool for improving the protein yield and the protein activity in the industry or scientific research, and is beneficial to scientific research, biological medicine industrial production and preparation of anti-infectious disease vaccines. Besides, glutathione S transferase (GST) is a protein containing 211 amino acids, is the most common stable polypeptide expressed by the protein, but has great effect difference on different target proteins. The identified SsppH1 leading stabilizing element can provide a new choice so as to obtain ideal expression of active protein.
Owner:SHANXI UNIV
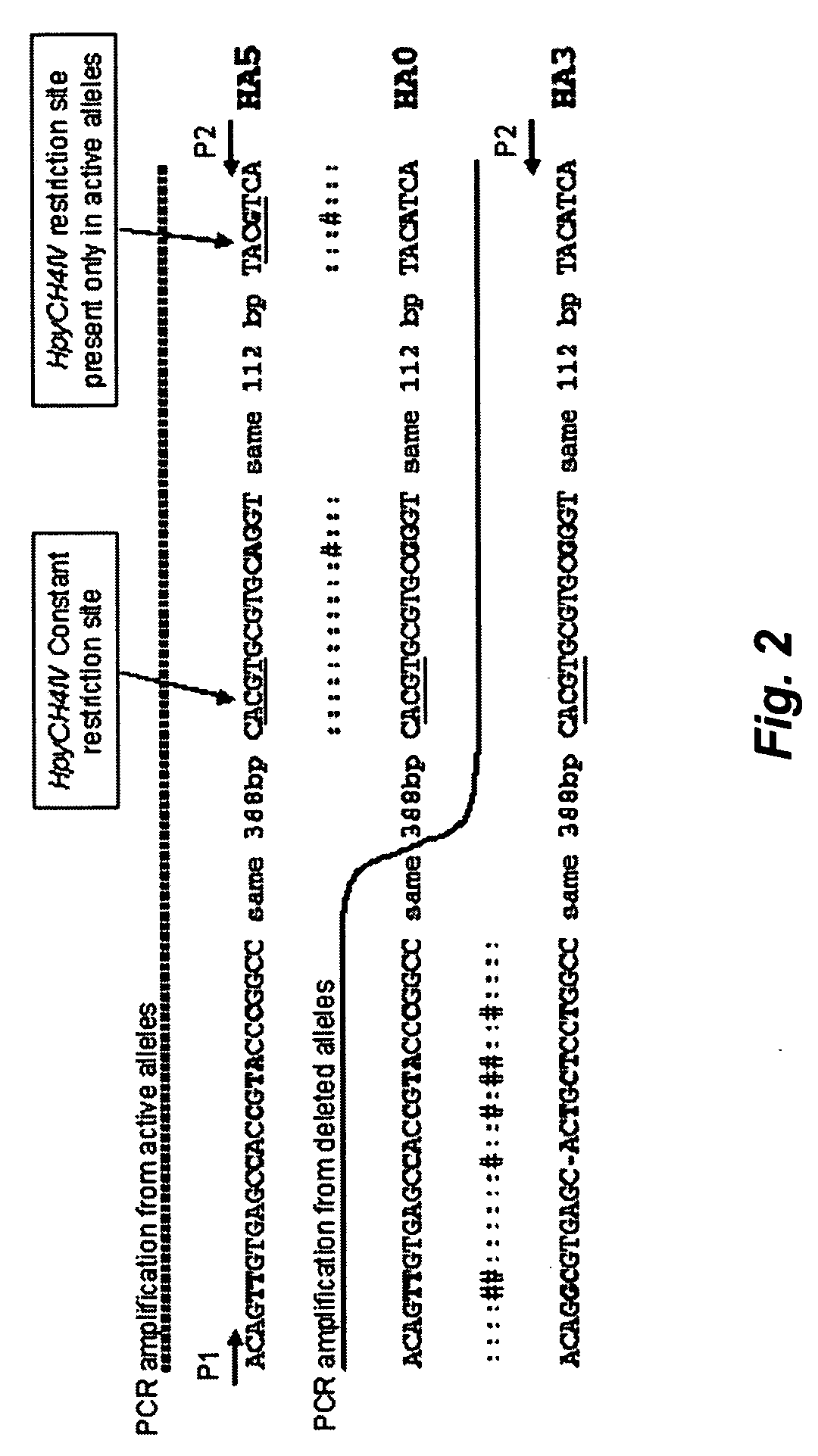
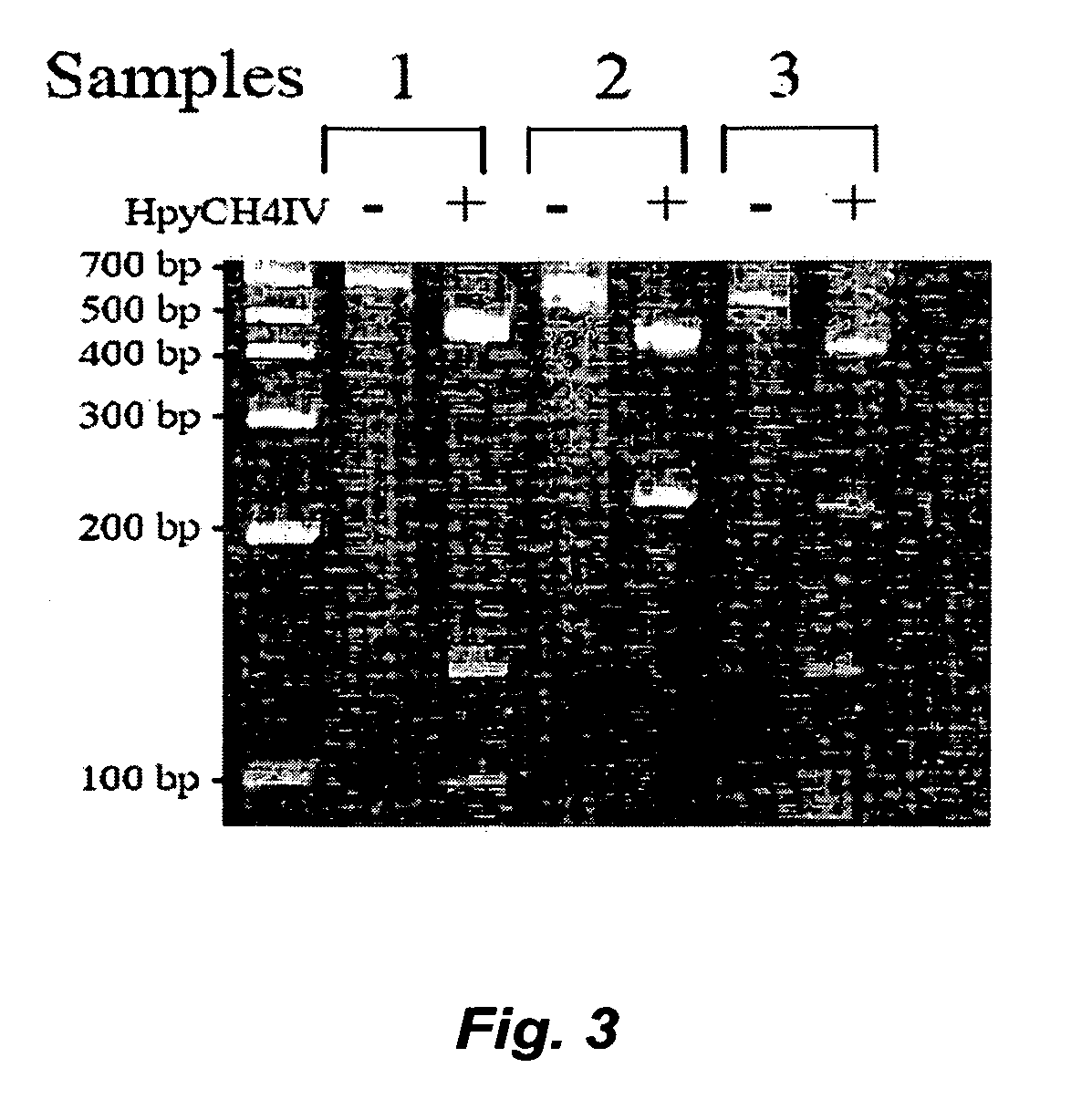
High throughput detection of glutathione s-transferase polymorphic alleles
InactiveUS20060194200A1Shorten the timeLow costMicrobiological testing/measurementFermentationCost effectivenessGenetic alleles
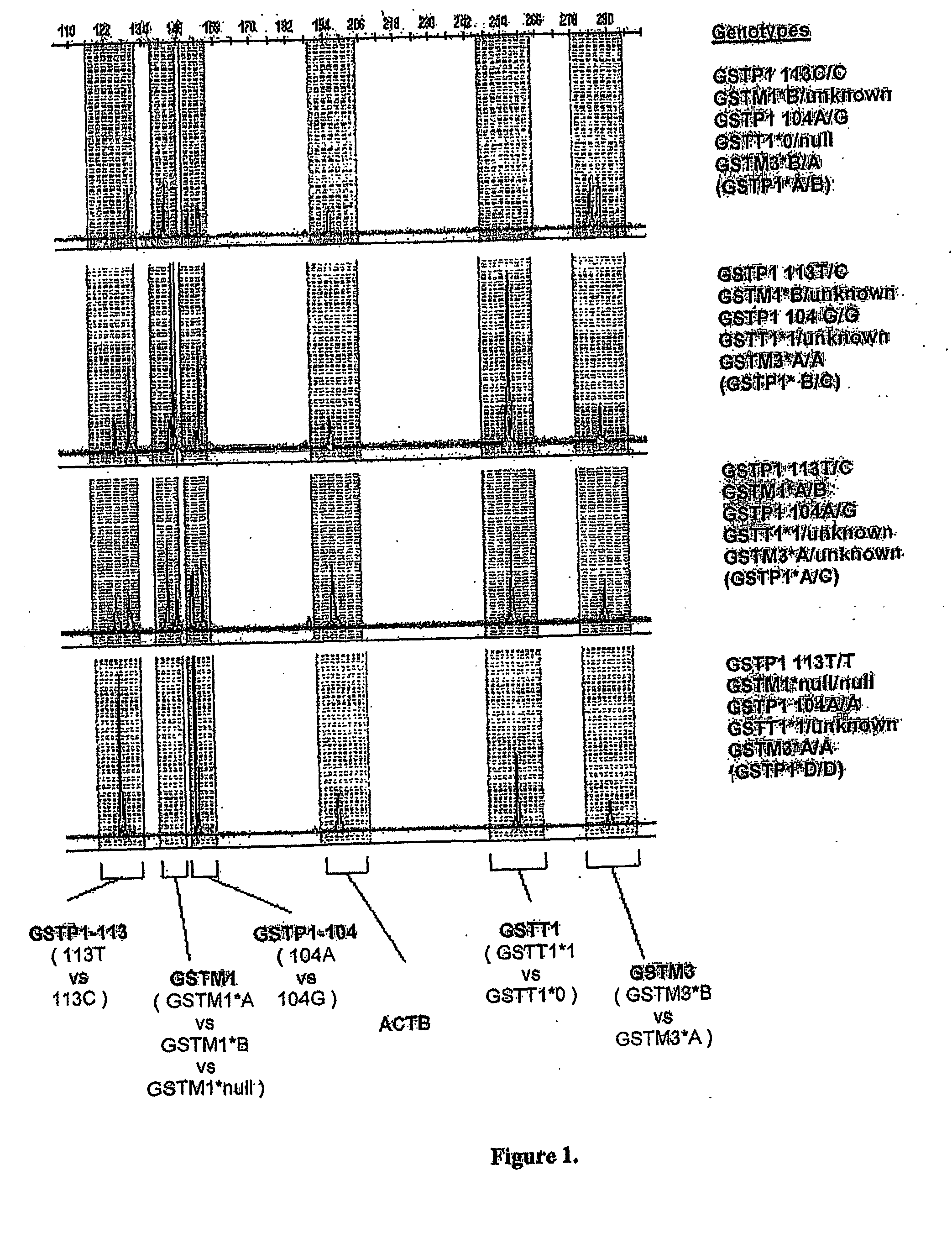
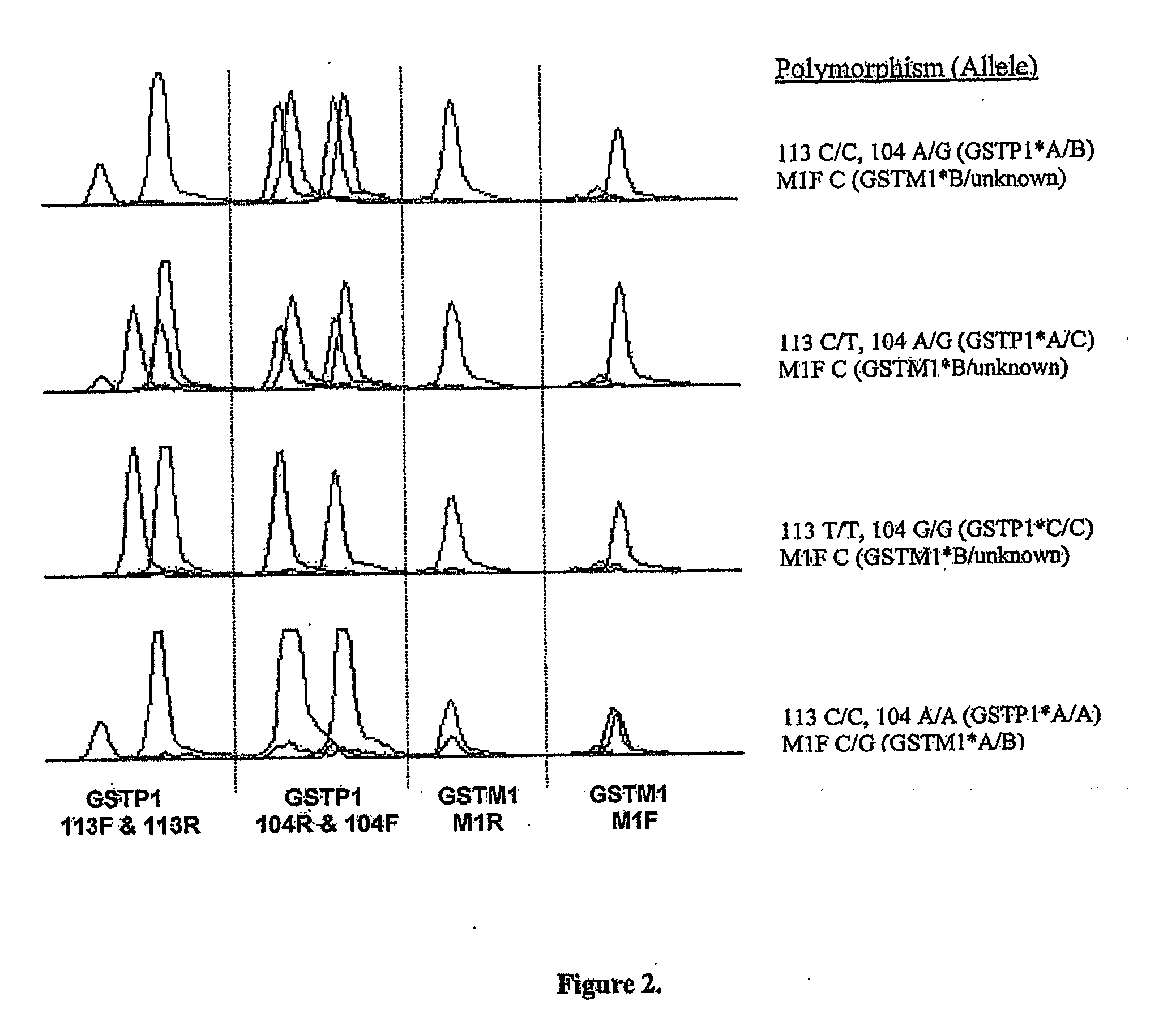
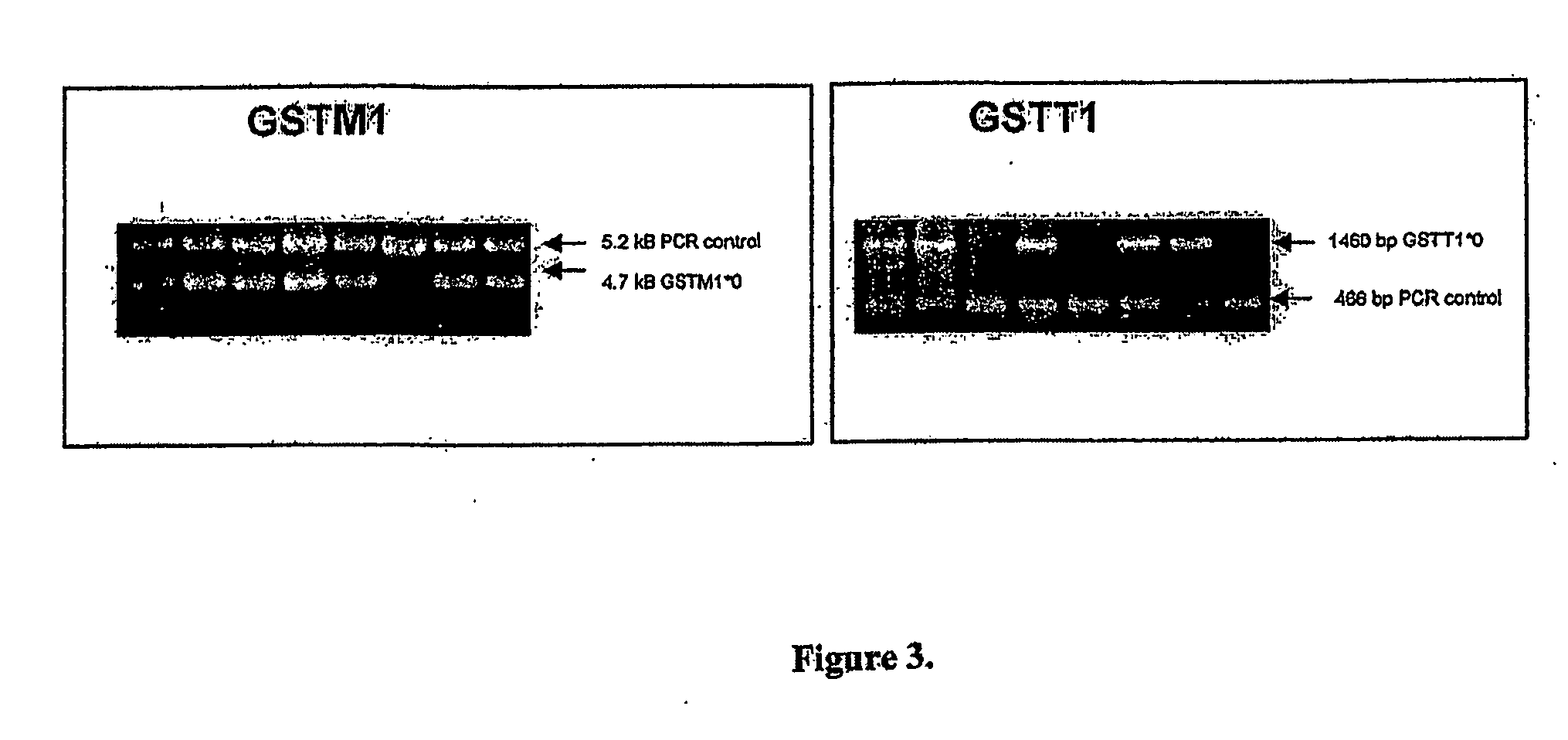
A high-throughput assay for characterizing a subject's genetic makeup is disclosed. Specifically, a high-throughput assay utilizing PCR is disclosed that permits the rapid and accurate characterization of a subject's inherited alleles of the polymorphic glutathione S-transferase (GST) genes GSTM1, GSTM3, GSTP1, and GSTT1. This method allows detection of the specific alleles inherited, including the gene dosage of GSTM1 and GSTT1 while not requiring restriction endonuclease digestion of the PCR products in order to detect length differences. Further, the method allows all analyses to be performed simultaneously in the same gel lane, thus further adding efficiency and cost-effectiveness.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Tobacco glutathione transferase gene and application thereof
InactiveCN105039277AHigh detoxification efficiencyMicrobiological testing/measurementTransferasesBiotechnologyNicotiana tabacum
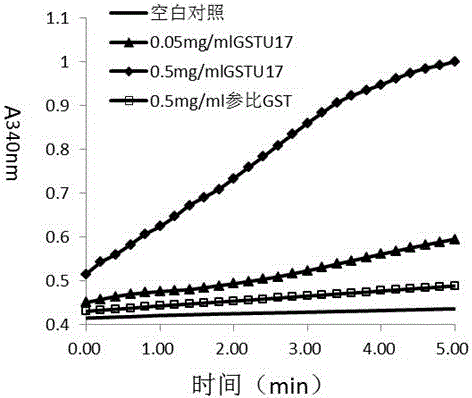
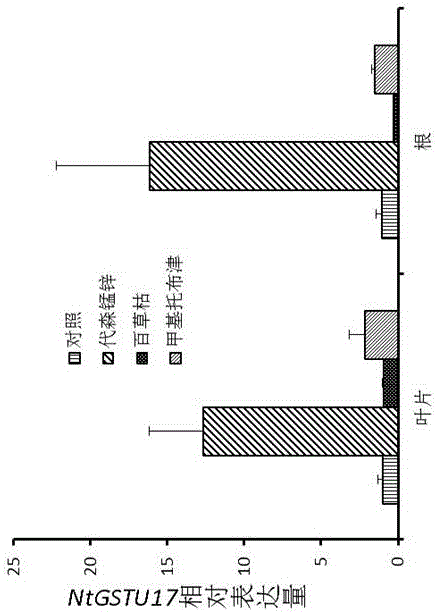
The invention discloses tobacco glutathione transferase protein, the effect of a tobacco glutathione transferase gene NtGSTU17 in detoxication metabolism, and application of the tobacco glutathione transferase gene in tobacco pesticide safener screening, and belongs to the technical field of biology. The amino acid sequence of the tobacco glutathione transferase gene can be seen in SEQ ID NO:1. Glutathione transferase (GST) is a key enzyme generally existing in the organism detoxication metabolism process, the tobacco NtGSTU17 protein has high detoxication activity, the encoding gene of the tobacco NtGSTU17 protein is expressed in tobacco in a constitutive mode and is remarkably expressed in an up regulation mode after being processed through some pesticide, and it shows that the gene has application value and economic value on the aspects of tobacco pesticide detoxication and screening.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
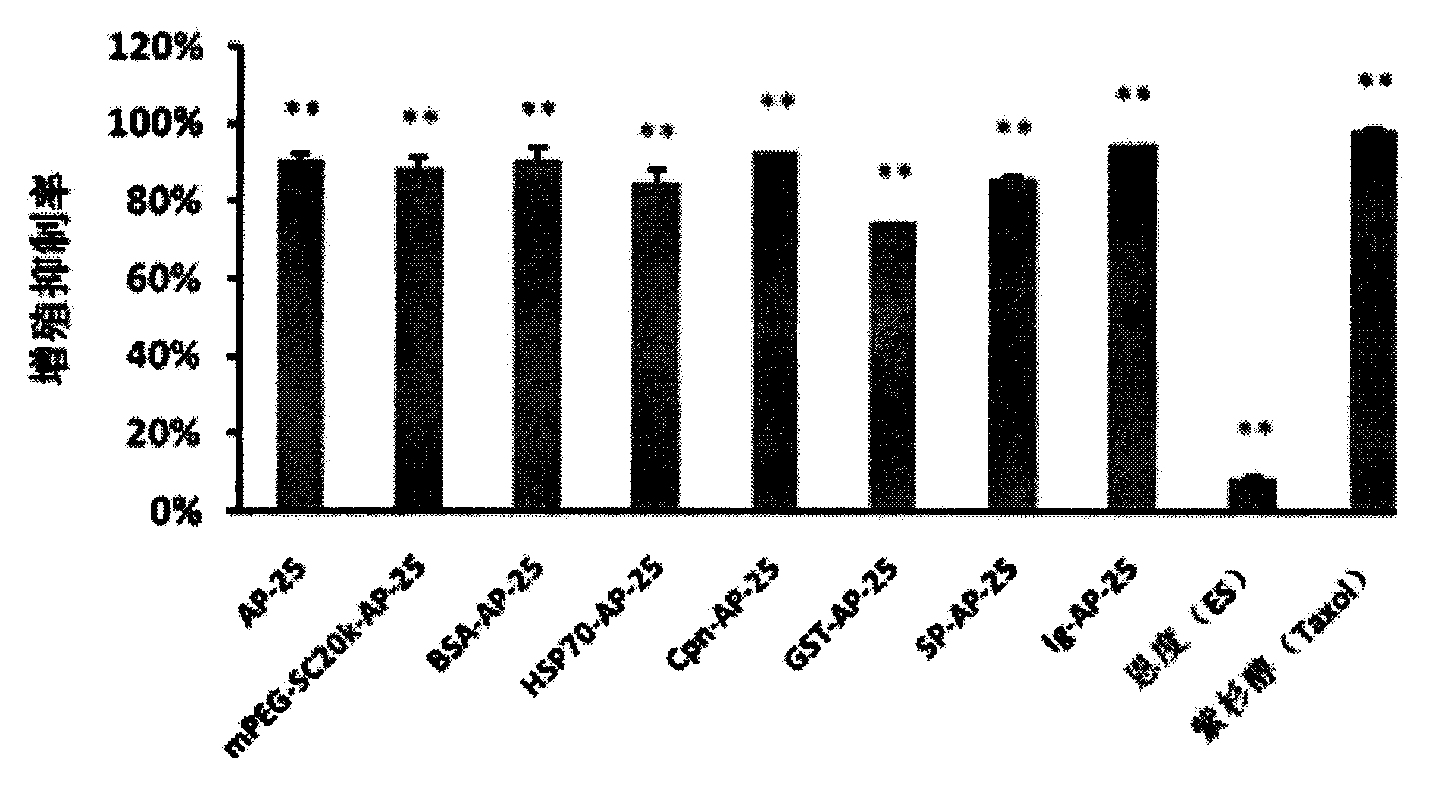
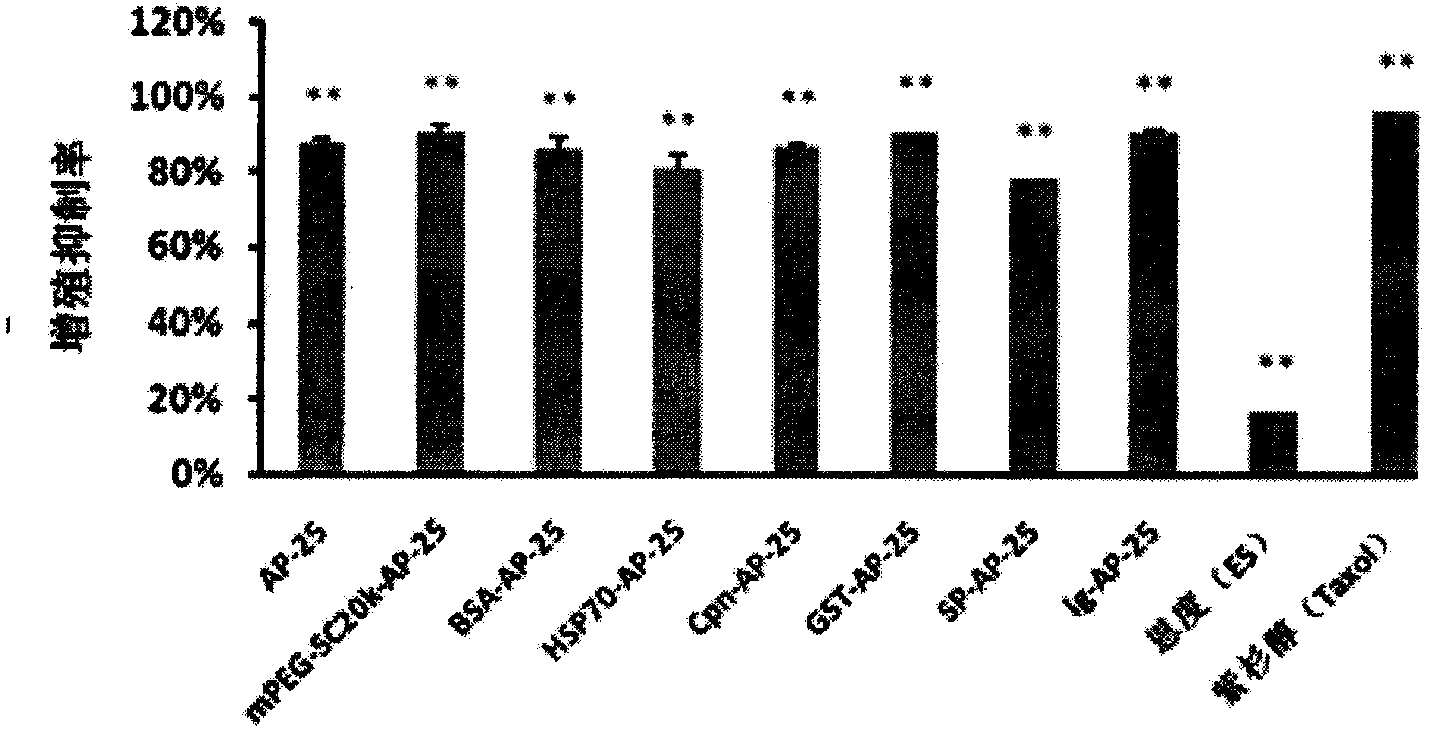
Integrin blocking agent AP-25 expressed by modification of polyethylene glycol and protein fusion and its application
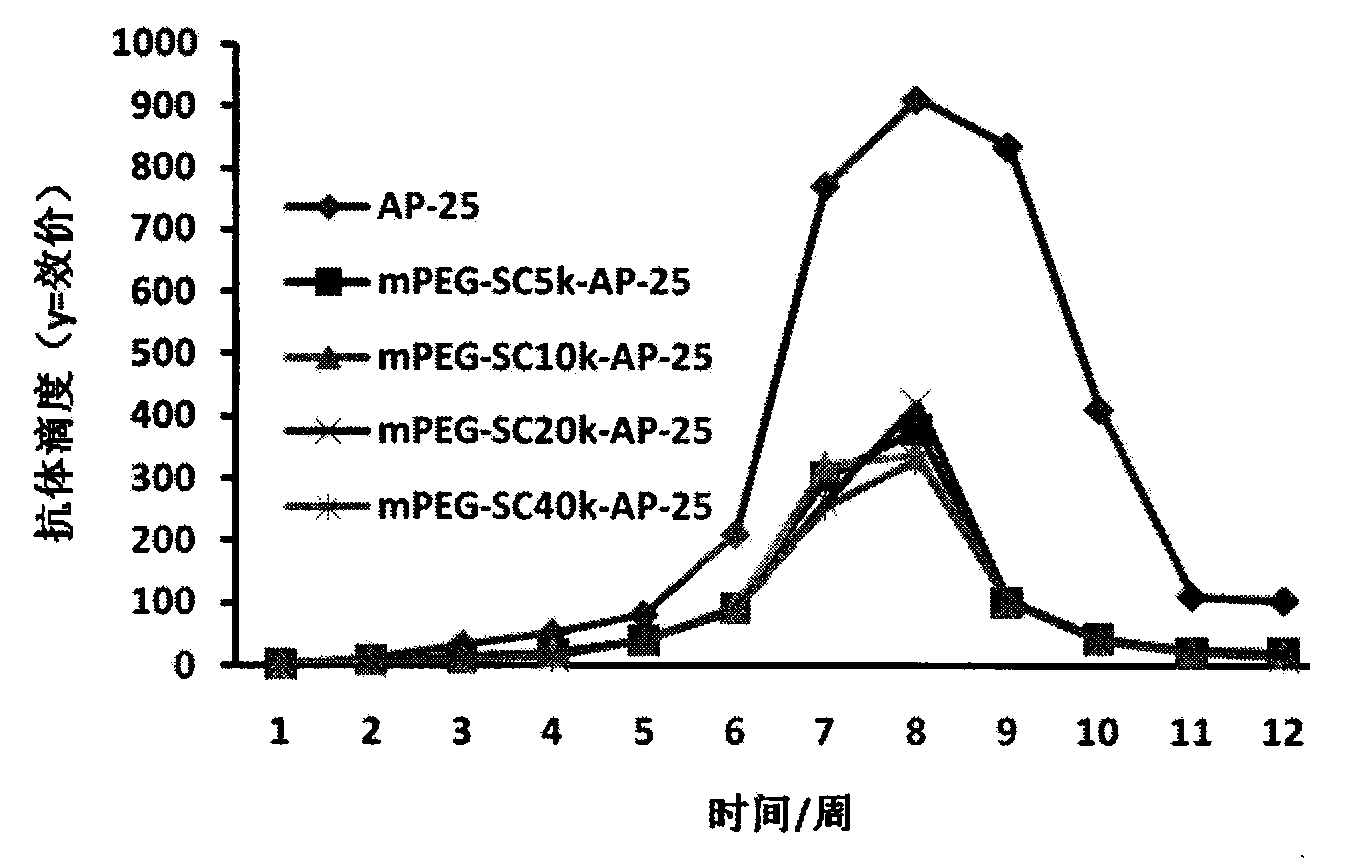
InactiveCN103819542AExtended half-lifeDoes not affect the activity in vivo and in vitroSenses disorderPeptide/protein ingredientsSequence signalHalf-life
The invention relates to the medicine field, and more specifically relates to an integrin blocking agent capable of inhibiting tumor vessel generation, having integrin element endophilicity and combination capability, the blocking agent is a polypeptide, and the polypeptide is modified by polyethylene glycol, after modifying, the half-life period of the polypeptide is prolonged, immunogenicity is reduced, and the blocking agent enables fusion expression with bovine serum albumin, heat shock protein, chaperonin, glutathione transferase (GST), signal peptide and immunoglobulin, and is in favor of correct formation of disulfide bond, the half life period can be prolonged, a certain special function of fusion protein is simultaneously increased. The integrin blocking agent polypeptide after modification and fusion expression can be used in treatment of solid tumor. The designed integrin blocking agent polypeptide has the advantages of scientific, reasonable, feasible and effective performances, and can be a treatment medicine for treating solid tumor of human, the treatment spectrum of the integrin blocking agent can be greatly developed, and the integrin blocking agent has obviously social value and market value.
Owner:CHINA PHARM UNIV
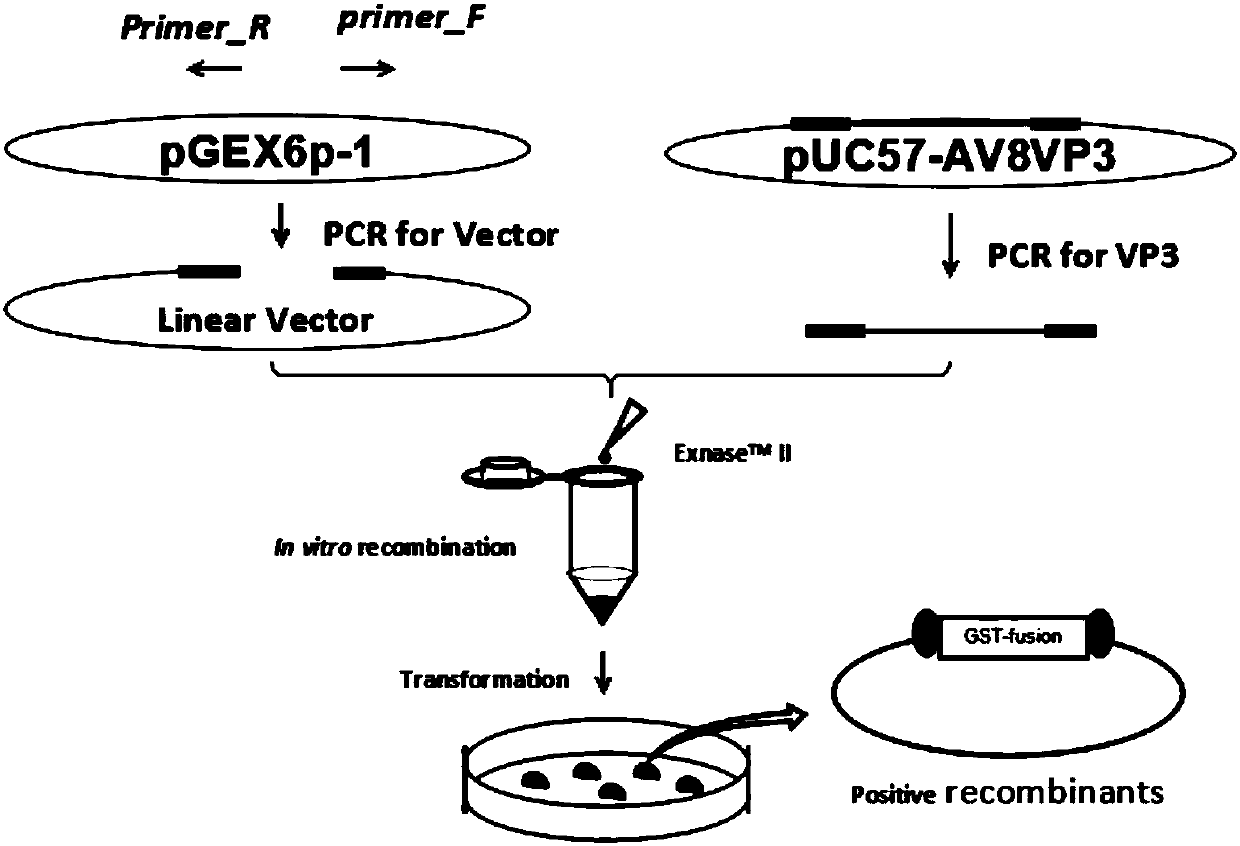
Preparation method of soluble protein of GyV8 gyrovirus VP3
The invention relates to a preparation method of soluble protein of novel GyV8 gyrovirus VP3. The preparation method comprises the following steps of using a pGEX-6p-1 linear carrier and a primer of a GyV8 virus VP3 gene segment to directly and quickly recombine and clone VP3 in vitro through recombinase ExnaseTMII without enzyme-cut and link up reaction, converting colibacillus coli, and performing IPTG (isopropyl-beta-d-thiogalactoside) inducing expression to realize the fusion soluble expression of GyV8 VP3 protein and GST (glutathione transferase), and obtain purified VP3 soluble protein. The preparation method has the advantages that the GyV8 virus VP3 gene is cloned by the recombinase ExnaseTM II in-vitro recombinant technique without relying on the enzyme cutting site and restriction endonuclease, the cloning process is simplified, and the quick cloning and expression of VP3 gene is realized.
Owner:YANGZHOU UNIV
Biomarker for evaluating zinc nutrition state of individual and application thereof
The invention discloses a biomarker for evaluating the zinc nutrition state of an individual and application of the biomarker. Wherein the biomarker is glutathione S-transferase Omega1 (GSTO1). Differential biomarkers (proteins and metabolites) in blood and liver of rats lack of zinc are screened through a multi-omics combined technology based on high-flux chromatography-mass spectrometry, such as proteomics and metabonomics; enrichment analysis is carried out on the multi-omics metabolic pathways to screen the most significant metabolic pathways with zinc deficiency and candidate biomarkers with zinc deficiency are screened; the screened candidate zinc deficiency biomarkers are verified through zinc deficiency sensitive population; and finally, the specific zinc deficiency biomarker of the crowd is determined through a random contrast zinc supplement intervention experiment of the zinc deficiency crowd, a rapid, sensitive and specific standard method system capable of being used for zinc nutrient state evaluation is tried to be established based on the screened biomarker, and help is provided for evaluating the zinc nutrient state and intervention measures of individuals.
Owner:HARBIN MEDICAL UNIVERSITY
Method for analyzing residual agricultural chemical
InactiveUS7655430B2High detection sensitivityHigh activityAnalysis using chemical indicatorsMicrobiological testing/measurementCarbamateCarbofuran
The present invention relates to a method for analyzing residual agricultural chemicals which comprises the steps of acting a reduced glutathione as a reactive substrate and a glutathione transferase serving as a catalyst for the reaction on a carbofuran derivative or a methomyl derivative as a carbamate type agricultural chemical of a new series to thus derivatize the agricultural chemical into a substance having a high choline esterase-inhibitory activity; reacting the substances formed through the derivatization reaction with a choline esterase; and then detecting the presence of the agricultural chemical as the new series of carbamate type one included in a sample to be examined on the basis of the changes in the choline esterase activity thus detected. The method of the present invention may serve as a powerful tool for the detection of the residual agricultural chemicals in grains such as rice and the detection of the content of agricultural chemicals remaining in agricultural products such as vegetables and fruits.
Owner:SATAKE CORP +1
A kind of gene expression product blsj-1 of Brucella diagnostic mark function and preparation method thereof
ActiveCN106749562BSignificant differential diagnosisDepsipeptidesBiological testingWestern blotBrucella
The invention relates to a gene expression product BLSJ-1 with a diagnostic marker function of Brucella and a preparation method thereof. The product is prokaryotically expressed from the gene of Brucella S2 strain with gene number (GI) 489054867, and is purified by glutathione S-transferase (GST) affinity chromatography column and glutathione gradient elution method. The product can be used as a coating antigen and can be used as a diagnostic antigen to differentiate between animal Brucella vaccine immunity and natural infection serum detection. The antigen was subjected to Western-blot with vaccine immune antibodies and natural infection antibodies respectively, and the differences were obvious. The BLSJ‑1 antigen was used as an indirect enzyme-linked immunosorbent assay (ELISA) coating antigen to detect hundreds of brucellosis clinical serum samples, and the differential diagnosis effect was significant.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
A method for high-throughput screening of single-domain antibodies using ispla and its application
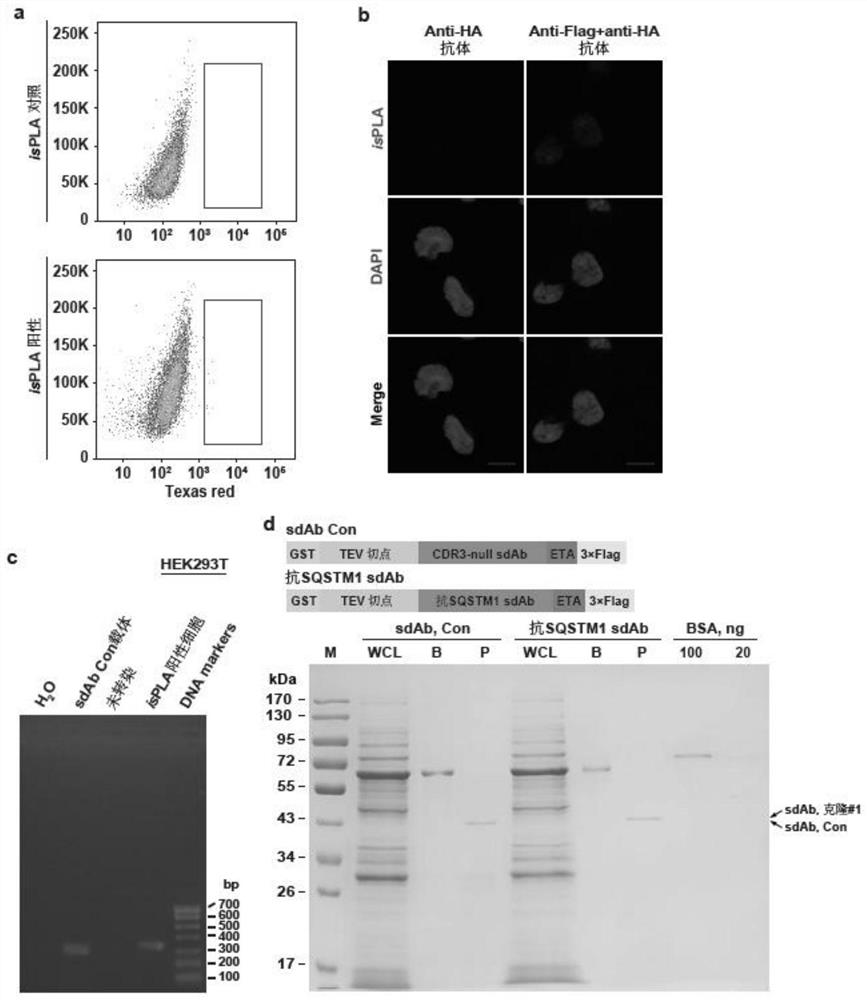
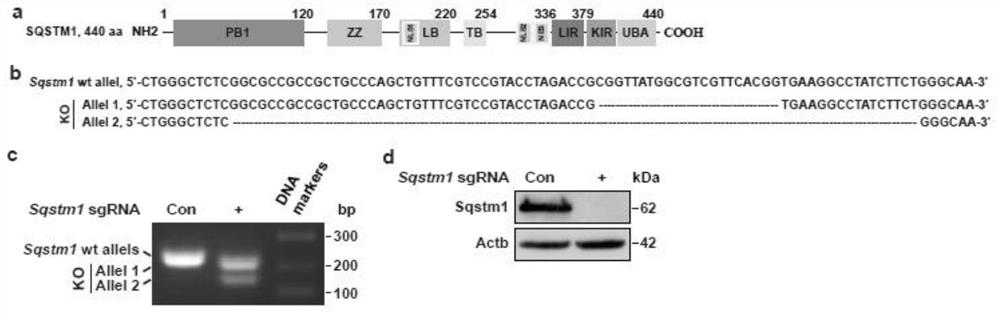
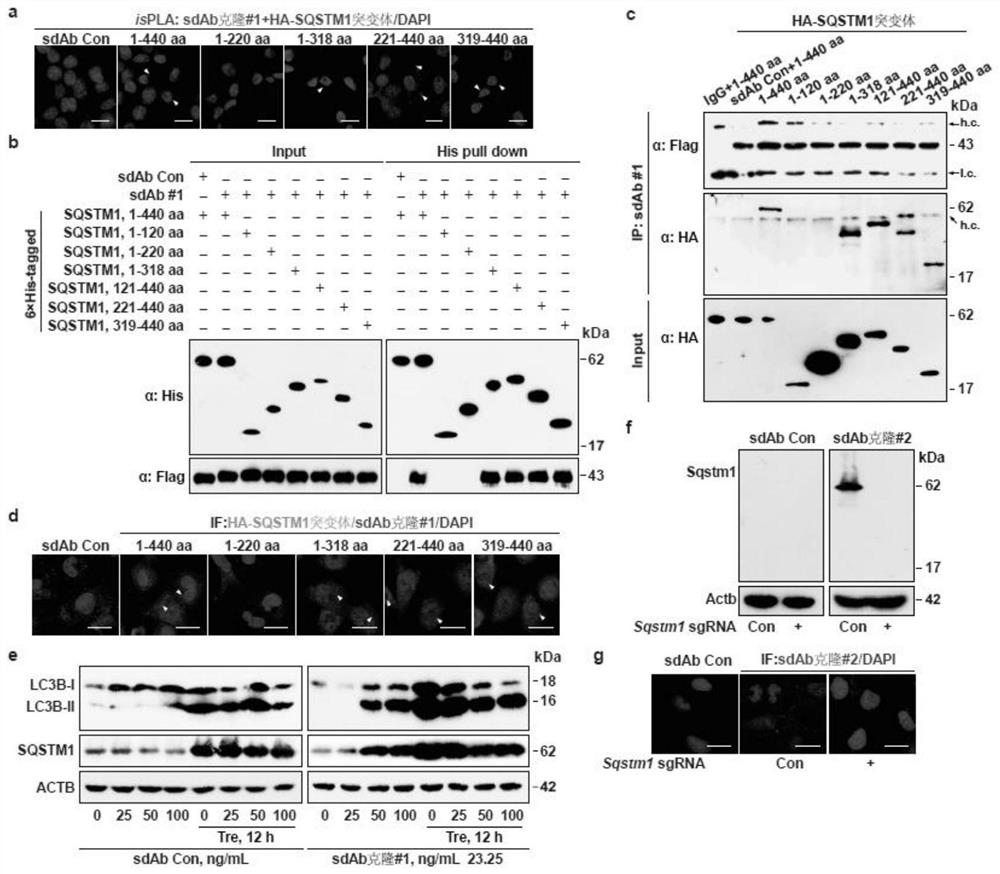
ActiveCN113528569BNo preparation involvedHigh affinityAntibody mimetics/scaffoldsLibrary screeningAntigenPseudomonas aeruginosa exotoxin A
The invention discloses a method for high-throughput screening of single-domain antibodies using isPLA. The steps are as follows: constructing an sdAb library containing 21 random amino acid sequences in the CDR3 region, and fusing a 3×Flag tag to the C-terminus of the sequence; co-transfection Cells, fixed cells for isPLA; followed by sorting, amplification, and recombination, and after multiple rounds of screening and sequencing, constructs that sequentially contain glutathione S-transferase GST, tobacco mosaic virus TEV protease cleavage site, and recognize SQSTM1. The sdAb sequence, the translocation domain of Pseudomonas exotoxin ETA and the 3×Flag-tagged recombinant protein were expressed in E. coli, followed by purification and enzyme digestion. The method can screen sdAbs that specifically recognize different antigenic determinants from the constructed high-capacity sdAb library, and has low cost and high efficiency. No preparation of animal samples or hybridomas is involved.
Owner:TIANJIN MEDICAL UNIV
Method for biotransformation of trichothecene
The present invention relates to a novel method for detoxification of trichothecene contaminated materials. More specifically, the present invention relates to a method of bioconverting trichothecene by contacting a material contaminated with trichothecene with an exogenous non-animal glutathione-S-transferase (GST) having substrate specificity for the epoxy ring of trichothecene. The invention also relates to recombinant GST as well as to transgenic plants and animals expressing said GST.
Owner:帝斯曼奥地利有限公司
A kind of gene expression product blsj-3 of Brucella diagnostic marker function and preparation method thereof
ActiveCN106749563BSignificant differenceSignificant differential diagnosisBiological material analysisDepsipeptidesWestern blotBrucella
The invention relates to a gene expression product BLSJ-3 with Brucella diagnosis and identification effect, and a preparation method thereof. According to the preparation method, the gene expression product is obtained via gene prokaryotic expression of Brucella S2 strain with GI of 490819668, and via glutathione S transferase (GST) affinity chromatographic column and glutathione gradient elution purifying. The gene expression product can be taken as a coating antigen, and as a diagnosis antigen of animal Brucella vaccine immunity and naturally infected serum detection. When the BLSJ-3 antigen is subjected to western blot with vaccine immune antibodies and natural infection antibodies, the difference is obvious. The gene expression product BLSJ-3 is taken as an indirect enzyme-linked immune adsorption assay (ELISA) coating antigen to detect hundreds of brucellosis clinical serum samples, and differential diagnosis effect is excellent.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Method for biotransformation of trichothecenes
PendingUS20220298521A1Reduce the amount requiredTransferasesAnimal feeding stuffBiotechnologyTrichothecene
The present invention relates to a novel method for detoxification of trichothecene contaminated material. More specifically the present invention pertains to a method for biotransformation of a trichothecene by contacting material contaminated with trichothecenes with an exogenous non-animal glutathione-S-transferase (GST) having substrate specificity for the epoxide ring of the trichothecene. The invention further relates to recombinant GSTs and transgenic plants and animals expressing said GSTs.
Owner:DSM AUSTRIA GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com