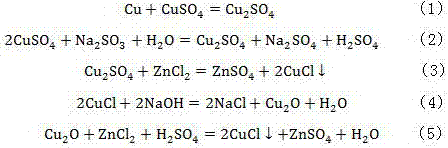Patents
Literature
81results about How to "Guaranteed reducibility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
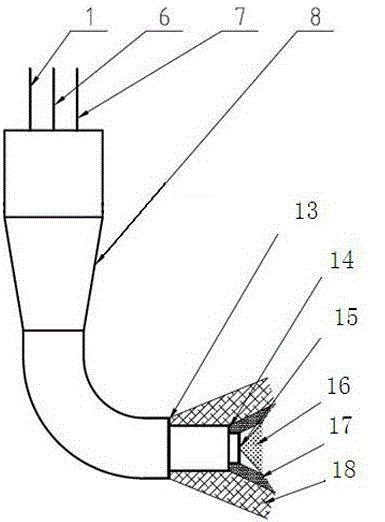
De-oxidation modifying agent for steel ladle top slag
ActiveCN103374642AThe modifier has good reducibility and continuous stabilityImprove reducibilityProcess efficiency improvementLadle furnace slagSlag
The invention relates to steel ladle furnace slag modifying agent in a converter steel tapping process. The de-oxidation modifying agent for the steel ladle top slag comprises the following main components in percentage by weight: 30%-50% of Al, 50%-30% of CaO, 5.0%-8.0% of Al2O3, not more than 3.0% of SiO2, not more than 0.07% of S+P, 0.7%-1.4% of C, 3.0%-8.0% of CaFe2 and not more than 0.2% of H2O. The modifying agent disclosed by the invention is a reductive modifying agent which can be used for solving an oxidation problem of the steel ladle top slag in a low-carbon pipeline steel production process, so that guarantee is provided for vacuum desulfurization and pure production of the pipeline steel.
Owner:BAOSHAN IRON & STEEL CO LTD
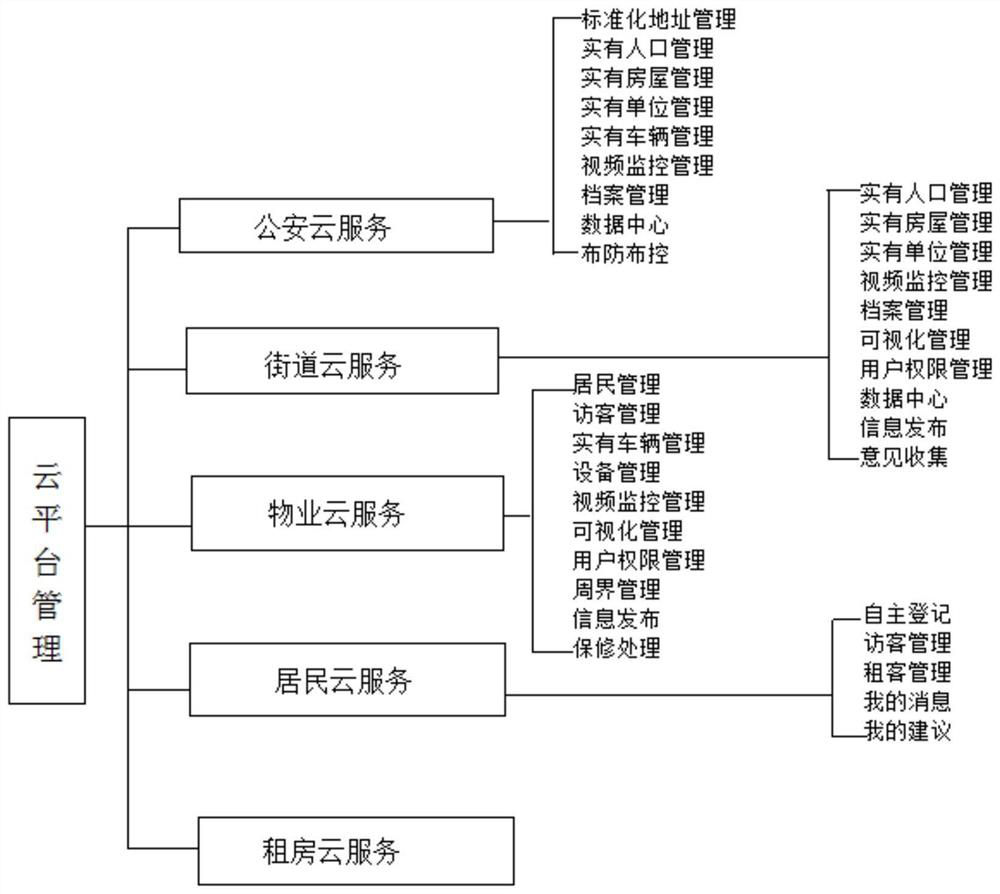
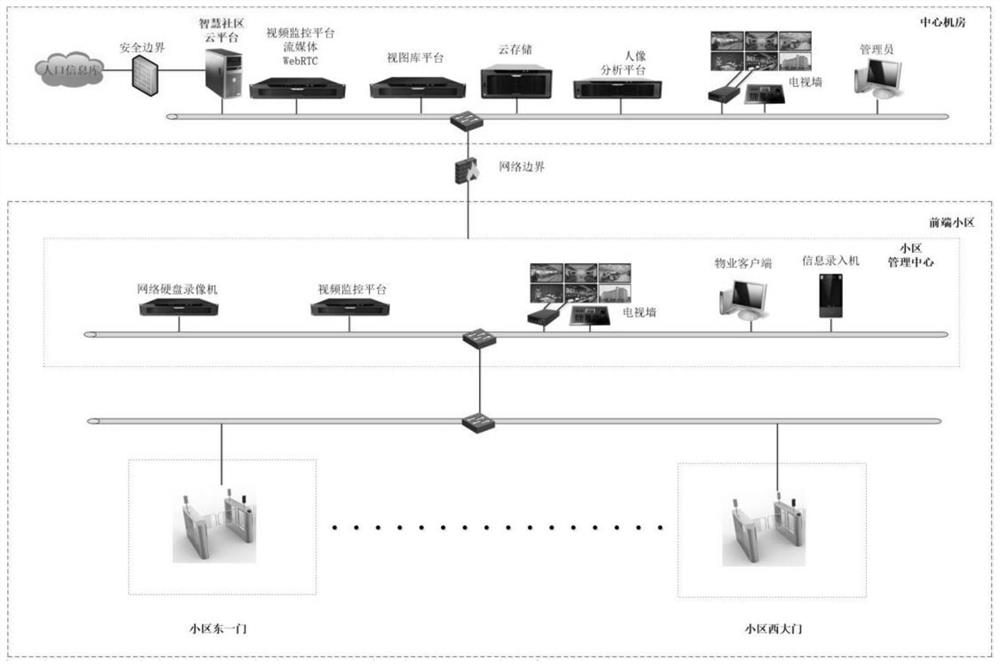
Cloud platform management system for smart community
PendingCN112116503ARealize interoperability and sharingRealize managementData processing applicationsTicket-issuing apparatusShared resourceSmart community
The invention belongs to the technical field of intelligent management, and relates to a cloud platform management system for a smart community. The system comprises a public security cloud service subsystem, a street cloud service subsystem, a property cloud service subsystem, a resident cloud service subsystem and a house renting cloud service subsystem which are in network connection to form the cloud platform management system. Cloud computing, big data and Internet of Things technologies are adopted, so that integration and analysis of community front-end management system data and modular management of multiple subsystems are achieved; artificial intelligence, deep learning and other technologies are adopted, convenience and resident-benefiting services are provided for community residents, a comfortable ecological environment is created, and a sustainable smart community is created; and meanwhile, accurate service is provided for the residents, and management is carried out in the service; through information sharing and resource integration, transformation and upgrading of community management are realized.
Owner:QINGDAO SONLI SOFTWARE INFORMATION TECH
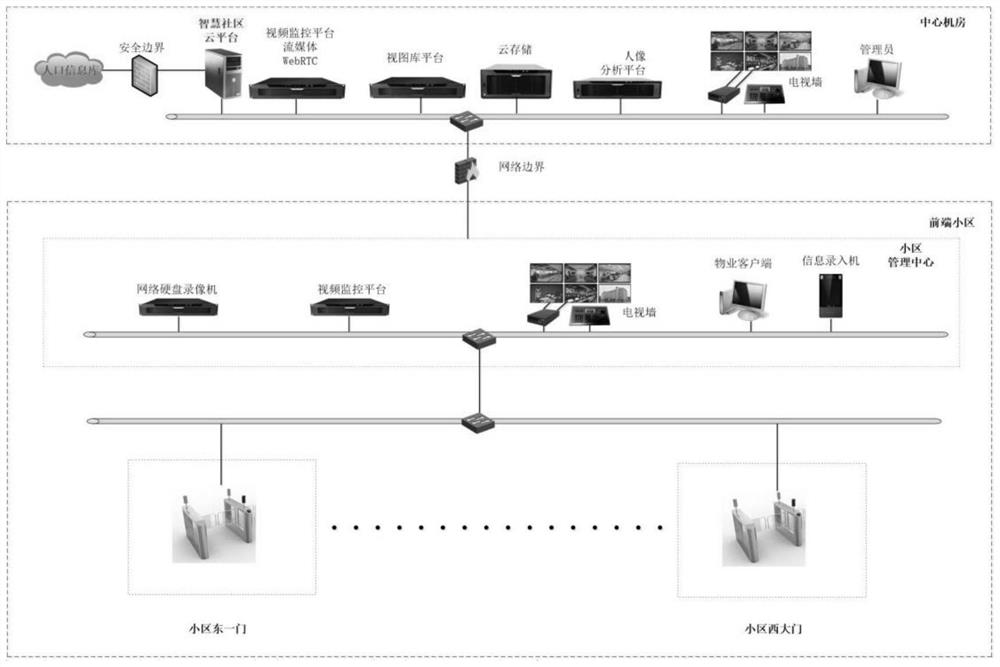
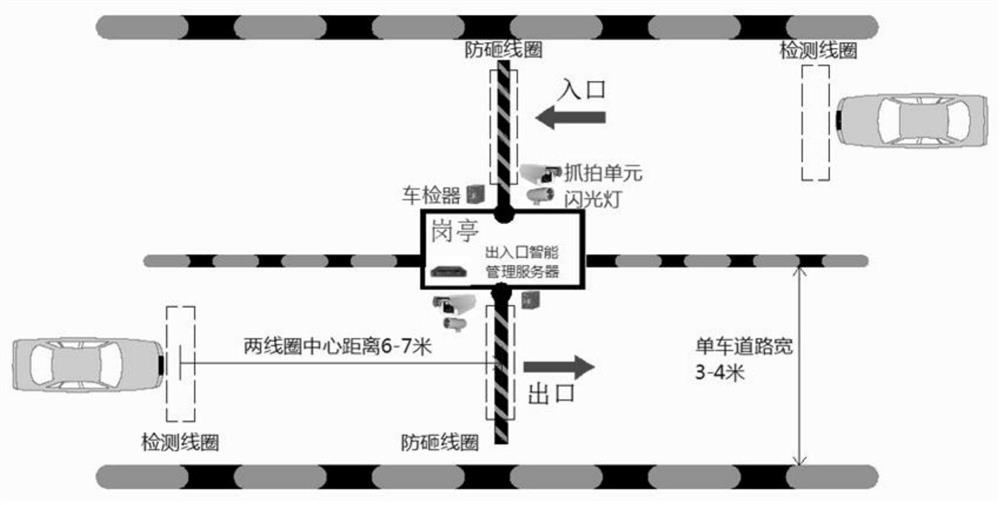
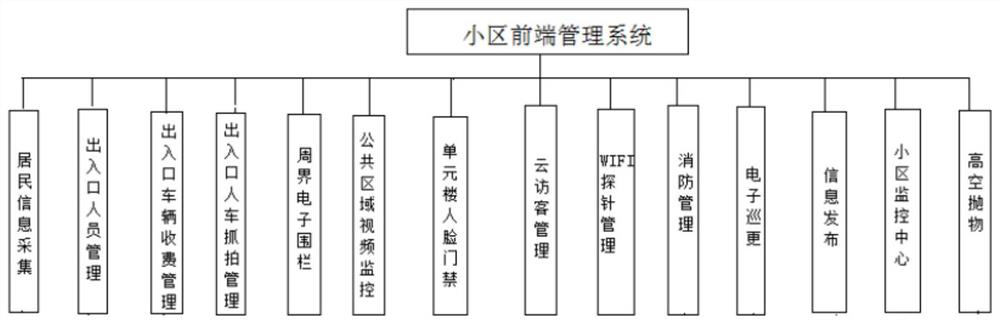
Smart community front-end management system
InactiveCN112383581APrevent internal theft and external theftPrevent cheatingTicket-issuing apparatusIndividual entry/exit registersVideo monitoringCommunity resident
The invention belongs to the technical field of intelligent management, and relates to a smart community front-end management system. The system comprises a resident information acquisition subsystem,an entrance and exit personnel management subsystem, an entrance and exit vehicle charging management subsystem, an entrance and exit person and vehicle snapshot management subsystem, a perimeter electronic fence subsystem, a public area video monitoring subsystem, an apartment face access control subsystem, a cloud visitor management subsystem, a WIFI probe management subsystem, a fire protection management subsystem, an electronic patrol subsystem, an information publishing subsystem, a community monitoring center and a high-altitude parabolic subsystem. Subsystems exist in an independent mode, data collected by the subsystems are collected to the cloud server in network connection with the subsystems to be analyzed and processed, convenience, benefit and benefit services are provided for community residents, a comfortable ecological environment is created, a sustainable smart community is created, meanwhile, precise services are provided for the residents, and management is carriedout in the services. Through information sharing and resource integration, transformation and upgrading of community management are realized.
Owner:QINGDAO SONLI SOFTWARE INFORMATION TECH
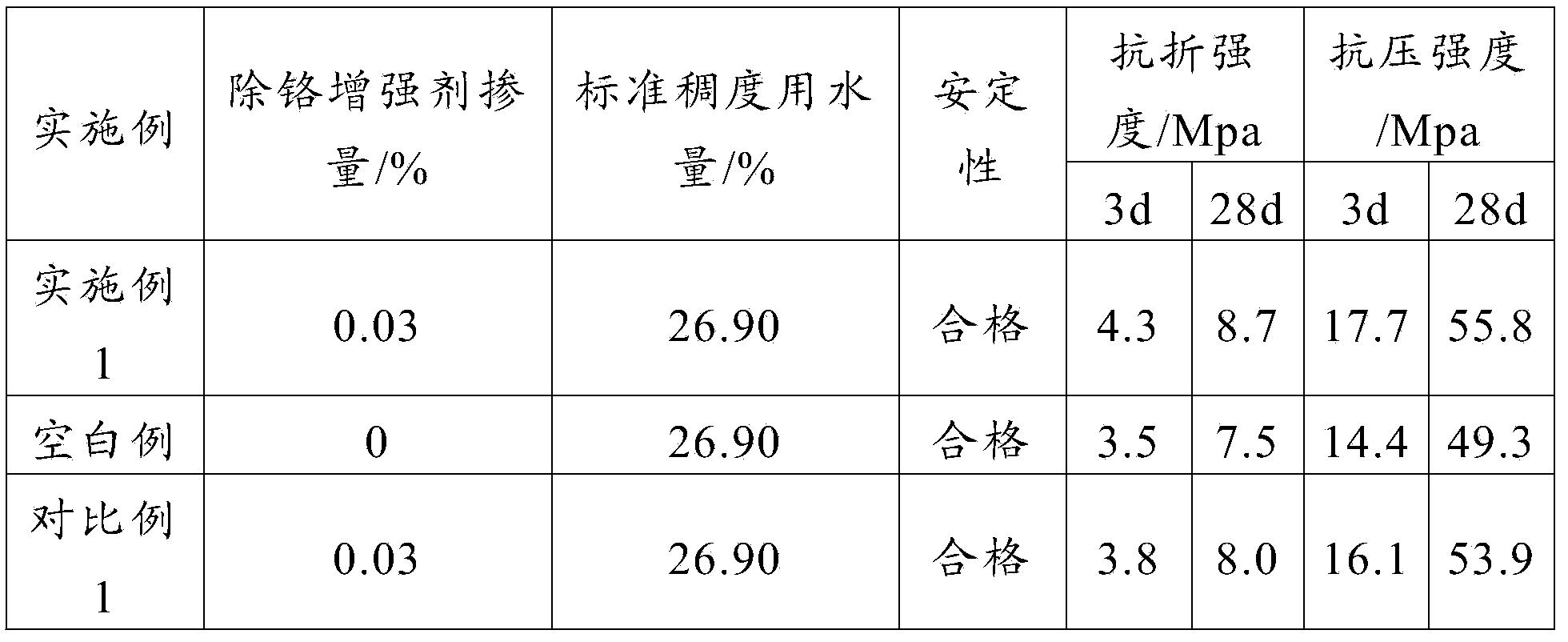
Chromium removal enhancer and preparation method thereof
The invention provides a method for preparing a chromium removal enhancer. The method comprises the following steps: (a) calcining kaolin, mixing the calcined kaolin and an acid solution, performing suction filtration, and washing and drying the obtained solid; (b) mixing the kaolin obtained in the step (a) and an mixed solution, and reacting to obtain the chromium removal enhancer, wherein the mixed solution is a mixed solution of a reducing agent, an antioxidant, an enhancer and water. According to the chromium removal enhancer prepared by the invention, the kaolin is taken as a raw material, the reducing agent, the antioxidant and the enhancer are mixed with the modified kaolin, and the chromium removal enhancer is obtained after the reaction. According to the chromium removal enhancer prepared by the invention, due to dual effects of adsorption and reduction, hexavalent chromium in cement is effectively removed, and physical properties of the cement are improved. The invention also provides the chromium removal enhancer prepared according to the method.
Owner:SHANDONG HONGYI TECH
Airborne multi-angle tilted camera
InactiveCN105898119AWide camera angleImprove camera efficiencyTelevision system detailsColor television detailsImaging qualityAngular degrees
The invention relates to an airborne multi-angle tilted camera. The airborne multi-angle tilted camera includes a housing, shooting windows and cameras, wherein a shooting window is arranged at the center position of the lower end of the housing; the center camera is arranged vertically downward in the shooting window; a plurality of shooting windows which are arranged in a tilted way are uniformly distributed at the periphery of the shooting window at the center position in the radial direction; the plurality of shooting windows which are arranged in a tilted way are each provided with a tilted camera; and the orientation of each tilted camera is identical to the orientation of each shooting window. The airborne multi-angle tilted camera utilizes the plurality of tilted cameras and one vertical camera to perform synchronous shooting, so that the shooting angle is larger and the shooting efficiency is improved. Besides, compared with a previous individual camera, the shooting effect of the airborne multi-angle tilted camera is greatly improved, and the shot image quality of the airborne multi-angle tilted camera is high.
Owner:天津全华时代航天科技发展有限公司
Catalyst for splitting decomposition of biomass tar and preparation method thereof
InactiveCN103551182AGood dispersionHigh activityMolecular sieve catalystsHydrocarbon oil crackingMolecular sievePtru catalyst
The invention discloses a catalyst for splitting decomposition of biomass tar. The catalyst comprises the following components according to weight percent: 0.1-1% of RuO2, 5-15% of NiO, 1-6% of CuO, 5-20% of CeO2, and the balance of catalyst carrier. The invention also discloses a preparation method for the catalyst. The method comprises steps of adding an HZSM-5 molecular sieve into an aluminum oxide binder, adding a pore former and an assisting extrusion agent, kneading and grinding into balls, extruding into strips, drying, and roasting to obtain the catalyst carrier; then respectively loading metallic elements through a saturated impregnation method, drying, and roasting to obtain the catalyst. The catalyst has high activity in tar splitting decomposition, and has strong stability and anti-carbon accumulation performance; the catalyst carrier is of a hollow strip shape, so that the device is guaranteed to run smoothly; the catalyst is obtained by reduction of feed gas generated by biomass gasification, so that the processes are greatly simplified, and the catalyst is applicable to industrial production.
Owner:ENERGY RES INST CO LTD HENAN ACADEMY OF SCI +1
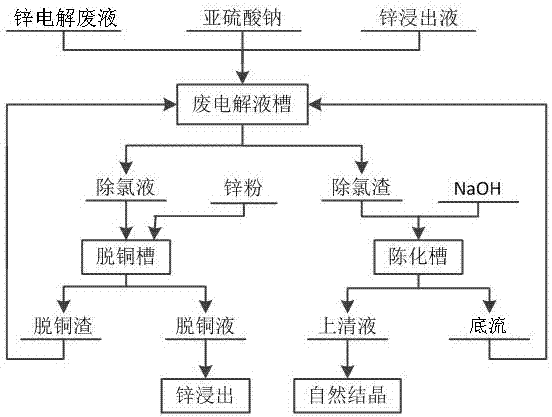
Method for removing chlorine in waste zinc electrolyte
InactiveCN107354484AEasy to operatePracticalPhotography auxillary processesElectrolysis componentsIonHydrometallurgy
The invention discloses a method for removing chlorine in waste zinc electrolyte. According to the method, excessive sodium sulfite is added into the waste zinc electrolyte to enable the whole waste zinc electrolyte to be in a reducing state; Cu2+ in the waste zinc electrolyte is fully reduced to Cu+, the reducibility of the waste zinc electrolyte is guaranteed, and Cu+ in the subsequent dechlorinated slag and dechlorinated slag treatment underflow is prevented from being oxidized; decoppered slag, a zinc leaching solution and the dechlorinated slag treatment underflow are added; an authigene product has a reduction reaction and a precipitation reaction in the solution through ingenious application of a zinc hydrometallurgy process, and chloride ions in a sulfuric acid containing zinc sulfate solution are removed. The content of chlorine returning to the chlorine in the leaching process is reduced. Accordingly, the requirement that the content of chlorine in the waste zinc electrolyte is lower than 300 mg / L home and abroad is met. The chlorine removal rate can reach up to 80% or above. Operation is easy. The practicality is high. At the same time, zinc is enriched in a decoppering solution, and the recovery rate of zinc is increased.
Owner:NORTHWEST RES INST OF MINING & METALLURGY INST
Method for preparing carbon-coated LiFePO4 for lithium ion batteries
InactiveCN101764205AGuaranteed reducibilityNot oxidizedElectrode manufacturing processesNew energyPhosphate ion
The invention relates to a method for preparing carbon-coated LiFePO4 (lithium iron phosphate) for lithium ion batteries, belonging to the technical field of new energy materials. The synthesis process of the invention comprises the following steps: mixing and drying ultra-fine iron powder, phosphoric acid, simple organic matter and doping element compounds with the molar ratio of phosphate ions, iron ions and doping element ions being 1:y:z, wherein y is larger than or equal to 0.95 and smaller than or equal to 1, and the sum of y and z is 1; adding lithium-source compounds and water to the mixture in sequence, remixing and drying, wherein the molar ratio between the lithium ions and the phosphate ions is x:1, and x is larger than or equal to 0.95 and smaller than or equal to 1.05; and sintering the mixture on a microwave temperature at 500 to 950 DEG C for 5 to 40 minutes in the vacuum atmosphere or the non-oxidizing atmosphere. The ultra-fine iron powder is used as microwave absorption media and iron source materials, so as to the iron powder is capable of rapidly absorbing the microwave energy to cause the solid-phase reaction to be rapidly conducted. Compared with the prior art, the invention has the advantages of simple technological process and easily controllable operation; and the final product has the advantages of high purity, complete crystallization, high capacity and good cycle stability.
Owner:孙琦
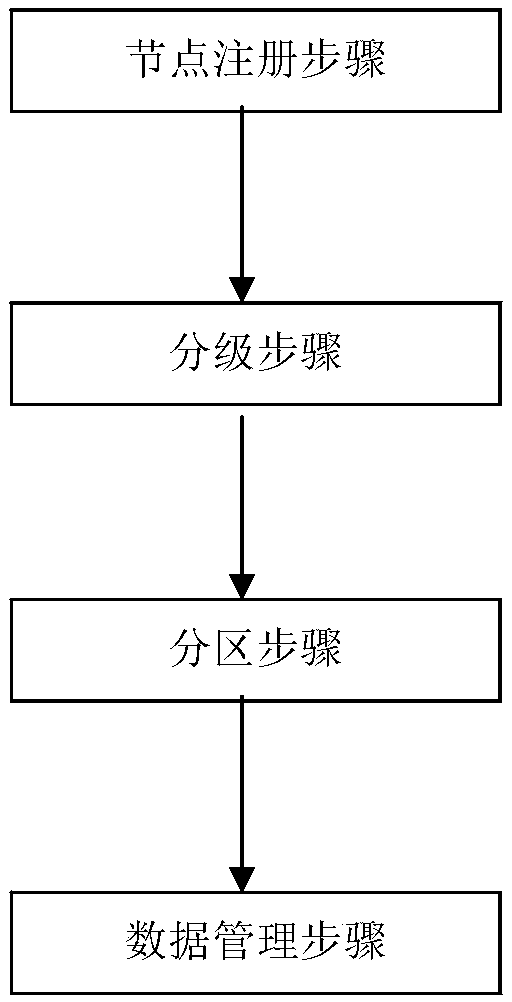
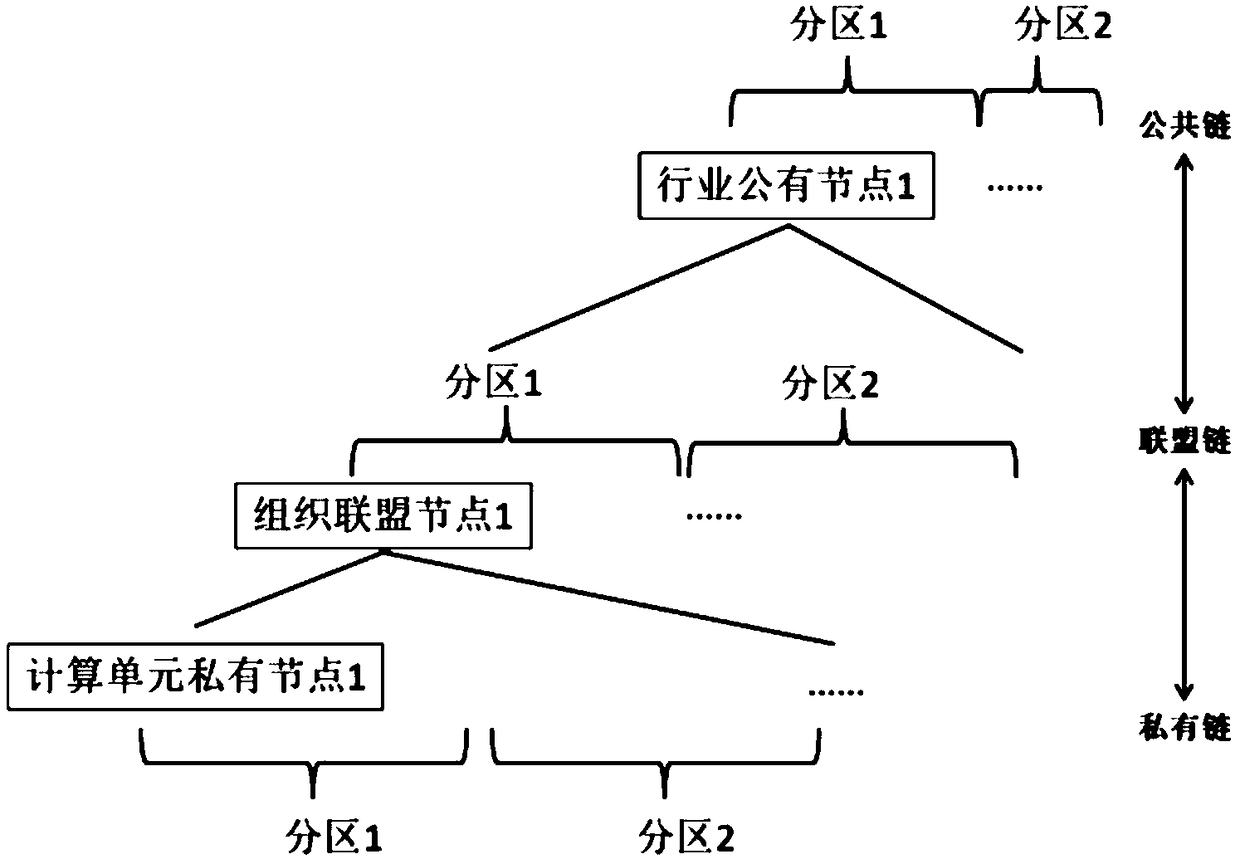
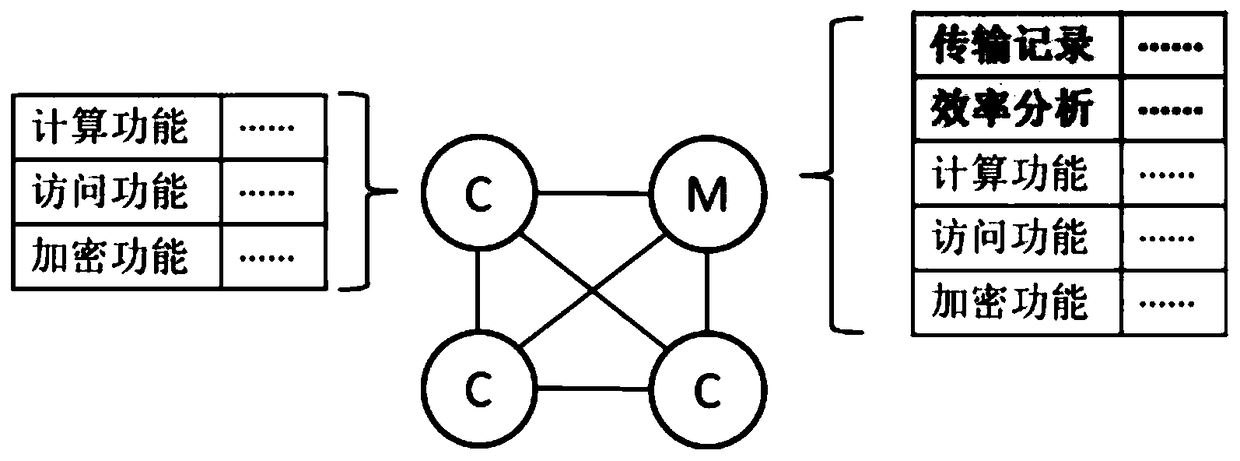
Layering and partitioning management method for nodes
InactiveCN108920723AImprove data transfer efficiencyImprove computing efficiencySpecial data processing applicationsStatistical analysisData transmission
The invention discloses a layering and partitioning management method for nodes. In order to effectively perform the function of blockchain data and give consideration to data transmission efficiency,the grading and partitioning management of the blockchain is carried out, each hierarchy of blockchain has a partitioning self-growth function, and a management node is arranged in each partition tocarry out statistics and analysis on data transmission efficiency in nodes. When efficiency achieves a transmission upper limit, partitioning segmentation is carried out, a new management node and a data backup are added, a tree structure organization is adopted between hierarchies, the next hierarchy of data interface is taken as an upper hierarchy of nodes, and partitioning management is carriedout in each hierarchy. Meanwhile, a verification step needs to be carried out for adding nodes, and the nodes can be added into the blockchain after voting. The management method is suitable for databackup management in the industry and specifically comprises an industrial public chain and an industrial internal organization alliance chain, wherein a private chain with a database structure is arranged in the organization.
Owner:HENGBAO
Augmented reality glasses and method for performing furniture displaying through same
InactiveCN106814457AGuaranteed fidelityEasy to displayOptical elementsUses eyeglassesComputer graphics (images)
The invention provides augmented reality glasses. The augmented reality glasses comprises a frame, lens assemblies, an embedded processor, display screens, and a camera arranged on the frame, wherein the lens assemblies, the embedded processor and the display screens are arranged in the frame. The camera has a wide-angle lens. The embedded processor is connected with the camera and the display screens, and are used for processing an image which is acquired by the camera, establishing or calling a digital model for being overlapped on the image, and displaying the image on the display screens. The lens assemblies are arranged in front of the display screens. Each lens assembly comprises a lens and an optical filter and is used for forming a three-dimensional display device together with the display screen, performing coupling adjustment on the image which is displayed on the display screen and projecting the adjusted image to an eye of a user. The invention further provides a method for performing furniture displaying through the augmented reality glasses. Through applying virtual reality technology on the augmented reality glasses, furniture displaying effect and shopping experience of a customer are improved.
Owner:杭州青杉奇勋科技有限公司
Method for utilizing sulfate slag and red mud comprehensively
The invention discloses a method for utilizing sulfate slag and red mud comprehensively. The method comprises the steps that the sulfate slag and the red mud are mixed with a reducing agent so as to obtain a mixed material; roasting is carried out on the mixed material so as to obtain a reduced iron product; the reduced iron product is separated so as to obtain iron powder. By the utilization of the method, the two metallurgical solid waste resources, namely the red mud and the sulfate slag are utilized comprehensively, so that the iron product with good indexes is obtained.
Owner:JIANGSU PROVINCE METALLURGICAL DESIGN INST
Preparation method of amorphous nickel sulfide
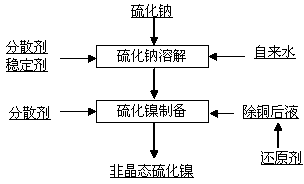
ActiveCN103864157AReduce the chance of collisionAvoid formingNickel sulfidesPurification methodsPolysulfide
The invention relates to a chemical purification method, and in particular relates to a preparation method of amorphous nickel sulfide. The preparation method provided by the invention is obviously different from the traditional nickel sulfide preparation method and is characterized in that a stabilizer is added when sodium sulfide is dissolved, so that the oxidation of the sodium sulfide is avoided and the system reducibility is ensured at the same time; a dispersant is added, so that a polysulfide is prevented from formed in the sodium sulfide; and a reducing agent is added into a copper suspension, so that the reducibility of the system for preparing the nickel sulfide is presented. When the amorphous nickel sulfide is prepared, the adding quantity of the sodium sulfide is controlled and the even stirring is carried out, so that the local excess sulfur source is avoided, namely, the polysulfide formed due to the local excess sulfur source is avoided; and meanwhile, the dispersant is added, so that the collision probability among the nickel sulfides is reduced, namely, the activity of nickel sulfide is maintained for a long time. According to the traditional method, after the prepared nickel sulfide is placed in the air for 24 hours, the copper removal activity of the nickel sulfide is gradually decreased; and after the prepared nickel sulfide is placed in the air for 48 hours, the copper removal activity of the nickel sulfide is decreased to 15% to 20%. According to the method provided by the invention, after the amorphous nickel sulfide is placed in the air for 7 days, the copper removal activity of the amorphous nickel sulfide can be still maintained at 80% to 90%.
Owner:JINCHUAN GROUP LIMITED +1
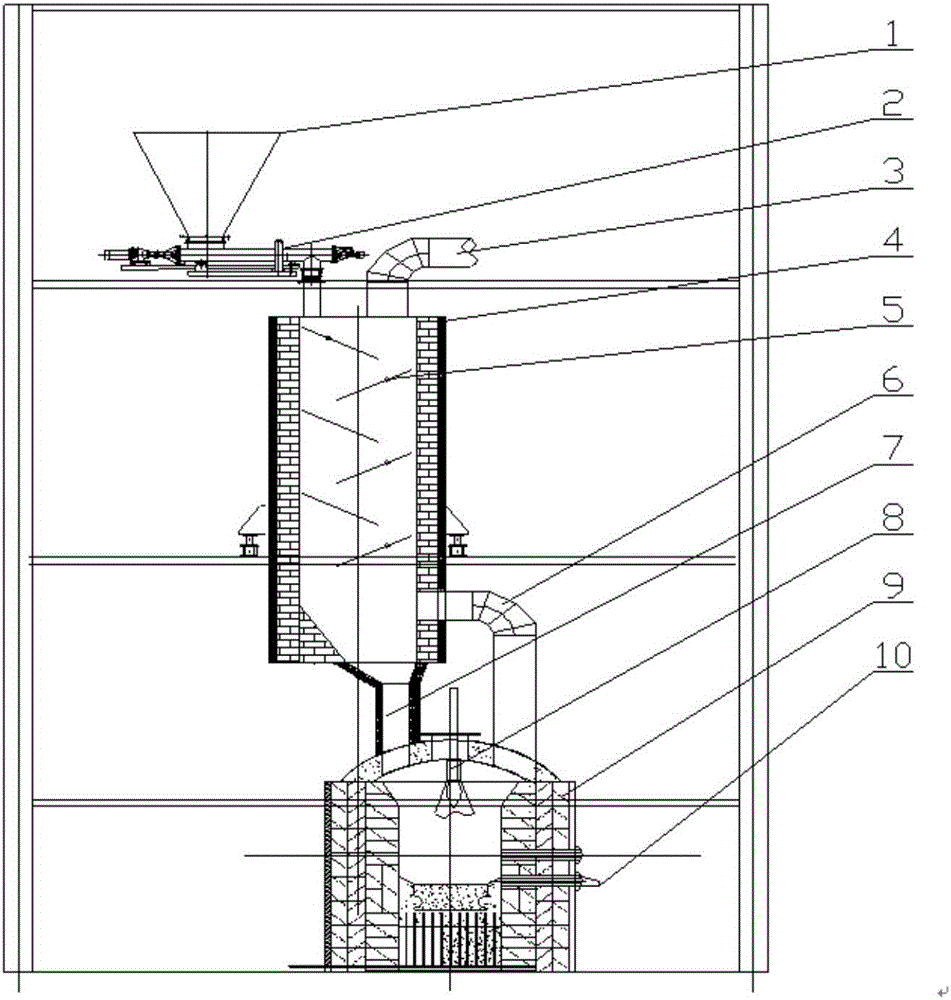
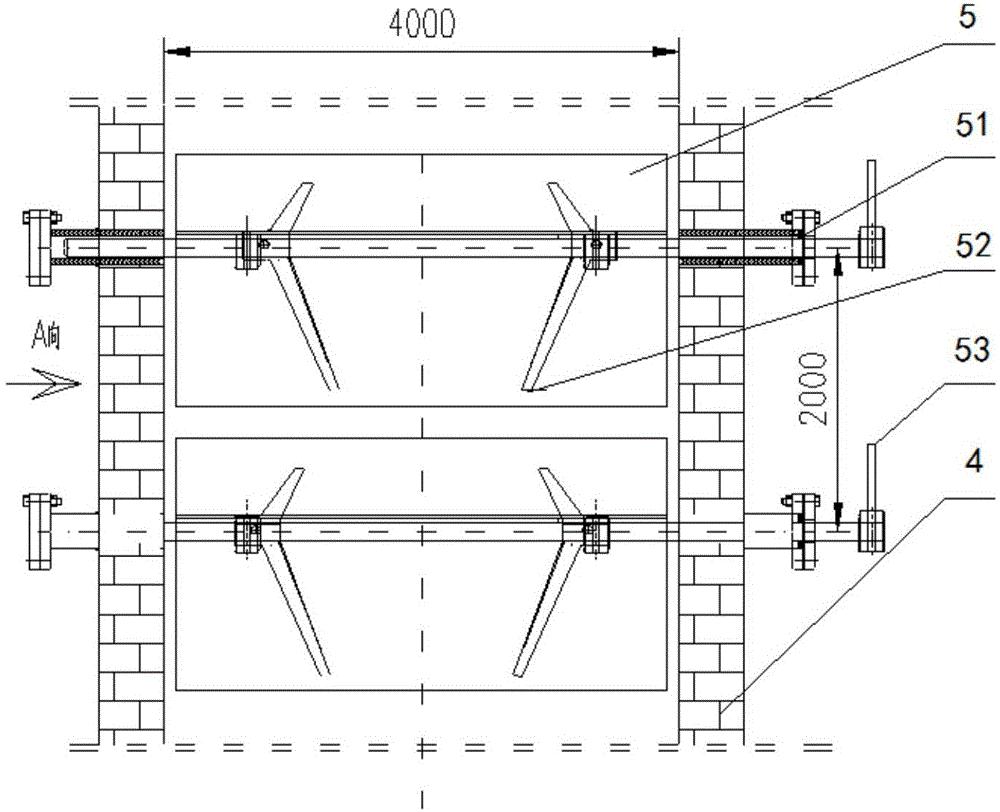
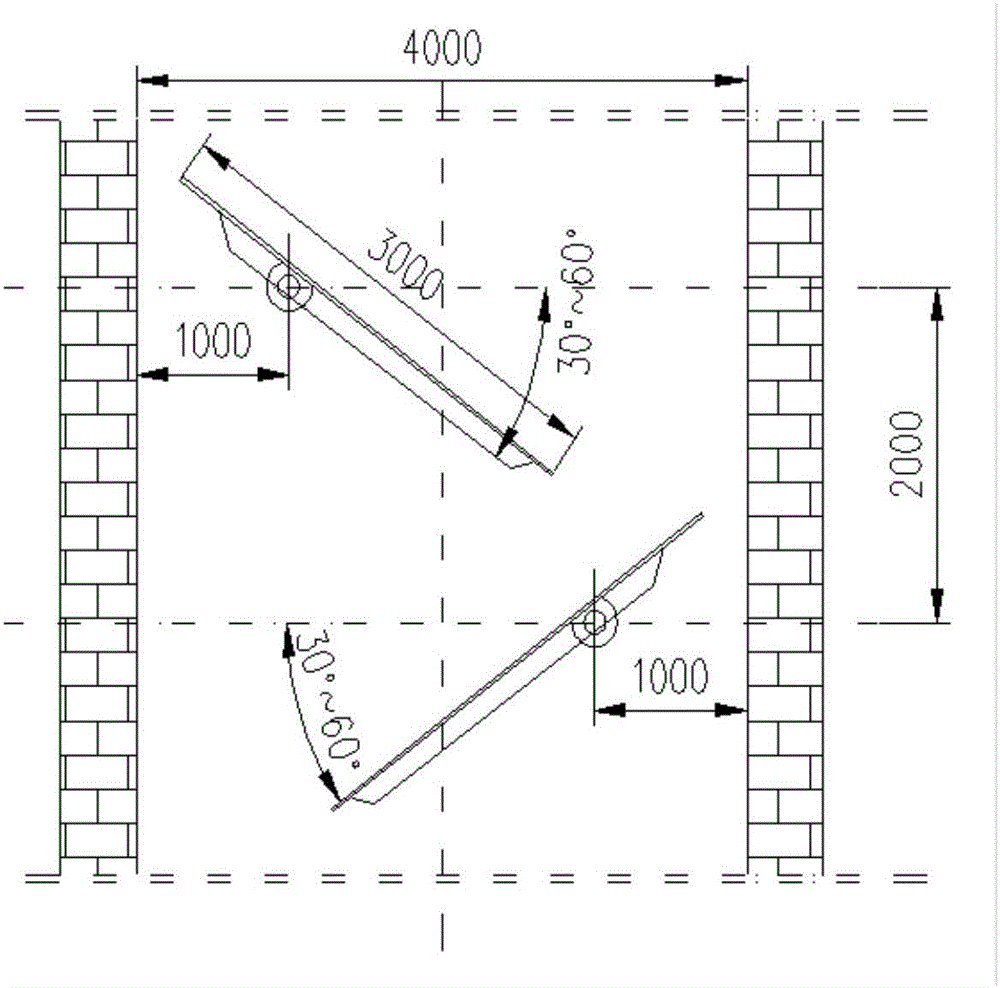
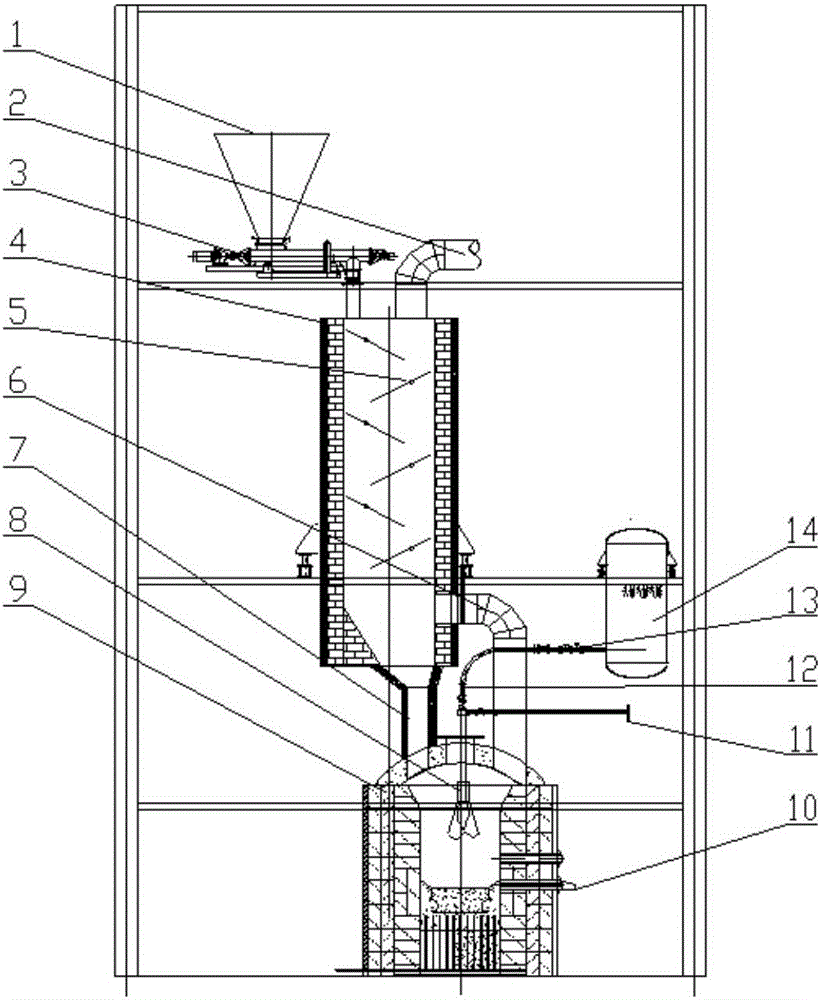
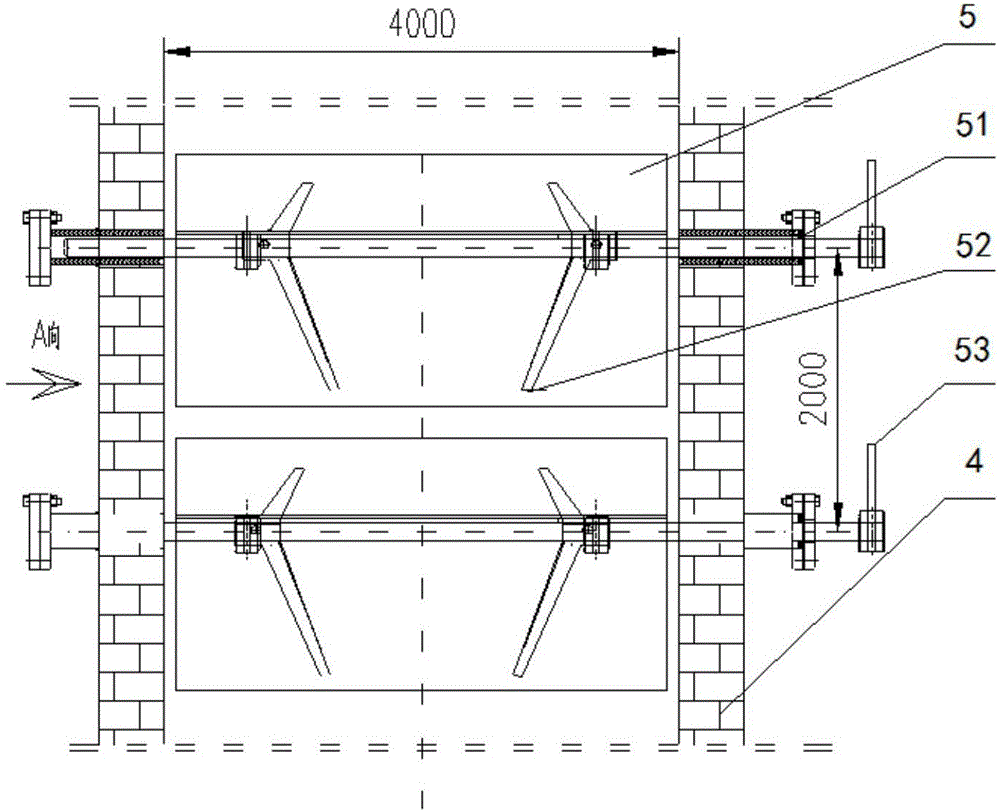
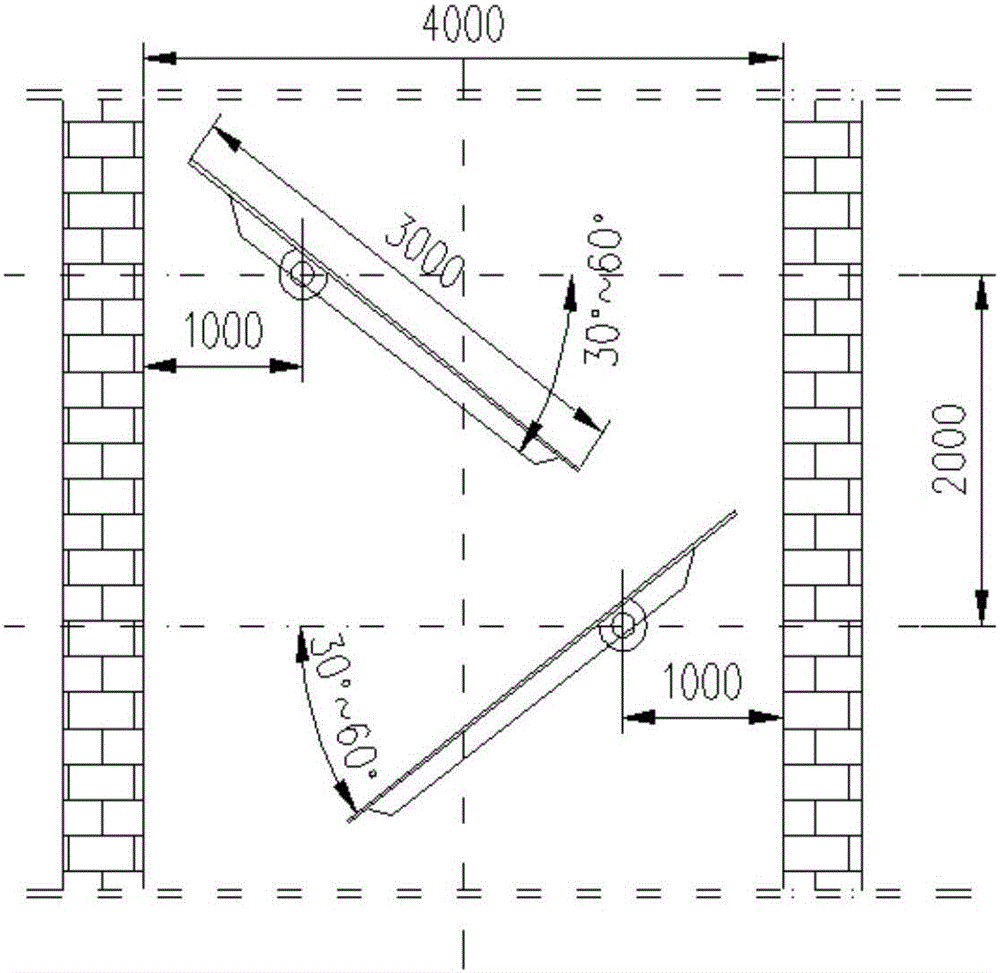
Melting and separating furnace reduction system and method for schreyerite
PendingCN106086283AMeet the needs of meltingSatisfy the conditions of high temperature reductionOxygenSchreyerite
The invention relates to a melting and separating furnace reduction system and method for schreyerite. The system comprises a reduction vertical furnace, a melting and separating furnace and an oxygen coal lance. The reduction vertical furnace comprises ore powder, a solvent feeding port, a discharging port and a smoke inlet. A baffle with the inclination angle capable of being adjusted is arranged in a hearth of the reduction vertical furnace, and the baffle and a supported rotating shaft are of a hollow structure. The melting and separating furnace comprises a feeding port, a smoke outlet and a discharging port, wherein the feeding port communicates with the discharging port of the reduction vertical furnace, and the smoke outlet communicates with the smoke inlet of the reduction vertical furnace. A spray lance head of the oxygen coal lance is arranged in the smelting and separating furnace. The oxygen coal lance stretches into the smelting and separating furnace from the furnace top of the smelting and separating furnace. According to the melting and separating furnace reduction system and method for the schreyerite, due to the fact that the baffle with the inclination angle capable of being adjusted is arranged in the reduction vertical furnace, the falling speed of and the staying time of the ore powder can be controlled, and the reduction effect of the ore powder is ensured.
Owner:SHENWU TECH GRP CO LTD
Smelting separation furnace reduction system and method based on natural gas-oxygen heating
PendingCN106148627AComplete restorationReduce usageShaft furnaceGas emission reductionFlue gasOxygen
The invention relates to a smelting separation furnace reduction system and method based on natural gas-oxygen heating. The system comprises a reduction shaft furnace, a smelting separation furnace and a natural gas-oxygen gun, wherein the reduction shaft furnace comprises a mineral powder-flux feeding port, a dropping port and a flue gas inlet; inclination-adjustable baffle plates are arranged in the hearth of the reduction shaft furnace; the smelting separation furnace comprises a feeding port, a flue gas outlet and a discharging port; the feeding port communicates with the dropping port of the reduction shaft furnace; the flue gas outlet communicates with the flue gas inlet of the reduction shaft furnace; a spray gun head of the natural gas-oxygen gun is arranged in the smelting separation furnace; and the natural gas-oxygen gun extends into the smelting separation furnace from the furnace top of the smelting separation furnace. According to the system and the method provide by the invention, the mineral powder dropping speed and residence time can be controlled through arrangement of the inclination-adjustable baffle plates in the reduction shaft furnace, thereby guaranteeing the mineral powder reduction effect.
Owner:SHENWU TECH GRP CO LTD
Reduced glutathione percutaneous absorption preparation and preparation method thereof
ActiveCN102100904BImprove bioavailabilityGuaranteed reducibilitySenses disorderMetabolism disorderHalf-lifePercutaneous absorption
The invention relates to a reduced glutathione percutaneous absorption preparation and a preparation method thereof, and belongs to the field of medicinal preparations. In the technical problem, the invention provides a reduced glutathione percutaneous absorption preparation which has the sustained-release effect. The reduced glutathione percutaneous absorption preparation is prepared from an auxiliary material bag and reduced glutathione, wherein an auxiliary material is at least one of gamma-cyclodextrin, a derivative of the gamma-cyclodextrin, alpha-cyclodextrin and a derivative of the alpha-cyclodextrin. The reduced glutathione percutaneous absorption preparation has the advantages of quick response, long half-life period and high bioavailability, is convenient to use and does not have pain. The reduced glutathione provides a new preparation, and has a wide application prospect.
Owner:CHENGDU JOY YOUNG BIOTECH CO LTD
Preparation method of narrow-hardenability pinion steel
ActiveCN102400052BImprove hardenabilityHardenability improved and stabilizedManufacturing convertersProcess efficiency improvementWear resistanceImpurity
The invention provides a narrow-hardenability pinion steel which comprises the following components in percentage by weight: 0.18%-0.22% of C, 0.20%-0.30% of Si, 0.90%-1.15% of Mn, less than or equal to 0.030% of P, less than or equal to 0.030% of S, 1.00%-1.35% of Cr, 0.04%-0.08% of Ti, 0.004%-0.005% of B, 0.025%-0.038% of W, less than or equal to 11.5*10<-6> of [O], less than or equal to 1.5*10<-6> of [H], 35*10<-6>-45*10<-6> of [N] and the balance of Fe and inevitable impurities. The invention provides a preparation method of the narrow-hardenability pinion steel. The preparation method comprises the following steps: 1) primary smelting based on a top-bottom blowing converter; 2) ladle deoxidizing and alloying; 3) LF (ladle furnace) refining; 4) RH refining; and 5) continuous casting. Compared with the prior art, the narrow-hardenability pinion steel provided by the invention has the characteristics of better wear resistance and high temperature resistance, higher hardenability andnarrower jominy hardenability.
Owner:WANGUAN SMELTING MOLD CASTING
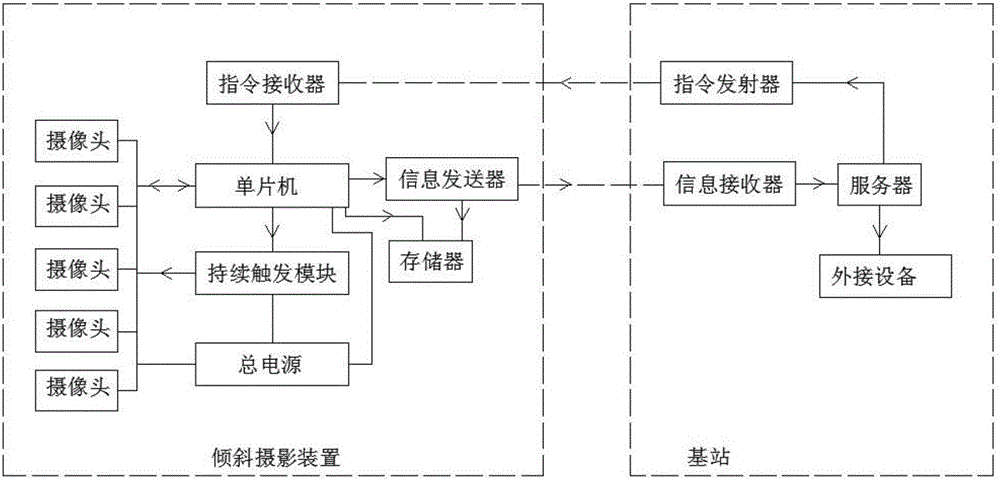
Intelligent work controlling oblique shooting system
InactiveCN105898142AWide camera angleImprove camera efficiencyTelevision system detailsPicture taking arrangementsData synchronizationEngineering
The invention relates to an intelligent work controlling oblique shooting system. The intelligent work controlling oblique shooting system includes an oblique shooting device and a base station, wherein the oblique shooting device includes a command receiver, a singlechip, an information sender, a memory and a plurality of cameras; an input end of the command receiver is in wireless connection with a command emitter of the base station; an output end of the command receiver is connected with an input end of the singlechip; an output end of the singlechip is connected with an input end of the memory; an output end of the memory is connected with an input end of the information sender; the information sender is in wireless connection with an information receiver of the base station; a transmitting-receiving end of the singlechip is connected with the plurality of cameras in parallel; the plurality of cameras perform synchronous shooting; and the data acquired by the plurality of cameras are transmitted synchronously. For the intelligent work controlling oblique shooting system, as the plurality of cameras work synchronously, the synchronism of shot frames can be guaranteed so that the restoration effect of frames can be guaranteed; and as the stability of a vidicon is high, the cameras are not required to rotate to find a view during the shooting process, the practicality is high and the quality of the shot frames is high.
Owner:天津全华时代航天科技发展有限公司
Anti-crystallization device for carbamide nozzle of selective catalytic reduction (SCR) system
ActiveCN105041432AReduced risk of crystal pluggingPromote pyrolysis reactionExhaust apparatusSilencing apparatusSolenoid valveHeating effect
Disclosed is an anti-crystallization device for a carbamide nozzle of a selective catalytic reduction (SCR) system. The anti-crystallization device comprises a solenoid valve, a vortex tube assembly and a spray head assembly. A gas inlet of the vortex tube assembly is provided with the solenoid valve. One end of the vortex tube assembly is connected with the spray head assembly through a cool gas pipeline, and the other end of the vortex tube assembly is connected with the spray head assembly through a hot gas pipeline. The spray head assembly is further provided with a carbamide inlet. The tail end of the spray head assembly is provided with a carbamide nozzle orifice, a hot gas nozzle orifice and a cool gas nozzle orifice. The carbamide nozzle orifice is nested in the cool gas nozzle orifice. The cool gas nozzle orifice is nested in the hot gas nozzle orifice. Compressed gas can be transformed into cool gas flow and hot gas flow. The cool gas flow can be used for cooling components in the spray head assembly. The hot gas flow can be used for heating carbamide. The cool gas flow and the hot gas flow finally produce gas curtains through the nozzle orifices and the gas curtains isolate carbamide mist and high-temperature tail gas at the nozzle, and carbamide crystallization produced at the nozzle is avoided to the greatest extent. At the same time, cooling and heating effects are achieved in different positions simply and reliably; the production and maintenance cost of the system is low.
Owner:山东艾泰克环保科技股份有限公司
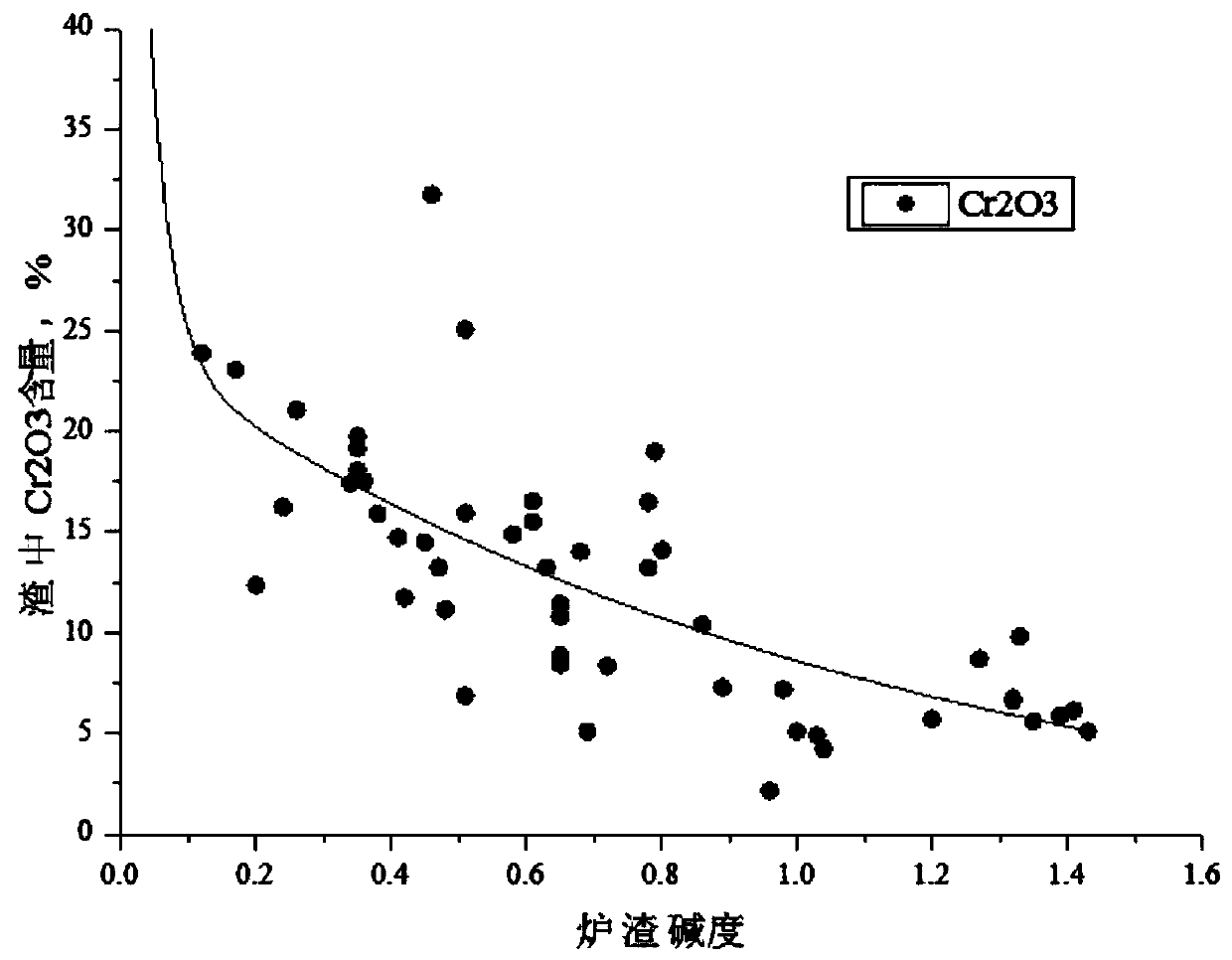
Electric furnace for producing Cr13 series stainless steel and smelting control method for refining furnace
ActiveCN109897938AReduce control difficultyReduce the probability of splash accidentsElectric furnaceProcess efficiency improvementChromiumCr element
The invention provides an electric furnace for producing Cr13 series stainless steel and a smelting control method for a refining furnace, wherein the process flow of the control method is as follows:raw material preparation - electric furnace trough type tapping - VOD blowing process - refining furnace process - molding process. The invention has the following beneficial effect: the lectric furnace for producing the Cr13 series stainless steel and the smelting control method for the refining furnace provided by the invention have the following advantages: the Cr element metal yield of the prepared Cr13 series stainless steel is greater than or equal to 95%, and compared with the prior art, the smelting time is shortened by more than 30min ; the smelting cost of Cr13 series stainless steel is greatly reduced, avoids genearating aluminum-chromium spinel inclusions in molten steel, has a significant improvement on the purity of molten steel, and can meet the increasingly quality requirement of stainless steel products in the downstream industries of steel enterprises.
Owner:XINING SPECIAL STEEL
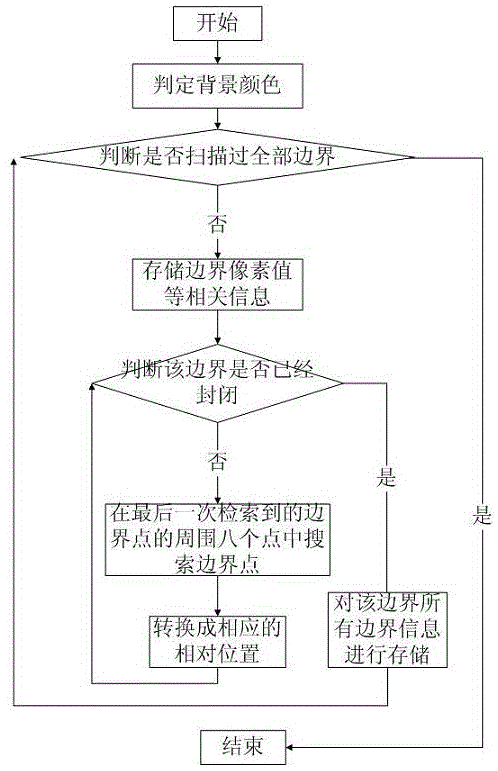
Medical image lossless compression method based on boundary extraction
ActiveCN105069821AIncrease the compression ratioGuaranteed reducibilityImage analysisImage codingComputer imageLossless compression
The invention relates to a medical image lossless compression method based on boundary extraction, which belongs to the field of computer image processing. The medical image lossless compression method based on boundary extraction comprises the steps of extracting all boundaries exist in a medical image at first, and storing relative positions of boundary points by adopting a bit operation manner. The medical image lossless compression method has the advantages that a compression ratio of the image is increased aiming at features that the medical image is a grey-scale map and contour hierarchy is clear, and each step of the method is reversible, thereby ensuring recoverability of compression. The medical image lossless compression method based on boundary extraction can alleviate pressure on storage as well as pressure on network caused by increasing number of medical images fundamentally.
Owner:JILIN UNIV
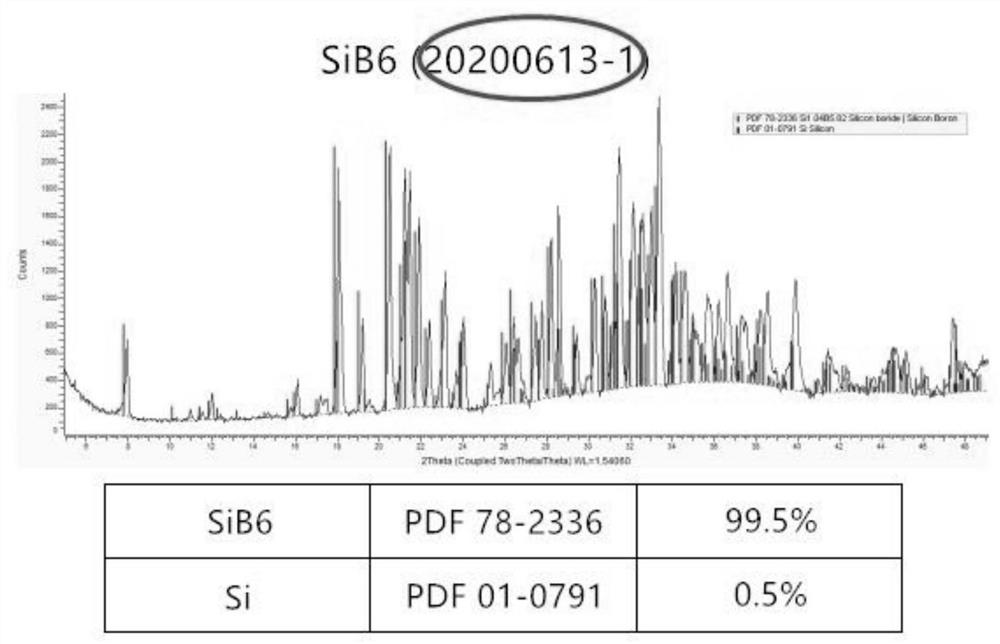
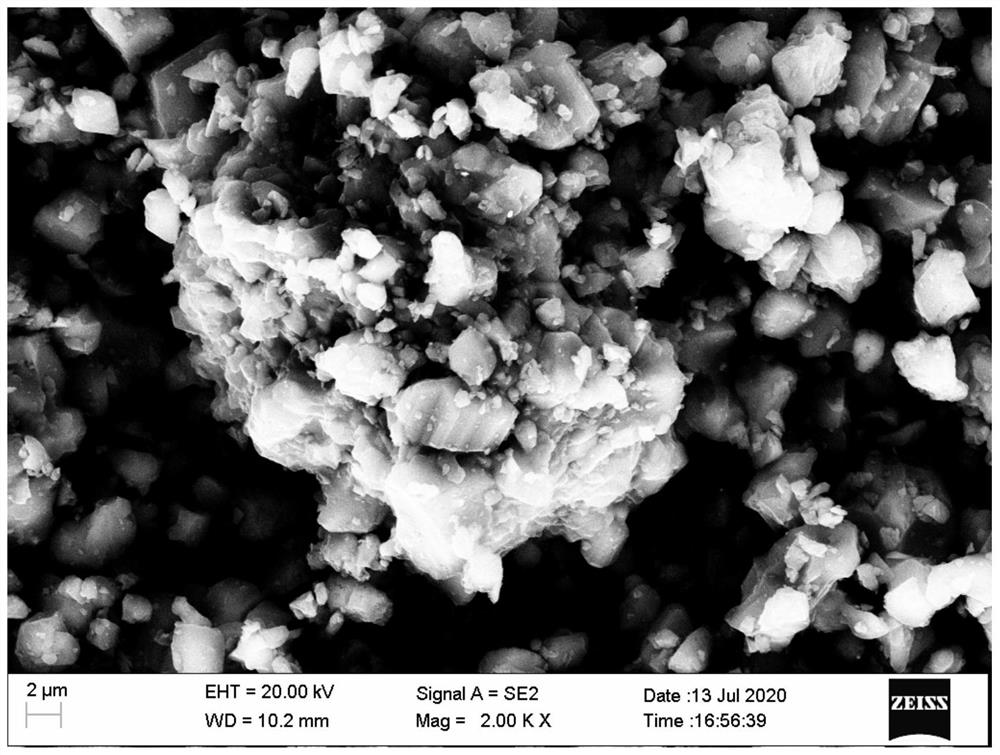
Low-cost high-purity silicon hexaboride production process
The invention relates to a low-cost high-purity silicon hexaboride production process, which comprises the following steps: pressing diboron trioxide and potassium borohydride into blocks after ball-milling and mixing under the protection of argon, putting the blocks into a vacuum carbon tube furnace, performing vacuumizing, and keeping the temperature at 750DEG C for 5 hours; conducting heating to 1250DEG C, continuously keeping the temperature until the pressure in the furnace is slightly positive and the reduction reaction is completely finished, putting the obtained monomer boron and potassium hydroxide mixture in a graphite crucible into distilled water, and conducting heating, cleaning and drying to obtain boron powder; conducting ball-milling and mixing on the boron powder and silicon powder, putting the mixture into a self-propagating combustion reaction kettle, vacuumizing the reaction kettle to a vacuum degree of 1Pa, powering on a heating tungsten wire, igniting zirconium powder, carrying out high-temperature self-propagating combustion combination reaction, and after the reaction is finished, performing cooling and removing impurities to obtain the high-purity silicon hexaboride. Diboron trioxide and potassium borohydride are used as the raw materials, the cost of the raw materials is relatively low, the whole process is reasonable and controllable, the purity of the produced silicon hexaboride is 99.5%, and the method is suitable for industrial production.
Owner:辽宁中色新材科技有限公司
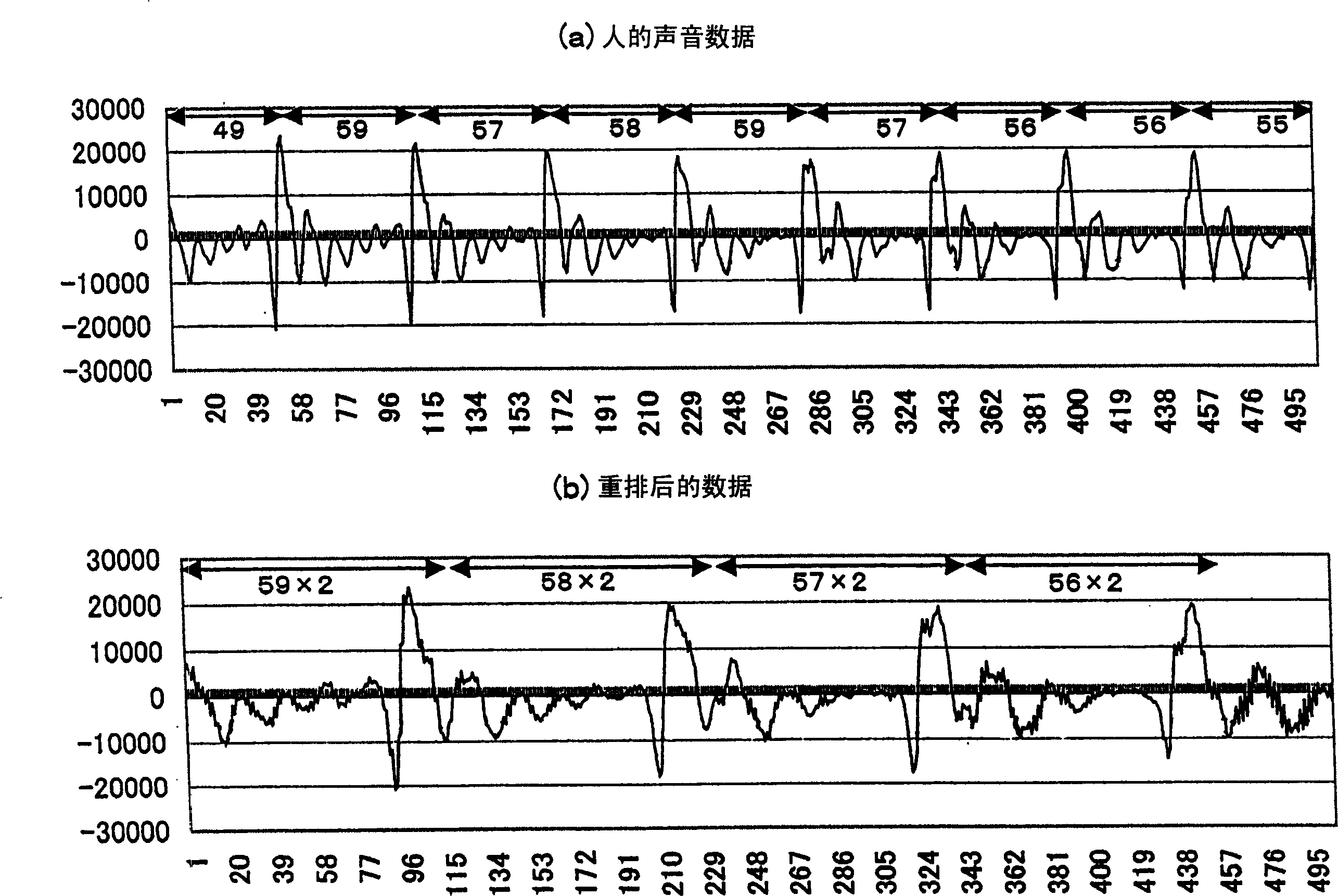
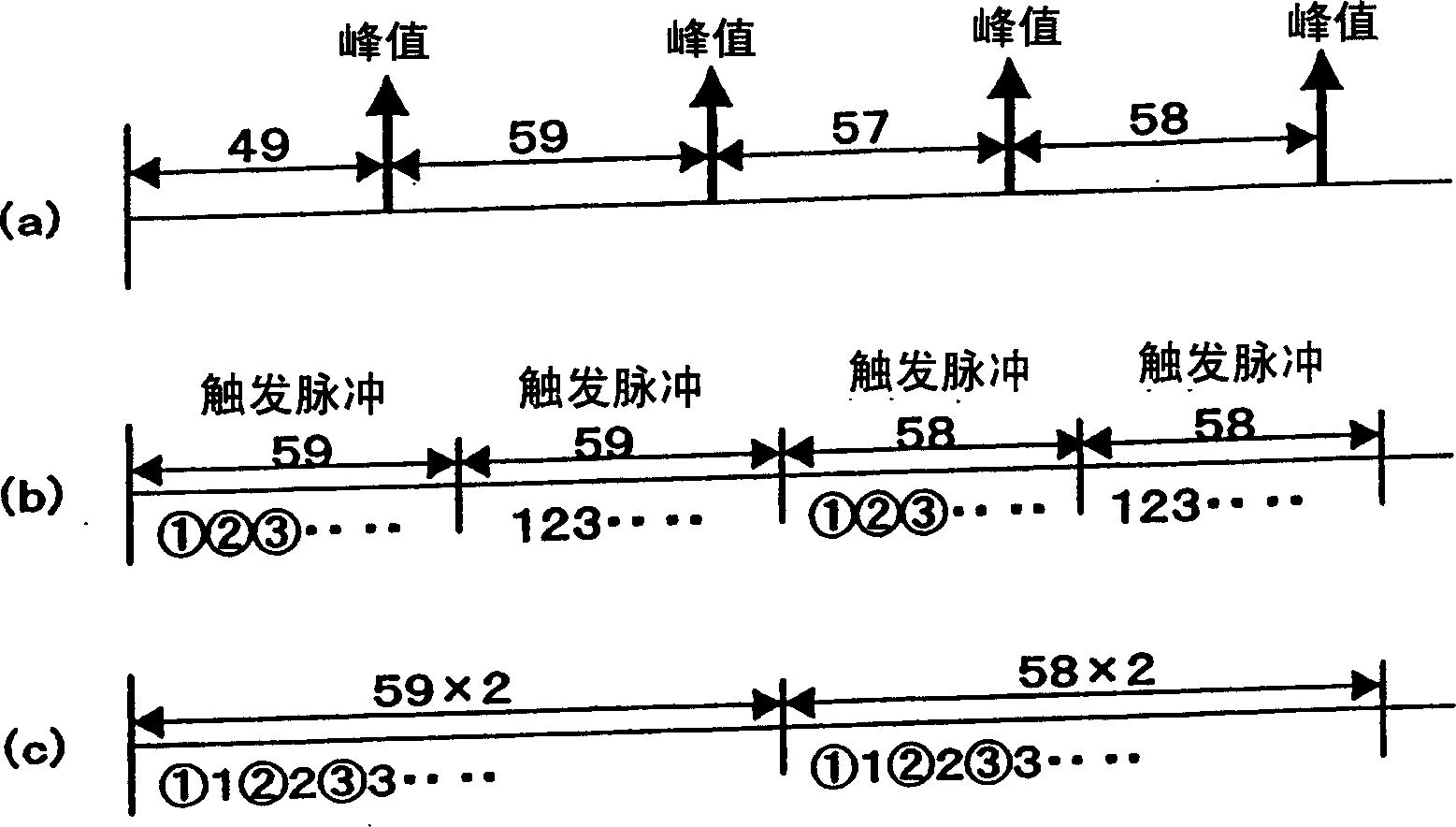
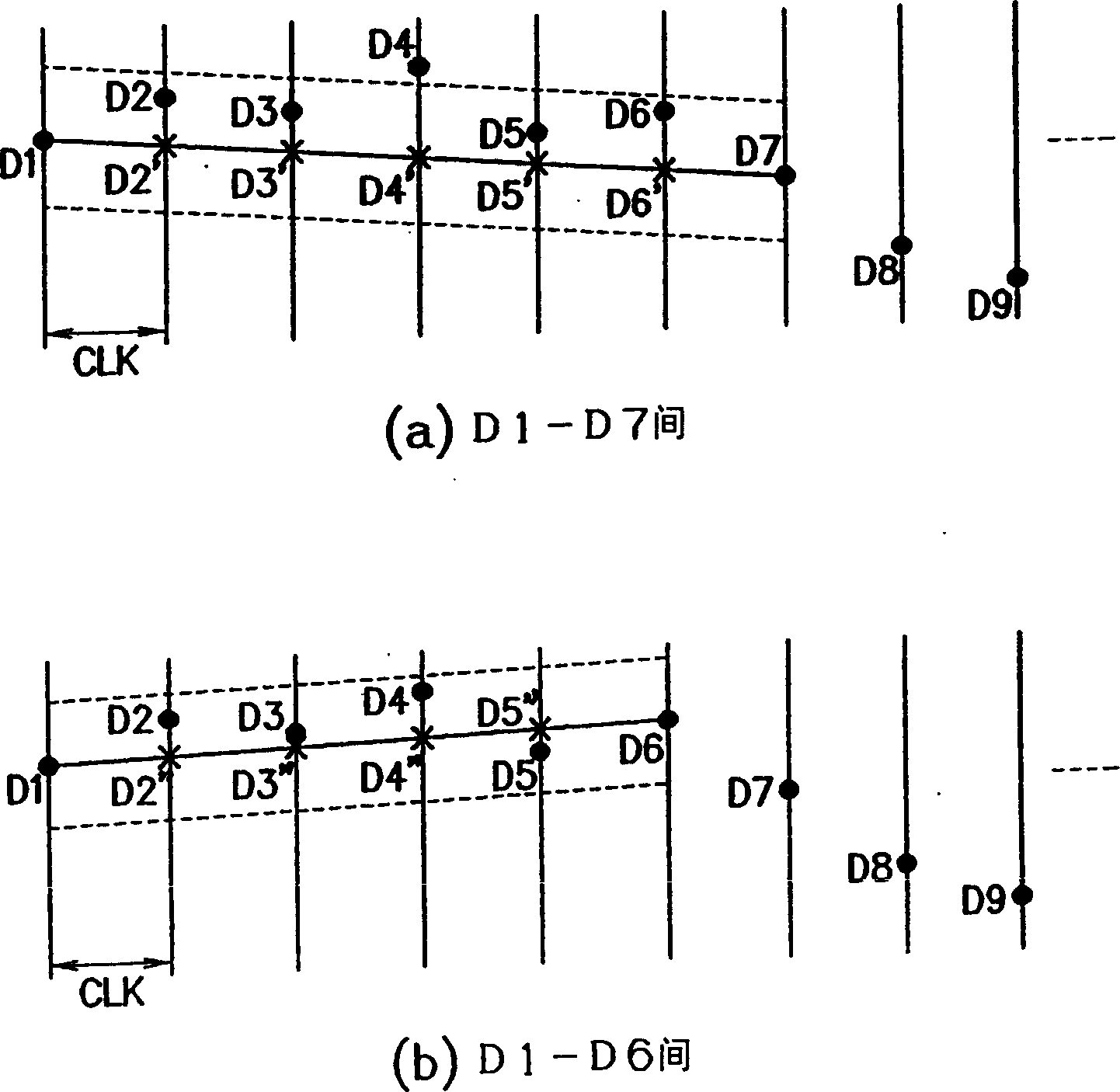
Compression method and apparatus, decompression method and apparatus, compression/decompression system, peak detection method, program and recording medium
InactiveCN1461466AQuality improvementImprove compression ratioSpeech analysisCode conversionHigh frequencyCompression ratio
For data to be compressed and having periodicity, two windows of identical size are set for two intervals in accordance with peaks appearing almost periodically and sample data is alternately rearranged between the windows of the identical size, thereby replacing the frequency of data having periodicity with approximately a half frequency without deteriorating reproducibility into original data, so that the replaced data of a low frequency is subjected to compression. This rearrangement processing may be applied to a compression having a characteristic that the compression ratio cannot be increased in a high frequency region, so as to increase the compression ratio without deteriorating the quality of the reproduced data obtained by decompression.
Owner:酒井康江
Melt separation furnace reducing system for blowing oxygen and method
The invention relates to a melt separation furnace reducing system for blowing oxygen and a method. The system comprises a reducing shaft furnace, a melt separation furnace and an oxygen gun. The reducing vertical furnace comprises a mineral powder and solvent feeding opening, a pulverized coal feeding opening, a discharging opening and a smoke inlet. A baffle with the adjustable dip angle is arranged in a hearth of the reducing vertical furnace, and the baffle and a supported rotary shaft are of hollow structures. The melt separation furnace comprises a feeding opening, a smoke outlet and a discharging opening, the feeding opening communicates with the discharging opening of the reducing vertical furnace, and the smoke outlet communicates with the smoke inlet of the reducing vertical furnace. A spray gun head of the oxygen gun is arranged in the melt separation furnace, and the oxygen gun stretches into the melt separation furnace from the top of the melt separation furnace. According to the melt separation furnace reducing system for blowing the oxygen and the method, the baffle with the adjustable dip angle is arranged in the reducing vertical furnace to control the mineral powder descending speed and staying time, and the reducing effect of mineral powder is guaranteed.
Owner:SHENWU TECH GRP CO LTD
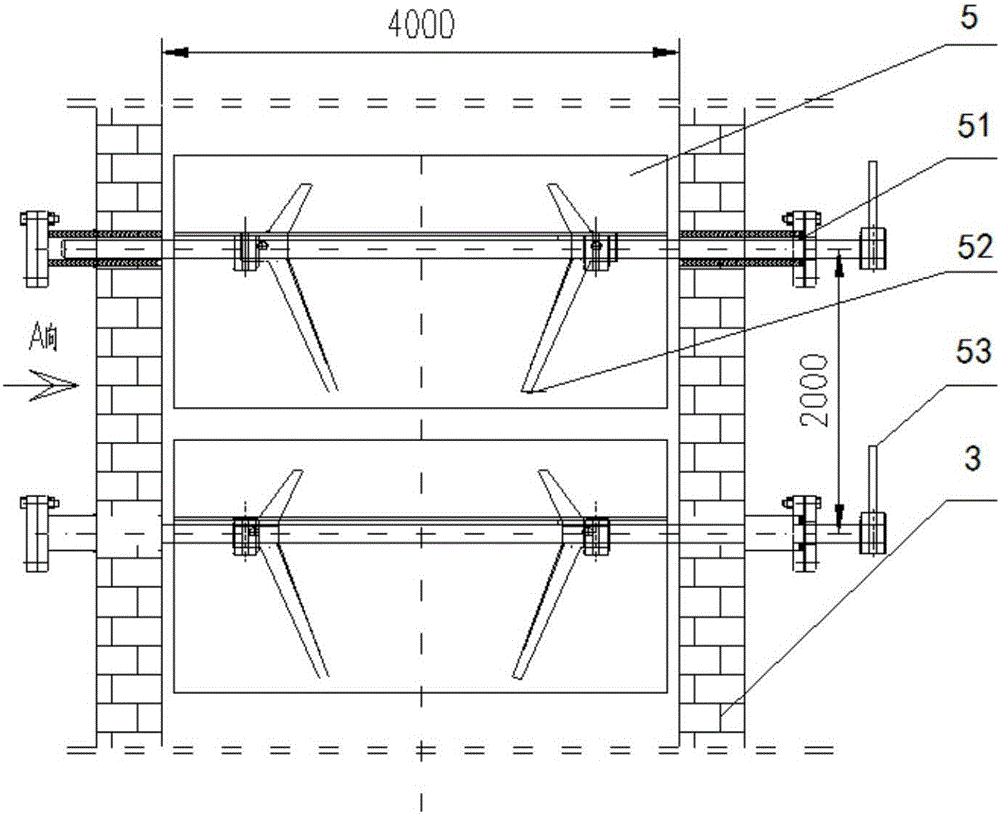
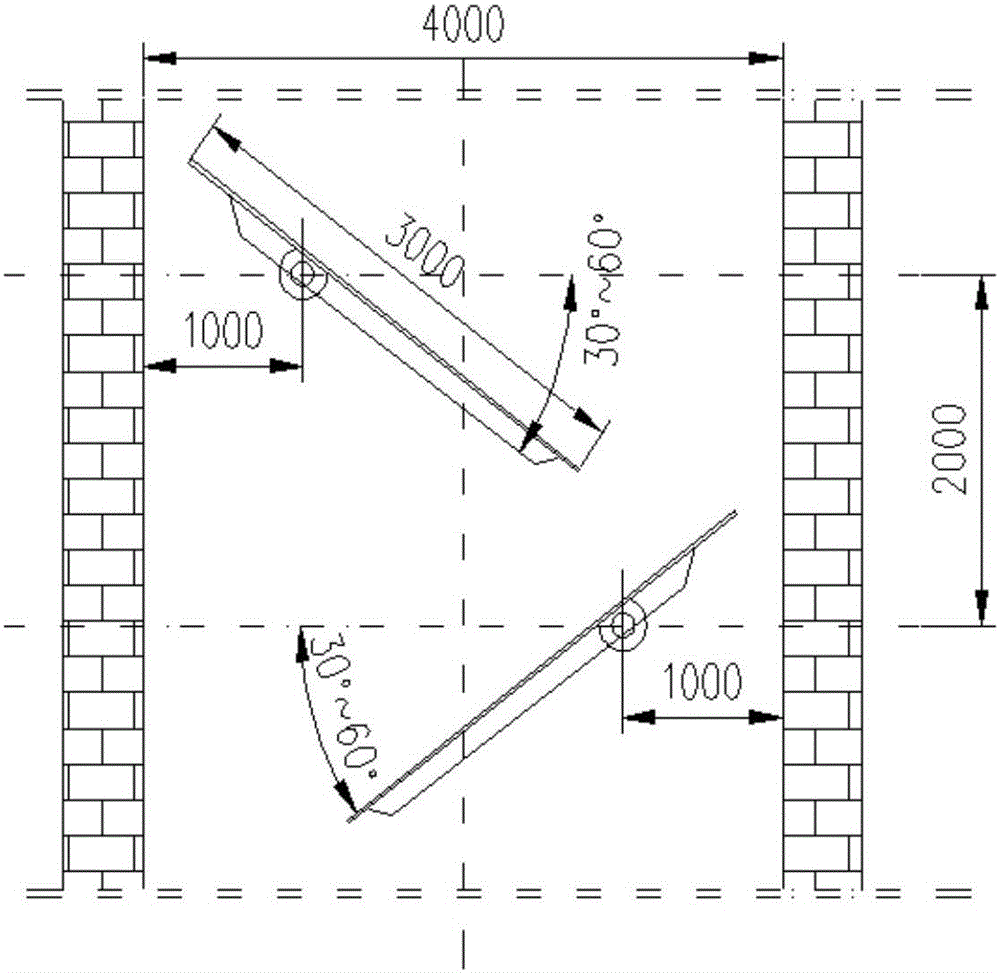
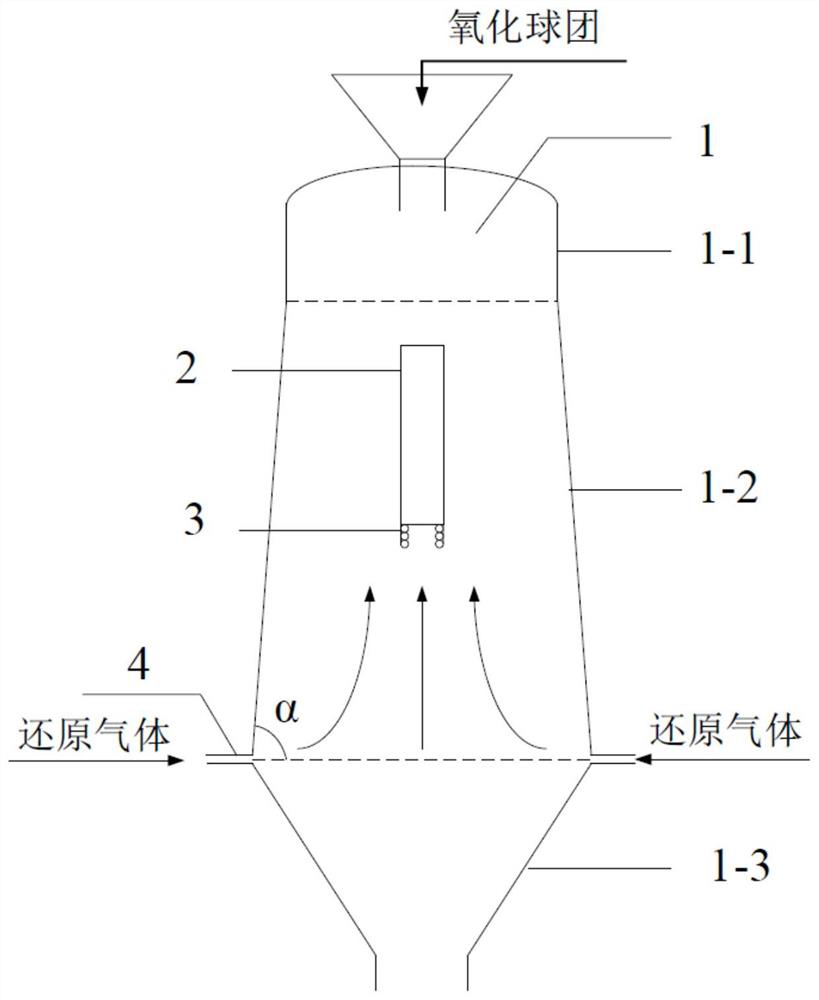
Gas-based reduction shaft furnace
ActiveCN112143846AReduce cakingReduce extrusion pressureProcess efficiency improvementShaft furnaceThermodynamicsEngineering
The invention belongs to the technical field of gas-based direct reduction iron-making, and particularly discloses a gas-based reduction shaft furnace. A shaft furnace body comprises a preheating section, a reduction section and a cooling section, wherein the reduction section is in a big-end-down circular truncated cone shape; the bottom of the reduction section is connected with the cooling section; the upper portion of the reduction section is connected with the preheating section; and an air guide wall is arranged in the reduction section and coincides with the axis of the reduction section. By changing the angle between the reduction section furnace body and the horizontal plane, the furnace profile adapts to volume expansion during pellet reduction, extrusion force between pellets and a furnace wall as well as between the pellets can be reduced, and the situation that liquid generated by the pellets with high temperature is consecutive and bonded into blocks is effectively restrained; the air permeability in the shaft furnace is enhanced, so that the shaft furnace runs smoothly; and the air guide wall is arranged in the reduction section, so that reduction air flow can be uniformly distributed in the shaft furnace.
Owner:XI'AN UNIVERSITY OF ARCHITECTURE AND TECHNOLOGY
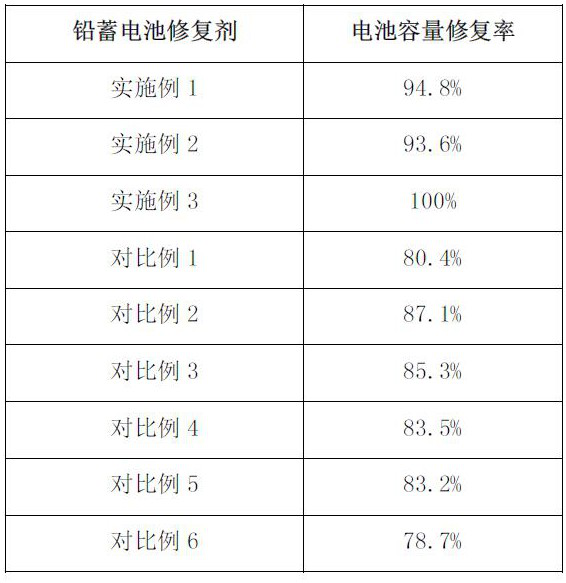
Lead storage battery repairing agent and repairing method
ActiveCN112259812AAvoid decompositionReduce churnLead-acid accumulatorsElectrolyte/reactants regenerationGellan gumSulfate
The invention belongs to the technical field of lead storage batteries, and particularly relates to a lead storage battery repairing agent and a repairing method. The lead storage battery repairing agent is prepared from the following raw materials in parts by weight: 10-15 parts of ammonium acetate, 0.1-3 part of graphene, 2-5 parts of mercaptoethanol, 1-10 parts of ferrous sulfate, 3-8 parts ofethylenediamine tetraacetic acid disodium salt, 0.01-0.05 part of gellan gum, 1-5 parts of copper sulfate and 20-45 parts of sulfuric acid solution. The lead storage battery repairing agent can significantly improve the battery capacity.
Owner:天长市易诚电子科技有限公司
Nitrogen-doped mesoporous carbon supported platinum catalyst as well as preparation method and application thereof
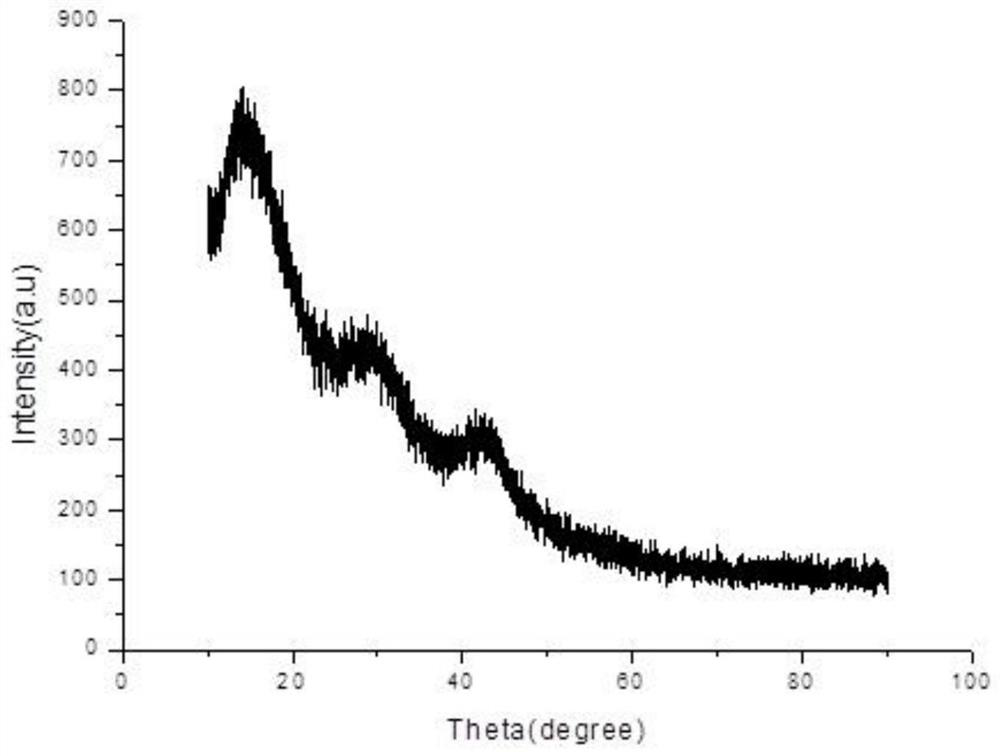
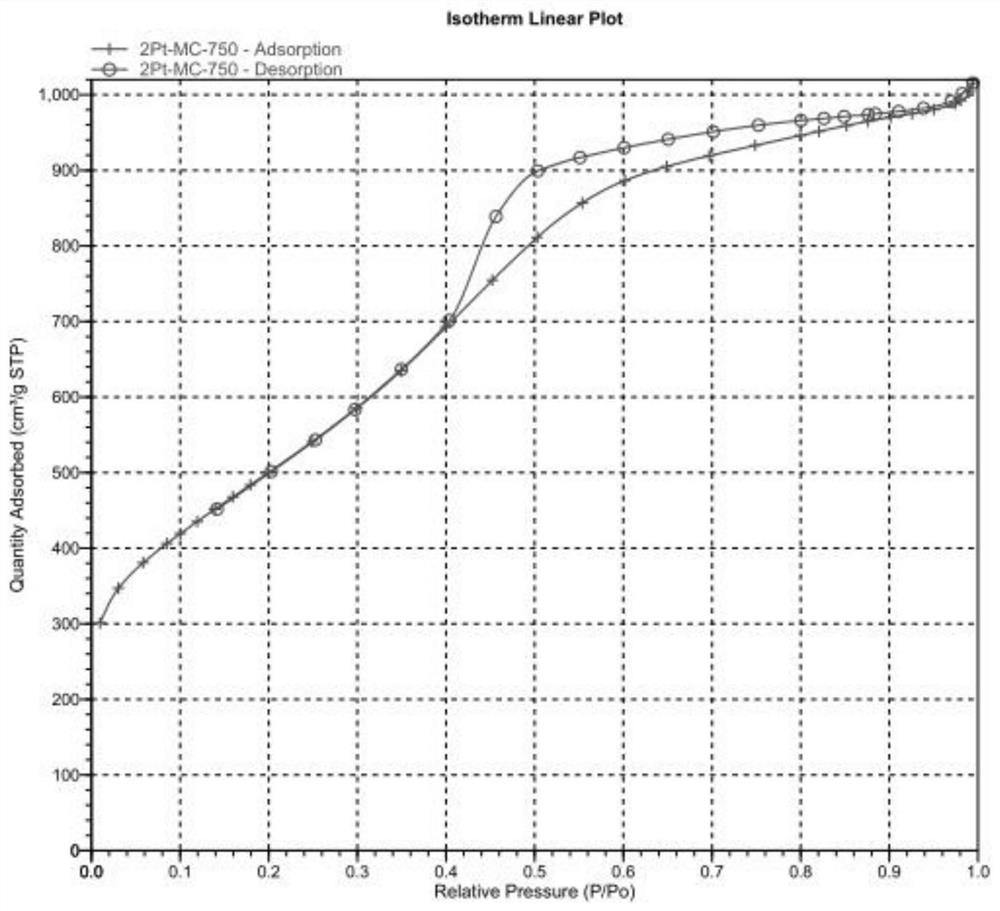
InactiveCN113509948AGuaranteed reducibilityLarge specific surface areaPhysical/chemical process catalystsOrganic compound preparationNitro compoundPtru catalyst
The invention belongs to the technical field of catalysts, and particularly relates to a nitrogen-doped mesoporous carbon supported platinum catalyst as well as a preparation method and application thereof. The preparation method of the nitrogen-doped mesoporous carbon loaded platinum catalyst provided by the invention comprises the following steps of: mixing a magnesium source solution with a nitrogen source, and sequentially carrying out evaporation and foaming treatment to obtain a foaming body; sequentially crushing and calcining the foaming body to obtain a calcined material; sequentially acid-pickling, washing and drying the calcined material to obtain a nitrogen-doped porous carbon material; and sequentially carrying out Pt loading and reduction treatment on the nitrogen-doped porous carbon material to obtain the nitrogen-doped mesoporous carbon loaded platinum catalyst. Experimental data show that the nitrogen-doped mesoporous carbon supported platinum catalyst provided by the invention has a mesoporous structure, and when the used in a nitro compound hydrogenation reaction, the nitrogen-doped mesoporous carbon supported platinum catalyst is high in catalytic activity, high in chemical selectivity as high as 99.9%, high in catalytic selectivity and good in catalytic effect.
Owner:SHANGHAI UNIV
De-oxidation modifying agent for steel ladle top slag
ActiveCN103374642BImprove slag removal effectThe modifier has good reducibility and continuous stabilityProcess efficiency improvementLadle furnace slagSlag
The invention relates to steel ladle furnace slag modifying agent in a converter steel tapping process. The de-oxidation modifying agent for the steel ladle top slag comprises the following main components in percentage by weight: 30%-50% of Al, 50%-30% of CaO, 5.0%-8.0% of Al2O3, not more than 3.0% of SiO2, not more than 0.07% of S+P, 0.7%-1.4% of C, 3.0%-8.0% of CaFe2 and not more than 0.2% of H2O. The modifying agent disclosed by the invention is a reductive modifying agent which can be used for solving an oxidation problem of the steel ladle top slag in a low-carbon pipeline steel production process, so that guarantee is provided for vacuum desulfurization and pure production of the pipeline steel.
Owner:BAOSHAN IRON & STEEL CO LTD
Surface printing technology for multi-edge-angle polygonal flexible hose
ActiveCN103587266AGuaranteed reducibilityEnsure adhesion fastnessOther printing apparatusContact pressurePrinting press
The invention discloses a surface printing technology for a multi-edge-angle polygonal flexible hose. The surface printing technology includes the following steps that (1), a relief printing plate is manufactured and fixed to a printing plate roller of a printing machine through double faced adhesive tape, and rubber blanket and a gasket are fixed to an impression cylinder of the printing machine at the same time; (2), the multi-edge-angle polygonal flexible hose is arranged on a printing core rod in a sleeved mode, and the diameter of the printing core rod is slightly larger than that of the flexible hose, so that the multi-edge-angle polygonal flexible hose is bulged to be a round flexible hose; (3), the machine is started to perform printing, the round flexible hose arranged on the core rod in the sleeved mode is then sent to the position between the printing plate roller and the impression cylinder in the step (1), contact pressure between the printing plate roller and the impression cylinder is adjusted, and therefore it is ensured that oil ink is evenly transferred to the surface of a flexible hose body in a covering mode in the process of printing. The printing technology ensures that the oil ink is evenly transferred to the surface of the multi-edge multi-side hose body in the covering mode.
Owner:上海雅思达亮亮包装材料科技有限公司
Micro-concave roller pressing formation device
The invention discloses a micro-concave roller pressing formation device which comprises a framework base. A pneumatic tool rest mechanism and a floating support mechanism are arranged on the framework base, the pneumatic tool rest mechanism comprises a tool rest platform with lifting adjusting displacement, a horizontal adjusting seat is arranged on the tool rest platform, a driving cylinder is arranged on the horizontal adjusting seat, a telescopic end of the driving cylinder is provided with a tool rest frame, a support end of the floating support mechanism is provided with horizontal self-rotation displacement and vertical floating displacement, and the tool rest frame is opposite to the support end. Pressure is accurately controlled by a pneumatic mode, a balancing weight is omitted,the device is provided with a stepless regulation section, precision adjustment is realized, product reducibility can be ensured, and manpower cost is reduced. By the design of the floating support mechanism, abnormal stress of a roller can be eliminated, large bounce and deformation of the roller are prevented, pressing stability and pressing precision are improved, and product qualification ratio is greatly increased. Overall design is compact, reliable and easy to implement, and market requirements are met.
Owner:昆山新合宇制辊有限公司
Temperature control device for forging alloy steel
PendingCN106843324AGuaranteed reducibilityHigh yieldAuxillary controllers with auxillary heating devicesForge furnacesTemperature controlHydrogen
The invention discloses a temperature control device for alloy steel forging, which comprises a base and a console. The top of the base is rotatably connected with a conveying roller, and the top of the base is fixedly connected with a heating furnace through bolts. The heating furnace One side of the heating furnace is hinged with a feed door, and the other side of the heating furnace is hinged with a discharge door. The heating furnace is provided with a heating section, a heat preservation section and an overaging section. The middle part of the front of the heating furnace is fixed with a pressure Sensors and temperature sensors, oxygen pipelines, gas pipelines, nitrogen-hydrogen gas pipelines and waste gas pipelines are connected above the heating furnace, heaters are installed inside the heating furnace, so that the temperature of each section in the heating furnace can be effectively detected, Furthermore, the temperature in the furnace is adjusted by controlling the opening of the oxygen control valve and the gas control valve, so that the temperature is controllable, the production efficiency is effectively improved, and the production cost is saved. This structure is easy to operate and has high operability, while saving production cost and improving production efficiency.
Owner:江苏瑞格高合金材料有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com