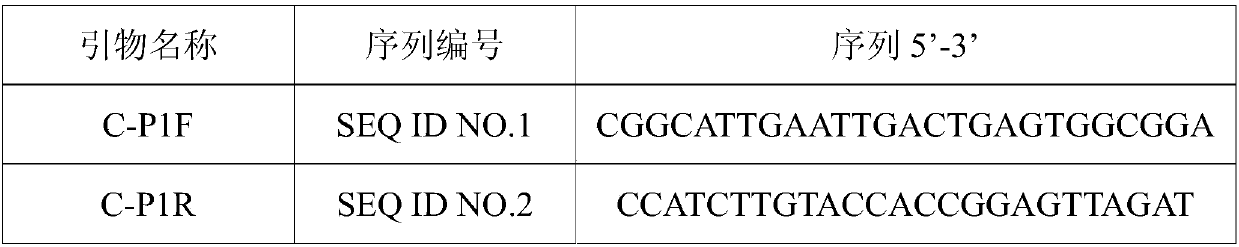
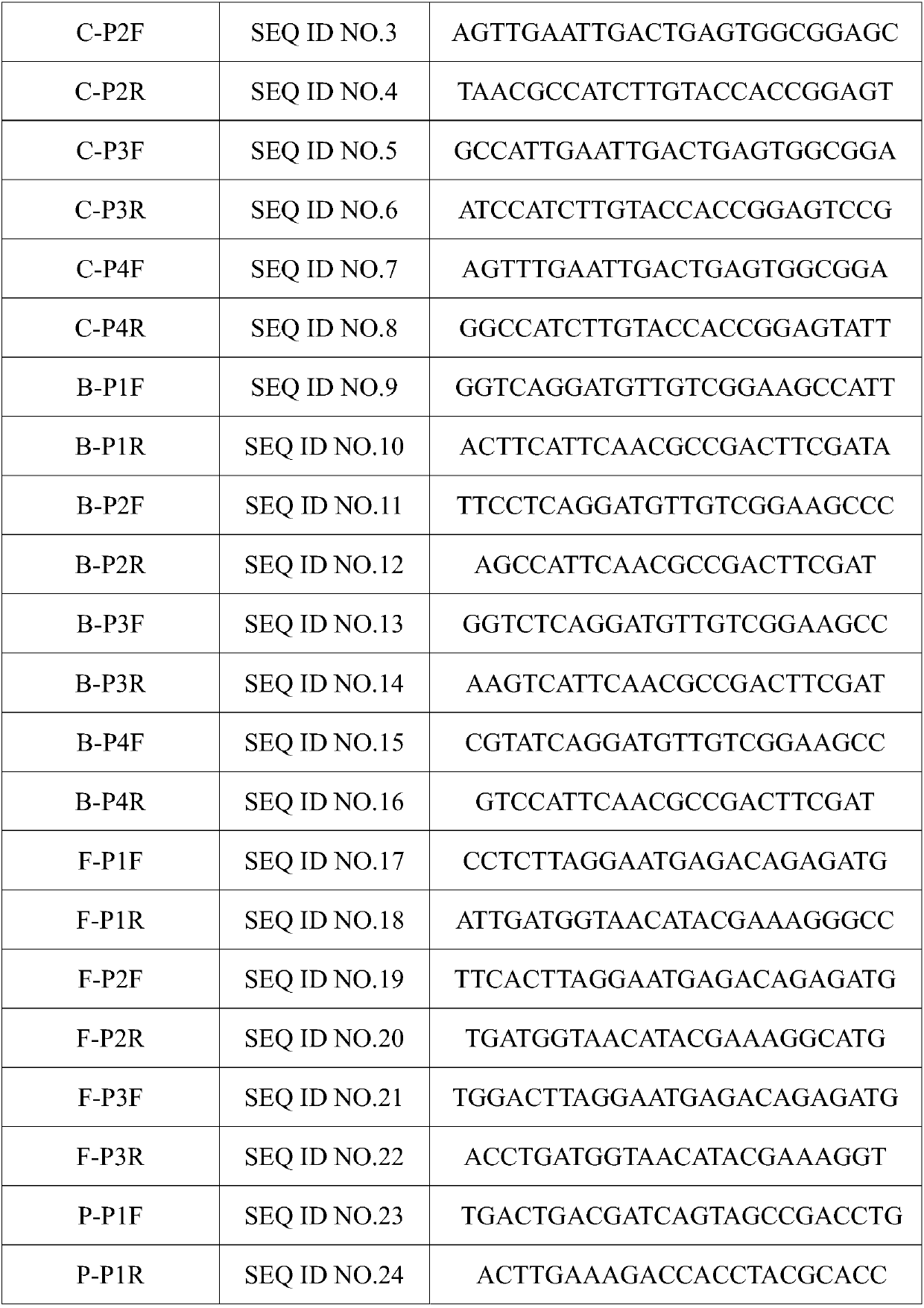
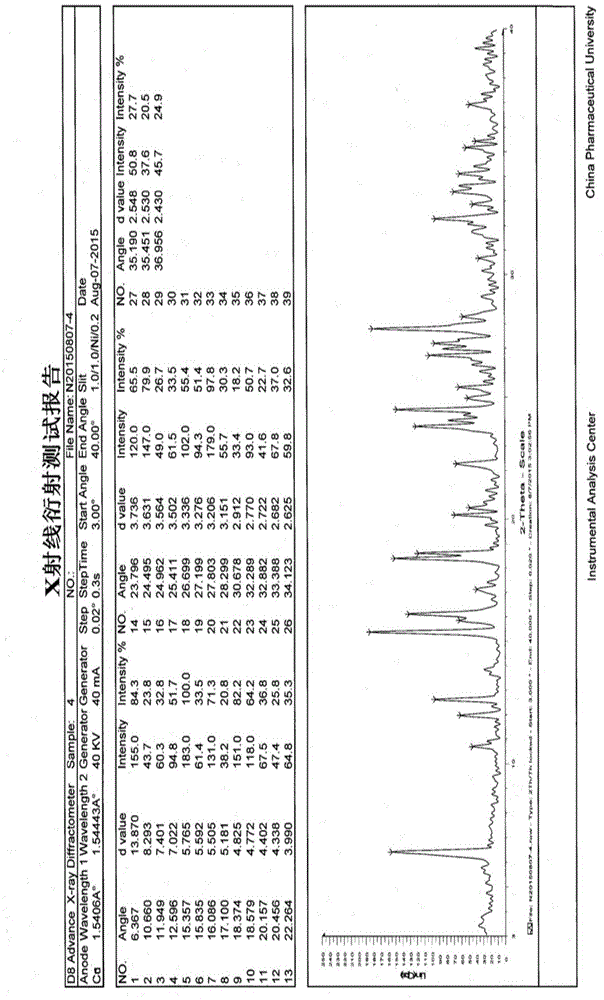
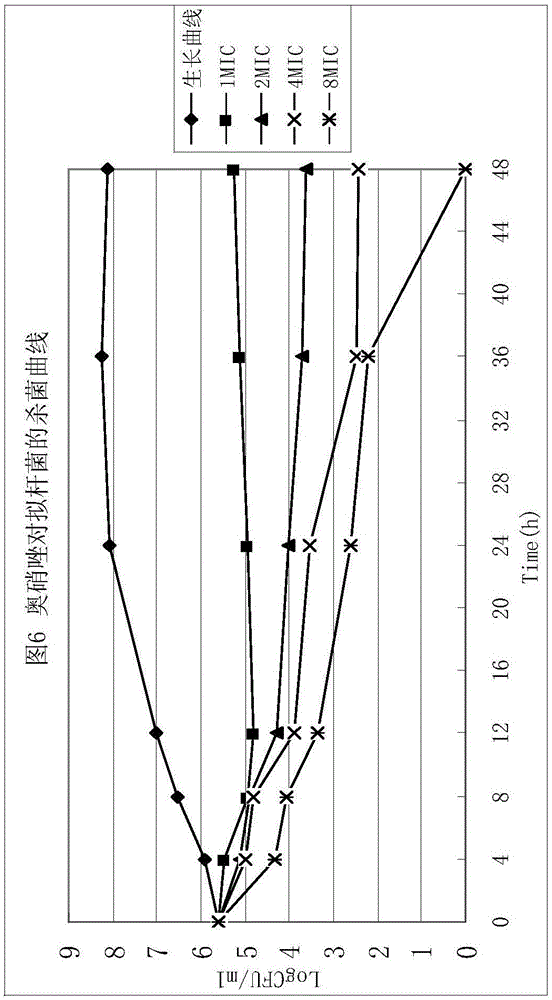
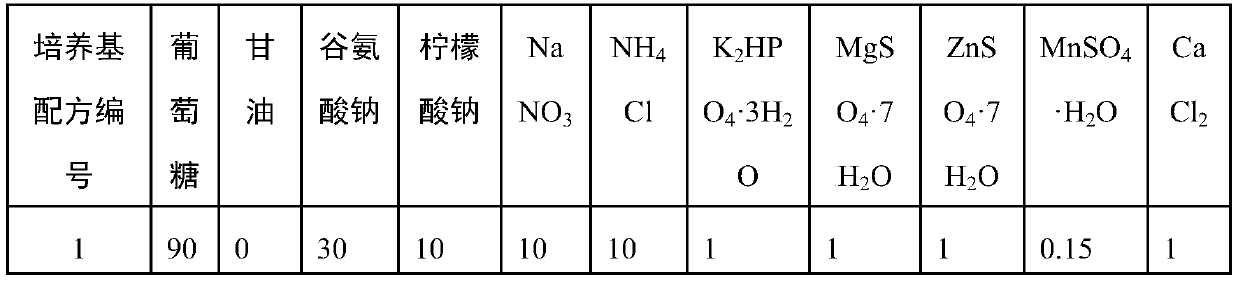
Patents
Literature
59 results about "Peptostreptococcus prevotii" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
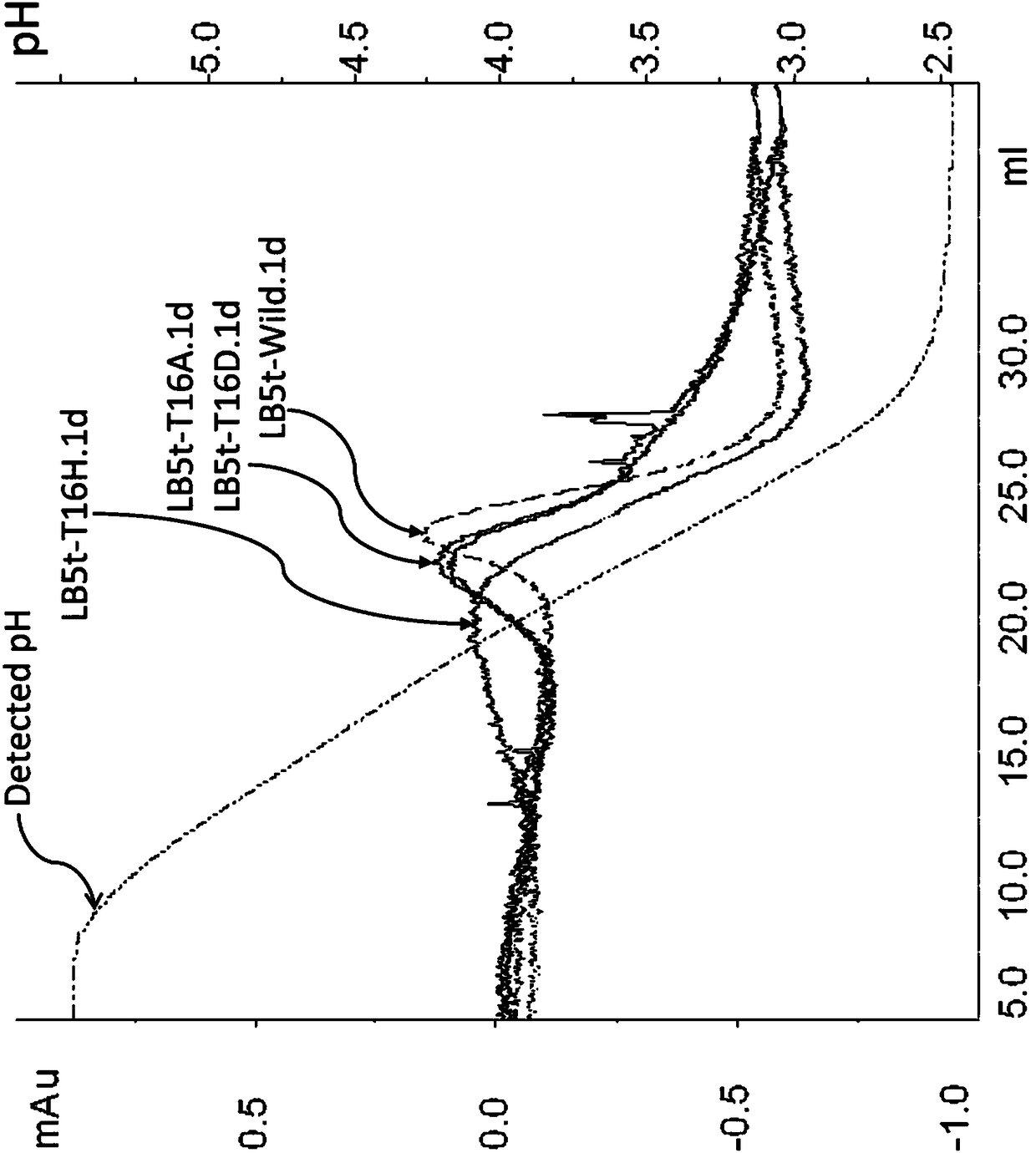
Peptostreptococcus is a genus of anaerobic, Gram-positive, non-spore forming bacteria. The cells are small, spherical, and can occur in short chains, in pairs or individually. They typically move using cilia. Peptostreptococcus are slow-growing bacteria with increasing resistance to antimicrobial drugs.
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E. Coli, Gemmiger, Desulfamonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
Multi-species universal ELISA kit for differential diagnosis of foot and mouth disease virus infection
The invention discloses a multi-species universal AGL-ELISA kit for differential diagnosis of foot and mouth disease virus infection. The multi-species universal AGL-ELISA kit comprises an ELISA plate coated with 8BF protein and recombinant protein [AGL]n undergoing enzyme labeling. The recombinant protein [AGL]n is a multi-copy recombinant protein formed by performing gene optimization and series connection on three protein structural domains which are a B structural domain in a staphylococcus aureus A protein combination structural domain, a C2 structural domain in a streptococcus protein G protein immune globulin combination structural domain and a B3 structural domain in a peptostreptococcus magnus protein L protein immune globulin combination structural domain, wherein n is 1, 2 or 3. The recombinant protein [AGL]n undergoes enzyme labeling, an enzyme labeling product can be combined with multi-species mammal immune globulin through western-blot and ELISA detection, can serve as a universal antibody for serologic detection of various mammals, and can replace species specific antibodies to perform multi-species anthropozoonosis detection. According to an AGL-ELISA, various animal foot-and-mouth disease infections can be detected, vaccine immunity, naturally injected animals and persistently infected animals can be identified.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Slow release agent containing aminoglycosides antibiotic and its uses
InactiveCN1843330AIncrease concentrationReduce concentrationOrganic active ingredientsMetabolism disorderBacillus acnesAbscess
The invention relates to a slow-releasing agent of antibiotics containing aminoglycoside, comprising slow-releasing micro-sphere containing slow-releasing findings and aminoglycoside antibiotics, and special dissolvent containing suspending adjuvant agent such as sodium carboxymethyl cellulose with the adhesive degree being 100cp-3000cp (at 20-30 Deg. C). The slow-releasing findings comprises EVAc, polyphenyl, PLA, PLGA, decanedioic acid copolymer, albumen glue and gelatin; the slow-releasing micro-sphere can be produced to slow-releasing implanting agent and unguent agent. When the slow-releasing implanting agent and injection is placed or injected into bacteria, the medicine can be released slowly for more than 5-30 days, and toxicity is dramatically reduced at the same time when effective medicine concentration is got and maintained. The said slow-releasing agent is specially effective for chronic medullitis, bedsore, intractable ulcer on skin, diabetes femoral head necrosis and other abscessus, which are caused by Staphylococcus, Streptococcus, Streptococcus, acne Propionibacteriaceae, Enterobacter, Enterobacter, gonotoxin or parameningococcus.
Owner:SHANDONG LANJIN PHARMA +1
Slow release injection containing cefradine
InactiveCN1843360AIncrease concentrationReduce concentrationAntibacterial agentsOrganic active ingredientsAdjuvantTreatment effect
Disclosed is a slow release injection containing cephalosporin, which comprises slow release microballoons and dissolvent, wherein the slow release microballoons include slow release auxiliary materials and cephalosporin, the dissolvent being specific one containing suspension adjuvant such as sodium carboxymethyl cellulose, the slow release auxiliary materials are selected from EVAc, PLA, PLGA, sebacylic acid copolymer, albumen glue or gelatin, the slow release microballoons can also be made into slow release implantation agent or ointment.
Owner:SHANDONG LANJIN PHARMA +1
Topically applied sustained-release antibiotic preparation
InactiveCN1883706AIncrease concentrationReduce concentrationAntisepticsSaccharide peptide ingredientsTreatment effectWhole body
Disclosed is a topical applicated antibiotics slow release agent as a slow release injection or a slow release implantation agent, comprising slow release microspheres and menstruum. Said release microsphere comprises slow release assist materials and antibiotics, and said menstruum is a special menstruum comprising suspending agents of sodium carboxymethylcellulose, etc, with a viscosity of 100cp-3000cp(at 20-30 DEG C). Said slow release assist materials are selected from the group of EVAc, polifeprosan, PLA, PLGA, sebacic acid copolymer, albumen glue, gelatin, and etc. Said slow release implantation agent is prepared with slow release microspheres or by melt method, etc. The disclosed slow release agent can release drugs for 10 days by topical applicated or injected at a focus, decreasing whole body toxicity obviously while obtaining and sustaining effective topical drug concentration at the focus.
Owner:JINAN KANGQUAN PHARMA TECH
Medicament combination preparation of medicinal compound containing cy-ethyl razepam
The invention relates to medicament combination preparation of medicinal compound taking cy-ethyl razepam medicinal compound containing cy-ethyl razepam or stereoisomer, pro-medicinal, medicinal salt, complex salt or / and salvation as effective constituent. The medicament combination preparation adopts the technical scheme that the cy-ethyl razepam medicinal compound, which has a general formula (1) structure and contains the cy-ethyl razepam or the stereoisomer, the pro-medicinal, the medicinal salt, the complex salt and the solvation, is taken as the effective constituent and forms the medicament combination preparation with a medicament carrier capable of being received on the pharmacy; and the preparation formulation is any preparation formulation said on the pharmacy. The invention also discloses an application of the medicament combination preparation in the process of treating the infection of bacteria taken as gram-positive bacteria like staphylococcus, pneumococcus, enterococcus faecalis, streptococcus, streptococcus bovis, streptococcus pneumoniae, peptostreptococcus, festering streptococcus pneumoniae, festering streptococcus pneumoniae, streptococcus pyogenes, streptococcus agalactiae, viridans streptococci, streptococcus bovis, streptococcus agalactiae B, group green streptococcus, corynebacterium diphtheriae and other bacteria.
Owner:LIAONING LIFENG SCI & TECH DEV
Slow-release injecta containing amino glucosides antibiotics and use thereof
InactiveCN1850044AIncrease concentrationReduce concentrationOrganic active ingredientsAntisepticsDiseaseAdjuvant
The present invention relates to a slow-released injection containing aminoglycoside antibiotics. It composed of slow-released microsphere and solvent. Said slow-released microsphere contains slow-released auxiliary material and aminoglycoside antibiotics, and the solvent is special solvent containing suspension adjuvant of carboxymethyl cellulose sodium, its viscosity is 100 cp-3000 cp (20 deg.C-30 deg.C), the slow-released auxiliary material is selected from EVAc, PLA, PLGA, sebacic acid copolymer, albumin glue and gelatin, etc. The slow-released microsphere also can be made into slow-released implant preparation and ointment preparation. Said invention also provides its application range and can obtain obvious therapeutic effect for curing various infective diseases.
Owner:JINAN SHUAIHUA PHARMA TECH
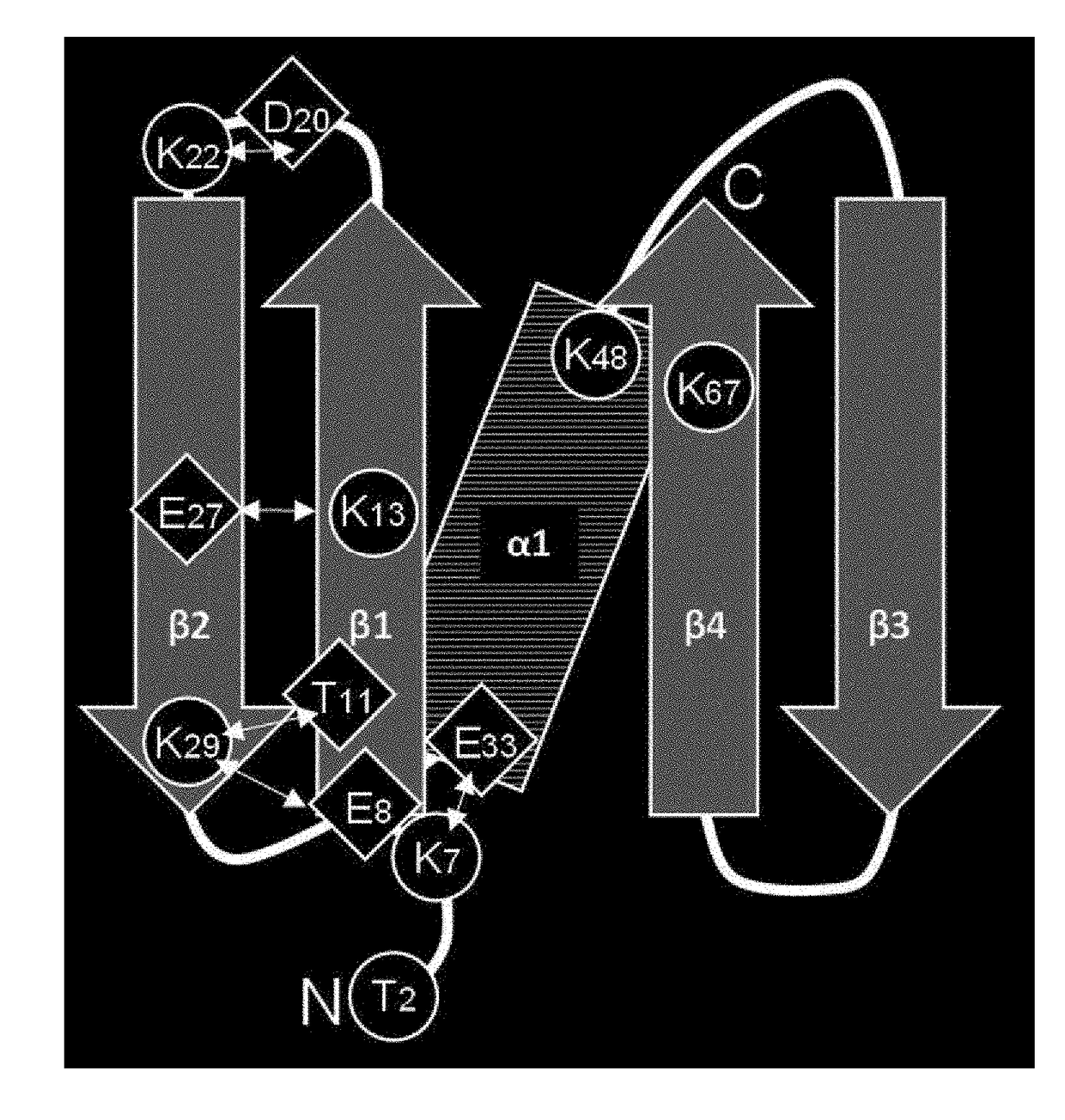
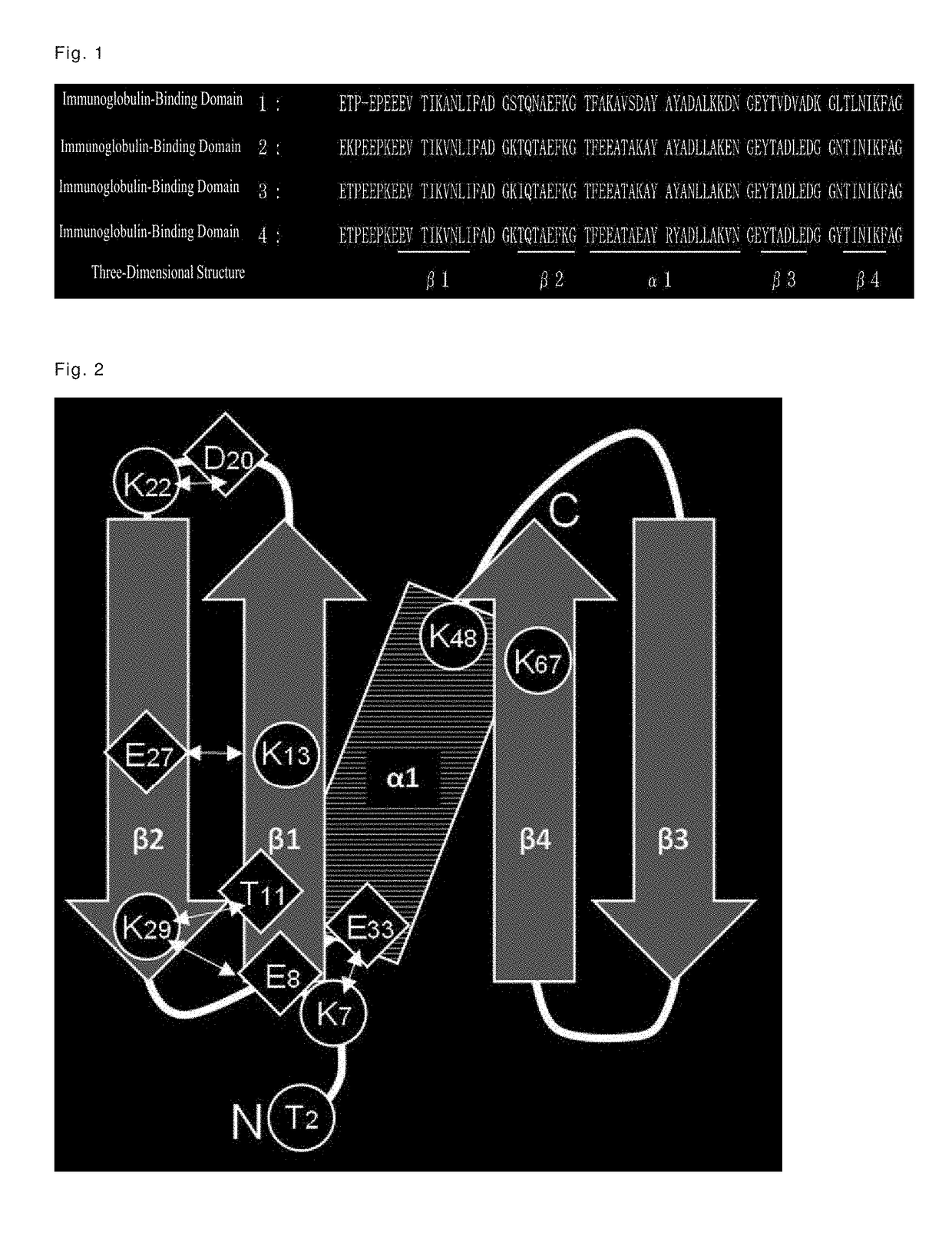
Immunoglobulin-binding polypeptide
ActiveUS20180305414A1Improve bindingReduce the binding forceAntibody mimetics/scaffoldsPeptide preparation methodsImmunoglobulin kappa-ChainsPeptostreptococcus magnus
An object of the present invention is to provide a polypeptide having a high binding capacity for an immunoglobulin kappa chain, and having excellent alkali stability, by modifying an amino acid sequence of an immunoglobulin-binding domain of Protein L derived from Peptostreptococcus magnus. A polypeptide having a high binding capacity for an immunoglobulin kappa chain, and having excellent alkali stability can be obtained by substituting specific lysine residues in an immunoglobulin-binding domain of Protein L derived from Peptostreptococcus magnus 3316 strain, with a basic amino acid or a hydroxyl group-containing amino acid.
Owner:PROTENOVA
Primer compound for identifying intestinal microecological state and application thereof
ActiveCN109576386AEfficient and convenient identificationHigh detection specificityMicrobiological testing/measurementMicroorganism based processesBacteroidesClostridium symbiosum
The invention provides a primer compound for identifying intestinal microecological state and an application thereof. The primer compound is a specific primer designed for intestinal bacterium 16S rRNA; the specific primer includes random basic groups; the quantity of random basic groups is 3-5; the intestinal bacterium includes any one of or combination of at least two of symbiotic clostridia, bifidobacterium adolescentis, fusobacterium nucleatum, peptostreptococcus anaerobius or klebsiella. A kit researched and developed on the basis of the primer compound provided by the invention has a specificity for detecting intestinal microecological imbalance reaching up to 92.5% and a sensitivity reaching up to 80.43%.
Owner:SUZHOU PRECISION BIOTECH CO LTD
A sustained release injection containing cephalosporin and application thereof
InactiveCN1879627AEasy to operateGood repeatabilityAntibacterial agentsOrganic active ingredientsBacillus acnesWhole body
The invention relates to a release injection which contains cefradine, wherein it is formed by release micro ball and dissolvent; the release micro ball contains release findings and penicillin antibiotic; the dissolvent is the special one contains sodium carboxymethyl cellulose as suspension agent; the viscosity is 100cp-3000cp (20Deg. C-30Deg. C); the release agent is selected from EVAc, polyphenyl, PLA, PLGA, sebacic acid polymer, protein pastern, and dope; the release micro ball can be made into release implant agent; the release implant agent and release injection is laid on bacteria stove or injected, to release the medicine for 5-30 days, to keep the effective medicine density and reduce the whole toxicity. The invention can be used to cure the infection caused by staphylococcus, streptococcus, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Crystal form of (s)-ornidazole phosphate disodium and preparation method thereof, and application of pharmaceutical compositions
InactiveCN106467558AEasy to prepareEase of industrial applicationAntibacterial agentsOrganic active ingredientsPhosphatePhosphoric acid
The invention provides a crystal form of (s)-ornidazole phosphate disodium, and a preparation method for the crystal form of the (s)-ornidazole phosphate disodium. In addition, pharmaceutical compositions containing the crystal form of the (s)-ornidazole phosphate disodium are applied in treatment of a plurality of infectious diseases caused by sensitive anaerobic bacteria like bacteroides fragilis, bacteroides distasonis, bacteroides ovatus, bacteroides thetaiotaomicron, bacteroides vulgatus, clostridium colinum, eubacterium, peptococcus and peptostreptococcus, helicobacter pylori, bacteroides melaninogenicus, fusobacterium, capnocytophaga and bacteroides gingivalis.
Owner:HC SYNTHETIC PHARMA CO LTD
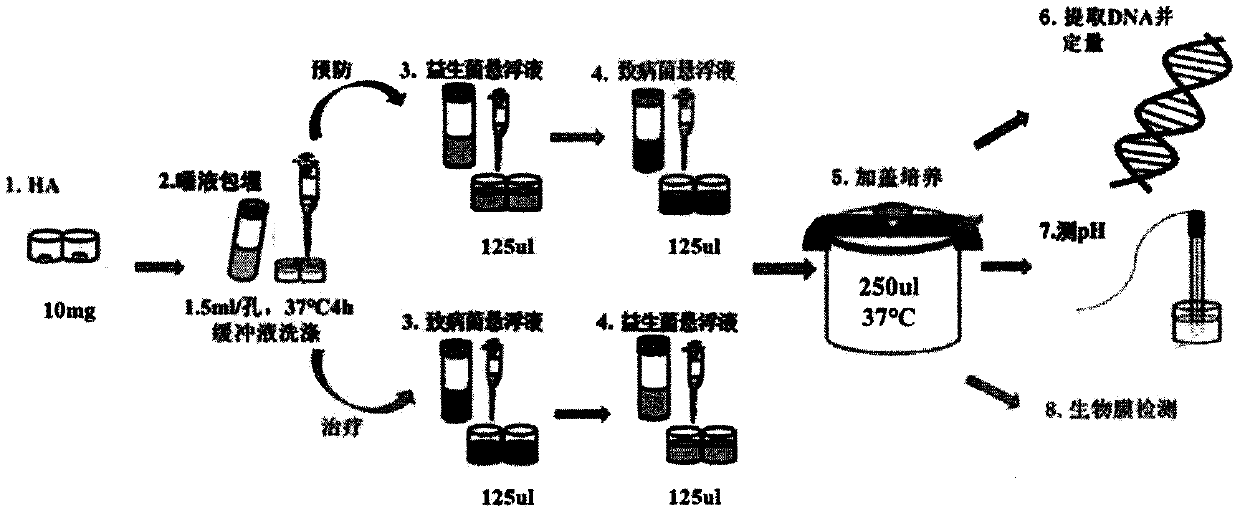
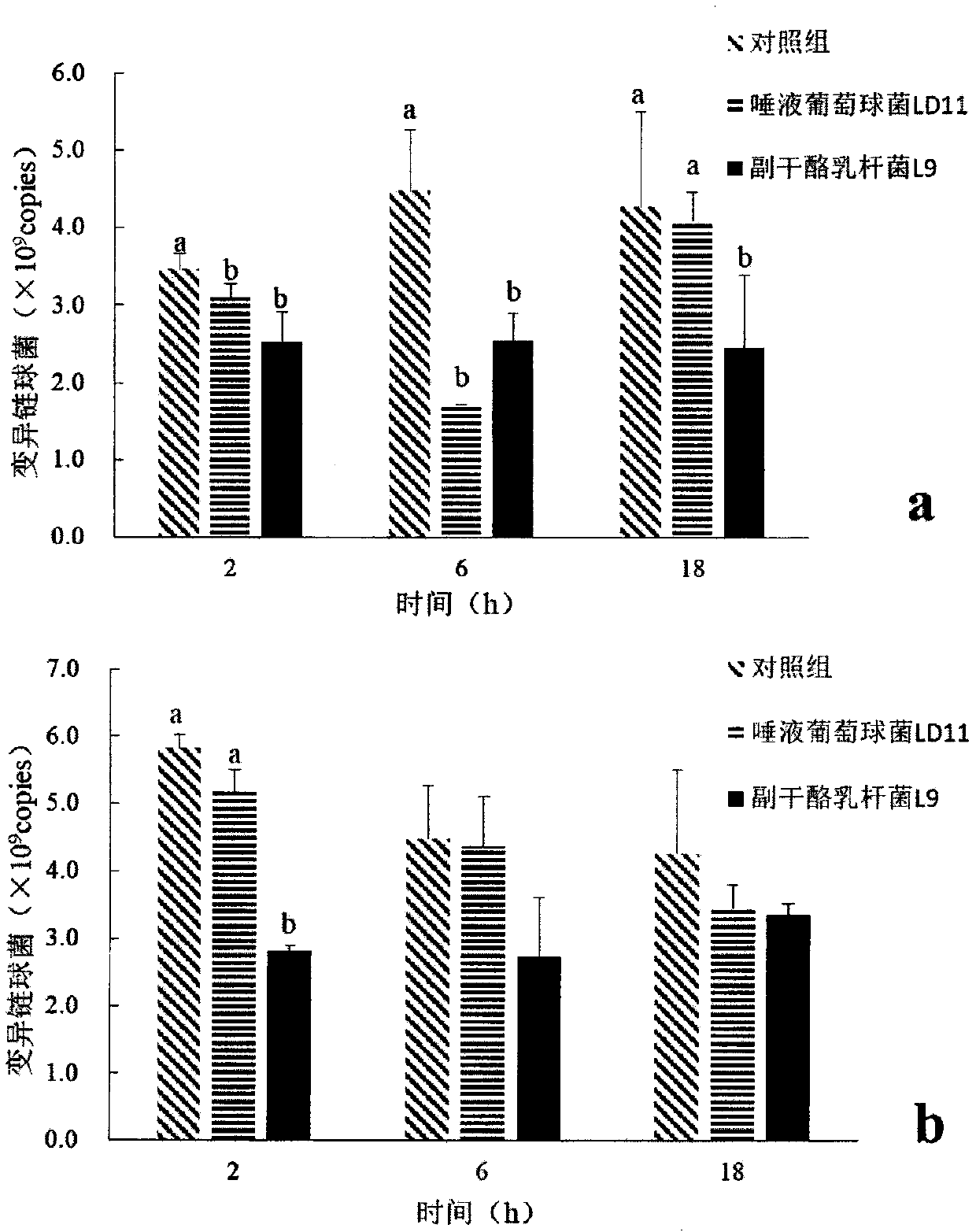
Application of lactobacillus paracasei L9 to prevention or treatment oral disease and regulation of oral cavity flora
InactiveCN110339217AInhibition of adhesionReduce adhesionCosmetic preparationsMilk preparationOral diseasePeptostreptococcus
The invention discloses an application of lactobacillus paracasei L9 to prevention or treatment oral disease and regulation of oral cavity flora. L9 can be used for preparing a medical composition anda food for preventing or treating dental caries. Research proves that L9 can notably relieve generation degree of occlusal surface E-level dental caries, after the LP is taken, the structure of the oral cavity flora is changed notably, particularly actinobacteria is notably increased, the actinobacteria of L9 group is obviously reduced, klebsiella, aerococcus, Gemella, corynebacterium and pseudomonas aeruginosa on the classification level are notably reduced, and the oral disease risk is reduced. When L9 is taken, the saliva species richness is notably reduced, the relative abundance of oraldisease relevant fungi of streptococcus salivarius, actinomycetaceae, peptostreptococcus and the like is notably reduced, and the L9 can have relieving effects on periodonitis diseases. The dental plaque flora species diversity is notably reduced, the relative abundance of leptothrix, fusobacteriaceaeand flavobacterium is notably reduced, and the L9 can have relieving effects on oral diseases of periodontal disease, halitosis, dental caries and the like.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
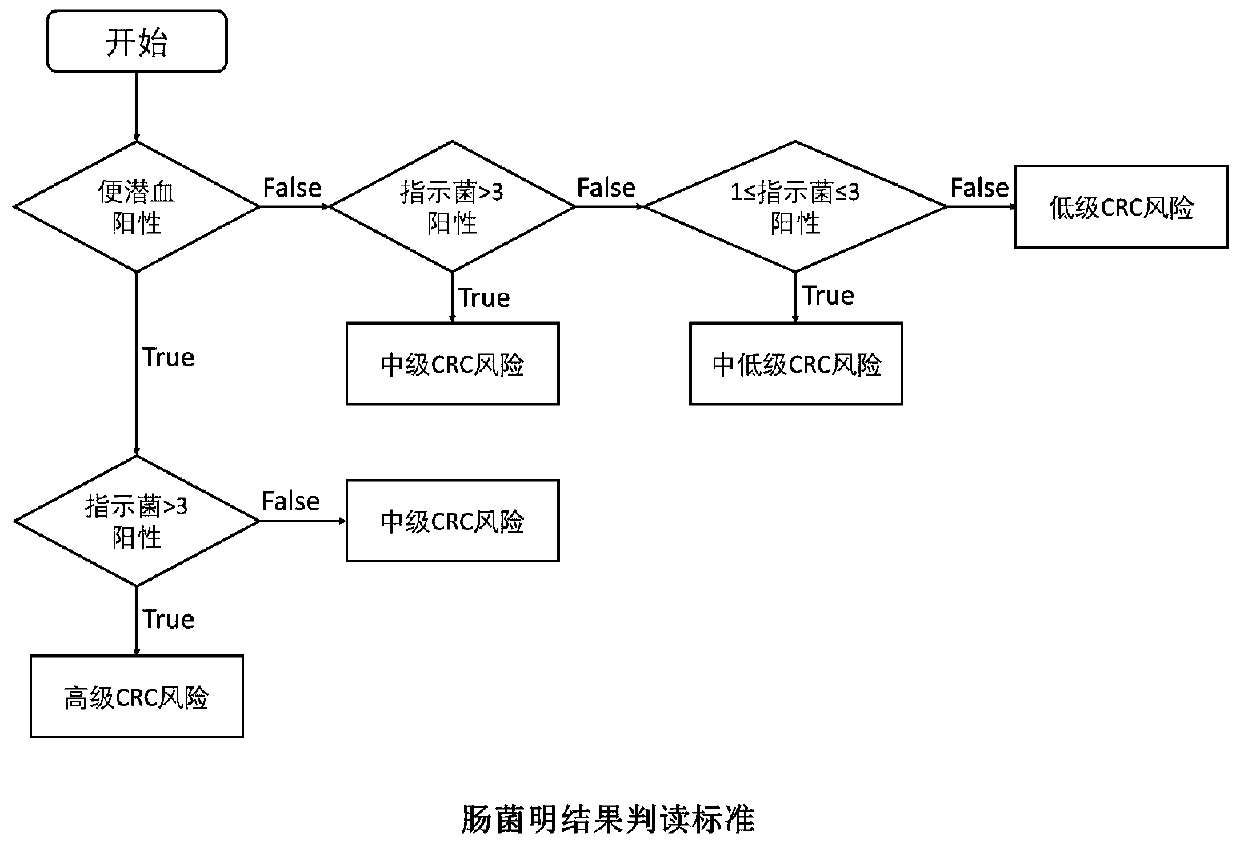
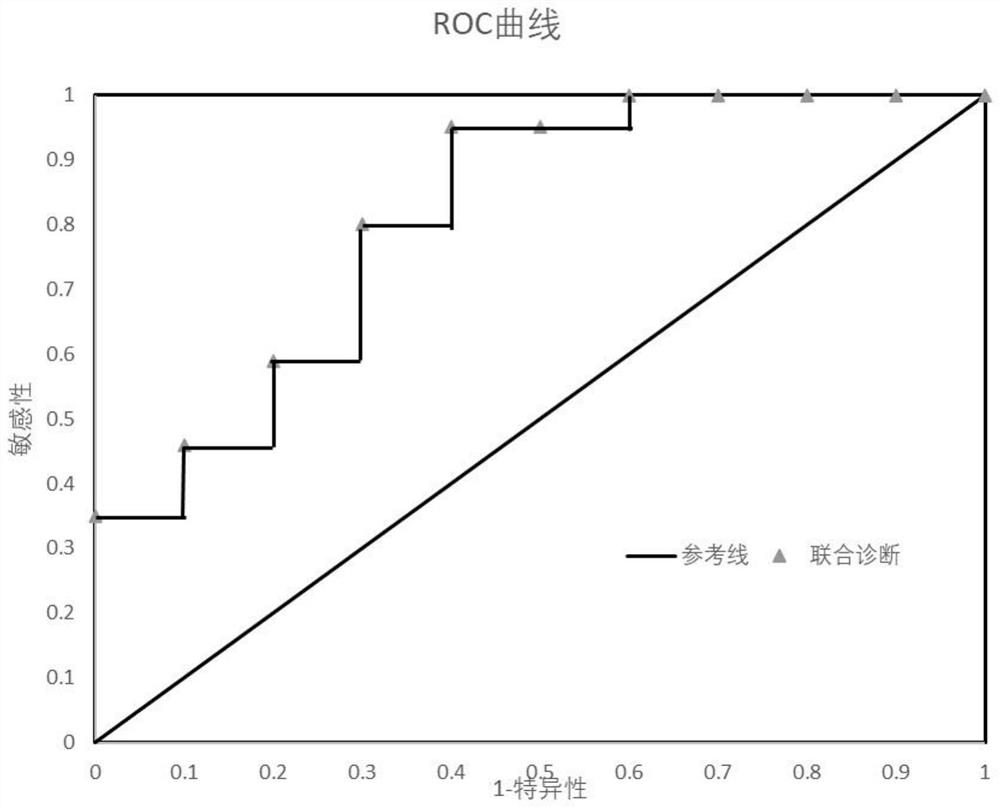
Kit for identifying colorectal cancer and application thereof
PendingCN111334590AHigh sensitivityMicrobiological testing/measurementMicroorganism based processesFecesFusobacteria
The invention discloses a kit for identifying colorectal cancer. The kit is characterized by comprising a specific primer combination for identifying indicating bacteria, wherein the primer combination is shown as SEQ ID NO.1-12, and the indicating bacteria are a combination of F. nucleatum, P. anaerobius, C. symbiosum, E. faecalis, P. asaccharolytica and S. salivaricus. The invention also discloses an application of the kit. The kit has the beneficial effects that risk prediction of colorectal cancer can be carried out only by collecting stool samples. The principle is to detect colorectal cancer pathogenic bacteria in feces, wherein the risk assessment sensitivity can reach 92% and the specificity is 95%.
Owner:南京派森诺基因科技有限公司
Pharmaceutical composition and food for preventing or treating oral diseases
InactiveCN110448578AInhibition of adhesionReduce adhesionAntibacterial agentsBacteria material medical ingredientsOral diseasePeptostreptococcus
The invention discloses a pharmaceutical composition and food for preventing or treating oral diseases. Both the composition and food comprise lactobacillus paracasei L9 and staphylococcus salivariusLD11. Studies show that the L9 and the LD11 can significantly alleviate the occurrence degree of E-level dental caries of an occlusal surface, after the composition and food containing the L9 and LD11are taken, the structures of oral floras change significantly, especially actinomycetes are significantly increased, and klebsiella, aerococcus, Gemella, corynebacterium and pseudomonas aeruginosa are significantly reduced in the category classification level. Taking of the L9 and LD11 can significantly reduce saliva species richness, the relative abundances of bacteria related to the oral diseases such as staphylococcus salivarius, actinomycetaceae and peptostreptococcus are significantly reduced, and periodontitis diseases may be alleviated; the species diversity of a dental plaque flora issignificantly reduced, the relative abundances of leptotrichia, fusobacterium and flavobacterium are all significantly reduced, and the oral diseases such as periodontal diseases, ozostomia and dental caries may be alleviated.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
A sustained release injection containing penicillins antibiotic and application thereof
InactiveCN1879620AEasy to operateGood repeatabilityAntibacterial agentsSenses disorderBacillus acnesMicrosphere
The invention relates to a release injection which contains mycin antibiotic, wherein the invention is formed by release micro ball and dissolvent; the release micro ball contains release findings and penicillin antibiotic; the dissolvent is the special one contains sodium carboxymethyl cellulose as suspension agent; the viscosity is 100cp-3000cp (20Deg. C-30Deg. C); the release agent is selected from EVAc, polyphenyl, PLA, PLGA, sebacic acid polymer, protein pastern, and dope; the release micro ball can be made into release implant agent; the release implant agent and release injection is laid on bacteria stove or injected, to release the medicine for 5-30 days, to keep the effective medicine density and reduce the whole toxicity. The invention can be used to cure the infection caused by staphylococcus, streptococcus, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Slow release formulation containing antibiotic and its uses
InactiveCN1843331AIncrease concentrationReduce concentrationPharmaceutical delivery mechanismCarbohydrate active ingredientsBacillus acnesTherapeutic effect
The invention relates to a slow-releasing agent or rslow-releasing implanting agent containing antibiotics. The injection comprises slow-releasing micro-sphere and dissolvent. The slow-releasing micro-sphere contains slow-releasing findings and antibiotics, and dissolvent contains suspending adjuvant agent such as sodium carboxymethyl cellulose with the adhesive degree being 100cp-3000cp (at 20-30 Deg. C). The slow-releasing findings comprises EVAc, polyphenyl, PLA, PLGA, decanedioic acid copolymer, albumen glue and gelatin; the slow-releasing implanting agent is prepared with slow-releasing micro-sphere or through other methods. When said antibiotics is placed or injected into bacteria, the medicine can be released slowly for more than 10 days, and toxicity is dramatically reduced at the same time when effective medicine concentration is got and maintained. The said slow-releasing agent is specially effective for chronic medullitis, bedsore, intractable ulcer on skin, diabetes femoral head necrosis and other abscessus, which are caused by Staphylococcus, Streptococcus, Streptococcus, acne Propionibacteriaceae, Enterobacter, Enterobacter, gonotoxin or parameningococcus.
Owner:SHANDONG LANJIN PHARMA +1
Sustained-release injection containing antibiotic lincomycin and application thereof
InactiveCN101283971AIncrease concentrationReduce concentrationAntibacterial agentsOrganic active ingredientsPeptostreptococcusBacillus acnes
The invention provides a sustained-release injection or sustained-release implant containing lincomycin antibiotic. The sustained-release injection comprises sustained-release microspheres and solvent. The microspheres contain sustained-release adjuvants and antibiotics, and the solvent is a special solvent containing suspending agent such as sodium carboxymethylcellulose and having viscosity of 100-3000cp (20-30 DEG C); and the sustained-release adjuvants are selected from poly(ethylene-co-vinylacetate) (EVAc), polifeprosan, poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), sebacic acid copolymer, albumin glue and gelatin. The sustained-release implant is made from microspheres or by other methods. The sustained-release implant or injection can be locally placed or injected into foci to locally sustained-release of drug for more than 10 days, so as to obtain and maintain local drug effective concentration while remarkably reduce the systemic toxicity of the drug. The sustained-release injection has distinct and unique therapeutic effect on local infection diseases caused by Staphylococci, Streptococci, Peptostreptococcus, Propionibacterium acnes, Enterobacter, Mycobacterium tuberculosis, Gonococcus or Meningococcus, such as chronic osteomyelitis, severe decubital ulcer, refractory skin ulcer, diabetic foot, femoral head necrosis, abscess, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Novel immunochromatography test paper for detecting human brucellosis antibody and preparing method thereof
InactiveCN104360058AEasy to readStrong specificityBiological material analysisEscherichia coliPeptostreptococcus
The invention relates to the field of zoonosis immunologic diagnosis and discloses a brucellosis antibody test strip and a preparing method thereof. The brucellosis antibody test strip is prepared in a way that colloidal gold with a grain diameter of 30nm is first prepared, used as an indication mark for marking and reconstructing large peptostreptococcus proteins L, dissolved in a gold mark work solution and sprayed on glass fibers to make a gold mark pad; VirB5 genes are cloned from a brucella genome and connected to pET-28a to form an expression vector; the expression vector is converted into escherichia coli, VirB5 proteins are expressed and coat on a nitrocellulose membrane after separation and purification as a detection line, IgGs (Immunoglobulin G) bonded with the proteins L are coated on the nitrocellulose membrane as a quality control line, and an immunochromatography detection device is made. The brucella immunochromatography device has the characteristics of strong specificity, high sensitivity, great stability, simplicity, economy, rapidness and the like and has extremely significant actual application value in the monitoring and epidemic control of zoonosis.
Owner:SHIHEZI UNIVERSITY
Kit for detecting colorectal cancer indicator bacteria
InactiveCN110643721AHigh sensitivityMicrobiological testing/measurementMicroorganism based processesClostridium symbiosumFusobacteria
The invention discloses a kit for detecting colorectal cancer indicator bacteria. The kit is characterized by comprising the following components: a specific primer combination for identifying the indicator bacteria, wherein the primer combination is shown as SEQ ID NO. 1-14, and the indicator bacteria are the combination of fusobacterium nucleatum (F.nucleolus), anaerobic digestion streptococcus(P.anaerobicus), clostridium symbiosum (C.symbiolum), Porphyromonas saccharolytica (P.asaccharolytica), Prevotella intermedia (P.intermedia), Bacteroides fragilis (B.fragilis) and Streptococcus salivarius (S.salivarius). The kit for detecting colorectal cancer indicator bacteria disclosed by the invention has the beneficial effects that the colorectal cancer can be identified only by collecting astool sample. The principle is that the aim of identifying the colorectal cancer by detecting the colorectal cancer pathogenic bacteria in excrement, the sensitivity can reach 92.9%, and the specificity is 92.6%.
Owner:SHANGHAI PASSION BIOTECHNOLOGY CO LTD
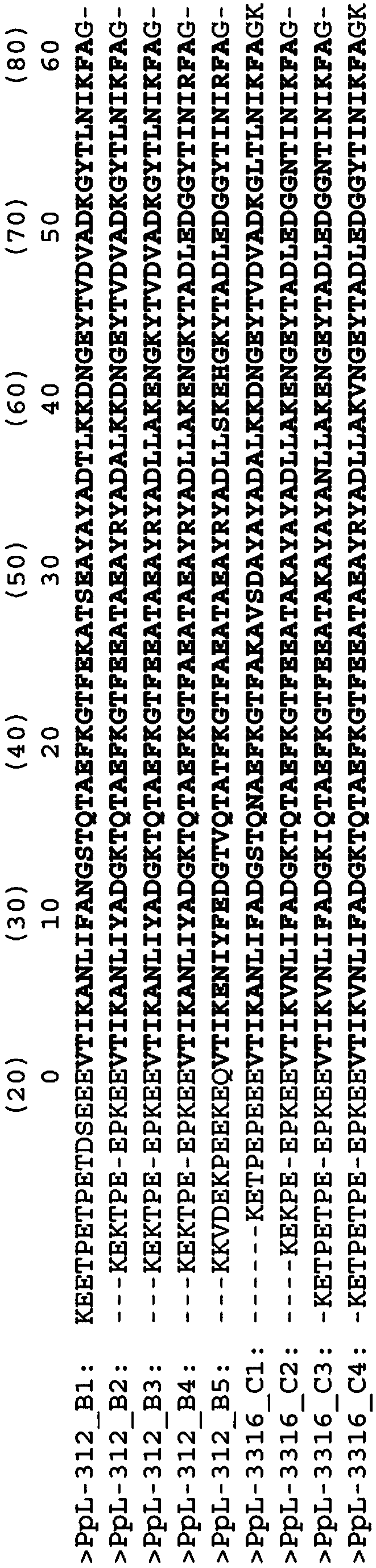
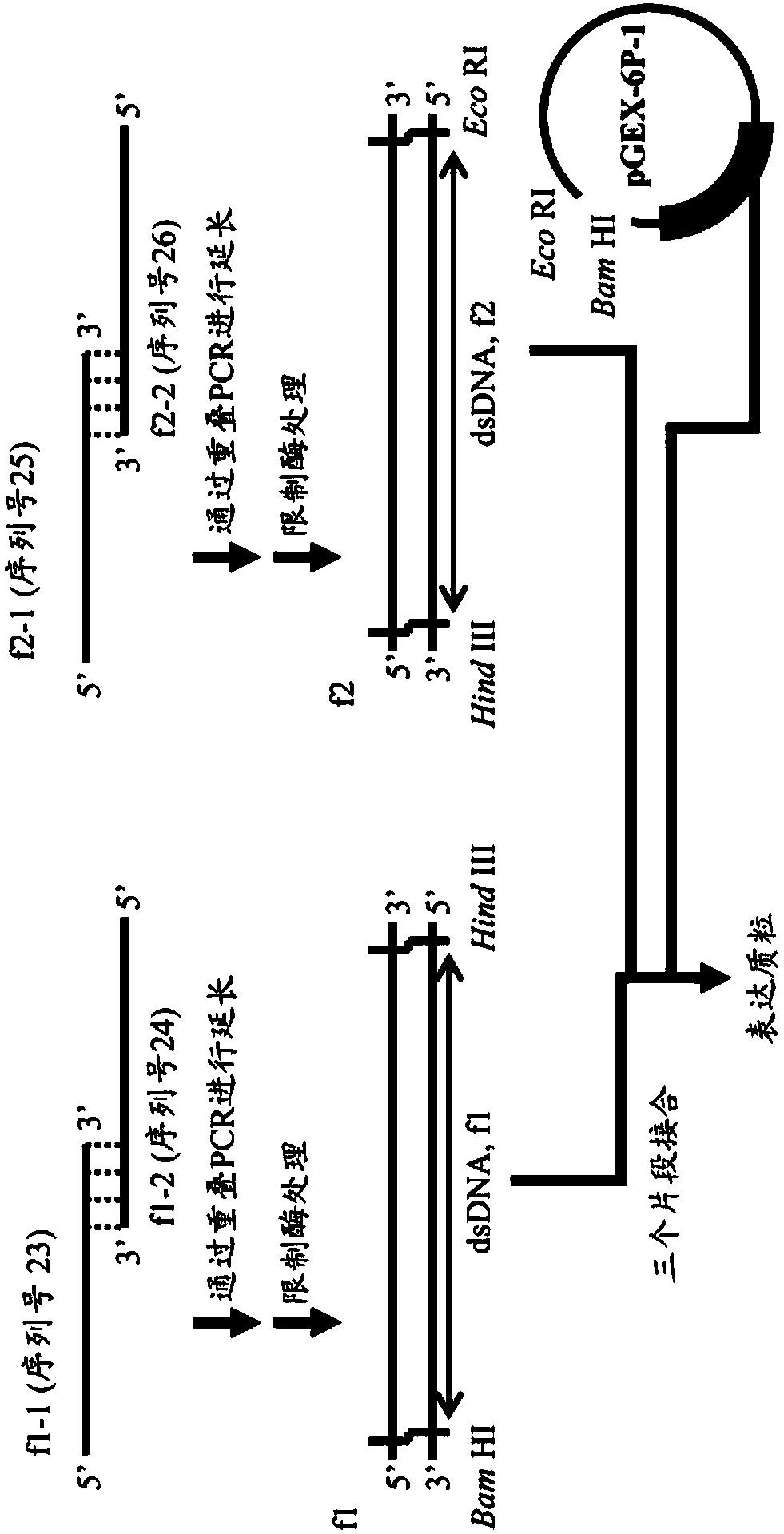
Modified Kappa Light Chain-Binding Polypeptides
ActiveUS20170327545A1Improve stabilityHighly selectivePolypeptide with localisation/targeting motifSolid sorbent liquid separationPeptostreptococcusBinding domain
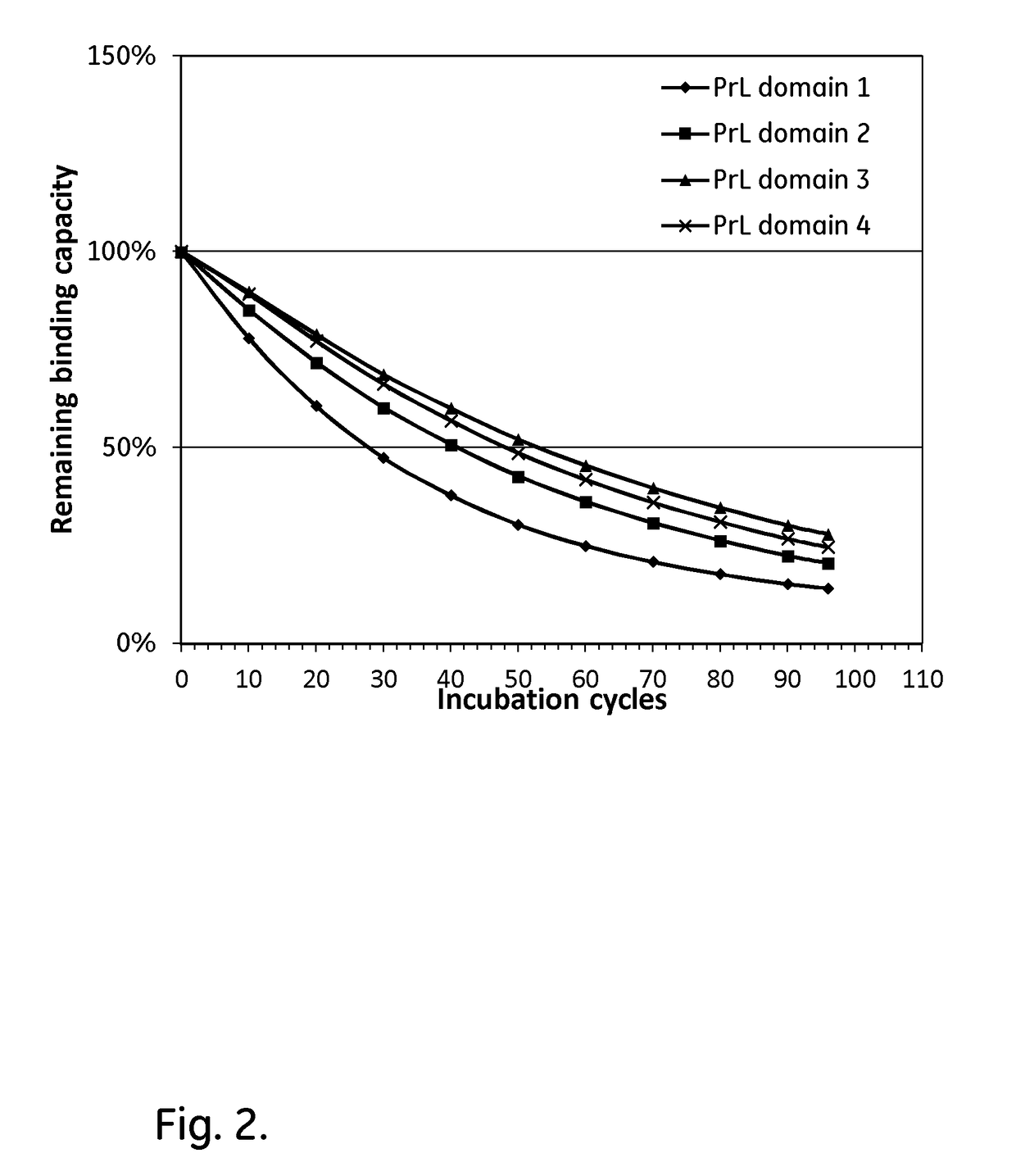
A kappa light chain-binding polypeptide comprising or consisting essentially of one or more binding domains of Peptostreptococcus Protein L, each of said domains being selected from the group consisting of domain 2, domain 3 and domain 4.
Owner:CYTIVA BIOPROCESS R&D AB
Slow-release preparation containing quinolones antibiotics
The present invention relates to an antibiotic slow-released preparation containing quinolones, including slow-released injection or slow-released implant preparation. Said injection is formed from slow-released microsphere and solvent, the slow-released microphere contains slow-released auxiliary material and antibiotic, the solvent is special solvent containing suspension adjuvant of sodium cellulose glycollate, its viscosity is 100 cp-3000 cp (20 deg.C-30 deg.C), the slow-released auxiliary material is selected from EVAc, PLA, PLGA, sebacic aid copolymer, albumin glue and gelatin. The slow-released implant preparation is prepared by using slow-released microsphere or adopting melting process. Besides, said invention also provides its application range for curing various diseases.
Owner:JINAN KANGQUAN PHARMA TECH
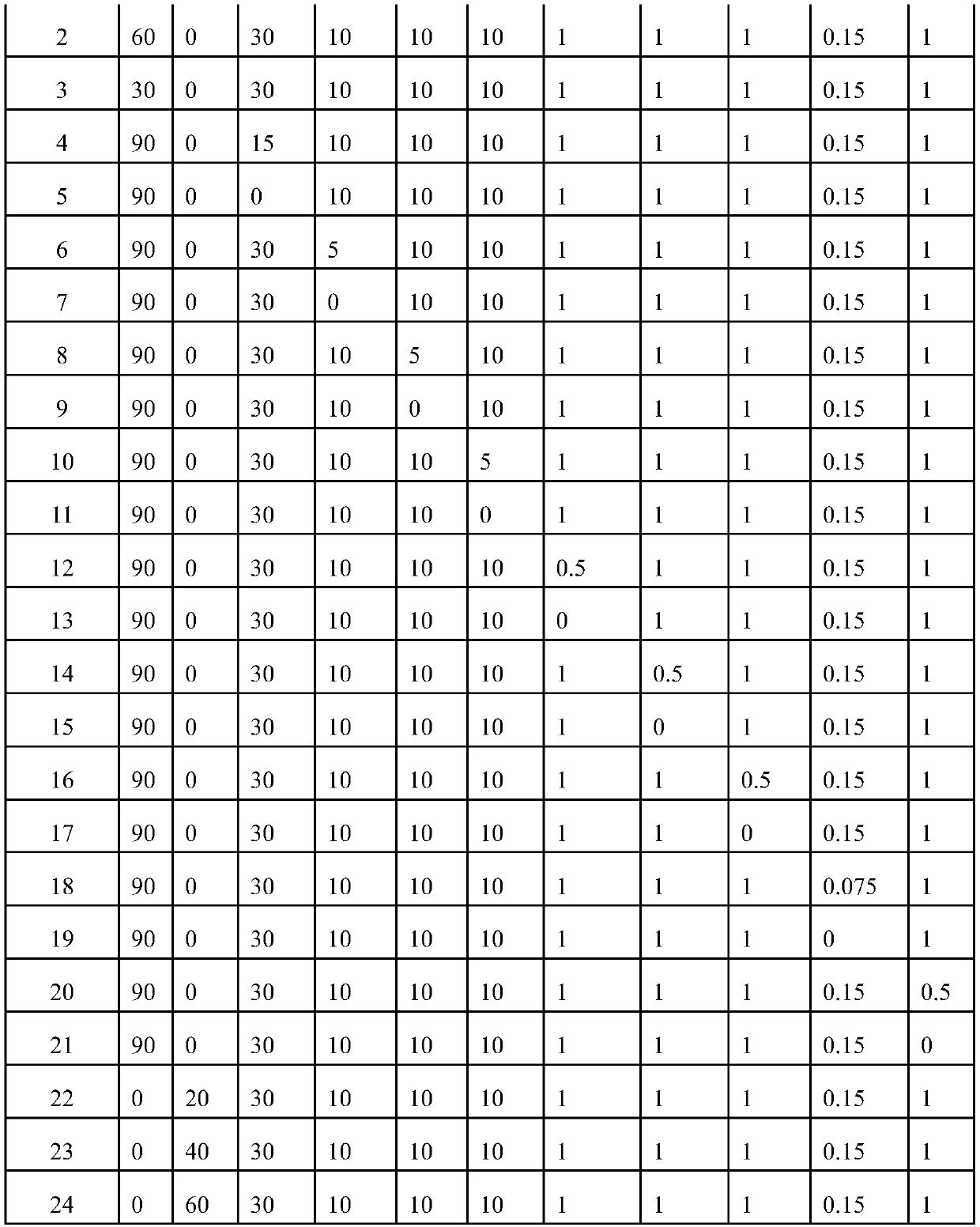
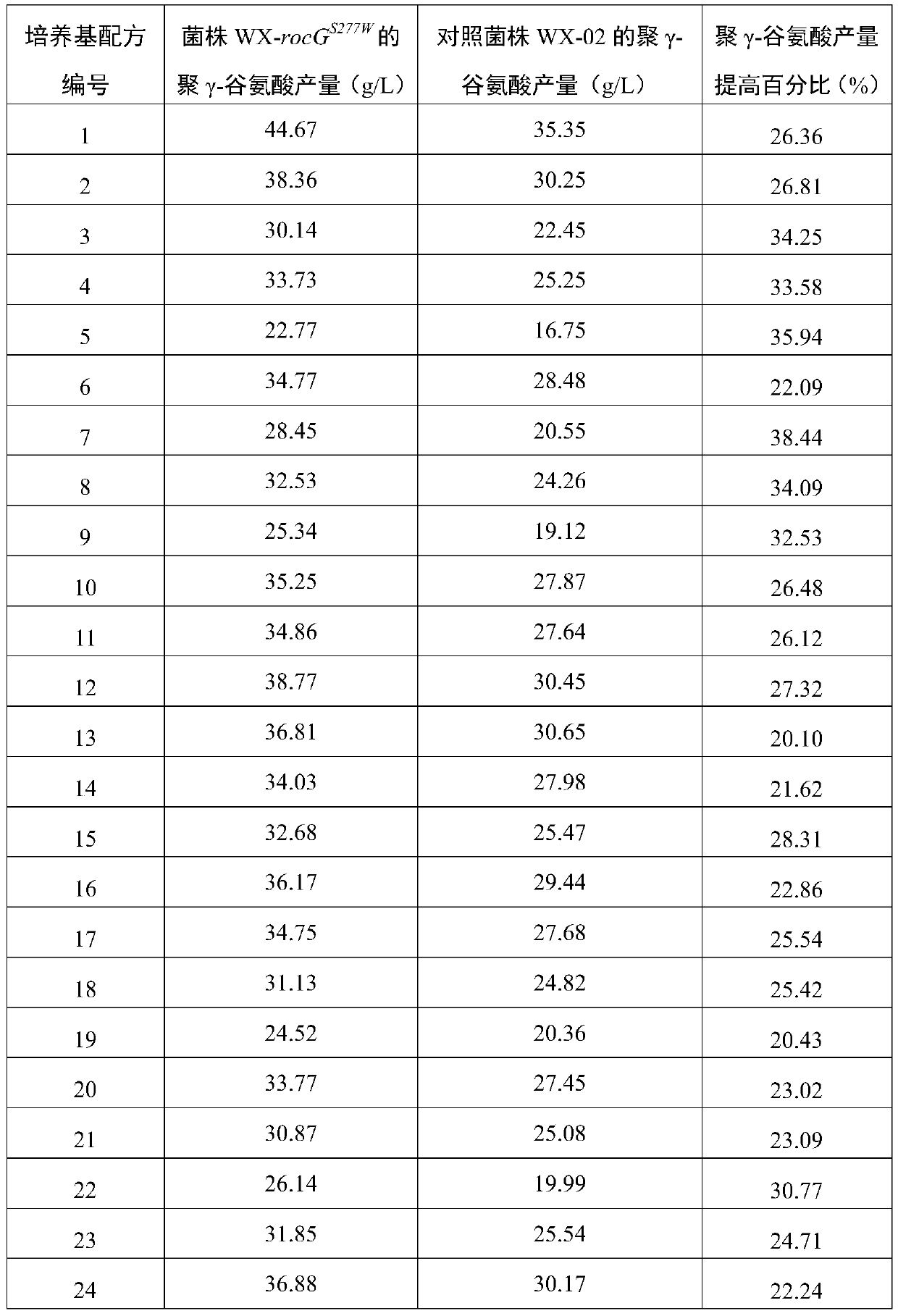
Application of Peptostreptococcus asaccharoly glutamate dehydrogenase (GdhA) in increasing yield of Bacillus licheniformis poly-gamma-glutamic acid
ActiveCN110951797ASolve the shortage of supplyIncrease synthesis levelBacteriaMicroorganism based processesPeptostreptococcusBacillus licheniformis
The invention belongs to the technical field of gene engineering and enzyme engineering, and discloses application of Peptostreptococcus asaccharoly glutamate dehydrogenase (GdhA) in increasing the yield of Bacillus licheniformis poly-gamma-glutamic acid. According to the invention, with a homologous recombination mode, glutamate dehydrogenase (GdhA) derived from Peptostreptococcus asaccharoly replaces glutamate dehydrogenase of Bacillus licheniformis (WX-02), the synthesis level of the Bacillus licheniformis poly-gamma-glutamic acid is remarkably improved, and the yield of the obtained strainpoly-gamma-glutamic acid is at least increased by 20% or above compared with that of a control strain. The invention provides a new strategy for efficient production of poly gamma-glutamic acid.
Owner:HUBEI UNIV
Sustained-release agent containing mezlocillin and uses thereof
InactiveCN101301272AIncrease concentrationReduce concentrationAntibacterial agentsSolution deliveryPeptostreptococcusBacillus acnes
The invention discloses a sustained release injection containing mezlocillin, which consists of sustained release microspheres and menstruum, wherein, the sustained release microspheres contain sustained release excipient and penicillin antibiotics, the menstruum is special menstruum containing suspending agents such as sodium carboxymethyl cellulose and so on, and the viscosity is between 100 and 3000cp (at the temperature of between 20 and 30 DEG C); the sustained release excipient is selected from EVAc, polifeprosan, PLA, PLGA, decanedioic acid copolymers, albumin glue, gelatin and so on; and the sustained release microspheres can also be prepared into sustained release implant. The sustained release implant and the sustained release injection are locally placed or injected on a bacteria focus, which can slowly release medicine at a local lesion for 5 to 30 days and apparently decrease the systemic toxicity of the medicine when the effective medicine concentration of the local lesion is effectively obtained and maintained. The sustained release injection has a remarkable and unique treatment effect on infections caused by staphylococcus, streptococcus, peptostreptococcus, acne propionate bacillus, enterobacter, tubercle bacillus, gonococcus or meningococcus and so on, particularly on local lesions such as chronic osteomyelitis, severe bedsore, refractory skin ulcer, diabetic foot and femoral head necrosis of diabetes, and various abscesses and so on.
Owner:SHANDONG LANJIN PHARMA +1
Mutant immunoglobulin kappa chain variable region-binding peptide
InactiveCN108064286AStrong selective adsorption capacityReduce capacityFungiBacteriaImmunoglobulin kappa-ChainsPeptostreptococcus magnus
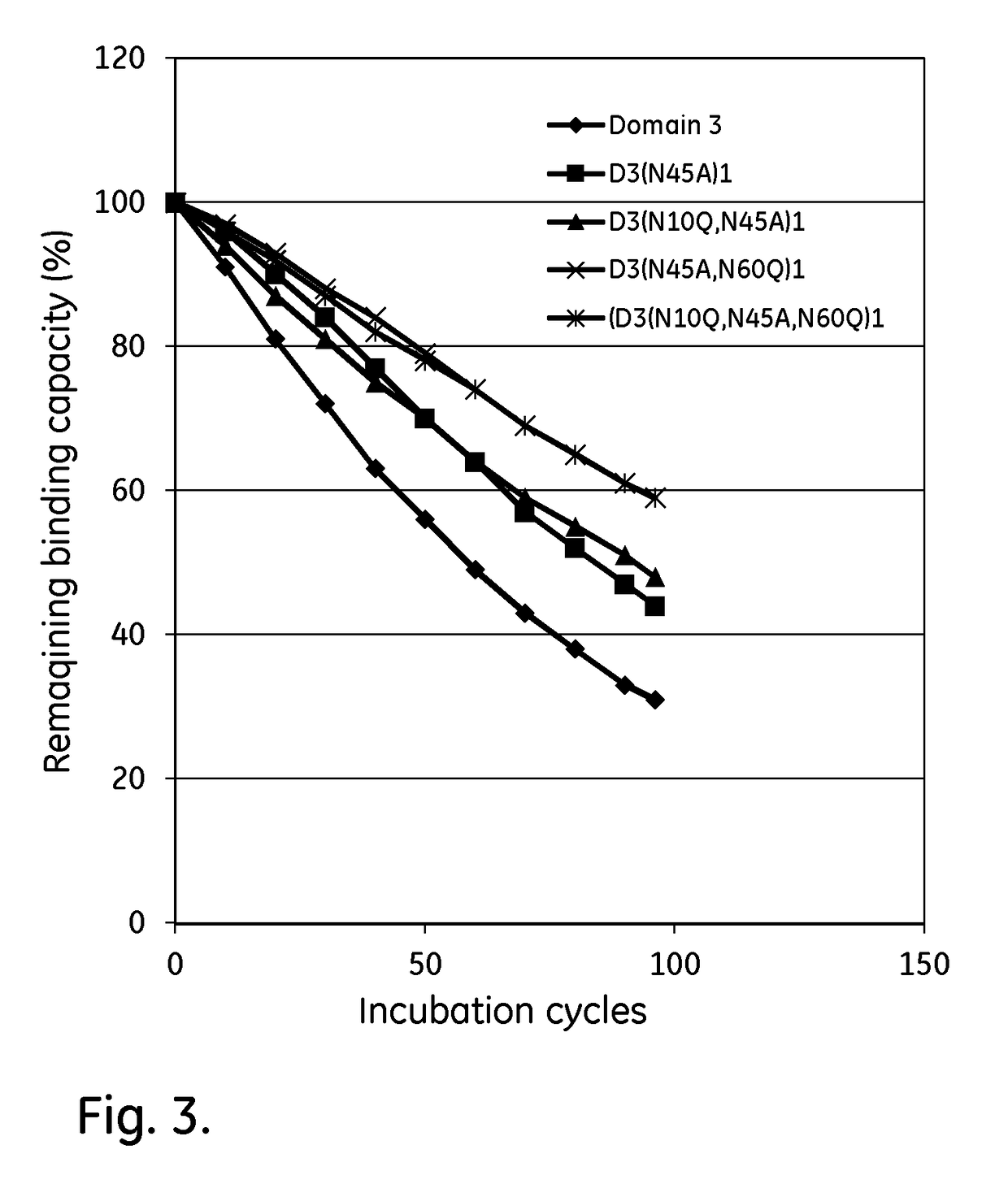
The purpose of the present invention is to provide: a mutant immunoglobulin kappa chain variable region-binding peptide characterized by the acid dissociation pH of an immunoglobulin kappa chain variable region-containing protein being shifted to the neutral side compared to prior to the introduction of a mutation; an affinity separation matrix comprising the peptide as a ligand; and a method forproducing a kappa chain variable region-containing protein using the affinity separation matrix. Another purpose of the present invention is to provide DNA that encodes the peptide, a vector containing the DNA, and a transformant that is transformed by the vector. The problem addressed by the present invention is solved by using a peptide into which a mutation has been introduced at an appropriatesite as an affinity ligand for a kappa chain variable region binding domain of protein L derived from a Peptostreptococcus magnus strain.
Owner:KANEKA CORP
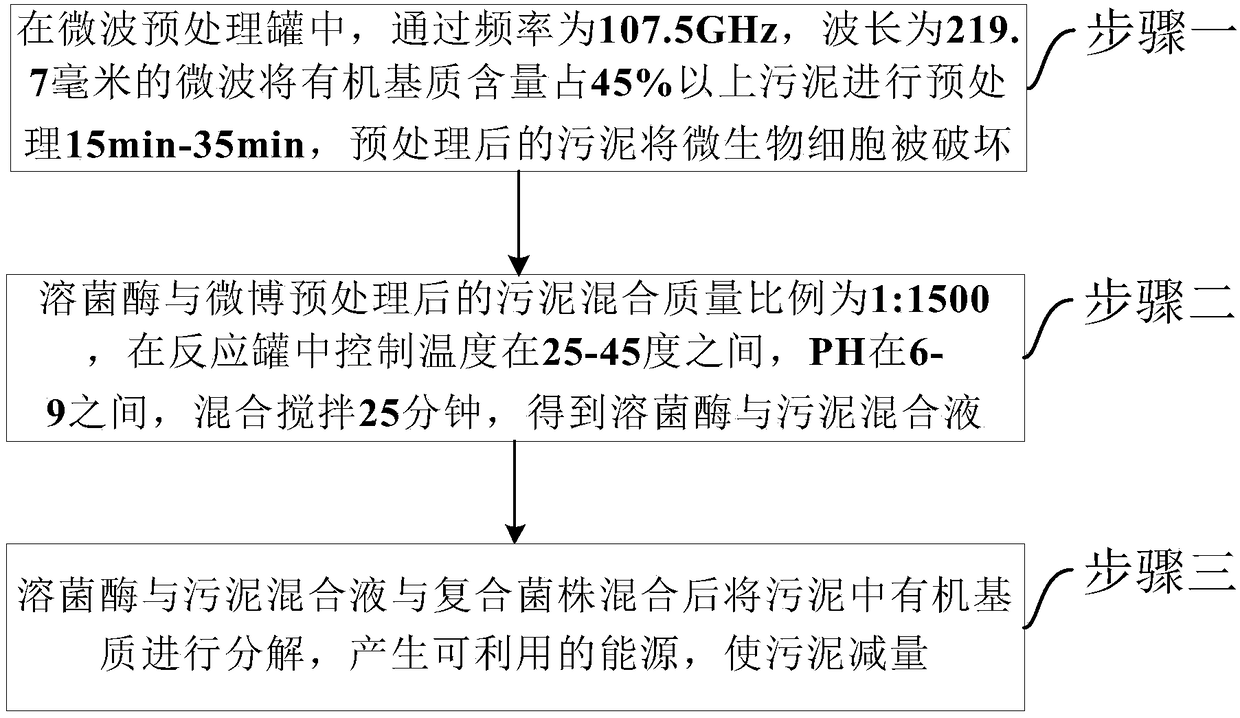
Sludge decrement method by combining microwaves, biological enzyme and microorganisms
ActiveCN108439744ASolve efficiency problemsSolve the investment costSludge treatmentSpecific water treatment objectivesPeptostreptococcusMethanosarcina barkeri
The invention provides a sludge decrement method by combining microwaves, biological enzyme and microorganisms. The method comprises the steps that sludge of which the content of an organic medium is45% or above is pretreated for 15-35 min in a microwave pretreatment tank; the mixing mass ratio of lysozyme to the sludge after microwave pretreatment is 1:1,500, and a mixed solution of lysozyme andthe sludge is obtained in a reaction tank; after the mixed solution of lysozyme and the sludge is mixed with composite bacterial strains, the organic medium in the sludge is decomposed, available energy is generated, the sludge amount is decreased, and the composite bacterial strains are peptostreptococcus, veillonella, propionibacteria, fire source methanococcus lactobacillus, methanosarcina barkeri, extreme thermophilic archaea and anaerobic ammonium oxidation bacteria. According to the sludge decrement method, the microwaves, special-effect enzyme and microorganisms are adopted for completely decreasing the amount of organic medium sludge generated by a biochemical system, and the sludge is converted into the available energy through a technology; the method has the ideal decrement effect on biochemical sludge generated in the process of treating municipal wastewater and industrial wastewater.
Owner:北京赛富威环境工程技术有限公司
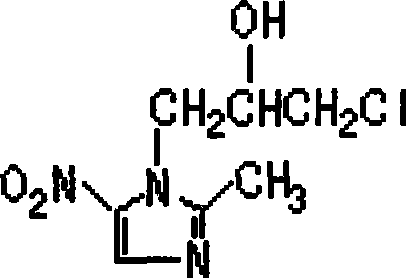
Preparation method of ornidazole gastric floating tablets and use thereof
InactiveCN101455664AImprove stabilityQuality improvementAntibacterial agentsOrganic active ingredientsMyometritisBrain infection
The invention discloses a preparation method and applications of gastric floating tablets containing ornidazole, belonging to a novel technical field of medicament and relating to a novel medicament form of ornidazole. 1. The gastric floating tablets are used to treat a plurality of diseases caused by bacteroides fragilis, bacteroides distasonis, bacteroides oval window, etc.; (2) oral cavity infections: pericoronitis, periapical periodontitis, pericoronitis, acute ulcerouse gingivitis etc.; (3) gynae infections: endometritis, myometritis, oviduct or ovarian abscess, pelvic cavity soft tissue infection, haemophilis vaginitis etc.; (4) surgical infections: wound infection, abscess of epidermis, bedsore ulcerouse infection, cellulites, gas gangrene etc.; (5) brain infections: meningitis, brain abscess; (6) septicaemia and severe general infections etc. 2. The gastric floating tablets are used to prevent infections before an operation and treat anaerobic infections after an operation. 3. The gastric floating tablets are used to treat urogenital canal trichomonas and giardia lamblia infections, such as trichomoniasis etc. 4. The gastric floating tablets are used to treat alimentary system amebiasis, such as amebic dysentery, amoebic liver abscess etc. The new medicament form is characterized in that the surface thereof is hydrated into gel after contacting with gastric juice at body temperature so as to cause volume expansion because the gastric floating tablets contain a plurality of hydrophilic macromolecule materials. Weight of the tablets is less than floating force of the gastric juice, so the tablets float on the gastric juice so as to prolong time in a stomach.
Owner:BEIJING HOPE HUGE PHARM SCI
Application of intestinal flora in nerve injury detection
ActiveCN112708686AEasy to operateStrong specificityMicrobiological testing/measurementMicroorganism based processesGut floraIntestino-intestinal
The invention relates to an application of intestinal flora in nerve injury detection. The intestinal flora is pseudomonas aeruginosa, veillonella and streptococcus digestive. According to the invention, the horizontal change of the specific flora in nerve injury is firstly discovered, the nerve injury diagnosis can be achieved by detecting the change of the specific flora, and with the application of the microbial marker to diagnose, the operation is simple, the specificity is high, and the sensitivity is strong.
Owner:温州医科大学慈溪生物医药研究院
Sustained-release injection containing antibiotic and application thereof
InactiveCN101283970AIncrease concentrationReduce concentrationAntibacterial agentsSolution deliveryPeptostreptococcusBacillus acnes
The invention provides a sustained-release injection or sustained-release implant containing antibacterial drugs. The sustained-release injection comprises sustained-release microspheres and solvent. The microspheres contain sustained-release adjuvants and antibiotics, and the solvent is a special solvent containing suspending agent such as sodium carboxymethylcellulose and having viscosity of 100-3000cp (20-30 DEG C); and the sustained-release adjuvants are selected from EVAc, polifeprosan, poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), sebacic acid copolymer, albumin glue and gelatin. The sustained-release implant is made from microspheres or by other methods. The sustained-release implant or injection can be locally placed or injected into foci to locally sustained-release of drug for more than 10 days, so as to obtain and maintain local drug effective concentration while remarkably reduce the systemic toxicity of the drug. The sustained-release injection has distinct and unique therapeutic effect on local infection diseases caused by Staphylococci, Streptococci, Peptostreptococcus, Propionibacterium acnes, Enterobacter, Mycobacterium tuberculosis, Gonococcus or Meningococcus, such as chronic osteomyelitis, severe decubital ulcer, refractory skin ulcer, diabetic foot, femoral head necrosis, abscess, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Sustained-release injection containing antibiotic
InactiveCN101283981AIncrease concentrationReduce concentrationAntibacterial agentsTetracycline active ingredientsPeptostreptococcusBacillus acnes
The invention provides a sustained-release injection or sustained-release implant containing antibiotics. The sustained-release injection comprises sustained-release microspheres and solvent. The microspheres contain sustained-release adjuvants and antibiotics, and the solvent is a special solvent containing suspending agent such as sodium carboxymethylcellulose and having viscosity of 100-3000cp (20-30 DEG C); and the sustained-release adjuvants are selected from poly(ethylene-co-vinylacetate) (EVAc), polifeprosan, poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), sebacic acid copolymer, albumin glue and gelatin. The sustained-release implant is made from microspheres or by other methods. The sustained-release implant or injection can be locally placed or injected into foci to locally sustained-release the drug for more than 10 days, so as to obtain and maintain local drug effective concentration while remarkably reduce the systemic toxicity of the drug. The sustained-release injection has distinct and unique therapeutic effect on local infection diseases caused by Staphylococci, Streptococci, Peptostreptococcus, Propionibacterium acnes, Enterobacter, Mycobacterium tuberculosis, Gonococcus or Meningococcus, such as chronic osteomyelitis, severe decubital ulcer, refractory skin ulcer, diabetic foot, femoral head necrosis and abscess.
Owner:JINAN SHUAIHUA PHARMA TECH
Bacterium with increased tolerance to butyric acids
Owner:LANZATECH NEW ZEALAND LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com