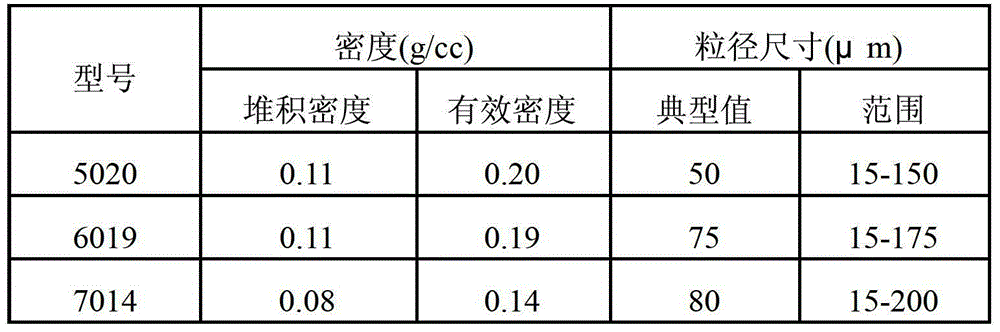
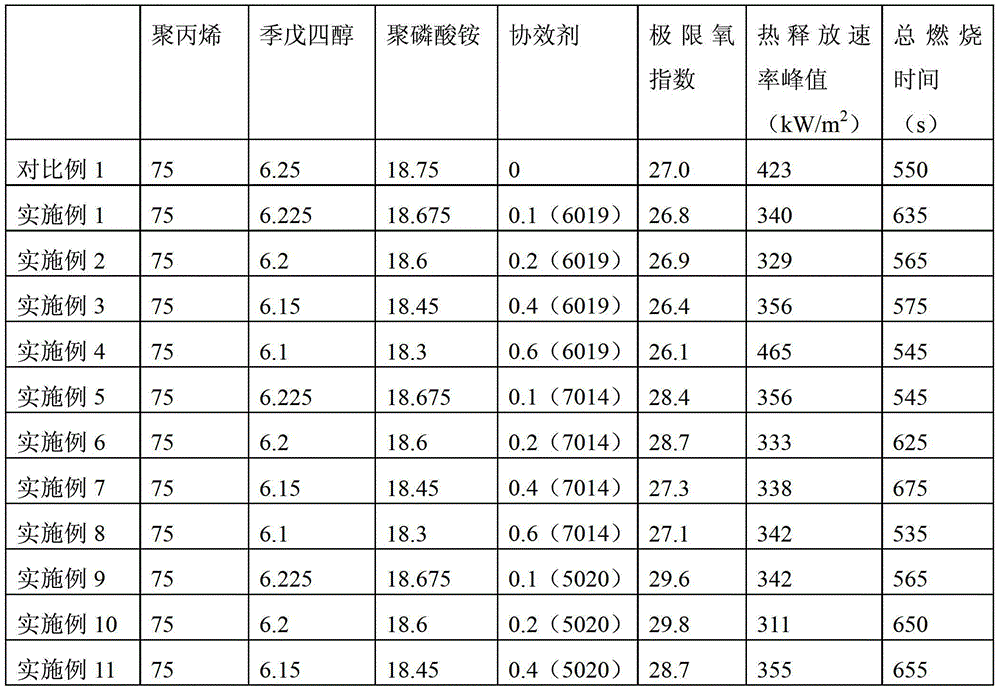
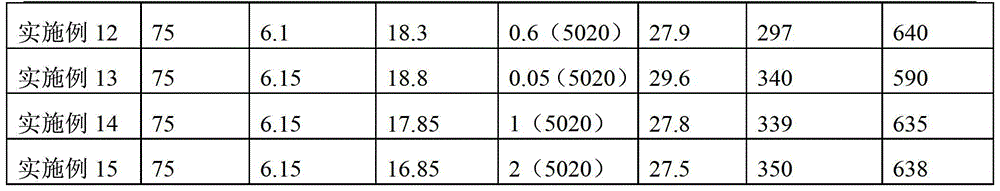
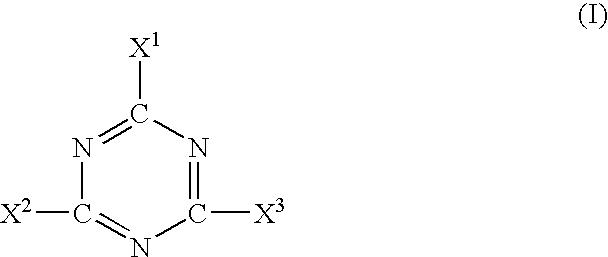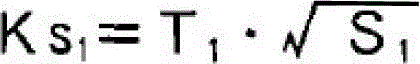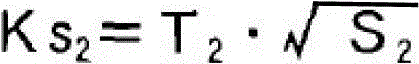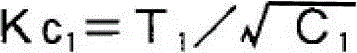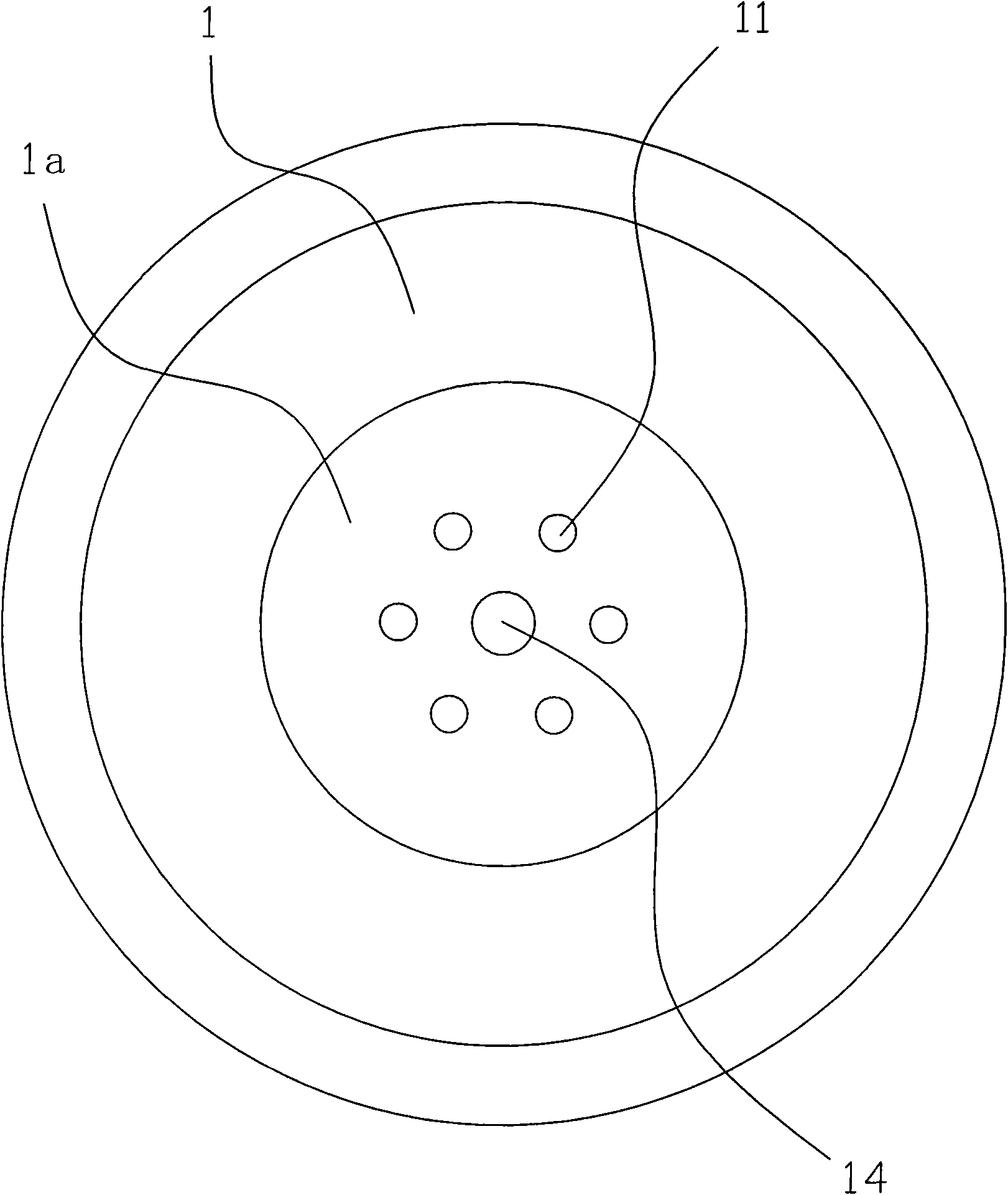Patents
Literature
59 results about "Flame test" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A flame test is an analytic procedure used in chemistry to detect the presence of certain elements, primarily metal ions, based on each element's characteristic emission spectrum. The color of flames in general also depends on temperature; see flame color.
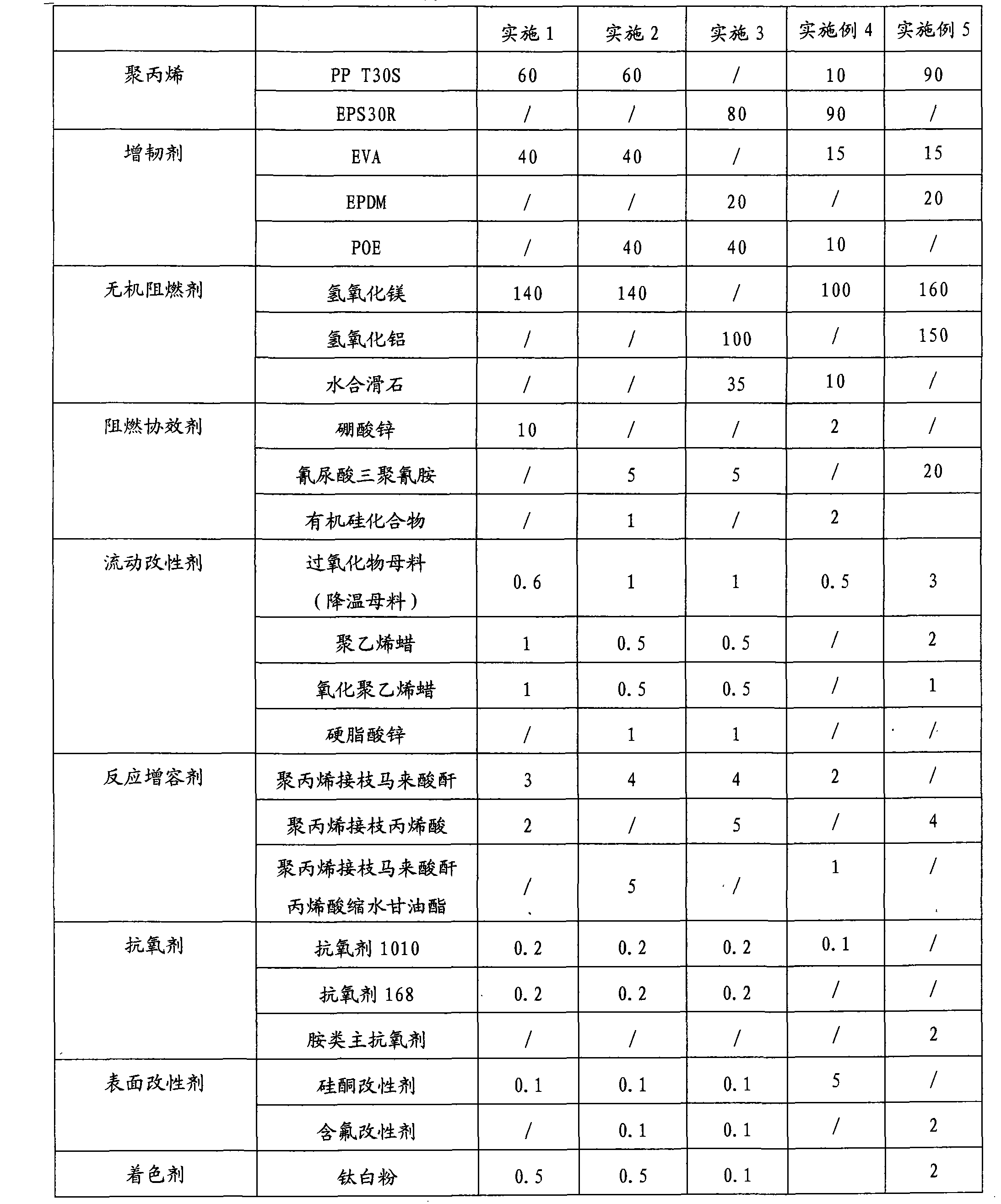
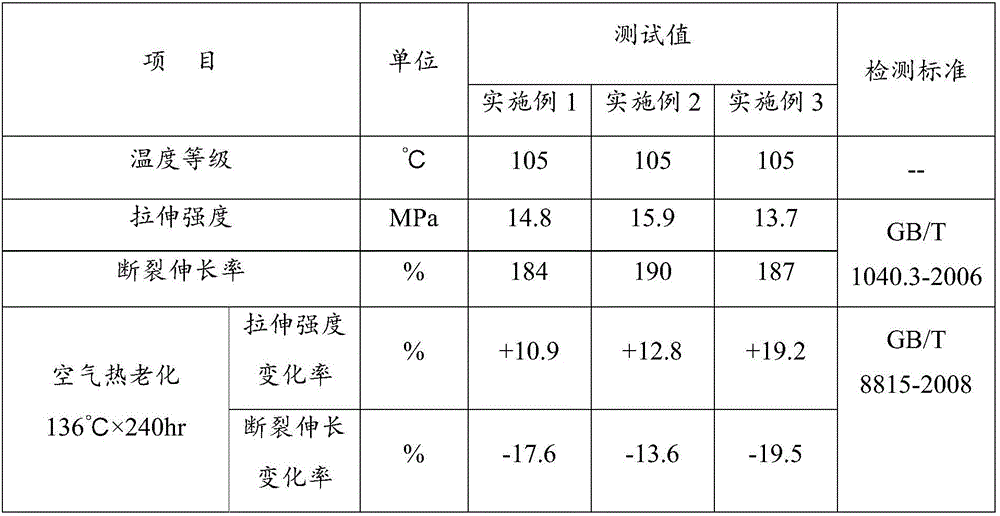
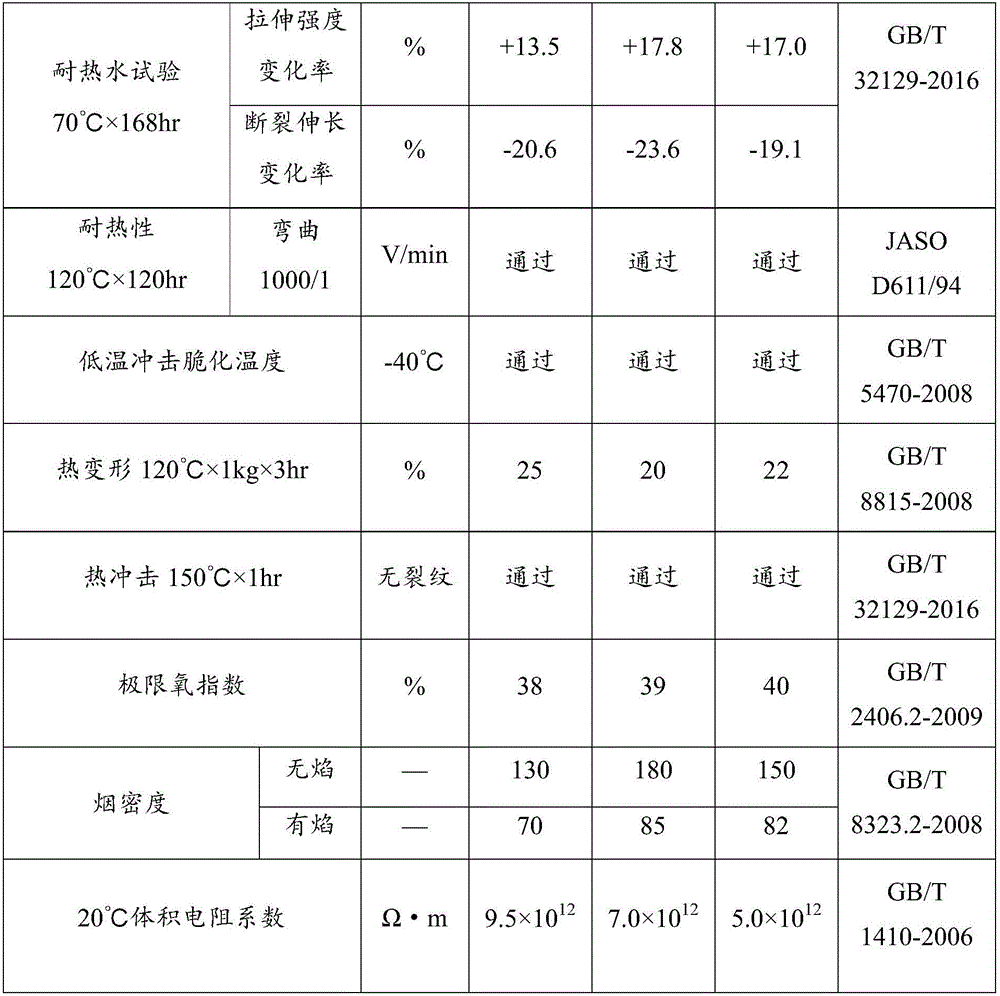
High tenacity low smoke zero halogen flame retardant polypropylene and preparation method thereof
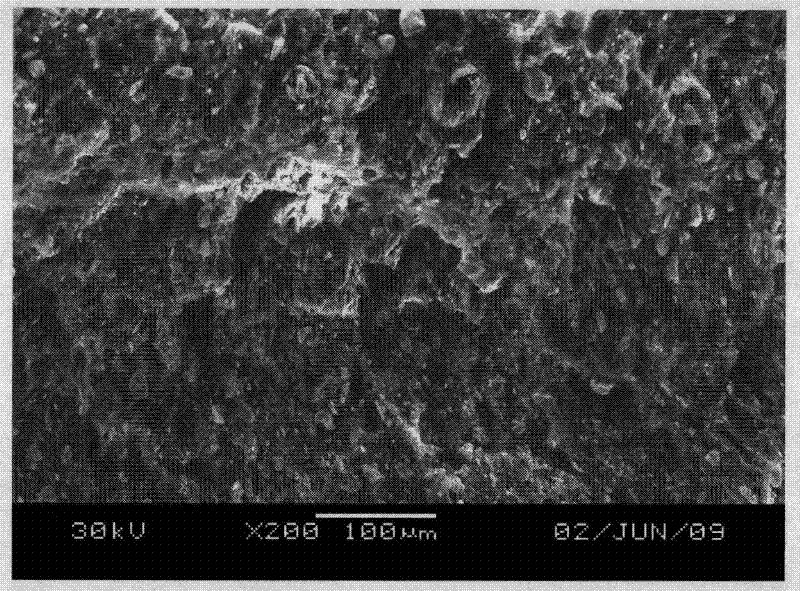
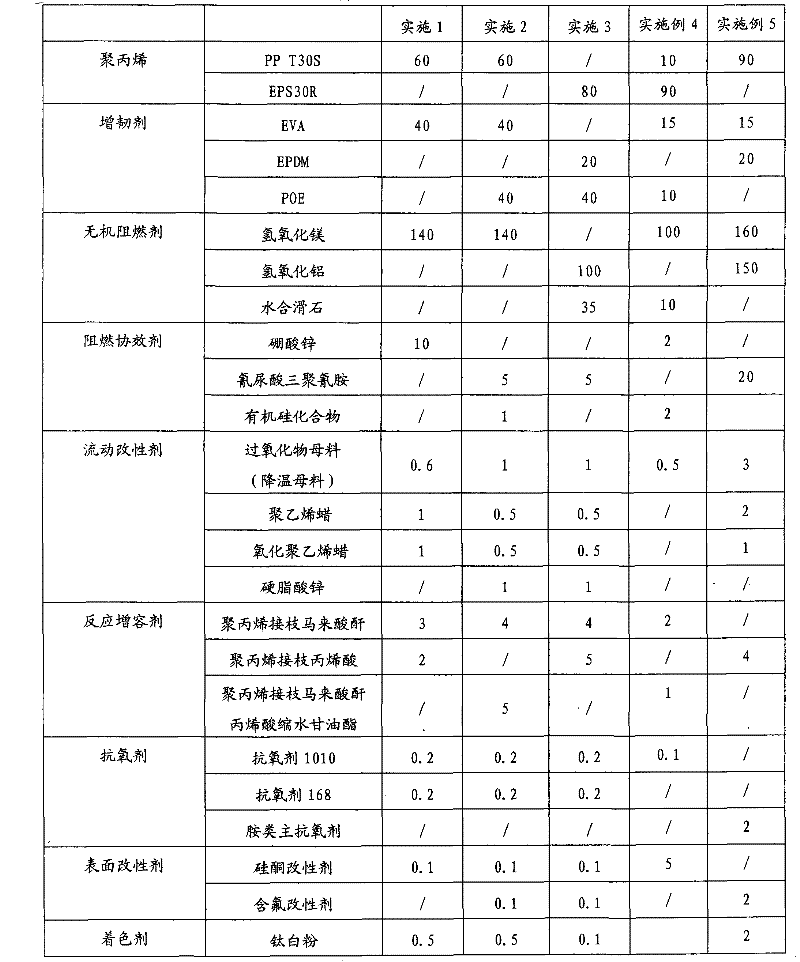
The invention discloses a high tenacity low smoke zero halogen flame retardant polypropylene and a preparation method thereof and the polypropylene comprises the following components according to the parts by weight; 10-90 parts of polypropylene, 10-90 parts of flexibilizer, 100-160 parts of inorganic flame retardant, 2-20 parts of flame-retardant synergist, 2-5 parts of reactive compatibilizer, 0.1-2 parts of antioxidant, 0.5-5 parts of flow modifier, 0.1-5 parts of surface modifier and a defined amount of coloring agent. The low smoke zero halogen flame retardant polypropylene has the advantage of good mechanical properties, good flame resistance (the vertical flame test of the polypropylene passes UL94V-0 level), low smoke density which is not more than 20%, good aging resistance and low cost, and can meet the processing demands for injection and extrusion molding.
Owner:内蒙古睿达鑫科技有限责任公司
Fire resistant mattress
A fire resistant mattress includes a fastener that allows a mattress cover to be opened to expose a mattress core having at least one combustible component. The fastener is preferably a slide fastener or a hook and pile fastener. A flap of fire resistant material extends downwardly from the top surface of the mattress cover and covers the fastener during normal use to protect it against an open flame. The mattress meets the open flame test requirements of Technical Bulletin 603 of the California Department of Consumer Affairs.
Owner:BOYD DENNIS
Flame resistant matelasse fabrics utilizing spun and filament flame resistant yarns
InactiveUS20070004302A1Efficient use ofReduce flammabilityWeft knittingDomestic upholsteryConus californicusUpholstered furniture
The invention relates to the use of a flame resistant (FR) three-layer double-knit fabric, also know as a matelasse fabric. The top layer is of standard non-FR face yarn, the middle layer is of a FR filler spun yarn and the bottom layer is of a FR spun yarn or FR filament yarn. This FR matelasse fabric can be used to protect a mattress, foundation, upholstery cushion, pillow, office panel, transportation seat or any other article requiring FR protection. In this invention, a matelasse fabric is formed by circular double knitting a FR spun or FR filament yarn into the bottom portion of the fabric, utilizing a heavy cotton count FR filler spun yarn for the middle layer and using conventional non-FR yarns for the top layer. The invention has particular applicability in the formation of FR mattresses and foundations that require passage of large open flame tests such as CPSC's 16 CFR Part 1633, California's Test Bulletin 603 and Test Bulletin 129 and in the formation of FR upholstered furniture that requires passage of California's Test Bulletin 133 or British Standard 5852 using the crib 5 ignition source or higher.
Owner:MCKINNON LAND
Chemical protective garment with added flash fire protection
ActiveUS20090255039A1High protection against chemicalHigh mechanical strengthChemical protectionHeat protectionFiberPersonal protective equipment
Owner:ANSELL PROTECTIVE SOLUTIONS
Compound intumescent flame retardant (IFR) and application thereof to carrying out flame retardance on polypropylene (PP) and polyurethane (PU)
InactiveCN103333364AOvercome the disadvantages of large addition amount and low flame retardant efficiencyReduce peak heat release rateThermoplasticCarbon layer
The invention relates to a compound intumescent flame retardant (IFR) and an application thereof to carrying out flame retardance on polypropylene (PP) and polyurethane (PU) elastomers. The compound IFR comprises hollow glass microspheres and IFRs. The vertical flame test level of the IFR PP and thermoplastic PU (TPU) elastomer composite material prepared by adopting the compound IFR is raised to V-0, and a dense intumescent carbon layer is formed on the surface of a sample of the IFR PP and TPU elastomer composite material, so that the IFR PP and TPU elastomer composite material has a good flame retardant effect. By using the hollow glass microspheres and the IFRs to carry out synergistic flame retardance on the PP and TPU elastomers, not only is the oxygen index of the composite material increased and the vertical flammability reflecting melting and dropping resistance obviously improved, can the heat release rate be well reduced and are obvious smoke suppression effects achieved, but also the viscosity of the polymer melts can be obviously reduced and the processability can be improved.
Owner:QINGDAO UNIV OF SCI & TECH
Resin Composition And Molding Thereof
A resin composition comprising 100 parts by mass of lactic acid resin (A), 50 to 100 parts by mass of metal hydroxide (B) and 0.1 to 30 parts by mass of fiber (C), wherein the fiber (C) is a natural fiber or / and a fiber derived from natural material. Moldings thereof satisfy the V-0 standard or VTM-0 standard according to UL94 vertical flame test (UL94V, UL94VTM).
Owner:SONY CORP +1
Flame-resistant, high visibility, Anti-static fabric and apparel formed therefrom
InactiveUS20140041107A1High tensile strengthImprove energy absorptionChemical protectionHeat protectionFiberYarn
A fabric for use in safety apparel comprising a first set of yarns comprising at least 60 percent modacrylic fibers, and a second set of yarns comprising at least some anti-static carbon filaments. The fabric meets at least the high visibility standard ANSI / ISEA-107-2010; the vertical flame test ASTM 1506-10w; and the Federal Test Method Standard 191A, Method 5931 for electrostatic decay, and the Electrostatic Discharge Association Advisory ADV11.2-1995 voltage potential.
Owner:GLEN RAVEN INC
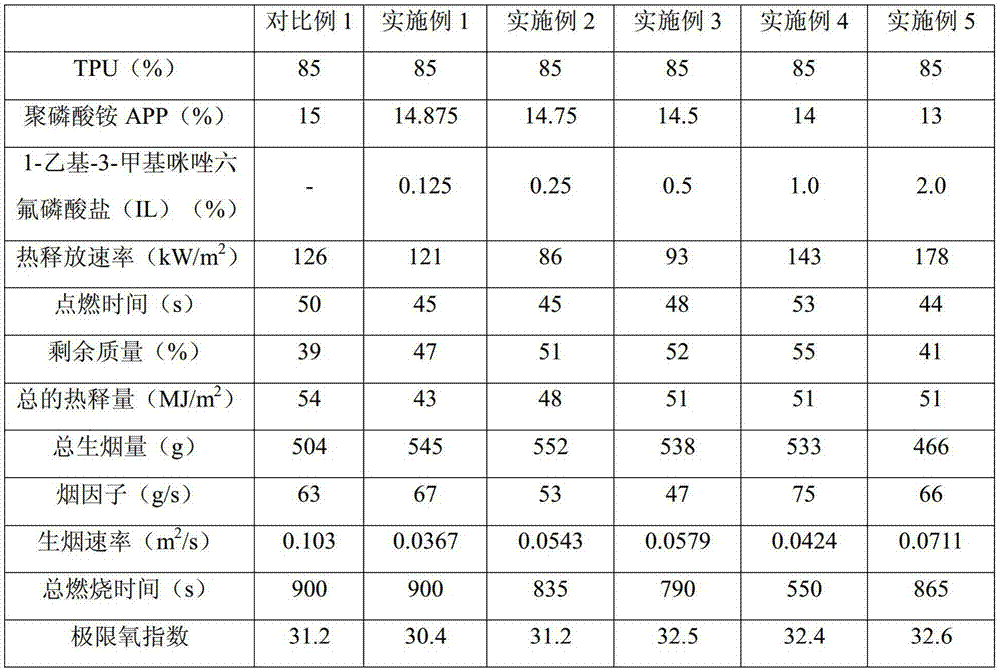
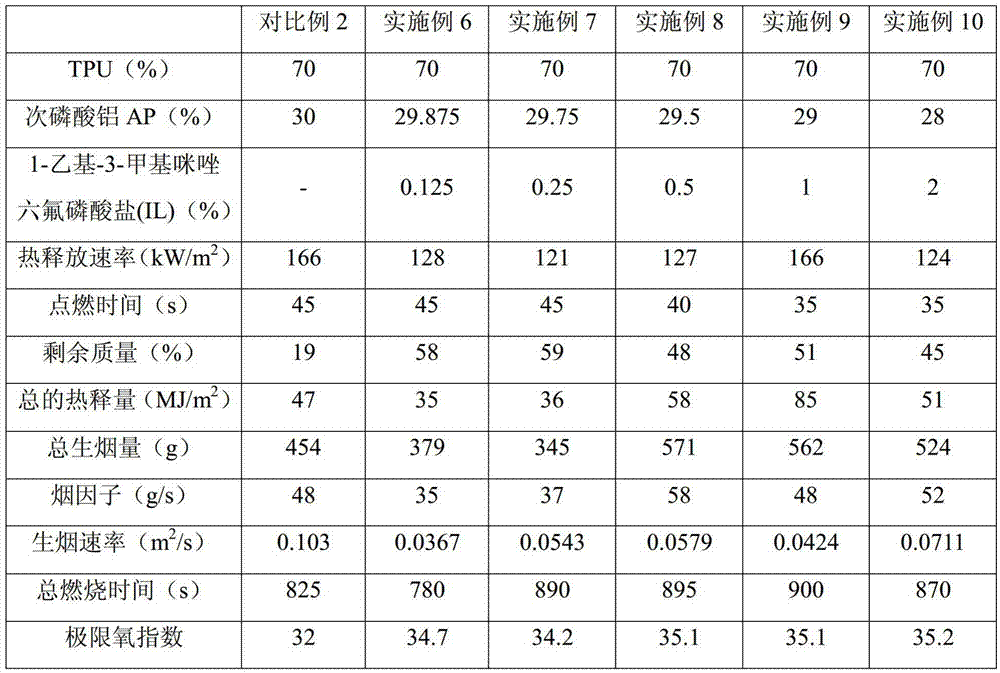
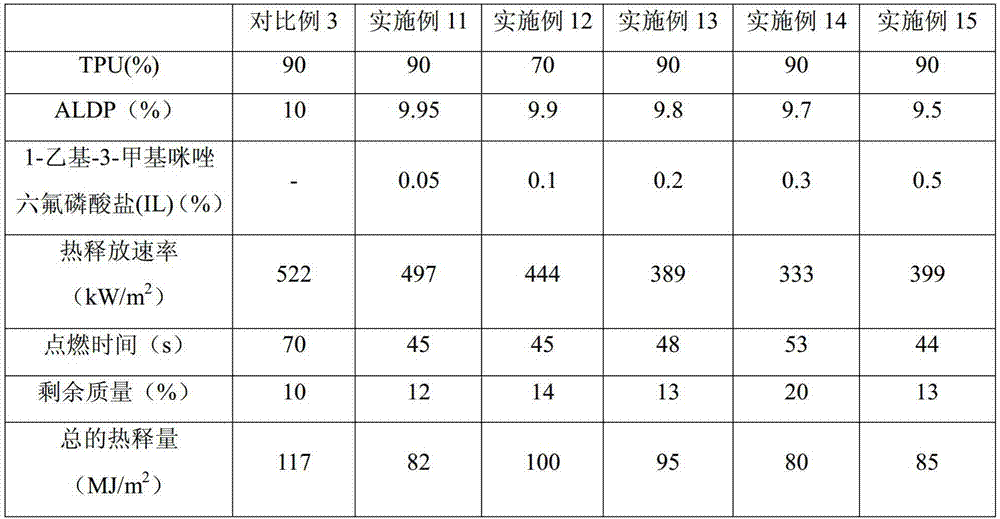
Flame retardant thermoplastic polyurethane elastomer (TPU) and preparation method thereof
ActiveCN105440652AHigh oxygen indexIncreased vertical burn test levelThermoplastic polyurethaneOxygen
The invention relates to a flame retardant thermoplastic polyurethane elastomer (TPU) and a preparation method thereof. The flame retardant TPU comprises the following components in parts by mass: 70-90 parts of TPU, 9.7-29.875 parts of intumescent flame retardants and 0.025-3.75 parts of ionic liquids. The intumescent flame retardant TPU composite material is prepared by adopting the composite intumescent flame retardant. The vertical flame test level of the composite material is increased to V-0. A dense intumescent char layer is formed on the specimen surface of the composite material, so that the composite material has good flame retardant effects. Under the condition of small additive amount of the ionic liquids, the oxygen index of the ionic liquid and intumescent flame retardant synergistic flame retardant TPU composite material is increased, so that the vertical flame performance embodying molten drop resistance is obviously improved, the heat release rate and total heat release can be well reduced, the charring performance during flaming is improved and the composite material has certain effects on smoke suppression.
Owner:盛鼎高新材料有限公司
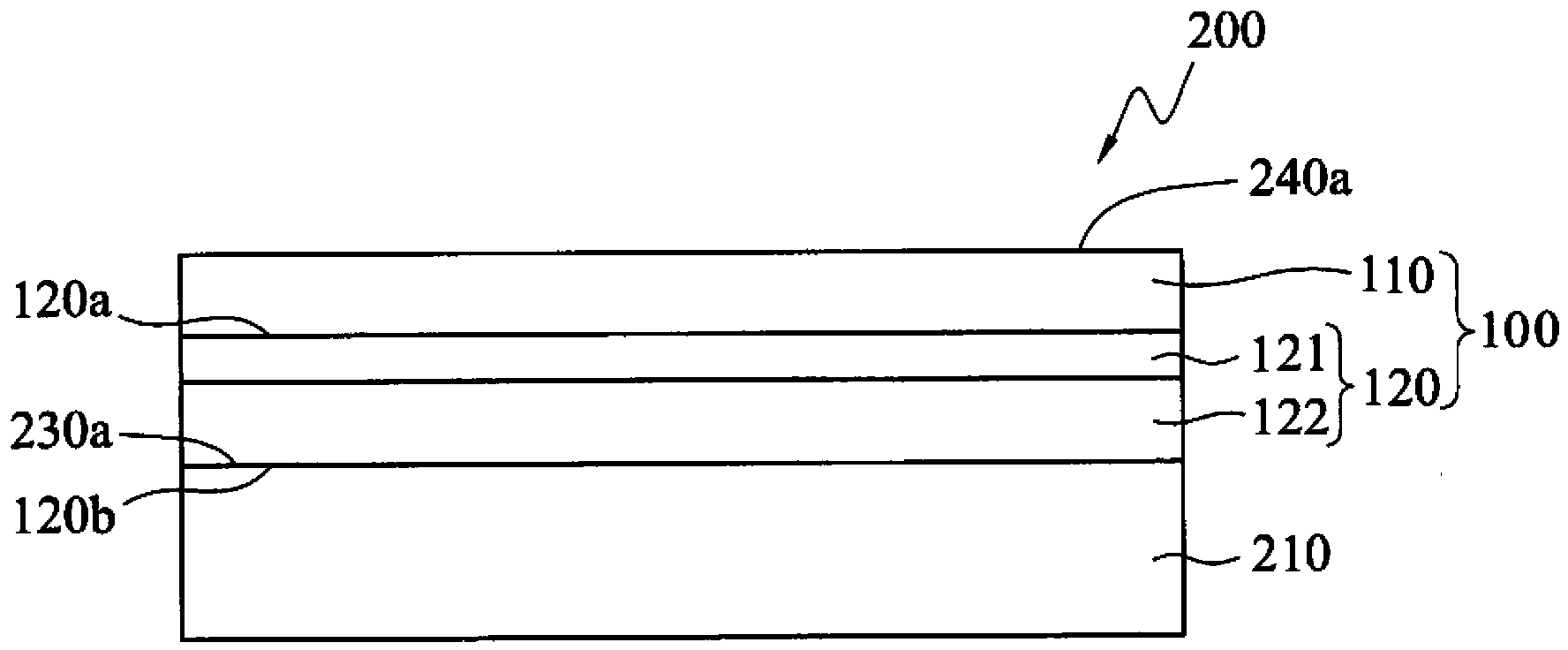
Electromagnetic wave shielding structure and flexible printed circuit board with same
InactiveCN103687459AImprove thermal stabilityImprove insulation performancePrinted circuit detailsMagnetic/electric field screeningComposite filmElectromagnetic electron wave
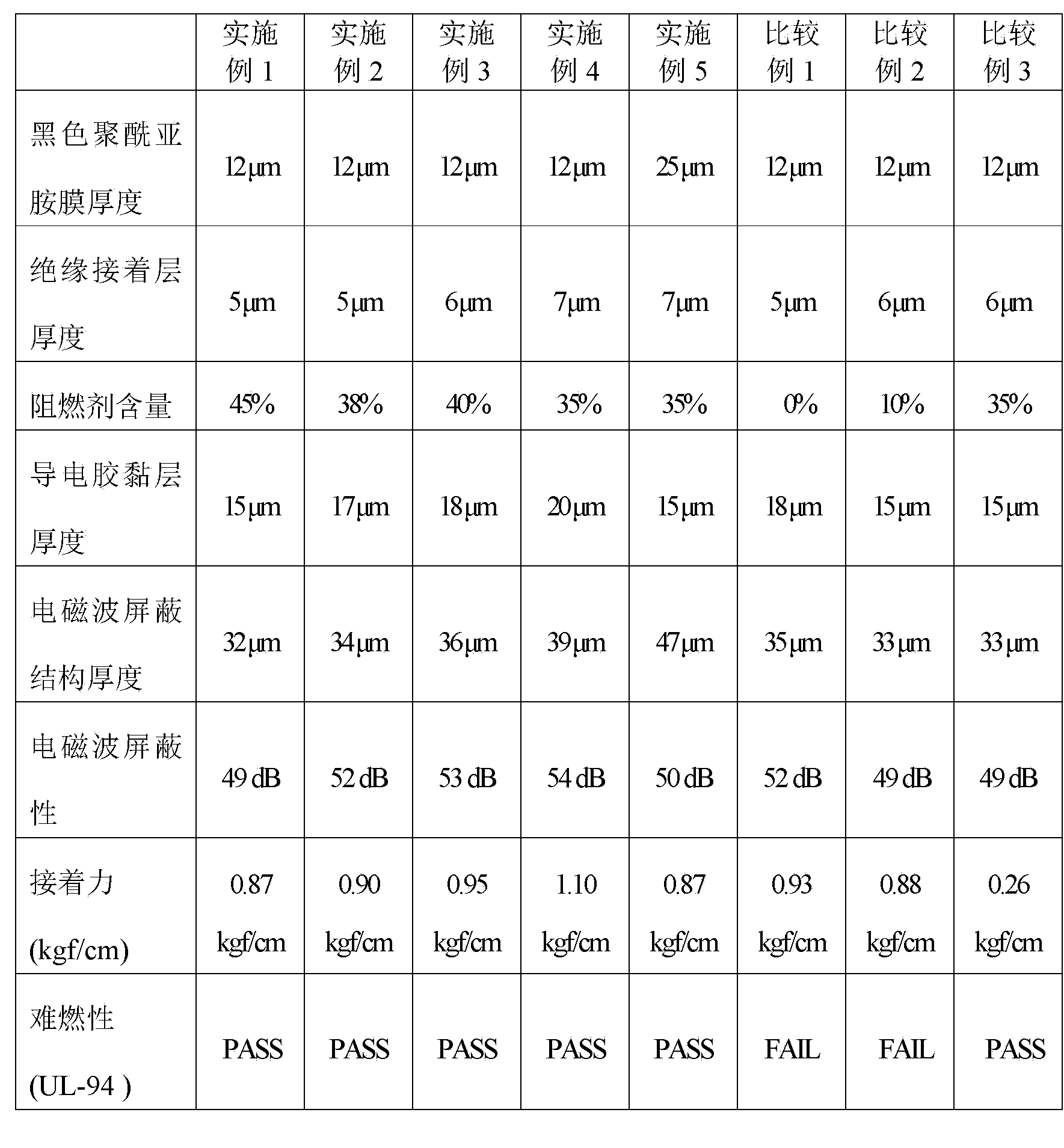
The invention discloses an electromagnetic wave shielding structure and a flexible printed circuit board with same. The electromagnetic wave shielding structure comprises a conducting composite film and a black polyimide film, wherein the conducting composite film comprises an insulating bonding layer and a conducting adhesive layer which are mutually overlapped. Compared with common thermoplastic industrial plastic, the black polyimide film not only has the characteristics of high thermostability, excellent insulating property, mechanical strength and resistance to chemical corrosion and the like, but also has non-bright anti-rotation property, so that circuit patterns are effectively prevented from being plagiarized by the same industry, moreover, as the conducting composite film comprises the insulating bonding layer and the conducting adhesive layer, and the conducting adhesive layer contains fire retardant, fire retardant is not required to be added to the conducting adhesive layer, the effects of optimal UL94 vertical flame test and VTM-0 flame retardant characteristic specification requirements can be achieved, and the flexible printed circuit board with the electromagnetic wave shielding structure can have optimal fire resistance.
Owner:KUSN APLUS TEC CORP
Flame resistant matelasse fabrics
The invention relates to the use of a flame resistant three-layer double-knit or woven fabric, also know as a matelasse fabric. The top layer is of standard non-flame resistant face yarn, the middle layer is of flame resistant filler yarn and the bottom layer is of flame resistant core wrap spun yarn. This flame resistant matelasse fabric can be used to protect a mattress, foundation, mattress pad, pillow, comforter, upholstery cushion, pillow, office panel, transportation seat or any other article requiring flame resistant protection. In this invention, a matelasse fabric is formed by circular double knitting or weaving a flame resistant core spun yarn into the bottom portion of the fabric, utilizing a heavy cotton count flame resistant filler yarns for the middle layer and using conventional non-flame resistant yarns for the top layer. The invention has particular applicability in the formation of flame resistant mattresses and foundations that require passage of large open flame tests such as CPSC's 16 CFR part 1633, California's Test Bulletin 603 and Test Bulletin 129 and in the formation of flame resistant upholstered furniture that requires passage of California's Test Bulletin 133 or British Standard 5852 using the crib 5 ignition source or higher.
Owner:MCKINNON LAND
Experimental system of coal mine gas explosion blocking device
InactiveCN106198086AVerify validityFind design flawsStructural/machines measurementGas cylinderData acquisition
The invention relates to an experimental system of a coal mine gas explosion blocking device. The experimental system comprises a gas accumulation device, an explosion ignition device, sensors, a data acquisition device, experimental pipelines and a gas explosion secondary disaster blocking device; testing gas is supplied by a gas cylinder, the gas accumulation quantity is controlled by a gas flowmeter and a gas valve, a partition plate partitions a gas accumulation pipeline and the experimental pipelines, and after the gas concentration reaches the experimental requirement, the gas valve is switched off; gas in the gas accumulation device is detonated by an electric spark generator to simulate gas explosion under a coal mine well; the sensors are installed at the two ends of the experimental pipeline of the gas explosion secondary disaster blocking device, temperature, pressure and flame test data simulating gas explosion is acquired by the data acquisition device, stored in a computer and analyzed by software; the experimental pipelines are made from tempered glass and comprise the experimental pipeline of the blocking device and the experimental pipeline of a monitoring segment. The experimental system can verify the validity of the gas explosion blocking device and show the defects existing in gas explosion blocking device design.
Owner:ANHUI UNIV OF SCI & TECH
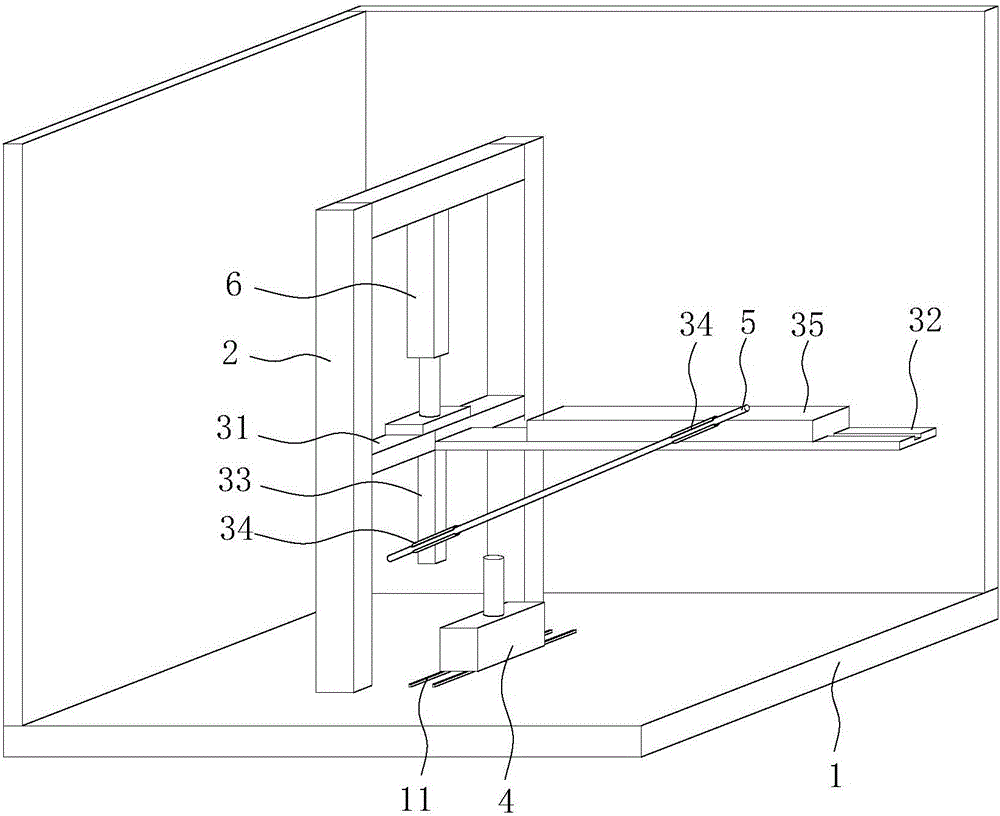
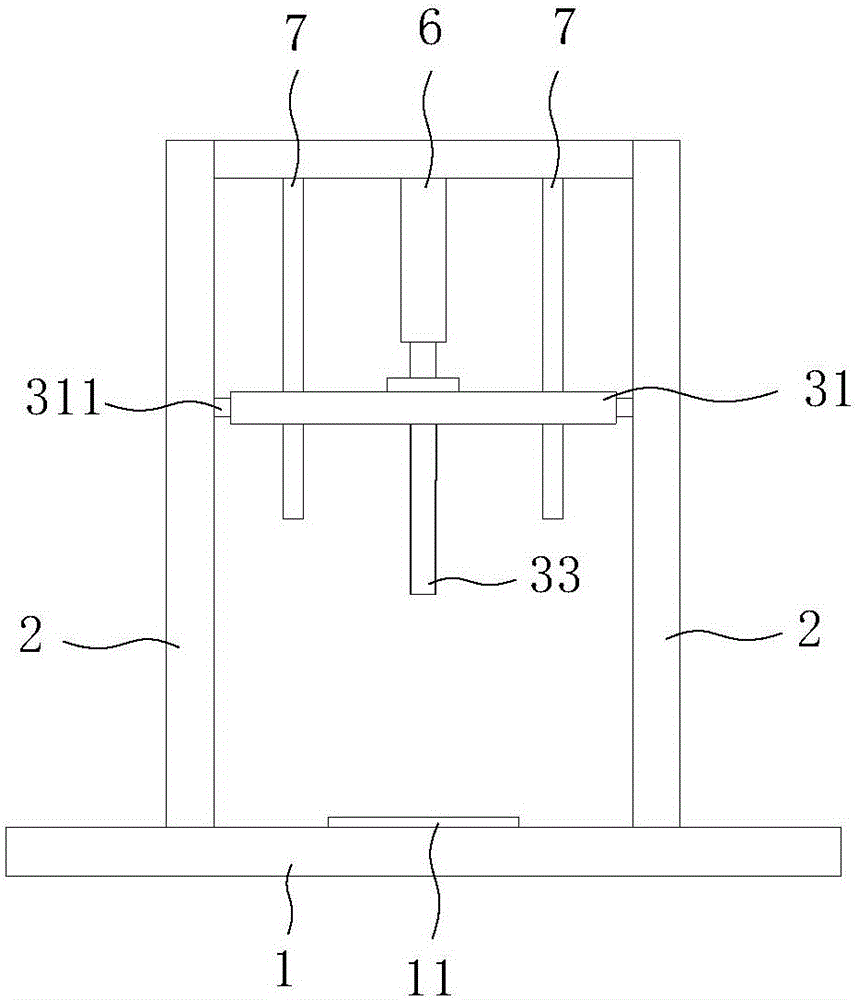
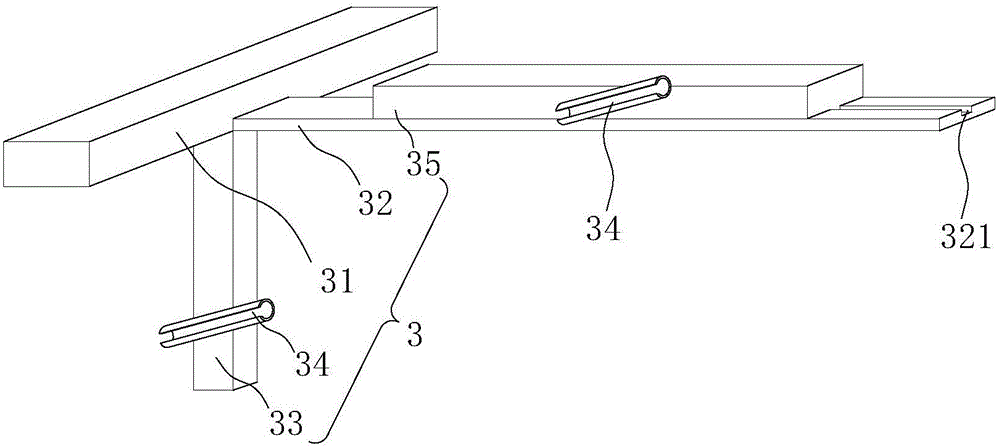
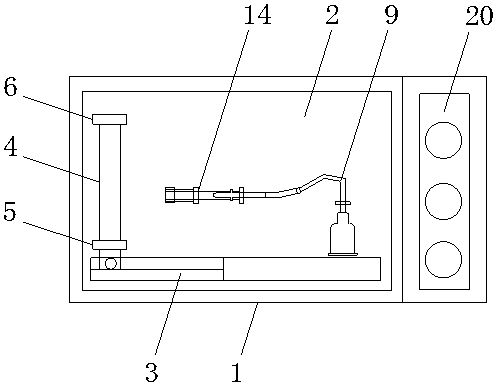
Slanting specimen flame test device for cable
InactiveCN105929105ACombustion test realizedSimple structureChemical analysis using combustionCombustorOblique angle
The invention discloses an slanting specimen flame test device for a cable. The slanting specimen flame test device for the cable comprises a box body, a bracket, a fixing device and a combustor, wherein the bracket is arranged in the box body; the fixing device is arranged on the inner side of the bracket and is used for obliquely fixing the cable; the combustor is arranged below the fixing device; the fixing device comprises a connecting rod horizontally connected to the bracket, a transverse rod which is perpendicularly connected with the side surface of the connecting rod and is further horizontally arranged, a regulating rod which can horizontally slide along the transverse rod, and a longitudinal rod which is perpendicularly connected with the bottom surface of the connecting rod and is further vertically arranged; during a test, one end of the cable is connected to the regulating rod, and the other end of the cable is connected to the longitudinal rod. By using the test device, combustion tests, at different oblique angles, on the cable can be realized; moreover, test results are reliable.
Owner:DEKAI INTEGRATED KUNSHAN DETECTION CO LTD
Flame retardant experimental facility for textile flame retardant fabric
The invention discloses a flame retardant experimental facility for a textile flame retardant fabric. The flame retardant experimental facility for the textile flame retardant fabric comprises a combustion testing box, wherein a base is arranged at the bottom end of the inside of the combustion testing box; a vertical placement rack is arranged on one side of the upper end of the base; a first clamping rod is fixedly mounted at the lower end of the placement rack; a second clamping rod is slidably connected to the upper end of the placement rack; a fixing nut is arranged on the other side, which is close to the second clamping rod, of the placement rack; a flame retardant fabric combustion time sensor is arranged on the second clamping rod; an ignition device is arranged on the other sideof the upper end of the base; a combustion device is arranged at the other end of the ignition device; and a flame temperature sensor is arranged on the inner wall of a combustion tube. By arrangementof the combustion testing box, the base, the placement rack, the ignition device, the combustion device, the flame temperature sensor and the flame retardant fabric combustion time sensor, the problem that the traditional flame retardant experimental facility is inaccurate in experiment, complicated in operation and inconvenient to use is solved.
Owner:新乡市新科防护科技有限公司
Plastic for low-smoke halogen-free high flame-retardant electric wire and cable
ActiveCN101328294AGood compatibilityImprove mechanical propertiesInsulated cablesInsulated conductorsEngineeringMechanical property
The invention provides plastics for low smoke halogen-free highly fire-retardant wire cables. The raw material formula of the plastics in weight portion is as follows: 50 to 75 portions of polyolefine, 25 to 50 portions of maleic anhydride grafting olefinic copolymer and 60 to 70 portions of humidity resistance phosphorus-nitrogen system expanding type fire retardant material. In the invention, the fire retardant performance, the mechanical and physical performance and the product hardness are comprehensively considered in the whole formula, the tensile strength is more than and equal to 13.8MPa and the extension rate is more than and equal to 300 percent; when the plastics is used for the production of the wire cables, the wire cables can pass the VW-1 vertical wire flame test, and after the wire cables are subject to the aging in an air oven with the temperature of 180 DEG C for 168h, the retention rates of the tensile strength and the elongation at break of the wire cables are more than or equal to 80 percent; when the wire cables are dipped in water at the room temperature for 7 days, at the temperature of 20 DEG C, the volume resistivity is less than 10 times reduced, therefore, the plastics is a macro molecular hybrid material for the highly fire-retardant environment-friendly polyolefine wire cables, which is good in mechanical property.
Owner:中广核三角洲(江苏)塑化有限公司 +1
Multipurpose gauge
ActiveCN104713452AEasy to operateQuick fixAngles/taper measurementsMechanical depth measurementsCombustorDistance detection
The invention provides a multipurpose gauge which comprises an electrode spacing detection part (11) used for detecting the distance between two electrodes in tracking test, a first distance detection part (31) used for detecting the distance between a nozzle end of a combustor and the bottom surface of a sample in needle flame test, a second distance detection part (32) used for detecting the distance between the nozzle end of the combustor and a side face of the sample in needle flame test, and a flame height detection part (21) used for detecting the height of flame in needle flame test. The multipurpose gauge provided by the invention is more convenient to operate.
Owner:GREE ELECTRIC APPLIANCES INC
Needle flame grade semitransparent PC (polycarbonate) material for anti-collision strip and preparation method thereof
The invention discloses a needle flame grade semitransparent PC (polycarbonate) material for an anti-collision strip and a preparation method thereof. The needle flame grade semitransparent PC material for the anti-collision strip consists of the following components in percentage by weight: 87-92% of polycarbonate, 8-12% of flame retardant, 0.3-0.5% of flame-retarding synergist and 0.2-0.4% of antioxidant, and the flame retardant is bromotriazine. The needle flame grade semitransparent PC material for the anti-collision strip, which is prepared by the preparation method, has excellent over-all properties, high strength, good ductility and high heat resistance; grains and a workpiece after injection molding are semitransparent; during a needle flame test, the PC material extinguishes once the PC material leaves fire; further, combusting cotton does not drip; and the PC material has quite high safety performance.
Owner:SHENZHEN KEJU NEW MATERIAL
Detection device used for production of lighters and use method of detection device used for production of lighters
InactiveCN112403930AImprove securityImprove pass rateDetection of fluid at leakage pointSortingBaseboardConveyor belt
The invention discloses a detection device used for production of lighters and a use method of the detection device used for production of the lighters. The detection device used for production of thelighters comprises a baseboard, a first conveyor belt, a second conveyor belt, a first air cylinder, a gas testing assembly, a flame testing assembly and a feeding assembly, wherein the first conveyor belt is fixedly connected with one side of the top of the baseboard, and an installation box is fixedly connected with the portion, located at one side of the first conveyor belt, of the top of thebaseboard. The detection device used for production of the lighters is compact in structure and simple and convenient to operate, the first conveyor belt, the second conveyor belt and the first air cylinder are used in cooperation, thus automatic loading can be achieved, reduction of the labor cost is facilitated, through the gas testing assembly, the produced lighters can be subjected to gas leakage detection, thus improvement of the storage safety of the lighters is facilitated, through the flame testing assembly, the lighters can be subjected to ignition detection, thus the lighters are picked, a qualified rate of the lighters is raised, improvement of use satisfaction is facilitated, and an original manual feeding mode can be replaced by the feeding assembly, so that improvement of theproduction efficiency of the lighters is facilitated.
Owner:湖南精专自动化技术有限公司
Fabric for protective clothing, and spun yarn for same
InactiveCN102884232AImprove heat resistanceImprove flame retardant performanceWoven fabricsYarnYarnPolyetherimide
This heat-resistant, flame-retardant fabric for protective clothing is constituted from a uniform blended spun yarn, and when the fabric contains 100% by mass of the spun yarn, the spun yarn includes 25-75% by mass of a polyetherimide fiber, 20-50% by mass of at least one fiber selected from wool and flame retardant rayon, and 5-25% by mass of a para-aramid fiber. When the fabric is exposed to a heat flux of 80 kW / m2+ / -5% for three seconds in accordance with the ISO 9151 Determination of Heat Transmission on Exposure to Flame test, there is no thermal shrinkage, and in a combustion test in accordance with JIS L 1091A-4, the char length in both the vertical and the horizontal direction is 10 cm or less. Consequently provided are a fabric for protective clothing, and a spun yarn for use therein, said protective clothing remaining comfortable even if the wearer perspires while working or wears the clothing in hot weather, having high heat resistance and flame retardancy, having good dyeability, and low production costs.
Owner:JAPAN WOOL TEXTILE +1
Non-halogen flame-retardant resin composition, and insulated electric wire and tube using the same
ActiveUS20130312998A1Increase in diameter of tubeIncrease the diameterRubber insulatorsWrappers shrinkageCross-linkElastomer
Provided are a non-halogen flame-retardant resin composition that contains a base polymer mainly containing a polyolefin-based resin, that passes a flame test, and that has good heat resistance and good heat-aging resistance which can satisfy a 150° C. rating, and an insulated electric wire and a tube using the non-halogen flame-retardant resin composition. The non-halogen flame-retardant resin composition contains (A) a base polymer containing 90% by mass or more of a mixture of a polyolefin-based resin and a styrene-based elastomer; (B) a metal phosphinate; and (C) a nitrogen-based flame retardant, and is used as an insulating coating layer. The insulating coating layer and the tube are preferably cross-linked by irradiation with ionizing radiation.
Owner:SUMITOMO ELECTRIC IND LTD
Polyolefin communication cable material used in rail transportation, and preparation method thereof
InactiveCN106519543AHigh strengthImprove wear resistancePlastic/resin/waxes insulatorsPolyolefinAntioxidant
The invention discloses a polyolefin communication cable material used in rail transportation, and a preparation method thereof. The polyolefin communication cable material is composed of, by weight, 40 to 60 parts of ethylene-vinyl acetate copolymer, 40 to 60 parts of propylene-ethylene block copolymer, 10 to 20 parts of an interface compatilizer, 140 to 180 parts of a fire retardant, 1 to 2 parts of a composite antioxidant, 5 to 10 parts of an antiwear additive, 1 to 2 parts of an ultraviolet light absorber, 2 to 4 parts of a lubricating agent, 2 to 3 parts of a water resistance agent, and 1 to 2 parts of a metal deactivator. The polyolefin communication cable material is high in strength, and excellent in wear resistance; possesses petroleum butter resistance, environmental stress crack resistance, hydrophobicity performance, watertightness, weatherability, excellent physical and mechanical properties, electrical performance, and excellent extrusion processability; and is capable of resisting a temperature as high as 105 DEG C; the preparation method and formula are simple; no toxic substance or waste gas is generated in production process; the compatibility of the above ingredients is excellent, and processing fluidity is excellent; the flame resistance is capable of satisfying requirements in UL-94 V-0 vertical flame test; and oxygen index value is equal to or larger than 38%.
Owner:WUXI JAKE PLASTIC
Enhancing the strength, moisture resistance of wood, timber, lumber, similar plant-derived construction and building materials, and other cellulosic material
InactiveUSRE40517E1High strengthReduce solubilityRadiation/waves wood treatmentRadiation applicationsElemental compositionCellulose
Materials variously treated with sodium silicate were studied until enough information was obtained to find a way to solve the problems that have prevented sodium silicate from being the used as a fire retardant. These problems are: 1) water solubility (miscible with water), which results in extensive leaching when exposed to water, 2) cracking, chipping and peeling of treated surfaces, and 3) surface granulation. During flame tests it was discovered that sodium silicate formed a foam-like material, and this material was found to have become water insoluble, yet its elemental composition had remained virtually identical to that of the unmodified sodium silicate. This investigator proposes that under the influence of heat and dehydration, sodium silicate undergoes a polymerization process resulting in particles sizes too large to dissolve in water, and then developed a mechanism to explain how the process could occur. The temperature and moisture conditions in treated samples were then manipulated to cause the polymerization process to occur while protecting the wood from damage. Thus samples were prepared that were both water insoluble, and possessed effective fire retardant properties. These samples also proved to be stronger than untreated wood, thus providing an improved product that was fire retardant and moisture resistant. Since aqueous sodium silicate can be combined with other inorganic fire retardants, this technique is a potential method for making any inorganic fire retardants moisture resistant. This represents a potential breakthrough in fire retardants that has been sought for approximately 100 years. In addition, sodium silicate treated samples were made moisture resistant by the application of a micro-thin layer of silicon monoxide to the surface of samples. This technique, also never tried before, represents a second method for providing moisture resistant, fire retardant substances.
Owner:T2EARTH HLDG LLC
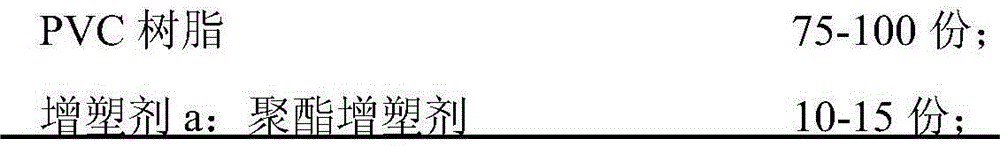
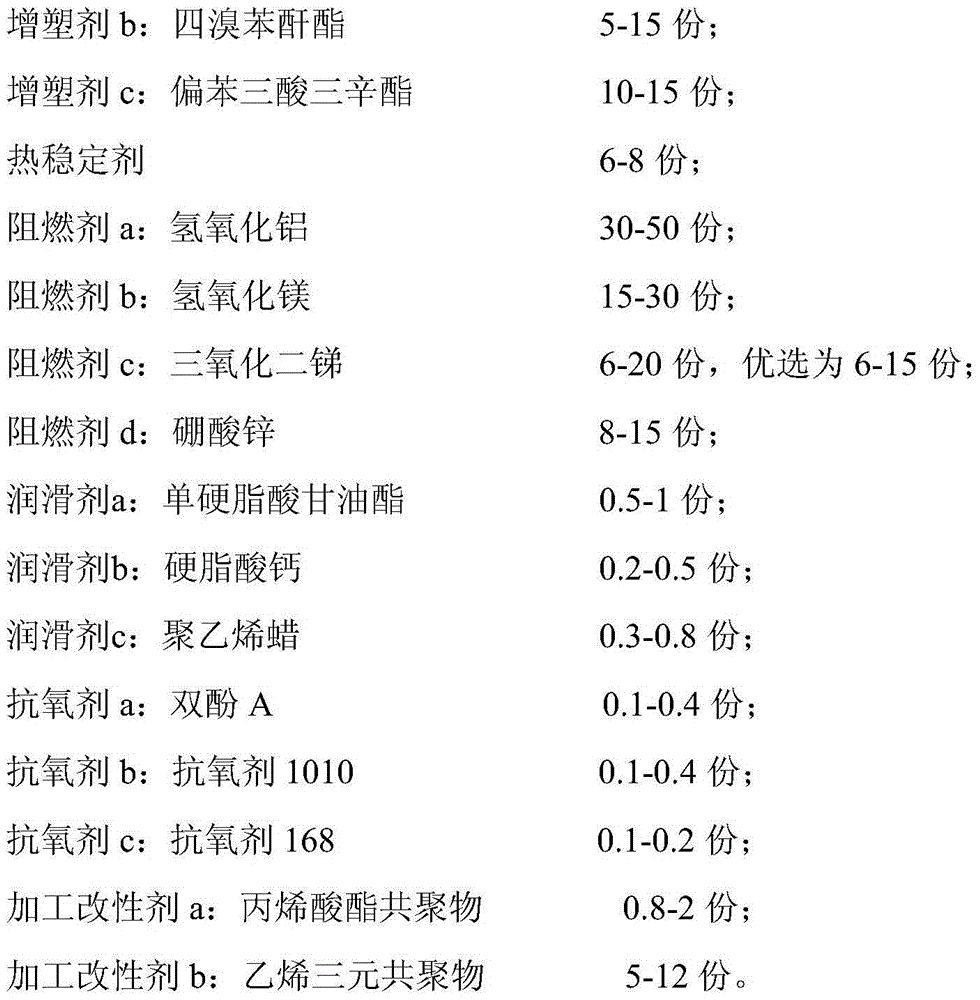
Environment-friendly, CMR-grade and high-flame-retardance PVC (Polyvinyl Chloride) sheath material, and preparation method and use of sheath material
InactiveCN105778327AGood extrusion processing performanceThe surface of the product is smooth and delicatePlastic/resin/waxes insulatorsInsulated cablesPolyesterAntimony trioxide
The invention relates to the field of flame-retardant cable sheath materials and particularly relates to an environment-friendly, CMR-grade and high-flame-retardance PVC (Polyvinyl Chloride) sheath material, and a preparation method and use of the sheath material. The environment-friendly, CMR-grade and high-flame-retardance PVC sheath material provided by the invention is prepared from the following ingredients in parts by weight: PVC resin, a polyester plasticizer, tetrabromophthalic anhydride, trioctyl trimellitate, a thermal stabilizer, aluminum hydroxide, magnesium hydroxide, antimony trioxide, zinc borate, glyceryl monostearate, calcium stearate, polyethylene wax, bisphenol A, an antioxidant 1010, an antioxidant 168, an acrylate copolymer and an ethylene terpolymer. The sheath material provided by the invention is in line with related technical indicators and requirements of UL1581 and UL1666, has environment-friendly performance, is in line with standards RoHS and REACH, also has good extrusion processability and excellent flame retardance and can pass a UL1666 intensive-flame test.
Owner:SHANGHAI KAIBO SPECIAL CABLE FACTORY
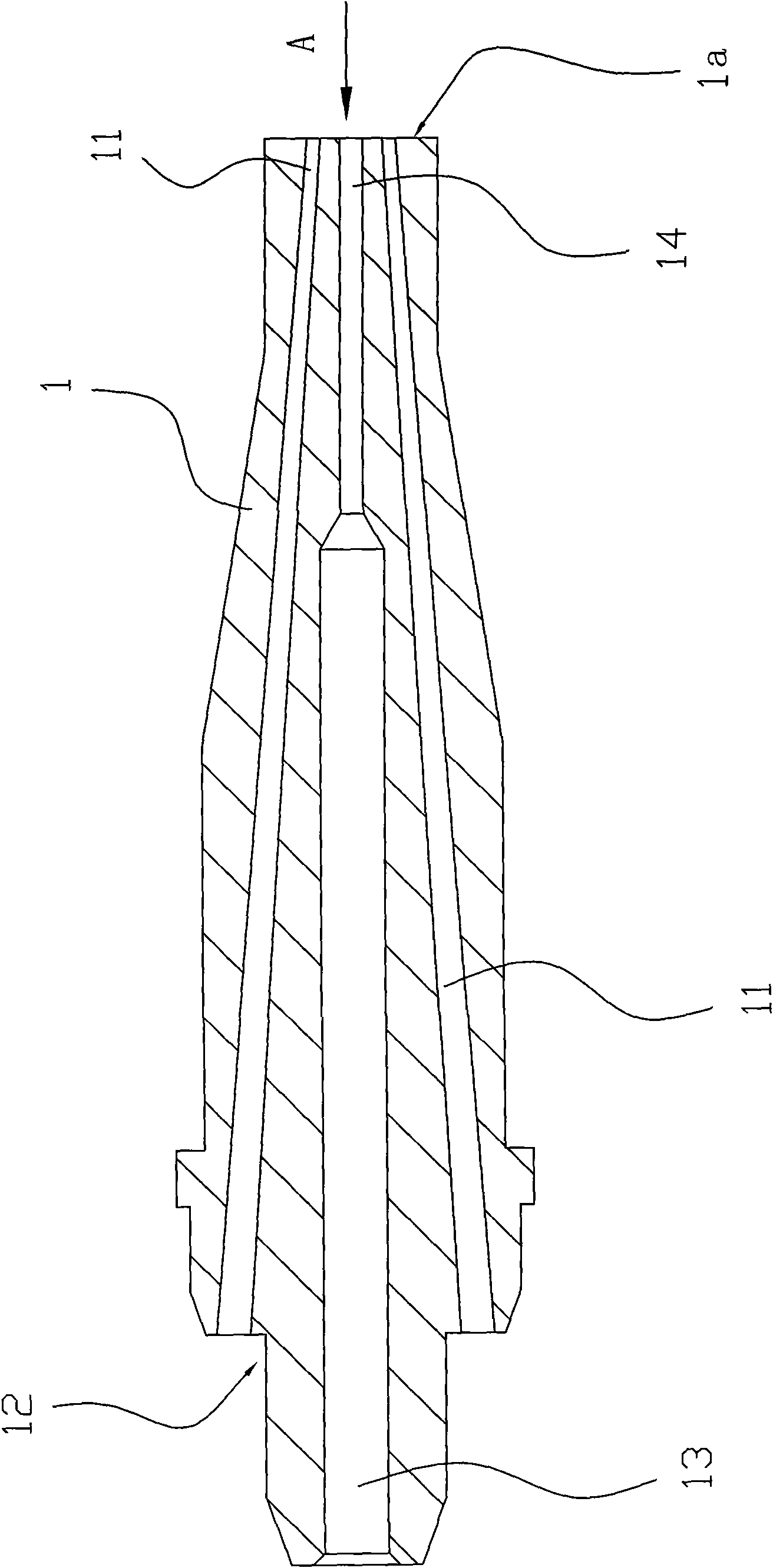
Cutting nozzle applied to welding gun and manufacturing method thereof
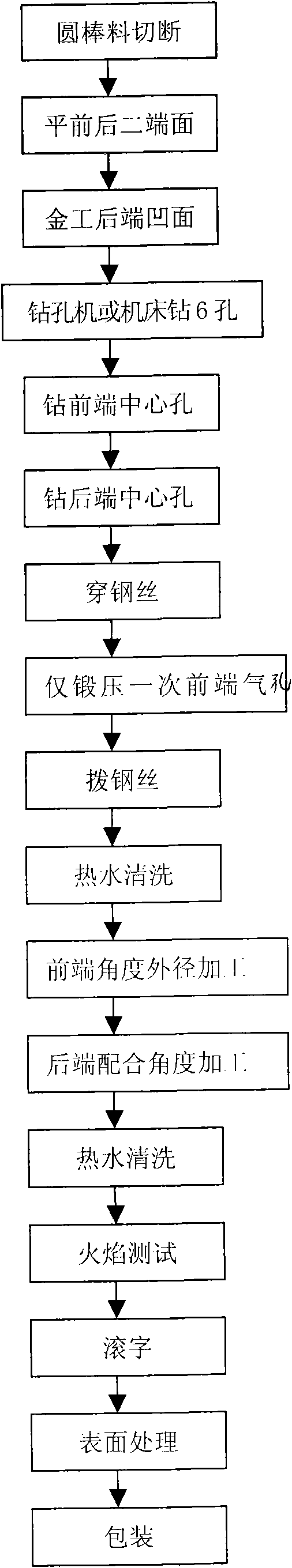
The invention relates to a cutting nozzle applied to a welding gun, which comprises a cutting nozzle body (1), wherein the cutting nozzle (1) is made from a tellurium copper material and vent holes on the cutting nozzle body (1) are drilled by a drilling machine. The method for manufacturing the cutting nozzle comprises the following steps: material selection: selecting a tellurium copper round bar, cutting off the tellurium copper round bar, flattening the front end face and the rear end face, and performing metalworking on a concave surface (12) of the rear end; hole drilling: drilling holes on a blank with the concave surface (12) through the metalworking; forging and stamping: performing once forging and stamping on the vent holes at the front end; dimension processing: performing angle outer diameter processing at the front end, performing matched angle processing at the rear end, and performing washing with hot water; and finished product: performing flame test on the processed cutting nozzle (1), rolling characters, performing surface treatment and obtaining the finished product through packaging. The cutting nozzle has the advantages that: by adopting the tellurium copper as the raw material instead of red copper, the fastening performance of the tellurium copper is good; the stickness is lower than that of the red copper; the vent holes are not easy to block; the tellurium copper has better heat resistance and long service life; the drilling machine hole drilling is adopted to replace artificial hole drilling and most of the forging and stamping process steps so that the workload is greatly reduced, and the quality of the formed vent holes is ensured effectively, the drilled vent holes are more normative, and the drill ways are smoother; and the manufacturing procedures are reduced.
Owner:NINGBO LONGXING WELDING CUTTING TECH STOCK
Formula and a preparation process of novel indoor and outdoor cold firework
The invention relates to a formula and a preparation process of novel indoor and outdoor cold firework. The novel indoor and outdoor cold firework comprises the following components in percentage by mass: 20%-70% of energetic substances, 10%-40% of an oxidizing agent, 10%-40% of one or more kinds of metal powder, 0%-20% of a flame color reaction material and 0%-5% of a binder. The preparation process comprises the steps of material mixing, particle sieving, firework charging and barrel packaging. Compared with the prior art, the novel indoor and outdoor cold firework of the invention has the following characteristics that elementary substances, compounds and mixtures capable of generating flame color reaction with fireworks during combustion are added, so that the novel indoor and outdoorcold firework has the characteristics of wide color gamut (covering the wavelength range of 400 nm-760 nm), multiple colors, narrow electromagnetic wave full width at half maximum, high contrast ratioof set-off patterns and the like, ensures reasonable cost, and can be widely accepted by consumers when being used for civil entertainment firework products or used for various celebrations or parties and other festival occasions.
Owner:湖南坤普科技有限公司
Automatic sample injection device for flame retardance tests
The invention discloses an automatic sampling device for flame retardancy testing, which comprises a base, a support seat is arranged on the top of the base, an electric control slide rail is arranged on the top of the support seat, and an electric control slide rail is arranged on the bottom of the electric control slide rail. An electric push rod, one side of the electric push rod is fixedly connected with a first pole, the bottom of the first pole is provided with an infrared emitter, the bottom of the electric push rod is provided with a mechanical claw, and one side of the base is provided with Sample compartment. In the present invention, by setting an infrared transmitter and an infrared receiver, the control device controls the mechanical claws to pick up the substance to be detected, and the electric control slide rail moves the electric push rod to the side of the sample chamber. After receiving the signal, the electrical signal is transmitted to the control device, the control device stops the electronically controlled slide rail, the mechanical claws are opened, and the substance to be detected is placed on the storage board, which is beneficial to conveniently determine the placement position and improve the positioning accuracy.
Owner:ZHEJIANG ZHONGTIAN TEXTILE TESTING
Automatic production line for common lighter
ActiveCN102168860ASafe and reliable workRealize closed-loop controlIncandescent ignitionFuel lightersProduction lineFlame test
The invention discloses an automatic production line for a common lighter, which comprises a frame, and a controller. A transmission line is arranged on the frame; one end of the transmission line is provided with a main feed mechanism used for automatically feeding during circular working; a shell conveying station, an automatic flame test station, a manual flame test station, an outer handpiecelocating and assembling station, a handpiece pressing station used for assembling finished products, and a final flame exhausting station used for adjusting the flames of finished lighters into a uniform height and screening defective products are respectively arranged along the transmission line. As the automatic production line for the common lighter adopts automatic assembly and flame test, the production efficiency is greatly improved, and the product quality is more stable.
Owner:刘健萍
High tenacity low smoke zero halogen flame retardant polypropylene and preparation method thereof
The invention discloses a high tenacity low smoke zero halogen flame retardant polypropylene and a preparation method thereof and the polypropylene comprises the following components according to the parts by weight; 10-90 parts of polypropylene, 10-90 parts of flexibilizer, 100-160 parts of inorganic flame retardant, 2-20 parts of flame-retardant synergist, 2-5 parts of reactive compatibilizer, 0.1-2 parts of antioxidant, 0.5-5 parts of flow modifier, 0.1-5 parts of surface modifier and a defined amount of coloring agent. The low smoke zero halogen flame retardant polypropylene has the advantage of good mechanical properties, good flame resistance (the vertical flame test of the polypropylene passes UL94V-0 level), low smoke density which is not more than 20%, good aging resistance and low cost, and can meet the processing demands for injection and extrusion molding.
Owner:内蒙古睿达鑫科技有限责任公司
Open cell spray fire-retardant foam
Embodiments of the present technology may include an open cell spray polyurethane foam. The foam may include a polymer. The polymer may formed by the polymerization of a reaction product of (1) a saccharide with an epoxide and (2) an isocyanate. The reaction product may have greater than 25 weight percent and less than 99 weight percent of the saccharide. The foam may exhibit a fire retardancy sufficient to pass flame tests in accordance with Appendix X and / or ASTM E-4.
Owner:JOHNS MANVILLE CORP
Hydrogen-flame coloring device
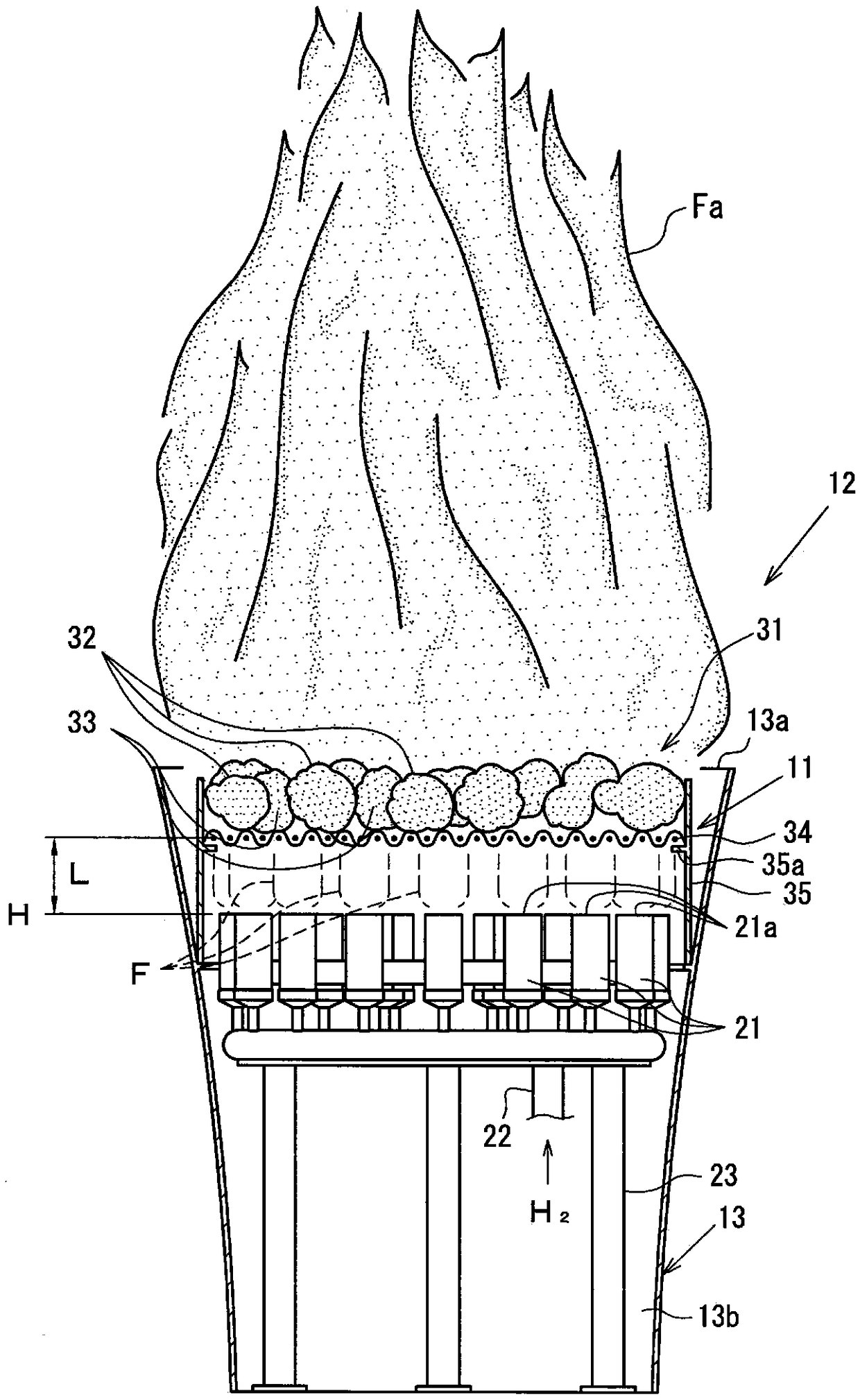
ActiveCN109196276AGood colorKeep hair colorDomestic stoves or rangesCooking fumes removalCombustorHydrogen
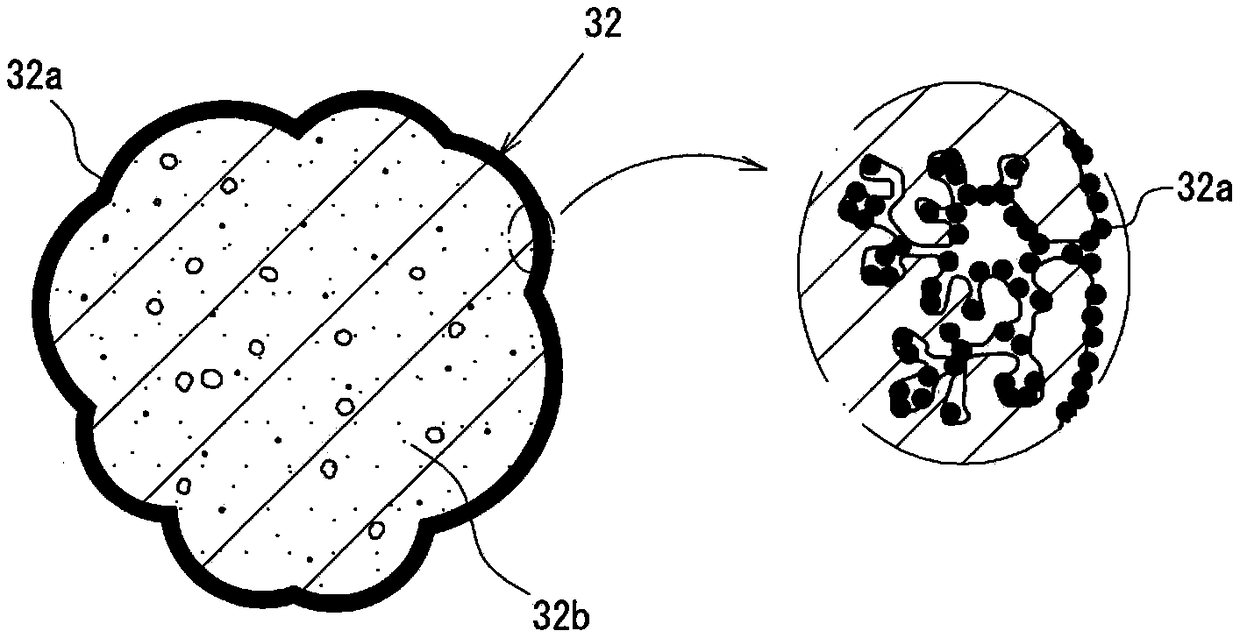
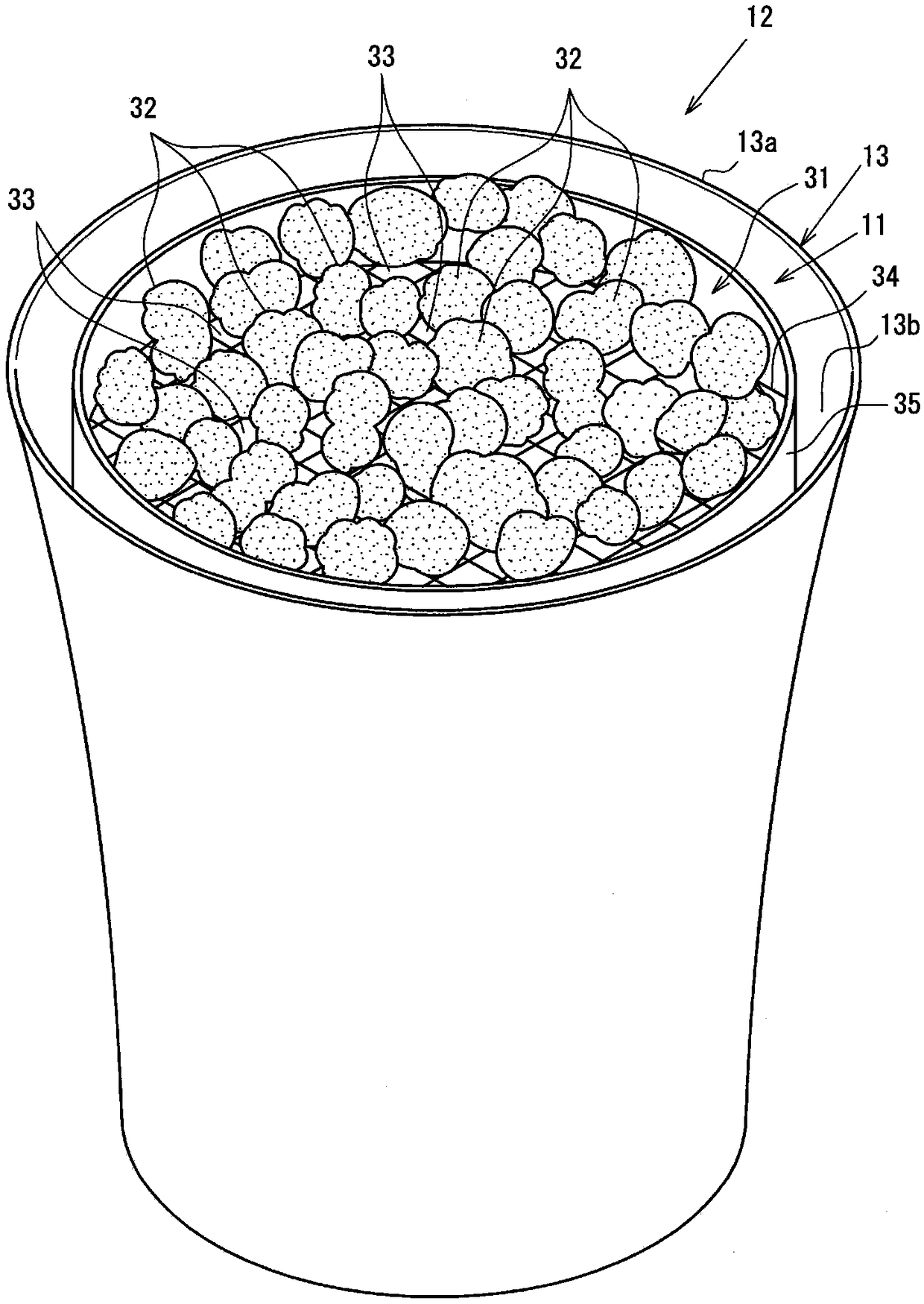
The present invention can excellently color a transparent hydrogen flame with high brightness, and can maintain a color developed state of the hydrogen flame for a long time period. The present invention is provided with: a plurality of burners (21) that generate a hydrogen flame (F) by burning hydrogen; and a coloring part (31) disposed at a position toward which the hydrogen flame (F) is generated from flame ports (21a) of the burners (21). The coloring part (31) is formed of color developing members (32) and through portions (33) through which the hydrogen flame (F) passes. The color developing members (32) are each formed by causing a porous body (32b) made of aggregated lava to carry a color developing material (32a) which gives a flame reaction. The through portions (33) are formed at spaces generated among the arranged color developing members (32).
Owner:IWATANI CORP
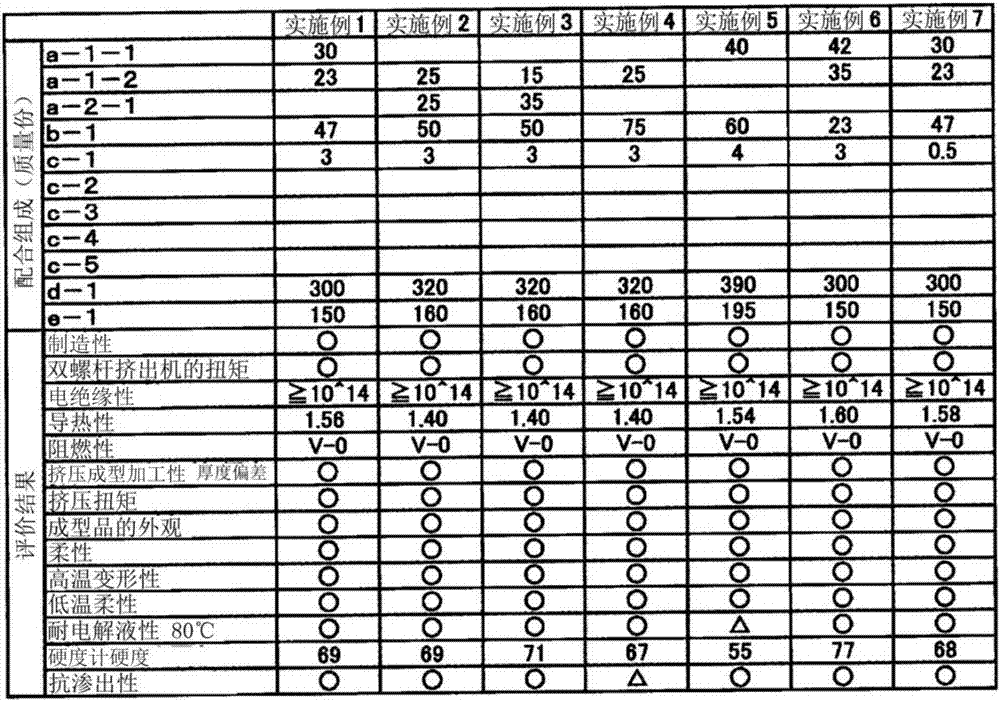
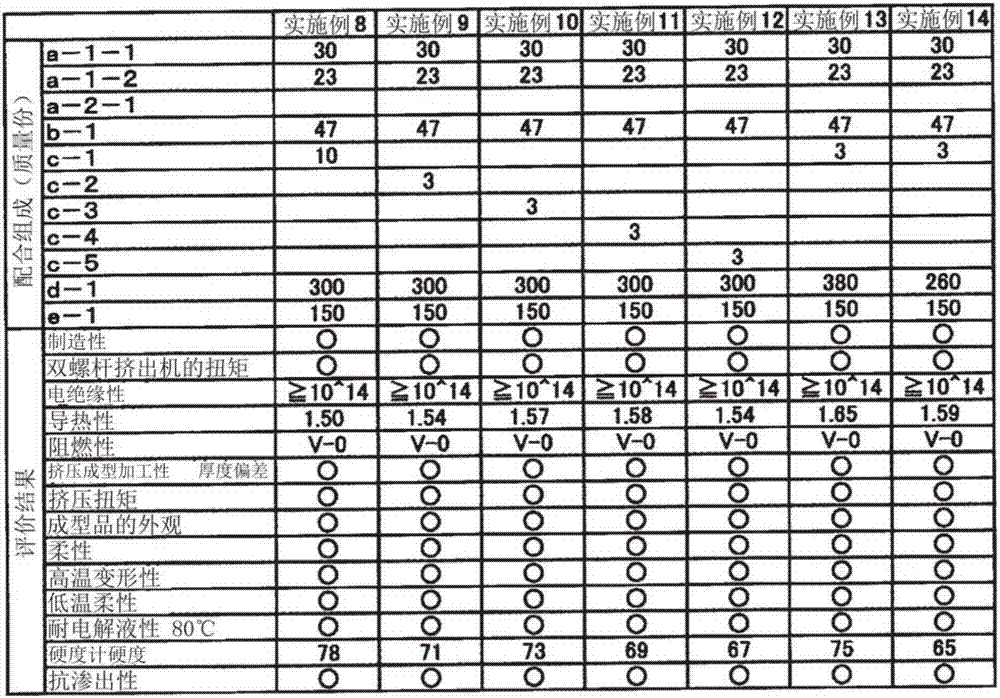
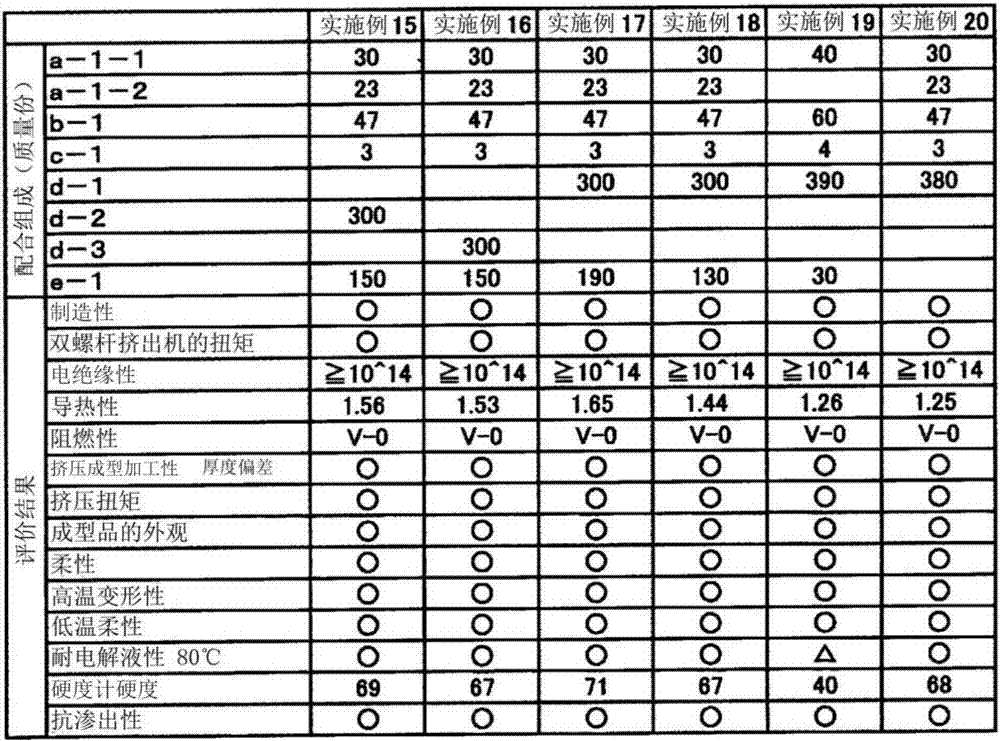
Thermoplastic elastomer composition for battery pack protective member
InactiveCN107431146AImprove flexibilityImprove thermal conductivityJackets/cases materialsElastomerElectrolytic agent
The purpose of the present invention is to provide a thermoplastic elastomer composition that shows outstanding flexibility over a wide temperature range from normal temperatures to low temperatures, has rank V-0 flame retardancy in a UL94 vertical flame test without the use of halogen-based flame retardants, has outstanding thermal conductivity, electrical insulation performance, and electrolysis resistance, and can be suitably used as a protective member for a soft battery pack. The present invention provides a thermoplastic elastomer composition for a battery pack protective member, the composition containing: 100 parts by mass of a resin composition that comprises (a) 20-80% by mass of a hydrogen additive that is a copolymer of an aromatic vinyl compound and a conjugated diene compound and (b) 80-20% by mass of a non-aromatic rubber softener, with the total of (a) and (b) being 100% by mass; (c) 0.1-10 parts by mass of a modified resin; and (d) 250-400 parts by mass of magnesium hydroxide.
Owner:RIKEN TECHNOS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com