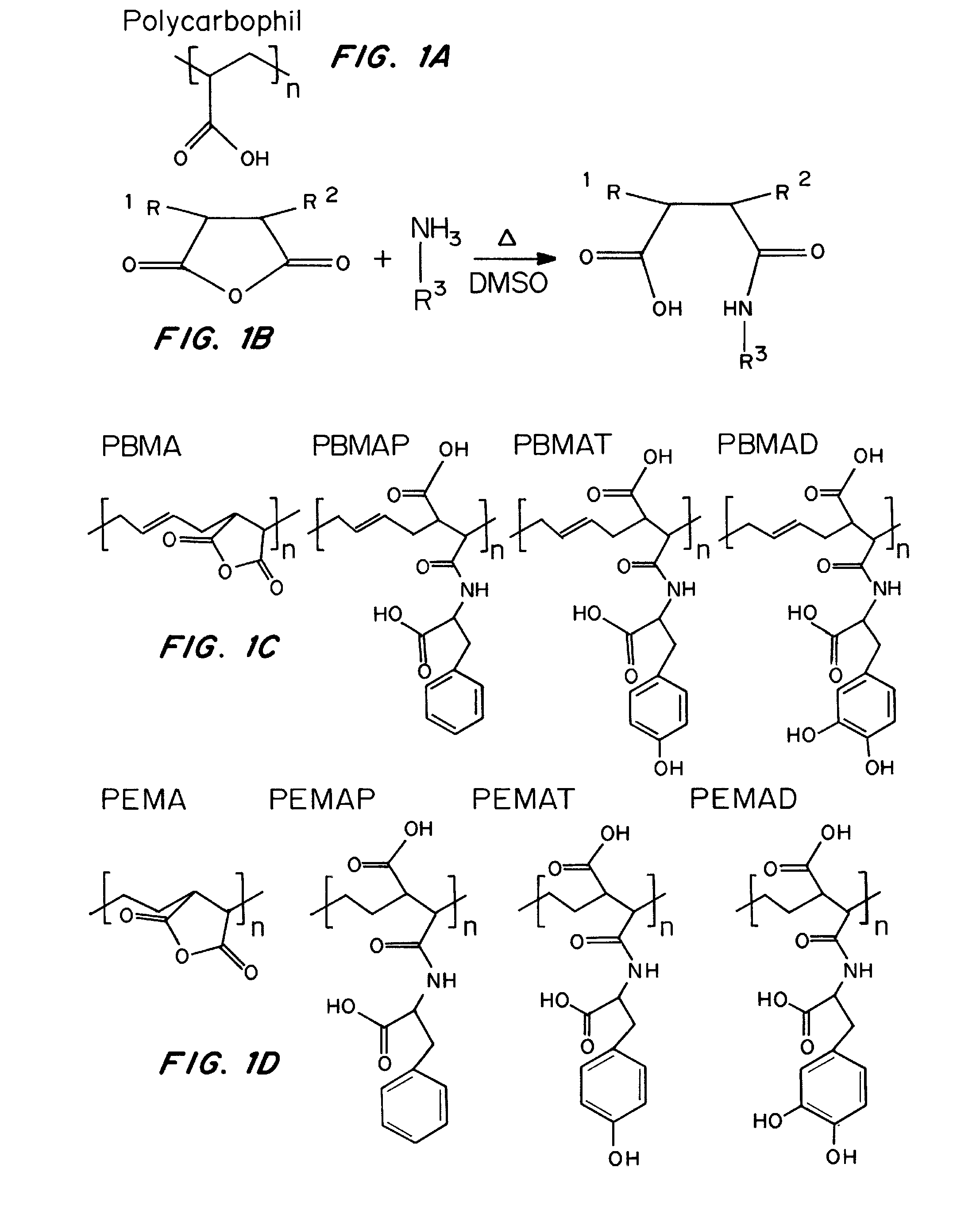
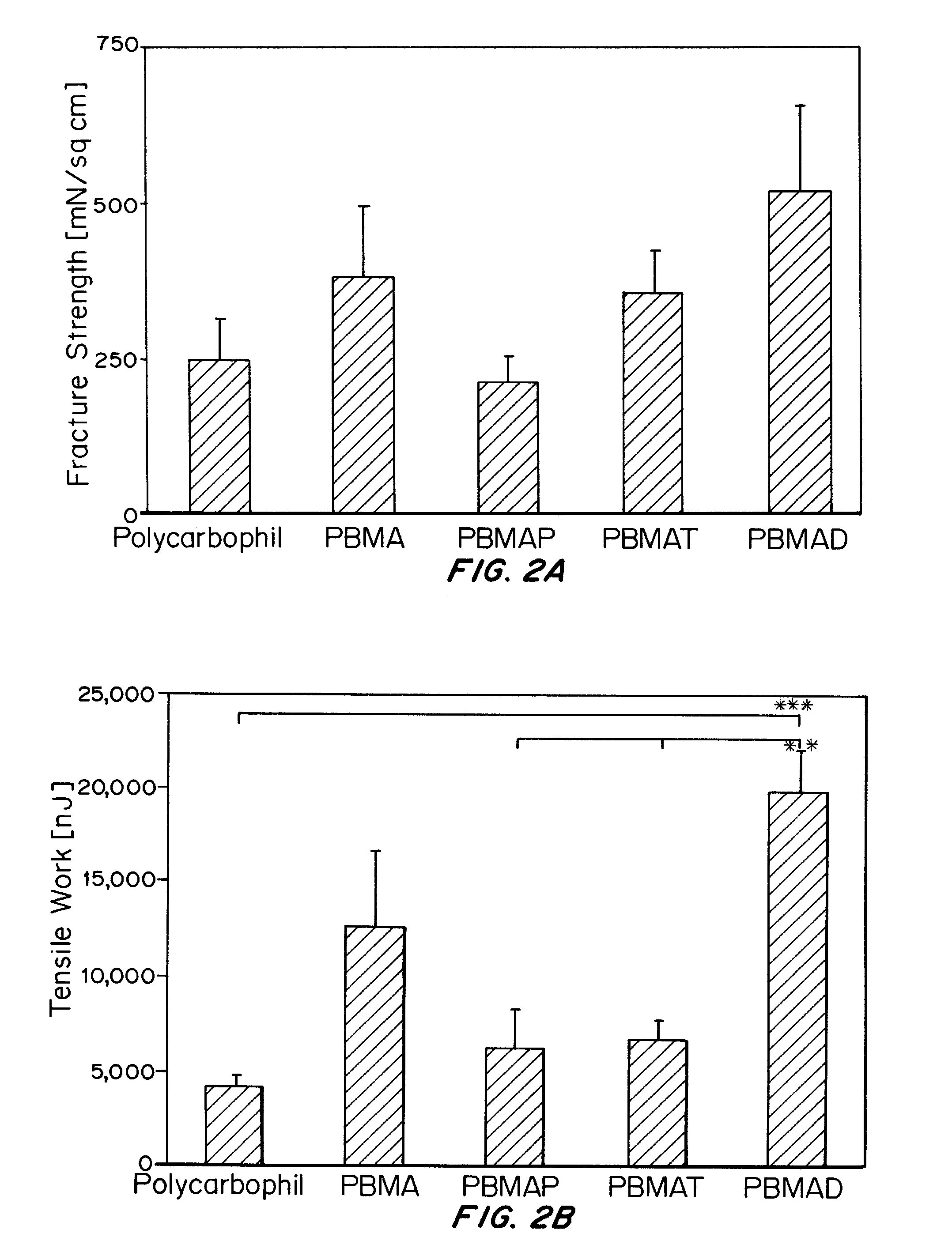
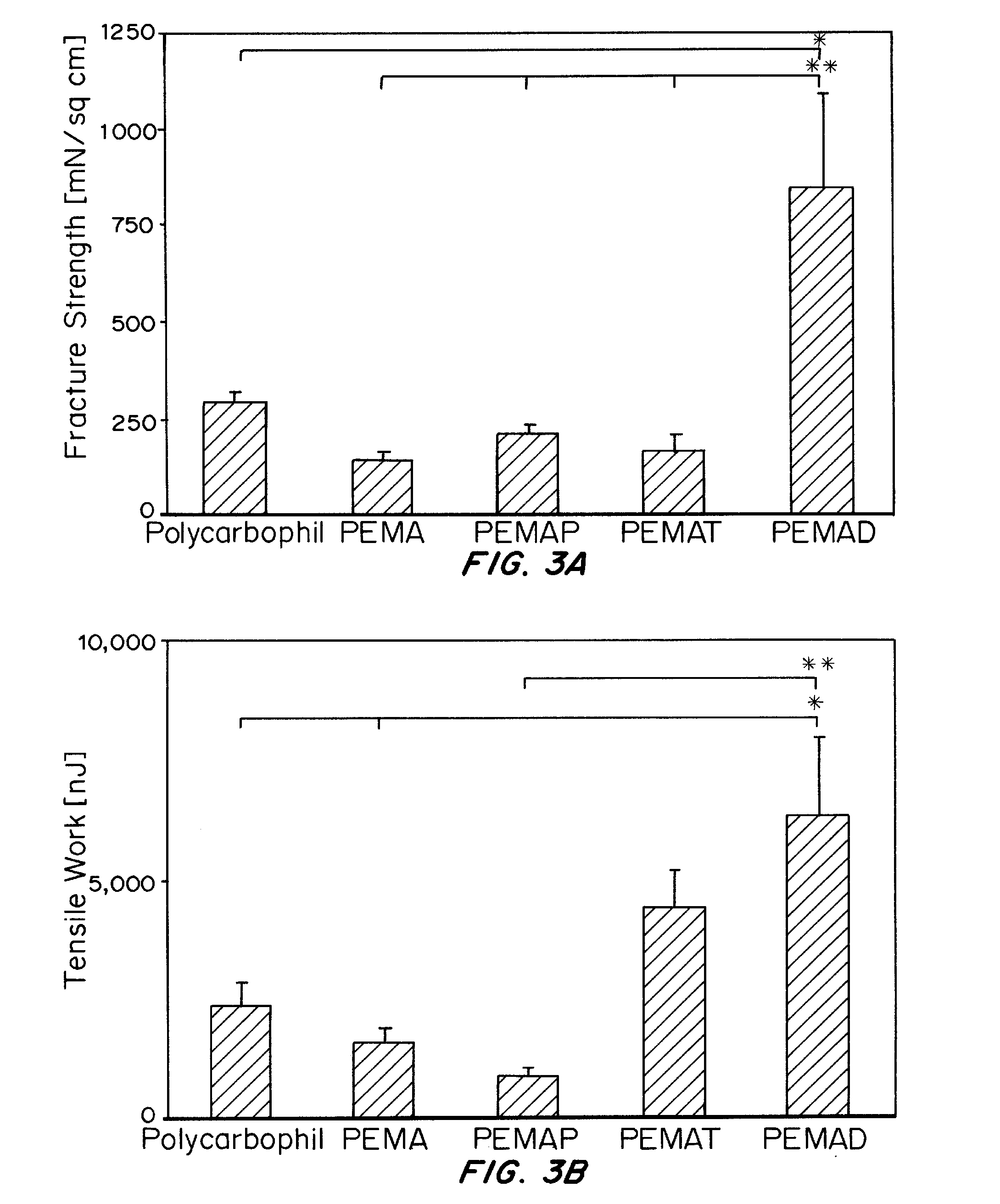
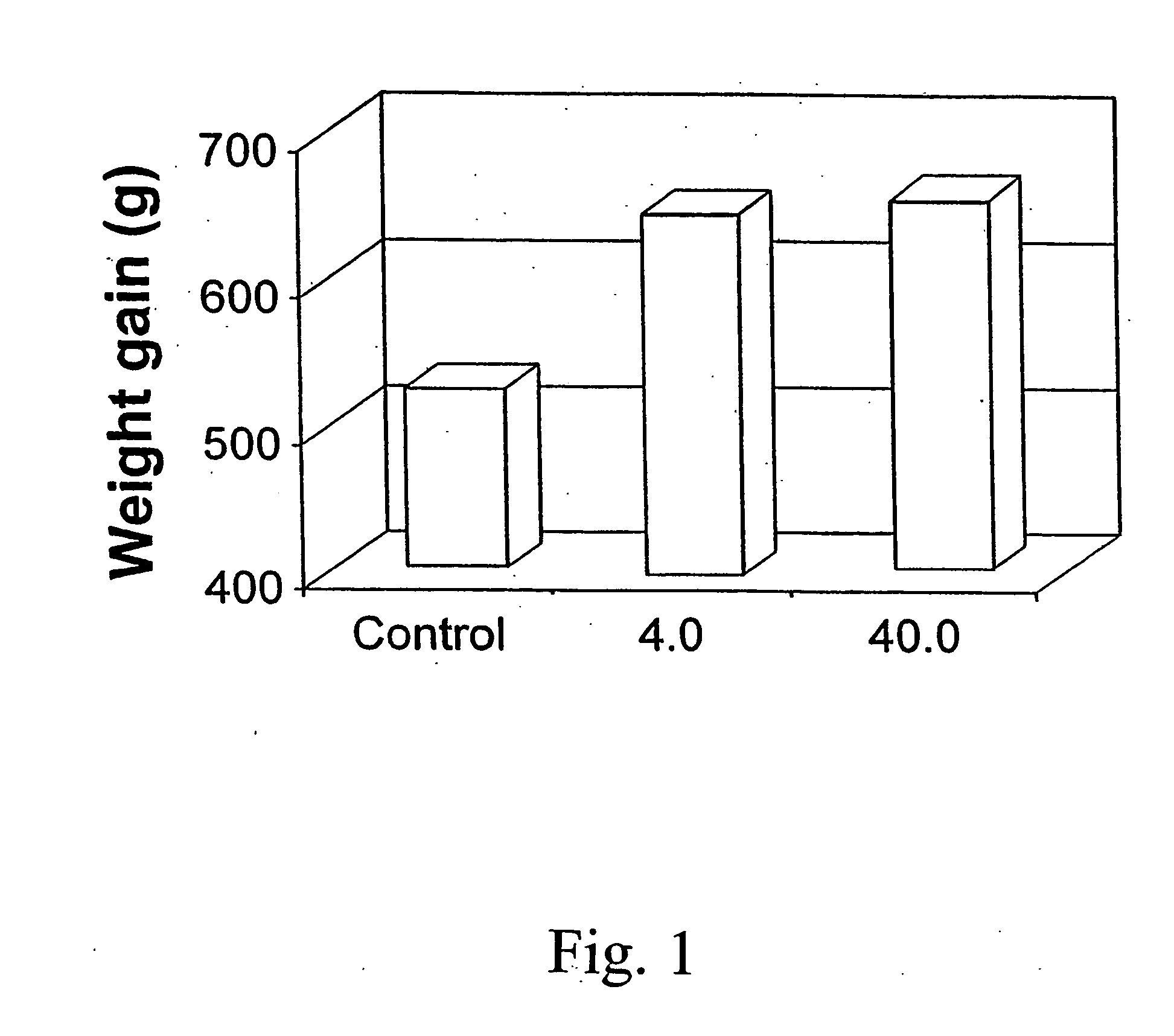
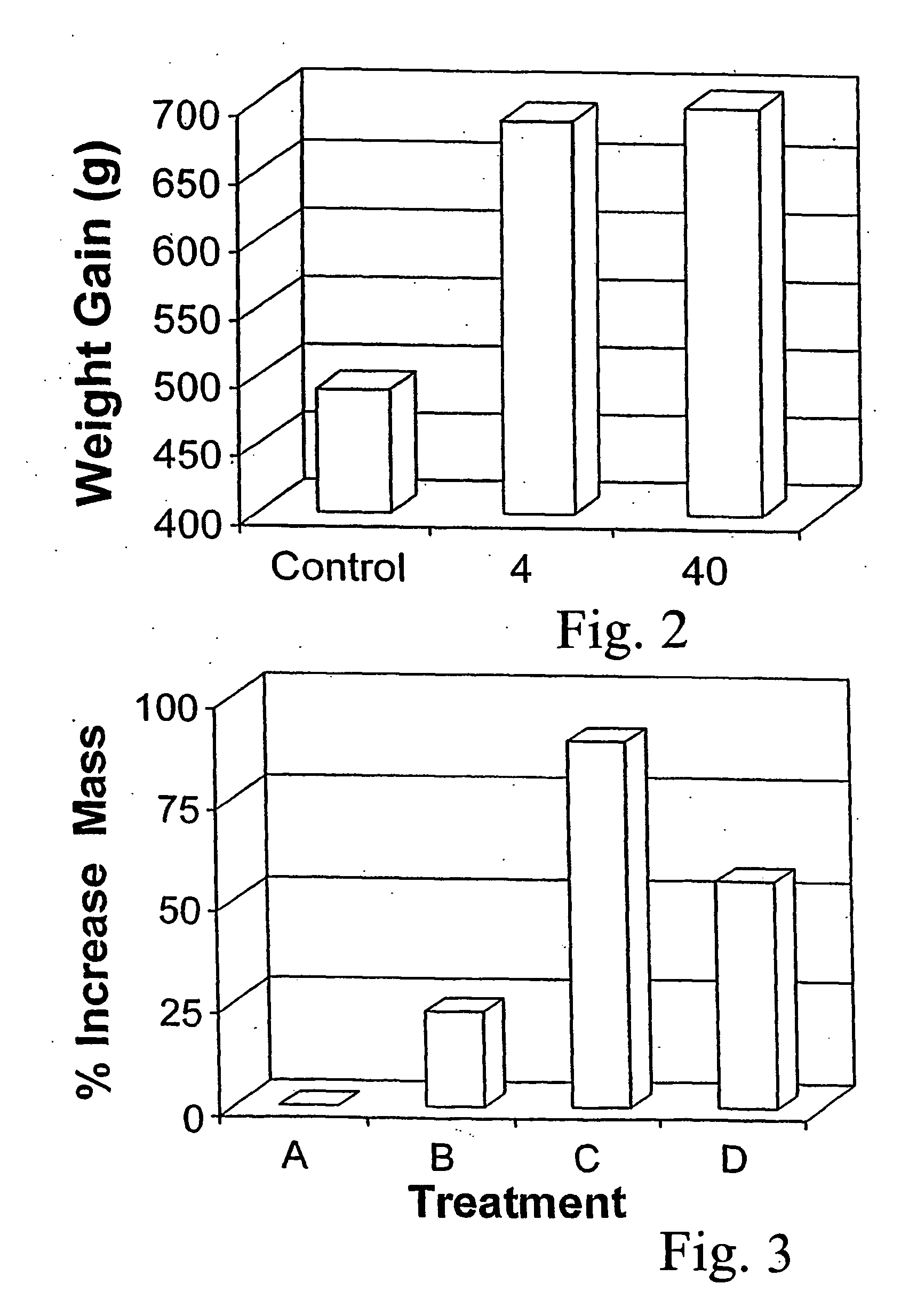
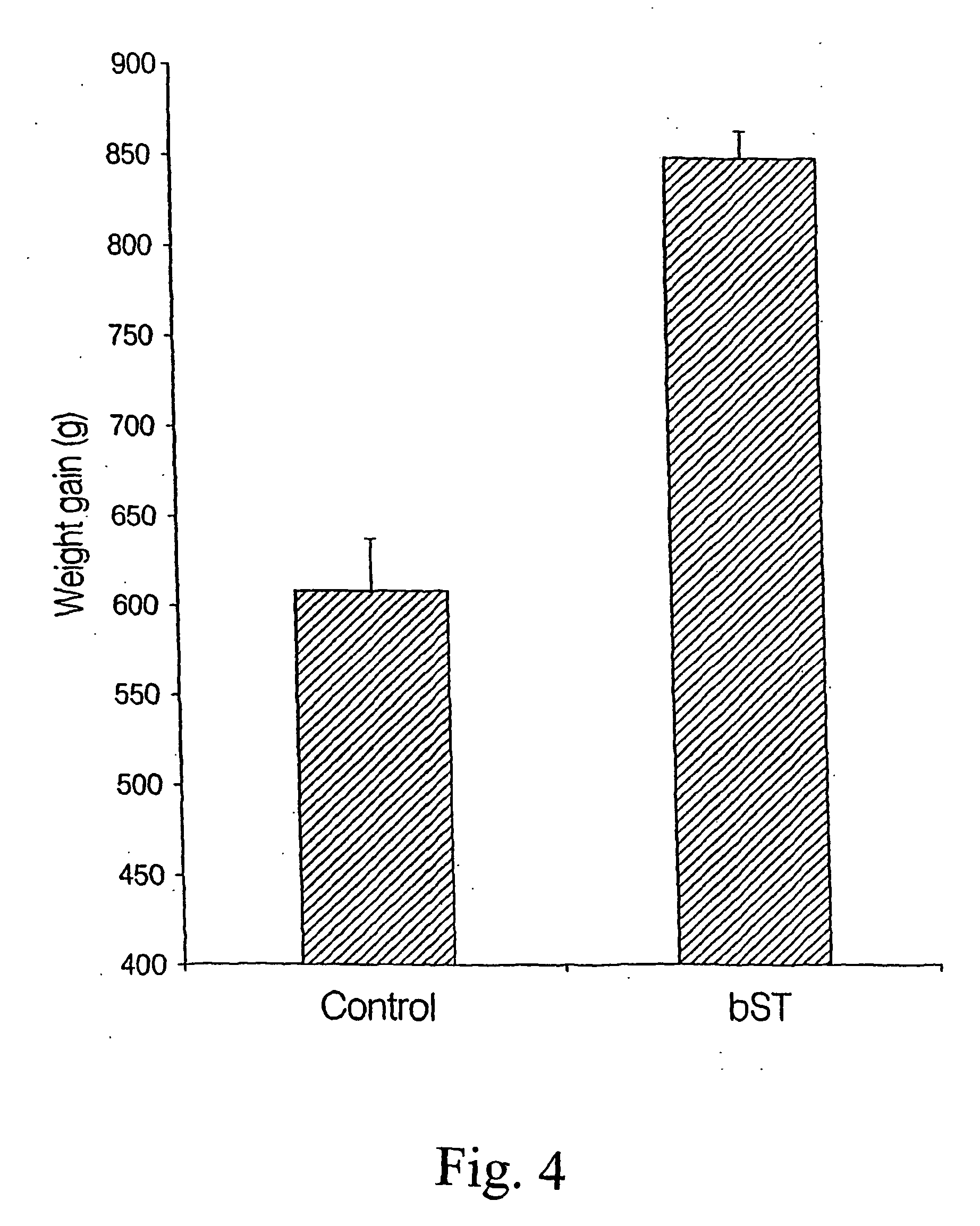
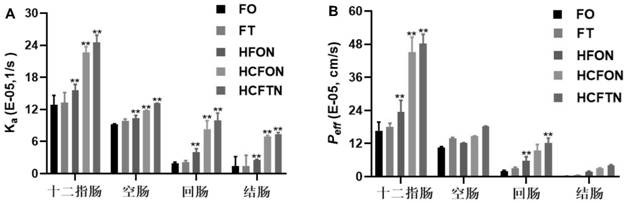
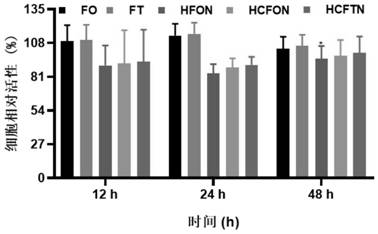
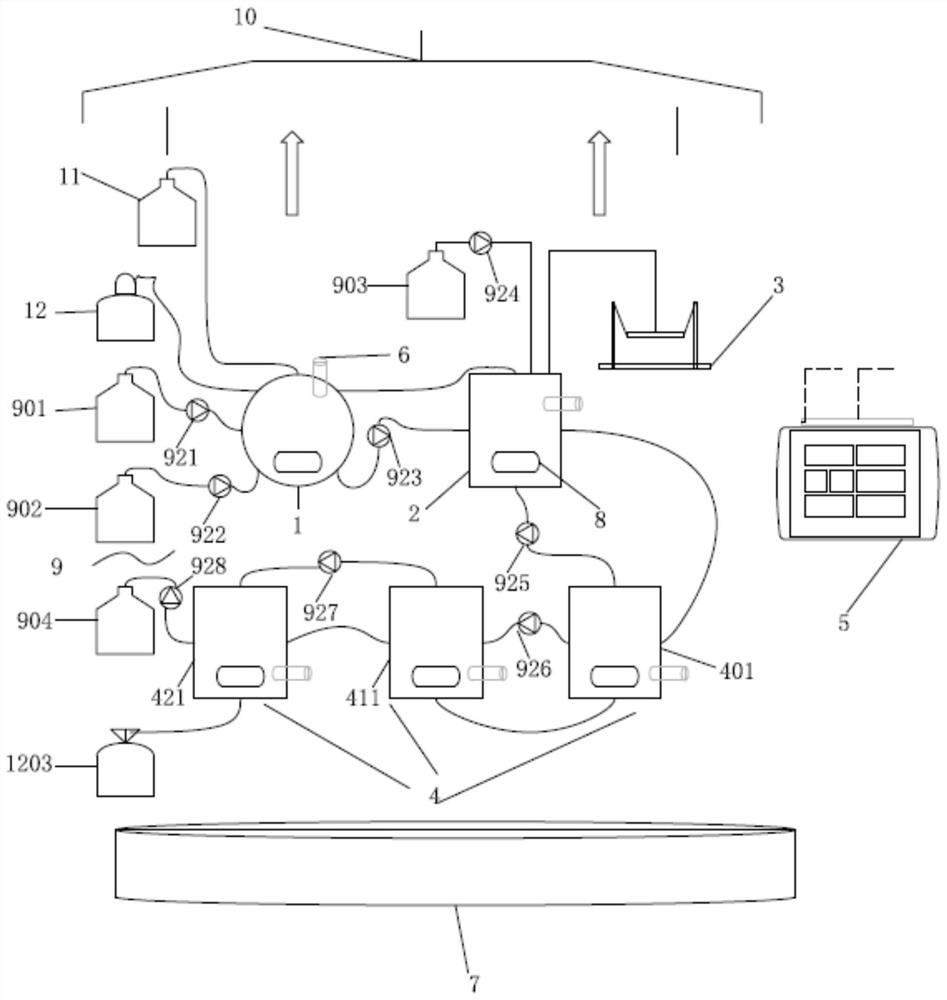
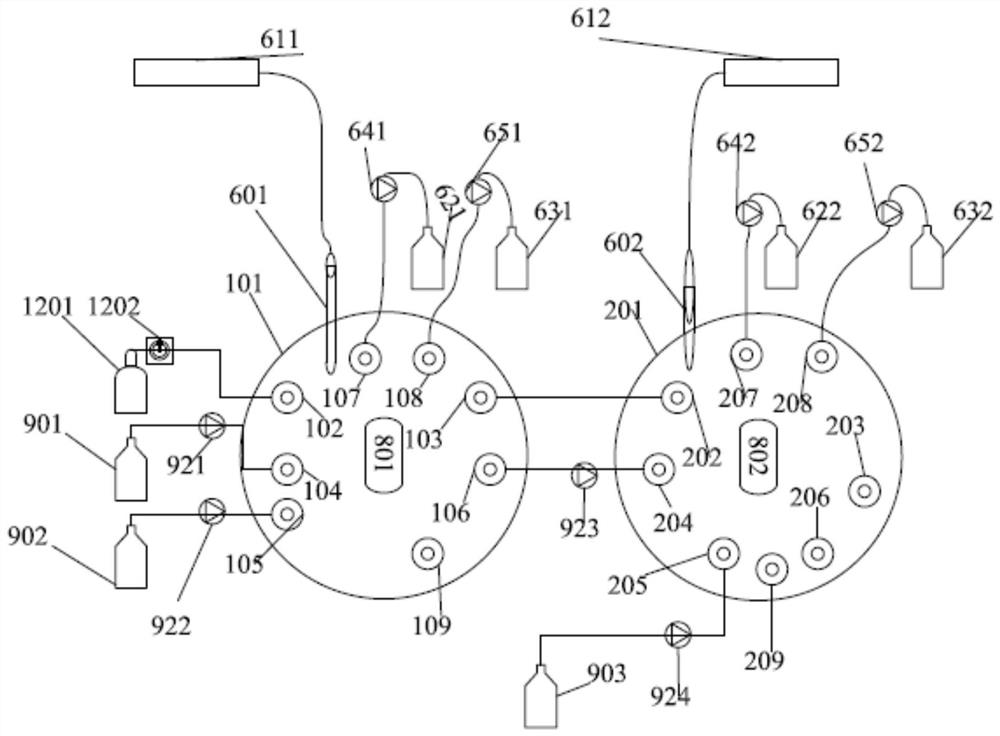
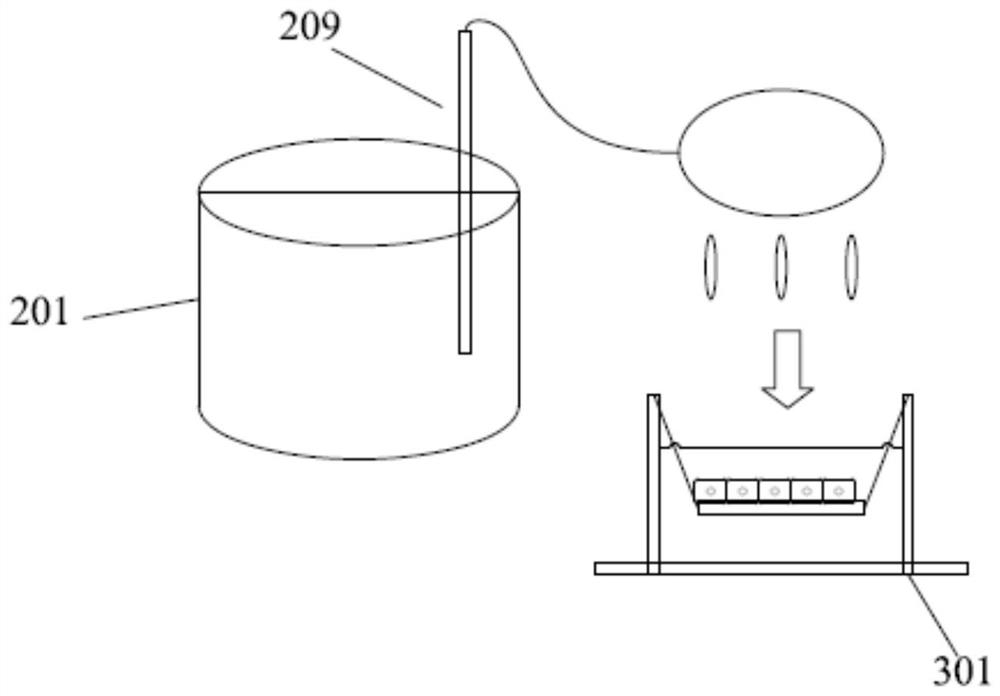
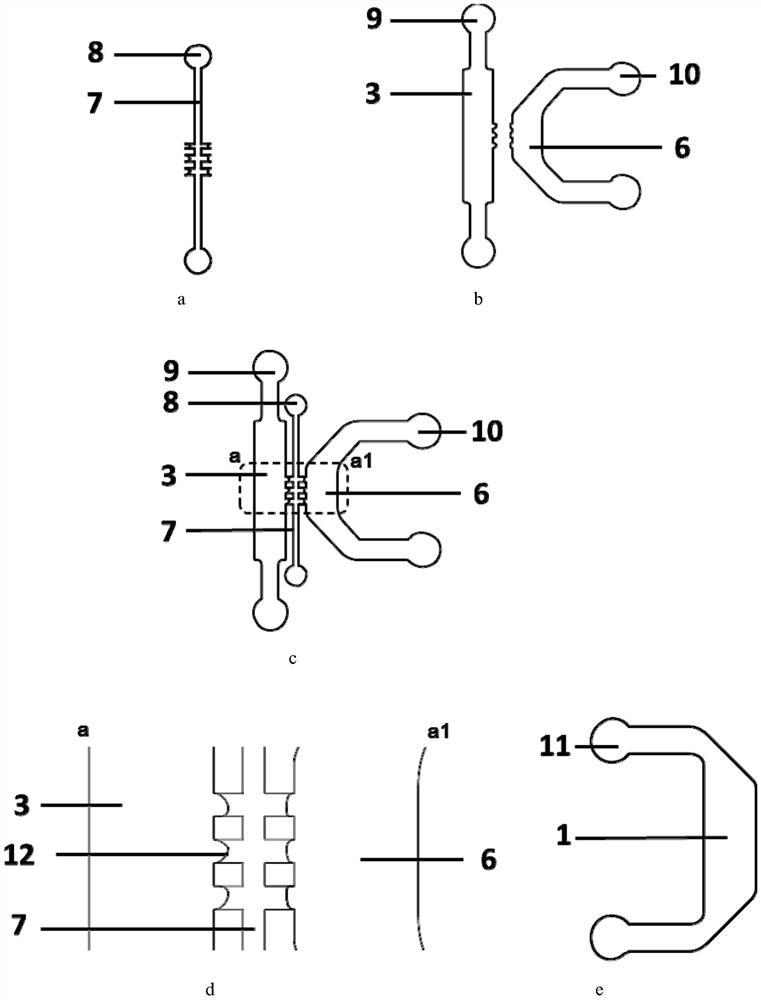
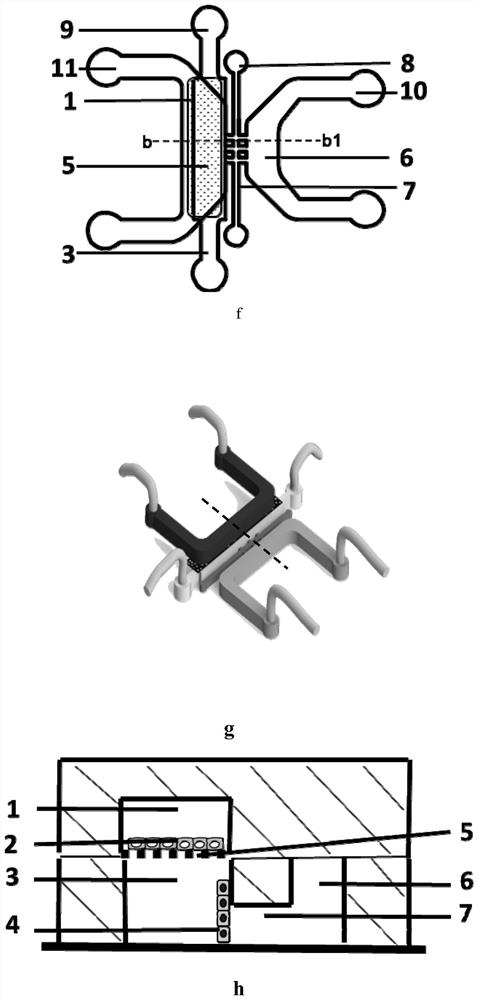
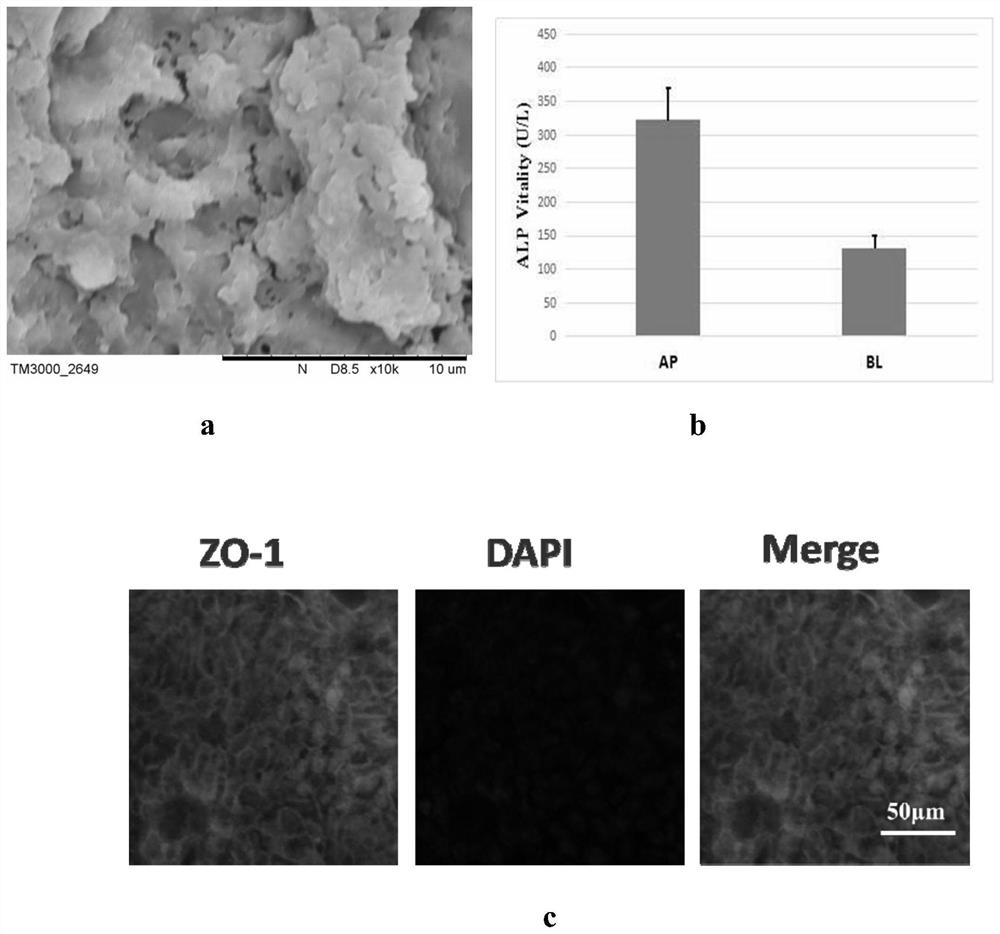
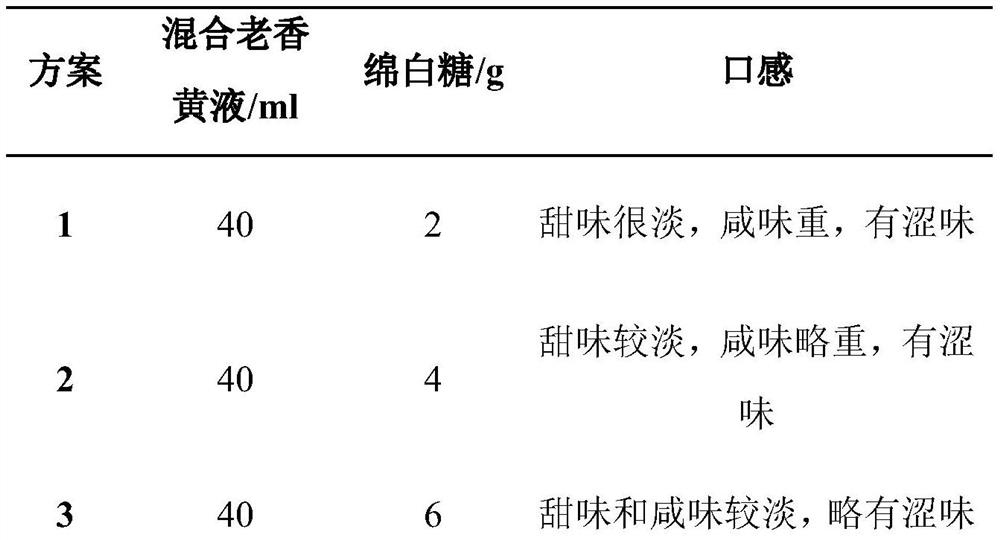
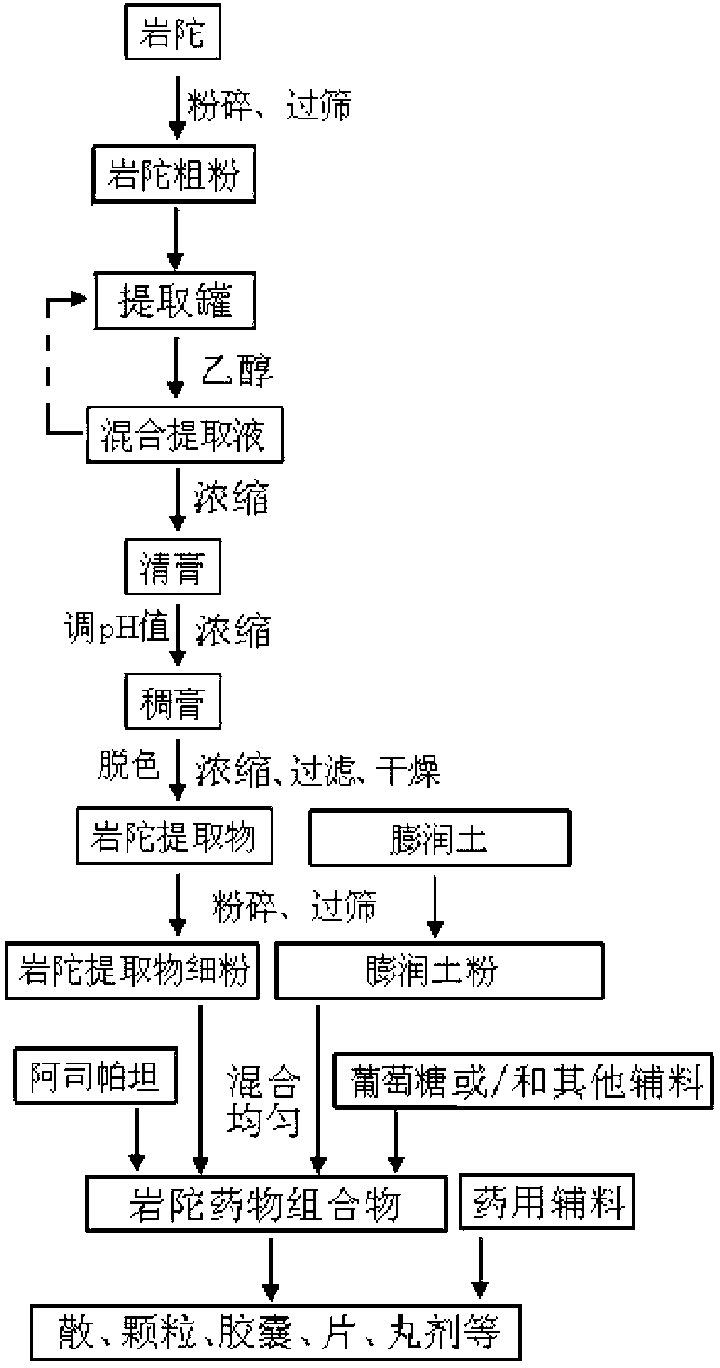
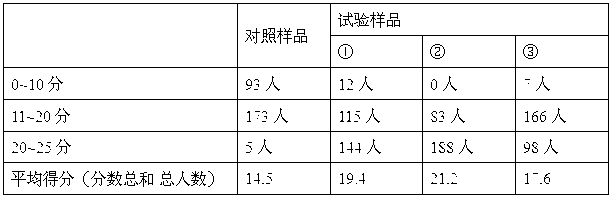
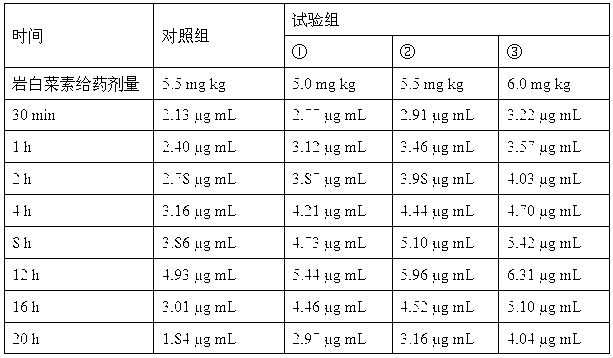
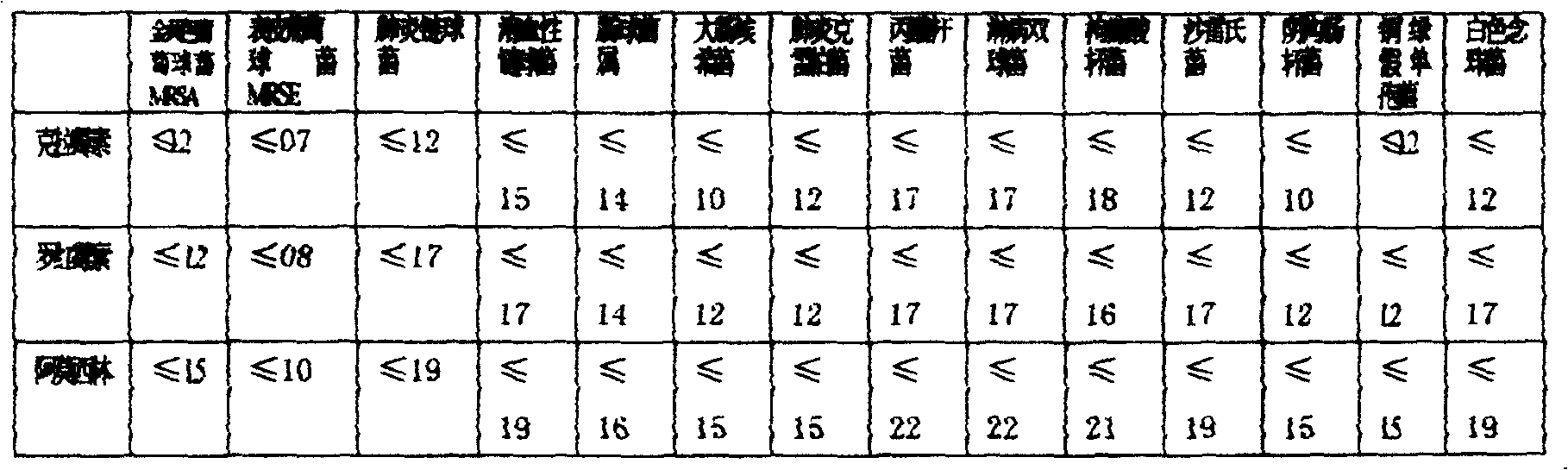
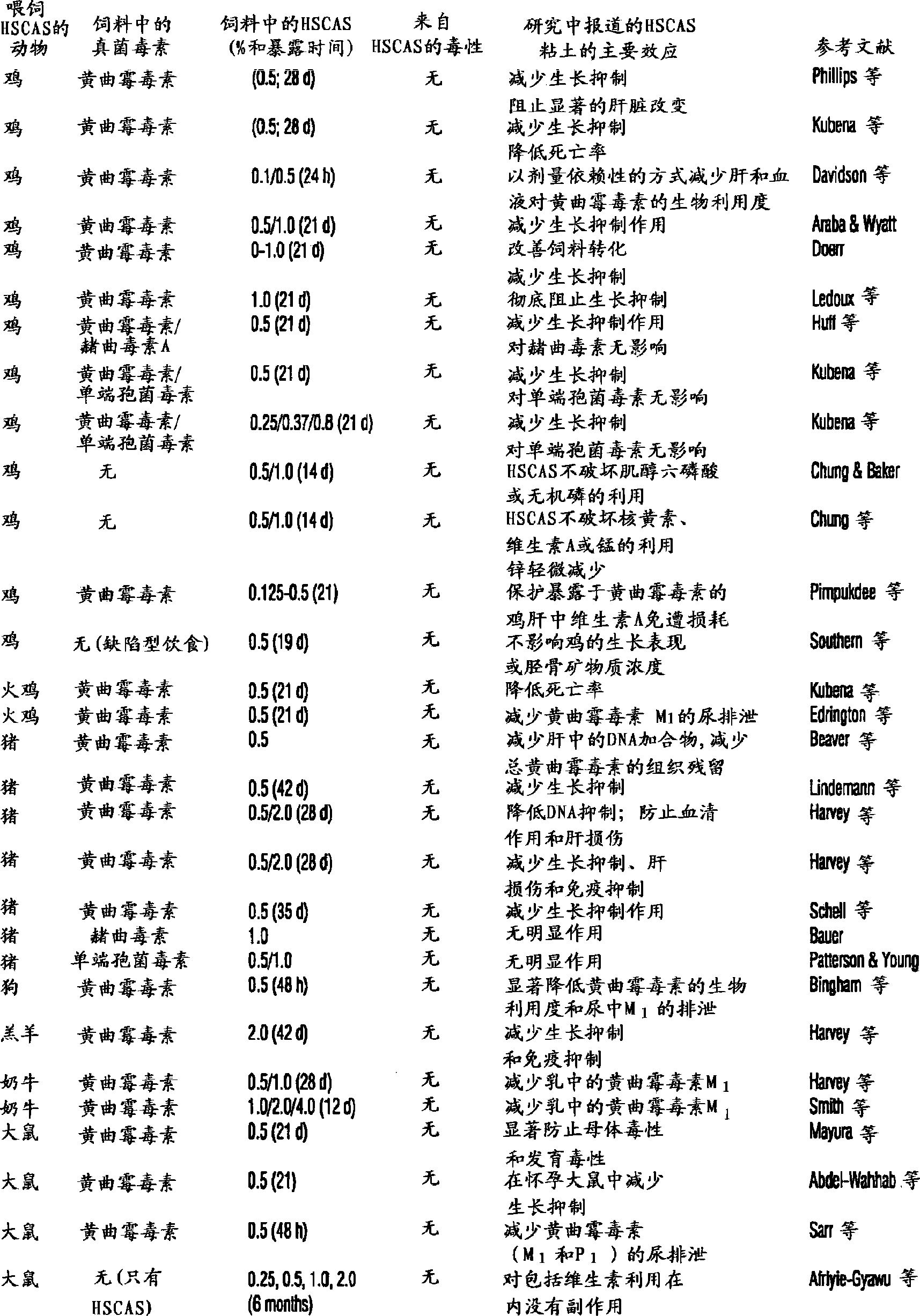
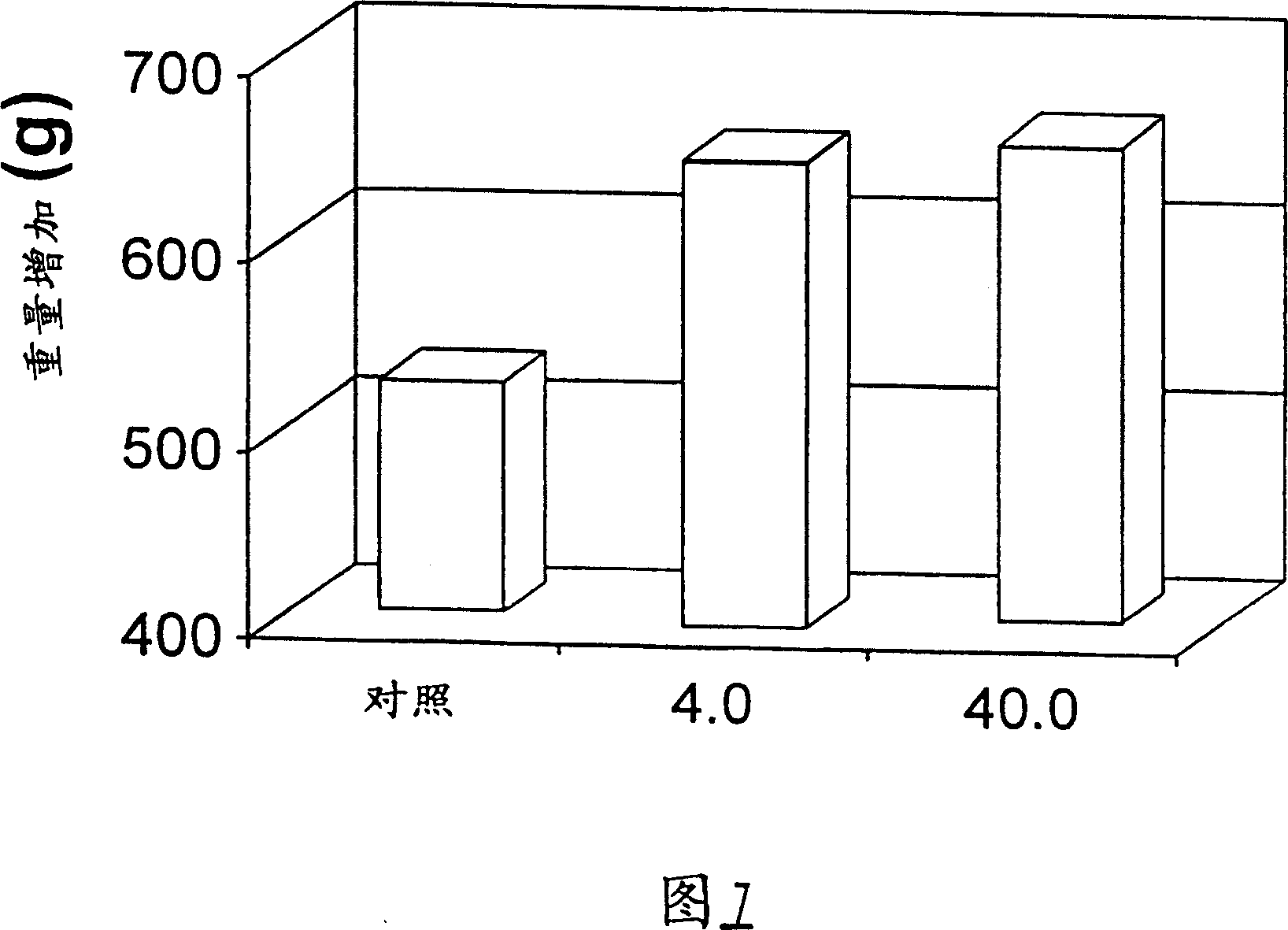
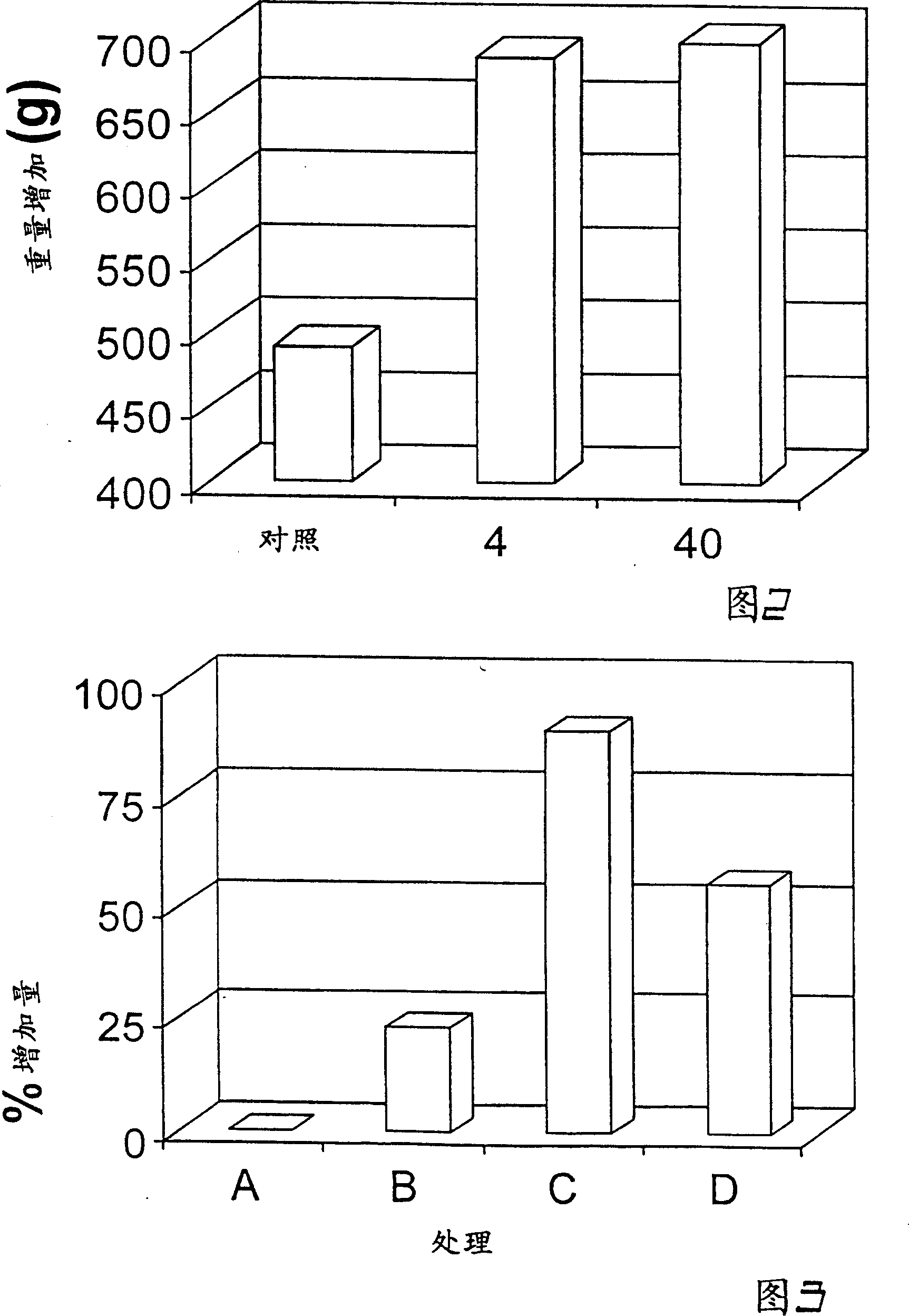
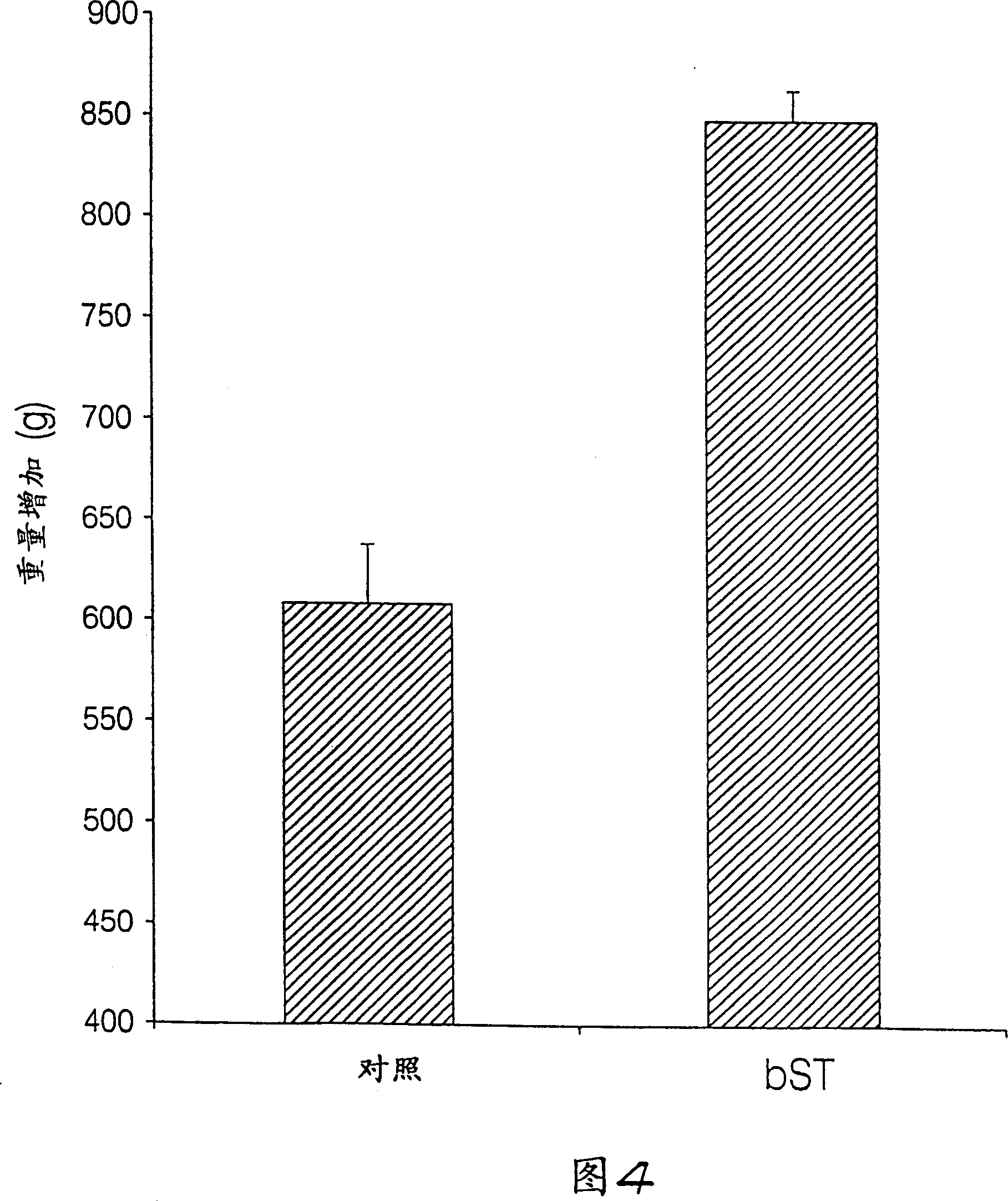
Patents
Literature
40 results about "Enterosorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adsorption of substances from the gastrointestinal tract onto an orally administered sorbent medium like activated charcoal. This technique is used to eliminate toxic and some biologically active substances and serves to modify the lipid and amino acid spectrum of the intestinal contents.
Nanoparticle compositions and methods for improved oral delivery of active agents
ActiveUS20120009267A1Minimize burst effectPromote absorptionOrganic active ingredientsBiocideEnteral administrationOral medication
Nanoparticles, compositions, and methods for the improved uptake of active agents are disclosed herein. The compositions contain a monodisperse population of nanoparticles, preferably including an active agent, where the nanoparticles are formed from a polymeric material possessing specified bioadhesion characteristics. Following enteral administration, preferably oral administration, the nanoparticles exhibit total intestinal uptakes of greater than 20%, preferably greater than 45%, more preferably greater than 65%. When compared to uptake of the same composition in the absence of the bioadhesive polymeric material, the nanoparticles have significantly increased uptake with intestinal uptake of the increased by more than 100%, preferably even greater than 500%. Further disclosed herein is a method of producing multi-walled nanoparticles, as well as methods of using thereof. Multi-walled particles prepared using the method disclosed herein are useful for controlling the release of active agents.
Owner:BROWN UNIVERSITY
Preparation method of Ovatodiolide and application of Ovatodiolide in anti-tumor drugs
InactiveCN104072513ASimple processEasy to operateOrganic active ingredientsOrganic chemistryWilms' tumorPollution
The invention discloses a preparation method of Ovatodiolide and an application of Ovatodiolide in anti-tumor drugs. The preparation method comprises the following steps: (1) taking Indian epimeredi herb leaf coarse powder, and performing continuous counter-current ultrasonic extraction by using a 90% ethanol solution to obtain an extracting solution; (2) concentrating the extracting solution, and then performing aluminum oxide column chromatography; (3) performing preparative liquid chromatography separation; (4) performing acetone recrystallization to obtain a high-purity Ovatodiolide crystallization product. The preparation method disclosed by the invention is simple and convenient in process operation, high in yield and small in pollution, and is suitable for producing high-purity Indian epimeredi herb dilactone. Indian epimeredi herb dilactone has an anti-tumor effect, and an intestinal absorption promoter is added into an oral formula of Indian epimeredi herb dilactone so as to facilitate the improvement of bioavailability.
Owner:NANJING ZELANG MEDICAL TECH
Composition for modulating a physiological reaction or inducing an immune response
InactiveUS20050175724A1Sufficient amountReasonable benefit/risk ratioAntibacterial agentsDispersion deliveryEnzymatic digestionActive agent
The present invention relates to a new composition, uses and method for modulating a physiological reaction or inducing an immune response after oral administration to a human or an animal of a physiologically active agent comprising (at least one) neutralizing agents to increase pH in the digestive system to prevent denaturation, (one) inhibitors of digestive enzymes to substantially prevent enzymatic digestion, and at least (one) uptake-increasing agents which increases intestinal absorption of a physiologically active agent, a drug and / or a nutrient.
Owner:PEROS SYST TECH
Edible nutritive health product for heart-dioscorea pig heart
InactiveCN101053395AIncrease coronary blood flowPrevent atherosclerosisFood preparationCardiovascular disorderNutritionTryptophan
A nutritive food dioscorea pig heart containing peltate yam, fresh pig heart, fresh peach kernel, and fresh rehmannia root etc., according to the nutrition characteristics, the character and taste, traits and the rate of its trace elements, amino acids, composes by compatibility inversion method special used for heart. Its characteristic is that in order to promote nutrient absorption in vivo according to characteristics of each component to adopt plant diversity physical and chemical method, to design specific preparing technology and processing, with compatibility balance method to protect the gastrointestinal absorption, to bring active function into full play. The present invention is the first time adopted net density dehydration technique, electrostatic electrolysis technology, soft magnetization technology, and uses traditional Chinese medicine processing technology, dish cooking technology, food can preparation technology and isoflux synthetic technology at the same time, with negative pressure vacuum technology and radiation technology to implement sterilization and preservative treatment. The present invention makes up the defects of traditional formula and preparation process in health food products. The present invention can participate in multi-physicochemical in the body, biochemical metabolism, effectively participate in the amino acid synthesis and decomposition, enhance protein biological activity, advance the impact of glutamic acid and tryptophan, promote central neurotransmitter synthesis and degradation, Enhance heart contractility and energy and oxygen supplying for myocardial, maintenance coronary patency, keep heart activity. The present invention also benefits liver and kidney during biotransformation and metabolism in vivo.
Owner:王晓光
Medicine for treating anorexia, limosis and preparing method thereof
InactiveCN101229331AIncrease secretionIncrease acidityAntibacterial agentsDigestive systemNormal functioningRhizome
The invention pertains to the traditional Chinese medicine field, which provides a medicine used for curing anorexia and limosis and is produced by the following raw medicines with portions by weight: 9-10 portions of corn, 9-10 portions of yam, 3-4 portions of corium stomachichum galli, 3-4 portions of atractylodes rhizome, 2-3 portions of monascus, 0.5-1 portions of rhubarb and 2-3 portions of black soybeans. The medicine of the invention gathers the health preserving and hygiene, treatment and rehabilitation in integral whole, promotes stomach reception, splenic normal functioning and intestinal absorption, and can achieve the aims of effectively curing the anorexia and the limosis through opsonizing human intestinal flora.
Owner:魏贤辉
Oral recombinant helicobacter pylori protein vaccine nanoparticles and preparation method thereof
ActiveCN113648405AGood water solubilityGastroprotectiveAntibacterial agentsBacterial antigen ingredientsHelicobacterNanoparticle
The invention belongs to the field of pharmaceutical preparations, and relates to a recombinant protein vaccine alpha 1,3-fucosyltransferase nanoparticle and a preparation method thereof. The recombinant protein vaccine alpha 1,3-fucosyltransferase nanoparticles prepared by the invention not only can improve the absorption of alpha 1,3-fucosyltransferase in body intestines, but also can improve the ability of macrophages to take in alpha 1,3-fucosyltransferase, and can be used for preventing and treating infection caused by helicobacter pylori.
Owner:CHONGQING MEDICAL UNIVERSITY
Integrated human gastrointestinal tract digestive absorption in-vitro substitution model
ActiveCN112029663AImprove stabilityGood repeatabilityBioreactor/fermenter combinationsBiological substance pretreatmentsBiotechnologyGastric digestion
The invention provides an integrated human gastrointestinal tract digestive absorption in-vitro substitution model. 12 systems are integrated into an integrated in-vitro simulation digestive model. The systems include a stomach digestion simulation system, a small intestine digestion simulation system, a small intestine absorption simulation system, a large intestine decomposition simulation system, a central intelligent control system, a pH control system, a temperature control system, a stirring rate control system, a conveying system, a central purification system, an automatic cleaning anddisinfection system and an anaerobic system. Through the synergistic effect of the systems, the in-vitro substitution model not only simulates the digestive absorption process of gastrointestinal tracts in vitro, but also simulates the fermentation and decomposition process of intestinal microbial communities at the same time. The model is suitable for accurately quantifying the in-vivo metabolicprocess and toxic effect of nutrients or pollutants in a food matrix to obtain related data such as biological accessibility, biological effectiveness, metabolism trend and microorganism interactionof the nutrients or pollutants, and accurate risk assessment is realized.
Owner:SOUTHERN MEDICAL UNIVERSITY +1
Preparation process for cyclosporine A-pH sensitive nanoparticle
InactiveCN104434804AThe preparation process is fastImprove conversion ratePowder deliveryCyclic peptide ingredientsPh sensitive nanoparticlesOrganosolv
Belonging to the field of chemical technologies, the invention relates to a preparation process for a cyclosporine A-pH sensitive nanoparticle, and more specifically relates to improvement of the preparation process of the cyclosporine A-pH sensitive nanoparticle. The preparation process for the cyclosporine A-pH sensitive nanoparticle is characterized by low cost and high synthesis rate, and the product has good intestinal absorption effect. The preparation process for the cyclosporine A-pH sensitive nanoparticle includes the steps of: weighing F68 and dissolving it in 125mL distilled water to serve as the water phase; dissolving 50mg of CyA and a proper amount of EudragitS100 in anhydrous ethanol to prepare an organic phase; injecting the organic phase into the water phase stirred at a speed of 400r.min with a No. 7 support marrow puncture needle quickly; and then conducting stirring continuously and transferring the mixture into 60DEG C water bath, and volatilizing the organic solvent completely, thus obtaining a CyA-NP colloidal solution.
Owner:李志刚
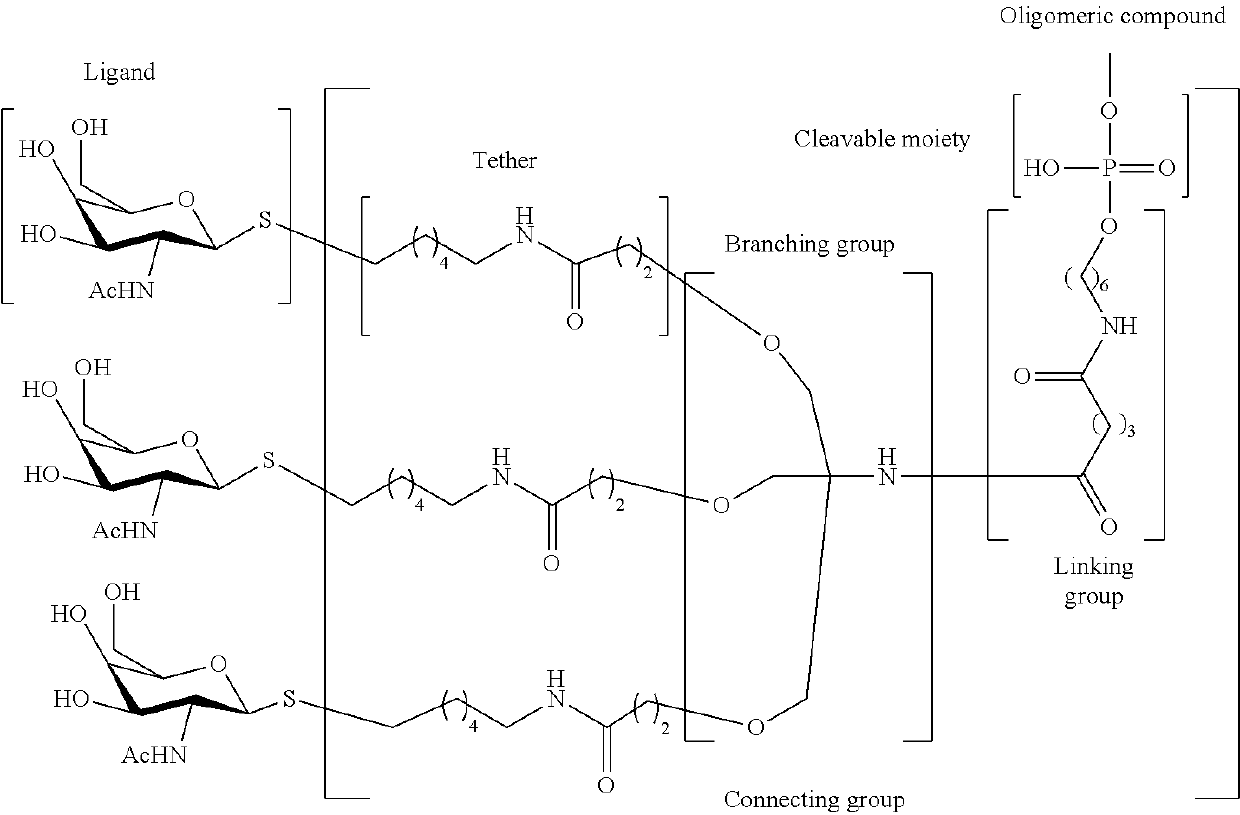
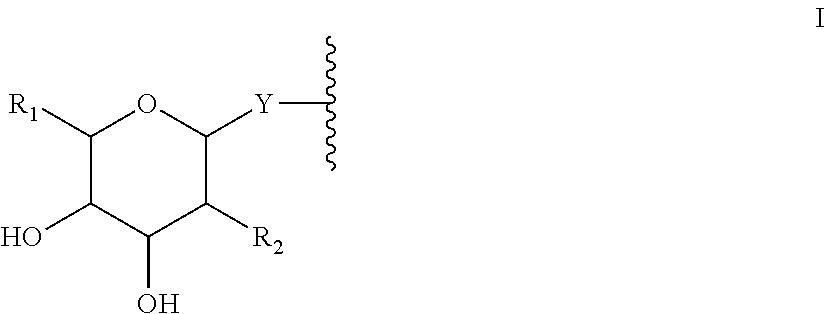
Compositions and methods for enhanced intestinal absorption of conjugated oligomeric compounds
InactiveUS20200157548A1Improve propertiesHigh potencyOrganic active ingredientsSpecial deliveryCombinatorial chemistryOrganic chemistry
Provided herein are compositions and methods for non-parenteral delivery of conjugated oligomeric compounds. In certain embodiments, compositions and methods are provided for oral delivery of conjugated oligomeric compounds. In certain embodiments, the oligomeric compounds are conjugated to one or more N-acetylgalactosamines or N-acetylgalactosamine analogues.
Owner:IONIS PHARMA INC
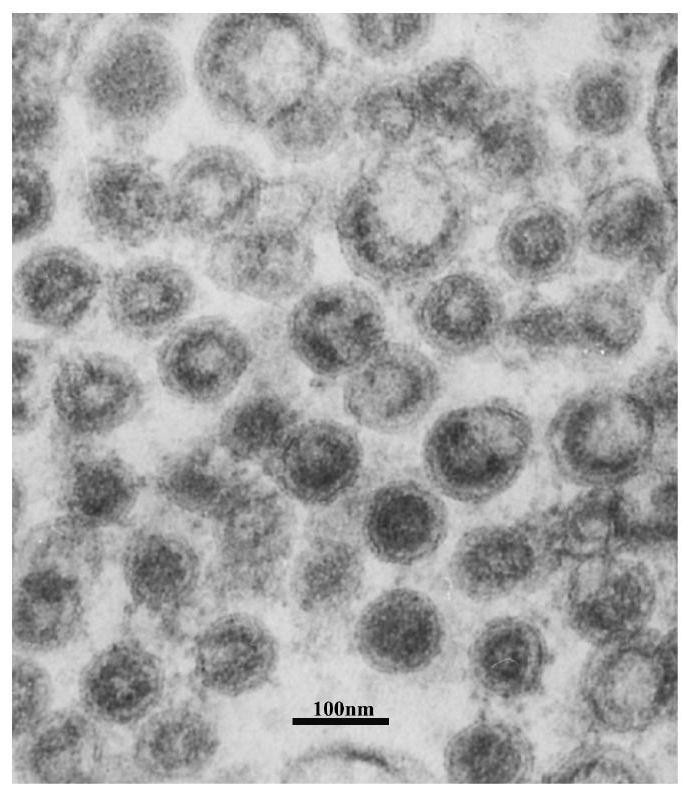
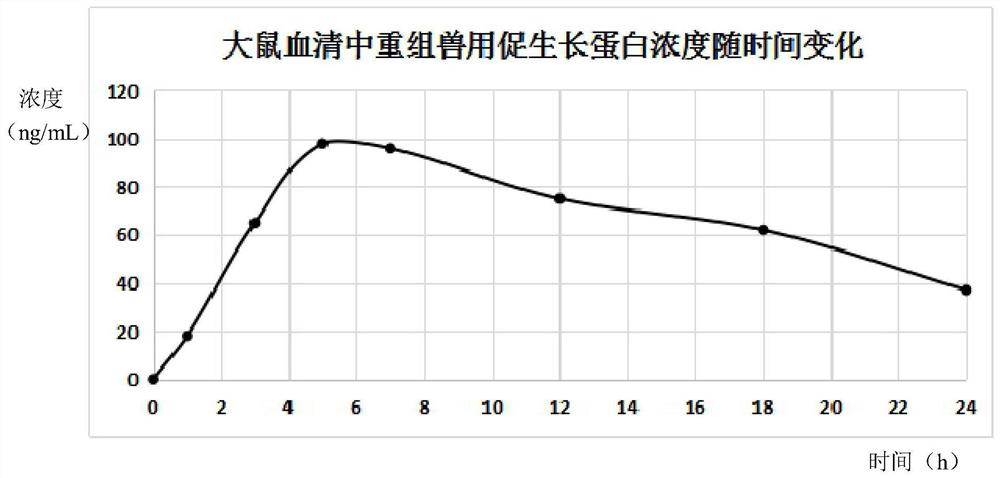
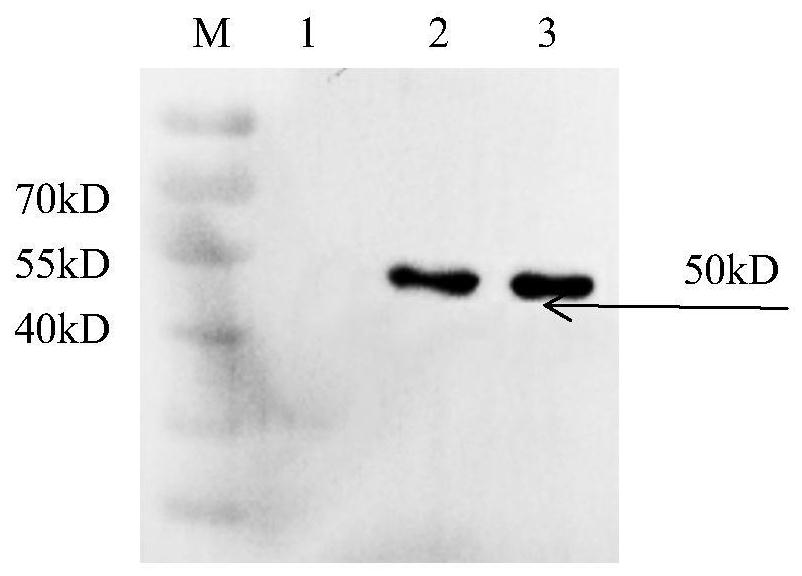
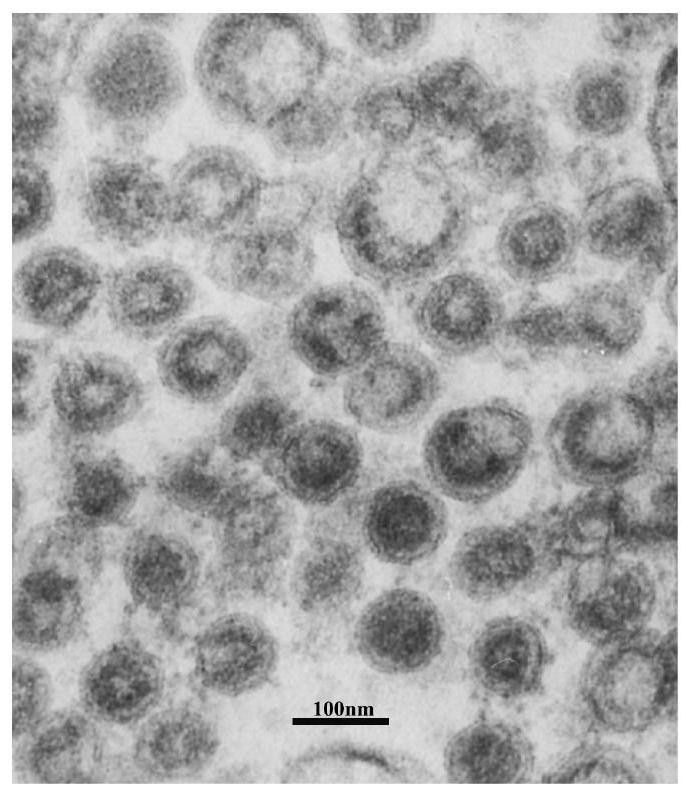
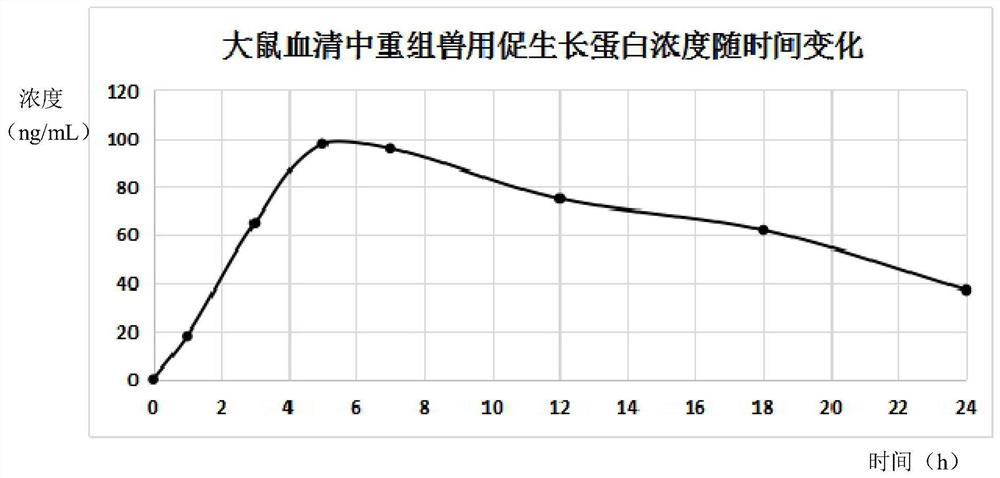
Liposome-encapsulated recombinant veterinary growth-promoting protein as well as preparation method and application of liposome-encapsulated recombinant veterinary growth-promoting protein
ActiveCN112724260APromote growthShort half-lifePeptide/protein ingredientsMetabolism disorderAnimal scienceSomatotropic hormone
The invention relates to the technical field of gene recombination, and particularly discloses lipidosome-encapsulated recombinant growth-promoting protein for animals as well as a preparation method and application of the lipidosome-encapsulated recombinant growth-promoting protein for animals, the nucleotide sequence of a coding gene of the recombinant growth-promoting protein for animals is shown as SEQ ID NO: 1, and the recombinant growth-promoting protein is fusion protein of pig growth hormone and chicken growth hormone and can simultaneously play a role in promoting growth of pigs and chickens; the molecular weight of the protein is large, and the problem of short half-life period can be well solved; the recombinant fusion protein is encapsulated in the liposome, so that decomposition of gastrointestinal tract protease can be avoided, and more importantly, the liposome has an effect of promoting absorption and can guide intestinal absorption of the protein. The veterinary growth-promoting hormone is produced in a recombination mode. Compared with an extraction method, the method is simple and low in cost and can be widely applied to feed additives.
Owner:安徽中起生物科技有限公司
An integrated in vitro surrogate model of human gastrointestinal digestion and absorption
ActiveCN112029663BImprove stabilityGood repeatabilityBioreactor/fermenter combinationsBiological substance pretreatmentsGastric digestionIntestinal microorganisms
The invention provides an integrated human gastrointestinal digestion and absorption in vitro substitution model, which is integrated by 12 systems into an integrated in vitro simulation digestion model, including a gastric digestion simulation system, a small intestine digestion simulation system, a small intestine absorption simulation system, a large intestine Decomposition simulation system, central intelligent control system, pH control system, temperature control system, stirring rate control system, conveying system, central purification system, automatic cleaning and disinfection system and anaerobic system. Through the synergy of various systems, this in vitro surrogate model not only simulates the digestion and absorption process of the gastrointestinal tract in vitro, but also simulates the fermentation and decomposition process of the intestinal microbial community. This model is suitable for accurately quantifying the metabolic process and toxic effect of nutrients or pollutants in the food matrix in the body, and obtaining relevant data such as bioaccessibility, bioavailability, metabolic fate and interaction with microorganisms of nutrients or pollutants. Realize precise risk assessment.
Owner:SOUTHERN MEDICAL UNIVERSITY +1
Human lysozyme suppository, preparation method and application
ActiveCN1593654ASafe and non-toxic side effectsRich sourcesAntibacterial agentsPeptide/protein ingredientsIntestinal structureMedicine
The invention relates to the use of human lysozyme in biological medicines targeting drug resisting bacterium and viruses, the invention also discloses its preparation and use thereof. The human lysozyme film agent contains active 300U - 3 million U / ml of human lysozyme, the suppository prepared thereby can be administered directly through rectal intestine, thus can prevent the gastric mucosa from being stimulated, it can facilitate administration to infant and children by using this method.
Owner:安米
Paclitaxel oral polymeric micelle and preparation method and application thereof
ActiveCN111053740AWell-formed micellesUniform sizeOrganic active ingredientsPharmaceutical non-active ingredientsCritical micelle concentrationOral medication
The invention discloses a paclitaxel oral polymeric micelle. Paclitaxel is used as a model drug, and a GA-CS-TPGS copolymer is used as a carrier for oral medication. The critical micelle concentrationof the GA-CS-TPGS copolymer is 0.0109 mg / mL to 0.0203 mg / mL, and the partical size of the paclitaxel oral polymeric micelle is controlled to be 98-182nm, wherein the molecular structural formula of the GA-CS-TPGS copolymer is shown as a formula (1). The invention also discloses a preparation method and application of the paclitaxel oral polymeric micelle. The paclitaxel oral polymeric micelle hasgood adhesion and intestinal absorption, the bioavailability of paclitaxel is improved, and the requirement of oral medication of paclitaxel can be met.
Owner:GUANGXI UNIV OF CHINESE MEDICINE +1
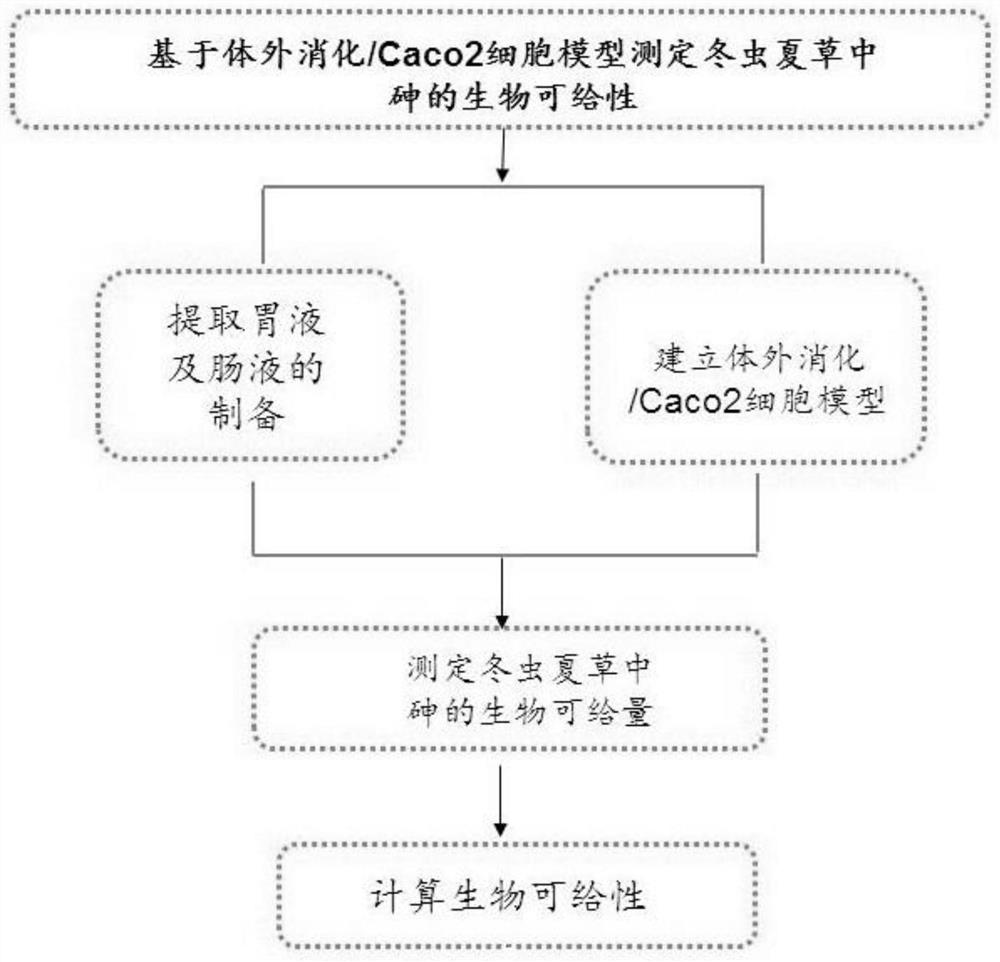
Method for determining bioavailability of arsenic in cordyceps sinensis based on in-vitro digestion/Caco2 cell model
PendingCN112557495ASave resourcesPreparing sample for investigationMaterial analysis by electric/magnetic meansBiotechnologyHuman body
The invention provides a method for determining bioavailability of arsenic in cordyceps sinensis based on an in-vitro digestion / Caco2 cell model, and belongs to the technical field of heavy metal biological availability detection. According to the method, a Caco-2 intestinal absorption model is established by adopting transwell culture, the in-vitro digestion / Caco-2 cell model is established by adopting a two-step digestion method for simulating gastric and intestinal digestion in vitro, and the biological availability of arsenic in cordyceps sinensis is determined by adopting an ICP-MS method, so a basis is provided for scientifically and objectively evaluating health risks of heavy metals in traditional Chinese medicines and modifying limit standards. Experiments prove that after Caco-2cells in cordyceps sinensis are transferred, the biological availability of arsenic is 1.5%-13.0%. According to the method, the development of the traditional Chinese medicine industry is promoted, resources are saved; meanwhile, a new thought is provided for objectively and scientifically evaluating the health risk of the heavy metal in the traditional Chinese medicine to the human body, and a basis is provided for formulating a scientific and reasonable heavy metal limit standard.
Owner:NAT INST FOR FOOD & DRUG CONTROL
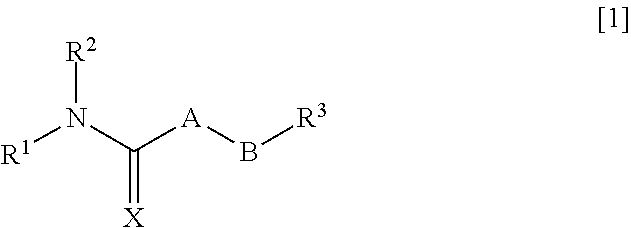
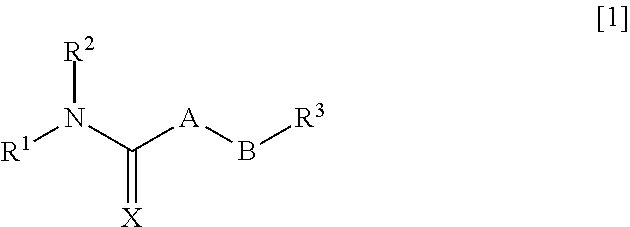
Pharmaceutical composition improving intestinal absorption
InactiveUS20110201655A1Good entryPromote intestinal absorptionBiocideSenses disorderChemical compoundPharmaceutical Substances
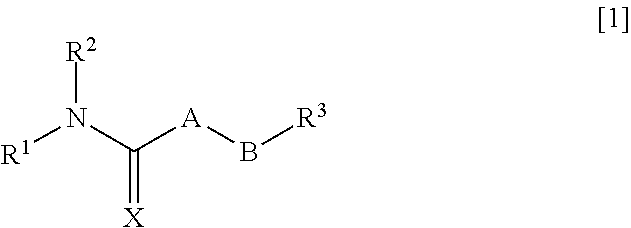
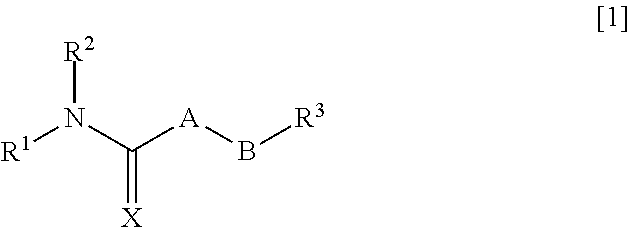
An object of the present invention is to provide a pharmaceutical composition that improves intestinal absorption of a compound having a structure represented by the general formula [1]. The composition containing a compound represented by the general formula [1] or a salt thereof and (b) a lipophilic substance improves intestinal absorption of the compound. In the formula, A represents —(NR4)—, —(CR5R6)— or the like; B represents an alkylene group or an alkenylene group; R1 represents an alkyl group, an alkenyl group or the like; R2 represents an adamantylalkyl group or the like; R3 represents an unsaturated heterocyclic ring; R4, R5 and R6 each represent a hydrogen atom or the like; and X represents an oxygen atom or the like.
Owner:AYUMI PHARMA
Pharmaceutical composition for improving intestinal absorption
InactiveUS8686006B2Good entryPromote intestinal absorptionBiocideSenses disorderChemical compoundPharmaceutical Substances
An object of the present invention is to provide a pharmaceutical composition that improves intestinal absorption of a compound having a structure represented by the general formula [1]. The composition containing a compound represented by the general formula [1] or a salt thereof and (b) a lipophilic substance improves intestinal absorption of the compound. In the formula, A represents —(NR4)—, —(CR5R6)— or the like; B represents an alkylene group or an alkenylene group; R1 represents an alkyl group, an alkenyl group or the like; R2 represents an adamantylalkyl group or the like; R3 represents an unsaturated heterocyclic ring; R4, R5 and R6 each represent a hydrogen atom or the like; and X represents an oxygen atom or the like.
Owner:AYUMI PHARMA
Pharmacological method of drug-containing liver incubation liquid and preparation method of drug-containing liver incubation liquid of ephedra, semen armeniacae amarae, gypsum and liquorice decoction
PendingCN112899217AConditions are strictly controllableReduce distractionsMicrobiological testing/measurementArtificial cell constructsPharmacologic actionPharmacometrics
The invention discloses a pharmacological method of drug-containing liver incubation liquid and a preparation method of the drug-containing liver incubation liquid of ephedra, semen armeniacae amarae, gypsum and liquorice decoction. The preparation method comprises the following steps of: processing orally-administrated traditional Chinese medicines according to a clinical method, taking decoction, obtaining drug-containing intestinal absorption liquid by adopting an everted enteric capsule method, taking the drug-containing intestinal absorption liquid and liver cell suspension for short-time incubation, and then preparing the drug-containing liver incubation liquid for pharmacological tests of in-vitro tissues and organs, cells and the like. The drug-containing liver incubation liquid prepared by the preparation method disclosed by the invention can reflect the actual migration or transformation status of chemical components of a traditional Chinese medicine compound entering a body more truly, has the advantages of strict and controllable conditions, less interference, high content of active ingredients, small standard deviation between groups and the like, and is used for in-vitro pharmacological research without the interference of endogenous components of serum, so that the drug-containing liver incubation liquid is high in stability and good in reproducibility and is more suitable for discussion of in-vitro pharmacological effects of the traditional Chinese medicine compound and mechanism thereof.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Pegylated phenol red, preparation method and application thereof
ActiveCN111217993ANo pollution in the processEasy to makeBiological particle analysisPermeability/surface area analysisPharmaceutical SubstancesPolymer
The invention belongs to the technical field of medicines, and relates to pegylated phenol red, a preparation method and application thereof, wherein the polymer is formed by connecting a small molecular drug phenol red and one end of polyethylene glycol (PEG) through a covalent bond, the phenolic hydroxyl group on the phenol red is covalently connected with the long chain of the polyethylene glycol derivative through an ether bond, the structure of the polymer is in the specification under different pH conditions, and n and R are defined in the claims and the specification. The invention alsoprovides a preparation method of the pegylated phenol red. The invention further discloses that the pegylated phenol red has an accurate moisture correction effect when being applied to a body intestinal absorption test. The method has the advantages of easily available raw materials, simple operation, wide application range and environmental protection reaction.
Owner:SHENYANG PHARMA UNIVERSITY
Intestine-kidney system for simulating drug absorption process in vivo based on microfluidic chip
ActiveCN107955790BReduce failure rateIn line with the actual situationCompound screeningApoptosis detectionNephrotoxicityIn vivo absorption
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Medicine for treating anorexia, limosis and preparing method thereof
InactiveCN101229331BIncrease secretionIncrease acidityAntibacterial agentsDigestive systemMonascus ankaAnorectic
Owner:魏贤辉
A kind of Laoxianghuang paste and preparation method thereof
ActiveCN109275909BEasy to brewShorten the timeFood ingredient as taste affecting agentFood ingredient as flavour affecting agentCelluloseMedicinal herbs
Owner:HANSHAN NORMAL UNIV
Rodgersia sambucifolia hemsl medicinal composition with good stability and high bioavailability as well as preparation method and application
ActiveCN102836185ALow hygroscopicityImprove stabilityDigestive systemAluminium/calcium/magnesium active ingredientsAdjuvantDrugs preparations
The invention discloses a rodgersia sambucifolia hemsl medicinal composition with good stability and high bioavailability as well as a preparation method and an application of the medicinal composition. The composition provided by the invention comprises 1.5 to 5.0% by weight of rodgersia sambucifolia hemsl extract, 5.0 to 24.0% by weight of flavoring agent and the balance of bentonite. The method provided by the invention comprises the processes of active component extracting, ingredient processing and mixing; the active component extracting means grinding the rodgersia sambucifolia hemsl into coarse powder, extracting with ethanol, concentrating to obtain a paste, adjusting the pH value, filtrating and concentrating to obtain a thick paste, diluting, decoloring, filtrating and concentrating to obtain a concentrated thick paste, standing, filtrating the precipitate, and drying to obtain a rodgersia sambucifolia hemsl active component extract; crushing and screening the bentonite; and mixing a main material and the adjuvants to obtain a target composition. According to the invention, the rodgersia sambucifolia hemsl extract is taken as an active component of the medicinal composition, and the formula is reasonably arranged to completely give full play to the effect of the adjuvants on improving the physical and chemical property of a medicinal preparation and the gastrointestinal absorption capacity of the human bodies, so that the stability and the bioavailability of the preparation are improved, the taste is improved, and the crowds with fear of sugars suitable for the composition can be expanded.
Owner:YUNNAN SHIPURUI BIOLOGICAL ENG
Human lysozyme suppository, preparation method and application
ActiveCN100420484CAntibacterial agentsPeptide/protein ingredientsSuppository drugPharmaceutical drug
Owner:安米
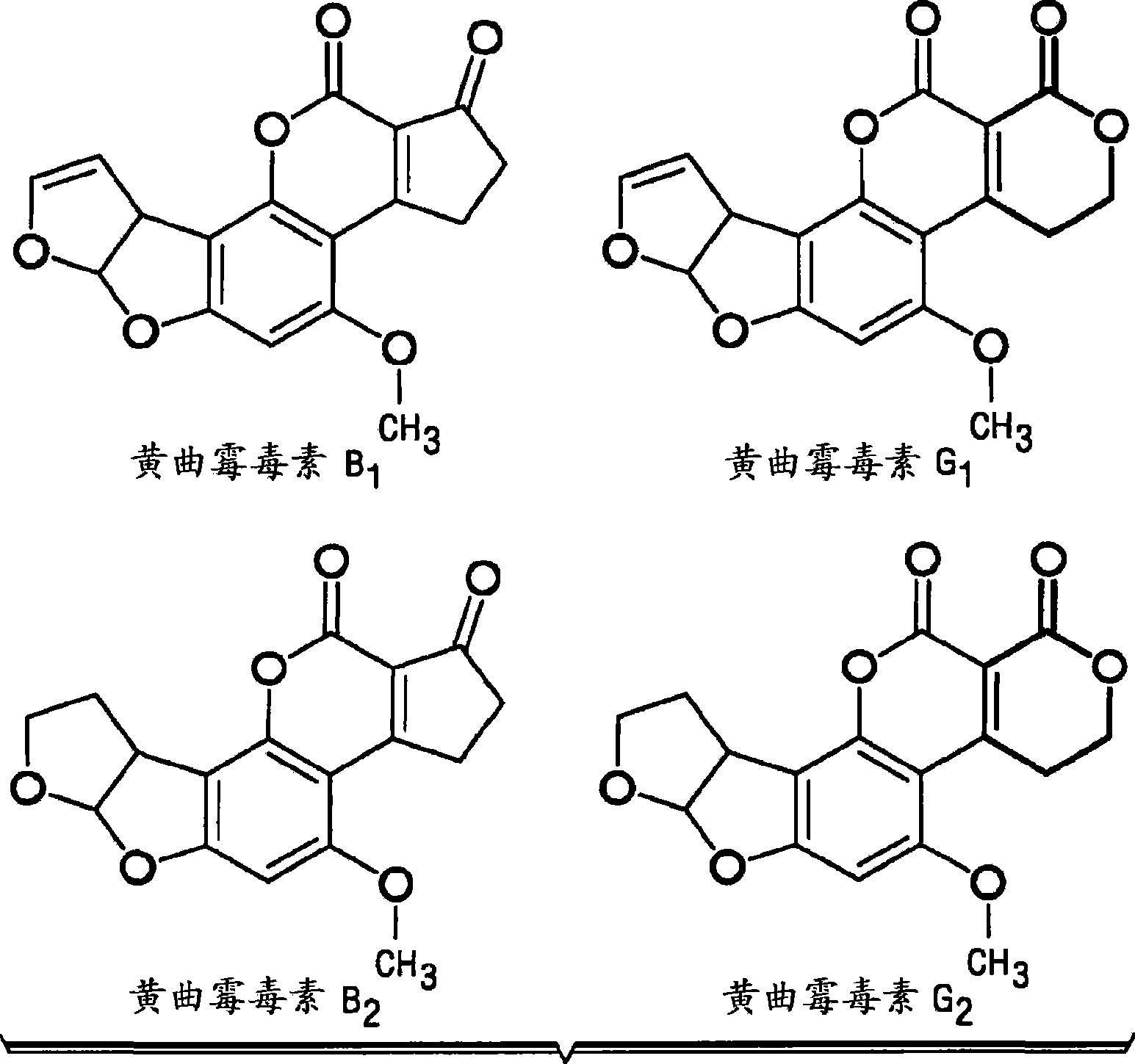
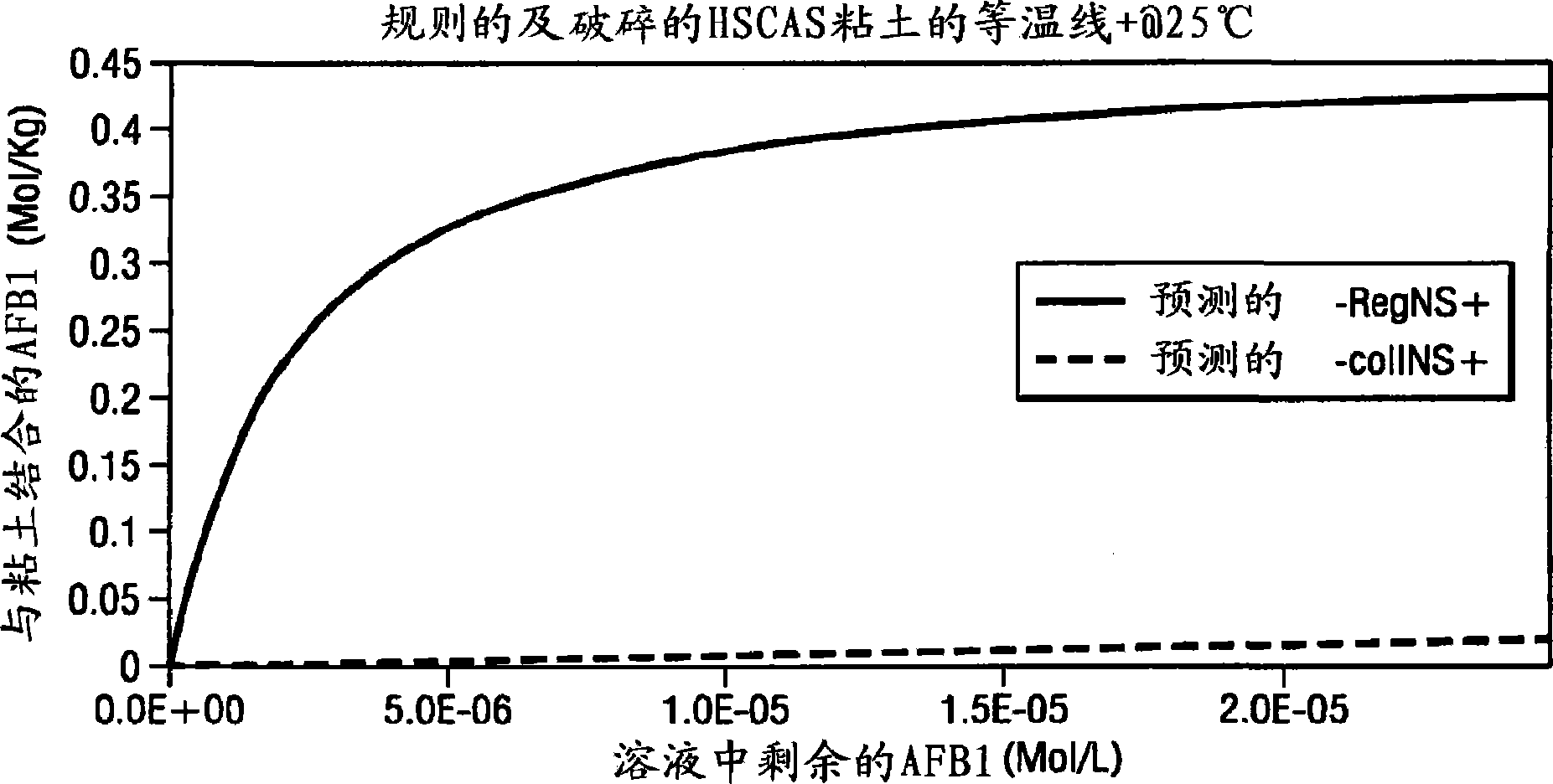
Composition and methods for the enterosorption and management of toxins
InactiveCN101522203AEliminate the effects ofOrganic active ingredientsInanimate material medical ingredientsCalcium silicateHepatocellular carcinoma
A composition and method for use as a preventive therapy to mitigate the effects of environmental toxins, and particularly aflatoxins in a subject. The subject may be at risk for hepatocellular carcinoma and aflatoxicosis. The composition comprising: an effective amount of an isolated low sodium, calcium aluminosilicate clay in a powder form, wherein the isolated low sodium, calcium aluminosilicate clay contains acceptable levels of dioxins and priority toxic heavy metal contamination, and is capable of preferentially binding aflatoxins in the gastrointestinal tract.
Owner:TEXAS A&M UNIVERSITY +1
Composition for intestinal delivery
The present invention relates to a new composition, use and method for oral administration to a human or an animal of a physiologically active agent comprising neutralizing agents to increase pH in the digestive system to prevent denaturation, inhibitors of digestive enzymes to substantially prevent enzymatic digestion, and at least uptake-increasing agents which increases intestinal absorption of a physiologically active agent, a drug and / or a nutrient.
Owner:佩罗斯美国公司
A liposome-encapsulated recombinant veterinary growth-promoting protein and its preparation method and application
ActiveCN112724260BPromote growthShort half-lifePeptide/protein ingredientsMetabolism disorderAnimal scienceSomatotropic hormone
The present invention relates to the technical field of genetic recombination, and specifically discloses a liposome-encapsulated recombinant veterinary growth-promoting protein and its preparation method and application. The nucleotide sequence of the gene encoding the recombinant veterinary growth-promoting protein is shown as SEQ ID NO Shown in: 1, it is the fusion protein of porcine somatotropin and chicken somatotropin, which can act on the growth promotion of pigs and chickens simultaneously; the molecular weight of this protein is relatively large, and can better solve the problem of short half-life; adopt liposome Encapsulating the recombinant fusion protein in it can avoid the decomposition of protease in the gastrointestinal tract, and more importantly, the liposome has the function of promoting absorption and can guide the intestinal absorption of the protein. The invention uses recombination to produce animal growth-stimulating hormone, which is simpler and lower in cost than the extraction method, and can be widely used in feed additives.
Owner:安徽中起生物科技有限公司
Special feed compound additive for anglerfish
The invention provides a special feed compound additive for squid, which belongs to the technical field of squid breeding, including (I) oligosaccharide composition; and / or (II) carboxylic acid ester; and (III) protease complex; the (III) protease The complex is a protease complex comprising a potent protease mixture obtained by culturing Myces lanuginosa. The invention also provides a special feed for squid containing the basic feed and compound additives for the special feed for squid. The beneficial effects are: the special feed compound additive for squid can improve the intestinal flora of squid and can promote the regeneration of intestinal villi, improve the efficiency of intestinal absorption, maintain the health of gastrointestinal tract and / or reduce the demand for antibiotics, and reduce the Abuse of antibiotics has significantly reduced the morbidity and mortality of goblins.
Owner:ZHEJIANG OCEAN UNIV
Preparation method and antitumor application of pendulone
InactiveCN104072466ASimple processEasy to operateOrganic active ingredientsOrganic chemistryAcetic acidEthyl ester
The invention discloses a preparation method and application of a plant extract and particularly relates to a preparation method and antitumor application of pendulone. The preparation method comprises the following steps of smashing rattans of millettia dielsiana, adding 5-10 times of 50-99% methanol solution, carrying out ultrasonic extraction 2-3 times, and adsorbing in a macroporous resin column after extracting and concentrating; eluting by using 3-9 times of 10-50% methanol solution, and then, eluting by using 5-10 times of 60-90% methanol solution; concentrating the eluate, and regulating the pH value of the solution to 3-5; adding ethyl acetate to sufficiently stir, collecting an ethyl acetate layer, and concentrating to obtain a solid; separating the solid by using a high-speed countercurrent chromatograph, collecting the fraction, and carrying out vacuum drying to obtain pendulone. The preparation method disclosed by the invention is simple and convenient is process operation, high in yield, little in pollution and suitable for producing high-purity pendulone. Pendulone has an antitumor promotion effect; an intestinal absorption enhancer is added in the formula of an oral liquid of pendulone, so that the bioavailability of pendulone is favorably increased.
Owner:NANJING ZELANG MEDICAL TECH
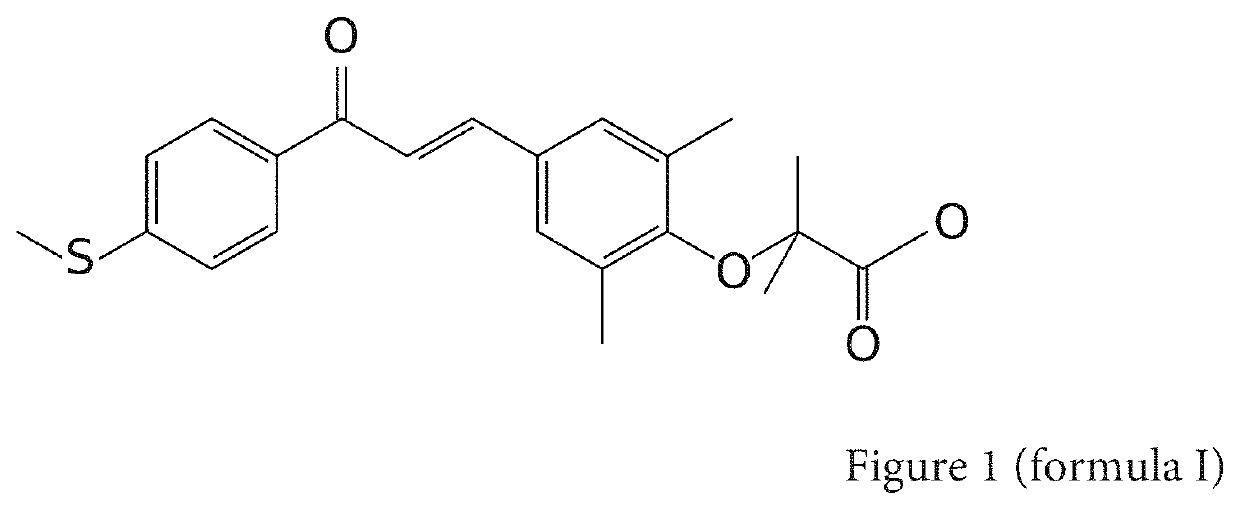
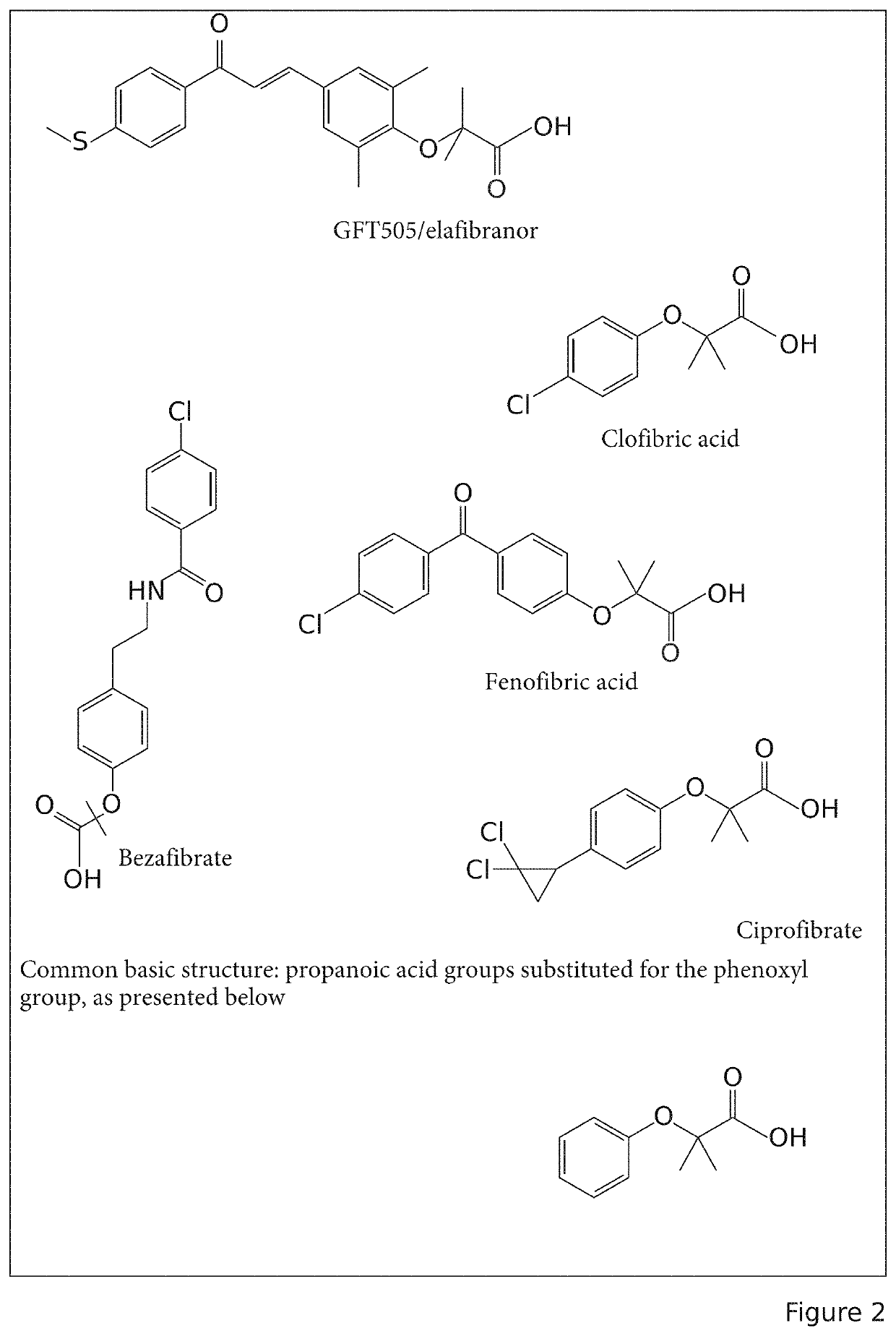
Composition comprising at least one water-soluble pharmaceutically acceptable salt of elafibranor having improved intestinal absorption
ActiveUS11185520B2Fast absorptionImprove bioavailabilityPowder deliveryOrganic active ingredientsEthanolaminesPharmaceutical medicine
A composition comprising, as an active principle, a pharmaceutically acceptable salt of elafibranor, characterised in that the pharmaceutically acceptable salt of elafibranor is chosen from at least a salt of choline, of ethanolamine, or of diethanolamine, or of L-lysine, or of piperazine, or of calcium, or of tromethamine. More particularly, one or more embodiments relate to the use of elafibranor salts with a view to improving stability and solubility compared with elafibranor in the basic form thereof. These salts make it possible to establish pharmaceutical formulations in various advantageous forms as intravenous injections or formulations by enteral route having quicker and less variable absorption and consequently better bioavailability.
Owner:NASHPHARM
Pharmaceutical Oral Dose Formulation and Composition of Matter
InactiveUS20180071214A1Low systemic bioavailabilityPrevents intestinal uptakePowder deliveryHydroxy compound active ingredientsQuercitrinEnterosorption
A method of formulating low solubility, low permeability and / or p-glycoprotein efflux transporter substrate drugs or herbal extracts for increased intestinal absorption and a composition of matter for said drugs or herbal extracts. The method utilizes a water, alcohol or organic solvent to complex a low solubility, low permeability and / or p-glycoprotein efflux transporter substrate drug or herbal extract, the p-glycoprotein inhibitors quercitin and piperine, with a carbohydrate. The result is a microemulsion of the drug or herbal extract which has both higher solubility and permeability than if dosed alone; as well as resistance to p-glycoprotein mediated efflux, once absorbed into the intestinal lumen.
Owner:THROWER DAVID W
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com