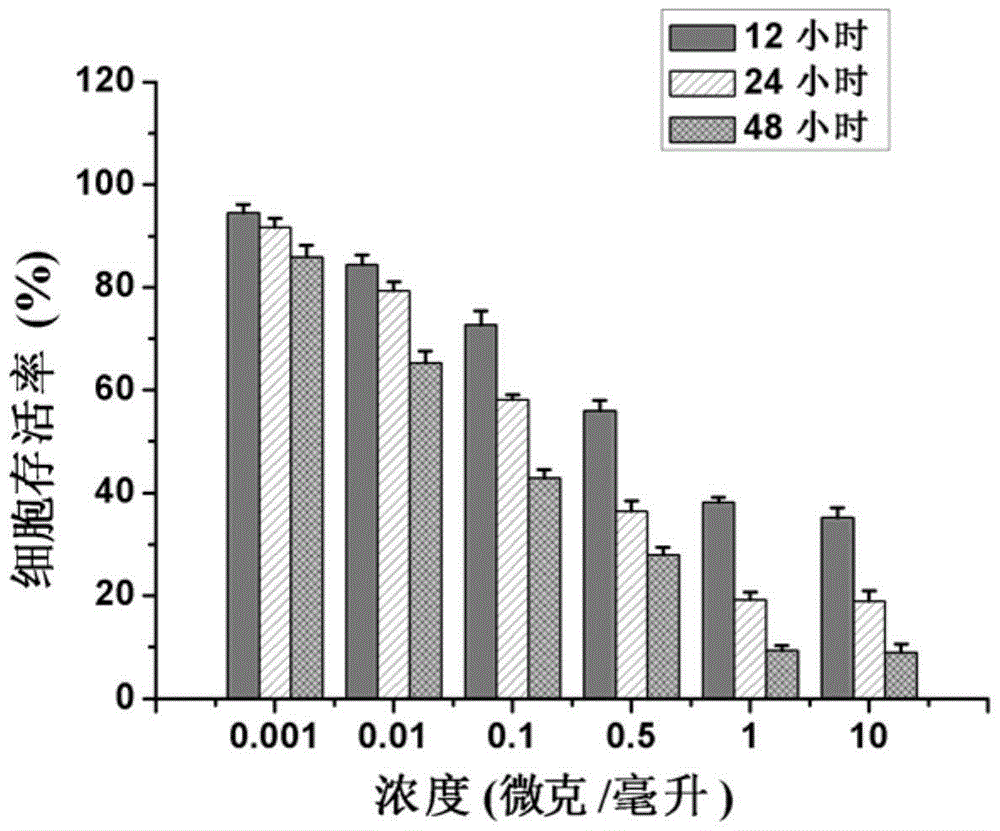
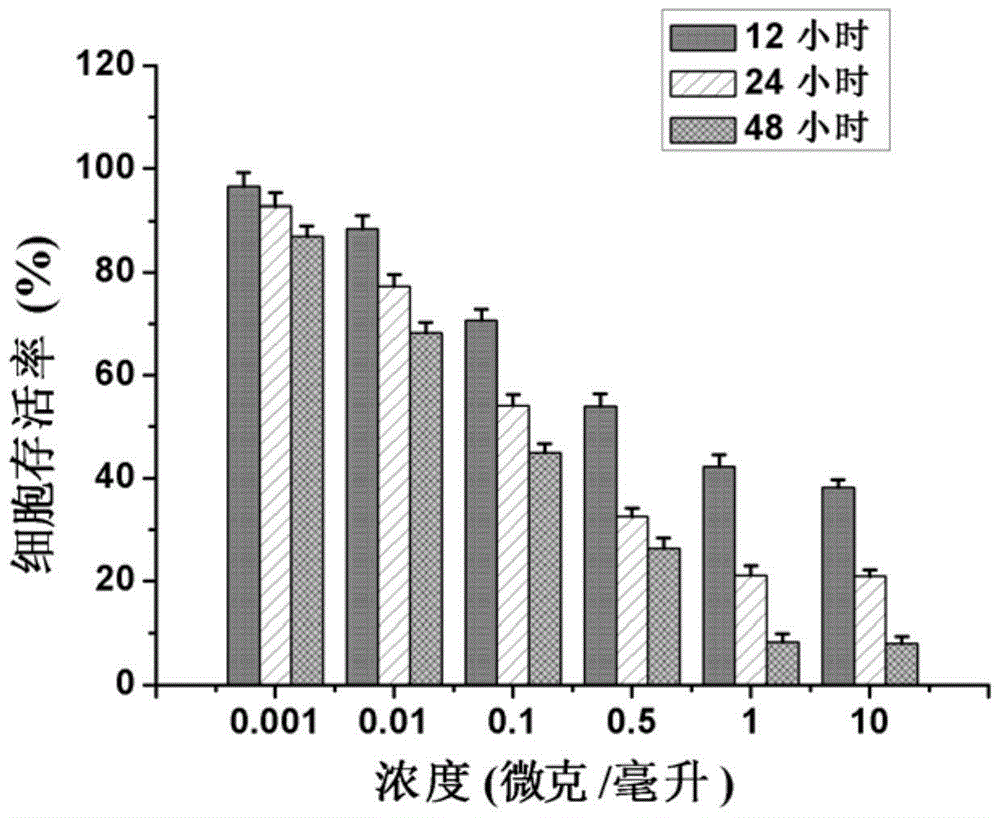
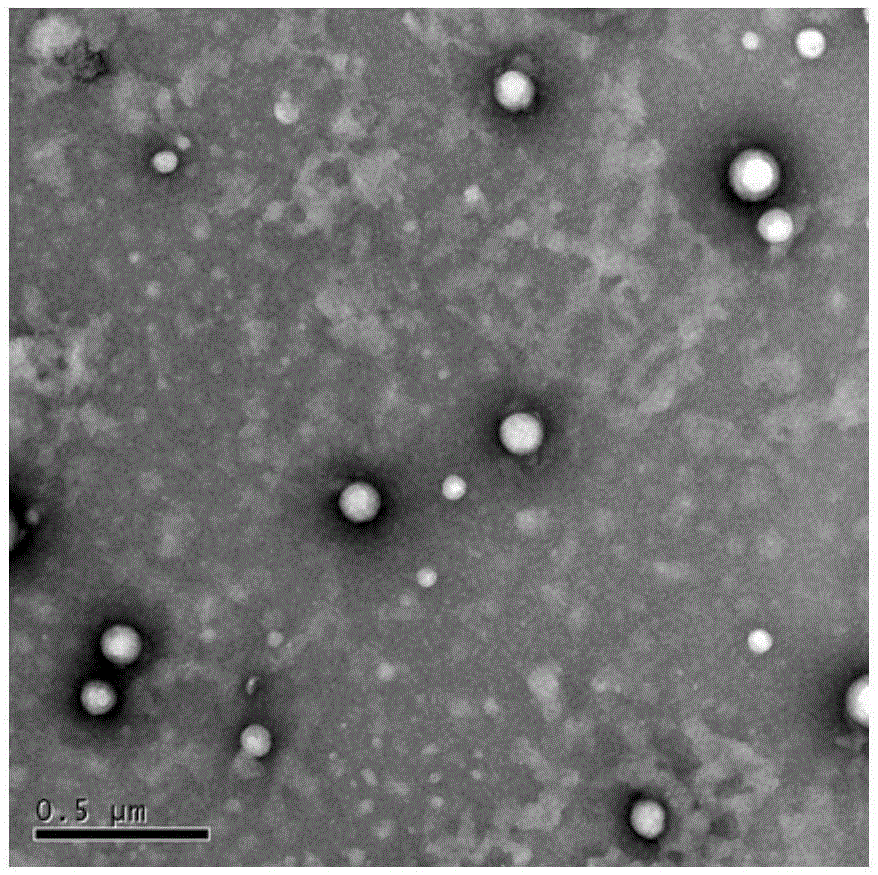
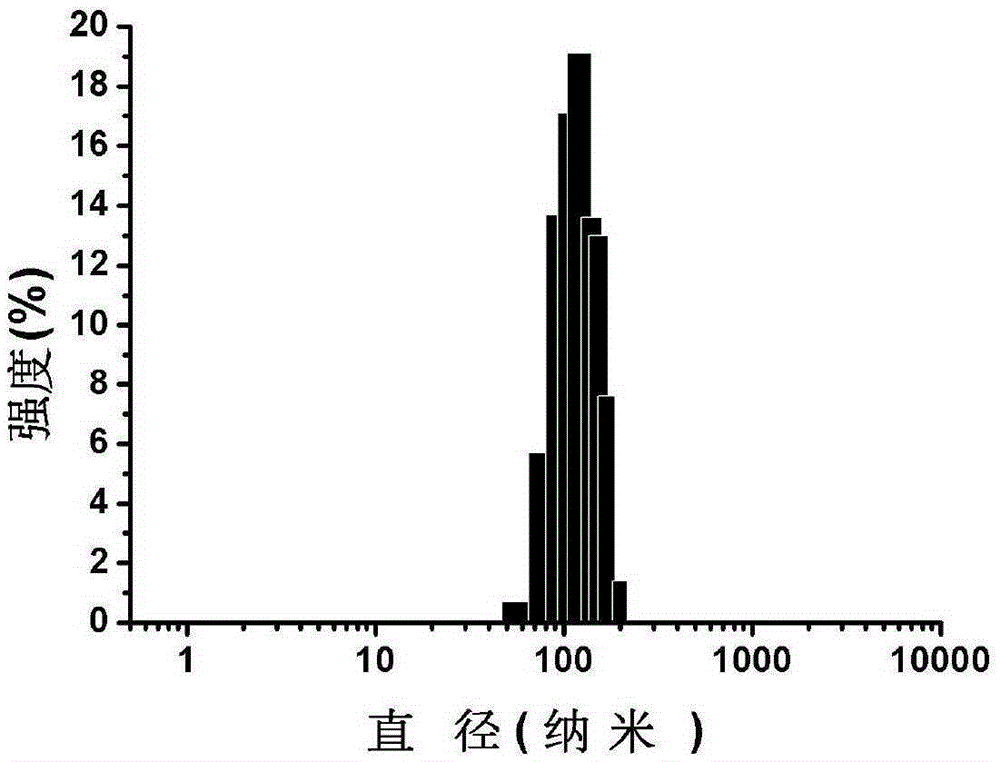
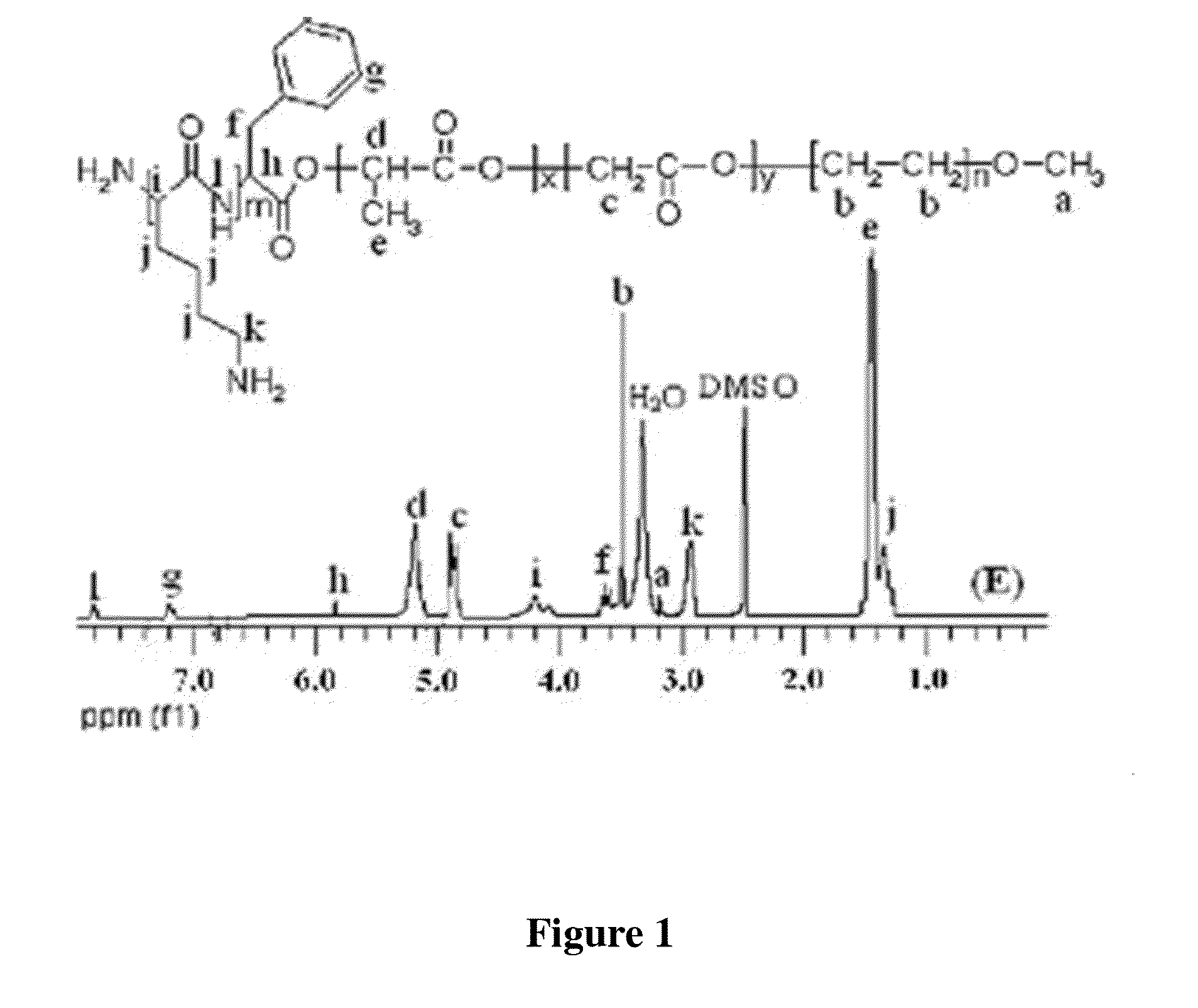
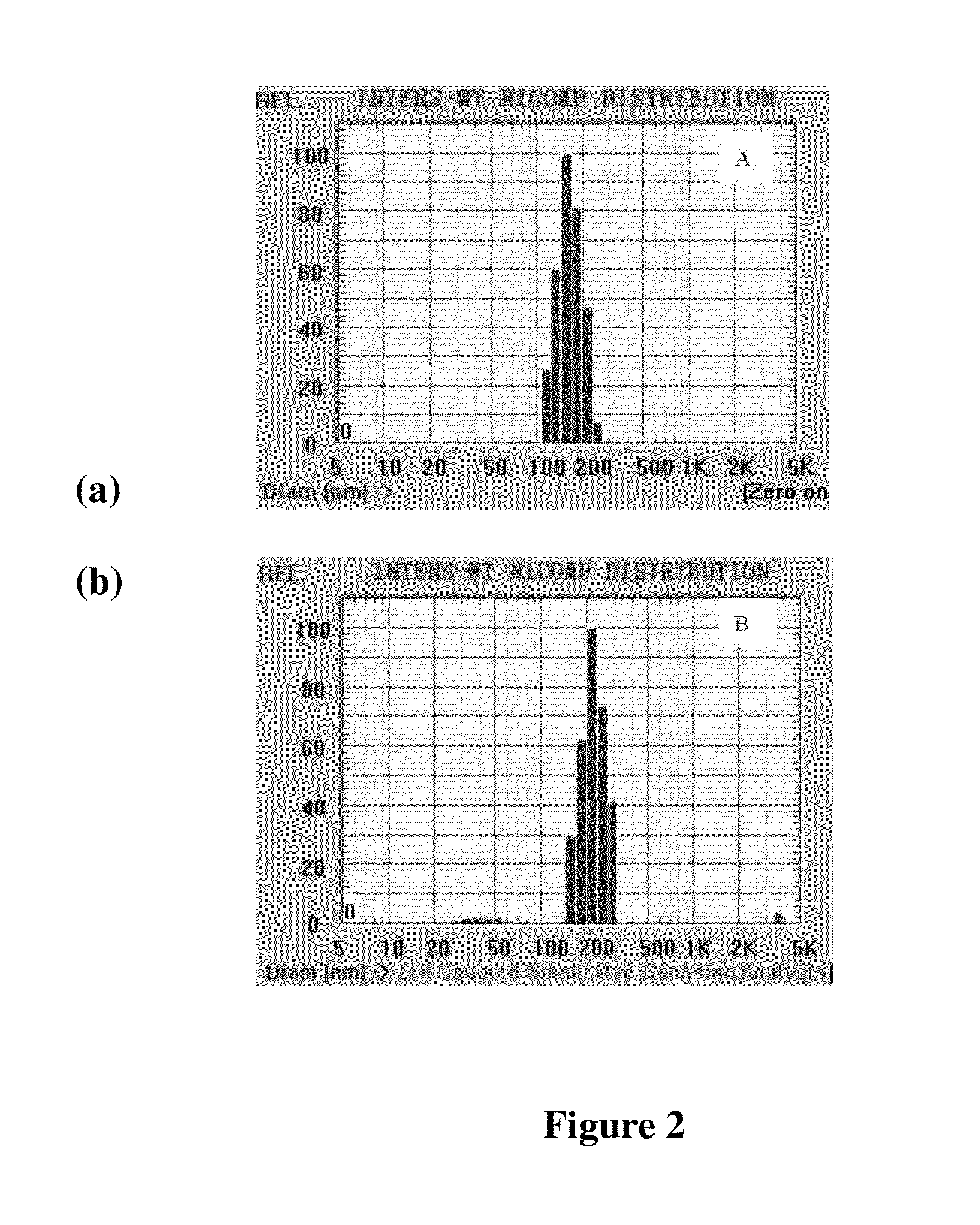
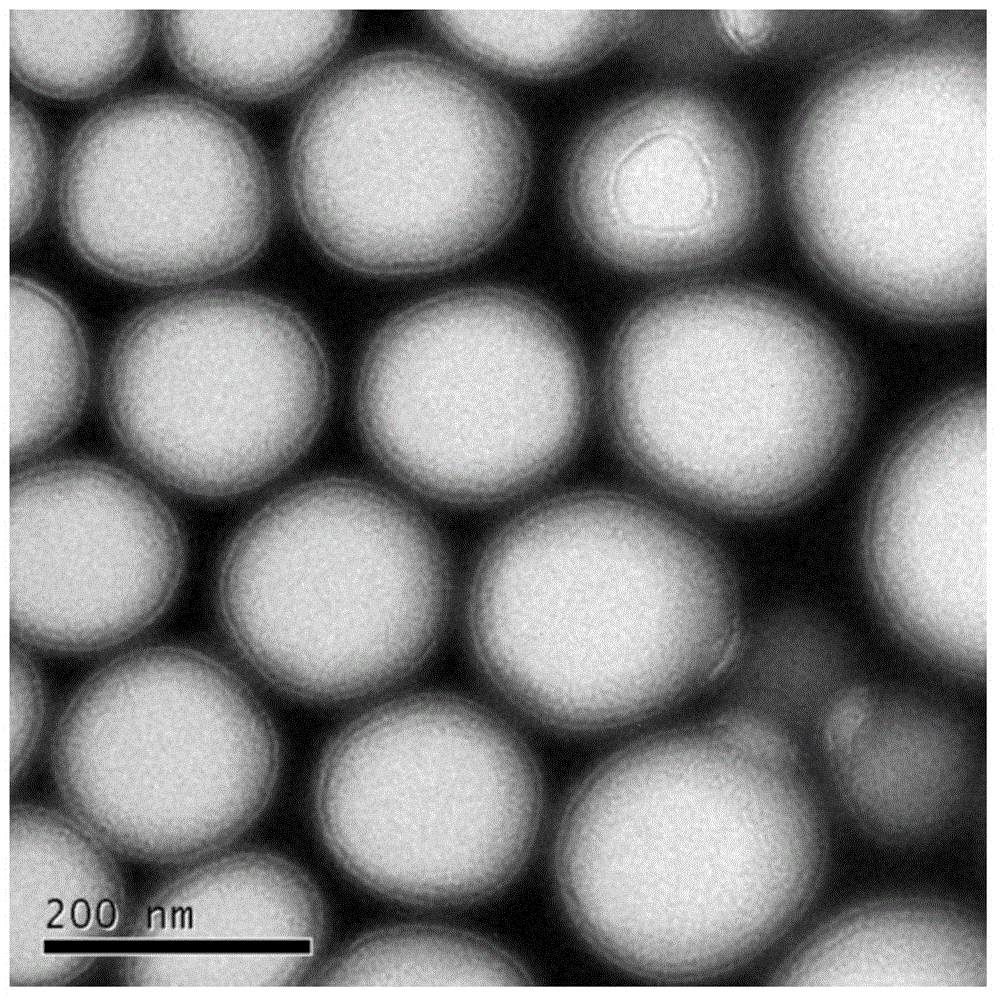
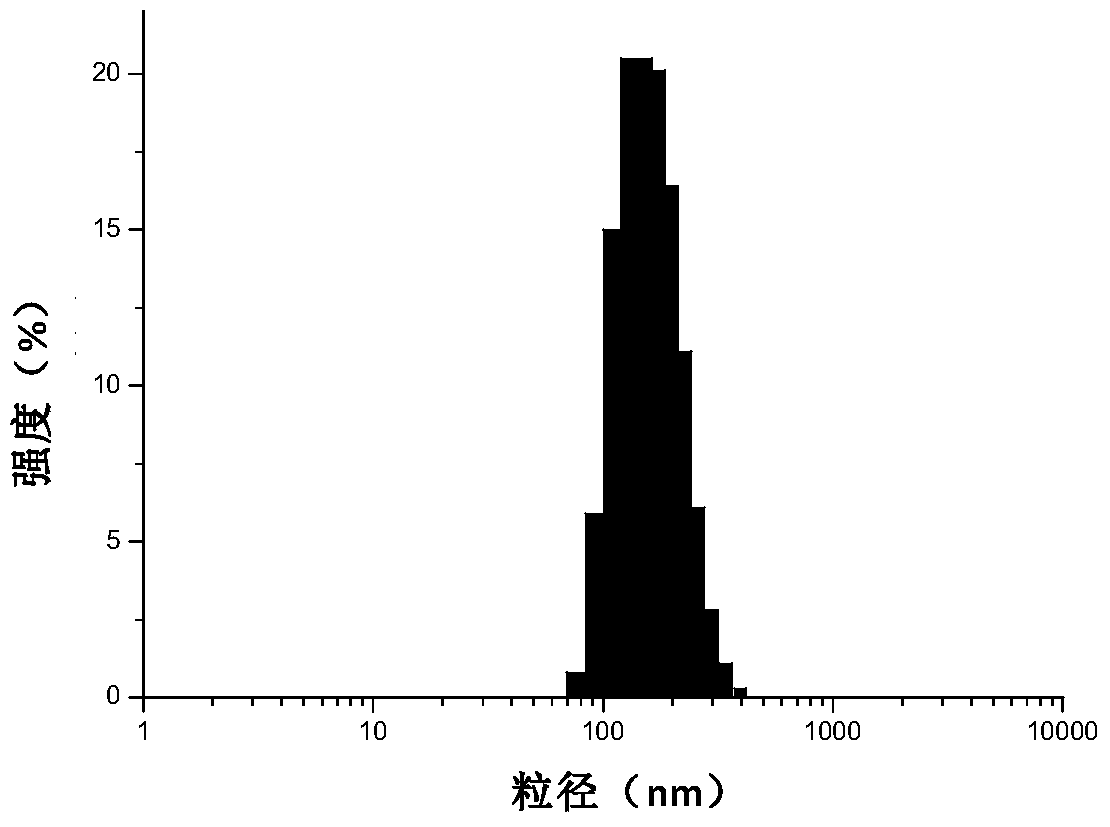
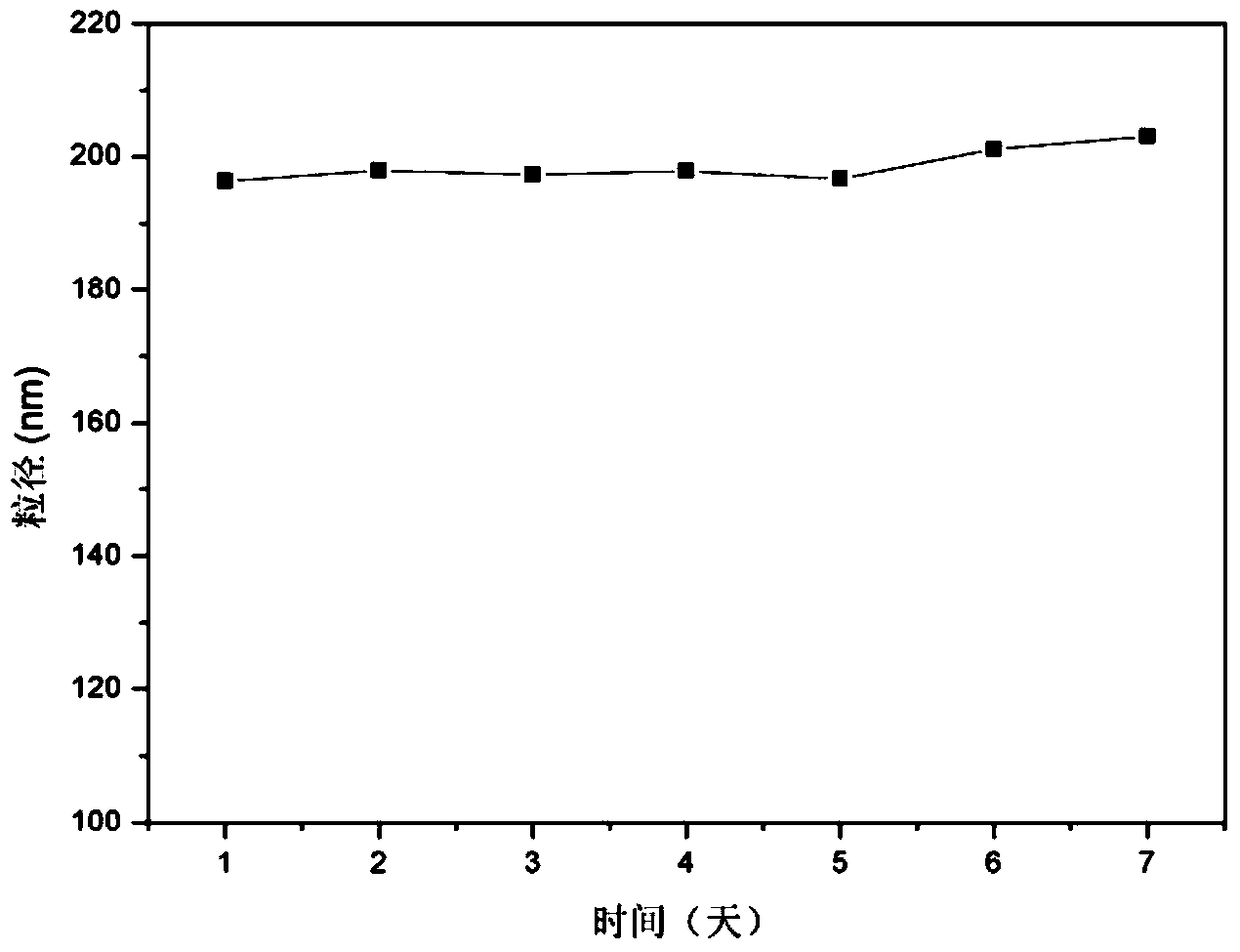
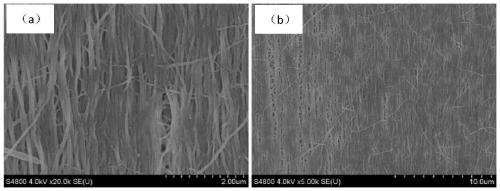
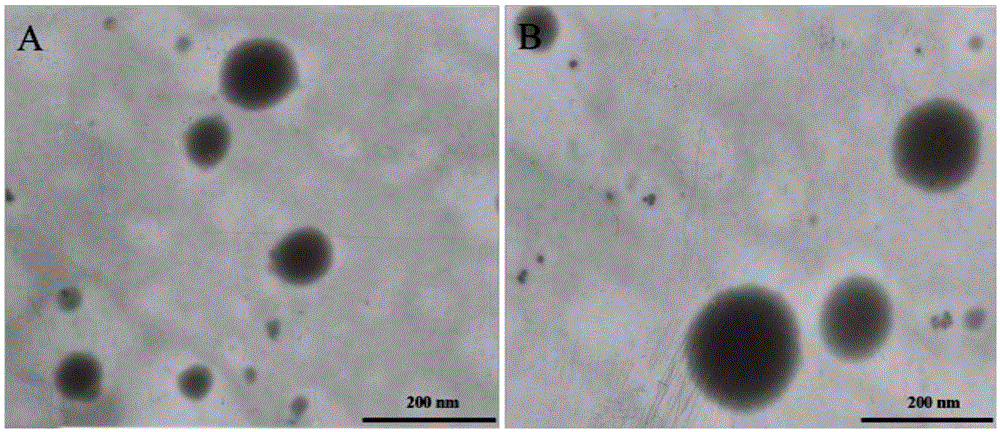
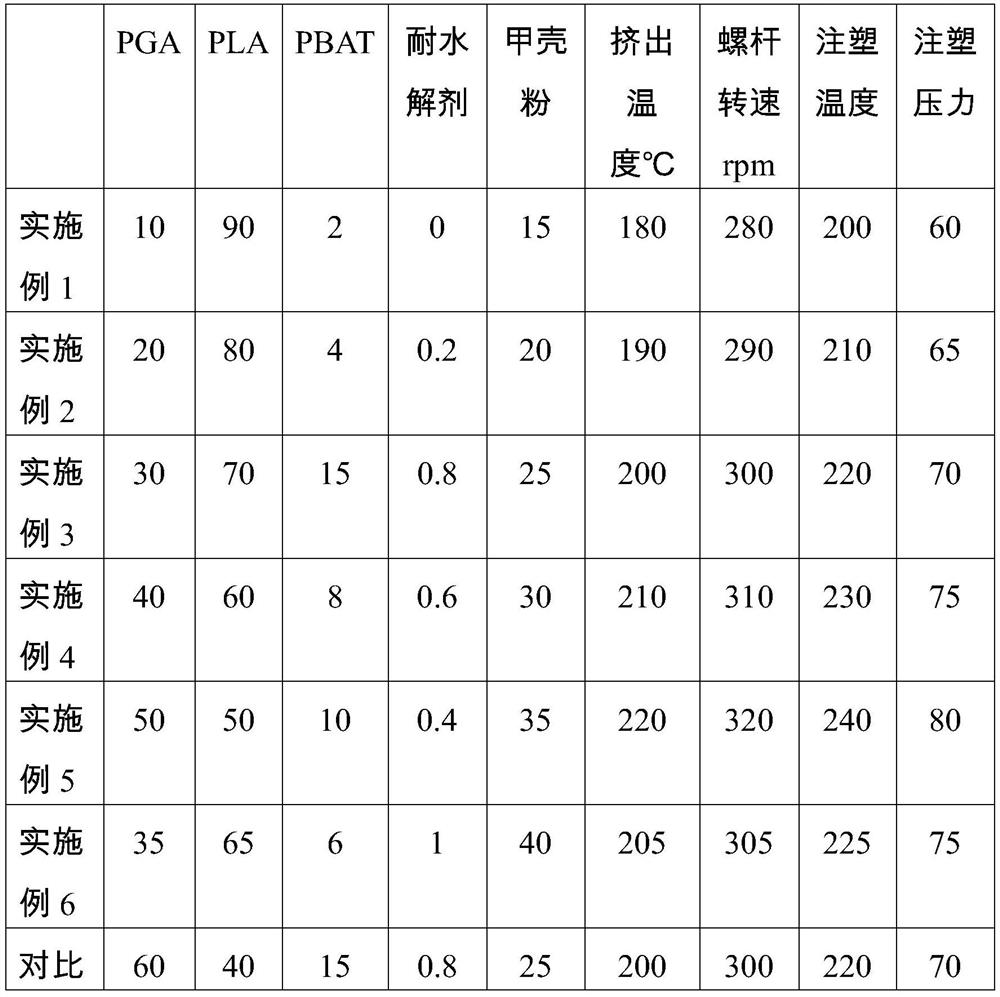
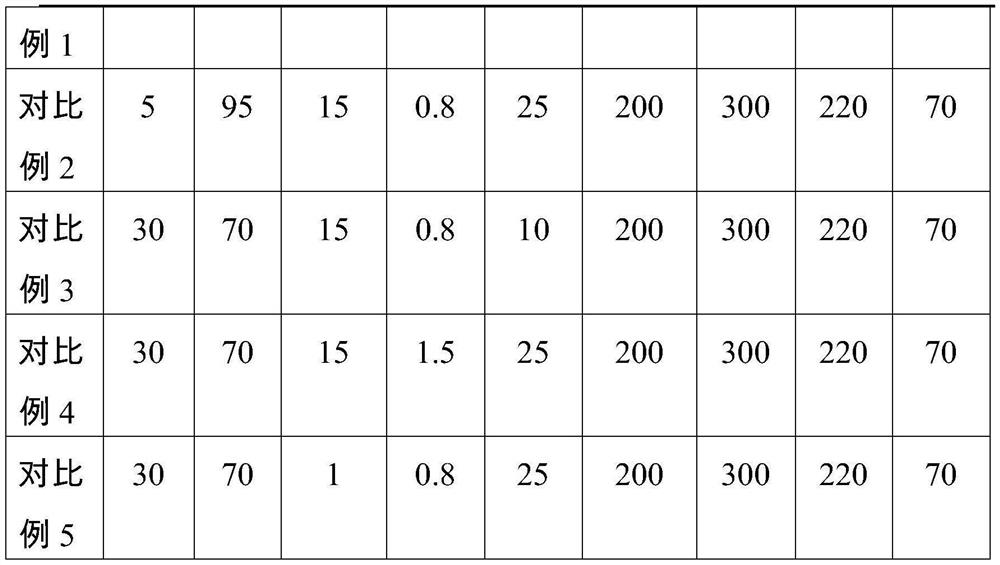
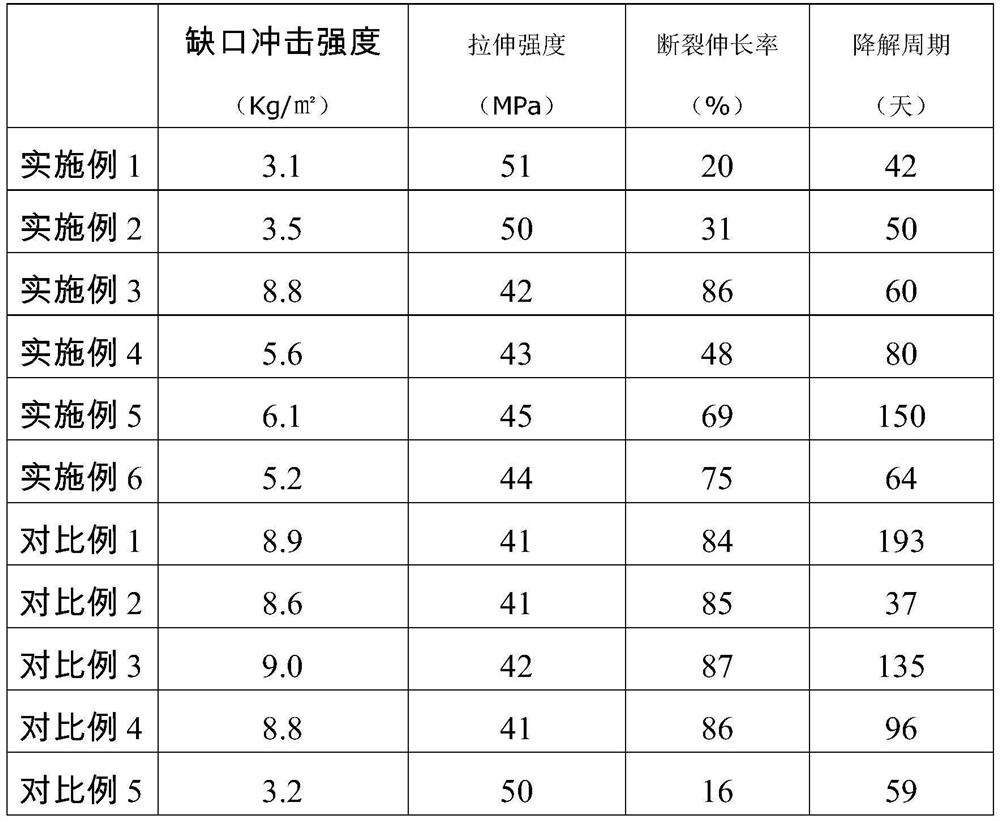
Patents
Literature
37 results about "Peg plga" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
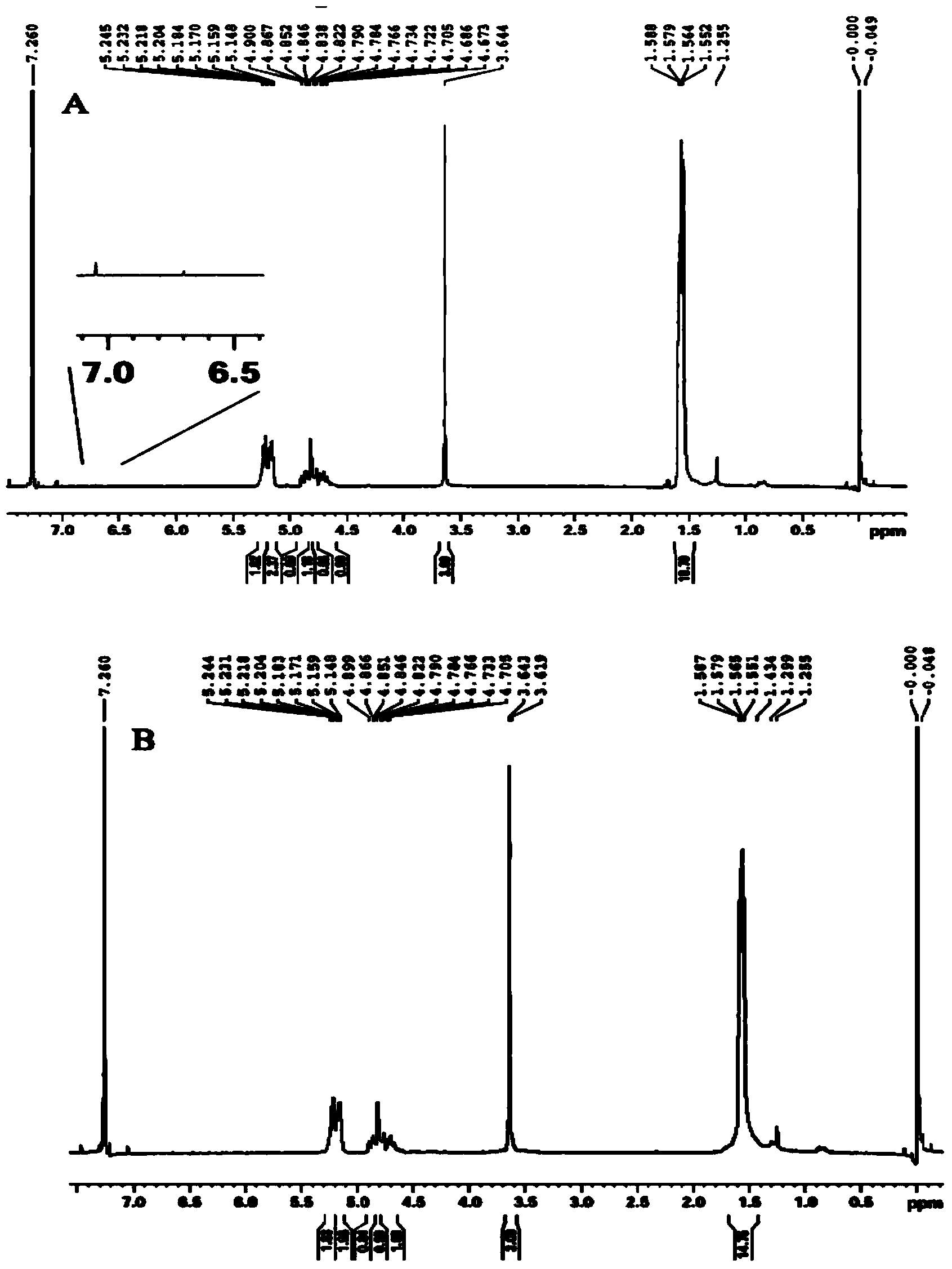
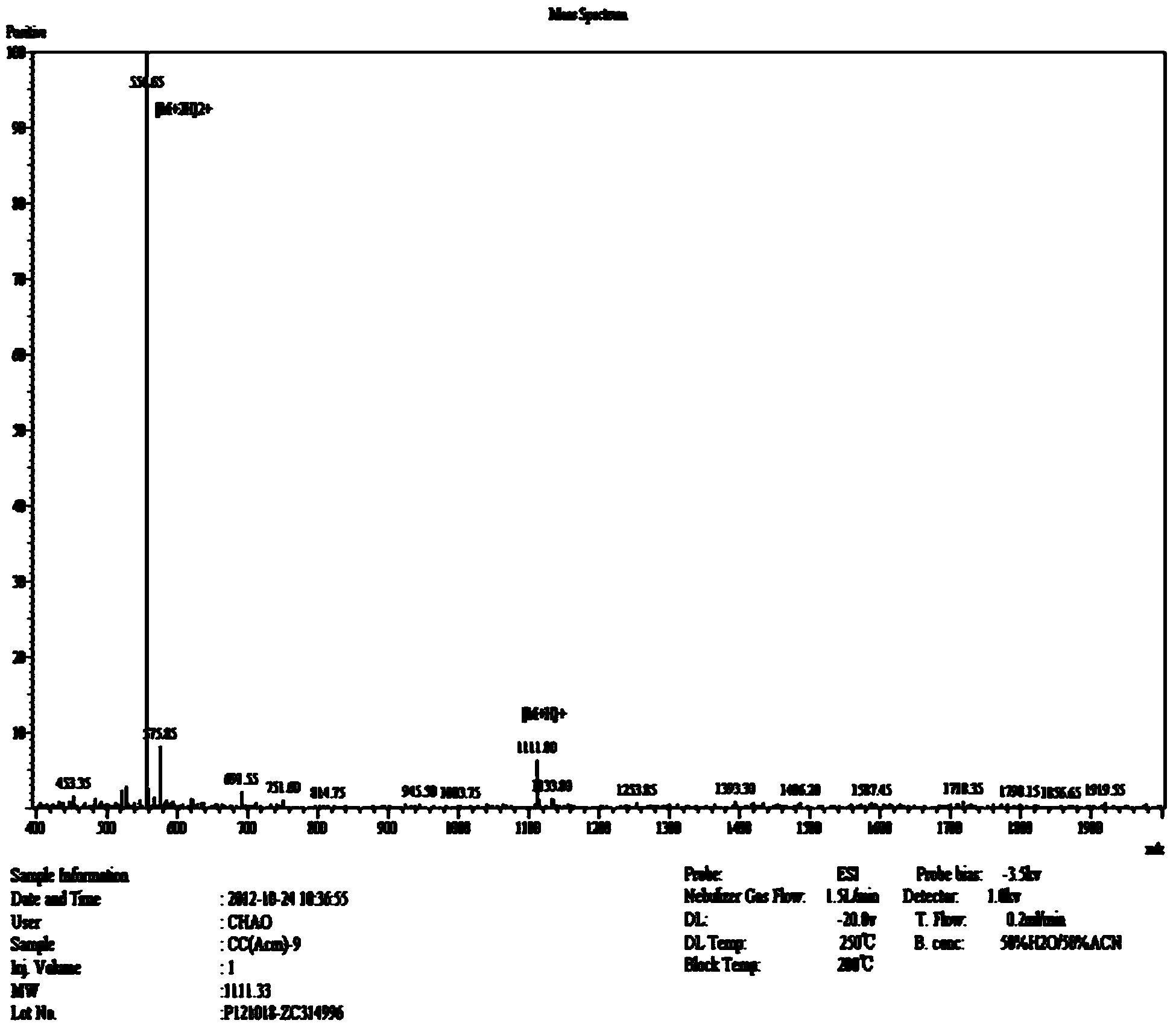
PLGA-PEGs is one of the most commonly used biodegradable ABCs for drug delivery application. PEG is the hydrophilic part and PLGA is the hydrophobic part.
Nano drug carrier particles for improving bioavailability of rapamycin and preparation method thereof
InactiveCN102871966AGood water solubilityGood biocompatibilityPowder deliveryOrganic active ingredientsUltrafiltrationBiocompatibility Testing
The invention discloses nano drug carrier particles for improving the bioavailability of rapamycin and a preparation method thereof, so as to carry out a drug effect optimization. The nano drug carrier particles are prepared in the following steps that according to the principle of an emulsion solvent evaporation method, a determined amount of PEG-PLGA and rapamycin are respectively dissolved in acetone; after being mixed uniformly, the PEG-PLGA, the rapamycin and the acetone are slowly added into water to be stirred by magnetic force; after a certain period of time, the obtained liquid is ultrasonically homogenated, and organic phase is removed in a vacuum dryer; the obtained water phase removes free medicine in a centrifugal tube; and then an ultrafiltration tube is used for concentration, and nano particles are obtained after freezing and drying. The method has the advantages of convenience in operation, simplicity and feasibility, good repeatability and the like. The prepared nano drug carrier particles can improve the utilization rate of the medicine by improving the absorptivity of the medicine and prolonging the cycling time in a human body. Meanwhile, the nano particles which are prepared through the method have good biocompatibility, and surface active groups can further modify ligands or targeted groups.
Owner:SOUTHEAST UNIV
Pep-1 peptide modified gliomas targeted nano drug delivery system and preparation method thereof
InactiveCN103655517AHigh fluorescence intensityIncrease savingsOrganic active ingredientsMacromolecular non-active ingredientsMonomethoxypolyethylene glycolTherapeutic effect
The invention discloses a Pep-1 peptide modified gliomas targeted nano drug delivery system and a preparation method thereof. The nano drug delivery system comprises polymer nano particles prepared by using an amphiphilic block copolymer (Pep-PEG-PLGA) as a carrier material, paclitaxel wrapped and carried by the polymer nano particles, and modified ligand Pep-1 polypeptide on the surfaces of the polymer nano particles. The amphiphilic block copolymer (Pep-PEG-PLGA) is composed of Male-PEG-PLGA and MePEG-PLGA, Pep-1 is adopted as a molecule with targeting function, the amphiphilic block copolymer PEG-PLGA is taken as a carrier material, the Pep-1 polypeptide is modified on the carrier material via covalent binding, and gliomas targeted polymer nano particles are prepared. According to the Pep-1 peptide modified gliomas targeted nano drug delivery system, gliomas targeting can be voluntarily performed, and the uptaking and accumulation of an anti-tumour drug in the gliomas part can be improved, as a result, the gliomas therapeutic effect is improved.
Owner:NANJING MEDICAL UNIV
LID-PEG-PLGA controlled-release nano microsphere and preparation method thereof
InactiveCN101961316AImprove hydrophobicityHigh encapsulation efficiencyPowder deliveryOrganic active ingredientsWater bathsMatrix solution
The invention discloses an LID-PEG-PLGA controlled-release nano microsphere and a preparation method thereof. The microsphere is the controlled-release microsphere which contains medicinal lidocaine and a degradable carrier. The degradable carrier contains polylactic-glycolic acid and PEG-2000. The mass percentage of the lidocaine in the controlled-release microsphere is 30 to 35 percent. The preparation method comprises the following steps of: preparing the carrier into matrix solution; dispersing the lidocaine into the matrix solution and preparing the lidocaine into an oil phase; mixing the oil phase and the aqueous solution of polyvinyl alcohol, and performing ultrasonic emulsification on the mixture under a water bath condition to obtain W / O-type protogala; mixing the W / O-type protogala and the aqueous solution of polyvinyl alcohol again, and further emulsifying the mixture into W / O / W-type complex emulsion; volatilizing the emulsion by reducing pressure at the normal temperature to obtain cured lidocaine-carried nano microsphere; and scattering, blending, packaging, freezing, sterilizing and the like. The medicament loading rate of the controlled-release nano microsphere can be up to 15 to 22 percent; the entrapment rate can be up to 68 to 78 percent; and the half-life period can be prolonged to 3 to 4 days. Therefore, the microsphere has relatively good effect of burst in the first day after the microsphere is taken and good effect of slow release in later days.
Owner:ARMY MEDICAL UNIV
Application of ursolic-acid nano medicine-carrying microspheres for treating tumors and preparation
InactiveCN102670525AExtend cycle timeExcellent anti-tumor efficacyPowder deliveryOrganic active ingredientsMpeg plgaPolyethylene glycol
The invention relates to the application of ursolic-acid nano medicine-carrying microspheres for treating tumors. The ursolic-acid nano medicine-carrying microspheres contain ursolic acid and a medicine-carrying material which are mixed, wherein the medicine-carrying material is a biparental segmented copolymer synthesized by one of polycaprolactone, polylactic acid or polyglycolic acid and polyethylene glycol, i.e. polycaprolactone-polyethylene glycol (mPEG-PCL), polylactic acid-polyethylene glycol (mPEG-PLA) or polyglycolic acid-polyethylene glycol (mPEG-PLGA); and the ursolic-acid nano medicine-carrying microspheres comprise 5 to 35wt% of ursolic acid and 65 to 95wt% of medicine-carrying material which are mixed.
Owner:李晓林
Multifunctional nano-drug composition and preparation method thereof
ActiveCN105233282AHigh drug loadingNarrow distributionOrganic active ingredientsEnergy modified materialsPorphyrinMpeg plga
The invention provides a multifunctional nano-drug combination and a preparation method thereof. The combination comprises a carrier and active ingredients loaded on the carrier, wherein the carrier is a polymer formed by mPEG-PLGA and / or PEG-PLGA and a porphyrin compound through covalent-bond connection, and the active ingredients include hydrophobic and hydrophilic drugs. Preferentially, the carrier and the active ingredients loaded on the carrier form particles with the nano size of 50-1000 nm. The multifunctional nano-drug combination has photo-thermal effect so that the treatment efficiency can be improved based on traditional chemotherapy in combination with photo-thermal therapy, and the multifunctional nano-drug combination can be applied to cancer therapy drugs.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
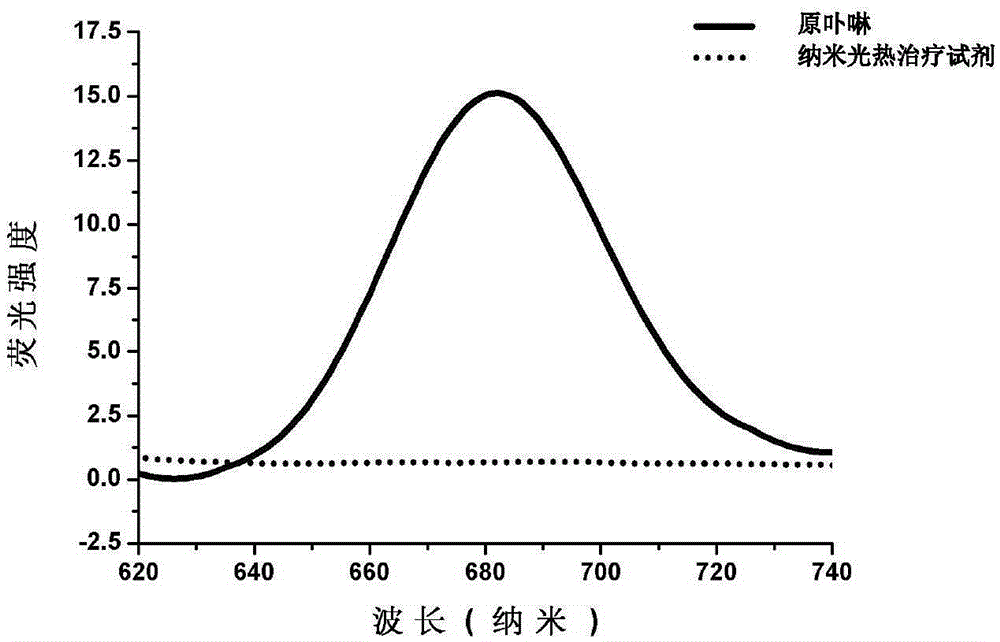
Nanometer photo-thermal therapy reagent and preparing method thereof
The invention provides a nanometer photo-thermal therapy reagent and a preparing method thereof. The nanometer photo-thermal therapy reagent is nano-particles formed by a polymer obtained through covalent bond connection of mPEG-PLGA and / or PEG-PLGA and porphyrins, and preferably, the size of the nano-particles is 50-1000 nm. The preparing method comprises the steps of preparing the polymer for modifying porphyrins, and then preparing the nano-particles. The nanometer photo-thermal therapy reagent has certain application potential in photo-thermal therapy reagents.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Segmented copolymer modified by polyethylene glycol 1000 vitamin E succinic acid ester, preparation method and applications thereof
ActiveCN101880381APromote degradationGood biocompatibilityOrganic active ingredientsPharmaceutical non-active ingredientsPolyesterPolymer science
The invention discloses a caprolactone-lactic acid-glycollic acid segmented copolymer modified by polyethylene glycol 1000 vitamin E succinic acid ester, a preparation method and applications thereof. The segmented copolymer is prepared by forming an ester bond by combing hydroxyl in polyethylene glycol 1000 vitamin E succinic acid ester and carboxyl in a construction unit A; and the construction unit A is a polymer by connecting polycaprolactone, polylactic acid and polyglycolic acid through the ester bond. The copolymer has excellent biodegradability, biocompatibility and nontoxicity, improves and regulates hydrophilcity and biodegradation rate of aliphatic polyester. The segmented copolymer is a new biodegradable polymer, and has wide biomedical applications.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Biodegradable liquid ink composition for ink jet printing
Biodegradable ink composition for ink jet printing, liquid at room temperature, comprising: a) a binder comprising at least 0.5% by weight relative to the total weight of the ink, of at least one biodegradable polymer having a weight average molecular weight less than 70 kDa, preferably less than 60 kDa, more preferably less than 50 kDa, further preferably less than 40 kDa, expressed as polystyrene equivalents; b) an organic vehicle or solvent comprising, preferably composed of, one or more organic solvent compound (s), and optionally water; c) at least 0.1% by weight relative to the total weight of the ink of one or more dye(s) and / or pigment (s); wherein the biodegradable polymer is chosen from among polyhydroxyalkanoates (PHA), polyesteramides (PEA), poly (glycolic acid) s, poly (lactic acid) s (PLA), polycaprolactones (PCL), the copolymers thereof; and aliphatic copolyesters such as succinates, adipates and terephtalates; and wherein the ink composition has a viscosity of 1 to 20 mPa.s, preferably of 2 to 10 mPa.s at 20°C. Process for marking a substrate using said ink composition. Substrate provided with marking obtained by drying and / or absorption of said ink composition.
Owner:MARKEM IMAJE HLDG
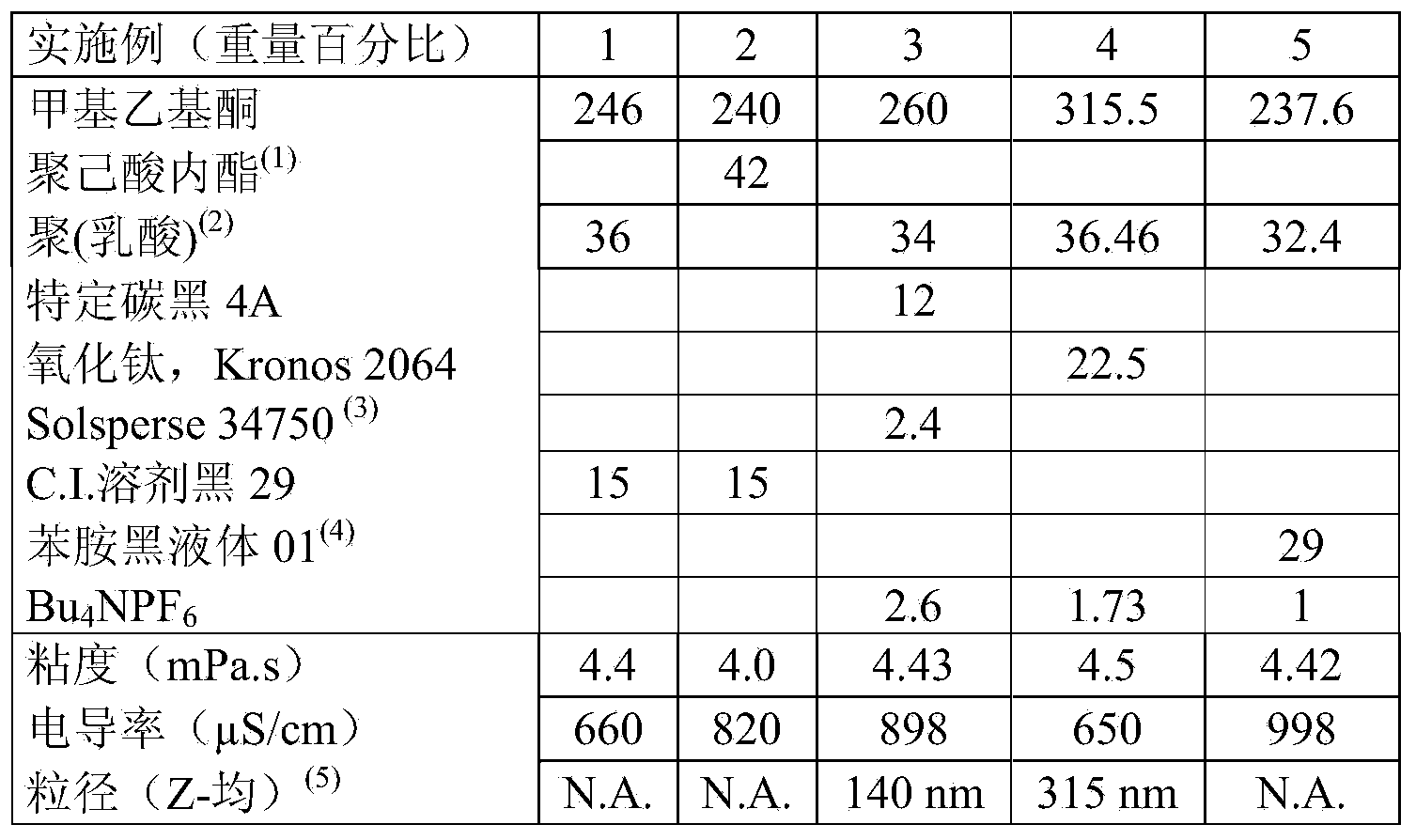
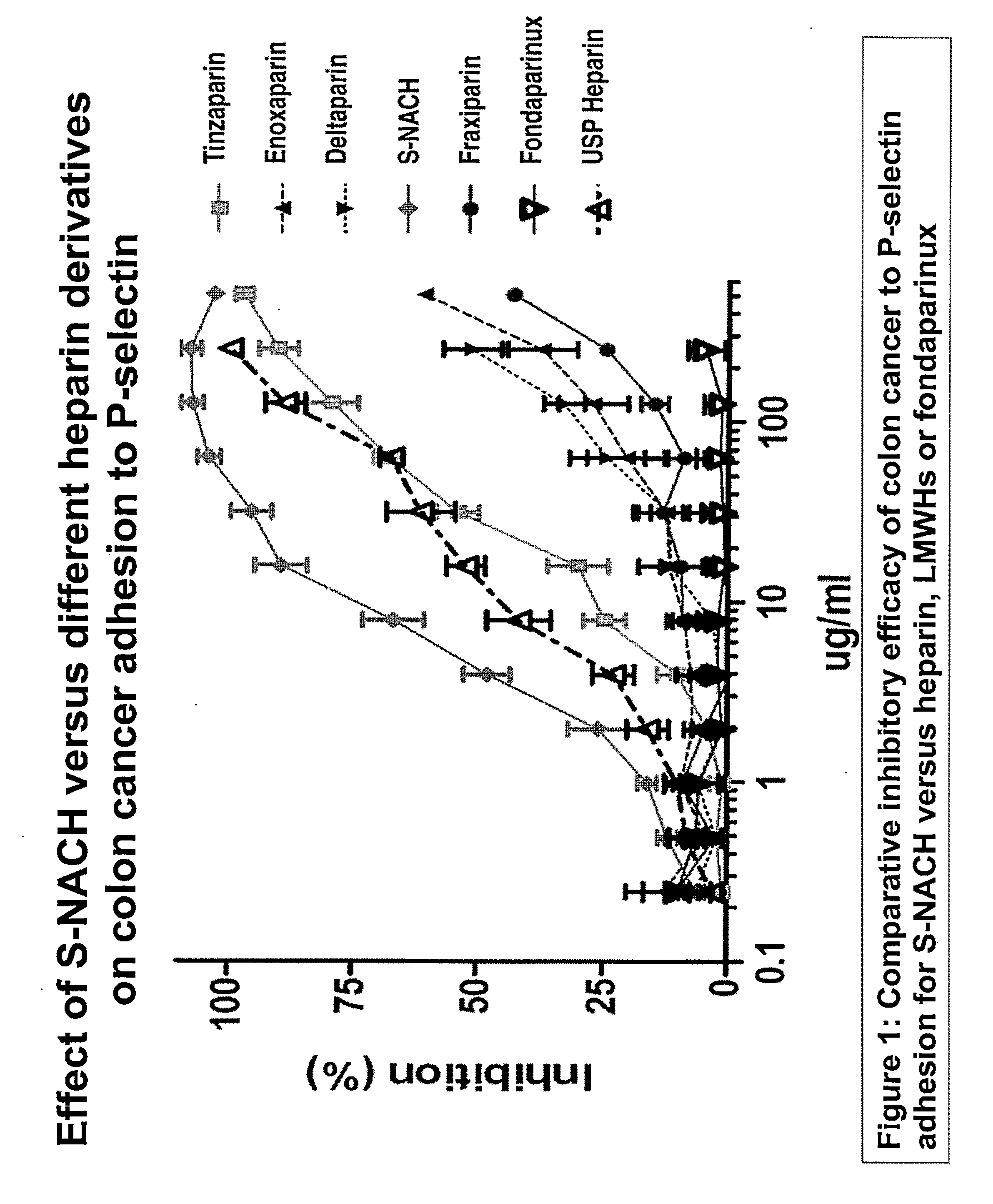
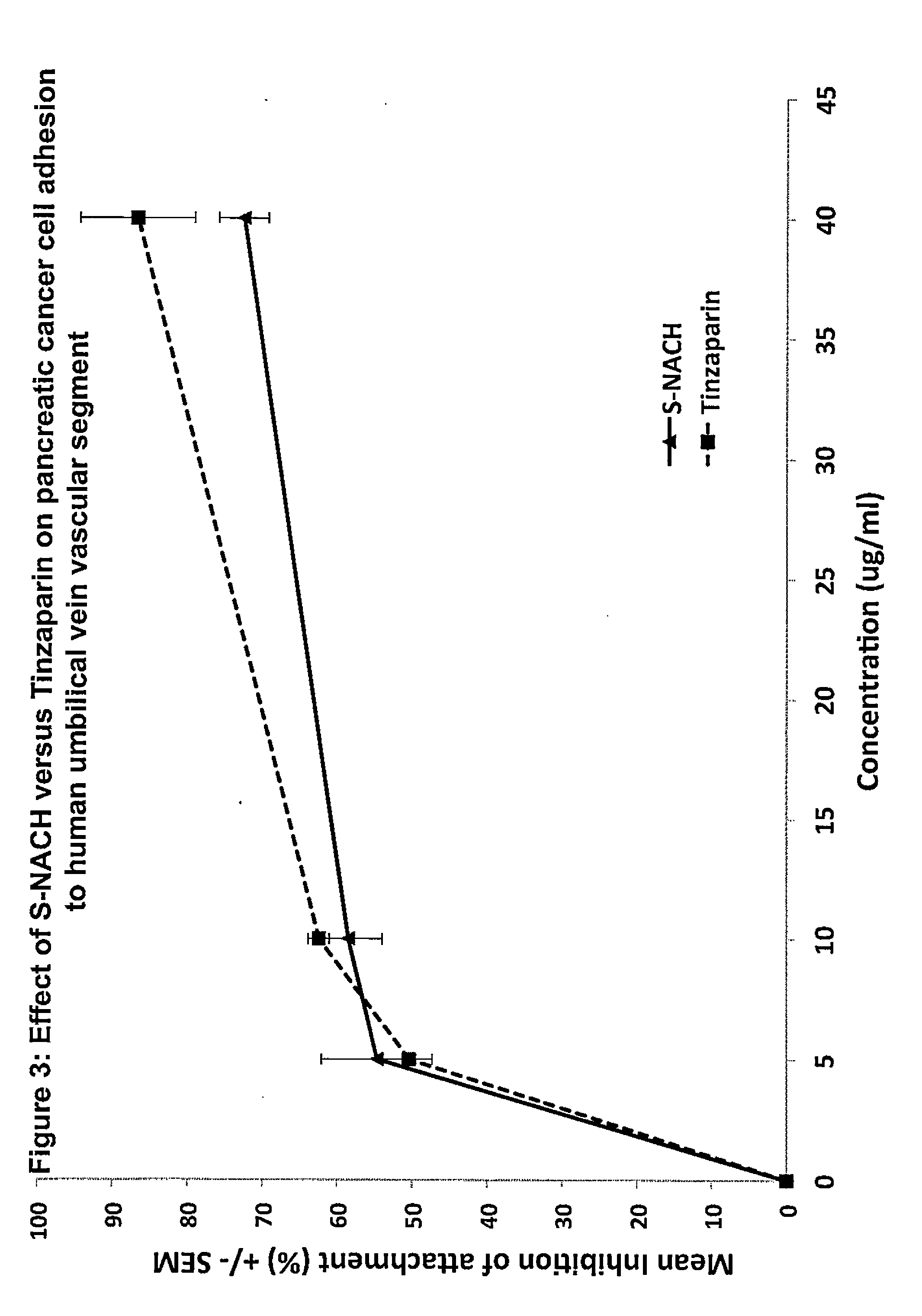
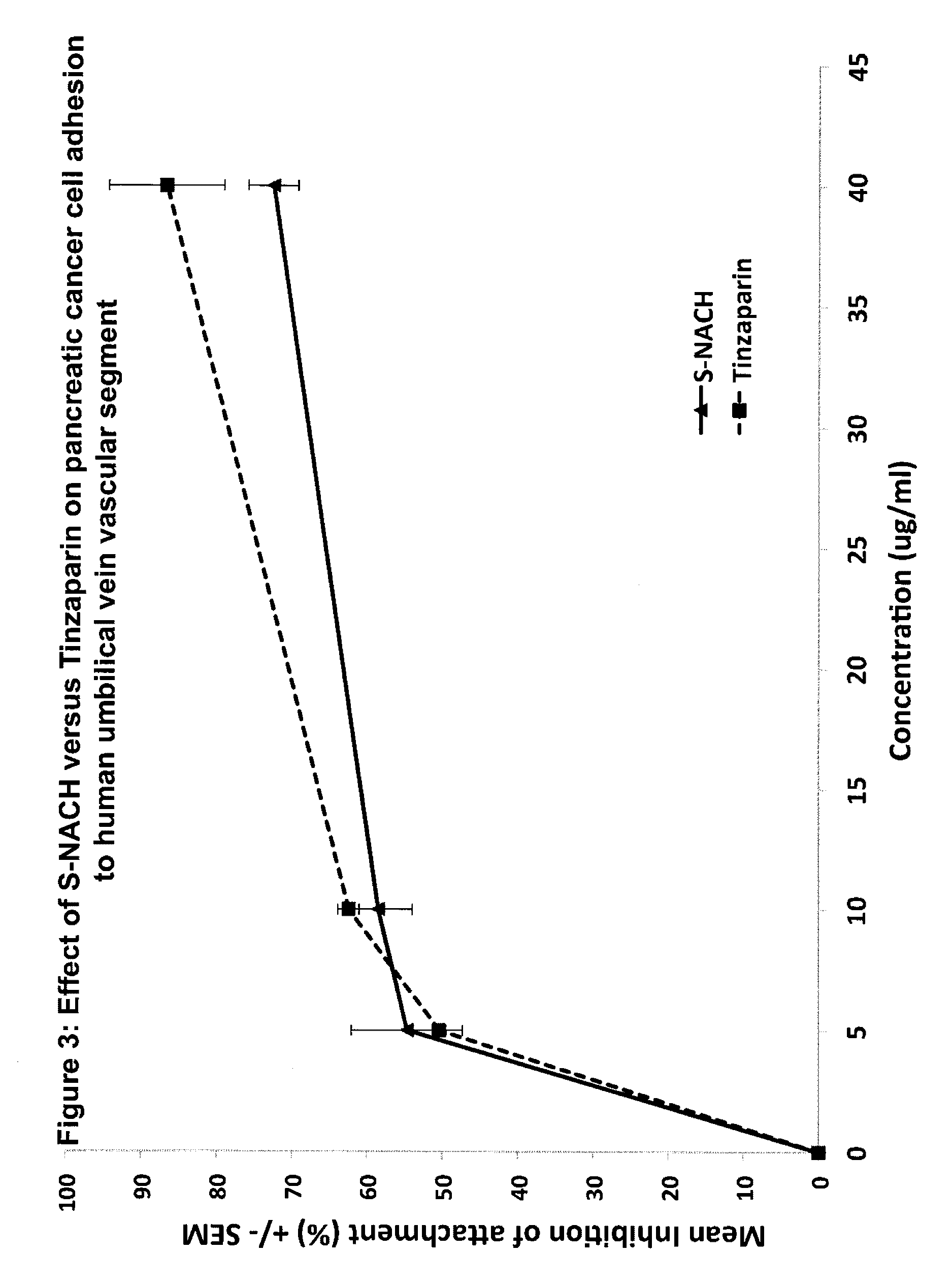
Composition and method for sulfated non-anticoagulant low molecular weight heparins in cancer and tumor metastasis
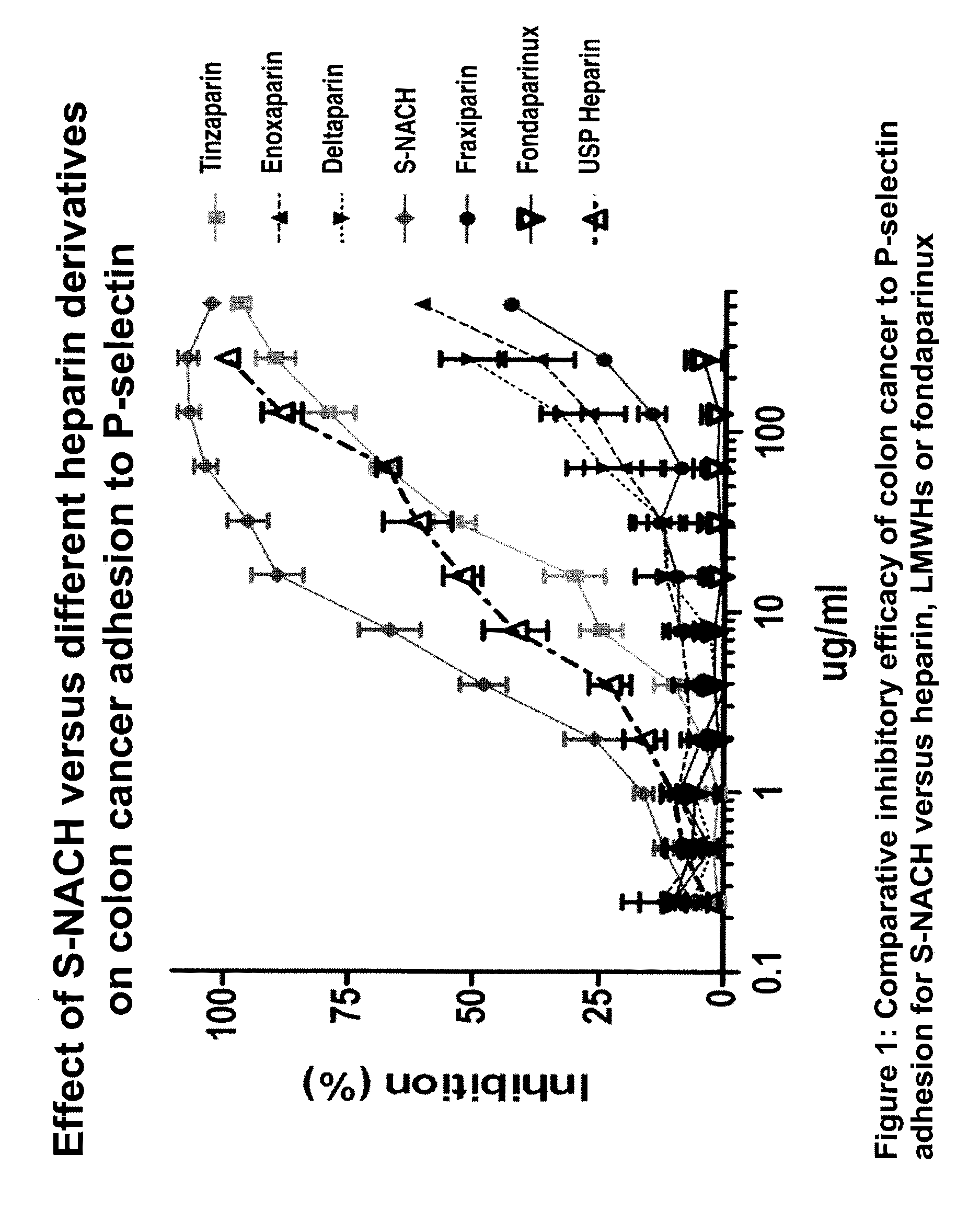
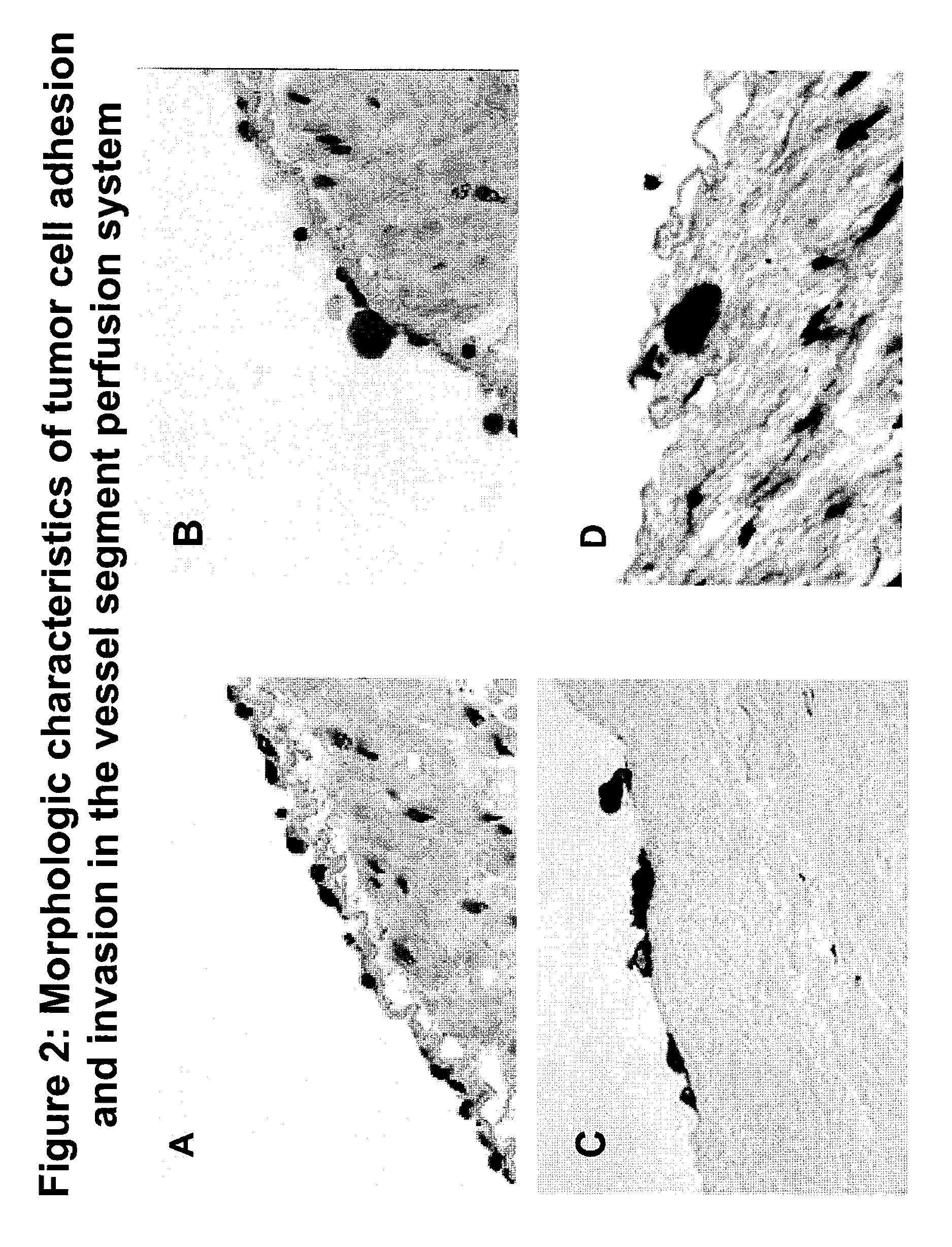
A nanoformulation that includes nanoparticles. Each nanoparticle includes a shell in which a glycosaminoglycan (GAG is encapsulated. The GAG is ionically or covalently bonded to the shell. The GAG is selected from the group consisting of sulfated non-anticoagulant heparin (SNACH), super-sulfated non-anticoagulant heparin (S-SNACH), and a combination thereof. The shell includes Poly (lactic-co-glycolic acid) (PLGA), Polyethylene Glycol (PEG)-PLGA, maleimide-PEG-PLGA, chitosan, chitosan-PLGA, methoxy-polyethyleneglycol-poly (lactide-co-glycolide) (MPEG-PLGA)-(maleimide-PEG-PLGA), PLGA-Polycaprolate, or calcium alginate. A method of using the nanoformulation to treat a cancer in a subject includes administering to the patient a therapeutically effective amount of the nanoformulation for treating the cancer.
Owner:MOUSA SHAKER A
A preparing method of drug carrying nanoparticles for targeted treatment of malignant lymphoma
InactiveCN109106952AAvoid damageImprove bioavailabilityPowder deliveryOrganic active ingredientsAptamerChemical reaction
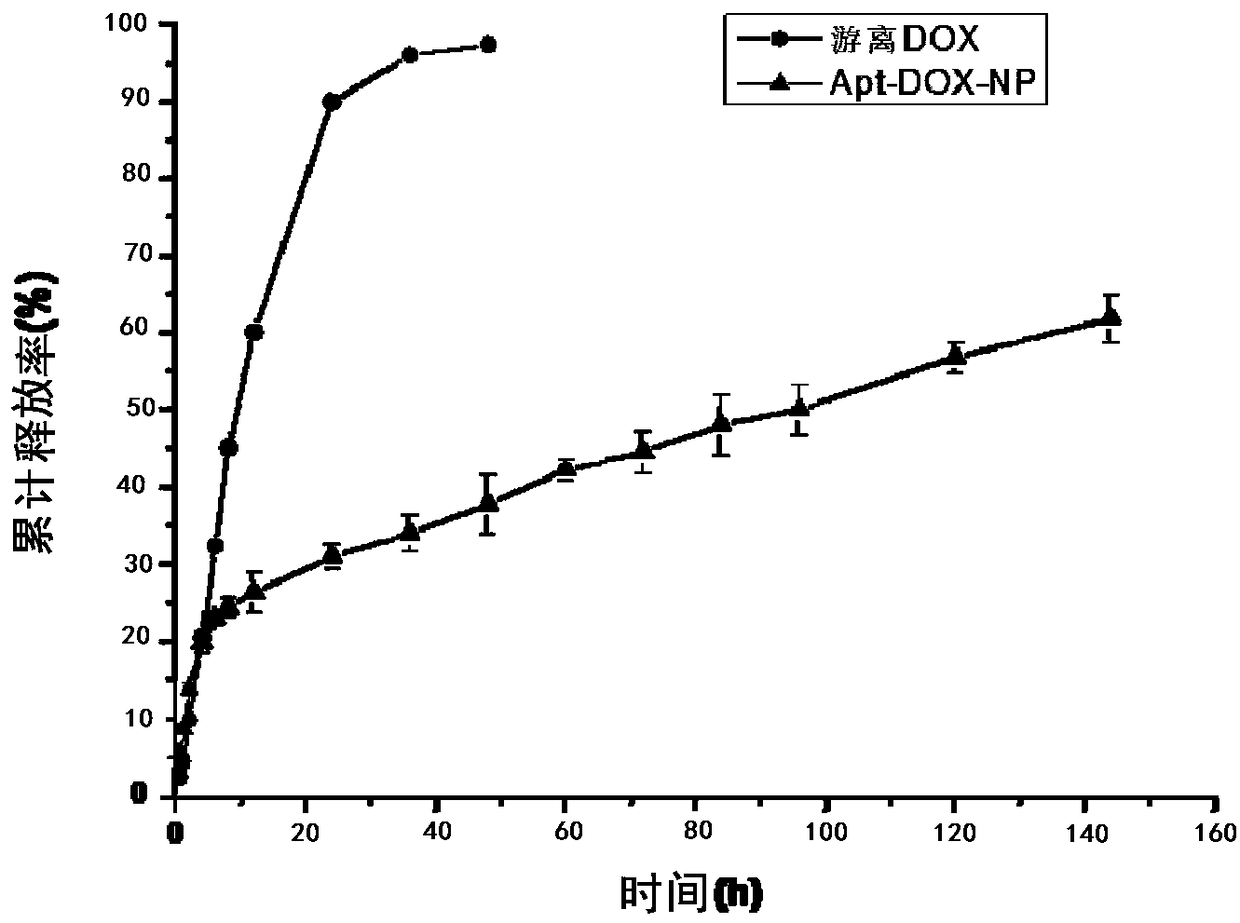
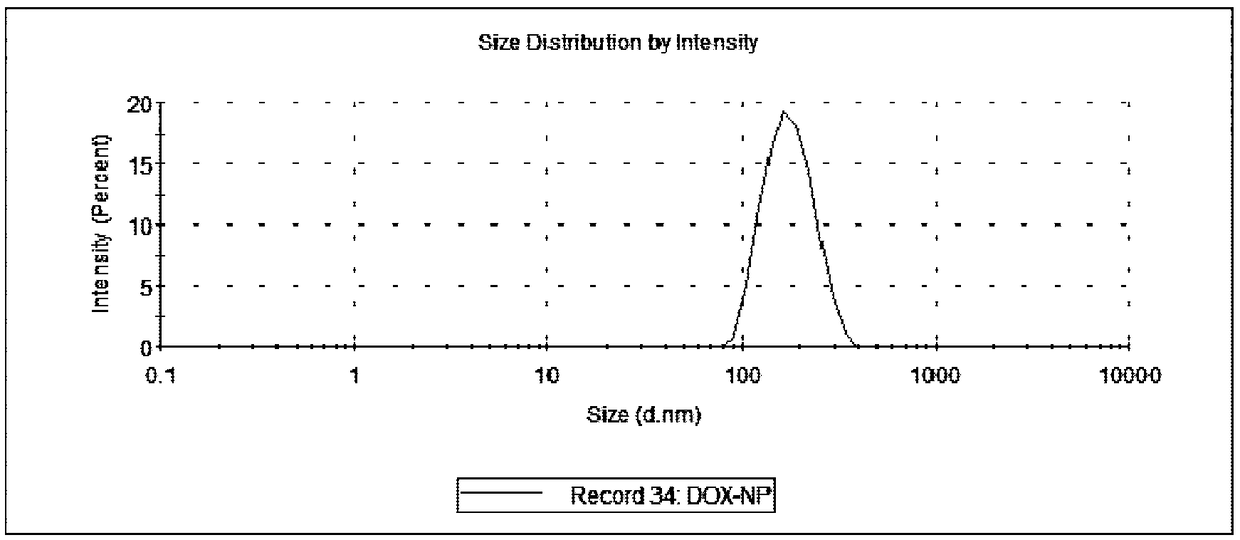
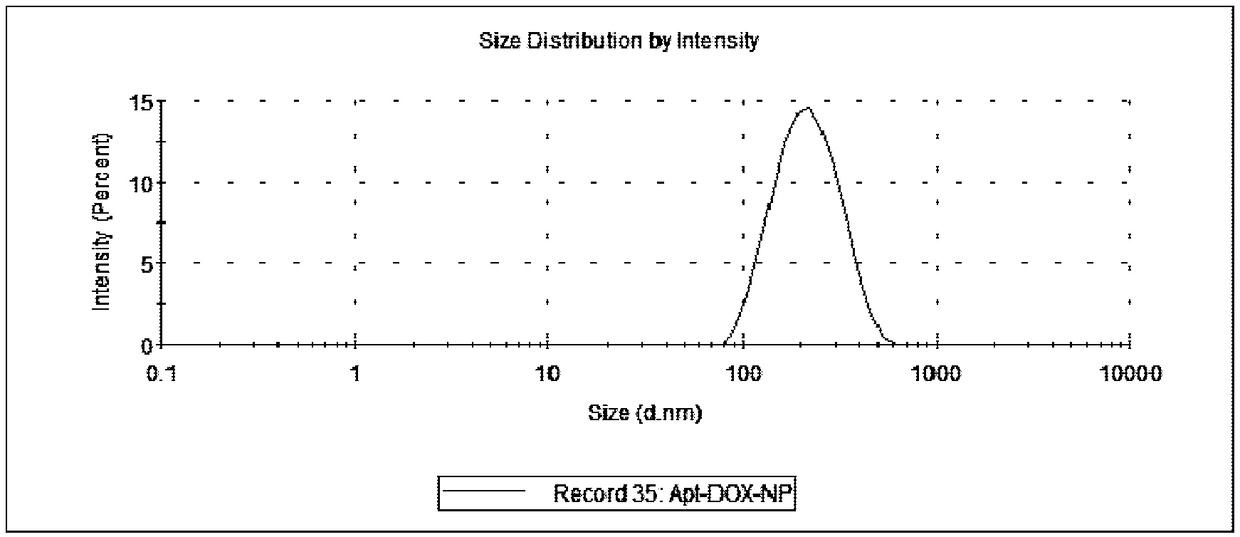
A preparing method of drug carrying nanoparticles for targeted treatment of malignant lymphoma is disclosed. An amphoteric polymer PEG-PLGA having good biocompatibility is adopted as a carrier center,a hydrophobic segment covers and carries a hydrophobic drug that is adriamycin (DOX), and carboxyl exposed at the outer end is activated through a chemical reaction and is boned with a nucleic acid aptamer having amino. The drug carrying nanoparticles are rapidly delivered to a tumor position by utilizing the targeting function of the nucleic acid aptamer, and the drug is released slowly by utilizing the sustained release function of the PEG-PLGA nanoparticles, thus achieving long-acting efficient treatment of the malignant lymphoma, and reducing side effects caused by common therapies such as chemotherapy.
Owner:SOUTH CHINA UNIV OF TECH
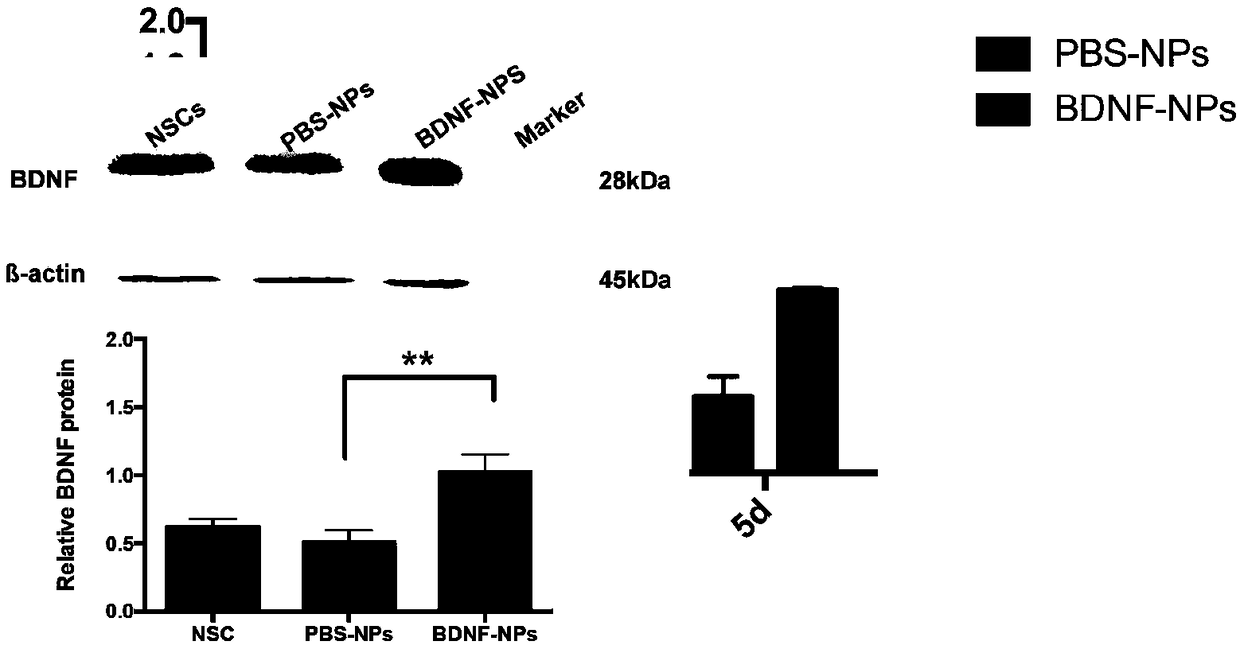
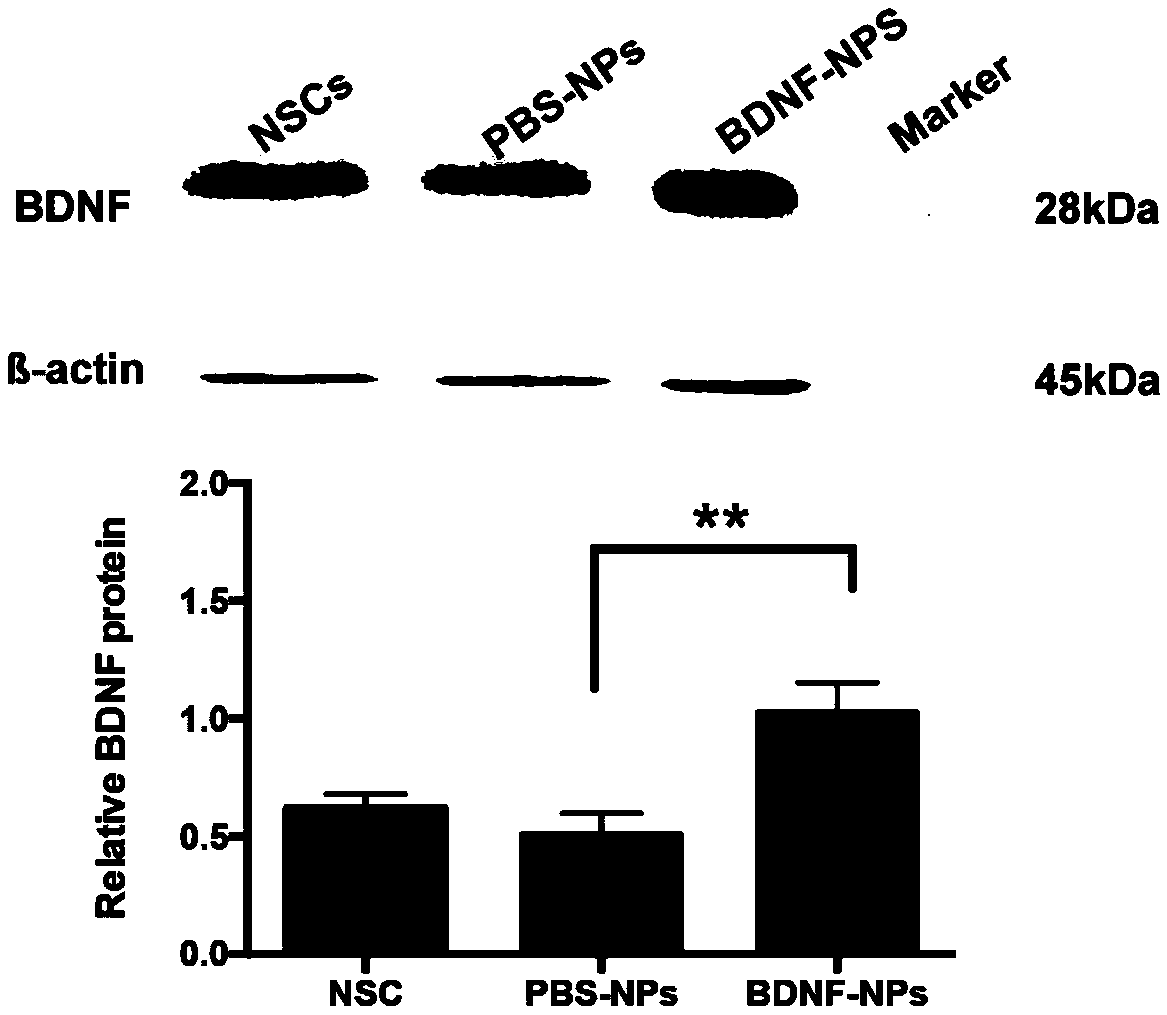
PEG-PLGA nanosphere loading BDNF gene plasmid as well as preparation method and application thereof
ActiveCN109157530ASmall particle sizeFlat surfaceNervous disorderPeptide/protein ingredientsMicrospherePolyethylene glycol
The invention discloses a PEG-PLGA nanosphere loading a BDNF gene plasmid as well as a preparation method and application thereof. The nanosphere is obtained after the BDNF gene plasmid is loaded by apoly(ethylene glyco)-b-poly(lactic-co-glycolic acid) (PEG-PLGA) nanoparticle. The nanosphere provided by the invention, which has the advantages of uniform particle size distribution, good dispersion, excellent ability in transfecting neural stem cells and high expression of brain derived neurotrophic factor (BDNF) functional protein after transfecting the neural stem cells, is applicable to protection and repairing of the function of nerve cells and can play an important role in the medical field as a transfection material for the neural stem cells, thereby having a broad application prospect.
Owner:SOUTHEAST UNIV
PEG-PLGA sustained release microsphere with encapsulated buprenorphine and preparation method thereof
ActiveCN105596298AGood biocompatibilitySmall particle sizeOrganic active ingredientsPharmaceutical product form changeBuprenorphine HydrochloridePatient compliance
The invention discloses a PEG-PLGA sustained release microsphere with encapsulated buprenorphine and a preparation method thereof. The preparation method is as below: conducting debydrochlorination on buprenorphine to prepare alkali, and conducting a single emulsion method to obtain the PEG-PLGA sustained release microsphere with encapsulated buprenorphine. The weight ratio of buprenorphine (calculated by buprenorphine hydrochloride) to polyethylene glycol-polylactic acid-glycolic acid copolymer is 2-6:10, and the buprenorphine sustained release microsphere has particle size of 10-50 micron. The invention employs for the first time the hydrochloric acid-single emulsion method to prepare the buprenorphine sustained release microspheres with high drug loading, obvious sustained-release effect and good biocompatibility, and can develop a variety of administration approaches, such as intravenous injection, and subcutaneous and intramuscular non-intravenous administration approaches. The invention has good application prospect, and can improve patient compliance.
Owner:SHANDONG UNIV
Peg-plga-pll polymer and method for preparing and using the same as the drug and gene carrier
InactiveUS20130052134A1Suitable for mass productionSimple manufacturing methodPowder deliveryGenetic material ingredientsAntisense nucleic acidSide chain
This invention belongs to the nanotechnology field, and discloses a nano drug delivery system with polyethyleneglycol-poly(lactic-co-glycolic acid)-poly-L-lysine (PEG-PLGA-PLL) polymer as the skeleton. The carrier can have the function of passive targeting through control of the carrier particle size. The polymer skeleton is modified through introducing side chains and specific targeting groups, so as to adjust and improve the carrier performance, and enable the carrier to have the function of active targeting. Such carrier material also has the functions of transporting active substances, tumor treatment and diagnosis, ultrasonic contrast, reversing or reducing drug resistance and so on. It is mainly applied to (1) Targeting preparation of anticancer drugs; (2) preparation to reverse or reduce the drug resistance of the tumor; (3) reagent for tumor diagnosis and contrast; (4) reagent to transfect DNA plasmids; (5) pharmaceutical preparation for cancer gene therapy; (6) reagent used to transfect antisense nucleic acid and siRNA (RNA interference); (7) pharmaceutical preparation used to prepare antisense nucleic acid and siRNA (RNA interference).
Owner:SHANGHAI INST OF ONCOLOGY
PEG-PLGA encapsulated hemoglobin
ActiveCN104644613AParticle size andUniform particle size distributionPeptide/protein ingredientsAntinoxious agentsLactideWhole body
The invention discloses hemoglobin oxygen carrier-PEG-PLGA encapsulated hemoglobin. The PEG-PLGA encapsulated hemoglobin is obtained by encapsulating the hemoglobin with polyethylene glycol-poly(lactide-co-glycolide) (PEG-PLGA) nanoparticles. The hemoglobin is uniform in particle size distribution, good in dispersibility and high in oxygen appetency and has good oxygen supply effect and can be used for the system with general or partial anoxia or the system needing in vitro oxygen supply. The hemoglobin oxygen carrier-PEG-PLGA encapsulated hemoglobin plays an important role in the medical field when serving as a blood substitute and has a broad application prospect.
Owner:ACADEMY OF MILITARY MEDICAL SCI
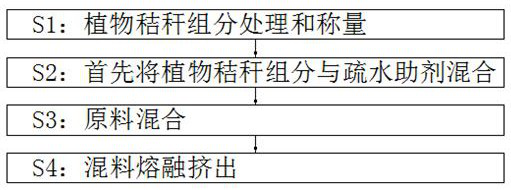
Biodegradable material and preparation method thereof
The invention belongs to the technical field of degradable materials, particularly relates to a biodegradable material and a preparation method, and provides the following scheme aiming at the problems that an existing degradable material is relatively high in cost and a preparation method is complicated. The degradable plastic material comprises the following important components: 40-50 parts of a plant straw component, 6-8 parts of modified starch, 3-6 parts of degradable plastic resin, 3-6 parts of polystyrene, 2-4 parts of polyglycolic acid, 1.2-2.4 parts of a flexibilizer, 3-6 parts of a hydrophobic auxiliary agent, 2-5 parts of a liquid thickener, 0.3-0.6 part of an antioxidant and 0.3-0.6 part of a preservative, and the straw component comprises corn straw powder, wheat straw powder, rice straw powder and soybean straw powder. The mass ratio of the corn straw powder to the wheat straw powder to the rice straw powder to the soybean straw powder is 1: 1: 1: 1. The material is prepared from waste plant straw components so that the cost can be reduced, and the material has an environment-friendly function; the preparation method is simple.
Owner:WENZHOU AOPU IND
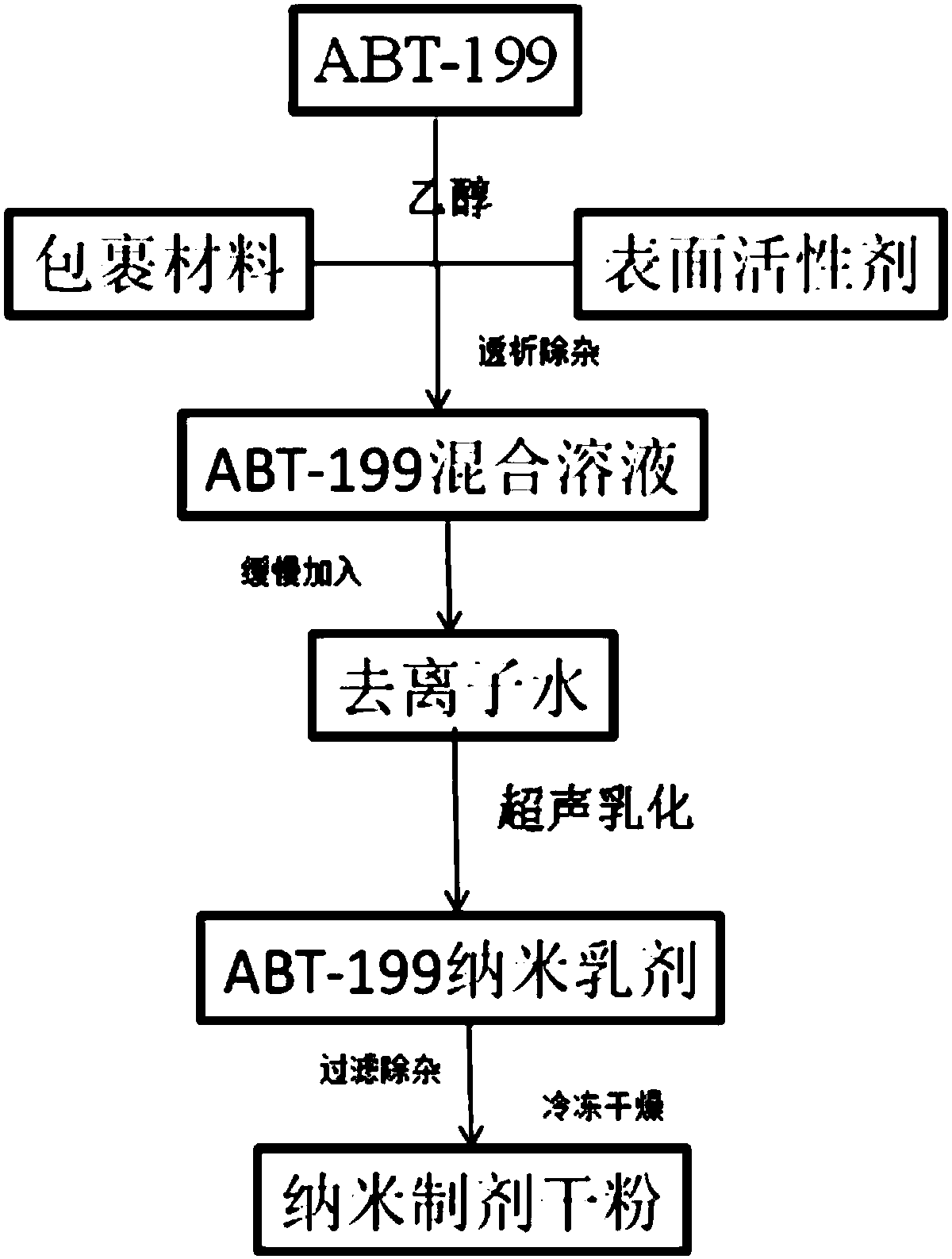
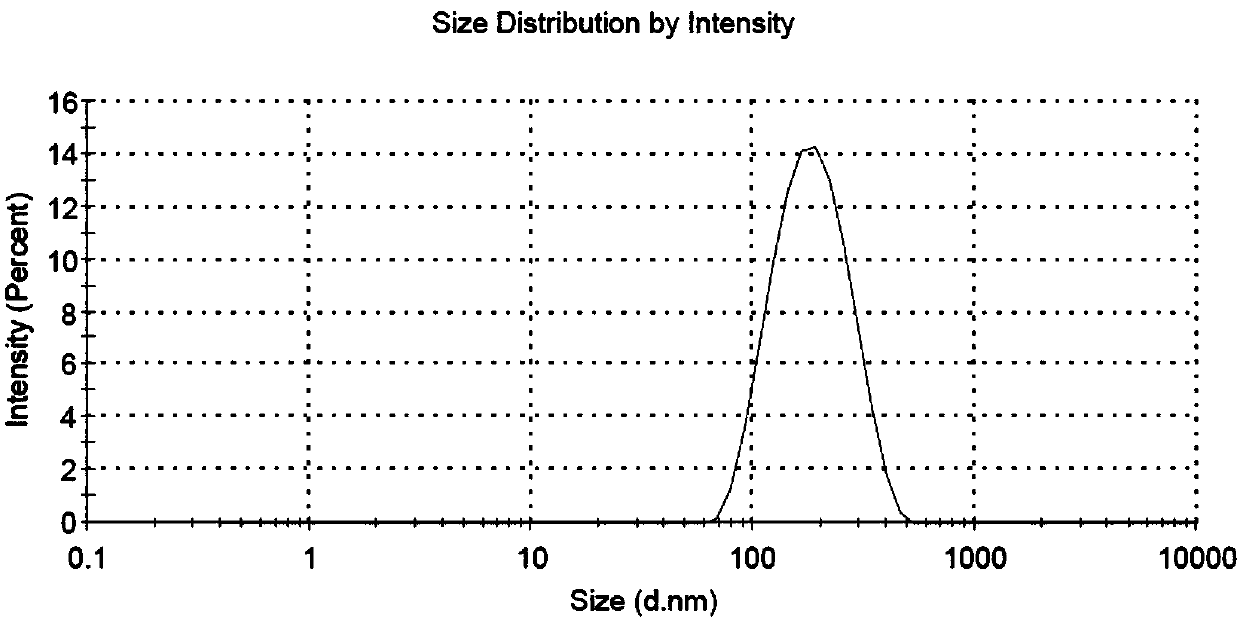
ABT-199 nano-emulsion dispersing agent and preparation method thereof
InactiveCN108042498AImprove packaging efficiencyImprove stabilityPowder deliveryOrganic active ingredientsSolubilitySide effect
The invention discloses an ABT-199 nano-emulsion dispersing agent which comprises, in total weight percent of 100%, 4%-8% of ABT-199, 28%-40% of wrapping materials, 20%-34% of surfactants and 29%-38%of cryoprotectants. The wrapping materials are one or mixture of soybean phospholipid, lecithin and cholesterol, the surfactants are nonionic surfactants and include one or mixture of Tween 80, poloxamer 188, PEG-PLGA (polyethylene glycol-polylactic acid), and the cryoprotectants are one or mixture of mannitol and glucose. The invention further discloses a preparation method of the ABT-199 nano-emulsion dispersing agent. The problems of poor water solubility, low bioavailability and strong in-vivo toxic and side effects of a compound in the prior art are solved.
Owner:XIAN MEDICAL UNIV
Nanoparticles preparation encapsulated with carfilzomib, and preparation method thereof
InactiveCN105919972ALow impurity contentGood water solubilityPowder deliveryPeptide/protein ingredientsSolubilityPEG-PLGA-PEG
The invention provides a nanoparticles preparation loaded with carfilzomib. The preparation includes, by weight, 1-20 parts of carfilzomib, 0.5-10 parts of lecithin, 10-100 parts of polyethylene glycol copolymer, which is a PEG-PLGA-PEG triblock copolymer with number-average molecular weight of 5000-50000 and / or a PEG-PLGA block copolymer with the number-average molecular weight of 5000-50000. The invention selects the specific polyethylene glycol copolymer, and uses lecithin as a surfactant. The prepared nanoparticles encapsulated with carfilzomib have water solubility better than carfilzomib encapsulated with cyclodextrin, have high water solubility, uniform particle size, and lower impurity content.
Owner:LIANGJIANG MEDICINE CO LTD
Hepatoma-targeted nano medicine delivery system and preparation method and application thereof
InactiveCN109503848ASimple preparation processEfficient killingOrganic active ingredientsDigestive systemActivity assessmentPolyethylene glycol
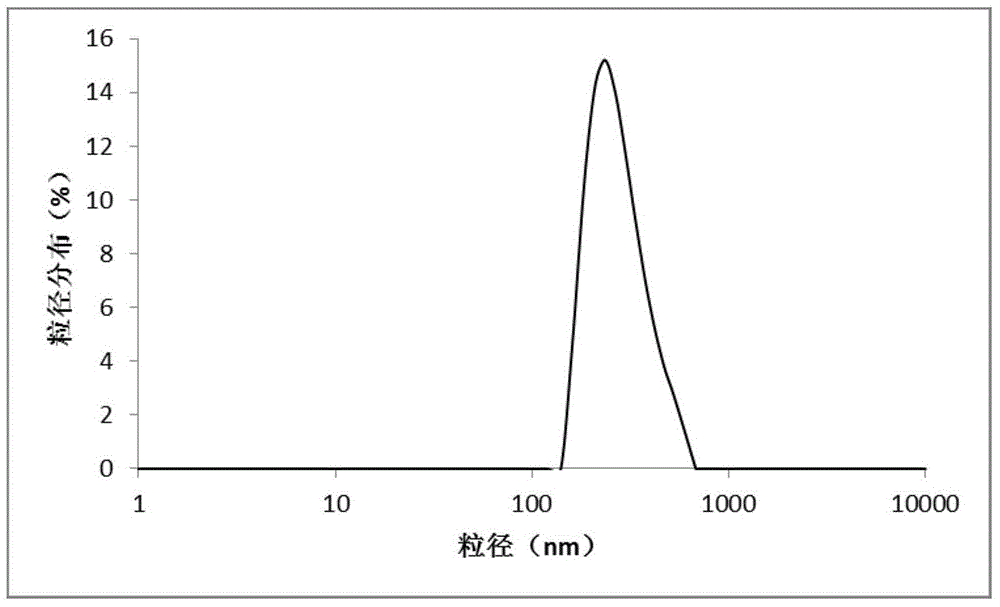
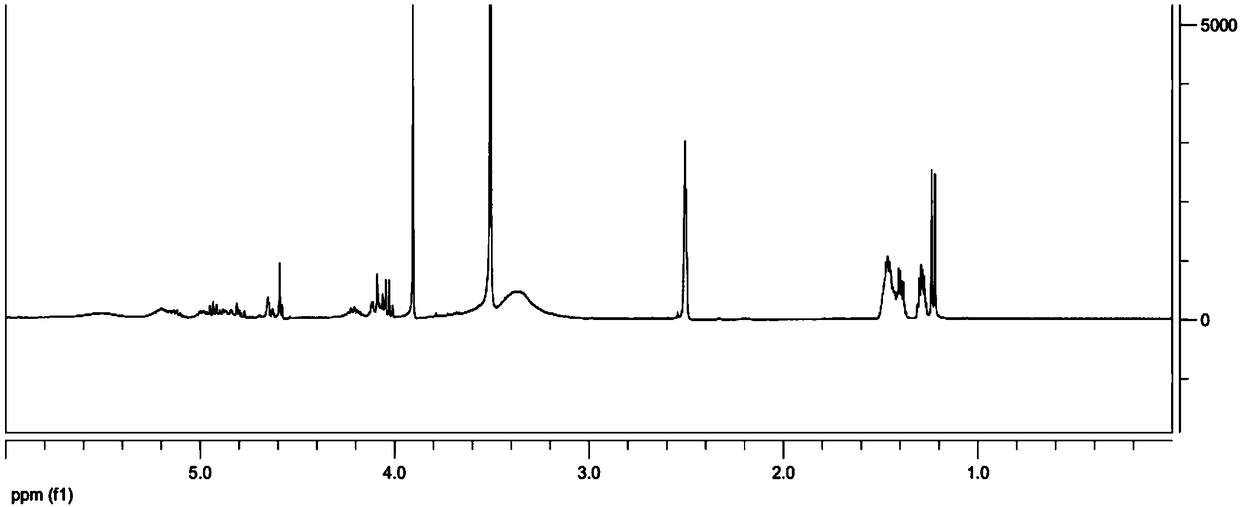
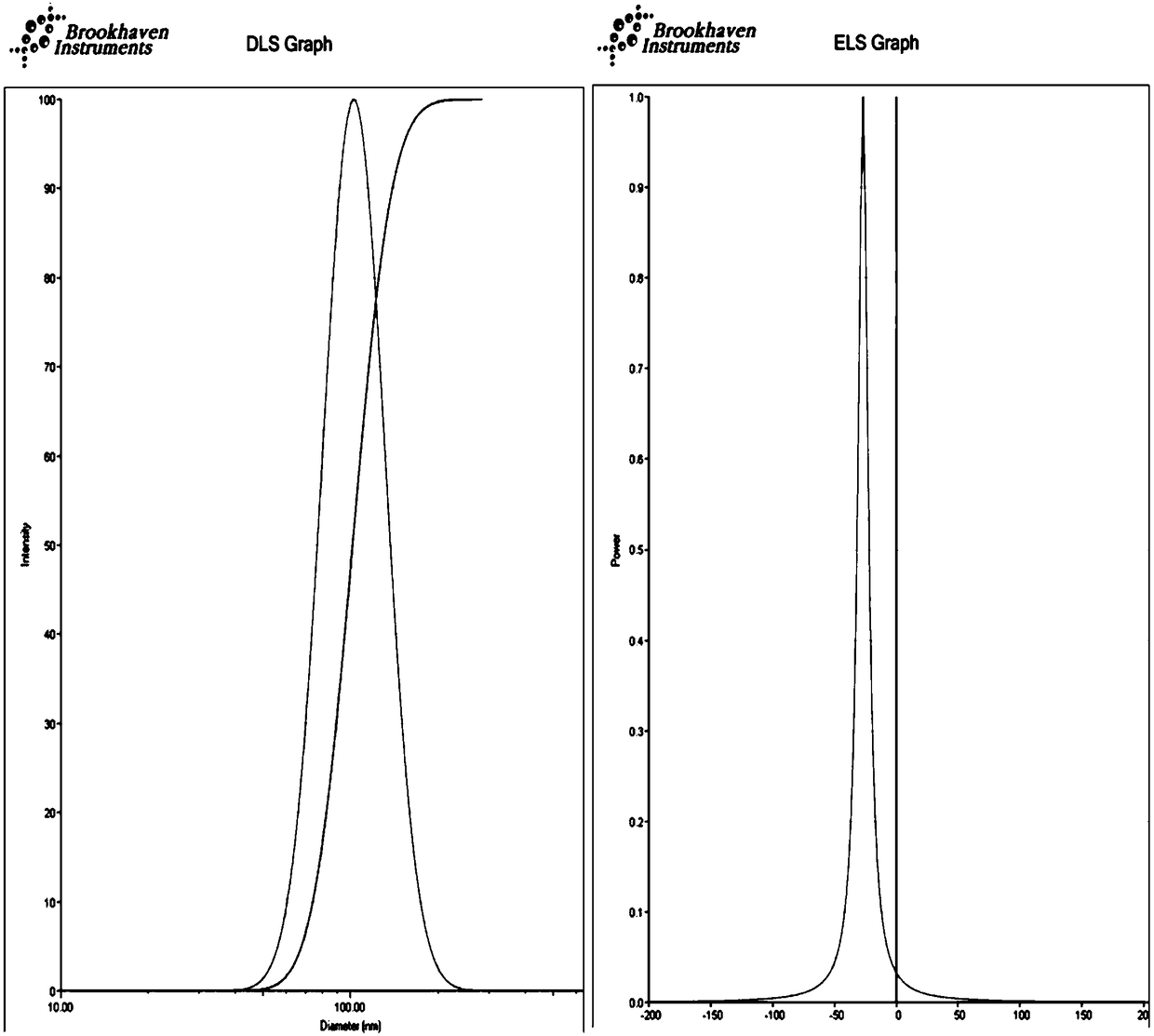
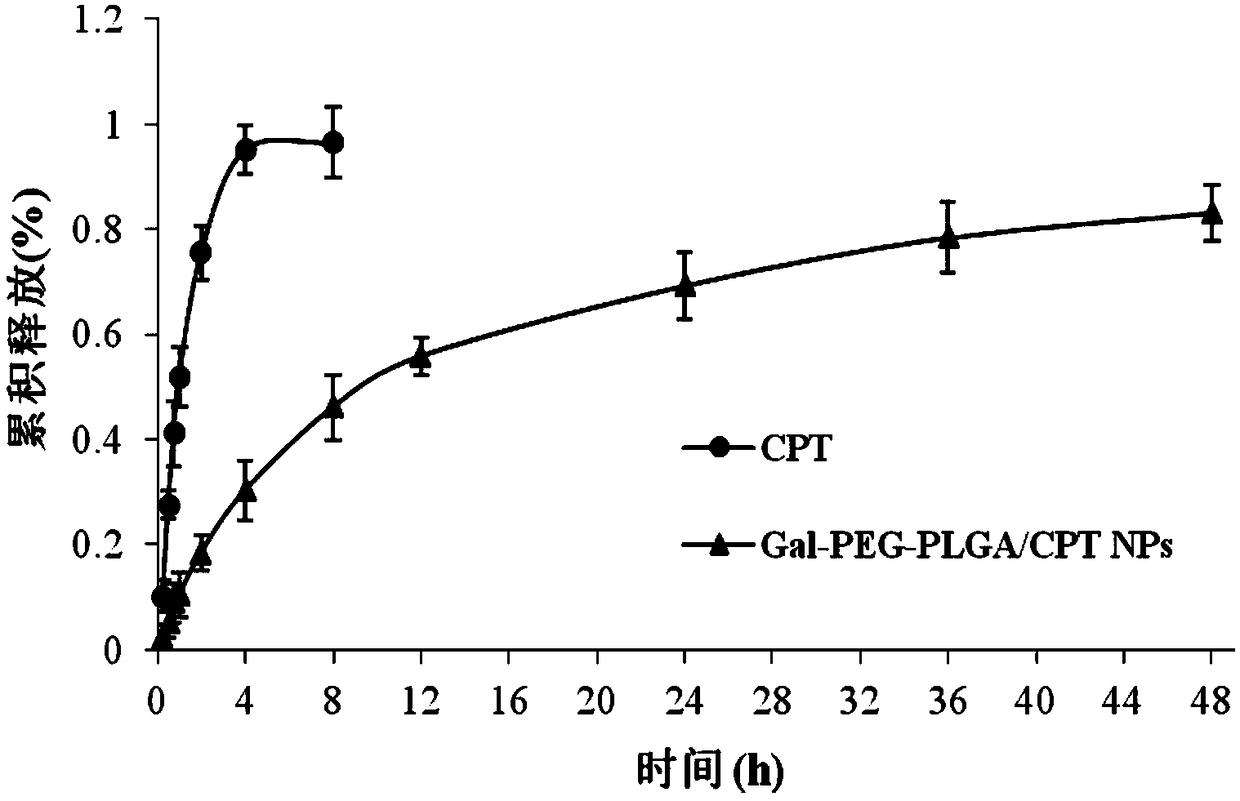
The invention discloses a hepatoma-targeted nano medicine delivery system and a preparation method and application thereof, and particularly relates to preparation and application of hepatoma-targetednano particles formed by a copolymer material-loaded anti-hepatoma medicine (camptothecin / 10-hydroxycamptothecine) prepared from galactose, polyethylene glycol and PLGA. The medicine delivery systemis prepared from a polymer material Gal-PEG-PLGA and the anti-hepatoma medicine (camptothecin / 10-hydroxycamptothecine) through a coprecipitation method or a dialysis method. The prepared material Gal-PEG-PLGA has hepatoma targeting, biocompatibility and biodegradability, the preparation technology of the nano particles is simple, and in vitro and vivo targeting and activity assessment proves thatthe medicine delivery system can deliver the camptothecin / 10-hydroxycamptothecine to hepatoma cells targetedly and effectively kill the hepatoma cells.
Owner:YICHUN UNIVERSITY
PEG-PLGA nanoparticle carrying recombinant human vascular endothelial inhibitor and preparation method thereof
InactiveCN101889983AFlat surfaceNo adhesionPowder deliveryPeptide/protein ingredientsNanoparticleMedicine
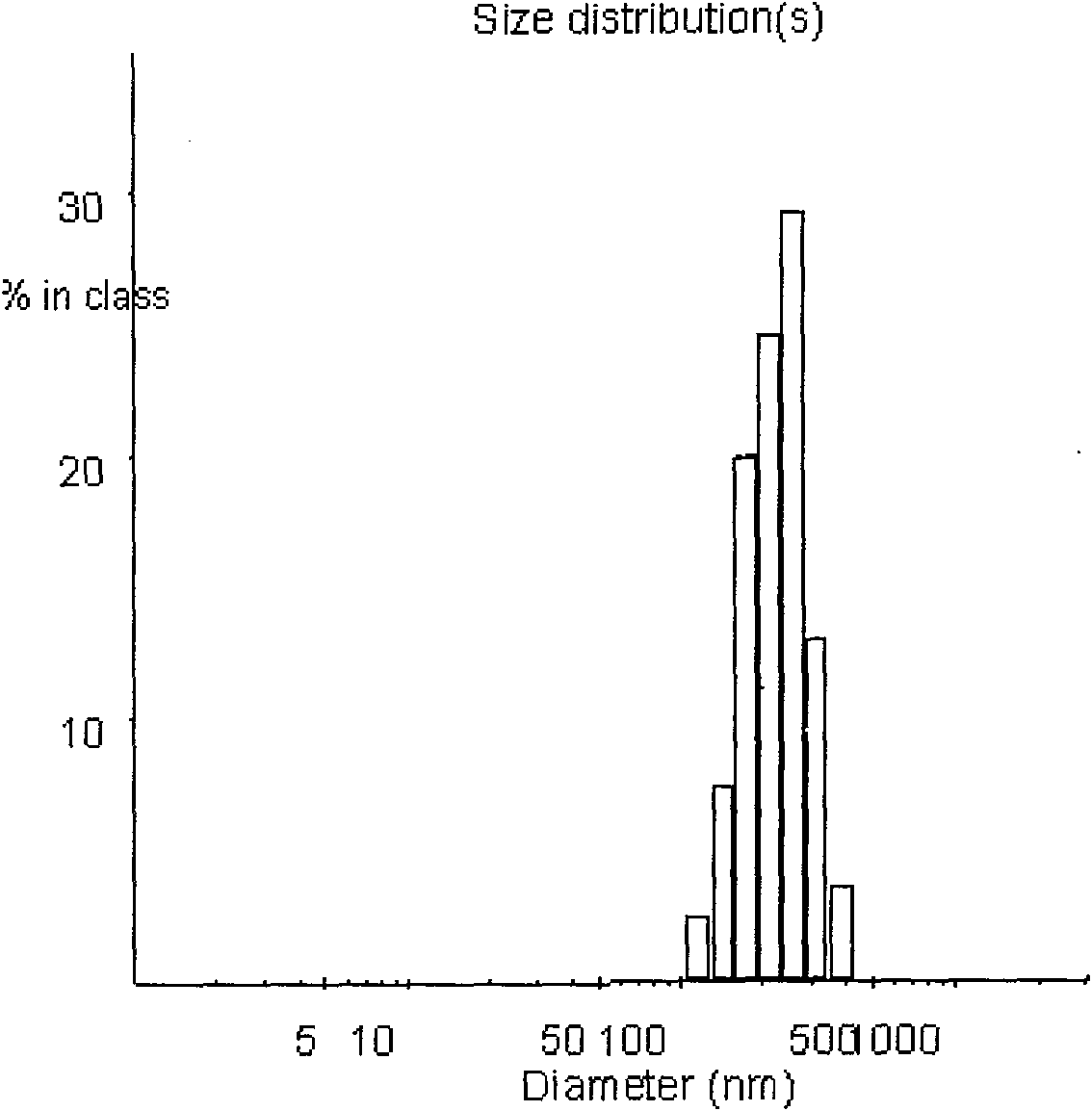
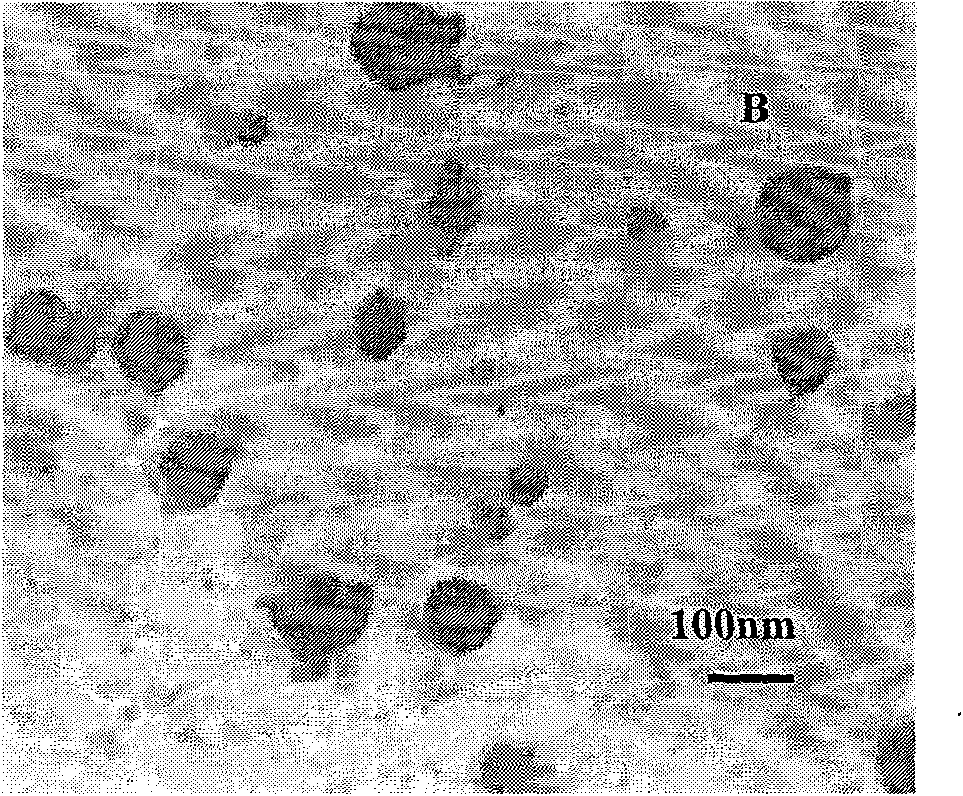
The invention discloses a PEG-PLGA nanoparticle carrying a recombinant human vascular endothelial inhibitor and a preparation method thereof. The nanoparticle comprises the following components in percentage by weight: 1 to 9.8 percent of recombinant human vascular endothelial inhibitor and 90.2 to 99 percent of PEG-PLGA; and the particle size of the nanoparticle is 50 to 500 nm and the polydispersity index is 0.28 to 1. The PEG-PLGA nanoparticle carrying the recombinant human vascular endothelial inhibitor has the advantages of relatively smooth surface of the nanoparticle, no adhesion, controllable nanoparticle size, small particle size, narrow particle size range, convenient intravenous injection and good sustained release effect; moreover, a carrier material modified by PEG has good medicament encapsulating effect, and the hydrophilicity of the carrier material can prevent the carrier material from being captured by a reticuloendothelial system and make the carrier material long-circulated.
Owner:SHANDONG UNIV QILU HOSPITAL
Hydrophilic membrane and preparation method thereof
The invention discloses a hydrophilic membrane. The hydrophilic membrane is characterized by comprising a hydrophilic fiber membrane and a hydrophilic coating, wherein the hydrophilic fiber membrane is coated with the hydrophilic coating; the hydrophilic fiber membrane is prepared from the following raw materials in parts by mass: 40 to 60 parts of modified starch resin, 20 to 30 parts of polyglycolic acid and 1 to 4 parts of cyanoguanidine; the modified starch resin comprises the following raw materials in parts by mass: 30-50 parts of glutinous rice starch, 20-30 parts of acrylic resin, 0.5-1 part of an initiator, 30 parts of a solvent and 20 parts of a NaOH solution; the hydrophilic coating comprises the following raw materials in parts by mass: 100 parts of an acrylic copolymer, 30-50 parts of deionized water and 20-30 parts of polyvinyl alcohol. The invention also discloses a preparation method of the hydrophilic membrane. Compared with the prior art, the hydrophilic membrane disclosed by the invention is safe, healthy, good in water absorption and water retention effects and degradable.
Owner:NINGBO SOKEN CHEM
Biodegradable material and suturing nail
The invention provides a biodegradable material which comprises a polyester biodegradable material and an active component, and the active component comprises at least one of a surfactant and bioactive glass; based on the total mass percentage of the polyester biodegradable material, the content of the active component is less than 40%, and the content of the surfactant is less than or equal to 15%; the polyester biodegradable material comprises a blending modification component, and the blending modification component comprises at least one of polyglycolic acid, glycolide, caprolactone, lactide and polyethylene glycol, so that the biodegradable material has good degradation performance, biological activity, biocompatibility, mechanical performance and degradation controllability at the same time. The invention further provides a suturing nail, and the suturing nail is prepared from the biodegradable material, so that the suturing nail has good degradation performance and mechanical performance at the same time.
Owner:HEFEI BREATH MEDICAL CO LTD
Preparation method of abrasion-resistant plastic package material and application thereof
The invention discloses a preparation method of an abrasion-resistant plastic package material and application thereof. The preparation method comprises the following steps of heating polyethylene glycol acrylate, alkyd resin, nanometer cerium oxide and propyl acetate, and stirring, so as to obtain premixed liquid; treating polyglycolic acid, bamboo fiber, vinyl bistearic amide, and melamine polyphosphate by ultrasonic waves, adding polybenzimidazole, performing warming reaction under the nitrogen protection, filtering, washing, and drying, so as to obtain a composite reaction product; addingpolyvinylidene chloride, sodium polystyrenesulfonate, methyl salicylate, the premixed liquid and the composite reaction product into a high-speed kneading machine, and kneading; adding a crosslinkingagent, a plasticizer and a photo-stabilizer, melting and mixing; finally, enabling a screw film blowing machine to perform extruding, blow injection and forming, and standing at high temperature, so as to obtain the finished product, namely the package material. The prepared plastic package material has the advantages that the abrasion-resistant property is excellent, and the application prospectis good when the plastic package material is used as the outer package of an electronic product.
Owner:WUJIANG YINGLIDA PLASTIC PACKAGING
Ocular surface drip anti-angiogenesis eye-drops preparation and preparation method thereof
InactiveCN109157511AAvoid side effectsGood dispersionOrganic active ingredientsSenses disorderSide effectTumor therapy
The invention discloses an ocular surface drip anti-angiogenesis eye-drops preparation formed by loading hydrophobic small molecule drugs on amphiphilic self-assembled polymers with excellent compatibility. The preparation can be self-assembled to form a core-shell structure at a critical micelle concentration or higher. The invention further provides a preparation method of the ocular surface drip anti-angiogenesis eye-drops preparation. Preparation of eye drops is completed by loading the hydrophobic small molecule drugs on the amphiphilic self-assembled polymers with excellent compatibility, the amphiphilic polymers (such as PEG (Polyethylene Glycol)-PCL (Polycaprolactone), PEG-PLGA and common DSPE-PEG) can complete loading of anti-angiogenesis drugs in the process of being assembled into nanoparticles, a signal path of angiogenesis is inhibited, a small-molecule tyrosine kinase inhibitor applicable to tumor therapy is combined with an increasingly mature nano drug-loading technology, and a novel candidate drug used for ocular surface angiogenesis resistance is prepared. The novel drug is economic and safe, and provides an excellent solution for avoiding side effects of hormonaldrugs.
Owner:WENZHOU MEDICAL UNIV
Resveratrol and temozolomide double-medicine-loaded nanospheres, as well as application and preparation method thereof
InactiveCN102988361AEnhanced anti-glioma efficacyHighlight stabilityPowder deliveryHydroxy compound active ingredientsMicrospherePolyethylene glycol
The invention discloses resveratrol and temozolomide double-medicine-loaded nanospheres, which are prepared by mixing temozolomide (TEM), resveratrol (RES) and a medicine-loading material, wherein the medicine-loading material is an amphiphilic segmented copolymer, namely, polycaprolactone-polyethyleneglycol (mPEG-PCL) or polylactic acid-polyethyleneglycol (mPEG-PLA) or polyglycolic acid-polyethyleneglycol (mPEG-PLGA) synthesised by polycaprolactone or polylactic acid or polyglycolic acid and polyethyleneglycol. The preparation method comprises the following steps of: dissolving the two raw material constituents, namely, the resveratrol (RES) and the medicine-loading material, in a water-soluble non-toxic organic solvent, and dissolving temozolomide in pure water, and stirring; filtering the obtained light-blue dispersion liquid by a microfiltration membrane having a mesh diameter of 220+30 nm to obtain anti-tumour double-medicine-loaded nanosphere solution having a particle diameter of 80-120 nm; and heat-drying or freeze-drying.
Owner:徐华娥 +1
peg-plga encapsulated hemoglobin
ActiveCN104644613BParticle size andUniform particle size distributionPeptide/protein ingredientsAntinoxious agentsWhole bodyPolyethylene glycol
The invention discloses a hemoglobin oxygen carrier, PEG-PLGA, to encapsulate hemoglobin. The PEG-PLGA encapsulated hemoglobin of the present invention is the encapsulated hemoglobin obtained by encapsulating the hemoglobin with polyethylene glycol-polylactic acid glycolic acid block copolymer (PEG-PLGA) nanoparticles, the particle size distribution is uniform, the dispersibility is good, and the oxygen It has high affinity and good oxygen supply effect, and can be used in systemic or local hypoxia, or in systems that require oxygen supply in vitro. The hemoglobin oxygen carrier-PEG-PLGA encapsulated hemoglobin of the present invention will play an important role in the medical field as a blood substitute, and has broad application prospects.
Owner:ACADEMY OF MILITARY MEDICAL SCI
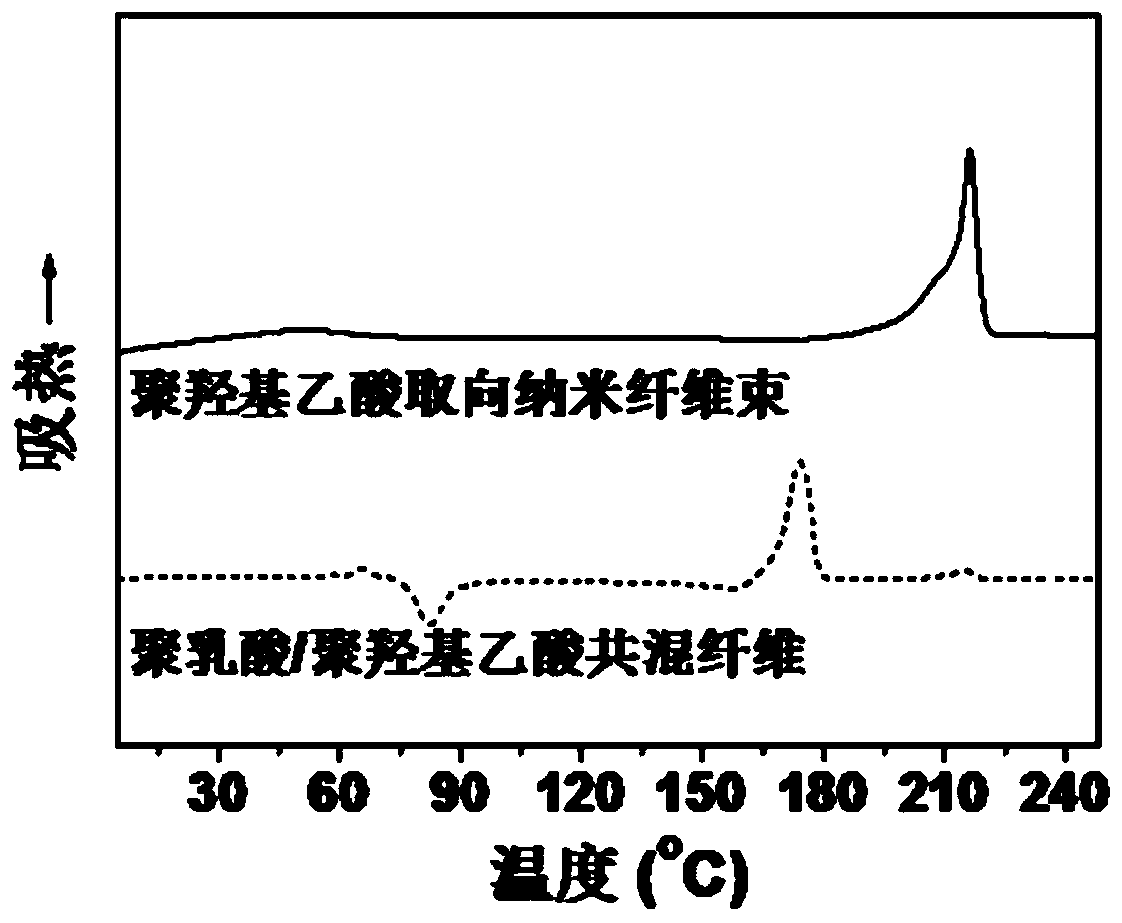
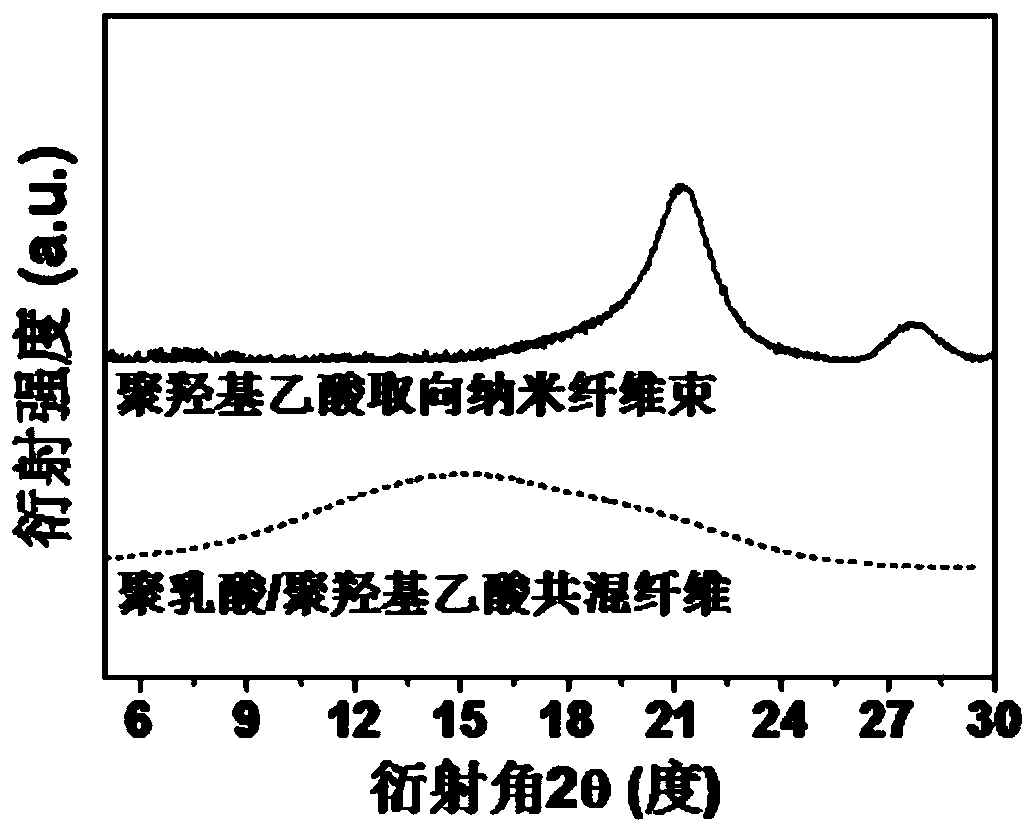
A kind of polyglycolic acid oriented nanofiber bundle and preparation method thereof
ActiveCN109457309BImprove resistance to degradationHigh crystallinityArtificial filament washing/dryingFilament/thread formingPolymer scienceSpinning
The invention discloses a polyglycolic-acid-orientation nanometer fiber bundle and a preparing method thereof. The fiber bundle is formed by polyglycolic acid nanometer fibers wrapped with a polylactic acid-hydroxyacetic acid copolymer. According to the preparing method, the molecular weight, the optical isomer content and the mixing ratio of polylactic acid and polyglycolic acid are preferred, and polylactic acid / polyglycolic acid blend fibers are produced at the suitable spinning temperature and the spinning speed. Under the specific conditions such as the preferred raw material components and sufficiently-strong stretch flow fields, the polyglycolic acid nanometer fibers (the average diameter is 50 nanometers to 200 nanometers) are in-situ formed in the forming process of the polylacticacid / polyglycolic acid blend fibers. The directional-arrangement polyglycolic-acid-orientation nanometer fiber bundle has excellent heat resistance, can keep the stability of the size and the orientation structure at the temperature of being higher than 120 DEG C, and has good degradability resistance, and a performance bottleneck that a conventional polyglycolic-acid nanometer fiber bundle is easy to disorientate and easy to degrade is broken through.
Owner:宁波康飞乐新材料有限公司
A targeted nano drug delivery system mediated by glioma homing peptide and its preparation method
InactiveCN103655517BHigh fluorescence intensityIncrease savingsOrganic active ingredientsMacromolecular non-active ingredientsMonomethoxypolyethylene glycolTherapeutic effect
The invention discloses a Pep-1 peptide modified gliomas targeted nano drug delivery system and a preparation method thereof. The nano drug delivery system comprises polymer nano particles prepared by using an amphiphilic block copolymer (Pep-PEG-PLGA) as a carrier material, paclitaxel wrapped and carried by the polymer nano particles, and modified ligand Pep-1 polypeptide on the surfaces of the polymer nano particles. The amphiphilic block copolymer (Pep-PEG-PLGA) is composed of Male-PEG-PLGA and MePEG-PLGA, Pep-1 is adopted as a molecule with targeting function, the amphiphilic block copolymer PEG-PLGA is taken as a carrier material, the Pep-1 polypeptide is modified on the carrier material via covalent binding, and gliomas targeted polymer nano particles are prepared. According to the Pep-1 peptide modified gliomas targeted nano drug delivery system, gliomas targeting can be voluntarily performed, and the uptaking and accumulation of an anti-tumour drug in the gliomas part can be improved, as a result, the gliomas therapeutic effect is improved.
Owner:NANJING MEDICAL UNIV
Chitin-reinforced PGA-PLA composite material with controllable degradation period as well as preparation method and application thereof
The invention discloses a chitin reinforced PGA-PLA composite material with a controllable degradation period as well as a preparation method and application thereof. The composite material comprises the following components in parts by weight: 10-50 parts of polyglycolic acid (PGA), 50-90 parts of polylactic acid (PLA), 2-15 parts of a toughening agent, 0-1 part of a hydrolysis-resistant agent and 10-40 parts of carapace powder. The composite material disclosed by the invention not only has excellent processability and mechanical property, but also has a controllable degradation period, so that the composite material can be used in fields with corresponding requirements on the degradation period.
Owner:湖南捷立康科技有限公司
LID-PEG-PLGA controlled-release nano microsphere and preparation method thereof
InactiveCN101961316BImprove hydrophobicityHigh encapsulation efficiencyPowder deliveryOrganic active ingredientsWater bathsMatrix solution
The invention discloses an LID-PEG-PLGA controlled-release nano microsphere and a preparation method thereof. The microsphere is the controlled-release microsphere which contains medicinal lidocaine and a degradable carrier. The degradable carrier contains polylactic-glycolic acid and PEG-2000. The mass percentage of the lidocaine in the controlled-release microsphere is 30 to 35 percent. The preparation method comprises the following steps of: preparing the carrier into matrix solution; dispersing the lidocaine into the matrix solution and preparing the lidocaine into an oil phase; mixing the oil phase and the aqueous solution of polyvinyl alcohol, and performing ultrasonic emulsification on the mixture under a water bath condition to obtain W / O-type protogala; mixing the W / O-type protogala and the aqueous solution of polyvinyl alcohol again, and further emulsifying the mixture into W / O / W-type complex emulsion; volatilizing the emulsion by reducing pressure at the normal temperature to obtain cured lidocaine-carried nano microsphere; and scattering, blending, packaging, freezing, sterilizing and the like. The medicament loading rate of the controlled-release nano microsphere can be up to 15 to 22 percent; the entrapment rate can be up to 68 to 78 percent; and the half-life period can be prolonged to 3 to 4 days. Therefore, the microsphere has relatively good effect of burst in the first day after the microsphere is taken and good effect of slow release in later days.
Owner:ARMY MEDICAL UNIV
Composition and method for sulfated non-anticoagulant low molecular weight heparins in cancer and tumor metastasis
A nanoformulation that includes nanoparticles. Each nanoparticle includes a shell in which a glycosaminoglycan (GAG is encapsulated. The GAG is ionically or covalently bonded to the shell. The GAG is selected from the group consisting of sulfated non-anticoagulant heparin (SNACH), super-sulfated non-anticoagulant heparin (S-SNACH), and a combination thereof. The shell includes Poly (lactic-co-glycolic acid) (PLGA), Polyethylene Glycol (PEG)-PLGA, maleimide-PEG-PLGA, chitosan, chitosan-PLGA, methoxy-polyethyleneglycol-poly (lactide-co-glycolide) (MPEG-PLGA)-(maleimide-PEG-PLGA), PLGA-Polycaprolate, or calcium alginate. A method of using the nanoformulation to treat a cancer in a subject includes administering to the patient a therapeutically effective amount of the nanoformulation for treating the cancer.
Owner:MOUSA SHAKER A
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com