Patents
Literature
36 results about "Pentylone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pentylone (β-Keto-Methylbenzodioxolylpentanamine, βk-Methyl-K, βk-MBDP, methylenedioxypentedrone, or 1‐(3,4‐methylenedioxyphenyl)‐2‐(methylamino)pentan‐1‐one) is a stimulant developed in the 1960s. It is a substituted cathinone (a type of substituted phenethylamine). It has been identified in some samples of powders sold as "NRG-1", along with varying blends of other cathinone derivatives including flephedrone, MDPBP, MDPV and 4-MePPP. It was also found in combination with 4-MePPP being sold as "NRG-3". Reports indicate side effects include feelings of paranoia, agitation and inability to sleep, with effects lasting for several days at high doses.
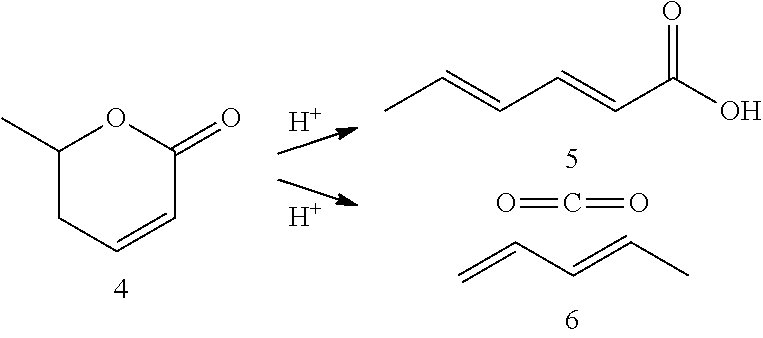
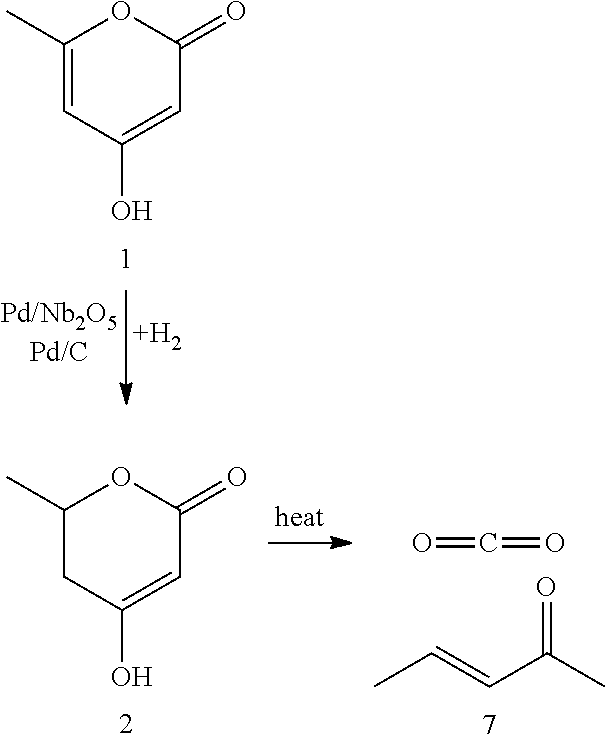
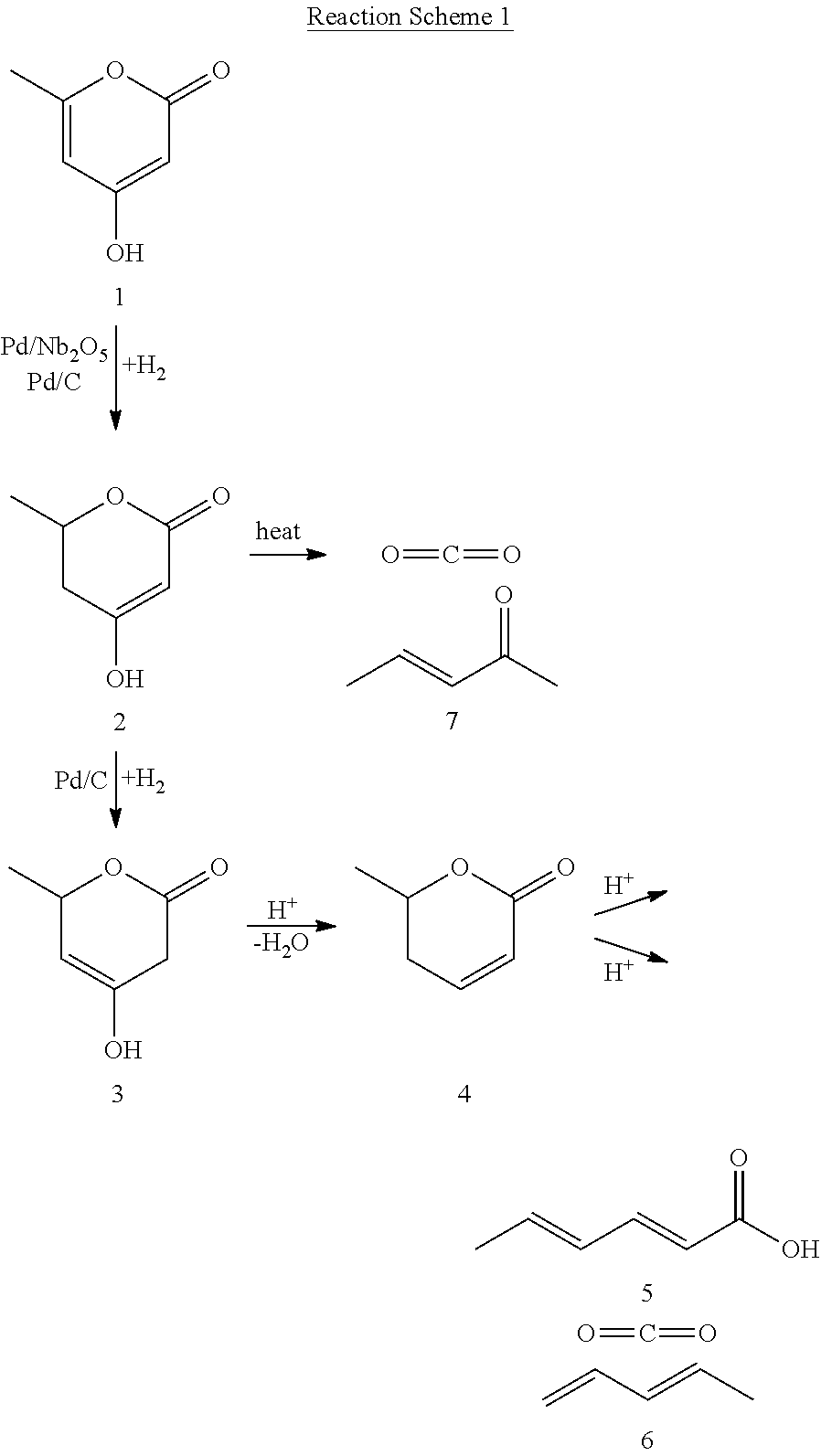
Production of 2,4-hexadienoic acid and 1,3-pentadiene from 6-methyl-5,6-dihydro-2-pyrone
ActiveUS20120116119A1Organic compound preparationPreparation from carboxylic acid esters/lactonesPtru catalystHexadienoic Acid
Described is a method of making sorbic acid, pentadiene, or 3-penten-2-one. The method includes partially hydrogenating 4-hydroxy-6-methyl-2-pyrone (HMP) to yield 5,6-dihydro-4-hydroxy-6-methyl-2H-pyran-2-one (4-DHMMP). Then, if 3-penten-2-one is desired, thermally decomposing the 4-DHMMP to yield 3-penten-2-one. If sorbic acid or pentadiene are desired, the 4-DHMMP is hydrogenated to yield 4-hydroxy-6-methyltetrahydro-2-pyrone (4-HMTHP). The 4-HMTHP is then dehydrated by contacting it with a solid acid catalyst to yield parasorbic acid (PSA). The PSA can then be ring-opened by contacting it with a solid acid catalyst. The reaction conditions of the ring-opening reaction can be controlled to yield sorbic acid and / or pentadiene.
Owner:WISCONSIN ALUMNI RES FOUND
Preparation method for Ni, Cu and Zn-loaded mesoporous MnO2 catalyst and 4, 4-dimethyl-1-(4-p-chlorophenyl)-3-pentanone
ActiveCN104998648ALarge specific surface areaHigh catalytic activityOrganic compound preparationCarbonyl compound preparationPtru catalystChlorobenzene
The invention relates to a preparation method for a Ni, Cu and Zn-loaded mesoporous MnO2 catalyst and 4, 4-dimethyl-1-(4-p-chlorophenyl)-3-pentanone. The catalyst comprises a mesoporous MnO2 carrier, Ni, Cu and Zn, wherein Ni, Cu and Zn are loaded on the mesoporous MnO2 carrier. The molar ratio of Ni, Cu and Zn is 1:0.3-1:0.1-0.3. The mass ratio of the sum of Ni, Cu and Zn to the mesoporous MnO2 carrier is 1-3:10. The catalyst prepared through the above method is high in catalytic activity, low in cost, simple in preparation technology and long in service life. The catalyst is applied to the hydrogenation reaction of 4, 4-dimethyl-1-(4-p-chlorophenyl)-1-pentene-3-ketone for preparing 4, 4-dimethyl-1-(4-p-chlorophenyl)-3-pentanone. The yield and the purity are up to 99%.
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD
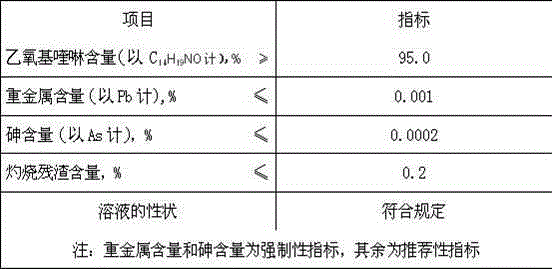
Method for preparing ethoxy quinoline
The invention relates to a method for preparing ethoxy quinoline and belongs to the field of fodder and food. The preparation of the ethoxy quinoline is divided into two steps, namely the preparation of midbody 4-methyl-3-penten-2-ketone and the preparation of a finished product ethoxy quinoline. The method comprises the steps that firstly acetone is transformed into the midbody 4-methyl-3-penten-2-ketone, the potential safety hazards to an operator due to the fact that in the reaction process, the acetone is not reacted completely and volatilized under heating are prevented, and in the transformation process of the 4-methyl-3-penten-2-ketone, by using a catalyst, namely 4%-6% of Mg2+mesoporous molecular sieve, the high-purity 4-methyl-3-penten-2-ketone midbody can be obtained; methylbenzene serves as solvent as well as a water-carrying agent, 'azeotropy' is conducted through the methylbenzene and water generated in reaction, water can be separated, so that the reaction is conducted toward the positive reaction direction, meanwhile, by adding the methylbenzene solvent, an 'inert' solvent gas environment is formed inside a reaction tank, and oxidation of materials is avoided; by means of the combined action of the methylbenzene and toluenesulfonic acid, the produced ethoxy quinoline can reach the standards of feed grade ethoxy quinoline.
Owner:TAIXING RUITAI CHEM
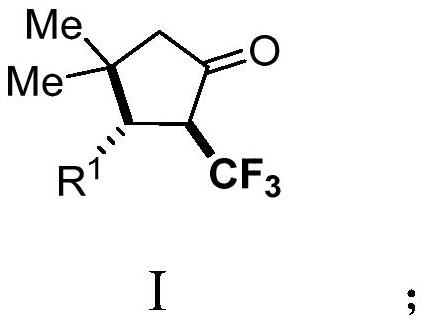
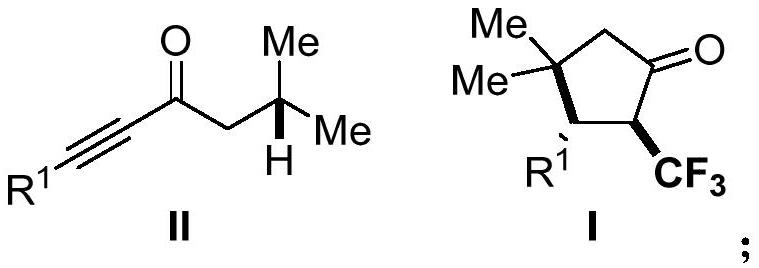
Derivative of 2-trifluoromethyl cyclopentanone and preparation method thereof
PendingCN114057578AImplement the buildAchieve trifluoromethylationOrganic compound preparationOrganic chemistry methodsAlkyneElectron transfer
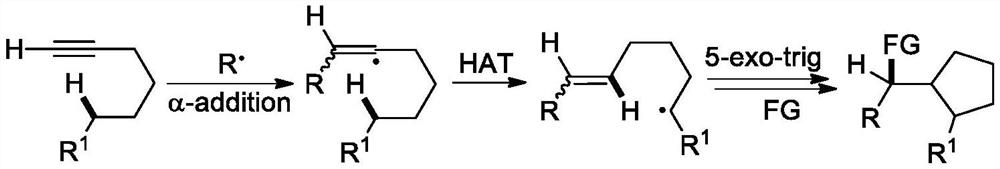
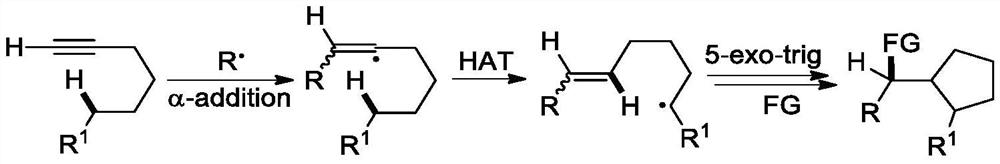
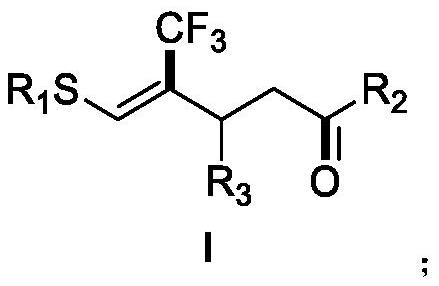
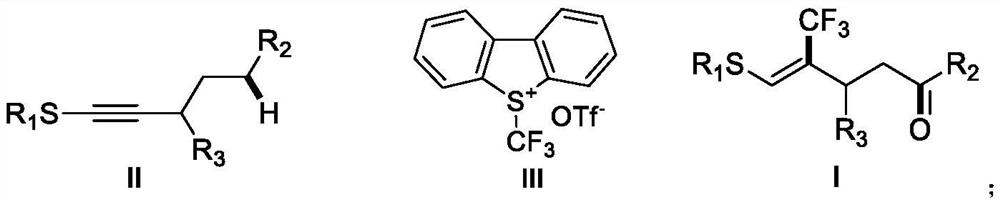
The invention discloses a 2-trifluoromethyl cyclopentenone derivative and a preparation method of the 2-trifluoromethyl cyclopentenone derivative. The preparation method comprises the following steps: adding a photocatalyst and a trifluororeagent into N,N-dimethylformamide, then adding the reactant into an acetylenic ketone compound with a structure as shown in a formula II, and forming a reaction system in a certain reaction environment; and after the reaction is completed, performing post-treatment to obtain the 2-trifluoromethyl cyclopentanone derivative with the structure shown in the formula I in the claim 1. According to the ingenious design, carbonyl is used as a guiding group, a trifluoromethyl free radical region is selectively added to internal alkyne, and then hydrogen migration, 5-internal cyclization, electron transfer and proton transfer are carried out to start the reaction. Besides, the reaction condition is mild, the substrate application range is wide and the operation is simple. A new way is provided for synthesis of the complex 2-trifluoromethyl cyclopentanone derivative.
Owner:ZHEJIANG NORMAL UNIVERSITY
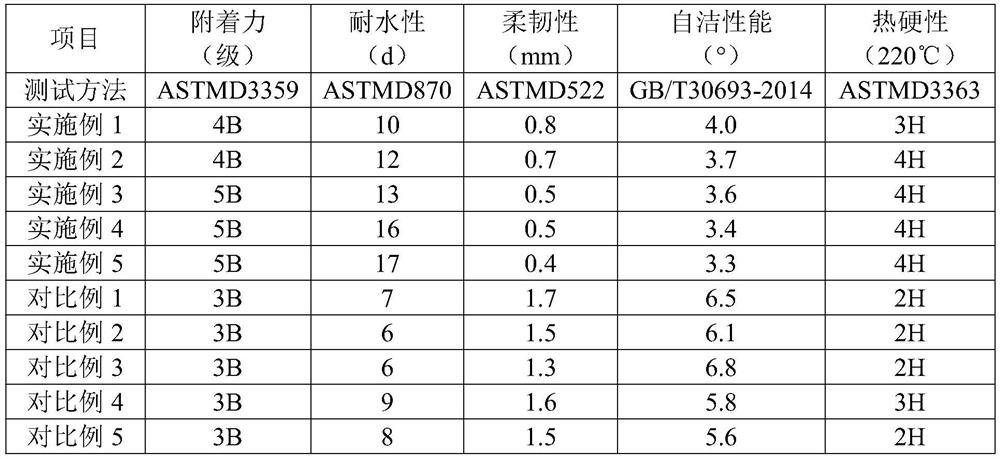
Self-cleaning flame-retardant corrosion-resistant fluorosilicone powder coating
ActiveCN111925707AWide variety of sourcesLow equipment requirementsFireproof paintsAntifouling/underwater paintsMeth-Boronic acid
The invention discloses a self-cleaning flame-retardant corrosion-resistant fluorine-silicon powder coating. The coating is prepared from the following components in parts by weight: 50-60 parts of afilm-forming copolymer, 4-8 parts of amino-terminated hyperbranched compound quaternary ammonium salt, 1-3 parts of lignosulfonic acid, 0.1-0.2 part of phosphorus pentoxide, 3-7 parts of a functionalaid, 10-15 parts of an inorganic filler and 1-3 parts of rare earth fluoride nanofibers, wherein the film-forming copolymer is prepared from 2-trimethylsiloxy-1,1,1,5,5,5-hexafluoro-2-penten-4-one, (E)-4-(2-cyano-3-ethoxy-3-oxo-1-propenyl)phenylboronic acid pinacol ester, trimethyl 4-phosphonobutenoate, N-hydroxysuccinimide methacrylate and 1,3-bis(oxiranylmethyl)-5-(2-propenyl)1,3,5-triazinyl-2,4,6(1H,3H,5H)-trione through a free radical polymerization reaction. The powder coating disclosed by the invention is remarkable in self-cleaning effect, good in performance stability, corrosion resistance, weather resistance, wear resistance, film-forming property and flame retardance, excellent in bonding property with a base material, superior in comprehensive performance, and safe and environment-friendly to use.
Owner:东莞市正荣实业有限公司
Environment-friendly conformal coating and preparation method thereof
ActiveCN110951382ASimple manufacturing methodReduce manufacturing costFireproof paintsAntifouling/underwater paintsPolymer scienceMeth-
The invention discloses an environment-friendly conformal coating which is characterized by comprising the following raw materials in parts by weight: 3-6 parts of octa-aminophenyl POSS, 10-15 parts of epoxy-terminated polyurethane, 20-30 parts of 3-azabicyclo [3,1,0] hexane-2-formic acid modified amino-terminated polyurethane, 15-25 parts of 2-vinyl-3H-4-quinazolinone / 2-(5-chloro-2H-benzothiazole-2-yl)-6-(1,1-dimethylethyl)-4-ethylphenol / 2-trimethylsiloxy-1,1,1,5,5,5-hexafluoro-2-pentene-4-one / 3-(2-propenyl seleno)-l-alanine / glycidyl acrylate copolymer, 3-6 parts of polyethylene glycol dicarboxylic acid modified nano zinc oxide, 3-5 parts of a drier, 1-3 parts of a defoamer, 0.5-1.5 parts of an antistatic additive, 25-35 parts of isopropanol and 0.5-1.5 parts of an emulsifier. The invention also discloses a preparation method of the environment-friendly conformal coating. The environment-friendly conformal coating has better comprehensive performance, better moisture-proof, fog-proofand mildew-proof effects, better electrical insulation performance, wear resistance, high temperature resistance, solvent resistance and chemical resistance, higher adhesive force, and safe and environment-friendly preparation and use processes.
Owner:刘艳蕊
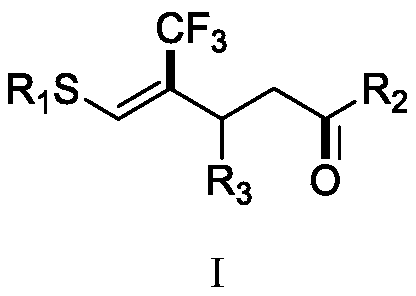
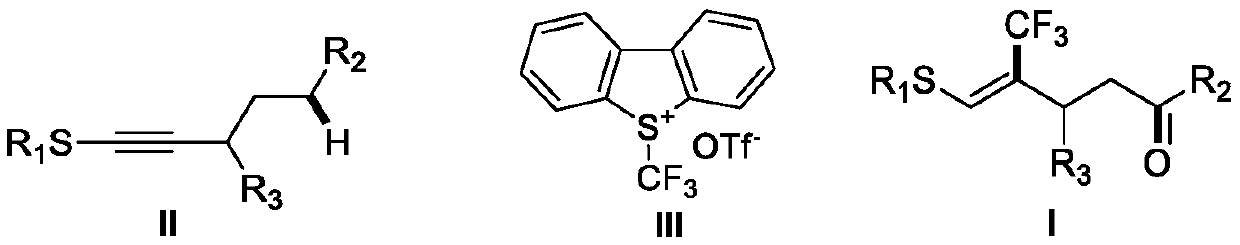
(Z)-4-trifluoromethyl-5-thio-alkyl-4-pentenone derivative and preparation method therefor
ActiveCN110698313AAchieving a cascade reactionExpand areaGroup 4/14 element organic compoundsOrganic free radical generationThio-Alkyne
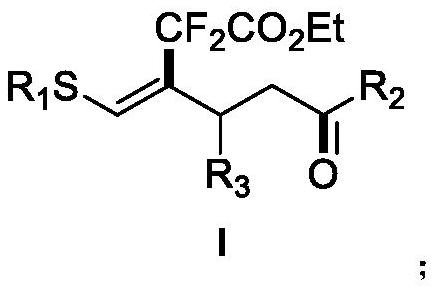
The invention discloses a (Z)-4-trifluoromethyl-5-thio-alkyl-4-pentenone derivative and a preparation method therefor. The method comprises the steps of adding and mixing alkyne thioether with a structure represented by a formula II, S-(trifluoromethyl)dibenzothiophene onium trifluoromethyl sulfonate with a structure represented by a formula III, ruthenium tri(2,2-bipyridyl)dichloride, alkali anda solvent, forming a reaction system under blue-light irradiation, and carrying out aftertreatment after a reaction is completed, thereby obtaining the (Z)-4-trifluoromethyl-5-thio-alkyl-4-pentenone derivative with a structure represented by a formula I. According to the method, the stereoselective synthesis of the (Z)-4-trifluoromethyl-5-thio-alkyl-4-pentenone derivative is achieved in one step.The reaction conditions are mild, the substrate applicable range is broad, the reaction yield is good, the operation is simple, and a new way is provided for synthesis of trifluoromethyl containing pentenone compounds.
Owner:ZHEJIANG NORMAL UNIVERSITY
Production method for cyclopentenone derivative
ActiveUS20210024444A1Cheap productionInexpensive and rapid supplyPreparation from heterocyclic compoundsBiotechnologyAgricultural science
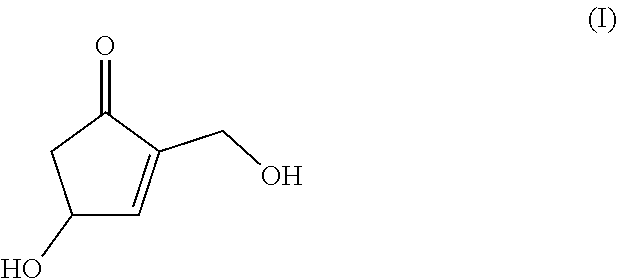
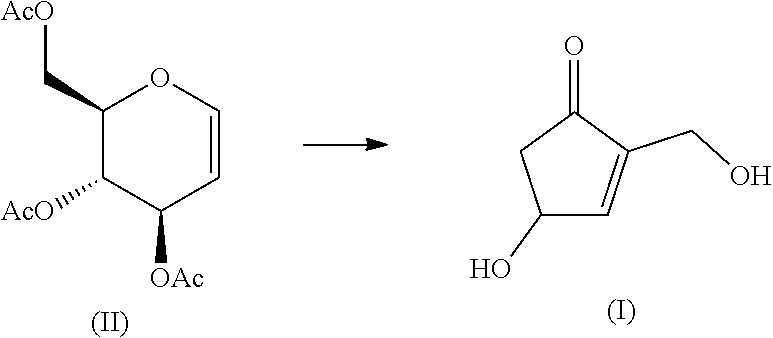
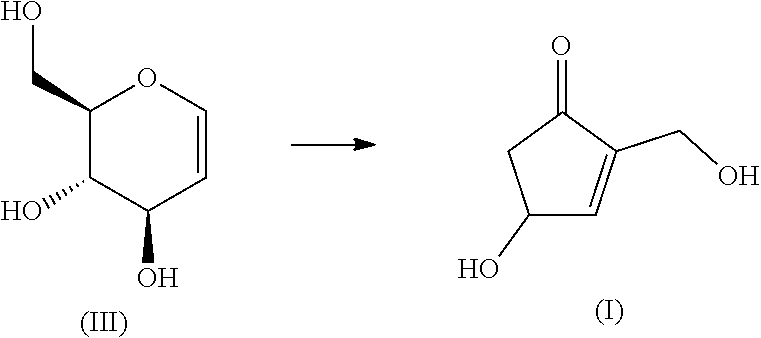
[Problem]The present invention provides an industrially-preferable, cost-efficient, low-cost production method for 4-hydroxy-2-hydroxymethyl-2-cyclopenten-1-one (a compound represented by formula (I)) useful as a medicine, an agricultural chemical, or a raw material or intermediate of a medicine, an agricultural chemical, or the like.[Solution]According to the present invention, this compound represented by formula (I) is produced by subjecting an easily available compound represented by formula (II) (tri-O-acetyl-D-glucal) to a heating reaction in pressurized water.
Owner:TOHOKU UNIV +1
Method for continuous production of 3-methyl-3-penten-2-one in micro-channel reactor
ActiveCN110790651AContinuous and efficient productionHigh yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystOrganic synthesis
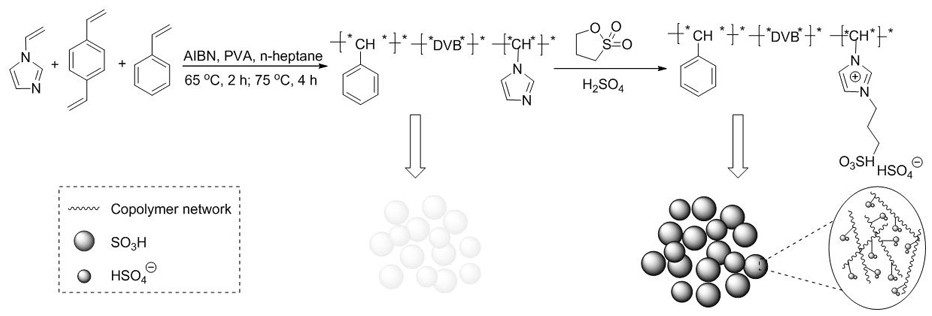
The invention discloses a method for continuous production of 3-methyl-3-penten-2-one in a micro-channel reactor and belongs to the technical field of organic synthesis processes. The method comprisesthe following specific steps: mixing 2-butanone with an ionic liquid [Ps-min]HSO4; simultaneously pumping a raw material acetaldehyde and a mixed solution of 2-butanone and a catalyst into a preheating module through metering pumps respectively, and introducing the components into an integral micro-channel mixer; performing uniform mixing, further introducing the components into a micro-channel reactor, and performing a reaction; and introducing the components into a cooling module, performing sample, and performing separation so as to obtain 3-methyl-3-penten-2-one. By adopting the method, aproduct can be efficiently and rapidly synthesized; the ionic liquid [Ps-min]HSO4 replaces a conventional inorganic acid to be used as a catalyst of the reaction, the reaction condition is mild, andthe product yield is high; meanwhile, the catalyst is easily separated from reaction products, the acid-base neutralization process of a conventional process is avoided, production of wastewater can be reduced, and the environment can be protected.
Owner:FUZHOU UNIV
Continuous production of methyl pentenone using cation exchange resin in a fixed bed reactor
ActiveUS20220089517A1Expensive to operateExpensive equipmentIon-exchange column/bed processesOrganic compound preparationPtru catalystFixed bed
Provided herein is a method for producing methyl pentenone (MPO) in high yield in a continuous mode in a fixed bed reactor having a plurality of sidewall injecting ports by reacting excess methyl ethyl ketone (MEK) with acetaldehyde in presence of a cation exchange resin catalyst, wherein the acetaldehyde is injected from the plurality of sidewall injecting ports of the reactor. The method is also effective in reducing the complete consumption of the catalyst during the course of the reaction.
Owner:HARMONY ORGANICS PTE LTD +1
Method for distinguishing special-grade virgin olive oil from other olive oil based on flavor markers
PendingCN114280196AAvoid interferenceAvoid lossComponent separationBiotechnologyIon-mobility spectrometry
The invention provides a method for distinguishing special-grade virgin olive oil from other olive oil based on a flavor marker, which adopts a headspace gas phase ion mobility spectrometry analysis method, and utilizes the peak area of the flavor marker in the olive oil and the fingerprint response spectrum difference to distinguish. The related flavor markers are (E)-2-hexenal, (E)-3-pentene-2-ketone, 3-pentanone, 1-pentene-3-ketone, 2-methylbutyraldehyde, isovaleraldehyde and methyl acetate, and can also be used as characteristic flavor markers of special virgin olive oil. According to the distinguishing method, only simple headspace treatment is needed, interference of external solvents is avoided, the detection accuracy is improved, instrument conditions are correspondingly controlled, it can be guaranteed that volatile matter in olive oil is volatilized to the maximum extent, useful information loss caused by disintegration of flavor marker substance structures due to excessive conditions can be avoided, and the method is suitable for popularization and application. The method is simple and rapid to operate, has no pollution to the environment, and is completely visualized in the characteristic region. And artificial sensory evaluation can be supported.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
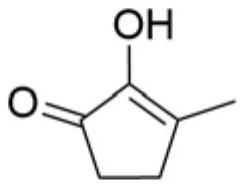
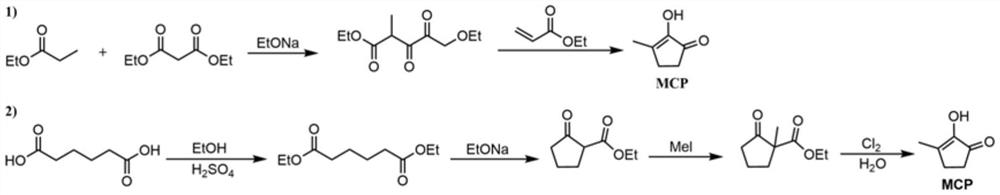
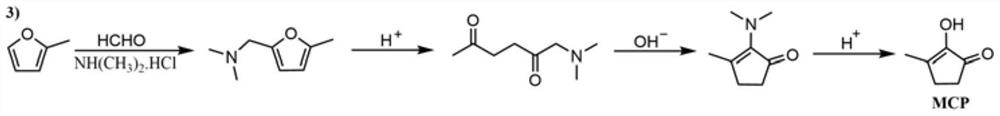
Synthetic method of methyl cyclopentenolone
PendingCN112142582AReduce processingEasy to operateOrganic compound preparationCarbonyl compound preparationCyclopenteneFuran
The invention relates to a preparation method of methyl cyclopentenolone, which comprises the following steps: (1) controlling the pH value and temperature of a reaction system, and reacting a dimethylamine hydrochloride, a formaldehyde aqueous solution and 2-methyl furan to generate N, N-dimethyl-5-methyl furfuryl amine; (2) when it is monitored that the content of the 2-methyl furan is not reduced any more, adjusting the pH value of the reaction solution to be less than 1 by using an acid solution, and reacting to obtain a product 1-dimethyl amino-2, 5-hexanedione; and (3) adjusting the pH value of the reaction solution to 12-13 by using an alkali, reacting, extracting the reaction solution, concentrating to recover the solvent, and rectifying to obtain a product 2-(dimethylamino)-3-methyl-2-cyclopentene-1-one; and (4) mixing 2-(dimethylamino)-3-methyl-2-cyclopentene-1-one with hydrochloric acid, reacting, hydrolyzing, crystallizing and purifying to obtain methyl cyclopentenolone, and concentrating and recycling a reaction mother liquor Compared with the prior art, the method has the advantages that remote and automatic operation is facilitated, wastewater treatment is reduced, and the raw material dimethylamine hydrochloride can be reused.
Owner:SHANGHAI INSTITUTE OF TECHNOLOGY
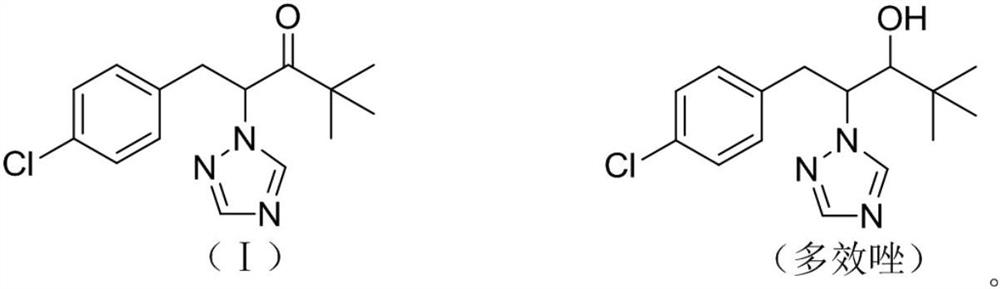
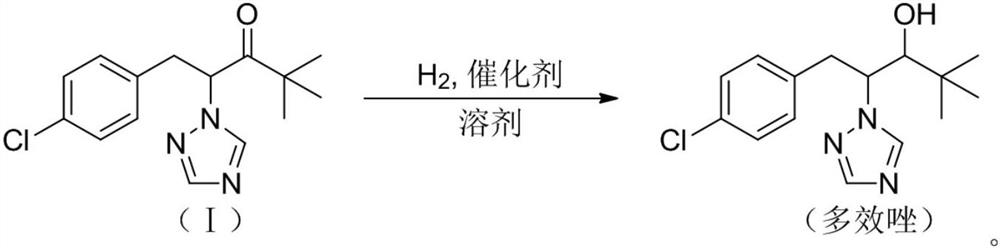
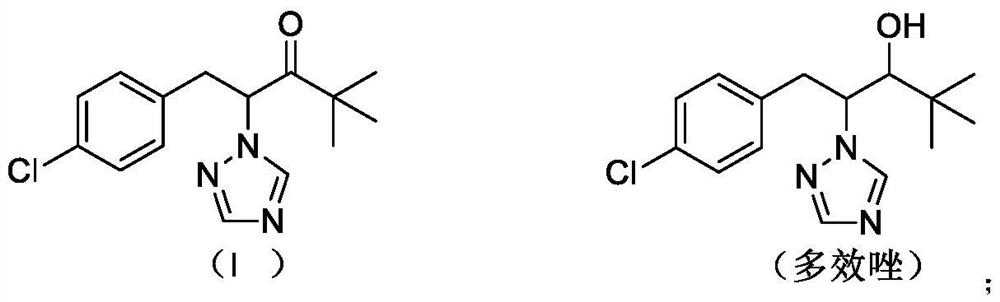
A kind of preparation method of paclobutrazol
Owner:JIANGSU SEVENCONTINENT GREEN CHEM CO LTD +1
Continuous production of methylpentenone using cation exchange resin in fixed bed reactor
PendingCN114249640AReduce investmentIncrease productionIon-exchange column/bed processesOrganic compound preparationPtru catalystFixed bed
The present application provides a process for the high yield production of methylpentenone (MPO) in a continuous mode by reacting an excess amount of methyl ethyl ketone (MEK) with acetaldehyde in the presence of a cation exchange resin catalyst in a fixed bed reactor having a plurality of sidewall injection ports, wherein acetaldehyde is injected from a plurality of sidewall injection ports of the reactor. The method also effectively reduces the complete consumption of the catalyst in the reaction process.
Owner:INDIAN INSTITUTE OF TECHNOLOGY BOMBAY +1
A kind of (z)-4-difluoroalkyl-5-sulfanyl-4-pentenone derivative and its preparation method
ActiveCN110627696BAchieving a cascade reactionImprove compatibilityGroup 4/14 element organic compoundsOrganic chemistry methodsEthylic acidAlkyne
The invention discloses a (Z)-4-difluoroalkyl-5-sulfanyl-4-pentenone derivative and a preparation method thereof. The preparation method comprises following steps: an alkyne thioether with a structureshown in formula II, monobromo difluoroacetate, fac-Ir(ppy)3, an alkali and a solvent are mixed, a reaction system is formed under blue light irradiation, and the (Z)-4-difluoroalkyl-5-sulfanyl-4-pentenone derivative with a structure shown in formula I is obtained through aftertreatment. According to the invention, the addition-hydrogen migration-noncyclization cascade reaction of free radicalson non-terminal alkyne is realized for the first time, and the stereoselective synthesis of the (Z)-4-difluoroalkyl-5-sulfanyl-4-pentenone derivative is realized in one step. The reaction conditions are mild, the substrate application range is wide, the reaction yield is good, the operation is simple, and a new way is provided for synthesis of fluorine-containing pentenone compounds.
Owner:ZHEJIANG NORMAL UNIVERSITY
A kind of (z)-4-trifluoromethyl-5-sulfanyl-4-pentenone derivative and its preparation method
ActiveCN110698313BAchieving a cascade reactionExpand areaGroup 4/14 element organic compoundsOrganic free radical generationRuthenium chlorideMethyl palmoxirate
Owner:ZHEJIANG NORMAL UNIVERSITY
1-phenyl-4-penten-1-one derivative and its synthesis method and application
ActiveCN110128257BAtom economy is highHigh regional selectivitySilicon organic compoundsAntineoplastic agentsPtru catalystOrganosolv
The invention discloses a 1-phenyl-4-penten-1-one derivative represented by formula (1) and a synthesis method thereof, using diazo compounds, allyl alcohol derivatives and alkyl alcohols as raw materials, The 1-phenyl-4-penten-1-one derivative is obtained by using metal palladium, metal rhodium and an organic phosphine ligand as catalysts in an organic solvent through one-step reaction. The synthesis method of the 1-phenyl-4-pentene-1-ketone derivative of the present invention has the advantages of high atom economy, high regioselectivity, low catalyst consumption, simple and safe operation, and the obtained 1-phenyl-4 ‑Penten‑1‑one derivatives are important chemical and pharmaceutical intermediates.
Owner:EAST CHINA NORMAL UNIV
Synthesis method of cis-jasmonone
PendingCN114409519AShort process routeHigh selectivityOrganic compound preparationOrganic chemistry methodsCyclopenteneMethylmagnesium chloride
The invention discloses a synthesis method of cis-jasmone, which specifically comprises the following steps: carrying out Grignard reaction on 1, 4-dichlorobutene and methylmagnesium chloride in a THF (tetrahydrofuran) medium to obtain 1-chloro-2-pentene; the method comprises the following steps: carrying out cyclization reaction on 2, 5-hexanedione in a dibromomethane medium under a strong alkaline condition to obtain 3-methyl-2-cyclopentene-1-ketone. According to the method, 1-chloro-2-pentene and 3-methyl-2-cyclopentene-1-ketone are subjected to an addition / elimination reaction in an ethanol medium under an alkaline condition, and cis-jasmonone is obtained. Compared with other synthesis methods, the method has the advantages of short process route, high reaction selectivity and conversion rate, high product yield, low cost and the like.
Owner:江苏宏邦化工科技有限公司
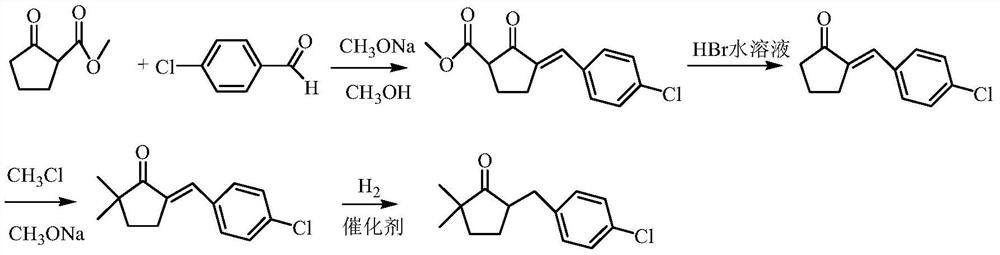
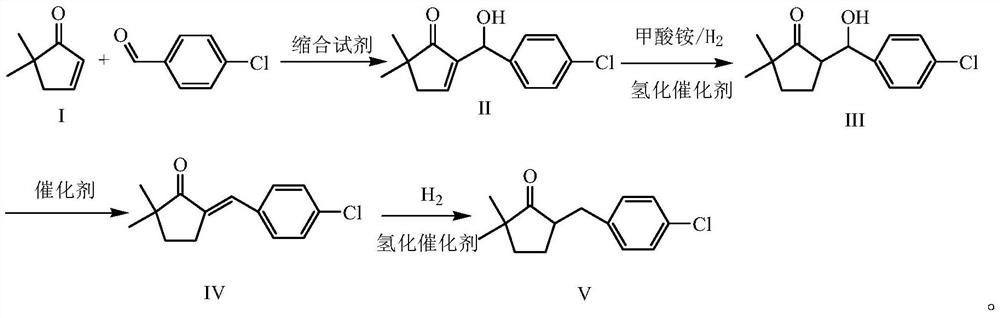
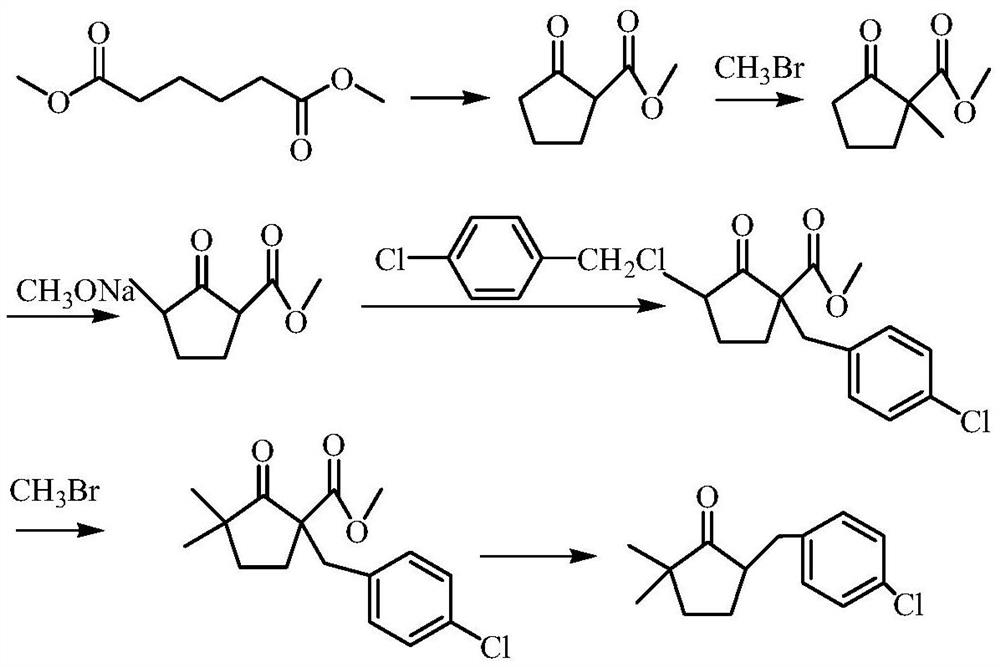
A kind of preparation method of 2,2-dimethyl-5-(4-chlorobenzyl) cyclopentanone
ActiveCN106045830BIncreased process safetySimple and fast operationOrganic compound preparationCarbonyl compound preparation by condensationChemical industryChlorobenzene
The invention discloses a preparation method of 2,2-dimethyl-5-(4-chlorobenzyl) cyclopentanone, which uses p-chlorobenzaldehyde and 2,2-dimethyl-4-cyclopentanone En-1-ketone, that is, the compound of formula I, is used as the initial raw material. After aldol condensation, hydrogenation, hydroxyl removal and further hydrogenation, the compound of formula V is obtained, which is the intermediate 2,2-dimethyl-5 of metconazole ‑(4‑chlorobenzyl) cyclopentanone, compared with the prior art, the inventive method avoids the use of high-risk compound sodium hydride, avoids operations such as highly toxic methylation, and the reaction is moderate and easy to control. The target product 2, The 2-dimethyl-5-(4-chlorobenzyl) cyclopentanone has a purity greater than 95%, a total yield of over 65%, meets the requirements of green chemical industry, and is suitable for industrial production.
Owner:SHANGHAI SHISI CHEM PROD
(1S,4aS,8aS)-Decahydro-5,5,8a-trimethyl-2-methylene-1-naphthaleneacetaldehyde-based spice and preparation process thereof
InactiveCN111848375AImprove production efficiencyAvoid pollutionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsO-Phosphoric AcidPtru catalyst
The invention discloses a (1S,4aS,8aS)-Decahydro-5,5,8a-trimethyl-2-methylene-1-naphthaleneacetaldehyde-based spice and a preparation process thereof. According to the method, butanone and acetaldehyde are used as raw materials for aldehyde ketone condensation; then, myrcene and 3-methyl-3-pentene-2-one are used for carrying out diene addition; and finally, a diene addition product is cyclizedto obtain the product. A catalyst is prepared in the cyclization process; the catalyst is prepared by the following steps: polymerizing a styrene micromonomer to prepare a first matrix; sulfonating the first matrix under the action of sulfuric acid to obtain a second matrix; and soaking the second matrix in a ferric chloride solution to produce the catalyst. The catalyst is a cation exchange resincatalyst, compared with the prior art, phosphoric acid is not used for catalysis, environmental pollution is prevented, metal iron ions are loaded on the cation exchange resin, sulfonic acid acid acid groups on the surface of the catalyst can be combined with the iron ions through complexation, then a stronger acid center is formed, and the preparation efficiency of the (1S,4aS,8aS)-Decahydro-5,5,8a-trimethyl-2-methylene-1-naphthaleneacetaldehyde is greatly improved.
Owner:ANHUI HYEA AROMAS
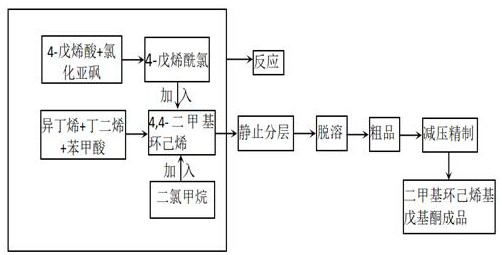
Synthesis method of dimethyl cyclohexenyl pentenone
PendingCN114349622ASafe and stable operationRatio and reaction temperature optimizationCarbonyl compound preparation by condensationSodium bicarbonatePtru catalyst
The invention discloses a synthesis method of dimethyl cyclohexenyl pentenone. The method comprises the following steps: synthesizing 4, 4-dimethyl cyclohexene from isobutene and butadiene under medium pressure by taking n-hexane as a solvent and p-toluenesulfonic acid as a catalyst; 4-pentenoic acid reacts with thionyl chloride to produce 4-pentenoyl chloride; dichloromethane is used as a solvent, 4, 4-dimethyl cyclohexene and 4-pentenoyl chloride are synthesized in a saturated sodium bicarbonate aqueous solution, and dimethyl cyclohexenyl pentenone is obtained. According to the method, the raw material ratio and the reaction temperature of the reaction are optimized at the same time, so that high yield of the reaction is achieved, safe and stable operation of the test is effectively guaranteed, and unexpected effects are achieved; according to the invention, the preparation process of the 4-pentenoyl chloride is improved, so that the yield of the 4-pentenoyl chloride is greatly improved, and the yield of the whole preparation process is further improved.
Owner:TENGZHOU TIANSHUI BIO TECH CO LTD
The preparation method of 4-furfurylthiopentanone-2
ActiveCN110357840BReduce manufacturing costImprove securityOrganic chemistryPhosphorous acidEngineering
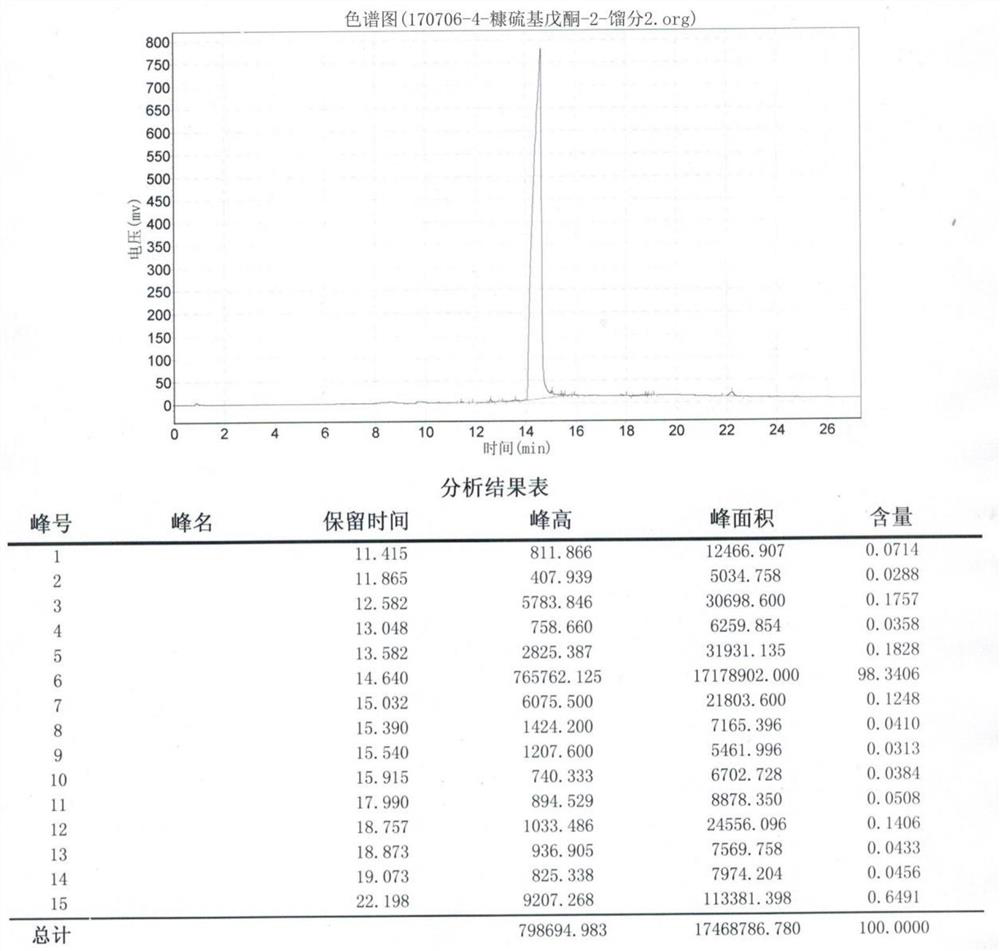
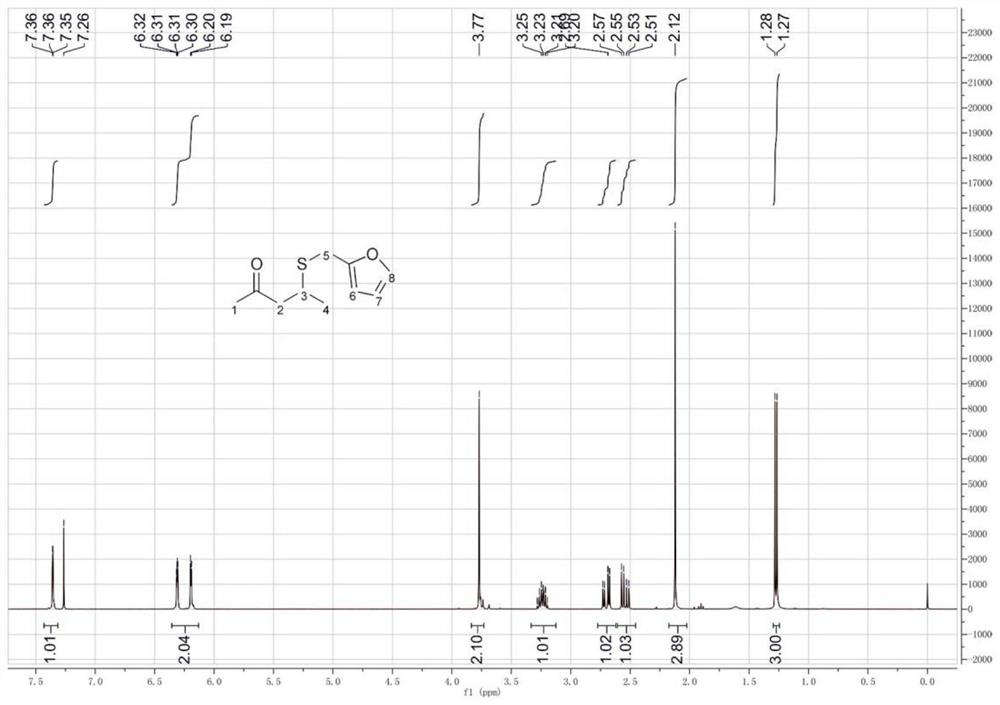
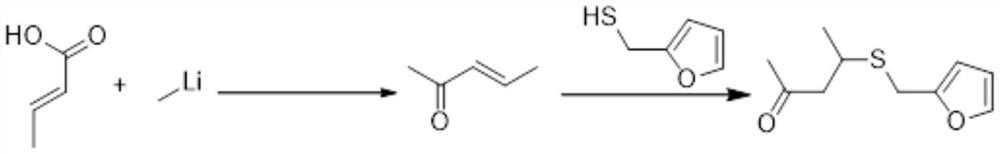
The invention belongs to the technical field of fine chemistry, and provides a preparation method of 4-furfurylthiopentanone-2, which specifically comprises the following steps: adding raw material chloroacetone into a toluene solution of triphenylphosphine or adding triethyl phosphite , the temperature is raised and refluxed to obtain a phosphorus salt; the obtained phosphorus salt is dissolved in an organic solvent, a strong base is added, and stirred for 2 to 3 hours, the reaction is completed, and phosphorus ylide is obtained; Stir until the reaction is completed to obtain 3-pentene-2-ketone; piperidine is added to 3-pentene-2-ketone, the dichloromethane solution of furfuryl mercaptan is added dropwise, and the temperature of the system is controlled to be 35~ 40° C., after the dropwise addition, the mixture was stirred until the reaction was completed to obtain the product 4-furfurylthiopentanone-2. The invention solves the problems in the prior art that the synthesis route of 4-furfuryl pentanone-2 is high in danger, high in cost and unsuitable for industrial production.
Owner:石家庄和中科技有限公司
A kind of environment-friendly three anti-paint and preparation method thereof
ActiveCN110951382BSimple manufacturing methodReduce manufacturing costFireproof paintsAntifouling/underwater paintsPolymer scienceMeth-
The invention discloses an environment-friendly conformal paint, which is characterized in that it comprises the following raw materials in parts by weight: 3-6 parts of octaaminophenyl POSS, 10-15 parts of epoxy-terminated polyurethane, 3-azabicyclo [3,1,0]Hexane-2-carboxylic acid modified amino-terminated polyurethane 20-30 parts, 2-vinyl-3H-4-quinazolone / 2-(5-chloro-2H-benzothiazole- 2-yl)-6-(1,1-dimethylethyl)-4-vinylphenol / 2-trimethylsiloxy-1,1,1,5,5,5-hexafluoro-2- Pentene-4-ketone / 3-(2-propenylselenyl)-L-alanine / glycidyl acrylate copolymer 15-25 parts, polyethylene glycol dicarboxylic acid modified nano-zinc oxide 3-6 3-5 parts of driers, 1-3 parts of defoamers, 0.5-1.5 parts of antistatic additives, 25-35 parts of isopropanol, and 0.5-1.5 parts of emulsifiers. The invention also discloses a preparation method of the environment-friendly three anti-paint. The environment-friendly three-proof paint disclosed by the invention has better overall performance, better moisture-proof, anti-fog, and mildew-proof effects, better electrical insulation performance, wear resistance, high temperature resistance, solvent resistance and chemical resistance, and better adhesion. Higher, safe and environmentally friendly in the process of preparation and use.
Owner:刘艳蕊
A kind of sila-beta-dynasty ketone and its preparation method
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
A kind of 4-methyl-4-phenylcyclopentenone compound and preparation method thereof
ActiveCN110759819BGet efficientlySimple process conditionsSulfonic acid amide preparationCarbonyl compound preparation by condensationLithium hydroxideTosylhydrazone
Owner:NANJING TECH UNIV
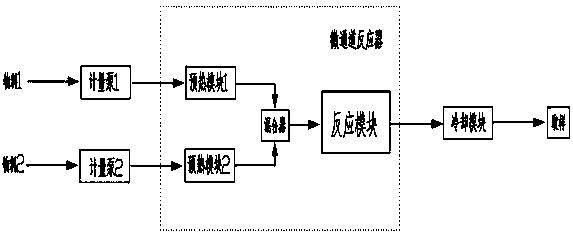
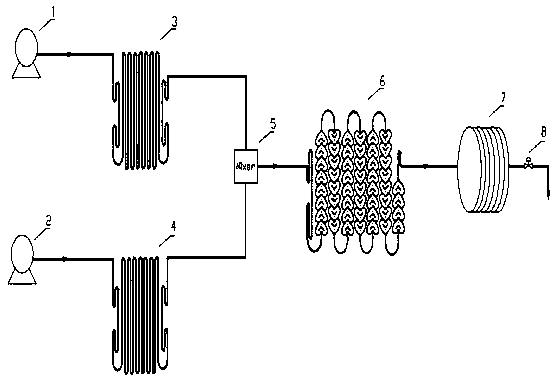
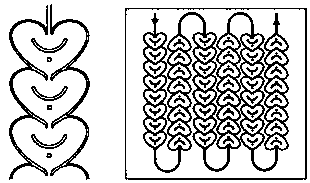
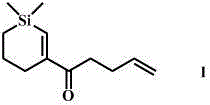
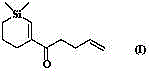
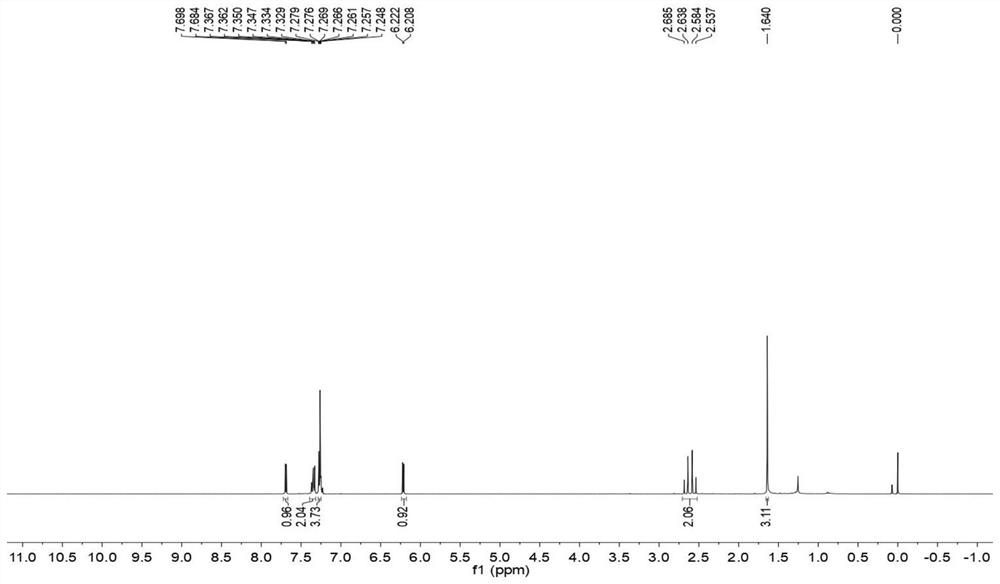
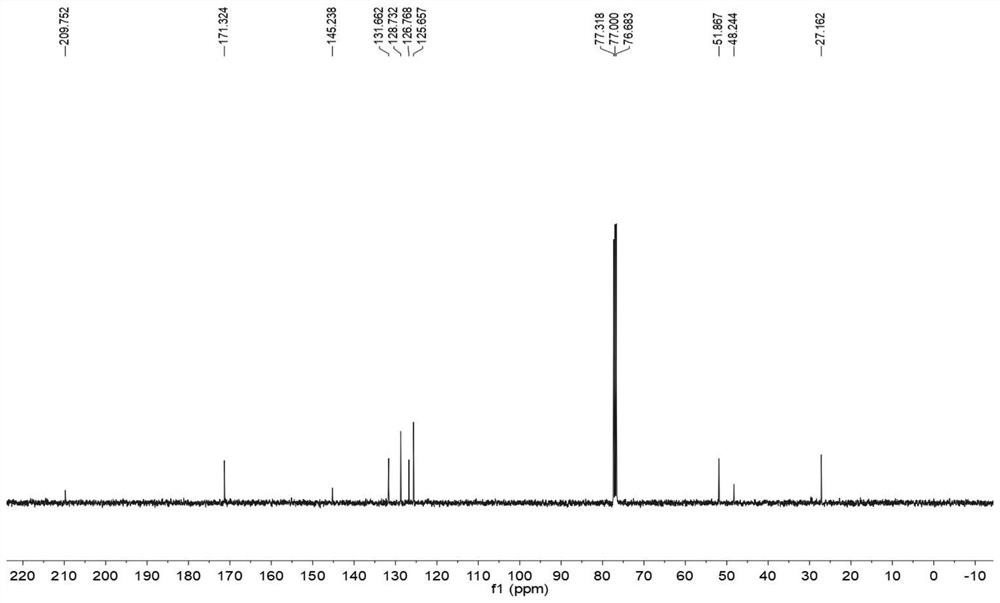
Convenient Michaal addition reaction of 6-phenylimidazo[2, 1-b]thiazole
The invention relates to a convenient Michaal addition reaction of 6-phenylimidazo[2, 1-b]thiazole. The method comprises the following steps: adding 6-phenylimidazo[2, 1-b]thiazole, 1-pentene-3-one, Lewis acid and a solvent into a reaction container, stirring and reacting at 40-150 DEG C for 1-10 hours, cooling to room temperature after the reaction is finished, filtering the obtained reaction liquid, evaporating the solvent under reduced pressure to obtain a crude product, and purifying by column chromatography to obtain the Michal addition product. The convenient Michael addition reaction of 6-phenylimidazo[2, 1-b]thiazole has the characteristics of convenient operation, mild conditions, easily available raw materials, good functional group tolerance and the like, and has important research value on the Michael addition reaction of sulfur-containing heterocycles such as imidazo[2, 1-b]thiazole.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
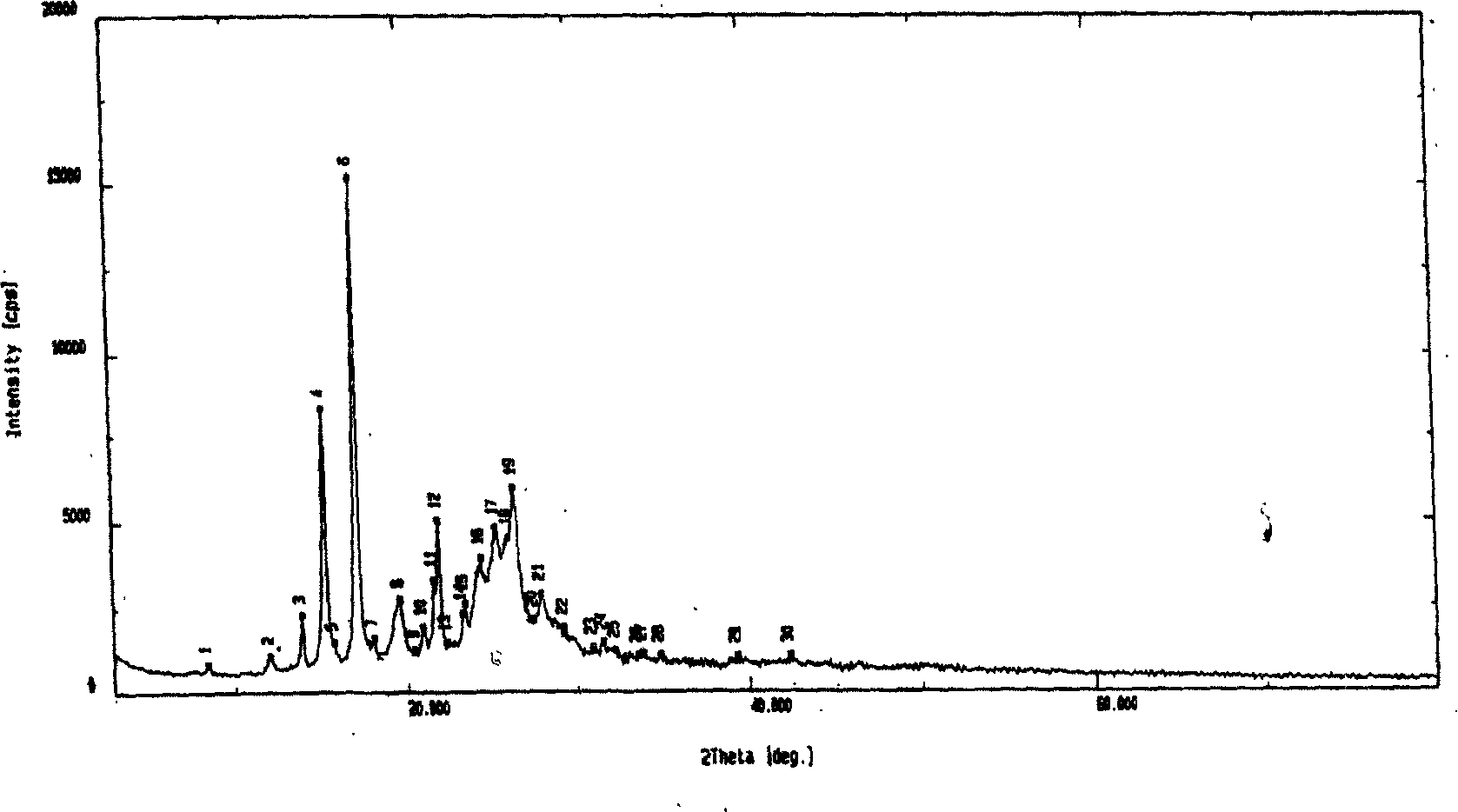
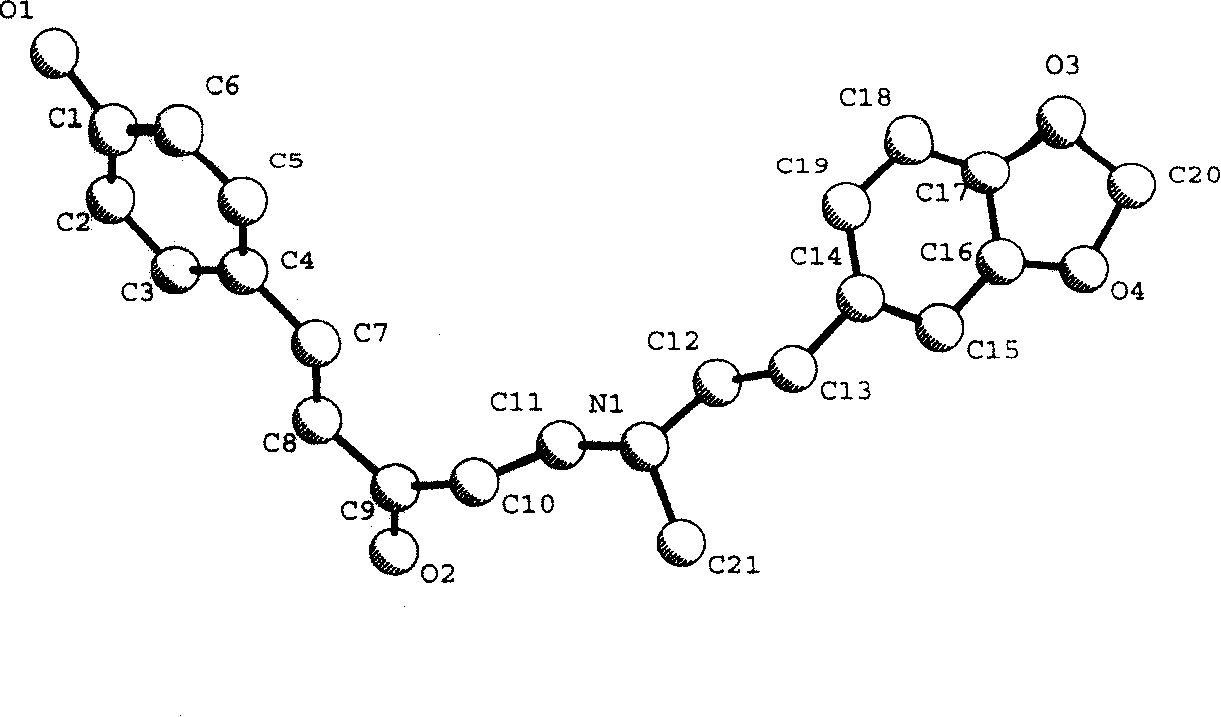
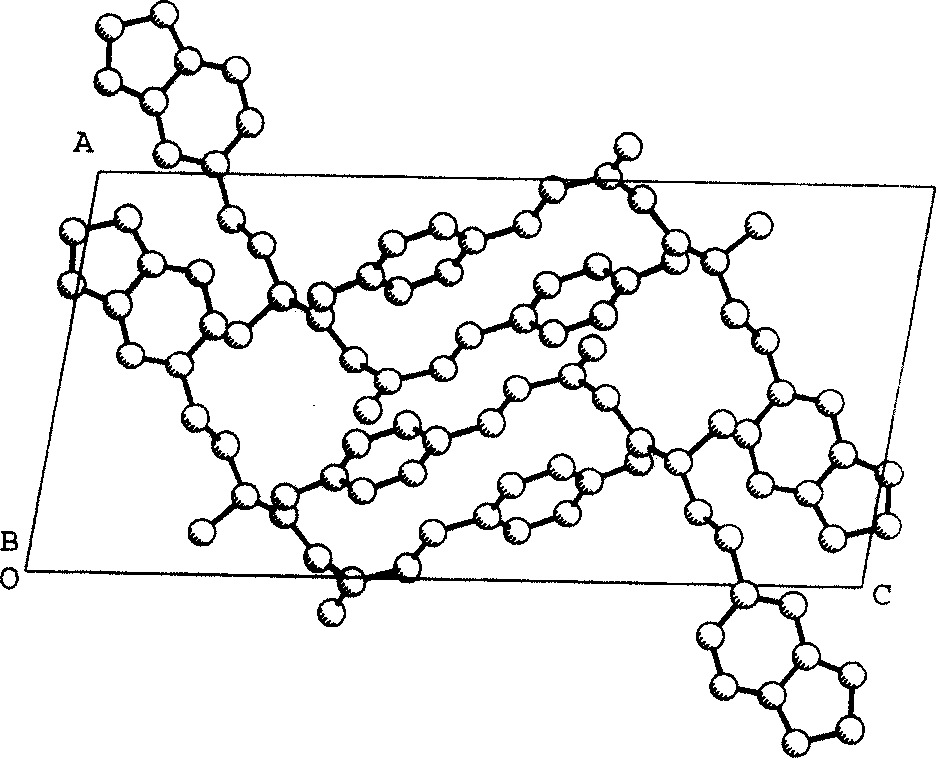
Xanthiphenylketamine crystal and its preparation method and medical use
The invention relates to a peperphentonamine crystal represented as (E)-5-{(3, 4-methylenedioxy phenylethyl) dimethylamino}-1-p-hydroxybenzene-1-amylene-3-ketone, which formula is represented as above, wherein the crystal powder X diffraction has characteristic peaks under the diffraction angles as 2-theta of 12.06, 15.28, 17.16 and 21.88, the single crystal is colorless, transparent and sheet belonging to monoclinic system, the space group is P21 / a, the molecule number Z in lattice is 4, and the fusion point is 148 to 151 DEG C. The invention further provides a single crystal preparation method, an agent prepared from the crystal and a relative drug application. The inventive peperphentonamine crystal has the advantages of high purity, activity and stable storage or the like, with better agent shape, solubility and clarity.
Owner:江西国能医药科技有限公司
A self-cleaning flame-retardant corrosion-resistant fluorosilicone powder coating
ActiveCN111925707BWide variety of sourcesLow equipment requirementsFireproof paintsAntifouling/underwater paintsMeth-Boronic acid
The invention discloses a self-cleaning, flame-retardant and corrosion-resistant fluorosilicon powder coating, which comprises the following components in parts by weight: 50-60 parts of film-forming copolymer, 4-8 parts of quaternary ammonium salt of amino-terminated hyperbranched compound , 1-3 parts of lignosulfonic acid, 0.1-0.2 parts of phosphorus pentoxide, 3-7 parts of functional additives, 10-15 parts of inorganic fillers, 1-3 parts of rare earth fluoride nanofibers; the film-forming copolymer is 2-trimethylsiloxy-1,1,1,5,5,5-hexafluoro-2-pentene-4-one, (E)-4-(2-cyano-3-ethoxy 3-oxo-1-propenyl) pinacol phenylboronate, trimethyl 4-phosphocrotonate, N-hydroxysuccinimidyl methacrylate, 1,3-bis(oxirane (methyl)-5-(2-propenyl)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione by free-radical copolymerization. The powder coating disclosed by the invention has remarkable self-cleaning effect, good performance stability, corrosion resistance, weather resistance, wear resistance, film forming property, and flame retardancy, good bonding performance with the base material, excellent comprehensive performance, and safe use Environmental friendly.
Owner:东莞市正荣实业有限公司
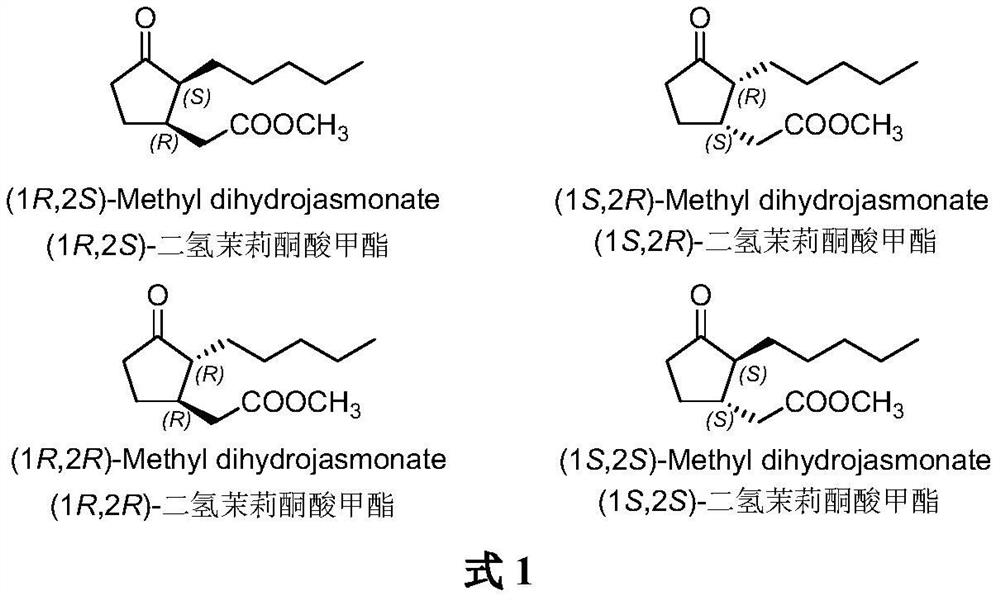
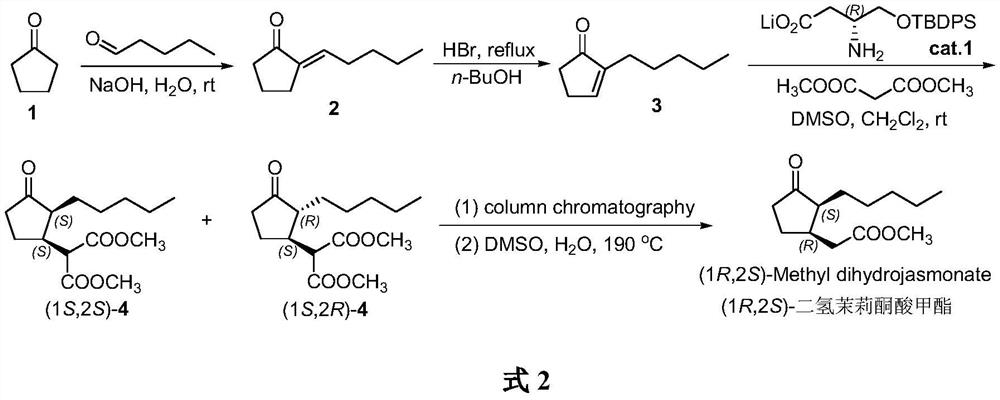
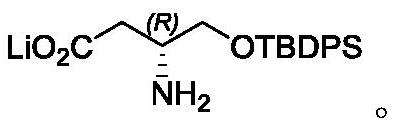
A kind of method for synthesizing (1r, 2s)-methyl dihydrojasmonate
The invention discloses a new method for synthesizing (1R, 2S)-dihydrojasmonate methyl ester by using an asymmetric Michael addition reaction. In this method, cyclopentanone is used as a starting material, and an aldol condensation reaction occurs with n-valeraldehyde under alkaline conditions to generate 2-pentylidene cyclopentanone 2, and then double bond transposition occurs under acidic conditions to obtain 2 ‑n-Pentyl‑2‑cyclopentenone 3. Then, under the catalysis of chiral amino acid lithium salt, a Michael addition reaction occurs with dimethyl malonate, and after two silica gel column chromatography separations, (1S, 2S)-2-n-pentyl-3-malonate di Methoxycyclopentanone 4. Finally, through hydrolysis and decarboxylation reaction, (1R,2S)-methyl dihydrojasmonate is obtained. The synthesis route of the invention is simple and convenient, the reaction conditions are mild, and the target compound can be prepared by only four steps of reaction.
Owner:北京安胜瑞力科技有限公司
A method for reactive distillation to continuously produce 3-methyl-3-penten-2-one
ActiveCN109867596BImprove conversion rateHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystKetone
The invention discloses a method for the continuous production of 3-methyl-3-pentene-2-ketone by reactive distillation. The raw materials are 2-butanone and acetaldehyde, and a novel microspherical polymer ionic liquid P(VB-VS ) HSO 4 As a catalyst, acetaldehyde and 2-butanone undergo an aldol condensation reaction in the reactive distillation tower to generate 3-methyl-3-penten-2-ketone, and then the reaction solution is sent to the vacuum tower for rectification The reactants and products are separated to obtain high-purity 3-methyl-3-penten-2-one. The invention adopts new microspherical polymer ionic liquid instead of the traditional strong acid and strong base as the reaction catalyst, the reaction conditions are mild, the conversion rate and selectivity of acetaldehyde are high, and the acid in the traditional process is also omitted while reducing side reactions. The alkali neutralization process greatly reduces energy loss and waste water treatment, is energy-saving and environmentally friendly, and the excess 2-butanone can continue to be used as a reaction raw material after separation, reducing resource loss.
Owner:FUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

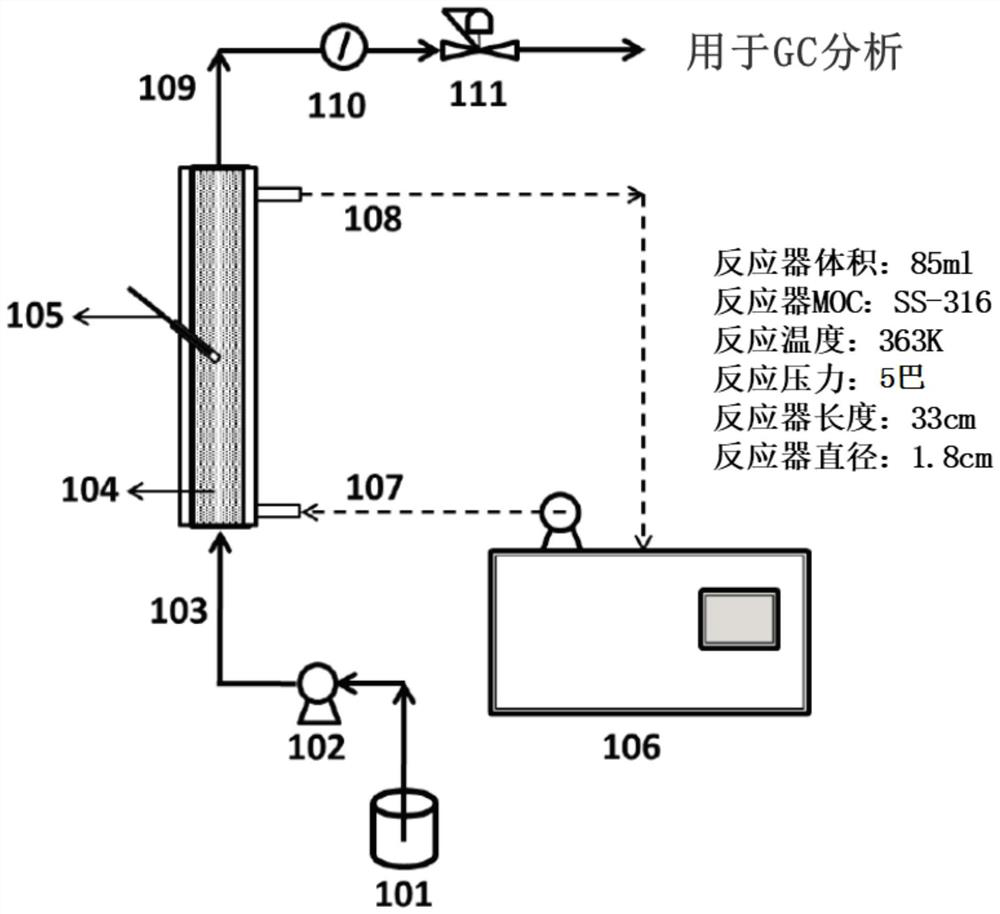
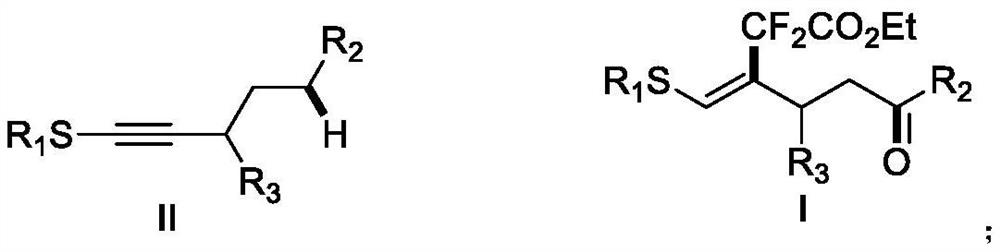
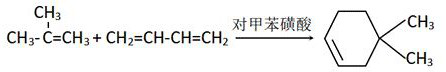
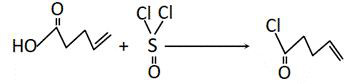
![Convenient Michaal addition reaction of 6-phenylimidazo[2, 1-b]thiazole Convenient Michaal addition reaction of 6-phenylimidazo[2, 1-b]thiazole](https://images-eureka.patsnap.com/patent_img/a4231876-c6e4-4302-9ebe-dac9b7273f8c/HDA0003251283490000011.png)
![Convenient Michaal addition reaction of 6-phenylimidazo[2, 1-b]thiazole Convenient Michaal addition reaction of 6-phenylimidazo[2, 1-b]thiazole](https://images-eureka.patsnap.com/patent_img/a4231876-c6e4-4302-9ebe-dac9b7273f8c/HDA0003251283490000012.png)
![Convenient Michaal addition reaction of 6-phenylimidazo[2, 1-b]thiazole Convenient Michaal addition reaction of 6-phenylimidazo[2, 1-b]thiazole](https://images-eureka.patsnap.com/patent_img/a4231876-c6e4-4302-9ebe-dac9b7273f8c/FDA0003251283470000011.png)