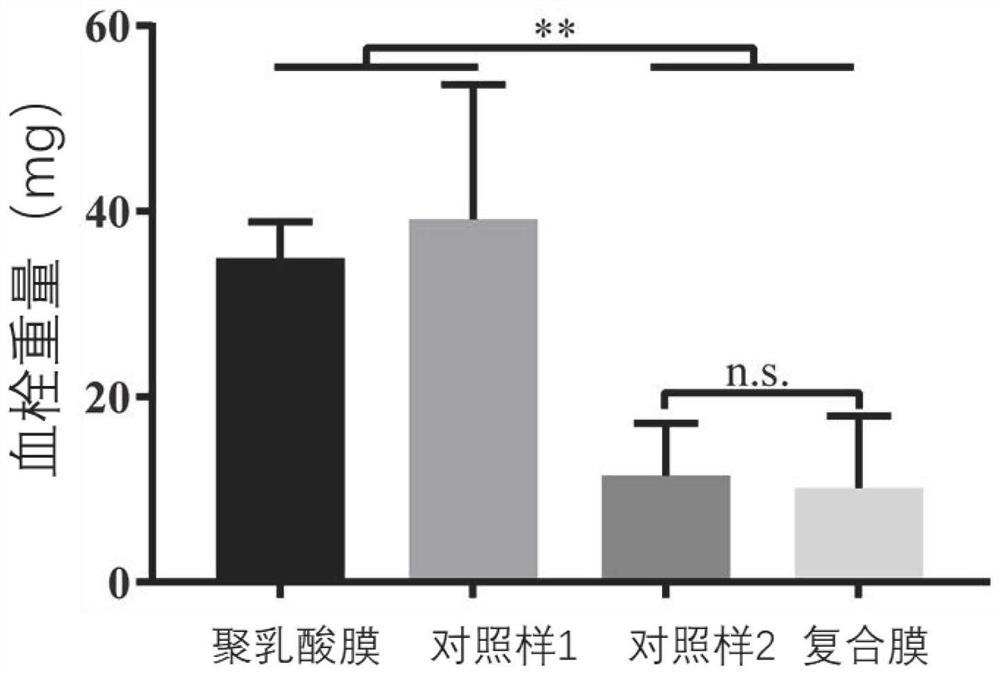
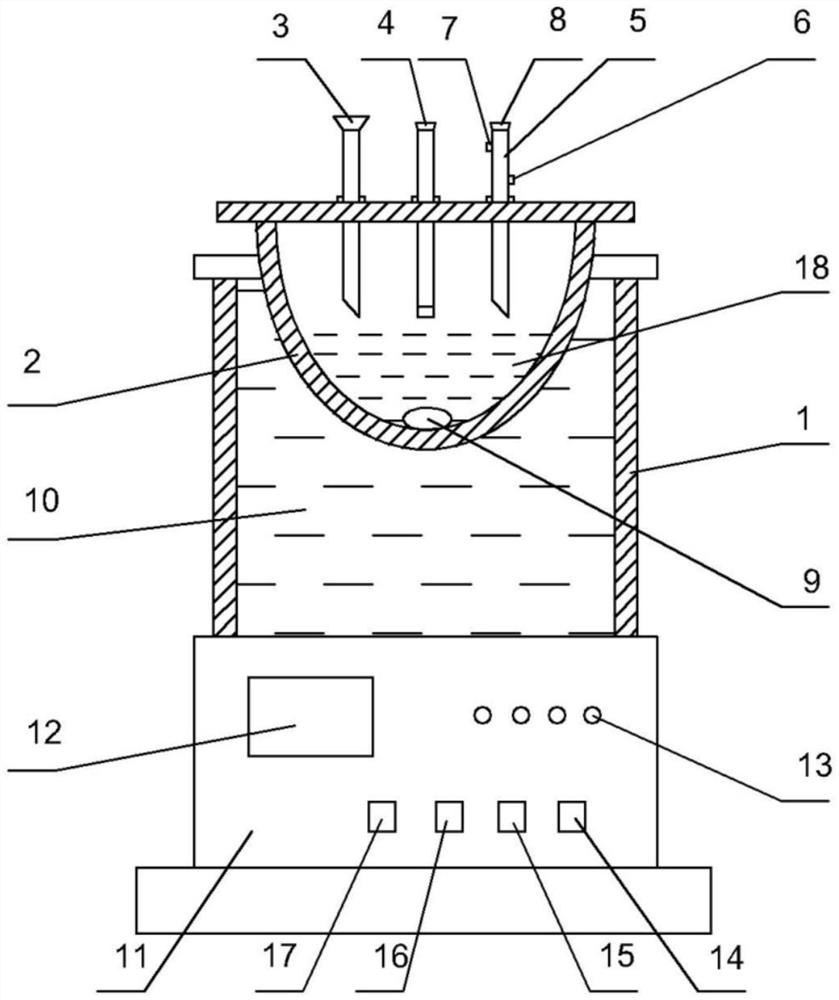
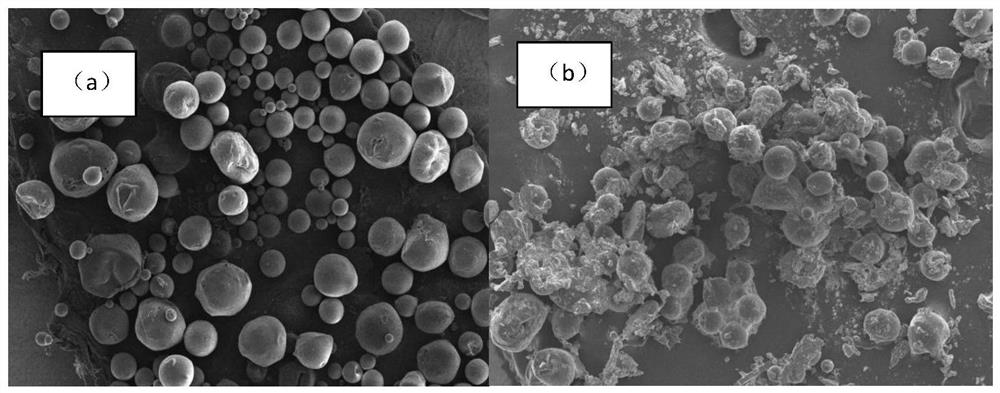
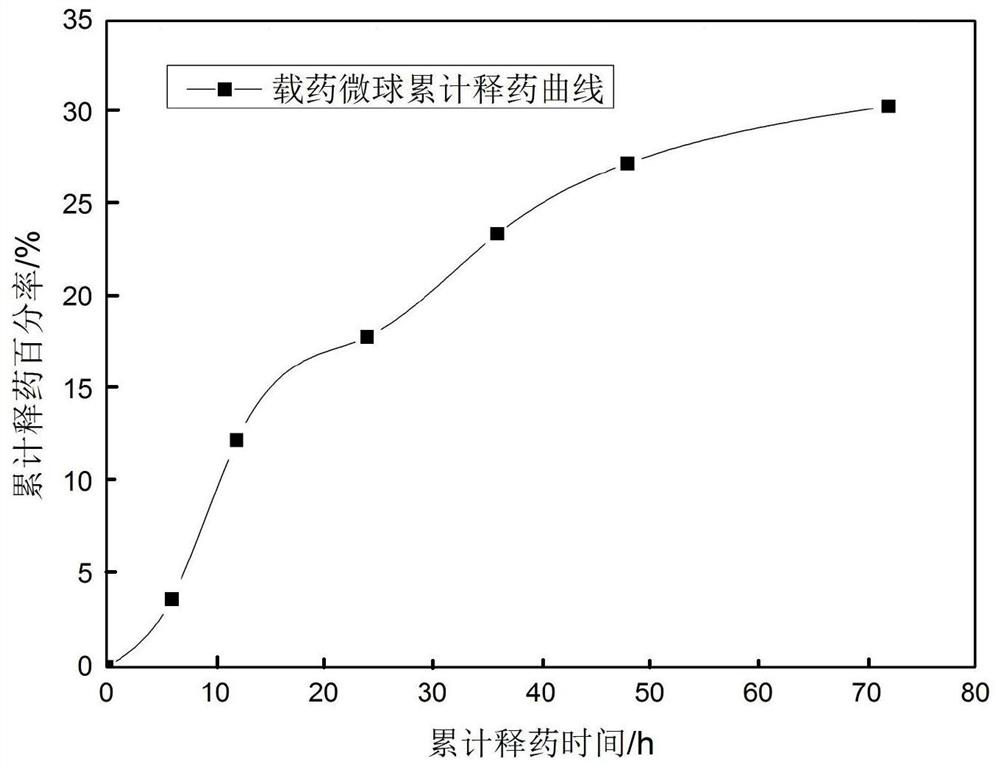
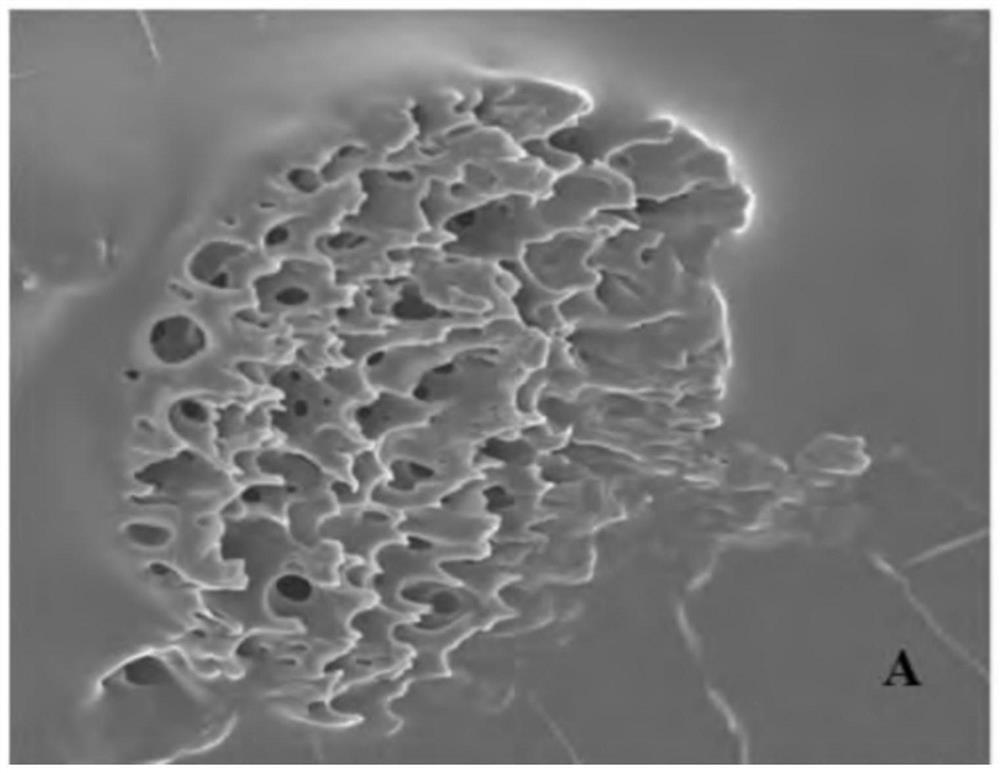
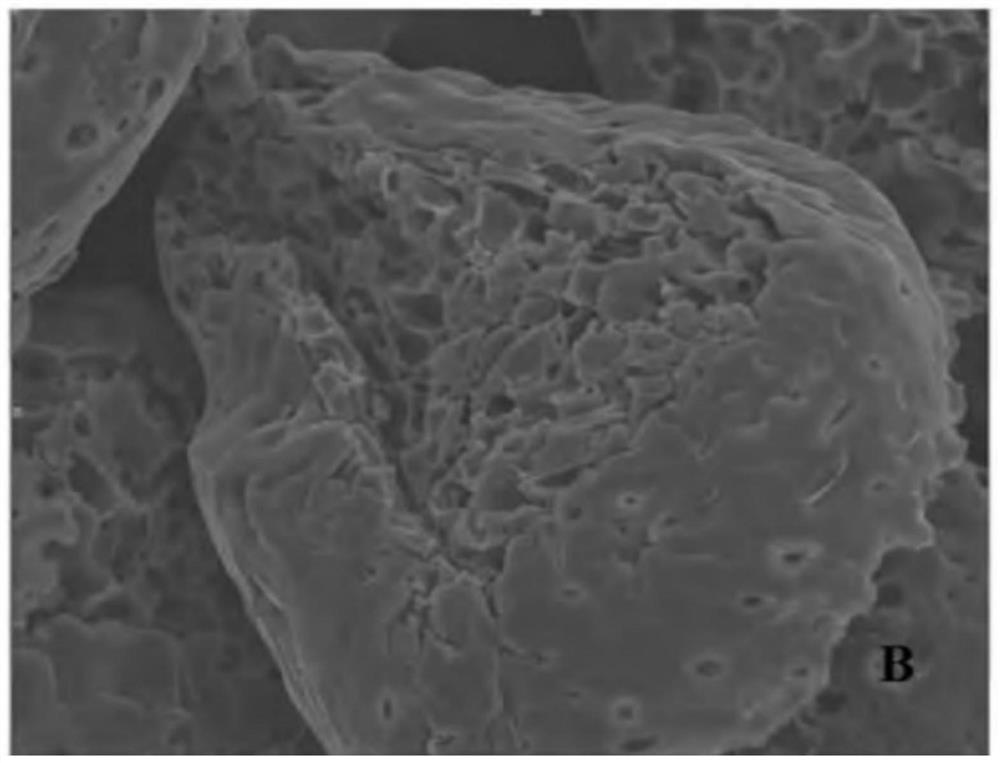
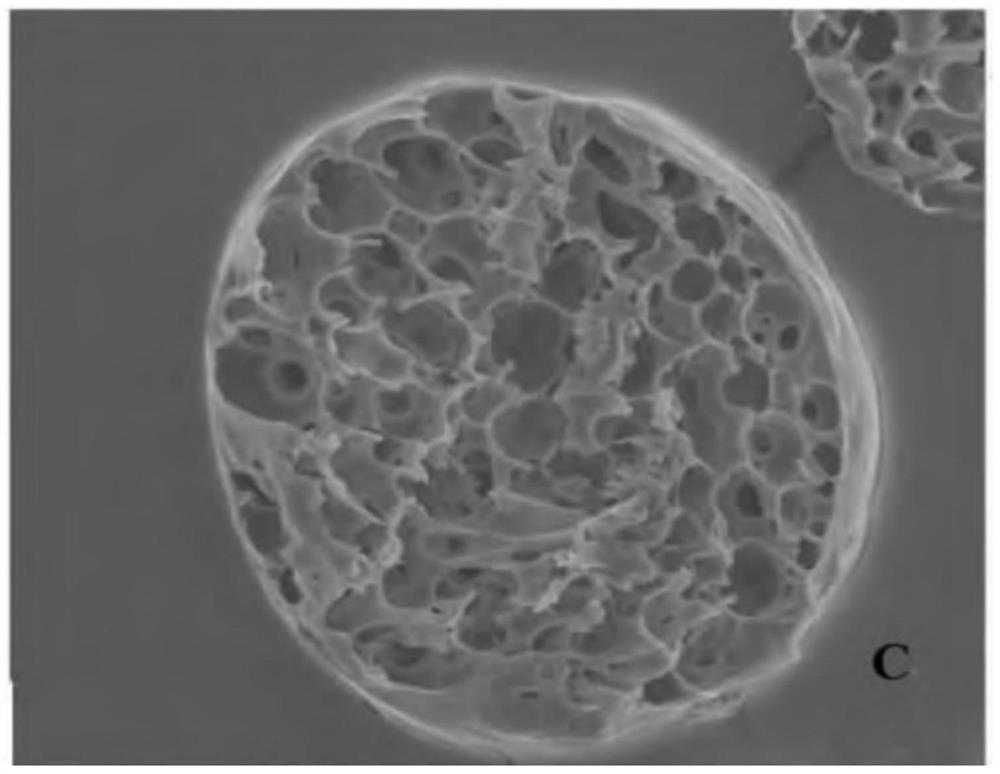
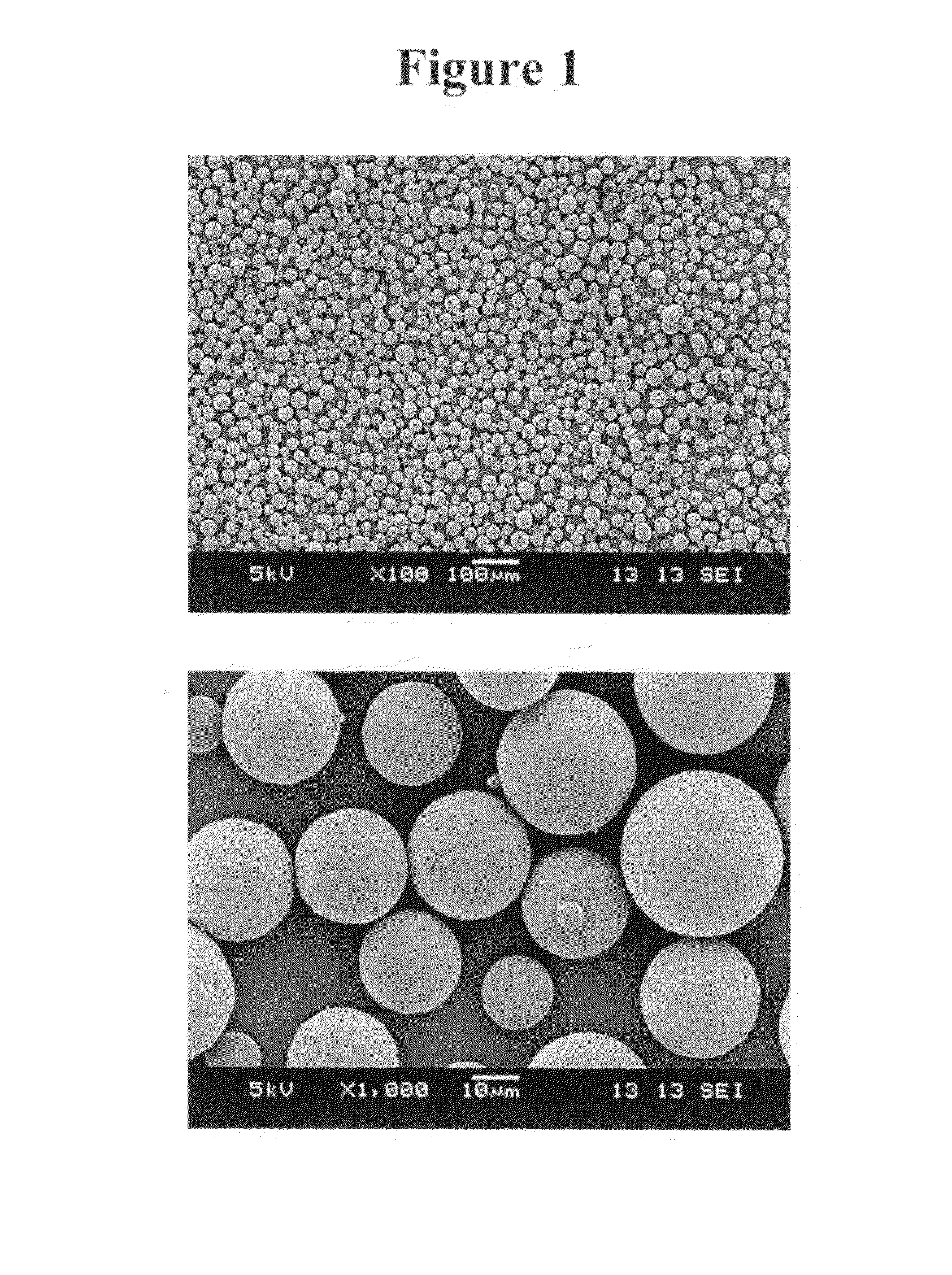
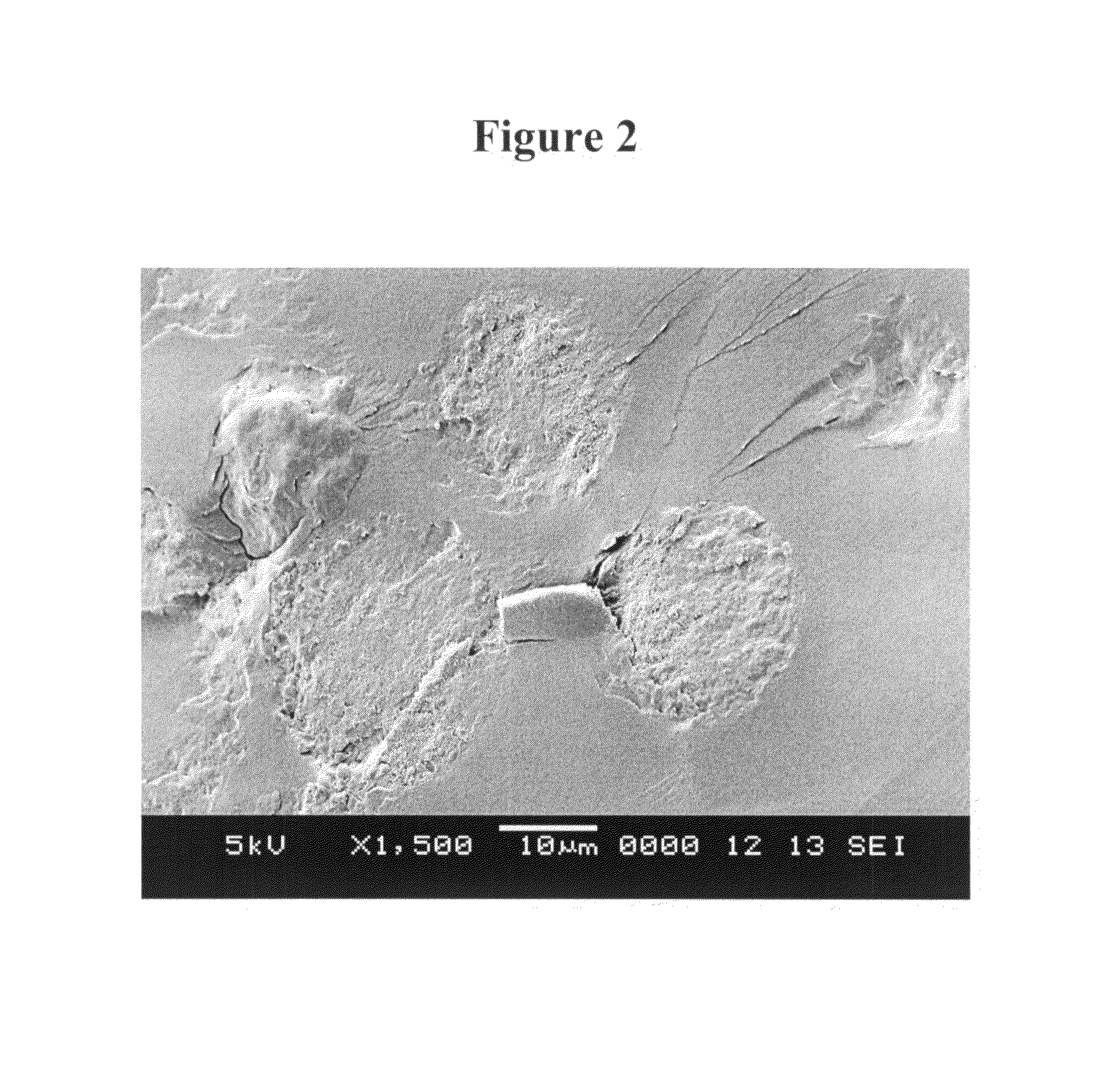
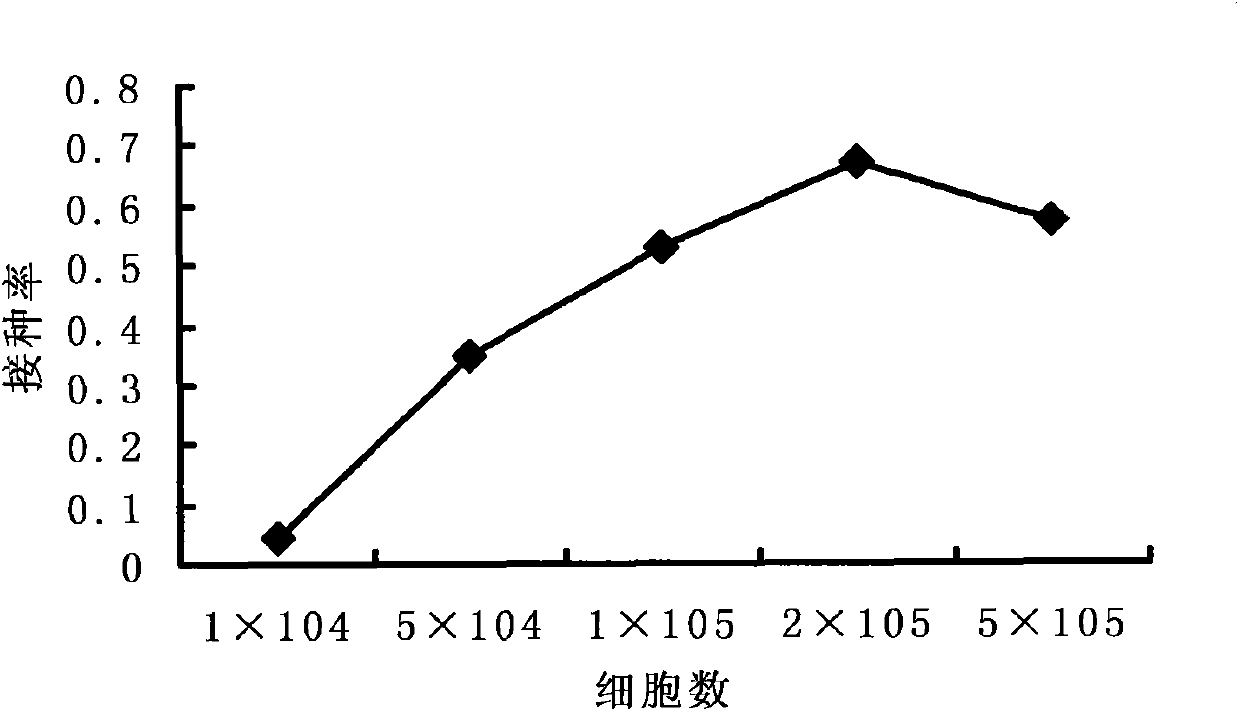
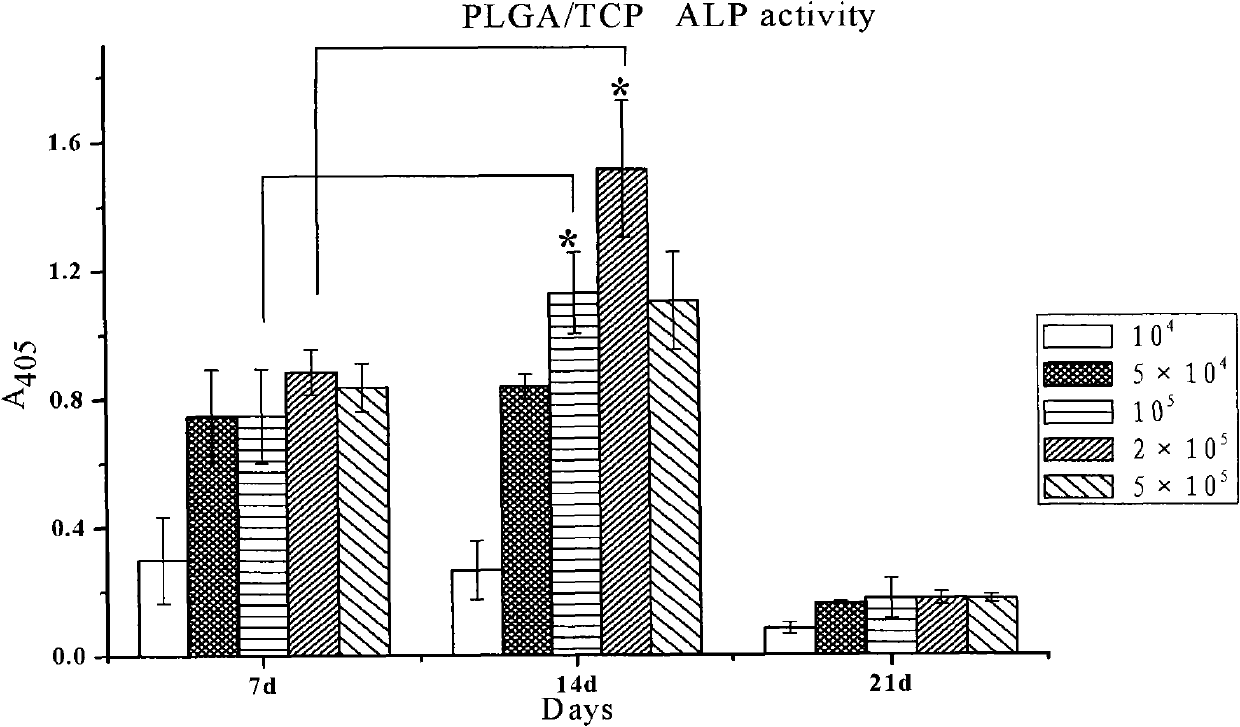
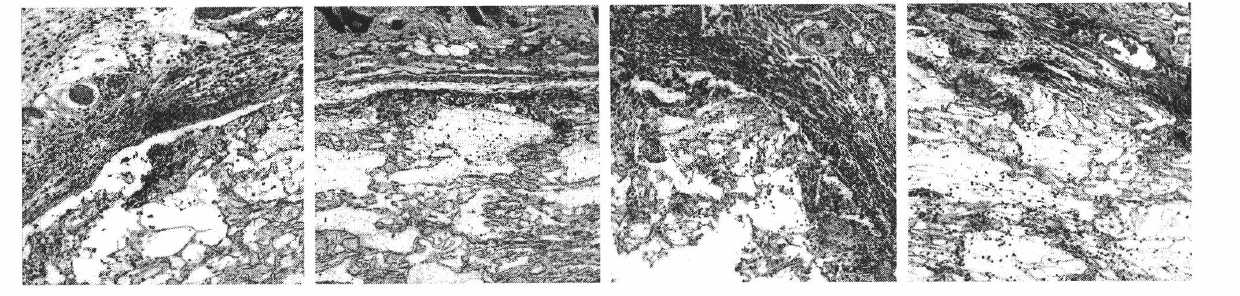
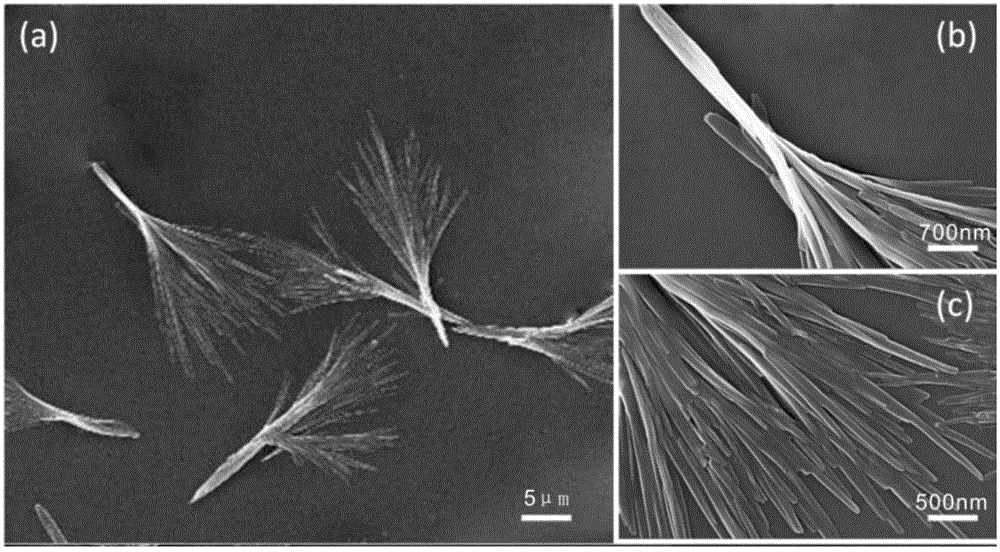
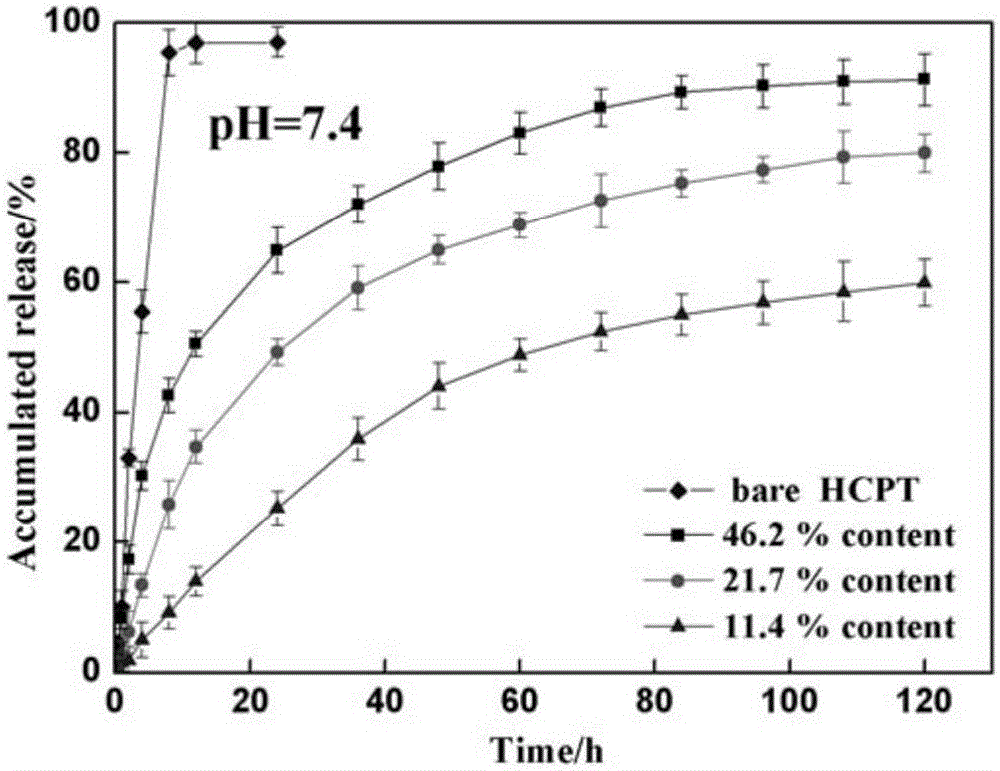
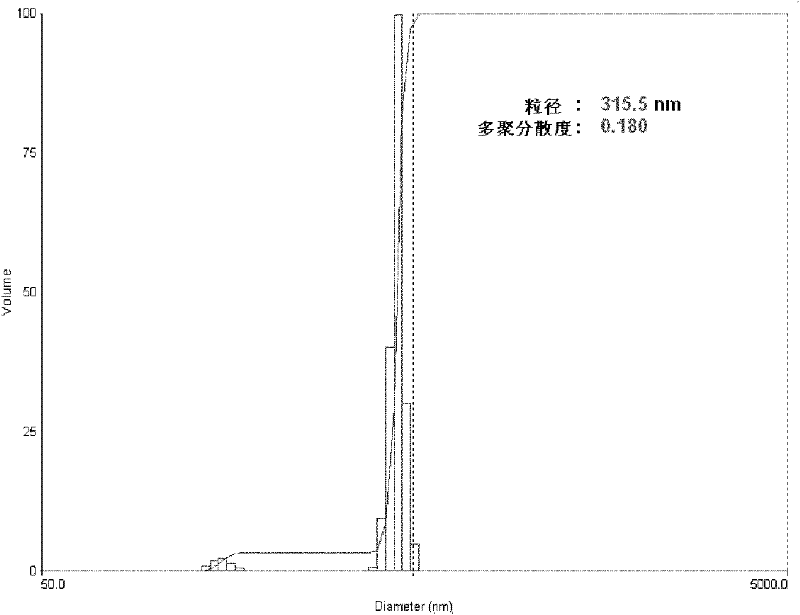
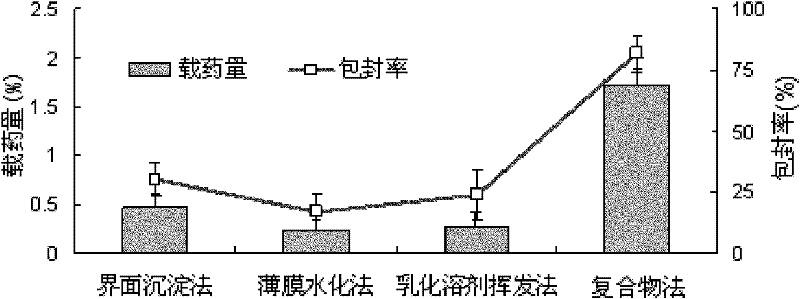
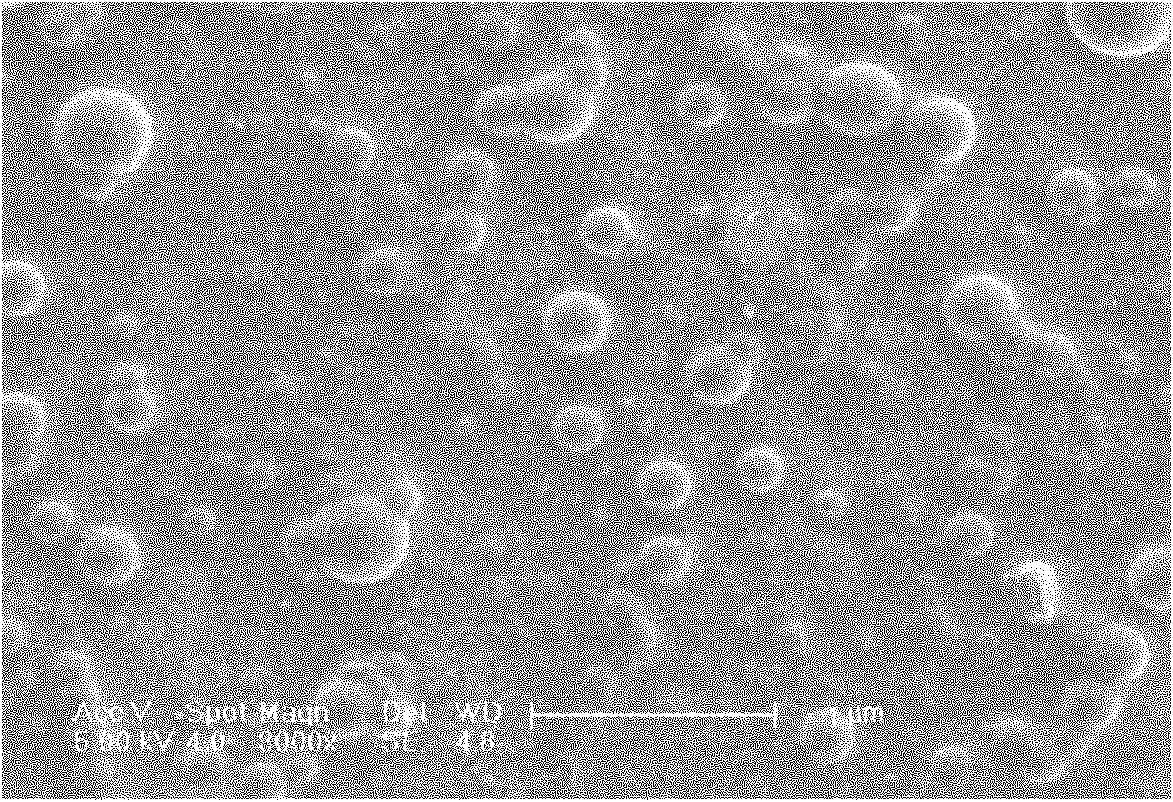Patents
Literature
33 results about "Poly-L-lactic-polyglycolic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
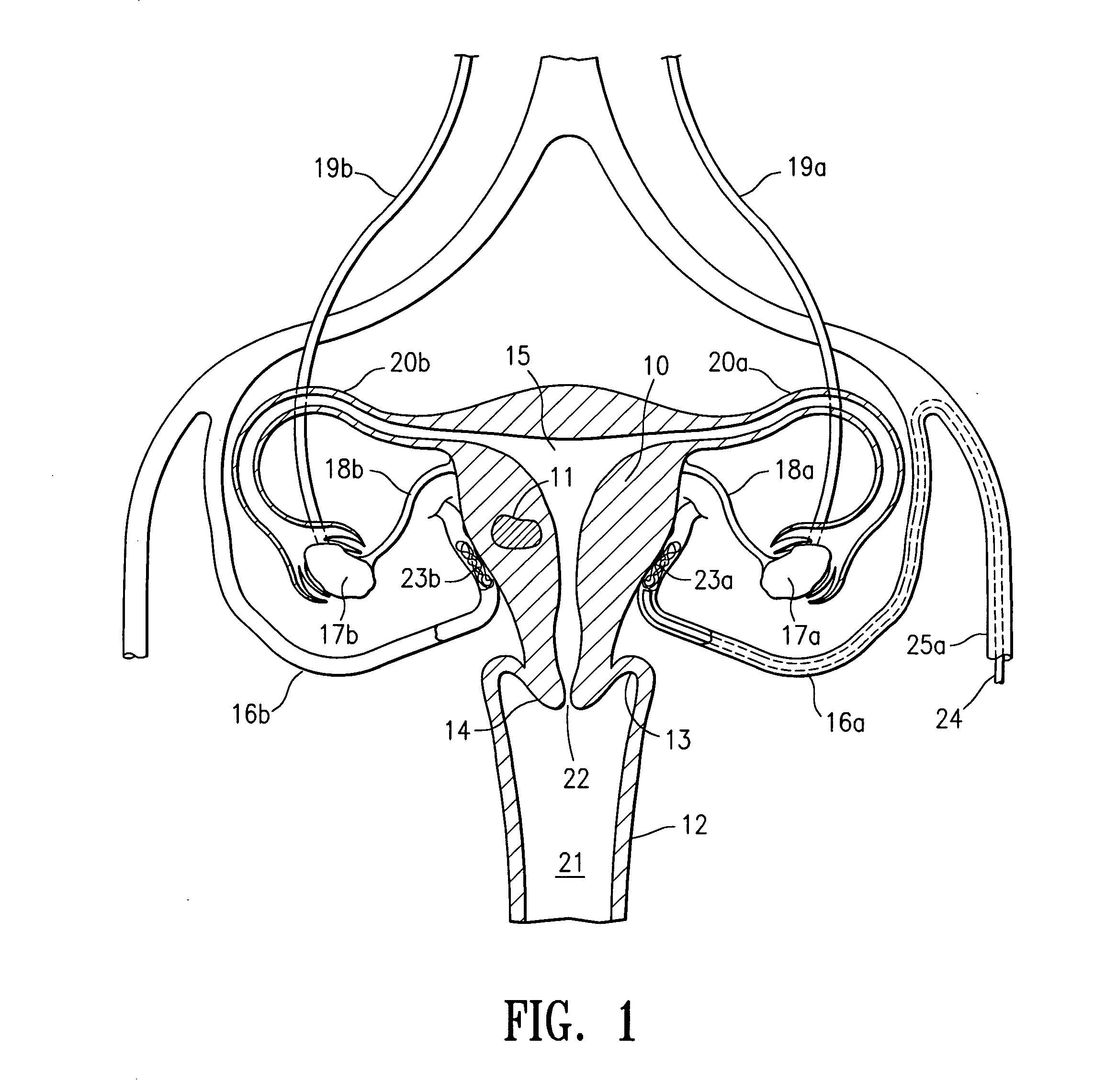
Embolic occlusion of uterine arteries
InactiveUS20040202694A1Decreased blood flowSlow and stop flowSurgical adhesivesFemale contraceptivesWater basedUterine Disorder
A treatment procedure is disclosed which involves the short term, non-permanent occlusion of the patient's blood vessels by depositing a bioabsorbable embolic mass within the patient's blood vessel. The procedure is particularly suitable for treating uterine disorders by occluding a patient's uterine arteries. A therapeutically effective time period for occlusion of a uterine artery is from about 0.5 to about 48 hours, preferably about 1 to about 24 hours, with occlusion times of about 1 to about 8 hours being suitable in many instances. The embolic mass may bioabsorbable particulate with minimum transverse dimensions of about 100 to about 2000 micrometers, preferably about 300 to about 1000 micrometers. The particulate may be a polymeric material formed of polylactic acid, polyglycolic acid or copolymers thereof, or a swellable copolymer of lactic acid and polyethylene glycol. The embolic material may be delivered to an intracorporeal site as a biocompatible solution containing a solute which is relatively insoluble in a water based fluid and a solvent which is relatively soluble in the water based fluid, where the solute forms the embolic mass which occludes or partially occludes a body lumen or fills or partially fills a body cavity.
Owner:VASCULAR CONTROL SYST
Controlled release microparticles
ActiveUS20070292475A1Extended durationReduce burstOrganic active ingredientsPowder deliveryControlled releaseMedicine
Formulations for controlled, sustained release of biologically active agents for the treatment of ocular disorders have been developed. These formulations are based on solid microparticles formed of the combination of biodegradable, synthetic polymers such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and copolymers thereof. The microparticles are characterized by low burst levels and efficient drug loading and sustained release.
Owner:EYETECH
Drawn Biodegradable Micro-Filament
InactiveUS20070222104A1Simple and convenient meanEasy to getMonocomponent synthetic polymer artificial filamentNon-woven fabricsPolymer scienceInfrared beam
The invention resides in enabling biodegradable filament of polylactic acid, polyglycolic acid and the like to manufacture biodegradable micro-filament by simple and convenient means without needing special, high-accuracy and high-level apparatus; it is characterized in that highly molecular oriented micro-filament those of 12 μm or less and generally from 2 μm to 3 μm can be obtained by heating biodegradable filament by infrared beam and the heated original filament is drawn to 100 times or more by tension of 10 MPa or less.
Owner:UNIVERSITY OF YAMANASHI
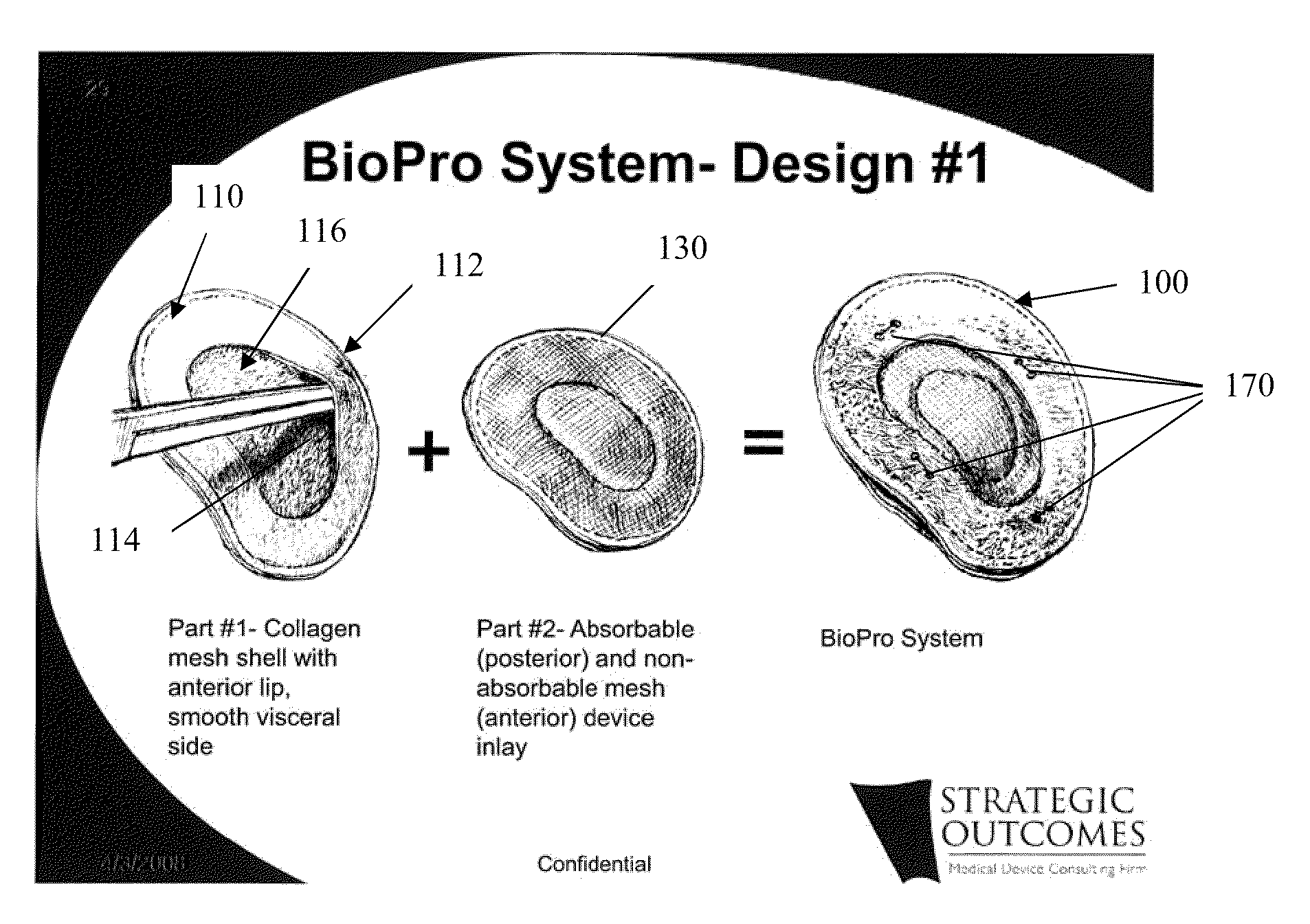
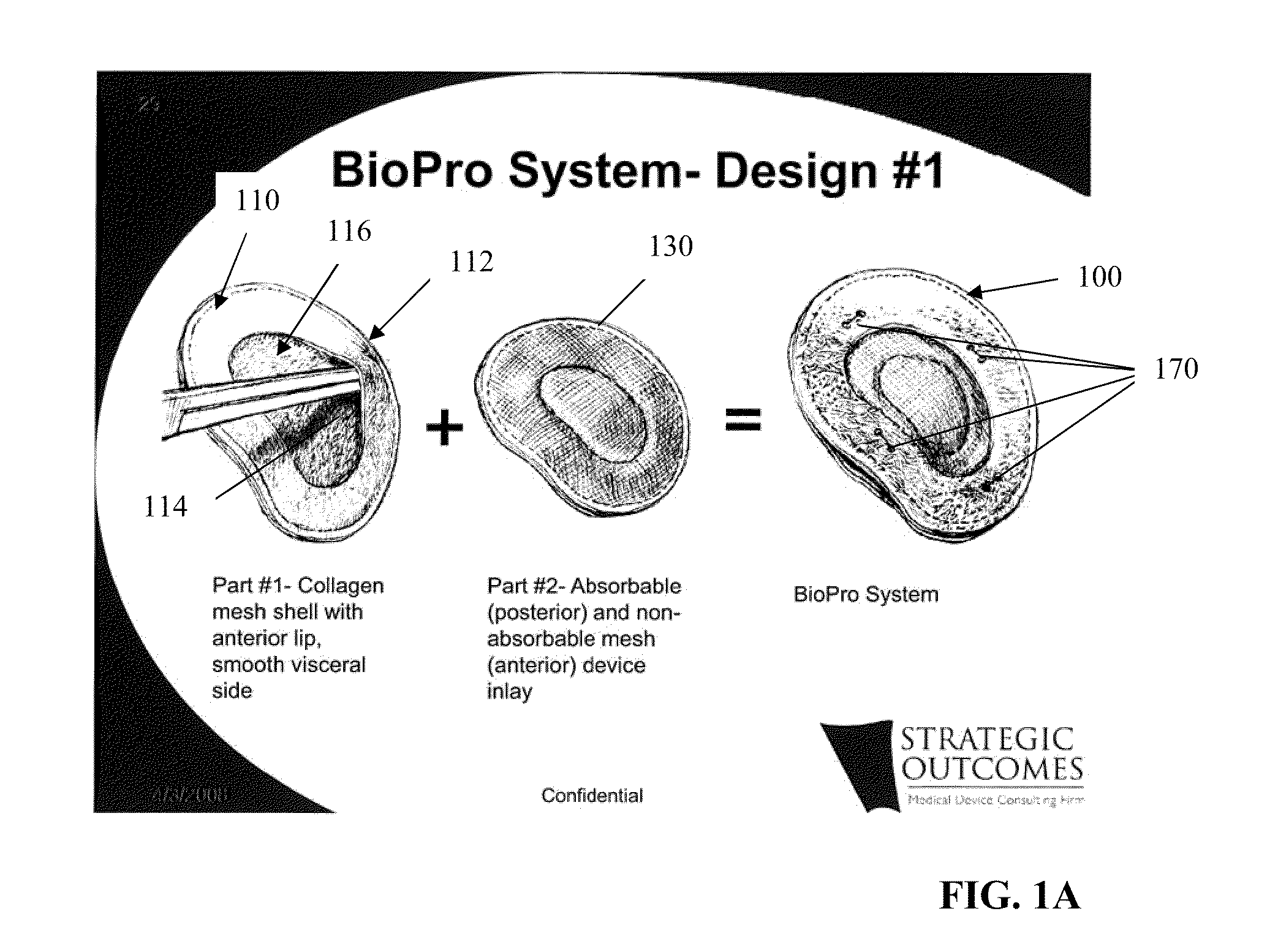
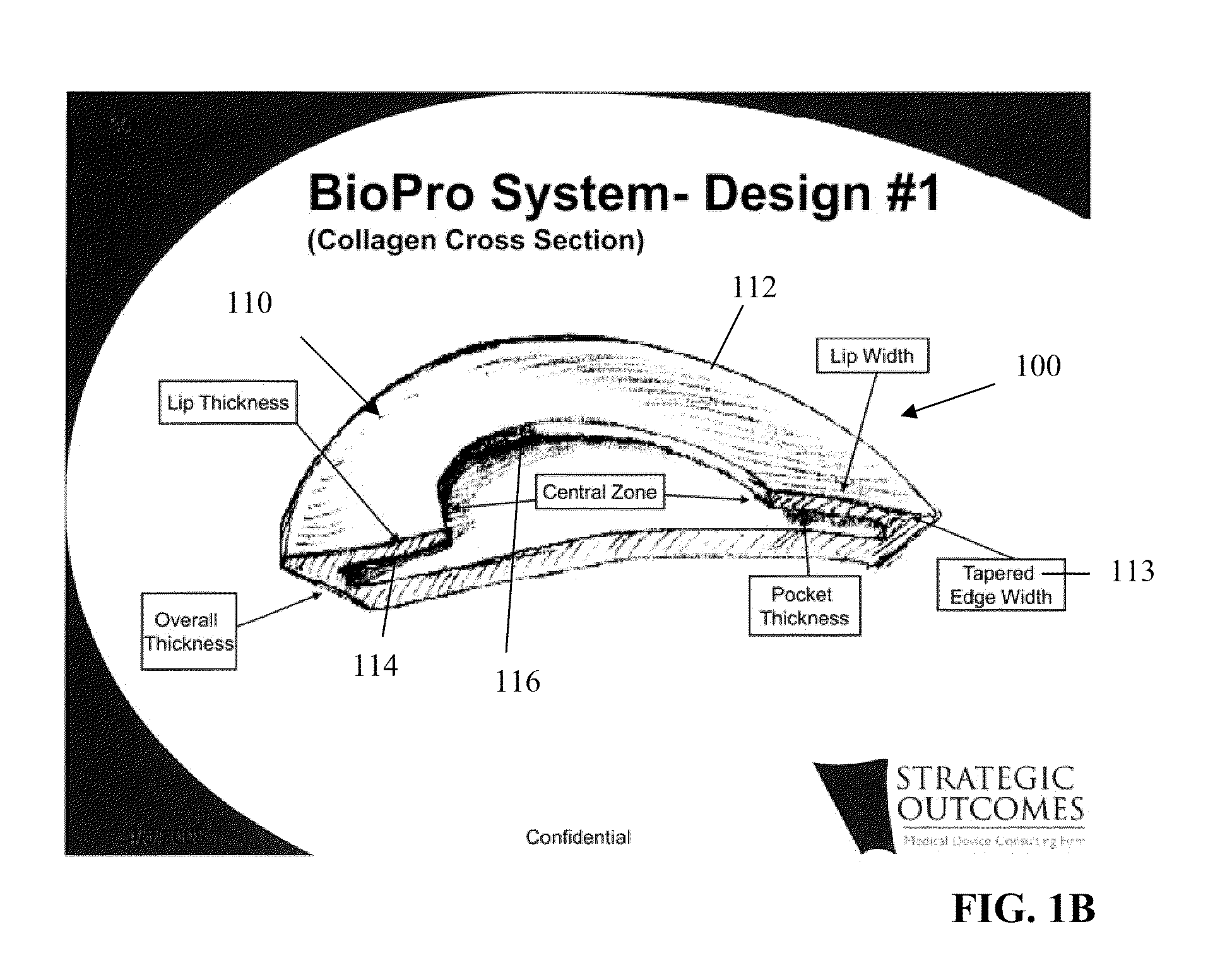
Tissue Reconstruction Devices and Methods
InactiveUS20090312843A1Reliable in-growthReduce chanceLaminationLamination apparatusPolypropylene meshTissue reconstruction
The present invention provides an implantable prosthesis comprising a viscera separating barrier that defines a shell having a pocket with an opening that receives an insert. It is contemplated that the insert comprises at least one of an absorbable mesh, a non-absorbable mesh, or an absorbable and non-absorbable mesh combination inserted into the pocket through the opening. In preferred embodiments the viscera separating barrier is acellular collagen, and the absorbable mesh is selected from at least one of a polyglactin, a polyglycolic acid, a polyglactin and a polylactic acid, and the non-absorbable mesh comprises a polypropylene mesh. It is contemplated that the viscera separating barrier acts to reduce or even eliminate attachment of viscera to the prosthesis to encourage rapid cell penetration and revascularization; the non-absorbable mesh will provide immediate, reliable in-growth; and the absorbable mesh will give stability to the prosthesis.
Owner:FORD STEVEN PALMER +1
Bioabsorbable polymer blends
The invention provides a bioabsorbable blend of (i) a polymer component comprising polylactic acid (PLA), polyglycolic acid (PGA), a copolymer of PLA and PGA, or any combination thereof and (ii) thermoplastic polyurethane (TPU) tailored to a medical application, and a process for making the same. In some embodiments the TPU comprises units derived from a diol chain extender, a diisocyanate, and a polyol formulated to provide a set biodegradation rate in combination with at least one physical property. The blend of the invention provides useful materials for medical applications that have the individual benefits of polylactic acid and TPU while moderating each material's typical limitations.
Owner:LUBRIZOL ADVANCED MATERIALS INC
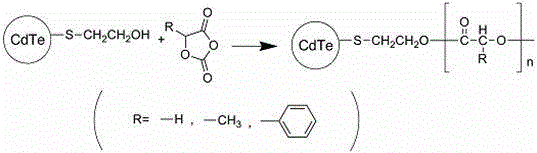
Poly-a-hydroxy acid modified CdTe quantum dot and preparation method thereof
InactiveCN104371730ARaw materials are easy to getLow costLuminescent compositionsPolymer modifiedMandelic acid
The invention discloses a preparation method of a poly-a-hydroxy acid modified CdTe quantum dot. The preparation method comprises the following steps: preparing a CdTe quantum dot with a mercaptoethanol modified surface by taking the mercaptoethanol as a ligand and adopting a water-phase method; generating a-hydroxy acid oxygen acid anhydride polymerization by adopting hydroxy contained in the surface of the CdTe quantum dot; and introducing poly-a-hydroxy acid, namely polylactic acid, polyglycolic acid, poly (mandelic acid) and the like, to the surface of the CdTe quantum dot so as to realize the polymer modification on the CdTe quantum dot. The poly-a-hydroxy acid modified CdTe quantum dot has the advantages of good hydrophilicity and stability, excellent fluorescence property, good biocompatibility and the like and can be widely applied to the field of biomedicine as a novel fluorescent nanoprobe.
Owner:YUNNAN MINZU UNIV
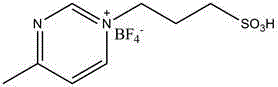
Catalyst for polylactone preparation
The invention discloses a catalyst for polylactone preparation. A raw material lactone monomer undergoes ring-opening polymerization under the action of a difunctional group ionic liquid catalyst to obtain polylactone, and the catalyst is 1-(4-sulfo)butyl-3-methylpyrazinyltetrafluoroborate or 1-(4-sulfo)butyl-3-methylimidazolyltetrafluoroborate. The catalyst can catalyze lactone ring-opening polymerization to obtain polylactic acid, polyglycolic acid, poly(trimethylene carbonate) or other degradable polymers.
Owner:YUNNAN MINZU UNIV
Bone screw
ActiveCN104887305AReduce intensityIncreased micromotionPharmaceutical delivery mechanismFastenersMetallic materialsScrew cap
The invention relates to a bone screw. The bone screw comprises a screw pole and a screw cap, the screw pole comprises a polish rod section and a screw rod section, a degradable material layer is compounded on the peripheral circumferential surface of the polish rod section, and the outer diameter of the degradable material layer is less than or equal to the large diameter of the screw rod section. The screw pole and screw cap are made of metal material; the degradable material layer is prepared from one or the polymer of polylactic acid, polyglycolic acid, poly trimethylene carbonate, polydioxanone and poly epsilon-caprolactone. Through operation, the bone screw is stable in fixing effect and avoided from sliding after the fracture part of a patient is fixed and before the degradable material layer is degraded; in the later degradation process of the degradable material layer and after the complete degradation of the degradable material layer, the bone moves slightly to stimulate the full healing of the bone, the achievement ratio of operation is improved, and the healing effect of the fracture part is better.
Owner:HEBEI RUINUO MEDICAL INSTR CO LTD
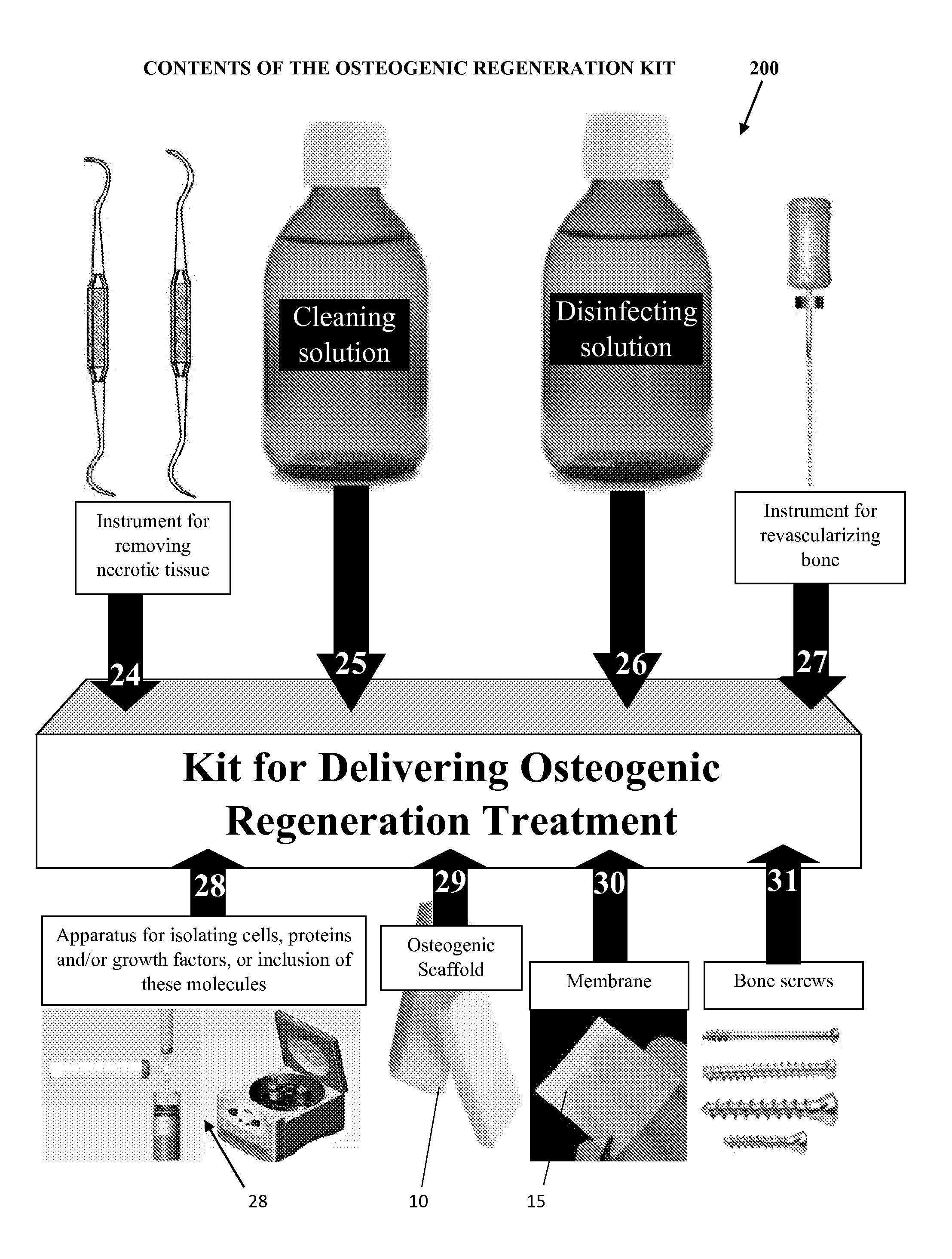
Osteogenic regenerative scaffold matrix composition
InactiveUS20170014550A1Good adhesionAntipyreticBone implantPhosphodiesterase Type 4Matrix composition
The implantable scaffold matrix composition for osteogenic regeneration has a biodegradable polymer material selected from the group consisting of one or more of polylactic acid, polyglycolic acid, or polycaprolactone, or any combination thereof to provide a time release delivery of an osteogenic effect and further has a pharmaceutical composition selected from the group consisting of one or more of ibuprofen, non-steroidal anti-inflammatory drugs (NSAIDS), acetaminophen, or naproxen sodium or any combination thereof and wherein the implantable composition has a primary and a secondary time release. The primary time release being one of the polymer compositions; each time release extending occurring between 0 days and 19 days, both not being at 0 days. In a preferred composition, the scaffold has an organic material for inhibiting osteoclast selected from a group consisting of one or more of Galardin, Decorin, Actinonin, Marimastat, Batimastat, and phosphodiesterase type 4 or any combination of osteoclast inhibitors.
Owner:NOVA SOUTHEASTERN UNIVERSITY
Preparation method for granula made of alpha-alcoholic acid resin ,and its use
InactiveCN1481902ASmall initial burst effectAchieve controlled releasePowder deliveryPharmaceutical non-active ingredientsControlled releaseGlycolic acid
The present invention belongs to the field of resin particle preparation. The resin particle is prepared with polylactic acid, polyglycolic adid, low molecular weight lactic acid-glycolic acid copolymer or their mixture as base material. The resin particle may be used in coating or carrying hydrophilic or hydrophobic medicine, bioactive macro molecule, cell, vaccine, and other chemical matter, such as cosmetics, pesticide, paint and dye. The present invention makes it possible to realize the control release of coated medicine in the period of one week to one month or even to one year with the low molecular weight poly-alpha-hydroxy acid resin to prepare medicine carrying particle.
Owner:天津麦凯泰生物制品有限公司
Methods and compositions for enhanced delivery of bioactive molecules
InactiveUS20080292712A1Reduce doseLow immunogenicityPowder deliveryNervous disorderPolymer scienceHydrophilic polymers
Formulations for controlled, prolonged release of bioactive molecules such as therapeutic proteins, peptides and oligonucleotides have been developed. These formulations are based on solid microparticles or nanoparticles formed of the combination of biodegradable, synthetic polymers such as poly (lactic acid) (PLA), poly (glycolic acid) (PGA), and copolymers thereof. Bioactive molecules are coupled to hydrophilic polymers such as polyethylene glycol or polypropylene glycol and formulated to provide controlled release. The bioactive molecules are more stable, less immunogenic and have improved release rate profiles with lower burst levels and increased drug loading relative to the same bioactive molecules lacking coupled hydrophilic polymers. The controlled release formulations can be administered by injection, by inhalation, nasally, or orally.
Owner:PR PHARMA
Slow release microsphere containing hormone kind anti cancer medicine and its application
InactiveCN1824313AEnhanced inhibitory effectOrganic active ingredientsSolution deliveryMicrosphereWhole body
A slow-release microball of the anticancer medicine containing hormone component for preparing the slow-release implant or slow-release injection is composed of the active anticancer component chosen from hormone-type anticancer medicine and its synergist and antimetabolic anticancer medicine, and the medicinal additive chosen from polylactic acid, the copolymer of polyoxyacetic acid and hydroxyacetic acid, ethene-vinyl acetate copolymer and polifeprosan.
Owner:JINAN KANGQUAN PHARMA TECH
Daidzein-entrapped PLGA nanoparticles and preparation method thereof
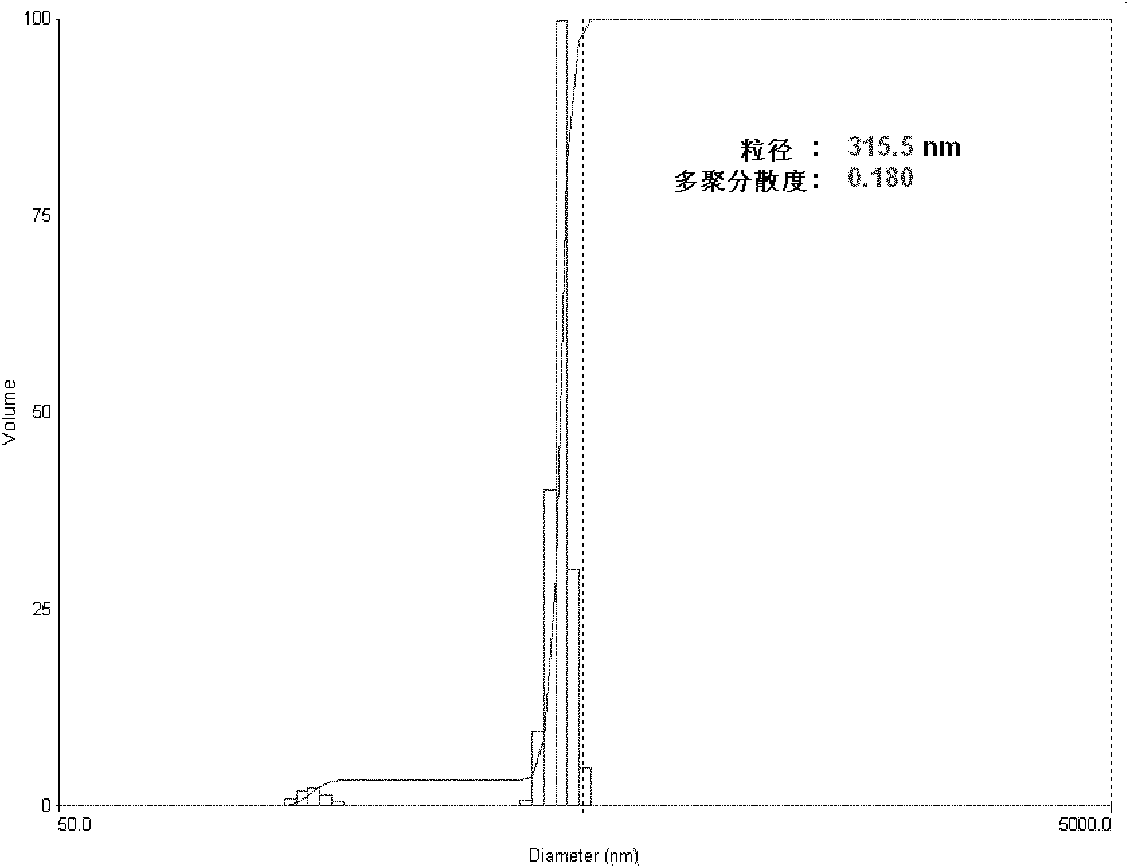
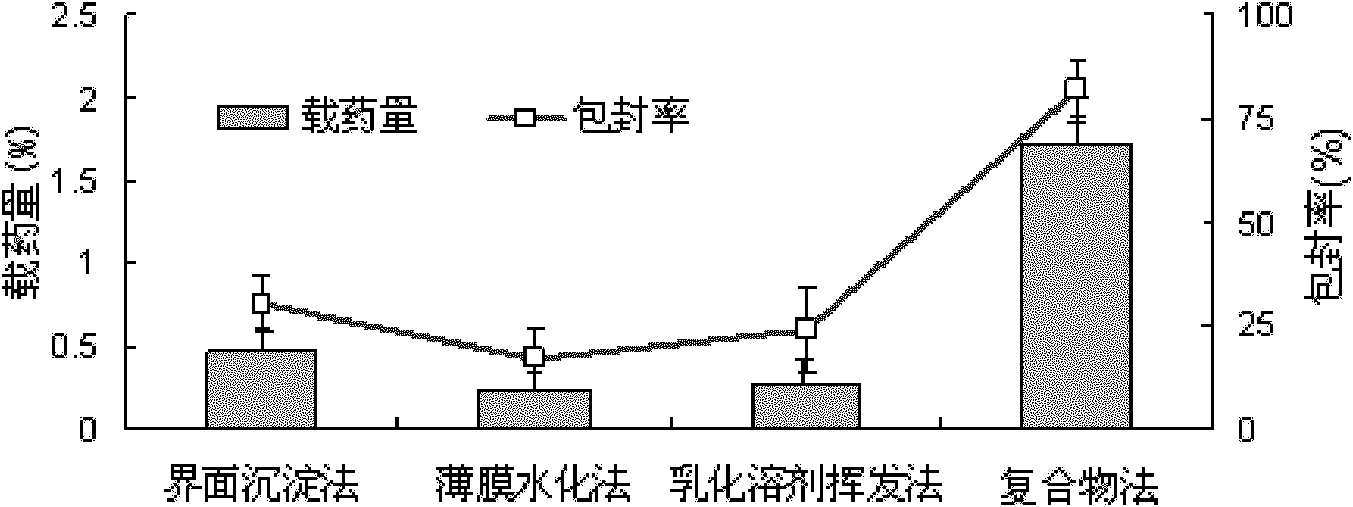
The invention relates to daidzein-entrapped polylactic polyglycolic acid (PLGA) nanoparticles and a preparation method thereof in the technical field of nanomedicines. The nanoparticles comprise the following components in percentage by mass: 0.1 to 45 percent of daidzein, 25 to 95 percent of polylactic polyglycolic acid and 1 to 65 percent of pharmaceutic adjuvant, wherein the molecular weight of the PLGA is between 8,000 and 15,000, the polylactic polyglycolic acid is long-chain macromolecules in which the ratio of the lactic acid to glycollic acid is 50:50, and the pharmaceutic adjuvant is lecithin, cyclodextrin or polyvinyl alcohol (PVA). The daidzein-entrapped PLGA nanoparticles have the particle size of between 280 and 330 nanometers and concentrated particle size distribution, redissolved freeze-dried powder is not adhered, and the medicine loading rate of the PLGA nanoparticles is between 1 and 2 percent, and the encapsulating rate is between 80 and 84 percent.
Owner:SHANGHAI JIAO TONG UNIV
Sustained-release injection containing methotrexate and synergist thereof
InactiveCN101396338AOrganic active ingredientsPharmaceutical delivery mechanismTreatment effectWhole body
The invention relates to a sustained release injection containing methotrexate, consisting of sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise effective anti-cancer component and sustained release auxiliary material; and the menstruum is ordinary menstruum or special menstruum containing suspending agent. The effective anti-cancer component is the combination of the methotrexate and the methotrexate synergist selected from platinum compound, topoisomerase inhibitor and / or tetrazine compound. The sustained release auxiliary material is selected from one or the combination of the copolymer of polylactic acid, polyglycolic acid and glycolic acid, ethylene vinyl acetate copolymer and the copolymer of polifeprosan, FAD and decanedioic acid. The suspending agent is selected from carboxymethyl cellulose, etc. The suspending agent is used for suspending the effective anti-cancer component or sustained release particle or microsphere containing the effective anti-cancer component, so as to be made into the sustained release injection. The sustained release injection is injected into tumor, which can not only reduce the general toxic reaction of the drug, but also improve the partial drug concentration at the tumour selectively and enhance the treatment effect of non-operative treatments, such as chemotherapeutic drug, radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Use of porous polymer film with regular pores for preparing anti-adhesion film
ActiveCN114634641AImprove mechanical propertiesArranged in orderBio-packagingPolymer sciencePolymer thin films
The invention belongs to the technical field of polymer materials, and particularly relates to application of a porous polymer film with regular pores to preparation of an anti-adhesion film. The shape memory polyurethane provided by the invention is formed by polymerizing diisocyanate and a soft segment polymer, the soft segment polymer is polylactic acid, polyglycolic acid, polycaprolactone, polypolyol or a copolymer of at least two of the polylactic acid, the polyglycolic acid, the polycaprolactone and the polypolyol. The shape memory polyurethane material is combined with a water drop template method, and a porous polymer film with a crystal structure can be contained. The porous film material has excellent mechanical properties, can meet the performance requirements of application scenes such as artificial periosteum and anti-adhesion membranes, and has a good application prospect.
Owner:CHONGQING UNIV
Broom-shaped hydroxycamptotecin-loaded sustained-release particle and preparation method thereof
The invention discloses a broom-shaped hydroxycamptotecin-loaded sustained-release particle and a preparation method thereof. The preparation method comprises the following steps: dissolving hydroxycamptothecine (10-HCPT) and methoxyl-terminated polyethylene glycol- poly(dl-lactide-co-glycolide) (MePEG-PLGA) in an organic solvent, injecting into a disperse phase of a membrane emulsifier, and injecting an aqueous solution containing little polyvinyl acetate (PVA) into a continuous phase of the membrane emulsifier; leading the solution of the disperse phase to quickly enter the continuous phase at nitrogen pressure by adopting a porous glass membrane as a template to obtain suspension of the hydroxycamptotecin-loaded sustained release particle (MePEG-PLGA-HCPT); distilling the suspension in vacuum, removing the organic solvent, removing uncoated square medicinal crystal by ultrafiltration of a microporous filter membrane, and lyophilizing the suspension in vacuum in a lyophilizer for 24 hours to obtain the broom-shaped MePEG-PLGA-HCPT sustained-release particle. The method is reliable, and convenient to operate; the prepared irregular particle is high in drug-loading amount and encapsulation rate, is excellent in sustained release effect, and is particularly suitable for tumor local medication and tumor post-operation medication.
Owner:XIAMEN UNIV
Slow released injection containing blood vessel inhibitor and its synergist
The slow released injection containing blood vessel inhibitor and its synergist consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is special solvent containing suspending agent. The effective anticancer component is blood vessel inhibitor carboxyl amino triazole, Irotinat, Lapatinat, etc and / or blood vessel inhibitor synergist selected from toporase inhibitor and tetrazin compound. The slow releasing supplementary material is one of difatty acid-sebacic acid copolymer, poly(erucic acid dipolymer-sebacic acid), poly(fumaric acid-sebacic acid), polylactic acid, etc or their composition. The suspending agent is carboxymethyl cellulose, etc. and has viscosity of 100-3000 cp at 20-30 deg.c. The slow released microsphere may be also prepared into slow released implanting agent for improving the treating effect of chemotherapeutic and / or radiotherapeutic medicine.
Owner:JINAN SHUAIHUA PHARMA TECH
Preparation method of nano-particle rapamycin-loaded drug coating balloon
InactiveCN113813449AGood compatibilityImprove encapsulation efficiencyCatheterCoatingsFluorescenceMesoporous silica
The invention discloses a preparation method of a nano-particle rapamycin-loaded drug coating balloon, and belongs to the technical field of drug manufacturing. The surface of the balloon is coated with hollow mesoporous silica nano-particles (MSNs) loaded with Rapamycin (RPM) through polylactic acid-polyglycolic acid (PLGA). The polylactic acid-polyglycolic acid mixes the mesoporous silica nano-particles loaded with rapamycin into the surface of the balloon, the surface of the balloon is coated with the polylactic acid-polyglycolic acid, nano-particles are bridged to prepare the nano-particle rapamycin-loaded drug coating balloon which is excellent in histocompatibility, biodegradable, high in encapsulation efficiency, stable in drug loading capacity control, small in particle size and constant in-vitro drug release, the effective action concentration of the drug can be reached and maintained in local tissue under the condition that blood flow is affected as little as possible, the negative influence of a drug loading matrix on vascular repair can be overcome, and the anti-endometrial hyperplasia and anti-restenosis effects of rapamycin are improved. The inorganic nano-particles can be compounded with an up-conversion rare earth luminescent fluorescent agent, 4f electrons of a rare earth inner shell layer can emit light under laser irradiation, and therefore the nano-particle rapamycin-loaded drug coating balloon is used for simulating in-vitro conveying and expansion of the balloon in plasma and monitoring the concentration of the nano-particles left in the plasma and used for detecting the drug loading stability of the balloon in the early stage.
Owner:孟繁宇
Ophthalmic Pharmaceutical Composition, Ophthalmic Kit, and Pharmaceutical Application Thereof
PendingUS20200215079A1Improve stabilityExtended shelf lifeOrganic active ingredientsSenses disorderGlutaric acidPolythylene glycol
The present invention relates to an ophthalmic pharmaceutical composition, comprising 0.06 to 40 parts by weight of a solid matrix, 0.4 to 55 parts by weight of a liquid matrix and 1 part by weight of an active pharmaceutical ingredient; the solid matrix is selected from the group consisting of polylactic acid, polyglycolic acid, polylactic acid-co-glycolic acid copolymer, and polyethylene glycol with a number average molecular weight of 800 to 10,000; the liquid matrix is a first liquid matrix and / or a second liquid matrix; the first liquid matrix is selected from the group consisting of diethyl succinate, diethyl tartrate, dimethyl glutarate and glycerol triacetate; when the liquid matrix is the first and second liquid matrixes, the second liquid matrix is selected from the group consisting of glycerol, propylene glycol, and polyethylene glycol with a number average molecular weight 70-750; when the liquid matrix is the second liquid matrix, the second liquid matrix is glycerol and / or propylene glycol, or a combination thereof with polyethylene glycol having a number average molecular weight of 70 to 750.
Owner:SHENYANG XINGQI PHARM CO LTD
Application of porous polymer film with regular pores to preparation of artificial periosteum
ActiveCN114656678AGood mechanical propertiesArranged in orderPharmaceutical delivery mechanismTissue regenerationPolymer sciencePolymer thin films
Owner:CHONGQING UNIV
Degradable composite film for heart occluder as well as preparation method and application of degradable composite film
ActiveCN114652366AImprove adhesionHigh tensile strengthSurgeryPharmaceutical delivery mechanismPolymer scienceMetal oxide nanoparticles
The invention relates to a degradable composite film as well as a preparation method and application thereof. The degradable composite membrane comprises a high polymer material membrane, the upper surface and the lower surface of the high polymer material membrane are covered with hydrogel membranes respectively, and the high polymer material membrane and the hydrogel membranes are connected through covalent bonds; a high polymer material in the high polymer material film is selected from polylactic acid, polyglycolic acid, a polylactic acid-glycolic acid copolymer, polyhydroxyalkanoate, poly (p-dioxanone), polycaprolactone and a polyurethane film; the hydrogel film contains a zwitterionic polymer, the cross-linking agent is gelatin derivative modified metal oxide nanoparticles, and the metal oxide nanoparticles and the gelatin derivative are connected through coordinate bonds or ionic bonds; the gelatin derivative contains a catechol group and an unsaturated double-bond group at the same time. The composite membrane is good in anticoagulation performance and X-ray developing capacity, can promote adhesion and growth of endothelial cells, and is beneficial to generation of normal new tissues on the surface of the membrane.
Owner:SICHUAN UNIV
Slow release injection comprising anticancer antibiotics and booster thereof
Disclosed is an anticancer slow release injection containing anticancer antibiotics and synergistic agents, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer active constituents include anticancer antibiotics and / or anticancer antibiotic synergistic agent selected from enzyme inhibitor and / or tetrazine-group compounds, the slow release auxiliary materials are selected from the following or their combination: Polifeprosan, di-aliphatic acid and sebacylic acid copolymer, poly(erucic aciddipolymer-sebacylic acid) copolymer, poly(fumaric acid-sebacylic acid) copolymer, poly(lactic acid), polyglycolic acid and glycolic acid copolymer, ethylene-vinylacetate copolymer or their combination. The viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The slow release microspheres can also be prepared into slow release implanting agent for injection or placement in or around tumor with improved treating effects of chemotherapy.
Owner:JINAN KANGQUAN PHARMA TECH
A preparation method of polylactic acid/polyglycolic acid drug-loaded microspheres with controllable drug release
ActiveCN109662957BEffective control of degradationAlleviate the burst phenomenonOrganic active ingredientsPharmaceutical non-active ingredientsMicrosphereDrug release
The invention relates to a method for preparing polylactic acid / polyglycolic acid drug-loaded microspheres with controllable drug release. For a case of a low drug release efficiency, polylactic acid,polyglycolic acid, tea polyphenol, dichloromethane, gelatin polysorbate / 80 and deionized water are used as raw materials, a solution is prepared, the drug-loaded microspheres are prepared, suction filtering and vacuum drying are conducted to prepare the polylactic acid / polyglycolic acid drug-loaded microspheres with the controllable drug release, the degradation of drugs is effectively controlled, the phenomenon of sudden release of the drugs is relieved, the action and utilization of the drugs are effectively prolonged, and the method is advanced in process, accurate and detailed in data andhigh-purity in the prepared drugs and is an advanced preparation method for a drug slow release agent.
Owner:TAIYUAN UNIV OF TECH
Microsphere frozen section as well as preparation method and application thereof
PendingCN114705709ARaw materials are easy to obtainThe preparation method is simple and easyMaterial analysis using wave/particle radiationMicroballoon preparationMicrosphereFreeze-drying
The invention provides a microsphere frozen section as well as a preparation method and application thereof. The preparation method of the microsphere frozen section comprises the following steps: dissolving bovine serum albumin and a pore-foaming agent in a poloxamer 188 solution to obtain an inner water phase; adding the mixture into an oil phase containing polylactic acid and polyglycolic acid, and stirring to form a primary emulsion; then adding into an outer water phase, stirring to form a multiple emulsion, curing, centrifugally collecting, and freeze-drying to obtain microspheres; then, suspending in a gelatin-glycerol embedding medium for soaking treatment, and freezing and solidifying to obtain an embedded block; and slicing the microspheres to obtain frozen microspheres. The method disclosed by the invention has the characteristics of simplicity, rapidness, no need of repairing an embedding block, low consumable price, difficulty in fragmentation, easiness in sheet expansion and the like, and the prepared microspheres have flat sections and clear pore structures, are suitable for observation of a scanning electron microscope, and can be used for calculating the porosity of the microspheres by image processing software; the frozen microballoon slice also provides reference for the slicing technology of microballoons, polymer porous scaffolds and the like prepared from other materials.
Owner:SUZHOU CANCERCELL BIOTECH
Controlled release microparticles
ActiveUS8877229B2Reduce burstExtended durationPowder deliveryOrganic active ingredientsActive agentEngineering
Formulations for controlled, sustained release of biologically active agents for the treatment of ocular disorders have been developed. These formulations are based on solid microparticles formed of the combination of biodegradable, synthetic polymers such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and copolymers thereof. The microparticles are characterized by low burst levels and efficient drug loading and sustained release.
Owner:EYETECH
Preparation consisting of PLGA (Polylactic Acid-Polyglycolic Acid)/TCP (Tris Calcium Polymer) and MSC (Mesenchymal Stem Cells) of compound MSC and application thereof
InactiveCN101991880AReduce infiltrationSpleen nodule size and decreaseMammal material medical ingredientsImmunological disordersTri calcium phosphateBiological materials
The invention discloses a preparation consisting of a PLGA (Polylactic Acid-Polyglycolic Acid) / TCP(Tris Calcium Polymer) and MSC (Mesenchymal Stem Cells) of a compound MSC and application thereof. Active components of the preparation comprise a substance (1) and a substance (b) which are separately packed, wherein the substance (1) is a biological compound sclerite compounded by the MSC and the PLGA / TCP; and the substance (2) is the MSC. Proved by experiments, the intravenous infusion of the substance (2), i.e. the MSC, can be used for lightening immune reactions caused by the heteroplastic transplantation of the compound sclerite.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
A broom-shaped hydroxycamptothecin-loaded sustained-release particle and its preparation method
The invention discloses a broom-shaped hydroxycamptotecin-loaded sustained-release particle and a preparation method thereof. The preparation method comprises the following steps: dissolving hydroxycamptothecine (10-HCPT) and methoxyl-terminated polyethylene glycol- poly(dl-lactide-co-glycolide) (MePEG-PLGA) in an organic solvent, injecting into a disperse phase of a membrane emulsifier, and injecting an aqueous solution containing little polyvinyl acetate (PVA) into a continuous phase of the membrane emulsifier; leading the solution of the disperse phase to quickly enter the continuous phase at nitrogen pressure by adopting a porous glass membrane as a template to obtain suspension of the hydroxycamptotecin-loaded sustained release particle (MePEG-PLGA-HCPT); distilling the suspension in vacuum, removing the organic solvent, removing uncoated square medicinal crystal by ultrafiltration of a microporous filter membrane, and lyophilizing the suspension in vacuum in a lyophilizer for 24 hours to obtain the broom-shaped MePEG-PLGA-HCPT sustained-release particle. The method is reliable, and convenient to operate; the prepared irregular particle is high in drug-loading amount and encapsulation rate, is excellent in sustained release effect, and is particularly suitable for tumor local medication and tumor post-operation medication.
Owner:XIAMEN UNIV
Slow-release injecta containing platinums compound and its synergist
The present invention relates to a slow-released injection containing platinum compound and its synergist. It is composed of slow-released microsphere and solvent, the slow-released microsphere includes anticancer effective component and slow-released auxiliary material, the solvent is general one or special solvent containing suspension adjuvant. The anticancer effective component includes platinum compound and platinum compound synergist, the platinum compound synergist is topoisomerase inhibitor, guanine analogues and / or tetrazine compound, the slow-released auriliary material is one selected from polylactic acid, copolymer of polyglycollic acid and glycolic acid, ethylene-vinylacetate copolymer, difatty acid and sebacic acid copolymer, poly (erucic acid dimmer-sebacic acid) copolymer and poly (fumaric acid-sebacic acid) copolymer or their combination. The viscosity of suspension adjuvant is 100 cp-3000 cp (20 deg.C-30 deg.C), and said suspension adjuvant is selected from sodium cellulose glycollate. The slow-released microsphere also can be made into the slow-released implant preparation.
Owner:JINAN SHUAIHUA PHARMA TECH
Daidzein-entrapped PLGA nanoparticles and preparation method thereof
The invention relates to daidzein-entrapped polylactic polyglycolic acid (PLGA) nanoparticles and a preparation method thereof in the technical field of nanomedicines. The nanoparticles comprise the following components in percentage by mass: 0.1 to 45 percent of daidzein, 25 to 95 percent of polylactic polyglycolic acid and 1 to 65 percent of pharmaceutic adjuvant, wherein the molecular weight of the PLGA is between 8,000 and 15,000, the polylactic polyglycolic acid is long-chain macromolecules in which the ratio of the lactic acid to glycollic acid is 50:50, and the pharmaceutic adjuvant islecithin, cyclodextrin or polyvinyl alcohol (PVA). The daidzein-entrapped PLGA nanoparticles have the particle size of between 280 and 330 nanometers and concentrated particle size distribution, redissolved freeze-dried powder is not adhered, and the medicine loading rate of the PLGA nanoparticles is between 1 and 2 percent, and the encapsulating rate is between 80 and 84 percent.
Owner:SHANGHAI JIAOTONG UNIV
Special starch/PLA-PGA-PBAT biodegradable compostable material and preparation method thereof
The invention discloses a special starch / PLA-PGA-PBAT biodegradable compostable material and a preparation method thereof. The special starch / PLA-PGA-PBAT biodegradable compostable material comprises the following components: starch, polylactic acid, polyglycolic acid, poly (butylene adipate-co-terephthalate), ethylene glycol, calcium stearate, diphenylmethane diisocyanate, an antioxidant and titanium dioxide. The diphenylmethane diisocyanate is used as a compatilizer and is prepared by reacting free amine with phosgene, and the diphenylmethane diisocyanate is used for reducing the interfacial tension among polylactic acid, polyglycolic acid and starch and enhancing the interphase binding force, so that the effect of improving the mechanical property is achieved.
Owner:杨如麟 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com