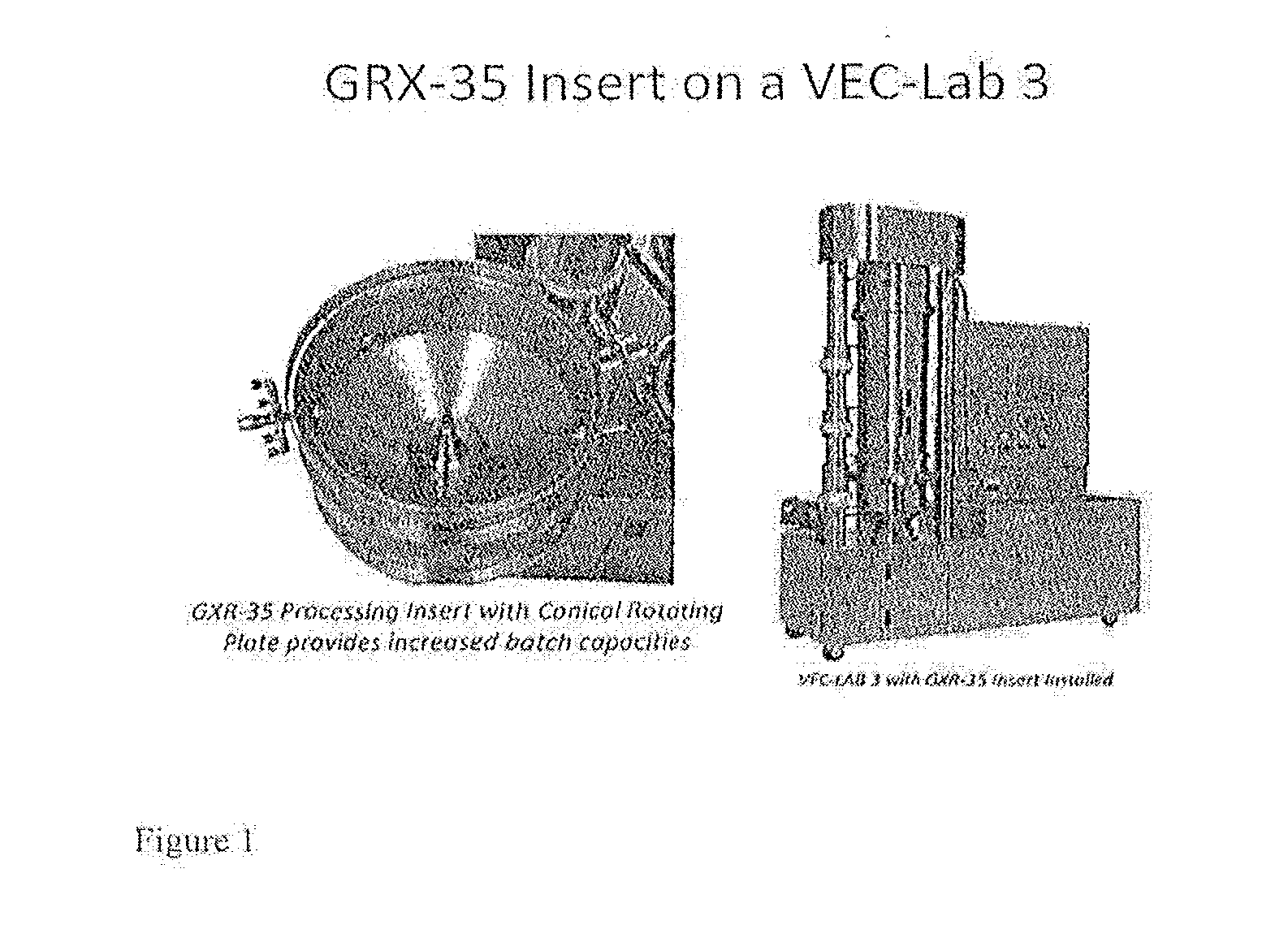
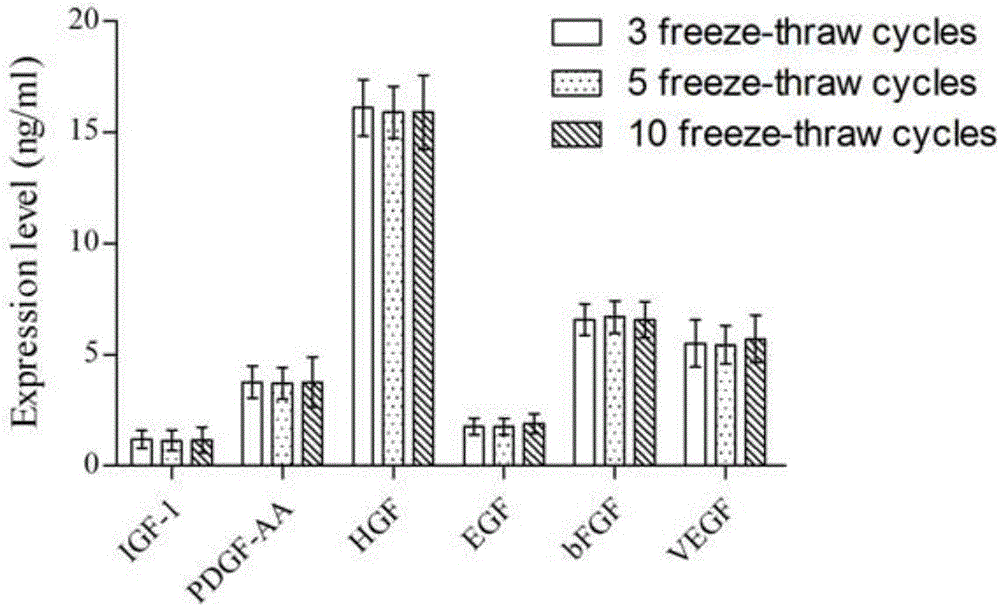
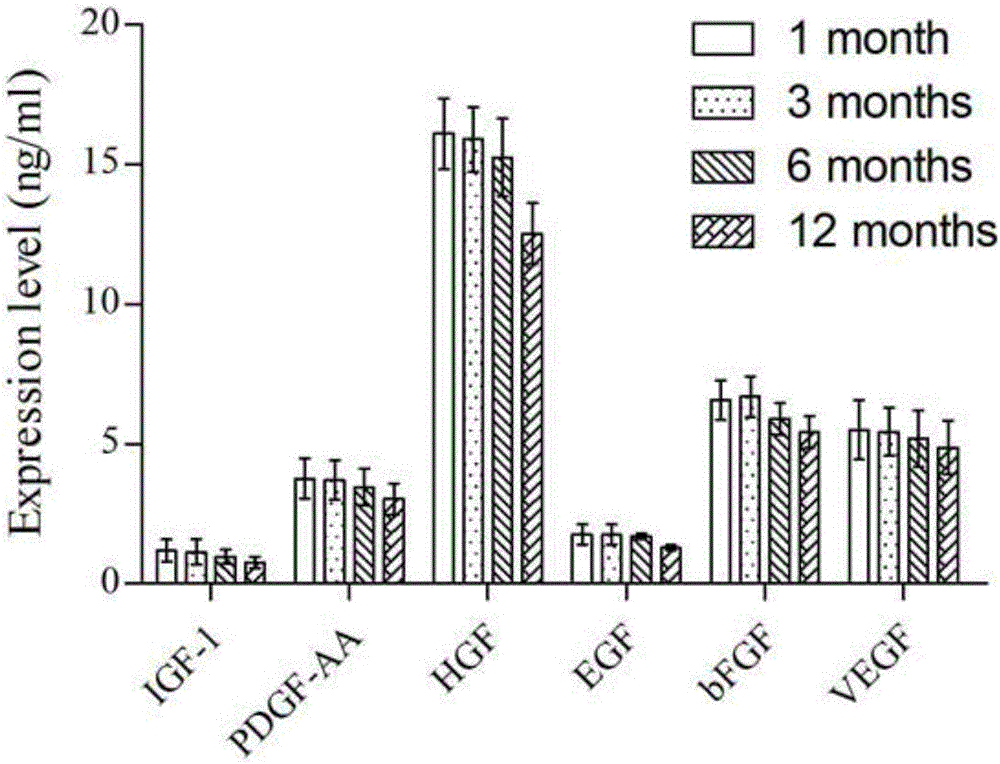
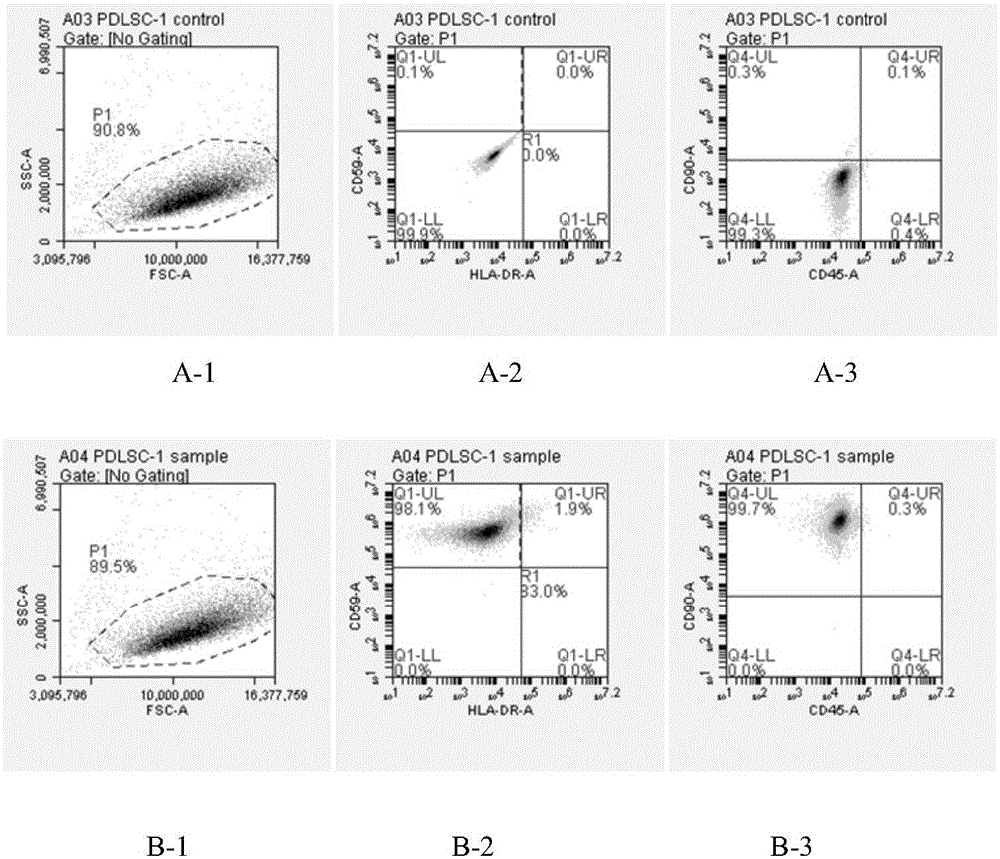
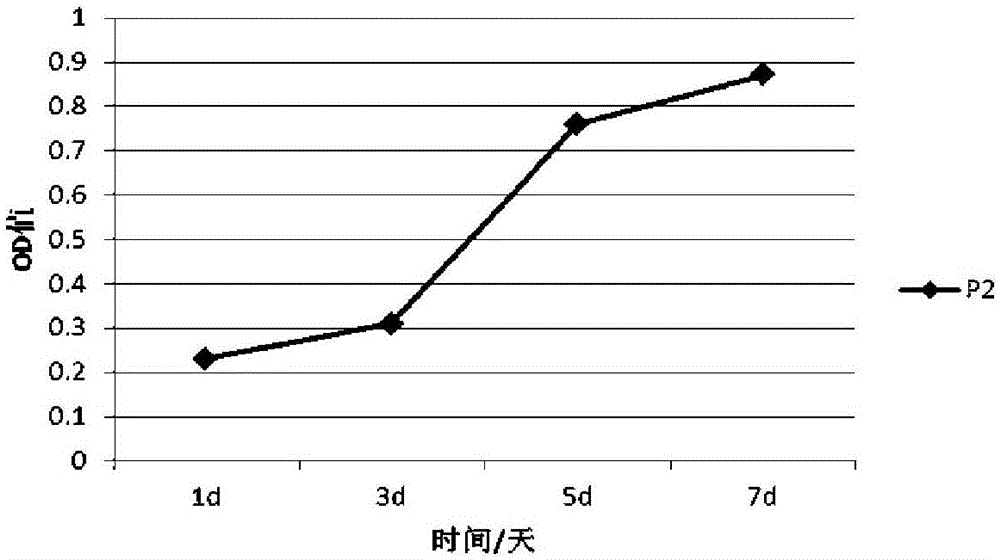
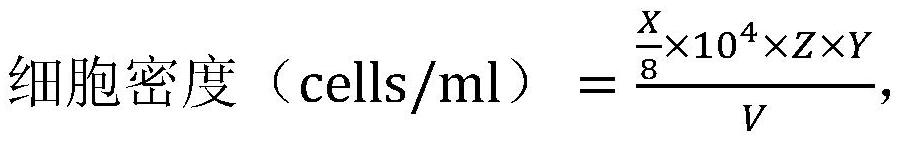Patents
Literature
102 results about "Pancreatinum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stable digestive enzyme compositions
InactiveUS20090117180A1Minimal loss of activityComposition is stablePowder deliveryHydrolasesDiseasePancrelipase
Compositions of the present invention, comprising at least one digestive enzyme (e.g., pancrelipase) are useful for treating or preventing disorders associated with digestive enzyme deficiencies. The compositions of the present invention can comprise a plurality of coated particles, each of which is comprised of a core coated with an enteric coating comprising at least one enteric polymer and 4-10% of at least one alkalinizing agent, or have moisture contents of about 9% or less or 3% or less, water activities of about 0.6 or less, or exhibit a loss of activity of no more than about 25%, about 20%, about 15% or about 10% after six months of accelerated stability testing and the titer level of a viral contaminant present in the pancreatin is at least about 1000 times less than the titer level of the viral contaminant present in a preparation from which the pancreatin is obtained.
Owner:APTALIS PHARMA
Decellularized heterogeneous corneal stroma carrier and its preparation method and application
The invention discloses a decellularized heterogeneous corneal stromal carrier and its preparation method and application. The carrier is an animal lamellar cornea from which epithelial cells and stromal cells have been removed through hypertonic solution combined with enzyme digestion. The preparation method of the carrier is as follows: firstly, take Fresh animal eyeballs are aseptically operated under an operating microscope, and the lamellar cornea with a thickness of 150 μm to 400 μm is drilled with a graduated trephine drill with a diameter of 5 mm to 12 mm, and then removed under the combined action of hypertonic solution and trypsin / pancreatin substitute The cells are finally dehydrated and dried to obtain the decellularized heterogeneous corneal stroma carrier, which is stored for future use. The decellularized heterogeneous corneal stroma carrier can be used as a corneal transplant donor to directly perform therapeutic corneal transplantation, and can also be used as an artificial biological corneal scaffold to construct a full-layer or lamellar artificial biological cornea. The decellularized heterogeneous corneal stroma carrier prepared by the invention has the following characteristics: the collagen is neatly arranged, similar to normal corneal tissue, and has good transparency after rehydration.
Owner:陕西省眼科研究所
Pancreatic Enzyme Compositions and Methods for Treating Pancreatitis and Pancreatic Insufficiency
Compositions of the present invention, comprising the combination of enterically coated and uncoated pancreatic enzyme-containing beads are useful for treating or preventing pancreatitis pain, and optionally disorders associated with digestive enzyme deficiencies.
Owner:SOC DES PROD NESTLE SA
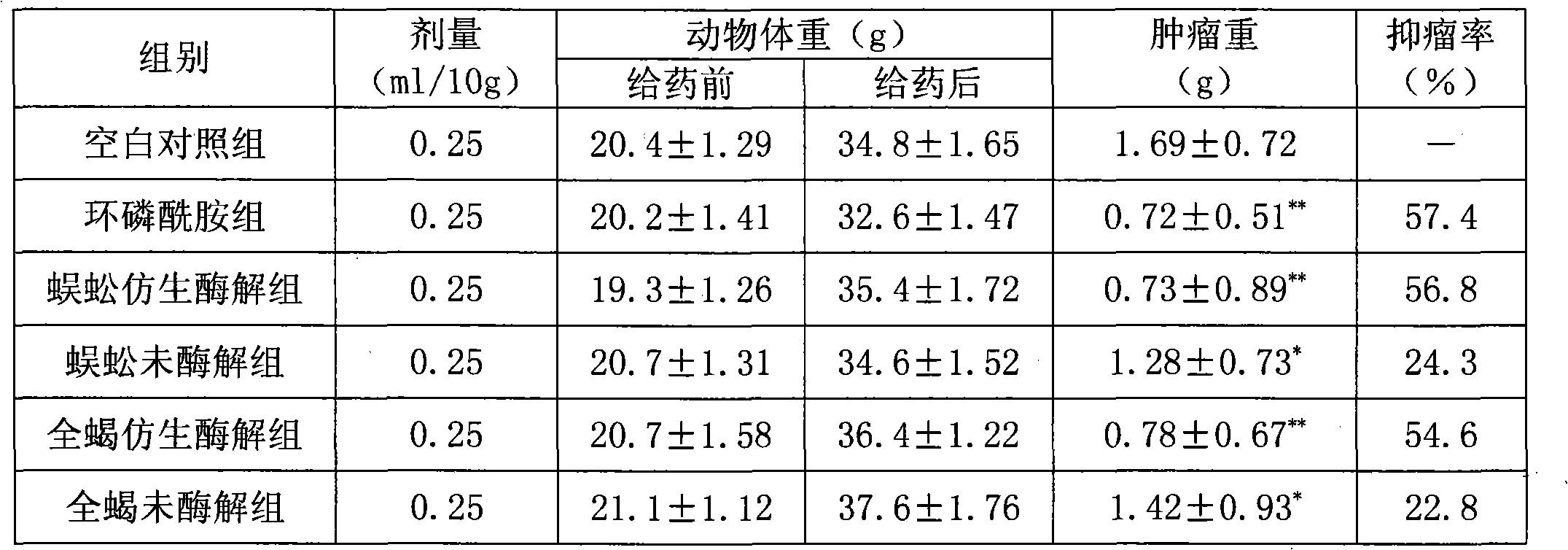
Bionic enzymatic product of animal medicament and application thereof
The invention discloses a bionic enzymatic product of animal medicament and application thereof, which belong to the field of Chinese medicament. The bionic enzymatic product is characterized in thata bionic enzymatic method is adopted, and comprises the following steps: taking animal medicament; grinding the animal medicament into fine powders; adding water into the fine powders; evenly stirringthe mixture; under appropriate condition, performing heat preservation by using pepsin; performing heat preservation by using pancreatin or trypsase; and preparing obtained zymolyte into preparationsaccording to different preparation requirements. The product of the invention has good effects in the aspects of resisting hepatitis virus, reducing blood press and resisting cancer.
Owner:BEIJING HERUN INNOVATION PHARMA TECH DEV
Micropellet compositions comprising pancreatin containing digestive enzyme mixture
The present invention relates to a small particle size composition comprising pancreatin containing digestive enzymes for use in patients in need, including pediatric, geriatric, and adult patients, particularly those patients with dysphagia or wherein enteral administration using such composition would be suitable. In addition, the invention is directed to the composition as particles, such as micropellets or microgranules having a high potency, high useable yield and at least 10%-90% of 400-800 μm. Furthermore, the composition optionally has an improved enteric coating and concomitant improved stability and enzyme activity compared to conventional prepared enterically coated pancreatic enzyme particles.
Owner:APTALIS PHARMATECH +1
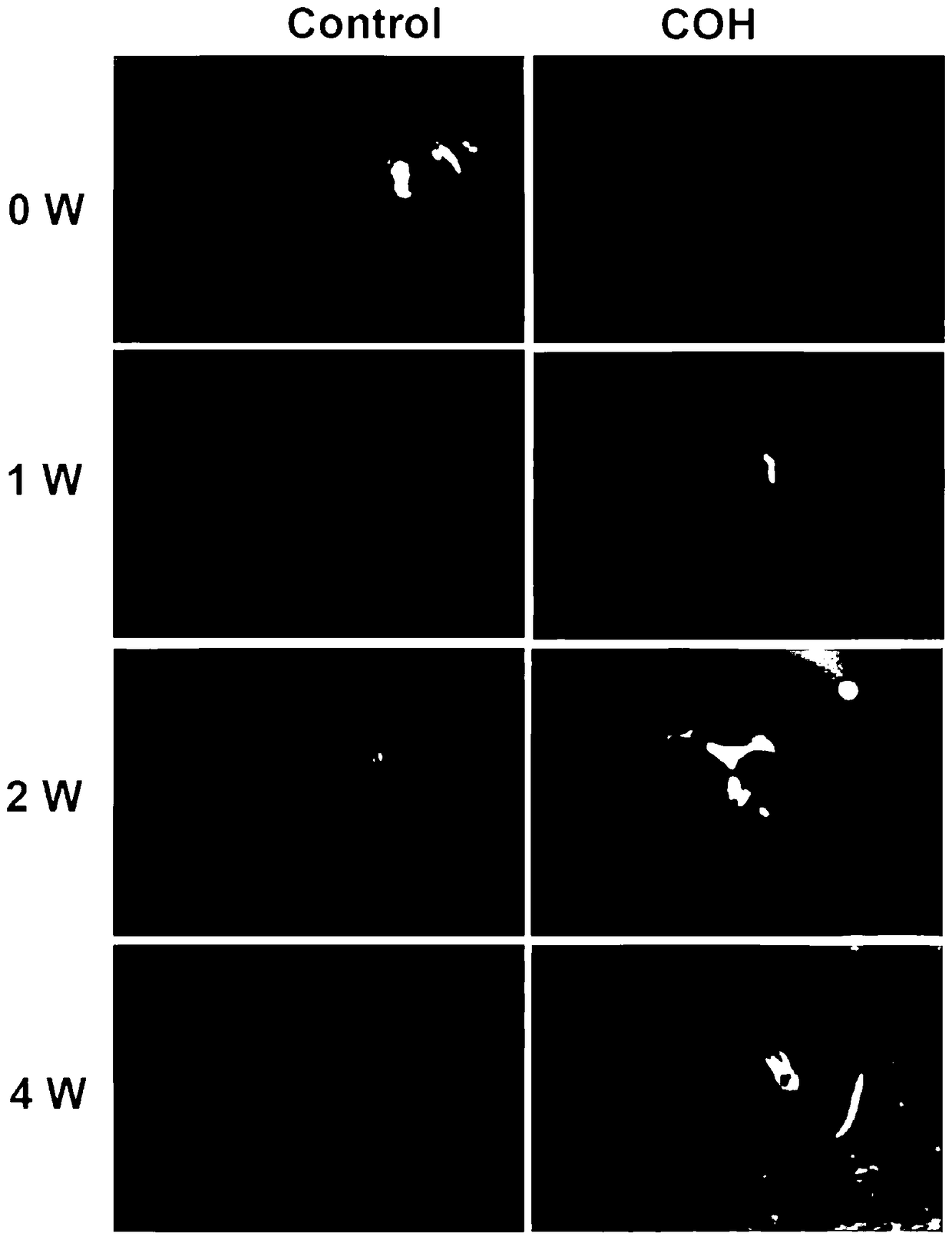
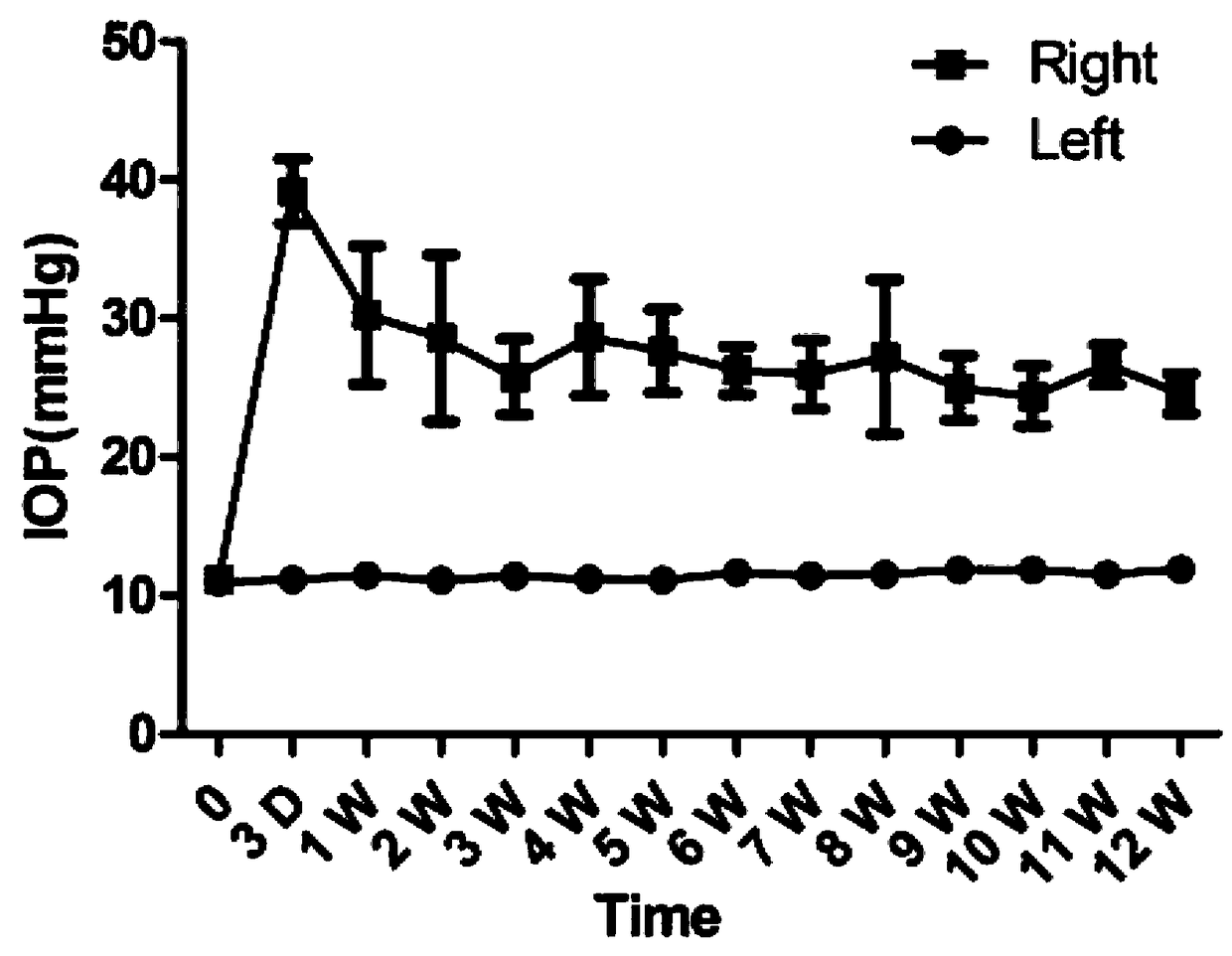
Chronic glaucoma animal model establishing method and application thereof
InactiveCN108210520AGood repeatabilityLow costCompounds screening/testingNervous system cellsConjunctivaSingle cell suspension
The invention discloses a chronic glaucoma animal model establishing method. The method comprises the following steps: (1) collecting conjunctiva tissues of GFP-SD rats, sterilizing, digesting, separating a conjunctiva epithelial layer, and obtaining a conjunctiva matrix tissue block; (2) digesting the conjunctiva matrix tissue block in pancreatin, terminating the digestion by using a cell culturesolution, blowing and beating to form a single-cell suspension, filtering by virtue of a cell sieve, centrifuging, and removing supernatant, thus obtaining conjunctiva matrix cell precipitates; (3) adding an appropriate amount of cell culture solution, blowing and beating to form single cells, counting, performing the in-vitro culture, and obtaining a remarkable amount of conjunctiva matrix cells; (4) digesting the conjunctiva matrix cells by using pancreatin, processing by utilizing the method of the step (2), obtaining the conjunctiva matrix cell precipitate, adding an appropriate amount ofcell culture solution, and obtaining cell suspension; and (5) injecting 10 micro liter of cell suspension by utilizing a micro injector into the anterior chamber of the right eye of a rat, and sufficiently and uniformly mixing. A glaucoma animal model obtained by using the method has the advantages of long-term stability, simplicity, ease, good repetition, and low cost.
Owner:XIAMEN EYE CENTER OF XIAMEN UNIVERSITY CO LTD
Pure natural cerebroprotein hydrolysate raw material preparation method
ActiveCN104096215AOvercome the defect of insufficient clarityComplete enzymatic hydrolysisNervous disorderHydrolysed protein ingredientsHydrolysateUltrafiltration
The invention discloses a pure natural cerebroprotein hydrolysate raw material preparation method. The method comprises the steps as follows: a, protein purification, to be specific, a cryopreserved fresh pig brain is defrosted, cleaned, homogenized, and subjected to centrifugal separation for obtaining precipitated brain protein; b, pepsin enzymolysis, to be specific, pepsin is added in the precipitated brain protein and stirred, then concentrated hydrochloric acid is added and stirred, and then the centrifugal separation carried out, so that a pepsin enzymatic hydrolysate is obtained; c, acid hydrolysis, to be specific, sulfuric acid is added into the pepsin enzymatic hydrolysate for hydrolyzing, so that an acid hydrolysis liquid is obtained; d, trypsin enzymolysis, to be specific, the pH value is adjusted to 7-9, and pancreatin with the weight 0.1% to 0.4% of that of the pig brain is added for hydrolyzing, and the centrifugal separation is carried out, so that a pancreatin enzymatic hydrolysate is obtained; e, chromatography, to be specific, the pH value is adjusted to 6.5-7.5, and resolving is carried out by using ammonia water, so that a chromatography liquid is obtained; f, concentration, to be specific, the vacuum concentration is carried out, the pH value is adjusted to 6.5-7.5, and then frozen storage is carried out; g, impurity removing, to be specific the condensed frozen liquid is defrosted, and then subjected to centrifugal separation as well as ultrafiltration for obtaining natural cerebroprotein hydrolysate raw material.
Owner:HUNAN LINUO BIOLOGICAL PHARMA
Pancreatic enzyme compositions and methods for treating pancreatitis and pancreatic insufficiency
Compositions of the present invention, comprising the combination of enterically coated and uncoated pancreatic enzyme-containing beads are useful for treating or preventing pancreatitis pain, and optionally disorders associated with digestive enzyme deficiencies.
Owner:SOC DES PROD NESTLE SA
Velvet antler polypeptide mixture and preparation method and application thereof
InactiveCN105031606APossesses anti-inflammatory activityLow costDipeptide ingredientsAntipyreticAnimal foodHydrolysate
The invention relates to the field of polypeptide biological drug, and particularly provides a velvet antler polypeptide mixture and a preparation method and application thereof. The preparation method includes the following steps: 1), sequentially using pepsase and pancreatin to hydrolyze velvet antler protein, and obtaining velvet antler protein hydrolate after enzyme deactivation; 2), separating polypeptide components with molecular weight smaller than 3kDa from the velvet antler protein hydrolate; 3), obtaining the velvet antler and polypeptide mixture through separation and purification, wherein main components of the velvet antler and polypeptide mixture include Ala-His-Gly, Ala-His-Trp-Lys, Val-His, Leu-Ala-Gln, Ile-Ala, Ala-Tyr, Ala-Leu and Thr-Leu. The velvet antler polypeptide mixture has anti-inflammation activity, thereby being capable of being used for preventing and treating inflammation, has the advantages of being natural, safe, efficient and low in cost, thereby being capable of being used in the fields of functional food, healthcare products, animal food and feed, has wide market prospect and considerable economic benefit and is easy for development and utilization.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
Method for preparing antioxidant peptide by utilizing pancreatin to hydrolyze rice residue
InactiveCN103805666AImprove cleanlinessImproves antioxidant activityFermentationFreeze-dryingAmino acid composition
The invention discloses a method for preparing antioxidant peptide by utilizing pancreatin to hydrolyze rice residue. The antioxidant peptide is obtained by adopting rice residue, namely a byproduct of aginomoto production as a raw material, concentrating the rice residue to extract rice residue protein by virtue of an pickling method, hydrolyzing the rice residue protein by adopting pancreatin, centrifuging, hyperfiltering and freeze drying the rice residue, the enzymolysis condition is as follows: after the rice residue protein is mixed with water at a mass-to-volume ratio of (1:6)-(1:9), the pH value is adjusted to 7.5 to 8.0 by adding hydrochloric acid, and the pancreatin which accounts for 6-8wt% of the rice residue protein is added at the temperature of 45-55 DEG C to enzymolyze the rice residue protein for 5-6 hours. The production cost is low, the process is simple, environmental friendliness is realized, the obtained antioxidant peptide has the characteristics of reasonability in ammonia acid constitution, low irritability, safety, reliability and the like, the content of total phenols is 34.47mg / g, the IC50 value of DPPH free radical and hydroxyl free radical is respectively 2.27mg / mL and 2.98mg / mL, and the elimination capacity is good, so that the antioxidant peptide is strong in antioxidant activity and a functional food base material with potential.
Owner:FUZHOU UNIV
Preparation method and application of mesenchymal stem cell cytokines
InactiveCN106520689AIncrease contentAvoid degradationCosmetic preparationsPeptide/protein ingredientsFreeze thawingCentrifugation
The invention provides a preparation method for mesenchymal stem cell cytokines. The preparation method comprises the following steps: (1) extraction of mesenchymal stem cells; (2) culture; (3) continued culture under low-oxygen conditions; (4) digestion with a 0.25% pancreatin-EDTA solution; (5) centrifugation and cleaning with 0.9% normal saline; (6) adjustment of cell concentration with 0.9% normal saline and addition of EDTA with a concentration of 1 to 3 mg / mL and Vc with a concentration of 5 to 10 mg / mL; (7) freezing-thawing; and (8) centrifugation and filtering. The mesenchymal stem cell cytokines prepared in the invention can effectively overcome the problems that artificially prepared cytokine compositions in the prior art cannot simulate the variety and ratio of cytokines in a cell microenvironment and incur adverse reaction during usage.
Owner:四川华皓生物科技有限公司
Linolenic acid pancreatin fancy soap and production process thereof
InactiveCN1827761AFewer raw materialsReduce moisture contentSoap detergent compositionsDistilled waterSugar
The linolenic acid pancreaticenzyme perfumed soap of this invention belongs to a functional soap developed by adopting biotechnology. The main materials consist of neat soap, pepertree seed oil and pancreatic powder. The process for linolenic acid pancreaticenzyme perfumed soap contains six technics comprising neat soap disintegrating, distilled water agitator toning agent, mixing and concocting, grinding, squeezing and packing. The invention is characterized in that the product materials are few, the cost is low, the process for producing is simple, the water content is low, the quality-assured period is long, at the same time, it possesses some effects that it can cool, relieve itching, treat dermatopathy and reduce voltage, fat and sugar, and is good for brain and can improve acuity of vision.
Owner:鱼新民
Bionic enzymatic hydrolysate for scorpion and application thereof
InactiveCN101647824AImprove curative effectEasy to absorbNervous disorderAnthropod material medical ingredientsChemistryDisease
The invention discloses bionic enzymatic hydrolysate for scorpion and application thereof, and belongs to the field of Chinese medicaments. The invention adopts the technical scheme that: a bionic enzymolysis method is adopted; the scorpion is taken; and after water is added in the scorpion so as to form homogenate, under proper conditions, the heat preserving enzymolysis is carried out firstly bypepsin, and then the heat preserving enzymolysis is carried out by pancreatin or trypsase. The obtained enzymatic hydrolysate is prepared into preparations according to different preparation requirements. The bionic enzymatic hydrolysate has excellent effects in aspects of treating cardiovascular and cerebrovascular diseases, tumors, rheumatism, epilepsia and the like.
Owner:北京凯瑞创新医药科技有限公司
Preparation method of pancreatic enzyme powder
The invention provides a method for preparing pancreatic enzyme powder. The method comprises the following steps of (1) collecting pancreas; (2) preserving the pancreas at a low temperature; (3) carrying out thawing, impurity removing and mincing; (4) extracting the pancreas so as to obtain pancreas slurry, and filtering by utilizing a frame filter press, so as to obtain an extracting solution; (5) adding ethanol into the extracting solution, standing, filtering by utilizing the frame filter press, and collecting a filter cake; (6) washing and centrifuging the filter cake to obtain sediments; (7) drying the sediments in vacuum and crushing, so as to obtain the pancreatic enzyme powder. According to the method, the bovine pancreatic enzyme powder accordant with a Mohammedanism rule is provided, and the market blank is made up; a method for preparing the industrial pancreatic enzyme powder by virtue of pig pancreas is technically improved. The method has the advantages that the production cost is low, the pollution to a final product and the environment is avoided, and the production security is greatly improved; the operation process is simplified, the consumed time is short, the time for preparing each batch of product only lasts for 45-48 hours, and the method is easy to industrialize. The product prepared by utilizing the method has the advantages that three enzyme activities are high and the yield reaches 11.0%.
Owner:马忠仁 +1
Primary separation and culture method for periodontal ligament stem cells
ActiveCN105062960AHigh affinityLow costArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsPeriodontiumPeriodontal ligament stem cells
The invention discloses a primary separation and culture method for periodontal ligament stem cells, which comprises the following steps: separating periodontal ligaments, carrying out enzymolysis on periodontal ligament tissues by a mixed enzyme of collagenase V of which the mass-volume concentration is 0.05-0.25% and pancreatin of which the mass-volume concentration is 0.05-0.25%, carrying out primary culture on periodontal ligament stem cells, and separating the periodontal ligament stem cells by adopting a limiting dilution method. The primary separation and culture method disclosed by the invention has the following advantages: (1) the cost is reduced because the cost of the pancreatin is lower than that of dispase; (2) the digestion time is shortened, the periodontal ligaments are dense connective tissues, the affinity of the collagenase V to the connective tissues is superior to that of collagenase I, and the collagenase V and the pancreatin are combined, so that the enzymolysis effect is obviously improved.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Serum-free suspension domestication method of Vero cells
InactiveCN110904025ALarge number of proliferation and expansionThe cultivation process is easy to scale upCulture processArtificial cell constructsBiotechnologyPancreatinum
The invention relates to the field of biology. A serum-free suspension domestication method of Vero cells comprises the steps of step I, performing adherent culture on the Vero cells until the convergence rate reaches 80%, discarding supernatant, adding D-PBS free from calcium and magnesium ions, and performing washing; step II, discarding the D-PBS, adding pancreas enzymes, discarding trypsinization liquid, remaining a few pancreas enzymes which are enough to cover cells, performing incubation until the cells become round or performing incubation for definite time, and patting the bottom of aflask to dissociate cells; step III, performing re-suspension with a Vero-S suspension serum-free culture medium; step VI, discarding the supernatant, and performing re-suspension with Vero-S suspension serum-free culture medium; step V, diluting the density of the cells, performing inoculation to a disposable shake flask, and performing culture; step VI, when the density of the cells reaches 3*10<6> cells / ml, performing cell passage; and step VII, once the density of the cells can reach 3*10<6> cells / ml or above in 48h for continuous 10 generations or above and the motility rate of the cellsis not less than 95%, thinking that the cells are adapted to suspension culture.
Owner:上海源培生物科技股份有限公司
Method for quickly separating Schistosoma japonicum immature egg
InactiveCN101055234AImprove work efficiencyHelp removeAnimal cellsPreparing sample for investigationAntigenDrug biological activity
The invention discloses a method of fast separating Japan schistosome unfledged spawn, which employs technique of combining repeat sieving and pancreatin processing and completes in two hours. Purity of the obtained spawn is above 99%, structure of the spawn is integrated and good biological activity is remained, which can satisfy basic need of antigen extraction and analysis. Comparing to exist technique, yield of the spawn is largely increased.
Owner:汪世平 +1
Method for separate-culturing cardiomyocytes
PendingCN111154715AStrong action temperatureAvoid overdigestionCell dissociation methodsSkeletal/connective tissue cellsMedicineHeart Muscle Cell
The invention provides a method for separate-culturing cardiomyocytes. The method includes the following steps that (1) heart tissue is taken, and PBS liquid is used to wash away residual blood; the heart tissue is cut into tissue blocks of 0.1-1mm<3> with ophthalmic scissors, 0.5-5ml of collagenase I is added, and shaking digesting is carried out at 36-37.5 DEG C for 0.5-3 hours; after digestion,2.5-50mL of PBS is added for dilution and stop, centrifuging is carried out at 1200rpm / min for 5 min, and a supernatant is discarded to obtain a cell pellet; (2) the cell pellet is resuspended with 0.5-5mL pancreatin, an elbow pipette is used for blowing-beating for 10-300 times, after digestion fluid is turbid, a culture medium is added to stop pancreatin digestion, and centrifuging is carried out at 1200rpm / min for 5 min, and a supernatant is discarded to obtain primary cardiomyocytes; and (3) after the primary cardiomyocytes is resuspended in the culture medium, the primary cardiomyocytesare inoculated into the culture medium according to 0.1-10x10<5> cells / cm<2>, and the obtained mixture is placed in a culture incubator of 37 DEG C and 5%CO2 for culturing to obtain the cardiomyocytes.
Owner:GUANGDONG BOXI BIO TECH CO LTD
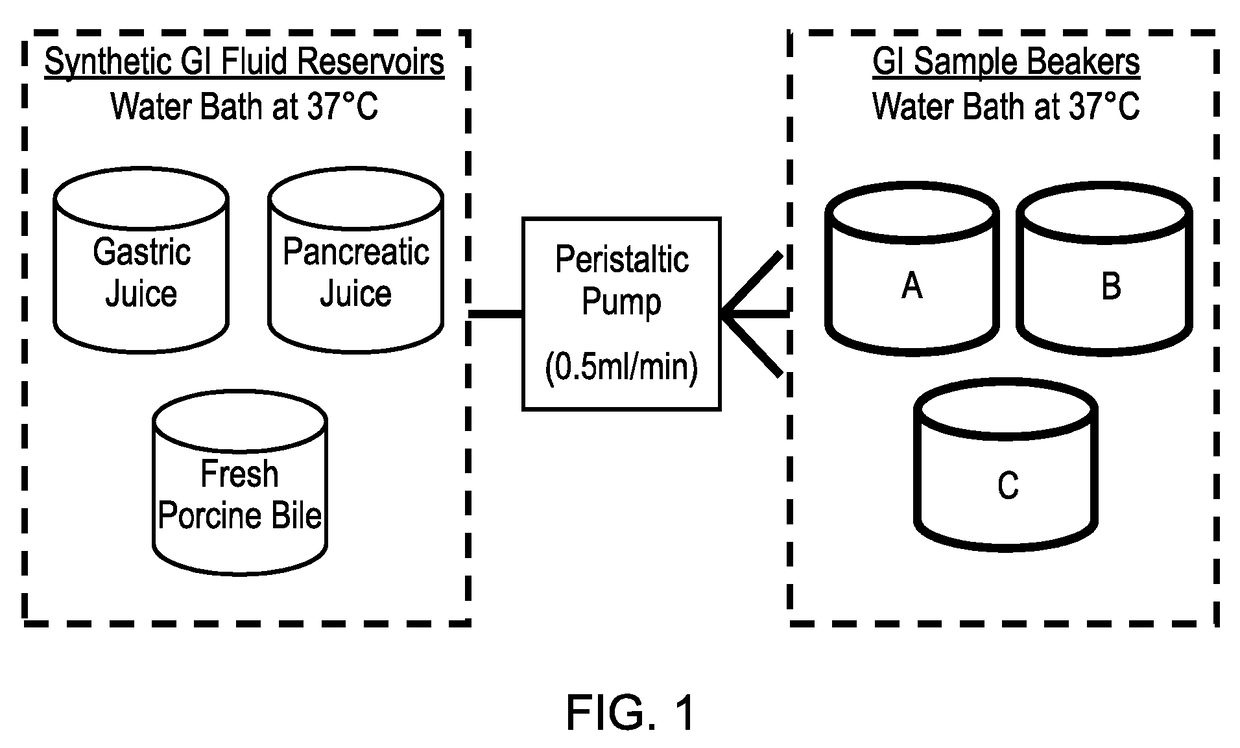
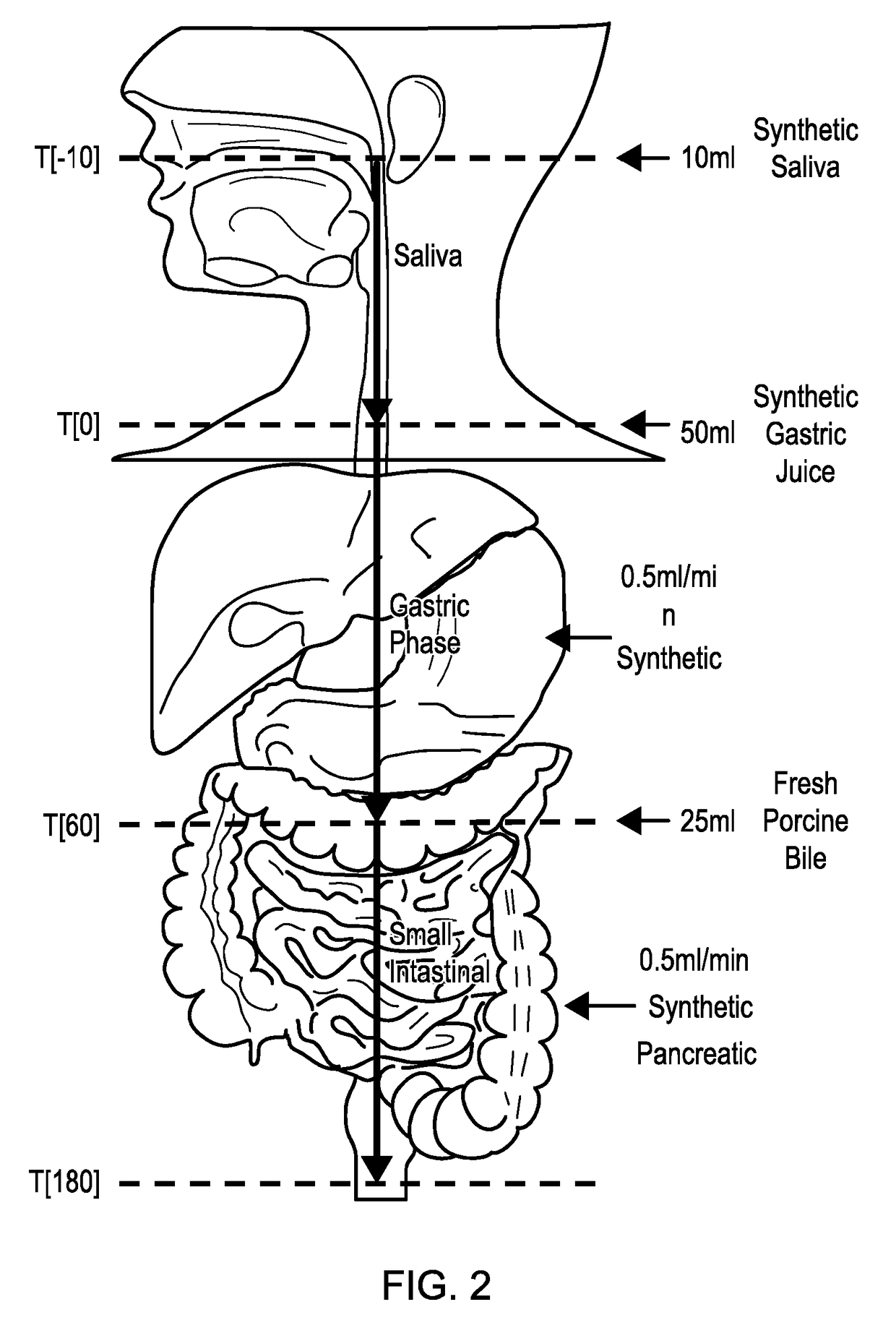
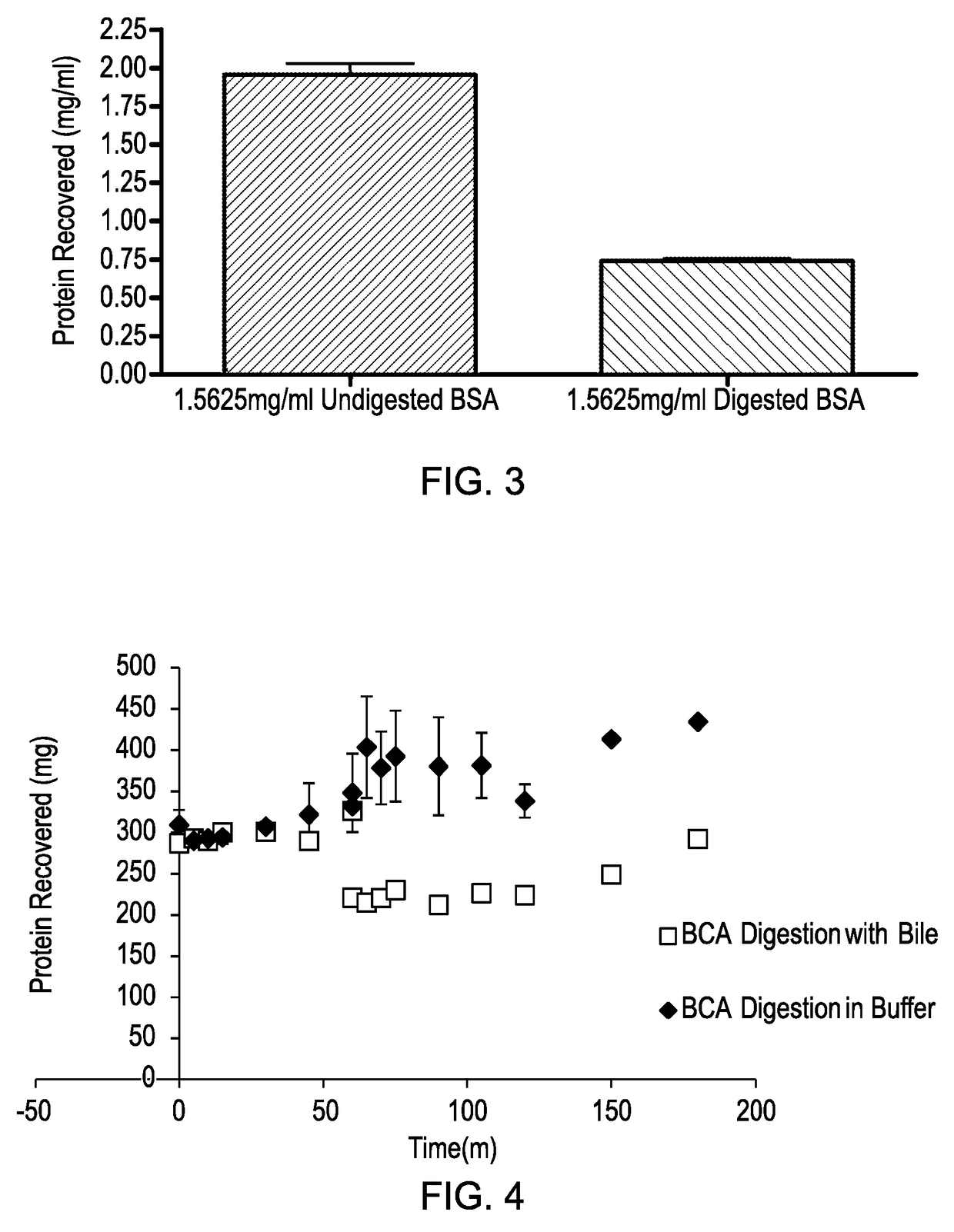
Model gut system
ActiveUS20170160283A1Cost prohibitiveSmall scaleCosmonautic condition simulationsMicrobiological testing/measurementPancreatic juiceDiluent
The invention relates to a Model Gut System (MGS) comprising a pancreatic phase consisting essentially of synthetic pancreatic juice comprising pancreatin and one or more suitable pancreatic diluent(s) at a pH from about 7 to about 9, preferably about 7.9 to about 8.2, and porcine bile.
Owner:NEWCASTLE UNIV
Cell large-scale culture method based on porous nanoscale temperature-sensitive soft colloid
PendingCN112442484APromote amplificationAmplification realizedBioreactor/fermenter combinationsBiological substance pretreatmentsDigestionBiology
The invention belongs to the technical field of biomedical materials, and particularly relates to a cell large-scale culture method based on porous nanoscale temperature-sensitive soft colloid. The method comprises the following steps: mixing the porous nanoscale temperature-sensitive soft colloid with a cell or tissue culture to obtain a mixture, conveying the mixture in a liquid state into a hollow column reactor, and performing curing at 32-37 DEG C for culture. According to the culture method, pancreatin digestion is not needed in the cell elution process, cells are not affected at all, and the cells can be conveniently amplified into reactors of more levels. Generally, the density of the cells after first-stage amplification can reach 108 per milliliter, the cells carried by the colloid are digested together through temperature changes, a previous inoculation method is repeated to input the cells into other reactors to realize amplification, or the diameter of a hollow column cavity is increased to realize volume expansion, and process amplification and culture volume amplification are realized by increasing the number of hollow fiber columns.
Owner:DALIAN PRACTICAL BIOTECH
Medicine composition pancreatin enteric capsule and preparation method thereof
ActiveCN103397013AImprove stabilityLow costPeptide/protein ingredientsDigestive systemCelluloseSucrose
The invention provides pancreatin and an enteric capsule thereof. An amino acid sequence of the pancreatin is shown in SEQIDNO:2 and the like; a particle in the pancreatin enteric capsule is composed of a pill core and a coating wrapped outside the pill core, wherein the pill core is composed of the pancreatin, cane sugar, dextrin and hydroxypropylated methylcellulose, and the coating is composed of polyacrylic resin, triethyl citrate and talcum powder.
Owner:浙江华润三九众益制药有限公司
High potency pancreatin pharmaceutical compositions
PendingCN105392496AMaintain digestive propertiesReduce volumePeptide/protein ingredientsDigestive systemBiochemistryPharmaceutical Substances
Owner:APTALIS PHARMA
Lactoalbumin hydrolysate and preparing method and application thereof
InactiveCN106086136ABiologically activeIncrease added valueCulture processArtificial cell constructsBiotechnologyHydrolysate
The invention provides a method for preparing lactoalbumin hydrolysate. Pancreatin is added to casein aqueous solution for enzymolysis lasting 4-24 h at 100-150 MPa and 40-50 DEG C, then enzymatic reaction is stopped, aftertreatment is conducted on enzymolysis products, and then lactoalbumin hydrolysate is obtained. The invention further provides lactoalbumin hydrolysate obtained with the method and the application of lactoalbumin hydrolysate. The molecular weight of the product obtained with the method is smaller than 10 kDa, and the product contains rich amino acid and polypeptide, has bioactivity, is suitable for cell culture, can serve as an additive of a low-serum or serum-free medium, and can be widely applied to food nutrition, pharmacy, healthcare food and cell culture.
Owner:NORTHWEST UNIVERSITY FOR NATIONALITIES
Composite culture medium for rapidly detecting total number of moulds and saccharomycetes and preparation method thereof
PendingCN112481350ABig shapeImprove the detection rateFungiMicrobiological testing/measurementBiotechnologyColony morphology
The invention relates to a composite culture medium for rapidly detecting total number of moulds and saccharomycetes. Every 100 g of distilled water in the composite culture medium contains 0.8-1.0 gof casein pancreatin digests, 1.0-4.0 g of glucose, 0.2-0.3 g of potassium dihydrogen phosphate, 0.05-0.2 g of sodium chloride, 0.4-0.8 g of a yeast extract, 0.4-0.8 g of rose bengal, 1.5-3 g of agar,0.1-0.2 g of chloramphenicol, 0.2-0.4 g of magnesium sulfate, 0.015-0.03 g of manganese chloride, 0.05-0.2 g of calcium chloride, and 0.1-0.25 g of Tween 60. By adopting the composite culture medium,the total number of molds and saccharomycetes in various foods can be rapidly detected, the detection rate is 20% higher than that of potato agar and rose bengal agar, the bacterial colony morphologyis large, counting is easy, and bacterial colonies are basically formed and basically grow completely within 72 hours.
Owner:NANJING ZHONGKE GRP CORP LTD +1
Injectable temperature-sensitive gel preparation for treating acute pancreatitis
InactiveCN105342984ASmall systemic side effectsSimple preparation processOrganic active ingredientsPeptide/protein ingredientsAcute pancreatitisPolyvinyl alcohol
Critical conditions of acute pancreatitis have high mortality. Trypsinization Theory is one of important pathogeneses of acute pancreatitis. Pancreatin inhibitor drugs, such as gabexate mesylate, ulinastatin and aprotinin, are selectively clinically applied against the mechanism. The routine administration mode of the drugs is intravenous injection, and has certain disadvantages. The invention provides an injectable temperature-sensitive gel preparation for treating acute pancreatitis. The dosage form of the above gel comprises a poloxamer polymer, a polylactide-co-glycolide / polyethylene glycol block copolymer, a polycaprolactam / polyethylene glycol block copolymer, a chitosan / beta-sodium glycerophosphate system, a chitosan / polyvinyl alcohol system or a chitosan-sodium bicarbonate system, or an arbitrary combination thereof. The gel has a reverse gelling characteristic, is a liquid at a low temperature, and converts to a semisolid state at a human body temperature. The invention also provides a preparation method of the injectable temperature-sensitive gel preparation for treating acute pancreatitis.
Owner:GENERAL HOSPITAL OF PLA
Mouse ovarian granulosa cell separation, culture and in-vitro injury model construction method
PendingCN112226403AHigh purityIncrease the number ofCell dissociation methodsCulture processPhysiologyGranular leucocyte
The invention discloses a mouse ovarian granulosa cell extraction and in-vitro chemotherapy injury model construction method. The method mainly comprises the following steps of 1) injecting PMSG to promote ovulation of a young mouse with age of 3-4 weeks; 2) performing mouse ovary separation and follicle breakage: dissecting the abdomen of the mouse subjected to ovulation induction for 48 hours, taking out ovaries on two sides, stripping fat tissues and connective tissues around the ovaries, puncturing follicles by using a 1 mL syringe needle under a microscope to release ovarian granulosa cells, sieving the cells with a 200-mesh sieve, and then putting the cells into a 15 mL centrifuge tube for later use; 3) digesting the ovaries with 0.25% pancreatin: putting ovary tissues after folliclebreakage into the 0.25% pancreatin, digesting theovary tissues in a water bath kettle of 37 DEG C for 30 minutes, sieving the ovary tissues with the 200-mesh sieve, putting the ovary tissues into the15 mL centrifuge tube, and centrifuging the ovary tissues to obtain a large amount of ovarian granulosa cells with higher purity; and 4) constructing an ovarian granulosa cell in-vitro chemotherapy injury model: treating the ovarian granulosa cells with cyclophosphamide (CTX) with the concentration of 5 mg / mL for 24 hours to obtain the ovarian granulosa cell chemotherapy injury model.
Owner:NANCHANG UNIV
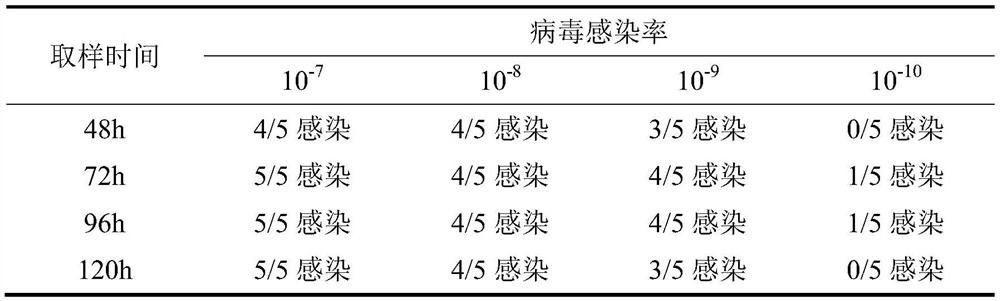
Method for determining content of Newcastle disease virus by using full-suspension cells
PendingCN112359142AShort training timeHigh sensitivityMicrobiological testing/measurementMicroorganism based processesNewcastle disease virus NDVViral test
The invention provides a method for determining content of Newcastle disease virus by using full-suspension cells, and belongs to the field of virus detection. The method mainly comprises the following steps: 1) diluting the newcastle disease virus into different dilutions; 2) inoculating into full-suspension duck embryo retina cells for culturing; and 3) determining HA titers with different dilutions and calculating virus titer; wherein in the step 2), pancreatin needs to be added during virus inoculation; wherein the final concentration of pancreatin is 10-20 [mu]g / ml. Through comparison, the method disclosed by the invention can be used for detecting the Newcastle disease virus in a relatively short time, and is superior to a traditional chick embryo detection method.
Owner:成都史纪生物制药有限公司
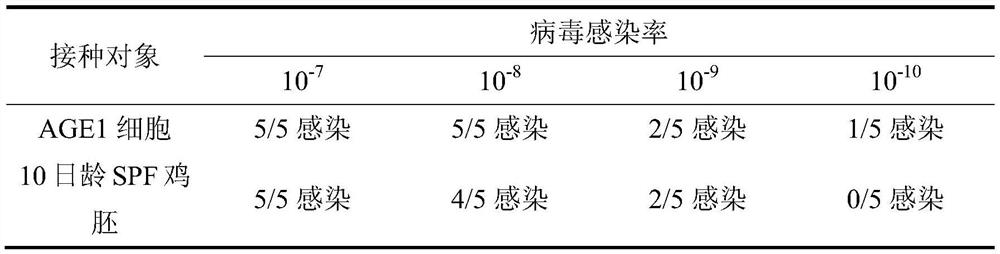
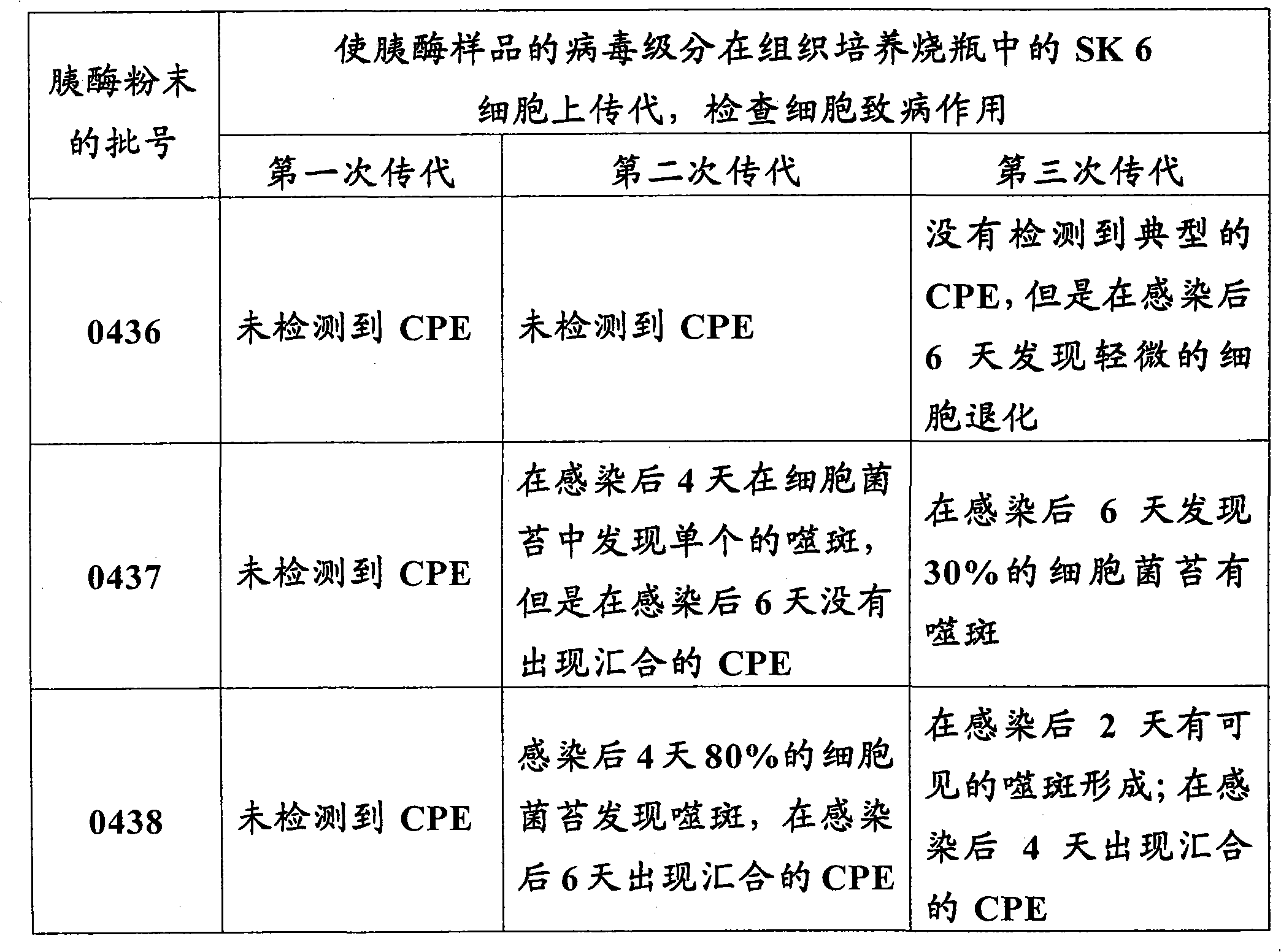
Novel process for separating and determining the viral load in a pancreatin sample
ActiveCN101821389AMicrobiological testing/measurementPancreatinBiochemical engineeringQuantitative determination
Processes for separating a viral load from a pancreatin sample and for quantitatively determining the viral load in a pancreatin sample with high sensitivity are described herein.
Owner:ABBOTT LAB INC
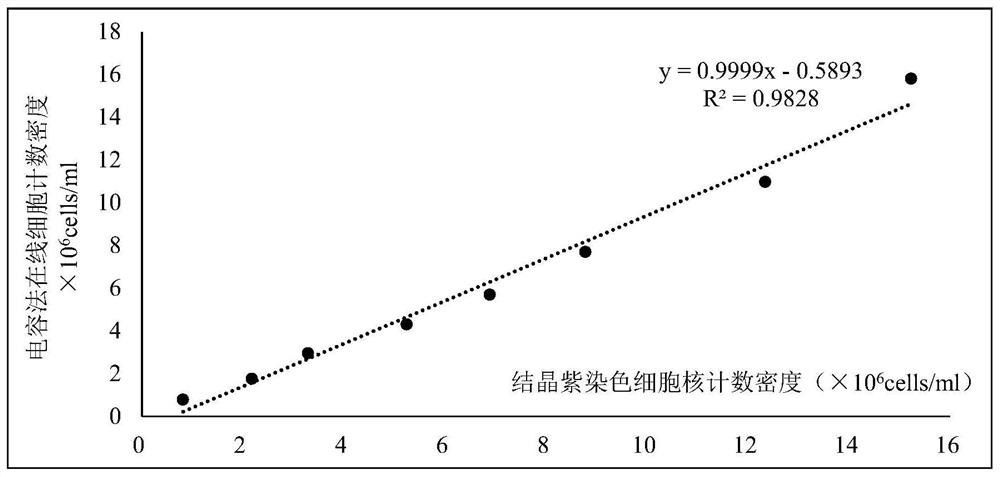
Digestion staining solution and crystal violet staining cell nucleus counting method
PendingCN112393961AReduce counting errorsPreparing sample for investigationIndividual particle analysisStainingDigestion
The invention belongs to the technical field of biochemistry, and particularly relates to a digestion staining solution and a crystal violet staining cell nucleus counting method. In order to solve the problems that pancreatin digestive juice digestion time is not easy to control in a sheet-shaped carrier adherent culture mode, and digested cells cannot be completely separated from a net-shaped space in the prior art, the invention provides the digestive staining solution which is a mixed solution of crystal violet, citric acid and triton X100. The crystal violet staining cell nucleus countingmethod comprises the following steps of: (1) mixing a carrier carrying cells with the digestion staining solution, and staining and digesting the mixture to obtain a staining solution; and (2) carrying out cell nucleus counting on the staining solution. The invention further provides application of triton X100 in digesting adherent cells in a cell nucleus counting process. According to the crystal violet staining cell nucleus counting method, the phenomena of cell damage and even death caused by overlong time in the digestion process can be reduced, so that the accuracy of cell counting is improved.
Owner:成都柏奥特克生物科技股份有限公司
Method for culturing kidney cancer organ
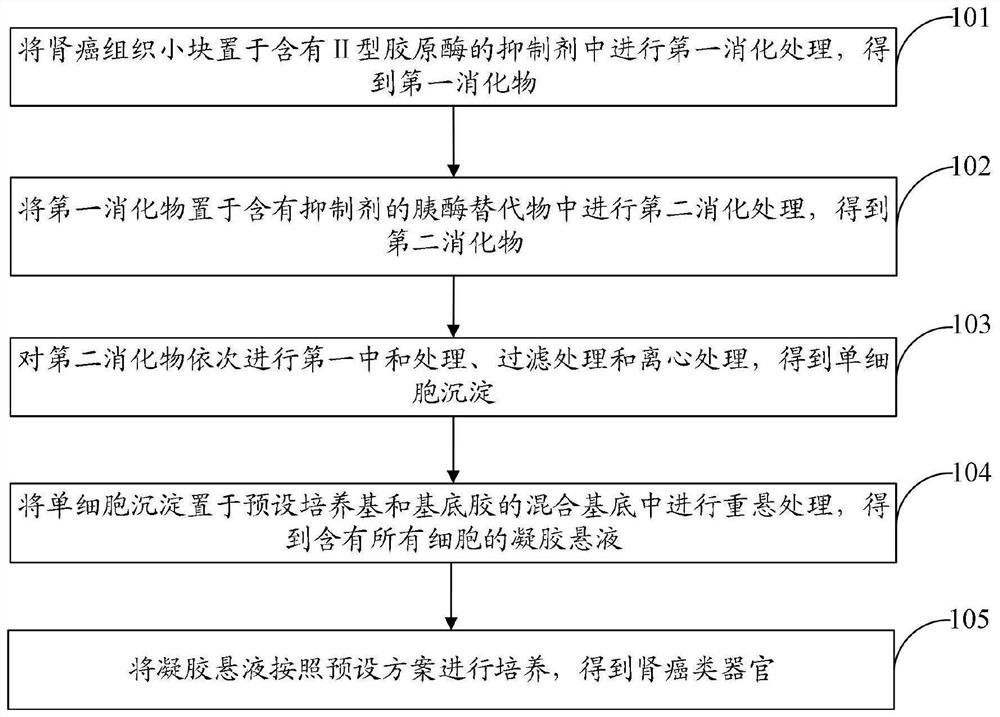
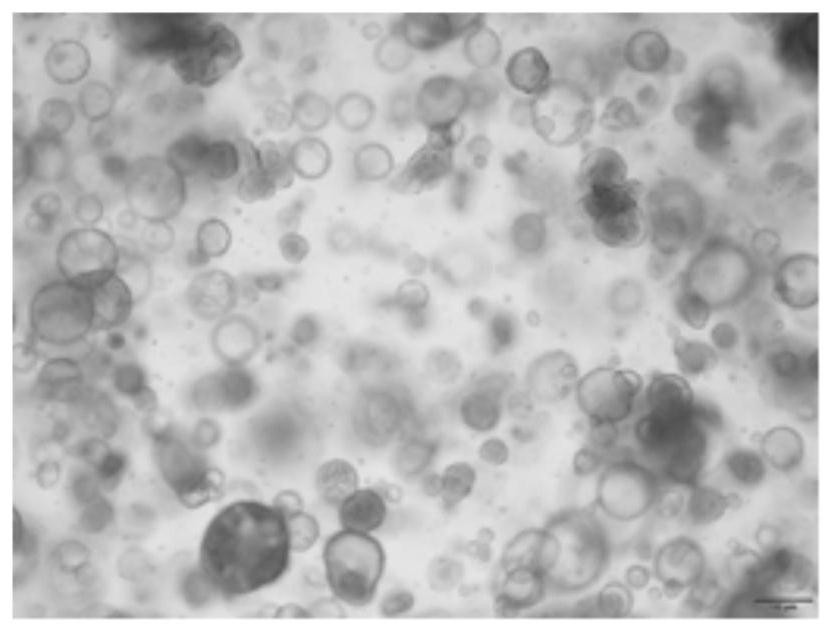
InactiveCN113717926ARetain heterogeneityImprove accuracyCell dissociation methodsArtificial cell constructsSmall kidneysKidney cancer
The invention discloses a method for culturing a kidney cancer organ. The method comprises the following steps: placing small kidney cancer tissue blocks in an inhibitor containing type II collagenase, and carrying out first digestion treatment to obtain a first digested substance; placing the first digested substance in a pancreatin substitute containing an inhibitor, and carrying out second digestion treatment to obtain a second digested substance; sequentially carrying out first neutralization treatment, filtering treatment and centrifugal treatment on the second digested substance to obtain single-cell precipitate; placing the single-cell precipitate in a mixed substrate of a preset culture medium and substrate glue for resuspension treatment to obtain a gel suspension containing all cells; and culturing the gel suspension according to a preset scheme, thereby obtaining the kidney cancer organ. According to the scheme, the accuracy of an expected treatment result can be improved.
Owner:深圳明澳生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com