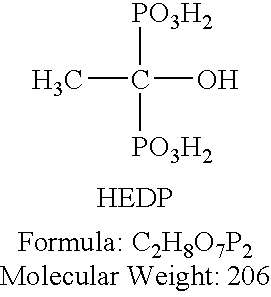Patents
Literature
61 results about "Tetrapotassium pyrophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
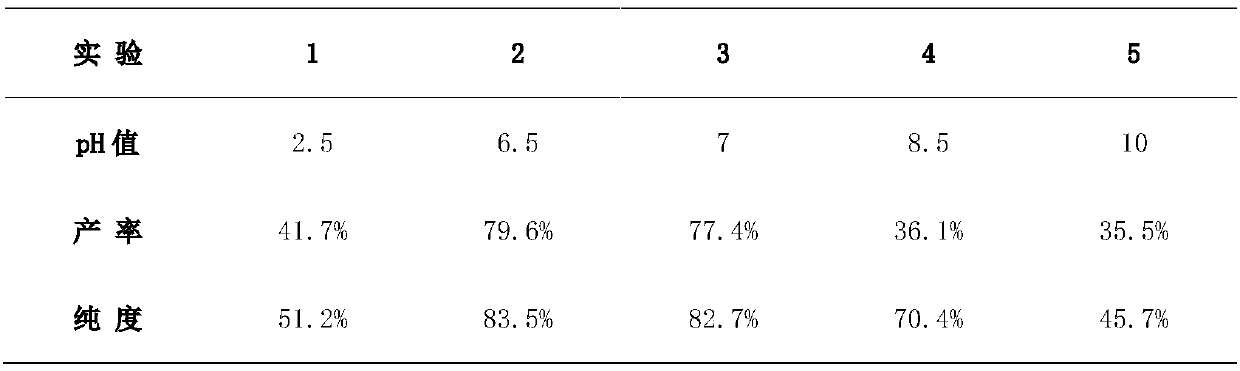
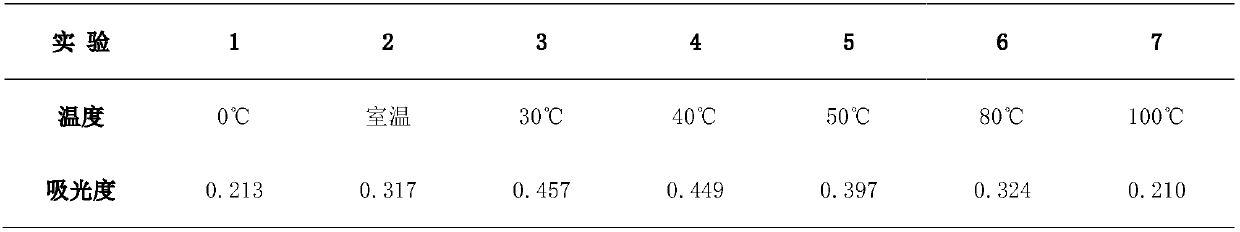
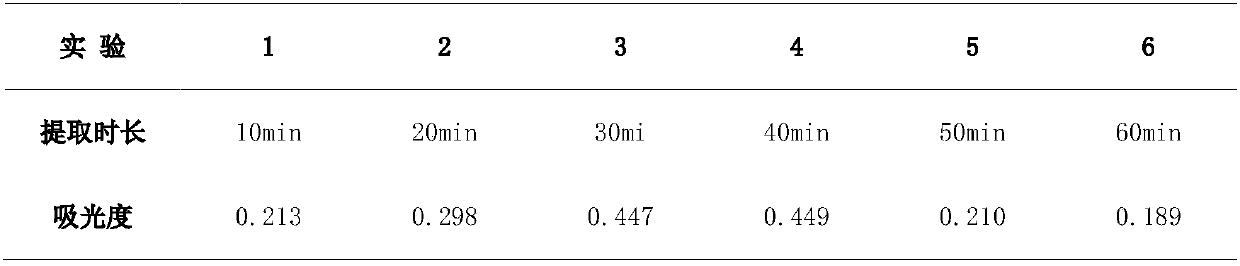
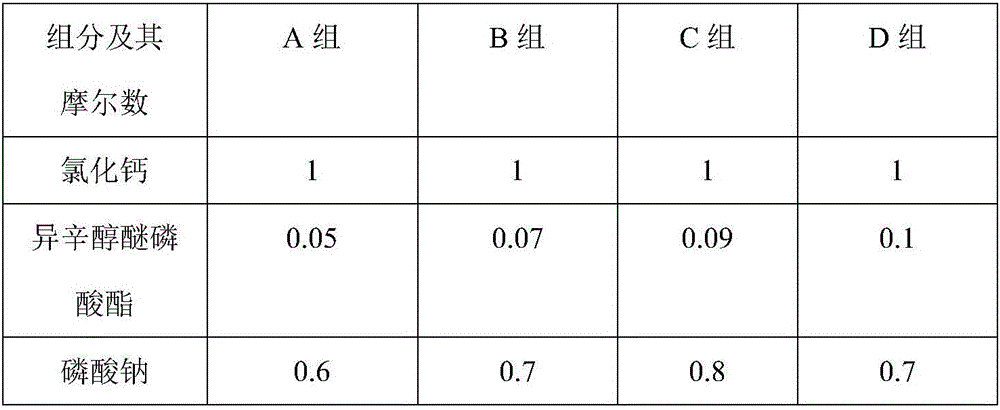
Tetrapotassium Pyrophosphate (TKPP) is a potassium phosphate derivative with excellent chelating capabilities and a variety of food and industrial uses.
Methods and compositions for enhancing palatability of animal feed using tetrapotassium pyrophosphate
InactiveUS20050037108A1Improve palatabilityLow production costAnimal feeding stuffAccessory food factorsPotassiumTetrapotassium pyrophosphate
Compositions and methods for enhancing the palatability of dry and semi-dry animal feed compositions, particularly extruded cat food compositions, are described. In one embodiment, the palatability enhancer is a dry cat food coating including tetrapotassium pyrophosphate at about 0.1% to about 1.0% by weight of the finished pet food product. In the case of extruded pet food products, the palatability enhancer is applied after the extrusion process to the extruded particles or pieces of pet food.
Owner:MEOW MIX CO THE
Solar cell silicon slice detergent and method for using same
InactiveCN101735891ANo harmNo pollution in the processInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsSodium metasilicatePolyvinyl alcohol
The invention provides a solar cell silicon slice detergent and a method for using the same and belongs to the technical field of solar cell production. The detergent is prepared by DZ1, DZ2 and water in a volume ratio of 5 to 15:1 to 10:100, wherein the DZ1 comprises sodium alkyl benzene sulfonate, sodium metasilicate pentahydrate, polyvinyl alcohol, polyethylene glycol and the water in a mol ratio of 5:7:3:7:6:72; and the DZ2 comprises ammonium hydroxide, hydrogen peroxide and the water the mol ratio of 5:15:80. The method for using the detergent comprises the following steps: placing a cut mono-crystalline silicon piece in the detergent and ultrasonically cleaning the cut mono-crystalline silicon piece at the temperature of between 40 and 70 DEG C for 5 to 20 minutes. The cleaning time and the cleaning temperature are accordant with the concentration of DZ1 and DZ2 in the detergent, so surface grease produced by linear cutting of the mono-crystalline silicon and metallic impurities in the silicon slice are effectively removed.
Owner:浙江向日葵聚辉新能源科技有限公司
Alkaline chlorine-containing foam detergent
InactiveCN104531382ASolve the problem of easy breeding of microorganismsSolve difficultyOrganic detergent compounding agentsAmpholytes/electroneutral surface-active compoundsAlkanePotassium
The invention relates to an alkaline chlorine-containing foam detergent which comprises the following components in percentage by mass: 0.1-2 percent of 60% secondary alkane sulphonate sodium, 2-8 percent of 50% dodecyl ether bisulphonate sodium, 0-2 percent of OP-10, 0-8 percent of 35% N-oxidized-N,N-dimethyl-1-lauryl amine (dodecyl dimethyl amine oxide), 0.3-5 percent of 30% N,N-dimethyl decylalkyl-N-amine oxide, 0-1 percent of tetrapotassium pyrophosphate, 40 percent of laurinol polyoxyethylene ether sulphate sodium, 0.8-6 percent of dodecyl polyoxyethylene ether sodium sulfate, 2-20 percent of potassium hydroxide, 0.9-6 percent of 48% polyacrylic acid of which the molecular weight is 4500, 12-40 percent of 12% sodium hypochlorite, 0-1 percent of EDTA and 1-81.9 percent of water, wherein the summation of the weight percentages of all components is 100%. The alkaline chlorine-containing foam detergent disclosed by the invention can be used for solving the problem of washing of food processing equipment which is easy to generate microorganisms, hard to wash and irregular in a washing surface, has the effects of being easy to wash and improving the washing efficiency, is quite high in re-sedimentation resistance, and can be used for dissolving organic matters such as milk fat and protein in production dirt residuals.
Owner:INNER MONGOLIA HEXI AEROSPACE TECH DEV
Corrosion inhibitor
InactiveUS20060043341A1Reduce and prevent formationAvoid it happening againOther chemical processesAmmonium nitrate fertilisersAcetodiphosphonic acidO-Phosphoric Acid
A corrosion inhibitor for use with Urea Ammonium Nitrate solutions is disclosed, comprising a blend of molybdate and one or more of the inorganic phosphates (including phosphates, polyphosphates, and pyrophosphates) and organic phosphates or phosphonates. Inorganic phosphates include, but are not limited to, SHMP (Sodium Hexametaphosphate) and TKPP (Tetra-Potassium Pyrophosphate). Phosphoric acid may also be utilized to provide phosphate ions, by itself or in combination with other phosphorous containing compounds as a secondary inhibitor. There are numerous other inorganic phosphates that will also serve as suitable secondary inhibitors. Organic phosphates or phosphonates include, but are not limited to, HEDP (1-Hydroxyethylidine-1,1-diphosphonic acid; also known as ethanol diphosphonate, acetodiphosphonic acid, or etidronic acid), ATMP (aminotri(methylenephosphonic acid)), PBTC (Phosphonobutane tricarboxylic acid), DETPMP (Diethylenetriaminepenta(methylene phosphonic acid)), and HPA (hydroxyphosphono acetic acid). There are numerous other organic phosphates and phosphonates that will also serve as suitable secondary inhibitors. The amount of molybdate can range from 1 ppm to 500 ppm by weight of fertilizer solution, with the preferred range from 10 ppm to 200 ppm. Amounts of inorganic or organic phosphate can also range from levels as low as 1 ppm up to 500 ppm, with the preferred range being from 5 ppm to 50 ppm.
Owner:TRAHAN SCOTT DAVID +1
Alkaline cleaner for yoghourt equipment
InactiveCN104479920AAvoid depositionEasy to rinseOrganic non-surface-active detergent compositionsInorganic non-surface-active detergent compositionsPotassiumWater quality
The invention relates to an alkaline cleaner for yoghourt equipment. The alkaline cleaner for yoghourt equipment is mainly used for cleaning the yoghourt equipment. The alkaline cleaner for yoghourt equipment comprises the following composition components in percentage by mass: 20-40% of sodium hydroxide, 1-8% of potassium hydroxide, 2-10% of tetrapotassium pyrophosphate, 0.5-4% of nitrilotriacetic acid, 0.1-1.5% of sodium gluconate, 0-8% of ethylenediamine tetraacetic acid, 0.2-2% of maltodextrin, 1-8% of peregal-20, 0-1% of OP-10, 0-2% of sodium tripolyphosphate and 15.5-75.2% of water, wherein the total sum of the percentages by mass of the various components is 100%. A preparation method comprises uniformly mixing and stirring the various components. The invention aims at providing an efficient and safe alkaline cleaner for yoghourt equipment in the dairy industry. The alkaline cleaner for yoghourt equipment disclosed by the invention is few in foams, easy to wash, capable of improving cleaning efficiency, suitable for water qualities with different hardness, and capable of preventing incrustation sedimentation; the compound is high in content and provides a suspension action on insoluble dirt, thus achieving a great re-sedimentation-resisting capacity; organic substances such as milk fats, proteins and mammary calculus in production residues can be dissolved.
Owner:INNER MONGOLIA HEXI AEROSPACE TECH DEV
Novel cleaning agent for rubber tyres
InactiveCN103205330ACationic surface-active compoundsSurface-active non-soap compounds and soap mixture detergentsPotassiumCleansing Agents
The invention relates to a novel cleaning agent for rubber tyres. A formula of the novel cleaning agent comprises 5.0 parts of sodium dimethyl benzene sulfonate (40%), 3.0 parts of potassium silicate, 20.0 parts of alkylbenzene sulfonic acid, 1.0 part of CMC-Na, 7.2 parts of diethanolamine, 0.1 part of flourescent brightener, 120 parts of tetrapotassium pyrophosphate, and the balance being purified water. The novel cleaning agent has a very good cleaning function, does no harm to coatings on the surface of the rubber, can play a role of polishing, and has an environment protection function.
Owner:SHANGHAI SHILU INFORMATION TECH
Alkaline cleaner and preparation method thereof
InactiveCN107099395AReduce economic costsEasy to cleanInorganic/elemental detergent compounding agentsNon-ionic surface-active compoundsSodium metasilicatePotassium
The invention discloses an alkaline cleaner and a preparation method thereof. The alkaline cleaner is prepared from 50-80g of fatty alcohol-polyoxyethylene ether, 80-100g of coconut oil alcohol acylamide, 20-40g of triethanolamine oleate, 80-100g of monoethanolamine, 10-20g of benzotriazole, 10-20g of EDTA disodium, 20-30g of liquid citric acid, 16-18g of silicon-potassium acid, 14-15g of alkyl benzene sulfonic acid, 25-27g of tetrapotassium pyrophosphate, 13-14g of sodium metasilicate, 30-34g of diethanol amine, 7-9g of sodium dimethyl benzene sulfonate, 11-12g of sodium hydroxide and 700-800g of water. The alkaline cleaner has the characteristics that the alkaline cleaner is environment-friendly and nontoxic and safe, the economic cost is low and the cleaning effect is good.
Owner:广州市极合技术咨询有限公司
Alkaline foam detergent
InactiveCN107574029AComplete biodegradationCompletely degradedInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsTetrapotassium pyrophosphateSodium hydroxide
The invention discloses an alkaline foam detergent. The alkaline foam detergent is prepared from the following components in percentage by mass: 12.0 to 15.0 percent of tea seed powder, 3.0 to 5.0 percent of sodium lauryl polyoxyethylene ether sulfate, 3.0 to 5.0 percent of N-oxidized-N,N-dimethyl-1-dodecylamine, 3.0 to 5.0 percent of sodium hydroxide, 2.0 to 5.0 percent of sodium dodecyl diphenylether disulfonate, 2.0 percent to 3.5 percent of tetrapotassium pyrophosphate, 1.0 to 2.0 percent of N,N-dimethyldecylamine-N-amine oxide, 0.5 to 1.0 percent of seconary alkane sulphonate sodium, 0.5to 1.0 percent of PVP, 0.5 to 1.0 percent of OP-10 and the balance of water, totally being 100 percent. By adopting the detergent disclosed by the invention, foaming power (the height after 5 minutes) is more than 160mm, detergency is more than 98 percent, and the time requiredfor enabling the biodegradability to be more than 90 percent is less than 24 hours; moreover, the bio-degradation is carried out completely and the degradation speed is high; the antiredeposition ability is greatly improved, and the alkaline foam detergent has an obvious sterilization and disinfection effect.
Owner:CHENGDU MINSHENG DISINFECTANT
Vehicle scratch cleaning agent
InactiveCN104404540AStrong ability to break down stainsImprove removal effectSodium acetateSodium phosphates
The invention discloses a vehicle scratch cleaning agent, which comprises the following components: tetrapotassium pyrophosphate, potassium hydroxide, potassium silicate, sodium perchlorate, citric acid, dimethyl tetradecyl amine oxide, sodium cumenesulfonate, magnesium chloride, glucose monocaprate, diglycolamide laurate, sulfonated potassium succinate, ether sodium, monoethanolamine, bentonite, sodium sulfite, sodium nitrilotriacetic acid, sodium tripolyphosphate, sodium disilicic acid, alkyl polyoxyethylene ether, sodium perborate, lauryl dimethyl amine oxide, sodium chloride, potassium sorbate, soybean lecithin, hexaglycerol monolaurate, tetrasodium ethylenediamine tetraacetate and sodium polyacrylate. The vehicle scratch cleaning agent has the beneficial effects that the vehicle scratch cleaning agent has better removal effects on multiple stains, high stain decomposition capability and a broad market prospect, toxicity and harms are eliminated in production and using processes, and chemical residues after long-term use are avoided.
Owner:卢家雄
Industrial heavy oil contamination cleaning agent
InactiveCN104893833AEasy to cleanFast cleaningInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsTransformerPotassium
The invention relates to an industrial heavy oil contamination cleaning agent. The industrial heavy oil contamination cleaning agent comprises 50-70 parts of water, 100-120 parts of tetrapotassium pyrophosphate, 7-20 parts of diethanolamine, 11-25 parts of alkyl benzene sulfonic acid, 20-50 parts of surfactant, 6-20 parts of essence, 5-12 parts of sodium hydroxide, 8-20 parts of lipase and 3-10 parts of potassium silicate. The industrial heavy oil contamination cleaning agent has the advantages that the industrial heavy oil contamination cleaning agent has an excellent cleaning effect, is little in dosage and can be used for cleaning heavy oil contamination on surfaces of various processing machines, machine tools, transformers, power distribution cabinets, water grindstones and the like, the clearance ratio of oil contamination can reach 95%, the cleaning speed of users is accelerated by using the cleaning agent, all the used raw materials are wide in sources and low in cost, the preparation method is simple, practicable, and convenient to implement, and the industrial heavy oil contamination cleaning agent has stable quality and can be stored for a long time.
Owner:时修官
Carrageenan and konjac glucomannan special composite gum for meat products and preparation process thereof
The invention provides carrageenan and konjac glucomannan special composite gum for meat products and a preparation process thereof, the carrageenan and konjac glucomannan special composite gum comprises kappa carrageenan, konjac glucomannan and tetrapotassium pyrophosphate, the weight part addition ratio of kappa carrageenan to konjac glucomannan is 5: 5-9: 1, the added amount of the tetrapotassium pyrophosphate by weight is 10-20% of the total weight of kappa carrageenan and konjac glucomannan. The preparation process comprises the steps of wet mixing, drying, crushing and the like. The technical problems that in the prior art, carrageenan and konjac glucomannan are easy to flow when heated in meat products, and the synergistic effect is greatly weakened are solved. The preparation process is reasonable in design, the prepared carrageenan and konjac glucomannan special composite gel is high in strength and not prone to flowing after being heated, and meat products are high in quality in all aspects.
Owner:青岛海之林生物科技开发有限公司
Tableware detergent and preparation method thereof
InactiveCN104762143AGood decontamination and washing effectNourish the skinAmpholytes/electroneutral surface-active compoundsDetergent compounding agentsAmylaseBetaine
The invention provides a tableware detergent and a preparation method thereof. The detergent comprises betaine, trisodium citrate, ethylene glycol monobutyl ether, sodium chloride, amylase, pectase, elastin hydrolysate, a tetrapotassium pyrophosphate aqueous solution, coconut oil diethanolamine condensate, hydroxylated acylated soybean lecithin, decyl glucoside, myristic amide propyl amine oxide, palmitic acid, and deionized water. The preparation method comprises: adding betaine, trisodium citrate, ethylene glycol monobutyl ether, sodium chloride, and the tetrapotassium pyrophosphate aqueous solution into one part of the deionized water to obtain a mixed solution I; adding amylase, pectase, elastin hydrolysate, and hydroxylated acylated soybean lecithin into the other part of the deionized water to obtain a mixed solution II; finally, mixing the mixed solution I and the mixed solution II; then, adding the left components; and performing stirring to obtain the final product. According to the invention, the detergent not only has an excellent decontamination and washing effect, but also can effectively nourish, soften, and moisten the hand skin.
Owner:SUZHOU GULI BIOTECH
Preparation method of instant and highly-efficient phosphate for food ingredients
InactiveCN110236171AInstantImprove qualityInorganic compound food ingredientsFood dryingDipotassium hydrogen phosphateSodium phosphates
The invention discloses a preparation method of instant and highly-efficient phosphate for food ingredients, and relates to the technical field of phosphate preparation. The preparation method includes the following steps: mixing sodium phosphate monomer, dispersing cosolvent potassium phosphate and water into homogeneous phase material liquid, subjecting the homogeneous phase material liquid to spraying granulation and drying dehydration, and finally obtaining the instant highly-efficient phosphate product for food ingredients. The sodium phosphate monomer is one or two or more than two kinds of monomer sodium phosphate; the dispersing cosolvent potassium phosphate is potassium triphosphate, potassium pyrophosphate, potassium polymetaphosphate, potassium dihydrogen phosphate, dipotassium hydrogen phosphate, tribasic potassium phosphate or potassium polyphosphate; the addition of dispersing cosolvent potassium phosphate accounts for 5%-10% of the total weight of sodium phosphate monomer and dispersing cosolvent potassium phosphate. The product obtained by the method can reach homogeneous phase mixing at the molecular level, and the obtained product is easy to disperse, free of agglomeration or absorption of moisture, good in mouthfeel, excellent in dissolution speed and capable of giving full play to a synergistic effect.
Owner:江苏恒世食品科技有限公司
Environmentally-friendly metal product cleaner and preparation method thereof
The invention provides an environment-friendly cleaning agent for metal products and a preparation method thereof, relating to the field of cleaning agents, comprising the following components: 15-22 parts of sodium polyacrylate, 13-17 parts of trichlorethylene, and 5-8 parts of fatty alcohol polyoxyethylene ether , 8‑10 parts of tetrapotassium phosphate, 5‑9 parts of co-emulsifier, 5‑10 parts of sodium sucrose fatty acid, 12‑16 parts of sodium pyrophosphate, 4‑6 parts of trisodium phosphate, 5‑8 parts of sodium citrate, poly 5-8 parts of carboxylic acid water-based dispersant, 2-5 parts of pH regulator, 4-6 parts of toluene, 1-2 parts of sodium stearate, 10-20 parts of polyvinyl alcohol, 8-12 parts of fatty alcohol polyoxyethylene ether 5-8 parts of glycerol, 2-5 parts of anionic surfactant, 1-5 parts of corrosion inhibitor, 23-35 parts of deionized water; Dust, sand, bird droppings, shellac, and other dirt on component glass; the cleaning agent of the present invention is environmentally friendly, non-toxic and non-polluting, and the method of use of the present invention is simple. It only needs to be sprayed on the surface of metal products, and then wipe dry with cotton cloth after slightly drying That's it, no need to rinse with water.
Owner:四会市启德信息咨询服务有限公司
Cleaning agent for damping rubber pad
InactiveCN109706003AEasy to cleanImprove cleanlinessInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsPhosphatePotassium
The invention discloses a cleaning agent for a damping rubber pad. The cleaning agent is composed of acetic acid, polydimethylsiloxane, potassium silicate, copper sulfate pentahydrate, tetrapotassiumpyrophosphate, phosphate, a surfactant, ethanol, citric acid, butyl acetate and water. A raw material composition comprises the following components in parts by weight: 15-25 parts of acetic acid, 6-12 parts of polydimethylsiloxane, 8-10 parts of potassium silicate, 5-10 parts of copper sulfate pentahydrate, 6-10 parts of potassium pyrophosphate, 15-20 parts of phosphate, 8-12 parts of a surfactant, 6-9 parts of ethanol, 10-15 parts of citric acid, 6-8 parts of butyl acetate and 45-65 parts of water. The cleaning agent prepared by the invention can be used to effectively clean out oil stains and dust on the damping pad, and neatness of the damping pad is improved, so that competitiveness of an enterprise is improved. In addition, hands and rubber products are not damaged, the smell is fresh and non-pungent, environment friendliness is achieved, use is safe, and the cleaning effect is good.
Owner:JIANGSU LUOSHI DAMPING MEMBER CO LTD
Paper coating formulation
InactiveUS20140295173A1Increase brightnessCoatings with pigmentsSynthetic resin layered productsOptical propertyMedicine
The present invention relates to a laminate comprising coated or uncoated paperboard coated with a film that comprises a polymeric binder, TiO2, and tetrapotassium pyrophosphate. Paper or paperboard coated with a film containing tetrapotassium pyrophosphate shows excellent optical properties.
Owner:DOW GLOBAL TECH LLC
Efficient environment-friendly cleaning agent
InactiveCN106987435AEasy to cleanReduce pollutionInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsTetrapotassium pyrophosphateEnvironmental chemistry
The invention discloses an efficient environment-friendly cleaning agent. The efficient environment-friendly cleaning agent comprises xylenesulfonic acid sodium salt, pyrophosphoric acid, silicon formic acid, alkylphenol ethoxylates, rose otto, acid blue fluorescent powder, and N,N-bis(2-Hydroxyethyl) coco-amide. Through the method, in the efficient environment-friendly cleaning agent, after mutual coordination of all kinds of components, the cleaning effect is good, obstinate dirt can be clearly cleaned, the efficient environment-friendly cleaning agent is poisonless and harmless, causes less pollution to the environment after being used and discharged to the environment, and thus the efficient environment-friendly cleaning agent has no influence on the environment and is beneficial to the development of the human being.
Owner:SUZHOU YONGCHUANGDA ELECTRONICS
Laundry soap
InactiveCN104804912AEasy to removeReduce stimulationSurface-active non-soap compounds and soap mixture detergentsSulfite saltDioxyethylene Ether
Laundry soap is prepared from the following raw materials in parts by weight: 6-8 parts of sodium carbonate, 7-9 parts of sodium lauryl sulfate, 3-6 parts of fatty alcohol-polyoxyethylene ether, 4-11 parts of sorbierite, 2-4 parts of a preservative, 8-12 parts of tetrapotassium pyrophosphate, 3-6 parts of sodium chloride, 7-11 parts of polyacrylic acid, 6-10 parts of thiourea dioxide, 3-8 parts of anhydrous sodium sulphate, 2-6 parts of heptahydrate sodium sulfite, 1-3 parts of propylene glycol, 4-12 parts of soluble essence, 2-6 parts of alkyl glycoside, 2-4 parts of mature vinegar, 2-6 parts of mica powder, 9-16 parts of lard oil, 8-14 parts of fatty acid diethanol amide and 2-6 parts of a soap base. The laundry soap has the benefits that the germs on clothes can be well cleaned, meanwhile the clothes are enabled to be soft and glossy, and irritation to a human body is less.
Owner:QINGDAO KELIKE INFORMATION TECH
Cleaning agent for rubber product
InactiveCN107779272AEasy to cleanThe smell is fresh and not pungentInorganic/elemental detergent compounding agentsSurface-active detergent compositionsAcetic acidPolymer science
The invention discloses a cleaning agent for rubber products, which is composed of the following raw materials: 25-35 parts of acetic acid, 15-18 parts of citric acid, 0.2-0.8 parts of ethanol and 12-12 parts of surfactant 14 parts, the phosphate content is 20-27 parts, the tetrapotassium pyrophosphate content is 18-20 parts, and the water content is 65-80 parts. The cleaning agent for rubber products of the present invention can effectively clean off oil stains and dust on the surface of rubber products, does not harm hands and rubber products, has fresh and non-pungent taste, is safe to use, and has good cleaning effect.
Owner:南通市亨大利橡胶制品有限公司
Fruity oil stain cleaning agent and preparation method thereof
ActiveCN110184127AImprove decontamination abilityLowering the freezing pointInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsPotassiumSolvent
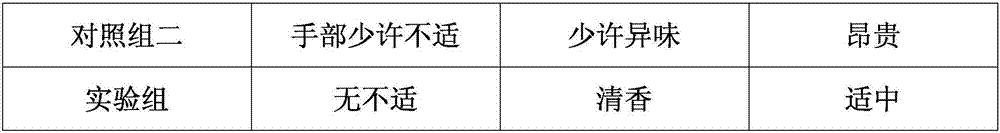
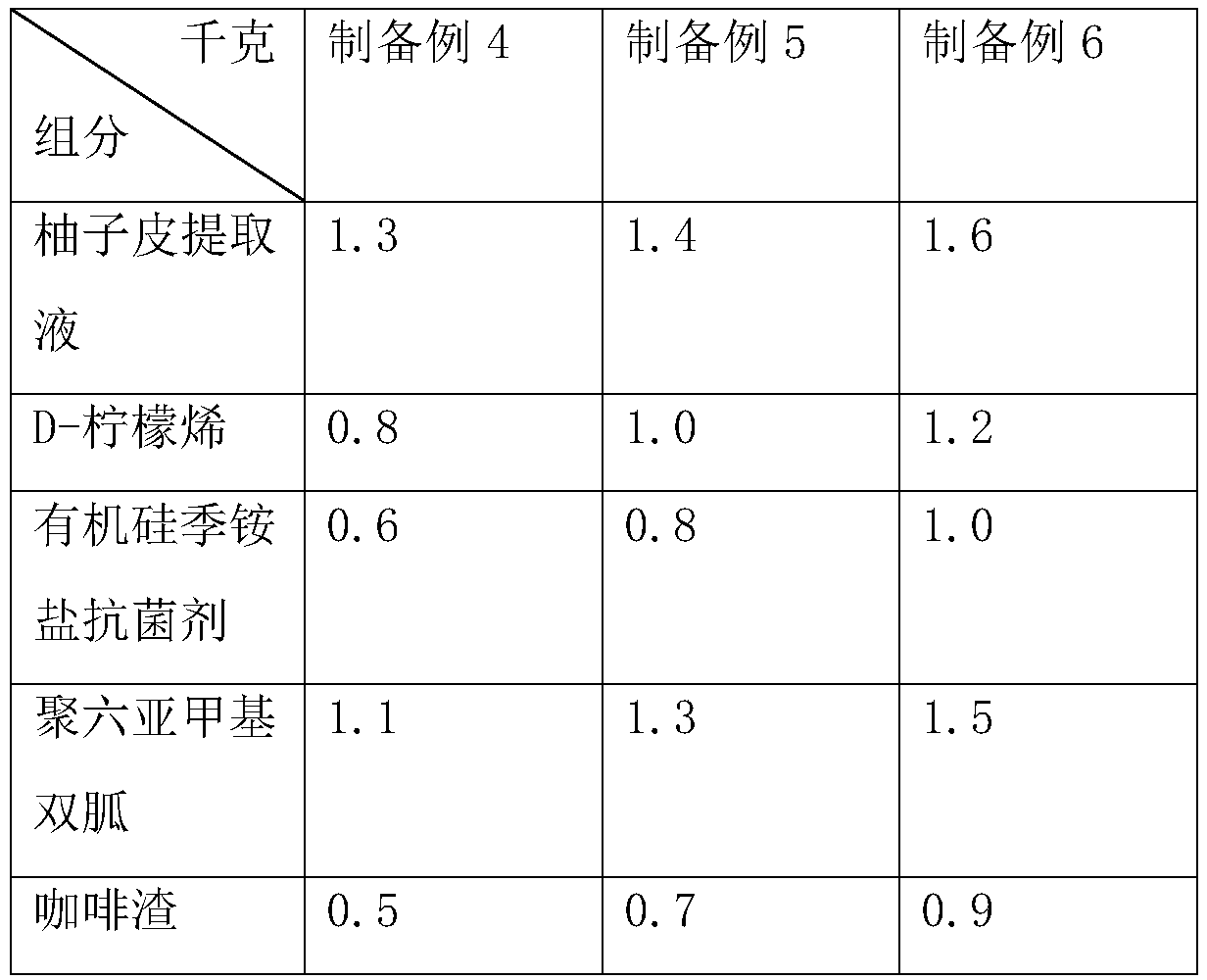
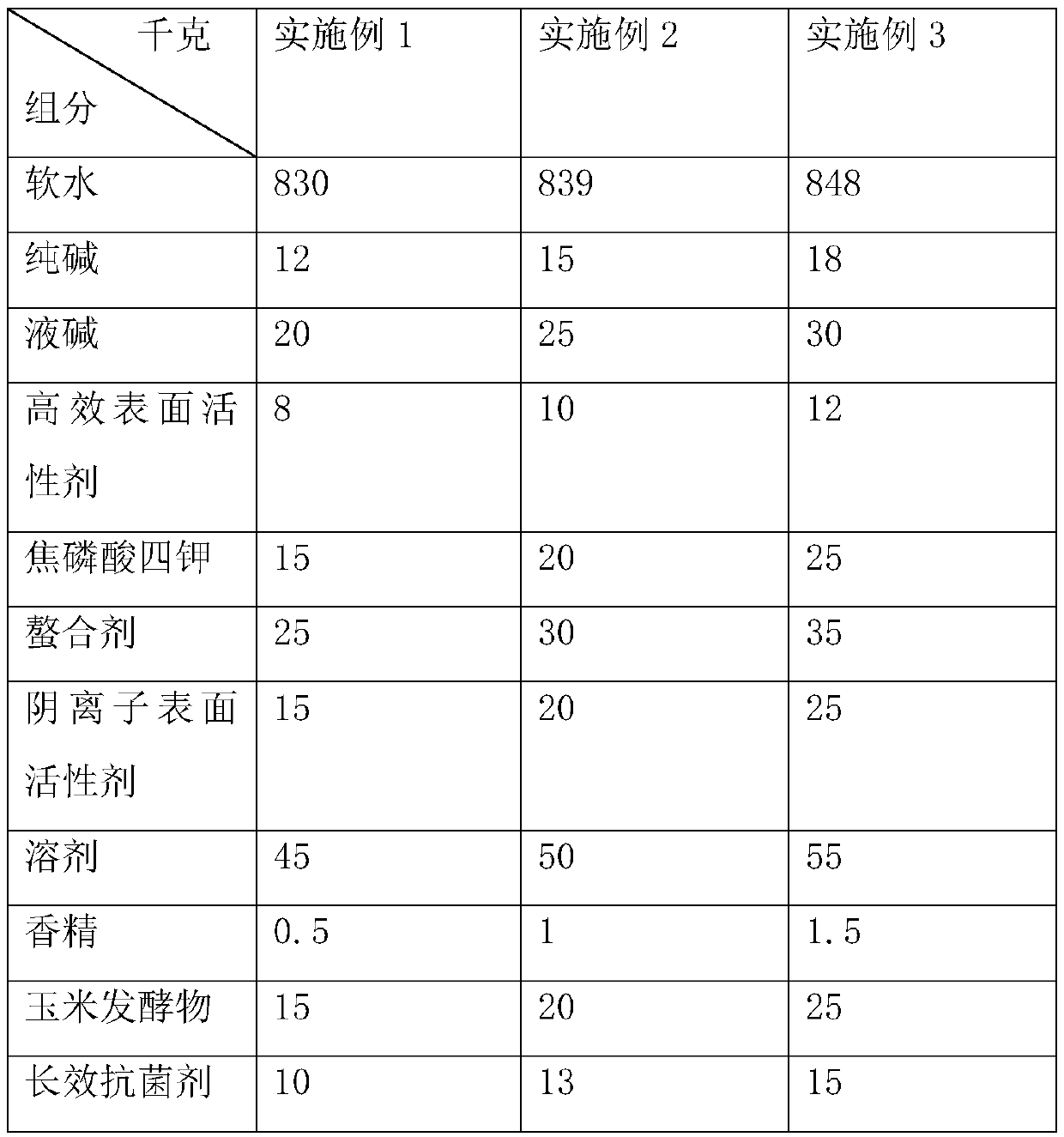
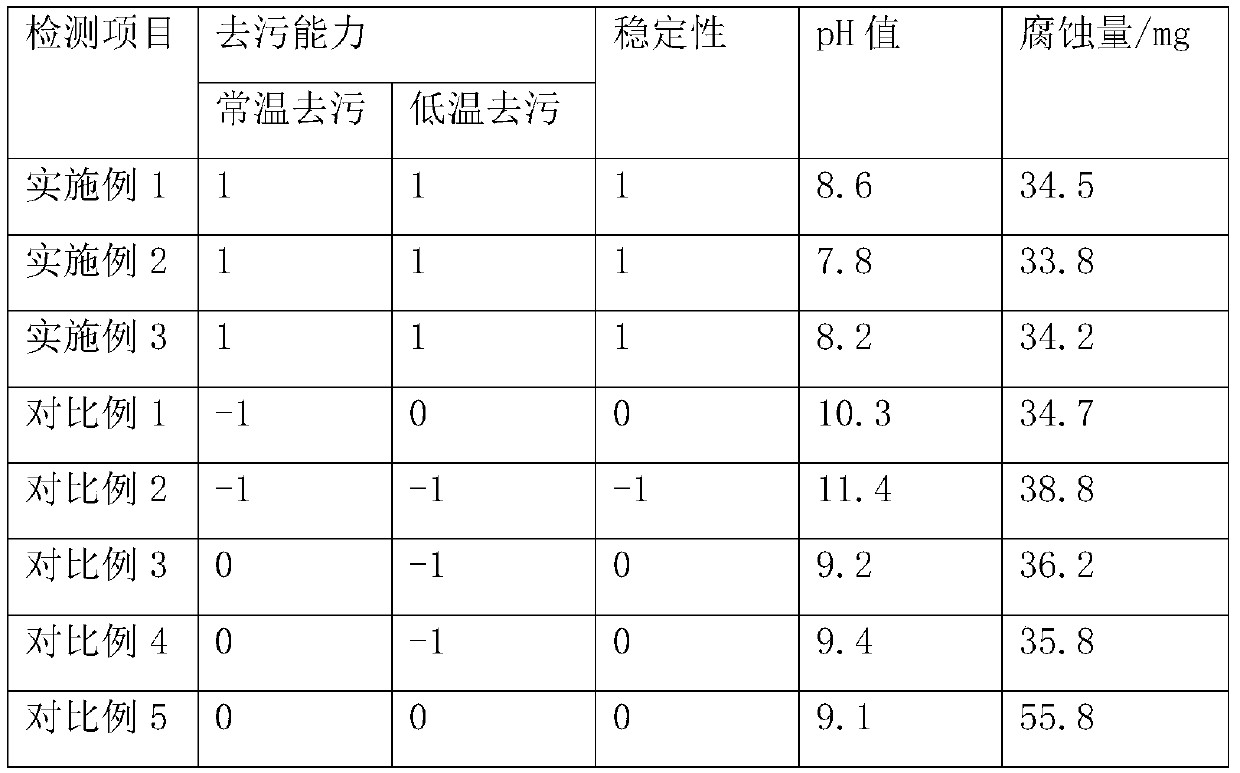
The invention discloses a fruity oil stain cleaning agent and a preparation method thereof. The fruity oil stain cleaning agent is prepared from the following components in parts by weight: 830-848 parts of soft water, 12-18 parts of soda ash, 20-30 parts of liquid alkali, 8-12 parts of high performance surfactants, 15-25 parts of tetrapotassium pyrophosphate, 25-35 parts of chelating agents, 15-25 parts of anionic surfactants, 45-55 parts of solvents, 0.5-1.5 parts of essences, 15-25 parts of corn ferment, and 10-15 parts of long-acting antibacterial agents; the long-acting antibacterial agents are prepared from the following components in parts by weight: 1.3-1.6 parts of grapefruit peel extract, 0.8-1.2 parts of D-limonene, 0.6-1.0 part of silicone quaternary ammonium antibacterial agents, 1.1-1.5 parts of polyhexamethylene biguanide, and 0.5-0.9 parts of coffee grounds. The fruity oil stain cleaning agent disclosed by the invention has the advantages of long-term sterilization andbetter cleaning effect in a low temperature environment.
Owner:杭州卓妙实业有限公司
N-2, 3-dimethyl-2-isopropyl butyrylamide dental plaque preventing mouth wash
InactiveCN101732176AReduce contaminationPlaque suppressionCosmetic preparationsToilet preparationsGlycerolPotassium
The invention relates to N-2, 3-dimethyl-2-isopropyl butyrylamide dental plaque preventing mouth wash comprising cetylpyridinium chloride, tetrapotassium pyrophosphate, alcohol, sulfuric acid (10%), glycerin, essence, N-2, 3-dimethyl-2-isopropyl butyrylamide, polyoxyethylene (20) sorbitan monostearate, saccharin and purified water. The dental plaque preventing mouth wash is spit out after being contained in a mouth for one minute, has the pH value of 7.0-9.5, and can restrain the dental plaques and reduce the calculus.
Owner:高庆亮
Banana peel yellow pigment and extraction method thereof
ActiveCN110484018AReduce the degree of oxidationReduce lossesNatural dyesFiltrationResource utilization
The invention belongs to the technical field of pigment extraction, and discloses banana peel yellow pigment and an extraction method thereof. The extraction method comprises (1) chopping clean bananapeel with the soft part in the inner layer removed, then soaking the chopped banana peel in a specially-made color protection solution, then soaking the banana peel in a tetrapotassium pyrophosphateaqueous solution, and performing filtration to obtain filter dregs; (2) sequentially subjecting the filter dregs to extraction using ethanol, acetone, ethyl acetate, petroleum ether, and other solvents, and subjecting solid to repeated extraction with a recovered mixed solvent until extract liquid is colorless; and (3) combining the extracting liquid, concentrating the mixture into paste, adding water into the paste concentrate, ultrasonically mixing the paste concentrate and the water, carrying out rotary evaporation to a viscous state, freezing the product at low temperature, and carrying out vacuum drying to obtain yellow pigment particles. The extraction method disclosed by the invention is simple to operate, low in cost, and high in extraction efficiency, the extracted pigment is stable and not easy to oxidize, the development and utilization value of the banana peel is improved, the resource utilization of the waste banana peel is promoted, and the yellow pigment has relatively high economic value and social value.
Owner:椰枫堂(广州)生物科技有限公司
Retarder for desulfurization building gypsum
The invention relates to a retarder for desulfurization building gypsum. The retarder for the desulfurization building gypsum is made of raw material by proportion: 20-30% of citric acid, 10-15% of tetrapotassium pyrophosphate, 2-5% of sodium alkyl sulfate and 50-68% of edible salt. The retarder for the desulfurization building gypsum is a retarder which is obtained by using the citric acid, the tetrapotassium pyrophosphate and the sodium alkyl sulfate as main coagulation delaying ingredients and adding the edible salt, contains few additives, is good in retarding effect, and simultaneously causes little loss to strength of the gypsum.
Owner:GUANGXI HUILONG SCI & TECH CO LTD
Automobile polishing agent
InactiveCN108084890AStrong ability to break down stainsImprove removal effectInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsSodium BentoniteSulfite salt
The invention discloses an automobile polishing agent. The automobile polishing agent comprises tetrapotassium pyrophosphate, potassium hydroxide, potassium silicate, sodium perchlorate, citric acid,dimethyltetradecylamine oxide, sodium cumenesulfonate, gluconic monodecanoate, lauroyl diethanolamide, potassium sulfonated succinate, sodium ether sulfate, monoethanolamine, bentonite, sodium sulfite, sodium nitrilotriacetate, sodium tripolyphosphate, sodium mesosilicate, alkyl polyoxyethylene ether, sodium perborate, lauryl dimethylamine oxide, sodium chloride, potassium sorbate, soya lecithin,hexaglycerol monolaurate, tetrasodium EDTA and sodium polyacrylate. The automobile polishing agent has strong stain removal effects and strong ability of decomposing stains, is non-toxic and harmlessduring production and use, prevents chemical residues after long-term use and has a good market prospect.
Owner:HAIAN KEHAO TEXTILE CO LTD
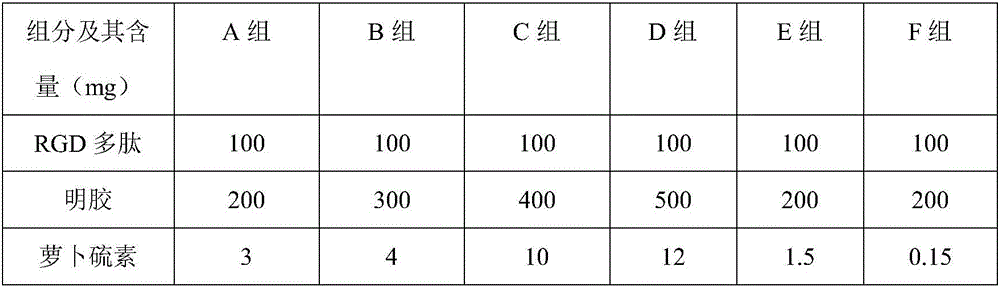
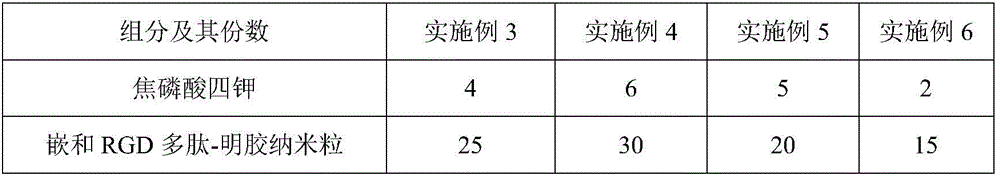
Anti-tartar toothpaste and preparation method thereof
InactiveCN106806164AImprove solubilityEfficient removalCosmetic preparationsToilet preparationsMicrospherePotassium
The invention relates to anti-tartar toothpaste and a preparation method thereof. The toothpaste is prepared from the following components in parts by mass: 2 to 6 parts of tetrapotassium pyrophosphate, 15 to 30 parts of nested RGD polypeptide-gelatin nanparticles, 1 to 4 parts of polymethylacrylic acid 2-hydroxyl ethyl ester, 10 to 25 parts of amorphous calcium phosphate microspheres, 1 to 4 parts of lipoic acid, 1 to 4 parts of a surfactant, 1 to 3 parts of distannous citrate and 60 to 80 parts of a matrix. The toothpaste provided by the invention is extremely high in cleaning capacity for the oral cavity, is high in friction property and safety, can effectively clear tartars and whiten teeth, cannot injure the teeth and destroy enamels, and has a certain defense effect on decayed teeth and the tartars; furthermore, the toothpaste is uniform in texture, has a delicate taste, and is rich in foam and stable in property; even if the toothpaste body of the toothpaste is placed for a long time, no water appearance caused by layering is caused; if people use the toothpaste for a long time, a healthy oral cavity environment can be effectively retained, and the teeth can be protected.
Owner:缪来耿 +1
Rubber product cleaning agent
InactiveCN107779307AEasy to cleanThe smell is fresh and not pungentOrganic non-surface-active detergent compositionsInorganic non-surface-active detergent compositionsPolymer sciencePotassium
The invention discloses a cleaning agent for rubber products, which is composed of the following raw materials: 30-32 parts of acetic acid, 25-28 parts of citric acid, 12-18 parts of polydimethylsiloxane, silicon The content of potassium sulfate is 12-14 parts, the content of copper sulfate pentahydrate is 20-27 parts, the content of tetrapotassium pyrophosphate is 8-10 parts, and the water content is 70-80 parts. The cleaning agent for rubber products of the present invention can effectively clean off oil stains and dust on the surface of rubber products, and does not harm hands and rubber products, has a fresh and non-pungent taste, is safe to use, and has good cleaning effect.
Owner:南通通江橡胶制品有限公司
Mild laundry soap
InactiveCN103834502AWith whitening effectAvoid damageAlkali/ammonium soap compositionsPotassiumLaundry
The invention relates to the field of detergents, and particularly relates to mild laundry soap which is prepared by mixing the following raw materials in parts by weight: 9-15 parts of sodium oleate, 18-25 parts of tetrapotassium pyrophosphate, 1-5 parts of brightening agent, 5-10 parts of sodium chloride, 10-18 parts of lauric acid, 7-20 parts of lard oil, 1-3 parts of essence and 5-10 parts of silicate. According to the mild laundry soap provided by the invention, the formula is reasonable, the cleaning efficacy is good, the cleaning power is much stronger than that of common laundry soap, and a brightening effect is realized on the fabric while little injury is caused to the hands.
Owner:马俊杰
Vehicle scratch cleaning agent
InactiveCN106675831AStrong ability to break down stainsImprove removal effectInorganic/elemental detergent compounding agentsNon-ionic surface-active compoundsSodium BentoniteSulfite salt
The invention discloses a vehicle scratch cleaning agent which comprises the following components: potassium pyrophosphate, potassium hydroxide, potassium silicate, sodium perchlorate, citric acid, oxidized N,N-dimethyltetradecylamine, sodium cumenesulfonate, glucose monodecanoate, lauroyl diethanolamide, sulfonated potassium bisuccinate, sodium ether sulfate, monoethanolamine, bentonite, sodium sulfite, sodium nitrilo triacetate, sodium tripolyphosphate, sodium silicate, alkyl polyoxyethylene ether, sodium perborate, lauryl dimethyl amine oxide, sodium chloride, potassium sorbate, soya bean lecithin, hexaglycerol monolaurate, EDTA (ethylenediaminetetraacetic acid) tetrasodium and sodium polyacrylate. The invention has the beneficial effects that the vehicle scratch cleaning agent provided by the invention has a strong effect of removing various stains, is high in stain decomposing capacity, non-toxic and harmless in the production and usage process and excellent in market prospects, and does not have any chemical residue even if used for a long time.
Owner:NANTONG BOTAI ART PATTERN DESIGN CO LTD
Novel silk fabric dry cleaning fluid and preparation method thereof
InactiveCN104862090ADoes not damage the internal structureDoes not change glossOrganic detergent compounding agentsSurface-active detergent compositionsPotassiumTetrapotassium pyrophosphate
The invention relates to a novel silk fabric dry cleaning fluid and a preparation method thereof. The dry cleaning fluid comprises the following components, by mass: 24-36 parts of hydroxyethyl dimethyl stearyl ammonium p-toluenesulfonate, 1.8-5 parts of xylene, 0.6-1.3 parts of diethanolamide laurate, 5-13 parts of triethylene glycol, 6-15 parts of sodium dioctylsulfosuccinate, 5-13 parts of lightweight sodium silicate, 1-4 parts of hydrogenated tallow primary amine, 0.5-3 parts of sodium perborate, 2-5 parts of sodium chloride, 0.08-0.18 parts of methyl paraben, 3-7 parts of Chinese insect wax, 5-10 parts of tetrapotassium pyrophosphate, and 1-5 parts of water. With the dry cleaning fluid, silk fabric surface stains can be effectively removed without damaging the internal structure of silk. The reinfection resistance is good. With the dry cleaning fluid, the original gloss of the silk fabric is not changed.
Owner:湖州市石淙万红丝织厂
Novel resin solvent capable of being used for recovery of aged motor vehicle lampshade
The invention relates to a novel resin solvent capable of being used for recovery of an aged motor vehicle lampshade. The novel resin solvent comprises the following components: 35 of No. 0 white oil, 12 of tetrapotassium pyrophosphate, 35-40 of vegetable oil, a small amount (less than 2) of triethanolamine, 0.1% of silicone oil, 1% of a varnish, 7.2 of diethanolamine, 0.1 of a fluorescent brightener, and the balance being water. The novel resin solvent capable of being used for the recovery of the aged motor vehicle lampshade has the main characteristics that a finished product has strong lubrication degree after precipitating for 24 hours according to the above formula, has very strong maintenance function, can repair the aged motor vehicle lampshade to shine like a new, has low cost, etc. Besides, the finished product is not likely to stick ashes.
Owner:SHANGHAI SHILU INFORMATION TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com