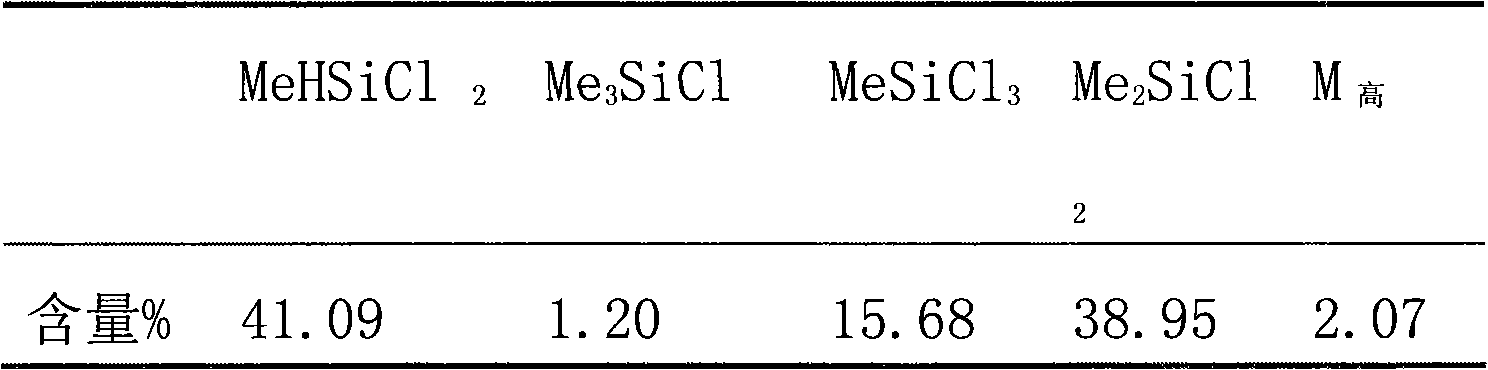
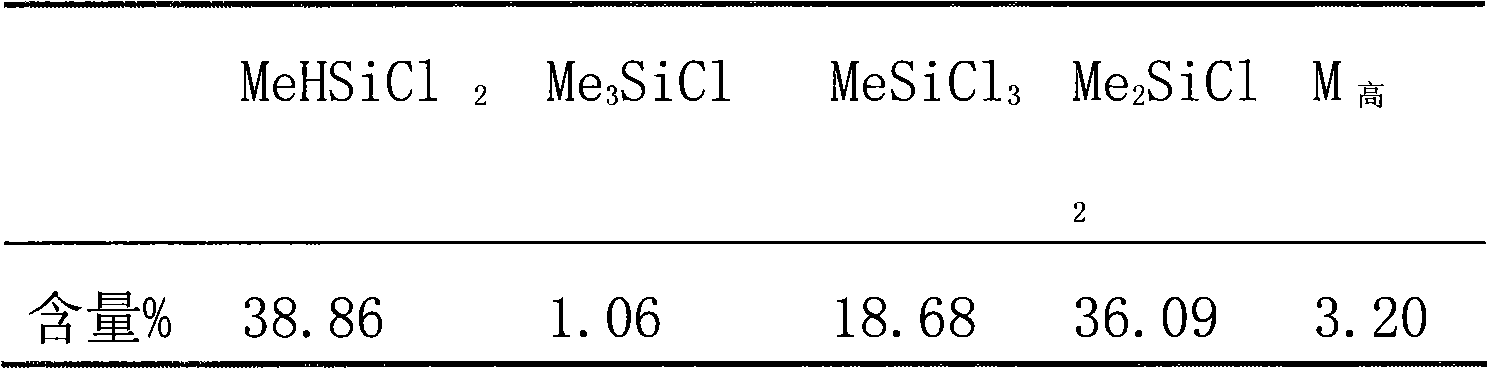
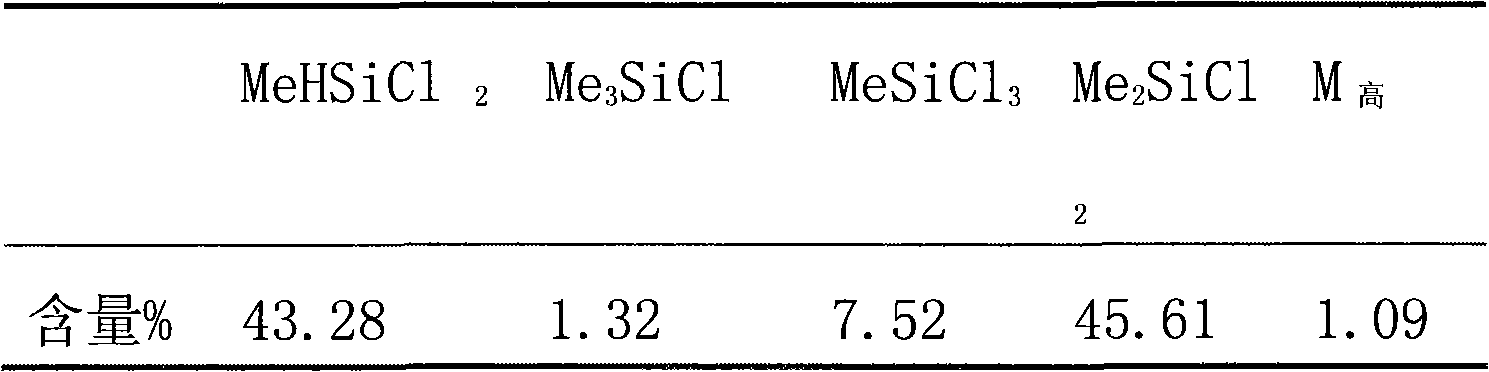
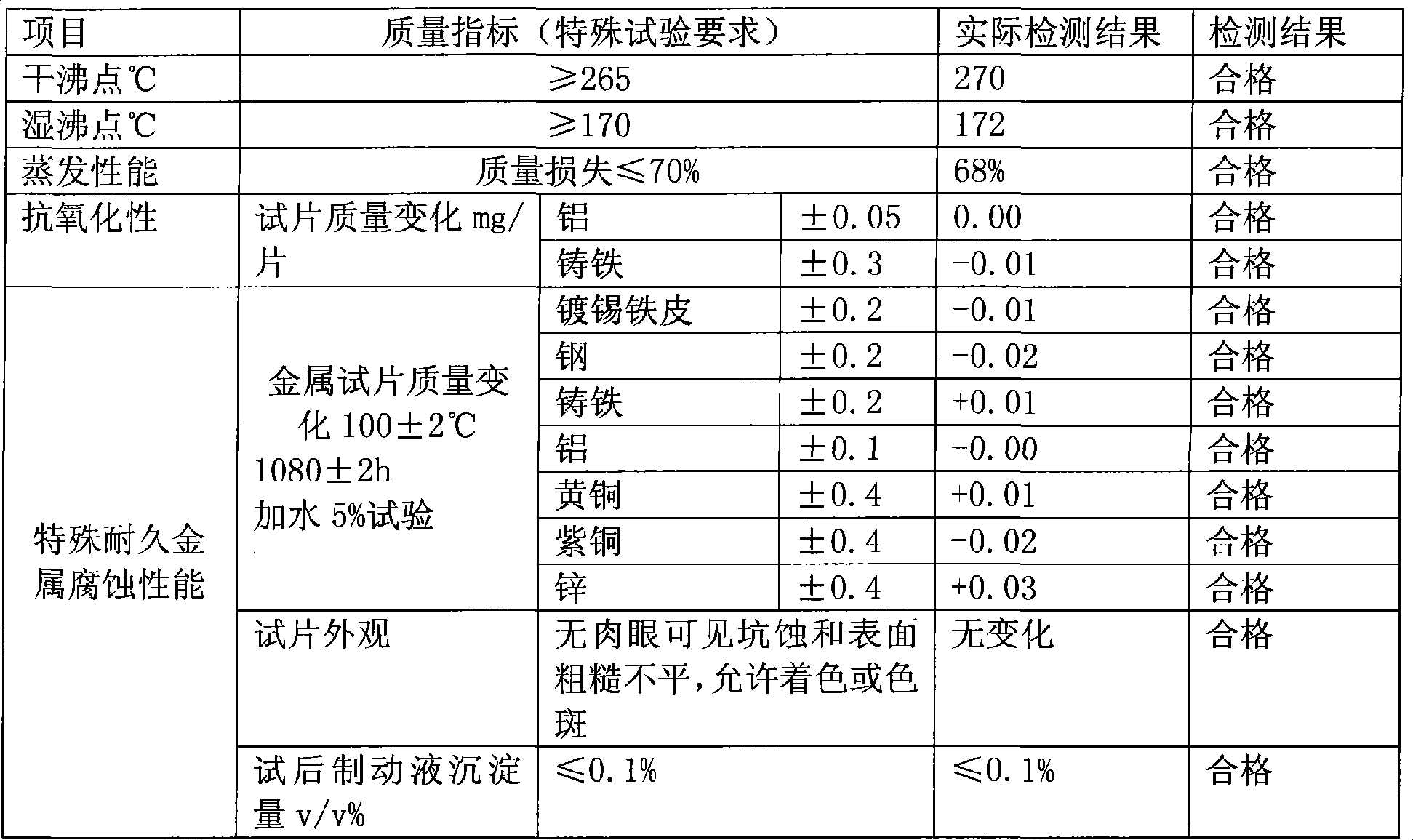
Patents
Literature
80 results about "Tri-n-butylamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
High-efficiency multifunctional water-base fire resistant fluid and preparation method thereof
InactiveCN101560400AGood water solubilityImprove solubilityFibre treatmentPaper/cardboardWater basedPolyester
The invention provides a high-efficiency multifunctional water-base fire resistant fluid and a preparation method thereof. The high-efficiency multifunctional water-base fire resistant fluid is characterized by comprising the following raw materials in portion by mass: 10 to 20 portions of ammonium phosphate salts, 4 to 8 portions of boron compounds, 1 to 3 portions of tri-n-butylamine, 2 to 8 portions of glucose, 3 to 5 portions of tertiary amine, 3 to 5 portions of urea, 1 to 3 portions of penetrant, 1 to 2 portions of surfactant, 0 to 1 portion of softener and 40 to 80 portions of water. The high-efficiency multifunctional water-base fire resistant fluid has the characteristics of clearness, achromatism, transparency, neutrality, low smoke, non-toxicity, nonirritant, noncorrosiveness, low cost and high efficiency. Inflammable materials such as polyester-cotton blend, cotton fabrics, ribless corduroy, bourette, silk-ramie, knitgoods, canvases, paper sheets, cartons and the like with hygroscopicity become fire-resistant materials after being treated by the high-efficiency multifunctional water-base fire resistant fluid, and reach the fire-resistant B1 standard stated by the country. The high-efficiency multifunctional water-base fire resistant fluid can be widely used for top-grade indoor finishing such as curtains, carpets, wall paper, paper-based finishing, wooden furniture, ceiling and the like, simultaneously has the advantages of simple construction and low cost, is a new generation of high-tech environment-friendly product, and has wide market prospect.
Owner:KUNMING UNIV OF SCI & TECH
Method for cracking treatment of organic silicon waste residue slurry
The invention relates to method for the cracking treatment of waste residues slurry produced in a synthesis process of an organic silicone monomer. The comehtod comprises: adding a high-boiling material of the same mass into a organic silicon waste residue slurry liquid and solid mixture containing 20 percent of solid to prepare a cracking feed solution, wherein a catalyst is tri-n-butylamine andthe cracking reaction temperature is 80 to 160 DEG C; and introducing HCl gas, wherein the ratio of the feed speeds of the hydrogen chloride and the raw material mixed liquid is 1:1.05-1:1.12. The method has the advantages that: the residue slurry containing 20 percent of solid is used in combination with the high-boiling material, so a monosilane product can be separated directly in a cracking process; the conversion rate is over 70 percent; the selectivity of dimethyldichlorosilane is over 35 percent; the selectivity of methylsilane is over 40 percent; and the slurry residue obtained after cracking having certain liquidity and can be further treated.
Owner:PETROCHINA CO LTD
Lithium ion battery separating membrane, preparing method thereof and applications of the separating membrane
InactiveCN104112833ALower internal resistanceImprove mechanical propertiesSecondary cellsCell component detailsPolyvinylidene fluorideAdhesive
The invention relates to a lithium ion battery separating membrane, a preparing method thereof and applications of the separating membrane. The separating membrane comprises a separating membrane substrate and an inorganic electrolyte coating covering one side or two sides of the separating membrane substrate. The inorganic electrolyte coating comprises 1-20% by weight of an inorganic component, 0.2-4% by weight of an adhesive, 0.1-1% by weight of an additive, and 75-98% by weight of a solvent. The inorganic component comprises at least one selected from LiPON, L2S-P2S5, Li10GeP2S12, Li2S-SiS2-Li3PO4, and La<0.5>Li<0.5>TaO3 solid particles. The particle size of the solid particles is 0.05-20 [mu]m. The adhesive comprises at least one of polyvinylidene fluoride, polymethyl methacrylate and polyacrylic acid (PAA). The additive comprises at least one of gamma-methacryloxypropyltrimethoxysilane, N-methylformamide, dimethylformamide, butyl butyrate and tributylamine. The solvent comprises at least one of N-methyl-2-pyrrolidone, acetone, methanol, n-heptane, isopropanol and dimethylacetamide. The lithium ion battery separating membrane has high electrical conductivity.
Owner:ZHUHAI COSMX BATTERY CO LTD
Cracking process of organosilicon high-boiling components
The invention relates to a cracking process of organosilicon high-boiling components.. The cracking process is characterized in that organosilicon high-boiling components are used as raw materials and are subjected to impurity removal, N,N-dimethylaniline, N,N-diethylformamide or tri-n-butylamine are selected as a catalyst, the catalytic cracking is performed inside a reaction vessel to which hydrogen chloride gas is introduced, and finally, dimethyldichlorosilane, monomethyltrichlorosilane, monomethyldichlorosilane and trimethylchlorosilane are respectively recovered through a layered cooling tower at different temperatures. The cracking process has the advantages that the matching is reasonable, the operation is simple, the recovery ratio is high, and more than 60% of dimethyldichlorosilane product with high added value can be obtained.
Owner:JIANGXI BLUESTAR XINGHUO SILICONE CO LTD
DOT4 boric acid ester type brake fluid
ActiveCN101418249ASolve the problem of long-term storage hydrolytic stabilityAdditivesBase-materialsMorpholinePhenol
The invention discloses a DOT4 boric acid ester type brake fluid, which comprises the following components in weight percentage: 4.3 to 4.7 percent of triethylene glycol methyl ether, 15 to 17 percent of tetraethylene glycol methyl ether, 1.9 to 2.1 percent of diethylene glycol, 74 to 76 percent of alcohol ether borate, 0.19 to 0.21 percent of benzotriazole, 0.33 to 0.37 percent of N-phenyl morpholine, 0.76 to 0.84 percent of tri-n-butylamine, 0.57 to 0.63 percent of 2, 2-di (4-hydroxyl phenyl) propane (bisphenol A), 0.057 to 0.063 percent of thiodipropionic acid didodecyl alcohol ester, 0.19 to 0.21 percent of 2.6-di-tert-butyl-4-methyl phenol, 0.095 to 0.105 percent of mercapto styrene-acrylic thiazole, and 0.38 to 0.42 percent of dodecatylene butane diacid (T746). The DOT4 boric acid ester type brake fluid has the advantages that the DOT4 boric acid ester type brake fluid can thoroughly solve the problem of hydrolytic stability of boric acid ester for long term storage by using a formulation technology of combining organic substances and additives.
Owner:张家港迪克汽车化学品有限公司
Antirust coating for steel plate and preparation method for antirust coating
The invention discloses antirust coating for steel plates and a preparation method for the antirust coating. The preparation method comprises the following steps: weighing isopropanol, nitrocellulose, sodium nitrite, methyl ethyl ketone, nitrilo-triethanolamine, alkyd resin, tributylaminem, cellosolve, petroleum asphalt, n-butyl alcohol, glycerin rosin, methylisobutylketone, heavy benzene and pigment, and filtering and packaging after mixing; the product hardness is 0.5-0.7, and the impact strength is 45-65cm; the flow time is 45-65s, and the adhesive force is first level; the water tolerance of the antirust coating is good, so that if immersed in water of 35 DEG C for 2-6d, the antirust coating does not bubble or fall off; surface dry is 1-3h, and hard dry is 8-10h.
Owner:WUXI EPIC TECH
Method for preparing methylchlorosilane with high boiling organosilicon
InactiveCN101314606AHigh selectivitySimple processGroup 4/14 element organic compoundsDimethylaniline N-oxideMethyltrichlorosilane
The invention relates to a method for preparing methyl chlorosilane from organosilicon high-boiling products. The method comprises the steps as follows: the whole composition of the high-boiling products which are the byproduct in the production process of dimethyl dichlorosilane as the organosilicon monomer is adopted as the raw material and reacts with hydrogen chloride in the presence of N,N-dimethylanlilne and tri-n-butylamine as a catalyst in a cracking kettle with a fractionating tower to obtain a mixed monomer mainly containing dimethyl dichlorosilane, trimethylchlorosilane, methyldichlorosilane and methyltrichlorosilane; and the mixed monomer is collected via the overhead condenser of the fractionating tower. The method can improve the conversion of the high-boiling products. The conversion is higher than 90%, the dimethyl dichlorosilane allows a selectivity of higher than 50%, and the selectivety of trimethylchlorosilane reaches 10%. The organosilicon monomer can be prepared at low cost.
Owner:SHANDONG DONGYUE ORGANIC SILICON MATERIAL
Antirust coating, antirust method and application of antirust coating
InactiveCN102690544AAccelerated corrosionImprove rust resistancePretreated surfacesAnti-corrosive paintsNitriteWater dispersible
The invention relates to an antirust coating, an antirust method and application of the antirust coating. The antirust coating comprises the following components: a compound with sulfo-carbonyl, water-dispersible silicon dioxide, nitrite, tri-n-butylamine and nitrilo-triethanolamine. The antirust method comprises the following steps of: applying the antirust coating on an object to be coated, and then heating the coated object by hot air to be dried; or heating the object to be coated, applying the antirust coating on the heated object, and drying the object by heat retained in the object. The antirust coating is suitable for being applied on zinc-plated steel or aluminum-plated steel or non-plated steel so as to perform antirust treatment on the zinc-plated steel or the aluminum-plated steel or the non-plated steel. The non-chrome antirust coating provided by the invention has the advantages of excellent storage stability, relatively low environmental toxicity and high antirust performance.
Owner:TIANCHANG JULONG TRAVEL PAINT
Sealing material, parts for plasma treating equipment having said sealing material, and process for preparing said sealing material
InactiveUS20100239867A1Improve plasma resistanceExcellent sealing propertyLiquid surface applicatorsOther chemical processesMetallurgyInorganic materials
The present invention provides a sealing material having excellent plasma resistance, sealing property and non-sticking property, parts for plasma treating equipment having the sealing material, and a process for preparing the sealing material. The sealing material comprises a fluoroelastomer sealing material and a coating film formed by using an inorganic material on the surface of the fluoroelastomer sealing material, wherein a ratio of weight reduction of the sealing material after dipping it in perfluoro tri-n-butylamine at 60° C. for 70 hours and then taking it out and drying it at 90° C. for 5 hours, at 125° C. for 5 hours, and then at 200° C. for 10 hours, is not more than 0.4% by weight.
Owner:DAIKIN IND LTD
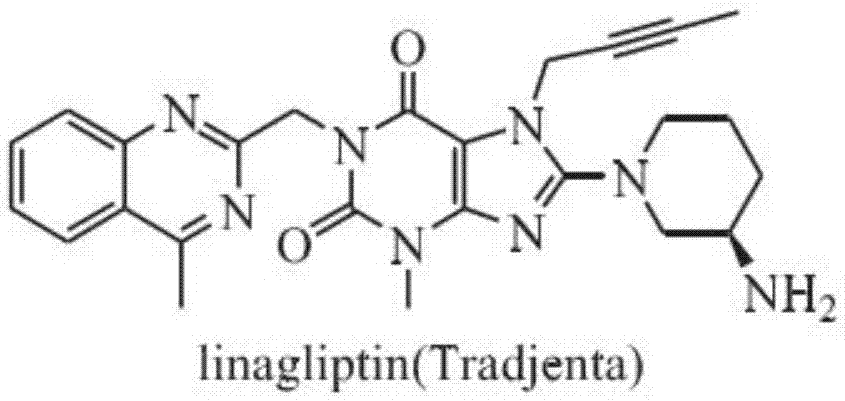
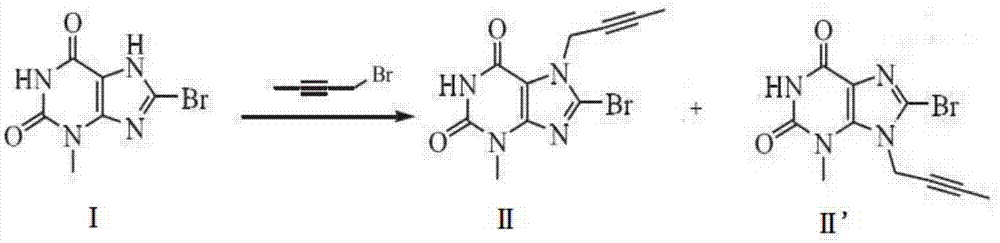
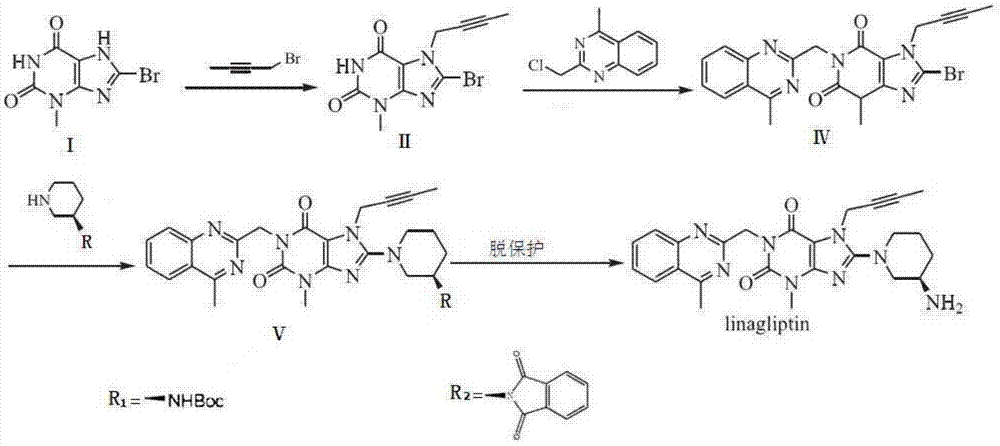
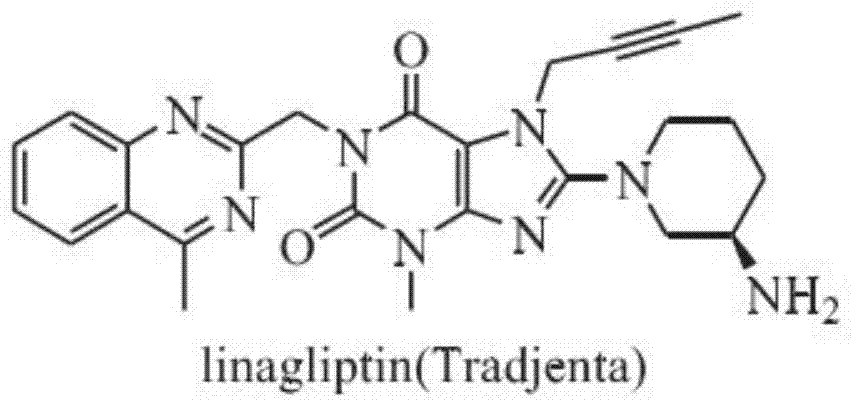
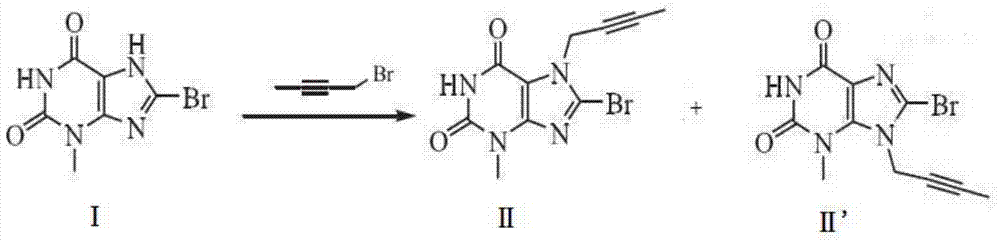
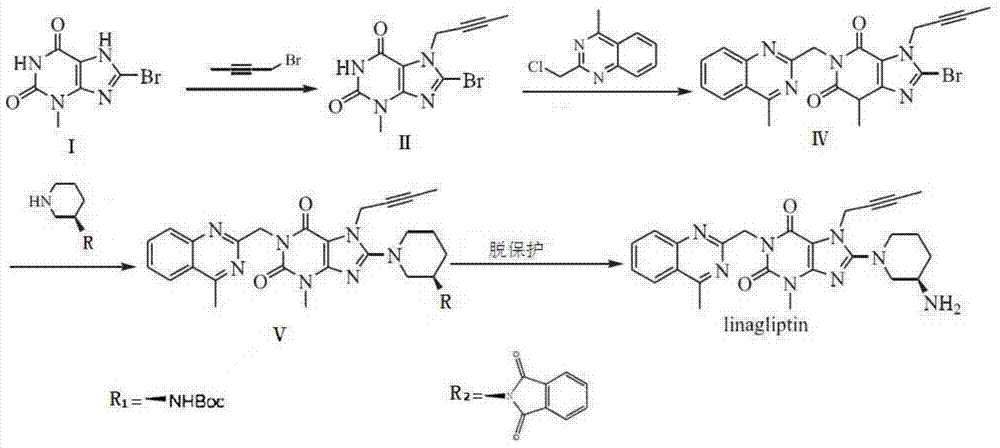
Preparation method of linagliptin and intermediate thereof
ActiveCN105440034ASolve the problem of simultaneous generation of N-9 and N-7 isomersMild reaction conditionsOrganic chemistrySolventTri-n-butylamine
The invention belongs to the field of pharmaceutical chemicals, and in particular relates to a preparation method of linagliptin and an intermediate thereof. The preparation method comprises the following steps: carrying out Mitsunobu reaction on a compound I and 2-butyne-1-alcohol in an organic solvent in the presence of trialkyl phosphine and an azo-reagent to obtain a linagliptin intermediate compound II; carrying out substitution reaction on the compound II and (R)-3-aminopiperdine by taking isopropanol as a solvent and tri-n-butylamine as an acid-binding agent to obtain a compound III; and carrying out alkylation reaction on the compound III and 4-methyl-2-chloro-methyl-quinazolin to obtain linagliptin. According to the preparation method provided by the invention, the Mitsunobu reaction is innovatively adopted to prepare the compound II, so that the preparation method has the advantages of mild reaction condition, reasonable operation, high selectivity, high product quality and the like. Moreover, by controlling the reaction condition, the compound III and R-3-aminopiperdine or an inorganic acid salt or an organic acid salt thereof are directly subjected to nucleophilic substitution to generate linagliptin, and the method does not have protection and de-protection processes of amino groups, so that the reaction steps are reduced, the reaction process is simpler, the cost is reduced, and the purity of an obtained product is relatively improved.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
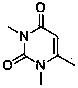
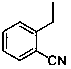
Synthesis method of alogliptin benzoate
ActiveCN104193726AMild reaction conditionsEasy to operateCarboxylic acid salt preparationBenzoic acidBenzoyl bromide
The invention discloses a synthesis method of alogliptin benzoate. The synthesis method comprises the following steps: putting 2-cyano benzyl bromide, 3-3metyl-6-chlorouracil and tri-n-butylamine in methylbenzene and stirring for reaction; cooling, adding water and stirring for crystallization; filtering and washing with water to obtain 2-(6-chlorine-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimdine-1-metyl)-benzonitrile; adding 2-(6-chlorine-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimdine-1-metyl)-benzonitrile, (R)-3-piperidinamine dihydrochloride and alkali into ethyl alcohol and stirring for reaction; purifying and salifying with benzoic acid to obtain alogliptin benzoate. The synthesis method disclosed by the invention is mild in condition, easy to control, non-toxic, environment-friendly, high in purity and high in yield.
Owner:JIANGSU DEYUAN PHARMA
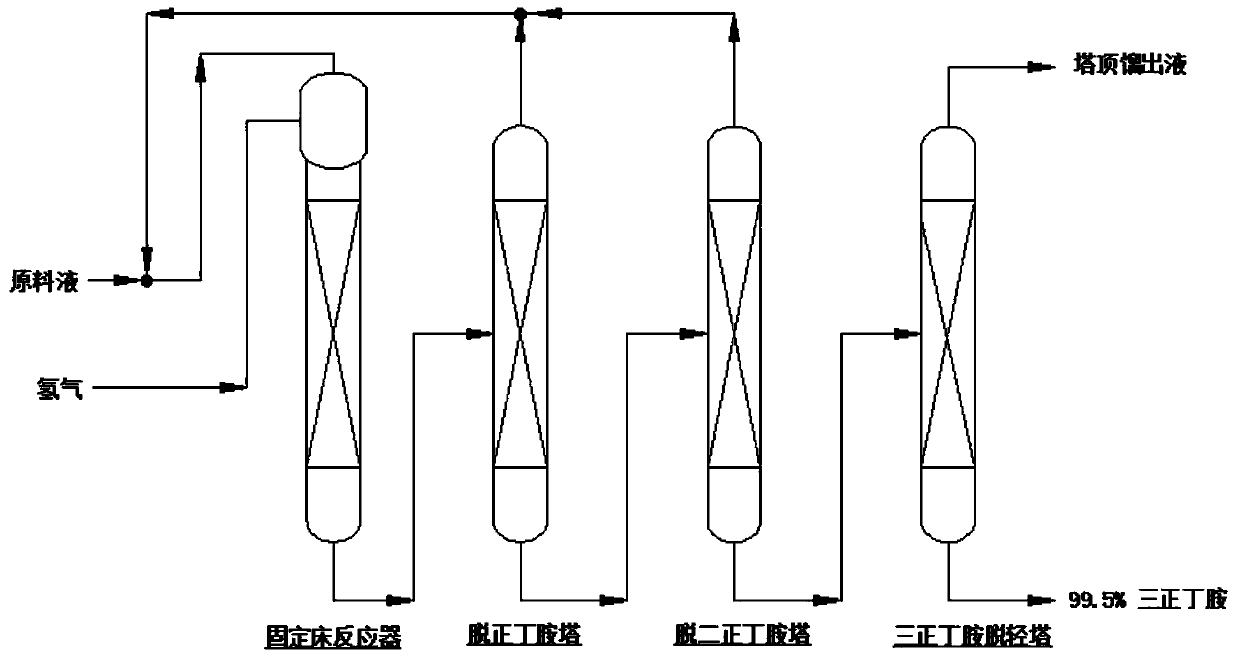
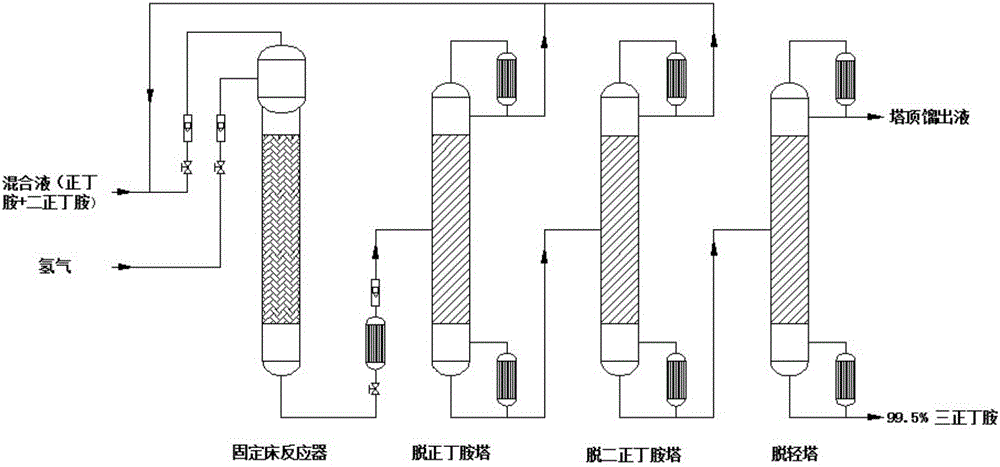
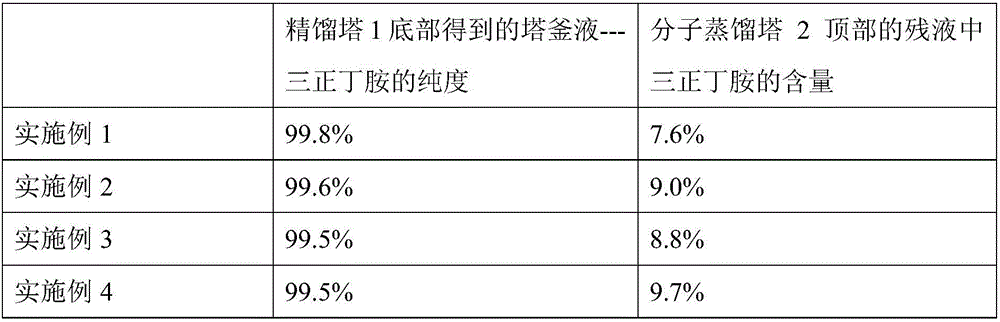
Device and method for producing tri-n-butylamine by extractive rectification heavy component removal
ActiveCN109694327AIncrease concentrationGuaranteed qualityAmino compound purification/separationDistillationFixed bed
The invention discloses a device for producing tri-n-butylamine by extractive rectification heavy component removal. The device comprises a fixed bed reactor, an n-butylamine removal tower, a di-n-butylamine removal tower, a tri-n-butylamine light component removal tower, a molecular distillation tower, an extractive rectification tower and an extractant regeneration tower; the bottom outlet of the molecular distillation tower, an extractant supplementing pipeline and the tower bottom liquid outlet of the extractant regeneration tower are joined by a cross tee, and then are communicated with the feed port of the extractive rectification tower; the top outlet of the molecular distillation tower is a light impurity outlet, and the tower top distillate port of the extractant regeneration tower is a heavy impurity outlet; and the tower bottle liquid outlet of the tri-n-butylamine light component removal tower is a tri-n-butylamine discharging tube. The present invention also discloses a method for producing tri-n-butylamine by extractive rectification heavy component removal through using the device. Heavy impurities (mainly including N-butylbutyramide) are discharged from the tri-n-butylamine production system in an extractive rectification manner, so the quality of the tri-n-butylamine product is ensured, and the energy consumption of the device is reduced.
Owner:ZHEJIANG JIANYE CHEM
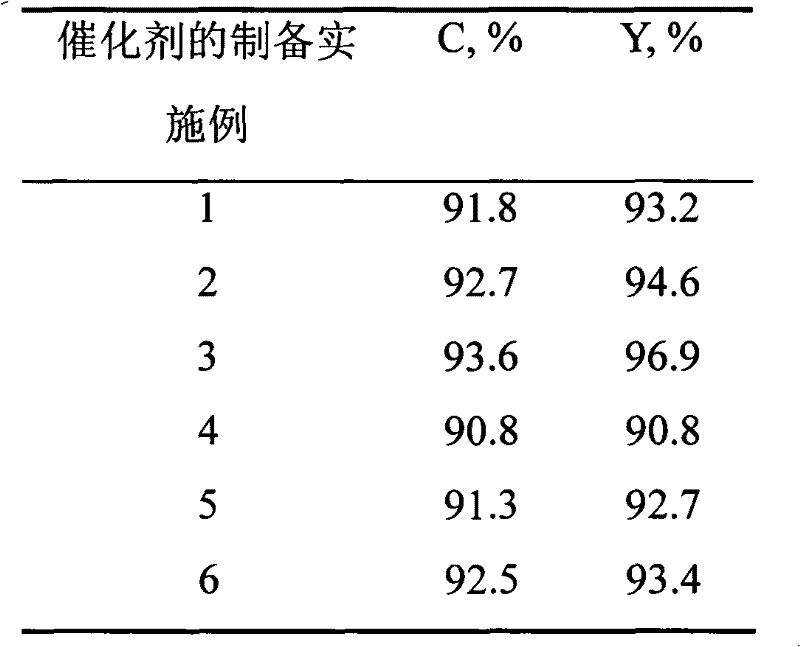
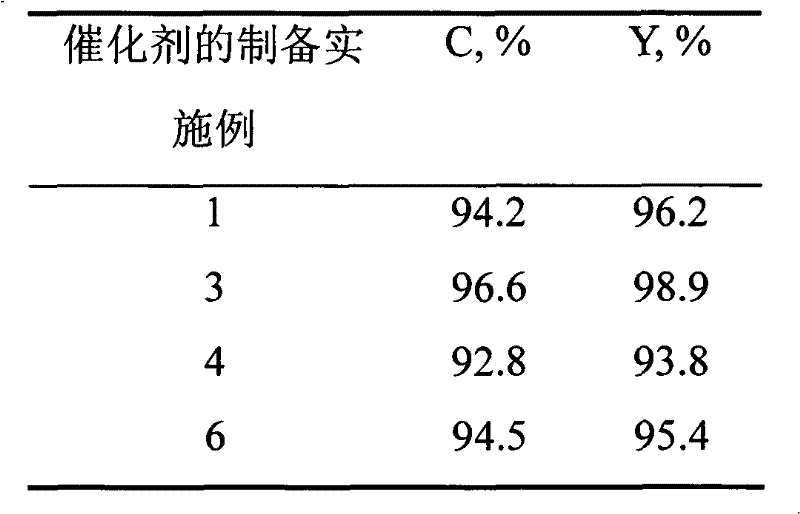
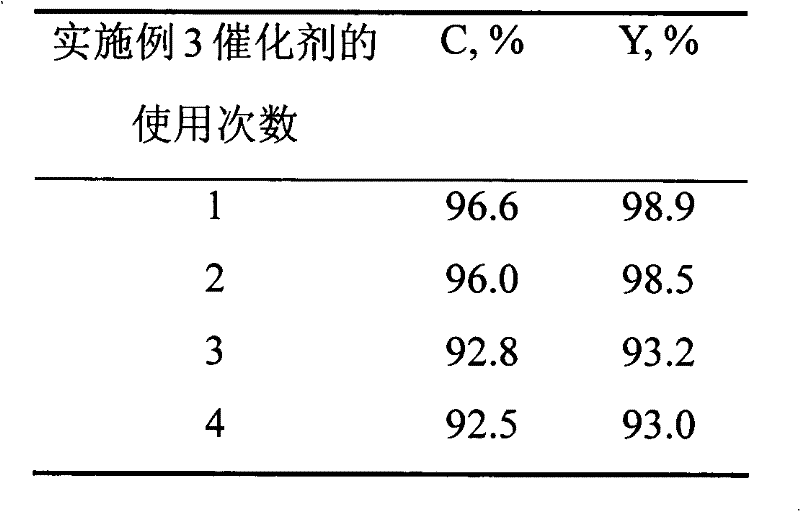
Method for pyrolysis of organosilicone high-boiling substance under catalysis of solid alkali
ActiveCN104130279AHigh activityGood compatibilityGroup 4/14 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalysts(Hydroxyethyl)methacrylateMethyltrichlorosilane
The invention discloses a method for pyrolysis of organosilicone high boiling substance under catalysis of solid alkali. The method comprises the steps of subjecting tri-n-butylamine, dioctadecyl amine, perfluorotriethylamine and macroporous crosslinked poly(hydroxyethyl methacrylate) resin to a load reaction so as to obtain a compound catalyst after the load reaction is finished; adding the organosilicone high-boiling substance and compound catalyst with impurities removed in a pyrolysis kettle with a fractionating tower; then introducing hydrogen chloride gas into the pyrolysis kettle; reacting to generate mixed products mainly including four monomers such as dimethyldichlorosilane, trimethylchlorosilane, methyldichlorosilane and methyltrichlorosilane; and condensing and collecting the above mixed products through a condenser at the top of the fractioning tower. The catalyst has good activity and can be used circularly, and the method has few three wastes (waste gas, waste water and industrial residue) and is environment-friendly.
Owner:山东时代新材料科技有限公司
Organic luminescent material phenylene ethynylene dimmer, synthesizing method and use
The invention provides a 2-polyphenylene ethynylene luminescent material of organic molecules as well as a preparation method and an application thereof; the preparation method comprises the steps that: paratrimethyl silicane acetylene iodobenzene substituted by short chain alkyls is taken as a raw material; the paratrimethyl silicane acetylene iodobenzene substituted by short chain alkyls, and para ethanethioyl phenylacetylene conduct a Sonogashira coupling reaction in tetrahydrofuran for 20 hours to 50 hours with 2-(triphenylphosphine) palladium chloride / cuprous iodide as a catalyst and under the action of tri-n-butylamine to obtain phenylene ethynylene dipolymer series; the phenylene ethynylene dipolymer series can be applied to the luminescent layer or the electron transport layer of the organic LED.
Owner:NANJING UNIV OF TECH
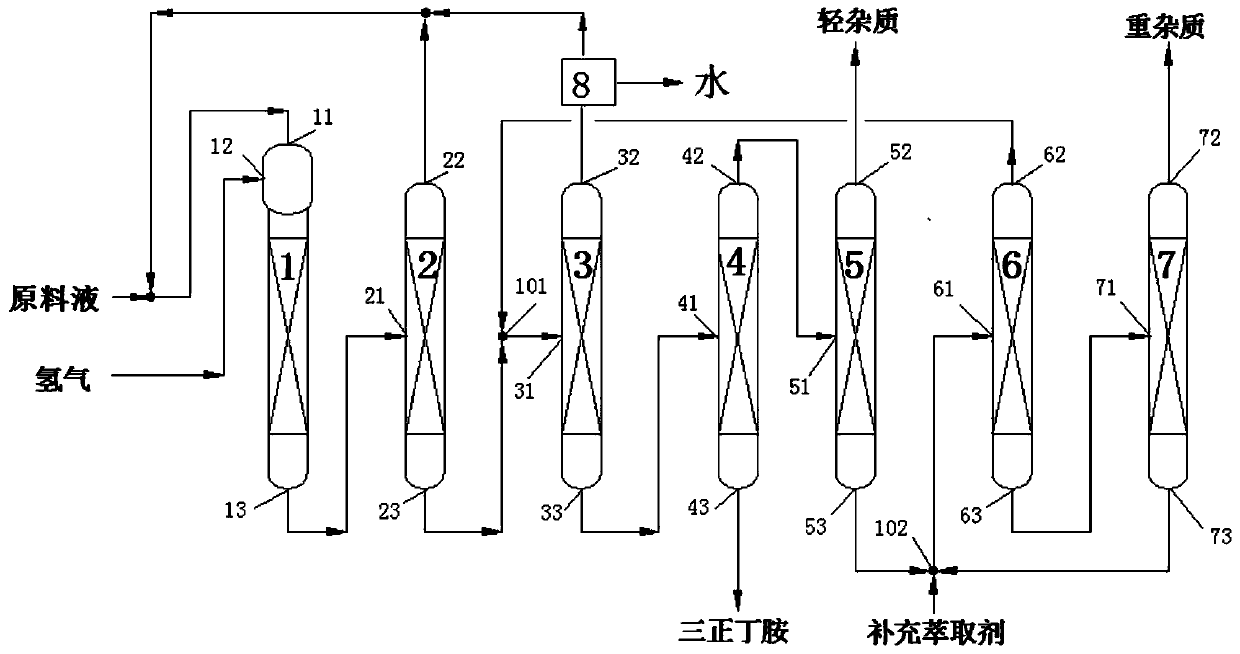
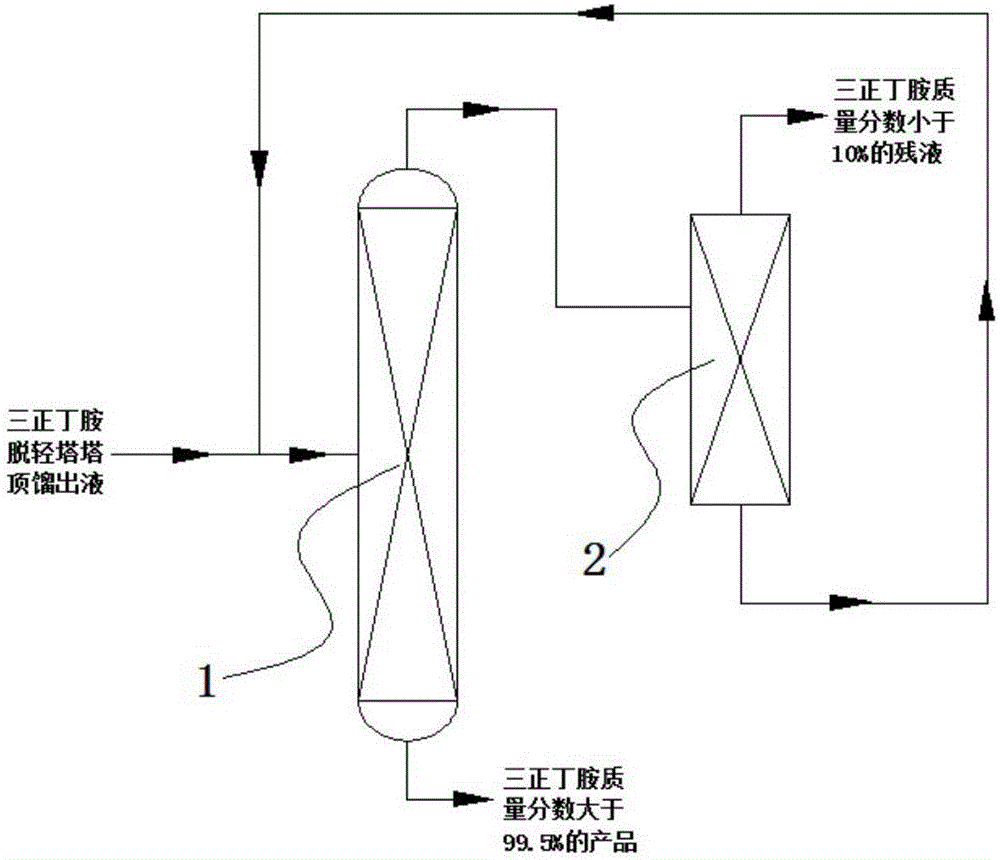
Tri-n-butylamine recycling method and recycling device used thereby
ActiveCN106748811AAmino compound purification/separationDistillation regulation/controlDistillationProcess engineering
The invention discloses a tri-n-butylamine recycling device and a tri-n-butylamine recycling method implemented by using the recycling device. The method is as follows: taking tower top distillate of a tri-n-butylamine light-component removing tower as a raw material at the starting time, and the tower top distillate of the tri-n-butylamine light-component removing tower and tower kettle liquid of a molecular distillation tower are mixed to serve as a mixed raw material at the subsequent time; the volume ratio of the tower top distillate of the tri-n-butylamine light-component removing tower to the tower kettle liquid of the molecular distillation tower in the mixed raw material is (1-5):1; the raw material / mixed raw material enters a rectifying tower from a feeding port of the rectifying tower to perform rectification, and tower kettle liquid obtained from the bottom of the rectifying tower is tri-n-butylamine; tower top distillate of the rectifying tower enters the molecular distillation tower from a feeding port of the molecular distillation tower to perform molecular distillation; and residual liquid is obtained from the top of the molecular distillation tower, and the tower kettle liquid of the distillation tower is obtained from the bottom of the molecular distillation tower.
Owner:ZHEJIANG JIANYE CHEM +1
Ionic liquid supported molecular sieve catalyst for synthesizing biodiesel fuel and preparation method of catalyst
ActiveCN102513155AFatty acid esterificationOrganic-compounds/hydrides/coordination-complexes catalystsBiodieselPtru catalyst
The invention discloses an ionic liquid supported molecular sieve catalyst for synthesizing biodiesel fuel and a preparation method of the ionic liquid supported molecular sieve catalyst. Carriers of the ionic liquid supported molecular sieve catalyst comprise a chloropropyl functionalized HMS(CP-HMS) molecule sieve and a chloropropyl functionalized MCM-41(CP-MCM-41) molecule sieve, and a component needed by supporting is triethylamine (TEA), tripropylamine (TPA) or tributylamine (TBA). The preparation method comprises the steps of: in a process of synthesizing the molecular sieves, carrying out chloropropyl functionalization on the molecular sieves, synthesizing the ionic liquid by using the TEA and the functionalized molecular sieves in a tetrahydrofuran solvent to prepare the catalyst. The invention has the advantages that the prepared catalyst is used for synthesizing the biodiesel fuel, has high activity, can be repeatedly use, and can be expected to be a competitive clean process route.
Owner:JIANGSU JINMA OIL TECH DEV
Anti-rust drawing oil and preparation method thereof
InactiveCN102899148AMeet the needs of lubricationMeet the needs of anti-rustAdditivesEnvironmental resistanceBenzotriazole
The invention belongs to the fields of hardware product lubrication and anti-rust technologies, and discloses anti-rust drawing oil and a preparation method thereof. The drawing oil consists of the following components in percentage by weight: 7 percent of barium petroleum sulfonate, 1 percent of benzotriazole tri-n-butylamine, 1-2 percent of lead naphthenate, 15-25 percent of vaseline and 65-76 percent of diesel oil. The drawing oil can meet the requirement of lubrication during stamping (such as drawing or bending), and can also meet the anti-rust requirement between the steps; the anti-rust period of a stamped workpiece is over seven days; and rust protection between the steps is not required additionally. The preparation method comprises the steps of: directly mixing the raw materials; heating through a heating tube; mechanically stirring; and collecting. The preparation method has the advantages of easiness, convenience, environmental friendliness, low cost and easiness in implementation.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing methyl chlorosilane by utilizing organosilicon high-boiling residues
InactiveCN102153581AHigh selectivitySimple processGroup 4/14 element organic compoundsDimethylaniline N-oxideMonomer
The invention relates to a method for preparing methyl chlorosilane by utilizing organosilicon high-boiling residues, which is realized in a way that: full components of high-boiling residues, which are the byproducts in the production process of organosilicon monomer dimethyl dichlorosilane, are used as the raw material, and react with chlorine hydride in a cracking kettle provided with a fractionating tower under the catalytic action of N,N-dimethylaniline and tri-n-butylamine to generate a monomer mixture mainly comprising dimethyl dichlorosilane, trimethyl monochlorosilane, monomethyl dichlorosilane and monomethyl trichlorosilane, and the monomer mixture is condensed and collected by a condenser on the top of the fractionating tower. The method can increase the conversion rate of high-boiling residues (greater than 90%), and produce organosilicon monomers at low cost; and the selectivity of the dimethyl dichlorosilane is up to higher than 50%, and the selectivity of the trimethyl monochlorosilane is up to 10%.
Owner:于淑芳
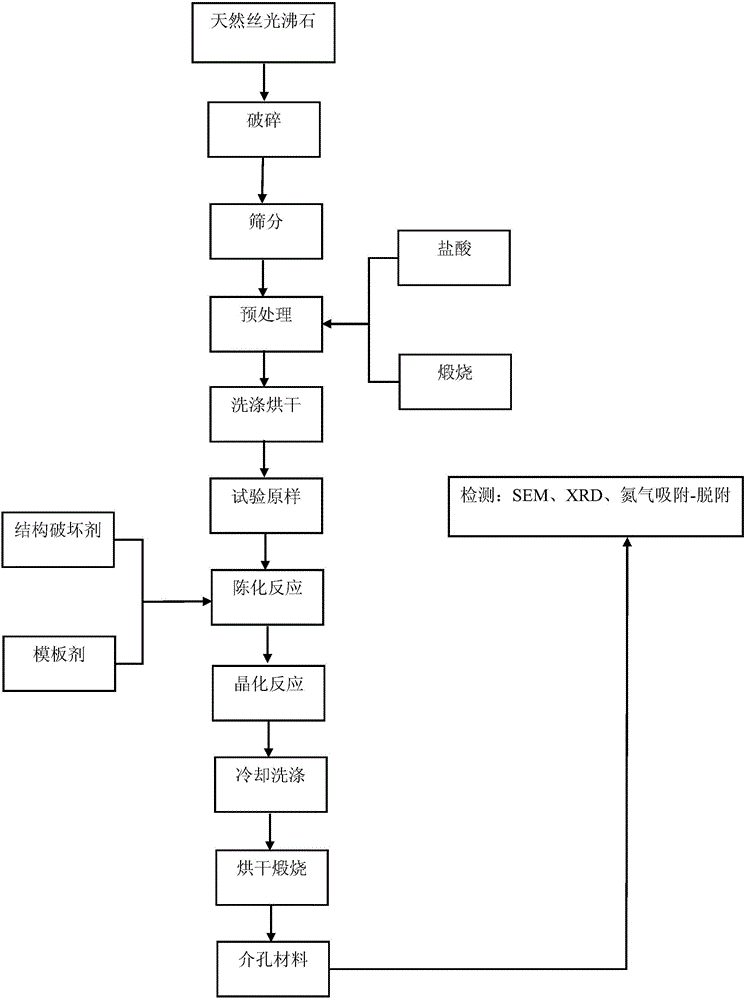
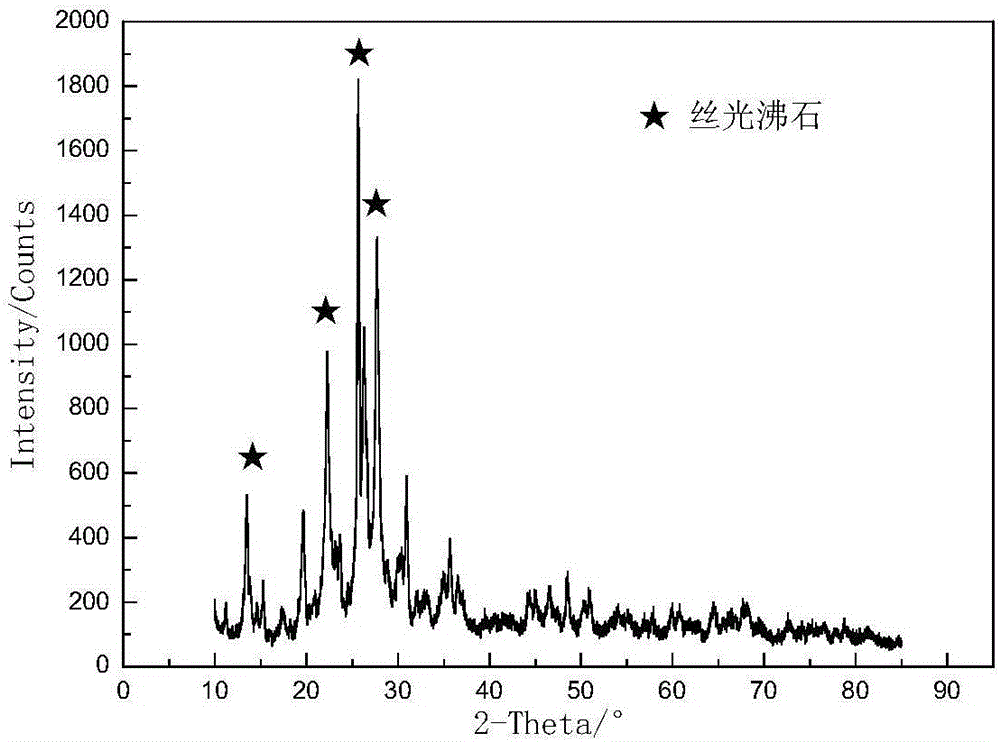
Method for preparing meso-porous material from natural mordenite
ActiveCN105948071AGood acid and alkali resistanceImprove performanceAluminium silicatesCrystalline aluminosilicate zeolitesWater bathsAlkalinity
The invention discloses a method for preparing a meso-porous material from natural mordenite, and belongs to the field of mineral processing. The method comprises the following steps: 1, breaking natural mordenite, calcining the broken natural mordenite, immersing the calcined natural mordenite in HCl, and drying the immersed calcined natural mordenite to obtain internal tunnel cleaned natural mordenite particles; 2, mixing the natural mordenite particles, an ammonium fluoride solution, a sodium hydroxide solution and a tri-n-butylamine solution, and carrying out constant-temperature water bath ageing; 3, crystallizing the obtained aged solution; and 4, washing the obtained crystallized solution until the crystallized solution is neutral, carrying out pumping filtration drying, and calcining the obtained material to prepare the meso-porous material. The method allows the meso-porous material with good alkalinity or acidity to be prepared from zeolite; and the method has the advantages of simple production process and device, high product yield, low cost, and easy realization of industrial production.
Owner:LIAONING TECHNICAL UNIVERSITY
External protective coating layer of caterpillar bands
InactiveCN107629653AImprove wear resistancePolyurea/polyurethane coatingsFilm-forming agentCaterpillar
The invention provides an external protective coating layer of caterpillar bands. The external protective coating layer of caterpillar bands comprises, by weight, 50 to 80 parts of polyurethane resin,5 to 12 parts of mica powder, 2 to 6 parts of a surfactant, 10 to 15 parts of diatomite, 3 to 5 parts of tri-n-butylamine, 5 to 9 parts of a film forming agent, and 1 to 3 parts of an antifoaming agent. The external protective coating layer is capable of improving the wear resistance of caterpillar bands greatly.
Owner:ZHENJIANG SHEN WARD MACHINERY CO LTD
Waterproof and antirust coating for metal
InactiveCN106147591AThe appearance of the coating film is smooth and brightEasy to prepareAnti-corrosive paintsPolyamide coatingsPolyamideMaterials science
The invention discloses a waterproof and antirust paint for metal. The raw materials are: water, silver paste powder, graphite powder, polyamide, tri-n-butylamine, wollastonite, potassium dichromate, ammonium molybdate, titanium dioxide and carbonic acid Calcium; used for anti-rust and anti-corrosion of medical appliances, the coating film has a smooth and bright appearance, and has a viscosity of 60-80s; first-class adhesion, no abnormality in salt spray resistance for 10-30d, water resistance for 20-60d without foaming or falling off; salt resistance The properties are 30-50d without abnormality, the surface dryness is 1-4h, and the actual dryness is 18-22h, the preparation method is simple, the raw materials are simple and easy to obtain, and it can be widely produced and continuously replace the existing materials.
Owner:任新根
A kind of preparation method of linagliptin and its intermediate
ActiveCN105440034BMild reaction conditionsEasy to operateOrganic chemistrySolventNucleophilic substitution
The invention belongs to the field of pharmaceutical chemicals, and in particular relates to a preparation method of linagliptin and an intermediate thereof. The preparation method comprises the following steps: carrying out Mitsunobu reaction on a compound I and 2-butyne-1-alcohol in an organic solvent in the presence of trialkyl phosphine and an azo-reagent to obtain a linagliptin intermediate compound II; carrying out substitution reaction on the compound II and (R)-3-aminopiperdine by taking isopropanol as a solvent and tri-n-butylamine as an acid-binding agent to obtain a compound III; and carrying out alkylation reaction on the compound III and 4-methyl-2-chloro-methyl-quinazolin to obtain linagliptin. According to the preparation method provided by the invention, the Mitsunobu reaction is innovatively adopted to prepare the compound II, so that the preparation method has the advantages of mild reaction condition, reasonable operation, high selectivity, high product quality and the like. Moreover, by controlling the reaction condition, the compound III and R-3-aminopiperdine or an inorganic acid salt or an organic acid salt thereof are directly subjected to nucleophilic substitution to generate linagliptin, and the method does not have protection and de-protection processes of amino groups, so that the reaction steps are reduced, the reaction process is simpler, the cost is reduced, and the purity of an obtained product is relatively improved.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Method for cracking treatment of organic silicon waste residue slurry
The invention relates to method for the cracking treatment of waste residues slurry produced in a synthesis process of an organic silicone monomer. The comehtod comprises: adding a high-boiling material of the same mass into a organic silicon waste residue slurry liquid and solid mixture containing 20 percent of solid to prepare a cracking feed solution, wherein a catalyst is tri-n-butylamine andthe cracking reaction temperature is 80 to 160 DEG C; and introducing HCl gas, wherein the ratio of the feed speeds of the hydrogen chloride and the raw material mixed liquid is 1:1.05-1:1.12. The method has the advantages that: the residue slurry containing 20 percent of solid is used in combination with the high-boiling material, so a monosilane product can be separated directly in a cracking process; the conversion rate is over 70 percent; the selectivity of dimethyldichlorosilane is over 35 percent; the selectivity of methylsilane is over 40 percent; and the slurry residue obtained after cracking having certain liquidity and can be further treated.
Owner:PETROCHINA CO LTD
Rubber mold coating and preparation method thereof
InactiveCN107805454ANot prone to depositionAvoid it happening againCoatingsPolymer scienceLiquid state
The invention discloses a rubber mold coating and a preparation method thereof. The rubber mold coating comprises the following components in parts by weight: 100-150 parts of liquid state polysiloxane, 20-30 parts of titanium dioxide powder, 10-25 parts of ferroferric oxide powder and 0.2-0.4 part of tri-n-butylamine. Due to adoption of the liquid state polysiloxane and the titanium dioxide powder, sulfide deposition is not easily generated on the surface of a mold; due to adoption of the titanium dioxide powder and the tri-n-butylamine, the rubber demolding convenience is improved. The rubber mold coating is prepared by using a special method, so that the coating is prevented from a great number of bubbles, and adverse results in the spraying process are avoided. The preparation method disclosed by the invention is reasonable in step, feasible to operate and easy in on-scale production.
Owner:荣成市华诚橡胶有限公司
Purification method for removing diethyl ether from trimethyl antimony
ActiveCN113831367AEfficient removalImprove reaction efficiencyAntimony organic compoundsProcess efficiency improvementEthyl groupDiethyl ether
The invention provides a purification method for removing diethyl ether from trimethyl antimony, the method comprises the following steps: using one of trimethylaluminum, trimethyl indium and triethyl gallium as an additive I, forming a high-boiling-point complex with diethyl ether, distilling and collecting the trimethyl antimony and a small amount of the additive I, then adding one of high-boiling-point tri-n-octylamine, tri-n-butylamine, MBDA and crown ether as an additive II to coordinate with the additive I to form a high-boiling-point coordination compound, and finally rectifying and purifying to obtain the 6N trimethyl antimony product. The method is low in cost, high in reaction efficiency and high in safety performance, diethyl ether can be effectively removed, harsh reaction conditions are not needed, and the operation is simple.
Owner:安徽亚格盛电子新材料有限公司
Antirust agent for coating
The invention relates to an antirust agent for a coating, which is characterized by containing the following components in parts by weight: 5-20 parts of citric acid, 5-30 parts of sodium silicate, 4-16 parts of ammonium nitrate, 10-20 parts of silicon dioxide particles, 10-20 parts of tri-n-butylamine, 5-25 parts of nitrilo-triethanolamine, and 10-50 parts of water. The antirust agent for a coating, prepared according to the invention, has excellent antirust property.
Owner:ZHENJIANG TAILANG NEW MATERIAL TECH CO LTD
DOT4 boric acid ester type brake fluid
ActiveCN101418249BSolve the problem of long-term storage hydrolytic stabilityAdditivesBase-materialsMorpholineTert butyl
The invention discloses a DOT4 boric acid ester type brake fluid, which comprises the following components in weight percentage: 4.3 to 4.7 percent of triethylene glycol methyl ether, 15 to 17 percent of tetraethylene glycol methyl ether, 1.9 to 2.1 percent of diethylene glycol, 74 to 76 percent of alcohol ether borate, 0.19 to 0.21 percent of benzotriazole, 0.33 to 0.37 percent of N-phenyl morpholine, 0.76 to 0.84 percent of tri-n-butylamine, 0.57 to 0.63 percent of 2, 2-di (4-hydroxyl phenyl) propane (bisphenol A), 0.057 to 0.063 percent of thiodipropionic acid didodecyl alcohol ester, 0.19to 0.21 percent of 2.6-di-tert-butyl-4-methyl phenol, 0.095 to 0.105 percent of mercapto styrene-acrylic thiazole, and 0.38 to 0.42 percent of dodecatylene butane diacid (T746). The DOT4 boric acid ester type brake fluid has the advantages that the DOT4 boric acid ester type brake fluid can thoroughly solve the problem of hydrolytic stability of boric acid ester for long term storage by using a formulation technology of combining organic substances and additives.
Owner:张家港迪克汽车化学品有限公司
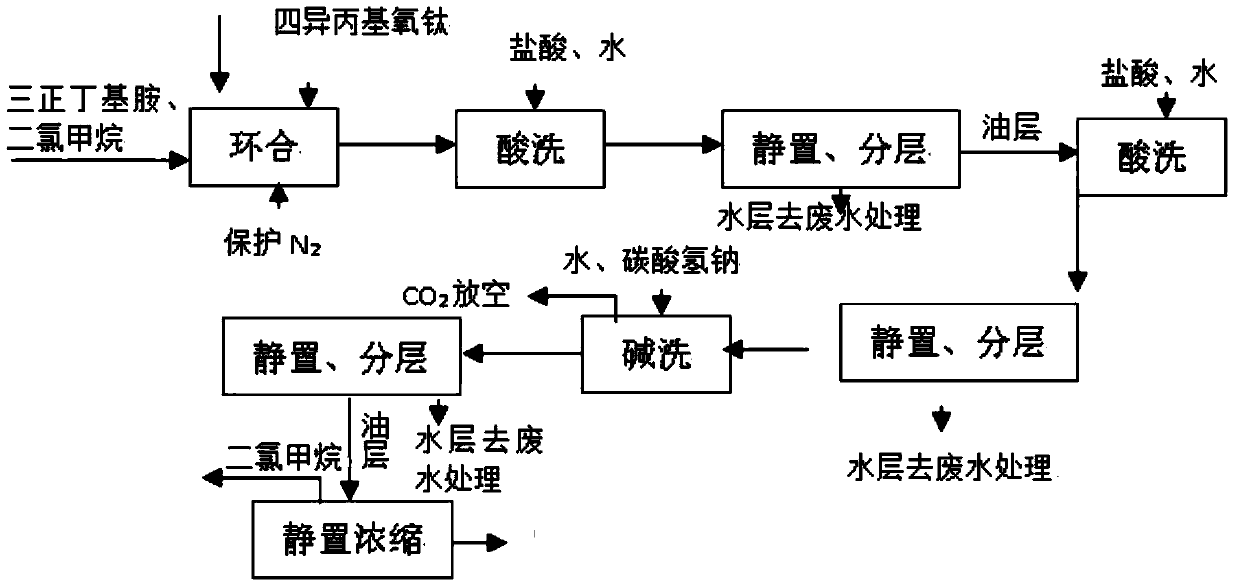
Method for improving N-(4-methoxyphenyl)-4-acetyl-3-(1-hydroxyl)ethylazetidinone-2 synthesis conversion rate
A disclosed method for improving N-(4-methoxyphenyl)-4-acetyl-3-(1-hydroxyl)ethylazetidinone-2 synthesis conversion rate comprises: under the protection of nitrogen, successively adding tri-n-butylamine and dichloromethane, controlling the temperature at -35 DEG C to -25 DEG C, adding titanium tetraisopropanolate, then adding an amide solution at -35 DEG C to -25 DEG C and keeping warm for 5 h, adding hydrochloric acid with the mass percent of 31% and the molar quantity equal to that of the amide into the reaction solution, controlling the temperature at -5 DEG C, stirring the material solution for 30 min, standing, layering, performing wastewater removing processing on the acidic water layer, and again adding hydrochloric acid with the mass percent of 31% and the molar quantity equal to that of the amide into the organic layer for secondary acid cleaning, standing and layering, performing wastewater removing processing on the acidic water layer, transferring the organic layer to a washing pan, then adding sodium bicarbonate and water for base cleaning, stirring for 1 h, standing for 1 h, layering, performing wastewater removing processing on the water layer, performing reduced-pressure distillation on the organic layer, concentrating to obtain a solid, then adding DMF to dissolve for direct next-step usage. According to the method, the reaction time is shortened, and the main reaction conversion rate is improved by 10-13%.
Owner:JIANGSU RUIKE MEDICAL SCI & TECH CO LTD
Organic luminescent material 9-(4-aryl ethynyl phenyl) carbazoles compounds, synthesis method and uses
The present invention provides an organic luminescent material 9-(4-aryl ethynyl phenyl) carbazoles, prepartion method and uses. The invention uses 9-(4-iodo-benzene) carbazole or derivates thereof as raw materials, under the action of tri-n-butylamine, using dual-triphenylphosphine palladium dichloride / cuprous iodide as catalyst to proceed a Sonogashira coupling reaction with the terminal aryne in tetrahydrofuran for 20 to 50 hours to obtain series of 9-(4-aryl ethynyl phenyl) carbazoles compounds. The compounds can by used as organic luminescent materials.
Owner:NANJING UNIV OF TECH
Method for Purification of Optically Active alpha-Fluorocarboxylic Acid Esters
ActiveUS20110021809A1Easily and efficiently reduceEasy and efficient removalOrganic compound preparationCarboxylic acid esters preparationPurification methodsDistillation
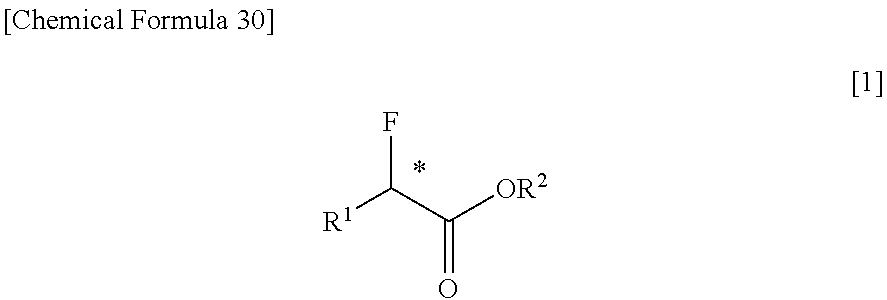
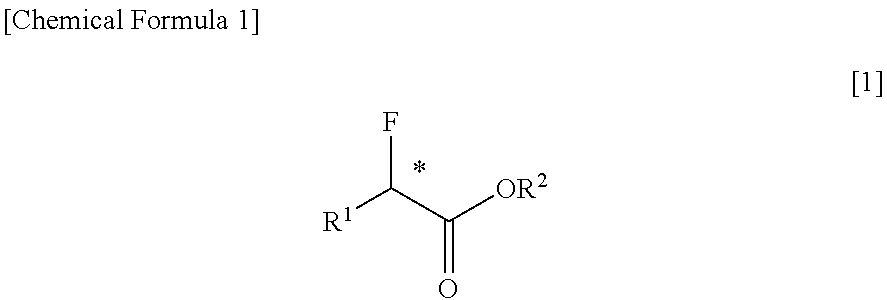
Disclosed is a purification method of reducing and removing fluoride ions contained in an optically active α-fluorocarboxylic acid ester represented by formula [1][in the formula, R1 represents a C1-6 alkyl group, R2 represents a C1-4 alkyl group, and * represents an asymmetric carbon], the purification method of the optically active α-fluorocarboxylic acid ester being characterized by that a distillation is conducted in the presence of an organic base. By this method, it is possible to greatly reduce the concentration of fluoride ion traces contained in the optically active α-fluorocarboxylic acid ester by a relatively easy operation. Of the organic base, a tertiary amine is preferable, and above all tri-n-butylamine is particularly preferable.
Owner:CENT GLASS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com